Summary
Immunocompromised hematology patients are vulnerable to severe COVID-19 and respond poorly to vaccination. Relative deficits in immunity are, however, unclear, especially after 3 vaccine doses. We evaluated immune responses in hematology patients across three COVID-19 vaccination doses. Seropositivity was low after a first dose of BNT162b2 and ChAdOx1 (∼26%), increased to 59%–75% after a second dose, and increased to 85% after a third dose. While prototypical antibody-secreting cells (ASCs) and T follicular helper (Tfh) cell responses were elicited in healthy participants, hematology patients showed prolonged ASCs and skewed Tfh2/17 responses. Importantly, vaccine-induced expansions of spike-specific and peptide-HLA tetramer-specific CD4+/CD8+ T cells, together with their T cell receptor (TCR) repertoires, were robust in hematology patients, irrespective of B cell numbers, and comparable to healthy participants. Vaccinated patients with breakthrough infections developed higher antibody responses, while T cell responses were comparable to healthy groups. COVID-19 vaccination induces robust T cell immunity in hematology patients of varying diseases and treatments irrespective of B cell numbers and antibody response.
Keywords: SARS-CoV-2, COVID-19 vaccines, hematology, B cells, antibody-secreting cells, T follicular helper cells, CD4+ T cells, CD8+ T cells, tetramer-specific, memory T cells
Graphical abstract
Highlights
-
•
COVID-19 vaccines elicit robust SARS-CoV-2-specific T cells in hematology patients
-
•
Prevalent TCRαβ motifs occur within tetramer+ T cells in hematological patients
-
•
Conversely, perturbed antibody responses and skewing of Tfh and ASC/Tfh kinetics occur
-
•
Breakthrough infection patients have higher antibodies and comparable T cell responses
Nguyen et al. define antibody, B, and T cell responses following COVID-19 vaccination in patients with hematological malignancy that are immunocompromised and vulnerable to severe COVID-19 infection. COVID-19 vaccination induces robust T cell immunity in hematology patients with diseases and treatments impacting B cell immunity irrespective of B cell numbers and antibody responses.
Introduction
Patients with hematological malignancies such as chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) and those in the early period following hematopoietic stem cell transplantation (HCT) and chimeric antigen receptor T (CAR-T) therapy are at higher risk for viral respiratory tract infections, including COVID-19.1,2,3 Up to half of hematology patients with COVID-19 present with severe disease and require hospital admission, 15% require intensive care, and mortality rates can reach 30%–40%.1,2 Furthermore, immune suppression from underlying disease, immune reconstitution following cellular therapies (HCT, CAR-T), and ongoing treatments such as anti-CD20 monoclonal antibody therapies continue to drive risk for COVID-19 infection but concurrently impact protective responses from vaccination.4,5,6 To prevent severe SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infection in this immunosuppressed high-risk group of patients, there is an urgent need to comprehensively profile their immune response to COVID-19 vaccination to better understand the interplay between underlying disease, treatment and optimal correlations of protection so that vaccination strategies can be enhanced.
Detection of circulating SARS-CoV-2-specific receptor-binding domains (RBDs) and neutralizing antibodies are widely utilized as surrogate endpoints for evaluation of vaccine efficacy in hematology patients,7,8 yet it is unclear whether these are appropriate endpoints, especially in the setting of B cell depletion. Patients with B cell hematological malignancies such as CLL, patients in the early period following HCT and CAR-T therapy, and those receiving active therapy and B cell-depleting therapies (anti-CD20, BTK inhibitors) within 12 months have low humoral response rates to SARS-CoV-2 vaccination.7,9
Serological endpoints offer only a glimpse of the potential breadth of immune response to vaccination and may not be the best predictor of efficacy. Cellular responses reported to date relied on limited measurements of SARS-CoV-2-specific T cell responses.9,10,11 Early data suggest a lack of correlation between humoral and cellular responses in patients with hematological malignancy, and cellular responses tend to be higher in B cell-depleted patients than non-B cell-depleted patients.9,12 Furthermore, the majority of studies in hematology patients were performed following 2 COVID-19 vaccine doses.13,14,15
In hematology patients hospitalized with COVID-19, robust CD8+ T cell responses correlated with better outcomes, including among those treated with anti-CD20 therapy.16 Similarly, in patients with multiple sclerosis (MS), SARS-CoV-2-specific antibody and memory B cell responses were reduced in patients on monoclonal anti-CD20 treatment following SARS-CoV-2 mRNA vaccination; however, all patients generated robust spike-specific CD4+ and CD8+ T cell responses.17
We have previously compared and contrasted ex vivo SARS-CoV-2-specific CD8+ and CD4+ T cell responses and their T cell receptor (TCR) repertoires in SARS-CoV-2-infected children and adults,18,19,20,21 pre-pandemic children and adults,18,19 and following COVID-19 mRNA vaccination versus infection,22,23 revealing diverse TCR repertoires with prominent TCRαβ motifs that were shared between different individuals.18,20,21,22,23,24 Whether these prominent TCRαβ signatures are observed in hematology patients following COVID-19 vaccination remains to be elucidated, particularly in those with B cell malignancies or following ChAdOx1 vaccination.
We evaluate the breadth of immune responses following COVID-19 vaccination in hematology patients with diseases and treatments impacting B cell immunity, where scant data exist after the third dose. Our study shows that hematology patients who fail to seroconvert and generate memory B cell responses post-vaccination can still generate robust SARS-CoV-2-specific T cell immunity to protect against severe and fatal COVID-19.
Results
COVID-19 vaccination cohort
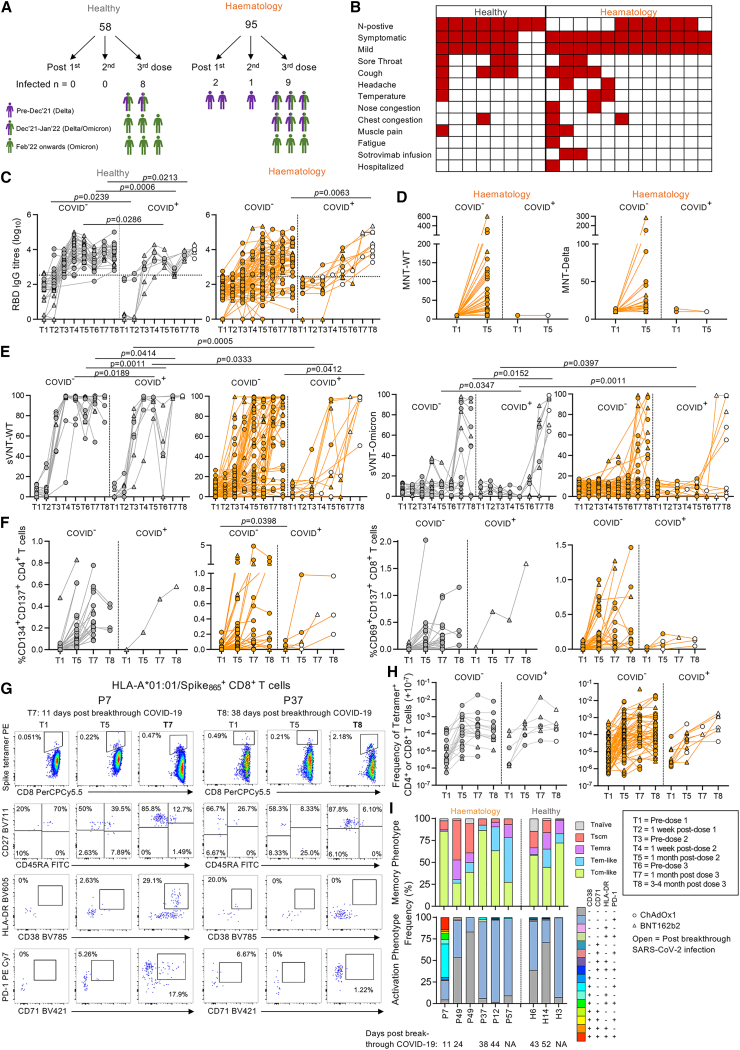
To assess immunological responses toward COVID-19 vaccines in hematology patients, 95 SARS-CoV-2-unexposed, seronegative patients were recruited between April and December 2021 (Figure 1A; Table S1). Patients were predominantly male compared with healthy individuals (71% versus 29%). For comparison, 58 healthy SARS-CoV-2-unexposed, seronegative participants were recruited during the same period (26 ChAdOx1, 32 BNT162b2). During the study, 12 patients and 8 healthy individuals had breakthrough SARS-CoV-2 infection (Table S1). ChAdOx1 and BNT162b2 vaccine responses were analyzed separately or grouped together with different symbols. Subjects labeled as having BNT162b2 or ChAdOx1 were based on their two doses.
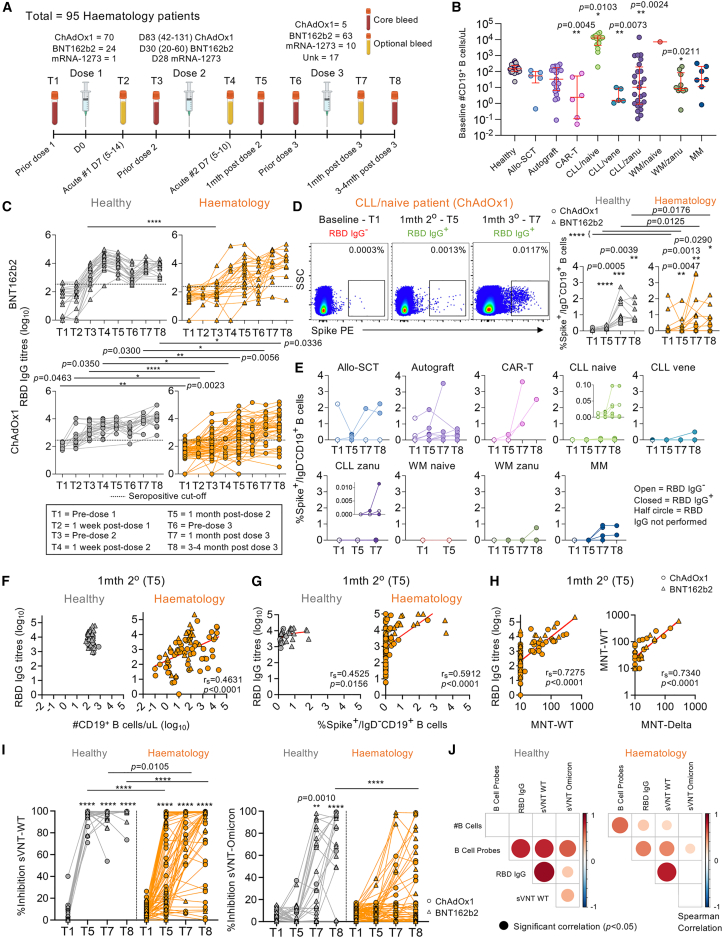
Figure 1.
RBD-specific IgG antibodies, neutralizing antibodies, and memory spike-specific B cell responses following COVID-19 vaccination
(A) Study design and sampling timepoints.
(B) B cell numbers per μL with median and interquartile range (IQR) shown (hematology n = 94; healthy n = 39). Statistical significance determined by Dunn’s multiple comparisons set on healthy versus all other disease groups.
(C) Endpoint IgG titers of ancestral RBD antibodies (hematology n = 94, 24 BNT162b2, 70 ChAdOx1; healthy n = 53, 27 BNT162b2, 26 ChAdOx1). Seropositive cutoff defined by baseline mean + 2 SD per group.
(D and E) Spike-probe staining of spike-specific memory B cells in (D) healthy (n = 16) and hematology groups (n = 64) and (E) per malignancy and treatment groups.
(F and G) Spearman’s correlation (rs) of RBD IgG titers with (F) B cell numbers (hematology n = 82; healthy n = 45) and (G) spike-specific memory B cells (hematology n = 64; healthy n = 16).
