Summary
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines demonstrate reduced protection against acquisition of BA.5 subvariant but are still effective against severe disease. However, immune correlates of protection against BA.5 remain unknown. We report the immunogenicity and protective efficacy of vaccine regimens consisting of the vector-based Ad26.COV2.S vaccine and the adjuvanted spike ferritin nanoparticle (SpFN) vaccine against a high-dose, mismatched Omicron BA.5 challenge in macaques. The SpFNx3 and Ad26 + SpFNx2 regimens elicit higher antibody responses than Ad26x3, whereas the Ad26 + SpFNx2 and Ad26x3 regimens induce higher CD8 T cell responses than SpFNx3. The Ad26 + SpFNx2 regimen elicits the highest CD4 T cell responses. All three regimens suppress peak and day 4 viral loads in the respiratory tract, which correlate with both humoral and cellular immune responses. This study demonstrates that both homologous and heterologous regimens involving Ad26.COV2.S and SpFN vaccines provide robust protection against a mismatched BA.5 challenge in macaques.
Keywords: SARS-CoV-2, Omicron, BA.5, vaccine, macaque, antibody, T cell, B cell
Graphical abstract

Highlights
-
•
Ad26.COV2.S and SpFN induce robust neutralizing antibodies and T cell responses
-
•
Ad26.COV2.S and SpFN provided robust protection against mismatched BA.5 challenge
-
•
Both humoral and cellular immune responses correlated with BA.5 protection
Yu et al. demonstrate protection against SARS-CoV-2 Omicron BA.5 infection by Ad26.COV2.S and adjuvanted spike ferritin nanoparticle (SpFN) vaccines in macaques after a long-term booster.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron BA.5 has been the dominant global subvariant as a result of its high transmissibility and substantial escape from neutralizing antibody responses.1 All COVID-19 vaccines have shown reduced protection against infection with BA.5 compared with prior variants in humans, although protection against severe disease remains robust.2 It remains to be determined whether updated COVID-19 vaccines currently deploying in the US that target BA.53 will improve protective efficacy compared with the original vaccines. To the best of our knowledge, vaccine protection against BA.5 has not yet been reported in non-human primates.
The Ad26.COV2.S (Ad26) vaccine, developed by Johnson & Johnson, is a recombinant replication-incompetent adenovirus vector-based vaccine that expresses the pre-fusion stabilized SARS-CoV-2 WA1/2020 spike protein.4,5 Ad26.COV2.S immunization has been shown to induce durable antibody and CD8 T cell responses that protect both macaques and humans.4,5,6,7 In particular, Ad26.COV2.S provided 70% and 82% protection against hospitalization and intensive care unit (ICU) admission, respectively, during the Omicron surge in South Africa.8 The spike ferritin nanoparticle (SpFN) vaccine, developed by the Walter Reed Army Institute of Research (WRAIR), is a nanoparticle-based protein subunit vaccine, displaying the WA1/2020 strain of SARS-CoV-2 spike protein with S-2P modifications genetically fused to H. pylori ferritin adjuvanted with Army Liposomal Formulation QS21 (ALFQ). SpFN has been shown to induce robust antibody responses and protect macaques against SARS-CoV-2 challenge.9,10 SpFN is currently in phase 1 clinical trials (ClinicalTrials.gov: NCT04784767). In the present study, we evaluated homologous and heterologous regimens involving Ad26.COV2.S and SpFN vaccines against a high-dose, mismatched SARS-CoV-2 Omicron BA.5 challenge in macaques.
Results
Humoral immune responses
We immunized 24 adult cynomolgus macaques with homologous or heterologous vaccine regimens involving SpFN adjuvanted with ALFQ (SpFN) and Ad26.COV2.S (Ad26) (n= 6/group; Figure 1). Groups of animals received three immunizations by the intramuscular route with 5 μg SpFN or 5 × 105 vp Ad26 at weeks 0, 8, and 32. Group 1 received three SpFN immunizations (SpFNx3), group 2 was primed with Ad26 and boosted twice with SpFN (Ad26 + SpFNx2), group 3 received three Ad26 immunizations (Ad26x3), and group 4 received sham immunizations.
Figure 1.
Study schema
Vaccine groups and timing of immunization and challenge are shown.
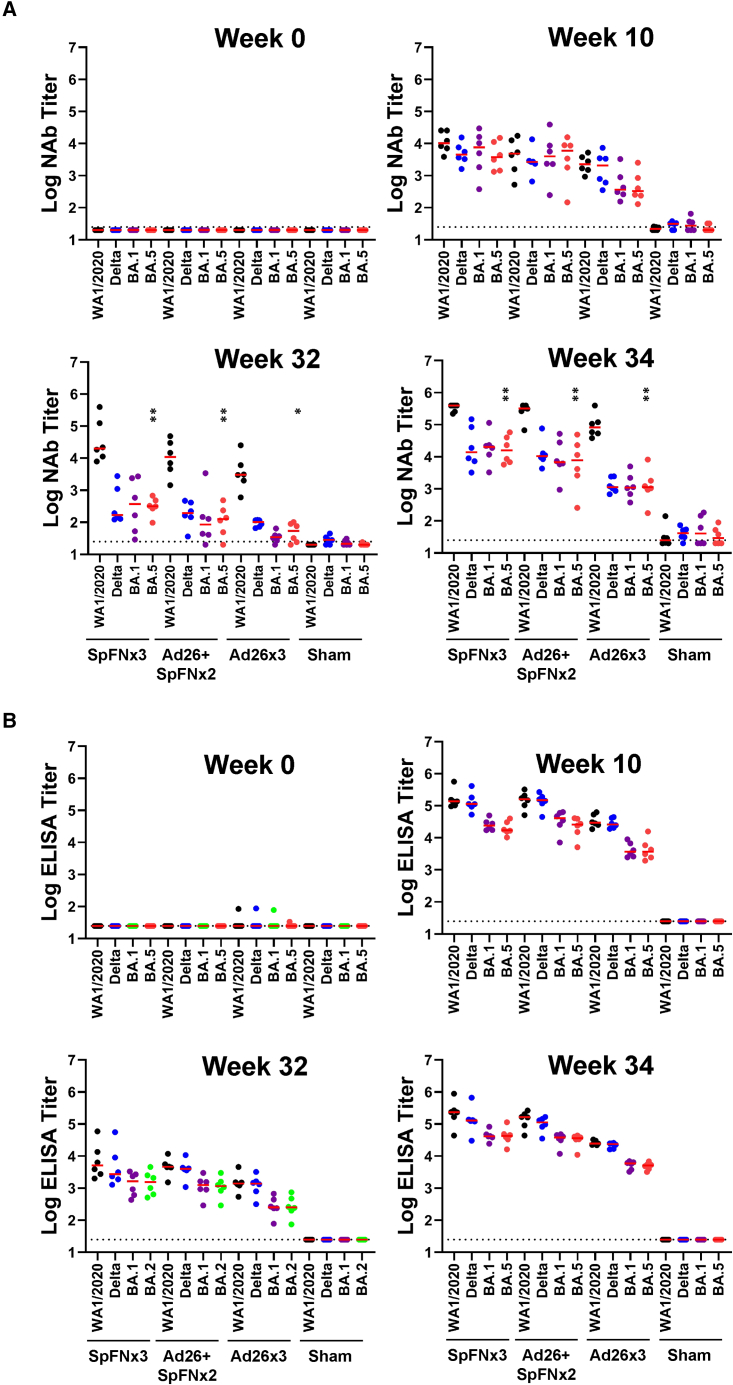
We evaluated the neutralizing antibody (NAb) titers by luciferase-based pseudovirus-NAb assay.11 NAb responses against WA1/2020, Delta, and Omicron BA.1 were induced in all animals at week 10 (Figure 2A). By week 32, Delta, BA.1, and BA.5 NAb titers in the SpFNx3-, Ad26 + SpFNx2-, and Ad26x3-vaccinated groups were still detectable (p = 0.0007 for each vaccine group compared with sham controls, two-tailed Mann-Whitney tests). At week 34, 2 weeks after the third immunization, NAb titers against all variants increased substantially (Table S1). Median WA1/2020 NAb NT50 titers were 393,660, 323,279, 82,755, and 25 in the SpFNx3, Ad26 + SpFNx2, Ad26x3, and sham groups, respectively. Median Omicron BA.5 NAb titers at week 34 were 15,856, 7,781, 1,128, and 29, respectively, reflecting a 20- to 70-fold reduction compared with WA1/2020 NAb titers (Table S1).
