Abstract
Uranium is a naturally occurring element found in the environment as a mixture of isotopes with differing radioactive properties. Enrichment of mined material results in depleted uranium waste with substantially reduced radioactivity but retains the capacity for chemical toxicity. Uranium mine and milling waste are dispersed by wind and rain leading to environmental exposures through soil, air, and water contamination. Uranium exposure is associated with numerous adverse health outcomes in humans, yet there is limited understanding of the effects of depleted uranium on the immune system. The purpose of this review is to summarize findings on uranium immunotoxicity obtained from cell, rodent and human population studies. We also highlight how each model contributes to an understanding of mechanisms that lead to immunotoxicity and limitations inherent within each system. Information from population, animal, and laboratory studies will be needed to significantly expand our knowledge of the contributions of depleted uranium to immune dysregulation, which may then inform prevention or intervention measures for exposed communities.
Keywords: Uranium, Immunotoxicity, Human exposure
1. Introduction
Uranium is a naturally occurring element found in the soil that consists of three isotopes: 238U (99.27%), 235U (0.72%), and 234U (0.0054%) (Agency for Toxic Substance and Disease Registry, 2013; US EPA, O, 2018). Natural uranium (NU) is the source material for enriched 235U (EU) used in nuclear power generation and for military applications. Depleted uranium (DU) is a byproduct of EU production that has both military and civilian uses (US EPA, O, 2018). DU and NU are radioactive and mainly emit alpha particle radiation. Alpha particles do not penetrate the skin but can exert radiological toxicity if ingested, inhaled, or embedded into tissue (Agency for Toxic Substance and Disease Registry, 2013; US EPA, O, 2018). NU and DU also display chemical toxicity that is less well characterized than the radiation effects.
NU located close to the surface is mined from open pits, which removes the overlying rock as well as the rock surrounding the uranium deposit. Deeper deposits are mined from underground tunnels. Milling and leaching are procedures to remove uranium from the ore. Uranium mining and processing leave behind waste contaminated with elevated levels of NU, DU and other metals that can be dispersed by the wind and rain leading to soil, air and water contamination (Agency for Toxic Substance and Disease Registry, 2013; Ravalli et al., 2022; US EPA, O, 2018; US EPA, R. 09, 2016a; World Health Organization, 2001). There are many examples of environmental uranium contamination associated with mining. High levels of uranium were found in surface sediments downstream of uranium mining and milling areas in the Guangdong Provence of China (Liu et al., 2015) and in soil samples taken at the old Mortórios uranium mine in central Portugal (Neiva et al., 2019). Moreover, distribution of uranium from that mine created hot spots of uranium southeast of the mine site speaking to contaminant mobility in the environment. Uranium mining in the Western US has left behind ~5000 abandoned uranium mines (AUMs) (Lewis et al., 2015; US EPA, R. 09, 2016b) and the United States Environmental Protection Agency (EPA) estimates that AUMs have contaminated 40% of the headwaters of surface waterways in the West (United States Environmental Protection Agency, 2000). Uranium levels as high as 772 μg/L were measured in the Rio Paguate downstream of the Jackpile mine on Laguna Pueblo (Blake et al., 2017). The levels of uranium are elevated in unregulated water sources on the Navajo Nation with 12.5% of the measured sites exceeding the maximum contaminant level (Hoover et al., 2017; Lewis et al., 2017). Fifty-two percent of the wells in the Nambe region of northern New Mexico exceeded the EPA-recommended drinking water standard for uranium (Hakonson-Hayes et al., 2002). A recent study reviewed compliance with EPA-regulated metal standards in community water sources including 128,000 records for uranium (Ravalli et al., 2022). The study reported that 2.1% of the sources had average uranium concentrations exceeding the EPA maximum contaminant limit of 30 μg/L and uranium was detected in 63.1% of the records of community water sources (Ravalli et al., 2022). Nolan and Weber estimated uranium in excess of the maximum contamination limit across an expansive area (22,375 km) of the High Plains (Colorado, Kansas, Nebraska, New Mexico, Oklahoma, South Dakota, Texas, and Wyoming) and Central Valley (California) aquifers, potentially exposing 1.9 million residents in nearby communities to contaminated groundwater (Nolan and Weber, 2015). These finding demonstrate that uranium contamination of public drinking water is more widespread than previously appreciated.
Human environmental exposure to uranium is largely through ingestion of contaminated drinking water and food, and inhalation of uranium containing dusts or soil particulates (Agency for Toxic Substance and Disease Registry, 2013). DU exposures are also apparent in areas of military conflict where DU was used in munitions or embedded DU-containing material (Ma et al., 2020). The chronic uranium exposures from living near AUMs or associated uranium waste sites raises questions regarding the potential health consequences (Brugge and Buchner, 2011; Lewis et al., 2017; Ma et al., 2020). As one example, biomonitoring data obtained from the Navajo Birth Cohort Study confirms that median levels of urinary uranium are elevated two to three-fold in residents of the Navajo Nation when compared to values obtained from the National Health and Nutrition Examination Survey (Dashner-Titus et al., 2018; Hoover et al., 2020). Exposure to NU or DU is associated with a wide range of adverse health effects including those related to the renal, respiratory and cardiovascular systems (Agency for Toxic Substance and Disease Registry, 2013; Brugge and Buchner, 2011; Lewis et al., 2017). Increased risk of kidney disease, diabetes and hypertension was associated with uranium exposure during the mining era and with exposures related to unremediated AUMs in Navajo communities (Hund et al., 2015; Lewis et al., 2017). Much of the experimental research on uranium has been conducted in cells and tissues reflecting possible exposure, accumulation or excretion routes such as kidney, liver and lung (Asic et al., 2017; Ma et al., 2020). The impact of uranium exposure on the immune system has received less attention.
The purpose of this review is to summarize the reported immunotoxic effects of NU and DU on immune cells in vitro, rodent models and human populations with an emphasis on the chemical toxicity of uranium. This literature review was conducted by identifying peer-reviewed publications through PubMed that assessed the effects of uranium on immune cells or functions. Search terms included “uranyl”, “uranium”, “depleted uranium,” or “natural uranium” and the following terms “immune or immunotoxicity,” “T-cell,” “B-cell”, “macrophages”, “DNA damage”, “genotoxicity”, oxidative stress”, “apoptosis”, “cytokine”, “cell + uptake”, “inflammation”, “rat”, “mouse”, “human health”, and “population or exposure”. We focused on studies that evaluated the immunotoxicity of a quantified uranium exposure either through defined treatment doses, environmental survey or biomonitoring protocols including urinary analysis. Papers that focused on radiation toxicity were excluded.
2. Effects of uranium on immune cells in vitro
Cell culture models provide an important method to assess the potential molecular mechanisms of toxic agents. In vitro studies investigating the effects of uranium have been conducted using various immune cells including immortalized or tumor-derived T-cell (e.g. Jurkat) and monocytic cell lines (e.g. THP-1) and mixed populations of lymphocytes or immune cell subtypes isolated from mice or humans then treated ex vivo. Depending on the study, primary cells were isolated from blood, specific immune organs such as thymus or spleen, or anatomical sites such as the peritoneal cavity or lung (Fig. 1). The following section will focus largely on results obtained from immortalized cell lines and primary cells isolated from blood or immune organs.
Fig. 1.
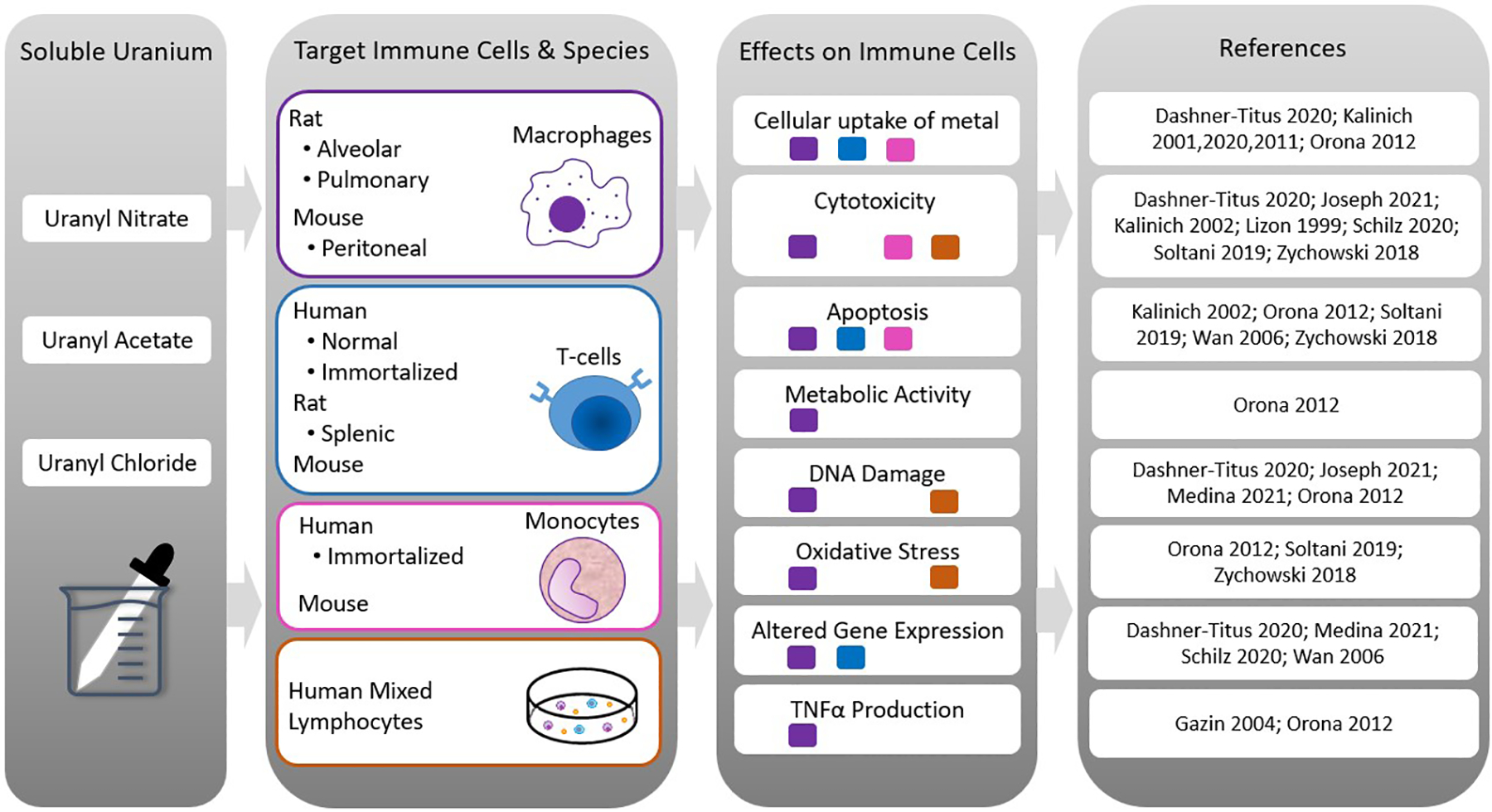
Summary of results from in vitro studies. The first column lists the most commonly used soluble uranium forms. The second column lists the species of origin and target immune cells used for the in vitro studies. The third column summarizes results from the in vitro treatments. The fourth column contains the corresponding results references. Squares under the stated effect correspond to the target immune cells displaying the response (purple = macrophages; blue = T-cells; pink = monocytes; orange = human mixed lymphocytes).
