Key Points
Question
Which individuals are at risk of developing post−COVID-19 condition (PCC)?
Findings
This systematic review and meta-analysis of 41 studies including 860 783 patients found that female sex, older age, higher body mass index, smoking, preexisting comorbidities, and previous hospitalization or ICU admission were risk factors significantly associated with developing PCC, and that SARS-CoV-2 vaccination with 2 doses was associated with lower risk of PCC.
Meanings
The findings of this systematic review and meta-analysis provide a profile of the characteristics associated with increased risk of developing PCC and suggest that vaccination may be protective against PCC.
Abstract
Importance
Post−COVID-19 condition (PCC) is a complex heterogeneous disorder that has affected the lives of millions of people globally. Identification of potential risk factors to better understand who is at risk of developing PCC is important because it would allow for early and appropriate clinical support.
Objective
To evaluate the demographic characteristics and comorbidities that have been found to be associated with an increased risk of developing PCC.
Data sources
Medline and Embase databases were systematically searched from inception to December 5, 2022.
Study Selection
The meta-analysis included all published studies that investigated the risk factors and/or predictors of PCC in adult (≥18 years) patients.
Data Extraction and Synthesis
Odds ratios (ORs) for each risk factor were pooled from the selected studies. For each potential risk factor, the random-effects model was used to compare the risk of developing PCC between individuals with and without the risk factor. Data analyses were performed from December 5, 2022, to February 10, 2023.
Main Outcomes and Measures
The risk factors for PCC included patient age; sex; body mass index, calculated as weight in kilograms divided by height in meters squared; smoking status; comorbidities, including anxiety and/or depression, asthma, chronic kidney disease, chronic obstructive pulmonary disease, diabetes, immunosuppression, and ischemic heart disease; previous hospitalization or ICU (intensive care unit) admission with COVID-19; and previous vaccination against COVID-19.
Results
The initial search yielded 5334 records of which 255 articles underwent full-text evaluation, which identified 41 articles and a total of 860 783 patients that were included. The findings of the meta-analysis showed that female sex (OR, 1.56; 95% CI, 1.41-1.73), age (OR, 1.21; 95% CI, 1.11-1.33), high BMI (OR, 1.15; 95% CI, 1.08-1.23), and smoking (OR, 1.10; 95% CI, 1.07-1.13) were associated with an increased risk of developing PCC. In addition, the presence of comorbidities and previous hospitalization or ICU admission were found to be associated with high risk of PCC (OR, 2.48; 95% CI, 1.97-3.13 and OR, 2.37; 95% CI, 2.18-2.56, respectively). Patients who had been vaccinated against COVID-19 with 2 doses had a significantly lower risk of developing PCC compared with patients who were not vaccinated (OR, 0.57; 95% CI, 0.43-0.76).
Conclusions and Relevance
This systematic review and meta-analysis demonstrated that certain demographic characteristics (eg, age and sex), comorbidities, and severe COVID-19 were associated with an increased risk of PCC, whereas vaccination had a protective role against developing PCC sequelae. These findings may enable a better understanding of who may develop PCC and provide additional evidence for the benefits of vaccination.
Trial Registration
PROSPERO Identifier: CRD42022381002
This systematic review and meta-analysis evaluates the findings of 41 studies with a total of 860 783 patients to identify risk factors associated with post−COVID-19 condition.
Introduction
Since the first SARS-CoV-2 infections were identified in December 2019, the COVID-19 pandemic has significantly increased morbidity and mortality around the world.1 Previous epidemics of viruses from the coronavirus family, such as SARS-CoV and the Middle East Respiratory Syndrome coronavirus (MERS-CoV), have developed into persistent symptoms in infected individuals, including severe fatigue, decreased quality of life (QOL), and shortness of breath, as well as behavioral and psychological problems.1 These persistent postviral symptoms have been associated with a substantial burden to health care systems.
Similarly, a constellation of various clinical symptoms has been described in a subset of patients who have survived the acute phase of SARS-CoV-2−induced COVID-19.1 This constellation of symptoms has received many labels, including post-acute COVID-19 syndrome, persistent post-COVID-19 syndrome, and Long COVID-19. These terms have been used interchangeably for several years. The UK National Institute for Health and Care Excellence proposed Long COVID to describe the presence of symptoms that persist for 4 or more weeks after acute COVID-19 infection.2 The World Health Organization (WHO) defined post−COVID-19 condition (PCC) as having symptoms usually 3 months from the onset of COVID-19 with a duration of at least 2 months.3 Typical clinical symptoms include dyspnea, fatigue, autonomic dysfunction, headache, and persistent loss of smell and/or taste—although a wide range of symptoms has been described.1,4 Given that individuals with PCC may need long-term clinical support,4 the economic consequences have been estimated to be substantial.5
Not only is it important to recognize which individuals may be at high risk of developing PCC and to offer follow-up care; it is imperative to plan population-level public health measures. Several studies have been published investigating clinical and epidemiologic risk factors and/or predictors of PCC.5,6,7,8 However, these studies often had relatively few patients. Furthermore, wide discrepancy exists among published data, yielding uncertainty on the clinical utility of their findings. Therefore, the aim of this study was to search the available literature for published studies that found clinical and epidemiologic risk factors associated with the development of PCC and to pool their results.
Methods
This study was exempt from ethics review because it used only previously published data; informed consent was also waived for this reason. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.9
Search Strategy and Selection Criteria
MEDLINE and Embase databases were systematically searched for studies investigating the risk factors or clinical predictors for PCC in patients diagnosed with COVID-19, from inception to December 5, 2022. Search terms included “long-COVID-19,” “post-COVID-19,” and “chronic COVID-19,” as well as the corresponding MeSH (Medical Subjects Heading) terms. Only peer-reviewed articles were included; preprints were excluded. The full search strategy is available in the eMethods in Supplement 1.
Data Extraction
Search results were imported for abstract screening; duplicates and irrelevant studies were removed based on predetermined inclusion and exclusion criteria. All studies that investigated the risk factors or predictors of PCC, as defined by the WHO definition (≥1 symptom for ≥3 months), in a cohort of adult (≥18 years) patients were included. The risk factors evaluated for this meta-analysis were: age; biological sex; body mass index (BMI), calculated as weight in kilograms divided by height in meters squared; smoking status; comorbidities including anxiety and/or depression, asthma, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), diabetes, immunosuppression, and ischemic heart disease (IHD); and COVID-19 vaccination status. Studies were excluded if they investigated persistent COVID-19 symptoms of less than 3 months duration; did not provide data for any of the risk factors listed; or used only univariate regression because we wanted to identify independent risk factor association.
Subsequently, full texts of studies were retrieved and scrutinized against the criteria. The relevant data from the included studies were independently extracted by 2 authors (H.E., V.T.) who were blinded to the authors and institutions involved. Any disagreements were resolved by discussion with the senior author (V.V.). Some cohorts were studied and/or published more than once, for example, neurological PCC and respiratory PCC were evaluated in the same cohort in some studies.10,11,12 To avoid double-counting patients of these cohorts, we initially meta-analyzed all the PCC symptoms and produced a single odds ratio (OR) for the specific cohort; this OR was then used in the meta-analysis. The Newcastle-Ottawa Scale,13 a 9-point measure assessing the quality of cohort studies and case-control studies or case series, was used to evaluate the observational studies included.
Statistical Analysis
Quantitative synthesis of included studies was performed using RStudio 2022.07.1 + 554 and R, version 4.0.5 (The R Foundation for Statistical Computing). The ORs for each risk factor were pooled with the random-effects model. This was deemed more appropriate than the fixed-effects model because the studies included in this meta-analysis represented samples from different populations. For studies reporting rate ratios, those were converted to ORs using the methods defined in the Cochrane Handbook for Systematic Reviews of Interventions.14 Summary statistics were expressed as ORs and 95% CIs. Prediction intervals were also reported. Statistical heterogeneity was assessed using the I2statistic. Publication bias was assessed qualitatively by visual inspection of inverted funnel plot asymmetry and Egger test was performed to assess small study effects. Statistical tests were 2-tailed, and the statistical significance threshold was P < .05. Data analyses were performed from December 5, 2022, to February 10, 2023.
Results
The search of MEDLINE and Embase databases yielded a total of 5334 records. After removal of duplicates, 3363 were screened at title and abstract level, and 255 studies underwent full-text evaluation. Of those, 41 records with a total of 860 783 patients met the inclusion criteria and were included in the meta-analysis. The PRISMA flowchart for study selection is available in eFigure 1 in Supplement 1. The Table summarizes the population cohorts and the study design characteristics of all the included studies.10,11,12,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61 Of the 41 observational studies, 30 were ranked as high quality and 11 moderate quality on the Newcastle-Ottawa Scale (eTable 1 in Supplement 1).
Table. Characteristics of Included Studies of Risk Factors Associated With Post−COVID-19 Condition (PCC), 2021 to 2022.
