Abstract
Although highly effective at durably suppressing plasma HIV-1 viremia, combination antiretroviral therapy (ART) treatment regimens do not eradicate the virus, which persists in long-lived CD4+ T cells. This latent viral reservoir serves as a source of plasma viral rebound following treatment interruption, thus requiring lifelong adherence to ART. Additionally, challenges remain related not only to access to therapy but also to a higher prevalence of comorbidities with an inflammatory etiology in treated HIV-1+ individuals, underscoring the need to explore therapeutic alternatives that achieve sustained virologic remission in the absence of ART. Natural killer (NK) cells are uniquely positioned to positively impact antiviral immunity, in part due to the pleiotropic nature of their effector functions, including the acquisition of memory-like features, and, therefore, hold great promise for transforming HIV-1 therapeutic modalities. In addition to defining the ability of NK cells to contribute to HIV-1 control, this review provides a basic immunologic understanding of the impact of HIV-1 infection and ART on the phenotypic and functional character of NK cells. We further delineate the qualities of “memory” NK cell populations, as well as the impact of HCMV on their induction and subsequent expansion in HIV-1 infection. We conclude by highlighting promising avenues for optimizing NK cell responses to improve HIV-1 control and effect a functional cure, including blockade of inhibitory NK receptors, TLR agonists to promote latency reversal and NK cell activation, CAR NK cells, BiKEs/TriKEs, and the role of HIV-1-specific bNAbs in NK cell–mediated ADCC activity against HIV-1-infected cells.
Keywords: memory NK cells, adaptive NK cells, innate immune memory, broadly neutralizing antibodies (bNAbs), dendritic cells (DCs), HIV
1. Introduction
As innate lymphocytes, natural killer (NK) cells serve on the front line of immunity, mounting immediate and powerful defenses against cells displaying molecular signals of stress, transformation, or infection.1–4 In fact, the term natural killer stems from their ability to spontaneously lyse target cells in the absence of priming or prior sensitization.1,2,5 Subsequent experiments in mouse models of bone marrow graft rejection prompted the development of the “missing-self” hypothesis, a notion that NK cells would kill any cell lacking self-major histocompatibility complex (MHC) class I molecules.6–8 This laid the framework for our current, although nascent, understanding that NK cell activation is dictated by the integration of multiple activating and inhibitory signals, with unique combinations of receptors determining the response potential and activation threshold of individual NK cells.9,10
When the balance shifts to net positive signaling, NK cells are released from inhibition, resulting in lysis of engaged target cells.11 Of the activating NK receptors (NKRs), NKp46, NKG2D, and CD16 have emerged as significant in protecting against and controlling HIV-1 infection by triggering NK cell cytotoxic activity.12–16 NK cells are important not only for the elimination of infected cells but also for the generation of antigen-specific immunity through their local interaction with dendritic cells (DCs) in infected tissues, promoting the development of mature DCs (mDCs) with an enhanced ability to produce IL-12 and to induce type-1 immune responses.17,18
However, HIV-1 has developed targeted means to cripple NK cell cytolytic activity and to interrupt NK-DC crosstalk, consequently curbing the capacity of DCs to promote effective antiviral T cell responses.19–24 In addition to causing NK cell dysfunction, HIV-1 infection is associated with expanded populations of “memory” NK cells.25–29 Although these “memory” subsets are poised for enhanced responses to antibody-mediated signaling, they have a limited ability to rapidly respond to innate factors,29–37 which under certain situations negatively impacts the quality of their interactions with DCs.38 It is our position that improving our understanding of the magnitude of dysfunction suffered by NK cells during HIV-1 infection will be integral in leveraging their strengths and maximizing the effectiveness of HIV-1 therapies. Therefore, in this review, we will dissect the interplay between NK cells and HIV-1, as well as the phenotypic and functional characteristics of “memory” NK cells, highlighting the promise of NK cell-based therapies and providing insight into novel strategies for effecting a functional HIV cure.
2. Interplay between NK cells and HIV-1
2.1. NK cell control of HIV-1 infection
Epidemiologic and functional studies have revealed the impact of NK cells on HIV-1 infection, with particular KIR-HLA combinations heavily influencing their effectiveness in protecting against acquisition of infection39,40 and in delaying disease progression.41,42 The epistatic interaction between the activating KIR allele KIR3DS1 and the HLA-B Bw4-80I allele in the setting of chronic HIV-1 infection is associated with slower depletion of CD4+ T cells and delayed progression to AIDS.41 This epidemiological association may be explained by the ability of KIR3DS1+ NK cells to strongly inhibit in vitro HIV-1 replication in target cells expressing HLA-B Bw4-80I,43 in addition to conferring enhanced function via CD107a and IFNγ in early HIV-1 infection.44 Moreover, a combined genotype of inhibitory KIR3DL1 high-expressing alleles and HLA-B*57 grants protection against disease progression and lowers the risk of HIV-1 infection in exposed uninfected individuals.39,42 These data suggest that binding of KIRs with their cognate ligands impacts the natural course of HIV-1 disease by defining the activation threshold and protective efficacy of NK cell responses.
Additionally, downregulation of MHC class I molecules by HIV-1 accessory proteins to avoid recognition by cytotoxic T lymphocytes (CTLs) simultaneously enhances the susceptibility of infected cells to NK cell-mediated killing.23,45 HIV-1 Nef downmodulates HLA-A and HLA-B molecules,46 while the viral Vpu protein downregulates expression of HLA-C,47 theoretically offering the missing-self trigger for NK cell activation. Indeed, this downmodulation of cell surface HLA expression on HIV-1-infected cells promotes NK cell activation through missing-self mechanisms, but the ability of NK cells to ultimately kill these infected cells depends on the extent of virus-mediated MHC class I downregulation, as well as the strength of inhibitory KIR (iKIR)-HLA interactions.48 In particular, the strength of this interaction determines education potency and influences the magnitude of NK cell effector responses against target cells.49 However, the highly polymorphic nature of KIR and HLA genes lends to incredible diversity, replete with a myriad of allotype combinations characterized by distinct binding specificities and affinities.50–53 For example, the inhibitory KIR3DL1 interacts with a subset of HLA-A and -B molecules containing the serological motif Bw4,54,55 with a stronger avidity toward Bw4*80I than Bw4*80T allotypes resulting in a higher degree of education and responsiveness.50,56–58 By contrast, KIR2DL2 and KIR2DL3 variants preferentially interact with HLA-C group 1 antigens, whereas KIR2DL1 allotypes display a stronger avidity for HLA-C group 2 ligands.51,59 HLA-C-educated NK cells sense alterations of HLA-C expression,48 and the combinations of KIR2DL2/HLA-C*12:02 and KIR2DL2/HLA-C*14:03 exert protective effects by suppressing HIV-1 replication. The enhanced functional activity of KIR2DL2+ NK cells is influenced by reduced expression of the peptide-HLA complex on the target cell surface, highlighting that the ability of NK cells to control HIV-1 replication is determined by the strength of KIR-HLA interactions.60 Kiani et al. confirmed that the potency of NK cell education is directly related to the response magnitude of each NK cell by demonstrating that educated KIR2DL+ NK cells more frequently respond to the reduction of their cognate HLA ligands on autologous HIV-1-infected cells by producing CCL4, IFNγ, and CD107a.49 Since KIR molecules dictate the activation status of NK cells, the potential exists to exploit the significance of KIR-HLA interactions to improve immunotherapies targeting HIV-1, including iKIR blockade and highly efficient chimeric antigen receptor (CAR)-engineered NK cells, as detailed in the sections to follow.
We have recently come to appreciate that primary HIV-1 strains can also differentially modulate HLA-E surface levels on infected CD4+ T cells. This process is mediated primarily by HIV-1 Nef by targeting the cytoplasmic tail of HLA-E.61 HLA-E is a nonclassical MHC class Ib molecule that engages the inhibitory NKG2A receptor, as well as its activating counterpart, NKG2C, in a peptide-dependent manner62–65—although signaling through NKG2A prevails over that of NKG2C.66,67 Expression of HLA-E is dependent on the stable binding of a signal peptide derived from the leader sequence of other MHC class I molecules containing a methionine at position two of the signal peptide (−21 M).64,68,69 Thus, reduced surface expression of MHC class I molecules is significantly associated with downregulation of HLA-E surface levels on HIV-1-infected CD4+ T cells, potentially rendering them more susceptible to the cytotoxicity of NKG2A+ NK cells,61 which display high activity against HIV-1-infected cells.70,71 NKG2A is also predominantly found on CD56bright NK cells, concomitant with resting expression of CCR7 and high-density expression of CD62L,72–74 supporting their preferential homing to lymph nodes—a main site of HIV-1 persistence.75–78
Another HIV-1 accessory protein, Vpr, upregulates cell-surface expression of UL16 binding proteins (ULBPs) on infected cells, thereby promoting NK cell-mediated killing through the activating NKG2D receptor79,80 (Fig. 1A). This NKG2D-dependent cytotoxicity is further enhanced by priming NK cells with IFNα.16 On a similar note, CD16 engagement strongly activates NK cell effector functions, leading to lysis of target cells and the release of polarizing cytokines (Fig. 1B). The potency of this response is highlighted by the fact that NK cell-mediated antibody dependent cellular cytotoxicity (ADCC) has been implicated in vaccine-induced protective immunity against acquisition of infection, phenotypes of viral control, and slower disease progression.12–15 Finally, NK cells participate in HIV-1 control by releasing the chemokines CCL3, CCL4, and CCL5, which are ligands for the HIV-1 coreceptor CCR5. These β-chemokines presumably inhibit viral entry into target cells by blocking the binding of HIV-1 envelope protein to CCR581 (Fig. 1C).
Fig. 1.
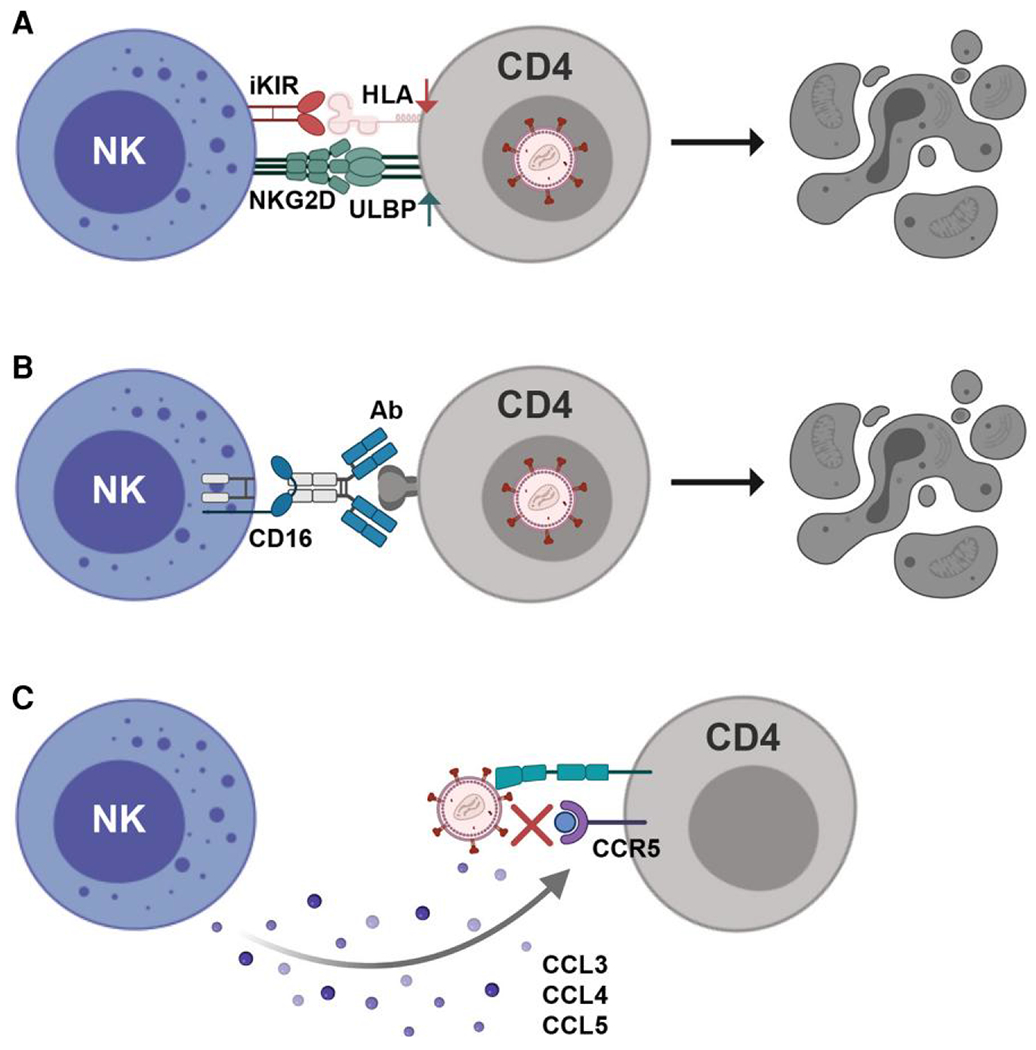
NK cell control of HIV-1 infection. (A) HIV-1 accessory proteins cause downregulation of MHC class I molecules and upregulation of stress ligands, activating NK cell cytolytic activity and promoting elimination of infected CD4+ T cells. (B) NK cells are also activated via CD16, resulting in ADCC-mediated elimination of infected cells and potent control of HIV-1 infection. (C) Secretion of CCL3, CCL4, and CCL5 by NK cells inhibits viral entry by blocking binding of HIV-1 to the coreceptor CCR5. Figure created with BioRender.com.
2.2. Impact of HIV-1 infection on NK cells
With such a diverse array of effector functions, from cytotoxicity to immune modulation, the importance of NK cells in the anti-HIV-1 immune response is clear; however, NK cells are defective at controlling the virus due to the profound impact of HIV-1 on NK cell phenotype and function, including the pathologic redistribution of dysfunctional NK cell subsets.19–21,82 The sequential deregulation of NK cell subset distribution begins during acute HIV-1 infection. Initially, the absolute number of circulating NK cells increases, accompanied by a relative expansion of the CD56dimCD16+ subset and early depletion of CD56brightCD16− NK cells.20 Ongoing viral replication induces the expansion of a dysfunctional CD56−CD16+ (CD56−) NK cell subset at the expense of cytotoxic CD56dimCD16+ NK cells.19,20,82,83 Importantly, CD56− NK cells do not appear in chronic HIV-1-infected individuals with undetectable plasma viremia or in acute HIV-1-infected individuals with high viral loads, underscoring the role of chronic HIV-1 viremia in driving the expansion of this aberrant population.20,83,84 Based on studies reporting that the percentage and/or absolute number of total NK cells in the peripheral blood decreased during active chronic HIV-1 infection, it was originally hypothesized that high levels of ongoing viral replication were contributing to the reduction in circulating NK cells through early NK cell death or preferential distribution to peripheral tissues.85,86 However, identification of the expanded pathologic CD56− subset demonstrates that chronic HIV-1 viremia is associated with a signification redistribution of NK cell populations rather than an absolute decline of total NK cells in the peripheral blood.19–21,82,83,87
Chronic HIV-1 viremia is also characterized by significantly reduced expression of all three natural cytotoxicity receptors (NCRs)—NKp30, NKp44, and NKp46—yet heightened levels of inhibitory NK receptors (iNKRs).21,88–90 NKG2A, conversely, is the only iNKR marked by decreased expression on NK cells from chronic viremic HIV-1+ individuals,19,21 which results in an inversion of the NKG2A/NKG2C ratio.91 These phenotypic abnormalities are especially pronounced within the CD56− population.19 By contrast, Siglec-7, an iNKR constitutively expressed on the majority of NK cells, represents a marker that is highly sensitive to high levels of HIV-1 viremia in the earliest stages of infection and precedes CD56 in its downmodulation. The differential kinetics of Siglec-7 and CD56 downregulation allow for the detection of two sequential pathologic NK cell subsets: Siglec-7−CD56+ NK cells, which preferentially expand during the initial phases of infection, and Siglec-7−CD56− NK cells, which only become detectable in the setting of chronic HIV-1 viremia83 (Fig. 2).
Fig. 2.
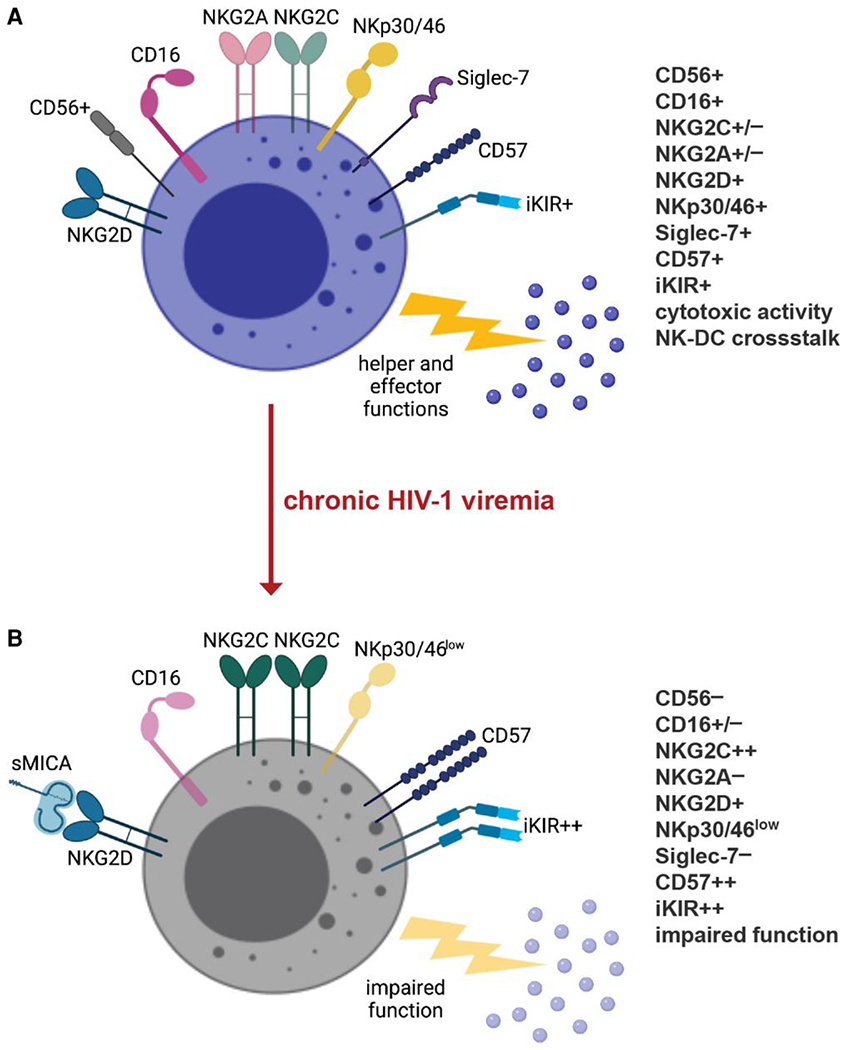
Impact of HIV-1 infection on NK cells. Chronic HIV-1 viremia causes a sequential loss of Siglec-7 and CD56, leading to an accumulation of Siglec-7−CD56− NK cells. This highly dysfunctional subset preferentially expresses iKIRs over activating NKRs, including NKp30 and NKp46. Chronic HIV-1 viremia is also associated with increased expression of NKG2C, combined with loss of NKG2A, resulting in an inversion of the NKG2A to NKG2C ratio. NK cell cytolytic activity is further impaired by cleavage of NKG2D ligands from the surfaced of infected CD4+ T cells by MMPs, which are highly expressed in viremic HIV-1-infected individuals. This proteolytic activity allows HIV-1-infected cells to evade NKG2D-mediated antiviral responses both by decreasing surface expression of NKG2D ligands on infected cells and by inactivating NKG2D on NK cells via the soluble ligands. Figure created with BioRender.com.
Perturbations in NK cell compartments resulting from chronic HIV-1 viremia profoundly hinder NK cell antiviral properties in a progressive fashion until the expansion of the Siglec-7−CD56− subset signals a maximal loss of NK cell function. Concomitant with the dysregulated expression of activating and inhibitory receptors are functional abnormalities that severely limit cytotoxicity.19–21,23 Of note, despite downregulated expression of MHC class I molecules on endogenously HIV-1-infected CD4+ T cells, NK cells from individuals with chronic HIV-1 viremia display poor cytolytic activity against these autologous targets, and this aberrant effector function is closely linked to defective surface expression and engagement of NCRs and to the high frequency of dysfunctional CD56− NK cells.23 The increased proportion of CD56− NK cells in chronic viremic HIV-1 infection is also associated with weak ADCC responses,19,92 and the degree of functional cytotoxic activity correlates inversely with levels of HIV-1 plasma viremia.93 Anergic CD56− NK cells further contribute to a dysregulated immune response by disrupting the mutually beneficial interactions between NK cells and DCs (Fig. 2). First, the failure of CD56− NK cells to secrete IFNγ, TNFα, and GM-CSF interferes with their ability to promote optimal DC maturation.19–22 These inadequately matured DCs are then substantially defective at secreting IL-12 and priming neighboring NK cells. Second, the expanded CD56− population is responsible for deficiencies in NK cell–mediated editing of immature or improperly matured DCs among HIV-1-infected individuals with persistent viremia. This phenomenon, attributed to compromised signaling through NKp30,24 likely underlies downstream ineffective T cell priming against HIV-1.