2.1. Uptake
There is limited information on uranium uptake into cultured immune cells in vitro. Orona and Tasat (2012) visualized uranium using a pyridylazo indicator dye and found permeation following ex vivo treatment of rat alveolar macrophage with 100 μM uranyl nitrate (UN) for 24 h (Orona and Tasat, 2012). The same staining method detected uptake of uranium into the J774 mouse macrophage cell line but not into the MOLT-4 human T-cell line or the REH human B-cell line (Kalinich and McClain, 2001; Kalinich, 2011; Kalinich et al., 2002). Uranium uptake by J774 macrophages was also detectable after 2 h treatment with 100 μM uranyl chloride (UC) (Kalinich et al., 2002). Quantification of uranium by inductively coupled plasma mass spectrometry in immortalized T-cells (Jurkats) after 24 h exposure to 3 μM and 30 μM uranyl acetate (UA) showed 1.62 and 766.8 parts per billion (ppb) uranium per million cells, respectively (Dashner-Titus et al., 2020). The study found substantially lower levels of cell-associated uranium in immortalized monocytes (THP-1 cells) compared to Jurkat cells under the same exposure conditions (Dashner-Titus et al., 2020). Dose-dependent uranium uptake has been reported for other non-immune cell lines (Paredes et al., 2018; Yellowhair et al., 2018), but the mechanisms of cellular uptake remain unclear. There is evidence that uranium uptake can occur through an endocytic mechanism (Hémadi et al., 2011; Liu et al., 2015; Muller et al., 2008) that may involve the iron acquisition pathway (El Hage Chahine et al., 2012; Hémadi et al., 2011) or by sodium-dependent phosphate co-transport as reported for kidney cells (Muller et al., 2006, 2008). However, these proposed mechanisms have not yet been tested in immune cells. Further research on the mechanism of uranium uptake may be important for understanding cell and tissue type differences in toxicity.
2.2. Cytotoxicity, apoptosis and DNA damage
Several studies tested the cytotoxicity of uranium in immune cells using soluble forms such as UA or UN. UA caused a dose-dependent reduction of viability in Ficoll isolated human lymphocytes with a calculated half maximal inhibitory concentration (IC50) value of 0.8 mM after 6 h of exposure (Soltani et al., 2019). A study using phytohemagglutinin-stimulated normal human lymphocytes treated with 128 or 1280 μM UN for 96 h reported 5% and 50% cell death, respectively (Joseph et al., 2021). Normal human CD4+ T-cells activated with CD3/CD28 and exposed to 30 μM UA for 72 h showed no significant cytotoxicity when compared to the no-treatment control (Schilz et al., 2020). Similarly, human Jurkat and THP-1 cells remained viable following exposure to 100 μM UA for 24 or 48 h (Dashner-Titus et al., 2020), whereas 24 h treatment of mouse macrophage J774 cells with 100 μM UC decreased viability by approximately 30% (Kalinich et al., 2002). The cytotoxicity of uranium-containing particulate matter (PM) was tested with particles derived from uranium mine waste (Zychowski et al., 2018). Exposures of 1.5 μg/mL for 24 h were cytotoxic to THP-1 cells as measured by LDH release, and the magnitude of toxicity increased with higher exposure levels (Zychowski et al., 2018).
Uranium-stimulated apoptosis has been reported in immune cells. Exposure of mouse J774 macrophages to UC led to dose-dependent annexin V labeling that was significantly elevated at the 10 μM dose with maximal response observed at 100 μM UC compared to untreated control (Kalinich et al., 2002). Similarly, treatment of rat alveolar macrophages ex vivo with 25–100 μM UN for 24 h significantly increased the percentage of active caspase 3-positive cells versus control (Orona and Tasat, 2012). Under the same conditions, mitochondrial metabolic activity was decreased by UN concentrations of ≥100 μM (Orona and Tasat, 2012). In contrast, ex vivo treatment of rat alveolar macrophages with 100 μM UA for 3d was not cytotoxic, but significant cell death occurred at higher doses of 500 and 1000 μM (live fraction <5%). The observed cytotoxicity at the highest concentrations of UN was attributed to insoluble forms of uranium (Lizon, 1999). One study compared apoptotic response in pooled peritoneal macrophages and CD4+ splenic T-cells isolated from mice then treated with UN ex vivo for 24 h (Wan et al., 2006). Annexin V and propidium iodide staining was significantly increased in the peritoneal macrophage at UN doses of 100 and 200 μM, whereas for T-cells the apoptotic response was evident only at 500 μM UN with a lower proportion of cells positive for apoptotic markers relative to peritoneal macrophages (Wan et al., 2006). Macrophage function as determined by phagocytic index was also impaired by UN treatment (Wan et al., 2006). Significant mitochondrial membrane potential collapse has been reported in mixed human lymphocytes, but only at UA doses of ≥400 μM (Soltani et al., 2019). At 100 μg/mL, uranium-containing particulate matter (PM) significantly increased the fraction of THP-1 cells expressing caspase-1 compared to background PM and phagocytosis was significantly decreased at lower concentrations (Zychowski et al., 2018).
Excess DNA damage contributes to cytotoxicity and apoptosis. An increase in DNA strand breaks as measured by single-cell gel electrophoresis (comet assay) was detected after exposure of normal human lymphocytes to 250 μM UN for 96 h (Joseph et al., 2021) but not in Jurkat cells treated with 100 μM UA for 6 h as detected by phospho-Histone H2A.X staining (Dashner-Titus et al., 2020). Enzymatic activity of poly-ADP-ribose polymerase-1 (PARP-1) is stimulated in response to DNA damage, so increased PARP activity may be an indirect indicator of DNA damage (Ray Chaudhuri and Nussenzweig, 2017). Significant increases in PARP-1-positive cells were detected in rat alveolar macrophages treated ex vivo with 25–100 μM UN for 24 h compared to control (Orona and Tasat, 2012). Primary mouse thymus cells exposed ex vivo to 5 or 50 μM UA for 18 h displayed a modest increase in PARP-1 activity (Medina et al., 2021).
Based on the limited evidence available, the cytotoxic, apoptotic and DNA damaging effects of uranium in immune cells treated in vitro or ex vivo are not generally detected after short term exposure to low micro-molar concentrations but may manifest at concentrations ≥ ~100 μM. The current EPA MCL for uranium is 30 μg/L and uranium levels detected in well water in the High Plains (Colorado, Kansas, Nebraska, New Mexico, Oklahoma, South Dakota, Texas, and Wyoming) and Central Valley (California) aquifers were as high as 89-fold and 180-fold the MCL respectively (Nolan and Weber, 2015). Therefore, in vitro treatments of 100 μM are approximately 4-fold greater than the upper range of environmental concentrations in the US. Research in other cell types and experimental models indicates that DU has cytotoxic and genotoxic potential (Asic et al., 2017; Ma et al., 2020). There is evidence for accumulation of uranium in the nucleus (Guéguen et al., 2015; Yellowhair et al., 2018), and DU can cause oxidative DNA damage and DNA adducts in cell-free systems without radiation (Miller et al., 2002; Wilson et al., 2014). It is interesting to note that cytotoxic and genotoxic effects of UA are more evident in cells with certain defined DNA repair deficiencies when compared to parental cells (Coryell and Stearns, 2006; Yellowhair et al., 2018). Research also suggests that during V(D)J recombination mammalian lymphoid cells generate DNA double-strand breaks and that DNA repair deficiencies or mutations in these pathways could alter the development of T and B cells (Prochazkova and Loizou, 2016). This suggests that some individuals may be more sensitive to uranium based on underlying DNA repair capacity.
2.3. Oxidative stress response
Reactive oxygen species (ROS) is a broad term for derivatives of molecular oxygen that are reactive and when in excess may lead to cellular damage (Sies and Jones, 2020). Metal-induced oxidative stress can be due to generation of ROS, depletion of the antioxidant defense system or by impairing mitochondrial function (Ma et al., 2020). ROS may alter cellular redox homeostasis and regulate numerous cellular functions including signal transduction and transcriptional response (Reczek and Chandel, 2015; Sies and Jones, 2020).
Oxidative stress is a common cellular response to metal exposure (Valko et al., 2016), and uranium-induced ROS has been reported in several experimental cell models (Daraie et al., 2012; Garmash et al., 2014; Ma et al., 2020; Periyakaruppan et al., 2007). There is limited information on uranium and oxidative stress in immune cells. Soltani et al. (2019) demonstrated a time- and dose-dependent increase in ROS production and decrease in glutathione levels using Ficoll-isolated human lymphocytes treated with UA (Soltani et al., 2019). In addition, lipid peroxidation and lysosomal membrane destabilization were evident at each UA concentration tested (0.4, 0.8 and 1.6 mM). Orona and Tasat (2012) reported an increase in superoxide anion release in rat alveolar macrophages at concentrations as low as 12.5 μM UN compared to untreated control (Orona and Tasat, 2012). Uranium-containing PM enhanced oxidative stress over control PM as measured in THP-1 cells as measured by dihydroethidium staining and lipid peroxidation (Zychowski et al., 2018). Thus, there is evidence that both soluble and particulate uranium can stimulate an oxidative stress response.
Production of ROS stimulates signal transduction leading to gene expression through the redox sensitive nuclear factor-erythroid factor 2-related factor 2 (Nrf-2) and the nuclear factor kappa B (NF-kB) pathways (He et al., 2020; Lingappan, 2018; Ma, 2013; Sies and Jones, 2020). There was no evidence for an increase in Nrf-2 nuclear localization nor induction of the Nrf-2 target oxidative stress genes HMOX1 or NQO1 after treatment of Jurkat cells with 30 μM UA for up to 24 h (Dashner-Titus et al., 2020). Furthermore, in human CD4+ T-cells activated with CD3/CD28 in the presence of 3 or 30 μM UA, there were no significantly altered activation-associated genes or changes in HMOX1 and NQO1 expression (Schilz et al., 2020). Treatment of primary mouse thymus cells ex vivo with 0.5 and 5 μM UA for 4 h revealed no increase in Hmox1 expression (Medina et al., 2021). These findings suggest that low concentrations of UA are insufficient to stimulate expression of these oxidative stress response genes in vitro.
2.4. Cytokines
Uranium-induced cytokine secretion or expression has been evaluated in immune cells in vitro. Gene expression analysis by microarray of mouse peritoneal macrophages exposed to 100 μM UN for 24 h reported significant alteration of 29/514 genes (Wan et al., 2006). Twenty-four genes were up-regulated including Rela (signal transduction), Mdk (cancer progression marker) and Il10 (implicated in Th2 shift of T-cell populations). Five genes were down-regulated including Ptgs2, Cash and Sdf5 which are all apoptosis-related genes (Wan et al., 2006). An increase in tumor necrosis factor alpha (TNFα) secretion was detected after 24 h treatment of rat alveolar macrophages with 100 or 200 μM UN (Orona and Tasat, 2012). TNFα production in response to UA was tested in rat pulmonary macrophages (Gazin et al., 2004). UA promoted a time- and dose-dependent increase in TNFα production. TNFα was elevated after 24 h exposure to 50 μM UA and plateaued at the 300 μM dose with no changes in interleukin (IL)-1β or IL-10. A significant increase in TNFα production over untreated control was evident after 14 h treatment with 100 μM UA and TNFα levels continued to increase over a 48 h time period (Gazin et al., 2004). The effects of lower dose and longer exposure duration was tested in the rat alveolar macrophage cell line NR8383. Treatment of NR8383 cells with 10 μM UA for 5 days increased TNFα production, and the response was partially mediated by both p38 mitogen-activated protein kinase and protein kinase C (Gazin et al., 2004).
