Abstract
Cancer remains a leading cause of death worldwide, partly owing to late detection which entails limited and often ineffective therapeutic options. Most cancers lack validated screening procedures, and the ones available disclose several drawbacks, leading to low patient compliance and unnecessary workups, adding up the costs to healthcare systems. Hence, there is a great need for innovative, accurate, and minimally invasive tools for early cancer detection. In recent years, multi-cancer early detection (MCED) tests emerged as a promising screening tool, combining molecular analysis of tumor-related markers present in body fluids with artificial intelligence to simultaneously detect a variety of cancers and further discriminate the underlying cancer type. Herein, we aim to provide a highlight of the variety of strategies currently under development concerning MCED, as well as the major factors which are preventing clinical implementation. Although MCED tests depict great potential for clinical application, large-scale clinical validation studies are still lacking.
Keywords: cancer screening, multi-cancer early detection, MCED, liquid biopsy, biomarkers
1. Introduction
Cancer represents a major public health concern, being the leading cause of death in most countries. Indeed, 10 million deaths and 19.3 million new cancer cases were estimated worldwide in 2020 [1]. This high mortality rate is mostly due to late detection, finding cancer when it has already progressed and metastasized, which significantly reduces effective treatment options. It is estimated that at least 15% of cancer-related deaths within 5 years could be avoided by early disease detection [2]. Hence, cancer screening and early detection should be prioritized, preventing cancer development by removing pre-cancerous lesions and avoiding its progression by effective treatment of localized disease [3,4]. Nonetheless, only a handful of cancer types have recommended screening procedures. The United States Preventive Services Task Force (USPSTF) recommends population-based screening for lung (in high-risk individuals), colorectal, breast, and cervical cancer, while in European countries, only the latter three tumor types have approved screening programs [5,6]. In addition, prostate cancer screening is available in the US, although on an individual basis [7]. Thus, more than 60% of cancer-related deaths are caused by malignancies for which there is no screening test available [1].
Although the adoption of screening programs has indeed contributed to increased survival rates in those cancer types, many factors are hampering screening from reaching higher level efficacy. For instance, lung and breast cancers are detected by low-dose CT and mammography, respectively, which, besides exposing individuals to radiation, eventually lead to some overdiagnosis and false positive results [8,9]. The same applies to cervical and prostate cancer screening, based on cytology/HPV and serum PSA testing, respectively. [10,11]. Contrarily, colonoscopy allows for a very accurate detection of colorectal cancer, as well as its precursor lesions and their subsequent removal. However, it is a rather invasive and uncomfortable procedure, requiring prior preparation, which results in low patient compliance [12] (Figure 1).
Figure 1.
Currently available cancer screening options: mammography for breast cancer; low-dose CT for lung cancer; colonoscopy for colorectal cancer; cytology and HPV testing for cervical cancer; serum PSA testing for prostate cancer. Colored organs represent those with available screening; grey organs represent those without any current screening option (not all cancer types are represented). Created with Biorender.com.
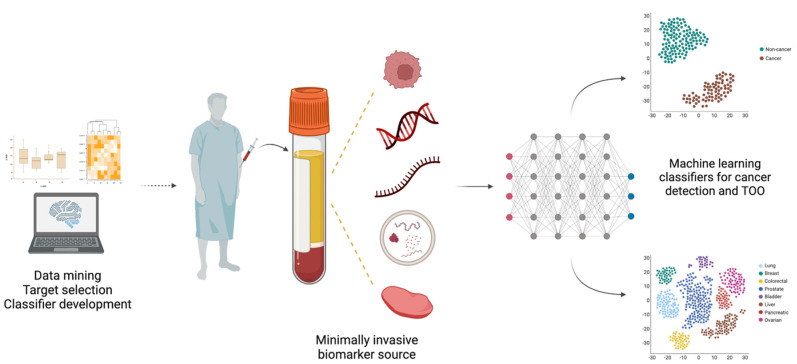
At present, following an abnormal finding in a screening procedure, a tissue biopsy must be conducted for histopathological evaluation and eventual cancer diagnosis. In fact, tissue sampling has been the gold standard approach for cancer diagnosis and prognostication, but several disadvantages can be pointed out to the use of this biological material: (1) it requires an invasive collection procedure; (2) some tumors are not easily accessible due to their anatomical location; (3) it has limited ability to be used as an early detection tool; (4) it has limitations in the evaluation of treatment efficacy and monitoring of tumor progression; and (5) it does not fully represent tumor heterogeneity [13,14]. Thus, minimally invasive techniques allowing for improved disease detection and monitoring are desirable. Recently, liquid biopsies have emerged as tools to overcome these challenges. Consisting in the analysis of disease-related markers from body fluids, such as blood or urine, liquid biopsies comprise a variety of analytes, namely, circulating cell-free DNA (cfDNA), cell-free RNA (cfRNA), circulating tumor cells (CTCs), extracellular vesicles (EVs), tumor-educated platelets (TEPs), proteins and metabolites [15,16]. The analysis of these biomarkers enables the identification of tumor-related information and, consequently, tumor burden real-time monitoring, thereby having great potential to improve routine clinical practice [17]. Furthermore, because tumors shed these analytes into the circulation early in their development, liquid biopsies have the capacity to detect cancer even when symptoms are not present or tumor masses are not detectable by imaging techniques [18,19]. Considering the hurdles faced by current cancer screening paradigms, a blood-based test that might simultaneously detect multiple cancer types, at early stages, and even be applied to high-risk population-based screening, constitutes an exciting and clinically valuable tool. Moreover, a pan-cancer approach might be the only cost-effective option for screening of low prevalent cancers [20]. Ideally, such a multi-cancer early detection (MCED) test should have high sensitivity for early-stage disease detection, high specificity to avoid false-positive results, and the ability to discriminate the tissue of origin (TOO) of the detected cancer [20].
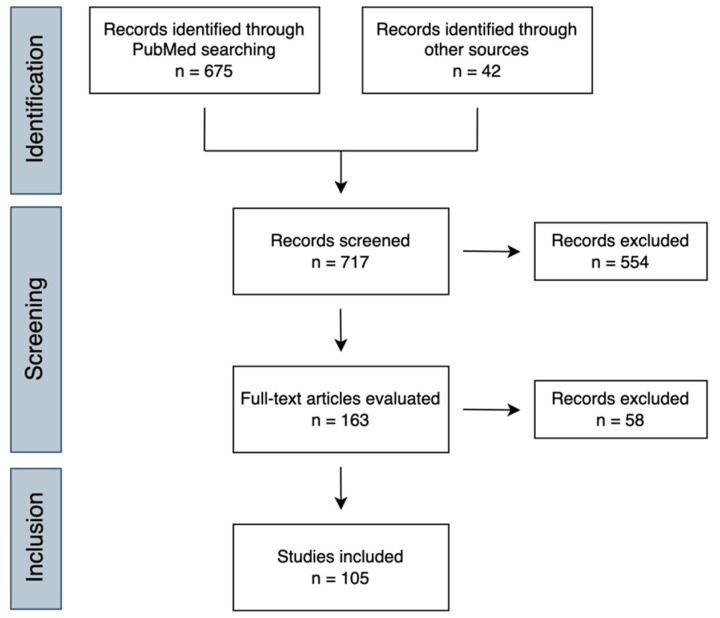
Having this in mind, we conducted a literature review aiming to explore the diversity of strategies currently under development for multi-cancer early detection. Thus, a PubMed search was performed with the query (pan-cancer OR multi-cancer) AND (detection OR screening OR diagnosis) with no time interval restrictions. In total, 675 results were retrieved and imported to Rayyan, an intuitive website for title and abstract screening [21]. Additionally, 42 articles found from other sources were included. All abstracts were critically evaluated to select only those providing relevant information related to the topic of interest. Furthermore, only articles written in English, presenting original data and reporting biomarker performance metrics (AUC, sensitivity, specificity, etc.) were considered. A summary of the methodology is shown in Figure 2. The information gathered from the included studies is displayed in Table 1 and Table 2, showing multi-cancer detection strategies validated in human clinical specimens or based on data mining, respectively. Finally, a search was conducted on the ClinicalTrials.gov webpage to look for relevant clinical studies evaluating MCED tests, and the respective results are shown in Table 3.
Figure 2.
Flow diagram of the conducted search methodology for this review.
Table 1.
Multi-cancer early detection tests validated on human samples.
| Biomarker | Source | Tumor Types | Sample | Methods | Main Findings | Test/ Company |
Ref. |
|---|---|---|---|---|---|---|---|
|
DNA
methylation |
Tissue | lung, breast, colorectal, esophagus, liver, pancreatic, gastric, cervical, head and neck | 120 tumor tissue 123 normal tissue |
Bisulfite pyrosequencing |
TCGA methylation data mining identified HIST1H4F as hypermethylated in 17 tumor types. Methylation analysis in tissue samples of 9 cancer types showed AUCs above 0.87 for all cancers and above 0.90 for all except pancreatic cancer. |
-- | [121] |
| Tissue | lung, breast, colorectal, prostate, pancreas, glioblastoma, and B cell chronic lymphocytic leukemia | 83 tumor tissue 54 normal tissue |
Bisulfite pyrosequencing |
Methylation levels at 27 CpGs of the GHSR gene showed a higher average methylation degree in all tumor samples compared to normal samples. 27 CpG-signature displayed an AUC of 0.8789 for discriminating cancer from normal tissue. |
-- | [122] | |
| Tissue | colorectal, gastric, and esophageal | 229 tumor and normal-adjacent tissue | Bisulfite sequencing PCR |
TCGA methylation data mining identified differentially methylated regions (DMRs) in the SST gene. 7 CpG sites were shown to be hypermethylated in all 3 cancers. A combination of 2 CpGs (+18 and +129) displayed the best AUC of 0.698, with 59.3% sensitivity and 72.8% specificity for detecting the 3 gastrointestinal cancers. |
-- | [123] | |
| Tissue | lung, breast, colon, gastric, and endometrial | 184 tumor tissue 34 normal tissue |
Bisulfite amplicon sequencing |
Designed a 302-bp PCR amplicon, covering the ZNF154 tumor-specific hypermethylated region, and methylation patterns were used to develop a multi-cancer classifier. AUC of 0.96 for discriminating cancer from normal tissue. Computational simulation of ctDNA displayed AUCs of up to 0.79. |
-- | [124] | |
| Plasma | colon, pancreatic, liver, and ovarian | 71 cancer patients 20 healthy individuals |
DREAMing | TCGA methylation data from white blood cells revealed that ZNF154 locus remains unmethylated, even in older individuals, showing the potential for the development of a blood test for cancer detection. AUC values ranged from 0.75 to 0.87 for discriminating cancer patients from healthy individuals, except for liver cancer which displayed an AUC of 0.48. |
-- | [42] | |
| Plasma | lung and prostate | 323 cancer patients 136 healthy individuals |
qMSP | “PanCancer” panel (FOXA1, RARβ2, and RASSF1A) detected cancer with 64.3% sensitivity, 69.8% specificity and 66.4% accuracy. “CancerType” panel (GSTP1 and SOX17) discriminated between lung and prostate cancer with 93% specificity. |
-- | [44] | |
| Plasma | lung, breast, and colorectal |
253 cancer patients 103 healthy individuals |
qMSP | “PanCancer” panel (APC, FOXA1, RASSF1A) detected cancer with 72.4% sensitivity, 73.5% specificity and 72.8% accuracy. “CancerType” panel (SCGB3A1, SEPT9, and SOX17) discriminated TOO with 80.0%, 98.9%, and 85.1% specificity for breast, colorectal, and lung cancer, respectively. |
-- | [43] | |
| Serum | lung, breast, colorectal, gastric, pancreatic, and hepatocellular | 70 cancer patients 10 healthy individuals |
MSP | Methylation levels of a 4 gene-panel (RUNX3, p16, RASSF1A, and CDH1) showed 89% sensitivity and 100% specificity for cancer detection. | -- | [125] | |
| Plasma | colorectal and pancreatic | 60 cancer patients 60 healthy individuals |
Methylation array | Found a 7 gene panel (MDR1, SRBC, VHL, MUC2, RB1, SYK, and GPC3) that detects colorectal and pancreatic cancers with 63.16% sensitivity, 84% specificity, and AUC of 0.8177. | -- | [126] | |
| Plasma | lung, breast, and liver | 46 cancer patients 32 healthy individuals |
Bisulfite sequencing |
Developed CancerLocator, a test based on cfDNA bisulfite sequencing combined with a probabilistic model for cancer detection and TOO discrimination. CancerLocator uses TCGA methylation data as features to estimate the fraction of ctDNA in the plasma and the likelihood of coming from each tumor type. TOO discrimination showed a low error rate of 0.265 (99.7% accuracy). |
Cancer Locator |
[127] | |
| Plasma | liver but applicable to any cancer | 33 cancer patients 36 healthy individuals |
Bisulfite sequencing |
Developed CancerDetector, a test based on cfDNA bisulfite sequencing combined with a probabilistic model that joints methylation states of multiple adjacent CpG sites on an individual sequencing read, for cancer detection. 94.8% sensitivity and 100% specificity were obtained. |
Cancer Detector |
[128] | |
| Plasma | > 50 cancer types | 2482 cancer patients 4207 healthy individuals |
Bisulfite sequencing |
Developed a targeted methylation assay combined with a machine learning classifier for detecting and discriminating TOO in more than 50 cancer types using cfDNA. 54.9% sensitivity and 99.3% specificity were obtained in the validation set. 93% accuracy for TOO prediction. |
Galleri (GRAIL) | [54] | |
| 2823 cancer patients 1254 healthy individuals |
Developed a refined assay and classifiers optimized for screening purposes and performed clinical validation. 51.5% sensitivity and 99.5% specificity were obtained. 88.7% accuracy for TOO prediction. PPV of 44.4% and NPV of 99.4% for cancer detection. |
[55] | |||||
| Plasma | colorectal, hepatocellular, esophageal, gastric, and pancreatic | 254 cancer patients 46 healthy individuals |
Bisulfite sequencing |
Developed EpiPanGI Dx, a cfDNA methylation-based test combining bisulfite sequencing and machine learning, for detecting and discriminating TOO of gastrointestinal cancers. AUC of 0.88 for detecting gastrointestinal cancers. Accuracy of 0.85–0.95 for TOO prediction. |
EpiPanGI Dx | [45] | |
| Plasma | lung, colorectal, gastric, liver, and esophageal | 191 pre-diagnosis cancer samples 223 post-diagnosis cancer samples 414 healthy samples |
Bisulfite sequencing (using semi-targeted PCR libraries) |
Developed PanSeer, a blood test combining the analysis of 477 cancer-specific differentially methylated regions with machine learning for cancer detection. 87.6% sensitivity for post-diagnosis samples, 94.9% sensitivity for pre-diagnosis samples and 96.1% specificity were obtained in the testing set. Cancer can be detected by PanSeer up to 4 years before conventional diagnosis with 95.7% sensitivity. |
PanSeer (Singlera Genomics) |
[49] | |
| Plasma | lung, pancreatic, and acute myeloid leukemia | 137 cancer patients 62 healthy individuals |
cfMeDIP-seq | Developed cfMeDIP-seq, an immunoprecipitation-based protocol for methylation profiling in cfDNA and combined it with machine learning algorithms to discriminate TOO. AUC values ranged from 0.92 to 0.98 for discriminating TOO. |
Adela, Inc. | [64] | |
| Plasma | lung, breast, colorectal, and melanoma | 78 cancer patients 66 healthy individuals |
Bisulfite sequencing |
Developed a targeted methylation sequencing assay to analyze the methylation status of 9 223 cancer related CpG sites, combined with a novel algorithm that converts sequencing data into a methylation score, for cancer detection and TOO discrimination. 83.8% sensitivity and 100% specificity were obtained for cancer detection. 78.9% accuracy for TOO discrimination. |
-- | [129] | |
| Plasma | Lung, breast, colorectal, and liver | Not available | NGS | Developed IvyGeneCORE Test, a blood test analyzing cfDNA methylation levels at specific genes combined with artificial intelligence for cancer detection. 84% sensitivity and 90% specificity were obtained for discriminating cancer from healthy individuals. |
IvyGeneCORE (Laboratory for Advanced Medicine) |
[47] | |
| Plasma | lung, colorectal, pancreatic, liver, esophageal, and ovarian | 625 cancer patients 483 healthy individuals |
ELSA-seq | Developed ELSA-seq, a targeted methylation sequencing assay combined with machine learning for cancer detection and TOO discrimination. 80.6% sensitivity and 98.3% specificity were obtained in validation set. 81.0% accuracy for TOO discrimination. |
OverC (Burning Rock Dx) |
[130] | |
| Plasma | 14 cancer types | 549 cancer patients 80 healthy individuals |
Targeted sequencing |
Developed a cancer detection model based on 37 methylation-correlated blocks (MCB). 72.86% sensitivity, 96.67% specificity, and AUC of 0.86 were obtained in the validation set. |
GENECAST | [131] | |
| Plasma | lung, breast, colorectal, pancreatic, gastric, esophageal, liver, and ovarian | 598 cancer patients 302 healthy individuals |
Targeted sequencing |
Developed a cancer detection and TOO discrimination model based on 135 MCB. 66.3% sensitivity, 95.5% specificity, and AUC of 0.85 were obtained in the validation set. 75.4% accuracy for TOO discrimination. |
[132] | ||
| Plasma | lung, breast, colorectal, and pancreatic | 101 cancer patients 71 healthy individuals |
MSRE-qPCR | Developed a 10-marker panel for cancer detection and a 16-marker panel for TOO discrimination. 79% sensitivity, 90% specificity, and AUC of 0.89 were obtained for cancer detection. TOO discrimination accuracy was 80% for colorectal, 78% for lung, 75% for pancreatic, and 62% for breast cancer. |
Signal-X (Universal Dx) |
[133] | |
| Plasma | Lung, colorectal, bladder, and pancreatic | >1500 cancer patients >1800 healthy individuals |
5mC enrichment and targeted sequencing |
Developed a blood test based on cfDNA methylation signatures for early cancer detection and TOO discrimination. 90% and 87% sensitivity at 90% specificity for stage I/II colorectal and lung cancer detection. 73% and 52% sensitivities at 95% specificity for stage I/II pancreatic and bladder cancer detection. At 98% specificity, TOO accuracy was 99% for colorectal, 94% for lung, 88% for bladder, and 86% for pancreatic cancer. |
LUNAR (Guardant Health) |
[134] | |
| Plasma | lung, breast, colorectal, prostate, pancreatic, liver, and ovarian | 111 cancer patients 55 healthy individuals |
Targeted sequencing |
Developed Omni1, a targeted methylation sequencing panel comprising around 3000 cancer-specific hypermethylation markers for cancer early detection. 65% sensitivity for stage I cancers, 75% sensitivity for stage II cancers, and 89% specificity were obtained. |
Omni1 (Avida Biomed) |
[135] | |
| Plasma | lung, breast, colorectal, gastric, esophageal, and liver | 269 cancer patients 170 healthy individuals |
Bisulfite sequencing |
Developed Aurora, a blood test based on cancer specific cfDNA methylation signatures for detecting 6 major cancer types. AUCs of 0.90, 0.98, and 0.92 were obtained for lung, breast and colorectal cancer detection, respectively. |
Aurora (AnchorDx) |
[136] | |
| 203 cancer patients 206 healthy individuals |
Improved to Aurora 2.0, a targeted methylation sequencing assay. AUCs of 0.94 and 0.935 were obtained for gastric and esophageal cancer detection, respectively. AUCs of 0.973, 0.962, and 0.92 were obtained for lung, breast, and colorectal cancer detection, respectively. |
[137] | |||||
| 1000 cancer patients 505 healthy individuals |
AUCs of 0.973, 0.962 and 0.92, 0.94, and 0.935 were obtained for lung, breast, colorectal, gastric and esophageal cancer detection, respectively. At 99% specificity, 84%, 75%, 82%, 85%, and 78% sensitivity were obtained for lung, breast, colorectal, gastric, and esophageal cancer, respectively. |
[138] | |||||
| Tissue Plasma |
breast, colorectal, prostate, and lymphoma | 72 tumor and 31 normal tissues 100 cancer and 45 healthy plasmas |
Electrochemical assays | Developed electrochemical and colorimetric assays that can detect methylation differences between cancer and healthy genomes based on the level of DNA adsorption on planar and colloidal gold surfaces. DNA adsorption levels could discriminate between cancer patients and healthy individuals with an AUC of 0.887 using an electrochemical assay. DNA adsorption levels could discriminate between cancer patients and healthy individuals with an AUC of 0.785 using a colorimetric assay. |
-- | [65] | |
| Stool | colorectal and gastric | 105 cancer patients 113 healthy individuals |
Hi-SA | Developed a method combining single-step sodium bisulfite modification and fluorescence PCR to measure RASSF2 and SFRP2 methylation status in fecal DNA. DNA recovery from feces showed an AUC of 0.78 for distinguishing cancer from non-advanced lesions (adenomas, polyps and healthy). Methylation levels showed an AUC of 0.78. A combination score showed the best AUC of 0.81. |
-- | [139] | |
|
DNA
methylation and circulating proteins |
Plasma Serum |
lung, pancreatic, gastric, esophageal, liver, and ovarian | 180 cancer patients 257 healthy individuals |
Multiplex PCR and LQAS | Developed a multi-analyte blood test based on 26 methylation markers and 5 circulating proteins combined machine learning algorithms for cancer detection. 83% sensitivity, 94% specificity, and AUC of 0.96 were obtained in the validation set. |
