Abstract
Widespread use and the continuous increase in consumption has intensified the presence of food additives and their metabolites in the environment. The growing awareness that newly identified compounds in the environment may cause a real threat, both to the environment and to future generations due to the transformation they undergo in ecosystems, makes this topic a leading problem of engineering and environmental protection. This manuscript highlights the relevance of finding these compounds in water. The exposure routes and the threat, both to human health and to the aquatic environment, have been discussed. The research presented in the article was aimed at determining the degree of contamination of swimming pools with food additives. Thirteen food additives have been identified in ten tested pools. The most frequently found were antioxidants (E320, E321) and preservatives (E211, E210), which were present in all of the tested swimming pools, both public and in private backyards. Ascorbic acid (E300) and citric acid (E330) occurred in all of the tested private swimming pools, while aspartame (E951, sweetener) and canthaxanthin (E161g, colour) were identified only in private pools. The hazard statements according to the European Chemicals Agency indicate that the identified compounds may cause both immediate effects (skin or eye irritation, allergic reactions) and also long-lasting effects, e.g., damaged fertility or genetic defects.
Keywords: food additives, organic micropollutants, swimming pools, ecotoxicity, health risk
1. Introduction
Food additives are substances that are not normally consumed as food itself but are added to food intentionally for a technological purposes. According to EU Regulations on food additives [1], they must be safe when used. Most of them are only permitted to be used in certain foods and are subject to specific quantitative limits in conjunction with the appropriate legislation. Among those approved in the EU, the Food Standards Agency distinguishes substances belonging to groups of compounds, such as colours, preservatives, antioxidants, sweeteners, emulsifiers, stabilizers thickeners and other types. These kinds of substances only partially undergo a biotransformation processes in particular phases of metabolism in the human body [2]. Therefore, they get into the sewage and sewage treatment plants in unchanged form, or their metabolites do. In the meantime, under the influence of many different factors, they undergo numerous transformations and chemical reactions, such as oxidation or photodegradation processes. Traditional sewage treatment systems are not able to retain most of these contaminants and their by-products, which results in the accumulation of these organic micropollutants in water, soil, air (in the case of volatile substances), plants and animal bodies. The continuous increase in consumption has intensified the presence of food additives and their metabolites in the environment. As a result, the latest literature [3,4] classifies food additives as one of the groups of so-called Contaminants of Emerging Concern (CECs), defined by the United States Environmental Protection Agency (USEPA) and United States Geological Survey (USGS) as any chemical substance detected in particular elements of the environment and previously not naturally occurring in them [5,6]. The development of analytical techniques allows for the separation of chemicals with very low concentrations from environmental samples with increasing efficiency, causing a growing awareness that newly identified compounds in the environment classified as CECs may cause a real threat, both to the environment and to future generations. The exposure of organisms to environmental factors (including organic pollution) is the main cause of numerous dysfunctions, diseases and premature death. This applies to both plant and animal organisms, as well as the human population. This makes CECs a leading problem of engineering and environmental protection. This group of contaminants cover such a broad classification framework that it is impossible to clearly indicate the number of compounds currently included in this group. Meijer et al. [7]. developed counting software that identified, in total, 69,526 compounds with a CAS number and 306,279 different metabolites of them occurring in the environment.
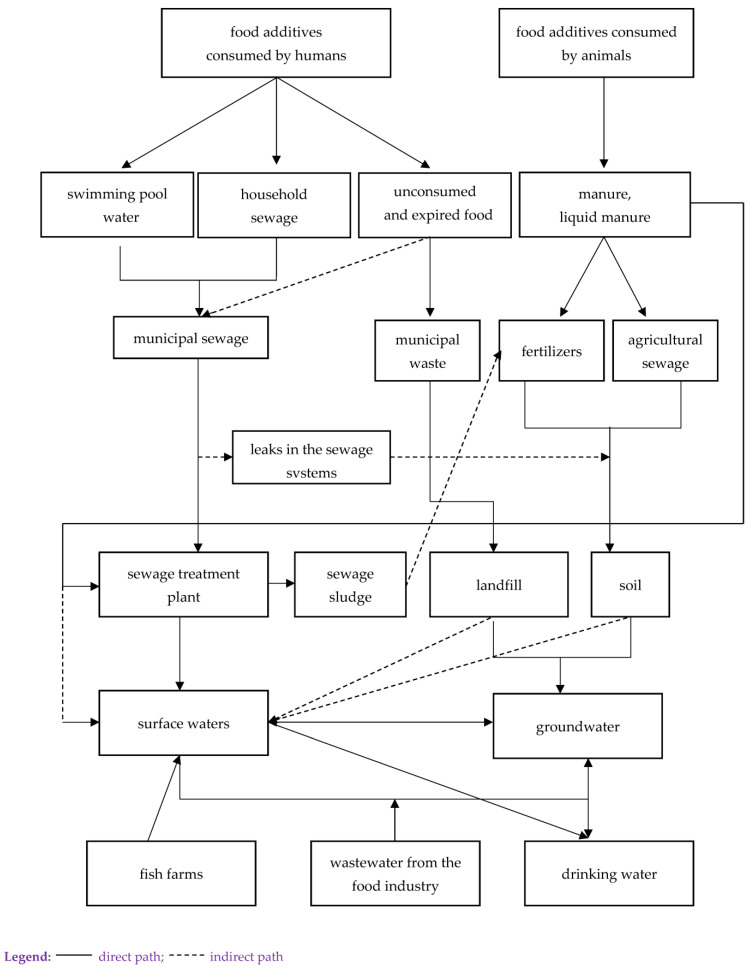
Migration paths of CECs (including food additives) into the environment are very different. Generally, their sources are divided into point and non-point types [8]; the main and most common of them are municipal, agricultural and industrial sewage, as well as leachate from landfills and the food industry. One possible means of food additives getting into the environment as its pollutant are shown in Figure 1.
Figure 1.
Pathways of food additive penetration into the environment.
Significant loads of food additives introduced into the environment in an uncontrolled way are carried by sewage from swimming pools. This applies to both the discharge of water from swimming pool basins and the discharge of washings from the backwashing of filters [9,10,11]. These are very special types of water streams because a number of different anthropogenic pollutants introduced by swimmers then get into the pool water, including not only food additives, but also Pharmaceuticals and Personal Care Products (PPCPs) [12,13], industrial additives and flame retardants [14,15]. Due to the specificity of the operation of swimming pool installations and treatment plants, in this particular water environment, pollutant accumulation processes, many physical transformations and chemical reactions occur (i.e., oxidation, chlorination, photodegradation), leading to the formation of very harmful by-products [16], including disinfection by-products (DBP), for example trichloromethane or pentachlorophenol, that are not only classified as priority substances in the field of water policy [17] particularly harmful to the environment but may also pose a serious threat to human health [18,19,20] due to their genotoxic and carcinogenic properties. Nowadays, the literature already describes over 600 harmful DBPs formed in swimming pools. These include: haloacetic acids (mostly di- and trichloroacetic acids), haloacetonitriles (dichloroacetonitrile, dibromoacetonitrile, trichloroacetonitrile), halobenzoquinones, halonitromethanes, N-nitrosamines, as well as chloral hydrate and bromine hydrate, cyanide halides and chloropicrin. In addition, when inorganic bromides are present in pool water (e.g., seawater pools or brines), they may oxidize and participate in the reaction, leading to the formation of brominated by-products. Food additives due to their chemical structure, if present in swimming pool water, may have the potential to form these types of potentially harmful compounds.
The research presented in this paper was aimed at determining the degree of contamination of swimming pools with food additives and their transformation products. In addition, an assessment of the threat caused to the environment by this phenomenon and the human exposure routes have been discussed. Determination of the extent of living organisms’ exposure to chemical contaminants is crucial to the evaluation of their adverse effects on both the environment and human health.
2. Materials and Methods
Samples of swimming pool water collected from 10 different outdoor swimming pools were subjected to broad-spectrum, non-target chromatographic analysis (NTCA), the aim of which was to identify as many compounds as possible by comparing the obtained mass spectra with reference spectra collected in the commercial NIST v.17 database. Food additives were selected from the identified chemical compounds, and then swimming pool water samples were subjected to qualitative targeted chromatographic analysis (TCA), during which their presence in the tested pools was confirmed by the injection of every identified compound’s analytical reference standard. For compounds with confirmed occurrence in the tested pools, a quantitative analysis was carried out, determining their concentration levels in swimming pool water.
Swimming pool fill water (fresh water) was also collected to assess the source of swimming pool water contamination.
2.1. Materials and Equipment
Chromatographic analyses were carried out using Gas Chromatograph with Mass Spectrometry detector (GC-MS) by Agilent Technologies (Santa Clara, CA, USA) equipped with capillary columns by Sigma-Aldrich (Poznań, Poland).
Disposable SupercleanTM extraction tubes by Merck KGaA (Darmstadt, Germany) and organic solvents methanol (MeOH), acetonitrile (ACN) and dichloromethane (DCM) with a purity over 99% from Avantor Performance Materials Poland S.A. (Gliwice, Poland) were used for Solid Phase Extraction (SPE).
The deionized water was obtained from a laboratory water distillation station Arium Comfort II UV by Sartorius AG (Göttingen, Germany).
The analytical reference standards of food additives used during targeted chromatographic analysis (TCA) were delivered by Merck KGaA (Darmstadt, Germany).
The obtained mass spectra were compared with the United States National Institute of Standards and Technology NIST v17 Mass Spectral Library using MassHunter software.