| Source and study design | Population | COVID-19 test type | Aims/parameter | Follow-up | Main findings | Analysis methods |
|---|---|---|---|---|---|---|
| Abdelrahman et al10 | ||||||
| Prospective cohort study | 172 Hospitalized and nonhospitalized patients | Positive SARS-CoV-2 test | Telephone interview | 8-10 mo | Age was a risk factor for persistent symptoms | Multivariate logistic regression analysis to detect potential risk factors associated with persistence of symptoms |
| Aranda et al15 | ||||||
| Multicenter prospective cohort study | 150 Hospitalized patients | RT-PCR-proven SARS-CoV-2 infection | Clinical assessment | 12 mo | Female sex, COPD, smoking were independent risk factors for persistent dyspnea | Multivariate logistic models to identify factors associated with persistent dyspnea |
| Asadi-Pooya et al16 | ||||||
| Observational cohort study | 2696 Hospitalized patients | RT-PCR-proven SARS-CoV-2 infection | Interview, questionnaire | ≥3 mo Postacute illness | Female sex, respiratory problems at onset, ICU admission were significantly associated with PCC “brain fog” | Significant variables from univariate analyses entered into the logistic regression analysis model |
| Augustin et al17 | ||||||
| Longitudinal prospective analysis | 958 Nonhospitalized patients with mild COVID-19 | RT-PCR-proven SARS-CoV-2 infection | Persistent symptoms (anosmia, ageusia, fatigue, shortness of breath) | 4 mo and 7 mo Postinfection | Sex not correlated with PCC risk. Lower baseline level of SARS-CoV-2 associated with higher risk of developing PCC. Anosmia and diarrhea in acute COVID-19 were independent predictors for PCC after 7 mo | Unadjusted and adjusted ORs with 95% CIs from logistic regression reported for various baseline clinical data and patient characteristics |
| Ayoubkhani et al18 | ||||||
| Cross-sectional study | 3090 Nonhospitalized patients | RT-PCR-proven SARS-CoV-2 infection or positive swab test in national testing programs | PCC incidence by vaccination status, UK COVID-19 Infection Survey | ≥12 wk Postinfection | Unvaccinated individuals had higher risk of developing PCC | Double-vaccinated and unvaccinated participants, 1:1 propensity score matched for single year of age, sex, ethnicity, country/region of residence, area deprivation quintile group, and preexisting health/disability status |
| Baruch et al19 | ||||||
| National cross-sectional survey | 2665 Hospitalized and nonhospitalized patients | RT-PCR-proven SARS-CoV-2 infection | Online questionnaire | 3-6 mo Postpositive test | Female sex, hospitalization, and initial symptoms were associated with higher odds of fatigue, shortness of breath, cough, anxiety, sadness, memory loss | Logistic regression. Age, sex, wave number, health care worker status, initial symptoms, and hospitalization for COVID-19 were used as covariates |
| Bellan et al20 | ||||||
| Prospective cohort study | 238 Hospitalized patients | 232 RT-PCR positive swab, 1 bronchoalveolar lavage positive, 5 SARS-CoV-2 antibodies, suggestive radiologic findings | Pulmonary function, physical performance, psychological symptoms tests | 4 mo Posthospital discharge | No significant association between sex, age, diabetes, CAD, obesity, CKD, COPD, and functional impairment | Univariate analysis to identify associations with different end points. All associations with P < .20 were included in logistic regression model |
| Blomberg et al21 | ||||||
| Prospective cohort study | 312 Hospitalized and nonhospitalized (home-isolated) patients | Positive SARS-CoV-2 antigen or antibody test | Collection of demographic and clinical data and blood samples | 6 mo Postinfection | At 6 mo, 61% (189/312) of all patients had persistent symptoms independently associated with severity of initial illness, increased convalescent antibody titers, and preexisting chronic lung disease | Multivariable analysis was performed by binary logistic regression for dichotomous outcome variables. Negative binomial regression to analyze factors associated with numeric outcome variables |
| Chudzik et al22 | ||||||
| Retrospective observational study (STOP COVID registry) | 2218 Hospitalized and nonhospitalized patients | Positive RT-PCR test and/or antigen test | Clinical assessment | 3 mo Postinfection | Female sex, severe acute COVID-19 infection, dyspnea, chest pain were risk factors for developing PCC | Multivariate logistic regression models (explanatory variables were duration and number of symptoms, severity of COVID-19 infection, blood pressure, diarrhea, arthralgia, headache, leg pain, hearing dysfunction) |
| Daitch et al23 | ||||||
| Multicenter prospective cohort study | 2333 Hospitalized patients | Positive RT-PCR test | Clinic review, history, physical examination, spirometry | 5 mo Postdisease onset (median) | Independent risk factors for PCC fatigue and dyspnea were female sex, obesity, and closer proximity to COVID-19 diagnosis | Multivariable analysis |
| Debski et al24 | ||||||
| Cross-sectional study | 1487 Hospitalized and nonhospitalized patients | Positive RT-PCR test | Data collection/analysis from NHS digital databases | Persistent symptoms ≥12 wk | Female sex, BMI were risk factors significantly associated with PCC | Multivariable logistic regression analysis. Full model with all covariates was assessed. Backward variable selection used to identify most important variables |
| Dias et al25 | ||||||
| Prospective cohort study | 1042 Hospitalized patients | Laboratory-confirmed COVID-19 | Telephone interview | ≥3 mo Posthospital discharge | Female sex, higher BMI, ICU admission, longer length of stay were independent predictors of PCC | Multivariable logistic model with all characteristics as predictors and PCC as the outcome |
| Emecen et al26 | ||||||
| Cross-sectional study | 5610 Hospitalized and nonhospitalized patients | Positive RT-PCR test | Telephone interview | 3 mo and 6 mo after the first positive test | Older age, female sex, low economic status, current smoking, vaccination status, underlying comorbidities, and hospitalization were associated with PCC | Multivariate generalized estimating equation regression model used to further evaluate the factors associated with reporting symptoms 1, 3, and 6 mo after diagnosis |
| Estrada-Codecido et al27 | ||||||
| Retrospective cohort study | 206 Hospitalized and nonhospitalized patients | Laboratory-confirmed COVID-19 | Email survey, clinical assessment, EHS | 90 d Postinfection | Persistent symptoms were more common in older patients, those diagnosed in hospital, and those with initial constitutional and rheumatologic symptoms | Multivariable logistic regression with prespecified predictor variables (age, sex, cardiorespiratory comorbidity—asthma, CVD, or chronic lung disease) presence/absence of each symptom at baseline assessment, location (outpatient vs in-hospital), and hospital admission during illness |
| Fernández-de-Las-Peñas et al28a | ||||||
| Multicenter cohort study | 1950 Hospitalized patients | Positive RT-PCR test | Prevalence data and associated risk factors of PCC cough | 1 y Posthospital discharge (mean, 11.2 mo) | No association between PCC cough and other PCC symptoms. Regression analysis did not reveal any clinical variable associated with the presence of PCC cough | Multivariate Poisson regression prediction and risk models to identify variables independently associated with presence of cough as a PCC symptom |
| Fernández-de-Las-Peñas et al29a | ||||||
| Multicenter case-control study (2:1) | 145 Patients with diabetes and 290 controls hospitalized with COVID-19 (age- and sex- matched) | Positive RT-PCR test | List of PCC symptoms systematically evaluated. HADS and PSQI to assess anxiety and depressive symptoms and sleep quality | 7.2 mo Posthospital discharge (mean) | Diabetes was not a risk factor for PCC. Most prevalent symptoms were fatigue, dyspnea on exertion, and pain. No between-group differences in any PCC symptom observed | Multivariable conditional logistic regression models to identify the variables associated with the presence of diabetes |
| Fernández-de-Las-Peñas et al30a | ||||||
| Multicenter case-control study (2:1) | 88 Patients with obesity and 176 controls hospitalized with COVID-19 (age- and sex-matched) | Positive RT-PCR test | List of PCC symptoms was systematically evaluated. HADS and PSQI to assess anxiety and depressive symptoms and sleep quality | 7.2 mo Posthospital discharge (mean) | Obesity was independently associated with a greater number of PCC symptoms and poor sleep quality | Multivariable conditional logistic regression models were applied to identify those variables independently associated with obesity |
| Fernández-de-Las-Peñas et al31a | ||||||
| Multicenter observational study | 1142 Hospitalized patients | Positive RT-PCR test | List of PCC symptoms was systematically evaluated | 7.0 mo Posthospital discharge (mean) | Female sex, number of days at hospital, previous comorbidities, and number of symptoms at hospital admission were associated with higher number of PCC symptoms. Fatigue, hair loss, and dyspnea were most prevalent symptoms | Multivariate Poisson regression prediction and risk models to identify clinical and hospitalization variables associated with number of PCC symptoms |
| Fernández-de-Las-Peñas et al32a | ||||||
| Multicenter cohort study | 1969 Hospitalized patients | Positive RT-PCR test | Assessed differences between COVID-19 −related symptoms and PCC symptoms between male and female COVID-19 survivors | 8.4 mo Posthospital discharge (mean) | Female sex was a risk factor for development of some PCC symptoms—mood disorders, fatigue, dyspnea, pain, hair loss, ocular problems, depressive levels, worse sleep quality | Multivariate logistic regression analysis for PCC symptoms adjusted by all variables collected at hospital admission (age, height, weight, preexisting medical comorbidities, COVID-19 onset symptoms at hospital admission, ICU admission, hospital stay) |
| Fernández-de-Las-Peñas et al33a | ||||||
| Multicenter cohort study | 1969 Hospitalized patients | Positive RT-PCR test | List of PCC symptoms systematically evaluated | 8.4 mo Posthospital discharge (mean) | Female sex, a greater number of symptoms at hospital admission, a greater number of preexisting comorbidities, and longer hospital stay were risk factors for developing more long-term PCC | Multivariate logistic regressions to analyze associations between clinical and hospitalization variables with the number of symptoms after COVID (dependent variable) using Python library stats model 0.11.1 |
| Fernández-de-Las-Peñas et al11a | ||||||
| Multicenter cohort study | 1593 Hospitalized patients | Positive RT-PCR test | Prevalence of musculoskeletal post-COVID pain | 8 mo and 13 mo Postdischarge | Female sex, previous history of pain symptoms, pain symptoms at onset, and days of hospital stay were factors associated with musculoskeletal pain 1 y after hospitalization | Multivariate logistic regression analysis |
| Ioannou et al34 | ||||||
