Summary
Background
Lifestyle interventions for weight loss are currently not individualised to underlying pathophysiology and behavioral traits in obesity. We aim to compare the outcome of a standard lifestyle intervention (SLI) to phenotype-tailored lifestyle interventions (PLI) on weight loss, cardiometabolic risk factors and physiologic variables contributing to obesity.
Methods
This 12-week, single-centre non-randomised proof-of-concept clinical trial including men and women aged 18–65 years with a body mass index (BMI) greater than 30 without history of any bariatric procedure, and current use of any medication known to affect weight. Participants lived anywhere in the United States, and underwent in-person testing in Rochester, MN at a teaching hospital. All participants completed in-person phenotype testing at baseline and after 12 weeks. Participants were assigned to their intervention based on their period of enrollment. In the first phase, participants were assigned to SLI with a low-calorie diet (LCD), moderate physical activity, and weekly behavioral therapy sessions. In the second phase, other participants were assigned to PLI according to phenotype: abnormal satiation (time-restricted volumetric LCD); abnormal postprandial satiety (LCD with pre-meal protein supplementation); emotional eating (LCD with intensive behavioral therapy); and abnormal resting energy expenditure (LCD with post-workout protein supplementation and high-intensity interval training). The primary outcome was total body weight loss in kg at 12 weeks using multiple imputation for missing data. Linear models estimated the association of study group allocation and study endpoints adjusting for age, sex, and baseline weight. This study was registered with ClinicalTrials.gov, NCT04073394.
Findings
Between July 2020 and August 2021, 211 participants were screened, and 165 were assigned to one of the two treatments in the two phases: 81 SLI (mean [SD] age 42.9 [12] years; 79% women; BMI 38.0 [6.0]) and 84 PLI (age 44.8 [12.2] years; 83% women; BMI 38.7 [6.9]); 146 completed the 12-week programs. The weight loss was −7.4 kg (95%CI, −8.8, −6.0) with PLI vs. −4.3 kg (95%CI, −5.8, −2.7) with SLI (difference, −3.1 kg [95%CI, −5.1 to −1.1]; P = 0.004). No adverse events were reported in any group.
Interpretation
Phenotype-tailored lifestyle interventions may result in significant weight loss, but a randomised controlled trial is required to confirm causality.
Funding
Mayo Clinic; NIH (K23-DK114460).
Keywords: Obesity phenotypes, Lifestyle interventions, Weight loss diet
Research in context.
Evidence before this study
We searched PubMed, MEDLINE, Scopus, Embase and Google scholar databases from the inception of the database to September 1, 2022, for articles published in English language using the terms: “lifestyle interventions”, “diet”, “obesity”, “overweight’, “obesity phenotypes”, “satiation”, “postprandial satiety”, “emotional eating”, and “energy expenditure”. Lifestyle interventions for weight loss are currently not individualised to underlying pathophysiology and behavioral traits in obesity. There is currently no consensus on a particular diet or lifestyle intervention that has constantly showed better weight loss outcomes in patients with overweight or obesity.
Added value of this study
Here, we aimed to compare the outcome of a standard lifestyle intervention (SLI) to phenotype-tailored lifestyle interventions (PLI) on weight loss, cardiometabolic risk factors and physiologic variables contributing to obesity pathophysiology. We demonstrated that PLI contributed to better obesity treatment outcomes than a SLI with a greater weight loss and, greater reduction in waist circumference, triglycerides, daily caloric intake, and anxiety, more significant increase in lean mass percentage, and lesser decrease in resting energy expenditure compared with the SLI.
Implications of all the available evidence
In adults with obesity, the phenotype-tailored lifestyle intervention resulted in more weight loss than with the standard lifestyle intervention. The results of this non-randomised proof-of-concept study suggest that a phenotype-tailored diet and lifestyle intervention may benefit patients with obesity and highlight the relevance of characterizing the underlying pathophysiology of obesity as a complex, multifactorial disease. Confirmation of these findings in a randomised trial would lay the foundation to phenotype-tailored, precision nutrition approaches for obesity.
Introduction
Obesity is a chronic multifactorial disease associated with multiple metabolic and cardiovascular complications.1 Even moderate weight loss can lower the risk of cardiovascular disease,2 improve metabolic parameters,3 and enhance the quality of life.4 Lifestyle interventions for weight loss are the cornerstone of obesity management. However, long-term weight loss is difficult to maintain and there is high variability in weight loss response.1
Most professional societies recommend a caloric deficit coupled with moderately intense physical activity.5 However, studies comparing different diets do not document superiority of any specific diet for weight loss,6 and long-term benefit with any intervention depends on diet adherence.7 Diet interventions based on macronutrients or meal timing8 have also shown no benefit compared to standard treatment. Furthermore, when matching diet to genetic predisposition there was no effect on weight loss9; nevertheless, the POUNDS lost trial post-hoc analysis demonstrated that genetic data can influence the selection of a personalised weight loss diet and may improve the metabolic profile.10 Diet interventions have mainly focused on obesity-related complications, such as managing and preventing type 2 diabetes,11 hypertension12,13 or cardiovascular risk.14 However, none of these interventions have been tailored to the underlying pathophysiological and behavioral traits identifiable in patients with obesity.
Thus, there is a need to develop a diet and lifestyle intervention that addresses the underlying pathophysiological and behavioral features of obesity. Due to the complexity of energy balance regulation and obesity pathogenesis, such an intervention should optimally be comprehensive, yet personalised depending on the most predominant underlying pathogenic factor of the individual.15 Obesity phenotypes based on pathophysiologic and behavioral components include three main domains: homeostatic eating, hedonic eating behavior and abnormal energy expenditure.16 These domains can be categorised in four actionable phenotypes: Abnormal satiation (measured by calories ingested to experiencing postprandial fullness), abnormal postprandial satiety (duration of fullness), emotional eating behavior and abnormal resting energy expenditure. In prior studies, these phenotypes explain 85% of the variance in obesity and a phenotype-guided anti-obesity pharmacotherapy selection in a clinical setting may enhance weight loss outcomes.16 However, it is unknown whether diet and lifestyle interventions tailored to these different obesity phenotypes would be superior to a standard lifestyle intervention.
