Abstract
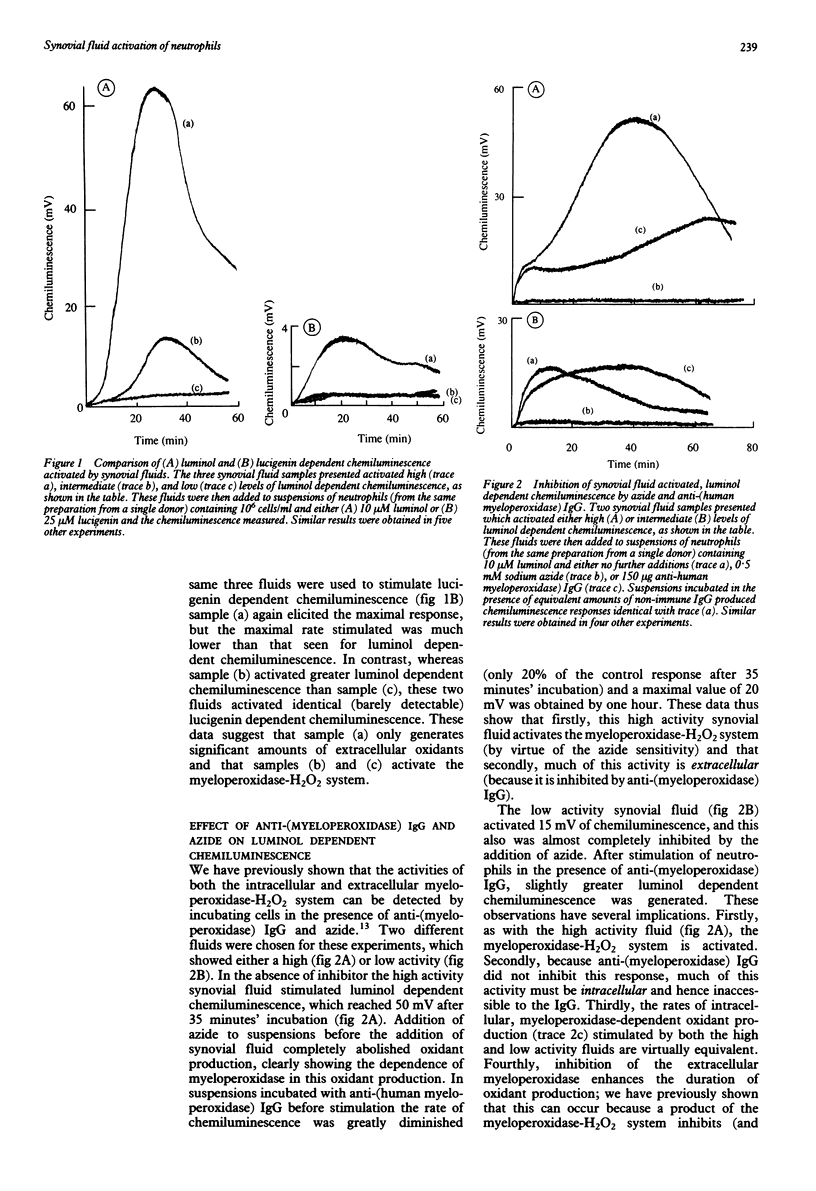
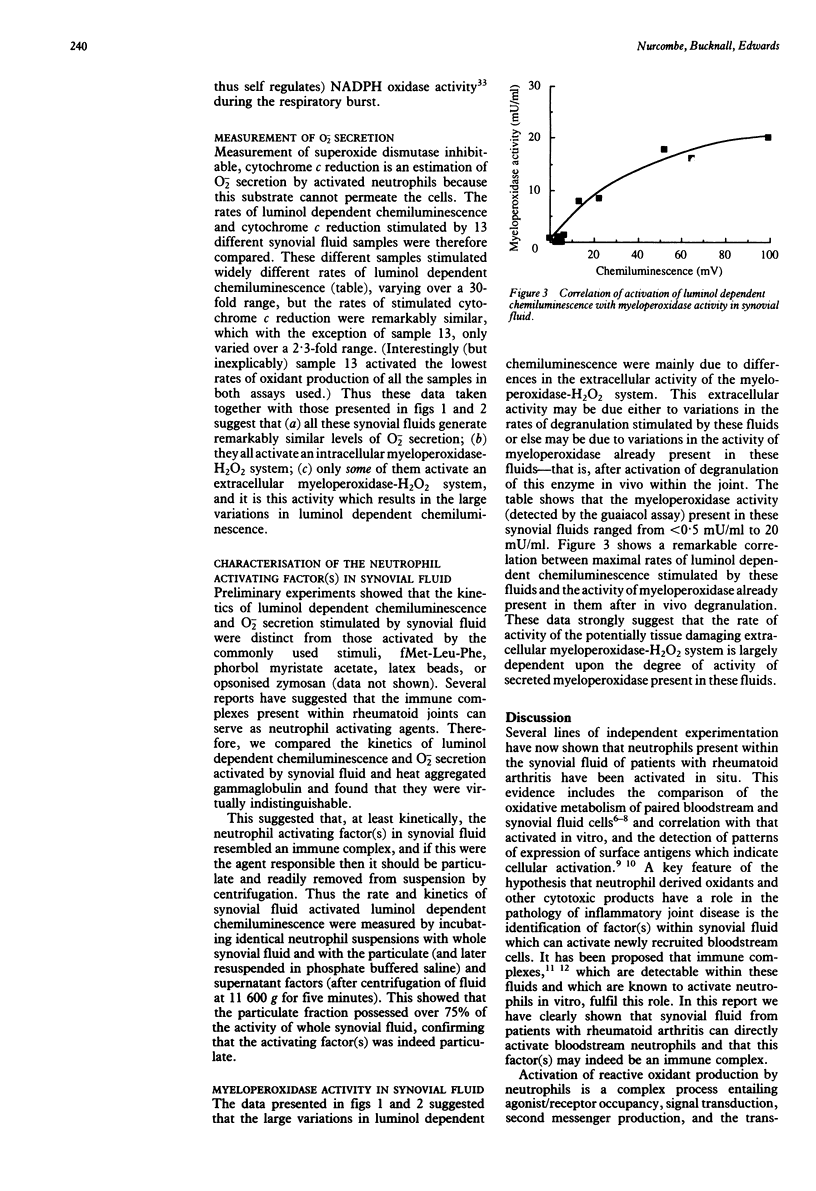
Synovial fluid isolated from 16 patients with rheumatoid arthritis activated luminol dependent chemiluminescence in bloodstream neutrophils, and the maximal activity stimulated varied over a 50-fold range. In contrast, these same fluids only activated a much lower range (two- to threefold) of maximal rates of lucigenin dependent chemiluminescence and cytochrome c reduction, two assays which only measure oxidant secretion which is independent of myeloperoxidase. Over 95% of the luminol dependent chemiluminescence activated by all samples was inhibited by azide (indicating its dependence upon myeloperoxidase), but anti-(myeloperoxidase) IgG (which specifically inhibits only the extracellular activity of this enzyme) only inhibited the response stimulated by some samples: those fluids which activated the highest luminol dependent chemiluminescence also stimulated the greatest activity of an extracellular myeloperoxidase-H2O2 system. A clear correlation was shown to exist between the activity of myeloperoxidase already present in the fluids (after its secretion from neutrophils in situ within the rheumatoid joint) and the ability of the fluid to activate luminol dependent chemiluminescence. It is concluded, therefore, that all synovial fluid samples tested possess almost equivalent levels of a factor(s) which activated O2-/H2O2 secretion and that the variations in the measured activity of the extracellular myeloperoxidase-H2O2 system are dependent upon the level of degranulation which had occurred within the joint.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Bender J. G., Van Epps D. E., Searles R., Williams R. C., Jr Altered function of synovial fluid granulocytes in patients with acute inflammatory arthritis: evidence for activation of neutrophils and its mediation by a factor present in synovial fluid. Inflammation. 1986 Dec;10(4):443–453. doi: 10.1007/BF00915828. [DOI] [PubMed] [Google Scholar]
- Blackburn W. D., Jr, Koopman W. J., Schrohenloher R. E., Heck L. W. Induction of neutrophil enzyme release by rheumatoid factors: evidence for differences based on molecular characteristics. Clin Immunol Immunopathol. 1986 Aug;40(2):347–355. doi: 10.1016/0090-1229(86)90039-5. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Treby D. A., Halliwell B., Gutteridge J. M. Protection against superoxide and hydrogen peroxide in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1981 Oct;61(4):483–486. doi: 10.1042/cs0610483. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C., Lafeber G. J., van den Barselaar M. T., van Dissel J. T., Leijh P. C. Phagocytosis and intracellular killing of Staphylococcus aureus by polymorphonuclear cells from synovial fluid of patients with rheumatoid arthritis. Arthritis Rheum. 1986 Feb;29(2):166–173. doi: 10.1002/art.1780290203. [DOI] [PubMed] [Google Scholar]
