Abstract
Background:
Smoking cessation rates after stroke and TIA are suboptimal, and smoking-cessation interventions are underutilized. We performed a cost-effectiveness analysis of smoking-cessation interventions in this population.
Methods:
We constructed a decision tree and used Markov models that aimed to assess the cost effectiveness of varenicline, any pharmacotherapy with intensive counseling, and monetary incentives, compared to brief counseling alone in the secondary stroke prevention setting. Payer and societal costs of interventions and outcomes were modeled. The outcomes were recurrent stroke, myocardial infarction, and death using a lifetime horizon. Estimates and variance for the base case (35% cessation), costs and effectiveness of interventions, and outcome rates were imputed from the stroke literature. We calculated incremental cost-effectiveness ratios (ICER) and incremental net-monetary benefits (NMB). An intervention was considered cost effective if the ICER was less than the willingness-to-pay threshold of $100,000 per quality-adjusted life year (QALY) or when the incremental NMB was positive. Probabilistic Monte Carlo simulations modeled the impact of parameter uncertainty.
Results:
From the payer perspective, varenicline and pharmacotherapy with intensive counseling were associated with more QALYs (0.67 and 1.00, respectively) at less total lifetime costs compared to brief counseling alone. Monetary incentives were associated with 0.71 more QALYs at an additional cost of $120 compared to brief counseling alone, yielding an ICER of $168/QALY. From the societal perspective, all three interventions provided more QALYs at less total costs compared to brief counseling alone. In 10,000 Monte Carlo simulations, all three smoking-cessation interventions were cost-effective in >89% of runs.
Conclusion:
For secondary stroke prevention, it is cost effective and potentially cost saving to deliver smoking cessation therapy beyond brief counseling alone.
Keywords: smoking, secondary prevention, cerebrovascular disease/stroke
Subject Terms: Transient Ischemic Attack (TIA)
Graphical Abstract
Introduction
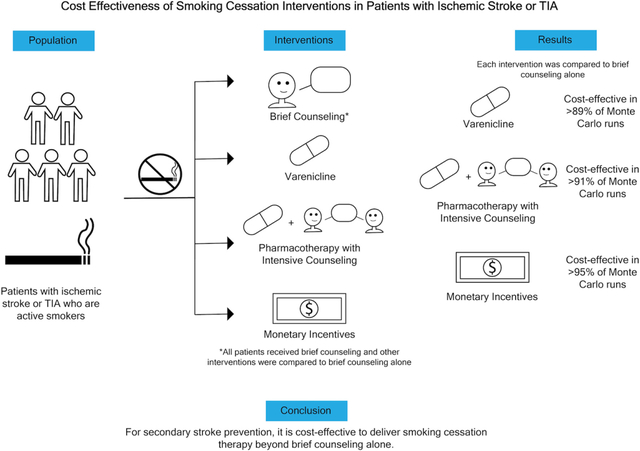
Patients who continue to smoke cigarettes after ischemic stroke and transient ischemic attack (TIA) face an increased risk of recurrent stroke and cardiovascular events.1-3 Smoking cessation is an important part of secondary prevention.4 Based on several estimates, only approximately 40% of people quit smoking after stroke and there are emerging data that smoking cessation strategies are under-utilized after TIA and ischemic stroke.3,5,6 An array of evidence-based smoking-cessation interventions are available. In addition to counseling and behavioral interventions, several pharmacotherapies have been shown to increase the likelihood of successful smoking cessation, including nicotine replacement therapy, varenicline, and bupropion.7,8 Monetary incentives, in the form of direct payments to patients for successful cessation, are uncommonly used but also an evidenced-based strategy.9 The cost effectiveness of these interventions in the secondary prevention setting after stroke and TIA has not been evaluated. Therefore, we performed a cost effectiveness analysis evaluating three smoking-cessation interventions for patients with ischemic stroke and TIA in the United States. Second, we used our models to estimate the maximum acceptable costs of effective smoking-cessation interventions for this population to better inform secondary prevention paradigms.
Methods
Design
The aim was to assess the cost effectiveness of three smoking-cessation interventions (varenicline, any pharmacotherapy with intensive counseling, and monetary incentives), as compared to brief counseling alone, for secondary stroke prevention in patients with TIA or ischemic stroke who are active smokers. To do this, we used decision tree models combined with Markov models. Our analysis does not constitute human subjects research. We adhered to best practices in cost effectiveness analysis, as codified by the Consolidated Health Economic Evaluation Reporting Standards statement guidelines.10 The data that support the findings of this study are available from the corresponding author upon reasonable request.
