Abstract
Purpose
Salmonella infection is a key global public health concern and has lead to an increased economic burden on society. We investigated the epidemiological characteristics and antimicrobial resistance profiles of clinically isolated Salmonella strains in Guangzhou Women and Children's Medical Center.
Patients and methods
This was a retrospective study of 1,338 Salmonella strains collected from children in Guangzhou Women and Children's Medical Center during 2016 to 2021.
Results
The results revealed that 1,338 cases of Salmonella were mainly isolated from feces and blood samples. The age distribution was dominated by infants under 3 years old. The seasonal distribution was high in summer and autumn. 48 serotypes were detected, and S. typhimurium (78.7%) was the predominant serogroup. The results of antimicrobial susceptibility showed that the highest resistance was observed in ampicillin (84.5%), while lower resistance was observed in piperacillin/tazobactam, cefoperazone/sulbactam and ciprofloxacin. The antimicrobial resistance rate of fecal isolates was higher than that of blood isolates. The five-year average detection rate of multi-drug resistant Salmonella was 8.5% (114/1338) and the MDR rate of S. typhimurium was the lowest (6.9%; 73/1053).
Conclusion
We concluded that antibacterial treatment should be carefully selected according to serotype and antimicrobial sensitivity results in children. Antimicrobial resistance monitoring for multi-drug resistant Salmonella is still required.
Keywords: children, salmonella, bacterial resistance rate, multi-drug resistant, serotypes
Introduction
Salmonella is a rod-shaped, gram-negative bacterium with no capsule and spore and is a member of the Enterobacterales (1). It can be transmitted via contaminated food products including meat, beef, potatoes and cucumbers, which may lead to foodborne outbreaks, and results in about 23,000 deaths every year (2–4). Moreover, Salmonella is the main pathogenic cause of diarrhea in children, with the largest proportion of infections in pediatric patients < 5 years old and most commonly occurs between summer and autumn (5, 6). Currently, more than 2,500 Salmonella serotypes have been reported and among them, over 20 serotypes can cause zoonotic diseases (7). S. typhimurium and S. enteritidis are the major Salmonella serotypes responsible for gastroenteritis and diarrhea in both children and adults (8).
The pathogenicity of Salmonella is mainly related to its toxic factors, including pathogenic islands, toxic plasmids, pili and enterotoxins (9, 10). Different serotypes carrying different virulence genes can exhibit different sensitivity to antibiotics (11). In recent years, studies have shown that Salmonella antibiotic resistance has markedly increased, due to long-term antibiotic use in the poultry industry and animal laboratory products (12, 13). Furthermore, there are many contraindications to clinical medication for children, which brings great challenges to clinical diagnosis and treatment. In order to understand Salmonella serotype distribution and antimicrobial resistance in Guangzhou, Southern China and to identify potential control strategies for formulating prevention and treatment plans, a retrospective study of 1,338 Salmonella strains isolated in Guangzhou Women and Children's Medical Center (National Children's Medical Center for South Central Region) from 2016 to 2021 was conducted in this study.
Materials and methods
Ethical approval
This study has been approved by the Ethics Committee of Guangzhou Women's and Children's Medical Center (No. 176A01, 2021).
Sample, clinical data collection and isolation of Salmonella strains
Between January 2016 and December 2021, 1,388 Salmonella were isolated from 12,361 pediatric patients samples at Guangzhou Women and Children's Medical Center (National Children's Medical Center for South Central Region). Clinical information, including gender, age, main clinical manifestations, laboratory examinations, etiology, and antimicrobial sensitivity test results was collected in electronic medical review system from January 2016 to December 2021.
The diagnostic criteria for diarrhea refer to the “Standards for the Diagnosis and Treatment of Acute Infectious Diarrheal Diseases in Children” issued in 2020. Within 2 weeks of the disease course, changes in fecal characteristics and increased fecal frequency can be diagnosed for acute diarrhea. Exclusion criteria is 1) incomplete clinical medical records; 2) children with diarrhea caused by nosocomial infection during hospitalization due to other diseases; 3) if the same child has the same strains isolated from the multiple fecal culture during the same hospitalization period, only the first result was included for analysis, and duplicate strains was eliminated.
Salmonella identification and antimicrobial susceptibility testing
Salmonella culture was performed according to the methods previously described (14). Briefly, clinical samples were inoculated in culture plates. Then, suspected colonies were tested using standard biochemical methods and colonies considered to be Salmonella had the following biochemical phenotypes: growth on tripler-sugar-iron agar (acid from glucose, gas, production of H2S); negative for lactose, sucrose, urease, oxidase,salicin, -galactosidase and indole production; positive for nitrate reduction and lysine decarboxylase (14). Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF-MS) and VITEK 2-Compact automatic bacterial identification antimicrobial susceptibility system were used for identification and antimicrobial susceptibility testing(ampicillin; piperacillin/ tazobactam; aztreonam; ceftazidime; ceftriaxone; cefotaxime; cefepime; cefoperazone/sulbactam; ciprofloxacin; imipenem; chloramphenicol). The K-B antimicrobial susceptibility test method and Clinical and Laboratory Standards Institute (CLSI, 2021) on Antimicrobial Susceptibility Testing breakpoints were used to assess the results. Escherichia coli ATCC 25,922, Pseudomonas aeruginosa ATCC 27,853 and Salmonella ATCC 14,028 (all from the Clinical Laboratory Center of the Ministry of Health) were used as the quality control strains for validation of antimicrobial susceptibility testing. Multidrug-resistant (MDR) strains were defined by resistance to three or more antimicrobial classes.
