Abstract
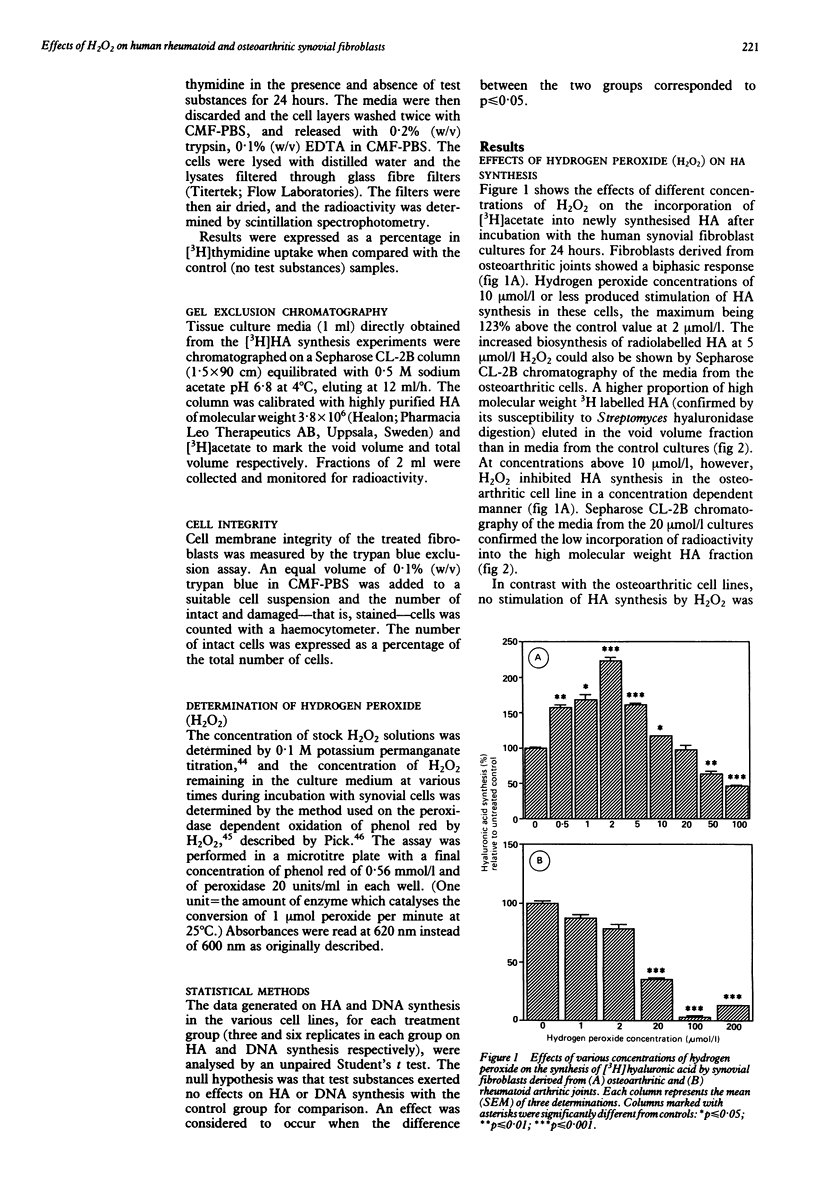
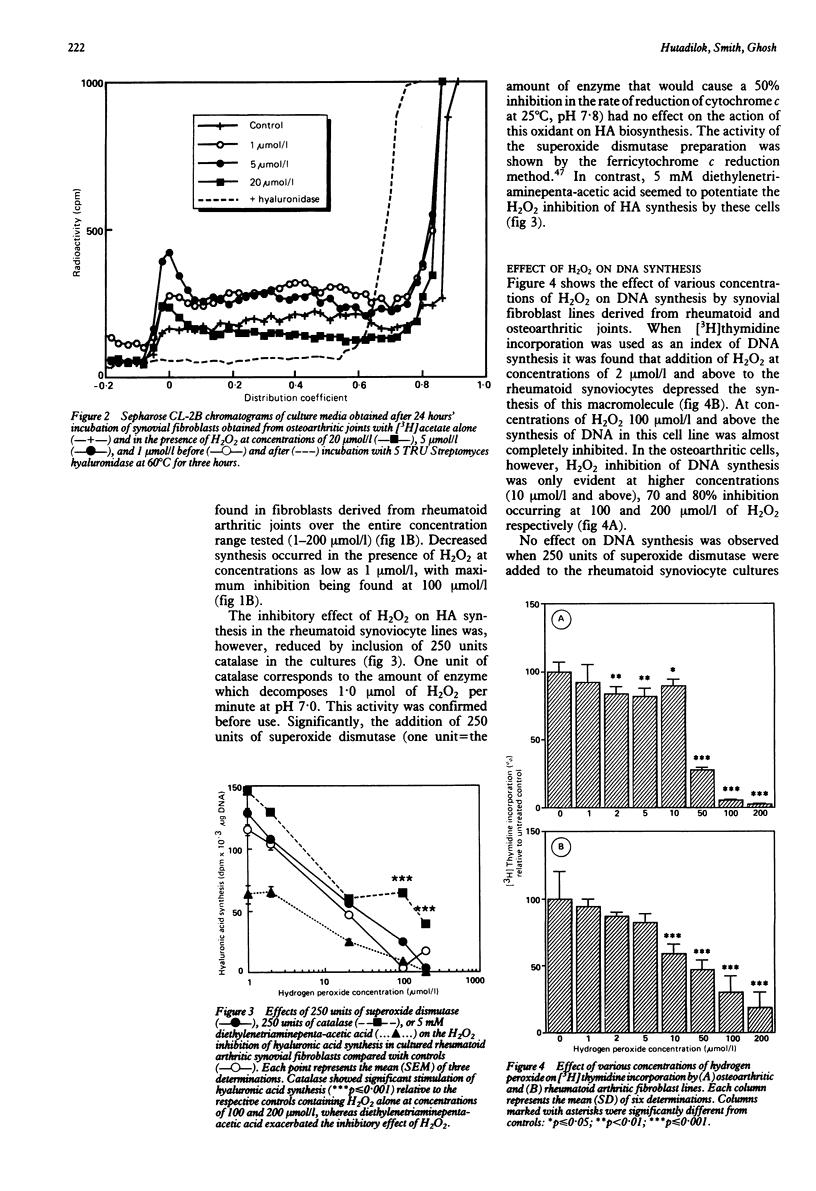
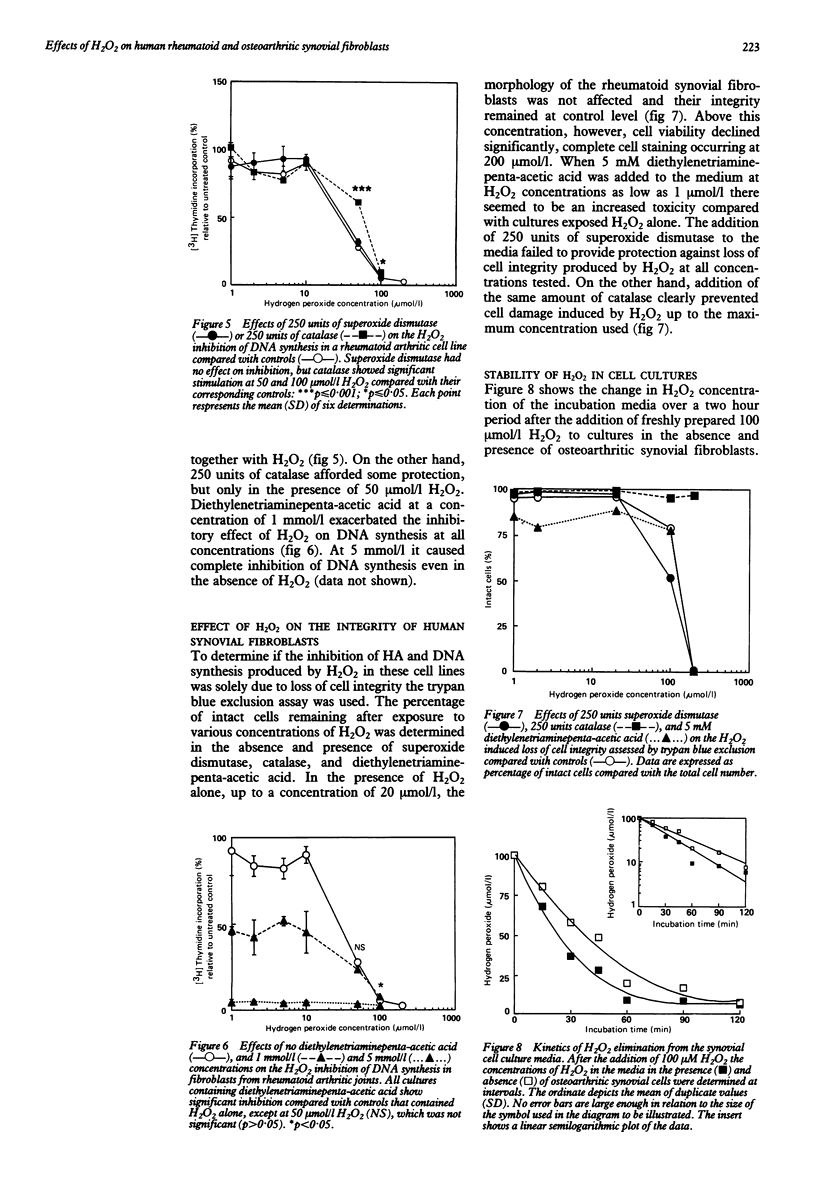
The effects of hydrogen peroxide (H2O2) on the metabolism of cultured human synovial fibroblasts derived from joints of four patients with rheumatoid arthritis and three with osteoarthritis have been investigated. The exposure of rheumatoid cell cultures to this oxygen derived species at sublethal concentrations (1-100 mumol/l) induced a dose related inhibition of both hyaluronic acid (HA) and DNA synthesis. In contrast, in osteoarthritic cell lines a biphasic response was shown. At low concentrations of H2O2 (less than 10 mumol/l) a stimulatory effect on HA synthesis was noted, whereas in the presence of higher concentrations (greater than 10 mumol/l) a significant inhibition of synthesis occurred. These deleterious effects of H2O2 were partially reduced by the addition of catalase to the culture media. The finding that both HA and DNA synthesis were inhibited at concentrations of H2O2 less than those which caused loss of cell integrity (greater than 200 mumol/l) suggests oxidation of intracellular components, such as glyceraldehyde-3-phosphate dehydrogenase, and subsequent depletion of ATP concentrations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L. Differential effects of hydrogen peroxide on indices of endothelial cell function. J Exp Med. 1984 Feb 1;159(2):592–603. doi: 10.1084/jem.159.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley U. P., Chakrabarti B. Role of singlet oxygen in the degradation of hyaluronic acid. Biochem Biophys Res Commun. 1983 Sep 30;115(3):894–901. doi: 10.1016/s0006-291x(83)80019-9. [DOI] [PubMed] [Google Scholar]
- Baker M. S., Feigan J., Lowther D. A. Chondrocyte antioxidant defences: the roles of catalase and glutathione peroxidase in protection against H2O2 dependent inhibition of proteoglycan biosynthesis. J Rheumatol. 1988 Apr;15(4):670–677. [PubMed] [Google Scholar]
- Bartold P. M., Wiebkin O. W., Thonard J. C. The effect of oxygen-derived free radicals on gingival proteoglycans and hyaluronic acid. J Periodontal Res. 1984 Jul;19(4):390–400. doi: 10.1111/j.1600-0765.1984.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Harper G. S., Lowther D. A., Preston B. N. Effect of oxygen-derived reactive species on cartilage proteoglycan-hyaluronate aggregates. Biochem Int. 1984 May;8(5):629–637. [PubMed] [Google Scholar]
