Key Points
Question
Does treatment with second-line liposomal irinotecan (nal-IRI) plus fluorouracil and leucovorin (FU/LV) improve outcomes of patients with previously treated advanced biliary tract cancer (BTC) compared with FU/LV alone?
Findings
In this multicenter, randomized, open-label phase 2b clinical trial that included 174 patients with previously treated advanced BTC, adding nal-IRI to treatment with FU/LV significantly improved progression-free survival by masked independent central radiologic review. The median progression-free survival of patients treated with nal-IRI plus FU/LV (4.2 months) was shorter than that in the previous report (7.1 months).
Meaning
The trial results suggest that nal-IRI plus FU/LV could be considered by physicians for use as a second-line treatment option for patients with previously treated advanced BTC.
Abstract
Importance
The NIFTY trial demonstrated the benefit of treatment with second-line liposomal irinotecan (nal-IRI) plus fluorouracil (FU) and leucovorin (LV) for patients with advanced biliary tract cancer (BTC).
Objective
To report the updated efficacy outcomes from the NIFTY trial with extended follow-up of 1.3 years with reperformed masked independent central review (MICR) with 3 newly invited radiologists.
Design, Setting, and Participants
The NIFTY trial was a randomized, multicenter, open-label, phase 2b clinical trial conducted between September 5, 2018, and December 31, 2021, at 5 tertiary referral centers in South Korea. Patients with advanced BTC whose disease progressed while receiving first-line gemcitabine plus cisplatin with at least 1 measurable lesion per Response Evaluation Criteria in Solid Tumors, version 1.1, were eligible. Data analysis was completed on May 9, 2022.
Interventions
Patients were randomized 1:1 to receive LV, 400 mg/m2, bolus and FU, 2400 mg/m2, for a 46-hour infusion intravenously every 2 weeks with or without nal-IRI, 70 mg/m2, before LV intravenously. Patients were treated until disease progression or unacceptable toxic effects.
Main Outcomes and Measures
Primary end point was progression-free survival (PFS) as assessed by MICR. Secondary end points were PFS as assessed by the investigator, overall survival, and objective response rate.
Results
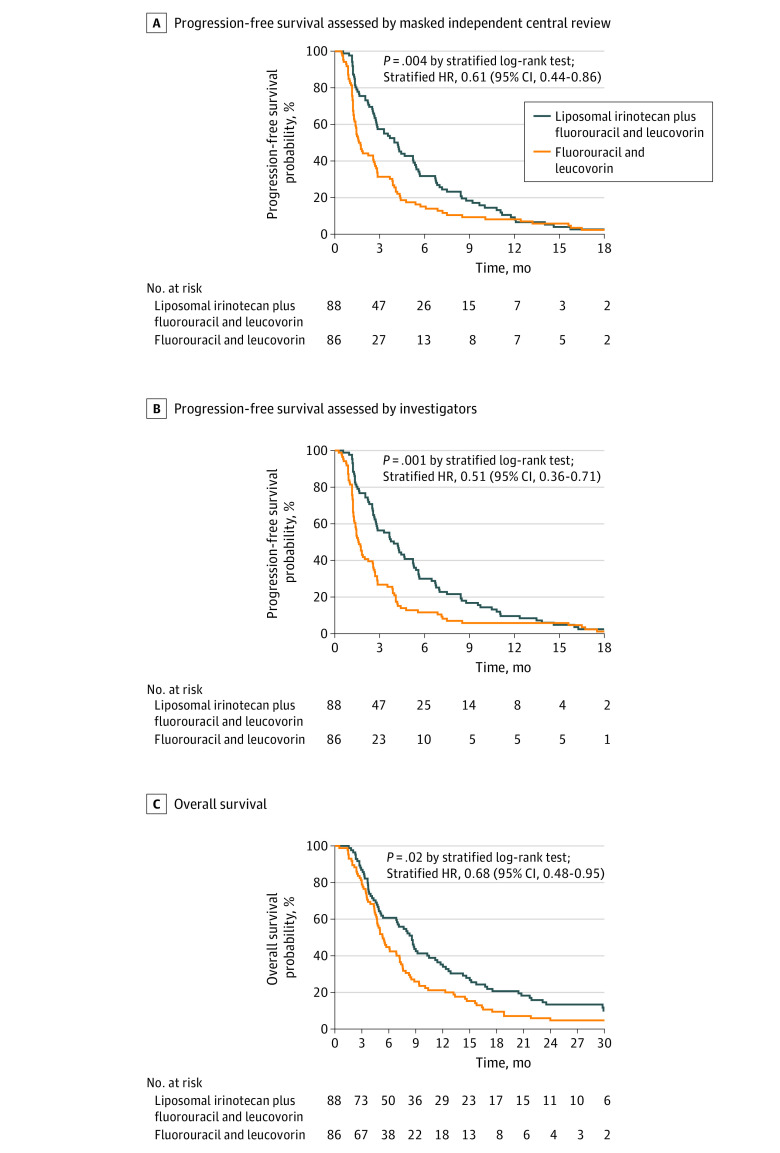
A total of 178 patients (75 women [42.1%]; median [IQR] age, 64 [38-84] years) were randomly assigned, and 174 patients were included in the full analysis set (88 patients [50.6%] in the nal-IRI plus FU/LV group vs 86 patients [49.4%] in the FU/LV alone group). In this updated analysis, the median MICR-assessed PFS was 4.2 months (95% CI, 2.8-5.3) for the nal-IRI plus FU/LV group and 1.7 months (95% CI, 1.4-2.6) for the FU/LV alone group (hazard ratio, 0.61; 95% CI, 0.44-0.86; P = .004), in contrast to the 7.1 and 1.4 months reported in the previous study, respectively. The discordance rate for tumor progression date between the MICR and investigators was 17.8% (vs 30% in the previous study).