Salmonella serotyping
Salmonella isolates serotyping was conducted by using a classic slide agglutination assay with anti-Salmonella (A∼F), anti-O and anti-H serum (Tianrun Bio-Pharmaceutical, Ningbo, China). We used Phosphate Buffered Saline (PBS) as negative control and Salmonella ATCC 14,028 as positive control.
Statistical analysis
WHONET 5.6 was used to analyze antimicrobial susceptibility data. SPSS 23.0 was used for statistical analysis. Briefly, we performed a normality test on the measurement data, and those that did not conform to the normal distribution were described by the median (interquartile range). The counting data was expressed as number of cases and percentages. The comparison used χ2 -value test or Fisher's exact probability method. P < 0.05 indicates statistically significant differences.
Results
Clinical information and distribution of Salmonella isolates
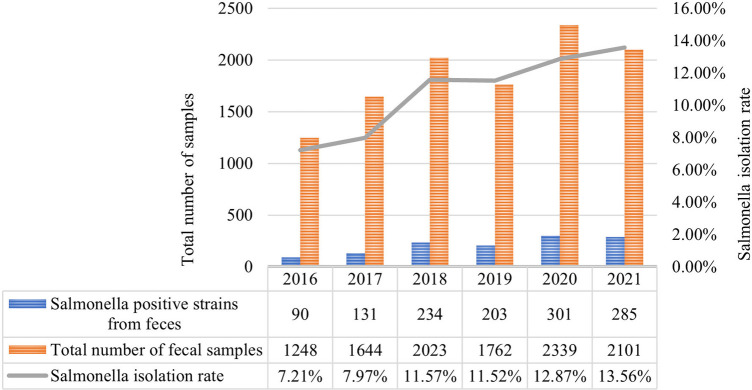
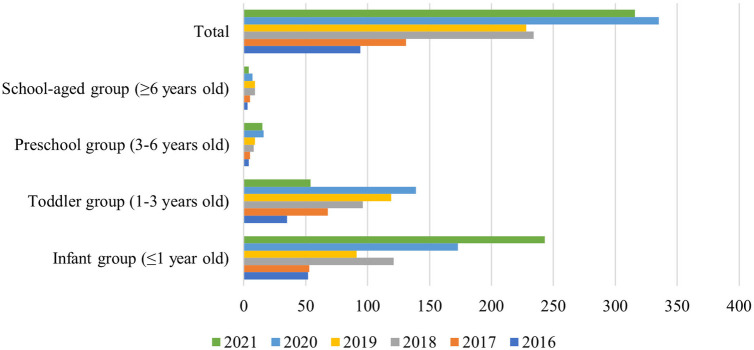
From January 2016 to December 2021, a total of 1,338 Salmonella strains were isolated from 12,361 children samples at in Guangzhou Women and Children's Medical Center (Figure 2). As shown in Figure 1, the majority of Salmonella isolates were collected from children under 3 years old [92.9% (1244/1338)], with an average age of 13 (6.3–34.7) months (Table 2). The isolate numbers of all serogroups increased dramatically over time. The isolate number in 2020 was 335 strains and 316 strains in 2021 (Figure 1). As shown in Table 1, the total isolation number was 93 in spring, 356 in summer, 608 in autumn, and 281 in winter.
Figure 2.
Annual distribution of Salmonella isolated from feces from 2016 to 2021. The bar chart represents the total number of fecal samples and Salmonella positive strains from feces. The Salmonella isolation rate of 6 years is shown in the line graph. Salmonella exhibited an increasing trend year by year.
Figure 1.
Age distribution of children with 1,338 Salmonella strains. Children are divided into four groups by age. The different bars represent different years. Age distribution of children with Salmonella infection was mostly under 3 years old.
Table 2.
The clinical information of 1,338 Salmonella isolates.
| Variable | Value |
|---|---|
| Sex | na (%) |
| Male | 826 (61.73%) |
| Female | 512 (38.27%) |
| Source | n (%) |
| Inpatient | 670 (50.07%) |
| Outpatient | 668 (49.93%) |
| Age | Median (range)b |
| 13 (6.3–34.7) | |
| Clinical symptoms | n (%) |
| Fever | 832 (62.18%) |
| Diarrhea | 805 (60.16%) |
| Fever and diarrhea | 363 (27.13%) |
| Vomiting | 598 (44.69%) |
| Respiratory symptoms | 164 (12.25%) |
| Leukemia | 72 (5.38%) |
Represents the number of strains.
