Abstract
Background
Mild cognitive impairment (MCI) is a heterogeneous condition with high individual variabilities in clinical outcomes driven by patient demographics, genetics, brain structure features, blood biomarkers, and comorbidities. Multi-modality data-driven approaches have been used to discover MCI subtypes, however disease comorbidities have not been included as a modality though multiple diseases including hypertension are well-known risk factors for Alzheimer’s disease (AD). The aim of this study was to examine MCI heterogeneity in the context of AD-related comorbidities along with other AD-relevant features and biomarkers.
Methods
A total of 325 MCI subjects with 32 AD-relevant comorbidities and features were considered. Mixed-data clustering is applied to discover and compare MCI subtypes with and without including AD-related comorbidities. Finally, the relevance of each comorbidity-driven subtype was determined by examining their MCI to AD disease prognosis, descriptive statistics, and conversion rates.
Results
We identified four (five) MCI subtypes: poor-, average-, good- and best-AD prognosis by including comorbidities (without including comorbidities). We demonstrated that comorbidity-driven MCI subtypes differed from those identified without comorbidity information. We further demonstrated the clinical relevance of comorbidity-driven MCI subtypes. Among the four comorbidity-driven MCI subtypes there were substantial differences in the proportions of participants who reverted to normal function, remained stable, or converted to AD. The groups showed different behaviors, having significantly different MCI to AD prognosis, significantly different means for cognitive test-related & plasma features, and by the proportion of comorbidities.
Conclusions
Our study indicates that AD comorbidities should be considered along with other diverse AD-relevant characteristics to better understand MCI heterogeneity.
Keywords: Alzheimer’s disease, Mild cognitive impairment, Subtypes, Mixed-data clustering, Comorbidity, Heterogeneity
1. Introduction
Mild cognitive impairment (MCI) is characterized by cognitive impairment that does not significantly interfere with independent living [1]. However, it may be a transitional phase between normal cognitive aging and Alzheimer’s disease (AD). The annual conversion rate from MCI to dementia varies from 5 to 10% and around 33.6% of subjects with MCI are at risk of having early-stage AD [2,3]. Some individuals with MCI stay stable after 10-years’ follow-up or even return to normal cognitive status by timely interventions [4], whereas others progress to AD and die within three years after diagnosis [5]. Understanding which persons with MCI will progress rapidly to AD would allow physicians to advise patients and families to plan appropriately for financial matters and living arrangements, and to redouble interventions to slow progression, such as lifestyle changes or medication compliance, or to initiate therapeutics intended to delay cognitive decline. Moreover, there are currently no cures for AD and current treatments are limited even in slowing the progression of the disease [3,6,7]. Many attempts have been made to discover new therapeutics, but more than 100 new drugs for AD have either been abandoned in development or failed in clinical trials [8]. Clinical trials in MCI or AD are difficult not only because of outcomes which have high variability, but also because of the substantial heterogeneity of the patient population. The ability to stratify patients according to expected progression of disease may allow better focused clinical trials that require less time and fewer subjects, and thereby accelerate progress toward effective control of the disease.
Patients with MCI and AD often live with multiple comorbid medical conditions, including cardiovascular conditions, obesity, diabetes, and depression, some of which may accelerate clinical development of AD and complicate the management [9–11]. Indeed, hypertension is associated with AD and MCI and increases conversion from MCI to AD, and better control of hypertension delays progression to AD [12,13]. Diabetes and depression in MCI are associated with increased incidence of dementia [14–16]. Obesity indices were associated with poorer performance in memory, and verbal fluency tasks [17]. One study [18] showed a significant association between total cholesterol and cognitive measures. Also, active smoking and excessive alcohol consumption confer significantly increased risk for AD [19,20]. Therefore, the comorbidities may represent modifiable risk factors for disease progression. For these reasons, an investigation of MCI heterogeneity in the context of AD-related comorbidities is important for better understanding AD mechanisms and for personalized prevention, treatment, and long-term care for AD patients.
Several investigators have undertaken subtyping approaches to sort out the heterogeneity of MCI [21,22]. Data-driven subtyping approaches have been proposed to understand heterogeneity of MCI [22,23], using single modality data like neuropsychological features [24,25], measures of atrophy derived from structural magnetic resonance imaging (MRI) [26,27] or use multi-modal data with invasive, high-cost biomarkers such as cerebrospinal fluid [28,29,30]. In [31], genetic polymorphism and gene expression data have been integrated to identify the subtypes of MCI patients. In another [29], multiple baseline and prognostic characteristics were used to discover two homogenous clusters of the MCI cohort. The identified clusters were with different prognostic cognitive trajectories, but the MCI subjects who converted to AD were present in both clusters, and some MCI patients did not fit into either cluster. In two studies [30,32], multidimensional features were considered to discover latent classes among MCI subjects. Patients with MCI who converted to AD were present in all groups, even in the low-risk group, and persons who reverted from MCI to normal cognitive function were present even in at-risk groups. Thus, there remains a need for better discriminants to classify patients with MCI and predict progression to AD. We undertook such a study, using clinically relevant, readily available features of the disease, including comorbidities, to find classifications of patients with MCI that are highly predictive of disease progression to AD.
In this study, we classified MCI patients into clinically relevant subtypes based on features including AD-related comorbidities, life-styles, demographics, imaging, genetics, cognitive scores, blood biomarkers derived from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [33]. The ADNI data set has been widely used in many research studies on subtype analysis [26,29,32], however, none of these published studies incorporated AD-related comorbidities in studying MCI heterogeneity. We compared the differences in MCI subtypes before and after including comorbidity information and demonstrated the importance of incorporating disease comorbidity data in stratifying MCI patients.
2. Data and Methods
Data used in this study was obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study (adni.loni.usc.edu). The primary goal of ADNI has been to test whether serial MRI, positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. In this study, all ADNI-1, ADNI-2/ADNI-GO MCI subjects with at least one post-baseline visit data were included. ADNI3 research data was not included as it does not include detailed data of smoking history, alcohol use, and medical history of participants. For each subject, a total of 32 features were included for MCI subtype analysis, including AD-related comorbidities, life-styles, and other AD-relevant data modalities at baseline have been considered and described below.
A total of 8 AD-related comorbidities [9,10] were extracted from patient medical and medication history. The details of search terms and drugs used for AD-related comorbidities are provided below.
- Hypertension –
- search terms (hypertension, high blood pressure, and HTN).
- treatment with antihypertensive medication.
- Diabetes –
- search terms (diabetes, type2 diabetes)
- the use of glucose-lowering medication.
- High cholesterol –
- search terms (Hypercholesterolemia, high cholesterol, elevated cholesterol, Hyperlipidemia)
- treatment with lipid-lowering medication.
- Depression –
- geriatric depression scale (GDS-15) of 10.
- treatment with an antidepressant.
Obesity – It was defined as body mass index (BMI) >30. BMI was calculated from measured body weight (in kilograms) divided by measured height (in meters) squared.
- Cardiovascular disease –
- search terms (stroke, coronary artery disease, congestive heart failure, cerebrovascular disease, carotid artery stenosis, peripheral vascular disease, or Atrial fibrillation).
- Hearing Loss –
- search terms hearing, auditory, ear, deaf, presbycusis.
- TBI –
- search terms (concussion and/or head injury).
