Abstract
This Scientific Opinion addresses a European Commission request on the welfare of calves as part of the Farm to Fork strategy. EFSA was asked to provide a description of common husbandry systems and related welfare consequences, as well as measures to prevent or mitigate the hazards leading to them. In addition, recommendations on three specific issues were requested: welfare of calves reared for white veal (space, group housing, requirements of iron and fibre); risk of limited cow–calf contact; and animal‐based measures (ABMs) to monitor on‐farm welfare in slaughterhouses. The methodology developed by EFSA to address similar requests was followed. Fifteen highly relevant welfare consequences were identified, with respiratory disorders, inability to perform exploratory or foraging behaviour, gastroenteric disorders and group stress being the most frequent across husbandry systems. Recommendations to improve the welfare of calves include increasing space allowance, keeping calves in stable groups from an early age, ensuring good colostrum management and increasing the amounts of milk fed to dairy calves. In addition, calves should be provided with deformable lying surfaces, water via an open surface and long‐cut roughage in racks. Regarding specific recommendations for veal systems, calves should be kept in small groups (2–7 animals) within the first week of life, provided with ~ 20 m2/calf and fed on average 1 kg neutral detergent fibre (NDF) per day, preferably using long‐cut hay. Recommendations on cow–calf contact include keeping the calf with the dam for a minimum of 1 day post‐partum. Longer contact should progressively be implemented, but research is needed to guide this implementation in practice. The ABMs body condition, carcass condemnations, abomasal lesions, lung lesions, carcass colour and bursa swelling may be collected in slaughterhouses to monitor on‐farm welfare but should be complemented with behavioural ABMs collected on farm.
Keywords: calf welfare, veal, cow–calf contact, individual housing, husbandry systems, iron
Summary
Background and European Commission's request
The European Commission requested the European Food Safety Authority (EFSA) to provide an independent view on the welfare of calves that reflected the most recent scientific knowledge on the topic. This mandate was received in the context of the comprehensive evaluation of the animal welfare legislation undertaken by the European Commission in the framework of its Farm to Fork strategy (including the Council Directive 2008/119/EC of 18 December 2008 laying down minimum standards for the protection of calves), and of the European Citizen Initiative ‘End the Cage Age’ registered by the European Commission in 2018 calling for a ban on the use of cages or individual stalls in several farmed species.
The mandate requested a description of the husbandry systems currently used to keep calves, and the identification of relevant welfare consequences and of animal‐based measures (ABMs) as indicators of the welfare consequences. EFSA was also requested to identify hazards leading to the welfare consequences and to provide recommendations to prevent, mitigate or correct them. A scientific assessment concerning this part of the request is presented as ‘general Terms of Reference (TORs)’; a similar request was received by EFSA on other farmed species (e.g. pigs, broilers chickens and laying hens). In addition to the general ToRs, the mandate included three requests specific to this mandate: (1) the assessment of the welfare of male dairy calves raised for producing ‘white’ veal and the risks associated with individual housing, insufficient space and feed restriction (iron and fibre); (2) the assessment of ABMs collected in the slaughterhouse to monitor the level of on‐farm welfare, and (3) the welfare of dairy calves and the risks associated with limited cow–calf contact. These scenarios are referred to as ‘Specific Scenarios’. The mandate specified that the animal category of interest was calves up to 6 months of age, but for the purposes of Scenario 1 the upper age limit was extended to 8 months to include animals slaughtered at an older age and marketed as veal. Further details on the background and the request received by EFSA are presented in the main body of this document.
Assessment ‐ Husbandry systems
The sources of data used for the identification of husbandry systems were expert knowledge and grey literature. Eleven husbandry systems to rear calves were identified: individual housing in dairy farms; individual housing in veal farms; group housing of veal calves in small groups with milk feeding by bucket/trough; group housing of veal calves in large groups with automatic milk feeding; group housing in small groups with milk feeding by bucket/trough in dairy farms; group housing in large groups and automatic milk feeding; and systems with cow–calf contact. Systems to rear calves after weaning were group housing in pens with fully or partially slatted floor without bedding; in fully or partly littered pens; in pens with cubicles, and in outdoor feedlots. The main features and common husbandry practices in each system were described and are presented in the main body of the scientific opinion.
Assessment ‐ Welfare consequences
The method used to identify the highly relevant welfare consequences in each system was a classification procedure based on expert opinion: a list of 25 welfare consequences potentially affecting calves was used as a starting point, and each welfare consequence was classified into one of three classes (high, medium or low relevance) taking into consideration the prevalence, severity and duration of the welfare consequence in each system. There was no maximum number of welfare consequences that could be assigned to each category.
The highly relevant welfare consequences of individual housing of calves (in dairy and veal farms) were inability to perform exploratory or foraging behaviour, inability to perform sucking behaviour, gastroenteric disorders, respiratory disorders, restriction of movement, isolation stress and inability to perform play behaviour. Calves in individual pens in dairy farms may also experience prolonged hunger.
The highly relevant welfare consequences of the two systems used to rear calves in dairy farms in groups before weaning were: inability to perform exploratory or foraging behaviour, inability to perform sucking behaviour, gastroenteric disorders, respiratory disorders, prolonged hunger and inability to perform play behaviour. Group stress was observed in calves kept in large groups with automatic milk feeding.
The highly relevant welfare consequences of housing veal calves in group pens (in small and large groups) were inability to perform exploratory or foraging behaviour, inability to perform sucking behaviour (especially in small groups), gastroenteric disorders, respiratory disorders, inability to chew and ruminate, resting problems, group stress and metabolic disorders (anaemia).
The highly relevant welfare consequences of all group pen systems used after weaning (in dairy farms) were respiratory disorders, inability to perform exploratory and foraging behaviour, group stress (especially in large groups), restriction of movement (when no bedding is provided or when animals are kept in cubicle pens), resting problems and, when animals are kept on slatted floors, inability to perform play behaviour.
Highly relevant welfare consequences of cow–calf contact systems were respiratory disorders, gastroenteric disorders, group stress, handling stress and separation stress.
Following the selection of the highly relevant welfare consequences, ABMs relevant to each were identified based on the sensitivity and specificity of the ABMs with reference to the welfare consequence concerned. Relevant hazards and corresponding preventive measures were also identified, based on peer‐reviewed literature and expert knowledge.
The main hazards observed in individual housing were restricted space allowance, limited contact with peers/dam, a barren environment (mostly in veal farms), a low number of milk meals and, in calves kept in dairy farms, the provision of restricted amounts of milk. Low space allowance, lack of bedding and slatted floors were recurrent hazards in group housing. Recommendations to improve current husbandry practices include keeping calves in stable groups with other calves and/or their dams from an early age onwards, increasing the space allowance per animal, allowing dedicated lying areas with deformable lying surfaces (preferably bedding) and keeping calves in buildings with good ventilation. If kept outdoors, calves should be protected from heat and cold by having access to shade or insulated shelter and with the provision of dry, deformable, insulating bedding if in cold regions.
Feeding recommendations include provision of large amounts of milk (~ 20% body weight per day until at least 4 weeks of life), long roughage in racks and permanent access to drinking water. Abrupt weaning should be avoided by gradually decreasing milk amounts; and weaning should be carried out preferably on an individual basis (e.g. depending on solid feed intake). In addition, transport events, commingling and regrouping should be avoided as much as possible by fattening calves in the farm of origin or in units close by. If calves are still to be transported, long journeys (i.e. longer than 8 h) should be avoided, and animals should not go through auction markets.
Assessment ‐ Specific Scenario 1 ‐ Welfare of calves raised for producing white veal meat
Specific Scenario 1 referred to the welfare of male dairy calves raised for producing white veal and the risks associated with individual housing, insufficient space and feed (iron and fibre) restriction. The mandate requested quantitative recommendations where possible; for this reason, each aspect named in the mandate (e.g. individual housing) was translated into quantifiable questions of interest. The data sources used for this part of the assessment were data published in peer‐reviewed studies and expert knowledge.
Individual housing – Group size and age at grouping
The objective of the assessment was to assess how calf's welfare is affected by keeping them in groups compared with individual housing.
A literature review was carried out on welfare consequences of individual and group housing and on how welfare is affected by age at grouping and group size. Natural behaviour and immunity development of young calves were reviewed as a starting point to provide an understanding of the positive effects of social housing on young calves' social competences, learning ability, feeding behaviour and affective states, as well as the potential negative effects of early group housing on health. Conclusions and recommendations for age at grouping were based on literature review and consensus among the group, while for the group size, an adapted Expert Knowledge Elicitation exercise was carried out to estimate the relationship between group size and prevalence of respiratory disorders.
The outcomes of the assessment indicated that positive effects of early group housing (e.g. from day 3) compared with grouping at a later age included more developed social behaviour, higher learning ability, social buffering (less reaction to stressful events), more positive affective states, and a greater solid feed intake. Negative effects of housing calves in groups during the second week of life compared with the third week were higher prevalence of respiratory disorders.
Regarding the negative effect of group size on calf welfare, evidence from literature showed that calves kept in large groups had a higher risk of exposure to infectious disease agents (respiratory and gastroenteric disorders) and of being exposed to group stress and cross‐sucking. In view of this, an adapted expert elicitation exercise was carried to estimate the relationship between group size and respiratory disorders. The elicited median prevalence of respiratory disorders in veal calves housed in groups of 2–3 animals was similar to that of individually housed calves and to that of group pens of 4–7 calves, and considerably higher in groups of 12–18 calves and in groups of 30–40 calves. This suggested that keeping young calves in small groups would not substantially increase the risk of disease exposure compared with keeping them individually.
Calf's immune status may have to be considered regarding the timing of introduction to social housing. If colostrum management is adequate, the level of passive immunity is highest in the first week of life, and because the calf's own active immunity builds up slowly, calf immunity is at its lowest at 2–3 weeks of age. In addition to age at grouping and immune status, husbandry and management of group‐housed calves also play an important role. Rearing calves in stable groups results in higher daily gain and a lower incidence of disease than dynamic group management.
Recommendations are to keep calves in pairs or small groups (2–7 animals) within the first week of life, and to keep them in stable groups from that point onwards. This allows calves to be exposed to benefits of social housing (more developed social behaviour, higher learning ability, social buffering and more positive affective states) without substantially increasing the likelihood of health disorders such as respiratory disease.
Space allowance
The objective of the assessment was to understand how calf welfare is affected by restricted space allowances. Welfare consequences experienced by calves kept in pens with limited space include restriction of movement, resting problems and inability to perform play behaviour. Calves are intrinsically motivated to carry our locomotor play behaviour. This type of behaviour is associated with positive affective states. For this reason, this welfare consequence was taken as a reference to estimate space allowance needs through an estimation of the play behaviour expressed by calves under no space restrictions. The relationship between space allowance and play behaviour was estimated via an adapted expert elicitation procedure. It was concluded that an individually housed calf needs ~ 30 m2 of space allowance to show the full extent of locomotor play behaviour, and 20 m2 per animal when in group pens (the difference is due to the shared space effects in group pens). From the literature, other behaviours (such as resting behaviour) that can be expressed at different space allowances were also considered. It was concluded that a calf housed in a group pen shows increased lying in a relaxed posture (stretched legs) and increased synchronous resting when given a lying area of 1.5 or 2 m2 compared with a lying area of 1 m2 per animal (at a total space allowance of 3 m2 per animal). It was also concluded that a calf housed in a group pen at or slightly below the current minimum legislated space allowance (i.e. ~ 1.8 m2 per animal) is expected to have higher risk of respiratory diseases, compared with a space allowance higher than 1.8 m2 per animal. There were no data in the literature on welfare effects of space allowances between 4 and 20 m2.
To allow the full extent of locomotor play behaviour, group housed calves should be provided with at least 20 m2/calf. Less preferable from an animal welfare perspective, but still allowing lying relaxed and increased activity and a degree of locomotor play behaviour, 3 m2 could be suggested as a minimum requirement.
Iron
In white veal farming, the iron content of diets administered to calves is purposefully kept low to achieve a pale meat colour and therefore an increased price per kilogram of meat. The risks associated with the deprivation of iron include anaemia. Considering that the haemoglobin (Hb) concentrations resulting from iron provision would more closely relate to the welfare state, the objective of the assessment was to evaluate the effects of different Hb concentrations on the welfare of calves. ABMs associated with lower levels of Hb include impaired immunity, higher prevalence of diarrhoea and respiratory diseases, low weight gains, increased cardiovascular and respiratory responses to physical effort (measured by oxygen consumption, lactate production, heart rate and respiratory frequency).
While Hb levels below 4.5 mmol/L (minimum value as stated in the legislation currently in place) are associated with impaired immunity, higher prevalence of diarrhoea, respiratory diseases and low weight gains, there are fewer data on the range between 4.5 and 5.3 mmol/L, and welfare effects are not as obvious. However, studies reported increased cardiovascular and respiratory responses to physical effort (measured by oxygen consumption, lactate production, heart rate and respiratory frequency) in calves with 5.3 compared with 7.76 mmol/L, and lower mean weight in calves with 4.6 compared with 6 mmol/L. Although there are limited data, the AHAW panel recommends that measures should be implemented to avoid Hb levels under 5.3 mmol/L in veal calves. Mechanisms for collection, record keeping and accessibility of Hb data on white veal production systems at farm and abattoir levels should be implemented for a better understanding of welfare effects of Hb values between 4.5 and 5.3 mmol/L.
Anaemia should be prevented through the provision of highly bioavailable iron through diet rather than corrected with iron injections. The provision of roughage with highly available iron content such as hay should be preferred to ensure a high iron intake rather than the provision of a solid feed composed of straw, cereals and grains or iron‐fortified milk replacer. It is also recommended to put research efforts into the validity of non‐invasive methods for assessing anaemia prevalence on the farm (e.g. mucosa colour) and at the abattoir (e.g. carcass colour assessment) for future monitoring purposes.
Fibre – Amounts of NDF
Fibrous feedstuff often relates to feed materials with a high amount of cellulose, hemicellulose or lignin, which are commonly denominated as the non‐detergent component of fibre (NDF). In this assessment ‘fibre’ was characterised in terms of NDF composition. The inability to chew and ruminate was identified as the most important welfare consequence experienced by calves provided with a limited amount of fibre in their diets. This is demonstrated by the work they are willing to do to perform these activities and by the occurrence of abnormal oral behaviours (such as tongue rolling) when the opportunity to chew and ruminate is limited. The fibre content of the feed influences the time a calf spends ruminating. Other identified gastroenteric disorders relevant for veal calves are poor rumen development and rumen hyperkeratinisation but due to lack of data on the quantity and type of fibre associated with these welfare consequences, these were not further considered.
The relationship between NDF amount and rumination behaviour was estimated via an adapted expert elicitation procedure. It was estimated that a mean daily intake of 1 kg of NDF is needed for calves aged 2 weeks to 6 months, to show the full extent of rumination behaviour that would be observed in a calf with no restriction of fibre. It was estimated that, when provided a restricted amount of fibre (assumed as on average 0.19 kg NDF/day), calves would spend on average 5.5 less hours ruminating than if provided fibre ad libitum.
It is recommended that from 2 to 8 weeks of age, calves are provided with a total of 11 kg of NDF, between weeks 9 and 18 a total of 65 kg of NDF, and between weeks 18 and 25 a total of 90 kg of NDF, reaching a total of 166 kg per rearing cycle.
Fibre with a minimum of 40–50% NDF and in long‐cut form (minimum 4–5 cm long) should be provided to allow for chewing and manipulation behaviours. Straw should not be provided as the only ad libitum roughage due to its coarseness and potential detrimental effects on the abomasum. Importantly, additional factors, other than fibre, can influence levels of rumination, such as type of feed, calf breed or time of the day.
Specific Scenario 2 ‐ Assessment of ABM in slaughterhouses
The objective of the assessment was to assess ABMs collected at slaughterhouses to monitor the level of on‐farm welfare of male dairy calves raised for producing white veal. To select the relevant ABMs, a semi‐quantitative consensus exercise was developed. The selected ABMs were body condition score (assessed ante‐mortem), carcass condemnations, carcass colour, lung lesions, abomasal lesions and bursa swelling (assessed post‐mortem). Carcass condemnations, lung lesions and abomasal lesions are useful to detect the most prevalent health‐related welfare consequences experienced by veal calves, i.e. respiratory and gastroenteric disorders. Carcass colour, body condition score and bursa swelling reflect issues related with anaemia, general health disorders/inability to cope with rearing conditions, and resting problems, respectively. There are no ABMs to be collected at slaughter to detect problems on farm related to the inability to perform exploratory and foraging behaviour, or restriction of movement. It was noted that the estimation of prevalence of these health‐related welfare consequences at the abattoir will be an underestimation of the prevalence on farm because calves that get sick and recover or die on farm are not detected at abattoir level.
The use of the selected ABMs of calf welfare is not routinely implemented in EU slaughterhouses, but some ABMs are already collected for food safety (such as carcass condemnation rate and presence of lung lesions) or commercial purposes (carcass colour). Automated systems for easy and standardised collection of data are unavailable for most ABMs, because the technology readiness index of veal ABMs at slaughterhouses is currently very low. Carcass colour assessment is the only routinely implemented ABM that is used by abattoir operators; however, these data are not publicly accessible.
Automated systems for easy and standardised collection and recording of data need to be implemented, including reliability testing, for a system to monitor welfare of calves based on the identified ABMs. The AHAW panel also recommends that for a comprehensive welfare assessment, ABMs collected at slaughter should be complemented with data on behavioural ABMs collected on farm and information of on‐farm mortality. In addition, data already collected for commercial purposes, such as carcass colour, should be made available to allow incorporation of these indicators in welfare monitoring systems.
Specific Scenario 3 ‐ Welfare of calves kept in systems with cow‐calf contact
The objective of the assessment was to understand how calf's welfare is affected by limited cow–calf bond. The methods used for this assessment were literature review and an adapted Expert Knowledge Elicitation exercise.
The great majority of dairy farms separate dam and calf immediately after birth. Evidence suggests that the calf experiences little or no separation stress if separated shortly after birth from the dam, but calves will not be able to experience the positive benefits that the contact to the dam bring. Data from experimental studies indicate that the benefits of cow–calf contact for the calf increase with the duration of contact: a positive impact on calf vitality can already be observed after some hours of contact with the dam; a positive effect on weight gain is observed after 4 days of contact; a reduced prevalence of diarrhoea is observed after 2 weeks of contact; and development of social competence improves after 12 weeks (there is, however, a lack of evidence on welfare effects potentially observed during 3–8 weeks of age). The negative welfare consequences from the absence of contact with the dam will also depend on the rearing method after separation: calves kept in groups experience inability to perform natural sucking behaviour (from an udder) and inability to perform play behaviour with the dam; if calves are individually housed, they will also experience isolation stress and inability to perform play behaviour with other calves.
However, if calves and cows have contact for a prolonged period, separation stress can occur when calves and cow are eventually separated. Separation stress is most severe after the cow–calf bond is formed (at 4 days post‐partum) and until 6–10 weeks of age. Hereafter, separation results in fewer reactions the older the calves are, but there is a lack of scientific evidence on the age at which separation responses start to decline.
For the calf to benefit from the positive effects of contact with the dam but without experiencing severe separation stress, the AHAW panel recommends that the calf should be kept with the dam for a minimum of ~ 24 h (before the bond is formed) and be housed with another calf after that. This will improve the current situation in which calves are mostly separated from the cow shortly after birth and housed individually afterwards. From a welfare point of view, prolonged cow–calf contact should increasingly be implemented due to the benefits for calf and cow, so that in the future, calves should have contact with the dam during the whole pre‐weaning period. Prolonged contact to a foster cow can be an alternative, but will not be as beneficial as dam‐rearing because of risks such as failed adoption, aggression or limited milk intake. However, further research is still needed to better understand how to implement such contact in a larger scale and to identify the best options in practice.
Public consultation
The results, conclusions and recommendations of Specific Scenarios 1 and 3 were made available for consultation and commenting by the public and EFSA stakeholders between September and November 2021. These were the sections considered to be most relevant to gather feedback on because they included specific and quantitative welfare recommendations on white veal farming and cow–calf contact. The remaining draft text was not included for public consultation due to time constraints. In total, 177 comments were received, with most comments containing several points, from the following affiliations: Non‐governmental organisations, Industry (small or medium‐sized enterprise), Industry (multinational), Academia/research institute, Public authority in EU Member state, EFSA registered stakeholder and Other. EFSA carefully reviewed the comments and answered each point raised; scientific publications mentioned in the comments were considered in the assessment when relevant, and changes carried out in the text for consistency and completeness when needed. The full list of answers to the comments is provided in Annex A.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Under its Farm to Fork strategy, the Commission will start a comprehensive evaluation of the animal welfare legislation. This will include the following acts:
Council Directive 98/58/EC of 20 July 1998 on the protection of animals kept for farming purposes 1 ;
Council Directive 1999/74/EC of 19 July 1999 laying down minimum standards for the protection of laying hens 2 ;
Council Directive 2008/119/EC of 18 December 2008 laying down minimum standards for the protection of calves 3 (Codified version);
Council Directive 2008/120/EC of 18 December 2008 laying down minimum standards for the protection of pigs 4 (Codified version);
Council Directive 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production 5 ;
Council Regulation (EC) No 1/2005 of 22 December 2004 on the protection of animals during transport and related operations and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97 6 ;
Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. 7
These acts are based on scientific opinions that are outdated. In the context of possible drafting of legislative proposals, the Commission needs new opinions that reflect the most recent scientific knowledge.
As the EFSA has already accepted mandates on the protection of animals at the time of killing, no opinion is requested on this topic.
Furthermore, a European Citizen Initiative (ECI) ‘end the cage age’ was registered in September 2018. The ECI calls for banning the use of cages or individual stalls in particular for laying hens, pigs and calves, where specific EU legislation exists.
The concept of ‘cage’ is not precisely defined in the legislation. In its common meaning ‘cage’ means a box or enclosure having some openwork (e.g. wires, bares) for confining or carrying animals. It can cover either individually confined animals or animals kept in group in a limited space.
Against this background, the Commission would like to request the EFSA to review the available scientific publications and possibly other sources to provide a sound scientific basis for future legislative proposals.
This request is about the protection of calves (bovine animals up to 6 months old).
The latest Scientific Opinion that was used for the current legislation was published in 1997. Since then, the EFSA has adopted opinions on the welfare of calves in 2006 8 and 2012. 9
1.1.1. Terms of Reference (ToRs)
The Commission therefore considers it opportune to request EFSA to give an independent view on the protection of calves.
The killing of animals on the farm is not part of the request.
For this request, the EFSA will:
Describe, based on existing literature and reports, the current husbandry systems and practices of keeping them.
Describe the relevant welfare consequences. Relevance will not need to be based on a comprehensive risk assessment, but on EFSA's expert opinion regarding the severity, duration and occurrence of each welfare consequence.
Define qualitative or quantitative measures to assess the welfare consequences (ABMs).
Identify the hazards leading to these welfare consequences.
Provide recommendations to prevent, mitigate or correct the welfare consequences (resource‐based and management‐based measures). The current legislation requires calves to be kept in groups after the age of 8 weeks. In the context of the ECI ‘end the cage age’, the EFSA will explore scientific information that supports the feasibility of further increasing the period of time during which calves can be kept in groups in a way that improves their overall welfare conditions.
For the following scenarios, the Commission has identified practical difficulties or insufficient information in ensuring the welfare of animals. At least for them, EFSA will propose detailed ABMs and preventative and corrective measures with, where possible, either qualitative (yes/no question) or quantitative (minimum/maximum) criteria (i.e. requirements to prevent and/or mitigate the welfare consequences):
The welfare of male dairy calves raised for producing ‘white’ veal meat and the risks associated with individual housing, insufficient space and feed restriction (such as deprivation of iron and fibres).
The assessment of ABMs collected in slaughterhouses to monitor the level of on‐farm welfare of male dairy calves raised for producing ‘white’ veal meat.
The welfare of dairy calves and the risks associated with limited cow–calf bond.
1.2. Interpretation of the Terms of Reference
This Scientific Opinion (SO) concerns the welfare of calves on farm only – welfare aspects of transport, slaughter or on‐farm killing of calves are not discussed. It was considered that the scope of this mandate is the welfare of calves born on dairy farms and kept as replacements or reared for white veal. Rosé veal systems are also not discussed because the mandate focuses on white veal systems, nor welfare aspects of calves in beef suckler herds.
The definition of ‘calf’ used in this document was the same as the definition provided in Council Directive 2008/119/EC – ‘bovines up to a maximum of six months old’. However, an upper age limit of 30 rather than 24 weeks was considered for the purposes of assessment of Scenario 1, which focuses on calves reared for white veal, to align it with the age range defined in regulation EC 700/2007.
With regard to the first part of the mandate, which requested to:
describe, based on existing literature and reports, the current husbandry systems and practices of keeping them;
describe the relevant welfare consequences;
define qualitative or quantitative measures to assess the welfare consequences (ABMs);
identify the hazards leading to these welfare consequences,
it was decided to identify the ‘current husbandry systems’ more relevant in a European context following the methodology described in the guidance document developed by the AHAW Panel for the ‘Farm to Fork’ (F2F) mandates (EFSA AHAW Panel, 2022a).The same methodology was used for the description of ‘relevant welfare consequences’ in each husbandry system and identification of hazards and preventive measures.
Specific Scenarios 1 and 3 of the mandate are the following:
Specific Scenario 1: The welfare of male dairy calves raised for producing ‘white’ veal meat and the risks associated with individual housing, insufficient space and feed restriction (such as deprivation of iron and fibres)
Specific Scenario 1 specifically requested an assessment of the welfare of ‘male dairy calves’ reared for white veal. While most calves reared for white veal are indeed males, a proportion is comprised by females that are not kept as herd replacements and fattened for white veal meat. It was considered that the outputs of the welfare assessment under this Specific Scenario would equally apply to females because male and female animals are managed in the same way in veal farms. Sex differences are not expected because animals are slaughtered before reaching puberty.
This scenario lists four major factors potentially leading to welfare issues in white veal production systems: insufficient space, individual housing and restriction of iron and fibre in the diet. In the context of this assessment, these factors were considered ‘exposure variables’, i.e. any condition to which calves are exposed to (e.g. fibre restriction) and that may be associated with an impact on their welfare (e.g. inability to ruminate). To perform the assessment, the factors named in the mandate were translated into measurable factors to allow comparisons of their impact on welfare and to provide recommendations on variables with a potential to be regulated. For instance, ‘space restriction’ was defined as ‘space allowance’ (number of square metres available per calf), ‘deprivation of fibre’ as ‘amount of fibre [neutral detergent fibre – NDF]’, ‘iron restriction’ as ‘haemoglobin concentration’ and ‘individual housing’ was considered both in terms of ‘age at grouping’ and ‘group size’. While some of these issues can be related (e.g. individual housing is often linked with insufficient space, as in the case of individual pens in veal farms), for clarity it was assumed that these aspects would be assessed separately, and each be interpreted as an exposure variable.
For clarity, it was deemed necessary to define some of the terms used. The term ‘commingling’ was defined as gathering and grouping of calves from different origins at auction markets and at the veal unit, and ‘regrouping’ as grouping of unfamiliar calves from the same farm. ‘Forage’ and ‘roughage’ are often used interchangeably in the scientific literature, but using the term ‘roughage’ was preferred and was defined as ‘high fibre feeds obtained by cutting and preserving the whole plant (except roots) or as a crop residue or a by‐product’ (Harris et al., 2017). Further details on the definitions used for each exposure variable are provided in the relevant sections.
Specific Scenario 2: The assessment of ABMs collected in slaughterhouses to monitor the level of on farm welfare of male dairy calves raised for producing ‘white’ veal meat
This Specific Scenario focuses on the ABMs that can be collected in slaughterhouses to monitor the level of welfare of veal calves on farm. Although the mandate only mentions male calves, all veal calves were considered regardless of sex.
Specific Scenario 3: The welfare of dairy calves and the risks associated with limited cow–calf bond
Specific Scenario 3 requests an assessment of the welfare risks of restricted limited cow–calf bond. Cow–calf bond was considered to be a function of the type and length of contact between cow and calf. The risks were assessed mostly from the perspective of the calf, because this is the animal category that the mandate focuses on, and because there are few data on the impact of separation on the dam's welfare.
In this document, systems involving cow–calf contact (CCC) included calves reared by the dam or by a foster cow, and the definitions of ‘nurse’, ‘suckle’ and ‘suck’ were adopted from Sirovnik et al. (2020): ‘nursing for cows allowing the calves to suckle their udder’, ‘suckling for the behaviour of the young while consuming milk from the udder’ and sucking ‘for feeding from a milk feeder’. The definitions of the same paper were adopted for defining full‐time contact, part‐time contact and restricted suckling.
This scientific opinion is structured as follows: the data and methodologies are presented in Section 2 and the results of the assessment in Section 3, including conclusions and recommendations.
2. Data and methodologies
2.1. Data
2.1.1. Data from literature
Data from previous EFSA outputs (e.g. EFSA AHAW Panel, 2012), from relevant papers obtained from the literature searches and from additional scientific and grey literature identified by EFSA experts, were used to address the common and specific ToRs (see relevant chapters of the assessment). Details on the literature searches can be found in Appendix E.
2.1.2. Expert opinion
Expert opinion was used at different phases of the assessment as detailed below. Expert opinion was mainly elicited via group discussion to gather consensus on each topic, namely:
identification of most common and relevant husbandry systems to rear veal calves;
identification and categorisation of welfare consequences in terms of relevance (high, medium and low) in each system;
identification of ABMs and qualitative assessment of their sensitivity and specificity to detect the welfare consequences of interest;
identification of hazards, and preventive, corrective and mitigation measures;
implementation of the F2F model to address Specific Scenarios 1 and 3, which relies on expert judgements to estimate the values of the model parameters.
2.1.3. Data from public consultation
EFSA launched a public consultation from 29 September to 4 November 2022 to consult interested parties and stakeholders and gather feedback on the results of the assessment, including conclusions and recommendations on Specific Scenarios 1 and 2. Any relevant publications suggested during the public consultation were considered by the WG in their assessment, but preference was given to published, peer‐reviewed publications. EFSA thanks the stakeholders who took time to read the draft scientific output and to provide comments.
2.2. Methodologies
2.2.1. Describing calf welfare
2.2.1.1. Negative affective states and welfare consequences
The methodological approach used in this Scientific Opinion had been previously defined to provide a common framework for the welfare assessment of the different species covered by the F2F mandates (EFSA AHAW Panel, 2022a). To carry out the assessment, the EFSA experts considered eight negative affective states that can be experienced by cattle and other species (fear, pain, discomfort, fatigue, stress and distress, frustration and boredom) (for the list and definitions, please refer to Appendix A). These negative affective states were the basis for the definition of welfare consequences, which allow a more precise estimation of welfare risks. The welfare consequences were phrased in a negative manner to follow the general risk assessment framework, which has hazards as starting points. Accordingly, positive welfare aspects were also considered but phrased negatively (e.g. ‘inability to perform play behaviour’), to align it with the general methodologies in risk assessment. The final list used in this scientific opinion (Appendix A) comprises only welfare consequences relevant for an assessment of the welfare of calves on farm; aspects from the initial list that were not relevant for the scope of this mandate were left out (e.g. relevant for assessment of welfare during transport, or relevant only for other species). Regarding the behaviour‐related welfare consequences, aspects of calf natural behaviour were considered, such as maternal, social and feeding behaviour (Whalin et al., 2021). More detailed descriptions of natural behaviour of calves are provided in relevant sections of the scientific opinion.
2.2.2. Identification of husbandry systems, highly relevant welfare consequences and ABMs
The most frequent husbandry systems to rear calves, including dairy calves for replacement, calves for white veal and systems with CCC, are described in this document (Sections 3.1–3.12). Relevant systems were identified through discussion and characterised in relation to animal category and production stage, feeding practices, flooring, general housing infrastructure and main husbandry practices. Given the limited published data on husbandry practices and physical structures of each housing each system, sources of information for this description included grey literature, technical recommendations of livestock institutes and expert knowledge.
The highly relevant welfare consequences for calves in each husbandry system were then identified based on a procedure described in EFSA AHAW Panel (2022a) in Section 3.1.1.4 of that document. Welfare consequences of medium and low relevance in each system are presented in Appendix C. In Appendix D, a summary table of the welfare consequences, hazards, ABMs and preventive measures in each system is presented.
Regarding identification of ABMs, only those ABMs feasible to be collected during a farm visit by a welfare inspector through direct observation were considered. This includes, for instance, ABMs that can be collected through observation of animals for a certain period of time (e.g. 30 min), and excludes ABMs that would require further tools (e.g. blood sampling to evaluate for presence of anaemia) or very prolonged periods of observation (e.g. several days).
Sensitivity and specificity of an ABM to detect a welfare consequence
The method described in EFSA AHAW Panel (2022a) was followed for a qualitative assessment of the sensitivity and specificity of an ABM to measure a welfare consequence. Further information on the assessment of ABMs is presented in Appendix B.
2.2.3. Provision of quantitative criteria for Specific Scenarios 1 and 3
The mandate included a request for the provision of qualitative (yes/no) or quantitative criteria to prevent and/or mitigate relevant welfare consequences for specific scenarios. To address these requests, a risk assessment model based on structured Expert Knowledge Elicitation (EKE) was developed by EFSA (EFSA AHAW Panel, 2022a) and applied to the different F2F mandates received by EFSA. For simplicity, this model is referred to in this scientific opinion as ‘F2F EKE model'. For more details on the general principles of the methodological framework, please see Appendix B).
The suitability of the model to assess each exposure variable of the Specific Scenarios was assessed case‐by‐case, depending on the nature of the exposure (quantitative (e.g. space allowance) vs qualitative (e.g. types of contact between the dam and the calf)) and data availability. A quantitative assessment based on the F2F EKE model (was carried out where a clear question could be identified and where sufficient data were available from experimental studies to estimate the relationship between an exposure variable and an outcome (welfare consequence). For simplicity, the welfare consequences resulting from each exposure variable were assessed independently, and each assessment was thus carried out separately for each, even though certain factors could be interlinked and interacting (e.g. individual housing and space allowance). Welfare consequences were selected based on their sensitivity to the exposure variable of interest, and ABMs on their sensitivity and specificity to assess the welfare consequence. Published information was considered to assess the availability of data on different welfare consequences and ABMs relevant to each exposure variable. Extensive literature searches (ELS) were carried out to identify peer‐reviewed publications of relevance to the exposure variables identified; details on ELS conducted for each exposure variable are presented in Appendix B. In cases where there were no sufficient quantitative data available in the literature, a literature review was carried out and the F2F EKE model was not formally applied. Table 1 shows an overview of the approaches that have been adopted to assess the exposure variables listed in the Specific Scenarios 1 and 3.
Table 1.
Overview of the approaches to assess the mandate Specific ToRs. Only information relative to the scenarios discussed in this document (1 and 3) is provided
| Scenario | Aspect assessed | Approach/type of assessment | Section | |
|---|---|---|---|---|
| #1 | The welfare of male dairy calves raised for producing ‘white’ veal meat and the risks associated with individual housing, insufficient space and feed restriction (such as deprivation of iron and fibres) | Age at grouping | Literature review | 3.16.1 |
| Group size | F2F EKE model | 3.16.1 | ||
| Amount of space | F2F EKE model | 3.16.2 | ||
| Provision of iron | Literature review | 3.16.3 | ||
| Amount of fibre | F2F EKE model | 3.16.4 | ||
| #2 | The assessment of ABMs collected in slaughterhouses to monitor the level of on farm welfare of male dairy calves raised for producing ‘white’ veal meat | Slaughter ABMs reflecting on‐farm welfare | Semi‐quantitative elicitation | 3.17 |
| #3 | The welfare of dairy calves and the risks associated with limited cow–calf bond | Duration of dam–calf contact | Literature review and F2F EKE model | 3.18 |
2.2.4. The assessment of ABMs collected in slaughterhouses to monitor the level of on farm welfare of male dairy calves raised for producing ‘white’ veal meat – Specific Scenario 2
This ToR requested the identification of ABMs collected at slaughter to evaluate the welfare of animals on farm. As a common request was included in other mandates received by EFSA under the Farm to Fork strategy (e.g. protection of pigs, broilers and laying hens), EFSA developed a dedicated methodology for the selection of ABMs. A set of ABMs was selected based on their association with welfare consequences, current use and potential to be used as a standard method. For details on the methodology and steps followed please refer to Section 3.17 (Specific Scenario 2). The details of the literature searches carried out are presented in Appendix A.
2.2.5. Uncertainty assessment
The overall methodology to assess uncertainty in this Scientific Opinion followed the approach described in Sections 3.2.1 and 3.2.2 of EFSA AHAW Panel (2022a). Accordingly, the main sources of uncertainty associated with each assessment stage were identified and are presented in each relevant section of the SO. Where the assessment of the exposure variable was carried out by applying the F2F EKE model (Table 1), the uncertainty around each point estimate was expressed in terms of credibility ranges obtained from the elicitation.
For those conclusions based on scientific literature and not resulting directly from the F2F EKE model, a judgement on the certainty of each conclusion was carried out. The certainty ranges were derived from three predefined certainty ranges from EFSA (2019) (Table 2). A group discussion took place during which experts had the chance to explain the rationale behind their judgement, and a consensus on the category better reflecting the overall certainty was reached. When a certainty range was placed at the end of a paragraph in the conclusions, it was considered that it applies to all sentences within that paragraph.
Table 2.
Certainty ranges used to classify the certainty of conclusion statements
| Certainty range | 50–100% | 66–100% | 90–100% |
|---|---|---|---|
| Expression of certainty | More likely than not | From likely to almost certain | From very likely to almost certain |
Table 3.