Represents months.
Table 1.
Seasonal distribution of 1,338 Salmonella strains.
| Seasons | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Total |
|---|---|---|---|---|---|---|---|
| Spring (Jan. to Mar.) | 5 | 19 | 6 | 22 | 13 | 28 | 93 |
| Summer (Apr. to Jun.) | 19 | 39 | 76 | 63 | 74 | 85 | 356 |
| Autumn (Jul. to Sept.) | 55 | 43 | 105 | 99 | 171 | 135 | 608 |
| Winter (Oct. to Dec.) | 15 | 30 | 47 | 44 | 77 | 68 | 281 |
| Total | 94 | 131 | 234 | 228 | 335 | 316 | 1338 |
Among the isolated strains, 92.8% (1241/1338) of strains were collected from feces, 5.2% (71/1338) from blood, 1.0% (13/1338) from pus, and 1.0% (13/1338) from other kinds of samples (Figure 2). As shown in Table 2, 670 strains were isolated from inpatients and 668 strains from outpatients; 826 from male and 512 from female (ratio 1.6:1). Symptoms were mainly fever and diarrhea, with some patients experiencing vomiting, respiratory infection and leukemia (Table 2).
Distribution of serotypes for Salmonella isolates
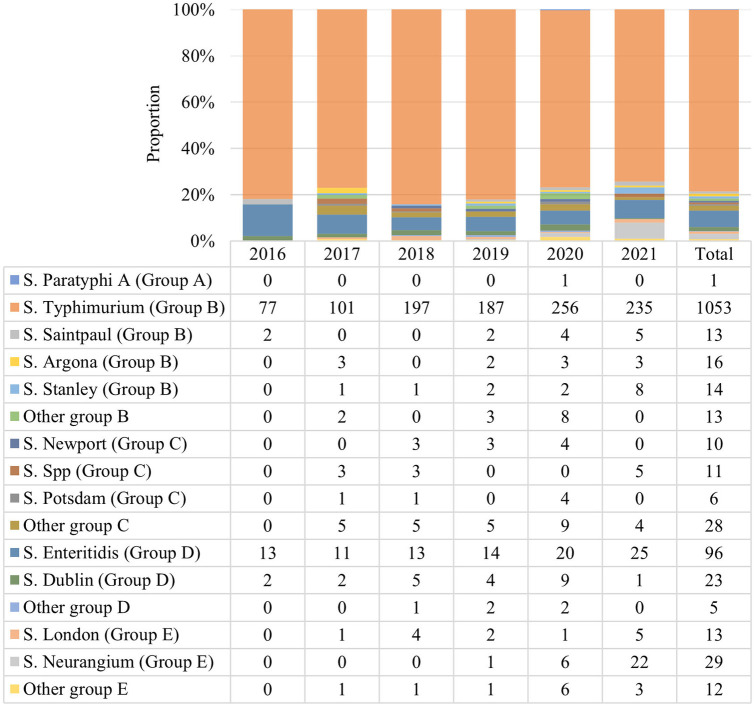
Among the identified 1,338 Salmonella isolates, 48 serotypes were detected, with group B (1110, 82.88%) and D (124, 9.27%) the top 2 predominant serogroups. The S. typhimurium (group B) serotype was detected in 78.69% (1053/1338), followed by 7.17% (96/1338) of S. enteritidis (group D) and 2.17% (29/1338) of S. neurangium (group E). Other rare serotypes were also detected, including 23 strains of S. dublin, 16 strains of S. Argona, 14 strains of S. stanley, 13 strains each of S. saintpaul and S. london, 11 strains of S. spp, 10 strains S. newport, and 6 strains S. potsdam (Figure 3).
Figure 3.
Serotype distribution of 1,338 Salmonella strains. 48 Salmonella serotypes were detected. This chart displays the major serotypes with different bar colors. Groups B and D were the top 2 predominant serogroups and S. Typhimurium was the predominant strain.
Phenotypic antimicrobial resistance
As shown in Table 3, the resistance rate of Salmonella isolated from children from 2016 to 2021 to ampicillin (AMP) was 84.5%. Resistance rates against 3rd generation cephalosporins were as follows: ceftriaxone (CRO, 40.1%), cefotaxime (CTX, 35.4%), ceftazidime (CAZ, 26.7%) and cefepime (FEP, 23.5%). Lower resistance rates were observed in piperacillin/tazobactam (PIP, 4.8%), cefoperazone/sulbactam (SCF, 4.1%) and ciprofloxacin (CIP, 6.7%). All strains were sensitive to imipenem (IPM) (Table 3). 97 strains of Salmonella isolated from blood and sterile body fluids demonstrated higher resistance to AMP (69.7%), and lower resistance to SCF (2.3%) and PIP (1.4%). 1,241 strains of Salmonella isolated from feces also exhibited the highest resistance to AMP (83.6%) and the lowest to SCF (3.8%) and PIP (3.6%). For all strains, the resistance rate of fecal isolates to the tested antibiotic agent was higher than that of blood and sterile body fluid isolates (Table 4).