Figure 2.
Humoral immune responses following vaccination
Antibody responses at weeks 0 (baseline), 10 (post-first boost), 32 (at second boost), and 34 (post-second boost) following vaccination with SpFNx3, Ad26 + SpFNx2, Ad26x3, or sham (N = 24; n = 6/group).
(A) Neutralizing antibody (NAb) titers (NT50) by a luciferase-based pseudovirus neutralization assay. Dotted lines represent limits of quantitation. Medians (red bars) are shown. BA.5-specific NAbs in the vaccinated groups were compared with sham control group by two-sided Mann-Whitney tests. Only statistically significant differences ∗p < 0.05 and ∗∗p < 0.01 are presented.
(B) Receptor-binding domain (RBD)-specific endpoint binding antibody titers by ELISA. Responses were measured against the SARS-CoV-2 WA1/2020 (black), B.1.617.2 (Delta; blue), B.1.351 (Beta; purple), and BA.1 (green) variants and BA.5 (bright red). All values are plotted on a logarithmic scale. Dotted lines represent limits of quantitation. Red bars represent medians of 6 biological replicates.
Receptor-binding domain (RBD)-specific binding antibodies were assessed by ELISA. Similar to NAb titers, ELISA titers were detected in all animals at week 10, declined by week 32, and increased at week 34 after the final boost (Figure 2B; Table S1). Binding antibody titers were consistently lower against BA.5 than WA1/2020. Similar trends were observed in spike- and RBD-specific electrochemiluminescence assays (ECLAs) (Figure S1). These data show that homologous and heterologous boosting substantially increased binding and NAb responses in all groups, with BA.5-specific antibody responses 10- to 70-fold lower than WA1/2020 antibody responses (Table S1).
Cellular immune responses
We next assessed spike-specific CD8+ and CD4+ T cell responses in response to pooled peptides by multiparameter flow cytometry. At week 10, spike-specific interferon γ (IFNγ)+CD4+ T cell responses were detected in all animals, with higher responses in animals that received Ad26 + SpFN compared with those that received either two homologous doses in group 1 (SpFN) or group 3 (Ad26) (Figure 3A). At week 34, 2 weeks after the third immunization, median WA1/2020 spike-specific IFN-γ+CD4+ T cell responses were 0.330%, 1.890%, 0.098%, and 0.004% in the SpFNx3, Ad26 + SpFNx2, Ad26x3, and sham groups, respectively. BA.5 spike-specific IFN-γ+CD4+ T cell responses were similar to BA.1 responses at 0.345%, 1.550%, 0.104%, and 0.002%, respectively. BA.5 spike-specific IFN-γ+CD4+ T cell responses were 4.5- and 15-fold higher in the Ad26 + SpFNx2-vaccinated animals compared with the SpFNx3- and Ad26x3-vaccinated animals (Figure 3A).
Figure 3.
Cellular immune responses following vaccination
(A and B) T cell responses at week 4 (post-prime), 10 (post-first boost), and 34 (post-second boost) following vaccination with SpFNx3, Ad26 + SpFNx2, Ad26x3, or sham (N = 24; n = 6/group). Pooled peptide spike-specific IFN-γ (A) CD4+ T cell responses and (B) CD8+ T cell responses by intracellular cytokine staining assays. Responses were measured against the SARS-CoV-2 WA1/2020 (black), B.1.617.2 (Delta; blue), B.1.351 (Beta; purple), and BA.5 (bright red) variants. All values are plotted on a logarithmic scale. Dotted lines represent limits of quantitation. Red bars represent medians of 6 biological replicates.
WA1/2020 spike-specific IFN-γ+CD8+ T cell responses were greater at week 10 in the Ad26-primed groups compared with the SpFNx2 group (Figure 3B). At week 34, median WA1/2020 spike-specific IFNγ+CD8+ T cell responses were 0.00627%, 0.195%, 0.1055%, and 0.00878% in the SpFNx3, Ad26 + SpFNx2, Ad26x3, and sham groups, respectively. BA.5 spike-specific IFNγ+CD8+ T cell responses were 0.00773%, 0.155%, 0.0425%, and 0.00539%, respectively (Figure 3B). In all groups, WA1/2020, Delta, and Omicron CD4+ and CD8+ T cell responses were comparable, indicative of substantial T cell cross-reactivity across variants as previously reported.12,13,14,15
At weeks 10 and 34, we also assessed memory immunoglobulin G (IgG)+ B cells in peripheral blood by multiparameter flow cytometry (Figure S2). Both WA1/2020 and Omicron BA.1 RBD-specific memory B cells were induced in all vaccinated groups, with SpFNx3 and Ad26 + SpFNx2 B cell numbers trending higher than Ad26x3. BA.1 RBD-specific memory B cells among all groups were approximately 25-fold lower than WA1/2020 RBD-specific memory B cells.