ABMs for restriction of movement in individual pens in dairy farms
| ABM | Comments |
|---|---|
| Slipping |
Definition: Loss of balance in which the calf loses foothold, or one or more hooves slide on the floor surface. No other body parts except hooves and/or legs are in contact with the floor surface (Welfare Quality®, 2009) Sensitivity: High for impairment of movement that results from slippery floors, but low for restriction of movement caused by low space allowances Specificity: High |
| Falling |
Definition: Loss of balance in which parts of the body other than the feet and legs get in contact with floor surface (Welfare Quality®, 2009) Sensitivity: High for impairment of movement that results from slippery floors, but low for restriction of movement due to low space allowance. The sensitivity is considered high in this case because although not all calves experiencing restriction of movement will show falling, the fact that some do indicates a restriction of movement problem in the herd due to slippery floors Specificity: High |
| Galloping in unrestricted conditions |
Definition: A rebound of galloping is seen when released in large area after 3 days of confinement in individual pen (Jensen, 2001) Sensitivity: High Specificity: Low. Galloping in unrestricted conditions can also be observed in situations where calves respond to other stimuli, e.g. fleeing behaviour |
3. Assessment
3.1. Husbandry and management of calves
In the sections below, general considerations on housing of calves in dairy and veal farms are provided. Information on each specific husbandry system is provided in Sections 3.2–3.15.
3.1.1. General husbandry considerations: dairy farms
This section aims at describing the common rearing practices of calves during their first weeks of life. The welfare implications of such practices are not discussed here because such welfare consequences are described in detail in the section on individual housing of dairy calves (Section 3.1.3). Exceptions to this are the welfare effects of disbudding and restricted water provision, which are hence discussed in this section.
Calving
Calving typically takes place in deep bedded individual or group calving pens, but cows may also calve in tie‐stalls (Jensen and Tolstrup, 2021), in a loose housing barn (Mülleder and Waiblinger, 2004; Wageningen UR Livestock Research, 2010) or on pasture. Hygiene of the calving area is a major determinant of calf health. Therefore, use of individual calving pens (Svensson et al., 2003), which are cleaned between each calving (Klein‐Jöbstl et al., 2014), and protocols for cleaning of group calving pens (Hyde et al., 2021) are recommended.
Early separation and colostrum management
In conventional dairy farms, calves are separated from their dams shortly after birth (e.g. within 1 h) (Klein‐Jöbstl et al., 2015) and moved to an individual pen. Since calves are prevented from ingesting colostrum directly from the dam's udder, their health largely depends on timely provision of sufficient high‐quality colostrum (Godden et al., 2019) via an artificial teat or tube feeding (hereafter named ‘artificial rearing’). Absorption of immunoglobulins in calves is optimal in the first 4 h after birth, declines rapidly after 12 h and ceases approximately 24 h postpartum. Therefore, calves should be fed colostrum as soon as possible after birth. Calves should be provided with high‐quality colostrum (i.e. specific gravity > 1.050 and colostrum IgG concentrations > 50 g/L) corresponding to 12% body weight to ensure a sufficient absorption of immunoglobulins and subsequently reduce the risk of disease and mortality (reviewed by Weaver et al. (2000)). When calves are not provided with sufficient quantities of high‐quality colostrum after birth, the transfer of immunity is impaired, and calves become more susceptible to endemic enteric and respiratory diseases. Further details on development of immunity in calves and the importance of colostrum ingestion are provided in the EFSA scientific opinion on the transport of cattle (EFSA AHAW Panel, 2022b).
Physical infrastructure: individual/group housing and flooring
The relevant EU legislation in place at the time of publication of this document (Council Directive 2008/119/EC) states that calves must not be housed in individual pens after the age of 8 weeks (with exceptions granted for holdings with less than six calves or in farms where calves are kept with their dams for suckling). While most dairy farms house calves individually at least in the first couple of weeks, many holdings keep calves in group pens during the first months of the calf's life. Other types of housing, such as outdoor igloos or open‐fronted barns are also common in this phase. Although pen design, space allowance and type of floor vary across farms, generally the pen features are simple and the environment relatively barren. For instance, brushes are often not provided and the opportunities for self‐grooming by scratching are limited (e.g. by pen fixtures). The time spent in these group pens can go up to 1 year and will vary according to breed and production purpose. For more details on individual and group housing of calves in dairy farms, please refer to Sections 3.2 and 3.6, respectively.
Feeding
Individually housed calves are typically fed milk from open or teat buckets. The milk fed to calves can be either milk replacer, whole milk from the bulk tank, milk from recently calved cows or milk of lower quality (e.g. waste milk from cows with mastitis (Hayer et al., 2021) or from cows treated with antimicrobials (Mahendran et al., 2022)), although this is not good agricultural practice. According to recent surveys, in 72.4% of farms in western Germany calves were fed waste milk (Hayer et al., 2021), and in 3.7% of farms in UK calves were provided with waste milk containing antimicrobials (Mahendran et al., 2022). Traditionally, calves in artificial rearing systems have been provided with a daily milk allowance corresponding to ~ 10% of their bodyweight, but there is increasing evidence that these amounts restrict calf's growth and are not sufficient particularly in situations of low temperatures (Palczynski et al., 2020). Hence, it is currently recommended to feed calves under the age of 4 weeks a milk allowance equivalent to 20% body weight of the calf (Khan et al., 2011; Costa et al., 2019). The common practice in dairy farms is to feed calves twice a day, but due to labour costs a milk‐feeding frequency of once daily has received interest recently (Jongman et al., 2020) (see Section 3.2.6). There are also systems in which calves are fed milk ad libitum (defined as unrestricted amounts of milk available at all times of the day), although intake may be limited by deliberately acidifying the milk. As with regard to solid feed, calves should be provided with roughage/concentrate from the second week of life onwards as stated in current legislation (Council Directive 2008/119/EC).
Water provision
The EU legislation Council Directive 2008/119/EC states that calves over 2 weeks of age must have access to sufficient water at all times. A review on this topic pointed out that, although the importance of water provision for calf welfare and calf growth is well recognised, survey data indicate that calves were not provided with water at all times in a high percentage of cattle holdings (data from Denmark, Norway, the USA, Chile and Canada) (Jensen and Vestergaard, 2021). Calves fed restricted amounts of milk had a higher water intake than calves fed milk for ad libitum intake, but the latter group also drank substantial amounts of water, suggesting that even ad libitum access to milk does not fully cover water requirements (Jensen and Vestergaard, 2021). As regards provision of water to calves, water should be provided through an open water surface, i.e. not through bottles nor nipple drinkers, because cattle are suction drinkers (Hepola et al., 2008). In group pens, 2.5–7 cm of linear waterer space per animal is usually provided, or several water bowls.
Weaning management
In conventional dairy farms, weaning occurs much earlier compared with when cow and calf are kept in extensive conditions or in cow–calf systems. Gradual weaning may be initiated as early as 4–6 weeks of life, but replacement dairy calves are commonly weaned off milk at 8–12 weeks of age (Drackley, 2008; Johnsen et al., 2021; Mahendran et al., 2022). Weaning strategies can be strictly age‐related but individual weaning schemes are also used, taking calf weight, concentrate intake or a combination hereof into account. A greater solid feed consumption during the weaning process will contribute to rumen development and bodyweight gain after weaning (Drackley, 2008).
Disbudding
Disbudding is commonly carried out in dairy farms to facilitate cattle management and cattle handling. The recommendations from the Council of Europe state that calves should not be disbudded after the age of 4 weeks (Council of Europe Standing Committee, 1998).
To prevent horn‐bud growth, calves are either disbudded by a hot iron or caustic paste. In either case the procedures cause severe pain (Knierim et al., 2015). Therefore, it is compulsory in some MSs to disbud under anaesthesia and to administer analgesic drugs to reduce post‐disbudding pain (Graf and Senn, 1999; Faulkner and Weary, 2000; Mintline et al., 2013). However, the efficacy of local anaesthesia depends on the compound used; for example in some cases cornual nerve block with procaine has been shown to be insufficient (Thomsen et al., 2021). Careful assessment of the anaesthesia efficacy should therefore be done by needle‐pricking the horn‐bud base. If pain is still present, additional measures should be taken (e.g. subcutaneous infiltration around the bud). Thermocautery (i.e. hot iron) disbudding is performed on calves up to ~ 8 weeks of age, when horn buds are 5–10 mm thick and ~ 2 cm in diameter (Stafford and Mellor, 2005). Disbudding age is important as younger animals have smaller horn buds and thus a smaller iron can be used, causing less tissue damage and a faster healing of the wound (Taschke and Fölsch, 1997; Adcock and Tucker, 2018). In case horns need to be removed at a later age, amputation needs to be performed and this is an even more painful procedure (Stilwell et al., 2007).
Caustic paste disbudding is done in younger calves (~ 2 weeks old). This method causes severe pain, and it is not easily controlled by anaesthesia (Stilwell et al., 2007; Stilwell et al., 2009). It can be associated with complications such as damage to surrounding skin and/or the eyes if runoff occurs. Additionally, destruction of the horn bud is often incomplete so that horns may grow or develop a divergent shape, and later might have to be removed by amputation (Weaver et al., 1986).
Independently of the method and the anaesthesia protocol, there is compelling evidence that pain lasts for longer than the effect of the analgesics usually given (Adcock and Tucker, 2018).
Selection of polled cattle is an alternative that will reduce and eventually eliminate the need for disbudding. Polledness is a dominant autosomal trait. The availability and the genetic merit of polled bulls will probably increase in the future, leading to a reduction in the frequency of the horned allele in the dairy cattle population (Mueller et al., 2019).
Differences between organic and conventional farming
Organic dairy farms represented ~ 4% of the total farms in the EU in 2019 (EUROSTAT, 2022). In organic farming, individual housing is only allowed during the first week of life, in contrast to 8 weeks in conventional farming. In addition, the minimum milk feeding period is 90 days, with no minimum feeding period being determined for conventional conditions. While still rare in absolute numbers, CCC systems are more common in organic farming compared with conventional farms. In CCC systems, a degree of contact with the dam is allowed (Sirovnik et al., 2020), for a shorter (e.g. 2 weeks) or longer (e.g. 2 months) period, depending on the management practices of the farm (Eriksson et al., 2022).
3.1.2. Introduction to veal systems
At ~ 2–5 weeks of age, male calves and some female calves not kept for herd replacement are moved from the dairy farm of origin to auction markets/assembly centres or transported directly to specialised veal units for further fattening (EFSA AHAW Panel, 2022b). Typically, these calves are of Holstein/Friesian breeds, but crossbreds can also be reared as veal calves. In some countries, calves can be, alternatively, fattened in the farm of birth, as it is the case of some herds in France (breeder‐fatteners).
The diets of calves reared for white veal are restricted in iron to produce meat that is light in colour (hence the name ‘white’ veal) and are comprised mostly of milk replacer, grains and a small amount of roughage (Magrin et al., 2020). Compared with earlier feeding practices, a larger amount of solid feed than what is legally required, has been provided to calves in recent years; however, there is still a tendency to provide solid feed in the form of small particles. Calves are usually fed milk replacer in open troughs or from open buckets without a teat and are not weaned until slaughter. The exact duration of the fattening varies depending on the production country, with France having shorter cycles compared with the Netherlands and Italy (150–175 days vs 190–200 days). In France, the amounts of solid feed and milk replacer depend on the strategy of the farm but are around 200–300 kg per animal. In the Netherlands, Germany and Italy, the amount of solid feed has increased (≥ 400 kg to up to 500 kg per cycle) while the amount of milk replacer has decreased in recent years.
Compared with white veal, the production of ‘rosé’ veal differs – these calves are weaned off milk at about 3–4 months of age and slaughtered at 8–12 months of age. The different slaughter age is reflected in the carcass classification for commercialisation (‘V’ and ‘Z’ categories), which is regulated through the Commission Regulation (EC) No 566/2008. Rosé veal systems are not further discussed in detail in this document because they are considered to be out of the scope of the mandate.
In 2021, ~ 4.08 million white veal calves were slaughtered in the EU‐27 accounting for ~ 620,000 t carcass per year. In addition, ~ 400,000 rosé calves are raised every year (accounting for ~ 76,000 t‐equivalent carcass per year). The Netherlands represents 33% (1,359 million calves), France 29% (1,200 million), Italy 14% (590,000), Germany 8% (307,000), Belgium 7% (288,000) and other Member States the 9% remaining (340,000) (IDELE, 2021) of total veal calves production.
3.1.3. Husbandry systems described in this document
A detailed description of the characteristics of each system (Figure 1) and an assessment of the most relevant welfare risks are presented in Sections 3.2–3.12.
Figure 1.
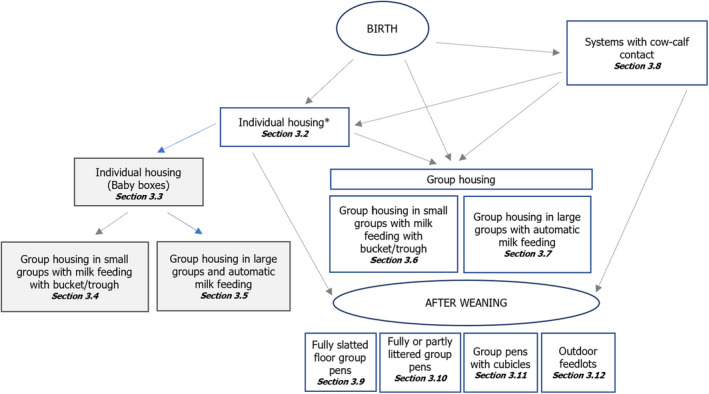
- *: In calves > 8 weeks only permitted in small farms with < 6 calves. Veal systems are showed in grey.
3.2. Welfare of calves kept in individual housing (at dairy farms)
3.2.1. Description of the system
After separation from the dam, calves are typically moved to individual housing, which comprises either a hutch (igloo) and a small outside run, an individual crate elevated from the ground with no access to an outdoor run, or a small indoor pen typically with some bedding, e.g. straw (Figures 2, 3, 4–2, 3, 4). However, systems with no provision of bedding may occur after a certain age. In the outdoor igloos, calves may be exposed to thermal discomfort, in particular during summer if the area provides no shade or protection from high temperatures.
Figure 2.
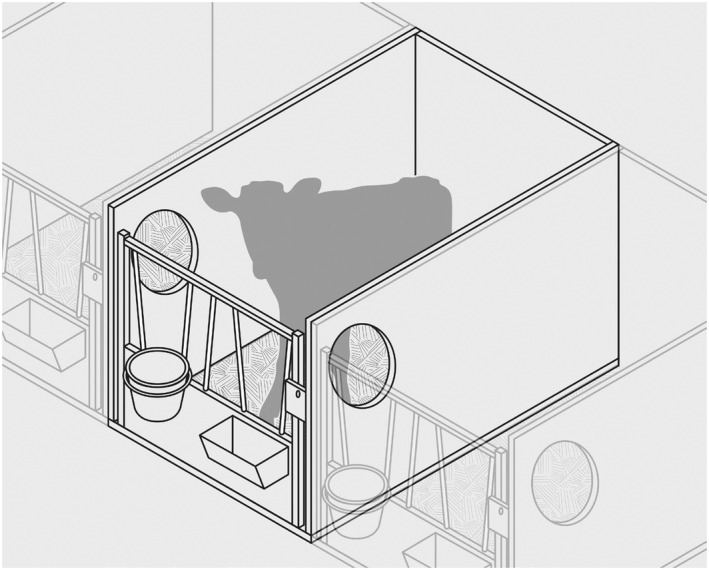
Schematic representation of an individual pen in a dairy farm
Figure 3.

Individual calf pens in a dairy farm. © George Stilwell
Figure 4.

Calf in an outdoor individual pen (‘igloo’). © JUNIA – France
Depending on the intended purpose of the calf, i.e. veal/beef or replacement dairy heifer, the duration of the stay in individual housing varies between 2–5 weeks (veal) and 0–8 weeks (dairy). Some farmers may keep calves in individual pens for a period of ~ 3 days only, after which they are housed in pairs or groups. In small, non‐organic farms with less than six calves, the animals can be kept for longer than 8 weeks in individual pens (Council Directive 2008/119/EC). When housed individually, calves must be able to see and touch other calves. In the EU there is a general increasing tendency of housing calves younger than 8 weeks in groups (Marcé et al., 2010; Johnsen et al., 2021; Mahendran et al., 2022), although figures from specific MSs indicate a different picture. For instance, a survey from Austria indicated that approximately 90% of farms housed calves individually after birth and 23% of these did so for longer than 6 weeks (Klein‐Jöbstl et al., 2015), a survey from the Czechia indicated that 97% of farms housed calves individually for a median of 8 weeks (Staněk et al., 2014), and a study from the UK indicated that the most prevalent initial type of housing used for newborn calves is individual housing (83/216, 38.4%), with pair housing used by 23.1% (50/216), and group housing of greater than 2 calves used by 35.6% (77/216) of the holdings (Mahendran et al., 2022). Another characteristic of this type of system is that individually reared calves are more likely to make contact with stockpersons (Webb et al., 2022).
3.2.2. Welfare consequence ‘Restriction of movement’
3.2.2.1. Description
Restriction of movement is defined as negative affective states such as pain, fear, discomfort and/or frustration experienced by the animal because it is unable to move freely or is unable to walk comfortably. Cattle are considered a hider species, meaning that, when possible, the dam isolates from the herd to calve. The newborn calf spends the first days of life hidden under bushes or tall grass with the dam grazing nearby and returning regularly to the calf to nurse (Kiley‐Worthington and Plain, 1983; Vitale et al., 1986). After some days, dam and calf re‐join the herd and the calf interacts with other calves of the group and engages in activities such as exploring the environment and playing while the dam is grazing (Wood‐Gush et al., 1984; Vitale et al., 1986). After some days, dam and calf re‐join the herd and the calf interacts with other calves of the group and engages in activities such as exploring the environment and playing while the dam is grazing (Wood‐Gush et al., 1984; Vitale et al., 1986). Besides play behaviours (for considerations on play behaviour please refer to Section 3.2.5), typical behaviours are walking, exploring and social interactions with dam and other calves. When in individual pens, calves are unable to perform motivated behaviour (e.g. to seek resources or to avoid fear eliciting stimuli) or hindered in their movements (e.g. when attempting to perform play behaviour) due to the insufficient space available. Additionally, floor properties may impede movements resulting in reduced locomotor activity, unsteady gait, as well as slipping or falling, which may lead to injury and pain.
3.2.2.2. ABMs
Measuring locomotor activity provides direct information on restriction of movement but requires long‐term observations or automatic data recording, e.g. using accelerometers. Alternatively, assessment of space allowance may be considered a proxy. The ABMs listed in the table below focus mostly on aspects related to impaired movement due to floor quality and less on spatial constraints.
3.2.2.3. Hazards
A listing of key hazards for this welfare consequence is presented below. Details on how the hazards lead to the welfare consequence here described are provided in the following section on preventive and corrective measures; the same approach was followed in all sections on hazards of the described husbandry systems.
Low space allowance
Slatted or slippery floors
3.2.2.4. Preventive and corrective measures
The quality of the floor has received little attention in unweaned calves, but studies on dairy cows showed that cattle housed in pens with rubber covered floor in the alleys had longer stride length (Telezhenko and Bergsten, 2005) indicating a better foothold on a rubber floor. Bulls housed in pens with rubber‐covered concrete slats performed more social behaviour (Brščić et al., 2015) and had less lying down interruptions (Gygax et al., 2007; Absmanner et al., 2009; Brščić et al., 2015) than animals in pens with concrete floor, and bulls on slats covered with rubber had more lying bouts than bulls in corresponding concrete slatted floor pens (Platz et al., 2007). These studies suggest that rubber flooring reduces some of the problems growing animals have in terms of lying down and getting up due to better traction. However, when bulls were given a choice between concrete, rubber‐covered concrete and deep bedded floor, they choose the deep bedded floor (Lowe et al., 2001) indicating that the rubber does not provide as good a grip and lying comfort as straw bedding.
Addition of deep bedding corrects the restriction of movement caused by slatted or slippery floors.
Housing of calves in pairs, or groups provides a larger shared space at the same space allowance per animal and has been shown to increase the level of locomotor play behaviour (Jensen et al., 1998), but increasing the space allowance per animal in group pens also increases locomotor behaviour (Jensen and Kyhn (2000); see also Section 3.2.5).
3.2.3. Welfare consequence ‘Isolation stress’
3.2.3.1. Description
Isolation stress is defined as negative affective states such as frustration and/or fear resulting from the absence of or from limited social contact with conspecifics. Among unweaned calves, the absence of or limited social contact with comparable age calves (and dam) increases calf's general fearfulness and results in inappropriate social responses. From 1 week old, calves have been shown to associate more with other calves if they are pair‐housed compared with individually housed with physical contact (Duve and Jensen, 2012), and calves housed in pairs or small groups were quicker to approach and interact with an unfamiliar calf in a social test than individually housed calves (Jensen et al., 1997; Duve and Jensen, 2011; De Paula Vieira et al., 2012). From 1 week old, calves have been shown to associate more with other calves if they are pair‐housed compared with individually housed with physical contact (Duve and Jensen, 2012), and calves housed in pairs or small groups were quicker to approach and interact with an unfamiliar calf in a social test than individually housed calves (Jensen et al., 1997; Duve and Jensen, 2011; De Paula Vieira et al., 2012). Comparing various levels of social contact, calves housed in pairs were the least fearful, isolated calves were the most fearful, while individually housed calves with physical contact were intermediate (Jensen and Larsen, 2014). Once individually housed calves have made first contact to another calf (either in a social test or at grouping) they engaged in more aggressive social interactions than pair housed calves (De Paula Vieira et al., 2010; Duve and Jensen, 2011), indicating poorer social competences. Individually housed calves were also more fearful than socially housed calves when introduced to a novel environment (Jensen et al., 1997; Jensen and Larsen, 2014) and being housed alone in a small pen was shown to be likely associated with stress, as also supported by an increased physiological stress response in individually housed compared with pair‐housed calves (Raussi et al., 2003).
3.2.3.2. ABMs
ABMs of isolation stress in this system are presented in Table 4.
Table 4.
ABMs for isolation stress in individual pens in dairy farms
| ABM | Comments |
|---|---|
| Response in standard social approach test |
Definition: Latency to approach an unfamiliar calf. Higher latency to approach an unfamiliar calf indicates a higher fear to other calves due to isolation (Jensen and Larsen, 2014) Sensitivity: High Specificity: High |
| Abnormal oral behaviours |
Definition: Oral manipulation, including excessive licking, of non‐feed items such as pen fixtures or bedding (Jensen and Larsen, 2014) Sensitivity: Low, because not all calves experiencing isolation will show abnormal behaviours Specificity: Low, because this behaviour can be due to other causes (e.g. inability to suck) |
| Fear response |
Definition: Startle response (e.g. withdrawal or freezing) when presented with a sudden external visual and/or auditory stimulus, e.g. the rapid opening of an umbrella (Boissy et al., 2001) Sensitivity: High Specificity: Low, because this ABM could also occur in situations of poor animal handling |
3.2.3.3. Hazards
Individual housing.
3.2.3.4. Preventive and corrective measures
Providing calves opportunity for full social contact with comparable age peers (pair or group housing), or their dam/foster cow, results in improved social skills, which are evident from less fearfulness in a standard social approach test (peers: Jensen and Larsen (2014)), the attainment of higher social rank (dam: Le Neindre (1989); peers: Veissier et al. (1994)), submissive behaviour as a response to threats (dam or foster: Buchli et al. (2017)), less aggressive after (re)grouping (peers: Jensen and Larsen (2013)) and encountering less aggression when introduced to the lactating herd as heifer (dam in CCC: Wagner et al. (2012)).
3.2.4. Welfare consequence ‘Inability to perform sucking behaviour’
3.2.4.1. Description
The inability to perform sucking behaviour is defined as negative affective states such as frustration resulting from the thwarting of the motivation to suck from a teat. Calves have a high motivation to suck in connection with milk intake. Sucking on a teat represents a behavioural need, here defined as a normal behaviour for which the animals have a high motivation and for which thwarting of the behaviour causes abnormal behaviour and/or stress responses (Dawkins, 1990). In the short term, the motivation to suck is elicited by the taste of milk, it is reduced by the performance of sucking, but also declines spontaneously 20–30 min after milk ingestion, even if calves are not allowed to suck the milk (reviewed by de De Passillé and Rushen (1997)). In the longer term the motivation is also affected by hunger in that calves on a low milk allowance perform non‐nutritive sucking for longer after ingestion of milk (De Passillé and Rushen, 1997). In these studies, the individually housed calves were given access to a dry teat to which they could direct non‐nutritive sucking. However, in the absence of a suitable sucking object, individually housed calves suck pen fixtures, or the head and neck of neighbouring calves (cross‐sucking). This behaviour is redirected and thus an abnormal behaviour. The inability to suck has also been related to the stereotypic behaviour tongue rolling, which occurs at a later age. Veal calves offered milk in a bucket showed more tongue rolling than calves offered milk in an automatic milk feeder (AMF) (Webb et al., 2015). Results of a cross‐sectional study in dairy farms also indicated that non‐nutritive sucking behaviour decreased when milk was provided with a teat compared with no teat (via bowl or trough) (Reipurth et al., 2020).
When full‐time dam‐reared, calves suckle their dam 5–9 times a day during the first weeks, the frequency and daily time suckling decreasing with age of the calves to 3–5 daily bouts (e.g. Fröberg and Lidfors, 2009); and each sucking bout takes ~ 7 min (e.g. Lidfors et al., 2010). In contrast, drinking milk from an open bucket, or sucking milk via a teat of a teat bucket or an automatic milk dispenser takes approximately half of the time in case of ad libitum milk feeding (Johns et al., 2011) and less in the case of restricted milk feeding. During the first 4 days of life, calves provided milk twice daily from a bucket spent sucking milk for only ~ 8 min per day compared with 576 min when sucking the dam (Krohn et al., 1999). Some of the time spent suckling the dam represents non‐nutritive sucking (Lidfors et al., 2010). If calves ingest the milk via an artificial teat and have access to suck the teat after the milk is ingested, they can continue performing non‐nutritive sucking and are thereby provided an outlet for the sucking motivation.
Some breeds are more prone to perform cross‐sucking. For instance, cross‐sucking was a larger problem on Simmental farms compared with Brown Swiss‐ and Holstein Frisian farms (Rinnhofer and Fürst‐Waltl, 2008; Ural et al., 2021), and crossed Montbéliarde × Holstein calves showed significantly more cross‐sucking than pure Holstein or Swedish Red × Holstein calves (Mateus, 2014).
3.2.4.2. ABMs
Table 5 shows ABMs of inability to perform sucking behaviour and estimates of their sensitivity and specificity. When used in combination, the overall sensitivity and specificity of the ABMs to inability to perform sucking behaviour increases.
Table 5.
ABMs for inability to perform sucking behaviour in individual pens in dairy farms
| ABM | Comments |
|---|---|
| Sucking of pen fixtures |
Definition: Mouth open and in physical contact with pen fixtures (not including artificial teat), with visible mouth movements (Horvath et al., 2020). A larger time spent sucking pen fixtures typically indicates a more severe inability to perform sucking behaviour Sensitivity: Low. The sensitivity of this ABM increases when there is restricted contact with neighbouring animals Specificity: High. Sucking is very specific to the need to suck |
| Cross‐sucking |
Definition: The calf is sucking on the skin of any body part of another calf, typically muzzle, ears and neck. The sucking movements are performed with the body part in the mouth (Jensen and Budde, 2006) Sensitivity: High. The sensitivity of this ABM increases when calves are hungry. The best observation time is the first 30 min after milk feeding. Importantly, this ABM is sensitive only if animals have sufficient contact with neighbouring animals Specificity: High |
| Loss of hair and inflammation of skin in the muzzle/ears area |
Definition: Observation of hair loss based on clinical examination (Lidfors, 1993) Sensitivity: Low, because cross‐sucking behaviour not always results in loss of hair or inflammation of skin Specificity: High |
3.2.4.3. Hazards
Offering milk in open buckets (Mounier et al., 2006)
Removing teat buckets too quickly after the milk ration is ingested (Jung and Lidfors, 2001)
Offering low milk allowances (Roth et al., 2009b)
Low dry matter intake (de Passillé et al., 2010) and negative energy balance during weaning (Keil and Langhans, 2001)
Breed (Ural et al., 2021)
Early separation from dam (reviewed by Meagher et al., 2019)
3.2.4.4. Preventive and corrective measures
Offering the milk via a teat, for instance using a teat bucket or an automatic milk dispenser, reduced the occurrence of cross‐sucking compared with when the milk is offered in an open bucket or trough. When calves ingested the milk via a teat, they spent more time ingesting the milk, they sucked the teat after the milk was ingested, and they performed less cross‐sucking of other calves after the milk was drunk compared with calf's ingesting milk from an open surface (Mounier et al., 2006).
The teats must be left with the calves for ~ 20 min after the milk is drunk to reduce cross‐sucking (Jung and Lidfors, 2001). Milk feeding via a teat may also be achieved by use of a floating teat placed in an open bucket or trough (Loberg and Lidfors, 2001). However, due to calf's propensity to butt the teat when the milk flow declines, floating teats may be pushed out of the calf's reach before the sucking motivation has subsided. The use of dry teats in combination with feeding the milk in open buckets has also been suggested to reduce cross‐sucking (de Passillé and Caza, 1997), but calves must learn to switch to the dry teats when the milk is drunk. This is probably easier if the dry teats are placed directly above the milk bucket, as placing dry teats away from the bucket in individual hutches did not affect the time calves spent sucking on pen fixtures (Pempek et al., 2017). In addition, dry teats were more attractive when dipped in milk (Jung and Lidfors, 2001), and sucking on another calf, whose mouth is smeared in milk, may be more attractive than a dry teat. Therefore, feeding the milk via a teat by using a teat bucket is preferable and most likely to prevent cross‐sucking when manual milk feeding is applied. Because the motivation to suck declines spontaneously within 20–30 min of milk allocation (De Passillé and Rushen, 1997), confining calves for this period of time, e.g. in closable individual feed stalls, reduced cross‐sucking (Größbacher et al., 2018). However, no studies have investigated whether dry teats can reduce cross‐sucking to the same extent as milk feeding via a teat. One study comparing teat buckets and open buckets plus a Braden bottle (teat bottle filled with calf starter) found that teat buckets reduced cross‐sucking the most (Salter et al., 2021), which may be because calves have to learn to switch to the dry teat when the milk is drunk and because dry teats are less attractive than milk teats (Jung and Lidfors, 2001). Therefore, feeding the milk via a teat by using a teat bucket, or an automatic milk dispenser, provides a better outlet for the sucking motivation and is thus more likely to reduce cross‐sucking than floating teats and dry teats.
A few studies have examined the effect of milk allowance during the pre‐weaning period on cross‐sucking and they found no consistent effect (Mounier et al., 2006; De Paula Vieira et al., 2008). However, after weaning of milk, cross‐sucking was negatively related to the duration of feeding on solid feed suggesting that calves with a low energy intake perform more cross‐sucking when weaned (Keil and Langhans, 2001). Gradual weaning off milk facilitates the transition from milk to solid feed, and gradual weaning off milk resulted in less cross‐sucking compared with abrupt weaning (Nielsen et al., 2008a).
Finally, another preventive measure is to rear calves with their dam, or a foster cow, i.e. allowing the calves to suck milk from an udder. Artificially reared calves fed milk via an AMF performed more cross‐sucking compared with the calves sucking a cows' udder (Fröberg et al., 2008; Roth et al., 2009b). In studies in which calves experienced full‐time contact to the dam, cross‐sucking was not observed (Fröberg and Lidfors, 2009; Roth et al., 2009b), and cross‐sucking was rare in a system with restricted suckling (Margerison et al., 2003; Fröberg et al., 2008). It is unclear if dam rearing ensures a better satisfaction of the sucking need than rearing by foster cows. There is a risk that foster cows do not accept the calves or prefer specific calves and this may lead to reduced sucking opportunities for some calves (Wieczorreck and Hillmann, 2022).
3.2.5. Welfare consequence ‘Inability to perform play behaviour’
3.2.5.1. Description
Inability to perform play behaviour is defined as negative affective states of frustration resulting from the thwarting of the motivation to engage in social, locomotor or object play. Animals actively seek opportunities to play, and the performance of play behaviour is rewarding (Wood‐Gush and Vestergaard, 1991). Play behaviour in calves is typically seen in a social context either as locomotor play or play fighting (Reinhardt, 1980). Locomotor play includes jumping, kicking and galloping, often interrupted by sudden halts and continued locomotor play in a new direction (Jensen et al., 1998). Locomotor play is typically performed in parallel by several calves at the same time but does per definition not involve physical contact. Rotations of the head is categorised as locomotor play (Jensen et al., 1998), but this element possibly functions as an invitation to social play (Bertelsen and Jensen, 2019). Play fighting involves two or more calves pushing and butting while facing each other and unlike aggressively motivated fights, play fighting is terminated without submission, flight or chase. Play behaviour also includes butting and pushing objects, as well as ground play, where the calf rubs the neck and head against the ground while kneeling down (Jensen et al., 1998). A final type of social play is playful mounting (Reinhardt et al., 1978; Vitale et al., 1986). The motivation to perform locomotor play behaviour increased within 3–4 days where calves could not perform play (Jensen, 2001; Bertelsen and Jensen, 2019), but an increase in this time frame was not seen for social play behaviour (Bertelsen and Jensen, 2019). The control treatment in the latter study was 1 day and it is unclear if social play is not subject to rebound, or if the motivation builds up already within 24 h.
3.2.5.2. ABMs
Play behaviour occurs sporadically in short episodes and it is therefore time‐consuming to record by direct observation. Locomotor play may be stimulated in the home pen by adding fresh straw (Duve et al., 2012), but this measure did not give the same result as data based on 48 h continuous video observations (Jensen et al., 2015). Also release in a large area outside the home pen stimulates locomotor play (Mintline et al., 2012) but because locomotor play is subject to rebound (Jensen and Kyhn, 2000), a high level of play during release in an arena may be due to low space allowances and the inability to play in the home environment. A low level of arena play can, however, not be taken as indicative of high welfare because this may also be due to poor health or malnutrition (Krachun et al., 2010; Bertelsen and Jensen, 2019), and thus arena test data are unsuitable as an ABM to compare between different housing systems. The performance and duration of locomotor play behaviour in dairy calves may be recognised and estimated using leg attached accelerometers. The pattern of acceleration of calves galloping, trotting and walking differ and inter‐peak intervals can be used to differentiate between them (de Passillé et al., 2010). When measured in an arena with a high incidence of running, but a low incidence of jumping/kicking, the summed acceleration strongly correlated with the duration of running, but only moderately correlated with the frequency of jumping/kicking (Rushen and de Passillé, 2012). A recent study on locomotor play of calves in their home environment found that peak acceleration measured at a rate of 1 Hz to be a promising estimate of locomotor play, although it did underestimate the occurrence among calves with a high level of play. However, this study also found that a method resembling one–zero recording at 10‐s intervals based on discriminant analysis of acceleration measures yielded less biased estimates across various levels of play (Größbacher et al., 2020). However, the authors caution the use of these methods to compare levels of play between calves housed in different housing conditions which an ABM must be able to do. This method holds great potential for an ABM of locomotor play behaviour but requires further development and validation. Furthermore, measures of acceleration have not been related to social play behaviour.
No ABMs of locomotor play behaviour are suitable for individual pens. In this type of pen, space poses severe restrictions on locomotor play and calves cannot perform social play (no partner). Only a few elements of locomotor play can be expressed (Jensen et al., 1998).
3.2.5.3. Hazards
Individual housing and restriction of movement due to low space allowances (Jensen et al., 1998)
Disease (Bertelsen and Jensen, 2019)
Injury, malnutrition (Krachun et al., 2010)
Low ambient temperature
Slippery surfaces (Sutherland et al., 2013) and dark environments (Dannenmann et al., 1985)
Thus, a lack of motivation to play is a sign of poor welfare. In individual housing, social play behaviour is not possible. In individual pens and in group pens with a low space allowance, the calves may be motivated, but unable to perform locomotor and social play and thus deprived of potential positive emotion experienced during play behaviour.
3.2.5.4. Preventive and corrective measures
Preventive measures to avoid the inability to perform play behaviour are firstly to ensure that calves thrive and are motivated to play, and secondly to keep calves in physical and social environments that enables them to perform the behaviour. With respect to thriving, calves that are healthy (Bertelsen and Jensen, 2019), well‐nourished (Jensen et al., 2015) and in their thermal comfort zone are more motivated to perform play behaviour than calves that are not. With respect to the physical environment, keeping calves in group housing from the outset of the rearing cycle enables calves to perform social play (Jensen et al., 2015). Housing calves in spacious group pens that allows the simultaneous performance of locomotor play behaviour by all calves in the group (e.g. Jensen and Kyhn (2000)), gives a good opportunity for both locomotor play behaviour in addition to social play. The quality of the floor is also important as the performance of the vigorous elements of locomotor and social play behaviour is supported by a solid, non‐slip surface (Sutherland et al., 2013). Finally, environmental change such as the daily provision of straw bedding, or novel environmental stimuli may also stimulate play (Jensen et al., 2015).
