Abstract
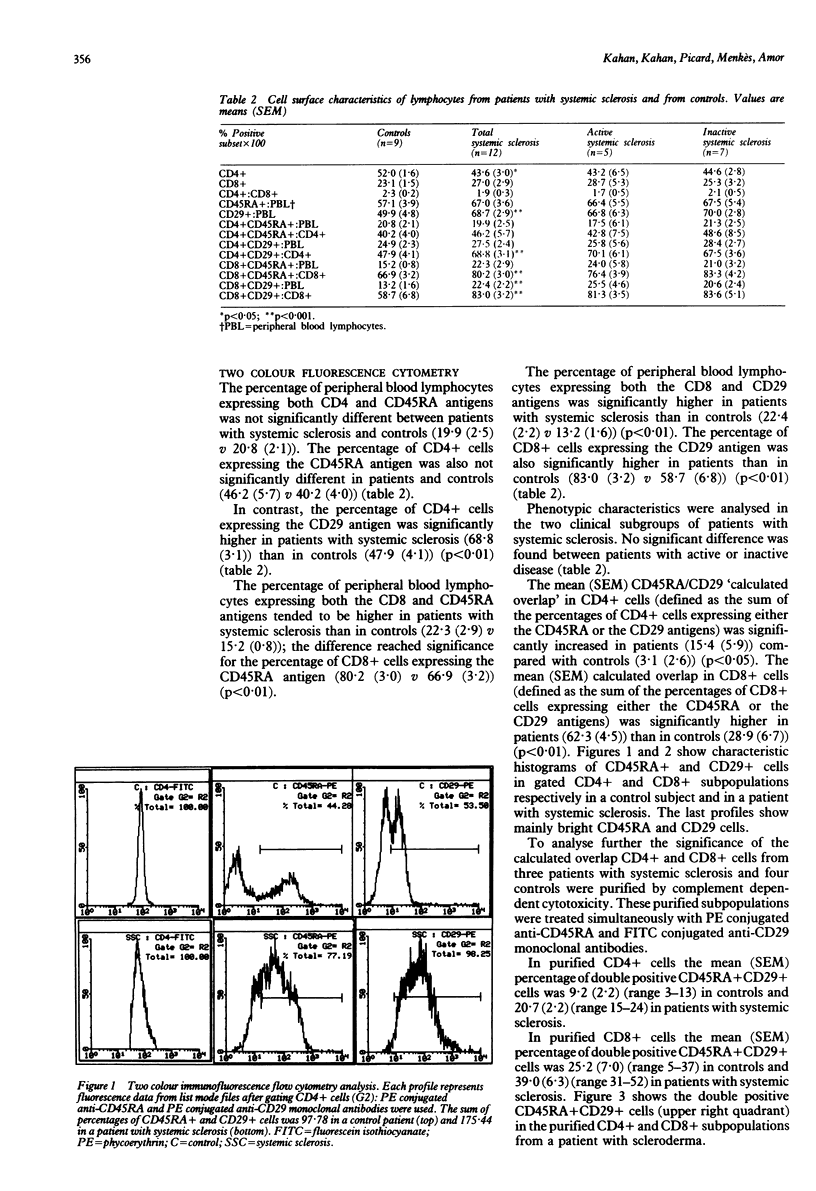
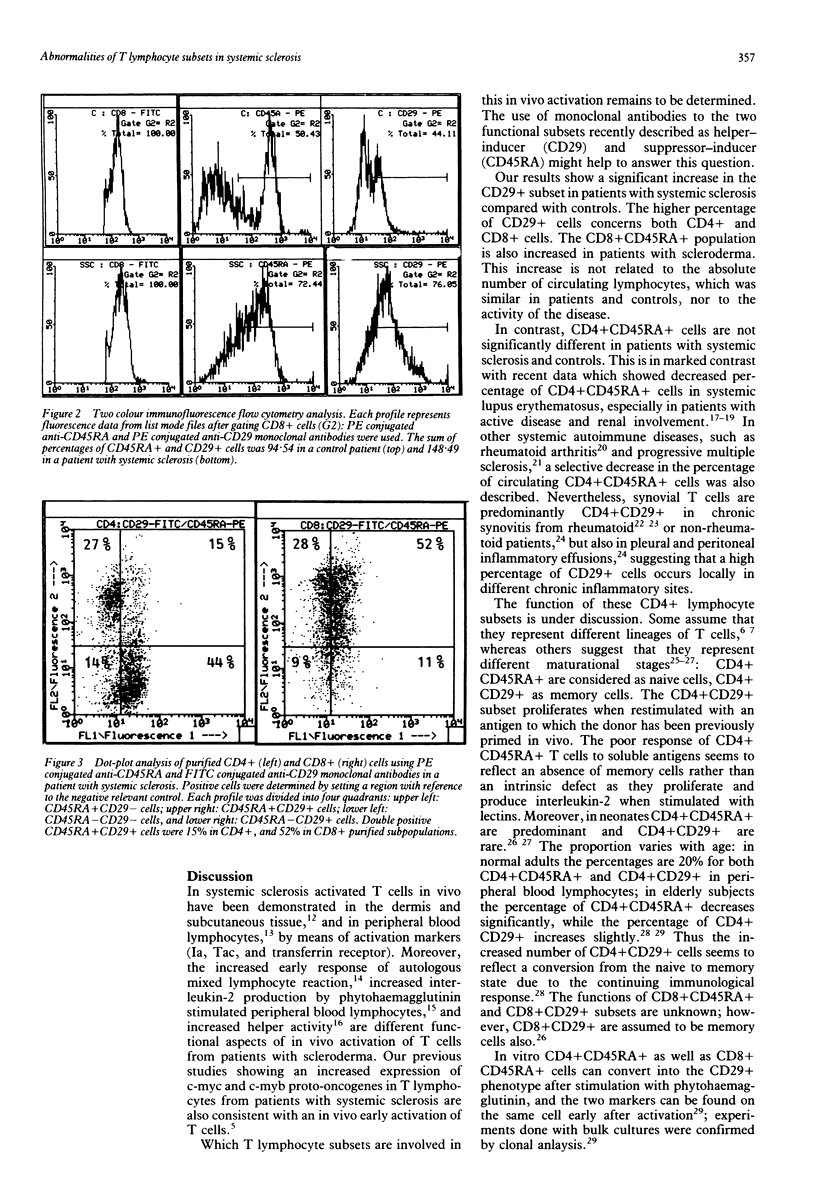
T cell subpopulations were assessed by two colour flow cytometry with phycoerythrin conjugated anti-CD45RA and anti-CD29 and fluorescein conjugated anti-CD4 and anti-CD8 monoclonal antibodies, on peripheral blood lymphocytes from 12 patients with systemic sclerosis and from nine control subjects. The percentage of CD4+CD29+ cells was significantly higher in patients with systemic sclerosis than in controls (mean (SEM) 68.8 (3.1) v 47.9 (4.1) respectively). CD4+CD45RA+ cells were not significantly different in patients and controls. CD8+CD29+ and CD8+CD45RA+ subpopulations were significantly higher in patients with systemic sclerosis than in controls (83.0 (3.2) v 58.7 (6.8) and 80.2 (3.0) v 66.9 (3.2) respectively). The increase in the percentage of CD29+ cells suggests an activation of memory cells in patients with systemic sclerosis, which may play an important part in the pathogenesis of the disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Alcocer-Varela J., Laffón A., Alarcón-Segovia D., Ibañez de Kasep G. Early proliferative response in the human autologous mixed lymphocyte reaction in scleroderma. J Rheumatol. 1984 Feb;11(1):48–52. [PubMed] [Google Scholar]
- Clement L. T., Yamashita N., Martin A. M. The functionally distinct subpopulations of human CD4+ helper/inducer T lymphocytes defined by anti-CD45R antibodies derive sequentially from a differentiation pathway that is regulated by activation-dependent post-thymic differentiation. J Immunol. 1988 Sep 1;141(5):1464–1470. [PubMed] [Google Scholar]
- De Paoli P., Battistin S., Santini G. F. Age-related changes in human lymphocyte subsets: progressive reduction of the CD4 CD45R (suppressor inducer) population. Clin Immunol Immunopathol. 1988 Sep;48(3):290–296. doi: 10.1016/0090-1229(88)90022-0. [DOI] [PubMed] [Google Scholar]
- Emery P., Gentry K. C., Mackay I. R., Muirden K. D., Rowley M. Deficiency of the suppressor inducer subset of T lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1987 Aug;30(8):849–856. doi: 10.1002/art.1780300802. [DOI] [PubMed] [Google Scholar]