Table 3.
Antimicrobial resistance rates of the 1,338 Salmonella strains.
| Antibiotic agent | 2016 (n = 94) | 2017 (n = 131) | 2018 (n = 234) | 2019 (n = 228) | 2020 (n = 335) | 2021 (n = 316) | 2016–2020 (n = 1338) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | R (%) | No. | R (%) | No. | R (%) | No. | R (%) | No. | R (%) | No. | R (%) | No. | R (%) | |
| AMP | 72 | 76.6 | 113 | 86.3 | 201 | 85.9 | 191 | 83.8 | 301 | 89.9 | 253 | 80.1 | 1131 | 84.5 |
| ATM | 0 | 0 | 47 | 35.9 | 59 | 25.2 | 73 | 32 | 69 | 20.6 | 51 | 16.1 | 299 | 22.30% |
| PIP | 24 | 25.5 | 11 | 8.4 | 16 | 6.8 | 10 | 4.4 | 0 | 0 | 3 | 0.9 | 64 | 4.80% |
| CAZ | 32 | 34 | 37 | 28.2 | 75 | 32.1 | 53 | 23.2 | 71 | 21.2 | 48 | 15.2 | 357 | 26.7 |
| CRO | 41 | 43.6 | 64 | 48.9 | 107 | 45.7 | 81 | 35.5 | 100 | 29.9 | 77 | 24.4 | 536 | 40.1 |
| CTX | 46 | 48.9 | 67 | 51.1 | 140 | 59.8 | 83 | 36.4 | 129 | 38.5 | 65 | 20.6 | 473 | 35.4 |
| FEP | 32 | 34 | 45 | 34.4 | 81 | 34.6 | 55 | 24.1 | 47 | 14 | 35 | 11.1 | 314 | 23.5 |
| SCF | 6 | 6.4 | 11 | 8.4 | 20 | 8.5 | 14 | 6.1 | 10 | 3 | 0 | 0 | 55 | 4.1 |
| CIP | 7 | 7.4 | 12 | 9.2 | 23 | 9.8 | 24 | 10.5 | 49 | 14.6 | 16 | 5.1 | 90 | 6.7 |
| IPM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CHL | 29 | 30.9 | 57 | 43.5 | 101 | 43.2 | 118 | 51.8 | 192 | 57.3 | 157 | 49.7 | 654 | 48.9 |
AMP, ampicillin; PIP, piperacillin/tazobactam; ATM, aztreonam; CAZ, ceftazidime; CRO, ceftriaxone; CTX, cefotaxime; FEP, cefepime; SCF, cefoperazone/sulbactam; CIP, ciprofloxacin; IPM, imipenem; CHL, chloramphenicol; R, resistant; No. represents the number of resistant strains.
Table 4.
Analysis of antimicrobial resistance rate of Salmonella isolates from blood and feces.
| Antibiotic agent | Feces (n = 1241) | Blood and sterile body fluids (n = 97) | χ 2 | P value | ||
|---|---|---|---|---|---|---|
| No. | R (%) | No. | R (%) | |||
| AMP | 1037 | 83.6 | 68 | 69.7 | 15.322 | <0.001 |
| ATM | 299 | 24.1 | 7 | 7.1 | 1.641 | 0.518 |
| PIP | 45 | 3.6 | 1 | 1.4 | 5.663 | 0.017 |
| CAZ | 257 | 20.7 | 15 | 15.1 | 5.778 | 0.016 |
| CRO | 397 | 32 | 20 | 20.3 | 16.188 | <0.001 |
| CTX | 417 | 33.6 | 0 | 0 | 12.856 | <0.001 |
| FEP | 249 | 20.1 | 16 | 16.7 | 6.599 | 0.01 |
| SCF | 47 | 3.8 | 2 | 2.3 | 1.717 | 0.307 |
| CIP | 103 | 8.3 | 16 | 16 | 0.955 | 0.328 |
| IPM | 0 | 0 | 0 | 0 | / | / |
| CHL | 612 | 49.3 | 16 | 16.7 | 21.232 | <0.001 |
R, resistant; No. represents the number of resistant strains.
Multidrug-resistant (MDR) Salmonella isolates
A total of 114 MDR strains were detected between 2016 and 2021, with a detection rate of 8.5% (114/1338). Among them, the MDR rate of S. typhimurium was the lowest at 6.9% (73/1053), and it was statistically significant compared to S. enteritidis (12/97, 12.4%) and other serotypes of Salmonella (χ2 = 21.855, P < 0.01) (Table 5).
Table 5.
