Summary
Background
Mechanisms contributing to COVID-19 severity in people with HIV (PWH) are poorly understood. We evaluated temporal changes in plasma proteins following SARS-CoV-2 infection and identified pre-infection proteomic markers associated with future COVID-19.
Methods
We leveraged data from the global Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Antiretroviral therapy (ART)-treated PWH with clinical, antibody-confirmed COVID-19 as of September 2021 were matched on geographic region, age, and sample timing to antibody negative controls. For cases and controls, pre COVID-19 pandemic specimens were obtained prior to January 2020 to assess change over time and relationship to COVID-19 severity, using false-discovery adjusted mixed effects modeling.
Findings
We compared 257 unique plasma proteins in 94 COVID-19 antibody-confirmed clinical cases and 113 matched antibody-negative controls, excluding COVID-19 vaccinated participants (age 50 years, 73% male). 40% of cases were characterized as mild; 60% moderate to severe. Median time from COVID-19 infection to follow-up sampling was 4 months. Temporal patterns of protein changes differed based on COVID-19 disease severity. Among those experiencing moderate to severe disease vs. controls, NOS3 increased whereas ANG, CASP-8, CD5, GZMH, GZMB, ITGB2, and KLRD1 decreased. Higher pre-pandemic levels of granzymes A, B and H (GZMA, GZMB and GZMH) were associated with the future development of moderate-severe COVID-19 and were related to immune function.
Interpretation
We identified temporal changes in proteins closely linked to inflammatory, immune, and fibrotic pathways which may relate to COVID-19-related morbidity among ART-treated PWH. Further we identified key granzyme proteins associated with future COVID-19 in PWH.
Funding
This study is supported through NIH grants U01HL123336, U01HL123336-06 and 3U01HL12336-06S3, to the clinical coordinating center, and U01HL123339, to the data coordinating center as well as funding from Kowa Pharmaceuticals, Gilead Sciences, and a grant award through ViiV Healthcare. The NIAID supported this study through grants UM1 AI068636, which supports the AIDS Clinical Trials Group (ACTG) Leadership and Operations Center, and UM1 AI106701, which supports the ACTG Laboratory Center. This work was also supported by NIAID through grant K24AI157882 to MZ. The work of IS was supported by the intramural research program of NIAID/NIH.
Keywords: COVID-19, SARS-CoV-2, Human immunodeficiency virus, Granzyme
Research in context.
Evidence before this study
COVID-19 may be more severe in people with HIV (PWH). However, underlying biological mechanisms associated with the development of COVID-19 and its clinical severity among antiretroviral therapy (ART) treated PWH are largely unknown.
Added value of this study
Our results comprehensively applied serial proteomic profiling to a large, international cohort of PWH, assessing predictive protein pathways and differential temporal protein expression to SARS-CoV-2 infection in this population. Our analysis of the differential temporal expression of proteins over time among PWH may help identify relevant adaptive and maladaptive changes in inflammatory, immune, and fibrotic pathways underlying moderate-to-severe COVID-19. Further we found increased pre-pandemic circulating concentrations of granzymes A, B, and H to be an independent risk factor for the development of subsequent moderate-to-severe COVID-19 among well treated PWH receiving ART. These results shed light on critical biological responses to COVID-19 which may contribute to disease severity.
Implications of all the available evidence
Our results provide unique insights into the biological susceptibility and responses to COVID-19 infection in PWH. Our findings guide future directions with respect to: 1) identification of PWH who are at greatest risk of developing moderate-severe COVID-19; 2) understanding potentially maladaptive changes in immune-regulatory proteins post-COVID, that may aid in future targeting of such proteins to mitigate disease severity and/or the development of post-acute sequelae of COVID-19 in PWH.
Introduction
To date, more than half a billion individuals worldwide have had coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Beyond the devastating effects on mortality, the post-acute sequelae following COVID-19 (PASC, also called long-COVID) affect the lives of millions,2,3 and increase the morbidity and mortality burden of non-communicable diseases.4
The effects of COVID-19 on the immune and inflammatory systems5, 6, 7, 8 are especially concerning in people with HIV (PWH) considering their immunocompromised state and chronic inflammation. Some studies indicate that while PWH are at equal risk of acquiring COVID-19, they may be more susceptible to severe symptoms and outcomes.9,10 However, the underlying mechanisms are poorly understood. Furthermore, information on baseline levels and the temporal changes in soluble proteomic markers following SARS-CoV-2 infection may help to better understand immune and tissue responses following COVID-19 in PWH.
The current analysis leverages data collected from the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE; NCT02344290) an ongoing global primary cardiovascular prevention clinical trial among PWH on antiretroviral therapy (ART) assessing the utility of a statin strategy.11 The availability of plasma samples from regular study visits provided a unique opportunity to conduct a case–control study to evaluate the effects of SARS-CoV-2 infection on the temporal profiles of plasma proteins. Primary objectives of this analysis were to assess the differential expression of proteomic markers following SARS-CoV-2 infection with respect to symptom severity. Additionally, we assessed potential differences in baseline protein values with respect to future COVID-19 disease and severity.
Methods
Study population
REPRIEVE enrolled 7769 participants between 2015 and 2019, for longitudinal follow-up of cardiovascular events. REPRIEVE-related site staff administered a standardized COVID-19 related questionnaire at each visit to assess COVID-19-related adverse events. Targeted COVID-19 symptom and severity assessments were performed every 4 months with each study visit in REPRIEVE. Information on SARS-CoV-2 vaccination status was also obtained from the concomitant medication logs. Participants were asked to report any adverse events at each visit, including any clinical diagnosis of COVID-19 and/or a positive SARS-CoV-2 rapid antigen detection test or polymerase chain reaction (PCR) test. Adverse events were graded for severity on the ordinal scale of mild, moderate, severe, potentially life-threatening, or resulting in death, as per the Division of AIDS table for Grading the Severity of Adult and Pediatric Adverse Events (DAIDS AE Grading Table, Version 2.1).
We identified all participants who reported clinical COVID-19 based on adverse event reporting from May 2020 to September 2021. A pre- and post-COVID specimen was obtained from cases and temporally matched to controls. We obtained a post-COVID-19 plasma specimen for each case from the subsequent visit following COVID-19 infection during this timeframe. In addition, we obtained a paired plasma specimen from each case, which was the latest pre COVID-19 specimen from an annual visit occurring prior to January 2020. We then identified a cohort of control participants, for whom a COVID-19 diagnosis was not entered on the adverse events log, using frequency matching to cases based on timing of sampling, geographic region, and age groups. Paired specimens were chosen for each control from a plasma specimen available before January 2020, in an analogous manner to the cases.
In constructing the final analytical cohort, participants who underwent COVID-19 vaccination between the two sampling periods were excluded. Further, both cases and controls underwent antibody testing using receptor binding domain-specific IgG and IgA SARS-CoV-2 assays (see below). We excluded any cases without positive antibody result, and any control participant with a positive antibody result. Finally, all analyses were limited to those in whom protein expression data passed quality control (detailed below). The final analytical cohort included 94 COVID-19 antibody positive cases and 113 COVID-19 antibody negative controls, with protein data that passed quality control at both sampling timepoints. See Participant Selection diagram (Fig. 1) for further details. Race, ethnicity, and natal sex were self-reported.11
Fig. 1.
Participant Selection diagram. Overall, 207 individuals were in our final cohort.
Ethics
The REPRIEVE trial was approved by the National Institutes of Health. The analysis plan and publication of this manuscript were further approved by the REPRIEVE Executive and Publications Committees. The Mass General Brigham Human Research Committee approved the study (MGH IRB # 2013P001898), and each clinical research site obtained institutional review board/ethics committee approval and any other applicable regulatory entity approvals. All participants were provided with study information, including discussion of risks and benefits and signed the approved declaration of informed consent. Results are presented in compliance with the STROBE guidelines.12
Proteomic measurements and quality control
Fasting plasma samples were drawn during annual study visits and stored at −80 °C. Three commercially available multiplex immunoassays were used (Olink Target 96 Cardiovascular III, Immuno-oncology, and Cardiometabolic) to quantify 275 unique proteins at two timepoints for each individual. Definitions, reproducibility, and validation information regarding measurements can be found at: https://www.olink.com. We excluded individuals (Fig. 1) if all measurements on one of the panels at either timepoint were flagged with warnings (n = 7).13 Based on standard analysis procedures, we excluded proteins from our analysis if ≥50% of the study samples had values below the limit of detection.13,14 This resulted in 18 proteins being excluded from analysis (included and excluded proteins are summarized in Supplemental Tables S1 and S2, respectively). Protein name abbreviations used in the manuscript are presented in Table 1. Therefore, 257 unique proteins were analyzed at both timepoints in both groups.
