Abstract
Simple Summary
REV7 is a multifunctional protein involved in various biological processes including DNA damage response and mutagenesis, cell cycle regulation, primordial germ cell maintenance, and cancer cell biology. Although human REV7 was originally discovered as a homologous molecule to a mutagenic protein, Rev7, and a spindle assembly checkpoint protein, Mad2, in the yeast Saccharomyces cerevisiae, investigations of vertebrate REV7 identified several novel biological networks surrounding REV7. In addition, studies using human cancer tissues and cancer cell lines revealed the significance of REV7 in cancer biology, which makes REV7 an attractive target molecule in cancer management. This review focuses on the functions of REV7 in human cancers and discusses the utility of REV7 in cancer management.
Abstract
DNA repair and cell cycle regulation are potential biological fields to develop molecular targeting therapies for cancer. Human REV7 was originally discovered as a homologous molecule to yeast Rev7, which is involved in DNA damage response and mutagenesis, and as the second homolog of yeast Mad2, involved in the spindle assembly checkpoint. Although REV7 principally functions in the fields of DNA repair and cell cycle regulation, many binding partners of REV7 have been identified using comprehensive analyses in the past decade, and the significance of REV7 is expanding in various other biological fields, such as gene transcription, epigenetics, primordial germ cell survival, neurogenesis, intracellular signaling, and microbial infection. In addition, the clinical significance of REV7 has been demonstrated in studies using human cancer tissues, and investigations in cancer cell lines and animal models have revealed the greater impacts of REV7 in cancer biology, which makes it an attractive target molecule for cancer management. This review focuses on the functions of REV7 in human cancer and discusses the utility of REV7 for cancer management with a summary of the recent development of inhibitors targeting REV7.
Keywords: cancer management, cell cycle, DNA repair, inhibitor, protein–protein interactions, REV7
1. Introduction
REV7 was originally identified as a critical gene of the yeast Saccharomyces cerevisiae (S. cerevisiae), rev7, a “reversionless” mutant that shows a less mutagenic phenotype after UV irradiation [1,2,3]. The rev7 mutant shows a phenotype that is closely similar to another reversionless mutant, rev3, and both genes are required for misrepair mutagenesis [1,2]. It was soon revealed that S. cerevisiae Rev7 and Rev3 proteins form a complex, resulting in mutagenic DNA polymerase ζ (Polζ), in which Rev3 is the catalytic subunit and Rev7 is the accessory subunit [4]. Polζ is involved in translesion DNA synthesis (TLS), which is a DNA damage-tolerance mechanism that synthesizes DNAs through damaged sites of the template DNAs [5,6]. Human REV7 (also known as MAD2L2, MAD2B, MAD2β, and FANCV) was identified as a homologous gene to spindle assembly checkpoint gene MAD2, as well as to yeast REV7; therefore, research into REV7 was first progressed in the fields of TLS and cell cycle checkpoint [7,8,9]. Then, REV7 was studied in mice, Xenopus, Drosophila, Neurospora, plants (Arabidopsis), and chickens, as well as in humans and S. cerevisiae [10,11,12,13,14,15,16]. Identification of new binding partners of REV7 with comprehensive analyses using the yeast two-hybrid assay and GST pull-down or co-immunoprecipitation assays, followed by mass spectrometry analyses, contributed to determining its significance in various biological processes other than TLS and cell cycle checkpoint. These include double-strand break (DSB) repair, homologous recombination (HR), telomere maintenance, the Fanconi anemia (FA) pathway, gene transcription, epigenetics, primordial germ cell (PGC) survival, neurogenesis, intracellular signaling, and microbial infection. In addition, investigations using human cancer tissues and cancer cell lines revealed the significance of REV7 in cancer development, proliferation, and progression, as well as its utility in cancer management. Recently, several excellent reviews describing the biological functions of REV7 have been published [17,18]; therefore, this review focuses on the significance of REV7 in cancer development, progression, and treatment, with an overview of REV7 functions associated with cancer biology.
2. Protein Structure of REV7
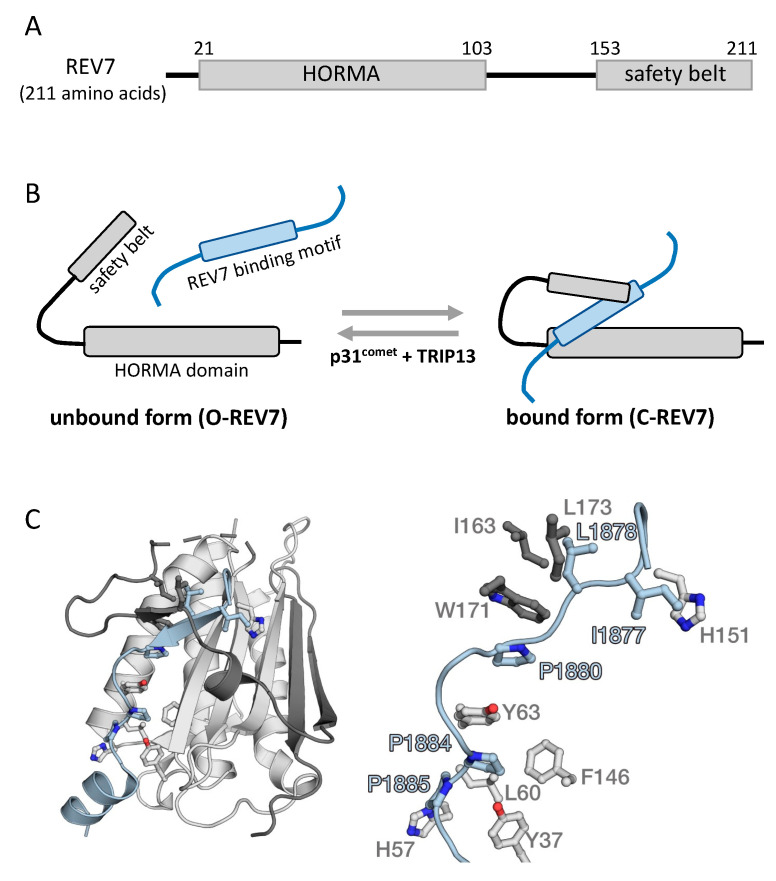
The human REV7 protein is composed of 211 amino acid residues, with a HORMA domain in its middle region but without any catalytically active domains (Figure 1A) [19]. The HORMA domain was originally proposed for its sequence similarity in yeast Hop1, Rev7, and Mad2 proteins [20]. The HORMA domain is supposed to be a domain for protein–protein interactions, and several HORMA family proteins have been identified in humans, including REV7 in DNA repair, MAD2 and p31comet in spindle assembly checkpoint, HORMAD1 and HORMAD2 in meiosis, and ATG13 and ATG101 in autophagy [19,20,21,22,23,24,25,26]. The HORMA domain is important for the interactions of REV7 with many effector proteins. The three-dimensional structure of REV7 in complex with the effector proteins demonstrates that REV7 possesses a “safety belt” region in its C-terminus, which plays a critical role in catching the interacting peptides (Figure 1A) [25,27,28]. When REV7 binds to other proteins, the safety belt structure closes (C-REV7), and when it unbinds from the proteins, the safety belt structure opens (O-REV7) (Figure 1B). The crystal structure of REV7 interacting with a REV3 fragment demonstrates that the C-terminal region of REV7 wraps the REV3 fragment, thereby resulting in C-REV7 (Figure 1C). The interactions with binding partners are quite important for the function of REV7; however, what triggers the conformational change between C-REV7 and O-REV7 and controls its activity is still under investigation. Recently, it was revealed that p31comet and thyroid hormone receptor-interacting protein 13 (TRIP13), an AAA+ ATPase, physically interact with REV7 and catalyze the conformational change from C-REV7 to O-REV7, controlling the functional activity of REV7 (Figure 1B) [29,30]. REV7 has numerous binding partners in various biological fields, and the consensus sequence for REV7 binding is proposed as ΦΦxPxxxpP, in which Φ is aliphatic, x is any residue, and proline p is less significant than proline P [31]. For example, REV3 has two REV7 binding sites: ILKPLMSPPS at amino acids 1877–1886 and VIMPCKCAPSR at amino acids 1993–2003, both of which are consistent with the REV7 consensus binding sequence [32].
Figure 1.
Structure of REV7 in complex with binding partners. (A) Domain structure of REV7. REV7 is composed of 211 amino acid residues, with a HORMA domain in its middle region and a “safety belt” region in its C-terminus [27]. (B) Bound and unbound forms of REV7. When REV7 unbinds its interaction partners, the safety belt structure is open (O-REV7), and when it binds its interaction partners, the safety belt structure is closed (C-REV7). The conformational change from C-REV7 to O-REV7 is catalyzed by p31comet and TRIP13. TRIP13, thyroid hormone receptor-interacting protein 13. (C) Structure of human REV7(R124A) in complex with a human REV3 fragment including residues 1877–1885 (PDB entry 3ABD). REV7 and REV3 are shown in gray and light blue, respectively. The C-terminal region of REV7 is shown in a darker color. A detail of the interactions between REV7 and REV3 is shown in the right panel. I1877, L1878, P1880, P1884, and P1885 of REV3 in the consensus sequence for REV7 binding, ΦΦxPxxxpP, are shown as ball-and-stick models and labeled. Amino acid residues of REV7 involved in the interactions with the REV3 fragment are also shown as ball-and-stick models and labeled.
