Abstract
Pediatric intracranial ependymoma has seen a recent exponential expansion of biological findings, rapidly dividing the diagnosis into several subgroups, each with specific molecular and clinical characteristics. While such subdivision may complicate clinical conclusions from historical trials, this knowledge also provides an opportunity for interrogating the major clinical and biological questions preventing near-term translation into effective therapy for children with ependymoma. In this article, we briefly review some of the most critical clinical questions facing both patient management and the construct of future trials in childhood ependymoma, as well as explore some of the current barriers to efficient translation of preclinical discovery to the clinic.
Keywords: Pediatric brain cancer, Childhood ependymoma, Translational barriers, Anaplasia, Chemotherapy, Radiation
Introduction
The management of childhood intracranial ependymomas remains an ongoing challenge for pediatric neuro-oncologists. Although these tumors are relatively common, their heterogeneity, in both clinical presentation and biology, has clouded best practice guidelines and left some clinical questions unanswered. The ongoing molecular classification of ependymomas, which has allowed for the correlation of molecular findings to clinical outcomes, suggests that subsets of these tumors may require radically different therapeutic approaches.
While the current ambiguity in treatment guidelines may understandably create apprehension for both families and providers, the recent discoveries in ependymoma also present an opportunity to dramatically shift the landscape of these challenging tumors. Through better understanding of ependymoma biology, careful analysis of clinical data, and thoughtful trial design, physicians and researchers can shape the future treatment of ependymomas and provide evidence-based care that is uniquely tailored to each individual case. Comprehensive biological and clinical reviews have been recently published and are beyond the scope of this article; [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11] instead, this review, from the Pacific Pediatric Neuro-oncology Consortium (PNOC) ependymoma working group, will aim to briefly highlight some of the clinical and biological questions currently being actively investigated and describe some barriers to translational therapeutic efforts.
General background
Ependymoma is the third most common pediatric central nervous system (CNS) tumor, accounting for 8-10% of all diagnoses [12], and occurring most frequently in the posterior fossa, followed by the supratentorial area and, less commonly, the spinal cord [13]. Unfortunately, outcomes for ependymomas are suboptimal, with five-year progression-free and overall survival of 23-45% and 50-64%, respectively [14], [15], [16], [17]. The current standard of care for ependymoma is maximal surgical resection followed by radiotherapy to the primary site. Management, outcomes, and tumor biology are distinct from adult tumors, typically with more rapid and prevalent recurrence, which may be delayed although portend poor outcomes.
Tumor location may play an important role in patient outcomes due to its impact on neurosurgical management as well as location-associated molecular drivers that determine the biological aggressiveness of these tumors [12]. Due to difficulties with obtaining complete surgical resection, ependymomas arising from the floor and the lateral aspect of the fourth ventricle have a worse prognosis than those arising from the roof [8]. Supratentorial ependymomas are typically seen in younger patients (age 0-12 years) and grow more uncommon with age [18], but with an increased ability to achieve gross-total resection, as well as distinct biological drivers, they are associated with an improved prognosis [8]. Because of the strong impact of extent of resection on prognosis, second look surgeries or cytoreductive chemotherapy should strongly be considered to achieve a complete response, especially as, upon review, they do not appear to increase mortality or permanent morbidity and provide an avenue to significantly reduce tumor volume [19]. Following surgical resection of their ependymoma, patients are commonly treated with radiotherapy with possible adjuvant chemotherapy, although some patients with completely resected, more indolent entities such as supratentorial ependymomas or posterior fossa B-type tumors may be able to be managed with observation alone [2].
Over the past decade, molecular features have revealed substantial differences in driver biology and epigenetic characteristics, leading to significant revisions in tumor stratification and identification of novel potential targets. While a comprehensive review of the current landscape is best approached in already-published articles [1], currently there exist at least six different intracranial subtypes in pediatrics, including posterior fossa ependymoma group A (PFA or PF-EPN-A), or B (PFB or PF-EPN-B), supratentorial ependymoma YAP1 (ST-EP-YAP1), supratentorial ependymoma RELA (ST-EP-RELA, now termed ZFTA), and both posterior fossa or supratentorial subependymoma [20]. Some subtypes are particularly distinctive in their outcomes or biology. For instance, within the posterior fossa, distinct cytogenetic patterns have been associated with prognostic outcomes. Tumors with a chromosome 1q gain and other partial structural chromosomal alterations have a worse prognosis, while those displaying a balanced profile without alterations have a slightly better prognosis [8,[21], [22], [23]]. These findings have, for example, opened the possibility for distinct treatment strategies that may achieve improved efficacy and reduce the significant morbidity associated with ependymoma treatment.
There is currently no standard of care for recurrent ependymoma in children. Children who receive incomplete resections of their tumor are at much higher risk for recurrence than those who receive a gross-total resection. Treatment strategies for children with recurrent ependymoma include re-resection and re-irradiation when feasible. [22] Chemotherapy has typically not demonstrated significant benefit in patients with recurrent tumor. [23] There have been documented long-term responses to metronomic anti-angiogenic regimens, but these treatments are generally not felt to be curative [24,25]. As understanding of the molecular and biologic features of pediatric ependymoma improves, targeted therapies for relapsed ependymoma may be identified and investigated through early-phase clinical trials.
Current questions in pediatric ependymoma management
As understanding of the heterogeneity of ependymoma matures, questions about interpretation of clinical results lacking molecular annotation present an obstacle to subtype-specific treatment strategies. Interpretation of small and large retrospective studies containing molecular annotation but lacking a cohesive common treatment strategy also present difficulties regarding sweeping conclusions in appropriate management. In the following section, a few of the more commonly debated questions will be addressed.
Chemotherapy: is it helpful in ependymoma?
Chemotherapy has long held a prominent role in the treatment of childhood cancer; however, its utility in ependymoma has been questioned. Historically, although not in the context of known molecular subtypes, several general chemotherapy-induced responses generated hope for potential benefit. The Children's Cancer Group (CCG) CCG9942 study showed that many of the 34 patients with residual disease who received pre-radiation chemotherapy (cisplatin, vincristine, cyclophosphamide, etoposide) had significant responses (42% complete response, 18% partial response, 26% stable disease), although in this study 15% progressed prior to irradiation; further, the 3-year event-free survival of these patients (58%) was not significantly different from patients who received a gross total resection (GTR) followed by irradiation (62%). [24] These results were replicated in the International Society for Pediatric Oncology (SIOP) Ependymoma I trial, with a 65% response rate in newly diagnosed, subtotally-resected ependymoma. [25] Other studies likewise demonstrated some promise, primarily to platinum-based chemotherapy regiments, although these were typically in small series of young patients seeking to avoid irradiation [26,27]. One of the more promising was the Pediatric Oncology Group (POG) 9233/34 study in infants with ependymoma (n=82), demonstrating a 2-year improved event free survival (EFS) with chemotherapy, 42.1% vs 19.6%; [28] together, these studies highlighted the possibility of chemosensitivity, especially as a tool to obtain complete response (and subsequent improved EFS), or to delay irradiation in very young children.