2.2. Research Objective
Environmental samples of swimming pool water were taken in accordance with the guidelines of the PN-EN ISO 5667-3:2018-08 standard [21], from outdoor swimming pools (five public and five private backyards facilities, SP1–SP10). The characteristic parameters of the sampled swimming pools are listed in Table 1. Water samples were collected in dark glass bottles, secured for transport in accordance with the procedures [21] and immediately transported to the Center of New Technologies of the Silesian University of Technology, where they were immediately prepared for analysis.
Table 1.
Characteristics of the tested swimming pool facilities.
| Parameter | Public Outdoors Swimming Pools | Private Backyards Swimming Pools | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SP1 | SP2 | SP3 | SP4 | SP5 | SP6 | SP7 | SP8 | SP9 | SP10 | |
| Function of Basin |
Sport pool | Water Playground |
Recreational | Children Pool |
Recreational | Recreational | Children Pool |
Children Pool |
Children Pool |
Recreational |
| Dimensions of the pool basin (m × m) | 25 × 12.5 | 25 × 20 | 25 × 12.5 | 25 × 20 | 11.5 × 10 | ⌀4.5 | ⌀5 | 5 × 10 | 6 × 3 | 4 × 2 |
| Depth of the pool basin (m) |
1.2 | 0.1 | 0.8–1.2 | 0.1–0.6 | 1.2 | 1.2 | 1.2 | 0.8–1.3 | 1.55 | 1.2 |
| Attendance (person/day) |
544 | 240 | 476 | 368 | 144 | 1 | 5 | 3 | 3 | 4 |
| Water temperature (°C) |
26 | 28 | 30 | 30 | 30 | 29 | 28 | 28 | 27 | 29 |
| pH | 7.0 | 7.1 | 7.0 | 7.1 | 7.0 | 6.9 | 7.2 | 7.2 | 7.4 | 6.8 |
| periodicity of changing of water |
1 year (according to requirements) | 3 months (summer season) | ||||||||
| input of fresh water | depending on water losses related to splashing and evaporation of water (an average of approx. 30 L/1 user per day) |
|||||||||
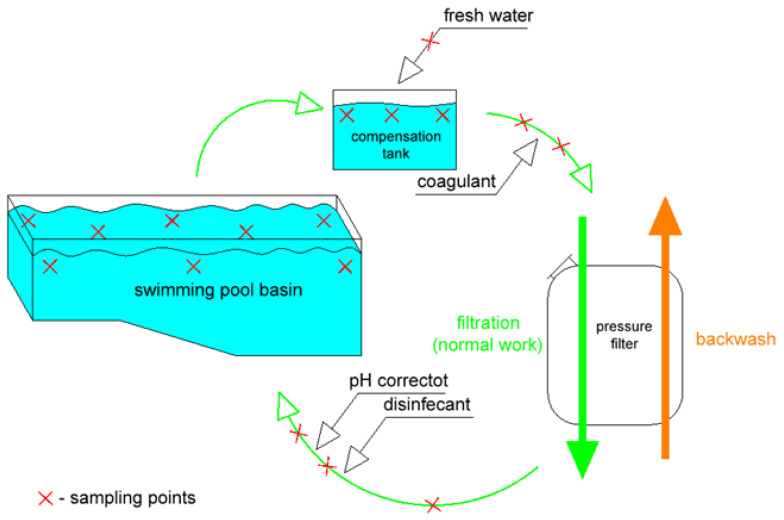
Due to the experience from earlier authors’ studies [22,23] and the dependence of chemical concentrations on the sampling point location in swimming pool basins [12,23], samples were taken from various characteristic points of both the basin, the installation and the pool water treatment system (Figure 2), and an average mixed sample was used for the analyses. The sampling points were selected in such a way as to ensure the reliability of the reported results, as during the processes taking place in the swimming pool water system, changes of micropollutants may occur.
Figure 2.
Sampling points of water for research.
Swimming pool fill water (fresh water) samples were analysed separately.
The pools tested in the presented study were selected in such a way that the water treatment procedures (disinfection, coagulation, filtration and pH adjustment) were comparable in all of them. This means that sodium hypochlorite was used at the disinfection stage, no methods of disinfection support were used, the same type of coagulant and pH corrector was used, and filtration was carried out in pressure filters filled with a classic sand bed.
2.3. Chromatographic Determination of Food Additives in Swimming Pool Water
The collected swimming pool water samples were subjected to chromatographic analysis using GC-MS, which was preceded by solid phase extraction (SPE) according to the authors’ own procedure [24] that allows for the extraction of the most possible analytes present in the sample.
Detailed parameters of the extraction processes are presented in Table 2 and conditions of GC-MS analysis are presented in Table 3.
Table 2.
Detailed parameters of the extraction processes.
| SupercleanTM Extraction Tubes Properties | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tube type | Envi-8 | Envi-18 | LC-8 | LC-18 | LC-CN | LC-Ph | ||
| Bed type | C8 (octyl) | C18 (octadecyl) | C8 (octyl) | C18 (octadecyl) | Cyano | Phenyl | ||
| Bed mass (mg) | 1000 | 1000 | 500 | 1000 | 500 | 500 | ||
| Tube volume (mL) | 6 | 6 | 6 | 6 | 6 | 3 | ||
| Carbon Loading (%) | 14 | 17 | 7 | 11.5 | 7 | 5.5 | ||
| Solid Phase Extraction Steps | ||||||||
| Conditioning | solvents | 5 mL MeOH | 5.0 mL ACN 5.0 mL MeOH |
3.0 mL DCM 3.0 mL ACN 3.0 mL MeOH |
5.0 mL ACN 5.0 mL MeOH |
5.0 mL ACN 5.0 mL MeOH |
5.0 mL ACN 5.0 mL MeOH |
5.0 mL ACN 5.0 mL MeOH |
| velocity | 10 mL/min | |||||||
| Bed washing | matrix | 5.0 mL deionized water | ||||||
| velocity | 10 mL/min | |||||||
| Sample flow | volume | 100 mL | ||||||
| velocity | 1 mL/min | |||||||
| Drying | 5 min under vacuum | |||||||
| Elution | solvents | 3 mL MeOH | 1.5 mL ACN 1.5 mL MeOH |
2.0 mL DCM 1.5 mL ACN 1.5 mL MeOH |
1.5 mL ACN 1.5 mL MeOH |
1.5 mL ACN 1.5 mL MeOH |
1.5 mL ACN 1.5 mL MeOH |
1.5 mL ACN 1.5 mL MeOH |
| velocity | 10 mL/min | |||||||
Table 3.
Conditions of chromatographic analysis.
| Carrier Gas | Helium 6.0 from SIAD (Ruda Śląska, Poland) | |
|---|---|---|
| Injection velocity | 3 mL/min | |
| Injection temperature | 250 °C | 325 °C |
| Column Type | SLBTM—5 ms | HP—5ms |
| Column size | 30 m × 0.25 mm | |
| Column film thickness | 0.25 μm | |
|
Oven temperature
program |
80 °C held for 6 min | 40 °C for 2 min |
| 5 °C/min up to 260 °C | ||
| 20 °C/min up to 300 °C held for 2 min |
10 °C/min up to 300 °C held 10 min |
|
| Transfer line temperature | 250 °C | 325 °C |
| Ion trap temperature | 150 °C | |
| Ion source temperature | 230 °C | |
| Ion registration mode | TIC | |
| Ion registration range (m/z) | 50 ÷ 600 | |
2.4. The Decomposition Processes for Toxicity Change Evaluation
The laboratory experiments were conducted in model conditions in order to show the changes in the toxicity of aqueous solutions of food additives during the decomposition processes taking place in swimming pool water installations. In swimming pool water, the toxicity effect is always 100% for each sample due to the presence of free chlorine. This is why the toxicity tests for real conditions are not applicable.
For this purpose, a laboratory swimming pool water treatment system was used, equivalent to the scheme of the tested pools (Figure 2), including coagulation, filtration, disinfection and pH adjustment. The sodium thiosulfate was used to stop the chlorination process and to avoid the influence of the presence of free chlorine on the toxicity of the samples.
Toxicity effect have been measured by the use of Microtox® bioassay based on the measurement of the changes in the intensity of light emission by selected strains of the bioluminescent bacteria Aliivibrio fischeri. These bacteria are widely used bioindicators due to their high sensitivity for a broad range of toxicants, including different groups of organic micropollutants. Details of the procedure are given in [15]. The test was conducted according to the Screening Test procedure, which allow for the estimation of the toxic effect of tested water samples comparative to a reference nontoxic sample. The reference sample was a 2% NaCl solution. The obtained test results of the toxicological effect of the compound water solutions allowed us to classify them into particular toxicity classes.
3. Results
As a result of qualitative NTCA, several hundred different mass spectra were obtained, the comparison of which, with reference spectra collected in the commercial database NIST v.17, allowed the identification of over 100 different organic micropollutants with a probability of a correct match of over 70%. They included not only food additives but also pharmaceuticals, personal care products, industrial additives and others. In addition, a group of micropollutants, which seem to be intermediate or final products of transformations or processes taking place in swimming pool installations, was separately identified.
The list of selected compounds from the group of food additives identified in the analysed samples with a match probability above 70% is presented in the Table 4. They have been classified according to the classification given by the Food Standards Agency [25] as colours, preservatives, antioxidants, sweeteners and others. No compounds from the group of emulsifiers, stabilizers, thickeners and gelling agents have been identified in this study.
Table 4.
