Abstract
Depression is a major public health concern. Unfortunately, the present antidepressants often are insufficiently effective, whilst the discovery of more effective antidepressants has been extremely sluggish. The objective of this review was to combine the literature on depression with the pharmacology of antidepressant compounds, in order to formulate a conceivable pathophysiological process, allowing proposals how to accelerate the discovery process. Risk factors for depression initiate an infection-like inflammation in the brain that involves activation microglial Toll-like receptors and glycogen synthase kinase-3β (GSK3β). GSK3β activity alters the balance between two competing transcription factors, the pro-inflammatory/pro-oxidative transcription factor NFκB and the neuroprotective, anti-inflammatory and anti-oxidative transcription factor NRF2. The antidepressant activity of tricyclic antidepressants is assumed to involve activation of GS-coupled microglial receptors, raising intracellular cAMP levels and activation of protein kinase A (PKA). PKA and similar kinases inhibit the enzyme activity of GSK3β. Experimental antidepressant principles, including cannabinoid receptor-2 activation, opioid μ receptor agonists, 5HT2 agonists, valproate, ketamine and electrical stimulation of the Vagus nerve, all activate microglial pathways that result in GSK3β-inhibition. An in vitro screen for NRF2-activation in microglial cells with TLR-activated GSK3β activity, might therefore lead to the detection of totally novel antidepressant principles with, hopefully, an improved therapeutic efficacy.
Keywords: depression risk factor, microglia, toll-like receptor, GSK3β, NRF2, GS-coupled receptor, ketamine, cannabinoid CBR2, psilocybin, 5-HT2B
1. Introduction
Major depressive disorder is a psychiatric syndrome involving persistent low mood, anhedonia, fatigue, loss of energy, sleep disturbances, and deficits in the cognitive domain, including impaired ability to concentrate, poor attention, and memory [1]. The view on major depression has changed during the last three decades from being a mental disorder caused by a lack of monoamine neurotransmitters [2] to a concept where the mental disorder is secondary to an immune activation in the brain [3,4]. The earliest antidepressants were serendipitously discovered, and, as a common denominator, they all raise the extracellular levels of dopamine, noradrenaline, and serotonin (5-hydroxytryptamine) in the brain [5,6,7]. The indirect monoaminergic activity [8] provides a moderately effective, central anti-inflammatory effect and initiates a slowly-developing improvement in the physical and mental symptoms of depression [9]. Major depression ranks high on the list of diseases posing the strongest burden on society [10], and unfortunately, the global situation has further deteriorated recently due to infections with SARS-Cov2 [11]. The discovery of antidepressants with a novel mode of action has stagnated for many decades, and recent progress is very modest. There is a pressing need to identify novel antidepressant principles and develop alternatives to the currently available insufficiently effective medications. Of course, there has been a been continuous progress in the understanding of the pathophysiology of depression and the mechanism of action of existing antidepressants. In the current review the novel insights in the pathophysiology of major depression and the pharmacology of antidepressants are integrated. Based on this, it is possible to propose a relatively simple in vitro screening method to identify new pharmacological principles with anti-inflammatory and antidepressant potential.
2. Activation of Microglia Cells by Risk Factors for Depression
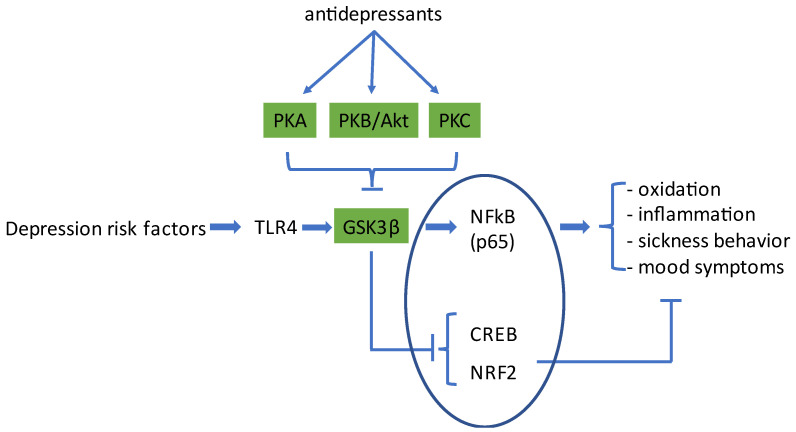
Risk factors for depression (see below) activate the immune system in the brain [4,9,12]. These risk factors activate Toll-like receptors (TLRs) expressed by the brain’s resident immune cells (microglia) and trigger an intracellular signaling pathway involving Nuclear Factor-κB (NFκB) [13] that promotes the transcription of genes that encode pro-oxidative and pro-inflammatory mediators. The serine/threonine kinase glycogen-synthase kinase 3 (GSK3) acts as a master switch between, on the one hand, a pro-inflammatory/pro-oxidative and, on the other hand, a proliferative, protective, and restorative biological program [14,15]. This process, which is discussed in more detail below, is depicted in Figure 1.
Figure 1.
Schematic representation of the process by which risk factors for depression increase inflammatory and pro-oxidative processes in the brain and lead to physical and psychological symptoms of depressive disorder. Depression risk factors activate Toll-like receptor-4 (TLR4) on microglia cells, which causes activation of the kinase GSK3β and an increase in gene transcription by NFκB (p65/RelA). Antidepressant compounds, via activation of different members of the AGC protein-kinase family, including protein kinase A (PKA), PKB/Akt, and PKC, inhibit the enzyme activity of GSK3β. This, on the one hand, inhibits NFκB-signaling and, on the other hand, increases gene transcription by CREB and NRF2, two transcription factors that promote the transcription of neuroprotective, anti-inflammatory, and anti-oxidant proteins. This way, antidepressants neutralize the negative effects evoked by TLR4 activation. CREB: cyclic-AMP responsive element binding protein; NRF2: nuclear factor erythroid 2-related factor 2. The transcription factors are enclosed in an ellipse. For further details, the reader is referred to the text.
The evidence that inflammation plays a subtle role in the pathophysiology of major depressive disorder (MDD) comes from three observations: (1) one-third of those with major depression show elevated peripheral inflammatory biomarkers, even in the absence of an additional medical illness; (2) inflammatory illnesses are associated with greater rates of MDD; and (3) patients treated with pro-inflammatory cytokines are at greater risk for developing major depressive illness (reviewed by [16,17]).
Infection [18] and inflammatory disorders such as IBD (inflammatory bowel disease) [19], metabolic disorders [19,20], cardiovascular disorders [21], neurological disorders [22,23,24,25,26], autoimmune diseases [18], and even alcohol abuse [27,28] are among the likely causes of immune dysfunction that contribute to the pathogenesis of depression [29]. In these diseases, affected cell populations generate and release so-called “danger associated molecular-pattern molecules” (DAMPs), which activate the innate immune system [30]. For instance, DAMPs such as HMGB1, modified lipids, heat shock proteins, S100, or hyaluronan-oligosaccharides activate the same signaling cascade as LPS, a cell wall fragment from Gram-negative bacteria [29,30,31]. Pathogens are recognized by specific immune receptors called Toll-like receptors (TLRs). Like LPS [30], DAMPs activate TLR2 and, in particular, TLR4 [32,33]. Notably, alcohol triggers a TLR4 response too [34].
Inflammation is thought to affect brain signaling causing mood symptoms, cognition dysfunction, and the production of a constellation of symptoms, termed ‘sickness behavior’. Sickness behavior is a set of symptoms (fatigue, immobility, fever, and loss of appetite) induced by an infection and mediated by pro-inflammatory cytokines. It is conceptualized as an adaptive response that enhances recovery by conserving energy to combat acute inflammation [35,36]. Mood symptoms in depression are anxiety, apathy, general discontent, guilt, hopelessness, loss of interest, anhedonia, and sadness [35].
Chronic stress greatly contributes to the development of major depressive disorder [14,37,38,39,40]. The pathophysiological sequelae to chronic stress have been evaluated in great detail in an array of rodent models, including chronic mild stress, social defeat stress, restraint stress, prenatal stress, and others (for review, see [41,42]). In each of these models, microglial cells were activated in a manner that resembled a bacterial infection [41,43,44,45,46,47,48], in particular in mood-relevant areas such as the hippocampus, frontal cortex, nucleus accumbens, and amygdala [31,38,49,50,51]. Chronic stress in laboratory animals thus increased the expression of pro-inflammatory cytokines and chemokines (IL1β, IL6, TNFα, TLR4, CCL2, and CX3CR1) by microglia and elevated the levels of corticosterone and IL6 in the blood circulation [52,53]. In further studies in chronic stress models, it was noted that stress increased inflammation markers (CD14, CD86, and TLR4) on microglia and macrophages [53,54]. Moreover, the morphology of microglia changed from ramified to hypertrophic/activated, with short and thick processes [42,53,55]. Similar to stress, chronic exposure to glucocorticoids also provoked microglial activation in rodents [39]. The process by which stress results in activation of microglia may involve the release of cellular distress signals such as HMGB1 (an agonist at TLR2 and TLR4 [32,56]) and ATP by abnormally active neurons [41,56]. Since attempts to understand the pathophysiology of depression have traditionally focused on neuronal dysfunction, the functionality of other types of brain cells has received (too) little attention [42]. The firm awareness that microglial cells play a crucial role in the development and maintenance of major depression has developed only quite recently [41,42,57,58,59].
Transcription in cells is limited by two important histone acetyltransferases, CBP and p300. In microglia cells, inflammatory signaling is to a large extent determined by the competition for CBP/p300-occupancy between the pro-inflammatory transcription factor NFκB (in particular the p65/RelA subunit) and the anti-inflammatory transcription factors, NRF2 and CREB [60,61,62,63,64]. As mentioned above, the balance between these pro- and anti-inflammatory pathways, which is essential for major depressive disorder [65,66], is influenced by the kinase activity of GSK3. Active GSK3 enhances the affinity of p65 for CBP and p300 [61] and, on the other hand, promotes the nuclear export of NRF2 [67], inhibits the nuclear import of CREB [60], and promotes the catabolism of CREB and NRF2 [68]. Although GSK3 comes in two distinct isoforms, modulation of CREB and NRF2 seems to be mediated by the GSK3β isoform only [60]. NRF2 increases the transcription of genes involved in oxidant-defense [63] and factors that stimulate mitochondrial biosynthesis [66,69]. Downstream of CREB are anti-inflammatory mediators such as brain-derived neurotrophic factor (BDNF), IL1-receptor antagonist (IL1-RA), IL10, and DUSP1 [62,67]. Notably, DUSP1 is an inhibitor of the pro-inflammatory kinase p38 [70,71,72]. Activation of NRF2 provides transcriptional repression of TNFα, IL1β, IL6, IL8, and CCL2 in microglia, monocytes, and macrophages [66,69]. For instance, activation of NRF2 reduced TLR4-induced IL1β, IL6, iNOS, and COX2 expression in primary mouse peritoneal macrophages [66,69]. Stimulation of Toll-like receptors promotes the enzyme activity of GSK3β [14] (for review, see [73]), whereas active GSK3 inhibits the production of the anti-inflammatory cytokine IL10, of IL1-RA, BDNF, and DUSP1 [72,74]. Activators of TLR-signaling are mutant presenilin-1 [75], α-synuclein fibrils [76], ethanol [77], and pathogens [73]. Importantly, repeated stress results in activation of GSK3 [73]. A group of kinases with similar substrate preferences, the so-called ‘AGC’ kinases are able to phosphorylate a serine residue in the N-terminal part of GSK3 (Ser-9 in GSK3β), which generates a pseudo-substrate for GSK3 and thereby inhibits the phosphorylation of other protein substrates (so inhibits GSK3 activity). AGC-kinases include the protein kinases PKA, PKB/Akt, PKC, PKG, S6K, and others [15,78,79]. Data from patients indicate that the cAMP-PKA pathway is hypoactive in MDD [80,81,82], so GSK3 is tendentially hyperactive [83]. This prediction is consistent with observations in MDD patients that both the nuclear translocation of NRF2 and the NRF2-mediated gene transcriptions are diminished [84], whereas biomarkers reflecting oxidative damage are elevated [85,86].