Exact Sciences |
[140] |
| 160 cancer patients 315 healthy individuals |
85% sensitivity, 95% specificity, and AUC of 0.96 were obtained in the validation set. | [141] | |||||
|
DNA
methylation and copy number variations (CNVs) |
Plasma | lung, breast, hepatocellular, nasopharyngeal, smooth muscle sarcoma, and neuroendocrine tumor | 46 cancer patients 32 healthy individuals |
Bisulfite sequencing |
Performed bisulfite sequencing to analyze genome-wide hypomethylation combined with copy number alterations in cfDNA and developed algorithms for cancer detection. If a sample was positive if either hypomethylation or CNAs were observed, 85% sensitivity, and 88% specificity were obtained. If a sample was positive if both hypomethylation and CNAs were observed, 60% sensitivity, and 94% specificity were obtained. |
-- | [142] |
|
DNA
methylation, fragmentation, CNVs and microbial composition |
Plasma | lung, colon, gastric, and liver | 275 cancer patients 204 healthy individuals |
cfMethyl-Seq | Developed CancerRadar, a test based on genome-wide methylation profiling of cfDNA combined with machine learning for cancer detection and TOO discrimination. 85.6% sensitivity and 99% specificity for cancer detection. 91.5% accuracy for TOO discrimination. |
Cancer Radar (Early Diagnostics) |
[76] |
|
DNA
hydroxymethylation |
Plasma | lung, breast, colorectal, gastric, esophageal, and liver | 2241 cancer patients 2289 healthy individuals |
5hmC-Seal profiling | Used the 5hmC-Seal technology to profile genome-wide 5hmC in cfDNA and combined it with machine learning for cancer detection and TOO discrimination. 79.3% sensitivity and 95% specificity were obtained in training set. 67.6% sensitivity and 98.2% specificity were obtained in the testing set. 83.2% accuracy for TOO discrimination. |
Epican Genetech | [67] |
| Plasma | lung, breast, prostate, and pancreatic | 188 cancer patients 180 healthy individuals |
5hmC sequencing |
Developed a novel 5hmC enrichment technology coupled with sequencing and machine learning for cancer detection. AUCs of 0.89, 0.84, 0.95, and 0.83 were obtained for breast, lung, pancreatic and prostate cancer detection, respectively. |
BlueStar Genomics |
[69] | |
|
Genetic
variants |
Plasma | lung, breast, colorectal, prostate, bladder, pancreatic, and liver | 260 cancer patients 415 healthy individuals |
NGS | Developed DEEPGENTM, an assay based on NGS combined with machine learning for cancer detection. 57% sensitivity at 95% specificity, 43% sensitivity at 99% specificity, and AUC of 0.90 were obtained. |
DEEPGEN (Quantgene) |
[28] |
| Stool | colorectal, pancreatic, gastric, biliary, and oropharyngeal | 69 cancer patients 69 healthy individuals |
Digital melt curve method | Identified target mutations in genes commonly mutated in gastrointestinal cancer by sequencing tumor tissues. Target mutation analysis in stool detected cancer with 68% sensitivity and 100% specificity. |
-- | [24] | |
|
Genetic
variants and cfDNA fragmentation |
Plasma | lung, breast, colorectal, GIST, ovarian, Hodgkin lymphoma, diffuse large B-cell lymphoma, and multiple myeloma | 558 cancer patients 367 healthy individuals |
WGS | Developed GIPXplore, a method combining cfDNA whole-genome sequencing profiles with machine learning for cancer detection and TOO discrimination. 92% sensitivity, 98% specificity, and AUC of 0.99 were obtained for discriminating hematological cancers from healthy samples. 85% accuracy for TOO prediction. 55% sensitivity, 95% specificity, and AUC of 0.83 were obtained for discriminating solid cancers from healthy samples. 69% accuracy for TOO prediction. |
-- | [143] |
| Plasma | 17 tumor types | 200 cancer patients 65 healthy individuals |
WGS | Analysis of mutations in size-selected cfDNA fragments improved diagnostic capacity. Combined fragmentation and mutation analysis provided an AUC > 0.99 compared to AUC <0.80 without using fragmentation features. |
-- | [75] | |
|
cfDNA
fragmentation |
Plasma | Lung, colorectal, and liver | 971 cancer patients 243 healthy individuals |
WGS | Used cfDNA fragmentation profiles combined with machine learning for cancer early detection and TOO discrimination. 95.5% sensitivity, 95% specificity, and AUC of 0.983 were obtained. 93.1% accuracy for TOO prediction. |
-- | [72] |
| Plasma | lung, breast, colorectal, pancreatic, gastric, bile duct, and ovarian | 236 cancer patients 245 healthy individuals |
WGS | Developed DELFI, a test based on cfDNA fragmentation patterns combined with machine learning for cancer detection and TOO discrimination. 73% sensitivity, 98% specificity, and AUC of 0.94 were obtained for discriminating cancer from healthy samples. 61% accuracy for TOO prediction. Combining DELFI with mutant ctDNA, sensitivity for cancer detection increased to 91%, and TOO accuracy increased to 75%. |
DELFI (Delfi Diagnostics) |
[74] | |
|
Circulating proteins and cfDNA
mutations |
Plasma | lung, breast, colorectal, pancreas, gastric, liver, esophageal, and ovarian | 1005 cancer patients 812 healthy individuals |
Targeted sequencing and Bead-based immunoassay |
Developed CancerSEEK, a blood test based on cfDNA mutations on 16 genes and 8 circulating proteins combined with machine learning for cancer detection and TOO discrimination. 62% sensitivity, 99% specificity, and AUC of 0.91 were obtained for discriminating cancer from healthy samples. 63% accuracy for TOO prediction. |
CancerSEEK (Exact Sciences) |
[29] |
| -- | 9911 women not previously known to have cancer | Evaluated the feasibility of CancerSEEK testing combined with PET-CT to detect cancer in a prospective cohort. The blood test was considered positive for 134 participants. 127 were further evaluated by PET. 64 showed imaging concerning for cancer. 26 were proven to have cancer by biopsy or other method. 27.1% sensitivity, 98.9% specificity, and 19.4% PPV were obtained for blood testing alone. 15.6% sensitivity, 99.6% specificity, and 28.3% PPV were obtained for blood testing combined with PET. |
[30] | ||||
| Circulating Hsp90α | Plasma | lung, breast, colorectal, stomach, liver, pancreatic, esophageal, and lymphoma | 661 cancer patients 308 non-cancer patients 331 healthy individuals |
ELISA | Hsp90α levels in plasma discriminated cancer from non-cancer controls (healthy + at-risk). AUC of 0.893, 81.72% sensitivity, and 81.03% specificity were obtained in the validation set. |
-- | [144] |
|
Gene
expression |
Whole blood | lung, breast, colorectal, pancreatic, hepatobiliary, and glioblastoma | 228 cancer patients 55 healthy individuals |
RNA sequencing |
Identified 2246 differentially expressed mRNAs in tumor-educated-platelets (TEPs). 1072 mRNAs were selected for developing a machine learning classifier for multi-cancer detection and TOO prediction. 97% sensitivity, 94% specificity, and 96% accuracy were obtained in the validation set. 71% accuracy for TOO prediction. |
thromboDx BV | [86] |
| Bone marrow | leukemias, myelodysplastic syndrome, myeloproliferative neoplasm, and lymphoma | 136 cancer patients | RNA sequencing |
Developed RANKING, a machine learning algorithm applied to RNA-seq data for the identification of hematological cancers. Accuracy of 100% for acute myelocytic leukemia and acute lymphocytic leukemia classification. |
-- | [145] | |
| Whole blood | breast, rectum, colon, esophagus, stomach, thyroid, and uterus | 45 cancer patients 30 healthy individuals |
RNA sequencing |
Developed a machine learning classifier that uses RNA-seq data for cancer detection. Identified 900 differentially expressed genes that were used for constructing the classifier. 0.77 accuracy and 0.72 precision were obtained in the testing set. Another classifier based only on very long intergenic non-coding RNAs (vlincRNAs) outperformed the previous with 0.86 accuracy and precision. vlincRNAs demonstrated superior performance compared with mRNAs for cancer status identification. |
-- | [80] | |
| Whole blood | 22 tumor types | 500 cancer patients 500 non-cancer patients |
qRT-PCR | Developed the HrC scale, using OCT-4A expression in 120 samples (based on fold increase), for cancer detection and staging. 100% sensitivity, 100% specificity, and AUC of 1 were obtained. |
-- | [87] | |
| lncRNA | Serum | 15 tumor types | 900 cancer patients 450 patients with benign conditions 450 healthy individuals |
qRT-PCR | AUC values ranged from 0.826 to 0.966 for discriminating cancer patients from healthy individuals. AUC values ranged from 0.723 to 0.896 for discriminating cancer from benign conditions. LOC553103 expression was not able to discriminating cancer from benign conditions in pancreatic, ovarian and thyroid cancer. |
-- | [81] |
| Serum | 12 tumor types | 360 cancer patients 360 patients with benign conditions 360 healthy individuals |
qRT-PCR | AUC values ranged from 0.833 to 0.967 for discriminating cancer patients from healthy individuals. AUC values ranged from 0.7 to 0.81 for discriminating cancer from benign conditions. BLACAT1 expression was not able to discriminating cancer from benign conditions in breast, ovarian, prostate, and nasopharyngeal cancer. |
-- | [82] | |
| microRNA | Serum | 14 tumor types | 112 cancer patients 48 patients with benign conditions 8 healthy individuals |
qRT-PCR | Higher miR-93 expression was observed for all cancers compared to healthy controls, except for colorectal, bladder, gastric, renal, cervical, and ovarian cancer. AUC values ranged from 0.86 to 1.00, sensitivities from 63% to 100% and specificities from 81% to 100% for discriminating cancer patients from healthy individuals. |
-- | [83] |
| Serum | 13 tumor types | 254 cancer patients 27 healthy individuals |
microRNA chip | miR-1307-3p levels showed 98% sensitivity, 85% specificity, and AUC of 0.98 for cancer detection in the validation set. | -- | [84] | |
| Epitope detection in monocytes (EDIM) | Whole blood | 17 tumor types | 240 cancer patients 117 healthy individuals |
Flow cytometry |
EDIM-TKTL1 test is based on the detection of activated macrophages that present the TKTL1 antigen intracellularly. 94% sensitivity, 81% specificity and AUC of 0.89 were obtained. |
PanTumDetect® (Zyagnum AG) |
[107] |
| oral squamous cell carcinoma, breast, and prostate | 213 cancer patients 74 healthy individuals |
Combination of EDIM-TKTL1 and EDIM-Apo10 tests showed 95.8% sensitivity and 97.3% specificity for cancer detection. | [108] | ||||
| cholangiocellular, pancreatic and colorectal | 62 cancer patients 13 patients with inflammatory conditions 16 healthy individuals |
Combination of EDIM-TKTL1 and EDIM-Apo10 tests showed 100% sensitivity, 96.2% specificity and an AUC of 0.9934 for cancer detection. A positive result was seen for 100% of all cancer patients, 0% of healthy individuals, and 7.7% of individuals with inflammation. |
[109] | ||||
|
Circulating ensembles of tumor-associated cells
(C-ETACs) |
Whole blood | 18 cancer types | 5509 cancer patients 10,625 healthy individuals |
Immuno-staining | C-ETACs were detected in 4944 out of 5509 cancer patients as well as in 255 of the 8493 individuals with no abnormal findings in routine screening procedures. This reflects an 89.8% sensitivity and 97% specificity. C-ETACs were detected in 137 out of 2132 asymptomatic individuals with abnormal findings in routine screening procedures. Assuming that cancer will not clinically manifest in none of the asymptomatic individuals positive for C-ETACs results in a maximum false-positive rate of 3.7%. |
-- | [94] |
| Whole blood | 27 cancer types | 9416 cancer patients 6725 individuals with suspected cancer 13,919 healthy individuals |
Immunocytochemistry | Additional organ-specific markers were profiled aiming to predict TOO. C-ETACs were detected in 91.8% of the 9416 cancer patients. Of the 6725 symptomatic individuals, 6025 were diagnosed with cancer and C-ETACs were detected in 92.6% of these. This resulted in a sensitivity of 92.1%. C-ETACs were undetectable in 13,408 of the 13,919 healthy individuals, resulting in a specificity of 96.3%. 93.1% accuracy for TOO prediction. |
-- | [95] | |
| Circulating tumor cells (CTCs) | Whole blood | lung, colorectal, gastric, liver, and esophageal | 174 cancer patients 32 non-cancer patients 25 healthy individuals |
Magnetic enrichment and immunofluorescence |
CTCs count in cancer patients was significantly higher compared to non-cancer patients with high-risk conditions and healthy individuals (p < 0.001). The average CTCs count was 7.3 for cancer patients, 2.4 for non-cancer patients, and 0.9 for healthy individuals. CTCs count superior to 5 could be indicative of cancer status. |
-- | [92] |
|
Extracellular
vesicles (EVs) |
Plasma | breast, lung, acute myelocytic leukemia, and acute lymphocytic leukemia | 53 cancer patients 15 healthy individuals |
Digital Profiling of Proteins on Individual EV (DPPIE) |
Developed an ultrasensitive assay of digital profiling of proteins on individual EV (DPPIE), based on DNA aptamer recognition of specific EV proteins and rolling circle amplification reactions, that produce fluorescent signals on each single EV. DPPIE showed an AUC of 1.0, with specificity and sensitivity of 100% for carcinomas. |
-- | [104] |
| Serum | lung, breast, prostate, liver, ovarian, and lymphoma | 145 cancer patients 27 healthy individuals |
Thermophoretic aptasensor (TAS) |
Developed TAS, an assay based on DNA aptamer recognition of 7 EV proteins and thermophoretic enrichment for cancer detection and TOO discrimination. 95% sensitivity, 100% specificity were obtained for cancer detection and 68% accuracy for TOO discrimination in the validation set. |
-- | [105] | |
| Plasma | pancreatic, bladder, and ovarian | 139 cancer patients 184 healthy individuals |
Verita™ and bead-based immunoassay | Developed an EV-based blood test combining alternating current electrokinetics (Verita™ System) for EVs isolation, immunoassays for protein quantification, and machine learning algorithms for early cancer detection. 71.2% sensitivity, 99.5% specificity, and AUC of 0.95 were obtained for discriminating cancer from healthy samples. |
-- | [101] | |
| Plasma | 16 cancer types | 77 cancer patients 43 healthy individuals |
Mass spectrometry | Analysis of tumor-specific extracellular vesicles and particles (EVP) proteomes combined with machine learning allowed cancer detection and TOO discrimination. Based on a 47-protein panel, 95% sensitivity and 90% specificity were obtained in the testing set. Based on all 372 tumor-related proteins, 100% sensitivity and 90% specificity were obtained. 30 protein panel discriminated TOO with very high accuracy. |
-- | [103] | |
| Serum | 15 cancer types | 133 cancer patients 45 healthy individuals |
qRT-PCR | Exossomal hTERT expression levels detected cancer with 62% sensitivity and 100% specificity. | -- | [100] | |
| Glycosaminoglycans | Plasma Urine |
14 cancer types | 753 plasma samples (460 cancers and 293 healthy) 559 urine samples (219 cancers and 340 healthy) |
Mass spectrometry | Measured the levels of glycosaminoglycans in plasma and urine samples and combined it with machine learning for cancer detection. AUC of 0.78 was obtained in the validation set for discriminating 5 cancer types from healthy individuals using urine glycosaminoglycans. AUC of 0.84 was obtained in the validation set for discriminating 14 cancer types from healthy individuals using plasma glycosaminoglycans. |
GAGome (Elypta) |
[114] |
| Metabolites | Serum | lung, colorectal, pancreatic, gastric, liver, and thyroid | 950 cancer patients 233 healthy individuals |
Mass spectrometry | Developed a laser desorption/ionization mass spectrometry-based liquid biopsy for multi-cancer detection and classification (MNALCI). MNALCI showed 93% sensitivity and 91% specificity for cancer detection in the internal validation cohort and 84% sensitivity and specificity in the external validation cohort. 92% accuracy for TOO discrimination in the internal validation cohort and 85% in the external cohort. |
-- | [112] |
| Serum | breast, endometrial, cervical, and ovarian | 1119 cancer patients 250 healthy individuals |
Mass spectrometry | Developed a method combining untargeted serum metabolomics with machine learning to identify metabolite signatures that allow early stages cancer detection. 98% sensitivity, 98.3% specificity, and 98% accuracy were obtained. TOO discrimination with 94.4% accuracy for breast, 91.6% for endometrial, 87.6% for cervical, and 92% for ovarian cancer. |
-- | [113] | |
|
Plasma
denaturation profiles |
Plasma | glioma but applicable to any cancer | 84 cancer patients 63 healthy individuals |
Differential scanning fluorimetry |
Applied nanoDSF, a differential scanning fluorimetry method for analyzing protein denaturation profiles, to plasma samples and combined it with a machine learning algorithm for distinguish the denaturation profiles of cancer patients. All 5 machine learning algorithms showed accuracies above 87%. Neural Networks (NN) algorithm performed the best, showing 92% sensitivity, 93% specificity and 92.5% accuracy. |
-- | [146] |
| Metallobalance | Serum | breast, colorectal, prostate, pancreatic, gastric, liver, bile duct, thyroid, ovarian, cervical, and endometrial | 1856 cancer patients 5327 healthy individuals |
Mass spectrometry | Applied a mass spectrometry-based technology to evaluate the serum profile of 17 elements as a cancer detection tool. AUC of 0.830 for discriminating cancer patients from healthy individuals. Classical markers (CEA, CA19-9, CA125, PSA) alone could not discriminating cancer patients from healthy individuals. However, for individuals with a normal CEA reading, the levels of Na, K, Cu, Fe Co, and Mo displayed differences in cancer over healthy samples, and the same applied to the other classical markers. |
-- | [116] |
|
Serum
spectral profile |
Serum | lung, breast, colorectal, prostate, pancreatic, renal, ovarian, and brain | 1543 cancer patients 460 symptomatic non-cancer patients 91 healthy individuals |
Infrared spectroscopy | Developed Dxcover®, an infrared spectroscopy-based blood test for the early detection of cancer and TOO discrimination. 90% sensitivity with 61% specificity (adjusted for higher sensitivity), 56% sensitivity with 91% specificity (adjusted for higher specificity), and AUC of 0.86 were obtained for cancer detection. AUC values ranged from 0.74 to 0.91 for TOO discrimination. |
Dxcover® | [117] |
|
Tumor-
activatable minicircles |
Whole blood | Applicable to any cancer |
-- | Luminescence measurement in the blood | Developed engineered non-viral vectors (minicircles) by coupling SEAP expression to activation of the Survinin promoter, resulting in luminescence production when tumor cells uptake the vectors. Minicircles were injected into tumor-bearing and control mice and SEAP was measured in the blood. AUC of 0.918 was obtained for discriminating cancer from healthy mice. |
-- | [119] |
|
Engineered
macrophages |
Whole blood | Applicable to any cancer |
-- | Luminescence measurement in the blood | Developed engineered macrophages by coupling luciferase expression to activation of the Arginase-1 promoter, resulting in luminescence production when macrophages adopt an M2 tumor-associated phenotype. Engineered macrophages were injected into tumor-bearing and control mice and luciferase was measured in the blood. 100% sensitivity and specificity were obtained for discriminating cancer from healthy mice. |
-- | [120] |
Abbreviations: AUC—Area under ROC curve; cfDNA—cell-free DNA; cfMeDIP-seq—cell-free methylated DNA immunoprecipitation and sequencing; CpG—Cytosine-phosphate-Guanine; ctDNA—circulating tumor DNA; DREAMing—Discrimination of Rare EpiAlleles by Melt; ELISA—enzyme-linked immunosorbent assay; Hi-SA—high-sensitivity assay for bisulfite DNA; LQAS—long probe quantitative amplified signal; MSP—methylation-specific PCR; MSRE-qPCR—methylation-sensitive restriction enzyme -based quantitative PCR; NGS—next generation sequencing; NPV—negative predictive value; PPV—positive predictive value; qMSP—quantitative methylation-specific PCR; qRT-PCR—real-time quantitative reverse transcription PCR; TCGA—The Cancer Genome Atlas; TOO—tissue of origin; WGS—whole genome sequencing; 5hmC—5-hydroxymethylcytosine; 5mC—5-methylcytosine.