List of food additives identified in tested swimming pool water samples.
| Group | Abbreviation | Compound | CAS | Molar Mass (g/mol) |
|---|---|---|---|---|
| Antioxidants | E320 | Butylated Hydroxyanisole | 25013-16-5 | 180.24 |
| E321 | Butylated Hydroxytoluene | 128-37-0 | 220.35 | |
| E319 | TertiaryButylhydroquinone | 1948-33-0 | 166.22 | |
| E300 | Ascorbic Acid | 50-81-7 | 176.12 | |
| Preservatives | E211 | SodiumBenzoate | 532-32-1 | 144.10 |
| E210 | Benzoic Acid | 65-85-0 | 122.12 | |
| Sweeteners | E954 | Saccharin | 81-07-2 | 183.18 |
| E951 | Aspartame | 22839-47-0 | 294.30 | |
| E420 | Sorbitol | 50-70-4 | 182.17 | |
| Colours | E101 | Riboflavin | 83-88-5 | 376.36 |
| E161g | Canthaxanthin | 514-78-3 | 564.84 | |
| Others | E330 | Citric Acid | 77-92-9 | 192.12 |
| E270 | Lactic Acid | 50-21-5 | 90.08 |
No food additives were detected in any of the fill water (fresh water) samples.
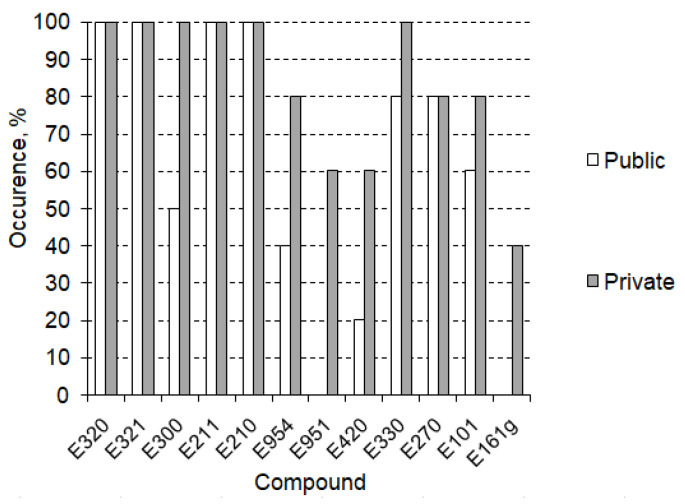
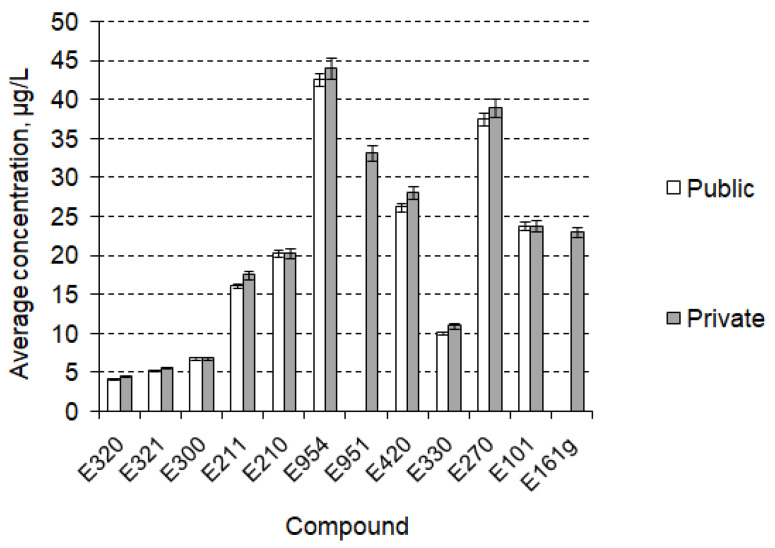
The further stage of research, including qualitative TCA, confirmed the presence of the listed compounds in the tested pools (Figure 3) and allowed the determination of their concentration levels, as presented in the Figure 4. Concentration levels are expressed a mean values calculated as the arithmetic mean of the measured concentrations in the pools where the compound was identified. Each concentration had been measured as the average of three consecutive concentration measurement replicates. The measurement errors marked on the chart are the standard deviation of the repetitions made. Error values did not exceed 5%.
Figure 3.
Food additives occurrence in public and private backyard swimming pools (N = 10).
Figure 4.
Concentration levels of food additives identified in tested swimming pools.
The values of the validation parameters for the determination of the concentrations of the tested compounds are summarized in Table 5. The Instrumental Detection Limit (IDL) for the tested compounds was determined on the basis of the quotient of the signal from a given compound recorded by the chromatograph detector and the noise from the coastline (Signal to Noise Ratio, SNR) equal to 3. The Recovery (R) was determined using the optimal extraction procedure for each test compound. The validation of the method was carried out for various concentrations of compounds, enabling the determination of the Coefficient of Variation (CV, value in the range of 1–3% confirms the high repeatability of the method) and the Limit of Quantification (LOQ), for which the SNR was assumed equal to 10.
Table 5.
Validation parameters of the method used for the targeted chromatographic analysis.
| Abbreviation | Compound | R ± SD, % | CV | IDL, µg/L | LOQ, µg/L |
|---|---|---|---|---|---|
| E320 | Butylated Hydroxyanisole | 96 ± 3 | 0.02 | 0.04 × 10−3 | 3.0 |
| E321 | Butylated Hydroxytoluene | 99 ± 1 | 0.01 | 0.01 × 10−3 | 2.0 |
| E319 | Tertiary Butylhydroquinone | 97 ± 1 | 0.01 | 0.03 × 10−3 | 2.7 |
| E300 | Ascorbic Acid | 97 ± 2 | 0.02 | 0.11 × 10−3 | 15.0 |
| E211 | SodiumBenzoate | 95 ± 1 | 0.01 | 0.01 × 10−3 | 1.8 |
| E210 | Benzoic Acid | 98 ± 1 | 0.02 | 0.36 × 10−3 | 41.2 |
| E954 | Saccharin | 94 ± 3 | 0.03 | 0.24 × 10−3 | 28.2 |
| E951 | Aspartame | 95 ± 1 | 0.01 | 0.18 × 10−3 | 20.4 |
| E420 | Sorbitol | 95 ± 2 | 0.01 | 0.07 × 10−3 | 6.2 |
| E101 | Riboflavin | 96 ± 2 | 0.02 | 0.31 × 10−3 | 36.7 |
| E161g | Canthaxanthin | 95 ± 3 | 0.02 | 0.22 × 10−3 | 23.3 |
| E330 | Citric Acid | 97 ± 2 | 0.03 | 0.09 × 10−3 | 12.3 |
| E270 | Lactic Acid | 95 ± 2 | 0.02 | 0.04 × 10−3 | 3.0 |
R—Recovery, CV—Coefficient of Variation, IDL—Instrumental Detection Limit, LOQ—Limit of Quantification.
4. Discussion
Food additives were found to be above the LOQs in all of the tested swimming pools, while they all were below the LOQs in all of the fill water samples. This implies that contamination of swimming pool water by food ingredients is occurring within the swimming pools themselves and is likely due to human-derived sources, such as through swimmers’ excretion of body fluids (accidental urinary excretion or sweat) or accidentally putting food in the water (e.g., falling into the swimming pool).
In general, food additives occurred more often in private swimming pools than in public ones. This results from the different specificity of the use of these two types of facilities, a lesser sanitary regime in private pools and more advanced methods of water treatment in public ones. The most frequently identified compounds in the studied basins were antioxidants (E320, E321) and preservatives (E211, E210); they were present in all of the tested swimming pools, in both public and private backyards, while the antioxidants’ average concentration levels were the lowest of all tested contaminants (respectively, 4.1 ± 0.3 µg/L and 5.2 ± 0.2 µg/L). Ascorbic acid (E300) and citric acid (E330) occurred in all of the studied private swimming pools and over half of public ones (3 of 5 and 4 of 5, respectively), while aspartame (E951, sweetener) and canthaxanthin (E161g, colour) were identified only in private pools (respectively, 3 of 5 and 2 of 5). The highest concentration level (42.6 ± 0.4 µg/L) was measured for saccharin (E954), which was present in 60% of tested swimming pools (40% of publics and 80% of privates). Sorbitol occurred in 40% of tested pools (1 of 5 public and 4 of 5 private) with average concentrations equal to 26.2 ± 0.3 µg/L. Lactic Acid was observed in 80% of facilities (4 of 5 of both public and private) at a concentration level of 37.5 ± 0.4 µg/L and Riboflavin in 70% (3 of 5 public and 4 of 5 private) with a concentration of 23.8 ± 0.2 µg/L.
The levels of the measured and presented concentrations in this paper are likely affected by many factors, including the function of the pool basin, water temperature, the number and demography of users, types of activities carried out, exposure of basin to sunlight and the type of disinfection used (such as the incorporation of UV disinfection) or the type of tested compound. This was similarly observed in studies on the presence of micropollutants from other groups in swimming pool water [26,27,28]. Variation of food additive concentrations during the day was also observed, described by Teo et al. on the example of caffeine [29].
All of the detected compounds are approved for use in food, which means they are relatively safe for human health. However, for a number of reasons, their presence in the environment is of concern. The phenomenon of the co-occurrence of compounds from different groups simultaneously in tested pool basins is alarming. Some studies were focused on the potential interactions and synergies of food additives [30]. For example, McCannet et al. [31] pointed out that a mixture of sodium benzoate with colourings can cause increased hyperactivity in children.
