Abstract
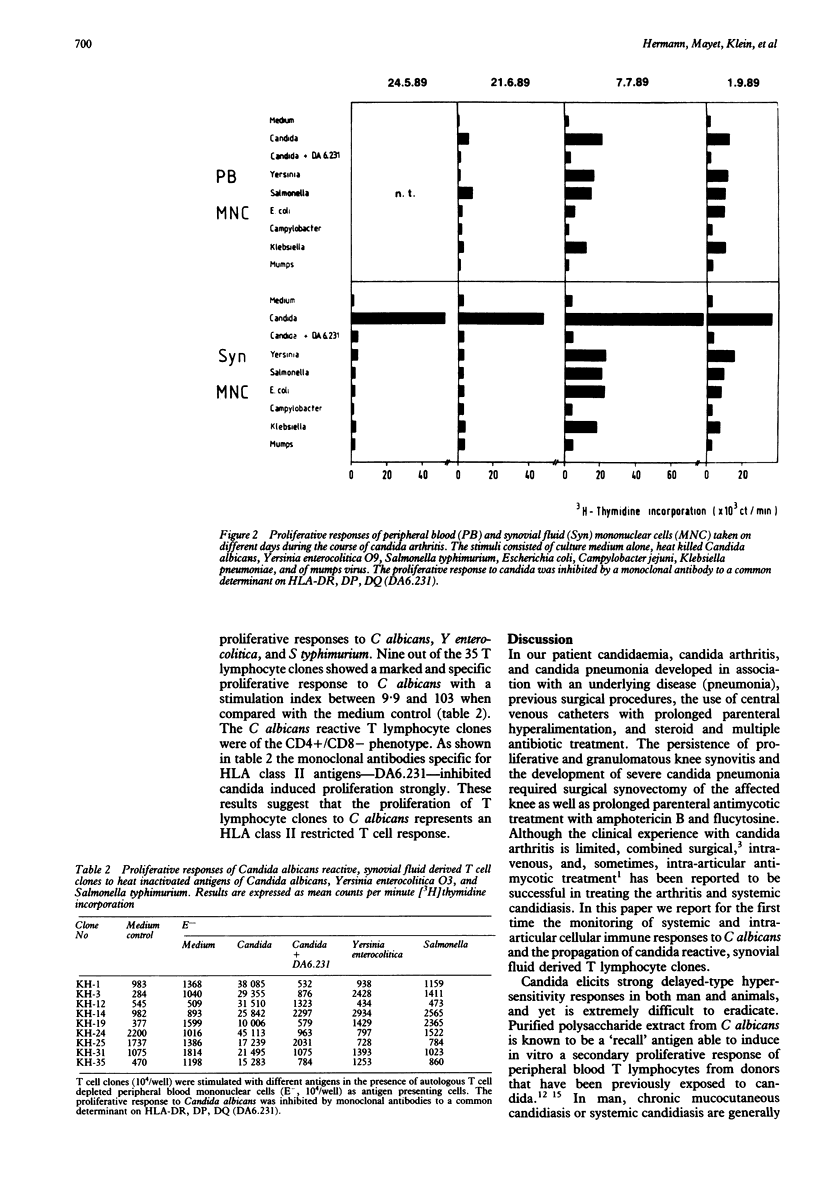
A case of septic Candida albicans arthritis of the knee in a patient with systemic candidiasis is presented. Systemic and intra-articular cellular immune responses to C albicans and various bacterial antigens were monitored for 15 weeks. It is shown that the candida induced blastogenesis of synovial fluid lymphocytes was much more stimulated than that of peripheral blood lymphocytes, and that the proportion of activated cells expressing HLA class II antigens was markedly increased in the synovial fluid. Strong cellular immune responses to Candida albicans could still be shown many weeks after the synovial fluid aspirates had become sterile. For the first time synovial fluid derived, CD4 positive T lymphocyte clones with specificity for candida antigens were characterised and further propagated in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B. Mouse candidiasis. II. Host responses are T-cell dependent and regulated by genes in the major histocompatibility complex. Immunogenetics. 1987;25(3):200–203. doi: 10.1007/BF00344035. [DOI] [PubMed] [Google Scholar]
- Ausiello C. M., Spagnoli G. C., Boccanera M., Casalinuovo I., Malavasi F., Casciani C. U., Cassone A. Proliferation of human peripheral blood mononuclear cells induced by Candida albicans and its cell wall fractions. J Med Microbiol. 1986 Nov;22(3):195–202. doi: 10.1099/00222615-22-3-195. [DOI] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986 Feb;51(2):668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry C. R., Quie P. G. Fungal septicemia in patients receiving parenteral hyperalimentation. N Engl J Med. 1971 Nov;285(22):1221–1225. doi: 10.1056/NEJM197111252852203. [DOI] [PubMed] [Google Scholar]
- Dupont B., Drouhet E. Cutaneous, ocular, and osteoarticular candidiasis in heroin addicts: new clinical and therapeutic aspects in 38 patients. J Infect Dis. 1985 Sep;152(3):577–591. doi: 10.1093/infdis/152.3.577. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Non-specific propagation of human antigen-dependent T lymphocyte clones. J Immunol Methods. 1988 May 9;109(2):215–219. doi: 10.1016/0022-1759(88)90245-1. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Granfors K., Merilahti-Palo R., Bailey L., Consalvey S., Toivanen A., Bacon P. A. Synovial T lymphocyte recognition of organisms that trigger reactive arthritis. Clin Exp Immunol. 1989 Jun;76(3):348–353. [PMC free article] [PubMed] [Google Scholar]