| Retrospective cohort study | 198 610 Hospitalized and nonhospitalized patients | Positive RT-PCR test | EHR data | ≥3 mo Postacute infection | Factors significantly associated with documented PCC were older age, Black or American Indian/Alaska Native race, Hispanic ethnicity, geographic region, high CCI score, documented symptoms at the time of acute infection, and requiring hospitalization or mechanical ventilation. Fully vaccinated patients less likely to receive PCC care | Multivariable logistic regression with adjustment for age, sex, self-reported race, self-reported ethnicity, urban vs rural residence, CCI score, VA Integrated Service Network, time period of infection (categorized by pandemic waves), and number of primary care, mental health, and specialty care encounters in the 2 y before infection |
| Jones et al35 | ||||||
| Observational study | 310 Hospitalized and nonhospitalized patients | Self- or clinician- diagnosed or test-confirmed COVID-19 | Patient-reported online questionnaire | 4 mo (data for ≥12 y used in meta-analysis) | PCC risk predictors were age ≥40 y, female sex, frailty, visit to emergency department, hospital admission for COVID-19 symptoms | Multivariable regression analyses (adjusted for demographic variables, hospital visit for COVID-19, frailty, comorbidities, COVID-19 status) to compare characteristics and symptoms in patients with PCC vs symptoms of shorter duration |
| Kisiel et al36 | ||||||
| Prospective longitudinal cohort studies | 366 Nonhospitalized patients | Positive RT-PCR test | Questionnaire/survey | 1 y Postpositive test result (51-54 wk) | Predictors of persistent symptoms were being born abroad, lower physical fitness vs peers before COVID-19, BMI >25, co-occurring hypertension and chronic pain, and having >7 COVID-19 symptoms at onset | Predictors of symptoms after 12 mo calculated with RRs and 95% CIs |
| Kostev et al37 | ||||||
| Retrospective cohort study | 51 630 Nonhospitalized patients | Positive RT-PCR test or clinician diagnosed | EHR data | 3-12 mo Postdiagnosis | Age >30 y and female sex were significantly associated with PCC | Multivariable logistic regression model (covariates included age, sex, and comorbidities) |
| Menezes et al38 | ||||||
| Prospective observational study | 108 Hospitalized and nonhospitalized patients | Positive RT-PCR test (105 patients), IgG antibody test (2 patients), CT (1 patient) | Clinical evaluation and self-administered questionnaire | 12 wk | Simultaneous presence of ≥15 COVID-19 symptoms, age >45 y, and obesity associated with higher probability of severe COVID-19 | Binary logistic regression analysis with stepwise variable filtering |
| Munblit et al39 | ||||||
| Longitudinal cohort study | 2649 Hospitalized patients | Positive RT-PCR test or clinical diagnosis (when laboratory test negative, inconclusive, or unavailable) | Telephone interview/questionnaire | 218 d Postdischarge (median) | Half of adults admitted to hospital with COVID-19 reported persistent symptoms 6-8 mo after discharge. Fatigue and respiratory symptoms were most common. Female sex associated with PCC | Multivariable logistic regression to investigate associations of demographic characteristics, comorbidities, and severity of acute phase COVID-19 with physical symptoms at follow-up |
| Pazukhina et al40 | ||||||
| Prospective cohort study | 1013 Hospitalized patients—only adult cohort data were used in meta-analysis | Positive RT-PCR test | Telephone interviews | 6 mo and 12 mo Posthospital discharge | Female sex and preexisting hypertension were risk factors of PCC | Multivariable logistic regression analysis. Selection of variables was: COVID-19 severity as exposure; PCC as outcome; comorbidities as covariates; sex and age as effect modifiers |
| Peghin et al41 | ||||||
| Bidirectional cohort study | 599 Hospitalized and nonhospitalized patients | Positive RT-PCR test and IgG antibody test | Telephone interview/questionnaire | 6 mo Postdisease onset | Female sex, a proportional increase in the number of symptoms at the onset of COVID-19 and ICU admission were all independent risk factors for PCC | Multivariable logistic regression performed. All clinically/microbiologically relevant variables or those significant at P < .10 in univariable analysis were included |
| Peters et al42 | ||||||
| Cross-sectional survey of employees in health or social facilities | 1930 Hospitalized and nonhospitalized patients | Positive RT-PCR test and/or clinical diagnosis | Questionnaire | ≥3 mo Duration of symptoms | Risk factors for persistent symptoms were older age, female sex, previous illness, many/ severe symptoms during the acute phase, outpatient medical care | Binary logistic regression model used to identify risk factors for persistent symptoms, and ORs with associated 95% CIs were calculated |
| Petersen et al43 | ||||||
| Prospective longitudinal study | 170 Nonhospitalized patients | Positive RT-PCR test | Telephone interview, clinical examination, questionnaire | 3 mo | PCC was more common in people reporting daily medication use. Age, smoking status, BMI were not risk factors for PCC | Multivariable logistic regression analyses (adjusted for sex, groups, BMI categories, smoking status, self-reported daily medication use) |
| Righi et al44 | ||||||
| Prospective cohort study | 465 Hospitalized and nonhospitalized patients | Positive RT-PCR test and clinical symptoms | Telephone interview | 9 mo +/− 2 Postdisease onset | Patients with older age, ICU stay, or multiple symptoms at onset were more likely to have long-term symptoms | Multivariable Cox proportional hazards model |
| Silverberg et al45 | ||||||
| Observational study | 390 Nonhospitalized patients | Anti-SARS-CoV-2 IgG antibody testing | Electronic survey | 11 mo (median) | Female sex, severity of acute phase, higher anti-SARS-CoV-2 IgG levels associated with highest risk of having PCC | Multivariable models included all variables tested in bivariable models (age, sex, household size, household sick contacts), state of residence as potential confounders per theoretical differences in exposures and mitigation strategies |
| Štěpánek et al46 | ||||||
| Observational cohort study | 305 Patient-health care workers | Positive RT-PCR test | Clinical assessment | ≥12 wk Postonset of symptoms | Statistically significant predictors of PCC were female sex and increasing age | Logistic regression analysis with PCC symptoms as a dependent (response) variable was applied to explore relationships between symptoms and other variables |
| Subramanian et al47 | ||||||
| Retrospective matched cohort study | 486 149 Nonhospitalized patients | Positive COVID-19 test | UK-based primary care database | ≥12 wk Postinfection | PCC risk factors were female sex, ethnic minority, socioeconomic deprivation, smoking, obesity, comorbidities and increased with increasing age | Cox proportional hazards model, adjusting for age, sex, ethnic group, socioeconomic status, index year, vaccination status, symptoms before COVID-19, and comorbidities |
| Thompson et al48 | ||||||
| Analyses of 10 UK established population-based longitudinal studies49,50,51,52,53,54,55,56,57 and EHRs (OpenSAFELY data set of primary care)b | 6907 Nonhospitalized patients from LS and 4189 from EHR (only LS data meta-analyzed) | Self-reported COVID-19 infection | COVID-19 questionnaire and analysis EHR data | ≥12 wk | Older age, female sex, White race, poor prepandemic general/mental health, overweight/obesity, asthma associated with prolonged symptoms in both LS and EHR data; findings for other factors, inconclusive | Logistic regression analysis to assess if PCC was associated with each sociodemographic or prepandemic health characteristic. Confounders analysis adjusted for age (as categorical variable), sex, ethnicity |
| Tleyjeh et al58 | ||||||
| Prospective cohort study | 222 Hospitalized patients | Positive RT-PCR test | Telephone interviews | 122 d Postdischarge; 4 mo postacute infection (median) | Female sex, preexisting hypertension, and length of hospital stay were associated with increased risk of new or persistent symptoms | Multivariate Cox proportional hazards model to identify factors associated with persistence of symptoms at follow-up with time-dependent days since discharge |
| Whitaker et al59 | ||||||
| Cross-sectional survey | 55 730 Hospitalized and nonhospitalized patients | Self-reported symptomatic COVID-19 infection | Data from rounds 3-5 (main analysis) and round 6 (replication of the REACT-2) | 12 wk Postdiagnosis | Female sex, older age, obesity, smoking, vaping, hospitalization with COVID-19, deprivation, being a health care worker associated with higher probability PCC | Multivariate logistic regression model |
| Wu et al60 | ||||||
| Cross-sectional survey | 308 Hospitalized and nonhospitalized patients | Positive laboratory test or diagnosed by clinician | Online survey | 12 wk Postdiagnosis | PCC more likely in patients with obesity, hair loss, headache, sore throat during infection. No association with age, sex, race/ethnicity, education, smoking, comorbidities | Multivariate logistic regression model was used to identify sociodemographic and health-related risk factors associated with PCC |
| Zhang et al61 | ||||||
| Retrospective cohort study | 2433 Hospitalized patients | Laboratory-confirmed COVID-19 | Telephone interviews (questionnaire and COPD assessment test) | 12 mo Posthospital discharge | Older age, female sex, severe disease associated with higher risks of fatigue. Older age, severe disease associated with higher risk of ≥3 symptoms | Univariate logistic regression analysis to identify potential risk factors with P < .10. Then used in stepwise selection process in multivariate logistic regression model |
| Zisis et al12 | ||||||
| Retrospective study | 25 225 Patients | Positive RT-PCR test | Data collection/analysis from EHS | 3 mo Postdiagnosis | COVID-19 vaccine protective against PCC symptoms, new onset of health conditions, and mortality | Propensity score matching (1:1) using greedy nearest-neighbor method used to balance 2 cohorts on age, sex, race, comorbidities |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EHR, electronic health care records; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; LS, longitudinal studies; NHS, UK National Health Service; PSQI, Pittsburgh Sleep Quality Index; RT-PCR, reverse transcription-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Where more than 1 study by the same authors is mentioned, only 1 was included in the meta-analysis (each to the relevant subgroup meta-analysis).
The data meta-analyzed was obtained solely from the study by Thompson et al,48 which analyzed 10 longitudinal studies that included patients aged 18-96 years old: ALSPAC G0 (Avon Longitudinal Study of Parents and Children-Generation 0), ALSPAC G1 (Avon Longitudinal Study of Parents and Children-Generation 1), BCS70 (British Cohort Study 1970), GS (Generation Scotland: the Scottish Family Health Study), MCS (Millennium Cohort Study), NCDS (National Child Development Study), NS (Next Steps, formerly known as Longitudinal Study of Young People in England), USOC (Understanding Society: the UK Household Longitudinal Survey), TwinsUK (the UK Adult Twin Registry).
All previously identified risk factors for PCC were evaluated, including patient age, sex, BMI, smoking status, comorbidities (ie, anxiety/depression, asthma, CKD, COPD, diabetes, immunosuppression, IHD), and hospitalization or ICU admission for COVID-19. In addition, the role of vaccination as a risk factor for PCC was evaluated. Funnel plots for all the meta-analyses are shown in eFigure 2 in Supplement 1. Race and ethnicity were not evaluated as this information was not provided consistently across the included studies.