Table 1.
Protein names abbreviated in the manuscript.
| Abbreviation | Uniport | Name |
|---|---|---|
| ANG | P03950 | Angiogenin |
| CASP-8 | Q14790 | Caspase-8 |
| CCL18 | P55774 | C–C motif chemokine 18 |
| CCL23 | P55773 | C–C motif chemokine ligand 23 |
| CD5 | P06127 | T-cell surface glycoprotein CD5 |
| CFHR5 | Q9BXR6 | Complement factor H-related protein 5 |
| CNDP1 | Q96KN2 | Beta-ala-his dipeptidase |
| COL18A1 | P39060 | Collagen alpha-1 (XVIII) chain |
| FASLG | P48023 | Tumor necrosis factor ligand superfamily member 6 |
| GZMA | P12544 | Granzyme A |
| GZMB | P10144 | Granzyme B |
| GZMH | P20718 | Granzyme H |
| IGFBP-2 | P18065 | Insulin-like growth factor-binding protein 2 |
| ITGB2 | P05107 | Integrin beta-2 |
| KLDR1 | Q13241 | Natural killer cells antigen CD94 |
| MMP7 | P09237 | Matrix metalloproteinase-7 |
| NOS3 | P29474 | Nitric-oxide synthase 3 |
| PDGF subunit B | P01127 | Platelet-derived growth factor subunit B |
| RARRES2 | Q99969 | Retinoic acid receptor responder protein 2 |
| SAA4 | P35542 | Serum amyloid A-4 protein |
| SCGB3A2 | Q96PL1 | Secretoglobin family 3A member 2 |
| ST6AL1 | P15907 | Beta-galactoside alpha-2,6-sialyltransferase 1 |
| TR | P02786 | Transferrin receptor protein 1 |
| TR-AP | P13686 | Tartrate-resistant acid phosphatase type 5 |
COVID-19 severity grading
Due to the relatively small number of COVID-19 diagnoses graded severe, the moderate and severe grades were combined into one group.
Antibody testing
The SARS-CoV-2 specific ELISA was developed at the Ragon Institute of Massachusetts Institute of Technology and Harvard, allowing for the detection of receptor binding domain specific IgG and IgA in an automated manner.15 The assay has been previously evaluated against EUA-approved ELISAs with >99.5% specificity.15,16 Conversion from OD values to μg/ml concentrations were performed on every ELISA plate via 12 two-fold dilution curves, starting at 625 ng/ml of a SARS-CoV-2 receptor binding domain specific monoclonal IgG1 (clone: CR3022). The sample concentration was then interpolated from the standard curve.15 A sample was considered positive if it equaled the mean of the negative-control wells on each respective plate plus five times the standard deviation of the concentration from negative plasma samples. Background-corrected concentrations were divided by the cutoff to generate signal-to-cutoff (S/CO) ratios.
Statistics
Continuous variables are presented as median and interquartile ranges, while categorical variables are presented using counts and percentages. To assess change over time in the proteins in each group, we modeled the temporal change in protein expression using linear mixed models. All models were corrected for ASCVD risk score at enrollment and included a random intercept per participant (assumed to be normally distributed) to model intra-individual temporal changes, and the time difference between the two samples to account for general temporal changes. Since ASCVD risk score contains data on age, sex, race, diabetes, smoking, blood pressure, lipids, and other relevant variables for COVID infection, we did not control for these variables individually but did perform additional stratified analyses on age, sex and CD4 in analyses assessing the association of change in proteins to COVID severity as outlined below. Multiple comparisons were adjusted for using the false discovery rate (FDR) method by Benjamini and Hochberg.17
First, we included COVID-19 infection as a binary predictor variable for cases. We used the beta coefficient of this binary predictor variable to evaluate the differential expression level of the proteins in cases as compared to controls.
Next, we assessed whether changes in protein expression levels were different depending on the degree of clinical COVID-19 severity. To do this we included COVID-19 as a categorical variable into our models as mild (DAIDS Grade 1) or moderate-severe (DAIDS Grade 2–3) and compared these categories to controls as reference. Since REPRIEVE was designed prior to the COVID pandemic and was a large ongoing trial, we could not implement the WHO grading scale. We used the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) knowledge-based protein–protein interaction database to understand the potential biological background of proteins showing similar or different expression with respect to COVID-19 severity.18 Spearman correlation was used to assess the association between baseline expression of proteins showing significant associations with COVID-19 in our analyses. Enrichment analysis was not done due to the lack of sufficient background proteins.
To further evaluate differences in protein expression with respect to clinical COVID-19 grade severity, we stratified our population based on: 1) nadir CD4 < or ≥200 cells/mL, to assess the effect of immune status; 2) timing of COVID-19 infection (< or ≥ the median of 4 months from time of infection to the time of the post COVID-19 sampling), to assess the effect of proximity of infection to testing; 3) age at baseline measurement < or ≥ the median age, to assess the effect of age on the observed temporal changes, and 4) natal sex, to assess the effects of sex.
To assess whether baseline protein expression levels differed between those who later acquired COVID-19 and those who did not, we conducted multivariable linear regression analyses. To account for potential variables that may influence subsequent acquisition of COVID, we controlled for immune and metabolic variables, including CD4, CD8, BMI, presence of metabolic syndrome and glucose.19 Model selection was not based on a specific algorithm but on clinical knowledge of the factors affecting COVID severity. We used Spearman correlation to assess the associations between baseline proteins showing significant associations with later COVID-19 and clinical, immune and metabolic variables.
We utilized a two-sided FDR corrected p value <0.1 to identify proteins of potential mechanistic interest and differentiate proteins meeting a <0.1 vs. <0.05 FDR threshold. All statistical analyses were done using R (version 4.1.3) and the lme4 package.20,21
This is a convenience sample of COVID cases and controls with available specimens pre and post COVID infection. Due to the unavailability of prior literature data using pre-COVID specimens to estimate temporal changes in proteomic markers in PWH or other populations, we were unable to perform a priori power calculations.
Role of the funding source
The funders had no role in study design, data collection, data analyses, interpretation, or writing of this report. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute or the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; or the US Department of Health and Human Services.
Results
Study population
We identified 94 antibody confirmed clinical cases. Cases were compared to 113 antibody negative controls. See CONSORT diagram (Fig. 1). The unblinded REPRIEVE study statistician confirmed that the difference in allocation to statin vs. placebo was minimal, with only a 1.5% difference in the percentage allocated to statin therapy in the COVID cases and controls.
Participant characteristics
The median age at enrollment was 50 years (interquartile range: 45–54 years), 73% were male. Among the cases, 40% had mild COVID-19, while 60% had moderate-severe COVID-19, with 12 serious cases requiring hospitalization. Relatively few patients received therapy for COVID-19. Cases and controls were comparable regarding demographics, median ASCVD risk scores, body mass index, and duration of HIV and ART use (Table 2). Baseline characteristics of the complete REPRIEVE population have been published.22
Table 2.
Demographics of the final analytical cohort.