Conclusions and Relevance
The NIFTY randomized clinical trial demonstrated significant improvement in PFS with treatment with nal-IRI plus FU/LV compared with FU/LV alone for patients with advanced BTC after progression to gemcitabine plus cisplatin. The combination of nal-IRI plus FU/LV could be considered as a second-line treatment option for patients with previously treated advanced BTC.
Trial Registration
clinicaltrials.gov Identifier: NCT03524508
This randomized clinical trial examines the addition of treatment with liposomal irinotecan to fluorouracil and leucovorin for patients with advanced biliary tract cancer.
Introduction
Biliary tract cancer (BTC) is an aggressive cancer arising from the intrahepatic bile duct, extrahepatic bile duct, and gallbladder.1 Although the incidence is higher in endemic regions, including East Asia, the incidence of BTC, especially intrahepatic cholangiocarcinoma, is rising in Western countries.1,2 For patients with unresectable or metastatic advanced BTC, systemic chemotherapy with gemcitabine plus cisplatin is the standard first-line treatment.3,4 Recently, the phase 3 TOPAZ-1 clinical trial showed substantial improvement in survival outcomes with the addition of durvalumab to gemcitabine plus cisplatin as first-line treatment and has been approved for treating patients with advanced BTC.5
Until recently, there was a lack of solid evidence supporting second-line treatment options for patients who experienced progression while receiving first-line gemcitabine plus cisplatin.1,6,7 The phase 3 ABC-06 trial showed the survival benefit of second-line chemotherapy with fluorouracil and leucovorin plus oxaliplatin (FOLFOX) compared with active symptom control alone (median overall survival [OS], 6.2 vs 5.3 months).8,9 Several targeted agents are recommended for patients with actionable molecular alterations as second-line or later-line treatment options, including fibroblast growth factor receptor (FGFR) inhibitors and isocitrate dehydrogenase–1 (IDH1) inhibitors; however, few patients harbor such aberrations, with 13% to 30% of patients with intrahepatic cholangiocarcinoma having IDH1 variants and approximately 20% of intrahepatic cholangiocarcinoma–harboring FGFR2 fusions or rearrangements.1,10,11,12,13,14,15
The NIFTY trial was a multicenter, open-label, randomized, phase 2b clinical trial investigating the efficacy of treatment with liposomal irinotecan (nal-IRI) plus fluorouracil and leucovorin (FU/LV) compared with FU/LV alone for patients with advanced BTC who experienced progression after receiving first-line gemcitabine plus cisplatin.16 A total of 174 patients were included in the primary analysis (as required), and the primary end point was met by showing significant improvement in progression-free survival (PFS) with nal-IRI plus FU/LV compared with FU/LV alone by the masked independent central review (MICR) with a hazard ratio (HR) of 0.56 (95% CI, 0.39-0.81; stratified log-rank P = .002) as of data cutoff (September 1, 2020).16 Although there was a higher incidence of grade 3 to 4 adverse events in the nal-IRI plus FU/LV group, including neutropenia and fatigue or asthenia, there was no significant difference in quality of life during treatment.16 Currently, nal-IRI plus FU/LV is recommended as a subsequent treatment option for patients with advanced BTC who experience progression while receiving first-line treatment.9
In this article, we report the final analysis of the phase 2b NIFTY trial that evaluated the long-term survival outcomes at an extended follow-up of 1.3 years (data cutoff: December 31, 2021) along with post hoc exploratory analysis for prognostic factors of patients with advanced BTC. As there was concern about the large discrepancy rate (30%) in the date of tumor progression between MICR and investigator review in the previous study, MICR was performed again by including new independent radiologists with more experience in clinical trials and through external monitoring.
Methods
Study Design and Participants
The NIFTY trial was a multicenter, open-label, randomized phase 2b study that was conducted at 5 tertiary centers in South Korea between September 5, 2018, and December 31, 2021. Key eligibility criteria were histologically or cytologically confirmed diagnosis of advanced BTC, including age of 19 years or older; disease progression as assessed by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, while receiving first-line gemcitabine plus cisplatin; at least 1 measurable lesion as defined by RECIST, version 1.1; an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; and adequate organ function.