Conversely, other studies have failed to demonstrate a significant benefit of chemotherapy. In cases of first recurrence, 138 patients in the German HIT-REZ studies (HIT-REZ 97 and HIT-REZ 2005) failed to demonstrate response to over 40 different chemo- and targeted agents of systemic therapy, including high-dose chemotherapy with autologous stem cell rescue, although some responses were recorded. [29] Similarly, several small case series demonstrated only small numbers of responses in children with relapsed ependymoma, with roughly 10% of patients exhibiting response. [30]
Thus, while evidence of response in some patients exists, data regarding actual progression-free or overall survival benefit remains elusive. This analysis is further complicated by the clusters of late relapses which may fall outside the survival windows for historical study outcomes. While initial reports from the Children's Oncology Group (COG) study ACNS0831, which enrolled 451 eligible patients and randomized to receipt of maintenance chemotherapy, showed that there might be a potential role for chemotherapy in some patients, subsequent analysis is required for definitive conclusions across the entire cohort and within subgroups. [6] These study results, which employed molecular phenotyping in a prospective trial, are eagerly awaited.
In conclusion, use of chemotherapy to obtain complete response prior to irradiation (with the known caveat of potential interval progression) or in the context of a radiation-delaying or sparing strategy in infants seem to have some consensus utility; however, chemotherapy's use as a consolidative strategy is less clear. ACNS0831 results combined with an evolving understanding of molecularly high-risk ependymoma, however, may push some providers to offer either intensive or metronomic chemotherapy, although the data to support their use currently remains limited.
Anaplasia: controversy in ependymoma
Anaplastic histology has variably been supported in past clinical trials as a marker of high-risk disease. This was in large part due to considerable literature describing anaplastic ependymoma as having worse clinical outcomes, leading to the incorporation of anaplasia as a high-risk stratification marker [31], [32], [33], [34] in clinical care as well as in prospective trial design. However, with the advent of molecular phenotyping and the separation of ependymoma into compartment- and biologically-relevant groups, the utility of anaplasia as an independent risk factor has been debated.
One motive for continued review was concern about reproducibility. In a retrospective study, five separate experienced neuropathologists reviewed the pathology of 229 children with ependymoma enrolled on four separate European trials (SIOP CNS9204, CNS9904; Societe Francaise d'Oncologie Pediatrique (SFOP), Assoociazone Italiana Ematologia Oncologia Pediatrica (AIEOP)). [35] This study showed significant variability in calling grade 2 versus grade 3 amongst the expert neuropathologists, reinforcing concerns about the reliability of a histopathological characteristic which may not be reliably reproduced. Subsequent recommendations by several neuropathology teams leaned heavily towards location- and molecularly-based assessment.
The 2021 World Health Organization has abandoned the usage of ‘anaplasia’ to define ependymoma, instead relying on location-based molecular phenotyping with allowance for grading, but specifically noting that the impact of anaplasia on prognosis was controversial when considered in the context of molecular information (n.b., the newest version of the WHO criteria also removed histologically-described ependymoma from its lexicon: papillary, tanycytic, and clear cell) [3,11].
Currently, while anaplastic ependymoma may represent a molecularly aggressive phenotype, in the context of complete molecular phenotyping, there is no clear data that it represents an additional independent risk factor. Thus, clinical practice has begun to shift away from responding to anaplasia to drive clinical decisions, and future therapeutic trials are likely to hinge more directly on molecular biology.
Ultra-high-risk Ependymoma: time to consider early phase trials at diagnosis?
Of all the genetic alterations frequently seen in pediatric posterior fossa ependymoma, the gain of chromosome 1q and loss of chromosome 6q are by far the most clinically impactful. Multiple series have reported 1q gain to be the most common structural aberration in pediatric ependymoma (17-20% of all cases), followed by 6q loss (8-9%) [11,26,27]. Unfortunately, these rather common alterations also confer a poorer prognosis, which is further compounded when both occur within the same tumor. In a cohort of 663 posterior fossa ependymoma type A (PF-EPN-A) tumors, the 5-year progression-free survival (PFS) was strikingly worse tumors harboring 1q gain or 6q loss, with a 5-year PFS of 50% (95% CI 45%-55%) for balanced tumors, compared with 32% (95% CI 24%-44%) for 1q gain only, 7.3% (95% CI 2.0%-27%) for 6q loss only and 0% for both 1q gain and 6q loss [27]. Tumors demonstrating 1q gain/6q loss were found also to progress rapidly with a median PFS of 0.75 years compared to 2.42 years for those with 1q gain who were 6q balanced. Recurrence of tumors with 1q gain/6q loss is often metastatic (38.5% local, 53.8% distant only, 7.7% combined, P = .03).
The aggressive behavior of this molecularly-distinct subgroup has earned it the unofficial label of “ultra-high- risk” ependymoma. These tumors account for approximately 10% of all PF-EPN-A tumors, making them a substantial contributor to the mortality seen in this tumor group [27]. The rapid progression and extremely high rate of relapse in tumors with 1q gain/6q loss has prompted discussions about initiating a radically new upfront treatment approach for this group, as current treatment standards are clearly ineffective.
The ethics of enrolling on early phase clinical trials has revolved around patients who otherwise do not have a reasonable expectation of cure or prolonged disease stabilization, engaging with the ethical concept of ‘beneficence.’ These situations are typically clearest in the context of relapsed childhood brain cancer, or in rarer situations where survival is poor, even at diagnosis. The subgroup of tumors described here replicate the significant mortality of some of the worse pediatric brain cancers, such as diffuse midline glioma, TP53-mutant sonic hedgehog medulloblastoma, and MYC-amplified Group 3 medulloblastoma. Thus, akin to those scenarios, prioritization of children with ultra-high-risk ependymoma may be considered for participation in Phase I studies at diagnosis. Furthermore, the dramatic prognostic implications of these (and other) molecular findings highlight the importance of genetic profiling of ependymoma at diagnosis.
Radiation: required for everyone?
Radiation has clearly been demonstrated to be an effective modality of treatment in ependymoma, with most studies demonstrating prolonged EFS with adjuvant radiotherapy. However, irradiation also may cause significant long-term morbidity, including secondary malignancy, vasculopathy, and cognitive impairment [36]. With the advent of molecular subgroups and refined risk stratification based on patient outcomes, some patients have excellent outcomes and may be considered for radiation-sparing treatment strategies.