The pathologic redistribution of NK cell subsets is only one mechanism by which HIV-1 subverts NK cell functional responses. Countermeasures employed by HIV-1 to facilitate immune evasion abound, thereby affecting NK cell–mediated control of infection and the overall quality of the immune response.92,94,95 Given the protective immunity conferred by specific KIR-HLA combinations, HIV-1 circumvents this NK cell–mediated immune pressure by selecting for sequence polymorphisms that enhance the binding of iKIRs to infected CD4+ T cells. Thus, NK cells directly contribute to viral evolution, with the outcome being reduced antiviral activity of KIR-expressing NK cells.96 Furthermore, KIR+ NK cells are not only rarely detected in steady-state lymph nodes,74,97 but they are also noticeably absent in lymph nodes during HIV-1 infection despite active viral replication. The failure of KIR+ NK cells to accumulate in secondary lymphoid tissue (SLT) during early HIV-1 infection is due to reduced expression of chemokine and lymph node–homing receptors, including CX3CR1 and CD62L. This potentially allows the virus to escape early NK cell control and hence to replicate profusely in an environment enriched with target cells.98
Although Nef-mediated downmodulation of HLA-E targets HIV-1-infected cells for killing by NKG2A+ NK cells, the reverse has also been reported, with HIV-1 infection leading to increased HLA-E levels on the cell surface. HIV-1 encodes at least one peptide in the Gag protein (p2414-22) that is recognized by T cells but that can also bind to HLA-E, stabilizing its cell surface expression and thus inhibiting NKG2A+ NK cells.99 Since HLA-E expression requires stable binding of a signal peptide derived from the leader sequence of MHC class I molecules, the extent to which HLA-E expression is altered highly depends on HLA polymorphism, which determines both peptide-binding specificity and variation in expression levels, particularly for HLA-A alleles. Therefore, elevated HLA-A expression impairs HIV-1 control through enhanced HLA-E expression, resulting in increased inhibition of NKG2A-expresing cells.100 The importance of this, in terms of HIV-1 control, is perhaps best exemplified by the contrast between African green monkeys (AGMs), natural hosts of SIV, and macaques, the nonhuman primate model of HIV. Before SIV infection, in both AGMs and macaques, memory CD4+ T cells and T follicular helper (Tfh) cells in lymph nodes preferentially express HLA-E. After SIV infection in macaques, the frequency of HLA-E+ memory CD4+ T cells and the expression intensity of HLA-E on CD4+ T cells increases; these changes are particularly apparent in Tfh cells.101 AGMs efficiently control viral replication in SLT, and this viral control is mediated predominantly by NK cells capable of trafficking within lymph node follicles,102 as well as the expansion in SLT of terminally differentiated NKG2Alow NK cells, characterized by increased HLA-E-restricted cytotoxicity in response to SIV Env peptides. Conversely, SIV-infected macaques generally show no viral control in SLT, and NK cell terminal differentiation is blocked, resulting in a predominance of less differentiated, IFNγ-producing NK cells with decreased HLA-E-restricted cytotoxicity in response to SIV Env peptides.101,103 By providing insights into an effective NK cell response during a controlled viral infection in lymph nodes, these findings offer key guidance for optimizing NK cell–based immunotherapies to improve HIV-1 control.
To counteract the Vpr-dependent upregulation of NKG2D ligands,79,80 HIV-1 Nef protein reduces surface expression of MICA and ULBPs, dampening NKG2D-mediated killing of infected cells.104 HIV-1 accessory proteins, likewise, block the expression on infected cells of ligands important for enhancing NK cell activation, including those that bind to the NKRs DNAM-1 and NTB-A.105,106 The NCRdull phenotype characteristic of chronic HIV-1 infection,21,88 together with defective NKG2D-mediated killing104 and an incomplete pattern of activation,107 negatively impacts NK cell function and immune surveillance, allowing for HIV-1 disease progression and development of opportunistic infections and malignancies.81,85,108 Furthermore, matrix metalloproteinases (MMPs), which are highly expressed in chronic HIV-1 infection,109 cleave NKG2D ligands from the surface of infected CD4+ T cells, resulting in an accumulation of the soluble form of NKG2D ligands in the plasma of viremic HIV-1-infected individuals.110,111 This proteolytic activity allows HIV-1-infected cells to evade NKG2D-mediated antiviral responses both by decreasing surface expression of NKG2D ligands on infected cells and by inactivating NKG2D on circulating effector cells via the soluble ligands110,112 (Fig. 2). MMPs also release CD16 from the surface of NK cells, consequently limiting HIV-1-specific ADCC activity.113,114 Moreover, HIV-1-induced IL-10 promotes aberrant elimination of immature DCs (iDCs) by NK cells but renders mDCs more susceptible to NKG2D-mediated lysis, leading to the accumulation of poorly immunogenic iDCs in lymph nodes of infected individuals.115 This, again, interferes with the development of an effective T cell–centric immune response.
It is unclear whether the aforementioned NK cell phenotypic and functional abnormalities occur as a result of direct interactions between NK cells and HIV-1 or the establishment of chronic inflammation through persistent antigenic exposure. Fresh NK cells purified ex vivo from human peripheral blood mononuclear cells (PBMCs) express the HIV-1 chemokine coreceptors CXCR4 and CCR589,116; however, they lack surface expression of CD4,21,117 which translates into NK cells being unlikely targets of productive HIV-1 infection. Supporting this notion, CD4− NK cells purified ex vivo from the peripheral blood of HIV-1-infected individuals have been shown to not harbor HIV-1 proviral DNA, even in the case of active HIV-1 viremia.21 In a conflicting report, though, Valentin et al. have identified a subset of circulating NK cells that express CD4, as well as CCR5 and CXCR4, and that remain persistently infected in HIV-1+ individuals even after one to two years of suppressive antiretroviral therapy (ART). Based on these data, direct infection of NK cells may account, at least partially, for the NK cell dysfunction observed in HIV-1 infection, and infected NK cells may serve as an in vivo reservoir of HIV-1.116 This discrepancy could potentially be explained by differences in the activation status of the purified NK cells, as peripheral blood–derived NK cells, subjected to maximal activating conditions in vitro, acquire de novo expression of CD4 and become susceptible to HIV-1 infection.117,118 By contrast, NK cells freshly isolated from SLT express higher levels of CD4 on their surface relative to NK cells in the periphery,119 raising the possibility that NK cells residing in SLT are susceptible to HIV-1 infection. Regardless of the mechanism, NK cells do not escape unscathed and suffer debilitating deficiencies as a consequence of HIV-1 infection that impair both their direct and indirect antiviral effector functions.
2.3. Does ART rescue NK cell phenotype and function?
Following consistent viral suppression with effective ART, NK cells experience, to varying degrees, a recovery of phenotype and function. Just as chronic HIV-1 viremia induces the sequential loss of Siglec-7 and CD56, ART-mediated viral suppression gradually restores surface expression of these molecules. Within the first 18 months of therapy, NK cells regain expression of Siglec-7, resulting in a shift from Siglec-7−CD56− to Siglec-7+CD56− NK cells,83 and a complete recovery of CD56 expression occurs only after 24 months of suppressive ART.19,83 Normalization of the NKG2A/NKG2C ratio to values greater than one similarly requires a minimum of 24 months of suppressive ART. This phenomenon occurs despite enduring elevated frequencies of NKG2C+ NK cells, therefore reflecting a return of surface expression of NKG2A.91 Given their sensitivity to HIV-1 viremia, normalization of the NKG2A/NKG2C ratio and NK cell subset distribution have been proposed as biomarkers for gauging the effectiveness of chronic suppression of HIV-1 replication by ART.94 Importantly, expression of inhibitory and activating receptors on NK cells derived from aviremic HIV-1-infected individuals compares to that of uninfected donors, and this reversal of phenotypic abnormalities through ART-mediated viral suppression correlates with a recovery of NK cell cytolytic function.21 In addition to rescuing NK cell–mediated cytotoxicity against iDCs and tumor cell targets,21,24,120 control of HIV-1 viremia by ART improves ADCC activity, the extent of which corresponds to the timing of ART initiation, with the most pronounced responses observed in individuals beginning treatment either prior to seroconversion or their CD4+ T cell counts dipping below 350 cells/μl.121,122 NK cells, indeed, benefit phenotypically and functionally from ART-mediated viral suppression, but maximal restoration of NK cell effector functions also requires properly functioning DCs. As an example, since HIV-1 interferes with DC functional maturation,123,124 mDCs from viremic HIV-1-infected individuals fail to activate NK cells to secrete adequate amounts of IFNγ,24 accentuating the dependence of both cell types on each other for optimal activation and functionality.
Notwithstanding the significant benefits associated with adherence to ART regimens, including undetectable levels of plasma viremia, a reduction in AIDS-associated mortality, and an increase in circulating CD4+ T cells, aviremic HIV-1+ individuals show signs of residual immune dysfunction, particularly within the innate immune system.125–127 Whereas ART successfully reverses HIV-1-related activation of T cells and monocytes, NK cell activation persists, demonstrated by elevated proportions of CD38+HLA-DR+ NK cells and heightened spontaneous degranulation in virologically suppressed HIV-1-infected individuals.128,129 Although the duration of this effect and its impact on comorbid disease remain unknown,128 chronic immune activation is the hallmark of HIV-1 infection leading to premature aging and apoptosis of immune cells.130 It is highly plausible, therefore, that persistence of inflammation and NK cell activation contribute to a higher prevalence of comorbidities with an inflammatory etiology in treated HIV-1+ individuals; more specifically, markers of innate immune activation are associated with cardiovascular disease, non-AIDS cancers, and neurocognitive disorders.131–135 Additionally, ART enhances the terminal differentiation of CD56dimCD16+ NK cells, as evidenced by elevated expression of CD57.136 This increase in terminally differentiated NK cells is likely due to an expansion of human cytomegalovirus (HCMV)-driven NKG2C+ NK cells, which also co-express CD57.27,91 NK cells expressing CD57 represent a mature and stable subset with retained cytolytic capacity, whose frequency increases with age27,137,138; however, expression of CD57 also signifies terminal differentiation and less functional activity,87 correlating with abrogated proliferative and IFNγ responses to cytokine stimulation.137 These data indicate an enduring effect of HIV-1 infection on NK cells despite effective ART. The clinical consequences of this persistent NK cell dysfunction, including an acceleration toward terminal differentiation, are poorly understood.
3. Adaptive and memory-like features of NK cells
Although NK cells are restricted to the expression of germline-encoded receptors, the combinatorial expression patterns of activating and inhibitory receptors give rise to a diverse NK cell repertoire.139–141 Reprogramming events in response to local inflammatory milieu, as well as genetic and environmental factors, further contribute to their phenotypic and functional heterogeneity.68,139,141,142 Mounting evidence also indicates that cumulative pathogen exposures elicit dynamic shifts in the repertoire of NKRs that potentially impact the quality of NK cell responses to subsequent infections,140 with recent studies even challenging the notion that memory is uniquely confined to adaptive immunity.143 In fact, NK cells, defying their classification as innate lymphoid cells, exhibit features of traditional immunological memory, including antigen-specific clonal-like expansions, heritable cell-intrinsic modifications, and the generation of long-lived populations capable of heightened recall responses. As detailed in the sections to follow, these “memory” NK cells can develop in either a cytokine- or antigen-dependent manner.29–31,144–148
3.1. Cytokine-induced memory-like NK cells
While CD56dim NK cells are widely recognized for their cytolytic abilities, they also play a critical role as immune helper cells, providing innate alarm signals that shape and regulate the adaptive immune response. IL-18, in particular, has been shown to have a unique ability to induce a helper pathway of differentiation in the CD56dim population, promoting upregulation of CD25 and transient expression of CD83.149,150 IL-18 also programs CD56dim NK helper cells to express CCR7, allowing them to migrate in response to CCL21 and to home to SLT, where they are primed to immediately produce IFNγ upon subsequent exposure to secondary stimuli, including IL-12, IL-2, or IFNα.150 This suggests that IL-18-primed CD56dim NK helper cells act not only on DCs in the periphery but also migrate to lymph nodes to modulate adaptive immune responses in a manner similar to the CD56bright NK cell subset and CD4+ T helper cells.
Subsequent studies in mice and humans have described a similar NK cell population, arising following a brief in vitro exposure to IL-12, IL-15, and IL-18. Murine cytokine-induced memory-like NK cells are detectable up to three weeks post-transfer into naïve hosts; despite having a resting phenotype similar to that of naïve cells, they produce significantly more IFNγ upon restimulation with cytokines or tumor cell lines. Notably, these amplified responses are heritable across multiple generations, highlighting the ability of NK cells to retain an intrinsic memory of prior activation. By contrast, cytokine-induced memory-like NK cells do not demonstrate enhanced cytotoxic activity compared with control-treated NK cells.144 The programming of murine NK cells into a helper population with robust IFNγ production in the presence of IL-18 corroborates the finding in human NK cells that the helper pathway of differentiation is not associated with enhancement of cytolytic activity. Whereas IL-2 selectively promotes the cytotoxic effector functions of NK cells, IL-18-primed NK helper cells support DC-mediated induction of Th1 responses via IFNγ.150 Human NK cells display analogous functional properties, with cytokine exposure inducing a memory-like pool characterized by an enhanced ability to rapidly produce IFNγ in response to restimulation with cytokines or tumor targets. Phenotypically, cytokine-induced memory-like NK cells feature increased levels of NKG2A and NKp46, as well as weak expression of NKG2C145 (Fig. 3A).
Fig. 3.
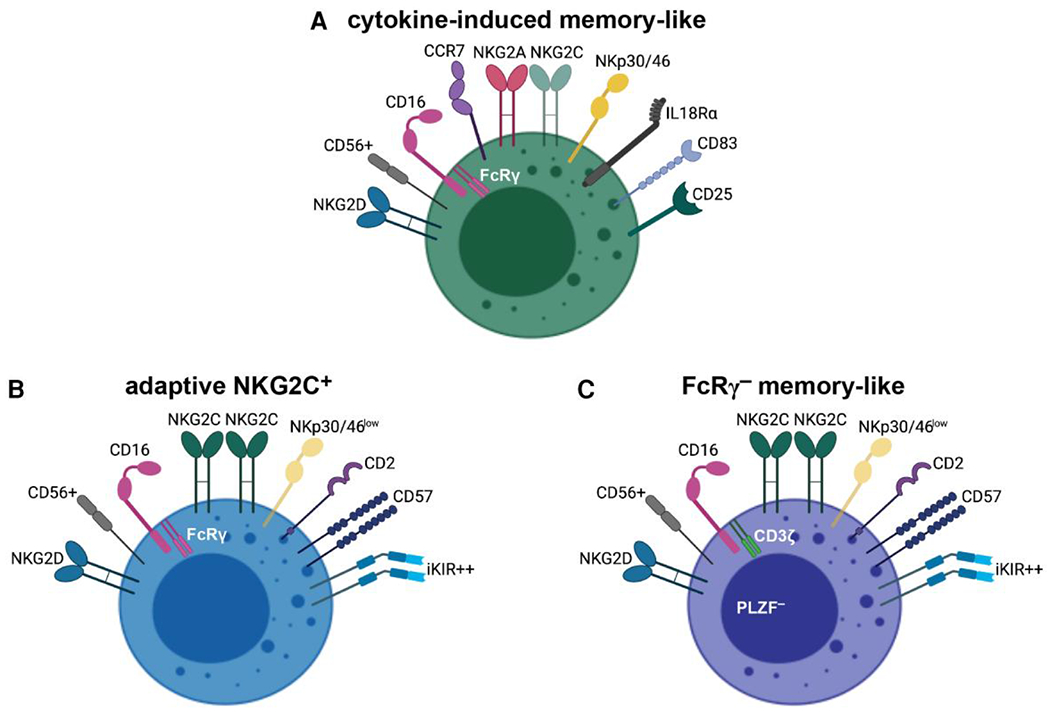
NK cell “memory” populations are phenotypically and functionally distinct. (A) Cytokine-induced memory-like NK cells, with high surface expression of cytokine receptors and CCR7, efficiently integrate immunostimulatory cytokines to drive their differentiation and subsequent recall responses. (B, C) Adaptive NKG2C+ and FcRγ− memory-like NK cells share numerous features, including high-density surface expression of CD57 and iKIRs and potent ADCC responses; however, initial programming and expansion of adaptive NKG2C+ NK cells result from interactions between NKG2C and specific HLA-E–peptide complexes (B). For FcRγ− NK cells, preferential expansion occurs upon encounter with HCMV-infected cells in the presence of HCMV-specific antibodies. They are notably deficient for FcRγ and the transcription factor PLZF and are not restricted by the expression of NKG2C (C). Epigenetic modifications underlie the unique attributes of “memory” observed in all three of these NK cell populations. Figure created with BioRender.com.
The mechanisms underlying the programming of memory-like NK cells through this combination of cytokines are complex and incompletely understood. However, epigenetic remodeling of the conserved noncoding sequence (CNS) at the IFNG locus, induced by in vitro cytokine priming, coincides with increased transcription of IFNG.151,152 Furthermore, IL-18 and IL-12 synergize to upregulate expression of CD25,153 resulting in high responsiveness to IL-2 receptor stimulation.149 IL-18, together with IL-15, also activates the mTORC1 pathway, which promotes glycolytic reprogramming and upregulation of glycolytic enzymes in NK cells,154,155 presumably supporting the energy demands of elevated production of IFNγ.156 Importantly, cytokine-induced memory-like responses can be distinguished from priming and arming based on response length, heritability, and activation status. Whereas priming and arming depend on direct signaling and conceptually represent events that occur just prior to NK cell effector functions, cytokine-induced memory-like responses are heritable and detectable weeks after initial activation. Additionally, cytokine-induced memory-like NK cells do not constitutively express high levels of activating receptors, granzyme B, or cytokines, suggesting priming and arming are the initial, complementary components of long-term memory-like responses.143
3.2. Adaptive NK cells in CMV
In the case of antigen-driven memory, cytomegalovirus (CMV) engineers a lasting imprint on NK cells, inducing the preferential expansion of a population with attributes of traditional immunological memory.146–148 Antigen-specific NK cells were first described in a murine model, whereby naïve NK cells expressing Ly49H clonally expand in response to the murine CMV (MCMV) antigen m157 and subsequently contract, forming a large pool of long-lived cells that reside in lymphoid and nonlymphoid organs for several months. In naïve neonatal hosts, adoptively transferred Ly49H+ NK cells confer a more efficient defense against subsequent infection, whereas adoptive transfer of the same quantity of naïve NK cells results in only 25% survival.148 Paralleling these findings, human NK cells adapt in response to HCMV infection and reactivation following solid organ and hematopoietic stem cell transplantation, resulting in the preferential expansion and accumulation of an NK cell subset marked by high-density surface expression of NKG2C.146,147,157–159 Adaptive NKG2C+ NK cells in HCMV-seropositive individuals display a skewed phenotype that includes elevated expression of CD57 and CD2, together with low expression of NKG2A.32,34,146,147,160,161 Moreover, the narrow expression patterns of otherwise stochastically distributed iKIRs suggest HCMV drives an oligoclonal or clonal-like expansion of NK cells32,34 (Fig. 3B; Table 1).
Table 1.
Phenotypic comparison of mature CD56dim, adaptive NKG2C+, and FcRγ− memory-like NK cells.
| Mature CD56dim | Adaptive NKG2C+ | FcRγ− memory-like | |
|---|---|---|---|
| CD56 | + | + | + |
| CD57 | + | ++ | ++ |
| PLZF | + | + | − |
| Activating Receptors | |||
| CD16 | ++ | ++ | + |
| NKp30 | + | low | low |
| NKp46 | + | low | low |
| NKG2C | +/− | ++ | ++a |
| NKG2D | + | + | + |
| Inhibitory Receptors | |||
| NKG2A | − | − | − |
| KIR | + | ++ | ++ |
| Siglec-7 | + | − | − |
| Co-stimulatory | |||
| Molecules | |||
| CD2 | +/− | + | + |
| CD7 | + | low | low |
| Signaling Molecules | |||
| FcRγ | + | + | − |
| CD3ζ | + | + | + |
| Syk | + | + | − |
| DAB2 | + | + | − |
| EAT-2 | + | − | − |
When present on FcRγ− NK cells, NKG2C is expressed at a high surface density but is not a defining feature of this memory-like population.
Belonging to the same C-type lectin family as NKG2A, NKG2C likewise forms a heterodimer with CD94 but conversely signals through the ITAM-bearing adaptor protein DAP12, thereby functioning in a stimulatory capacity.162,163 NKG2A and NKG2C share ligand specificity for HLA-E,62,64,65 whose cell-surface stabilization requires bound peptide derived from host proteins, including leader sequences of classical MHC class I molecules and the heat-shock protein HSP60.63,164 These stabilizing nonameric peptides share conserved amino acid residues at positions two and nine,63 facilitating their loading within HLA-E molecules. By contrast, subtle changes in the HLA-E peptide ligands, particularly substitutions at positions five and eight, influence the binding affinities of NKG2A and NKG2C.67,165–170 As the inhibitory receptor, though, NKG2A generally displays a higher affinity for HLA-E.66,67 Accordingly, HCMV stabilizes expression of HLA-E by providing UL40-derived peptides that mimic HLA leader sequences, thereby protecting infected cells against lysis by NKG2A-expressing NK cells despite a downregulation of classical MHC class I molecules.171–174 Adaptive NK cells, typically lacking NKG2A, circumvent this evasion strategy, preferentially expanding in response to HCMV infection through NKG2C.160 Beyond interacting with HLA-E to drive the expansion of an adaptive subset,175,176 NKG2C demonstrates exquisite peptide specificity, with adaptive NK cells differentially recognizing HCMV strains. Interestingly, UL40 sequence variations, specifically a single amino acid substitution at position eight, control the degree of activation and proliferation of NKG2C+ NK cells37,160 (e.g. peptides with a low potency elicit suboptimal activation of adaptive NK cells and rely on co-stimulatory signaling via CD2 to trigger polyfunctional responses). Furthermore, the combination of IL-18 and IL-12 synergizes with high potency peptides to drive the accumulation and differentiation of adaptive NKG2C+ NK cells from HCMV-seronegative donors.160 NKG2C, in other words, is not merely a defining feature of this population; rather, its interactions with HLA-E–peptide complexes are critical for the function and expansion of adaptive NK cells.