- Freundlich B., Jimenez S. A. Phenotype of peripheral blood lymphocytes in patients with progressive systemic sclerosis: activated T lymphocytes and the effect of D-penicillamine therapy. Clin Exp Immunol. 1987 Aug;69(2):375–384. [PMC free article] [PubMed] [Google Scholar]
- Inoshita T., Whiteside T. L., Rodnan G. P., Taylor F. H. Abnormalities of T lymphocyte subsets in patients with progressive systemic sclerosis (PSS, scleroderma). J Lab Clin Med. 1981 Feb;97(2):264–277. [PubMed] [Google Scholar]
- Kahan A., Gerfaux J., Kahan A., Joret A. M., Menkès C. J., Amor B. Increased proto-oncogene expression in peripheral blood T lymphocytes from patients with systemic sclerosis. Arthritis Rheum. 1989 Apr;32(4):430–436. doi: 10.1002/anr.1780320412. [DOI] [PubMed] [Google Scholar]
- Kahan A., Kahan A., Menkes C. J., Amor B. Defective Epstein-Barr virus specific suppressor T cell function in progressive systemic sclerosis. Ann Rheum Dis. 1986 Jul;45(7):553–560. doi: 10.1136/ard.45.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky H. P., Bauer K., Pope R. M. Increased helper inducer and decreased suppressor inducer phenotypes in the rheumatoid joint. Arthritis Rheum. 1988 Jan;31(1):52–59. doi: 10.1002/art.1780310108. [DOI] [PubMed] [Google Scholar]
- Moore K., Nesbitt A. M. Functional heterogeneity of CD4+ T lymphocytes: two subpopulations with counteracting immunoregulatory functions identified with the monoclonal antibodies WR16 and WR19. Immunology. 1987 Jun;61(2):159–165. [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Hafler D. A., Weiner H. L., Letvin N. L., Hagan M., Daley J., Schlossman S. F. Selective loss of the suppressor-inducer T-cell subset in progressive multiple sclerosis. Analysis with anti-2H4 monoclonal antibody. N Engl J Med. 1987 Jan 8;316(2):67–72. doi: 10.1056/NEJM198701083160202. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Morimoto C., Steinberg A. D., Letvin N. L., Hagan M., Takeuchi T., Daley J., Levine H., Schlossman S. F. A defect of immunoregulatory T cell subsets in systemic lupus erythematosus patients demonstrated with anti-2H4 antibody. J Clin Invest. 