(H) rs of RBD IgG titers with ancestral MNT and ancestral versus Delta MNT (hematology n = 82).
(I) Percentage sVNT inhibition assay against wild-type (WT) ancestral and Omicron strains (haematology n = 63; healthy n = 29).
(J) rs matrix of B cell and antibody parameters at T1/T5/T7/T8.
Statistical significance determined by Wilcoxon test for time point comparisons against T1 or by Mann-Whitney for comparisons between healthy and patient time points. ∗∗∗∗p < 0.0001. Experiments were performed once for each sample. Refer to Figure S2A.
Hematology patients have reduced RBD-specific IgG antibodies, memory spike-specific B cells, and neutralizing antibodies following COVID-19 vaccination
Given the heterogeneity in the hematology cohort, blood B cell numbers varied in patients compared with healthy individuals (Figure 1B), with higher numbers in patients with CLL not on treatment but lower numbers in CAR-T patients, patients with CLL on venetoclax, patients with CLL on zanubrutinib, and patients with Waldenstrom macroglobulinemia on zanubrutinib. ELISA immunoglobulin G (IgG) antibody responses directed at RBDs corresponding to the ancestral vaccine strain (herein called RBD IgG) were lower in BNT162b2-vaccinated hematology patients compared with healthy individuals prior to the second vaccine dose (T3) but were comparable after the second and third doses (Figure 1C). RBD IgG antibody responses in ChAdOx1-vaccinated patients were lower than healthy individuals at all time points. Patient seropositivity was lower after first dose (26%, T3, n = 87), then increased to 65% 1 month after the second dose (T5, n = 82) and 87% 1 month after the third dose (T7, n = 30). Seropositivity remained stable at 84% ∼3–4 months post-third dose (T8, n = 56), indicating that a small proportion of patients, particularly patients with CLL on zanubrutinib (CLL/zanu), still did not have any robust RBD IgG antibody responses after 3 COVID-19 vaccinations. Healthy individuals were 91% seropositive after the primary vaccination and 100% seropositive after the second- (T5) and third-dose vaccinations (T7 and T8). Heterogeneity of RBD IgG antibody responses were observed when hematology patients were grouped by low, normal, or high B cell numbers or by disease/treatment group (Figures S1A–S1C).
COVID-19 vaccination induced gradual significant increases in memory spike probe-specific IgD− B cells in both healthy individuals and hematology patients of the same magnitude range (Figures 1D and S2A). However, hematology patients’ spike-specific B cell responses were significantly lower than healthy individual responses after vaccination due to some of the patients not having detectable memory spike-specific B cell responses, particularly those with B cell malignancies (CLL, Waldenstrom macroglobulinemia [WM], MM) (Figures 1D and 1E) or abnormal B cell numbers (Figure S1D). Total B cell numbers and frequencies of memory spike-specific B cells correlated with RBD IgG antibody titers in hematology patients ∼1 month after the second vaccine, but only memory spike-specific B cell frequencies correlated with antibody titers in healthy individuals given the narrower range in B cell numbers (Figures 1F and 1G). Patients’ RBD IgG antibody titers strongly correlated with microneutralizing titers (MNTs) against the ancestral strain (Figure 1H) and, to a lesser extent, the Delta strain (rs = 0.5994, p < 0.0001, data not shown), whereas ancestral and Delta MNTs were strongly correlative (Figure 1H).
Ancestral surrogate virus neutralization test (sVNT) neutralizing antibodies increased following COVID-19 vaccination in both healthy individuals and hematology patients, but increases in Omicron (B.1.1.529; BA.1-like) sVNT antibodies were only observed in healthy individuals (Figure 1I). Neutralizing sVNT responses were lower in hematology patients compared with healthy individuals after the second and third doses using the ancestral strain and were also lower ∼3–4 months after the third dose using the B.1.1.529 Omicron strain.
Correlation matrix of antibody and B cell responses showed B cell numbers only correlating with spike-specific memory B cells in hematology patients (Figure 1J). Correlations of spike-specific memory B cells with antibody responses were also stronger for healthy individuals compared with patients. Thus, antibody and B cell responses following COVID-19 vaccination were reduced compared with otherwise healthy individuals, likely due to their disease state and immunosuppressive treatment, thus explaining the low B cell numbers.
COVID-19 vaccination induces non-prototypical activation of ASCs, Tfh cells, and CD8+ T cells in hematology patients
Transient activation of CD27hiCD38hi antibody-secreting cells (ASCs) and circulating PD-1+ICOS+ CXCR5+ T follicular helper (Tfh) cells peak in the blood ∼7–10 days after SARS-CoV-2 infection using whole-blood flow cytometry assays.25,26 Induction of activated Tfh cells also occurs in the blood and draining lymph nodes following COVID-19 mRNA vaccination.22,27 Dual ASC and Tfh cell responses have also been observed following influenza virus infection28 and influenza vaccination,29 with the predominant Tfh subset being Tfh type 1 cells (Tfh1 cells; CXCR3+CCR6−). We performed whole-blood staining on a subset of 30 hematology patients that underwent intense blood sampling to capture transient acute responses following first and second COVID-19 vaccination doses (Figures 2A and S2B).
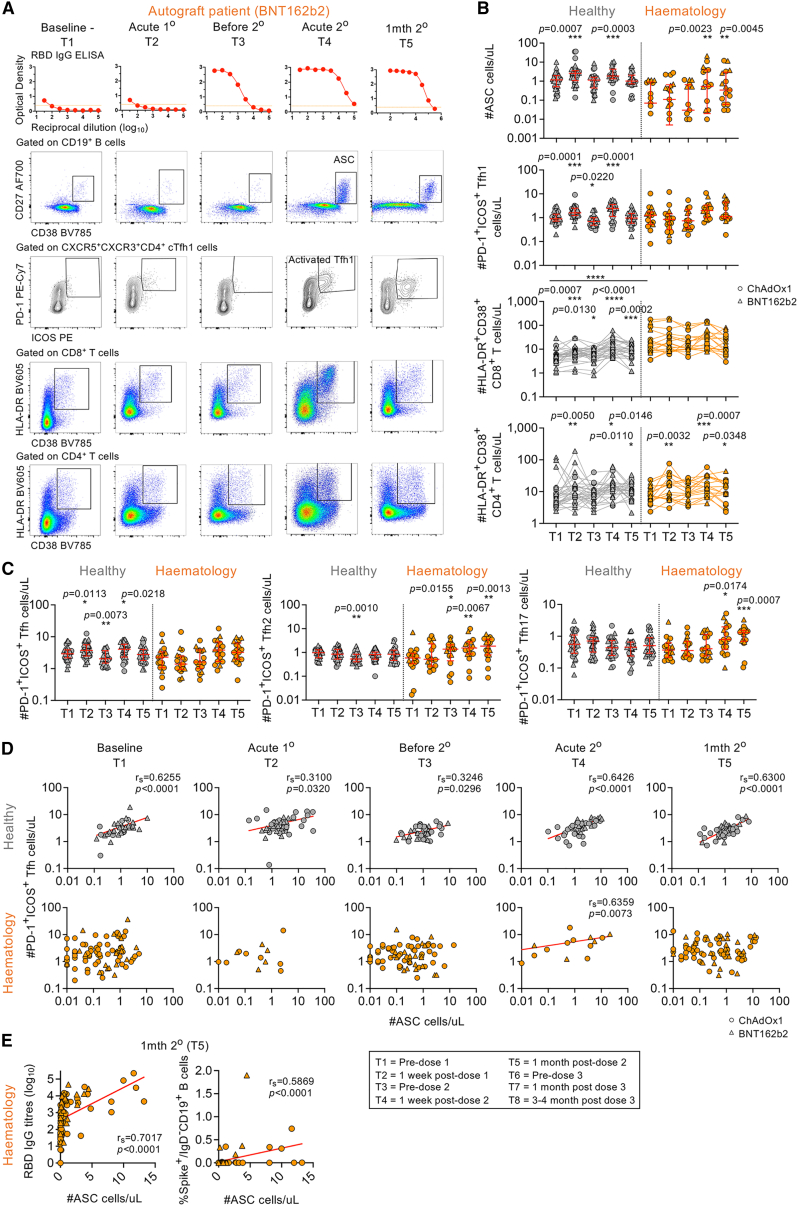
Figure 2.
Whole-blood analyses of acute ASC, Tfh cell, and activated CD8+ and CD4+ T cell responses
(A) RBD IgG ELISA titration curves and fluorescence-activated cell sorting (FACS) plots of ASCs and activated Tfh1/CD8+/CD4+ T cells. Orange dotted lines indicate endpoint titer cutoffs.
(B) Numbers of ASCs and activated Tfh1 cells and CD8+ and CD4+ T cells per μL (hematology n = 17; healthy n = 39).
(C) Numbers of Tfh, Tfh2, and Tfh17 subsets per μL.
(D) rs of ASCs and activated Tfh1 cells per time point T1–T5 (T1/T3/T5 hematology n = 94; T2/T4 hematology n = 17). Statistical significance determined by Wilcoxon test for time point comparisons against T1 (floating values) or by Mann-Whitney for comparisons between healthy and patient time points (connecting line). ∗∗∗∗p < 0.0001. Experiments were performed once for each sample. Refer to Figures S2B and S3. Zero data points not shown but included in statistics.
Healthy individuals showed prototypical vaccine-induced responses with transient increases in CD27hiCD38hi ASCs, PD-1+ICOS+-activated Tfh1 cells, and HLA-DR+CD38+-activated CD8+ and CD4+ T cells acutely observed at ∼7 days after the first (T2) and second COVID-19 vaccination doses (T4) when compared with baseline T1 levels (Figure 2B). In hematology patients, ASCs only increased at ∼7 days after the second dose (T4) and remained high at ∼1 month post-second dose (T5) (Figures 2A and 2B). PD-1+ICOS+ Tfh responses were skewed toward type 2 (Tfh2; CXCR3−CCR6−) and type 17 subsets (Tfh17; CXCR3−CCR6+), which similarly increased and remained high after the second dose (Figure 2C). Since hematology patients had higher baseline levels of activated HLA-DR+CD38+CD8+ T cells compared with healthy individuals, HLA-DR+CD38+CD8+ T responses were not further induced by two doses of COVID-19 vaccination (Figure 2B). In contrast, HLA-DR+CD38+ CD4+ T cell responses were prototypical of those observed in healthy individuals with acute transient increases after the first and second doses (Figure 2B).
Numbers of ASCs typically correlated with Tfh numbers across all time points measured (T1–T5) in healthy individuals, but this correlation was only observed at ∼7 days post-second dose (T4) in hematology patients (Figure 2D). At T4, ASCs correlated with each Tfh subset in healthy individuals, whereas Tfh1 cells, but not Tfh2 or Tfh17 cells, correlated with ASCs in hematology patients (Figure S3A), even though we did not observe significant increases in Tfh1 responses but rather observed significant increases in the other Tfh2/17 subsets. HLA-DR+CD38+ CD8+ and CD4+ T cells correlated with each other across all time points in both healthy individuals and hematology patients except for ∼7 days after the first vaccine dose (T2) in hematology patients (Figure S3B).
Overall, hematology patients needed two vaccination doses to generate ASC and Tfh responses, but there was prolonged activation of ASCs, skewing of Tfh2/17 responses, and high basal levels of activated CD8+ T cells.
Comparable spike-specific CD4+ and CD8+ T cell responses in hematology patients and healthy individuals post-COVID-19 vaccination
To evaluate COVID-19 vaccine-induced spike-specific T cell responses, we performed activation-induced marker (AIM) and intracellular cytokine staining (ICS) assays (Figures 3A, S2C, S2D, and S4A). AIM frequency of CD134+CD137+CD4+ and CD69+CD137+ CD8+ cells increased at ∼1 month post-second dose (T5) compared with baseline (T1) by a mean 32- and 31-fold for healthy individuals and a higher mean 87- and 75-fold for patients, respectively (Figure 3B), which was not due to any differences in baseline threshold levels at T1. These increases in AIM CD4+ and CD8+ T cell responses were maintained after the third dose (T7 and T8) in both healthy individuals and hematology patients. Unlike antibody and memory B cell responses, there was no difference in AIM responses between healthy individuals and hematology patients at any time point (Figure 3B).
