Abstract
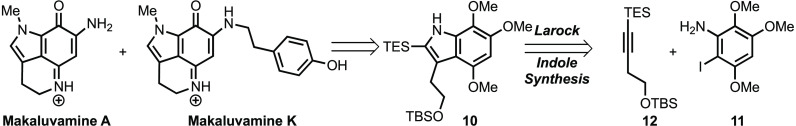
Herein, an efficient, scalable, and concise approach to an advanced pyrroloiminoquinone synthetic intermediate (6b) by way of a Larock indole synthesis is reported. The synthetic utility of this intermediate is demonstrated by its ready conversion to makaluvamines A (1) and K (4).
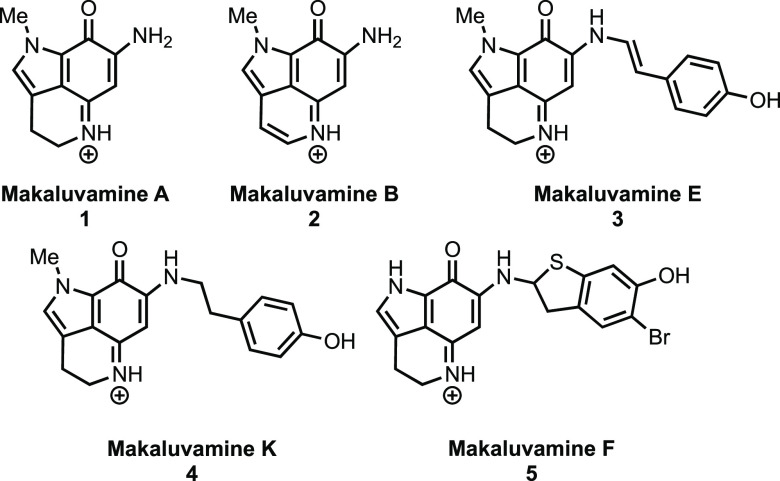
Numerous natural products containing pyrroloiminoquinone core structures have been isolated from marine sponges, and several of these, due to their potent biological activities and unique structural features, have captured the attention of the synthetic community.1 In particular, the makaluvamines, isolated from Fijian sponges of the genus Zyzzya, were found to possess inhibitory activity toward topoisomerase II along with cytotoxic activity against HCT-116 human colon cancer cells.2−4 Representative members of this family are shown in Figure 1.
Figure 1.
Members of the makaluvamine family.
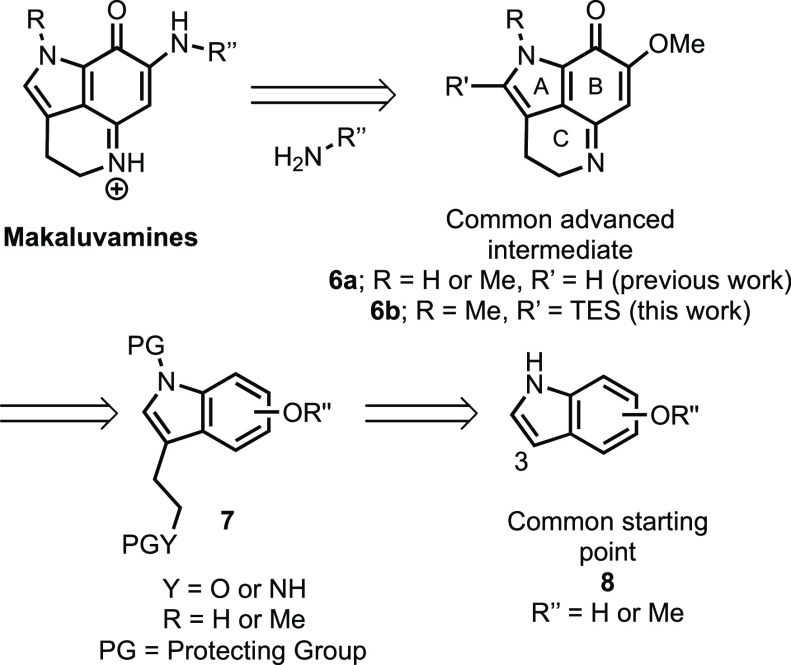
To date, the synthetic approaches toward pyrroloiminioquinones have, in most cases, proceeded via aminolysis of a corresponding vinylogous imidate (e.g., 6a, Scheme 1) with an appropriate amine.5−10 The imidate has generally been accessed from the corresponding tryptamine (7, Y = NH) or tryptophol 7 (Y = O) by oxidation to an indoloquinone followed by cyclodehydration to form the C-ring. The requisite 3-substituted indoles (7) are typically prepared from the indole precursors (8) by regioselective electrophilic aromatic substitution at C3.
