Abstract
Syndemics are a framework that documents health inequities and vulnerabilities in populations with rheumatic diseases. Compared with other approaches, syndemics are able to conjunctly consider epidemiological, biological, sociodemographic and economic factors, and their interactions.
Objective
To estimate health inequity and vulnerability among Indigenous and non-Indigenous populations with rheumatic and musculoskeletal diseases (RMD) in Latin America using the syndemic approach.
Design
This is a secondary analysis of a previously published large-scale study on the prevalence of RMD.
Setting
Studies carried out in five Latin American countries (Argentina, Colombia, Ecuador, Mexico and Venezuela). Health inequity and vulnerability in RMD were identified through a syndemic approach using network and cluster analysis.
Participants
A total of 44 560 individuals were studied: 29.78% self-identified as Indigenous, 60.92% were female, the mean age was 43.25 years. Twenty clusters were identified in the Indigenous population and 17 in the non-Indigenous population.
Results
The variables associated with RMD among Indigenous populations were rurality, public health system, high joint biomechanical stress, greater pain, disability and alcoholism; and among non-Indigenous people they were being a woman, urban origin, older age, private health system, joint biomechanical stress, greater pain and disability. We identified different health inequities among patients with RMD (ie, lower educational attainment, more comorbidities), associated with factors such as Indigenous self-identification and rural residence.
Conclusions
A syndemic approach enables us to identify health inequities in RMD, as shown by higher prevalence of comorbidities, disability and socioeconomic factors like lower educational attainment. These inequities exist for the overall population of patients with RMD, although it is more evident in Indigenous groups with added layers of vulnerability.
Keywords: epidemiology, public health, rheumatology, statistics & research methods
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Syndemics are a framework using strategies from artificial intelligence to perform complex analyses that document health inequities.
The analysis of clusters and networks groups individuals by variables to document inequity, the principal objective of this study.
The cross-sectional nature of this study is a limitation to establish causality.
Introduction
Rheumatic and musculoskeletal diseases (RMD) are a significant cause of morbidity and mortality worldwide1; they produce substantial socioeconomic impact and deterioration of quality of life in patients, who represent approximately 10% of the general population.2 Since 2000, WHO has recognised RMD as a relevant health problem, due to the increase in secondary disability and a greater demand for health resources.3
There is now a greater need to define global strategies for the timely access of patients with RMD to health systems,4 including the evaluation of social determinants, such as gender, education, work, income level, ethnicity and place of residence.5
Latin America is an extensive geographic area made up of 26 countries, characterised by multiculturalism and great contrasts in political, social and economic aspects.4 6 Significant social inequity has been documented, with marked disparities in health coverage for individuals and social groups; these inequities are observed within and among countries in the region.7 Epidemiological studies have documented a prevalence of RMD between 23% and 46.5% in Latin America, with more aggressive presentations (higher morbidity and mortality) among Indigenous populations. Genetic predisposition to systemic lupus erythematosus has also been identified among Indigenous groups,8 as well as a high prevalence of rheumatoid arthritis (RA) among Indigenous Mayan groups of Yucatan, Mexico9 and the Qom of Argentina.10 11
Despite the high prevalence of RMD in the Latin American region, these diseases continue to have a low priority in the planning of health policies.4 Overall, the healthcare system in Latin America is highly fragmented and disconnected. For rheumatology care specifically, 33.5% of rheumatologists work in public/government hospitals, 28.8% in private practice, 20.8% in private hospitals and 15.5% in university hospitals, most of them distributed in large urban areas, with a significant lack in small cities and none in rural areas.12–16
These differences in disease prevalence and distribution of health resources which limit access to rheumatology care in Latin America can be understood as health inequities. Health inequity is not synonymous with inequality. Inequity implies the idea of injustice and of lack of actions to avoid preventable differences. On the other hand, inequality describes differences in health outcomes that are not fundamentally unfair.12 Health inequity is deeply connected to vulnerability. From a biomedical perspective, vulnerability means being susceptible to certain diseases or to environmental risk. However, vulnerability can also be understood as a product of the interaction between available resources (personal, family, community, cultural, economic, institutional), the sociocultural context of the patient, structural elements and exposure to risk.12 17–20 Therefore, vulnerability is a result of health inequity.
To document inequity in health, the syndemic model has proven useful to analyse the interaction of disease with social determinants that condition inequality in health, and how these lead to increased physical and environmental vulnerability.17 18 21 22 Syndemics aggregate the interaction of two or more concurrent diseases, as well as the sociocultural and healthcare contexts which can exacerbate the negative effects of this interaction on the health of individuals, communities and societies.21 The syndemic framework evaluates the interaction of any type of disease in conditions of health inequality caused by poverty, stigmatisation, stress or structural violence.21–23 Thus, syndemics encompass social determinants, vulnerabilities and inequities and inequalities in health as well.
Previous studies have shown that syndemics are a good comprehensive model to document inequity and inequality in health. In a study of RMD in Indigenous populations in Latin America, as well as a study of patients with low back pain, disease is associated with being a woman, belonging to an indigenous population, and having low educational attainment. It is also exacerbated by the presence of comorbidities, especially those within the mental health domain.8 24
Given the intricacy of a syndemic approach, conventional statistical methodologies are insufficient. Instead, using strategies from graph theory (network analysis) and machine learning (cluster analysis) is necessary to perform complex analyses that document health inequities comprehensively. The syndemic approach is useful to identify health inequities and vulnerabilities in different population groups.
We hypothesise that there is a syndemic in Latin American populations suffering from rheumatic diseases, associated with comorbidities such as diabetes and hypertension, and living in a fragmented healthcare context. We also hypothesise that this phenomenon is more significant in vulnerable populations such as Indigenous peoples. Therefore, we proposed the following study to measure syndemics comparatively between Indigenous and non-Indigenous populations with RMD in Latin America.
Materials and methods
Design
This is a secondary analysis, based on multilevel network analysis using a syndemic framework, of a previously published large-scale cross-sectional study on the prevalence of RMD in five Latin American countries.
Data sources
We used a database compiled by Grupo de Estudios Epidemiológicos de Enfermedades Músculo Articulares, Community Oriented Programme for Control of Rheumatic Diseases-Latin America (COPCORD-LATAM) and Grupo Latino Americano de Estudios de Enfermedades Reumáticas en Pueblos Originarios (GLADERPO).
GLADERPO recorded information on the Qom and Wichí Indigenous populations of Argentina,10 25 Saraguro of Ecuador,26 Yucatec-Maya and Mixtec of Mexico8 9 and the Chaimas, Kariñas and Warao of Venezuela.27
COPCORD-LATAM was developed with the results of epidemiological studies conducted on the non-Indigenous populations of Colombia,28 Ecuador,29 Mexico30 and Venezuela,31 using COPCORD methodology, culturally and linguistically adapted to the different communities studied and subsequently validated in each country.
The COPCORD methodology consists of trained health personnel administering a questionnaire house to house, which identifies patients with pain of non-traumatic origin, historical and in the last 7 days. The participation of certified rheumatologists allowed for the diagnosis of RMD.29–33
The same measurements were collected in all the studies: sociodemographic variables, joint biomechanical stress, comorbidities, physical disability and accessibility to local healthcare.
Sociodemographic variables such as age, gender, self-defined ethnicity according to the laws of each country (Indigenous and non-Indigenous), formal schooling (numbers of years studied in the official education system) and place of residence (urban/rural).
Level of joint biomechanical stress was classified according to self-reported occupation. Individuals were asked for a visual recreation of their activity, according to the degree of effort and the body regions involved. Following a survey on the level of physical load repetitiveness, each occupation was classified into three levels of joint biomechanical stress in the workplace: high (eg, farmers, homemakers, machine operators), medium (eg, artisans, drivers, technicians) and low (eg, merchants, professionals, students, teachers, retirees).
Comorbidities were self-reported,32 33 while physical disability was measured with the Health Assessment Questionnaire-Disability Index (HAQ-DI), validated for each country and with an established cut-off point of >0.8.34
Accessibility to the local healthcare system was classified by conducting an exercise of comparisons and equivalences among the researchers from the five participating countries.