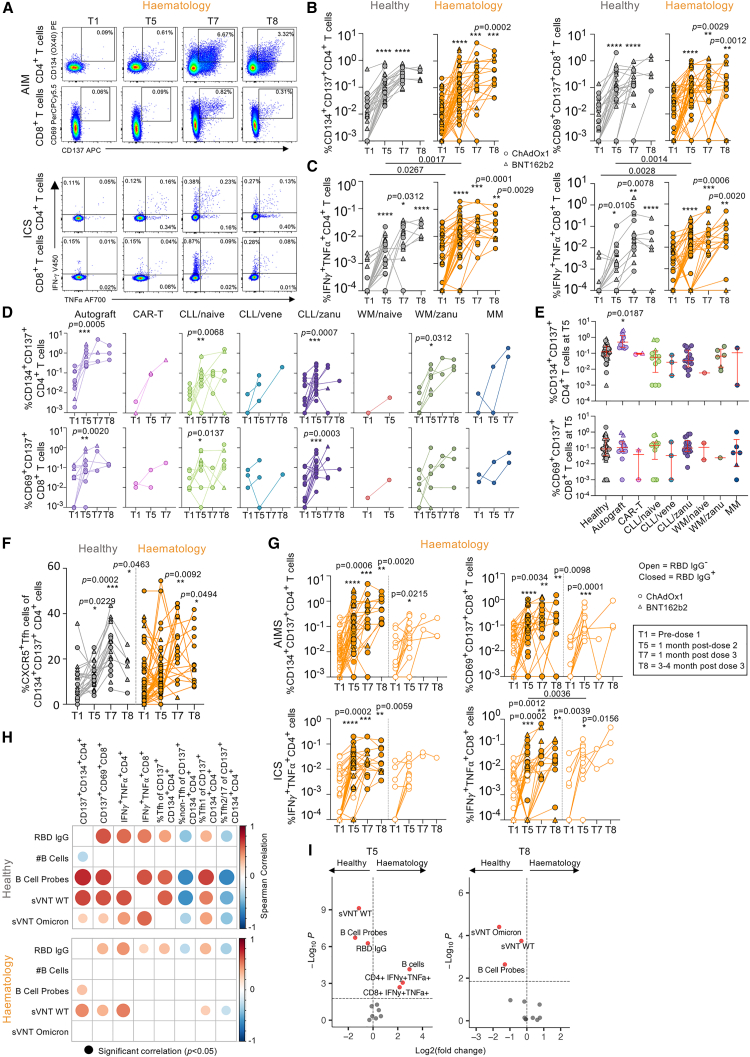
Figure 3.
Comparable spike-specific CD4+ and CD8+ T cell responses between hematology patients and healthy individuals
(A) AIM and ICS FACS plots.
(B and C) AIM (B) and ICS (C) frequencies of CD4+ and CD8+ T cells in healthy (n = 35, 23 BNT162b2, 12 ChAdOx1) and hematology groups (n = 56, 8 BNT162b2, 48 ChAdOx1).
(D and E) AIM frequency (D) per malignancy and treatment group and (E) at T5 where median and IQR are shown. Statistical significance determined by Dunn’s multiple comparisons set on healthy versus all other disease groups.
(F) CXCR5+CD4+ Tfh response of total CD134+CD137+ CD4+ T cells.
(G) AIM and ICS frequency between IgG RBD+ and RBD− patients.
(H) rs matrix of antibody/B cell and T cell responses.
(I) Volcano plots at T5 and T8 comparing healthy individuals and hematology patients.
Statistical significance determined by Wilcoxon test for time point comparisons against T1 (floating values) or by Mann-Whitney for comparisons between healthy and patient time points (connecting line). ∗∗∗∗p < 0.0001. Experiments were performed once for each sample. Refer to Figures S2C, S2D, and S4–S6.
Similarly, functional ICS assay showed increased interferon γ (IFNγ)+tumor necrosis factor α (TNF-α)+ CD4+ and CD8+ T cell responses for both healthy individuals and hematology patients at all time points post-COVID-19 vaccination (T5, T7, and T8) with mean 3- to 19-fold and 6- to 36-fold increases for healthy individuals and mean 11- to 31-fold and 10- to 44-fold for hematology patients, respectively (Figure 3C). Frequencies of IFNγ+TNF-α+ CD4+ and CD8+ T cells were higher for patients at baseline (T1) and ∼1 month post-second dose (T5) compared with healthy individuals, but frequencies were comparable after the third dose. Importantly, T cell responses were observed across disease groups by AIM (Figure 3D) or ICS assay (Figure S4B). Autograft patients had overall higher CD134+CD137+ and IFNγ+TNF-α+ CD4+ T cell frequencies at ∼1 month post-second dose (T5) when compared with healthy individuals, whereas CLL/naive patients had higher IFNγ+TNF-α+ CD4+ and CD8+ T cell frequencies (Figures 3E and S4C).
Correlations of CD4+ versus CD8+ T cell responses and of AIM versus ICS responses were more observed in healthy individuals compared with hematology patients (Figure S4D). AIM Tfh responses showed increases in CXCR5+ Tfh and CXCR5+CXCR3+ Tfh1 responses in both groups following COVID-19 vaccination, while CXCR5+CXCR3− Tfh2/17 responses decreased (Figures 3F and S5A). Based on serostatus, RBD IgG+ patients generated more robust AIM and ICS CD4+ and CD8+ T cell responses compared with RBD IgG− patients (Figure 3G). Correlations of T cell responses against antibody and B cell responses were stronger and more frequent in healthy individuals compared with hematology patients (Figure 3H).
Spike-specific CD4+ and CD8+ T cell responses were fairly similar between ChAdOx1- or BNT162b2-vaccinated patients and compared with healthy groups (Figures S5B and S5C). T cell responses were also comparable between hematology patients with low, normal, or high B cell numbers (Figure S1E). This was exemplified by volcano plot analyses combining antibodies with spike-specific memory B and T cell responses (Figures 3I and S6), where humoral responses were more enriched in healthy individuals after the second and third doses compared with hematology patients, irrespectively of whether they had low or normal to high B cells.
Following three-dose COVID-19 vaccination, hematology patients of varying diseases and immunosuppressive treatments, including those with B cell deficiencies, can still generate robust and functional spike-specific T cell responses.
SARS-CoV-2 peptide-HLA tetramer-specific T cell responses increase post-COVID-19 vaccination in hematology patients
To define SARS-CoV-2 epitope-specific CD4+ and CD8+ T cell responses, peptide-HLA tetramers combined with tetramer-associated magnetic enrichment (TAME)18,21 (Figures 4A and S2E) were used to measure directly ex vivo CD4+ T cell responses directed against the prominent DPB4/S167 epitope21,22 and CD8+ T cell responses against 6 immunodominant CD8+ T cell epitopes (A1/S865, A2/S269, A3/S378, A24/S1208, B15/S919, and B35/S321).18,19,20,23,30,31 These epitopes originated from the ancestral SARS-CoV-2 spike and are highly conserved across variant of concern (VOC) strains.21,32 B15/S919 shares similar homology and is highly cross-reactive to common cold coronaviruses (HKU1/OC43).23
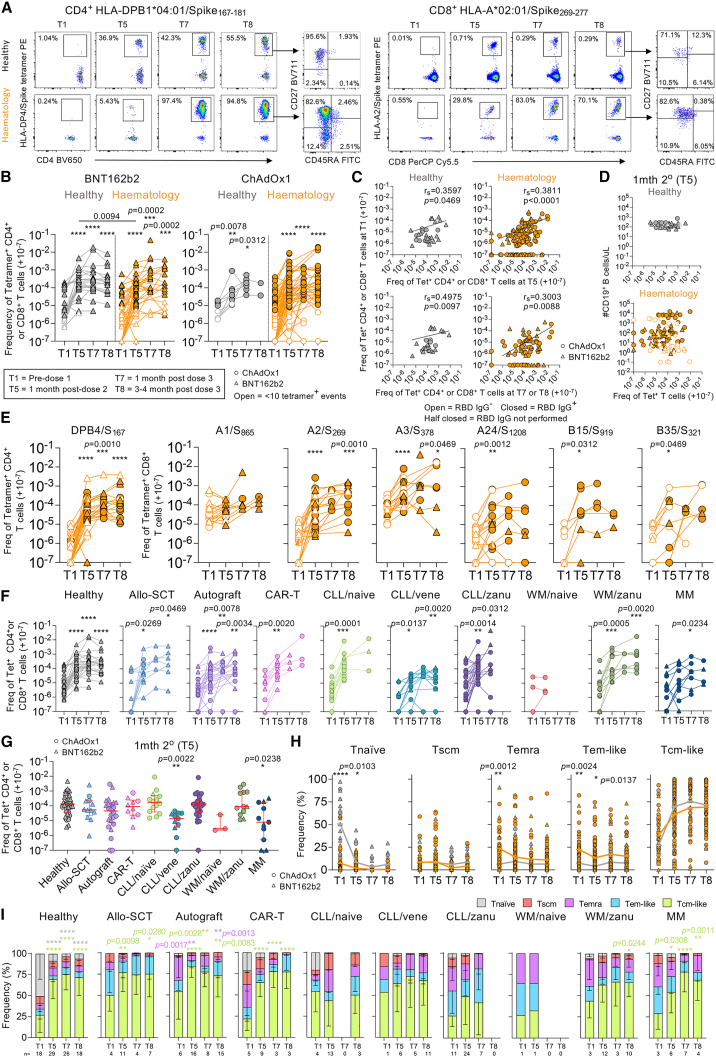
Figure 4.
COVID-19 vaccination induces expansion of SARS-CoV-2-specific tetramer+ T cell responses
(A) FACS plots of TAME-enriched CD4+ and CD8+ tetramer populations.
(B) Tetramer CD4+ and CD8+ T cell frequencies of healthy (n = 16, ChAdOx1 = 4, BNT162b2 = 12) and hematology groups (n = 54, ChAdOx1 = 35, BNT162b2 = 19) per vaccine type. Any samples with <10 tetramer+ events are shown as open symbols. 1–3 tetramer responses shown per donor.
(C) rs of T1 tetramer frequencies versus T5 or T7/T8.
(D) rs of tetramer frequencies versus B cell numbers at T5.
(E–G) Tetramer frequencies per (E) epitope, (F) malignancy and immunosuppressive treatment, or (G) at T5 where median and IQR are shown.
(H) Phenotype frequencies of tetramer+ cells from healthy (gray line) and hematology patients (orange line).
(I) Tetramer+ phenotype per malignancy and immunosuppressive treatment.
The frequency of tetramer+ cells are right shifted by 10−7 (i.e., no detected tetramer+ events displayed as 10−7). Only samples with 10 or more tetramer+ events are included for (H) and (I). Statistical significance determined by Wilcoxon test for time point comparisons against T1 (floating values); Mann-Whitney for comparisons between healthy and patient timepoints (connecting line); (G) Dunn’s multiple comparisons set on healthy versus all other disease groups; and (H) Sidak’s multiple comparison test and (I) Dunnett’s multiple comparison test for time point comparisons against T1. ∗∗∗∗p < 0.0001. Experiments were performed once for each sample. Refer to Figures S2E and S7.