3. Classical Antidepressants Activate PKA to Inhibit GSK3β
Interestingly, as in MDD patients, also in the chronic mild stress depression model there is a reduction in cortical cAMP-PKA signaling and consequently a decrease in CREB activation and BDNF transcription [39]. Chronic but not acute treatment with ‘classical’ antidepressants attenuates the decrease in BDNF [8]. Classical antidepressants are typically inhibitors of monoamine reuptake, inhibitors of monoamine metabolism, or antagonists at presynaptic monoamine receptors, so their overall effect is an increase in extracellular levels of monoamine neurotransmitters. It is conceivable that this will lead to stimulation of monoamine receptors on microglia. Some of these receptors (dopamine D1/D5 [76,87,88,89], noradrenaline β2 [90,91], and serotonin 5HT7 [92]) are coupled to GS and generate high cAMP levels. Elevated cAMP levels activate PKA, which in turn inhibits GSK3β. As outlined above and depicted in Figure 1, the balance between pro-inflammatory TLR-NFκB signaling and NRF2/CREB signaling would be shifted away from inflammation (seen as a reduction in pro-inflammatory cytokines) and towards transcription growth factors such as insulin-like growth factor-1, BDNF, and anti-inflammatory cytokines such as IL10 and IL1-RA [93,94]. In accordance with this, it has been proposed that classical antidepressants limit the sequelae of TLR-activation by activation of cAMP-PKA signaling [8,95,96] and reduce the production of pro-inflammatory cytokines and oxygen radicals by microglia cells [52,96,97,98,99,100]. Importantly, chronic antidepressants also ameliorate the behavioral effects of stress [42,52]. So, these antidepressants limit an ongoing inflammation in the brain, and this is presumably the reason why they reduce depression symptoms. However, since the classical antidepressants elevate monoamine levels in the brain, any inflammatory process in the periphery will remain unopposed [67]. It is conceivable that a continuing peripheral inflammation will hinder full recovery and might represent a precipitating factor for relapses [16].
GS-coupled monoamine receptors that are expressed by microglial cells are D1 and D5 [88], β1, and β2, and 5HT7 (notably two other GS-coupled serotonin receptors, 5HT4 and 5HT6, are not expressed by microglia [101,102]). Direct agonists for these microglial receptors would provide anti-inflammatory and antidepressant activity, possibly with a more rapid onset of action. In this context, it is worth noting that already in 1996, Shimizu and colleagues suggested that activation of 5HT7 receptors might underlie the therapeutic response to classical antidepressants [95]. However, there is no reason for a restriction to monoamine receptors only. Extensive reviews have been published that report on the types of G-protein-coupled receptors (GPCRs) expressed by microglia [101,103,104]. Those GPCRs that couple to GS might offer alternative targets for novel anti-inflammatory and antidepressant drugs. In fact, there is not even a reason to stick to only the cAMP-PKA pathway. Essential is the activity of GSK3β, and this enzyme can be inhibited by, for instance, PKB or PKC. The next paragraphs deal with compound classes with certain evidence for clinical efficacy against depression, for which activation of PKB or PKC could represent the underlying mechanism for therapeutic activity.
4. Activation of PKB/Akt to Inhibit GSK3β
Electrical stimulation of the vagus nerve is an exploratory method for the treatment of depression and inflammation [105,106,107,108]. The mode of action involves activation of nicotinic α7 receptors [109,110,111], which results in increases in Ser9-phosphorylation of GSK3β [112,113,114]. This pharmacological response involves a pathway where stimulation of the α7 receptor activates the kinase JAK2, which leads to subsequent activation of PI3K, PKB, and phosphorylation/inhibition of GSK3 [115,116]. Consistent with the inhibition of GSK3, NRF2 signaling was promoted, as seen by increases in gene transcription of antioxidant and anti-apoptotic genes and by a reduction in the levels of pro-inflammatory cytokines [112,115,116]. These effects occur in microglia cells [117,118]. Importantly, treatment with an experimental nicotinic α7 agonist mitigated both the biochemical changes and the depressive behavior of mice subjected to the chronic mild stress procedure [119]. It may be noted that responses to nicotinic-α7 agonists resemble the effects of the anti-inflammatory cytokine IL10. Both activate the JAK2/STAT3 and the PI3K-Akt pathways to improve NRF2-mediated HO-1 (heme oxygenase-1) transcription, and both inhibit NFκB and suppress the hypertrophic/activated microglia polarization [116].
BDNF is produced by neurons [120,121,122], astrocytes [123,124], endothelial cells [93], as well as by reparative microglia cells [125,126]. The receptor for BDNF, TrkB (tropomyosin kinase receptor B), is expressed by neurons [120,122], astrocytes, and microglia [127,128,129,130]. TrkB signaling leads to activation of the PI3K-Akt pathway [122,131,132] and, as a consequence thereof, to inhibition of GSK3β [133] and activation of CREB and NRF2 [134]. Since long, BDNF is suspected to play an important role in the antidepressant effect of both classical and novel antidepressant medications [135,136,137,138,139]. HDAC inhibition by valproate, butyrate, trichostatin, or fingolimod increases the expression of BDNF [121,140,141]. HDAC inhibitors may act as antidepressants [142,143], which is consistent with their proposed effect on the BDNF-TrkB-Akt-GSK3 signal transduction cascade.
5. Gq-PLC-PKC-Induced GSK3β-Inhibition
5.1. Fatty Acids and Endocannabinoids
Fatty acids and their oxidized metabolites can form endogenous cannabinoids when they are esterified to ethanolamine, glycerin, or amino acids such as glycine or serine. These mediators are produced by astrocytes and microglia [144] and activate several GPCRs [145]. Microglia and macrophages particularly express CBR2 and the ‘orphan’ cannabinoid receptor, GPR18 [144,146,147,148]. Activation of both receptors inhibits the production of pro-inflammatory cytokines and increases the levels of IL10 and IL1-RA [147,148,149]. GPR18 seems to couple to GS [150,151], whereas the CB2 receptor causes PLC activation [147], although GS-cAMP-PKA signaling has been reported too [152]. The CB2 receptor is inducible and becomes expressed in microglia cells following inflammation or injury (reviewed by [153]). Overexpression of CBR2 in mice resulted in resistance to chronic mild stress-induced depression [39,154]. The ethanolamines (EA’s) formed from the omega-3 polyunsaturated fatty acids DHA and EPA (DHA-EA and EPEA, respectively) display anti-inflammatory activity in microglia, both in vitro and in vivo [155]. DHA-EA (known also as “synaptamide”) activates cAMP-PKA signaling in microglia and is a potent suppressor of LPS-induced neuroinflammation in mice [156,157,158]. Synaptamide is an agonist at the Gs-coupled orphan receptor GPR110/ADGRF1 [159]. Likewise, the epoxides of EPA and DHA (EEQ and EDP, respectively) can be esterified to ethanolamine, generating the endogenous cannabinoids EEQ-EA and EDP-EA. These products are produced by BV-2 microglial cells and are known to inhibit IL6 and NO production while raising the levels of IL10 [160]. These responses are in part mediated through CBR2 [160], however, any agonistic activity at GPR18 or GPR110 remains to be tested. Such experiments are indicated, as there is an evident discrepancy between the potent anti-inflammatory effects of 19,20-EDP-EA and its weak CB2 affinity in the data reported by McDougle et al. [160]. To summarize, the data collected in this paragraph indicate that agonists for microglial endocannabinoid receptors could represent novel approaches for the treatment of depression.
5.2. Opioid μ-Receptor Agonists
Other examples of receptors that are Gq-coupled and activate PLC-PKC are the opioid μ-receptor and the class of 5HT2-receptors (see Table 1).
Table 1.
Gq-coupled receptors that mediate anti-inflammatory and antidepressant activity.
| Receptor | Citations for Expression by Microglia | Citations for Gq-PLC-PKC Coupling | Citations for Inflammatory Cytokines Decrease | Citation for Antidepressant Activity |
|---|---|---|---|---|
| Cannabinoid CBR2 | [146,153] | [147,161] | [148,149,161] | [144,162] |
| Opioid μ-receptor | [103,163] | [164] | [165] | [166,167,168] |
| Serotonin 5HT2B | [101,102] | [169,170,171] | [101,102] | [172,173,174] |
Literature citations for the expression of the cannabinoid receptor CBR2, the opioid μ receptor, and the serotonin receptor 5HT2B by microglia cells. Agonists at these receptors activate phospholipase-C (PLC) and protein kinase-C (PKC), which results in inhibition of brain inflammation and improvement of depression symptoms.
The endogenous opioid system is known to play a central role in the pathophysiology of affective disorders [175,176,177]. Opioid μ-receptors, in contrast to δ- or κ-opioid receptors, are involved in immune modulation in the brain [178]. Furthermore, depression-scores in healthy subjects were inversely correlated with the binding capacity of an μ-receptor PET-ligand in cortical and subcortical brain regions [179]. Microglia cells express μ-receptors [103,163], and the prototypic opioid receptor agonist morphine decreases the production of IL1β, IL2, TNFα, and IFNγ, and increases TGFβ1 and IL10 [163]. Similarly, tianeptine, a selective μ-receptor receptor agonist [180], inhibited LPS-induced microglia M1-polarization and reduced the LPS-induced expression of IL1β, IL18, IL6, TNFα, and CCL2, as well as the production of NO and ROS [165]. In animal models, tianeptine reversed stress-induced dystrophy of hippocampal dendrites and produced antidepressant-like effects [176]. These effects were blocked by a selective μ-receptor antagonist and were absent in mice with a genetic μ-receptor deficiency [176]. In contrast to morphine, which has off-target pro-inflammatory activity via TLR4 in BV-2 microglia cells [181], tianeptine did not induce tolerance to the analgesic and anti-inflammatory effects [176]. Tianeptine reduces the depression symptoms in patients with mild to moderate-to-severe major depression [167], but given its inherent abuse liability, the risk-benefit ratio of tianeptine remains uncertain [182]. The mixed μ-receptor agonist/κ-antagonist buprenorphine is antidepressant too, and similar to tianeptine, it remains a therapeutic option for patients with treatment-resistant major depression [166,168]. The μ-receptor primarily couples to Gi/G0 but additionally to Gq-mediated PLC-activation [164]. Activation of PLC leads to PKC activity and thereby, to phosphorylation/inhibition of GSK3.
5.3. Hallucinogenic 5HT2 Agonists
Anecdotal reports of the antidepressant activity of psilocybin and LSD [173] have led to the exploration of the antidepressant effects of psilocybin in randomized clinical trials [172,174]. Although the psychedelic effect precludes an effective study blinding, the antidepressant effect was encouraging. The hallucinogenic activity is ascribed to stimulation of central 5HT2A receptors [183,184], and in the neuron-centric view on depression, 5HT2A receptor stimulation is considered the likely mechanism for the improvement of mood [173,185]. Notably, serotonergic hallucinogens are potent activators of the 5HT receptor of the rat stomach fundus [186], a model for 5HT2B receptor activation of PKC [170]. Importantly, the 5HT2B receptor is strongly expressed in microglia [101], and its stimulation suppresses the release of pro-inflammatory cytokines [187]. It is conceivable that the antidepressant activity of psilocybin is related to the activation of 5HT2B receptors on microglia. This would imply that it should be possible to dissociate the hallucinogenic activity from the antidepressant activity by increasing the 5HT2B/5HT2A selectivity.