| Characteristic | Total (N = 207) | Case (N = 94) | Control (N = 113) |
|---|---|---|---|
| Demographics | |||
| GBD Super Region | |||
| High Income | 95 (46%) | 41 (44%) | 54 (48%) |
| Latin America and Caribbean | 83 (40%) | 34 (36%) | 49 (43%) |
| South Asia | 9 (4%) | 7 (7%) | 2 (2%) |
| Sub-Saharan Africa | 20 (10%) | 12 (13%) | 8 (7%) |
| Age at enrollment (years) | |||
| Median (Q1-Q3) | 50 (45–54) | 49 (44–54) | 50 (45–54) |
| Average (SD) | 50 (6) | 50 (6) | 50 (6) |
| 40–49 | 102 (49%) | 49 (52%) | 53 (47%) |
| 50–59 | 87 (42%) | 37 (39%) | 50 (44%) |
| 60+ | 18 (9%) | 8 (9%) | 10 (9%) |
| Natal sex | |||
| Male | 152 (73%) | 73 (78%) | 79 (70%) |
| Female | 55 (27%) | 21 (22%) | 34 (30%) |
| Race | |||
| White | 80 (39%) | 33 (35%) | 47 (42%) |
| Black or African American | 79 (38%) | 34 (36%) | 45 (40%) |
| Asian | 11 (5%) | 8 (9%) | 3 (3%) |
| Other | 37 (18%) | 19 (20%) | 18 (16%) |
| Ethnicity | |||
| Hispanic or Latino | 21 (22%) | 12 (29%) | 9 (16%) |
| Not Hispanic or Latino | 76 (78%) | 29 (71%) | 47 (84%) |
| Cardiometabolic risk factors | |||
| ASCVD risk score (%) | |||
| Median (Q1-Q3) | 3.9 (2.2–6.2) | 4.1 (2.8–6.7) | 3.9 (2.1–5.9) |
| Average (SD) | 4.6 (3.1) | 4.8 (3.0) | 4.4 (3.3) |
| 0−<2.5 | 56 (27%) | 23 (24%) | 33 (29%) |
| 2.5−<5 | 68 (33%) | 29 (31%) | 39 (35%) |
| 5–10 | 71 (34%) | 38 (40%) | 33 (29%) |
| >10 | 12 (6%) | 4 (4%) | 8 (7%) |
| BMI (kg/m2) | |||
| Median (Q1-Q3) | 25.9 (23.2–29.5) | 26.3 (24.0–30.7) | 25.6 (22.6–28.6) |
| Average (SD) | 26.9 (5.5) | 27.6 (5.9) | 26.3 (5.2) |
| Hypertension at entry | |||
| Yes | 63 (30%) | 30 (32%) | 33 (29%) |
| Ever been on a statin | |||
| Yes | 20 (10%) | 9 (10%) | 11 (10%) |
| History of diabetes | |||
| Yes | 3 (1%) | 0 (0%) | 3 (3%) |
| Smoking status | |||
| Current | 41 (20%) | 17 (18%) | 24 (21%) |
| Former | 62 (30%) | 25 (27%) | 37 (33%) |
| Never | 104 (50%) | 52 (55%) | 52 (46%) |
| HIV-related health status | |||
| Duration of HIV (years) | |||
| <5 | 25 (12%) | 12 (13%) | 13 (12%) |
| 5–10 | 66 (32%) | 36 (38%) | 30 (27%) |
| >10 | 116 (56%) | 46 (49%) | 70 (62%) |
| Total ART use (years) | |||
| <5 | 48 (23%) | 23 (24%) | 25 (22%) |
| 5–10 | 58 (28%) | 28 (30%) | 30 (27%) |
| 10+ | 101 (49%) | 43 (46%) | 58 (51%) |
| ART regimen (by class) | |||
| NRTI + NNRTI | 101 (49%) | 52 (55%) | 49 (43%) |
| NRTI + INSTI | 53 (26%) | 20 (21%) | 33 (29%) |
| NRTI + PI | 35 (17%) | 16 (17%) | 19 (17%) |
| NRTI-sparing | 4 (2%) | 2 (2%) | 2 (2%) |
| Other NRTI-containing | 14 (7%) | 4 (4%) | 10 (9%) |
| Nadir CD4 count (cells/mm3) | |||
| <50 | 33 (17%) | 15 (16%) | 18 (17%) |
| 50–99 | 25 (13%) | 11 (12%) | 14 (13%) |
| 100–199 | 41 (21%) | 20 (22%) | 21 (19%) |
| 200–349 | 49 (25%) | 23 (25%) | 26 (24%) |
| 350–499 | 27 (14%) | 13 (14%) | 14 (13%) |
| 500+ | 25 (13%) | 9 (10%) | 16 (15%) |
| HIV-1 RNA (copies/mL) | |||
| <LLQ | 144 (92%) | 59 (95%) | 85 (90%) |
| LLQ−< 400 | 12 (8%) | 3 (5%) | 9 (10%) |
| CD4 count (cells/mm³) | |||
| Median (Q1-Q3) | 633 (485–852) | 606 (483–795) | 690 (494–908) |
| Average (SD) | 690 (306) | 650 (296) | 723 (311) |
| <200 | 4 (2%) | 2 (2%) | 2 (2%) |
| 200–349 | 20 (10%) | 11 (12%) | 9 (8%) |
| 350–499 | 32 (15%) | 14 (15%) | 18 (16%) |
| 500+ | 151 (73%) | 67 (71%) | 84 (74%) |
| CD8 count (cells/mm³) | |||
| Median (Q1-Q3) | 728 (544–1021) | 773 (546–1006) | 676 (515–1023) |
| Average (SD) | 812 (375) | 850 (401) | 778 (348) |
| CD4:CD8 ratio | |||
| Median (Q1-Q3) | 0.9 (0.7–1.2) | 0.9 (0.5–1.1) | 0.9 (0.7–1.4) |
| Average (SD) | 1.0 (0.5) | 0.9 (0.5) | 1.1 (0.5) |
| Soluble CD14 (ng/mL) | |||
| Median (Q1-Q3) | 1687 (1435–1987) | 1602 (1389–1820) | 1782 (1493–2151) |
| Average (SD) | 1734 (449) | 1617 (387) | 1825 (474) |
| Antibody status | |||
| IgG result | |||
| Positive | 85 (41%) | 85 (90%) | 0 (0%) |
| IgA result | |||
| Positive | 52 (25%) | 52 (55%) | 0 (0%) |
| Clinical COVID-19 and medicationin cases only | |||
| Severity grade of COVID-19 case | |||
| Mild | N/A | 38 (40%) | N/A |
| Moderate | N/A | 44 (47%) | N/A |
| Severe | N/A | 12 (13%) | N/A |
| Received corticosteroids | |||
| Yes | N/A | 9 (10%) | N/A |
| Received antivirals | |||
| Yes | N/A | 6 (6%) | N/A |
| Received immune sera and immunoglobulins | |||
| Yes | N/A | 1 (1%) | N/A |
| Received immunosuppressants | |||
| Yes | N/A | 1 (1%) | N/A |
Association of COVID-19 infection with temporal changes in protein expression
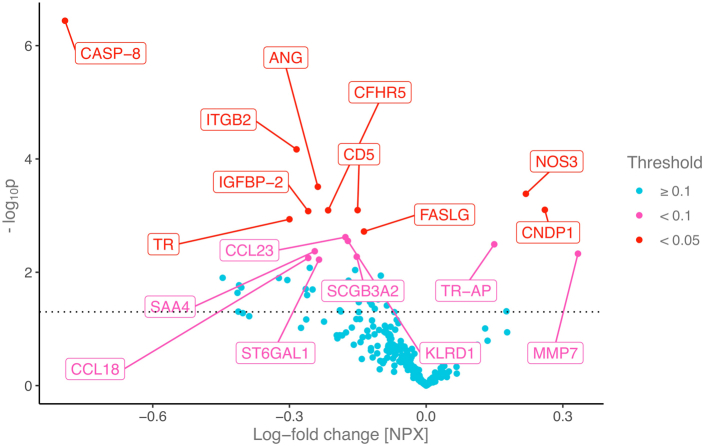
The median time between the two blood samples was 2 years (interquartile range: 1.9–2.2 years). The median time from the COVID-19 diagnosis to the follow-up samples was 4 months (interquartile range: 2–7 months) for the cases. Comparing the 94 cases to the 113 controls, 18 proteins were found to be differentially expressed following COVID-19. This included ANG, CASP-8, CCL18, CCL23, CD5, CFHR5, CNDP1, FASLG, IGFBP-2, ITGB2, KLRD1, MMP7, NOS3, SAA4, SCGB3A1, ST6AL1, TR and TR-AP (see Table 1 for abbreviations). Among these proteins, relative to changes observed in controls, CNDP1, MMP7, NOS3 and TR-AP demonstrated a pattern of increased expression over time among cases, while other proteins demonstrated a pattern of decreased protein expression, compared to pre-pandemic protein levels (Fig. 2 and Supplemental Table S3).
Fig. 2.
Temporal changes in protein expression following COVID-19 infection. Each point represents the change in protein expression following COVID-19 (x axis) for a given protein and it’s corresponding -log10p value (y axis). The further away a point is from the 0 value on the x axis, the larger the change in protein expression following COVID-19 as compared to controls, while the higher the point is, the smaller the p value. The dotted horizontal line indicates a nominal p = 0.05. The proteins with FDR corrected p < 0.1 are labeled and colored according to FDR corrected p values. All linear mixed models were corrected for ASCVD risk score and the time difference between the two measurements and included a random intercept per patient. Protein name abbreviations can be found in Table 1. ASCVD: atherosclerotic cardiovascular disease; COVID-19: Coronavirus disease 2019.
