Abstract
Nearly all adults are infected with one or more herpes viruses. The most common are herpes simplex virus (HSV)-1 and HSV-2, which upon reactivation can cause painful skin and mucosal erosions. Patients who are immune compromised often experience frequent, atypical, or chronic lesions and thus a greatly diminished quality of life. Pellino1 (Peli1) is a ubiquitin ligase involved in IL-1 and Toll-like receptor signaling; however, the role of Pellino1 in skin immunity against HSV is unknown. Here, using the mouse flank HSV-1 skin infection model, we demonstrate that Pellino1 has several critical functions during active viral replication. Peli1−/− mice succumb more than wild type to systemic disease and develop larger zosteriform skin lesions along affected dermatomes. In Pellino1 deficient mice, the virus spread extensively through the epidermis, follicular infundibulum into sebaceous glands where sebocytes were found positive for the virus. The latter did not appear to involve a shift in how the virus migrated through the nervous system. Immunohistochemistry revealed delayed recruitment of myeloid and T cells to the infected epidermis in Peli1−/− mice. This was associated with decreased expression of the cytokine mRNAs Il1a, Il36b and 2610528A11Rik, also known as Gpr15l. In conclusion, Pellino1 plays important roles in restricting viral dissemination, and the involved pathways may represent novel therapeutic targets in patients with frequent or chronic HSV infections.
The herpes simplex viruses HSV-1 and HSV-2 are common human pathogens that establish life-long latent infections of trigeminal and dorsal root ganglions. Reactivation of the viral lytic cycle results in migration of viral particles to the skin or mucosa leading to often painful oral, genital, and anal sores as the immune system clears the active infection. Immune suppression or dysregulation increases the risk of frequent and severe disease. Furthermore, long-term use of immunosuppressive medications in transplant patients is associated with development of atypical chronic lesions and the emergence of drug resistant HSV strains (Frobert et al., 2014, Fujimoto et al., 2010, Ilyas et al., 2017, Labrunie et al., 2019, Schimming et al., 2014). Thus, there is a continued need for improving our understanding of host-pathogen-drug interactions in the skin.
Pellino proteins are ubiquitin ligases involved in intracellular signaling, relaying extracellular cues from the cellular microenvironment to the nucleus. There are three Pellino proteins, Pellino1–3 (also known as Peli1–3), encoded by distinct genes. Pellino1 is 70 and 79% identical to Pellino2 and Pellino3, respectively, while human and mouse Pellino1 differ from each other by only a single amino acid. This degree of conservation suggests critical physiological functions. In vitro Pellino1 mediates signaling from the IL-1 receptor IL-1R1 and Toll-like receptors (Humphries and Moynagh, 2015, Medvedev et al., 2015), including TLR3, a receptor for double stranded RNA that is often generated during viral infections. Both beneficial and detrimental functions of Pellino1 during in vivo infections with RNA viruses have been reported (Huang et al., 2021, Luo et al., 2020, Luo et al., 2018, Marsh et al., 2020, Xiao et al., 2015). Some of the detrimental effects are linked to pro-inflammatory activity.
The previous in vivo studies focused on the lungs and the CNS, which are frequent targets for RNA viruses. It is of interest that HSV-1, a DNA virus, is the most common cause of sporadic encephalitis. Detrimental mutations in TLR3 are associated with HSV-1 encephalitis (Jouanguy et al., 2020), while other mutations contribute to disseminated and/or severe skin and eye disease (Cummings et al., 2021, Jouanguy et al., 2020). The role of Pellino1 during HSV-1 infection is unknown. To elucidate its role, we took advantage of the flank skin HSV infection model, which we previously employed to demonstrate important functions of the IL-1 and IL-36 cytokines (Milora et al., 2014, Milora et al., 2017, Wang et al., 2019). Here we report our findings that Pellino1 plays a critical role in restricting viral dissemination.
RESULTS
Pellino1 is important for survival during HSV-1 infection
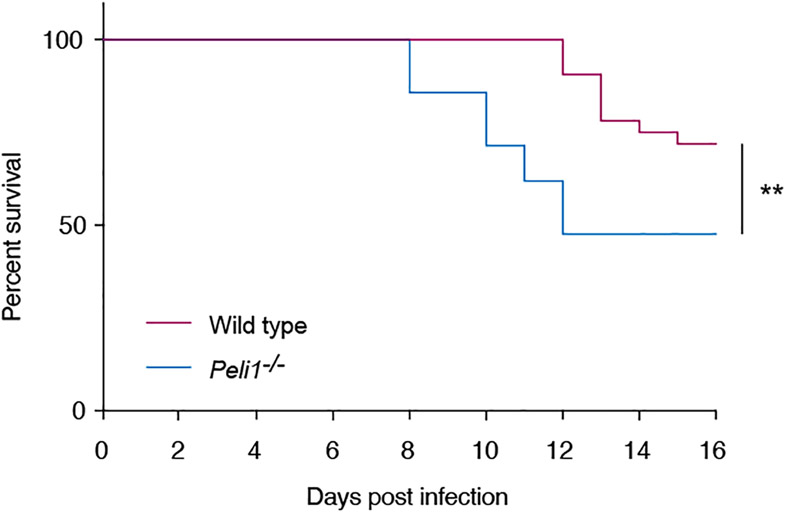
Depending on the experimental model, Pellino1 can have beneficial or detrimental effects during infections with RNA viruses (Huang et al., 2021, Luo et al., 2020, Luo et al., 2018, Marsh et al., 2020, Xiao et al., 2015). Peli1 is expressed by multiple cell types within the skin, including keratinocytes in the epidermis (Supplementary Fig. S1) (Joost et al., 2020). Keratinocytes are the primary target for HSV during active symptomatic viral replication in skin or mucosa; however, the role of Pellino1 in the skin and in immune responses directed against HSV has not been examined. The flank skin mouse model of HSV infection is a valuable tool for examining how herpes viruses infect epithelial cells and disseminate systemically. For example, the model has been used to identify viral factors involved in neuronal egress (Diefenbach et al., 2016), while we used it to demonstrate the important roles for IL-1 and IL-36 in protective immunity against HSV-1 (Milora et al., 2014, Milora et al., 2017, Wang et al., 2019). Since Pellino1 is a signaling factor activated by IL-1 (Jiang et al., 2003), we compared disease progression in Peli1−/− and wild type mice. While the median survival rate was greater than 16 days in wild type mice, it was significantly (p < 0.01) diminished to only 12 days in the knockout (KO) mice (Fig. 1). Thus, Pellino1 clearly plays an important role in protecting against lethal outcome of HSV infection.