Table 2.
Data mining studies of multi-cancer early detection.
| Biomarker | Database | Tumor Types | Main Findings | Ref. |
|---|---|---|---|---|
|
DNA
methylation |
TCGA GEO |
26 tumor types | Identified 7 informative CpG sites capable of discriminating tumor from normal samples. AUC of 0.986 was obtained in the training set. Validation using GEO datasets of breast, colorectal cancer, and prostate cancer obtained AUCs of 0.97, 0.95, and 0.93, respectively. Validation set comprising the remaining cancer types obtained an AUC of 0.94. Identified 12 CpG sites capable of discriminating each tumor type with an AUC of 0.98. |
[168] |
| TCGA GEO |
27 tumor types | Identified 12 CpG markers and 13 promoter markers and constructed diagnostic models by deep learning. CpG marker model achieved 98.1% sensitivity, 99.5% specificity, and 98.5% accuracy on training set, while achieving 92.8% sensitivity, 90.1% specificity, and 92.4% accuracy on testing set. Promoter marker model achieved 96.9% sensitivity, 99.9% specificity, and 97.8% accuracy on testing set, while achieving 89.8% sensitivity, 81.1% specificity, and 88.3% accuracy on testing set. |
[156] | |
| TCGA GEO |
27 tumor types | Developed the CAncer Cell-of-Origin (CACO) methylation panel comprising 2 572 cytosines that are significantly hypermethylated in tumor tissues compared with normal tissues and healthy blood samples. CACO panel identified TOO with AUC ranging from 0.856 to 0.998 in discovery cohort and 0.854 to 0.998 in validation cohort. CACO panel could identify TOO in liquid biopsies and unknown primary carcinoma samples. |
[169] | |
| TCGA | 14 tumor types | Combined genome-wide differential methylation profiling with machine learning to detect cancer and discriminate TOO. Set of 4 CpGs detected cancer with an AUC of 0.95 in the discovery set and an AUC of 0.96 in the validation set. Set of 20 CpGs discriminated TOO with AUC values ranging from 0.87 to 0.99; 12 out of 14 cancer types were discriminated with sensitivities and specificities above 90%. |
[157] | |
| TCGA GEO |
3 tumor types | Developed a machine learning algorithm to detect and discriminate TOO in 3 urological cancers (prostate, bladder, and kidney) using 128 methylation markers. 99.1% accuracy in training set; 97.6% accuracy in 2 independent validation sets. |
[170] | |
| TCGA GEO |
33 tumor types | Identified a 12-market set that can detect all 33 cancers in TCGA database with AUCs > 0.84. Identified sets of 6 markers that can discriminate TOO with AUCs ranging from 0.969 to 1. |
[171] | |
| TCGA | 12 tumor types | While performing genome-wide methylation analysis for pancreatic cancer biomarker discovery, identified SST as hypermethylated in pancreatic tumors compared to normal tissue and showed an AUC of 0.89 for pancreatic cancer detection in cfDNA. SST methylation and expression in 11 other cancer types showed significant hypermethylation and downregulation of expression when compared to the respective normal tissue (p < 0.0001). |
[154] | |
| TCGA GEO |
14 tumor types | Identified 6 CpGs in the GSDME gene differentially methylated between tumor and normal samples and used them for developing a machine learning algorithm for cancer identification. 98.8% sensitivity, 94.2% specificity, and AUC of 0.86 in the training set. AUC of 0.85 in validation set. 6 CpG model showed TOO discrimination capacity. |
[172] | |
|
DNA
methylation, gene expression and somatic mutations |
TCGA | 13 tumor types | Developed EAGLING, a model that expands the Illumina 450K array data to cover about 30% of CpGs in the genome. Used this expanded methylation data combined with gene expression and somatic mutation data to identify genes with differential patterns in various cancer types (triple-evidenced genes). Developed a machine learning algorithm, using the identified triple-evidenced genes, for cancer detection. AUC of 0.85 was obtained; 95.3% accuracy was obtained for TOO discrimination. TNXB, RRM2, CELSR3, SLC16A3, FANCI, MMP9, MMP11, SIK1, and TRIM59 showed great capacity for cancer diagnosis. |
[158] |
| Gene mutations | TCGA | 5 tumor types | Based on a tumor’s mutations and their respective GO terms and KEGG pathways, a machine learning algorithm was developed for TOO discrimination; 62% accuracy was obtained for discriminating TOO in 5 cancer types. | [173] |
| Gene expression | GEO | 10 tumor types | Developed a deep learning classifier for multi-cancer diagnosis using transcriptomic data termed DeepDCancer. 90% accuracy was obtained for distinguishing cancer from normal samples, while accuracies ranged from 86 to 98% (94% average) for discriminating individual cancer types. 96% accuracy was obtained for distinguishing cancer from normal samples using an improved classifier, DeepDCancer. |
[159] |
| TCGA | 40 tumor types | Developed SCOPE, a machine learning algorithm that uses RNA-seq data for TOO prediction. SCOPE achieved 97% accuracy in training set and 99% in testing set. SCOPE showed the ability to identify TOO in cancers of unknown primary. |
[174] | |
| TCGA | 11 tumor types | Developed GeneCT, a deep learning algorithm that uses RNA-seq data for cancer identification and TOO prediction. Known cancer-related genes were used for cancer status identification and transcription factors for TOO prediction. 100% sensitivity and 99.6% specificity for cancer identification in training set. 96.0% sensitivity and 96.1 specificity for cancer identification in validation set. 99.6% accuracy for TOO prediction in training set and 98.6% in validation set. |
[160] | |
| TCGA | 33 tumor types | 5 machine learning algorithms were compared on their performance for cancer classification. Linear support vector machine (SVM) showed the best accuracy of 95.8%. |
[175] | |
| TCGA | 5 tumor types | Developed a deep learning model for TOO discrimination using RNA-seq data among the 5 most common cancers in women. LASSO feature selection reduced all 14,899 genes to only 173 relevant genes. 99.45% accuracy was obtained for discriminating TOO in 5 cancer types. |
[176] | |
| TCGA GTEx |
28 tumor types | Identified differentially expressed genes (DEGs) that were shared in various cancer types and constructed a diagnostic model using 10 upregulated DEGs (CCNA2, CDK1, CCNB1, CDC20, TOP2A, BUB1B, AURKB, NCAPG, CDC45, and TTK). AUC of 0.894 was obtained for discriminating cancer from normal samples. |
[177] | |
| TCGA | 15 tumor types |
MMP11 and MMP13 expression was significantly higher in most cancer types compared to tissue matched controls. Each cancer type featured at least one MMP with an AUC greater than 0.9, except prostate cancer; 6 cancer types featured 4 or more MMPs with AUC > 0.9. If serum detection is possible, upregulated MMP11 or MMP13 could serve as a multi-cancer biomarker. |
[178] | |
| TCGA | 9 tumor types | Hsp90α expression was significantly higher in 8 cancers compared to tissue matched controls, except for prostate cancer which displayed significant lower expression. AUC values ranged from 0.63 to 0.94 for individual cancer types. |
[155] | |
| TCGA GTEx |
33 tumor types | Claudin-6 was significantly overexpressed in 20 cancer types. AUC > 0.7 were obtained for detecting 15 cancer types. AUC > 0.9 were obtained for detecting acute myeloid leukemia, testicular, ovarian, and uterine cancer. |
[179] | |
| TCGA GTEx |
33 tumor types |
YTHDC2 expression was significantly downregulated in most cancers compared with normal tissues. YTHDC2 displayed high diagnostic value (AUC > 0.90) for 7 cancer types and moderate diagnostic value (AUC > 0.723) in 8 cancer types. |
[180] | |
| TCGA GTEx |
24 tumor types |
PAFAH1B expression was significantly upregulated in most cancers compared with normal tissues. PAFAH1B displayed high diagnostic value (AUC > 0.90) for 15 cancer types and moderate diagnostic value (AUC > 0.75) in 9 cancer types. |
[181] | |
| TCGA GTEx |
20 tumor types |
SHC1 expression was significantly upregulated in most cancers compared with normal tissues. SHC1 displayed high diagnostic value (AUC > 0.90) for 4 cancer types and moderate diagnostic value (AUC > 0.70) in 16 cancer types. Strong diagnostic capability for KICH (AUC = 0.92), LIHC (AUC = 0.95), and PAAD (AUC = 0.95). |
[182] | |
| TCGA GTEx |
29 tumor types |
GPC2 expression was significantly upregulated in 12 early-stage cancers compared with normal tissues. GPC2 displayed high diagnostic value (AUC > 0.90) for 6 cancer types, moderate diagnostic value (AUC > 0.70) in 16 cancer types, and low diagnostic value (AUC > 0.50) in 7 cancer types. |
[183] | |
| ncRNA | TCGA | 26 tumor types | Developed algorithms to remove all the factor effects (genetic, epidemiological, and environmental variables) from big data and revealed 56 ncRNAs as universal markers for 26 cancer types. Used these 56 ncRNAs as markers and employed machine learning algorithms to discriminating cancer from normal samples and identify TOO. AUC of 0.963 for discriminating cancer from normal samples. AUC values ranged from 0.99 to 1 for detecting individual cancer types. 82.15% accuracy for discriminating TOO. |
[161] |
| lncRNA | TCGA GEO |
9 tumor types | CRNDE expression was significantly higher in 9 cancers compared to tissue matched controls. AUC values ranged from 0.855 to 0.984, sensitivities from 70 to 97% and specificities from 75 to 100%. Meta-analysis from 6 studies showed a pooled sensitivity of 77%, specificity of 90%, and AUC of 0.87. |
[184] |
| TCGA GEO |
12 tumor types | Identified 6 differently expressed long intergenic noncoding RNAs (lincRNAs) (PCAN-1 to PCAN-6) and applied machine learning algorithms for cancer detection using 5 of them. AUC of 0.947 was obtained in the training set. AUC of 0.947, 81.7% sensitivity, and 97% specificity were obtained in the testing set. |
[185] | |
| TCGA GEO |
8 tumor types | Using RNA-seq and methylation data from TCGA, identified 9 epigenetically regulated lncRNAs (lncRNAs regulated by methylation) that can predict cancer. Developed a score based on expression and methylation data of these 9 genes (PVT1, PSMD5-AS1, FAM83H-AS1, MIR4458HG, HCP5, GAS5, CTD2201E18.3, HCG11, and AC016747.3) that was applied to all cancer and normal samples. AUC values ranged from 0.741 to 0.992 for detecting 8 cancer types. AUC values ranged from 0.712 to 1 in an independent validation set. |
[186] | |
| TCGA | 33 tumor types | SNHG3 expression was significantly upregulated in 16 (out of 33) cancers compared with normal tissues. 72% sensitivity, 87% specificity, and an AUC of 0.89 was observed for cancer detection. |
[187] | |
| microRNA | TCGA | 21 tumor types | Used machine learning algorithms to develop a multi-cancer diagnostic method based on microRNA expression. Support vector machine (SVM) classifier was chosen, since it provided the highest accuracy of 97.2%, sensitivities over 90%, and specificities of 100% for most cancers. | [188] |
| GEO | 11 tumor types | Developed a computational pipeline for extracellular microRNA-based cancer detection and classification. All classifiers showed accuracies over 95%. SVM classifier performed the best, with 99% accuracy. Identified a 10 microRNA-signature capable of TOO discrimination. |
[162] | |
| TCGA | 4 tumor types | Identified 3 differentially expressed miRNAs (miR-552, miR-490, and miR-133a-2) with diagnostic potential for digestive tract cancers. 3 miRNAs showed high diagnostic value in rectal cancer (AUC > 0.961) and moderate diagnostic value in esophageal (AUC > 0.826), gastric (AUC > 0.798), and colon cancer (AUC > 0.797). |
[189] | |
| GEO | 12 tumor types | Developed a serum-based 4-microRNA diagnostic model (has-miR-5100, has-miR-1343-3hashsa-miR-1290hasnd hsa-miR-4787-3p) for cancer early detection. Sensitivities ranging from 83.2 to 100% for biliary tract, bladder, colorectal, esophageal, gastric, glioma, liver, pancreatic, and prostate cancers were obtained, while reasonable sensitivities of 68.2 and 72.0% for ovarian cancer and sarcoma, respectively, with 99.3% specificity. |
[190] | |
| GEO | 12 tumor types | Developed a m6A target miRNAs serum signature, based on 18 microRNAs combined with machine learning, for cancer detection. 93.9% sensitivity, 93.3% specificity, and AUC of 0.979 in training set. 94.2% sensitivity, 91.6% specificity, and AUC of 0.976 in internal validation set. 90.8% sensitivity, 84.7% specificity, and AUC of 0.936 in external validation set. |
[191] | |
| Progenitorness score | TCGA GEO |
17 tumor types | Selected 77 progenitor genes and formulated a score to quantify the progenitorness of a sample using its expression profile data. Tumor samples showed significantly higher progenitorness scores than normal tissues for all cancer types, with AUC ranging from 0.746 to 1.000. For the majority of cancers, AUC was above 0.90. |
[192] |
Table 3.