Patient Sex
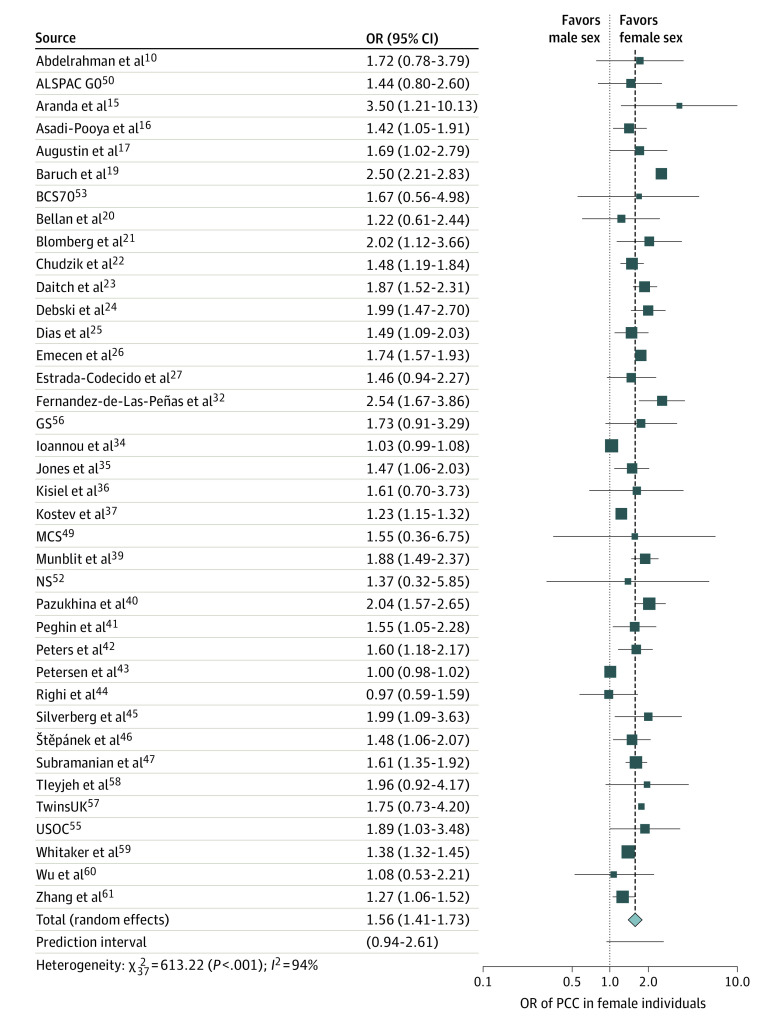
Of the 41 studies, 38 studies including a total of 727 630 patients investigated sex as a risk factor for PCC. Overall, the pooled ORs showed that female sex was significantly associated with PCC (OR, 1.56; 95% CI, 1.41 to 1.73; I2 = 94%; Figure 1). However, the 95% prediction interval (95% PI, 0.94 to 2.61) suggested that this may not be demonstrated in all future studies. To investigate this further, we undertook subgroup analysis separating the studies that included only hospitalized patients from those that included only nonhospitalized and those that included a mixture of hospitalized and nonhospitalized patients (eFigure 3 in Supplement 1). This showed that heterogeneity was lower in studies that included only hospitalized or only nonhospitalized patients compared with those that included patients from both settings (58%, 24%, and 97%, respectively), with the correlation remaining significant and the prediction intervals showing evidence supporting this significance for future studies. Subgroup analysis was also performed by study quality (high vs moderate) per the Newcastle-Ottawa Scale, with no significant between-group differences demonstrated (eFigure 4 in Supplement 1). Meta-regression analysis by study size showed no significance (effect size = 0.0001; 95% CI, −0.0001 to 0.0001; P = .26). Egger test for small study effects was not significant (intercept = 0.36, 95% CI, 0.21 to 0.50; P = .15).
Figure 1. Association of Sex With Post−COVID-19 Condition (PCC), 2021 to 2022.
Female sex was shown to have a statistically significant association with high risk of developing PCC. The dotted line represents the point where there is no difference between the 2 groups; the dashed line represents the average effect of all studies when pooled together. Note that when >1 study by the same author(s) was identified, only 1 was included in the meta-analysis (in the relevant subgroup meta-analysis). Data for 10 longitudinal studies49,50,51,52,53,54,55,56,57 of patients 18-96 years old were obtained solely from a single study by Thompson et al,48 including ALSPAC G0, which refers to the Avon Longitudinal Study of Parents and Children-Generation 0; GS, the Generation Scotland−the Scottish Family Health Study; MCS, Millennium Cohort Study; NS, Next Steps (formerly the Longitudinal Study of Young People in England); BCS70, the British Cohort Study 1970; NCDS, the National Child Development Study; TwinsUK, the UK Adult Twin Registry; and USOC, Understanding Society−the UK Household Longitudinal Survey. OR indicates odds ratio.
Patient Age
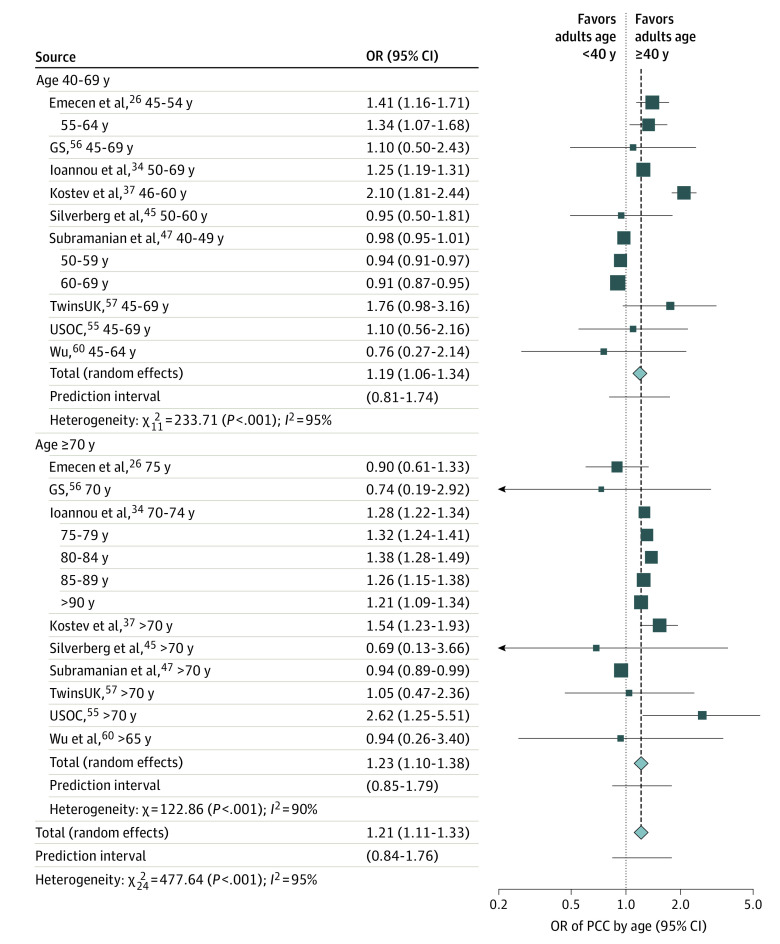
Of the 41 studies, 9 studies including a total of 324 950 patients investigated age as a risk factor for PCC. For the meta-analysis, the risk of PCC among 3 age groups (40-69 years and ≥70 years vs 18-40 years) was analyzed. We found that patients in both of the older groups had a significantly higher risk of PCC when compared with adult patients younger than 40 years, with no significant between-group differences (OR, 1.21; 95% CI, 1.11 to 1.33; I2 = 95%; Figure 2). However, this may not be demonstrated in all future studies (95% PI, 0.84-1.76). Subgroup analysis by study size demonstrated a high rate of heterogeneity in the group of large studies (eFigure 5 in Supplement 1). Meta-regression analysis by study size was significant (effect size = −0.0001; 95% CI, −0.0002 to −0.0001; P = .02) indicating that study size may have influenced the results (eFigure 6 in Supplement 1). Egger test for small study effects was not significant (intercept = 0.20; 95% CI, 0.06 to 0.50; P = .34). Subgroup analysis by study population (not hospitalized patients vs combined settings) showed no significant between-group differences (eFigure 7 in Supplement 1), whereas sensitivity analysis by study quality demonstrated that high quality studies have higher heterogeneity (eFigure 8 in Supplement 1).
Figure 2. Association of Age With Post−COVID-19 Condition (PCC), 2021 to 2022.
Older individuals (40-69 and ≥70 y) had a significantly higher risk of ongoing persistent PCC symptoms compared with adults <40 years old. The dotted line represents the point of no difference between the 2 groups, and the dashed line represents the average effect of all studies when pooled together. Data for 10 longitudinal studies49,50,51,52,53,54,55,56,57 of patients 18-96 years old were obtained solely from a single study by Thompson et al,48 including GS, which refers to the Generation Scotland−the Scottish Family Health Study; TwinsUK, the UK adult Twin Registry; and USOC, the Understanding Society−the UK Household Longitudinal Survey. OR indicates odds ratio.
Body Mass Index
Of 41 studies, 16 studies including a total of 701 807 patients investigated obesity (high BMI, defined as ≥30) as a risk factor for PCC. Obesity was found to be significantly associated with PCC (OR, 1.15; 95% CI, 1.08 to 1.23; I2 = 91%; eFigure 9 in Supplement 1). However, this significant correlation may not be shown in all future studies (95% PI, 0.94 to 1.42). Subgroup analysis by study population (hospitalized vs nonhospitalized vs combined) showed that the correlation remained significant in all 3 subgroups; however, the studies of nonhospitalized patients had the lowest heterogeneity (eFigure 10 in Supplement 1). Subgroup analysis by study quality showed that the significant correlation between obesity and PCC was evident only in high quality studies (eFigure 11 in Supplement 1). Egger test was found to be significant (intercept = .06; 95% CI, −0.02 to 0.15; P < .001), suggesting publication bias as shown in the funnel plot (eFigure 2C in Supplement 1). Meta-regression analysis by study size was not significant (effect size = −0.0001; 95% CI −0.00001 to 0.0001; P = .86).
