Abstract
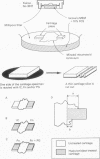
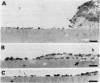
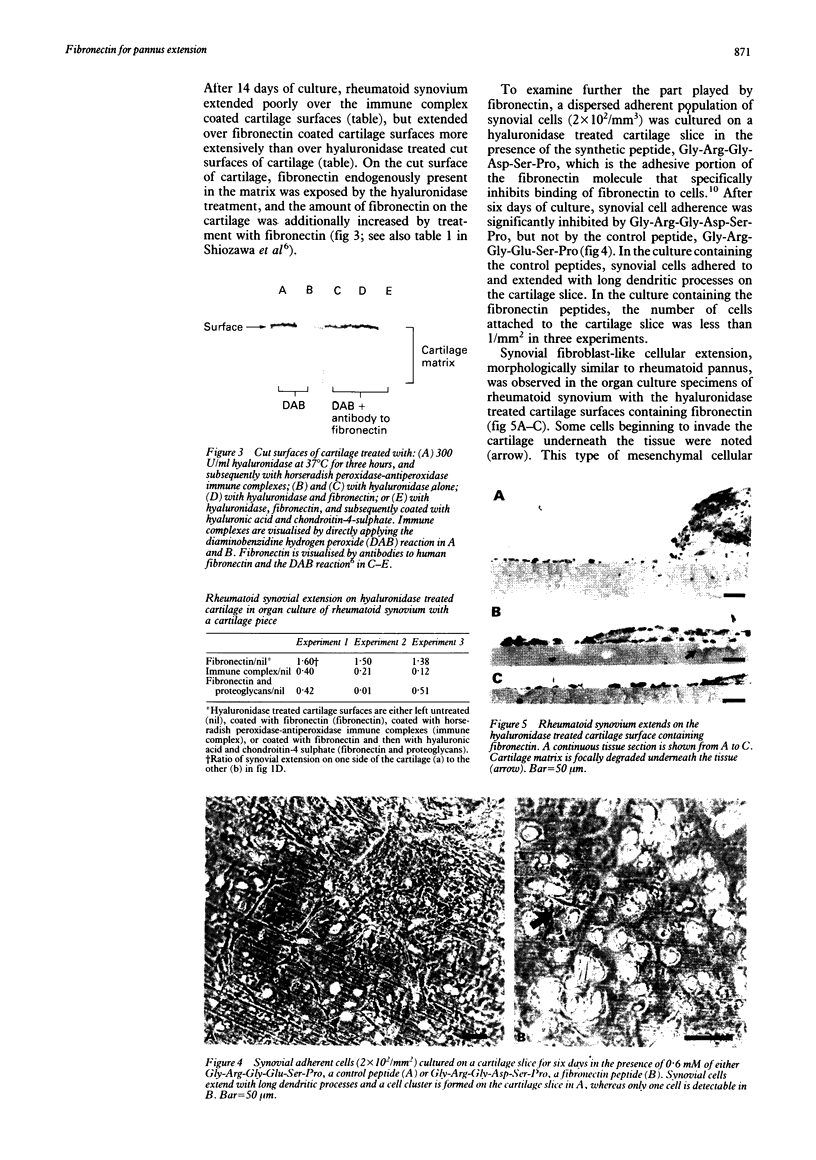
To identify the local factors in cartilage that are responsible for the induction of pannus invasion, a 14 day organ culture study in which rheumatoid synovium was grown in contact with cartilage pieces was carried out. Rheumatoid synovium preferentially extended over hyaluronidase treated cartilage pieces, but detached from untreated pieces. Rheumatoid synovium extended over hyaluronidase treated cartilage surfaces containing fibronectin more extensively than over surfaces treated with hyaluronidase only. Extension over hyaluronidase treated cartilage surfaces containing immune complexes was small. The adherence of synovial cells to hyaluronidase treated cartilage slices in vitro was specifically inhibited by the synthetic peptide, Gly-Arg-Gly-Asp-Ser-Pro, which is the adhesive portion of the fibronectin molecule. Furthermore, synovial fibroblast-like cellular extension, morphologically similar to rheumatoid pannus, was observed in the organ culture experiments in which rheumatoid synovium grew over hyaluronidase treated cartilage surfaces containing fibronectin. Synovial tissue extension over fibronectin coated surfaces was inhibited when hyaluronic acid and chondroitin-4-sulphate, major components of cartilage proteoglycans, were present on the cartilage surface. These findings suggest that fibronectin present in the superficial region of cartilage potentiates rheumatoid synovial extension and proteoglycans and immune complexes inhibit rheumatoid synovial extension. It is likely that fibronectin deposited on the eroded surface of articular cartilage induces pannus formation in rheumatoid arthritis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Carsons S., Mosesson M. W., Diamond H. S. Detection and quantitation of fibronectin in synovial fluid from patients with rheumatic disease. Arthritis Rheum. 1981 Oct;24(10):1261–1267. [PubMed] [Google Scholar]
- Cooke T. D., Hurd E. R., Jasin H. E., Bienenstock J., Ziff M. Identification of immunoglobulins and complement in rheumatoid articular collagenous tissues. Arthritis Rheum. 1975 Nov-Dec;18(6):541–551. doi: 10.1002/art.1780180603. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., ROTSTEIN J. Rheumatoid arthritis and agammaglobulinemia. Bull Rheum Dis. 1960 Jan;10:203–206. [PubMed] [Google Scholar]
- Ishikawa H., Smiley J. D., Ziff M. Electron microscopic demonstration of immunoglobulin deposition in rheumatoid cartilage. Arthritis Rheum. 1975 Nov-Dec;18(6):563–576. doi: 10.1002/art.1780180606. [DOI] [PubMed] [Google Scholar]