Figure 1: Mortality is increased in peli1−/− mice following HSV-1 skin infection.
Wild type (magenta line, n = 32) and Pellino-1 KO (blue line, n = 21) mice were infected with HSV-1 and survival monitored for 16 days. Data is pooled from 4 independent experiments. **, p < 0.01 (Gehan-Breslow-Wilcoxon test).
Pellino1 limits disease severity in the skin
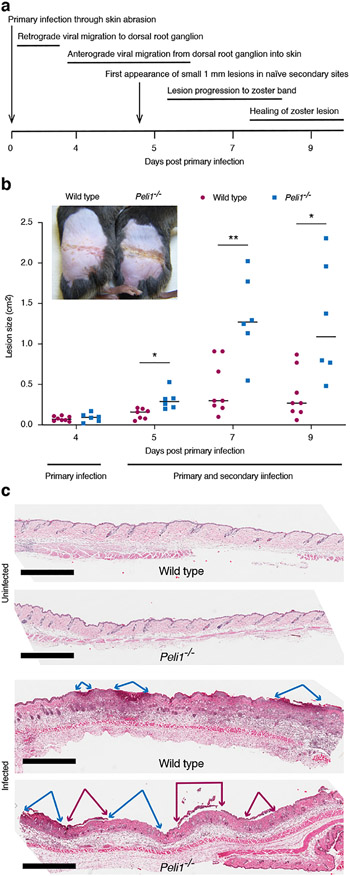
Mortality in the flank model arises as the virus disseminates through the nervous system to internal organs. An external sign of this virus migration through nerve axons is the appearance of zosteriform skin lesions along the dermatome infected during initial inoculation of the virus on the flank (previously illustrated in (Milora et al., 2017)). These lesions resemble herpes zoster, commonly known as shingles, and first start to appear in virus naïve sites on the flank on day 5 post-infection (Fig. 2a). In addition to observing reduced survival in the Pellino1 KO mice (Fig. 1), we also found that these zosteriform lesions became larger than those in wild type mice (Fig. 2b). Histologically these lesions revealed early loss of the epidermis, which was more extensive in the Peli1−/− than wild type mice (Fig. 2c). No discernable differences were found between the two strains in the absence of infection (Fig. 2c and Supplementary Fig. S2). Hence, HSV infection causes greater tissue damage in Pellino1 deficient mice.
Figure 2: HSV-1 causes larger lesions in Peli1−/− mice.
Wild type and Pellino-1 KO mice were infected with HSV-1. (a), Timing and progression of HSV infection in the mouse flank model is illustrated. (b), Lesions on the flanks were photographed up to day 9 and sized using ImageJ. Representative data and images from one of at least 3 independent experiments (n = 4-5 per group) is shown. Each dot represents a single mouse. *, p < 0.05; **, p < 0.01 (t test), (c), Skin area with early zosteriform sites above and below the primary infection site were collected 5 days post-primary infection and histology evaluated alongside uninfected skin by H&E staining. Scale bar = 1 mm. Blue and magenta arrows mark regions with absent and detaching epidermis, respectively.
Pellino1 is involved in restricting viral replication in the epidermis
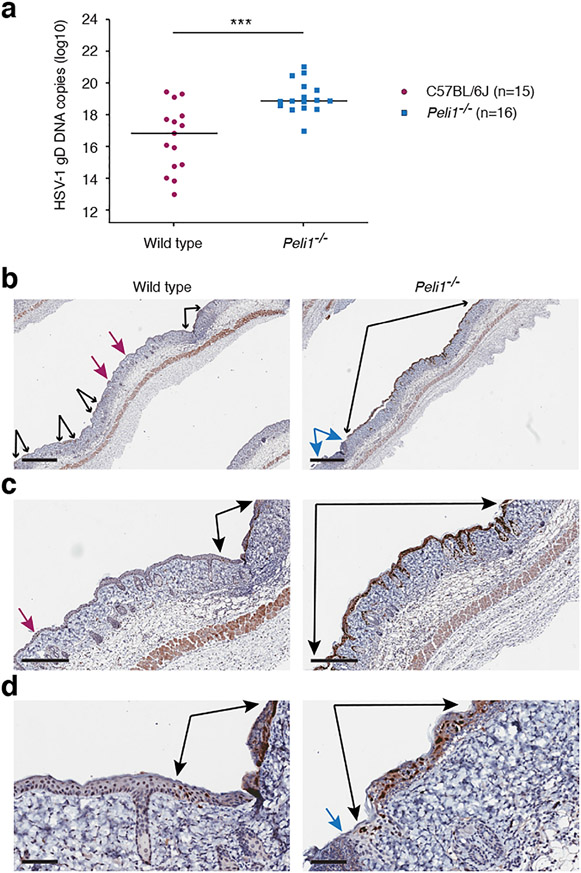
To further understand why HSV-1 causes more damage to the skin and epidermis in Pellino1 KO mice, as the zosteriform lesions emerged on day 5 of the infection, we examined viral load in the skin around the dermatome (Fig. 3). Significantly higher levels of viral DNA were detected in Peli1−/− skin than wild type (Fig. 3a). In agreement with this, immunohistochemistry revealed extensive presence of HSV-1 through the remaining epidermis in Pellino1 KO mice, while in the wild type mice the virus was more restricted within smaller regions of the epidermis (Fig. 3b). These findings strongly suggest that Pellino1 is critical for restricting replication and/or spread of HSV-1 within the epidermis.