Target Population
We constructed a decision model to evaluate different smoking cessation strategies for adult patients with TIA or ischemic stroke who are active smokers. The target population was based on patients from the Oxford Vascular Study with stroke or TIA (mean age, 73.9 years; 51% women; 14% diabetes; 19% mRS >2).11 This population was used to represent a typical stroke population encountered in clinical practice, and because key metrics needed for this analysis (rates of recurrent stroke, MI, death) were available for the Oxford Vascular Study.11
Comparators
Three interventions were compared to brief smoking-cessation counseling alone: varenicline, any pharmacotherapy with intensive counseling, and monetary incentives.7-9 We chose these smoking-cessation interventions, delivered in addition to brief counseling alone, to encompass a variety of effective approaches. Our standalone pharmacotherapy intervention was varenicline because it is endorsed by current stroke guidelines and considered first-line pharmacotherapy by some professional societies.4,12 Combining any pharmacotherapy (nicotine replacement therapy, bupropion, or varenicline) with intensive longitudinal counseling has been found to be an effective approach to smoking cessation after all-cause hospitalization.8,13 Monetary incentives for abstinence from tobacco use, in the form of direct payments to patients for successful cessation, have been shown to improve smoking cessation rates in hospitalized patients.14 Brief counseling was chosen as the base-case intervention because, although guidelines recommend smoking-cessation counseling with or without pharmacotherapy, in clinical practice patients commonly do not receive pharmacotherapy after stroke/TIA.5 In contrast, general smoking-cessation counseling is regularly provided to these patients.4,15
Model Construction
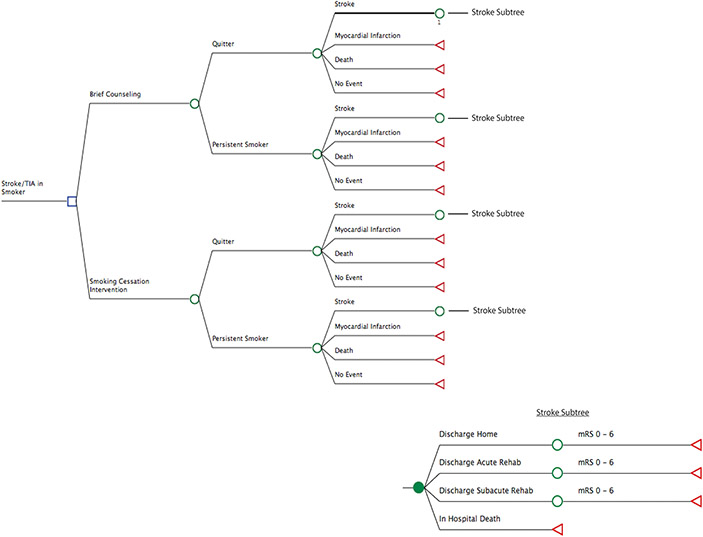
In our decision-analytic model, the main decision node was whether brief counseling alone was used or if one of the three smoking-cessation interventions were used in addition to brief counseling (Figure 1). A second decision node was whether the patient continued to smoke or quit smoking after each intervention. Model outcomes (end nodes) included no event, recurrent stroke, myocardial infarction, and all-cause death, the probabilities of which differed based on whether there was smoking cessation (Table 1). For patients who experienced a recurrent stroke, modified Rankin Scale (mRS) score distributions were estimated based on published population-based outcomes studies.15,16 This allowed for estimation of quality-adjusted life years (QALYs) based on mRS score.17-19
Figure 1.
Decision tree for cost effectiveness analysis of smoking cessation interventions. The branch entitled “Smoking Cessation Intervention” included each of the three interventions strategies plus brief counseling versus brief counseling alone. Abbreviations: TIA, transient ischemic attack; mRS, modified Rankin scale
Table 1.
Decision model input parameters
| Variable | Base Case Value |
Distribution type, uncertainty |
Reference |
|---|---|---|---|
| Probability of smoking cessation | |||
| Brief counseling alone | 0.35 | β, 0.32-0.37 | 3 |
| Varenicline | 0.51 | β, 0.48-0.78 | 7, 20,26 |
| Pharmacotherapy + intensive counseling | 0.59 | β, 0.39-0.89 | 9 |
| Monetary incentives | 0.52 | β, 0.44-0.60 | 8,24 |
| Risk of events in patients who quit smoking | |||
| Recurrent ischemic stroke | 14.4% | β, 12.7%-16.0% | 1,11 |
| Myocardial infarction | 3.5% | β, 2.6%-4.4% | 1,11 |
| Death | 19.0% | β, 17.4%-20.6% | 1,11 |
| Recurrent stroke after MI | 4.4% | β, 2.6% - 4.4% | 28,31 |
| MI after MI | 13.8% | β, 6.3%-13.8% | 28, 30 |
| Death after MI | 9.7% | β, 5.0%-17.0% | 27 |
| Risk of events in patients who continue smoking | |||
| Recurrent ischemic stroke | 18.3% | β, 16.2%-20.4% | 11 |
| Myocardial infarction | 4.3% | β, 3.2%-5.4% | 11 |
| Death | 31.7% | β, 29.0%-34.4% | 11 |
| Recurrent stroke after MI | 5.9% | β, 3.4%-5.9% | 1, 28 |
| MI after MI | 20.8% | β, 9.5%-20.8% | 29 |
| Death after MI | 15.4% | β, 8%-27% | 27, 32 |
| Discharge destination after stroke | |||
| Home | 58.3% | β, 49.0%-58.0% | 15 |
| Skilled nursing facility | 16.5% | β, 16.5%-19.9% | 15 |
| Rehabilitation facility | 18.2% | β, 17.9%-18.3% | 15 |
| In-hospital death | 6.5% | β, 6.1%-8.0% | 15 |
| mRS distribution after recurrent stroke | |||
| mRS 0 | 0.21 | NA | 16 |
| mRS 1 | 0.23 | NA | 16 |
| mRS 2 | 0.09 | NA | 16 |
| mRS 3 | 0.09 | NA | 16 |
| mRS 4 | 0.07 | NA | 16 |
| mRS 5 | 0.06 | NA | 16 |
| mRS 6 | 0.23 | NA | 16 |
| Outcome QALYs | |||
| mRS 0 | 0.85 | NA | 17,18,19 |
| mRS 1 | 0.80 | NA | 17,18,19 |
| mRS 2 | 0.70 | NA | 17,18,19 |
| mRS 3 | 0.51 | NA | 17,18,19 |
| mRS 4 | 0.30 | NA | 17,18,19 |
| mRS 5 | 0.15 | NA | 17,18,19 |
| Myocardial infarction | 0.84 | NA | 25 |
| Death | 0 | NA | 25 |
Abbreviations: mRS, modified rankin scale; MI, myocardial infarction; QALY, quality-adjusted life year
Our model used a lifetime horizon to estimate quality-adjusted life years and costs. We constructed a Markov state transition model, using a cycle length of five years. A cycle length of five years was used to be consistent with the time horizon linked to many of the input parameters.11 A patient who is a current smoker enters the model after experiencing a TIA or ischemic stroke and receives brief counseling alone, or one of the smoking cessation interventions in addition to brief counseling. The patient then either does not experience a recurrent cardiovascular event, experiences a recurrent stroke, experiences a myocardial infarct, or dies. After the first five-year cycle, the patient enters the Markov model and cycles through different health states; they can remain in their current health state, experience another recurrent stroke, experience a myocardial infarction, or die (Figure S1). A patient progresses through the model until death (absorbing state).