Two doses of mRNA COVID-19 vaccination have previously been shown to induce robust CD4+ and CD8+ tetramer+ T cell responses.22,23,33 We observed robust expansions of pooled tetramer+ CD4+ and CD8+ T cell frequencies after the third dose with either vaccine (Figure 4B). Baseline tetramer precursor frequencies weakly correlated with tetramer responses at T5 and T7/T8 (Figure 4C). There was no correlation between tetramer-specific T cell responses and B cell numbers at T1 or T5, with weak correlations based on RBD serostatus (Figures 4D and S7A). Patients’ tetramer+ T cell frequencies increased following three-dose COVID-19 vaccination, irrespective of RBD IgG serostatus (Figure 4E) or B cell numbers (Figure S1F), similar to healthy individuals (Figure S7B). Cross-reactive B15/S919 epitope had comparable or lower baseline frequency (mean 3.26 × 10−6) than other CD8+ epitopes in patients and healthy individuals (4.47 × 10−5), suggesting no numerical cross-reactive advantage. Importantly, increase and maintenance of SARS-CoV-2-epitope T cell responses following COVID-19 vaccination were observed across all malignancy and treatment groups, except for the WM/naive group, which was small in number and not followed up with after the second dose (Figure 4F). Most patient groups generated similar response magnitudes to healthy individuals at T5, except for CLL/venetoclax (vene) patients and patients with MM (Figure 4G). CLL/vene patients also had lower tetramer+ T cell frequencies at T1 baseline and ∼3 months post-third dose (T8) (Figure S7C). Venetoclax also targets T cells, which may explain the lower responses for CLL/vene patients.
Baseline tetramer+ phenotypes differed between healthy and hematology patients but converged post-vaccination (Figures 4H and S7D). This was attributed by overall baseline CD4+/CD8+ T cell phenotypes (Figure S7E) rather than individual epitope specificities (Figure S7F). Baseline tetramer+ T cell profiles from healthy individuals were more of a prototypical CD45RA+CD27+CD95− naive-like phenotype compared with those of hematology patients, which gradually decreased post-vaccination. Tetramer+ cells from hematology patients displayed activated profiles, with elevated baseline CD45RA−CD27− T effector memory (Tem)-like and CD45RA+CD27− T effector memory CD45RA (Temra)-like features. Nevertheless, both groups had increasing CD45RA−CD27+ T central memory (Tcm)-like tetramer+ populations post-vaccination (Figure 4H), which is highly desirable for any T cell-based vaccine. Differences in phenotype profiles were also somewhat related to certain disease and treatment groups (Figure 4I).
Overall, hematology patients can generate robust SARS-CoV-2-specific T cell responses to a range of immunodominant spike epitopes following vaccination.
Hematology patients with COVID-19 breakthrough infections generate higher antibody responses than non-COVID-19 patients
During our study, 12 hematology patients had COVID-19 breakthrough infections (Figure 5). 2, 1, and 9 patients had COVID-19 after the first, second, and third vaccine doses, respectively. 3 patients were infected during the Delta period (June–July 2022), 6 during the emergence of Omicron (strain unknown, December 2021–January 2022), and 3 during the BA.1 Omicron wave (post-February 2022) where Delta was absent from circulation (Figure 5A). 8 healthy individuals had breakthrough COVID-19 after the third vaccine dose, which occurred during or after the emergence of BA.1 Omicron. All had mild COVID-19 infections, although 1 patient was hospitalized but not treated with any monoclonal antibodies, and 2 were treated with monoclonal antibody sotrovimab as outpatients (Figure 5B).
Figure 5.
Vaccine responses between non-COVID-19 and breakthrough COVID-19
(A) COVID-19 following SARS-CoV-2 vaccination.
(B) Clinical symptoms and monoclonal antibody treatment for SARS-CoV-2 breakthrough infections.
(C) Endpoint IgG titers of ancestral RBD antibodies (44 COVID− and 8 COVID+ healthy individuals; 83 COVID− and 12 COVID+ patients). Seropositive cutoff defined by baseline mean + 2 SD per group.
(D) MNT titers at T5 against WT ancestral and Delta strains (74 COVID− and 2 COVID+ patients).
(E) Percentage of inhibition by sVNT assay against WT ancestral and Omicron strains (44 COVID− and 8 COVID+ healthy individuals; 83 COVID− and 12 COVID+ patients).
(F) AIM between COVID− and COVID+ groups (33 COVID− and 1 COVID+ healthy individuals; 51 COVID− and 5 COVID+ patients).
(G) TAME-enriched FACS plots depicting tetramer and memory and activation phenotypes.
(H) Tetramer frequencies between COVID− and COVID+ groups (12 COVID− and 3 COVID+ healthy individuals; 49 COVID− and 5 COVID+ patients). 1–2 tetramer responses per donor.
(I) CD8+tetramer+ phenotypes from individuals with breakthrough COVID-19.
The frequency of tetramer+ cells has been right shifted by 10−7 (i.e., no detected tetramer+ events displayed as 10−7).
Statistical significance determined by Mann-Whitney for comparisons between COVID− and COVID+ time points (connecting line). ∗∗∗∗p < 0.0001. Experiments were performed once for each sample. Refer to Figures S8 and S9.
COVID-19+ patients had higher RBD IgG antibodies than COVID-19− patients with 100% seropositivity at T8, consistent with an anamnestic response to the infection (Figure 5C). This was still significant when the 2 COVID-19+ patients treated with sotrovimab were excluded from the analysis (p = 0.0077, data not shown). There were no differences in MNT and sVNT neutralizing responses (Figures 5D and 5E), although COVID-19+ patients had higher sVNT neutralization toward the ancestral strain at T8 compared with COVID-19− patients. Spike-specific T cell responses were comparable between COVID-19− and COVID-19+ healthy individuals and hematology patients (Figures 5F, 5G, 5H, S8A, and S8B). Tetramer+ phenotypes were comparable between breakthrough COVID-19+ patients and healthy individuals, although the activation status of one patient sampled during acute infection showed increased PD-1 expression (Figures 5I and S8C). Our hematology patient data agree with previous reports in healthy individuals,23 showing that vaccination or vaccination plus infection can elicit robust spike epitope-specific T cell responses.
We also performed tetramer staining directed against non-spike epitopes for 3 breakthrough patients and 3 healthy participants (Figure S9A). Spike, ORF1a, and nucleocapsid-specific CD8+ T cells increased in breakthrough patients, while spike CD8+ T cell frequencies were maintained in healthy participants (Figure S9B) with phenotype changes post-infection (Figure S9C) but similar activation profiles for spike and non-spike-specific CD8+ T cells (Figure S9D).
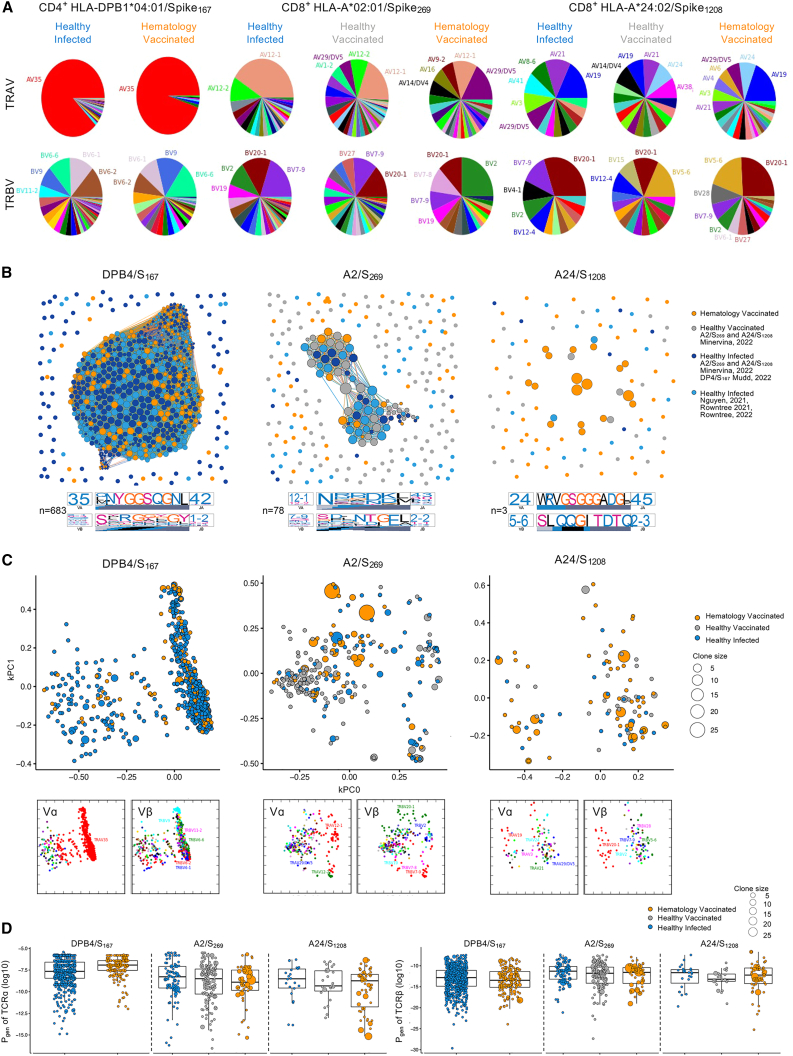
SARS-CoV-2-specific T cells in hematology patients display prominent gene segment usage
TCRαβ repertoires of dominant SARS-CoV-2 T cell epitopes have been identified as sharing similar motifs and gene usage in immunocompetent healthy individuals following SARS-CoV-2 infection18,20,21,22,23,24 and COVID-19 mRNA vaccination.23 To determine whether the molecular signatures underpinning epitope-specific T cell responses were conserved in our cohort of COVID-19-vaccinated hematology patients, we determined TCRαβ repertoires for 3 of the most prominent spike-specific CD4+ and CD8+ T cell epitopes, DPB4/S167, A2/S269, and A24/S1208, using single-cell TCRαβ multiplex-nested RT-PCR after ex vivo tetramer enrichment.34 A total of 637 paired TCRαβ clonotypes from up to 17 hematology patients were analyzed across DPB4/S167 (n = 17), A2/S269 (n = 17), and A24/S1208 (n = 12) epitopes in terms of their clonotype composition and clonal expansion. These TCRαβ repertoires were compared with previously published TCR datasets from healthy SARS-CoV-2-infected (DPB4/S167, A2/S269, and A24/S1208) and COVID-19-vaccinated (A2/S269 and A24/S1208 only) individuals.18,21,22,23
In line with previous reports,21,22 DPB4/S167-specific CD4+ T cells in hematology patients displayed a heavy bias for TRAV35/TRAJ42 gene segments (Figures 6A and S10; Table S2), which were paired with different TRBV/TRBJ genes. The A2/S269-specific TCR repertoire in healthy infected and vaccinated cohorts is generally dominated by TRAV12-1 pairing with TRBV20-1 or TRBV7-9.18,21,23,24 In contrast, TRAV29 had the highest number of different clonotypes observed in hematology patients, followed by TRAV12-1, both of which were paired with TRBV2. In addition, both TRAV29 and TRAV12-1 were only observed in 4/17 donors tested with very little clonal expansions, while some hematology patients had very large clonal expansions of non-TRAV12-1 clonotypes pairing with the common TRBV20-1 gene (donor P46: 86% TRAV8-1; donor P51: 96% TRAV13-2) (Table S2). The A24/S1208-specific CD8+ TCR repertoire was more diverse in hematology patients, which has been observed previously20; however, all 3 cohorts shared common TRAV19, TRAV21, and TRBV20-1 genes.
Figure 6.
TCR sequence similarity network identifies sharing of dominant motifs between the groups
(A) Pie charts of TRAV and TRBV usage for TCRαβ clonotypes specific to DPB4/S167 (n = 18 patients), A2/S269 (n = 18 patients), and A24/S1208 (n = 13 patients). Clonally expanded TCRs were reduced to a single data point for this analysis.