In addition to AD-related comorbidities, additional 6 data modalities were selected for each subject to study MCI heterogeneity. These features were selected based on their reported associations with the risk for MCI to AD conversion: Demographic factors like age, gender, and education can influence the progression to AD [1,34], APOE ɛ4 has been shown to be associated with age at onset of AD and to be a strong predictor of clinical progression in patients with MCI [35], MRI volumes such as hippocampal and entorhinal volumes have also been associated with the MCI to AD conversion process [29,36,37,38], cognitive evaluations have strong predictive power of MCI to AD conversion [29,39,40], and plasma Nfl and p-tau181 shown to have good ability to predict progression to AD [41,42].
Demographic data (Age, Sex), Education in years, Family history.
APOE gene status: Apolipoprotein E (APOE) ɛ4 allele frequency was included as a genetic marker of AD.
The MRI volumetric features: Total ventricular volume, hippocampal volume (left plus right), middle temporal gyral volume (left plus right), total entorhinal and fusiform volume. These MRI volumes were normalized by total intracranial volume (ICV).
Cognitive tests: The 11-item score and 13-item score from the Alzheimer’s Disease Assessment Scale (ADAS); total score from the Clinical Dementia Rating scale Sum of Boxes (CDRSB); total score from the Functional Assessment Questionnaire (FAQ); total score from the Mini-Mental State Examination (MMSE); and immediate score, learning score, forgetting score, and percentage forgetting score from the Rey’s Auditory Verbal Learning Test (RAVLT).
plasma Nfl and p-tau181.
Lifestyle factors: status of alcohol drinking, smoking.
Method
The MCI subtype analysis was outlined in Figure 1 and described as follows. First, a total of 32 patient attributes (AD-comorbidities and other features) were extracted, and all the continuous features were z-score normalized (except for the integer and categorical data). Next, mixed-data clustering [43,44] was applied to discover the MCI clusters. MCI clusters identified using combined features of comorbidity and non-comorbidity vs. using non-comorbidity features only were compared, and the importance of including comorbidity information for MCI subtype analysis was demonstrated. Finally, the clinical relevance of each comorbidity-driven cluster was determined by examining the MCI to AD disease prognosis of subjects in the cluster based on the Kaplan-Meier survival analysis [45]. Log-rank test [46] was used to test for statistical differences between survivals of different clusters, where survival indicates that patients maintain a diagnosis of MCI rather than progress to AD. Furthermore, statistical differences between features of the subtypes were computed using the Chi-square test for categorical variables and ANOVA for continuous variables. Post hoc analysis was performed for multiple comparisons using Bonferroni method. P values lower than .05 were considered significant.
Figure 1.
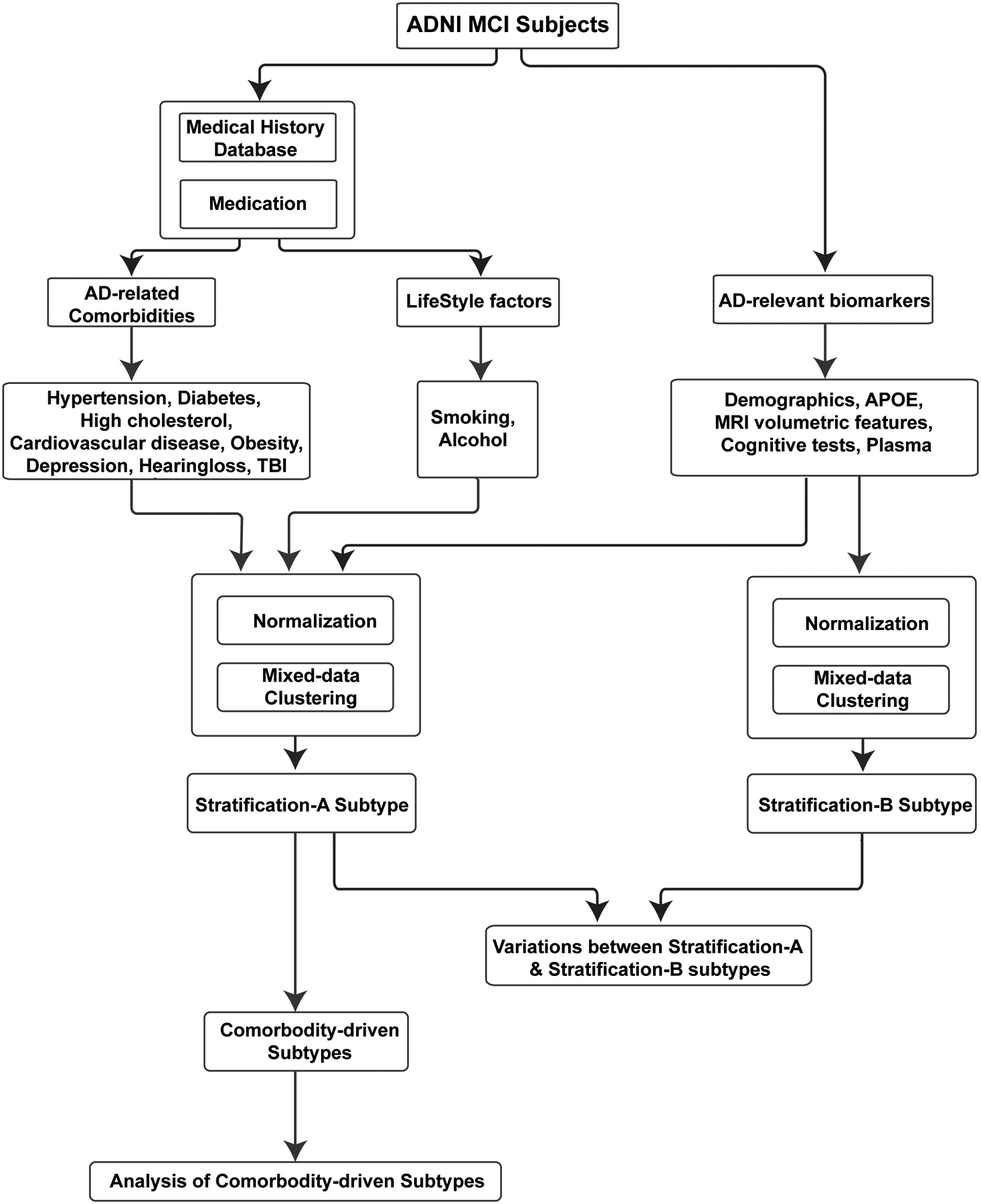
The overall methodology of stratification of MCI subjects.
Model-based clustering in the presence of mixed types of data has been used to identify the clusters of MCI subjects [43,44]. This method assumes a specific distribution to define each cluster and effectively models the distribution of each variable separately depending on the type of data being used [44]. Model-based clustering models the distribution of a continuous (integer and categorical) variable, using Gaussian (Poisson and multinomial respectively) distribution. Finally, the method models the distribution of the observed variables with a mixture of these parametric distributions. The maximum penalized likelihood estimates for the model was computed using the EM algorithm [47] and the optimal number of clusters was identified by evaluating the Bayesian Information Criterion (BIC) [48]. BIC introduces a penalty term for the number of parameters in the model thus selecting models with a better fit. The optimal number of clusters is determined by the model with the lowest absolute value of the BIC. Clustering was performed using the VarSelLCM R package [49], and the number of clusters tried and varied between 2 and 10.
