Abstract
Previous studies have strongly suggested an association between glycosaminoglycans and tissue deposits of amyloid. The present study was aimed at studying this association in purified preparations of hepatic amyloid fibrils obtained from human AA type secondary amyloidosis. Glycosaminoglycans were isolated by gradient ion exchange chromatography of purified amyloid fibrils treated with pronase. Degradation with specific enzymes identified the glycosaminoglycans as chondroitin sulphate, dermatan sulphate, and heparin/heparan sulphate. The total amount of glycosaminoglycans specifically coisolated with the amyloid fibrils was 15 micrograms/mg fibril weight. The presence of glycosaminoglycans in amyloid may play a part in the incorporation of structurally diverse protein precursors into amyloid fibrils of identical ultrastructure.
Full text
PDF

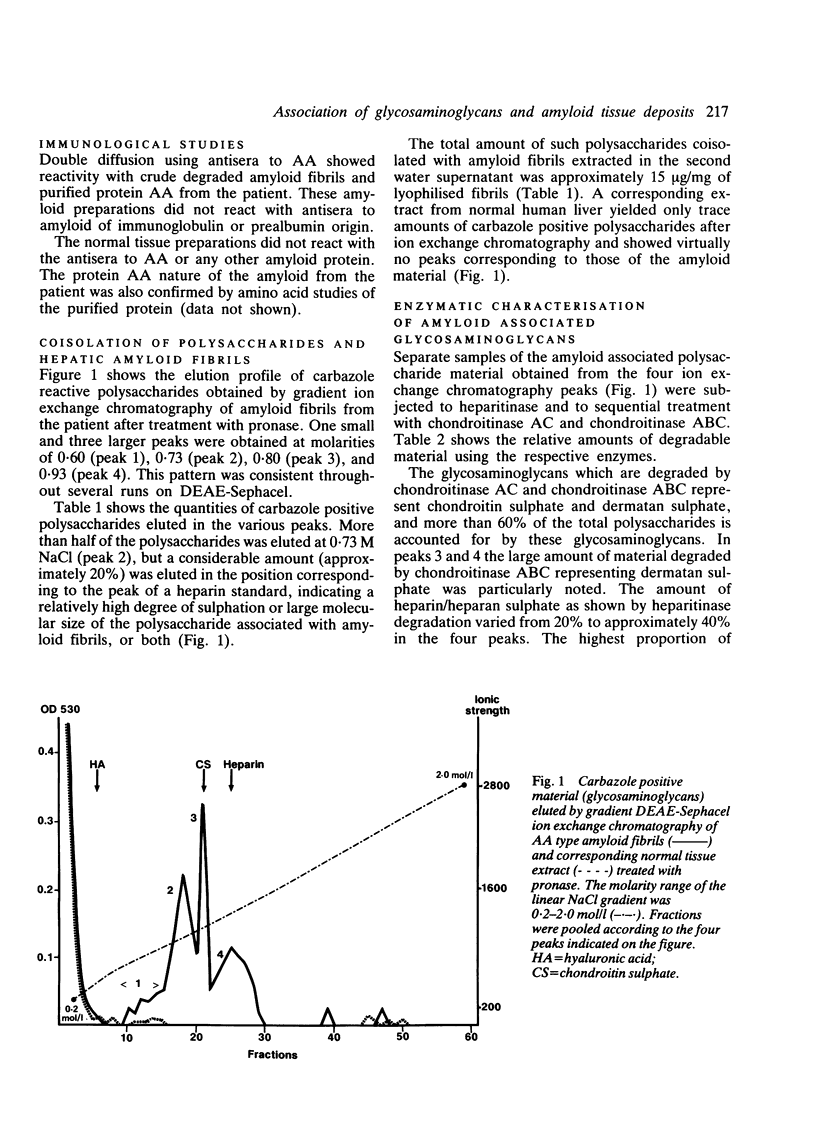

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad M. A., Kisilevsky R., Willmer J., Chen S. J., Skinner M. Further characterization of amyloid-enhancing factor. Lab Invest. 1982 Aug;47(2):139–146. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hermodson M. A., Ericsson L. H. The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971 Dec 1;19(2):169–173. doi: 10.1016/0014-5793(71)80506-9. [DOI] [PubMed] [Google Scholar]
- Bitter T., Muir H. Mucopolysaccharides of whole human spleens in generalized amyloidosis. J Clin Invest. 1966 Jun;45(6):963–975. doi: 10.1172/JCI105412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. S., Connors L. H. The pathogenesis and biochemistry of amyloidosis. J Pathol. 1987 Jan;151(1):1–10. doi: 10.1002/path.1711510102. [DOI] [PubMed] [Google Scholar]
- Dalferes E. R., Jr, Radhakrishnamurthy B., Berenson G. S. Acid mucopolysaccharides of amyloid tissue. Arch Biochem Biophys. 1967 Feb;118(2):284–291. doi: 10.1016/0003-9861(67)90350-5. [DOI] [PubMed] [Google Scholar]
- Dalferes E. R., Jr, Radhakrishnamurthy B., Berenson G. S. Glycosaminoglycans in experimental amyloidosis. Proc Soc Exp Biol Med. 1968 Mar;127(3):925–929. doi: 10.3181/00379727-127-32836. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Hallén A. Chromatography of acidic glycosaminoglycans on DEAE-cellulose. J Chromatogr. 1972 Aug 23;71(1):83–91. doi: 10.1016/s0021-9673(01)85691-0. [DOI] [PubMed] [Google Scholar]
- Husby G., Natvig J. B. Immunological characterization of amyloid fibrils in tissue sections. Clin Exp Immunol. 1972 Jul;11(3):357–366. [PMC free article] [PubMed] [Google Scholar]