Model Parameters
All parameter inputs are found in Table 1.1,3,7-9,11,15-32 We used a quit rate of 35% with brief counseling alone, the usual care strategy that each intervention is compared to, based on population-based estimates from the United States and meta-analyses of cohort studies.3,6 Quit rates associated with each of the three comparator interventions, used in addition to brief counseling, were based on meta-analyses and randomized trials that examined the effect of each of the three interventions on smoking cessation.7-9,20,24,26 Key parameters for the Markov model were recurrent event rates that differed by smoking status.1,11,15,27-32 Recurrent event rates for patients who quit smoking were calculated by applying the relative risk reduction of smoking cessation on recurrent cardiovascular events reported in a post-hoc analysis of the Insulin Resistance Intervention After Stroke (IRIS) trial to the recurrent event rates seen in the Oxford Vascular Study.1,11 We estimated that the beneficial impact of smoking cessation on recurrent event rates in a trial population overstates the real-world benefit. 1,2 Thus, we discounted the beneficial impact of smoking cessation by 25% as a conservative strategy to avoid overestimating the cost-effectiveness of smoking cessation interventions to account for potentially attenuated benefits of smoking cessation outside of a trial population. Additionally, input parameters for analyses that directly used the recurrent event rates in people who quit versus continued smoking in the IRIS trial are provided in the Supplemental Materials (Table S1).
Cost Calculations
We constructed our model from the payer and societal perspectives (Table 2). The payer perspective includes only direct healthcare costs (e.g., costs of medications and healthcare). Healthcare costs of interventions were estimated based on previously reported costs from the literature. We estimated the cost of varenicline to be $473 for a three-month supply of medication, which is the standard course of treatment.7 The cost of any pharmacotherapy with intensive counseling was estimated to be $9048; this was the average cost of a post-discharge cessation program that included a three-month supply of any pharmacotherapy (nicotine replacement, bupropion, etc.) with three months of telephone-based counseling sessions. The cost of an average monetary incentive, in the form of one-time direct payments to patients for successful cessation, was estimated to be $912.9 These costs were varied in sensitivity analyses. We assumed no cost difference for brief counseling alone, which we assumed all patients received, as this is commonly provided verbally in standard clinical care. Healthcare costs associated with stroke, myocardial infarction, and death were estimated based on the literature.17,22,23 For the societal perspective, we accounted for indirect costs caused by stroke, myocardial infarction, and death. These were assessed based on a human capital approach. The societal costs were estimated based on previously reported costs for reduced productivity caused by stroke and myocardial infarction, lost productivity due to death, and costs of informal care given by family members.33-41 All costs were adjusted for inflation to 2021 US dollars using the Consumer Price Index.42 All costs and QALYs were discounted at 3% per year in the Markov model.43
Table 2.
Costs
| Variable | Base Case Value |
Distribution type, uncertainty |
Reference |
|---|---|---|---|
| Payer Costs | |||
| Brief counseling alone | $0 | NA | 1 |
| Varenicline | $473 | NA | 21 |
| Pharmacotherapy with intensive counseling | $904 | Normal, $551-$1,256 | 8 |
| Monetary Incentives | $912 | Normal, $555-$1,268 | 9 |
| Hospitalization, non-disabling stroke (mRS 0-2) | $17,663 | Normal, $15,665 - $19,613 | 22 |
| Hospitalization, disabling stroke (mRS 3-5) | $26,886 | Normal, $20,394- $33,624 | 22 |
| 5-year stroke cost after discharge (mRS 0-2) | $34,315 | Normal, $27,450 - $41,170 | 17 |
| 5-year stroke cost after discharge (mRS 3-5) | $87,890 | Normal, $70,310 - $105,465 | 17 |
| Hospitalization death | $38,146 | Normal, $26,355 - $51,027 | 22 |
| Myocardial infarction | $17,904 | Normal, $10,885 - $24,922 | 23 |
| Societal Costs | |||
| Average annual earnings of employed | $63,888 | NA | 33 |
| Population employment rate | 0.619 | NA | 34 |
| Relative earnings of stroke survivors | 0.825 | NA | 40 |
| 5-year earnings after initial stroke | $100,162 | NA | 33,34,36,40 |
| Probability return to work after stroke mRS 0 | 0.63 | NA | 39 |
| Probability return to work after stroke mRS 1 | 0.72 | NA | 39 |
| Probability return to work after stroke mRS 2 | 0.49 | NA | 39 |
| Probability return to work after stroke mRS 3 | 0.19 | NA | 39 |
| Probability return to work after stroke mRS 4 | 0.14 | NA | 39 |
| Probability return to work after stroke mRS 5 | 0 | NA | 39 |
| 5-year relative earnings lost after stroke, mRS 0 | $37,060 | Normal, $22,542-$51,587 | 39 |
| 5-year relative earnings lost after stroke, mRS 1 | $28,045 | Normal, $17,051-$39,038 | 39 |
| 5-year relative earnings lost after stroke, mRS 2 | $51,083 | Normal, $31,058-$71,107 | 39 |
| 5-year relative earnings lost after stroke, mRS 3 | $81,131 | Normal, $49,328-$112,933 | 39 |
| 5-year relative earnings lost after stroke, mRS 4 | $86,139 | Normal, $52,373-$119,904 | 39 |
| 5-year relative earnings lost after stroke, mRS 5 | $100,162 | Normal, $60,899-$139,424 | 39 |
| 5-year earnings lost after MI | $50,830 | Normal, $30,905-$70,755 | 35 |