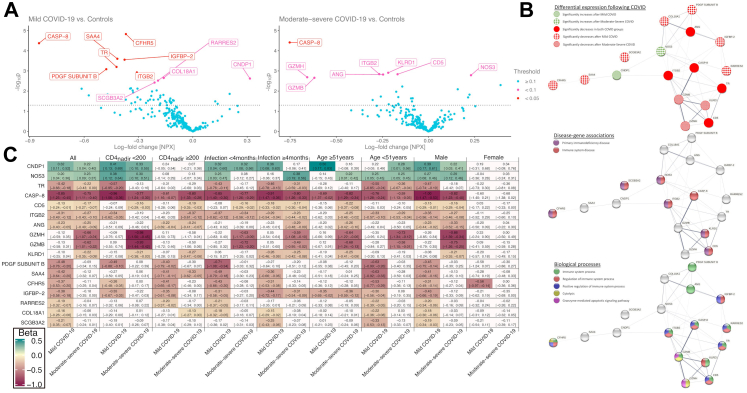
Association of COVID severity with differential temporal changes in protein
Differential patterns of protein changes were seen among those with mild disease vs. controls and among those experiencing moderate-severe disease vs. controls (Fig. 3A). Among those experiencing mild disease vs. controls CNDP1 increased whereas CASP-8, CFHR5, COL18A1, IGFBP-2, ITGB2, PDGF Subunit B, RARRES2, SAA4, SCGB3A2 and TR decreased. Among those experiencing moderate to severe disease vs. controls, NOS3 increased whereas ANG, CASP-8, CD5, GZMH, GZMB, ITGB2, and KLRD1 decreased (Fig. 3A and Supplemental Table S4).
Fig. 3.
A) Association between temporal changes in protein expression and COVID-19 severity. Each point represents the change in protein expression following COVID-19 (x axis) for a given protein and it’s corresponding -log10p value (y axis). The further away a point is from the 0 value on the x axis, the larger the change in protein expression following COVID-19 as compared to controls, while the higher the point is the smaller the p value. The dotted horizontal line indicates a nominal p = 0.05. The proteins with FDR corrected p < 0.1 for coefficient are labeled and colored according to FDR corrected p values. All linear mixed models were corrected for ASCVD risk score and the time difference between the two measurements and included a random intercept per patient. B) Protein–protein interaction network of significant proteins. Nodes represent proteins showing significant differential expression following mild and/or moderate-severe COVID-19 as compared to controls in the final analytical cohort. Edges represent the confidence level of the inter-relatedness where the width is proportional to the confidence level based on all interaction sources. Panel A shows which proteins were associated with the different COVID-19 severity grades. Panel B shows the disease–gene associations of the proteins. Panel C shows the biological process gene ontology terms. C) Differential expression of proteins between mild COVID-19 and moderate-severe COVID-19 vs. control in the different stratification groups. Beta coefficients and 95% confidence intervals from linear mixed models of proteins showing significant changes in expression levels following mild and/or moderate-severe COVID-19 vs. control in the final analytical cohort and for each stratification group. All linear mixed models were corrected for ASCVD risk score and the time difference between the two measurements and included a random intercept per patient. Boxes are colored if nominal p < 0.05 and the intensity of the color represents the magnitude of the beta coefficient. Protein name abbreviations can be found in Table 1. ASCVD: atherosclerotic cardiovascular disease; COVID-19: Coronavirus disease 2019.
Knowledge-based interaction analysis focused on proteins demonstrating temporal change following COVID-19
Most proteins were associated with immune functions and processes in gene ontology, while GZMB, GZMH and KLRD1 are associated with granzyme-mediated apoptotic signaling and natural killer cell regulation in the STRING knowledge-based protein interaction database. CASP-8, CD5, CFHR5, ITGB2, KLRD1, NOS3 and SCGB3A2 play roles in immune system and primary immunodeficiency diseases. Network graphs of the significant proteins are shown in Fig. 3B. Different patterns of protein interactions were seen among those with mild diseases vs. control as opposed to those with moderate-severe disease vs. control.
Stratified analyses of change in protein expression by immune function, time since infection, age, and sex
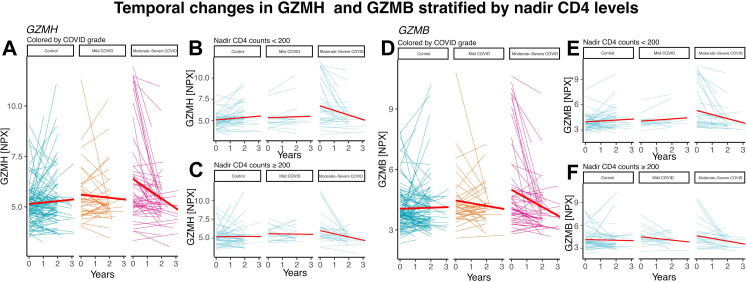
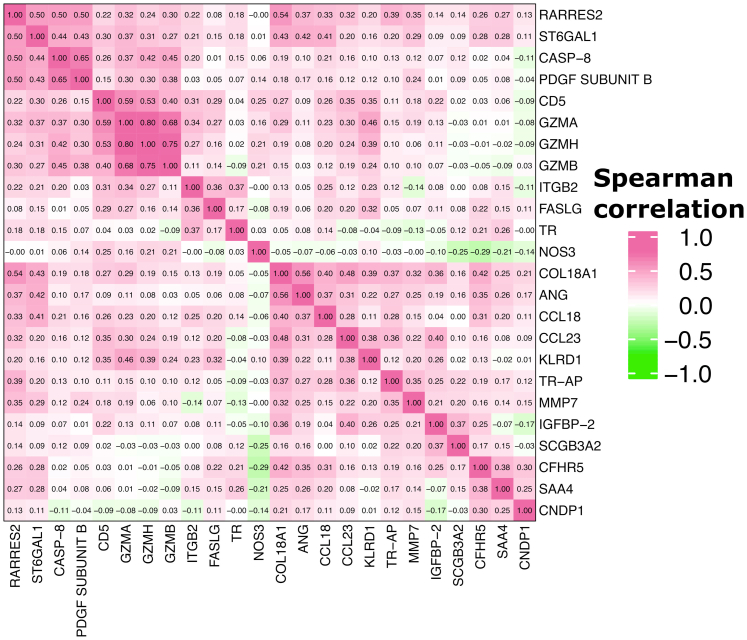
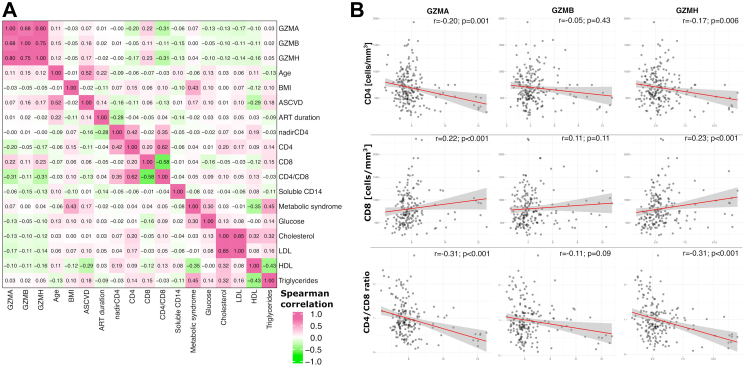
CASP-8 showed decrease in expression both for mild and moderate-severe cases vs. controls in all stratified analyses (Fig. 3C). GZMH and GZMB was decreased in moderate-severe cases most prominently in the CD4nadir <200 group (Beta coefficients −0.98 [CI: −1.50; −0.45] p < 0.001, −0.93 [CI: −1.44; −0.42] p < 0.001, respectively), while showing borderline associations in the CD4nadir ≥200 (−0.58 [CI: −1.17; 0.01] p = 0.05, −0.50 [CI: −1.06; 0.06] p = 0.08). The decreases in GZMH and GZMB, were not seen in mild disease. Temporal trend plots of GZMB and GZMH levels are shown in Fig. 4. Among proteins only differentially expressed in mild cases, we found more prominent effect sizes in those who had acquired COVID-19 within 4 months of the second sample, and also in younger individuals (Fig. 3C). In sex-stratified analyses, findings for GZMH and GZMB were most prominent for males. Correlation heatmap of baseline expression levels of significant proteins can be found in Fig. 5.
Fig. 4.