Considering all characteristics of the healthcare systems, the three subgroups used to classify accessibility were: partial coverage, involving a public system that covers physician appointments, laboratory tests and basic but not high specialty medications; full coverage, involving a social security system that covers all health expenses and private coverage, where patients pay fully for their care.
Analysis
A multiphase analysis was performed.
Phase I
We applied inferential statistics (ie, bivariate analysis) to explore associations between ethnicity (Indigenous or non-Indigenous) and country of origin, and sociodemographic characteristics (ie, age, gender, formal schooling, urban/rural residence), rheumatic diagnoses, comorbidities, disability (HAQ-DI) and levels of accessibility (partial, total and private coverage).
Phase II
We performed simple logistic regression models to identify factors (ie, sociodemographic, comorbidities, disability, accessibility and joint biomechanical stress) associated with RMD diagnosis (ie, present or absent) as a dependent variable by ethnicity (Indigenous vs non-Indigenous). We estimated ORs, along with 95% CIs and significance (p).
Phase III
We used a network analysis approach35 to generate groups with similar characteristics (eg, sociodemographic, country, comorbidities, diagnoses, etc) called clusters. These clusters helped to determine the negative characteristics associated with disease and disability using the syndemic framework. The network analysis method requires the definition of a characteristic that allows creation of connections between subjects; a measure of similarity was defined to create these. The similarity measure determined the relationships between the different subjects within the database. The measure of similarity evaluated the number of similarities between two subjects regarding the results of their evaluations. To construct the first part of the similarity measure, a vector was defined with the following variables: (a) accessibility level, (b) level of joint biomechanical stress and (c) urban/rural residence. Using the cosine similarity method, this vector was used to calculate a similarity index for each individual concerning the rest of the population.36 The final similarity index was obtained by applying a weighted difference by years of education between each individual.
The similarity index was used to determine an individual’s degree of similarity to the rest of the population and to build the relations between individuals. In the network definition, each individual is a node; an axis of relations is generated when the similarity index between two individuals is greater than the average of the similarity indices plus the SD of the whole population.37 The network obtained is simulated in Gephi38 and the final position of the nodes or individuals is used to define the new groups using the Density-Based Spatial Clustering of Applications with Noise (DBSCAN) method.39
Due to the complexity of the representation of the clusters, we conducted a consensus process among all researchers to select the most relevant clusters regarding socio-economic and clinical impact, which included healthcare access, disability, educational level and type of RMD. Selected clusters were further analysed in network analysis, including the following factors in a hierarchical order of importance: (1) prevalence of RMD, (2) prevalence of RA and (3) the number of individuals comprising the cluster. All researchers assigned every cluster a weighted score for each of the three selected variables. Finally, six clusters were selected per group (ie, Indigenous and non-Indigenous) according to their amount of representation of health inequity factors.
Phase IV
We conducted a sensitivity analysis to confirm no biases using a randomly selected weighted sample of Indigenous/non-Indigenous populations from the three countries that studied both at the same time (Ecuador, Mexico and Venezuela), and two countries that only had samples of Indigenous (Argentina) or non-Indigenous (Colombia) populations. The clusters obtained through this analysis were defined by factors such as living in a rural setting, lower health coverage and greater disability, which went beyond our initial Indigenous/non-Indigenous classification and impacted the management of rheumatic diseases. These emerging differences can be used to document inequity insofar as they highlight the variables which negatively affect the health of people with RMD.
Patient and public involvement
Patients or the public were not involved in the design or conduct, or reporting, or dissemination plans of our research. The members of the public were involved at original stages of each study including as cultural liaisons. We disseminated the main results to all participants and health authorities to improve health conditions.
Results
A total of 44 560 individuals from five Latin American countries (Argentina, Colombia, Ecuador, Mexico and Venezuela) were studied. Of these, 29.78% (13 269) self-identified as Indigenous and 27 145 (60.92%) were female, with an average age of 43.25 (SD=18.02) years and a mean of 8.06 (SD=5.02) years of schooling. RMD was diagnosed in 13 528 (30.36%) individuals. Rheumatic regional pain syndromes (RRPS) was the rheumatic diagnosis with the highest prevalence (6100, 13.69%) followed by osteoarthritis (3690, 8.28%), while RA was reported in (877, 1.97%) individuals (table 1).
Table 1.
Comparison of sociodemographic characteristics, country, health coverage, rheumatic diagnosis, pain, disability and comorbidities between Indigenous and non-Indigenous groups
| Indigenous n=13 269 (29.78) |
Non-Indigenous n=31 291 (70.22) |
Total n=44 560 (100.00) |
P value | |
| Gender (female) | 8010 (60.37) | 19 135 (61.15) | 27 145 (60.92) | 0.123 |
| Age (years), mean (SD) | 42.23 (18.17) | 43.69 (17.94) | 43.25 (18.02) | <0.001 |
| Urban setting | 3877 (29.22) | 24 331 (77.76) | 28 208 (63.30) | <0.001 |
| Educational level, mean number of years (SD) | 7.13 (5.07) | 8.46 (4.95) | 8.06 (5.02) | <0.001 |
| Countries | ||||
| Argentina | 2295 (17.30) | 0 (0.00) | 2295 (5.15) | <0.001 |
| Colombia | 234 (1.76) | 6454 (20.63) | 6688 (15.01) | <0.001 |
| Ecuador | 2682 (20.21) | 4858 (15.53) | 7540 (16.92) | <0.001 |
| Mexico | 6525 (49.17) | 16 085 (51.40) | 22 610 (50.74) | <0.001 |
| Venezuela | 1533 (11.55) | 3894 (12.44) | 5427 (12.18) | <0.01 |
| Health coverage* | ||||
| Full | 3481 (26.23) | 4493 (14.36) | 7974 (17.89) | <0.001 |
| Partial | 7441 (56.08) | 18 314 (58.53) | 25 755 (57.80) | <0.001 |
| Private | 795 (5.99) | 1741 (5.56) | 2536 (5.69) | 0.079 |
| Other† | 330 (2.49) | 221 (0.71) | 551 (1.24) | <0.001 |
| Joint biomechanical stress‡ | ||||
| High | 5000 (37.68) | 10 199 (32.59) | 15 199 (34.11) | <0.001 |
| Medium | 1538 (11.59) | 4720 (15.08) | 6258 (14.04) | <0.001 |
| Low | 4014 (30.25) | 9213 (29.44) | 13 227 (29.68) | 0.090 |
| Unspecified | 1815 (13.68) | 2784 (8.90) | 4599 (10.32) | <0.001 |
| Rheumatic disease | ||||
| Total | 4012 (30.24) | 9516 (30.41) | 13 528 (30.36) | 0.721 |
| Osteoarthritis | 1433 (10.80) | 2257 (7.21) | 3690 (8.28) | <0.001 |
| Rheumatoid arthritis | 278 (2.10) | 599 (1.91) | 877 (1.97) | 0.223 |
| Back pain | 1548 (11.67) | 1281 (4.09) | 2829 (6.35) | <0.001 |
| RRPS | 505 (3.81) | 5595 (17.88) | 6100 (13.69) | <0.001 |
| Musculoskeletal disorders | 521 (3.93) | 664 (2.12) | 1185 (2.66) | <0.001 |
| Fibromyalgia | 181 (1.36) | 212 (0.68) | 393 (0.88) | <0.001 |
| Other§ | 45 (0.34) | 118 (0.38) | 163 (0.37) | 0.602 |
| Pain | ||||
| Historical pain | 5408 (40.76) | 11 780 (37.65) | 17 188 (38.57) | <0.001 |
| Non-traumatic pain (7 days) | 2258 (17.02) | 8024 (25.64) | 10 282 (23.07) | <0.001 |
| Physical disability (HAQ-DI) | ||||
| HAQ-DI≥0.8 | 761 (5.74) | 2558 (8.17) | 3319 (7.45) | <0.001 |
| Comorbidities | ||||
| Diabetes mellitus | 814 (6.13) | 2279 (7.28) | 3093 (6.94) | <0.001 |
| High blood pressure | 1649 (12.43) | 5613 (17.94) | 7262 (16.30) | <0.001 |
| Cardiovascular disease | 415 (3.13) | 1106 (3.53) | 1521 (3.41) | 0.033 |
| Smoking | 1138 (8.58) | 4996 (15.97) | 6134 (13.77) | <0.001 |
| Alcoholism | 1751 (13.20) | 1068 (3.41) | 2819 (6.33) | <0.001 |
| Anxiety/Depression | 2304 (17.36) | 3727 (11.91) | 6031 (13.53) | <0.001 |
| No comorbidities | 6391 (48.16) | 14 450 (46.18) | 20 841 (46.77) | <0.001 |
*Missing data: 1222 (9.21%) Indigenous and 6522 (20.84%) non-Indigenous, total 7744 (17.38%).