Scheme 1. Common Synthetic Approach to Makaluvamines.
Although the previous synthetic approaches have proven effective for preparing an array of makaluvamines, they have typically required several linear steps to install the proper alkyl chain at the C3 position of the indole and oxidation pattern on the aromatic ring.7−10 The shortest synthesis, reported to date, of pyrroloiminoquinones was reported by Ishibashi and co-workers starting from commercially available 6-methoxy indole (1 g/$170).7 Although this synthetic route required only nine steps to prepare makaluvamine A and K, we were reluctant to utilize this approach due to the cost of the starting material. Therefore, as part of a program targeting the preparation of pyrroloiminoquinone-containing alkaloids of greater structural complexity, we set out to investigate alternative strategies to access 6. Herein, we report the highly efficient preparation of a versatile pyrroloiminoquinone intermediate (6b) and demonstrate its synthetic utility via the total synthesis of makaluvamines A (1) and K (4).
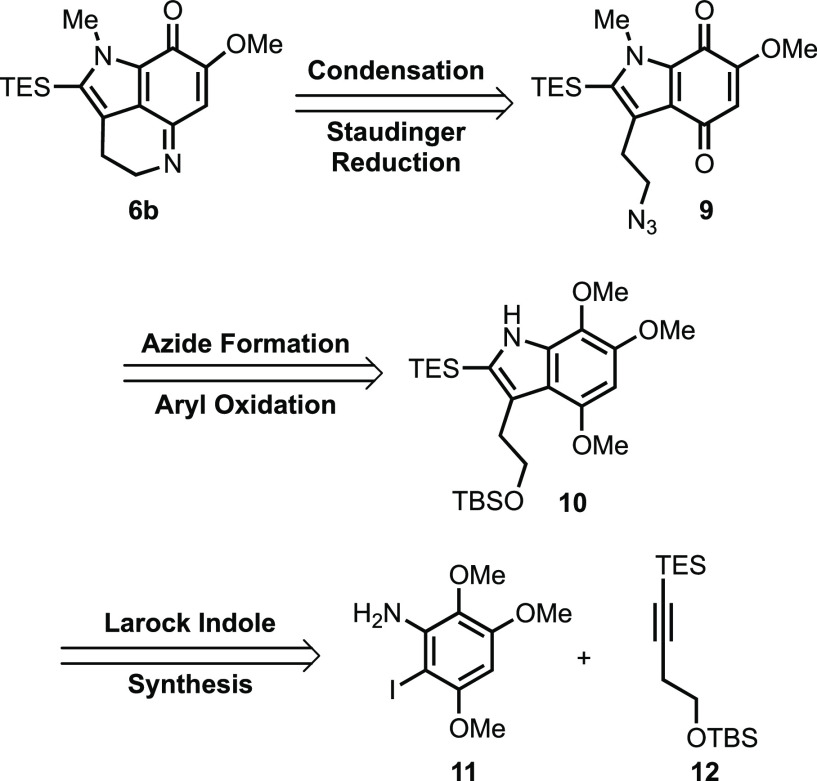
Our approach to common advanced intermediate 6b is illustrated retrosynthetically in Scheme 2. As illustrated, we envisioned accessing 6b from azide 9 utilizing a Staudinger reduction to generate a transient primary amine, which we hoped would undergo spontaneous cyclodehydration to form the tricyclic pyrroloiminoquinone skeleton. The azide 9 would, in turn, be prepared from indole 10 by oxidation of the trimethoxy arene to the indoloquinone and subsequent azidation. We envisioned assembling indole 10, with the desired functionality at the C3 position and oxidation pattern about the aromatic ring, in a convergent fashion by combining iodoaniline 11 and silylated internal alkyne 12 via Larock’s indole synthesis. Although the Larock approach has been utilized in the synthesis of several tryptophan analogues and natural products, to the best of our knowledge it has not been employed in the preparation of pyrroloiminoquinone alkaloids.11−15 This was surprising given the flexibility and efficiency this approach provides in accessing an array of C3-substituted indoles.