In supratentorial ependymoma, small case series also provided rationale for potential radiation sparing in gross totally resected, non-anaplastic disease. Initially reported in 1998, ten patients with supratentorial ependymoma were observed after GTR, with only three patients experiencing relapse, and two of those being salvaged with repeat resection and focal irradiation [37]. The possibility of supratentorial ependymoma surviving without radiotherapy was seemingly confirmed in a SEER database study that included children and adults with supratentorial EP after GTR, half of whom received radiotherapy, with no statistically significant outcome differences [38]. Later, ACNS0121 utilized observation for supratentorial ependymoma after a GTR, accruing eleven patients. In this study, 5-year EFS was 61.4%, similar to the general population, with over half of the patients with durable response and only one patient experiencing distant failure; the group exhibited a -year OS of 100% [2]. Conversely, a study of Canadian centers found that of 14 ST ependymoma that received GTR alone, 57% experienced relapse, most occurring within 1 year of diagnosis, although the pattern of relapse mimicked that of other studies, with only one patient exhibiting distant relapse not salvageable by re-resection and focal irradiation [39]. Further, follow-up for these studies was relatively brief given the possibility of delayed progression for ependymoma, lending some equipoise to the concept of surgery-only treatment in GTR ST ependymoma. However, it is worthwhile to note that molecularly analyzed cases hypothesize that ST-EPN-YAP1 in particular, may be amenable to this strategy.
Similarly, in patients with posterior fossa group B tumors who receive a total resection, survival without irradiation may be feasible. This data is reported in both relatively small case series, such as a report with 9 patients (no failures), [36] as well as a large combined series collating the Global Ependymoma Network of Excellence (GENE), St Jude RT1, and Collaborative Ependymoma Research Network (CERN) cases and demonstrating that, in the context of a GTR, patients with non-irradiated PFB tumors harbored a 10 year OS of 82.3%, although the PFS was only 45.1% [40]. Thus, while irradiation did increase 10-year PFS to 74%, this data suggested that at least some children with completely-resected PFB ependymoma were curable with surgery alone. Subsequent analysis further refined PFB ependymoma, postulating that 13-q balanced PFB had an even better survival, supporting the intriguing hypothesis of a surgery-only initial approach [1,41].
Coupled with clear and significant declines in full scale IQ from even focal upfront conformal radiotherapy, consideration for radiation-sparing trials in selected cases of ependymoma seem reasonable. However, due to the relatively small overall numbers and some conflicting data, investigators at an ependymoma consensus meeting concluded that radiation-sparing strategies should be tested in clinical trials in these relatively low-risk groups in order to reduce the rate of treatment-related morbidity without sacrificing survival [42].
The advent of advanced radiation delivery, such as with proton beam irradiation, must also be considered when weighing the benefits and consequences of primary or repeat irradiation. Thus far, proton-based irradiation does not seem to compromise efficacy, [43] and may improve negative cognitive and other late effects. Other questions regarding optimal radiation treatment remain, such as whether craniospinal radiation is necessary given some reports of distant relapse [43,44], whether radiation doses may be mitigated in some children to <59Gy2,43and other compelling questions outside the scope of this article.
Regardless, the significant survival differences discussed in these brief vignettes, namely, extremely poor prognoses in patients with 1q+/6q-, contrasted against the extremely good prognosis of ST-EPN-YAP1 and PFB ependymoma, highlight one agreed upon conclusion: upfront molecular phenotyping is essential for both clinical care and design in future clinical trials.
Challenges in therapeutic translation
The explosion of knowledge regarding ependymoma subtypes and biology has led to significant revisions in clinical perspective. However, translation of this data into improved outcomes for children with ependymoma has not yet materialized.
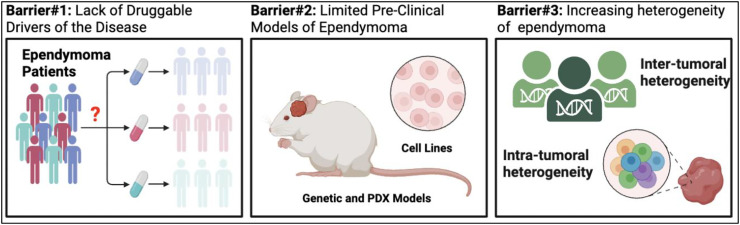
Several major challenges have hampered development of clinical trials and treatments for ependymoma (Fig. 1). Few genetic and patient-derived xenograft models of disease have limited pre-clinical studies and efforts to delineate the molecular basis of ependymoma. A lack of druggable-driver alterations has prevented application of precision medicine-based approaches [45]. Finally, despite few genetic alterations, the transcriptional heterogeneity of ependymoma is recognized as increasingly diverse both between patients and within a given tumor sample [20,41,42,[46], [47], [48]]. Recent advancements in animal modeling epigenomics, and single cell genomics have attempted to address some of these hurdles, providing new therapeutic targets and an improved understanding of the disease [4,5,7,[49], [50], [51], [52], [53]]. We will review recent advances in ependymoma biology according to the following key barriers and discuss opportunities for future advancement and discovery.
Fig 1.
Major obstacles to rapid translation of novel biologic findings to effective clinical interventions.
Barrier 1: lack of druggable drivers of the disease
Like most pediatric cancers, ependymomas are characterized by relative ‘quiet’ genomes [45,52]. In the case of supratentorial ependymoma, ZFTA or YAP1 associated gene fusions are sufficient to initiate tumors, with co-occurring CDKN2A deletions occurring in about 20-30% of cases [40]. There are currently no small molecules capable of inhibiting or degrading ZFTA gene fusion proteins, likely due to their highly disordered protein structure (commonly observed in transcription factors). However, the presence of at least 1-3 C2H2 zinc finger binding domains in ZFTA may provide a potential opportunity for molecular glues to be utilized in a protein degradation type strategy [52]. Before drug discovery approaches are initiated, however, a key unanswered question is whether ZFTA- and YAP1- fusions are still required for tumor progression or rather these gene fusions serve as initiating events. Alternate (although indirect) targeting strategies may be effective such as probing the dependency on transcriptional co-regulators critical for maintaining the neoplastic transcriptional state [7,50]. These include inhibitors of chromatin regulators such as BET bromodomain proteins, EP300/CBP lysine acetyl-transferases, and transcriptional elongation factors [54], [55], [56]. A targetable strategy may exist for YAP1 fusion driven ependymoma, with the advancement of TEAD inhibitors and degraders. Such approaches could be readily tested in novel genetic models of YAP1 fusion ependymoma (see next section) [57].