3.2.6. Welfare consequence ‘Inability to perform exploratory or foraging behaviour’
3.2.6.1. Description
The inability to perform exploratory or foraging behaviour defined as stress and negative affective states such as frustration and/or boredom resulting from the thwarting of the motivation to investigate the environment or to seek for food (i.e. extrinsic and intrinsic exploration). The thwarting of foraging and exploratory motivation result in frustration and possibly boredom. Exploratory behaviour may be motivated by curiosity (intrinsic exploration) or it may be appetitive behaviour (extrinsic exploration) motivated by the same as the corresponding consummatory behaviour, such as hunger (appetitive foraging; Wood‐Gush and Vestergaard, 1989). Curiosity can be further subdivided into response to novelty (inspective exploration) and novelty seeking (inquisitive exploration); the function of novelty seeking being to keep the animal informed about the availability of resources in a changing environment. Even though the causal factors are different, these two types of exploratory behaviour often share behavioural elements.
Evidence of the importance of novelty seeking is found in operant tests, in which calves will work for access to resources that are also freely available, because it gives them information about the availability of hidden resources (Van Os et al., 2018) referred to as contra freeloading (Inglis et al., 2001). Evidence of the importance of (appetitive) foraging opportunities is the development of abnormal oral behaviours when foraging opportunities are suboptimal (Webb et al., 2012, 2015, 2017).
The term enrichment is used to describe the addition of a biologically relevant feature to a captive environment. The feature can function as occupational or nutritional enrichment (Mandel et al., 2016), providing an outlet for curiosity and appetitive behaviour, or physical enrichment, for instance a brush providing an outlet for grooming motivation.
Calves are often housed in barren environments where feeding events are few, typically twice per day, and the feed is typically offered in a processed and concentrated form, limiting search and manipulation time. Calves at pasture start eating small amounts of vegetation within the first few weeks of age, suggesting that they are motivated to explore and ingest feed such as grass from an early age. Providing pre‐weaned calves with access to hay not only leads to more time spent eating solid feed and ruminating, but also reduces the time spent self‐grooming, tongue flicking and performing non‐nutritive oral manipulation including licking, chewing and sucking of fixtures (Downey et al., 2022).
The typical barren environments also provide little outlet for exploratory behaviour and calves housed in pens provided with various objects do interact with these. Recent studies have included a brush for grooming as enrichment, and results suggest that a brush may serve as both an enrichment and as a grooming substrate. Access to a brush not only reduced scratching against fixtures, but calves also used the brush for exploration (Zhang et al., 2022), and a study on calves in groups of four, found that calves provided with a brush showed reduced pen‐directed sucking, and were scored with improved coat cleanliness compared with calves provided with no brush (Horvath et al., 2020). In this study, the 4‐ to 7‐week‐old calves used the brush for 20.5 ± 6.1 min/12 h. Another study found daily use (27 min/20 h) of brushes by 11‐day old calves and calves spent a similar amount of time interacting with manila rope (Zobel et al., 2017). In another study, calves housed in so‐called furnished hutches (with two artificial teats, a stationary brush, a calf 'lollie’ and a rubber chain link) spent the most time using the brush and increased their use of the brush from about 2.5 min/12 h to 9 min/12 h from week 1 to 7 (Pempek et al., 2017). The ‘enriched’ calves engaged in more locomotor play compared with calves placed in a barren pen, but no differences in the amount of time spent sucking pen fixtures were detected. Indicating that enrichment may also stimulate solid feed intake, a recent study found that the combination of enrichment in the form of rubber teats, plastic chains, brushes and strawberry scented hay (in addition to unscented hay) and pair housing improved calf's average daily gain after weaning when compared with either component alone (Zhang et al., 2022).
3.2.6.2. ABMs
ABMs of the inability to express exploratory or foraging behaviour can be found in Table 6.
Table 6.
ABMs of inability to express exploratory or foraging behaviour in individual pens in dairy farms
| ABM | Comments |
|---|---|
| Non‐nutritive oral manipulation |
Definition: Licking, chewing or sucking directed towards bars, hutch, bedding or empty bottle (Downey et al., 2022). Sensitivity: High for foraging behaviour. Sensitivity is considered high especially when there is restricted contact with neighbouring animal. Specificity: Low. Abnormal oral behaviours can also occur in situations of isolation stress or inability to perform sucking behaviour. |
| Tongue flicks |
Definition: Tongue extends out of the mouth without touching other objects or forming a full or partial circular motion or extends up to the nose before retracting back into mouth and repeating at least once more within 1 s. This can occur while eating and ruminating (Downey et al., 2022). Sensitivity: Low. Not all animals experiencing inability to forage or explore will show this behaviour. Sensitivity increases in young calves (the current hypothesis is that this behaviour is a precursor of tongue rolling). Specificity: High. |
| Tongue rolling |
Definition: Tongue is held in a full or partial circular position or moves in a full or partial circular motion; this can occur when the tongue is held within the mouth or extended outside it (adapted from Downey et al., 2022). Sensitivity: Low (see tongue flicks). Although it can be more frequent in older calves, it can also occur in young calves (< 8 weeks of age). Specificity: High. |
3.2.6.3. Hazards
3.2.6.4. Preventive and corrective measures
The inability to perform exploratory behaviour can be prevented by keeping calves in an enriched environment, i.e. an environment with biologically relevant features that stimulates exploration and natural foraging behaviours, and which may lead to the fulfilment of behavioural needs to explore and to forage.
In calves, one option is to provide long roughage that requires manipulation; roughage in racks that necessitates curling the tongue around it and pulling to get it out; access to outdoor areas or pasture; and provision of physical enrichment, e.g. mechanical or stationary brushes (Mandel et al., 2016). Increasing administration of solid feed frequency stimulates chewing (Webb et al., 2013, 2014a, b).
The mentioned preventive measures will also correct the welfare consequence when it is present. However, correction may not be possible in cases of emancipated stereotypies (Mason and Latham, 2004).
3.2.7. Welfare consequence ‘Prolonged hunger’
3.2.7.1. Description
Prolonged hunger is defined as the craving or urgent need for food or a specific nutrient, accompanied by an uneasy sensation (a negative affective state), and eventually leading to a weakened condition as metabolic requirements are not met. Negative affect specifically related to inappropriate sucking, chewing and rumination opportunities are described in other sections (inability to perform sucking behaviour and inability to perform exploratory and foraging behaviour).
In dairy calves kept in individual housing, prolonged hunger may occur when the restricted amount of feed offered does not enable the calves to reach satiety. Calves have little capacity to digest solid feed before 3–4 weeks of age and thus rely mainly on milk for nutrients (Diaz et al., 2001). Young dairy calves receiving milk corresponding to 10–15% of their body weight (BW)/day(d) performed more high‐pitch vocalisations (Thomas et al., 2001), directed frequent butting towards the empty buckets after milk ingestion (Herskin et al., 2010) and showed more unrewarded visits to an AMF (Jensen and Holm, 2003; De Paula Vieira et al., 2008) compared with calves fed milk corresponding to ~ 20% of their BW/day, indicating that the restrictively fed calves were hungry.
When calves are weaned off milk, which typically occurs at ~ 8 weeks of age, they may experience hunger if the milk is removed abruptly or reduced gradually before the calves are able to ingest enough solid feed to compensate for the loss of milk. Weaning calves gradually is preferred to abrupt weaning, because it enables the calves to gradually increase the intake of solid feed. Weaning according to solid feed intake was most efficient in achieving continued growth throughout the gradual weaning process and likely resulted in less hunger in relation to weaning (De Passillé et al., 2011; de Passillé and Rushen, 2016).
When calves are weaned off milk, a sufficient amount and quality of solid feed must be available for the calves to be able to compensate for the loss of milk and to maintain/increase their nutrient intake.
Next to the amount of milk provided to young calves, a concern is the type of feed provided and the frequency of this provision. When with the dam, calves suckle 5–9 times daily during the first weeks of life, decreasing to 3–5 times per day when 2–3 months old (reviewed by Lidfors and Jensen (2003)). Calves offered a high milk allowance with limited restriction on meal patterning in an AMF, also ingested the milk in fewer and larger meals as they grew older, while calves offered a low allowance ingested the milk as soon as it became available to them (Jensen, 2009).
Studies investigating the effect of reducing milk feeding frequency from twice to once daily in calves offered a milk allowance corresponding to 10% of BW/day on BW gain (Stanley et al., 2002; Kehoe et al., 2007; Saldana et al., 2019) found that average BW gain, calculated based on weekly weighing, did not differ, suggesting that on average over the milk feeding period, calves can ingest the milk in one meal. However, when looking at calves 3–8 days old, a recent study found that milk intake was reduced by ~ 50% on day 3, ~ 20% on day 4 and 10% on days 6–8 among calves fed once per day, compared with calves fed twice daily (Jongman et al., 2020). There are very few studies investigating the effect of feeding frequency at the recommended level corresponding to 20% of BW/day. In a small study, calves (n = 26) were offered free access to whole milk either twice or once daily from 3 days to 52 days of age. Here, twice daily feeding resulted in higher milk intake during the first 4 weeks (e.g. at 2 weeks of age, calves ingested on average ~ 8 L/day and 6 L/day in twice and once daily feeding management, respectively (Muya and Nherera, 2014). This may be due to 2‐week‐old calves not being able to ingest more than approximately 6 L of milk in one meal. In accordance with this suggestion, Ellingsen et al. (2016) found that 3‐week‐old to 4‐week‐old calves ingested on average 3.8, 4.9 and 5.4 L in one meal (corresponding to 8, 10 and 11% of BW in one meal, respectively). However, a large individual variation in milk uptake was recorded (3.5 L to 6.4 L on test day 3) and therefore the results only partly support that 3‐week‐old to 4‐week‐old calves can ingest up to 6 L (approximately equivalent to 10% of BW) in one milk meal. Only a few studies have examined the effect of feeding frequency, but given the available evidence, one daily milk feeding of calves under the age of 4 weeks likely results in prolonged hunger (EFSA AHAW Panel, 2022b).
3.2.7.2. ABMs
ABMs of prolonged hunger can be found in Table 7.
Table 7.
ABMs for prolonged hunger in individual pens in dairy farms
| ABM | Comments |
|---|---|
| Body condition score (BCS) |
Definition: Poor body condition (protruding bones, sharp ribs etc.). For estimation of BCS categories, the following criteria are taken into account (adapted from the Welfare Quality® protocol for veal calves (2009)):
Sensitivity: Low. The calf will only show low body condition if group stress occurs over a long period of time and the animal is prevented from accessing feed sources over this period. Specificity: Low. The specificity of this indicator decreases in cases of disease (chronically ill animals may also have low BCS). |
| Non‐nutritive sucking |
Definition: Sucking equipment, e.g. a teat bucket, a dry teat, or pen fixtures (Herskin et al., 2010), or sucking the head or neck of a neighbouring calf (Mounier et al., 2006). Sensitivity: Low, because not all animals experiencing prolonged hunger will show non‐nutritive sucking. Specificity: Low. This ABM can also be present in cases of inability to perform sucking behaviour. |
| Number of vocalisations |
Definition: Every single open mouthed ‘muh’ vocalisation with inhalation between two occurrences (Johnsen et al., 2015). Sensitivity: High. Specificity: Low. It can also occur in situations of for example separation stress and handling stress. |
| Restlessness, i.e. increased activity and decreased lying |
Definition: Expression of behaviours like stepping, kicking, foot‐lifting (Gygax et al., 2008), leg stomping, weight shifting, repositioning, head swinging (Cooper et al., 2007). Sensitivity: High. Specificity: Low. Can also occur, e.g. in situations of acute hunger. |
3.2.7.3. Hazards
3.2.7.4. Preventive and corrective measures
Offering milk ad libitum or in amounts and in types that meet nutrient requirements, i.e. a daily milk allowance equivalent to 20% of body weight until at least 4 weeks of age (Thomas et al., 2001; Jensen and Holm, 2003; De Paula Vieira et al., 2008; Herskin et al., 2010) prevents prolonged hunger in calves. Offering this allowance in at least two daily milk feedings until at least 4 weeks of age, or until gradual weaning is initiated, ensures that the calves can ingest the allotted milk (see references in Section 3.2.7.1). If prolonged hunger is present, it can be corrected by increasing amounts of feed and feeding frequency. Recommendations for amounts of fibre (NDF) to be provided to calves to prevent (or correct) prolonged hunger are presented in Section 3.16.4.5.
During the weaning period, prolonged hunger can be prevented by weaning calves gradually (see Section 3.2.7.1).
3.2.8. Welfare consequence ‘Gastroenteric disorders’
3.2.8.1. Description
The WC is defined as ‘negative affective states experienced by the animal such as discomfort, pain and/or distress due to impaired function or lesion of the gastrointestinal tract resulting from, for example nutritional deficiency, infectious, parasitic or toxigenic agents.
In young calves, the most important and prevalent gastroenteric disorders are grouped under the name of neonatal calf diarrhoea (NCD) or calf scours (Naylor, 2009). NCD is the most common disorder in pre‐weaned dairy calves, and accounts for more than half of all calf mortality on dairy farms (Sivula et al., 1996; Foster and Smith, 2009; Torsein et al., 2011; Walker et al., 2012). This is a syndrome caused mainly by the following infectious agents, acting solely or together: bacteria (enterotoxigenic and enteropathogenic Escherichia coli; Clostridium perfringens types A, B, C and E), viruses (corona and rotavirus) or protozoa (Cryptosporidium parvum, Giardia duodenalis) (Van Metre et al., 2008; Foster and Smith, 2009; Blanchard, 2012). Other infectious types of diarrhoea are due to Salmonella spp., Campylobacter jejuni and Yersinia enterocolitica. Diarrhoea may also have dietary origins such as poor‐quality milk replacers and/or management errors, such as miscalculating milk replacer concentration (Van Metre et al., 2008; Naylor, 2009; Blanchard, 2012). NCD can affect calves from few days of age (mainly E. coli K99+ or ETEC), up to 1–2 months.
Independently of the causative agent, the disease can cause variable degrees of dehydration, metabolic acidosis (mainly due to absorption of d‐lactate from the gastrointestinal tract), hypoglycaemia, hypothermia, visceral pain (colic), apathy and depression (Foster and Smith, 2009; Olson et al., 2019). Death due to dehydration and acidosis is not unusual in non‐treated calves.
Another notable gastroenteric disease, which cause very poor welfare, is abomasitis. Several studies have reported a high prevalence of abomasal lesions in veal farms in Europe (70–93% detected at slaughter) (Bähler et al., 2010; Brščić et al., 2011a,b). Calf abomasitis is considered a multifactorial disease (Gitter and Austwick, 1957; Jensen et al., 1994; Jelinski et al., 1995; Bus et al., 2019; Guarnieri et al., 2020) that may include inadequate milk management (e.g. large feedings), cold milk, poor milk replacers, poor hygiene, stress, mineral deficiencies such as copper, hair balls and infection. Possible microorganisms are Clostridium perfringens, Sarcina spp., Escherichia coli, Lactobacillus sp., Campylobacter sp., Aspergillus and zygomycosis. Bloat that occurs especially in ruminal drinkers (Simpson et al., 2018; Bus et al., 2019). This last condition is related to the absence or incomplete oesophageal groove reflex so that milk flows partially or completely to the undeveloped rumen where it ferments and eventually rots. It often results from inadequate milk feeding (e.g. excessive volume, inadequate calf head position when sucking, milk replacers' low quality) (Stocker et al., 1999). These conditions may cause severe visceral pain (colic), depression, ill thrift and death (Stocker et al., 1999; Van Metre et al., 2008; Simpson et al., 2018).
Calves with little access to fibre may show rumen under‐development, presence of rumen plaques and hyperkeratinisation, as well as abomasal lesions (ulcers), mainly in the pyloric region (Brščić et al., 2011a,b; Bus et al., 2019). This is most probably due to ruminal acidosis that results from very high ruminal volatile fatty acids (VFAs) and lactic acid concentrations. Calves exposed to a very high concentrate/roughage ratio diet (42.7% starch, 15.1% NDF vs 35.3% starch, 25.3% NDF) exhibited signs of poor rumen health similar to what happens in adult cattle including reduced appetite, reduced growth, altered feeding rate, chronic bloat and rumen epithelial lesions (Gelsinger et al., 2020). Decrease in ruminal pH has also been associated with immunosuppression and inflammation (Kleen et al., 2003; Gozho et al., 2005) moderate to severe depression and eventually death by asphyxiation.
3.2.8.2. ABMs
While most of the ABMs presented in Table 8 are not sensitive to detect gastroenteric disorders when used separately, their sensitivity increases when used in combination.
Table 8.
ABMs of gastroenteric (GE) disorders in calves kept in individual housing
| ABM | Comments |
|---|---|
| Diarrhoea |
Definition: Stool on the floor or on the bedding material. Character and colour of the faeces vary but are usually voluminous, watery or very loose, white, yellow or greenish. Watery faeces may also cover the tail, perineum and hind legs (Van Metre et al., 2008). Sensitivity: High. Specificity: High. |
| Hair loss in the perineum and hind legs |
Definition: Small to extensive areas without any hair are seen where diarrhoea caused the death of hair follicles (Van Metre et al., 2008). Sensitivity: Low. Not all calves experiencing GE disorders will show hair loss in the perineum and hind legs. Specificity: High. |
| Mortality > 1% |
Definition: Mortality due to Neonatal Diarrhoea above 1% of all calves born alive, should trigger careful investigation (Van Metre et al., 2008). Sensitivity: Low. Farms with GE problems may have low mortality due to good disease management. Specificity: Low. Mortality can also be due to other problems (e.g. respiratory disorders). |
| Bloat |
Definition: A calf with an obvious distended and tensed belly, more convex than the shape of the ribs (Panciera et al., 2007; Marshall, 2009). Sensitivity: High. Specificity: Low. There are other causes for bloat that are not related to ruminal drinking (e.g. chronic respiratory disease). |
3.2.8.3. Hazards
For NCD:
Poor colostrum quality, or poor colostrum management (Berge et al., 2009; Naylor, 2009; Hammon et al., 2020)
Poor hygiene in maternity pen, calf box and bucket/teats (Van Metre et al., 2008; Pithua et al., 2009; Klein‐Jöbstl et al., 2014; Medrano‐Galarza et al., 2018; Mohammed et al., 2019)
Poor biosecurity (Frank and Kaneene, 1993), including presence of other animal species on the farm (Klein‐Jöbstl et al., 2014)
Calf stocking density (Bendali et al., 1999)
Inadequate positioned or conceived bucket or teat
Poor quality milk replacers.
3.2.8.4. Preventive and corrective measures
Gastroenteric disorders can be prevented by good colostrum management. The ingestion of 10–12% of body weight of good quality colostrum up to 6 h after birth will ensure adequate immunity transfer (Cortese, 2009; Godden et al., 2019). Verification of plasma immunity in calves not artificially fed will allow to identify calves needing further administration of colostrum. In addition, vaccinating cows at the end of gestation against neonatal diarrhoea agents will increase the concentration of specific immunoglobulins, thus ensuring better protection (Crouch et al., 2001; Van Metre et al., 2008; Meganck et al., 2015).
Further important measures to prevent GE disorders in very young calves include removing bed material and thoroughly washing and disinfecting pens in‐between calves, ensuring strict biosecurity measures in calf area (Van Metre et al., 2008; Olson et al., 2019) including access of other animals (e.g. dogs, pigeons) and of outside people (Klein‐Jöbstl et al., 2014). Providing fresh and good quality water also prevents contamination of calves with agents such as E. coli and Cryptosporidium. Routinely (twice daily) monitoring of calves will increase early detection of sick animals, prompt treatment and reduction in the risk of outbreaks of the disease. Correction of GE disorders involves provision of appropriate clinical care (Meganck et al., 2014).
3.2.9. Welfare consequence ‘Respiratory disorders’
3.2.9.1. Description
Respiratory disorders are defined as negative affective states such as discomfort, pain, air hunger and/or distress due to impaired function or lesion of the lungs or airways. In pre‐weaned calves, respiratory disorders are second to gastroenteric disorder in terms of morbidity and mortality.
An important type of respiratory disorder is bovine respiratory disease (BRD). BRD is a complex of lower respiratory pathological states and is a common cause of mortality in dairy calves before weaning. BRD is a multifactorial disorder caused by bacteria (such as Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis) with possible involvement of viruses (such as bovine respiratory syncytial virus (BRSV), BoHV‐1 (IBR), PI3, coronaviruses and bovine viral diarrhoea virus (BVD) (Woolums and Step, 2020). It has been demonstrated that stress significantly alters the viral‐bacterial synergy resulting in fatal BRD (Hodgson et al., 2005; Taylor et al., 2010). Clinical signs of BRD may vary from mild or even unapparent to very severe symptoms. When lately diagnosed or mistreated, BRD can result in chronic pneumonia which can lead to suffering. Death can occur after several days but may also be sudden. This condition is much more common in calves housed in groups than in calves housed in individual pens (see Section 3.16.1, Specific Scenario 1).
Other respiratory disorders affecting young dairy calves, are sporadic or accidental in nature. These include pharynx or larynx trauma after incorrect intubation for colostrum, milk or electrolyte provision, oral necrobacillosis and diphtheria, traumatised ribs after dystocia, aspiration pneumonia.
3.2.9.2. ABMs
A combination of ABMs can be useful to detect respiratory disorders. The Universities of Wisconsin and California created a scoring system using five clinical signs (cough, altered breathing, nasal discharge, eye discharge, ear position and increased rectal temperature) that allows for a more practical and early detection of BRD and have increased sensitivity and specificity compared with use of single ABMs. In the scoring system described by (Svensson and Liberg, 2006), clinical respiratory‐tract disease (CRTD) was defined as either coughing or sneezing for more than 2 days, as severely increased respiratory sounds at lung auscultation or as moderately increased respiratory sounds together with other clinical signs such as coughing, sneezing or nasal discharge. Lung auscultation findings were scored as normal respiratory sounds or as mildly, moderately or severely increased respiratory sound. When used in combination, the overall sensitivity and specificity of the ABMs to diagnose respiratory disease increase.
The clinical signs and most important ABM are common to all husbandry systems and are presented in detail in Table 9.
Table 9.
ABMs of respiratory disorders in individual pens in dairy farms
| ABM | Comments |
|---|---|
| Rectal Temperature |
Definition: Rectal temperature above 39.7°C is considered a sign of infection. Sensitivity: High. Sensitivity of high rectal temperature to diagnose respiratory disease is considered to be high in acute cases. Specificity: Low. Other health disorders in calves of the same age may result in high temperature. |
| Respiratory sounds at lung auscultation* |
Definition: Increased respiratory sounds at lung auscultation. Sensitivity: Low. Upper respiratory disease may not always cause increased respiratory sounds Specificity: High. |
| Coughing* |
Definition: Brisk expel of air from the lungs by sudden contraction of the diaphragm and intercostal muscles in response to irritation of the lower respiratory tract. Sensitivity: High in acute cases of BRD. Specificity: High. |
| Nasal discharge* |
Definition: Clearly visible flow from the nostril. Can be transparent, yellow or green (Welfare quality® protocol cattle). Upper and lower respiratory tract infection will cause an increase in mucous or purulent discharge. Sensitivity: Low. Animals with respiratory disease do not always show nasal discharge. Specificity: High. |
| Ocular discharge* |
Definition: Usually bilateral mucous or purulent discharge, because some BRD infectious agents will also cause conjunctivitis. Sensitivity: High. Specificity: Low. Also seen in eye diseases and in dusty environments. |
| Droopy ears* |
Definition: Droopy and sometimes asymmetrical‐positioned ears (additionally, some of the BRD agents will cause otitis). Sensitivity: Low. Not all calves with respiratory disease will show droopy ears. Specificity: Low. Calves with conditions causing apathy, dullness and poor overall condition can also show droopy ears. |
ABMs included in scoring systems for detection of bovine respiratory disorders.
3.2.9.3. Hazards
The hazards for BRD are multifactorial (host, management, environment) but all these factors can occur simultaneously.
Poor colostrum management at the farm of origin increases BRD prevalence even after weaning (Stilwell and Carvalho, 2011; Pardon et al., 2015). There is some evidence that male dairy calves that will be fattened for veal receive poorer colostrum compared with females (Fecteau et al., 2002; Renaud et al., 2020; Reed et al., 2022).
Provision of non‐pasteurised waste milk (Stabel et al., 2004).
Stressful events, such as separation from dam, transport (EFSA AHAW Panel, 2022b) poor handling, mutilations and weaning (Hodgson et al., 2005; Taylor et al., 2010).
Environmental conditions, such as poor ventilation leading to high concentrations of ammonia (> 20 ppm) and other noxious gases, or high temperatures (Carroll and Forsberg, 2007). Calves reared outside typically develop less respiratory disease (Earley et al., 2004), and show a more active immune response (Cobb et al., 2014), compared with those kept indoors.
The quality of bedding is also an important hazard as moist bedding will lead to higher concentration of gases and will reduce calf comfort (Lago et al., 2006; Gorden and Plummer, 2010).
Keeping calf's pens close to adult or older cattle is a hazard for BRD in young calves (Gorden and Plummer, 2010).
3.2.9.4. Preventive and corrective measures
Good colostrum management (time of provision, quantity and quality) is important to prevent BRD. Calves should ingest 10–12% of body weight of good quality colostrum up to 6 h after birth to ensure adequate immunity transfer. Failed transfer of passive immunity (FTPI) remains a widespread problem in dairy farms and female calves are often given more attention than male calves (Boyle and Mee, 2021). Colostrum antibodies may protect calves for long periods (up to 6 months) against some virus (BVDV and IBR); however, they will have a short half‐life for other pathogens such as BRD bacteria, BRSV and PI3 (Fulton et al., 2004; Prado et al., 2006; Chamorro et al., 2014). This can be mitigated by the use of intra‐nasal vaccination against BRSV and PI3 in very young calves (Masset et al., 2020; Sandelin et al., 2020). Appropriate feeding of calves is another key aspect. Pasteurisation of waste milk has been shown to effectively reduce pathogenic bacteria associated with respiratory disease (Stabel et al., 2004). In addition, vaccination of dams against respiratory viruses and proper navel disinfection after birth reduces BRD prevalence throughout the first months of life. Another preventive measure is the avoidance of stressful events (see stress causes in the hazards section above), or at least of the simultaneous occurrence of these. If possible, avoid mutilation procedures, such as castration and disbudding. Provide training to stockpersons to reduce stress induced by handling, good practices at weaning and avoiding early weaning may also reduce BRD prevalence (Losinger and Heinrichs, 1996; Bach et al., 2010).
Ensuring proper ventilation and adequate temperature‐humidity index is another important preventive measure. Good ventilation without exposing animals to too high or too low temperatures and to drafts, and isolating calves showing clinical signs of BRD, may be the key elements in preventing respiratory disease (Hillman et al., 1992; Lago et al., 2006). Individual calf hutches should be situated where weather effects are minimal and away from features that can contaminate the calf's environment, such as building exhaust fan vents, manure or runoff from other farm buildings (Gorden and Plummer, 2010; Taylor et al., 2010; Engelken, 2020). When possible, calves should be kept outside in sheltered hutches as those reared indoors commonly develop more severe respiratory disease (Earley et al., 2004; Lorenz et al., 2011).
When prevention is not effective and calves develop BRD, antimicrobials are still a crucial tool to limit the impact of the disease and increase the chances of survival of diseased calves. Judicious, early and competent use of antimicrobials by veterinarians should be guaranteed for calves affected by BRD. Rational use of non‐steroidal‐anti‐inflammatory drugs (NSAID) is essential when treating acute cases to reduce life‐threatening lung inflammation. These drugs may also be beneficial because of the anti‐pyretic and analgesic activity. However, reducing inflammation is not always desirable and the adverse effects of NSAIDs (e.g. ulcerogenic potential) should be taken into account. Metaphylactic antimicrobial treatment of calves when there is an outbreak of BRD may reduce morbidity and mortality (Frank et al., 2000; Cusack, 2004). It is recommended to treat all animals when ≥ 10% of a group are found to be affected. However, veterinarians should carefully evaluate the effectiveness of this practice, as it may lead to increased antimicrobial resistance (Gorden and Plummer, 2010).
3.3. Welfare of veal calves kept in individual housing (i.e. so‐called baby boxes)
3.3.1. Description of the system
Calves fattened for veal are transported to the veal farm at ~ 14–35 days of age (for details on the impact on welfare of transport of unweaned calves please refer to EFSA AHAW Panel, 2022b). At arrival, calves are placed in individual pens within a large pen, using removable barriers made from tubular metal bars (Figures 5 and 6). These individual pens must by law at least be as wide as the height of the calf at the withers and as long as the length of the calf multiplied by 1.1, according to the Council Directive 2008/119/EC. This phase may last between 3 and 6 weeks.
Figure 5.
Schematic representation of individual pens in a veal farm
Figure 6.

Calves kept in an individual pen in a white veal farm. © Marta Brščić
Visual and tactile contact with other calves is possible with neighbouring calves through and over the barrier and/or trough/bucket. According to the different features of the group pens, most of the calves are positioned in individual pens in the front facing the manger corridor, whereas ~ 20% of the calves are positioned in individual pens in the back. Towards the end of the individual pen phase, these calves are larger and physical head‐to‐head contact is no longer possible neither with the pen mates nor the calves from the adjacent pens, because the bucket is placed in front of them thereby limiting any access to other calves. Calves are typically fed twice a day milk replacer in open buckets, sometimes with floating teats, and provided with solid feed (see Section 3.1.1 for a description of feeding practices). This system is in place to enable easy health checks and minimise the spread of diseases from mixing calves from various locations. The floor tends to be the same as the floor of the larger pen (see sections below) and hence comprises most often of wooden or concrete slatted floors, which are occasionally covered in rubber, while not blocking the gaps between the slats.
The most relevant welfare consequences identified for this system were restriction of movement, isolation stress, inability to perform play behaviour, inability to perform sucking behaviour, inability to perform exploratory or foraging behaviour, respiratory disorders, gastroenteric disorders and resting problems.
3.3.2. Welfare consequence ‘Restriction of movement’
3.3.2.1. Description
Please refer to Section 3.2.2.1 for a description of this welfare consequence in calves kept in individual housing in dairy farms. Individual pens in veal farms usually have a very small area (~ 1.8 m2) and hence cause severe restriction of movement. Activities such as walking, running, jumping and self‐grooming are not possible, neither is adoption of relaxed lying postures (i.e. lateral recumbency – lying on the flank with legs extended). At the end of the individual housing period, calves cannot turn around anymore, due to the limited space allowance. In addition, the floor of these pens usually does not have bedding, which can provoke calves to slip due to lack of good grip, thereby constituting another type of restriction of movement.
3.3.2.2. ABMs
See Section 3.2.2.2 (calves in individual pens in dairy farms).
3.3.2.3. Hazards
The same hazards as individual pens in dairy farms (Section 3.2.2.3) apply in this system.
3.3.2.4. Preventive and corrective measures
See Section 3.2.2.4 for further information on prevention of restriction of movement. Additional preventive measures include increasing space allowances and provision of bedding on a solid floor. For a specific assessment of the risks of limited space allowance and recommendations on this, see Section 3.16.2.
3.3.3. Welfare consequence ‘Isolation stress’
3.3.3.1. Description
Calves are social animals that learn their social role in hierarchical groups early and develop relationships with their dam, other calves and adults from birth (Raussi et al., 2010). Isolation impairs their cognitive and learning abilities and the quality of their social interactions throughout their lives. Isolation also reduces their ability to cope with novelty resulting in fear and calves being less prone to explore the environment. Calf growth may also be impaired leading to lower weight gain (see Section 3.2.3.1 for more details).
While most of the calves in the veal sector are not fully isolated because they can see and touch other calves through the metal bars of the individual pens, calves positioned in the back sometimes cannot interact except for having access to the rear part of the pen mates (Figure 5). This is especially frequent towards the end of this phase. For a detailed description of impacts on welfare of individual housing as opposed to group housing, see Section 3.16.1.
3.3.3.2. ABMs
For a list of ABMs of isolation stress, see Section 3.2.3.2.
3.3.3.3. Hazards
Individual housing
Narrow size and position of openings between individual pens preventing contact between calves.
3.3.3.4. Preventive and corrective measures
Group housing with no use of individual pens and direct inclusion of calves in pairs or small groups of pen mates of the same age and size would allow all calves the possibility to interact with the calves in their own pen and with those in adjacent pen(s) through the metal bars. This would allow an opportunity for all calves to establish their role in the social group and would omit the frustration due to the impossibility of behaving in an allelomimetic way for the calves positioned in the baby boxes in the back of the pens. Please refer to Section 3.16 (Specific Scenario 1) for further recommendations on group housing for veal calves.
3.3.4. Welfare consequence ‘Inability to perform play behaviour’
3.3.4.1. Description
See Section 3.2.5.1 for a description of this welfare consequence. Individual pens in veal farms limit space and social contact, and may involve a slippery floor surface, such as wooden slatted floors. These characteristics limit play behaviour, particularly locomotor and social play. Only few aspects of locomotor play are possible in small individual pens and social play is prevented (Jensen et al., 1998). The calves may be motivated, but unable to perform locomotor play and thus deprived of potential positive emotions while performing play behaviour.
3.3.4.2. ABMs
There are no available direct measures of an inability to display play behaviours (see Section 3.2.5.2). Play behaviours can be monitored in individual pens and compared in duration and frequency with the play behaviours of calves in loose housing and/or with a non‐slip surface. If calves are prevented from performing locomotor behaviour, this may result in a rebound of the behaviour when the calves are released in a larger area (Mintline et al., 2012).
3.3.4.3. Hazards
See Section 3.2.5.3 (calves in individual pens in dairy farms) for a list of hazards for inability to perform play behaviour.
3.3.4.4. Preventive and corrective measures
See preventive measures in Section 3.2.5.4.
3.3.5. Welfare consequence ‘Inability to perform sucking behaviour’
3.3.5.1. Description
Calves have a high motivation to suck in connection with milk intake as described in Section 3.2.4. Veal calves in individual pens are typically fed milk out of an open bucket. While milk feeding via a teat provides an outlet for the motivation to suck, milk feeding in open buckets increases the risk of calves directing their non‐nutritive sucking towards pen fixtures, or the head and neck of neighbouring calves (cross‐sucking; Lidfors and Jensen, 2003). This behaviour is redirected and thus an abnormal behaviour. If the calves show gastroenteric issues or have trouble drinking, the farmer may provide the calves with floating teats. These floating teats do provide the opportunity to suck milk, but do not allow the natural position of the calf head during milk ingestion, thereby potentially limiting the oesophageal reflex and thus facilitate ruminal drinking (Jones and Heinrichs, online).
3.3.5.2. ABMs
See Section 3.2.4.2 (calves in individual pens in dairy farms).
3.3.5.3. Hazards
Offering milk in open buckets (Mounier et al., 2006), or a trough
Absence of dry teats (rubber teats) to which sucking behaviour can be directed.
3.3.5.4. Preventive and corrective measures
See Section 3.2.4.4.
3.3.6. Welfare consequence ‘Inability to perform exploratory or foraging behaviour’
3.3.6.1. Description
See Section 3.2.6.1 (calves in individual pens in dairy farms) for a general description of inability to perform exploratory or foraging behaviour. When in individual pens, calves are housed in a barren environment with limited space. They receive some sort of chopped or pelleted solid feed, which does allow for some foraging. The provision of hay to calves was shown to induce natural feeding behaviours, particularly rumination and chewing, and to reduce abnormal behaviours such as tongue flicks, oral manipulation of pen fixtures and self‐grooming (Downey et al., 2022). These calves have limited possibility for exploration due to the barren nature of the environment and cannot forage on pasture or graze, thereby limiting their foraging opportunities.
3.3.6.2. ABMs
See Section 3.2.6.2.
3.3.6.3. Hazards
See Section 3.2.6.3.
3.3.6.4. Preventive and corrective measures
See Section 3.2.6.4.
3.3.7. Welfare consequence ‘Resting problems’
3.3.7.1. Description
Calves need to rest and sleep in order to recover. They use several postures which include one in which they rest the head on the legs and another in which the legs are fully stretched out (De Wilt, 1985; Ketelaar‐de Lauwere and Smits, 1989; Ketelaar de Lauwere and Smits, 1991). Sleep disruption may occur if comfortable lying positions cannot be adopted or if there is disturbance to lying animals because they are trodden on or otherwise disturbed by other calves. Young calves rest for much of the day, mainly while lying on the sternum: this occupies ~ 50% of the day during the first 3 months of age (Hänninen et al., 2005). Some data suggest that inadequate lying times reduce growth (Mogensen et al., 1997; Hänninen et al., 2005). Lying behaviour also has been used as indicator of how well calves adjust to new housing (Veissier et al., 1989; von Keyserlingk et al., 2011).
Little time is spent resting while lying on the side; time in this occupies 1–2% of the day during the first 3 months (Le Neindre, 1993; Hänninen et al., 2005). The function of different resting postures is not clear. A thermoregulatory function may be involved as lying on the side increases the exposure of the body surface to the atmosphere and may increase heat loss (Redbo et al., 1996; Hänninen et al., 2005) and time spent resting on the side by unweaned calves is shorter on cool or draughty floors (Hänninen et al., 2005). In cold or cool environments, calves also choose to rest curled up in order to reduce heat loss through conduction (Redbo et al., 1996; Hänninen et al., 2005). In addition, the available space (Le Neindre, 1993) and the degree of softness of the floor have effect on resting behaviour (Camiloti et al., 2012). In all veal systems, the slatted flooring, often made of wood, may restrict the amount of time spent resting or the resting postures, if it is wet, cold, with draught. In individual pens in particular, as described in the section on restriction of movement (Section 3.3.2.1), certain lying positions may be hindered (e.g. lying on the flank with legs extended), veal calves in crates spent more time lying with all legs bent (Andrighetto et al., 1999).
