Abstract
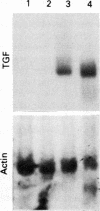
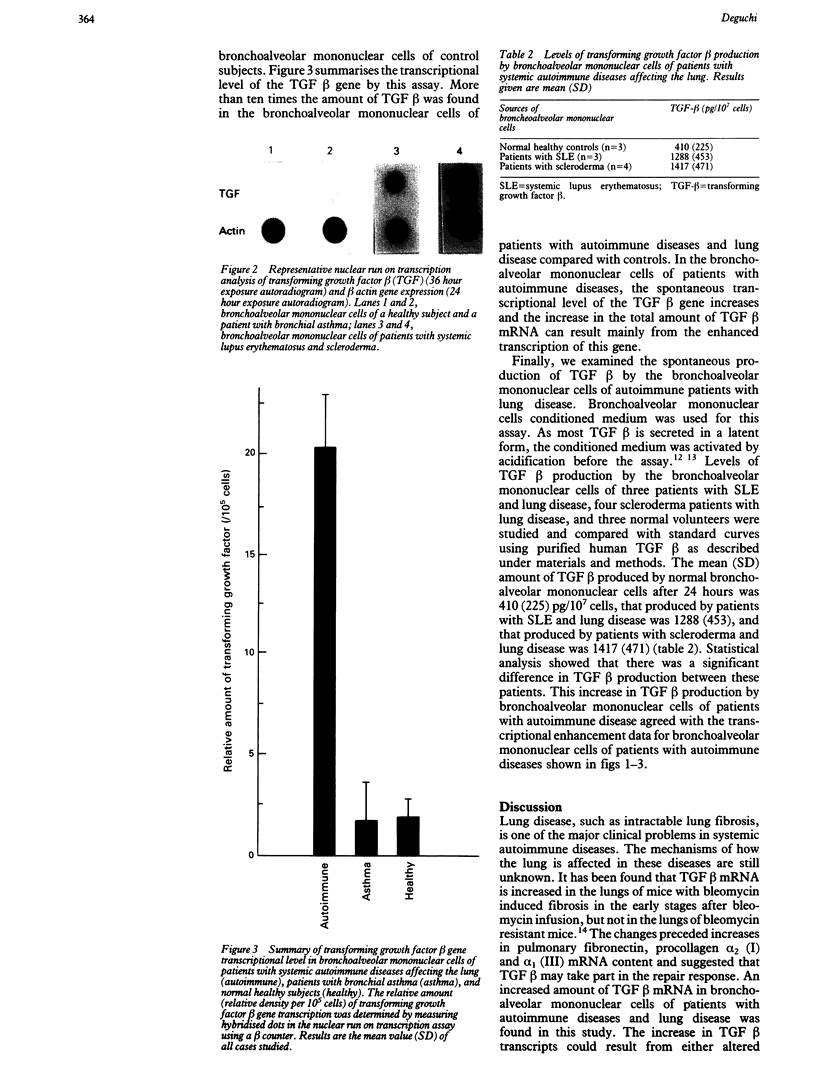
The spontaneous increase in the transcription of the transforming growth factor beta (TGF beta) gene in bronchoalveolar mononuclear cells of patients with autoimmune diseases affecting the lung has been shown by northern blot assay and a nuclear run on transcription assay. Transcription of the TGF beta gene in bronchoalveolar mononuclear cells of patients with autoimmune diseases affecting the lung was increased 10 times compared with normal healthy subjects or patients with bronchial asthma as controls. This observation, confirmed by protein data, suggests that TGF beta, a potent mitogen for fibroblasts, may be produced in bronchoalveolar mononuclear cells during an active immune response in such patients and may be involved in autoimmune related changes of the pathophysiology of cytokine networks when the lung is affected, such as in lung fibrosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett A. J. Scleroderma (progressive systemic sclerosis): progress and course based on a personal series of 118 cases. Med J Aust. 1978 Aug 12;2(4):129–134. doi: 10.5694/j.1326-5377.1978.tb131413.x. [DOI] [PubMed] [Google Scholar]
- Deguchi Y., Negoro S., Kishimoto S. Age-related changes of heat shock protein gene transcription in human peripheral blood mononuclear cells. Biochem Biophys Res Commun. 1988 Dec 15;157(2):580–584. doi: 10.1016/s0006-291x(88)80289-4. [DOI] [PubMed] [Google Scholar]
- Fine A., Goldstein R. H. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987 Mar 15;262(8):3897–3902. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Jiménez S. A. Cellular immune dysfunction and the pathogenesis of scleroderma. Semin Arthritis Rheum. 1983 Aug;13(1 Suppl 1):104–113. doi: 10.1016/0049-0172(83)90029-x. [DOI] [PubMed] [Google Scholar]
- Khalil N., Bereznay O., Sporn M., Greenberg A. H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989 Sep 1;170(3):727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Heldin C. H. Role for carbohydrate structures in TGF-beta 1 latency. Nature. 1989 Mar 9;338(6211):158–160. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Kapanci Y., Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989 Sep 1;170(3):655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Tsujimoto Y., Alpers J. D., Croce C. M., Nowell P. C. Regulation of bcl-2 proto-oncogene expression during normal human lymphocyte proliferation. Science. 1987 Jun 5;236(4806):1295–1299. doi: 10.1126/science.3495884. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]