We hypothesise that an individualised intervention tailored to obesity-related phenotypes may enhance weight loss. The aim of this proof-of-concept study was to develop and evaluate the outcomes of a phenotype-tailored lifestyle intervention on weight loss, cardiometabolic risk factors, and physiologic parameters, in adults with obesity. These results were compared to those observed in a separate cohort of adults with obesity treated with a standard lifestyle intervention.
Methods
Study design
This is a single-centre, non-randomised trial with a single intervention team. To restrict the research team's impact, the design was divided into two phases. In the first, all individuals got the standard lifestyle intervention (SLI). After the study team was trained in adapting lifestyle intervention to measured phenotypes, all participants recruited in the second phase underwent phenotype-tailored lifestyle intervention (PLI). Participants were assigned to their intervention based on their period of enrollment (Appendix Fig. S1). The first phase lasted from August 2020 to February 2021, and the second from March 2021 to September 2021. The intervention team was unaware of the PLI method until February 2021, and only received training on the PLI approach in March 2021 (Appendix Fig. S1).
Each phase consisted of a screening phase, in-person physiologic testing visit, 2-day training on the weight loss program, and 12-weeks of assigned lifestyle treatment. Body weight, vital signs, waist and hip circumferences, laboratory parameters, and 24-h dietary recall questionnaire were collected at baseline, week 4, and week 12. Phenotype parameters were measured at baseline and at week 12. Manuscript reported per CONSORT 2010 guidelines for pilot or feasibility trials.
Participants
Participants were recruited by advertising the study on the Mayo Clinic's classifieds website, targeted social media ad campaigns, and the Mayo Clinic Weight Management and Nutrition Clinic between August 2020 and September 2021, and data collection was completed in March 2022. This study was conducted with approval from the institutional research review board at the Mayo Clinic, Rochester, MN. The study was registered on ClinicalTrials.gov (NCT04073394). All participants provided written informed consent for study participation. This study was conducted with approvals from the institutional review board.
Eligible participants were men and women aged 18 years or older, with a body mass index (BMI) of 30 kg/m2 or higher. Participants were excluded if they had a self-reported body weight change greater than 3% in the previous three months, history of any bariatric procedure, untreated psychiatric disorders, and current use of medications known to affect body weight.
Procedures
Phenotype assessment and identification
Participants underwent extensive in-person phenotype testing at the Clinical Research Trials Unit at the Mayo Clinic, Rochester, MN and at the Mayo Clinic Healthy Living Centre.16 Details are provided in the supplement and appendix Fig. S2. Phenotype identification was based on key energy balance variables including ad libitum meal calorie intake (CTF), gastric emptying (GE T½), anxiety measured by the anxiety sub-scale of the hospital anxiety and depression scale (HADS),17 body composition measured by dual-energy x-ray absorptiometry (DEXA), and measured resting energy expenditure (REE) divided by predicted REE based on Harris Benedict equation. The HADS comprises 14 items, seven related to anxiety symptoms and seven related to depressive symptoms. The scores for anxiety and depression can therefore vary from 0 to 21, depending on the presence and severity of the symptoms. The cutoffs of each measurement16 were as follows: abnormal satiation defined as abnormal CTF for females >894 kcal and males >1376 kcal; abnormal postprandial satiety defined as accelerated GE T½ for females <101 min and males <86 min; abnormal emotional eating defined as abnormal anxiety score ≥7 points on the HADS-A for both genders; abnormal REE defined as measured REE lower-than-expected for females <96% and males <94% of predicted REE.
Interventions
Standard lifestyle intervention
The study SLI program (control group) included recommendations for calorie and macronutrient intake, physical activity, and behavioral skills. They were delivered by a multidisciplinary team of obesity experts in a two-day program with a 12-week structured follow up.18 Diets were prescribed for each participant with a 500 kcal/day deficit calculated from measured resting energy expenditure. Diet composition was guided based on the United States Dietary Association that focused on stressing the value of whole grains, avoiding sugar, fat-free or low-fat dairy and boosting fiber without any specific macronutrient composition recommendation. Participants were prescribed 150 min of moderate physical activity per week (spread across the week) along with a recommendation to reach 10,000 steps daily. Participants received 12 individual sessions with a health and wellness coach, who worked in partnership with the participant to set weekly diet, physical activity, and behavioral goals. Self-monitoring and stimulus control were utilised as cognitive-behavioral approaches. (Further SLI details in Appendix).
Phenotype-tailored lifestyle intervention (PLI)
The concept of the phenotype-tailored diet was derived from several nutritional studies that showed targeted-specific physiological or metabolical benefits after/during a unique intervention. For example, Watson and colleagues19 showed that a protein preload delayed gastric emptying and improved glycemia. Consequently, supported by appropriate literature, our study team matched those post-intervention findings to pre-intervention “abnormalities” or phenotypes, and we conceived a priori defined diet for each phenotype.
-
•
Abnormal satiation phenotype: characterised by an abnormal fullness. The intervention aimed to keep the brain hunger centre “switched off” for longer periods of time, by reducing the allowed period of caloric intake during a day20; the intervention was also tailored to produce maximal gastric distention to induce the sensation of fullness using a volumetric diet,21 and if desired, a healthy second serving of fruits or vegetables, helping participants to reach satiation.22
-
•
Abnormal postprandial satiety phenotype: characterised by accelerated gastric emptying and increased postprandial hunger.23 The intervention's purpose was to deliver protein preloads to increase the early release of gastrointestinal hormones,24 delaying gastric emptying.19
-
•
Abnormal emotional eating phenotype: characterised by negative mood, high anxiety, and reward-seeking behaviors in relation to negative and positive emotions. Anxiety is highly correlated with negative perceptions to food and emotional eating.25 The behavioral intervention was structured to improve emotional regulation, self-efficacy, goal-setting, self-monitoring, and stimulus control through the use of a targeted mindfulness-based motivational approach.26
-
•
Abnormal resting energy expenditure phenotype: characterised by reduced REE, and muscle mass. Low REE was suggested to play a role in the development of obesity, contributing toward positive energy balance and subsequent weight gain.27, 28, 29, 30 Body composition is the most important driver of REE, particularly in metabolically active tissues such as lean mass.31 The intervention was based on a structured exercise plan to increase lean (muscle) mass, which accounts for most of the overall energy expenditure ratio32; implement a high-intensity resistance training to enhance muscle strength and size to boost the total energy expenditure,33 and with a protein supplement post-exercise for muscle mass.34
Further details are described in the supplemental material.