†Other: traditional healthcare.
‡Missing data: 902 (7.01%) Indigenous and 4375 (13.80%) non-Indigenous.
§Others: Indigenous: 29 ankylosing spondylitis, 9 gout, 4 scleroderma and 3 psoriasis. Non-Indigenous: 39 ankylosing spondylitis, 74 gout, 1 scleroderma and 4 psoriasis.
HAQ-DI, Health Assessment Questionnaire-Disability Index; RRPS, rheumatic regional pain syndromes.
A lower urban origin (18.71%) and less years of formal schooling (6.74, SD=5.71) were observed in the Indigenous population, while the non-Indigenous population had a predominance of private coverage (10.89%). High joint biomechanical stress (47.01%) and historical pain (39.99%) were more frequent in Indigenous populations. The prevalence of RMD was similar between populations studied; RA was more prevalent in Indigenous people (2.26% vs 1.74%), but not significantly. Non-Indigenous people had greater disability (8.15% with HAQ≥0.8) and higher prevalence of diabetes mellitus, high blood pressure and smoking (7.09%, 18.59% and 15.16%, respectively). Among Indigenous people, alcohol consumption and anxiety/depression were more prevalent (13.98% and 19.55%) (see online supplemental table).
bmjopen-2022-069246supp001.pdf (45.5KB, pdf)
In terms of the subanalysis by country, Argentina had the youngest individuals (35.98, SD=14.25); Ecuador and Colombia recorded a higher level of schooling (9.31, SD=5.49) and a higher prevalence of RMD (47.69% and 40.76%); Argentina and Mexico had the highest prevalence of RA (3.01% and 2.22%); Colombia had a higher prevalence of historical and non-traumatic pain (73.95% and 43.94%) and Ecuador had the highest number of disabled people (8.70% with HAQ≥0.8) (table 2).
Table 2.
Comparison of sociodemographic characteristics, health coverage, rheumatic diagnosis, pain, disability and comorbidities between populations of five Latin American countries
| Argentina, n (%) n=2295 (5.15) |
Colombia, n (%) n=6688 (15.01) |
Ecuador, n (%) n=7540 (16.92) |
Mexico, n (%) n=22 610 (50.74) |
Venezuela, n (%) n=5427 (12.18) |
Total*, n (%) n=44 560 (100.00) |
P value | |
| Ethnicity (Indigenous) | 2295 (100.00) | 234 (3.50) | 2682 (35.57) | 6525 (28.86) | 1533 (28.25) | 13 269 (29.78) | <0.001 |
| Gender (female) | 1393 (60.70) | 4280 (64.00) | 4590 (60.88) | 13 634 (60.30) | 3248 (59.85) | 27 145 (60.92) | <0.001 |
| Age (years), mean (SD) | 35.98 (14.25) | 46.41 (18.35) | 43.39 (18.60) | 43.08 (17.93) | 42.98 (17.63) | 43.25 (18.02) | <0.001 |
| Urban setting | 0 (0.00) | 6688 (100.00) | 3384 (44.88) | 14 242 (62.99) | 3894 (71.75) | 28 208 (63.30) | <0.001 |
| Educational level, mean number of years (SD) | 5.43 (3.60) | 9.19 (4.00) | 9.31 (5.49) | 7.41 (4.98) | 8.77 (5.27) | 8.06 (5.02) | <0.001 |
| Health coverage* | |||||||
| Total | 2295 (100.00) | 6527 (97.60) | 5453 (72.32) | 17 114 (75.70) | 5427 (100.00) | 36 816 (82.62) | |
| Full | 29 (1.26) | 1920 (28.71) | 3148 (41.75) | 2877 (12.72) | 0 (0.00) | 7974 (17.89) | <0.001 |
| Partial | 2053 (89.46) | 4465 (66.76) | 405 (5.37) | 13 674 (60.48) | 5158 (95.04) | 25 755 (57.80) | <0.001 |
| Private | 183 (7.97) | 39 (0.58) | 1482 (19.66) | 563 (2.49) | 269 (4.96) | 2536 (5.69) | <0.001 |
| Other† | 30 (1.31) | 103 (1.54) | 418 (5.54) | 0 (0.00) | 0 (0.00) | 551 (1.24) | <0.001 |
| Joint biomechanical stress‡ | |||||||
| Total | 1698 (74.00) | 6686 (99.97) | 7440 (98.67) | 20 253 (89.58) | 3206 (59.08) | 39 283 (88.16) | |
| High | 420 (18.30) | 3511 (52.50) | 3382 (44.85) | 6667 (29.49) | 1219 (22.46) | 15 199 (34.11) | <0.001 |
| Medium | 159 (6.93) | 1569 (23.46) | 516 (6.84) | 3438 (15.21) | 576 (10.61) | 6258 (14.04) | <0.001 |
| Low | 120 (5.23) | 1604 (23.98) | 3510 (46.55) | 6684 (29.56) | 1309 (24.12) | 13 227 (29.68) | <0.001 |
| Unspecified | 999 (43.53) | 2 (0.03) | 32 (0.42) | 3464 (15.32) | 102 (1.88) | 4599 (10.32) | <0.001 |
| Rheumatic disease | |||||||
| Total | 705 (30.72) | 2726 (40.76) | 3596 (47.69) | 5092 (22.52) | 1409 (25.96) | 13 528 (30.36) | <0.001 |
| Osteoarthritis | 88 (3.83) | 521 (7.79) | 470 (6.23) | 1797 (7.95) | 814 (15.00) | 3690 (8.28) | <0.001 |
| Rheumatoid arthritis | 69 (3.01) | 84 (1.26) | 120 (1.59) | 501 (2.22) | 103 (1.90) | 877 (1.97) | <0.001 |
| Back pain | 460 (20.04) | 237 (3.54) | 474 (6.29) | 1357 (6.00) | 301 (5.55) | 2829 (6.35) | <0.001 |
| RRPS | 41 (1.79) | 2726 (40.76) | 2671 (35.42) | 461 (2.04) | 201 (3.70) | 6100 (13.69) | <0.001 |
| Musculoskeletal disorders | 50 (2.18) | 0 (0.00) | 62 (0.82) | 1013 (4.48) | 60 (1.11) | 1185 (2.66) | <0.001 |
| Fibromyalgia | 3 (0.13) | 27 (0.40) | 214 (2.84) | 126 (0.56) | 23 (0.42) | 393 (0.88) | <0.001 |
| Other§ | 2 (0.09) | 26 (0.39) | 32 (0.42) | 80 (0.35) | 23 (0.42) | 163 (0.37) | 0.179 |
| Pain | |||||||
| Historical pain | 938 (40.87) | 4946 (73.95) | 3420 (45.36) | 6141 (27.16) | 1743 (32.12) | 17 188 (38.57) | <0.001 |
| Non-traumatic pain (7 days) | 402 (17.52) | 2939 (43.94) | 1525 (20.23) | 4204 (18.59) | 1212 (22.33) | 10 282 (23.07) | <0.001 |
| Physical disability (HAQ-DI) | |||||||
| HAQ≥0.8 | 95 (4.14) | 400 (5.98) | 656 (8.70) | 1741 (7.70) | 427 (7.87) | 3319 (7.45) | <0.001 |
| Comorbidities | |||||||
| Diabetes mellitus | 125 (5.45) | 428 (6.40) | 382 (5.07) | 1898 (8.39) | 260 (4.79) | 3093 (6.94) | <0.001 |
| High blood pressure | 379 (16.51) | 1591 (23.79) | 1046 (13.87) | 3078 (13.61) | 1168 (21.52) | 7262 (16.30) | <0.001 |
| Cardiovascular disease | 144 (6.27) | 435 (6.50) | 250 (3.32) | 471 (2.08) | 221 (4.07) | 1521 (3.41) | <0.001 |
| Smoking | 497 (21.66) | 2409 (36.02) | 1587 (21.05) | 1080 (4.78) | 561 (10.34) | 6134 (13.77) | <0.001 |
| Alcoholism | 379 (16.51) | 0 (0.00) | 470 (6.23) | 1523 (6.74) | 447 (8.24) | 2819 (6.33) | <0.001 |
| Anxiety/Depression | 123 (5.36) | 1463 (21.88) | 1843 (24.44) | 2185 (9.66) | 417 (7.68) | 6031 (13.53) | <0.001 |
| No comorbidities | 882 (38.43) | 2483 (37.13) | 2460 (32.63) | 12 471 (55.16) | 2545 (46.90) | 20 841 (46.77) | <0.001 |
*Missing data: 7744 (17.38).
