Abstract
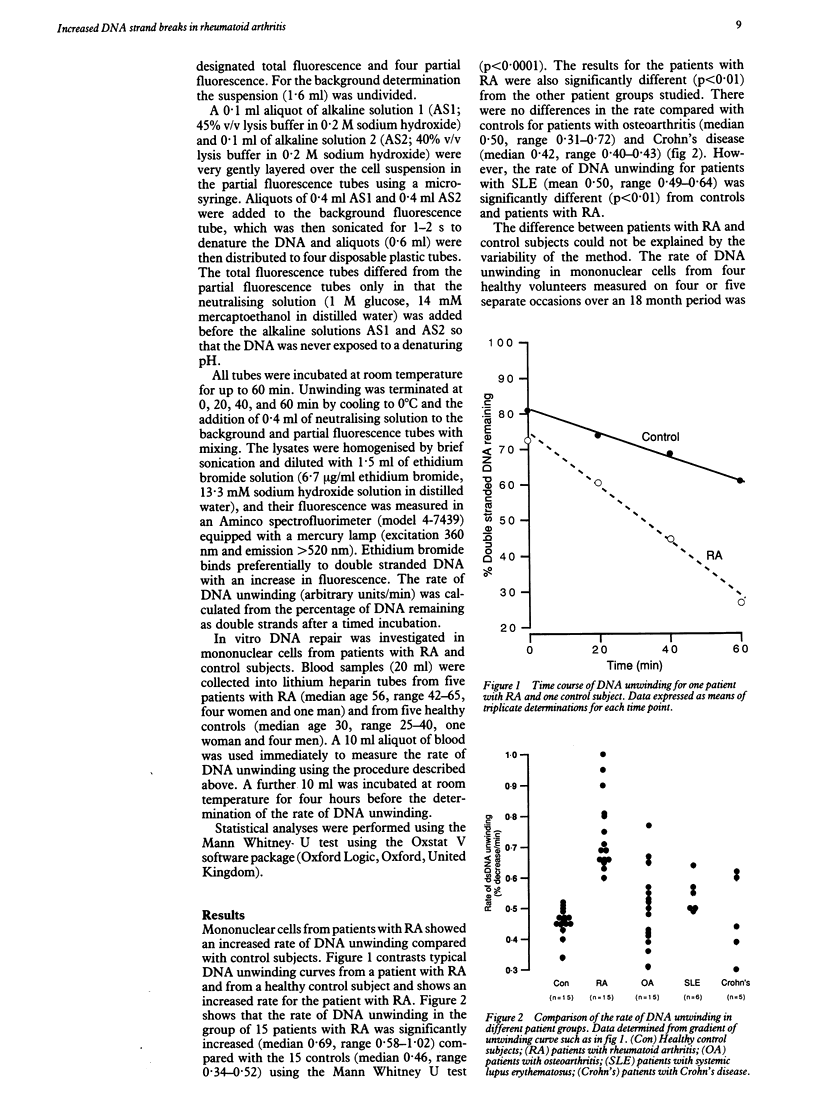
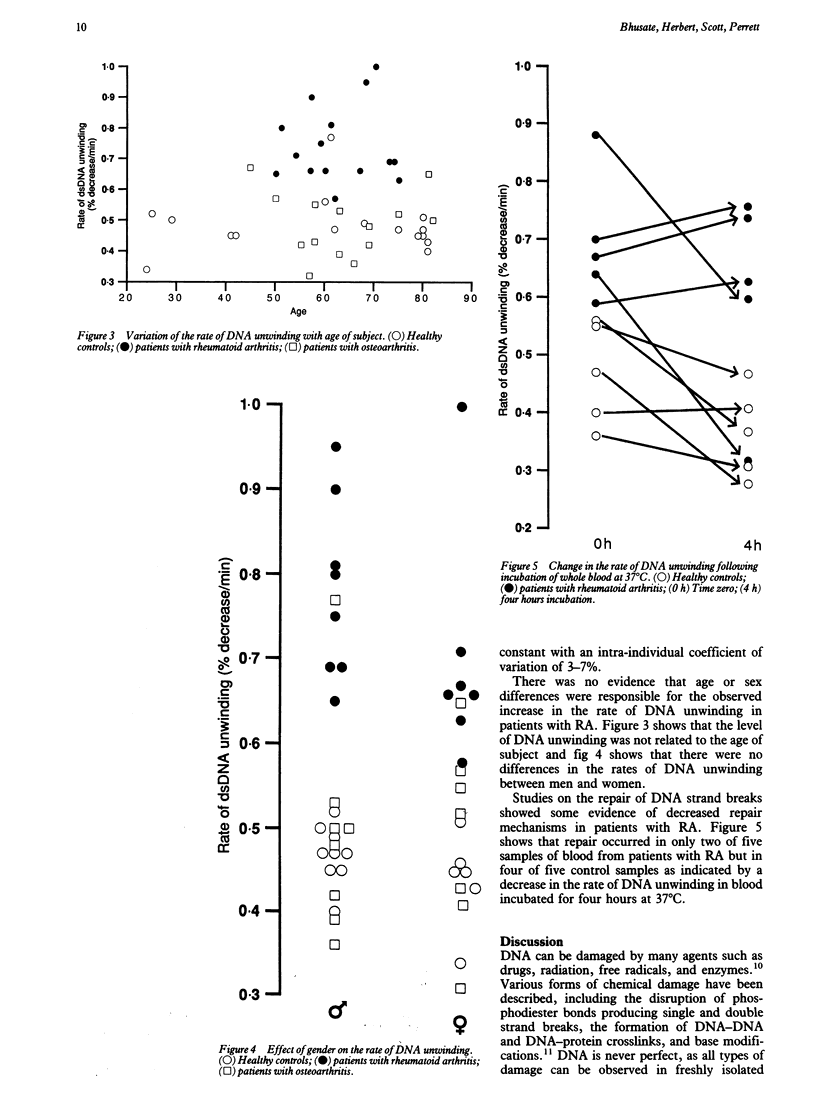
Immune dysfunction is linked with lymphocyte DNA metabolism. In particular, DNA damage may impair lymphocyte function and induce increased cell turnover; such changes are of relevance to the pathogenesis of rheumatoid arthritis. The rate of DNA unwinding in alkaline solution was used as a measure of the number of DNA strand breaks in mononuclear cells freshly isolated from peripheral blood. The rate of DNA unwinding was significantly increased in cells from patients with rheumatoid arthritis compared with those from healthy subjects and from patients with other autoimmune and connective tissue diseases. These findings support the hypothesis that DNA damage is increased in patients with rheumatoid arthritis and it is one factor contributing to immune dysfunction in this disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B., Dizdaroglu M. Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase. J Biol Chem. 1989 Aug 5;264(22):13024–13028. [PubMed] [Google Scholar]
- Bhusate L. L., Herbert K. E., Perrett D. Application of a fluorimetric method for measuring DNA strand breaks in purified DNA. Biochem Soc Trans. 1990 Aug;18(4):676–677. doi: 10.1042/bst0180676. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Carson D. A., Seto S., Wasson D. B., Carrera C. J. DNA strand breaks, NAD metabolism, and programmed cell death. Exp Cell Res. 1986 Jun;164(2):273–281. doi: 10.1016/0014-4827(86)90028-5. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Application of capillary gas chromatography-mass spectrometry to chemical characterization of radiation-induced base damage of DNA: implications for assessing DNA repair processes. Anal Biochem. 1985 Feb 1;144(2):593–603. doi: 10.1016/0003-2697(85)90158-7. [DOI] [PubMed] [Google Scholar]