(B) TCRαβ sequence similarity networks. Each vertex is a different clonotype, and edges connect clonotypes with highly similar amino acid TCRαβ sequences (TCR distance [TCRdist] ≤ 120). Size of the vertex is proportional to number of neighbors. TCRdist sequence logos for TCRα and TCRβ chains are shown for central largest cluster for each panel. Each TCR motif depicts the V (left side) and J (right side) gene frequencies, the CDR3 amino acid sequence (middle), and the inferred rearrangement structure (bottom bars colored by source region; V-region, light gray; insertions, blue; diversity [D]-region, black; and J-region dark gray).
(C) TCR landscapes displayed using kernel principal-component analysis (PCA) projections. Vα/Vβ usage shown below.
(D) Pgen for TCRα and TCRβ. Boxplots represent the median (middle bar), 75% quantile (upper hinge), and 25% quantile (lower hinge), with whiskers extending 1.5 times the IQR.
Experiments were performed once for each sample. Refer to Figures S10 and S11.
TCR sequence similarity network for each epitope identified prominent sharing of dominant motifs between vaccinated hematology and healthy infected cohorts for DPB4/S167, followed by a smaller network for A2/S269 connecting all 3 cohorts available, and the smallest network was for A24/S1208 (Figure 6B). The DPB4/S167 network generated a common TRAV35/TRAJ42 sequence motif that represented both vaccinated hematology and healthy infected cohorts when motifs were analyzed separately per cohort (Figures 6B and S11), whereas TCRβ motifs were very different between groups. Interestingly, the TCRα and TCRβ network motifs for A2/S269 were only observed in the healthy vaccinated and infected cohorts and not in vaccinated hematology patients, which mainly comprised of TRAV29/TRAJ45 and TRBV2-TRBJ2-2 motifs. The A24/S1208 TCRα and TCRβ network motifs were only observed in vaccinated hematology patients, given the low number of sequences identified, while another TRBV5-6 motif was observed for healthy vaccinated individuals (Figures 6B and S11).
Strikingly, clonal expansions of diverse TCR clonotypes were most evident in hematology patients for A2/S269 and A24/S1208 CD8+ T cells compared with healthy infected and healthy vaccinated cohorts. However, similarly to the network analyses, these clonotypes did not cluster as closely with each other compared with the DPB4/S167 repertoire, which was closely clustered and sharing the same gene usage but had less evidence of clonal expansions from either vaccinated hematology and healthy infected cohorts (Figure 6C). Nevertheless, the probability to generate TCRs were comparable across the cohort groups for each epitope (Figure 6D).
Overall, following COVID-19 vaccination, hematology patients could generate robust SARS-CoV-2-specific T cells that shared many common TCR signatures with healthy vaccinated and infected cohorts.
Discussion
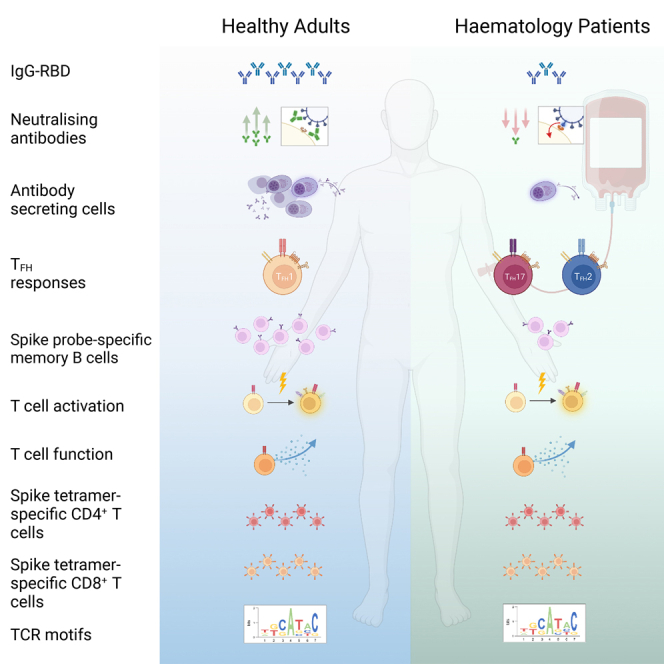
Our study provides comprehensive insights into humoral and cellular immune responses following COVID-19 vaccination in hematology patients with malignancies and treatments expected to eradicate or profoundly impair B cell function. We demonstrate that hematology patients mount effective SARS-CoV-2-specific T cell responses to COVID-19 vaccines irrespective of their B cell malignancy or B cell-depleting therapies, with which B cell numbers are greatly affected. Importantly, these T cell responses, detected directly ex vivo with peptide-HLA tetramers, are comparable to healthy individuals with respect to the magnitude, phenotype, TCRαβ diversity, clonal composition, and motifs. Conversely, B cell numbers relating to disease status markedly impacted SARS-CoV-2-specific antibody levels and memory spike-specific B cell responses, together with ASC and Tfh skewing.
Our cohort is representative of a hematological malignancy population with B cell deficiencies arising from disease and/or therapies. Apart from patients with CLL, WM, and myeloma, other hematological malignancies and prior treatments are well represented in our post-cellular therapies cohort (allogeneic transplant, autologous transplant, and CAR-T therapy), including patients with non-Hodgkin’s lymphoma treated with prior anti-CD20 antibody exposure. While our patient group is heterogeneous, breakdown of immune response is provided by individual disease/treatment group in the individual figures to allow a reader to consider/assess responses by a particular group of interest (Figure S12).
Consistent with previous studies, hematology patients had lower seroconversion rates following the first and second doses compared with healthy individuals. We highlight that the third dose observed close to 90% seropositivity by RBD IgG in hematology patients, which is an excellent outcome for immunocompromised patients and comparable to the seropositivity rates observed after 2 doses in healthy individuals.
RBD-specific IgG antibody responses toward two doses of ChAdOx1 vaccine in hematology patients were lower than BNT162b2-vaccinated patients, when compared with healthy individuals, and remained lower after the third mRNA dose. This could partially be explained by the age difference, where ChAdOx1-vaccinated patients were older than BNT162b2-vaccinated patients or healthy individuals. Another explanation could be related to the type of vaccine, as it has been reported that two-dose ChAdOx1 vaccination provides lower protective efficacy (∼60%) in terms of neutralizing antibodies compared with BNT162b2 (∼90%).35 A recent study comparing antibody and memory B cell responses following two-dose ChAdOx1, two-dose mRNA, or combined ChAdOx1/mRNA vaccination also observed inferior antibody responses with the ChAdOx1 vaccine.36 Importantly, there were no differences in T cell responses between vaccine type.
Spike-specific CD4+ and CD8+ T cell responses following COVID-19 vaccination were also measured by AIM and ICS assays, as previously used in other SARS-CoV-2 infection studies.37,38 The skewing of PD-1+ICOS+-activated Tfh2/Tfh17 in the whole-blood assay aligned with our AIM data, where the majority of the AIM Tfh response was made up of Tfh2/Tfh17 cells rather than Tfh1 cells. Our data support Apostolidis et al.’s study17 where all patients with MS on anti-CD20 monoclonal antibody treatment (n = 20) generated robust spike-specific CD4+ and CD8+ T cell responses following BNT162b2 or mRNA-1273 mRNA vaccination in the absence of B cells.
Two doses of mRNA COVID-19 vaccination can induce robust CD4+ and CD8+ tetramer+ T cell responses.22,23,33 Here, both hematology patients and healthy individuals can generate robust CD4+ and CD8+ T cell responses to 7 immunodominant spike-specific epitopes after two doses of ChAdOx1 or BNT162b2 vaccines and after the third dose. Importantly, patients lacking RBD-specific IgG antibodies could still generate robust epitope-specific T cell responses. Furthermore, TCR repertoires from hematology patients shared common TCR signatures with healthy vaccinated and infected cohorts.
Breakthrough SARS-CoV-2 infections are increasingly becoming more common in the vaccinated community. Although SARS-CoV-2-infected patients had higher RBD-specific IgG antibody responses, T cell responses were indistinguishable between infected and non-infected individuals in both patient and healthy groups, which aligns with previous reports in healthy individuals,23 showing that vaccination alone or vaccination followed by subsequent infection can elicit comparable spike epitope-specific T cell responses.
Overall, our study shows that hematology patients who fail to seroconvert and generate memory B cell responses post-vaccination can nevertheless generate robust SARS-CoV-2-specific T cell immunity. Our findings are particularly relevant for reducing disease severity in hematology patients who acquire breakthrough infections since robust CD8+ T cell responses correlate with better outcomes in hematology patients hospitalized with COVID-19, including those treated with anti-CD20 therapy, while B cells had no impact on survival.16 Our data also support development of vaccines targeted to immunocompromised patients, such as those that have shown to induce potent and prolonged T cell responses to multiple SARS-CoV-2 antigens in healthy individuals.39 Therefore, COVID-19 vaccination can still be immunogenic, especially with respect to SARS-CoV-2-specific CD4+ and CD8+ T cell responses, in hematology patients of varying diseases and treatment.