Gene expression in splenic murine CD4+ T-cells exposed to 100 μM UN for 24 h differed from peritoneal macrophages isolated from the same animals (Wan et al., 2006). In the T-cells, only 14/514 genes were significantly altered as detected by microarray. Four genes including Il5 (Th2 type cytokine) and Mdk (cancer progression marker) were up-regulated and ten genes including Cmkbr4 and Scyd1 (chemokine related genes) and Lif (cytokine and receptor related gene) were down-regulated (Wan et al., 2006). Treatment of human CD4+ T-cells activated in the presence of 30 μM UA revealed no significant changes in gene expression as determined by RNA sequencing when compared to activation alone (Schilz et al., 2020).
The findings regarding uranium’s stimulation of ROS, ROS signaling pathways, and changes in cytokine production or gene expression in rodent or human immune cells are mixed. Differences in dose, form of uranium (soluble form or particulate) and cell species source may account for some discordant findings such as those detected for gene expression changes in mouse versus human CD4+ T-cells (Schilz et al., 2020; Wan et al., 2006). In vitro evidence in other cell types and in vivo tissue studies indicate uranium induces ROS, signaling and gene expression (Daraie et al., 2012; Linares et al., 2007; Lu et al., 2021; Shaki et al., 2013; Yi et al., 2019; Yue et al., 2018). Additional studies will be required to delineate the effects of uranium exposure more clearly on mixed and defined immune cell types.
3. Uranium exposure and immune effects in rodent models
Animal models are often used to gain insights into the potential consequences of human exposure to various metals beyond what can be elucidated from in vitro cellular systems. In contrast to other toxic environmental metals such as arsenic, there are relatively few studies reporting the immune effects of in vivo uranium exposures in rodent models. In this section, we will review and summarize findings on uranium distribution to immune tissues and the impact of uranium exposure on immunologic endpoints.
3.1. Uranium distribution to immune organs
Distribution of uranium to immune tissues has been reported following chronic uranium exposure in mice (Bolt et al., 2018; Hao et al., 2013). A dose-dependent accumulation of uranium in spleen and thymus was noted in Kunming mice after 4 months of exposure to UN in food (3, 30 and 300 mg/kg feed). At each dose, the levels of uranium in spleen and thymus were increased significantly above the no-treatment control. At the highest dose of 300 mg/kg in feed, the uranium concentration in spleen and thymus was elevated but approximately 10-fold lower than detected in bone and kidney (Hao et al., 2013). Measurements in other immune organs were not reported (Hao et al., 2013). A more recent study by Bolt et al. (2018) evaluated the tissue distribution of uranium in blood, bone marrow, spleen, and thymus and compared the values to those obtained in femur, kidney, and liver tissue. Male and female mice were exposed to UA in drinking water for 60 days at concentrations of 5 and 50 ppm (ppm) (Bolt et al., 2018). In contrast to the results reported by Hao et al., there was no significant accumulation of uranium in the spleen or thymus at either dose. However, uranium was detected in the blood and bone marrow above control levels at the 50-ppm dose (0.6 and 16.8 ng/g, respectively) in male mice only. Greater than 80% of uranium accumulation was in bone and kidney with some differences evident between sexes (Bolt et al., 2018). Though the majority of uranium uptake was observed in bone and kidney, both studies demonstrate detectable levels of uranium in immune cell organs that might account for alterations in immune cells as described in Fig. 2.
Fig. 2.
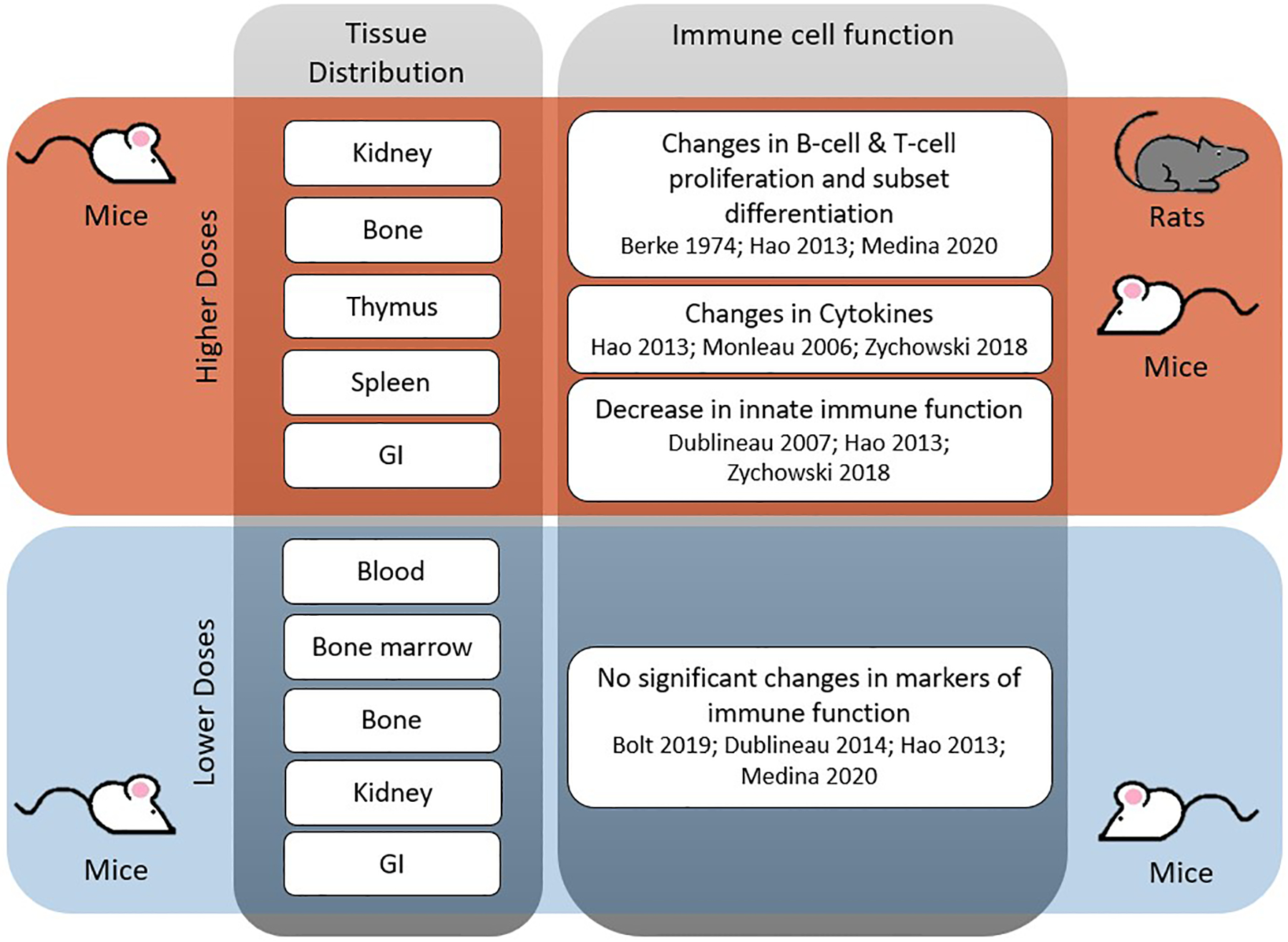
Summary of in vivo tissue distribution and immune cell function alterations by dose and model species. Grey and colored boxes create quadrants where red indicates exposure to higher uranium doses and blue indicates exposure to lower uranium doses. Column one lists tissues where uranium accumulation was detected in the corresponding species. Accumulation of uranium in immune tissues has been reported by Bolt et al., 2018 and Hao et al., 2013. Column two lists the changes in immune cell function that were measured with either higher (red quadrant) or lower (blue quadrant) doses in the corresponding species (references listed below each immune cell function).
3.2. Impact of uranium exposure on immune cell function
The impact of DU on immune cell function in vivo has been investigated. An early study found that intraperitoneal injection of UN at doses between 0.1 and 3 mg/kg in Sprague-Dawley rats induced a blastogenic effect in peripheral blood lymphocytes (Berke and Palazzolo, 1974). The effect was dose-dependent, rapid (evident within 24 h) and peaked approximately seven days after exposure (Berke and Palazzolo, 1974). Subsequent studies characterized proliferative response. Opposing findings were observed for B- or T- lymphocytes isolated from the spleens of mice chronically exposed to UN in feed (Hao et al., 2013). A dose-dependent increase in proliferation index was detected in splenic B-cells stimulated with lipopolysaccharide, whereas the T-cell proliferation index after stimulation with concanavalin A was decreased (Hao et al., 2013). In contrast, at lower dose exposures of 5 and 50 ppm UA in drinking water for 60 days, there were no significant differences in mitogen-induced proliferation of B- or T-cells in either male or female mice (Bolt et al., 2019). These doses of UA did not alter either the number or viability of cells isolated from spleen or thymus or the total or differential (lymphocyte, monocyte, or neutrophil) white blood cell counts. The authors concluded that exposure to 50 ppm UA did not lead to overt cytotoxicity of immune cells (Bolt et al., 2019). Similarly, male Sprague-Dawley rats treated with up to 120 mg/L UN in drinking water for 9 months displayed no significant differences in circulating white blood cells, lymphocytes, granulocytes, or monocytes or bone marrow progenitor frequencies as measured by burst-forming units erythroid and colony-forming units-granulocyte macrophages (Bolt et al., 2019; Dublineau et al., 2014).
Hao et al. reported changes in multiple markers of immune cell function following chronic dietary exposures to UN in male mice (Hao et al., 2013). Several measures of innate immune function were decreased in mice exposed to the highest dose of UN (300 mg/kg in feed) for 4 months including decreased secretion of nitric oxide, IL-1β, IL-18 and TNF-α in peritoneal macrophages and reduced cytotoxic activity of natural killer cells isolated from the spleen (Hao et al., 2013). There were also disruptions in humoral and cellular immune functions. These included an increase in total serum immunoglobulin levels with changes evident for IgG and IgE, changes in CD3+ positive cells, a decrease in the CD4+/CD8+ ratio, and a decrease in delayed-type hypersensitivity response to sheep red blood cells (Hao et al., 2013). Notably, these broad effects of UN on the immune system were not evident or minimal at the lower doses of 3 mg/kg or 30 mg/kg in feed. The minimal effect of uranium at lower doses was confirmed by Bolt et al. (2019), where exposure to 5 or 50 ppm UA in drinking water for 60d had no detectable impact on T-dependent antibody response to sheep red blood cells; cytokine measurements were not reported in this study (Bolt et al., 2019).