Scheme 2. Retrosynthetic Analysis of Common Advanced Intermediate 6b.
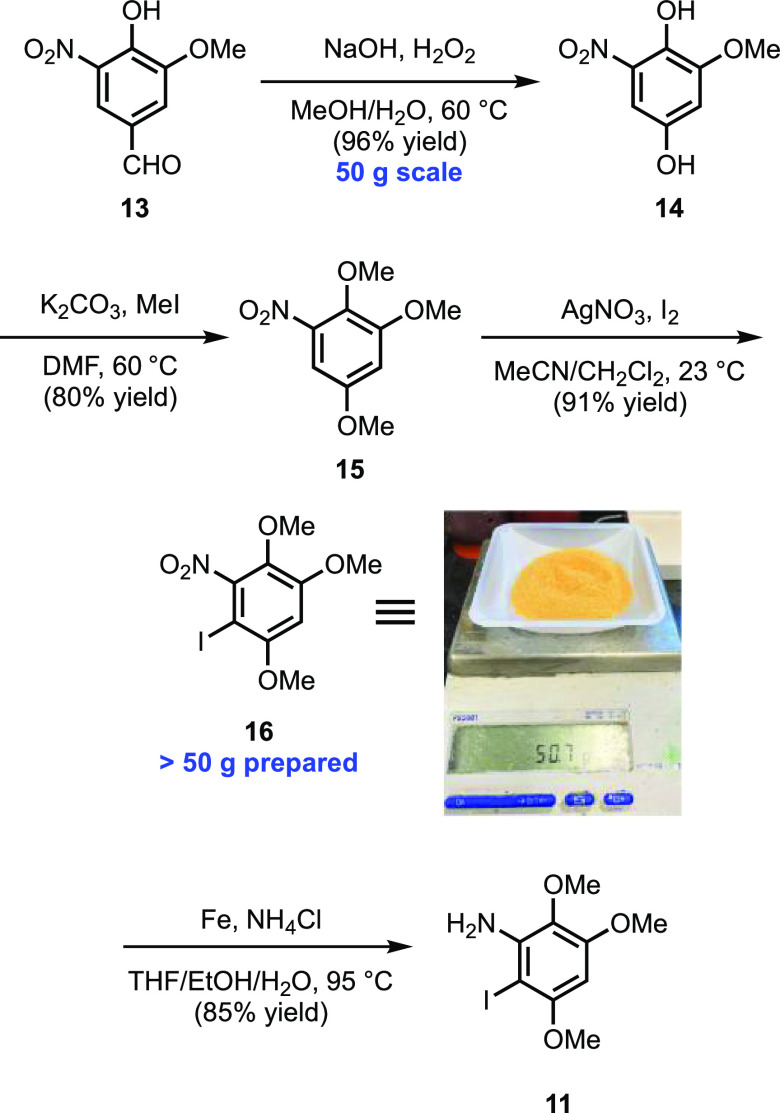
In accord with our retrosynthetic analysis, we initially turned our attention to the synthesis of iodoaniline 11. As shown in Scheme 3, treatment of commercially available 5-nitrovanillin (13) under Dakin oxidation conditions provided hydroquinone 14 in excellent yield.16 Subsequent methylation of 14 furnished 15 which, upon silver mediated iodination, produced 16 as the sole regioisomer.17 Overall, this three-step sequence proved highly efficient and was readily performed on multigram scale in a single pass. Reduction of 16 proceeded smoothly with iron powder in a mildly acidic medium to provide the desired aniline 11 in 85% yield.17 Notably, this final reduction was the only step requiring chromatographic purification. All the previous intermediates were isolated and carried forward after simple aqueous workup and/or filtration.