3. Results
A total of 325 MCI subjects from the ADNI study were included in the current analysis. A total of 32 AD-relevant characteristics (8 AD-related comorbidities, 20 AD-relevant features, 2 lifestyle factors) were considered (Table 1). To demonstrate the importance of comorbidities in MCI subtyping, we developed two strategies using both comorbidity and non-comorbidity features (stratification-A subtype/subtype solution-A) or using only non-comorbidity features (stratification-B subtype/subtype solution-B). The survival plots for MCI subjects in subtypes identified by stratification-A and stratification-B are provided in Figures 2 and 3, respectively. In survival analysis, there were patients who progressed to AD; patients who maintained a diagnosis of MCI or those who were censored during the same time interval. Here, the censored data are information from subjects who were lost to follow-up before conversion was detected. Stratification-A identified four MCI subtypes: 1,2,3,4 (ordered as poor-prognosis to better-prognosis). Stratification-B identified five subtypes: 1,2,3,4,5 (ordered from poor to better prognosis). In both figures, the numbers below the plot represent the number of subjects who are at risk at given time point. Subtype-4 in Figure 2 (Subtype-5 in Figure 3) is having the constant probability, and the change in the number of patients at risk at different time points is because of the censored subjects. Subtype 4 initially had 63 subjects; in the time interval of 500 days, 9 subjects were censored (they had follow-up times below 500 days); and so, at 500 days’ time point, 54 (63–9) subjects were there at risk, and so on. The stratification-A subtyping strategy produced statistically significant survivals for all 4 subtypes or clusters of MCI subjects (P < .001). The stratification-B subtype produces statistically significant survival (P < .001), but between Subtype-3 and Subtype-4, there is no significant difference, with P = .45.
Table 1:
Statistics of the MCI population.
| Feature | MCI (N=325) |
|---|---|
| Female (%) | 44.9 |
| Age (years, mean±SD) | 71.08 ± 7.30 |
| Education in years (years, mean±SD) | 16.18 ± 2.68 |
| Family History (%) | 65.23 |
| APOE ɛ4 (% of >=1 allele) | 47.38 |
| The MRI volumetric features (volumes in mm3, mean±SD) | |
| Ventricles | 0.024 ± 0.012 |
| Hippocampus | 0.005 ± 8e-04 |
| Middle temporal gyrus | 0.013 ± 0.0016 |
| Entorhinal | 0.002 ± 4e-04 |
| Fusiform | 0.012 ± 0.0014 |
| Cognitive tests (scores, mean±SD) | |
| ADAS11 | 9.08 ± 4.22 |
| ADAS13 | 14.55 ± 6.41 |
| CDRSB | 1.48 (0.89) |
| FAQ | 2.73 (3.77) |
| MMSE | 28.11 (1.67) |
| RAVLT immediate | 37.10 (10.85) |
| RAVLT learning | 4.71 (2.52) |
| RAVLT forgetting | 4.63 (2.54) |
| RAVLT % forgetting | 54.94 (31.48) |
| Plasma biomarkers (pg/mL, mean±SD) | |
| Nfl | 37.17 (18.20) |
| P-tau181 | 18.00 (12.24) |
| Lifestyle factors | |
| Alcoholic (%) | 4.0 |
| Smoking (%) | 37.23 |
| Comorbidities | |
| Hypertension (%) | 45.84 |
| Diabetes (%) | 11.07 |
| Cholesterol (%) | 51.07 |
| Depression (%) | 28.61 |
| Obesity (%) | 24.30 |
| Cardiovascular (%) | 67.07 |
| Hearing Problems (%) | 17.53 |
| TBI (%) | 4.62 |
NOTE: Mean and standard deviation (SD) for continuous variables and percentages for categorical variables were computed.
Abbreviations: N-number of subjects. ADAS - Alzheimer’s Disease Assessment Scale; CDRSB - Clinical Dementia Rating scale Sum of Boxes. FAQ - Functional Assessment Questionnaire. MMSE - Mini-Mental State Examination. RAVLT - Rey’s Auditory Verbal Learning Test.
Figure 2:
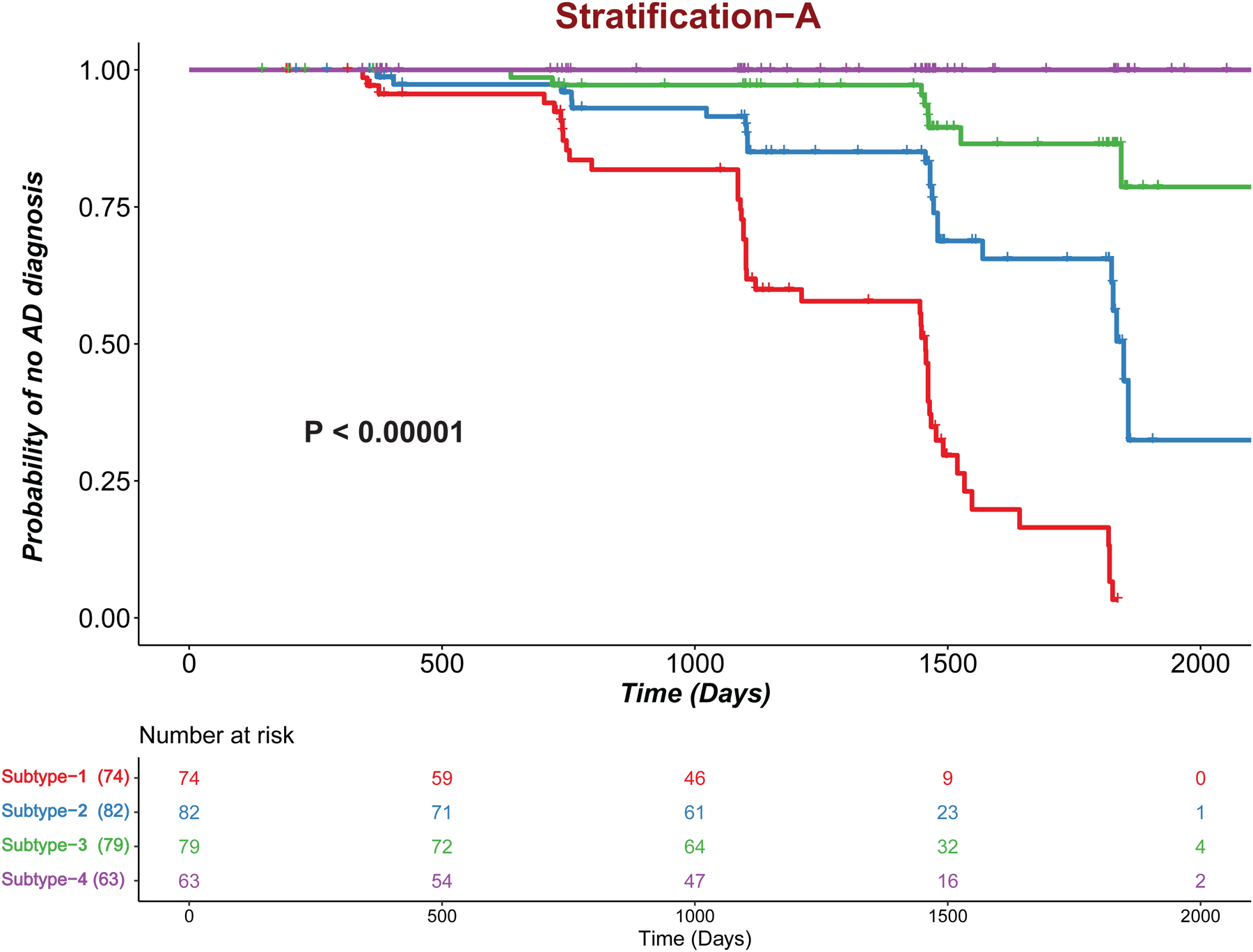
Kaplan-Meier plots for the stratification-A subtype. Tick marks represent censored patients.