DU toxicity has been detected following inhalation exposures. Aspiration of dissolved UA (0.22 μmole) in mice led to an increase in neutrophils, but not macrophages, in the bronchoalveolar lavage fluid 24 h after exposure (Zychowski et al., 2018). In this exposure paradigm, UA increased multiple cytokines in the bronchoalveolar lavage fluid including IL-1β and TNFα (Zychowski et al., 2018) which were decreased in peritoneal macrophages following chronic oral exposure in feed (Hao et al., 2013). Inhalation of DU as uranium dioxide caused DNA strand breaks and an increase in inflammatory cytokines including TNFα in a predominantly alveolar macrophage cell population derived from bronchoalveolar fluid (Monleau et al., 2006). These findings indicate that ingestion and respiratory routes of exposure both evoke responses in immune cells.
3.3. Effects of uranium on immune cells in the gastrointestinal tract
There is evidence that uranium exposure promotes expression of inflammatory cytokines in tissues along exposure routes such as the lung and the gastrointestinal tract (Dublineau et al., 2006, 2007; Medina et al., 2020; Monleau et al., 2006; Zychowski et al., 2018). The intestine is of particular interest because uranium uptake occurs largely through the small intestine following oral exposure (Dublineau et al., 2005). Gut-associated lymphoid structures called Peyer’s patches were found to be a preferential site of uranium accumulation in the gastrointestinal tract (Dublineau et al., 2006). Exposure of Sprague-Dawley rats to 40 mg/L UN in water for 3 to 9 months revealed accumulation of uranium in Peyer’s patches relative to the intestinal epithelium (Dublineau et al., 2006). Cytokine expression was unchanged in Peyer’s patches following uranium exposure; however, several measures of inflammatory response were modified in the ileum (Dublineau et al., 2006, 2007). There was a decrease in macrophage density and an increase in neutrophils in addition to elevation of cyclooxygenase-2, IL-1β and IL-10 expression (Dublineau et al., 2007). Uranium also inhibited the nitric oxide pathway after long-term exposure (6 months). The authors speculated that chronic uranium exposure may contribute to increased hypersensitivity or decreased defense against pathogens (Dublineau et al., 2007).
More recent work investigated the effects of uranium exposure on intraepithelial and innate immune cells in the small intestine of mice (Medina et al., 2020). Male and female mice were exposed to UA in drinking water at 5 and 50 ppm for 45 or 60 days. In contrast to the minimal effects of uranium on splenic immune cells (Bolt et al., 2019), uranium exposure led to a significant dose-dependent suppression of CD4− intraepithelial lymphocyte subsets (TCRαβ+ and TCRγδ+) in the small intestine of male, but not female, mice (Medina et al., 2020).
Overall, the findings indicate that uranium exposure may alter immune cell functions (Fig. 2) although differences in study design in terms of mouse strain or rodent species, the form of uranium, exposure route, and exposure duration make it difficult to draw firm conclusions. Uranium dose appears to be a significant determinant of response (Fig. 2). At the high dose of 300 mg/kg in feed, multiple markers of altered immune function were detected (Hao et al., 2013). These changes were not detected at lower doses employed in multiple independent studies (Bolt et al., 2019; Dublineau et al., 2014; Hao et al., 2013). Interestingly, there is evidence to suggest that disruption of immune function may be more pronounced and evident with lower doses at anatomical sites of uranium absorption such as the GI tract or lung than evident at other sites. Future studies on the immune regulatory effects of uranium beyond the classical immune organs and tissues may be necessary to gain a more complete picture of uranium immunotoxicity.
4. Evidence for uranium immunotoxicity in human populations
Human exposures to uranium in the environment occur through water, food, dust and soil. The magnitude of exposure varies based on local geology and proximity to uranium mining, processing, or manufacturing facilities (Agency for Toxic Substance and Disease Registry, 2013; Bjørklund et al., 2017). The human health effects of DU exposure have been reported (Asic et al., 2017; Brugge and Buchner, 2011; Faa et al., 2018) including the consequences of exposures due to military uses of DU (Institute of Medicine, 2008; National Research Council, 2008). However, the effects of uranium on the immune system remain an understudied topic.
Biomarkers of uranium immunotoxicity commonly measured in population studies include DNA damage in cells isolated from blood, immune cell genotoxicity, immune cell phenotyping and levels of circulating cytokines (Fig. 3). The consequences of immunotoxicity may be evident through changes in immune-driven functions such as hypersensitivity, chronic inflammation, immune suppression or stimulation, and autoimmunity. We discuss evidence for immune dysregulation in association with uranium exposures in the following sections.
Fig. 3.
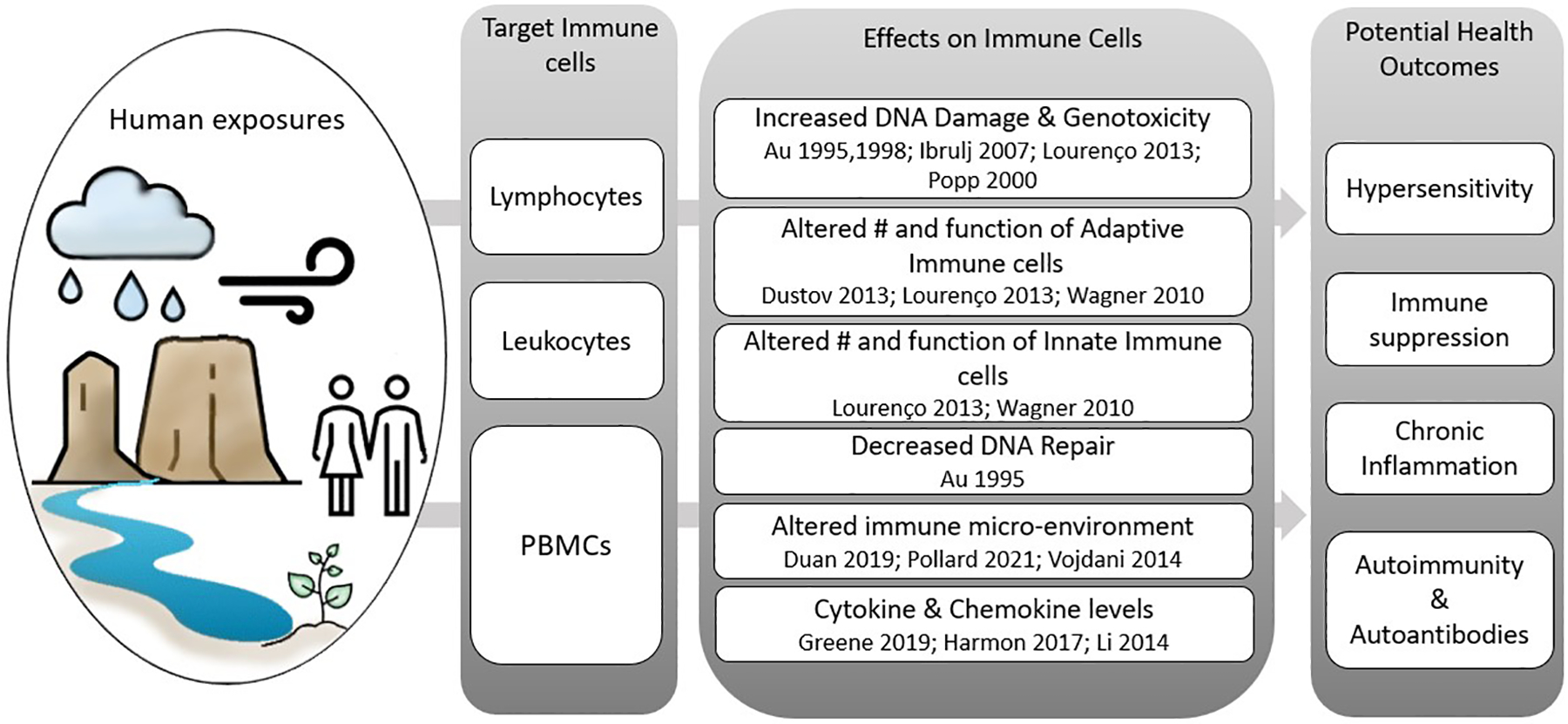
Summary of molecular changes observed in target immune cells due to uranium exposure and potential health outcomes. Column one lists target immune cell categories including lymphocytes (T-cells, B-cells, and natural killer cells), leukocytes (all white blood cells) and peripheral blood mononuclear cells or PBMCs (T-cells, B-cells, natural killer cells and dendritic cells). Column two summarizes the effects reported for the target immune cells (references listed below each reported immune cell effect). Column three lists potential health outcomes that may arise due to reported effects on target immune cells.
4.1. DNA damage and genotoxicity
Evidence for DNA damage, cytogenic aberrations, mutations, and defects in DNA repair has been reported in miners and residents living in proximity to uranium mine waste sites (Au et al., 1995, 1998; Lourenço et al., 2013). Gender- and age-matched nonsmokers living away from, or in proximity to, uranium mining activities in Texas were tested for chromosomal aberrations (Au et al., 1995). The exposed group had an increased frequency of abnormal lymphocytes and chromosomal deletions compared to the control group that neared statistical significance (Au et al., 1995). After cells were exposed to ionizing radiation, ex vivo DNA damage was significantly elevated in cells isolated from participant blood samples in the exposure group compared to controls. This finding was viewed as evidence of reduced DNA repair capacity in the exposed population (Au et al., 1995, 1998). Another community study was conducted in individuals living near a deactivated uranium mine in Cunha Baixa, Portugal, and a control site (Lourenço et al., 2013). A significant increase in DNA strand breaks was reported in peripheral blood samples from the exposed group as detected by single-cell gel electrophoresis (comet assay). Elevated uranium in whole blood was confirmed in the samples from Cunha Baixa village when compared to the unexposed group (Lourenço et al., 2013). Additional evidence for the genotoxic potential of uranium is based on an increase in chromosomal aberrations in subjects from an area with likely DU exposure in Boznia and Herzegovina compared to control populations (Ibrulj et al., 2007). These findings from three exposed communities are similar to those reported for East German former uranium miners (Popp et al., 2000). DNA damage measured in blood lymphocytes by comet assay was elevated in former uranium miners compared to the control group, but the results did not achieve statistical significance. However, chromosomal aberrations were significantly increased in the uranium miners without measurable differences in lymphocyte micronuclei (Popp et al., 2000). In contrast, a study to measure genotoxicity due to exposure to uranium and radon in areas of Brazil with high NU content found no evidence of increased micronuclei, chromosomal aberrations, or DNA damage by comet assays in individuals from the three study areas compared to a control population (Guimãraes et al., 2010). One limitation of the population studies is that it is difficult to make direct comparisons due to different methods used for estimating uranium exposure.
A limited number of studies have investigated mutational frequencies or specific mutational events as a measure of genotoxicity. Blood samples from individuals living near the Fernald uranium processing site and a control group were tested for mutations. There were no significant differences in somatic gene mutations in T-lymphocytes using the hypoxanthine phosphoribosyl transferase assays nor two other assays related to mutational outcomes (Wones et al., 1995). Fluorescence in situ hybridization analysis did not detect BCL2 translocation or MYC gene amplification in a Brazilian population exposed to NU (Guimarães et al., 2010). More recent studies analyzed the mutation spectrum or mutational frequencies of the HPRT gene in circulating T-cells from Gulf War veterans and found no significant differences based on their DU exposures (Albertini et al., 2015; Nicklas et al., 2019). Similarly, there were no consistent correlations in phosphatidylinositol glycan biosynthesis class A protein mutations based on uranium dose in Gulf War veterans (Nicklas et al., 2019). More research is needed to resolve the mutational potential of DU in immune cells and tissues.