3.3.7.2. ABMs
ABMs of resting problems in this system are presented in Table 10.
Table 10.
ABMs of resting problems in calves housed in individual pens in veal farms
| ABM | Comments |
|---|---|
| Number of lying bouts |
Definition: Count of lying bouts. A high number of lying bouts suggests interrupted resting. Sensitivity: High. Specificity: Low. High number of lying bouts may be due to other causes, for instance, interruptions resultant from group stress. |
| Time spent standing |
Definition: Number of minutes standing (Bokkers and Koene, 2001). A long time standing in the individual pen suggests resting problems. Sensitivity: Low. Not all animals that experience resting problems will show prolonged standing. Specificity: Low. Hunger or group stress may lead to extending periods of standing. |
| Time spent in lateral recumbency |
Definition: Lying with legs extended (relaxed posture) (Ketelaar de Lauwere and Smits, 1991; Færevik et al., 2008). Lack of adoption of this type of posture suggests resting problems. Sensitivity: High. Specificity: Low. Calves may not lie down in a relaxed posture due to cold stress issues. |
3.3.7.3. Hazards
Slatted floor of wood or concrete
Wet floor
Low space allowance per animal
Low or high temperature.
3.3.7.4. Preventive and corrective measures
Rubber on slats, or better, provision of bedding prevents resting problems: straw and rubber flooring solutions offer better thermal comfort and avoid potential health risks for calves exposed to a cold environment (Hänninen et al., 2005; Brščić et al., 2012). Dry bedding is likely to be important for calves because it can reduce heat loss through conduction, helping the animals to cope with cold environments. The lower critical temperature for young calves is 18°C when they lie down on concrete versus 6°C when they lie down on deep dry straw (Wathes et al., 1983; Webster et al., 1985). When calves were free to lie down on either kiln‐dried wood bedding or bare concrete, they never chose the latter (Camiloti et al., 2012).
In addition, large pens permit to adopt resting postures with one or more legs outstretched which are considered more natural and comfortable (Ketelaar de Lauwere and Smits, 1991). A large pen can allow the lateral recumbency with legs extended, which increases the exposure of the body surface to the atmosphere and may increase heat loss (Redbo et al., 1996; Hänninen et al., 2005). Another measure to reduce cold stress, calves may benefit during the winter months from paired or group housing because they can rest next to each other in physical contact to improve thermoregulation (Hänninen et al., 2005; Hepola et al., 2006). Another measure is that air temperature and humidity should be appropriate to give calves suitable thermal comfort. For this reason, dedicated cooling systems are necessary, especially during summer in hot and humid climates (Cozzi et al., 2009).
3.3.8. Welfare consequence ‘Respiratory disorders’
3.3.8.1. Description
See Section 3.2.9.1 for a detailed description of respiratory disorders. This condition is relevant in veal farms because calves arrive on veal farms coming from many dairy farms and carrying infectious agents against which other calves will have no immunity (Autio et al., 2007). Grouping these animals of different origins, following the challenge of transport which leads to poor resilience and heightened vulnerability, may promote severe outbreaks of the disease (see EFSA's scientific opinion on the transport of cattle for more details – EFSA AHAW Panel, 2022b)).
3.3.8.2. ABMs
The clinical signs and most important ABMs are common to all systems and are presented in detail in Section 3.2.9.2.
3.3.8.3. Hazards
See Section 3.2.9.3 for hazards of respiratory disorders in calves. Hazards specific to veal calves are:
Transport from many different dairy farms, often going through auctions, plus loading and unloading, on‐vehicle‐stocking density and commingling (Trunkfield and Broom, 1990; Bernardini et al., 2012; Hulbert and Moisá, 2016; Masmeijer et al., 2021; EFSA AHAW Panel, 2022b).
Long distance transport, poor appetite after arrival and diet changes will also cause a reduction in bodyweight (~ 10%), further compromising the immune system (Marcato et al., 2020; EFSA AHAW Panel, 2022b).
Size (determined by age and weight) of calves when transported to fattening units is also a hazard for BRD, with lighter animals being more prone to pneumonia (Brščić et al., 2012; Stilwell et al., 2013; Winder et al., 2016; Santman‐Berends et al., 2018).
Poor air quality in the veal farm may occur in completely closed barns with fully slatted floors (Cozzi et al., 2009) but air quality depends on the ventilation system. A threshold of 10 ppm ammonia was recommended for cattle by the Scientific Committee on Animal Health and Animal Welfare (SCAHAW, 2001) and adopted by the Swedish animal welfare legislation (Lundborg et al., 2005). A more recent paper confirmed an increased antimicrobial treatment incidence when ammonia concentrations were > 10 ppm (Schnyder et al., 2019), but a previous study reported a lower risk of respiratory disease with ammonia concentrations < 6 ppm (Lundborg et al., 2005). A more recent paper confirmed an increased antimicrobial treatment incidence when ammonia concentrations were > 10 ppm (Schnyder et al., 2019), but a previous study reported a lower risk of respiratory disease with ammonia concentrations < 6 ppm (Lundborg et al., 2005). Here, 20% of farms had at least one ammonia measure exceeding the recommended level of 10 ppm (Brščić et al., 2010) and the highest ammonia concentrations recorded in veal calf's barns reached the maximum value of 15 ppm, comparable to the level recorded in group housed calves on slats in the Netherlands (Koerkamp et al., 1998).
Poor ventilation, high concentration of noxious gases and high THI.
3.3.8.4. Preventive and corrective measures
See Section 3.2.9.4 for general measures to prevent the disease in calves. Preventive measures specific for veal systems include avoiding, or reducing as much as possible, long journeys (maximum 8 h long) and multiple loading and unloading (for a detailed assessment of welfare of unweaned calves during transport, please refer to the EFSA scientific opinion on this topic; EFSA AHAW Panel, 2022b). In addition, promote management to keep together animals from same farm of origin. Measures should be taken to ensure that both female and male dairy calves receive high‐quality colostrum on time at the farm of origin. In addition, ensure good ventilation and monitor regularly noxious gases concentration in closed barns.
3.3.9. Welfare consequence ‘Gastroenteric disorders’
3.3.9.1. Description
Diarrhoea is a common gastroenteric disorder observed in veal calves in this system and it is an important cause of morbidity and mortality at this age. In a survey with 4,825 veal calves in Canada, mortality was related to clinical signs such as high dehydration score, diarrhoea, sunken flanks and navel infection at arrival at the veal farm (Renaud et al., 2018). Research suggests that major stressful events faced by calves during the transition from the dairy to the veal farm, such as transport to the veal farm and dietary and environmental changes, seem to cause impaired immune function and loss of gut barrier function, resulting in enteritis (reviewed by Timmerman et al., 2005; and by EFSA AHAW Panel (2022b)). A recent study has confirmed that transport‐related hazards have an impact on short‐term veal calf diarrhoea, but such transport‐related effects on calf health were not observed in the long run (Marcato et al., 2020).
White veal calves also tend to develop gastroenteric disorders that are not clinically evident in vivo but are retrospectively found at slaughter. Up to 70% of veal calves slaughtered showed abomasal lesions alterations as post‐mortem findings (reviewed by Bus et al., 2019). Although the whole abomasum may be affected by lesions of different severity, lesions in the pyloric area are prevalent (Bähler et al., 2010). Proposed factors involved in the aetiology of abomasal lesions include the abrasive coarse fibre and hairballs in the abomasum (reviewed by Bus et al. (2019)). This review proposed two explanations behind abomasal lesions; one linked with abomasal overloading due to fast drinking of milk, and another coupled to low abomasal pH (Bus et al., 2019). It is possible that several factors interact and play a role in the development of the lesions, with stress also being involved (Bus et al., 2019), but the exact mechanisms are not well understood.
Other gastroenteric disorders affecting white veal calves include rumen under‐development (limited number of papillae in atrium and ventral and dorsal rumen), presence of rumen plaques (multiple patches with coalescing papillae covered by a sticky mass and hair) and rumen hyperkeratinisation (hardened rumen papillae) (Brščić et al., 2011a,b; Bus et al., 2019). For a general description of gastroenteric disorders in young calves, see also Section 3.2.8.1.
3.3.9.2. ABMs
Relevant ABMs of gastroenteric disorders in veal calves kept in small groups are presented in Table 11. In addition, other relevant ABMs include diarrhoea, bloat (as an ABM of ruminal drinking), hair loss in the perineum and on hind legs and increased mortality (see Section 3.2.8.2. These ABMs are not sensitive when used in isolation, but their sensitivity increases when used in combination.
Table 11.
ABM of gastroenteric disorders in veal calves kept in small groups. The assessment of abomasal lesions, ruminal plaques and rumen underdevelopment can only be carried post‐mortem at abattoir level
| ABM | Comments |
|---|---|
| Abomasal lesions |
Definition: Presence of lesions in the abomasum (torus pylorus, pyloric area), classified according to size and severity (for a description of scoring systems of abomasal lesions, see Section 3.17.4.3). Sensitivity: Low. Not all calves with GE disorders will show abomasal lesions. Specificity: High. |
| Ruminal plaques |
Definition: Presence of plaques (multiple patches with coalescing papillae covered by a sticky mass and hair), hyperkeratosis (hardened rumen papillae) and underdeveloped rumen mucosa (almost no papillae in atrium and ventral and dorsal rumen) (Suárez et al., 2006). Sensitivity: Low. Not all calves with GE disorders will show ruminal plaques. Specificity: High. Ruminal plaques are a specific indicator of low‐quality fibre. |
| Rumen under‐development |
Definition: Small, underdeveloped rumen mucosa with almost no papillae in atrium and ventral and dorsal areas (Suárez et al., 2006; Brščić et al., 2011a,b). Sensitivity: Low. Not all calves with GE disorders will show ruminal plaques. Specificity: High. |
3.3.9.3. Hazards
The exact mechanisms behind abomasal ulcers and erosions are not yet fully understood (Bus et al., 2019). The presence for abomasitis and other pathological conditions affecting the abomasum of calves (Brščić et al., 2011a,b; Webb et al., 2013) seem to result from the simultaneous action of different factors:
Milk replacer‐only diets (although this is no longer practised in the EU)
Abomasal overloading
Coarse roughage
Little water provision
Stressful events, such as transport to the veal farm (Bus et al., 2019; Marcato et al., 2020)
Low frequency of large milk meals combined with little structure in the solid feed (Bus et al., 2019).
Hazards for ruminal diseases (parakeratosis and plaques) include:
concentrate diets with small particle size and low abrasive value (Brščić et al., 2011a,b)
high concentrate/fibre ratio (Laarman and Oba, 2011).
For hazards of diarrhoea, see Section 3.2.8.3.
3.3.9.4. Preventive and corrective measures
Provision of lower amounts of milk fed more frequently has been proposed as a preventive measure for abomasal lesions (Bus et al., 2019). Abomasal lesions and poor rumen development tend to be linked to each other: better rumen development protects to some extent against abomasal lesions due to minimisation of entry of undigested fibres in the abomasum (Bus et al., 2019).
3.4. Welfare of veal calves kept in group housing in small groups with milk feeding by bucket/trough
3.4.1. Description of the system
This system is typically used to rear calves for ‘white veal’ production. Following the individual pen phase (Section 3.3), calves are released into group pens holding typically 5–7 calves, but in some instances up to 10 animals are kept together. In group pens, full social contact with pen mates is possible (Figures 7, 8, 9–7, 8, 9). Calves are kept in this system from 4–7 weeks of age until slaughter at 21–28 weeks. In France, holdings tend to have shorter cycles (160–165 days) in France compared with Italy, Germany and the Netherlands (190–200 days).
Figure 7.
Schematic representation of calves in small‐group pens in a veal farm
Figure 8.

Calves housed in a small‐group pen (5–7 animals) with slatted floors and milk trough in a veal unit. © JUNIA – France
Figure 9.

Calves housed in small groups in a veal unit. © JUNIA – France
Calves reared for veal are typically provided with the minimum EU space allowance per animal (i.e. 1.8 m2 per calf) and housed on slatted floors made of wood or very rarely concrete, though rubber flooring on top of wooden or concrete slats is also used on some farms. No enrichment is provided.
Because the white veal industry aims for meat of a pale colour, calves in this system are not weaned off milk and are fed limited amounts of solid feed (and hence limited iron) to keep the meat colour light, which can result in consequences for calf's welfare (see Section 3.4.9). Solid feed is usually comprised of concentrate mixed with chopped straw and/or maize silage, with feed being harvested and processed specifically to minimise iron content (e.g. harvesting maize higher above the ground). In recent years, the amount of solid feed has increased (mostly more than 200 kg and sometimes up to 400–500 kg solid feed per cycle) and the amount of milk replacer has decreased. Haemoglobin (Hb) levels are usually controlled two to three times throughout the fattening period by venepuncture (of jugular, tail or ear) and calves with Hb lower than 4.5 mmol/L (7.25 g/dL) are required to be provided with iron via injection.
The level of automation of the veal units varies, with some producers opting for the automation of the filling of the troughs for milk distribution, others using non‐automatised (hand filling with a hose) troughs and others using buckets to feed calves. The bucket‐feeding system is the more labour‐intensive, with calves being fed individually their portion of milk. Units using common troughs tend to regroup the calves more often (e.g. every 1 or 2 weeks), depending on the calf's drinking speed and body weight, to achieve a homogeneous group in terms of weight and thus reduced drinking competition. Frequent regrouping of calves may lead to higher group stress. There is an indication that veal farms are working on reducing this practice, but it is unknown whether this is true for independent veal farmers.
3.4.2. Welfare consequence ‘Inability to perform exploratory or foraging behaviour’
3.4.2.1. Description
See Section 3.3.6.1 for a description of this welfare consequence. The situation in this system is similar to that described for veal calves in individual pens (Section 3.3.6.1): despite the pen being larger and access to pen mates is possible, the environment remains barren, and the feeding schedule remains the same.
3.4.2.2. ABMs
The ABMs are the same as for calves in in individual pens (Section 3.3.6.2), with the exception of tongue flicks, which are mostly observed in younger calves and are therefore less relevant in this age range.
3.4.2.3. Hazards
See Section 3.3.6.3 (veal calves in individual pens).
3.4.2.4. Preventive and corrective measures
See Section 3.3.6.4 (veal calves in individual pens).
3.4.3. Welfare consequence ‘Inability to perform sucking behaviour’
3.4.3.1. Description
As mentioned in Section 3.2.4.1, calves have a high motivation to suck in connection with milk intake. When calves cannot perform sucking behaviour in connection with ingestion of milk, they may re‐direct the sucking behaviour to pen fixtures and other calves. This abnormal behaviour, which is termed cross‐sucking, may be injurious to the calves that are sucked due to loss of hair and inflammation of sucked body parts (De Passillé, 2001; Lidfors, 2003). Cross‐sucking is most intense during the first 10–20 min after milk ingestion (Lidfors, 1993), but also occurs independently of milk intake (Nielsen et al., 2008a; Roth et al., 2009b), likely motivated by hunger. Some breeds are more prone to perform cross‐sucking; for instance, cross‐sucking was a larger problem on Simmental farms compared with Brown Swiss and Holstein Frisian farms (Rinnhofer and Fürst‐Waltl, 2008; Ural et al., 2021).
The inability to suck has also been related to the stereotypic behaviour tongue rolling in veal calves (Webb et al., 2015).
3.4.3.2. ABMs
3.4.3.3. Hazards
3.4.3.4. Preventive and corrective measures
See Section 3.2.8.4 (calves in individual housing in dairy farms) for general preventive measures of inability to perform sucking behaviour.
3.4.4. Welfare consequence ‘Inability to chew and ruminate’
3.4.4.1. Description
The inability to chew and ruminate is defined as negative affective states such as frustration experienced by the animal resulting from the thwarting of the motivation to ingest sufficient amounts of effective fibres.
Calves have a motivation to chew and ruminate. This is demonstrated by the work they are willing to perform for the opportunity to chew and ruminate (Webb et al., 2014b) and by the appearance of abnormal oral behaviours when the opportunity to chew and ruminate is too limited (Webb et al., 2012, 2013, 2015). Calves will select for structure in their diet when given the opportunity to do so (Webb et al., 2014a). In veal systems, the tendency was to provide large milk meals and small solid feed meals, which consisted for a large part of pelleted or concentrated feed, limiting the opportunity to chew and ruminate (Prevedello et al., 2012). Groups of calves fed in this manner develop repetitive, invariant and seemingly functionless (i.e. stereotypic) behaviours including licking and biting the pen structure, grooming the coat of other calves and rolling their tongue inside or outside of their mouths.
3.4.4.2. ABMs
ABMs of inability to chew and ruminate in this system are presented in Table 12.
Table 12.
ABMs of inability to chew and ruminate in veal calves kept in small groups
| ABM | Comments |
|---|---|
|
Non‐nutritive oral manipulation |
Definition: Licking, chewing or sucking directed towards bars etc. (modified after Downey et al., 2022). Sensitivity: Low. Specificity: Low, because non‐nutritive oral manipulation can also be due to inability to perform sucking behaviour. More relevant for foraging behaviour. Non‐nutritive oral manipulation can also be due to inability to perform sucking behaviour, prolonged hunger and inability to perform exploratory or foraging behaviour. |
| Tongue rolling |
Definition: Tongue is held in a full or partial circular position or moves in a full or partial circular motion; this can occur when the tongue is held within the mouth or extended outside it (adapted from Downey et al., 2022). Sensitivity: Low. Not all animals experiencing inability to forage or explore will show this behaviour. Specificity: High. Although it is more frequent in older calves, it can also occur in young calves (< 8 weeks of age). |
3.4.4.3. Hazards
Limited structure in the solid feed.
Limited amount of solid feed.
Low frequency of feeding.
3.4.4.4. Preventive and corrective measures
Measures to prevent inability to chew and ruminate are provision of ad libitum, ideally long, roughage in a rack to stimulate rumination and foraging behaviour.
3.4.5. Welfare consequence ‘Resting problems’
3.4.5.1. Description
See Section 3.3.7.1 (veal calves in individual pens).
3.4.5.2. ABMs
See Section 3.3.7.2 (veal calves in individual pens).
3.4.5.3. Hazards
See Section 3.3.7.3.
3.4.5.4. Preventive and corrective measures
See Section 3.3.7.4 (veal calves in individual pens).
3.4.6. Welfare consequence ‘Group stress’
3.4.6.1. Description
Calves mostly do not develop clear dominance relations in the first 3–4 months of life (Reinhardt, 1982; Canali et al., 1986), but show aggressive interactions in case of competition for resources already at the age of a few weeks (Canali et al., 1986; von Keyserlingk et al., 2004). Commingling (i.e. mixing unfamiliar calves) increases the level of displacements at the feeding place for 1–2 days with interactions mainly between unfamiliar calves, while familiar animals are more tolerant to each other (Bouissou et al., 2001; Færevik et al., 2007); see also below). Feeding and lying time is lower the day of commingling as compared with 1 week later (Færevik et al., 2010). Competition for access to milk can also reduce feeding time and milk intake of calves (von Keyserlingk et al., 2004) and lighter calves may be affected most. Drinking speed differs between calves and without restraint, quick drinkers may displace others from buckets or open troughs. In contrast to feed competition, the size of the lying area hardly affects the level of displacements in this area and is not increased after commingling (Færevik et al., 2007, 2008), but insufficient lying space reduces synchronicity of calf's behaviour (Færevik et al., 2008). Already in the first few weeks of life, calves develop social affiliative, preferential relationships, i.e. social bonds (Reinhardt, 1982; Duve and Jensen, 2011) which can be long‐lasting, up to several years (Reinhardt, 1982). Calves prefer proximity to familiar calves compared with unfamiliar calves and the presence of a familiar companion can reduce stress reactions in a challenging situation more than the presence of an unfamiliar calf (Færevik et al., 2006), which is in line with results from adult cattle and other species. Being with one or more peers, to whom a preferential relationship is developed, may help in coping with stress more generally. Repeated regrouping of calves preclude formation of or lead to disruption of social bonds and has some long‐term effects on calf behaviour and physiology: regular regrouping of pair‐housed calves increased behavioural reactivity to challenging situations (Boissy et al., 2001), led to some changes in daily rhythm of activity and an enhanced sensitivity of the adrenal gland to ACTH (Veissier et al., 2001). A higher level of aggression in the first hours after mixing as compared with stable pairs has also been reported; however, this effect decreased after several regroupings of calves (Veissier et al., 2001). Calves formerly kept individually reduced milk intake for 1 day after mixing into a group of three other calves (O'Driscoll et al., 2006). Negative welfare effects of regrouping are likely to be higher in groups of 5–8 calves as compared with pairs because the numbers of potential competitors and encounters are higher.
In veal calves kept in small groups, group stress can be caused by competition for access to feed as well as regrouping, performed in different frequency.
3.4.6.2. ABMs
Group stress can be assessed by direct observation of the number of aggressive interactions involving physical contact (head butts, pushing) and displacement by other calves during times of expected highest level of competition or density (Table 13).
Table 13.
ABMs of group stress in veal calves kept in small groups
| ABM | Comments |
|---|---|
| Aggressive interactions with physical contact |
Definition: Aggressive interactions involving direct, physical contact (head butts, pushing) with or without displacement of other calves during times of expected highest level of competition/density (Færevik et al., 2007; Færevik et al., 2008). Sensitivity: High. Specificity: High. |
|
Body condition score (BCS) |
Definition: Poor body condition (protruding bones, sharp ribs etc.). For estimation of BCS categories, the following criteria are taken into account (adapted from the Welfare Quality® protocol for calves): Calves should be assessed on the basis of the quantity of muscle, the estimated weight and physical appearance characteristics including: (i) visibility of the ribs, (ii) the extent to which the backbone protrudes, and (iii) the size of the belly. Calves are compared with the mean level of the batch. Severe lower condition: the calf is 30% below the average weight or condition of the batch. Sensitivity: Low. The calf will only show low body condition if group stress occurs over a long period of time and the animal is prevented from accessing feed sources over this period. Specificity: Low. Situations of prolonged hunger or chronic disease may also result in low weight gain. |
3.4.6.3. Hazards
Low space allowance in general and especially at trough.
Open trough and no individual feeding place during milk feeding (no fixation).
Repeated regrouping.
3.4.6.4. Preventive and corrective measures
Measures to prevent group stress in calves include provision of sufficient space for lying to enable synchronous resting (Færevik et al., 2008) and individual feeding places with a possibility to fixate calves during milk feeding to avoid competition for milk (e.g. von Keyserlingk et al., 2004). The latter measure also makes regrouping due to different speed of drinking milk unnecessary. Keeping groups stable will avoid disturbance of the group stability and hierarchy. However, regrouping may be appropriate in the case of very weak calves to avoid too high competition for milk.
3.4.7. Welfare consequence ‘Respiratory disorders’
3.4.7.1. Description
See Section 3.3.8.1 (veal calves in individual housing).
3.4.7.2. ABMs
See Section 3.2.9.2 (calves in individual housing in dairy farms).
3.4.7.3. Hazards
See Section 3.3.8.3 (veal calves in individual housing).
Overstocking (Woolums, 2013; Cobb et al., 2014) and large group sizes. Risk for BRD was significantly higher for AMF‐fed calves housed in groups of 10–30 calves/pen compared with manually fed calves housed individually (Svensson et al., 2003; Lundborg et al., 2005). Similarly, other studies have shown less morbidity and mortality associated with respiratory disease in groups of 3–8 calves/pen (Lundborg et al., 2005), < 7 calves (Losinger and Heinrichs, 1996) or < 10 calves (Losinger and Heinrichs, 1996). A review of group housing of dairy calves with different feeding systems concluded that group housing increases the risk of infection, especially in larger groups (Hepola, 2003). For a more detailed assessment of the relationship between group size and prevalence of respiratory disorders, please see Section 3.16.1.
Commingling of unfamiliar calves as well as regrouping familiar animals after a period of separation leads to an increase in aggressive interactions and probably group stress. Housing calves with a difference in age of more than 8 weeks together in the same group increases the risk of respiratory disorders (Gulliksen et al., 2009b).
In addition, the prevalence of both diarrhoea and respiratory disease was more than twice as high among calves in dynamic compared with stable groups (Pedersen et al., 2009; Gulliksen et al., 2009b).
3.4.7.4. Preventive and corrective measures
Limiting group size and avoiding overstocking has been shown to result in the best overall welfare for calves kept in groups (Svensson and Liberg, 2006; Gulliksen et al., 2009b; Torsein et al., 2011). In addition, commingling of calves from different farms should be avoided as much as possible. Calves should be kept in stable groups of similar age and size (Pedersen et al., 2009; Gulliksen et al., 2009b; Lorenz et al., 2011).
3.4.8. Welfare consequence ‘Gastroenteric disorders’
3.4.8.1. Description
See Section 3.3.9.1 (veal calves in individual pens) for information on gastroenteric disorders affecting veal calves.
3.4.8.2. ABMs
See Section 3.3.9.2 (veal calves in individual pens).
3.4.8.3. Hazards
See Section 3.3.9.3 (veal calves in individual pens).
3.4.8.4. Preventive and corrective measures
See Section 3.3.9.4 for preventive measures of diarrhoea, bloat and ruminal drinking in veal calves, and Section 3.2.8.4 for general preventive measures of gastroenteric disorders.
3.4.9. Welfare consequence ‘Metabolic disorders’
3.4.9.1. Description
The most relevant metabolic disorder observed in veal calves is iron deficiency leading to anaemia. Hb is checked in veal calves several times during the fattening period and calves below the minimum Hb levels (current minimum regulated values are Hb 4.5 mmol/L) will receive an injection of iron. In humans, iron deficiency leads to weakness, dizziness and headache (Afari and Buchwald, 2003; Meng et al., 2021), but the impact of iron deficiency in calves is not well understood. Reported negative effects of severe iron deficiency include impaired immune function (Gygax et al., 1993), higher infection rates (Gygax et al., 1993), low cardiovascular performance and fatigue when exposed to physical effort (Lindt and Blum, 1993) and poor weight gains (Sarkozy et al., 1984; Lindt and Blum, 1993). For a detailed discussion of the effects of iron‐deficiency anaemia in calves, see 3.16.3 (Specific Scenario 1), in which a whole Section is dedicated to this topic.
3.4.9.2. ABMs
ABM of metabolic disorders (anaemia) in this system are presented in Table 14.
Table 14.
ABM of metabolic disorders (anaemia) in veal calves kept in small groups
| ABM | Comments |
|---|---|
| Fatigue |
Definition: Animals experiencing fatigue exhibit tachypnoea and exhaustion; exhaustion being defined as inability to stand up and reluctance to movement (adapted from EFSA AHAW Panel, 2020)). Sensitivity: Low. Fatigue may not be apparent in cases of disease, and chronic anaemia where physiological adaptations to low haemoglobin levels occurred. Specificity: Low. Fatigue may also be caused by disease conditions leading to general poor state. |
|
Haemoglobin concentration |
Definition: Concentration of haemoglobin in blood (g/L). Sensitivity: High. Specificity: High. |
3.4.9.3. Hazards
Low iron content in the diet.
Insufficient amount of solid feed.
Other hazards for anaemia in calves exist (e.g. hemoparasitoses), but they are not strictly related to management of veal calves and are hence outside the scope of this assessment.
3.4.9.4. Preventive and corrective measures
The provision of diet with a high iron content is effective to prevent iron‐deficiency anaemia. A good diet component would be hay, which has a high iron concentration and provides other benefits to calves: it is highly palatable (Webb et al., 2014a), it improves rumen development (Khan et al., 2011) and abomasal health (Webb et al., 2013), and it promotes chewing and rumination (Webb et al., 2014b). Compared with hay, wheat straw has a decreased bioavailability of iron from solid feeds rich in structured NDF (Cozzi et al., 2002; Prevedello et al., 2012). Hence, provision of ad libitum, long hay to veal calves would be the preferred option and would address many welfare consequences linked to the veal sector. To correct anaemia in veal calves, provision of iron dextran in calves has been showed to be effective (Allan et al., 2020) but this practice also has welfare consequences linked to venepuncture and handling stress, as discussed in Section 3.16.4, and hence preventive measures are preferred from a welfare point of view. For further recommendations on iron‐deficiency anaemia see Section 3.16.3 and on fibre provision, see Section 3.16.4 (Specific Scenario 1).
3.5. Welfare of veal calves kept in group housing in large groups and automatic milk feeding
3.5.1. Description of the system
Systems keeping calves in large groups are not common but occur in a small proportion of farms in the Netherlands, France and Italy. In the Netherlands, this system is used in a small number of farms for production of white veal and, more rarely, rosé veal.
Veal calves are, following the baby box phase or immediately after arrival at the veal farm, released into large group pens holding 40–70 calves, usually with the minimum EU space allowance per animal (i.e. 1.8 m2 per calf) (Bokkers and Koene, 2001) (Figures 10 and 11). Full social contact with pen mates is possible. The calves are typically housed on slatted floors made of wood or concrete, although rubber flooring on top of wooden or concrete slats is used on some farms, possibly even more common in this system compared with the smaller pens described above. In France, straw bedding is used as well. The calves may receive enrichment, such as a ball hanging from a chain from the ceiling, fixed brushes on the walls and some form of dry teats to suck/chew on.
Figure 10.
Schematic representation of calves in large‐group pens in a veal farm
Figure 11.

Veal calves kept in large groups. © Marta Brščić
The calves are typically fed milk replacer via automated milk feeders, which allocates the milk replacer evenly in several (e.g. up to 5) periods per day. The milk flow tends to be rather high to maximise the speed of ingesting milk thus maximising the number of calves that can be served by one machine. Milk allowance can be controlled at individual level, but regrouping decisions still tend to be based on weight. The solid feed management and assessment of blood Hb levels are the same as for the small pens described in Section 3.4.1 (veal calves kept in small groups) and in Section 3.16.3 (Specific Scenario on risk associated with iron restriction), respectively.
3.5.2. Welfare consequence ‘Inability to perform exploratory or foraging behaviour’
3.5.2.1. Description
See Section 3.2.6.1 (calves in individual pens in dairy farms) for a general description of inability to perform exploratory or foraging behaviour. White veal calves kept in large groups have more total space available compared with calves reared in individual pens/small groups, and often some enrichment objects such as teats, brushes and balls. The diet is similar to the other veal systems described above, so the ability to perform foraging behaviour is similar.
3.5.2.2. ABMs
The ABMs are the same as for calves in individual housing (Section 3.3.6.2), with the exception of tongue flicks, which are mostly observed in younger calves and are therefore less relevant in this age range.
3.5.2.3. Hazards
See Section 3.3.6.3 (veal calves in individual housing).
3.5.2.4. Preventive and corrective measures
See Section 3.3.6.4 (veal calves in individual housing).
3.5.3. Welfare consequence ‘Inability to chew and ruminate’
3.5.3.1. Description
The same of Section 3.4.4.1 (veal calves in small groups) applies to veal calves in large groups.
3.5.3.2. ABMs
See Section 3.4.4.2 (veal calves in small groups).
3.5.3.3. Hazards
See Section 3.4.4.3 (veal calves in small groups).
3.5.3.4. Preventive and corrective measures
See Section 3.4.4.4 (veal calves in small groups).
3.5.4. Welfare consequence ‘Respiratory disorders’
3.5.4.1. Description
See Section 3.2.9.1 for a general description of respiratory diseases and Section 3.3.8.1 (veal calves in individual housing) for details of the disease in veal.
3.5.4.2. ABMs
The clinical signs and most important ABM are common to all systems and are presented in detail in Section 3.2.9.2.
3.5.4.3. Hazards
See Section 3.2.9.3 (calves in individual housing in dairy farms) for a general description of respiratory diseases and Section 3.3.8.3 (veal calves in individual housing) for hazards specific to veal calves. Further considerations on the relationship between respiratory disease and group size are presented in Section 3.16.1 (Specific Scenario 1).
3.5.4.4. Preventive and corrective measures
See Section 3.4.7.4 for a general description of preventive measures of respiratory diseases and Section 3.3.8.4 (veal calves in individual housing) for preventive measures specific to veal calves.
3.5.5. Welfare consequence ‘Group stress’
3.5.5.1. Description
A general description of the development of social behaviour in calves, as well as effects of competition and regrouping, can be found in Sections 3.4.6.1 (veal calves kept in small groups) and 3.9.6.1 (calves kept from weaning in fully or partially slatted floors without bedding). In larger groups, competition for access to milk is higher due to a higher number of calves per AMF (Jensen, 2004). Calves had to wait longer for access to the milk feeder and were more often disturbed during sucking at the AMF in groups of 24 as compared with 12; in consequence calves in the larger group showed an increased milk intake rate (Jensen, 2004). In addition, keeping calves in large groups may lead to disturbance or interruption of behaviours. In such groups, displacement at the AMF and disruption of resting (Færevik et al., 2008) can be expected. This is particularly relevant as calves grow bigger and the pen size remains the same.
Health disorders might be caused at least partly by increased social stress due to high social competition, especially over access to the milk dispenser, as well as due to high density throughout the pen. Increased respiratory disorders and reduced growth rate have been observed in larger (> 10 animals) compared with smaller groups (< 10; Svensson et al., 2003 Svensson and Liberg (2006)). However, further studies specifically on large veal calf groups (> 30 animals) are lacking.
3.5.5.2. ABMs
See Section 3.4.6.1. In addition, one easily standardisable measure for calves kept in large groups would be to count displacements from the AMF (high specificity and high sensitivity).
3.5.5.3. Hazards
High calf‐milk feeder ratio.
Open stalls at the AMF.
Low space allowance.
Regrouping.
3.5.5.4. Preventive and corrective measures
Preventive measures of group stress can be applied when designing the pen and when determining stocking rates. A lower number of animals per AMF, by decreasing the total group size and/or increasing the number of AMF per pen, can reduce the level of competition. Disturbance and displacement of calves at the AMF area can be reduced by a feeding stall that closes when a calf enters (Weber and Wechsler, 2001).
Higher space allowance also reduces disturbance during resting. Structuring the pen into a designated lying area, e.g. with further structuring could reduce disturbance as suggested by results in adult cattle (Menke et al., 2015). In addition, keeping groups stable also prevents group stress because the social bonds can be maintained throughout the fattening period.
3.5.6. Welfare consequence ‘Resting problems’
3.5.6.1. Description
Veal calves kept in large groups have limited space and it is quite common to remove some calves and/or include new animals. Space allowance and changes in the group can affect resting time and lying position. The space of the lying area may influence the resting of calves. For instance, a reduction of the lying space allowance from 1.25 to 0.75 m2 per animal for calves with a live weight up to 100 kg and a reduction from 1.50 to 1.00 m2 per animal for calves with a live weight up to 150 kg, decreased the occurrence of synchronous resting, lowered the calf's possibility to lie in a relaxed recumbent posture with legs stretched out and increased the occurrence of calves resting in close proximity to others (Færevik et al., 2008).
3.5.6.2. ABMs
See Section 3.3.7.2 (veal calves in individual pens).
3.5.6.3. Hazards
Although in large group systems the total space allowance is larger, the space available per animal is similar to that observed in white veal husbandry systems (e.g. 1.8 m2 per animal) and hence still very limited (Bokkers and Koene, 2001).
3.5.6.4. Preventive and corrective measures
A preventive measure is higher space allowance: a larger lying area increased synchronous resting and improved the calf's possibility to choose a recumbent resting posture; synchronisation of resting behaviour is seen to be more sensitive to changes in space allowance than total lying time (Færevik et al., 2008). Another measure is keeping the group stable avoiding inserting new subjects.
3.5.7. Welfare consequence ‘Gastroenteric disorders’
3.5.7.1. Description
See Section 3.2.8.1 for an overall description of gastroenteric disorders and Section 3.4.8.1 for a description of gastroenteric issues in veal calf systems. Compared with calves fed via open troughs or buckets, calves housed in group pens with automatic milk feeding will have access to more frequent, smaller milk meals.
3.5.7.2. ABMs
The relevant ABMs are similar to those described in Section 3.2.8.2 (calves in individual pens in dairy farms) and Section 3.4.8.2 (veal calves in small groups).
3.5.7.3. Hazards
See Section 3.2.8.3 for hazards of gastroenteric disorders in young calves and Section 3.3.9.3 for hazards of gastroenteric disorders in veal calves. In addition, hazards relevant to calves kept in large groups and fed with AMF are:
Poor teat hygiene (Van Metre et al., 2008; Pithua et al., 2009; Klein‐Jöbstl et al., 2014; Medrano‐Galarza et al., 2018; Mohammed et al., 2019);
Slatted concrete floor in group pens (Gulliksen et al., 2009a);
Heterogeneous (size and age) groups, which will increase the probability of stress and poor immunity (Gorden and Plummer, 2010; Cho and Yoon, 2014; Medrano‐Galarza et al., 2018);
Poor quality milk replacers (Van Metre et al., 2008; Blanchard, 2012).
3.5.7.4. Preventive and corrective measures
Additional to the prevention measures recommended for other systems, calves kept in large groups with automated milk feeder present several particularities. Because many calves will use the same teat and the AMF will be feeding animals all day, maintenance is crucial. Thorough washing and disinfection of all the components of the AMF can prevent GE disorders as bacteria usually flourish in soured milk remaining in tubes, teats, filters, etc. Ensuring strict biosecurity rules especially in large groups is especially important due to the higher risk of exposure to infectious agents (Van Metre et al., 2008; Olson et al., 2019). Early detection of sick calves kept in large groups may be difficult, so frequent and routine monitoring of calves is highly recommended (Van Metre et al., 2008). Strategies to detect these animals should include indicators collected by automated milk feeders (Borderas et al., 2009; Morrison et al., 2021; Conboy et al., 2022).