The administrative process, trial site monitoring, and data management were conducted by the Academic Research Office at Asan Medical Center (Seoul, Korea). This study was performed according to the International Conference on Harmonization of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.17 Ethics approval of the study protocol was performed by the institutional review board of each participating center, and all patients provided written informed consent before enrollment. This trial is registered in ClinicalTrials.gov (NCT03524508), and the complete study protocol with statistical analysis plan is available in Supplement 1. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines for randomized clinical trial reporting.18
Randomization and Masking
Patients were randomly assigned at a 1:1 ratio to receive either nal-IRI plus FU/LV or FU/LV and were stratified by the primary tumor location (intrahepatic vs extrahepatic vs gallbladder), previous surgery with curative intent (yes vs no), and participating centers. As this trial was an open-label study, treatment allocation was not masked to the investigators, patients, and those analyzing the data.
Study Procedures
The procedure of the NIFTY trial was described previously.16 Patients received 400 mg/m2 of LV for 30 minutes and 2400 mg/m2 of FU for 46 hours intravenously every 2 weeks. Patients assigned to the nal-IRI plus FU/LV group received 70 mg/m2 of nal-IRI for 90 minutes intravenously before the administration of LV and FU. The treatment was continued until the patient showed radiologic disease progression, which was determined by the investigator per RECIST, version 1.1, unacceptable toxic effects, or withdrawn consent.
Assessment of radiologic disease response was performed according to RECIST, version 1.1, every 6 weeks, with a fixed schedule from day 1 of cycle 1 by computed tomography or magnetic resonance imaging. The decision for the continuation of the study treatment was based on radiologic assessment by the local investigator. Patients who discontinued the study treatment for reasons other than radiologic disease progression were assessed for disease response every 6 weeks until disease progression or the initiation of a new treatment. In this final analysis, we reperformed MICR (as specified in the eMethods in Supplement 2) with 3 newly invited independent radiologists with experience in clinical trials, including a US board-certified cancer imaging radiologist. The procedure was managed by Asan Image Metrics at the Clinical Trial Center of Asan Medical Center.
Outcomes
The primary end point was PFS by MICR assessment per RECIST, version 1.1. Progression-free survival was defined as the time from the date of randomization to the date of disease progression or death of any cause. Secondary end points included PFS by investigator assessment per RECIST, version 1.1; objective response rate (ORR) per RECIST, version 1.1, by either investigator or MICR assessment; OS defined as the time from the date of randomization to the date of death of any cause; safety profiles; and quality of life as per the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30. The quality of life assessments, along with safety profiles, were reported previously and not included in the final analysis.16
Statistical Analysis
This study was designed to detect an HR of 0.6 with a power of 80% with a 2-sided type 1 error of 5% for PFS. Assuming a follow-up loss rate of 10%, 174 patients with 87 patients per each group with 131 PFS events were required for the analysis of the primary end point. The efficacy analysis for PFS, OS, and ORR was performed in the full analysis set, as previously described.16 The full analysis set was selected for the main analysis as there may have been some patients assigned to the FU/LV group who dropped out before the first dosing, and these patients may have had inferior outcomes compared with those who remained in the study and received FU/LV because, to our knowledge, there was no proven effective treatment for BTC at the time of the study. Efficacy outcomes were also analyzed in the intention-to-treat and per-protocol populations.
Patients were censored in terms of PFS if the patient did not experience a PFS event, received poststudy treatment before disease progression, or did not undergo response evaluation after randomization. Patients were also censored in terms of OS if the patient did not experience death during the study. The Kaplan-Meier method was used to estimate PFS and OS, which were compared between the 2 treatment groups by the stratified log-rank test. The HR with 95% CIs for PFS and OS was estimated with a stratified Cox proportional hazards model using the stratification factors during randomization. The ORR was compared between the 2 groups by stratified Cochran-Mantel-Haenszel test. Post hoc comparison of disease control rate per RECIST, version 1.1, was also performed by stratified Cochran-Mantel-Haenszel test. Prespecified subgroup analysis of the MICR-assessed PFS and OS was done to evaluate interactions between the study treatments and subgroups by stratified Cox proportional hazards model, as previously described, and adjustment for multiplicity was not performed for this subgroup analysis.16
Additionally, we performed a post hoc exploratory analysis to evaluate prognostic factors associated with outcomes of patients with previously treated advanced BTC in terms of PFS by MICR and OS by multivariable Cox proportional hazards regression model. All statistical analysis was conducted using SAS, version 9.4 (SAS Institute), and 2-sided P < .05 was considered statistically significant.
Results
Participants
A total of 193 patients were assessed for eligibility between September 5, 2018, and February 18, 2020, and 178 patients were randomized for the study treatments. For the efficacy analysis of the primary end point, 174 patients were included as the full analysis set (88 [50.6%] in the nal-IRI plus FU/LV group and 86 [49.4%] in the FU/LV group) (Figure 1). The median age of the full analysis set was 64 years (IQR, 38-84), 75 (43%) were female, 74 (43%) had intrahepatic cholangiocarcinoma, 47 (27%) had extrahepatic cholangiocarcinoma, and 53 (30%) had gallbladder cancer. The baseline clinical characteristics were well balanced between the 2 groups (Table 1).