Scheme 3. Synthesis of Iodoaniline 11.
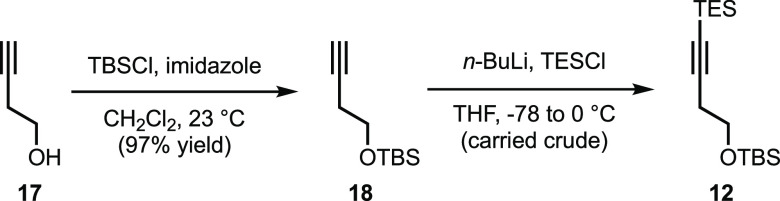
Having developed an efficient, scalable, and operationally simple sequence to 11, we focused our attention on the preparation of alkyne 12. As shown in Scheme 4, 12 was prepared from commercially available 3-butyn-1-ol (17) via a two-step sequence. In the event, TBS-protection of the primary alcohol of 17 was found to provide a near-quantitative yield of TBS ether 18, which upon conversion to the lithium acetylide with n-BuLi, followed by quenching with TESCl, afforded 12 in sufficient purity to be used directly in the forthcoming Larock sequence.18
Scheme 4. Synthesis of Silylated Internal Alkyne 12.
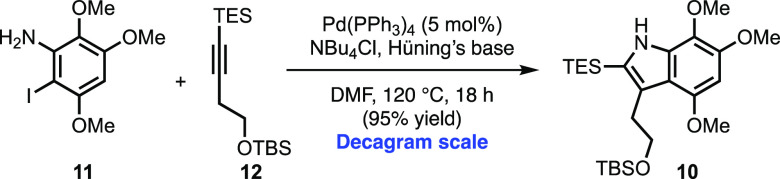
With both coupling partners in hand, we directed our attention to constructing indole 10 under Larock conditions (Scheme 5). Although a survey of the literature revealed numerous applications of the Larock chemistry, there were relatively few examples utilizing similar electron-rich anilines.19,20 Thus, our expectations were tempered as we speculated that aniline 11 may perform poorly due to sluggish oxidative addition of the active Pd catalyst to the aryl iodide.
Scheme 5. Larock Indole Synthesis.
In the event, we were delighted to observe that the coupling of 11 and 12, under standard Larock conditions, proceeds smoothly to give the desired indole (10) in excellent yield.19,21 Moreover, in contrast to previous reports employing electron-rich anilines, we observed that lowering the catalyst loading from 20 to 5 mol % Pd and shortening the reaction time from 24 to 18 h did not adversely affect the yield.19 Additionally, no loss in efficiency was observed when the reaction was conducted on decagram scale.