3. Role of REV7 in Translesion DNA Synthesis and DNA Damage-Induced Mutagenesis
TLS is a DNA damage-tolerance mechanism that saves cells from the adverse consequences of replication arrest raised by DNA damage [5,33]. DNA damage is largely repaired by the DNA repair system before replication, but it sometimes escapes from the DNA repair network and remains unrepaired. When the replication fork encounters an unrepaired lesion on the template DNA, replicative DNA polymerase δ/ε (Polδ/ε) cannot replicate DNA through the lesion. In this situation, several specialized low-fidelity DNA polymerases proceed with the replication throughout the lesion on behalf of Polδ/ε, which is called translesion DNA synthesis (TLS). Finally, DNA replication completes through the lesion without removing the damage. However, in compensation for saving cells from replication fork collapse, error-prone TLS sometimes incorporates an incorrect nucleotide opposite the DNA lesion, resulting in the introduction of mutations.
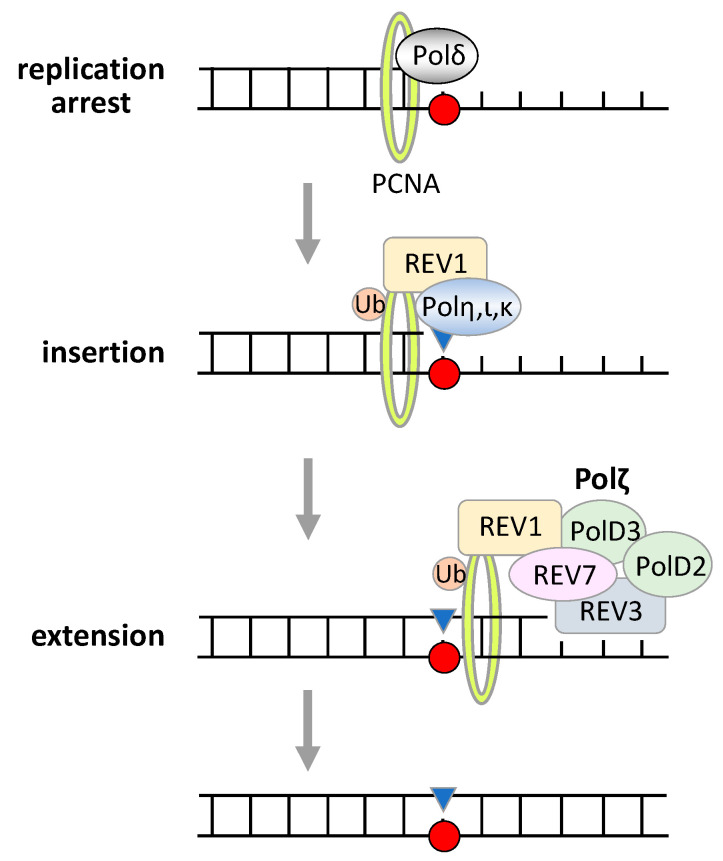
REV7 is a subunit of Polζ, a low-fidelity TLS polymerase, which is composed of REV3, REV7, Pol31, and Pol32 in the yeast S. cerevisiae, and of REV3, REV7, PolD2, and PolD3 in humans (Figure 2) [4,34,35,36,37]. REV3 possesses polymerase activity, while the other three proteins are non-catalytic subunits required for the efficient TLS activity of Polζ [38]. The biological activity of Polζ in TLS has been mainly studied in the yeast S. cerevisiae because human REV3 is a huge protein of approximately 350 kDa and is difficult to be analyzed in vitro [9,39,40,41,42]. The TLS activity of Polζ in synthesizing DNA past a thymine–thymine cis-syn cyclobutane dimer was first demonstrated in the yeast S. cerevisiae [4]; it was revealed that the TLS activity of yeast Polζ is very limited and that another TLS polymerase is required to complete TLS in cooperation with Polζ [43,44,45,46]. Now, the consensus about the mechanism of TLS is that when the replication fork stops at a DNA lesion, an “inserter” TLS polymerase, such as Y family DNA polymerase Polη, ι, κ, or REV1, incorporates a nucleotide opposite the damaged site, and then Polζ proceeds with the subsequent extension from the improperly paired DNA end as a “mispair extender” (Figure 2) [43,44,45,46]. This mechanism of TLS has been also achieved using human Polζ in cisplatin bypass [37].
Figure 2.
Mechanism of translesion DNA synthesis (TLS). When the replication fork stops at a DNA lesion (replication arrest), REV1 binds to monoubiquitinated proliferating cell nuclear antigen (PCNA) at the lesion and recruits inserter polymerases Polη, ι, and κ to the damaged site to incorporate a nucleotide opposite the damaged site (insertion). Then, REV1 recruits Polζ via interaction with REV7 or PolD3, and Polζ proceeds with the subsequent extension from the improperly paired DNA end (extention). Finally, TLS completes without removing the damage. Ub, ubiquitin.
The REV7 subunit of Polζ also interacts with REV1, which is required for the efficient TLS activity of Polζ (Figure 2) [47,48,49]. REV1 also binds to other inserter polymerases Polη, ι, κ, and the PolD3 subunit of Polζ, and REV1 itself possesses deoxycytidyl transferase activity; thus, REV1 is thought to be a hub protein of the TLS machinery [50,51,52]. When the replication fork encounters damage on template DNA, REV1 binds to monoubiquitinated proliferating cell nuclear antigen (PCNA) at the lesion and recruits inserter polymerases Polη, ι, and κ to the damaged site, as well as Polζ via interaction with REV7 or PolD3, although the mechanistic details remain controversial (Figure 2) [6,53,54,55,56]. The transcription factor TFII-I also binds to PCNA and REV7 and promotes TLS by connecting Polζ with PCNA [57]. Conversely, the physical interaction of p31comet and TRIP13 with REV7 facilitates the dissociation of REV7 from REV3 by promoting a conformational change from C-REV7 to O-REV7, resulting in inhibition of TLS activity of Polζ [29,30].
Polζ, together with REV1, is responsible for spontaneous and DNA damage-induced mutagenesis events via error-prone TLS [58,59]. The involvement of REV7 in damage-induced mutagenesis has been demonstrated using yeast and mammalian cells. S. cerevisiae rev7 mutants showed a deficiency in UV-induced reversion mutations [1,2], while depletion in REV7 by RNA interference in human fibroblasts or nasopharyngeal carcinoma cells decreases UV-induced or chemotherapy-induced mutation frequencies of HPRT, respectively [60,61], indicating that REV7 is involved in DNA damage-induced mutagenesis.
4. REV7 in Other DNA Repair Pathways
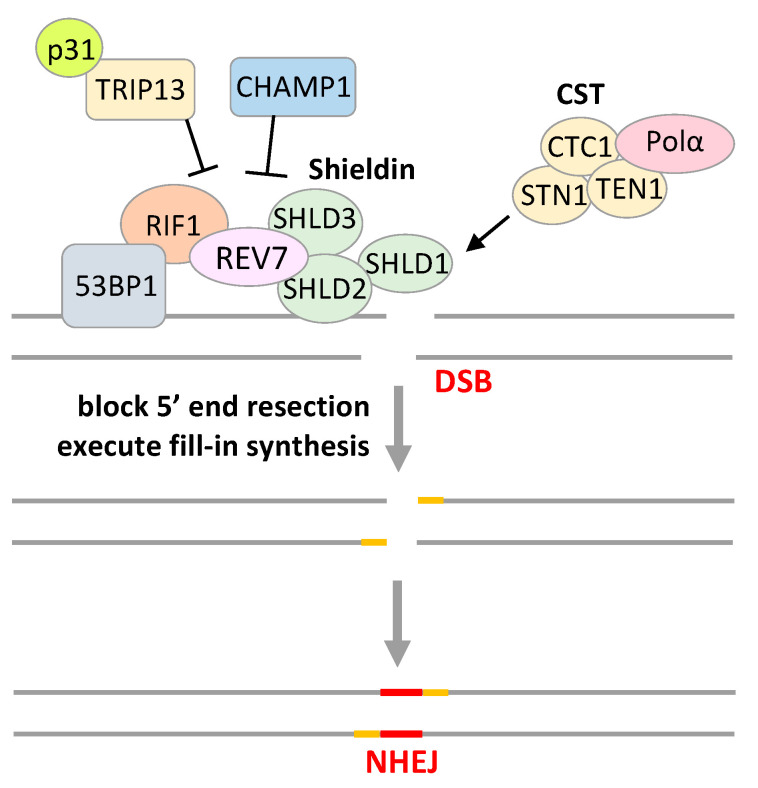
Recent progress in REV7 research revealed the importance of REV7 function in the DNA damage response other than TLS. BRCA1-mutated, HR-deficient breast or ovarian cancer cells are sensitive to poly(ADP-ribose) polymerase (PARP) inhibitors, and resistance to PARP inhibitors in BRCA1-mutated cells is induced by restoration of HR, which is triggered by REV7 depletion [62]. REV7 is recruited to DSBs under the control of TP53-binding protein 1 (53BP1) and RAP1 interacting factor 1 (RIF1), and inhibits 5’ end resection at DSB sites, resulting in the inhibition of HR and the facilitation of non-homologous end joining (NHEJ) (Figure 3). REV7 loss restores the 5’ end resection by CTBP-interacting protein (CtIP) at DSBs, which causes NHEJ inhibition and HR restoration, resulting in PARP inhibitor resistance [62]. In addition, REV7 is also recruited to uncapped telomere ends and inhibits 5’ end resection at telomere ends, facilitating NHEJ-mediated telomere fusion [63]. REV7 depletion promotes 5’ end resection and 3’ overhang at uncapped telomere ends, preventing telomere fusion [63]. Both studies discovered an important biological function of REV7 in DSB repair choice. At DSB lesions or uncapped telomere ends, 53BP1 is recruited with RIF1 under ataxia telangiectasia mutated (ATM)-or RAD3-related (ATR) signaling, and then 53BP1–RIF1 recruits the Shieldin complex, composed of C20orf196 (SHLD1), FAM35A (SHLD2), CTC-534A2.2 (SHLD3), and REV7, which acts at DSB lesions to block 5’ end resection and 3’ single-stranded overhang generation [64,65,66,67,68]. Shieldin also recruits CST complex, composed of CTC1, STN1, and TEN1, together with DNA polymerase α (Polα) to execute fill-in synthesis, resulting in the inhibition of HR and facilitation of NHEJ (Figure 3) [69,70]. The 53BP1-recruited Shieldin complex is also required for immunoglobulin class-switch recombination by NHEJ [64,65,66,67,68,69,70]. Inhibitory effects on REV7 are provoked by chromosome alignment-maintaining phosphoprotein 1 (CHAMP1) and p31comet–TRIP13. CHAMP1, which is involved in microtubule organization, binds to REV7 and reduces the Shieldin complex activity to promote 5’ end resection and HR repair at DSBs [71,72]. p31comet-TRIP13 binds to REV7 in the Shieldin complex and promotes its dissociation from SHLD3 by inducing O-REV7 from C-REV7, resulting in a reduction in the Shieldin complex activity (Figure 3) [29,30].