Figure 1. Consolidated Standards of Reporting Trials Diagram.
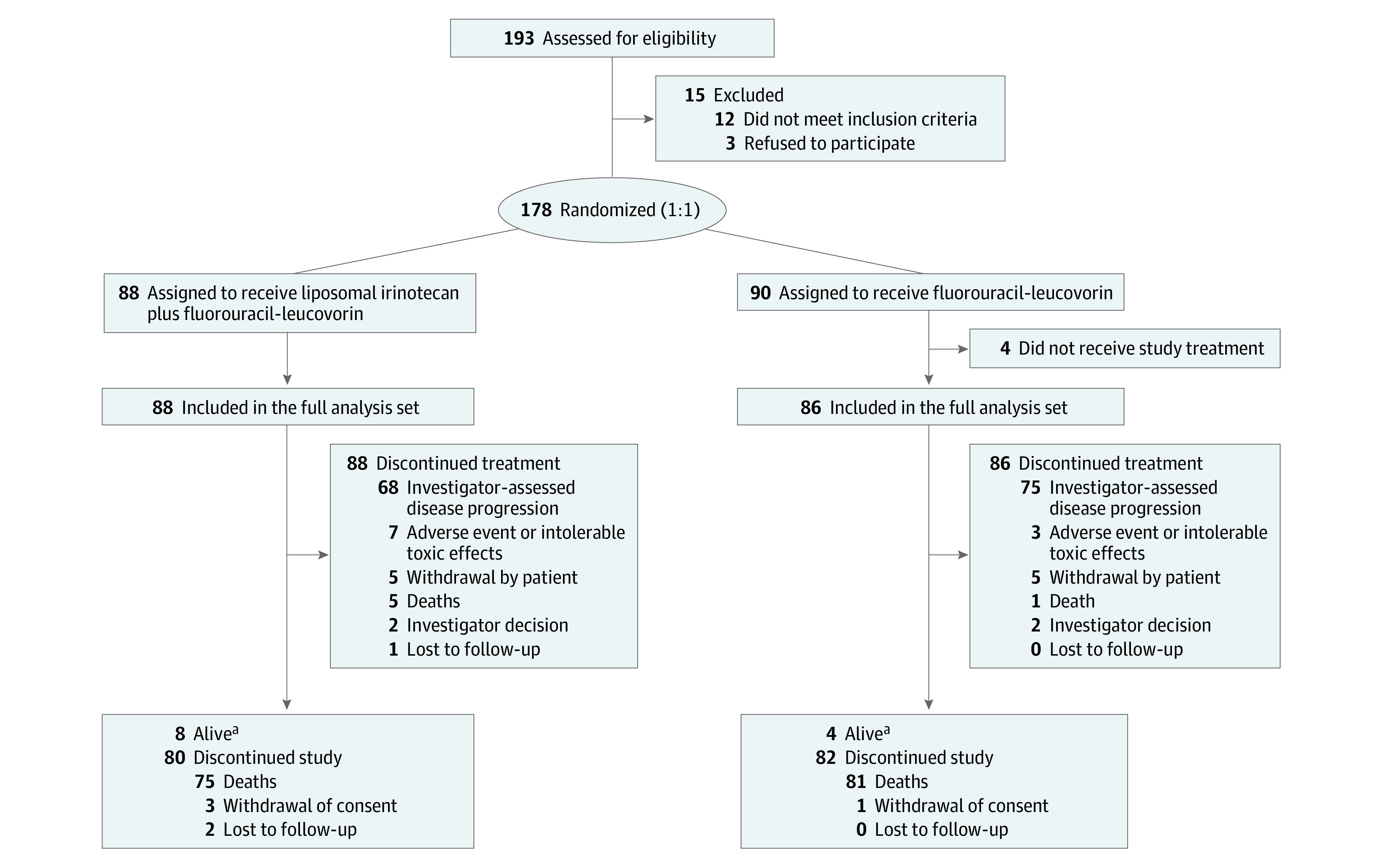
aAs of the date of data cutoff, December 31, 2021.