Temporal trend plots of GZMB and GZMH levels stratified by COVID-19 severity. Spaghetti plots show the intra-individual changes in protein expression stratified by COVID-19 severity. Red lines indicate temporal regression lines for the given group. GZMB, GZMH: Granzyme B, H.
Fig. 5.
Heatmap of Spearman correlation values of baseline expression levels of significant proteins in our analyses. Abbreviations can be found in Table 1.
Differences in baseline protein expression among participants who went on to acquire COVID-19 (cases) vs. controls
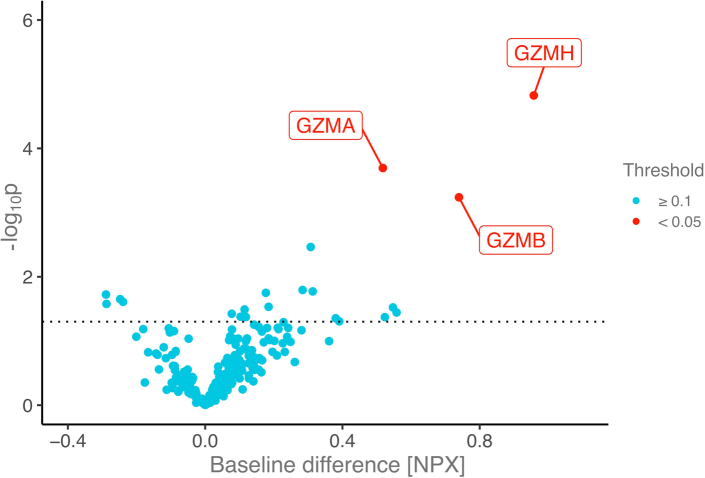
Among the 257 proteins assessed, expression of GZMA, GZMB and GZMH was significantly higher at baseline among participants who went on to acquire COVID-19 as compared to controls (0.51 [CI: 0.24; 0.79] p < 0.001; 0.73 [CI: 0.32; 1.15]) p < 0.001; 0.95 [CI: 0.53; 1.38] p < 0.001, respectively). Baseline expression of other proteins was not associated with future COVID-19 (Fig. 6).
Fig. 6.
Differences in baseline protein expression between those who later acquire COVID-19 vs. controls. Each point represents the difference in baseline protein expression value (x-axis) between those who will later acquire COVID-19 and those who will not (controls), and the corresponding -log10p value (y axis). The further away a point is from the 0 value on the x axis, the larger the difference in protein expression between future cases and controls, while the higher the point is the smaller the p value. The dotted horizontal line indicates a nominal p = 0.05. The proteins with FDR corrected p < 0.1 are labeled and colored according to FDR corrected p values. All linear regression models were corrected for ASCVD risk score. Protein name abbreviations can be found in Table 1. ASCVD: atherosclerotic cardiovascular disease; COVID-19: Coronavirus disease 2019. GZMA, GZMB, GZMH: Granzyme A, B, H.
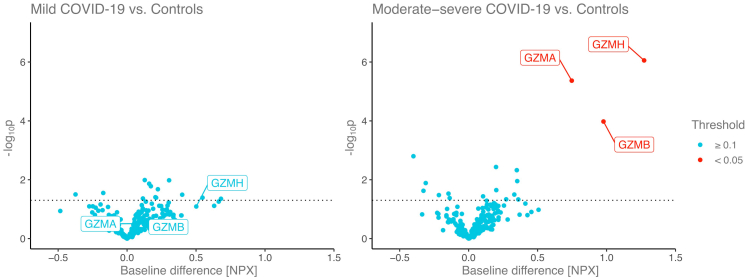
Baseline levels of GZMA, GZMB and GZMH were associated with the later development of moderate-severe grade COVID-19 (0.75 [CI: 0.44; 1.06] p < 0.001; 0.98 [CI: 0.49; 1.47] p < 0.001; 1.28 [CI: 0.78; 1.77] p < 0.001, respectively), but not significantly associated with mild COVID-19 (0.18 [CI: −0.17; 0.54] p = 0.31; 0.39 [CI: −0.16; 0.95] p = 0.16; 0.50 [CI: −0.06; 1.06] p = 0.08, respectively) (Fig. 7). Baseline GZMA, GZMB and GZMH values showed very modest correlations with age and ASCVD risk score and other metabolic variables (Fig. 8A). GZMA and GZMH were inversely correlated with CD4 count and CD4/CD8 ratio, and positively correlated with CD8 counts (Fig. 8B). In additional multivariate regression analysis, baseline GZMA, GZMB and GZMH remained significantly associated with later development of moderate to severe COVID-19 adjusting for CD4, CD8, age, sex and Global Burden of Disease region, and ASCVD score, as well as for BMI, metabolic syndrome, and duration of ART (Table 3, Supplementary Table S5).
Fig. 7.
Differences in baseline protein expression with regards to COVID-19 severity grade. Each point represents the difference in baseline protein expression value (x-axis) between those who will later acquire COVID-19 and have mild grade disease and those who will not (controls) or moderate-severe grade disease and controls, and the corresponding -log10p value (y axis). The further away a point is from the 0 value on the x axis, the larger the difference in protein expression future cases and controls, while the higher the point is the smaller the p value. The dotted horizontal line indicates a nominal p = 0.05. The proteins with FDR corrected p < 0.1 are labeled and colored according to FDR corrected p values. GZMA, B, H and are highlighted on the mild COVID-19 vs. control plot to show the difference in effect size and p values as compared to the moderate-severe group vs. controls. All linear regression models were corrected for ASCVD risk score. ASCVD: atherosclerotic cardiovascular disease; COVID-19: Coronavirus disease 2019; GZMA, GZMB, GZMH: Granzyme A, B, H.
Fig. 8.
A) Correlation between baseline GZMA, GZMB and GZMH with clinical, metabolic, lipid and immunological parameters. B) Scatter plots between baseline GZMA, GZMB, GZMH and CD4, CD8 and CD4/CD8 levels. Red lines indicate regression lines with 95% confidence bounds indicated in gray between the given proteins and CD4, CD8 and CD4/CD8 levels. Spearman correlation values and corresponding p values are reported for each graph. ART: antiretroviral therapy; ASCVD: atherosclerotic cardiovascular disease; BMI: Body mass index; GZMA, GZMB, GZMH: Granzyme A, B, H; HDL: high-density lipoprotein; LDL: low density lipoprotein.
Table 3.
Association between baseline GZMA, GZMB and GZMH with later development of COVID-19.
| Assay | Mild COVID-19 vs. controls |
Moderate-severe COVID-19 vs. controls |
||||
|---|---|---|---|---|---|---|
| β | p | 95% CI | β | p | 95% CI | |
| Model-I | ||||||
| GZMA | 0.13 | 0.48 | −0.23; 0.48 | 0.71 | <0.0001 | 0.40; 1.02 |
| GZMB | 0.35 | 0.22 | −0.21; 0.91 | 0.95 | <0.001 | 0.46; 1.44 |
| GZMH | 0.43 | 0.14 | −0.13; 0.99 | 1.22 | <0.0001 | 0.73; 1.71 |
| Model-II | ||||||
| GZMA | 0.08 | 0.63 | −0.24; 0.39 | 0.38 | 0.01 | 0.09; 0.67 |
| GZMB | 0.29 | 0.25 | −0.21; 0.78 | 0.47 | 0.04 | 0.02; 0.93 |
| GZMH | 0.37 | 0.15 | −0.14; 0.87 | 0.74 | 0.002 | 0.28; 1.20 |
| Model-III | ||||||
| GZMA | 0.12 | 0.53 | −0.26; 0.49 | 0.70 | <0.0001 | 0.37; 1.03 |
| GZMB | 0.37 | 0.22 | −0.23; 0.97 | 0.96 | <0.001 | 0.44; 1.49 |
| GZMH | 0.41 | 0.30 | −0.18; 1.00 | 1.15 | <0.0001 | 0.64; 1.67 |
Model-I: Model corrected for ASCVD risk score at enrollment and CD4.
Model-II: Model corrected for Age at baseline proteomic sample, natal sex, GBD super region, CD4, body mass index, metabolic syndrome, and fasting glucose.
Model-III: Model corrected for ASCVD risk score at enrollment and CD4, CD8, time on anti-retroviral therapy.
GZMA, GZMB, GZMH: Granzyme A, B, H.