- Briheim G., Stendahl O., Dahlgren C. Intra- and extracellular events in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1984 Jul;45(1):1–5. doi: 10.1128/iai.45.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. A., McCarthy D., Perry J. D., Dumonde D. C. Reduction of the surface charge of blood polymorphonuclear cells by rheumatoid sera and heat induced aggregated human IgG (HAGG). Ann Rheum Dis. 1988 May;47(5):359–363. doi: 10.1136/ard.47.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. A. The polymorphonuclear cell in rheumatoid arthritis. Br J Rheumatol. 1988 Apr;27(2):150–155. doi: 10.1093/rheumatology/27.2.150. [DOI] [PubMed] [Google Scholar]
- Dahlgren C., Aniansson H., Magnusson K. E. Pattern of formylmethionyl-leucyl-phenylalanine-induced luminol- and lucigenin-dependent chemiluminescence in human neutrophils. Infect Immun. 1985 Jan;47(1):326–328. doi: 10.1128/iai.47.1.326-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B., Edwards S. W. Inhibition of myeloperoxidase by salicylhydroxamic acid. Biochem J. 1989 Mar 15;258(3):801–806. doi: 10.1042/bj2580801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dularay B., Elson C. J., Dieppe P. A. Enhanced oxidative response of polymorphonuclear leukocytes from synovial fluids of patients with rheumatoid arthritis. Autoimmunity. 1988;1(3):159–169. doi: 10.3109/08916938808997161. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Hughes V., Barlow J., Bucknall R. Immunological detection of myeloperoxidase in synovial fluid from patients with rheumatoid arthritis. Biochem J. 1988 Feb 15;250(1):81–85. doi: 10.1042/bj2500081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W. Luminol- and lucigenin-dependent chemiluminescence of neutrophils: role of degranulation. J Clin Lab Immunol. 1987 Jan;22(1):35–39. [PubMed] [Google Scholar]
- Edwards S. W., Say J. E., Hart C. A. Oxygen-dependent killing of Staphylococcus aureus by human neutrophils. J Gen Microbiol. 1987 Dec;133(12):3591–3597. doi: 10.1099/00221287-133-12-3591. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Swan T. F. Regulation of superoxide generation by myeloperoxidase during the respiratory burst of human neutrophils. Biochem J. 1986 Jul 15;237(2):601–604. doi: 10.1042/bj2370601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., Lopez A. F., Burns G. F., Vadas M. A. Synovial fluid neutrophils of patients with rheumatoid arthritis have membrane antigen changes that reflect activation. Ann Rheum Dis. 1988 Jan;47(1):34–39. doi: 10.1136/ard.47.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale R., Bertouch J. V., Bradley J., Roberts-Thomson P. J. Direct activation of neutrophil chemiluminescence by rheumatoid sera and synovial fluid. Ann Rheum Dis. 1983 Apr;42(2):158–162. doi: 10.1136/ard.42.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Hopkins S. J., Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol. 1988 Jul;73(1):88–92. [PMC free article] [PubMed] [Google Scholar]