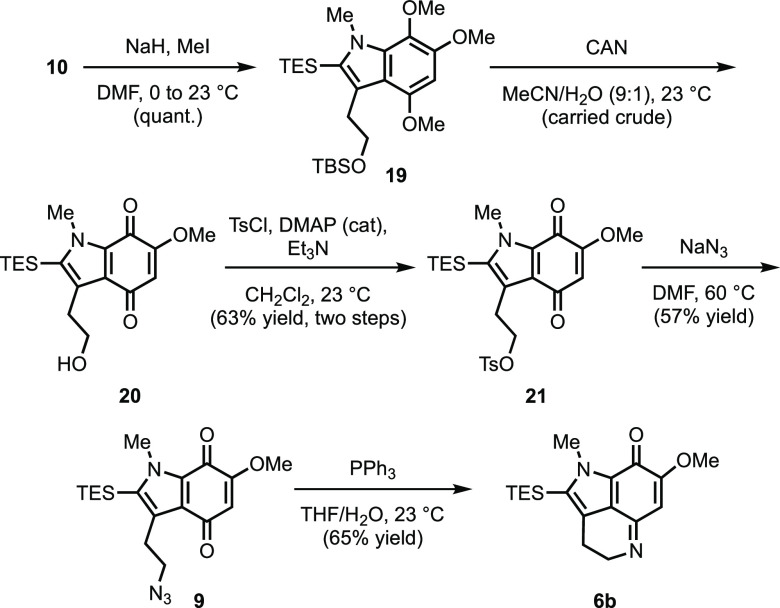
Having accessed indole 10, which contains all the requisite carbon atoms, the stage was set for accessing common intermediate 6b via a series of functional group interconversions. As illustrated in Scheme 6, these efforts began with methylation of indole 10 under standard conditions to give 19 in quantitative yield.11 Subsequent treatment of 19 with CAN induced not only oxidative demethylation of the trimethoxy arene to the corresponding methoxyquinone but also desilylation of the primary TBS-ether to deliver indoloquinone 20.22 Conversion of alcohol 20 to the corresponding tosylate (21) followed by exposure to sodium azide provided azidoindoloquinone 9.
Scheme 6. Completion of Common Advanced Intermediate 6b.
Much to our delight, subjection of 9 to Staudinger reduction conditions led directly to the desired vinylogous imidate 6b.22 This was an interesting outcome, as several literature examples of similar cyclodehydrations were conducted under either acidic or forcing conditions.9,24−27,29
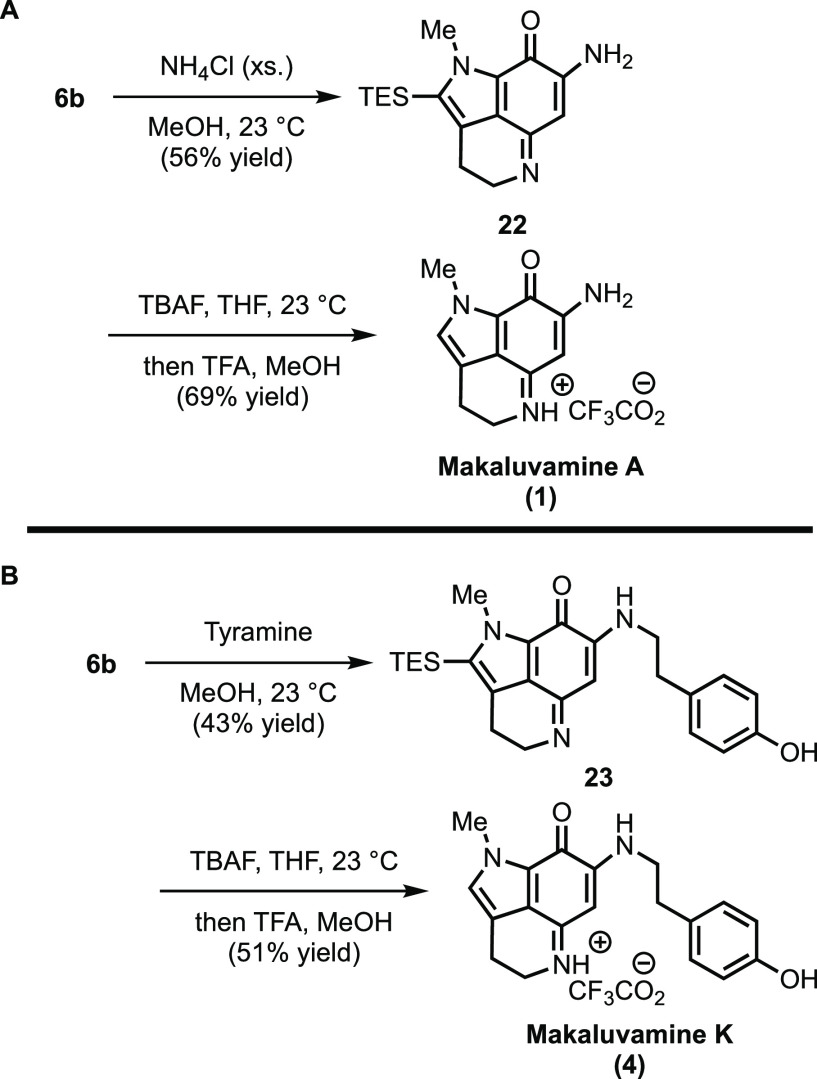
With 6b in hand, we turned our attention toward completing the synthesis of makaluvamines A (1) and K (4). As illustrated in Scheme 7A, aminolysis of 6b with NH4Cl furnished vinylogous amidine 22. Gratifyingly, desilylation of 22 with TBAF, in contrast to 6b, proceeded smoothly and subsequent acidification with TFA produced makaluvamine A (1) as a TFA salt.2,28 A similar sequence (Scheme 7B), wherein NH4Cl is replaced with tyramine, was found to deliver the TFA salt of makaluvamine K (4).8,30
Scheme 7. Total Synthesis of Makaluvamines A (1) and K (4).