Figure 3: Pellino1 limits viral replication in the epidermal keratinocytes.
Five days post-HSV-1 infection, skin was collected from above and below the primary infection site and examined by QPCR for HSV-1 DNA (a) or immunohistochemistry for HSV-1 protein (b). (a), Each symbol represents a single mouse. Data is pooled from 3 independent experiments. ***, p < 0.001 (t test), (b-d), Black arrows flank continuous epidermal regions with HSV-1 positive staining. Blue arrows flank lesion site with absent epidermis. Magenta arrows identify small HSV-1 positive foci. Larger magnifications of images in (b) are shown in (c) and (d). Scale bars represent 700 μm (b), 300 μm (c), and 70 μm (d), respectively. Images are representative from 2 independent experiments each involving 4-5 mice per group.
HSV-1 infects more sebaceous glands and sebocytes in Peli1−/− skin
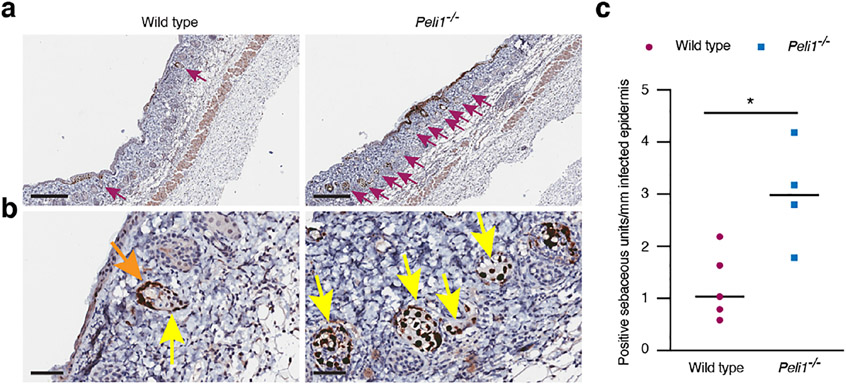
In addition to observing overall higher levels of HSV-1 in Peli1−/− mice, we also noticed more frequent involvement of the sebaceous glands (Fig. 4a). HSV-1 positive cells were found both in the basal keratinocytes that line the sebaceous gland (orange arrow, Fig. 4b) and in the sebocytes (yellow arrows, Fig. 4b). Since infection was much more extensive in the epidermis of Pellino1 KO mice (Fig. 3), we further determined the number of infected sebaceous glands relative to the length of skin with HSV-1 involvement. Even with this standardization, the number of HSV-1 positive sebaceous glands was higher in the Peli1−/− mice (Fig. 4c). Hence, Pellino1 appears to not only limit systemic viral dissemination (Fig. 1), but also restricts viral spread locally within the skin.
Figure 4: Pellino1 restricts dissemination to the sebaceous glands.
Wild type and Pellino1 KO mice were infected with HSV-1 and secondary zosteriform skin areas collected 5 days later. (a-b) Skin was examined by immunohistochemistry for HSV-1. Magenta, orange, and yellow arrows identify HSV-1 positive sebaceous glands, basal keratinocytes in the sebaceous units lining and sebocytes, respectively. Scale bars represent 300 μm (a) and 50 μm (b), respectively. (c) Number of HSV-1 positive sebaceous glands were counted and standardized against the length of HSV-1 positive interfollicular epidermis. Each dot represents a single mouse. *, p < 0.05 (t test). Images and data are representative from 2 independent experiments each involving 4-5 mice per group.
HSV-1 disseminates to sebaceous glands through the hair follicle infundibulum
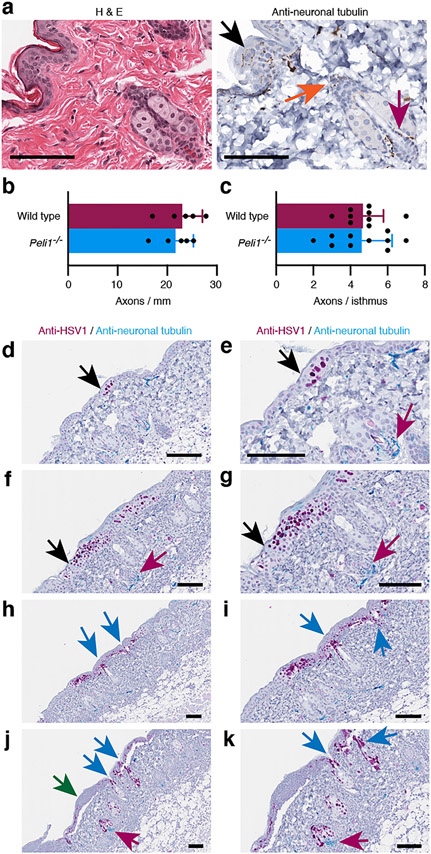
It is generally believed that following reactivation in ganglia, HSV emerges from free nerve endings in the external epithelial lining of the oral and genital mucosa, and the skin (black arrow Fig. 5a). In contrast, the related varicella-zoster virus (chickenpox) migrates through mechanosensory nerves that wrap around the hair follicles (orange and magenta arrows, Fig. 5a) and initiates active replication in the isthmus of the hair follicle below the sebaceous gland (Muraki et al., 1996). We found that the Pellino1 deficient mice had normal numbers of nerve endings crossing from the dermis into the interfollicular epidermis (Fig. 5b). There was also no significant difference in the number of axons at the isthmus in wild type and Peli1−/− mice (Fig. 5c). Nerve fibers around the infundibulum were rare and too few to quantify.