In the case of PF-A ependymoma, over 90% of cases lack a detectable recurrently mutated gene [45]. Despite EZH2 interacting protein (EZHIP) over-expression in nearly all PF-A tumors, EZHIP is mutated in less than 10% of cases, and the functional contribution of those mutations still unclear [48,58]. Similar to ZFTA/YAP1 fusions the continued dependence on EZHIP is an outstanding question. Expression of EZHIP phenocopies the effects of H3K27M mutation seen in diffuse midline glioma (DMG), specifically the global loss of the histone repressive mark, H3K27me3 [58], [59], [60], [61]. EZHIP over-expression and the subsequent hypomethylation on H3K27 interestingly is only found in PF-A ependymomas, and in stark contrast to the more differentiated and clinically less aggressive PF-B ependymomas. Tied to wide-spread epigenomic alterations in PFA ependymoma are significant alterations to metabolic pathways and a dependency on several nodes such as methionine pathway, glucose, and glutamine regulation [9,55]. These nutrient pathways may represent important vulnerabilities and sources for drug discovery against the most clinically aggressive forms of PFA ependymoma. Furthermore, PFA ependymomas have been shown to grow preferentially in “hypoxic” environments, highlighting a sensitivity to changes in oxygen tension. These distinct epigenomic and metabolic programs observed in PFA ependymomas have revealed new opportunities for treatment including agents that modulate glucose regulation (Metformin [9]), H3K27me3 modifications (EZH2 inhibitors [45]), H3 acetylation (HDAC inhibitors such as valproic acid in the SIOP Ependymoma Program II)and global methylation levels (5-Azacytidine, [62] and MAT2A inhibitors [55]). Drugs potentially closest to translation include Metformin, which has been shown to extend PFA ependymoma survival in a mouse model, through restoration of H3K27me3 levels. Furthermore, EZH2 inhibitors are in clinical trial evaluation for other brain tumors including atypical teratoid rhabdoid tumors. However, expansion of pre-clinical studies is needed for these promising drugs beyond single cell lines and patient-derived xenograft (PDX) models to establish broad applicability of these findings. Finally, investigating key downstream transcriptional targets of ependymoma by mining the chromatin landscape and cellular architecture of these tumors has yielded new targets and insights about drivers of the disease [48]. Active chromatin mapping has revealed essential transcriptional circuits critical for ependymoma tumor initiation, as well as novel dependency genes that are amenable to small molecule inhibition such as FGFR inhibitors [63]. While traditional application of precision medicine approaches that pairs gene mutation with a specific drug is likely to be ineffective against ependymoma, recognition of the distinct epi-metabolic networks of these unique tumors is an active area of therapeutic discovery. A small number of PF ependymomas cluster separately from PFA and PFB tumors on methylation, and typically have ACVR1 mutations, while lacking EZHIP expression [64]. While targeted therapies have yet to achieve significant clinical effect, these varied approaches remain in development and are summarized in Table 1.
Table 1.
Current molecularly-targeted strategies in development for ependymoma.
| Ependymoma Driver Gene |
Cellular Dependency |
Druggable? | Candidate Therapeutic Approaches |
|---|---|---|---|
| ZFTA Fusion Variants | Not Determined | Not Yet, Disordered Protein Structure |
|
| YAP1 Fusion Variants | Not Determined | Possibly |
|
| EZHIP | Not Determined | Not Yet, Disordered Protein Structure |
|
| MYCN | Not Determined | Not Yet, Disordered Protein Structure |
|
| ACVR1 | Not Determined | Yes |
|
Despite the challenges of druggable driver identification, other areas of therapeutic innovation that rely less on cellular signaling may also be helpful, such as novel immunotherapeutic approaches. Ependymomas that display prolonged stability have been shown to have upregulation of immune-related genes, increased T cell infiltration [65], and increased PDL1 expression. [66] For instance, expression of HER2 has been described in a large number of pediatric ependymomas with only limited expression on normal brain cells. Identification of these cellular targets may provide a vulnerability towards adoptive cellular therapy, such as chimeric antigen receptor (CAR) T cells, and has been shown preclinical to be potentially useful [67,68], and has generated several currently active clinical trials. Similarly, oncolytic virotherapy [69], vaccines [70], and immune checkpoint blockade [71] are currently being developed for ependymoma.
Barrier 2: limited pre-clinical models of ependymoma
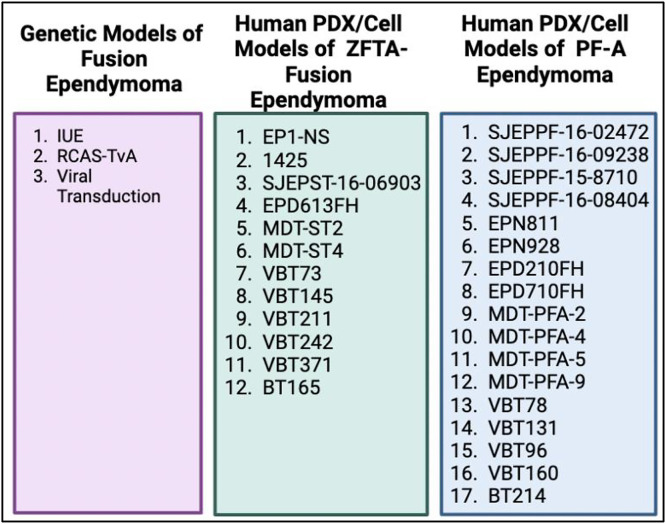
Compared to other pediatric brain tumors such as medulloblastoma and diffuse midline glioma (DMG), genetic models of ependymoma have only recently been developed (Fig. 2). ZFTA (formerly C11orf95) gene fusions constitute the majority of supratentorial ependymomas and are sufficient to drive tumorigenesis when expressed in mouse neural stem cells through viral transduction, in utero electroporation (IUE), or the RCAS-TVa system [7,[51], [52], [53]]. In similar vein, YAP1 gene fusions have also been modeled using similar approaches and are also sufficient to initiate the disease [57]. Advancements of ZFTA driven genetic models have revealed new insight into the role of ZFTA fusion proteins and their importance in regulating oncogenic gene expression [7,[50], [51], [52], [53]]. These new animal models have also provided a framework for investigating the ependymoma microenvironment and contribution of distinct tumor cell populations, immune cells, and normal neural cells that may contribute to disease pathogenesis. In vivo modeling of ZFTA fusion driven ependymomas has also incorporated gene editing based approaches to study genetic dependencies, such as the role of master (i.e., core regulatory circuit) transcription factors and their key roles in ependymoma initiation [48]. A key example includes SOX9, which was found to be one of the most active transcription factors (TFs) in ependymoma and essential for ependymoma cell proliferation and tumor initiation [72].