Prevalence of multidrug-resistant (MDR) Salmonella isolates from 2016 to 2021.
| Years | All serotypes of Salmonella | S. Typhimurium | S. Enteritidis | Other serotypes of Salmonella | χ2 | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| MDR/Total | % | MDR/Total | % | MDR/Total | % | MDR/Total | % | |||
| 2016 | 1/94 | 1.1 | 1/77 | 1.3 | 0/13 | 0 | 0/4 | 0 | 21.86 | <0.01 |
| 2017 | 9/131 | 6.9 | 3/101 | 3 | 2/11 | 18.2 | 3/19 | 15.8 | ||
| 2018 | 69/234 | 29.5 | 44/197 | 22.3 | 6/13 | 46.2 | 18/27 | 66.7 | ||
| 2019 | 7/228 | 3.1 | 4/187 | 2.1 | 1/15 | 6.7 | 2/25 | 8 | ||
| 2020 | 5/335 | 1.5 | 4/256 | 1.6 | 0/20 | 0 | 1/57 | 1.8 | ||
| 2021 | 23/316 | 7.3 | 17/235 | 7.2 | 1/25 | 4 | 5/56 | 8.9 | ||
| Total | 114/1338 | 8.5 | 73/1053 | 6.9 | 12/97 | 12.4 | 29/188 | 15.4 | ||
Discussion
Salmonella is a zoonotic intestinal pathogen with many serotypes, mainly causing food-borne infections which lead to corresponding clinical symptoms (15). Salmonella infections are primarily divided into typhoidal and non-typhoidal (16). The former manifests as bloodstream infections, which are more common in adults; While the latter usually manifests as intestinal infections, which cause symptoms such as diarrhea, fever and abdominal pain and are more common in children (17, 18). This study demonstrated that the main specimen type of children with Salmonella infection was feces (92.75%), and fecal Salmonella isolation rate was increasing year by year, mainly in infants and toddlers under 3 years old (92.97%), consistent with domestic reports (19). This may be related to the fact that children are more susceptible than adults due to their lower immunity and weak gastrointestinal resistance (20). On the other hand, infant milk products have been reported as related to Salmonella outbreaks worldwide (21). This study also showed that Salmonella infections in children tend to have a high incidence in summer and autumn, which was consistent with domestic reports (22). However, the winter Salmonella infection rate in Guangzhou is still relatively high compared with that in northern China. This may be related to the geographical location of Guangzhou, which has a south subtropical maritime monsoon climate, with an average annual temperature of 21.5–22.2°C and abundant rainwater resources. Hot and humid weather promotes the growth of Salmonella and insufficient heat treatment of food can increase the risk of Salmonella infection.
In this study, we conducted a study of the clinical manifestations of Salmonella infection in children. 1,338 children with intestinal Salmonella infection had fever (62.18%) and diarrhea (60.16%) as prominent symptoms, but some children did not present with gastrointestinal symptoms. Previous reports of domestic Salmonella invasive infections mainly concentrated on typhoid or paratyphoid bloodstream infections (23, 24). In recent years, improvements in living standards and sanitation facilities have maintained Salmonella typhoid and paratyphoid infections at a low level. The increase in detection rate of invasive non-typhoid Salmonella (iNTS) has gradually attracted attention. The main diagnosis of invasive Salmonella infection is based on systemic multi-system clinical manifestations, symptoms of infection and poisoning, and Salmonella cultured in sterile body fluids (25). Extraintestinal infections caused by iNTS have also been reported, and the clinical features are similar to those of typhoid fever. It usually does not cause diarrhea, but causes bloodstream infections (such as bacteremia) or secondary systemic focal infections (such as meningitis), and failure to treat can often lead to death. The main reported serotypes are typhimurium, enteritidis, dublin and swine cholera. S. typhimurium ST313 is the main epidemic type of iNTS internationally, especially in Africa (26, 27). Specific susceptible populations of iNTS include HIV patients, immunodeficiency, hematological malignancies, young children, and malnourished or anemic adolescents, with a fatality rate as high as 20% (28). Unlike common NTS infections, patients rarely displayed gastroenteritis symptoms, but all had fever symptoms. Combined with the gradual increase in detection rate of Salmonella in recent years, the trend of increasing iNTS in Guangzhou cannot be ruled out. In addition, this study showed that there some children experienced diarrhea with clinical manifestations similar to viral diarrhea, no symptoms of high fever and diarrhea, routine stool microscopy, a significant increase in the number of stools, accompanied by obvious electrolyte metabolism disorders, and fecal culture detects Salmonella positive. Therefore, the necessity of early fecal culture detection in the diagnosis and treatment of children with diarrhea should be emphasized to avoid misdiagnosis.
