Multiple myeloma (MM) is a complex disease with highly heterogeneous tumor biology, especially involving cytogenetic abnormalities.1 Consequently, MM patients display markedly diverse clinical characteristics, therapeutic responses, and outcomes.2,3 In this context, the international staging system (ISS) was developed to predict the prognosis of MM patients in 2005,4 which was later updated and named revised ISS (R-ISS) in 2015.5 Although the R-ISS has widely been used as a powerful tool to guide daily practice till present, some considerable limitations (eg, a large proportion of R-ISS II patients with varied outcomes, not reflecting the significance of 1q gain/amplification and concurrent high-risk cytogenetic abnormalities [HRCAs]) have been emerging.6–9 In this context, several new prognostic scoring systems have been reported,10,11 the most recent of which is the second revision of ISS (R2-ISS) updated by the European Myeloma Network (EMN).12
In the R2-ISS,12 5 risk variates with the highest impact on both progression-free survival (PFS) and overall survival (OS) were weighted according to their OS impact, including ISS III 1.5, ISS II 1.0, del(17p) 1.0, lactate dehydrogenase high 1.0, t(4;14) 1.0, and 1q+ (either 1q gain or amplification) 0.5. However, t(14;16) was not included because patients with this HRCA had only a trend toward a shorter PFS than those without it, but not statistically significant, although its role was significant in predicting OS. According to the R2-ISS, patients could be categorized into 4 groups with different risk scores that is, 0 (I), 0.5 to 1.0 (II), 1.5 to 2.5 (III), and 3.0 to 5.0 (IV). In 2 independent cohorts, the R2-ISS was able to sharply stratify newly diagnosed MM (NDMM) patients into these 4 groups with significantly different OS and PFS, including R2-ISS I (19.2% or 11.1% of all patients), II (30.8% or 26.5%), III (41.2% or 51.6%), and IV (8.8% or 10.7%). Moreover, the R2-ISS was able to predict the OS of both transplant-eligible and transplant-ineligible patients, and patients who received different upfront treatments (eg, proteasome inhibitors [PIs], immunomodulatory drugs [IMiDs], or both). Of note, this scoring system could further discriminate OS and PFS of R-ISS II patients, underlining its value in restratifying this large heterogeneous group of NDMM patients. With these advantages, this new simple algorithm would be expected to be applied soon in clinical practice to improve the performance of the currently using risk stratification systems such as R-ISS (especially further stratification of R-ISS II patients). However, since the R2-ISS was developed and validated in the European population of NDMM patients enrolled