3.5.8. Welfare consequence ‘Metabolic disorders’
3.5.8.1. Description
See Section 3.4.9.1 (veal calves in small groups).
3.5.8.2. ABMs
See Section 3.4.9.2 (veal calves in small groups).
3.5.8.3. Hazards
See Section 3.4.9.3 (veal calves in small groups).
3.5.8.4. Preventive and corrective measures
See Section 3.4.9.4 (veal calves in small groups).
3.6. Welfare of calves kept from birth until weaning in group housing in small groups with milk feeding by bucket/trough
3.6.1. Description of the system
Dairy calves housed in small groups are typically housed in small group pens (2–8 animals) bedded with straw or sawdust. The pens may be situated indoor with sides made from tubular metal bars, or be outdoor group hutches, e.g. made from wood, with an open front and/or an outdoor area (Figures 12 and 13). Two individual outdoor calf hutches may also be placed next to each other, and the pair‐housed calves share an outdoor area. Calves in small groups are fed milk (whole milk or milk replacer), often by use of manual milk feeding systems. This may be teat buckets or a teat bar (buckets or a shared trough fitted with rubber teats through which the calves suck the milk; at least one teat per calf), or open buckets or a shared open trough from which the calves drink the milk from the surface. The latter may be combined with access to rubber teats, either floating teats through which the calves suck the milk from the surface, or ‘dry’ rubber teats that the calves have access to suck on after the milk is drunk.
Figure 12.
Schematic representation of calves in an outdoor group pen with littered floor
Figure 13.

Outdoor group pen with littered floor. © George Stilwell
3.6.2. Welfare consequence ‘Inability to perform sucking behaviour’
3.6.2.1. Description
See Section 3.2.4.1 (calves in individual pens in dairy farms)
3.6.2.2. ABMs
See Section 3.2.4.2 (calves in individual pens in dairy farms).
3.6.2.3. Hazards
See Section 3.2.4.3 (calves in individual pens in dairy farms).
3.6.2.4. Preventive and corrective measures
For an overview of measures to prevent inability to perform sucking behaviour, see Section 3.2.4.4. Offering milk via a teat, for instance in a teat bucket, reduces the occurrence of cross‐sucking compared with when milk is offered in an open bucket or trough.
When calves ingest milk from a teat bucket, they spend more time ingesting the milk, they suck the teat after the milk is ingested, and they perform less cross‐sucking after the ingestion of milk (Jung and Lidfors, 2001; Mounier et al., 2006). To prevent calves from displacing each other from their individual teat bucket, a barrier can be placed between the teats separating calf's heads and shoulders (Jensen et al., 2008). Among calves fed in teat bars (a common trough with 2–6 teats), provision of more than one teat per calf has also been shown to reduce displacements from teats, and thus competition (von Keyserlingk et al., 2004). Milk feeding via a teat can also be achieved by use of a floating teat placed in an open bucket or trough (Loberg and Lidfors, 2001). For a discussion on the use of dry teats to reduce cross‐sucking, please see Section 3.2.4.4.
The motivation to suck declines spontaneously within 20–30 min of milk allocation (De Passillé and Rushen, 1997) and confining calves for this period of time, e.g. in closable individual feed stall, has been shown to reduce cross‐sucking (Größbacher et al., 2018) but calves should have access to a teat; otherwise the motivation to suck persists until it wanes towards the end of the confinement period. Gradual weaning and insurance of a good nutrient supply through solid feed during weaning (Roth et al., 2009a), as well as ad libitum access to roughage (Keil and Langhans, 2001), have been found to reduce cross‐sucking after weaning.
Finally, another preventive measure is to rear calves with their dam, or a foster cow, i.e. allowing the calves to suck milk from an udder. Calves suckling a cows' udder performed less cross‐sucking compared with calves fed milk via an AMF (Fröberg et al., 2008; Roth et al., 2009b). In studies where calves experienced full‐time contact to the dam, cross‐sucking was not observed at all (Fröberg and Lidfors, 2009; Roth et al., 2009b), and it was rare in restricted suckling (Fröberg et al., 2008). It is unclear if dam rearing ensures a better satisfaction of the sucking need than use of foster cows. This will likely depend on how strongly cow and calf are bonded (Wieczorreck and Hillmann, 2022).
3.6.3. Welfare consequence ‘Inability to perform exploratory or foraging behaviour’
3.6.3.1. Description
See Section 3.2.6.2 (calves in individual pens in dairy farms) for a general description of inability to perform exploratory or foraging behaviour. The inability to perform exploratory or foraging behaviour experienced by calves in small groups from birth to weaning and fed with a bucket/trough is comparable to that described for calves kept in individual pens, with the exception that in this system the calves have the possibility of social contacts with other calves which may promote foraging behaviour.
3.6.3.2. ABMs
See Section 3.2.6.2 (calves in individual pens in dairy farms).
3.6.3.3. Hazards
See Section 3.2.6.3 (calves in individual pens in dairy farms).
3.6.3.4. Preventive and corrective measures
See Section 3.2.6.4 (calves in individual housing in dairy farms).
3.6.4. Welfare consequence ‘Inability to perform play behaviour’
3.6.4.1. Description
See Section 3.2.5.1 for a description of this welfare consequence.
3.6.4.2. ABMs
Spontaneous play behaviour occurs sporadically in short episodes and is therefore time consuming to record by direct observation. Locomotor play stimulated by the addition of straw (Duve et al., 2012) or through the release in a large area outside the home pen (Mintline et al., 2012) are unsuitable as ABMs to compare between different housing systems because they do not reflect play behaviour during the 24 h in the home environment. A promising ABM is the duration of locomotor play behaviour estimated using leg attached accelerometers (de Passillé et al., 2010; Rushen and de Passillé, 2012; Größbacher et al., 2020). However, this method requires further development and validation (Table 15).
Table 15.
ABMs for inability to play behaviour in group housing in small groups with milk feeding by bucket/trough
| ABM | Comments |
|---|---|
| Time spent in locomotor play |
Definition: Duration of locomotor play behaviour estimated using leg attached accelerometers (Größbacher et al., 2020). Sensitivity: High Specificity: High Further considerations: Requires further development and validation. Sensitivity and specificity measures will largely depend on the classification algorithm performance. |
3.6.4.3. Hazards
Low space allowance – In group pens with a low space allowance, the calves may be motivated, but unable to perform locomotor play and thus deprived of potential positive emotion while performing play behaviour.
3.6.4.4. Preventive and corrective measures
See Section 3.2.5.4 (calves kept in individual pens in dairy farms).
3.6.5. Welfare consequence ‘Prolonged hunger’
3.6.5.1. Description
See Section 3.2.7.1 (calves in individual pens in dairy farms). An increasing tendency for once‐a‐day feeding in these systems has been reported (Jongman et al., 2020) which can aggravate prolonged hunger.
3.6.5.2. ABMs
See Section 3.2.7.2 (calves in individual pens in dairy farms).
3.6.5.3. Hazards
See Section 3.2.7.3 (calves in individual pens in dairy farms).
3.6.5.4. Preventive and corrective measures
See Section 3.2.7.4 (calves in individual pens in dairy farms).
3.6.6. Welfare consequence ‘Gastroenteric disorders’
3.6.6.1. Description
See Section 3.2.8.1 (calves in individual pens in dairy farms).
3.6.6.2. ABMs
See Section 3.2.8.2 (calves in individual pens in dairy farms).
3.6.6.3. Hazards
See Section 3.2.8.3 (calves in individual pens in dairy farms).
3.6.6.4. Preventive and corrective measures
See Section 3.2.8.4 (calves in individual pens in dairy farms).
3.6.7. Welfare consequence ‘Respiratory disorders’
3.6.7.1. Description
See Section 3.2.9.1 for a description of respiratory diseases.
3.6.7.2. ABMs
The clinical signs and most important ABM are common to all systems and are presented in detail in Section 3.2.9.2.
3.6.7.3. Hazards
See Section 3.2.9.3 for hazards for respiratory disorders.
3.6.7.4. Preventive and corrective measures
See Section 3.2.9.4 for general measures to prevent the disease in calves.
3.7. Welfare of calves kept from birth till weaning in group housing in large groups and automatic milk feeding
3.7.1. Description of the system
In Europe, the group size for unweaned dairy calves housed in large groups typically ranges from nine to 20 (Marcé et al., 2010) calves housed in group pens bedded with straw. The pens may be indoors with sides made from tubular metal bars, or outdoor group hutches, e.g. made from wood and with an open front. The calves are fed milk using an automatic milk feeding (AMF) system. Through ear tags, or collars, the identity of each calf is established in the automatic feeder and the calf is offered milk via a rubber teat in two to several daily milk portions depending on milk allowance and age.
3.7.2. Welfare consequence ‘Inability to perform sucking behaviour’
3.7.2.1. Description
See Section 3.2.4.1 (individual housing in dairy farms)
3.7.2.2. ABMs
See Section 3.2.4.2 (individual housing in dairy farms).
3.7.2.3. Hazards
See Section 3.2.4.3 (individual housing in dairy farms).
3.7.2.4. Preventive and corrective measures
For an overview of measures to prevent inability to perform sucking behaviour, see Section 3.2.4.4. Contrarily to manual milk feeding methods, where all calves in a group are fed milk at the same time, only one calf can ingest milk at a time from an AMF. Depending on number of calves per feeder and management of the feeder, calves compete for access to the feeder, and calves may be displaced from the feeder before they have had time to satisfy the behavioural need to suck. Furthermore, the teat must be available for the calf for the duration of the sucking motivation (20 min; Lidfors, 1993) and not be retracted as soon as the milk is drunk. When AMFs are used, a preventive measure is to have a low number of calves per feeder. For instance, with 12 calves per feeder, calves were disturbed by other calves during 10% of the time they spent in the feeder compared with 50% with 24 calves per feeder, which resulted in more time spent on both nutritive and non‐nutritive sucking among calves when there were 12 calves per feeder (Jensen, 2004) and thus a lower risk of cross‐sucking. Disturbance and displacement of calves at the AMF and the occurrence of cross‐sucking can also be reduced by use of protected AMFs, i.e. a stall that closes when a calf enters (Weber and Wechsler, 2001).
3.7.3. Welfare consequence ‘Group stress’
3.7.3.1. Description
Please refer to Section 3.4.6.1 for a general description of group stress, and Section 3.5.5.1 (veal calves kept in large groups) on aspects related with competition for the AMF.
3.7.3.2. ABMs
See Section 3.4.6.2 for ABMs of group stress. In addition, one easily standardisable measure for calves kept in large groups would be to count displacements from the AMF (high specificity and high sensitivity).
3.7.3.3. Hazards
High number of calves per automated milk feeder.
Feeder that cannot be closed or with no lateral barriers.
Low space allowance per calf.
Heterogeneous group composition in terms of age.
3.7.3.4. Preventive and corrective measures
See preventive measures for group stress in Section 3.4.6.4. Additional measures specific of this system are low calves‐feeder ratio and small group sizes (Jensen, 2004), closable feeders or with lateral barriers and access to teat after milk intake (Weber and Wechsler, 2001; Ude et al., 2011).
3.7.4. Welfare consequence ‘Inability to perform exploratory or foraging behaviour’
3.7.4.1. Description
See Section 3.2.6.1 (calves in individual pens in dairy farms) for a general description of inability to perform exploratory or foraging behaviour. Compared with individual pens and calves housed in small groups, calves in this housing system have access to a larger space but the environment remains relatively barren.
3.7.4.2. ABMs
See Section 3.2.6.2 for a list of ABMs of inability to perform exploratory or foraging behaviour.
3.7.4.3. Hazards
See Section 3.2.6.3.
3.7.4.4. Preventive and corrective measures
See Section 3.2.6.4.
3.7.5. Welfare consequence ‘Respiratory disorders’
3.7.5.1. Description
See Section 3.2.9.1 for a description of respiratory diseases.
3.7.5.2. ABMs
The clinical signs and most important ABM are common to all systems and are presented in detail in Section 3.2.9.2 (calves in individual housing in dairy farms).
3.7.5.3. Hazards
See Section 3.2.9.3 (calves in individual housing in dairy farms) for hazards of respiratory disorders in calves. Sharing a common teat in group feeding allows for an increased transmission of pathogens from calf to calf leading to a higher prevalence of respiratory problems (Curtis et al., 2016).
3.7.5.4. Preventive and corrective measures
See Section 3.2.9.4 (calves in individual housing in dairy farms) for general measures to prevent the disease in calves.
3.7.6. Welfare consequence ‘Prolonged hunger’
3.7.6.1. Description
Prolonged hunger is a common issue in this system, even if calves have fed through an AMF. See Section 3.2.7.1 for a description of prolonged hunger in calves.
3.7.6.2. ABMs
Please refer to Section 3.2.7.2 (calves kept in individual pens in dairy farms) for ABMs of prolonged hunger.
3.7.6.3. Hazards
Low milk allowance
High number of calves per automated milk feeder
Heterogeneous group composition in terms of age.
3.7.6.4. Preventive and corrective measures
See Section 3.2.7.4.
3.7.7. Welfare consequence ‘Gastroenteric disorders’
3.7.7.1. Description
See Section 3.2.8.1 (calves in individual pens in dairy farms).
3.7.7.2. ABMs
See Section 3.2.8.2 (calves in individual pens in dairy farms).
3.7.7.3. Hazards
The hazards are the same as identified in Section 3.2.8.3, plus poor teat hygiene (Van Metre et al., 2008; Medrano‐Galarza et al., 2018) and poor‐quality milk replacers (Lallès et al., 1995; Miqueo et al., 2017).
3.7.7.4. Preventive and corrective measures
See Section 3.5.7.4.
3.8. Welfare of calves kept in systems with cow–calf contact
3.8.1. Description of the system
Calves are allowed physical contact with either their dam or a foster cow (Sirovnik et al., 2020) for a varying period of time (Figure 14). This period may comprise the whole milk feeding period or only part of it, if separation from the dam or foster cow happens before weaning off milk (combined rearing; Sirovnik et al., 2020). The type of physical contact can be full contact (all social behaviours such as suckling, licking, playing are possible) or partial contact (e.g. fence‐line contact or nose‐flaps hindering suckling (Sirovnik et al., 2020). In full dam–calf contact rearing, the calves are allowed to suckle their dam for at least some time. Furthermore, daily duration of contact may vary from whole day CCC to part‐time contact (mainly day‐time or night‐time contact) or short‐time contact for only two or more short periods a day (Sirovnik et al., 2020). In whole‐ or part‐time contact rearing, cow and calf may be integrated in the herd of all lactating cows or in a smaller group of only cow–calf pairs. Housing systems may include cubicle housing, deep litter and straw flow pens, often with a creep area available where calves can lie, and feed separated from cows. In dam–calf contact rearing, the cow and calf pair usually is kept separately for some days after calving (often an individual calving pen), to enable bonding, before transition into a whole day, part‐ or short‐time contact system. On some farms, these cows are left in the group of pre‐parturient cows or in a group calving pen. On some farms, the calves can go outdoors with their dam/foster cow or to a separate outdoor pasture for calves. The calves typically do not follow the cow into the milking parlour or milking robot, but some farmers milk some or all cows with the calves present to try to enhance milk let‐down. Irrespectively of having access to the cows' solid feed or not, calves typically have their own area for feeding, although this differs between farms/systems. Permanent separation from the cow and weaning off milk can occur at the same time (abrupt separation without access to another milk source) or can be disconnected in case of two‐step weaning, i.e. fence‐line separation or using a nose‐flap for some time before permanent separation or combined rearing (i.e. calves obtaining milk from an artificial source after weaning off the dam/foster cow) (Sirovnik et al., 2020). Other cow–calf systems used to keep calves over the first weeks of life exist (e.g. seasonal calving, pasture‐based systems) but these are not described in detail.
Figure 14.

Dairy cow–calf contact system for the whole milk feeding period under organic conditions, i.e. until 3 months of age. © Susanne Waiblinger
3.8.2. Welfare consequence ‘Separation stress’
3.8.2.1. Description
Calves experience separation stress when they are separated from their dam/foster cow following a period of close contact. Separation stress is more severe if separation occurs after the bond between dam and calf is fully formed (i.e. after 4 days from birth) (Stěhulová et al., 2008). The response of bonded young dairy calves to abrupt separation is characterised by restlessness and attempts to re‐establish contact. In CCC dairy production systems where the dam/foster cow and calf were kept together for 8–12 weeks, abrupt separation resulted in increased activity and high pitch calls by the calves (Veissier et al., 2013; Johnsen et al., 2015). Stress‐related behaviours were lowered if permanent separation from the cow and weaning off milk were disconnected (Johnsen et al., 2015; Johnsen et al., 2018). For a more detailed description of welfare aspects of cow–calf separation, please refer to Section 3.18 (Specific Scenario 3 – Risks of cow–calf separation).
3.8.2.2. ABMs (Table 16)
Table 16.
ABMs of separation stress in cow–calf contact systems
| ABM | Comments |
|---|---|
| Vocalisations |
Definition: Increased frequency of vocalisations (Johnsen et al., 2015; Green et al., 2021). Sensitivity: High. Specificity: Low. Vocalisations may also occur in cases of prolonged hunger or isolation stress. |
| Heart rate after separation |
Definition: Increased heart rate (heart beats per minute) (Loberg et al., 2008; Stěhulová et al., 2008). Sensitivity: High. Specificity: Low. High heart rate could also result from isolation stress or heat stress. |
| Restlessness behaviour |
Definition: Behaviours such as head‐out behaviour, movements (Flower and Weary, 2001; Stěhulová et al., 2008). Sensitivity: High. Specificity: Low. Restlessness behaviour could also result from isolation stress or prolonged hunger. |
3.8.2.3. Hazards
Simultaneous separation from dam and weaning off milk.
Young age at weaning.
Simultaneous separation from the dam and additional change in the social and/or physical environment (separation from other peers and change of housing).
3.8.2.4. Preventive and corrective measures
Generally, separation of cow and calf is easier the older and less reliant on milk the calf is (see for a review on this topic). In calves of 3 to 4 months of age, gradual weaning off milk (Wenker et al., 2022), or disconnecting separation from weaning of milk (e.g. fenced‐line, nose flaps (Loberg et al., 2007) has been found to reduce the stress responses of calf and cow. Also maintaining the social group of calves intact after the separation from cow(s) may provide a social buffer (Rault, 2012), mitigating the stress experienced by the calves at weaning.
If calves are separated from the cow before weaning off milk (and are offered milk after the separation), then fence line‐separation reduced the stress response (Johnsen et al., 2015).
3.8.3. Welfare consequence ‘Group stress’
3.8.3.1. Description
Calves that live with their dam in a group with other cows experience some aggression by other cows (Waiblinger et al., 2020). This per se does not indicate social stress but can contribute to improve social competence (Wagner et al., 2012; Buchli et al., 2017) and reduce social stress later in life (Wagner et al., 2012). However, aggression by cows may lead to stress when calves cannot react appropriately with withdrawal, a situation that may occur more often in case of high stocking densities, constricted space and lack of a calf creep area. No studies so far found indication of group stress in calves in CCC; often cows seem to be quite tolerant to calves. If there are too many calves per (foster) cow, there may be competition for access to the cows leading to calf‐calf aggression and hindered access for smaller calves. Further, cows may kick alien calves that suckle or try to do so in foster cow systems (Wieczorreck and Hillmann, 2022).
3.8.3.2. ABMs
Measuring group stress can be assessed by direct observation of the number of physical aggression (head butts, pushing) calves receive from cows or physical displacement from other calves during times of expected highest level of competition. In addition, injurious events that are a consequence of cow aggression can be observed, i.e. bumping into equipment, being pressed into equipment or falling as a consequence of aggression, although this has not been reported in scientific studies. Reduced growth in otherwise healthy calves may be a sign of group stress eventually leading to hindered access of the dam (Table 17).
Table 17.
ABMs of group stress in cow–calf contact systems
| ABM | Comments |
|---|---|
| Headbutts, displacements and kicks from cow |
Definition: A calf receives a head butt or is displaced from a cow (i.e. any interaction involving physical contact where the cow is butting, hitting, thrusting, striking or pushing the calf with forehead, horns or horn base with a forceful movement and the calf is either staying at its position (=head butt) or giving up its position (=displacement); modified from WQ® 2009) or is kicked by a cow (i.e. the cow lifts its leg, moves it quickly in the direction of the calf and finally strikes the calf with the leg); interactions without physical contact, i.e. threats or kicking movements without striking the calf, are not counted. The number of head butts, displacements and kicks (=aggressions) per calf per hour is calculated (Waiblinger et al., 2020). Sensitivity: High. Specificity: High. |
| Injurious events |
Definition: Calf is either falling (see Table 3 for falling definition) or bumping into equipment such as a cubicle partition when withdrawing from an aggression from a cow or is being pushed or pressed into the equipment by the cow. Sensitivity: Low. Group stress does not always result in injurious events. Specificity: High. |
| Displacements by calves |
Definition: A calf is displaced by another calf (for the definition of displacement, see above). Sensitivity: High. Specificity: High. |
3.8.3.3. Hazards
High stocking density, restricted space and lack of a creep area likely prevent withdrawal of calves from alien cows, and this may lead to group stress.
Too many calves per (foster) cow likely increases competition for access to the dam's/foster cow's udder and may result in calf‐calf aggression and hindered access for smaller calves.
Poor bond between calf and dam, e.g. due to mismothering events in group calving pens (Edwards, 1983), or poor bond between calf and foster cow (Wieczorreck and Hillmann, 2022), e.g. due to poor bonding in individual pen before the introduction to the larger group (Perez et al., 1985).
3.8.3.4. Preventive and corrective measures
Agonistic interactions can be prevented by the provision of high space allowance and by avoiding dead ends, spatial bottlenecks as well as small alleys (Smulders and Algers, 2009). This also likely reduces aggression of cows towards calves. In addition, a freely accessible calf creep area offering sufficient space for all calves allows calves to lie down, feed and drink water while undisturbed by cows (e.g. used in Roth et al., 2009a; Wagner et al., 2013). The number of calves per foster cow need to be adapted to the milk yield and length of suckling period (weaning age). The behaviour and weight gain of calves need to be monitored regularly. Further, it should be ensured that the foster cow bonds to the calves, e.g. by keeping them together for sufficient time in individual pens (Perez et al., 1985).
3.8.4. Welfare consequence ‘Handling stress’
3.8.4.1. Description
Some CCC systems require regular handling of calves, e.g. short‐time contact systems where calves are moved to meet their cows twice daily. Depending on the human–animal relationship on the farm, and thus on farmer/stockperson behaviour and frequency and type of human‐animal interactions, these handling events can cause fear and stress (Waiblinger et al., 2006; Hosey and Melfi, 2018) and may cause injury. Injurious events were lower in a handling situation involving calves from farms with improved human‐animal relationship (defined as having a high frequency of farmer‐animal gentle contacts and a low frequency of rough contacts) than calves from farms with a poorer human‐animal relationship (Lensink et al., 2001). It has been shown that calves reared in CCC systems did not generally have a higher fear of humans compared with artificially reared calves (Mogensen et al., 1999; Bieber et al., 2022; Wenker et al., 2022). However, the risk of handling stress depends on the amount of human contact provided (Waiblinger et al., 2020). Dam‐reared calves that experience less human contact may have higher levels of fear of humans than artificially reared calves (Waiblinger et al., 2020; Webb et al., 2022). Dam‐reared calves that experience less human contact may have higher levels of fear of humans than artificially reared calves (Waiblinger et al., 2020; Webb et al., 2022).
3.8.4.2. ABMs
Handling stress can be assessed by observing the behaviour of calves during handling and counting the number of injurious events (e.g. Lensink et al., 2001) as well as by assessing the quality of their relationship with humans using avoidance distance (e.g. Lürzel et al., 2016) as a proxy and by checking for occurrence of aversive handling (Table 18).
Table 18.
ABMs of handling stress in cow–calf contact systems
| ABM | Comments |
|---|---|
| Injurious events during handling |
Definition: Calf is falling, stumbling, bumping into equipment or another calf in reaction to handling (adapted from Welfare Quality®). Sensitivity: High. Specificity: Low. Not all calves experiencing handling stress may fall or bump into equipment. |
| Avoidance distance |
Definition: % of animals that avoid touching attempt. Calf escape test (5‐point score, Welfare Quality® protocol; Bokkers et al., 2009; Leruste et al., 2012). Sensitivity: High. Specificity: High. |
3.8.4.3. Hazards
A bad quality human‐animal relationship – characterised by insufficient contact and/or inappropriate handling and consequently animals with fear of humans – makes handling of cows and calves more difficult and stressful.
Lack of knowledge of and training of farmers and stock people in appropriate handling.
Large number of animals per stockperson and thus less time per animal can reduce contact and hinder habituation of animals.
Time pressure and high workload can lead to stress during handling.
3.8.4.4. Preventive and corrective measures
Training of stock‐people in appropriate animal handling can improve their attitudes and behaviour, thus improving the human‐animal relationship and avoiding stress during calf handling (for a review, see Hemsworth and Coleman (2011)). If regular handling is required in CCC systems, this offers the opportunity to improve calf's relationship with humans compared with systems without such regular human–calf contact (Boivin et al., 1992; Waiblinger et al., 2006). Gentle human contact reduces a calf's fear of humans (Lensink et al., 2001; Lürzel et al., 2015) and calves are especially sensitive during the first 5 days of life (Krohn et al., 2001). Gentle interactions with calves early in life can improve their relationship to humans also in CCC systems and thus avoid stress (Probst et al., 2012; Waiblinger et al., 2020). Gentle human contact during feeding in the first 5 days of life (i.e. bottle feeding of colostrum and assisting calves in suckling their dam in CCC systems) seem especially effective to improve calf's relationship to humans and thus avoid handling stress (Krohn et al., 2003; Waiblinger et al., 2020). Appropriate behaviour of humans during handling can prevent or minimise stress also in calves showing some level of fear of humans (Grandin, 2014). Certain environmental conditions support ease of handling and help to prevent handling stress through minimisation of stimuli that elicit fear and stress, such as loud noises, quick movements, sharp and narrow turns, spatial bottlenecks, dazzling light or darkness.
3.8.5. Welfare consequence ‘Gastroenteric disorders’
3.8.5.1. Description
For a general description of gastroenteric disorders please refer to Section 3.2.8.1.
Diarrhoea can occur in CCC systems and can be caused by infectious diseases or nutritive reasons such as drinking large amount of milk (Roth et al., 2009a). However, in CCC, there is reduced risk of ruminal drinking because the calves can suckle from the udder in a more natural way in terms of head and neck position and the number of feeding bouts.
While the contact to older animals (cows, older calves) in CCC may enhance the risk of transmission of infectious agents including those causing diarrhoea, contact to the dam and suckling may have an immune‐strengthening effect (Selman et al., 1971; Stott et al., 1979; Quigley III et al., 1995), but this depends on a successful passive immune‐transfer from the cow to the calf after birth. Failure of passive transfer in neonate calves occurred as often in CCC as in calves provided with supplementary colostrum artificially (i.e. through a bottle) and depended mainly on colostrum IgG level (Johnsen et al., 2019). In an epidemiological study on 39 farms with either CCC or artificial rearing, diarrhoea was observed more often in artificially reared calves in summer, autumn and winter, especially compared with calves with cow‐contact for longer than 14 days, but in spring the prevalence of diarrhoea was higher in CCC. However, farmers' records over 1 year did not confirm differences (Hillmann et al., 2019). Diarrhoea is also dependent from other management factors, such as hygiene levels, which can explain the lack of, or low improvement, of the condition in calves reared in CCC.
3.8.5.2. ABMs
See Section 3.2.8.2 (calves in individual pens in dairy farms).
3.8.5.3. Hazards
Failure of adequate colostrum intake
Low hygienic levels on the farm.
3.8.5.4. Preventive and corrective measures
See Section 3.2.8.4. Preventive measures specific of this system are keeping maternity pens with high hygiene levels (Svensson et al., 2003; Pithua et al., 2009; Klein‐Jöbstl et al., 2014) and confirmation of adequate sucking to ensure colostrum intake (Godden et al., 2019; Lorenz, 2021).
3.8.6. Welfare consequence ‘Respiratory disorders’
3.8.6.1. Description
See Section 3.2.9.1 (calves in individual pens in dairy farms).
3.8.6.2. ABMs
The clinical signs and most important ABM are common to all systems and are presented in detail in Section 3.2.9.2.
3.8.6.3. Hazards
See Section 3.2.9.3 (calves in individual pens in dairy farms). Comparing to veal housing, calves housed in CCC systems are less exposed to air containing high concentration of noxious gases. Lack of bedding is also not an issue in CCC systems.
3.8.6.4. Preventive and corrective measures
See Section 3.2.9.4 (calves in individual pens in dairy farms).
3.9. Welfare of calves kept from weaning to 6 months in fully or partially slatted floor group pens without bedding
3.9.1. Description of the system
Fully slatted floor systems are used for replacement calves or calves for fattening. There are variations in the design, but the common feature is to keep calves on a concrete surface, with no or little bedding, and draining slurry and manure through floor gaps into an underground concrete structure. There are also some variation in the proportion of the pen floor that is slatted: (i) fully slatted floor (Figure 15), (ii) partially slatted floors, in which only some of the occupied area is slatted with the rest being solid concrete, covered or not with rubber mats; (iii) ‘sloped floors’ in which the floors are predominantly solid concrete sloping towards narrow channels covered with slats for slurry drainage.
Figure 15.

Calves kept from weaning to six months in fully slatted floor group pens without bedding. © George Stilwell
The most frequent barn outline consists of a central feeding corridor (3.5–4 m wide) with troughs and pens running on both sides. The length of the feed trough is typically 30–50 cm per calf, depending on calf's weight and also the type of diet. Depending on the geographical region, the barn may be fully closed and insulated, or may be open on one side and/or at the ends, to provide ventilation.
Minimum space allowance in current European legislation (Council Directive 2008/119/EC) is 1.8 m2 for calves of a live weight of up to 220 kg.
An improvement for the fully slatted system is to cover the concrete slats with specially manufactured rubber mats designed with spaces to match the opening in the slats.
Some facilities may have a small open outdoor yard that is not covered by a roof, but generally not larger than 10% of the entire pen space.
3.9.2. Welfare consequence ‘Resting problems’
3.9.2.1. Description
A suitable floor is very important for calves as adequate rest is essential for the good welfare of animals. Positive correlations between the resting time and growth rates have been observed for growing cattle (Mogensen et al., 1997; Hänninen et al., 2005). In fattening cattle, the type of surface affected the movements of getting up and lying down as well as lying and resting behaviour. Mayer (2007) and Absmanner et al. (2009) found a higher prevalence of abnormal standing and lying movements in beef cattle kept on slatted floors than on straw. Reduced number of lying bouts, abnormal standing up and abnormal lying down were seen more often in bulls accommodated on concrete slats (Ruis‐Heutinck et al., 2000; Mayer, 2007; Rouha‐Muelleder et al., 2012). When cattle can choose between different floor types, they prefer deep litter to slatted floor especially for resting (reviewed by Wechsler, 2011). Space allowance is another trait that can affect resting behaviour; disturbances in the lying behaviour of heifers between 18 months old and 21 months old were observed when the space allowance in the lying area per animal was low (1.8 m2 per heifer compared with 2.7 or 3.6 m2) (Nielsen et al., 1997). For further details on this welfare consequence, see Section 3.3.7.1.
3.9.2.2. ABMs
See Section 3.3.7.2.
3.9.2.3. Hazards
Concrete or slatted floor
Wet floor
Low space allowance
Commingling and regrouping
Low or high temperature.
3.9.2.4. Preventive and corrective measures
Partial rubberisation or rubber mats on concrete floors, especially for lying areas, reduces the prevalence of lesions to claws and joints (EFSA AHAW Panel, 2012), and has been shown to reduce abnormal standing up and lying down, and slips (Platz et al., 2007). A preventive measure for calves housed in slatted concrete is to provide access to a bedded area (EFSA AHAW Panel, 2012). Calves should be provided with adequate floor space in order to ensure that they are not disturbed when lying. Fattening bulls with increasing space allowance had more lying bouts per day and lay for longer periods (Gygax et al., 2007).
3.9.3. Welfare consequence ‘Inability to perform exploratory or foraging behaviour’
3.9.3.1. Description
See Section 3.2.6.1 (calves in individual pens in dairy farms) for a general description of inability to perform exploratory or foraging behaviour. Calves in this system are exposed to a very barren environment due to the slatted floors and low space allowances.
3.9.3.2. ABMs
See Section 3.2.6.2 (calves in individual pens in dairy farms).
3.9.3.3. Hazards
See Section 3.2.6.3 (calves in individual pens in dairy farms).
3.9.3.4. Preventive and corrective measures
See Section 3.2.6.4 (calves in individual pens in dairy farms).
3.9.4. Welfare consequence ‘Inability to perform play behaviour’
3.9.4.1. Description
See Section 3.2.5.1 for a general description of play behaviour. In calves kept in groups, play behaviour is predominantly locomotor play or play fighting (social play). Locomotor play peaks earlier than social play behaviour and locomotor play is reduced by weaning (Krachun et al., 2010). There are few studies of play behaviour after weaning in dairy cattle; however, generally social behaviour becomes more frequent around puberty and social play may be as frequent as locomotor play at this age. Play fighting involves two or more calves pushing and butting while facing each other and unlike serious fights, play fighting is terminated without submission, flight or chase. However, around puberty what starts as social play may develop into serious fights (Reinhardt, 1980; Reinhardt, 1982).
3.9.4.2. ABMs
See Section 3.6.4.2 (calves kept from birth to weaning in group housing in small pens with feeding by bucket/trough) for a list of ABMs on the inability to perform play behaviour in this system.
3.9.4.3. Hazards
3.9.4.4. Preventive and corrective measures
See Section 3.2.5.4 for preventive measures of inability to perform play behaviour.
3.9.5. Welfare consequence ‘Restriction of movement’
3.9.5.1. Description
For a description of this welfare consequence, please see Section 3.2.2.1. In group pens with fully or partially slatted floor, movements may be impeded resulting in an unsteady gait, slipping or falling, which may lead to injury and pain.
3.9.5.2. ABMs
See Section 3.2.2.3 (calves in individual housing in dairy farms).
3.9.5.3. Hazards
Slippery nature of slats/concrete
Poor integrity (state of maintenance) of slats
Too wide, uneven or missing slats
Low space allowance.
3.9.5.4. Preventive and corrective measures
Preventive measures of restriction of movement in this system include increasing space allowance. It is not possible to correct the effects of slats on restriction of movement without changing the husbandry system and use a system with deep bedding or rubber slats. For more details on preventive measures of restriction of movement, see Section 3.3.2.4.
3.9.6. Welfare consequence ‘Group stress’
3.9.6.1. Description
See Section 3.4.6.1 (veal calves kept in small groups) for a general description of group stress.
Regrouping is a source of group stress because new dominance relationships have to be established based on aggressive encounters and because it may break preferential social relationships thus impairing social support (Rault, 2012). There is little research on calves between weaning and 6 months but regrouping of 9‐month‐old bulls resulted in more aggressive behaviour and more competition at the feed manger (Mounier et al., 2005, 2006) compared with stable groups. These studies also showed that among individuals previously housed in the same group, regrouped calves showed more affiliative behaviour and less aggression. Low space allowance is also a source of group stress. For example, studies in heifers have shown that increasing the space allowance in the lying area to 3.6 m2/animal reduced the number of aggressive interactions as compared with a space allowance of 1.8 and 2.7 m2/animal (Wechsler, 2011). Low space allowances and little space at the feed manger means that there is competition for resources and these impacts younger calves in the group more than older ones. For instance, in dairy heifers, aggressive interactions were higher in groups with a heterogeneous weight composition than in groups with a homogeneous composition, especially when concentrates were offered separately and not as part of a total mixed ration (Hindhede et al., 1999). In addition, younger calves in age‐heterogeneous groups gained less weight after weaning than calves in age‐homogeneous groups (Færevik et al., 2010).
3.9.6.2. ABMs
See Section 3.5.5.2 (calves housed in large groups with automatic milk feeding).
3.9.6.3. Hazards
Low space allowance
Regrouping
Low feed place to animal ratio (Keys et al., 1978).
3.9.6.4. Preventive and corrective measures
Lowering the group size and/or reducing the number of calves per feeding place can reduce the level of competition for feed. Increasing space allowance reduces disturbance during resting as indicated by results from studies in finishing bulls (Gygax et al., 2007). Structuring the pen into a designated lying area, possibly with further structuring, could reduce disturbance as suggested by results for adult cattle (Menke et al., 2015) and other species (goats: Aschwanden et al., 2009; Nordmann et al., 2015; horses: Pollmann, 2003). Regrouping should be avoided so that social bonds can be maintained throughout the fattening period, affiliative relationships are characterised by enhanced tolerance in competitive situations (Bouissou et al., 2001) thus reducing aggression and social stress.
3.9.7. Welfare consequence ‘Respiratory disorders’
3.9.7.1. Description
See Section 3.2.9.1 (calves in individual housing in dairy farms) for an overview of respiratory disease in calves. Another less common respiratory disease in weaned calves is Atypical Interstitial Pneumonia (AIP). The clinical signs usually associated with AlP are dyspnoea, loud expiratory grunts, frothing at the mouth, mouth‐breathing, tachypnoea and coughing.
3.9.7.2. ABMs
The clinical signs and most important ABM are common to all systems and are presented in detail in Section 3.2.9.2.