| 5-year caregiving costs after stroke, mRS 0-1 | $8,095 | Normal, $4,921-$11,268 | 38 |
| 5-year caregiving costs after stroke, mRS 2-5 | $40,490 | Normal, $24,418-$56,361 | 38 |
| 5-year caregiving costs after MI | $365 | Normal, $222-$508 | 37, 41 |
Abbreviations: mRS, modified Rankin scale
Statistical Analysis
Effectiveness was measured in QALYs, and costs were measured in US dollars. We used the incremental cost-effectiveness ratio (ICER) and the incremental net monetary benefit (NMB) to evaluate cost effectiveness. We calculated the ICER by dividing the difference in total costs (incremental cost) by the difference in effectiveness (incremental effect) between each intervention individually and brief counseling alone. Interventions could be cost effective in two ways: (1) an intervention was considered cost-effective if the ICER was < $100,000 per QALY gained, a commonly used willingness-to-pay (WTP) threshold for the United States;44 (2) interventions with a negative ICER were both less expensive and more effective as compared to brief counseling alone and thus considered the dominant strategy. Additionally, interventions were evaluated at a WTP threshold of $50,000/QALY in sensitivity analyses. We also calculated the incremental NMB for each intervention. The NMB combines QALYs and costs into one composite outcome: NMB = ([QALYs x WTP] – costs). The incremental NMB for each intervention was then calculated by subtracting the NMB of brief counseling alone from the NMB of the intervention. An intervention was considered favorable if the incremental NMB was positive.
Sensitivity Analyses
We performed probabilistic sensitivity analyses for each of the three interventions using Monte Carlo simulations to evaluate the impact of parameter uncertainty (Table 1, Table 2). For example, we widely varied the smoking cessation rate achieved by the “pharmacotherapy with intensive counseling” intervention in Monte Carlo sensitivity analyses to account for the effects of possible heterogeneity in pharmacotherapy. The Monte Carlo simulations were run 10,000 times to evaluate stability of the results.45 We evaluated the number of cost-effective Monte Carlo runs for each intervention at two WTP thresholds ($100,000/QALY, $50,000/QALY). We visualized probabilistic sensitivity analyses using scatterplots comparing brief counseling alone to each of the three interventions. Additionally, we conducted a two-way sensitivity analysis varying the payer cost and effectiveness of a smoking cessation intervention simultaneously; this analysis allows for the estimation of the maximum acceptable payer cost of an intervention given a particular effectiveness. All analyses were done by PMW using R and TreeAge Pro 2021 (TreeAge Software, Williamstown, MA).
Results
In the base case, all three smoking-cessation interventions were cost effective based on the ICER and the incremental NMB in both the payer and societal perspective (Table 3). From the payer perspective, the ICER for varenicline was -$133/QALY, -$1,496/QALY for pharmacotherapy with intensive counseling, and $168/QALY for monetary incentives (Table 3). The ICER values for varenicline and pharmacotherapy with intensive counseling were negative because though these interventions had more upfront costs, these interventions resulted in less total lifetime costs with more QALYs (dominant strategy) compared to brief counseling alone. The incremental NMB was $67,004 for varenicline, $101,874 for pharmacotherapy with intensive counseling, and $70,978 for monetary incentives (Table 3). From the societal perspective, all three interventions were the dominant strategy; the ICER for varenicline was -$20,809/QALY, -$20,615/QALY for pharmacotherapy with intensive counseling, and -$20,233/QALY for monetary incentives (Table 3). The incremental NMB was $80,840 for varenicline, $121,065 for pharmacotherapy with intensive counseling, and $85,483 for monetary incentives (Table 3).
Table 3.
Results of cost effectiveness analysis of smoking-cessation interventions in stroke secondary prevention with a lifetime horizon
| Brief counseling alone |
Varenicline | Pharmacotherapy with intensive counseling |
Monetary Incentives |
|
|---|---|---|---|---|
| Payer perspective | ||||
| Total Cost, $ | 255,674 | 255,585 | 254,173 | 255,794 |
| Total Effectiveness, QALY | 8.03 | 8.70 | 9.03 | 8.74 |
| ICER, $/QALY | - | −133* | −1,496* | 168 |
| Incremental NMB, $ | - | 67,004 | 101,874 | 70,978 |
| Cost effective Monte Carlo runs (WTP $100,000/QALY), % | - | 89.7% | 91.5% | 95.4% |
| Cost effective Monte Carlo runs (WTP $50,000/QALY), % | - | 88.7% | 90.6% | 94.6% |
| Societal perspective | ||||
| Total Cost, $ | 860,329 | 846,405 | 839,637 | 845,944 |
| Total Effectiveness, QALY | 8.03 | 8.70 | 9.03 | 8.74 |
| ICER, $/QALY | - | −20,809* | −20,615* | −20,233* |
| Incremental NMB, $ | - | 80,840 | 121,065 | 85,483 |
| Cost effective Monte Carlo runs (WTP $100,000/QALY), % | - | 98.1% | 96.4% | 99.9% |
| Cost effective Monte Carlo runs (WTP $50,000/QALY), % | - | 97.9% | 95.8% | 99.9% |
Total costs, total effectiveness, incremental cost-effective ratios, incremental net monetary benefits, and Monte Carlo results. Monte Carlo results are probabilistic sensitivity analyses.
Abbreviations: QALY, quality adjusted life year; ICER, incremental cost-effective ratio; NMB, net monetary benefit; WTP, willingness-to-pay
Negative ICER indicates intervention was the dominant strategy (less costly, more effective) compared to brief counseling alone. Interventions with a positive ICER less than the Willingness to Pay threshold are also cost-effective.
