Severe thrombocytopenia is a relatively uncommon event in myelodysplastic syndromes (MDS) and may occur in high-risk patients, advocating treatment with hypomethylating agents or with allogeneic bone marrow transplantation.1 In low-risk patients, thrombocytopenia may significantly influence the prognosis since a major bleeding may be a life-threatening complication. Since hypomethylating agents are not licensed in Europe for low-risk MDS, this condition remains an unmet need. Immunosuppressants, such as corticosteroids, cyclosporine, or antithymocyte globulin, showed some efficacy, particularly in hypocellular cases.2 The activity of thrombopoietin receptor agonists (TPO-RA) is under evaluation, and preliminary results showed that eltrombopag is effective in raising platelet (PLT) counts and reducing bleeding events in about half of the low-risk MDS patients.3 Similar data have been reported for Romiplostim,4 although a warning on possible blast progression deserves further investigation. Danazol is a synthetic steroid with antigonadotropic and antiestrogenic activities. It possesses androgenic, progestational, and glucocorticoid anabolic properties. The drug inhibits steroidogenic enzymes and the pituitary output of gonadotropins (follicle stimulating hormone and luteinizing hormone) both in males and females, by blocking the ovarian/testicular function. Its half-life is 12–18 hours. This drug is licensed for the treatment of endometriosis, fibrocystic mastopathy, and hereditary angioedema. Its efficacy in autoimmune cytopenias, particularly immune thrombocytopenia and aplastic anemia, is well documented.5 In MDS, danazol use was explored in retrospective reports,6–13 although some limitations (low number, heterogeneity of patient populations, variable target cytopenias, and different response criteria), did not allow definite conclusions.
Here, we present the results of a multicentre observational study exploring the efficacy and safety of danazol in patients with thrombocytopenic MDS or MDS/myeloproliferative neoplasms (MDS/MPN), focusing on predictors of response and impact on outcome. This was a retrospective multicenter study including MDS or MDS/MPN overlap patients with thrombocytopenia (PLT count <50 × 109/L) and an international prognostic scoring system (IPSS) score of low or intermediate-1 (for MDS only) treated with danazol at 6 centers, in northern Italy. No patient had received hypomethylating agents before danazol, based on the exclusion of these patients from 5-azacytidine treatment in Europe.
PLT and erythroid responses were evaluated according to the International Working Group-2018 criteria (Suppl. Materials and Methods). The study was approved by the local ethical committee and conducted according to the Helsinki Declaration.
As shown in Table 1, a total of 57 patients were included (51 MDS, 5 chronic myelomonocytic leukemia, and 1 MDS/MPN unclassified). They were mainly males (34/57) with a median age of 69 years (27–87). They had all IPSS low or intermediate-1, while IPSS-revised (IPSS-R) risk score was equally distributed between very low/low (45%) and intermediate/higher (55%). Notably, only 3 patients with an int-1 IPSS score had an IPSS-R risk score >3.5 and had not received hypomethylating therapy due to indication based on IPSS. Seventeen percent (9/43 evaluable) of patients had a hypocellular bone marrow (<25%), while 26% (11/43) had WHO grade 2 fibrosis. All patients were thrombocytopenic (median 20 × 109/L; range 2–49), and 30% (n = 17/57) had required at least 1 PLT pool transfusion in the previous 8 weeks (only 2/17, 12%, due to bleeding). One patient was receiving double antiplatelet therapy. Furthermore, 52% were anemic (median hemoglobin 11.1 g/dL, 6.9–15.7), 25% had received at least 1 red blood cell transfusion in the 16 weeks before danazol initiation, and 8 patients had severe neutropenia (median absolute neutrophil count in all cases 2 × 109/L; range 0.2–20).
Table 1.