A defining component of immunological memory is the ability to mount a quantitatively and/or qualitatively greater recall response.177 In this regard, the adaptive NK cell subset is characterized by superior effector functions in response to antibody-dependent and NKG2C-mediated signaling.32,34,37,147,178 Aside from persisting over time,32,158 NKG2C+ NK cells expand in vivo following clinical HCMV reactivation and demonstrate an increased capacity for target cell–induced cytokine production. Furthermore, NKG2C+ NK cells transplanted from HCMV-seropositive donors show more potent functional activity following a secondary HCMV event compared to NKG2C+ NK cells from HCMV-seronegative donors,147,157,158 indicative of secondary effector responses upon antigen re-exposure. By contrast, adaptive NKG2C+ NK cells respond poorly to stimulation with IL-18 and IL-12 alone,32–34 but they are capable of integrating IL-18 during target cell encounter.33 Moreover, IL-18 and IL-12 work in concert with specific HCMV peptides to strengthen the establishment of an adaptive NK cell population.160
Epigenetic imprinting underlies the adaptive features of NK cells, including reprogramming of the receptor repertoire and enhanced effector responses. Specifically, an open configuration at the IFNG CNS1, mirroring that of CD4+ Th1 and memory CD8+ T cells, promotes stable IFNγ competence.151,160 NKG2C+ NK cells also exhibit metabolic hallmarks of lymphocyte memory, including increased oxidative mitochondrial respiration, mitochondrial membrane potential, and spare respiratory capacity, as well as higher expression of genes related to the electron transport chain. These metabolic alterations involve epigenetic modifications and poise adaptive NK cells for survival and robust recall responses.179 In a further parallel to T cells, a three-signal mechanism—NKG2C engagement with specific HLA-E–peptide complexes, CD2 co-stimulation, and proinflammatory signaling via IL-18 and IL-12—is necessary to drive maximal expansion and functional activity of adaptive NKG2C+ NK cells through broad imprinting of the transcriptional landscape.151,160,161,180 Peptide recognition is crucial, though, as proinflammatory cytokines alone do not lead to the development of adaptive NKG2C+ NK cells, but instead to the generation of cytokine-induced memory-like NK cells,145,160 underscoring the potential for discrete, yet complementary, mechanisms to contribute to the differentiation of “memory” NK cell populations.
3.3. FcRγ− memory-like NK cells
Another subpopulation strongly associated with HCMV seropositivity requires antibodies to grant antigen specificity. These CD56dim memory-like NK cells are notably deficient in the adaptor protein FcRγ (FcRγ−), which is otherwise known as FcϵRIγ.30,31 FcRγ is an intracellular signaling protein that associates with NKp46, NKp30, and CD16 as a homodimer or as a heterodimer with CD3ζ. Phosphorylation of the ITAMs leads to the recruitment of Zap70 or Syk, which prompts a downstream signaling cascade, resulting ultimately in cytokine secretion, cytotoxicity, and/or calcium release.181 All mature NK cells were once believed to constitutively express FcRγ and CD3ζ,182 but a distinct subset of CD56dim NK cells deficient for FcRγ expression is readily detectable in approximately one-third of healthy individuals, correlating with HCMV seropositivity.30,31 FcRγ− NK cells are stably maintained, albeit at highly variable frequencies between individuals that range from 3% to 85% of the CD56dim population,30 through promoter DNA hypermethylation that silences FcRγ mRNA and protein expression.30,35,36 By contrast, they express CD3ζ at normal levels.30,35 The phenotypic profile of FcRγ− NK cells markedly resembles that of adaptive NK cells, notably increased levels of CD57, CD2, and iKIRs, with reduced NKG2A expression31,35,36; however, although expressed at high frequencies, NKG2C is not a defining feature of this subset.31,36 In comparison with conventional (CD56dimFcRγ+) NK cells, the FcRγ− population differs in the expression of FcRγ-associated receptors, including NKp30, NKp46, and CD16. FcRγ deficiency appears to dramatically affect cell-surface levels of the NCRs while having only a limited effect on CD16 expression, suggesting that FcRγ is required for the expression of both NKp30 and NKp46. By contrast, CD16 depends less on FcRγ, as its cell-surface expression is supported, to an extent, by CD3ζ.30
Epigenetic modifications further shape the FcRγ− population. On one hand, promoter DNA hypermethylation is associated with additional deficiencies for the signaling molecules Syk, DAB2, and EAT-2, as well as the transcription factor PLZF (Fig. 3C; Table 1), and reduced expression of this transcription factor is linked to minimal cytokine responsiveness in FcRγ− NK cells.35,36 On the other hand, FcRγ− NK cells display robust effector functions, particularly cytokine production, in response to CD16 engagement, including HCMV-infected cells in the presence of HCMV-specific antibodies.30,31,36 The enhanced functional capabilities of this memory-like subset compared with conventional NK cells can be explained by exclusive association of CD16 with CD3ζ in the absence of FcRγ, presumably delivering a stronger signal given that CD3ζ bears three ITAMs, whereas FcRγ contains only one such domain,30,182 and by hypomethylation of the IFNG and TNF regulatory regions. Interestingly, genome-wide DNA methylation patterns are strikingly similar between memory-like NK cells and CTLs but differ from those of conventional mature NK cells.35 Additionally, FcRγ− NK cells undergo preferential expansion upon encounter with HCMV-infected cells in the presence, but not in the absence, of seropositive plasma, and these expanded FcRγ− NK cells maintain superior CD16 responsiveness.36 Based on these data, Lee et al. proposed a model whereby HCMV infection directs stochastic epigenetic modifications, leading to FcRγ deficiency, with this particular pool of memory-like NK cells further selected and preferentially expanded in an antibody-dependent manner during HCMV reactivation.36 Although FcRγ− NK cells are a memory-like effector population specialized for antibody-dependent reactivity, their precise role in controlling HCMV reactivation or heterologous infections is not clearly defined.
Adaptive and memory-like NK cells relate in that they persist as stable populations and demonstrate enhanced recall responses due to remarkable remodeling of the epigenetic landscape. Furthermore, their intrinsic qualities are heritable across generations.35,36,151,152,160 “Memory” NK cell subsets are, however, distinct in phenotype and function. For example, each population is characterized by a differential degree of responsiveness to IL-18. The reduced ability of adaptive NKG2C+ and FcRγ− memory-like NK cells to produce IFNγ following cytokine stimulation suggests a level of distinction from cytokine-induced memory-like NK cells,33,35 for whom IL-18 is a key component of their identity.145,150 In spite of this, IL-18 does optimize the expansion and functionality of adaptive NKG2C+ NK cells.160 Moreover, Syk deficiency is largely confined to FcRγ−, but not NKG2C+, NK cells, and the FcRγ− population displays superior antibody-dependent responsiveness, regardless of NKG2C expression, compared with their conventional NK cell counterparts.36 These data reveal key differences between adaptive NKG2C+ and FcRγ− memory-like NK cells. Taken together, multiple pathways contribute to the differentiation and accumulation of distinct subsets of “memory” NK cells, and a deeper understanding of these mechanisms will unlock enormous opportunities to exploit the intrinsic features of adaptive and memory-like NK cells for future immunotherapies.
4. NK cell “memory” in HIV-1
4.1. Properties of NK cell “memory” populations in HIV-1
Antigen specificity is not exclusive to CMV, as NK cells from mice and nonhuman primates have been documented to demonstrate antigen-driven memory responses to HIV-1. In the first of such studies, following transfer into naïve Rag2−/−Il2rg−/− mice, liver NK cells from Rag1−/− mice immunized with viral-like particles containing HIV antigens mediate recall responses that are antigen-specific, occurring only in recipients challenged with HIV antigens, but not other viral antigens.183 More recently, Nikzad et al. discovered that human NK cells, isolated from livers of humanized mice previously vaccinated with HIV-encoded envelope protein, display vaccination-dependent, antigen-specific memory responses,184 both of which are hallmarks of adaptive immunity.185,186 Building on the concept of antigen specificity in the context of HIV, splenic and hepatic NK cells from rhesus macaques infected with or vaccinated against SIV and simian HIV (SHIV) specifically lyse Gag- and Env-pulsed DCs in an NKG2A/NKG2C-dependent fashion. Furthermore, vaccination elicits antigen-specific “memory” NK cell responses that are both durable and potent, with NK cells from vaccinated macaques efficiently killing only the antigen-matched targets five years post-vaccination.187 These data, especially, point to the possibility of inducing long-lived, antigen-specific NK cell “memory” in humans after infection and vaccination. In fact, cytotoxic NK cells with a tissue-resident phenotype infiltrate sites of varicella-zoster virus (VZV) skin test antigen challenge in VZV-experienced human volunteers decades after initial VZV exposure.184 Identification of human “memory” NK cells specific for HIV-1, together with a detailed understanding of their molecular signature, may help to inform efforts to develop preventative and therapeutic vaccines against HIV-1.
Despite known direct interactions between NK cells and HIV-1 peptides,96 clear evidence for HIV-1-specific NK cells in humans is lacking; nevertheless, adaptive NKG2C+ and FcRγ− memory-like NK cells are present at elevated frequencies in HIV-1-infected individuals.25–29 Loss of NKG2A+ NK cells in the setting of chronic HIV-1 viremia is accompanied by a dramatic expansion of NKG2C+ NK cells, whose frequency remains elevated even after prolonged ART-mediated viral suppression.91,188 The expansion of NKG2C+ NK cells occurs independently of HIV-1 viral load,188 and their inflated presence appears to positively contribute to viral dynamics during primary HIV-1 infection and early responses to ART.25 Among newly infected HIV-1+ individuals, a higher frequency of NKG2C+CD57+ NK cells correlates with lower viral set point and immune activation, along with better responses to ART, as those with high frequencies of NKG2C+CD57+ NK cells more rapidly reach undetectable levels of HIV-1 viremia.25,189 Furthermore, homozygous deletion of the NKG2C gene is associated with increased risk for HIV-1 infection and disease progression.190,191 Conversely, during antiviral responses, NK cell repertoire diversity increases, resulting in terminal differentiation, i.e. expression of CD57, and existing high repertoire diversity has been associated with increased risk for HIV-1 acquisition in African women with a high HIV-1 exposure risk.140 This suggests that diversity within the human NKR repertoire represents reduced plasticity to new challenging pathogens.
An expanded population of FcRγ-deficient NK cells has also been identified in viremic HIV-1+ individuals, and this population persists following virologic suppression with ART.28,29,128,129 In agreement with the findings in the context of HCMV seropositivity, FcRγ− NK cells in HIV-1+ individuals display altered phenotypic and functional properties, including superior ADCC activity and drastically reduced expression of NKG2A, NKp30, and NKp46.29 Using a rhesus macaque model, it has also been demonstrated that FcRγ− NK cells are distributed systemically but are inclined to migrate to mucosal sites.192 The response potential of FcRγ− NK cells in different phases of HIV-1 infection has yet to be thoroughly evaluated, but their enhanced ADCC activity and preferential homing to mucosal sites have potentially important implications for designing strategies aimed at generating immune responses that mediate early control of HIV-1 infection.
4.2. The confounding effect of HCMV
Given the high prevalence of HCMV seropositivity among HIV-1+ individuals (>90%), the profound skewing and adaptation of the NK cell repertoire following HIV-1 infection are likely confounded by co-infection with HCMV.29,128,193,194 In fact, changes in NKG2C expression on NK cells among HIV-1+ individuals are related to concomitant comorbidity with HCMV rather than HIV-1 infection alone, as inclusion of HCMV serological status in a multivariate regression model abolishes the positive correlation between high levels of NKG2C+ NK cells and HIV-1 infection.26 Subsequent reports have confirmed that HCMV co-infection is responsible for the expansion of NKG2C+ NK cells during the course of HIV-1 infection, with the proportion of NKG2C+CD57+ NK cells being extremely low or undetectable in HCMV-seronegative individuals, regardless of their HIV-1 status.27,91 Moreover, in HIV-1 infection, an inverse relationship exists between the extent of NKG2C+CD57+ NK cell expansion and the fraction of HCMV-specific CD8+ T cells expressing CD28.27 Loss of CD28 expression on T cells signifies effector memory status and progression toward senescence, which is thought to be driven by persistent exposure to antigen.195–197 This relationship may, in fact, reflect the common cumulative effect of chronic HCMV infection and periodic reactivation on responding NK and T cells in the setting of HIV-1 infection.27 Although the expansion of NKG2C+ NK cells has also been observed in response to other viral infections, including hepatitis B, hepatitis C, chikungunya, and hantavirus, this phenomenon is largely restricted to HCMV-seropositive individuals,34,198,199 suggesting infection with other viruses may reactivate HCMV from latency and trigger the proliferation of pre-existing NKG2C+ NK cells. However, as there is no evidence of HCMV reactivation during primary HIV-1 infection among HCMV-seropositive individuals,25 HCMV-primed NK cell subsets appear to expand in response to secondary viral infection alone.
On a related note, the enhanced antibody-dependent effector functions of FcRγ− NK cells are not limited to HCMV. Cultures with HSV-1- and influenza-infected target cells and virus-specific antibodies produce similarly superior responses,31,36 indicating that while these memory-like NK cells require initial programming by HCMV, their specialization for antibody-dependent reactivity is, importantly, not restricted to a particular pathogen, as antigen specificity is conferred through the antibody. Despite elevated frequencies of the FcRγ− subset in HIV-1 infection, reports suggest that HCMV exposure has a greater impact on inducing this population.29,128 Moreover, HCMV/HIV-1 co-infection is associated with higher HCMV antibody titers,29,128,194 and the proportion of FcRγ− NK cells correlates with levels of CXCL10.29 It is plausible, then, that the expansion of FcRγ− NK cells is inflated in HIV-1+ individuals as a result of ongoing immune activation and higher infectious burdens, including HCMV. The expanded NKG2C+ and FcRγ− NK cell “memory” populations found in HIV-1+ individuals share phenotypic and functional properties with those identified in association with HCMV infection, but they have not been characterized nearly as extensively, including comprehensive analyses of their transcriptional signatures and epigenetic modifications. Therefore, open questions remain related to the mechanism by which HIV-1 contributes to the expansion of “memory” NK cells, their role in protecting against HIV-1 acquisition and disease progression, and the clinical implications of their inflated expansions in chronic HIV-1 infection. Increased knowledge of the specialized nature of “memory” NK cells will provide opportunities for the creation of novel HIV-1 therapeutic modalities.
5. Harnessing the unique features of NK cells for a functional cure
Although effective at suppressing HIV-1 viremia, antiretrovirals as a combination therapy do not eradicate the virus. Consequently, a major barrier to curing HIV-1 is persistence of the latent viral reservoir in long-lived resting CD4+ T cells, which serve as a source of plasma viral rebound following treatment interruption. Successful management of HIV-1 as a chronic infection, therefore, requires lifelong adherence to a daily ART regimen, which has potential associations with long-term toxicity.200–205 Moreover, regardless of treatment, HCMV lingers as a significant cofactor in HIV-1 disease progression,206–208 strongly correlating with systemic inflammation,209–211 reduced immune resilience,212 and immune senescence,27,209,212 and individuals infected with HIV-1 continue to experience an increased burden of comorbidities such as cardiovascular disease and cancer.134,135,211,213 For these reasons, the development of therapeutic strategies aimed at combating HIV-1 and simultaneously improving long-term health outcomes remains a global priority. While interventions capable of achieving a sterilizing cure represent the ideal, the challenges associated with total elimination of replication-competent virus have prompted a shift in focus to designing therapeutic strategies that effect a functional cure, whereby the virus is not eradicated but rather silenced to undetectable levels in the absence of ongoing ART.214,215 One approach, referred to as the shock and kill or kick and kill, centers on inducing HIV-1 latency reversal during ART to expose infected cells, rendering them susceptible to immune-mediated clearance. However, latency reversal is not sufficient, as administration of latency-reversing agents increases cellular HIV-1 RNA but fails to decrease reservoir size.216–218 Furthermore, the CTL responses of ART-treated, HIV-1-infected individuals are incapable of efficiently clearing infected cells that exit latency due to deficiencies and/or dysfunction of HIV-1-specific CTLs,218,219 as well as the presence of CTL escape mutants in latent viral genomes.220 These shortcomings highlight the need to optimize the kill arm of the kick and kill strategy. NK cells are an untapped resource with enormous potential for boosting HIV-1 therapeutic modalities, given their importance in antiviral immunity and their ability to circumvent the limitations inherent to T cell approaches. In fact, with their distinct features and heightened response potentials, “memory” NK cell populations may be ideal candidates for immunotherapies to improve HIV-1 control.
5.1. Cytokine stimulation
As the efficacy of NK cell immunotherapy is dependent on the dose of NK cells administered or reached after infusion through in vivo expansion, large-scale NK cell expansion, whether autologous or allogeneic, is required to achieve a therapeutic effect in a patient. To achieve such high expansion rates, NK cells have traditionally been co-cultured with irradiated feeder cells, commonly K562, in the presence of IL-2 and IL-15.221 Recently, though, genetically engineered feeder cells have been developed—such as NKF, which overexpress membrane-bound IL-21, and K562 cells co-expressing membrane-bound IL-15 and 4-1BBL (K562-mb15-41BBL)—inducing robust expansion and sustained proliferation of highly cytotoxic NK cells.222,223 Further efforts to optimize clinical-scale production of NK cells center on the formation of plasma membrane (PM) vesicles from either K562-mb15-41BBL (PM15) or K562-mb21-41BBL (PM21) cells, with the latter resulting in an exponential NK cell expansion of 100 000-fold by day 28 of cultures initiated with PBMCs and PM21 particles.224,225 Because the success of NK cell–based immunotherapies hinges on efficient production of mass quantities of NK cells, methods for optimizing their expansion will continue to be of crucial importance.
In addition to being a component of the cocktail to induce NK cells into a memory-like phenotype,144,145 IL-15 alone is a powerful cytokine for NK cell development, proliferation, homeostasis, and antiviral immunity.226 ALT-803, also known as N-803, is a fusion complex of IL-15 and IL-15Rα with an IgG1 Fc region that demonstrates enhanced IL-15 biological activity.227,228 With ALT-803 able to augment both NK and T cell responses in patients with relapsed hematologic malignances,229 significant interest has been generated for utilizing this complex in SIV/HIV infection. ALT-803 has similarly been shown to drive virus-specific CD8+ T and NK cells to B cell follicles in macaques230,231 and to enhance NK cell–mediated killing of HIV-1-infected cells ex vivo,232 highlighting the synergizing effects of IL-15 on the innate and adaptive arms of immunity. Indeed, recent completion of a phase I clinical trial evaluating ALT-803 in ART-suppressed HIV-1+ individuals (NCT02191098) demonstrated that its administration is associated with the proliferation and/or activation of T and NK cells, as well as a significant decrease in the frequency of PBMCs with an inducible HIV-1 provirus, persisting for up to 6 months after therapy.233 In pathogenic SIV and HIV-1 infection, NK cells are inefficiently recruited to lymph nodes and germinal centers, where the virus preferentially replicates and persists, instead exhibiting a bias toward homing to intestinal mucosa.98,102,192 By contrast, accumulation of NK cells in lymph node follicles through the expression of CXCR5 is associated with the presence of high levels of IL-15 and represents a key parameter of viral control in the nonpathogenic AGM model of SIV infection.102 Interestingly, in vitro treatment of cells from SHIV-infected rhesus macaques with IL-12 and IL-15/IL-15Rα enhances the generation and proliferation of highly functional CXCR5+ NK cells.234 On a related note, sequential IL-21 and IFNα therapy in ART-treated, SIV-infected rhesus macaques promotes terminal differentiation of NK cells with the same NKG2a/clowCD16+ phenotype observed in the AGM model of nonpathogenic SIV infection.101,103 The frequency and activity of this NK cell population correlate with a reduction of replication competent SIV in lymph nodes during ART and time to viral rebound following analytical treatment interruption.103 The ability of cytokine therapy to generate CXCR5+ and NKG2a/clowCD16+ NK cells during pathogenic infection in macaques has important implications for HIV cure strategies due to the potential of these populations to infiltrate B cell follicles and target the viral reservoir.
5.2. Release of inhibition
Allogeneic haplo-mismatched stem cell transplantation studies in patients with acute myeloid leukemia (AML) have provided the first line of evidence for the clinical relevance of KIR inhibition, with mismatches between KIRs on donor NK cells and recipient MHC class I molecules promoting NK cell activation and correlating with improved relapse-free and overall survival. These results not only suggested that, in the absence of iKIR interactions, NK cells mediate more effective responses against leukemia,235 but also sparked the development of monoclonal antibodies (mAbs) to sterically block KIR-HLA interactions. The anti-KIR mAb IPH2101 binds with high affinity to KIR2D receptors and augments NK cell-mediated elimination of autologous HLA-C–expressing leukemic cells in vitro in a dose-dependent manner.236 Targeting of mAbs against iNKRs also represents a strategy for improving anti-HIV-1 responses of NK cells. Although most primary isolates of HIV-1 are capable of inducing HLA-C downmodulation,47 the functional potency of HLA-C–licensed NK cells depends on the extent to which HLA-C is downregulated, and residual KIR-mediated inhibitory signaling is associated with reduced antiviral activity.48 Since the variability in viral-mediated downregulation of cell surface HLA-C impacts NK cell antiviral activity, IPH2101 may prove especially relevant in HIV-1-infected individuals with KIR2DL+ NK cells—essentially mimicking a KIR-HLA mismatch and improving the elimination of infected cells.