Table 1. Baseline Clinical Characteristics.
| Characteristics | Patients, No. (%) | |
|---|---|---|
| Liposomal irinotecan plus fluorouracil-leucovorin (n = 88) | Fluorouracil-leucovorin (n = 86) | |
| Sex | ||
| Female | 37 (42) | 38 (44) |
| Male | 51 (58) | 48 (56) |
| Age, median (IQR), y | 63 (38-84) | 65 (37-80) |
| Primary tumor location | ||
| Intrahepatic | 35 (40) | 39 (45) |
| Extrahepatic | 22 (25) | 25 (29) |
| Gallbladder | 31 (35) | 22 (26) |
| Previous surgery with curative intent | 26 (30) | 29 (34) |
| Median duration of treatment with first-line gemcitabine plus cisplatin, mo | ||
| <6 | 57 (65) | 55 (64) |
| ≥6 | 31 (35) | 31 (36) |
| Median serum CA 19-9 concentration, U/mL | ||
| <172 | 48 (55) | 39 (45) |
| ≥172 | 40 (45) | 47 (55) |
| Site of metastasis | ||
| Liver | 59 (67) | 64 (74) |
| Lung | 22 (25) | 16 (19) |
| Lymph node | 57 (65) | 48 (56) |
| Peritoneum | 25 (28) | 20 (23) |
| Bone | 5 (6) | 9 (10) |
| ECOG performance status | ||
| 0 | 23 (26) | 15 (17) |
| 1 | 65 (74) | 71 (83) |
Abbreviations: CA, cancer antigen; ECOG, Eastern Cooperative Oncology Group.
Efficacy Outcomes
The median follow-up duration of the patients in the full analysis set was 33.2 months (IQR, 27.6-35.7), with 12 patients (6.9%; 8 in the nal-IRI plus FU/LV group and 4 in the FU/LV group) continuing follow-up for survival as of the data cut-off date (December 31, 2021). The MICR-assessed median PFS of the nal-IRI plus FU/LV group (4.2 months [IQR, 2.0-7.2]; 95% CI, 2.8-5.3) was significantly longer than the FU/LV group (1.7 months [IQR, 1.2-4.1]; 95% CI, 1.4-2.6) with a stratified HR of 0.61 (95% CI, 0.44-0.86; stratified log-rank P = .004; Figure 2A). The MICR-assessed 6-month PFS rate was 31.8% (95% CI, 21.7%-41.8%) for the nal-IRI plus FU/LV group and 15.1% (95% CI, 7.5%-22.7%) for the FU/LV group (Table 2). The investigator-assessed median PFS of the nal-IRI plus FU/LV group (3.9 months [IQR, 2.0-7.0]; 95% CI, 2.7-5.2) was also significantly longer than that of the FU/LV group (1.6 months [IQR, 1.2-3.8]; 95% CI, 1.3-2.2) with stratified HR of 0.51 (95% CI, 0.36-0.71; stratified log-rank P < .001; Figure 2B). Investigator-assessed 6-month PFS was 30.0% (95% CI, 20.2%-39.8%) for the nal-IRI plus FU/LV group and 11.6% (95% CI, 4.9%-18.4%) for the FU/LV group (Table 2). The discordance rate for tumor progression date between the MICR and investigator was 17.8% (eMethods in Supplement 2). The profiles of subsequent systemic anticancer therapy for patients who discontinued the study treatments are described in eTable 1 in Supplement 2.
Figure 2. Comparison of Survival Outcomes According to the Study Treatments in the Full-Analysis Set.
A, Progression-free survival as assessed by masked independent central review. B, Progression-free survival as assessed by investigators. C, Overall survival. HR indicates hazard ratio.
Table 2. Efficacy Outcomes in the Full Analysis Set Population.
| Review | Liposomal irinotecan plus fluorouracil and leucovorin (n = 88) | Fluorouracil and leucovorin (n = 86) | P value | HR (2-sided 95% CI)a |
|---|---|---|---|---|
| Masked independent central review | ||||
| Progression-free survival, median (95% CI), mo | 4.2 (2.8-5.3) | 1.7 (1.4-2.6) | .004b | 0.61 (0.44-0.86) |
| Progression-free survival at 6 mo, % (95% CI) | 31.8 (21.7-41.8) | 15.1 (7.5-22.7) | NA | NA |
| Objective response rate, % | 12.5 | 3.5 | .04c | NA |
| Investigator review | ||||
| Progression-free survival, median (95% CI), mo | 3.9 (2.7-5.2) | 1.6 (1.3-2.2) | <.001b | 0.51 (0.36-0.71) |
| Progression-free survival at 6 mo, % (95% CI) | 30.0 (20.2-39.8) | 11.6 (4.9-18.4) | NA | NA |
| Objective response rate, % | 19.3 | 2.3 | <.001c | NA |
| Overall survival | ||||
| Median (95% CI), mo | 8.6 (5.4-10.5) | 5.3 (4.7-7.2) | .02b | 0.68 (0.48-0.95) |
| Rate at 6 mo, % (95% CI) | 60.7 (50.3-71.2) | 44.7 (34.2-55.3) | NA | NA |
Abbreviations: HR, hazard ratio; NA, not applicable.
Using a stratified Cox regression of HR of the liposomal irinotecan plus fluorouracil and leucovorin vs the fluorouracil and leucovorin.
P value by stratified log-rank tests, as stratified by the randomization stratification factors.
P value by Cochran-Mantel-Haenszel test as stratified by the randomization stratification factors.