Smoking Status
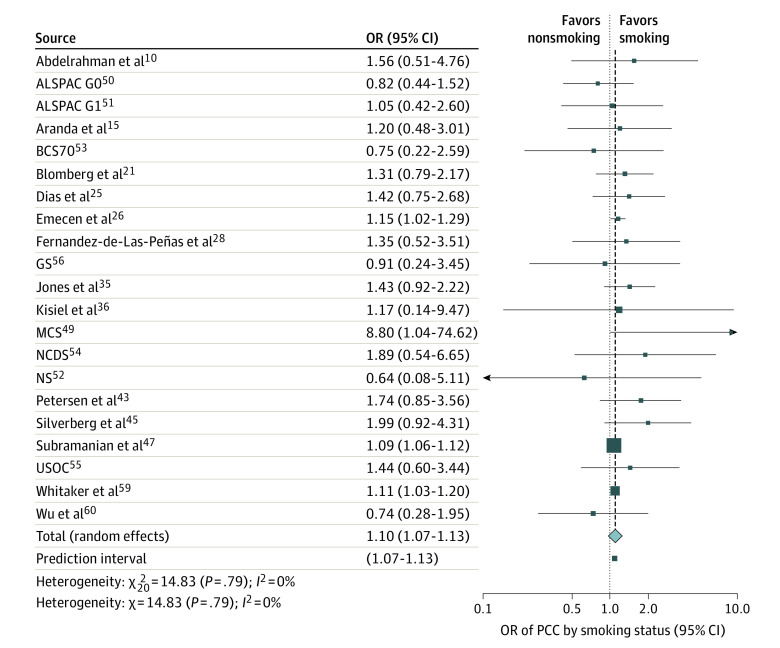
Of the 41 studies, 20 studies including a total of 455 204 patients investigated whether current smokers had higher risk of developing PCC compared with nonsmokers. Overall, the pooled ORs showed that smoking was significantly associated with PCC (OR, 1.10; 95% CI, 1.07 to 1.13; I2 = 0%; Figure 3). Subgroup analysis by study quality showed no significant differences (eFigure 12 in Supplement 1). Egger test suggested no significant publication bias (intercept = .08; 95% CI, 0.05 to 0.11; P = .07), and meta-regression analysis by study size also showed no significance (effect size = −0.0001; 95% CI, −0.0001 to 0.001; P = .14).
Figure 3. Association of Smoking Status With Post−COVID-19 Condition (PCC), 2021 to 2022.
Individuals who smoked had 1.10 times higher risk of developing PCC compared with individuals who did not smoke. The dotted line represents the point of no difference between the 2 groups, and the dashed line represents the average effect of all studies when pooled together. Data for 10 longitudinal studies49,50,51,52,53,54,55,56,57 of patients 18-96 years old were obtained solely from a single study by Thompson et al,48 including ALSPAC G0, which refers to the Avon Longitudinal Study of Parents and Children-Generation 0; ALSPAC G1, the Avon Longitudinal Study of Parents and Children-Generation 1; BCS70, British Cohort Study 1970; GS, Generation Scotland−the Scottish Family Health Study; MCS, the Millennium Cohort Study; NS, Next Steps (formerly the Longitudinal Study of Young People in England); NCDS, the National Child Development Study; and USOC, Understanding Society−the UK Household Longitudinal Survey. OR indicates odds ratio.
Comorbidities
Meta-analysis was performed for 34 studies that investigated the presence of comorbidities potentially associated with the risk of PCC syndrome. Specifics for each comorbidity follow.
Anxiety and/or Depression
Four studies including 634 734 patients investigated the risk of PCC in patients with anxiety and/or depression. Pooled analysis of these studies showed a significant association with PCC (OR, 1.19; 95% CI, 1.02 to 1.40; I2 = 96%; eFigure 24 in Supplement 1). Egger test for small study effects was not significant (intercept = 0.22; 95% CI, 0.03 to 0.41; P = .47). Meta-regression analysis for study size was not performed owing to the small number of studies in each group. Subgroup analysis by study quality is shown in eFigure 25 in Supplement 1.
Asthma
Meta-analysis of 13 studies including 639 397 patients showed that patients with asthma had significantly higher risk of developing PCC (OR, 1.24; 95% CI, 1.15 to 1.35; I2 = 53%; eFigure 13 in Supplement 1). All of these studies were of high quality; therefore, subgroup analysis for this factor was not conducted. Meta-regression analysis for study size showed significance (effect size = −0.0001; 95% CI, −0.0003 to −0.0001; P < .001), which was confirmed by subgroup analysis of studies by their sample size (eFigure 14 in Supplement 1). In this analysis, larger studies demonstrated a significant association between asthma and PCC, whereas smaller studies (<1000 patients) failed to reach significance. Egger test showed no significant publication bias (intercept = 0.23; 95% CI, 0.13 to 0.34; P = .51).
Chronic Kidney Disease
A pooled analysis of 8 studies with a total of 255 791 patients showed that CKD was not a significant risk factor for PCC (OR, 1.12; 95% CI, 0.98 to 1.28; I2 = 22%; eFigure 19 in Supplement 1). Subgroup analysis by study quality is shown in eFigure 20 in Supplement 1. Meta-regression analysis for study size showed no significance (effect size = 0.10; 95% CI, −0.05 to 0.25; P = .20), and Egger test showed no publication bias (intercept = 0.04; 95% CI, −0.21 to 0.29; P = .56).
Chronic Obstructive Pulmonary Disease
Analysis of 10 studies including 257 340 patients showed that COPD was a risk factor associated with persistent symptoms after COVID-19 infection (OR, 1.38; 95% CI, 1.08 to 1.78; I2 = 77%; eFigure 15 in Supplement 1). Nevertheless, this significance may not be shown in all future studies (95% PI, 0.70 to 2.74). Subgroup analyses by study quality is shown in eFigure 16 in Supplement 1. Meta-regression analysis for study size and Egger test were both nonsignificant (effect size = −0.0002; 95% CI, −0.0003 to 0.0001; P = .66; and intercept = 0.23; 95% CI, 0.14 to 0.33; P = .69, respectively).
Diabetes
Meta-analysis of 18 studies including 259 978 patients showed that patients with diabetes (OR, 1.06; 95% CI, 1.03 to 1.09; I2 = 0%) had a significant risk of PCC (eFigure 17 in Supplement 1). Subgroup analysis by study quality is shown in eFigure 18 in Supplement 1. Meta-regression analysis showed that study size did not have a significant effect (effect size = 0.001; 95% CI, −0.0002 to 0.0002; P = .15), and Egger test showed no publication bias (intercept = −0.008; 95% CI, −0.14 to 0.12; P = .34).
Immunosuppression
Three studies with a total of 967 patients evaluated whether patients with immunosuppression exhibited higher risk of PCC. Meta-analysis of these studies showed a significant association of immunosuppression with PCC (OR, 1.50; 95% CI, 1.05-2.15; I2 = 0%; eFigure 23 in Supplement 1). Egger test did not show significant publication bias. Owing to the small number of studies, subgroup analysis and meta-regression were not performed for these studies.
Ischemic Heart Disease
Five studies including 201 906 patients investigated the association of preexisting IHD. Meta-analysis of these studies showed that patients with IHD had 1.28 times higher risk of developing PCC (OR, 1.28; 95% CI, 1.19 to 1.38; I2 = 0%; eFigure 21 in Supplement 1). Subgroup analysis by study quality is shown in eFigure 22 in Supplement 1. Meta-regression analysis for study size and Egger test for small study effects did not show significance (effect size = −0.0001; 95% CI, −0.0003 to 0.001; P = .49, and intercept = 0.23; 95% CI, 0.14 to 0.33; P = .69, respectively).
Hospitalization and ICU Admission
Meta-analysis of 8 studies with a total of 265 466 patients previously hospitalized for COVID-19 infection was performed. The findings showed that patients who required hospitalization during the acute phase of COVID-19 had significantly higher risk of developing PCC (OR, 2.48; 95% CI, 1.97 to 3.13; I2 = 86%; eFigure 26 in Supplement 1). Subgroup analysis by study quality is shown in eFigure 27 in Supplement 1. Meta-regression analysis for study size and Egger test did not demonstrate statistical significance (effect size = 0.002; 95% CI, −0.001 to 0.0001; P = .73 and intercept = 0.96; 95% CI, 0.47 to 1.43; P = .78, respectively).
Similarly, a meta-analysis of 10 studies with a total of 213 441 patients showed that patients who required ICU admission during the acute phase were at higher risk for PCC (OR, 2.37; 95% CI, 2.18 to 2.56; I2 = 0%; eFigure 28 in Supplement 1). Subgroup analysis by study quality is shown in eFigure 29 in Supplement 1. Meta-regression analysis for study size showed that there was an effect (effect size = 0.0001; 95% CI, 0.0001 to 0.002; P = .01); however, subgroup analysis of studies by sample size did not demonstrate significant between-group differences (eFigure 30 in Supplement 1). Egger test showed no significance (intercept = 0.92; 95% CI, 0.82 to 1.02; P = .06).
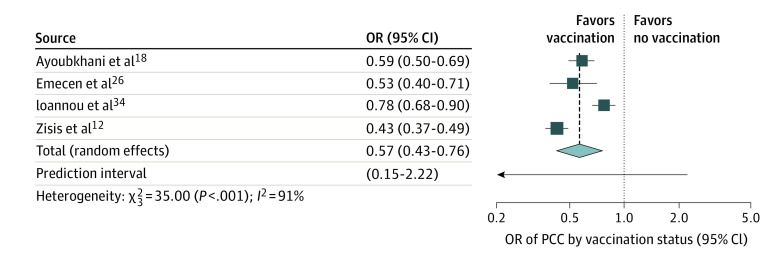
Vaccination Status
Four studies with a total of 249 788 patients evaluated the effect of vaccination status on the risk of developing PCC. Meta-analysis of these showed that individuals who had been vaccinated with 2 doses (in all included studies) had a 40% lower risk of developing PCC (OR, 0.57; 95% CI, 0.43 to 0.76; I2 = 91%; Figure 4). This may not be demonstrated in all future studies (95% PI, 0.15 to 2.22). Subgroup analysis by study quality and meta-regression for study size were not performed because all these studies were of high quality and included more than 1000 patients each. Egger test showed no significant publication bias (intercept = −0.44; 95% CI, −1.38 to 0.48; P = .80).
Figure 4. Association of Vaccination Status With Post−COVID-19 Condition (PCC), 2021 to 2022.
Individuals who were vaccinated against COVID-19 with 2 doses had a significantly lower risk of developing PCC than individuals who had not been vaccinated. The dotted line represents the point of no difference between the 2 groups, and the dashed line represents the average effect of all studies when pooled together. OR indicates odds ratio.
Sensitivity Analyses
One original publication48 of 10 longitudinal studies49,50,51,52,53,54,55,56,57 and another study35 included patients that were self- or clinician-diagnosed with COVID-19 during the acute phase. For this reason, in addition to the aforementioned analyses, we performed sensitivity analyses for all the risk factors excluding these studies (eFigure 31 in Supplement 1). Overall, there were no differences in the outcomes of any risk factor investigated. Additional sensitivity analyses were performed based on the studies that investigated 5 or more risk factors (eFigure 32 in Supplement 1). There were no changes noted in the outcomes of each risk factor. Meta-regression analyses by geographic location were also performed for the risk factors (eTable 2 in Supplement 1); however, given the limited geographic diversity (30 studies from Europe; only 1 from Africa, 6 from the Americas [Brazil, Canada, US], and 5 from Asia), the interpretation of results should be guarded.