Protective efficacy against BA.5 challenge
At week 36, all animals were challenged with a high dose of 2 × 106 PFU SARS-CoV-2 BA.5 by the intranasal and intratracheal routes. This challenge stock was generated in VeroE6-TMPRSS2 cells and was fully sequenced (hCOV-19/USA/COR-22-063113/2022; 1.9 × 107 PFU/mL in Vero-TMPRSS2; Mehul Suthar, Emory University). Following challenge, viral RNAs were assessed in bronchoalveolar lavage (BAL) and nasal swab (NS) samples by RT-PCR for E subgenomic RNA (sgRNA).16,17
Sham controls showed median virus levels of 4.02 (range 3.24–4.73) log sgRNA copies/mL in BAL on day 2, and these levels declined substantially by day 7 to median levels of 1.82 (range 1.70–2.60) log sgRNA copies/mL (Figure 4A). Nearly all vaccinated animals showed viral RNA in BAL, but all animals showed markedly reduced peak viral RNA on day 1 compared with sham controls and had undetectable viral RNA by days 2–4 (Figure 4A). In NSs, sham controls showed median virus levels of 4.68 (range 3.73–5.43) log sgRNA copies/swab on day 2, and these levels declined by day 7 to median levels of 3.20 (range 2.57–3.71) log sgRNA copies/swab (Figure 4B). Nearly all vaccinated animals showed low levels of viral RNA in NSs that resolved by days 4–7 (Figure 4B).
Figure 4.
Viral loads following SARS-CoV-2 Omicron BA.5 challenge
(A) Log subgenomic RNA (sgRNA) copies/mL in bronchoalveolar lavage (BAL) following SARS-CoV-2 Omicron BA.5 challenge.
(B) Log sgRNA copies/swab in nasal swabs (NSs) following SARS-CoV-2 Omicron challenge. Medians (red lines) are shown. The peak and day 4 viral loads were compared.
(C) Log sgRNA copies/mL in BAL at peak and on day 4 following SARS-CoV-2 BA.5 Omicron challenge.
(D) Log sgRNA copies/swab in NSs at peak and on day 4 following SARS-CoV-2 Omicron BA.5 challenge. Viral load values are plotted on a logarithmic scale. Dotted lines represent limits of quantitation. Medians (red bars) are shown. Vaccinated groups were compared with the sham controls by two-sided Mann-Whitney tests. ∗∗p < 0.01.
Median peak viral loads in BAL were reduced by 104-, 126-, and 28-fold in the SpFNx3, Ad26 + SpFNx2, and Ad26x3 groups, respectively, compared with sham controls (p = 0.0022, 0.0022, and 0.0022, respectively, two-tailed Mann-Whitney tests; Figure 4C). Median day 4 viral loads in BAL were also significantly reduced to undetectable levels in all groups compared with sham controls (p = 0.0022, 0.0022, and 0.0022, respectively, two-tailed Mann-Whitney tests; Figure 4C). Median peak viral loads in NSs were reduced by 132-, 123-, and 60-fold in the SpFNx3, Ad26 + SpFNx2, and Ad26x3 groups, respectively, compared with sham controls (p = 0.0022, 0.0022, and 0.0022, respectively, two-tailed Mann-Whitney tests; Figure 4D). Median day 4 viral loads in NSs were also reduced in all three groups compared with sham controls (p = 0.0022, 0.0022, and 0.0022, respectively, two-tailed Mann-Whitney tests; Figure 4D).
Correlates of protection
The diversity of immune responses prior to challenge and viral loads following challenge allowed for a detailed immune correlates analysis. BA.5-specific NAb titers and CD4 T cell responses showed a strong inverse correlation with viral RNA in BAL (Figure 5A) and NSs (Figure 5B). CD8 T cell responses and RBD-specific memory B cells also correlated with reduced sgRNA in BAL (Figure 5A) and NS (Figure 5B). Since CD8 T cell responses have been shown to contribute to vaccine protection against SARS-CoV-2 infection in non-human primates,18 we assessed the correlation of CD8 and CD4 T cell responses with NAb titers. CD4 T cell responses correlated strongly with NAb responses (R = 0.7969, p < 0.001), whereas CD8 T cell responses only correlate weakly with NAb responses (R = 0.4247; p = 0.0386) (Figure 6). These data suggest that CD4 T cell responses correlate with NAb responses, whereas CD8 T cell responses are largely independent. These data suggest that both humoral and cellular immunity likely contributed to virologic control of the BA.5 challenge.
Figure 5.
Immune correlates of protection analyses
(A and B) Correlations of week 34 NAb titers, CD8+ and CD4+ T cell responses, and memory B cell responses with peak sgRNA copies/mL in BAL (A) and peak sgRNA copies/swab in NSs (B) are shown. All values are plotted on a logarithmic scale. Correlations were assessed by two-sided Spearman rank-correlation tests. R and p values and a regression line of best fit are shown.
Figure 6.
Additional immune correlates analyses
(A) Correlations of week 34 CD8 T cell responses with NAb, CD4 T cell responses, and memory B cell responses.
(B) Correlations of week 34 NAb titers, CD4+ T cell responses, and memory B cell responses are shown. All values are plotted on a logarithmic scale. Correlations were assessed by two-sided Spearman rank-correlation tests. R and p values and a regression line of best fit are shown.
Discussion
The emergence of the Omicron BA.5 subvariant has led to renewed questions about the efficacy of current COVID-19 vaccines and the immune correlates of protection. Although clinical vaccine efficacy data against BA.5 remain limited, the ability of BA.5 to escape NAb responses and its increased transmissibility appear to reduce the efficacy of COVID-19 vaccines in humans. In this study, we show that homologous and heterologous regimens involving Ad26.COV2.S and ALFQ-adjuvanted SpFN5,9 induced robust humoral immunity with partial cross-reactivity to BA.5 and cellular immunity with substantial cross-reactivity to BA.5. All three vaccine regimens provided minimal protection against acquisition of infection, despite robust BA.5-specific NAb titers, but rapid and robust control of viral replication in the upper and lower respiratory tracts following challenge. Peak viral loads correlated with both humoral and cellular immune responses. These data suggest that COVID-19 vaccines based on the WA1/2020 strain may continue to provide robust protection against severe disease from mismatched current and future variants even if they do not protect against infection.