The serotypes of 1,338 Salmonella strains in this study covered groups A to E, mainly group B (1110, 82.88%) and group D (124, 9.27%), of which S. typhimurium had the highest detection rate (1053, 78.69%), followed by S. enteritidis (7.17%) and S. neurangium (2.17%). The detection rate of S. typhimurium was higher than that reported by the CHINET Bacterial Resistance Surveillance Network (27.4%), and also higher than that of Tongji Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology (51.6%) and the Affiliated Children's Hospital to Zhengzhou University (36.5%) (29, 30). In addition, the serotypes detected in this study were different to serotypes collected in Wuhan, Beijing, Zhengzhou and other locations, indicative of regional differences in Salmonella serotypes. A total of 48 serotypes were detected in this study and the distribution was diversified, with some rare serotypes. Different Salmonella serotypes exhibit differences in antibiotic resistance. AMP resistance was mainly manifested in Salmonella typhimurium, enteritidis, indiana and delby as AMP was the earliest and most widely used antibiotic in livestock in China (31). The diversification of serotype distribution will lead to broadening of the MDR phenotype and antimicrobial resistance spectrum, and the trend of pan-drug resistance will become more evident. In previous studies, MDR strains mainly existed in S. typhimurium, but this study demonstrated that S. typhimurium had the lowest MDR rate among MDR Salmonella. Therefore, it is necessary to continuously and regularly monitor Salmonella serotypes in children to detect changes in the main types as early as possible.
Due to the excessive use of antibiotics in animal husbandry and clinical practice, Salmonella resistance is increasing, especially to AMP and 3rd generation cephalosporins (32). Generally, Salmonella has been resistant to previously used first-line antimicrobial drugs such as AMP, CHL, sulfamethoxazole, and amoxicillin. In this study, Salmonella presented the highest resistance rate against AMP of 84.5%, which was greater than other domestic scholars and regions to AMP. The resistance rate to 3rd generation cephalosporins (CRO, CTX, CAZ) was about 40.0%. Salmonella mainly produces ESBL, extended-spectrum β-lactamase and AmpC enzymes to generate resistance to cephalosporin antimicrobial drugs. Synthesis of Salmonella ESBL will indirectly reduce its sensitivity to quinolones, leading to the generation of multi-drug resistant strains resistant to cephalosporins and quinolones (33). Although the antimicrobial resistance rate of CIP in this study was low (6.7%), due to the side effects of central nervous and joint toxicity, it is no longer a suitable drug for children with Salmonella infection. CLSI 2020 recommends that only AMP, quinolones and trimethoprim-sulfamethoxazole are required for regular reports for fecal-isolated Salmonella. According to the antimicrobial sensitivity results of this study and the poor therapeutic effect of AMP in clinical use for S. Typhimurium, AMP is unsuitable for clinical promotion and application. At present, Salmonella still maintains high sensitivity to enzyme inhibitor compound preparations, 4th generation cephalosporins and IPM. When choosing antimicrobial treatment, drugs with antibacterial activity against Salmonella should be selected. Antimicrobial spectrum does not include gastrointestinal infections and drugs with unknown antimicrobial activity against Salmonella may lead to drug resistance. In order to decrease antibiotic consumption and AMR, the majority of gastroenteritis caused by Salmonella should not be treated with antibiotics (23, 34). In general, the current resistance rate of 3rd generation cephalosporins and quinolone antibiotics is still low, but there is an upward trend. Antimicrobial susceptibility studies to azithromycin are lacking.
There have been disagreements regarding the selection, use, and timing of antibiotics for Salmonella infection in children (35). At present, most of the 3rd generation cephalosporins are the first choice for clinical use, with azithromycin as an alternative. Quinolone antibiotics are generally not recommended for children, but can be considered for severe infections when no other antibiotics can be substituted. For MDR Salmonella, carbapenem antibiotics are suggested. The use of multiple antibiotics in combination is not recommended, and specific antibiotic selection should be based on the local epidemiology and antimicrobial susceptibility results. recommended course of treatment for intestinal infections is within 1 week, while the course of treatment for extra-intestinal infections needs to be extended. It is worth noting that there are few studies with large samples on the comparison of treatment effects between azithromycin, 3rd generation cephalosporins and quinolone antibiotics in children's NTS, and more extensive and in-depth research is required.