Clinical trials conducted/ongoing for validation of multi-cancer early detection tests.
| Trial ID | Trial Name | MCED Test | Sponsor | Status (as of July 2022) |
|---|---|---|---|---|
| NCT02889978 | CCGA | Galleri | GRAIL, LLC | Active, not recruiting |
| NCT03085888 | STRIVE | Galleri | GRAIL, LLC | Active, not recruiting |
| NCT03934866 | SUMMIT | Galleri | University College London and GRAIL | Active, not recruiting |
| NCT04241796 | PATHFINDER | Galleri | GRAIL, LLC | Active, not recruiting |
| NCT05155605 | PATHFINDER 2 | Galleri | GRAIL, LLC | Recruiting |
| NCT05205967 | REFLECTION | Galleri | GRAIL, LLC | Recruiting |
| NCT05235009 | LEV87A | GAGome | Elypta | Recruiting |
| NCT05295017 | LEV93A | GAGome | Elypta | Recruiting |
| NCT05227534 | PREVENT | OverC | Guangzhou Burning Rock Dx | Recruiting |
| NCT04825834 | DELFI-L101 | DELFI | Delfi Diagnostics Inc. | Recruiting |
| NCT04213326 | ASCEND | CancerSEEK | Exact Sciences | Completed |
| NCT03756597 | PAN | ReCIVA | Owlstone Ltd. | Unknown |
| NCT03517332 | -- | DEEPGEN | Quantgene Inc. | Unknown |
| NCT03967652 | -- | Na-nose | Anhui Medical University | Not yet recruiting |
| NCT05366881 | CAMPERR | -- | Adela, Inc. | Recruiting |
| NCT05254834 | Vallania | -- | Freenome Holdings Inc. | Recruiting |
| NCT04972201 | PROMISE | -- | Chinese Academy of Medical Sciences (and Burning Rock Dx) | Recruiting |
| NCT04822792 | PRESCIENT | -- | Chinese Academy of Medical Sciences (and Burning Rock Dx) | Recruiting |
| NCT04820868 | THUNDER | -- | Shanghai Zhongshan Hospital (and Burning Rock Dx) | Recruiting |
| NCT04817306 | PREDICT | -- | Shanghai Zhongshan Hospital (and Burning Rock Dx) | Recruiting |
| NCT05227261 | K-DETEK | -- | Gene Solutions | Recruiting |
| NCT05159544 | FuSion | -- | Singlera Genomics Inc. | Recruiting |
| NCT04405557 | PREDICT | -- | Geneplus-Beijing Co. | Active, not recruiting |
| NCT02662621 | EXODIAG | -- | Centre Georges Francois Leclerc | Completed |
| NCT04197414 | -- | -- | Yonsei University | Recruiting |
| NCT03951428 | -- | -- | LifeStory Health Inc. | Unknown |
| NCT03869814 | -- | -- | Bluestar Genomics Inc. | Active, not recruiting |
| NCT02612350 | -- | -- | Pathway Genomics | Completed |
2. Multi-Cancer Early Detection (MCED) Tests: State of the Art
2.1. Mutation-Based MCED Tests
Molecular profiling of driver mutations in tumor tissue has been the main strategy to assess cancer prognosis, treatment response monitoring, and resistance detection, as well as to detect disease recurrence. Accordingly, the current major clinical application of liquid biopsies is the detection of these mutations in tumor-derived cfDNA, i.e., circulating tumor DNA (ctDNA), to replace multiple puncturing with multiple blood draws [22,23]. Not surprisingly, MCED strategies have also relied on the detection of tumor-specific genetic variants in body fluids. As early as 2009, Zou et al. performed targeted mutation analysis in stool from several gastrointestinal cancer patients and showed that pan-gastrointestinal cancer detection was feasible with 68% sensitivity and 100% specificity [24]. In fact, stool is also a non-invasive source of cancer biomarkers but mostly limited to tumors of the digestive system. Interestingly, a study evaluating patients’ perceptions about stool-based multi-cancer detection reported that 98% of participants would use such a test, preferring it over conventional colorectal cancer screening, and highlighted its pan-cancer feature as the most relevant [25]. Subsequently, Quantgene Inc. developed DEEPGENTM, a blood test based on next-generation sequencing (NGS) that detects low-frequency genetic abnormalities at a variant allele frequency of 0.09% [26,27]. When applied to the detection of seven cancer types, this assay displayed 43% sensitivity at 99% specificity with an area under ROC curve (AUC) of 0.90. Remarkably, an AUC of 0.88 was obtained for stage I cancer detection [28]. Cohen et al. also reported another blood test, CancerSEEK, for detecting eight common cancers (lung, breast, colorectal, pancreatic, gastric, hepatic, esophageal, and ovarian) based on the analysis of mutations in 16 genes combined with the circulating levels of eight proteins. Methodologically, this test consists of a multiplex PCR and a single immunoassay, constituting a simple workflow, easily applicable to clinical practice, with an estimated price of around USD 500. When applied to 1005 cancer patients and 812 healthy controls, CancerSEEK disclosed 62% sensitivity at a specificity greater than 99% for discriminating cancer from healthy samples. Concerning early-stage detection, a median sensitivity of 43% was observed for stage I, 73% for stage II, and 78% for stage III. Additionally, TOO discrimination was accomplished with 63% accuracy [29]. However, it is noteworthy that protein biomarkers were the major contributors to cancer type identification following a positive test result. A refined version of CancerSEEK was then developed in combination with PET-CT imaging to evaluate the test performance for prospectively detecting cancer in a study (DETECT-A) involving 10,006 women not known to harbor cancer. For that purpose, participants were blood tested, and, if abnormal, a second blood collection was conducted for confirmation and, if confirmed positive, a full body PET-CT was performed. Test results were considered positive for 134 participants, out of which 127 were further evaluated by PET. Sixty-four depicted suspicious imaging findings and 26 were proven to have cancer. This resulted in 27.1% sensitivity and 98.9% specificity for blood testing alone, while sensitivity decreased to 15.6% and specificity increased to 99.6% for blood testing combined with PET-CT imaging [30].
Therefore, although mutation-based MCED tests have demonstrated great capacity for cancer detection, even in early stages, these might not be the ideal standalone approach, since accurate TOO identification is difficult, due to a lack of tissue-specific gene driver mutations [31]. In fact, TOO discrimination is an essential feature of a MCED test, otherwise, individuals with a positive test would have to undergo additional costly exams for full body examination, instead of a confirmatory localized search [32,33]. Contrarily, epigenetic signatures are unique to each differentiated cell type, regulating its gene expression profile, thereby constituting a cell- and tissue-specific trait [34]. Indeed, DNA methylation patterns have demonstrated the capacity to distinguish tumor types in tissue samples [35] and also body fluids [36,37], as cfDNA fragments carry the methylation patterns of their cell of origin.
2.2. DNA Methylation-Based MCED Tests
DNA methylation, the most well studied epigenetic mechanism, consists in the addition of a methyl group to the 5-carbon of cytosines within CpG dinucleotides. While most CpG dinucleotides are scattered across gene coding regions and repetitive sequences, CpG clusters can be found in the so-called CpG islands, which are mostly present in gene promoters and first exons. In normal cells, CpG islands tend to be unmethylated, while coding and repetitive sequences are methylated. However, this methylation pattern is reversed in cancer cells, with promoters becoming hypermethylated, leading to tumor suppressor genes silencing, along with global hypomethylation, entailing genomic instability [38,39,40]. This aberrant methylation is thought to occur very early in the carcinogenic process, rendering DNA methylation an attractive biomarker for early cancer detection, alongside its TOO discrimination capacity and easy access through liquid biopsies [41]. Remarkably, about 50% of the studies selected for this review (Table 1) used DNA methylation as their approach for MCED.
Whether analyzing a single gene [42] or gene panels [43,44], cfDNA methylation levels have demonstrated the feasibility of using minimally invasive procedures to detect multiple cancers and further identify their anatomical location. Nonetheless, these approaches fall short regarding sensitivity values. Moreover, sequencing-based methylation profiling of cfDNA has shown more promising results, through the use of machine learning algorithms that convert the complex data acquired into classifiers that discriminate cancer from healthy individuals and further identify its origin. For instance, Kandimalla et al. reported EpiPanGI Dx, an assay that simultaneously detected gastrointestinal cancers with an AUC of 0.88 and 85–95% accuracy for TOO prediction [45]. Focusing on four major cancers (lung, breast, colorectal, and liver), the IvyGeneCORE® Test developed by the Laboratory for Advanced Medicine demonstrated that methylation analysis of target genes discovered by data mining could detect these cancers with 84% sensitivity and 90% specificity [46,47]. Similarly, the PanSeer assay developed by Singlera Genomics [48] uses semi-targeted PCR libraries followed by sequencing for analyzing 477 differentially methylated regions (DMRs). This blood test was evaluated using samples from the Taizhou Longitudinal Study, in which healthy individuals provided plasma samples and were monitored for cancer development, allowing for a retrospective take on early detection viability. Concerning five tumor types (lung, colorectal, gastric, liver, and esophageal), 87.6% sensitivity and 96.1% specificity were observed, with similar sensitivity between early- and late-stage disease. Remarkably, using pre-diagnostic samples, PanSeer showed that cancer may be detected up to 4 years before medical diagnosis with 95.7% sensitivity [49]. Nevertheless, no results regarding TOO prediction were reported. At the time of writing, a clinical trial sponsored by Singlera Genomics (NCT05159544) was recruiting for a prospective study aiming to evaluate a multi-omics blood test for pan-cancer screening (Table 3).
A company that revolutionized the cancer screening paradigm and emphasized the wide variety of cancers that can be simultaneously detected through liquid biopsy is GRAIL, a spin-off of Illumina, that received around USD 1 billion in funding for the sole goal of developing a blood test for early cancer detection [50,51]. For such purpose, the Circulating Cell-free Genome Atlas Study (CCGA) (NCT02889978), divided into three sub-studies, was conducted and recruited over 15000 participants with and without cancer that were longitudinally followed up. In the first CCGA sub-study, three different sequencing assays were evaluated and, ultimately, whole-genome bisulfite sequencing outperformed whole-genome sequencing and targeted mutation analysis, demonstrating, once more, the superiority of DNA methylation analysis for early cancer detection [52,53]. Therefore, in the second sub-study, a targeted methylation assay was developed, trained, and validated using 6689 participants, for simultaneous detection and TOO discrimination of more than 50 cancer types. In this study, 54.9% sensitivity and 99.3% specificity were disclosed for all cancer stages, whereas 43.9% sensitivity was observed in early stages. Furthermore, when focusing on a set of 12 high-signal cancers (based on Surveillance, Epidemiology, and End Results (SEER) mortality data) sensitivity was 67.3%. Notably, 93% accuracy was displayed for TOO localization [54]. In the third and final sub-study, carried out to further validate an improved test version specific for screening purposes, an independent validation set of 5309 participants was used and resulted in 51.5% sensitivity, 99.5% specificity, and 88.7% accuracy for TOO prediction [55]. Considering the prospective nature of CCGA, the prognostic value of this blood test was also assessed. By following-up cancer patients from the second sub-study for 3 years, it was observed that cancers not detected by the test had significantly better overall survival (OS) than those detected by the MCED test. Additionally, detection sensitivity was higher for participants who died than in those who were alive, indicating that this test may improve the detection of aggressive cancers, thus being less prone to overdiagnosis [56]. Currently, this blood test is commercially available as Galleri® at the price of USD 949, upon request to health care providers [57]. In addition to CCGA, other clinical trials are being conducted by GRAIL to ripen the tests’ potential as a screening tool (Table 3): STRIVE (NCT03085888) is evaluating the test performance to detect breast and other invasive cancers in women undergoing screening mammography; SUMMIT (NCT03934866) is evaluating the test performance to detect invasive cancers in individuals at high risk of lung and other cancers due to a significant smoking history; PATHFINDER (NCT04241796, NCT05155605) is assessing the implementation of the test in clinical practice; REFLECTION (NCT05205967) aims to understand the performance of the test in specific clinical settings and its impact on patients and healthcare professionals. Some results from the PATHFINDER study have already been reported. Aiming to evaluate the time and number of additional procedures required to achieve a final diagnosis following a positive test result, it was observed that a cancer signal was detected in 1.5% of participants, of which 65% reached a diagnostic resolution. The median time for diagnosis was 78 days, with 93% of participants undergoing imaging tests and 72% being submitted to an invasive diagnostic procedure. Remarkably, only 18% of participants with a final non-cancer diagnosis had to go through an invasive diagnostic procedure [58,59].
Most PCR- and sequencing-based methods for methylation analysis rely on sodium-bisulfite modification and it has been proven that this chemical treatment causes DNA degradation and fragmentation, hindering the analysis of large CpG islands, especially in cfDNA which is already highly fragmented [60]. As an alternative, immunoprecipitation of methylated DNA (MeDIP), i.e., the use of antibodies that target 5-methylcytosine (5mC) for the enrichment of methylated DNA fragments, followed by sequencing can be used [61]. Following such reasoning, Adela Inc. is developing a sensitive technology for the enrichment of methylated fragments from low input samples, like cfDNA, followed by sequencing of cancer-related regions (cfMeDIP-seq) [62,63]. When applied to cancer detection, by combining the above-described assay with machine learning, AUC values of 0.980, 0.918, 0.971, and 0.969 were depicted for discriminating acute myeloid leukemia, pancreatic cancer, lung cancer, and healthy individuals, respectively. Moreover, early- and late-stage cancer detection depicted similar values [64]. Interestingly, the CAMPERR study (NCT05366881) was, at the time of writing, recruiting patients with any of 20 tumor types, plus healthy individuals to validate the cfMeDIP-seq assay (Table 3).
Several other methylation-based MCED tests using a variety of methodologies are being currently developed by different companies (Table 1). Many of them are also conducting clinical trials for prospective assessment of test performance (Table 3).
Remarkably, methylation analysis showed potential for cancer detection even beyond its molecular analysis. Aberrant DNA methylation patterns in cancer also modify the physicochemical properties of DNA, which led Sina et al. to develop simple, fast analysis and low-input electrochemical and colorimetric assays, achieving AUC values of 0.887 and 0.785 in differentiating breast and colorectal cancer from control plasma samples, respectively [65]. Nonetheless, as only advanced-stage samples were used, although promising, these prototypes require validation in early-stage cancer as well as in more tumor types.
In addition to 5mC, 5-hydroxymethylcytosine (5hmC), another DNA pyrimidine base resulting from 5mC oxidation catalyzed by Ten-Eleven Translocation (TET) enzymes [66], was also proposed as a pan-cancer biomarker by Li et al. [67]. Using genome-wide 5hmC analysis, 67.6% sensitivity and 98.2% specificity were attained for cancer detection and 83.2% accuracy for TOO discrimination in six cancer types [67]. Additionally, BlueStar Genomics is also conducting a study (NCT03869814) for the development of a 5hmC-based MCED test and has already reported some promising preliminary results [68,69].
2.3. Fragmentation-Based MCED Tests
The entire population of cfDNA found in the blood of an individual may arise from a wide variety of cell types and its proportions are also dependent on the physiological status. The cfDNA of a healthy individual is primarily derived from dead blood cells, whereas a pathological tissue, such as a tumor tissue, may contribute and release larger amounts of DNA into the circulation [70]. Furthermore, the mechanisms of cell death causing DNA shedding are variable, reflecting different fragmentation patterns, which is also a cell- and tissue-dependent mechanism, reflecting nucleosome positioning in the nucleus [31,70,71]. Thereby, tumor-derived cfDNA fragments carry distinct features that may allow for cancer detection and further TOO identification.
Indeed, this has been confirmed by Bao et al., who showed that combining machine learning algorithms with cfDNA fragmentation profiles enabled lung, colorectal, and liver cancer detection with 95.5% sensitivity and 95% specificity, as well as 93.1% accuracy for TOO prediction, with consistent results even for early-stage and small-size tumors [72]. In this vein, DELFI Diagnostics developed the DELFI assay, which, using genome-wide fragmentation analysis in 236 cancer patients (lung, breast, colorectal, pancreatic, gastric, bile duct, and ovarian) and 245 healthy individuals, displayed 73% sensitivity and 98% specificity for discriminating cancer from healthy subjects, and 61% accuracy for TOO [73,74]. Notably, when combining mutation analysis with fragmentation, DELFI showed an increase in sensitivity to 91% and of TOO accuracy to 75% [74]. Similarly, Mouliere et al. also reported that mutation analysis in size-selected cfDNA fragments detected several cancer types with an AUC over 0.99 [75]. Interestingly, CancerRadar, a multi-omics approach combining cfDNA fragmentation with methylation, copy number variations, and microbial composition depicted a remarkable 85.6% sensitivity and 99% specificity for lung, colon, gastric, and liver cancer detection and 91.5% accuracy for TOO [76].
2.4. Gene Expression/Non-Coding RNA-Based MCED Tests
Given their potential as minimally invasive biomarkers for several disorders, the identification of cfRNAs has attracted significant interest in recent years. Circulating microRNAs (miRs) have been the primary focus of cfRNA studies, due to their high abundance and stability in body fluids, as they are often protected by protein complexes and/or within EVs cargo. However, only a small number of miRs exhibit tissue-specificity. Contrarily, messenger RNA (mRNA) and long non-coding RNA (lncRNA) disclose numerous tissue- and disease-specific gene expression patterns and constitute a larger portion of the transcriptome, being easily assessed through RNA sequencing (RNA-seq) [77,78,79].
Supporting the evidence for MCED using whole-transcriptome data, Qi et al. performed RNA-seq in blood samples of 45 cancer patients and 30 healthy individuals and identified 900 differentially expressed genes that were used for constructing a machine learning classifier, which resulted in 0.77 accuracy and 0.72 precision for detecting seven tumor types. Interestingly, when considering only very long intergenic non-coding RNAs (vlincRNAs), the classifier showed 0.86 accuracy and precision, outperforming mRNA-based cancer detection [80]. Other lncRNAs, such as LOC553103 and BLACAT1, also showed the capacity for pan-cancer detection with AUC values ranging from 0.826 to 0.966 and 0.833 to 0.967 for individual cancer types, respectively. Furthermore, discriminating cancer from benign conditions was also achievable in some cases [81,82]. Concerning microRNAs, circulating miR-93 levels were able to detect a variety of different malignancies with 63% to 100% sensitivity and of 81% to 100% specificity for individual cancer types [83]. Moreover, miR-1307-3p also showed 98% sensitivity and 85% specificity in discriminating 13 cancer types from healthy individuals [84]. One advantage of these single-target approaches is that only a simple quantitative PCR reaction is needed, thus favoring clinical implementation.
It has been known for a long time that platelets interact with cancer cells and promote the metastatic cascade at all its phases. Nonetheless, since the interaction between the tumor and the platelets results in the “education” of these particles (tumor-educated platelets, TEPs), altering their transcriptional profile, RNA-seq of TEPs might open a window of new cancer biomarkers [85]. Indeed, Best et al. performed RNA-seq on TEPs from 228 cancer patients and 55 healthy individuals and identified 2246 differentially expressed mRNAs, of which 1072 were selected for constructing a machine learning classifier. This classifier achieved 97% sensitivity, 94% specificity, and 96% accuracy for distinguishing six cancer types from controls, as well as 71% accuracy for TOO prediction [86].
Another interesting approach to multi-cancer detection was reported by Tripathi et al., who developed a scale for scoring individuals as non-cancer, inflammatory, high-risk or stage I–IV cancer. Such a scale was based on OCT-4A expression, a marker of pluripotency, thereby targeting cancer stem cells (CSCs). Remarkably, by enriching CSCs from the blood of 500 cancer patients and 500 non-cancer controls, OCT-4A expression levels detected and staged 22 tumor types with a perfect sensitivity and specificity [87].
2.5. Circulating Tumor Cell-Based MCED Tests
CTCs are cells released from the primary tumor into the circulation as a part of the metastatic process. Although usually scarce, the increasing number of CTCs found in the blood has been associated with poor patient prognosis, but its diagnostic and early detection potential remains largely unexplored [88]. Nonetheless, CTCs have been found in the circulation of patients with localized tumors or even prior to the detection of a primary tumor by imaging, thus indicating a putative value in early cancer detection if the right tools are applied [88,89,90,91].
Using EpCAM+/Vimentin+ specific immunomagnetic beads for CTC isolation from 174 cancer patients (118 stage I/II), Huang et al. showed that the mean CTC count in lung, colorectal, gastric, liver, and esophageal cancers was significantly higher when compared to healthy individuals and non-cancer patients with high-risk conditions, also discriminating between the latter two groups of samples [92]. Notably, their technology showed a CTC capture rate higher than 80%, being superior to that of the FDA-approved CellSearch device, which is around 70% [92,93]. Moreover, no significant differences were seen in CTC count between the different cancer types, suggesting a potential multi-cancer detection biomarker, although TOO identification was not possible. Another strategy that has been followed is the analysis of circulating ensembles of tumor-associated cells (C-ETACs), defined as cell clusters with at least 3 cells positive for EpCAM and pan-cytokeratin immunostaining, regardless of CD45 status. C-ETACs detection discriminated cancer patients (18 cancer types) from healthy individuals with 89.8% sensitivity and 97% specificity, outperforming conventional CTCs-based methodologies [94]. Furthermore, the addition of cancer-specific markers to cell staining allowed for TOO identification with 93.1% accuracy [95].