Figure 3:
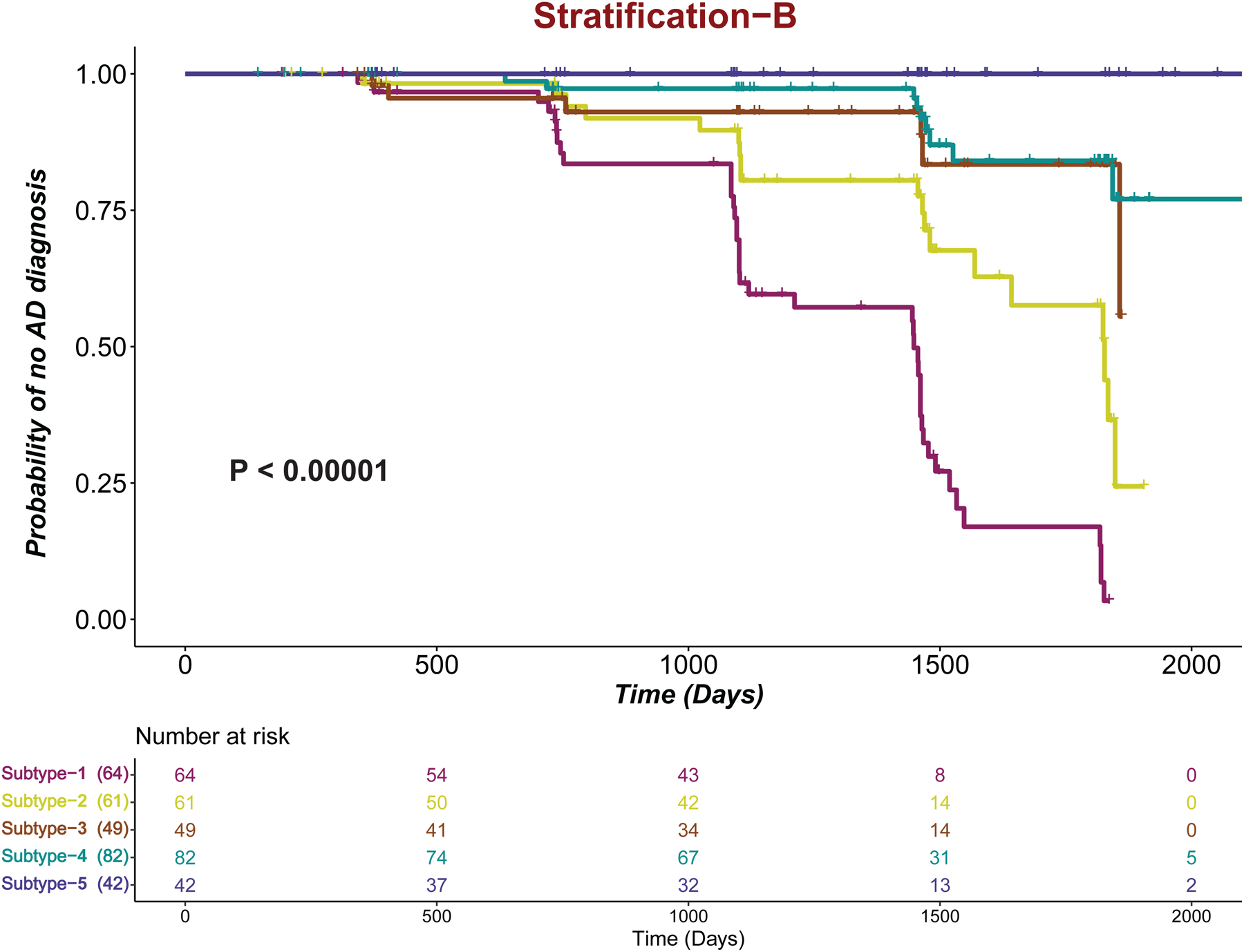
Kaplan-Meier plots for the stratification-B subtype. Tick marks represent censored patients.
The variations and subject flow between subtypes or clusters that generated by stratification-A and stratification-B were provided in Figure 4. The number of subjects in each cluster, and the number of subjects flow between clusters generated by two stratification strategies is also provided. The sizes of the clusters in the survival plots (Figures 2,3) and in Figure 4 are not the same, as the survivals were plotted using MCI subjects who remain stable and who converted to AD, but not the subjects who reverted to normal cognition, but the clusters in Figure 4 show all MCI subjects who remain stable, who converted to AD, and who reverted to normal cognition. As shown in Figure 4, the flow of the subjects was mostly between the same level prognosis clusters or poor-prognosis cluster of stratification-A to better-prognosis cluster of stratification-B (for example, Subtype-1 to Subtype-1,2; Subtype-3 to Subtype-3,4). Only in one case, we observed subject flow from poor-prognosis cluster of stratification-A to better-prognosis cluster of stratification-B (Subtype 4 to Subtype-3). This indicates that without considering comorbidities, MCI subject with poor prognosis could be mis-classified as having a better prognosis, even though comorbidities such as cardiovascular diseases and type 2 diabetes are well known to affect the cognitive decline and risk for AD. In summary, the above analysis demonstrates that it would be important to include comorbidity information along with AD biomarkers to analyze MCI heterogeneity and identify clinically relevant subtypes.
Figure 4:
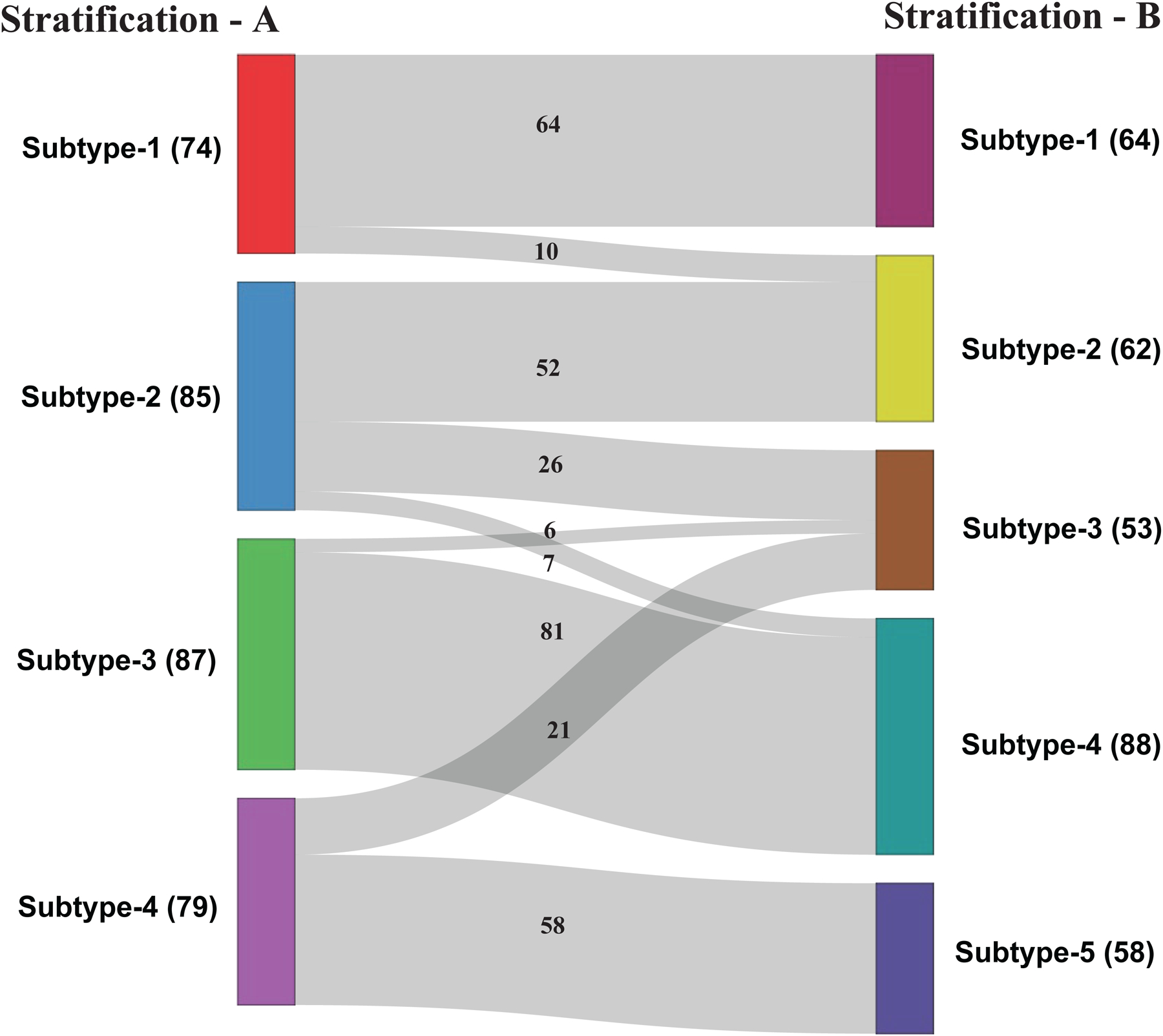
Subjects flow between two stratification subtypes (stratification-A subtype vs. stratification-B subtype).
