Abstract
Chemical post-translational methods allow convergent side-chain editing of proteins without needing to resort to genetic intervention. Current approaches that allow the creation of constitutionally native side chains via C–C bond formation, using off-protein carbon-centered C· radicals added to unnatural amino acid radical acceptor (SOMOphile, singly occupied molecular orbital (SOMO)) “tags” such as dehydroalanine, are benign and wide-ranging. However, they also typically create epimeric mixtures of d/l-residues. Here, we describe a light-mediated desulfurative method that, through the creation and reaction of stereoretained on-proteinl-alanyl Cβ· radicals, allows Cβ–Hγ, Cβ–Oγ, Cβ–Seγ, Cβ–Bγ, and Cβ–Cγ bond formation to flexibly generate site-selectively edited proteins with full retention of native stereochemistry under mild conditions from a natural amino acid precursor. This methodology shows great potential to explore protein side-chain diversity and function and in the construction of useful bioconjugates.
Short abstract
Cys-arylation and then C−S bond scission creates on-proteinl-alanyl Cβ· radicals that allow Cβ−Hγ, Cβ−Oγ, Cβ−Seγ, Cβ−Bγ, and Cβ−Cγ bond formation to edit proteins with full retention of native stereochemistry.
Introduction
In nature, post-translational protein modification enables and mediates various essential biological processes.1 For example, glycosylation can drive immune responses,2 phosphorylation activates enzymes,3 and ubiquitination triggers protein degradation.4 While such natural post-translational modifications (PTMs) greatly extend the complexity of protein structures and also increase the diversity of gene product/protein function, they do not cover all possible chemical space and, so, in principle useful unnatural functionality in biology remains undiscovered. The editing of proteins to create such “chemical PTMs” could efficiently bridge that gap.5 Moreover, the recapitulation of PTM (and other protein) function through precise structural design allows causal links to be established to putative mechanisms.6
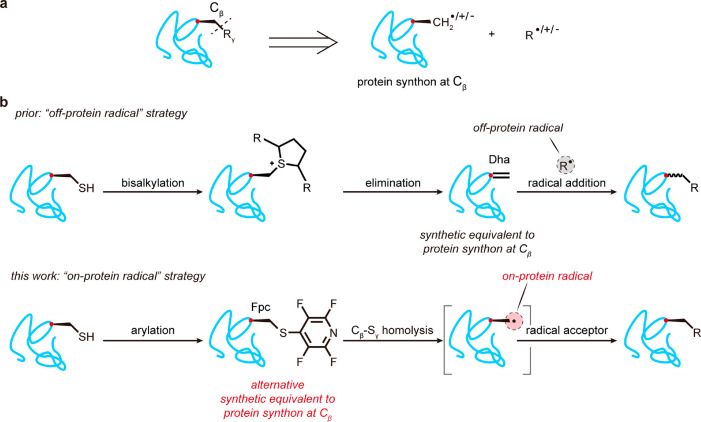
Classical strategies for protein modification often feature bonds to heteroatoms (noncarbon) made at the γ (Cys Sγ, Thr Oγ, Ser Oγ) or ω (Lys Nω, Tyr Oω) positions of side chains.7,8 These have valuably allowed technological and translational development of novel diagnostic and medical tools as well as the interrogation and the manipulation of biological processes.5,9,10 However, constructing Cβ–X bonds via Cβ, which is present in all amino acid side chains, is a rare but potentially far-reaching disconnection in synthetic and chemical biology (Figure 1a).11 From a retrosynthetic viewpoint,12 the major difficulty of utilizing Cβ to create C–X bonds is that appropriate synthetic equivalents of a protein synthon at Cβ are difficult to generate under mild conditions using classical heterolytic/2e– chemistries as the C+ or C– equivalents (Figure 1a) that are required are typically quenched.
Figure 1.
Strategies for Cβ–Xγ bond formation on proteins. (a) Retrosynthetic analysis of in situ side chain Cβ–Xγ bond formation. (b) Contrasting methods for the use of synthetic equivalents to a Cβ protein synthon using prior off-protein or this work on-protein carbon-centered C· radicals. The use of on-protein radicals, derived here from a tetrafluoropyridyl-Cys (Fpc) intermediate, allows the potential for retention of stereochemistry via a configurationally defined l-alanyl radical intermediate, arbitrarily denoted in protein image “cartoons” throughout this manuscript via the use of a “wedged” bond.
To address this problem, we11−14 and others15 have considered the use of homolytic/1e– chemistries that may be used in combination with readily generated15−19 dehydroalanine (Dha) residues on proteins. In this way, Dha, by acting as a singly occupied molecular orbital (SOMO) acceptor (“radical acceptor” or “SOMOphile”), can serve as a synthetic equivalent of a protein synthon at Cβ by reacting with off-protein carbon-centered C· radical species. This has allowed selective Cβ–Cγ bond formation to introduce a wide variety of side chains into several protein scaffolds.12−14 Although this “off-protein radical” strategy (radical acceptor (Dha) on-protein reacts with C· radical species generated off-protein) allows ready exploration of protein side-chain diversity, modification state, and consequent function, the native l-stereochemistry at the modified residue is erased (Figure 1b, top) and typically regenerated with low diastereoselectivity. This results in the creation of a mixture of d/l-epimers in diastereomeric ratios (d.r.s) of typically ∼1:1 (and <3:1). Moreover, while function can often be reliably inferred from such epimeric mixtures,12,14,20 access to a stereodefined chemical method would undoubtedly be advantageous in removing the ambiguity of the potential role of the d-epimer in such analyses and in considering synthetic chemical routes to pure protein products. Here, we describe a readily applied, stereoretentive method for achieving this through the strategic inversion of this homolytic/1e– disconnection to allow efficient use of on-protein Cβ· radicals, which retain their l-configuration (Figure 1b, bottom).
Results
Design of a Method for Selectively Creating On-Protein Cβ· Radicals
In re-examining methods for the refunctionalization of Cβ in proteins to create putative synthons, we noted that, for one synthetic equivalent, Dha, one of the most prevalent methods exploits the elimination of an activated sulfonium intermediate, generated via the chemoselective alkylation of a Cys residue.16 The strategic success of this method therefore relies in part on the ability to chemoselectively access a suitable synthetic equivalent (that itself can then be manipulated chemoselectively). For Dha formation via Cys, this relies in part on the ability to react free Cys in the presence of unreacted cystinyl S–S bonds.
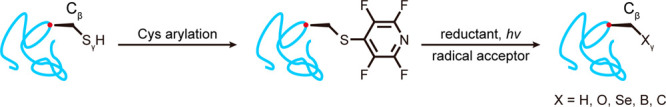
We considered that if a preactivated (e.g., through suitable modification or “alkylation”) Cys-derived Cβ–Sγ bond underwent 1e– homolysis instead of an heterolytic 2e– E1cB process,21 then an l-alanyl radical Cβ· might be generated without influencing the configuration of the stereogenic l-Cα center. This l-alanyl radical Cβ· might then be subsequently trapped by radical acceptors (SOMO-philes) to form new side chains. This “on-protein radical” (radical acceptor is off the protein, and radical is generated on the protein) strategy could theoretically avoid the loss of native stereochemistry (Figure 1b, bottom).
Notably, it has been proposed for over 60 years that alanyl radicals may be intermediates in Cys desulfurization reactions,22,23 and such reactions are now commonly exploited in so-called “traceless native chemical ligation”24−26 to convert Cys to desulfurized Ala residues (Figure S18). In peptidic systems, alanyl radicals generated in this way have shown promise by taking advantage of phosphine to activate the Cβ–Sγ bond.27,28 Such prior strategies for desulfurization at cysteine, cystine, or selenenylcysteines proceed via a seemingly complex or likely multiple-manifold process29 involving the likely intermediate formation of thiophosphoranyl radical adducts as precursors to C· radicals formed upon β-scission (Figure S18a).22,23 The requirement in these systems for use of phosphines or other P(III) reagents, which are strongly reducing, effectively precludes more general use in typical protein systems since these are commonly used to disrupt disulfides (e.g., TCEP; see also Figure S16) at the concentrations required to effect desulfurization via thiophosphoranyl. This and the sometimes additional requirement of organic (co)solvent have therefore prevented their efficient use in a general manner in protein systems beyond their use in “traceless” ligation methods via C–H bond formation prior to refolding. We have shown that eliminative mechanisms to Dha may compete in some phosphine mediated desulfurization manifolds, thereby raising the potential for loss of stereochemistry or a side reaction (Figure S18a).16,30
We have also shown that such on-protein C· radicals, when stabilized by α-fluoro-substitution as C(F)n·, allow reactivity that enables C–Se, C–O, and C–C bond formation.14 Nonetheless, despite the promise suggested by all of these above methods, they all require either the use of conditions that are not compatible with typical proteins or the creation of unnatural (e.g., fluorine substituted) side chain precursors to access C· radicals (Figure S18b).