†Other: traditional healthcare.
‡Missing data: 5277 (11.84).
§Others: ankylosing spondylitis, gout, scleroderma, psoriasis.
HAQ-DI, Health Assessment Questionnaire-Disability Index; RRPS, rheumatic regional pain syndromes.
A logistic regression analysis was performed by ethnicity. In the Indigenous population, the variables significantly associated with RMD diagnosis were living in a rural setting, younger age, relying on the public health system for treatment, high levels of joint biomechanical stress, greater pain and greater disability. In turn, the variables associated with RMD diagnosis in the non-Indigenous population were being a woman, living in an urban setting, older age, relying on the private sector for treatment, more frequent joint biomechanical stress regardless of the level, greater pain, greater disability and less association with having diabetes mellitus (table 3).
Table 3.
Logistic regression
| Indigenous | Non-Indigenous | |||
| OR (95% CI two-sided) | P value | OR (95% CI two-sided) | P value | |
| Intercept | 0.02 (0.01 to 0.03) | <0.01 | 0.10 (0.08 to 0.12) | <0.01 |
| Gender (female) | 1.10 (0.96 to 1.25) | 0.164 | 1.19 (1.11 to 1.27) | <0.01 |
| Age (years) | 0.49 (0.41 to 0.59) | <0.01 | 1.49 (1.37 to 1.62) | <0.01 |
| Urban setting | 1.02 (1.02 to 1.02) | <0.01 | 1.00 (1.00 to 1.01) | <0.01 |
| Educational level | 0.99 (0.97 to 1.00) | 0.051 | 1.01 (1.00 to 1.01) | 0.081 |
| Health coverage | ||||
| Full | 1.46 (1.11 to 1.91) | <0.01 | 0.82 (0.74 to 0.91) | <0.01 |
| Partial | 1.15 (0.88 to 1.50) | 0.322 | 0.59 (0.55 to 0.64) | <0.01 |
| Private | 1.55 (1.10 to 2.19) | 0.013 | 1.43 (1.25 to 1.64) | <0.01 |
| Other | 1.36 (0.87 to 2.13) | 0.172 | 0.98 (0.70 to 1.36) | 0.900 |
| Level of joint biomechanical stress | ||||
| High | 1.18 (1.00 to 1.40) | 0.054 | 1.55 (1.41 to 1.69) | <0.01 |
| Medium | 1.22 (0.96 to 1.56) | 0.110 | 1.31 (1.17 to 1.46) | <0.01 |
| Low | 1.17 (0.97 to 1.42) | 0.101 | 1.52 (1.38 to 1.66) | <0.01 |
| Pain | ||||
| Historical pain | 27.77 (24.09 to 32.01) | <0.01 | 3.84 (3.59 to 4.11) | <0.01 |
| Non-traumatic pain (7 days) | 2.51 (2.18 to 2.89) | <0.01 | 2.26 (2.11 to 2.43) | <0.01 |
| Physical disability (HAQ-DI) | ||||
| HAQ≥0.8 | 1.25 (1.00 to 1.56) | 0.045 | 1.37 (1.23 to 1.52) | <0.01 |
| Comorbidities | ||||
| Diabetes mellitus | 0.95 (0.75 to 1.20) | 0.653 | 0.82 (0.73 to 0.93) | <0.01 |
| High blood pressure | 0.98 (0.82 to 1.18) | 0.842 | 0.95 (0.87 to 1.03) | 0.226 |
| Cardiovascular disease | 0.83 (0.62 to 1.12) | 0.219 | 1.06 (0.91 to 1.24) | 0.433 |
| Smoking | 0.93 (0.74 to 1.16) | 0.504 | 1.06 (0.97 to 1.16) | 0.217 |
| Alcoholism | 0.78 (0.64 to 0.94) | <0.01 | 1.15 (0.97 to 1.37) | 0.107 |
| Anxiety/Depression | 0.99 (0.84 to 1.17) | 0.926 | 1.05 (0.96 to 1.16) | 0.266 |
| No comorbidities | 0.87 (0.74 to 1.03) | 0.111 | 0.73 (0.67 to 0.80) | <0.01 |
Dependent variable: a rheumatic disease. Independent variables: gender, place of residence, age, schooling, health coverage, biomechanical stress, pain, functional capacity and comorbidities.
HAQ-DI, Health Assessment Questionnaire-Disability Index.
Twenty clusters were identified in the Indigenous population and 17 in the non-Indigenous population. In order to best represent the results, six clusters were selected for each group, using consensus and weighing as described in the methodology.
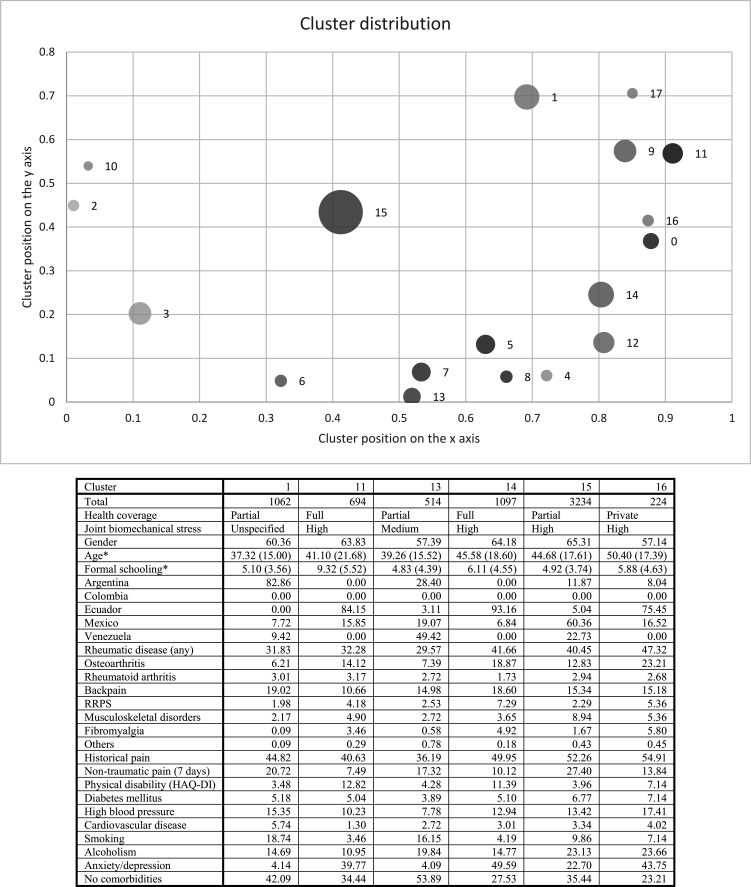
The six clusters selected from the Indigenous population were: cluster 1 was represented by individuals with partial coverage, younger, with lower educational attainment, higher prevalence of RA and low back pain and higher pain and smoking. Cluster 11 included individuals with full coverage, greater functional limitation and higher prevalence of RA and anxiety/depression. Cluster 13 was represented by individuals with less schooling and a high percentage of smoking and alcoholism. Cluster 14 was represented by individuals with full coverage, high prevalence of RMD and higher percentage of anxiety/depression and pain. Cluster 15 was the largest, with partial coverage, high level of joint biomechanical stress and higher prevalence of RMD and associated pain. Lastly, cluster 16 was the smallest and included individuals with private coverage, high level of joint biomechanical stress, older age, anxiety/depression and alcoholism and the highest prevalence of RMD and associated pain out of all the clusters (figure 1).
