Introduction
Obesity is a clinical and public health epidemic. In most populations, women contribute more to the prevalence of obesity in adults than their male counterparts. The biopsychosocial changes experienced during various stages of the reproductive cycle might implicate excessive weight gain in women.[1] One such high-risk reproductive stage for gaining weight is the menopause transition experienced during midlife. In addition to biological transition, midlife women experience changes in their everyday lifestyle-related habits in a way that might promote unhealthy eating habits, sedentary lifestyles, and distress in the face of household and work responsibilities.[2] Further, weight gain promotes menopausal symptom severity, making it difficult to maintain healthy lifestyle-related behaviours and health-related quality of life.[3]
The introduction of corrective lifestyle-related habits has proven beneficial in managing weight and menopausal health.[4] Corrective lifestyle habits such as healthy dietary behaviour, physically active lifestyle, and psychological well-being are necessary for successful weight management. Most midlife women lack the motivation to initiate corrective practices as they prioritise their roles as mothers, homemakers, and workers over their own health. Besides, factors including poor musculoskeletal health, limited physical function, menstrual irregularities, and psychological distress interfere with adopting healthy lifestyle practices.[5] Considering these factors, it becomes necessary to incorporate strategies to address midlife-specific barriers while managing obesity in this group of women to initiate and sustain weight loss efforts.
Generally, midlife women receive generic weight management advice from healthcare professionals.[6] This advice is less actionable, so it rarely contributes towards correcting the existing lifestyle-related behaviours in midlife women. In contrast, comprehensive obesity care also lacks specificity to address issues faced by midlife women in managing weight and overall health. This care can be provided by a team of clinicians such as gynaecologists, physicians, and allied healthcare professionals, including nutritionists and exercise physiologists. The availability of such a team in the healthcare setting might be limited, especially in developing countries.[7] This underscores the need for the development of evidence and consensus-based recommendations related to diet, physical activity, and behaviour, which can be used by clinicians and/or any other healthcare provider for opportunistic management of overweight and obesity in midlife women.
Methods
The development and validation of the guideline were initiated to address the need for protocolised weight management measures specific to midlife women that can be implemented in day-to-day clinical practice at different healthcare settings. The guidelines were developed in two phases using standardised methodology as per the National Health and Medical Research Council (NHMRC): (i) development of recommendations and (ii) validation of the developed recommendations. Firstly, an exhaustive list of key clinical questions through literature search, expert opinion, and Delphi method was identified to be addressed by the guideline. In Phase I, a systematic review of the evidence, grading, and expert opinion were undertaken to formulate clinical practice recommendations for each clinical question. In Phase II, the clinical practice recommendations were peer-reviewed and validated using the Delphi method and graded using the GRADE approach via the experts participating in the Guideline Development Group (GDG).
Guideline Development Group (GDG)
Expert representatives from multidisciplinary fields including medicine, gynecology, psychology, psychiatry, physical medicine and rehabilitation, nutrition, and exercise physiology were part of the GDG. Further, chairperson and field experts from prominent national organisations such as the Department of Science and Technology, the Federation of Obstetric and Gynaecological Societies of India, Indian Menopause Society, Association of Physicians of India, Academy of Family Physicians of India, Association of Obstetricians & Gynaecologists of Delhi, Indian Dietetic Association, and the Indian Society of Clinical Nutrition also participated in the GDG. From academia, senior professors across five leading medical colleges of the country participated in the proceedings of GDGs. The role and responsibilities of a member of the GDG included finalisation and prioritisation of key clinical questions to be addressed in guideline, review of the available evidence, providing expert opinion, development of the recommendation for each clinical question, validation of the developed recommendations, and grading of the final recommendations.
Development and prioritization of key clinical question
Initially, a series of exploratory focus group discussions were conducted to identify the risk factors, barriers, and facilitators for appropriate weight management among midlife women.[8] Secondly, a comprehensive, valid and reliable screening tool was designed to evaluate the lifestyle-related practices and barriers faced in maintaining healthy eating, physical activity, and sleep practices. Finally, a cross-sectional survey on 504 midlife women was conducted to identify the daily lifestyle-related patterns and their association with menopausal symptom severity. These steps guided the team in identifying key critical areas that should be addressed for appropriate weight management in midlife women. In addition, an exhaustive literature review was conducted to determine key clinical areas of interest for translation into key clinical questions. Based on the stages of weight management, a comprehensive list of key clinical questions was classified into four domains: (i) initiation of discussion for weight management, (ii) screening and risk assessment of the target population, (iii) management of weight, and (iv) follow-up for weight sustenance. A series of online meetings with GDG were organised to prioritise and finalise the list of key clinical questions that can be addressed in this guideline document. The list of key clinical questions was peer-reviewed for its necessity in clinical practice, relevance, face, and content validity under two levels. At the first level review, key clinical questions were reviewed, modified, and finalised by a group of four to five topic-specific experts. The modified clinical questions were subjected to a second-level peer-review done by a larger group of experts, including experts from different disciplines, journal editors, and senior professors from leading organisations. Finally, 19 key clinical questions were prioritised for appropriate midlife health care.
Review of evidence to answer the clinical questions
To develop the recommendations, an exhaustive and systematic literature review was conducted independently for each clinical question.
Search for evidence: A search string was developed for each clinical question. The keywords related to each clinical question were identified through initial literature search, recommendations from experts, and discussion amongst the evidence review team. Three electronic databases (PubMed, Wiley, and Cochrane) were searched to extract relevant evidence.
Selection criteria: Studies published in peer-reviewed English-language journals and on human participants were selected. Methodological filters related to the study design were not applied at this stage to ensure an extensive and exhaustive search. The evidence team further performed the title, abstract, and full-text screening of articles. Any disagreements on selecting a manuscript were resolved by consensus among the evidence team members.
Eligibility criteria
Inclusion Criteria: Existing practice guidelines, position statement, consensus statement, systematic reviews on weight management and menopausal health and randomised control trials (RCT), experimental and observational studies recruiting midlife women at different menopausal stages, i.e. perimenopause, menopause, or post-menopause were included.
Exclusion Criteria: Studies reported in non-English language, published in non-peer-reviewed journals, and with limited access were excluded.
Data extraction and synthesis: The following study characteristics were extracted: year of publication, country, author, study design, sample size, and sample characteristics specific to the clinical question. The findings of the studies were reported in tables to form a write-up for a summary statement.
Development of clinical practice recommendations
The extracted high-quality data for each clinical question was presented with a summary of evidence supplemented by a narrative table. The evidence was circulated amongst the experts for evaluation to form necessary clinical practice recommendations. Experts were given the training to develop recommendations through online meetings on the following criteria: reviewing evidence, formulating evidence-based recommendations, providing expert opinion, reaching a consensus whenever there is a lack of evidence, and grading the recommendation based on quality. This led to the development of two types of recommendations that is, Recommendations Based on Evidence (RBE) and Recommendations Based on Opinion (RBO). The developed recommendations were subjected to a two-level peer-review process. The first review was conducted by a small group of topic-specific experts, and the second review with a large group of experts, including field experts, academicians and journal editors.
GRADE approach
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) evidence profile was constructed to determine the quality of evidence obtained for clinical questions [Tables 1 and 2]. Four grades: high, moderate, low, and very low were used to rate the quality of evidence.[9]
Table 1.
Quality of evidence
| Quality of evidence | Description |
|---|---|
| I | High-quality evidence |
| Based on evidence gathered from the literature search, there is substantial certainty that the true effect lies within the estimated effect. | |
| The high-quality evidence will include: | |
| (i) Well-designed and executed randomised control trials (RCTs) consisting of adequate randomisation, allocation and blinding, sufficient power and intention-to-treat analysis, and adequate measures for follow-up | |
| (ii) Meta-analysis including high-quality RCTs is also included | |
| (iii) Previously published good quality recommendations/consensus statements and/or position statements were given by an organisation or working group consisting of experts in that field. The quality of the recommendations should be established on the basis of the Appraisal Guideline for Research and Evaluation[6] | |
| II | Moderate quality evidence |
| Based on evidence gathered from the literature search, it is possible that the true effect lies close to the estimated effect. The moderate-quality evidence includes: | |
| (i) Well-designed and executed RCTs with minor methodological limitations impacting the confidence in the estimated effect | |
| (ii) Quasi-randomised trials with good methodological quality | |
| (iii) Systematic and meta-analysis of low-quality RCTs with limited quality | |
| III | Low-quality evidence |
| Based on the evidence gathered from the literature search, there is little certainty that the true effect is close to the estimated effect. | |
| The low quality of evidence includes: | |
| (i) Well-designed and executed RCTs with major methodological limitations affecting the confidence in estimated effect. | |
| (ii) Well-designed and executed non-randomised trials including intervention studies, cohort, and quasi-experimental studies, case-control studies with minor methodological limitations | |
| (ii) Observational studies with minor methodological limitations | |
| IV | Expert opinion |
| Very uncertain that the true effect is close to the estimated effect. | |
| Based on clinical experience, reasoning, and suggestions. | |
| There might be a small net benefit from the suggestion. Based on the feasibility, healthcare providers may incorporate the suggestion for weight management. |
Table 2.
Grades for the strength of recommendation
| Strength of recommendation | Description |
|---|---|
| A | Strong recommendation: |
| Quantum of benefit expected >>> Resource requirement/logistic needs | |
| Certainly, the net benefits, (i.e. the benefits in comparison to resources required/logistics needed for the service/intervention) outweigh the resource requirement for achieving optimal weight loss outcome. | |
| Clinicians and allied healthcare providers should universally adopt these recommendations as a standard practice to prevent and manage overweight and obesity in women at an individual, clinical, and public health level. | |
| B | Moderate recommendation: |
| Quantum of benefit expected >> Resource requirement/logistic needs | |
| It is moderately certain that the net benefit from the recommendation is moderate to substantial. | |
| These recommendations might not be a mandatory part of a standard weight management clinical practice; however, their implementation can prove beneficial in attaining significant weight loss outcomes. The implementation of these recommendations should be as per an individual’s preference, values, and settings. | |
| C | Weak recommendation: |
| Quantum of benefit expected > = < Resource requirement/logistic needs | |
| It is at least certain that there might be a small net benefit from the recommendation. | |
| These recommendations should be incorporated on the basis of resource availability, feasibility, cost-effectiveness and acceptability in the weight management program. |
Results
Section I: Initiation of discussion for weight management
1.1 When should a healthcare provider initiate structured counselling regarding weight management in midlife women?
Background
The menopausal transition is marked by the onset of irregularity in the menstrual cycle and menopausal symptoms, including hot flushes, physical decline, and emotional volatility.[2,10] In midlife, chronological ageing coupled with menopausal transition leads to several biological changes predisposing women to weight gain. The independent and interactive contribution of increasing age and menopausal transition remains debatable. Nevertheless, both these factors impact the daily lifestyle behaviours, including eating, activity, and sleep habits of midlife women in a way that might make them prone to excessive weight gain.[6] There is a need to understand at which stage during the menopausal transition should weight gain be identified as a potential detriment to the overall health and quality of life. Identifying these critical phases during midlife can help in the early initiation of weight management support by the healthcare provider.
Summary of evidence
In India, the mean age of initiation of the menopausal transition is 44 years, whereas 46 years is the mean age at menopause, which is lower than Caucasian women.[11] Similar findings are reported by a systematic review reporting that the age at menopause was 46.24 ± 3.38 years in Indian women.[12] Further, the mean age of early menopause due to premature ovarian insufficiency was 38 years, and late menopause was beyond 54 years. Some studies reported a positive association of menopause with an overall increase in body weight status.[13,14] However, a direct association of the overall increase in the weight status with hormonal changes during the menopausal transition could not be established. Menopause transition was related to an increase in visceral adiposity.[15,16] Women experienced a steady increase in weight and body fat during perimenopause and menopausal phases. During the menopausal transition, the accelerated gain in fat mass doubled, whereas a decline in lean muscle mass was observed. The rate of change in the body weight and fat accumulation stabilised before initiation of the post-menopausal phase.[17] Postmenopausal women with overweight and obesity have shown greater circulating estrogen levels than non-obese counterparts. This increase in estrogen is contributed by the peripheral aromatisation of the androgens in adipose tissues. It was previously hypothesised that excessive circulating estrogen levels are beneficial for maintaining menopausal and metabolic health. However, recent studies found out that the increase in estrogen levels is not associated with the protective cardiometabolic effects.[16]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 1.1.1 | Opportunistic screening and management of obesity should be delivered all through the lifespan of a woman. | IV A |
| 1.1.2 | In the late 30s, before menopause transition, women should be counselled about the added risk of menopause-related weight gain and body fat distribution. Opportunistic screening and management of obesity should be continued. | II A/B |
| 1.1.3 | In the early 40s, when women experience menopausal transition, intensive customised weight management counselling should be given. The emphasis should be on corrective lifestyle behaviour and handling health issues specific to menopausal transition such as menopausal symptoms, sleep disturbances, psychological distress, bone and joint health and other comorbidities. | II A |
Discussion
There is a lack of consistent literature on the independent and interactive nature of chronological ageing and menopausal transition on the weight status of menopausal women. Considering the mean age at menopause as 46 years, it is crucial to engage midlife women in weight regulation before menopausal transition.[11] Ideally, opportunistic screening and management should be delivered across the women’s lifespan (as presented in Table 1.1.1). Similarly, as per the Anklesaria staging menopausal system, at stage 1 during the perimenopausal phase, the advice should be shared to create awareness and manage obesity and other menopause-related health risks amongst midlife women.[18] In the later 30s, women should be made aware of experiencing a probable increase in their weight and total body fat status with the onset of the menopausal transition. At this phase, the healthcare providers should counsel women regarding the changes in the menstrual cycle and its probable association with weight gain. Because the age at menopause can vary amongst women belonging to different population groups, the experts have given a dual strength of recommendation to this clinical practice. In the late 40s, an opportunistic screening of weight and health status, followed by customised weight management, should be initiated and continued with the aim of achieving normal weight status. The healthcare provider should advise dietary and physical activity modifications using behavioural techniques for managing weight. In addition, other menopause-related health issues, including psychological distress, sarcopenia, and bone health, should also be addressed during weight management. It should be noted that the evidence for the recommendations mentioned above is derived mainly from studies on midlife women in the west. To our knowledge, there are no longitudinal studies that assess the changes in weight status and body composition during the menopausal transition in midlife women from India. The association of menopausal transition (independent of chronological ageing) and weight status needs to be assessed for the engagement of Indian women in weight-management practices. Thus, the recommendations should be revisited and revised every 5 to 10 years in the face of new evidence on the age of menopause in Indian women, changes in body composition during menopausal transition, and effective weight management practices.
Table 1.1.1.
Opportunistic screening and management of weight during reproductive stages
| Reproductive stage | Key opportunities to screen and discuss weight |
|---|---|
| Adolescents | Engagement in school, family, and community-based health education programs |
| Screening of adolescent visiting regarding other health conditions for weight status (as a vital clinical sign) at clinical set-ups (Criteria: overweight: BMI ≥85th percentile, obese: <95th percentile) | |
| Adolescent girls presenting with obesity-related complications such as polycystic ovary syndrome (PCOS) and metabolic disorder should be enrolled in a multidisciplinary program | |
| Pregnancy and delivery | Weight counselling during regular antenatal check-up |
| Gynaecologists should counsel women regarding postpartum weight retention and its health risk | |
| Encouraging women to enrol in post-delivery weight management program | |
| Postpartum | Post-delivery weight management programs as part of future gynaecologist visits |
| Promotion of breastfeeding as a preventive strategy for post-partum weight gain | |
| Paediatrician involved in immunisation of child should reinforce weight management | |
| Support groups: Discussion weight control during post-pregnancy | |
| Premenopausal | Screening of women at clinical set-ups for weight status (as a vital clinical sign) |
| Engagement in community and workplace weight management programs | |
| Referring women with obesity and associated metabolic complications to specialist or multidisciplinary teams | |
| Menopausal transition | Screening midlife women at different clinical departments such as medicine, obstetrics and gynaecology, orthopaedics, physical medicine and rehabilitation, and endocrinology |
| Engagement in community and workplace-specific healthcare intervention | |
| Provision of screening at religious centres | |
| Referring women with obesity and associated metabolic complications to specialist or multidisciplinary teams | |
| Post-menopausal | Screening of midlife women at different clinical departments such as geriatric medicine, obstetrics and gynaecology, orthopaedics, physical medicine and rehabilitation, and endocrinology |
| Engagement in community-based healthcare intervention | |
| Referring women with obesity and associated metabolic complications to specialist or multidisciplinary teams |
1.2: What are the components of knowledge, attitude and practice (KAP) that should be evaluated to plan a personalised weight management intervention in midlife women?
Background
The successful management of obesity and other lifestyle-related disorders depends on adopting behavioural techniques for correcting eating, activity, and other lifestyle-related habits.[19] The behavioural practices are motivated by pre-existing knowledge and attitude of an individual. In the literature, knowledge is defined as information and skills acquired using experience and education. Attitude is a value, belief, and feeling that predisposes an individual towards a particular behaviour and behavioural practices are habits and patterns associated with the maintenance of weight status.[20,21] KAP assessment is common in lifestyle-related diseases in clinical and community settings.[22] The clinician needs to understand the KAP of midlife women that promotes obesity. The KAP of core components associated with obesity in midlife women should include lifestyle practices, menopausal symptoms, bone health, and psychological distress.[2] Generally, the KAP can be assessed before the initiation of lifestyle management to identify the risk factors specific to middle-aged women that the clinician should attempt to modify during weight management. A few qualitative studies and cross-sectional surveys have independently assessed KAP for different obesity-related factors in midlife women.[23-25] In qualitative studies, the influence of social, cultural, and economic constructs on obesity-related behaviours has been highlighted. Across cross-sectional studies, KAP was assessed using self-developed or validated questionnaires that rate an individual’s competence in the three domains and correlate with weight and metabolic status.[26-28] A comprehensive assessment of KAP on lifestyle practices, menopausal symptoms, bone health, and psychological distress as risk factors for obesity in midlife is lacking in the current literature. Hence, this clinical question addressed the present evidence on KAP of critical components associated with the management of obesity in midlife women to deliver women-centric management of obesity.
Summary of evidence
The critical components evaluated for the initiation of management of obesity in midlife women included: weight and health-related measures, menopausal symptoms, bone health and psychological distress. The KAP of these components is considered necessary for customising the weight management regime for a midlife woman.[24,28-30] For weight and lifestyle-related parameters, women reported a lack of knowledge regarding corrective weight-related practices, lack of time, social eating, indulging in high-fat, sugar, and salt foods (HFSS), and reduced daily activity, led to obesity.[31,32] Four studies reported the interplay between the menopausal symptoms severity and psychological distress, leading to the consumption of energy-dense foods and limited physical activity in midlife women. The difficulty in sleeping due to hot flashes and mood disorders was associated with weight gain in midlife women.[33-35] Menopause led joint pain was the primary reason for limited participation in dedicated physical activity. Moreover, women had limited knowledge regarding age-related osteoporosis and measures that can be taken to maintain bone health such as getting a regular bone check-up, consuming foods rich in calcium, and consulting doctors for guidance on supplementation such as vitamin D and calcium for bone health.[27,30] In addition to limited knowledge, most women prioritised their roles as homemakers, working women and child care providers over their own health issues.[36,37] This self-sacrificing attitude coupled with limited support from family and friends in maintaining health-related behaviours in their day-to-day lifestyle should also be addressed for maintaining continuous and sustainable efforts for weight management and allied comorbidities.[24,36]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 1.2.1 | Assessment of KAP related to risk factors and consequences of obesity on the holistic health of women is recommended. | III A |
| 1.2.2 | Different modalities for weight management, barriers, and facilitators in their implementation should be evaluated and accounted for in the management plan. | III B |
Discussion
A comprehensive assessment of KAP should be done to identify key areas that the interventionist should attempt to modify during lifestyle intervention to achieve clinically significant weight loss. The recommendations were based on well-known, lifestyle and midlife-related risk factors experienced during the menopausal transition. However, the evidence was from independent studies on a particular risk factor (e.g., diet, mental health, joint health etc.). There were no data on a comprehensive assessment of KAP related to risk factors of obesity in midlife women, which would be relevant to guide future clinical and research practice. Besides, the effectiveness of KAP in providing customised weight management advice for achieving significant weight loss should be assessed using longitudinal studies or RCTs in future research. Considering the importance of KAP in customising weight management approaches in midlife women, experts agreed that a comprehensive assessment of KAP as a component of risk assessment of obesity in midlife women should be incorporated into clinical practice (shown in Table 1.2.1). Based on available resources in the healthcare or community setting, healthcare professionals can assess KAP via history taking, group discussions and/or interview schedule with a structured questionnaire. The key questions that can be assessed in resource-constrained settings are presented in Table 1.2.2.
Table 1.2.1.
KAP regarding obesity, associated risk factors for weight gain, and appropriate weight management in midlife women
| Knowledge | Attitude | Practice |
|---|---|---|
| Knowledge associated with vegetables and fruit consumption Awareness of weight gain and health Osteoporosis and its complications Calcium-rich dietary sources Perceived benefits of exercise, adequate calcium intake, regular physical activity, dairy intake Management of menopausal symptoms Contraception use and weight gain |
Easy to gain weight but difficult to lose Dissatisfaction with one’s appearance Body shape concerns Self-rated physical health Attitude towards menopause Health motivation |
Sedentary lifestyle Not engaging in any exercise Eating during socialisation High calorie and fat intake Having limited free time to manage diet and exercise Limited dairy intake Stress-eating Regular health check-ups Regular visits to the gynaecologist Maintaining menopausal hygiene Self-management of menopausal symptoms |
Table 1.2.2.
Questions for assessment of KAP regarding obesity in midlife women
| Domains | Key questions that can be enquired while initiating clinical management |
|---|---|
| Knowledge | How can obesity cause health-related complications in midlife women? What are the common menopausal symptoms and how can you manage them? What is the importance of bone health during the menopausal phase? What lifestyle modifications should you incorporate into your daily routine to avoid weight gain? |
| Attitude | How motivated are you to participate in a weight management program at this stage of your life? How ready are you to change your current dietary and physical activity habits at this point in time? How do you feel about your menopausal health during the transition? |
| Practice | What are the different food items that you usually consume in a day? What kind of activities do you participate in on a regular day? How do you maintain your bone health? What are common measures that you adopt for the management of menopausal symptoms? How do you cope with emotional distress? |
*These questions should be asked as an opportunity to initiate discussion. Healthcare providers should supplement the information received from these questions with appropriate facts through objective diagnosis and assessment, if and when required.
1.3 Which healthcare providers can be involved in the management of overweight and obesity in midlife women?
Background
Obesity is a complex and multisystem disorder.[38] According to the international guidelines, preventive and curative obesity treatment should be delivered to individuals across the healthcare system.[39] The comprehensive treatment of obesity and allied complications requires a multicomponent and multidisciplinary approach. A multidisciplinary approach manages treatment components with the help of different healthcare professionals including a primary care physician, family physician and/or a gynaecologist with expertise in pharmacotherapy, an exercise physiologist, a dietitian, and a psychologist to provide comprehensive weight management counselling.[40] Despite the consensus on promoting multicomponent and multidisciplinary approaches amongst obesity experts, there are no specific guidelines or position statements on the operationalisation of these treatment modalities in daily clinical practice.[41] The limited application of multidisciplinary approaches can be due to limited healthcare resources such as a low doctor-to-patient ratio, time constraints, lack of auxiliary healthcare teams, and infrastructure, especially in developing countries.[42] Considering the challenges in the management of obesity in healthcare settings, it becomes essential to identify the roles and responsibilities of clinicians and allied healthcare professionals that can make multidisciplinary teams. Furthermore, these teams should be educated and trained to equip healthcare professionals to assess obesity risk, provide brief weight counselling advice, and referrals specialists at healthcare centres.[43]
Summary of evidence
The comprehensive management of overweight and obesity includes three key components: diet, physical activity and behavioural strategies. In 29 pertinent weight-loss trials, the treatment modalities were managed by either a multidisciplinary team, a core team of two healthcare professionals or single-handedly by a dietitian. In seven studies, a qualified dietitian provided lifestyle counselling to the participants to manage their weight.[44,45,46] As per 11 studies, weight management teams commonly consist of two healthcare professionals from different specialities. Out of 11 studies, nine studies opted for a team of dietitians and exercise experts such as physiotherapists, exercise physiologists, exercise specialists or personal trainers for weight management.[47,48,49] In three studies, lifestyle advice was coupled with behavioural techniques counselled by a team of dietitians and behaviour management experts such as psychologists, behaviour science experts or clinical health psychologists.[50,51,52] It was observed that dietitians are an essential component of all the core teams. Nine studies reported that a multidisciplinary team consisting of a dietitian, exercise physiologist, and behavioural therapist were a part of a comprehensive weight management program.[53,54,55]
Clinical practice recommendations
| No. | Recommendation | Grade |
|---|---|---|
| 1.3.1 | The protocolised weight management module should be implemented by any healthcare worker who encounters a woman in her midlife for routine screening or specific health conditions. | IV A |
| 1.3.2 | Wherever feasible, a multi-disciplinary team consisting of primary care physicians, clinicians, dietitians, and exercise physiologists/physiotherapists should be involved in weight management of midlife women. Specialists including psychologists/psychiatrists, endocrinologist and orthopedicians should be involved, whenever indicated. | I A |
| 1.3.3 | All healthcare workers should be empowered with the knowledge and skills for the prevention, diagnosis and treatment of obesity in midlife women. | IV B |
Discussion
The effective management of weight in healthcare settings requires a multidisciplinary team. Despite the importance of multidisciplinary teams, the onus of effective preventive and curative obesity care lies on all the healthcare professionals who encounter midlife women with overweight and obesity. Often, midlife women meet primary care physicians and family physicians as the first point of contact in the healthcare system. The incorporation of family care physicians as a part of the core weight management team or an extended weight management team is vital for initiating effective management at the healthcare level. In literature, dietitians-led weight management was most commonly observed across weight-loss trials. The inclusion of dietitians in core weight management teams and/or frequent referrals to dietitians can be helpful to impart detailed lifestyle modification counselling that might not be feasible for a general practitioner or gynaecologist in a busy outpatient setting. Experts believe further attempts can be made based on the available healthcare resources to involve specialists and clinicians such as exercise physiologists, psychologists, and orthopaedics who have decisive roles in obesity management in midlife women. Considering the limited healthcare resources in developing countries such as India, a dedicated multidisciplinary approach can be limited to established tertiary healthcare set-ups. Experts suggest that interprofessional education and training sessions to discuss professional attitudes, skills and barriers faced in everyday clinical practice for obesity management can be the first step to mitigate this resource constraint. Every healthcare provider should be trained in opportunistic screening and generalised management of obesity in midlife women.
1.4: What could be the effective ways of delivering pertinent information to midlife women regarding the management of overweight and obesity?
Background
Health promotion counselling is a feasible, cost-effective and long-term solution for the effective management of obesity. The National Health and Medical Research Council (NHMRC) (2013) recommendations refer to the importance of health promotion and lifestyle advice to identify, assist and treat individuals with obesity.[56] The lifestyle counselling paradigm has evolved over the years from personalised counselling sessions to web-based interventions.[57,58] Personalised counselling can be defined as one-to-one counselling sessions with trained healthcare providers focused on managing an individual’s weight-related issues.[59] Group counselling provides an opportunity to discuss and share similar weight-related concerns amongst a group of participants in the presence of trained healthcare professionals in a real social setting.[60] In the current clinical practice, the traditional one-to-one counselling is supplemented with web-based interventions that help in improving compliance to the lifestyle advice by providing personalised feedback and addressing challenges in real-time.[61] Web-based counselling utilises technological components such as the internet, mobile application and online social support groups to counsel women on weight-related issues.[62] Nowadays, web-based counselling techniques have replaced the non-digital formats for nutrition education consisting of patient education material such as pamphlets, brochures, and e-newsletters. However, there is limited evidence on the mode of lifestyle counselling (independent or in combination) that will effectively manage weight in midlife women. Further, healthcare professionals need to understand the most effective and promising way of delivering information for counselling on weight management components (diet, physical activity and behaviour) for widespread acceptance of advice. To date, the role of different counselling techniques and modalities of communication in improving odds of achieving successful weight loss and prevention of weight gain remains unclear. Hence, this clinical question was undertaken to identify the most promising ways of counselling and delivery of patient education advice for effective management of obesity in clinical and community settings.
Summary of evidence
The pertinent studies identified used a range of counselling techniques, including personalised counselling sessions, group counselling, telephonic counselling sessions, or a combination to achieve significant weight loss. A systematic review comparing the role of group and individualised counselling for weight management reported that group counselling led by a psychologist was more effective for female participants.[63] It was also reported that in-person and more frequent contact were not associated with more significant weight loss.[64] In addition to these techniques, web-based counselling sessions were also opted for weight management. Four meta-analysis and systematic reviews assessed the role of technology-based interventions suggesting different web-based interventions were minimally effective in weight management. In comparison to traditional personalised counselling techniques, the efficacy of the web-based intervention was inconsistent.[65] A recent systematic review suggested that web-supported video conferencing sessions are effective in maintaining physical activity.[58]
In addition to counselling, various forms of nutrition education material have been used across trials to facilitate weight loss. Education material such as printed material, exercise videos and mobile application for self-monitoring is available.[44,66,67] A systematic review has reported that dietary counselling was mostly given as verbal advice coupled with handouts/brochures. There were no referrals for comprehensive weight management to the dietitians. Three-fourth of the participants were advised to walk for physical activity without providing any tailor-made activity plan.[68] It was suggested that a toolkit approach should be undertaken, including evidence-based and practical dietary, physical activity, and behavioural advice.
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 1.4.1 | A combination of face-to-face and technology-supported distance counselling should be planned for the management of weight in midlife women with overweight and obesity. | IV A |
| 1.4.2 | Internet and mobile-based applications should be used proactively for improving compliance by enhancing patient education, motivation, self-monitoring, personalised feedback, and managing challenges faced in appropriate weight management practices. | II A |
| 1.4.3 | A toolkit consisting of education material apprising midlife women about menopausal transition and its impact on weight status and key management strategies can be developed to be distributed and utilised across healthcare settings. | IV B |
Discussion
A combined counselling regime consisting of individualised and internet-based distance counselling sessions can help to improve the odds of achieving weight loss success in the general population and midlife women. Experts believed that healthcare providers should decide the mode of counselling based on several factors, including infrastructure, availability of resources, the expertise of the healthcare provider, clinician to patient ratio, and characteristics of the patient attending healthcare settings. It is important to note the advantages and disadvantages of each counselling method, education tools to customise the weight management approach (as depicted in Table 1.4.1 and 1.4.2). The experts recommended a uniform toolkit approach to provide nutrition, physical activity and behavioural strategies for management of weight in midlife women across health care settings. This toolkit should include health education material that can be easily used for counselling by allied healthcare professionals, especially in rural settings. Currently, social media has been identified as an upcoming cost-effective tool for increasing patient engagement, health promotion and social support at an individual and community level.[69]
Table 1.4.1.