Discussion
Through our study, we have gleaned insights from the application of serial proteomic phenotyping using a case–control design within a large international sample of PWH. First, we identified that levels of a set of proteins, including MMP7, NOS3, CNDP1, and TR-AP, increased more over time among PWH who experienced COVID-19 as compared to PWH who did not. Second, we found that key proteins involved in immune regulation, including CASP-8, decreased more over time in both mild and moderate-severe COVID-19 as compared to controls. Third, we identified that the granzyme proteins GZMB and GZMH decreased more over time among PWH who experienced moderate-severe COVID-19 (but not among PWH who experienced mild COVID-19) as compared to PWH who did not. Finally, we observed that baseline levels of the three proteins in the granzyme family—GZMA, GZMB, and GZMH—independently associated with ensuing development of moderate-severe COVID-19 among PWH. Taken together, our findings shed light on biologic pathways involved in predisposing PWH to symptomatic COVID-19 and increasing disease severity. Moreover, our work paves the way for ensuing studies exploring whether persistent biologic changes precipitated by COVID-19 among PWH are protective against future COVID-19 re-infection, or alternatively, whether such changes are maladaptive, potentially contributing to more severe acute COVID-19 or even to PASC symptomatology and other immune or tissue vulnerabilities.
The relationship between higher levels of granzyme proteins—particularly GZMB—and ensuing moderate-to-severe COVID-19 among PWH represents an intriguing and potentially clinically relevant finding. Prior studies have shown a sharp transition and changes in immune cell function and circulating proteins between mild and moderate disease,23 supporting the choice to separately compare those with mild and moderate/severe disease vs. controls. GZMB, a serine protease expressed by cytotoxic T lymphocytes (CTL) and natural killer (NK cells), participates in triggering apoptosis among cells expressing foreign antigens (e.g. in the context of an infection).24 Studies among individuals newly infected with HIV have suggested that cytotoxic activity effected by CTLs via granzymes A, B, and K (as well as by other effector proteins such as perforin) is critical to establishing the initial viral set point.25 Elevated levels of soluble granzymes have also been described in autoimmune diseases suggesting they are a biomarker of systemic inflammation.26 In general-population studies, increased GZMB expression by cytotoxic cells during COVID-19 infection has been directly related to disease severity.27,28 Studies assessing acute and convalescent serum in this regard have shown increased granzyme B production acutely,29 followed by a decline over time from symptom onset,30 and reduced production in convalescent specimens compared to uninfected controls.31 CD4 and NK cells demonstrate a reduced cytolytic response and intracellular Granzyme A, which is more pronounced among ICU COVID patients, related inversely to IL-6 concentrations, and improved with tocilizumab.32 Mathematical modeling has predicted peak cytokine response rates against structural SARS-CoV-2 structural proteins for GZMB of 7 days with declining but persistent GZMB production in 70% of cells in ex vivo assays one year after symptom onset.33 These observations are compatible with host efforts to lyse SARS-CoV-2 infected cells through increased cytotoxic cell granzyme expression and highlight differences with regard to phase of infection and severity. Moreover, these data suggest a reduced intracellular granzyme response in COVID-19, particularly in the setting of severe COVID and in convalescence.
Our data on circulating Granzyme levels, comparing pre and post specimens from COVID infected and non-infected PWH extend our understanding of the granzyme response in COVID-19. Sneller et al. demonstrated that circulating levels of GZMB levels were increased in COVID-19 vs. controls in the general population.34 Similarly, Filbin et al. demonstrated higher GZMB, A, and H in patients presenting acutely with COVID than in patients presenting symptomatically with other respiratory infections,35 using the same Olink assay as in the current study. In contrast, we show that granzyme levels decrease over time in PWH with severe COVID vs. those with mild. Given that our median specimen collection is 4 months post infection, this result demonstrates a decrease in convalescent serum of those with severe COVID, suggestive of a senescent or exhausted phenotype reminiscent of that seen in analyses of cellular immune function outlined above. Importantly, we make this observation in serial blood collected from the individual patients with samples available pre COVID, allowing for a comparison with a pre COVID time points. Moreover, using a baseline specimen available pre COVID, we show an association with future development of COVID-19. To our knowledge this is the first such observation in PWH. In addition, our analysis delineates a specific subgroup, those with moderate-severe COVID-19, in whom this relationship is most significant. In our data, the association between GZMB and development of future moderate-severe disease remains significant controlling for critical baseline parameters that might also influence the development of COVID-19. Moreover, recent interest has focused on the relationship of COVID severity with specific metabolic phenotypes, including hypermetabolic CD8 T cells,36 unique adaptive NK phenotypes and NK cells with early IFNα signatures.37,38 It will be important to relate granzyme levels to these immune phenotypes in future studies.
Why might increased circulating GZMB among PWH predict moderate or severe COVID-19? One intriguing possibility is that increased GZMB among individuals with chronic, ART-treated HIV reflects a form of immune dysfunction whereby enhanced cytotoxic activity is required to maintain a given virologic set-point. Increased CTL GZMB response to HIV viral proteins is primarily from CD8+ cells, relates to replication competent proviral DNA levels and helps to limit the HIV viral reservoir.39 CD8+ cells among PWH have a reduced functional capacity with reduced degranulation and cytotoxicity associated with inflammation and immune exhaustion.40 In Macaque simian immunodeficiency virus models, elevated GZMB expressing B cells relate to lower CD4 count.41 Increased GZMB responses from CD8+ stimulation have also been shown to be a marker of other co-infections in HIV, including tuberculosis.42 In addition to cytotoxic killing, extracellular granzymes are an important amplifier of the inflammatory response by promoting the release of inflammatory cytokines from monocytes and macrophages.43,44 Granzyme production thereby functions as part of a critical immune response to HIV as well as a generalized inflammatory response to pathogens and/or tissue repair. Here we show an inverse relationship between GZMB and the CD4/CD8 ratio among ART-treated PWH. We interpret our data to suggest that increased GZMB levels may either serve as a marker for immune dysfunction and/or potentially amplify an increased inflammatory response that predisposes to severe COVID-19 in PWH. Notably, these observations are hypothesis generating as we are not measuring specific CTL responses, but rather circulating levels of a few specific granzymes. Further studies are needed to explore the role of the granzyme system in the response to COVID-19 among PWH.
In our study, PWH who experienced antibody-confirmed COVID-19 (vs. PWH who did not) exhibited greater increase over time in levels of proteins including MMP7, CDNP1, NOS3, and TR-AP. Persistent elevation of MMP7 is particularly interesting, given that this matrix metalloproteinase may either contribute to fibrosis or promote resolution of fibrosis.45 General population studies of patients with persistent respiratory symptoms after acute COVID-19 have shown that levels of MMP7 were higher among patients with more severe COVID-19 and inversely correlated to measures of respiratory health (including forced vital capacity and diffusing capacity for carbon monoxide).46 Data from Su et al. support a finding of increased MMP7 over time with COVID-19.23 Whether MMP7 upregulation during COVID-19 is a pathologic or salutary response, persistent increases in this marker among PWH may be reflective of the burden of organ fibrosis incurred during the COVID-19 bout and thus potentially predictive of PASC symptomatology.
Among PWH who experienced antibody-confirmed COVID-19 (vs. PWH who did not), levels of numerous proteins—including CASP8 and others—declined to a greater extent. STRING knowledge-based protein interaction analysis revealed that several of these proteins participate in positive regulation of immune system processes. For example, CASP-8 is a protease integral to initiation of cellular apoptosis. In murine models, SARS-CoV-2 infection triggers innate immune responses leading to production of inflammatory cytokines such as TNF-a and IFN-gamma, which in-turn activate cellular JAK/stat signaling and a cascade towards CASP8-mediated inflammatory cell death.47 Meanwhile, general-population studies applying proteomic phenotyping to patients hospitalized with COVID-19 have revealed higher circulating CASP8 levels among patients with more severe disease.48 Why would COVID-19 prompt levels of proteins involved in positive regulation of the immune system to drop to levels below the pre-COVID baseline? Possibilities include adaptive downregulation vs. maladaptive immune exhaustion. With respect to the latter possibility, we noted in our cohort of PWH that levels of granzymes including GZMB significantly decreased only among the subset of participants who experienced moderate-to-severe COVID-19. Thus, among PWH, high levels of GZMB at baseline associate with ensuing moderate-to-severe COVID-19, relate inversely to measures of immune health (CD4/CD8 ratio), and decrease to below baseline levels post moderate-to-severe COVID-19 bout. Our study highlights a particular subset of PWH in whom granzyme levels are elevated prior to COVID-19 in relationship to lower CD4 and higher CD8, and who demonstrate decline with moderate-severe COVID-19. Taken together, these findings could be consistent with an immune exhaustion phenomenon among a subset of at-risk PWH.