Figure 1.
Network and cluster analysis to describe groups with shared variables according to the syndemic framework in the Indigenous population. HAQ-DI cut-off point of >0.8. *Age and formal schooling show mean value (SD). Circle size represents the number of individuals per cluster for visual comparison. The cluster positions are the result of the network simulation; the position of each cluster is obtained during the simulation depending on the similarity of the individuals. HAQ-DI, Health Assessment Questionnaire-Disability Index; RRPS, rheumatic regional pain syndromes.
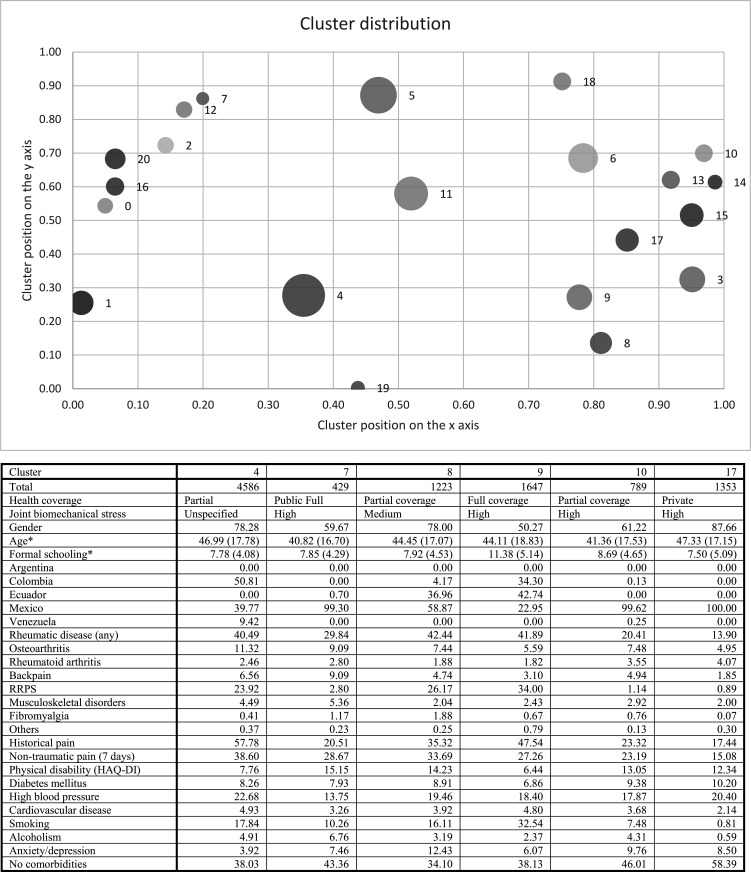
In the non-Indigenous population, the six selected clusters were: cluster 4 was the largest, represented by individuals with partial coverage, high level of joint biomechanical stress, higher percentage of pain and higher prevalence of RMD, high blood pressure and anxiety/depression. Cluster 7 was the smallest, with a low percentage of pain and RMD, but greater physical disability. Cluster 8 included individuals with less years of formal schooling, partial health coverage, higher prevalence of RMD and anxiety/depression, medium level of joint biomechanical stress and high physical disability.
Cluster 9 included individuals with higher educational attainment, full coverage, higher prevalence of RRPS, greater pain, greater level of smoking and less disability. Cluster 10 was represented by individuals with partial coverage, and lower prevalence of RMD and associated pain, but with greater limitation. Cluster 17 included only Mexican individuals with partial coverage, high level of joint biomechanical stress, lower educational attainment and higher prevalence of RA, diabetes mellitus and high blood pressure (figure 2).
Figure 2.
Network and cluster analysis to describe groups with shared variables according to the syndemic framework in the non-Indigenous population. HAQ-DI cut-off point of >0.8. *Age and formal schooling show mean value (SD). Circle size represents the number of individuals per cluster for visual comparison. The cluster positions are the result of the network simulation; the position of each cluster is obtained during the simulation depending on the similarity of the individuals. HAQ-DI, Health Assessment Questionnaire-Disability Index; RRPS, rheumatic regional pain syndromes.
Discussion
The syndemic approach analyses the synergistic inter-relationship between different biological and non-biological factors that lead to disease. The application of this approach to the area of health is relatively recent. Multiple studies describe how epidemiological and socioeconomic factors are related to disability and inequity in patients with RMD.40 However, there are few publications that evaluate inflammatory joint diseases and other chronic musculoskeletal conditions from a broader social and biocultural context, taking into consideration how the socioeconomic characteristics of the environment interact with the disease.
In the present study, a syndemic approach was used to identify factors associated with health inequity.41 The results obtained through a complex analysis of networks showed a greater clustering of patients with rheumatic diseases who shared common social determinants, such as rural setting and lower schooling. This coincides with the results published by Norton et al, who have described that the greater the comorbidities, the greater the risk of a negative impact on the evolution of RMD42 and, consequently, the greater the difficulty to adequately control the disease.43
This study identified factors associated with inequity in individuals with RMD in five Latin American countries with a syndemic approach. The clusters obtained through our analysis show differential negative impacts in the groups that were formed. The relevant emerging factors are living in rural communities, having lower educational attainment and depending on the public healthcare system, described as fragmented in all participating countries. Comorbidities such as smoking, alcoholism and those related to mental health (anxiety/depression) are most prevalent overall, and greater in the Indigenous population. The differences detected through the clusters can be considered health inequities, since they constitute avoidable differences such as low schooling and a healthcare system without full coverage. Furthermore, the clusters that have greater impact are those which include Indigenous people. All of the above attests to the inequity in RMD in low-income and middle-income countries in general, and even more so in historically vulnerable populations, such as Indigenous groups.