Heightened HLA-E levels, as a consequence of elevated HLA-A expression, similarly impair HIV-1 control through inhibition of NKG2A-expressing cells.100 Furthermore, CD56bright and cytokine-induced memory-like NK cells, in contrast to adaptive NKG2C+ and FcRγ− memory-like NK cells, express NKG2A, as well as CCR7—facilitating their migration to lymph nodes,72–74,145,150 where cells targeted by SIV and HIV-1 preferentially increase expression of HLA-E101—suggesting therapeutic benefit from blockade of NKG2A. Monalizumab, a mAb that binds to NKG2A to prevent inhibitory signaling, has already been shown to improve NK cell cytotoxicity and viral clearance in both mice and patients with chronic hepatitis B infection.237 The potential also exists for mAbs against NKG2A and KIR to boost CTL responses in a complementary fashion.238,239 Therefore, therapeutic blockade of NKG2A and iKIRs could improve HIV-1 control by enhancing both CTL and NK cell antiviral activity. However, given the potential for blockade of iNKRs to lower the activation threshold of NK cells against normal cells expressing low levels of stimulatory ligands, significant concerns have been raised related to autoreactivity and by-stander killing of activated uninfected CD4+ T cells. In spite of this, IPH2101 and lirilumab, a recombinant anti-KIR mAb that recognizes the same epitope as IPH2101,240 have demonstrated good clinical tolerability in hematological malignancies and solid tumors.240–242 In vitro experiments and in vivo tumor rejection models further indicate that full KIR occupancy is necessary for optimal enhancement of NK cell activity, but prolonged KIR inhibition may negatively impact NK cell education/licensing,240 a process that involves the acquisition of functional competence through interactions between KIRs and their cognate ligands.243–246 As such, a dosing schedule that supports transient, rather than continuous, full KIR occupancy may allow for optimal clinical effectiveness without impeding the development of new, fully competent NK cells.
Asde from NKG2A and iKIRs, NK cell activity is dampened through other regulatory receptors, including PD-1 and Siglec-9. Similar to T lymphocytes,247–249 NK cells can develop an exhausted phenotype, characterized by reduced proliferative capacity and functional activity, during chronic viral infections such as HIV-1.250–253 However, as immune checkpoint inhibition, including selective blockade of PD-1, can reinvigorate antigen-specific CD8+ T cells in the setting of cancer and HIV-1,254–257 this strategy holds promise for helping to also restore the functional potency of NK cells. Indeed, PD-1 and IL-10 blockade enhances cytokine secretion, degranulation, and killing capacity of NK cells via restored CD4+ T cell function in both chronically infected ART-naïve and ART-suppressed HIV-1+ individuals.258 Analogous to the PD-1 checkpoint on activated CD8+ T cells, Siglec-9 functions as a glyco-immune negative checkpoint on a subset of CD56dim NK cells.259–261 Despite being highly cytotoxic against HIV-1-infected targets, the Siglec-9+CD56dim NK cell population is restrained by the inhibitory effects of Siglec-9; however, blocking Siglec-9 enhances their cytotoxicity against infected cells.262 Together, these studies suggest potential for immune checkpoint inhibition to promote HIV-1 control by maximizing the functional capacity of NK cells, including their cooperation with other immune cells.
5.3. Toll-like receptor (TLR) agonists and NK cells
Belonging to the family of pattern recognition receptors, TLRs are potent enhancers of innate antiviral immunity and efficiently activate NK cells,263 highlighting the potential application of TLR agonists to enhance the effector functions of NK cells for HIV-1 immunotherapies. The selective TLR7 agonist GS-9620 potently inhibits HIV-1 replication in human PBMCs at a step coincident with or prior to the early stages of reverse transcription, with IFNα playing a dominant role.264 Triggering of TLR8 or TLR7/8 similarly interferes with HIV-1 replication after virus-cell fusion but before integration, and this anti-HIV-1 response is attributed to NK and CD8+ T cells.265,266 Moreover, a study involving HIV-1 serodiscordant couples showed that TLR3 activation stimulates more pronounced polyfunctional responses in NK cells from exposed seronegative individuals compared with unexposed controls.267 Combined knowledge of the natural mechanisms promoting resistance to HIV-1 infection, along with the tools available to inhibit acute HIV-1 infection, has important clinical implications for developing preventative strategies.
In addition to blocking HIV-1 replication, TLR agonists support antiviral immunity and function as latency-reversing agents. Ex vivo studies have demonstrated that GS-9620 and the TLR9 agonist MGN1703 activate HIV-1 from latency, thereby enhancing viral transcription in PBMCs from HIV-1+ individuals on suppressive ART.268,269 MGN1703 also induces strong innate immune responses, including copious production of IFNα, and boosts NK cell–mediated suppression of HIV-1 infection in autologous CD4+ T cells,269 presumably through increased expression of NKp46.16 Confirming these data, treatment of virally suppressed HIV-1-infected individuals with TLR9 agonists increases HIV-1 transcription, enhances activation of cytotoxic NK cells,270 and reduces the HIV-1 proviral reservoir.271 It is conceivable, based on these ex vivo and in vivo studies, that TLR agonists pose as a dual threat in the kick and kill strategy, reactivating expression of the latent HIV-1 reservoir while simultaneously supporting the elimination of newly exposed, latently infected cells via stimulation of effector NK cells.
An important challenge related to the kick and kill strategy as a therapeutic intervention is the identification of latency-reversing agents that induce HIV-1 expression without causing serious adverse events or compromising the effector functions of cells targeting activated reservoirs. While PKC agonists such as bryostatin are characterized by stronger latency-reversing activity in comparison with TLR agonists,268,272,273 bryostatin is highly toxic and has led to serious adverse events in phase II oncology clinical trials.274–276 Furthermore, some HDAC inhibitors have been shown to reduce the killing capacity of NK cells277 and to negatively impact antigen-specific CD8+ T cell functions, with transient exposure inducing the selective death of activated T cells.276,278,279 In contrast, TLR agonists have proven their safety and tolerability in clinical trials,280–283 making them an appealing alternative not only due to their safety profile but also for their ability to improve the killing of HIV-1-infected cells.
5.4. CAR-engineered NK cells
Since the seminal work by Medawar and colleagues,284 adoptive cell therapy has emerged as a powerful treatment for advanced cancers resistant to conventional agents. More specifically, CAR-engineered T cells have produced unprecedented clinical results in patients receiving autologous CD19-directed T cells for the treatment of relapsed or refractory B cell malignancies.285–292 CAR T cells are engineered to express an extracellular single-chain variable fragment (scFv) antibody, coupled to an intracellular signaling domain (e.g. CD3ζ) and one or more co-stimulatory domains (e.g. CD28 or 4-1BB), thus conferring antigen specificity in an MHC-independent fashion.293–295 Despite their resounding successes, CAR T cells have several limitations, including the feasibility and cost associated with generating clinically relevant doses of autologous product from heavily pretreated lymphopenic patients and the risk of prolonged toxic effects.291,296–299 On the other hand, NK cells offer an attractive alternative to T cells for CAR engineering. Allogeneic NK cells, for one, show little to no risk of inducing graft-versus-host disease, allowing for scalability and a low-cost, off-the-shelf cellular immunotherapeutic agent.300–302 Additionally, the administration of CAR NK cells is not associated with the development of neurotoxicity or cytokine release syndrome,303 and CAR NK cells retain their intrinsic capacity to recognize and kill target cells through their germline-encoded receptors, reducing the risk of immune escape even if acquired mutations and alternative splicing were to render targets resistant to the engineered CAR.298,304 Therefore, CAR-engineered NK cells are a promising immunotherapeutic tool in the HIV-1 field. To direct the selective targeting of HIV-1-infected CD4+ T cells, a CAR NK cell was created by fusing CD4 to CD3ζ, thereby enabling binding of HIV-1 gp120 through CD4 and signaling through CD3ζ. Although these CD4ζCAR NK cells effectively inhibit HIV-1 replication in vitro, they fail to enhance suppression of HIV-1 infection in vivo,305–307 with a potential explanation being lack of adequate co-stimulation.308 Indeed, including the co-stimulatory molecule 4-1BB in the chimeric anti-CD19–CD3ζ receptor markedly enhances NK cell–mediated killing of leukemic cells, and the cytotoxicity of NK cells expressing this construct uniformly exceeds that of NK cells lacking 4-1BB.309 Incorporation of DAP12—a signaling adaptor protein that associates with the activating NKRs NKG2C and KIR3DS1163,310—as opposed to CD3ζ, also improves the cytolytic activity of CAR NK cells targeting the prostate stem cell antigen, and the specific cytotoxicity of these CAR NK cells is further enhanced by KIR-HLA mismatches.311 More recently, Lim et al. have developed a universal CAR NK cell that recognizes 2,4-dinitrophenyl (DNP) and can subsequently be redirected to target various epitopes of HIV-1 gp160 (a complex between gp120 and gp41) using DNP-conjugated antibodies as adaptor molecules.296 One of the limitations of their universal approach, though, is that a small proportion (up to 1%) of naturally occurring human antibodies recognize DNP,312,313 which can compete with anti-DNP CAR NK cells in binding to the DNP-conjugated adaptor molecules. A possibility for addressing this problem is the design of higher affinity anti-DNP CARs to strengthen their interactions with DNP-conjugated adaptor molecules.296 Based on their findings, future research is warranted to validate the in vivo efficacy and toxicity of their construct and to optimize the potency of universal CAR NK cells through co-stimulatory and/or NK cell–specific signaling domains, including the advantageous KIR3DS1/DAP12 complex.41,43,44,310
5.5. Bi-specific and tri-specific killer cell engagers (BiKEs and TriKEs)
Another approach for improving NK cell functionality is through the use of immunomodulators termed BiKEs and TriKEs.314 These small molecules consist of a scFv from the heavy and light variable chains of an antibody, which is connected via a short, flexible polypeptide linker to another scFv or single-domain antibody of a differing specificity.315–317 One component is directed against an antigen expressed on the surface of the target cell of interest, and the other engages CD16 on NK cells, thus inducing NK cell–mediated directed cytotoxicity against the target cell through the creation of a synapse between the two cell types.315–320 Although BiKEs circumvent previous challenges associated with NK cell–based immunotherapies, including lack of specificity,314 TriKE molecules were later developed to also ensure maximal NK cell activation and survival in vivo by accommodating an IL-15 moiety as a functional linker between two scFv segments.316 As an example, a BiKE against CD16 and CD33, termed 1633 BiKE, was initially created to promote NK cell engagement with CD33+ AML tumor targets,318,320 and subsequent integration of a novel modified human IL-15 crosslinker led to the generation of the 161533 TriKE. In comparison with the 1633 BiKE, the 161533 TriKE mediates superior NK cell cytotoxicity against CD33+ targets and primary AML blasts and promotes enhanced antitumor function in immunodeficient mice by improving the in vivo expansion and persistence of human NK cells.316 Initial findings from a phase I/II clinical trial (NCT03214666) indicate that the 161533 TriKE (GTB-3550) drives robust NK cell proliferation without causing adverse toxicity, suggesting the design of the TriKE encourages direct delivery of IL-15 to CD16+ NK cells.321 To date, BiKEs and TriKEs have been created against CD19/CD22 on B cell non-Hodgkin’s lymphomas315,322; CD33 and CLEC12A on AML, myelodysplastic syndrome, and neoplastic mast cells316,320,323–325; and EpCAM, CD133, and B7-H3 on various solid tumor types.319,326–330 in the context of HIV-1 therapies, Li et al. have reported a BiKE consisting of CD16A-binding human antibody domains fused through a linker to an engineered one-domain soluble human CD4 that mediates specific, activating interactions with CD16 to effectively kill primary HIV-1-infected T cells.331 Importantly, the relatively small size of this anti-HIV-1 BiKE facilitates superior biodistribution, including diffusion within lymphoid tissue, where the majority of HIV-1 replication occurs.331–333 Although BiKEs and TriKEs show incredible promise as therapeutic interventions, their in vivo efficacy in HIV-1+ individuals remains to be determined.
5.6. Broadly neutralizing antibodies (bNAbs)
The natural development of therapeutically effective anti-HIV-1 bNAbs occurs in a fraction of HIV-1-infected individuals after years of infection and viral diversification.334–338 Although first-generation antibodies were safe and well tolerated, they showed limited breadth and activity, producing little, if any, measurable effects in viremic individuals and often leading to the rapid emergence of resistant viral variants.339–341 Advances in single cell cloning technologies have allowed for the isolation, expansion, and characterization of more potent next-generation bNAbs,342–347 which have gained prominence recently in HIV-1 research aiming to elicit a functional cure for their capacity to inhibit viral entry, block viral cell-cell transmission, and suppress HIV-1 replication,348–351 as well as their potential to promote sustained virologic remission in the absence of ART.352–360 These bNAbs bind to relatively conserved regions on the HIV-1 Env trimer, including the CD4 binding site, the V1/V2 and V3 glycan-dependent loops, the membrane-proximal external region, the Env silent face, the gp41-gp120 interface, and the fusion domain.336,338,345,361 The most commonly used bNAbs to treat humanized mice, macaques, and human patients belong to the classes targeting the CD4 binding site (VRC01362 and 3BNC117363) and the V3 glycan-dependent loop (PGT121364 and 10-1074365). Overall, bNAb monotherapy leads to transient suppression of viremia, followed by viral rebound reaching pretreatment levels and the emergence of antibody-resistant viral strains.354,356,366–369 There is one notable exception: in PGT121-treated, SHIV SF162P3-infected macaques, mutations conferring resistance were not detected, and suppression of viremia lasted 35 to greater than 100 days, with three of the 18 treated animals experiencing no viral rebound.355 In contrast to monotherapy, combination bNAb treatment promotes a longer duration of virus suppression and antibody-resistant populations emerge less frequently and generally only to one component of the administered bNAb mixture.352–354,366,370 To date, combination bNAb treatment studies have primarily been conducted in humanized mouse and macaque models340; however, analogous to the current standard of care with ART in HIV-1-infected individuals, combination bNAb therapy, as opposed to monotherapy, will likely be required for complete viremic control and to prevent the emergence of resistant viral strains.
One key advantage of bNAbs over ART is their ability to engage host immune effector pathways, such as binding to cell-free virions to expedite clearance and prevent their entry into target cells,348,371 forming immune complexes that activate DCs for priming of T cells,340 and attaching to HIV-1 Env on the surface of infected cells to promote killing via ADCC.372,373 In particular, passive immunization with bNAbs capable of inducing NK cell–mediated ADCC is garnering widespread interest due, in part, to the protective effect mediated by increased ADCC activity in the RV144 human vaccine trial.12 Not only do antibodies promote in vitro lysis of infected cells by Fc receptor (FcR)–mediated mechanisms,373–376 but their interactions with FcRs have also proven essential in HIV-1 prevention and therapy in vivo,372,377–379 with bNAbs decreasing viral rebound from latent reservoirs and accelerating clearance of infected cells in humanized mice in an FcR-dependent manner.352,380 Indeed, significantly higher HIV-1 viremia in the absence of FcR engagement demonstrates that the therapeutic efficacy of bNAbs highly depends on the binding of the Fc domain of bNAbs to FcγRs in immune effector cells.377 Additionally, in acutely treated, SHIV SF162P3-infected rhesus macaques, co-administration of PGT121 and the TLR7 agonist GS-9620 delays viral rebound following ART discontinuation, which correlates with NK cell activation. These findings suggest that the TLR agonist-bNAb combination enables NK cells to target the viral reservoir via antibody-mediated elimination of infected CD4+ T cells, prolonging the duration of virologic suppression in the absence of ART381 (Table 2). Not all bNAbs are equal, though, in their ability to bind to heterogeneous Env epitopes from different HIV-1 strains, to induce signaling through FcγRIIIA (CD16), or to kill infected cells via ADCC.373 However, alterations to the variable and Fc domains can be employed to optimize the functionality of bNAbs, with the potential to promote highly efficacious responses upon passive administration to humans. As enhanced in vivo potency of anti-HIV-1 bNAbs is associated with preferential engagement of activating FcγRs, Fc domain–engineered bNAb variants with selective binding capacity for activating FcγRs can be created to augment their in vivo protective effect.377 It is also possible to enhance FcR binding, thereby improving antiviral activity, by shifting the Fc domain of bNAbs from a global antibody-glycosylation profile toward agalactosylated glycoforms.376 Furthermore, combining Fc domain modifications with structure-based rational design may allow for heightened Fc effector function, as well as increased potency and breadth to different viral epitopes.382
Table 2.
Select bNAb studies with implications for NK cell–mediated ADCC.
| Class | bNAb | Population/Setting | Notes | Ref. |
|---|---|---|---|---|
| Various | Various | Human primary cells and cell lines (in vitro) | Identification of a subset of bNAbs that bind and kill HIV-1-infected cells through NK cell engagement. | 373 |
| CD4bs | b12 | Human primary cells and cell lines (in vitro) | Variants with increased affinity for FcγRIIIa show an increase in NK cell activation and the ability to mediate ADCC. | 374 |
| CD4bs | A32 | Human primary cells and cell lines (in vitro) | A32 potently mediates ADCC. An A32 Fab fragment blocks the majority of ADCC-mediating Ab activity in plasma of HIV-1+ individuals. | 375 |
| N/A | Abs purified from PWH | Human primary cells (in vitro) | Agalactosylated Abs are associated with enhanced FcR binding and Fc-mediated reduction of viral replication. | 376 |
| CD4bs V3 loop V2 loop |
3BNC117 10-1074 PG16 |
Humanized mouse model (in vivo) | bNAb tri-mix interferes with the establishment of a silent reservoir by Fc-FcR mediated mechanisms. bNAbs and a combination of viral inducers synergize to decrease the reservoir. | 352 |
| CD4bs V3 loop V2 loop |
3BNC117 10-1074 PG16 |
Humanized mouse model (in vivo) | Fc domain-engineered bNAb variants with selective binding capacity for activating FcγRs display augmented protective activity. | 377 |
| CD4bs V3 loop |
3BNC117 10-1074 |
Humanized mouse model (in vivo) | bNAbs accelerate the clearance of HIV-1-infected cells by a mechanism requiring FcγR engagement. | 380 |
| CD4bs | b12 | Rhesus macaques (in vivo) | In the absence of FcγR binding, protection against SHIV challenge is dramatically decreased. | 378 |
| CD4bs | VRC01 | Rhesus macaques (in vivo); human primary cells and cell lines (in vitro) | Variant with enhanced neonatal FcR binding improves protection against SHIV infection (in vivo) while retaining FcγRIIIa binding and ADCC activity (in vitro, human cells). | 372 |
| V3 loop | PGT121 | Rhesus macaques (in vivo) | Co-administration of PGT121 and a TLR7 agonist delays viral rebound following ART discontinuation. This delayed viral rebound correlates with NK cell activation. | 381 |
| CD4bs | 3BNC117 | Humans (in vivo) | A single infusion reduces the viral load in HIV-1+ individuals and viremia remains significantly reduced for 28 days. | 356 |
| CD4bs V3 loop |
3BNC117 10-1074 |
Humans (in vivo) | bNAbs maintain viral suppression and may accelerate the rate of reservoir decay, with changes in the size and composition of the intact proviral reservoir. | 358 |
| CD4bs V3 loop |
3BNC117 10-1074 |
Humans (in vivo) | bNAbs provide complete and sustained virological suppression in the absence of ART. | 359 |
| CD4bs | 3BNC117 | Humans (in vivo) | The combination of 3BNC117 with a LRA does not delay viral rebound during ATI. Latency reversal with a single agent and modulation of HIV-1-specific immunity by a single bNAb is insufficient to achieve remission. | 360 |
Ab, antibody; ATI, analytic treatment interruption; CD4bs, CD4 binding site; FcR, Fc receptor; LRA, latency-reversing agent; N/A, not applicable; PWH, people with HIV; Ref, reference.
On a final note, early bNAb therapy following mucosal SHIV challenge in a macaque model has been implicated in the generation of CD8+ T cell responses that mediate long-term control of viremia in the absence of ART, but the mechanism underlying this immune-mediated control is unclear.353 To date, no study has identified NK cells as the definitive link between bNAbs and durable CTL responses, as the aforementioned studies have merely focused on the classical killing effector function of NK cells.352,373,380,381 It is plausible, though, that NK cells utilize antibodies not only to kill infected cells via ADCC but also to enhance the function of DCs, leading to highly functional CTLs. Therefore, we sought to demonstrate the importance of this reciprocal crosstalk for the induction of durable cytotoxic T cell responses. NK cells from virally suppressed HIV-1-infected men, indeed, mediated superior ADCC against rituximab-opsonized Raji cells, with increasing ratios of effector to target cells corresponding with more robust cytolytic activity (Fig. 4A). Additionally, rituximab-opsonized Raji target cells synergized with the plasmacytoid DC-associated cytokine IFNα to promote NK cell helper activity through the production of IFNγ (Fig. 4B), resulting in the development of mDCs with a heightened ability to produce IL-12p70 (Fig. 4C). Importantly, DCs matured in the presence of NK cells generated highly functional HIV-1-specific cytotoxic T cells, as determined by IFNγ ELISPOT responses (Fig. 4D). These data demonstrate the capacity of NK cells to enhance DC-mediated immune responses to HIV-1 via antibody and, thus, highlight the potential for harnessing the reciprocal crosstalk between NK cells and DCs in the design of novel anti-HIV-1 bNAb-based therapies (Fig. 5).
Fig. 4.
NK cell enhancement of DC-mediated cellular immunity to HIV-1 is driven by antibody. (A) NK cells from HIV-1+ participants mediated superior ADCC against rituximab-opsonized Raji cells as determined by flow cytometry following a 6 h co-culture; n = 5 HIV-1+ and n = 5 HIV-1− participants. (B)NK cells from HIV-1+ participants required a second signal to produce IFNγ in the presence of rituximab-opsonized Raji target cells; n = 6 HIV-1+ participants. (C) Day-4 iDCs were cultured for 48 h either with opsonized Rajis + IFNα in the presence of autologous NK cells (1:1; NK-DC), only with opsonized Rajis + IFNα (DC0), or in the absence of NK cells and Rajis + IFNα (iDC). Two-signal activated NK cells induced the development of high IL-12-producing DCs compared with iDCs and DCs matured in the absence of NK cells (DC0); n = 6 HIV-1+ participants. (D)NK cells enhanced DC-mediated induction of cellular immunity to HIV-1 as demonstrated by CD8+ T cell IFNγ responses to HIV-1-derived 9mer peptide pools (Supplementary Table S1); n = 2 HIV-1+ participants from four independent experiments. Statistical significance was determined by the unpaired Student’s t-test (A), one-way ANOVA (B, C), or the paired Student’s t-test (D) (****P 0.0001; **P<0.01; *P<0.05). Extended methods available in the Online Supplemental Material.