Limitations of the study
Our patient cohort is heterogeneous, and so we were not always powered to statistically analyze the data per disease, treatment, or vaccine group for all assays. Some assays were not performed for all patients, but patient numbers per assay are included in figure legends. Larger prospective studies are needed to demonstrate protection against severe and fatal disease with patient stratification based on antibody/B cell and T cell responses. SARS-CoV-2 infections occurred after 1/2/3 vaccinations in patients but only after 3 vaccinations in healthy individuals, although numbers are small. ChAdOx1-vaccinated patients were older than BNT162b2-vaccinated or healthy individuals.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD71 M-A712 BV421 | BD Biosciences | Cat#562995; RRID: AB_2737939 |
| CD4 SK3 BV650 | BD Biosciences | Cat#563875; RRID: AB_2744425 |
| CD27 L128 BV711 | BD Biosciences | Cat#563167; RRID: AB_2738042 |
| CD38 HIT2 BV786 | BD Biosciences | Cat#563964; RRID: AB_2738515 |
| CCR7 150503 AF700 | BD Biosciences | Cat#561143; RRID: AB_10562031 |
| CD14 MΦP9 APC-H7 | BD Biosciences | Cat#560180; RRID: AB_1645464 |
| CD19 SJ25C1 APC-H7 | BD Biosciences | Cat#560177; RRID: AB_1645470 |
| CD45RA HI100 FITC | BD Biosciences | Cat#555488; RRID: AB_395879 |
| CD8a SK1 PerCP-Cy5.5 | BD Pharmingen | Cat#565310; RRID: AB_2687497 |
| CD95 DX2 PE-CF594 | BD Biosciences | Cat#562395; RRID: AB_11153666 |
| PD-1 EH12.1 PE-Cy7 | BD Biosciences | Cat#561272; RRID: AB_10611585 |
| CD3 OKT3 BV510 | BioLegend | Cat#317332; RRID: AB_2561943 |
| HLA-DR L243 BV605 | BioLegend | Cat#307640; RRID: AB_2561913 |
| CXCR5 RF8B2 BV421 | BD Biosciences | Cat#562747; RRID: AB_2737766 |
| CD19 SJ25C1 BV510 | BD BioSciences | Cat#562947; RRID: AB_2737912 |
| CCR6 11A9 BV650 | BD Biosciences | Cat#563922; RRID: AB_2738488 |
| CD20 2H7 BV711 | BD Biosciences | Cat#563126; RRID: AB_2313579 |
| CD27 M-T271 AF700 | BD Biosciences | Cat#560611; RRID: AB_1727454 |
| CD4 RPA-T4 APC-H7 | BD Biosciences | Cat#560158; RRID: AB_1645478 |
| CD3 UCHT1 PE-CF594 | BD Biosciences | Cat#562280; RRID: AB_11153674 |
| CXCR3 (CD183) 1C6 APC | BD Biosciences | Cat#550967; RRID: AB_398481 |
| CD8 SK1 FITC | BD Biosciences | Cat#555634; RRID: AB_395996 |
| CD45 HI30 PerCPCy5.5 | BD Biosciences | Cat#340953; RRID: AB_400194 |
| ICOS (CD278) DX29 PE | BD Biosciences | Cat#557802; RRID: AB_396878 |
| CD8a SK1 BV605 | BD Biosciences | Cat#564116; RRID: AB_2869551 |
| CD25 2A3 BV711 | BD Biosciences | Cat#563159; RRID: AB_2738037 |
| CXCR3 G025H7 BV785 | BioLegend | Cat#353738; RRID: AB_2565924 |
| APC (4-1BB) 4B4-1 CD137 | BioLegend | Cat#309810; RRID: AB_830672 |
| CD69 FN50 PerCPCy5.5 | BioLegend | Cat#310926; RRID: AB_2074956 |
| CD134 L106 PE | BD Biosciences | Cat#340420; RRID: AB_400027 |
| CD45RA L48 PECy7 | BD Biosciences | Cat#337167; RRID: AB_647424 |
| CD19 J4.119 ECD | Beckman Coulter | Cat#IM2708U; RRID:AB_130854 |
| IgM G20-127 BUV395 | BD Biosciences | Cat#563903; RRID:AB_2721269 |
| CD21 B-ly4 BUV737 | BD Biosciences | Cat#564437; RRID:AB_2738807 |
| IgD IA6-2 PE-Cy7 | BD Biosciences | Cat#561314; RRID:AB_10642457 |
| IgG G18-145 BV786 | BD Biosciences | Cat#564230; RRID:AB_2738684 |
| CD27 O323 BV605 | BioLegend | Cat#302829; RRID:AB_11204431 |
| Streptavidin PE | BD Biosciences | Cat#554061, RRID:AB_10053328 |
| Streptavidin APC | BD Biosciences | Cat#554067, RRID:AB_10050396 |
| Peroxidase AffiniPure goat anti-human IgG, Fcγ fragment specific | Jackson ImmunoResearch | Cat#109-035-098; RRID: AB_2337586 |
| Biological samples | ||
| Blood samples (peripheral blood mononuclear cells (PBMCs) and plasma samples) from COVID-19-vaccinated adult haematological malignancy patients and healthy control individuals | The Royal Melbourne Hospital, The Austin Hospital, St Vincent’s Hospital, and The Peter McCallum Cancer Center | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| 3,3′,5,5′-Tetramethylbenzidine (TMB) Liquid Substrate System for ELISA, peroxidase substrate | Sigma | Cat#T0440-1L |
| Alkaline phosphatase yellow (pNPP) liquid substrate for ELISA | Sigma | Cat#P7998-100ML |
| Pierce High Sensitivity Streptavidin-HRP | Thermo Fisher Scientific | Cat#21130 |
| SARS-CoV-2 RBD protein | Amanat et al.,40 | N/A |
| SARS-CoV-2 Spike protein | Juno et al.,37 | N/A |
| SARS-CoV-2 peptides – A1/ORF1a1637 TTDPSFLGRY; A1/S865-873 LTDEMIAQY; A2/S269 YLQPRTFLL; A3/N361 KTFPPTEPK; A3/S378 KTFPPTEPK; A24/S1208 QYIKWPWYI; B7/N105-113 SPRWYFYYL; B15/S919-927 NQKLIANQF; B35/S321-329 QPTESIVRF; and DPB4/S167 TFEYVSQPFLMDLE | GenScript | N/A |
| HLA-A∗01:01/S865 monomer (SARS-CoV-2, S865, LTDEMIAQY) | Peptide sequence30, monomer [Rossjohn Laboratory] | N/A |
| HLA-A∗01:01/ORF1a1637 monomer (SARS-CoV-2, ORF1a1637, TTDPSFLGRY) | Peptide sequence41, monomer21 | N/A |
| HLA-A∗03:01/S378 monomer (SARS-CoV-2, S378, KCYGVSPTK) | Peptide sequence41, monomer [Rossjohn Laboratory] | N/A |
| HLA-A∗03:01/N361 monomer (SARS-CoV-2, N361, KTFPPTEPK) | Peptide sequence42, monomer21 | N/A |
| HLA-B∗07:02/N105 monomer (SARS-CoV-2, N105, SPRWYFYYL) | Peptide sequence42, monomer18 | N/A |
| HLA-B∗15:01/S919 monomer (SARS-CoV-2, S919, NQKLIANQF) | Peptide sequence23, monomer [Rossjohn Laboratory] | N/A |
| HLA-A∗02:01/S269 monomer (SARS-CoV-2, S269, YLQPRTFLL) | Peptide sequence/monomer19 | N/A |
| HLA-B∗35:01/S321 monomer (SARS-CoV-2, S321, QPTESIVRF) | Peptide sequence33, monomer [Rossjohn Laboratory] | N/A |
| HLA-A∗24:02/S1208 monomer (SARS-CoV-2, S1208, QYIKWPWYI) | Peptide sequence31, monomer20 | N/A |
| HLA-DPA1∗01:03/DPB1∗04:01/S167 monomer (SARS-CoV-2, S167, TFEYVSQPFLMDLE) | Peptide sequence/monomer22 | N/A |
| Software and algorithms | ||
| R v4.2.1 | R Core Team,43 | https://cran.r-project.org |
| conga package | Schattgen et al.44 | https://github.com/phbradley/conga |
| igraph R package v1.3.2 | Csárdi and Nepusz45 | https://igraph.org/r/ |
| TCRdist pipeline | Dash et al.46 | https://github.com/phbradley/tcr-dist |
| Gephi v0.9.7 | Jacomy et al.47 | https://gephi.org/ |
| Corrplot v0.92 | Wei and Simko48 | https://github.com/taiyun/corrplot |
| rstatix pacakage v0.7.0 | Kassambara49 | https://CRAN.R-project.org/package=rstatix |
| EnhancedVolcano v1.14.0 | Blighe et al.50 | https://github.com/kevinblighe/EnhancedVolcano |
| FlowJo v10.5.3 | FlowJo | https://www.flowjo.com |
| Prism v8.3.1 or v9.1.0 | GraphPad | https://www.graphpad.com |
| BD FACS Diva v8.0.1 | BD Biosciences | https://www.bdbiosciences.com/en-us/instruments/research-instruments/research-software/flow-cytometry-acquisition/facsdiva-software |
| Other | ||
| Anti-PE Micro-Beads | Miltenyi Biotec | Cat# 130-048-801, RRID:AB_244373 |
| Anti-APC Micro-Beads | Miltenyi Biotec | Cat# 130-090-855, RRID:AB_244367 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Katherine Kedzierska (kkedz@unimelb.edu.au).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Victorian blood cancer patients and healthy volunteers that were scheduled for the COVID-19 vaccine, as part of Australia’s COVID-19 vaccine rollout in 2021, were recruited to the study. Patients with various haematological malignancies were enrolled at the Peter MacCallum Cancer Centre or St Vincent’s Hospital following ethics approval by the Peter MacCallum Cancer Centre Human Research Ethics Committee (HREC/74271/PMCC-2021, HREC/74260/PMCC-2021). Healthy volunteers were enrolled at the Royal Melbourne Hospital or the Austin Hospital with approvals from Melbourne Health (HREC/68355/MH-2020) and Austin Health (HREC/73256/Austin-2021), respectively. Human ethics was also approved by the University of Melbourne HREC (21817, 21711, 21626, 21560, 13344). All participants provided written informed consent. Patient and healthy cohort demographics are summarised in Table S1. Clinical information and limited immune data have been described for a subset of CLL patients.51
Participants were vaccinated with 2 doses of the BNT162b2 Comirnaty (Pfizer) vaccine scheduled ∼3 weeks apart or the ChAdOx1 (AstraZeneca) vaccine scheduled ∼8–12 weeks apart. One patient also received 2 doses of the mRNA-1273 (Moderna) vaccine. Heparinised peripheral blood and serum were collected prior to vaccination (T1), ∼1 week following the first dose (T2, optional bleed), just prior to the second dose (T3), ∼1 week following the second dose (T4, optional bleed), and ∼1 month (T5) following the second dose. Additionally, a subset of patients were bled prior to receiving a third dose of BNT162b2 Comirnaty or mRNA-1273 (T6), 1 month following the third dose (T7) and 3–4 months following the third dose (T8). Healthy vaccinated adults were recruited as controls. PBMCs were isolated by Ficoll-Paque separation for cellular assays, plasma was collected for measuring antibodies, serum for microneutralisation assays and DNA isolated from granulocytes for HLA typing by VTIS (Melbourne, Australia), essentially as described.28
The study was conducted in compliance with the conditions of the ethics committee approval, the NHMRC National Statement on ethical Conduct in Human Research (2007) and the Note for Guidance on Good Clinical Practice (CPMP/ICH-135/95).
Method details
Cellular activation in whole blood
The kinetics of ASCs and activation of Tfh/CD8+/CD4+ T cell subsets were measured at T1-T5 timepoints by directly staining whole blood with antibodies for flow cytometry, essentially as described.25,28,52
Assessment of SARS-CoV-2-specific antibodies and memory B cells
Spike and nucleocapsid antibodies were measured using Elecsys Anti-SARS-CoV-2 kit and Roche e601 analyser according to manufacturer’s instructions. Plasma antibodies against wildtype SARS-CoV-2 RBD protein (vaccine strain) were assessed by IgG ELISA as previously described in detail.18,40,53 MNT activity of serum samples (T1 and T5 only) was assessed as previously described with the wildtype and delta strains.26,37,54 The sVNT assay was performed with wildtype and B.1.1.529 omicron (Genscript Z03730) strains essentially as described.53 Spike-specific B cell responses from the vaccine strain were measured on thawed PBMCs or TAME-flow through fractions. Cells were stained with wildtype Spike recombinant probes conjugated to PE, fixed and acquired on a BD LSRII Fortessa, essentially as described.18,37
24-H stimulation with spike overlapping peptide pools
Thawed PBMCs were plated into a 96-well plate at 1e6 PBMCs/well. For AIM assay, cells were stimulated in complete-RPMI with 10 μg/ml SARS-CoV-2 Spike peptide pool (181 peptides, 0.06 μg/ml per peptide; BEI Resources, NIAID, NIH, SARS-Related Coronavirus 2 Spike (S) Glycoprotein, NR-52402) or DMSO (0.1%; Sigma), as a negative control, and cultured at 37°C/5% CO2 for 24 h. Cells were washed and stained with CXCR5-BV421 (562747; BD Biosciences), CD3-BV510 (317332; BioLegend), CD8-BV605 (564116; BD Biosciences), CD4-BV650 (563875; BD Biosciences), CD25-BV711 (563159; BD Biosciences), CXCR3-BV786 (353738; BD Biosciences), CD137-APC (309810; BioLegend), CD27-AF700 (560611; BD Biosciences), CD14/CD19-APC-H7 (560180/560252; BD Biosciences), Live/Dead NIR (L34976; Invitrogen), CD69-PerCPCy5.5 (310925; BioLegend), CD134-PE (340420; BD Biosciences), CD95-PE-CF594 (562395; BD Biosciences), CD45RA-PeCy7 (337167; BD Biosciences) before fixing with 1% PFA.