Figure 3.
Involvement of REV7 in double-strand break (DSB) repair. At a DSB lesion, 53BP1–RIF1 recruits the Shieldin complex (SHLD1, SHLD2, SHLD3, and REV7) to block 5’ end resection, and Shieldin also recruits the CST complex (CTC1, STN1, and TEN1) together with DNA polymerase α (Polα) to execute fill-in synthesis, resulting in the inhibition of HR and the facilitation of non-homologous end joining (NHEJ). CHAMP1 binds to REV7 promoting the decreased level of the Shieldin complex, and p31comet–TRIP13 binds to REV7 in the Shieldin complex and promotes its dissociation from SHLD3 by inducing O-REV7 from C-REV7, resulting in a reduction in the Shieldin complex activity.
Inconsistently, the human Polζ/REV1 complex is involved in HR repair. REV1-, REV3-, or REV7-depleted HeLa cells display enhanced chromosomal instability induced by ionizing radiation (IR) due to a deficiency in HR repair at IR-induced DSBs [73]. Similarly, chicken DT40 cells lacking REV1, REV3, and REV7 show suppressed levels of sister chromatid exchange (SCE), suggesting HR insufficiency, although DT40 cells with every single deletion show elevated levels of SCE [16]. These findings seem incompatible with the role of REV7 in the Shieldin complex, indicating that complicated roles of REV7 are involved in the DNA damage response via various binding partners.
REV7 also belongs to the FA genes, whose germline mutations cause an autosomal recessive disorder characterized by congenital abnormality, chromosomal instability, bone marrow failure, and cancer predisposition [74]. REV7 with a biallelic inactivating mutation encoding REV7-V85E mutant protein was identified in an FA patient. Patient-derived cells showed undetectable levels of REV7 with normal transcript levels, suggesting destabilization of the mutant protein, and displayed phenotypes of interstrand crosslink repair failure, which was restored by the expression of wild-type REV7, indicating that REV7 is the 21st FA gene, termed FANCV [74].
Accordingly, REV7 plays important roles in the replication stress response, which is associated with cancer biology [75]. REV7 is involved in both TLS and DSB repair, the former solves replication stress raised by various types of DNA damage by bypassing the lesion, and the latter solves replication stress raised by DSBs by facilitating NHEJ or HR. REV7 is also involved in the FA DNA repair pathway, which solves the replication stress of DNA interstrand crosslink. Thus, REV7 dysfunction should be associated with cancer biology, which will be discussed later.
5. REV7 in Cell Cycle Regulation and Gene Transcription
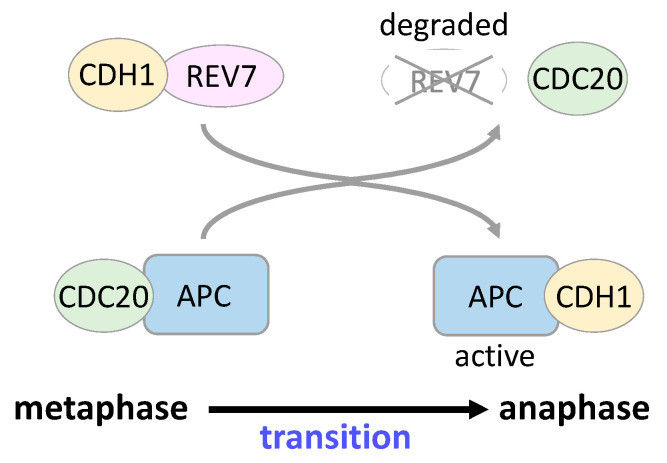
Human REV7 is a homologous molecule of S. cerevisiae Mad2, a spindle assembly checkpoint protein, and interacts with human MAD2 [9]. Xenopus Rev7 directly binds to anaphase-promoting complex (Apc) activator Cdc20 homolog 1 (Cdh1) and inhibits the E3 ubiquitin ligase activity of Cdh1–Apc [10,11]. Xenopus Rev7 also binds weakly to another Apc activator, Cdc20, and inhibits Cdc20–Apc activity [11]. Injection of Xenopus Rev7 into one cell of a two-cell Xenopus embryo causes cell cycle arrest in the injected cell [10]. Human REV7 binds to CDH1 to sequester it from APC in prometaphase, and REV7 is degraded by APC–CDC20 in early anaphase; released CDH1 activates APC, leading to a transition from metaphase to anaphase (Figure 4) [76]. REV7 is localized around the metaphase plates and mitotic spindles, and its depletion causes cell cycle arrest in the G2/M phase, exhibiting abnormal spindle formation and chromosomal misalignment [77]. In addition, REV7 interacts with the Ras-related nuclear protein (RAN) small GTPase, Clathrin light chain A, and CHAMP1, all of which play important roles in microtubule organization and proper progression of the G2/M phase [28,76,78,79]. These findings indicate the involvement of REV7 in cell cycle regulation.
Figure 4.
REV7 involvement in the mitotic checkpoint. REV7 binds to CDH1 to sequester it from APC in prometaphase, and APC–CDC20 degrades REV7 in early anaphase; released CDH1 activates APC, leading to a transition from metaphase to anaphase.
REV7 interacts with the ELK-1 transcription factor, promoting its phosphorylation by c-Jun N-terminal protein kinase (JNK) mitogen-activated protein (MAP) kinase and facilitating its transcription activity following DNA damage [80]. REV7 binds to WNT signal transducer T cell factor 4 (TCF4) and blocks TCF4-mediated transactivation of downstream target genes such as SLUG in colon cancer cells. REV7 depletion suppresses E-cadherin expression via upregulation of SLUG and promotes TCF4-mediated epithelial–mesenchymal transition (EMT) in colon cancer cells [81].
6. REV7 in Cancer
6.1. Possible Role of REV7 in Cancer Development
Cancer development is mainly caused by mutations in cancer-related genes or by epigenetic regulation; thus, molecules involved in mutagenesis are thought to be associated with cancer development. TLS is a DNA damage-tolerance mechanism and is a cause of DNA damage-induced and spontaneous mutations. Polζ is involved in most of the TLSs, including error-free and error-prone TLSs, playing an essential role in TLS [19,33]. The frequency of UV-induced mutations in human fibroblast cells with siRNA-mediated depletion in REV7, as well as REV3 and REV1, is significantly decreased compared with control cells, which may be caused by abrogation of Polζ activity [39,60,82]. Suppressed spontaneous and damage-induced mutagenesis, increased chromosomal aberrations, and H2AX phosphorylation also occur in cancer cells with REV7 depletion after treatment with various DNA-damaging reagents, indicating the involvement of REV7 in damage-induced mutagenesis in cancer cells [61]. Considering the biological role of REV7 in DNA damage-induced mutagenesis, dysregulated REV7 expression is supposed to promote cancer development via the introduction of mutations in cancer-related genes or in regions of epigenetic regulation, although there is no direct evidence showing the relationship between REV7 expression and cancer development thus far. Conversely, Rev7C70R/C70R mutant mice, in which mutant REV7 is unable to interact with REV3, causing germ cell aplasia after birth in both males and females, develop tubulostromal adenomas in the ovary, in which an increase in gonadotropin levels and accumulation of DNA damage may contribute to tumor development [83,84]. Notably, REV7 is a causative gene of FA, which shows a predisposition for the development of hematological malignancy [74].