Multiple reports describe disparity and inequity among patients with RMD. Although they contemplate the interaction of disease with epidemiological, biological and socioeconomic factors, most of the research of this phenomenon does not include a conjunct and comprehensive analysis of all factors as is achieved by syndemics.40
Another important finding of the study is the clusters with higher prevalence of comorbidities, particularly high blood pressure, tobacco and alcohol consumption, and those related to mental health (anxiety/depression). As previously reported, the greater the comorbidity, the greater the risk of negative impact on the evolution of RMD.42 The coexistence of two or more conditions prevents the proper control of disease activity, hindering the achievement of therapeutic goals like those proposed by the treat-to-target recommendations.43
The coexistence of several chronic conditions involving systemic inflammatory processes and deterioration in functional capacities leads to a greater impact on the quality of life and greater demand of health services, to which many populations in Latin America have no universal access. Indeed, the results of this analysis identified several clusters with partial or no access to medical care coinciding with greater comorbidity (clusters 1, 10, 11). The association between RMD severity and comorbidities as biological interactions is clear, but it is important to correlate these at a social level, since not having access to timely diagnoses or specialised care increases the possibility of greater comorbidity and complications. Additionally, it is important to address the interaction of certain prevalent comorbidities (smoking, alcoholism and mental health disorders) which contribute to the syndemic as both social and biological factors. While there is sufficient evidence to suggest the possibility of common pathophysiological mechanisms with inflammatory joint diseases, it has also been shown that states of anxiety and depression can be triggered by non-biological factors such as social isolation, poverty, mental health worldview or cultural stigmatisation and/or lack of access to healthcare.44
When comparing inequity between population groups, the poverty rate in Indigenous and rural communities is higher, as reported in this study: 29.78% of the population self-identified as Indigenous, with a higher level of individuals from rural areas and fewer years of schooling. The prevalence of RA specifically was more pronounced in the Indigenous population, with the highest rates in Argentina and Mexico (3.01% and 2.22%).8 10 Previous research has similarly found that RMD are more frequent in the Indigenous populations than in the non-Indigenous populations of Canada, Australia, New Zealand and the USA.17
The Indigenous population had a lower prevalence of disability despite presenting greater high level of joint biomechanical stress, historical pain and RA, which may be related to a worldview favouring normalisation or underestimation of symptoms. In addition, the interpretation of these symptoms may be one of the causes of delay in seeking specialised care.11 The relationship between ethnicity and health outcomes seems to be influenced by acculturation, that is, when one ethnic group is forced to adopt the beliefs and practices of another, the members develop negative health behaviours as coping mechanisms.45
Health systems in Latin America are diverse and complex. Individuals in this study are distributed among the spectrum of public (partial or full) and private systems. Most Indigenous communities have public health coverage, though this does not guarantee access or continuity of care and treatment. Limited access is due to economic barriers, and related to ethnic, cultural and geographical factors, among others.8 24 40 46 Indigenous communities are among the most vulnerable groups and, due to the conditions described above, their inclusion into the healthcare system is complex.11 46 47
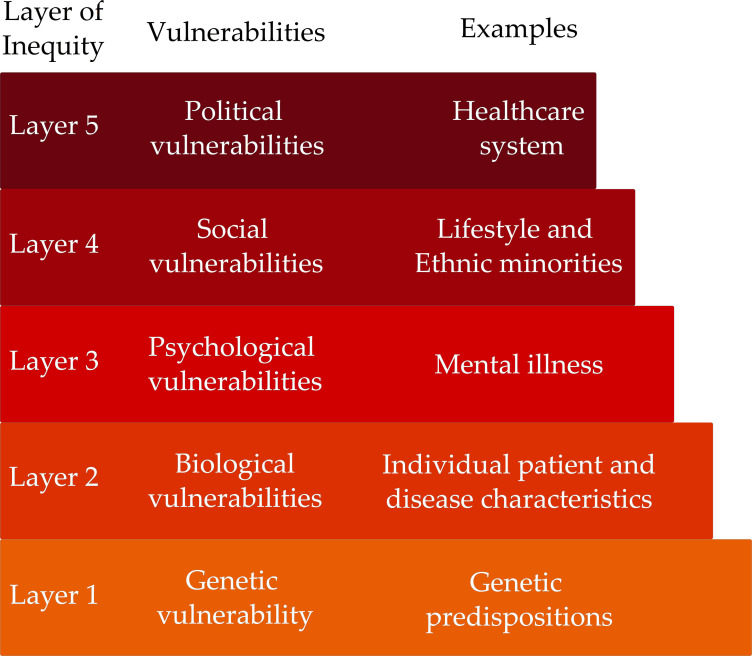
The inaccessibility of the healthcare system, socioeconomic conditions, presence of comorbidities involving mental health and RMD disease activity are all factors that exist in interacting layers to create specific conditions of vulnerability for different patient populations. A model of vulnerability in layers, called a palimpsest design,12 analyses how the determinants of health at different levels—genetic, biological, psychological, social and political—interact over time, creating barriers that lead to health inequity. The syndemic approach, in taking into consideration all factors and their interactions conjunctly, corresponds with a palimpsest model, providing evidence for the vulnerability of patients with RMD associated with social factors such as rurality, low educational attainment and greater reliance on the public health system (figure 3).
Figure 3.
Inequities and vulnerabilities in RMD: a palimpsest model. A model of vulnerability in layers analyses how the determinants of health at different levels—genetic, biological, psychological, social and political—interact over time, creating barriers that lead to health inequity.
Limitations
The cross-sectional nature of our study is a limitation to establish causality. However, the network and cluster analysis allowed the grouping of individuals by variables to document inequity, the principal objective of this study.
Another limitation is the documentation of comorbidities through self-reporting, which can condition a measurement error. However, an attempt was made to verify these reports through the medications that individuals informed having taken.
In conclusion, the complex analysis from a syndemic approach allowed us to identify the greatest inequity in the clusters that group younger individuals, residents of rural areas, those who self-identify as Indigenous, have lower educational attainment, higher prevalence of RMD and RA specifically, greater comorbidities especially related to mental health and high blood pressure and partial coverage in the public healthcare system. Given the above, we can assume that these social vulnerabilities and comorbidities lead to health inequities for populations living in countries in which RMD are not considered a priority, resulting in lack of coverage for prevention, diagnosis and management.
Supplementary Material
Acknowledgments
We thank all the participating communities and their authorities for allowing this study to be conducted. We also thank all primary care physicians who collaborated in the study. Amaranta Manrique de Lara for her critical reading, comments and editing. We also thank Arturo Velasco Gutierres.
Footnotes
BAP-E, IP-B and contributed equally.
Collaborators: GLADERPO group all the researchers: Argentina: Mario Goñi, Nora Mathern, Marisa Jorfen, Silvana Conti, Romina Nieto, Alvaro Sanabria, Cristina Prigione, Adriana MR Silvestre, Vanina García, Julio Miljevic, Daniel Dhair, Matias Laithe, Fadua Midauar, Maria Celeste Martin, Maria Cecilia Barrios, Vicente, María Elena Crespo, Mariana Aciar, Emilio Buschiazzo, Natalia L Cucchiaro, Eugenia Picco, Mario Ruiz, José Adolfo Sánchez, Rodolfo Franco, Natalia Estrella, Silvia Jorge, Cinthya Retamozo, Sofia Fernandez, Martina Fay, Cecilia Camacho, Graciela Gomez, Jazmin Petrelli, Andrés Honeri, Viviana Arenas Solórzano, Ana Bensi, Maria Elena Calvo, Marcela Valdata. Colombia: Rodrigo Giraldo, Ignacio Angarita, Jesus G Ballesteros, Sofia Arias, Andres Vásquez, Lina Valero, Ani Cortes, Estafania Castañeda, Elias Forero. Ecuador: Astrid Feicán, Fernando Vintimilla, Jaime Vintimilla, Veronica Ochoa, Jorge Delgado, Angelita Lliguisaca, Holger Dután. México: Mario H Cardiel, Jacqueline Rodríguez-Amado, Julio Casasola-Vargas, Conrado Garcia, Imelda García-Olivera, Natalia Santana, César Pacheco, Susana Aidee Gonzalez-Chávez, Hazel Garcia Morales, Arturo Velasco Gutierrez, JF Moctezuma-Rios, Everardo Álvarez-Hernández, Eduardo Navarro-Zarza, Angelia Angulo, Rosana Flores, Janeth Galván Padrón, Lorena Pérez B, Janett Riega Brenda Vaquez Fuentes, Miguel A Villarreal, Cassandra Skinner Taylor, Sara Marín, Dionicio GalarzaDelgado, Diana Flores Alvarado, Jorge A Esquivel Varerio, Luz Helena Sanín, Marco Maradiaga Ceceño, Jorge Zamudio Lerm. Venezuela: Ysabel Granados, Rosa Chacón, Ivan Stekman, Yanira Martínez, Gloris Sánchez, Celenia Rosillo, Ligia Cedeño.
Contributors: YG, AGS, JA-N, RQ, FJ-S, AMS, SG-P, AL-S, MVG-R, VJ, MAG-E, JCR, RB-V, JL, BAP-E, IP-B were involved in study conception, design, acquisition of data and drafting the manuscript. YG, AGS, JA-N, RQ, FJ-S, AMS, SG-P, AL-S, MVG-R, VJ, MAG-E and IP-B contributions to analysis and interpretation of data. YG and IP-B drafted the manuscript. All authors have read and approved the final version of the manuscript. AGS develop all statistical analysis and modelling. IP-B is the author acting as the guarantor for this study.