2.6. Extracellular Vesicle-Based MCED Tests
EVs are a heterogeneous population of lipid membrane vesicles comprising exosomes, microvesicles and apoptotic bodies, being categorized by size, biogenesis, and release mechanisms particularities [96]. These small vesicles are secreted by a variety of cell types, including cancer cells, and play a major role in mediating cell-cell communication, contributing to the modulation of a cancer-favorable microenvironment. Such a role can be attributed due to EVs transporting different cargo molecules, including nucleic acids, proteins, and metabolites, which are also appealing as cancer biomarkers [97,98].
Unlimited proliferation is a cancer hallmark, due to cancer cells expressing significant levels of telomerase, resulting in the extension of telomeric DNA which hinders cellular senescence [99]. Thus, Goldvaser et al. hypothesized that the presence of human telomerase reverse transcriptase (hTERT) mRNA, the catalytical subunit of telomerase, in exosomes could serve as a minimally invasive pan-cancer biomarker. Interestingly, their results sustained the theory, disclosing 62% sensitivity and 100% specificity for detecting 15 cancer types, including solid and hematological ones [100]. Despite the potential of the nucleic acid content of EVs, most studies on MCED have focused on the protein cargo.
Using the Verita™ platform for EV isolation, an alternative to the conventional ultracentrifugation protocols developed by Biological Dynamics, EV-protein profiling combined with machine learning detected stage I and II pancreatic, ovarian, and bladder cancers with 71.2% sensitivity, 99.5% specificity, corresponding to an AUC of 0.95 [101,102]. Focusing on the proteome from both extracellular vesicles and particles (EVPs), machine learning classifiers also discriminated 16 cancer types from healthy controls with 95% sensitivity and 90% specificity using only 47 proteins, while TOO was accurately predicted using a 30-protein classifier [103]. Interestingly, two studies also focused on EVs’ surface proteins and used DNA aptamer-based recognition of such proteins. Using a chip targeting CD9+ EVs and aptamer recognition of CD63/EpCAM/MUC1 (epithelial markers), carcinomas were detected with 100% sensitivity and specificity [104]. When targeting tumor-type-specific proteins in EVs’ surface, 95% sensitivity and 100% specificity were achieved for cancer detection and 68% accuracy for TOO discrimination in cancers of the lung, breast, prostate, liver and ovary, and lymphoma [105].
2.7. Other Approaches to MCED Tests
Besides CTCs, other circulating cells have demonstrated the ability to signal several cancer types. Considering that activated monocytes (or macrophages) phagocyte tumor cells or related structures, thereby presenting tumor material highly concentrated in their interior, the epitope detection in monocytes (EDIM) technology was developed [106]. Consisting in the analysis of tumor markers intracellularly of monocytes, this strategy can be easily applied using flow cytometry by targeting CD14+/CD16+ cells extracted from a whole-blood sample in addition to the markers of interest. In fact, this method can be applied to a wide range of diseases, since any epitope may be selected [106].
Combining the natural immune response to cancer with the fact that tumor cells have an altered metabolism, in 2012, Feyen et al. first reported the EDIM-TKTL1 blood test to evaluate if the transketolase-like-1 (TKTL1) protein could be detected in monocytes and allow for cancer detection. This protein was chosen because it is an enzyme involved in the pentose phosphate pathway and upregulated in tumors, thus promoting aerobic glycolysis. Using 240 patients with several malignancies and 117 healthy individuals, the EDIM-TKTL1 test showed 95% sensitivity and 88% specificity, depicting a superior ability to detect small tumors compared to FDG-PET-CT, an imaging technique also relying on cancers’ particular metabolism [107]. Later, Grimm et al. added Apo10, a marker of apoptosis resistance, and showed that the combined analysis of the 2 epitopes (EDIM-TKTL1/Apo10 blood test) detected oral squamous cell carcinoma, breast, and prostate cancer with 95.8% sensitivity and 97.3% specificity [108]. More recently, this technology was further tested on cholangiocellular, pancreatic, and colorectal cancer, showing 100% sensitivity and 96.2% specificity, with the false-positive results being due to individuals harboring inflammatory conditions [109]. In a prospective study involving 5114 asymptomatic individuals, this blood test demonstrated the capacity to be used as a screening tool followed by imaging, since TOO localization is not possible [110]. The EDIM technology has been developed by Zyagnum AG and is available for early cancer early detection as the PanTum Detect® blood test [111].
Pursuing the deregulated metabolism hallmark, metabolite profiling of body fluids may also give insight into the cancer status of individuals. Using different techniques for the analysis of serum metabolites, 84% sensitivity and specificity for detection and 85% accuracy for TOO identification were reported for six cancer types [112], whereas female cancers (breast, endometrial, cervical, and ovarian) could be detected at early stage with 98% sensitivity and 98.3% specificity as well as over 90% accuracy for TOO [113]. Plasma and urine glycosaminoglycans (GAGs) also showed MCED potential, with AUC values around 0.80 [114]. In fact, this GAGome approach to early cancer detection is being tested by Elypta, with two clinical trials currently ongoing (NCT05295017, NCT05235009) (Table 3) [115].
Other components of body fluids, such as metals, also demonstrated capacity for pan-cancer detection, showing an AUC of 0.83 for distinguishing cancer patients from healthy individuals [116]. Remarkably, these elements even disclosed significantly different levels in cancer patients with a normal reading for classical markers (CEA, CA19-9, CA125, PSA). The profile of serum resulting from infrared spectroscopy itself allowed for cancer detection with an AUC of 0.86 [117]. Interestingly, the Dxcover® platform of infrared spectroscopy is being developed as an MCED test, requiring only a blood drop for analysis [118].
When developing cancer biomarkers, it is inevitable not to focus on endogenous molecules, but an exogenous source can also shed light on cancer diagnosis. Although only tested in animal models, non-viral vectors [119] and macrophages [120], engineered to contain a luminescent reporter coupled to the promotor of a tumor-specific actionable gene, showed the ability to point out the presence of very small tumors by measuring luminescence levels in the blood.
3. Bioinformatics Meets Cancer Detection: Finding the Right Targets and Improving Biomarker Performance
With the development of The Cancer Genome Atlas (TCGA) project, a large-scale open-access database containing genomic, transcriptomic, epigenomic, and proteomic datasets across more than 30 tumor types, cancer research experienced a boost derived from the analysis of molecular features of individual tumor samples, not only increasing the knowledge on tumor heterogeneity but also on individual and shared profiles among different cancers [147]. Furthermore, given the complexity of sequencing- and array-generated data, many tools have been created to allow a more interactive and comprehensive mining of the different types of data, including the UCSC Cancer Genomics Browser [148], the cBioPortal for Cancer Genomics [149] and UALCAN [150], among others. In 2012, the TCGA Pan-Cancer analysis project was launched with the goal of providing new multi-omics data across multiple cancers, increasing the statistical power of the datasets, and making it easier to identify and analyze common cancer molecular abnormalities [147,151]. Nonetheless, performing differential analysis in the context of early detection and diagnosis also requires a significant amount of data from matched normal tissues. Despite several normal-adjacent tissue datasets being available in TCGA, the sample size is small, which is why many studies combine their analysis with GTEx samples, although only RNA-seq data is available [152]. The Gene Expression Omnibus (GEO) is another database containing many sets of high-throughput sequencing data, consisting in a repository where researchers upload their results and these become freely available to the entire scientific community [153].
The availability of such a large amount of molecular data has prompted data mining as the first step of biomarker discovery and, since the clinical data of the sequenced samples is also publicly available, possibilities range from early detection to prognosis and therapy response prediction. Indeed, many molecules have been proposed as detection biomarkers across several cancer types by data mining (Table 2). For instance, when mining the available methylome data of TCGA in search of pancreatic cancer biomarkers, Manoochehri et al. found DMRs in the first exon of the SST gene that were significantly hypermethylated in tissues of 11 cancers compared to para-cancerous tissues [154]. Interestingly, it was later reported that two CpG sites within SST’s first exon could detect colorectal, gastric, and esophageal cancer with 59.3% sensitivity and 72.8% specificity using tissue samples of 229 patients [123]. Similarly, the expression of Hsp90α was shown to significantly differ between 9 tumors and respective normal tissues by in silico analysis [155], and the circulating plasma levels of the Hsp90α protein were also reported to detect several cancer types with 81.72% sensitivity and 81.03% specificity [144]. Although without validation in biological specimens, many other markers have shown MCED potential, with the benefit of data mining allowing the reduction of an entire sequencing/array run data into single genes or proteins, enabling the design of a targeted validation assay. For example, Liu et al. used whole-genome methylation data to identify 12 CpG markers and then utilized them to construct a deep learning model that detected 27 cancer types with 92.8% sensitivity, 90.1% specificity, and 92.4% accuracy [156]. Likewise, Ibrahim et al. showed that the methylation levels of a set of 4 CpGs could detect 14 tumor types with an AUC of 0.96 and a set of 20 CpGs discriminated TOO with AUC values ranging from 0.87 to 0.99 [157]. Remarkably, this was possible by using machine learning algorithms that tested different combinations of CpGs to find the most informative ones. Aside from methylation, in silico analysis of the expression of several individual genes, miRs, and lncRNAs also displayed biomarker potential for several tumor types (Table 2), thus being easily validated by quantitative PCR in human samples.
Importantly, these databases also allow the development and training of machine learning algorithms to improve biomarkers’ detection capacity. Fan et al. developed a mathematical model to expand the Illumina 450K methylation array data to cover a larger percentage of the total CpG sites in the genome and combined such expanded data with genome-wide expression and mutational coverage into a random forest classifier that detected cancer with an AUC of 0.85. Additionally, a multi-class regression model was constructed to discriminate TOO in 13 tumors, showing 95.3% accuracy [158]. Applying neural network-based deep learning on transcriptomic data, Yuan et al. developed the DeepDCancer classifier that disclosed 90% accuracy for detecting and an average 94% for discriminating 10 cancer types [159]. Similarly, the GeneCT model was constructed by Sun et al. resulting in 96.0% sensitivity and 96.1% specificity for cancer identification, followed by 99.6% accuracy for TOO prediction [160]. This time focusing on ncRNA, Wang et al. showed that this deep learning approach detected 26 cancers with an AUC of 0.963 and discriminated between cancer types with 82.15% accuracy [161]. MicroRNA data can also be used for classifier construction, with Yuan et al. evaluating several algorithms and reporting accuracies over 95%, but ultimately, the support vector machine (SVM) performed the best with 99% accuracy in classifying 11 tumor types [162].
In fact, the benefits of combining machine learning with molecular analysis may be corroborated by the fact that practically all the studies above mentioned using human samples (Table 1) applied algorithms to the outputted data of the used methodologies and built classifiers for discriminating cancer from healthy and further detecting the underlying cancer type. Indeed, the remarkable results obtained with GRAIL’s MCED test were the result of a custom model based on two ensemble logistic regression (LR) algorithms, one to differentiate cancer from non-cancer and the other to identify the TOO [54]. CancerSEEK’s and PanSeer’s technology also relied on LR [29,49], while DELFI used a stochastic gradient boosting model [74]. This shows that several models exist and can be tested to disclose the most suitable, according to the type of data being used as model features and its final purpose.
Machine learning is a subset of artificial intelligence that uses mathematical and statistical methods to improve a computer’s performance in decision-making. Using large amounts of data, algorithms can be trained to learn certain tasks and then be tested to predict their behavior in a real-world scenario [163,164]. Moreover, deep learning is a subset of machine learning that uses supervised or unsupervised learning methods to train multilayered artificial neural networks. It has been shown to outperform even the best machine learning algorithms, due to performing better on big datasets, which may also be a drawback, since many biological samples are available in low quantity [163,164]. While classical statistics is based on probability and assumption, machine learning uses algorithms that are trained and improved with experience and increasing input data, thus being more effective in dealing with high-resolution data, such as biological data [165]. In fact, it is the complexity of the high-throughput molecular techniques’ data that led to the inclusion of machine learning as a part of biomarker research, in feature extraction of relevant biomarkers, as well as in validating these for sample classification [166]. Moreover, the capacity of developing multimodal algorithms, i.e., models containing not only molecular but also histological, radiological, and clinical data as input features, holds great promise for precision oncology [167].
4. What Is Stopping MCED Tests from Moving into Clinical Application?
MCED tests are paving the way for a shift in the current cancer screening paradigm, moving from single organ screening of a hand full of cancers to universal testing using a simple blood draw. Being a recent topic in cancer research, there are still no guidelines on how to evaluate and compare the performance of tests being developed. For that reason, Braunstein and Ofman proposed 9 criteria that should be taken into account in a MCED test: (1) target high-risk individuals; (2) detect the highest possible number of cancer types; (3) display a low false-positive rate (FPR); (4) possess an accurate TOO discriminating capacity; (5) limit detection to cancers that tend to be deadly during a typical lifetime; (6) display a balanced sensitivity and specificity; (7) be validated in prospective, multi-center, population based studies; (8) be evaluated in studies comprising several controls, namely, non-cancer individuals and those harboring benign and inflammatory conditions; (9) be cost-effective [193]. Still, even if such criteria are met, the performance of MCED tests in a real-life screening scenario remains unknown.
Thus far, MCED studies have only reported tests’ diagnostic capacity by using post-diagnosis samples in a retrospective case-control manner [194,195]. Hence, it is not possible to know if these tests will indeed detect cancer at early stages and, if so, if such stage-shift will result in clinical benefit and mortality reduction. Furthermore, there are no guidelines for confirming a positive test result and, consequently, there is no way to infer the real false-result rates and TOO accuracy, as well as the number of necessary additional procedures to reach a final diagnosis. Importantly, questions regarding when, how, and to whom to provide a MCED test also remain unanswered [194,195,196]. To obtain insights into such uncertainties, prospective randomized clinical trials, comparing screened and unscreened asymptomatic individuals, with the right endpoint being cancer-specific mortality, are demanded [194,195]. Nonetheless, these studies require many participants and a long follow-up to provide meaningful clinical information. Interestingly, Hackshaw and Berg suggested that the adoption of a nested randomized trial i.e., storing collected blood samples and only applying the test in those positive, whether in the screened group or in the control group, would allow for a more economic and less resource-consuming trial [197]. Currently, most of the developed MCED tests are under prospective trials, either in asymptomatic general or high-risk populations, to confirm their screening potential and shed light into all the above-mentioned concerns (Table 3).
Notably, many simulation studies have used the available performance data of published MCED tests to estimate their potential impact on health care systems. For instance, combining stage-specific incidence and survival data from SEER with GRAIL’s test performance, a 78% reduction in late-stage cancer incidence and 26% of all cancer-related deaths were estimated [198]. In addition, breast cancer detection might be incremented in 11% if a MCED test would be available during a routine examination [199]. When estimating the impact of pan-cancer screening in the US and UK, Hackshaw et al. reported that using such a strategy in someone without a cancer detected by conventional screening not only increased cancer detection rates, but also significantly reduced the cost of additional diagnostic workups [200]. Similarly, Tafazzoli et al. demonstrated an estimated USD 5421 in cost reduction per cancer treatment if, hypothetically, annual MCED testing was provided to the population between 50 and 79 years [201]. Focusing on the consequent harms and benefits, a favorable balance was depicted between the number of individuals exposed to unnecessary confirmatory tests and the number of detected cancers, with it being even improved if the test included more lethal cancers [202]. In fact, the harms of MCED testing, not only concerning overdiagnosis and needless procedures, but also the resulting anxiety and the overall perception of screening, should not be overlooked. To be cost-effective, population adherence to screening programs must be high, thus psychological and behavioral aspects also need to be taken into account, such as how the science behind the tests will be explained, the tests be delivered, and the results revealed, or further procedures be recommended [203]. Hence, for a successful implementation of MCED tests, adequate medical communication and public understanding is required [203].
Another concern regarding MCED tests is the use of high-throughput sequencing-based methodologies. Although allowing for the simultaneous screen of several genomic regions, which is beneficial when using a limiting material as liquid biopsies, such methods are highly costly and lengthy processes, requiring specialized data analysis [204,205]. As mentioned above, tests may reach USD 1000 per individual, which is not a feasible option for population-based screening. Thus, efforts should also be made to develop more targeted, fast workflow and cost-effective assays.
Notably, lack of standardization is a major issue encompassing the liquid biopsy research field. Pre-analytical variables and isolation methods have great impact on subsequent molecular results, precluding an accurate comparison between different tests and studies. Hence, initiatives such as Cancer-ID, now replaced by the European Liquid Biopsy Society (ELBS), and BloodPAC are key for standardizing and moving liquid biopsy testing from bench to beside [206,207].
5. Conclusions
Overall, by complementing the currently available screening and diagnostic approaches, MCED tests show great promise for reducing cancer mortality by shifting detection to earlier stages, in which curative options are most likely to be effective. DNA methylation-based tests are in forefront of development, being the most frequently chosen source of biomarkers, due to its features of aberrant tumor-specific patterns, tissue-specificity, and easiness to assess in cfDNA. By combining molecular analysis of liquid biopsies with artificial intelligence, the performance of MCED tests may be greatly improved, increasing not only the sensitivity of detection of multiple cancers but also the accuracy of discriminating among the different tumor types (Figure 3). MCED tests, however, still lack validation in prospective multicenter studies to enable their implementation into population-based screening programs and make their way into routine clinical practice.
Figure 3.
Schematic representation of the workflow for developing multi-cancer early detection tests. Data mining of big datasets, such as TCGA, are great tools for selecting biomarkers with utility for cancer detection. Liquid biopsies provide a minimally invasive way to obtain cancer-related information, namely, circulating tumor cells (CTCs), circulating nucleic acids (cfDNA and cfRNA), extracellular vesicles (EVs), and tumor-educated platelets (TEPs). The molecular analysis of these biomarkers combined with machine learning classifiers shows great potential for detecting multiple cancers simultaneously and discriminating tissue of origin (TOO). Created with BioRender.com.
