Abstract
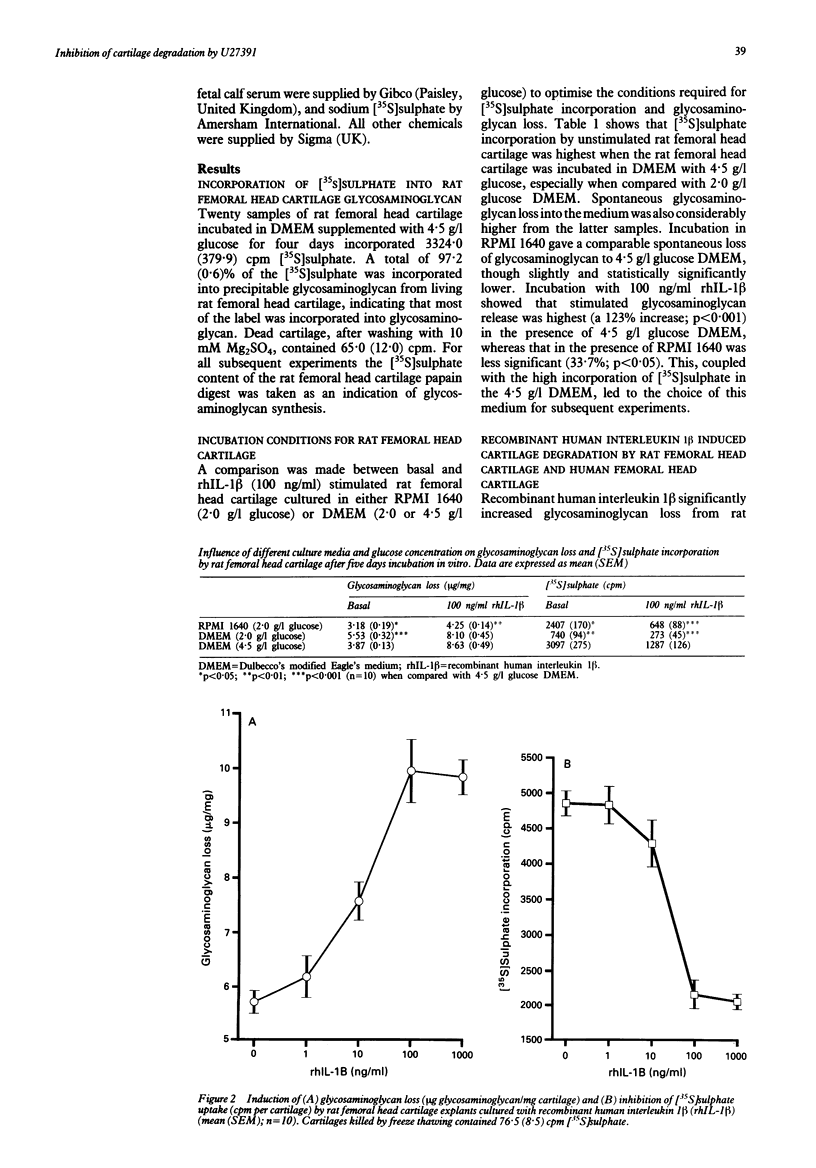
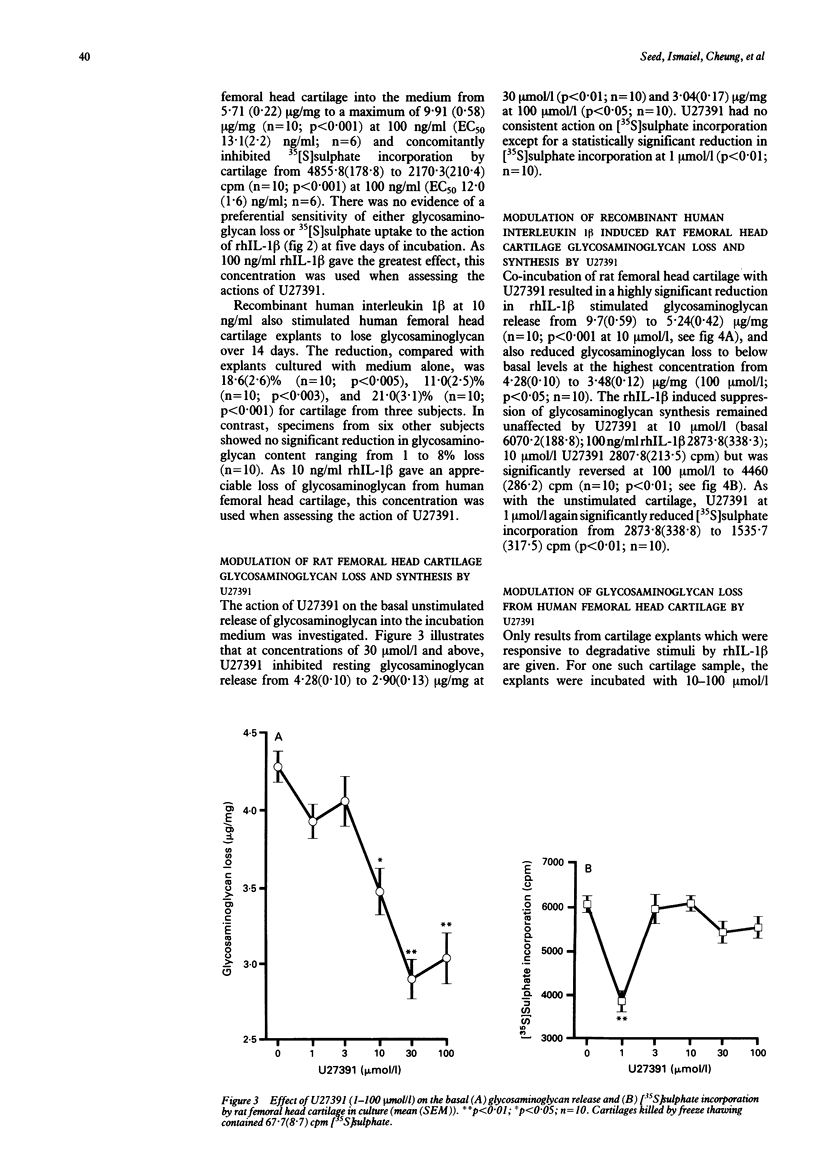
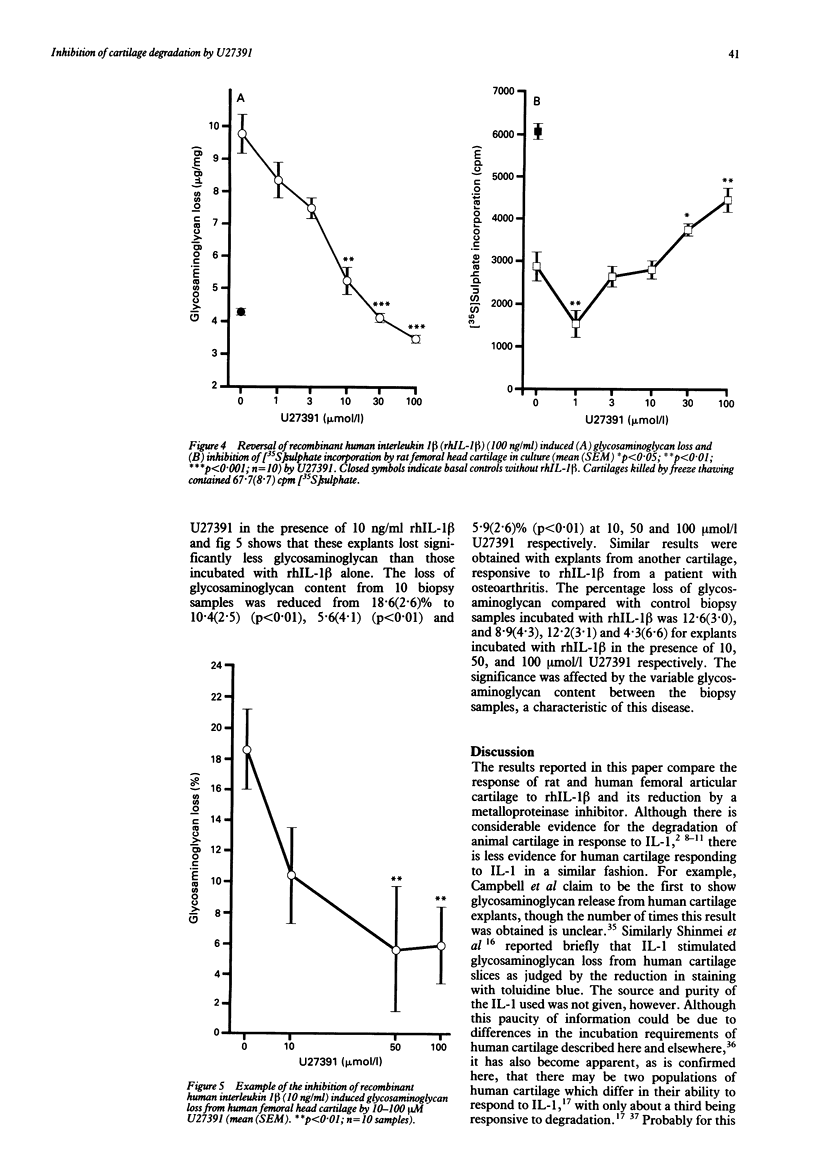
Interleukin 1 induced proteoglycan loss from cartilage in vitro was prevented by a biochemical inhibitor of metalloproteinase activity. The inhibitor also partially relieved the inhibition of proteoglycan synthesis caused by interleukin 1. The loss of glycosaminoglycan by rat and human femoral head cartilage in response to human recombinant interleukin 1 beta (rhIL-1 beta) was established, and the modulation of this loss by the metalloproteinase inhibitor U27391 was investigated. Rat femoral head cartilage consistently lost glycosaminoglycan in response to rhIL-1 beta whereas only a proportion (30%) of normal human femoral head cartilage did so. Concentrations of 10-100 mumol/l U27391 inhibited the action of rhIL-1 beta on rat femoral head cartilage, reversing both the loss of glycosaminoglycan and the inhibition of glycosaminoglycan synthesis. U27391 also prevented the reduction in glycosaminoglycan content of those human femoral head cartilage explants responsive to rhIL-1 beta. Metalloproteinase inhibition therefore prevents rhIL-1 beta induced glycosaminoglycan loss by rat and human femoral head cartilage, suggesting that inhibitors of such enzymes may prove to be of therapeutic benefit in erosive diseases in humans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttle D. J., Saklatvala J., Tamai M., Barrett A. J. Inhibition of interleukin 1-stimulated cartilage proteoglycan degradation by a lipophilic inactivator of cysteine endopeptidases. Biochem J. 1992 Jan 1;281(Pt 1):175–177. doi: 10.1042/bj2810175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. K., Piccoli D. S., Butler D. M., Singleton D. K., Hamilton J. A. Recombinant human interleukin-1 stimulates human articular cartilage to undergo resorption and human chondrocytes to produce both tissue- and urokinase-type plasminogen