Fig. 2.
A summary of available preclinical murine models of childhood ependymoma.
The successes in genetic modeling of supratentorial ependymoma have not been seen in the case of posterior fossa ependymoma. Efforts to this date have failed to generate EZHIP-driven mouse models, possibly due to several factors: i) Lack of knowledge of the cellular compartment of origin (and its existence in mice), and ii) Lack of additional genetic alterations that may participate with EZHIP unlike the TP53, ATRX, and PDFGRA alterations seen in DMG. These challenges may be overcome with advances in murine and human hindbrain-cerebellar organoids that may enable EZHIP driven transformation of cells within the correct cellular compartment. Furthermore, CRISPR-CAS9 based functional genomics screens may facilitate identification of co-operating events with EZHIP over-expression. Encompassing these proposed future studies is the finding that PFA ependymomas grow preferentially in hypoxic environments and exhibit dramatically altered cellular metabolism [55]. Mouse modeling of PFA ependymoma may depend on multiple factors coming together including oncogene expression in appropriate cell types, consideration of additional co-operating driver genes, and growth within specific nutrient availability and oxygen tension. Immunocompetent model development, essential for comprehensive development of immune-based treatment strategies, has likewise been challenging without identification of clear transformative genetic aberrations that can be translated to murine models with normal immune systems.
In the past, pre-clinical efforts to identify effective therapies for ependymoma have been limited by the lack of patient-derived xenograft models [42]. In recent years, multiple groups have established several ZFTA fusion driven models, which have enabled drug- and CRISPR- based genetic screening [73], [74], [75], [76]. While fewer models for PF-A ependymoma have been established, continued efforts have led to a growing number of representative models, particularly of the most aggressive forms of the disease, such as tumors harboring 1q gain and 6q loss [55,76]. One would anticipate with continued scientific community efforts to establish ependymoma PDX models, that additional PF-A models will be developed, but also including development of rare variants such as PF-B or YAP1 altered ependymoma. A major challenge is the length of time ependymoma mouse models take to grow in vivo, with some cell lines requiring 8 months to >1 year to develop brain tumors. This hurdle has made pre-clinical drug efficacy studies particularly challenging to perform in ependymoma, combined with the high variability in tumor formation within a given model.
Barrier 3: increasing heterogeneity of the disease that constitutes ependymoma
While our review focuses on the major subgroups of ependymoma, additional subtypes of ependymoma have been discovered such as ZFTA (non-RELA) gene fusions, and heterogeneity within PF-A, PF-B, and spinal tumors as defined by DNA methylation-based classification (Table 2) [40,77]. These subgroups/subtypes are directly tied to distinct clinical outcomes and will shape future clinical trial design for ependymoma [2,46,78,79]. Complicating the observed inter-tumoral (between patients) heterogeneity is a recognition of the diverse cellular landscape of ependymoma, composed of distinct tumor populations, microglia, infiltrating immune cells, and neurons [4,5,80]. The ependymoma microenvironment and functional interactions between cell types is poorly defined but may hold opportunities for identifying effective therapies and understanding the mechanisms of treatment resistance and relapse. Early lessons from single-cell genomic characterization of ependymoma has revealed distinct cell populations responsive to disparate receptor tyrosine kinase (RTK) inhibitors such as those that block the FGFR and IGFR pathways [5]. These data underscore the importance of tailored combinatorial strategies against different cellular populations within a given patient tumor. Furthermore, transcriptional trajectories that reflect ependymoma cell state and differentiation capacity may hold clues to understanding the mechanistic basis of ependymoma progression [4,81]. Understanding the functional consequences of these transitions, such as epithelial-to-mesenchymal and hypoxic programs, will be important to uncovering therapeutic vulnerabilities. Other stem-cell properties, like telomerase re-activation, might also be at play in ependymoma stem cells, and might represent not only a biomarker of the disease, but also a future therapeutic target [82,83]. These studies have also provided critical insight on the developmental basis of ependymoma, which are likely to improve our understanding to the processes of tumor initiation and lead to improved mouse modeling of the disease.
Table 2.
A brief overview of pediatric ependymoma classes and major drivers.
| Posterior Fossa Ependymoma |
Supratentorial Ependymoma |
Spinal Ependymoma |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PF-EPN-A (EZHIP+) |
PF-EPN-B |
SE |
ZFTA Fusion |
YAP1 Fusion |
SE |
MYCN |
SP-EPN |
MPE |
SE |
| Poor | Favorable | Varied | Favorable | Favorable | Poor | Favorable | |||
| Balanced Genome Chr 1q gain and Chr6q loss poor prognostic factors |
Chromosomal Instability |
Balanced Genome | Chromosome 11 Chromothripsis CDKN2A loss a poor prognostic factor |
Balanced Genome | Balanced Genome | MYCN Amplification |
NF2 Mutations |
Chromosomal Instability |
6q deletion |
Ependymoma (EPN), Sub-Ependymoma (SE), Myxopapillary Ependymoma (MPE)
Conclusion
The relatively recent advent of molecular studies has enabled paradigm-shifting assessment of childhood ependymoma, dividing a historically single-disease into many separate subtypes. This enhancement has engendered optimism for the development of more effective treatment for what is now widely understood to be a tumor with a poor overall prognosis and has highlighted the need for comprehensive molecular phenotyping in every child at diagnosis and, potentially, recurrence. While both scientific and clinical barriers to rapid improvement in outcomes exist, identification of these obstacles allows for appropriate resource investment and consensus building to propel the field towards rapid and rational improvements in survival and morbidity.