Funding: This work was supported by Federico Wilhelm Agricola Foundation, N/A. Argentina, Asociacion Colombiana de Reumatologia (ASOREUMA), No 156. Colombia, Colegio Mexicano de Reumatología, N/A. México, National Council for Science and Technology (CONACYT)-Mexico. Salud 2011-01-162154, Mexico, PDVSA East and SUELOPETROL, N/A. Venezuela and Universidad de Cuenca, N/A. Ecuador.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: on behalf of the Latin American Study Group of Rheumatic Diseases in Indigenous Peoples (GLADERPO), Amaranta Manrique de Lara, Mario Goñi, Nora Mathern, Marisa Jorfen, Silvana Conti, Romina Nieto, Alvaro Sanabria, Cristina Prigione, Adriana MR Silvestre, Vanina García, Julio Miljevic, Daniel Dhair, Matias Laithe, Fadua Midauar, Maria Celeste Martin, Maria Cecilia Barrios, María Elena Crespo, Mariana Aciar, Emilio Buschiazzo, Natalia L Cucchiaro, Eugenia Picco, Mario Ruiz, José Adolfo Sánchez, Rodolfo Franco, Natalia Estrella, Silvia Jorge, Cinthya Retamozo, Sofia Fernandez, Martina Fay, Cecilia Camacho, Graciela Gomez, Jazmin Petrelli, Andrés Honeri, Viviana Arenas Solórzano, Ana Bensi, Maria Elena Calvo, Marcela Valdata, Rodrigo Giraldo, Ignacio Angarita, Jesus G Ballesteros, Sofia Arias, Andres Vásquez, Lina Valero, Ani Cortes, Estafania Castañeda, Elias Forero, Astrid Feicán, Fernando Vintimilla, Jaime Vintimilla, Veronica Ochoa, Jorge Delgado, Angelita Lliguisaca, Holger Dután, Mario H Cardiel, Jacqueline Rodríguez-Amado, Julio Casasola-Vargas, Conrado Garcia, Imelda García-Olivera, Natalia Santana, César Pacheco, Susana Aidee Gonzalez-Chávez, Hazel Garcia Morales, Arturo Velasco Gutierrez, JF Moctezuma-Rios, Everardo Álvarez-Hernández, Eduardo Navarro-Zarza, Angelia Angulo, Rosana Flores, Janeth Galván Padrón, B Lorena Pérez, Janett Riega, Brenda Vaquez Fuentes, Miguel A Villarreal, Cassandra Skinner Taylor, Sara Marín, Dionicio Galarza Delgado, Diana Flores Alvarado, Jorge A Esquivel Varerio, Luz Helena Sanín, Marco Maradiaga Ceceño, Jorge Zamudio Lerm, Ysabel Granados, Rosa Chacón, Ivan Stekman, Yanira Martínez, Gloris Sánchez, Celenia Rosillo, and Ligia Cedeño
Data availability statement
Data are available on reasonable request. The data are available but must be requested from the researcher IP-B through a specific application request for the use of data, which will be evaluated by all groups.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
As the present investigation involves data collected as a part of prior studies, no specific study protocol approval was needed, as all institutional and ethics committees of each participating institution (Argentina: 1619/2010 and 0127/2011; Ecuador: 2016-129IN and Mexico: DI/11/4044B/3/123) had already approved pertinent studies and authorities from participating indigenous communities. Participants gave informed consent to participate in the study before taking part.
References
- 1.Bilsborrow JB, Peláez-Ballestas I, Pons-Estel B, et al. Global rheumatology research: frontiers, challenges, and opportunities. Arthritis Rheumatol 2022;74:1–4. 10.1002/art.41980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardiel MH. Present and future of rheumatic diseases in Latin America. are we prepared to face them? Reumatol Clin 2011;7:279–80. 10.1016/j.reuma.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 3.Brooks PM. The burden of musculoskeletal disease -- a global perspective. Clin Rheumatol 2006;25:778–81. 10.1007/s10067-006-0240-3 [DOI] [PubMed] [Google Scholar]
- 4.Briggs A, Slater H, Jordan J, et al. Towards a global strategy to improve musculoskeletal health. Sydney, Australia: Global Alliance for Musculoskeletal Health, 2021. [Google Scholar]
- 5.Commission on Social Determinants of Health . Subsanar las desigualdades en una generación: alcanzar la equidad sanitaria actuando sobre los determinantes sociales de la salud: informe final de la comisión sobre determinantes sociales de la salud. Organización Mundial de la Salud, 2009. Available: https://apps.who.int/iris/handle/10665/44084 [Google Scholar]
- 6.OECD . Health at a glance 2021: OECD indicators. Paris: Organisation for Economic Co-operation and Development, 2021. 10.1787/ae3016b9-en [DOI] [Google Scholar]
- 7.Linares-Pérez N, Arellano OL. La equidad en salud: propuestas conceptuales, aspectos críticos y perspectivas desde el campo de la salud colectiva. Med Soc 2008;3:247–59. [Google Scholar]
- 8.Peláez-Ballestas I, Granados Y, Quintana R, et al. Epidemiology and socioeconomic impact of the rheumatic diseases on indigenous people: an invisible syndemic public health problem. Ann Rheum Dis 2018;77:1397–404. 10.1136/annrheumdis-2018-213625 [DOI] [PubMed] [Google Scholar]
- 9.Loyola-Sanchez A, Richardson J, Pelaez-Ballestas I, et al. The impact of arthritis on the physical function of a rural maya-yucateco community and factors associated with its prevalence: a cross sectional, community-based study. Clin Rheumatol 2016;35 Suppl 1:25–34. 10.1007/s10067-015-3084-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintana R, Goñi M, Mathern N, et al. Rheumatoid arthritis in the indigenous qom population of rosario, argentina: aggressive and disabling disease with inadequate adherence to treatment in a community-based cohort study. Clin Rheumatol 2018;37:2323–30. 10.1007/s10067-018-4103-5 [DOI] [PubMed] [Google Scholar]
- 11.Quintana R, Fernández S, Orzuza SM, et al. « living with rheumatoid arthritis » in an indigenous qom population in argentina. A qualitative study. Reumatol Clin (Engl Ed) 2021;17:543–8. 10.1016/j.reumae.2020.04.006 [DOI] [PubMed] [Google Scholar]
- 12.Colmenares-Roa T, Figueroa-Perea JG, Pelcastre-Villafuerte B, et al. Vulnerability as a palimpsest: practices and public policy in a Mexican hospital setting. Health (London) 2022;26:753–76. 10.1177/1363459320988879 [DOI] [PubMed] [Google Scholar]
- 13.Londoño J, Peláez Ballestas I, Cuervo F, et al. Prevalencia de la enfermedad reumática en Colombia, según estrategia COPCORD-asociación Colombiana de reumatología. estudio de prevalencia de enfermedad reumática en población Colombiana mayor de 18 años. Revista Colombiana de Reumatología 2018;25:245–56. 10.1016/j.rcreu.2018.08.003 [DOI] [Google Scholar]
- 14.Dantés OG, Sesma S, Becerril VM, et al. Sistema de salud de México. Salud Pública México 2011;53:s220–32. [PubMed] [Google Scholar]
- 15.Soriano ER. Defining quality of rheumatolgic care: Argentina. J Clin Rheumatol 2017;23:207–8. 10.1097/RHU.0000000000000540 [DOI] [PubMed] [Google Scholar]
- 16.Pineda C, Sandoval H. Defining quality of rheumatologic care: Mexico. J Clin Rheumatol 2017;23:209–11. 10.1097/RHU.0000000000000532 [DOI] [PubMed] [Google Scholar]
- 17.Montenegro RA, Stephens C. Indigenous health in Latin America and the Caribbean. Lancet 2006;367:1859–69. 10.1016/S0140-6736(06)68808-9 [DOI] [PubMed] [Google Scholar]
- 18.Peláez-Ballestas I, Granados Y, Silvestre A, et al. Culture-sensitive adaptation and validation of the community-oriented program for the control of rheumatic diseases methodology for rheumatic disease in Latin American Indigenous populations. Rheumatol Int 2014;34:1299–309. 10.1007/s00296-014-2997-z [DOI] [PubMed] [Google Scholar]