Hazard statements of the food additives identified in the tested swimming pools, according to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), based on European Chemicals Agency (ECHA) data, are presented in Table 6. The collected data show that the tested compounds may affect different parts of the human body and also the aquatic organism. Human exposure via different routes may cause not only immediate effects, such as skin or eye irritation and allergic reactions, but also long-lasting effects, e.g., damaged fertility or genetic defects. The available literature data highlight three main ways of exposing swimmers to organic compounds (including food additives) and their by-products present in swimming pool water:
Oral route—by the direct swallowing of water. Studies presented in [32] showed that during 45 min of swimming, an average adult swallows 16 mL of pool water, while an average child swallows 37 mL;
Inhalation route—by inhalation of volatile or aerosolized substances dissolved. Among them, volatile by-products, which are formed as a result of the reaction of organic compounds (including food additives) with chlorine compounds. It should be noted that, due to the properties of these compounds and substances, they accumulate in swimming pools just above the water table, in the so-called swimmer’s breathing zone (exactly where the swimmer takes a breath). Research has proven the cause of respiratory illness (e.g., asthma) among professional swimmers. Literature reports indicate that even a short exposure to some of the chlorinated by-products causes coughing or severe irritation of the respiratory tract of swimmers. It can also cause changes in biomarkers in the lungs [33];
Dermal route—by direct contact or skin absorption. Some contaminants occurring in swimming pool water, or their by-products, can directly affect the skin, eyes or mucous membranes, and some can also penetrate the skin and be absorbed by the body. For example, E321 does penetrate the skin [34]. The extent of such absorption depends on a number of factors, including the time of contact with water, its temperature or the concentration of the absorbed chemical substance.
Table 6.
Health hazards classification of food additives identified in tested swimming pools [35].
| Abbreviation | Hazard Statement Code |
Hazard Class | Category |
|---|---|---|---|
| E320 | H302 | Harmful if swallowed | 4 |
| H315 | Causes skin irritation | 2 | |
| H317 | May cause an allergic skin reaction | 1 | |
| H319 | Causes serious eye irritation | 2 | |
| H335 | May cause respiratory irritation | 3 | |
| H351 | Suspected of causing cancer | 2 | |
| H361 | Suspected of damaging fertility or the unborn child | 2 | |
| H400 | Very toxic to aquatic life | 2 | |
| H411 | Toxic to aquatic life with long lasting effects | 1 | |
| E321 | H302 | Harmful if swallowed | 4 |
| H312 | Harmful in contact with skin | 4 | |
| H315 | Causes skin irritation | 2 | |
| H317 | May cause an allergic skin reaction | 1 | |
| H319 | Causes serious eye irritation | 2 | |
| H335 | May cause respiratory irritation | 3 | |
| H340 | May cause genetic defects | 1B | |
| H351 | Suspected of causing cancer | 2 | |
| H361 | Suspected of damaging fertility or the unborn child | 2 | |
| H370 | Causes damage to organs | 1 | |
| H373 | May cause damage to organs through prolonged or repeated exposure | 2 | |
| H400 | Very toxic to aquatic life | 1 | |
| H410 | Very toxic to aquatic life with long lasting effects | 1 | |
| H413 | May cause long lasting harmful effects to aquatic life | 4 | |
| E319 | H302 | Harmful if swallowed | 4 |
| H312 | Harmful in contact with skin | 4 | |
| H315 | Causes skin irritation | 2 | |
| H317 | May cause an allergic skin reaction | 1 | |
| H319 | Causes serious eye irritation | 2 | |
| H335 | May cause respiratory irritation | 3 | |
| H400 | Very toxic to aquatic life | 1 | |
| H410 | Very toxic to aquatic life with long lasting effects | 1 | |
| E300 | H314 | Causes severe skin burns and eye damage | 1 |
| H315 | Causes skin irritation | 2 | |
| H319 | Causes serious eye irritation | 2 | |
| H318 | Causes serious eye damage | 1 | |
| E211 | H319 | Causes serious eye irritation | 1 |
| E210 | H302 | Harmful if swallowed | 4 |
| H315 | Causes skin irritation | 2 | |
| H318 | Causes serious eye damage | 1 | |
| H319 | Causes serious eye irritation | 2 | |
| H372 | Causes damage to organs (lungs) through prolonged or repeated exposure by inhalation | 1 | |
| E954 | H315 | Causes skin irritation | 2 |
| H317 | Causes serious eye damage | 1 | |
| H318 | May cause an allergic skin reaction | 1 | |
| H341 | Suspected of causing genetic defects | 2 | |
| H351 | Suspected of causing cancer | 2 | |
| H361 | Suspected of damaging fertility or the unborn child | 2 | |
| E951 | H312 | Harmful in contact with ski | 4 |
| H332 | Harmful if inhaled | 4 | |
| H372 | Causes damage to organs through prolonged or repeated exposure | 1 | |
| E420 | Not Classified | - | - |
| E101 | H302 | Harmful if swallowed. | 0 |
| E161g | Not Classified | - | - |
| E330 | H302 | Acute Toxicity | 4 |
| H315 | Skin corrosion/irritation | 2 | |
| H318 | Eye damage/eye irritation | 1 | |
| H335 | May cause respiratory irritation | 3 | |
| E270 | H315 | Skin corrosion/irritation | 1 |
| H318 | Eye damage/eye irritation | 1 |
Swimming pool water is only one of many sources of environmental pollution with organic compounds classified as food additives and their by-products. Pollutants entering ecosystems in trace concentrations through various routes (including effluent from swimming pool facilities) accumulate in the environment, causing a continuous increase in environmental concentrations, as well as an increase in the exposure of plant, animal and human organisms.
Based on the methodology proposed by Fantuzzi et al. [36], for the determined concentration levels of the tested organic micropollutants, the human health risk assessment was carried out, for children (3 years), teenagers (14 years) and adults, dividing each age group by gender. The average body weight for the individual analysed groups was determined according to Cacciari et al. [37], taking into account the 50th percentile. The average volume of water swallowed by swimmers was adopted according to Dufour et al. [32]. The worst-case scenario was predicted assuming the maximum measured concentration of each micropollutants tested and assuming daily use of the pool by users. The hazard factors for swimmers of all ages and genders were less than 0.001, indicating that the health risk from oral exposure to the tested compounds in swimming pools is low, considering exposure to a single contamination with individual compounds. However, it should be highlighted and kept in mind that in swimming pool basins there is co-exposure to countless amount of different organic micropollutants, which is not taken into account in this health risk assessment methodology. It must also be taken into account that some of these food additives bioaccumulate, so with frequent use of the pool, the actual health risk may be much higher.
Table 7 provides single chemical environmental toxicity data of the identified compounds on aquatic species collected by the use of ECOTOX Knowledgebase of the United States Environmental Protection Agency EPA [38].
Table 7.
Toxicity of the tested food additives to aquatic organisms [38].
| Abbreviation | Species Name | Parameter | Value (mg/L) | Test Duration Time (Days) |
| E320 | Lepomis macrochirus | LC50 | 4.8 | 2 |
| Ictalurus punctatus | LC50 | 1.5 | 2 | |
| Oryziaslatipes | LC50 | 2.5 | 1 | |
| Oncorhynchus mykiss | LC50 | 1 | 2 | |
| Dreissenapolymorpha | EC50 | 3.4 | 2 | |
| Dreissenapolymorpha | LC50 | 65 | 2 | |
| E321 | Daphnia pulex | EC50 | 1.44 | 2 |
| Oryziaslatipes | LC50 | 5.3 | 1 | |
| Tetrahymena pyriformis | EC50 | 1.7 | 1 | |
| Dreissenapolymorpha | EC50 | 1.3 | 2 | |
| Daphnia pulex | EC50 | 1.44 | 2 | |
| E319 | Ictalurus punctatus | LC50 | 0.37 | 2 |
| Lepomis macrochirus | LC50 | 0.15 | 2 | |
| Oncorhynchus mykiss | LC50 | 0.37 | 2 | |
| Dreissenapolymorpha | EC50 | 1 | 2 | |
| Dreissenapolymorpha | LC50 | 118 | 2 | |
| E300 | Xenopuslaevis | EC50 | 11600 | 4 |
| Xenopuslaevis | LC50 | 19200 | 4 | |
| E211 | Asellus intermedius | LC50 | 100 | 4 |
| Gammarus fasciatus | LC50 | 100 | 4 | |
| Daphnia magna | LC50 | 100 | 4 | |
| Danio rerio | EC50 | 68.5 | 2 | |
| Danio rerio | LC50 | 461 | 2 | |
| Pimephalespromelas | LC50 | 100 | 4 | |
| Girardiatigrina | LC50 | 100 | 4 | |
| Lumbriculus variegatus | LC50 | 100 | 4 | |
| E210 | Raphidocelissubcapitata | EC50 | 36.39 | 2 |
| Anabaena variabilis | EC50 | 55 | 0.125 | |
| Anabaena cylindrica | EC50 | 60 | 0.125 | |
| Anabaena inaequalis | EC50 | 5 | 0.125 | |
| Chlorella pyrenoidosa | EC50 | 60 | 0.125 | |
| Anabaena cylindrica | EC50 | 30 | 0.2083 | |
| Chlorococcales | EC50 | 168 | 1 | |
| Raphidocelissubcapitata | EC50 | 207.5 | 2 | |
| Scenedesmusquadricauda | EC50 | 75 | 0.125 | |
| Microcystis aeruginosa | EC50 | 0.25 | 3 | |
| Chlorella vulgaris | EC50 | 0.14 | 3 | |
| Desmodesmussubspicatus | EC50 | 333 | 7 | |
| Xenopuslaevis | EC50 | 433 | 4 | |
| Thamnocephalusplatyurus | EC50 | 177 | 1 | |