6. Discussion
Stress and infection activate the NFκB pathway in phagocytic cells and lead to the transcription of pro-inflammatory cytokines and oxidizing enzymes such as COX2 and iNOS [44,188,189,190,191]. The opposite process is initiated by the transcription factors CREB and NRF2, which compete with NFκB for access to the gene transcription machinery and activate the production of anti-oxidant proteins and anti-inflammatory cytokines [192,193,194]. Both CREB and NRF2 are stabilized when the pro-inflammatory kinase GSK3β is inhibited. GSK3β becomes blocked when kinases such as PKA, PKB, and PKC phosphorylate a distinct serine residue (Ser-9) in the N-terminal part of GSK3β. Any process that activates PKA, PKB, or PKC therefore has the potential to limit the consequences of stress and infection. In the foregoing sections, several pharmacological principles with antidepressant potential were discussed that, in each case, inhibited GSK3β, either by activating PKA, PKB, or PKC in microglial cells. A screen in microglial cells for compounds and pharmacological principles that inhibit GSK3β or activate CREB and NRF2 transcription has the potential to discover novel antidepressants. The pharmacological principles that were discussed in the foregoing sections were mostly investigated in LPS-stimulated human or rodent microglia cell lines [92,128,195], rat and mouse microglia primary cultures, or primary microglia acutely isolated after in vivo exposure to LPS or stress [94,118]. A good alternative to consider would be to screen induced pluripotent stem cell-derived microglial precursor cells [196]. However, a simple screen in mouse BV-2 microglial cells would already detect α-lipoic acid [197], 9-cis-retinoic acid [198], stable cAMP analogues [94,199], PDE4 inhibitors [200,201], agonists of the fatty acid receptor FFAR1/GPR40 [202], the ‘specialized pro-resolving mediators’ lipoxin A4, resolvin D1 and resolvin E1 [59,203,204,205,206], and inhibitors of the enzyme soluble epoxide-hydrolase [207]. Presumably, even ketamine might be detected as a hit.
6.1. Ketamine
The mechanism by which ketamine exerts its antidepressant effects is still a matter of debate [208,209]. The two mechanisms that are discussed are glutamate NMDA-receptor blockade and opioid μ-receptor activation. The issue with NMDA-blockade is that other NMDA blockers do not induce a rapid antidepressant effect (for instance, memantine [210]), whereas the ketamine-metabolite 2R,6R-HNK provoked rapid antidepressant-like activity in preclinical models that was independent of NMDA-blockade [211]. Moreover, ketamine was still ‘antidepressant’ in transgenic mice that lack the essential NR1-subunit of the NMDA channel, whereas glycine-site agonists that activate the functioning of the NMDA-channel are also antidepressants (reviewed in [209]). Ketamine has a significant affinity for opioid receptors and is antinociceptive [210]. In a pilot study in humans with major depression, the antidepressant but not the dissociative effects of ketamine were blocked by naltrexone [212]. Notably, the antinociceptive effects of 2R,6R-HNK were not affected by the opioid μ-receptor antagonist naltrexone [213]. Independent of the precise mechanism of action, ketamine induced a robust inhibitory phosphorylation of GSK3 in preclinical models, whereas the antidepressant-like effect of ketamine was lost in transgenic mice that carried a non-inhibitable GSK3-variant [214,215]. Consistent with this activity, ketamine increased the expression of BDNF [216,217], increased the expression of the NRF2-target gene heme oxygenase-1 [218], suppressed chronic stress-induced depressive behaviors and pro-inflammatory cytokine levels [219,220,221], as well as LPS-induced depressive behaviors [222]. In a human monocyte cell line, ketamine inhibited NFκB and reduced pro-inflammatory cytokine levels [223,224]. Ketamine administration to humans before or during surgery inhibited the increase in post-operative IL6 and TNFα levels [225,226]. Similarly, in two small studies in patients suffering from MDD, blood IL6 levels decreased after ketamine treatment in the responders but not in the non-responders [227,228]. These results suggest that suppression of inflammation is relevant for the antidepressant effects of ketamine. The remaining question is: by what mechanism does ketamine provoke inhibition of GSK3β. An interesting proposal was made by Wray and co-authors [124]. GS is singly palmitoylated on its N-terminus, which targets the protein to lipid rafts. Ketamine and 2R,6R-HNK are lipophilic and remove Gs from the lipid raft. This has a positive effect on the interaction between GS and adenylate cyclase and thus on the production of cAMP, the activation of PKA, and the CREB-target gene BDNF [82,124]. The authors consider it likely that other lipid-soluble anesthetics act similarly to ketamine and 2R,6R-HNK. This proposal is consistent with the observation that intravenous and inhaled anesthetics (ketamine, propofol, opioids, isoflurane, sevoflurane, desflurane, etc.) to varying extents upregulate heme oxygenase-1 expression [218]. The ketamine-induced displacement of GS from the lipid rafts occurs much more rapidly than with, for instance, tricyclic antidepressants [82] and could be an explanation for ketamine’s faster onset of antidepressant activity. It is conceivable that microglial cells are the targets for these effects [41,229,230].
6.2. GSK3β Blockade by Antipsychotics
Although dopamine D2 receptor antagonists (antipsychotics) are known to elevate the levels of Ser-9 phosphorylated GSK3β in the brain [231], such compounds are not genuinely known to act as antidepressants. A conceivable explanation for this apparent discrepancy is provided hereafter.
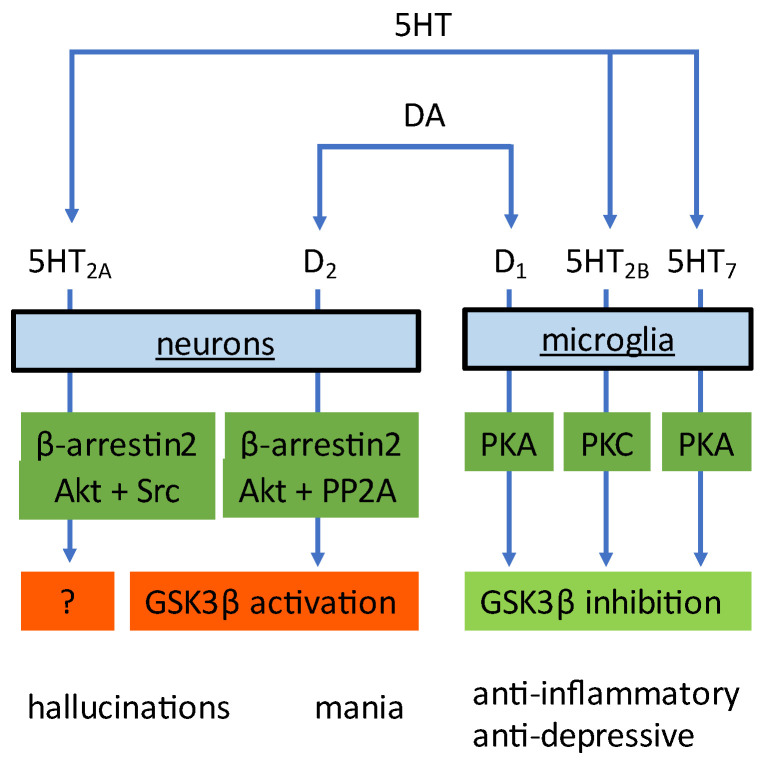
Dopamine and serotonin not only influence microglia cells, they also modulate the function of neurons. However, the downstream signaling pathways in neurons are distinctly different from those in microglia. Activation of dopamine D2 receptors expressed on neurons (by, for instance, amphetamine) leads to activation of GSK3. The signaling cascade involves the formation of a molecular complex consisting of β-arrestin2 (βArr2), PKB, and the phosphatase PP2A [232]. Formation of this complex leads to PP2A-mediated deactivation of PKB and thus to the disinhibition of GSK3 [232]. Conversely, D2-receptor antagonists such as haloperidol, raclopride, and atypical antipsychotics such as clozapine, risperidone, olanzapine, quetiapine, and ziprasidone (their common profile is dual antagonism of 5HT2A receptors and D2-receptors [233,234]) all induce inhibition of GSK3, owing to the inhibitory phosphorylation of GSK3 by PKB [235,236]. Similar to dopamine, activation of neuronal 5HT2A receptors involves the formation of a molecular complex, here consisting of βArr2, PKB, and Src [237] and ultimately results in activation of GSK3 [238]. In agreement, 5HT2A receptor antagonists provoke an inhibition of GSK3 [239]. Therefore, a GSK3 inhibitor will block amphetamine-induced mania (GSK3 inhibition in neurons) and will generate antidepressant responses (via GSK3 inhibition in microglia). The solution to the apparent discrepancy mentioned above is quite simple. Whereas a GKS3 inhibitor will presumably limit both the manic and depressive symptoms, a D2-receptor antagonist will affect the signaling in neurons [231], but will not produce a profound antidepressant effect (see Figure 2). Under the assumption that this explanation is proven to be correct, it would emphasize the point that only GSK3 blockade in microglia results in antidepressant activity.
Figure 2.
Dopamine (DA) and serotonin (5HT) activate distinct receptor subtypes and signaling pathways in microglia and neurons. In microglial cells, the functional outcome is an inhibition of GSK3β, whereas activation of the D2-receptor in neurons results in activation of GSK3β. Whether activation of the 5HT2A receptor in neurons leads to GSK3β activation has not been investigated.
6.3. Conclusions
Depression is a major public health concern. The risk-benefit ratio of the currently available antidepressants is quite unsatisfactory, since either their therapeutic effectiveness is insufficient or they cause unacceptable side effects. Consequently, there is a pressing need to discover better treatments. Literature data indicate that risk factors for depression initiate an infection-like inflammation in the brain that involves activation of microglial Toll-like receptors and glycogen synthase kinase-3β (GSK3β). GSK3β activity alters the balance between two major competing transcription factors, the pro-inflammatory/pro-oxidative transcription factor NFκB and the neuroprotective, anti-inflammatory, and anti-oxidative transcription factor NRF2. The negative consequences of depression risk factors could thus be counteracted by procedures that lead to GSK3β inhibition. As an example, the antidepressant activity of tricyclic antidepressants is assumed to involve activation of GS-coupled microglial receptors, raising intracellular cAMP levels, and activation of protein kinase A (PKA). PKA inhibits the enzyme activity of GSK3β. Importantly, PKA is just one of a series of AGC-kinases that is able to inhibit GSK3β. Literature information is provided to show that each pharmacological principle for which evidence for antidepressant activity in humans exists results in the inhibition of GSK3β in microglial cells, albeit via different signaling cascades and involving different AGC-kinases. Ketamine and lipid-soluble anesthetics act somewhat distinct in that they do lead to GSK3β inhibition; however, not via a pharmacological effect but in response to a physical property (lipid solubility) that alters the lipid raft composition. Furthermore, it is argued that in order to achieve antidepressant activity, GSK3β inhibition has to occur in microglia rather than in neurons. Based on these considerations, an in vitro screen testing for GSK3β inhibition/NRF2 activation in microglial cells with TLR4-stimulated GSK3β activity might ultimately lead to the detection of novel antidepressant principles. This would, hopefully, end the stagnation in the discovery and development of safe and effective treatments for major depressive disorders.