Intervention
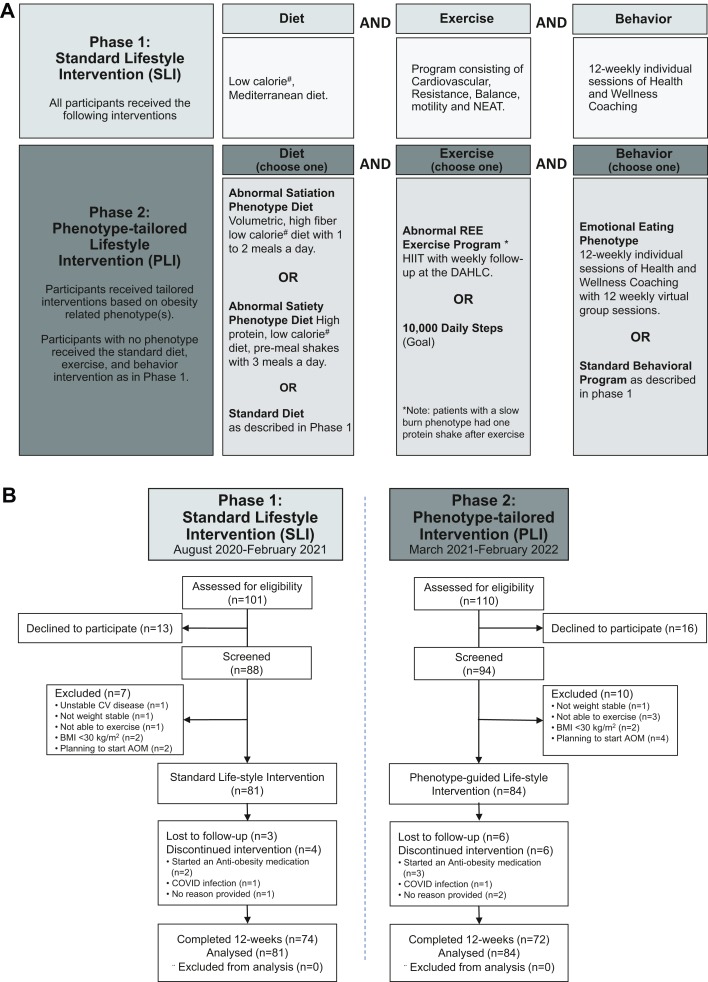
The PLI program included recommendations for calorie and macronutrient intake, physical activity level, and behavioral skills to be followed for the 12-week intervention period. The program was delivered by a multidisciplinary team of obesity experts as part of the Mayo Clinic HLP in a two-day program. All participants received a low-calorie diet with a 500 kcal/day deficit calculated from their measured REE. Meal timing, macronutrient intake, physical activity, and behavioral therapy were a priori determined for each obesity phenotype (Fig. 1A). Participants were provided a tailored approach for diet, physical activity, and behavioral intervention.
-
a.
Abnormal Satiation phenotype: participants were instructed to restrict their meals to one or two per day with a volumetric, high fiber diet.
-
b.
Abnormal Satiety phenotype: participants were instructed to have three meals per day with a pre-meal protein supplement.
-
c.
Emotional eating: participants were instructed to follow a standard diet (the same as that used in the standard life intervention), avoid snacks, and participate in a 12-session intensive behavioral group therapy co-led by two clinical health psychologists with expertise in weight management along with weekly check-in with their wellness coach.
-
d.
Abnormal REE: participants were instructed to follow a standard diet (same as controls), plus a post-work-out protein supplementation and to engage in at least 30 min of high-intensity interval training four to five times per week, with a weekly check-in with their physical therapist.
Fig. 1.
Phenotype-tailored Lifestyle Intervention (A) and CONSORT Flow Diagram (B). Phase 1 = Standard of Care Intervention (SLI) and Phase 2 = Phenotype-tailored Lifestyle Intervention (PLI). A)Interventions: Phase 1 – Standard Diet consisted of a diet, exercise (physical activity) and behavior program. Phase 2 – Phenotype-tailored Lifestyle intervention consisted in diet, exercise and behavior programs tailored to the obesity-related phenotypes. B) CONSORT Flow Diagram for Phase 1 and Phase 2 Interventions.
Other participants in the PLI group were advised to reach a goal of 10,000 steps daily (as controls) without a standard behavioral program.
Participants without an identified phenotype were instructed to follow the standard lifestyle intervention for diet, physical activity, and behavior change (as per the cohort of patients assigned to SLI).
Outcomes
The primary endpoint was total body weight loss in kg, defined as the change in body weight from baseline to the 12-week time point in kg. Secondary endpoints included weight loss in percentage, the proportions of participants achieving weight reductions of at least 5% or 10%, and the change from baseline to week 12 of the following variables: waist circumference, lean mass percentage measured using DEXA, changes in the phenotype-defining variables (GE T½ and percentage emptied at 120 min, CTF, HADS-A score, REE % of predicted), daily caloric intake from a 24-h dietary recall, plasma low-density lipoprotein (LDL) and high-density lipoprotein (LDL) cholesterol, triglycerides, C-reactive protein, glucoregulatory factors including fasting glucose, HbA1C, fasting insulin, blood pressure (systolic blood pressure [SBP] and diastolic blood pressure [DBP]), and heart rate.
Statistical analysis
The statistical analysis plan is available in the Supplement. Data are presented as mean (SD) or mean change from baseline (95% confidence interval). The primary approach for missing weight measurements due to drop-out (n = 19/165) or withdrawal in the primary analysis cohort was multiple imputation.35 Ten imputation data sets were created and analysed using the mice package in R. A sensitivity analysis of the 146 participants who completed the assigned 12-week program was also conducted. Analyses of secondary endpoints were conducted on those who had a 12-week measurement. All statistical tests were 2-sided with a 0.05 significance level. All effect estimates were reported with 95% confidence intervals. Linear and logistic models were used to estimate the association of PLI on study endpoints adjusting for age, sex, weight at baseline, and the baseline value. Similar models were used to assess the PLI outcomes on endpoints within phenotype subgroups. Multiple imputation was used in a parallel analysis for the secondary endpoints, available in the Supplement. All statistical analyses were performed in R version 4.1.2.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Results
Study participants
Between July 2020 and August 2021, 211 participants were screened, and 165 were assigned to one of the two treatments in the two phases: 81 received standard lifestyle intervention (SLI) and 84 phenotype-tailored lifestyle intervention (PLI). The first phase lasted from August 2020 to February 2021, and the second from March 2021 to September 2021. Overall, 146 participants (88.5%) had a 12-week weight assessment (study flow chart in Fig. 1B). The proportion of participants not completing the trial in the 2 groups was not significantly different (PLI, 14.3%; SLI, 8.6%; P = 0.26).