Fig. 5.
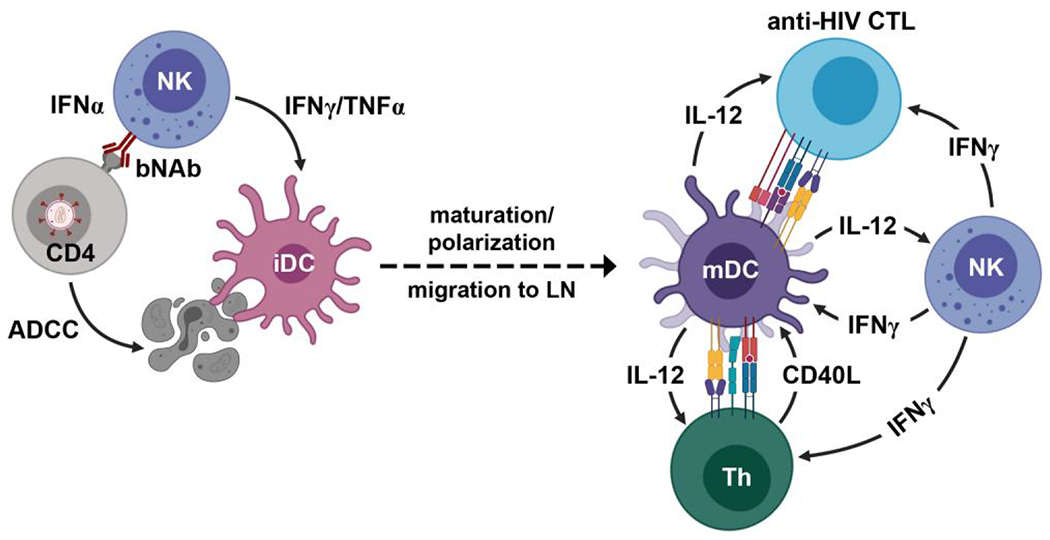
Schematic depicting the mechanism of NK cell help in DC-mediated adaptive immunity to HIV-1. bNAbs support NK cell-mediated killing of an HIV-1-infected CD4+ T cell via ADCC. IFNα synergizes with antibody-dependent signaling to activate the helper function of NK cells, promoting DC maturation and polarization. The DC, carrying environmental cues from the periphery, produces IL-12 in response to CD40L stimulation by a CD4+ T helper cell, and IL-12 acts with NK cell-derived IFNγ to drive cellular-mediated immunity to HIV-1. Figure created with BioRender.com.
6. Concluding remarks
The importance of NK cells in anti-HIV-1 immunity has already been widely established; specifically, ADCC activity has been implicated in both vaccine-induced protection from acquisition of infection and phenotypes of viral control.12–15 However, HIV-1 infection disrupts the delicate balance of the innate and adaptive arms of the immune system, and NK cells are particularly sensitive to these immunologic perturbations, resulting in pathologic subset redistribution, a skewed repertoire, and maintenance of an activated phenotype despite undetectable levels of plasma viremia.21,81,92,94,128,129 Furthermore, the presence of expanded populations of FcRγ− memory-like NK cells in treated chronic HIV-1 infection negatively impacts the quality of immune help delivered to DCs by NK cells in response to innate cytokine stimulation. This functional shortcoming of FcRγ− NK cells is counteracted, though, by their superior responsiveness to antibody-mediated signaling,383 a quality that can potentially be harnessed toward the goal of HIV cure given the growing interest in and success of bNAb therapies (Table 2). Notably, our preliminary results highlight that NK cells utilize antibodies not only to kill target cells but also to activate their helper function, resulting in the polarization of DCs with a heightened ability to induce highly functional CTLs (Fig. 4). Combining our findings with the knowledge that bNAbs lead to durable suppression of viremia via CTL-mediated mechanisms,353 we propose a novel HIV functional cure strategy that marries proviral reactivation and bNAb administration, with the latter engaging NK cells, namely the FcRγ− memory-like population, to enhance their antibody-mediated effector and helper functions. That is, NK cells will boost the kill component of the kick and kill approach directly by eliminating cells harboring reactivated latent HIV-1 via ADCC and indirectly by promoting highly effective DC-mediated cellular immunity to HIV-1 (Fig. 5).
Supplementary Material
Acknowledgments
The authors express their sincerest gratitude to the Multicenter AIDS Cohort Study (MACS), now the MACS/WIHS Combined Cohort Study (MWCCS), Principal Investigators Dr. Charles R. Rinaldo and Dr. Jeremy Martinson, William G. Buchanan, and the participants of the Pittsburgh site of the MWCCS for their tireless dedication in the fight against HIV/AIDS.
Funding
Data in this article were collected by the MWCCS, which is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). MWCCS Pittsburgh Principal Investigators: Dr. Charles R. Rinaldo and Dr. Jeremy Martinson (U01-HL146208); MWCCS Data Analysis and Coordination Center: Gypsyamber D’Souza, Stephen Gange, and Elizabeth Topper (U01-HL146193). This work was also supported by NIH/National Institute of Allergy and Infectious Diseases (NIAID) Grants R01-RAI152655A (RBM), R01-AI167711-01 (RBM), and T32-AI065380-14 (RRA), as well as CRDF Global Grant 65333 (RBM). RBM and RRA were supported through the American Association of Immunologists Careers in Immunology Fellowship Program. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the funding sources.
Abbreviations:
- ADCC
antibody dependent cellular cytotoxicity
- AGM
African green monkey
- AML
acute myeloid leukemia
- ART
antiretroviral therapy
- BiKE
bi-specific killer cell engager
- bNAb
broadly neutralizing antibody
- CAR
chimeric antigen receptor
- CMV
cytomegalovirus
- CNS
conserved noncoding sequence
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DNP
2,4-dinitrophenyl
- FcR
Fc receptor
- FMO
fluorescence minus one
- HCMV
human cytomegalovirus
- iDC
immature dendritic cell
- iKIR
inhibitory KIR
- iNKR
inhibitory NK receptor
- mAb
monoclonal antibody
- MCMV
murine cytomegalovirus
- mDC
mature dendritic cell
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase
- MWCCS
MACS/WIHS Combined Cohort Study
- NCR
natural cytotoxicity receptor
- NK
natural killer
- NKR
NK receptor
- PBL
peripheral blood lymphocyte
- PBMC
peripheral blood mononuclear cell
- PM
plasma membrane
- rh
recombinant human
- scFv
single-chain variable fragment
- SFU
spot forming unit
- SHIV
simian HIV
- SLT
secondary lymphoid tissue
- Tfh
T follicular helper
- TLR
toll-like receptor
- TriKE
tri-specific killer cell engager
- ULBP
UL16 binding protein
- VZV
varicella-zoster virus
Footnotes
Supplementary material
Supplementary material is available at Journal of Leukocyte Biology online.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975:16(2):230–239. 10.1002/ijc.2910160205 [DOI] [PubMed] [Google Scholar]
- 2.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975:5(2):117–121. 10.1002/eji.1830050209 [DOI] [PubMed] [Google Scholar]
- 3.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983:131(3):1531–1538. 10.4049/jimmunol.131.3.1531 [DOI] [PubMed] [Google Scholar]
- 4.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008:9(5):503–510. 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 5.Lanier LL, Phillips JH, Hackett J Jr, Tutt M, Kumar V. Natural killer cells: definition of a cell type rather than a function. J Immunol. 1986;137(9):2735–2739. 10.4049/jimmunol.137.9.2735 [DOI] [PubMed] [Google Scholar]
- 6.Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med. 1971:134(6):1513–1528. 10.1084/jem.134.6.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljunggren HG, Karre K. In search of the “missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990:11(7):237–244. 10.1016/0167-5699(90)90097-S [DOI] [PubMed] [Google Scholar]
- 8.Murphy WJ, Kumar V, Bennett M. Rejection of bone marrow allografts by mice with severe combined immune deficiency (SCID). Evidence that natural killer cells can mediate the specificity of marrow graft rejection. J Exp Med. 1987:165(4):1212–1217. 10.1084/jem.165.4.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanier LL. Face off: the interplay between activating and inhibitory immune receptors. Curr Opin Immunol. 2001:13(3):326–331. 10.1016/S0952-7915(00)00222-3 [DOI] [PubMed] [Google Scholar]
- 10.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004:306(5701):1517–1519. 10.1126/science.1103478 [DOI] [PubMed] [Google Scholar]
- 11.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006:6(7):520–531. 10.1038/nri1863 [DOI] [PubMed] [Google Scholar]
- 12.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012:366(14):1275–1286. 10.1056/NEJMoa1113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001:75(15):6953–6961. 10.1128/JVI.75.15.6953-6961.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wren LH, Chung AW, Isitman G, Kelleher AD, Parsons MS, Amin J, Cooper DA, Stratov I, Navis M, Kent SJ. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology. 2013:138(2):116–123. 10.1111/imm.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambotte O, Ferrari G, Moog C, Yates NL, Liao H-X, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009:23(8):897–906. 10.1097/QAD.0b013e328329f97d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomescu C, Mavilio D, Montaner LJ. Lysis of HIV-1-infected autologous CD4+primary T cells by interferon-alpha-activated NK cells requires NKp46 and NKG2D. AIDS. 2015:29(14):1767–1773. 10.1097/QAD.0000000000000777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, Storkus WJ, Kalinski P. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003:171(5):2366–2373. 10.4049/jimmunol.171.5.2366 [DOI] [PubMed] [Google Scholar]
- 18.Wong JL, Mailliard RB, Moschos SJ, Edington H, Lotze MT, Kirkwood JM, Kalinski P. Helper activity of natural killer cells during the dendritic cell-mediated induction of melanoma-specific cytotoxic T cells. J Immunother. 2011:34(3):270–278. 10.1097/CJI.0b013e31820b370b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, O MA, Kinter A, Kovacs C, Moretta A, et al. Characterization of CD56−/CD16+natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005:102(8):2886–2891. 10.1073/pnas.0409872102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H, Johnston MN, Staller KD, Zaman MT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005:106(10):3366–3369. 10.1182/blood-2005-03-1100 [DOI] [PubMed] [Google Scholar]
- 21.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, Marcenaro E, Bottino C, Moretta L, Moretta A, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003:100(25):15011–15016. 10.1073/pnas.2336091100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott-Algara D, Vuillier F, Cayota A, Dighiero G. Natural killer (NK) cell activity during HIV infection: a decrease in NK activity is observed at the clonal level and is not restored after in vitro long-term culture of NK cells. Clin Exp Immunol. 1992:90(2):181–187. 10.1111/j.1365-2249.1992.tb07925.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, Kovacs C, Follmann D, Pende D, Ward J, et al. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 2008:4(7):e1000101. 10.1371/journal.ppat.1000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavilio D, Lombardo G, Kinter A, Fogli M, La Sala A, Ortolano S, Farschi A, Follmann D, Gregg R, Kovacs C, et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006:203-(10):2339–2350. 10.1084/jem.20060894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gondois-Rey F, Cheret A, Granjeaud S, Mallet F, Bidaut G, Lécuroux C, Ploquin M, Müller-Trutwin M, Rouzioux C, Avettand-Fenoël V, et al. NKG2C(+) memory-like NK cells contribute to the control of HIV viremia during primary infection: Optiprim-ANRS 147. Clin Transl Immunol. 2017:6(7):e150. 10.1038/cti.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guma M, Cabrera C, Erkizia I, Bofill M, Clotet B, Ruiz L, López-Botet M. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006:194(1):38–41. 10.1086/504719 [DOI] [PubMed] [Google Scholar]
- 27.Heath J, Newhook N, Comeau E, Gallant M, Fudge N, Grant M. NKG2C(+)CD57(+) natural killer cell expansion parallels cytomegalovirus-specific CD8(+) T cell evolution towards senescence. J Immunol Res. 2016:2016:7470124 . 10.1155/2016/7470124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leeansyah E, Zhou J, Paukovics G, Lewin SR, Crowe SM, Jaworowski A. Decreased NK cell FcRgamma in HIV-1 infected individuals receiving combination antiretroviral therapy: a cross sectional study. PLoS One. 2010:5(3):e9643. 10.1371/journal.pone.0009643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Amran FS, Kramski M, Angelovich TA, Elliott J, Hearps AC, Price P, Jaworowski A. An NK cell population lacking FcRgamma is expanded in chronically infected HIV patients. J Immunol. 2015:194(10):4688–4697. 10.4049/jimmunol.1402448 [DOI] [PubMed] [Google Scholar]
- 30.Hwang I, Zhang T, Scott JM, Kim AR, Lee T, Kakarla T, Kim A, Sunwoo JB, Kim S. Identification of human NK cells that are deficient for signaling adaptor FcRgamma and specialized for antibody-dependent immune functions. Int Immunol. 2012:24-(12):793–802. 10.1093/intimm/dxs080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol. 2013:190(4):1402–1406. 10.4049/jimmunol.1203034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Björklund AT, Retière C, Sverremark-Ekström E, Traherne J, Ljungman P. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013:121(14):2678–2688. 10.1182/blood-2012-10-459545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammer Q, Ruckert T, Dunst J, Romagnani C. Adaptive natural killer cells integrate interleukin-18 during target-cell encounter. Front Immunol. 2017:8:1976. 10.3389/fimmu.2017.01976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debré P, et al. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012:42(2):447–457. 10.1002/eji.201141826 [DOI] [PubMed] [Google Scholar]
- 35.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SCC, Foley B, Mattsson K, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015:42(3):443–456. 10.1016/j.immuni.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Nitschke L, Kim M, Scott JM, Kamimura Y, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015:42(3):431–442. 10.1016/j.immuni.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolle A, Meyer M, Calderazzo S, Jager D, Momburg F. Distinct HLA-E peptide complexes modify antibody-driven effector functions of adaptive NK cells. Cell Rep. 2018:24(8):1967–1976.e4. 10.1016/j.celrep.2018.07.069 [DOI] [PubMed] [Google Scholar]
- 38.Anderko RR, Rinaldo CR, Mailliard RB. IL-18 defines exclusive “memory-like” NK cell populations. J Immunol. 2019:202(1 Supplement):76.8. 10.4049/jimmunol.202.Supp.76.8 [DOI] [Google Scholar]
- 39.Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, Bruneau J, Routy J-P, Tsoukas CM, Bernard NF. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS. 2008:22(12):1487–1491. 10.1097/QAD.0b013e3282ffde7e [DOI] [PubMed] [Google Scholar]
- 40.Boulet S, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS. 2008:22(5):595–599. 10.1097/QAD.0b013e3282f56b23 [DOI] [PubMed] [Google Scholar]
- 41.Martin MP, Gao X, Lee J-H, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002:31(4):429–434. 10.1038/ng934 [DOI] [PubMed] [Google Scholar]
- 42.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, Colombo S, Brown EE, Shupert WL, Phair J, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007:39(6):733–740. 10.1038/ng2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, Streeck H, Waring M, Meier A, Brander C, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007:204(12):3027–3036. 10.1084/jem.20070695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long BR, Ndhlovu LC, Oksenberg JR, Lanier LL, Hecht FM, Nixon DF, Barbour JD. Conferral of enhanced natural killer cell function by KIR3DS1 in early human immunodeficiency virus type 1 infection. J Virol. 2008:82(10):4785–4792. 10.1128/JVI.02449-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004:104(7):2087–2094. 10.1182/blood-2004-02-0696 [DOI] [PubMed] [Google Scholar]
- 46.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999:10(6):661–671. 10.1016/S1074-7613(00)80065-5 [DOI] [PubMed] [Google Scholar]
- 47.Apps R, Del Prete GQ, Chatterjee P, Lara A, Brumme ZL, Brockman MA, Neil S, Pickering S, Schneider DK, Piechocka-Trocha A, et al. HIV-1 Vpu mediates HLA-C downregulation. Cell Host Microbe. 2016:19(5):686–695. 10.1016/j.chom.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korner C, Simoneau CR, Schommers P, Granoff M, Ziegler M, Hölzemer A, Lunemann S, Chukwukelu J, Corleis B, Naranbhai V, et al. HIV-1-mediated downmodulation of HLA-C impacts target cell recognition and antiviral activity of NK cells. Cell Host Microbe. 2017:22(1):111–119.e4. 10.1016/j.chom.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiani Z, Dupuy FP, Bruneau J, Lebouche B, Retiere C, Geraghty DE, Bernard NF. The education of NK cells determines their responsiveness to autologous HIV-infected CD4 T cells. J Virol. 2019:93(23):e01185–19. 10.1128/JVI.01185-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carr WH, Pando MJ, Parham P. KIR3DL1 polymorphisms that affect NK cell inhibition by HLA-Bw4 ligand. J Immunol. 2005:175(8):5222–5229. 10.4049/jimmunol.175.8.5222 [DOI] [PubMed] [Google Scholar]
- 51.Hilton HG, Guethlein LA, Goyos A, Nemat-Gorgani N, Bushnell DA, Norman PJ, Parham P. Polymorphic HLA-C receptors balance the functional characteristics of KIR haplotypes. J Immunol. 2015:195(7):3160–3170. 10.4049/jimmunol.1501358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moesta AK, Norman PJ, Yawata M, Yawata N, Gleimer M, Parham P. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol. 2008:180(6):3969–3979. 10.4049/jimmunol.180.6.3969 [DOI] [PubMed] [Google Scholar]
- 53.Yawata M, Yawata N, Draghi M, Little AM, Partheniou F, Parham P. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med. 2006:203(3):633–645. 10.1084/jem.20051884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med. 1995:181(3):1133–1144. 10.1084/jem.181.3.1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saunders PM, Vivian JP, Baschuk N, Beddoe T, Widjaja J, O’Connor GM, Hitchen C, Pymm P, Andrews DM, Gras S, et al. The interaction of KIR3DL1*001 with HLA class I molecules is dependent upon molecular microarchitecture within the Bw4 epitope. J Immunol. 2015:194(2):781–789. 10.4049/jimmunol.1402542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boudreau JE, Mulrooney TJ, Le Luduec JB, Barker E, Hsu KC. KIR3DL1 and HLA-B density and binding calibrate NK education and response to HIV. J Immunol. 2016:196(8):3398–3410. 10.4049/jimmunol.1502469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Connor GM, Guinan KJ, Cunningham RT, Middleton D, Parham P, Gardiner CM. Functional polymorphism of the KIR3DL1/S1 receptor on human NK cells. J Immunol. 2007:178(1):235–241. 10.4049/jimmunol.178.1.235 [DOI] [PubMed] [Google Scholar]
- 58.O’Connor GM, Vivian JP, Widjaja JM, Bridgeman JS, Gostick E, Lafont BA, Anderson SK, Price DA, Brooks AG, Rossjohn J, et al. Mutational and structural analysis of KIR3DL1 reveals a lineage-defining allotypic dimorphism that impacts both HLA and peptide sensitivity. J Immunol. 2014:192(6):2875–2884. 10.4049/jimmunol.1303142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colonna M, Borsellino G, Falco M, Ferrara GB, Strominger JL. HLA-C is the inhibitory ligand that determines dominant resistance to lysis by NK1- and NK2-specific natural killer cells. Proc Natl Acad Sci U S A. 1993:90(24):12000–12004. 10.1073/pnas.90.24.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Z, Kuroki K, Kuse N, Sun X, Akahoshi T, Qi Y, Chikata T, Naruto T, Koyanagi M, Murakoshi H, et al. HIV-1 control by NK cells via reduced interaction between KIR2DL2 and HLA-C(*) 12:02/C(*)14:03. Cell Rep. 2016:17(9):2210–20. 10.1016/j.celrep.2016.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Stigt Thans T, Akko JI, Niehrs A, Garcia-Beltran WF, Richert L, Stürzel CM, Ford CT, Li H, Ochsenbauer C, Kappes JC, et al. Primary HIV-1 strains use Nef To downmodulate HLA-E surface expression. J Virol. 2019:93(20):e00719–19. 10.1128/JVI.00719-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998:187(5):813–818. 10.1084/jem.187.5.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braud V, Jones EY, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol. 1997:27(5):1164–1169. 10.1002/eji.1830270517 [DOI] [PubMed] [Google Scholar]
- 64.Braud VM, Allan DS, O’Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998:391(6669):795–799. 10.1038/35869 [DOI] [PubMed] [Google Scholar]
- 65.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty DE. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A. 1998:95(9):5199–5204. 10.1073/pnas.95.9.5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaiser BK, Barahmand-Pour F, Paulsene W, Medley S, Geraghty DE, Strong RK. Interactions between NKG2x immunoreceptors and HLA-E ligands display overlapping affinities and thermodynamics. J Immunol. 2005:174(5):2878–2884. 10.4049/jimmunol.174.5.2878 [DOI] [PubMed] [Google Scholar]
- 67.Vales-Gomez M, Reyburn HT, Erskine RA, Lopez-Botet M, Strominger JL. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J. 1999:18(15):4250–4260. 10.1093/emboj/18.15.4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horowitz A, Djaoud Z, Nemat-Gorgani N, Blokhuis J, Hilton HG, Béziat V, Malmberg K-J, Norman PJ, Guethlein LA, Parham P. Class I HLA haplotypes form two schools that educate NK cells in different ways. Sci Immunol. 2016:1(3):eaag1672. 10.1126/sciimmunol.aag1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol. 1998:160(10):4951–4960. 10.4049/jimmunol.160.10.4951 [DOI] [PubMed] [Google Scholar]
- 70.Davis ZB, Cogswell A, Scott H, Mertsching A, Boucau J, Wambua D, Le Gall S, Planelles V, Campbell KS, Barker E. A conserved HIV-1-derived peptide presented by HLA-E renders infected T-cells highly susceptible to attack by NKG2A/CD94-bearing natural killer cells. PLoS Pathog. 2016:12(2):e1005421. 10.1371/journal.ppat.1005421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lisovsky I, Isitman G, Song R, DaFonseca S, Tremblay-McLean A, Lebouche B, Routy J-P, Bruneau J, Bernard NF. A higher frequency of NKG2A+ than of NKG2A− NK cells responds to autologous HIV-infected CD4 cells irrespective of whether or not they coexpress KIR3DL1. J Virol. 2015:89(19):9909–9919. 10.1128/JVI.01546-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001:166(11):6477–6482. 10.4049/jimmunol.166.11.6477 [DOI] [PubMed] [Google Scholar]
- 73.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001:22(11):633–640. 10.1016/S1471-4906(01)02060-9 [DOI] [PubMed] [Google Scholar]
- 74.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003:101(8):3052–3057. 10.1182/blood-2002-09-2876 [DOI] [PubMed] [Google Scholar]
- 75.Fox CH, Tenner-Racz K, Racz P, Firpo A, Pizzo PA, Fauci AS. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J Infect Dis. 1991:164(6):1051–1057. [DOI] [PubMed] [Google Scholar]
- 76.Pantaleo G, Graziosi C, Butini L, Pizzo PA, Schnittman SM, Kotler DP, Fauci AS. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991:88(21):9838–9842. 10.1073/pnas.88.21.9838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013:210(1):143–156. 10.1084/jem.20121932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schacker T, Little S, Connick E, Gebhard-Mitchell K, Zhang ZQ, Krieger J, Pryor J, Havlir D, Wong JK, Richman D, et al. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J Infect Dis. 2000:181(1):354–357. 10.1086/315178 [DOI] [PubMed] [Google Scholar]