Advantages and disadvantages of counselling techniques
| Counseling technique | Advantage | Disadvantage |
|---|---|---|
| Individualised counseling | One-on-one attention | More expensive |
| Intense and comprehensive therapy | Lack of social support | |
| Focused and tailored treatment | Requires human resource | |
| Personalised feedback | Time intensive | |
| High level of confidentiality | ||
| Group counseling | Cost-effective | Requires space |
| Social support | Limited opportunities for managing more specific | |
| Resource-saving | individual needs | |
| New perspectives and diversity in opinion | Less confidentiality and trust | |
| Social support | Hesitancy | |
| Less focus and attention towards each individual | ||
| Web-based counseling | Cost-effective | Limited opportunities for attending to more specific |
| Time-efficient | individual needs | |
| Enhanced availability and easy accessibility | Data security issues | |
| Improved adherence | Lack of non-verbal signals | |
| Long-term efficacy | Need of technology | |
| Privacy and anonymity | Low need or desire for in-person contact | |
| Close contact with a healthcare professional at any time | ||
| Improved discretion and comfort | ||
| Reduced barriers related to patient disengagement, | ||
| geographical distance, socioeconomic status | ||
| Mass awareness | Cater to a broad-range audience | Lack of non-verbal signals |
| Cost-effective | Need of technology | |
| Time-saving | Lack of effectiveness | |
| Social support | No long-term effect | |
| Bridge geographical distance | Less opportunity for individual needs | |
| Anonymity |
Table 1.4.2.
Advantages and disadvantages of various modes used for counseling
| Modes used for counselling | Advantages | Disadvantages |
|---|---|---|
| Posters, brochure, pamphlet and leaflets | Cost-effective | No long-term effect |
| Easy to distribute | ||
| Time-saving | ||
| Connect with the target audience | ||
| Informative | ||
| Presentations and videos | Broad population reach | Limited interaction |
| Informative Easily accessible | ||
| Cost-effective | Requires time for planning and content | |
| Social media | Easy access | Lacks social-emotional connection |
| More flexibility | Availability and varied understanding of | |
| Anonymity | technology | |
| Cost-effective | Lacks feedback and follow-up | |
| Wider reachability | ||
| User friendly | ||
| Language friendly | ||
| E-counselling tools: websites, e-mail, group chat | Accessible | Time-delayed format |
| Convenient | Absence of visual and vocal cues | |
| Cater to masses as well as individuals | Greater potential for misinterpretation | |
| Bridge geographical distance | Varied understanding of technology | |
| Increased follow-up | ||
| Video conferencing | Accessible | Availability of technology |
| Convenient | Decreased sense of intimacy | |
| Real-time | ||
| More privacy | ||
| Bridge geographical distance |
Section II: Screening and risk assessment of midlife women
2.1: What BMI cut-off and other anthropometric parameters should determine the need to initiate weight management intervention in midlife women?
Background
Body mass index (BMI) is a universally accepted method for diagnosing overweight and obesity.[70] BMI is an index of weight and height, expressed as kg/m2. Ideally, BMI is used as a measure of adiposity. The internationally accepted BMI cut-offs and corresponding components of body weight (body fat, muscle mass and total water) are known to vary in different ethnic communities.[71] Because the internationally accepted BMI cut-offs are based on data from the western population, ethnic variation in BMI cut-offs for Asian Indians should be addressed. In addition to BMI, other anthropometric parameters such as waist and hip circumference and their ratios such as waist to height ratio (WHtR) and waist to hip ratio (WHR) can be used for the assessment of generalised and central obesity. Waist circumference and its ratios are important anthropometric parameters associated with visceral adiposity and cardiometabolic health.[72] In resource-rich settings, methods such as Bioelectric Impedence Analysis (BIA) and Dual X-ray Absorptiometry (DEXA) can be used for objective assessment of total body fat, skeletal and lean muscle mass.[73] The choice of anthropometric measure opted for diagnosis is dependent on several factors including technical know-how, availability of instruments, healthcare personnel, infrastructure, and the clinician to patient ratio. It is important to identify anthropometric parameters, which are simple, feasible, cost-effective and efficacious in identifying generalised and central obesity status in clinical practice.
Summary of evidence
The optimum BMI coupled with waist circumference cut-off has been identified across the literature to diagnose overweight and obesity. Globally, for the categorization of BMI, World Health Organisation (WHO) suggests an individual with a cut-off of 25–29.9 kg/m2 as overweight and ≥ 30 kg/m2 as obese. It was identified that the globally recommended cut-off for defining overweight and obesity should be lowered in different Asian population groups due to ethnic variation in the total body fat and fat-free mass. The Indian Consensus Group (2009) recommended a cut-off value of 23–24.9 kg/m2 as an indicator of overweight individuals and ≥25 kg/m2 as an indicator for obese individuals.[74] Across literature on lifestyle modification in menopausal women, the BMI range was 23–45 kg/m2.[75,76,77]
In addition to BMI, the consensus statement from a working group reported that waist circumference should be made a vital sign for clinical assessment.[72] According to the WHO, women with waist circumference > 88 cm can be at risk of at least one cardiometabolic risk factor.[78] For Asian Indians, a woman > 80 cm waist circumference should seek help from a healthcare practitioner for management of obesity-related risk factors and women with waist circumference > 72 cm should avoid gaining weight and incorporate physical activity to avoid having any cardiometabolic risk factor in later life.[74] We found six trials that assessed the waist circumference at the baseline. Out of which, four studies defined the waist circumference cut-off as 80 cm and two studies defined waist circumference cut-off as 88 cm.[79,80,81,82] We found three studies assessing baseline total body fat for midlife women enrolling in lifestyle modification intervention, with a cut-off of more than 32% in one study[83] and more than 35% in one RCT and systematic review. Only four studies assessed the total body fat percentage at the baseline in midlife women undergoing weight loss intervention.[75,79,83]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 2.1.1 | BMI and waist circumference should be used independently for population- and clinic-based cardiometabolic risk stratification and other obesity-related diseases. | I A |
| 2.1.2 | For initiating a weight management intervention, the class of generalised obesity should be identified according to the BMI-cut-off 18.5-22.9 kg/m2: Normal weight 23.0-24.9 kg/m2: Overweight ≥25 kg/m2: Obesity |
I A |
| 2.1.3 | For initiating weight management intervention, the cut-off for waist circumference should be as below: Waist Circumference less than or equal to 72 cm: normal Waist Circumference more than 72 cm but <80 cm: associated with one cardiometabolic risk factor-initiate weight management advice Waist Circumference more than 80 cm: associated with cardiometabolic comorbidities-initiate intensive weight management |
I A |
Discussion
We found robust evidence that suggests that BMI and waist circumference are associated with risk of general and abdominal adiposity, cardiometabolic risk and obesity-related diseases. The universally recommended BMI values for overweight (≥ 25 kg/m2) and obesity (≥ 30 kg/m2) were established on the basis of morbidity and mortality data from the white adult population.[70] In Asian Indians, higher body fat and higher cardiometabolic comorbidities are observed at a lower BMI status.[84] This raises concerns in clinical practice as these individuals who are metabolically obese and predisposed to developing hyperlipidaemia, diabetes, blood pressure and atherosclerosis are misclassified as individuals with normal BMI. Considering a greater cardiometabolic risk at a lower BMI, a revision of the BMI cut-off for Asian Indians was suggested by national recommendation bodies as 23–24.9 kg/m2 for overweight and 25 kg/m2 or more as obese. It should be noted that clinicians and healthcare providers should classify midlife women according to the Asian Indian specifications in their daily clinical practice, rather than the internationally accepted guidelines.
In addition to BMI, waist circumference should be utilised in the assessment of generalised and abdominal obesity, especially to identify women who are metabolically obese presenting with normal weight (MONW) in clinical settings. These anthropometric parameters can be used to screen women at high risk of cardiometabolic disease and obesity-related disorders for timely initiation of treatment. Considering the importance of waist circumference in assessing cardiometabolic risk factors in an individual, a consensus on cut-offs of waist circumference in the adult population was recommended. For women, a waist circumference of more than 88 cm was considered as an indicator of cardiometabolic risk.[78,85] In Asian women, a waist circumference of 80 cm was considered appropriate in identifying cardiovascular risk factors in comparison to 88 cm, which was internationally accepted.[74] Furthermore, ratios such as WtHR and WHR are used as an indicator of metabolic health. Evidence suggests that waist circumference alone is a vital responsive clinical sign of cardiometabolic health in comparison to WHR.[72]
Experts suggested that measurement of anthropometric parameters BMI and waist circumference should be made a part of routine clinical practice as a vital clinical sign for health assessment of midlife women.
In addition, clinicians should also stratify treatment at different BMI cut-offs for optimal utilization of resources for diagnosis and treatment. The intensity of weight management advice should be in accordance with deviation from the ideal weight and waist circumference. The use of pharmacotherapy should be incorporated in the treatment only as per indications by the treating clinician. In addition to cut-offs, the availability of resources should also be noted while planning obesity care, especially in resource-constrained settings such as anganwadi, community clinics and primary healthcare centres (PHC).
2.2: How should dietary practices of midlife women be evaluated?
Background
The assessment of lifestyle-related risk factors is an important step in managing weight in midlife women. In clinical practice, a detailed dietary history consisting of current caloric intake, adequacy of macronutrient composition (carbohydrate, protein and fat), consumption of HFSS foods, meal pattern, portion size, eating out, and nutritional deficiencies is a common method of dietary assessment.[86] For an objective assessment in terms of quantity and quality, traditional methods such as twenty-four-hour recall and food frequency questionnaires (FFQ) can be administered by trained healthcare professionals.[87] In addition, most weight-loss trials administered valid and reliable questionnaires to assess different aspects of eating behaviour.[45,88-90] The choice of the assessment method depends on a number of factors such as the aim of assessment, the expertise of the healthcare provider, availability of time, participant burden, infrastructure and method of interpretation of collected information. It is commonly observed that a majority of clinicians are involved in general dietary management as a part of midlife care.[2] It becomes important to identify simple, practical and cost-effective ways for dietary assessment, which can facilitate holistic dietary counselling, especially in resource-constrained settings with limited or no availability of a nutritionist or a dietitian.
Summary of evidence
A total of 15 instruments were identified for dietary assessment of the individuals participating in weight-loss interventions. Only one study assessed the dietary quality using the healthy eating index (HEI)-2010.[91] The usual dietary intake of the participants was most commonly assessed at baseline in weight loss trials. A total of nine studies assessed usual dietary intake by administering 24-hour dietary recall (three weekdays and one weekend)[92] Women’s Health Initiative FFQ,[89] Vioscreen (an electronic FFQ),[44] four-day food record[48] and dietary food logs.[83] The frequency, type and distribution of meals were assessed via a meal pattern assessment grid in two studies.[49,89] The frequency of intake of HFSS foods was assessed using two scales including fat-related intake questionnaires,[93] and Connor Diet Habit Survey.[94] The eating behaviour was assessed via a 51-item valid and reliable Three-Factor Eating Behavior Questionnaire(TFEQ) measuring three domains: Cognitive Restraint, Disinhibition, and Hunger on a four-point Likert scale.[88,95,96] Only two studies measured the barriers faced in maintaining healthy eating in an interview schedule to assess the factors such as emotional eating, daily mechanics of healthy eating and lack of social support on a five-point Likert scale.[97,98]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 2.2.1 | The detailed dietary evaluation should include an assessment of the usual meal pattern (including the number of food items consumed) and dietary habits (including skipping meals, typical frequency of HFSS usual frequency of eating out, emotional/stressful eating). | I A |
| 2.2.2 | Twenty-four-hour dietary recall and food frequency questionnaire for 3 days (2 weekdays and 1 weekend) should be used for dietary evaluation, if feasible. Energy, macronutrient, fibre intake should subsequently be calculated. Alternatively, a short validated questionnaire can be used.* | II B |
| 2.2.3 | Dietary intake of foods rich in protein, iron, and calcium should also be assessed while taking dietary history. | IV B |
| 2.2.4 | The barriers faced by midlife women to maintain a healthy diet in their daily lifestyle should be enquired. | IV B |
*Refer to Annexure 4.
Discussion
The recommendations were based on guidelines, position statements and RCTs in the general population and midlife women for the management of overweight and obesity. The evidence suggests that assessment of current caloric intake, dietary habits related to health risks (such as sarcopenic obesity, cardiometabolic distress, osteoarthritis), and micronutrient deficiencies is a crucial step in the dietary management for weight loss in midlife women.[99,100] Ideally, midlife women should be referred to dietitians and/or nutritionists to assess current dietary intake using a 24-h dietary recall method coupled with FFQ. For clinicians and researchers interested in utilizing traditional tools for dietary assessment, a detailed description of available tools with their advantages and disadvantages is presented in Table 2.2.1. However, these methods suffer from practical limitations such as the need for technical knowledge, are time-consuming, and are difficult to interpret, especially in resource-constrained settings. Experts believed that a detailed dietary history should be considered a simple, practical and feasible assessment method in daily clinical practice. For ease of practice, the details on specific points to be considered while taking the dietary history are presented in [Table 2.2.2]. Considering the resource availability, practitioners can opt for a combination of methods for dietary assessment that can provide them with complete information on current dietary practices in midlife women. Special attention should be given to assessing the regular consumption of foods rich in protein, iron, and calcium. Healthcare practitioners can assess the intake of foods rich in protein and other micronutrients in two ways: (i) quantitatively via 24-h dietary recall method or a semi-quantitative FFQ and/or (ii) qualitatively via dietary history. In the presence of any clinical symptoms related to micronutrient deficiencies, a detailed clinical history, assessment of clinical signs and confirmatory laboratory tests (if feasible) should be planned under the guidance of a clinician. Diet diversity can also be checked via an easy to administer checklist like the FIGO Nutrition checklist consisting of six questions on consumption of food groups to assess the dietary quality. This checklist can be completed before the doctor’s appointment at the outpatient setting.[101] This checklist can be customised and validated for Indian midlife women.
Table 2.2.1.
Advantages and disadvantages of traditional tools used for dietary assessment
| Tools/Description | Advantages | Disadvantages |
|---|---|---|
| 24-h recall (Retrospective assessment method of dietary intake over 24 h) |
Precise method if interviewed efficiently Less burden on the respondent Sensitive to ethnicity Captures eating habits such as meal pattern, preparation methods | Administered skilled interviewer Recall bias Time-consuming Accuracy depends on respondent’s literacy and ability to describe the food and portion size |
| Food frequency questionnaire (Retrospective method Assessment of frequency of foods consumption over time) Types: Qualitative: Frequency of food consumed only Semi-quantitative: Frequency along with portion size consumed, for example, small, average, large Quantitative: Portion size enquired |
Helpful for long term dietary intake estimation Capture food diversity, specific nutrient or food group Less burden on researcher and participant Cost-efficient More accurate and culture-specific Less intensive analysis | Relies on participant’s memory Chances of misreporting May lead to underreporting if the food list is not comprehensive |
| Food diary/records (Record of all food items consumed Amount of food consumed is either estimated or weighed) | More accurate Detailed information on dietary intake Good estimates of short term total dietary intake and total nutrient intake Little reliance on participants’ memory Cost-effective | High participant burden Applicable for trained/literate participant Labour intensive. Time-consuming analysis Chances of misreporting Estimated food diaries rely on one’s ability to describe portion size |
| Semi-quantitative weighed food record (Portion sizes quantified (weight of foods consumed in a day/estimation using household measures)) |
Direct and simple recording of all consumed foods Does not rely on respondent’s memory | High participant burden Accuracy depends on the motivation of the respondent Requires literate/trained participants Time-consuming Can lead to undertreatment |
Table 2.2.2.
Important points of consideration while taking a comprehensive dietary history
| Type of diet: Amount and quality of usual calorie intake should be assessed |
| Meal pattern: Assess eating habits in terms of frequency and timings of the meal consumptions. Unhealthy eating habits such as meal skipping habits, longer meal gaps and energy-dense meals should be marked. |
| Portion size: Estimation of accurate food portions. Identify large portion size, energy-dense products and second helpings. |
| Macronutrient intake: The proportion of fat, protein, fat and fibre intake should be noted. |
| Protein intake: Daily consumption of adequate portions of foods high in protein including legumes, meats, dairy and nuts. |
| Fat intake: Consumption of fried, processed foods and bakery products should be asked along with frequency and portion size. The amount and quality of oil on a daily basis should also be noted. |
| Fibre intake: Fiber intake includes fruits and vegetables, whole grains and legumes and nuts and seeds. |
| Meal preparation: Method of cooking, intake of processed and convenience food items should also be assessed. |
| Eating out habits: Frequency and quality of food consumed outside the home. |
Midlife women presenting with signs and symptoms of iron deficiency anaemia can be assessed through (i) examination of clinical signs and symptoms like pallor of eyelids, tongue, nail beds and palms and (ii) prescribing a complete blood count including assessment of haemoglobin (Hb), mean corpuscular haemoglobin (MCH), mean cell haemoglobin concentration (MCHC), and mean corpuscular volume (MCV) as a preliminary predictive test.[102] The blood level of serum ferritin is considered the most appropriate test for assessing iron deficiency anaemia.[103] The cut-off values for the assessment of iron deficiency anaemia are given in [annexure 1].
There are no reliable biochemical status markers for the assessment of calcium deficiency in adults.[104] In a resource-constrained setting with limited possibilities of performing a 24-hour recall for assessment of calcium intake, the daily dietary calcium intake can be assessed using a feasible National Osteoporosis Foundation (NOF) tool (as shown in Annexure 2).[105] The adequacy of calcium intake can be assessed by comparing the calculated daily dietary calcium intake with the recommended daily allowance (RDA). In addition, Vitamin D deficiency can be assessed by testing the serum 25-hydroxyvitamin D concentrations, especially in midlife women presenting with joint and bone-related issues.[104] The cut-offs for the assessment of vitamin D deficiency in adults are presented in Annexure 3.[106]
The final step in dietary assessment is to ascertain the barriers faced by midlife women in managing healthy eating practices in their day-to-day life. These barriers should be considered for providing personlised dietary modification advice. A valid and reliable questionnaire for assessing dietary practices and barriers to healthy eating in Indian midlife women can be accessed from [Annexure 4].
2.3: How should the level of daily physical activity of midlife women be evaluated?
Background
The assessment of daily physical activity status is important for determining the total caloric deficit required for achieving weight loss in midlife women. Daily activity assessment is aimed at calculating caloric expenditure. Daily physical activity can be comprehensively assessed under the following domains: exercise, occupational, household-related, leisure-related and sedentary activities.[107] Physical activity is defined as ‘bodily movement generated due to contraction of the muscle that raises the energy expenditure above resting metabolic equivalents (METs)’. It is generally characterised by type, intensity, and duration. Exercise is a sub-category of activity that is planned, structured and repeated for maintaining physical fitness.[108] Sedentary activities are defined as “any activity with an energy expenditure of ≤ 1.5 metabolic equivalents (METs) while in a sitting, reclining and lying posture”.[109] Several methods have been used for objective assessment (accelerometers, heart rate monitors, mobile applications) and subjective assessment (detailed activity history, physical activity questionnaires and activity monitoring applications).[51,110-113] The choice of assessment method in healthcare settings depends on efficacy and ease of use with respect to available time, expertise, and economics. In most healthcare settings, clinicians primarily engage midlife women in considering an active lifestyle. It is important to identify concise, valid and reliable assessment methods that can be taken up by the clinicians and other healthcare professionals for a time-bound and comprehensive assessment of daily physical activity.
Summary of evidence
Across weight-loss trials, daily physical activity was assessed using objective, subjective and a combination of both measures. Objective measures included an accelerometer and pedometer worn on the wrist, waist or hip to calculate the mean physical activity as MET values.[90,111,112] In subjective measures, a total of ten questionnaires were used to assess the total physical activity across different domains such as leisure, occupational, household chores, and sitting and screen time. Five studies assessed physical activity using a combination of objective measures such as accelerometer and subjective measures such as Stanford 7 day Physical Activity Recall, International Physical Activity Questionnaire (IPAQ), and Minnesota Physical Activity Questionnaire.[110,114,115] The questionnaires evaluating both sedentariness and activity status are IPAQ and Modifiable Activity Questionnaire (MAQ).[113,116] Other domain-specific activity questionnaires are the Leisure-Time Physical Activity Questionnaire and Allied Dunbar National Fitness Survey for leisure-related physical activity and work and non-work activity by Stanford Physical Activity Recall.[50,117] Sedentariness was measured using low level physical activity recall and Sitting Questionnaire.[118] The assessment can be reported as continuous scores (mean MET values per day or week) or categorical scores as low, moderate and high activity according to the cut-off MET values per day.
It was also observed that physical activity assessment should be done for activities that are specific to geographical, social and cultural constructs. Considering this, the physical activity assessment scale for the western population might not correctly measure the level of physical activity in midlife women in India. MPAQ is a recall measure specifically developed and validated in the Indian population to measure both the level of physical activity in different domains and sedentariness.[119] Although, the application of this measure in a busy outpatient setting is still limited. There is limited evidence on short and comprehensive physical activity assessment tools that can measure the level of physical activity as well as barriers faced by midlife women in managing daily physical activity.
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 2.3.1 | The detailed physical activity evaluation should include an assessment of dedicated physical exercise, domestic, work-related, leisure-related, transport-related and sedentary activities (screen and sitting time). | I B |
| 2.3.2 | Madras Diabetes Research Foundation- Physical Activity Questionnaire (MPAQ) should be used for the evaluation of physical activity, if feasible. Alternatively, a short validated questionnaire can be used.* | II B/C |
| 2.3.3 | Evaluation of the adequacy of physical exercise should be done by assessing: type of exercise (stretching/strengthening/aerobics/balance), intensity (light/moderate/vigorous), frequency (number of days per week) and duration (number of minutes per day). | IV B |
| 2.3.4 | Special attention should be given to assess the number of sedentary hours (especially, screen and sitting time) spent during the day. | IV A |
| 2.3.5 | Midlife women should be encouraged to discuss the barriers faced by them in maintaining an active lifestyle. | IV A |
*Refer to Annexure 2.
Discussion
Physical activity assessment should be made a standard step in routine care to design and promote strategies to maintain an active lifestyle.[120] The recommendations on baseline assessment of physical activity status in midlife women are derived from lifestyle modification RCTs aiming at achieving weight loss. The two methods identified for physical activity assessment are objective (activity monitors: accelerometer, pedometer) and subjective methods (questionnaires and activity history). In public health and clinical settings, subjective methods including self-reported PA questionnaires, daily activity logs and activity history schedules are commonly used to assess the level of daily activities. There is evidence that comprehensive assessment of daily activities across domains including physical activity, household chores, work, transport and sedentary activities is crucial to assess the total daily caloric expenditure.[80] Experts believed that healthcare practitioners should consider the feasibility of the assessment approach before including it in daily clinical practice. Based on resource availability, the assessment can be done using three possible methods of assessment: MPAQ, comprehensive questionnaire and/or detailed activity history. It is important to integrate the physical activity assessment into clinical practice.[121] If the application of the questionnaires is not feasible, some important points of consideration while taking activity history in midlife women are presented in [Table 2.3.1].
Table 2.3.1.
Important points to be considered for physical activity assessment
| Dedicated exercise (moderate to vigorous-intensity activity; such as yoga, aerobics, resistance or strength training exercises for 150 min/week): |
|---|
| •How often do you engage in dedicated physical activity in a week? |
| •How much time duration do you dedicate to participate in exercise? |
| •What types of exercises do you include in dedicated physical activity? |
| Household activities (cooking, cleaning, mopping, gardening, child care etc.): |
| •How often do you participate in household chores in a week? |
| •What household chores do you usually do on a regular day? |
| •How much time do you spend doing household chores in a day? |
| Occupation-related activities (manual tasks, sitting task, walking, working on a laptop, carrying or lifting objects): |
| •How many hours in a day do you usually spend doing sedentary work-related activities (sitting and screen time)? |
| Transport-related activities (self-driving, cycling and walking from one place to another): |
| •What kind of transport do you usually opt for while travelling from one place to another? |
| •How much time do you usually spend travelling? |
| Leisure time activities (discretional or recreational activities including hobbies, walking, gardening): |
| •What type of activities do you usually do in your free time? |
| •How much time do you usually spend on leisure activities? |
| Sedentary habits (includes such as sitting, reclining or lying posture, watching TV, playing video games, or using the computer): |
| •How much is your daily screen and sitting time? |
Considering the effect of dedicated activity in maintaining cardiometabolic, bone and cognitive health in midlife women, a detailed investigation focusing on the type of exercise (stretching/ strengthening/aerobics/balance), frequency of participation (number of days in a week) and duration of involvement (in minutes) is also recommended (as presented in Figure 2.3.1). Experts suggested that the participation of midlife women in household chores might be limited due to the shifting of the household-related responsibilities to younger women. Hence, it is important to assess the daily sitting and screen time as two main indicators of sedentary behaviours in this group of women.[122]
Figure 2.3.1.
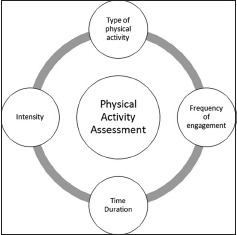
Key components of physical activity assessment
The application of objective methods of physical activity assessment such as wearable activity monitors (WAM) are more commonly used for self-monitoring of daily activity status. A number of technology-driven and consumer-oriented WAMs (e.g. Fitbit) and mobile applications are available for assessment of the number of steps taken per day. However, it is challenging for these methods to assess the intensity of physical activity.[123] The major limitation of this method is that it cannot assess physical activity compliance in accordance with the international activity guidelines of moderate-intensity physical activity.[121]
Experts believed that assessment of barriers faced in managing daily physical activity can help practitioners to devise a customised women-centric activity plan. A comprehensive tool for assessment of daily physical activity and barriers associated with maintaining daily activities in Indian midlife women is given in Annexure 4.
There is limited literature on comprehensive and practical tools that can be integrated into the clinical workflow with a limited burden on healthcare practitioners and patients. In current literature, the tools only account for the assessment of aerobic activity. As per the international recommendations for advising comprehensive physical activity, muscle strengthening and flexibility should be incorporated twice a week. Clinicians should include questions such as ‘How many times in a week do you engage in muscle-strengthening exercises such as bodyweight resistance?’ and ‘How many times do you participate in flexibility exercises such as stretching and yoga?’ in their routine assessment schedule.
2.4: How psychological and behavioural health should be evaluated in midlife women being engaged in the management of overweight and obesity?
Background
The question is important as psychological and behavioural health attributes may have an implication as it drives adherence and compliance amongst midlife women engaged in the management of overweight and obesity. The presence of diagnosed psychiatric disorders and clearly discernible psychological attributes may limit the ability of the woman to engage in structured weight-loss interventions. Behaviours such as stress and emotional eating may have an impact on the weight-loss trajectory or an increased probability to regain the initially lost weight. Thus, addressing psychological and behavioural health may be an important consideration in women who undergo weight-loss interventions.
The context of psychological and behavioural health holds importance as mental distress in midlife is common, especially depression and anxiety. Personality profile, stress appraisal and stress coping mechanisms may also influence adherence and outcome of weight management efforts. Moreover, eating disorders such as binge eating and food addiction may impair systematic efforts at weight loss by increasing proclivity towards sugar or fat-rich foods, which may lead to weight gain, or may impair weight reduction measures. Considering the role of psychological health in weight management, the assessment of psychological and behavioural health is of importance.
Summary of evidence
Psychological symptoms such as depression, anxiety, distress, deteriorated sleep and decline in cognitive abilities have been found to be significantly associated with middle-aged women.[124,125,126,127] Literature has also revealed that these symptoms become even worse from the perimenopausal to the post-menopausal transition period.[128] Several psychiatric disorders are also associated with an increase in weight. Common psychiatric disorders such as anxiety and depressive disorders can be associated with obesity. A meta-analysis of cross-sectional studies found anxiety disorders to be associated with 1.4 times the risk of obesity (95% confidence intervals of odds ratio ranging from 1.2 to 1.6).[129] Similarly, another meta-analysis suggested that the presence of depressive disorder was associated with greater odds of developing obesity in later life. Although controlling for baseline BMI and other factors, it was seen that those with depression had 1.18 times the risk of developing obesity.[130] Binge eating disorder is also associated with 3 to 6 times the risk of obesity as compared to those without any eating disorder.[131] Night-eating syndrome is also associated with obesity as per observational studies.[131] It has been seen that the use of psychotropic medications is also associated with obesity and weight gain. Different psychotropic medications have different propensities to cause weight gain. Among the antidepressants, amitriptyline, mirtazapine, and paroxetine are associated with greater weight gain as compared to other medications.[132] Similarly, antipsychotics are associated with weight gain, with clozapine and olanzapine being associated with the highest risk of weight gain.[133]
Psychological factors and psychiatric issues can have an impact on weight loss management, affecting the outcome of individuals undergoing weight-loss interventions. It has been seen that baseline mental health correlates with weight change in family physician-led interventions for weight management.[134] Another study found that women with significant weight loss (more than 10%) were more likely to have lower depression at baseline.[135] Similarly, other studies have found psychological issues to be predictors of impaired outcomes of weight-loss interventions.[136,137] Depression is common among individuals with obesity and RCTs have addressed comorbid depression and obesity (RAINBOW trial and the Be Active Trial).[138,139] Furthermore, certain weight management interventions have addressed psychological issues as a component of their overall strategy.[137,140] Thus, psychological issues are a relevant consideration for weight management.
The literature search resulted in 22 tools to evaluate psychological and behavioural health in midlife women engaged in managing their weight. Several tools with good psychometric properties are available to assess these psychological and behavioural changes. However, the limitation in using these tools is that they need to be administered by trained psychologists or mental health professionals. Therefore, it is necessary to identify the tools which are validated and can be self -administered. The Menopause-Specific Quality of Life (MENQOL) scale, Menopause Rating Scale (MRS), Women’s Health Questionnaire (WHQ), history taking and self-developed validated questionnaire are some of the tools which assess psychological domains including mood disorders, sleep quality, cognitive impairment and somatic symptoms. Two questionnaires specific to female sexual functionality were found to be the Female Sexual Distress Scale-Revised (FSDS-R) and Female Sexual Function Index (FSFI).[141] The Attitudes Toward Menopause Scale (ATMS) can be used to assess the overall perception towards the menopausal symptoms which is directly correlated with the quality of life. Evaluating these domains will help us identify the problematic psychological, social, emotional and behavioural domains and cater to the needs of these women in the management of obesity.
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 2.4.1 | Assessment should include inquiry into the presence of a diagnosed psychiatric disorder, especially depression, anxiety or eating disorder. | II A |
| 2.4.2 | If there is clinical suspicion, DASS-21 can be used as an initial screening tool for the assessment of depression, anxiety and stress.* | I B |
| 2.4.3 | In case a diagnosed psychiatric disorder is present, then the current condition of the psychiatric disorder and use of psychotropic medications should be asked for by the weight management team. | I A |
| 2.4.4 | The referral to a mental health professional should be considered if: Dietary history reveals abnormal eating practices to cope with stress. There is a sudden lack of motivation for weight reduction. There are persistent interpersonal difficulties with the weight management team. DASS-21 score indicates the possibility of depression, anxiety or stress. There is a history of diagnosed psychiatric disorders. There is clinical suspicion of a known psychiatric disorder. |
IV A |
*Refer to Annexure 5
Discussion
The clinical practice recommendations are based upon the pragmatic assessment of feasibility, along with the clinical relevance of the assessment. We suggest only one aspect of psychological and behavioural health should be routinely asked by anyone/any team providing intervention for weight management: enquiring about the history of psychiatric disorder and the medications being taken for the same. In cases with suspicion, the primary screening tool should be DASS-21 screening depression, anxiety or stress (refer to Annexure 5). The presence of a psychiatric disorder is not a contraindication for weight loss management intervention; however, this information can provide insights into a few issues: whether the disorder is likely to impair efforts at weight loss (depression can be associated with poor energy, lack of interest and altered eating patterns), or whether the psychotropics being used have an impact on the weight (antidepressants such as mirtazapine and antipsychotics such as olanzapine can increase weight).
Stress eating presents a unique challenge in weight management intervention and hence should be evaluated by a mental health professional. Such an evaluation can facilitate the implementation of alternate stress management techniques that may help to better deal with stress. Similarly, the presence of significant anxiety or depression may indicate an undiagnosed psychiatric illness, sudden lack of motivation for weight management may indicate adjustment difficulties or the onset of an illness, and persistent/recurrent interpersonal issues may indicate personality problems. All these may be evaluated better by a mental health professional.