in multiple clinical trials, its prognostic property remains to be verified in other populations and in a real-world setting. In this context, the R2-ISS has recently been validated using real-world data in Australian and New Zealand population.13 Considering the significance of 1q+ in the R2-ISS, the Chinese population of NDMM patients may be particularly susceptible to this scoring system due to their high frequency (~50% of NDMM patients) of 1q+ and its prognostic value as reported earlier by our group.14
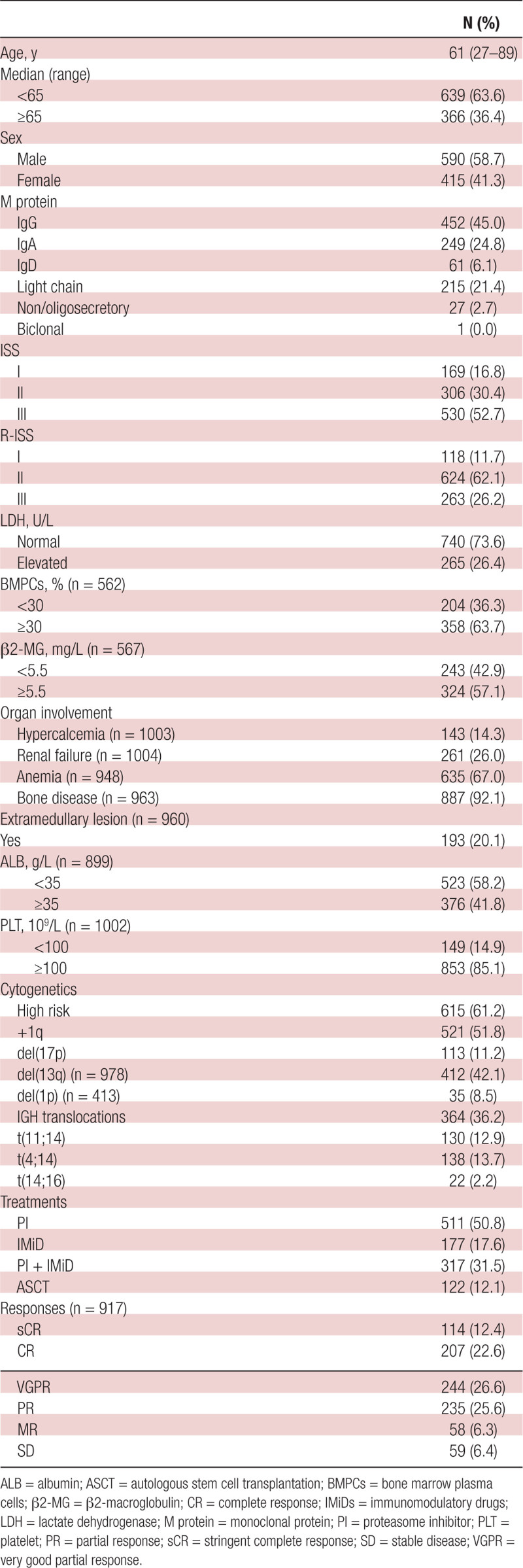
To this end, we carried out a retrospective analysis of the real-world data collected from daily practice to further validate the R2-ISS in a cohort of 1005 MM patients newly diagnosed between November 27, 2009 and November 20, 2019, at 6 centers nationwide in China (Table 1). This study was approved by the Institutional Review Boards of the First Hospital of Jilin University (Approval No. 2016-087). All patients had given written informed consent to the use of clinical data according to the Declaration of Helsinki. The inclusion criteria of this study were patients who had complete baseline information for R2-ISS scoring, particularly involving cytogenetics in CD138+ cells by fluorescence in-situ hybridization (FISH) that must include the probes for del(17p) (cutoff, 20%), t(4;14) (cutoff, 7.3%), and 1q+ (cutoff, 5.5%) including gain (3 copies) and amplification (≥4 copies) as described previously,14 and who must receive novel agents (PIs, IMiDs, or both) for upfront treatment. It is noteworthy that the cutoffs for some HRCAs such as t(4;14) and 1q+ was lower than those usually recommended, which might, at least in part, contribute to a relatively higher percentage of high-risk patients (eg, 1q+) in this cohort, thus representing one limitation of this study. All patients were treated in the real-world setting, of whom 511 (50.8%), 177 (17.6%), and 317 (31.5%) received PI-, IMiD-, and PI plus IMiD-based induction, and 122 (12.1%) received autologous stem cell transplantation (ASCT). According to the International Myeloma Working Group consensus criteria,15 114 (12.4%), 207 (22.6%), 244 (26.6%), 235 (25.6%), 58 (6.3%), and 59 (6.4%) of 917 evaluable patients had a stringent complete response, complete response, very good partial response, partial response, minimal response, and stable disease, respectively. PFS was defined as the time from diagnosis until disease progression, relapse, or death due to any cause. Patients who did not progress or relapse were censored on the last date when they were seen alive and event free. OS was defined as the time from diagnosis until death due to any cause or last follow-up.
Table 1.