Discussion
This meta-analysis of 41 studies that included a total of 860 783 patients demonstrates that there were certain epidemiologic and clinical risk factors that are associated with a higher risk of developing PCC. In particular, female sex, older age, higher BMI, and smoking were significantly associated with increased risk of persistent symptoms of 3 months or more after the acute phase of COVID-19 infection, ie, PCC. In addition, preexisting comorbidities, including anxiety and/or depression, asthma, COPD, diabetes, IHD, and immunosuppression were also found to be significantly associated with higher risk of PCC. Furthermore, patients who needed hospitalization or ICU care during the acute phase of COVID-19 infection were found to have more than twice the risk of developing PCC compared with those who were not. On the other hand, vaccination (with 2 doses) for COVID-19 was noted to have a protective role against PCC—vaccinated patients had a significantly lower risk of developing the persistent symptoms of PCC.
The aforementioned findings confirm that PCC is a multifactorial and complex clinical syndrome.62 These results strengthen the evidence available regarding the association of female sex with PCC.8,63,64 A previous meta-analysis by Maglietta and colleagues65 including 13 340 patients also highlighted that female sex was significantly associated with the persistent COVID-19 symptoms. A recent large analysis and meta-regression of more than 2 million patients64 confirmed this finding. Many authors have hypothesized mechanistic processes to explain the association between certain risk factors, including female sex, and PCC.1,66,67,68,69 For example, it has been suggested that hormones may play a role in perpetuating the hyperinflammatory status of the acute phase of COVID-19 even after recovery.66,67 Also, stronger IgG antibodies production in female individuals in the acute phase has been reported68 and could contribute to perpetuating disease manifestations.68,69
As previous research has suggested,48,65 older age appears to be an independent risk factor for PCC. Subgroup analysis showed that individuals 40 to 69 years old and those 70 years or older are at equally high risk of PCC when compared with younger patients. However, it is important to consider that the prevalence of PCC consists of individuals who have survived the acute phase of COVID-19 infection. Older individuals, possibly with multiple underlying comorbidities, may not survive the acute phase of COVID-19 because they are at higher risk of severe illness.70 As highlighted by Di Toro and colleagues,71 PCC reflects the population of COVID-19 survivors, not the epidemiologic characteristics of COVID-19.
Additionally, the results of our meta-analysis revealed that obesity and smoking were significantly associated with higher risk of developing PCC. These findings concur with recent evidence identifying these characteristics as important risk factors for PCC.48,72,73 Obesity and PCC share a metabolic proinflammatory state that promotes inflammatory processes and their associated signs and symptoms to linger for a prolonged period of time.74 Smoking has been shown to be a significant risk factor for both PCC and severe acute COVID-19 infection.75,76 However, it is unclear whether smoking per se or the associated severe illness predisposes this cohort of patients to higher risk of PCC.
Our meta-analysis revealed that patients who were hospitalized or admitted to the ICU had more than double risk of developing PCC. Severe illness has been found to be a significant risk factor for PCC in previous studies. In a multicenter cohort study that included 246 patients, 74.3% had ongoing physical symptoms 1 year after ICU admission for COVID-19.77 However, it should be noted that ICU survivors may experience postintensive care syndrome after an episode of critical care illness.78,79 Postintensive care syndrome is well-recognized and entails a variety of symptoms that may persist for months or years; therefore, there may be an important overlap with PCC sequelae. Nevertheless, the results of our meta-analysis and those of other studies highlight that patients with previous critical illness represent a high-risk population and their follow-up should reflect intensive plans for prevention, rehabilitation, and treatment of the ongoing debilitating symptoms of PCC.
The results of our study showed that vaccination for COVID-19 has a protective role against PCC, with vaccinated individuals having a significantly lower risk compared with unvaccinated individuals. This finding concurs with those of other studies and the recent report from the UK Office of National Statistics that found a 42% lower risk of PCC after 2 doses of a COVID-19 vaccine.80,81,82 Importantly, emerging evidence suggests that vaccination reduces the risk of PCC and its sequelae even in individuals with other risk factors, such as older age or high BMI,81 expanding the benefits of vaccination beyond the morbidity and mortality benefits seen during the acute COVID-19 phase.
Individuals with PCC may experience long-lasting adverse effects requiring long-lasting support. It has been reported that 15% of individuals with PCC were absent from work owing to illness.5 Follow-up outpatient services may be needed to manage this condition and to better understand the possible association between symptoms and residual organ impairment. Given that health care systems worldwide have been substantially burdened by the COVID-19 pandemic,83 routine follow-up may not be possible to all those living with PCC.
Limitations
This review had some limitations. Some of the meta-analyses performed had considerable statistical heterogeneity, which may have affected results. Large meta-epidemiologic studies have shown that studies at high risk of bias tend to overestimate the strength of associations. In addition, all the included studies were observational. Consequently, the results of the performed meta-analyses were based on observational data. Although the observational studies were of moderate or high quality per the Newcastle-Ottawa scale, the scale itself is not without limitations.84 Furthermore, by virtue of being observational, all the studies (even those with a high rating) have an unavoidable risk of bias. Despite this and considering that randomized studies (with the current COVID-19 strains) will not be undertaken, studies providing risk factors following multivariable regression allow us to draw important conclusions. Furthermore, as discussed previously, PCC is a clinically heterogenous condition with a range of manifestations and symptoms. In this analysis, we considered all the various manifestations as a single entity. For this meta-analysis, we relied on the diagnosis identified by the authors of the included studies, accepting that the definition of symptoms included among the different studies might not have been exactly the same. Lastly, the studies spanned across various COVID-19 variants, but were all pooled together independent of variant. It is possible that the various variants, including the effect of vaccination, could alter the absolute value of patients with PCC; however, it is unlikely that the risk factors associated with PCC would change.
Conclusions
The findings of this systematic review and meta-analysis demonstrated that certain demographic characteristics (eg, age and sex) and comorbidities were significantly associated with an increased risk of developing PCC, whereas vaccination had a protective role against developing PCC sequelae. Given these results, a holistic approach and integrated care pathways may enable suitable support for patients who develop PCC and may allow physicians to be better prepared to care for patients at high risk of developing PCC. Moreover, in addition to preventing and diminishing the acute phase of the infection, COVID-19 vaccination may protect against PCC, giving vaccination additional evidence of benefit.
eMethods. Search Strategy in PubMed
eTable1. Newcastle-Ottawa Quality Assessment table for included cohort studies
eTable 2. Meta-regression analysis by geographic location
eFigure 1. PRISMA flow diagram of the study selection process
eFigure 2. Funnel plots assessing publication bias for each group meta-analysis: A) Sex, B) Age, C) Body Mass Index, D) Smoking, E) Diabetes, F) IHD, G) COPD, H) CKD, I) Hospitalisation, J) ICU K) Vaccination
eFigure 3. Subgroup analysis of studies investigating the correlation of female sex and development of long COVID syndrome according to study population
eFigure 4. Subgroup analysis of studies investigating the correlation of female sex and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 5. Subgroup analysis of studies investigating the correlation of age and development of long COVID syndrome according to study size
eFigure 6. Meta-regression analysis bubble plot of studies investigating age as risk factor for long COVID syndrome.
eFigure 7. Subgroup analysis of studies investigating the correlation of age and development of long COVID syndrome according to study population
eFigure 8. Subgroup analysis of studies investigating the correlation of age and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 9. Forest plot showing the effect of BMI on Long COVID. Individuals with higher BMI have an increased risk of developing Long COVID syndrome.
eFigure 10. Subgroup analysis of studies investigating the correlation of obesity and development of long COVID syndrome according to study population
eFigure 11. Subgroup analysis of studies investigating the correlation of obesity and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 12. Subgroup analysis of studies investigating the correlation of smoking and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 13. Forest plot showing the effect of asthma on Long COVID. Individuals with asthma have an increased risk of developing Long COVID syndrome.
eFigure 14. Subgroup analysis of studies investigating the correlation of asthma and development of long COVID syndrome according to study size
eFigure 15. Forest plot showing the effect of COPD on Long COVID. Individuals with COPD have an increased risk of developing Long COVID syndrome.
eFigure 16. Subgroup analysis of studies investigating the correlation of COPD and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 17. Forest plot showing the effect of diabetes on Long COVID. Individuals with diabetes have an increased risk of developing Long COVID syndrome.
eFigure 18. Subgroup analysis of studies investigating the correlation of diabetes and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 19. Forest plot showing the effect of CKD on Long COVID. CKD was not significantly associated with Long COVID syndrome.
eFigure 20. Subgroup analysis of studies investigating the correlation of CKD and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 21. Forest plot showing the association of IHD with Long COVID. Individuals with IHD have an increased risk of developing Long COVID syndrome.
eFigure 22. Subgroup analysis of studies investigating the correlation of IHD and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 23. Forest plot showing the association of immunosuppression with Long COVID. Individuals with immunosuppresion have an increased risk of developing Long COVID syndrome.
eFigure 24. Forest plot showing the association of anxiety/depression with Long COVID. Individuals with anxiety/depression have an increased risk of developing Long COVID syndrome.
eFigure 25. Subgroup analysis of studies investigating the correlation of anxiety/ depression and development of long COVID syndrome according to study quality (as per the Newcastle-Ottawa Scale)
eFigure 26. Forest plot showing the association of hospitalisation with Long COVID. Individuals that were hospitalised during the acute infection had an increased risk of developing Long COVID syndrome.
eFigure 27. Subgroup analysis of studies investigating the correlation of hospitalisation and development of long COVID syndrome according to study quality (as per the Newcastle-Ottawa Scale)
eFigure 28. Forest plot showing the association of ICU admission with Long COVID. Individuals that required ICU admission during the acute infection had an increased risk of developing Long COVID syndrome.