Good psychological functioning can help midlife women to better engage with the interventions for weight loss management. However, the presence of a psychiatric disorder or significant psychological issues may adversely affect the engagement into intervention, continued efforts at weight management, and overall outcomes of the intervention. Clinicians and experts providing interventions for weight management in midlife women need to be sensitised to the need to consider mental health aspects, which may have an influence on weight gain and may impair the efforts at weight loss. Thus, routinely asking for mental health history can help in appropriate assessment, without significantly increasing the time/depth of assessment. Where major issues are identified as above, a more detailed assessment by a mental health professional can be done to help in ascertaining the mental health problem(s) and for suggesting ameliorative measures. Such assessment is likely to be required in a minority of the cases and may help to improve the outcomes of the midlife women attempting to lose weight.
2.5: What are the important comorbid conditions (cardiometabolic and other medical conditions) that should be evaluated before initiating management of overweight and obesity?
Background
Weight gain is an important concern among women in their midlife years. In addition to weight gain, midlife women are predisposed to cardiometabolic comorbidities and obesity-related complications.[6,142,143] In the Study of Women’s Health Across the Nation (SWAN) study, the chances of cardiometabolic comorbidities including diabetes, hypertension, dyslipidaemia are increased as women undergo menopausal transition.[144] Other obesity-related comorbidities includes non-fatty alcoholic liver (NAFLD), sleep apnoea, polycystic ovary syndrome (PCOS), infertility and osteoarthritis.[145] Studies suggest that midlife-specific factors such as low estrogen deficiency, limited activity, poor dietary habits (low protein and calcium intake) and low vitamin D levels contribute to conditions such as poor bone density, reduced muscle mass and strength, which consequently lead to osteoporosis and sarcopenia.[146,147] Although there is a need to assess and understand the cumulative role of comorbid conditions in weight management, such a comprehensive approach is rarely used in healthcare settings.[148] It becomes crucial to assess the consequence of comorbid conditions on achieving significant weight loss. The introduction of timely screening, diagnosis and action for prevention and treatment of allied comorbidities will help in improving the overall quality of life in later years.
Summary of evidence
Across the literature, weight gain has been associated with a number of comorbid conditions ranging from metabolic, mental, musculoskeletal and other obesity-related disorders. Out of 23 studies, nine studies reported a positive correlation of clustering of one or more components of metabolic syndrome (elevated lipid profile, insulin resistance and high blood pressure, and elevated inflammatory markers) with weight gain, total body fat and central adiposity.[142,149,150,151] A higher incidence of cardiovascular events was observed in six studies among obese menopausal women.[152,153,154]
Besides weight gain and fat accumulation, women in midlife are also subjected to declining lean mass, bone health and muscle strength leading to musculoskeletal diseases such as sarcopenia and osteoporosis. In addition, factors such as chronological ageing, post-menopausal status, perceived barriers for exercise and limited consumption of calcium-rich foods were also positively associated with osteoporosis.[155,156] Studies report a positive correlation between central obesity and increased risk of fractures especially hip fracture among menopausal women.[157]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 2.5.1 | In midlife women, lifestyle intervention should be initiated if they have any known cardiometabolic risk factors (such as abnormal blood sugar, increased blood pressure, dyslipidaemia) or obesity-related complications (such as non-alcoholic fatty liver and sleep apnoea). | I A |
Discussion
Weight gain during menopausal transition is associated with a range of non-communicable chronic conditions.[2] We identified some important comorbid conditions that are generally associated with obesity in midlife women: metabolic syndrome, diabetes, cardiovascular diseases such as heart attack and stroke, osteoporosis, osteoarthritis and sarcopenia.[158,159] It can be noted that multifactorial risk factors specific to midlife such as hormonal and physiological changes coupled with unhealthy lifestyle habits make these women prone to weight gain and consequently to increased cardiometabolic risk as compared to other reproductive phases.[160] The guidelines for screening of cardiovascular risk factors in adults can be referred from the American Heart Association Task Force on Clinical Practice Guidelines available from https://www.ahajournals.org/doi/10.1161/CIR.0000000000000678.[161]
Apart from cardiovascular diseases, women suffer from sarcopenic obesity marked by a reduction in muscle mass coupled with an increase in fat mass leading to reduced physical performance.[162] Poor bone mass and decreased bone mineral density also contribute to compromised mobility and physical functionality interfering with daily life activities.[147] Factors such as consumption of a diet low in protein and calcium, long-term physical inactivity and low exposure to sunlight are some factors related to musculoskeletal conditions.[163,164] According to the Asian Working Group for Sarcopenia (AWGS) Criteria, if feasible, bioelectric impedance can be used for assessing muscle strength and muscle mass. In addition, a detailed history of fractures, dietary habits, physical activity level, and alcohol consumption should also be taken. The consensus on diagnosis and management of sarcopenia in adults as per the AWGS working group is available at https://pubmed.ncbi.nlm.nih.gov/32033882/.[163]
In a resource-rich setting, bone health can be assessed using a Dual-energy X-ray machine (DXA) or vertebral imaging.[105] Furthermore, the clinical practice guidelines on screening and management of osteoporosis in midlife women are available at https://pubmed.ncbi.nlm.nih.gov/33281419/.[164]
2.6: How should menopause-related symptoms be evaluated in midlife women being engaged in the management of overweight and obesity?
Background
Menopause is a transition from a reproductive to a non-reproductive phase marked by the emergence of specific symptoms. The changes in menstrual cyclicity as a symptom is a clinical marker of the onset of the menopause transition at the later stages of the reproductive cycle.[165] The menopausal symptoms can be categorised as physical, psychological, vasomotor, genitourinary, and sexual symptoms. There can be variation in the type, frequency, and severity of menopausal symptoms experienced by midlife women. Vasomotor symptoms are considered as primary symptoms of menopause causing hot and cold sweats, especially in the upper body.[164] Somatic symptoms include musculoskeletal pains, which are associated with the menopausal transition status. Often, musculoskeletal pain is associated with BMI, fatigue, sleep disturbance, mood disorder, anxiety and stress.[166] Further, the biological decline in ovarian function is also associated with psychological distress leading to episodes of mood disturbances, irritability, anxiety, depression and poor sleep, especially during the menopausal transition.[165,167] Both vasomotor symptoms and psychological distress lead to poor sleep quality in menopausal women.[168]
Across the literature, menopausal symptoms have been associated with lifestyle-related practices in a way that can promote overweight and obesity in midlife women. Women with higher total menopausal symptom severity scores are known to have a higher caloric intake, especially from HFSS foods. It has been observed that midlife women having joint issues have limited participation in physical activity leading to weight gain over time.[169] Similarly, women reporting higher vasomotor symptom severity often report greater sleep disturbances and emotional volatility.[170] Considering the association between menopausal symptom severity with the key lifestyle practices associated with weight gain in menopausal women, an assessment of menopausal symptom severity should be undertaken by clinicians, especially in symptomatic women. The assessment should be done objectively to classify menopausal women into mild, moderate and severe categories.[171]
Summary of evidence
Across the literature, a total of seven questionnaires assessing the menopausal-related symptoms in middle-aged women were identified. The identified questionnaires are condition-specific questionnaires that assess symptoms experienced by middle-aged women undergoing menopausal transition. All the questionnaires can be self-administered to examine menopausal symptoms. Five questionnaires measured menopause-specific symptoms including vasomotor, physical, psychological, urogenital, and sexual symptoms. Other symptoms such as cognitive impairment, body image issues, sleep disorder, perceived loss of control, and numbness were assessed in three questionnaires: Women’s Health Questionnaire (36-items), Menopause Symptoms Severity Inventory (38-items), and Self-developed validated questionnaire (41-items).[35,172,173] On the basis of severity, the symptoms were rated on a Likert-scale response scale ranging from not severe (lowest score) to maximum severity (highest scores). Questionnaires such as Menopause Specific Quality of Life calculated domain-wise scores, whereas Menopause Rating Scale, Greene Climacteric scale and Kupperman’s index calculated total scores for all the menopausal symptoms.[174,175,176,177] The higher scores indicate severe menopausal symptoms and lower scores indicate mild menopausal symptoms.
These questionnaires are concise, valid and reliable measures for assessment of menopause symptoms severity in women. There is a lack of menopause symptom assessment scales specific to middle-aged Indian women. There is a need for the development and validation of simple, practical, and feasible tools to assess menopausal symptom severity in Indian women.
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 2.6.1 | Menopause Rating Scale (MRS) may be used for evaluation of menopausal symptoms which includes physical, psychological, vasomotor, genitourinary and sexual domains.* | I B |
| 2.6.2 | The impact of menopausal symptoms severity on weight-related behaviours (diet, physical activity and sleep) should also be assessed. | IV A |
*Refer to Annexure 6.
Discussion
Menopause is retrospectively diagnosed considering menstrual irregularities. The assessment of menopause using biochemical parameter follicle-stimulating hormone (FSH) is initiated in special cases or during infertility.[165] Further, the severity of menopausal symptoms can be assessed using a number of developed and validated questionnaires. Often, these scales assess menopause symptom severity in relation to the overall life quality in midlife women. The commonly used scales includes Menopause Rating Scale (MRS), Greene’s Climacteric Scale, and Menopause Specific Quality of Life (MENQOL).[172,174,175] According to the Practice Guideline on Menopause, MRS is the recommended self-administered tool for screening of the severity of the menopausal symptoms in midlife Indian women (refer to Annexure 6).[11] The MRS is also translated and validated in Hindi language for easier administration in Indian women.[178] However, the integration of MRS in clinical practice for the management of weight in midlife women is still lacking. An adapted version of MRS in Indian women is also given as a part of a comprehensive assessment tool for an overall assessment of menopausal symptoms and lifestyle-related factors for appropriate weight management in Indian women (shown in Annexure 4). According to the WHO, the categorization of the menopause symptom severity in midlife women is decided on the percentage of menopausal symptoms and can range from no problem (0-4%) to complete problem (95–100%).
Section III: Management of overweight and obesity
3.1: How stepwise weight loss goals should be set for midlife women being engaged in the management of overweight and obesity?
Background
Goal setting is a common behaviour change strategy that is considered a fundamental component of successful weight loss intervention.[179] Weight loss goals should be patient-centric, realistic, practical and mutually set with the help of a healthcare provider. Generally, a weight loss goal is set in terms of absolute or percentage reduction from baseline weight at the end of the intervention.[78] In addition to weight loss, incorporation of corrective lifestyle habits and improvement in cardiometabolic, menopausal and overall health should also be considered while setting weight loss goals in midlife women. The S.T.A.R.T criteria (Specificity, Timing, Acquisition, Rewards and feedback, and Tools) is considered useful for promoting health behaviour change to set goals that are specific, timely, self-determined, and supported by self-monitoring tools, feedback and rewards.[179] This can help in directing the midlife woman’s attention and efforts in defining relevant strategies for accomplishing her future weight and health goals.[180] However, the nature of weight loss goals for initiating lifestyle modification intervention and its association with achieving therapeutic weight loss has not been systematically explored. Hence, we undertook this question of clinical relevance to understand the type of weight loss and health goals that should be set for midlife women and their association with achieving weight loss success.
Summary of evidence
Across trials, weight loss goals were defined in terms of lost weight, improvement in cardiometabolic risk factors and menopausal symptom severity. The American Heart Association (AHA) guidelines suggested that a modest weight loss goal of 5–10% should be initially targeted for individuals seeking weight loss treatment. A modest weight loss of 5–10% was related to improvements in cardiometabolic risk factors and other obesity-related disorders. Most RCTs also aimed at reducing 5–10% in a duration of 6–12 months.[53,76,90] Similar consistent findings were reported in four systematic reviews where the achievement of 5–10% weight loss was associated with improvement in hypertension, hyperlipidaemia, and blood glucose fasting.[181-183] Any further improvement in weight correlated with graded improvement in obesity-related diseases such as non-fatty liver disease, and sleep apnoea. Weight loss was also associated with improvement in polycystic ovary disease, regularity of menstrual cycle and fertility in women.[182] A limited number of experimental studies reported that a weight loss of 10% can be associated with a reduction in menopause-related hot flushes.[46,184] The improvement in other comorbidities such as depression, mobility, sexual dysfunction, and urinary stress incontinence and overall quality of life was also associated with a reduction of 5–10% body weight. The further reduction of body weight correlates with a graded improvement in cardiometabolic and obesity-related disease status.
In addition to quantitative weight loss goals, attention should be given to setting realistic and patient-centric goals.[78,182] The weight loss goals should also be discussed with the woman in the context of any competing priorities in the woman’s everyday life that might hinder the achievement of the set goal. The woman’s readiness to achieve the weight and health goal can also be ascertained before initiating a comprehensive lifestyle management program.[78]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 3.1.1 | Healthcare providers should assess the readiness to engage in weight loss attempts by changing the current diet and physical activity using behavioural modification. | IV A |
| 3.1.2 | Realistic and sustainable patient-centric weight loss goals should be established after a detailed discussion with the midlife woman. If feasible, family members should be involved and similar health goals should be planned for them. | IV A |
| 3.1.3 | Overweight and obese women should be advised to reduce body weight to a normal BMI (18.5-22.5 kg/m2). | I A |
| 3.1.4 | A step-wise body-weight loss goal should be set with a target weight loss of 0.5 kg per week attaining a weight loss goal of 5-10% of baseline body weight over a period of 6 months (clinically significant weight loss). | I A |
Discussion
The recommended weight loss goals for midlife women seeking lifestyle modification treatment for weight loss are similar to previous recommendations for the adult population.[78,86] Initially, the readiness to change existing lifestyle-related behaviours, especially dietary and physical activity behaviours to lose weight should be assessed in midlife women. The transtheoretical model (TTM) of health behaviour change defines the ‘readiness of change’ across five stages: pre-contemplation, contemplation, preparation, action, and maintenance (as shown in Figure 3.1.1). Generally, the proportion of population at-risk is 40% in pre-contemplation, 40% in contemplation and 20% in the action stage.[185] An early understanding of the readiness and motivation to change can help clinicians and healthcare professionals in devising customised weight loss and health goals. For example: at the precontemplation stage, providing education on the consequences of weight gain in later life can be an optimum goal. At the contemplation stage, setting personal weight and health goals can be set in detailed discussion with the healthcare provider. Across the literature, a modest and realistic weight loss goal of 5-10% reduction from baseline body weight in 6 months is recommended as it has significant clinical benefits. For setting sustainable goals, healthcare providers should educate women that a target of 0.5-1 kg weight loss per week should be undertaken.[186] Ideally, the weight management efforts should be continued till the midlife woman has achieved the ideal weight status. Furthermore, healthcare providers should discuss lifelong weight management efforts for the sustenance of appropriate weight in the long term.[86] It should be noted that goal setting is an important component of the behavioural strategy for weight management. Currently, there is a lack of well-designed interventions assessing goal pursuits (goal setting and goal striving) amongst adults participating in weight loss programs.[187] Furthermore, across weight loss literature, goal setting is considered as an initial step rather than an ongoing process. Goal setting as a process includes finalizing weight loss goals, and reflecting on and receiving feedback from healthcare providers to successfully achieve the initially set goals during the lifestyle modification intervention. In addition, customization of weight loss plans to manage the barriers faced by the participant during the intervention should be incorporated as a part of goal striving.[179,188] The incorporation of goal pursuit can help to achieve and sustain therapeutic weight loss.
Figure 3.1.1.
Trans-theoretical model (TTM)
3.2: What type of dietary recommendations should be advised for improving weight loss, anthropometric and metabolic health outcomes?
Background
Dietary modification is the cornerstone of weight management. Dietary modification aims at reducing the caloric intake for creating a negative calorie balance to produce weight loss.[189] Other dietary recommendations include having a balanced diet, adequate protein intake, consumption of fruits and vegetables and limiting consumption of calorie-dense foods with a high proportion of fat, salt, and sugar to improve the quality of the meals.[190] Further, improvements in the meal pattern in terms of number, type, frequency and portions size of meals is also recommended.[191] Across the literature, different dietary modification techniques have been recommended for achieving weight loss, improving the cardiometabolic profile and menopausal health in midlife women. The understanding of a comprehensive dietary intervention that can prove effective in managing weight, metabolic and menopausal health in midlife women is still lacking. Moreover, the dietary interventions recommended across weight-loss trials can be mostly prescribed by trained healthcare professionals such as registered dietitians and nutritionists. The availability of trained professionals across healthcare settings in developing countries such as India might be difficult. There is a need for simple, practical and technically feasible recommendations that can be prescribed by healthcare professionals during patient encounters across healthcare settings. Hence, this clinical question was undertaken to identify effective dietary strategies for weight management in midlife Indian women.
Summary of evidence
Dietary interventions for managing overweight and obesity in midlife women were prescribed for an average duration of 6 months; ranging from 21 days to 48 months across trials.[94,192] The dietary interventions aims to achieve weight loss by creating a negative calorie balance. In all the dietary interventions, calorie restriction (500 kcal per day) from the baseline intake was prescribed after the estimation of an individual’s energy requirement.[114] Studies also specified the daily energy intake ranging from 1000–2000 kcal.[76,192] The ad libitum approaches of energy restriction were not prescribed, but lower caloric intake was prescribed by manipulation of macronutrient composition. A number of dietary approaches including macronutrient manipulation with caloric restriction were recommended: AHA style diet (fat <30% of total calories, <10% from saturated fat), Very Low-Calorie Diet, Low Carbohydrate, Glycemic-Load and Fat Diet, Mediterranean Diet and High Protein Diet.[78] The most common dietary interventions were based on a calorie-restricted diet with the following macronutrient composition: carbohydrate 50%-60%, fats 30%, and protein 10%-20%. In a low carbohydrate diet, carbohydrate intake ranged from 5-15% of the total calories.[66,83] The prescription on the incorporation of high dietary fibre (soluble and insoluble) foods was incorporated in four RCTs and one experimental study. A dietary fibre intake of 20 to 30 grams per day was prescribed from whole grains, nuts, oilseeds, fruits and vegetables. The average protein intake of 10-20% was prescribed in most diets with the focus on the consumption of at least one serving of lean meat sources in non-vegetarian diets.[193] Low Calorie, High Protein diet (25-30% of energy) was prescribed in three studies with the focus on the consumption of lean meats, non-fat dairy, nuts and pulses.[83,192,194] Low-fat diets are defined as diets with less than 30% of total energy intake from fats were prescribed in six trials. In addition, two studies reported Mediterranean style diet prescribing daily consumption limit of different fatty acids based on the proportion of total fat intake: saturated fats (<7%), polyunsaturated fatty acids (PUFA) (9%), monounsaturated fatty acids (MUFA) (20%), and cholesterol intake < 1% per day.[77,80] Other dietary prescriptions were based on recommended country-specific healthy dietary patterns (Dietary Recommendations for Americans) and landmark weight loss trials (Diabetes Prevention Program and Look AHEAD) focusing on improving the overall dietary quality.[50,195] These prescriptions were limited to healthy eating practices such as consumption of lean protein, limited fat, salt and sugar (≤10 g) intake and consumption of fruits and vegetables. Two trials assessed ways of calorie restriction via intermittent fasting with continuous calorie restriction as an approach for the management of weight and other biochemical parameters.[45,196] Only two studies focused on providing education on healthy dietary practices such as meal pattern, portion size, Eatwell plate, emotional eating, reading food labels as strategies for management of weight in midlife women.[49,110] In midlife women, dietary management included supplementation of phytoestrogen from soy-based products, omega-3 for essential fatty acid, and calcium and vitamin D for weight management.[75,83,113]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 3.2.1 | An individualised diet plan should be recommended considering eating preferences, food habits and health status of the patient. | I A |
| 3.2.2 | The meal pattern should be spread throughout the day preferably involving three major meals and two snacks. | II A |
| 3.2.3 | The daily dietary calorie intake should be based on the baseline caloric intake and level of physical activity of the midlife woman. | I A |
| 3.2.4 | The diet plan should incorporate an energy deficit of 500 kcal per day to achieve a weight loss of 0.5 kg body weight per week. | I A |
| 3.2.5 | Restricted carbohydrate, good quality fat and a high protein diet is recommended for midlife women. | II A |
| 3.2.6 | Women should be counselled to consume foods rich in protein, calcium and iron in their daily diet to meet the estimated average requirement (EAR). | I A |
| 3.2.7 | Dietary fibre intake of 20-30 grams per day should be prescribed from whole grains, legumes, nuts, oilseeds, fruits and vegetables. | I A |
| 3.2.8 | Restricted consumption of food products high in fat, sugar and salt (HFSS) should be emphasised. Salt intake should be limited to <5 g/day. | I A |
| 3.2.9 | Dietary intake of foods rich in phytoestrogen from the Indian diet should be encouraged. | II C |
Discussion
The dietary recommendations in midlife women are based on the principles of caloric restriction, balanced diet and healthy eating practices. The caloric restrictions should be individualised by accounting for the current dietary and activity habits, comorbidities and previous dieting attempts. A daily caloric restriction of 500–750 kcal is optimum for losing 0.5-1 kg weight per week.[197] Generally, in clinical practice, a hypocaloric balanced diet of around 1000-1200 kcal is prescribed in adult women.[2,194] A total of 15-30% caloric restriction with respect to habitual caloric intake is optimum for weight loss. However, clinicians should note that patients often underreport caloric intake and overestimate caloric expenditure.
The caloric restriction should be translated into optimum macronutrient manipulation. In the current literature, different diets based on acceptable macronutrient distribution range are low carbohydrate (45–55%), high protein (10–35%), low fat (20–30%) and low glycaemic index diet.[66,83,192] There is inconsistent evidence that these diets are beneficial over the hypocaloric balanced diet. However, these diets have been shown to improve the cardiometabolic risk factors such as dyslipidaemia, type 2 diabetes and blood pressure. In some cases, the total carbohydrates should be restricted (<26% per 2000 kcal caloric intake) and complex carbohydrates with a low glycaemic index should be incorporated.[198] The targeted daily fibre intake should be around 30 grams from fruits and vegetables (4-5 servings per day), legumes (1-2 servings), nuts and oilseeds (1–2 servings) and whole-grain flour. Special attention should be given to adequate protein intake as per the estimated average requirement for maintaining optimum muscle and bone health in midlife women.[199] A very low-calorie diet (500–800 kcal) might lead to micronutrient deficiency in the long term. These diets should preferably be recommended by trained healthcare professionals such as nutritionists and dietitians.
Healthcare providers should consider personal and cultural food habits while prescribing any dietary modification. Fixing the meal pattern can also be helpful in avoiding fasting and feeding cycles throughout the day. For the main meals, these women should be encouraged to adjust the servings of the foods according to the ‘EatWell’ plate.[200] The caloric manipulation should be supplemented with generic advice on healthy eating behaviours. Some generic advice can be: limit the consumption of HFSS, avoid skipping meals, adequate intake of water, decrease the portion size, avoid eating out, and manage episodes of emotional eating.[189]
In addition, the consumption of micronutrients should be recommended in accordance with the estimated average requirement (EAR). The daily intake of iron (29 mg), calcium (1000 mg) and other B-complex vitamins should be planned from dietary sources available in Indian diets (refer to Annexure 7).[199] The dietary sources of iron, calcium and phytoestrogen are presented in Annexure 8. If women are found deficient, supplementations can be recommended as per clinical indications. The detailed clinical practice guideline for iron deficiency anaemia in women (age > 15 years) can be accessed from:https://www.nhm.gov.in/images/pdf/programmes/child-health/guidelines/Control-of-Iron-Deficiency-Anaemia.pdf.[201] Considering the practical limitations for the consumption of Vitamin D from natural dietary sources, Vitamin D supplementations can be recommended in cases of deficiency as per clinical recommendations under the guidance of a clinician. The detailed clinical practice recommendations on the management of micronutrient deficiency (calcium and vitamin D) in midlife women are available from https://pubmed.ncbi.nlm.nih.gov/33281419/.[164] Women suffering from severe menopausal symptoms can also be prescribed supplementations of phytoestrogen from natural sources, especially soy-based products.[202] The reinforcement of healthy eating habits during intervention is important for the long-term sustenance of weight loss.
3.3: What type of physical activity recommendations should be advised for improving weight loss, anthropometric and metabolic health outcomes?
Background
Physical activity is defined as all movements that can lead to caloric expenditure.[203] The caloric expenditure is important for creating an energy deficit required to produce a reduction in weight. Generally, women with overweight and obesity are recommended to incorporate exercise in their daily regime to increase the caloric deficit. Exercise is structured and planned physical activity.[204] The midlife women can be suggested different types of exercises including aerobic exercise for maintaining cardiometabolic health, strength training for increasing bone and muscle mass and balance exercises for maintaining posture and flexibility.[205-207] Healthcare providers should recommend the type, intensity, duration and frequency of exercise in a patient-centric, simple and feasible manner.[208] Further, the activity recommendations should be menopause friendly, i.e. it should not aggravate any menopausal symptoms such as vasomotor symptoms or joint pains. It is important to understand the effective combination of exercises that can help in producing significant weight loss. In current literature, the effectiveness of independent exercise and/or a combination of exercise regimes in producing significant weight loss in midlife women is lacking. Hence, this clinical question was undertaken to identify the type and nature of exercises that can help in improving weight loss and overall health-related outcomes in midlife women.
Summary of evidence
Physical activity prescriptions for midlife women to manage overweight and obesity were based on pre-existing recommendations from the American College of Sports Medicine and National Strength and Conditioning Guidelines.[79,205] Most studies recommend different types of physical activity in collaboration with caloric restrictions for managing weight in midlife women. The total duration of interventions ranged from 10 weeks to 18 months.[209,210] During the intervention, an average of three exercise sessions were planned in a week. Only one study planned a stepwise increase in the number of sessions ranging from three sessions per week to five sessions per week.[51] Exercise sessions were planned in a phasic manner starting with a warm-up, exercise and cool down.[207] The duration of the exercise sessions varied from 50 to 65 min, except for one study which reported 90-minute walking sessions.[53] Studies have recommended different activities including aerobics, resistance training and endurance, stretching, and combined exercise circuits. Six trials recommended moderate (60-70% Heart Rate Maximum (HRmax) to vigorous (70-80% HRmax) intensity aerobic exercises including walking, nordic walking, jogging, swimming, bicycling, ergo cycling, treadmill and video-based aerobic exercise only one trial focused on moderate (HRmax 50-75%) intensity endurance exercises including upper limb, spine-stabilizing, deep muscle-forming, lower limb and balance-adjusting exercises.[79] Three studies focused on the role of resistance training of all major muscle groups and core exercises. The resistance training included different types of exercises such as leg press, leg extensions, calf raises, chest press, lateral pull-downs, shoulder press and triceps extension.[194,205,211] Five trials customised exercise circuits for management of weight in midlife women. The following exercise circuits were recommended: aerobics and resistance exercises, high-intensity interval training (HIIT) and resistance exercises, walking and weight-bearing exercises.[51,79,207] The aim of these circuits was to reduce the total body fat and visceral fat (especially HIIT), improve lean muscle mass, bone mineral density, physical capacity and maximum range of oxygen (VO2 max). Only one trial reported the importance of aerobic exercises in managing distress, hot flushes as climacteric symptoms and health-related overall quality of life (HRQoL).[212] An experimental study reported that involvement in 60 min of daily physical activity helps in improving climacteric symptoms and psychological distress.[213] Social support from coaches in maintaining regular engagement in physical activity did not contribute to an increased frequency of engagement in physical activity.[214]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 3.3.1 | Midlife women should be encouraged to avoid a sedentary lifestyle as far as possible by ensuring participation in dedicated exercise, household-related, work-related and leisure-related physical activities. | II A |
| 3.3.2 | A stepwise progressive and personalised physical exercise regime based on body weight, presence of cardiometabolic risk factors, bone, muscle and joint health should be prescribed. | II A |
| 3.3.3 | The barriers and facilitators for adopting different types of physical exercise should also be considered while prescribing a physical exercise regime. | IV A |
| 3.3.4 | Women should be encouraged to incorporate up to 300 min/week of moderate-intensity aerobic physical activity or 150 min/week of high-intensity aerobic physical activity or an identical combination of moderate and high-intensity exercise. | I A |
| 3.3.5 | Preferably, daily 60 min of physical activity should be recommended including a combination of moderate-intensity aerobic exercise (30 min moderate intensity), muscle strengthening engaging major muscle group or balance exercise (15 min), work-related and/or household-related activities (15 min). | I B |
| 3.3.6 | Women can participate in moderate-intensity aerobic activities in short bouts (10-15 min) throughout the day (2-3 times). | II B |
| 3.3.7 | The talk test should be used for self-monitoring the intensity of aerobic activities. | I A |
| 3.3.8 | Yogic practices (including physically intensive yoga) can be prescribed for weight management and overall well-being in midlife women. | III A |
Discussion
A personalised, progressive and stepwise exercise regime should be planned by the healthcare provider. According to the Australian pre-exercise screening guide, factors such as cardio-respiratory health, musculoskeletal strength, and pre-existing clinical conditions should be assessed before recommending an exercise regime.[215] The recommendations are derived from RCTs that incorporate both dietary restrictions and physical activity for weight regulation. Most studies comparing exercise alone vs exercise and diet as components of weight loss intervention report that exercise alone results in minimal weight loss. According to the position statement from the American College of Sports Medicine and American Diabetes Association, “up to 60 min/day physical activity alone is required to produce weight loss”.[216] Similarly, clinical recommendations from the American Academy of Clinical Endocrinologists (AACE) and the American College of Endocrinology also state that 150 minutes of moderate-intensity aerobic exercise improves weight loss outcomes and improvement in intensity and duration can lead to improved weight and fat loss.[217] The intensity of aerobic exercises can be assessed using the talk test: comfortable speech denotes light intensity, speech with some difficulty denotes moderate intensity and speech limited to phrases denotes vigorous intensity.[218] Besides, resistance and weight-bearing exercises are recommended twice a week for enhancing muscle and bone mass. It should be noted that exercise should be done for all major muscle groups, preferably with the help of a trainer.[219] The overall muscle functioning can also be improved with the help of stretching and flexibility exercises. Regular practice of yogic postures (asanas) can also help in improving flexibility. Midlife women can undertake yoga consisting of postures (asanas), controlled breathing (pranayama) and meditation (dhyana) for maintaining physical, mental and emotional well-being.[220]
A comprehensive exercise regime including moderate-intensity aerobic, resistance and flexibility exercises should be planned for overall weight loss and health benefits. For example: initiating warm-up (5–10 min) with stretching to ensure flexibility, engaging in moderate-intensity aerobic exercises (30–45 min) for increasing heart rate and improving cardiometabolic health, followed by resistance exercises (15–20 min) for muscle and bone strength and cool down (5–10 min) to relax the muscles.[219] Different activities that can be planned in each exercise category are presented in (Table 3.3.1).
Table 3.3.1.
Different types of activities that can be planned in exercise categories
| Aerobics/endurance exercises: Physical exercises that lead to increased heart rate and oxygen consumption. |
| Examples: walking, running, cycling, swimming, jumping, etc. |
| Strength exercise/resistance training: These exercises help in increasing muscle strength, carried out against an opposing force and/or one’s body weight. |
| Examples: weight lifting, using resistance bands, arm curls, etc. |
| Balance exercise: These exercises help in improving and maintaining balance. |
| Examples: Yoga asana, Tai chi |
| Flexibility exercises: Flexibility exercises help in keeping your muscles elastic and flexible and joints moving. |
| Example: Yoga, Tai-chi, pilates, stretching |
The management of exercise should also include mitigating barriers faced by midlife women to ensure optimum engagement in physical activity. Healthcare providers should ensure regular follow-up to understand women-centric barriers in managing physical activity.