Median OS was significantly longer for the nal-IRI plus FU/LV group (8.6 months [IQR, 3.8-15.7]; 95% CI, 5.4-10.5) compared with the FU/LV group (5.3 months [IQR, 3.4-9.4]; 95% CI, 4.7-7.2), with a stratified HR of 0.68 (95% CI, 0.48-0.95; stratified log-rank P = .02; Figure 2C). The 6-month OS rate was 60.7% (95% CI, 50.3%-71.2%) for the nal-IRI plus FU/LV group and 44.7% (95% CI, 34.2%-55.3%) for the FU/LV group (Table 2). The ORRs were significantly higher for the nal-IRI plus FU/LV group compared with the FU/LV group according to MICR (12.5% vs 3.5%; P = .04) and investigators (19.3% vs 2.3%; P < .001) (Table 2). The efficacy outcomes were consistent in the intention-to-treat and per-protocol populations (eTables 2-4 in Supplement 2). In the prespecified subgroup analysis, bone metastasis and primary tumor locations showed a significant interaction favoring nal-IRI plus FU/LV in patients with bone metastasis and gallbladder cancer for PFS and OS, respectively; however, this result should be interpreted cautiously considering the small number of patients included in each subgroup (eFigures 1 and 2 in Supplement 2). Otherwise, there was no statistically significant interaction, and all subgroups favored nal-IRI plus FU/LV in terms of PFS and OS.
Prognostic Factor Analysis
In the post hoc exploratory analysis for prognostic factors, treatment with nal-IRI plus FU/LV was independently associated with improved survival outcomes in terms of PFS per MICR and OS after adjustment for other clinical variables that were potentially associated with prognosis (Table 3). Also, in multivariable analysis, higher baseline levels of cancer antigen 19-9 and C-reactive protein were independently associated with poor PFS per MICR and OS, while male sex and more than 2 metastatic sites were independently associated with poor OS (Table 3).
Table 3. Multivariable Analysis Using Cox Proportional Hazards Regression According to the Study Group and Potential Prognostic Variables in Terms of PFS Assessed by the MICR and Overall Survival.
| Characteristic | PFS per MICR | OS | ||
|---|---|---|---|---|
| HR (95% CI) | Adjusted P value | HR (95% CI) | Adjusted P value | |
| Study treatment | ||||
| Fluorouracil and leucovorin | 1 [Reference] | NA | 1 [Reference] | NA |
| Liposomal irinotecan plus fluorouracil and leucovorin | 0.61 (0.44-0.83) | .002 | 0.65 (0.47-0.92) | .01 |
| Age (per 1-unit increase) | 0.99 (0.97-1.01) | .24 | 1.01 (0.99-1.03) | .19 |
| Sex | ||||
| Female | 1 [Reference] | NA | 1 [Reference] | NA |
| Male | 0.94 (0.67-1.32) | .74 | 1.48 (1.04-2.10) | .03 |
| ECOG performance status | ||||
| 0 | 1 [Reference] | NA | 1 [Reference] | NA |
| 1 | 0.78 (0.53-1.15) | .21 | 1.00 (0.67-1.51) | .99 |
| Primary tumor site | ||||
| Intrahepatic | 1 [Reference] | NA | 1 [Reference] | NA |
| Extrahepatic | 0.81 (0.53-1.23) | .32 | 0.93 (0.61-1.42) | .73 |
| Gallbladder | 0.76 (0.51-1.13) | .18 | 0.86 (0.57-1.29) | .46 |
| Prior surgery | ||||
| Yes | 1 [Reference] | NA | 1 [Reference] | NA |
| No | 0.83 (0.56-1.23) | .36 | 0.75 (0.50-1.12) | .16 |
| Duration of prior gem/cis (per 1-unit increase), mo | 0.98 (0.95-1.02) | .33 | 0.98 (0.95-1.01) | .24 |
| No. of metastatic lesion | ||||
| ≤2 | 1 [Reference] | NA | 1 [Reference] | NA |
| >2 | 1.40 (0.95-2.05) | .09 | 1.97 (1.30-2.98) | .001 |
| Baseline (per 1-unit increase) | ||||
| CA 19-9 level (per 1000-unit increase) | 1.01 (1.00-1.02) | .002 | 1.02 (1.01-1.03) | <.001 |
| CRP | 1.10 (1.03-1.17) | .003 | 1.19 (1.12-1.27) | <.001 |
| Albumin | 1.08 (0.75-1.55) | .68 | 0.80 (0.54-1.17) | .26 |
Abbreviations: CA, cancer antigen; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; gem/cis, gemcitabine plus cisplatin; HR, hazard ratio; MICR, masked independent central review; OS, overall survival; PFS, progression-free survival.
Discussion
In this final analysis of the NIFTY randomized clinical trial with extended follow-up, the significant benefit of additional nal-IRI to FU/LV in terms of MICR-assessed PFS (HR, 0.61; stratified log-rank P = .004) and OS (HR, 0.68; stratified log-rank P = .02) was affirmed. In the post hoc analysis, treatment with nal-IRI plus FU/LV showed significantly improved MICR-assessed PFS and OS after adjusting for potential prognostic factors. The NIFTY trial results suggest a potential role for nal-IRI as a second-line treatment for patients with advanced BTC in a biomarker-unselected population.