Characteristics of Patients With Newly Diagnosed Multiple Myeloma in Our Cohort (n = 1005)
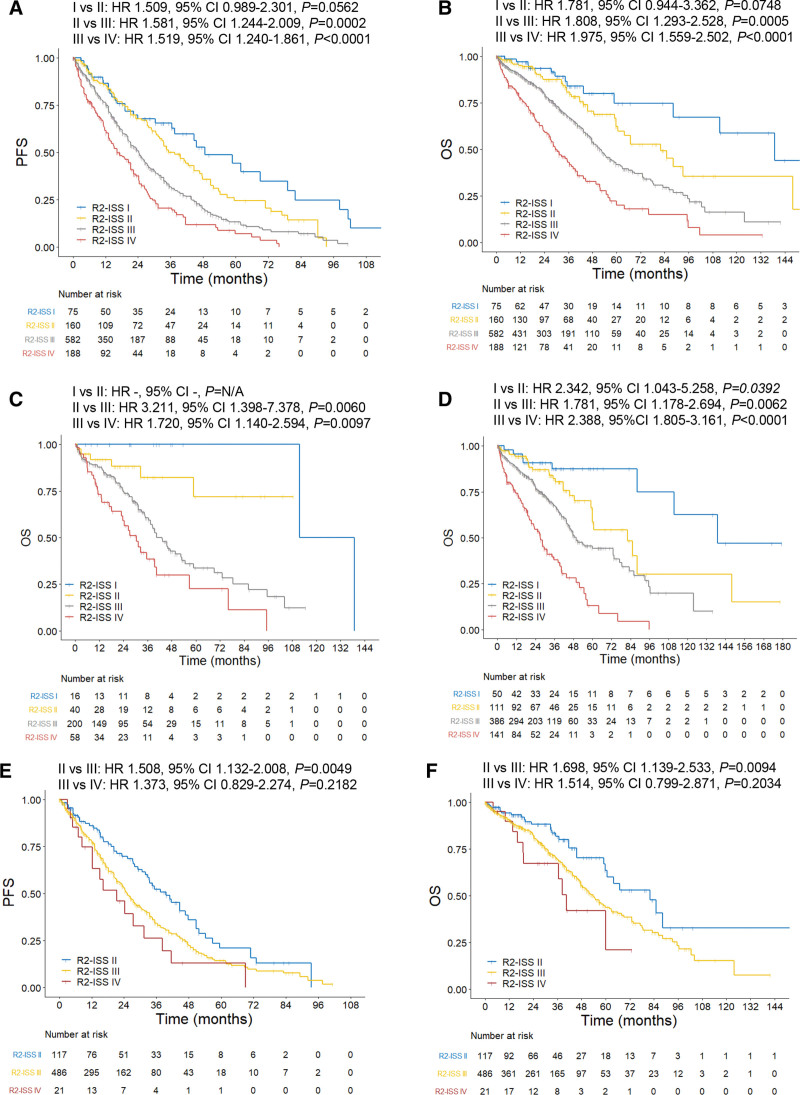
With a median follow-up of 35.5 months (95% confidence interval [CI], 32.8-38.2), median PFS and OS were 25.2 (95% CI, 23.1-27.3) and 53.0 (95% CI, 48.1-57.9) months, respectively. A total of 1005 patients with NDMM were categorized into R-ISS I (118, 11.7%), II (624, 62.1%), and III (263, 26.2%), with the largest proportion of patients in the R-ISS II group.12 As expected, the R-ISS could clearly stratify NDMM patients on both PFS (Suppl. Figure S1A) and OS (Suppl. Figure S1B), with P < 0.001 for each comparison between R-ISS I, II, and III. According to the R2-ISS,12 all patients (n = 1005) were then scored and divided into 4 groups: R2-ISS I (75, 7.5%), II (160, 15.9%), III (582, 57.9%), and IV (188, 18.7%). Compared with those reported by the EMN,12 there were relatively fewer patients with low (R2-ISS I) and low-intermediate risk (R2-ISS II) but more patients with intermediate-high (R2-ISS III) and high risk (R2-ISS IV) in this cohort. Moreover, every group of patients in this cohort had also relatively shorter PFS and OS, consistent with the fact that majority of patients had more advanced disease, primarily due to late diagnosis, but much fewer patients received ASCT (a main limitation of this study) mostly due to unaffordability, in our cohort than those reported by the EMN (Suppl. Table S1). Of note, the differences in PFS (Figure 1A) and OS (Figure 1B) were significant for almost all comparisons between the 2 groups (P < 0.001), except R-ISS I versus II (PFS, P = 0.056; OS, P = 0.075), probably due to a relatively small number of low-risk patients (eg, ISS or R-ISS I) in our cohort. Moreover, the R2-ISS largely remained its risk-stratifying property in the subgroups of patients with different treatments, particularly in distinguishing the patients with R2-ISS III or IV from those with R2-ISS I or II. In addition, the R2-ISS might work well in patients who did not receive ASCT, for either age >65 years (Figure 1C and Suppl. Figure S2A) or age ≤65 years (Suppl. Figure S2B, C), compared with those who received ASCT (Suppl. Figure S3A, B). Similarly, the R2-ISS also separated patients with different risk levels in the ones who received either PI- or IMiD-based induction (Figure 1D and Suppl. Figure S4A) better than those who received PI plus IMiD-based induction (Suppl. Figure S4B, C). However, due to the small number of patients in some subgroups, these results should be interpreted with caution. Nonetheless, these observations support that the R2-ISS could well discriminate NDMM patients with different risk levels and predict their outcomes (both PFS and OS) at diagnosis, although treatments might influence its performance.