Activation of the DNA damage response by genotoxic or environmental agents upregulates DNA repair mechanisms through release of numerous signaling molecules including damage sensors, transducer kinases and other effector molecules that interface closely with both the innate and adaptive arms of the immune system (Manolakou et al., 2021; Nastasi et al., 2020; Ye et al., 2021). DNA damage has been shown to promote type 1 interferon expression via the DNA sensor STING (Stimulator of interferon genes) (Härtlova et al., 2015). Upregulation of type 1 IFNs regulate both magnitude and quality of immune responses, with chronic upregulation of expression potentially leading to chronic inflammation and autoimmune outcomes (Gough et al., 2012). Production of other cytokines, such as IL-6, TNF-α, and IFN-γ have also been associated with activation of DNA damage response (Rodier et al., 2009; Shabrish and Mittra, 2021). These cytokines are secreted by a wide range of cell types, including T and B lymphocytes which have been shown to be highly sensitive to exogenously induced DNA damage (Heylmann et al., 2021). Thus, the molecular interactions between DNA damage and immune regulation provide a plausible link between uranium exposures, DNA damage and immune modulation in human populations.
4.2. Effects of uranium exposure on biomarkers of immune cell function
Changes in immune cell numbers and phenotypes have been reported as evidence for the immune modulatory effects of uranium exposure. In a population living near the Fernald, Ohio uranium processing facility, uranium exposure was associated with decreased white blood cell and lymphocyte counts and increased eosinophil counts (Wagner et al., 2010). Numerous differences in immune phenotypes were predominantly detected in younger individuals (<40 years old) in a population living near a deactivated uranium mine in Cunha Baixa, Portugal (Lourenço et al., 2013). The changes included increased plasmablast counts, decreased T lymphocytes (including CD4+ and CD8- T-cells), and decreased NK cells in men and women in the younger age group. In males over 60 years of age there was a significant decrease in the percentage of immature B cells (Lourenço et al., 2013). Changes in lymphocyte proportions were reported in a community study of individuals living in proximity to AUMs in Tajikistan. Exposed individuals had a decreased percentage of T lymphocytes with reduced T-helper cells and significantly increased cytotoxic lymphocytes in the exposed versus unexposed group (Dustov et al., 2013).
Serum cytokine and chemokine levels are often measured in population studies to detect immune alterations. Increased uranium exposure was associated significantly with decreased levels of CXCL11 and CXCL16 and increased levels of CCL7, CXCL6, IFNγ, IL1β, and MCP1/CCL2 in a group of women living in proximity to uranium mine waste in Fernald Ohio, when compared to the control group (Greene et al., 2019). The authors concluded that uranium exposure significantly alters inflammation. These findings are supported by a study of uranium miners categorized into low versus high exposure based on number of years of below-ground work activity in which numerous cytokines, including some associated with inflammatory response, were up-regulated. These included significant elevations of IL1α, IL1RI, IL-15, IL-3 and IL-10 concentrations and slight elevations of IFNγ, IL-10, IL-6 and TNFα (Li et al., 2014). Harmon et al. (2017) reported increased inflammatory potential of serum collected from residents of the Navajo Nation that was associated with living in proximity to AUMs (Harmon et al., 2017). The inflammatory potential analysis reflects the net effect of pro- and anti-inflammatory mediators in serum. Taken together, the findings in human population studies suggest that exposure to uranium can alter immune cell phenotypes and cytokine production, potentially leading to immune dysregulation and inflammation.
4.3. Uranium and autoimmunity
There is a growing recognition that environmental factors contribute to the development of autoimmunity and/or facilitate the progression of autoimmune diseases (AIDs) (Miller et al., 2012; Parks et al., 2014, 2017; Pestka et al., 2021; Pollard et al., 2018; Roberts and Erdei, 2020). Chronic inflammation related to certain xenobiotics and environmental exposures is believed to provide the underpinnings of autoimmunity in susceptible individuals (Duan et al., 2019; Pollard et al., 2021). Environmental exposures have been proposed to promote molecular changes associated with the appearance of autoimmune biomarkers in humans (Pollard et al., 2021; Vojdani et al., 2014). Greater than 80 distinct AIDs have been defined and include an array of clinical conditions such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), type 1 diabetes, multiple sclerosis, and a host of other diseases (U.S. National Institution of Health, 2005, 2014).
Evidence for potential involvement of uranium exposure to the development of AIDs was provided in a study of uranium miners (Conrad et al., 1996). Uranium miners had greater risk of developing systemic sclerosis and systemic lupus erythematosus (SLE) than the control population. A number of autoantibodies typical of SSc or SLE were higher in miners than the control population and associated with exposure (Conrad and Mehlhorn, 2000). The pattern of autoantibody production between idiopathic SLE/SSc versus those of the miners suggest that environmental factors contributed to autoimmune disease (Conrad and Mehlhorn, 2000). One potential caveat is that the miners were also exposed to silica, so the specific contribution of uranium to the elevated autoantibodies cannot be ascertained. An epidemiological and medical monitoring study of individuals living in proximity to the Fernald, Ohio uranium processing and enrichment facility indicated a relationship between the prevalence of SLE as defined by diagnostic criteria and higher uranium exposure (Lu-Fritts et al., 2014). Interestingly, there was no increase in rheumatoid arthritis, another common autoimmune disorder (Lu-Fritts et al., 2014).
Detection of anti-nuclear antibodies and specific autoantibodies can serve as biomarkers of autoimmune activity that may precede clinical diagnosis of AID by many years, even decades (Arbuckle et al., 2003; Pérez et al., 2018). Erdei et al. (2019) measured specific autoantibodies in Navajo Nation residents living in proximity to AUMs and mine waste or milling sites. Measurement of specific autoantibodies helps to distinguish between environmentally induced (denatured DNA and histone-specific) and idiopathic (native DNA and chromatin-specific) autoimmunity. In this population, the presence of serum autoantibodies to denatured DNA, native DNA and/or chromatin was associated with participants’ proximity to the uranium mine waste sites (Erdei et al., 2019). These autoantibodies were additionally linked to uranium consumption through drinking water for both men and women (Erdei et al., 2019). Five metals were measured in water sources, and only annual uranium consumption was predictive of autoantibodies (anti-native DNA and anti-chromatin-specific antibodies) commonly detected in SLE patients and identified in another uranium exposed population (Lu-Fritts et al., 2014). A smaller study of young and healthy Navajo and Nicaraguan men (mean age 29 years) found low overall prevalence of ANA positivity. This is in accordance with younger age and possible shorter exposure duration; however, a non-significant trend of association with higher tertiles of uranium exposure was observed (Scammell et al., 2020). Overall, the current evidence suggests that occupational and community level uranium exposures may contribute to generation of serum autoantibodies and perhaps development of AIDs.
5. Perspectives
When viewed in the aggregate, research findings support the conclusion that uranium exposure affects immune cells in a manner that may cause immune dysregulation with consequences for human health. It is also evident that no single model is sufficient to address the complexity of uranium immunotoxicity.
Cell-based studies measuring responses of specific immune cell types to DU and NU provide opportunities for mechanistic insights and define cellular targets. Although cytotoxicity is often measured, a greater focus on non-cytotoxic doses of uranium may reveal more subtle, yet meaningful effects. As noted by Yue et al. (2018), a non-cytotoxic exposure to DU may damage or alter immune functions by modulating gene expression or impinging on signal transduction mechanisms (Yue et al., 2018). This is predicted based on in vitro studies that revealed UA binding to multiple proteins including signaling proteins and DNA binding transcription factors (Dedieu et al., 2009; Hartsock et al., 2007). Fig. 1 summarizes evidence that certain forms and concentrations of uranium elicit changes in immune cells; however, an in vitro system is not sufficient to capture immune cell responses due to cell:cell interactions or factors released by other cells or tissues after uranium exposure. For example, activation and/or dysregulation of the DNA-damage response, accumulation of unrepaired DNA damage, or extracellular DNA increases inflammatory responses and has been associated with autoimmune diseases such as SLE (Duvvuri and Lood, 2019; Lou and Pickering, 2018; Nagata et al., 2010; Nastasi et al., 2020; Uchihara et al., 2021).
The studies conducted on DU immunotoxicity in rodent models provide evidence for uranium accumulation in immune tissues and at higher doses, changes in multiple aspects of immune cell function (Fig. 2). The substantial differences in uranium absorption between mammalian species make it difficult to directly extrapolate findings obtained in animal models to humans (Konietzka, 2015; Leggett and Harrison, 1995). The percent absorption of uranium from drinking water for mice and rats is 0.06 to 0.07% compared to estimates for humans of 0.2 to 2.1%. Higher uranium absorption values of 5.6–6.5% (male vs. female respectively) were reported when uranium was ingested with food. Despite many differences between studies in dose and route of exposure, the evidence indicates that uranium absorption in humans substantially exceeds that of rodents and significant adverse human impact may occur at lower exposures than defined in experimental studies in mice or rats.
Many of the findings obtained in immune cells obtained from human subjects overlap with observations in cell or animal studies (Fig. 3). Altered cytokine expression and DNA damage have been reported in isolated immune cells, animal models and in human populations, whereas changes in cell counts and phenotypes are reported in animal studies and human populations. Population studies provide evidence for adverse effects of uranium on the immune system, but many studies of environmental uranium exposures are hampered by small sample size (<100 exposed individuals) and different methods for estimating exposure. This points to the critical need for expanded population-based studies and longitudinal follow-up for accurate exposure assessment.
Another important consideration when comparing results obtained between different models is the chemical form of uranium and route(s) of exposure. Studies in mice or rats typically use oral exposures through water or food while humans are exposed through multiple, simultaneous routes including ingestion of food and water and inhalation of contaminated dusts or particulates. Evidence for changes in cytokines, inflammatory markers, or immune cells in anatomical sites of exposure such as gut and lung merit further study based on potential relevance to human toxicity (Dublineau et al., 2006, 2007; Medina et al., 2020; Monleau et al., 2006; Zychowski et al., 2018).
Although controlled exposures of specific forms of uranium are an important aspect of experimental studies, human populations experience co-exposures to other metals and toxicants in the environment that may complicate interpretation of findings or comparisons with experimental models. Many metals share mechanistic properties of oxidative stress and redox sensitive signaling that could converge or interact upon these pathways in metal mixtures. As one example of unexpected interaction, UA did not significantly alter activation associated gene expression in CD4+ human T-cells, but the mixture of UA with sodium arsenite modified the effect relative to sodium arsenite alone (Schilz et al., 2020). A greater understanding of the contributions of uranium to immune toxicity within metal mixtures is a high priority for future research.
The significant gaps in knowledge in the cellular and molecular mechanisms that lead to uranium immunotoxicity will require cell-based, animal model and population study approaches to resolve. There is an urgent need to better characterize and understand how low, but likely cumulative, community-level legacy uranium exposures affect human health. This knowledge would significantly improve our understanding of uranium immunotoxicity and provide an essential foundation for successful identification of preventative measures or community interventions.