- Kimura H., Tateishi H., Ziff M. Surface ultrastructure of rheumatoid articular cartilage. Arthritis Rheum. 1977 Jun;20(5):1085–1094. doi: 10.1002/art.1780200508. [DOI] [PubMed] [Google Scholar]
- Mitchell N., Shepard N. The ultrastructure of articular cartilage in rheumatoid arthritis. A preliminary report. J Bone Joint Surg Am. 1970 Oct;52(7):1405–1423. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Delamere J. P., Walton K. W. The distribution of fibronectin in the pannus in rheumatoid arthritis. Br J Exp Pathol. 1981 Aug;62(4):362–368. [PMC free article] [PubMed] [Google Scholar]
- Shiozawa K., Shiozawa S., Shimizu S., Fujita T. 1 alpha,25-dihydroxyvitamin D3 inhibits pokeweed mitogen-stimulated human B-cell activation: an analysis using serum-free culture conditions. Immunology. 1985 Sep;56(1):161–167. [PMC free article] [PubMed] [Google Scholar]
- Shiozawa K., Shiozawa S., Shimizu S., Fujita T. Fibronectin on the surface of articular cartilage in rheumatoid arthritis. Arthritis Rheum. 1984 Jun;27(6):615–622. doi: 10.1002/art.1780270603. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Jasin H. E., Ziff M. Absence of immunoglobulins in rheumatoid cartilage-pannus junctions. Arthritis Rheum. 1980 Jul;23(7):816–821. doi: 10.1002/art.1780230707. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Shiozawa K. A review of the histopathological evidence on the pathogenesis of cartilage destruction in rheumatoid arthritis. Scand J Rheumatol Suppl. 1988;74:65–72. doi: 10.3109/03009748809102940. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Shiozawa K., Fujita T. Morphologic observations in the early phase of the cartilage-pannus junction. Light and electron microscopic studies of active cellular pannus. Arthritis Rheum. 1983 Apr;26(4):472–478. doi: 10.1002/art.1780260404. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Tokuhisa T. Contribution of synovial mesenchymal cells to the pathogenesis of rheumatoid arthritis. Semin Arthritis Rheum. 1992 Feb;21(4):267–273. doi: 10.1016/0049-0172(92)90058-l. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Ziff M. Immunoelectron microscopic demonstration of fibronectin in rheumatoid pannus and at the cartilage-pannus junction. Ann Rheum Dis. 1983 Jun;42(3):254–263. doi: 10.1136/ard.42.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Vartio T., Vaheri A., Von Essen R., Isomäki H., Stenman S. Fibronectin in synovial fluid and tissue in rheumatoid arthritis. Eur J Clin Invest. 1981 Jun;11(3):207–212. doi: 10.1111/j.1365-2362.1981.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Reddi A. H. Appearance of fibronectin during the differentiation of cartilage, bone, and bone marrow. J Cell Biol. 1981 Mar;88(3):630–636. doi: 10.1083/jcb.88.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]