- 79.Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W, Collins KL. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nat Immunol. 2011:12(10):975–83. 10.1038/ni.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood. 2010:115(7):1354–1363. 10.1182/blood-2009-08-237370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005:5(11):835–843. 10.1038/nri1711 [DOI] [PubMed] [Google Scholar]
- 82.Hu PF, Hultin LE, Hultin P, Hausner MA, Hirji K, Jewett A, Bonavida B, Detels R, Giorgi JV. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16 + CD56 + cells and expansion of a population of CD16dimCD56− cells with low lytic activity. J Acquir Immune Defic Syndr Hum Retrovirol. 1995:10(3):331–340. [PubMed] [Google Scholar]
- 83.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, Moretta A, Mavilio D. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. 2009:114(18):3822–3830. 10.1182/blood-2009-06-226332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barker E, Martinson J, Brooks C, Landay A, Deeks S. Dysfunctional natural killer cells, in vivo, are governed by HIV viremia regardless of whether the infected individual is on antiretroviral therapy. AIDS. 2007:21(17):2363–2365. 10.1097/QAD.0b013e3282f1d658 [DOI] [PubMed] [Google Scholar]
- 85.Iannello A, Debbeche O, Samarani S, Ahmad A. Antiviral NK cell responses in HIV infection: II. viral strategies for evasion and lessons for immunotherapy and vaccination. J Leukoc Biol. 2008:84(1):27–49. 10.1189/jlb.0907649 [DOI] [PubMed] [Google Scholar]
- 86.Scott-Algara D, Paul P. NK cells and HIV infection: lessons from other viruses. Curr Mol Med. 2002:2(8):757–768. 10.2174/1566524023361781 [DOI] [PubMed] [Google Scholar]
- 87.Hong HS, Eberhard JM, Keudel P, Bollmann BA, Ballmaier M, Bhatnagar N, Zielinska-Skowronek M, Schmidt RE, Meyer-Olson D. HIV infection is associated with a preferential decline in less-differentiated CD56dim CD16+ NK cells. J Virol. 2010:84(2):1183–1188. 10.1128/JVI.01675-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, Moretta L. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur J Immunol. 2003:33(9):2410–2418. 10.1002/eji.200324141 [DOI] [PubMed] [Google Scholar]
- 89.Kottilil S, Shin K, Planta M, McLaughlin M, Hallahan CW, Ghany M, Chun T-W, Sneller MC, Fauci AS. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J Infect Dis. 2004:189(7):1193–1198. 10.1086/382090 [DOI] [PubMed] [Google Scholar]
- 90.Ahmad R, Sindhu ST, Tran P, Toma E, Morisset R, Menezes J, Ahmad A. Modulation of expression of the MHC class I-binding natural killer cell receptors, and NK activity in relation to viral load in HIV-infected/AIDS patients. J Med Virol. 2001:65(3):431–40. 10.1002/jmv.2053 [DOI] [PubMed] [Google Scholar]
- 91.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, Moretta A, Mavilio D. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS. 2010:24(1):27–34. 10.1097/QAD.0b013e3283328d1f [DOI] [PubMed] [Google Scholar]
- 92.Scully E, Alter G. NK cells in HIV disease. Curr HIV/AIDS Rep. 2016:13(2):85–94. 10.1007/s11904-016-0310-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmad A, Menezes J. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 1996:10(2):258–266. 10.1096/fasebj.10.2.8641559 [DOI] [PubMed] [Google Scholar]
- 94.Brunetta E, Hudspeth KL, Mavilio D. Pathologic natural killer cell subset redistribution in HIV-1 infection: new insights in pathophysiology and clinical outcomes. J Leukoc Biol. 2010:88(6):1119–1130. 10.1189/jlb.0410225 [DOI] [PubMed] [Google Scholar]
- 95.Mikulak J, Oriolo F, Zaghi E, Di Vito C, Mavilio D. Natural killer cells in HIV-1 infection and therapy. AIDS. 2017:31(17):2317–2330. 10.1097/QAD.0000000000001645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011:476(7358):96–100. 10.1038/nature10237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Münz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004:172(3):1455–1462. 10.4049/jimmunol.172.3.1455 [DOI] [PubMed] [Google Scholar]
- 98.Luteijn R, Sciaranghella G, van Lunzen J, Nolting A, Dugast AS, Ghebremichael MS, Altfeld M, Alter G. Early viral replication in lymph nodes provides HIV with a means by which to escape NK-cell-mediated control. Eur J Immunol. 2011:41(9):2729–2740. 10.1002/eji.201040886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nattermann J, Nischalke HD, Hofmeister V, Kupfer B, Ahlenstiel G, Feldmann G, Rockstroh J, Weiss EH, Sauerbruch T, Spengler U. HIV-1 infection leads to increased HLA-E expression resulting in impaired function of natural killer cells. Antivir Ther. 2005:10(1):95–107. 10.1177/135965350501000107 [DOI] [PubMed] [Google Scholar]
- 100.Ramsuran V, Naranbhai V, Horowitz A, Qi Y, Martin MP, Yuki Y, Gao X, Walker-Sperling V, Del Prete GQ, Schneider DK, et al. Elevated HLA-A expression impairs HIV control through inhibition of NKG2A-expressing cells. Science. 2018:359(6371):86–90. 10.1126/science.aam8825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huot N, Rascle P, Petitdemange C, Contreras V, Sturzel CM, Baquero E, Harper JL, Passaes C, Legendre R, Varet H, et al. SIV-induced terminally differentiated adaptive NK cells in lymph nodes associated with enhanced MHC-E restricted activity. Nat Commun. 2021:12(1):1282. 10.1038/s41467-021-21402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huot N, Jacquelin B, Garcia-Tellez T, Rascle P, Ploquin MJ, Madec Y, Reeves RK, Derreudre-Bosquet N, Müller-Trutwin M. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med. 2017:23(11):1277–1286. 10.1038/nm.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harper J, Huot N, Micci L, Tharp G, King C, Rascle P, Shenvi N, Wang H, Galardi C, Upadhyay AA, et al. IL-21 and IFNalpha therapy rescues terminally differentiated NK cells and limits SIV reservoir in ART-treated macaques. Nat Commun. 2021:12(1):2866. 10.1038/s41467-021-23189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, Santoni A, Doria M. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007:88(Pt 1):242–250. 10.1099/vir.0.82125-0 [DOI] [PubMed] [Google Scholar]
- 105.Matusali G, Potesta M, Santoni A, Cerboni C, Doria M. The human immunodeficiency virus type 1 Nef and Vpu proteins downregulate the natural killer cell-activating ligand PVR. J Virol. 2012:86(8):4496–4504. 10.1128/JVI.05788-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shah AH, Sowrirajan B, Davis ZB, Ward JP, Campbell EM, Planelles V, Barker E. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe. 2010:8(5):397–409. 10.1016/j.chom.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fogli M, Costa P, Murdaca G, Setti M, Mingari MC, Moretta L, Moretta A, De Maria A. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol. 2004:34(8):2313–2321. 10.1002/eji.200425251 [DOI] [PubMed] [Google Scholar]
- 108.Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009:265(1):29–42. 10.1111/j.1365-2796.2008.02045.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mastroianni CM, Liuzzi GM. Matrix metalloproteinase dysregulation in HIV infection: implications for therapeutic strategies. Trends Mol Med. 2007:13(11):449–459. 10.1016/j.molmed.2007.09.001 [DOI] [PubMed] [Google Scholar]
- 110.Matusali G, Tchidjou HK, Pontrelli G, Bernardi S, D’Ettorre G, Vullo V, Buonomini AR, Andreoni M, Santoni A, Cerboni C. Soluble ligands for the NKG2D receptor are released during HIV-1 infection and impair NKG2D expression and cytotoxicity of NK cells. FASEB J. 2013:27(6):2440–2450. 10.1096/fj.12-223057 [DOI] [PubMed] [Google Scholar]
- 111.Nolting A, Dugast AS, Rihn S, Luteijn R, Carrington MF, Kane K, Jost S, Toth I, Nagami E, Faetkenheuer G, et al. MHC class I chain-related protein A shedding in chronic HIV-1 infection is associated with profound NK cell dysfunction. Virology. 2010:406(1):12–20. 10.1016/j.virol.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baragano Raneros A, Suarez-Alvarez B, Lopez-Larrea C. Secretory pathways generating immunosuppressive NKG2D ligands: new targets for therapeutic intervention. Oncoimmunology. 2014:3(4):e28497. 10.4161/onci.28497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Q, Sun Y, Rihn S, Nolting A, Tsoukas PN, Jost S, Cohen K, Walker B, Alter G. Matrix metalloprotease inhibitors restore impaired NK cell-mediated antibody-dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J Virol. 2009:83(17):8705–8712. 10.1128/JVI.02666-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang C-C, Isitman G, Bruneau J, Tremblay C, Bernard NF, Kent SJ, Parsons MS. Phenotypical and functional profiles of natural killer cells exhibiting matrix metalloproteinase-mediated CD16 cleavage after anti-HIV antibody-dependent activation. Clin Exp Immunol. 2015:181(2):275–285. 10.1111/cei.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alter G, Kavanagh D, Rihn S, Luteijn R, Brooks D, Oldstone M, van Lunzen J, Altfeld M. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest. 2010:120(6):1905–1913. 10.1172/JCI40913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valentin A, Rosati M, Patenaude DJ, Hatzakis A, Kostrikis LG, Lazanas M, Wyvill KM, Yarchoan R, Pavlakis GN. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2002:99-(10):7015–7020. 10.1073/pnas.102672999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harada H, Goto Y, Ohno T, Suzu S, Okada S. Proliferative activation up-regulates expression of CD4 and HIV-1 co-receptors on NK cells and induces their infection with HIV-1. Eur J Immunol. 2007:37(8):2148–2155. 10.1002/eji.200737217 [DOI] [PubMed] [Google Scholar]
- 118.Bernstein HB, Wang G, Plasterer MC, Zack JA, Ramasastry P, Mumenthaler SM, Kitchen CMR. CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. Virology. 2009:387(1):59–66. 10.1016/j.virol.2009.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bernstein HB, Plasterer MC, Schiff SE, Kitchen CM, Kitchen S, Zack JA. CD4 expression on activated NK cells: ligation of CD4 induces cytokine expression and cell migration. J Immunol. 2006:177(6):3669–3676. 10.4049/jimmunol.177.6.3669 [DOI] [PubMed] [Google Scholar]
- 120.Weber K, Meyer D, Grosse V, Stoll M, Schmidt RE, Heiken H. Reconstitution of NK cell activity in HIV-1 infected individuals receiving antiretroviral therapy. Immunobiology. 2000:202(2):172–178. 10.1016/S0171-2985(00)80063-7 [DOI] [PubMed] [Google Scholar]
- 121.Jensen SS, Fomsgaard A, Borggren M, Tingstedt JL, Gerstoft J, Kronborg G, Rasmussen LD, Pedersen C, Karlsson I. HIV-specific antibody-dependent cellular cytotoxicity (ADCC)-mediating antibodies decline while NK cell function increases during antiretroviral therapy (ART). PLoS One. 2015:10-(12):e0145249. 10.1371/journal.pone.0145249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jensen SS, Hartling HJ, Tingstedt JL, Larsen TK, Nielsen SD, Pedersen C, Fomsgaard A, Karlsson I. HIV-specific ADCC improves after antiretroviral therapy and correlates with normalization of the NK cell phenotype. J Acquir Immune Defic Syndr. 2015:68(2):103–111. 10.1097/QAI.0000000000000429 [DOI] [PubMed] [Google Scholar]
- 123.Fantuzzi L, Purificato C, Donato K, Belardelli F, Gessani S. Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: a novel mechanism for AIDS pathogenesis. J Virol. 2004:78(18):9763–9772. 10.1128/JVI.78.18.9763-9772.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smed-Sorensen A, Lore K, Walther-Jallow L, Andersson J, Spetz AL. HIV-1-infected dendritic cells up-regulate cell surface markers but fail to produce IL-12 p70 in response to CD40 ligand stimulation. Blood. 2004:104(9):2810–2817. 10.1182/blood-2003-07-2314 [DOI] [PubMed] [Google Scholar]
- 125.Collier AC, Coombs RW, Schoenfeld DA, Bassett RL, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe VJ, et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N Engl J Med. 1996:334(16):1011–1018. 10.1056/NEJM199604183341602 [DOI] [PubMed] [Google Scholar]
- 126.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debré P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997:277(5322):112–116. 10.1126/science.277.5322.112 [DOI] [PubMed] [Google Scholar]
- 127.Palella FJ J, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998:338(13):853–860. 10.1056/NEJM199803263381301 [DOI] [PubMed] [Google Scholar]
- 128.Hearps AC, Agius PA, Zhou J, Brunt S, Chachage M, Angelovich TA, Cameron PU, Giles M, Price P, Elliott J, et al. Persistence of activated and adaptive-like NK cells in HIV(+) individuals despite 2 years of suppressive combination antiretroviral therapy. Front Immunol. 2017:8:731. 10.3389/fimmu.2017.00731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, Kramski M, Hearps AC, Cameron PU, Lewin SR, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012:189(3):1491–1499. 10.4049/jimmunol.1200458 [DOI] [PubMed] [Google Scholar]
- 130.Gougeon ML. Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol. 2003:3(5):392–404. 10.1038/nri1087 [DOI] [PubMed] [Google Scholar]
- 131.Casper C. The increasing burden of HIV-associated malignancies in resource-limited regions. Anna Rev Med. 2011:62(1):157–170. 10.1146/annurev-med-050409-103711 [DOI] [PubMed] [Google Scholar]
- 132.Crowe SM, Westhorpe CL, Mukhamedova N, Jaworowski A, Sviridov D, Bukrinsky M. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol. 2010:87(4):589–598. 10.1189/jlb.0809580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007:370(9581):59–67. 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- 134.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, Bertisch B, Bernasconi E, Weber R. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011:53(11):1130–1139. 10.1093/cid/cir626 [DOI] [PubMed] [Google Scholar]
- 135.Hearps AC, Martin GE, Rajasuriar R, Crowe SM. Inflammatory co-morbidities in HIV+ individuals: learning lessons from healthy ageing. Curr HIV/AIDS Rep. 2014:11(1):20–34. 10.1007/s11904-013-0190-8 [DOI] [PubMed] [Google Scholar]
- 136.Ahmad F, Tufa DM, Mishra N, Jacobs R, Schmidt RE. Terminal differentiation of CD56(dim)CD16(+) natural killer cells is associated with increase in natural killer cell frequencies after antiretroviral treatment in HIV-1 infection. AIDS Res Hum Retroviruses. 2015:31(12):1206–1212. 10.1089/aid.2015.0115 [DOI] [PubMed] [Google Scholar]
- 137.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Björklund AT, Flodström-Tullberg M, Michaëlsson J, Rottenberg ME, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010:116-(19):3853–3864. 10.1182/blood-2010-04-281675 [DOI] [PubMed] [Google Scholar]
- 138.Nielsen CM, White MJ, Goodier MR, Riley EM. Functional significance of CD57 expression on human NK cells and relevance to disease. Front Immunol. 2013:4:422. 10.3389/fimmu.2013.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013:5(208):208ra145. 10.1126/scitranslmed.3006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, Kinuthia J, Montgomery RR, John-Stewart G, Holmes S, et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med. 2015:7(297):297ra115. 10.1126/scitranslmed.aac5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Manser AR, Weinhold S, Uhrberg M. Human KIR repertoires: shaped by genetic diversity and evolution. Immunol Rev 2015:267(1):178–196. 10.1111/imr.12316 [DOI] [PubMed] [Google Scholar]
- 142.Stegmann KA, Robertson F, Hansi N, Gill U, Pallant C, Christophides T, Pallett LJ, Peppa D, Dunn C, Fusai G, et al. CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Sci Rep. 2016:6(1):26157. 10.1038/srep26157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol Rev 2010:235(1):297–305. 10.1111/j.0105-2896.2010.00891.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009:106(6):1915–1919. 10.1073/pnas.0813192106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012:120(24):4751–4760. 10.1182/blood-2012-04-419283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004:104(12):3664–3671. 10.1182/blood-2004-05-2058 [DOI] [PubMed] [Google Scholar]
- 147.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang S-M, Norris PJ, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011:108(36):14725–14732. 10.1073/pnas.1110900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009:457(7229):557–561. 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant. 2014:20(4):463–473. 10.1016/j.bbmt.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, Kalinski P. IL-18-induced CD83+ CCR7+ NK helper cells. J Exp Med. 2005:202(7):941–953. 10.1084/jem.20050128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luetke-Eversloh M, Hammer Q, Durek P, Nordstrom K, Gasparoni G, Pink M, Hamann A, Walter J, Chang H-D, Dong J, et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014:10(10):e1004441. 10.1371/journal.ppat.1004441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ni J, Holsken O, Miller M, Hammer Q, Luetke-Eversloh M, Romagnani C, Cerwenka A. Adoptively transferred natural killer cells maintain long-term antitumor activity by epigenetic imprinting and CD4(+) T cell help. Oncoimmunology. 2016:5(9):e1219009. 10.1080/2162402X.2016.1219009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee SH, Fragoso MF, Biron CA. Cutting edge: a novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells. J Immunol. 2012:189(6):2712–2716. 10.4049/jimmunol.1201528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Almutairi SM, Ali AK, He W, Yang DS, Ghorbani P, Wang L, Fullerton MD, Lee S-H. Interleukin-18 up-regulates amino acid transporters and facilitates amino acid-induced mTORC1 activation in natural killer cells. J Biol Chem. 2019:294(12):4644–4655. 10.1074/jbc.RA118.005892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marcais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, Rabilloud J, Mayol K, Tavares A, Bienvenu J, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014:15(8):749–757. 10.1038/ni.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stary V, Stary G. NK cell-mediated recall responses: memory-like, adaptive, or antigen-specific? Front Cell Infect Microbiol. 2020:10:208. 10.3389/fcimb.2020.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012:189(10):5082–5088. 10.4049/jimmunol.1201964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Vergès S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012:119(11):2665–2674. 10.1182/blood-2011-10-386995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008:112(3):914–915. 10.1182/blood-2008-05-157354 [DOI] [PubMed] [Google Scholar]
- 160.Hammer Q, Ruckert T, Borst EM, Dunst J, Haubner A, Durek P, Heinrich F, Gasparoni G, Babic M, Tomic A, et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol. 2018:19(5):453–463. 10.1038/s41590-018-0082-6 [DOI] [PubMed] [Google Scholar]
- 161.Liu LL, Landskron J, Ask EH, Enqvist M, Sohlberg E, Traherne JA, Hammer Q, Goodridge JP, Larsson S, Jayaraman J, et al. Critical role of CD2 co-stimulation in adaptive natural killer cell responses revealed in NKG2C-deficient humans. Cell Rep. 2016:15(5):1088–1099. 10.1016/j.celrep.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lazetic S, Chang C, Houchins JP, Lanier LL, Phillips JH. Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits. J Immunol. 1996:157(11):4741–4745. 10.4049/jimmunol.157.11.4741 [DOI] [PubMed] [Google Scholar]
- 163.Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998:8(6):693–701. 10.1016/S1074-7613(00)80574-9 [DOI] [PubMed] [Google Scholar]
- 164.Michaelsson J, Teixeira de Matos C, Achour A, Lanier LL, Karre K, Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002:196(11):1403–1414. 10.1084/jem.20020797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Heatley SL, Pietra G, Lin J, Widjaja JML, Harpur CM, Lester S, Rossjohn J, Szer J, Schwarer A, Bradstock K, et al. Polymorphism in human cytomegalovirus UL40 impacts on recognition of human leukocyte antigen-E (HLA-E) by natural killer cells. J Biol Chem. 2013:288(12):8679–8690. 10.1074/jbc.M112.409672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kaiser BK, Pizarro JC, Kerns J, Strong RK. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc Natl Acad Sci U S A. 2008:105(18):6696–6701. 10.1073/pnas.0802736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Llano M, Lee N, Navarro F, Garcia P, Albar JP, Geraghty DE, López-Botet M. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur J Immunol. 1998:28(9):2854–2863. <2854::AID-IMMU2854>3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- 168.Petrie EJ, Clements CS, Lin J, Sullivan LC, Johnson D, Huyton T, Heroux A, Hoare HL, Beddoe T, Reid HH, et al. CD94-NKG2A recognition of human leukocyte antigen (HLA)-E bound to an HLA class I leader sequence. J Exp Med. 2008:205(3):725–735. 10.1084/jem.20072525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stevens J, Joly E, Trowsdale J, Butcher GW. Peptide binding characteristics of the non-classical class Ib MHC molecule HLA-E assessed by a recombinant random peptide approach. BMC Immunol. 2001:2(1):5. 10.1186/1471-2172-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sullivan LC, Clements CS, Beddoe T, Johnson D, Hoare HL, Lin J, Huyton T, Hopkins EJ, Reid HH, Wilce MCJ, et al. The heterodimeric assembly of the CD94-NKG2 receptor family and implications for human leukocyte antigen-E recognition. Immunity. 2007:27(6):900–911. 10.1016/j.immuni.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 171.Wang EC, McSharry B, Retiere C, Tomasec P, Williams S, Borysiewicz LK, Braud VM, Wilkinson GWG. UL40-mediated NK evasion during productive infection with human cytomegalovirus. Proc Natl Acad Sci U S A. 2002:99(11):7570–7575. 10.1073/pnas.112680099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cerboni C, Mousavi-Jazi M, Wakiguchi H, Carbone E, Karre K, Soderstrom K. Synergistic effect of IFN-gamma and human cytomegalovirus protein UL40 in the HLA-E-dependent protection from NK cell-mediated cytotoxicity. Eur J Immunol. 2001:31-(10):2926–2935. <2926::AID-IMMU2926>3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- 173.Tomasec P, Braud VM, Rickards C, Powell MB, McSharry BP, Gadola S, Cerundolo V, Borysiewicz LK, McMichael AJ, Wilkinson GWG. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000:287(5455):1031–1033. 10.1126/science.287.5455.1031 [DOI] [PubMed] [Google Scholar]