- 19.Quintana R, Juárez V, Silvestre A, et al. Prevalencia de artrtitis reumatoide en DOS poblaciones originarias de Argentina. estudio de base comunitaria: ¿ “ DOS caras de Una misma moneda ”? Rev Fac Cs Méd UNR 2020;1:113–21. 10.35305/fcm.v1i.27 [DOI] [Google Scholar]
- 20.Peláez-Ballestas I, Pons-Estel BA, Burgos-Vargas R. Epidemiology of rheumatic diseases in indigenous populations in Latin-Americans. Clin Rheumatol 2016;35 Suppl 1:1–3. 10.1007/s10067-016-3298-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Bulled N, Ostrach B, et al. Syndemics and the biosocial conception of health. Lancet 2017;389:941–50. 10.1016/S0140-6736(17)30003-X [DOI] [PubMed] [Google Scholar]
- 22.Mendenhall E, Kohrt BA, Norris SA, et al. Non-communicable disease syndemics: poverty, depression, and diabetes among low-income populations. Lancet 2017;389:951–63. 10.1016/S0140-6736(17)30402-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willen SS, Knipper M, Abadía-Barrero CE, et al. Syndemic vulnerability and the right to health. Lancet 2017;389:964–77. 10.1016/S0140-6736(17)30261-1 [DOI] [PubMed] [Google Scholar]
- 24.Strozzi AG, Peláez-Ballestas I, Granados Y, et al. Syndemic and syndemogenesis of low back pain in Latin-American population: a network and cluster analysis. Clin Rheumatol 2020;39:2715–26. 10.1007/s10067-020-05047-x [DOI] [PubMed] [Google Scholar]
- 25.Juárez V, Quintana R, Crespo ME, et al. Prevalence of musculoskeletal disorders and rheumatic diseases in an Argentinean Indigenous wichi community. Clin Rheumatol 2021;40:75–83. 10.1007/s10067-020-05130-3 [DOI] [PubMed] [Google Scholar]
- 26.Guevara SV, Feicán EA, Peláez I, et al. Prevalence of rheumatic diseases and quality of life in the saraguro indigenous people, ecuador: a cross-sectional community-based study. J Clin Rheumatol 2020;26:S139–47. 10.1097/RHU.0000000000001131 [DOI] [PubMed] [Google Scholar]
- 27.Granados Y, Rosillo C, Cedeño L, et al. Prevalence of musculoskeletal disorders and rheumatic disease in the warao, kari’ña, and chaima indigenous populations of monagas state, venezuela. Clin Rheumatol 2016;35 Suppl 1:53–61. 10.1007/s10067-016-3194-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peláez Ballestas I, Santos AM, Angarita I, et al. Adecuación Y validación transcultural del cuestionario COPCORD: programa orientado a la comunidad para El control de las Enfermedades reumáticas en Colombia. Revista Colombiana de Reumatología 2019;26:88–96. 10.1016/j.rcreu.2019.01.004 [DOI] [Google Scholar]
- 29.Guevara-Pacheco S, Feicán-Alvarado A, Sanín LH, et al. Prevalence of musculoskeletal disorders and rheumatic diseases in cuenca, Ecuador: a WHO-ILAR COPCORD study. Rheumatol Int 2016;36:1195–204. 10.1007/s00296-016-3446-y [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Nemegyei J, Peláez-Ballestas I, Rodríguez-Amado J, et al. Prevalence of rheumatic regional pain syndromes in adults from Mexico: a community survey using COPCORD for screening and syndrome-specific diagnostic criteria. J Rheumatol Suppl 2011;86:15–20. 10.3899/jrheum.100953 [DOI] [PubMed] [Google Scholar]
- 31.Granados Y, Cedeño L, Rosillo C, et al. Prevalence of musculoskeletal disorders and rheumatic diseases in an urban community in monagas state, Venezuela: a COPCORD study. Clin Rheumatol 2015;34:871–7. 10.1007/s10067-014-2689-9 [DOI] [PubMed] [Google Scholar]
- 32.Darmawan J, World Health Organization-International League of Associations for Rheumatology Community Oriented Program for Control of Rheumatic Disease . Recommendations from the community oriented program for control of rheumatic disease for data collection for the measurement and monitoring of health in developing countries. Clin Rheumatol 2007;26:853–7. 10.1007/s10067-007-0553-x [DOI] [PubMed] [Google Scholar]
- 33.Muirden KD. Community oriented program for the control of rheumatic diseases: studies of rheumatic diseases in the developing world. Curr Opin Rheumatol 2005;17:153–6. 10.1097/01.bor.0000151402.11028.53 [DOI] [PubMed] [Google Scholar]
- 34.Bruce B, Fries JF. The health assessment questionnaire (HAQ). Clin Exp Rheumatol 2005;23:S14–8. [PubMed] [Google Scholar]
- 35.Chiesi AM. Network analysis. In: Smelser NJ, Baltes PB, eds. International encyclopedia of the social & behavioral sciences. Oxford, UK: Pergamon, 2001: 10499–502. 10.1016/B0-08-043076-7/04211-X [DOI] [Google Scholar]
- 36.Singhal A. Modern information retrieval: a brief overview. IEEE Data Eng Bull 2001;24:35–43. [Google Scholar]
- 37.Han J, Kamber M, Pei J. 2 - getting to know your data. In: Han J, Kamber M, Pei J, eds. Data mining. Third Edition. Boston: Morgan Kaufmann, 2012: 39–82. 10.1016/B978-0-12-381479-1.00002-2 [DOI] [Google Scholar]
- 38.Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. ICWSM 2009;3:361–2. 10.1609/icwsm.v3i1.13937 [DOI] [Google Scholar]
- 39.Ester M, Kriegel H-P, Sander J, et al. A density-based algorithm for discovering clusters in large spatial databases with noise. Proceedings of the Second International Conference on Knowledge Discovery and Data Mining; Portland, Oregon: AAAI Press, 1996:226–31 [Google Scholar]
- 40.Yip K, Navarro-Millán I. Racial, ethnic, and healthcare disparities in rheumatoid arthritis. Curr Opin Rheumatol 2021;33:117–21. 10.1097/BOR.0000000000000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caribe CE para AL y el . Panorama social de américa latina 2020. 2021. Available: https://www.cepal.org/es/publicaciones/46687-panorama-social-america-latina-2020
- 42.Norton S, Koduri G, Nikiphorou E, et al. A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology (Oxford) 2013;52:99–110. 10.1093/rheumatology/kes262 [DOI] [PubMed] [Google Scholar]
- 43.Radner H, Yoshida K, Frits M, et al. The impact of multimorbidity status on treatment response in rheumatoid arthritis patients initiating disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2015;54:2076–84. 10.1093/rheumatology/kev239 [DOI] [PubMed] [Google Scholar]
- 44.Nerurkar L, Siebert S, McInnes IB, et al. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry 2019;6:164–73. 10.1016/S2215-0366(18)30255-4 [DOI] [PubMed] [Google Scholar]
- 45.Ford ME, Kelly PA. Conceptualizing and categorizing race and ethnicity in health services research. Health Serv Res 2005;40:1658–75. 10.1111/j.1475-6773.2005.00449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massardo L, Pons-Estel BA, Wojdyla D, et al. Early rheumatoid arthritis in Latin America: low socioeconomic status related to high disease activity at baseline. Arthritis Care Res (Hoboken) 2012;64:1135–43. 10.1002/acr.21680 [DOI] [PubMed] [Google Scholar]
- 47.Gibson O, Lisy K, Davy C, et al. Enablers and barriers to the implementation of primary health care interventions for indigenous people with chronic diseases: a systematic review. Implement Sci 2015;10:71. 10.1186/s13012-015-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069246supp001.pdf (45.5KB, pdf)
Data Availability Statement
Data are available on reasonable request. The data are available but must be requested from the researcher IP-B through a specific application request for the use of data, which will be evaluated by all groups.