Demographic and baseline clinical characteristics were similar for the 2 groups participating in the two treatments (Table 1). Most participants were women (81.2%) and white (92%), with a mean (SD) age of 43.9 (11.9) years. Mean body weight was 110.6 (21.9) kg, mean BMI was 38.3 (6.5) kg/m2, and mean waist circumference was 116.2 (13.6) cm. At baseline, 76.8% of participants had 1 or more comorbidities, with depression being the most frequently reported among participants (32%). Baseline characteristics of participants with the specific phenotype were similar for the 2 groups (Appendix Table S1).
Table 1.
Baseline demographics and clinical characteristics by treatment group.
| Standard Lifestyle Intervention |
Phenotype-tailored Lifestyle Intervention |
p-value | |
|---|---|---|---|
| N = 81 | N = 84 | ||
| Demographics | |||
| Age, years | 42.9 (11.6) | 44.8 (12.2) | 0.32 |
| Sex, females | 64 (79%) | 70 (83.3%) | 0.48 |
| Race, white | 75 (92.6%) | 76 (90.5%) | 0.63 |
| Body composition | |||
| Weight, kg | 109 (19.9) | 112 (23.7) | 0.49 |
| Body mass index, kg/m2 | 38.0 (6.0) | 38.7 (6.9) | 0.49 |
| Waist circumference, cm | 116 (12.7) | 117 (14.5) | 0.72 |
| Hip circumference, cm | 126 (13.5) | 128 (14.7) | 0.36 |
| Fat mass, kg | 51.2 (13.6) | 53.5 (16.2) | 0.34 |
| Lean mass, kg | 54.2 (9.3) | 54.4 (9.7) | 0.89 |
| Glycemic factors | |||
| Fasting glucose, mg/dl | 104 (20.8) | 101 (11.9) | 0.31 |
| HbA1c (%) | 5.6 (0.7) | 5.5 (0.5) | 0.55 |
| Fasting insulin, mg/dl | 12.0 (8.6) | 9.8 (4.5) | 0.11 |
| Blood pressure and heart rate | |||
| Heart rate, bpm | 78.3 (11.1) | 78.9 (10.5) | 0.71 |
| Systolic blood pressure, mmHg | 121 (12.6) | 121 (11.8) | 0.95 |
| Diastolic blood pressure, mmHg | 80.2 (9.6) | 79.6 (7.6) | 0.62 |
| Plasma lipids and inflammation | |||
| LDL cholesterol, mg/dl | 113 (29.5) | 114 (31.7) | 0.88 |
| HDL cholesterol, mg/dl | 51.6 (15.4) | 50.2 (13.3) | 0.62 |
| Triglycerides, mg/dl | 112 (60.5) | 126 (52.3) | 0.18 |
| C-reactive protein HS, mg/dl | 4.1 (3.3) | 5.3 (4.7) | 0.14 |
| Comorbidities | |||
| Participants with ≥1 comorbidities, % | 64 (80) | 62 (74) | 0.35 |
| Phenotype characteristics | |||
| Calories to fullness, kcal | 924 (371) | 860 (330) | 0.24 |
| Gastric emptying T ½, minutes | 133 (33) | 128 (34) | 0.44 |
| Harris-Benedict, kcal/day | 1896 (317) | 1899 (343) | 0.95 |
| REE, kcal/day | 1890 (316) | 1858 (346) | 0.54 |
| REE predicted/measured, % | 101 (14) | 99 (14) | 0.39 |
| HADS anxiety, score | 4.2 (3.2) | 4.6 (3.4) | 0.42 |
| Phenotype distribution | |||
| Abnormal satiation, n | 30 (37.5%) | 28 (33.3%) | 0.58 |
| Abnormal postprandial satiety, n | 7 (8.6%) | 13 (15.5%) | 0.18 |
| Emotional eating, n | 18 (22.5%) | 26 (31.0%) | 0.22 |
| Abnormal REE, n | 31 (38.3%) | 38 (45.2%) | 0.36 |
| Completers, n | 74 (91.4%) | 71 (85.7%) | 0.23 |
Continuous data are summarized as mean and standard deviations. Categorical data are presented as frequencies and percentages. P-values were calculated with t-test or chi-square test as appropriate. All P-values <0.05 were considered significant.
HADS, hospital anxiety and depression scale; HDL, high density lipoprotein; HS, high sensitivity; LDL, low density lipoprotein; REE, resting energy expenditure.
For all participants, mean GE T ½ was 129.7 (33.8) min, mean CTF was 891.2 (351.1) kcal, and mean REE was 1874 (331) kcal/day. There were no significant differences in clinical characteristics between the 2 groups (Table 1). At baseline, 58 (35.4%) of participants had abnormal satiation; 20 (12.1%) participants had abnormal satiety; 45 (27.3%) participants had emotional hunger; 68 (41.4%) participants had reduced REE, and 49 (29.6%) participants did not have one of these specific phenotypes. There were no significant differences in the proportion of phenotypes in the 2 treatment phases (Table 1), and there were no significant differences in baseline characteristics within each phenotype between treatment groups (Appendix Table S1).