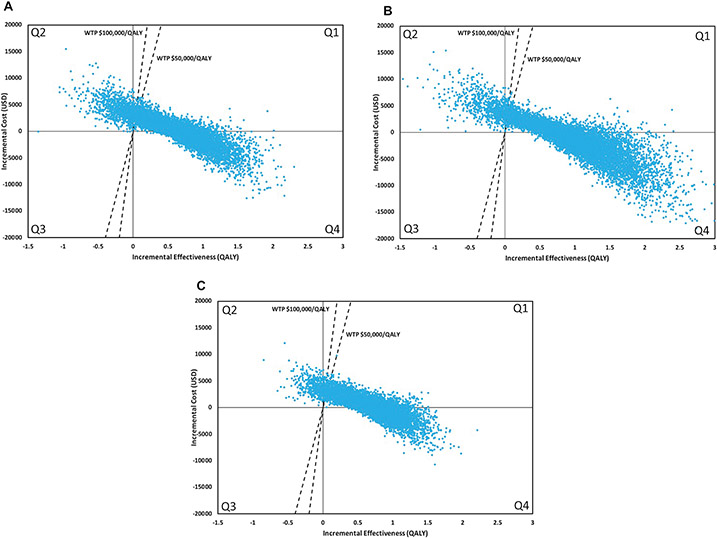
Results of the Monte Carlo probabilistic sensitivity analyses for the payer perspective are shown in Figure 2. Varenicline was cost-effective in 89.7% of runs at a WTP threshold of $100,000/QALY. Specifically, varenicline was the dominant strategy in 43.8% of runs (less total costs and more effective), and cost-effective (with an ICER below the WTP) in an additional 45.9% of runs. Pharmacotherapy with intensive counseling was cost-effective in 91.5% of runs (dominant runs, 59.3%; cost effective runs, 32.2%). Monetary incentives were cost-effective in 95.4% of runs (dominant runs, 37.8%; cost effective runs, 57.6%). Results were similar with a WTP threshold of $50,000/QALY and for the societal perspective (Table 3; Figure S2). Results were also similar using input parameters and event rates from the IRIS trial (Table S2, Figures S3-S4).
Figure 2.
Incremental cost-effectiveness scatterplot of smoking-cessation intervention with brief counseling versus brief counseling alone for the payer perspective. Each scatterplot includes a set of points representing pairs of incremental cost and effectiveness values from the simulation results (n=10,000). The comparator is brief counseling alone. The dashed line is the WTP threshold. Each scatterplot is divided into four quadrants. Points in Q1 indicate the intervention is more costly and more effective. Points in this quadrant below the WTP threshold are cost-effective and points above the willingness-to-pay threshold are not cost-effective. Points in Q2 indicate the intervention is more costly and less effective. Points in Q3 indicate the intervention is less costly and less effective. Points in Q4 indicate the intervention is less costly and more effective (dominant strategy). A. Varenicline B. Any pharmacotherapy with intensive counseling C. Monetary incentives. Abbreviations: WTP, willingness-to-pay; QALY, quality-adjusted life year.
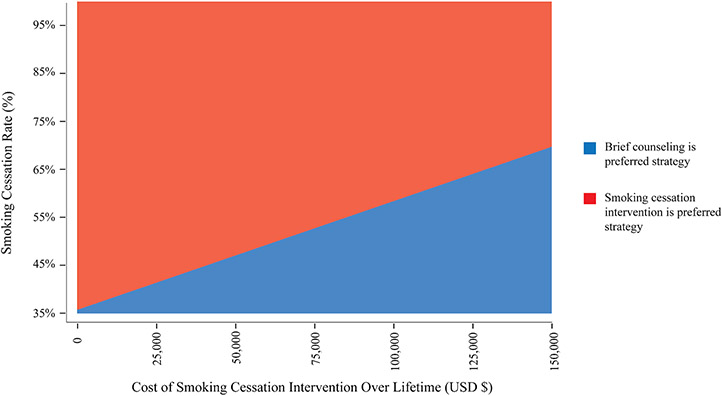
We performed a two-way sensitivity analysis across a range of different payer costs and effectiveness of smoking cessation interventions (Figure 3). This figure depicts the maximum allowable cost, from the payer perspective, for any hypothetical intervention that increases the smoking cessation rate above 35%, the base case rate. For example, an intervention that increases the smoking cessation rate to 45% can cost up to $41,001 in payer costs over a lifetime, and an intervention that increases the smoking cessation rate to 55% can cost up to $85,127 in payer costs over a lifetime, while remaining cost effective (Figure 3).
Figure 3.
Sensitivity analysis varying the payer cost and effectiveness of smoking cessation interventions. For an intervention at a certain payer cost and cessation rate, the red area represents the intervention is the preferred strategy and the blue area represents brief counseling alone is the preferred strategy. Preferred strategies are based on net monetary benefit. For example, an intervention that improves the smoking-cessation rate to 45% can cost up to $41,001 in payer costs.
Discussion
Our analysis demonstrated that varenicline, pharmacotherapy with intensive counseling, and monetary incentives were cost-effective smoking-cessation interventions for secondary prevention after stroke and TIA from both a payer and societal perspective. The robustness of these findings is supported by the results of our probabilistic sensitivity analyses, which showed that results were similar over a range of input parameters. Additionally, our model assesses the maximum acceptable payer cost of a smoking cessation intervention as a function of its effectiveness.