activator. Biochim Biophys Acta. 1988 Nov 17;967(2):183–194. doi: 10.1016/0304-4165(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Campbell I. K., Roughley P. J., Mort J. S. The action of human articular-cartilage metalloproteinase on proteoglycan and link protein. Similarities between products of degradation in situ and in vitro. Biochem J. 1986 Jul 1;237(1):117–122. doi: 10.1042/bj2370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. B., Sygowski L. A., Wolanin D. J., Patton S. P., Caccese R. G., Shaw A., Roberts R. A., DiPasquale G. Effect of synthetic metalloprotease inhibitors on cartilage autolysis in vitro. J Pharmacol Exp Ther. 1987 Feb;240(2):460–465. [PubMed] [Google Scholar]
- Clay K., Seed M. P., Clements-Jewery S. Studies on interleukin-1 beta induced glycosaminoglycan release from rat femoral head cartilage in-vitro. J Pharm Pharmacol. 1989 Jul;41(7):503–504. doi: 10.1111/j.2042-7158.1989.tb06514.x. [DOI] [PubMed] [Google Scholar]
- Deshmukh-Phadke K., Lawrence M., Nanda S. Synthesis of collagenase and neutral proteases by articular chondrocytes: stimulation by a macrophage-derived factor. Biochem Biophys Res Commun. 1978 Nov 14;85(1):490–496. doi: 10.1016/s0006-291x(78)80068-0. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Jubb R. W. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977 Sep-Oct;20(7):1359–1371. doi: 10.1002/art.1780200710. [DOI] [PubMed] [Google Scholar]
- Fontana A., Hengartner H., Weber E., Fehr K., Grob P. J., Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49–53. doi: 10.1007/BF00541245. [DOI] [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Hardingham T. E., Murphy G., Hamilton J. A. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991 Aug 25;266(24):15579–15582. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., Meats J. E., Russell R. G. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta. 1984 Feb 14;797(2):186–193. doi: 10.1016/0304-4165(84)90121-1. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Knight C. G., Dingle J. T., Barrett A. J. Evidence that extracellular cathepsin D is not responsible for the resorption of cartilage matrix in culture. Biochim Biophys Acta. 1982 Feb 2;714(2):307–312. doi: 10.1016/0304-4165(82)90338-5. [DOI] [PubMed] [Google Scholar]
- Hickery M. S., Vilim V., Bayliss M. T., Hardingham T. E. Effect of interleukin-1 and tumour necrosis factor-alpha on the turnover of proteoglycans in human articular cartilage. Biochem Soc Trans. 1990 Oct;18(5):953–954. doi: 10.1042/bst0180953. [DOI] [PubMed] [Google Scholar]
- Hughes C., Murphy G., Hardingham T. E. Metalloproteinase digestion of cartilage proteoglycan. Pattern of cleavage by stromelysin and susceptibility to collagenase. Biochem J. 1991 Nov 1;279(Pt 3):733–739. doi: 10.1042/bj2790733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaiel S., Atkins R. M., Pearse M. F., Dieppe P. A., Elson C. J. Susceptibility of normal and arthritic human articular cartilage to degradative stimuli. Br J Rheumatol. 1992 Jun;31(6):369–373. doi: 10.1093/rheumatology/31.6.369. [DOI] [PubMed] [Google Scholar]
- Ismaiel S., Hollander A. P., Atkins R. M., Elson C. J. Differential responses of human and rat cartilage to degrading stimuli in-vitro. J Pharm Pharmacol. 1991 Mar;43(3):207–209. doi: 10.1111/j.2042-7158.1991.tb06668.x. [DOI] [PubMed] [Google Scholar]
- Jubb R. W., Fell H. B. The effect of synovial tissue on the synthesis of proteoglycan by the articular cartilage of young pigs. Arthritis Rheum. 1980 May;23(5):545–555. doi: 10.1002/art.1780230505. [DOI] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Hughes C. E., Fosang A. J., Hardingham T. E. Role and regulation of metalloproteinases in connective tissue turnover. Biochem Soc Trans. 