Main Features, Doses, and Response Rates of MDS or MDS/MPN Patients Treated With Danazol
| Age and sex | |
| Age at diagnosis (y, range) | 69 (27–87) |
| Female (n, %) | 23/57 (40) |
| Male (n, %) | 34/57 (60) |
| WHO-2016 diagnosis | |
| MDS-MLD | 28/57 (49) |
| MDS-SLD | 7/57 (12) |
| MDS-EB1 | 11/57 (19) |
| MDS-EB2 | 2/57 (4) |
| MDS-RS-MLD | 1/57 (2) |
| CMML | 5/57 (9) |
| MDS/MPN (not CMML) | 1/57 (2) |
| MDS-U | 2/57 (4) |
| Bone marrow features at diagnosis (n, %) | |
| Available core biopsy at diagnosis | 43/57 (75) |
| Hypocellular (cellularity <25%) | 9/43 (21) |
| Fibrosis MF 0–1 | 30/43 (70) |
| Fibrosis MF 2 | 11/43 (26) |
| MF-not evaluated | 2/43 (5) |
| IPSS at diagnosis (n, %), only for MDS (n = 51) | |
| Low | 17/51 (33) |
| Int-1 | 34/51 (67) |
| IPSS-R at diagnosis (n, %), only for MDS (n = 51) | |
| Very low | 2/51 (4) |
| Low | 21/51 (41) |
| Intermediate | 15/51 (29) |
| High | 9/51 (18) |
| Very high | 4/51 (8) |
| Blood counts before treatment | |
| Hb (g/dL) | 11.1 (6.9–15.7) |
| PLT (103/μL) | 20 (2–49) |
| ANC (103/μL) | 2.00 (0.26–20.4) |
| Danazol dosing and timing | |
| Mean daily dose (mg, range) | 500 (88–667) |
| Dose reductions due to any cause (n, %) | 19/57 (33) |
| Median treatment duration (mo, range) | 12.0 (0.6–149.5) |
| Concomitant treatments at danazol start (n, %) | |
| Erythropoiesis stimulating agents | 5/57 (9) |
| Corticosteroids | 12/57 (21) |
| Cyclosporin A | 1/57 (2) |
| No concomitant treatments | 41/57 (72) |
| Response data | |
| Median follow-up (mo, range) | 16.2 (0.7–171) |
| PLT response (n, %) | 29/57 (51) |
| Median time to PLT response (mo, range) | 3.3 (1.3–24.6) |
| Erythroid hematological improvement (n, %) | 10/24 (42) |
| Neutrophil hematological improvement (n, %) | 4/9 (44) |
ANC = absolute neutrophil count; CMML = chronic myelomonocytic leukemia; Hb = hemoglobin; IPSS = International Prognostic Scoring System; IPSS-R = revised International Prognostic Scoring System; MDS/MPN = myelodysplastic syndrome/myeloproliferative neoplasms; MDS EB1 = myelodysplastic syndrome with excess blast 1; MDS EB2 = myelodysplastic syndrome with excess blast 2; PLT = platelet.
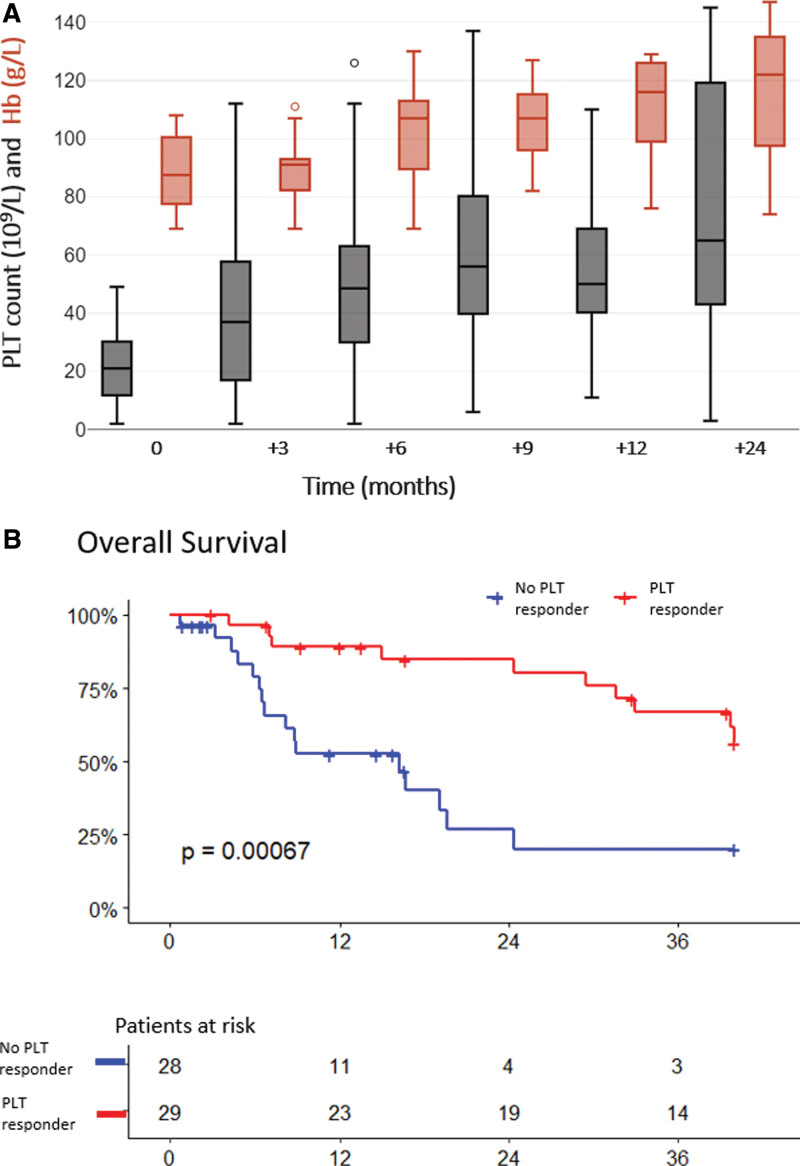