CRediT authorship contribution statement
Eugene I. Hwang: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Derek Hanson: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Mariella G. Filbin: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Stephen C. Mack: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Saleh AH, Samuel N, Juraschka K, Saleh MH, Taylor MD, Fehlings MG. The biology of ependymomas and emerging novel therapies. Nat. Rev. Cancer. 2022;22(4):208–222. doi: 10.1038/s41568-021-00433-2. [DOI] [PubMed] [Google Scholar]
- 2.Merchant TE, Bendel AE, Sabin ND, et al. Conformal radiation therapy for pediatric ependymoma, chemotherapy for incompletely resected ependymoma, and observation for completely resected, supratentorial ependymoma. J. Clin. Oncol. 2019;37(12):974–983. doi: 10.1200/JCO.18.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellison DW, Aldape KD, Capper D, et al. cIMPACT-NOW update 7: advancing the molecular classification of ependymal tumors. Brain Pathol. 2020;30(5):863–866. doi: 10.1111/bpa.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillen AE, Riemondy KA, Amani V, et al. Single-cell RNA sequencing of childhood ependymoma reveals neoplastic cell subpopulations that impact molecular classification and etiology. Cell Rep. 2020;32(6) doi: 10.1016/j.celrep.2020.108023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gojo J, Englinger B, Jiang L, et al. Single-cell RNA-seq reveals cellular hierarchies and impaired developmental trajectories in pediatric ependymoma. Cancer Cell. 2020;38(1):44–59. doi: 10.1016/j.ccell.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith A, Onar-Thomas A, Ellison D, et al. presented at: Society of Neuro-oncology; 2020. EPEN-54. ACNS0831, Phase III Randomized Trial of Post-Radiation Chemotherapy in Patients with Newly Diagnosed Ependymoma Ages 1 to 21 Years. [Google Scholar]
- 7.Arabzade A, Zhao Y, Varadharajan S, et al. ZFTA-RELA dictates oncogenic transcriptional programs to drive aggressive supratentorial ependymoma. Cancer Discov. 2021;11(9):2200–2215. doi: 10.1158/2159-8290.CD-20-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jünger ST, Timmermann B, Pietsch T. Pediatric ependymoma: an overview of a complex disease. Childs Nerv. Syst. 2021;37(8):2451–2463. doi: 10.1007/s00381-021-05207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panwalkar P, Tamrazi B, Dang D, et al. Targeting integrated epigenetic and metabolic pathways in lethal childhood PFA ependymomas. Sci. Transl. Med. 2021;13(614):eabc0497. doi: 10.1126/scitranslmed.abc0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batchu S, Patel K, Yu S, Mohamed AT, Karsy M. Single cell transcriptomics reveals unique metabolic profiles of ependymoma subgroups. Gene. 2022;820 doi: 10.1016/j.gene.2022.146278. [DOI] [PubMed] [Google Scholar]
- 11.Kresbach C, Neyazi S, Schüller U. Updates in the classification of ependymal neoplasms: The 2021 WHO Classification and beyond. Brain Pathol. 2022;32(4):e13068. doi: 10.1111/bpa.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen JC, Siffert J, Hukin J. Clinical manifestations of childhood ependymoma: a multitude of syndromes. Pediatr. Neurosurg. 1998;28(1):49–55. doi: 10.1159/000028619. [DOI] [PubMed] [Google Scholar]
- 13.McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J. Neurosurg. 2009;110(4):725–729. doi: 10.3171/2008.9.JNS08117. [DOI] [PubMed] [Google Scholar]
- 14.Marinoff AE, Ma C, Guo D, et al. Rethinking childhood ependymoma: a retrospective, multi-center analysis reveals poor long-term overall survival. J. Neurooncol. 2017;135(1):201–211. doi: 10.1007/s11060-017-2568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foreman NK, Love S, Thorne R. Intracranial ependymomas: analysis of prognostic factors in a population-based series. Pediatr. Neurosurg. 1996;24(3):119–125. doi: 10.1159/000121027. [DOI] [PubMed] [Google Scholar]
- 16.Pollack IF, Gerszten PC, Martinez AJ, et al. Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery. 1995;37(4):655–666. doi: 10.1227/00006123-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Horn B, Heideman R, Geyer R, et al. A multi-institutional retrospective study of intracranial ependymoma in children: identification of risk factors. J. Pediatr. Hematol. Oncol. 1999;21(3):203–211. doi: 10.1097/00043426-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Elsamadicy AA, Koo AB, David WB, et al. Comparison of epidemiology, treatments, and outcomes in pediatric versus adult ependymoma. Neurooncol. Adv. 2020;2(1) doi: 10.1093/noajnl/vdaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz AK, Munoz-Bendix C, Remke M, et al. Second-look surgery after pediatric brain tumor resection - single center analysis of morbidity and volumetric efficacy. Brain Spine. 2022;2 doi: 10.1016/j.bas.2022.100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across All CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. doi: 10.1016/j.ccell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilday JP, Mitra B, Domerg C, et al. Copy number gain of 1q25 predicts poor progression-free survival for pediatric intracranial ependymomas and enables patient risk stratification: a prospective European clinical trial cohort analysis on behalf of the Children's Cancer Leukaemia Group (CCLG), Societe Francaise d'Oncologie Pediatrique (SFOP), and International Society for Pediatric Oncology (SIOP) Clin. Cancer Res. 2012;18(7):2001–2011. doi: 10.1158/1078-0432.CCR-11-2489. [DOI] [PubMed] [Google Scholar]
- 22.Korshunov A, Witt H, Hielscher T, et al. Molecular staging of intracranial ependymoma in children and adults. J. Clin. Oncol. 2010;28(19):3182–3190. doi: 10.1200/JCO.2009.27.3359. [DOI] [PubMed] [Google Scholar]
- 23.Dyer S, Prebble E, Davison V, et al. Genomic imbalances in pediatric intracranial ependymomas define clinically relevant groups. Am. J. Pathol. 2002;161(6):2133–2141. doi: 10.1016/S0002-9440(10)64491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garvin JH, Selch MT, Holmes E, et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma. Children's Cancer Group protocol 9942: a report from the Children's Oncology Group. Pediatr. Blood. Cancer. 2012;59(7):1183–1189. doi: 10.1002/pbc.24274. [DOI] [PubMed] [Google Scholar]
- 25.Ritzmann TA, Chapman RJ, Kilday JP, et al. SIOP Ependymoma I: Final results, long-term follow-up, and molecular analysis of the trial cohort-A BIOMECA Consortium Study. Neuro. Oncol. 2022;24(6):936–948. doi: 10.1093/neuonc/noac012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason WP, Grovas A, Halpern S, et al. Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J. Clin. Oncol. 1998;16(1):210–221. doi: 10.1200/JCO.1998.16.1.210. [DOI] [PubMed] [Google Scholar]