Our generation of side chain on-protein carbon-centered radicals from sulfonyl precursors through the cleavage of Cγ–S(O)2R bonds through reductive initiation highlighted the feasibility of C–S bond scission for radical generation in proteins. While the redox potential and C–S bond strength to allow initiation at such sites may potentially be tuned,31 our attempts to selectively generate suitable alkyl sulfonyl Cβ–S(O)2R side chains at the β-carbon rather than γ-carbon directly from modified Cys residues proved challenging due, in part, to concomitant oxidation of other residues such as methionine.
Therefore, we turned to alternative methods for tuning the radical scission potential of the Cβ–S bond. The presence of electron-withdrawing substituents on S is known to enhance C–S bond cleavage via homolytic and mesolytic manifolds.32,33 In reductive initiation, this may stabilize appropriate radical anion intermediates formed upon single electron transfer (SET) and/or thiolates in mesolysis (Figure S18c). Such SET driven initiation may also be light-stimulated (either in the reductant, e.g., photoredox catalyst, or in the substrate).34
Initial36ab initio DFT calculations (see Supplementary Methods and Figure S14) suggested that the appropriate precursor MO energies and associated stability of a resulting radical anion might be effectively altered through the attachment of strongly electron withdrawing substituents on a simple sulfide and especially those allowing π-acidic, conjugation effects. These could potentially be derived directly from the free thiol SH of Cys if an effective method for selective modification could be established. This therefore suggested that the installation of an electron withdrawing π-system on Sγ might create a suitable Cys-modified precursor for a Cβ· radical via Cβ–Sγ bond homolysis; DFT calculations also suggested an associated bond lengthening of the alkyl S–Csp3 and not the aryl S–Csp2. While our calculations suggested different possible Cys-Sγ substituents might prove fruitful, we turned our attention to the installation of electron-poor aryl moieties that could be readily achieved with chemoselectivity via direct protein arylation (Figure 1b, bottom).
While we37 and others8 have shown that S-arylated motifs may be readily installed through metal-mediated arylation, we also considered more classical methods that allow SN2Ar-type reactions for installation of such moieties.38 For example, elegant studies have shown that benzenoid systems may be installed in such a way into proteins, even exploiting selectivity by proposed exploitation of interactions of local residues (a so-called “pi-clamp”).39 More recently, the tuning of these benzenoids with electron-withdrawing substituents has further allowed enhanced selectivities for such benzenoid conjugations.40 However, computation (see the Supporting Information) suggested that certain aryl moieties might fail to stabilize required intermediates on the putative pathway required for reductive initiation.
SN2Ar reactions at heteroarenes are often more greatly favored than their arene counterparts. One archetype, pentafluoropyridine (pyF5), possesses enhanced reactivity41−43 with respect to SN2Ar, attributed in part to the ability to delocalize developing negative charge in transition states around any Meisenheimer intermediate to the heteroatom.42 Such heteroatom-associated stabilization would likely also contribute to the pathway to initiation via a radical anion intermediate. This conclusion was not only supported by our computation (see the Supporting Information) but also further confirmed by the experimental measurement of half potentials for the reduction of pyF4-sulfides44 (PyF-S) to radical anion intermediates that might potentially allow initiation via Cys-Cβ–Sγ bond homolysis.
We therefore reasoned that, although unprecedented, by combining a two-step process of chemoselective arylation (with such an electron poor heteroaryl) with reductively driven initiation, we might create a ready and direct pathway to stereoretained, on-protein l-alanyl radical intermediates (via Cys-Cβ–Sγ cleavage) suitable for further addition reactions. Here, we demonstrate that either direct single electron transfer (SET) process or electron donor-electron acceptor (EDA) complexes in this way allow us to construct Cβ–Hγ, Cβ–Oγ, Cβ–Seγ, Cβ–Cγ, and Cβ–Bγ bonds on proteins (Figures 2 and 3).
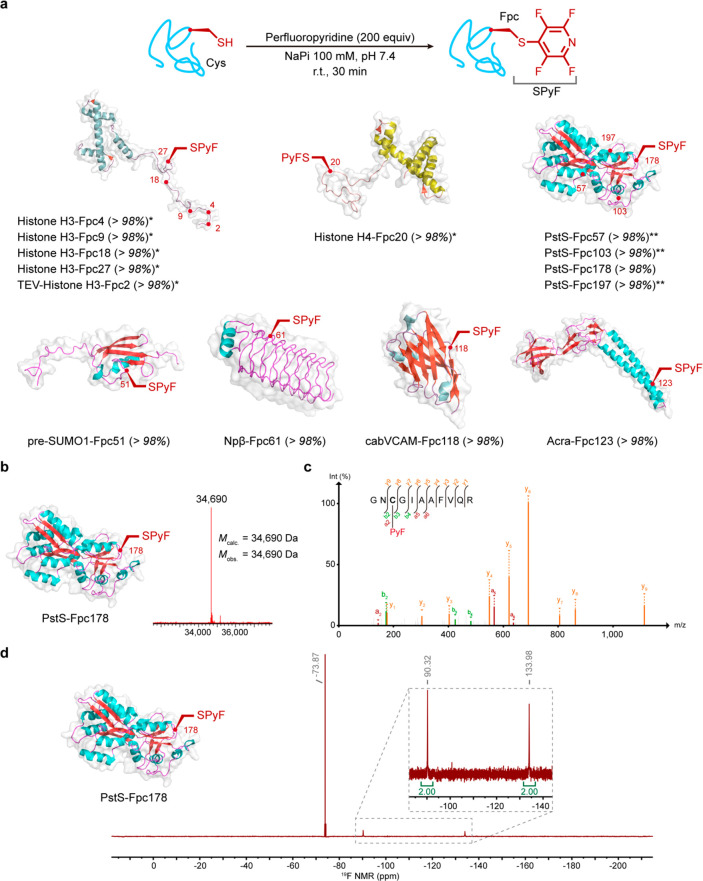
Figure 2.
Site-selective chemical introduction of the Fpc side chain into proteins. (a) The chemoselective modification of Cys allows site-selective introduction of Fpc at a range of sites in representative proteins with a varied scaffold type and with different secondary structure motifs. % conversion shown in parentheses. (b) Representative intact protein MS confirms excellent conversion to install Fpc, shown here for PstS-Fpc178. (c) Site-selectivity was confirmed by tryptic-MSMS analyses, shown here for PstS-Fpc178176–186 peptide. (d) Advantageously, intact protein 19F NMR, here with internal standard CF3COO–, allows sensitive assessment of reaction chemoselectivity in a “zero-background”. Characteristic chemical shifts (δF1 ∼−90.3, δF2 ∼−134 ppm)45 confirmed selective C–S product formation to generate Fpc, shown here for PstS-Fpc178. *: in Tricine buffer (100 mM, pH 7.4); **: in NaPi buffer (100 mM, pH 7.4 with Gdn·HCl 3 M).
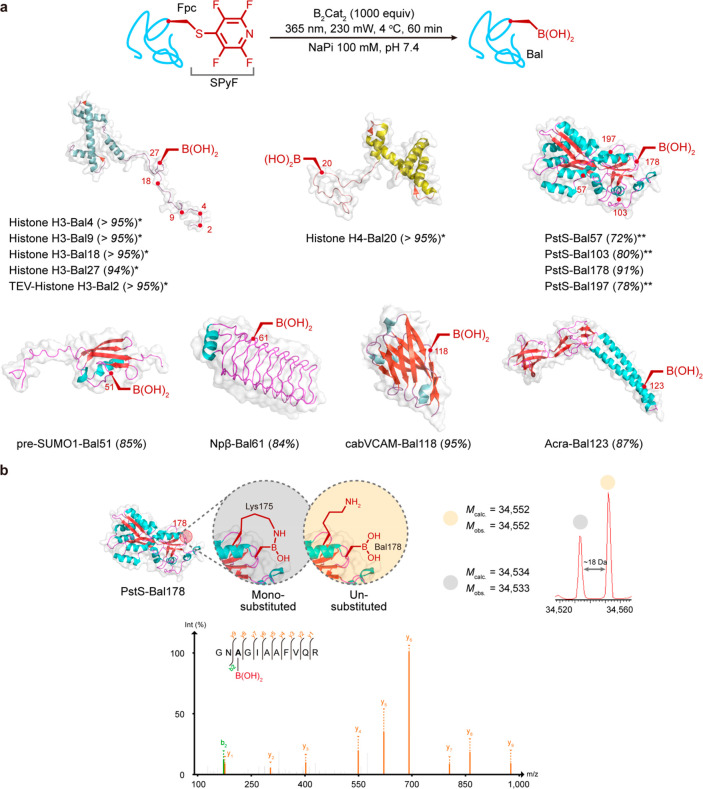
Figure 3.