Figure 1.
Survival of newly diagnosed multiple myeloma (NDMM) patients according to the R2-ISS. (A, B) PFS (A; median: 48.7, 37.5, 24.3, and 16.3 mo for R2-ISS I–IV, respectively) and OS (B; median: 139.0, 82.2, 51.8, 29.5 mo for R2-ISS I–IV, respectively) of all patients (n = 1005). (C) OS of patients with age >65 y (n = 314; median: 125.3, not reached [NR], 43.3, and 30.5 mo for R2-ISS I–IV, respectively). (D) OS of patients who received either proteasome inhibitor (PI)- or immunomodulatory drug (IMiD)-based induction (n = 688; median: 139.0, 82.8, 49.7, and 27.1 mo for R2-ISS I-IV, respectively). (E, F) PFS (E; median: 39.6, 25.3, and 21.3 mo for R2-ISS II–IV, respectively) and OS (F; median: 82.2, 53.8, and 40.5 mo for R2-ISS II–IV, respectively) of R-ISS II patients (n = 624). OS = overall survival; PFS = progression-free survival; R2-ISS = second revision of international staging system.
One of the main aims for developing the R2-ISS is to better stratify R-ISS II patients,12,13 which account for about 60% of patients with considerably heterogeneous outcomes in this cohort. We thus validated whether the R2-ISS would be able to restratify the R-ISS II patients and predict their outcomes more precisely. In R-ISS II patients (n = 624), there were 117 (18.8%), 486 (77.9%), and 21 (3.4%) patients with R2-ISS II, III, and IV (Suppl. Figure S4D). The differences in PFS (Figure 1E) and OS (Figure 1F) were statistically different between R2-ISS II versus III or IV (P < 0.01 for each comparison), consistent with those reported by the EMN.12 However, no significant difference was observed between R2-ISS III and IV (P > 0.05 for both PFS and OS), similar to the results from the training set, but not the validation set, in the study reported by the EMN.12 Moreover, a comparison of patient outcomes between R-ISS and R2-ISS suggests that the capability to further restratify R-ISS II patients might represent an advantage for the R2-ISS (Suppl. Table S2). Therefore, these observations support the notion that the R2-ISS could restratify patients with R-ISS II, particularly in the case of distinguishing R2-ISS III and IV from R2-ISS II.