Figure 5: HSV-1 disseminates from the surface epidermis through the infundibulum to the sebaceous gland.
Uninfected and HSV-1 infected skin was examined by H&E and single (a-c) or dual (d-k) immunohistochemistry for HSV-1 (magenta, d-k) and/or neuronal tubulin (brown, a; turquois, d-k). (c) Number of axons crossing the dermal/epidermal boundary (b) and wrapped around the isthmus. Images in e, g, i and k are larger magnifications of images in d, f, h and j, respectively. Black arrows identify free nerves in the epidermis. Orange and magenta arrows point to mechanosensory nerves wrapped around the infundibulum and isthmus, respectively. Blue arrows indicate positions of infected infundibulum. Green arrow points to infected epidermis where most infected keratinocytes have already died. Scale bars represent 50 μm.
To evaluate whether Pellino1 deficiency affected the HSV-1 migration pattern through nerve axons, we examined slides dual stained for HSV-1 and neuronal tubulin (Fig. 5d-k). In both wild type and Peli1−/− mice early HSV-1 lesions involving only a few infected cells could be found in the interfollicular epidermis (Fig. 5d-e). More advanced lesions were also found in the surface epidermis (Fig. 5f-g). HSV-1 staining in the infundibulum was only detected in regions also involving infection of the interfollicular epidermis (Fig. 5h-k). HSV-1 positive sebocytes above mechanosensory nerves wrapped around the isthmus of the hair follicle (Fig. 5j-k, turquois stain, magenta arrow) were also only detected in the proximity of infected epidermis (green arrow, Fig. 5j-k) in both wild type and Pellino1 KO mice. We did not identify HSV-1 positive cells in the isthmus (Fig. 5d-k) in either strain. Hence, our data strongly suggest that the increased susceptibility to infection and viral dissemination in the Peli1−/− mice is not due to a shift in involved neurons.
Early skin pathology in Peli1−/− mice is not due to increased neutrophilic inflammation
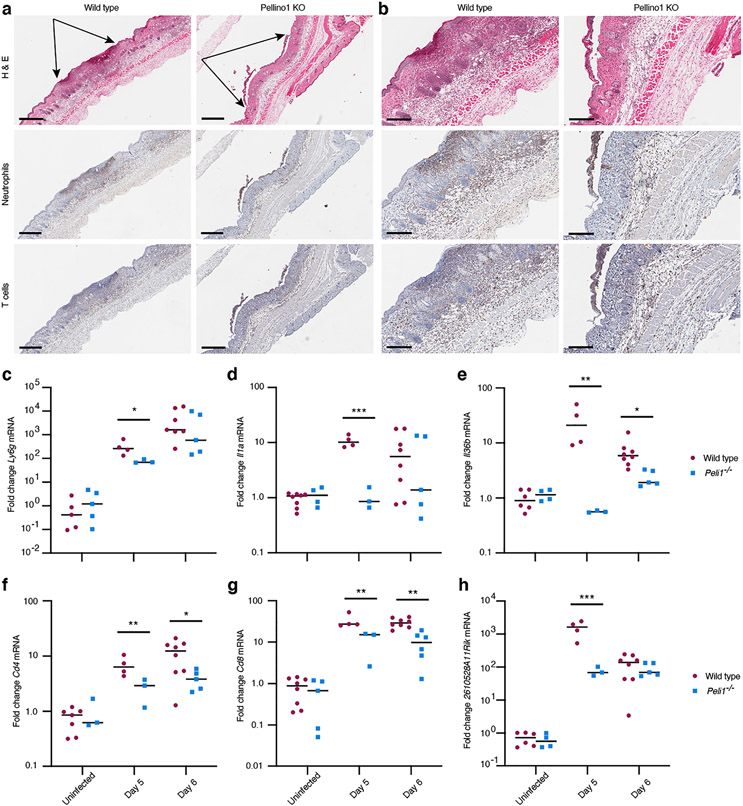
Previous studies involving the RNA viruses Zika, West Nile and influenza reported that Pellino1 promotes tissue damaging inflammation and thus Pellino1 KO mice exhibited less severe disease than wild type (Luo et al., 2020, Luo et al., 2018, Marsh et al., 2020). HSV lesions are known to be neutrophil rich, but the physiological significance of this cell population remains unclear. While some studies found no effect of eliminating neutrophils (Gardner et al., 2019, Hor et al., 2017, Wojtasiak et al., 2010), others have reported less severe disease (Lebratti et al., 2021). Here, immunohistochemical analyses of the neutrophil marker myeloperoxidase (MPO) revealed recruitment of cells to the epidermis, dermis, and subcutaneous fat in wild type mice (Fig. 6a-b). Histologically, the presence of these cells appeared reduced in Peli1−/− mice at day 5 post-primary infection when the secondary zosteriform lesions started to emerge in naïve sites (Fig. 6a-b). Quantitative RT-PCR analyzes revealed lower levels of the neutrophil marker Ly6g (Lee et al., 2013) in early 1 mm wide lesions at day 5 in Pellino1 KO mice compared to wild type mice (Fig. 6c).
Figure 6: Recruitment of immune cells is delayed in Pellino1 KO mice.