Author Contributions
Conceptualization, T.B.-R., V.C. and C.J.; literature review and manuscript writing, T.B.-R.; critical revision of manuscript, V.C., R.H. and C.J.; images were conceptualized and performed by T.B.-R.; supervision, R.H. and C.J. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Research Center-Portuguese Oncology Institute of Porto (grant PI 74-CI-IPOP-19-2015). T.B-R. received the support of a fellowship from LPCC-NRN 2023. V.C. received the support of a fellowship from “la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/DR20/11790013.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Clarke C.A., Hubbell E., Kurian A.W., Colditz G.A., Hartman A.R., Gomez S.L. Projected Reductions in Absolute Cancer-Related Deaths from Diagnosing Cancers Before Metastasis, 2006-2015. Cancer Epidemiol. Biomark. Prev. 2020;29:895–902. doi: 10.1158/1055-9965.EPI-19-1366. [DOI] [PubMed] [Google Scholar]
- 3.Smith R.A., Oeffinger K.C. The Importance of Cancer Screening. Med. Clin. N. Am. 2020;104:919–938. doi: 10.1016/j.mcna.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Guide to Cancer Early Aiagnosis. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 5.Barsouk A., Saginala K., Aluru J.S., Rawla P., Barsouk A. US Cancer Screening Recommendations: Developments and the Impact of COVID-19. Med. Sci. 2022;10:16. doi: 10.3390/medsci10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armaroli P., Villain P., Suonio E., Almonte M., Anttila A., Atkin W.S., Dean P.B., de Koning H.J., Dillner L., Herrero R., et al. European Code against Cancer, 4th Edition: Cancer screening. Cancer Epidemiol. 2015;39:S139–S152. doi: 10.1016/j.canep.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Grossman D.C., Curry S.J., Owens D.K., Bibbins-Domingo K., Caughey A.B., Davidson K.W., Doubeni C.A., Ebell M., Epling J.W., Jr., Kemper A.R., et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901–1913. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 8.Wu G.X., Raz D.J. Lung Cancer Screening. Cancer Treat Res. 2016;170:1–23. doi: 10.1007/978-3-319-40389-2_1. [DOI] [PubMed] [Google Scholar]
- 9.Marmot M.G., Altman D.G., Cameron D.A., Dewar J.A., Thompson S.G., Wilcox M. The benefits and harms of breast cancer screening: An independent review. Lancet. 2012;380:1778–1786. doi: 10.1038/bjc.2013.177. [DOI] [PubMed] [Google Scholar]
- 10.Force U.P.S.T. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674–686. doi: 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- 11.Barry M.J., Simmons L.H. Prevention of Prostate Cancer Morbidity and Mortality: Primary Prevention and Early Detection. Med. Clin. N. Am. 2017;101:787–806. doi: 10.1016/j.mcna.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Geneve N., Kairys D., Bean B., Provost T., Mathew R., Taheri N. Colorectal Cancer Screening. Prim. Care. 2019;46:135–148. doi: 10.1016/j.pop.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Mannelli C. Tissue vs Liquid Biopsies for Cancer Detection: Ethical Issues. J. Bioeth. Inq. 2019;16:551–557. doi: 10.1007/s11673-019-09944-y. [DOI] [PubMed] [Google Scholar]
- 14.Poulet G., Massias J., Taly V. Liquid Biopsy: General Concepts. Acta Cytol. 2019;63:449–455. doi: 10.1159/000499337. [DOI] [PubMed] [Google Scholar]
- 15.Marrugo-Ramírez J., Mir M., Samitier J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018;19:2877. doi: 10.3390/ijms19102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ignatiadis M., Sledge G.W., Jeffrey S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021;18:297–312. doi: 10.1038/s41571-020-00457-x. [DOI] [PubMed] [Google Scholar]
- 17.Lone S.N., Nisar S., Masoodi T., Singh M., Rizwan A., Hashem S., El-Rifai W., Bedognetti D., Batra S.K., Haris M., et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer. 2022;21:79. doi: 10.1186/s12943-022-01543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y., Agrawal N., Bartlett B.R., Wang H., Luber B., Alani R.M., et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014;6:224ra224. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alix-Panabières C., Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021;11:858–873. doi: 10.1158/2159-8290.CD-20-1311. [DOI] [PubMed] [Google Scholar]
- 20.Ahlquist D.A. Universal cancer screening: Revolutionary, rational, and realizable. NPJ Precis. Oncol. 2018;2:23. doi: 10.1038/s41698-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller L., Belloum Y., Wikman H., Pantel K. Clinical relevance of blood-based ctDNA analysis: Mutation detection and beyond. Br. J. Cancer. 2021;124:345–358. doi: 10.1038/s41416-020-01047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siravegna G., Marsoni S., Siena S., Bardelli A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 24.Zou H., Harrington J.J., Taylor W.R., Devens M.E., Cao X., Heigh R.I., Romero Y., Chari S.T., Petersen G.M., Roberts L.R., et al. T2036 Pan-Detection of Gastrointestinal Neoplasms By Stool DNA Testing: Establishment of Feasibility. Gastroenterology. 2009;136:A-625. doi: 10.1016/S0016-5085(09)62882-1. [DOI] [Google Scholar]
- 25.Yang D., Hillman S.L., Harris A.M., Sinicrope P.S., Devens M.E., Ahlquist D.A. Patient perceptions of stool DNA testing for pan-digestive cancer screening: A survey questionnaire. World J. Gastroenterol. 2014;20:4972–4979. doi: 10.3748/wjg.v20.i17.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermann B.T., Pfeil S., Groenke N., Schaible S., Kunze R., Ris F., Hagen M.E., Bhakdi J. DEEPGEN(TM)-A Novel Variant Calling Assay for Low Frequency Variants. Genes. 2021;12:507. doi: 10.3390/genes12040507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quantgene Inc. DEEPGEN™ R&D Solution. [(accessed on 20 August 2022)]. Available online: https://www.quantgene.com/deepgen-research-solutions.
- 28.Ris F., Hellan M., Douissard J., Nieva J.J., Triponez F., Woo Y., Geller D., Buchs N.C., Buehler L., Moenig S., et al. Blood-Based Multi-Cancer Detection Using a Novel Variant Calling Assay (DEEPGENTM): Early Clinical Results. Cancers. 2021;13:4104. doi: 10.3390/cancers13164104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J.D., Li L., Wang Y., Thoburn C., Afsari B., Danilova L., Douville C., Javed A.A., Wong F., Mattox A., et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennon A.M., Buchanan A.H., Kinde I., Warren A., Honushefsky A., Cohain A.T., Ledbetter D.H., Sanfilippo F., Sheridan K., Rosica D., et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020;369:6499. doi: 10.1126/science.abb9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Pol Y., Mouliere F. Toward the Early Detection of Cancer by Decoding the Epigenetic and Environmental Fingerprints of Cell-Free DNA. Cancer Cell. 2019;36:350–368. doi: 10.1016/j.ccell.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Tempero M. Pan-Cancer Screening: A Dream or a Nightmare. J. Natl. Compr. Canc. Netw. 2021;19:773. doi: 10.6004/jnccn.2021.0034. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava S., Hanash S. Pan-Cancer Early Detection: Hype or Hope? Cancer Cell. 2020;38:23–24. doi: 10.1016/j.ccell.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Inbar-Feigenberg M., Choufani S., Butcher D.T., Roifman M., Weksberg R. Basic concepts of epigenetics. Fertil. Steril. 2013;99:607–615. doi: 10.1016/j.fertnstert.2013.01.117. [DOI] [PubMed] [Google Scholar]
- 35.Lokk K., Modhukur V., Rajashekar B., Märtens K., Mägi R., Kolde R., Koltšina M., Nilsson T.K., Vilo J., Salumets A., et al. DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biol. 2014;15:3248. doi: 10.1186/gb-2014-15-4-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun K., Jiang P., Chan K.C.A., Wong J., Cheng Y.K.Y., Liang R.H.S., Chan W.-K., Ma E.S.K., Chan S.L., Cheng S.H., et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc. Natl. Acad. Sci. USA. 2015;112:E5503–E5512. doi: 10.1073/pnas.1508736112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo S., Diep D., Plongthongkum N., Fung H.-L., Zhang K., Zhang K. Identification of methylation haplotype blocks aids in deconvolution of heterogeneous tissue samples and tumor tissue-of-origin mapping from plasma DNA. Nat. Genet. 2017;49:635–642. doi: 10.1038/ng.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones P.A., Baylin S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 39.Kulis M., Esteller M. DNA methylation and cancer. Adv. Genet. 2010;70:27–56. doi: 10.1016/b978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 40.Baylin S.B., Jones P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Constâncio V., Nunes S.P., Henrique R., Jerónimo C. DNA Methylation-Based Testing in Liquid Biopsies as Detection and Prognostic Biomarkers for the Four Major Cancer Types. Cells. 2020;9:624. doi: 10.3390/cells9030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller B.F., Petrykowska H.M., Elnitski L. Assessing ZNF154 methylation in patient plasma as a multicancer marker in liquid biopsies from colon, liver, ovarian and pancreatic cancer patients. Sci. Rep. 2021;11:221. doi: 10.1038/s41598-020-80345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunes S.P., Moreira-Barbosa C., Salta S., Palma de Sousa S., Pousa I., Oliveira J., Soares M., Rego L., Dias T., Rodrigues J., et al. Cell-Free DNA Methylation of Selected Genes Allows for Early Detection of the Major Cancers in Women. Cancers. 2018;10:357. doi: 10.3390/cancers10100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Constâncio V., Nunes S.P., Moreira-Barbosa C., Freitas R., Oliveira J., Pousa I., Oliveira J., Soares M., Dias C.G., Dias T., et al. Early detection of the major male cancer types in blood-based liquid biopsies using a DNA methylation panel. Clin. Epigenet. 2019;11:175. doi: 10.1186/s13148-019-0779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandimalla R., Xu J., Link A., Matsuyama T., Yamamura K., Parker M.I., Uetake H., Balaguer F., Borazanci E., Tsai S., et al. EpiPanGI Dx: A Cell-free DNA Methylation Fingerprint for the Early Detection of Gastrointestinal Cancers. Clin. Cancer Res. 2021;27:6135–6144. doi: 10.1158/1078-0432.CCR-21-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao X., Luo H., Krawczyk M., Wei W., Wang W., Wang J., Flagg K., Hou J., Zhang H., Yi S., et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc. Natl. Acad. Sci. USA. 2017;114:7414–7419. doi: 10.1073/pnas.1703577114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.IvyGene Labs The IvyGeneCORE Test. [(accessed on 20 August 2022)]. Available online: https://www.ivygenelabs.co.za/the-ivygenecore-test/
- 48.Singlera Genomics Singlera Genomics’ PanSeer Assay Technology. [(accessed on 20 August 2022)]. Available online: https://singleraoncology.com/technology/
- 49.Chen X., Gole J., Gore A., He Q., Lu M., Min J., Yuan Z., Yang X., Jiang Y., Zhang T., et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat. Commun. 2020;11:3475. doi: 10.1038/s41467-020-17316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheridan C. Grail to pour $1 billion into blood test to detect early cancer. Nat. Biotechnol. 2017;35:101–102. doi: 10.1038/nbt0217-101. [DOI] [PubMed] [Google Scholar]
- 51.Fiala C., Diamandis E.P. Can Grail find the trail to early cancer detection? Clin. Chem. Lab. Med. 2019;57:403–406. doi: 10.1515/cclm-2018-1249. [DOI] [PubMed] [Google Scholar]
- 52.Klein E.A., Hubbell E., Maddala T., Aravanis A., Beausang J.F., Filippova D., Gross S., Jamshidi A., Kurtzman K., Shen L., et al. Development of a comprehensive cell-free DNA (cfDNA) assay for early detection of multiple tumor types: The Circulating Cell-free Genome Atlas (CCGA) study. J. Clin. Oncol. 2018;36:12021. doi: 10.1200/JCO.2018.36.15_suppl.12021. [DOI] [Google Scholar]
- 53.Liu M.C., Klein E., Hubbell E., Maddala T., Aravanis A.M., Beausang J.F., Filippova D., Gross S., Jamshidi A., Kurtzman K., et al. Plasma cell-free DNA (cfDNA) assays for early multi-cancer detection: The circulating cell-free genome atlas (CCGA) study. Ann. Oncol. 2018;29:viii14. doi: 10.1093/annonc/mdy269.048. [DOI] [Google Scholar]
- 54.Liu M.C., Oxnard G.R., Klein E.A., Swanton C., Seiden M.V., Liu M.C., Oxnard G.R., Klein E.A., Smith D., Richards D., et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020;31:745–759. doi: 10.1016/j.annonc.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klein E.A., Richards D., Cohn A., Tummala M., Lapham R., Cosgrove D., Chung G., Clement J., Gao J., Hunkapiller N., et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 2021;32:1167–1177. doi: 10.1016/j.annonc.2021.05.806. [DOI] [PubMed] [Google Scholar]
- 56.Chen X., Dong Z., Hubbell E., Kurtzman K.N., Oxnard G.R., Venn O., Melton C., Clarke C.A., Shaknovich R., Ma T., et al. Prognostic Significance of Blood-Based Multi-cancer Detection in Plasma Cell-Free DNA. Clin. Cancer Res. 2021;27:4221–4229. doi: 10.1158/1078-0432.CCR-21-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.GRAIL The Galleri® Test. [(accessed on 2 September 2022)]. Available online: https://www.galleri.com/the-galleri-test/request-the-test.
- 58.Nadauld L.D., McDonnell C.H., Beer T.M., Liu M.C., Klein E.A., Hudnut A., Whittington R.A., Taylor B., Oxnard G.R., Lipson J., et al. The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice. Cancers. 2021;13:3501. doi: 10.3390/cancers13143501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beer T.M., McDonnell C.H., Nadauld L., Liu M.C., Klein E.A., Reid R.L., Marinac C., Chung K., Lopatin M., Fung E.T., et al. Interim results of PATHFINDER, a clinical use study using a methylation-based multi-cancer early detection test. J. Clin. Oncol. 2021;39:3010. doi: 10.1200/JCO.2021.39.15_suppl.3010. [DOI] [Google Scholar]
- 60.Kint S., De Spiegelaere W., De Kesel J., Vandekerckhove L., Van Criekinge W. Evaluation of bisulfite kits for DNA methylation profiling in terms of DNA fragmentation and DNA recovery using digital PCR. PLoS ONE. 2018;13:e0199091. doi: 10.1371/journal.pone.0199091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sørensen A.L., Collas P. Immunoprecipitation of methylated DNA. Methods Mol. Biol. 2009;567:249–262. doi: 10.1007/978-1-60327-414-2_16. [DOI] [PubMed] [Google Scholar]
- 62.Adela Inc. A breakthrough approach to early detection. [(accessed on 21 August 2022)]. Available online: https://www.adelabio.com/
- 63.Shen S.Y., Burgener J.M., Bratman S.V., De Carvalho D.D. Preparation of cfMeDIP-seq libraries for methylome profiling of plasma cell-free DNA. Nat. Protoc. 2019;14:2749–2780. doi: 10.1038/s41596-019-0202-2. [DOI] [PubMed] [Google Scholar]
- 64.Shen S.Y., Singhania R., Fehringer G., Chakravarthy A., Roehrl M.H.A., Chadwick D., Zuzarte P.C., Borgida A., Wang T.T., Li T., et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579–583. doi: 10.1038/s41586-018-0703-0. [DOI] [PubMed] [Google Scholar]
- 65.Sina A.A.I., Carrascosa L.G., Liang Z., Grewal Y.S., Wardiana A., Shiddiky M.J.A., Gardiner R.A., Samaratunga H., Gandhi M.K., Scott R.J., et al. Epigenetically reprogrammed methylation landscape drives the DNA self-assembly and serves as a universal cancer biomarker. Nat. Commun. 2018;9:4915. doi: 10.1038/s41467-018-07214-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szyf M. The elusive role of 5′-hydroxymethylcytosine. Epigenomics. 2016;8:1539–1551. doi: 10.2217/epi-2016-0076. [DOI] [PubMed] [Google Scholar]
- 67.Li W., Zhang X., Lu X., You L., Song Y., Luo Z., Zhang J., Nie J., Zheng W., Xu D., et al. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic biomarkers for human cancers. Cell Res. 2017;27:1243–1257. doi: 10.1038/cr.2017.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bluestar Genomics Multi-Cancer Panels. [(accessed on 21 August 2022)]. Available online: https://www.bluestargenomics.com/multi-cancer-panels/
- 69.Bergamaschi A., Ning Y., Ku C.-J., Ellison C., Collin F., Guler G., Phillips T., McCarthy E., Wang W., Antoine M., et al. Pilot study demonstrating changes in DNA hydroxymethylation enable detection of multiple cancers in plasma cell-free DNA. medRxiv. 2020 doi: 10.1101/2020.01.22.20018382. [DOI] [Google Scholar]
- 70.Peng X., Li H.D., Wu F.X., Wang J. Identifying the tissues-of-origin of circulating cell-free DNAs is a promising way in noninvasive diagnostics. Brief Bioinform. 2021;22:bbaa060. doi: 10.1093/bib/bbaa060. [DOI] [PubMed] [Google Scholar]
- 71.Snyder M.W., Kircher M., Hill A.J., Daza R.M., Shendure J. Cell-free DNA Comprises an In Vivo Nucleosome Footprint that Informs Its Tissues-Of-Origin. Cell. 2016;164:57–68. doi: 10.1016/j.cell.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bao H., Wang Z., Ma X., Guo W., Zhang X., Tang W., Chen X., Wang X., Chen Y., Mo S., et al. Letter to the Editor: An ultra-sensitive assay using cell-free DNA fragmentomics for multi-cancer early detection. Mol. Cancer. 2022;21:129. doi: 10.1186/s12943-022-01594-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DELFI Diagnostics Our Science. [(accessed on 21 August 2022)]. Available online: https://delfidiagnostics.com/our-science/
- 74.Cristiano S., Leal A., Phallen J., Fiksel J., Adleff V., Bruhm D.C., Jensen S., Medina J.E., Hruban C., White J.R., et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570:385–389. doi: 10.1038/s41586-019-1272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mouliere F., Chandrananda D., Piskorz A.M., Moore E.K., Morris J., Ahlborn L.B., Mair R., Goranova T., Marass F., Heider K., et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018;10:466. doi: 10.1126/scitranslmed.aat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stackpole M., Zeng W., Li S., Liu C.-C., Zhou Y., He S., Yeh A., Wang Z., Sun F., Li Q., et al. Abstract 24: Multi-feature ensemble learning on cell-free dna for accurately detecting and locating cancer. Cancer Res. 2021;81:24. doi: 10.1158/1538-7445.AM2021-24. [DOI] [Google Scholar]