- Bates E. J., Johnson C. C., Lowther D. A. Inhibition of proteoglycan synthesis by hydrogen peroxide in cultured bovine articular cartilage. Biochim Biophys Acta. 1985 Feb 15;838(2):221–228. doi: 10.1016/0304-4165(85)90082-0. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Lowther D. A., Johnson C. C. Hyaluronic acid synthesis in articular cartilage: an inhibition by hydrogen peroxide. Biochem Biophys Res Commun. 1985 Oct 30;132(2):714–720. doi: 10.1016/0006-291x(85)91191-x. [DOI] [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., Koster J. F. Protective factors against oxygen free radicals and hydrogen peroxide in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1984 Jul;27(7):760–765. doi: 10.1002/art.1780270706. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Allen R. E., Lunec J. Free radicals in biological systems--a review orientated to inflammatory processes. Br Med Bull. 1987 Apr;43(2):371–385. doi: 10.1093/oxfordjournals.bmb.a072188. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Treby D. A., Halliwell B., Gutteridge J. M. Protection against superoxide and hydrogen peroxide in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1981 Oct;61(4):483–486. doi: 10.1042/cs0610483. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Winyard P., Lunec J., Williams A., Good P. A., Crewes S. J., Gutteridge J. M., Rowley D., Halliwell B., Cornish A. Cerebral and ocular toxicity induced by desferrioxamine. Q J Med. 1985 Jul;56(219):345–355. [PubMed] [Google Scholar]
- Chung M. H., Kesner L., Chan P. C. Degradation of articular cartilage by copper and hydrogen peroxide. Agents Actions. 1984 Oct;15(3-4):328–335. doi: 10.1007/BF01972367. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Chronic granulomatous disease. Adv Hum Genet. 1987;16:229–297. doi: 10.1007/978-1-4757-0620-8_4. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Roberts C. R., Forni L. G. Oxygen-centred free radicals can efficiently degrade the polypeptide of proteoglycans in whole cartilage. Biosci Rep. 1984 Dec;4(12):1017–1026. doi: 10.1007/BF01116694. [DOI] [PubMed] [Google Scholar]
- Del Maestro R. F., Arfors K. E., Lindblom R. Free radical depolymerization of hyaluronic acid. Influence of scavenger substances. Bibl Anat. 1979;(18):132–135. [PubMed] [Google Scholar]