The three vaccine regimens studied induced phenotypically different immune responses. SpFN induced high levels of NAbs but low CD8 T cell responses, whereas Ad26.COV2.S induced lower levels of NAbs but higher CD8 T cell responses. The heterologous prime-boost regimen Ad26 + SpFNx2 induced the highest CD4 T cell responses as well as robust NAb and CD8 T cell responses. These data suggest that heterologous prime-boost (“mix-and-match”) vaccine regimens may diversify immune responses. However, all three vaccine regimens showed comparable protection with markedly lower peak viral loads and more rapid viral clearance compared with sham controls. These findings suggest that both humoral and cellular immune responses likely contribute to protection against high-dose BA.5 challenge.
Previous studies have reported potential immunogenic benefits of heterologous prime-boost regimens.19 For instance, DNA/protein administration has been reported to elicit qualitatively and quantitatively superior HIV envelope (Env)-specific antibody responses than DNA- or protein-only regimens.20 We previously reported that viral vectored vaccine prime plus protein vaccine boost generated greater anti-HIV-1 humoral and cellular immune responses in non-human primates and humans than did homologous immunization.21 Moreover, a growing number of COVID-19 vaccine studies reported that heterologous prime/boost regimens elicited greater immune responses than homologous regimens.22,23,24 In the present study, the Ad26 + SpFNx2 regimen induced higher CD4 T cell responses compared with the SpFNx3 and Ad26x3 regimens but showed comparable protection following challenge.
Despite high BA.5-specific NAb titers (median NAb titers of 15,856 in the SpFNx3 group), all animals were infected by the high-dose BA.5 challenge. These data suggest that, dependent on exposure, complete protection against infection with this highly transmissible subvariant may require exceptionally high NAb titers. Instead, rapid control of virus in the lower respiratory tract is likely critical for prevention of severe disease. Moreover, following challenge, vaccinated animals developed “hybrid immunity” with particularly robust anamnestic BA.5 neutralizing and binding antibody responses (Figure S3) and T cell responses (Figure S4).
Correlates analyses suggest that both antibody and T cell responses likely contribute to protection against BA.5. NAb titers against SARS-CoV-2 have been reported to correlate with protection against homologous SARS-CoV-2 infection in humans25,26 and non-human primates,5,27 and CD8 T cell responses have also been shown to contribute to protection.28 In contrast to NAbs, vaccine-induced T cell responses appear highly cross-reactive against SARS-CoV-2 variants12,15 and thus likely have a critical role at preventing severe disease against viral variants.29 Our data suggest that CD4 T cell responses strongly correlate with NAb responses, whereas CD8 T cell responses are largely independent although still weakly correlated. We speculate that the importance of T cell responses for vaccine protection against SARS-CoV-2 will become increasingly important as variants emerge that are progressively more antibody evasive.
Little data exist to date on vaccine protection against BA.5, especially in non-human primate models. Our data demonstrate that homologous and heterologous prime-boost regimens with SpFN and Ad26 provide robust protection against Omicron BA.5 challenge in macaques, likely by inducing partially cross-reactive and high-titer BA.5-specific antibody responses and highly cross-reactive BA.5-specific T cell responses.
Limitation of the study
One limitation of this study is that the macaque model of SARS-CoV-2 infection may not fully recapitulate the human infection, given the mild symptoms observed in macaques. Another limitation is that we used viral loads as a biomarker for immune correlates of protection analysis; however, this may not apply to protection against clinical disease, which is also mild in macaques. In addition, this study does not evaluate vaccination protection against transmission, which serves as the major concern for SARS-CoV-2 Omicron subvariants.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-human IFN-γ monoclonal antibody | BD Pharmigen | Cat # 554699; RRID: AB_2313773 |
| Rabbit polyclonal anti-human IFN-γ Biotin | U-Cytech | Cat # CT243; RRID: AB_2313773 |
| Streptavidin-alkaline phosphatase antibody | Southern Biotech | Cat # 7100-04; RRID: AB_2313773 |
| Anti-macaque IgG HRP | Nonhuman Primate Reagent Resource | Cat# 1b3-HRP: 0320K235 / 070920SC; RRID: AB_2313773 |
| Mouse anti-human CD45 BUV805 | BD Pharmingen | Cat # 742055 RRID:AB_2871344 |
| Mouse anti-human CD11c Alexa700 | Affymetrix | Cat # 56-0116-42 RRID:AB_10547281 |
| Mouse anti-human CD123 Alexa700 | FISHER/NOVUS | Cat # NB6001185AF700; RRID: AB_2313773 |
| Mouse anti-human CD7 Alexa700 | BD Pharmingen | Cat # 561603 RRID:AB_10898348 |
| Mouse anti-human CD3 APC-Cy7 | BD Pharmingen | Cat # 557757 RRID:AB_396863 |
| Mouse anti-human CD14 BV570 | BIOLEGEND | Cat # 301832 RRID:AB_2563629 |
| Mouse anti-human CD21 BV605 | BD Pharmingen | Cat # 740395 RRID:AB_2740125 |
| Mouse anti-human CD95 BV711 | BIOLEGEND | Cat # 305644 RRID:AB_2632623 |
| Mouse anti-human CD80 BV786 | BD Pharmingen | Cat # 564159 RRID:AB_2738631 |