- 174.Ulbrecht M, Martinozzi S, Grzeschik M, Hengel H, Ellwart JW, Pla M, Weiss EH. Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J Immunol. 2000:164(10):5019–5022. 10.4049/jimmunol.164.10.5019 [DOI] [PubMed] [Google Scholar]
- 175.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, López-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006:107(9):3624–3631. 10.1182/blood-2005-09-3682 [DOI] [PubMed] [Google Scholar]
- 176.Rolle A, Pollmann J, Ewen EM, Le VT, Halenius A, Hengel H, Cerwenka A. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J Clin Invest. 2014:124-(12):5305–5316. 10.1172/JCI77440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Geary CD, Sun JC. Memory responses of natural killer cells. Semin Immunol. 2017:31:11–19. 10.1016/j.smim.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu Z, Sinzger C, Frascaroli G, Reichel J, Bayer C, Wang L, Schirmbeck R, Mertens T. Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. J Virol. 2013:87(13):7717–7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cichocki F, Wu CY, Zhang B, Felices M, Tesi B, Tuininga K, Dougherty P, Taras E, Hinderlie P, Blazar BR, et al. ARID5B regulates metabolic programming in human adaptive NK cells. J Exp Med. 2018:215(9):2379–2395. 10.1084/jem.20172168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rolle A, Halenius A, Ewen EM, Cerwenka A, Hengel H, Momburg F. CD2-CD58 interactions are pivotal for the activation and function of adaptive natural killer cells in human cytomegalovirus infection. Eur J Immunol. 2016:46(10):2420–2425. 10.1002/eji.201646492 [DOI] [PubMed] [Google Scholar]
- 181.Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol. 2003:15(3):308–314. 10.1016/S0952-7915(03)00039-6 [DOI] [PubMed] [Google Scholar]
- 182.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008:9(5):495–502. 10.1038/ni1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010:11(12):1127–35. 10.1038/ni.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nikzad R, Angelo LS, Aviles-Padilla K, Le DT, Singh VK, Bimler L, Vukmanovic-Stejic M, Vendrame E, Ranganath T, Simpson L, et al. Human natural killer cells mediate adaptive immunity to viral antigens. Sci Immunol. 2019:4(35):eaat8116. 10.1126/sciimmunol.aat8116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Paust S, Blish CA, Reeves RK. Redefining memory: building the case for adaptive NK cells. J Virol. 2017:91(20):e00169–17. 10.1128/JVI.00169-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011:12(6):500–508. 10.1038/ni.2032 [DOI] [PubMed] [Google Scholar]
- 187.Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, et al. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015:16(9):927–932. 10.1038/ni.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mela CM, Burton CT, Imami N, Nelson M, Steel A, Gazzard BG, Gotch FM, Goodier MR. Switch from inhibitory to activating NKG2 receptor expression in HIV-1 infection: lack of reversion with highly active antiretroviral therapy. AIDS. 2005:19(16):1761–1769. 10.1097/01.aids.0000183632.12418.33 [DOI] [PubMed] [Google Scholar]
- 189.Ma M, Wang Z, Chen X, Tao A, He L, Fu S, Zhang Z, Fu Y, Guo C, Liu J, et al. NKG2C(+)NKG2A(−) natural killer cells are associated with a lower viral set point and may predict disease progression in individuals with primary HIV infection. Front Immunol. 2017:8:1176. 10.3389/fimmu.2017.01176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Thomas R, Low HZ, Kniesch K, Jacobs R, Schmidt RE, Witte T. NKG2C deletion is a risk factor of HIV infection. AIDS Res Hum Retroviruses. 2012:28(8):844–851. 10.1089/aid.2011.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Comeau EM, Holder KA, Fudge NJ, Grant MD. Cytomegalovirus-driven adaption of natural killer cells in NKG2C(null) human immunodeficiency virus-infected individuals. Viruses. 2019:11(3):239. 10.3390/v11030239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shah SV, Manickam C, Ram DR, Kroll K, Itell H, Permar SR, Barouch DH, Klatt NR, Reeves RK. CMV primes functional alternative signaling in adaptive deltag NK cells but is subverted by lentivirus infection in rhesus macaques. Cell Rep. 2018:25(10):2766–2774.e3. 10.1016/j.celrep.2018.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Peppa D. Natural killer cells in human immunodeficiency virus-1 infection: spotlight on the impact of human cytomegalovirus. Front Immunol. 2017:8:1322. 10.3389/fimmu.2017.01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mela CM, Goodier MR. The contribution of cytomegalovirus to changes in NK cell receptor expression in HIV-1-infected individuals. J Infect Dis. 2007:195(1):158–159; author reply 9-60. 10.1086/509811 [DOI] [PubMed] [Google Scholar]
- 195.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schächter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994:29(6):601–609. 10.1016/0531-5565(94)90073-6 [DOI] [PubMed] [Google Scholar]
- 196.Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV. Shortened telomeres in the expanded CD28−CD8 + cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996:10(8):F17–F22. 10.1097/00002030-199607000-00001 [DOI] [PubMed] [Google Scholar]
- 197.Effros RB. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol. 1997:21(6):471–478. 10.1016/S0145-305X(97)00027-X [DOI] [PubMed] [Google Scholar]
- 198.Petitdemange C, Becquart P, Wauquier N, Beziat V, Debre P, Leroy EM, Vieillard V. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011:7(9):e1002268. 10.1371/journal.ppat.1002268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaëlsson J, Malmberg K-J, Klingström J, Ahlm C, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011:208(1):13–21. 10.1084/jem.20100762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997:94(24):13193–13197. 10.1073/pnas.94.24.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997:278-(5341):1295–300. 10.1126/science.278.5341.1295 [DOI] [PubMed] [Google Scholar]
- 202.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003:9(6):727–728. 10.1038/nm880 [DOI] [PubMed] [Google Scholar]
- 203.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997:278-(5341):1291–1295. 10.1126/science.278.5341.1291 [DOI] [PubMed] [Google Scholar]
- 204.Desimio MG, Covino DA, Doria M. Potential of the NKG2D/NKG2DL axis in NK cell-mediated clearance of the HIV-1 reservoir. Int J Mol Sci. 2019:20(18):4490. 10.3390/ijms20184490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Davey RT J, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A. 1999:96(26):15109–15114. 10.1073/pnas.96.26.15109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Adland E, Klenerman P, Goulder P, Matthews PC. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front Microbiol. 2015:6:1016. 10.3389/fmicb.2015.01016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Deayton JR, Prof Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet. 2004:363(9427):2116–2121. 10.1016/S0140-6736(04)16500-8 [DOI] [PubMed] [Google Scholar]
- 208.Patel EU, Gianella S, Newell K, Tobian AA, Kirkpatrick AR, Nalugoda F, Grabowski MK, Gray RH, Serwadda D, Quinn TC, et al. Elevated cytomegalovirus IgG antibody levels are associated with HIV-1 disease progression and immune activation. AIDS. 2017:31(6):807–813. 10.1097/QAD.0000000000001412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Freeman ML, Lederman MM, Gianella S. Partners in crime: the role of CMV in immune dysregulation and clinical outcome during HIV infection. Curr HIV/AIDS Rep. 2016:13(1):10–19. 10.1007/s11904-016-0297-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Freeman ML, Mudd JC, Shive CL, Younes SA, Panigrahi S, Sieg SF, Lee SA, Hunt PW, Calabrese LH, Gianella S, et al. CD8 T-cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin Infect Dis. 2016:62(3):392–396. 10.1093/cid/civ840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012:9(2):139–147. 10.1007/s11904-012-0118-8 [DOI] [PubMed] [Google Scholar]
- 212.Barrett L, Stapleton SN, Fudge NJ, Grant MD. Immune resilience in HIV-infected individuals seronegative for cytomegalovirus. AIDS. 2014:28(14):2045–2049. 10.1097/QAD.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 213.Bedimo R. Non-AIDS-defining malignancies among HIV-infected patients in the highly active antiretroviral therapy era. Curr HIV/AIDS Rep. 2008:5(3):140–149. 10.1007/s11904-008-0022-4 [DOI] [PubMed] [Google Scholar]
- 214.Chun TW, Fauci AS. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS. 2012:26(10):1261–1268. 10.1097/QAD.0b013e328353f3f1 [DOI] [PubMed] [Google Scholar]
- 215.Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. 2016:14(1):55–60. 10.1038/nrmicro.2015.5 [DOI] [PubMed] [Google Scholar]
- 216.Deeks SG. HIV: shock and kill. Nature. 2012:487(7408):439–440. 10.1038/487439a [DOI] [PubMed] [Google Scholar]
- 217.Spivak AM, Planelles V. HIV-1 eradication: early trials (and tribulations). Trends Mol Med. 2016:22(1):10–27. 10.1016/j.molmed.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Thorlund K, Horwitz MS, Fife BT, Lester R, Cameron DW. Landscape review of current HIV ‘kick and kill’ cure research: some kicking, not enough killing. BMC Infect Dis. 2017:17(1):595. 10.1186/s12879-017-2683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang H-C, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012:36(3):491–501. 10.1016/j.immuni.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015:517(7534):381–385. 10.1038/nature14053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Baek H-J, Kim J-S, Yoon M, Lee J-J, Shin M-G, Ryang D-W, Kook H, Kim S-K, Cho D. Ex vivo expansion of natural killer cells using cryopreserved irradiated feeder cells. Anticancer Res. 2013:33(5):2011–2019. [PubMed] [Google Scholar]
- 222.Lim D-P, Jang Y-Y, Kim S, Koh SS, Lee J-J, Kim J-S, Thi Phan M-T, Shin D-J, Shin M-G, Lee S-H, et al. Effect of exposure to interleukin-21 at various time points on human natural killer cell culture. Cytotherapy. 2014:16(10):1419–1430. 10.1016/j.jcyt.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 223.Ojo EO, Sharma AA, Liu R, Moreton S, Checkley-Luttge MA, Gupta K, Lee G, Lee DA, Otegbeye F, Sekaly R-P, et al. Membrane bound IL-21 based NK cell feeder cells drive robust expansion and metabolic activation of NK cells. Sci Rep. 2019:9(1):14916. 10.1038/s41598-019-51287-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Copik AJ, Oyer JL, Chakravarti N, Lee DA. PM21 particles to improve bone marrow homing of NK cells. Google Patents; 2020. [Google Scholar]
- 225.Copik AJ, Oyer JL, Igarashi RY, Altomare D. Methods and compositions for natural killer cells. Google Patents; 2017. [Google Scholar]
- 226.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002:100-(10):3633–3638. 10.1182/blood-2001-12-0293 [DOI] [PubMed] [Google Scholar]
- 227.Han KP, Zhu X, Liu B, Jeng E, Kong L, Yovandich JL, Vyas VV, Marcus WD, Chavaillaz P-A, Romero CA, et al. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine. 2011:56(3):804–810. 10.1016/j.cyto.2011.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rhode PR, Egan JO, Xu W, Hong H, Webb GM, Chen X, Liu B, Zhu X, Wen J, You L, et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res. 2016:4(1):49–60. 10.1158/2326-6066.CIR-15-0093-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, Weisdorf DJ, Blazar BR, Ustun C, DeFor TE, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018:131(23):2515–2527. 10.1182/blood-2017-12-823757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Webb GM, Li S, Mwakalundwa G, Folkvord JM, Greene JM, Reed JS, Stanton JJ, Legasse AW, Hobbs T, Martin LD, et al. The human IL-15 superagonist ALT-803 directs SIV-specific CD8(+) T cells into B-cell follicles. Blood Adv. 2018:2(2):76–84. 10.1182/bloodadvances.2017012971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Webb GM, Molden J, Busman-Sahay K, Abdulhaqq S, Wu HL, Weber WC, Bateman KB, Reed JS, Northrup M, Maier N, et al. The human IL-15 superagonist N-803 promotes migration of virus-specific CD8+ T and NK cells to B cell follicles but does not reverse latency in ART-suppressed, SHIV-infected macaques. PLoS Pathog. 2020:16(3):e1008339. 10.1371/journal.ppat.1008339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Garrido C, Abad-Fernandez M, Tuyishime M, Pollara JJ, Ferrari G, Soriano-Sarabia N, Margolis DM. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J Virol. 2018:92(12):e00235–18. 10.1128/JVI.00235-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Miller JS, Davis ZB, Helgeson E, Reilly C, Thorkelson A, Anderson J, Lima NS, Jorstad S, Hart GT, Lee JH, et al. Safety and virologic impact of the IL-15 superagonist N-803 in people living with HIV: a phase 1 trial. Nat Med. 2022:28(2):392–400. 10.1038/s41591-021-01651-9 [DOI] [PubMed] [Google Scholar]
- 234.Rahman SA, Billingsley JM, Sharma AA, Styles TM, Govindaraj S, Shanmugasundaram U, Babu H, Riberio SP, Ali SA, Tharp GK, et al. Lymph node CXCR5+ NK cells associate with control of chronic SHIV infection. JCI Insight. 2022:7(8):e155601. 10.1172/jci.insight.155601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999:94(1):333–339. 10.1182/blood.V94.1.333.413a31_333_339 [DOI] [PubMed] [Google Scholar]
- 236.Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Benson DM, Blaser BW, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009:114(13):2667–2677. 10.1182/blood-2009-02-206532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li F, Wei H, Wei H, Gao Y, Xu L, Yin W, Sun R, Tian Z. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013:144(2):392–401. 10.1053/j.gastro.2012.10.039 [DOI] [PubMed] [Google Scholar]
- 238.Alrubayyi A, Ogbe A, Moreno Cubero E, Peppa D. Harnessing natural killer cell innate and adaptive traits in HIV infection. Front Cell Infect Microbiol. 2020:10:395. 10.3389/fcimb.2020.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Andre P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018:175(7):1731–1743.e13. 10.1016/j.cell.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vey N, Karlin L, Sadot-Lebouvier S, Broussais F, Berton-Rigaud D, Rey J, Charbonnier A, Marie D, André P, Paturel C, et al. A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget. 2018:9(25):17675–17688. 10.18632/oncotarget.24832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012:120(22):4317–4323. 10.1182/blood-2012-06-437558 [DOI] [PubMed] [Google Scholar]
- 242.Benson DM Jr, Cohen AD, Jagannath S, Munshi NC, Spitzer G, Hofmeister CC, Efebera YA, Andre P, Zerbib R, Caligiuri MA. A phase I trial of the anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma. Clin Cancer Res. 2015; 21(18):4055–4061. 10.1158/1078-0432.CCR-15-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006:25(2):331–342. 10.1016/j.immuni.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 244.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010:115(6):1166–1174. 10.1182/blood-2009-09-245746 [DOI] [PubMed] [Google Scholar]
- 245.Yawata M, Yawata N, Draghi M, Partheniou F, Little AM, Parham P. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 2008:112(6):2369–2380. 10.1182/blood-2008-03-143727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song Y-J, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005:436(7051):709–713. 10.1038/nature03847 [DOI] [PubMed] [Google Scholar]
- 247.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006:443(7109):350–354. 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- 248.Macatangay BJC, Gandhi RT, Jones RB, McMahon DK, Lalama CM, Bosch RJ, Cyktor JC, Thomas AS, Borowski L, Riddler SA, et al. T cells with high PD-1 expression are associated with lower HIV-specific immune responses despite long-term antiretroviral therapy. AIDS. 2020:34(1):15–24. 10.1097/QAD.0000000000002406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006:203(10):2281–2292. 10.1084/jem.20061496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Norris S, Coleman A, Kuri-Cervantes L, Bower M, Nelson M, Goodier MR. PD-1 expression on natural killer cells and CD8(+) T cells during chronic HIV-1 infection. Viral Immunol. 2012:25(4):329–332. 10.1089/vim.2011.0096 [DOI] [PubMed] [Google Scholar]
- 251.Della Chiesa M, Pesce S, Muccio L, Carlomagno S, Sivori S, Moretta A, Marcenaro E. Features of memory-like and PD-1(+) human NK cell subsets. Front Immunol. 2016:7:351. 10.3389/fimmu.2016.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Schafer JL, Li H, Evans TI, Estes JD, Reeves RK. Accumulation of cytotoxic CD16+ NK cells in simian immunodeficiency virus-infected lymph nodes associated with in situ differentiation and functional anergy. J Virol. 2015:89(13):6887–6894. 10.1128/JVI.00660-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Schafer JL, Muller-Trutwin MC, Reeves RK. NK cell exhaustion: bad news for chronic disease? Oncotarget. 2015:6(26):21797–-21798. 10.18632/oncotarget.5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006:203(10):2223–2227. 10.1084/jem.20061800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel M-R, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006:12(10):1198–1202. 10.1038/nm1482 [DOI] [PubMed] [Google Scholar]
- 256.Brahmer JR, Tykodi SS, Chow LQ, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012:366(26):2455–2465. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012:366(26):2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Porichis F, Hart MG, Massa A, Everett HL, Morou A, Richard J, Brassard N, Veillette M, Hassan M, Ly NL, et al. Immune checkpoint blockade restores HIV-specific CD4 T cell help for NK cells. J Immunol. 2018:201(3):971–981. 10.4049/jimmunol.1701551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and −9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004:173(11):6841–6849. 10.4049/jimmunol.173.11.6841 [DOI] [PubMed] [Google Scholar]
- 260.Belisle JA, Horibata S, Jennifer GA, Petrie S, Kapur A, Andre S, Gabius H-J, Rancourt C, Connor J, Paulson JC, et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer. 2010:9(1):118. 10.1186/1476-4598-9-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007:7(4):255–266. 10.1038/nri2056 [DOI] [PubMed] [Google Scholar]
- 262.Adeniji OS, Kuri-Cervantes L, Yu C, Xu Z, Ho M, Chew GM, Shikuma C, Tomescu C, George AF, Roan NR, et al. Siglec-9 defines and restrains a natural killer subpopulation highly cytotoxic to HIV-infected cells. PLoS Pathog. 2021:17(11):e1010034. 10.1371/journal.ppat.1010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Noh J-Y, Yoon SR, Kim T-D, Choi I, Jung H. Toll-like receptors in natural killer cells and their application for immunotherapy. J Immunol Res. 2020;2020:2045860. 10.1155/2020/2045860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bam RA, Hansen D, Irrinki A, Mulato A, Jones GS, Hesselgesser J, Frey CR, Cihlar T, Yant SR. TLR7 agonist GS-9620 is a potent inhibitor of acute HIV-1 infection in human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2017:61(1):e01369–16. 10.1128/AAC.01369-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Schlaepfer E, Audige A, Joller H, Speck RF. TLR7/8 triggering exerts opposing effects in acute versus latent HIV infection. J Immunol. 2006:176(5):2888–2895. 10.4049/jimmunol.176.5.2888 [DOI] [PubMed] [Google Scholar]
- 266.Schlaepfer E, Speck RF. Anti-HIV activity mediated by natural killer and CD8+ cells after toll-like receptor 7/8 triggering. PLoS One. 2008:3(4):e1999. 10.1371/journal.pone.0001999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lima JF, Oliveira LMS, Pereira NZ, Duarte AJS, Sato MN. Polyfunctional natural killer cells with a low activation profile in response to Toll-like receptor 3 activation in HIV-1-exposed seronegative subjects. Sci Rep. 2017:7(1):524. 10.1038/s41598-017-00637-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tsai A, Irrinki A, Kaur J, Cihlar T, Kukolj G, Sloan DD, Murry JP. Toll-like receptor 7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J Virol. 2017:91(8):e02166–16. 10.1128/JVI.02166-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Offersen R, Nissen SK, Rasmussen TA, Østergaard L, Denton PW, Søgaard OS, Tolstrup M. A novel toll-like receptor 9 agonist, MGN1703, enhances HIV-1 transcription and NK cell-mediated inhibition of HIV-1-infected autologous CD4+ T cells. J Virol. 2016:90(9):4441–4453. 10.1128/JVI.00222-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Vibholm L, Schleimann MH, Hojen JF, Benfield T, Offersen R, Rasmussen K, Olesen R, Dige A, Agnholt J, Grau J, et al. Short-course toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin Infect Dis. 2017:64(12):1686–1695. 10.1093/cid/cix201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Winckelmann AA, Munk-Petersen LV, Rasmussen TA, Melchjorsen J, Hjelholt TJ, Montefiori D, Østergaard L, Søgaard OS, Tolstrup M. Administration of a toll-like receptor 9 agonist decreases the proviral reservoir in virologically suppressed HIV-infected patients. PLoS One. 2013:8(4):e62074. 10.1371/journal.pone.0062074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014:20(4):425–429. 10.1038/nm.3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Spivak AM, Bosque A, Balch AH, Smyth D, Martins L, Planelles V. Ex vivo bioactivity and HIV-1 latency reversal by ingenol dibenzoate and panobinostat in resting CD4(+) T cells from aviremic patients. Antimicrob Agents Chemother. 2015:59(10):5984–5991. 10.1128/AAC.01077-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lam AP, Sparano JA, Vinciguerra V, Ocean AJ, Christos P, Hochster H, Camacho F, Goel S, Mani S, Kaubisch A. Phase II study of paclitaxel plus the protein kinase C inhibitor bryostatin-1 in advanced pancreatic carcinoma. Am J Clin Oncol. 2010:33(2):121–124. 10.1097/COC.0b013e3181a31920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Morgan RJ Jr, Leong L, Chow W, Gandara D, Frankel P, Garcia A, Lenz H-J, Doroshow JH. Phase II trial of bryostatin-1 in combination with cisplatin in patients with recurrent or persistent epithelial ovarian cancer: a California cancer consortium study. Invest New Drugs. 2012:30(2):723–728. 10.1007/s10637-010-9557-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Clutton G, Xu Y, Baldoni PL, Mollan KR, Kirchherr J, Newhard W, Cox K, Kuruc JD, Kashuba A, Barnard R, et al. The differential short- and long-term effects of HIV-1 latency-reversing agents on T cell function. Sci Rep. 2016:6:30749. 10.1038/srep30749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pace M, Williams J, Kurioka A, Gerry AB, Jakobsen B, Klenerman P, Nwokolo N, Fox J, Fidler S, Frater J. Histone deacetylase inhibitors enhance CD4 T cell susceptibility to NK cell killing but reduce NK cell function. PLoS Pathog. 2016:12(8):e1005782. 10.1371/journal.ppat.1005782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Walker-Sperling VE, Pohlmeyer CW, Tarwater PM, Blankson JN. The effect of latency reversal agents on primary CD8+ T cells: implications for shock and kill strategies for human immunodeficiency virus eradication. EBioMedicine. 2016:8:217–229. 10.1016/j.ebiom.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jones RB, O’Connor R, Mueller S, Foley M, Szeto GL, Karel D, Lichterfeld M, Kovacs C, Ostrowski MA, Trocha A, et al. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog. 2014:10(8):e1004287. 10.1371/journal.ppat.1004287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gane EJ, Lim Y-S, Gordon SC, Visvanathan K, Sicard E, Fedorak RN, Roberts S, Massetto B, Ye Z, Pflanz S, et al. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J Hepatol. 2015:63(2):320–328. 10.1016/j.jhep.2015.02.037 [DOI] [PubMed] [Google Scholar]