6.2. Clinical Significance of REV7 in Cancer
The significance of REV7 expression has been studied using clinical materials from various cancer tissues. Although REV7 expression is low in most normal human tissues except for the testis, its expression is relatively high in various human tumor tissues, including colon, ovarian, breast, esophageal, lung, and skin cancers, gliomas, diffuse large B cell lymphomas, and testicular germ cell tumors (TGCTs) [85,86,87,88,89,90,91,92,93]. REV7 expression levels are correlated with cell proliferation ability represented by Ki-67 labeling indexes in small cell lung carcinomas and malignant melanomas, with metastases in breast and lung cancers, with tumor thickness in malignant melanomas, and with tumor sizes of gliomas [89,92,93,94,95]. There is a significant association between high levels of REV7 expression and poor prognosis in several tumors, including colon, breast, lung, gastric, and advanced ovarian cancers, diffuse large B cell lymphomas, and advanced bone and soft tissue sarcomas, suggesting the utility of REV7 as a biomarker for cancer prognosis [85,87,88,89,94,96,97]. The mechanisms for the upregulation of REV7 in tumor tissues have not been fully elucidated, although zinc finger protein with KRAB and SCAN domains 3 (ZKSCAN3), an oncogenic transcription factor, acts as a transcriptional repressor of REV7 in cultured tumor cells [98]. The contribution of REV7 to poor prognosis in various malignancies may be explained by the following: (1) REV7-high tumors exhibit the potential for high proliferation and tend to be more advanced [89,92,93,94,95]; and (2) REV7 expression affects cell mobility, intracellular signaling, EMT, and sensitivity to chemoradiotherapy, which will be discussed later. In support of this, a clinical study on advanced bone and soft tissue sarcomas demonstrated that REV7 expression is significantly high in non-responders to trabectedin and olaparib combination therapy [97]. A genetic polymorphism of REV7 rs746218GG is also associated with shorter progression-free survival in patients with lung cancer who have received platinum-based chemotherapy, probably due to affecting the expression of REV7 [99]. In contrast, high expression of REV7 is associated with favorable prognosis in colon cancer patients, and REV7 overexpression suppresses cell proliferation, migration, and clonogenicity in colorectal cancer cells via the degradation of nuclear receptor coactivator 3 (NCOA3), a transcriptional coactivator that interacts with REV7 [100].
6.3. REV7 in Cancer Cell Biology
The significance of REV7 in cancer cell biology has been studied using cultured cancer cells, most of which demonstrate the cancer-promoting effects of REV7, and a possible application of REV7 to cancer management has been proposed. Inactivation of REV7 with siRNA-mediated knockdown or CRISPR/Cas9 system-mediated gene silencing in a variety of cancer cells promotes the inhibition of cell growth, mobility, invasion, clonogenicity, and EMT in vitro, indicating that REV7 expression affects the nature of cancer cells [87,89,90,91,92,93,94,95,96,99]. Hypersensitivity to DNA-damaging agents such as cisplatin, carboplatin, melphalan, chlorethamine, doxorubicin, mitomycin c, or γ-irradiation, but not to H2O2, 5FU, taxol, or vincristine, decreased spontaneous and damage-induced mutation frequency, accumulation of DSBs and increased chromosomal aberrations after DNA damage, and suppressed cisplatin-induced SCE are promoted by REV7 depletion in nasopharyngeal carcinoma cells [61]. REV7 suppression also sensitizes malignant melanoma, TGCT, and colon cancer cells to cisplatin, doxorubicin, or oxaliplatin, and sensitizes gliomas and esophageal squamous cell carcinoma (SCC) cells to IR [86,90,91,101]. In addition, tumor-bearing mouse experiments with REV7-depleted cancer cells also demonstrated suppressed cell proliferation, increased apoptotic cells, and enhanced sensitivity to cisplatin in vivo in ovarian cancer and TGCT cells [87,91]. Resistance to 5FU or oxaliplatin correlates with the upregulation of REV7 and high TLS efficiency in colon cancer cells [102], and REV7 inactivation overcomes acquired resistance to cisplatin in TGCT cells and to 5FU and oxaliplatin in colon cancer cells in vitro and in vivo [91,102]. Consistently, the overexpression of REV7 promotes chemoresistance in lung cancer cells and radioresistance in esophageal SCC cells [90,94]. Mechanistically, REV7 activates intracellular signaling of the PI3K–AKT and RAS–MAPK pathways and promotes TGF-β1 expression, resulting in the acceleration of cell proliferation, migration, invasion, and EMT [89,93,95]. These results indicated that REV7 is involved in cell activity via modulating intracellular signaling and that its depletion promotes increased sensitivity to chemoradiotherapy, conceivably by abrogation of TLS-mediated DNA damage tolerance.
Several molecules mediating the tumor-promoting and chemoradioresistant effects of REV7 have been identified. REV7 interacts with peroxiredoxin 2 (PRDX2), an antioxidant protein, and disruption of REV7–PRDX2 interactions increases oxidative stress and DSB post-irradiation [90]. In lung cancer cells, REV7 promotes the expression of SLUG, which might be an effector molecule for the tumor-promoting effects of REV7 [94]. REV7 exhibits opposing effects on SLUG expression in colon cancer cells and lung cancer cells, the reason for which is not known [81,94]. Papillary renal cell carcinoma-associated protein (PRCC) interacts with REV7, promoting its translocation from the cytoplasm to the nucleus. The translocation is impaired by PRCCTFE3, which is a fusion protein of PRCC and the transcription factor enhancer 3 (TFE3), and is frequently observed in papillary renal cell carcinoma, resulting in mitotic checkpoint defects in tumor cells [103]. Another mechanism for the chemosensitizing effect of REV7 inactivation is cellular senescence. Tumor cells with REV7 deficiency exhibited enhanced sensitivity to cisplatin in a mouse model of non-small cell lung cancer (NSCLC), in which cellular senescence, but not apoptotic cell death, was induced by DNA damage, showing a flattened, vacuolized cell morphology and induction of senescence-associated β-galactosidase [104]. REV7 inactivation facilitates cisplatin sensitivity more than REV3 inactivation, suggesting that the chemosensitizing effect of REV7 inactivation is mediated not only by TLS defects but also by insufficiency of the other biological roles of REV7 [104].
6.4. Development of Inhibitors Targeting TLS Associated with REV7
Identification of small molecules to inhibit the TLS activity associated with REV7 has recently been attempted (Table 1). A small compound, JH-RE-06, has been identified using ELISA in the screening of small-molecule inhibitors targeting REV1–REV7 interactions [105]. REV7 interacts with the REV1 C-terminal domain (CTD) to form Polζ–REV1 complexes, and JH-RE-06 binds to the REV1 CTD to promote dimerization of the REV1 molecules, resulting in the disruption of REV1–REV7 interactions. The addition of JH-RE-06 to cell culture medium significantly sensitizes various human and mouse cancer cells to cisplatin or other DNA-damaging agents. The addition of JH-RE-06 also suppresses TLS over a cisplatin adduct and decreases the frequency of both spontaneous and cisplatin-induced mutations in HT1080 cells. Moreover, JH-RE-06 is also effective in vivo. The injection of cisplatin plus JH-RE-06 into subcutaneous xenografted tumors in tumor-bearing mouse models significantly suppressed tumor growth and improved their prognosis compared with the injection of saline, cisplatin, or JH-RE-06 alone, indicating that targeting REV1–REV7 interactions together with DNA-damaging treatment is an effective strategy for cancer therapy [105]. Unexpectedly, cancer cells treated with cisplatin plus JH-RE-06 do not have increased apoptotic cell death but exhibit an enhancement in senescence markers such as senescence-associated β-galactosidase and p21 expression, suggesting that the addition of TLS inhibitors to DNA-damaging chemotherapy alters the apoptotic cell death effects of chemotherapy to senescence-based anti-tumor effects [106]. It is very interesting that these senescence-based anti-tumor effects were also demonstrated following REV7 loss in an NSCLC mouse model [104].
Table 1.
List of inhibitors targeting REV1/Polζ-associated TLS.
| Inhibitor | Screening Target | Mechanism | Effect | References |
|---|---|---|---|---|
| JH-RE-06 | REV1 CTD-REV7 PPI | Promote REV1 dimerization Disrupt REV1-REV7 PPI |
Inhibition of cisplatin-induced mutagenic TLS Enhancement of cisplatin, BPDE, 4-NQO, and MMS cytotoxicity in vitro Enhancement of cisplatin chemosensitivity in vivo |
[105,106] |
| Compound 7 | REV3-REV7 PPI | Disrupt REV3-REV7 PPI | Inhibition of interstrand cross-link repair Enhancement of cisplatin cytotoxicity in vitro |
[107] |
| ZINC97017995, ZINC25496030 | REV7 homodimer | Disrupt REV1-REV7 PPI | Enhancement of cisplatin and doxorubicin cytotoxicity in vitro | [108] |
| Thiophene, piperazine, piperidine, and aryl piperazine compounds | REV1 CTD-Polκ RIR PPI | Disrupt REV1 CTD-RIR PPI | Enhancement of cisplatin and UV cytotoxicity in vitro Inhibition of cisplatin-induced mutagenesis |
[109,110] |
| Phenazopyridine compounds | REV1 CTD | Disrupt REV1 CTD-RIR PPI | Enhancement of cisplatin cytotoxicity in vitro | [111,112,113] |
CTD: C terminal domain, PPI: protein–protein interaction, RIR: REV1-interacting region, TLS: translesion DNA synthesis, BPDE: benzo[a]pyrene diol epoxide, 4-NQO: 4-nitroquinolone 1-oxide, MMS: methyl methanesulfonate, UV: ultraviolet.