In current literature, the effect of ‘exercise alone’ interventions on achieving significant weight loss and cardiometabolic outcomes is still lacking. Furthermore, the effect of exercise intensity, frequency and duration on weight loss outcomes can be explored for devising better exercise regimes for weight loss in midlife women.
3.4: What are the behaviour modification techniques that should be incorporated in weight-management advice?
Background
Lifestyle modification for weight management focuses on changes in dietary habits, physical activity patterns and behavioural counselling. While incorporating dietary and activity-related modifications, women face a range of barriers associated with adoption and long-term compliance consequently leading to unsuccessful outcomes.[221] It is important to empower patients with behavioural modification skills which may help them to overcome their challenges in adopting healthy lifestyle modifications.[222] Behavioural modification techniques can play a pivotal role in restructuring an individuals’ lifestyle habits.[223] These behavioural modification strategies can be inculcated with the help of a behavioural specialist and/or psychologist in collaboration with a multidisciplinary team of doctors, nutritionists and exercise specialists.[224] Some of the effective strategies suggested in the literature include goal-setting, self-monitoring, coping strategies, stimulus control, cognitive restructuring, and relapse prevention.[54,115,225,226] Midlife women should be educated to apply these behavioural strategies to mitigate the challenges they face while trying to incorporate healthy lifestyle practices. However, there is a lack of comprehensive list of behavioural strategies established particularly for addressing issues faced by midlife women. Hence, effective behavioural strategies should be identified to counsel women in midlife during the weight management process.
Summary of evidence
Comprehensive weight management in midlife women was based on three components: diet, physical activity and behavioural modification recommendations. The behavioural intervention can be delivered via web-based, group-based and individualised sessions for a duration ranging from 3 months to 60 months.[54,225,227] These sessions were delivered by nutritionists, doctors and behavioural therapists. Only one study had a multidisciplinary team for behavioural weight loss counselling.[228] A number of behavioural strategies were recommended to enhance compliance to dietary and activity advice in the long term: goal setting, motivational interviewing, self-monitoring, positive reinforcement, thought process, coping skills, problem-solving, and social support. During the initiation of weight management, goal setting was used as a behavioural strategy to set realistic and practical weight reduction goals.[54,55,229] During the intervention, motivational interviewing as a technique was used to advise healthy eating behaviours including fibre consumption and activity habits including completing 10,000 steps.[48,115] Dietitian-led counselling sessions or seminars were planned for improvement in eating behaviours such as reading labels, eating out, coping skills for emotional eating, recipe substitution, and selection of foods.[226,227,230] At follow-up, self-monitoring of diet, activity and weight was the most common behavioural strategy that was adopted for managing corrective eating and activity.[54,55,118,229]
Across the literature, web-based behavioural lifestyle modification in midlife women produced significant weight reduction up to -1.34 kg over 12 months.[225] On-site exercise sessions under the supervision of a trained interventionist resulted in greater weight loss than those produced by usual care (limited provision of dietary or activity advice and/or educational materials).[46,116,230] Only three studies reported a significant reduction in the visceral adipose tissue observed as a reduction in waist circumference.[116,226] Limited studies assessed changes in metabolic parameters such as blood pressure, HbA1c, serum insulin, C-peptide, and HOMA-IR leading to inconclusive evidence on the role of comprehensive behavioural lifestyle intervention on improvement in metabolic status.[225,230]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 3.4.1 | Behaviour modification techniques such as realistic goal setting, motivational interviewing, and self-monitoring strategies should be used. | II B |
| 3.4.2 | Women should be trained regarding problem-solving skills such as defining problems, creating solutions and opting for the best possible choice. | II B/C |
| 3.4.3 | Cognitive restructuring skills such as identifying, challenging, and correcting the negative thoughts and emotions related to weight management should be imparted. | II B/C |
| 3.4.4 | Feedback on the accomplishments, achievements and scope for better progress should be given regularly. | II B |
Discussion
The recommendations derived from the current systematic reviews and RCTs reveal that behavioural modification strategies have a crucial role in weight management programs.[115,225,228] Across the literature, a number of behavioural techniques were identified at different stages of weight management. Strategies such as setting realistic goals and motivational interviewing during initiation; problem-solving and cognitive restructuring during the intervention; self-monitoring and relapse prevention during the follow-up have proven to be effective.[54,229] Initially, when patients are confronted with the need to change lifestyle-related behaviour they might face some ambivalence in incorporating this behaviour change. Healthcare providers can resolve this ambivalence through motivational interviewing, reflective listening and asking open-ended questions to stimulate involvement towards a behaviour change.[231] The counselling of problem-solving skills provides patients with an opportunity to discuss barriers experienced in day-to-day life for maintaining corrective dietary and activity behaviour.[55,230] Problem solving skills include identifying the problem, finding possible solutions and adopting the best solution that can help in sustaining the corrective lifestyle habits for a long time period.[232] During follow-up sessions, self-monitoring of weight, diet and activity status should also be included as a key component for maintaining weight loss. Thus, a combination of behavioural approaches can be used in addition to lifestyle intervention for successful and sustainable weight loss outcomes.
In clinical settings, weight loss programs focus only on prescribing corrective dietary and physical activity behaviours as primary modalities of weight loss. Healthcare providers should also consider incorporating behavioural modification strategies as an important modality of successful weight loss outcome.[223] Ideally, behavioural strategies should be advised by a trained behavioural therapist and/or psychologist. Often in healthcare settings, there is limited availability of dedicated behavioural therapists for weight management. Other healthcare providers should also be trained in providing preliminary behavioural strategies to mitigate barriers faced by midlife women for sustainable weight loss.
3.5: What is the role of menopausal hormone therapy (MHT) in a weight management program for midlife women?
Background
Menopausal hormone therapy (MHT) is an effective treatment for the management of menopausal symptoms, especially for vasomotor and genitourinary symptoms. MHT is indicated in women with oestrogen deficiency. It is a systematic treatment with a group of preparations consisting of oestrogen and/or tibolone.[233] Almost 600 million women-years of MHT use have been reported in the period between 1970-2019.[234] Because there is high utilisation of MHT during the menopausal transition, it becomes important to understand its role in maintaining weight and health in midlife women. There is inconsistent literature on the effect of MHT use on body fat and changes in its distribution.[235] Besides, accelerated decline in lean muscle mass leading to sarcopenia has been associated with the menopausal transition. In midlife women using MHT, no positive or detrimental impact on muscle mass can be observed in the literature. In addition to alleviating menopausal symptoms, the possible health effects of MHT have been inconsistently reported across the literature.[236] Due to inconsistencies in literature it is important to realise the effect of MHT on weight status in midlife women undergoing lifestyle interventions. Hence, this clinical question was undertaken to understand the role of MHT in managing weight status, body composition and metabolic parameters in midlife women.
Summary of evidence
MHT has been evaluated in the management of osteoporosis, cognitive function, emotional volatility, sleep disturbances, cardiometabolic disorders and sexual dysfunction, especially in early menopausal women in the age group of 40 - 45 years.[237,238] The International Menopause Society recommends customising and prescribing the lowest dose of hormone replacement drugs after considering the severity of menopausal symptoms, age of onset of menopause, family and personal history, route of administration and woman’s preference.[238] In comparison to a conventional dose, low MHT doses led to a reduction in the levels of low-density lipoprotein (LDL), triglycerides and an increase in high-density lipoprotein (HDL). In addition, no effect on weight and corresponding BMI was observed in comparison to placebo.[239,240] Only one prospective experimental study reported a reduction in body weight, fat mass and waist to hip ratio on the administration of oestradiol valerate plus dienogest at 6 and 12 months.[241] Similar inconsistencies have been reported on the protective role of MHT in the management of metabolic risk factors such as blood glucose, blood pressure, and serum lipid level in midlife women administered MHT in comparison to placebo.[242,243] This calls for more research in this area of midlife health to provide conclusive recommendations on the impact of MHT on the body composition of midlife women.
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 3.5.1 | Menopausal Hormone Therapy (MHT) is not indicated solely for weight management in midlife women. | II A |
| 3.5.2 | Weight management by lifestyle interventions may help in improving the overall well-being of midlife women with menopausal symptoms. | II A |
Discussion
The recommendations suggest that MHT is not solely prescribed for weight management in midlife women. Experts believe that MHT is indicated in women with severe menopausal symptoms. The menopausal symptoms are known to impact the daily lifestyle of midlife women in a way that promotes weight gain. MHT should be primarily prescribed to manage menopausal symptoms severity, which in turn might improve the adherence towards healthy eating and activity behaviours, leading to better weight loss outcomes. Lifestyle modification should be recommended as the primary treatment modality in midlife women with overweight and obesity. The incorporation of corrective eating and activity behaviour can also help in mitigating the menopausal symptom severity and managing weight status.
Section IV: Follow-up for Sustainable Weight Loss
4.1: Which other parameters are of importance in determining the overall improvement in midlife women after undergoing weight management?
Background
Generally, most weight-loss interventions are primarily aimed at achieving clinically significant weight loss determined by a reduction of 5-10% from the baseline weight. The achievement of clinically significant weight loss through corrective lifestyle modification techniques is associated with improvement in cardiometabolic risk factors, obesity-related disease, menopausal and overall HRQoL in midlife women.[182] It is observed that a 10% reduction from body weight in an individual can promote significant improvement in circulating metabolic parameters including blood glucose, insulin, LDL, triglyceride and inflammatory markers such as C-reactive protein.[244] Some community-based interventions also aim at a reduction in waist circumference as an indicator for improvement in cardiometabolic health.[196,245] Other anthropometric parameters such as skeletal muscle mass, lean muscle mass and bone mineral density are also important outcome measures for women transitioning towards menopause. The improvement in menopausal symptoms such as vasomotor symptoms and psychological distress is associated with enhanced overall HRQoL, especially in later years.[158] A comprehensive assessment of health-related outcome measures in addition to weight change is essential for understanding holistic health benefits associated with weight loss. However, the success of lifestyle modification intervention is mainly associated with weight loss. Hence, this clinical question was undertaken to identify the relevant outcome measures that should be taken into account to determine the success of a weight-loss intervention.
Summary of evidence
Across thirty-seven pertinent weight-loss trials, parameters such as anthropometric variables, cardiometabolic risk factors, bone mass density, menopausal symptoms, psychological distress and overall quality of life were assessed for improvement in women completing the lifestyle modification interventions. The most commonly assessed parameters were anthropometric variables including a reduction in fat mass or percentage body fat across 22 studies, followed by a reduction in visceral fat mass or waist circumference assessed in 15 studies.[66,75,196,246] The increase in lean mass or muscle mass was an outcome measure in eleven studies.[111,247,248] The improvement in cardiometabolic risk factors such as HDL, LDL, fasting blood glucose was considered in ten weight loss trials.[80,193,210] The changes in satiety and hunger hormones were assessed in three studies.[82,194,207] Other parameters such as psychological distress in the form of anxiety, depression, insomnia, stress and quality of life were also assessed.[111,212,249,250,251] Three studies assessed improvement in bone mineral density and menopausal symptoms.[53,75,214,252]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 4.1.1 | In addition to weight management, midlife women with obesity should be appraised and sensitised about the benefits of a healthy lifestyle on bone health, muscle strength and menopausal symptoms. | II B |
Discussion
Midlife women measure successful weight loss outcomes mainly in terms of achieving clinically significant weight loss.[253] Women should be sensitised regarding the role of weight loss in managing cardiometabolic, musculoskeletal, menopausal and mental health. In addition to weight, waist circumference as an outcome indicator of cardiometabolic health is easier to assess in public health and community settings. The cut-off values for biochemical parameters associated with cardiometabolic health are presented in Annexure 9. In addition to biochemical improvement, the changes in the clinical signs and symptoms related to menopausal health should also be evaluated post intervention. The improvement in menopausal symptoms such as joint pain and distress has been associated with improved compliance to corrective eating and activity behaviour. Clinicians should utilise improvement in health-related parameters as a motivator to maintain healthy eating and activity behaviour in the long term.[254] Hence, healthcare practitioners should consider weight management programs as an opportunity for improvement in overall health and quality of life in later years.
4.2: What should be the duration, frequency and mode of follow-up for midlife women during the intervention?
Background
Regular and timely follow-ups is an integral component of a successful weight loss program. Considering that a substantial proportion of adults do not adhere to weight loss programs, providing supervision during intervention helps to facilitate adherence to corrective lifestyle-related behaviours. During the intervention, follow-up is an opportunity for healthcare providers to assess a patient’s progress, evaluate health-related outcomes, mitigate barriers faced during weight management and customise lifestyle modification strategies.[255,256]
Follow-up meetings can be planned in individual or group sessions or combinations of both. Use of technology such as web-based follow-ups, text messages, motivational messages, or sending newsletters with recent updates can be utilised for maintaining appropriate contact with the patients.[257-259] Factors such as target weight loss goal, type of intervention (modality, duration, intensity) and level of adherence are associated with planning the follow-up during weight loss intervention. The frequency, mode and duration of follow-up varies across different types of weight-loss interventions.[260] However, the optimum duration, frequency and mode of follow-up to be planned for midlife women for achieving weight loss success has not been studied. Hence, this clinical question was undertaken to understand the effective combination of mode and frequency of follow-up during the intervention that can help in producing significant and sustainable weight loss outcomes.
Summary of evidence
The duration and frequency of follow-up were mentioned across 26 pertinent weight-loss trials; including two systematic reviews on weight maintenance.[261,262] The total duration of follow-up ranged from 3 - 48 months.[67,263] The most common follow-up duration was between 12 and 18 months reported across nine weight-loss trials. Long follow-up duration (≥ 30 months) was reported across six studies.[67,260,264,265] Similar findings were reported by the two systematic reviews with a total follow-up duration ranging from 6 - 36 months for weight maintenance studies.[261,262] As per one of the reviews, there was a vast difference in the frequency of contact made with the participants during the follow-up phase ranging from no/minimal contact to biweekly contact.[262] Similar findings were observed in independent weight-loss trials. The frequency of contact ranged from biannual to biweekly contact with the participants enrolled in the intervention group.[67,257] In ten studies, a monthly contact was established with the participants. Some studies used a combination approach by establishing frequent contact at the initial intervention stage followed by limited contact at the later intervention stage.[260,266] In the control groups, follow-up included no contact to web-based contact, contact via untrained staff and delivery of electronic health promotion material.[266,267] The most common mode of contact was face-to-face, individualised sessions with a trained interventionist such as a dietitian, physician or behaviour therapist.[268-270] Group discussions as a mode of contact were opted by five studies and two studies each used telephonic, email and text messages as their way of maintaining follow-up contact with the participants.[271-274]
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 4.2.1 | The time schedule for the intervention phase of weight management should be decided on the basis of target weight loss to be achieved in the patient. | IV A |
| 4.2.2 | The duration of the intervention phase should be planned such that the patient can lose 5-10% body weight every six months till the target body weight is achieved. | I A |
| 4.2.3 | Bimonthly contact should be planned in the initial stages of the intervention phase that can be reduced to monthly contact in the later phase. | II A/B |
| 4.2.4 | A combination of physical (face-to-face and/or group counselling) and online meeting modalities/telephonic contacts can be used for the follow-up | II A |
Discussion
Regular contact with health care practitioners helps the patient to stay motivated and focused, improving the compliance towards corrective eating and activity advice.[275] The adherence to lifestyle practices is a key factor for improved weight loss outcomes.[276] Generally, the duration of the intervention is planned to achieve the target weight loss of 5-10% in six months. Follow-up is an important strategy for achieving target weight loss and long-term sustenance. A follow-up of 6 to 12 months is commonly considered for achieving the optimum weight loss goal. In addition to target weight loss, factors such as nature of the intervention, readiness to change, available social support and feasibility should be kept in mind while planning the follow-up meetings.[67,268,270]
Weight loss achieved at the initial stage of the intervention is a key motivator of compliance and adherence to corrective lifestyle-related behaviours in the long term.[19] Frequent bimonthly follow-up sessions can be planned during the initial stages of intervention to achieve early weight loss and improve compliance. Further, frequent initial contact can help in providing information on corrective lifestyle habits and customise lifestyle interventions required for improving weight loss efforts.[255] This information can be provided using a combination of face-to-face sessions and technological modalities.[266,268] With advanced technologies, the use of mobile applications for self-monitoring and providing real-time feedback can facilitate weight loss outcomes.[264] Mostly, a combination of individualised and technological sessions are planned across weight-loss interventions. Healthcare providers should identify the most feasible, interactive and sustainable method to maintain contact with midlife women enrolled for lifestyle modification.
4.3: What should be the duration, frequency and mode of contact during the maintenance phase of the weight-management program for a midlife woman?
Background
The maintenance of lost weight is more challenging than achieving clinically significant weight loss across treatment modalities.[254] Weight maintenance is defined as a weight change of less than 3% for at least a period of one year.[277] Midlife women can experience fluctuations in weight status during the weight maintenance phase. Some common issues faced by midlife women during the maintenance phase are weight plateau, weight cycling, and relapse of lost weight.[278,279] Studies suggest that regular contact of patients with healthcare providers helps in weight maintenance and long-term health-related outcomes.[255] Follow-up meetings during the maintenance phase can act as check points for adherence, compliance and challenges faced by the midlife woman. However, there are limited studies suggesting the frequency and duration of follow-up for effective weight maintenance. Hence, it is important to understand the best combination of duration, frequency and mode of follow-up for midlife women undergoing lifestyle intervention programs for successful long-term maintenance.
Summary of evidence
Across weight management trials, the duration of follow-up during the maintenance phase ranged from 3 to 48 months.[260,263,264] Most studies had a maintenance phase for 12 months.[257,266,267,280,281] Apart from the duration of follow-up, variation in the frequency and mode of contact with the patient was also observed. The frequency of follow-up in 26 maintenance studies ranged from biweekly to yearly follow-up visits. Most studies had a monthly follow-up visit along with different forms of contact using various technological modalities such as biweekly transmission of information using newsletters, weekly website logins, and telephonic calls with trained staff.[265] Group-based sessions were organised in five studies and others studies planned an individualised follow-up session.[67,260,266,272] A study by Leahey et al.[267] also used monetary rewards for better follow-up and reduced dropout rates.
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 4.3.1 | The weight maintenance phase should be continued throughout life with sequential incorporation of parameters related to holistic well-being. | III A |
| 4.3.2 | Face-to-face contact can be maintained every three months coupled with a monthly contact using technological components such as text messages, telephonic calls and mobile applications. | II A |
Discussion
Ideally, the follow-up of a woman achieving a weight loss target should be continued throughout her lifespan. The follow-up should include regular monitoring of parameters including weight status, lifestyle-related behaviours, cardiometabolic risk factors, menopausal and musculoskeletal health. Parameters such as BMI and waist circumference can be used as initial indicators to plan health care during maintenance.[282] In case of relapse of lost weight, the BMI, waist circumference and presence of cardiometabolic risk factors should be referred to plan the intensity of future care. The action-points at different anthropometric and health-related parameters during weight maintenance are shown in [Table 4.3.1]. Weight maintenance advice should include behavioural strategies to address the barriers faced by midlife women in maintaining corrective eating and activity habits required for weight loss. The weight management advice can be delivered through a combination of individualised and long-distance technology-based sessions as per the availability of resources. In addition to physical follow-up at three months, the use of technological modalities such as telephonic counselling, virtual counselling sessions, text messages, emails and social media groups have proven effective for weight maintenance.
Table 4.3.1.
Assessment and advice during follow-up visits
| Parameters | Cut-off value | Follow-up actions |
|---|---|---|
| BMI | ≥23 kg/m2 | To be counselled for weight gain prevention strategies |
| ≥25 kg/m2 | To be enrolled in a lifestyle intervention program for weight loss | |
| Waist circumference | ≥72 cm | To be counselled for weight gain prevention strategies |
| ≥80 cm | To be enrolled in a lifestyle intervention program for weight loss | |
| Elevated cardiometabolic parameters (Blood glucose, low-density lipoprotein, cholesterol, blood pressure) |
Present | To be counselled for behavioural lifestyle modification Appropriately referred to a specialist (if required) |
| Other obesity-related disease (Osteoarthritis, sleep apnoea, NAFLD) |
To be referred to a specialist for proper medical and lifestyle management |
BMI: body mass index, NAFLD: non-alcoholic fatty liver disease, LDL: low-density lipoprotein
4.4: What advice should be given during the follow-up phase for maintenance of weight in midlife women?
Background
Weight maintenance aims at the management of physiological, behavioural, psychological and environmental factors to sustain weight in the long term.[283,284] Even women who lose significant weight during the intervention phase find maintenance of weight through corrective eating and activity habits as difficult.[254] Weight maintenance counselling is an essential part of a sustainable weight management program.
Weight maintenance advice includes adherence to healthy dietary habits, physically active routine, adequate sleep and other behavioural modifications important for further weight loss and/or weight maintenance.[261] These lifestyle habits should be reinforced to the patients at regular intervals during the maintenance phase to improve compliance towards a healthy lifestyle that can result in weight maintenance.[285] Behavioural modification strategies such as self-monitoring, problem-solving, relapse prevention, stimulus control and stress management should be considered as vital components of the counselling plan.[254,286] Thus, a comprehensive plan should be devised to impart sustainable and successful weight maintenance advice among midlife women.
Summary of evidence
Weight maintenance advice can be extended to the participants after completion of the weight loss program with the aim to lose more weight, maintain the lost weight and prevent weight regain. The weight maintenance advice was based on dietary modification, the introduction of physical activity regime, cognitive behavioural therapy, social support, technological support and pharmacotherapy. In a systematic review, behavioural modification advice led to greater weight loss in comparison to pharmacological intervention. Six studies reported a combination of lifestyle modification with cognitive behavioural techniques to manage the challenges experienced during the weight maintenance phase.[67,270,281] Participants involved in new healthy behaviours during weight maintenance were able to maintain the lost weight for a longer duration in comparison to the control group following healthy dietary and activity habits.[67,268,271] Almost all studies included various forms of dietary modification advice for weight maintenance. Dietary advice for weight maintenance included low-calorie diets, meal replacement, alternate day fasting, replacing sweetened beverages with water, and reinforcement of healthy dietary habits.[258,260,261,266,268,287] Some studies used key behavioural strategies for weight loss maintenance including problem-solving techniques, stimulus control, relapse prevention and overcoming barriers to healthy eating and physical activity provided across self-directed groups.[257,265,288] In five studies, the technological component was also compared with social support and positive reinforcement provided by a coach in the weight maintenance phase.[264,267,274] These interventions were associated with greater weight maintenance and less regain. The improvement in cardiometabolic risk factors such as fasting blood glucose, triglyceride, insulin and waist circumference were inconsistent across dietary interventions.[263,264]
Self-monitoring techniques were used to track the dietary habits, level of physical activity, weight, and behavioural change goals during the weight loss intervention or weight maintenance phase.[289-292] Across studies, the self-monitoring techniques were used to regulate the dietary, activity, sleep and weight-related behaviours by providing personal feedback, health promotion education, maintaining contact with interventionist, peer group support and frequent behavioural prompters.[290,293-296] Four modes were identified for promoting self-monitoring techniques: digital platform, minimal digital platform, wearable technologies and non-digital platform or usual care.[297] Self-monitoring intervention most commonly uses digital platforms including mobile and web-based applications, text messages, and personal digital assistants followed by minimally used digital platforms including health promotion information, e-consultations, e-newsletter, email, etc. for regulating healthy lifestyle behaviours.[293,298-300] The application-based dietary monitors tracked the amount of food and beverage consumed, daily calorie and fat intake, photographs of food items, bite counters and assigned a rating to foods consumed with comments and reviews from the interventionist.[264,289,301,302] Physical activity was tracked by wearable technologies and applications, which provided insights on the daily duration of moderate physical activity, number of steps per day, and information on exercise plans.[294,299,303] Across systematic reviews and weight-loss trials, application-based self-monitoring strategies improved adherence to healthy dietary and activity advice.[290,296,297,298] The improved adherence to healthy lifestyle habits for a longer duration contributed to a significant decrease in weight in application-based monitoring techniques in comparison to traditional monitoring techniques such as pen and paper-based daily diet and activity logs.[292,293,304] There is limited conclusive evidence on the impact of different combinations, durations and frequencies of digital self-monitoring techniques on weight loss outcomes.
Clinical practice recommendations
| No. | Recommendations | Grade |
|---|---|---|
| 4.4.1 | At every contact, healthcare providers should reinforce healthy eating, physical activity and sleep habits and address barriers and challenges faced during this phase. | II A |
| 4.4.2 | During follow-ups, self-monitoring through technological devices should be encouraged to maintain weight, dietary, physical activity and sleep routine records. | II A |
| 4.4.3 | Throughout weight maintenance, special attention should be given to the behavioural strategies such as enhancing motivation, social support, self-efficacy, problem-solving, relapse prevention and addressing individualised barriers. | II B |
| 4.4.4 | Clinical and biochemical parameters such as blood glucose, lipid profile and blood pressure measurements should be done as per the standard guidelines and/or advice by the treating doctor. | IV A |
| 4.4.5 | Adequate and appropriate care of bone, muscle, joints and menopausal symptoms should be ensured during follow-up contacts to ensure holistic well-being. | IV B |
Discussion
Lifestyle counselling acts as the cornerstone for sustainable weight management during the maintenance phase. Healthcare providers should reinforce compliance towards healthy dietary and activity practices using behavioural skills to prevent weight regain. Dietary strategies such as adequate protein intake, consumption of low glycemic foods, high fibre diet and low-fat dairy are associated with maintenance of weight.[189,305] Patients should also be counselled to maintain an active lifestyle by including a combination of aerobic, resistance and balance exercises for at least 150 minutes/week.[306] The adherence to healthy eating and activity status should be facilitated by behavioural strategies such as problem-solving, stimulus control, and relapse prevention.[266,268,307]
Self-monitoring is central to the process of weight maintenance and includes deliberate attention to dietary and activity behaviours, associated environmental cues and immediate and long-term health benefits.[308] Regular self-weighing is also a recommended strategy for weight maintenance. Self-weighing is defined as weighing oneself over a definite period of time such as daily, weekly or monthly.[309] Nowadays, the use of technical assistance through mobile and web-based applications, text messages, and personal digital assistants has proven effective for self-monitoring.[293,297-300] In addition to regular monitoring, healthcare providers should focus on providing frequent personalised feedback using technological aid.
In literature, the individual and interactive impact of different lifestyle modification strategies in weight maintenance is lacking. Future research should also focus on factors associated with the management of overall health in association with maintenance of weight status.
Discussion
During midlife, women face multiple challenges that make them prone to weight gain, abdominal adiposity and poor HRQoL.[2,6] There is a need to address these midlife-specific challenges to facilitate appropriate weight management in this group of women. To address these challenges, the experts from various fields came together to develop evidence and consensus-based guidelines for women-centric weight management in midlife women. The guidelines focus on clinical practice recommendations on behavioural lifestyle modification as first-line treatment of overweight and obesity in midlife women. In addition to lifestyle modification, areas specific to managing an adequate quality of life in midlife including metabolic, menopausal and musculoskeletal health was also included to provide a comprehensive treatment module.
The guidelines include clinical practice points for healthcare providers to modify the existing dietary and activity behaviours in a way that promotes weight loss. The recommendations include stepwise assessment, management and monitoring of key dietary and activity behaviours to facilitate effective weight management. Dietary recommendations include counselling balanced dietary intake and corrective eating behaviours, focusing on macronutrient intake, consumption of HFSS foods and sugar sweetened beverages (SSBs), eating out and emotional eating. Physical activity recommendations help in customising a progressive exercise regime including aerobics, strength training, flexibility for managing weight, musculoskeletal and distress in midlife women. A mutually agreeable duration, intensity and frequency of exercise regime is important for long-term participation.[189] The recommendations also highlight different behavioural techniques including goal setting, problem-solving, managing triggers, and self-monitoring for sustainable weight loss. Behavioural modification can help in improving the compliance to corrective dietary and activity recommendations required to maintain ideal weight in the long term.[223] In clinical practice, the behavioural lifestyle modifications should be recommended in consideration with the maintenance of the overall health of midlife women.