- 77.Giraldez M.D., Spengler R.M., Etheridge A., Goicochea A.J., Tuck M., Choi S.W., Galas D.J., Tewari M. Phospho-RNA-seq: A modified small RNA-seq method that reveals circulating mRNA and lncRNA fragments as potential biomarkers in human plasma. Embo J. 2019;38:e101695. doi: 10.15252/embj.2019101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Larson M.H., Pan W., Kim H.J., Mauntz R.E., Stuart S.M., Pimentel M., Zhou Y., Knudsgaard P., Demas V., Aravanis A.M., et al. A comprehensive characterization of the cell-free transcriptome reveals tissue- and subtype-specific biomarkers for cancer detection. Nat. Commun. 2021;12:2357. doi: 10.1038/s41467-021-22444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ludwig N., Leidinger P., Becker K., Backes C., Fehlmann T., Pallasch C., Rheinheimer S., Meder B., Stähler C., Meese E., et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44:3865–3877. doi: 10.1093/nar/gkw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qi F., Gao F., Cai Y., Han X., Qi Y., Ni J., Sun J., Huang S., Chen S., Wu C., et al. Complex Age- and Cancer-Related Changes in Human Blood Transcriptome-Implications for Pan-Cancer Diagnostics. Front. Genet. 2021;12:746879. doi: 10.3389/fgene.2021.746879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan H., Sun L., Wang W., Qin Z., Tang L., Gu X., Zhang J., He B. Serum long non-coding RNA LOC553103 as non-specific diagnostic and prognostic biomarker for common types of human cancer. Clin. Chim. Acta. 2020;508:69–76. doi: 10.1016/j.cca.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 82.Chen X., Dai M., Zhu H., Li J., Huang Z., Liu X., Huang Y., Chen J., Dai S. Evaluation on the diagnostic and prognostic values of long non-coding RNA BLACAT1 in common types of human cancer. Mol. Cancer. 2017;16:160. doi: 10.1186/s12943-017-0728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao Y., Deng K., Liu X., Dai M., Chen X., Chen J., Chen J., Huang Y., Dai S., Chen J. Molecular mechanism and role of microRNA-93 in human cancers: A study based on bioinformatics analysis, meta-analysis, and quantitative polymerase chain reaction validation. J. Cell Biochem. 2019;120:6370–6383. doi: 10.1002/jcb.27924. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto K., Inada M., Yamamoto Y., Ochiya T. Preliminary evaluation of miR-1307-3p in human serum for detection of 13 types of solid cancer using microRNA chip. Heliyon. 2021;7:e07919. doi: 10.1016/j.heliyon.2021.e07919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Varkey J., Nicolaides T. Tumor-Educated Platelets: A Review of Current and Potential Applications in Solid Tumors. Cureus. 2021;13:e19189. doi: 10.7759/cureus.19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Best M.G., Sol N., Kooi I., Tannous J., Westerman B.A., Rustenburg F., Schellen P., Verschueren H., Post E., Koster J., et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tripathi V., Bhartiya D., Vaid A., Chhabria S., Sharma N., Chand B., Takle V., Palahe P., Tripathi A. Quest for Pan-Cancer Diagnosis/Prognosis Ends with HrC Test Measuring Oct4A in Peripheral Blood. Stem Cell Rev. Rep. 2021;17:1827–1839. doi: 10.1007/s12015-021-10167-1. [DOI] [PubMed] [Google Scholar]
- 88.Vasseur A., Kiavue N., Bidard F.C., Pierga J.Y., Cabel L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2021;15:1647–1666. doi: 10.1002/1878-0261.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flores B.C.T., Correia M.P., Rodríguez J.G., Henrique R., Jerónimo C. Bridging the Gaps between Circulating Tumor Cells and DNA Methylation in Prostate Cancer. Cancers. 2021;13:4209. doi: 10.3390/cancers13164209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crook T., Leonard R., Mokbel K., Thompson A., Michell M., Page R., Vaid A., Mehrotra R., Ranade A., Limaye S., et al. Accurate Screening for Early-Stage Breast Cancer by Detection and Profiling of Circulating Tumor Cells. Cancers. 2022;14:3341. doi: 10.3390/cancers14143341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ilie M., Hofman V., Long-Mira E., Selva E., Vignaud J.-M., Padovani B., Mouroux J., Marquette C.-H., Hofman P. “Sentinel” Circulating Tumor Cells Allow Early Diagnosis of Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. PLoS ONE. 2014;9:e111597. doi: 10.1371/journal.pone.0111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang C., Ding S., Huang C., Pan F., Liu X., Zhang H., Zhou J., Liang X., Wang X., Song P. Distribution and Clinical Analysis of EpCAM+/Vimentin+ Circulating Tumor Cells in High-Risk Population and Cancer Patients. Front Oncol. 2021;11:642971. doi: 10.3389/fonc.2021.642971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hillig T., Horn P., Nygaard A.B., Haugaard A.S., Nejlund S., Brandslund I., Sölétormos G. In vitro detection of circulating tumor cells compared by the CytoTrack and CellSearch methods. Tumour Biol. 2015;36:4597–4601. doi: 10.1007/s13277-015-3105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Akolkar D., Patil D., Crook T., Limaye S., Page R., Datta V., Patil R., Sims C., Ranade A., Fulmali P., et al. Circulating ensembles of tumor-associated cells: A redoubtable new systemic hallmark of cancer. Int. J. Cancer. 2020;146:3485–3494. doi: 10.1002/ijc.32815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gaya A., Crook T., Plowman N., Ranade A., Limaye S., Bhatt A., Page R., Patil R., Fulmali P., Datta V., et al. Evaluation of circulating tumor cell clusters for pan-cancer noninvasive diagnostic triaging. Cancer Cytopathol. 2021;129:226–238. doi: 10.1002/cncy.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Urabe F., Kosaka N., Ito K., Kimura T., Egawa S., Ochiya T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020;318:C29–C39. doi: 10.1152/ajpcell.00280.2019. [DOI] [PubMed] [Google Scholar]
- 98.Yu W., Hurley J., Roberts D., Chakrabortty S.K., Enderle D., Noerholm M., Breakefield X.O., Skog J.K. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021;32:466–477. doi: 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 100.Goldvaser H., Gutkin A., Beery E., Edel Y., Nordenberg J., Wolach O., Rabizadeh E., Uziel O., Lahav M. Characterisation of blood-derived exosomal hTERT mRNA secretion in cancer patients: A potential pan-cancer marker. Br. J. Cancer. 2017;117:353–357. doi: 10.1038/bjc.2017.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hinestrosa J.P., Kurzrock R., Lewis J.M., Schork N.J., Schroeder G., Kamat A.M., Lowy A.M., Eskander R.N., Perrera O., Searson D., et al. Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Commun. Med. 2022;2:29. doi: 10.1038/s43856-022-00088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biological Dynamics Early Cancer Detection Program. [(accessed on 21 August 2022)]. Available online: https://biologicaldynamics.com/early-cancer-detection.
- 103.Hoshino A., Kim H.S., Bojmar L., Gyan K.E., Cioffi M., Hernandez J., Zambirinis C.P., Rodrigues G., Molina H., Heissel S., et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell. 2020;182:1044–1061.e1018. doi: 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J., Shi J., Zhang H., Zhu Y., Liu W., Zhang K., Zhang Z. Localized fluorescent imaging of multiple proteins on individual extracellular vesicles using rolling circle amplification for cancer diagnosis. J. Extracell Vesicles. 2020;10:e12025. doi: 10.1002/jev2.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu C., Zhao J., Tian F., Cai L., Zhang W., Feng Q., Chang J., Wan F., Yang Y., Dai B., et al. Low-cost thermophoretic profiling of extracellular-vesicle surface proteins for the early detection and classification of cancers. Nat. Biomed. Eng. 2019;3:183–193. doi: 10.1038/s41551-018-0343-6. [DOI] [PubMed] [Google Scholar]
- 106.Coy J.F. EDIM-TKTL1/Apo10 Blood Test: An Innate Immune System Based Liquid Biopsy for the Early Detection, Characterization and Targeted Treatment of Cancer. Int. J. Mol. Sci. 2017;18:878. doi: 10.3390/ijms18040878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feyen O., Coy J.F., Prasad V., Schierl R., Saenger J., Baum R.P. EDIM-TKTL1 blood test: A noninvasive method to detect upregulated glucose metabolism in patients with malignancies. Future Oncol. 2012;8:1349–1359. doi: 10.2217/fon.12.98. [DOI] [PubMed] [Google Scholar]
- 108.Grimm M., Schmitt S., Teriete P., Biegner T., Stenzl A., Hennenlotter J., Muhs H.J., Munz A., Nadtotschi T., König K., et al. A biomarker based detection and characterization of carcinomas exploiting two fundamental biophysical mechanisms in mammalian cells. BMC Cancer. 2013;13:569. doi: 10.1186/1471-2407-13-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saman S., Stagno M.J., Warmann S.W., Malek N.P., Plentz R.R., Schmid E. Biomarkers Apo10 and TKTL1: Epitope-detection in monocytes (EDIM) as a new diagnostic approach for cholangiocellular, pancreatic and colorectal carcinoma. Cancer Biomark. 2020;27:129–137. doi: 10.3233/CBM-190414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burg S., Audrey L., Grust, Feyen O., Failing K., Banat G.-A., Coy J., Grimm M., Gosau M., Smeets R. Clinical Study Blood-Test Based Targeted Visualization Enables Early Detection of Premalignant and Malignant Tumors in Asymptomatic Individuals. J. Clin. Med. Images. 2022;6:1–12. [Google Scholar]
- 111.Zyagnum AG EDIM® TECHNOLOGY. [(accessed on 21 August 2022)]. Available online: https://www.zyagnum.com/en/edim-technology/
- 112.Zhang H., Zhao L., Jiang J., Zheng J., Yang L., Li Y., Zhou J., Liu T., Xu J., Lou W., et al. Multiplexed nanomaterial-assisted laser desorption/ionization for pan-cancer diagnosis and classification. Nat. Commun. 2022;13:617. doi: 10.1038/s41467-021-26642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gupta A., Sagar G., Siddiqui Z., Rao K.V.S., Nayak S., Saquib N., Anand R. A non-invasive method for concurrent detection of early-stage women-specific cancers. Sci. Rep. 2022;12:2301. doi: 10.1038/s41598-022-06274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gatto F., Bratulic S., Cavarretta I.T.R., Alfano M., Maccari F., Galeotti F., Volpi N., Edqvist P.-H., Levin M., Nyman J., et al. Detection of any-stage cancer using plasma and urine glycosaminoglycans. J. Clin. Oncol. 2021;39:3034. doi: 10.1200/JCO.2021.39.15_suppl.3034. [DOI] [Google Scholar]
- 115.Elypta Systems Biology to Identify Biomarkers of Cancer Metabolism—The GAGome. [(accessed on 21 August 2022)]. Available online: https://www.elypta.com/science.
- 116.Kusakabe M., Sato M., Nakamura Y., Mikami H., Lin J., Nagase H. Elemental analysis by Metallobalance provides a complementary support layer over existing blood biochemistry panel-based cancer risk assessment. PeerJ. 2021;9:e12247. doi: 10.7717/peerj.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cameron J.M., Sala A., Antoniou G., Brennan P.M., Conn J.J.A., Connal S., Palmer D.S., Smith B.R., Baker M.J. Abstract 5920: Multi-cancer early detection with a spectroscopic liquid biopsy platform. Cancer Res. 2022;82:5920. doi: 10.1158/1538-7445.AM2022-5920. [DOI] [Google Scholar]
- 118.Dxcover Limited The Dxcover Platform for Detection of Cancer. [(accessed on 21 August 2022)]. Available online: https://www.dxcover.com/technology.
- 119.Ronald J.A., Chuang H.Y., Dragulescu-Andrasi A., Hori S.S., Gambhir S.S. Detecting cancers through tumor-activatable minicircles that lead to a detectable blood biomarker. Proc. Natl. Acad. Sci. USA. 2015;112:3068–3073. doi: 10.1073/pnas.1414156112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aalipour A., Chuang H.Y., Murty S., D’Souza A.L., Park S.M., Gulati G.S., Patel C.B., Beinat C., Simonetta F., Martinić I., et al. Engineered immune cells as highly sensitive cancer diagnostics. Nat. Biotechnol. 2019;37:531–539. doi: 10.1038/s41587-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dong S., Li W., Wang L., Hu J., Song Y., Zhang B., Ren X., Ji S., Li J., Xu P., et al. Histone-Related Genes Are Hypermethylated in Lung Cancer and Hypermethylated HIST1H4F Could Serve as a Pan-Cancer Biomarker. Cancer Res. 2019;79:6101–6112. doi: 10.1158/0008-5472.CAN-19-1019. [DOI] [PubMed] [Google Scholar]
- 122.Moskalev E.A., Jandaghi P., Fallah M., Manoochehri M., Botla S.K., Kolychev O.V., Nikitin E.A., Bubnov V.V., von Knebel Doeberitz M., Strobel O., et al. GHSR DNA hypermethylation is a common epigenetic alteration of high diagnostic value in a broad spectrum of cancers. Oncotarget. 2014;6:4418. doi: 10.18632/oncotarget.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dai X., Sun X., Wu Y., Lv Z., Yu Z., Yuan Y., Sun L. Site-Specific Hypermethylation of SST 1stExon as a Biomarker for Predicting the Risk of Gastrointestinal Tract Cancers. Dis. Mrk. 2022;2022:4570290. doi: 10.1155/2022/4570290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Margolin G., Petrykowska H.M., Jameel N., Bell D.W., Young A.C., Elnitski L. Robust Detection of DNA Hypermethylation of ZNF154 as a Pan-Cancer Locus with in Silico Modeling for Blood-Based Diagnostic Development. J. Mol. Diagn. 2016;18:283–298. doi: 10.1016/j.jmoldx.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan S.H., Ida H., Lau Q.C., Goh B.C., Chieng W.S., Loh M., Ito Y. Detection of promoter hypermethylation in serum samples of cancer patients by methylation-specific polymerase chain reaction for tumour suppressor genes including RUNX3. Oncol. Rep. 2007;18:1225–1230. doi: 10.3892/or.18.5.1225. [DOI] [PubMed] [Google Scholar]
- 126.Melson J., Li Y., Cassinotti E., Melnikov A., Boni L., Ai J., Greenspan M., Mobarhan S., Levenson V., Deng Y. Commonality and differences of methylation signatures in the plasma of patients with pancreatic cancer and colorectal cancer. Int. J. Cancer. 2014;134:2656–2662. doi: 10.1002/ijc.28593. [DOI] [PubMed] [Google Scholar]
- 127.Kang S., Li Q., Chen Q., Zhou Y., Park S., Lee G., Grimes B., Krysan K., Yu M., Wang W., et al. CancerLocator: Non-invasive cancer diagnosis and tissue-of-origin prediction using methylation profiles of cell-free DNA. Genome Biol. 2017;18:53. doi: 10.1186/s13059-017-1191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li W., Li Q., Kang S., Same M., Zhou Y., Sun C., Liu C.C., Matsuoka L., Sher L., Wong W.H., et al. CancerDetector: Ultrasensitive and non-invasive cancer detection at the resolution of individual reads using cell-free DNA methylation sequencing data. Nucleic Acids Res. 2018;46:e89. doi: 10.1093/nar/gky423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu L., Toung J.M., Jassowicz A.F., Vijayaraghavan R., Kang H., Zhang R., Kruglyak K.M., Huang H.J., Hinoue T., Shen H., et al. Targeted methylation sequencing of plasma cell-free DNA for cancer detection and classification. Ann. Oncol. 2018;29:1445–1453. doi: 10.1093/annonc/mdy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gao Q., Li B., Cai S., Xu J., Wang C., Fang S., Qiu F., Su J., Xu F., Wen X., et al. LBA3 Early detection and localization of multiple cancers using a blood-based methylation assay (ELSA-seq) Ann. Oncol. 2020;31:S1358. doi: 10.1016/j.annonc.2020.10.292. [DOI] [Google Scholar]
- 131.Han T., Hong Y., Zhihua P., Xiaofeng S., Yu J., Chen G., Chen W., He J. An ultrasensitive method for noninvasive pan-cancer early detection based on targeted methylation sequencing of cell-free DNA. J. Clin. Oncol. 2021;39:10544. doi: 10.1200/JCO.2021.39.15_suppl.10544. [DOI] [Google Scholar]
- 132.Han T., Liu T., Suxing L., Li X., Zhang Q., Yu J., Hong Y., Li Y., Huang Y.S., He J., et al. An ultrasensitive approach for cancer screening and tissue of origin prediction based on targeted methylation sequencing of cell-free DNA. J. Clin. Oncol. 2022;40:10553. doi: 10.1200/JCO.2022.40.16_suppl.10553. [DOI] [Google Scholar]
- 133.Kinross J., Kruusmaa K., Bitenc M., Chersicola M., Pulverer W., Weinhaeusel A. 97P A panel of methylation markers for multi-cancer detection from plasma. Ann. Oncol. 2020;31:S280. doi: 10.1016/j.annonc.2020.08.218. [DOI] [Google Scholar]
- 134.Valouev A., Zotenko E., Snyder M., Eid C., Nguyen N., Min J., He Y., Jaimovich A., Axelrod H., Natarajan P., et al. Abstract 2141: Development of a highly-sensitive targeted cell-free DNA epigenomic assay for early-stage multi-cancer screening. Cancer Res. 2022;82:2141. doi: 10.1158/1538-7445.AM2022-2141. [DOI] [Google Scholar]
- 135.Zhao G.Q., Bao Y., Wang H., Vu P., Patel S.P., Lin S. A novel NGS kit solution for multi-cancer early detection using circulating cell free DNA based methylation analysis. J. Clin. Oncol. 2022;40:10542. doi: 10.1200/JCO.2022.40.16_suppl.10542. [DOI] [Google Scholar]
- 136.Xu L., Wang J., Yang T., Tao J., Liu X., Ye Z., Zhao D., Jiang Z., Chen Z., Fan J.-B. Abstract 4601: Toward the development of a $100 screening test for 6 major cancer types. Cancer Res. 2020;80:4601. doi: 10.1158/1538-7445.AM2020-4601. [DOI] [Google Scholar]
- 137.Xu L., Wang J., Ma W., Liu X., Yang T., Li S., Hu Q., Tao J., Ye Z., Chen Z., et al. Abstract 2610: A high performance blood test for multiple cancer early screening. Cancer Res. 2021;81:2610. doi: 10.1158/1538-7445.AM2021-2610. [DOI] [Google Scholar]
- 138.Xu L., Wang J., Ma W., Liu X., Li S., Chen S., Hu Q., Yang T., Ye Z., Chen Z., et al. Validation of a high performing blood test for multiple major cancer screenings. J. Clin. Oncol. 2021;39:10561. doi: 10.1200/JCO.2021.39.15_suppl.10561. [DOI] [Google Scholar]
- 139.Nagasaka T., Tanaka N., Cullings H.M., Sun D.-S., Sasamoto H., Uchida T., Koi M., Nishida N., Naomoto Y., Boland C.R., et al. Analysis of Fecal DNA Methylation to Detect Gastrointestinal Neoplasia. JNCI J. Natl. Cancer Inst. 2009;101:1244–1258. doi: 10.1093/jnci/djp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Katerov S., Vaccaro A., Hennek J., Carlson J., Taylor W.R., Mahoney D., Kisiel J.B., Allawi H.T. Abstract 111: Accurate multi-cancer detection using methylated DNA markers and proteins in plasma. Cancer Res. 2021;81:111. doi: 10.1158/1538-7445.AM2021-111. [DOI] [Google Scholar]