- Hermann E., Fleischer B., Mayet W. J., Poralla T., Meyer zum Büschenfelde K. H. Response of synovial fluid T cell clones to Yersinia enterocolitica antigens in patients with reactive Yersinia arthritis. Clin Exp Immunol. 1989 Mar;75(3):365–370. [PMC free article] [PubMed] [Google Scholar]
- Katzenstein D. Isolated Candida arthritis: report of a case and definition of a distinct clinical syndrome. Arthritis Rheum. 1985 Dec;28(12):1421–1424. doi: 10.1002/art.1780281216. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H. Host factors in defense against fungal infections. Am J Med. 1984 Oct 30;77(4D):1–12. [PubMed] [Google Scholar]
- Lertratanakul Y., Glassford G. H., Rubinstein H. M. Arthritis and osteomyelitis due to Candida albicans: a case report. J Rheumatol. 1977 Autumn;4(3):317–320. [PubMed] [Google Scholar]
- Lindström F. D., Lindholm T. Candida albicans arthritis treated with flucytosine. Ann Intern Med. 1973 Jul;79(1):131–131. doi: 10.7326/0003-4819-79-1-131_1. [DOI] [PubMed] [Google Scholar]
- Lombardi G., Piccolella E., Vismara D., Colizzi V., Asherson G. L. Candida albicans polysaccharide extract (MPPS) and PPD stimulate the production of interleukin-1 and lymphocyte proliferation. Clin Exp Immunol. 1984 Dec;58(3):581–586. [PMC free article] [PubMed] [Google Scholar]
- Noyes F. R., McCabe J. D., Fekety F. R., Jr Acute candida arthritis. Report of a case and use of amphotericin B. J Bone Joint Surg Am. 1973 Jan;55(1):169–176. [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Human lymphocyte-activating properties of a purified polysaccharide from Candida albicans: B and T cell cooperation in the mitogenic response. J Immunol. 1980 Nov;125(5):2082–2088. [PubMed] [Google Scholar]
- Poplack D. G., Jacobs S. A. Candida arthritis treated with amphotericin B. J Pediatr. 1975 Dec;87(6 Pt 1):989–990. doi: 10.1016/s0022-3476(75)80926-7. [DOI] [PubMed] [Google Scholar]
- van Heyningen V., Guy K., Newman R., Steel C. M. Human MHC class II molecules as differentiation markers. Immunogenetics. 1982;16(5):459–469. doi: 10.1007/BF00372104. [DOI] [PubMed] [Google Scholar]