- 27.White L, Kellie S, Gray E, et al. Postoperative chemotherapy in children less than 4 years of age with malignant brain tumors: promising initial response to a VETOPEC-based regimen. A Study of the Australian and New Zealand Children's Cancer Study Group (ANZCCSG) J. Pediatr. Hematol. Oncol. 1998;20(2):125–130. doi: 10.1097/00043426-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Strother DR, Lafay-Cousin L, Boyett JM, et al. Benefit from prolonged dose-intensive chemotherapy for infants with malignant brain tumors is restricted to patients with ependymoma: a report of the Pediatric Oncology Group randomized controlled trial 9233/34. Neuro. Oncol. 2014;16(3):457–465. doi: 10.1093/neuonc/not163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adolph JE, Fleischhack G, Mikasch R, et al. Local and systemic therapy of recurrent ependymoma in children and adolescents: short- and long-term results of the E-HIT-REZ 2005 study. Neuro. Oncol. 2021;23(6):1012–1023. doi: 10.1093/neuonc/noaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouffet E, Foreman N. Chemotherapy for intracranial ependymomas. Childs Nerv. Syst. 1999;15(10):563–570. doi: 10.1007/s003810050544. [DOI] [PubMed] [Google Scholar]
- 31.Tihan T, Zhou T, Holmes E, Burger PC, Ozuysal S, Rushing EJ. The prognostic value of histological grading of posterior fossa ependymomas in children: a Children's Oncology Group study and a review of prognostic factors. Mod. Pathol. 2008;21(2):165–177. doi: 10.1038/modpathol.3800999. [DOI] [PubMed] [Google Scholar]
- 32.Merchant TE, Jenkins JJ, Burger PC, et al. Influence of tumor grade on time to progression after irradiation for localized ependymoma in children. Int. J. Radiat. Oncol. Biol. Phys. 2002;53(1):52–57. doi: 10.1016/s0360-3016(01)02801-2. [DOI] [PubMed] [Google Scholar]
- 33.Korshunov A, Golanov A, Sycheva R, Timirgaz V. The histologic grade is a main prognostic factor for patients with intracranial ependymomas treated in the microneurosurgical era: an analysis of 258 patients. Cancer. 2004;100(6):1230–1237. doi: 10.1002/cncr.20075. [DOI] [PubMed] [Google Scholar]
- 34.Tamburrini G, D'Ercole M, Pettorini BL, Caldarelli M, Massimi L, Di Rocco C. Survival following treatment for intracranial ependymoma: a review. Childs Nerv. Syst. 2009;25(10):1303–1312. doi: 10.1007/s00381-009-0874-y. [DOI] [PubMed] [Google Scholar]
- 35.Ellison DW, Kocak M, Figarella-Branger D, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat. Result. Biomed. 2011;10:7. doi: 10.1186/1477-5751-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zapotocky M, Beera K, Adamski J, et al. Survival and functional outcomes of molecularly defined childhood posterior fossa ependymoma: Cure at a cost. Cancer. 2019;125(11):1867–1876. doi: 10.1002/cncr.31995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hukin J, Epstein F, Lefton D, Allen J. Treatment of intracranial ependymoma by surgery alone. Pediatr. Neurosurg. 1998;29(1):40–45. doi: 10.1159/000028683. [DOI] [PubMed] [Google Scholar]
- 38.Ghia AJ, Mahajan A, Allen PK, et al. Supratentorial gross-totally resected non-anaplastic ependymoma: population based patterns of care and outcomes analysis. J. Neurooncol. 2013;115(3):513–520. doi: 10.1007/s11060-013-1254-8. [DOI] [PubMed] [Google Scholar]
- 39.Ailon T, Dunham C, Carret AS, et al. The role of resection alone in select children with intracranial ependymoma: the Canadian Pediatric Brain Tumour Consortium experience. Childs Nerv. Syst. 2015;31(1):57–65. doi: 10.1007/s00381-014-2575-4. [DOI] [PubMed] [Google Scholar]
- 40.Ramaswamy V, Hielscher T, Mack SC, et al. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: a retrospective multicohort analysis. J. Clin. Oncol. 2016;34(21):2468–2477. doi: 10.1200/JCO.2015.65.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witt H, Mack SC, Ryzhova M, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20(2):143–157. doi: 10.1016/j.ccr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133(1):5–12. doi: 10.1007/s00401-016-1643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters S, Merta J, Schmidt L, et al. Evaluation of dose, volume, and outcome in children with localized, intracranial ependymoma treated with proton therapy within the prospective KiProReg Study. Neuro. Oncol. 2022;24(7):1193–1202. doi: 10.1093/neuonc/noab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Indelicato DJ, Bradley JA, Rotondo RL, et al. Outcomes following proton therapy for pediatric ependymoma. Acta Oncol. 2018;57(5):644–648. doi: 10.1080/0284186X.2017.1413248. [DOI] [PubMed] [Google Scholar]
- 45.Mack SC, Witt H, Piro RM, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506(7489):445–450. doi: 10.1038/nature13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavalli FMG, Hübner JM, Sharma T, et al. Heterogeneity within the PF-EPN-B ependymoma subgroup. Acta Neuropathol. 2018;136(2):227–237. doi: 10.1007/s00401-018-1888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mack SC, Agnihotri S, Bertrand KC, et al. Spinal myxopapillary ependymomas demonstrate a warburg phenotype. Clin. Cancer Res. 2015;21(16):3750–3758. doi: 10.1158/1078-0432.CCR-14-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mack SC, Pajtler KW, Chavez L, et al. Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature. 2018;553(7686):101–105. doi: 10.1038/nature25169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu R, Norris GA, Willard N, et al. Spatial transcriptomic analysis delineates epithelial and mesenchymal subpopulations and transition stages in childhood ependymoma. Neuro. Oncol. 2022 doi: 10.1093/neuonc/noac219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kupp R, Ruff L, Terranova S, et al. Translocations constitute ependymoma chromatin remodeling and transcription factors. Cancer Discov. 2021;11(9):2216–2229. doi: 10.1158/2159-8290.CD-20-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozawa T, Arora S, Szulzewsky F, et al. A De Novo mouse model of C11orf95-RELA fusion-driven ependymoma identifies driver functions in addition to NF-κB. Cell Rep. 2018;23(13):3787–3797. doi: 10.1016/j.celrep.2018.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker M, Mohankumar KM, Punchihewa C, et al. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature. 2014;506(7489):451–455. doi: 10.1038/nature13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng T, Ghasemi DR, Okonechnikov K, et al. Cross-species genomics reveals oncogenic dependencies in ZFTA/C11orf95 fusion-positive supratentorial ependymomas. Cancer Discov. 2021;11(9):2230–2247. doi: 10.1158/2159-8290.CD-20-0963. [DOI] [PubMed] [Google Scholar]