Conclusion
In summary, under the current pressure of antibiotic selection, the typing and continuous monitoring of antimicrobial resistance of intestinal Salmonella is important research work to provide clear epidemiological evidence for clinical diagnosis, treatment, and prevention of infection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
GF designed the study. LB, LY, and HZ: performed the PCR, MLST, and antibiotic sensitivity tests. ZS and DQ: conducted bacterial culture and isolation. ZH, LY, and WJ: prepared the samples. FG: wrote the manuscript. LB, XZ, and FG: revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Zhao X, Hu M, Zhang Q, Zhao C, Zhang Y, Li L, et al. Characterization of integrons and antimicrobial resistance in salmonella from broilers in shandong, China. Poult Sci. (2020) 99(12):7046–54. 10.1016/j.psj.2020.09.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, et al. World health organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. (2015) 12(12):e1001920. 10.1371/journal.pmed.1001920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall KEH, Tewell M, Tecle S, Leeper M, Sinatra J, Kissler B, et al. Protracted outbreak of salmonella newport infections linked to ground beef: possible role of dairy cows—21 states, 2016–2017. MMWR Morb Mortal Wkly Rep. (2018) 67(15):443–46. 10.15585/mmwr.mm6715a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughlin M, Bottichio L, Weiss J, Higa J, McDonald E, Sowadsky R, et al. Multistate outbreak of poona infections associated with imported cucumbers, 2015–2016. Epidemiol Infect. (2019) 147:e270. 10.1017/S0950268819001596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng X, Ran L, Wu S, Ke B, He D, Yang X, et al. Laboratory-based surveillance of non-typhoidal salmonella infections in guangdong province, China. Foodborne Pathog Dis. (2012) 9(4):305–12. 10.1089/fpd.2011.1008 [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Wang L-P, Yu J-X, Chen X, Wang R-N, Yang X-Z, et al. Prevalence of enteropathogens in outpatients with acute diarrhea from urban and rural areas, southeast China, 2010–2014. Am J Trop Med Hyg. (2019) 101(2):310–18. 10.4269/ajtmh.19-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achtman M, Wain J, Weill F-X, Nair S, Zhou Z, Sangal V, et al. Multilocus sequence typing as a replacement for serotyping in salmonella enterica. PLoS Pathog. (2012) 8(6):e1002776. 10.1371/journal.ppat.1002776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi X, Li P, Xu X, Yuan Y, Bu S, Lin D. Epidemiological and molecular investigations on responsible for gastrointestinal infections in the southwest of Shanghai from 1998 to 2017. Front Microbiol. (2019) 10:2025. 10.3389/fmicb.2019.02025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mebrhatu MT, Cenens W, Aertsen A. An overview of the domestication and impact of the salmonella mobilome. Crit Rev Microbiol. (2014) 40(1):63–75. 10.3109/1040841X.2012.755949 [DOI] [PubMed] [Google Scholar]
- 10.D'Aoust JY. Pathogenicity of foodborne salmonella. Int J Food Microbiol. (1991) 12(1):17–40. 10.1016/0168-1605(91)90045-Q [DOI] [PubMed] [Google Scholar]
- 11.Shen H, Chen H, Ou Y, Huang T, Chen S, Zhou L, et al. Prevalence, serotypes, and antimicrobial resistance of salmonella isolates from patients with diarrhea in Shenzhen, China. BMC Microbiol. (2020) 20(1):197. 10.1186/s12866-020-01886-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Fu Y, Xiong Z, Ma Y, Wei Y, Qu X, et al. Highly prevalent multidrug-resistant from chicken and pork meat at retail markets in guangdong, China. Front Microbiol. (2018) 9:2104. 10.3389/fmicb.2018.02104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Z, Ke B, Deng X, Liang J, Ran L, Lu L, et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal salmonella from human patients in guangdong province, China, 2009-2012. BMC Infect Dis. (2015) 15:53. 10.1186/s12879-015-0784-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang B, Xie Y, He S, Mai J, Huang Y, Yang L, et al. Prevalence, serotypes, and drug resistance of nontyphoidal salmonella among paediatric patients in a tertiary hospital in Guangzhou, China, 2014–2016. J Infect Public Health. (2019) 12(2):252–57. 10.1016/j.jiph.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 15.Cai Y, Tao J, Jiao Y, Fei X, Zhou L, Wang Y, et al. Phenotypic characteristics and genotypic correlation between salmonella isolates from a slaughterhouse and retail markets in yangzhou, China. Int J Food Microbiol. (2016) 222:56–64. 10.1016/j.ijfoodmicro.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Vargas FM, Abu-El-Haija MA, Gómez-Duarte OG. Salmonella infections: an update on epidemiology, management, and prevention. Travel Med Infect Dis. (2011) 9(6):263–77. 10.1016/j.tmaid.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 17.Lo H-Y, Lai F-P, Yang Y-J. Changes in epidemiology and antimicrobial susceptibility of nontyphoid salmonella in children in southern Taiwan, 1997–2016. J Microbiol Immunol Infect. (2020) 53(4):585–91. 10.1016/j.jmii.