1990 Oct;18(5):812–815. doi: 10.1042/bst0180812. [DOI] [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Roughley P. J., Mort J. S. Degradation of proteoglycan aggregate by a cartilage metalloproteinase. Evidence for the involvement of stromelysin in the generation of link protein heterogeneity in situ. Biochem J. 1989 Apr 1;259(1):61–67. doi: 10.1042/bj2590061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon J. S., Bottomley K. M., Broadhurst M. J., Brown P. A., Johnson W. H., Lawton G., Marley J., Sedgwick A. D., Wilkinson S. E. Potent collagenase inhibitors prevent interleukin-1-induced cartilage degradation in vitro. Int J Tissue React. 1991;13(5):237–241. [PubMed] [Google Scholar]
- Pasternak R. D., Hubbs S. J., Caccese R. G., Marks R. L., Conaty J. M., DiPasquale G. Interleukin-1 stimulates the secretion of proteoglycan- and collagen-degrading proteases by rabbit articular chondrocytes. Clin Immunol Immunopathol. 1986 Dec;41(3):351–367. doi: 10.1016/0090-1229(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A., Tyler J. A., Hardingham T. E. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem J. 1986 Sep 1;238(2):571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Pilsworth L. M., Sarsfield S. J., Gavrilovic J., Heath J. K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984 Dec 1;224(2):461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Boynton R. E., Flannery C. R. Analysis of the catabolism of aggrecan in cartilage explants by quantitation of peptides from the three globular domains. J Biol Chem. 1991 May 5;266(13):8198–8205. [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Sapolsky A. I., Keiser H., Howell D. S., Woessner J. F., Jr Metalloproteases of human articular cartilage that digest cartilage proteoglycan at neutral and acid pH. J Clin Invest. 1976 Oct;58(4):1030–1041. doi: 10.1172/JCI108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky A. I., Malemud C. J., Norby D. P., Moskowitz R. W., Matsuta K., Howell D. S. Neutral proteinases from articular chondrocytes in culture. 2. Metal-dependent latent neutral proteoglycanase, and inhibitory activity. Biochim Biophys Acta. 1981 Mar 13;658(1):138–147. doi: 10.1016/0005-2744(81)90257-6. [DOI] [PubMed] [Google Scholar]