Danazol was administered for a median of 12.0 months (0.6–149.5) at a daily dose of 500 mg (range 88–667). Concomitant treatments at danazol start included corticosteroids (21%), recombinant erythropoietin (rEPO 9%), and cyclosporine in 1 patient. Overall, PLT response was obtained in 51% of evaluable patients (29/57). Median time to response was 3.3 months (1.3–24.6) from treatment start. PLT count progressively increased at each time point as shown in Figure 1A: from a median of 21 × 109/L at baseline to 37, 50, and 66 × 109/L at 3, 6, 9 months, respectively, and it stabilized thereafter. Among those who met response criteria, the median (range) increase in PLTs was +38 × 109/L (+20 to +341 × 109/L) at the time of first response and +50 × 109/L (+23 to +341 × 109/L) at the time of best response. Among those who did not meet response criteria, the median PLT count variation at 3 months was +2 × 109/L (−12 to +23 × 109/L). Registered bleeding events were mainly grade 1–2 (11%) and all occurred in nonresponders. One nonresponding patient died due to intracranial bleeding. Notably, 23.5% of patients (n = 4/17) who had received at least 1 PLT transfusion in the 8 weeks preceding danazol resolved their transfusion need thereafter. Similarly, 28.5% of patients (n = 4/14) with history of packed red cell transfusion in the previous 8 weeks reached a transfusion-free status after danazol start. PLT response was long-lasting, with 52% maintaining PLT response, only 14% relapses (n = 4/29) and 34% (n = 10/29) of responders who were lost in follow-up while on response, after a median of 17.2 months. We observed higher PLT response rates in MDS/MPN versus MDS patients (6/6, 100% versus 23/51, 45%, respectively; P = 0.02). Myelodysplastic syndrome with excess blast 1 or 2 trended to lower responses (4/13, 31%), although not significantly.
Figure 1.
Blood counts and survival of MDS or MDS/MPN patients after danazol start. (A) PLT values (gray box plots) and Hb levels (red box plots) overtime from danazol start. Only Hb values from patients with baseline Hb <110 g/L were reported. (B) Overall survival of PLT-responders vs nonresponders. Survival was censored at 40 mo from danazol start. Hb = hemoglobin; PLT = platelet.
Erythroid improvement, evaluated in a subgroup of patients with anemia, was obtained in 10 of 24 (42%) patients after a median of 6.8 months (2.5–23.4), independently of concomitant rEPO or steroids administration (Suppl. Table S1). Erythroid improvement occurred only in patients with baseline low transfusion burden (n = 3/10, all major responses) or transfusion-free at baseline (n = 7/11).