eFigure 29. Subgroup analysis of studies investigating the correlation of ICU admission and development of long COVID syndrome according to study quality (as per the Newcastle-Ottawa Scale)
eFigure 30. Subgroup analysis of studies investigating the correlation of ICU admission and development of long COVID syndrome according to study size
eFigure 31. Sensitivity analysis of all the risk factors examined using only the studies that included patients with laboratory-confirmed COVID-19 infection
eFigure 32. Sensitivity analysis of all the risk factors with studies that investigated ≥5 variables
Data Sharing Statement
References
- 1.Chippa V, Aleem A, Anjum F. Post-Acute Coronavirus (COVID-19) Syndrome. StatPearls. Published online June 19, 2022. https://www.ncbi.nlm.nih.gov/books/NBK570608/
- 2.UK National Institute for Health and Care Excellence . COVID-19 rapid guideline: managing the long-term effects of COVID-19. Published online December 18, 2020. Accessed March 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK567264/ [PubMed]
- 3.World Health Organization . A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. Accessed November 28, 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
- 4.Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine. 2021;36:100899. doi: 10.1016/j.eclinm.2021.100899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013-1022. doi: 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 6.Halpin S, O’Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol. 2021;93(3):1242-1243. doi: 10.1002/jmv.26587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430-436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelrahman MM, Abd-Elrahman NM, Bakheet TM. Persistence of symptoms after improvement of acute COVID-19-infection, a longitudinal study. J Med Virol. 2021;93(10):5942-5946. doi: 10.1002/jmv.27156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-de-Las-Peñas C, Cancela-Cilleruelo I, Moro-López-Menchero P, et al. Exploring the trajectory curve of long-term musculoskeletal post-COVID pain symptoms in hospitalized COVID-19 survivors: a multicenter study. Pain. 2023;164(2):413-420. doi: 10.1097/j.pain.0000000000002718 [DOI] [PubMed] [Google Scholar]
- 12.Zisis SN, Durieux JC, Mouchati C, Perez JA, McComsey GA. The protective effect of Coronavirus Disease 2019 (COVID-19) vaccination on postacute sequelae of COVID-19: a multicenter study from a large national health research network. Open Forum Infect Dis. 2022;9(7):ofac228. doi: 10.1093/ofid/ofac228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed November 28, 2022. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 14.Higgins JPT, Thomas J, Chandler J, et al. , eds. Chapter 6: choosing effect measures and computing estimates of effect. In: Cochrane Handbook for Systematic Reviews of Interventions. Accessed November 28, 2022. https://training.cochrane.org/handbook/current/chapter-06
- 15.Aranda J, Oriol I, Feria L, et al. Persistent COVID-19 symptoms 1 year after hospital discharge: a prospective multicenter study. PLoS One. 2022;17(10):e0275615. doi: 10.1371/journal.pone.0275615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asadi-Pooya AA, Akbari A, Emami A, et al. Long COVID syndrome-associated brain fog. J Med Virol. 2022;94(3):979-984. doi: 10.1002/jmv.27404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augustine R, S A, Nayeem A, et al. Increased complications of COVID-19 in people with cardiovascular disease: Role of the renin-angiotensin-aldosterone system (RAAS) dysregulation. Chem Biol Interact. 2022;351:109738. doi: 10.1016/j.cbi.2021.109738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayoubkhani D, Bosworth ML, King S, et al. Risk of long COVID in people infected with severe acute respiratory syndrome coronavirus 2 after 2 doses of a coronavirus disease 2019 vaccine: community-based, matched cohort study. Open Forum Infect Dis. 2022;9(9):ofac464. doi: 10.1093/ofid/ofac464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baruch J, Zahra C, Cardona T, Melillo T. National long COVID impact and risk factors. Public Health. 2022;213:177-180. doi: 10.1016/j.puhe.2022.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. doi: 10.1001/jamanetworkopen.2020.36142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blomberg B, Mohn KGI, Brokstad KA, et al. ; Bergen COVID-19 Research Group . Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607-1613. doi: 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chudzik M, Babicki M, Kapusta J, et al. Long-COVID clinical features and risk factors: a retrospective analysis of Patients from the STOP-COVID registry of the PoLoCOV Study. Viruses. 2022;14(8):1755. doi: 10.3390/v14081755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daitch V, Yelin D, Awwad M, et al. ; ESCMID study group for infections in the elderly (ESGIE) . Characteristics of long-COVID among older adults: a cross-sectional study. Int J Infect Dis. 2022;125:287-293. doi: 10.1016/j.ijid.2022.09.035 [DOI] [PubMed] [Google Scholar]
- 24.Debski M, Tsampasian V, Haney S, et al. Post-COVID-19 syndrome risk factors and further use of health services in East England. PLOS Glob Public Health. 2022;2(11):e0001188. doi: 10.1371/journal.pgph.0001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dias MB, Medeiros APV, de Melo SS, et al. ; CO-FRAIL Study Group . The long and winding road of COVID-19 in survivors of hospitalisation: symptoms trajectory and predictors of long COVID. J Intern Med. 2023;293(2):264-268. doi: 10.1111/joim.13583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emecen AN, Keskin S, Turunc O, et al. The presence of symptoms within 6 months after COVID-19: a single-center longitudinal study. Ir J Med Sci. 2022;(0123456789):1-10. doi: 10.1007/s11845-022-03072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estrada-Codecido J, Chan AK, Andany N, et al. Prevalence and predictors of persistent post−COVID-19 symptoms. J Assoc Med Microbiol Infect Dis Can. 2022;7(3):208-219. doi: 10.3138/jammi-2022-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-de-Las-Peñas C, Guijarro C, Plaza-Canteli S, Hernández-Barrera V, Torres-Macho J. Prevalence of post-COVID-19 cough one year after SARS-CoV-2 infection: a multicenter study. Lung. 2021;199(3):249-253. doi: 10.1007/s00408-021-00450-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-de-Las-Peñas C, Guijarro C, Torres-Macho J, et al. Diabetes and the risk of long-term post-COVID symptoms. Diabetes. 2021;70(12):2917-2921. doi: 10.2337/db21-0329 [DOI] [PubMed] [Google Scholar]
- 30.Fernández-de-Las-Peñas C, Torres-Macho J, Elvira-Martínez CM, Molina-Trigueros LJ, Sebastián-Viana T, Hernández-Barrera V. Obesity is associated with a greater number of long-term post-COVID symptoms and poor sleep quality: a multicentre case-control study. Int J Clin Pract. 2021;75(12):e14917. doi: 10.1111/ijcp.14917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Long-term post-COVID symptoms and associated risk factors in previously hospitalized patients: a multicenter study. J Infect. 2021;83(2):237-279. doi: 10.1016/j.jinf.2021.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández-de-Las-Peñas C, Martín-Guerrero JD, Pellicer-Valero ÓJ, et al. Female sex is a risk factor associated with long-term post-COVID related-symptoms but not with COVID-19 symptoms: the LONG-COVID-EXP-CM multicenter study. J Clin Med. 2022;11(2):413. doi: 10.3390/jcm11020413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández-de-Las-Peñas C, Pellicer-Valero ÓJ, Navarro-Pardo E, et al. Symptoms experienced at the acute phase of SARS-CoV-2 infection as risk factor of long-term post-COVID symptoms: the LONG-COVID-EXP-CM multicenter study. Int J Infect Dis. 2022;116:241-244. doi: 10.1016/j.ijid.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ioannou GN, Baraff A, Fox A, et al. Rates and factors associated with documentation of diagnostic codes for long COVID in the National Veterans Affairs Health Care System. JAMA Netw Open. 2022;5(7):e2224359. doi: 10.1001/jamanetworkopen.2022.24359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones R, Davis A, Stanley B, et al. Risk predictors and symptom features of long COVID within a broad primary care patient population including both tested and untested patients. Pragmat Obs Res. 2021;12:93-104. doi: 10.2147/POR.S316186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kisiel MA, Janols H, Nordqvist T, et al. Predictors of post-COVID-19 and the impact of persistent symptoms in non-hospitalized patients 12 months after COVID-19, with a focus on work ability. Ups J Med Sci. 2022;127. doi: 10.48101/ujms.v127.8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostev K, Smith L, Koyanagi A, Jacob L. Prevalence of and factors associated with post-coronavirus disease 2019 (COVID-19) condition in the 12 months after the diagnosis of COVID-19 in adults followed in general practices in Germany. Open Forum Infect Dis. 2022;9(7):ofac333. doi: 10.1093/ofid/ofac333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menezes AS Jr, Botelho SM, Santos LR, Rezende AL. Acute COVID-19 syndrome predicts severe long COVID-19: an observational study. Cureus. 2022;14(10):e29826. doi: 10.7759/cureus.29826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munblit D, Bobkova P, Spiridonova E, et al. ; Sechenov StopCOVID Research Team . Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy. 2021;51(9):1107-1120. doi: 10.1111/cea.13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pazukhina E, Andreeva M, Spiridonova E, et al. ; Sechenov StopCOVID Research Team . Prevalence and risk factors of post-COVID-19 condition in adults and children at 6 and 12 months after hospital discharge: a prospective, cohort study in Moscow (StopCOVID). BMC Med. 2022;20(1):244. doi: 10.1186/s12916-022-02448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27(10):1507-1513. doi: 10.1016/j.cmi.2021.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters C, Dulon M, Westermann C, Kozak A, Nienhaus A. Long-term effects of COVID-19 on workers in health and social services in Germany. Int J Environ Res Public Health. 2022;19(12):6983. doi: 10.3390/ijerph19126983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen MS, Kristiansen MF, Hanusson KD, et al. Prevalence of long COVID in a national cohort: longitudinal measures from disease onset until 8 months’ follow-up. Int J Infect Dis. 2022;122:437-441. doi: 10.1016/j.ijid.2022.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Righi E, Mirandola M, Mazzaferri F, et al. Determinants of persistence of symptoms and impact on physical and mental wellbeing in long COVID: a prospective cohort study. J Infect. 2022;84(4):566-572. doi: 10.1016/j.jinf.2022.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silverberg JI, Zyskind I, Naiditch H, et al. Predictors of chronic COVID-19 symptoms in a community-based cohort of adults. PLoS One. 2022;17(8):e0271310. doi: 10.1371/journal.pone.0271310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Štěpánek L, Nakládalová M, Janošíková M, Štěpánek L, Kabrhelová K, Boriková A. Predictors and characteristics of post-acute COVID-19 syndrome in healthcare workers. Infect Dis (Lond). 2023;55(2):125-131. doi: 10.1080/23744235.2022.2136750 [DOI] [PubMed] [Google Scholar]
- 47.Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi: 10.1038/s41591-022-01909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson EJ, Williams DM, Walker AJ, et al. ; OpenSAFELY Collaborative . Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13(1):3528. doi: 10.1038/s41467-022-30836-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzsimons E, Brown M, Sanchez A, et al. MCS (Millennium Cohort Study). Accessed November 11, 2023. https://cls.ucl.ac.uk/cls-studies/millennium-cohort-study/