- Schnyder J., Payne T., Dinarello C. A. Human monocyte or recombinant interleukin 1's are specific for the secretion of a metalloproteinase from chondrocytes. J Immunol. 1987 Jan 15;138(2):496–503. [PubMed] [Google Scholar]
- Seed M. P., Thomson T. A., Gardner C. R. Investigation of the role of metalloproteinases in recombinant human interleukin-1 beta-induced degradation of rat femoral head cartilage. Drugs Exp Clin Res. 1991;17(7):355–361. [PubMed] [Google Scholar]
- Shinmei M., Kikuchi T., Masuda K., Shimomura Y. Effects of interleukin-1 and anti-inflammatory drugs on the degradation of human articular cartilage. Drugs. 1988;35 (Suppl 1):33–41. doi: 10.2165/00003495-198800351-00008. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Rohloff N. A., Sam L. M., Justen J. M., Deibel M. R., Cornette J. C. Recombinant human interleukin-1 alpha and recombinant human interleukin-1 beta stimulate cartilage matrix degradation and inhibit glycosaminoglycan synthesis. Inflammation. 1989 Aug;13(4):367–382. doi: 10.1007/BF00914921. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Hamerman D. Release of interleukin-1 from human synovial tissue in vitro. Arthritis Rheum. 1985 Aug;28(8):853–862. doi: 10.1002/art.1780280804. [DOI] [PubMed] [Google Scholar]
- van de Loo A. A., van Beuningen H. M., van Lent P. L., van den Berg W. B. Direct effect of murine rIL-1 on cartilage metabolism in vivo. Agents Actions. 1989 Jan;26(1-2):153–155. doi: 10.1007/BF02126590. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B., van de Loo F. A., Zwarts W. A., Otterness I. G. Effects of murine recombinant interleukin 1 on intact homologous articular cartilage: a quantitative and autoradiographic study. Ann Rheum Dis. 1988 Oct;47(10):855–863. doi: 10.1136/ard.47.10.855. [DOI] [PMC free article] [PubMed] [Google Scholar]