1987 Mar;79(3):762–768. doi: 10.1172/JCI112882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Murphy J., Panayi G. Abnormal distribution of the helper-inducer and suppressor-inducer T-lymphocyte subsets in the rheumatoid joint. Clin Immunol Immunopathol. 1987 Nov;45(2):252–258. doi: 10.1016/0090-1229(87)90040-7. [DOI] [PubMed] [Google Scholar]
- Roumm A. D., Whiteside T. L., Medsger T. A., Jr, Rodnan G. P. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984 Jun;27(6):645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Sato K., Miyasaka N., Yamaoka K., Okuda M., Yata J., Nishioka K. Quantitative defect of CD4+2H4+ cells in systemic lupus erythematosus and Sjögren's syndrome. Arthritis Rheum. 1987 Dec;30(12):1407–1411. doi: 10.1002/art.1780301212. [DOI] [PubMed] [Google Scholar]
- Serra H. M., Krowka J. F., Ledbetter J. A., Pilarski L. M. Loss of CD45R (Lp220) represents a post-thymic T cell differentiation event. J Immunol. 1988 Mar 1;140(5):1435–1441. [PubMed] [Google Scholar]
- Takeuchi T., Schlossman S. F., Morimoto C. The T4 molecule differentially regulating the activation of subpopulations of T4+ cells. J Immunol. 1987 Aug 1;139(3):665–671. [PubMed] [Google Scholar]
- Takeuchi T., Tanaka S., Steinberg A. D., Matsuyama T., Daley J., Schlossman S. F., Morimoto C. Defective expression of the 2H4 molecule after autologous mixed lymphocyte reaction activation in systemic lupus erythematosus patients. J Clin Invest. 1988 Oct;82(4):1288–1294. doi: 10.1172/JCI113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara H., Kumagai S., Ishida H., Suginoshita T., Maeda M., Imura H. Enhanced production of interleukin-2 in patients with progressive systemic sclerosis. Hyperactivity of CD4-positive T cells? Arthritis Rheum. 1988 Mar;31(3):401–407. doi: 10.1002/art.1780310312. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Kumagai Y., Roumm A. D., Almendinger R., Rodnan G. P. Suppressor cell function and T lymphocyte subpopulations in peripheral blood of patients with progressive systemic sclerosis. Arthritis Rheum. 1983 Jul;26(7):841–847. doi: 10.1002/art.1780260704. [DOI] [PubMed] [Google Scholar]