| Daphnia magna | EC50 | 100 | 2 | |
| Gambusiaaffinis | LC50 | 180 | 4 | |
| Leuciscusidus ssp. melanotus | LC50 | 460 | 2 | |
| Pimephalespromelas | EC50 | 2809 | 0.0833 | |
| Meloidogyne arenaria | LC50 | 290.6 | 1 | |
| E954 | Xenopuslaevis | EC50 | 0.0141 | 4 |
| Xenopuslaevis | LC50 | 0.01303 | 4 | |
| Danio rerio | EC50 | 4753.6 | 5.8333 | |
| Danio rerio | LC50 | 7272.3 | 1.8333 | |
| Danio rerio | LC50 | 5585.2 | 5.8333 | |
| Danio rerio | LC50 | 7272.3 | 2.8333 | |
| E420 | Lemnagibba | EC50 | 27143.9 | 7 |
| E101 | Raphidocelissubcapitata | EC50 | 12 | 2 |
| E161g | Leuciscusidus | LC50 | 10 | 4 |
| E330 | Daphnia magna | EC50 | 1535 | 1 |
| Carcinusmaenas | LC50 | 160 | 2 | |
| Leuciscusidus ssp. melanotus | LC50 | 440 | 2 | |
| Pimephalespromelas | EC50 | 2942 | 0.0833 | |
| Oncorhynchus mykiss | EC50 | 653.2 | 1 | |
| Azumiobodohoyamushi | EC50 | 100 | 1 | |
| E270 | Moinamicrura | LC50 | 329.12 | 4 |
| Oreochromismossambicus | LC50 | 257.73 | 4 | |
| Meloidogyne arenaria | LC50 | 4503.94 | 1 | |
| Branchiurasowerbyi | LC50 | 50.82 | 4 |
Butylated hydroxyanisole (E320), butylated hydroxytoluene (E321) and tertiary butylhydroquinone (E319) are the most widely used food antioxidants due to their low cost, high performance, and wide availability. They can be found in a great variety of products, e.g., oils, margarines and fat-containing products. They are approved food antioxidants in the European Union, the United States, Australia, New Zealand and many other regions [39,40]. Previous studies show that these antioxidants and their transformation products have been found in a concentration level of 10÷2000 ng/L in the water environment (rivers, ocean, ground water and wastewater, sewage, sludge, sediment). They have also been detected in indoor dust, sludge, sediments, molluscs and human plasma and nails [39,41,42,43,44,45,46]. Human exposure to synthetic phenolic antioxidants (including E319, E320 and E321) is of high interest due to their reported toxicity effects. For example, E320 can disrupt the endocrine system [47,48] and according to the release in2021 of the Fifteenth Edition of the Report on Carcinogens by the U.S. Department of Health and Human Services, it is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals [49]. It has also been found that E321 interferes with satiety signals sent from the digestive system to the brain, which may cause individuals to eat more than they otherwise would, potentially leading to obesity [43]. In addition, both E320 and E321 can easily be transformed to tert-butylhydroquinone (E319) by oxidation reaction [39] and further degrade due to irradiation. Accumulation ofE319 in body tissues is negligible; however, it is noteworthy that it possibly leads to nutritional disorders and chronic diseases and adverse biological effects on human health at high doses or in the long-term. E319 can have side effects on human health through activation of inflammatory routes, generation of reactive species, induction of CYP1A1, activation of caspases and decreases in GSH/ATP levels, and triggering of the gradual development of cancers [50].
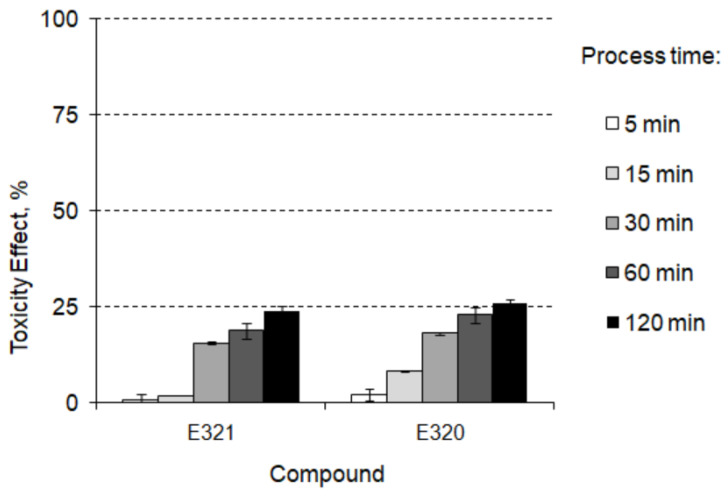
The laboratory experiment conducted in model conditions showed the increase in the toxicity of aqueous solution during the decomposition processes taking place in swimming pool water installations (Figure 5) due to the formation of, among others, 2,6-di-tert-butylhydroquinone and2,6-di-tert-butylbenzoquinone that can be cytotoxic and genotoxic to diverse cells and animals [51].
Figure 5.
The toxicity change in the by-product formation pathway in swimming pool water installation.
An interesting phenomenon is the presence of ascorbic acid in swimming pool water. The studies have shown it to be the fourth most frequently consumed of all food additives [52] that, in addition to its antioxidant and nutritional properties, has been investigated as a means for reducing residual halogen-based oxidants [47]. However, the safety for human health of ascorbic acid presence in swimming pool water is questionable and under discussion. Research by the Environmental Protection Agency (EPA) shows that the use of ascorbic acid is one of the effective methods for the dechlorination of water [53]. For this reason, many not fully aware swimming pool users (especially private backyard ones) decide to deliberately introduce this compound into their swimming pools. However, it should be emphasized that such action is not surely beneficial for the quality of pool water and the safety of its use. First of all, chlorine is introduced into the pool to kill bacteria, viruses and microorganisms. Attempting to achieve dechlorination is thus achieving the opposite effect. Moreover, ascorbic acid lowers the pH of the water, which may result in corrosion of the elements, such as ladders, nozzles, skimmers, foil, etc. In addition, it may irritate the eyes and skin of swimmers. It has also been shown that ascorbic acid can be rapidly oxidized to dehydroascorbic acid when added to bicarbonate rich (buffered) copper-contaminated drinking water [54]. Thus, swimmer will most likely ingest dehydroascorbic acid that has been shown to cause oxidative stress and apoptosis in pancreatic and neural cells by depleting their intracellular store of reduced glutathione [55,56,57]. The impact of the long-term intake of dehydroascorbic acid, the oxidized form of ascorbic acid, on human health, still remains to be studied [54].
Sodium benzoate (E211) and Benzoic Acid (E210) are two of the most popular preservatives that can be used in various food products [58]. These are compounds with abroad safety profile and dose-dependent effects that are almost always adverse in the case of high doses [59]. From the use pattern, it can be expected that benzoic acid is released to surface waters, leaching water and groundwater, while no information on the environmental transport and distribution of sodium benzoate could be identified [60]. Owing toits use pattern, which is similar to that of benzoic acid, most of the amounts released to the environment are also expected to be emitted to aquatic compartments (e.g., surface waters). From their physical/chemical properties, they are not expected to volatilize from water and soil to the atmosphere or to adsorb to sediment or soil particles. They both exhibited low to moderate toxicity to aquatic organisms. The lowest reported EC50 value of9 mg/L was determined in a chronic study (14 days) for cell multiplication inhibition by benzoic acid in the cyan bacterium Anabaena inadequacies. EC50/LC50 values for the other aquatic species tested were in the range of 17–1291 mg/L [60]. However, it is believed that benzoates can be transformed by decarboxylation into toxic benzene, especially in combination with ascorbic acid(also detected in tested swimming pools) and then become a compound of high toxicity, mutagenicity and teratogenicity [61]. The hydroxyl radical, formed by the metal-catalysed reduction in O2 and H2O2 by ascorbic acid, can attack benzoic acid to form benzene [62], and the heat and light can increase the rate of benzene formation [63]. It has also been reported that sodium benzoate has a mutagenic and genotoxic effect [64], generates oxidative stress and has an adverse effect on the immune system, liver, kidneys and fertility.
Saccharin, aspartame and sorbitol, due to their environmental persistence and common detection in the environment, have been recognized as compounds of emerging concern [65]. They are some of the most popular artificial sweeteners used in various food products [66], so their presence in the pool water environment seems to be inevitable. Although comprehensive toxicological tests have been conducted and they appear to be nontoxic to humans within regulated concentrations, their unintended presence in the environment still causes considerable concern. They have previously been proven to occur ubiquitously in surface waters, with concentrations ranging from 0.10 mg/L to 0.12 mg/L [67]. They are recognized as a new class of environmental contaminants due to their extreme persistence and ubiquitous nature. The continuous introduction of artificial sweeteners into the aquatic environments is attributed to their resistant behaviour to wastewater treatment processes [68]. However, their behaviour, fate and long-term ecotoxicological contribution in water resources is, by large, still unknown.
The real impact of aspartame on human health is still unclear. The studies conducted in these field focused mainly on animals [69] and indicate a carcinogenic impact on many species [70]. However, Borghoff et al. [71], after an extensive review of the literature, pointed out that there were no clear or consistent signals of carcinogenicity following aspartame. Bandyopadhyay et al. [72] showed that compounds such as aspartame and sorbitol could induce DNA damage in bone marrow cells. A recent study has also reported that aspartameisis toxic to Lemna minor, Sinapis alba, Daphnia magna, Enchytraeuscrypticus, Desmodesmussubspicatus and Lactuca sativa (100 mg/kg) and disrupts the reproduction of Enchytraeidae [73].