- Dormandy T. L. In praise of peroxidation. Lancet. 1988 Nov 12;2(8620):1126–1128. doi: 10.1016/s0140-6736(88)90535-1. [DOI] [PubMed] [Google Scholar]
- Emerit I., Levy A., Camus J. P. Monocyte-derived clastogenic factor in rheumatoid arthritis. Free Radic Biol Med. 1989;6(3):245–250. doi: 10.1016/0891-5849(89)90051-8. [DOI] [PubMed] [Google Scholar]
- Felder M., Doré C. J., Knight S. C., Ansell B. M. In vitro stimulation of lymphocytes from patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1985 Nov;37(2):253–261. doi: 10.1016/0090-1229(85)90157-6. [DOI] [PubMed] [Google Scholar]
- Harris G., Cramp W. A., Edwards J. C., George A. M., Sabovljev S. A., Hart L., Hughes G. R., Denman A. M., Yatvin M. B. Radiosensitivity of peripheral blood lymphocytes in autoimmune disease. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Jun;47(6):689–699. doi: 10.1080/09553008514550931. [DOI] [PubMed] [Google Scholar]
- Harris G., Holmes A., Sabovljev S. A., Cramp W. A., Hedges M., Hornsey S., Hornsey J. M., Bennett G. C. Sensitivity to X-irradiation of peripheral blood lymphocytes from ageing donors. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Oct;50(4):685–694. doi: 10.1080/09553008614551091. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P. Rejoining of DNA strand breaks is an early nuclear event during the stimulation of quiescent lymphocytes. Eur J Biochem. 1984 Apr 16;140(2):401–406. doi: 10.1111/j.1432-1033.1984.tb08116.x. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Topper R., Denman A. M., Hylton W., Hill I. D., Harris G. Increased sensitivity of lymphocytes from patients with systemic autoimmune diseases to DNA alkylation by the methylating carcinogen N-methyl-N-nitrosourea. Ann Rheum Dis. 1988 Jun;47(6):445–451. doi: 10.1136/ard.47.