Our results show that smoking-cessation interventions are similar in cost effectiveness to pharmacologic secondary stroke prevention interventions, such as antiplatelet and anticoagulant therapies, for which ICERs are estimated to be $5,000-$25,000/QALY.46-48 There are less data regarding cost effectiveness of behavioral interventions for secondary stroke prevention specifically. Weight loss interventions have been shown to be cost-effective in reducing cardiovascular disease associated with obesity, including cerebrovascular disease.49,50 A multipronged text-messaging based program encouraging tobacco cessation, exercise, and dietary changes was shown to be cost-effective in patients with coronary artery disease.51 The results from our study are in line with these studies in the context of cardiovascular disease more broadly, and this study is the first we know of to investigate the cost-effectiveness of behavioral interventions, specifically smoking cessation, for stroke secondary prevention.
Our study supports the notion that it is reasonable to devote resources to intensive smoking-cessation interventions after stroke/TIA. All three of the smoking-cessation interventions were similarly cost-effective in sensitivity analyses. Therefore, no one specific smoking cessation intervention should be preferred from a cost effectiveness perspective. Interventions should be chosen to maximize effectiveness for smoking cessation in this patient population. In contrast to the secondary stroke prevention realm, considerable resources have been devoted to smoking cessation for patients with other smoking-related conditions, such as cancer.52 The National Cancer Institute’s Cancer Center Cessation Initiative’s program costs have been shown to be favorable.53 Given the results of our current study, whether a similar program targeted for stroke centers would benefit patients with cerebrovascular disease could be evaluated.
Our study has several limitations. First, our study relied on data from a population of stroke patients included in the Oxford Vascular Study. Although these patients were mostly similar to the general stroke population in the United States in terms of age and comorbidities, there were demographic differences.11,15 Future studies should investigate the cost effectiveness of these smoking cessation interventions in more diverse stroke populations. Second, we applied the relative risk reductions associated with smoking cessation reported in the IRIS trial population to our study population. This may reduce certainty around cost effectiveness estimates. However, to avoid overestimating cost effectiveness, because observations from a trial population may overestimate the expected benefits of smoking cessation in the real world, we attenuated the benefit of smoking cessation by 25% in our analyses. Additionally, results were similar in analyses that more directly modeled the IRIS trial population, supporting our overall findings. Third, we performed our cost effectiveness analysis using input parameters and assumptions typically used in high resource settings. The cost effectiveness of these interventions in low- and middle-income countries may differ.
Conclusion
Smoking cessation interventions, specifically varenicline, pharmacotherapy with intensive counseling, and monetary incentives, were cost-effective in the secondary prevention setting after stroke and TIA as compared to brief counseling alone.
Supplementary Material
Sources of Funding
Dr. Parikh is supported by the NIH/NIA (K23 AG073524), the Florence Gould Endowment for Discovery in Stroke, and was supported by the New York State Empire Clinical Research Investigator Program for this work. Dr. Liberman is supported by the NIH/NINDS (K23NS107643). This content is solely the responsibility of the authors and does not represent the official views of the National Institute of Health (NIH).
Disclosures
Drs. Wechsler, Liberman, Abramson, and Navi: none.
Dr. Parikh has received research funding from the Leon Levy Foundation unrelated to this work.
Dr. Kamel serves as a PI for the NIH-funded ARCADIA trial (NINDS U01NS095869), which receives in-kind study drug from the BMS-Pfizer Alliance for Eliquis® and ancillary study support from Roche Diagnostics; as Deputy Editor for JAMA Neurology; on clinical trial steering/executive committees for Medtronic, Janssen, and Javelin Medical; and on endpoint adjudication committees for AstraZeneca, Novo Nordisk, and Boehringer Ingelheim. He has an ownership interest in TETMedical, Inc.
Abbreviations
- TIA
transient ischemic attack
- QALY
quality adjusted life year
- ICER
incremental cost-effectiveness ratio
- NMB
net monetary benefit
- WTP
willingness-to-pay
- IRIS
Insulin Resistance Intervention After Stroke trial
- mRS
modified Rankin scale
References
- 1.Epstein KA, Viscoli CM, Spence JD, Young LH, Inzucchi SE, Gorman M, Gerstenhaber B, Guarino PD, Dixit A, Furie KL, et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology. 2017;89:1723–1729. doi: 10.1212/WNL.0000000000004524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Li S, Zheng K, Wang H, Xie Y, Xu P, Dai Z, Gu M, Xia Y, Zhao M, et al. Impact of Smoking Status on Stroke Recurrence. J Am Heart Assoc. 2019;8:e011696. doi: 10.1161/JAHA.118.011696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noubiap JJ, Fitzgerald JL, Gallagher C, Thomas G, Middeldorp ME, Sanders P. Rates, Predictors, and Impact of Smoking Cessation after Stroke or Transient Ischemic Attack: A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Dis. 2021;30:106012. doi: 10.1016/j.jstrokecerebrovasdis.2021.106012 [DOI] [PubMed] [Google Scholar]
- 4.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 5.Parikh NS, Zhang Y, Restifo D, Abramson E, Carpenter MJ, Navi BB, Kamel H. Prescription smoking-cessation medication pharmacy claims after stroke and transient ischemic attack. Prev Med Rep. 2022;25:101682. doi: 10.1016/j.pmedr.2021.101682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh NS, Parasram M, White H, Merkler AE, Navi BB, Kamel H. Smoking Cessation in Stroke Survivors in the United States: A Nationwide Analysis. Stroke. 2022; 53: 1285–1291. doi: 10.1161/STROKEAHA.121.036941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, Iskander A, Lauzon C, Srivastava N, Clarke A, et al. Varenicline for Smoking Cessation in Hospitalized Patients With Acute Coronary Syndrome. Circulation. 2016;133:21–30. doi: 10.1161/CIRCULATIONAHA.115.019634 [DOI] [PubMed] [Google Scholar]