In summary, this study provides the evidence for the value of the R2-ISS recently updated by the EMN in the risk stratification of NDMM patients, particularly those categorized as R-ISS II by the R-ISS staging system, in an entirely independent cohort of patients with considerable differences in baseline characteristics (eg, more advanced disease and HRCAs) and treatments (ie, in the real-world setting, rather than clinical trials in the EMN study12). Thus, this new simple prognostic tool warrants further attention in future investigations and practice.
AUTHOR CONTRIBUTIONS
PY, FZ, YD(1), GG, HX, XL, SY, WX, YM, XQ, ML, YD(2), and FJ performed the research. FJ, PY, and YD(2) analyzed and interpreted the data. FJ and YD(2) conceived and planned the study design, and wrote the article. All authors approved the article.
DATA AVAILABILITY
Original data are available upon reasonable request to corresponding authors.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81471165, 81670190, 81670189, 81870160, 81971108, and 82270207) and Science and Technology Development Program of the Jilin Province (Grant Nos. 20190201042JC, 20190201163JC, and 20210509010RQ), and Interdisciplinary Integration and Innovation Project of Jilin University.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES
- 1.Kumar SK, Rajkumar V. The multiple myelomas — current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol. 2018;15:409–421. [DOI] [PubMed] [Google Scholar]
- 2.van de Donk NWCJ, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397:410–427. [DOI] [PubMed] [Google Scholar]
- 3.Atrash S, Flahavan EM, Xu T, et al. Treatment patterns and outcomes according to cytogenetic risk stratification in patients with multiple myeloma: a real-world analysis. Blood Cancer J. 2022;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greipp PR, Miguel JS, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. [DOI] [PubMed] [Google Scholar]
- 5.Antonio P, Hervé A-L, Stefania O, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinhold N, Salwender HJ, Cairns DA, et al. Chromosome 1q21 abnormalities refine outcome prediction in patients with multiple myeloma - a meta-analysis of 2,596 trial patients. Haematologica. 2021;106:2754–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garifullin A, Voloshin S, Shuvaev V, et al. Significance of modified risk stratification mSmart 3.0 and autologous stem cell transplantation for patients with newly diagnosed multiple myeloma. Blood. 2019;134:5593–5593. [Google Scholar]
- 8.Orgueira AM, Pérez MSG, Arias JAD, et al. Prognostic stratification of multiple myeloma using clinicogenomic models: Validation and performance analysis of the IAC-50 model. HemaSphere. 2022;6:e760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corre J, Munshi NC, Avet-Loiseau H. Risk factors in multiple myeloma: is it time for a revision? Blood. 2021;137:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrot A, Lauwers-Cances V, Tournay E, et al. Development and validation of a cytogenetic prognostic index predicting survival in multiple myeloma. J Clin Oncol. 2019;37:1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdallah NH, Binder M, Rajkumar SV, et al. A simple additive staging system for newly diagnosed multiple myeloma. Blood Cancer J. 2022;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Agostino M, Caims DA, Lahuerta JJ, et al. Second revision of the International Staging System (R2-ISS) for overall survival in multiple myeloma: a European Myeloma Network (EMN) report within the HARMONY project. J Clin Oncol. 2022;40:3406–3418. doi:10.1200/JCO.21.02614. [DOI] [PubMed] [Google Scholar]
- 13.Tan JLC, Wellard C, Moore EM, et al. The second revision of the International Staging System (R2-ISS) stratifies progression-free and overall survival in multiple myeloma: real world data results in an Australian and New Zealand Population. Br J Haematol. 2022;200:e17–e21. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Chen H, Liang X, et al. Proposed risk-scoring model for estimating the prognostic impact of 1q gain in patients with newly diagnosed multiple myeloma. Am J Hematol. 2023;98:251–263. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data are available upon reasonable request to corresponding authors.