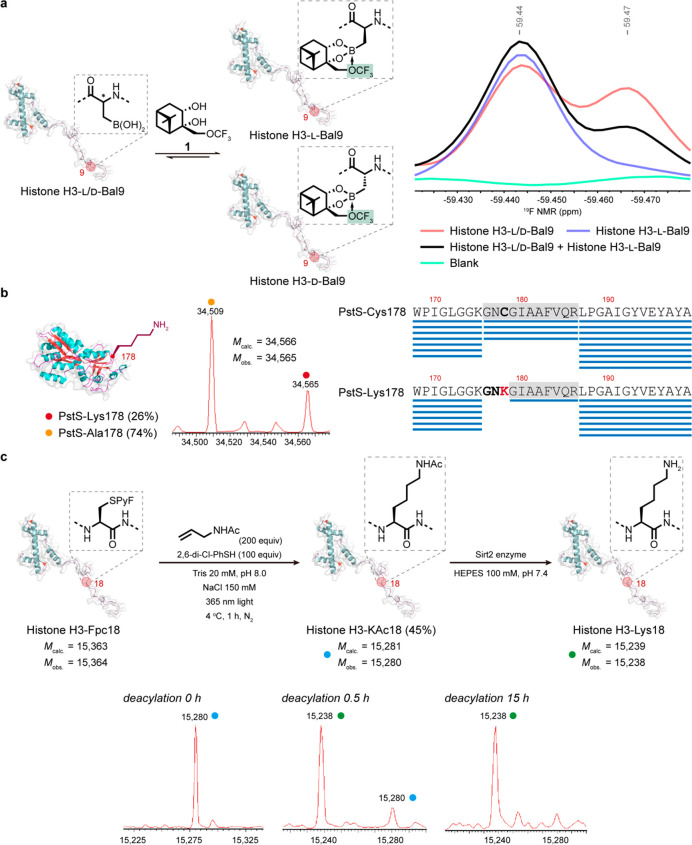
Chemical introduction of l-boronoalanine (l-Bal) into proteins. (a) Dual initiation and trapping from Fpc allows the introduction of Bal into diverse protein sites and scaffolds. % conversion shown in parentheses. (b) Consistent with prior observations,58 the insertion of Bal is observed in multiple states via intact protein MS concomitant with internal ligated boronates in intact protein (top), attributed here speculatively to nearby Lys175 as an illustrative example only. Bal can be observed directly via tryptic-MSMS (bottom). Data shown here for representative system PstS-Bal178. *: in Tricine buffer (100 mM, pH 7.4); **: in NaPi buffer (100 mM, pH 7.4 with Gdn·HCl 3 M).
Site-Selective Chemical Introduction of TetraFluoropyridyl-Cys (Cys-S-PyF or Fpc) into Proteins
Perhaps due to its known enhanced SN2Ar reactivity in classical small molecule systems,42 pyF5 has been perceived to be a nonselective modification reagent,38 in part based on exploration of peptidic systems in DMF.45 While this is a reactivity that can be tuned by the use of protic solvent trifluoroethanol,46 its use in aqueous systems has not been exploited. The creation of tetrafluoropyridyl-Cys (Cys-S-PyF/Fpc) in proteins is therefore unexplored.
The reaction between a model protein containing a single cysteine, AcrA-Cys123, and pyF5 was evaluated as an initial model. AcrA is a challenging model substrate membrane protein that would allow us to test the limits of this method. While initial attempts explored the use of lower temperature (4 °C) to control reactivity, this ultimately proved unnecessary. Instead, pH proved an important determinant. Thus, while under neutral or even mildly acidic conditions (pH 6), the reaction proceeded only slowly; strikingly, perfluoroheteroarylation with pyF5 proceeded efficiently in different buffers at pH > 7.0 (Table S1). Optimized conditions (pH 7.4, 200 equiv. of pyF5, 25 °C, 30 min) were effective in a range of buffers (NaPi, tricine, or Tris) and allowed full conversion to AcrA-Fpc123.
With a reliable method in hand, a variety of proteins were screened, resulting in all cases in the full conversion to tetrafluoropyridyl-cysteine containing proteins (Figure 2): histones H3 and H4, small α-helical nuclear proteins; PstS, a protein involved in bacterial phosphate transport;47 pre-SUMO1 (SUMO, small ubiquitin-like modifier), a small globular protein containing α-helices and β-sheets; cabVCAM, a cross-reactive nanobody against human and murine VCAM1;48 Npβ, a β-helical pentapeptide repeat;49 AcrA, a membrane protein. Notably, cabVCAM also contains an internal disulfide that allowed testing of the compatibility our methods with such key structural motifs. Characterization (including intact protein mass spectrometry (MS) and proteolytic/tandem MS (MS/MS): Figure 2b–d and the Supporting Information) confirmed that proteins were successfully and site-selectively modified with perfluoropyridine.
Notably, the strong dual 19F resonances in the Fpc side chain also allowed unequivocal confirmation of the formation of C–S product formation via direct use of 19F protein NMR (δF1 = −90.32, δF2 = −133.98 ppm) wholly consistent with observations in peptidic systems (δF1 = −90.37, δF2 = −134.15 ppm)45 and highlighting a lack of modification of other putative protein nucleophiles (e.g., Lys: δF1 = −98.17, δF2 = −165.54; Tyr: δF1 = −91.32, δF2 = −155.98; Ser: δF1 = −97.10, δF2 = −165.92).45 The ability to use this 19F signal in the “zero-signal” background of native proteins is a striking additional advantage of the Fpc side chain as an intermediate protein “tag” state (Figure 2d).
Light-Mediated Cβ–Sγ Bond Cleavage Testing
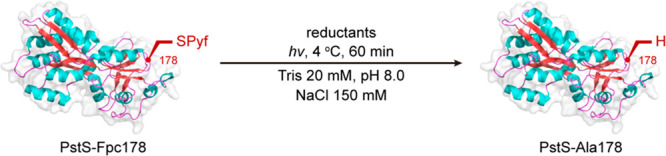
With the establishment of a reliable method to install Fpc into a range of protein substrates, we then tested its potential in light-mediated Cβ–Sγ bond cleavage on a protein scaffold. Our prior generation of on-protein radicals had successfully exploited reductive initiation.14 Multiple reductants were screened to drive putative reductive initiation using protein PstS-Fpc178 as a test substrate (Table 1 and Figure S1).
Table 1. Light-Mediated Cβ–Sγ Bond Cleavage in Fpc-Containing Proteinsa.
| entry | reductants | hν/nm | conversion/% |
|---|---|---|---|
| 1 | Ir(dtppy)(bpy)2BF4 (10 equiv), FeSO4 (200 equiv) | 365 | 90 |
| 2 | Ir(dtppy)(bpy)2BF4 (10 equiv), FeSO4 (200 equiv) | 385 | 90 |
| 3 | Ir(dtppy)(bpy)2BF4 (10 equiv), FeSO4 (200 equiv) | 405 | 71 |
| 4 | Ir(dtppy)(bpy)2BF4 (10 equiv), FeSO4 (200 equiv) | 420 | 77 |
| 5 | Ir(dtppy)(bpy)2BF4 (10 equiv), FeSO4 (200 equiv) | 445 | 35 |
| 6 | Ru(bpy)3Cl2 (10 equiv), FeSO4 (200 equiv) | 365 | 52 |
| 7 | Ru(bpy)3Cl2 (10 equiv), FeSO4 (200 equiv) | 385 | 63 |
| 8 | Ru(bpy)3Cl2 (10 equiv), FeSO4 (200 equiv) | 405 | 66 |
| 9 | Ru(bpy)3Cl2 (10 equiv), FeSO4 (200 equiv) | 420 | 75 |
| 10 | Ru(bpy)3Cl2 (10 equiv), FeSO4 (200 equiv) | 445 | 51 |
| 11 | 4-Me-PhSH (100 equiv) | 365 | >98 |
| 12 | 4-Me-PhSH (100 equiv) | 385 | 0 |
| 13 | 4-Me-PhSH (100 equiv) | 405 | 0 |
| 14 | 4-Me-PhSH (100 equiv) | 420 | 0 |
| 15 | 4-Me-PhSH (100 equiv) | 445 | 0 |
| 16 | 2-Cl-6-F-PhSH (100 equiv) | 365 | >98 |
| 17 | B2Cat2 (100 equiv) | 365 | >98b |
| 18 | B2Cat2 (100 equiv) | 385 | 0 |
| 19 | B2Cat2 (100 equiv) | 405 | 0 |
| 20 | B2Cat2 (100 equiv) | 420 | 0 |
| 21 | B2Cat2 (100 equiv) | 445 | 0 |
Exploration of varied SET reagents revealed differing modes of reductive initiation and optimal conditions. Conditions (see also scheme): in a glovebox at <10 ppm of O2, PstS-Fpc178 (15 μM, 50 μL); reductants were mixed, and the reaction was irradiated at 4 °C for 60 min. The reaction mixture was then analyzed by LC-MS.