In conclusion, we have developed an efficient and scalable approach to access intermediate 6b by way of the Larock indole synthesis. In addition, we have demonstrated the synthetic utility of this intermediate by advancing it to the tricyclic pyrroloiminoquinone natural products makaluvamines A (1) and K (4).
Acknowledgments
The authors gratefully acknowledge financial support from Baylor University, the Welch Foundation (Chair, AA-006), the Cancer Prevention Research Institute of Texas (CPRIT, R1309), the National Science Foundation (NSF, CHE-1764240), and the National Institute of General Medical Sciences of the National Institutes of Health (NIGMS-NIH, R01GM136759).
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c00350.
Experimental procedures, compound characterization, and NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Antunes E. M.; Copp B. R.; Davies-Coleman M.; Samaai T. Pyrroloiminoquinone and related metabolites from marine sponges. Nat. Prod. Rep. 2005, 22, 62–72. 10.1039/b407299p. [DOI] [PubMed] [Google Scholar]
- Radisky D. C.; Radisky E. S.; Barrows L. R.; Copp B. R.; Kramer R. A.; Ireland C. M. Novel cytotoxic topoisomerase II inhibiting pyrroloiminoquinones from Fijian sponges of the genus Zyzzya. J. Am. Chem. Soc. 1993, 115, 1632–1638. 10.1021/ja00058a003. [DOI] [Google Scholar]
- Schmidt E. W.; Harper M. K.; Faulkner D. J. Makaluvamines H-M and Damirone C from the Pohnpeian Sponge Zyzzya fuliginosa. J. Nat. Prod. 1995, 58, 1861–1867. 10.1021/np50126a008. [DOI] [PubMed] [Google Scholar]
- Molinski T. F. Marine pyridoacridine alkaloids: structure, synthesis, and biological chemistry. Chem. Rev. 1993, 93, 1825–1838. 10.1021/cr00021a009. [DOI] [Google Scholar]
- Fujioka H.; Kita Y.. Marine Pyrroloiminoquinone Alkaloids, Makaluvamines and Discorhabdins, and Marine Pyrrole-Imidazole Alkaloids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat K. G., Mérillon J., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp 251–283. [Google Scholar]
- Peat A. J.; Buchwald S. L. Novel Syntheses of Tetrahydropyrroloquinolines: Applications to Alkaloid Synthesis. J. Am. Chem. Soc. 1996, 118, 1028–1030. 10.1021/ja953080t. [DOI] [Google Scholar]
- Iwao M.; Motoi O.; Fukuda T.; Ishibashi F. New synthetic approach to pyrroloiminoquinone marine alkaloids. Total synthesis of makaluvamines A, D, I, and K. Tetrahedron 1998, 54, 8999–9010. 10.1016/S0040-4020(98)00543-2. [DOI] [Google Scholar]
- Sadanandan E. V.; Pillai S. K.; Lakshmikantham M. V.; Billimoria A. D.; Culpepper J. S.; Cava M. P. Efficient Syntheses of the Marine Alkaloids Makaluvamine D and Discorhabdin C: The 4,6,7-Trimethoxyindole Approach. J. Org. Chem. 1995, 60, 1800–1805. 10.1021/jo00111a043. [DOI] [Google Scholar]