- 8.Rigotti NA, Regan S, Levy DE, Japuntich S, Chang Y, Park ER, Viana JC, Kelley JH, Reyen M, Singer DE. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312:719–728. doi: 10.1001/jama.2014.9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, Loewenstein G, Brennan TA, Asch DA, Volpp KG. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372:2108–2117. doi: 10.1056/NEJMoa1414293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29:117–122. doi: 10.1017/S0266462313000160 [DOI] [PubMed] [Google Scholar]
- 11.Li L, Yiin GS, Geraghty OC, Schulz UG, Kuker W, Mehta Z, Rothwell PM, Oxford Vascular S. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14:903–913. doi: 10.1016/S1474-4422(15)00132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leone FT, Zhang Y, Evers-Casey S, Evins AE, Eakin MN, Fathi J, Fennig K, Folan P, Galiatsatos P, Gogineni H, et al. Initiating Pharmacologic Treatment in Tobacco-Dependent Adults. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5–e31. doi: 10.1164/rccm.202005-1982ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladapo JA, Jaffer FA, Weinstein MC, Froelicher ES. Projected cost-effectiveness of smoking cessation interventions in patients hospitalized with myocardial infarction. Arch Intern Med. 2011;171:39–45. doi: 10.1001/archinternmed.2010.479 [DOI] [PubMed] [Google Scholar]
- 14.Berlin I, Berlin N, Malecot M, Breton M, Jusot F, Goldzahl L. Financial incentives for smoking cessation in pregnancy: multicentre randomised controlled trial. BMJ. 2021;375:e065217. doi: 10.1136/bmj-2021-065217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwamm LH, Fonarow GC, Reeves MJ, Pan W, Frankel MR, Smith EE, Ellrodt G, Cannon CP, Liang L, Peterson E, et al. Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119:107–115. doi: 10.1161/CIRCULATIONAHA.108.783688 [DOI] [PubMed] [Google Scholar]
- 16.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ, Suri MF. Discharge destination as a surrogate for Modified Rankin Scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil. 2012;93:1408–1413.e1401. doi: 10.1016/j.apmr.2012.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earnshaw SR, Jackson D, Farkouh R, Schwamm L. Cost-effectiveness of patient selection using penumbral-based MRI for intravenous thrombolysis. Stroke. 2009;40:1710–1720. doi: 10.1161/STROKEAHA.108.540138 [DOI] [PubMed] [Google Scholar]
- 18.Gage BF, Cardinalli AB, Owens DK. Cost-effectiveness of preference-based antithrombotic therapy for patients with nonvalvular atrial fibrillation. Stroke. 1998;29:1083–1091. doi: 10.1161/01.str.29.6.1083 [DOI] [PubMed] [Google Scholar]
- 19.Nelson RE, Okon N, Lesko AC, Majersik JJ, Bhatt A, Baraban E. The cost-effectiveness of telestroke in the Pacific Northwest region of the USA. J Telemed Telecare. 2016;22:413–421. doi: 10.1177/1357633X15613920 [DOI] [PubMed] [Google Scholar]
- 20.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013:CD009329. doi: 10.1002/14651858.CD009329.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard P, Knight C, Boler A, Baker C. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO Simulation model: application to a population of US adult smokers. Pharmacoeconomics. 2008;26:497–511. doi: 10.2165/00019053-200826060-00004 [DOI] [PubMed] [Google Scholar]
- 22.Joo H, Wang G, George MG. Age-specific Cost Effectiveness of Using Intravenous Recombinant Tissue Plasminogen Activator for Treating Acute Ischemic Stroke. Am J Prev Med. 2017;53:S205–S212. doi: 10.1016/j.amepre.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kauf TL, Velazquez EJ, Crosslin DR, Weaver WD, Diaz R, Granger CB, McMurray JJ, Rouleau JL, Aylward PE, White HD, et al. The cost of acute myocardial infarction in the new millennium: evidence from a multinational registry. Am Heart J. 2006;151:206–212. doi: 10.1016/j.ahj.2005.02.028 [DOI] [PubMed] [Google Scholar]
- 24.Notley C, Gentry S, Livingstone-Banks J, Bauld L, Perera R, Hartmann-Boyce J. Incentives for smoking cessation. Cochrane Database Syst Rev. 2019;7:CD004307. doi: 10.1002/14651858.CD004307.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420. doi: 10.1177/0272989X06290495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Windle SB, Dehghani P, Roy N, Old W, Grondin FR, Bata I, Iskander A, Lauzon C, Srivastava N, Clarke A, et al. Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital. CMAJ. 2018;190:E347–E354. doi: 10.1503/cmaj.170377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerber Y, Rosen LJ, Goldbourt U, Benyamini Y, Drory Y, Infarction ISGoFAM. Smoking status and long-term survival after first acute myocardial infarction a population-based cohort study. J Am Coll Cardiol. 2009;54:2382–2387. doi: 10.1016/j.jacc.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 28.Li S, Peng Y, Wang X, Qian Y, Xiang P, Wade SW, Guo H, Lopez JAG, Herzog CA, Handelsman Y. Cardiovascular events and death after myocardial infarction or ischemic stroke in an older Medicare population. Clin Cardiol. 2019;42:391–399. doi: 10.1002/clc.23160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med. 2002;137:494–500. doi: 10.7326/0003-4819-137-6-200209170-00009 [DOI] [PubMed] [Google Scholar]
- 30.Shah AM, Pfeffer MA, Hartley LH, Moyé LA, Gersh BJ, Rutherford JD, Lamas GA, Rouleau JL, Braunwald E, Solomon SD. Risk of all-cause mortality, recurrent myocardial infarction, and heart failure hospitalization associated with smoking status following myocardial infarction with left ventricular dysfunction. Am J Cardiol. 2010;106:911–916. doi: 10.1016/j.amjcard.2010.05.021 [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Lichtman JH, Dharmarajan K, Masoudi FA, Ross JS, Dodson JA, Chen J, Spertus JA, Chaudhry SI, Nallamothu BK, et al. National trends in stroke after acute myocardial infarction among Medicare patients in the United States: 1999 to 2010. Am Heart J. 2015;169:78–85.e74. doi: 10.1016/j.ahj.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta-analysis of cohort studies. Arch Intern Med. 2000;160:939–944. doi: 10.1001/archinte.160.7.939 [DOI] [PubMed] [Google Scholar]
- 33.US Census Bureau (Current Population Survey). https://www.census.gov/topics/income-poverty/income/data/tables/cps.html. Accessed April 15, 2022.