Small-molecule compounds identified with the screening of molecules that inhibit the interaction of REV7(R124A) and REV3(1875–1895) exhibit inhibition of interstrand crosslink repair and have a chemosensitization effect [107]. The small molecules ZINC97017995 and ZINC25496030, identified in a screen for molecules interfering with the interaction between REV1 and the REV7 dimer, directly bind to the REV7 dimer and disrupt REV7–REV1 interactions in vitro, enhancing the cell death effect of cisplatin in lung cancer cell lines [108].
These findings, together with the results from other similar attempts targeting the REV1-CTD, indicate that targeting the REV1/Polζ-associated TLS with small-molecule inhibitors is a promising strategy for cancer therapy [109,110,111,112,113].
7. Conclusions
In the past decade, many REV7 functions have been discovered, and we now understand REV7 to be a multifunctional protein involved in not only TLS and cell cycle regulation but also in many biological fields. However, many questions remain to be answered about REV7. Although REV7 has numerous binding partners and controls their activities, it is not clear what factors trigger the transition from the open to closed forms of REV7, and what happens to the binding partners through interactions with REV7. Regarding cancer biology and management, it is not clear whether mutagenic TLS involving REV7 really contributes to de novo tumor development via the introduction of mutations. High levels of REV7 expression are detected in some malignant tumor cells, but we do not understand the mechanisms that control REV7 expression in vivo, which may serve as important information to block REV7 expression for molecular targeting therapy. Although small molecules targeting REV1–REV7 interactions are effective in vitro and in vivo, an inhibitor targeting the global activity of REV7 may become another attractive tool for cancer therapy, because REV7 functions not only in TLS but also in other tumor-promoting conditions. In addition, it is important to elucidate the roles of REV7 in developmental biology, especially in PGC survival and differentiation, which is not discussed in this review [83,114,115].
Although more than 20 years have passed since human REV7 was identified, the number of identified REV7 interaction partners is still growing, and the significance of mammalian REV7 has been expanding in various biological fields. As the multifunctionality of REV7 becomes evident, the biological studies of REV7 become more complicated. However, recent progress in basic and clinical cancer research reveals REV7 to be a possible predictive biomarker for the effectiveness of DNA-damaging chemotherapy or PARP inhibitors and for the prognosis of cancer patients; it is also an attractive molecular target for the enhancement of DNA-damaging chemotherapy. Future research on REV7 may provide a new tool to treat cancer.
Acknowledgments
We apologize to those whose work is not cited in this review due to space constraints.
Author Contributions
Y.M.: Conceptualization, writing–original draft, funding acquisition; H.H.: Figure drawing, writing–review and editing; Y.S., T.K., and M.I.: Writing–review and editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by The Uehara Memorial Foundation (No. 202120092).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lawrence C.W., Das G., Christensen R.B. REV7, a new gene concerned with UV mutagenesis in yeast. Mol. Gen. Genet. 1985;200:80–85. doi: 10.1007/BF00383316. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence C.W., Nisson P.E., Christensen R.B. UV and chemical mutagenesis in rev7 mutants of yeast. Mol. Gen. Genet. 1985;200:86–91. doi: 10.1007/BF00383317. [DOI] [PubMed] [Google Scholar]
- 3.Torpey L.E., Gibbs P.E., Nelson J., Lawrence C.W. Cloning and sequence of REV7, a gene whose function is required for DNA damage-induced mutagenesis in Saccharomyces cerevisiae. Yeast. 1994;10:1503–1509. doi: 10.1002/yea.320101115. [DOI] [PubMed] [Google Scholar]
- 4.Nelson J.R., Lawrence C.W., Hinkle D.C. Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 5.Sale J.E. Translesion DNA synthesis and mutagenesis in eukaryotes. Cold Spring Harb. Perspect. Biol. 2013;5:a012708. doi: 10.1101/cshperspect.a012708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shilkin E.S., Boldinova E.O., Stolyarenko A.D., Goncharova R.I., Chuprov-Netochin R.N., Khairullin R.F., Smal M.P., Makarova A.V. Translesion DNA Synthesis and Carcinogenesis. Biochemistry. 2020;85:425–435. doi: 10.1134/S0006297920040033. [DOI] [PubMed] [Google Scholar]
- 7.Cahill D.P., da Costa L.T., Carson-Walter E.B., Kinzler K.W., Vogelstein B., Lengauer C. Characterization of MAD2B and Other Mitotic Spindle Checkpoint Genes. Genomics. 1999;58:181–187. doi: 10.1006/geno.1999.5831. [DOI] [PubMed] [Google Scholar]
- 8.Nelson K.K., Schlondorff J., Blobel C.P. Evidence for an interaction of the metalloprotease—Disintegrin tumour necrosis factor a convertase (TACE) with mitotic arrest deficient 2 (MAD2), and of the metalloprotease—Disintegrin MDC9 with a novel MAD2-related protein, MAD2β. Biochem. J. 1999;343:673–680. doi: 10.1042/bj3430673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakumo Y., Roth T., Ishii H., Rasio D., Numata S., Croce C.M., Fishel R. A Human REV7 Homolog That Interacts with the Polymerase ζ Catalytic Subunit hREV3 and the Spindle Assembly Checkpoint Protein hMAD2. J. Biol. Chem. 2000;275:4391–4397. doi: 10.1074/jbc.275.6.4391. [DOI] [PubMed] [Google Scholar]
- 10.Pfleger C.M., Salic A., Lee E., Kirschner M.W. Inhibition of Cdh1-APC by the MAD2-related protein MAD2L2: A novel mechanism for regulating Cdh1. Genes Dev. 2001;15:1759–1764. doi: 10.1101/gad.897901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J., Fang G. MAD2B is an inhibitor of the anaphase-promoting complex. Genes Dev. 2001;15:1765–1770. doi: 10.1101/gad.898701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai W., Wada Y., Naoi Y., Ishii C., Inoue H. Isolation and genetic characterization of the Neurospora crassa REV1 and REV7 homologs: Evidence for involvement in damage-induced mutagenesis. DNA Repair. 2003;2:337–346. doi: 10.1016/S1568-7864(02)00223-9. [DOI] [PubMed] [Google Scholar]
- 13.Guo C., Fischhaber P.L., Luk-Paszyc M.J., Masuda Y., Zhou J., Kamiya K., Kisker C., Friedberg E.C. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 2003;22:6621–6630. doi: 10.1093/emboj/cdg626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi R., Oshige M., Uchida M., Ishikawa G., Takata K., Shimanouchi K., Kanai Y., Ruike T., Morioka H., Sakaguchi K. Purification of Drosophila DNA polymerase ζ by REV1 protein-affinity chromatography. Biochem. J. 2004;382:535–543. doi: 10.1042/BJ20031833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi S., Sakamoto A., Sato S., Kato T., Tabata S., Tanaka A. Roles of Arabidopsis AtREV1 and AtREV7 in translesion synthesis. Plant Physiol. 2005;138:870–881. doi: 10.1104/pp.105.060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada T., Sonoda E., Yoshimura M., Kawano Y., Saya H., Kohzaki M., Takeda S. Multiple roles of vertebrate REV genes in DNA repair and recombination. Mol. Cell. Biol. 2005;25:6103–6111. doi: 10.1128/MCB.25.14.6103-6111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clairmont C.S., D’Andrea A.D. REV7 directs DNA repair pathway choice. Trends Cell Biol. 2021;31:965–978. doi: 10.1016/j.tcb.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Krijger I., Boersma V., Jacobs J.J.L. REV7: Jack of many trades. Trends Cell Biol. 2021;31:686–701. doi: 10.1016/j.tcb.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakumo Y. The property of DNA polymerase ζ: REV7 is a putative protein involved in translesion DNA synthesis and cell cycle control. Mutat. Res. 2002;510:37–44. doi: 10.1016/S0027-5107(02)00250-6. [DOI] [PubMed] [Google Scholar]