- Tao X. L.; Cheng J. F.; Nishiyama S.; Yamamura S. Synthetic Studies on Tetrahydropyrroloquinoline-Containing Natural Producs: Syntheses of Discorhabdin C, Batzelline C, and Isobatzelline C. Tetrahedron 1994, 50, 2017–2028. 10.1016/S0040-4020(01)85065-1. [DOI] [Google Scholar]
- Hu J.; Fan H.; Xiong J.; Wu S. Discorhabdins and Pyrroloiminoquinone-Related Alkaloids. Chem. Rev. 2011, 111, 5465–5491. 10.1021/cr100435g. [DOI] [PubMed] [Google Scholar]
- Ma C.; Liu X.; Li X.; Flippen-Anderson J.; Yu S.; Cook J. M. Efficient Asymmetric Synthesis of Biologically Important Tryptophan Analogues via a Palladium-Mediated Heteroannulation Reaction. J. Org. Chem. 2001, 66, 4525–4542. 10.1021/jo001679s. [DOI] [PubMed] [Google Scholar]
- Newhouse T.; Baran P. S. Total Synthesis of (±)-Psychotrimine. J. Am. Chem. Soc. 2008, 130, 10886–10887. 10.1021/ja8042307. [DOI] [PubMed] [Google Scholar]
- Garfunkle J.; Kimball F. S.; Trzupek J. D.; Takizawa S.; Shimamura H.; Tomishima M.; Boger D. L. Total Synthesis of Chloropeptin II (Complestatin) and Chloropeptin I. J. Am. Chem. Soc. 2009, 131, 16036–16038. 10.1021/ja907193b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breazzano S. P.; Boger D. L. Synthesis and Stereochemical Determination of Complestatin A and B (Neuroprotectin A and B). J. Am. Chem. Soc. 2011, 133, 18495–18502. 10.1021/ja208570q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nesic M.; Ryffel D. B.; Maturano J.; Shevlin M.; Pollack S. R.; Gauthier D. R. J.; Trigo-Mouriño P.; Zhang L.; Schultz D. M.; McCabe Dunn J. M.; Campeau L.; Patel N. R.; Petrone D. A.; Sarlah D. Total Synthesis of Darobactin A. J. Am. Chem. Soc. 2022, 144, 14026–14030. 10.1021/jacs.2c05891. [DOI] [PubMed] [Google Scholar]; b Lin Y.; Schneider F.; Eberle K. J.; Chiodi D.; Nakamura H.; Reisberg S. H.; Chen J.; Saito M.; Baran P. S. Atroposelective Total Synthesis of Darobactin A. J. Am. Chem. Soc. 2022, 144, 14458–14462. 10.1021/jacs.2c05892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissner A.; Floyd M. B.; Johnson B. D.; Fraser H.; Ingalls C.; Nittoli T.; Dushin R. G.; Discafani C.; Nilakantan R.; Marini J.; Ravi M.; Cheung K.; Tan X.; Musto S.; Annable T.; Siegel M. M.; Loganzo F. 2-(Quinazolin-4-ylamino)-[1,4]benzoquinones as Covalent-Binding, Irreversible Inhibitors of the Kinase Domain of Vascular Endothelial Growth Factor Receptor-2. J. Med. Chem. 2005, 48, 7560–7581. 10.1021/jm050559f. [DOI] [PubMed] [Google Scholar]
- Zou Y.; Li X.; Yang Y.; Berritt S.; Melvin J.; Gonzales S.; Spafford M.; Smith A. B. III Total Synthesis of (−)-Nodulisporic Acids D, C, and B: Evolution of a Unified Synthetic Strategy. J. Am. Chem. Soc. 2018, 140, 9502–9511. 10.1021/jacs.8b04053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon H. F.; Gaunt M. J.; Ley S. V. Addition of Dithiols to Bis-Ynones: Development of a Versatile Platform for the Synthesis of Polyketide Natural Products. Org. Lett. 2003, 5, 1147–1150. 10.1021/ol034248f. [DOI] [PubMed] [Google Scholar]