- 54.Garcia K, Gingras AC, Harvey KF, Tanas MR. TAZ/YAP fusion proteins: mechanistic insights and therapeutic opportunities. Trend. Cancer. 2022;8(12):1033–1045. doi: 10.1016/j.trecan.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michealraj KA, Kumar SA, Kim LJY, et al. Metabolic regulation of the epigenome drives lethal infantile ependymoma. Cell. 2020;181(6):1329–1345. doi: 10.1016/j.cell.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Servidei T, Meco D, Martini M, et al. The BET inhibitor OTX015 exhibits in vitro and in vivo antitumor activity in pediatric ependymoma stem cell models. Int. J. Mol. Sci. 2021;22(4) doi: 10.3390/ijms22041877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pajtler KW, Wei Y, Okonechnikov K, et al. YAP1 subgroup supratentorial ependymoma requires TEAD and nuclear factor I-mediated transcriptional programmes for tumorigenesis. Nat. Commun. 2019;10(1):3914. doi: 10.1038/s41467-019-11884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hübner JM, Müller T, Papageorgiou DN, et al. EZHIP/CXorf67 mimics K27M mutated oncohistones and functions as an intrinsic inhibitor of PRC2 function in aggressive posterior fossa ependymoma. Neuro. Oncol. 2019;21(7):878–889. doi: 10.1093/neuonc/noz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pajtler KW, Wen J, Sill M, et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol. 2018;136(2):211–226. doi: 10.1007/s00401-018-1877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piunti A, Smith ER, Morgan MAJ, et al. CATACOMB: An endogenous inducible gene that antagonizes H3K27 methylation activity of Polycomb repressive complex 2 via an H3K27M-like mechanism. Sci. Adv. 2019;5(7):eaax2887. doi: 10.1126/sciadv.aax2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain SU, Rashoff AQ, Krabbenhoft SD, et al. H3 K27M and EZHIP impede H3K27-methylation spreading by inhibiting allosterically stimulated PRC2. Mol. Cell. 2020;80(4):726–735. doi: 10.1016/j.molcel.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandberg DI, Yu B, Patel R, et al. Infusion of 5-Azacytidine (5-AZA) into the fourth ventricle or resection cavity in children with recurrent posterior Fossa Ependymoma: a pilot clinical trial. J. Neurooncol. 2019;141(2):449–457. doi: 10.1007/s11060-018-03055-1. [DOI] [PubMed] [Google Scholar]
- 63.Lötsch D, Kirchhofer D, Englinger B, et al. Targeting fibroblast growth factor receptors to combat aggressive ependymoma. Acta Neuropathol. 2021;142(2):339–360. doi: 10.1007/s00401-021-02327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pratt D, Lucas CG, Selvam PP, et al. Recurrent ACVR1 mutations in posterior fossa ependymoma. Acta Neuropathol. 2022;144(2):373–376. doi: 10.1007/s00401-022-02435-2. [DOI] [PubMed] [Google Scholar]
- 65.Donson AM, Birks DK, Barton VN, et al. Immune gene and cell enrichment is associated with a good prognosis in ependymoma. J. Immunol. 2009;183(11):7428–7440. doi: 10.4049/jimmunol.0902811. [DOI] [PubMed] [Google Scholar]
- 66.Nambirajan A, Malgulwar PB, Sharma A, et al. Clinicopathological evaluation of PD-L1 expression and cytotoxic T-lymphocyte infiltrates across intracranial molecular subgroups of ependymomas: are these tumors potential candidates for immune check-point blockade? Brain Tumor Pathol. 2019;36(4):152–161. doi: 10.1007/s10014-019-00350-1. [DOI] [PubMed] [Google Scholar]
- 67.Donovan LK, Delaidelli A, Joseph SK, et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020;26(5):720–731. doi: 10.1038/s41591-020-0827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khatua S, Cooper LJN, Sandberg DI, et al. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro. Oncol. 2020;22(8):1214–1225. doi: 10.1093/neuonc/noaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kieran MW, Goumnerova L, Manley P, et al. Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro. Oncol. 2019;21(4):537–546. doi: 10.1093/neuonc/noy202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pollack IF, Jakacki RI, Butterfield LH, Okada H. Ependymomas: development of immunotherapeutic strategies. Expert. Rev. Neurother. 2013;13(10):1089–1098. doi: 10.1586/14737175.2013.840420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Witt DA, Donson AM, Amani V, et al. Specific expression of PD-L1 in RELA-fusion supratentorial ependymoma: Implications for PD-1-targeted therapy. Pediatr. Blood. Cancer. 2018;65(5):e26960. doi: 10.1002/pbc.26960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sardar D, Chen HC, Reyes A, et al. Sox9 directs divergent epigenomic states in brain tumor subtypes. Proc. Natl. Acad. Sci. U. S. A. 2022;119(29) doi: 10.1073/pnas.2202015119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith KS, Xu K, Mercer KS, et al. Patient-derived orthotopic xenografts of pediatric brain tumors: a St. Jude resource. Acta Neuropathol. 2020;140(2):209–225. doi: 10.1007/s00401-020-02171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brabetz S, Leary SES, Gröbner SN, et al. A biobank of patient-derived pediatric brain tumor models. Nat. Med. 2018;24(11):1752–1761. doi: 10.1038/s41591-018-0207-3. [DOI] [PubMed] [Google Scholar]
- 75.Yu L, Baxter PA, Voicu H, et al. A clinically relevant orthotopic xenograft model of ependymoma that maintains the genomic signature of the primary tumor and preserves cancer stem cells in vivo. Neuro. Oncol. 2010;12(6):580–594. doi: 10.1093/neuonc/nop056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson DB, Manouchehri A, Haugh AM, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J. Immunother. Cancer. 2019;7(1):134. doi: 10.1186/s40425-019-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 78.Baroni LV, Sundaresan L, Heled A, et al. Ultra high-risk PFA ependymoma is characterized by loss of chromosome 6q. Neuro. Oncol. 2021;23(8):1360–1370. doi: 10.1093/neuonc/noab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuoka K, Kanemura Y, Shofuda T, et al. Significance of molecular classification of ependymomas: C11orf95-RELA fusion-negative supratentorial ependymomas are a heterogeneous group of tumors. Acta Neuropathol. Commun. 2018;6(1):134. doi: 10.1186/s40478-018-0630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vladoiu MC, El-Hamamy I, Donovan LK, et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature. 2019;572(7767):67–73. doi: 10.1038/s41586-019-1158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aubin RG, Troisi EC, Montelongo J, et al. Pro-inflammatory cytokines mediate the epithelial-to-mesenchymal-like transition of pediatric posterior fossa ependymoma. Nat. Commun. 2022;13(1):3936. doi: 10.1038/s41467-022-31683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gojo J, Lötsch D, Spiegl-Kreinecker S, et al. Telomerase activation in posterior fossa group A ependymomas is associated with dismal prognosis and chromosome 1q gain. Neuro. Oncol. 2017;19(9):1183–1194. doi: 10.1093/neuonc/nox027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castelo-Branco P, Choufani S, Mack S, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14(6):534–542. doi: 10.1016/S1470-2045(13)70110-4. [DOI] [PubMed] [Google Scholar]