- Edwards S. W., Hallett M. B., Campbell A. K. Oxygen-radical production during inflammation may be limited by oxygen concentration. Biochem J. 1984 Feb 1;217(3):851–854. doi: 10.1042/bj2170851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Flohé L., Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Falchuk K. H., Zeiger L. S., Sullivan A. L., Hebert C. L., Adams J. P., Decker J. L. A physiological approach to the assessment of disease activity in rheumatoid arthritis. J Clin Invest. 1971 Jun;50(6):1167–1180. doi: 10.1172/JCI106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Hoffstein S. T., Gennaro D. E., Meunier P. C. Cytochemical demonstration of constitutive H2O2 production by macrophages in synovial tissue from rats with adjuvant arthritis. Am J Pathol. 1988 Jan;130(1):120–125. [PMC free article] [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Halsey W. A., Jr, Schraufstätter I. U., Sauerheber R. D., Spragg R. G., Jackson J. H., Cochrane C. G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem. 1988 Feb 5;263(4):1665–1675. [PubMed] [Google Scholar]
- Lund-Olesen K. Oxygen tension in synovial fluids. Arthritis Rheum. 1970 Nov-Dec;13(6):769–776. doi: 10.1002/art.1780130606. [DOI] [PubMed] [Google Scholar]
- Marklund S. L., Bjelle A., Elmqvist L. G. Superoxide dismutase isoenzymes of the synovial fluid in rheumatoid arthritis and in reactive arthritides. Ann Rheum Dis. 1986 Oct;45(10):847–851. doi: 10.1136/ard.45.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McNeil J. D., Wiebkin O. W., Betts W. H., Cleland L. G. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann Rheum Dis. 1985 Nov;44(11):780–789. doi: 10.1136/ard.44.11.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Suppression of fibroblast proliferation by activated macrophages: involvement of H2O2 and a non-prostaglandin E product of the cyclooxygenase pathway. Cell Immunol. 1986 Jul;100(2):501–514. doi: 10.1016/0008-8749(86)90048-1. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Arrick B. A., Murray H. W., DeSantis N. M., Cohn Z. A. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J Exp Med. 1981 Apr 1;153(4):766–782. doi: 10.1084/jem.153.