While a previous systematic review with several retrospective studies and phase 2 studies showed insufficient evidence to recommend second-line chemotherapy for patients with advanced BTC, the phase 3 ABC-06 trial to our knowledge was the first to prove the benefit of second-line chemotherapy compared with active symptom control alone in terms of OS for patients with advanced BTC who were previously treated with first-line gemcitabine plus cisplatin.6,8 With a comparably large sample size (174 patients) as in the ABC-06 trial (162 patients), median PFS by MICR assessment and OS for patients treated with nal-IRI plus FU/LV was 4.2 months and 8.6 months, respectively, which are favorably comparable with the outcomes of patients treated with second-line FOLFOX in the ABC-06 trial (median PFS of 4.0 months and OS of 6.2 months).8 Although several differences exist between the 2 trials, including baseline characteristics and the presence of measurable lesions, nal-IRI plus FU/LV and FOLFOX showed comparable efficacy and could be sequentially used for medically fit patients among a biomarker-unselected population. As FGFR inhibitors and IDH1 inhibitors have been recently approved, targeted therapies should be preferentially considered for patients with FGFR2 fusions or rearrangements and IDH1, respectively.9,12,13,14,15
The recent German phase 2 NALIRICC trial, which had a similar trial design to the NIFTY trial, showed no improvement in survival outcomes with the addition of nal-IRI to FU/LV despite improvement in terms of ORR (14.3% vs 3.9%).19 The discrepancy between the 2 trials may be attributed to differences in sample size (174 vs 100 patients) and the proportion of patients with intrahepatic cholangiocarcinoma (64% vs 43%).19 Also, differences in ethnicity may explain the discrepancy, as the current study was conducted in South Korea, while the NALIRICC study was conducted in Germany. From a pharmacokinetics study of treatment with nal-IRI in patients with heterogeneous solid tumors, ethnicity (East Asian vs White) was significantly associated with pharmacokinetic profiles, including total plasma irinotecan levels and SN-38, the active metabolite of irinotecan,20 which may underlie the higher incidence of grade 3 to 4 diarrhea (15% vs 5%) in the NALIRICC trial than the NIFTY trial. In a post hoc analysis of the phase 3 NAPOLI trial for patients with pancreatic adenocarcinoma, an Asian population treated with nal-IRI plus FU/LV showed longer median OS compared with the overall intention-to-treat population, with a comparable safety and tolerability profile.21
In this updated analysis of the NIFTY trial, we reperformed MICR with 3 newly invited radiologists who specialized in gastrointestinal cancer with more experience in clinical trials. Differences in the assessment of radiologic disease response per RECIST, version 1.1, between MICR and investigators were inevitable, as independent reviewers selected the target lesions after reviewing images from every point.16 However, the discrepancy rate for the date of tumor progression between MICR and investigators was reduced from 30% in the previous study to 17.8%.16 Median PFS by MICR assessment was 4.2 months in the nal-IRI plus FU/LV group and 1.7 months for the FU/LV group, whereas the same was 7.1 months and 1.4 months in the previous study, respectively. These differences in the discordance rates might be attributed to the qualification of radiologists as reviewers for clinical trials. In the prior analysis, 2 primary readers were abdominal radiologists who specialized in hepatobiliary imaging but had less experience in oncology trials.16 In this final analysis, 2 primary readers were radiologists with specialties in cancer imaging and abundant experience in oncology clinical trials (eMethods in Supplement 2). The cancer imaging radiologists tended to select target lesions that could represent the change in overall tumor burden, and the assessment of disease response of cancer imaging radiologists showed a similar tendency in determining tumor progression date with site investigators (ie, oncologists). Radiologic evaluation of BTC is challenging in selecting target lesions, as tumors often show infiltrative growth along the bile duct without discrete mass formation and the selected target lesions at baseline may not represent the overall disease course.22 An unclear extent of the tumor hinders the evaluation of nontarget lesions, and defining unequivocal progression or new lesions is more difficult compared with other types of solid tumors. There may be obstructive cholangitis or liver abscess at the progression of the tumor, and small abscesses may not be distinguished from new nodular lesions.23 Moreover, there may be no measurable lesions in some cases per RECIST, version 1.1, especially for extrahepatic cholangiocarcinoma.24
Limitations
This study was conducted in a single country, and all patients shared the same ethnicity, which may limit the generalizability of its results. In addition, there were discrepancies in the values of MICR-assessed PFS between the current study and the previous study, which may be due to the traits of BTC.
Conclusions
The NIFTY phase 2b randomized clinical trial demonstrated a significant improvement in PFS and OS with treatment with second-line nal-IRI plus FU/LV compared with FU/LV alone for patients with advanced BTC who experienced progression while receiving gemcitabine plus cisplatin. Treatment with nal-IRI plus FU/LV could be considered as an option for patients with previously treated advanced BTC.