Abbreviations
5HT (5-hydroxytryptamine); ATP (adenosine triphosphate); BDNF (brain-derived neurotrophic factor); cAMP (cyclic adenosine monophosphate); CCL2 (CC-chemokine ligand-2); CNS (central nervous system); CBP (CREB-binding protein); COX2 (cyclooxygenase-2); CREB (cyclic-AMP responsive element binding protein); CX3CR1 (CX3C motif chemokine receptor-1); DHA (docosahexaenoic acid); DUSP1 (dual specificity phosphatase-1); EA (ethanolamine); EPA (Eicosapentaenoic acid); FFAR1 (free fatty acid receptor-1); GPCR (G-protein coupled receptor); GSK3β (glycogen synthase kinase-3β); HDAC (histone deacetylase); HMGB1 (high mobility group box-1); HO-1 (heme oxygenase-1); IFNγ (interferon-γ); IL (interleukin); IL1-RA (interleukin-1 receptor antagonist); iNOS (inducible nitric oxide synthase); JAK2 jJanus kinase-2); LPS (lipopolysaccharide/TLR4 agonist); MDD (major depressive disorder); NFκB (nuclear Factor-κB); NMDA (N-methyl-D-aspartate); NO (nitric oxide); NRF2 (nuclear factor erythroid 2-related factor-2); PDE4 (phosphodiesterase-4); PET (positron emission tomography); PKA (protein kinase-A); PKB (protein kinase-B); PKC (protein kinase-C); PLC (phospholipase-C); PP2A (protein phosphatase-2A); ROS (reactive oxygen species); TGFβ1 (transforming growth factor-β1); TLR (toll-like receptor); TNFα (tumor necrosis factor-α); TrkB (tropomyosin kinase receptor-B).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Malhi G.S., Mann J.J. Depression. Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 2.Schildkraut J.J. The Catecholamine Hypothesis of Affective Disorders: A Review of Supporting Evidence. Am. J. Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 3.Capuron L., Miller A.H. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berk M., Williams L.J., Jacka F.N., O’Neil A., Pasco J.A., Moylan S., Allen N.B., Stuart A.L., Hayley A., Byrne M.L., et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ögren S.O., Fuxe K., Agnati L. The Importance of Brain Serotonergic Receptor Mechanisms for the Action of Antidepressant Drugs. Pharmacopsychiatry. 1985;18:209–213. doi: 10.1055/s-2007-1017366. [DOI] [PubMed] [Google Scholar]
- 6.Palazidou E., Beer M.S., Checkley S., Stahl S.M. Pharmacologic exploitation of neurotransmitter receptors for the design of novel antidepressant drugs. Drug Des. Deliv. 1988;2:247–256. [PubMed] [Google Scholar]
- 7.Broekkamp C.L.E., Leysen D., Peeters B.W., Pinder R.M. Prospects for Improved Antidepressants. J. Med. Chem. 1995;38:4615–4633. doi: 10.1021/jm00023a001. [DOI] [PubMed] [Google Scholar]
- 8.Kenis G., Maes M. Effects of antidepressants on the production of cytokines. Int. J. Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- 9.Haroon E., Raison C.L., Miller A.H. Psychoneuroimmunology Meets Neuropsychopharmacology: Translational Implications of the Impact of Inflammation on Behavior. Neuropsychopharmacology. 2011;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GBD 2019 Mental Disorders Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.COVID-19 Mental Disorders Collaborators Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S., Jeong J., Kwak Y., Park S.K. Depression research: Where are we now? Mol. Brain. 2010;3:8. doi: 10.1186/1756-6606-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo J.W., Russo S.J., Ferguson D., Nestler E.J., Duman R.S. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. USA. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Y., Pardo M., Armini R.D.S., Martinez A., Mouhsine H., Zagury J.-F., Jope R.S., Beurel E. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav. Immun. 2016;53:207–222. doi: 10.1016/j.bbi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duda P., Hajka D., Wójcicka O., Rakus D., Gizak A. GSK3β: A Master Player in Depressive Disorder Pathogenesis and Treatment Responsiveness. Cells. 2020;9:727. doi: 10.3390/cells9030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnadas R., Cavanagh J. Depression: An inflammatory illness? J. Neurol. Neurosurg. Psychiatry. 2012;83:495–502. doi: 10.1136/jnnp-2011-301779. [DOI] [PubMed] [Google Scholar]
- 17.Strawbridge R., Arnone D., Danese A., Papadopoulos A., Vives A.H., Cleare A.J. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 2015;25:1532–1543. doi: 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Benros M.E., Waltoft B.L., Nordentoft M., Østergaard S.D., Eaton W.W., Krogh J., Mortensen P.B. Autoimmune Diseases and Severe Infections as Risk Factors for Mood Disorders: A nationwide study. JAMA Psychiatry. 2013;70:812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- 19.Bhandari S., Larson M.E., Kumar N., Stein D. Association of Inflammatory Bowel Disease (IBD) with Depressive Symptoms in the United States Population and Independent Predictors of Depressive Symptoms in an IBD Population: A NHANES Study. Gut Liver. 2017;11:512–519. doi: 10.5009/gnl16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beurel E., Toups M., Nemeroff C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron. 2020;107:234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalkman H.O. The Association Between Vascular Inflammation and Depressive Disorder. Causality, Biomarkers and Targeted Treatment. Pharmaceuticals. 2020;13:92. doi: 10.3390/ph13050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vezzani A. Epilepsy and Inflammation in the Brain: Overview and Pathophysiology. Epilepsy Curr. 2014;14:3–7. doi: 10.5698/1535-7511-14.s2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung Y.-C., Ko H.-W., Bok E.-G., Park E.-S., Huh S.-H., Nam J.-H., Jin B.-K. The role of neuroinflammation on the pathogenesis of Parkinson’s disease. BMB Rep. 2010;43:225–232. doi: 10.5483/BMBRep.2010.43.4.225. [DOI] [PubMed] [Google Scholar]
- 24.Ouchi Y., Yagi S., Yokokura M., Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Park. Relat. Disord. 2009;15((Suppl. S3)):S200–S204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- 25.Tan Z.S., Seshadri S. Inflammation in the Alzheimer’s disease cascade: Culprit or innocent bystander? Alzheimer’s Res. Ther. 2010;2:6. doi: 10.1186/alzrt29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandberg M., Patil J., D’Angelo B., Weber S.G., Mallard C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan L.E., Fiellin D.A., O’Connor P.G. The prevalence and impact of alcohol problems in major depression: A systematic review. Am. J. Med. 2005;118:330–341. doi: 10.1016/j.amjmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Fergusson D.M., Boden J.M., Horwood L.J. Tests of Causal Links Between Alcohol Abuse or Dependence and Major Depression. Arch. Gen. Psychiatry. 2009;66:260–266. doi: 10.1001/archgenpsychiatry.2008.543. [DOI] [PubMed] [Google Scholar]
- 29.Beurel E., Jope R.S. Inflammation and lithium: Clues to mechanisms contributing to suicide-linked traits. Transl. Psychiatry. 2014;4:e488. doi: 10.1038/tp.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mogensen T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann F.N., Costa A.P., Ghisleni G., Diaz A.P., Rodrigues A.L.S., Peluffo H., Kaster M.P. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav. Immun. 2017;64:367–383. doi: 10.1016/j.bbi.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Park J.S., Svetkauskaite D., He Q., Kim J.Y., Strassheim D., Ishizaka A., Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 33.Kim S.Y., Son M., Lee S.E., Park I.H., Kwak M.S., Han M., Lee H.S., Kim E.S., Kim J.-Y., Lee J.E., et al. High-Mobility Group Box 1-Induced Complement Activation Causes Sterile Inflammation. Front. Immunol. 2018;9:705. doi: 10.3389/fimmu.2018.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alfonso-Loeches S., Pascual-Lucas M., Blanco A.M., Sanchez-Vera I., Guerri C. Pivotal Role of TLR4 Receptors in Alcohol-Induced Neuroinflammation and Brain Damage. J. Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maes M., Berk M., Goehler L., Song C., Anderson G., Gałecki P., Leonard B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kendler K.S., Hettema J.M., Butera F., Gardner C.O., Prescott C.A. Life Event Dimensions of Loss, Humiliation, Entrapment, and Danger in the Prediction of Onsets of Major Depression and Generalized Anxiety. Arch. Gen. Psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- 38.Calcia M.A., Bonsall D.R., Bloomfield P.S., Selvaraj S., Barichello T., Howes O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. 2016;233:1637–1650. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willner P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress. 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohleder N. Stimulation of Systemic Low-Grade Inflammation by Psychosocial Stress. Psychosom. Med. 2014;76:181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 41.Yirmiya R., Rimmerman N., Reshef R. Depression as a Microglial Disease. Trends Neurosci. 2015;38:637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Kreisel T., Frank M.G., Licht T., Reshef R., Ben-Menachem-Zidon O., Baratta M.V., Maier S.F., Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 43.Beumer W., Gibney S.M., Drexhage R.C., Pont-Lezica L., Doorduin J., Klein H.C., Steiner J., Connor T.J., Harkin A., Versnel M.A., et al. The immune theory of psychiatric diseases: A key role for activated microglia and circulating monocytes. J. Leukoc. Biol. 2012;92:959–975. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- 44.Hinwood M., Morandini J., Day T.A., Walker F.R. Evidence that Microglia Mediate the Neurobiological Effects of Chronic Psychological Stress on the Medial Prefrontal Cortex. Cereb. Cortex. 2012;22:1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- 45.Delpech J.-C., Madore C., Nadjar A., Joffre C., Wohleb E.S., Layé S. Microglia in neuronal plasticity: Influence of stress. Neuropharmacology. 2015;96:19–28. doi: 10.1016/j.neuropharm.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 46.Lehmann M.L., Cooper H.A., Maric D., Herkenham M. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J. Neuroinflamm. 2016;13:224. doi: 10.1186/s12974-016-0672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehmann M.L., Weigel T.K., Poffenberger C.N., Herkenham M. The Behavioral Sequelae of Social Defeat Require Microglia and Are Driven by Oxidative Stress in Mice. J. Neurosci. 2019;39:5594–5605. doi: 10.1523/JNEUROSCI.0184-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang J., Yu W., Chen S., Gao Z., Xiao B. Microglia Polarization and Endoplasmic Reticulum Stress in Chronic Social Defeat Stress Induced Depression Mouse. Neurochem. Res. 2018;43:985–994. doi: 10.1007/s11064-018-2504-0. [DOI] [PubMed] [Google Scholar]