- Humphreys J. M., Davies B., Hart C. A., Edwards S. W. Role of myeloperoxidase in the killing of Staphylococcus aureus by human neutrophils: studies with the myeloperoxidase inhibitor salicylhydroxamic acid. J Gen Microbiol. 1989 May;135(5):1187–1193. doi: 10.1099/00221287-135-5-1187. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Meuer S. C., Blohm D., Mertelsmann R. H., Herrmann F. Granulocyte/macrophage colony-stimulating factor induces interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1988 Feb 1;140(3):837–839. [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Ziegler-Heitbrock H. W., Mertelsmann R., Herrmann F. Granulocyte-macrophage colony-stimulating factor induces cytokine secretion by human polymorphonuclear leukocytes. J Clin Invest. 1989 Apr;83(4):1308–1312. doi: 10.1172/JCI114016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McKenna R. M., Wilkins J. A., Warrington R. J. Lymphokine production in rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 1988 Nov;15(11):1639–1642. [PubMed] [Google Scholar]
- Miossec P. The role of interleukin 1 in the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 1987 Oct-Dec;5(4):305–308. [PubMed] [Google Scholar]
- Nurcombe H. L., Bucknall R. C., Edwards S. W. Neutrophils isolated from the synovial fluid of patients with rheumatoid arthritis: priming and activation in vivo. Ann Rheum Dis. 1991 Mar;50(3):147–153. doi: 10.1136/ard.50.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe H. L., Edwards S. W. Role of myeloperoxidase in intracellular and extracellular chemiluminescence of neutrophils. Ann Rheum Dis. 1989 Jan;48(1):56–62. doi: 10.1136/ard.48.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Rafter G. W. Interleukin 1 and rheumatoid arthritis. Med Hypotheses. 1988 Nov;27(3):221–224. doi: 10.1016/0306-9877(88)90147-8. [DOI] [PubMed] [Google Scholar]
- Steinbeck M. J., Roth J. A. Neutrophil activation by recombinant cytokines. Rev Infect Dis. 1989 Jul-Aug;11(4):549–568. doi: 10.1093/clinids/11.4.549. [DOI] [PubMed] [Google Scholar]
- Yamagata N., Kobayashi K., Kasama T., Fukushima T., Tabata M., Yoneya I., Shikama Y., Kaga S., Hashimoto M., Yoshida K. Multiple cytokine activities and loss of interleukin 2 inhibitor in synovial fluids of patients with rheumatoid arthritis. J Rheumatol. 1988 Nov;15(11):1623–1627. [PubMed] [Google Scholar]
- Yocum D. E., Esparza L., Dubry S., Benjamin J. B., Volz R., Scuderi P. Characteristics of tumor necrosis factor production in rheumatoid arthritis. Cell Immunol. 1989 Aug;122(1):131–145. doi: 10.1016/0008-8749(89)90154-8. [DOI] [PubMed] [Google Scholar]