SUMMARY OF RECOMMENDATION
| S. No. | Recommendations | Grade$ |
|---|---|---|
| Initiation of discussion for weight management | ||
|
| ||
| 1.1: When should a healthcare provider initiate structured counseling regarding weight management in midlife women? | ||
|
| ||
| 1.1.1 | Opportunistic screening and management of obesity should be delivered all through the lifespan of a woman. | IV A |
| 1.1.2 | In the late thirties, before menopause transition, women should be counselled about the added risk of menopause-related weight gain and body fat distribution. Opportunistic screening and management of obesity should be continued. | II A/B |
| 1.1.3 | In the early forties, when women experience menopausal transition, intensive customised weight management counselling should be given. The emphasis should be on corrective lifestyle behaviour and handling health issues specific to menopausal transition such as menopausal symptoms, sleep disturbances, psychological distress, bone and joint health and other comorbidities. | II A |
|
| ||
| 1.2: What are the components of Knowledge, Attitude and Practice (KAP) that should be evaluated to plan a personalised weight management intervention in midlife women? | ||
|
| ||
| 1.2.1 | Assessment of KAP related to risk factors and consequences of obesity on the holistic health of women is recommended. | III A |
| 1.2.2 | Different modalities for weight management, barriers and facilitators in their implementation should be evaluated and accounted for in the management plan. | III B |
|
| ||
| 1.3: Who are the healthcare providers that can be involved in the management of overweight and obesity in midlife women? | ||
|
| ||
| 1.3.1 | The protocolised weight management module should be implemented by any healthcare worker who encounters a woman in her midlife for routine screening or specific health conditions. | IV A |
| 1.3.2 | Wherever feasible, a multi-disciplinary team consisting of primary care physicians, clinicians, dietitians and exercise physiologists/physiotherapists should be involved in weight management of midlife women. Specialists including psychologists/psychiatrists, endocrinologists, orthopaedics and physiatrists should be involved, whenever indicated. | I A |
| 1.3.3 | All the healthcare workers should be empowered with the knowledge and skills for prevention, diagnosis and treatment of obesity in midlife women. | IV B |
|
| ||
| 1.4: What could be effective ways of delivering pertinent information to midlife women regarding the management of overweight and obesity? | ||
|
| ||
| 1.4.1 | A combination of face-to-face and technology-supported distance counselling should be planned for the management of weight in midlife women with overweight and obesity. | IV A |
| 1.4.2 | Internet and mobile-based applications should be used proactively for improving compliance by enhancing patient education, motivation, self-monitoring, personalised feedback, and managing challenges faced in appropriate weight management practices. | II A |
| 1.4.3 | A toolkit consisting of education material apprising midlife women about menopausal transition, its impact on weight status and key management strategies can be developed, distributed and utilised across healthcare settings. | IV B |
|
| ||
| Screening and risk assessment of midlife women | ||
|
| ||
| 2.1: What BMI cut-off and other anthropometric parameters should determine the need to initiate weight management intervention in midlife women? | ||
| 2.1.1 | BMI and Waist Circumference should be used independently for population- and clinic-based cardiometabolic risk stratification and other obesity-related diseases. | I A |
| 2.1.2 | For initiating a weight management intervention, the class of generalised obesity should be identified according to the BMI-cut-off: 18.5-22.9 kg/m2: normal weight 23.0-24.9 kg/m2: overweight ≥25 kg/m2: obesity | I A |
| 2.1.3 | For initiating weight management intervention, the cut-off reference for waist circumference should be as below: Waist circumference less than or equal to 72 cm: normal Waist circumference >72 cm but <80 cm: associated with one cardiometabolic risk factor-initiate weight management advice Waist circumference >80 cm: associated with cardiometabolic comorbidities-initiate intensive weight management | I A |
|
| ||
| 2.2: How should dietary practises of midlife women be evaluated ? | ||
|
| ||
| 2.2.1 | The detailed dietary evaluation should include an assessment of the usual meal pattern (including the number of food items consumed) and dietary habits (including skipping meals, typical frequency of consumption of HFSS foods, emotional/stress eating). | I A |
| 2.2.2 | Twenty-four-hour dietary recall and food frequency questionnaire for three days (two weekdays and one weekend) should be used for dietary evaluation, if feasible. Energy, macronutrient, fibre intake should subsequently be calculated. Alternatively, a short validated questionnaire can be used.** | II B |
| 2.2.3 | Dietary intake of foods rich in protein, iron, and calcium should also be assessed while taking dietary history. | IV B |
| 2.2.4 | The barriers faced by midlife women to maintain a healthy diet in their daily lifestyle should be evaluated. | IV B |
|
| ||
| 2.3: How should the level of daily physical activity of midlife women be evaluated? | ||
|
| ||
| 2.3.1 | The detailed physical activity evaluation should include an assessment of dedicated physical exercise and domestic, work-related, leisure-related, transport-related and sedentary activities (screen and sitting time). | I B |
| 2.3.2 | Madras Diabetes Research Foundation-Physical Activity Questionnaire (MPAQ) should be used to evaluate the level of physical activity, if feasible. Alternatively, a short validated questionnaire can be used.** | II B/C |
| 2.3.3 | Evaluation of the adequacy of physical exercise should be done by assessing: type (stretching/strengthening/aerobics/balance), intensity (light/moderate/vigorous), frequency (number of days per week) and duration (number of minutes per day) of exercise. | IV B |
| 2.3.4 | Special attention should be given to assess the number of sedentary hours (especially screen and sitting time) spent during the day. | IV A |
| 2.3.5 | Midlife women should be encouraged to discuss the barriers faced by them in maintaining an active lifestyle. | IV A |
|
| ||
| 2.4: How psychological and behavioural health should be evaluated in midlife women being engaged in the management of overweight and obesity? | ||
|
| ||
| 2.4.1 | Assessment should include inquiry into the presence of a diagnosed psychiatric disorder, especially depression, anxiety or eating disorder. | II A |
| 2.4.2 | If there is clinical suspicion, DASS-21 can be used as an initial screening tool for the assessment of depression, anxiety and stress. | I B |
| 2.4.3 | In case a diagnosed psychiatric disorder is present, the current condition of the psychiatric disorder and use of psychotropic medications should be asked for by the weight management team. | I A |
| 2.4.4 | The referral to a mental health professional should be considered if: Dietary history reveals abnormal eating practices to cope with stress. There is a sudden lack of motivation for weight reduction. There are persistent interpersonal difficulties with the weight management team. DASS-21 score indicates the possibility of depression, anxiety or stress. There is a history of diagnosed psychiatric disorders. There is clinical suspicion of a known psychiatric disorder. |
IV A |
|
| ||
| 2.5: What are the important comorbid conditions (cardio-metabolic and other medical conditions) that should be evaluated before initiating management of overweight and obesity? | ||
|
| ||
| 2.5.1 | In midlife women, lifestyle intervention should be initiated if they have any known cardiometabolic risk factors (such as abnormal blood sugar, increased blood pressure, dyslipidaemia) or obesity-related complications (such as non-alcoholic fatty liver, sleep apnoea, etc.). | I A |
|
| ||
| 2.6: How should menopause-related symptoms be evaluated in midlife women being engaged in the management of overweight and obesity? | ||
|
| ||
| 2.6.1 | Menopause Rating Scale (MRS) may be used for the evaluation of menopausal symptoms, which includes physical, psychological, vasomotor, genitourinary and sexual domains. | I B |
| 2.6.2 | The impact of menopausal symptoms severity on weight-related behaviours (diet, physical activity and sleep) should also be assessed. | IV A |
|
| ||
| Management of overweight and obesity | ||
|
| ||
| 3.1: How stepwise weight loss goals should be set for midlife women being engaged in the management of overweight and obesity? | ||
|
| ||
| 3.1.1 | Healthcare providers should assess the readiness to engage in weight loss attempts by changing the current diet and physical activity using behavioural modification. | IV A |
| 3.1.2 | Realistic and sustainable patient-centric weight loss goals should be established after a detailed discussion with the midlife woman. If feasible, family members should be involved and similar health goals should be planned for them. | IV A |
| 3.1.3 | Overweight and obese women should be advised to reduce body weight to a normal BMI (18.5-22.9 kg/m2). | I A |
| 3.1.4 | A step-wise body-weight loss goal should be set with a target weight loss of 0.5 kg per week attaining a weight loss of 5-10% of baseline body weight over a period of 6 months (clinically significant weight loss). | I A |
|
| ||
| 3.2: What type of dietary recommendations should be advised for improving weight loss, anthropometric and metabolic health outcomes? | ||
|
| ||
| 3.2.1 | An individualised diet plan should be recommended considering eating preferences, food habits and the health status of the participant. | I A |
| 3.2.2 | The meal pattern should be spread throughout the day preferably involving three major meals and two snacks. | II A |
| 3.2.3 | The daily dietary calorie intake should be based on the baseline caloric intake and level of physical activity of the midlife woman. | I A |
| 3.2.4 | The diet plan should incorporate an energy deficit of 500 kcal per day to achieve a weight loss of 0.5 kg body weight per week. | I A |
|
| ||
| 3.2: What type of dietary recommendations should be advised for improving weight loss, anthropometric and metabolic health outcomes? | ||
|
| ||
| 3.2.5 | Restricted carbohydrate, good quality fat and a high protein diet is recommended for midlife women. | II A |
| 3.2.6 | Women should be counselled to consume foods rich in protein, calcium and iron in their daily diet to meet the estimated average requirement (EAR). | I A |
| 3.2.7 | Dietary fibre intake of 20-30 g per day should be prescribed from whole grains, legumes, nuts, oilseeds, fruits and vegetables. | I A |
| 3.2.8 | Restricted consumption of food products high in fat, sugar and salt (HFSS) should be emphasised. Salt intake should be limited to less than 5 g/day. | I A |
| 3.2.9 | Dietary intake of foods rich in phytoestrogen from the Indian diet should be encouraged. | II C |
|
| ||
| 3.3: What type of physical activity recommendations should be advised for improving weight loss, anthropometric and metabolic health outcomes? | ||
|
| ||
| 3.3.1 | Midlife women should be encouraged to avoid a sedentary lifestyle as far as possible by ensuring participation in dedicated exercise, household-related, work-related and leisure-related physical activities. | II A |
| 3.3.2 | A stepwise progressive and personalised physical exercise regime based on body weight, presence of cardiometabolic risk factors, bone, muscle and joint health should be prescribed. | II A |
| 3.3.3 | The barriers and facilitators for adopting different types of physical exercises should also be considered while prescribing a physical exercise regime. | IV A |
| 3.3.4 | Women should be encouraged to incorporate up to 300 min/week of moderate-intensity aerobic physical activity or 150 min/week of high-intensity aerobic physical activity or an identical combination of moderate and high-intensity exercise. | I A |
| 3.3.5 | Preferably, daily 60 min of physical activity should be recommended including a combination of moderate-intensity aerobic exercise (30 min), muscle strengthening engaging major muscle groups or balance exercise (15 min), and work-related and/or household-related moderate physical activities (15 min). | I B |
| 3.3.6 | Women can participate in moderate-intensity aerobic activities in short bouts (10-15 min) throughout the day (2-3 times). | II B |
| 3.3.7 | The talk test* should be used for self-monitoring the intensity of aerobic activities. | I A |
| 3.3.8 | Yogic practices (including physically intensive yoga) can be prescribed for weight management and overall well-being in midlife women. | III A |
|
| ||
| 3.4: What are the behaviour modification techniques that should be incorporated in weight management advice? | ||
|
| ||
| 3.4.1 | Behaviour modification techniques such as realistic goal setting, motivational interviewing, and self-monitoring strategies should be used. | II B |
| 3.4.2 | Women should be trained regarding problem-solving skills such as defining problems, creating solutions and opting for the best possible choice. | II B/C |
| 3.4.3 | Cognitive restructuring skills such as identifying, challenging, and correcting the negative thoughts and emotions related to weight management should be imparted. | II B/C |
| 3.4.4 | Feedback on the accomplishments, achievements and scope for better progress should be given regularly. | II B |
|
| ||
| 3.5: What is the role of menopausal hormone therapy (MHT) in a weight management program for midlife women? | ||
|
| ||
| 3.5.1 | Menopausal hormone therapy (MHT) is not indicated solely for weight management in midlife women. | II A |
| 3.5.2 | Weight management by lifestyle interventions may help in improving the overall well-being of midlife women with menopausal symptoms. | II A |
|
| ||
| Follow-up for sustainable weight loss | ||
|
| ||
| 4.1: What are the other important parameters in determining the overall improvement in midlife women after undergoing weight management? | ||
|
| ||
| 4.1.1 | In addition to weight management, midlife women with obesity should be appraised and sensitised about the benefits of a healthy lifestyle on bone health, muscle strength and menopausal symptoms. | II B |
|
| ||
| 4.2: What should be the duration, frequency and mode of follow-up of midlife women during the intervention? | ||
|
| ||
| 4.2.1 | The time schedule for the intervention phase of weight management should be decided on the basis of target weight loss to be achieved in the patient. | IV A |
| 4.2.2 | The duration of the intervention phase should be planned such that the patient can lose 5-10% body weight every 6 months till the target body weight is achieved. | I A |
| 4.2.3 | Bimonthly contact should be planned in the initial stages of the intervention phase that can be reduced to monthly contact in the later phase. | II A/B |
| 4.2.4 | A combination of physical (face-to-face and/or group counselling) and online meeting modalities/telephonic contacts can be used for the follow-up | II A |
|
| ||
| 4.3: What should be the duration, frequency and mode of contact during the maintenance phase of the weight management program for a midlife woman? | ||
|
| ||
| 4.3.1 | Weight maintenance phase should be continued throughout life with sequential incorporation of parameters related to holistic well-being. | III A |
| 4.3.2 | Face-to-face contact can be maintained every three months coupled with a monthly contact using technological components such as text messages, telephonic calls and mobile applications. | II A |
|
| ||
| 4.4: What advice should be given during the follow-up phase for maintenance of weight in midlife women? | ||
|
| ||
| 4.4.1 | At every contact, healthcare providers should reinforce healthy eating, physical activity and sleep habits and address barriers and challenges faced during this phase. | II A |
| 4.4.2 | During follow-up visits, self-monitoring through technological devices should be encouraged to maintain weight, dietary, physical activity and sleep routine records. | II A |
| 4.4.3 | Throughout weight maintenance, special attention should be given to behavioural strategies such as enhancing motivation, social support, self-efficacy, problem-solving, relapse prevention and addressing individualised barriers. | II B |
| 4.4.4 | Clinical and biochemical parameters such as blood glucose, lipid profile and blood pressure measurements should be done as per the standard guidelines and/or advice by the treating doctor. | IV A |
| 4.4.5 | Adequate and appropriate care of bone, muscle, joints and menopausal symptoms should be ensured during follow-up contacts to ensure holistic well-being. | IV B |
$Grade: Quality of evidence+strength of recommendation. Quality of evidence. I: High-quality evidence; II: Moderate-quality evidence; III: Low-quality evidence; IV: Expert opinion. Strength of recommendation. A: Strong recommendation; B: Moderate recommendation; C: Weak recommendation. *Kumari A, Chopra S, Ranjan P, Verma A, Malhotra A, Upadhyay A, Sharma AK, Vikram NK. Development and validation of Comprehensive evaluation tool for weight management in midlife women. Under publication. **Talk test can be used to assess the intensity of aerobic exercises: comfortable speech denotes light intensity, speech with some difficulty denotes moderate intensity and speech limited to phrases denotes vigorous intensity.[9]
Benefit to healthcare fraternity
The protocolised weight management module depicted in [Box 1] is a practical clinical tool for healthcare providers to deliver appropriate weight management in midlife women. Generally, healthcare providers encounter midlife women for other health-related issues such as diabetes, blood pressure, joint pain, and distress.[10] Healthcare providers should counsel midlife women regarding the role of managing weight as an effective strategy for maintaining overall health. They can also refer them to a multidisciplinary weight management team for customising a lifestyle modification approach.[57] The multidisciplinary team can use the recommendations to facilitate a well-rounded approach in the management of obesity and associated adiposity-related comorbidities.
Box 1.
Women-centric weight management module for midlife women
| Step 1: Weight management counselling at midlife |
| WHO should get it? |
| Opportunistic screening and management for all women throughout the lifespan |
| Women in the late thirties before menopause transition should be made aware regarding obesity management |
| Women in the early forties experiencing menopausal transition |
| From WHERE ? |
| Different Health Clinics: Medical OPDs, Gynaecology, Community health care centres, etc. |
| Community: Resident welfare association and institutes such as schools, colleges, corporate offices |
| By WHOM? |
| Primary Care Physicians, Family Physicians |
| Specialists: Orthopedicians, Gynaecologists, Endocrinologists, Internists |
| Community Health Nurses |
| Registered Dietitians/Nutritionists |
| Behavioural Health Specialists/Psychologists/Psychiatrists |
| WHAT Type of advice? |
| Weight management advice related to diet, physical activity and behavioural modification |
| Awareness regarding appropriate weight maintenance |
| BY WHAT MODE? |
| Message/advice disseminated through short videos, PowerPoint presentations, posters, booklets, diet charts, recipes etc. |
| Step 2: Initiation of discussion |
| Ensure privacy and confidentiality while initiating discussion |
| Introduce obesity as a health issue |
| Ask for woman’s perception and problems associated with weight gain |
| Explain benefits of weight reduction |
| Use patient-centric language |
| Avoid blaming and be supportive |
| Step 3: Assess the readiness and motivation to initiate weight management counselling |
| If the participant is eager to initiate: |
| Counsel the woman for the next stages of lifestyle modification (screening, management and follow-up) |
| Evaluate the participant’s degree of obesity, associated risk factors and lifestyle-related behaviour |
| If the participant is indecisive and reluctant to initiate lifestyle modification: |
| Be supportive and invite for discussion |
| Reinforce the benefits of weight loss |
| Assess the reason associated with unwillingness |
| Try to solve the individual’s issues and motivate her to initiate lifestyle modification |
| Step 4: Anthropometric assessment |
| Measure height, weight, body fat, and waist circumference. |
| Calculate body mass index (BMI) using the height and weight measurements, |
| BMI=Weight (kg)/Height2 (m2) |
| Identify the following indications for initiating weight management: |
| BMI in the overweight category (23-24.9 kg/m2) or obese category (≥25kg/m2), and/or; |
| Body fat >30%, and/or; |
| Waist circumference >80 cm |
| Step 5: Detailed clinical assessment |
| Medical history: |
| Assess the previous history of weight gain, women-centric risk factors, previous weight loss attempts, family history, etc. |
| Assess cardiometabolic risk factors (abnormal blood sugar, increased blood pressure, dyslipidaemia). |
| Identify obesity-related comorbidities (non-alcoholic fatty liver, sleep apnoea, joint issues, polycystic ovary syndrome, etc). |
| Biochemical assessment: Assess biochemical parameters such as lipid profile, blood glucose level, thyroid test, C- reactive protein, as per indications. |
| Menopausal health assessment: |
| Assess the presence and severity of menopausal symptoms using the Menopause Rating Scale (MRS) to evaluate physical, psychological, vasomotor, genitourinary and sexual domains. |
| Evaluate the impact of menopausal symptoms severity on weight-related lifestyle practices. |
| Step 6: Dietary, physical activity and psychosocial assessment |
| Dietary assessment: |
| Assess dietary intake using 24-h recall. |
| Assess dietary diversity via intake of foods from various food groups. |
| Evaluate dietary behaviour and barriers in maintaining a healthy diet via a validated questionnaire. |
| Physical activity assessment |
| Assess detailed physical activity habits using Madras Diabetes Research Foundation - Physical Activity Questionnaire (MPAQ). Assess the type, frequency, duration and intensity of physical activity |
| Assess the number of sedentary hours (screen and sitting time) spent in the entire day. |
| Validated questionnaire to assess barriers faced in being physically active by midlife women. |
| Psychological assessment: |
| Assess the presence of a diagnosed psychiatric disorder (Depression, Anxiety or Eating Disorder). |
| Use DASS-21 for initial screening of depression, anxiety and stress. |
| Refer to a mental health professional, if indicated. |
| Management of overweight and obesity |
| Step 7: Goal setting: |
| Establish realistic, sustainable and patient-centric weight loss goals. |
| Advice to reduce body weight to the BMI equal to or <23 kg/m2. |
| Step-wise body-weight loss goal of 0.5 kg per week acquiring 5-10% of clinically significant weight loss. |
| Step 8: Lifestyle management advice |
| Dietary management: |
| Customise diet plan considering eating preferences, food habits and health status of the patient |
| Plan energy deficit diet of 500 kcal/day for 0.5 kg body weight loss per week |
| Advise a restricted carbohydrate, good quality fat and high protein diet |
| Spread meal pattern throughout the day (Three major meals and Two snacks) |
| Counsel women to consume foods rich in protein, calcium and iron |
| Prescribe dietary fibre intake of 20-30 g/day (whole grains, legumes, nuts, oilseeds, fruits and vegetables) |
| Encourage dietary intake of foods rich in phytoestrogen |
| Emphasise restricted consumption of food products high in fat, sugar and salt (HFSS foods) |
| Encourage self-monitoring of dietary behaviour using a food diary |
| Physical activity management: |
| Prescribe stepwise progressive and personalized physical activity regime based on body weight, cardio-metabolic, bone, muscle and joint health |
| Encourage to incorporate up to 300 min/week of moderate-intensity aerobic physical activity or 150 min/week of high-intensity aerobic physical activity |
| Recommend a combination of moderate-intensity aerobic exercise (30 min), muscle strengthening (15 min), work-related and/or household-related activities (15 min) |
| Encourage women to participate in the aerobic exercise in short bouts (10-15 min) throughout the day (2-3 times) |
| Consider barriers and facilitators while prescribing physical activity advice |
| Prescribe intensive yoga for weight management and overall well-being |
| Behavioral modification management: |
| Use progressive realistic goal setting, motivational interviewing and self-monitoring strategies |
| Train women for improving problem-solving skills and cognitive structure skills |
| Give feedback on accomplishment, achievement and scope for better progress |
| Step 9: Follow-up |
| Plan duration, frequency and mode of follow-up: |
| Duration: 12 months, ranging from 6-18 months based on the targeted weight loss. |
| Frequency: Once or twice a month at initial stages followed by every 2-3 months at a later stage. |
| Mode: Face-to-face individualised/group counselling coupled with telephonic contacts. |
| Plan advise to be provided during follow-ups: |
| Reinforce advice related to a healthy diet and physically active behaviour. |
| Motivate and provide support to improve adherence for maintaining healthy lifestyle behaviour. |
| Encourage self-monitoring through technological devices such as mobile applications, pedometers etc. |
| Follow up checklist: |
| Were you able to lose 0.5-1 kg weight per week? |
| Have you followed a balanced diet comprising foods from various food groups such as fruits, vegetables, high fiber cereals and legumes and low-fat dairy? |
| Have you included protein-rich foods in your diet? |
| Have you restricted/avoided high fat, sugar and salt (HFSS) foods? |
| Have you initiated an exercise regime including aerobic and/or strengthening exercises? |
| Are you mindful of the sitting and screen time? |
In resource-constrained settings, the availability of experts from multidisciplinary teams might be limited.[310] The clinician and/or healthcare provider encountering midlife women with obesity are required to provide obesity care.[311] Mostly, midlife women visit primary care physicians, family physicians, internists, gynaecologists, endocrinologists, and orthopaedicians for other health-related issues.[312] The recommendations provide clinical practice points for opportunistic screening and management of obesity in midlife women that can be incorporated by clinicians and allied healthcare providers from different healthcare specialties in their daily clinical practice.
Further, a significant proportion of women belonging to lower socio-economic strata and/or residing in rural settings might have access only to local primary healthcare centres and anganwadi. These healthcare settings offer suboptimal obesity care due to a lack of multidisciplinary teams, resources and time.[313] The woman’s preference to initiate weight loss, motivation, education status, access to resources, daily lifestyle and income level becomes important in determining the appropriate weight management approach. The protocolised treatment plan supplemented with health education material can help the primary care provider to initiate optimum obesity care with limited resource availability.
The recommendations can help in capacity building by providing evidence and consensus-based weight management practices at both clinical and community level. The clinical practice recommendations are simple, practical, and actionable statements to manage obesity in midlife women. This will ensure uniform and appropriate obesity treatment practices for midlife women within various healthcare settings.
The guideline promotes preventive obesity care in midlife women. The current guideline highlights the following key areas: when to initiate and engage midlife women for weight management, who should provide obesity care, and which mode should be used to deliver healthcare. These recommendations promote a proactive approach in managing obesity-related disorders in midlife women. The practical application of these guidelines can help to manage the ever-growing burden of obesity and associated cardiometabolic diseases in different healthcare systems of the country.
Suggestions for policy-makers
A substantial increase in the prevalence of overweight and obesity, especially in rural residents and older adults is projected in India by 2040.[314] The policy makers should disseminate recommended clinical practice points at medical, curriculum and public health levels to manage the burden of obesity and allied comorbidities. The policy makers should ensure resources for the application of the evidence-based clinical practice points at different levels of healthcare settings. The clinicians and allied healthcare professionals should be provided with knowledge regarding appropriate obesity care including lifestyle modification, pharmacological and surgical interventions. Healthcare providers in resource-constrained settings should be appraised about the additional challenges in managing obesity. In addition, the practitioners should empower midlife women with the knowledge related to appropriate eating, physical activity and other lifestyle-related behaviours through providing health education material (like posters, brochures, newsletters, etc.), providing necessary and appropriate referrals and managing support groups at the clinical settings. Healthcare settings should establish ‘women wellness clinics’ for multidisciplinary management of obesity and allied health disorders and ensure active and regular participation of the clinicians from different specialties and allied healthcare professionals.
In addition to caregivers, midlife women should also be empowered with the correct knowledge to maintain healthy lifestyle-related behaviours. These women experience a number of biopsychosocial changes. Poor health literacy at this stage hampers the ability of midlife women to understand and implement health-related information to manage overall health.[315] The promotion of the use of artificial intelligence to support weight loss-related behaviour change in adults through improving self-regulation, decision making, problem-solving and pattern identification can help in improving KAP of midlife women towards weight management. The policy makers should introduce policies and programs to educate midlife women experiencing menopausal transitions and associated health implications including weight gain, musculoskeletal and cardiometabolic health. Some initiatives like Club 35+, and Midlife Healthcare Clinics taken up by the Indian Menopausal Society can be replicated to empower midlife women with appropriate medical advice with the support of healthcare professionals.
Policy makers should use mass-media campaigns to transform weight-related behaviour. This will help in exposing a significant proportion of women with corrective weight-related information using print media (leaflets, posters, brochures) and social media (websites, newsletters, television). These campaigns focus on simple and actionable corrective eating behaviours such as consumption of milk, pulses and fruits and vegetables, optimum water intake and limited consumption of HFSS foods.[316] Generally, campaigns promote regular participation in physical activities like walking, jogging, dancing and aerobics. Although campaigns promote positive health behaviours in the short term, often their results do not translate in the long term due to competing cues for unhealthy behaviours enforced by an obesogenic environment.[317] Hence, a more comprehensive and integrated approach is required to help address overweight and obesity in midlife women, as has been discussed in this document.
Statement
Considering its potential for widespread public health impact and general interest, the guidelines in multiple versions (comprehensive, concise, executive summary, good clinical practice points) have been submitted for simultaneous publication and/or co-publication (either fully or a part of it) in Diabetes and Metabolic Syndrome: Clinical Research and Reviews, National Medical Journal of India, Journal of Midlife Health, Journal of Family Medicine and Primary Care, JAPI: Journal of the Association of Physicians of India, and Indian Journal of Medical Specialities Journal of Obstetrics and Gynaecology of India. The guidelines can be submitted to some more journals for publication. Besides, the guidelines can be published on the government’s website and AIIMS, New Delhi’s website.
Financial support and sponsorship
The study was financially supported by the SEED Division, Department of Science and Technology, Government of India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The guidelines have been developed in association with the following National Bodies and Societies: (i) Federation of Obstetric and Gynaecological Societies of India, (ii) Indian Menopause Society, (iii) Association of Physicians of India, (iii) Academy of Family Physicians of India, (iv) Association of Obstetricians & Gynaecologists of Delhi and (v) Indian Dietetic Association.
Annexures
Annexure 1.