Acknowledgements
We would like to thank T.R. Duncan, PhD for contributions to the initial literature review. We also want to express deep gratitude for the innumerable contributions made by Dr. Scott W. Burchiel to the field of toxicology and his leadership in the area of immunotoxicology. Dr. Burchiel had boundless energy and enthusiasm for science and training the next generation of researchers. He was an inspiration to all that knew him, and he is keenly missed.
Funding sources
This work was funded by grants from the National Institutes of Health, United States including: P42 ES025589 (NIEHS), P50 MD015706 (NIMHD/NIEHS) and P30 CA118100 (NCI). The funders had no role in preparation of the manuscript.
Abbreviations:
- AID
autoimmune disease
- AUM
abandoned uranium mines
- DU
depleted uranium
- EPA
Environmental Protection Agency
- IL
interleukin
- NU
natural uranium
- PARP
poly-ADP-ribose polymerase
- PM
particulate matter
- ROS
Reactive oxygen species
- SLE
systemic lupus erythematosus
- TNFα
tumor necrosis factor alpha
- UA
uranyl acetate
- UC
uranyl chloride
- UN
uranyl nitrate
Footnotes
CRediT authorship contribution statement
Jodi R. Schilz: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. Erica J. Dashner-Titus: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. Karen A. Simmons: Investigation, Writing – original draft, Writing – review & editing. Esther Erdei: Investigation, Writing – original draft, Writing – review & editing. Alicia M. Bolt: Writing – review & editing. Debra A. MacKenzie: Investigation, Funding acquisition, Writing – original draft, Writing – review & editing. Laurie G. Hudson: Conceptualization, Investigation, Visualization, Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- Agency for Toxic Substance and Disease Registry, 2013. Toxicological Profile for Uranium [WWW Document]. URL. https://www.atsdr.cdc.gov/toxprofiles/tp150.pdf. [PubMed]
- Albertini RJ, Vacek PM, Carter EW, Nicklas JA, Squibb KS, Gucer PW, Engelhardt SM, McDiarmid MA, 2015. Mutagenicity monitoring following battlefield exposures: longitudinal study of HPRT mutations in gulf war I veterans exposed to depleted uranium. Environ. Mol. Mutagen 56, 581–593. 10.1002/em.21955. [DOI] [PubMed] [Google Scholar]
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB, 2003. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med 349, 1526–1533. 10.1056/nejmoa021933. [DOI] [PubMed] [Google Scholar]
- Asic A, Kurtovic-Kozaric A, Besic L, Mehinovic L, Hasic A, Kozaric M, Hukic M, Marjanovic D, 2017. Chemical toxicity and radioactivity of depleted uranium: the evidence from in vivo and in vitro studies. Environ. Res 156, 665–673. 10.1016/j.envres.2017.04.032. [DOI] [PubMed] [Google Scholar]
- Au WW, Lane RG, Legator MS, Whorton EB, Wilkinson GS, Gabehart GJ, 1995. Biomarker monitoring of a population residing near uranium mining activities. Environ. Health Perspect 103, 466–470. 10.1289/ehp.95103466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au WW, McConnell MA, Wilkinson GS, Ramanujam VM, Alcock N, 1998. Population monitoring: experience with residents exposed to uranium mining/milling waste. Mutat. Res 405, 237–245. 10.1016/s0027-5107(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Berke HL, Palazzolo MJ, 1974. The blastogenic effect of uranyl nitrate on the peripheral lymphocyte of the rat. Am. Ind. Hyg. Assoc. J 35, 207–217. 10.1080/0002889748507024. [DOI] [PubMed] [Google Scholar]
- Bjørklund G, Christophersen OA, Chirumbolo S, Selinus O, Aaseth J, 2017. Recent aspects of uranium toxicology in medical geology. Environ. Res 156, 526–533. 10.1016/j.envres.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Blake JM, De Vore CL, Avasarala S, Ali A-M, Roldan C, Bowers F, Spilde MN, Artyushkova K, Kirk MF, Peterson E, Rodriguez-Freire L, Cerrato JM, 2017. Uranium mobility and accumulation along the Rio Paguate, Jackpile mine in Laguna Pueblo, NM. Environ Sci Process Impacts 19, 605–621. 10.1039/c6em00612d. [DOI] [PubMed] [Google Scholar]
- Bolt AM, Medina S, Lauer FT, Xu H, Ali A-M, Liu KJ, Burchiel SW, 2018. Minimal uranium accumulation in lymphoid tissues following an oral 60-day uranyl acetate exposure in male and female C57BL/6J mice. PLoS One 13, e0205211. 10.1371/journal.pone.0205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt AM, Medina S, Lauer FT, Liu KJ, Burchiel SW, 2019. Minimal uranium immunotoxicity following a 60-day drinking water exposure to uranyl acetate in male and female C57BL/6J mice. Toxicol. Appl. Pharmacol 372, 33–39. 10.1016/j.taap.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge D, Buchner V, 2011. Health effects of uranium: new research findings. Rev. Environ. Health 26, 231–249. [DOI] [PubMed] [Google Scholar]
- Conrad K, Mehlhorn J, 2000. Diagnostic and prognostic relevance of autoantibodies in uranium miners. Int. Arch. Allergy Immunol 123, 77–91. 10.1159/000024426. [DOI] [PubMed] [Google Scholar]
- Conrad K, Mehlhorn J, Lüthke K, Dörner T, Frank KH, 1996. Systemic lupus erythematosus after heavy exposure to quartz dust in uranium mines: clinical and serological characteristics. Lupus 5, 62–69. 10.1177/096120339600500112. [DOI] [PubMed] [Google Scholar]
- Coryell VH, Stearns DM, 2006. Molecular analysis ofhprt mutations generated in Chinese hamster ovary EM9 cells by uranyl acetate, by hydrogen peroxide, and spontaneously. Mol. Carcinog 45, 60–72. 10.1002/mc.20155. [DOI] [PubMed] [Google Scholar]
- Daraie B, Pourahmad J, Hamidi-Pour N, Hosseini M-J, Shaki F, Soleimani M, 2012. Uranyl acetate induces oxidative stress and mitochondrial membrane potential collapse in the human dermal fibroblast primary cells. Iran. J. Pharm. Res 11, 495–501. [PMC free article] [PubMed] [Google Scholar]
- Dashner-Titus EJ, Hoover J, Li L, Lee J-H, Du R, Liu KJ, Traber MG, Ho E, Lewis J, Hudson LG, 2018. Metal exposure and oxidative stress markers in pregnant Navajo birth cohort study participants. Free Radic. Biol. Med 124, 484–492. 10.1016/j.freeradbiomed.2018.04.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashner-Titus EJ, Schilz JR, Simmons KA, Alvarez SC, Duncan TR, Hudson LG, 2020. Differential response of human T-lymphocytes to arsenic and uranium. Toxicol. Lett 333, 269–278. 10.1016/j.toxlet.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedieu A, Bérenguer F, Basset C, Prat O, Quéméneur E, Pible O, Vidaud C, 2009. Identification of uranyl binding proteins from human kidney-2 cell extracts by immobilized uranyl affinity chromatography and mass spectrometry. J. Chromatogr. A 1216, 5365–5376. 10.1016/j.chroma.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Duan L, Rao X, Sigdel KR, 2019. Regulation of inflammation in autoimmune disease. J Immunol Res 2019, 1–2. 10.1155/2019/7403796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dublineau I, Grison S, Baudelin C, Dudoignon N, Souidi M, Marquette C, Paquet F, Aigueperse J, Gourmelon P, 2005. Absorption of uranium through the entire gastrointestinal tract of the rat. Int. J. Radiat. Biol 81, 473–482. 10.1080/09553000500196029. [DOI] [PubMed] [Google Scholar]
- Dublineau I, Grison S, Grandcolas L, Baudelin C, Tessier C, Suhard D, Frelon S, Cossonnet C, Claraz M, Ritt J, Paquet P, Voisin P, Gourmelon P, 2006. Absorption, accumulation and biological effects of depleted uranium in Peyer’s patches of rats. Toxicology 227, 227–239. 10.1016/j.tox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Dublineau I, Grandcolas L, Grison S, Baudelin C, Paquet F, Voisin P, Aigueperse J, Gourmelon P, 2007. Modifications of inflammatory pathways in rat intestine following chronic ingestion of depleted uranium. Toxicol. Sci 98, 458–468. 10.1093/toxsci/kfm132. [DOI] [PubMed] [Google Scholar]
- Dublineau I, Souidi M, Gueguen Y, Lestaevel P, Bertho J-M, Manens L, Delissen O, Grison S, Paulard A, Monin A, Kern Y, Rouas C, Loyen J, Gourmelon P, Aigueperse J, 2014. Unexpected lack of deleterious effects of uranium on physiological systems following a chronic oral intake in adult rat. Biomed. Res. Int 2014, 181989 10.1155/2014/181989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustov A, Mirojov G, Yakubova M, Umarov S, Ishankulova D, Eliasziw M, Brugge D, 2013. Uranium mine proximity, immune function, and Helicobacter pylori infection in Tajikistan. J. Toxicol. Environ. Health A 76, 1261–1268. 10.1080/15287394.2013.836694. [DOI] [PubMed] [Google Scholar]
- Duvvuri B, Lood C, 2019. Cell-free DNA as a biomarker in autoimmune rheumatic diseases. Front. Immunol 10, 502. 10.3389/fimmu.2019.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage Chahine J-M, Hémadi M, Ha-Duong N-T, 2012. Uptake and release of metal ions by transferrin and interaction with receptor 1. Biochim. Biophys. Acta Gen. Subj 1820, 334–347. 10.1016/j.bbagen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Erdei E, Shuey C, Pacheco B, Cajero M, Lewis J, Rubin RL, 2019. Elevated autoimmunity in residents living near abandoned uranium mine sites on the Navajo nation. J. Autoimmun 99, 15–23. 10.1016/j.jaut.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faa A, Gerosa C, Fanni D, Floris G, Eyken PV, Lachowicz JI, Nurchi VM, 2018. Depleted uranium and human health. Curr. Med. Chem 25, 49–64. 10.2174/0929867324666170426102343. [DOI] [PubMed] [Google Scholar]
- Garmash SA, Smirnova VS, Karp OE, Usacheva AM, Berezhnov AV, Ivanov VE, Chernikov AV, Bruskov VI, Gudkov SV, 2014. Pro-oxidative, genotoxic and cytotoxic properties of uranyl ions. J. Environ. Radioact 127, 163–170. 10.1016/j.jenvrad.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Gazin V, Kerdine S, Grillon G, Pallardy M, Raoul H, 2004. Uranium induces TNFα secretion and MAPK activation in a rat alveolar macrophage cell line. Toxicol. Appl. Pharmacol 194, 49–59. 10.1016/j.taap.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Gough DJ, Messina NL, Clarke CJP, Johnstone RW, Levy DE, 2012. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36, 166–174. 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AD, Kendziorski JA, Buckholz JM, Niu L, Xie C, Pinney SM, Burns KA, 2019. Elevated serum chemokines are independently associated with both endometriosis and uranium exposure. Reprod. Toxicol 84, 26–31. 10.1016/j.reprotox.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guéguen Y, Suhard D, Poisson C, Manens L, Elie C, Landon G, Bouvier-Capely C, Rouas C, Benderitter M, Tessier C, 2015. Low-concentration uranium enters the HepG2 cell nucleus rapidly and induces cell stress response. Toxicol. in Vitro 30, 552–560. 10.1016/j.tiv.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Guimarães AC, Antunes LMG, Ribeiro HF, dos Santos AKR, dos Cardoso PCS, de Lima PL, Seabra AD, Pontes TB, Pessoa C, de Moraes MO, Cavalcanti BC, Sombra CML, de Bahia MO, Burbano RR, 2010. Cytogenetic biomonitoring of inhabitants of a large uranium mineralization area: the municipalities of Monte Alegre, Prainha, and Alenquer, in the state of Pará, Brazil. Cell Biol. Toxicol 26, 403–419. 10.1007/s10565-010-9152-8. [DOI] [PubMed] [Google Scholar]