- 281.Lopatin U, Wolfgang G, Tumas D, Frey CR, Ohmstede C, Hesselgesser J, Kearney B, Moorehead L, Subramanian GM, McHutchison JG. Safety, pharmacokinetics and pharmacodynamics of GS-9620, an oral toll-like receptor 7 agonist. Antivir Ther. 2013:18(3):409–418. 10.3851/IMP2548 [DOI] [PubMed] [Google Scholar]
- 282.Schmidt M, Hagner N, Marco A, Konig-Merediz SA, Schroff M, Wittig B. Design and structural requirements of the potent and safe TLR-9 agonistic immunomodulator MGN1703. Nucleic Acid Ther. 2015:25(3):130–140. 10.1089/nat.2015.0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wittig B, Schmidt M, Scheithauer W, Schmoll HJ. MGN1703, an immunomodulator and toll-like receptor 9 (TLR-9) agonist: from bench to bedside. Crit Rev Oncol Hematol. 2015:94(1):31–44. 10.1016/j.critrevonc.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 284.Billingham RE, Brent L, Medawar PB. Quantitative studies on tissue transplantation immunity. II. The origin, strength and duration of actively and adoptively acquired immunity. Proc R Soc Lond B Biol Sci. 1954:143(910):58–80. 10.1098/rspb.1954.0054 [DOI] [PubMed] [Google Scholar]
- 285.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013:368(16):1509–1518. 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015:385(9967): 517–528. 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014:371(16):1507–1517. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Porter DL, Hwang W-T, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015:7(303):303ra139. 10.1126/scitranslmed.aac5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011:365(8):725–733. 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013:5(177):177ra38. 10.1126/scitranslmed.3005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014:6(224):224ra25. 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan D-AN, Lanier BJ, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010:116(20):4099–4102. 10.1182/blood-2010-04-281931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989:86(24):10024–10028. 10.1073/pnas.86.24.10024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991:64(5):891–901. 10.1016/0092-8674(91)90314-O [DOI] [PubMed] [Google Scholar]
- 295.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016:16(9):566–581. 10.1038/nrc.2016.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lim RM, Rong L, Zhen A, Xie J. A universal CAR-NK cell targeting various epitopes of HIV-1 gp160. ACS Chem Biol. 2020:15(8):2299–2310. 10.1021/acschembio.0c00537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Herzig E, Kim KC, Packard TA, Vardi N, Schwarzer R, Gramatica A, Deeks SG, Williams SR, Landgraf K, Killeen N, et al. Attacking latent HIV with convertibleCAR-T cells, a highly adaptable killing platform. Cell. 2019:179(4):880–894.e10. 10.1016/j.cell.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. 2018:9:283. 10.3389/fimmu.2018.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Locke FL, Lin Y, Jain N, Daver N, Gulbis AM, Adkins S, et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit “ALL.” Nat Rev Clin Oncol. 2018:15(4):218. 10.1038/nrclinonc.2018.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005:105(8):3051–3057. 10.1182/blood-2004-07-2974 [DOI] [PubMed] [Google Scholar]
- 301.Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011:118(12):3273–3279. 10.1182/blood-2011-01-329508 [DOI] [PubMed] [Google Scholar]
- 302.Mehta RS, Randolph B, Daher M, Rezvani K. NK cell therapy for hematologic malignancies. Int J Hematol. 2018:107(3):262–270. . 10.1007/s12185-018-2407-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020:382(6):545–553. 10.1056/NEJMoa1910607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015:5(12):1282–1295. 10.1158/2159-8290.CD-15-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ni Z, Knorr DA, Bendzick L, Allred J, Kaufman DS. Expression of chimeric receptor CD4zeta by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo. Stem Cells. 2014:32(4):1021–1031. 10.1002/stem.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tran AC, Zhang D, Byrn R, Roberts MR. Chimeric zeta-receptors direct human natural killer (NK) effector function to permit killing of NK-resistant tumor cells and HIV-infected T lymphocytes. J Immunol. 1995:155(2):1000–1009. 10.4049/jimmunol.155.2.1000 [DOI] [PubMed] [Google Scholar]
- 307.Zhen A, Kamata M, Rezek V, Rick J, Levin B, Kasparian S, Chen ISY, Yang OO, Zack JA, Kitchen SG. HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol Ther. 2015:23(8):1358–1367. 10.1038/mt.2015.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Abate-Daga D, Davila ML. CAR models: next-generation CAR modifications for enhanced T-cell function. Mol Ther Oncolytics. 2016:3:16014. 10.1038/mto.2016.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005:106(1):376–383. 10.1182/blood-2004-12-4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Carr WH, Rosen DB, Arase H, Nixon DF, Michaelsson J, Lanier LL. Cutting edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J Immunol. 2007:178(2):647–651. 10.4049/jimmunol.178.2.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Topfer K, Cartellieri M, Michen S, Wiedemuth R, Muller N, Lindemann D, Bachmann M, Füssel M, Schackert G, Temme A. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol. 2015:194(7):3201–3212. 10.4049/jimmunol.1400330 [DOI] [PubMed] [Google Scholar]
- 312.Farah FS. Natural antibodies specific to the 2,4-dinitrophenyl group. Immunology. 1973:25(2):217–226. [PMC free article] [PubMed] [Google Scholar]
- 313.Karjalainen K, Makela O. Concentrations of three hapten-binding immunoglobulins in pooled normal human serum. Eur J Immunol. 1976:6(2):88–93. 10.1002/eji.1830060204 [DOI] [PubMed] [Google Scholar]
- 314.Tay SS, Carol H, Biro M. TriKEs and BiKEs join CARs on the cancer immunotherapy highway. Hum Vaccin Immunother. 2016:12-(11):2790–2796. 10.1080/21645515.2016.1198455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, Panoskaltsis-Mortari A, Weiner LM, Vallera DA, Miller JS. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012:11(12):2674–2684. 10.1158/1535-7163.MCT-12-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, Zhang B, Lenvik AJ, Panoskaltsis-Mortari A, Verneris MR, et al. IL15 Trispecific Killer Engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res. 2016:22(14):3440–3450. 10.1158/1078-0432.CCR-15-2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Felices M, Lenvik TR, Davis ZB, Miller JS, Vallera DA. Generation of BiKEs and TriKEs to improve NK cell-mediated targeting of tumor cells. Methods Mol Biol. 2016:1441:333–346. 10.1007/978-1-4939-3684-7_28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33 + targets. Blood. 2014:123(19):3016–3026. 10.1182/blood-2013-10-533398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Vallera DA, Zhang B, Gleason MK, Oh S, Weiner LM, Kaufman DS, McCullar V, Miller JS, Verneris M. Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. Cancer Biother Radiopharm. 2013:28(4):274–282. 10.1089/cbr.2012.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B, et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 (33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013:19(14):3844–3855. 10.1158/1078-0432.CCR-13-0505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Warlick E, Weisdorf D, Vallera D, Wangen R, Lewis D, Knox J, Schroeder M, Felices M, Miller JS. GTB-3550 TriKETM for the treatment of high-risk myelodysplastic syndromes (MDS) and refractory/relapsed acute myeloid leukemia (AML) safely drives natural killer (NK) cell proliferation at initial dose cohorts. Blood. 2020:136(Suppl 1):7–8. 10.1182/blood-2020-136398 [DOI] [Google Scholar]
- 322.Felices M, Kodal B, Hinderlie P, Kaminski MF, Cooley S, Weisdorf DJ, Vallera DA, Miller JS, Bachanova V. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv. 2019:3(6):897–907. 10.1182/bloodadvances.2018029371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Arvindam US, van Hauten PMM, Schirm D, Schaap N, Hobo W, Blazar BR, Vallera DA, Dolstra H, Felices M, Miller JS. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia. 2021:35(6):1586–1596. 10.1038/s41375-020-01065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Felices M, Lenvik TR, Kodal B, Lenvik AJ, Hinderlie P, Bendzick LE, Schirm DK, Kaminski MF, McElmurry RT, Geller MA, et al. Potent cytolytic activity and specific IL15 delivery in a second-generation trispecific killer engager. Cancer Immunol Res. 2020:8(9):1139–1149. 10.1158/2326-6066.CIR-19-0837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yun HD, Felices M, Vallera DA, Hinderlie P, Cooley S, Arock M, Gotlib J, Ustun C, Miller JS. Trispecific killer engager CD16xIL15xCD33 potently induces NK cell activation and cytotoxicity against neoplastic mast cells. Blood Adv. 2018:2(13):1580–1584. 10.1182/bloodadvances.2018018176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Schmohl JU, Felices M, Oh F, Lenvik AJ, Lebeau AM, Panyam J, Miller JS, Vallera DA. Engineering of anti-CD133 trispecific molecule capable of inducing NK expansion and driving antibody-dependent cell-mediated cytotoxicity. Cancer Res Treat. 2017:49(4):1140–1152. 10.4143/crt.2016.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Schmohl JU, Felices M, Taras E, Miller JS, Vallera DA. Enhanced ADCC and NK cell activation of an anticarcinoma bispecific antibody by genetic insertion of a modified IL-15 cross-linker. Mol Ther. 2016:24(7):1312–1322. 10.1038/mt.2016.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Schmohl JU, Felices M, Todhunter D, Taras E, Miller JS, Vallera DA. Tetraspecific scFv construct provides NK cell mediated ADCC and self-sustaining stimuli via insertion of IL-15 as a cross-linker. Oncotarget. 2016:7(45):73830–73844. 10.18632/oncotarget.12073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Schmohl JU, Gleason MK, Dougherty PR, Miller JS, Vallera DA. Heterodimeric bispecific single chain variable fragments (scFv) killer engagers (BiKEs) enhance NK-cell activity against CD133+ colorectal cancer cells. Target Oncol. 2016:11(3):353–361. 10.1007/s11523-015-0391-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Vallera DA, Ferrone S, Kodal B, Hinderlie P, Bendzick L, Ettestad B, Hallstrom C, Zorko NA, Rao A, Fujioka N, et al. NK-cell-mediated targeting of various solid tumors using a B7-H3 tri-specific killer engager in vitro and in vivo. Cancers (Basel). 2020:12(9):2659. 10.3390/cancers12092659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Li W, Wu Y, Kong D, Yang H, Wang Y, Shao J, Feng Y, Chen W, Ma L, Ying T, et al. One-domain CD4 Fused to human anti-CD16 antibody domain mediates effective killing of HIV-1-infected cells. Sci Rep. 2017:7(1):9130. 10.1038/s41598-017-07966-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990:50(3 Suppl):814s–819s. [PubMed] [Google Scholar]
- 333.Li W, Yang H, Dimitrov DS. Identification of high-affinity anti-CD16A allotype-independent human antibody domains. Exp Mol Pathol. 2016:101(2):281–289. 10.1016/j.yexmp.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014:28(2):163–169. 10.1097/QAD.0000000000000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol. 2011:85(10):4828–4840. 10.1128/JVI.00198-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kwong PD, Mascola JR. HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity. 2018:48(5):855–871. 10.1016/j.immuni.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 337.Haynes BF, Shaw GM, Korber B, Kelsoe G, Sodroski J, Hahn BH, Borrow P, McMichael AJ. HIV-host interactions: implications for vaccine design. Cell Host Microbe. 2016:19(3):292–303. 10.1016/j.chom.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Haynes BF, Burton DR, Mascola JR. Multiple roles for HIV broadly neutralizing antibodies. Sci Transl Med. 2019:11(516):eaaz2686. 10.1126/scitranslmed.aaz2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Jaworski JP, Vendrell A, Chiavenna SM. Neutralizing monoclonal antibodies to fight HIV-1: on the threshold of success. Front Immunol. 2016:7:661. 10.3389/fimmu.2016.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Nishimura Y, Martin MA. Of mice, macaques, and men: broadly neutralizing antibody immunotherapy for HIV-1. Cell Host Microbe. 2017:22(2):207–216. 10.1016/j.chom.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Stephenson KE, Barouch DH. Broadly neutralizing antibodies for HIV eradication. Curr HIV/AIDS Rep. 2016:13(1):31–37. 10.1007/s11904-016-0299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009:83(14):7337–7348. 10.1128/JVI.00110-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Doria-Rose NA, Klein RM, Daniels MG, O’Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. 2010:84(3):1631–1636. 10.1128/JVI.01482-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009:83(2):757–769. 10.1128/JVI.02036-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Landais E, Moore PL. Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers. Retrovirology. 2018:15(1):61. 10.1186/s12977-018-0443-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009:15(8):866–870. 10.1038/nm.1949 [DOI] [PubMed] [Google Scholar]
- 347.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009:458(7238):636–640. 10.1038/nature07930 [DOI] [PubMed] [Google Scholar]
- 348.Chun TW, Murray D, Justement JS, Blazkova J, Hallahan CW, Fankuchen O, Gittens K, Benko E, Kovacs C, Moir S, et al. Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proc Natl Acad Sci U S A. 2014:111(36):13151–-13156. 10.1073/pnas.1414148111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, Scheid JF, Eden C, Mouquet H, Nussenzweig MC, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med. 2013:210(13):2813–2821. 10.1084/jem.20131244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Reh L, Magnus C, Schanz M, Weber J, Uhr T, Rusert P, Trkola A. Capacity of broadly neutralizing antibodies to inhibit HIV-1 cell-cell transmission is strain- and epitope-dependent. PLoS Pathog. 2015:11(7):e1004966. 10.1371/journal.ppat.1004966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Pietzsch J, Gruell H, Bournazos S, Donovan BM, Klein F, Diskin R, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, et al. A mouse model for HIV-1 entry. Proc Natl Acad Sci U S A. 2012:109(39):15859–15864. 10.1073/pnas.1213409109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014:158(5):989–999. 10.1016/j.cell.2014.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Nishimura Y, Gautam R, Chun TW, Sadjadpour R, Foulds KE, Shingai M, Klein F, Gazumyan A, Golijanin J, Donaldson M, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017:543(7646):559–563. 10.1038/nature21435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012:492(7427):118–122. 10.1038/nature11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang H-W, Shekhar K, Gupta S, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013:503(7475):224–228. 10.1038/nature12744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015:522(7557):487–491. 10.1038/nature14411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suárez I, Oliveira TY, Lorenzi JCC, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018:561(7724):479–484. 10.1038/s41586-018-0531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Gaebler C, Nogueira L, Stoffel E, Oliveira TY, Breton G, Millard KG, Turroja M, Butler A, Ramos V, Seaman MS, et al. Prolonged viral suppression with anti-HIV-1 antibody therapy. Nature. 2022:606(7913):368–374. 10.1038/s41586-022-04597-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Sneller MC, Blazkova J, Justement JS, Shi V, Kennedy BD, Gittens K, Tolstenko J, McCormack G, Whitehead EJ, Schneck RF, et al. Combination anti-HIV antibodies provide sustained virological suppression. Nature. 2022:606(7913):375–381. 10.1038/s41586-022-04797-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Gruell H, Gunst JD, Cohen YZ, Pahus MH, Malin JJ, Platten M, Millard KG, Tolstrup M, Jones RB, Conce Alberto WD, et al. Effect of 3BNC117 and romidepsin on the HIV-1 reservoir in people taking suppressive antiretroviral therapy (ROADMAP): a randomised, open-label, phase 2A trial. Lancet Microbe. 2022:3(3):e203–e214. 10.1016/S2666-5247(21)00239-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sok D, Burton DR. Recent progress in broadly neutralizing antibodies to HIV. Nat Immunol. 2018:19(11):1179–1188. 10.1038/s41590-018-0235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Do Kwon Y, Scheid JF, Shi W, Xu L, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010:329-(5993):811–817. 10.1126/science.1192819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011:333(6049):1633–1637. 10.1126/science.1207227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011:477(7365):466–470. 10.1038/nature10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A. 2012:109-(47):E3268–E3277. 10.1073/pnas.1217207109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Lifson JD, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013:503-(7475):277–280. 10.1038/nature12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JCC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016:535(7613):556–560. 10.1038/nature18929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med. 2016:375(21):2037–2050. 10.1056/NEJMoa1608243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015:7(319):319ra206. 10.1126/scitranslmed.aad5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A. 2013:110(41):16538–16543. 10.1073/pnas.1315295110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Igarashi T, Brown C, Azadegan A, Haigwood N, Dimitrov D, Martin MA, Shibata R. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nat Med. 1999:5(2):211–216. 10.1038/5576 [DOI] [PubMed] [Google Scholar]
- 372.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014:514(7524):642–645. 10.1038/nature13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, Donahue DA, Lorin V, Casartelli N, Noäl N, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016:7(1):10844. 10.1038/ncomms10844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Moldt B, Schultz N, Dunlop DC, Alpert MD, Harvey JD, Evans DT, Poignard P, Hessell AJ, Burton DR. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcgamma receptors to define the role of effector functions in protection against HIV. J Virol. 2011:85(20):10572–10581. 10.1128/JVI.05541-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, et al. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol. 2011:85-(14):7029–7036. 10.1128/JVI.00171-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast A-S, Heizen EL, Ercan A, Choi I, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013:123(5):2183–2192. 10.1172/JCI65708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014:158(6): 1243–1253. 10.1016/j.cell.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CMS, Landucci G, Forthal D. Parren PWHI, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007:449(7158):101–104. 10.1038/nature06106 [DOI] [PubMed] [Google Scholar]
- 379.Boesch AW, Brown EP, Ackerman ME. The role of Fc receptors in HIV prevention and therapy. Immunol Rev. 2015:268(1):296–310. 10.1111/imr.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016:352(6288):1001–1004. 10.1126/science.aaf1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu WH, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, et al. Publisher correction: antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. 2018;564(7734):E8. 10.1038/s41586-018-0721-y [DOI] [PubMed] [Google Scholar]
- 382.Diskin R, Scheid JF, Marcovecchio PM, West AP Jr, Klein F, Gao H, Gnanapragasam PNP, Abadir A, Seaman MS, Nussenzweig MC, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011; 334(6060):1289–1293. 10.1126/science.1213782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Anderko RR, Rinaldo CR, Mailliard RB. IL-18 responsiveness defines limitations in immune help for specialized FcRgamma(−) NK cells. J Immunol. 2020:205(12):3429–3442. 10.4049/jimmunol.2000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