Trial protocol
eMethods.
eTable 1. Post-study anti-cancer therapy in the full analysis set
eTable 2. Progression-free survival and overall survival in intention-to treat population and per protocol population
eTable 3. Best overall response according to the blinded independent central review
eTable 4. Best overall response according to the investigator
eFigure 1. Forest plot of prespecified subgroup analyses in terms of progression-free survival by the blinded independent central review in the full analysis set
eFigure 2. Forest plot of prespecified subgroup analyses in terms of overall survival in the full analysis set
Data sharing statement
References
- 1.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428-444. doi: 10.1016/S0140-6736(21)00153-7 [DOI] [PubMed] [Google Scholar]
- 2.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472-477. doi: 10.1016/j.jhep.2003.11.030 [DOI] [PubMed] [Google Scholar]
- 3.Valle J, Wasan H, Palmer DH, et al. ; ABC-02 Trial Investigators . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 4.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469-474. doi: 10.1038/sj.bjc.6605779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh D-Y, He AR, Qin S, et al. Durvalumab plus gemcitabine and xcisplatin in advanced biliary tract cancer. NEJM Evidence. 2022;1(8):EVIDoa2200015. doi: 10.1056/EVIDoa2200015 [DOI] [PubMed] [Google Scholar]
- 6.Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328-2338. doi: 10.1093/annonc/mdu162 [DOI] [PubMed] [Google Scholar]
- 7.Kim BJ, Yoo C, Kim KP, et al. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: retrospective analysis of 321 patients. Br J Cancer. 2017;116(5):561-567. doi: 10.1038/bjc.2016.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamarca A, Palmer DH, Wasan HS, et al. ; Advanced Biliary Cancer Working Group . Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690-701. doi: 10.1016/S1470-2045(21)00027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network . Hepatobiliary cancer version 1.2022. Accessed May 29, 2022. http://www.nccn.org
- 10.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154-4161. doi: 10.1158/1078-0432.CCR-18-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol. 2019;10(4):751-765. doi: 10.21037/jgo.2019.03.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671-684. doi: 10.1016/S1470-2045(20)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javle M, Roychowdhury S, Kelley RK, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6(10):803-815. doi: 10.1016/S2468-1253(21)00196-5 [DOI] [PubMed] [Google Scholar]
- 14.Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796-807. doi: 10.1016/S1470-2045(20)30157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu AX, Macarulla T, Javle MM, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021;7(11):1669-1677. doi: 10.1001/jamaoncol.2021.3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo C, Kim KP, Jeong JH, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021;22(11):1560-1572. doi: 10.1016/S1470-2045(21)00486-1 [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63(8):834-840. doi: 10.1016/j.jclinepi.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 19.Vogel A, Wenzel P, Folprecht G, et al. 53MO Nal-IRI and 5-FU/LV compared to 5-FU/LV in patients with cholangio- and gallbladder carcinoma previously treated with gemcitabine-based therapies (NALIRICC – AIO-HEP-0116). Ann Oncol. 2022;33:S563-S564. doi: 10.1016/j.annonc.2022.07.081 [DOI] [Google Scholar]
- 20.Adiwijaya BS, Kim J, Lang I, et al. Population pharmacokinetics of liposomal irinotecan in patients with cancer. Clin Pharmacol Ther. 2017;102(6):997-1005. doi: 10.1002/cpt.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang YJ, Li CP, Lee KH, et al. Liposomal irinotecan in metastatic pancreatic adenocarcinoma in Asian patients: Subgroup analysis of the NAPOLI-1 study. Cancer Sci. 2020;111(2):513-527. doi: 10.1111/cas.14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung YE, Kim MJ, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29(3):683-700. doi: 10.1148/rg.293085729 [DOI] [PubMed] [Google Scholar]
- 23.Kim JE, Kim HO, Bae K, Cho JM, Choi HC, Choi DS. Differentiation of small intrahepatic mass-forming cholangiocarcinoma from small liver abscess by dual source dual-energy CT quantitative parameters. Eur J Radiol. 2017;92:145-152. doi: 10.1016/j.ejrad.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 24.Keenan BP, Kelley RKK. Key challenges for drugs in clinical development for cholangiocarcinoma. Expert Opin Investig Drugs. 2021;30(4):285-290. doi: 10.1080/13543784.2021.1880565 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods.
eTable 1. Post-study anti-cancer therapy in the full analysis set
eTable 2. Progression-free survival and overall survival in intention-to treat population and per protocol population
eTable 3. Best overall response according to the blinded independent central review
eTable 4. Best overall response according to the investigator
eFigure 1. Forest plot of prespecified subgroup analyses in terms of progression-free survival by the blinded independent central review in the full analysis set
eFigure 2. Forest plot of prespecified subgroup analyses in terms of overall survival in the full analysis set
Data sharing statement