- Husby G., Sletten K. Chemical and clinical classification of amyloidosis 1985. Scand J Immunol. 1986 Mar;23(3):253–265. doi: 10.1111/j.1365-3083.1986.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Husby G., Sletten K., Michaelsen T. E., Natvig J. B. Antigenic and chemical characterization of non-immunoglobulin amyloid proteins. Scand J Immunol. 1972;1(4):393–400. doi: 10.1111/j.1365-3083.1972.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Husebekk A., Skogen B., Husby G., Marhaug G. Transformation of amyloid precursor SAA to protein AA and incorporation in amyloid fibrils in vivo. Scand J Immunol. 1985 Mar;21(3):283–287. doi: 10.1111/j.1365-3083.1985.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Kisilevsky R. From arthritis to Alzheimer's disease: current concepts on the pathogenesis of amyloidosis. Can J Physiol Pharmacol. 1987 Sep;65(9):1805–1815. doi: 10.1139/y87-282. [DOI] [PubMed] [Google Scholar]
- Linker A., Carney H. C. Presence and role of glycosaminoglycans in amyloidosis. Lab Invest. 1987 Sep;57(3):297–305. [PubMed] [Google Scholar]
- Niewold T. A., Hol P. R., van Andel A. C., Lutz E. T., Gruys E. Enhancement of amyloid induction by amyloid fibril fragments in hamster. Lab Invest. 1987 May;56(5):544–549. [PubMed] [Google Scholar]
- Nordlie M., Sletten K., Husby G., Ranløv P. J. A new prealbumin variant in familial amyloid cardiomyopathy of Danish origin. Scand J Immunol. 1988 Jan;27(1):119–122. doi: 10.1111/j.1365-3083.1988.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Pras M., Nevo Z., Schubert M., Rotman J., Matalon R. The significance of mucopolysaccharides in amyloid. J Histochem Cytochem. 1971 Jul;19(7):443–448. doi: 10.1177/19.7.443. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Skinner M., Shirahama T., Cohen A. S., Deal C. L. The association of amyloid P-component (AP) with the amyloid fibril: an updated method for amyloid fibril protein isolation. Prep Biochem. 1982;12(5):461–476. doi: 10.1080/10826068208070597. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R., Stephens C., Anastassiades T. Characterization of tissue and plasma glycosaminoglycans during experimental AA amyloidosis and acute inflammation. Qualitative and quantitative analysis. Lab Invest. 1987 Jun;56(6):665–675. [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R. Temporal relationship between glycosaminoglycan accumulation and amyloid deposition during experimental amyloidosis. A histochemical study. Lab Invest. 1985 Jul;53(1):37–44. [PubMed] [Google Scholar]
- Snow A. D., Willmer J., Kisilevsky R. A close ultrastructural relationship between sulfated proteoglycans and AA amyloid fibrils. Lab Invest. 1987 Dec;57(6):687–698. [PubMed] [Google Scholar]
- Varga J., Flinn M. S., Shirahama T., Rodgers O. G., Cohen A. S. The induction of accelerated murine amyloid with human splenic extract. Probable role of amyloid enhancing factor. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(3):177–185. doi: 10.1007/BF02899027. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Trotter J. A. The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll Relat Res. 1987 Jun;7(2):105–114. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]