- 50.Timpson N, Molloy L, Ring S, et al. ALSPAC G0 (Avon Longitudinal Study of Parents and Children): Children of the 90s. University of Bristol. Accessed November 11, 2023. http://www.bristol.ac.uk/alspac/
- 51.Timpson N, Molloy L, Ring S, et al. ALSPAC G1 (Avon Longitudinal Study of Parents and Children): Children of the Children of the 90s. University of Bristol. Accessed November 11, 2023. http://www.bristol.ac.uk/alspac/participants/our-participants/coco90s/
- 52.Henderson M, Brown M, Sanchez A, et al. NS (Next Steps). Accessed November 11, 2023. https://cls.ucl.ac.uk/cls-studies/next-steps/
- 53.Ploubidis G, Brown M, Sanchez A, et al. BCS70 (1970. British Cohort Study). Accessed November 11, 2023. https://cls.ucl.ac.uk/cls-studies/1970-british-cohort-study/
- 54.Ploubidis G, Brown M, Sanchez A, et al. 1958. National Child Development Study. Accessed November 11, 2023. https://cls.ucl.ac.uk/cls-studies/1958-national-child-development-study/
- 55.Benzeval M, Al Baghal T, Kneeshaw J, et al. USOC (Understanding Society). Accessed November 11, 2023. https://www.understandingsociety.ac.uk/
- 56.Sudlow C, Porteous D, McIntosh A, et al. GS (Generation Scotland). Accessed November 11, 2023. https://www.ed.ac.uk/generation-scotland/
- 57.Spector T, Hart D, Stevens C, et al. TwinsUK. Accessed November 11, 2023. https://twinsuk.ac.uk/
- 58.Tleyjeh IM, Saddik B, AlSwaidan N, et al. Prevalence and predictors of Post-Acute COVID-19 Syndrome (PACS) after hospital discharge: a cohort study with 4 months median follow-up. PLoS One. 2021;16(12):e0260568. doi: 10.1371/journal.pone.0260568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitaker M, Elliott J, Chadeau-Hyam M, et al. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat Commun. 2022;13(1):1957. doi: 10.1038/s41467-022-29521-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Q, Ailshire JA, Crimmins EM. Long COVID and symptom trajectory in a representative sample of Americans in the first year of the pandemic. Sci Rep. 2022;12(1):11647. doi: 10.1038/s41598-022-15727-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4(9):e2127403. doi: 10.1001/jamanetworkopen.2021.27403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sylvester SV, Rusu R, Chan B, Bellows M, O’Keefe C, Nicholson S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Curr Med Res Opin. 2022;38(8):1391-1399. doi: 10.1080/03007995.2022.2081454 [DOI] [PubMed] [Google Scholar]
- 64.Wulf Hanson S, Abbafati C, Aerts JG, et al. ; Global Burden of Disease Long COVID Collaborators . Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604-1615. doi: 10.1001/jama.2022.18931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11(6):1541. doi: 10.3390/jcm11061541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116(14):2197-2206. doi: 10.1093/cvr/cvaa284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohamed MS, Moulin TC, Schiöth HB. Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine. 2021;71(1):3-8. doi: 10.1007/s12020-020-02536-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng F, Dai C, Cai P, et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020;92(10):2050-2054. doi: 10.1002/jmv.25989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28(4):611.e9-611.e16. doi: 10.1016/j.cmi.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romero Starke K, Reissig D, Petereit-Haack G, Schmauder S, Nienhaus A, Seidler A. The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis. BMJ Glob Health. 2021;6(12):e006434. doi: 10.1136/bmjgh-2021-006434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Toro A, Bozzani A, Tavazzi G, et al. Long COVID: long-term effects? Eur Heart J Suppl. 2021;23(suppl E):E1-E5. doi: 10.1093/eurheartj/suab080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tenforde MW, Kim SS, Lindsell CJ, et al. ; IVY Network Investigators; CDC COVID-19 Response Team . Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network: United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993-998. doi: 10.15585/mmwr.mm6930e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Evans RA, Leavy OC, Richardson M, et al. ; PHOSP-COVID Collaborative Group . Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med. 2022;10(8):761-775. doi: 10.1016/S2213-2600(22)00127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Florencio LL, Fernández-de-Las-Peñas C. Long COVID: systemic inflammation and obesity as therapeutic targets. Lancet Respir Med. 2022;10(8):726-727. doi: 10.1016/S2213-2600(22)00159-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barthélémy H, Mougenot E, Duracinsky M, et al. Smoking increases the risk of post-acute COVID-19 syndrome: results from a French community-based survey. Tob Induc Dis. 2022;20(June):59. doi: 10.18332/tid/150295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clift AK, von Ende A, Tan PS, et al. Smoking and COVID-19 outcomes: an observational and Mendelian randomisation study using the UK Biobank cohort. Thorax. 2022;77(1):65-73. doi: 10.1136/thoraxjnl-2021-217080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;327(6):559-565. doi: 10.1001/jama.2022.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colbenson GA, Johnson A, Wilson ME. Post-intensive care syndrome: impact, prevention, and management. Breathe (Sheff). 2019;15(2):98-101. doi: 10.1183/20734735.0013-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gardashkhani S, Ajri-Khameslou M, Heidarzadeh M, Rajaei Sedigh S. Post-intensive care syndrome in COVID-19 patients discharged from the intensive care unit. J Hosp Palliat Nurs. 2021;23(6):530-538. doi: 10.1097/NJH.0000000000000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.UK Office for National Statistics . Self-reported long COVID after two doses of a coronavirus (COVID-19) vaccine in the UK. Accessed December 1, 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidaftertwodosesofacoronaviruscovid19vaccineintheuk/26january2022
- 81.Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22(1):43-55. doi: 10.1016/S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Notarte KI, Catahay JA, Velasco JV, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine. 2022;53:101624. doi: 10.1016/j.eclinm.2022.101624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gebru AA, Birhanu T, Wendimu E, et al. Global burden of COVID-19: situational analysis and review. Hum Antibodies. 2021;29(2):139-148. doi: 10.3233/HAB-200420 [DOI] [PubMed] [Google Scholar]
- 84.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategy in PubMed
eTable1. Newcastle-Ottawa Quality Assessment table for included cohort studies
eTable 2. Meta-regression analysis by geographic location
eFigure 1. PRISMA flow diagram of the study selection process
eFigure 2. Funnel plots assessing publication bias for each group meta-analysis: A) Sex, B) Age, C) Body Mass Index, D) Smoking, E) Diabetes, F) IHD, G) COPD, H) CKD, I) Hospitalisation, J) ICU K) Vaccination
eFigure 3. Subgroup analysis of studies investigating the correlation of female sex and development of long COVID syndrome according to study population
eFigure 4. Subgroup analysis of studies investigating the correlation of female sex and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 5. Subgroup analysis of studies investigating the correlation of age and development of long COVID syndrome according to study size
eFigure 6. Meta-regression analysis bubble plot of studies investigating age as risk factor for long COVID syndrome.
eFigure 7. Subgroup analysis of studies investigating the correlation of age and development of long COVID syndrome according to study population
eFigure 8. Subgroup analysis of studies investigating the correlation of age and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 9. Forest plot showing the effect of BMI on Long COVID. Individuals with higher BMI have an increased risk of developing Long COVID syndrome.
eFigure 10. Subgroup analysis of studies investigating the correlation of obesity and development of long COVID syndrome according to study population
eFigure 11. Subgroup analysis of studies investigating the correlation of obesity and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 12. Subgroup analysis of studies investigating the correlation of smoking and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 13. Forest plot showing the effect of asthma on Long COVID. Individuals with asthma have an increased risk of developing Long COVID syndrome.
eFigure 14. Subgroup analysis of studies investigating the correlation of asthma and development of long COVID syndrome according to study size
eFigure 15. Forest plot showing the effect of COPD on Long COVID. Individuals with COPD have an increased risk of developing Long COVID syndrome.
eFigure 16. Subgroup analysis of studies investigating the correlation of COPD and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 17. Forest plot showing the effect of diabetes on Long COVID. Individuals with diabetes have an increased risk of developing Long COVID syndrome.
eFigure 18. Subgroup analysis of studies investigating the correlation of diabetes and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 19. Forest plot showing the effect of CKD on Long COVID. CKD was not significantly associated with Long COVID syndrome.
eFigure 20. Subgroup analysis of studies investigating the correlation of CKD and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 21. Forest plot showing the association of IHD with Long COVID. Individuals with IHD have an increased risk of developing Long COVID syndrome.
eFigure 22. Subgroup analysis of studies investigating the correlation of IHD and development of long COVID syndrome according to study quality (as per the Newcastle- Ottawa Scale)
eFigure 23. Forest plot showing the association of immunosuppression with Long COVID. Individuals with immunosuppresion have an increased risk of developing Long COVID syndrome.
eFigure 24. Forest plot showing the association of anxiety/depression with Long COVID. Individuals with anxiety/depression have an increased risk of developing Long COVID syndrome.
eFigure 25. Subgroup analysis of studies investigating the correlation of anxiety/ depression and development of long COVID syndrome according to study quality (as per the Newcastle-Ottawa Scale)
eFigure 26. Forest plot showing the association of hospitalisation with Long COVID. Individuals that were hospitalised during the acute infection had an increased risk of developing Long COVID syndrome.
eFigure 27. Subgroup analysis of studies investigating the correlation of hospitalisation and development of long COVID syndrome according to study quality (as per the Newcastle-Ottawa Scale)
eFigure 28. Forest plot showing the association of ICU admission with Long COVID. Individuals that required ICU admission during the acute infection had an increased risk of developing Long COVID syndrome.
eFigure 29. Subgroup analysis of studies investigating the correlation of ICU admission and development of long COVID syndrome according to study quality (as per the Newcastle-Ottawa Scale)
eFigure 30. Subgroup analysis of studies investigating the correlation of ICU admission and development of long COVID syndrome according to study size
eFigure 31. Sensitivity analysis of all the risk factors examined using only the studies that included patients with laboratory-confirmed COVID-19 infection
eFigure 32. Sensitivity analysis of all the risk factors with studies that investigated ≥5 variables
Data Sharing Statement