Adverse events (AEs) were observed in 68% of cases, mainly grade 1–2, the most common being creatinine increase (45%; 5% grade 3), transaminitis (35%; 6% grade 3), and mood disorders (7%) (Suppl. Table S2). AEs required dose reduction in 11 (19%) and interruption in 5 (9%) of the patients.
Progression to acute myeloid leukemia equally affected PLT-responders (n = 3/29, 10%, at +6, +18, and +38 months from danazol start) and nonresponders (n = 4/28, 14%; at +2, +14, +19, and +20 months from danazol, start). Twenty-eight patients died during the follow-up. Responders had a significantly lower mortality rate compared with nonresponders (34% versus 64%, P = 0.035) and a longer overall survival (P < 0.001; Figure 1B). The occurrence of a PLT response was significantly associated with lower risk of death by multivariate analysis (hazard ratio, 0.24; 95% CI, 0.09-0.64), independently of other features.
This is one of the largest series ever reported on the use of danazol in thrombocytopenic MDS patients in the real world. In fact, patients included were treated at Italian centers outside clinical trials and with a relatively inexpensive and easily available medication. We observed a PLT response in half of cases, which was long-lasting in more than half of the subjects after a median of 16.2-month follow-up. Treatment was well tolerated with mainly low-grade AEs and a small percentage of discontinuation.
Such response rate well compares with historical data about danazol use in MDS14 and with response rates to TPO-RA eltrombopag3 and romiplostim.4 An interesting result is the positive association of PLT response with survival, although the retrospective nature of the study does not allow definitive conclusions. However, we note that only 1 bleeding-related death was reported in nonresponders (ie, an intracranial bleeding in a patient who did not respond to danazol, with PLT counts persistently below 10 × 109/L), while no bleeding deaths occurred among responders.
Notably, response requires time to establish, with a median time to PLT improvement of 3.3 months from treatment start and some responses occurring even after >1 year. This suggests that danazol therapy should be continued for a minimum of 6 months before deciding to withdraw it. The low rate of thrombocytopenia relapse is also a valuable outcome to take into account. The mechanism of action is unclear. Danazol efficacy in autoimmune thrombocytopenia has been associated with an impairment of the clearance of IgG-sensitized PLTs by reticuloendothelial cells.15 In MDS, along with megakaryocytic dysplasia, an increased destruction of PLT has also been reported, and danazol may act at this stage. Another possible mechanism relies on regulation of telomerase genes expression, with reduction of stem cell apoptosis, as reported in telomere diseases.16 A further consideration is the ability of danazol to induce erythroid improvement in anemic patients, with a rate of 42% in our series. These data suggest considering this therapy in low-risk anemic MDS patients who failed or are not candidate to erythropoietin, but also, in those with 5q deletion who failed lenalidomide, or in MDS with ring sideroblasts after luspatercept. The potential synergism of danazol in combination with TPO-RA and immunosuppressants for thrombocytopenia as well as with ESAs, lenalidomide, and luspatercept for anemia may be hypothesized and deserve future investigation.
Regarding safety, grade 3 or 4 AEs were rare, requiring danazol discontinuation in only 5 patients, and dose reductions in 11. A careful monitoring of creatinine and transaminases is advised during treatment, these being the more frequently altered parameters. Additionally, prostate-specific antigen dosing and, possibly, urologic referral are advised in male patients before starting the treatment, as well as abdominal echography to rule out focal lesions in the liver. Pregnancy should also be avoided while on treatment.
Danazol appears to be a good treatment option for thrombocytopenic MDS patients, particularly in low-risk setting. The rate of response well compared with newer drugs, particularly with TPO mimetics, while side effects seem manageable with proper monitoring. Notably, responses may require some months to establish, but appear long-lasting and are associated with improved survival. The retrospective nature of our findings and the limited number of patients may limit definite conclusions about danazol efficacy. However, our real-life data highlight the benefits of such old-fashioned drug and its potential as a valuable option for this neglected patient-population, often excluded from clinical trials of novel therapies.17
AUTHOR CONTRIBUTIONS
MR, BF, and AM equally contributed to study design. All authors followed patients, collected data, and created the dataset. AB and BF organized and analyzed the data. MR, BF, AB, and AM wrote the article. All authors revised the article for important intellectual content.