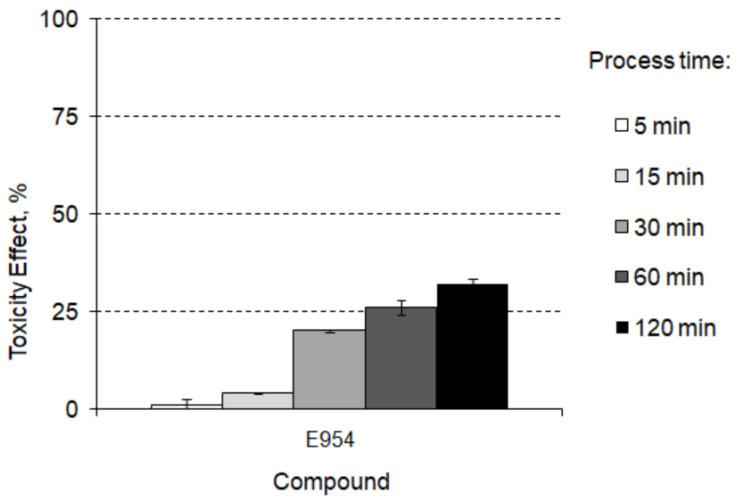
Luo et al. [74] indicated that saccharincan causes a hazard and risk potential to aquatic organisms, which may also affect human health. Uçar and Yilmaz [75] noted that this type of artificial sweetener may be a weak carcinogen, causing cancer of the urinary tract of male rats. Other studies have proved that saccharin can induce liver inflammation in mice [76] or could be one of the main factors causing paediatric inflammatory bowel disease [77].Studies conducted on the assessment of the possibility of saccharin and other artificial sweeteners decomposition in the chlorination process have shown that most of those compounds were persistent and not transformed by the action of reactive chlorine radicals [78].This may be related toa lack of electron-rich sites for oxidation in the compound molecule [79]. In addition, the action of ultraviolet (UV)irradiation, which is used in the swimming pool water treatment technology as a supporting disinfection process [80], does not allow for a significant increase in the concentration of saccharin in the aquatic environment [81]. On the other hand, Davididou et al. [82] proved the degradation of saccharin under solar radiation. However, this process leads to the production of toxic decomposition by-products with –OH and =O groups. The toxicity change in the saccharine by-product formation pathway in swimming pool water installation is shown in Figure 6.
Figure 6.
The toxicity change in the saccharine by-product formation pathway in swimming pool water installation.
5. Conclusions
The presented study investigated the occurrence of 13 selected micropollutants, classified as Contaminants of Emerging Concern, from the group of food additives in water samples collected from 10 swimming pool systems. The study area was selected based on the lack of available information regarding suspected contamination of swimming pool water by food additives. The variety and concentration of chemical compounds in these aquatic systems can be quite diversified, presenting a challenge in terms of both purification and quality control. This paper provides insights into the concentrations and variability of food additives in various types of swimming pools. The presence of these compounds and the possibility of their accumulation and transformation in swimming pool installations raise questions about the potential threat to the health of swimming pool users.
Thirteen food additives have been identified in the tested pools. They have been classified as colours (Riboflavin, Canthaxanthin), preservatives (Sodium Benzoate, Benzoic Acid), antioxidants (Butylated Hydroxyanisole, Butylated Hydroxytoluene, Tetiary Butylhydroquinone), sweeteners (Saccharin, Aspartame, Sorbitol) and others (Citric Acid, Lactic Acid). No compounds from the group of emulsifiers, stabilizers, thickeners and gelling agents have been identified in this study. In general, food additives were found more often in private than in public swimming pools. All of the food additives identified in the swimming pools were below the LOQs in all of the fill water (fresh water) samples. This implies that contamination of swimming pool water by food ingredients is occurring within the swimming pools themselves.
There are three main ways of exposing swimmer to food additives and their by-products present in swimming pool water (oral route by water swelling, inhalation and direct contact route). The determined hazard factors for swimmers of all ages and genders indicated that the health risk from oral exposure to the food additives present in swimming pools is low, considering exposure to a single contamination with individual compounds. However, it should be highlighted and kept in mind that in swimming pool basins there is co-exposure to countless amounts of different organic micropollutants, which is not taken into account in the implemented health risk assessment methodology. The hazard statements of food additives identified in tested swimming pools indicate that human exposure via different routes may cause not only immediate effects, such as skin or eye irritation and allergic reactions, but also long-lasting effects, e.g., damaged fertility or genetic defects.
Author Contributions
Conceptualization, A.L.-R., E.K., K.B. and M.D.; methodology, A.L.-R. and E.K.; validation, A.L.-R., E.K., Ł.L. and R.R.; software E.K, R.R. and Ł.L.; formal analysis, A.L.-R., E.K., K.B., R.R. and Ł.L.; investigation, A.L.-R., E.K., K.B., R.R. and Ł.L.; resources, A.L.-R., E.K., Ł.L. and R.R.; data curation, M.D.; writing—original draft preparation, A.L.-R. and E.K.; writing—review and editing, K.B. and M.D.; visualization, A.L.-R., E.K., Ł.L. and R.R.; supervision, K.B. and M.D.; project administration, A.L.-R., E.K. and M.D.; funding acquisition, A.L.-R. and M.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Funding Statement
This research was funded by the National Science Centre in Poland, No. 2018/29/N/ST8/01352.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Official Journal of the European UnionL 354/16. [(accessed on 15 January 2023)]. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:354:0016:0033:EN:PDF.
- 2.Birch G.F., Drage D.S., Thompson K., Eaglesham G., Mueller J.F. Emerging contaminants (pharmaceuticals, personal care products, a food additive and pesticides) in waters of Sydney estuary, Australia. Mar. Pollut. Bull. 2015;97:56–66. doi: 10.1016/j.marpolbul.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Salimi M., Esrafili A., Gholami M., JonidiJafari A., RezaeiKalantary R., Farzadkia M., Kermani M., Sobhi H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017;189:414. doi: 10.1007/s10661-017-6097-x. [DOI] [PubMed] [Google Scholar]
- 4.Han Y., Hu L.-X., Liu T., Liu J., Wang Y.-Q., Zhao J.-H., Liu Y.-S., Zhao J.-L., Ying G.-G. Non-target, suspect and target screening of chemicals of emerging concern in landfill leachates and groundwater in Guangzhou, South China. Sci. Total Environ. 2022;837:155705. doi: 10.1016/j.scitotenv.2022.155705. [DOI] [PubMed] [Google Scholar]
- 5.EPA U.S. Environmental Protection Agency: Contaminants of Emerging Concern Including Pharmaceuticals and Personal Care Products. [(accessed on 15 January 2023)]; Available online: https://www.epa.gov/wqc/contaminants-emerging-concern-including-pharmaceuticals-and-personal-care-products.
- 6.USGS: Emerging Contaminants. [(accessed on 15 January 2023)]; Available online: https://www.usgs.gov/mission-areas/water-resources/science/emerging-contaminants.
- 7.Meijer J., Lamoree M., Hamers T., Antignac J.P., Hutinet S., Debrauwer L., Covaci A., Huber C., Krauss M., Walker D.I., et al. An annotation database for chemicals of emerging concern in exposome research. Environ. Int. 2021;152:106511. doi: 10.1016/j.envint.2021.106511. [DOI] [PubMed] [Google Scholar]
- 8.Tijani J.O., Fatoba O.O., Babajide O.O., Petrik L.F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environ. Chem. Lett. 2016;14:27–49. doi: 10.1007/s10311-015-0537-z. [DOI] [Google Scholar]
- 9.Wyczarska-Kokot J., Lempart A. The reuse of washings from pool filtration plants after the use of simple purification processes. ACEE. 2018;11:136–170. doi: 10.21307/acee-2018-049. [DOI] [Google Scholar]
- 10.Wyczarska-Kokot J., Lempart-Rapacewicz A. The Influence of the Filtration Bed type in the Pool Water Treatment System on Washings Quality. Ecol. Chem. Eng. S. 2019;26:535–545. doi: 10.1515/eces-2019-0039. [DOI] [Google Scholar]
- 11.Wyczarska-Kokot J., Lempart-Rapacewicz A., Dudziak M., Łaskawiec E. Impact of swimming pool water treatment system factors on the content of selected disinfection by-products. Environ. Monit. Assess. 2020;192:722. doi: 10.1007/s10661-020-08683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lempart A., Kudlek E., Dudziak M. Concentration levels of selected pharmaceuticals in swimming pool water. Desalin. Water Treat. 2018;117:353–361. doi: 10.5004/dwt.2018.22552. [DOI] [Google Scholar]
- 13.Lempart A., Kudlek E., Lempart M., Dudziak M. The presence of compounds from the Personal Care Products group in swimming pool water. J. Ecol. Eng. 2018;19:29–37. doi: 10.12911/22998993/85377. [DOI] [Google Scholar]
- 14.Lempart A., Kudlek E., Dudziak M. Nanofiltration treatment of swimming pool water in the aspect of the phenolic micropollutants elimination. Desalin. Water Treat. 2018;128:306–313. doi: 10.5004/dwt.2018.22876. [DOI] [Google Scholar]
- 15.Lempart A., Kudlek E., Dudziak M. The potential of the organic micropollutants emission from swimming accessories into pool water. Environ. Int. 2020;136:105442. doi: 10.1016/j.envint.2019.105442. [DOI] [PubMed] [Google Scholar]
- 16.Kudlek E., Lempart-Rapacewicz A., Dudziak M. Identification of Potential Harmful Transformation Products of Selected Micropollutants in Outdoor and Indoor Swimming Pool Water. Int. J. Environ. Res. Public Health. 2022;19:5660. doi: 10.3390/ijerph19095660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC and 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Official Journal of the European UnionL 348/84. [(accessed on 15 January 2023)]. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:348:0084:0097:EN:PDF.