(a) Early zosteriform lesion areas above and below the primary infection site were collected at day 5 post-primary infection from wild type and Pellino1 KO mice. The skin was examined by H&E and immunohistochemical staining for neutrophils and T cells. Representative images focused on skin regions with approximately equally wide involvement of the epidermis are shown flanked by black arrows (a). Larger magnifications of images in (a) are shown in (b). Scale bars represent 500 μm (a) and 200 μm (b), respectively. (c-h) Wild type (red circles) and Pellino1 KO (blue squares) mice were left uninfected or infected with HSV-1, and skin collected at the indicated times. On day 5, 1 mm secondary lesions were collected with 4 mm punch biopsies. On day 6, 8 mm punch biopsies across the entire secondary regions were collected. Expression of Ly6g (c), Il1a (d), Il36b (e), Cd4 (f), Cd8 (g), and 2610528A11Rik (Gpr15l) (h) mRNAs were examined by RT-PCR. Each symbol represents a single mouse. Data for each timepoint is representative of 3 independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test).
We previously showed the important roles of IL-1α and IL-36β in the HSV flank model (Milora et al., 2014, Milora et al., 2017, Wang et al., 2019). These cytokines are potent activators of neutrophil recruitment into the skin (Calabrese et al., 2022, Gardner et al., 2019), and we therefore examined their expression in our model. In the Peli1−/− mice, we observed reduced levels of both the Il1a and the Il36b mRNAs in early secondary 1 mm zosteriform lesions on day 5 post-primary infection (Fig. 6d-e). Levels of Il36b were also lower in fully developed Peli1−/− zosteriform lesions on day 6 compared to wild type lesions (Fig. 6e). Thus, while Peli1−/− mice have more severe disease (Figs. 1-2), overall neutrophilic inflammation is delayed in the Pellino1 deficient mice (Fig. 2c and Fig. 6a-e).
Pellino1 is important for T cell recruitment and expression of Gpr15l
T cells play diverse roles in immune responses against HSV in epithelial tissues (Peng et al., 2021); thus, their timely recruitment to infected sites is critical for effective restriction of HSV replication. Using immunohistochemistry, we observed reduced presence of T cells in wounds, dermis and sub-cutaneous fat in regions of HSV infection in Pellino1 KO mice compared to wild type (Fig. 6a-b). Further quantitative RT-PCR for helper and cytotoxic T cells revealed lower levels of the CD4 and CD8 markers in Peli1−/− mice (Fig. 6f-g).
The 2610528A11Rik mouse gene (human: C10orf99) encodes a protein recently identified as a ligand for the orphan receptor GPR15 on T cells; thus, prompting the proposed name GPR15L for the 2610528A11Rik gene product. GPR15L has been shown to promote T cell recruitment in, for example, skin and colon epithelial tissues (Ocón et al., 2017, Suply et al., 2017). Here, we observed less dramatic induction of Gpr15l in early Peli1−/− lesions at day 5 compared to wild type mice (Fig. 6h). This reduced expression of Gpr15l (Fig. 6h) may explain the observed diminished recruitment of T cells into infected sites in Pellino1 deficient mice (Fig. 6a-b, f-g). Thus, Pellino1 appears to play a critical role in expression of the T cell activating cytokine GPR15L and T cell recruitment during active HSV replication in the skin.
DISCUSSION
HSV-1 is a ubiquitous human pathogen that can greatly affect quality of life as its reactivation causes ulcerating blisters. The mouse HSV flank model recapitulates recrudescence of viral replication in the skin and as such is a valuable tool for examining how the skin immune system senses and restricts viral infection. Here we find that Pellino1 plays a critical role in limiting HSV-1 disease severity. Specifically, survival was greatly diminished in Peli1−/− mice compared to wild type (Fig. 1). Furthermore, in the skin Pellino1 is essential for limiting HSV-1 replication in the epidermis (Fig. 2 and Fig. 3) and dissemination to the sebaceous glands (Figs. 4-5). Pellino1 is also involved in recruitment of immune cells including T cells (Fig. 6) that may play a role in restricting the virus. Thus, we have here established an important physiological function of Pellino1 in skin antiviral immunity.
Frequent reactivation of HSV in immune compromised patients greatly affect quality of life. While acyclovir and related drugs are effective in many patients, drug resistant viral strains do emerge, especially in transplant patients (Frobert et al., 2014, Labrunie et al., 2019). Interestingly, transplant patients are also a group of people in which lesions can progress in an atypical manner involving sebaceous glands (Fujimoto et al., 2010, Schimming et al., 2014). The functions of sebaceous glands beyond secretion of skin moisturizing oils remain poorly understood; however, production of antimicrobial peptides by sebocytes suggest active immune functions (Nguyen and Soulika, 2019). Pellino1 appears to play an important role in limiting replication and spread of HSV through the interfollicular epidermis into the hair follicles and sebaceous glands (Figs. 4-6). An improved understanding of how Pellino1 restricts this viral dissemination may underpin development of alternative strategies to manage HSV in patient populations at increased risk, including transplant recipients.
Prompted by in vitro work associating Pellino1 with TLR3 signaling and production of antiviral type I IFN, numerous studies of Pellino1 functions during viral infections have focused on RNA viruses affecting the lungs and central nervous system. However, very divergent outcomes of inhibiting or deleting Peli1−/− have been observed. While Pellino1 is important for IFNβ production in response to Sendai virus in fibroblasts (Ordureau et al., 2013), it does not appear essential for induction of IFN by rhinovirus in bronchial epithelial cells (Bennett et al., 2012). A third group found that Pellino1 inhibits type I IFN in astrocytes following infection with vesicular stomatitis virus (Xiao et al., 2015). The latter also reported improved viral control and survival of Peli1−/− mice compared to wild type (Xiao et al., 2015). Conflicting results have also been reported for RNA virus infections of lungs in mice. In one study Pellino1 did not appear essential for restricting viral replication of influenza or rhinoviruses (Marsh et al., 2020), while another study reported enhanced clearance of influenza in connection with Pellino1 deletion (Huang et al., 2021). The former is in agreement with independent in vitro work on rhinovirus (Bennett et al., 2012), while the latter outcome may be associated with a specialized role for Pellino1 in preventing T follicular helper cell differentiation and c-Rel activation, and the adoptive transfer model used (Huang et al., 2021).