9% conversion to PstS-Ala178; 91% conversion to PstS-Bal178.
Pleasingly, both strongly reducing [Ir(dtbbpy)(ppy)2]PF6 (Eox −1.51 V vs SCE) and less reducing Ru(bpy)3Cl2 (Eox −1.33 V vs SCE) photostimulated outer-sphere SET metal complexes (“photoredox” catalysts) drove the reaction at a range of wavelengths (365–445 nm) in the presence of Fe(II) as coreductant14 to give PstS-Ala178 (Table 1). While the conversions with [Ir(dtbbpy)(ppy)2]PF6 proved typically higher (up to 90%), even the milder Ru(bpy)3Cl2 proved effective (up to 75% conversion); the former also generated some apparent oxidative damage in proteins, consistent with prior observations.14 The implied breadth of the action spectrum for photoreaction is consistent with the broad absorption excitation region of both of these complexes. As we have noted previously, control of dissolved oxygen levels (e.g., <10 ppm) in associated buffers (e.g., through prior equilibration under low oxygen conditions in a glovebox or other means such as sparging) also proved apparently beneficial.
While these reactions proved successful, the associated issues of conversion and damage led us to consider alternative systems and, so, alternative reductants. Two nonmetal chemical reductant types were therefore considered: aryl thiols and diboron(IV) compounds. Both classes importantly encompassed the use of reagents with the potential to act in a photostimulated manifold via putative charge-transfer complexes.50,51
With both arylthiols, para-tolyl-SH (pTol-SH) and 2-chloro-6-fluoro-phenyl-SH (ClFΦ-SH) gave excellent conversions under mild conditions of PstS-Fpc178 → PstS-Ala178. It should be noted that while these aryl thiols represent differently tuned acidities (pKas pTol-SH = 6.82 in water,52 ClFΦ-SH = 5.27 predicted in water53) neither are sufficiently potent nucleophilic reductants to disrupt protein S–S bonds.54 In all cases, the activated Cβ–Sγ bond in the Fpc side chain could be successfully cleaved under 365 nm light but not at other wavelengths (Table 1). Notably, this narrow implied action spectrum, in contrast with the observed outer-sphere metal complexes (see above), was consistent with the formation of a corresponding charge-transfer complex enabling donor–acceptor55 SET (see below) as well as the putative reductive capacity of, for example, the thiolate/thiyl half reaction.56
Finally, the aryl diboron(IV) compound bis(catecholato)diboron (B2Cat2) was also tested. This too proved effective under mild conditions (Table 1) in driving cleavage of the activated Cβ–Sγ bond in PstS-Fpc178, also with a narrow action spectrum (365 nm). Notably, however, this reaction of PstS-Fpc178 led to not only the formation of PstS-Ala178 but also the concomitant formation of boronylated PstS-Bal178 (containing boronoalanine residue (Bal)) as, indeed, the major product. Excitingly, this implied not only the potential for reductive initiation of PstS-Fpc178 but also the trapping of a putative intermediate on-protein Cβ· radical by B2Cat2 through coincident B–B bond cleavage allowing direct on-protein Cβ–Bγ bond formation.
Stereoretentive Introduction of l-Boronoalanine (Bal) into Proteins Using Dual Initiation-Trapping
This observed, dual, light-mediated reductive initiation and trapping using B2Cat2 led us to test the greater breadth of such concomitant on-protein l-alanyl trapping, here in Cβ–Bγ formation. While organoboronic acids and their esters are vital building blocks that play a pivotal role in organic synthesis,57 there are few chemical methods to introduce boronic acid groups to proteins.58 Currently, the minimal borono amino acid boronoalanine (Bal) cannot be introduced without dilution of homochirality at Cα.58,59 Since use of epimeric d/l-Bal on proteins already exhibits the benefits of de novo binding function in expanding biological function,58 the ability to control such function at a homochiral residue could allow more precise dissection of associated mechanisms and ligand sequestration.
Strikingly, application of a 1000 equiv excess of B2Cat2 combined with photostimulated reductive initiation at 365 nm led, under a reduced oxygen atmosphere (<10 ppm), to concomitant trapping and hydrolysis to form l-Bal (see also below) directly in proteins with excellent efficiency on seven different protein scaffolds at multiple predetermined sites (Figures 3 and S2). Importantly, these included the generation of cabVCAM-Bal118, where complete retention of the internal disulfide was observed, highlighting the benign nature of this editing method (Figure S16a). This was notably in complete contrast to detected disruption when treated with the phosphine TCEP (Figure S16b).
Testing the Breadth of Cβ· Alanyl Radical-Trapping
This promising indication of C–B bond formation via reaction of an on-protein Cβ· radical led us to test the breadth and utility of l-alanyl C· trapping. Given the observed dual reduction-trapping activity of B2Cat2, we sought first to separate reductive initiation from trapping. To accomplish this, we tested the utility of aryl thiols, pTol-SH, 2,6-dichlorophenyl-SH (Cl2Φ-SH) and ClFΦ-SH, as reagents that could be varied in not only their pKa but also potentially their associated SET and hydrogen atom transfer (HAT) activities via tuning of their substituents60 in concert with several different kinds of representative radical acceptors that would allow formation of varied Cβ–Xγ bonds.
First, using pTol-SH as reductant, we explored direct Cβ–Oγ formation using 2,2,6,6-tetramethyl-1-piperidine-1-oxyl (TEMPO) as a persistent radical that might trap. Pleasingly, this combination enabled separation of reductive initiation (by pTol-SH) from Cβ· trapping allowing conversion in 90% (Figures 4a,b and S3) from the alanyl radical with formation of a Cβ–Oγ bond.
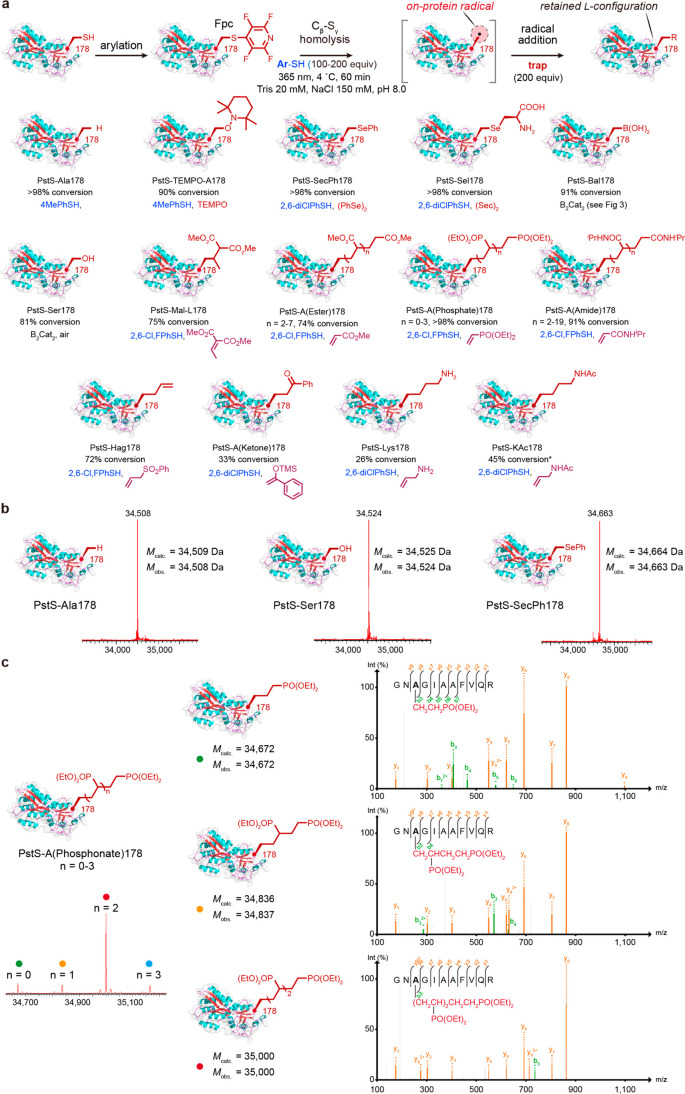
Figure 4.