For ICS, cells were stimulated in complete-RPMI with 100 μg/mL overlapping Spike peptide pool (181 peptides, 0.6 μg/ml per peptide; BEI Resources, NR-52402) or DMSO (1%), as a negative control, in combination with anti-CD28/CD49d (1:100, 347690; BD Biosciences) and 10U/ml IL-2 (11147528001; Roche), with Brefeldin A (½000 dilution; 555029; BD Biosciences) added after 6 h. Following further 18 h of the stimulation, cells were washed twice with MACS buffer (PBS/0.5% BSA, 2mM EDTA), then stained with surface antibodies: CD3-BV510 (317332; BioLegend), CD4-BV650 (563875; BD Biosciences), CD8-PerCPCy5.5 (565310; BD Biosciences) and Live/Dead NIR (L34976; Invitrogen) for 30 min on ice. Cells were washed twice, then fixed using the BD Cytofix/Cytoperm kit (554723; BD Biosciences) according to the manufacturer’s instructions, washed twice and intracellularly stained with IFNγ-v450 (560371; BD Biosciences), MIP-1β-APC (560656; BD Biosciences) and TNFα-AF700 (557996; BD Biosciences) for 30 min on ice. Following two further washes, lymphocytes were resuspended in MACS buffer and acquisition was on an LSRII Fortessa. Data were analyzed using FlowJo v10. Values obtained for PBMCs cultured with DMSO under the same conditions (negative controls) were subtracted from peptide-stimulated values. As a negative control, PBMCs cultured with DMSO also had IL-2, thus this control accounts for any background cytokine production triggered by IL-2 by CD4+ and CD8+ T cells.
Spike and non-spike epitope-specific tetramer+ T cell responses
HLA class I tetramers HLA-A∗01:01/S86534 (LTDEMIAQY), HLA-A∗01:01/ORF1a163741 (TTDPSFLGRY), HLA-A∗02:01/S26919 (YLQPRTFLL), HLA-A∗03:01/S37841 (KCYGVSPTK), HLA-A∗03:01/N36142 (KTFPPTEPK), HLA-A∗24:02/S120835 (QYIKWPWYI), HLA-B∗07:02/N10542 (SPRWYFYYL), HLA-B∗15:01/S91923 (NQKLIANQF) and HLA-B∗35:01/S32133 (QPTESIVRF) were generated by Rossjohn laboratory and validated as previously described.18,19,20 HLA class II tetramer HLA-DPA1∗01:03/DPB1∗04:01/S167 (TFEYVSQPFLMDLE) was generated by Rossjohn laboratory and validated as previously described.22
Cryopreserved PBMCs (5-10x106) underwent tetramer-associated magnetic enrichment (TAME) following staining with a class I and/or DP4 class II Spike tetramer on PE and/or another class I tetramer on APC as described.18 Class I and class II tetramers on PE were exclusively stained on CD8+ or CD4+ T cells, respectively, with minimal to zero non-specific binding. Flow-through fractions were cryopreserved for Spike-specific B cell probe analysis, as previously described21 which were negative for tetramer+ cells. Samples were acquired on an LSRII Fortessa using the software BD FACS DIVA v8.0.1 and flow cytometry data were analyzed using FlowJo v10 software.
Enriched HLA-A∗02:01/S269, HLA-A∗24:02/S1208 and HLA-DPB1∗04:01/S167 tetramer+ T cells were indexed single-cell sorted on a BD FACSAria III for TCR analysis essentially as described.18,21 CDR3α and CDR3β regions from single cells were amplified using multiplex-nested RT-PCR18,34 analyzed by IMGT/V-QUEST.
As in our study, we had a limited number of PBMCs from hematology patients (and healthy controls), our baseline T1 tetramer-positive T cell population correspond to a few events (<10 events) for some individuals. For transparency, we show any samples with <10 tetramer+ events as open symbols. Only samples with more than 10 tetramer+ events are included in the phenotypic analysis (Figures 4H and 4I).
Quantification and statistical analysis
TCRαβ statistical analysis
Single-chain alpha and beta TCR sequences were analyzed by TCRdist for modeling amino acid motifs, TCR landscapes, neighbor distance distribution and probabilities of generation (Pgen).46 For comparisons, published TCR datasets from unvaccinated SARS-CoV-2-infected individuals in the blood were sourced from Rowntree et al.,21 Nguyen et al.,18 Rowntree et al.20 (HLA-A∗02:01/S269, HLA-A∗24:02/S1208 and HLA-DPB1∗04:01/S167), Minervina et al.23 (HLA-A∗02:01/S269 and HLA-A∗24:02/S1208) and Mudd et al.22 (HLA-DPB1∗04:01/S167). Published TCR datasets from COVID-19-vaccinated uninfected individuals were sourced from Minervina et al.23 (HLA-A∗02:01/S269 and HLA-A∗24:02/S1208). TCRdist46 was used to calculate pairwise distances between clonotypes, sequence logos was created with conga python package44 network was generated with igraph R package (v. 1.3.2)45 and visualized with Gephi (v.0.9.7).47
Statistical analysis
Statistical significance of nonparametric datasets (two-tailed) were determined using GraphPad Prism v9 software. Mann-Whitney U-test (unpaired) and Wilcoxin signed-rank test (paired) were used for comparisons between two groups. Kruskal-Wallis test (unmatched) with Dunn’s multiple comparisons was used to compare more than two groups. Tukey’s multiple comparison test compared row means between more than two groups, while Sidak’s multiple comparison test compared column means between multiple groups. Correlation matrices were prepared in R using corrplot version 0.92.48 Volcano plots were generated within healthy individuals and haematology patients, and in patients with differing B cell ranges by Wilcoxon Rank-Sum test with Benjamini-Hochberg adjustment for multiple comparisons, using the rstatix version 0.7.0 package49 within R version 4.2.1,43 and plotted using EnhancedVolcano version 1.14.0.50 Volcano plots display log2(fold change) vs. -log10(unadjusted p value), with horizontal dashed line representing the adjusted p threshold. Parameters used for the volcano plots included sVNT, AIM, IgG RBD; log MNT (T5 only); B cell number and B cell probes (all participants and med-high B cell participants only).
Acknowledgments
We thank B. Cox, M. Fezollari, E. Shilling, and L. Davies for support with the healthy cohort; G. Au-Yeung for support with patients; Melbourne Cytometry Platform for technical assistance; and BEI Resources, NIAID, NIH, for providing the peptide array, SARS-related coronavirus 2 spike (S) glycoprotein, NR-52402. This work was supported by NHMRC L1 to K.K. (#1173871) and M.A.S. (#1173791); NHMRC L2 to K.S. (#1177174); NHMRC EL1 to T.H.O.N. (#1194036) and A.K.W. (#1173433); NHMRC EL2 to B.W.T. (#1195894) and D.A.W. (#1174555); Research Grants Council of the Hong Kong Special Administrative Region, China (#T11-712/19-N) to K.K.; the Victorian government (S.J.K. and A.K.W.); a MRFF Award (#2016062) to K.K., T.H.O.N., L.C.R., A.K.W., S.J.K., J.R., and B.W.T.; a MRFF award (#2002073) to S.J.K. and A.K.W.; a MRFF Award (#1202445) to K.K.; a MRFF Award (#2005544) to K.K., S.J.K., and A.K.W.; NHMRC program grant 1149990 (S.J.K.); NHMRC project grant 1162760 (A.K.W.); NHMRC Synergy grant 2011100 (M.A.S.); and NIH contract CIVC-HRP (HHS-NIH-NIAID-BAA2018) to P.G.T. and K.K. E.B.C. is supported by a NHMRC Peter Doherty Fellowship (#1091516). W.Z., S.Y.C and J.R.H were supported by a Melbourne Research Scholarship from the University of Melbourne. S.J.K. is supported by NHMRC Senior Principal Research Fellowship (#1136322). J.R. is supported by an ARC Laureate Fellowship. J.C.C. and P.G.T. are supported by NIH NIAID R01 AI136514-03 and ALSAC at St. Jude. We acknowledge BeiGene for supporting a part of the study. C.S.T. receives research funding from the CLL Global Research Foundation.
Author contributions
K.K. led the study. K.K., B.W.T., C.S.T., M.A.S., and T.H.O.N. supervised the study. K.K., T.H.O.N., L.C.R., L.F.A., B.Y.C., L.K., L.H., J.A.N., F.L.M., H.-X.T., K.S., T.K., S.N., and D.A.W. designed the experiments. T.H.O.N., L.C.R., L.F.A., B.Y.C., L.K., E.B.C., W.Z., S.Y.C., J.R.H., X.J., L.H., J.A.N., F.L.M., H.-X.T., A.F.C., and T.K. performed and analyzed experiments. H.A.M., T.M., A.A.M., M.V.P., A.F.C., J.C.C., and P.G.T. analyzed data. P.C., J.P., A.K.W., S.J.K., K.S., and J.R. provided crucial reagents. C.L., M.L., G.S.T., N.R.S., M.C., L.C., J.F.S., M.A.A., A.W., K.A.T., M.A.S., C.S.T., and B.W.T. recruited the vaccinated hematology patients. G.G., F.J., J.M.T., J.A.T., J.M., B.C., K.A.B., and D.A.W. recruited the healthy vaccinated cohorts. T.H.O.N., L.C.R., L.F.A., B.W.T., and K.K. wrote the manuscript. All authors reviewed and approved the manuscript.
Declaration of interests
The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines, which list F.K. as co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. F.K. has consulted for Merck and Pfizer (before 2020) and is currently consulting for Pfizer, Seqirus, and Avimex. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. H.A.M. and B.Y.C. are currently consulting for Ena Respiratory. B.W.T. has received research funding from MSD, Seqirus, and Sanofi and is on the advisory board for Moderna, CSL-Behring, and Takeda.
Published: March 27, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101017.
Contributor Information
Benjamin W. Teh, Email: ben.teh@petermac.org.
Katherine Kedzierska, Email: kkedz@unimelb.edu.au.