- 49.McKim D.B., Niraula A., Tarr A.J., Wohleb E.S., Sheridan J.F., Godbout J.P. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. J. Neurosci. 2016;36:2590–2604. doi: 10.1523/JNEUROSCI.2394-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohgidani M., Kato T.A., Sagata N., Hayakawa K., Shimokawa N., Sato-Kasai M., Kanba S. TNF-α from hippocampal microglia induces working memory deficits by acute stress in mice. Brain Behav. Immun. 2016;55:17–24. doi: 10.1016/j.bbi.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y.-L., Han Q.-Q., Gong W.-Q., Pan D.-H., Wang L.-Z., Hu W., Yang M., Li B., Yu J., Liu Q. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflamm. 2018;15:21. doi: 10.1186/s12974-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramirez K., Sheridan J.F. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav. Immun. 2016;57:293–303. doi: 10.1016/j.bbi.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C., Zhang Y.-P., Li Y.-Y., Liu B.-P., Wang H.-Y., Li K.-W., Zhao S., Song C. Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav. Brain Res. 2019;356:348–357. doi: 10.1016/j.bbr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Ślusarczyk J., Trojan E., Głombik K., Budziszewska B., Kubera M., Lasoń W., Popiołek-Barczyk K., Mika J., Wędzony K., Basta-Kaim A. Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Front. Cell. Neurosci. 2015;9:82. doi: 10.3389/fncel.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wohleb E.S., Hanke M.L., Corona A.W., Powell N.D., Stiner L.M., Bailey M.T., Nelson R.J., Godbout J.P., Sheridan J.F. β-Adrenergic Receptor Antagonism Prevents Anxiety-Like Behavior and Microglial Reactivity Induced by Repeated Social Defeat. J. Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber M.D., Frank M.G., Tracey K.J., Watkins L.R., Maier S.F. Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J. Neurosci. 2015;35:316–324. doi: 10.1523/JNEUROSCI.3561-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng S.-L., Chen J.-G., Wang F. Microglia: A Central Player in Depression. Curr. Med. Sci. 2020;40:391–400. doi: 10.1007/s11596-020-2193-1. [DOI] [PubMed] [Google Scholar]
- 58.Wang H., He Y., Sun Z., Ren S., Liu M., Wang G., Yang J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022;19:132. doi: 10.1186/s12974-022-02492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahimian R., Belliveau C., Chen R., Mechawar N. Microglial Inflammatory-Metabolic Pathways and Their Potential Therapeutic Implication in Major Depressive Disorder. Front. Psychiatry. 2022;13:871997. doi: 10.3389/fpsyt.2022.871997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin M., Rehani K., Jope R.S., Michalek S.M. Toll-like receptor–mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viatour P., Merville M.-P., Bours V., Chariot A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Wen A.Y., Sakamoto K.M., Miller L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010;185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gill A.J., Kolson D.L. Dimethyl Fumarate Modulation of Immune and Antioxidant Responses: Application to HIV Therapy. Crit. Rev. Immunol. 2013;33:307–359. doi: 10.1615/CritRevImmunol.2013007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S.-W., Lee H.-K., Shin J.-H., Lee J.-K. Up-down Regulation of HO-1 and iNOS Gene Expressions by Ethyl Pyruvate via Recruiting p300 to Nrf2 and Depriving It from p65. Free. Radic. Biol. Med. 2013;65:468–476. doi: 10.1016/j.freeradbiomed.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 65.Morris G., Walker A.J., Walder K., Berk M., Marx W., Carvalho A.F., Maes M., Puri B.K. Increasing Nrf2 Activity as a Treatment Approach in Neuropsychiatry. Mol. Neurobiol. 2021;58:2158–2182. doi: 10.1007/s12035-020-02212-w. [DOI] [PubMed] [Google Scholar]
- 66.Zuo C., Cao H., Song Y., Gu Z., Huang Y., Yang Y., Miao J., Zhu L., Chen J., Jiang Y., et al. Nrf2: An all-rounder in depression. Redox Biol. 2022;58:102522. doi: 10.1016/j.redox.2022.102522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maes M., Fišar Z., Medina M., Scapagnini G., Nowak G., Berk M. New drug targets in depression: Inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates—Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. 2012;20:127–150. doi: 10.1007/s10787-011-0111-7. [DOI] [PubMed] [Google Scholar]
- 68.Rada P., Rojo A.I., Chowdhry S., McMahon M., Hayes J.D., Cuadrado A. SCF/β-TrCP Promotes Glycogen Synthase Kinase 3-Dependent Degradation of the Nrf2 Transcription Factor in a Keap1-Independent Manner. Mol. Cell. Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dinkova-Kostova A.T., Kostov R.V., Kazantsev A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018;285:3576–3590. doi: 10.1111/febs.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kondoh K., Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2007;1773:1227–1237. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Kasuya Y., Umezawa H., Hatano M. Stress-Activated Protein Kinases in Spinal Cord Injury: Focus on Roles of p38. Int. J. Mol. Sci. 2018;19:867. doi: 10.3390/ijms19030867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoppstädter J., Ammit A. Role of Dual-Specificity Phosphatase 1 in Glucocorticoid-Driven Anti-inflammatory Responses. Front. Immunol. 2019;10:1446. doi: 10.3389/fimmu.2019.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jope R.S., Cheng Y., Lowell J.A., Worthen R.J., Sitbon Y.H., Beurel E. Stressed and Inflamed, Can GSK3 Be Blamed? Trends Biochem. Sci. 2017;42:180–192. doi: 10.1016/j.tibs.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beurel E., Michalek S.M., Jope R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 2010;31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engel T., Hernández F., Avila J., Lucas J.J. Full Reversal of Alzheimer’s Disease-Like Phenotype in a Mouse Model with Conditional Overexpression of Glycogen Synthase Kinase-3. J. Neurosci. 2006;26:5083–5090. doi: 10.1523/JNEUROSCI.0604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo H., Callaway J.B., Ting J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qin L., He J., Hanes R.N., Pluzarev O., Hong J.-S., Crews F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflamm. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forlenza O.V., Spink J.M., Dayanandan R., Anderton B.H., Olesen O.F., Lovestone S. Muscarinic agonists reduce tau phosphorylation in non-neuronal cells via GSK-3β inhibition and in neurons. J. Neural Transm. 2000;107:1201–1212. doi: 10.1007/s007020070034. [DOI] [PubMed] [Google Scholar]
- 79.Pearce L.R., Komander D., Alessi D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 80.Dwivedi Y., Pandey G.N. Adenylyl cyclase-cyclicAMP signaling in mood disorders: Role of the crucial phosphorylating enzyme protein kinase A. Neuropsychiatr. Dis. Treat. 2008;4:161–176. doi: 10.2147/NDT.S2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujita M., Richards E.M., Niciu M., Ionescu D.F., Zoghbi S.S., Hong J., Telu S., Hines C.S., Pike V.W., Zarate C.A., et al. cAMP signaling in brain is decreased in unmedicated depressed patients and increased by treatment with a selective serotonin reuptake inhibitor. Mol. Psychiatry. 2017;22:754–759. doi: 10.1038/mp.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schappi J.M., Rasenick M.M. Gαs, adenylyl cyclase, and their relationship to the diagnosis and treatment of depression. Front. Pharmacol. 2022;13:1012778. doi: 10.3389/fphar.2022.1012778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karege F., Perroud N., Burkhardt S., Fernandez R., Ballmann E., La Harpe R., Malafosse A. Protein levels of β-catenin and activation state of glycogen synthase kinase-3β in major depression. A study with postmortem prefrontal cortex. J. Affect. Disord. 2012;136:185–188. doi: 10.1016/j.jad.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 84.Bansal Y., Singh R., Parhar I., Kuhad A., Soga T. Quinolinic Acid and Nuclear Factor Erythroid 2-Related Factor 2 in Depression: Role in Neuroprogression. Front. Pharmacol. 2019;10:452. doi: 10.3389/fphar.2019.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forlenza M.J., Miller G.E. Increased Serum Levels of 8-Hydroxy-2′-Deoxyguanosine in Clinical Depression. Psychosom. Med. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- 86.Savage K., Gogarty L., Lea A., Deleuil S., Nolidin K., Croft K., Stough C. The Relationship between F2-Isoprostanes Plasma Levels and Depression Symptoms in Healthy Older Adults. Antioxidants. 2022;11:822. doi: 10.3390/antiox11050822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan Y., Jiang W., Liu L., Wang X., Ding C., Tian Z., Zhou R. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 88.Wang B., Chen T., Li G., Jia Y., Wang J., Xue L., Chen Y. Dopamine Alters Lipopolysaccharide-Induced Nitric Oxide Production in Microglial Cells via Activation of D1-Like Receptors. Neurochem. Res. 2019;44:947–958. doi: 10.1007/s11064-019-02730-7. [DOI] [PubMed] [Google Scholar]
- 89.Perreault M.L., Jones-Tabah J., O’Dowd B.F., George S.R. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int. J. Neuropsychopharmacol. 2013;16:477–483. doi: 10.1017/S1461145712000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fujita H., Tanaka J., Maeda N., Sakanaka M. Adrenergic agonists suppress the proliferation of microglia through β2-adrenergic receptor. Neurosci. Lett. 1998;242:37–40. doi: 10.1016/S0304-3940(98)00003-2. [DOI] [PubMed] [Google Scholar]
- 91.Russo C.D., Boullerne A.I., Gavrilyuk V., Feinstein D.L. Inhibition of microglial inflammatory responses by norepinephrine: Effects on nitric oxide and interleukin-1β production. J. Neuroinflamm. 2004;1:9. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mahé C., Loetscher E., Dev K.K., Bobirnac I., Otten U., Schoeffter P. Serotonin 5-HT7 receptors coupled to induction of interleukin-6 in human microglial MC-3 cells. Neuropharmacology. 2005;49:40–47. doi: 10.1016/j.neuropharm.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 93.Nakahashi T., Fujimura H., Altar C., Li J., Kambayashi J.-I., Tandon N.N., Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/S0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 94.Ghosh M., Xu Y., Pearse D.D. Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. J. Neuroinflamm. 2016;13:9. doi: 10.1186/s12974-015-0463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimizu M., Nishida A., Zensho H., Yamawaki S. Chronic antidepressant exposure enhances 5-hydroxytryptamine7 receptor-mediated cyclic adenosine monophosphate accumulation in rat frontocortical astrocytes. J. Pharmacol. Exp. Ther. 1996;279:1551–1558. [PubMed] [Google Scholar]
- 96.Tynan R.J., Weidenhofer J., Hinwood M., Cairns M.J., Day T.A., Walker F.R. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav. Immun. 2012;26:469–479. doi: 10.1016/j.bbi.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 97.Hashioka S., Klegeris A., Monji A., Kato T., Sawada M., McGeer P.L., Kanba S. Antidepressants inhibit interferon-γ-induced microglial production of IL-6 and nitric oxide. Exp. Neurol. 2007;206:33–42. doi: 10.1016/j.expneurol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 98.Bielecka A.M., Paul-Samojedny M., Obuchowicz E. Moclobemide exerts anti-inflammatory effect in lipopolysaccharide-activated primary mixed glial cell culture. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010;382:409–417. doi: 10.1007/s00210-010-0535-4. [DOI] [PubMed] [Google Scholar]
- 99.Wang L., Wang R., Liu L., Qiao D., Baldwin D.S., Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: A systematic review and meta-analysis. Brain Behav. Immun. 2019;79:24–38. doi: 10.1016/j.bbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 100.Dionisie V., Filip G.A., Manea M.C., Manea M., Riga S. The anti-inflammatory role of SSRI and SNRI in the treatment of depression: A review of human and rodent research studies. Inflammopharmacology. 2021;29:75–90. doi: 10.1007/s10787-020-00777-5. [DOI] [PubMed] [Google Scholar]
- 101.Krabbe G., Matyash V., Pannasch U., Mamer L., Boddeke H.W., Kettenmann H. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav. Immun. 2012;26:419–428. doi: 10.1016/j.bbi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 102.de las Casas-Engel M., Domínguez-Soto A., Sierra-Filardi E., Bragado R., Nieto C., Puig-Kroger A., Samaniego R., Loza M., Corcuera M.T., Gómez-Aguado F., et al. Serotonin Skews Human Macrophage Polarization through HTR2B and HTR7. J. Immunol. 2013;190:2301–2310. doi: 10.4049/jimmunol.1201133. [DOI] [PubMed] [Google Scholar]