Cut-off for haemoglobin and serum ferritin in the assessment of iron deficiency anaemia in women
| Hemoglobin level | Anaemia status |
|---|---|
| 12.0 to 15 g/dL | Normal |
| 11-11.9 g/dL | Mild anaemia |
| 8-10.9 g/dL | Moderate anaemia |
| <8 g/dL | Severe anaemia |
| Serum ferritin concentration | |
| (<15 g/L) | Depleted iron stores |
| Severe risk of iron overload | >150 |
World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. World Health Organization; 2011
Annexure 2
Quick assessment of approximate dietary calcium intake per day
| Source | Calcium (mg) | Number of serving* |
|---|---|---|
| Dairy products (Milk, curd, paneer, chhena, khoya, cheese, milk powder) | 300-525 | |
| Non-dairy products (Non-dairy milk and milk products, ragi, bengal gram, soy and soy products, green leafy vegetables, nuts and oilseeds) | 200-300 | |
| Approximate total calcium intake (mg) = (Number of servings from dairy products×300-525) + (number of servings from non-dairy products ×200-300) | ||
| RDA for adult Indian women: 800 mg/day; post-menopausal woman: 1200 mg/day | ||
| *Number of servings: To be filled according to the daily intake of the midlife woman. | ||
Source: Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, National Osteoporosis Foundation et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014 Oct; 25 (10):2359-81. doi: 10.1007/s00198-014-2794-2. Epub 2014 Aug 15. Erratum in: Osteoporos Int. 2015
Annexure 3
Cut off for serum 25-hydroxyvitamin D
| Serum 25-hydroxyvitamin D (ng/mL) | Associated health status |
|---|---|
| <20 | Deficiency of vitamin D |
| 21-29 | Insufficient vitamin D levels |
| ≥30 | Sufficient vitamin D levels |
| >150 | Toxicity |
Source: Aparna P, Muthathal S, Nongkynrih B, Gupta SK. Vitamin D deficiency in India. J Family Med Prim Care. 2018 Mar-Apr; 7 (2):324-330. doi: 10.4103/jfmpc.jfmpc_78_18. PMID: 30090772; PMCID: PMC6060930
Annexure 4
A comprehensive assessment tool for appropriate weight management for midlife women
| Menopause is a natural life event marked by the final menstrual period. Perimenopause is the transitional phase that leads to menopause, usually experienced at the age of 43 to 55 years. | |||||
| This questionnaire has been developed to assess the interplay of the presence and severity of menopausal changes, weight.related perceptions and lifestyle practices including eating habits, physical activity and sleep patterns among women going through the menopausal transition. | |||||
| This is a valid and reliable tool for assessment of: Dietary practices (6B-10B) and barriers associated with practicing healthy eating behavior (11B) in everyday life of midlife women. | |||||
| Daily physical activity (12B-14B) and barriers associated with an inability to follow a physically active routine (15B) in everyday life of midlife women. | |||||
| Menopausal symptoms experienced by menopausal women (6B-10B). | |||||
|
| |||||
| Section A: Sociodemographic profile | |||||
|
| |||||
| 1. Name: | 2. Age: | 3. Phone no.: | |||
| 4. House address: | |||||
| 5. Marital status: | |||||
| Single◻ Married◻ Widow◻ Divorced◻ Separated◻ | |||||
| 6. Education: | |||||
| Profession or Honours◻ Graduate◻ Intermediate or diploma◻ High school◻ Middle-school certificate◻ Primary school certificate◻ Illiterate◻ | |||||
| 7.Education of the head of family: | |||||
| Profession or Honours◻ Graduate◻ Intermediate or diploma◻ High-school certificate◻ Middle-school certificate◻ Primary school certificate◻ Illiterate◻ | |||||
| 8. Occupation: | |||||
| Unemployed◻ Housewife◻ Working from home◻ Going to work as usual◻ Any other, please specify ………………. | |||||
| 9. Occupation of the head of family | |||||
| Legislators◻ Senior officials and managers◻ Professionals◻ Technicians and associate professionals◻ Clerks◻ Skilled workers, shop and market sales workers◻ Skilled agricultural and fishery workers◻ Craft and related trade workers◻ Plant and machine operators and assemblers◻ Elementary occupation/unemployed◻ | |||||
| 10. Total monthly income of the family: | |||||
| ≥199,862◻ 99,931-199,861◻ 74,756-99,930◻ 49,962-74,755◻ 29,973-49,961◻ 10,002-29,972◻ ≤ 10,001◻ | |||||
| 11. Medical history (any chronic illness with which you are suffering): Diabetes Hypertension Heart diseases Hypothyroidism Arthritis Depression Cancer Any other:_______________ | |||||
| 12. History of operations………. | |||||
| 13. Current menstrual status: | |||||
| I am having regular periods (each cycle occurs every 21 to 35 days)◻ | |||||
| I am having irregular periods, but I have not gone 12 months in a row without periods◻ | |||||
| My periods were stopped but now having periods as I am taking hormones◻ | |||||
| My periods have completely stopped◻ | |||||
| 14. Status of menopause: | |||||
| Normal periods◻ Using hormone replacement therapy◻ Surgical (removal of the ovaries/uterus)◻ Natural stopping of periods with age◻ | |||||
| Menopausal symptoms | |||||
| With the onset of the perimenopause stage, women experience menopausal (Items 1 & 2), physical (Items 3 to 5) and psychological (Items 6 to 8) symptoms. Please indicate the extent to which you are bothered by any of these symptoms: | |||||
|
| |||||
| Name of symptoms/severity | None | Mild | Moderate | Severe | Very severe |
|
| |||||
| 1.Irregular periods (heavy bleeding, blood clotting) | |||||
| 2.Hot flushes (excessive sweating) | |||||
| 3.Physical discomfort (fatigue, heaviness of body, body pain, swelling) | |||||
| 4.Heart discomfort (heart racing, skipping of a beat) | |||||
| 5.Joint and muscular discomfort (Pain in joints, rheumatoid complaints) | |||||
| 6.Emotional volatility (feeling nervous, inner tension, aggression, depression) | |||||
|
| |||||
| Anthropometric profile | |||||
|
| |||||
| Weight: | Height: | ||||
|
| |||||
| Section B: Lifestyle-related behaviour | |||||
|
| |||||
| 1B. Which statement (in your opinion) does define your body weight status? | |||||
| (i) Prefer not to comment | |||||
| (ii) My weight is slightly less | |||||
| (iii) My weight is about right | |||||
| (iv) My weight is slightly more | |||||
| (v) My weight is significantly more | |||||
| 2B. In the past, how many focussed attempts did you make to lose or maintain appropriate weight? | |||||
| (i) One attempt every year | |||||
| (ii) None | |||||
| (iii) 1-2 attempts | |||||
| (iv) 3-4 attempts | |||||
| (v) 5-6 attempts | |||||
| 3B. What has been the usual outcome of the weight loss attempts that you have made in the past? | |||||
| (i) Not applicable | |||||
| (ii) Mostly unsuccessful | |||||
| (iii) Initially successful (initial weight loss followed by regain) | |||||
| (iv) Minimal success in losing weight (considerable weight loss followed by some weight regain) | |||||
| (v) Mostly successful (considerable weight loss with no weight regain) | |||||
| 4B. With the onset of the perimenopausal phase, weight gain experienced by the women can be controlled by appropriate lifestyle measures. | |||||
| (i) Definitely | |||||
| (ii) Probably | |||||
| (iii) Can not say | |||||
| (iv) Probably not | |||||
| (v) Definitely not | |||||
| 5B. I intend to initiate lifestyle intervention methods (diet, exercise, etc.) to keep my body weight appropriate. | |||||
| (i) Eager to initiate | |||||
| (ii) Somewhat eager to initiate | |||||
| (iii) Undecided | |||||
| (iv) Somewhat not eager to initiate | |||||
| (v) Not eager to initiate | |||||
| 6B. Balanced diet consists of the right proportions of whole wheat, pulses, legumes, eggs, nuts, fruits and vegetables. How often are you able to consume a balanced diet? | |||||
| (i) Not routinely | |||||
| (ii) One to two times per week | |||||
| (iii) Three to four times a week | |||||
| (iv) Five to six times a week | |||||
| (v) Daily | |||||
| 7B. How often do you consume 2-3 servings of protein-rich foods (dairy, legumes, nuts and chicken)? | |||||
| (i) Not routinely | |||||
| (ii) One to two times per week | |||||
| (iii) Three to four times a week | |||||
| (iv) Five to six times a week | |||||
| (v) Daily | |||||
| 8B. I snack on foods high in calories, fat, sugar and salt in between my meals. | |||||
| (i) Not routinely | |||||
| (ii) One to two times per week | |||||
| (iii) Three to four times a week | |||||
| (iv) Five to six times a week | |||||
| (v) Daily | |||||
| 9B. I believe mood swings and stress in my everyday life leads to__________ | |||||
| (i) Increase in my food intake | |||||
| (ii) Somewhat increase in my food intake | |||||
| (iii) No change | |||||
| (iv) Somewhat decrease in my food intake | |||||
| (v) Decrease in my food intake | |||||
| 10B. How frequently do you eat out at a canteen, restaurant or at social gatherings? | |||||
| (i) Not routinely | |||||
| (ii) One to two times every month | |||||
| (iii) One to two times every fortnight | |||||
| (iv) One to two times every week | |||||
| (v) Almost daily | |||||
|
| |||||
|
| |||||
| Section B: Lifestyle-related behaviour | |||||
|
| |||||
|
| |||||
| 11B. Reasons associated with inability to follow a healthy dietary pattern. Please select a response that appropriately defines the extent to which these factors impact your daily efforts to maintain a healthy diet. | |||||
|
| |||||
| Reasons | Strongly agree | Agree | Neither agree nor disagree | Disagree | Strongly disagree |
|
| |||||
| 1. My food intake has increased during the perimenopausal phase. | |||||
| 2. I tend to overeat due to food cravings. | |||||
| 3. I often disengage from healthy eating patterns around my periods. | |||||
| 5. My involvement with work and family responsibilities leaves me with less time to focus on healthy eating. | |||||
| 6. I feel healthy food products are either not readily available or costly. | |||||
| 8. I find myself eating out of anxiousness, boredom or restlessness. | |||||
| 9. I eat my favourite foods to make me feel better. | |||||
| 10. I tend to eat energy-dense food during festive and/or religious fasting. | |||||
| 11. My friends and family often offer me foods rich in fat, sugar and salt while eating out or at social gatherings. | |||||
| 12B. Participation in a moderate aerobic exercise (walking, jogging, swimming, and cycling) for about 150 min/week is recommended to maintain optimum health. How closely do you match your weekly physical activity level with the recommendations? | |||||
| (i) 100% | |||||
| (ii) 75% | |||||
| (iii) 50% | |||||
| (iv) 25% | |||||
| (v) Not applicable | |||||
| 13B. I spend………. (h) of a day on sedentary activities (sitting, desk job, using phone, watching TV) ? | |||||
| (i) <2 h | |||||
| (ii) 2-4 h | |||||
| (iii) 4-6 h | |||||
| (iv) 6-8 h | |||||
| (v) More than 8 h | |||||
| 14B. I feel doing household activities (alone) contributes to an adequate amount of physical activity in a day to maintain a healthy weight for my | |||||
| age group. | |||||
| (i) Strongly agree | |||||
| (ii) Agree | |||||
| (iii) Neither agree nor disagree | |||||
| (iv) Disagree | |||||
| (v) Strongly disagree | |||||
| 15B: Reasons associated with inability to follow a physically active routine. Physically active lifestyle includes participation in exercise, household chores, commuting and leisure-related activities. Please select a response that appropriately defines the extent to which these factors impact your daily efforts to maintain a physically active lifestyle. | |||||
|
| |||||
| Factors | Strongly agree | Agree | Neither agree nor disagree | Disagree | Strongly disagree |
|
| |||||
| 1. My involvement with personal and professional commitments leaves me with less time for dedicated physical activity. | |||||
| 2. I find it difficult to engage in physical activities due to joint pain/body pain/excessive tiredness. | |||||
| 3. I find it difficult to engage in physical activities around my periods. | |||||
| 4. My friends and family members show little interest in maintaining an active lifestyle. | |||||
| 5. I do not have access to parks, fitness centres and gyms. | |||||
| 16B. On average, I am able to get a sleep of ………… h in the night. | |||||
| (i) <6 | |||||
| (ii) 6 -8 | |||||
| (iii) >8 | |||||
| 17B. I would rate my quality of sleep as…………….. | |||||
| (i) Excellent | |||||
| (ii) Very good | |||||
| (iii) Good | |||||
| (iv) Poor | |||||
| (v) Very poor | |||||
| 18B: Reasons associated with an inability to follow sleep pattern. Please select the appropriate response to the following factors according to the extent to which these factors contribute to disturbed sleep patterns. | |||||
|
| |||||
| Section B: Lifestyle-related behaviour | |||||
|
| |||||
| Factors | Strongly agree | Agree | Neither agree nor disagree | Disagree | Strongly disagree |
|
| |||||
| 1. I face restlessness and discomfort during the night due to sweating and hot flashes leading to disturbed sleep. | |||||
| 2. I face difficulty in falling asleep due to stress and anxiety. | |||||
| 3. Mismatched sleep routines of family members negatively affect my sleep pattern as well. | |||||
Source: Kumari A, Chopra S, Ranjan P, Verma A, Malhotra A, Upadhyay AD, Sharma KA, Vikram NK. Development and validation of Comprehensive evaluation tool for weight management in midlife women. Journal of Mid-life Health (under publication)
Annexure 5
DASS-21 for initial screening of anxiety, depression and stress
| Please read each statement and circle a number 0, 1, 2 or 3 that indicates how much the statement applied to you over the past week. There are no right or wrong answers. Do not spend too much time on any statement. | ||||||||
| The rating scale is as follows: | ||||||||
| 0 Did not apply to me at all | ||||||||
| 1 Applied to me to some degree, or some of the time | ||||||||
| 2 Applied to me to a considerable degree or a good part of time | ||||||||
| 3 Applied to me very much or most of the time | ||||||||
| 1 (s) I found it hard to wind down. | 0 | 1 | 2 | 3 | ||||
| 2 (a) I was aware of the dryness of my mouth. | ||||||||
| 3 (d) I could not seem to experience any positive feeling at all. | ||||||||
| 4 (a) I experienced breathing difficulty (e.g. excessively rapid breathing, breathlessness in the absence of physical exertion). | ||||||||
| 5 (d) I found it difficult to work up the initiative to do things. | ||||||||
| 6 (s) I tended to over-react to situations. | ||||||||
| 7 (a) I experienced trembling (e.g. in the hands). | ||||||||
| 8 (s) I felt that I was using a lot of nervous energy. | ||||||||
| 9 (a) I was worried about situations in which I might panic and make a fool of myself. | ||||||||
| 10 (d) I felt that I had nothing to look forward to. | ||||||||
| 11 (s) I found myself getting agitated. | ||||||||
| 12 (s) I found it difficult to relax. | ||||||||
| 13 (d) I felt down-hearted and blue. | ||||||||
| 14 (s) I was intolerant of anything that kept me from getting on with what I was doing. | ||||||||
| 15 (a) I felt I was close to panic. | ||||||||
| 16 (d) I was unable to become enthusiastic about anything. | ||||||||
| 17 (d) I felt I was not worth much as a person. | ||||||||
| 18 (s) I felt that I was rather touchy. | ||||||||
| 19 (a) I was aware of the action of my heart in the absence of physical exertion (e.g. sense of heart rate increase, heart missing a beat) | ||||||||
| 20 (a) I felt scared without any good reason. | ||||||||
| 21 (d) I felt that life was meaningless. | ||||||||
|
| ||||||||
| DASS-21 scoring instructions | ||||||||
| The DASS-21 should not be used to replace a face to face clinical interview. If you are experiencing significant emotional difficulties you should contact your GP for a referral to a qualified professional. | ||||||||
| Depression, Anxiety and Stress Scale-21 Items (DASS-21) | ||||||||
| The Depression, Anxiety and Stress Scale-21 Items (DASS-21) is a set of three self-report scales designed to measure the emotional states of depression, anxiety and stress. | ||||||||
| Each of the three DASS-21 scales contains seven items, divided into subscales with similar content. The depression scale assesses dysphoria, hopelessness, devaluation of life, self-deprecation, lack of interest/involvement, anhedonia and inertia. The anxiety scale assesses autonomic arousal, skeletal muscle effects, situational anxiety, and subjective experience of anxious affect. The stress scale is sensitive to levels of chronic non-specific arousal. It assesses difficulty relaxing, nervous arousal, and being easily upset/agitated, irritable/over-reactive and impatient. Scores for depression, anxiety and stress are calculated by summing the scores for the relevant items. | ||||||||
| DASS-21 is based on a dimensional rather than a categorical conception of psychological disorder. The assumption on which the DASS-21 development was based (and which was confirmed by the research data) is that the differences between the depression, anxiety and stress experienced by normal subjects and clinical populations are essentially differences of degree. The DASS-21, therefore, has no direct implications for the allocation of patients to discrete diagnostic categories postulated in classificatory systems such as the DSM and ICD. | ||||||||
| Recommended cut-off scores for conventional severity labels (normal, moderate, severe) are as follows: NB Scores on the DASS-21 will need to be multiplied by 2 to calculate the final score. | ||||||||
| Depression | Anxiety | Stress | |
|---|---|---|---|
| Normal | 0-9 | 0-7 | 0-14 |
| Mild | 10-13 | 8-9 | 15-18 |
| Moderate | 14-20 | 10-14 | 19-25 |
| Severe | 21-27 | 15-19 | 26-33 |
| Extremely severe | 28+ | 20+ | 34+ |
Source: Lovibond, SH & Lovibond PF (1995). Manual for the Depression Anxiety & Stress Scales. (2nd ed.) Sydney: Psychology Foundation
Annexure 6
Menopause Rating Scale: standardised scale to assess the severity of menopausal symptoms among menopausal women
| Description |
| Hot flushes, sweating (episode of sweating) |
| Heart discomfort (unusual awareness of heartbeat, heart skipping, heart racing, and tightness) |
| Sleep problems (difficulty falling asleep, difficulty in sleeping through the night, and waking up too early) |
| Depressive mood (feeling “down” sad, on the verge of tears, lack of derive, and mood swings) |
| Irritability (feeling nervous, inner tension, and feeling aggressive) |
| Anxiety (inner restlessness, feeling “panicky”) |
| Physical and mental exhaustion (general decrease in performance, impaired memory, decrease in concentration, forgetfulness, fatigue, headache, and dizziness) |
| Sexual problems (change in sexual desire, in sexual activity, and satisfaction) |
| Bladder problems (difficulty in urinating, increased need to urinate, and bladder incontinence) |
| Dryness of the vagina (sensation of dryness or burning in the vagina, difficulty with sexual intercourse) |
| Joint and muscular discomfort (joint pain, muscle pain, and backache) |
(b): According to the World Health Organization (WHO) standards, the degree of severity is consistent with the following:
| Symptoms | Degree | Percentage |
|---|---|---|
| No problem | None, absent, negligible | 0-4 |
| Mild problem | Slight, low | 5-24 |
| Moderate problem | Medium, fair | 25-49 |
| Severe problem | High, extreme | 50-95 |
| Complete problem | Total | 95-100 |
Source:Heinemann LA, Potthoff P, Schneider HP. International versions of the Menopause Rating Scale (MRS). Health Qual Life Outcomes 2003;1:28
Annexure 7
Estimated average requirement for adult Indian women
| Nutrient | Value |
|---|---|
| Iron (mg/day) | 29 |
| Calcium for non-pregnant-non lactating women (mg/day) | 1000 |
| Calcium for postmenopasual women (mg/day) | 1200 |
| Vitamin D (IU/day) | 600 |
Source: ICMR-NIN Expert Group on Nutrient Requirement for Indians, recommended Dietary Allowances (RDA) and Estimated Average Requirements (EAR)-2020. Available From: https://www. nin.res.in/RDA_short_Report_2020.html. Accessed on: 1st December 2021.
Annexure 8
Dietary sources of calcium, iron and phytoestrogen
| (i) Dietary sources of calcium | ||
|---|---|---|
|
| ||
| Food products | Servings/portion size (mL/g) | Calcium (mg) |
| Milk/curd (whole, buffalo) | 1 glass (250 mL) | 520 |
| Milk/curd (whole, cow) | 1 glass (250 mL) | 300 |
| Paneer | 30 | 96 |
| Khoya | 100 | 600 |
| Cheese | 20 | 160 |
| Amaranth seeds | 100 | 162 |
| Ragi | 100 | 360 |
| Rajma | 30 | 40.2 |
| Soyabean | 30 | 72 |
| Bengal gram | 30 | 45 |
| Black gram/chickpeas | 30 | 30-36 |
| Bathua leaves | 100 | 211 |
| Fenugreek leaves (methi leaves) | 100 | 270 |
| Mustard leaves | 100 | 190 |
| Radish leaves | 100 | 234 |
| Spinach | 100 | 83 |
| Lady finger | 100 | 85 |
| Beans | 100 | 50-70 |
| Cabbage | 100 | 58 |
| Jackfruit | 100 | 45 |
| Dates | 25 | 17.75 |
| Fig | 25 | 20 |
| Almonds | 25 | 60 |
| Walnuts | 25 | 52.5 |
| Sesame seeds/flax seeds | 15 | 90 |
Source: Longvah T, AnINLINEantanINLINEI, Bhaskarachary K, Venkaiah K, Longvah T. Indian food composition tables. Hyderabad: National Institute of Nutrition, Indian Council of Medical Research; 2017 May
| (ii) Dietary sources of phytoestrogen | ||
|---|---|---|
|
| ||
| Food groups | Food sources | Phytoestrogen content (µg/100 g) |
| Oil seeds and nuts | Sesame seed | 8008 |
| Flax seed | 379 | |
| Dried dates | 329 | |
| Sunflower seed | 216 | |
| Chestnuts | 210 | |
| Olive oil | 181 | |
| Almonds | 131 | |
| Peanuts | 34.5 | |
| Soy and soy products | Soy beans | 103920 |
| Tofu | 27151 | |
| Soy yogurt | 10275 | |
| Soy milk | 2958 | |
| Breads | Flax bread | 7540 |
| Multigrain bread | 4799 | |
| Pulses and products | Hummus | 993 |
| Mung bean sprouts | 495 | |
| Alfalfa sprouts | 442 | |
| Vegetables | Garlic | 604 |
| Green bean | 106 | |
| Onion | 32 | |
| Fruits | Dried apricots | 445 |
| Blueberry | 17.5 | |
Adapted from Gupta C, Prakash D, Gupta S. Phytoestrogens as pharma foods. Adv Food Technol Nutr Sci Open J. 2016;2 (1):19-3
Annexure 9
Important metabolic parameters for assessment of overall improvement
| Metabolic parameters | Cut-off points |
|---|---|
| Fasting blood glucose (mg/dL) | 100 |
| Insulin (μU/mL) | 15 |
| HOMA-IR | 3.0 |
| CRP (mg/L) | |
| Low-risk | <1 |
| medium-risk | 1-2.99 |
| High-risk | ≥3.0 |
| Total cholesterol (mg/dL) | 200 |
| LDL-cholesterol (mg/dL) | 130 |
| non-HDL-C (mg/dL) | 160 |
| HDL-C (mg/dL) | 60 |
| Triglycerides (mg/dL) | 150 |
| Waist circumference (cm) | 80 |
| Waist to height ratio | 0.5 |
| Bone mineral density (T-score) | |
| Normal | Above−1.0 |
| Low bone mass (osteopenia) | −1.0-−2.5 |
| Osteoporosis | Less than equal to−2.5 |
| Severe osteoporosis | Below −2.5 with fragility fracture |
| Appendicular skeletal muscle mass (ASM) | |
| Dual-energy X-ray absorptiometry for females (kg/m2) | <5.4 |
| Bioelectrical impedance analysis for females (kg/m2) | <5.7 |
Source: Dow CA, Thomson CA, Flatt SW, Sherwood NE, Pakiz B, Rock CL. Predictors of improvement in cardiometabolic risk factors with weight loss in women. Journal of the American Heart Association. 2013 Dec 18;2(6):e000152; Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-307.e2. doi: 10.1016/j.jamda.2019.12.012. Epub 2020 Feb 4. PMID: 32033882; Meeta M, Harinarayan CV, Marwah R, Sahay R, Kalra S, Babhulkar S. Clinical Practice Guidelines on Postmenopausal Osteoporosis: *An Executive Summary and Recommendations - Update 2019-2020. J Midlife Health. 2020 Apr-Jun;11(2):96-112. doi: 10.4103/jmh.JMH_143_20. Epub 2020 Aug 10. PMID: 33281419; PMCID: PMC7688018.
References
- 1.Garawi F, Devries K, Thorogood N, Uauy R. Global differences between women and men in the prevalence of obesity:Is there an association with gender inequality? Eur J Clin Nutr. 2014;68:1101–6. doi: 10.1038/ejcn.2014.86. [DOI] [PubMed] [Google Scholar]
- 2.Chopra S, Sharma KA, Ranjan P, Malhotra A, Vikram NK, Kumari A. Weight management module for perimenopausal women:A practical guide for gynecologists. J Midlife Health. 2019;10:165–72. doi: 10.4103/jmh.JMH_155_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurston RC, Ewing LJ, Low CA, Christie AJ, Levine MD. Behavioral weight loss for the management of menopausal hot flashes:A pilot study. Menopause. 2015;22:59–65. doi: 10.1097/GME.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathnayake N, Alwis G, Lenora J, Mampitiya I, Lekamwasam S. Effect of health-promoting lifestyle modification education on knowledge, attitude, and quality of life of postmenopausal women. Biomed Res Int. 2020;2020:3572903. doi: 10.1155/2020/3572903. doi: 10.1155/2020/3572903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumsden MA, Hor K. Impact of obesity on the health of women in midlife. TOG. 2015;17:201–8. [Google Scholar]
- 6.Kapoor E, Collazo-Clavell ML, Faubion SS. Weight gain in women at midlife:A concise review of the pathophysiology and strategies for management. Mayo Clin Proc. 2017;92:1552–8. doi: 10.1016/j.mayocp.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Muzammil S, Lopes G. Lack of primary health care services in developing countries during pandemic:An urgent reminder! J Fam Med Dis Prev. 2021;7:138. [Google Scholar]
- 8.Chopra S, Ranjan P, Malhotra A, Verma A, Kumari A, Sharma AK, et al. Perceived risk factors for weight gain, barriers and facilitators related to weight loss experienced by perimenopausal women:Focus Group study and thematic analysis. Menopause. 2021;29 doi: 10.1097/GME.0000000000001909. [doi:10.1097/GME.0000000000001909] [DOI] [PubMed] [Google Scholar]
- 9.Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines:10. Considering resource use and rating the quality of economic evidence. JClin Epidemiol. 2013;66:140–50. doi: 10.1016/j.jclinepi.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am. 2015;44:497–515. doi: 10.1016/j.ecl.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meeta M, Digumarti L, Agarwal N, Vaze N, Shah R, Malik S. Clinical practice guidelines on menopause:*An executive summary and recommendations:Indian menopause society 2019-2020. J Midlife Health. 2020;11:55–95. doi: 10.4103/jmh.JMH_137_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad JB, Tyagi NK, Verma P. Age at menopause in India:A systematic review. Diabetes Metab Syndr. 2021;15:373–7. doi: 10.1016/j.dsx.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Greendale GA, Sternfeld B, Huang M, Han W, Karvonen-Gutierrez C, Ruppert K, et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019;4:e124865. doi: 10.1172/jci.insight.124865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montazeri SA, Tehrani FR, Yarandi RB, Erfani H, Mansournia MA, Azizi F. Effect of aging, menopause, and age at natural menopause on the trend in body mass index:A 15-year population-based cohort. FertilSteril. 2019;111:780–6. doi: 10.1016/j.fertnstert.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Karvonen-Gutierrez C, Kim C. Association of mid-life changes in body size, body composition and obesity status with the menopausal transition. Healthcare (Basel) 2016;4:42. doi: 10.3390/healthcare4030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leeners B, Geary N, Tobler PN, Asarian L. Ovarian hormones and obesity. Hum Reprod Update. 2017;23:300–21. doi: 10.1093/humupd/dmw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko SH, Kim HS. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients. 2020;12:202. doi: 10.3390/nu12010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anklesaria BS. The staging of menopause. J South Asian Feder Menopause Soc. 2013;1:1–3. [Google Scholar]
- 19.Chopra S, Malhotra A, Ranjan P, Vikram NK, Sarkar S, Siddhu A, et al. Predictors of successful weight loss outcomes amongst individuals with obesity undergoing lifestyle interventions:A systematic review. Obes Rev. 2021;22:e13148. doi: 10.1111/obr.13148. [DOI] [PubMed] [Google Scholar]
- 20.Altmann TK. Attitude: A concept analysis. 2008;43:144–50. doi: 10.1111/j.1744-6198.2008.00106.x. doi:10.1111/j.1744-6198.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 21.Lytle LA, Nicastro HL, Roberts SB, Evans M, Jakicic JM, Laposky AD, et al. Accumulating data to optimally predict obesity treatment (ADOPT) core measures:Behavioral domain. Obesity. 2018;26:S16–24. doi: 10.1002/oby.22157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reethesh SR, Ranjan P, Arora C, Kaloiya GS, Vikram NK, Dwivedi SN, et al. Development and validation of a questionnaire assessing knowledge, attitude, and practices about obesity among obese individuals. Indian J Endocrinol Metab. 2019;23:102–10. doi: 10.4103/ijem.IJEM_487_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Vriendt T, Matthys C, Verbeke W, Pynaert I, De Henauw S. Determinants of nutrition knowledge in young and middle-aged Belgian women and the association with their dietary behaviour. Appetite. 2009;52:788–92. doi: 10.1016/j.appet.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Su MC, Lin HR, Chu NF, Huang CH, Tsao LI. Weight loss experiences of obese perimenopausal women with metabolic syndrome. JClin Nurs. 2015;24:1849–59. doi: 10.1111/jocn.12806. [DOI] [PubMed] [Google Scholar]
- 25.Vallance JK, Murray TC, Johnson ST, Elavsky S. Understanding physical activity intentions and behavior in postmenopausal women:an application of the theory of planned behavior. Int J Behav Med. 2011;18:139–49. doi: 10.1007/s12529-010-9100-2. [DOI] [PubMed] [Google Scholar]
- 26.Alharbi RD, Mahrus AM, Alarabi AA, Alraddadi DM. Assessment of menopausal symptoms severity among female school teachers and their knowledge, attitudes, and practices. Obstet Gynecol. 2021;9:299–316. [Google Scholar]
- 27.Khashayar P, Qorbani M, Keshtkar A, Khashayar P, Ziaee A, Larijani B. Awareness of osteoporosis among female head of household:An Iranian experience. Arch Osteoporos. 2017;12:36. doi: 10.1007/s11657-017-0330-7. [DOI] [PubMed] [Google Scholar]
- 28.Mohd Hatta NNKN, Nurumal MS, Isa MLM, Daud A, Ibrahim M, Sharifudin MA, et al. Knowledge and attitudes of maintaining bone health among post-menopausal women in Malaysia. Cent Asian J Glob Health. 2019;8:348. doi: 10.5195/cajgh.2019.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prairie BA, Wisniewski SR, Luther J, Hess R, Thurston RC, Wisner KL, et al. Symptoms of depressed mood, disturbed sleep, and sexual problems in midlife women:Cross-sectional data from the study of women's health across the nation. J Womens Health (Larchmt) 2015;24:119–26. doi: 10.1089/jwh.2014.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Hage C, Hallit S, Akel M, Dagher E. Osteoporosis awareness and health beliefs among Lebanese women aged 40 years and above. Osteoporos Int. 2019;30:771–86. doi: 10.1007/s00198-019-04901-2. [DOI] [PubMed] [Google Scholar]
- 31.McNeil J, Liepert M, Brenner DR, Courneya KS, Friedenreich CM. Behavioral predictors of weight regain in postmenopausal women:Exploratory results from the breast cancer and exercise trial in Alberta. Obesity. 2019;27:1451–63. doi: 10.1002/oby.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharifi N, Mahdavi R, Ebrahimi-Mameghani M. Perceived barriers to weight loss programs for overweight or obese women. Health Promot Perspect. 2013;3:11–22. doi: 10.5681/hpp.2013.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali AM, Ahmed AH, Smail L. Psychological climacteric symptoms and attitudes toward menopause among Emirati women. Int J Environ Res Public Health. 2020;17:5028. doi: 10.3390/ijerph17145028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwak EK, Park HS, Kang NM. Menopause knowledge, attitude, symptom and management among midlife employed women. J Menopausal Med. 2014;20:118–25. doi: 10.6118/jmm.2014.20.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pimenta F, Leal I, Maroco J, Ramos C. Menopause Symptoms'Severity Inventory (MSISSI-38):Assessing the frequency and intensity of symptoms. Climacteric. 2012;15:143–52. doi: 10.3109/13697137.2011.590617. [DOI] [PubMed] [Google Scholar]
- 36.McArthur D, Dumas A, Woodend K, Beach S, Stacey D. Factors influencing adherence to regular exercise in middle-aged women:A qualitative study to inform clinical practice. BMC Womens Health. 2014;14:49. doi: 10.1186/1472-6874-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan CY, Subramaniam S, Chin KY, Ima-Nirwana S, Muhammad N, Fairus A, et al. Levels of knowledge, beliefs, and practices regarding osteoporosis and the associations with bone mineral density among populations more than 40 years old in Malaysia. IntJEnviron ResPublic Health. 2019;16:4115. doi: 10.3390/ijerph16214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamid TKA. New York: Springer; 2009. Thinking in Circles About Obesity: Applying Systems Thinking to Weight Management. [Google Scholar]
- 39.Wolf AM, Woodworth KA. Obesity prevention:Recommended strategies and challenges. Am J Med. 2009;122((4 Suppl 1)):S19–23. doi: 10.1016/j.amjmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Foster D, Sanchez-Collins S, Cheskin LJ. Multidisciplinary team-based obesity treatment in patients with diabetes:Current practices and the state of the science. Diabetes Spectr. 2017;30:244–9. doi: 10.2337/ds17-0045. doi: 10.2337/ds17-0045. Erratum in: Diabetes Spectr 2018;31:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cochrane AJ, Dick B, King NA, Hills AP, Kavanagh DJ. Developing dimensions for a multicomponent multidisciplinary approach to obesity management:A qualitative study. BMC Public Health. 2017;17:814. doi: 10.1186/s12889-017-4834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearce C, Rychetnik L, Wutzke S, Wilson A. Obesity prevention and the role of hospital and community-based health services:A scoping review. BMC Health Serv Res. 2019;19:453. doi: 10.1186/s12913-019-4262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Ramirez DC, Long H, Mowat S, Hein C. Obesity education for front-line healthcare providers. BMC Med Educ. 2018;18:278. doi: 10.1186/s12909-018-1380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson KM, Weinhold KR, Andridge R, Arnold K, Chu PP, Orchard TS. Associations of erythrocyte polyunsaturated fatty acids with inflammation and quality of life in post-menopausal women with obesity completing a pilot dietary intervention. Nutrients. 2019;11:1589. doi: 10.3390/nu11071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oustric P, Beaulieu K, Casanova N, O'Connor D, Gibbons C, Hopkins M, et al. Food liking but not wanting decreases after controlled intermittent or continuous energy restriction to ≥5% weight loss in women with overweight/obesity. Nutrients. 2021;13:182. doi: 10.3390/nu13010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroenke CH, Caan BJ, Stefanick ML, Anderson G, Brzyski R, Johnson KC, et al. Effects of a dietary intervention and weight change on vasomotor symptoms in the Women's Health Initiative. Menopause. 2012;19:980–8. doi: 10.1097/gme.0b013e31824f606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hintze LJ, Messier V, Lavoie MÈ, Brochu M, Lavoie JM, Prud'homme D, et al. A one-year resistance training program following weight loss has no significant impact on body composition and energy expenditure in postmenopausal women living with overweight and obesity. Physiol Behav. 2018;189:99–106. doi: 10.1016/j.physbeh.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Hollis JL, Williams LT, Young MD, Pollard KT, Collins CE, Morgan PJ. Compliance to step count and vegetable serve recommendations mediates weight gain prevention in mid-age, premenopausal women. Findings of the 40-Something RCT. Appetite. 2014;83:33–41. doi: 10.1016/j.appet.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Kong A, Beresford SAA, Alfano CM, Foster-Schubert KE, Neuhouser ML, Johnson DB, et al. Self-monitoring and eating-related behaviors are associated with 12-month weight loss in postmenopausal overweight-to-obese women. J Acad Nutr Diet. 2012;112:1428–35. doi: 10.1016/j.jand.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ilich JZ, Kelly OJ, Liu PY, Shin H, Kim Y, Chi Y, et al. Role of calcium and low-fat dairy foods in weight-loss outcomes revisited:Results from the randomized trial of effects on bone and body composition in overweight/obese postmenopausal women. Nutrients. 2019;11:1157. doi: 10.3390/nu11051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grossman JA, Arigo D, Bachman JL. Meaningful weight loss in obese postmenopausal women:A pilot study of high-intensity interval training and wearable technology. Menopause. 2018;25:465–70. doi: 10.1097/GME.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 52.Shin H, Shin J, Liu PY, Dutton GR, Abood DA, Ilich JZ. Self-efficacy improves weight loss in overweight/obese postmenopausal women during a 6-month weight loss intervention. Nutr Res. 2011;31:822–8. doi: 10.1016/j.nutres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 53.Rossi AP, Muollo V, Fantin F, Masciocchi E, Urbani S, Taylor M, et al. Effects of diet combined with Nordic walking or walking programme on weight loss and arterial stiffness in postmenopausal overweight and obese women:The Walking and Aging Verona pilot study. Eur J Prev Cardiol. 2020;27:2208–11. doi: 10.1177/2047487319877712. [DOI] [PubMed] [Google Scholar]