3.9.7.3. Hazards
See Section 3.2.9.3 (calves in individual pens in dairy farms).
Allergens can occur when calves start eating hay or large amounts of concentrate.
3.9.7.4. Preventive and corrective measures
See Section 3.2.9.4 (calves in individual housing in dairy farms) for general measures to prevent the disease in calves.
3.10. Welfare of calves kept from weaning to 6 months in fully or partly littered group pens
3.10.1. Description of the system
Calves kept in group pens with fully or partly littered floor typically have a higher space allowance than calves kept in fully slatted group pens. In fully littered pens, the pen area is used for feeding as well as lying (Figure 16). In partly littered pens, the lying area is littered, while the surface in the feeding area is typically solid, or slatted, concrete. The litter is typically straw, but also, e.g. wood shavings, sawdust and coconut fibres may be used. Dry litter is regularly added over wet bedding. Removal of bedding can be done regularly or only once at the end of the fattening period (deep bedding). Calves are typically fed at a feed manger. The size of the feed manger varies, and it may not allow all calves to feed at the same time. In partly littered pens, the feed space can be smaller or larger depending on the shape of the entire pen with narrower pens usually providing less space per animal at the feeder than wide pens. Calves are typically offered water via water bowls.
Figure 16.

Calves kept in a group pen with littered floor. © BOKU
3.10.2. Welfare consequence ‘Inability to perform exploratory or foraging behaviour’
3.10.2.1. Description
See Section 3.3.6.1 (calves in individual housing in dairy farms). This welfare consequence can be more or less severe, depending on the absence or presence of bedding provided, respectively.
3.10.2.2. ABMs
See Section 3.3.6.2 (calves in individual housing in dairy farms).
3.10.2.3. Hazards
See Section 3.3.6.3.
3.10.2.4. Preventive and corrective measures
See Section 3.3.6.4.
3.10.3. Welfare consequence ‘Group stress’
3.10.3.1. Description
See Section 3.4.6.1 (veal calves in small groups).
3.10.3.2. ABMs
See Section 3.4.6.2.
3.10.3.3. Hazards
See Section 3.9.6.3 (calves kept from weaning to 6 months in fully or partially slatted floor group pens without bedding).
3.10.3.4. Preventive and corrective measures
See Section 3.9.6.4 for a list of preventive and corrective measures of group stress.
3.10.4. Welfare consequence ‘Respiratory disorders’
3.10.4.1. Description
See Section 3.2.9.1 for a general description of respiratory disorders.
3.10.4.2. ABMs
The clinical signs and most important ABM are common to all systems and are presented in detail in Section 3.2.9.2.
3.10.4.3. Hazards
The same hazards apply in variable degrees to all systems to where cattle are moved to after weaning. Compared with slatted floor, littered pens may have less noxious gases related problems, but all depends on ventilation and manure and barn management.
3.10.4.4. Preventive and corrective measures
See Section 3.9.7.4 (calves in group pens after weaning with fully/partially slatted floors).
3.11. Welfare of calves kept from weaning to 6 months in group pens with cubicles
3.11.1. Description of the system
Cubicle loose housing systems (Figure 17) are mostly used for female replacement calves. These systems are currently used in MSs such as Austria and their use is increasing in Denmark and Germany. They usually consist of single‐row systems with one row of cubicles facing a wall, an adjacent alley and a feed barrier on the side opposite to the cubicle row. Floors in the alleys are slatted or solid, and most often made of concrete. The cubicle base is either littered (with straw, other organic material, or sand) or covered with rubber mats or mattresses. Cubicle dimensions vary from pen to pen trying to account for animal size. This requires frequent moving and/or regrouping of animals. Barn buildings may range from insulated buildings to open‐barn systems.
Figure 17.

Calves in cubicle housing. © Susanne Waiblinger
3.11.2. Welfare consequence ‘Restriction of movement’
3.11.2.1. Description
For a description of restriction of movement in calves, see Section 3.2.2.1. The use of group pens with cubicles may result in restriction of movement as the only available area for moving is the alleys between cubicles and the feeding alley, i.e. the area at the feeding places, which usually offers limited space. Alleys are typically made of concrete (slatted or plain) and thus the calves can walk only on a hard surface posing restrictions to comfortable walking as their claws evolved for walking on softer surfaces (Tranter and Morris, 1992). A slippery surface and/or slatted floors in the alleys further aggravate the situation and can also result in additional restriction of movement and injuries (Stefanowska et al., 2002); in dairy cows: (Telezhenko et al., 2017).
3.11.2.2. ABMs
See Section 3.2.2.2 (individual pens in dairy farms) for ABMs for restriction of movement. In cubicle systems, part of the floor area is occupied by cubicles which does not leave much free space for the calves to walk. A potential ABM for restriction of movement could be locomotory activity but this requires long‐term observations or access to automatic data recording, e.g. use of accelerometers. Alternatively, the assessment of space allowance may be considered a proxy. The ABMs listed in the table below focus mostly on aspects related to impaired movement due to floor quality and less on spatial constraints (Table 19).
Table 19.
ABMs for restriction of movement in group pens with cubicles in dairy farms
| ABM | Comments |
|---|---|
| Slipping |
Definition: Loss of balance in which the animal loses its foothold or the hooves slide on the floor surface. No other body parts except hooves and/or legs are in contact with the floor surface (Welfare Quality®, 2009) Sensitivity: High for impairment of movement that results from slippery floors and low for slipping for restriction of movement due to low space allowances Specificity: High. |
| Falling |
Definition: Loss of balance in which parts of the body other than the feet and legs get in contact with floor surface (Welfare Quality®, 2009) Sensitivity: High. Specificity: High. |
3.11.2.3. Hazards
In addition to the hazards identified in Section 3.4.6.4, hazards specific of cubicle systems are:
slippery floors due to slats/concrete in the alleys;
cubicle size and design not adapted to calf size.
3.11.2.4. Preventive and corrective measures
Prevention of restriction of movement in group pens with cubicles can be achieved by providing more spacious alleys and/or additional loafing/walking areas. A higher space allowance per calf can also increase any opportunities for movement. Provision of non‐slippery; e.g. plain rubber flooring, prevents slipping and falling and thus helps to avoid injuries.
3.11.3. Welfare consequence ‘Resting problems’
3.11.3.1. Description
In cubicle systems the total time spent lying is affected by many aspects of stall design (dimensions), floor and type of bedding etc. (Cook et al., 2004; Tucker and Weary, 2004; Drissler et al., 2005). When sleeping, cattle adopt a lying posture. Sleep was found to occur most often when calves rested with their neck relaxed and the head resting on the back or the surface (Hänninen et al., 2008). A reduction in lying time due to poor housing is likely to have a more severe effect on animal welfare if the time spent sleeping is also reduced. Thus, a stall or cubicle design that prevents calves from adopting the head postures associated with sleep could have a strong effect on animal welfare (EFSA AHAW Panel, 2009).
3.11.3.2. ABMs
See Section 3.4.5.1 (veal calves in individual pens).
3.11.3.3. Hazards
More calves than cubicles.
Absence or shallow, non‐deformable bedding.
Inadequate cubicle dimension.
Slatted or wet floor.
3.11.3.4. Preventive and corrective measures
See Section 3.4.5.4 (veal calves in individual pens). At least one cubicle per animal should be provided to allow fully synchronous lying behaviour.
3.11.4. Welfare consequence ‘Inability to perform exploratory or foraging behaviour’
3.11.4.1. Description
See Section 3.2.6.1 (calves in individual pens in dairy farms) for a general description of inability to perform exploratory or foraging behaviour. Although space allowance is larger in this system compared with individual pens, in group pens with cubicles the opportunities to explore are still very limited. In some cases, bedding is provided. Some farms offer the possibility to access an outdoor alley.
3.11.4.2. ABMs
See Section 3.2.6.2 (calves in individual housing in dairy farms).
3.11.4.3. Hazards
See Section 3.2.6.3.
3.11.4.4. Preventive and corrective measures
See Section 3.2.6.4.
3.11.5. Welfare consequence ‘Group stress’
3.11.5.1. Description
See Section 3.4.6.1 (veal calves in small groups).
3.11.5.2. ABMs
See Section 3.4.6.2.
3.11.5.3. Hazards
See Section 3.4.6.3.
3.11.5.4. Preventive and corrective measures
See Section 3.4.6.4.
3.11.6. Welfare consequence ‘Inability to perform play behaviour’
3.11.6.1. Description
For a description of the inability to perform play behaviour, see Sections 3.2.5.1 and 3.9.4.1. Play fighting involves two or more calves pushing and butting while facing each other and unlike serious fights, play fighting is terminated without submission, flight or chase. However, calves may obtain information on their own and others' competitive abilities through social play and around puberty what starts as social play may develop into serious fights (Reinhardt, 1980; Reinhardt, 1982).
3.11.6.2. ABMs
See Section 3.6.4.1 (calves kept from birth until weaning in group housing in small groups with milk feeding by bucket/trough).
3.11.6.3. Hazards
See Section 3.2.5.4. for preventive measures for the inability to perform play behaviour. Low space allowances (Jensen et al., 1998) and slippery surfaces in the alleys reduce the performance of locomotor play behaviour. There are limited data on the influence of floor type on calf's locomotor behaviour. Concrete floors generally reduce the activity level (in dairy cows: Telezhenko et al., 2007), and thus likely also reduce locomotor play behaviour. Concrete floors reduce social interactions generally (e.g. in finishing beef cattle: Brščić et al., 2015), and thus are likely to reduce social play behaviour. However, the time spent running in the home pen did not differ for calves reared on stones or sawdust (Sutherland et al., 2014b) as well as in animals reared on pea gravel, rubber chips, sand, or wood shavings (Sutherland et al., 2017).The increased motivation for locomotor play in an arena test in calves kept on stones (Sutherland et al., 2014a) indicates that other factors than rearing substrate may play a role.
3.11.6.4. Preventive and corrective measures
Good health and nutrition, as well as thermal comfort. Increased space allowance, non‐slippery rubber coated or straw bedded surfaces.
3.11.7. Welfare consequence ‘Respiratory disorders’
3.11.7.1. Description
See Section 3.2.9.1 (calves in individual housing in dairy farms) for an overview of respiratory disease in calves.
3.11.7.2. ABMs
The clinical signs and most important ABM are common to all systems and are presented in detail in Section 3.2.9.2.
3.11.7.3. Hazards
See Section 3.2.9.3 for hazards of respiratory disorders in calves.
3.11.7.4. Preventive and corrective measures
See Section 3.2.9.4 for general measures to prevent the disease in calves.
3.12. Welfare of calves kept from weaning to 6 months in outdoor feedlots
3.12.1. Description of the system
In outdoor feedlots, calves are kept in paddocks of 400–5,000 m2 with a soil surface, typically involving a space allowance per calf of above 9 m2 (Figure 18).
Figure 18.

Calves kept from weaning to 6 months in outdoor feedlots. © George Stilwell
The paddocks' perimeter is fenced and, in some cases, an internal fencing may also be present. Although most outdoor feedlots are uncovered, some paddocks may have an adjoining roofed building with concrete floor and straw bedding. Alternatively, nets or trees are often used to provide shade.
Outdoor feedlots usually have feed troughs lined along one side where feed can be provided. Total mixed ration, based on bought‐in or home‐grown feed, is the diet most often used. Troughs can be made of wood, metal or concrete and keep feed from being scattered. In most cases, the trough area is covered by a roof and the adjacent floor is concrete, making animal access easier, allowing calves to stand on a level surface while feeding and protecting the feed from rain or direct sunlight.
In Europe, outdoor feedlots are mainly found in southern regions (e.g. in Portugal). Elsewhere, outdoor paddocks are used for the periods when grazing is not possible (e.g. in Ireland).
3.12.2. Welfare consequence ‘Resting problems’
3.12.2.1. Description
For a description of resting problems in calves, see Section 3.3.7.1.
3.12.2.2. ABMs
See Section 3.3.7.2 (veal calves in individual pens).
3.12.2.3. Hazards
Lack of bedded lying area.
Deep mud during rainy season
Low space allowance.
3.12.2.4. Preventive and corrective measures
Provision of bedded lying area and shelter had a positive effect on the welfare of outdoor housed bulls (Tuomisto et al., 2009).
3.12.3. Welfare consequence ‘Heat stress’
3.12.3.1. Description
Heat stress is defined as stress or negative affective states such as discomfort or distress experienced by an animal exposed to a high effective temperature. Heat stress does not result solely from air temperature but is influenced by relative humidity (usually considered together with air temperature in form of a temperature–humidity index [THI]), air movement, cloud cover, rainfall, presence of shade and the possibility of ingesting cold water (Gaughan et al., 2002). Conditions of high relative humidity increase heat load by reducing the efficiency of sweating and panting as a means of heat loss. Metabolic heat increases with feed intake and so animals on the highest rations are usually more sensitive to heat. Dark coats absorb more heat than light coats when exposed to solar radiation.
Heat stress leads to a reduction in appetite, changes or inhibition of social and eating behaviours (increasing the number of meals and decreasing their duration) (Morand‐Fehr and Doreau, 2001), impaired immunity and health, discomfort and even death in extreme conditions (heat stroke) (Hubbard et al., 1999). Prolonged thermal panting, a behaviour shown by animals under heat stress, may lead to dehydration and alkalosis (Sparke et al., 2001). A threshold of 25°C has been proposed as the Upper Critical Temperature in cattle (EFSA AHAW Panel, 2022b). The number of days where the THI exceeds the comfort threshold for cattle is increasing in Europe (Schär et al., 2004; Segnalini et al., 2011; Renaudeau et al., 2012).
3.12.3.2. ABMs
Behavioural responses of heat stressed calves include increased respiration rate, panting, sweating, high body temperature, modifying drinking and feed intake, increased standing time and shade seeking, and decreased activity and movement (Polsky and von Keyserlingk, 2017). Respiration rate has been shown to be a good indicator of thermal stress (Brown‐Brandl et al., 2005; Brown‐Brandl et al., 2006). Because evaporative heat dissipation is achieved in part by sweating but mainly by increased respiration rate, it has been recommended to use respiration rate and panting (rapid shallow breathing) scores as a way of measuring heat stress (Eigenberg et al., 2000; Gaughan et al., 2008; Islam et al., 2020; EFSA AHAW Panel, 2022b) (Table 20).
Table 20.
ABMs for heat stress in outdoor feedlots
| ABM | Comments |
|---|---|
| Respiration rate (RR) |
Description: Frequency of breathing, usually measured by counting the movements of the flank manually and converting it into breaths per minute. Sensitivity: High. Specificity: Low. Pneumonia can also cause high respiratory rate. |
| Panting score |
Description: A panting score was described by with 5 grades (0 – normal respiration; 1 – elevated respiration; 2 – moderate panting and/or presence of drool or small amount of saliva; 3 – heavy open‐mouthed panting; saliva usually present; 4 – severe open‐mouthed panting accompanied by protruding tongue and excessive salivation; usually with neck extended forward (Mader et al., 2006). Sensitivity: High. Specificity: Low. Pneumonia or exercise can also cause panting. |
| Body temperature |
Description: Body temperature will increase above 39.5°C in animals in heat stress. Rectal temperature is the conventional way to measure temperature in cattle, but technology has provided alternatives such as thermography or intra‐ruminal bolus (Stygar et al., 2021). Sensitivity: High. Specificity: Low. Body temperature may also increase in cases of infectious disease. |
| Sweating |
Description: Loss of heat through evaporation (Idris et al., 2021). Visual signs of sweating in cattle are wet patches along animals' backs and shoulders. Sensitivity: Low. Cows sweat at a low rate so that it may be imperceptible (Gebremedhin et al., 2008; Wang et al., 2020). Specificity: High. |
3.12.3.3. Hazards
High temperature‐humidity index, high solar radiation and low wind speed
Lack of shade especially at the hotter times of the day
Lack of access to cold water, because of exposure of troughs to sunlight, insufficient water points, strong competition for these with weaker calves having difficulty to access water.
3.12.3.4. Preventive and corrective measures
Shade should be provided to calves kept in open pens. Fresh, clean and cold water should be available in several water troughs to reduce competition. Water flow should be sufficient for several animals drinking at the same time. Water should not be continuously exposed to sunlight in the hot seasons.
3.12.4. Welfare consequence ‘Respiratory disorders’
3.12.4.1. Description
See Section 3.2.9.1 (calves in individual housing in dairy farms) for an overview of respiratory disease in calves.
3.12.4.2. ABMs
The clinical signs and most important ABM are common to all systems and are presented in detail in Section 3.2.9.2.
3.12.4.3. Hazards
In addition to the hazards described in Section 3.2.9.3, calves kept in outdoor feedlots may be exposed to extreme weather conditions.
heat stress, and less frequently cold stress, may predispose calves to BRD by causing immunodepression, higher breathing frequency and inspiration duration;
very hot or very cold.
In contrast, by being outdoor and usually in very low animal‐density conditions, noxious gases, dust and bacteria circulation, will play a smaller role.
3.12.4.4. Preventive and corrective measures
See Section 3.2.9.4 (calves in individual housing in dairy farms) for general measures to prevent the disease in calves.
3.13. Overview of highly relevant welfare consequences in housing systems used to rear calves
Table 21 presents an overview of the welfare consequences ranked as highly relevant in each husbandry system. Welfare consequences ranked as medium, low, and not relevant in each system, are presented in Appendix A, as well quantitative results of the ranking procedure.
Table 21.
Summary of assessment of highly relevant welfare consequences per system (marked with an x/red cells)
| Calves – before weaning at dairy farm | Veal calves | CCC systems | Calves after weaning | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Section | 3.2 | 3.6 | 3.7 | 3.3 | 3.4 | 3.5 | 3.8 | 3.9 | 3.10 | 3.11 | 3.12 |
| Individual housing | Kept from birth till weaning in group housing in small groups with milk feeding by bucket/trough | Kept from birth till weaning in group housing in large groups and automatic milk feeding | Individual housing | Group housing in small groups with milk feeding by bucket/trough | Group housing in large groups and automatic milk feeding | Systems with cow–calf contact | From weaning to 6 months in fully or partially slatted floor group pens without bedding | From weaning to 6 months in fully or partly littered group pens | From weaning to 6 months in group pens with cubicles | From weaning to 6 months in outdoor feedlots | |
| Respiratory disorders | x | x | x | x | x | x | x | x | x | x | |
| Inability to perform exploratory or foraging behaviour | x | x | x | x | x | x | x | x | x | ||
| Gastroenteric disorders | x | x | x | x | x | x | x | ||||
| Inability to perform sucking behaviour | x | x | x | x | x | ||||||
| Group stress | x | x | x | x | x | x | x | ||||
| Inability to chew and ruminate | x | x | |||||||||
| Resting problems | x | x | x | x | x | x | |||||
| Inability to perform play behaviour | x | x | x | x | x | ||||||
| Restriction of movement | x | x | x | x | |||||||
| Prolonged hunger | x | x | x | ||||||||
| Isolation stress | x | x | |||||||||
| Metabolic disorders | x | x | |||||||||
| Separation stress | x | ||||||||||
| Heat stress | x | ||||||||||
| Handling stress | x | ||||||||||
3.14. Sources of uncertainty in the assessment of housing systems (Table 22)
Table 22.
Sources of uncertainty in the assessment of housing systems
| Topic | Sources of uncertainty | Impact of the uncertainty on the assessment |
|---|---|---|
| Identification and description of most prevalent housing systems |
|
No data were found in the literature on the prevalence of the different housing systems in the EU. Hence the selection of ‘most prevalent’ systems was based on expert knowledge and may have been over or underestimated. |
| Identification of welfare consequences (prevalence, severity, duration), hazards, ABMs and preventive measures |
Ranking of relevance of welfare consequences
|
Due to the sources of uncertainty listed, the number of relevant welfare consequences, ABMs and/or hazards may have been underestimated. |
3.15. Conclusions and recommendations general ToR
These conclusions and recommendations refer to general aspects of housing systems used to rear veal and dairy calves. Conclusions and recommendations for certain specific aspects (individual/group housing, space allowance, iron and fibre provision, CCC and ABMs at slaughter) are provided in Sections 3.16–3.18.
For the methodology on the certainty assessment, please refer to Section 2.2.5.
Conclusions on housing systems
The most common systems for rearing dairy calves from birth to weaning are individual housing, small group pens with milk feeding via bucket/trough and large group pens with automatic milk feeding. After weaning, calves are kept in group pens either with fully/partially slatted floors without bedding, in group pens with littered floor, in group pens with cubicles or in outdoor feedlots (certainty 90–100%).
Common veal calf housing systems are individual housing (‘individual pens’) (from their arrival to the veal farm to maximum 8 weeks), small group pens with milk feeding via bucket/trough and large group pens with automatic milk feeding (certainty 90–100%).
Highly relevant welfare consequences of individual housing of calves in dairy and veal farms are the inability to perform exploratory or foraging behaviour, inability to perform sucking behaviour, gastroenteric disorders, respiratory disorders, restriction of movement, isolation stress and inability to perform play behaviour. Calves in individual pens in dairy farms also experience prolonged hunger (certainty 90–100%).
Highly relevant welfare consequences of housing systems used to rear calves in groups before weaning in dairy farms are inability to perform exploratory or foraging behaviour, inability to perform sucking behaviour, gastroenteric disorders, respiratory disorders and prolonged hunger. Inability to perform play behaviour also affects calves kept in small groups, and group stress is observed in calves kept in large groups with automatic milk feeding (certainty 90–100%).
Highly relevant welfare consequences of housing veal calves in group pens (small and large groups), and under the typical feeding management in veal production, are inability to perform exploratory or foraging behaviour, inability to perform sucking behaviour (especially in small groups), gastroenteric disorders, respiratory disorders, inability to chew and ruminate, resting problems, group stress and metabolic disorders (anaemia) (certainty 90–100%).
Highly relevant welfare consequences for calves housed in group pens after weaning are respiratory disorders, inability to perform exploratory and foraging behaviour, group stress (especially in large groups), restriction of movement (when no bedding is provided or when animals are kept in cubicle pens), resting problems and the inability to perform play behaviour when calves are kept in group pens with slatted floors (certainty 90–100%).
Highly relevant welfare consequences of CCC systems are respiratory disorders, gastroenteric disorders, group stress, handling stress and separation stress (certainty 90–100%).
Respiratory disorders are a highly relevant welfare consequence of all systems assessed. The risk of respiratory disorders is minimised by ensuring adequate colostrum intake and good ventilation (to reduce exposure to noxious gases and dust), minimising stressful events linked with transport as well as commingling and alterations of group composition, avoiding exposing calves to cold and heat stress or by applying preventive vaccination (certainty 90–100%).
The inability to perform exploratory or foraging behaviour is a highly relevant welfare consequence in all the housing systems assessed with the exception of outdoor feedlots (after weaning) and CCC systems. The risk of the inability to perform exploratory or foraging behaviour is reduced by access to outdoor area, provision of enrichment or access to ad libitum roughage (certainty 90–100%).
Prolonged hunger is a welfare consequence commonly observed in dairy calves before weaning except in CCC systems. Prolonged hunger can be prevented by providing calves with milk corresponding to approximately 20% of their body weight per day until at least 4 weeks of life (certainty 90–100%).
Recommendations on housing systems
For each housing system, preventive measures to the hazards have been identified and are presented in Sections 3.2–3.12.
Measures to improve current husbandry practices include keeping calves from an early age onwards in stable groups with other calves and/or their dam, increase the space allowance per animal, allow dedicated lying areas with deformable lying surfaces (preferably bedding), and keeping calves in buildings with good ventilation. If kept outdoors, calves should have protection from heat and cold by having access to shade or insulated shelters and with the provision of dry, deformable, insulating bedding if in cold regions.
Adequate colostrum management and provision of a least 4–5 cm long roughage in racks (see Section 3.16.3).
Provision of brushes is recommended, but further research is needed regarding the welfare effects of ratio of calves per brush or brush type.
Compared with current practice, milk feeding practices of dairy calves should include provision of large milk amounts (~ 20% body weight per day until at least 4 weeks of life). Milk should be distributed to dairy and veal calves over at least 3 meals a day and provided through a teat. Abrupt weaning should be avoided by gradually (over at least 1 week) decreasing milk amounts and carried out preferably on an individual basis (e.g. depending on solid feed intake).
Transport events, commingling and regrouping should be avoided through the fattening of calves in the farm of origin or in units close by. If calves are still transported, long journeys should be avoided and animals should not go through auction markets.
Water should be provided through an open surface. Even if provided with milk, calves should have permanent access to drinking water because milk does not cover water requirements.
Occurrence of respiratory disorders is an issue of all housing systems and efforts should be made to house calves in an environment with sufficient air volume and well‐managed ventilation. Further research is needed for specific recommendations on these parameters. Calves kept outdoors are less exposed to poor‐quality air but should be protected from inclement weather conditions which are also risk factors for respiratory disease.
3.16. Specific Scenario 1 – The welfare of male dairy calves raised for producing ‘white’ veal meat and the risks associated with individual housing, insufficient space and feed restriction (such as deprivation of iron and fibres)
3.16.1. Risks associated with individual housing (conclusions on group size and age at grouping)
3.16.1.1. Assessment scope and assumptions
The ToR requests an assessment of the welfare risks of individual housing. To address this request, the following subquestions of interest were formulated:
Is group housing of young calves beneficial from a welfare point of view, compared with individual housing?
If yes, at what age should calves be grouped to maximise positive effects of social housing and minimise the negative effects related to occurrence of disease?
If yes, at which group size should calves be kept to maximise the positive effects of social housing and minimise the negative effects related to exposure to disease?
To address these questions, a literature review was carried out on welfare consequences from individual and group housing and how welfare is affected by age at grouping and group size. Natural behaviour and immunity development of young calves were reviewed as a starting point to provide an understanding of the positive effects of social housing on young calf's social competence, learning abilities, feeding behaviours and affective states, as well as the potential negative effects of early group housing on health. Conclusions and recommendations for the first question (age at grouping) were based on a discussion of the literature review and consensus among the group, while for the second question (group size), an EKE was carried out to estimate the relationship between group size and prevalence of respiratory disorders.
3.16.1.2. Natural behaviour and physiology of calves during early life
In a natural setting, the dam often stays in the periphery of the herd in the first days after parturition. The calf typically ‘hides’ in vegetation, while the dam grazes close by (Vitale et al., 1986) or stands over their calf guarding it (Kiley‐Worthington and Plain, 1983). The dam is therefore the main social partner of the calf during the first week(s) of life, and it is during this period that the maternal‐filial bond is established (von Keyserlingk and Weary, 2007). After the ‘hiding’ phase, the dam and calf re‐joins the herd, and the calf interacts with same‐age calves in addition to the continued contact with the dam (Wood‐Gush et al., 1984; Vitale et al., 1986; Sato et al., 1987). The duration of the contact between calf and dam decreases with age as the suckling bouts become fewer (Vitale et al., 1986), but when kept together, the dam and calf maintain a preferential social relationship even after the birth of a new calf (Veissier et al., 1990).
During early life, changes in the calf's immunity occur. At birth, calves are immunologically naïve, and ingestion of colostrum within the first hours of life is essential for acquiring maternal antibodies (passive immunity) (Chase and Kaushik, 2019). Between ~ 2 and 3 weeks of age, the passive immunity starts to fade but the calf's active immunity is not yet fully developed, which puts calves at higher risk of acquiring infectious diseases during this immunological gap phase (between ~ 3 and 7 weeks of life; Hulbert and Moisá, 2016). Additional details on GE aetiology and clinical signs observed in calves are described in Section 3.2.8.
3.16.1.3. Is group housing of young calves beneficial compared with individual housing?
This section summarises the main outcomes of a thorough review of the welfare impacts of isolation or individual housing on calf's social behaviour (available in Jensen (2018)), learning ability, affective states and feeding behaviour. Because negative welfare consequences can also be observed in calves reared in groups, such as respiratory disorders, gastroenteric disorders and group stress, these are also discussed below.
The main reasons for keeping calves in individual pens is the easier management of the animal for monitoring general health status and milk intake, reduction of cross‐sucking and lower risk of transmission of disease.
Impact of individual housing on social behaviour . Unweaned dairy calves associate with other calves if they have this opportunity. For instance, individually housed calves sniffed and licked the neighbouring calf through pen partitions, but the level of social behaviour was lower than that of pair‐housed calves that could perform all aspects of social behaviour (Duve and Jensen, 2012). It is also known that social contact reduces pre‐weaned calf's fearfulness of other calves; calves housed in pairs or small groups more readily approached and interacted with an unfamiliar calf in a social test than individually housed calves (Jensen et al., 1997; Duve and Jensen, 2011; De Paula Vieira et al., 2012). The level of social contact matters: calves that could not see and touch other calves were the most fearful, pair housed calves were the least fearful, while individually housed calves with tactile contact were intermediate (Jensen and Larsen, 2014). Although individually housed calves are more reluctant to approach and interact with an unfamiliar calf, once they have made contact (either in a social test, or at regrouping) these calves engaged in more agonistic social interactions than pair housed calves (De Paula Vieira et al., 2010; Duve and Jensen, 2011; Jensen and Larsen, 2014). According to the current legislation, calves in individual pens must have visual and tactile contact. The findings that individually housed calves with visual and tactile contact are more fearful of other calves during a test (Jensen and Larsen, 2014) and more aggressive after regrouping (Jensen and Larsen, 2013) than pair housed calves, illustrate that full social interaction is a prerequisite for the development of social skills.
Compared with individually housed calves, pair or group housed calves form social bonds. For instance, they prefer a pen mate to an unfamiliar calf in a preference test (Færevik et al., 2006; Lindner et al., 2022), and this preference is stronger the longer they have been housed together (Duve and Jensen, 2011). Furthermore, previously pair‐housed calves spent more time lying within 1 m of another calf after regrouping than individually housed calves (Lindner et al., 2021).
In addition to being more fearful of other calves, individually housed calves are also more fearful than socially housed calves when introduced to a novel environment (Jensen et al., 1997; Jensen and Larsen, 2014). Being housed alone in a small pen is likely to be associated with stress, as also supported by a lower cortisol response to ACTH (adrenocorticotropic hormone) in individually housed compared with pair housed calves (Raussi et al., 2003). This indicates that social housing improves the calf's ability to cope with challenges such as weaning, regrouping and other common management practices.
Impact of individual housing on learning ability . Isolation has negative effects on learning ability of calves, with calf's cognitive skills being improved by early social housing. The proportion of calves learning a reversal task at 7 weeks of age was higher in calves paired at 6 days old, intermediate in calves paired at 6 weeks of age and lowest for individually reared calves (Meagher et al., 2015). Reversal learning was also superior in pair‐housed calves compared with individually housed calves (Gaillard et al., 2014). Calves housed in dam‐calf groups were also less neophobic (Costa et al., 2014).
Impact of individual housing on affective states. Calf's affective states are positively affected by group housing. Pair‐housed calves judged ambiguous cues more positively in a judgement bias test (Bučková et al., 2019) compared with individually housed calves, indicating more positive affective states. Social housing with peers also reduced calf's responses to stressors and thus provided social buffering (Rault, 2012) when calves were separated from the group (Færevik et al., 2006), during blood sampling (Duve et al., 2012) and during the period of weaning (De Paula Vieira et al., 2010; Bolt et al., 2017). Thus, social housing appears not only to provide the opportunity for positive experiences, but also to mitigate the effects of negative experiences.
Impact of individual housing on feeding behaviour. Several studies have found social housing to stimulate solid feed intake in the pre‐weaning period. It could be assumed that such an effect could depend on the milk‐feeding level, e.g. the higher feeding motivation in low‐fed calves overrides the effect of social housing, it was found studies with high as well as low level of milk allowance (e.g. low milk allowance: Babu et al., 2004; Phillips, 2004; Hepola et al., 2006; Tapki, 2007; high milk allowance: Costa et al., 2015; Whalin et al., 2018; and ad libitum feeding: De Paula Vieira et al., 2010; Miller‐Cushon and DeVries, 2016; Overvest et al., 2018). However, some studies failed to find such an effect both when offering milk ad libitum (Chua et al., 2002) and when offering 6 L/day (Jensen, 2004; Bolt et al., 2017). During the first 3–4 weeks calves can only digest small amounts of solid feed (Kristensen et al., 2007), but low intakes early on may enhance intake later when the calf's nutritional needs are no longer covered by milk and when the calf is weaned off milk.
Among calves housed in large groups (here: in groups of 8–18 calves) and fed via an AMF, calves that were group housed when they were 6 days old were more inactive and required more assistance to learn to use the milk feeder than calves that were group housed when 14 days old (Rasmussen et al., 2006; Jensen, 2007). In a similar set‐up, the earlier the calf was group housed in a large group between 5 and 14 days old (Fujiwara et al., 2014) and between 1 and 5 days of age (Medrano‐Galarza et al., 2018), the longer it took before it visited a milk feeder. This indicates that early housing of a calf in a large group may be detrimental to its welfare. An age effect can however not be ruled out, as there is a lack of studies using smaller groups due to the fact that AMF are not used with small group sizes.
With regard to influence of age at grouping on concentrate intake, Costa et al. (2015) found that calves paired at 6 days of age consumed more concentrate (in the period from 3 to 10 weeks) than calves paired at the age of 6 weeks and individually housed, which suggests that early social housing, and thus early social facilitation of concentrate intake, is important for later concentrate intake. Mahendran et al. (2021) saw a higher concentrate intake in paired calves compared with individually housed animals, but no difference between calves paired days after birth and paired at 21 days old. These results are in line with outcomes from a study that found no difference in concentrate intake (measured at 3–7 weeks) between calves paired within 3 days of birth and at 14 days old (Jensen and Larsen, 2014), or between calves paired within 3 days of birth and at 21 days old (Duve and Jensen, 2012). Bolt et al. (2017) also did not find a difference between calves paired at day 28 compared with calves paired at day 5. Studies comparing more different ages at grouping are needed but based on the above it may be hypothesised that to obtain a positive effect of social housing on concentrate intake, calves should be socially housed at some point between 2 and 6 weeks of age. It should be noted, however, that differences in milk allowance may also play a role on solid feed intake as Jensen et al. (2015) found that pair housed calves ingested more concentrate than individually housed calves when the calves were fed 9 L/day during the first 4 weeks, but not when fed 6 L/day throughout. Although early social facilitation of feeding on concentrates is likely important, the differences in intake may not be evident until the calves start eating a substantial amount. For instance, Miller‐Cushon and DeVries (2016) did not detect difference in intakes between individual and pair housed calves until week 6. The comparison between the studies is complicated by differences in milk allowance, weaning management and the period of data collection.
In summary, the effect of social contact on intake of solid feed is likely due to social facilitation of feeding, and this may play a bigger role in calves that have their nutritional need covered by milk during the first weeks. Because calves fed high volumes of milk do not eat much solid feed, it is typically a challenge to wean these calves off milk. However, social housing, through social facilitation of feeding on solid feeds, may help solve this problem, especially if calves can eat at the same time. When pair housed calves could eat at the same time, they consumed more concentrates than if only one calf of the pair could eat at the time (Miller‐Cushon et al., 2014).
Respiratory disorders and gastroenteric disorders may occur due to higher exposure to infectious agents than in individual housing. Upper and lower (pneumonia) respiratory tract disease, independently of its cause, is traditionally designated BRD and may result in severe illness. Studies have found poorer calf health in groups compared with individual housing (Gulliksen et al., 2009b; Karle et al., 2019), and that larger groups have a higher risk of respiratory disease (Svensson et al., 2003; Svensson and Liberg, 2006; Karle et al., 2019).
Another relevant welfare consequence experienced by calves kept in group pens is group stress. Group stress can be especially relevant in veal farms when calves are grouped with unfamiliar calves from different farm origins. Some veal farms also frequently regroup animals to achieve comparable growth rates and a homogeneous weight in each pen, which may be an additional source of stress. It is well established that stress is a predisposing factor for BRD (Hodgson et al., 2012; Griebel et al., 2014). Grouping and constant change in group composition will induce stress that may lead to lower immune function and increase the susceptibility to infection by calves (Bach et al., 2010; Hulbert and Ballou, 2012).
In summary, there is a large body of evidence against individual housing of calves due to detrimental effects on social behaviour development, learning ability, feeding behaviour and affective states. Group housing has, however, been linked to a higher prevalence of respiratory, GE disorders and group stress.
3.16.1.4. At what age should calves be grouped to maximise positive effects of social housing and minimise negative effects related with occurrence of disease?
3.16.1.4.1. Summary of available evidence in the literature
The main results from the literature on the effects of grouping age on calf's welfare are summarised in Table 23.
Table 23.
Summary of main welfare effects reported in the literature depending on age at grouping
| Age at grouping | Positive effects on welfare | Negative effects on welfare | Reference |
|---|---|---|---|
| Group housed between 3 and 6 days old |
ABM – Vocalisations Calves paired between 3 and 6 days vocalised less in the post‐weaning period compared with calves group housed at day 28. |
Bolt et al. (2017) | |
| Group housed from 6 days old |
ABM – Learning ability More calves group‐housed from day 6 learned a reversal task compared with calves housed individually ABM – Mixed ration (grain, silage and hay) intake No differences in intake between calves paired at 6 days and 6 weeks. |
Meagher et al. (2015) | |
|
ABM – Concentrate intake Calves paired at 6 days consumed more concentrate than calves paired at 6 weeks |
Costa et al. (2015) | ||
| Group housed at 9–12 days |
ABM – Respiratory disease Prevalence of respiratory disease was higher in calves group housed at 9–12 days compared with calves group housed 1 week later (at 13–19 days old) |
Svensson and Liberg (2006) | |
| Group housed from 3 weeks old |
ABM – Social behaviour Calves housed in pairs from 3 weeks onwards performed more social behaviour than calves that remained in individual pens |
Duve and Jensen (2012) |
Most studies on pair or small group housing implemented this treatment from 3 to 6 days of age, as the establishment of pairs or group requires two to several calves to be born.