- 18.Chowdhury S., Alhooshani K., Karanfil T. Disinfection Byproducts in Swimming Pool: Occurrences, Implications and Future Needs. Water Res. 2014;53:68–109. doi: 10.1016/j.watres.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Pándics T., Hofer Á., Dura G., Vargha M., Szigeti T., Tóth E. Health Risk of Swimming Pool Disinfection By-Products: A Regulatory Perspective. J. Water Health. 2018;16:947–957. doi: 10.2166/wh.2018.178. [DOI] [PubMed] [Google Scholar]
- 20.Barna Z., Kádár M. The Risk of Contracting Infectious Diseases in Public Swimming Pools. A Review. Ann. Ist. Super. Sanita. 2012;48:374–386. doi: 10.4415/ANN_12_04_05. [DOI] [PubMed] [Google Scholar]
- 21.Water Quality–Sampling–Part 3: Fixation and Handling of Water Samples. Polish Committee forStandardization (PCS); Warsaw, Poland: 2018. p. 66. [Google Scholar]
- 22.Wyczarska-Kokot J., Lempart A., Marciniak M. Research and evaluation of water quality in outdoor swimming pools, E3S Web of Conferences. E3S Web Conf. 2019;100:00089. doi: 10.1051/e3sconf/201910000089. [DOI] [Google Scholar]
- 23.Wyczarska-Kokot J., Lempart-Rapacewicz A., Dudziak M. Analysis of free and combined chlorine concentrations in swimming pool water and an attempt to determine a reliable water sampling point. Water. 2020;12:311. doi: 10.3390/w12020311. [DOI] [Google Scholar]
- 24.Lempart A., Kudlek E., Dudziak M. Determination of Micropollutants in Water Samples from Swimming Pool Systems. Water. 2018;10:1083. doi: 10.3390/w10081083. [DOI] [Google Scholar]
- 25.Food Standards Agency, Approved Additives and E Numbers. [(accessed on 29 January 2023)]; Available online: https://www.food.gov.uk/
- 26.Weng S.C., Sun P., W-w B., Huang C.H., Lee L.T., Blatchley E.R. The presence of pharmaceuticals and personal care products (PPCPs) in swimming pools. Environ. Sci. Technol. Lett. 2014;1:495–498. doi: 10.1021/ez5003133. [DOI] [Google Scholar]
- 27.Suppes L.M., Huang C.H., Lee W.N., Brockman K.J. Sources of pharmaceuticals and personal care products in swimming pools. J. Water Health. 2017;15:829–833. doi: 10.2166/wh.2017.004. [DOI] [PubMed] [Google Scholar]
- 28.Ekowati Y., Buttiglieri G., Ferrero G., Valle-Sistac J., Diaz-Cruz M.S., Barceló D., Petrovic M., Villagrasa M., Kennedy M.D., Rodríguez-Roda I. Occurrence of pharmaceuticals and UV filters in swimmingpools and spas. Environ. Sci. Pollut. Res. Int. 2016;23:14431–14441. doi: 10.1007/s11356-016-6560-1. [DOI] [PubMed] [Google Scholar]
- 29.Teo T.L.L., Coleman H.M., Khan S.J. Occurrence and daily variability of pharmaceuticals and personal care products in swimming pools. Environ. Sci. Pollut. Res. 2016;23:6972–6981. doi: 10.1007/s11356-015-5967-4. [DOI] [PubMed] [Google Scholar]
- 30.Elmore S.A., Rehg J.E., Schoeb T.R., Everitt J.I., Bolon B. Pathologists’ perspective on the study design, analysis, and interpretation of proliferative lesions in lifetime and prenatal rodent carcinogenicity bioassays of aspartame. Food Chem. Toxicol. 2023;171:113504. doi: 10.1016/j.fct.2022.113504. [DOI] [PubMed] [Google Scholar]
- 31.McCann D., Barrett A., Cooper A., Crumpler D., Dalen L., Grimshaw K., Kitchin E., Lok K., Porteous L., Prince E., et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet. 2007;370:1560–1567. doi: 10.1016/S0140-6736(07)61306-3. [DOI] [PubMed] [Google Scholar]
- 32.Dufour A.P., Evans O., Behymer T.D., Cantú R. Water ingestion during swimming activities in a pool: A pilot study. J. Water Health. 2006;4:425–430. doi: 10.2166/wh.2006.0026. [DOI] [PubMed] [Google Scholar]
- 33.Włodyka-Bergier A., Gajewska D. Trichloramina w wodzie basenowej [Trichloramine in swimmingpoolwater] PływalnieiBaseny. 2018;28:90–92. [Google Scholar]
- 34.Final Report on the Safety Assessment of BHT. Int. J. Toxicol. 2002;21:19–94. doi: 10.1080/10915810290096513. [DOI] [PubMed] [Google Scholar]
- 35.European Chemicals Agency (ECHA) [(accessed on 15 January 2023)]. Available online: https://echa.europa.eu/web/guest/legal-notice.
- 36.Fantuzzi G., Aggazzotti G., Righi E., Predieri G., Castiglioni S., Riva F., Zuccato E. Illicit drugs and pharmaceuticals in swimming pool waters. Sci. Total Environ. 2018;635:956–963. doi: 10.1016/j.scitotenv.2018.04.155. [DOI] [PubMed] [Google Scholar]
- 37.Cacciari E., Milani S., Balsamo A., Spada E., Bona G., Cavallo L., Cerutti F., Gargantini L., Greggio N., Tonini G., et al. Italian cross-sectional growth charts for height, weight and bmi (2 To 20 Yr) J. Endocrinol. Investig. 2014;29:579–580. doi: 10.1007/BF03344156. [DOI] [PubMed] [Google Scholar]
- 38.United States Environmental Protection Agency, ECOTOX Knowledgebase. [(accessed on 15 January 2023)]; Available online: https://cfpub.epa.gov/ecotox/search.cfm.
- 39.Wang Y., Li X., Sun X. The transformation mechanism and eco-toxicity evaluation of butylated hydroxyanisole in environment. Ecotoxicol. Environ. Saf. 2022;231:113179. doi: 10.1016/j.ecoenv.2022.113179. [DOI] [PubMed] [Google Scholar]
- 40.García-García R., Searle S.S. Preservatives: Food Use. In: Caballero B., Finglas P.M., Toldrá F., editors. Encyclopedia of Food and Health. Academic Press; Cambridge, MA, USA: 2016. pp. 505–509. [Google Scholar]
- 41.Ohta M., Narita M., Miyoshi T., Itoyama T., Kimura M., Kobayashi M., Ochi R., Sekiguchi Y., Koiguchi S., Hirahara Y. Analysis of BHA, BHT and TBHQ in chewing gum by GC and GC/MS. J. Food Hyg. Soc. Jpn. 2009;38:78–84. doi: 10.3358/shokueishi.38.78. [DOI] [Google Scholar]
- 42.Wang Y., He L., Lv G., Liu W., Liu J., Ma X., Sun X. Distribution, transformation and toxicity evaluation of 2,6-Di-tert-butyl-hydroxytotulene in aquatic environment. Environ. Pollut. 2019;255:113330. doi: 10.1016/j.envpol.2019.113330. [DOI] [PubMed] [Google Scholar]
- 43.Liu R., Lin Y., Ruan T., Jiang G. Occurrence of synthetic phenolic antioxidants and transformation products in urban and rural indoor dust. Environ. Pollut. 2017;221:227–233. doi: 10.1016/j.envpol.2016.11.069. [DOI] [PubMed] [Google Scholar]
- 44.Liu R., Mabury S.A. Unexpectedly high concentrations of 2,4-di-tert-butylphenol in human urine. Environ. Pollut. 2019;252:1423–1428. doi: 10.1016/j.envpol.2019.06.077. [DOI] [PubMed] [Google Scholar]
- 45.Liu R., Mabury S.A. Synthetic phenolic antioxidants and transformation products in dust from different indoor environments in Toronto, Canada. Sci. Total Environ. 2019;672:23–29. doi: 10.1016/j.scitotenv.2019.03.495. [DOI] [PubMed] [Google Scholar]
- 46.Liu R.Z., Ruan T., Song S.J., Lin Y.F., Jiang G.B. Determination of synthetic phenolic antioxidants and relative metabolites in sewage treatment plant and recipient river by high performance liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A. 2015;1381:13–21. doi: 10.1016/j.chroma.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 47.Lu Z., Smyth S.A., De Silva A.O. Distribution and fate of synthetic phenolic antioxidants in various wastewater treatment processes in Canada. Chemosphere. 2019;219:826–835. doi: 10.1016/j.chemosphere.2018.12.068. [DOI] [PubMed] [Google Scholar]
- 48.Yang X.X., Song W.T., Liu N., Sun Z.D., Liu R.R., Liu Q.S., Zhou Q.F., Jiang G.B. Synthetic phenolic antioxidants cause perturbation in steroidogenesis in vitro and in vivo. Environ. Sci. Technol. 2018;52:850–858. doi: 10.1021/acs.est.7b05057. [DOI] [PubMed] [Google Scholar]
- 49.The U.S. Department of Health and Human Services, 15th Report on Carcinogens. [(accessed on 29 January 2023)]; Available online: https://ntp.niehs.nih.gov/whatwestudy/assessments/cancer/roc/index.html.