| Mouse anti-human IgM BUV395 | BD Pharmingen | Cat # 563903 RRID:AB_2721269 |
| Mouse anti-human CD27 BUV563 | BD Pharmingen | Cat # 741366 RRID:AB_2870866 |
| Mouse anti-human IgG BUV737 | BD Pharmingen | Cat # 612819 RRID:AB_2870143 |
| Goat anti-human IgD PE | Southern Biotec | Cat # 2030-09 RRID:AB_2795630 |
| Mouse Anti-Bcl-6 PE-CF594 | BD Pharmingen | Cat # 562401; RRID: AB_2313773 |
| SARS CoV Nucleoprotein / NP mouse monoclonal antibody (Clone MM05) | Sino Biological | Cat # 40143-MM05 RRID:AB_2827977 |
| Biological samples | ||
| Non-human primate SARS-CoV2 infected lung tissue | Bioqual, Inc. | N/A |
| Bronchoalveolar lavage from Non-Human Primates | Bioqual, Inc. | N/A |
| Nasal swabs from Non-Human Primates | Bioqual, Inc. | N/A |
| EDTA, SST, Paxgene collection tubes with whole blood, from Non-Human Primates | Bioqual, Inc. | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Peptides for WA1/2020, Omicron, Delta, Beta | 21st Century Biochemicals | Custom |
| Nitroblue Tetrazolium Chloride/5-bromo-4-chloro 3 ‘indolyl phosphate p-toludine salt | Thermo Scientific | Cat #34042 |
| SARS-CoV-2 Receptor Binding Domain protein (WA1/2020, Omicron, Delta, Beta) | Aaron Schmidt Laboratory; Harvard Medical School | https://doi.org/10.1126/sciimmunol.abe0367 |
| Biotinylated SARS-CoV-2 (WA1/2020) RBD | Sino Biological | Cat # 40592-V08B-B |
| SARS-CoV-2 (WA1/2020) RBD | Sino Biological | Cat # 40592-V08B |
| SARS-CoV-2 (Omicron) RBD | Sino Biological | Cat # 40592-V08H121 |
| BV650 streptavidin | BD Pharmingen | Cat # 563855 RRID:AB_2869528 |
| Fixation Medium (Medium A) | ThermoFisher Scientific | Cat # GAS001S100 |
| Permeabilization Medium (Medium B) | ThermoFisher Scientific | Cat # GAS002S100 |
| Citrate buffer antigen retrieval | ThermoFischer Scientific | Cat # AP-9003-500 |
| Protein block | Biocare | Cat # BP974M |
| DaVinci Green Antibody diluent | Biocare | Cat # PD900M |
| Mach-2 Mouse HRP-Polymer | Biocare | Cat # MHRP520L |
| Critical commercial assays | ||
| V-PLEX SARS-CoV-2 Panel 22 Kit – Human IgG | Meso Scale Discovery | K15559U |
| V-PLEX SARS-CoV-2 Panel 23 Kit – Human IgG | Meso Scale Discovery | K15567U |
| Experimental models: Cell lines | ||
| HEK293T-hACE2 | This paper | N/A |
| Experimental models: Organisms/strains | ||
| Macaca mulatta | This paper | N/A |
| Oligonucleotides | ||
| Primer:sgLeadSARSCoV2-F Forward: CGATCTCTTGTAGATCTGTTCTC | Wolfel et al., 2020 | ThermoFisher Scientific:4448510 |
| Primer: E_Sarbeco_R Reverse: ATATTGCAGCAGTACGCACACA | Wolfel et al., 2020 | ThermoFisher Scientific:4448510 |
| Probe:E_Sarbeco_P1 : VIC-ACACT AGCCATCCTTACTGCGCTTCG-MGBNFQ |
Wolfel et al., 2020 | ThermoFisher Scientific:4448510 |
| Primer:pcDNA.T7.NdeI.Fwd Forward: TGATCTCATATGGAACCCACTGCTTACTG | This paper | Integrated DNA Technologies |
| Primer: pcDNA.T7.Rev Reverse: CACTGTGCTGGATATCTGC | This paper | Integrated DNA Technologies |
| Recombinant DNA | ||
| psPAX2 | AIDS Resource and Reagent Program | Cat# 11348 |
| pLenti-CMV Puro-Luc | Addgene | Cat# 17477 |
| pcDNA3.1-SARS CoV-2 SΔCT | This paper | N/A |
| Software and algorithms | ||
| GraphPad Prism 8.4.2 | GraphPad Software | http://www.graphpad.com/scientific-software/prism/ |
| FlowJo | BD Bioscience | http://www.flowjo.com/ |
| Diva SoftMax Pro 6.5.1 |
SoftMax Pro Software | www.moleculardevices.com/products/microplate-readers/acquisition-and-analysis-software/softmax-pro-software |
| BioRender | BioRender | https://biorender.com/ |
| Other | ||
| Ad26.COV2.S | Janssen | JNJ-78436735 |
| SpFN | WRAIR | N/A |
| RNA Standard: SARS-CoV-2 E gene subgenomic RNA (sgRNA) | This paper | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author, Dr. Dan H. Barouch (dbarouch@bidmc.harvard.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Cell lines
HEK293T (ATCC CRL-11268) and VeroE6-TMPRSS2 was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 0.5% penicillin-streptomycin and 5% fetal bovine serum (FBS). HEK293T-hACE2 cells were maintained in DMEM supplemented with 0.5% penicillin-streptomycin, 5% FBS, and 1 μg/ml puromycin (Sigma).
Animals and study design
24 outbred adult male and female cynomolgus macaques ages 4-12 years old were randomly allocated to 4 experimental groups (N=6/group; Figure 1). All animals were singly housed at Bioqual, Inc. (Rockville, MD). Groups of animals were primed with either a single immunization of 5 μg SpFN30 adjuvanted with ALFQ31 or 5x1010 vp Ad26.COV2.S at week 0. At week 8, animals were boosted with either 5 μg SpFN adjuvanted with ALFQ or 5x1010 vp Ad26.COV2.S. At week 36, all animals were challenged with 2x106 PFU SARS-CoV-2 Omicron BA.5 by the intranasal and intratracheal routes in a total volume of 2 mls. This challenge stock was generated in VeroE6-TMPRSS2 cells and was fully sequenced (hCOV-19/USA/COR-22-063113/2022; 1.9x107 PFU/mL in Vero-TMPRSS2; Mehul Suthar, Emory University). Following challenge, viral loads were assessed in bronchoalveolar lavage (BAL) and nasal swab (NS) samples by RT-PCR for E subgenomic RNA (sgRNA). Animals were sacrificed on day 9 or 10 following challenge. Immunologic and virologic assays were performed blinded. All animal studies were conducted in compliance with all relevant local, state, and federal regulations and were approved by the Bioqual Institutional Animal Care and Use Committee (IACUC).