- 20.Aravind L., Koonin E.V. The HORMA domain: A common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem. Sci. 1998;23:284–286. doi: 10.1016/S0968-0004(98)01257-2. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 22.Xia G., Luo X., Habu T., Rizo J., Matsumoto T., Yu H. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 2004;23:3133–3143. doi: 10.1038/sj.emboj.7600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y., Rosenberg S.C., Kugel C.L., Kostow N., Rog O., Davydov V., Su T.Y., Dernburg A.F., Corbett K.D. The chromosome axis controls meiotic events through a hierarchical assembly of HORMA domain proteins. Dev. Cell. 2014;31:487–502. doi: 10.1016/j.devcel.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki H., Kaizuka T., Mizushima N., Noda N.N. Structure of the Atg101-Atg13 complex reveals essential roles of Atg101 in autophagy initiation. Nat. Struct. Mol. Biol. 2015;22:572–580. doi: 10.1038/nsmb.3036. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg S.C., Corbett K.D. The multifaceted roles of the HORMA domain in cellular signaling. J. Cell Biol. 2015;211:745–755. doi: 10.1083/jcb.201509076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prince J.P., Martinez-Perez E. Functions and Regulation of Meiotic HORMA-Domain Proteins. Genes. 2022;13:777. doi: 10.3390/genes13050777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara K., Hashimoto H., Murakumo Y., Kobayashi S., Kogame T., Unzai S., Akashi S., Takeda S., Shimizu T., Sato M. Crystal structure of human REV7 in complex with a human REV3 fragment and structural implication of the interaction between DNA polymerase ζ and REV1. J. Biol. Chem. 2010;285:12299–12307. doi: 10.1074/jbc.M109.092403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara K., Taharazako S., Ikeda M., Fujita H., Mikami Y., Kikuchi S., Hishiki A., Yokoyama H., Ishikawa Y., Kanno S., et al. Dynamic feature of mitotic arrest deficient 2-like protein 2 (MAD2L2) and structural basis for its interaction with chromosome alignment-maintaining phosphoprotein (CAMP) J. Biol. Chem. 2017;292:17658–17667. doi: 10.1074/jbc.M117.804237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clairmont C.S., Sarangi P., Ponnienselvan K., Galli L.D., Csete I., Moreau L., Adelmant G., Chowdhury D., Marto J.A., D’Andrea A.D. TRIP13 regulates DNA repair pathway choice through REV7 conformational change. Nat. Cell Biol. 2020;22:87–96. doi: 10.1038/s41556-019-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarangi P., Clairmont C.S., Galli L.D., Moreau L.A., D’Andrea A.D. p31comet promotes homologous recombination by inactivating REV7 through the TRIP13 ATPase. Proc. Natl. Acad. Sci. USA. 2020;117:26795–26803. doi: 10.1073/pnas.2008830117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanafusa T., Habu T., Tomida J., Ohashi E., Murakumo Y., Ohmori H. Overlapping in short motif sequences for binding to human REV7 and MAD2 proteins. Genes Cells. 2010;15:281–296. doi: 10.1111/j.1365-2443.2009.01380.x. [DOI] [PubMed] [Google Scholar]
- 32.Tomida J., Takata K., Lange S.S., Schibler A.C., Yousefzadeh M.J., Bhetawal S., Dent S.Y., Wood R.D. REV7 is essential for DNA damage tolerance via two REV3L binding sites in mammalian DNA polymerase ζ. Nucleic Acids Res. 2015;43:1000–1011. doi: 10.1093/nar/gku1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dash R.C., Hadden K. Protein-Protein Interactions in Translesion Synthesis. Molecules. 2021;26:5544. doi: 10.3390/molecules26185544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson R.E., Prakash L., Prakash S. Pol31 and Pol32 subunits of yeast DNA polymerase δ are also essential subunits of DNA polymerase ζ. Proc. Natl. Acad. Sci. USA. 2012;109:12455–12460. doi: 10.1073/pnas.1206052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baranovskiy A.G., Lada A.G., Siebler H.M., Zhang Y., Pavlov Y.I., Tahirov T.H. DNA polymerase δ and ζ switch by sharing accessory subunits of DNA polymerase δ. J. Biol. Chem. 2012;287:17281–17287. doi: 10.1074/jbc.M112.351122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makarova A.V., Stodola J.L., Burgers P.M. A four-subunit DNA polymerase ζ complex containing Pol δ accessory subunits is essential for PCNA-mediated mutagenesis. Nucleic Acids Res. 2012;40:11618–11626. doi: 10.1093/nar/gks948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y.S., Gregory M.T., Yang W. Human Pol ζ purified with accessory subunits is active in translesion DNA synthesis and complements Pol η in cisplatin bypass. Proc. Natl. Acad. Sci. USA. 2014;111:2954–2959. doi: 10.1073/pnas.1324001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin S.K., Wood R.D. DNA polymerase ζ in DNA replication and repair. Nucleic Acids Res. 2019;47:8348–8361. doi: 10.1093/nar/gkz705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbs P.E., McGregor W.G., Maher V.M., Nisson P., Lawrence C.W. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase ζ. Proc. Natl. Acad. Sci. USA. 1998;95:6876–6880. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao W., Lechler T., Chow B.L., Fontanie T., Agustus M., Carter K.C., Wei Y.F. Identification, chromosomal mapping and tissue-specific expression of hREV3 encoding a putative human DNA polymerase ζ. Carcinogenesis. 1998;19:945–949. doi: 10.1093/carcin/19.5.945. [DOI] [PubMed] [Google Scholar]
- 41.Morelli C., Mungall A.J., Negrini M., Barbanti-Brodano G., Croce C.M. Alternative splicing, genomic structure, and fine chromosome localization of REV3L. Cytogenet. Cell Genet. 1998;83:18–20. doi: 10.1159/000015157. [DOI] [PubMed] [Google Scholar]
- 42.Lin W., Wu X., Wang Z. A full-length cDNA of hREV3 is predicted to encode DNA polymerase ζ for damage-induced mutagenesis in humans. Mutat. Res. 1999;433:89–98. doi: 10.1016/S0921-8777(98)00065-2. [DOI] [PubMed] [Google Scholar]
- 43.Yuan F., Zhang Y., Rajpal D.K., Wu X., Guo D., Wang M., Taylor J.S., Wang Z. Specificity of DNA lesion bypass by the yeast DNA polymerase η. J. Biol. Chem. 2000;275:8233–8239. doi: 10.1074/jbc.275.11.8233. [DOI] [PubMed] [Google Scholar]
- 44.Johnson R.E., Washington M.T., Haracska L., Prakash S., Prakash L. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature. 2000;406:1015–1019. doi: 10.1038/35023030. [DOI] [PubMed] [Google Scholar]
- 45.Guo D., Wu X., Rajpal D.K., Taylor J.S., Wang Z. Translesion synthesis by yeast DNA polymerase ζ from templates containing lesions of ultraviolet radiation and acetylaminofluorene. Nucleic Acids Res. 2001;29:2875–2883. doi: 10.1093/nar/29.13.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao B., Xie Z., Shen H., Wang Z. Role of DNA polymerase η in the bypass of abasic sites in yeast cells. Nucleic Acids Res. 2004;32:3984–3994. doi: 10.1093/nar/gkh710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson J.R., Lawrence C.W., Hinkle D.C. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996;382:729–731. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- 48.Murakumo Y., Ogura Y., Ishii H., Numata S., Ichihara M., Croce C.M., Fishel R., Takahashi M. Interantions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J. Biol. Chem. 2001;276:35644–35651. doi: 10.1074/jbc.M102051200. [DOI] [PubMed] [Google Scholar]
- 49.Masuda Y., Ohmae M., Masuda K., Kamiya K. Structure and enzymatic properties of a stable complex of the human REV1 and REV7 proteins. J. Biol. Chem. 2003;278:12356–12360. doi: 10.1074/jbc.M211765200. [DOI] [PubMed] [Google Scholar]
- 50.Acharya N., Haracska L., Johnson R.E., Unk I., Prakash S., Prakash L. Complex formation of yeast Rev1 and Rev7 proteins: A novel role for the polymerase-associated domain. Mol. Cell. Biol. 2005;25:9734–9740. doi: 10.1128/MCB.25.21.9734-9740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pustovalova Y., Bezsonova I., Korzhnev D.M. The C-terminal domain of human Rev1 contains independent binding sites for DNA polymerase η and Rev7 subunit of polymerase ζ. FEBS Lett. 2012;586:3051–3056. doi: 10.1016/j.febslet.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pustovalova Y., Magalhães M.T., D’Souza S., Rizzo A.A., Korza G., Walker G.C., Korzhnev D.M. Interaction between the Rev1 C-Terminal Domain and the PolD3 Subunit of Polζ Suggests a Mechanism of Polymerase Exchange upon Rev1/Polζ-Dependent Translesion Synthesis. Biochemistry. 2016;55:2043–2053. doi: 10.1021/acs.biochem.5b01282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garg P., Stith C.M., Majka J., Burgers P.M. Proliferating cell nuclear antigen promotes translesion synthesis by DNA polymerase ζ. J. Biol. Chem. 2005;280:23446–23450. doi: 10.1074/jbc.C500173200. [DOI] [PubMed] [Google Scholar]