Scope of l-alanyl radical-trapping. (a) Diverse bond-forming proves possible through trapping of the on-protein radical generated from PstS-Fpc178 to generate varied side chains. Please also see the Supplementary Methods where the acceptors used have been described in detail with individual schemes and structures for each. (b) Representative examples of Cβ–Hγ, Cβ–Oγ, and Cβ–Seγ bond formation proceed with excellent conversions. (c) Cβ–Cγ bond formation allows differing modes of bond formation. For example, on-protein C–C polymerization of vinyl phosphonate (which gives rise to a stabilized adduct radical product that can react further via matched polarity) can be observed via individual oligomer states using both intact protein MS (bottom left) and precisely mapped by tryptic-MSMS analyses (right). Direct Cβ–Cγ trapping without polymerization may also be achieved with differing substrates. In all cases, conversions are essentially full and the major side product formed in lower yielding reactions is the reduced Ala product. *: for KAc formation in PstS, the formation of KAc includes 40% “dimer” formation; interestingly, in histone H3 at site 18, only KAc product is observed (see Figure 5).
Diselenides could also trap the alanyl radical to form the Cβ–Seγ bond, creating modified selenocysteine residues; selenocysteines can exhibit relevant, typically redox, activities in natural systems61 (Figure 4a,b). Notably, despite the strong potential for direct nucleophilic heterolytic reduction of the diselenides by thiol, the use of Cl2Φ-SH as a tuned SET reductant proved effective, allowing essentially complete conversion to both PstS-SecPh178 (using PhSe-SePh) and the selenolanthionine (Sel) adduct PstS-Sel178 (Figures S4 and S5).
Most importantly, Cβ–Cγ bonds could be constructed, providing one of nature’s most important side chain structural motifs (see above), when treated with appropriate polarized olefins as radical acceptor traps (Figure 4a,b). Notably, use again of low nucleophilicity thiols ClFΦ-SH and Cl2Φ-SH allowed reaction without an apparent concomitant inhibitory side reaction (e.g., Michael-type). The consequent reactivity was also determined by the nature of the radical acceptor. Thus, while expectedly62 vinyl phosphonate, acrylate, and acrylamide, which give rise to stabilized adduct radical products that can react further via matched polarity, allowed on-protein polymerization (n = 1–4, Figure 4c), acceptors that give rise to more fleeting intermediate radicals or ones with nonmatched polarities or lower consequent addition allowed simple addition.
In this way, dimethyl ethylidenemalonate, 1-phenyl-1-trimethylsiloxyethylene, and phenyl allylsulfone also allowed ready formation of Cβ–Cγ bonds as direct or indirect adducts (Figures S6–S8). The indirect adducts thereby allowed the generation of side chains with functional groups that may be further reacted as chemoselective “tags” in protein chemistry. The former provides a reactive acetophenone carbonyl-containing moiety with the consequent potential for further application in diverse bioconjugation.63 The latter allows chemical generation of l-homoallylglycine (Hag), an archetypal “tag” side chain for thiyl-ene ligations.64 The ability to introduce this chemically now complements its typical introduction via sense-codon reassignment.
While typically poor electrophiles for protein modification, some of these Cβ–Cγ bond-forming reagent C acrylates and acrylamides nonetheless have the potential to nonspecifically modify protein nucleophiles. Notably, in control reactions in the absence of arylthiol, protein substrate was not consumed. Moreover, when arylthiol alone was used to first reduce Pst-178Fpc to Pst-178Ala and then treated with further portions of arylthiol and acrylate or acrylamide, no further reaction was detected. These control experiments suggest that any such competing side reactions are negligible and that only radical-trapping reactivity is seen under these conditions that we describe here (see also Discussion).
Importantly, such Cβ–Cγ bond formation also allowed direct access also to fully native l-residues or their post-translationally modified variants. Thus, through the use of allylic amines (allylamine or its acetamide), fully native l-lysine and l-N-acetyl-lysine residues were generated not only in a natural Lys site in histone H3 (H3-KAc18; see Figure 5 and the Supporting Information) but also in an unnatural site in PstS (PstS-Lys178 and PstS-KAc178, Figures S9 and S10), albeit in more modest yields. Interestingly, the reactivity of the acetamide appears to sit at a cusp under these conditions that leads to some concomitant dimer formation (presumably through trapping of the radical intermediate by a second equivalent of allylacetamide) at some sites (e.g., unnatural site 178 in PstS but not at natural site 18 in H3).
Figure 5.
Functional and spectroscopic characterization of the retention of native l-stereochemistry. Different sites, proteins, and side chains were assessed for the configuration of the residues edited into corresponding proteins. (a) NMR “chiral shift” reagent, diol 1 allowed the use of intact protein 19F NMR to assess the configuration of Bal introduced into histone H3-Bal9. A comparison of that introduced with poorly stereoselective methods58 (mixture of d/l-, red) with that formed using the methods described here (blue), as well as the corresponding “mutual spike” (black), suggests full formation of l-Bal using the editing methods described here within detection limits. (b) The observation of full cleavage of chemically edited Lys178 in PstS-Lys178 by stereospecific enzyme trypsin through its binding of Lys178 as an S1 residue in proteolysis suggests the full formation of l-Lys using the editing methods described here within detection limits. Sequence coverage of the region of the precursor PstS-Cys178 is compared with that following editing to PstS-Lys178; this leads to tryptic cleavage C-terminal to the edited Lys178. Accordingly, the results of the tandem LCMS analysis of the modified protein were processed using the protein sequence database in which wild-type PstS was changed to PstS-Lys178. (c) The observation of full deacetylation of chemically edited KAc18 in histone H3-KAc18 by stereospecific HDAC enzyme Sirt2 to generate H3-Lys18 suggests the full formation of l-KAc using the editing methods described here within detection limits.
Finally, the ability to install Bal through dual initiation-trapping using B2Cat2 (see above) was used to further extend the range of methods that would allow access to native residues. Thus, when conducted in open air, l-Ser was formed as a consequence of a three-step, one-pot initiation-trapping-migratory oxidation pathway as another example of a Cβ–Oγ bond formation. In this way, the original l-Cys residue was mutated (via l-Fpc and, without isolation, l-Bal) to l-Ser (Figures 4a,b and S11) in 80% conversion as a further example of the use of post-translational editing in allowing chemical protein mutagenesis.
Functional and Spectroscopic Characterization of the Retention of Native l-Stereochemistry
To probe the configuration of the residues formed from on-protein radical-trapping, three differing systems were tested with complementary and orthogonal spectroscopic and functional methods. First, intact protein 19F NMR experiments were conducted on a histone H3 variant (H3-Bal9) in which Bal had been installed at site 9 (Figure 5a). We have previously shown that chiral NMR shift reagent 1 is able to distinguish epimers of d- and l-Bal following installation of epimeric d/l-Bal into proteins.58 The resulting fluorinated diol boronate ester adducts formed through binding to the Bal residue on proteins therefore reveals d/l-configuration information via 19F NMR analysis.58 When an epimeric sample of H3–d/l-Bal9 (formed using Cu(II)-catalyzed boronylation58) was mixed with chiral shift reagent 1, a ratio of approximately 1:1 was detected after integration of the corresponding CF3 resonances (Figure 5a, red line) consistent with the known poor diastereoselectivity. However, when a sample of putative H3–l-Bal9, prepared using the methods described here, was mixed with chiral shift reagent 1, only a single resonance was detected (Figure 5a, blue line). When these two protein samples were combined in equal amounts (mutually “spiked”), a ratio of >2:1 was detected after integration following enhancement of only one of the resonances in the H3–d/l-Bal9 epimeric mixture (Figure 5a, black line), consistent with the enhancement of concentration of only one epimer. Together, these data strongly indicated that the native l-stereochemistry at the modified residue was preserved during the reaction to form H3–l-Bal9 from H3–l-Cys9 via H3–l-Fpc9.