* Footnote: The number of MCI subjects in each subtype are provided with in the brackets. Each subtype consists of all MCI subjects who remain stable, who converted to AD, and who reverted to normal cognition.
We then examined how each data modality contributed to MCI subtypes. The comorbidity-driven Stratification-A subtypes 1 through 4 represent the AD poor-, average-, good- and best-prognosis (PP, AP, GP, BP) subtypes, respectively. The descriptive statistics of the biomarkers for these four subtypes was provided in Table 2. As shown in the table, subtypes differed significantly in age and frequency of females. Post hoc testing further indicated that the subjects in PP and AP subtypes were significantly older than those in GP and BP subtypes. PP subtype members had less education and higher frequency of the APOE ε4 allele compared with other subtypes. These results are consistent with the fact that age and APOE ε4 are two strong risk-factors for AD prognosis. BP subtype had the highest frequency of females. All 4 MCI subtypes showed significantly different plasma Nfl. PP and AP had significantly more plasma Nfl levels than the other two subtypes. Six of the nine cognitive tests significantly differed among the four MCI subtypes. On these tests, mostly PP subtype had the most severe impairment, AP and GP were in the middle, and BP subtype had the best cognitive scores. Two of the five MRI volumetric features, hippocampus, entorhinal volumes, were differed among subtypes. Lifestyle factors did not show statistical differences among the four subtypes. Among 8 comorbidities examined, four (hypertension, cardiovascular, hearing problems, and obesity) showed significant differences among identified MCI subtypes, with higher prevalence in the PP, AP (poorer prognosis of MCI to AD conversion) subtypes.
Table 2:
Subtype-wise distribution of biomarkers.
| Feature | PP (Subtype 1) N=74 |
AP (Subtype 2) N=85 |
GP (Subtype 3) N=87 |
BP (Subtype 4) N=79 |
P-val |
|---|---|---|---|---|---|
| Female (%) | 27.03 | 37.65 | 42.53 | 72.15 | <.0013,5,6 |
| Age (years, mean±SD) | 76.25 (6.87) | 73.11 (5.90) | 68.27 (6.49) | 66.71 (5.77) | <.051,2,3,4,5 |
| Education (years, mean±SD) | 15.70 (2.89) | 16.37 (2.82) | 16.28 (2.50) | 16.30 (2.49) | .14 |
| Family History (%) | 62.16 | 55.29 | 67.81 | 75.94 | <.055 |
| APOE ɛ4 (% of >=1 allele) | 54.05 | 51.77 | 47.13 | 36.71 | .13 |
| The MRI volumetric features (volumes in mm3, mean±SD) | |||||
| Ventricles | 0.03 (0.013) | 0.031 (0.0131) | 0.017 (0.0064) | 0.018 (0.0092) | .44 |
| Hippocampus | 0.004 (7e-04) | 0.004 (6e-04) | 0.005 (5e-04) | 0.005 (4e-04) | <.052,3,4,5 |
| Middle temporal gyrus | 0.012 (0.001) | 0.013 (0.0014) | 0.014 (0.0012) | 0.014 (0.0013) | .14 |
| Entorhinal | 0.002 (5e-04) | 0.002 (4e-04) | 0.003 (4e-04) | 0.003 (3e-04) | <.052,3,4,5 |
| Fusiform | 0.011 (0.0013) | 0.012 (0.0011) | 0.013 (0.0013) | 0.013 (0.0011) | .17 |
| Cognitive tests (scores, mean±SD) | |||||
| ADAS11 | 13.37 (4.52) | 9.55 (2.98) | 8.49 (2.56) | 5.22 (2.25) | <.0011,2,3,5,6 |
| ADAS13 | 21.27 (6.08) | 15.69 (4.88) | 13.60 (3.68) | 8.08 (2.83) | <.0011,2,3,4,5,6 |
| CDRSB | 2.40 (0.94) | 1.18 (0.60) | 1.26 (0.73) | 1.18 (0.65) | <.0011,2,3 |
| FAQ | 7.95 (4.26) | 1.14 (1.28) | 1.54 (2.05) | 0.88 (1.44) | <.0011,2,3 |
| MMSE | 27.17 (1.79) | 27.89 (1.75) | 28.37 (1.46) | 28.94 (1.13) | .1 |
| RAVLT forgetting | 5.01 (2.09) | 5.29 (2.45) | 5.31 (2.47) | 2.82 (2.24) | <.0011,2,3,5,6 |
| RAVLT immediate | 27.82 (6.63) | 34.1 (7.42) | 35.27 (6.34) | 50.98 (7.35) | .9 |
| RAVLT learning | 2.77 (1.96) | 4.54 (2.41) | 4.51 (1.97) | 6.92 (1.92) | <.051,2,3,5,6 |
| RAVLT % forgetting | 76.76 (27.41) | 61.87 (25.98) | 59.15 (26.09) | 22.40 (18.40) | .91 |
| Plasma (pg/mL, mean±SD) | |||||
| Nfl | 47.64 (18.60) | 42.78 (21.63) | 31.50 (11.93) | 26.86 (9.84) | <.052,3,4,5 |
| P-tau181 | 22.60 (16.96) | 22.30 (12.49) | 15.89 (7.82) | 11.41 (5.30) | .4 |
| Lifestyle factors | |||||
| Smoking (%) | 32.43 | 38.82 | 32.18 | 45.57 | .24 |
| Alcoholic (%) | 6.75 | 4.7 | 2.29 | 2.53 | .44 |
| Comorbidities | |||||
| Hypertension (%) | 67.05 | 54.05 | 34.48 | 27.84 | <.0012,3,5 |
| Diabetes (%) | 10.81 | 14.11 | 11.49 | 7.595 | .61 |
| Cholesterol (%) | 51.35 | 61.17 | 47.12 | 44.30 | .14 |
| Depression (%) | 29.73 | 20 | 36.78 | 27.84 | .11 |
| Obesity (%) | 27.02 | 15.29 | 24.13 | 31.64 | <.055 |
| Cardiovascular (%) | 67.56 | 83.52 | 63.21 | 53.16 | <.0014,5 |
| Hearing Problems (%) | 22.97 | 28.23 | 14.94 | 3.79 | <.0013,5 |
| TBI (%) | 5.40 | 5.88 | 4.59 | 2.53 | .75 |
NOTE: The Mean, Standard Deviation (Mean (SD)) for continuous features and percentages for categorical variables were computed in each subtype. MRI volumes presented as a fraction of intracranial volume.