- 103.Kettenmann H., Hanisch U.-K., Noda M., Verkhratsky A. Physiology of Microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 104.Harry G.J. Microglia during development and aging. Pharmacol. Ther. 2013;139:313–326. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rush A.J., Sackeim H.A., Marangell L.B., George M.S., Brannan S.K., Davis S.M., Lavori P., Howland R., Kling M.A., Rittberg B., et al. Effects of 12 Months of Vagus Nerve Stimulation in Treatment-Resistant Depression: A Naturalistic Study. Biol. Psychiatry. 2005;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 106.Bajbouj M., Merkl A., Schlaepfer T., Frick C., Zobel A., Maier W., O’Keane V., Corcoran C., Adolfsson R., Trimble M., et al. Two-Year Outcome of Vagus Nerve Stimulation in Treatment-Resistant Depression. J. Clin. Psychopharmacol. 2010;30:273–281. doi: 10.1097/JCP.0b013e3181db8831. [DOI] [PubMed] [Google Scholar]
- 107.Howland R.H. Vagus Nerve Stimulation. Curr. Behav. Neurosci. Rep. 2014;1:64–73. doi: 10.1007/s40473-014-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bonaz B., Sinniger V., Hoffmann D., Clarençon D., Mathieu N., Dantzer C., Vercueil L., Picq C., Trocmé C., Faure P., et al. Chronic vagus nerve stimulation in Crohn’s disease: A 6-month follow-up pilot study. Neurogastroenterol. Motil. 2016;28:948–953. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- 109.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 110.De Jonge W.J., Van Der Zanden E.P., The F.O., Bijlsma M.F., Van Westerloo D.J., Bennink R.J., Berthoud H.-R., Uematsu S., Akira S., van den Wijngaard R.M., et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 111.Bencherif M., Lippiello P.M., Lucas R., Marrero M.B. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell. Mol. Life Sci. 2011;68:931–949. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rehani K., Scott D.A., Renaud D., Hamza H., Williams L.R., Wang H., Martin M. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2008;1783:375–382. doi: 10.1016/j.bbamcr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Bitner R.S., Nikkel A.L., Markosyan S., Otte S., Puttfarcken P., Gopalakrishnan M. Selective α7 nicotinic acetylcholine receptor activation regulates glycogen synthase kinase3β and decreases tau phosphorylation in vivo. Brain Res. 2009;1265:65–74. doi: 10.1016/j.brainres.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 114.Echeverria V., Zeitlin R., Burgess S., Patel S., Barman A., Thakur G., Mamcarz M., Wang L., Sattelle D.B., Kirschner D.A., et al. Cotinine Reduces Amyloid-β Aggregation and Improves Memory in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2011;24:817–835. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- 115.Bertrand D., Lee C.-H.L., Flood D., Marger F., Donnelly-Roberts D. Therapeutic Potential of α7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2015;67:1025–1073. doi: 10.1124/pr.113.008581. [DOI] [PubMed] [Google Scholar]
- 116.Egea J., Buendia I., Parada E., Navarro E., León R., Lopez M.G. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem. Pharmacol. 2015;97:463–472. doi: 10.1016/j.bcp.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 117.Parada E., Egea J., Buendia I., Negredo P., Cunha A.C., Cardoso S., Soares M.P., López M.G. The Microglial α7-Acetylcholine Nicotinic Receptor Is a Key Element in Promoting Neuroprotection by Inducing Heme Oxygenase-1 via Nuclear Factor Erythroid-2-Related Factor 2. Antioxid. Redox Signal. 2013;19:1135–1148. doi: 10.1089/ars.2012.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Navarro E., Gonzalez-Lafuente L., Pérez-Liébana I., Buendia I., López-Bernardo E., Sánchez-Ramos C., Prieto I., Cuadrado A., Satrustegui J., Cadenas S., et al. Heme-Oxygenase I and PCG-1α Regulate Mitochondrial Biogenesis via Microglial Activation of Alpha7 Nicotinic Acetylcholine Receptors Using PNU282987. Antioxidants Redox Signal. 2017;27:93–105. doi: 10.1089/ars.2016.6698. [DOI] [PubMed] [Google Scholar]
- 119.Zhao D., Xu X., Pan L., Zhu W., Fu X., Guo L., Lu Q., Wang J. Pharmacologic activation of cholinergic alpha7 nicotinic receptors mitigates depressive-like behavior in a mouse model of chronic stress. J. Neuroinflamm. 2017;14:234. doi: 10.1186/s12974-017-1007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kerschensteiner M., Gallmeier E., Behrens L., Leal V.V., Misgeld T., Klinkert W.E.F., Kolbeck R., Hoppe E., Oropeza-Wekerle R.-L., Bartke I., et al. Activated Human T Cells, B Cells, and Monocytes Produce Brain-derived Neurotrophic Factor In Vitro and in Inflammatory Brain Lesions: A Neuroprotective Role of Inflammation? J. Exp. Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yasuda S., Liang M.-H., Marinova Z.I., Yahyavi A., Chuang D.-M. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol. Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 122.Ghosal S., Bang E., Yue W., Hare B., Lepack A.E., Girgenti M.J., Duman R.S. Activity-Dependent Brain-Derived Neurotrophic Factor Release Is Required for the Rapid Antidepressant Actions of Scopolamine. Biol. Psychiatry. 2018;83:29–37. doi: 10.1016/j.biopsych.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiese S., Karus M., Faissner A. Astrocytes as a Source for Extracellular Matrix Molecules and Cytokines. Front. Pharmacol. 2012;3:120. doi: 10.3389/fphar.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wray N.H., Schappi J.M., Singh H., Senese N.B., Rasenick M.M. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol. Psychiatry. 2019;24:1833–1843. doi: 10.1038/s41380-018-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen P.-S., Peng G.-S., Li G., Yang S., Wu X., Wang C.-C., Wilson B.A., Lu R.-B., Gean P.-W., Chuang D.-M., et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol. Psychiatry. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- 126.Cherry J.D., Olschowka J.A., O’Banion M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakajima K., Kikuchi Y., Ikoma E., Honda S., Ishikawa M., Liu Y., Kohsaka S. Neurotrophins regulate the function of cultured microglia. Glia. 1998;24:272–289. doi: 10.1002/(SICI)1098-1136(199811)24:3<272::AID-GLIA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 128.Zhang J., Geula C., Lu C., Koziel H., Hatcher L.M., Roisen F.J. Neurotrophins regulate proliferation and survival of two microglial cell lines in vitro. Exp. Neurol. 2003;183:469–481. doi: 10.1016/S0014-4886(03)00222-X. [DOI] [PubMed] [Google Scholar]
- 129.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., 3rd, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.-B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gomes C., Ferreira R., George J., Sanches R., Rodrigues D.I., Gonçalves N., A Cunha R. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J. Neuroinflamm. 2013;10:16. doi: 10.1186/1742-2094-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang Y., Wei N., Zhu J., Lu T., Chen Z., Xu G., Liu X. Effects of Brain-Derived Neurotrophic Factor on Local Inflammation in Experimental Stroke of Rat. Mediat. Inflamm. 2010;2010:372423. doi: 10.1155/2010/372423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Porcher C., Medina I., Gaiarsa J.-L. Mechanism of BDNF Modulation in GABAergic Synaptic Transmission in Healthy and Disease Brains. Front. Cell. Neurosci. 2018;12:273. doi: 10.3389/fncel.2018.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li X., Frye M.A., Shelton R.C. Review of Pharmacological Treatment in Mood Disorders and Future Directions for Drug Development. Neuropsychopharmacology. 2012;37:77–101. doi: 10.1038/npp.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tao X., Finkbeiner S., Arnold D.B., Shaywitz A.J., Greenberg M.E. Ca2+ Influx Regulates BDNF Transcription by a CREB Family Transcription Factor-Dependent Mechanism. Neuron. 1998;20:709–726. doi: 10.1016/S0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 135.Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee B.-H., Kim Y.-K. BDNF mRNA expression of peripheral blood mononuclear cells was decreased in depressive patients who had or had not recently attempted suicide. J. Affect. Disord. 2010;125:369–373. doi: 10.1016/j.jad.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 137.McEwen B.S., Chattarji S., Diamond D.M., Jay T.M., Reagan L.P., Svenningsson P., Fuchs E. The neurobiological properties of tianeptine (Stablon): From monoamine hypothesis to glutamatergic modulation. Mol. Psychiatry. 2010;15:237–249. doi: 10.1038/mp.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schmidt H.D., Shelton R.C., Duman R.S. Functional Biomarkers of Depression: Diagnosis, Treatment, and Pathophysiology. Neuropsychopharmacology. 2011;36:2375–2394. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Björkholm C., Monteggia L.M. BDNF—A Key Transducer of Antidepressant Effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hait N.C., Wise L., Allegood J.C., O’Brien M., Avni D., Reeves T.M., Knapp P., Lu J., Luo C., Miles M.F., et al. Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat. Neurosci. 2014;17:971–980. doi: 10.1038/nn.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.di Nuzzo L., Orlando R., Tognoli C., Di Pietro P., Bertini G., Miele J., Bucci D., Motolese M., Scaccianoce S., Caruso A., et al. Antidepressant activity of fingolimod in mice. Pharmacol. Res. Perspect. 2015;3:e00135. doi: 10.1002/prp2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Covington H.E., 3rd, Vialou V.F., LaPlant Q., Ohnishi Y.N., Nestler E.J. Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci. Lett. 2011;493:122–126. doi: 10.1016/j.neulet.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lima I.V.D.A., Almeida-Santos A.F., Ferreira-Vieira T.H., Aguiar D.C., Ribeiro F.M., Campos A.C., de Oliveira A.C.P. Antidepressant-like effect of valproic acid—Possible involvement of PI3K/Akt/mTOR pathway. Behav. Brain Res. 2017;329:166–171. doi: 10.1016/j.bbr.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 144.Boorman E., Zajkowska Z., Ahmed R., Pariante C.M., Zunszain P.A. Crosstalk between endocannabinoid and immune systems: A potential dysregulation in depression? Psychopharmacology. 2016;233:1591–1604. doi: 10.1007/s00213-015-4105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Biringer R.G. Endocannabinoid signaling pathways: Beyond CB1R and CB2R. J. Cell Commun. Signal. 2021;15:335–360. doi: 10.1007/s12079-021-00622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Núñez E., Benito C., Pazos M.R., Barbachano A., Fajardo O., González S., Tolón R.M., Romero J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: An immunohistochemical study. Synapse. 2004;53:208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- 147.Turcotte C., Blanchet M.-R., LaViolette M., Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016;73:4449–4470. doi: 10.1007/s00018-016-2300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Benyó Z., Ruisanchez É., Leszl-Ishiguro M., Sándor P., Pacher P. Endocannabinoids in cerebrovascular regulation. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H785–H801. doi: 10.1152/ajpheart.00571.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roche M., Finn D.P. Brain CB2 Receptors: Implications for Neuropsychiatric Disorders. Pharmaceuticals. 2010;3:2517–2553. doi: 10.3390/ph3082517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chiang N., de la Rosa X., Libreros S., Serhan C.N. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol. 2017;198:842–851. doi: 10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chiang N., Dalli J., Colas R.A., Serhan C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015;212:1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tao Y., Li L., Jiang B., Feng Z., Yang L., Tang J., Chen Q., Zhang J., Tan Q., Feng H., et al. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav. Immun. 2016;58:118–129. doi: 10.1016/j.bbi.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 153.Bie B., Wu J., Foss J.F., Naguib M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr. Opin. Anaesthesiol. 2018;31:407–414. doi: 10.1097/ACO.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gutiérrez G., Pérez-Ortiz J., Gutiérrez-Adán A., Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB 2 receptors. Br. J. Pharmacol. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Meijerink J., Balvers M., Witkamp R. N-acyl amines of docosahexaenoic acid and other n-3 polyunsatured fatty acids—From fishy endocannabinoids to potential leads. Br. J. Pharmacol. 2013;169:772–783. doi: 10.1111/bph.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Park T., Chen H., Kevala K., Lee J.-W., Kim H.-Y. N-Docosahexaenoylethanolamine ameliorates LPS-induced neuroinflammation via cAMP/PKA-dependent signaling. J. Neuroinflamm. 2016;13:284. doi: 10.1186/s12974-016-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Park T., Chen H., Kim H.-Y. GPR110 (ADGRF1) mediates anti-inflammatory effects of N-docosahexaenoylethanolamine. J. Neuroinflamm. 2019;16:225. doi: 10.1186/s12974-019-1621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tyrtyshnaia A., Konovalova S., Bondar A., Ermolenko E., Sultanov R., Manzhulo I. Anti-Inflammatory Activity of N-Docosahexaenoylethanolamine and N-Eicosapentaenoylethanolamine in a Mouse Model of Lipopolysaccharide-Induced Neuroinflammation. Int. J. Mol. Sci. 2021;22:10728. doi: 10.3390/ijms221910728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee J.-W., Huang B.X., Kwon H., Rashid A., Kharebava G., Desai A., Patnaik S., Marugan J., Kim H.-Y. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat. Commun. 2016;7:13123. doi: 10.1038/ncomms13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McDougle D.R., Watson J.E., Abdeen A.A., Adili R., Caputo M.P., Krapf J.E., Johnson R.W., Kilian K.A., Holinstat M., Das A. Anti-inflammatory ω-3 endocannabinoid epoxides. Proc. Natl. Acad. Sci. USA. 2017;114:E6034–E6043. doi: 10.1073/pnas.1610325114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ma L., Jia J., Liu X., Bai F., Wang Q., Xiong L. Activation of murine microglial N9 cells is attenuated through cannabinoid receptor CB2 signaling. Biochem. Biophys. Res. Commun. 2015;458:92–97. doi: 10.1016/j.bbrc.2015.01.073. [DOI] [PubMed] [Google Scholar]
- 162.Yang B., Lin L., Bazinet R.P., Chien Y.-C., Chang J.P.-C., Satyanarayanan S.K., Su H., Su K.-P. Clinical Efficacy and Biological Regulations of ω–3 PUFA-Derived Endocannabinoids in Major Depressive Disorder. Psychother. Psychosom. 2019;88:215–224. doi: 10.1159/000501158. [DOI] [PubMed] [Google Scholar]
- 163.Ninković J., Roy S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids. 2013;45:9–24. doi: 10.1007/s00726-011-1163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee J.W., Joshi S., Chan J.S., Wong Y.H. Differential coupling of mu-, delta-, and kappa-opioid receptors to G alpha16-mediated stimulation of phospholipase C. J. Neurochem. 1998;70:2203–2211. doi: 10.1046/j.1471-4159.1998.70052203.x. [DOI] [PubMed] [Google Scholar]
- 165.Ślusarczyk J., Trojan E., Głombik K., Piotrowska A., Budziszewska B., Kubera M., Popiołek-Barczyk K., Lasoń W., Mika J., Basta-Kaim A. Targeting the NLRP3 Inflammasome-Related Pathways via Tianeptine Treatment-Suppressed Microglia Polarization to the M1 Phenotype in Lipopolysaccharide-Stimulated Cultures. Int. J. Mol. Sci. 2018;19:1965. doi: 10.3390/ijms19071965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Emrich H.M., Vogt P., Herz A. Possible Antidepressive Effects of Opioids: Action of Buprenorphine. Ann. N. Y. Acad. Sci. 1982;398:108–112. doi: 10.1111/j.1749-6632.1982.tb39483.x. [DOI] [PubMed] [Google Scholar]
- 167.Kasper S., McEwen B.S. Neurobiological and Clinical Effects of the Antidepressant Tianeptine. CNS Drugs. 2008;22:15–26. doi: 10.2165/00023210-200822010-00002. [DOI] [PubMed] [Google Scholar]
- 168.Peciña M., Karp J.F., Mathew S., Todtenkopf M.S., Ehrich E.W., Zubieta J.-K. Endogenous opioid system dysregulation in depression: Implications for new therapeutic approaches. Mol. Psychiatry. 2019;24:576–587. doi: 10.1038/s41380-018-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Loric S., Maroteaux L., Kellermann O., Launay J.M. Functional serotonin-2B receptors are expressed by a teratocarcinoma-derived cell line during serotoninergic differentiation. Mol. Pharmacol. 1995;47:458–466. [PubMed] [Google Scholar]
- 170.Cox D.A., Cohen M.L. 5-HT2B receptor signaling in the rat stomach fundus: Dependence on calcium influx, calcium release and protein kinase C. Behav. Brain Res. 1996;73:289–292. doi: 10.1016/0166-4328(96)00125-8. [DOI] [PubMed] [Google Scholar]
- 171.Naito K., Tanaka C., Mitsuhashi M., Moteki H., Kimura M., Natsume H., Ogihara M. Signal Transduction Mechanism for Serotonin 5-HT2B Receptor-Mediated DNA Synthesis and Proliferation in Primary Cultures of Adult Rat Hepatocytes. Biol. Pharm. Bull. 2016;39:121–129. doi: 10.1248/bpb.b15-00735. [DOI] [PubMed] [Google Scholar]
- 172.Carhart-Harris R., Giribaldi B., Watts R., Baker-Jones M., Murphy-Beiner A., Murphy R., Martell J., Blemings A., Erritzoe D., Nutt D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021;384:1402–1411. doi: 10.1056/NEJMoa2032994. [DOI] [PubMed] [Google Scholar]
- 173.Carhart-Harris R.L., Bolstridge M., Rucker J., Day C.M.J., Erritzoe D., Kaelen M., Bloomfield M., Rickard J.A., Forbes B., Feilding A., et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry. 2016;3:619–627. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- 174.Gukasyan N., Davis A.K., Barrett F.S., Cosimano M.P., Sepeda N.D., Johnson M.W., Griffiths R.R. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up. J. Psychopharmacol. 2022;36:151–158. doi: 10.1177/02698811211073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yovell Y., Bar G., Mashiah M., Baruch Y., Briskman I., Asherov J., Lotan A., Rigbi A., Panksepp J. Ultra-Low-Dose Buprenorphine as a Time-Limited Treatment for Severe Suicidal Ideation: A Randomized Controlled Trial. Am. J. Psychiatry. 2016;173:491–498. doi: 10.1176/appi.ajp.2015.15040535. [DOI] [PubMed] [Google Scholar]
- 176.Samuels B.A., Nautiyal K.M., Kruegel A.C., Levinstein M.R., Magalong V.M., Gassaway M.M., Grinnell S.G., Han J., Ansonoff M.A., E Pintar J., et al. The Behavioral Effects of the Antidepressant Tianeptine Require the Mu-Opioid Receptor. Neuropsychopharmacology. 2017;42:2052–2063. doi: 10.1038/npp.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Serafini G., Adavastro G., Canepa G., De Berardis D., Valchera A., Pompili M., Nasrallah H., Amore M. The Efficacy of Buprenorphine in Major Depression, Treatment-Resistant Depression and Suicidal Behavior: A Systematic Review. Int. J. Mol. Sci. 2018;19:2410. doi: 10.3390/ijms19082410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nelson C.J., Schneider G.M., Lysle D.T. Involvement of Central μ- but Not δ- or κ-Opioid Receptors in Immunomodulation. Brain Behav. Immun. 2000;14:170–184. doi: 10.1006/brbi.1999.0575. [DOI] [PubMed] [Google Scholar]
- 179.Nummenmaa L., Karjalainen T., Isojärvi J., Kantonen T., Tuisku J., Kaasinen V., Joutsa J., Nuutila P., Kalliokoski K., Hirvonen J., et al. Lowered endogenous mu-opioid receptor availability in subclinical depression and anxiety. Neuropsychopharmacology. 2020;45:1953–1959. doi: 10.1038/s41386-020-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gassaway M.M., Rives M.-L., Kruegel A.C., Javitch J.A., Sames D. The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Transl. Psychiatry. 2014;4:e411. doi: 10.1038/tp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang X., Loram L.C., Ramos K., de Jesus A.J., Thomas J., Cheng K., Reddy A., Somogyi A.A., Hutchinson M.R., Watkins L.R., et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci. USA. 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lutz P.-E., Kieffer B.L. Opioid receptors: Distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Halberstadt A.L., Geyer M.A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ling S., Ceban F., Lui L.M.W., Lee Y., Teopiz K.M., Rodrigues N.B., Lipsitz O., Gill H., Subramaniapillai M., Mansur R.B., et al. Molecular Mechanisms of Psilocybin and Implications for the Treatment of Depression. CNS Drugs. 2022;36:17–30. doi: 10.1007/s40263-021-00877-y. [DOI] [PubMed] [Google Scholar]
- 185.Desouza L.A., Benekareddy M., Fanibunda S.E., Mohammad F., Janakiraman B., Ghai U., Gur T., Blendy J.A., Vaidya V.A. The Hallucinogenic Serotonin2A Receptor Agonist, 2,5-Dimethoxy-4-Iodoamphetamine, Promotes cAMP Response Element Binding Protein-Dependent Gene Expression of Specific Plasticity-Associated Genes in the Rodent Neocortex. Front. Mol. Neurosci. 2021;14:790213. doi: 10.3389/fnmol.2021.790213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Glennon R.A., Rosecrans J.A. Speculations on the mechanism of action of hallucinogenic indolealkylamines. Neurosci. Biobehav. Rev. 1981;5:197–207. doi: 10.1016/0149-7634(81)90002-6. [DOI] [PubMed] [Google Scholar]
- 187.Szabo A., Gogolak P., Koncz G., Foldvari Z., Pazmandi K., Miltner N., Poliska S., Bacsi A., Djurovic S., Rajnavolgyi E. Immunomodulatory capacity of the serotonin receptor 5-HT2B in a subset of human dendritic cells. Sci. Rep. 2018;8:1765. doi: 10.1038/s41598-018-20173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bierhaus A., Wolf J., Andrassy M., Rohleder N., Humpert P.M., Petrov D., Ferstl R., von Eynatten M., Wendt T., Rudofsky G., et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bailey M.T., Engler H., Powell N.D., Padgett D.A., Sheridan J.F. Repeated social defeat increases the bactericidal activity of splenic macrophages through a Toll-like receptor-dependent pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1180–R1190. doi: 10.1152/ajpregu.00307.2007. [DOI] [PubMed] [Google Scholar]
- 190.Diz-Chaves Y., Pernía O., Carrero P., Garcia-Segura L.M. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J. Neuroinflamm. 2012;9:71. doi: 10.1186/1742-2094-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lindqvist D., Dhabhar F.S., James S.J., Hough C.M., Jain F.A., Bersani F.S., Reus V.I., Verhoeven J.E., Epel E.S., Mahan L., et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197–205. doi: 10.1016/j.psyneuen.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vilhardt F., Haslund-Vinding J., Jaquet V., McBean G. Microglia antioxidant systems and redox signalling. Br. J. Pharmacol. 2017;174:1719–1732. doi: 10.1111/bph.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bellezza I., Giambanco I., Minelli A., Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 194.Martín-Hernández D., Caso J.R., Meana J.J., Callado L.F., Madrigal J.L.M., García-Bueno B., Leza J.C. Intracellular inflammatory and antioxidant pathways in postmortem frontal cortex of subjects with major depression: Effect of antidepressants. J. Neuroinflamm. 2018;15:251. doi: 10.1186/s12974-018-1294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nadjar A., Leyrolle Q., Joffre C., Laye S. Bioactive lipids as new class of microglial modulators: When nutrition meets neuroimunology. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;79:19–26. doi: 10.1016/j.pnpbp.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 196.Hsiao C.-C., Sankowski R., Prinz M., Smolders J., Huitinga I., Hamann J. GPCRomics of Homeostatic and Disease-Associated Human Microglia. Front. Immunol. 2021;12:674189. doi: 10.3389/fimmu.2021.674189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang Q., Lv C., Sun Y., Han X., Wang S., Mao Z., Xin Y., Zhang B. The Role of Alpha-Lipoic Acid in the Pathomechanism of Acute Ischemic Stroke. Cell. Physiol. Biochem. 2018;48:42–53. doi: 10.1159/000491661. [DOI] [PubMed] [Google Scholar]