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E. Immunosuppression by D-penicillamine in vitro. Inhibition of human T lymphocyte proliferation by copper- or ceruloplasmin-dependent generation of hydrogen peroxide and protection by monocytes. J Clin Invest. 1984 Jan;73(1):53–65. doi: 10.1172/JCI111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Orrenius S., Jondal M. Cellular signalling in programmed cell death (apoptosis). Immunol Today. 1990 Apr;11(4):120–121. doi: 10.1016/0167-5699(90)90048-e. [DOI] [PubMed] [Google Scholar]
- Merry P., Winyard P. G., Morris C. J., Grootveld M., Blake D. R. Oxygen free radicals, inflammation, and synovitis: and synovitis: the current status. Ann Rheum Dis. 1989 Oct;48(10):864–870. doi: 10.1136/ard.48.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi Y., Yoshioka A., Imamura S., Niwa Y. Effect of sulphasalazine and its metabolites on the generation of reactive oxygen species. Gut. 1987 Feb;28(2):190–195. doi: 10.1136/gut.28.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Lanchbury J. S., Murphy J., Panayi G. S. Expression of HLA-DR, DQ and DP antigens and interleukin-2 receptor on synovial fluid T lymphocyte subsets in rheumatoid arthritis: evidence for "frustrated" activation. J Rheumatol. 1987 Aug;14(4):662–666. [PubMed] [Google Scholar]
- Smith M. D., Roberts-Thomson P. J. Lymphocyte surface marker expression in rheumatic diseases: evidence for prior activation of lymphocytes in vivo. Ann Rheum Dis. 1990 Feb;49(2):81–87. doi: 10.1136/ard.49.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Zoschke D. C., Kaja J. Suboptimal levels of hydrogen peroxide scavengers in synovial fluid: in vitro augmentation with slow acting antirheumatic drugs. J Rheumatol. 1989 Sep;16(9):1233–1240. [PubMed] [Google Scholar]