Phenotype-tailored lifestyle on body composition
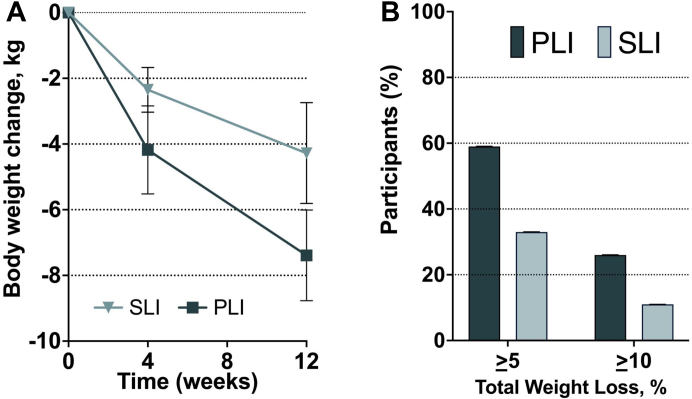
The mean total body weight loss from baseline was −7.4 kg (95%CI −8.8, −6.0) with PLI vs. −4.3 (95%CI −5.8, −2.7) with SLI (difference, −3.1 kg [95%CI −5.1, −1.1]; P = 0.004) (Fig. 2A and Table 2). After adjusting for age, sex and baseline weight, the mean difference was −3.0 kg (95%CI −5.0 to −1.1) (P = 0.003). In those who completed the 12-week visit, the adjusted weight loss was −8.0 kg (95%CI −9.2, −6.8) with PLI vs −3.7 kg [95%CI −4.8, −2.7] with SLI (mean difference, −4.3 kg, [95%CI −5.8 to −2.7]; P < 0.001). Changes in body composition between baseline and week 12 using multiple imputation analysis are reported in supplementary Table S2.
Fig. 2.
A) Total Body Weight loss (kg) in standard lifestyle intervention (SLI) and the phenotype-tailored lifestyle intervention (PLI). Error bars represent 95% confidence interval of the mean. B) Percentage of participants per treatment group with total body weight greater than 5 and 10% at 12 weeks.
Table 2.
Changes in body composition and cardiometabolic risk factors between baseline and week 12.
| Mean difference (95% confidence interval) |
p-valuea | |||
|---|---|---|---|---|
| Standard Lifestyle Intervention | Phenotype-tailored Lifestyle Intervention | Adjusteda group differences | ||
| Body composition | ||||
| Weight loss at 4 weeks, kgb | −2.35 (−3.03, −1.67) | −4.18 (−5.52, −2.84) | −1.64 (−2.96, −0.31) | 0.018 |
| Weight loss at 12 weeks, kgb | −4.28 (−5.81, −2.74) | −7.39 (−8.77, −6.01) | −3.04 (−4.99, −1.10) | 0.003 |
| Weight loss at 12 weeks, kg (completers only) | −3.72 (−4.77, −2.66) | −7.99 (−9.16, −6.81) | −4.06 (−5.61, −2.51) | <0.001 |
| Body mass index, kg/m2 | −1.45 (−1.99, −0.91) | −2.61 (−3.17, −2.04) | −1.14 (−1.83, −0.46) | 0.002 |
| Waist circumference, cm | −4.05 (−6.10, −2.00) | −7.90 (−9.80, −5.99) | −4.53 (−6.92, −2.15) | <0.001 |
| Hip circumference, cm | −3.31 (−5.17, −1.46) | −4.85 (−6.25, −3.45) | −1.50 (−3.43, 0.43) | 0.13 |
| Fat mass, kg | −3.49 (−4.53, −2.46) | −6.94 (−8.04, −5.84) | −3.29 (−4.81, −1.77) | <0.001 |
| Lean mass, kg | −0.27 (−0.64, 0.10) | −1.39 (−2.18, −0.61) | −1.05 (−1.89, −0.22) | 0.015 |
| Lean mass, % | 1.68 (1.15, 2.20) | 2.92 (2.11, 3.72) | 1.35 (0.41, 2.28) | 0.006 |
| Glucoregulatory factors | ||||
| Fasting glucose, mg/dl | −2.45 (−5.55, 0.65) | −1.59 (−3.69, 0.51) | 0.48 (−2.86, 3.83) | 0.78 |
| HbA1c (%) | −0.12 (−0.31, 0.06) | −0.14 (−0.21, −0.07) | −0.01 (−0.18, 0.15) | 0.87 |
| Fasting insulin, mg/dl | −3.02 (−5.52, −0.53) | −2.72 (−4.07, −1.37) | −1.46 (−3.30, 0.38) | 0.12 |
| Blood pressure and heart rate | ||||
| Heart rate, bpm | −4.03 (−6.70, −1.37) | −6.95 (−10.17, −3.72) | −2.75 (−6.76, 1.26) | 0.18 |
| Systolic blood pressure, mmHg | 0.36 (−2.12, 2.83) | 0.19 (−2.38, 2.76) | −0.06 (−3.28, 3.15) | 0.97 |
| Diastolic blood pressure, mmHg | −2.10 (−3.97, −0.23) | −3.33 (−5.70, −0.97) | −1.27 (−4.08, 1.54) | 0.38 |
| Plasma lipids and inflammation | ||||
| LDL cholesterol, mg/dl | −9.89 (−16.16, −3.63) | −9.56 (−17.86, −1.25) | −0.57 (−12.56, 11.42) | 0.93 |
| HDL cholesterol, mg/dl | 1.42 (−3.08, 5.93) | 1.43 (−2.98, 5.84) | −1.60 (−8.12, 4.92) | 0.63 |
| Triglycerides, mg/dl | 8.09 (−5.96, 22.14) | −18.91 (−30.64, −7.18) | −26.24 (−42.71, −9.77) | 0.002 |
| C-reactive protein HS, mg/dl | −0.58 (−1.46, 0.29) | 0.04 (−1.17, 1.25) | 0.57 (−1.12, 2.27) | 0.51 |
Data are summarized as mean change from baseline and 95% confidence interval (CI). All P-values <0.05 were considered significant.
bpm, beats per minute; HbA1c, hemoglobin A1c; HDL, high density lipoprotein; HS, high sensitivity; LDL, low density lipoprotein.
Mean group differences and p-values are adjusted for age, sex, baseline weight, and baseline measure of the response variable.
Multiple imputation was used to handle missing data.
Participants in the PLI group had significantly higher odds to have lost at least 5% and 10% of baseline body weight at week 12 vs the SLI group (P = 0.004 and P = 0.049 respectively). These thresholds were achieved by 59% vs. 33% and by 26% vs. 11% of participants in the PLI and the SLI groups, respectively (Fig. 2B). Participants in the PLI group had significantly higher odds to lose at least 10% of their body weight compared to the SLI group, after adjusting for age, sex, and baseline weight (odds ratio 3.2, [95%CI 1.1 to 9.4]; p = 0.035).