Post hoc Differences between PP and AP1; PP and GP2; PP and BP3; AP and GP4; AP and BP5; GP and BP6.
Abbreviations: N- number of subjects. PP - Poor Prognosis, AP - Average Prognosis, GP - Good Prognosis, BP -Best Prognosis. ADAS - Alzheimer’s Disease Assessment Scale; CDRSB - Clinical Dementia Rating scale Sum of Boxes. FAQ - Functional Assessment Questionnaire. MMSE - Mini-Mental State Examination. RAVLT - Rey’s Auditory Verbal Learning Test.
Table 3 provided the percentage of MCI subjects who reverted to normal (“reverters”) who stayed at MCI stage (“stablers”) and who progressed to AD (“converters”) for each of the four MCI subtypes. For PP subtype, no reverter from MCI to normal was observed. For BP subtype, no MCI converter to AD was observed. In the table, conversion times (average conversion time, first conversion time to AD) per subtype provided. Average AD-conversion time for PP subtype is much smaller than the other two AP, GP subtypes. First AD-conversion time among the subjects in PP group was 343 days, less than 1 year and for the other two groups, it was over 1 year. These results demonstrate that comorbidity-driven subtyping provided clinically relevant stratification of MCI subjects.
Table 3:
Distribution of Reverters to Normal, MCI stable, and Converters to AD for each of the four subtypes generated by comorbidity-driven Stratification-A strategy.
| PP (Subtype 1) | AP (Subtype 2) | GP (Subtype 3) | BP (Subtype 4) | |
|---|---|---|---|---|
| Reverters (%) | 0 | 3.53 | 11.2 | 18.25 |
| Stablers (%) | 40.54 | 68.24 | 79.6 | 81.75 |
| Converters (%) | 59.46 | 28.24 | 9.2 | 0 |
| Mean conversion time to AD (Days) | 1191.5 | 1320.58 | 1328.5 | - |
| First conversion time to AD (Days) | 343 | 371 | 636 | - |
PP – Poor Prognosis, AP Average Prognosis, GP - Good Prognosis, BP -Best Prognosis.
4. Discussion
Our study investigated comorbidity-driven multi-modal analysis of heterogeneities of MCI patients and classified MCI patients into clinically relevant subtypes based on the ADNI database. We utilized 8 AD-related comorbidities along with 24 other features from demographics, APOE genotype, brain volumetric data, cognitive measurements, plasma biomarkers, and behavioral factors to understand the variability in progression from MCI to AD. Using the mixed multi-modal data, we stratified MCI subjects into four subtypes, with poor-, average-, good- and best- AD prognosis. We showed that the subtyping strategy based on both AD-related comorbid conditions and other relevant data modalities was better in clustering MCI subjects with differing prognosis than the subtyping strategy only considering AD-relevant (non-comorbidity) data. From the analysis of the MCI groups identified, we observe that no MCI subject in the BP subtype progressed to AD during the observation period, and conversely, none of MCI subjects in the PP subtype reverted to normal. This clean separation between the best and the poorest prognosis subtypes gives confidence that the comorbidity-driven mixed-data subtyping strategy is able to separate MCI patients into groups with different clinical outcomes or prognosis with both high sensitivity and high specificity.
In a study in Mexico [32] using ADNI multimodal biomarker data without using comorbidity features, MCI subjects were classified into three groups, at-risk groups showed a different trajectory from the low-risk subtype. Patients with MCI who converted to AD were present in all groups, even in the low-risk group, and persons who reverted from MCI to normal cognitive function were present even in at-risk groups. The rate of conversion of the low-risk group was five times larger than that of the at-risk group. In our work, MCI subjects were classified into four groups, with zero conversions from MCI to AD in the BP group and zero participants who reverted to normal in the PP group. The rate of conversion of the PP subtype is six times larger than the rate of conversion of the BP subtype. In a different study [30], MCI subjects were classified into four groups (MCI 1,2, 3, and 4) using ADNI MRI and CSF biomarkers. MCI 2, 3, and 4 all differ significantly from MCI 1 but do not differ significantly from each other in time to conversion. MCI patients who converted to AD were present in all groups. Gamberger et al. [29] proposed to use a multilayer clustering algorithm based on MCI biomarker data and identified two homogenous clusters of MCI subjects with different prognostic cognitive trajectories. MCI converters to AD were present in both clusters, and a cluster of rapid decliners had a five times larger rate of conversion than that of a cluster of slow decliners. However, some MCI patients did not fit into either cluster. In our work, all patients were assigned to a specific subtype, and we were able to discover four subtypes with significantly different trajectories, and with better conversion rate differences between PP and BP groups.
Identifying MCI patients based on pre-existing and often actionable comorbidities who will have distinct clinical outcomes later on is important for designing clinical trials. The power of a clinical trial to detect a significant drug effect depends on the degree to which change in status occurs in the untreated group compared to the treated group (size of the treatment effect) as well as the variability of the change in status in each group, both factors that determine the number of participants required and the study duration needed to demonstrate a desired effect. In trials of AD or MCI, variability is a problem in conducting clinical trials, for clinical tests of cognition are themselves inherently variable, and the study participants represent an extremely heterogeneous group with respect to disease progression. Trials that include MCI patients with slow decline of cognitive function will need to enroll large numbers of patients to detect an effect, whereas trials that enroll patients who suffer rapid decline have the potential to demonstrate a treatment effect more rapidly with smaller number of participants, if the treatment is effective. Moreover, the mechanisms governing the decline of cognitive function may differ among MCI participants. Our proposed comorbidity-driven multi-modality method in clustering patients into subtypes with distinct future clinical outcomes has high potential for designing and conducting effective clinical trials and improve the ability to detect a real effect if it exists, especially if the effect is greatest in one of the study groups.
In studies of MCI, progression/conversion to AD is a frequent outcome of interest. Our methodology successfully separated the patients who converted to AD (PP, AP, BP subtypes) with higher percentage of converters being in PP subtype. Analysis of the subgroups indicates that both hypertension and hearing loss are significantly more frequent in the PP subgroup and the frequency progresses across subgroups with increasing probability of progression, so special effort should be made to modify these risk factors in any patient with MCI, but especially in those in the PP subgroup. Recent data from SPRINT-MIND shows that very tight control of blood pressure is superior to moderate control in limiting the probability of dementia. Although obesity, diabetes and cardiovascular conditions also differed among the subgroups, the progression from BP to PP was not consistent. Nevertheless, best practice would include control of comorbid conditions of any type. Other baseline features suggestive of AD in poorer prognosis subtypes are MRI hippocampus, entorhinal volumes, and cognitive tests, so activities shown to improve cognition such as exercise [50], may be recommended to improve their cognitive function abilities. Risk factors such as age or APOE status currently cannot be modified but may be helpful in further stratifying participants for clinical trials.