Method details
Pseudovirus neutralizing antibody assay
The SARS-CoV-2 pseudoviruses expressing a luciferase reporter gene were used to measure pseudovirus neutralizing antibodies.11 In brief, the packaging construct psPAX2 (AIDS Resource and Reagent Program), luciferase reporter plasmid pLenti-CMV Puro-Luc (Addgene) and spike protein expressing pcDNA3.1-SARS-CoV-2 SΔCT were co-transfected into HEK293T cells (ATCC CRL_3216) with lipofectamine 2000 (ThermoFisher Scientific). Pseudoviruses of SARS-CoV-2 variants were generated by using WA1/2020 strain (Wuhan/WIV04/2019, GISAID accession ID: EPI_ISL_402124), B.1.617.2 (Delta, GISAID accession ID: EPI_ISL_2020950), Omicron BA.1 (GISAID ID: EPI_ISL_7358094.2), BA.2 (GISAID ID: EPI_ISL_6795834.2), BA.2.12.1 (GISAID ID: EPI_ISL_12003853.1), and BA.4/BA.5 (GSAID ID: EPI_ISL_12268495.2). The supernatants containing the pseudotype viruses were collected 48h after transfection; pseudotype viruses were purified by filtration with 0.45-μm filter. To determine the neutralization activity of human serum, HEK293T-hACE2 cells were seeded in 96-well tissue culture plates at a density of 1.75 × 104 cells per well overnight. Three-fold serial dilutions of heat-inactivated serum samples were prepared and mixed with 50 μl of pseudovirus. The mixture was incubated at 37°C for 1 h before adding to HEK293T-hACE2 cells. After 48 h, cells were lysed in Steady-Glo Luciferase Assay (Promega) according to the manufacturer’s instructions. SARS-CoV-2 neutralization titers were defined as the sample dilution at which a 50% reduction (NT50) in relative light units was observed relative to the average of the virus control wells.
Enzyme-linked immunosorbent assay (ELISA)
SARS-CoV-2 receptor-binding domain (RBD)-specific binding antibodies in serum were assessed by ELISA. 96-well plates were coated with 1 μg/mL of similarly produced SARS-CoV-2 WA1/2020, B.1.617.2 (Delta), B.1.351 (Beta), or Omicron BA.1 and BA.5 RBD protein in 1× Dulbecco phosphate-buffered saline (DPBS) and incubated at 4 °C overnight. After incubation, plates were washed once with wash buffer (0.05% Tween 20 in 1× DPBS) and blocked with 350 μL of casein block solution per well for 2 to 3 hours at room temperature. Following incubation, block solution was discarded, and plates were blotted dry. Serial dilutions of heat-inactivated serum diluted in Casein block were added to wells, and plates were incubated for 1 hour at room temperature, prior to 3 more washes and a 1-hour incubation with a 1μg/mL dilution of anti–human IgG horseradish peroxidase (HRP) (Invitrogen, ThermoFisher Scientific) at room temperature in the dark. Plates were washed 3 times, and 100 μL of SeraCare KPL TMB SureBlue Start solution was added to each well; plate development was halted by adding 100 μL of SeraCare KPL TMB Stop solution per well. The absorbance at 450 nm was recorded with a VersaMax microplate reader (Molecular Devices). For each sample, the ELISA end point titer was calculated using a 4-parameter logistic curve fit to calculate the reciprocal serum dilution that yields an absorbance value of 0.2 at 450 nm. Interpolated end point titers were reported.
Electrochemiluminescence assay (ECLA)
ECLA plates (MesoScale Discovery SARS-CoV-2 IgG) were designed and produced with up to 10 antigen spots in each well, including spike and RBD from multiple SARS-CoV-2 variants. The plates were blocked with 50 uL of Blocker A (1% BSA in distilled water) solution for at least 30 minutes at room temperature shaking at 700 rpm with a digital microplate shaker. During blocking the serum was diluted to 1:5,000 in Diluent 100. The calibrator curve was prepared by diluting the calibrator mixture from MSD 1:9 in Diluent 100 and then preparing a 7-step 4-fold dilution series plus a blank containing only Diluent 100. The plates were then washed 3 times with 150 μL of Wash Buffer (0.5% Tween in 1x PBS), blotted dry, and 50 μL of the diluted samples and calibration curve were added in duplicate to the plates and set to shake at 700 rpm at room temperature for at least 2 h. The plates were again washed 3 times and 50 μL of SULFO-Tagged anti-Human IgG detection antibody diluted to 1x in Diluent 100 was added to each well and incubated shaking at 700 rpm at room temperature for at least 1 h. Plates were then washed 3 times and 150 μL of MSD GOLD Read Buffer B was added to each well and the plates were read immediately after on a MESO QuickPlex SQ 120 machine. MSD titers for each sample was reported as Relative Light Units (RLU) which were calculated using the calibrator.
Intracellular cytokine staining (ICS) assay
CD4+ and CD8+ T cell responses were quantitated by pooled peptide-stimulated intracellular cytokine staining (ICS) assays. Peptide pools were 16 amino acid peptides overlapping by 11 amino acids spanning the SARS-CoV-2 WA1/2020, B.1.617.2 (Delta), BA.1 or BA.5 spike proteins (21st Century Biochemicals). 106 peripheral blood mononuclear cells well were re-suspended in 100 μL of R10 media supplemented with CD49d monoclonal antibody (1 μg/mL) and CD28 monoclonal antibody (1 μg/mL). Each sample was assessed with mock (100 μL of R10 plus 0.5% DMSO; background control), peptides (2 μg/mL), and/or 10 ng/mL phorbol myristate acetate (PMA) and 1 μg/mL ionomycin (Sigma-Aldrich) (100μL; positive control) and incubated at 37°C for 1 h. After incubation, 0.25 μL of GolgiStop and 0.25 μL of GolgiPlug in 50 μL of R10 was added to each well and incubated at 37°C for 8 h and then held at 4°C overnight. The next day, the cells were washed twice with DPBS, stained with aqua live/dead dye for 10 mins and then stained with predetermined titers of monoclonal antibodies against CD279 (clone EH12.1, BB700), CD4 (clone L200, BV711), CD27 (clone M-T271, BUV563), CD8 (clone SK1, BUV805), CD45RA (clone 5H9, APC H7) for 30 min. Cells were then washed twice with 2% FBS/DPBS buffer and incubated for 15 min with 200 μL of BD CytoFix/CytoPerm Fixation/Permeabilization solution. Cells were washed twice with 1X Perm Wash buffer (BD Perm/WashTM Buffer 10X in the CytoFix/CytoPerm Fixation/ Permeabilization kit diluted with MilliQ water and passed through 0.22μm filter) and stained with intracellularly with monoclonal antibodies against Ki67 (clone B56, BB515), IL21 (clone 3A3-N2.1, PE), CD69 (clone TP1.55.3, ECD), IL10 (clone JES3-9D7, PE CY7), IL13 (clone JES10-5A2, BV421), IL4 (clone MP4-25D2, BV605), TNF-α (clone Mab11, BV650), IL17 (clone N49-653, BV750), IFN-γ (clone B27; BUV395), IL2 (clone MQ1-17H12, BUV737), IL6 (clone MQ2-13A5, APC), and CD3 (clone SP34.2, Alexa 700) for 30 min. Cells were washed twice with 1X Perm Wash buffer and fixed with 250μL of freshly prepared 1.5% formaldehyde. Fixed cells were transferred to 96-well round bottom plate and analyzed by BD FACSymphony™ system. Data were analyzed using FlowJo v9.9.