- 54.Perry CD, Degeneffe D, Davey C, Kollannoor-Samuel G, Reicks M. Weight gain prevention among midlife women:A randomized controlled trial to address needs related to the physical and social environment. Int J Environ Res Public Health. 2016;13:530. doi: 10.3390/ijerph13060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anton SD, Manini TM, Milsom VA, Dubyak P, Cesari M, Cheng J, et al. Effects of a weight loss plus exercise program on physical function in overweight, older women:A randomized controlled trial. Clin Interv Aging. 2011;6:141–9. doi: 10.2147/CIA.S17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.NHaMR C. National Health and Medical Research Council 2013; Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. [Google Scholar]
- 57.Khandelwal S. Obesity in midlife:Lifestyle and dietary strategies. Climacteric. 2020;23:140–7. doi: 10.1080/13697137.2019.1660638. [DOI] [PubMed] [Google Scholar]
- 58.Jahangiry L, Farhangi MA. Obesity paradigm and web-based weight loss programs:An updated systematic review and meta-analysis of randomized controlled trials. J Health Popul Nutr. 2021;40:16. doi: 10.1186/s41043-021-00240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KH, MacLeod J, et al. Nutrition therapy for adults with diabetes or prediabetes:A consensus report. Diabetes Care. 2019;42:731–54. doi: 10.2337/dci19-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kabir SM Essentials of Counseling. Abosar Prokashana Sangstha. 2017 [Google Scholar]
- 61.Teeriniemi AM, Salonurmi T, Jokelainen T, Vähänikkilä H, Alahäivälä T, Karppinen P, et al. A randomized clinical trial of the effectiveness of a web-based health behaviour change support system and group lifestyle counselling on body weight loss in overweight and obese subjects:2-year outcomes. J Intern Med. 2018;284:534–54. doi: 10.1111/joim.12802. [DOI] [PubMed] [Google Scholar]
- 62.Ghelani DP, Moran LJ, Johnson C, Mousa A, Naderpoor N. Mobile apps for weight management:A review of the latest evidence to inform practice. FrontEndocrinol. 2020;11:412. doi: 10.3389/fendo.2020.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paul-Ebhohimhen V, Avenell A. A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obes Facts. 2009;2:17–24. doi: 10.1159/000186144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hartmann-Boyce J, Johns DJ, Jebb SA, Aveyard P, Behavioural Weight Management Review Group Effect of behavioural techniques and delivery mode on effectiveness of weight management:systematic review, meta-analysis and meta-regression. Obes Rev. 2014;15:598–609. doi: 10.1111/obr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sorgente A, Pietrabissa G, Manzoni GM, Re F, Simpson S, Perona S, et al. Web-based interventions for weight loss or weight loss maintenance in overweight and obese people:A systematic review of systematic reviews. J Med Internet Res. 2017;19:e229. doi: 10.2196/jmir.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valsdottir TD, Øvrebø B, Falck TM, Litleskare S, Johansen EI, Henriksen C, et al. Low-carbohydrate high-fat diet and exercise:Effect of a 10-week intervention on body composition and CVD risk factors in overweight and obese women-A randomized controlled trial. Nutrients. 2020;13:110. doi: 10.3390/nu13010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vitolins MZ, Blackwell CS, Katula JA, Isom SP, Case LD. Long-term weight loss maintenance in the continuation of a randomized diabetes prevention translational study:The Healthy Living Partnerships to Prevent Diabetes (HELP PD) continuation trial. Diabetes Care. 2019;42:1653–60. doi: 10.2337/dc19-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walsh K, Grech C, Hill K. Health advice and education given to overweight patients by primary care doctors and nurses:A scoping literature review. Prev Med Rep. 2019;14:100812. doi: 10.1016/j.pmedr.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jane M, Hagger M, Foster J, Ho S, Pal S. Social media for health promotion and weight management:A critical debate. BMC Public Health. 2018;18:932. doi: 10.1186/s12889-018-5837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nuttall FQ. Body mass index:Obesity, BMI, and health:A critical review. Nutr Today. 2015;50:117–28. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced body composition assessment:from body mass index to body composition profiling. J Investig Med. 2018;66:1–9. doi: 10.1136/jim-2018-000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice:A consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16:177–89. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duren DL, Sherwood RJ, Czerwinski SA, Lee M, Choh AC, Siervogel RM, et al. Body composition methods:Comparisons and interpretation. J Diabetes Sci Technol. 2008;2:1139–46. doi: 10.1177/193229680800200623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 75.Mu Y, Kou T, Wei B, Lu X, Liu J, Tian H, et al. Soy products ameliorate obesity-related anthropometric indicators in overweight or obese Asian and non-menopausal women:A meta-analysis of randomized controlled trials. Nutrients. 2019;11:2790. doi: 10.3390/nu11112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Foster-Schumbert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012;20:1628–38. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lombardo M, Perrone MA, Guseva E, Aulisa G, Padua E, Bellia C, Della-Morte D, et al. Losing weight after menopause with minimal aerobic training and mediterranean diet. Nutrients. 2020;12:2471. doi: 10.3390/nu12082471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults:A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity society. Circulation. 2014;129:S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jamka M, Mądry E, Bogdański P, Kryściak J, Mądry R, Lisowska A, et al. The effect of endurance and endurance-strength training on bone mineral density and content in abdominally obese postmenopausal women:A randomized trial. Healthcare (Basel) 2021;9:1074. doi: 10.3390/healthcare9081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bajerska J, Chmurzynska A, Muzsik A, Krzyżanowska P, Mądry E, Malinowska AM, et al. Weight loss and metabolic health effects from energy-restricted Mediterranean and Central-European diets in postmenopausal women:A randomized controlled trial. Sci Rep. 2018;8:11170. doi: 10.1038/s41598-018-29495-3. doi:10.1038/s41598-018-29495-3. Erratum in:Sci Rep 2019;9:16077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brinkley TE, Ding J, Carr JJ, Nicklas BJ. Pericardial fat loss in postmenopausal women under conditions of equal energy deficit. Med Sci Sports Exerc. 2011;43:808–14. doi: 10.1249/MSS.0b013e3181fb512d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riesco E, Choquette S, Audet M, Lebon J, Tessier D, Dionne IJ. Effect of exercise training combined with phytoestrogens on adipokines and C-reactive protein in postmenopausal women:a randomized trial. Metabolism. 2012;61:273–80. doi: 10.1016/j.metabol.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 83.Kerksick CM, Roberts MD, Campbell BI, Galbreath MM, Taylor LW, Wilborn CD, et al. Differential impact of calcium and vitamin D on body composition changes in post-menopausal women following a restricted energy diet and exercise program. Nutrients. 2020;12:713. doi: 10.3390/nu12030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sen P, Das S, Hore S, Bhattacharjee S, Choudhuri D. Obesity and associated cardiometabolic risk among women from Tripura - A Northeastern state of India. J Midlife Health. 2017;8:110–7. doi: 10.4103/jmh.JMH_116_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–61. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts. 2015;89:402–24. 64. doi: 10.1159/000442721. doi:10.1159/000442721. Epub 2015 Dec 5. Erratum in: Obes Facts 2016;9:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.FAO. Dietary assessment: A resource guide to method selection and application in low resource settings. Food and Agriculture Organization of the United Nations; 2018. [Google Scholar]
- 88.Batra P, Das SK, Salinardi T, Robinson L, Saltzman E, Scott T, et al. Eating behaviors as predictors of weight loss in a 6 month weight loss intervention:Eating behavior constructs and weight loss. Obesity. 2013;21:2256–63. doi: 10.1002/oby.20404. [DOI] [PubMed] [Google Scholar]
- 89.Kong A, Beresford SA, Imayama I, Duggan C, Alfano CM, Foster-Schubert KE, et al. Adoption of diet-related self-monitoring behaviors varies by race/ethnicity, education, and baseline binge eating score among overweight-to-obese postmenopausal women in a 12-month dietary weight loss intervention. Nutr Res. 2012;32:260–5. doi: 10.1016/j.nutres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mason C, de Dieu Tapsoba J, Duggan C, Wang C-Y, Alfano CM, McTiernan A. Eating behaviors and weight loss outcomes in a 12-month randomized trial of diet and/or exercise intervention in postmenopausal women. Int J Behav Nutr Phys Act. 2019;16:113. doi: 10.1186/s12966-019-0887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Svetkey LP, Ard JD, Stevens VJ, Loria CM, Young DY, Hollis JF, et al. Predictors of long-term weight loss in adults with modest initial weight loss, by sex and race. Obesity. 2012;20:1820–8. doi: 10.1038/oby.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kartiko Sari I, Utari DM, Kamoshita S, Oktaviana D, Sakai S, Nishiyama H, et al. Increasing vegetable intake 400 g/day to control body weight and lipid profile in overweight hyperlipidemia menopausal women. J Public Health Res. 2020;9:1733. doi: 10.4081/jphr.2020.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Delahanty LM, Peyrot M, Shrader PJ, Williamson DA, Meigs JB, Nathan DM, et al. Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the Diabetes Prevention Program (DPP) Diabetes Care. 2013;36:34–40. doi: 10.2337/dc12-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barone Gibbs B, Kinzel LS, Pettee Gabriel K, Chang YF, Kuller LH. Short- and long-term eating habit modification predicts weight change in overweight, postmenopausal women:Results from the WOMAN study. J Acad Nutr Diet. 2012;112:1347–55.e2. doi: 10.1016/j.jand.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urbanek JK, Metzgar CJ, Hsiao PY, Piehowski KE, Nickols-Richardson SM. Increase in cognitive eating restraint predicts weight loss and change in other anthropometric measurements in overweight/obese premenopausal women. Appetite. 2015;87:244–50. doi: 10.1016/j.appet.2014.12.230. [DOI] [PubMed] [Google Scholar]
- 96.Grave RD, Calugi S, Corica F, Di Domizio S, Marchesini G, QUOVADIS Study Group Psychological variables associated with weight loss in obese patients seeking treatment at medical centers. J Am Diet Assoc. 2009;109:2010–20. doi: 10.1016/j.jada.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Ye L, Zheng Y, Burke LE. Impact of perceived barriers to healthy eating on diet and weight in a 24-month behavioral weight loss trial. J Nutr Educ Behav. 2015;47:432–6. doi: 10.1016/j.jneb.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng Y, Sereika SM, Danford CA, Imes CC, Goode RW, Mancino J, et al. Trajectories of weight change and predictors over 18-month weight loss treatment:Weight change trajectories and predictors. J Nurs Scholarsh. 2017;49:177–84. doi: 10.1111/jnu.12283. [DOI] [PubMed] [Google Scholar]
- 99.Brończyk-Puzoń A, Piecha D, Nowak J, Koszowska A, Kulik-Kupka K, Dittfeld A, et al. Guidelines for dietary management of menopausal women with simple obesity. PrzMenopauzalny. 2015;14:48–52. doi: 10.5114/pm.2015.48678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lambrinoudaki I, Ceasu I, Depypere H, Erel T, Rees M, Schenck-Gustafsson K, et al. EMAS position statement:Diet and health in midlife and beyond. Maturitas. 2013;74:99–104. doi: 10.1016/j.maturitas.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 101.Killeen SL, Callaghan SL, Jacob CM, Hanson MA, McAuliffe FM. “It only takes two minutes to ask”-A qualitative study with women on using the FIGO Nutrition Checklist in pregnancy. Int J Gynaecol Obstet. 2020;151((Suppl 1)):45–50. doi: 10.1002/ijgo.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011;4:177–84. doi: 10.1177/1756283X11398736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Short MW, Domagalski JE. Iron deficiency anemia:Evaluation and management. Am Fam Physician. 2013;87:98–104. [PubMed] [Google Scholar]
- 104.Bailey RL, Ard JD, Davis TA, Naimi TS, Schneeman BO, Stang JS, et al. A proposed framework for identifying nutrients and food components of public health relevance in the dietary guidelines for Americans. JNutr. 2021;151:1197–204. doi: 10.1093/jn/nxaa459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aparna P, Muthathal S, Nongkynrih B, Gupta SK. Vitamin D deficiency in India. J Family Med Prim Care. 2018;7:324–30. doi: 10.4103/jfmpc.jfmpc_78_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.World Health Organization. WHO guidelines on physical activity and sedentary behaviour: at a glance 2020. [PubMed] [Google Scholar]
- 108.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness:definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 109.Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, et al. Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Gemert WA, May AM, Schuit AJ, Oosterhof BY, Peeters PH, Monninkhof EM. Effect of weight loss with or without exercise on inflammatory markers and adipokines in postmenopausal women:The SHAPE-2 trial, A randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2016;25:799–806. doi: 10.1158/1055-9965.EPI-15-1065. [DOI] [PubMed] [Google Scholar]
- 111.Seimon RV, Wild-Taylor AL, Keating SE, McClintock S, Harper C, Gibson AA, et al. Effect of weight loss via severe vs moderate energy restriction on lean mass and body composition among postmenopausal women with obesity:The TEMPO diet randomized clinical trial. JAMA Netw Open. 2019;2:e1913733. doi: 10.1001/jamanetworkopen.2019.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pelclová J, Štefelová N, Hodonská J, Dygrýn J, Gába A, Zając-Gawlak I. Reallocating time from sedentary behavior to light and moderate-to-vigorous physical activity:What has a stronger association with adiposity in older adult women? Int J Environ Res Public Health. 2018;15:1444. doi: 10.3390/ijerph15071444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sadeghian M, Hosseini SA, Zare Javid A, Ahmadi Angali K, Mashkournia A. Effect of fasting-mimicking diet or continuous energy restriction on weight loss, body composition, and appetite-regulating hormones among metabolically healthy women with obesity:A randomized controlled, parallel trial. Obes Surg. 2021;31:2030–9. doi: 10.1007/s11695-020-05202-y. [DOI] [PubMed] [Google Scholar]
- 114.de Roon M, van Gemert WA, Peeters PH, Schuit AJ, Monninkhof EM. Long-term effects of a weight loss intervention with or without exercise component in postmenopausal women:A randomized trial. Prev Med Rep. 2016;5:118–23. doi: 10.1016/j.pmedr.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Williams LT, Hollis JL, Collins CE, Morgan PJ. Can a relatively low-intensity intervention by health professionals prevent weight gain in mid-age women?12-Month outcomes of the 40-Something randomised controlled trial. NutrDiabetes. 2014;4:e116. doi: 10.1038/nutd.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gabriel KK, Conroy MB, Schmid KK, Storti KL, High RR, Underwood DA, et al. The impact of weight and fat mass loss and increased physical activity on physical function in overweight, postmenopausal women:Results from the women on the move through activity and nutrition study. Menopause. 2011;18:759–65. doi: 10.1097/gme.0b013e31820acdcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yank V, Xiao L, Wilson SR, Stafford RS, Rosas LG, Ma J. Short-term weight loss patterns, baseline predictors, and longer-term follow-up within a randomized controlled trial:Short-term weight loss patterns in RCT. Obesity. 2014;22:45–51. doi: 10.1002/oby.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morgan PJ, Hollis JL, Young MD, Collins CE, Teixeira PJ. Workday sitting time and marital status:Novel pretreatment predictors of weight loss in overweight and obese men. Am J Mens Health. 2018;12:1431–8. doi: 10.1177/1557988316654866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anjana RM, Sudha V, Lakshmipriya N, Subhashini S, Pradeepa R, Geetha L, et al. Reliability and validity of a new physical activity questionnaire for India. Int J Behav Nutr Phys Act. 2015;12:40. doi: 10.1186/s12966-015-0196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mensah K, Maire A, Oppert JM, Dugas J, Charreire H, Weber C, et al. Assessment of sedentary behaviors and transport-related activities by questionnaire:A validation study. BMC Public Health. 2016;16:753. doi: 10.1186/s12889-016-3412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lobelo F, Rohm Young D, Sallis R, Garber MD, Billinger SA, Duperly J, et al. Routine assessment and promotion of physical activity in healthcare settings: A scientific statement from the American Heart Association. Circulation. 2018;137:e495–522. doi: 10.1161/CIR.0000000000000559. doi:10.1161/CIR.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 122.Thivel D, Tremblay A, Genin PM, Panahi S, Rivière D, Duclos M. Physical activity, inactivity, and sedentary behaviors:Definitions and implications in occupational health. Front Public Health. 2018;6:288. doi: 10.3389/fpubh.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lyons EJ, Lewis ZH, Mayrsohn BG, Rowland JL. Behavior change techniques implemented in electronic lifestyle activity monitors:A systematic content analysis. J Med Internet Res. 2014;16:e192. doi: 10.2196/jmir.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mulhall S, Andel R, Anstey KJ. Variation in symptoms of depression and anxiety in midlife women by menopausal status. Maturitas. 2018;108:7–12. doi: 10.1016/j.maturitas.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 125.Tamaria A, Bharti R, Sharma M, Dewan R, Kapoor G, Aggarwal A, et al. Risk assessment for psychological disorders in postmenopausal women. J Clin Diagn Res. 2013;7:2885–8. doi: 10.7860/JCDR/2013/7580.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Whiteley J, Wagner JS, Bushmakin A, Kopenhafer L, Dibonaventura M, Racketa J. Impact of the severity of vasomotor symptoms on health status, resource use, and productivity. Menopause. 2013;20:518–24. doi: 10.1097/GME.0b013e31827d38a5. [DOI] [PubMed] [Google Scholar]
- 127.Abdel-Salam DM, Mohamed RA, Alruwaili RR, Alhablani FS, Aldaghmi RM, ALghassab RE. Postmenopausal symptoms and their correlates among Saudi Women attending different primary health centers. Int J Environ Res Public Health. 2021;18:6831. doi: 10.3390/ijerph18136831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lima JE, Palacios S, Wender MC. Quality of life in menopausal women:A Brazilian Portuguese version of the Cervantes scale. ScientificWorldJournal. 2012;2012:620519. doi: 10.1100/2012/620519. doi:10.1100/2012/620519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gariepy G, Nitka D, Schmitz N. The association between obesity and anxiety disorders in the population:A systematic review and meta-analysis. IntJObesity. 2010;34:407–19. doi: 10.1038/ijo.2009.252. [DOI] [PubMed] [Google Scholar]
- 130.Blaine B. Does depression cause obesity?A meta-analysis of longitudinal studies of depression and weight control. JHealth Psychol. 2008;13:1190–7. doi: 10.1177/1359105308095977. [DOI] [PubMed] [Google Scholar]
- 131.McCuen-Wurst C, Ruggieri M, Allison KC. Disordered eating and obesity:Associations between binge eating-disorder, night-eating syndrome, and weight-related co-morbidities. Ann N Y AcadSci. 2018;1411:96–105. doi: 10.1111/nyas.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Serretti A, Mandelli L, Laura M. Antidepressants and body weight:A comprehensive review and meta-analysis. JClin Psychiatry. 2010;71:1259–72. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 133.Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain:A meta-analysis. PLoS One. 2014;9:e94112. doi: 10.1371/journal.pone.0094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tunay M, Kurdak H, Özcan S, Özdemir Ç, Özer ZY. Family physician-led group visits for lifestyle modification in women with weight problems:A pilot intervention and follow-up study. Obesity facts. 2018;11:1–4. doi: 10.1159/000486133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hageman PA, Mroz JE, Yoerger MA, Pullen CH. Weight loss is associated with improved quality of life among rural women completers of a web-based lifestyle intervention. PLoS One. 2019;14:e0225446. doi: 10.1371/journal.pone.0225446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Annesi JJ, Whitaker AC. Psychological factors discriminating between successful and unsuccessful weight loss in a behavioral exercise and nutrition education treatment. IntJBehav Med. 2010;17:168–75. doi: 10.1007/s12529-009-9056-2. [DOI] [PubMed] [Google Scholar]
- 137.Carson TL, Jackson BE, Nolan TS, Williams A, Baskin ML. Lower depression scores associated with greater weight loss among rural black women in a behavioral weight loss program. TranslBehav Med. 2017;7:320–9. doi: 10.1007/s13142-016-0452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ma J, Rosas LG, Lv N, Xiao L, Snowden MB, Venditti EM, et al. Effect of integrated behavioral weight loss treatment and problem-solving therapy on body mass index and depressive symptoms among patients with obesity and depression:The RAINBOW randomized clinical trial. JAMA. 2019;321:869–79. doi: 10.1001/jama.2019.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pagoto S, Schneider KL, Whited MC, Oleski JL, Merriam P, Appelhans B, et al. Randomized controlled trial of behavioral treatment for comorbid obesity and depression in women:The Be Active Trial. IntJ Obes. 2013;37:1427–34. doi: 10.1038/ijo.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Palmeira L, Pinto-Gouveia J, Cunha M. Exploring the efficacy of an acceptance, mindfulness &compassionate-based group intervention for women struggling with their weight (Kg-Free):A randomized controlled trial. Appetite. 2017;112:107–16. doi: 10.1016/j.appet.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 141.ter Kuile MM, Brauer M, Laan E. The Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale (FSDS):Psychometric properties within a Dutch population. J Sex Marital Ther. 2006;32:289–304. doi: 10.1080/00926230600666261. [DOI] [PubMed] [Google Scholar]
- 142.Kim HR, Kim HS. Optimal cutoffs of cardiometabolic risk for postmenopausal Korean women. Asian Nurs Res. 2017;11:107–12. doi: 10.1016/j.anr.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 143.Rocha T, Crespo RP, Yance VVR, Hayashida SA, Baracat EC, Carvalho F, et al. Persistent poor metabolic profile in postmenopausal women with ovarian hyperandrogenism after testosterone level normalization. J Endocr Soc. 2019;3:1087–96. doi: 10.1210/js.2018-00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome:The study of women's health across the nation. Arch Intern Med. 2008;168:1568–75. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kinlen D, Cody D, O'Shea D. Complications of obesity. QJM. 2018;111:437–43. doi: 10.1093/qjmed/hcx152. [DOI] [PubMed] [Google Scholar]
- 146.Monterrosa-Castro A, Ortiz-Banquez M, Mercado-Lara M. Prevalence of sarcopenia and associated factors in climacteric women of the Colombian Caribbean. Menopause. 2019;26:1038–44. doi: 10.1097/GME.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 147.Orprayoon N, Wainipitapong P, Champaiboon J, Wattanachanya L, Jaisamrarn U, Chaikittisilpa S. Prevalence of pre-sarcopenia among postmenopausal women younger than 65 years. Menopause. 2021;28:1351–7. doi: 10.1097/GME.0000000000001866. [DOI] [PubMed] [Google Scholar]
- 148.Maiello M, Zito A, Ciccone MM, Palmiero P. Prevalence of co-morbidities and clinical coexisting conditions among post-menopausal women affected by coronary artery disease:Data from the “Real World”. J Cardiol Clin Res. 2014;2:1033. [Google Scholar]
- 149.Aoi S, Miyake T, Iida T, Ikeda H, Ishizaki F, Chikamura C, et al. Association of changes in neck circumference with cardiometabolic risk in postmenopausal healthy women. J Atheroscler Thromb. 2016;23:728–36. doi: 10.5551/jat.31963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu PJ, Ma F, Lou HP, Zhu YN. Normal-weight central obesity is associated with metabolic disorders in Chinese postmenopausal women. Asia Pac J Clin Nutr. 2017;26:692–7. doi: 10.6133/apjcn.052016.08. [DOI] [PubMed] [Google Scholar]
- 151.Welsh P, Cezard G, Gill JM, Wallia S, Douglas A, Sheikh A, et al. Associations between weight change and biomarkers of cardiometabolic risk in South Asians:Secondary analyses of the PODOSA trial. Int J Obes (Lond) 2016;40:1005–11. doi: 10.1038/ijo.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mongraw-Chaffin ML, Peters SAE, Huxley RR, Woodward M. The sex-specific association between BMI and coronary heart disease:A systematic review and meta-analysis of 95 cohorts with 1·2 million participants. Lancet Diabetes Endocrinol. 2015;3:437–49. doi: 10.1016/S2213-8587(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Larsen BA, Allison MA, Laughlin GA, Araneta MR, Barrett-Connor E, Wooten WJ, et al. The association between abdominal muscle and type II diabetes across weight categories in diverse post-menopausal women. J Clin Endocrinol Metab. 2015;100:E105–9. doi: 10.1210/jc.2014-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tohidi M, Hatami M, Hadaegh F, Azizi F. Triglycerides and triglycerides to high-density lipoprotein cholesterol ratio are strong predictors of incident hypertension in Middle Eastern women. J Hum Hypertens. 2012;26:525–32. doi: 10.1038/jhh.2011.70. [DOI] [PubMed] [Google Scholar]
- 155.Thulkar J, Singh S, Sharma S, Thulkar T. Preventable risk factors for osteoporosis in postmenopausal women:Systematic review and meta-analysis. JMidlife Health. 2016;7:108–13. doi: 10.4103/0976-7800.191013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McLeod KM, Johnson CS. A systematic review of osteoporosis health beliefs in adult men and women. JOsteoporos. 2011;2011 doi: 10.4061/2011/197454. [doi:10.4061/2011/197454] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sadeghi O, Saneei P, Nasiri M, Larijani B, Esmaillzadeh A. Abdominal obesity and risk of hip fracture:A systematic review and meta-analysis of prospective studies. Adv Nutr. 2017;8:728–38. doi: 10.3945/an.117.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, et al. Understanding weight gain at menopause. Climacteric. 2012;15:419–29. doi: 10.3109/13697137.2012.707385. [DOI] [PubMed] [Google Scholar]
- 159.Khadilkar SS. Musculoskeletal disorders and menopause. J Obstet Gynecol India. 2019;69:99–103. doi: 10.1007/s13224-019-01213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jull J, Stacey D, Beach S, Dumas A, Strychar I, Ufholz LA, et al. Lifestyle interventions targeting body weight changes during the menopause transition:A systematic review. JObes. 2014;2014:824310. doi: 10.1155/2014/824310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Werner N, Nickenig G, Sinning JM. Complex PCI procedures:Challenges for the interventional cardiologist. Clin Res Cardiol. 2018;107((Suppl 2)):64–73. doi: 10.1007/s00392-018-1316-1. [DOI] [PubMed] [Google Scholar]
- 162.Reginster J-Y, Beaudart C, Buckinx F, Bruyère O. Osteoporosis and sarcopenia:Two diseases or one? Curr Opin Clin Nutr Metab Care. 2016;19:31–6. doi: 10.1097/MCO.0000000000000230. doi:10.1097/MCO.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia:2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 164.Meeta M, Harinarayan CV, Marwah R, Sahay R, Kalra S, Babhulkar S. Clinical practice guidelines on postmenopausal osteoporosis:*An executive summary and recommendations –Update 2019–2020. J Mid-life Health. 2020;11:96–112. doi: 10.4103/jmh.JMH_143_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the stages of reproductive aging workshop +10:Addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–68. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Watt FE. Musculoskeletal pain and menopause. Post Reprod Health. 2018 doi: 10.1177/2053369118757537. [doi:10.1177/2053369118757537] [DOI] [PubMed] [Google Scholar]
- 167.Gracia CR, Freeman EW. Onset of the menopause transition. Obstet Gynecol Clin North Am. 2018;45:585–97. doi: 10.1016/j.ogc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 168.Burleson MH, Todd M, Trevathan WR. Daily vasomotor symptoms, sleep problems, and mood:Using daily data to evaluate the domino hypothesis in middle-aged women. Menopause. 2010;17:87–95. doi: 10.1097/gme.0b013e3181b20b2d. [DOI] [PubMed] [Google Scholar]
- 169.Peeters G, Edwards KL, Brown WJ, Barker AL, Arden N, Redmond AC, et al. Potential effect modifiers of the association between physical activity patterns and joint symptoms in middle-aged women. Arthritis Care Res (Hoboken) 2018;70:1012–21. doi: 10.1002/acr.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Baker FC, Lampio L, Saaresranta T, Polo-Kantola P. Sleep and sleep disorders in the menopausal transition. Sleep Med Clin. 2018;13:443–56. doi: 10.1016/j.jsmc.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531–9. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Esposito Sorpreso IC, Laprano Vieira LH, Longoni Calió C, Abi Haidar M, Baracat EC, Soares JM., Jr Health education intervention in early and late postmenopausal Brazilian women. Climacteric. 2012;15:573–80. doi: 10.3109/13697137.2011.635915. [DOI] [PubMed] [Google Scholar]
- 173.Zhang L, Ruan X, Cui Y, Gu M, Mueck AO. Menopausal symptoms among Chinese peri- and postmenopausal women:A large prospective single-center cohort study. Gynecol Endocrinol. 2021;37:185–9. doi: 10.1080/09513590.2020.1832070. [DOI] [PubMed] [Google Scholar]
- 174.Chuni N, Sreeramareddy CT. Frequency of symptoms, determinants of severe symptoms, validity of and cut-off score for Menopause Rating Scale (MRS) as a screening tool:A cross-sectional survey among midlife Nepalese women. BMC Womens Health. 2011;11:30. doi: 10.1186/1472-6874-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rulu P, Sievert LL, Dhall M, Bertone-Johnson ER. Symptoms at midlife among women in Nagaland, India. Am J Hum Biol. 2021;33:e23456. doi: 10.1002/ajhb.23456. [DOI] [PubMed] [Google Scholar]
- 176.Sourouni M, Zangger M, Honermann L, Foth D, Stute P. Assessment of the climacteric syndrome:A narrative review. Arch Gynecol Obstet. 2021;304:855–62. doi: 10.1007/s00404-021-06139-y. [DOI] [PubMed] [Google Scholar]
- 177.Taşkıran G, Özgül S. Individual characteristics associated with menopausal symptom severity and menopause-specific quality of life:A rural perspective. Reprod Sci. 2021;28:2661–71. doi: 10.1007/s43032-021-00545-y. [DOI] [PubMed] [Google Scholar]
- 178.Malik R, Pokaria C, Singh S, Khera K. Hindi translated version of menopausal rating score questionnaire: A method to evaluate post menopausal symptoms in India. Int J Reprod Contraception Obstet Gynecol. 2019;8:3102. [Google Scholar]
- 179.Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults:A systematic literature review examining intervention components. Patient Educ Couns. 2012;87:32–42. doi: 10.1016/j.pec.2011.07.018. doi:10.1016/j.pec.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 180.Levack WMM, Weatherall M, Hay-Smith EJC, Dean SG, McPherson K, Siegert RJ. Goal setting and strategies to enhance goal pursuit for adults with acquired disability participating in rehabilitation. Cochrane Database Syst Rev. 2015:CD009727. doi: 10.1002/14651858.CD009727.pub2. doi:10.1002/14651858. CD009727.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zomer E, Gurusamy K, Leach R, Trimmer C, Lobstein T, Morris S, et al. Interventions that cause weight loss and the impact on cardiovascular risk factors:A systematic review and meta-analysis. ObesRev. 2016;17:1001–11. doi: 10.1111/obr.12433. [DOI] [PubMed] [Google Scholar]
- 182.Ryan DH, Yockey SR. Weight loss and improvement in comorbidity:Differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6:187–94. doi: 10.1007/s13679-017-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Aucott L, Gray D, Rothnie H, Thapa M, Waweru C. Effects of lifestyle interventions and long-term weight loss on lipid outcomes–A systematic review. Obes Rev. 2011;12:e412–25. doi: 10.1111/j.1467-789X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- 184.Huang AJ, Subak LL, Wing R, West DS, Hernandez AL, Macer J, et al. An intensive behavioral weight loss intervention and hot flushes in women. Arch Intern Med. 2010;170:1161–7. doi: 10.1001/archinternmed.2010.162. doi:10.1001/archinternmed.2010.162. Erratum in:Arch Intern Med 2010;170:1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McCusker M, Gregoski MJ. Instruments to measure readiness to lose weight:An integrative review. J Nurs Meas. 2015;23:142–62. doi: 10.1891/1061-3749.23.3.E142. [DOI] [PubMed] [Google Scholar]
- 186.Avery A, Langley-Evans SC, Harrington M, Swift JA. Setting targets leads to greater long-term weight losses and 'unrealistic'targets increase the effect in a large community-based commercial weight management group. J Hum Nutr Diet. 2016;29:687–96. doi: 10.1111/jhn.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Behr H, Ho AS, Mitchell ES, Yang Q, DeLuca L, Michealides A. How do emotions during goal pursuit in weight change over time?Retrospective computational text analysis of goal setting and striving conversations with a coach during a mobile weight loss program. Int J Environ Res PublicHealth. 2021;18:6600. doi: 10.3390/ijerph18126600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Knittle K, Nurmi J, Crutzen R, Hankonen N, Beattie M, Dombrowski SU. How can interventions increase motivation for physical activity?A systematic review and meta-analysis. Health Psychol Rev. 2018;12:211–30. doi: 10.1080/17437199.2018.1435299. [DOI] [PubMed] [Google Scholar]
- 189.Chopra S, Malhotra A, Ranjan P, Vikram NK, Singh N. Lifestyle-related advice in the management of obesity:A step-wise approach. J Educ Health Promot. 2020;9:239. doi: 10.4103/jehp.jehp_216_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sharma M, Kishore A, Roy D, Joshi K. A comparison of the Indian diet with the EAT-Lancet reference diet. BMC Public Health. 2020;20:812. doi: 10.1186/s12889-020-08951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rock CL, Flatt SW, Pakiz B, Barkai HS, Heath DD, Krumhar KC. Randomized clinical trial of portion-controlled prepackaged foods to promote weight loss. Obesity (Silver Spring) 2016;24:1230–7. doi: 10.1002/oby.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Joseph G, Arviv-Eliashiv R, Tesler R. A comparison of diet versus diet +exercise programs for health improvement in middle-aged overweight women. Womens Health (Lond) 2020;16:1745506520932372. doi: 10.1177/1745506520932372. doi:10.1177/1745506520932372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cheng CC, Hsu CY, Liu JF. Effects of dietary and exercise intervention on weight loss and body composition in obese postmenopausal women:A systematic review and meta-analysis. Menopause. 2018;25:772–82. doi: 10.1097/GME.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 194.Figueroa A, Vicil F, Sanchez-Gonzalez MA, Wong A, Ormsbee MJ, Hooshmand S, et al. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am J Hypertens. 2013;26:416–23. doi: 10.1093/ajh/hps050. [DOI] [PubMed] [Google Scholar]
- 195.Cespedes Feliciano EM, Tinker L, Manson JE, Allison M, Rohan T, et al. Change in dietary patterns and change in waist circumference and DXA trunk fat among postmenopausal women. Obesity (Silver Spring) 2016;24:2176–84. doi: 10.1002/oby.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Arguin H, Dionne IJ, Sénéchal M, Bouchard DR, Carpentier AC, Ardilouze JL, et al. Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women:A pilot study. Menopause. 2012;19:870–6. doi: 10.1097/gme.0b013e318250a287. [DOI] [PubMed] [Google Scholar]
- 197.Koliaki C, Spinos T, Spinou Μ, Brinia ΜE, Mitsopoulou D, Katsilambros N. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel) 2018;6:73. doi: 10.3390/healthcare6030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barber TM, Hanson P, Kabisch S, Pfeiffer AFH, Weickert MO. The low-carbohydrate diet:Short-term metabolic efficacy versus longer-term limitations. Nutrients. 2021;13:1187. doi: 10.3390/nu13041187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.ICMR-NIN Expert Group on Nutrient Requirement for Indians, recommended Dietary Allowances (RDA) and Estimated Average Requirements (EAR)-2020. [[Last accessed on2021 Dec 01]]. Available from: https://www.nin.res.in/RDA_short_Report_2020.html .