Supplemental information
Data and code availability
-
•
The published article includes all datasets generated or analyzed during the study.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Sharma A., Bhatt N.S., St Martin A., Abid M.B., Bloomquist J., Chemaly R.F., Dandoy C., Gauthier J., Gowda L., Perales M.A., et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet. Haematol. 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood W.A., Neuberg D.S., Thompson J.C., Tallman M.S., Sekeres M.A., Sehn L.H., Anderson K.C., Goldberg A.D., Pennell N.A., Niemeyer C.M., et al. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH research collaborative data hub. Blood Adv. 2020;4:5966–5975. doi: 10.1182/bloodadvances.2020003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch H.H., Martino R., Ward K.N., Boeckh M., Einsele H., Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin. Infect. Dis. 2013;56:258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mato A.R., Roeker L.E., Lamanna N., Allan J.N., Leslie L., Pagel J.M., Patel K., Osterborg A., Wojenski D., Kamdar M., et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chari A., Samur M.K., Martinez-Lopez J., Cook G., Biran N., Yong K., Hungria V., Engelhardt M., Gay F., García Feria A., et al. Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136:3033–3040. doi: 10.1182/blood.2020008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yri O.E., Torfoss D., Hungnes O., Tierens A., Waalen K., Nordøy T., Dudman S., Kilander A., Wader K.F., Ostenstad B., et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118:6769–6771. doi: 10.1182/blood-2011-08-372649. [DOI] [PubMed] [Google Scholar]
- 7.Teh J.S.K., Coussement J., Neoh Z.C.F., Spelman T., Lazarakis S., Slavin M.A., Teh B.W. Immunogenicity of COVID-19 vaccines in patients with hematologic malignancies: a systematic review and meta-analysis. Blood Adv. 2022;6:2014–2034. doi: 10.1182/bloodadvances.2021006333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haggenburg S., Lissenberg-Witte B.I., van Binnendijk R.S., den Hartog G., Bhoekhan M.S., Haverkate N.J.E., de Rooij D.M., van Meerloo J., Cloos J., Kootstra N.A., et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv. 2022;6:1537–1546. doi: 10.1182/bloodadvances.2021006917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiménez M., Roldán E., Fernández-Naval C., Villacampa G., Martinez-Gallo M., Medina-Gil D., Peralta-Garzón S., Pujadas G., Hernández C., Pagès C., et al. Cellular and humoral immunogenicity of the mRNA-1273 SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Adv. 2022;6:774–784. doi: 10.1182/bloodadvances.2021006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindemann M., Klisanin V., Thummler L., Fisenkci N., Tsachakis-Muck N., Ditschkowski M., Schwarzkopf S., Klump H., Reinhardt H.C., Horn P.A., Koldehoff M. Humoral and cellular vaccination responses against SARS-CoV-2 in hematopoietic stem cell transplant recipients. Vaccines. 2021;9 doi: 10.3390/vaccines9101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarisch A., Wiercinska E., Daqiq-Mirdad S., Hellstern H., Ajib S., Cremer A., Nguyen N.T.T., Dukat A., Ullrich E., Ciesek S., et al. SARS-CoV-2-specific T cells are generated in less than half of allogeneic HSCT recipients failing to seroconvert after COVID-19 vaccination. Eur. J. Immunol. 2022;52:1194–1197. doi: 10.1002/eji.202149771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., Del Molino Del Barrio I., Alaguthurai T., Domingo-Vila C., Hayday T.S., Graham C., Seow J., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavriatopoulou M., Terpos E., Ntanasis-Stathopoulos I., Briasoulis A., Gumeni S., Malandrakis P., Fotiou D., Migkou M., Theodorakakou F., Eleutherakis-Papaiakovou E., et al. Poor neutralizing antibody responses in 106 patients with WM after vaccination against SARS-CoV-2: a prospective study. Blood Adv. 2021;5:4398–4405. doi: 10.1182/bloodadvances.2021005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakkassery H., Carpenter E., Patten P.E.M., Irshad S. Immunogenicity of SARS-CoV-2 vaccines in patients with cancer. Trends Mol. Med. 2022;28:1082–1099. doi: 10.1016/j.molmed.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y., Cai C., Wullimann D., Niessl J., Rivera-Ballesteros O., Chen P., Lange J., Cuapio A., Blennow O., Hansson L., et al. Immunodeficiency syndromes differentially impact the functional profile of SARS-CoV-2-specific T cells elicited by mRNA vaccination. Immunity. 2022;55:1732–1746.e5. doi: 10.1016/j.immuni.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bange E.M., Han N.A., Wileyto P., Kim J.Y., Gouma S., Robinson J., Greenplate A.R., Hwee M.A., Porterfield F., Owoyemi O., et al. CD8(+) T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021;27:1280–1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen T.H.O., Rowntree L.C., Petersen J., Chua B.Y., Hensen L., Kedzierski L., van de Sandt C.E., Chaurasia P., Tan H.X., Habel J.R., et al. CD8(+) T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope display high naive precursor frequency and TCR promiscuity. Immunity. 2021;54:1066–1082.e5. doi: 10.1016/j.immuni.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habel J.R., Nguyen T.H.O., van de Sandt C.E., Juno J.A., Chaurasia P., Wragg K., Koutsakos M., Hensen L., Jia X., Chua B., et al. Suboptimal SARS-CoV-2-specific CD8(+) T cell response associated with the prominent HLA-A∗02:01 phenotype. Proc. Natl. Acad. Sci. USA. 2020;117:24384–24391. doi: 10.1073/pnas.2015486117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowntree L.C., Petersen J., Juno J.A., Chaurasia P., Wragg K., Koutsakos M., Hensen L., Wheatley A.K., Kent S.J., Rossjohn J., et al. SARS-CoV-2-specific CD8(+) T-cell responses and TCR signatures in the context of a prominent HLA-A∗24:02 allomorph. Immunol. Cell Biol. 2021;99:990–1000. doi: 10.1111/imcb.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowntree L.C., Nguyen T.H.O., Kedzierski L., Neeland M.R., Petersen J., Crawford J.C., Allen L.F., Clemens E.B., Chua B., McQuilten H.A., et al. SARS-CoV-2-specific T cell memory with common TCRalphabeta motifs is established in unvaccinated children who seroconvert after infection. Immunity. 2022;55:1299–1315.e4. doi: 10.1016/j.immuni.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mudd P.A., Minervina A.A., Pogorelyy M.V., Turner J.S., Kim W., Kalaidina E., Petersen J., Schmitz A.J., Lei T., Haile A., et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell. 2022;185:603–613.e15. doi: 10.1016/j.cell.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minervina A.A., Pogorelyy M.V., Kirk A.M., Crawford J.C., Allen E.K., Chou C.H., Mettelman R.C., Allison K.J., Lin C.Y., Brice D.C., et al. SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8(+) T cells. Nat. Immunol. 2022;23:781–790. doi: 10.1038/s41590-022-01184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goncharov M., Bagaev D., Shcherbinin D., Zvyagin I., Bolotin D., Thomas P.G., Minervina A.A., Pogorelyy M.V., Ladell K., McLaren J.E., et al. VDJdb in the pandemic era: a compendium of T cell receptors specific for SARS-CoV-2. Nat. Methods. 2022;19:1017–1019. doi: 10.1038/s41592-022-01578-0. [DOI] [PubMed] [Google Scholar]
- 25.Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B., et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koutsakos M., Rowntree L.C., Hensen L., Chua B.Y., van de Sandt C.E., Habel J.R., Zhang W., Jia X., Kedzierski L., Ashhurst T.M., et al. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Rep. Med. 2021;2:100208. doi: 10.1016/j.xcrm.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wragg K.M., Lee W.S., Koutsakos M., Tan H.X., Amarasena T., Reynaldi A., Gare G., Konstandopoulos P., Field K.R., Esterbauer R., et al. Establishment and recall of SARS-CoV-2 spike epitope-specific CD4(+) T cell memory. Nat. Immunol. 2022;23:768–780. doi: 10.1038/s41590-022-01175-5. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen T.H.O., Koutsakos M., van de Sandt C.E., Crawford J.C., Loh L., Sant S., Grzelak L., Allen E.K., Brahm T., Clemens E.B., et al. Immune cellular networks underlying recovery from influenza virus infection in acute hospitalized patients. Nat. Commun. 2021;12:2691. doi: 10.1038/s41467-021-23018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koutsakos M., Wheatley A.K., Loh L., Clemens E.B., Sant S., Nüssing S., Fox A., Chung A.W., Laurie K.L., Hurt A.C., et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci. Transl. Med. 2018;10:eaan8405. doi: 10.1126/scitranslmed.aan8405. [DOI] [PubMed] [Google Scholar]
- 30.Schulien I., Kemming J., Oberhardt V., Wild K., Seidel L.M., Killmer S., Sagar. Daul F., Salvat Lago M., Decker A., et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells. Nat. Med. 2021;27:78–85. doi: 10.1038/s41591-020-01143-2. [DOI] [PubMed] [Google Scholar]
- 31.Ferretti A.P., Kula T., Wang Y., Nguyen D.M.V., Weinheimer A., Dunlap G.S., Xu Q., Nabilsi N., Perullo C.R., Cristofaro A.W., et al. Unbiased screens show CD8+ T cells of COVID-19 patients recognize shared epitopes in SARS-CoV-2 that largely reside outside the spike protein. Immunity. 2020;53:1095–1107.e3. doi: 10.1016/j.immuni.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kedzierska K., Thomas P.G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Rep. Med. 2022;3:100562. doi: 10.1016/j.xcrm.2022.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 34.Valkenburg S.A., Josephs T.M., Clemens E.B., Grant E.J., Nguyen T.H.O., Wang G.C., Price D.A., Miller A., Tong S.Y.C., Thomas P.G., et al. Molecular basis for universal HLA-A∗0201-restricted CD8+ T-cell immunity against influenza viruses. Proc. Natl. Acad. Sci. USA. 2016;113:4440–4445. doi: 10.1073/pnas.1603106113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z., Muecksch F., Muenn F., Cho A., Zong S., Raspe R., Ramos V., Johnson B., Ben Tanfous T., DaSilva J., et al. Humoral immunity to SARS-CoV-2 elicited by combination COVID-19 vaccination regimens. J. Exp. Med. 2022;219:e20220826. doi: 10.1084/jem.20220826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juno J.A., Tan H.-X., Lee W.S., Reynaldi A., Kelly H.G., Wragg K., Esterbauer R., Kent H.E., Batten C.J., Mordant F.L., et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020;26:1428–1434. doi: 10.1038/s41591-020-0995-0. [DOI] [PubMed] [Google Scholar]
- 38.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiuppesi F., Zaia J.A., Faircloth K., Johnson D., Ly M., Karpinski V., La Rosa C., Drake J., Marcia J., Acosta A.M., et al. Vaccine-induced spike- and nucleocapsid-specific cellular responses maintain potent cross-reactivity to SARS-CoV-2 Delta and Omicron variants. iScience. 2022;25:104745. doi: 10.1016/j.isci.2022.104745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saini S.K., Hersby D.S., Tamhane T., Povlsen H.R., Amaya Hernandez S.P., Nielsen M., Gang A.O., Hadrup S.R. SARS-CoV-2 genome-wide T cell epitope mapping reveals immunodominance and substantial CD8(+) T cell activation in COVID-19 patients. Sci. Immunol. 2021;6:eabf7550. doi: 10.1126/sciimmunol.abf7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team . R Foundation for Statistical Computing; 2022. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 44.Schattgen S.A., Guion K., Crawford J.C., Souquette A., Barrio A.M., Stubbington M.J.T., Thomas P.G., Bradley P. Integrating T cell receptor sequences and transcriptional profiles by clonotype neighbor graph analysis (CoNGA) Nat. Biotechnol. 2022;40:54–63. doi: 10.1038/s41587-021-00989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Csárdi G., Nepusz T. The igraph software package for complex network research. InterJournal Complex Systems. 2006 [Google Scholar]
- 46.Dash P., Fiore-Gartland A.J., Hertz T., Wang G.C., Sharma S., Souquette A., Crawford J.C., Clemens E.B., Nguyen T.H.O., Kedzierska K., et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature. 2017;547:89–93. doi: 10.1038/nature22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacomy M., Venturini T., Heymann S., Bastian M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS One. 2014;9:e98679. doi: 10.1371/journal.pone.0098679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei T., Simko V. R package 'corrplot': visualization of a correlation matrix. 2021. https://github.com/taiyun/corrplot (Version 0.92)
- 49.Kassambara A. Rstatix: pipe-friendly framework for basic statistical tests. 2021. https://CRAN.R-project.org/package=rstatix R package version 0.7.0.
- 50.Blighe K., Rana S., Lewis M. EnhancedVolcano: publication-ready volcano plots with enhanced colouring and labeling. 2022. https://github.com/kevinblighe/EnhancedVolcano R package version 1.14.0.
- 51.Nguyen T.H.O., Lim C., Lasica M., Whitechurch A., Tennakoon S., Saunders N.R., Allen L.F., Rowntree L.C., Chua B.Y., Kedzierski L., et al. Prospective comprehensive profiling of immune responses to COVID-19 vaccination in patients on zanubrutinib therapy. EJHaem. 2023;4:216–220. doi: 10.1002/jha2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chappell K.J., Mordant F.L., Li Z., Wijesundara D.K., Ellenberg P., Lackenby J.A., Cheung S.T.M., Modhiran N., Avumegah M.S., Henderson C.L., et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2021;21:1383–1394. doi: 10.1016/S1473-3099(21)00200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowntree L.C., Chua B.Y., Nicholson S., Koutsakos M., Hensen L., Douros C., Selva K., Mordant F.L., Wong C.Y., Habel J.R., et al. Robust correlations across six SARS-CoV-2 serology assays detecting distinct antibody features. Clin. Transl. Immunology. 2021;10:e1258. doi: 10.1002/cti2.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang W., Chua B.Y., Selva K.J., Kedzierski L., Ashhurst T.M., Haycroft E.R., Shoffner-Beck S.K., Hensen L., Boyd D.F., James F., et al. SARS-CoV-2 infection results in immune responses in the respiratory tract and peripheral blood that suggest mechanisms of disease severity. Nat. Commun. 2022;13:2774. doi: 10.1038/s41467-022-30088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The published article includes all datasets generated or analyzed during the study.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.