Some of the above apparent divergent outcomes of Pellino1 deletion may be due to distinct immune evasive strategies deployed by specific viruses and the diverse impact such mechanisms may have in different cell types. Viral immune evasion strategies are highly varied, yet common immune responses antagonized are viral detection and IL-1 pathways (Danastas et al., 2020, Jensen, 2017, Unterholzner and Almine, 2019) in which Pellino1 is directly or indirectly involved (Humphries and Moynagh, 2015, Medvedev et al., 2015). Interestingly, Pellino1 itself appears to be targeted by the Japanese encephalitis virus, which upregulates mir155 (Rastogi and Singh, 2020). This in turn downregulates Pellino1 expression and thus induction of IL-6 and TNFα in infected microglia (Rastogi and Singh, 2020). How this affects viral replication and pathology in vivo remains to be determined. Viruses not only inhibit immune responses but also take advantage of host factors and cells. Through an unknown mechanism the West Nile virus utilizes Pellino1 for entry into macrophages, neurons, and microglia and thus Peli1−/− mice are more resistant to viral replication, brain inflammation and fatal outcome (Luo et al., 2018). These studies link Pellino1 to inflammatory responses in distinct viral specific ways. Pellino1 also modulates inflammatory responses associated with influenza and zika infections (Luo et al., 2020, Marsh et al., 2020). Here we observe reduced/delayed recruitment of immune cells to infected sites in Pellino-1 KO mice (Fig. 6). Inflammation is a double-edge sword during viral infections. While recruitment of immune cells is essential for viral control, exuberant inflammatory responses can lead to collateral tissue damage. In our model delayed mobilization of the immune system (Fig. 6) may allow HSV-1 to spread (Figs. 1 and 4-6), and thus more tissue is damaged by the virus and the immune responses (Figs. 1-2). Thus, the primary function of Pellino1 is, in this system, to promote protective immunity.
Clearly, the roles of Pellino1 in antiviral immune responses against RNA and DNA viruses is complex. Further in-dept mechanistic studies of both RNA and DNA viruses should shed light on not only the function of Pellino1 as an antiviral protein, but also its potential involvement in pathogenesis. Insight gained from such studies may facilitate the development of alternative strategies to alleviate diseases associated with viral infections.
METHODS
Mice
Wild type C57BL/6J mice were obtained from the Jackson Laboratory, Bar Harbor, ME, and bred in-house. The Peli1−/− strain (Mutant Mouse Resource & Research Centers, stock 036482-UNC) was crossed to C57BL/6J for more than 10 generations in house. Mice were genotyped using ear punches and a previously described protocol (Jensen et al., 2006). The following primers were used: mPELI1-2, CCTGACCAGTGAACAGAGATTTG; mPELI1-4, GATGGCATAAATACGGTCAC; mPELI1-8, GAGAACTCAGACAGCCTTAGAG and NEO3A, GCAGCGCATCGCCTTCTATCGCC. Mice were matched for sex and age in experiments; male and female mice were used. Mice were housed in specific pathogen free facilities and provided water and chow ad libitum. All animal procedures were approved by the Temple University (Philadelphia, PA) Institutional Animal Care and Use Committee and in compliance with the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Virus
The clinical HSV-1 isolate NS was originally obtained from Dr. Harvey M. Friedman, University of Pennsylvania, Philadelphia, US. The virus was propagated and titered in Vero cells (American Type Culture Collection, Manassas, Virginia) using standard virology methods as previously described (Milora et al., 2017).
Infections
All procedures were performed while mice were under isoflurane anesthesia. Mice were denuded using trimmer and epilating cream. The following day mice were infected with 1.5 × 106 PFU HSV-1 by abrading the skin on the right flank as previously described (Milora et al., 2017). On predetermined days mice were photographed next to a ruler and/or euthanized for tissue collection. Mice were monitored for pain and paralysis daily.
Quantification of DNA and mRNA
DNA was extracted from skin using the DNeasy Blood & Tissue kit (Qiagen, Germantown, Maryland). RNA was isolated using Direct-zol RNA preps (Zymo Research, Irvine, California). Reverse transcription and real-time PCR was performed with LUNAScript and Luna qPCR reagents (New England BioLabs, Ipswich, Massachusetts) using the following oligonucleotides: Cd4, TGCAAAGTTGAGTGGGAAGG and TTCTGGAACTGCACCGTGAC; Cd8, CCCGTGGCTCAGTGAAG and TGATCAAGGACAGCAGAAGG; Cxcl2, ACCAACCACCAGGCTACAG and CCCTTGAGAGTGGCTATGAC; Gpr15l, GGTTGTCAGCTTGGATCTAGG, CCTCTGTTTCTGCATTTTCTCC and FAM-TGAGTTTCA/ZEN/AGGACTTGGCAGGATGT-IABkFQ; HSV gD, CGACCAACTACCCCGAT, CACTATGACGACAAACAAAATCAC and VIC-CAGTTATCCTTAAGGTCTC-MGBNFQ; Il1a, AGTGAGCCAT AGCTTGCATC and TGAGTCGGCAAAGAAATCAAG; Il36b, CACTATGCATGGATCCTCAC and TGTCTCTACATGCTATCAAGC; Ly6g, TTGCGTTGCTCTGGAGATAG and CAGATGGGAAGGCAGAGATT; Tbp, CCAGAACTGAAAATCAACGCAG, TGTATCTACCGTGAATCTTGGC and SUN-ACTTGACCT/ZEN/AAAGACCATTGCACTTCGT-IABkFQ.