- 200.Damms-Machado A, Weser G, Bischoff SC. Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr J. 2012;11:34. doi: 10.1186/1475-2891-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gupta A, Kumar R, Mohan A, Rahi S. New Delhi: Ministry of Health and Family Welfare, Government of India; 2013. Guidelines for Control of Iron Deficiency Anaemia. [Google Scholar]
- 202.Chen LR, Ko NY, Chen KH. Isoflavone supplements for menopausal women:A systematic review. Nutrients. 2019;11:2649. doi: 10.3390/nu11112649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Westerterp KR. Physical activity and physical activity induced energy expenditure in humans:Measurement, determinants, and effects. Front Physiol. 2013;4:90. doi: 10.3389/fphys.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dasso NA. How is exercise different from physical activity?A concept analysis. NursForum. 2019;54:45–52. doi: 10.1111/nuf.12296. [DOI] [PubMed] [Google Scholar]
- 205.Hintze LJ, Messier V, Lavoie MÈ, Brochu M, Lavoie JM, Prud'homme D, et al. A one-year resistance training program following weight loss has no significant impact on body composition and energy expenditure in postmenopausal women living with overweight and obesity. Physiol Behav. 2018;189:99–106. doi: 10.1016/j.physbeh.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 206.Kendall KL, Fairman CM. Therapeutic physical activities for people with cancer. [[Last accessed on 2021 Oct 21]]. Available from: https://www.researchgate.net/profile/Ciaran-Fairman-2/publication/318207071_Therapeutic_Physical_Activities_for_People_with_Cancer/links/5988727645851560584f4b44/Therapeutic-Physical-Activities-for-People-with-Cancer.pdf .
- 207.Kang SJ, Kim JH, Gang Z, Yook YS, Yoon JR, Ha GC, et al. Effects of 12-week circuit exercise program on obesity index, appetite regulating hormones, and insulin resistance in middle-aged obese females. J Phys Ther Sci. 2018;30:169–73. doi: 10.1589/jpts.30.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, et al. Guide to the assessment of physical activity:clinical and research applications:A scientific statement from the American Heart Association. Circulation. 2013;128:2259–79. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 209.Yarizadeh H, Asadi S, Baharlooi H, Setayesh L, Kakavandi NR, Hambly C, et al. Beneficial impact of exercise on bone mass in individuals under calorie restriction:A systematic review and Meta-analysis of randomized clinical trials. Crit Rev Food Sci Nutr. 2021;61:553–65. doi: 10.1080/10408398.2020.1739620. [DOI] [PubMed] [Google Scholar]
- 210.Rashti BA, Mehrabani J, Damirchi A, Babaei P. The influence of concurrent training intensity on serum irisin and abdominal fat in postmenopausal women. Prz Menopauzalny. 2019;18:166–73. doi: 10.5114/pm.2019.90810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Choquette S, Dion T, Brochu M, Dionne IJ. Soy isoflavones and exercise to improve physical capacity in postmenopausal women. Climacteric. 2013;16:70–7. doi: 10.3109/13697137.2011.643515. [DOI] [PubMed] [Google Scholar]
- 212.Luoto R, Moilanen J, Heinonen R, Mikkola T, Raitanen J, Tomas E, et al. Effect of aerobic training on hot flushes and quality of life--A randomized controlled trial. Ann Med. 2012;44:616–26. doi: 10.3109/07853890.2011.583674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.David P, Buckworth J, Pennell ML, Katz ML, DeGraffinreid CR, Paskett ED. A walking intervention for postmenopausal women using mobile phones and Interactive Voice Response. J Telemed Telecare. 2012;18:20–5. doi: 10.1258/jtt.2011.110311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.de Azevedo Guimarães AC, Baptista F. Influence of habitual physical activity on the symptoms of climacterium/menopause and the quality of life of middle-aged women. Int J Womens Health. 2011;3:319–28. doi: 10.2147/IJWH.S24822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McHugh IJ, Williams AD, Fell JW. Practical application of the Sports Medicine Australia pre-exercise screening system. J Sci Med Sport. 2008;11:182–4. doi: 10.1016/j.jsams.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 216.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes:The American College of Sports Medicine and the American Diabetes Association joint position statement. Diabetes Care. 2010;33:e147–67. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. AACE/ACE comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22((Suppl 3)):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 218.Webster AL, Aznar-Laín S. Intensity of physical activity and the “talk test”. ACSM'S Health Fitness J. 2008;12:13–7. [Google Scholar]
- 219.Mishra N, Mishra VN, Devanshi Exercise beyond menopause:Dos and Don'ts. J Midlife Health. 2011;2:51–6. doi: 10.4103/0976-7800.92524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vaze N, Joshi S. Yoga and menopausal transition. J Midlife Health. 2010;1:56–8. doi: 10.4103/0976-7800.76212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle Grave R. Long-term weight loss maintenance for obesity:A multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37–46. doi: 10.2147/DMSO.S89836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Burgess E, Hassmén P, Welvaert M, Pumpa KL. Behavioural treatment strategies improve adherence to lifestyle intervention programmes in adults with obesity:A systematic review and meta-analysis. ClinObes. 2017;7:105–14. doi: 10.1111/cob.12180. [DOI] [PubMed] [Google Scholar]
- 223.Kelley CP, Sbrocco G, Sbrocco T. Behavioral modification for the management of obesity. Prim Care. 2016;43:159–75. doi: 10.1016/j.pop.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Buckworth J. Weight management behavioral strategies. ACSM's Health Fitness J. 2018;22:45–6. [Google Scholar]
- 225.Beishuizen CR, Stephan BC, van Gool WA, Brayne C, Peters RJ, Andrieu S, et al. Web-based interventions targeting cardiovascular risk factors in middle-aged and older people:A systematic review and meta-analysis. J Med Internet Res. 2016;18:e55. doi: 10.2196/jmir.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Idoate F, Ibañez J, Gorostiaga EM, García-Unciti M, Martínez-Labari C, Izquierdo M. Weight-loss diet alone or combined with resistance training induces different regional visceral fat changes in obese women. Int J Obes (Lond) 2011;35:700–13. doi: 10.1038/ijo.2010.190. [DOI] [PubMed] [Google Scholar]
- 227.Shin H, Shin J, Liu PY, Dutton GR, Abood DA, Ilich JZ. Self-efficacy improves weight loss in overweight/obese postmenopausal women during a 6-month weight loss intervention. Nutr Res. 2011;31:822–8. doi: 10.1016/j.nutres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 228.Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P, Behavioural Weight Management Review Group Diet or exercise interventions vs combined behavioral weight management programs:A systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114:1557–68. doi: 10.1016/j.jand.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Imayama I, Alfano CM, Kong A, Foster-Schubert KE, Bain CE, Xiao L, et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women:A randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:118. doi: 10.1186/1479-5868-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mason C, Foster-Schubert KE, Imayama I, Kong A, Xiao L, Bain C, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41:366–75. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Durrer Schutz D, Busetto L, Dicker D, Farpour-Lambert N, Pryke R, Toplak H, et al. European practical and patient-centred guidelines for adult obesity management in primary care. Obes Facts. 2019;12:40–66. doi: 10.1159/000496183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hooker S, Punjabi A, Justesen K, Boyle L, Sherman MD. Encouraging health behavior change:eight evidence-based strategies. FamPract Manag. 2018;25:31–6. [PubMed] [Google Scholar]
- 233.Fait T. Menopause hormone therapy:Latest developments and clinical practice. Drugs Context. 2019;8:212551. doi: 10.7573/dic.212551. doi:10.7573/dic.212551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.United Nations Population Division. World Population Prospects 2019. New York: United Nations Population Division; 2020. [[Last accessed on 2020 Nov 27]]. Available from: https://population.un.org/wpp/DataQuery/ [Google Scholar]
- 235.Papadakis GE, Hans D, Rodriguez EG, Vollenweider P, Waeber G, Marques-Vidal P, et al. Menopausal hormone therapy is associated with reduced total and visceral adiposity:The OsteoLaus cohort. J Clin Endocrinol Metab. 2018;103:1948–57. doi: 10.1210/jc.2017-02449. [DOI] [PubMed] [Google Scholar]
- 236.Zhang GQ, Chen JL, Luo Y, Mathur MB, Anagnostis P, Nurmatov U, et al. Menopausal hormone therapy and women's health:An umbrella review. PLoS Med. 2021;18:e1003731. doi: 10.1371/journal.pmed.1003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mishra GD, Chung HF, Cano A, Chedraui P, Goulis DG, Lopes P, et al. EMAS position statement:Predictors of premature and early natural menopause. Maturitas. 2019;123:82–8. doi: 10.1016/j.maturitas.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 238.Sturdee DW, Pines A, International Menopause Society Writing Group. Archer DF, Baber RJ, Barlow D, et al. Updated IMS recommendations on postmenopausal hormone therapy and preventive strategies for midlife health. Climacteric. 2011;14:302–20. doi: 10.3109/13697137.2011.570590. [DOI] [PubMed] [Google Scholar]
- 239.Casanova G, Bossardi Ramos R, Ziegelmann P, Spritzer PM. Effects of low-dose versus placebo or conventional-dose postmenopausal hormone therapy on variables related to cardiovascular risk:A systematic review and meta-analyses of randomized clinical trials. J Clin Endocrinol Metab. 2015;100:1028–37. doi: 10.1210/jc.2014-3301. [DOI] [PubMed] [Google Scholar]
- 240.Casanova G, Spritzer PM. Effects of micronized progesterone added to non-oral estradiol on lipids and cardiovascular risk factors in early postmenopause:A clinical trial. Lipids Health Dis. 2012;11:133. doi: 10.1186/1476-511X-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Paoletti AM, Lello S, Di Carlo C, Orrù M, Malune ME, Neri M, et al. Effect of Estradiol valerate plus dienogest on body composition of healthy women in the menopausal transition:A prospective one-year evaluation. Gynecol Endocrinol. 2016;32:61–4. doi: 10.3109/09513590.2015.1079175. [DOI] [PubMed] [Google Scholar]
- 242.Honisett SY, Tangalakis K, Wark J, Apostolopoulos V, Stojanovska L. The effects of hormonal therapy and exercise on bone turnover in postmenopausal women:A randomised double-blind pilot study. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2016;37:23–32. doi: 10.1515/prilozi-2016-0013. [DOI] [PubMed] [Google Scholar]
- 243.Golden SH, Kim C, Barrett-Connor E, Nan B, Kong S, Goldberg R, et al. The association of elective hormone therapy with changes in lipids among glucose intolerant postmenopausal women in the diabetes prevention program. Metabolism. 2013;Sep; 62:1313–22. doi: 10.1016/j.metabol.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Johnson WD, Brashear MM, Gupta AK, Rood JC, Ryan DH. Incremental weight loss improves cardiometabolic risk in extremely obese adults. Am J Med. 2011;124:931–8. doi: 10.1016/j.amjmed.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 245.Wasenius NS, Isomaa BA, Östman B, Söderström J, Forsén B, Lahti K, et al. Low-cost exercise interventions improve long-term cardiometabolic health independently of a family history of type 2 diabetes:A randomized parallel group trial. BMJ Open Diabetes Res Care. 2020;8:e001377. doi: 10.1136/bmjdrc-2020-001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gao HL, Gao HX, Sun FM, Zhang L. Effects of walking on body composition in perimenopausal and postmenopausal women:A systematic review and meta-analysis. Menopause. 2016;23:928–34. doi: 10.1097/GME.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 247.Funderburk L, Heileson J, Peterson M, Willoughby DS. Efficacy of L-Leucine supplementation coupled with a calorie-restricted diet to promote weight loss in mid-life women. J Am Coll Nutr. 2020:1–9. doi: 10.1080/07315724.2020.1815607. doi:10.1080/07315724.2020.1815607. [DOI] [PubMed] [Google Scholar]
- 248.Mason C, Xiao L, Imayama I, Duggan CR, Foster-Schubert KE, Kong A, et al. Influence of diet, exercise, and serum vitamin d on sarcopenia in postmenopausal women. Med Sci Sports Exerc. 2013;45:607–14. doi: 10.1249/MSS.0b013e31827aa3fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Abedi P, Nikkhah P, Najar S. Effect of pedometer-based walking on depression, anxiety and insomnia among postmenopausal women. Climacteric. 2015;18:841–5. doi: 10.3109/13697137.2015.1065246. [DOI] [PubMed] [Google Scholar]
- 250.Ponde K, Agrawal R, Hussaini SH. Effect of yoga therapy versus aerobic exercise on climacteric symptoms, perceived stress and quality of life in perimenopausal women. Int J Yoga Physiother Phys Educ. 2019;4:1–6. [Google Scholar]
- 251.Subrahmanyam N, Padmaja A. Effect of interventional package on quality of life of perimenopausal women residing in a rural community of Idukki District, Kerala. J Gynecol Obstetr. 2018;1:4–10. [Google Scholar]
- 252.Shorey S, Ang L, Lau Y. Efficacy of mind-body therapies and exercise-based interventions on menopausal-related outcomes among Asian perimenopause women:A systematic review, meta-analysis, and synthesis without a meta-analysis. J Adv Nurs. 2020;76:1098–110. doi: 10.1111/jan.14304. [DOI] [PubMed] [Google Scholar]
- 253.Millstein RA. Measuring outcomes in adult weight loss studies that include diet and physical activity:a systematic review. J Nutr Metab. 2014;2014:421423. doi: 10.1155/2014/421423. [doi:10.1155/2014/421423] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102:183–97. doi: 10.1016/j.mcna.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lemstra M, Bird Y, Nwank Stevenwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence:A meta-analysis. Patient Prefer Adherence. 2016;10:1547–59. doi: 10.2147/PPA.S103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Fruh SM. Obesity:Risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc of Nurse Pract. 2017;29:S3–14. doi: 10.1002/2327-6924.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hageman PA, Mroz JE, Yoerger MA, Pullen CH. User engagement associated with web-intervention features to attain clinically meaningful weight loss and weight maintenance in rural women. JObes. 2019;2019 doi: 10.1155/2019/7932750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Karimi G, Azadbakht L, Haghighatdoost F, Esmaillzadeh A. Low energy density diet, weight loss maintenance, and risk of cardiovascular disease following a recent weight reduction program:A randomized control trial. J Res Med Sci. 2016;21:32. doi: 10.4103/1735-1995.181992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Milsom VA, Middleton KM, Perri MG. Successful long-term weight loss maintenance in a rural population. Clin Interv Aging. 2011;6:303–9. doi: 10.2147/CIA.S25389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Christensen P, Henriksen M, Bartels EM, Leeds AR, Meinert Larsen T, Gudbergsen H, et al. Long-term weight-loss maintenance in obese patients with knee osteoarthritis:A randomized trial. Am J Clin Nutr. 2017;106:755–63. doi: 10.3945/ajcn.117.158543. [DOI] [PubMed] [Google Scholar]
- 261.Peirson L, Fitzpatrick-Lewis D, Ciliska D, Usman Ali M, Raina P, Sherifali D. Strategies for weight maintenance in adult populations treated for overweight and obesity:A systematic review and meta-analysis. CMAJ Open. 2015;3:E47–54. doi: 10.9778/cmajo.20140050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tussing-Humphreys LM, Fitzgibbon ML, Kong A, Odoms-Young A. Weight loss maintenance in African American women:A systematic review of the behavioral lifestyle intervention literature. J Obes. 2013;2013:437369. doi: 10.1155/2013/437369. doi:10.1155/2013/437369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang J, Wang S, Henning SM, Qin T, Pan Y, Yang J, et al. Mixed tree nut snacks compared to refined carbohydrate snacks resulted in weight loss and increased satiety during both weight loss and weight maintenance:A 24-week randomized controlled trial. Nutrients. 2021;13:1512. doi: 10.3390/nu13051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Conroy MB, Bryce CL, McTigue KM, Tudorascu D, Gibbs BB, Comer D, et al. Promoting weight maintenance with electronic health record tools in a primary care setting:Baseline results from the MAINTAIN-pc trial. ContempClin Trials. 2017;54:60–7. doi: 10.1016/j.cct.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 265.Brantley PJ, Stewart DW, Myers VH, Matthews-Ewald MR, Ard JD, Coughlin JW, et al. Psychosocial predictors of weight regain in the weight loss maintenance trial. J Behav Med. 2014;37:1155–68. doi: 10.1007/s10865-014-9565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dutton GR, Gowey MA, Tan F, Zhou D, Ard J, Perri MG, et al. Comparison of an alternative schedule of extended care contacts to a self-directed control:A randomized trial of weight loss maintenance. Int J Behav Nutr Phys Act. 2017;14:107. doi: 10.1186/s12966-017-0564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Leahey TM, Fava JL, Seiden A, Fernandes D, Doyle C, Kent K, et al. A randomized controlled trial testing an Internet delivered cost-benefit approach to weight loss maintenance. Prev Med. 2016;92:51–7. doi: 10.1016/j.ypmed.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Effects of cognitive behavioral therapy on weight maintenance after successful weight loss in women;A randomized clinical trial. Eur J Clin Nutr. 2020;74:436–44. doi: 10.1038/s41430-019-0495-9. [DOI] [PubMed] [Google Scholar]
- 269.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults:A randomized clinical trial. JAMA Intern Med. 2017;177:930–8. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kaikkonen KM, Saltevo SS, Korpelainen JT, Vanhala ML, Jokelainen JJ, Korpelainen RI, et al. Effective weight loss and maintenance by intensive start with diet and exercise. Med Sci Sports Exerc. 2019;51:920–9. doi: 10.1249/MSS.0000000000001855. [DOI] [PubMed] [Google Scholar]
- 271.Calugi S, Marchesini G, El Ghoch M, Gavasso I, Dalle Grave R. The association between weight maintenance and session-by-session diet adherence, weight loss and weight-loss satisfaction. Eat Weight Disord. 2020;25:127–33. doi: 10.1007/s40519-018-0528-8. [DOI] [PubMed] [Google Scholar]
- 272.Zwickert K, Rieger E, Swinbourne J, Manns C, McAulay C, Gibson AA, et al. High or low intensity text-messaging combined with group treatment equally promote weight loss maintenance in obese adults. Obes Res Clin Pract. 2016;10:680–91. doi: 10.1016/j.orcp.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 273.Champagne CM, Broyles ST, Moran LD, Cash KC, Levy EJ, Lin PH, et al. Dietary intakes associated with successful weight loss and maintenance during the weight loss maintenance trial. J Am Diet Assoc. 2011;111:1826–35. doi: 10.1016/j.jada.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gerber BS, Schiffer L, Brown AA, Berbaum ML, Rimmer JH, Braunschweig CL, et al. Video telehealth for weight maintenance of African-American women. J Telemed Telecare. 2013;19:266–72. doi: 10.1177/1357633X13490901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Teixeira PJ, Silva MN, Mata J, Palmeira AL, Markland D. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012;9:22. doi: 10.1186/1479-5868-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food:Observational cohort study. Am J Clin Nutr. 2012;96:953–61. doi: 10.3945/ajcn.112.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Stevens J, Truesdale K, McClain J, Cai J. The definition of weight maintenance. Int J Obes. 2006;30:391–9. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 278.Liebert KB. City University of New York: 2017. Weight loss maintenance:Women's experience during perimenopause. [Google Scholar]
- 279.Ulen CG, Huizinga MM, Beech B, Elasy TA. Weight regain prevention. Clin Diabetes. 2008;26:100–13. [Google Scholar]
- 280.Peters JC, Beck J, Cardel M, Wyatt HR, Foster GD, Pan Z, et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance:A randomized clinical trial. Obesity (Silver Spring) 2016;24:297–304. doi: 10.1002/oby.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pekkarinen T, Kaukua J, Mustajoki P. Long-term weight maintenance after a 17-week weight loss intervention with or without a one-year maintenance program:A randomized controlled trial. J Obes. 2015;2015:651460. doi: 10.1155/2015/651460. doi:10.1155/2015/651460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Casadei K, Kiel J. In:StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. Anthropometric Measurement. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537315/ [Updated 2021 Oct 01] [PubMed] [Google Scholar]
- 283.Lee A, Cardel M, Donahoo WT. Social and environmental factors influencing obesity. In: Feingold KR, Anawalt B, Boyce A, editors. Endotext [Internet] South Dartmouth (MA): MDText.com, Inc.; 2000. [[Lase accessed on 2019 Oct 12]]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278977/ [Google Scholar]
- 284.Greaves C, Poltawski L, Garside R, Briscoe S. Understanding the challenge of weight loss maintenance:A systematic review and synthesis of qualitative research on weight loss maintenance. Health Psychol Rev. 2017;11:145–63. doi: 10.1080/17437199.2017.1299583. [DOI] [PubMed] [Google Scholar]
- 285.Dalle Grave R, Calugi S, Centis E, El Ghoch M, Marchesini G. Cognitive-behavioral strategies to increase the adherence to exercise in the management of obesity. J Obes. 2011;2011:348293. doi: 10.1155/2011/348293. doi:10.1155/2011/348293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Soleymani T, Daniel S, Garvey WT. Weight maintenance:challenges, tools and strategies for primary care physicians. Obes Rev. 2016;17:81–93. doi: 10.1111/obr.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Arciero PJ, Edmonds R, He F, Ward E, Gumpricht E, Mohr A, et al. Protein-pacing caloric-restriction enhances body composition similarly in obese men and women during weight loss and sustains efficacy during long-term weight maintenance. Nutrients. 2016;8:476. doi: 10.3390/nu8080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Crain AL, Sherwood NE, Martinson BC, Jeffery RW. Mediators of weight loss maintenance in the keep it off trial. Ann Behav Med. 2018;52:9–18. doi: 10.1007/s12160-017-9917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Patel ML, Wakayama LN, Bennett GG. Self-monitoring via digital health in weight loss interventions:A systematic review among adults with overweight or obesity. Obesity (Silver Spring) 2021;29:478–99. doi: 10.1002/oby.23088. [DOI] [PubMed] [Google Scholar]
- 290.Dounavi K, Tsoumani O. Mobile health applications in weight management:A systematic literature review. Am J Prev Med. 2019;56:894–903. doi: 10.1016/j.amepre.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 291.Wang Y, Min J, Khuri J, Xue H, Xie B, A Kaminsky L, et al. Effectiveness of mobile health interventions on diabetes and obesity treatment and management:Systematic review of systematic reviews. JMIR Mhealth Uhealth. 2020;8:e15400. doi: 10.2196/15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Burke LE, Conroy MB, Sereika SM, Elci OU, Styn MA, Acharya SD, et al. The effect of electronic self-monitoring on weight loss and dietary intake:A randomized behavioral weight loss trial. Obesity (Silver Spring) 2011;19:338–44. doi: 10.1038/oby.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Cavero-Redondo I, Martinez-Vizcaino V, Fernandez-Rodriguez R, Saz-Lara A, Pascual-Morena C, Álvarez-Bueno C. Effect of behavioral weight management interventions using lifestyle mhealth self-monitoring on weight loss:A systematic review and meta-analysis. Nutrients. 2020;12:1977. doi: 10.3390/nu12071977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Willmott TJ, Pang B, Rundle-Thiele S, Badejo A. Weight management in young adults:Systematic review of electronic health intervention components and outcomes. J Med Internet Res. 2019;21:e10265. doi: 10.2196/10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Laitner MH, Minski SA, Perri MG. The role of self-monitoring in the maintenance of weight loss success. Eat Behav. 2016;21:193–7. doi: 10.1016/j.eatbeh.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Thomas JG, Raynor HA, Bond DS, Luke AK, Cardoso CC, Wojtanowski AC, et al. Weight loss and frequency of body-weight self-monitoring in an online commercial weight management program with and without a cellular-connected 'smart'scale:a randomized pilot study. Obes Sci Pract. 2017;3:365–72. doi: 10.1002/osp4.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Berry R, Kassavou A, Sutton S. Does self-monitoring diet and physical activity behaviors using digital technology support adults with obesity or overweight to lose weight?A systematic literature review with meta-analysis. Obes Rev. 2021;22:e13306. doi: 10.1111/obr.13306. [DOI] [PubMed] [Google Scholar]
- 298.Turner-McGrievy GM, Dunn CG, Wilcox S, Boutté AK, Hutto B, Hoover A, et al. Defining adherence to mobile dietary self-monitoring and assessing tracking over time:Tracking at least two eating occasions per day is best marker of adherence within two different mobile health randomized weight loss interventions. J Acad Nutr Diet. 2019;119:1516–24. doi: 10.1016/j.jand.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Turk MW, Elci OU, Wang J, Sereika SM, Ewing LJ, Acharya SD, et al. Self-monitoring as a mediator of weight loss in the SMART randomized clinical trial. Int J Behav Med. 2013;20:556–61. doi: 10.1007/s12529-012-9259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Shuger SL, Barry VW, Sui X, McClain A, Hand GA, Wilcox S, et al. Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss:A randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:41. doi: 10.1186/1479-5868-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Dunn CG, Turner-McGrievy GM, Wilcox S, Hutto B. Dietary self-monitoring through calorie tracking but not through a digital photography app is associated with significant weight loss:The 2SMART pilot study-a 6-month randomized trial. J Acad Nutr Diet. 2019;119:1525–32. doi: 10.1016/j.jand.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 302.Acharya SD, Elci OU, Sereika SM, Styn MA, Burke LE. Using a personal digital assistant for self-monitoring influences diet quality in comparison to a standard paper record among overweight/obese adults. J Am Diet Assoc. 2011;111:583–8. doi: 10.1016/j.jada.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Tronieri JS, Fabricatore AN, Wadden TA, Auerbach P, Endahl L, Sugimoto D, et al. Effects of dietary self-monitoring, physical activity, liraglutide 3.0 mg, and placebo on weight loss in the SCALE IBT trial. Obes Facts. 2020;13:572–83. doi: 10.1159/000511130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ho TJH, Lee CCS, Wong SN, Lau Y. Internet-based self-monitoring interventions for overweight and obese adolescents:A systematic review and meta-analysis. Int J Med Inform. 2018;120:20–30. doi: 10.1016/j.ijmedinf.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 305.Soeliman FA, Azadbakht L. Weight loss maintenance:A review on dietary related strategies. J Res Med Sci. 2014;19:268–75. [PMC free article] [PubMed] [Google Scholar]
- 306.Lee PG, Jackson EA, Richardson CR. Exercise prescriptions in older adults. AmFam Physician. 2017;95:425–32. [PubMed] [Google Scholar]
- 307.Castelnuovo G, Pietrabissa G, Manzoni GM, Cattivelli R, Rossi A, Novelli M, et al. Cognitive behavioral therapy to aid weight loss in obese patients:current perspectives. Psychol Res Behav Manag. 2017;10:165–73. doi: 10.2147/PRBM.S113278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss:A systematic review of the literature. J Am Diet Assoc. 2011;111:92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self-weighing in weight management:a systematic literature review. Obesity (Silver Spring) 2015;23:256–65. doi: 10.1002/oby.20946. [DOI] [PubMed] [Google Scholar]
- 310.Kasthuri A. Challenges to healthcare in India - The five A's. Indian J Community Med. 2018;43:141–3. doi: 10.4103/ijcm.IJCM_194_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Dietz WH. Obesity and excessive weight gain in young adults:New targets for prevention. JAMA. 2017;318:241–2. doi: 10.1001/jama.2017.6119. [DOI] [PubMed] [Google Scholar]
- 312.Berga SL, Garovic VD. Barriers to the care of menopausal women. Mayo Clin Proc. 2019;94:191–3. doi: 10.1016/j.mayocp.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Aboueid S, Bourgeault I, Giroux I. Nutrition care practices of primary care providers for weight management in multidisciplinary primary care settings in Ontario, Canada - A qualitative study. BMC Fam Pract. 2018;19:69. doi: 10.1186/s12875-018-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Luhar S, Timæus IM, Jones R, Cunningham S, Patel SA, Kinra S, et al. Forecasting the prevalence of overweight and obesity in India to 2040. PLoS One. 2020;15:e0229438. doi: 10.1371/journal.pone.0229438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Seifi B, Ghanizadeh G, Seyedin H. Disaster health literacy of middle-aged women. J Menopausal Med. 2018;24:150–4. doi: 10.6118/jmm.2018.24.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Pomerleau J, Lock K, Knai C, McKee M. Interventions designed to increase adult fruit and vegetable intake can be effective:A systematic review of the literature. J Nutr. 2005;135:2486–95. doi: 10.1093/jn/135.10.2486. [DOI] [PubMed] [Google Scholar]
- 317.Adler NE, Stewart J. Reducing obesity:Motivating action while not blaming the victim. Milbank Q. 2009;87:49–70. doi: 10.1111/j.1468-0009.2009.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