- 50.Rajamani U., Gross A.R., Ocampo C., Andres A.M., Gottlieb R.A., Sareen D. Endocrine disruptors induce perturbations in endoplasmic reticulum and mitochondria of human pluripotent stem cell derivatives. Nat. Commun. 2017;8:219. doi: 10.1038/s41467-017-00254-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Khezerlou A., pouyaAkhlaghi A., Mirza Alizadeh A., Dehghan P., Maleki P. Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review. Toxicol. Rep. 2022;9:1066–1075. doi: 10.1016/j.toxrep.2022.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chazelas E., Druesne-Pecollo N., Esseddik Y., de Edelenyi F.S., Agaesse C., De Sa A., Lutchia R., Rebouillat P., Srour B., Debras C., et al. Exposure to food additive mixtures in 106,000 French adults from the NutriNet-Santé cohort. Sci. Rep. 2021;11:19680. doi: 10.1038/s41598-021-98496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urbansky E.T. Ascorbic acid treatment to reduce residual halogen-based oxidants prior to the determination of halogenated disinfection byproducts in potable water. J. Environ. Monit. 1999;1:471–476. doi: 10.1039/a904574k. [DOI] [PubMed] [Google Scholar]
- 54.Wastewater Fact Sheet Dechlorination. Environmental Protection Agency; Washington, DC, USA: 2000. [Google Scholar]
- 55.Jansson P.J., Del Castillo U., Lindqvist C., Nordström T. Effects of iron on Vitamin C/copper-induced hydroxyl radical generation in bicarbonate-rich water. Free Radic. Res. 2005;39:565–570. doi: 10.1080/10715760400009233. [DOI] [PubMed] [Google Scholar]
- 56.Song J.H., Shin S.H., Wang W., Ross G.M. Involvement of oxidative stress in ascorbateinducedproapoptotic death of PC12 cells. Exp. Neurol. 2001;169:425–437. doi: 10.1006/exnr.2001.7680. [DOI] [PubMed] [Google Scholar]
- 57.Brown S., Georgatos M., Reifel C., Song J.H., Shin S.H., Hong M. Recycling processes of cellular ascorbate generate oxidative stress in pancreatic tissues in in vitro system. Endocrine. 2002;18:91–96. doi: 10.1385/ENDO:18:1:91. [DOI] [PubMed] [Google Scholar]
- 58.Song J.H., Shin S.H., Ross G.M. Oxidative stress induced by ascorbate causes neuronal damage in an in vitro system. Brain Res. 2001;895:66–72. doi: 10.1016/S0006-8993(01)02029-7. [DOI] [PubMed] [Google Scholar]
- 59.Walczyk-Nowicka Ł.J., Herbet M. Sodium Benzoate—Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review. Nutrients. 2022;14:1497. doi: 10.3390/nu14071497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wibbertmann A., Kielhorn J., Koennecker G., Mangelsdorf I., Melber C. Benzoic Acid and Sodiumbenzoate. World Health Organization; Geneva, Switzerland: 2000. pp. 1–46. [Google Scholar]
- 61.Piper J.D., Piper P.W. Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate. Compr. Rev. Food Sci. Food Saf. 2017;16:868–880. doi: 10.1111/1541-4337.12284. [DOI] [PubMed] [Google Scholar]
- 62.Gardner L.K., Lawrence G.D. Benzene Production from Decarboxylation of Benzoic Acid in the Presence of Ascorbic Acid and a Transition-Metal Catalyst. J. Agric. Food Chem. 1993;41:693–695. doi: 10.1021/jf00029a001. [DOI] [Google Scholar]
- 63.Nyman P.J., Wamer W.G., Begley T.H., Diachenko G.W., Perfetti G.A. Evaluation of Accelerated UV and Thermal Testing for Benzene Formation in Beverages Containing Benzoate and Ascorbic Acid. J. Food Sci. 2010;75:C263–C267. doi: 10.1111/j.1750-3841.2010.01536.x. [DOI] [PubMed] [Google Scholar]
- 64.Pongsavee M. Effect of Sodium Benzoate Preservative on Micronucleus Induction, Chromosome Break, and Ala40Thr Superoxide Dismutase Gene Mutation in Lymphocytes. BioMed Res. Int. 2015;2015:103512. doi: 10.1155/2015/103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pang L., Borthwick A.G.L., Chatzisymeon E. Determination, occurrence, and treatment of saccharin in water: A review. J. Clean. Prod. 2020;270:122337. doi: 10.1016/j.jclepro.2020.122337. [DOI] [Google Scholar]
- 66.Lange F.T., Scheurer M., Brauch H. Artificial sweeteners—A recently recognized class of emerging environmental contaminants: A review. Anal. Bioanal. Chem. 2012;403:2503–2518. doi: 10.1007/s00216-012-5892-z. [DOI] [PubMed] [Google Scholar]
- 67.Gan Z., Sun H., Feng B., Wang R., Zhang Y. Occurrence of seven artificial sweeteners in the aquatic environment and precipitation of Tianjin, China. Water Res. 2013;47:4928–4937. doi: 10.1016/j.watres.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 68.Naik A.Q., Zafar T., Shrivastava V.K. Environmental Impact of the Presence, Distribution, and Use of Artificial Sweeteners as Emerging Sources of Pollution. J. Environ. Public Health. 2021;2021:6624569. doi: 10.1155/2021/6624569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tibaldi E., Gnudi F., Panzacchi S., Mandrioli D., Vornoli A., Manservigi M., Sgargi D., Falcioni L., Bua L., Belpoggi F. Identification of aspartame-induced haematopoietic and lymphoid tumours in rats after lifetime treatment. Acta Histochem. 2020;122:151548. doi: 10.1016/j.acthis.2020.151548. [DOI] [PubMed] [Google Scholar]
- 70.Landrigan P.J., Straif K. Aspartame and cancer—New evidence for causation. Environ. Health. 2021;20:42. doi: 10.1186/s12940-021-00725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borghoff S.J., Cohen S.S., Jiang X., Lea I.A., Klaren W.D., Chappell G.A., Britt J.K., Rivera B.N., Choski N.Y., Wikoff D.S. Updated systematic assessment of human, animal and mechanistic evidence demonstrates lack of human carcinogenicity with consumption of aspartame. Food Chem. Toxicol. 2023;172:113549. doi: 10.1016/j.fct.2022.113549. [DOI] [PubMed] [Google Scholar]
- 72.Bandyopadhyay A., Ghoshal S., Mukherjee A. Genotoxicity Testing of Low-Calorie Sweeteners: Aspartame, Acesulfame-K, and Saccharin. Drug Chem. Toxicol. 2008;31:447–457. doi: 10.1080/01480540802390270. [DOI] [PubMed] [Google Scholar]
- 73.Kobeticova K., Mocova K.A., Mrhalkova L., Frycova Z., Koci V. Artificial sweeteners and the environment. Czech J. Food Sci. 2016;34:149–153. doi: 10.17221/220/2015-CJFS. [DOI] [Google Scholar]
- 74.Luo J., Zhang Q., Cao M., Wu L., Cao J., Fang F., Li C., Xue Z., Feng Q. Ecotoxicity and environmental fates of newly recognized contaminants-artificial sweeteners: A review. Sci. Total Environ. 2019;653:1149–1160. doi: 10.1016/j.scitotenv.2018.10.445. [DOI] [PubMed] [Google Scholar]
- 75.Uçar A., Yilmaz S. Saccharin genotoxicity and carcinogenicity: A review. Adv. Food Res. 2015;37:138–142. [Google Scholar]
- 76.Bian X., Tu P., Chi L., Gao B., Ru H., Lu K. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem. Toxicol. 2017;107:530–539. doi: 10.1016/j.fct.2017.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qin X. Impaired inactivation of digestive proteases in lower gut due to inhibition of gut bacteria by food additives such as saccharin and sucralose as main cause of inflammatory bowel disease: A two-decades-long hypothesis warrants testing. Inflamm. Bowel Dis. 2019;25:e141. doi: 10.1093/ibd/izz187. [DOI] [PubMed] [Google Scholar]
- 78.Soh L., Connors K.A., Brooks B.W., Zimmerman J. Fate of sucralose through environmental and water treatment processes and impact on plant indicator species. Environ. Sci. Technol. 2011;45:1363–1369. doi: 10.1021/es102719d. [DOI] [PubMed] [Google Scholar]
- 79.Scheurer M., Brauch H.-J., Lange F.T. Analysis and occurrence of seven artificial sweeteners in German waste water and surface water and in soil aquifer treatment (SAT) Anal. Bioanal. Chem. 2009;394:1585–1594. doi: 10.1007/s00216-009-2881-y. [DOI] [PubMed] [Google Scholar]
- 80.Torres C.I., Ramakrishna S., Chiu C.-A., Nelson K.G., Westerhoff P., Krajmalnik-Brown R. Fate of sucralose during wastewater treatment. Environ. Eng. Sci. 2011;28:325–331. doi: 10.1089/ees.2010.0227. [DOI] [Google Scholar]
- 81.Li S., Ren Y., Fu Y., Gao X., Jiang C., Wu G., Ren H., Geng J. Fate of artificial sweeteners through wastewater treatment plants and water treatment processes. PLoS ONE. 2018;13:e0189867. doi: 10.1371/journal.pone.0189867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davididou K., Chatzisymeon E., Perez-Estrada L., Oller I., Malato S. Photo-Fenton treatment of saccharin in a solar pilot compound parabolic collector: Use of olive mill wastewater as iron chelating agent, preliminary results. J. Hazard. Mater. 2019;372:137–144. doi: 10.1016/j.jhazmat.2018.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.