B cell staining
Fresh PBMCs were stained with Aqua live/dead dye (Invitrogen) for 20 min, washed with 2% FBS/DPBS buffer, and suspended in 2% FBS/DPBS buffer with Fc Block (BD) for 10 min, followed by staining with monoclonal antibodies against CD45 (clone D058-1283, BUV805), CD3 (clone SP34.2 , APC-Cy7), CD7 (clone M-T701, Alexa700), CD123 (clone 6H6, Alexa700), CD11c (clone 3.9, Alexa700), CD20 (clone 2H7, PE-Cy5), IgA (goat polyclonal antibodies, APC), IgG (clone G18-145, BUV737), IgM (clone G20-127, BUV396), IgD (goat polyclonal antibodies, PE), CD80 (clone L307.4, BV786), CD95 (clone DX2, BV711), CD27 (clone M-T271, BUV563), CD21 (clone B-ly4, BV605), CD14 (clone M5E2, BV570). Cells were also stained with SARS-CoV-2 antigens including biotinylated SARS-CoV-2 RBD proteins (Sino Biological) and SARS-CoV-2 RBD proteins (Sino Biological) labeled with FITC, DyLight 405 or APC (DyLight® 405 Conjugation Kit, FITC Conjugation Kit, APC Conjugation Kit, Abcam), at 4 °C for 30 min. After staining, cells were washed twice with 2% FBS/DPBS buffer, followed by incubation with BV650 streptavidin (BD Pharmingen) for 10min, then washed twice with 2% FBS/DPBS buffer. After staining, cells were washed and fixed by 2% paraformaldehyde. All data were acquired on a BD FACSymphony flow cytometer. Subsequent analyses were performed using FlowJo software (Treestar, v10.8.1). Immunologic assays were performed blinded.
Subgenomic RT-PCR assay
SARS-CoV-2 E gene subgenomic RNA (sgRNA) was assessed by RT-PCR using primers and probes as previously described.16 A standard was generated by first synthesizing a gene fragment of the subgenomic E gene. The gene fragment was subsequently cloned into a pcDNA3.1+ expression plasmid using restriction site cloning (Integrated DNA Technologies). The insert was in vitro transcribed to RNA using the AmpliCap-Max T7 High Yield Message Maker Kit (CellScript). Log dilutions of the standard were prepared for RT-PCR assays ranging from 1x1010 copies to 1x10-1 copies. Viral loads were quantified from bronchoalveolar lavage (BAL) fluid and nasal swabs (NS). RNA extraction was performed on a QIAcube HT using the IndiSpin QIAcube HT Pathogen Kit according to manufacturer’s specifications (Qiagen). The standard dilutions and extracted RNA samples were reverse transcribed using SuperScript VILO Master Mix (Invitrogen) following the cycling conditions described by the manufacturer. A Taqman custom gene expression assay (Thermo Fisher Scientific) was designed using the sequences targeting the E gene sgRNA. The sequences for the custom assay were as follows, forward primer, sgLeadCoV2.Fwd: CGATCTCTTGTAGATCTGTTCTC, E_Sarbeco_R: ATATTGCAGCAGTACGCACACA, E_Sarbeco_P1 (probe): VIC-ACACTAGCCATCCTTACTGCGCTTCG-MGBNFQ. Reactions were carried out in duplicate for samples and standards on the QuantStudio 6 and 7 Flex Real-Time PCR Systems (Applied Biosystems) with the thermal cycling conditions: initial denaturation at 95°C for 20 seconds, then 45 cycles of 95°C for 1 second and 60°C for 20 seconds. Standard curves were used to calculate subgenomic RNA copies per ml or per swab. The quantitative assay sensitivity was determined as 50 copies per ml or per swab.
Quantification and statistical analysis
Descriptive statistics and logistic regression were performed using GraphPad Prism 9.0.0, (GraphPad Software, San Diego, California). Immunologic data were generated in duplicate and were compared by two-sided Mann-Whitney tests. Correlations were assessed by two-sided Spearman rank-correlation tests. P values less than 0.05 were considered significant.
Acknowledgments
We thank Holly Thomasson, Katelyn Steingrebe, and Elyse Teow for animal procedures. We thank Jake Yalley-Ogunro, Mehtap Cabus, and Tim Moran for preparations of immunogens and processing. This work was partially executed through a cooperative agreement between the US Department of Defense and the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (W81XWH-18-2-0040). Research was conducted under an approved animal use protocol in an AAALAC International-accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition. The views expressed are those of the authors and should not be construed to represent the positions of the US Army, the Department of Defense, or the Henry M. Jackson Foundation (HJF). We further acknowledge support from National Institutes of Health grant CA260476, the Massachusetts Consortium for Pathogen Readiness, and the Ragon Institute (DHB).
Author contributions
D.H.B. and J.Y. designed the study and reviewed all data. P.V.T., W.C.C., E.G., P.A.R., C.E.P., A.H., W.-H.C., E.J.M., N.L.M., M.G.J., and K.M. provided the SpFN immunogen; E.H., C.L., H.G., G.R.M., and M.R. provided the ALFQ adjuvant; M. Sciacca, C.W., J.L., X.H., J.M., N.P.H., N.S., K.M., C.J.-D., O.P., K.H., J.B., D.H., C.R.H., and T.M. performed the immunologic and virologic assays; M.G.L. and H.A. led the animal work with the involvement of D.L.B., N.L.M., and K.M.; M. Suthar provided the SARS-CoV-2 BA.5 challenge stock. J.Y. and D.H.B. wrote the paper with the involvement of all co-authors.
Declaration of interests
D.H.B. is a co-inventor on provisional vaccine patents licensed to Janssen (63/121,482; 63/133,969; 63/135,182) and serves as a consultant to Pfizer. M.G.J. and K.M. are co-inventors on international patent application WO/2021/178971 A1 entitled “Vaccines against SARS-CoV-2 and other coronaviruses.” M.G.J. is a co-inventor on international patent application WO/2018/081318 and a US patent 10,960,070 entitled “Pre-fusion coronavirus spike proteins and their use.” K.M.’s current affiliation is Pfizer.
Published: March 27, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101018.