DATA AVAILABILITY
All data have been included in the article. Further information may be obtained upon reasonable request to the corresponding author.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
Publication charges were covered by the Italian Ministry of Health, Current Research Grant.
Supplementary Material
Footnotes
AM and BF have contributed equally to this work.
Ethics approval and consent to participate: The study was approved by the local ethical committee and patients gave informed consent.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
REFERENCES
- 1.Kantarjian H, Giles F, List A, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer. 2007;109:1705–1714. [DOI] [PubMed] [Google Scholar]
- 2.Stahl M, DeVeaux M, De Witte T, et al. The use of immunosuppressive therapy in MDS: clinical outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2:1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliva EN, Alati C, Santini V, et al. Eltrombopag versus placebo for low-risk myelodysplastic syndromes with thrombocytopenia (EQoL-MDS): phase 1 results of a single-blind, randomised, controlled, phase 2 superiority trial. Lancet Haematol. 2017;4:e127–e136. [DOI] [PubMed] [Google Scholar]
- 4.Giagounidis A, Mufti GJ, Fenaux P, et al. Results of a randomized, double-blind study of romiplostim versus placebo in patients with low/intermediate-1-risk myelodysplastic syndrome and thrombocytopenia. Cancer. 2014;120:1838–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn YS. Efficacy of danazol in hematologic disorders. Acta Haematol. 1990;84:122–129. [DOI] [PubMed] [Google Scholar]
- 6.Buzaid AC, Garewal HS, Lippman SM, et al. Danazol in the treatment of myelodysplastic syndromes. Eur J Haematol. 1987;39:346–348. [DOI] [PubMed] [Google Scholar]
- 7.Stadtmauer EA, Cassileth PA, Edelstein M, et al. Danazol treatment of myelodysplastic syndromes. Br J Haematol. 1991;77:502–508. [DOI] [PubMed] [Google Scholar]
- 8.Wattel E, Cambier N, Caulier MT, et al. Androgen therapy in myelodysplastic syndromes with thrombocytopenia: a report on 20 cases. Br J Haematol. 1994;87:205–208. [DOI] [PubMed] [Google Scholar]
- 9.Chan G, DiVenuti G, Miller K. Danazol for the treatment of thrombocytopenia in patients with myelodysplastic syndrome. Am J Hematol. 2002;71:166–171. [DOI] [PubMed] [Google Scholar]
- 10.Viniou N, Plata E, Terpos E, et al. Danazol therapy for thrombocytopenia in patients with myelodysplastic syndromes. Acta Haematol. 2002;107:234–236. [DOI] [PubMed] [Google Scholar]
- 11.Catalano L, Selleri C, Montuori N, et al. Danazol for myelodysplastic syndromes. Br J Haematol. 1993;85:230–231. [DOI] [PubMed] [Google Scholar]
- 12.Colunga-Pedraza PR, Colunga-Pedraza JE, Garza-Ledezma MA, et al. Danazol as first-line therapy for myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk. 2018;18:e109–e113. [DOI] [PubMed] [Google Scholar]
- 13.Choi EJ, Lee JH, Park HS, et al. Androgen therapy for patients with lower-risk myelodysplastic syndrome and significant cytopenia: a retrospective study. Br J Haematol. 2019;187:e4–e7. [DOI] [PubMed] [Google Scholar]
- 14.Aviles A, Rubio ME, Gomez J, et al. Randomized study of danazol vs. placebo in myelodysplastic syndromes. Arch Invest Med. 1989;20:183–188. [PubMed] [Google Scholar]
- 15.Schreiber AD, Chien P, Tomaski A, et al. Effect of danazol in immune thrombocytopenic purpura. N Engl J Med. 1987;316:503–508. [DOI] [PubMed] [Google Scholar]
- 16.Townsley DM, Dumitriu B, Liu D, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassanello G, Pasquale R, Barcellini W, et al. Novel therapies for unmet clinical needs in myelodysplastic syndromes. Cancers. 2022;14:4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data have been included in the article. Further information may be obtained upon reasonable request to the corresponding author.