Some limitations of the current study are recognized, which can be addressed in future work. First, the comorbidity information in our study was based on the self-reported medical history and medication usage of participants captured in the ADNI database, which may be incomplete. In the future, we plan to apply this strategy using patient electronic health records that contains more comprehensive data of patient medical diagnoses, medication, lab test, and demographics. Second, in the current study, only the status (presence/absence), but not the severity of concurrent medical conditions, is considered. The severity of comorbidities (e.g., stage 1 vs stage 2 hypertension) is important for clustering MCI subjects into groups with distinct clinical outcomes. For example, the SPRINT MIND study found that there was significantly less cognitive impairment in patients whose blood pressure was reduced more aggressively than in those whose blood pressure was subjected to looser control. In addition, the risk associations with MCI/AD may vary among the 8 comorbidities. In our study, we treated them the same (1 if they are present). In the future, we will further improve the comorbidity-driven subtyping algorithm by treating comorbidities as continuous instead of binary variables. Third, as chronic inflammation is a central mechanism of AD, in future work we will add another dimension based on inflammatory status, to include inflammatory biomarkers like CRP [51], other serum biomarkers related to inflammation, systemic inflammatory diseases including rheumatoid arthritis, psoriasis, ulcerative colitis, inflammatory bowel disease that are related to AD risk, and anti- or pro-inflammation medications such as TNF alpha inhibitors that our previous studies [52,53] have shown to be associated with significantly decreased risk for AD and dementia. Fourth, as our study used multi-level features and different level of features often partially correlate with each other, for example, age with cognitive functions and brain images, genotypes with biomarkers and brain images, and age with comorbidities. In future, we will consider such relations while stratifying the patients. Fifth, our current study is based on the ADNI database. Although ADNI is designed to develop clinical, imaging, genetic, and biochemical biomarkers for the early detection and tracking of AD and is one of the largest databases for AD research and contains multiple types of AD-related data, including MRI, PET, APOE, age, gender, and blood biomarkers, it is not a population-based study. Our findings need to be validated in other independent cohorts but the availability of such multi-modality databases with both MCI and AD subjects are limited. Finally, longitudinal follow-up changes in the comorbidity and non-comorbidity features were not considered, but the longitudinal changes may yield different results, especially given that the co-existing diseases may progress – or be treated – which may impact the subtypes in MCI. In the future, we will include such longitudinal data for MCI subtype analysis, and study conversion rates and patterns for each of the MCI subtypes.
5. Conclusion
We performed a multi-modal subtype analysis of the MCI population from the ADNI database utilizing comorbidity information along with other AD-relevant biomarkers. These results highlight the importance of considering the comorbidity information along with other AD-relevant data modalities for the stratification analysis of MCI with distinct disease prognosis. Based on both comorbidities and other AD-related features, MCI population has been stratified into four different subtypes with significantly different trajectories. These subtypes are characterized by substantial differences in patient characteristics and patient outcomes (in the proportions of participants who revert to normal cognition, remain stable, or convert to AD). Both comorbidity profiles/signatures and biomarkers could become helpful for patient stratification in clinical trials, monitoring of patients, and for personalizing therapies. Since many of these comorbidities are modifiable risk factors for AD such as hypertension, obesity and depression, clusters identified by the comorbidity-driven subtyping strategy can offer actionable strategies for both preventing and treating AD, for example, by managing comorbidities in Subtype 4 (BP). Appropriate management of these profiles/signatures might increase the possibility of delaying or even converting MCI to normal cognitive.
RESEARCH IN CONTEXT.
1. Systematic review:
We reviewed the literature using traditional methods and sources (e.g., Google Scholar, PubMed). Research suggests that the mild cognitive impairment (MCI) is a heterogeneous condition with high individual variability and several comorbidities co-existing with MCI have been associated with poorer cognitive decline and development of dementia. None of these published studies incorporated AD-related comorbidities in studying MCI heterogeneity and subtypes.
2. Interpretation:
Our findings highlight the need to consider the comorbidity information along with other AD-relevant biomarkers for the subtype analysis of MCI.
3. Futuredirections:
Further study including longitudinal follow up changes will be required as it may yield different results, especially given that the co-existing diseases may progress – or be treated – which may impact the subtypes in MCI. Our findings need to be validated in other independent cohorts.
Acknowledgments
We thank the Alzheimer’s Disease Neuroimaging Initiative (ADNI) for generously sharing clinical, imaging, genetic, and biochemical biomarkers for the early detection and tracking of Alzheimer’s disease (AD).
Funding
We acknowledge support from National Institute on Aging (grants nos. AG057557, AG061388, AG062272), National Institute on Alcohol Abuse and Alcoholism (grant no. R01AA029831), National Institute on Drug Abuse (grant no. UG1DA049435), the Clinical and Translational Science Collaborative (CTSC) of Cleveland (grant no. 1UL1TR002548-01).
References
- [1].Roberts R, Knopman DS. Classification and epidemiology of MCI. Clinics in geriatric medicine. 2013;29(4):753–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mitchell AJ, Shiri-Feshki M. Temporal trends in the long-term risk of progression of mild cognitive impairment: a pooled analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2008; 79(12):1386–1391. [DOI] [PubMed] [Google Scholar]
- [3].DeCarli C Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. The Lancet Neurology. 2003;2(1):15–21. [DOI] [PubMed] [Google Scholar]
- [4].Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia–meta‐analysis of 41 robust inception cohort studies. Acta psychiatrica scandinavica. 2009;119(4):252–265. [DOI] [PubMed] [Google Scholar]
- [5].Tifratene K, Robert P, Metelkina A, Pradier C, Dartigues JF. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology. 2015; 85(4): 331–338. [DOI] [PubMed] [Google Scholar]
- [6].Hampel H, Vergallo A, Perry G, Lista S. The Alzheimer precision medicine initiative. Journal of Alzheimer’s Disease. 2019;68(1):1–24. [DOI] [PubMed] [Google Scholar]
- [7].Reitz C Toward precision medicine in Alzheimer’s disease. Annals of translational medicine. 2016;4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mehta D, Jackson R, Paul G, Shi J, Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert opinion on investigational drugs. 2017;26(6):735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Campbell NL, Unverzagt F, LaMantia MA, Khan BA, Boustani MA. Risk factors for the progression of mild cognitive impairment to dementia. Clinics in geriatric medicine. 2013;29(4):873–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Santiago JA, Potashkin JA Potashkin. The impact of disease comorbidities in Alzheimer’s disease. Frontiers in aging neuroscience. 2021; 13:631770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Forti P, Maioli F, Pisacane N, Rietti E, Montesi F, Ravaglia G. Atrial fibrillation and risk of dementia in non-demented elderly subjects with and without mild cognitive impairment. Neurological research. 2006;28(6):625–629. [DOI] [PubMed] [Google Scholar]
- [12].Nelson L, Gard P, Tabet N. Hypertension and inflammation in Alzheimer’s disease: close partners in disease development and progression! Journal of Alzheimer’s Disease. 2014;41(2): 331–343. [DOI] [PubMed] [Google Scholar]