- Hakonson-Hayes AC, Fresquez PR, Whicker FW, 2002. Assessing potential risks from exposure to natural uranium in well water. J. Environ. Radioact 59, 29–40. 10.1016/S0265-931X(01)00034-0. [DOI] [PubMed] [Google Scholar]
- Hao Y, Ren J, Liu J, Yang Z, Liu C, Li R, Su Y, 2013. Immunological changes of chronic oral exposure to depleted uranium in mice. Toxicology 309, 81–90. 10.1016/j.tox.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Harmon ME, Lewis J, Miller C, Hoover J, Ali A-MS, Shuey C, Cajero M, Lucas S, Zychowski K, Pacheco B, Erdei E, Ramone S, Nez T, Gonzales M, Campen MJ, 2017. Residential proximity to abandoned uranium mines and serum inflammatory potential in chronically exposed Navajo communities. J. Expo. Sci. Environ. Epidemiol 27, 365–371. 10.1038/jes.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtlova A, Erttmann SF, Raffi FAM, Schmalz AM, Resch U, Anugula S, Lienenklaus S, Nilsson LM, Kröger A, Nilsson JA, Ek T, Weiss S, Gekara NO, 2015. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity 42, 332–343. 10.1016/j.immuni.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Hartsock WJ, Cohen JD, Segal DJ, 2007. Uranyl acetate as a direct inhibitor of DNA-binding proteins. Chem. Res. Toxicol 20, 784–789. 10.1021/tx600347k. [DOI] [PubMed] [Google Scholar]
- He F, Ru X, Wen T, 2020. NRF2, a transcription factor for stress response and beyond. IJMS 21, 4777. 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hémadi M, Ha-Duong N-T, El Hage Chahine J-M, 2011. Can uranium be transported by the iron-acquisition pathway? Ur uptake by transferrin. J. Phys. Chem. B 115, 4206–4215. 10.1021/jp111950c. [DOI] [PubMed] [Google Scholar]
- Heylmann D, Ponath V, Kindler T, Kaina B, 2021. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep 11, 2478. 10.1038/s41598-021-81058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover J, Gonzales M, Shuey C, Barney Y, Lewis J, 2017. Elevated arsenic and uranium concentrations in unregulated water sources on the Navajo nation, USA. Expos. Health 9, 113–124. 10.1007/s12403-016-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JH, Erdei E, Begay D, Gonzales M, Jarrett JM, Cheng P-Y, Lewis J, 2020. Exposure to uranium and co-occurring metals among pregnant Navajo women. Environ. Res 190, 109943 10.1016/j.envres.2020.109943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund L, Bedrick EJ, Miller C, Huerta G, Nez T, Ramone S, Shuey C, Cajero M, Lewis J, 2015. A Bayesian framework for estimating disease risk due to exposure to uranium mine and mill waste on the Navajo nation. J. R. Stat. Soc. A 178, 1069–1091. 10.1111/rssa.12099. [DOI] [Google Scholar]
- Ibrulj S, Haverić S, Haverić A, 2007. Chromosome aberrations as bioindicators of environmental genotoxicity. Bosn. J. Basic Med. Sci 7, 311–316. 10.17305/bjbms.2007.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine, 2008. Gulf War and Health: Updated Literature Review of Depleted Uranium. National Academies Press, Washington, D.C.. 10.17226/12183 [DOI] [PubMed] [Google Scholar]
- Joseph SJ, Arunachalam KD, Murthy PB, Ramalingam R, Musthafa MS, 2021. Uranium induces genomic instability and slows cell cycle progression in human lymphocytes in acute toxicity study. Toxicol. in Vitro 73, 105149. 10.1016/j.tiv.2021.105149. [DOI] [PubMed] [Google Scholar]
- Kalinich JF, 2011. Heavy metal-induced carcinogenicity: depleted uranium and heavy-metal tungsten alloy. In: Banfalvi G (Ed.), Cellular Effects of Heavy Metals. Springer, Netherlands, Dordrecht, pp. 221–236. 10.1007/978-94-007-0428-2_10. [DOI] [Google Scholar]
- Kalinich J, McClain D, 2001. Staining of intracellular deposits of uranium in cultured murine macrophages. Biotech. Histochem 76, 247–252. 10.1080/bih.76.5-6.247.252. [DOI] [PubMed] [Google Scholar]
- Kalinich JF, Ramakrishnan N, Villa V, McClain DE, 2002. Depleted uranium–uranyl chloride induces apoptosis in mouse J774 macrophages. Toxicology 179, 105–114. 10.1016/S0300-483X(02)00318-9. [DOI] [PubMed] [Google Scholar]
- Konietzka R, 2015. Gastrointestinal absorption of uranium compounds – a review. Regul. Toxicol. Pharmacol 71, 125–133. 10.1016/j.yrtph.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Leggett RW, Harrison JD, 1995. Fractional absorption of ingested uranium in humans. Health Phys. 68, 484–498. 10.1097/00004032-199504000-00005. [DOI] [PubMed] [Google Scholar]
- Lewis J, Gonzales M, Burnette C, Benally M, Seanez P, Shuey C, Nez H, Nez C, Nez S, 2015. Environmental exposures to metals in native communities and implications for child development: basis for the Navajo birth cohort study. J. Soc. Work Disabil. Rehabil 14, 245–269. 10.1080/1536710X.2015.1068261. [DOI] [PubMed] [Google Scholar]
- Lewis J, Hoover J, MacKenzie D, 2017. Mining and environmental health disparities in native American communities. Curr. Environ. Health Rep 4, 130–141. 10.1007/s40572-017-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Chen Y, Li X, Lei S, Chen Q, Liu J, Sun Q, 2014. Alteration of cytokine profiles in uranium miners exposed to long-term low dose ionizing radiation. ScientificWorldJournal 2014, 216408. 10.1155/2014/216408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares V, Sánchez DJ, Bellés M, Albina L, Gómez M, Domingo JL, 2007. Pro-oxidant effects in the brain of rats concurrently exposed to uranium and stress. Toxicology 236, 82–91. 10.1016/j.tox.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Lingappan K, 2018. NF-κB in oxidative stress. Curr. Opin. Toxicol 7, 81–86. 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang J, Li H, Shen C-C, Chen Y, Wang C, Ye H, Long J, Song G, Wu Y, 2015. Surface sediment contamination by uranium mining/milling activities in South China: surface sediment contamination by uranium mining/milling activities. Clean Soil Air Water 43, 414–420. 10.1002/clen.201300297. [DOI] [Google Scholar]
- Lizon F, 1999. Chemical toxicity of some actinides and lanthanides towards alveolar macrophages: an in vitro study. Int. J. Radiat. Biol 75, 1459–1471. 10.1080/095530099139322. [DOI] [PubMed] [Google Scholar]
- Lou H, Pickering MC, 2018. Extracellular DNA and autoimmune diseases. Cell. Mol. Immunol 15, 746–755. 10.1038/cmi.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço J, Pereira R, Pinto F, Caetano T, Silva A, Carvalheiro T, Guimarães A, Gonçalves F, Paiva A, Mendo S, 2013. Biomonitoring a human population inhabiting nearby a deactivated uranium mine. Toxicology 305, 89–98. 10.1016/j.tox.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Lu B, Ran Y, Wang S, Li J, Zhao Y, Ran X, Li R, Hao Y, 2021. Chronic oral depleted uranium leads to reproductive damage in male rats through the ROS-hnRNP A2/B1-COX-2 signaling pathway. Toxicology 449, 152666. 10.1016/j.tox.2020.152666. [DOI] [PubMed] [Google Scholar]
- Lu-Fritts P-Y, Kottyan LC, James JA, Xie C, Buckholz JM, Pinney SM, Harley JB, 2014. Association of systemic lupus erythematosus with uranium exposure in a community living near a uranium-processing plant: a nested case-control study. Arthritis Rheum. 66, 3105–3112. 10.1002/art.38786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, 2013. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol 53, 401–426. 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Wang R, Xu L, Xu M, Liu S, 2020. Emerging health risks and underlying toxicological mechanisms of uranium contamination: lessons from the past two decades. Environ. Int 145, 106107 10.1016/j.envint.2020.106107. [DOI] [PubMed] [Google Scholar]
- Manolakou T, Verginis P, Boumpas DT, 2021. DNA damage response in the adaptive arm of the immune system: implications for autoimmunity. IJMS 22, 5842. 10.3390/ijms22115842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina S, Lauer FT, Castillo EF, Bolt AM, Ali A-MS, Liu KJ, Burchiel SW, 2020. Exposures to uranium and arsenic alter intraepithelial and innate immune cells in the small intestine of male and female mice. Toxicol. Appl. Pharmacol 403, 115155 10.1016/j.taap.2020.115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina S, Zhou X, Lauer FT, Zhang H, Liu KJ, Lewis J, Burchiel SW, 2021. Modulation of PARP activity by Monomethylarsonous (MMA+3) acid and uranium in mouse thymus. Toxicol. Appl. Pharmacol 411, 115362 10.1016/j.taap.2020.115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AC, Stewart M, Brooks K, Shi L, Page N, 2002. Depleted uranium-catalyzed oxidative DNA damage: absence of significant alpha particle decay. J. Inorg. Biochem 91, 246–252. 10.1016/s0162-0134(02)00391-4. [DOI] [PubMed] [Google Scholar]
- Miller FW, Pollard KM, Parks CG, Germolec DR, Leung PSC, Selmi C, Humble MC, Rose NR, 2012. Criteria for environmentally associated autoimmune diseases. J. Autoimmun 39, 253–258. 10.1016/j.jaut.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monleau M, De Méo M, Paquet F, Chazel V, Duménil G, Donnadieu-Claraz M, 2006. Genotoxic and inflammatory effects of depleted uranium particles inhaled by rats. Toxicol. Sci 89, 287–295. 10.1093/toxsci/kfj010. [DOI] [PubMed] [Google Scholar]
- Muller D, Houpert P, Cambar J, Hengé-Napoli M-H, 2006. Role of the sodium-dependent phosphate co-transporters and of the phosphate complexes of uranyl in the cytotoxicity of uranium in LLC-PK1 cells. Toxicol. Appl. Pharmacol 214, 166–177. 10.1016/j.taap.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Muller DS, Houpert P, Cambar J, Hengé-Napoli M-H, 2008. Role of the sodium-dependent phosphate cotransporters and absorptive endocytosis in the uptake of low concentrations of uranium and its toxicity at higher concentrations in LLC-PK1 cells. Toxicol. Sci 101, 254–262. 10.1093/toxsci/kfm266. [DOI] [PubMed] [Google Scholar]
- Nagata S, Hanayama R, Kawane K, 2010. Autoimmunity and the clearance of dead cells. Cell 140, 619–630. 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Nastasi C, Mannarino L, D’Incalci M, 2020. DNA damage response and immune defense. Int. J. Mol. Sci 21, E7504. 10.3390/ijms21207504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2008. Review of Toxicologic and Radiologic Risks to Military Personnel from Exposure to Depleted Uranium During and After Combat. National Academies Press, Washington, D.C.. 10.17226/11979 [DOI] [Google Scholar]