- Gathergood N.; Scammells P. J. Preparation of the 4-Hydroxytryptamine Scaffold via Palladium-Catalyzed Cyclization: A Practical and Versatile Synthesis of Psilocin. Org. Lett. 2003, 5, 921–923. 10.1021/ol0341039. [DOI] [PubMed] [Google Scholar]
- Batail N.; Bendjeriou A.; Lomberget T.; Barret R.; Dufaud V.; Djakovitch L. First Heterogeneous Ligand- and Salt-Free Larock Indole Synthesis. Adv. Synth. Catal. 2009, 351, 2055–2062. 10.1002/adsc.200900386. [DOI] [Google Scholar]
- Larock R. C.; Yum E. K. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690. 10.1021/ja00017a059. [DOI] [Google Scholar]
- Jackson Y. A.; Billimoria A. D.; Sadanandan E. V.; Cava M. P. Regioselective Amination of Indole-4,7-quinones. J. Org. Chem. 1995, 60, 3543–3545. 10.1021/jo00116a049. [DOI] [Google Scholar]
- Hess W.; Burton J. W. Palladium-Catalysed Cyclisation of N-Alkynyl Aminomalonates. Chem.—Eur. J. 2010, 16, 12303–12306. 10.1002/chem.201001951. [DOI] [PubMed] [Google Scholar]
- Legentil L.; Benel L.; Bertrand V.; Lesur B.; Delfourne E. Synthesis and Antitumor Characterization of Pyrazolic Analogues of the Marine Pyrroloquinoline Alkaloids: Wakayin and Tsitsikammamines. J. Med. Chem. 2006, 49, 2979–2988. 10.1021/jm051247f. [DOI] [PubMed] [Google Scholar]
- Kita Y.; Tohma H.; Inagaki M.; Hatanaka K.; Yakura T. Total synthesis of discorhabdin C: a general aza spiro dienone formation from O-silylated phenol derivatives using a hypervalent iodine reagent. J. Am. Chem. Soc. 1992, 114, 2175–2180. 10.1021/ja00032a036. [DOI] [Google Scholar]
- Aubart K. M.; Heathcock C. H. A Biomimetic Approach to the Discorhabdin Alkaloids: Total Syntheses of Discorhabdins C and E and Dethiadiscorhabdin D. J. Org. Chem. 1999, 64, 16–22. 10.1021/jo9815397. [DOI] [PubMed] [Google Scholar]
- Rives A.; Delaine T.; Legentil L.; Delfourne E. Total synthesis of the marine pyrroloiminoquinone alkaloid tsitsikammamine A. Tetrahedron Lett. 2009, 50, 1128–1130. 10.1016/j.tetlet.2008.12.078. [DOI] [Google Scholar]
- Efforts to convert 6b to 6a proved difficult. Treatment of 6b under typical desilylation conditions failed to deliver the desired compound or any recognizable product. Therefore, we elected to advance 6b in the hope that removal of the TES group could be accomplished late-stage.
- Izawa T.; Nishiyama S.; Yamamura S. Total syntheses of makaluvamines A, B, C, D and E, cytotoxic pyrroloiminoquinone alkaloids isolated from marine sponge bearing inhibitory activities against topoisomerase II. Tetrahedron 1994, 50, 13593–13600. 10.1016/S0040-4020(01)85674-X. [DOI] [Google Scholar]
- Schmidt E. W.; Harper M. K.; Faulkner D. J. Makaluvamines H-M and Damirone C from the Pohnpeian Sponge Zyzzya fuliginosa. J. Nat. Prod. 1995, 58, 1861–1867. 10.1021/np50126a008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information