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–421. doi: 10.1016/s0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- Roberts C. R., Mort J. S., Roughley P. J. Treatment of cartilage proteoglycan aggregate with hydrogen peroxide. Relationship between observed degradation products and those that occur naturally during aging. Biochem J. 1987 Oct 15;247(2):349–357. doi: 10.1042/bj2470349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. R., Roughley P. J., Mort J. S. Degradation of human proteoglycan aggregate induced by hydrogen peroxide. Protein fragmentation, amino acid modification and hyaluronic acid cleavage. Biochem J. 1989 May 1;259(3):805–811. doi: 10.1042/bj2590805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. E., McLees B. D., Kerby G. P. Pathways of glucose metabolism in rheumatoid and nonrheumatoid synovial membrane. J Lab Clin Med. 1967 Sep;70(3):503–511. [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Ruch W., Cooper P. H., Baggiolini M. Assay of H2O2 production by macrophages and neutrophils with homovanillic acid and horse-radish peroxidase. J Immunol Methods. 1983 Oct 28;63(3):347–357. doi: 10.1016/s0022-1759(83)80008-8. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., van den Berg W. B., van de Putte L. B., Joosten L. A. Hydrogen peroxide suppresses the proteoglycan synthesis of intact articular cartilage. J Rheumatol. 1985 Apr;12(2):205–210. [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Smith M. M., Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7(3):113–122. doi: 10.1007/BF00270463. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Simon L. M. Importance of the glutathione redox cycle for the resistance of lung epithelial cells against a polymorphonuclear leukocyte-mediated oxidant attack. Biochem Pharmacol. 1986 Jul 1;35(13):2268–2270. doi: 10.1016/0006-2952(86)90605-2. [DOI] [PubMed] [Google Scholar]
- Verbruggen G., Veys E. M. Influence of sulphated glycosaminoglycans upon proteoglycan metabolism of the synovial lining cells. Acta Rhumatol Belg. 1977 Jan-Jun;1(1-2):75–92. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. F., Halliwell B., Richmond R., Skowroneck W. R. The role of superoxide and hydroxyl radicals in the degradation of hyaluronic acid induced by metal ions and by ascorbic acid. J Inorg Biochem. 1981 Apr;14(2):127–134. doi: 10.1016/s0162-0134(00)80033-1. [DOI] [PubMed] [Google Scholar]
- Zoschke D. C., Kaja J. Suboptimal levels of hydrogen peroxide scavengers in synovial fluid: in vitro augmentation with slow acting antirheumatic drugs. J Rheumatol. 1989 Sep;16(9):1233–1240. [PubMed] [Google Scholar]