Among our set of proteins changing differentially over time among those experiencing COVID-19 vs. controls CASP8, CCL23, CD5, MMP7, NOS3, PDGFB, KLRD1, were measured by Su et al. similarly using Olink technology, and shown to be higher in patient with COVID-19 vs. controls. Of these, CCL23, NOS3 and KLDR1 decreased, while MMP7 increased over the course of COVID-19 (at admission, acute phase of disease and 2–3 months after symptoms). Of note, our studies were comparing change from pre-COVID to convalescence, whereas Su et al. compared data from diagnosis to convalescence, limiting a direct comparison between the studies.23 Future studies can be performed with better understanding of chronic symptomatology to assess more comprehensively whether these protein changes relate to potential PASC in PWH and non PWH populations.
This study has a number of strengths as well as limitations. We are not aware of other studies, in any population, that have used banked specimens for proteomic assessment of pre and post COVID specimen in the same individual. We compared longitudinal proteomic responses over time among matched PWH with and without confirmed COVID in this study, focusing on a key patient group with increased COVID morbidity. We utilized a case–control design with careful matching to enhance the validity of our findings among PWH. However, given the unique design of the REPRIEVE trial and access to pre and post COVID-specimens, we were not able to obtain similar information in non-HIV nor able to perform a validation study, which will be important in the future. Nonetheless, we have compared data from the literature providing context to our observations of changes in granzyme activity in response to COVID. Other limitations, include inter-individual variation in the duration of time between COVID-19 and post-COVID blood sampling. However, we have endeavored to account for this potential pitfall through our analytic approach, and our average sampling duration of 4 months allows comparison with data of post convalescent sampling. Cases and controls were balanced with respect to statin assignment which had been ongoing for many years prior to the current analysis. We used a standard DAIDS severity grading scale that was available in REPRIEVE, but participants were not graded using WHO severity scales. Further classification of severity using more detailed grading scales may provide additional data in future studies. Participants in this protocol were all receiving ART, with generally good immune function. Greater perturbations in the proteins identified in this study may occur among PWH with more severe immune dysfunction. Also, this analysis included a limited number of participants with severe or life-threatening COVID-19, and further analyses of these proteins are necessary in this important subset. Although our analysis included 25% females, sex stratified analyses may nonetheless have been limited by these small numbers. Our study cannot fully address the issue of protein changes among PWH with long COVID. Only a limited number of proteins that we found to be changing most significantly over time have been assessed with respect to long COVID in comprehensive studies to date,23 and further studies assessing proteomic changes among PWH are necessary to assess the relevance of our findings with respect to long-COVID. We chose to evaluate soluble proteins that changed pre- and post-COVID with an FDR <0.1 in order not to miss important mechanistic signals.
Among ART-treated PWH, we identified proteins in the granzyme family which predict ensuing moderate-to-severe COVID-19, relate to baseline immune dysregulation, and decline significantly and in a lasting manner after moderate-to-severe disease. In this regard, it is possible that high granzyme levels represent a more sensitive measurement of residual immune dysfunction which impacts susceptibility to specific respiratory viruses in HIV. We also characterized a network of proteins involved in immune–regulatory processes which are known to be elevated during COVID-19, but which appear to decline to pre-COVID levels and remain persistently low. Finally, we discovered a set of proteins the levels of which rose more among PWH who experienced COVID-19 vs. PWH who did not. Our study represents the first to comprehensively apply serial proteomic profiling to a large, international cohort of PWH with vs. without COVID-19. These findings may help to guide future directions with respect to: 1) improving clinical prediction of which PWH are at greatest risk of developing severe COVID-19 so as to most effectively deploy preventive and therapeutic strategies; 2) understanding which persistent changes in immune-regulatory proteins post-COVID are maladaptive. These may potentially be either reflective of or contributing to pathology—including, but not limited to, either PASC symptomatology or immune dysfunction and concurrent susceptibility to other infectious complications.
Contributors
Study design: MK, CD, MVZ, SKG, HJR; Methodology: MK, CD, SKG, HJR; Analysis: MK, SM, HJR; Visualizations: MK; Supervision of statistics: HJR; Primary funding acquisition: SKG; Verification of data and results: MK, SM, SKG, HJR; Writing—draft: MK, MVZ, SKG; Writing—review & editing: CD, SM, KVF, MRD, ESF, HJR, CJF, JAA, CDM, JSC, JLC, FG, IS, PSD.
All authors read and approved the final version of the manuscript.
Data sharing statement
We have deposited the Proteomic data from this analysis including baseline and follow-up levels, as well as classifiers for cases and controls and disease severity in a publicly available database (URL: https://data.mendeley.com/datasets/r5sr3ryg83).
Declaration of interests
MK received financial support from the Ralph Schlaeger Fellowship Award from the Department of Radiology at Mass General Hospital (Boston, USA) outside of this project, and was also supported by the T32 fellowship 5T32HL076136 of the NIH.
CD reports no disclosures.
SM reports no disclosures.
KVF reports no disclosures.
MRD reports no disclosures.
ESF reports no disclosures.
HJR reports grant support from NIH/NIAID and NIH/NHLBI related to the conduct of the study, as well as grant support from NIH/NIAID, NIH/NHLBI, NIH/NIDDK, and NIH/NIA, outside the submitted work.
CJF reports grant support through his institution from Gilead Sciences, ViiV Healthcare, GSK, Janssen, Abbvie, Merck, Amgen, and Cytodyn, outside the submitted work; and personal fees from Theratechnologies and ViiV for consulting and participation on Advisory Board unrelated to REPRIEVE with Theratechnologies and ViiV, and role as Chair on DSMB for Intrepid Study, outside the submitted work.
JAA reports institutional research support for clinical trials from Atea, Emergent Biosolutions, Frontier Technologies, Gilead Sciences, GlaxoSmithKline, Janssen, MacroGenics, Merck, Pfizer, Regeneron, and ViiV Healthcare and personal fees for advisory boards from Glaxo Smith Kline/Viiv and Merck and participation on DSMB for Kintor Pharmaceuticals; all outside the submitted work.
CDM reports institutional research support by Lilly and honoraria from ViiV Healthcare and Gilead Sciences for Advisory Board membership, outside the submitted work.
JSC reports consulting fees from Merck and Company.
JLC reports honoraria for presentations for Gilead, MSD, and Janssen, and honoraria from Viiv Healthcare and Gilead Sciences for Advisory Board membership, all outside the submitted work.
FG has received compensation for lectures, presentations, speaker bureaus, educational events or advisory boards from Janssen-Cilag, Gilead Sciences and ViiV Healthcare, and support for attending meetings and/or travel from Janssen-Cilag and ViiV Healthcare, and participation on a DSMB or Advisory Board for Janssen-Cilag and ViiV Healthcare.
IS reports no disclosures.
PSD reports no disclosures.
MVZ reports being Principal Investigator of research grants from NIH (NIAID and NHLBI) and from Gilead Sciences to her institution, receiving support from CROI and International Workshop for HIV and Women Conferences when invited to be an abstract reviewer and/or speaker, and participating in a DSMB for NIH-funded studies involving no compensation.
SKG reports grant support through his institution from Kowa Pharmaceuticals America, Inc., Gilead Sciences, Inc., and ViiV Healthcare for the conduct of the study, as well as grants from Theratechnologies and Navidea and personal fees from Theratechnologies and ViiV, all outside the submitted work. He is a member of the Scientific Advisory Board of Marathon Asset management.
Acknowledgements
This study is supported through NIH grants U01HL123336, U01HL123336-06 and 3U01HL12336-06S3, to the clinical coordinating center, and U01HL123339, to the data coordinating center as well as funding from Kowa Pharmaceuticals and a grant award through ViiV Pharmaceuticals. The NIAID supported this study through grant UM1 AI068636, which supports the AIDS Clinical Trials Group (ACTG) Leadership and Operations Center.
This work was also supported by NIAID through grant K24AI157882 to MZ.
The study investigators thank the study participants, site staff, and study-associated personnel for their ongoing participation in the trial. In addition, we thank the following: the AIDS Clinical Trial Group (ACTG) for clinical site support; ACTG Clinical Trials Specialists for regulatory support; the data management center, Forensic Sciences Foundation, for data support; and the Center for Biostatistics in AIDS Research for statistical support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104538.