Next, we tested the stereospecific enzymatic reaction of residues in two other protein scaffolds and sites. Trypsin digests stereoselectively at l-Lys (rather than d-Lys) residues,65 including in polypeptides,66 thereby enabling tryptic digests for so-called peptide mapping in protein sequencing via MSMS methods. When PstS-Lys178, that had been formed using the methods described here, was subject to digestion, full and complete enzymatic digestion to the corresponding octapeptide PstS179–186 fragment was observed (Figure 5b). This arises from cleavage C-terminal to the Lys178 residue through the known selectivity of trypsin for basic P1 residues in its S1 pocket. This was further confirmed to be l-Lys specific through the notable absence of the same PstS179–186 peptide in any of the tryptic digests of the other PstS-178 protein variants formed in this study; these were instead formed as native, expected PstS176–186 undecapeptides.
Finally, after exploiting the stereospecificity of a protein-backbone-modifying enzyme, we then exploited the complementary stereospecificity of a protein-side chain-modifying enzyme in yet another protein scaffold and site, H3–l-KAc18. Acetylated lysine (KAc) is known to be stereospecifically deacetylated during the “erasing” of the acetyl post-translational modification at l-Lys18 in histone H3 by the histone deacetylation (HDAC) enzyme Sirt2.67,68 We have previously shown that this enzyme does not fully process mixed d/l-epimers of acetylated l-lysine or its analogues, also consistent with this l-stereospecificity and with failed deacetylation of acetylated d-KAc.14,20 Strikingly, when H3-KAc18, prepared using the methods described here, was treated with Sirt2 then complete deacetylation was observed, consistent with the presence only of an acetylated l-KAc residue (Figure 5c).
Finally, in a protease (TEV) cleavable variant of histone H3 into which Ser2 had been edited from a precursor Fpc2 residue, we also used the derivatization method of Marfey69 to analyze configuration. From the residues found in the cleaved N-terminal residue 1–8 fragment, no d-Ser was detected under the limits of these analyses, consistent with the presence only of an l-Ser residue (see Figure S17).
Together, this testing of configuration in four different, representative protein scaffold, sites and residue systems (H3–l-Bal9, PstS–l-Lys178, H3–l-KAc18, and clevable-H3–l-Ser2) suggested that l-configuration is well preserved through diverse reactions and substrates using the reactions we describe here in a stereoretentive manner.
Discussion
In summary, we have described an efficient on-protein free radical generation-and-trapping method via light-mediated Cβ–Sγ bond cleavage to now realize a general form of chemical mutagenesis via post-translational editing. Most importantly, we observe that the l-configuration of the stereogenic Cα at mutated residues is preserved during such editing.
Not all of the problems of this form of stereoretentive post-translational editing have been solved, and some limitations remain. For example, while many reactions are efficient in the trapping of the on-protein radicals that are formed, in other cases, lower conversions are observed for some of the proof-of-principle systems that we describe here. Notably, however, the secondary side products in these cases are the directly reduced inactive l-alanine variants (see above for examples with Sirt2 or trypsin). In this way, this form of editing even with lower conversions still allows ready chemical installation of altered residues for rapid functional scoping of protein activity in the background of a typically inactive (i.e., l-Ala) variant. In addition, in the case of phenyl vinyl sulfone, nonspecific modification of protein lysines was observed (Figure S15) in addition to on-protein radical-trapping, highlighting that in some cases competent radical acceptors may also have concomitant additional direct electrophilic reactivity that proves competitive.
While the full details of the mechanism of the reactions that we describe here are the subject of current experiments and we cannot discount other mechanisms, our initial data suggest that a pathway consistent with the formation of charge-transfer complexes is followed involving so-called electron acceptor-electron donor (EDA) species. Indeed, the observation of a weak charge-transfer (CT) band lower in energy than the parent molecular transitions was observed not only in model small molecule systems but also in protein systems during spectroscopic probing (via absorption spectra) of the preirradiation reaction mixture of bis(catecholato)diboron (B2Cat2 (electron donor, 0.1 mM)) and PstS-Fpc178 (0.02 mM, in Tris pH 8.0) (Figures S12 and S13).
The breadth of bond-forming reactions at diverse sites and in differing representative protein scaffolds used here suggests a true generality for this l-residue specific method that could enable numerous applications in protein science. To pick just three, the installation of l-Bal as a minimal homochiral precursor boronic acid may open yet further synthetic avenues. Now that such l-Bal boronic acids can be installed into proteins with site selectivity, it may be possible to take advantage of well-developed boronic acid transformations for further post-translational editing. Moreover, the functional utility that we demonstrate here now of an on-protein l-alanyl radical, without the need for additional stabilization,14 opens the door to further radical processing methods including metal-relayed trapping and so-called “sorting”70 methods with further potential for editing of biological systems. It has also not escaped our attention that this strategy should readily enable complementary methods for the creation of epimeric series not only of l-residues but also d-residues in proteins, and studies in this direction will be published in due course.
Acknowledgments
The authors would like to thank Tim Mollner for invaluable discussions and Matthew Davy for technical assistance. The authors are grateful to Nick Devoogdt for the provision of the cAbVCAM plasmid and Guy Salvesen for plasmids distributed via Addgene. The Next Generation Chemistry theme at the Franklin Institute is supported by the EPSRC (V011359/1 (P)).
Data Availability Statement
Raw LC-MS data are available in the open-access Pride database (PXD036570) and Zenodo depository (10.5281/zenodo.7011026).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.2c00991.
MS/MS analyses; spectral signatures of EDA complexation; frontier molecular orbitals; additional testing and optimization; experimental procedures; protein expression, purification, and Fpc generation; additional characterization; DFT calculations (PDF)
Transparent Peer Review report available (PDF)
Author Contributions
∇ X.-P.F. and Y.Y. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Walsh C. T.; Garneau-Tsodikova S.; Gatto G. J. Jr Protein Posttranslational Modifications: The Chemistry of Proteome Diversifications. Angew. Chem., Int. Ed. 2005, 44 (45), 7342–7372. 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- Ohtsubo K.; Marth J. D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126 (5), 855–867. 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Cohen P. The origins of protein phosphorylation. Nat. Cell Biol. 2002, 4 (5), E127–E130. 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- Swatek K. N.; Komander D. Ubiquitin modifications. Cell Research 2016, 26 (4), 399–422. 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutureira O.; Bernardes G. J. L. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115 (5), 2174–2195. 10.1021/cr500399p. [DOI] [PubMed] [Google Scholar]