- [13].Inzelberg R, Massarwa M, Schechtman E, et al. Estimating the risk for conversion from mild cognitive impairment to Alzheimer’s disease in an elderly Arab community. Journal of Alzheimer’s disease. 2015;45(3):865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pal K, Mukadam N, Petersen I, Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Social psychiatry and psychiatric epidemiology. 2018;53(11):1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ma F, Wu T, Miao R, Zhang W, Huang G. Conversion of mild cognitive impairment to dementia among subjects with diabetes: a population-based study of incidence and risk factors with five years of follow-up. Journal of Alzheimer’s Disease. 2015;43(4):1441–1449. [DOI] [PubMed] [Google Scholar]
- [16].Lee GJ, Lu PH, Hua X, et al. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s disease-related regions. Biological psychiatry. 2012;71(9):814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34(4):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Elias PK, Elias MF, D’agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosomatic medicine. 2005;67(1):24–30. [DOI] [PubMed] [Google Scholar]
- [19].Durazzo TC, Mattsson N, Weiner MW, Alzheimer’s Disease Neuroimaging Initiative. Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimer’s & Dementia. 2014;10: S122–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhou S, Zhou R, Zhong T, Li R, Tan J, Zhou H. Association of smoking and alcohol drinking with dementia risk among elderly men in China. Current Alzheimer Research. 2014;11(9):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zheng C, Xu R. Molecular subtyping of Alzheimer’s disease with consensus non-negative matrix factorization. Plos one. 2021;16(5): e0250278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dementia and geriatric cognitive disorders. 2006;22(4):312–319. [DOI] [PubMed] [Google Scholar]
- [23].Clark LR, Delano-Wood L, Libon DJ, McDonald CR, et al. Are empirically derived subtypes of mild cognitive impairment consistent with conventional subtypes? Journal of the International Neuropsychological Society. 2013;19(6):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Libon DJ, Xie SX, Eppig J, Wicas G, et al. The heterogeneity of mild cognitive impairment: A neuropsychological analysis. Journal of the International Neuropsychological Society. 2010;16(1):84–93. [DOI] [PubMed] [Google Scholar]
- [25].Damian M, Hausner L, Jekel K, Richter M, et al. Single-domain amnestic mild cognitive impairment identified by cluster analysis predicts Alzheimer’s disease in the European prospective DESCRIPA study. Dementia and geriatric cognitive disorders. 2013;36(1–2):1–19. [DOI] [PubMed] [Google Scholar]
- [26].Kwak K, Giovanello KS, Bozoki A, Styner M, et al. Subtyping of mild cognitive impairment using a deep learning model based on brain atrophy patterns. Cell Reports Medicine. 2021;2(12):100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ezzati A, Zammit AR, Habeck C, Hall CB, et al. Detecting biological heterogeneity patterns in ADNI amnestic mild cognitive impairment based on volumetric MRI. Brain imaging and behavior. 2020;14(5):1792–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dadar M, Shafiee N, Collins L, Camicioli R, Duchesne S. Subtyping mild cognitive impairment based on imaging and CSF biomarker levels. Alzheimer’s & Dementia. 2021;17: e054129. [Google Scholar]
- [29].Gamberger D, Lavrač N, Srivatsa S, Tanzi RE, Doraiswamy PM, et al. Identification of clusters of rapid and slow decliners among subjects at risk for Alzheimer’s disease. Scientific reports. 2017;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nettiksimmons J, DeCarli C, Landau S, Beckett L, Alzheimer’s Disease Neuroimaging Initiative. Biological heterogeneity in ADNI amnestic mild cognitive impairment. Alzheimer’s & Dementia. 2014;10(5):511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li HT, Yuan SX, Wu JS, Zhang XZ, et al. Molecular Subtyping of Mild Cognitive Impairment Based on Genetic Polymorphism and Gene Expression. The journal of prevention of Alzheimer’s disease. 2021;8(2):224–233. [DOI] [PubMed] [Google Scholar]
- [32].Nezhadmoghadam F, Martinez-Torteya A, Treviño V, Martínez E, et al. Robust Discovery of Mild Cognitive impairment subtypes and their Risk of Alzheimer’s Disease conversion using unsupervised machine learning and Gaussian Mixture Modeling. Current Alzheimer Research. 2021;18(7):595–606. [DOI] [PubMed] [Google Scholar]
- [33].Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s & Dementia. 2005. Jul 1;1(1):55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stern Y Cognitive reserve and Alzheimer disease. Alzheimer Disease & Associated Disorders. 2006;20: S69–S74. [DOI] [PubMed] [Google Scholar]
- [35].Petersen RC, Smith GE, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. Jama. 1995;273(16):1274–8. [PubMed] [Google Scholar]
- [36].Devanand DP, Bansal R, Liu J, Hao X, et al. MRI hippocampal and entorhinal cortex mapping in predicting conversion to Alzheimer’s disease. Neuroimage. 2012;60(3):1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Q, Li Y, Zheng C, Xu R. DenseCNN: A Densely Connected CNN Model for Alzheimer’s Disease Classification Based on Hippocampus MRI Data. AMIA Annual Symposium Proceedings. Vol. 2020.p 1277. American Medical Informatics Association. [PMC free article] [PubMed] [Google Scholar]
- [38].Katabathula S, Wang Q, Xu R. Predict Alzheimer’s disease using hippocampus MRI data: a lightweight 3D deep convolutional network model with visual and global shape representations. Alzheimer’s Research & Therapy. 2021;13(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu Y, Zhang X, He Y, et al. Predicting Alzheimer’s disease based on survival data and longitudinally measured performance on cognitive and functional scales. Psychiatry research 291 (2020): 113201. [DOI] [PubMed] [Google Scholar]
- [40].Isella V, Villa L, et al. Discriminative and predictive power of an informant report in mild cognitive impairment. Journal of Neurology, Neurosurgery & Psychiatry. 2006;77(2):166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang YL, Chen J, Du ZL, Weng H, et al. Plasma p-tau181 Level Predicts Neurodegeneration and Progression to Alzheimer’s Dementia: A Longitudinal Study. Frontiers in neurology. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lewczuk P, Ermann N, Andreasson U, Schultheis C, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s research & therapy. 2018;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jorgensen M, Hunt L. Mixture model clustering of data sets with categorical and continuous variables. Proceedings of the Conference ISIS. 1996; Vol. 96; pp. 375–384. [Google Scholar]
- [44].Marbac M, Sedki M, Patin T. Variable selection for mixed data clustering: application in human population genomics. Journal of Classification. 2020;37(1):124–142. [Google Scholar]
- [45].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in medicine. 1998;17(24):2815–2834. [DOI] [PubMed] [Google Scholar]
- [46].Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1996;50(3):163–170. [PubMed] [Google Scholar]
- [47].Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. Journal of the Royal Statistical Society: Series B (Methodological). 1977. Sep;39(1):1–22. [Google Scholar]
- [48].Schwarz G Estimating the dimension of a model. The annals of statistics. 1978;461–464. [Google Scholar]
- [49].Marbac M, Sedki M. VarSelLCM: an R/C++ package for variable selection in model-based clustering of mixed-data with missing values. Bioinformatics. 2019;35(7):1255–1257. [DOI] [PubMed] [Google Scholar]
- [50].Mandolesi L, Polverino A, Montuori S, et al. Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Frontiers in psychology. 2018; 9:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tao Q, Ang TF, DeCarli C, Auerbach SH, et al. Association of chronic low-grade inflammation with risk of Alzheimer disease in ApoE4 carriers. JAMA network open. 2018;1(6): e183597–e183597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhou M, Xu R, Kaelber DC, Gurney ME. Tumor Necrosis Factor (TNF) blocking agents are associated with lower risk for Alzheimer’s disease in patients with rheumatoid arthritis and psoriasis. PloS one. 2020;15(3): e0229819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zheng C, Fillmore NR, Ramos‐Cejudo J, Brophy M. Potential long‐term effect of tumor necrosis factor inhibitors on dementia risk: A propensity score matched retrospective cohort study in US veterans. Alzheimer’s & Dementia. 2022;18(6):1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]