- Neiva AMR, Albuquerque MTD, Antunes IMHR, Carvalho PCS, Santos ACT, Boente C, Cunha PP, Henriques SBA, Pato RL, 2019. Assessment of metal and metalloid contamination in soils trough compositional data: the old Mortórios uranium mine area, Central Portugal. Environ. Geochem. Health 41, 2875–2892. 10.1007/s10653-019-00347-x. [DOI] [PubMed] [Google Scholar]
- Nicklas JA, Vacek PM, Carter EW, McDiarmid M, Albertini RJ, 2019. Molecular analysis of glycosylphosphatidylinositol anchor deficient aerolysin resistant isolates in gulf war i veterans exposed to depleted uranium. Environ. Mol. Mutagen 60, 470–493. 10.1002/em.22283. [DOI] [PubMed] [Google Scholar]
- Nolan J, Weber KA, 2015. Natural uranium contamination in major U.S. aquifers linked to nitrate. Environ. Sci. Technol. Lett 2, 215–220. 10.1021/acs.estlett.5b00174. [DOI] [Google Scholar]
- Orona NS, Tasat DR, 2012. Uranyl nitrate-exposed rat alveolar macrophages cell death: influence of superoxide anion and TNF α mediators. Toxicol. Appl. Pharmacol 261, 309–316. 10.1016/j.taap.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Paredes E, Avazeri E, Malard V, Vidaud C, Reiller PE, Ortega R, Nonell A, Isnard H, Chartier F, Bresson C, 2018. Impact of uranium uptake on isotopic fractionation and endogenous element homeostasis in human neuron-like cells. Sci. Rep 8, 17163. 10.1038/s41598-018-35413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CG, Miller FW, Pollard KM, Selmi C, Germolec D, Joyce K, Rose NR, Humble MC, 2014. Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int. J. Mol. Sci 15, 14269–14297. 10.3390/ijms150814269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH, 2017. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol 31, 306–320. 10.1016/j.berh.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez D, Gilburd B, Cabrera-Marante Ó, Martínez-Flores JA, Serrano M, Naranjo L, Pleguezuelo D, Morillas L, Shovman O, Paz-Artal E, Shoenfeld Y, Serrano A, 2018. Predictive autoimmunity using autoantibodies: screening for anti-nuclear antibodies. Clin. Chem. Lab. Med. (CCLM) 56, 1771–1777. 10.1515/cclm-2017-0241. [DOI] [PubMed] [Google Scholar]
- Periyakaruppan A, Kumar F, Sarkar S, Sharma CS, Ramesh GT, 2007. Uranium induces oxidative stress in lung epithelial cells. Arch. Toxicol 81, 389–395. 10.1007/s00204-006-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Pollard KM, Rosenspire AJ, 2021. Editorial: the role of the environment in autoimmunity. Front. Immunol 12, 641171 10.3389/fimmu.2021.641171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM, Christy JM, Cauvi DM, Kono DH, 2018. Environmental xenobiotic exposure and autoimmunity. Curr. Opin. Toxicol 10, 15–22. 10.1016/j.cotox.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM, Cauvi DM, Mayeux JM, Toomey CB, Peiss AK, Hultman P, Kono DH, 2021. Mechanisms of environment-induced autoimmunity. Annu. Rev. Pharmacol. Toxicol 61, 135–157. 10.1146/annurev-pharmtox-031320-111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp W, Plappert U, Müller WU, Rehn B, Schneider J, Braun A, Bauer PC, Vahrenholz C, Presek P, Brauksiepe A, Enderle G, Wüst T, Bruch J, Fliedner TM, Konietzko N, Streffer C, Woitowitz HJ, Norpoth K, 2000. Biomarkers of genetic damage and inflammation in blood and bronchoalveolar lavage fluid among former German uranium miners: a pilot study. Radiat. Environ. Biophys 39, 275–282. 10.1007/s004110000072. [DOI] [PubMed] [Google Scholar]
- Prochazkova J, Loizou JI, 2016. Programmed DNA breaks in lymphoid cells: repair mechanisms and consequences in human disease. Immunology 147, 11–20. 10.1111/imm.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravalli F, Yu Y, Bostick BC, Chillrud SN, Schilling K, Basu A, Navas-Acien A, Nigra AE, 2022. Sociodemographic inequalities in uranium and other metals in community water systems across the USA, 2006–11: a cross-sectional study. Lancet Planet. Health 6, e320–e330. 10.1016/S2542-5196(22)00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Chaudhuri A, Nussenzweig A, 2017. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol 18, 610–621. 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek CR, Chandel NS, 2015. ROS-dependent signal transduction. Curr. Opin. Cell Biol 33, 8–13. 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MH, Erdei E, 2020. Comparative United States autoimmune disease rates for 2010–2016 by sex, geographic region, and race. Autoimmun. Rev 19, 102423 10.1016/j.autrev.2019.102423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppé J-P, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J, 2009. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol 11, 973–979. 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell MK, Sennett C, Laws RL, Rubin RL, Brooks DR, Amador JJ, López-Pilarte D, Ramirez-Rubio O, Friedman DJ, McClean MD, Cohort Study Team, N.B, Lewis J, Erdei E, 2020. Urinary metals concentrations and biomarkers of autoimmunity among Navajo and Nicaraguan men. Int. J. Environ. Res. Public Health 17, E5263. 10.3390/ijerph17155263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilz JR, Dashner-Titus EJ, Lou L, Simmons K, MacKenzie DA, Hudson LG, 2020. Co-Exposure of Sodium Arsenite and Uranyl Acetate Differentially Alters Gene Expression in CD3/CD28 Activated CD4+ T-Cells. 10.6073/pasta/f198331f536a56a696226405e4d9561f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabrish S, Mittra I, 2021. Cytokine storm as a cellular response to dsDNA breaks: a new proposal. Front. Immunol 12, 622738 10.3389/fimmu.2021.622738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaki F, Hosseini M-J, Ghazi-Khansari M, Pourahmad J, 2013. Depleted uranium induces disruption of energy homeostasis and oxidative stress in isolated rat brain mitochondria. Metallomics 5, 736–744. 10.1039/c3mt00019b. [DOI] [PubMed] [Google Scholar]
- Sies H, Jones DP, 2020. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol 21, 363–383. 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- Soltani M, Zarei MH, Salimi A, Pourahmad J, 2019. Mitochondrial protective and antioxidant agents protect toxicity induced by depleted uranium in isolated human lymphocytes. J. Environ. Radioact 203, 112–116. 10.1016/j.jenvrad.2019.03.009. [DOI] [PubMed] [Google Scholar]
- U.S. National Institution of Health, 2005. Progress in Autoimmune Disease Research (No. 05–5140) National Institute of Allergy and Infectious Disease. [Google Scholar]
- U.S. National Institution of Health, 2014. Biennial Report of the Director.
- Uchihara Y, Permata TBM, Sato H, Shibata A, 2021. Modulation of immune responses by DNA damage signaling. DNA Repair (Amst) 104, 103135. 10.1016/j.dnarep.2021.103135. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency, 2000. Liqid Assets 2000: America’s Water Resources at a Turning Point (Reports and Assessments No. EPA-840-B-00–001)
- US EPA, O, 2018. Depleted Uranium [WWW Document]. URL. https://www.epa.gov/radtown/depleted-uranium (accessed 5.9.22).
- US EPA, R. 09, 2016a. Abandoned Mines Cleanup [WWW Document]. URL. https://www.epa.gov/navajo-nation-uranium-cleanup/abandoned-mines-cleanup (accessed 5.9.22).
- US EPA, R. 09, 2016b. Federal Actions to Address Impacts of Uranium Contamination on the Navajo Nation [WWW Document]. URL. https://www.epa.gov/navajo-nation-uranium-cleanup/federal-plans-related-documents (accessed 6.9.22).
- Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K, 2016. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol 90, 1–37. 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Pollard KM, Campbell AW, 2014. Environmental triggers and autoimmunity. Autoimmune Dis. 2014, 798029 10.1155/2014/798029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner SE, Burch JB, Bottai M, Pinney SM, Puett R, Porter D, Vena JE, Hébert JR, 2010. Hypertension and hematologic parameters in a community near a uranium processing facility. Environ. Res 110, 786–797. 10.1016/j.envres.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan B, Fleming JT, Schultz TW, Sayler GS, 2006. In vitro immune toxicity of depleted uranium: effects on murine macrophages, CD4+ T cells, and gene expression profiles. Environ. Health Perspect 114, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J, Young A, Civitello ER, Stearns DM, 2014. Analysis of heat-labile sites generated by reactions of depleted uranium and ascorbate in plasmid DNA. J. Biol. Inorg. Chem 19, 45–57. 10.1007/s00775-013-1057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wones R, Radack K, Martin V, Mandell K, Pinney S, Buncher R, 1995. Do persons living near a uranium processing site have evidence of increased somatic cell gene mutations? A first study. Mutat. Res 335, 171–184. 10.1016/0165-1161(95)90053-5. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2001. Depleted Uranium: Sources, Exposure and Health Effects.
- Ye Z, Shi Y, Lees-Miller SP, Tainer JA, 2021. Function and molecular mechanism of the DNA damage response in immunity and cancer immunotherapy. Front. Immunol 12, 797880 10.3389/fimmu.2021.797880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellowhair M, Romanotto MR, Stearns DM, Clark Lantz R, 2018. Uranyl acetate induced DNA single strand breaks and AP sites in Chinese hamster ovary cells. Toxicol. Appl. Pharmacol 349, 29–38. 10.1016/j.taap.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Yuan Y, Zheng J, Zhao T, 2019. Hydrogen sulfide alleviates uranium-induced rat hepatocyte cytotoxicity via inhibiting Nox4/ROS/p38 MAPK pathway. J. Biochem. Mol. Toxicol 33, e22255 10.1002/jbt.22255. [DOI] [PubMed] [Google Scholar]
- Yue Y-C, Li M-H, Wang H-B, Zhang B-L, He W, 2018. The toxicological mechanisms and detoxification of depleted uranium exposure. Environ. Health Prev. Med 23, 18. 10.1186/s12199-018-0706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychowski KE, Kodali V, Harmon M, Tyler CR, Sanchez B, Ordonez Suarez Y, Herbert G, Wheeler A, Avasarala S, Cerrato JM, Kunda NK, Muttil P, Shuey C, Brearley A, Ali A-M, Lin Y, Shoeb M, Erdely A, Campen MJ, 2018. Respirable uranyl-vanadate-containing particulate matter derived from a legacy uranium mine site exhibits potentiated cardiopulmonary toxicity. Toxicol. Sci 164, 101–114. 10.1093/toxsci/kfy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