Research has demonstrated a wide range of beneficial effects for early group housing compared with individual housing, including increased social competences and social buffering (Bolt et al., 2017), more positive affective states and higher learning ability (see Costa et al., 2016) for review). However, a calf's immune status may have to be considered regarding the timing of the introduction to social housing. If colostrum management is good (please refer to Section 3.2.8.4 for a description of good colostrum management practices), the level of maternal immunity is highest in the first week of life and, because the calf's own active immunity builds up slowly, calf immunity is at its lowest at 2–3 weeks of age (Hulbert and Moisá, 2016). Besides age at grouping, husbandry and management of group‐housed calves also seem to play an important role. Rearing calves in stable groups resulted in higher daily gain and a lower incidence of disease than dynamic group management (i.e. regrouping; Pedersen et al., 2009).
In view of these effects, it was concluded that social housing in pairs or small stable groups within 1 week of age appears to pose no or little threat to calf welfare and seems the best trade‐off to allow calves to be exposed to the benefits of social housing without substantially increasing the likelihood of health disorders such as respiratory disorders.
Conclusions on this aspect were provided based on the literature and interpretation of past research studies; however, it is worth noting that the understanding the impact of age at grouping on welfare is limited by some data gaps. For instance, there is a lack of understanding of the effects of isolation of calves during their first week of life; in a natural situation, a calf would be isolated from the herd and interact with the dam only for the first few days of life, but currently it is not known to what extent interacting with a calf of a similar age can mitigate the lack of contact with the dam when early separation from the dam occurs. There are also limited data on the calf's ability to develop social relationships with other calves during the first week, and on the impact for animal welfare of housing a calf in a large group as opposed to a small group during the first weeks of life.
3.16.1.5. At what group size should calves be grouped to maximise the positive effects of social housing and minimise negative effects related with exposure to disease?
To provide an estimate of the relationship between group size and likelihood of development of health disorders, the WG has thoroughly reviewed past research on this topic and provided an estimate of the prevalence of respiratory disorders expected at each group size via EKE. Evidence from experimental studies published in peer‐reviewed journals served as a basis to estimate the relationship between these two variables.
3.16.1.5.1. EKE model parameters
A summary on the EKE parameters and components is presented in Table 24.
Table 24.
Summary of EKE parameters
| EKE components | Description |
|---|---|
| Animal category |
Calves reared for white veal meat. It was assumed that: calves left the dairy farm where they were born at the age of 2 weeks, were transported to the auction market and then to the veal farm or transported directly from the farm of origin to the veal farm. At arrival, they were placed either in an individual pens or in a group pen; transport and commingling increased the risk of respiratory disorders; and the prevalence of respiratory disorders peaked at approximately 6 weeks of age (approximately 3 weeks after arrival at the veal farm). Six weeks of age was the age of the calves considered in the EKE for the estimation of respiratory disorders prevalence. |
| Husbandry system | White veal rearing systems |
| Exposure variable of interest | Group size |
| Welfare consequence | Respiratory disorders |
| ABM |
% of animals affected by respiratory disorders in a group at the age of 6 weeks. The period of 6 weeks were selected as the age of interest for the EKE because it was assumed that a group pen would experience the peak of respiratory disorder prevalence at this stage. This was due to the fact that calves arrive at the veal farm at about 2–4 weeks old, during a time of low immunity, and respiratory disorder can take a couple of weeks to develop and become apparent. Respiratory disorder was defined as animals presenting one or more of the clinical signs: ‘Increased respiratory sounds at lung auscultation, or moderately increased respiratory sounds together with coughing and/or nasal discharge (increased respiratory sounds were defined as obviously increased bronchial (or vesicular) breath sounds or presence of adventitious sounds synchronous with breathing) or coughing or sneezing for > 2 days’ (adapted from Svensson and Liberg (2006). Animals showing one or more of these signs were considered to have respiratory disorders. |
3.16.1.5.2. Sources of uncertainty in the estimates
Additional factors, other than group size, can influence the occurrence of respiratory disorders, such as type of season, colostrum management at the source farm and group stability. These aspects were considered sources of uncertainty in the estimate because, although it is assumed that they can affect the prevalence of respiratory disorder, their exact impact is not well understood. A list of the sources of uncertainty identified can be found in Table 25.
Table 25.
Sources of uncertainty on the relationship between group size and prevalence of respiratory disorder estimated via EKE
| Sources of uncertainty | Reason |
|---|---|
| Housing type (e.g. igloo vs closed barn), including presence or absence of ventilation | Whether the animal is reared indoors or has access to an outdoor run; total air volume and the degree of ventilation can have an impact on the prevalence of respiratory diseases (Moser et al., 2020) |
| Humidity and temperature (temperature humidity index) | Seasonal effects are possible (e.g. colder temperatures may be associated with higher rate of respiratory disorders) |
| Stocking density | Larger stocking density can be associated with higher rate of respiratory disorders |
| Regrouping | Less stable groups (with higher rate of groups being rearranged, with animals being moved in and out) can be associated with a higher rate of respiratory disorders (Pedersen et al., 2009) |
| Immune system of the calf | A higher immune competence is thought to be associated with a lower prevalence of respiratory diseases (Langel et al., 2015). This will depend on the quality and quantity of colostrum ingested by the calf and on the vaccination status of the dam |
| Antibiotic use (metaphylactic) | Prevalence of respiratory disease can depend on whether metaphylactic application of antibiotics is carried out (Pardon et al., 2012; Lava et al., 2016) |
| Feeding system (bucket versus shared teats) | Automatic systems (with competition and teat sharing) can be associated with higher prevalence of disorders |
Although group size is a continuous variable, for the purposes of the estimation of the expected prevalence of respiratory disorder depending on group size, it was deemed to be more useful to consider some key group size categories; i.e. group size categories commonly used in veal farms and categories that could be interesting from a welfare point of view. The rationale behind the chosen categories is presented in Table 26.
Table 26.
Group size categories considered in the EKE
| Group size category | Rationale and comments |
|---|---|
| Individual pens | This housing system is common in veal farms in calves between 2 and 6 to 8 weeks of age. In this housing system, the effect of commingling (i.e. grouping animals from different sources in the same pen) is largely reduced because animals, although able to see and touch other calves, do not have full contact. |
| Pens of 2–3 animals | Although not common in veal farms, the estimation of prevalence on this housing system was deemed important because it was considered that this group size could provide the advantages of social housing and prevent the disease problems associated with larger groups. |
| Pens of 4–7 animals | Very common group size in veal farms. Regrouping is relatively common especially in pens with milk troughs where calf weight variation is higher than in automated milk feeding systems. |
| Pens of 12–18 animals | Not a very common group size in veal farms. Literature suggests that respiratory disorders in calves kept in groups larger than 10 tend to be higher. |
| Pens of 30–40 animals | This group size can be found in veal farms in the Netherlands (~ 5% of farms) and France (5–10% of farms). Regrouping is not common in this housing system. No data were found in the literature on respiratory disorder incidence for this group size. |
3.16.1.6. Results of assessment on group size
3.16.1.6.1. Summary of available evidence in the literature on the relationship between group size and respiratory disorder prevalence
This section summarises the results from studies investigating the relationship between group size and prevalence of respiratory disorders. Out of the studies identified, only one focused on veal calves (Abdelfattah et al., 2013) with all the others being carried out in calves on dairy farms (Svensson et al., 2003; Svensson and Liberg, 2006; Cobb et al., 2014; Karle et al., 2019).
A higher prevalence of respiratory disorders in large groups of calves compared with individual or small groups was reported throughout the reviewed studies. For instance, in an experimental study with 168 veal calves, animals kept in groups of eight showed significantly more coughing than calves kept in pairs during the first month of life (Abdelfattah et al., 2013). The same pattern was observed in experimental and observation studies in dairy calves. In 892 dairy calves kept in small (6–9 calves) and large (12–18 calves) groups in nine commercial dairy farms in Sweden, the odds of having increased respiratory sounds was smaller in small groups compared with large groups (odds ratio of 0.69) (Svensson and Liberg, 2006). The same trend was identified in a study investigating disease in heifer calves between 0 and 90 days of age from 122 dairy herds: the prevalence of respiratory disorder and increased respiratory sounds were 14.1% for calves housed in large‐group pens, 8.6% in small‐group pens and 5.4% in individual pens (Svensson et al., 2003). A lower prevalence of respiratory disorders in smaller group size was also identified in an observational study in the USA undertaken in 100 dairy farms, with 16% in pens with 8 animals or more, 9% in pens with 2–7 calves, and 7% in individual pens (Karle et al., 2019). Similarly, a previous study undertaken in the USA concluded that raising calves in groups with more than 7 calves was associated with a higher mortality but found no differences between farms with individually housed calves or groups of 6 or fewer (Losinger and Heinrichs, 1997).
The results from these studies were used as a basis for the EKE on the relationship between group size and the prevalence of respiratory disorders.
3.16.1.6.2. Results from EKE – respiratory disorder estimates for different group sizes
It was estimated that the prevalence of respiratory disorders in calves reared for veal increases with increasing group size. The shape of this relationship was assessed to be non‐linear (sigmoidal curve).
It was estimated that 6‐week‐old calves:
Housed in individual pens have a median prevalence of respiratory disorders of 12% (90% credible interval 2–28).
Housed in groups of 2–3 animals have a median prevalence of respiratory disorders of 12% (90% credible interval 2–28).
Housed in groups of 4–7 animals have a median prevalence of respiratory disorders of 13% (90% credible interval 4–27).
Housed in groups of 12–18 animals have a median prevalence of respiratory disorders of 30% (90% credible interval 10–53).
Housed in groups of 30–40 animals have a median prevalence of respiratory disorders of 35% (90% credible interval 8–62).
Figure 19 shows the prevalence of respiratory disorder in each group size for the different group size categories estimated via EKE.
Figure 19.
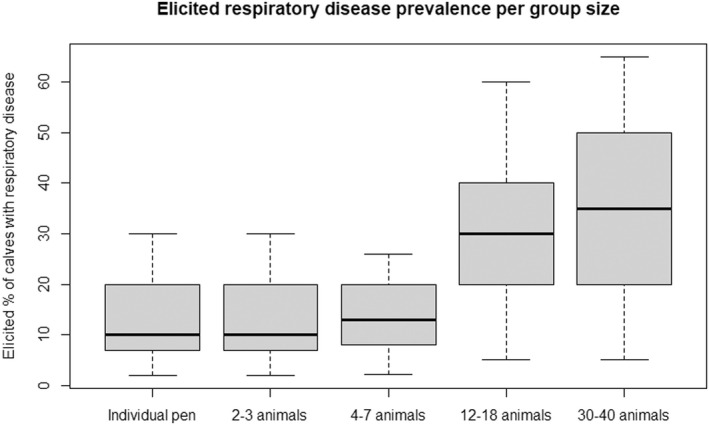
EKE estimates on prevalence of respiratory disorder depending on group size category. Median value, interquartile ranges and 1st and 99th percentile are represented via boxplots to represent the uncertainty around each value
The results of the literature review presented above and the EKE outcomes served as a basis for the conclusions below.
3.16.1.7. Conclusions on individual and group housing
Under natural conditions, the dam is the most important social partner of a calf in the first week after birth. The duration of the contact between the calf and the dam decreases progressively during the following weeks, during which the calf establishes social bonds with calves of similar age (certainty 90–100%).
Calves kept in individual pens for up to 8 weeks of age experience isolation stress which is reflected in heightened fear reactions to other calves and situations of novelty, as well as cognitive deficits. Other highly relevant negative welfare consequences of individual housing systems in veal farms and in dairy farms are the inability to perform exploratory behaviour, inability to perform play behaviour, restriction of movement, resting problems and the inability to perform sucking behaviour (certainty 90–100%).
Advantages of group housing include more developed social behaviour, higher learning ability, social buffering (less reaction to stressful events), more positive affective states and larger solid feed intake (certainty 90–100%).
The risk of respiratory disorders increases with increasing group size. The estimated median prevalence of respiratory disorders in veal calves housed in groups of 2–3 animals (12%; 90% credible interval 2–28) is similar to that of individually housed calves (12%; 90% credible interval 2–28), and to that of group pens of 4–7 calves (13%; 90% credible interval 4–27), and considerably higher in groups of 12–18 calves (30%; 90% credible interval 10–53) and in groups of 30–40 animals (35%; 90% credible interval 8–62).
There is limited understanding of the risk of respiratory disease in calves kept in groups between 7 and 12 animals.
Disadvantages of housing veal calves younger than 6 weeks old in large groups (> 7 animals) include higher exposure to infectious disease agents (certainty 90–100%) and risk of being exposed to group stress and cross‐sucking (certainty 66–100%).
Welfare benefits of group housing from the first week of life are more developed social behaviour, higher learning ability, social buffering and more positive affective states, compared with individual housing (certainty 90–100%).
If colostrum management is adequate, maternal immunity levels are high during the first weeks of life of the calf. However, maternal immunity is not fully protective if calves are highly exposed to pathogens originating from different farms, especially when calves are commingled in large groups (> 7 animals) (certainty 90–100%).
Compared with individual housing, housing calves in pairs or small groups (2–7 calves) from the first week of life allows them to experience the welfare benefits of contact with other calves without increasing the risk of infectious disorders by no more than ~ 1% (certainty 90–100%).
Certain common practices in the veal sector increase the risk of health disorders. Transport of calves from dairy farms to auction markets and to the veal farms when immunity competence is at its the lowest levels (2–4 weeks of age) at 14–21 days together with low immunity levels observed in this period, increases the susceptibility to disease (certainty 90–100%).
Commingling exposes calves to pathogens and increases the risk of infectious disease, increases stress resultant from the interaction with unknown calves and provokes disturbance of the group composition (certainty 90–100%).
Calves that remain with the same pen mates when moved from the dairy farm to the veal unit experience reduced levels of stress and increased social support compared with calves grouped with unfamiliar calves at arrival (certainty 66–100%).
There is a lack of understanding of the effects of isolation during the first week of life for calves. There are also limited data on the development of social relationships for calves under natural conditions and between same age calves and on the welfare impact of group size during the first week(s) of life (certainty 90–100%).
There is limited understanding of the risk of respiratory disease in calves kept in groups between 7 and 12 animals (certainty 90–100%).
3.16.1.8. Recommendations on individual and group housing
Unless they have contact with the dam, calves should be moved to and kept in pairs or small groups (2–7 animals) within the first week of life (i.e. before day 7). It is not recommendable to house young calves in large groups (> 7 animals) within the first week of life.
Calves should not be kept individually at the veal unit.
Veal calves should be housed in groups of maximum 7 animals at least until the age of 6 weeks.
If possible, calves should be kept with a familiar pen mate(s) from the dairy farm of origin after arrival at the veal unit.
On the veal farm, groups should be kept stable as much as possible.
Aspects such as ventilation and pen air volume should be well managed, but further research is needed for specific recommendations on these parameters.
3.16.2. Risks associated with insufficient space (conclusions on space allowance)
3.16.2.1. Assessment scope and assumptions
In the context of this Specific Scenario, ‘space’ was interpreted as the number of total square metres available to a calf.
3.16.2.2. Welfare consequences of restricted space allowance
The inability to perform locomotor play behaviour was identified as an important welfare issue experienced by calves kept in small pens. Young animals play when their physiological needs are met, indicating the absence of welfare threats. In addition, the performance of play is also believed to be associated with positive experiences and thus indicate positive welfare. Play behaviour can be categorised in different types and may be either locomotor, social play or object play. It has been shown that calves are intrinsically motivated to perform locomotor play behaviour, and this behaviour is affected by space available to the calf (e.g. Jensen and Kyhn, 2000). Research has shown that housing calves in small pens limits locomotor play and increasing space allowance increases the occurrence of locomotor play and allows the performance of more (or all) elements of the species‐specific locomotor play. There have been only a few studies looking at the behaviour displayed by calves (locomotor play and other behaviours) housed in environments with large space allowances (e.g. > 8 m2, Waiblinger et al., 2020) and most studies focused on space allowances between 1.5 and 4 m2/animal.
Other welfare consequences experienced by calves kept in pens with limited space include restriction of movement (Jensen, 1999), resting problems (Færevik et al., 2008) and the inability to perform play behaviour (Jensen et al., 1998; Jensen and Kyhn, 2000; Waiblinger et al., 2020).
3.16.2.2.1. EKE model parameters
A theoretical model integrating the general concept of a ‘non‐exposed’ population and based on expert estimates was applied to provide quantitative recommendations on space allowance for veal calves, as requested in the mandate. To provide an estimate of the relationship between space allowance and play behaviour, and give recommendations on space allowance that would allow a calf to play as much as it would if not restricted by space, a theoretical model integrating the general concept of a ‘non‐exposed’ population and based on expert estimates was applied (see Section 2.2.3 on Methodologies for more details).
It was noted that the size of the group, and therefore, the total space available to a calf, can influence the time a calf spends playing due to shared space effects. For this reason, two categories of group size were considered when providing quantitative recommendations:
Individual pens
Small groups (4–7 animals) pens, as this is a typical size of a veal calf group in the EU.
Large groups of veal calves (with approximately or more than 40 animals) can be found in the Netherlands and France. Because this is a production system not common elsewhere in the EU, no quantitative EKE estimates were provided for this scenario. An overview of the EKE parameters and components is presented in Table 27.
Table 27.
Background and assumptions of the EKE exercise on the effects of restricted space allowance on veal calf welfare
| EKE components | Definitions and assumptions |
|---|---|
| Exact wording of the Specific Scenario | The welfare of male dairy calves raised for producing ‘white’ veal meat and the risks associated with individual housing, insufficient space and feed restriction (such as deprivation of iron and fibres) |
| Interpretation of the scenario | This Specific Scenario requires to assess three separate aspects. In this section insufficient space will be addressed |
| Animal category to be considered | Veal calves (from 14 days to 6 months old) |
| Husbandry system | White veal calf production systems |
| The exposure variable of interest for the EKE |
Space allowance per animal (m2/calf) The larger the group of animals, the larger the shared space available in an enclosure. Both the space available per individual animal and the shared space are considered to influence locomotor behaviour |
| Selected welfare consequence | The welfare consequence assessed by EKE was the inability to perform play behaviour |
| ABM chosen for the exercise |
The ABM chosen for assessing inability to perform play behaviour was ‘% total time of locomotor play behaviour during the first 3 months’. The ABM locomotor play in calves includes galloping, jumping, hindleg kicking and fast turns. The fast gait trotting may be seen in connection with play but also in other connections and is not included in the definition. The motivational basis of social play may be different from locomotor play and therefore social play was not included. Object play is rare in calves and little researched and thus not included |
| Unexposed population | Defined as a hypothetical group of calves of an ‘average’ breed aged between 2 weeks and 3 months, kept in an enclosure with infinite space and non‐slippery flooring (e.g. bedded flooring). It was assumed that animals were kept at full (near ad libitum) milk allowance, were additionally provided energy through feed to reach the intended growth rates (or their genetic potential for growth), and were maintained at the physiological level for haemoglobin, ensuring that play behaviour was not impaired by poor nutrition nor disease. |
| Highly exposed population | Defined as a hypothetical group of calves reared under a standard white veal production system, aged between 2 weeks and 6 months, kept in pens of 1.8 m2/animal (minimum space allowed in current legislation). |
Evidence from experimental studies published in peer‐reviewed journals served as a basis to estimate the relationship between the space allowance (‘number of m2 available to the calf’) and the ‘percentage of time a calf aged between 2 and 3 months spends in locomotor play behaviour per day’. To the authors' knowledge, only two publications have reported locomotor play behaviour levels observed in calves with access to large (i.e. > 10 m2) space allowances (Waiblinger et al., 2020; Bailly‐Caumette et al., 2022). A short summary of the study conditions and context is provided. Bailly‐Caumette et al. (2022)) and others observed 48 Holstein calves housed in a 9 m × 7.5 m large straw‐bedded pens together with their dam and 3 other dam‐calf pairs. Of the total pen area, 12 m2 was designated to calf creeps, leaving 55.5 m2 of free pen space (13.9 m2 per cow–calf‐pair). Calves were observed at an average age of 21 days (range 3–36 days, week 3) and 49 days (range from 27 to 66 days, week 7) from video recordings obtained via cameras placed above each pen. The play behaviour of two randomly selected calves per pen was recorded continuously for 24 h. Recordings included both social play (frontal pushing) and locomotor play behaviour (defined according to (Jensen et al., 1998)). For the purpose of the EKE carried out by the working group, only data for locomotor play behaviour (individual and parallel locomotor play) was considered. Considering the total duration of locomotor play (parallel and individual locomotor play), calves performed on average (median and interquartile range) 3.89 (2.1–6.2) min per 24 h locomotor play behaviour when 3 weeks old and 2.71 (1.54–3.37) min per 24 h locomotor play behaviour when 7 weeks old. The space allowance in the present study is considered high from a practical perspective. However, when the cows left the pen, the calves had even more space at their disposal. More space leads to higher level of play behaviour and in line with this, full‐time and part‐time calves were observed playing more when the cows left the pen than during the rest of the 24 h period, which emphasises the importance of space for this behaviour. Waiblinger et al. (2020) compared the play behaviour and social interactions of dairy calves with either access to their dam and the cow herd or separated from their dam. Although the primary aim of this study was not to investigate effect of space allowance on play behaviour, data from locomotor play behaviour reported for different enclosure areas were useful for the EKE. Due to the dynamic group management the total individual space allowance in the calf area ranged from 3.5 to 16.8 m2 per calf depending on the actual group size. Average time spent in locomotor play was observed as 0.12% per observation period for calves with 4.2 m2 per animal and 0.38% for calves with > 10 m2 per animal. More data are reported in the tables below. All data points that were considered for the assessment of the relationship between space allowance and play behaviour are reported in Appendix G.
3.16.2.2.2. Sources of uncertainty in the estimates
In addition to space allowance, there are other aspects that may affect the time a calf spends playing, such as animal age, pen shape, season or floor types (Table 28). These aspects are considered sources of uncertainty in the estimate because, although it is known that they can affect time spent playing, their exact impact and their relationship with space and play are not fully understood.
Table 28.
Sources of uncertainty around EKE estimates on the relationship between space allowance and time spent in play behaviour
| Sources of uncertainty | Reason |
|---|---|
|
Group size and differences between shared and total space available in a pen |
For the same area available per animal (m2/animal), there is much more shared space available in a large group compared with a small group. The more shared space, the more locomotor play was shown by calves (Jensen et al., 1998) For this reason, two ‘scenarios’ were considered (small and large group) and recommendations provided for a) individual calves and b) groups of 4–7 animals. |
| Animal age |
Data available refer to animals up to 3 months of age. There is a larger uncertainty on play behaviour levels in older calves. Motivation to perform locomotor play decreases as age increases, but there is a lack of data on the degree of this reduction. Values estimated via the EKE were based on this age range (14 days to 3 months); values were, however, assumed to be applicable to the whole age period of interest (until 6 months of age). |
| Pen shape | On pasture, animals were observed to not necessarily run in a circle as observed in pens and seem to benefit from long straight running distances (Somers, 2012). Play behaviour appears to be influenced by the longest distance that animals can run free with longer distances and open spaces promoting play behaviour. Longest distances are influenced by pen shape (i.e. circular vs squared vs rectangular shapes). In arena tests, calves spent more time running in longer pens (twice the length compared with shorter pens; (Mintline et al., 2012)). |
| Period (morning/afternoon) | Data on locomotor play observation were collected in the afternoon (Waiblinger et al., 2020) or during the whole day (24 h) (Jensen and Kyhn, 2000). It is believed that play behaviour tends to occur more frequently in the morning, but there is lack of scientific data on the diurnal distribution of play behaviour. |
| Types of floor (variation) | No specific data were available on the effect of floor type on time spent in play behaviour. Bedded floor is considered to provide a good grip, and some types of rubber floor provide the same level of grip, in contrast with solid and slatted concrete floors, which are more slippery and tend to hamper demonstration of play behaviour. |
| Season (variation) | Play behaviour is likely to be less pronounced when calves are exposed to very low temperatures or to heat stress. |
| Presence of enrichment | No specific data available on the potential effect of enrichment on time spent in play behaviour. |
3.16.2.3. Results
The WG based their elicitation on scientific data reported on peer‐reviewed literature and on their expert knowledge.
3.16.2.3.1. Locomotor play behaviour expected in individual pens of different sizes
It was estimated that:
A calf aged between 14 days and 6 months, when kept in an enclosure with a very large or unlimited space, is expected to play on average for ~ 0.5% of 24 h (corresponding to 6 min and 38 s) (90% credible interval 0.3%–0.78%).
A space allowance of 29.5 m2 (90% credible interval 9.6–37.2) allows the average calf to show the same level of locomotor play (0.46% of 24 h) shown by the ‘median’ animal of the unexposed population.
A calf provided with the current minimum legislated space allowance (1.8 m2/animal) is expected to demonstrate virtually no locomotor play behaviour, which indicates that the animal is highly restricted in this situation.
The relationship between space and time spent in locomotor play behaviour in individual pens can be represented by y = 0.0002x − 2E‐05, assuming a linear relationship between the two variables, with x representing the space allowance (m2) and y the % total time of locomotor play behaviour during the first 3 months.
The model estimates are presented in Figure 20.
Figure 20.
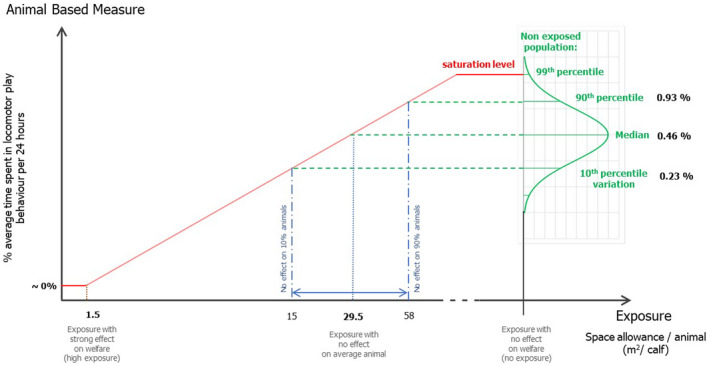
Schematic representation of theoretical model estimating space allowances and its relationship with time spent in locomotor play behaviour, showing order of elicited points (1st step, 2nd step, etc.) and elicited values for calves kept in individual enclosures (elicited values are shown in black). The green, vertical distribution represents the variability in play behaviour expected in a population of calves placed in a location with unrestricted space (e.g. large field, ‘no exposure’). A linear relationship between increasing space allowances (m 2 per animal) and % of time spent in play behaviour was assumed (red line)
3.16.2.3.2. Locomotor play behaviour expected in group pens (4–7 animals) of different sizes
It was estimated that a calf aged between 14 days and 6 months:
when kept in an enclosure with a very large, or unlimited, space (‘unexposed population’), is expected to play on average ~ 0.46% of 24 h (corresponding to 6 min and 38 s) (90% credible interval 0.3–0.78%).
a space allowance of 20 m2/calf (90% credible interval 11–35 m2) allows the average calf to show the same level of locomotor play shown by the median animal of the ‘unexposed’ population.
the relationship between space and time spent in locomotor play behaviour in individual pens can be represented by y = −0.0222x, with x being number of m2/calf, assuming a linear relationship between the two variables.
The model estimates are presented in Figure 21.
Figure 21.
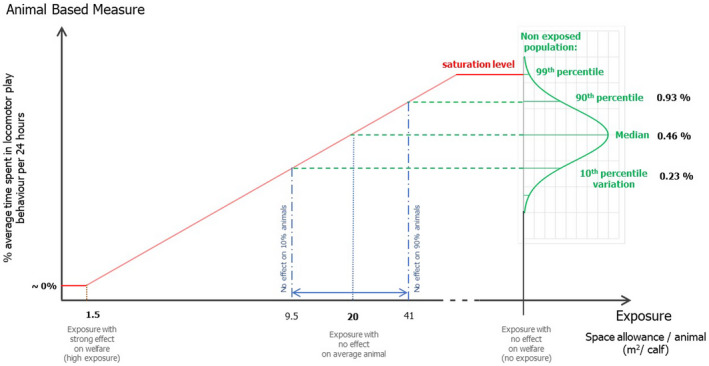
Schematic representation of F2F EKE model showing the results of an expert elicitation on the relationship between space allowances and time spent in locomotor play behaviour by calves
Most data on play behaviour originated from studies with animals of up to 3 months of age. This results in a larger uncertainty on play behaviour levels in calves older than 3 months. Although it is known that the calf's motivation to play decreases as it gets older, the degree of this reduction is unknown. However, it was considered that the expected decrease was not so pronounced to significantly impact space allowance requirements to perform locomotor play. These space allowance values were also considered to be sufficient for a larger calf to perform the level of estimated play behaviour even if heavier/larger in size. Therefore, the average value provided is expected to be applicable to the average calf between 14 days and 6 months of age with no expected impact on space allowance needs depending on the size or age of the calf.
3.16.2.3.3. Other relevant welfare consequences observed in calves reared in low space allowances
Low space allowance affects a range of other behaviours in calves under the age of 6 months, such as resting behaviour, feeding behaviour as well as health problems. For instance, if calves are given very little space, they tend to lie down a lot. Increasing the space allowance from 1.22 to 3.7 m2/calf in individual pens (Calvo‐Lorenzo et al., 2016) and from 1.0 and 1.5 to 2.0 m2 in group pens of 4 calves (Sutherland et al., 2014b) reduced the time spent lying down and increased activity in unweaned calves.
Conversely, if the space allowance in the lying area of a group pen is very low, this may reduce the lying down time. For instance, increasing the space in the designated lying area from 0.75 to 1.75 m2 per 100‐kg calf and from 1.00 m2 to 2.00 m2 per 150‐kg calf in group pens, increased the occurrence of synchronous resting, increased lying in a recumbent posture with legs stretched and reduced the occurrence of calves resting in close proximity to others. Furthermore, calves housed individually in hutches with 1.85 or 3.71 m2/calf compared with 1.23 m2/calf consumed feed at an earlier age. Respiratory problems were also observed in calves housed in small pens: a space allowance < 1.8 m2/calf was associated with an increased odds of respiratory disease in a survey (Calderón‐amor and Gallo, 2020); however, other factors such as pen ventilation and total pen volume may also play a role on the occurrence of respiratory disease.
Tables 29 and 30 present a summary of the welfare effects observed in calves kept at different space allowances in individual and group pens, respectively.
Table 29.
Relationships between available space and welfare effects observed in calves kept in individual pens, based on data reported in the literature and on the EKE estimates
| Space allowance (m2/calf) | Behaviours that can be expressed |
|---|---|
| 1.5 |
No locomotor play behaviour possible (EKE result) Calves spend most of time (70%) lying down (Calvo‐Lorenzo et al., 2016) (indicating low levels of general activity) |
| 1.8 |
No locomotor play behaviour possible (EKE result) Calves start ingesting feed at an earlier age, compared with when provided 1.25 m2 (Hulbert et al., 2019) because their feeding and exploratory behaviours are facilitated |
| 3 | No data in literature |
| 4 | Compared with a space allowance of 1.8 m2, calves at 3.7 m2 increase general activity (defined as any activity other than laying down) (Calvo‐Lorenzo et al., 2016) |
| 6 | No data in the literature |
| 10 | No data in the literature |
| 15 | No data in the literature |
| 29.5 | Calves are expected to show same levels of play as if not restricted by space. No data in the literature on other behaviours. |
Table 30.
Relationships between available space and welfare effects observed in calves kept in group pens, based on data reported in the literature and on the EKE outputs
| Space allowance (m2/calf) | Behaviours that can be expressed |
|---|---|
| 1.8 | Calves housed at < 1.8 m2 per animal showed an increased probability of respiratory diseases compared with calves housed at > 1.8 m2 per animal (Calderón‐amor and Gallo, 2020) |
| 2 | Calves reduced the time spent lying down and increase their activity when kept at 2 m2 compared with 1.5 m2 per animal (Sutherland et al., 2014a). |
| 3 | Increased lying in relaxed posture (stretched legs) and increased synchronous resting given a sufficiently sized lying area (1.5 or 2 m2 per animal depending on animal weight) (Færevik et al., 2008) |
| 4 | No data in the literature |
| 6 | No data in the literature |
| 10 | No data in the literature |
| 20 | No data in the literature. Calves are expected to show same levels of play as if not restricted by space. |
3.16.2.4. Conclusions on space allowance
Calves are intrinsically motivated to perform locomotor play behaviour (Jensen et al., 1998). Locomotor play is considered a good indicator of positive welfare in calves and a certain space allowance is necessary for a calf to demonstrate it on its full scale. Recommendations on space allowance were drawn based on the premise that, if only welfare arguments need to be taken into account, the available space of a pen should not impair the demonstration of such behaviour. Other behaviours that can be expressed at different space allowances were also considered.
For calves housed in individual pens
From the EKE, it is concluded that a calf housed in an individual pen at current minimum legislated space allowance (i.e. 1.5 m2 per animal) is likely not to carry out any locomotor play behaviour (90–100% certainty).
From the EKE, it is concluded that a space allowance of 29.5 m2 (90% credible interval 20–35 m2) is needed for a calf to show the full extent of locomotor play behaviour.
From the literature, it is concluded that a calf housed in an individual pen at current minimum legislated space allowance (i.e. 1.5 or 1.8 m2 per animal) is expected to spend most of its time lying down because no other activities can be performed (90–100% certainty).
From the literature, it is concluded that a calf housed in an individual pen shows substantially more general activity when space allowance is ~ 4 m2 (precisely 3.7 m2) compared with 1.8 m2 but no data on the response in terms of activity exist for the range 1.8–4 m2 (90–100% certainty).
For calves housed in group pens (4–7 animals)
From the EKE, it is concluded that a calf housed in group pens (4–7 animals) at current minimum legislated space allowance (i.e. 1.8 m2 per animal) is expected to carry out ~ 10% of the full extent of play behaviour (90–100% certainty).
From the EKE, it is concluded that a space allowance of 20 m2 per animal (90% credible interval 9–37 m2) is needed for calves in group pens to show the full extent of play behaviour.
From the literature, it is concluded that a calf housed in a group pen at or slightly below the current minimum legislated space allowance (i.e. 1.8 m2 per animal) is expected to show increased probability of respiratory diseases, compared with a space allowance higher than 1.8 m2 per animal (90–100% certainty).
From the literature, it is concluded that a calf housed in a group pen shows increased lying in a relaxed posture (stretched legs) and increased synchronous resting given a lying area of 1.5 or 2 m2 per animal (depending on animal weight) when total space allowance is 3 m2, compared with a lying area of 1 m2 per animal (90–100% certainty).
3.16.2.5. Recommendations on space allowance
The AHAW Panel recommends taking into consideration the following options to improve the current situation:
For calves housed in individual pens
To allow the full extent of locomotor play behaviour, it is recommended that individually housed calves should be provided with a space allowance of at least 29.5 m2. From the point of view of animal welfare, such large space allowances would be highly desirable.
The current minimum space allowance (i.e. 1.5 m2 per animal) should at least be doubled to reduce the welfare consequence of general behavioural restriction. This space allows calves to perform more general activity (defined as any activity other than lying down) compared with the minimum requirement.
The current minimum space allowance (i.e. 1.5 m2 per animal) should be increased four times to allow calves to perform 16% of the ‘full extent’ of locomotor play behaviour (i.e. amount of locomotor play that a calf would show in a situation without space restriction) in order to achieve a further improvement in welfare.
For calves housed in group pens
To allow the full extent of locomotor play behaviour, it is recommended that group housed calves should be provided with a space allowance of at least 20 m2 per animal. From the point of view of animal welfare, such large space allowances would be highly desirable.
the current minimum space allowance (i.e. 1.8 m2 per animal) should be increased to at least 3 m2 per animal. Three square metres per animal allow calves to perform 15% of the ‘full extent’ of locomotor play behaviour (i.e. the amount of locomotor play that a calf would show in a situation without space restriction) in order to achieve an improvement in welfare. A space allowance of 3 m2 per animal also allows to increase time spent lying in a relaxed posture and likely an increase in general activity.
3.16.3. Risks associated with iron restriction
3.16.3.1. Assessment scope and assumptions
The ToR requests an assessment of the welfare risks of ‘deprivation of iron’ due to ‘feed restriction’. Although it is possible to measure iron concentration in feedstuff, a high iron content in feedstuff does not always translate into high available iron for absorption due to different chelating properties of feedstuff components and digestion and absorption dynamics (Cozzi et al., 2002). It was therefore considered that this assessment should focus primarily on blood indicators of iron provision, such as Hb, as they will more closely relate to welfare state, rather than iron content in feedstuff. Variation of iron content of feedstuff is nevertheless briefly described, as well as current practices in veal farming in terms of solid feed administration.
Regarding units of measurement of Hb concentration, it was decided to report all result studies in mmol/L in this section. Although research articles often report Hb concentrations in g/dL, mmol/L are the units specified in the current legislation (Council Directive 2008/119/EC) and it was considered that using the same units would facilitate comparisons between current requirements and welfare effects associated with lower or higher values of Hb. When values were reported in scientific studies in g/L, values were converted to mmol/L.