Appendix ASupplementary data
References
- 1.WHO Coronavirus disease (COVID-19) dashboard. 2022. https://covid19.who.int/ Available from:
- 2.Subramanian A., Nirantharakumar K., Hughes S., et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706–1714. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 4.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 Multi-omics Blood Atlas (COMBAT) Consortium A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell. 2022;185(5):916–938.e58. doi: 10.1016/j.cell.2022.01.012. julian.knight@well.ox.ac.uk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georg P., Astaburuaga-Garcia R., Bonaguro L., et al. Complement activation induces excessive T cell cytotoxicity in severe COVID-19. Cell. 2022;185(3):493–512.e25. doi: 10.1016/j.cell.2021.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su Y., Chen D., Yuan D., et al. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020;183(6):1479–1495.e20. doi: 10.1016/j.cell.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulte-Schrepping J., Reusch N., Paclik D., et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182(6):1419–1440.e23. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown L.B., Spinelli M.A., Gandhi M. The interplay between HIV and COVID-19: summary of the data and responses to date. Curr Opin HIV AIDS. 2021;16(1):63–73. doi: 10.1097/COH.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spinelli M.A., Jones B.L.H., Gandhi M. COVID-19 outcomes and risk factors among people living with HIV. Curr HIV AIDS Rep. 2022;19(5):425–432. doi: 10.1007/s11904-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinspoon S.K., Fitch K.V., Overton E.T., et al. Rationale and design of the randomized trial to prevent vascular events in HIV (REPRIEVE) Am Heart J. 2019;212:23–35. doi: 10.1016/j.ahj.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 13.Kolossvary M., deFilippi C., Lu M.T., et al. Proteomic signature of subclinical coronary artery disease in people with HIV: analysis of the REPRIEVE mechanistic substudy. J Infect Dis. 2022;226(10):1809–1822. doi: 10.1093/infdis/jiac196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders-van Wijk S., Tromp J., Beussink-Nelson L., et al. Proteomic evaluation of the comorbidity-inflammation paradigm in Heart failure with preserved ejection fraction: results from the PROMIS-HFpEF study. Circulation. 2020;142(21):2029–2044. doi: 10.1161/CIRCULATIONAHA.120.045810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy V., Fischinger S., Atyeo C., et al. SARS-CoV-2-specific ELISA development. J Immunol Methods. 2020;484-485 doi: 10.1016/j.jim.2020.112832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilles E.J., Karlson E.W., Norman M., et al. Evaluation of three commercial and two non-commercial immunoassays for the detection of prior infection to SARS-CoV-2. J Appl Lab Med. 2021;6(6):1561–1570. doi: 10.1093/jalm/jfab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 18.Szklarczyk D., Gable A.L., Lyon D., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti K.G., Eckel R.H., Grundy S.M., et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national Heart, lung, and blood Institute; American Heart association; world Heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 20.R Foundation for Statistical Computing; 2019. R: a language and environment for statistical computing. 4.0: R core team. [Google Scholar]
- 21.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models Usinglme4. J Stat Softw. 2015;67(1):48. [Google Scholar]
- 22.Grinspoon S.K., Douglas P.S., Hoffmann U., Ribaudo H.J. Leveraging a landmark trial of primary cardiovascular disease prevention in human immunodeficiency virus: introduction from the REPRIEVE coprincipal investigators. J Infect Dis. 2020;222(Suppl 1):S1–S7. doi: 10.1093/infdis/jiaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Y., Yuan D., Chen D.G., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881–895.e20. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay Z.L.Z., Slansky J.E. Granzymes: the molecular executors of immune-mediated cytotoxicity. Int J Mol Sci. 2022;23(3) doi: 10.3390/ijms23031833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson S., Eller M., Teigler J.E., et al. Cooperativity of HIV-specific cytolytic CD4 T cells and CD8 T cells in control of HIV viremia. J Virol. 2015;89(15):7494–7505. doi: 10.1128/JVI.00438-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wensink A.C., Hack C.E., Bovenschen N. Granzymes regulate proinflammatory cytokine responses. J Immunol. 2015;194(2):491–497. doi: 10.4049/jimmunol.1401214. [DOI] [PubMed] [Google Scholar]
- 27.Zenarruzabeitia O., Astarloa-Pando G., Terren I., et al. T cell activation, highly armed cytotoxic cells and a shift in monocytes CD300 receptors expression is characteristic of patients with severe COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.655934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M., Guo W., Dong Y., et al. Elevated exhaustion levels of NK and CD8+ T cells as indicators for progression and prognosis of COVID-19 disease. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.580237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q., Yu B., Yang Y., et al. Immunological and inflammatory profiles during acute and convalescent phases of severe/critically ill COVID-19 patients. Int Immunopharmacol. 2021;97 doi: 10.1016/j.intimp.2021.107685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malengier-Devlies B., Filtjens J., Ahmadzadeh K., et al. Severe COVID-19 patients display hyper-activated NK cells and NK cell-platelet aggregates. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.861251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J., Yang X., Wang H., et al. Analysis of the long-term impact on cellular immunity in COVID-19-recovered individuals reveals a profound NKT cell impairment. mBio. 2021;12(2) doi: 10.1128/mBio.00085-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzoni A., Salvati L., Maggi L., et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. 2020;130(9):4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J., Law R., Korosec C.S., et al. Longitudinal assessment of SARS-CoV-2-specific T cell cytokine-producing responses for 1 Year reveals persistence of multicytokine proliferative responses, with greater immunity associated with disease severity. J Virol. 2022;96(13) doi: 10.1128/jvi.00509-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sneller M.C., Liang C.J., Marques A.R., et al. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann Intern Med. 2022;175(7):969–979. doi: 10.7326/M21-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filbin M.R., Mehta A., Schneider A.M., et al. Longitudinal proteomic analysis of severe COVID-19 reveals survival-associated signatures, tissue-specific cell death, and cell-cell interactions. Cell Rep Med. 2021;2(5) doi: 10.1016/j.xcrm.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J.W., Su Y., Baloni P., et al. Integrated analysis of plasma and single immune cells uncovers metabolic changes in individuals with COVID-19. Nat Biotechnol. 2022;40(1):110–120. doi: 10.1038/s41587-021-01020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shemesh A., Su Y., Calabrese D.R., et al. Diminished cell proliferation promotes natural killer cell adaptive-like phenotype by limiting FcepsilonRIgamma expression. J Exp Med. 2022;219(11) doi: 10.1084/jem.20220551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer B., Knoll R., Bonaguro L., et al. Early IFN-alpha signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19. Immunity. 2021;54(11):2650–2669.e14. doi: 10.1016/j.immuni.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue F.Y., Cohen J.C., Ho M., et al. HIV-specific granzyme B-secreting but not gamma interferon-secreting T cells are associated with reduced viral reservoirs in early HIV infection. J Virol. 2017;91(8) doi: 10.1128/JVI.02233-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perdomo-Celis F., Velilla P.A., Taborda N.A., Rugeles M.T. An altered cytotoxic program of CD8+ T-cells in HIV-infected patients despite HAART-induced viral suppression. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotb A., Klippert A., Daskalaki M., Sauermann U., Stahl-Hennig C., Neumann B. Elevated granzyme B+ B-cell level in SIV-infection correlate with viral load and low CD4 T-cell count. Immunol Cell Biol. 2017;95(3):316–320. doi: 10.1038/icb.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkar P., Mitra S., Pant P., et al. Granzyme B as a diagnostic marker of tuberculosis in patients with and without HIV coinfection. Diagn Microbiol Infect Dis. 2016;85(1):47–52. doi: 10.1016/j.diagmicrobio.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Cullen S.P., Brunet M., Martin S.J. Granzymes in cancer and immunity. Cell Death Differ. 2010;17(4):616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- 44.Metkar S.S., Menaa C., Pardo J., et al. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity. 2008;29(5):720–733. doi: 10.1016/j.immuni.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Craig V.J., Zhang L., Hagood J.S., Owen C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;53(5):585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chun H.J., Coutavas E., Pine A.B., et al. Immunofibrotic drivers of impaired lung function in postacute sequelae of SARS-CoV-2 infection. JCI Insight. 2021;6(14) doi: 10.1172/jci.insight.148476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karki R., Sharma B.R., Tuladhar S., et al. Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184(1):149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haljasmagi L., Salumets A., Rumm A.P., et al. Longitudinal proteomic profiling reveals increased early inflammation and sustained apoptosis proteins in severe COVID-19. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-77525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.