The PLI group had a significant reduction in waist circumference at week 12 compared SLI group (−7.9 cm [95%CI −9.8, −6.0] vs. −4.1 cm [95%CI −6.1, −2.0]; P < 0.001) (Table 2). Fat mass was significantly reduced in the PLI group (−6.9 kg [95%CI −8.0, −5.8]) compared to the SLI group (−3.5 kg [95%CI −4.5, −2.5]; P < 0.001). Lean mass percentage was significantly increased (p = 0.006) in the PLI group (2.9% [95%CI 2.1, 3.7) compared to the SLI group (1.7% [95%CI 1.2, 2.2]).
Phenotype-tailored lifestyle on cardiometabolic risk factors
At week 12, fasting triglycerides were significantly lower in the PLI group (−19 mg/dl [95%CI −31, −7]) compared to the SLI group (8 mg/dl [95%CI −6, 22], p = 0.002). Both groups had a significant reduction in LDL cholesterol from baseline (PLI group −10 mg/dl [95%CI −18, −1] and SLI group −10 mg/dl [95%CI −16, −4]), without any difference between the groups. There were no significant changes in glycemic factors, blood pressure, heart rate or inflammatory markers (Table 2). Changes in cardiometabolic risk factors between baseline and week 12 using multiple imputations are reported in supplementary Table S2.
Phenotype-tailored lifestyle on physiological, metabolic and behavioral variables
Total daily caloric intake was significantly reduced (p = 0.002) in the PLI group (−752 kcal [95%CI −1051, −453]) compared to the SLI group (−148 kcal [95%CI −331, 35]). There was no significant difference in calories consumed to reach fullness at the ad libitum meal 12-week test among the groups. Both groups consumed less calories at 12 weeks compared to the baseline test (PLI group: −127 kcal [95%CI −179, −76], SLI group: −95 kcal [95%CI −157, −33]). Gastric content emptied at 120 min was significantly reduced (p = 0.014) in the PLI group (−4.9% min [95%CI −8.0, −1.8]) compared to the SLI group (2.8% [95%CI −1.2, 6.9]), while there was no significant difference in mean improvement in T1/2 time (8.2 vs. −0.7 min, p = 0.11). There was no significant difference in pre-meal hunger from baseline among the groups (Table 3).
Table 3.
Changes in physiological, metabolic and behavioral variables between baseline and week 12.
| Mean difference (95% confidence interval) |
p-valuea | |||
|---|---|---|---|---|
| Standard lifestyle intervention | Phenotype-tailored lifestyle intervention | Adjusteda group differences (95% CI) | ||
| Food intake | ||||
| Daily caloric intake, kcal | −148 (−331, 35) | −752 (−1051, −453) | −397 (−644, −150) | 0.002 |
| Ad libitum meal, kcal | −95 (−157, −33) | −127 (−179, −76) | −21 (−95, 53) | 0.57 |
| Gastric function | ||||
| Gastric Emptied 120 min, % | 2.8 (−1.2, 6.9) | −4.9 (−8.0, −1.8) | −6.0 (−10.7, −1.3) | 0.014 |
| Gastric Emptying T1/2, min | −0.7 (−8.6, 7.3) | 8.2 (1.1, 15.3) | 8.1 (−1.9, 18.0) | 0.11 |
| Appetite sensations (VAS) | ||||
| Hunger pre-meal, mm | −0.4 (−6.0, 5.3) | 5.9 (−1.7, 13.5) | 2.5 (−5.5, 10.4) | 0.54 |
| Resting energy expenditure | ||||
| REE, kcal/day | −208 (−270, −146) | −116 (−182, −49) | 48 (−30, 125) | 0.23 |
| REE predicted/measured, % | −9.2 (−12.6, −5.7) | −2.0 (−5.6, 1.5) | 4.4 (0.4, 8.5) | 0.034 |
| Behavioral questionnaires | ||||
| HADS Anxiety Score | 0.65 (0.11, 1.18) | −0.73 (−1.58, 0.13) | −1.15 (−2.06, −0.25) | 0.014 |
| TFEQ Emotional Score | −2.33 (−3.37, −1.28) | −3.15 (−4.60, −1.70) | −0.54 (−2.12, 1.04) | 0.50 |
Data are summarized as mean change from baseline and 95% confidence interval (CI). All P-values <0.05 were considered significant.
CI, confidence interval; HADS, hospital anxiety and depression scale; REE, resting energy expenditure; VAS, visual analogue scale; TFEQ, three factor eating questionnaire.
Mean group differences and p-values are adjusted for age, sex, baseline weight, and baseline measure of the response variable.
REE was significantly reduced from baseline in both groups (PLI, −116 kcal/day [95%CI −182, −49], and SLI, −208 kcal/day [95%CI −270, −146]). There was no difference in the absolute value of REE in the PLI group compared to the SLI group (adjusted difference 48 kcal/day [95%CI −30, 125]; P = 0.23). But, when compared with predicted energy expenditure values, measured REE at 12 weeks showed a significantly smaller reduction from baseline in the PLI group compared to the SLI group (adjusted difference 4.4% [95%CI 0.5%, 8.5%], p = 0.034) (Table 3).
Anxiety levels (measured by the anxiety component of the HADS) improved in the PLI group compared to the SLI group (adjusted difference, −1.2 points [95%CI −2.1, −0.3]; P = 0.014). Both groups had an improvement in the TFEQ emotional domain score with no significant difference in improvement between the groups (Table 3). Changes in physiological, metabolic and behavioral variables between baseline and week 12 using multiple imputation analysis are reported in supplementary Table S2.
Discussion
Based on our data in adults with obesity, phenotype-tailored lifestyle interventions may result in more weight loss than standard lifestyle intervention. Participants in the phenotype-tailored lifestyle intervention group not only lost more weight but also had greater decrease in waist circumference, fat mass, gastric emptying, anxiety score, and triglyceride levels compared to the standard lifestyle intervention group. They also had a greater increase in lean mass percentage and a lower reduction in REE. The results of this non-randomised proof-of-concept study suggest that a phenotype-tailored diet and lifestyle intervention may benefit patients with obesity and highlight the relevance of characterizing the underlying pathophysiology of obesity as a complex, multifactorial disease. Confirmation of these findings in a randomised trial would lay the foundation for phenotype-tailored, precision nutrition approaches for obesity.