Histology and Immunohistochemistry
Skin was fixed in formaldehyde and embedded in paraffin. Tissue sections were examined by standard H&E staining or immunohistochemistry. Immunohistochemical staining was performed on a VENTANA Discovery XT automated staining instrument (Ventana Medical Systems) using VENTANA reagents according to the manufacturer's instructions. Slides were de-paraffinized using EZ Prep solution (950–102) for 16 min at 72 °C. Epitope retrieval was accomplished with EDTA for 32 min. The following antibodies were used: anti-HSV-1 (20.7.1), sc-57863, Santa Cruz Biotechnology, Santa Cruz, US; anti-Tubulin β 3 (TUBB3), clone TUJ1, BioLegend, San Diego, US; anti-Cd3, A0452, Agilent Technologies Inc, (formerly Dako), Santa Clara, US; anti-MPO, A0398, Dako. Primary antibodies were titered with a TBS antibody diluent. Immune complex was detected using the Ventana OmniMap anti-Rabbit detection kit (760-4311) and developed using the VENTANA ChromMap DAB detection kit (760-159) according to the manufacturer’s instructions. Slides were then counterstained with hematoxylin II (790-2208) for 8 min, followed by Bluing reagent (760-2037) for 4 min.
Barrier analyses
Newborn pups (less than 12 hours) from Peli1− heterozygous x heterozygous matings were euthanized and genotyped as described above. Homozygous Pellino1 KO and homozygous wildtype littermates were sequentially immersed for 10 min in 25%, 50%, 75%, 100%, 75%, 50% and 25% methanol. Pups were rinse in PBS and stained in 1% toluidine blue in PBS for 10 min. Pups were rinse in PBS for 10 min and photographed.
Digital analyses
Stained slides were scanned using a Leica Aperio CS2 scanner. Display images were acquired using Aperio ImageScope 12.4.3 for Windows 10 Professional (https://www.leicabiosystems.com/digital-pathology/manage/aperio-imagescope/) or ObjectiveView 1.4.8 software for Mac (https://www.objectivepathology.com/objectiveview-download. External lesion photographs were analyzed using the ImageJ software https://imagej-nih-gov.libproxy.temple.edu/ij/ and the depicted ruler for scaling.
Statistical analyses
Experiments were performed at least twice with similar outcomes. Statistical significance was determined using the GraphPad Prism software version 9 (GraphPad Software, San Diego, CA). Survival curves were examined using Gehan-Breslow-Wilcoxon test. Lesion sizes, viral loads and infected sebaceous gland numbers were examined using the t test.
Supplementary Material
Acknowledgements
This study was made possible through funding from The National Institute of Allergy and Infectious Diseases grant R01 AI125111 to LEJ. The Histopathology facility at Fox Chase Cancer Center was supported by The National Cancer Institute core grant P30 CA006927. The funding agencies played no role in the study design, data acquisition and interpretation, or manuscript preparation and submission.
Footnotes
Conflict of interests
The authors have no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
No large datasets were generated or analyzed during this study.
References
- Bennett JA, Prince LR, Parker LC, Stokes CA, de Bruin HG, van den Berge M, et al. Pellino-1 selectively regulates epithelial cell responses to rhinovirus. J Virol 2012;86:6595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese L, Fiocco Z, Satoh TK, Peris K, French LE. Therapeutic potential of targeting interleukin-1 family cytokines in chronic inflammatory skin diseases. Br J Dermatol 2022;186:925–41. [DOI] [PubMed] [Google Scholar]
- Cummings L, Tucker M, Gibson M, Myers A, Pastinen T, Johnston J, et al. Rare genetic variants in immune genes and neonatal herpes simplex viral infections. Pediatrics 2021;147. [DOI] [PubMed] [Google Scholar]
- Danastas K, Miranda-Saksena M, Cunningham AL. Herpes simplex virus type 1 interactions with the interferon system. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach RJ, Davis A, Miranda-Saksena M, Fernandez MA, Kelly BJ, Jones CA, et al. The basic domain of herpes simplex virus 1 pUS9 recruits kinesin-1 to facilitate egress from neurons. J Virol 2016;90:2102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobert E, Burrel S, Ducastelle-Lepretre S, Billaud G, Ader F, Casalegno J-S, et al. Resistance of herpes simplex viruses to acyclovir: An update from a ten-year survey in France. Antiviral research 2014;111:36–41. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Wakabayashi M, Tanaka T, Minamiguchi H, Hodohara K. Herpes folliculosebaceous ulcer in a patient with chronic lymphocytic leukaemia: an ulcerative variant of herpes folliculitis associated with herpesvirus invasion of folliculosebaceous units in immunocompromised hosts. Clin Exp Dermatol 2010;35:447–9. [DOI] [PubMed] [Google Scholar]
- Gardner JK, Swaims-Kohlmeier A, Herbst-Kralovetz MM. IL-36γ is a key regulator of neutrophil infiltration in the vaginal microenvironment and limits neuroinvasion in genital HSV-2 infection. J Immunol 2019;203:2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hor JL, Heath WR, Mueller SN. Neutrophils are dispensable in the modulation of T cell immunity against cutaneous HSV-1 infection. Sci Rep 2017;7:41091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Hao S, Liu J, Huang Y, Liu M, Xiao C, et al. The ubiquitin ligase Peli1 inhibits ICOS and thereby Tfh-mediated immunity. Cell Mol Immunol 2021;18:969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries F, Moynagh PN. Molecular and physiological roles of Pellino E3 ubiquitin ligases in immunity. Immunol Rev 2015;266:93–108. [DOI] [PubMed] [Google Scholar]
- Ilyas M, Maganty N, Sharma A. Cutaneous infections from viral sources in solid organ transplant recipients. J Clin Virol 2017;97:33–7. [DOI] [PubMed] [Google Scholar]