- 198.Xu J., Drew P.D. 9-Cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J. Neuroimmunol. 2006;171:135–144. doi: 10.1016/j.jneuroim.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Suh H.-S., Zhao M.-L., Derico L., Choi N., Lee S.C. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: Differential regulation by inflammatory mediators. J. Neuroinflamm. 2013;10:37. doi: 10.1186/1742-2094-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang B., Yang L., Konishi Y., Maeda N., Sakanaka M., Tanaka J. Suppressive effects of phosphodiesterase type IV inhibitors on rat cultured microglial cells: Comparison with other types of cAMP-elevating agents. Neuropharmacology. 2002;42:262–269. doi: 10.1016/S0028-3908(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 201.Dinarello C.A. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nishimura Y., Moriyama M., Kawabe K., Satoh H., Takano K., Azuma Y.-T., Nakamura Y. Lauric Acid Alleviates Neuroinflammatory Responses by Activated Microglia: Involvement of the GPR40-Dependent Pathway. Neurochem. Res. 2018;43:1723–1735. doi: 10.1007/s11064-018-2587-7. [DOI] [PubMed] [Google Scholar]
- 203.Wang Y.-P., Wu Y., Li L.-Y., Zheng J., Liu R.-G., Zhou J.-P., Yuan S.-Y., Shang Y., Yao S.-L. Aspirin-triggered lipoxin A4attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-κB and MAPKs in BV-2 microglial cells. J. Neuroinflamm. 2011;8:95. doi: 10.1186/1742-2094-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rey C., Nadjar A., Buaud B., Vaysse C., Aubert A., Pallet V., Layé S., Joffre C. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav. Immun. 2016;55:249–259. doi: 10.1016/j.bbi.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 205.Gu Z., Lamont G.J., Lamont R.J., Uriarte S.M., Wang H., Scott D.A. Resolvin D1, resolvin D2 and maresin 1 activate the GSK3β anti-inflammatory axis in TLR4-engaged human monocytes. J. Endotoxin Res. 2016;22:186–195. doi: 10.1177/1753425916628618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tylek K., Trojan E., Leśkiewicz M., Regulska M., Bryniarska N., Curzytek K., Lacivita E., Leopoldo M., Basta-Kaim A. Time-Dependent Protective and Pro-Resolving Effects of FPR2 Agonists on Lipopolysaccharide-Exposed Microglia Cells Involve Inhibition of NF-κB and MAPKs Pathways. Cells. 2021;10:2373. doi: 10.3390/cells10092373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hung T.-H., Shyue S.-K., Wu C.-H., Chen C.-C., Lin C.-C., Chang C.-F., Chen S.-F. Deletion or inhibition of soluble epoxide hydrolase protects against brain damage and reduces microglia-mediated neuroinflammation in traumatic brain injury. Oncotarget. 2017;8:103236–103260. doi: 10.18632/oncotarget.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Abdallah C.G., Sanacora G., Duman R.S., Krystal J.H. The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation? Pharmacol. Ther. 2018;190:148–158. doi: 10.1016/j.pharmthera.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zanos P., Gould T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry. 2018;23:801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sanacora G., Schatzberg A.F. Ketamine: Promising Path or False Prophecy in the Development of Novel Therapeutics for Mood Disorders? Neuropsychopharmacology. 2015;40:259–267. doi: 10.1038/npp.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lumsden E.W., Troppoli T.A., Myers S.J., Zanos P., Aracava Y., Kehr J., Lovett J., Kim S., Wang F.-H., Schmidt S., et al. Antidepressant-relevant concentrations of the ketamine metabolite (2 R,6 R )-hydroxynorketamine do not block NMDA receptor function. Proc. Natl. Acad. Sci. USA. 2019;116:5160–5169. doi: 10.1073/pnas.1816071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Williams N.R., Heifets B., Blasey C., Sudheimer K., Pannu J., Pankow H., Hawkins J., Birnbaum J., Lyons D.M., Rodriguez C.I., et al. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am. J. Psychiatry. 2018;175:1205–1215. doi: 10.1176/appi.ajp.2018.18020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yost J.G., Wulf H.A., Browne C.A., Lucki I. Antinociceptive and Analgesic Effects of (2R,6R)-Hydroxynorketamine. J. Pharmacol. Exp. Ther. 2022;382:256–265. doi: 10.1124/jpet.122.001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Beurel E., Song L., Jope R.S. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol. Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhou W., Dong L., Wang N., Shi J.-Y., Yang J.-J., Zuo Z.-Y., Zhou Z.-Q. Akt Mediates GSK-3β Phosphorylation in the Rat Prefrontal Cortex during the Process of Ketamine Exerting Rapid Antidepressant Actions. Neuroimmunomodulation. 2014;21:183–188. doi: 10.1159/000356517. [DOI] [PubMed] [Google Scholar]
- 216.Choi M., Lee S.H., Park M.H., Kim Y.-S., Son H. Ketamine induces brain-derived neurotrophic factor expression via phosphorylation of histone deacetylase 5 in rats. Biochem. Biophys. Res. Commun. 2017;489:420–425. doi: 10.1016/j.bbrc.2017.05.157. [DOI] [PubMed] [Google Scholar]
- 217.Hashimoto K. Essential Role of Keap1-Nrf2 Signaling in Mood Disorders: Overview and Future Perspective. Front. Pharmacol. 2018;9:1182. doi: 10.3389/fphar.2018.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hoetzel A., Schmidt R. Regulatory role of anesthetics on heme oxygenase-1. Curr. Drug Targets. 2010;11:1495–1503. doi: 10.2174/1389450111009011495. [DOI] [PubMed] [Google Scholar]
- 219.Wang N., Yu H.-Y., Shen X.-F., Gao Z.-Q., Yang C., Yang J.-J., Zhang G.-F. The rapid antidepressant effect of ketamine in rats is associated with down-regulation of pro-inflammatory cytokines in the hippocampus. Upsala J. Med. Sci. 2015;120:241–248. doi: 10.3109/03009734.2015.1060281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tan S., Wang Y., Chen K., Long Z., Zou J. Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice. Biol. Pharm. Bull. 2017;40:1260–1267. doi: 10.1248/bpb.b17-00131. [DOI] [PubMed] [Google Scholar]
- 221.Qu Y., Shan J., Wang S., Chang L., Pu Y., Wang X., Tan Y., Yamamoto M., Hashimoto K. Rapid-acting and long-lasting antidepressant-like action of (R)-ketamine in Nrf2 knock-out mice: A role of TrkB signaling. Eur. Arch. Psychiatry Clin. Neurosci. 2021;271:439–446. doi: 10.1007/s00406-020-01208-w. [DOI] [PubMed] [Google Scholar]
- 222.Walker A.K., Budac D.P., Bisulco S., Lee A.W., Smith R.A., Beenders B., Kelley K.W., Dantzer R. NMDA Receptor Blockade by Ketamine Abrogates Lipopolysaccharide-Induced Depressive-Like Behavior in C57BL/6J Mice. Neuropsychopharmacology. 2013;38:1609–1616. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wu G.-J., Chen T.-L., Ueng Y.-F., Chen R.-M. Ketamine inhibits tumor necrosis factor-α and interleukin-6 gene expressions in lipopolysaccharide-stimulated macrophages through suppression of toll-like receptor 4-mediated c-Jun N-terminal kinase phosphorylation and activator protein-1 activation. Toxicol. Appl. Pharmacol. 2008;228:105–113. doi: 10.1016/j.taap.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 224.Welters I.D., Hafer G., Menzebach A., Mühling J., Neuhäuser C., Browning P., Goumon Y. Ketamine Inhibits Transcription Factors Activator Protein 1 and Nuclear Factor-κB, Interleukin-8 Production, as well as CD11b and CD16 Expression: Studies in Human Leukocytes and Leukocytic Cell Lines. Anesth. Analg. 2010;110:934–941. doi: 10.1213/ANE.0b013e3181c95cfa. [DOI] [PubMed] [Google Scholar]
- 225.Beilin B., Rusabrov Y., Shapira Y., Roytblat L., Greemberg L., Yardeni I.Z., Bessler H. Low-dose ketamine affects immune responses in humans during the early postoperative period. Br. J. Anaesth. 2007;99:522–527. doi: 10.1093/bja/aem218. [DOI] [PubMed] [Google Scholar]
- 226.Zunszain P.A., Horowitz M.A., Cattaneo A., Lupi M.M., Pariante C.M. Ketamine: Synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol. Psychiatry. 2013;18:1236–1241. doi: 10.1038/mp.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yang J.-J., Wang N., Yang C., Shi J.-Y., Yu H.-Y., Hashimoto K. Serum Interleukin-6 Is a Predictive Biomarker for Ketamine’s Antidepressant Effect in Treatment-Resistant Patients With Major Depression. Biol. Psychiatry. 2015;77:e19–e20. doi: 10.1016/j.biopsych.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 228.Zhan Y., Zhou Y., Zheng W., Liu W., Wang C., Lan X., Deng X., Xu Y., Zhang B., Ning Y. Alterations of multiple peripheral inflammatory cytokine levels after repeated ketamine infusions in major depressive disorder. Transl. Psychiatry. 2020;10:246. doi: 10.1038/s41398-020-00933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yuskaitis C.J., Jope R.S. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell. Signal. 2009;21:264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.VanderZwaag J., Halvorson T., Dolhan K., Šimončičová E., Ben-Azu B., Tremblay M. The Missing Piece? A Case for Microglia’s Prominent Role in the Therapeutic Action of Anesthetics, Ketamine, and Psychedelics. Neurochem. Res. 2022:1–38. doi: 10.1007/s11064-022-03772-0. [DOI] [PubMed] [Google Scholar]
- 231.Urs N.M., Snyder J.C., Jacobsen J.P.R., Peterson S.M., Caron M.G. Deletion of GSK3β in D2R-expressing neurons reveals distinct roles for β-arrestin signaling in antipsychotic and lithium action. Proc. Natl. Acad. Sci. USA. 2012;109:20732–20737. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Beaulieu J.-M. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J. Psychiatry Neurosci. 2012;37:7–16. doi: 10.1503/jpn.110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Meltzer H.Y., Matsubara S., Lee J.C. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol. Bull. 1989;25:390–392. [PubMed] [Google Scholar]
- 234.Kapur S., Zipursky R.B., Remington G. Clinical and Theoretical Implications of 5-HT2 and D2 Receptor Occupancy of Clozapine, Risperidone, and Olanzapine in Schizophrenia. Am. J. Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 235.Alimohamad H., Rajakumar N., Seah Y.-H., Rushlow W. Antipsychotics alter the protein expression levels of β-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol. Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 236.Li X., Rosborough K.M., Friedman A.B., Zhu W., Roth K. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int. J. Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 237.Schmid C.L., Bohn L.M. Serotonin, But Not N-Methyltryptamines, Activates the Serotonin 2A Receptor Via a β-Arrestin2/Src/Akt Signaling Complex In Vivo. J. Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Li X., Zhu W., Roh M.-S., Friedman A.B., Rosborough K., Jope R.S. In Vivo Regulation of Glycogen Synthase Kinase-3β (GSK3β) by Serotonergic Activity in Mouse Brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Polter A.M., Li X. Glycogen Synthase Kinase-3 is an Intermediate Modulator of Serotonin Neurotransmission. Front. Mol. Neurosci. 2011;4:31. doi: 10.3389/fnmol.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.