- Davis B. G. Mimicking Posttranslational Modifications of Proteins. Science 2004, 303 (5657), 480–482. 10.1126/science.1093449. [DOI] [PubMed] [Google Scholar]
- Wright T. H.; Vallée M. R. J.; Davis B. G. From Chemical Mutagenesis to Post-Expression Mutagenesis: A 50 Year Odyssey. Angewandte Chemie (International ed. in English) 2016, 55 (20), 5896–5903. 10.1002/anie.201509310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Vinogradova E. V.; Spokoyny A. M.; Buchwald S. L.; Pentelute B. L. Arylation Chemistry for Bioconjugation. Angew. Chem., Int. Ed. 2019, 58 (15), 4810–4839. 10.1002/anie.201806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer C. D.; Davis B. G. Selective chemical protein modification. Nat. Commun. 2014, 5 (1), 4740. 10.1038/ncomms5740. [DOI] [PubMed] [Google Scholar]
- Shadish J. A.; DeForest C. A. Site-Selective Protein Modification: From Functionalized Proteins to Functional Biomaterials. Matter 2020, 2 (1), 50–77. 10.1016/j.matt.2019.11.011. [DOI] [Google Scholar]
- Chalker J. M.; Davis B. G. Chemical mutagenesis: selective post-expression interconversion of protein amino acid residues. Curr. Opin. Chem. Biol. 2010, 14 (6), 781–789. 10.1016/j.cbpa.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Wright T. H.; Bower B. J.; Chalker J. M.; Bernardes G. J. L.; Wiewiora R.; Ng W.-L.; Raj R.; Faulkner S.; Vallée M. R. J.; Phanumartwiwath A.; et al. Posttranslational mutagenesis: A chemical strategy for exploring protein side-chain diversity. Science 2016, 354, aag1465. 10.1126/science.aag1465. [DOI] [PubMed] [Google Scholar]
- Wright T. H.; Davis B. G. Post-translational mutagenesis for installation of natural and unnatural amino acid side chains into recombinant proteins. Nat. Protoc. 2017, 12, 2243–2250. 10.1038/nprot.2017.087. [DOI] [PubMed] [Google Scholar]
- Josephson B.; Fehl C.; Isenegger P. G.; Nadal S.; Wright T. H.; Poh A. W. J.; Bower B. J.; Giltrap A. M.; Chen L.; Batchelor-McAuley C.; et al. Light-driven post-translational installation of reactive protein side chains. Nature 2020, 585 (7826), 530–537. 10.1038/s41586-020-2733-7. [DOI] [PubMed] [Google Scholar]
- Yang A.; Ha S.; Ahn J.; Kim R.; Kim S.; Lee Y.; Kim J.; Söll D.; Lee H.-Y.; Park H.-S. A chemical biology route to site-specific authentic protein modifications. Science 2016, 354, 623–626. 10.1126/science.aah4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker J. M.; Gunnoo S. B.; Boutureira O.; Gerstberger S. C.; Fernández-González M.; Bernardes G. J. L.; Griffin L.; Hailu H.; Schofield C. J.; Davis B. G. Methods for converting cysteine to dehydroalanine on peptides and proteins. Chemical Science 2011, 2 (9), 1666–1676. 10.1039/c1sc00185j. [DOI] [Google Scholar]
- Seebeck F. P.; Szostak J. W. Ribosomal Synthesis of Dehydroalanine-Containing Peptides. J. Am. Chem. Soc. 2006, 128 (22), 7150–7151. 10.1021/ja060966w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Schiller S. M.; Schultz P. G. A Biosynthetic Route to Dehydroalanine-Containing Proteins. Angew. Chem., Int. Ed. 2007, 46 (36), 6849–6851. 10.1002/anie.200702305. [DOI] [PubMed] [Google Scholar]
- Lai K.-Y.; Galan S. R. G.; Zeng Y.; Zhou T. H.; He C.; Raj R.; Riedl J.; Liu S.; Chooi K. P.; Garg N.; et al. LanCLs add glutathione to dehydroamino acids generated at phosphorylated sites in the proteome. Cell 2021, 184 (10), 2680–2695.E26. 10.1016/j.cell.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker J. M.; Lercher L.; Rose N. R.; Schofield C. J.; Davis B. G. Conversion of Cysteine into Dehydroalanine Enables Access to Synthetic Histones Bearing Diverse Post-Translational Modifications. Angew. Chem., Int. Ed. 2012, 51 (8), 1835–1839. 10.1002/anie.201106432. [DOI] [PubMed] [Google Scholar]
- Galan S. R. G.; Wickens J. R.; Dadova J.; Ng W.-L.; Zhang X.; Simion R. A.; Quinlan R.; Pires E.; Paton R. S.; Caddick S.; et al. Post-translational site-selective protein backbone α-deuteration. Nat. Chem. Biol. 2018, 14 (10), 955–963. 10.1038/s41589-018-0128-y. [DOI] [PubMed] [Google Scholar]
- Walling C.; Rabinowitz R. THE REACTION OF THIYL RADICALS WITH TRIALKYL PHOSPHITES1. J. Am. Chem. Soc. 1957, 79 (19), 5326–5326. 10.1021/ja01576a077. [DOI] [Google Scholar]
- Walling C.; Basedow O. H.; Savas E. S. Some Extensions of the Reaction of Trivalent Phosphorus Derivatives with Alkoxy and Thiyl Radicals; a New Synthesis of Thioesters1. J. Am. Chem. Soc. 1960, 82 (9), 2181–2184. 10.1021/ja01494a023. [DOI] [Google Scholar]
- Wan Q.; Danishefsky S. J. Free-Radical-Based, Specific Desulfurization of Cysteine: A Powerful Advance in the Synthesis of Polypeptides and Glycopolypeptides. Angew. Chem., Int. Ed. 2007, 46 (48), 9248–9252. 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]
- Haase C.; Rohde H.; Seitz O. Native Chemical Ligation at Valine. Angew. Chem., Int. Ed. 2008, 47 (36), 6807–6810. 10.1002/anie.200801590. [DOI] [PubMed] [Google Scholar]
- Rohde H.; Seitz O. Ligation—Desulfurization: A powerful combination in the synthesis of peptides and glycopeptides. Peptide Science 2010, 94 (4), 551–559. 10.1002/bip.21442. [DOI] [PubMed] [Google Scholar]
- Griffiths R. C.; Smith F. R.; Long J. E.; Williams H. E. L.; Layfield R.; Mitchell N. J. Site-Selective Modification of Peptides and Proteins via Interception of Free-Radical-Mediated Dechalcogenation. Angew. Chem., Int. Ed. 2020, 59 (52), 23659–23667. 10.1002/anie.202006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. C.; Smith F. R.; Long J. E.; Scott D.; Williams H. E. L.; Oldham N. J.; Layfield R.; Mitchell N. J. Site-Selective Installation of Nϵ-Modified Sidechains into Peptide and Protein Scaffolds via Visible-Light-Mediated Desulfurative C-C Bond Formation. Angew. Chem., Int. Ed. 2022, 61 (2), e202110223 10.1002/anie.202110223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metanis N.; Keinan E.; Dawson P. E. Traceless Ligation of Cysteine Peptides Using Selective Deselenization. Angew. Chem., Int. Ed. 2010, 49 (39), 7049–7053. 10.1002/anie.201001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes G. J. L.; Grayson E. J.; Thompson S.; Chalker J. M.; Errey J. C.; El Oualid F.; Claridge T. D. W.; Davis B. G. From Disulfide- to Thioether-Linked Glycoproteins. Angew. Chem., Int. Ed. 2008, 47 (12), 2244–2247. 10.1002/anie.200704381. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Ni C.; Deng L.; Hu J. Electrochemical reduction of fluoroalkyl sulfones for radical fluoroalkylation of alkenes. Chem. Commun. 2021, 57 (70), 8750–8753. 10.1039/D1CC03258E. [DOI] [PubMed] [Google Scholar]
- Fleming S. A.; Jensen A. W. Photocleavage of benzyl-sulfide bonds. Journal of Organic Chemistry 1993, 58 (25), 7135–7137. 10.1021/jo00077a041. [DOI] [Google Scholar]
- Maslak P.; Theroff J. Intrinsic Barriers of the Alternative Modes of Mesolytic Fragmentations of C-S Bonds. J. Am. Chem. Soc. 1996, 118 (30), 7235–7236. 10.1021/ja960735x. [DOI] [Google Scholar]
- Gao J.; Feng J.; Du D. Shining Light on C-S Bonds: Recent Advances in C-C Bond Formation Reactions via C-S Bond Cleavage under Photoredox Catalysis. Chemistry - An Asian Journal 2020, 15 (22), 3637–3659. 10.1002/asia.202000905. [DOI] [PubMed] [Google Scholar]
- For more detailed analyses of similar pathways, see:; Costentin C.; Robert M.; Savéant J.-M. Activation Barriers in the Homolytic Cleavage of Radicals and Ion Radicals. J. Am. Chem. Soc. 2003, 125 (1), 105–112. 10.1021/ja027287f. [DOI] [PubMed] [Google Scholar]
- Willwacher J.; Raj R.; Mohammed S.; Davis B. G. Selective Metal-Site-Guided Arylation of Proteins. J. Am. Chem. Soc. 2016, 138 (28), 8678–8681. 10.1021/jacs.6b04043. [DOI] [PubMed] [Google Scholar]