- Jensen LE. Interleukin-36 cytokines may overcome microbial immune evasion strategies that inhibit interleukin-1 family signaling. Science Signaling 2017;10:eaan3589. [DOI] [PubMed] [Google Scholar]
- Jensen LE, Etheredge AJ, Brown KS, Mitchell LE, Whitehead AS. Maternal genotype for the monocyte chemoattractant protein 1 A(−2518)G promoter polymorphism is associated with the risk of spina bifida in offspring. Am J Med Genet A 2006;140A:1114–8. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Johnson HJ, Nie H, Qin J, Bird TA, Li X. Pellino 1 is required for Interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-Tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J Biol Chem 2003;278:10952–6. [DOI] [PubMed] [Google Scholar]
- Joost S, Annusver K, Jacob T, Sun X, Dalessandri T, Sivan U, et al. The molecular anatomy of mouse skin during hair growth and rest. Cell Stem Cell 2020;26:441–57.e7. [DOI] [PubMed] [Google Scholar]
- Jouanguy E, Béziat V, Mogensen TH, Casanova JL, Tangye SG, Zhang SY. Human inborn errors of immunity to herpes viruses. Curr Opin Immunol 2020;62:106–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrunie T, Ducastelle S, Domenech C, Ader F, Morfin F, Frobert E. UL23, UL30, and UL5 characterization of HSV1 clinical strains isolated from hematology department patients. Antiviral Res 2019;168:114–20. [DOI] [PubMed] [Google Scholar]
- Lebratti T, Lim YS, Cofie A, Andhey P, Jiang X, Scott J, et al. A sustained type I IFN-neutrophil-IL-18 axis drives pathology during mucosal viral infection. Elife 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PY, Wang J-X, Parisini E, Dascher CC, Nigrovic PA. Ly6 family proteins in neutrophil biology. J Leukoc Biol 2013;94:585–94. [DOI] [PubMed] [Google Scholar]
- Luo H, Li G, Wang B, Tian B, Gao J, Zou J, et al. Peli1 signaling blockade attenuates congenital zika syndrome. PLoS Pathog 2020;16:e1008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Winkelmann ER, Zhu S, Ru W, Mays E, Silvas JA, et al. Peli1 facilitates virus replication and promotes neuroinflammation during West Nile virus infection. J Clin Invest 2018;128:4980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EK, Prestwich EC, Williams L, Hart AR, Muir CF, Parker LC, et al. Pellino-1 regulates the responses of the airway to viral infection. Front Cell Infect Microbiol 2020; 10:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE, Murphy M, Zhou H, Li X. E3 ubiquitin ligases Pellinos as regulators of pattern recognition receptor signaling and immune responses. Immunol Rev 2015;266:109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milora KA, Miller SL, Sanmiguel JC, Jensen LE. Interleukin-1α released from HSV-1 infected keratinocytes acts as a functional alarmin in the skin. Nat Commun 2014;5:5230-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milora KA, Uppalapati SR, Sanmiguel JC, Zou W, Jensen LE. Interleukin-36β provides protection against HSV-1 infection, but does not modulate initiation of adaptive immune responses. Sci Rep 2017;7:5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki R, Iwasaki T, Sata T, Sato Y, Kurata T. Hair follicle involvement in herpes zoster: pathway of viral spread from ganglia to skin. Virchows Arch 1996;428:275–80. [DOI] [PubMed] [Google Scholar]
- Nguyen AV, Soulika AM. The dynamics of the skin's immune system. Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocόn B, Pan J, Dinh TT, Chen W, Ballet R, Bscheider M, et al. A mucosal and cutaneous chemokine ligand for the lymphocyte chemoattractant receptor GPR15. Front Immunol 2017;8:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordureau A, Enesa K, Nanda S, Le Francois B, Peggie M, Prescott A, et al. DEAF1 is a Pellino1-interacting protein required for interferon production by Sendai virus and double-stranded RNA. J Biol Chem 2013;288:24569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Phasouk K, Sodroski CN, Sun S, Hwangbo Y, Layton ED, et al. Tissue-resident-memory CD8(+) T cells bridge innate immune responses in neighboring epithelial cells to control human genital herpes. Front Immunol 2021;12:735643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi M, Singh SK. Japanese Encephalitis Virus exploits microRNA-155 to suppress the non-canonical NF-κB pathway in human microglial cells. Biochim Biophys Acta Gene Regul Mech 2020;1863:194639. [DOI] [PubMed] [Google Scholar]
- Schimming TT, Griewank KG, Esser S, Schadendorf D, Hillen U. Angioplasmacellular hyperplasia - a new histopathologic clue for anogenital herpes simplex recidivans in immunocompromised patients? Am J Dermatopathol 2014;36:822–6. [DOI] [PubMed] [Google Scholar]
- Suply T, Hannedouche S, Carte N, Li J, Grosshans B, Schaefer M, et al. A natural ligand for the orphan receptor GPR15 modulates lymphocyte recruitment to epithelia. Sci Signal 2017;10. [DOI] [PubMed] [Google Scholar]
- Unterholzner L, Almine JF. Camouflage and interception: How pathogens evade detection by intracellular nucleic acid sensors. Immunol 2019;156:217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Gamero AM, Jensen LE. IL-36 promotes anti-viral immunity by boosting sensitivity to IFN-alpha/beta in IRF1 dependent and independent manners. Nat Commun 2019;10:4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtasiak M, Pickett DL, Tate MD, Bedoui S, Job ER, Whitney PG, et al. Gr-1+ cells, but not neutrophils, limit virus replication and lesion development following flank infection of mice with herpes simplex virus type-1. Virology 2010;407:143–51. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Jin J, Zou Q, Hu H, Cheng X, Sun SC. Peli1 negatively regulates type I interferon induction and antiviral immunity in the CNS. Cell Biosci 2015;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No large datasets were generated or analyzed during this study.