Contributor Information
M. Gordon Joyce, Email: gjoyce@eidresearch.org.
Dan H. Barouch, Email: dbarouch@bidmc.harvard.edu.
Supplemental information
Data and code availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. There is no code associated with the paper. Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.
References
- 1.Hachmann N.P., Miller J., Collier A.R.Y., Ventura J.D., Yu J., Rowe M., Bondzie E.A., Powers O., Surve N., Hall K., Barouch D.H. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022;387:86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC_Data_Tracker . 2022. Rates of laboratory-confirmed COVID-19 hospitalizations by vaccination status.https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalizations-vaccination [Google Scholar]
- 3.Peter Marks C. 2022. Coronavirus (COVID-19) Update: FDA Recommends Inclusion of Omicron BA.4/5 Component for COVID-19 Vaccine Booster Doses.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-recommends-inclusion-omicron-ba45-component-covid-19-vaccine-booster [Google Scholar]
- 4.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson K.E., Le Gars M., Sadoff J., De Groot A.M., Heerwegh D., Truyers C., Atyeo C., Loos C., Chandrashekar A., McMahan K., et al. Immunogenicity of the Ad26. COV2. S vaccine for COVID-19. JAMA. 2021;325:1535–1544. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch D.H., Stephenson K.E., Sadoff J., Yu J., Chang A., Gebre M., McMahan K., Liu J., Chandrashekar A., Patel S., et al. Durable humoral and cellular immune responses 8 Months after Ad26.COV2.S vaccination. N. Engl. J. Med. 2021;385:951–953. doi: 10.1056/NEJMc2108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray G., Collie S., Goga A., Garrett N., Champion J., Seocharan I., Bamford L., Moultrie H., Bekker L.G. Effectiveness of Ad26.COV2.S and BNT162b2 vaccines against omicron variant in South Africa. N. Engl. J. Med. 2022;386:2243–2245. doi: 10.1056/NEJMc2202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce M.G., King H.A.D., Elakhal-Naouar I., Ahmed A., Peachman K.K., Macedo Cincotta C., Subra C., Chen R.E., Thomas P.V., Chen W.H., et al. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates. Sci. Transl. Med. 2022;14:eabi5735. doi: 10.1126/scitranslmed.abi5735. [DOI] [PubMed] [Google Scholar]
- 10.Johnston S.C., Ricks K.M., Lakhal-Naouar I., Jay A., Subra C., Raymond J.L., King H.A.D., Rossi F., Clements T.L., Fetterer D., et al. A SARS-CoV-2 spike ferritin nanoparticle vaccine is protective and promotes a strong immunological response in the cynomolgus macaque coronavirus disease 2019 (COVID-19) model. Vaccines. 2022;10:e50717. doi: 10.3390/vaccines10050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J., Li Z., He X., Gebre M.S., Bondzie E.A., Wan H., Jacob-Dolan C., Martinez D.R., Nkolola J.P., Baric R.S., Barouch D.H. Deletion of the SARS-CoV-2 spike cytoplasmic tail increases infectivity in pseudovirus neutralization assays. J. Virol. 2021;95:e00044–e00121. doi: 10.1128/JVI.00044-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Chandrashekar A., Sellers D., Barrett J., Jacob-Dolan C., Lifton M., McMahan K., Sciacca M., VanWyk H., Wu C., et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603:493–496. doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y., Cai C., Grifoni A., Müller T.R., Niessl J., Olofsson A., Humbert M., Hansson L., Österborg A., Bergman P., et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 2022;28:472–476. doi: 10.1038/s41591-022-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeton R., Tincho M.B., Ngomti A., Baguma R., Benede N., Suzuki A., Khan K., Cele S., Bernstein M., Karim F., et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603:488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarke A., Coelho C.H., Zhang Z., Dan J.M., Yu E.D., Methot N., Bloom N.I., Goodwin B., Phillips E., Mallal S., et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–859.e11. doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dagotto G., Mercado N.B., Martinez D.R., Hou Y.J., Nkolola J.P., Carnahan R.H., Crowe J.E., Jr., Baric R.S., Barouch D.H. Comparison of subgenomic and total RNA in SARS-CoV-2 challenged rhesus macaques. J. Virol. 2021;95:e02370–e02420. doi: 10.1128/JVI.02370-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu J., Yu J., McMahan K., Jacob-Dolan C., He X., Giffin V., Wu C., Sciacca M., Powers O., Nampanya F., et al. CD8 T cells contribute to vaccine protection against SARS-CoV-2 in macaques. Sci. Immunol. 2022;7 doi: 10.1126/sciimmunol.abq7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaine M., Wang S., Hackett A., Arthos J., Lu S. Antibody responses elicited through homologous or heterologous prime-boost DNA and protein vaccinations differ in functional activity and avidity. Vaccine. 2010;28:2999–3007. doi: 10.1016/j.vaccine.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barouch D.H., Tomaka F.L., Wegmann F., Stieh D.J., Alter G., Robb M.L., Michael N.L., Peter L., Nkolola J.P., Borducchi E.N., et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19) Lancet. 2018;392:232–243. doi: 10.1016/S0140-6736(18)31364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., Jentzsch S., Helbig E.T., Lippert L.J., Tscheak P., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir. Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collier A.R.Y., Yu J., McMahan K., Liu J., Chandrashekar A., Maron J.S., Atyeo C., Martinez D.R., Ansel J.L., Aguayo R., et al. Differential kinetics of immune responses elicited by covid-19 vaccines. N. Engl. J. Med. 2021;385:2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., Abu-Omar A., Ziegler L., Guckelmus C., Urschel R., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., Zhou H., Houchens C.R., Martins K., Jayashankar L., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., Dold C., Fuskova M., Gilbert S.C., Hirsch I., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Nkolola J.P., Liu J., Li Z., Chandrashekar A., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barouch D.H. Covid-19 vaccines — immunity, variants, boosters. N. Engl. J. Med. 2022;387:1011–1020. doi: 10.1056/NEJMra2206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joyce M.G., Chen W.H., Sankhala R.S., Hajduczki A., Thomas P.V., Choe M., Martinez E.J., Chang W.C., Peterson C.E., Morrison E.B., et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck Z., Matyas G.R., Alving C.R. Detection of liposomal cholesterol and monophosphoryl lipid A by QS-21 saponin and Limulus polyphemus amebocyte lysate. Biochim. Biophys. Acta. 2015;1848:775–780. doi: 10.1016/j.bbamem.2014.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. There is no code associated with the paper. Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.