The results of this proof-of-concept study further support the need for an actionable phenotype-based classification, rather than reliance only on anthropometric parameters or obesity-related comorbidities. As evident in this study, participants assigned to the phenotype-tailored lifestyle intervention had a significant improvement in some of these targeted traits (specifically abnormal satiation and emotional eating), in contrast to the physiological and metabolic adaptations that confound the results during a calorie-restricted intervention.36 Long-term, confirmatory randomised studies are needed to answer this important question and substantiate whether the positive metabolic results seen here can persist during weight maintenance.37 Additionally, further studies may need to incorporate other physiological and metabolic variables to understand the participants with no identified phenotype in our current study. Such advances may enhance the tailored approach proposed from our data.
Participants in the PLI group showed some improvement in cardiometabolic risk factors including waist circumference, fat mass, LDL cholesterol and fasting triglycerides; with no improvement in blood pressure, glycemia, HDL or inflammatory markers. However, the latter markers were normal at baseline in our population. Except for triglycerides, there were no changes in improvement in lipid profile when compared to SLI. It is important to note that this may be explained by a change in diet composition, despite no explicit recommendations, but by emphasizing the need to avoid sweets, increase fiber, and increase protein recommendations, which may result in reduced consumption of carbohydrates overall. Future studies of a phenotype-tailored lifestyle intervention should also focus on patients with obesity and other cardiometabolic diseases such as type 2 diabetes and cardiovascular disease.
This study has several limitations. First, this is a proof-of-concept, short-term study that was designed as a sequential two-phase trial, with non-randomised allocation based on enrollment time. However, while randomization minimises bias, there were no significant baseline characteristic differences between groups. Furthermore, this design permitted participants in both groups to be treated by the same team and deliver the interventions during the COVID-19 pandemic. However, there were several circumstances related to the pandemic that we were unable to control for that may have influenced the results. Second, given the non-randomised, non-blinded nature of this study, there is an inherent treatment bias. To minimise this, we trained the study team delivering the interventions on the PLI interventions after all participants for the SLI completed the intervention. We recognise that a randomised clinical trial would be the most suitable method to address this limitation. Third, most of the participants were white women and therefore, it is unknown how these findings would apply to a more diverse population. Fourth, as a proof-of-concept study, this study was conducted over only a 12-week period of time. However, most studies suggest that after 6 months of therapy, weight loss slows considerably, with more than half of the weight loss occurring in the first 12 weeks.38 Furthermore, it is now recognised that weight loss and weight maintenance are distinct behavioral and physiological processes, since additional metabolic, physiological, and behavioral adaptations may be seen beyond this timeframe. Thus, the effects of therapy on these two processes must be examined independently. Finally, participants with an emotional eating component received a more intense intervention, as they had a total of 24 behavior modification sessions provided to them (12 group session and 12 individual sessions). However, this intervention is necessary to address the underlying phenotypic trait that may have a central role in obesity development. Otherwise, the two interventions were very similar in terms of intensity for other phenotypes. As per the methods section, in addition to the 2-day instruction session on diet and exercise, the standard program included a 12-session behavior modification program. Patients in the phenotype-tailored intervention also had a 2-day instruction session, but these patients had more specific advice on dietary recommendations and exercise interventions based on the presence or absence of the phenotypic trait as per described in methods.
In conclusion, among adults with obesity, a phenotype-tailored lifestyle intervention compared with a standard weight loss intervention may result in significantly greater weight loss after a 12-week intervention. Further research is needed to assess the long-term effect of this innovative approach in a larger and randomised cohort.
Contributors
LC: Collected the data, conceived, and designed the analysis, contributed to the analysis, and wrote the paper. WG, FF, AC, DS, and ADLR: Collected the data, contributed to the analysis, contributed to writing the paper. MDH, MMC, MC, AM, TR, SM, JE, JF, and KG: contributed to writing the paper. RJL: performed the analysis. DHD and AA obtained founding, contributed to writing the paper and provided supervision. LC, AA, and RL have accessed and verified the underlying data. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Data sharing statement
Data from this study can be requested from AA (Acosta.andres@mayo.edu) after publication of this study. Deidentified participant data, data dictionary and other specified data sets can be requested. The study protocol, statistical analysis plan, and informed consent form will also be made available upon request. Specific requests for data will require approval of a proposal and signed data access agreement.
Declaration of interests
AA is a stockholder in Gila Therapeutics and Phenomix Sciences; he served as a consultant for Rhythm Pharmaceuticals, General Mills, Amgen, Bausch Health, RareStone; has contracts with Vivus Inc, Satiogen Pharmaceutical, and Rhythm pharmaceutical; and has a patent submitted for biomarkers of phenotype tailored diet. MC is a stockholder in Phenomix Sciences. No other disclosure to be reported. DHD receives royalties from the book, The Mayo Clinic Diet and consulting fees from the app that includes the Mayo Clinic Diet. All other authors declare no competing interests.
Acknowledgments
This study was funded by Mayo Clinic. Dr. Acosta is supported by NIH (NIH K23-DK114460).
We thank participants in the study, the nurses, and the Mayo Clinic Clinical Research Trials Unit (supported by Mayo Clinic Centre for Clinical and Translational Science [CCaTS] grant UL1-TR000135). This research used REDCap for data collection and management, supported by NCRR grants 5M01-RR00095, G12RR03051, 5M01RR000058-45, and 1 UL1 RR024975 from NCRR/NIH.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101923.
Contributor Information
Donald D. Hensrud, Email: Hensrud.donald@mayo.edu.
Andres Acosta, Email: acosta.andres@mayo.edu.