- 54.Guo C., Sonoda E., Tang T.S., Parker J.L., Bielen A.B., Takeda S., Ulrich H.D., Friedberg E.C. REV1 protein interacts with PCNA: Significance of the REV1 BRCT domain in vitro and in vivo. Mol. Cell. 2006;23:265–271. doi: 10.1016/j.molcel.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 55.Guo C., Tang T.S., Bienko M., Parker J.L., Bielen A.B., Sonoda E., Takeda S., Ulrich H.D., Dikic I., Friedberg E.C. Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Mol. Cell. Biol. 2006;26:8892–8900. doi: 10.1128/MCB.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Souza S., Waters L.S., Walker G.C. Novel conserved motifs in Rev1 C-terminus are required for mutagenic DNA damage tolerance. DNA Repair. 2008;7:1455–1470. doi: 10.1016/j.dnarep.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fattah F.J., Hara K., Fattah K.R., Yang C., Wu N., Warrington R., Chen D.J., Zhou P., Boothman D.A., Yu H. The transcription factor TFII-I promotes DNA translesion synthesis and genomic stability. PLoS Genet. 2014;10:e1004419. doi: 10.1371/journal.pgen.1004419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajpal D.K., Wu X., Wang Z. Alteration of ultraviolet-induced mutagenesis in yeast through molecular modulation of the REV3 and REV7 gene expression. Mutat. Res. 2000;461:133–143. doi: 10.1016/S0921-8777(00)00047-1. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence C.W., Maher V.M. Mutagenesis in eukaryotes dependent on DNA polymerase zeta and Rev1p. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:41–46. doi: 10.1098/rstb.2000.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McNally K., Neal J.A., McManus T.P., McCormick J.J., Maher V.M. hRev7, putative subunit of hPolζ, plays a critical role in survival, induction of mutations, and progression through S-phase, of UV((254nm))-irradiated human fibroblasts. DNA Repair. 2008;7:597–604. doi: 10.1016/j.dnarep.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheung H.W., Chun A.C., Wang Q., Deng W., Hu L., Guan X.-Y., Nicholls J.M., Ling M.-T., Wong Y.C., Tsao S.W., et al. Inactivation of human MAD2B in nasopharyngeal carcinoma cells leads to chemosensitization to DNA-damaging agents. Cancer Res. 2006;66:4357–4367. doi: 10.1158/0008-5472.CAN-05-3602. [DOI] [PubMed] [Google Scholar]
- 62.Xu G., Chapman J.R., Brandsma I., Yuan J., Mistrik M., Bouwman P., Bartkova J., Gogola E., Warmerdam D., Barazas M., et al. REV7 counteracts DNA double-strand break resection and affects PARP inhibition. Nature. 2015;521:541–544. doi: 10.1038/nature14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boersma V., Moatti N., Segura-Bayona S., Peuscher M.H., van der Torre J., Wevers B.A., Orthwein A., Durocher D., Jacobs J.J.L. MAD2L2 controls DNA repair at telomeres and DNA breaks by inhibiting 5′ end resection. Nature. 2015;521:537–540. doi: 10.1038/nature14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta R., Somyajit K., Narita T., Maskey E., Stanlie A., Kremer M., Typas D., Lammers M., Mailand N., Nussenzweig A., et al. DNA Repair Network Analysis Reveals Shieldin as a Key Regulator of NHEJ and PARP Inhibitor Sensitivity. Cell. 2018;173:972–988.e23. doi: 10.1016/j.cell.2018.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noordermeer S.M., Adam S., Setiaputra D., Barazas M., Pettitt S.J., Ling A.K., Olivieri M., Álvarez-Quilón A., Moatti N., Zimmermann M., et al. The shieldin complex mediates 53BP1-dependent DNA repair. Nature. 2018;560:117–121. doi: 10.1038/s41586-018-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghezraoui H., Oliveira C., Becker J.R., Bilham K., Moralli D., Anzilotti C., Fischer R., Deobagkar-Lele M., Sanchiz-Calvo M., Fueyo-Marcos E., et al. 53BP1 cooperation with the REV7-shieldin complex underpins DNA structure-specific NHEJ. Nature. 2018;560:122–127. doi: 10.1038/s41586-018-0362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Findlay S., Heath J., Luo V.M., Malina A., Morin T., Coulombe Y., Djerir B., Li Z., Samiei A., Simo-Cheyou E., et al. SHLD2/FAM35A co-operates with REV7 to coordinate DNA double-strand break repair pathway choice. EMBO J. 2018;37:e100158. doi: 10.15252/embj.2018100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Setiaputra D., Durocher D. Shieldin—The protector of DNA ends. EMBO Rep. 2019;20:e47560. doi: 10.15252/embr.201847560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirman Z., Lottersberger F., Takai H., Kibe T., Gong Y., Takai K., Bianchi A., Zimmermann M., Durocher D., de Lange T. 53BP1-RIF1-shieldin counteracts DSB resection through CST- and Polα-dependent fill-in. Nature. 2018;560:112–116. doi: 10.1038/s41586-018-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mirman Z., Sasi N.K., King A., Chapman J.R., de Lange T. 53BP1-shieldin-dependent DSB processing in BRCA1-deficient cells requires CST-Polα-primase fill-in synthesis. Nat. Cell. Biol. 2022;24:51–61. doi: 10.1038/s41556-021-00812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fujita H., Ikeda M., Ui A., Ouchi Y., Mikami Y., Kanno S.I., Yasui A., Tanaka K. CHAMP1-POGZ counteracts the inhibitory effect of 53BP1 on homologous recombination and affects PARP inhibitor resistance. Oncogene. 2022;41:2706–2718. doi: 10.1038/s41388-022-02299-6. [DOI] [PubMed] [Google Scholar]
- 72.Li F., Sarangi P., Iyer D.R., Feng H., Moreau L., Nguyen H., Clairmont C., D’Andrea A.D. CHAMP1 binds to REV7/FANCV and promotes homologous recombination repair. Cell Rep. 2022;40:111297. doi: 10.1016/j.celrep.2022.111297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma S., Hicks J.K., Chute C.L., Brennan J.R., Ahn J.Y., Glover T.W., Canman C.E. REV1 and polymerase ζ facilitate homologous recombination repair. Nucleic Acids Res. 2012;40:682–691. doi: 10.1093/nar/gkr769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bluteau D., Masliah-Planchon J., Clairmont C., Rousseau A., Ceccaldi R., d’Enghien C.D., Bluteau O., Cuccuini W., Gachet S., de Latour R.P., et al. Biallelic inactivation of REV7 is associated with Fanconi anemia. J. Clin. Investig. 2016;126:3580–3584. doi: 10.1172/JCI88010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.da Costa A.A.B.A., Chowdhury D., Shapiro G.I., D’Andrea A.D., Konstantinopoulos P.A. Targeting replication stress in cancer therapy. Nat. Rev. Drug. Discov. 2023;22:38–58. doi: 10.1038/s41573-022-00558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Listovsky T., Sale J.E. Sequestration of CDH1 by MAD2L2 prevents premature APC/C activation prior to anaphase onset. J. Cell Biol. 2013;203:87–100. doi: 10.1083/jcb.201302060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhat A., Wu Z., Maher V.M., McCormick J.J., Xiao W. Rev7/Mad2B plays a critical role in the assembly of a functional mitotic spindle. Cell Cycle. 2015;14:3929–3938. doi: 10.1080/15384101.2015.1120922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Medendorp K., van Groningen J.J.M., Vreede L., Hetterschijt L., van den Hurk W.H., de Bruijn D.R.H., Brugmans L., van Kessel A.G. The Mitotic Arrest Deficient Protein MAD2B Interacts with the Small GTPase RAN throughout the Cell Cycle. PLoS ONE. 2009;4:e7020. doi: 10.1371/journal.pone.0007020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Medendorp K., Vreede L., van Groningen J.J.M., Hetterschijt L., Brugmans L., Jansen P.A.M., van den Hurk W.H., de Bruijn D.R.H., van Kessel A.G. The Mitotic Arrest Deficient Protein MAD2B Interacts with the Clathrin Light Chain A during Mitosis. PLoS ONE. 2010;5:e15128. doi: 10.1371/journal.pone.0015128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang L., Yang S.H., Sharrocks A.D. Rev7/MAD2B links c-Jun N-terminal protein kinase pathway signaling to activation of the transcription factor Elk-1. Mol. Cell. Biol. 2007;27:2861–2869. doi: 10.1128/MCB.02276-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong C.F., Chou Y.T., Lin Y.S., Wu C.W. MAD2B, a novel TCF4-binding protein, modulates TCF4-mediated epithelial-mesenchymal transdifferentiation. J. Biol. Chem. 2009;284:19613–19622. doi: 10.1074/jbc.M109.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gibbs P.E., Wang X.D., Li Z., McManus T.P., McGregor W.G., Lawrence C.W., Maher V.M. The function of the human homolog of Saccharomyces cerevisiae REV1 is required for mutagenesis induced by UV light. Proc. Natl. Acad. Sci. USA. 2000;97:4186–4191. doi: 10.1073/pnas.97.8.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khalaj M., Abbasi A., Yamanishi H., Akiyama K., Wakitani S., Kikuchi S., Hirose M., Yuzuriha M., Magari M., Degheidy H.A., et al. A missense mutation in Rev7 disrupts formation of Polζ, impairing mouse development and repair of genotoxic agent-induced DNA lesions. J. Biol. Chem. 2014;289:3811–3824. doi: 10.1074/jbc.M113.514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abbasi A., Khalaj M., Akiyama K., Mukai Y., Matsumoto H., Acosta T.J., Said N., Yoshida M., Kunieda T. Lack of Rev7 function results in development of tubulostromal adenomas in mouse ovary. Mol. Cell. Endocrinol. 2015;412:19–25. doi: 10.1016/j.mce.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 85.Rimkus C., Friederichs J., Rosenberg R., Holzmann B., Siewert J.-R., Janssen K.-P. Expression of the mitotic checkpoint gene MAD2L2 has prognostic significance in colon cancer. Int. J. Cancer. 2006;120:207–211. doi: 10.1002/ijc.22155. [DOI] [PubMed] [Google Scholar]