- 34.US Bureau of Labor Statistics. https://www.bls.gov/cps/cpsaat03.pdf. Accessed April 15, 2022.
- 35.Bishu KG, Lekoubou A, Kirkland E, Schumann SO, Schreiner A, Heincelman M, Moran WP, Mauldin PD. Estimating the Economic Burden of Acute Myocardial Infarction in the US: 12 Year National Data. Am J Med Sci. 2020;359:257–265. doi: 10.1016/j.amjms.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 36.Cucchiara B, Elm J, Easton JD, Coutts SB, Willey JZ, Biros MH, Ross MA, Johnston SC. Disability After Minor Stroke and Transient Ischemic Attack in the POINT Trial. Stroke. 2020;51:792–799. doi: 10.1161/STROKEAHA.119.027465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunbar SB, Khavjou OA, Bakas T, Hunt G, Kirch RA, Leib AR, Morrison RS, Poehler DC, Roger VL, Whitsel LP, et al. Projected Costs of Informal Caregiving for Cardiovascular Disease: 2015 to 2035: A Policy Statement From the American Heart Association. Circulation. 2018;137:e558–e577. doi: 10.1161/CIR.0000000000000570 [DOI] [PubMed] [Google Scholar]
- 38.Hickenbottom SL, Fendrick AM, Kutcher JS, Kabeto MU, Katz SJ, Langa KM. A national study of the quantity and cost of informal caregiving for the elderly with stroke. Neurology. 2002;58:1754–1759. doi: 10.1212/wnl.58.12.1754 [DOI] [PubMed] [Google Scholar]
- 39.Tanaka H, Toyonaga T, Hashimoto H. Functional and occupational characteristics predictive of a return to work within 18 months after stroke in Japan: implications for rehabilitation. Int Arch Occup Environ Health. 2014;87:445–453. doi: 10.1007/s00420-013-0883-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vyas MV, Hackam DG, Silver FL, Laporte A, Kapral MK. Lost Productivity in Stroke Survivors: An Econometrics Analysis. Neuroepidemiology. 2016;47:164–170. doi: 10.1159/000454730 [DOI] [PubMed] [Google Scholar]
- 41.US Bureau of Labor Statics. https://www.bls.gov/oes/current/oes311120.htm. Accessed April 15, 2022.
- 42.Bureau of Labor Statistics. U.S. Department of Labor. CPI Inflation Calculator. https://www.bls.gov/data/inflation_calculator.htm. 2019.
- 43.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 44.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 45.Hatswell AJ, Bullement A, Briggs A, Paulden M, Stevenson MD. Probabilistic Sensitivity Analysis in Cost-Effectiveness Models: Determining Model Convergence in Cohort Models. Pharmacoeconomics. 2018;36:1421–1426. doi: 10.1007/s40273-018-0697-3 [DOI] [PubMed] [Google Scholar]
- 46.Kamel H, Easton JD, Johnston SC, Kim AS. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79:1428–1434. doi: 10.1212/WNL.0b013e31826d5fe8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamel H, Johnston SC, Easton JD, Kim AS. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke. 2012;43:881–883. doi: 10.1161/STROKEAHA.111.641027 [DOI] [PubMed] [Google Scholar]
- 48.Pan Y, Wang A, Liu G, Zhao X, Meng X, Zhao K, Liu L, Wang C, Johnston SC, Wang Y, et al. Cost-effectiveness of clopidogrel-aspirin versus aspirin alone for acute transient ischemic attack and minor stroke. J Am Heart Assoc. 2014;3:e000912. doi: 10.1161/JAHA.114.000912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forster M, Veerman JL, Barendregt JJ, Vos T. Cost-effectiveness of diet and exercise interventions to reduce overweight and obesity. Int J Obes (Lond). 2011;35:1071–1078. doi: 10.1038/ijo.2010.246 [DOI] [PubMed] [Google Scholar]
- 50.Harrison S, Dixon P, Jones HE, Davies AR, Howe LD, Davies NM. Long-term cost-effectiveness of interventions for obesity: A mendelian randomisation study. PLoS Med. 2021;18:e1003725. doi: 10.1371/journal.pmed.1003725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burn E, Nghiem S, Jan S, Redfern J, Rodgers A, Thiagalingam A, Graves N, Chow CK. Cost-effectiveness of a text message programme for the prevention of recurrent cardiovascular events. Heart. 2017;103:893–894. doi: 10.1136/heartjnl-2016-310195 [DOI] [PubMed] [Google Scholar]
- 52.Croyle RT, Morgan GD, Fiore MC. Addressing a Core Gap in Cancer Care - The NCI Moonshot Program to Help Oncology Patients Stop Smoking. N Engl J Med. 2019;380:512–515. doi: 10.1056/NEJMp1813913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salloum RG, D'Angelo H, Theis RP, Rolland B, Hohl S, Pauk D, LeLaurin JH, Asvat Y, Chen LS, Day AT, et al. Mixed-methods economic evaluation of the implementation of tobacco treatment programs in National Cancer Institute-designated cancer centers. Implement Sci Commun. 2021;2:41. doi: 10.1186/s43058-021-00144-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.