Appendix A. Supplementary data
References
- 1.Heymsfield S.B., Wadden T.A. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 2.Powell-Wiley T.M., Poirier P., Burke L.E., et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeBlanc E.L., Patnode C.D., Webber E.M., Redmond N., Rushkin M., O'Connor E.A. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: an updated systematic review for the US preventive services task force. Agency for Healthcare Research and Quality (US); Rockville, MD: 2018. U.S. Preventive services task force evidence syntheses, formerly systematic evidence reviews. [PubMed] [Google Scholar]
- 4.Payne M., Porter Starr K., Orenduff M., et al. Quality of life and mental health in older adults with obesity and frailty: associations with a weight loss intervention. J Nutr Health Aging. 2018;22(10):1259–1265. doi: 10.1007/s12603-018-1127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acosta A., Streett S., Kroh M.D., et al. White paper AGA: POWER - practice guide on obesity and weight management, education, and resources. Clin Gastroenterol Hepatol. 2017;15(5):631–649.e10. doi: 10.1016/j.cgh.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Tobias D.K., Chen M., Manson J.E., Ludwig D.S., Willett W., Hu F.B. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(12):968–979. doi: 10.1016/S2213-8587(15)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Corral P., Bryan D.R., Garvey W.T., Gower B.A., Hunter G.R. Dietary adherence during weight loss predicts weight regain. Obesity. 2011;19(6):1177–1181. doi: 10.1038/oby.2010.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu D., Huang Y., Huang C., et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386(16):1495–1504. doi: 10.1056/NEJMoa2114833. [DOI] [PubMed] [Google Scholar]
- 9.Gardner C.D., Trepanowski J.F., Del Gobbo L.C., et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667–679. doi: 10.1001/jama.2018.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bray G.A., Krauss R.M., Sacks F.M., Qi L. Lessons learned from the POUNDS Lost study: genetic, metabolic, and behavioral factors affecting changes in body weight, body composition, and cardiometabolic risk. Curr Obes Rep. 2019;8(3):262–283. doi: 10.1007/s13679-019-00353-1. [DOI] [PubMed] [Google Scholar]
- 11.Knowler W.C., Barrett-Connor E., Fowler S.E., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appel L.J., Moore T.J., Obarzanek E., et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 13.Sacks F.M., Svetkey L.P., Vollmer W.M., et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 14.Rosato V., Temple N.J., La Vecchia C., Castellan G., Tavani A., Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2019;58(1):173–191. doi: 10.1007/s00394-017-1582-0. [DOI] [PubMed] [Google Scholar]
- 15.Gordon-Larsen P., French J.E., Moustaid-Moussa N., et al. Synergizing mouse and human studies to understand the heterogeneity of obesity. Adv Nutr. 2021;12(5):2023–2034. doi: 10.1093/advances/nmab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acosta A., Camilleri M., Abu Dayyeh B., et al. Selection of antiobesity medications based on phenotypes enhances weight loss: a pragmatic trial in an obesity clinic. Obesity. 2021;29(4):662–671. doi: 10.1002/oby.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Hensrud D.D., Clinic Mayo. 2nd ed. Mayo Clinic; Rochester, MN: 2017. The Mayo Clinic diet; p. 334. [Google Scholar]
- 19.Watson L.E., Phillips L.K., Wu T., et al. A whey/guar “preload” improves postprandial glycaemia and glycated haemoglobin levels in type 2 diabetes: a 12-week, single-blind, randomized, placebo-controlled trial. Diabetes Obes Metabol. 2019;21(4):930–938. doi: 10.1111/dom.13604. [DOI] [PubMed] [Google Scholar]
- 20.Ravussin E., Beyl R.A., Poggiogalle E., Hsia D.S., Peterson C.M. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity. 2019;27(8):1244–1254. doi: 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G.-J., Tomasi D., Backus W., et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39(4):1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Warrilow A., Mellor D., McKune A., Pumpa K. Dietary fat, fibre, satiation, and satiety—a systematic review of acute studies. Eur J Clin Nutr. 2019;73(3):333–344. doi: 10.1038/s41430-018-0295-7. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Izundegui D., Campos A., Calderon G., et al. Association of gastric emptying with postprandial appetite and satiety sensations in obesity. Obesity. 2021;29(9):1497–1507. doi: 10.1002/oby.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchison A.T., Piscitelli D., Horowitz M., et al. Acute load-dependent effects of oral whey protein on gastric emptying, gut hormone release, glycemia, appetite, and energy intake in healthy men. Am J Clin Nutr. 2015;102(6):1574–1584. doi: 10.3945/ajcn.115.117556. [DOI] [PubMed] [Google Scholar]
- 25.Cifuentes L., Campos A., Silgado M.L.R., et al. Association between anxiety and eating behaviors in patients with obesity. Obes Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katterman S.N., Kleinman B.M., Hood M.M., Nackers L.M., Corsica J.A. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: a systematic review. Eat Behav. 2014;15(2):197–204. doi: 10.1016/j.eatbeh.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Roberts S.B. Abnormalities of energy expenditure and the development of obesity. Obes Res. 1995;3(Suppl 2):155s–163s. doi: 10.1002/j.1550-8528.1995.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 28.Hume D.J., Yokum S., Stice E. Low energy intake plus low energy expenditure (low energy flux), not energy surfeit, predicts future body fat gain. Am J Clin Nutr. 2016;103(6):1389–1396. doi: 10.3945/ajcn.115.127753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faria S.L., Faria O.P., Menezes C.S., de Gouvêa H.R., de Almeida Cardeal M. Metabolic profile of clinically severe obese patients. Obes Surg. 2012;22(8):1257–1262. doi: 10.1007/s11695-012-0651-y. [DOI] [PubMed] [Google Scholar]
- 30.Ravussin E., Lillioja S., Knowler W.C., et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318(8):467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 31.Nelson K.M., Weinsier R.L., Long C.L., Schutz Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr. 1992;56(5):848–856. doi: 10.1093/ajcn/56.5.848. [DOI] [PubMed] [Google Scholar]
- 32.Hall K.D., Sacks G., Chandramohan D., et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seynnes O.R., de Boer M., Narici M.V. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol. 2007;102(1):368–373. doi: 10.1152/japplphysiol.00789.2006. [DOI] [PubMed] [Google Scholar]
- 34.Morton R.W., Murphy K.T., McKellar S.R., et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Buuren S., Oudshoorn C.G. TNO; Leiden: 2000. Multivariate imputation by chained equations. [Google Scholar]
- 36.Hall K.D. Metabolic adaptations to weight loss. Obesity. 2018;26(5):790–791. doi: 10.1002/oby.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall K.D., Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102(1):183–197. doi: 10.1016/j.mcna.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster G.D., Wyatt H.R., Hill J.O., et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.