- 86.Zhao J., Liu S., Wang H., Zhang X., Kang T., Li Z., Deng H., Yue W., Cao S. Mitotic arrest deficient protein MAD2B is overexpressed in human glioma, with depletion enhancing sensitivity to ionizing radiation. J. Clin. Neurosci. 2011;18:827–833. doi: 10.1016/j.jocn.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 87.Niimi K., Murakumo Y., Watanabe N., Kato T., Mii S., Enomoto A., Asai M., Asai N., Yamamoto E., Kajiyama H., et al. Suppression of REV7 enhances cisplatin sensitivity in ovarian clear cell carcinoma cells. Cancer Sci. 2014;105:545–552. doi: 10.1111/cas.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Okina S., Yanagisawa N., Yokoyama M., Sakurai Y., Numata Y., Umezawa A., Higashihara M., Murakumo Y. High expression of REV7 is an independent prognostic indicator in patients with diffuse large B-cell lymphoma treated with rituximab. Int. J. Hematol. 2015;102:662–669. doi: 10.1007/s12185-015-1880-3. [DOI] [PubMed] [Google Scholar]
- 89.Feng L., Wei W., Heng Z., Yantao H., Chunbo W. Knockdown of REV7 Inhibits Breast Cancer Cell Migration and Invasion. Oncol. Res. 2016;24:315–325. doi: 10.3727/096504016X14666990347590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gu C., Luo J., Lu X., Tang Y., Ma Y., Yun Y., Cao J., Cao J., Huang Z., Zhou X., et al. REV7 confers radioresistance of esophagus squamous cell carcinoma by recruiting PRDX2. Cancer Sci. 2019;110:962–972. doi: 10.1111/cas.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sakurai Y., Ichinoe M., Yoshida K., Nakazato Y., Saito S., Satoh M., Nakada N., Sanoyama I., Umezawa A., Numata Y., et al. Inactivation of REV7 enhances chemosensitivity and overcomes acquired chemoresistance in testicular germ cell tumors. Cancer Lett. 2020;489:100–110. doi: 10.1016/j.canlet.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Sanoyama I., Sakurai Y., Ichinoe M., Hoshino A., Kesen Y., Kato T., Numata Y., Umezawa A., Jiang S.X., Murakumo Y. Increased expression of REV7 in small cell lung carcinomas and its association with tumor cell survival and proliferation. Pathol. Int. 2021;71:15–23. doi: 10.1111/pin.13040. [DOI] [PubMed] [Google Scholar]
- 93.Hoshino A., Nakayama C., Jiang S.X., Sakurai Y., Kato T., Numata Y., Umezawa A., Ichinoe M., Murakumo Y. Upregulation of REV7 correlates with progression of malignant melanoma. Pathol. Int. 2022;72:14–24. doi: 10.1111/pin.13174. [DOI] [PubMed] [Google Scholar]
- 94.Zhang H., He X., Yu W., Yue B., Yu Z., Qin Y. Mitotic Arrest-Deficient Protein 2B Overexpressed in Lung Cancer Promotes Proliferation, EMT, and Metastasis. Oncol. Res. 2019;27:859–869. doi: 10.3727/096504017X15049209129277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J., Ding W., Wang X., Xiang Y. Knockdown of DNA polymerase ζ relieved the chemoresistance of glioma via inhibiting the PI3K/AKT signaling pathway. Bioengineered. 2021;12:3924–3933. doi: 10.1080/21655979.2021.1944027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pernicone N., Peretz L., Grinshpon S., Listovsky T. MDA-MB-157 Cell Line Presents High Levels of MAD2L2 and Dysregulated Mitosis. Anticancer Res. 2020;40:5471–5480. doi: 10.21873/anticanres.14558. [DOI] [PubMed] [Google Scholar]
- 97.Merlini A., Centomo M.L., Ferrero G., Chiabotto G., Miglio U., Berrino E., Giordano G., Brusco S., Pisacane A., Maldi E., et al. DNA damage response and repair genes in advanced bone and soft tissue sarcomas: An 8-gene signature as a candidate predictive biomarker of response to trabectedin and olaparib combination. Front. Oncol. 2022;12:844250. doi: 10.3389/fonc.2022.844250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho Y.E., Kim J.H., Che Y.H., Kim Y.J., Sung J.Y., Kim Y.W., Choe B.G., Lee S., Park J.H. Role of the WNT/β-catenin/ZKSCAN3 Pathway in Regulating Chromosomal Instability in Colon Cancer Cell lines and Tissues. Int. J. Mol. Sci. 2022;23:9302. doi: 10.3390/ijms23169302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J.-Y., Zou T., Yin J.-Y., Wang Z., Liu C., Huang H.-X., Ding F.-X., Lei M.-R., Wang Y., Liu M., et al. Genetic Variants in Double-Strand Break Repair Pathway Genes to Predict Platinum-Based Chemotherapy Prognosis in Patients With Lung Cancer. Front. Pharmacol. 2022;13:915822. doi: 10.3389/fphar.2022.915822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Y., Li L., Chen M., Yu X., Gu Z., Qiu H., Qin G., Long Q., Fu X., Liu T., et al. MAD2L2 inhibits colorectal cancer growth by promoting NCOA3 ubiquitination and degradation. Mol. Oncol. 2018;12:391–405. doi: 10.1002/1878-0261.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma L., Li X., Zhao X., Sun H., Kong F., Li Y., Sui Y., Xu F. Oxaliplatin promotes siMAD2L2-induced apoptosis in colon cancer cells. Mol. Med. Rep. 2021;24:629. doi: 10.3892/mmr.2021.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun X., Hou W., Liu X., Chai J., Guo H., Yu J. Targeting REV7 effectively reverses 5-FU and oxaliplatin resistance in colorectal cancer. Cancer Cell Int. 2020;20:580. doi: 10.1186/s12935-020-01668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weterman M.A., van Groningen J.J., Tertoolen L., van Kessel A.G. Impairment of MAD2B-PRCC interaction in mitotic checkpoint defective t(X;1)-positive renal cell carcinomas. Proc. Natl. Acad. Sci. USA. 2001;98:13808–13813. doi: 10.1073/pnas.241304198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vassel F.M., Bian K., Walker G.C., Hemann M.T. Rev7 loss alters cisplatin response and increases drug efficacy in chemotherapy-resistant lung cancer. Proc. Natl. Acad. Sci. USA. 2020;117:28922–28924. doi: 10.1073/pnas.2016067117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wojtaszek J.L., Chatterjee N., Najeeb J., Ramos A., Lee M., Bian K., Xue J.Y., Fenton B.A., Park H., Li D., et al. A Small Molecule Targeting Mutagenic Translesion Synthesis Improves Chemotherapy. Cell. 2019;178:152–159. doi: 10.1016/j.cell.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chatterjee N., Whitman M.A., Harris C.A., Min S.M., Jonas O., Lien E.C., Luengo A., Vander Heiden M.G., Hong J., Zhou P., et al. REV1 inhibitor JH-RE-06 enhances tumor cell response to chemotherapy by triggering senescence hallmarks. Proc. Natl. Acad. Sci. USA. 2020;117:28918–28921. doi: 10.1073/pnas.2016064117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Actis M.L., Ambaye N.D., Evison B.J., Shao Y., Vanarotti M., Inoue A., McDonald E.T., Kikuchi S., Heath R., Hara K., et al. Identification of the first small-molecule inhibitor of the REV7 DNA repair protein interaction. Bioorg. Med. Chem. 2016;24:4339–4346. doi: 10.1016/j.bmc.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pernicone N., Elias M., Onn I., Tobi D., Listovsky T. Disrupting the MAD2L2-Rev1 Complex Enhances Cell Death upon DNA Damage. Molecules. 2022;27:636. doi: 10.3390/molecules27030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sail V., Rizzo A.A., Chatterjee N., Dash R.C., Ozen Z., Walker G.C., Korzhnev D.M., Hadden M.K. Identification of Small Molecule Translesion Synthesis Inhibitors That Target the Rev1-CT/RIR Protein-Protein Interaction. ACS Chem. Biol. 2017;12:1903–1912. doi: 10.1021/acschembio.6b01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ozen Z., Dash R.C., McCarthy K.R., Chow S.A., Rizzo A.A., Korzhnev D.M., Hadden M.K. Small molecule scaffolds that disrupt the Rev1-CT/RIR protein-protein interaction. Bioorg. Med. Chem. 2018;26:4301–4309. doi: 10.1016/j.bmc.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 111.Dash R.C., Ozen Z., Rizzo A.A., Lim S., Korzhnev D.M., Hadden M.K. Structural Approach To Identify a Lead Scaffold That Targets the Translesion Synthesis Polymerase Rev1. J. Chem. Inf. Model. 2018;58:2266–2277. doi: 10.1021/acs.jcim.8b00535. [DOI] [PubMed] [Google Scholar]
- 112.Dash R.C., Ozen Z., McCarthy K.R., Chatterjee N., Harris C.A., Rizzo A.A., Walker G.C., Korzhnev D.M., Hadden M.K. Virtual Pharmacophore Screening Identifies Small-Molecule Inhibitors of the Rev1-CT/RIR Protein-Protein Interaction. ChemMedChem. 2019;14:1610–1617. doi: 10.1002/cmdc.201900307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McPherson K.S., Zaino A.M., Dash R.C., Rizzo A.A., Li Y., Hao B., Bezsonova I., Hadden M.K., Korzhnev D.M. Structure-Based Drug Design of Phenazopyridine Derivatives as Inhibitors of Rev1 Interactions in Translesion Synthesis. ChemMedChem. 2021;16:1126–1132. doi: 10.1002/cmdc.202000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Watanabe N., Mii S., Asai N., Asai M., Niimi K., Ushida K., Kato T., Enomoto A., Ishii H., Takahashi M., et al. The Rev7 Subunit of DNA Polymerase ζ Is Essential for Primordial Germ Cell Maintenance in the Mouse. J. Biol. Chem. 2013;288:10459–10471. doi: 10.1074/jbc.M112.421966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pirouz M., Pilarski S., Kessel M. A critical function of Mad2l2 in primordial germ cell development of mice. PLoS Genet. 2013;9:e1003712. doi: 10.1371/journal.pgen.1003712. [DOI] [PMC free article] [PubMed] [Google Scholar]