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 18.Mahmoudi S, Pourakbari B, Moradzadeh M, Eshaghi H, Ramezani A, Haghi Ashtiani MT, et al. Prevalence and antimicrobial susceptibility of salmonella and shigella spp. Among children with gastroenteritis in an Iranian referral hospital. Microb Pathog. (2017) 109:45–8. 10.1016/j.micpath.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 19.Wang L-P, Zhou S-X, Wang X, Lu Q-B, Shi L-S, Ren X, et al. Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat Commun. (2021) 12(1):2464. 10.1038/s41467-021-22551-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S-X, Zhou Y-M, Tian L-G, Chen J-X, Tinoco-Torres R, Serrano E, et al. Antibiotic resistance and molecular characterization of diarrheagenic escherichia coli and non-typhoidal salmonella strains isolated from infections in southwest China. Infect Dis Poverty. (2018) 7(1):53. 10.1186/s40249-018-0427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jourdan-da Silva N, Fabre L, Robinson E, Fournet N, Nisavanh A, Bruyand M, et al. Ongoing nationwide outbreak of agona associated with internationally distributed infant milk products, France, December 2017. Euro Surveill. (2018) 23(2):17-00852. 10.2807/1560-7917.ES.2018.23.2.17-00852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng C-F, Chiu N-C, Huang C-Y, Huang DT-N, Chang L, Kung Y-H, et al. The epidemiology of non-typhoidal salmonella gastroenteritis and campylobacter gastroenteritis in pediatric inpatients in northern Taiwan. J Microbiol Immunol Infect. (2019) 52(3):449–55. 10.1016/j.jmii.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 23.Qiu Y, Yang J, Chen Y, Yang J, Zhu Q, Zhu C, et al. Microbiological profiles and antimicrobial resistance patterns of pediatric bloodstream pathogens in China, 2016-2018. Eur J Clin Microbiol Infect Dis. (2021) 40(4):739–49. 10.1007/s10096-020-04069-2 [DOI] [PubMed] [Google Scholar]
- 24.Tennant SM, Toema D, Qamar F, Iqbal N, Boyd MA, Marshall JM, et al. Detection of typhoidal and paratyphoidal salmonella in blood by real-time polymerase chain reaction. Clin Infect Dis. (2015) 61(Suppl 4):S241–S50. 10.1093/cid/civ726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang M, Wang H-M, Zhou G-L, Chen Y-S, Deng J-K. Invasive salmonella infections among children in Shenzhen, China: a five-year retrospective review. Pediatr Infect Dis J. (2022) 41(9):684–9. 10.1097/INF.0000000000003588 [DOI] [PubMed] [Google Scholar]
- 26.Uche IV, MacLennan CA, Saul A. A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal salmonella (ints) disease in Africa (1966 to 2014). PLoS Negl Trop Dis. (2017) 11(1):e0005118. 10.1371/journal.pntd.0005118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balasubramanian R, Im J, Lee J-S, Jeon HJ, Mogeni OD, Kim JH, et al. The global burden and epidemiology of invasive non-typhoidal infections. Hum Vaccin Immunother. (2019) 15(6):1421–26. 10.1080/21645515.2018.1504717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert MJ, Bulach D, Alfouzan W, Izumiya H, Carter G, Alobaid K, et al. Non-typhoidal salmonella blood stream infection in Kuwait: clinical and microbiological characteristics. PLoS Negl Trop Dis. (2019) 13(4):e0007293. 10.1371/journal.pntd.0007293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L, Zhang H, Chen J, Chen L, Qi X, Zhang R. Epidemiology of foodborne disease outbreaks caused by nontyphoidal in zhejiang province, China, 2010–2019. Foodborne Pathog Dis. (2021) 18(12):880–86. 10.1089/fpd.2021.0006 [DOI] [PubMed] [Google Scholar]
- 30.Gong X-H, Wu H-Y, Li J, Xiao W-J, Zhang X, Chen M, et al. Epidemiology, aetiology and seasonality of infectious diarrhoea in adult outpatients through active surveillance in Shanghai, China, 2012–2016: a cross-sectional study. BMJ Open. (2018) 8(9):e019699. 10.1136/bmjopen-2017-019699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Xu L, Xu X, Zhu Y, Suo Y, Shi C, et al. Antimicrobial resistance and molecular characterization of salmonella enterica serovar enteritidis from retail chicken products in Shanghai, China. Foodborne Pathog Dis. (2018) 15(6):346–52. 10.1089/fpd.2017.2387 [DOI] [PubMed] [Google Scholar]
- 32.Zhan Z, Xu X, Gu Z, Meng J, Wufuer X, Wang M, et al. Molecular epidemiology and antimicrobial resistance of invasive non-typhoidal in China, 2007–2016. Infect Drug Resist. (2019) 12:2885–97. 10.2147/IDR.S210961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katiyo S, Muller-Pebody B, Minaji M, Powell D, Johnson AP, De Pinna E, et al. Epidemiology and outcomes of nontyphoidal salmonella bacteremias from England, 2004 to 2015. J Clin Microbiol. (2019) 57(1):e01189-18. 10.1128/JCM.01189-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oneko M, Kariuki S, Muturi-Kioi V, Otieno K, Otieno VO, Williamson JM, et al. Emergence of community-acquired, multidrug-resistant invasive nontyphoidal salmonella disease in rural western kenya, 2009–2013. Clin Infect Dis. (2015) 61(Suppl 4):S310–S16. 10.1093/cid/civ674 [DOI] [PubMed] [Google Scholar]
- 35.Chang Y-J, Chen Y-C, Chen N-W, Hsu Y-J, Chu H-H, Chen C-L, et al. Changing antimicrobial resistance and epidemiology of non-typhoidal infection in Taiwanese children. Front Microbiol. (2021) 12:648008. 10.3389/fmicb.2021.648008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.