- 141.Allawi H.T., Katerov S., Vaccaro A., Fleming H.L., Rugowski D.E., Otto B., Heilberger J., Cassel J., Hennek J., Taylor W., et al. Abstract 631: Validation of a panel of methylated DNA and protein markers for multi-cancer detection in plasma. Cancer Res. 2022;82:631. doi: 10.1158/1538-7445.AM2022-631. [DOI] [Google Scholar]
- 142.Chan K.C.A., Jiang P., Chan C.W.M., Sun K., Wong J., Hui E.P., Chan S.L., Chan W.C., Hui D.S.C., Ng S.S.M., et al. Noninvasive detection of cancer-associated genome-wide hypomethylation and copy number aberrations by plasma DNA bisulfite sequencing. Proc. Natl. Acad. Sci. USA. 2013;110:18761–18768. doi: 10.1073/pnas.1313995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Che H., Jatsenko T., Lenaerts L., Dehaspe L., Vancoillie L., Brison N., Parijs I., Van Den Bogaert K., Fischerova D., Heremans R., et al. Pan-Cancer Detection and Typing by Mining Patterns in Large Genome-Wide Cell-Free DNA Sequencing Datasets. Clin. Chem. 2022;68:1164–1176. doi: 10.1093/clinchem/hvac095. [DOI] [PubMed] [Google Scholar]
- 144.Liu W., Li J., Zhang P., Hou Q., Feng S., Liu L., Cui D., Shi H., Fu Y., Luo Y. A novel pan-cancer biomarker plasma heat shock protein 90alpha and its diagnosis determinants in clinic. Cancer Sci. 2019;110:2941–2959. doi: 10.1111/cas.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Lange K., de Boer E.N., Bosga A., Alimohamed M.Z., Johansson L.F., Mulder A.B., Vellenga E., van Diemen C.C., Deelen P., van den Berg E., et al. Targeted RNA-Sequencing Enables Detection of Relevant Translocations and Single Nucleotide Variants and Provides a Method for Classification of Hematological Malignancies-RANKING. Clin. Chem. 2020;66:1521–1530. doi: 10.1093/clinchem/hvaa221. [DOI] [PubMed] [Google Scholar]
- 146.Tsvetkov P.O., Eyraud R., Ayache S., Bougaev A.A., Malesinski S., Benazha H., Gorokhova S., Buffat C., Dehais C., Sanson M., et al. An AI-Powered Blood Test to Detect Cancer Using NanoDSF. Cancers. 2021;13:1294. doi: 10.3390/cancers13061294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tomczak K., Czerwińska P., Wiznerowicz M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp. Oncol. 2015;19:A68–A77. doi: 10.5114/wo.2014.47136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goldman M., Craft B., Swatloski T., Ellrott K., Cline M., Diekhans M., Ma S., Wilks C., Stuart J., Haussler D., et al. The UCSC Cancer Genomics Browser: Update 2013. Nucleic Acids Res. 2013;41:D949–D954. doi: 10.1093/nar/gks1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chandrashekar D.S., Bashel B., Balasubramanya S.A.H., Creighton C.J., Ponce-Rodriguez I., Chakravarthi B., Varambally S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weinstein J.N., Collisson E.A., Mills G.B., Shaw K.R., Ozenberger B.A., Ellrott K., Shmulevich I., Sander C., Stuart J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Human Genomics The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Barrett T., Troup D.B., Wilhite S.E., Ledoux P., Rudnev D., Evangelista C., Kim I.F., Soboleva A., Tomashevsky M., Edgar R. NCBI GEO: Mining tens of millions of expression profiles--database and tools update. Nucleic Acids Res. 2007;35:D760–D765. doi: 10.1093/nar/gkl887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Manoochehri M., Wu Y., Giese N.A., Strobel O., Kutschmann S., Haller F., Hoheisel J.D., Moskalev E.A., Hackert T., Bauer A.S. SST gene hypermethylation acts as a pan-cancer marker for pancreatic ductal adenocarcinoma and multiple other tumors: Toward its use for blood-based diagnosis. Mol. Oncol. 2020;14:1252–1267. doi: 10.1002/1878-0261.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen W., Li G., Peng J., Dai W., Su Q., He Y. Transcriptomic analysis reveals that heat shock protein 90α is a potential diagnostic and prognostic biomarker for cancer. Eur. J Cancer Prev. 2020;29:357–364. doi: 10.1097/CEJ.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 156.Liu B., Liu Y., Pan X., Li M., Yang S., Li S.C. DNA Methylation Markers for Pan-Cancer Prediction by Deep Learning. Genes. 2019;10:778. doi: 10.3390/genes10100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ibrahim J., Op de Beeck K., Fransen E., Peeters M., Van Camp G. Genome-wide DNA methylation profiling and identification of potential pan-cancer and tumor-specific biomarkers. Mol. Oncol. 2022;16:2432–2447. doi: 10.1002/1878-0261.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fan S., Tang J., Li N., Zhao Y., Ai R., Zhang K., Wang M., Du W., Wang W. Integrative analysis with expanded DNA methylation data reveals common key regulators and pathways in cancers. NPJ Genom. Med. 2019;4:2. doi: 10.1038/s41525-019-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yuan B., Yang D., Rothberg B.E.G., Chang H., Xu T. Unsupervised and supervised learning with neural network for human transcriptome analysis and cancer diagnosis. Sci. Rep. 2020;10:19106. doi: 10.1038/s41598-020-75715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun K., Wang J., Wang H., Sun H. GeneCT: A generalizable cancerous status and tissue origin classifier for pan-cancer biopsies. Bioinformatics. 2018;34:4129–4130. doi: 10.1093/bioinformatics/bty524. [DOI] [PubMed] [Google Scholar]
- 161.Wang A., Hai R., Rider P.J., He Q. Noncoding RNAs and Deep Learning Neural Network Discriminate Multi-Cancer Types. Cancers. 2022;14:352. doi: 10.3390/cancers14020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yuan F., Li Z., Chen L., Zeng T., Zhang Y.H., Ding S., Huang T., Cai Y.D. Identifying the Signatures and Rules of Circulating Extracellular MicroRNA for Distinguishing Cancer Subtypes. Front. Genet. 2021;12:651610. doi: 10.3389/fgene.2021.651610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huang S., Yang J., Fong S., Zhao Q. Artificial intelligence in cancer diagnosis and prognosis: Opportunities and challenges. Cancer Lett. 2020;471:61–71. doi: 10.1016/j.canlet.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 164.Shimizu H., Nakayama K.I. Artificial intelligence in oncology. Cancer Sci. 2020;111:1452–1460. doi: 10.1111/cas.14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu C., Jackson S.A. Machine learning and complex biological data. Genome Biol. 2019;20:76. doi: 10.1186/s13059-019-1689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ledesma D., Symes S., Richards S. Advancements within Modern Machine Learning Methodology: Impacts and Prospects in Biomarker Discovery. Curr. Med. Chem. 2021;28:6512–6531. doi: 10.2174/0929867328666210208111821. [DOI] [PubMed] [Google Scholar]
- 167.Boehm K.M., Khosravi P., Vanguri R., Gao J., Shah S.P. Harnessing multimodal data integration to advance precision oncology. Nat. Rev. Cancer. 2022;22:114–126. doi: 10.1038/s41568-021-00408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ding W., Chen G., Shi T. Integrative analysis identifies potential DNA methylation biomarkers for pan-cancer diagnosis and prognosis. Epigenetics. 2019;14:67–80. doi: 10.1080/15592294.2019.1568178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shimizu D., Taniue K., Matsui Y., Haeno H., Araki H., Miura F., Fukunaga M., Shiraishi K., Miyamoto Y., Tsukamoto S., et al. Pan-cancer methylome analysis for cancer diagnosis and classification of cancer cell of origin. Cancer Gene Ther. 2022;29:428–436. doi: 10.1038/s41417-021-00401-w. [DOI] [PubMed] [Google Scholar]
- 170.Peng D., Ge G., Xu Z., Ma Q., Shi Y., Zhou Y., Gong Y., Xiong G., Zhang C., He S., et al. Diagnostic and prognostic biomarkers of common urological cancers based on aberrant DNA methylation. Epigenomics. 2018;10:1189–1199. doi: 10.2217/epi-2018-0017. [DOI] [PubMed] [Google Scholar]
- 171.Vrba L., Futscher B.W. A suite of DNA methylation markers that can detect most common human cancers. Epigenetics. 2018;13:61–72. doi: 10.1080/15592294.2017.1412907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ibrahim J., Op de Beeck K., Fransen E., Peeters M., Van Camp G. The Gasdermin E Gene Has Potential as a Pan-Cancer Biomarker, While Discriminating between Different Tumor Types. Cancers. 2019;11:1810. doi: 10.3390/cancers11111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang S., Cai Y. Identification of the functional alteration signatures across different cancer types with support vector machine and feature analysis. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:2218–2227. doi: 10.1016/j.bbadis.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 174.Grewal J.K., Tessier-Cloutier B., Jones M., Gakkhar S., Ma Y., Moore R., Mungall A.J., Zhao Y., Taylor M.D., Gelmon K., et al. Application of a Neural Network Whole Transcriptome-Based Pan-Cancer Method for Diagnosis of Primary and Metastatic Cancers. JAMA Netw. Open. 2019;2:e192597. doi: 10.1001/jamanetworkopen.2019.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hsu Y.H., Si D. Cancer Type Prediction and Classification Based on RNA-sequencing Data; Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Honolulu, HI, USA. 18–21 July 2018; pp. 5374–5377. [DOI] [PubMed] [Google Scholar]
- 176.Mohammed M., Mwambi H., Mboya I.B., Elbashir M.K., Omolo B. A stacking ensemble deep learning approach to cancer type classification based on TCGA data. Sci. Rep. 2021;11:15626. doi: 10.1038/s41598-021-95128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhu L., Miao Y., Xi F., Jiang P., Xiao L., Jin X., Fang M. Identification of Potential Biomarkers for Pan-Cancer Diagnosis and Prognosis Through the Integration of Large-Scale Transcriptomic Data. Front Pharm. 2022;13:870660. doi: 10.3389/fphar.2022.870660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gobin E., Bagwell K., Wagner J., Mysona D., Sandirasegarane S., Smith N., Bai S., Sharma A., Schleifer R., She J.-X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer. 2019;19:581. doi: 10.1186/s12885-019-5768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang C., Guo C., Li Y., Liu K., Zhao Q., Ouyang L. Identification of Claudin-6 as a Molecular Biomarker in Pan-Cancer Through Multiple Omics Integrative Analysis. Front. Cell Dev. Biol. 2021;9:726656. doi: 10.3389/fcell.2021.726656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang C., Guo C., Li Y., Ouyang L., Zhao Q., Liu K. The role of YTH domain containing 2 in epigenetic modification and immune infiltration of pan-cancer. J. Cell Mol. Med. 2021;25:8615–8627. doi: 10.1111/jcmm.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yuan Y., Jiang X., Tang L., Wang J., Duan L. Comprehensive Analysis of the Prognostic and Immunological Role of PAFAH1B in Pan-Cancer. Front. Mol. Biosci. 2021;8:799497. doi: 10.3389/fmolb.2021.799497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen J., Gao G., Li L., Ding J., Chen X., Lei J., Long H., Wu L., Long X., He L., et al. Pan-Cancer Study of SHC-Adaptor Protein 1 (SHC1) as a Diagnostic, Prognostic and Immunological Biomarker in Human Cancer. Front. Genet. 2022;13:817118. doi: 10.3389/fgene.2022.817118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen G., Luo D., Zhong N., Li D., Zheng J., Liao H., Li Z., Lin X., Chen Q., Zhang C., et al. GPC2 Is a Potential Diagnostic, Immunological, and Prognostic Biomarker in Pan-Cancer. Front. Immunol. 2022;13:857308. doi: 10.3389/fimmu.2022.857308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hongzhen Z., Yanyu L., Xuexiang L., Meiyu D., Xiaoli C., Yun G., Jingfan C., Shengming D. The diagnostic and prognostic significance of long non-coding RNA CRNDE in pan-cancer based on TCGA, GEO and comprehensive meta–analysis. Pathol. Res. Pract. 2019;215:256–264. doi: 10.1016/j.prp.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 185.Ching T., Peplowska K., Huang S., Zhu X., Shen Y., Molnar J., Yu H., Tiirikainen M., Fogelgren B., Fan R., et al. Pan-Cancer Analyses Reveal Long Intergenic Non-Coding RNAs Relevant to Tumor Diagnosis, Subtyping and Prognosis. EBioMedicine. 2016;7:62–72. doi: 10.1016/j.ebiom.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xu D., Wang L., Pang S., Cao M., Wang W., Yu X., Xu Z., Xu J., Wang H., Lu J., et al. The Functional Characterization of Epigenetically Related lncRNAs Involved in Dysregulated CeRNA-CeRNA Networks Across Eight Cancer Types. Front. Cell Dev. Biol. 2021;9:649755. doi: 10.3389/fcell.2021.649755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang D., Zou L., Luo J., Zhang C., Feng H., Qin G. Potential diagnostic and prognostic value of the long non-coding RNA SNHG3 in human cancers: A systematic review and meta-analysis. Int. J. Biol. Mrk. 2022;37:3–12. doi: 10.1177/03936155221077121. [DOI] [PubMed] [Google Scholar]
- 188.Cheerla N., Gevaert O. MicroRNA based Pan-Cancer Diagnosis and Treatment Recommendation. BMC Bioinform. 2017;18:32. doi: 10.1186/s12859-016-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lai C.H., Liang X.Z., Liang X.Y., Ma S.J., Li J.G., Shi M.F., Zhu X., Lan H.H., Zeng J.H. Study on miRNAs in Pan-Cancer of the Digestive Tract Based on the Illumina HiSeq System Data Sequencing. Biomed. Res. Int. 2019;2019:8016120. doi: 10.1155/2019/8016120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang A., Hu H. A Novel Blood-Based microRNA Diagnostic Model with High Accuracy for Multi-Cancer Early Detection. Cancers. 2022;14:1450. doi: 10.3390/cancers14061450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang B., Chen Z., Tao B., Yi C., Lin Z., Li Y., Shao W., Lin J., Chen J. m(6)A target microRNAs in serum for cancer detection. Mol. Cancer. 2021;20:170. doi: 10.1186/s12943-021-01477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ji X., Cui Q. Ancient genes can be served as pan-cancer diagnostic and prognostic biomarkers. J. Cell Mol. Med. 2020;24:6908–6915. doi: 10.1111/jcmm.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Braunstein G.D., Ofman J.J. Criteria for Evaluating Multi-cancer Early Detection Tests. Touchrev. Oncol. Haematol. 2021;17:3–6. doi: 10.17925/OHR.2021.17.1.3. [DOI] [Google Scholar]
- 194.Etzioni R., Gulati R., Weiss N.S. Multicancer Early Detection: Learning From the Past to Meet the Future. J. Natl. Cancer Inst. 2022;114:349–352. doi: 10.1093/jnci/djab168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Loomans-Kropp H.A., Umar A., Minasian L.M., Pinsky P.F. Multi-Cancer Early Detection Tests: Current Progress and Future Perspectives. Cancer Epidemiol. Biomark. Prev. 2022;31:512–514. doi: 10.1158/1055-9965.EPI-21-1387. [DOI] [PubMed] [Google Scholar]
- 196.Putcha G., Gutierrez A., Skates S. Multicancer Screening: One Size Does Not Fit All. JCO Precis. Oncol. 2021;5:574–576. doi: 10.1200/PO.20.00488. [DOI] [PubMed] [Google Scholar]
- 197.Hackshaw A., Berg C.D. An efficient randomised trial design for multi-cancer screening blood tests: Nested enhanced mortality outcomes of screening trial. Lancet Oncol. 2021;22:1360–1362. doi: 10.1016/S1470-2045(21)00204-7. [DOI] [PubMed] [Google Scholar]
- 198.Hubbell E., Clarke C.A., Aravanis A.M., Berg C.D. Modeled Reductions in Late-stage Cancer with a Multi-Cancer Early Detection Test. Cancer Epidemiol. Biomark. Prev. 2021;30:460–468. doi: 10.1158/1055-9965.EPI-20-1134. [DOI] [PubMed] [Google Scholar]
- 199.Hathaway C., Paetsch P., Li Y., Wu J., Asgarian S., Parker A., Welsh A., Deverka P., Cohain A. Association of Breast Cancer Screening Behaviors with Stage at Breast Cancer Diagnosis and Potential for Additive Multi-Cancer Detection via Liquid Biopsy Screening: A Claims-Based Study. Front. Oncol. 2021;11:688455. doi: 10.3389/fonc.2021.688455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hackshaw A., Cohen S.S., Reichert H., Kansal A.R., Chung K.C., Ofman J.J. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br. J. Cancer. 2021;125:1432–1442. doi: 10.1038/s41416-021-01498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tafazzoli A., Ramsey S.D., Shaul A., Chavan A., Ye W., Kansal A.R., Ofman J., Fendrick A.M. The Potential Value-Based Price of a Multi-Cancer Early Detection Genomic Blood Test to Complement Current Single Cancer Screening in the USA. Pharmacoeconomics. 2022;40:1107–1117. doi: 10.1007/s40273-022-01181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jiao B., Gulati R., Katki H.A., Castle P.E., Etzioni R. A Quantitative Framework to Study Potential Benefits and Harms of Multi-Cancer Early Detection Testing. Cancer Epidemiol. Biomark. Prev. 2022;31:38–44. doi: 10.1158/1055-9965.EPI-21-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Marlow L.A.V., Schmeising-Barnes N., Brain K., Duncombe S., Robb K.A., Round T., Sanderson S.C., Waller J. Multi-cancer early detection tests for cancer screening: A behavioural science perspective. Lancet Oncol. 2022;23:837–839. doi: 10.1016/S1470-2045(22)00161-9. [DOI] [PubMed] [Google Scholar]
- 204.Ewalt M.D., West H., Aisner D.L. Next Generation Sequencing—Testing Multiple Genetic Markers at Once. JAMA Oncol. 2019;5:1076. doi: 10.1001/jamaoncol.2019.0453. [DOI] [PubMed] [Google Scholar]
- 205.Soto J., Rodriguez-Antolin C., Vallespín E., de Castro Carpeño J., Ibanez de Caceres I. The impact of next-generation sequencing on the DNA methylation-based translational cancer research. Transl. Res. 2016;169:1–18. doi: 10.1016/j.trsl.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 206.Rossi G., Ignatiadis M. Promises and Pitfalls of Using Liquid Biopsy for Precision Medicine. Cancer Res. 2019;79:2798–2804. doi: 10.1158/0008-5472.CAN-18-3402. [DOI] [PubMed] [Google Scholar]
- 207.Connors D., Allen J., Alvarez J.D., Boyle J., Cristofanilli M., Hiller C., Keating S., Kelloff G., Leiman L., McCormack R., et al. International liquid biopsy standardization alliance white paper. Crit. Rev. Oncol./Hematol. 2020;156:103112. doi: 10.1016/j.critrevonc.2020.103112. [DOI] [PubMed] [Google Scholar]