- Brittain W. D. G.; Coxon C. R. Perfluoroaryl and Perfluoroheteroaryl Reagents as Emerging New Tools for Peptide Synthesis, Modification and Bioconjugation. Chem.—Eur. J. 2022, 28 (7), e202103305 10.1002/chem.202103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Welborn M.; Zhu T.; Yang N. J.; Santos M. S.; Van Voorhis T.; Pentelute B. L. π-Clamp-mediated cysteine conjugation. Nat. Chem. 2016, 8 (2), 120–128. 10.1038/nchem.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embaby A. M.; Schoffelen S.; Kofoed C.; Meldal M.; Diness F. Rational Tuning of Fluorobenzene Probes for Cysteine-Selective Protein Modification. Angew. Chem., Int. Ed. 2018, 57 (27), 8022–8026. 10.1002/anie.201712589. [DOI] [PubMed] [Google Scholar]
- Banks R. E.; Burgess J. E.; Cheng W. M.; Haszeldine R. N. 93. Heterocyclic polyfluoro-compounds. Part IV. Nucleophilic substitution in pentafluoropyridine: the preparation and properties of some 4-substituted 2,3,5,6-tetrafluoropyridines. Journal of the Chemical Society (Resumed) 1965, (0), 575–581. 10.1039/jr9650000575. [DOI] [Google Scholar]
- Brooke G. M. The preparation and properties of polyfluoro aromatic and heteroaromatic compounds. J. Fluorine Chem. 1997, 86 (1), 1–76. 10.1016/S0022-1139(97)00006-7. [DOI] [Google Scholar]
- Sandford G.Pentafluoropyridine. In Encyclopedia of Reagents for Organic Synthesis; Wiley, 2005. [Google Scholar]
- Zubkov M. O.; Kosobokov M. D.; Levin V. V.; Kokorekin V. A.; Korlyukov A. A.; Hu J.; Dilman A. D. A novel photoredox-active group for the generation of fluorinated radicals from difluorostyrenes. Chemical Science 2020, 11 (3), 737–741. 10.1039/C9SC04643G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez D.; Mooney C. A.; Dose A.; Sandford G.; Coxon C. R.; Cobb S. L. The application of perfluoroheteroaromatic reagents in the preparation of modified peptide systems. Organic & Biomolecular Chemistry 2017, 15 (19), 4086–4095. 10.1039/C7OB00283A. [DOI] [PubMed] [Google Scholar]
- Gimenez D.; Dose A.; Robson N. L.; Sandford G.; Cobb S. L.; Coxon C. R. 2,2,2-Trifluoroethanol as a solvent to control nucleophilic peptide arylation. Organic & Biomolecular Chemistry 2017, 15 (19), 4081–4085. 10.1039/C7OB00295E. [DOI] [PubMed] [Google Scholar]
- Qi Y.; Kobayashi Y.; Hulett F. M. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the pho regulon. J. Bacteriol. 1997, 179 (8), 2534–2539. 10.1128/jb.179.8.2534-2539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broisat A.; Hernot S.; Toczek J.; De Vos J.; Riou L. M.; Martin S.; Ahmadi M.; Thielens N.; Wernery U.; Caveliers V.; et al. Nanobodies Targeting Mouse/Human VCAM1 for the Nuclear Imaging of Atherosclerotic Lesions. Circ. Res. 2012, 110 (7), 927–937. 10.1161/CIRCRESAHA.112.265140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetting M. W.; Hegde S. S.; Hazleton K. Z.; Blanchard J. S. Structural characterization of the fusion of two pentapeptide repeat proteins, Np275 and Np276, from Nostoc punctiforme: Resurrection of an ancestral protein. Protein Sci. 2007, 16 (4), 755–760. 10.1110/ps.062637707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.; Yang H.; Fu H. An N-(acetoxy)phthalimide motif as a visible-light pro-photosensitizer in photoredox decarboxylative arylthiation. Chem. Commun. 2016, 52 (87), 12909–12912. 10.1039/C6CC06994K. [DOI] [PubMed] [Google Scholar]
- Fawcett A.; Pradeilles J.; Wang Y.; Mutsuga T.; Myers E. L.; Aggarwal V. K. Photoinduced decarboxylative borylation of carboxylic acids. Science 2017, 357 (6348), 283–286. 10.1126/science.aan3679. [DOI] [PubMed] [Google Scholar]
- Bordwell F. G.; Hughes D. L. Thiol acidities and thiolate ion reactivities toward butyl chloride in dimethyl sulfoxide solution. The question of curvature in Broensted plots. Journal of Organic Chemistry 1982, 47 (17), 3224–3232. 10.1021/jo00138a005. [DOI] [Google Scholar]
- Advanced Chemistry Development (ACD/Labs) Software V11.02; ACD/Labs, 2022.
- Singh R.; Whitesides G. M.. Reagents for rapid reduction of disulfide bonds in proteins. In Techniques in Protein Chemistry; Crabb J. W., Ed.; Vol. 6; Academic Press, 1995; pp 259–266. [Google Scholar]
- Lima C. G. S.; de M. Lima T.; Duarte M.; Jurberg I. D.; Paixão M. W. Organic Synthesis Enabled by Light-Irradiation of EDA Complexes: Theoretical Background and Synthetic Applications. ACS Catal. 2016, 6 (3), 1389–1407. 10.1021/acscatal.5b02386. [DOI] [Google Scholar]
- Surdhar P. S.; Armstrong D. A. Reduction potentials and exchange reactions of thiyl radicals and disulfide anion radicals. J. Phys. Chem. 1987, 91 (26), 6532–6537. 10.1021/j100310a022. [DOI] [Google Scholar]
- Hall D. G.Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials; Wiley, 2012. [Google Scholar]
- Mollner T. A.; Isenegger P. G.; Josephson B.; Buchanan C.; Lercher L.; Oehlrich D.; Hansen D. F.; Mohammed S.; Baldwin A. J.; Gouverneur V.; et al. Post-translational insertion of boron in proteins to probe and modulate function. Nat. Chem. Biol. 2021, 17 (12), 1245–1261. 10.1038/s41589-021-00883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries R. H.; Viel J. H.; Kuipers O. P.; Roelfes G. Rapid and Selective Chemical Editing of Ribosomally Synthesized and Post-Translationally Modified Peptides (RiPPs) via CuII-Catalyzed β-Borylation of Dehydroamino Acids. Angew. Chem., Int. Ed. 2021, 60 (8), 3946–3950. 10.1002/anie.202011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dénès F.; Pichowicz M.; Povie G.; Renaud P. Thiyl Radicals in Organic Synthesis. Chem. Rev. 2014, 114 (5), 2587–2693. 10.1021/cr400441m. [DOI] [PubMed] [Google Scholar]
- Mousa R.; Notis Dardashti R.; Metanis N. Selenium and Selenocysteine in Protein Chemistry. Angew. Chem., Int. Ed. 2017, 56 (50), 15818–15827. 10.1002/anie.201706876. [DOI] [PubMed] [Google Scholar]
- Flory P. J. The Mechanism of Vinyl Polymerizations1. J. Am. Chem. Soc. 1937, 59 (2), 241–253. 10.1021/ja01281a007. [DOI] [Google Scholar]
- Imiołek M.; Isenegger P. G.; Ng W.-L.; Khan A.; Gouverneur V.; Davis B. G. Residue-Selective Protein C-Formylation via Sequential Difluoroalkylation-Hydrolysis. ACS Central Science 2021, 7 (1), 145–155. 10.1021/acscentsci.0c01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd N.; Vijayakrishnan B.; Koeppe J. R.; Davis B. G. Thiyl Glycosylation of Olefinic Proteins: S-Linked Glycoconjugate Synthesis. Angew. Chem., Int. Ed. 2009, 48 (42), 7798–7802. 10.1002/anie.200903135. [DOI] [PubMed] [Google Scholar]
- Purdie J. E.; Demayo R. E.; Seely J. H.; Benoiton N. L. The trypsin-catalysed hydrolysis of d-lysine and d-arginine ethyl esters. Biochimica et Biophysica Acta (BBA) - Enzymology 1972, 268 (2), 523–526. 10.1016/0005-2744(72)90348-8. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Yang X.; Yi D.; Wang S.; Chen Y. Genetic incorporation of d-lysine into diketoreductase in Escherichia coli cells. Amino Acids 2012, 43 (6), 2553–2559. 10.1007/s00726-012-1311-1. [DOI] [PubMed] [Google Scholar]
- Jamonnak N.; Hirsch B. M.; Pang Y.; Zheng W. Substrate specificity of SIRT1-catalyzed lysine Nε-deacetylation reaction probed with the side chain modified Nε-acetyl-lysine analogs. Bioorganic Chemistry 2010, 38 (1), 17–25. 10.1016/j.bioorg.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Hirsch B. M.; Zheng W. Sirtuin mechanism and inhibition: explored with Nε-acetyl-lysine analogs. Molecular BioSystems 2011, 7 (1), 16–28. 10.1039/C0MB00033G. [DOI] [PubMed] [Google Scholar]
- Marfey P. Determination of D-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Research Communications 1984, 49 (6), 591. 10.1007/BF02908688. [DOI] [Google Scholar]
- Sakai H. A.; MacMillan D. W. C. Nontraditional Fragment Couplings of Alcohols and Carboxylic Acids: C(sp3)-C(sp3) Cross-Coupling via Radical Sorting. J. Am. Chem. Soc. 2022, 144 (14), 6185–6192. 10.1021/jacs.2c02062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw LC-MS data are available in the open-access Pride database (PXD036570) and Zenodo depository (10.5281/zenodo.7011026).