Haemoglobin levels in white veal systems
In white veal farming, the iron content of diets administered to calves is purposefully kept low to achieve a pale meat colour and an increased price per kilogram of this product (‘white veal’). Although the type of milk provided to veal calves (milk replacer) is higher in iron content (varying concentrations, from 60 to 150 mg/kg) than whole milk (0.5 mg of iron/kg), the total iron intake is still low due to the low iron content of the solid feed provided to veal calves. This solid feed is mostly composed of grains, which are relatively low in iron, and often washed to remove soil and minerals to further decrease their iron content. It has been shown that calves provided with grains as the main component of the solid feed fraction in their diet are at risk of developing iron‐deficiency anaemia (Prevedello et al., 2009; Prevedello et al., 2012).
As in other domestic species, a combination of blood indicators is often used to evaluate the presence of anaemia in calves. Anaemia is generally defined as a decrease in Hb, red blood cells (RBC) and packed cell volume (PCV) in the blood stream (Ježek et al., 2009; Bhardwaj et al., 2010). The laboratory diagnosis can also be based on parameters such as RBC and total proteins (McFarlane et al., 1988; Mohri et al., 2010). However, the indicators more frequently used in the literature to assess the anaemia status in calves are Hb concentration and haematocrit, defined as the volume percentage of erythrocytes in blood. There are other indicators of iron intake, but their relationship with physiological and welfare indicators are difficult to assess due to the complexity of iron metabolism. For instance, serum iron concentration is not a good indicator of the amount of iron present in the body, and ferritin has been suggested as a promising possible parameter for the diagnosis of iron deficiency anaemia in calves (Joerling and Doll, 2019) but validation studies for this indicator are still lacking. Hence, due to the wider availability of studies focusing on Hb evaluation and evidence on the links between low Hb levels and welfare consequences for calves, it was decided to focus this assessment on Hb and not on other parameters.
A review of studies looking at Hb concentration indicated that there is no clear agreement on the cut‐off under which a calf is considered anaemic (Table 31). Studies from the 1980s and 1990s defined anaemia as, for instance, an Hb concentration below 5.59 mmol/L (Welchman et al., 1988) or 4.3 mmol/L (Schwartz, 1990; Stull and McDonough, 1994), but more recently, authors considered calves with having Hb values of 5.34 (Ramin et al., 2014) or 4.65 mmol/L (Allan et al., 2020) having mild or subclinical anaemia. It is worth nothing that laboratory techniques have been optimised in the last decades and this may hinder direct comparisons across studies' results carried out at different points in time. However, it is evident from the studies that establishing a precise Hb cut‐off is not straightforward; for this reason, a detailed literature review was conducted to understand the impacts of different Hb levels on calf's physiology and health. Most evidence was extracted from old studies because most research on this topic dates from the 1970s to the 1990s, but evidence from recent studies was also considered when available.
Table 31.
Values of haemoglobin, haematocrit and iron concentration reported in the literature for different anaemia classes (e.g. subclinical, mild, moderate)
| Parameter | Value | Classification of level of anaemia as reported in the papers | Animal category | Age (weeks) | Sample size | Reference |
|---|---|---|---|---|---|---|
| Haemoglobin concentration (mmol/L) | 5.03 | Anaemia | Veal | 6 | 6 |
Bremner and Dalgarno (1973) |
| 4.03 | Anaemia | Veal | 12 | 6 |
Bremner and Dalgarno (1973) |
|
| 4.84–5.71 | Veal | 16–20 | 166 | Welchman et al. (1988) | ||
| 4.34 | Clinical anaemia | Veal | Schwartz (1990) | |||
| 4.34–4.90 | Marginal anaemia | Veal | 16 | 550 | Stull and McDonough (1994) | |
| 4.65 | Very low/subclinical anaemia | Dairy | 8–12 | 237 | Allan et al. (2020) | |
| 4.65 | Moderate | Dairy | 12 | 167 | Ramin et al. (2014) | |
| 5.34 | Mild | Dairy | 12 | 167 | Ramin et al. (2014) | |
| Haematocrit (PCV) | 18.8% | Moderate | Dairy | 12 | 167 | Ramin et al. (2014) |
| 21.8% | Mild | Dairy | 12 | 167 | Ramin et al. (2014) |
While there is no publicly available data on the extent of anaemia in the white veal sector in Europe, it is likely that many calves reared in this type of system have low haemoglobin levels. Research conducted in Austria with 107 white veal calves concluded that the haemoglobin values of 93% of the animals was above 4.5 mmol/L (current minimum as reported in EU legislation) but 82% of the animals had values below 6 mmol/L (considered lower bound of the physiological range in the study) (Wittek et al., 2014).
3.16.3.2. Welfare consequences of low haemoglobin levels
As mentioned above, Hb is the indicator most frequently reported in the scientific literature used to evaluate whether calf's blood parameters related to iron intake fall within the physiological range. However, when evaluating calf's blood parameters, it is necessary to consider the age of the animal at the time of sampling because Hb concentration in blood varies during the first weeks of a calf's life. For instance, a study measuring Hb at 14, 28, 42, 56, 70 and 84 days of age concluded that Hb concentration significantly decreased from birth to day 28 and then significantly increased up to day 84 (Mohri et al., 2007). Other studies have reported variation even within the same age range (e.g. Bouda and Jagoš, 1984; Panousis et al., 2018); these differences are likely to be due to different animal categories studied, different study conditions or different laboratory tests used. Details on the Hb values reported in each study are presented in Appendix H.
The changes in Hb concentration during the first weeks following birth are a consequence of calf's dietary changes during their early life and are considered physiological. During the first 3 weeks, the iron reserve in the liver is sufficient to compensate for low iron intakes from milk (Heidarpour Bami et al., 2008), which has a low iron content (0.5 (0.3–0.6) mg of iron/kg) (INRA, 2018). After that, the progressive ingestion of roughage, which is much richer in iron compared with milk [iron concentration in hay being 250–525 mg/kg dry matter (DM); (INRA, 2018), tends to compensate the low iron levels from milk and results in increasing levels of haemoglobin (Egli and Blum, 1998; Heidarpour Bami et al., 2008; Hunt and Nielsen, 2009). For this reason, calves fed whole milk alone, or mostly whole milk, and little amounts of feedstuff high in iron such as roughage, are at risk of developing iron deficiency anaemia (Reif et al., 2019). The limited intake of roughage provided to calves is associated with iron‐deficient anaemia in calves reared for white veal, with calves reared for rosé veal not presenting the same extent of haemoglobin deficit.
Even though anaemia is the main consequence of iron deficiency, it is not the only outcome of such deficiency (de Passillé and Rushen, 2016). Negative effects of iron deficiency have been observed on immune functions, infection rates, cardiovascular performance, physical effort and weight gains. These effects are described below and summarised in Table 32.
Table 32.
Summary of welfare effects of haemoglobin levels between 3.72 and 6.01 mmol/L reported in experimental studies
| Haemoglobin (mmol/L) | Observations as reported in the studies | Effects | Reference |
|---|---|---|---|
| 3.49 | Higher prevalence of diarrhoea in calves with 3.49 mmol/L compared with 5.71 mmol/L | Higher diarrhoea prevalence | Prodanović et al. (2019) |
| 3.72 | Less weight gain in calves with 3.7 mmol/L compared with 3.9, 6.4 and 6.8 mmol/L [mean 1,152, 1,384, 1,465 and 1,449 g/day in each respective group) (p < 0.05) | Impaired weight gain | Lindt and Blum (1994) |
| 4.3 |
Lower total serum proteins in calves with 4.3 compared with 5.5 mmol/L (p < 0.05) Lower globulins concentration in calves with 4.3 compared with 5.5 mmol/L (p < 0.05) |
Impaired immunity |
Sarkozy et al. (1984) |
| 4.3 | Lower mean weight in calves with 4.3 compared with 5.5 mmol/L (p < 0.05) | Impaired weight gain |
Sarkozy et al. (1984) |
| 4.34 |
Less phagocytosis observed in serum from calves with Hb of 4.34 mmol/L compared with calves with 6.28 mmol/L (p < 0.05) Less myeloperoxidase activity of neutrophils in calves in serum from calves with mean Hb of 4.34 compared with 6.28 mmol/L (p < 0.05) IgG concentration lower in calves with 4.34 mmol/L compared with 6.01 mmol/L (p < 0.05) |
Impaired immunity | Gygax et al. (1993) |
| 4.34 |
More frequent hyperthermia in calves with 4.34 mmol/L compared with 6.01 mmol/L (p < 0.05) More frequent infections and antibiotic treatments in calves with 4.34 mmol/L compared with 6.01 mmol/L (p < 0.05) |
Higher infection rates | Gygax et al. (1993) |
| 4.5 | Current minimum haemoglobin value as reported in the legislation | ||
| 4.6 | Lower mean weight in calves with 4.6 compared with 6 mmol/L [474 g/day vs 615 g/day] | Impaired weight gain | Gygax et al. (1993) |
| 4.9 | No statistically significant differences on total serum proteins between calves with 4.9 and 6.4 mmol/L |
Sarkozy et al. (1984) |
|
| 5.27 | No significant differences observed on phagocytosis levels nor on myeloperoxidase activity between calves with mean Hb of 5.27 compared with calves with 6.01 mmol/L | Gygax et al. (1993) | |
| 5.3 |
Oxygen consumption rate from physical effort on a treadmill much lower in calves with 5.3 mmol/L compared with 7.76 and 8.6 mmol/L Higher lactate production in calves with 5.3 mmol/L compared with 7.76 and 8.6 mmol/L Heart rate and respiratory frequency higher in calves with 5.3 mmol/L compared with 7.76 and 8.6 mmol/L |
Higher physical effort | Lindt and Blum (1993) |
| 6.01 | No significant differences on milk replacer intake, number of meals refused and gain/feed ratios between calves with 6.01 and 7.2 mmol/L |
Hostettler‐Allen et al. (1993) |
|
3.16.3.2.1. Effects on immune functions and susceptibility to infections
The effects on immune function resulting from feeding calves with a milk replacer containing 10 or 50 mg of iron/kg were investigated in a study on 56 male calves. At 10 weeks, calves with higher iron intake showed a significantly higher Hb level (6.83 mmol/L) and higher phagocytosis compared with the group with the lower iron intake (mean Hb level of and 4.34 mmol/L). The same effect was not observed at 5 weeks, when the Hb levels were, respectively, 6.8 mmol/L and 5.28 mmol/L (Gygax et al., 1993). A significantly higher level of IgG following vaccination in calves with Hb concentration of 5.5 mmol/L compared with calves with 4.3 mmol/L was observed in another study (Sarkozy et al., 1984). A recent study reported that calves with Hb < 5.21 mmol/L showed a significant increase in haemolysate (malondialdehyde) concentration compared with calves with higher values of Hb indicating an impairment of the antioxidant defence system (Rajabian et al., 2017).
A higher prevalence of diarrhoea and a higher faecal pH in neonatal calves with Hb concentration of 3.49 mmol/L compared with 5.71 mmol/L was reported by (Prodanović et al., 2019). In this study, iron deficiency has been hypothesised to cause lower gastric secretion and impaired gut function, but these hypotheses have not been further investigated.
3.16.3.2.2. Effects on weight gain
Lower growth rates and lower weights have been reported in calves with Hb < 4.6 mmol/L compared with calves with higher blood Hb concentration (Sarkozy et al., 1984; Hostettler‐Allen et al., 1993; Lindt and Blum, 1994). A recent study showed that calves showed increased growth rates following injection of iron dextran (Allan et al., 2020).
3.16.3.2.3. Effects on physical performance and heart rate
Calves with haemoglobin levels of 5.3 and 5.4 mmol/L were shown to have less oxygen consumption and higher lactate production compared with calves with 7.76 and 8.6 mmol/L suggesting that these calves are less apt to withstand a physical workload than non‐anaemic calves (Lindt and Blum, 1993). Table 32 presents an overview of the welfare effects of varying Hb concentrations reported in experimental studies. Haemoglobin concentration in those studies was mostly influenced by different feeding strategies. In summary, Hb levels between 3.73 and 5.3 mmol/L have been shown to be associated with impaired weight gains, impaired immunity, higher infection rates and higher physical effort compared with higher levels of haemoglobin.
3.16.3.2.4. Measures to prevent low haemoglobin levels
Provision of roughage will generally increase the iron available to calves; however not all sources of roughage are equally rich in available iron. The binding of iron to low digestible compounds of the roughage cell wall and the presence of insoluble complexes of phytates, tannins or phosphates results in less bioavailable iron for absorption (Cozzi et al., 2002; Prevedello et al., 2012). For instance, the iron provided by wheat straw has been suggested to be poorly bioavailable compared with hay (Cozzi et al., 2002). Due to the complexity of iron digestion and absorption dynamics, from a welfare point of view, it is considered that the provision of roughage with high availability of iron content such as hay should be preferred to ensure a high iron intake rather than the provision of a solid feed composed of straw, cereals and grains.
A common practice in veal units is to monitor the concentration of Hb in individual calves several times during the fattening period to ensure compliance with the current legal minimum value of 4.5 mmol/L and administer an iron dextran injection when the average values of sampled animals are below the legislated threshold. A calf can go through up to three Hb checks during its life (Marcato et al., 2018). To the AHAW Panel's knowledge, no studies have evaluated the welfare impacts of monitoring of Hb concentration in veal calves through repeated handling nor the pain associated with frequent puncturing the skin for blood sampling and injection of iron. Less invasive monitoring systems, such as the assessment of ocular mucosa or carcass colour, could prove useful to reduce/prevent this practice. The FAMACHA© eye colour chart is typically used in sheep, but two studies have evaluated its reliability in calves by comparing the sensitivity and specificity of the test in animals with different PCV values. It was concluded that the overall sensitivity and specificity of the scoring system is ~ 30% and 90% respectively, with higher scores (indicating higher anaemia) presenting higher specificity and lower sensitivity than lower scores (Grace et al., 2007; Dorny et al., 2011).
Although post‐mortem identification of anaemia through carcass colour evaluation would come too late to allow the correction of Hb levels in the affected calves, and hence not be effective to prevent on‐farm welfare consequences of anaemia, the implementation of a monitoring system at abattoir level could allow to signal holdings with a consistent proportion of carcasses with a pale colour and needing implementation of preventive measures. A correlation of 0.5–0.6 between Hb measured before slaughter and lightness of certain muscles (e.g. rectus abdominis) have been reported in previous studies (Scheeder et al., 1999), but no studies have validated this method.
3.16.3.2.5. Correction of metabolic disorders (anaemia) through iron administration
The evidence on the administration of iron on Hb and serum iron levels is mixed. While some studies reported no clear efficacy of these treatments (Völker and Rotermund, 2000; Mohri et al., 2007; Mohri et al., 2010) others have reported an increased serum iron concentration in dairy calves following parenteral or oral administration (Mohri et al., 2004; Heidarpour Bami et al., 2008; Moosavian et al., 2010; Allan et al., 2020). However, frequent parenteral administration of iron can be associated with distress due to handling and pain due to puncturing of the skin, and hence preventive provision of iron through feedstuff is preferable.
3.16.3.3. Conclusions on haemoglobin levels
There is a physiological and age‐related variation in Hb levels in calves during the first weeks of life. The range of mean values for non‐anaemic animals between weeks 1 and 30 of age is between 5.64 and 7.11 mmol/L (certainty 90–100%).
There are very few accessible data on the prevalence of anaemia in the white veal farming sector (certainty 90–100%).
Feeding practices based on solid feed low in bioavailable iron and restricted roughage, as commonly applied in white veal farming, result in anaemia (certainty 66–100%).
Current minimum legislated values for calves (Hb concentration 4.5 mmol/L) are well below the physiological range (certainty 90–100%).
Hb levels below 4.5 mmol/L are associated with impaired immunity, higher prevalence of diarrhoea and respiratory disease and low weight gains (certainty 90–100%).
Increased cardiovascular and respiratory responses to physical efforts observed with Hb values between 4.5 and 5.3 mmol/L indicate a reduction in welfare (certainty 66–100%).
Studies on the effect of Hb levels of between 4.5 and 5.3 mmol/L on other welfare indicators such as disease prevalence or behaviour are lacking (certainty 90–100%).
In the white veal sector, it is common practice to monitor Hb sampling animals by venipuncture up to three times per production cycle to check compliance with legislation and market requirements regarding meat colour. This results in handling stress and pain from puncturing the skin (certainty 90–100%).
There are no validated methods for non‐invasive monitoring of Hb levels ante‐mortem as well as for post‐mortem assessment (certainty 90–100%).
3.16.3.4. Recommendations on haemoglobin levels
Based on a precautionary principle, measures should be implemented to avoid Hb of less than 5.3 mmol/L in veal calves.
Procedures for collection, record keeping and accessibility of Hb data from white veal production systems at farm and abattoir levels should be implemented for a better understanding of the welfare effects of Hb values between 4.5 and 5.6 mmol/L.
The diet of veal calves should be composed of feedstuff high in iron such as roughage (e.g. hay).
From an animal welfare perspective, anaemia should be prevented through provision of highly bioavailable concentration of iron through diet, such as roughage with high availability of iron content such as hay, rather than corrected with iron injections.
Research should investigate the validity of non‐invasive methods for assessing anaemia prevalence at farm (e.g. mucosa colour) and at abattoir (e.g. carcass colour assessment).
3.16.4. Risks of fibre restriction
3.16.4.1. Assessment scope and assumptions
In the context of this Specific Scenario, ‘fibre’ can have different interpretations. Fibrous feedstuff often relates to feed materials with a high amount of cellulose, hemicellulose or lignin, which are commonly denominated as the non‐detergent component of fibre, or NDF (Van Soest et al., 1991). Fresh grass, hay, straw or silage usually contain high amounts of NDF. Other physicochemical properties of fibre such as fermentability, solubility, viscosity or water binding capacity have been identified (Bach Knudsen, 2001), but their precise impact on calf's physiology or welfare has not yet been determined. In this scientific opinion, fibre was characterised in terms of NDF composition only.
3.16.4.2. Welfare consequences of fibre restriction
The inability to chew and ruminate was identified as the most important welfare issue experienced by calves provided with a limited amount of fibre in their diets. Chewing and ruminating is important to calves: this is demonstrated by the work they are willing to do to perform these activities and by the occurrence of abnormal oral behaviours (such as tongue rolling) when the opportunity to chew and ruminate is too limited (Leruste et al., 2014; Webb et al., 2015). The fibre content of the feed influences the time a calf spends ruminating: the higher the fibre component in the feed, the longer rumination time is required. The length and structure of the feedstuff particle are also important, with long particle size (e.g. > 5 cm) feedstuff tending to be associated with longer chewing times (Webb et al., 2013) and provision of manipulation opportunities.
Gastroenteric disorders such as pyloric lesions are very frequent among veal calves. In a survey of 179 veal farms in the Netherlands, Italy and France, 74% of the inspected rumens had abomasal lesions in the pyloric area (Brščić et al., 2011a,b). Although their aetiology has not yet been precisely determined, research results point to a combination of low fibre diets, large and infrequent milk meals, and ingestion of coarse fibre (EFSA AHAW Panel, 2012; Bus et al., 2019)). However, the role of quantity of solid feed and fibre in the formation of such lesions is not well understood. Past research has generally shown that the feeding of straw, a very coarse roughage, exacerbates abomasal lesions (Mattiello et al., 2002; Bus et al., 2019).
Other gastroenteric disorders relevant for veal calves identified were poor rumen development and rumen hyperkeratinisation but due to lack of data on the quantity and type of fibre on these welfare consequences, these were not further considered.
3.16.4.2.1. EKE model parameters
A theoretical model integrating the general concept of a ‘non‐exposed’ population and based on expert estimates (see Section 2.2.3 for more details) was applied to provide quantitative recommendations on fibre amounts for veal calves, as requested in the mandate. This approach involves the selection of relevant welfare consequences and ABM to be considered in the model. In this case, the welfare consequence and ABM selected were, respectively, the ‘inability to chew and ruminate’ and ‘time spent ruminating’ (high sensitivity and high specificity). For the purposes of the assessment of the effect of fibre on calf welfare, fibre amount was defined as ‘kilograms of NDF ingested per day’. The NDF content of feedstuff provided to calves varied markedly: as an example, hay and wheat straw contain ~ 60% and 80% of NDF, fresh grass 52–59% NDF, and wheat and barley grains 16% and 22% NDF respectively (Appendix I).
Evidence from experimental studies published in peer‐reviewed journals served as a basis to estimate the relationship between fibre content of feed and rumination times.
There are very limited recent data on rumination times of calves with access to ad libitum, or unrestricted, pasture or roughage. The available studies were from the 1950s and 1960s (Swanson and Harris, 1958; Chambers, 1959; Hutchison et al., 1962) with animal age ranging from 2.5 weeks to 6 months of age. These data suggested that the time spent ruminating on pasture increased substantially from ~ 2 weeks of age to 3 months, reaching a plateau and remaining relatively constant until the age of 6 months. Recent data exist for the distribution of rumination activity during the day: a study measuring behaviour and activity of 96 Holstein calves using ear‐tag accelerometers showed that rumination activity was approximately equally distributed throughout the day and night (Dennis et al., 2018). Scientific data also reports limited rumination times in veal calves fed limited amounts of fibre compared with calves fed ad libitum amounts of roughage. For instance, calves between 11 and 29 weeks of age fed 80% concentrate, 10% maize and 10% straw (with an estimated mean value of NDF being approximately 0.15 kg/day) were ruminating approximately 12% of time per day (Webb et al., 2015). Data from experimental studies on NDF amounts and rumination activity (Mattiello et al., 2002; Webb et al., 2012, 2013, 2014b, 2015; Brščić et al., 2014) suggest an approximately linear relationship between amount of NDF and percentage of time a calf spends ruminating per day. Details on these studies can be found in Appendix J.
Research has also looked at the effect of different lengths of roughage on calf behaviour. When given both options, calves preferred to ingest fibre in the form of ad libitum chopped roughage (i.e. > 4–5 cm) compared with roughage provided in a ground or pelleted form (Webb et al., 2014a; Jahani‐Moghadam et al., 2015). Longer form roughage is associated with longer rumination times (Webb et al., 2014b) and provides more chewing and manipulation opportunities. In relation to the effect of type of fibre on welfare, previous studies have suggested that straw can have detrimental effects at the abomasal level (Mattiello et al., 2002), especially if provided in a long form. Additionally, feeding solely maize silage as the starter feed to calves was shown to stunt the growth of rumen papillae and to impair intestinal morphology (Kehoe et al., 2019).
A summary on the EKE parameters and components is presented in Table 33.
Table 33.
Background and assumptions of the EKE exercise on the effects of restricted fibre on veal calf welfare
| EKE components | Definitions and assumptions |
|---|---|
| Animal category | Calves reared for white veal meat, aged between 2 weeks and 6 months |
| Husbandry system | White veal rearing systems |
| Exposure variable of interest | Kilograms of NDF provided to the calf per day |
| Welfare consequence | Inability to chew and ruminate |
| ABM |
Percentage of time a calf aged between 2 weeks and 6 months spends ruminating per day. Rumination was defined as the ‘process of returning newly eaten feed to the mouth for further chewing’. |
| Unexposed population | Defined as a group of calves in a suckler herd, aged between 2 weeks and 6 months, with ad libitum access to pasture and fibre, and continuous and unlimited access to the dam's milk. It was assumed that the calf would ingest increasing amounts of pasture and grass as time went by. It was also assumed that at younger ages, the calf would perform numerous, small bouts of milk intake, and, as the calf grew older, the milk meals would become fewer and larger. |
| Highly exposed population |
Defined as a group of calves reared under a conventional white veal production system, aged between 2 weeks and 6 months, with restricted access to solid feed (total of 270–300 kg dry matter (DM) per rearing cycle). The standard white veal calf's diet is composed of mostly corn with some protein complementation, or of a ‘muesli’ feed made of corn mixture plus fibrous material such as chopped straw. These calves are fed milk twice a day through a bucket or a trough or through an automatic milk dispenser. Calves in these systems are routinely provided with on average ~ 0.19 kg DM NDF per day. It was also assumed that these calves are never weaned and are slaughtered at ~ 22–30 weeks. For the purposes of the EKE it was assumed that calves ingest between 1.1% and 2.4% of body weight of DM per day when aged between 2 weeks and 6 months. |
3.16.4.2.2. Sources of uncertainty in the estimates
Additional factors, other than fibre, can influence the levels of rumination, such as type of feed, calf breed or time of day. These aspects were considered sources of uncertainty in the estimate because, although it is assumed that they can affect the time a calf spends ruminating, their exact impact is not well understood. The list of sources of uncertainty can be found in Table 34.
Table 34.
Sources of uncertainty in the estimation of the relationship between NDF amount provided to the calf and percentage of time spent ruminating
| Sources of uncertainty | Reason |
|---|---|
| Climate | Hot weather is expected to result in lower rumination times |
| Breed | Different breeds can have different rumination behaviour |
| Type of feed (e.g. grass, pasture, roughage, other) | Different feed types can potentially result in different rumination times. Coarse fibre is expected to result in longer rumination times. |
| Sex | Possible effect of sex on rumination times not determined |
3.16.4.3. Results
3.16.4.3.2.1 Rumination times expected when calves have unrestricted and unrestricted access to fibre
It was estimated through EKE that:
a calf aged between 14 days and 6 months, when provided with an unrestricted amount of fibre (NDF), is expected to spend 30% of a day ruminating (7.2 h; median value of the green distribution in Figure 22), with 90% credible intervals ranging from 20% and 39%.
an ingestion of 1 kg of NDF per day (90% uncertainty interval between 0.64 kg and 1.36 kg) is estimated to allow a calf to show the same level of rumination shown by the ‘median’ animal of the unexposed population.
a calf aged between 14 days and 6 months provided a standard low fibre diet (assumed as containing on average 0.19 kg NDF per day) is expected to spend 10% of time per day (90% uncertainty interval 7–14%) ruminating, corresponding to ~ 2.4 h. The relationship between NDF amount and time spent ruminating can be expressed by y = 0.247x + 5.31, with x representing the NDF amount (g DM) and y the daily percentage of time spent ruminating.
Figure 22.
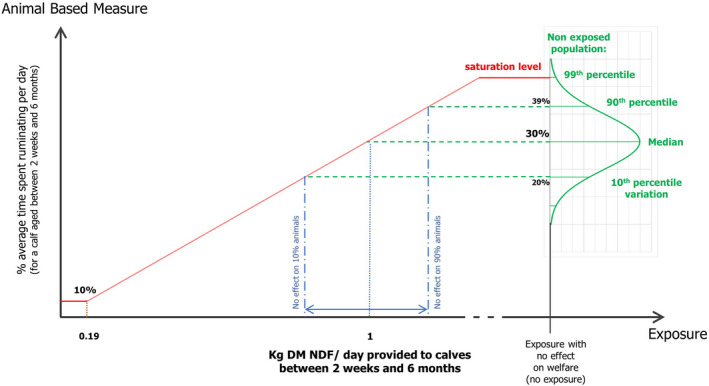
Elicited values on the relationship between rumination times shown by calves aged between 2 weeks and 6 months and amount of fibre provided in the diet during the same period (measured in kg NDF DM/day). The figures estimated via the EKE are shown in black
Taking the ‘average calf’ of the unexposed population as the reference that is assumed to demonstrate the ‘full extent of rumination’ behaviour when provided 1 kg of NDF per day, it is possible to calculate the degree of reduction of rumination shown by calves provided with a restricted amount of NDF. When provided a restricted amount of fibre (assumed as on average 0.19 kg NDF/day), calves spend 5.5 h less than what they would ruminate if they were provided fibre ad libitum. Other estimates for rumination times depending on NDF amounts can be calculated, as reported in Table 35.
Table 35.
Relationships between NDF provided in the diet, time spent ruminating and extent of rumination, based on the EKE estimates for the ‘average’ animal and assuming a linear relationship between time spent ruminating and fibre content of the daily diet (kg NDF/day). The values estimated via EKE are shown in the grey cells; the remaining values were estimated via linear interpolation
| NDF (kg)/day | Corresponding grams of hay (DM), assuming 60% NDF content (kg) | % time spent ruminating per day | Number of hours |
|---|---|---|---|
| 0.19 | 0.32 | 10 | 2.4 |
| 0.5 | 0.83 | 18 | 4.2 |
| 0.7 | 1.17 | 23 | 5.4 |
| 1 | 1.67 | 30 | 7.2 |
3.16.4.3.2.2 Amounts of NDF to be provided to calves 2–8 weeks, 9–18 weeks and 18–25 weeks old
An experimental study investigating feed preferences of calves showed that the voluntary intake of solid feed increased almost linearly as the calf aged when calves were provided different options (milk, hay, concentrate, maize and straw) (Webb et al., 2014a). Based on these data, a linear increase in ingested NDF as the calf grows older was assumed. Through an EKE procedure, it was estimated that a mean amount of 1 kg NDF per day should be fed to calves aged between 2 weeks and 6 months, but the fibre material should be increased gradually over time. Based on these assumptions, the linear increase of NDF to be provided to calves at different ages could be represented by y = 0.012–0.168, with x being the calf's age in days (14 days represents ‘day 0’ as this is the assumed earliest age calves can arrive at the veal farm) and y the daily amount of NDF (Figure 23).
Figure 23.
Daily amount of NDF (kg) to be provided to veal calves, at different ages, according to the expert elicitation outcomes. A linear increase in ingested solid feed over time was assumed based on voluntary intake research results (Webb et al., 2014a,b)
Table 36 shows the total amounts of NDF to be provided to calves between 2 and 8, 9–18 and 19–25 weeks.
Table 36.
Amount of NDF in kilograms recommended to be provided to veal calves aged between 2–8 weeks, 9–18 weeks and 18–25 weeks. For each age category, mean live weights are also provided (kg live weight, LW)
| Calves age and mean live weight | 2–8 weeks/40 kg LW | 9–18 weeks/80 kg LW | 19–25 weeks/130–300 kg LW | Total |
|---|---|---|---|---|
| Total recommended NDF to be provided in this period (kg) | 11 | 65 | 90 | 166 |
3.16.4.4. Conclusions on fibre amounts
From the EKE, it was concluded that a mean daily intake of 1 kg of NDF (DM) (90% credible interval 0.64–1.36) is needed for calves aged between 2 weeks and 6 months to show the ‘full extent of rumination time’ (i.e. time spent ruminating when calves are not restricted on fibre) which was estimated as 30% of time per day.
From the EKE, it was concluded that a calf provided with on average 0.19 kg NDF/day (current practice in white veal farms) is expected to spend ruminating only approximately one third of the ‘full extent of rumination time’ (90–100% certainty).
From the literature, it was concluded that, provision of fibre in the form of ad libitum long‐cut hay is preferred (90–100% certainty). Straw does not provide the same benefits and can have detrimental effects at abomasal level especially if provided in a long form (66–100% certainty).
From the literature, it was concluded that provision of long or chopped roughage (i.e. > 4–5 cm) is preferred over ground or pelleted roughage. Longer forms of roughage provide more chewing and manipulation opportunities and are associated with longer rumination times (90–100% certainty).
From the literature, it was concluded that feeding solely maize silage as starter feed to calves is not advisable as it has been shown to stunt the growth of rumen papillae and tends to impair intestinal morphology (90–100% certainty).
3.16.4.5. Recommendations on fibre amounts
To allow the full extent of rumination, is recommended that calves between 2 weeks and 6 months of age are provided a mean NDF amount of 1 kg/day. To achieve this amount of NDF (mean value of 1 kg/day), solid feed with a minimum of 40–50% of NDF should be provided.
The AHAW Panel recommends increasing progressively the amount of NDF provided to calves they age. From 2 to 8 weeks of age, it is recommended to provide a total of 11 kg of NDF, between weeks 9 and 18 a total of 65 kg of NDF and between weeks 18 and 25 a total of 90 kg of NDF, achieving a total of 166 kg. In case the fattening period is extended beyond 25 weeks, a proportional increase in NDF in the diet is recommended.
Long‐cut roughage (minimum 4–5 cm long) should be provided. Straw should not be provided as the only ad libitum roughage due to its coarseness and potential detrimental effects on the abomasum.
3.16.5. Representation of system incorporating recommendations of Specific Scenario 1
Specific Scenario 1 included a detailed assessment of the risks associated with individual housing, insufficient space, and iron and fibre restriction experienced by white veal calves. The recommendations to prevent these risks are provided in each individual section (see Sections 3.16.1–3.16.4). If combined and applied in practice, the resulting rearing system would be similar to that represented in Figure 24. The system includes the provision of separated functional areas; a bedded area for lying, a feeding area with access to the feed manger and water trough, and access to an outdoor area. Calves are also provided with a hack with ray and a brush.
Figure 24.
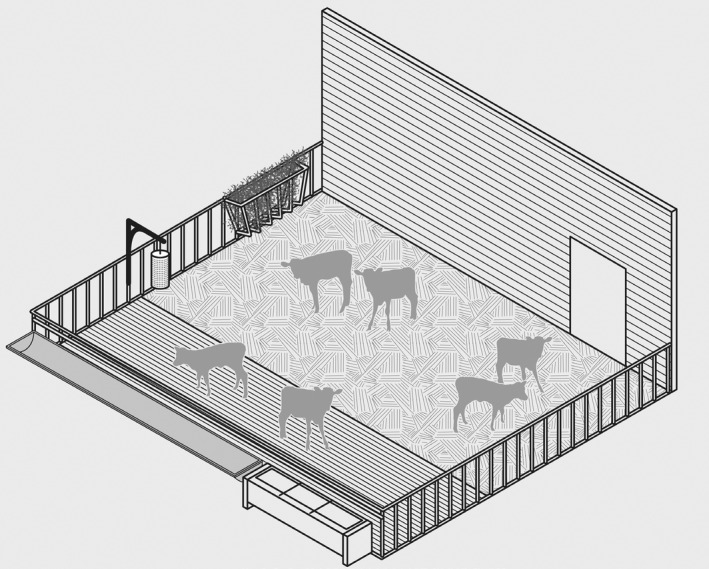
Representation of a pen incorporating the recommendations on space, group size, and fibre and iron provision from the assessment carried out under Specific Scenario 1
3.17. Specific Scenario 2 – The assessment of ABMs collected in slaughterhouses to monitor the level of on‐farm welfare of male dairy calves raised for producing ‘white’ veal meat
3.17.1. Introduction
This ToR requested an identification of ABMs collected at the slaughterhouse before and after slaughter that could be used to monitor the welfare of veal calves during their life on the farm. These ABMs should provide information on the overall welfare state of a certain population in a herd, farm, or region/country. In this section, the used methodology, the assessment results and the list of identified ABMs are presented.
3.17.2. Methodology
There has been very limited research on the use of ABMs collected ante‐mortem or post‐mortem at slaughterhouses to monitor the welfare of calves on farms. A recent paper proposed a list of such ABMs relevant for calf health and welfare (Boyle and Mee, 2021). The calf age of interest in the study was, however, different (i.e. dairy calves less than 1 month old) from the age range considered in the ToR (veal calves aged between 5 and 7 months). Some of the ABMs identified in the paper were, however, considered useful for application to older calves as well.
Considering the limited availability of published data on this topic, a procedure based on expert opinion was developed. The starting point was a list of 24 ABMs (11 ante‐mortem and 13 post‐mortem) potentially relevant for measurement at slaughter in veal calves up to 7 months of age. These ABMs were identified and described on the basis of existing literature (Welfare Quality®, 2009; EFSA AHAW Panel, 2012; Boyle and Mee, 2021) and complemented with knowledge from representatives from MS part of the EFSA AHAW Network during the 2021 network meeting. For the list of ABMs, full details on methodology and results of this exercise, see EFSA AHAW Network, 2021. This list includes a comprehensive, initial list of ABMs mentioned at the 2021 AHAW network meeting that could potentially be assessed in slaughterhouses. Some of these ABMs may reflect, however, transport‐related effects (e.g. lameness, bruises) and not be indicative of poor welfare on the farm. Nevertheless, as these ABMs were mentioned at the 2021 EFSA AHAW network meeting, they were kept in the initial list considered by the WG.
During the discussion it was decided to merge two of the ABMs (pneumonia and pleuritis), because they were both related to lung lesions; this combined ABM was rephrased to ‘Lung lesions – pneumonia and pleuritis’. Table 37 shows the final list of the 23 ABMs assessed. The definitions of the ABMs can be found in Appendix G.
Table 37.
Initial list of ABMs that can be assessed in slaughterhouses at ante‐ or post‐mortem
| ABMs in veal calves | |||
|---|---|---|---|
| Ante‐mortem | Post‐mortem | ||
| 1 | Body condition | 1 | Lung lesions – pneumonia and pleuritis (b) |
| 2 | Lameness | 2 | Pericarditis |
| 3 | Skin lesions – wounds/injuries | 3 | Skin lesions – bruises |
| 4 | Skin lesions – abscesses | 4 | Abscesses (in other locations than on skin) |
| 5 | Manure on the body | 5 | Bursa swelling (hygroma) |
| 6 | Couching/sneezing | 6 | Abomasal lesions |
| 7 | Nasal discharge (a) | 7 | Rumen lesions |
| 8 | Pumping/laboured breathing | 8 | Rumen disorders |
| 9 | Rectal prolapse | 9 | Intestinal disorders |
| 10 | Hernia | 10 | Carcass colour |
| 11 | Diarrhoea | 11 | Carcass condemnation** |
| 12 | Carcass aspect | ||
From the ABMs listed in Table 37 a semi‐quantitative consensus exercise was carried out to identify those ABMs that best reflect the overall animal welfare state on the farm. The exercise consisted of two steps: (i) Screening and (ii) Selection (Figure 25).
Figure 25.
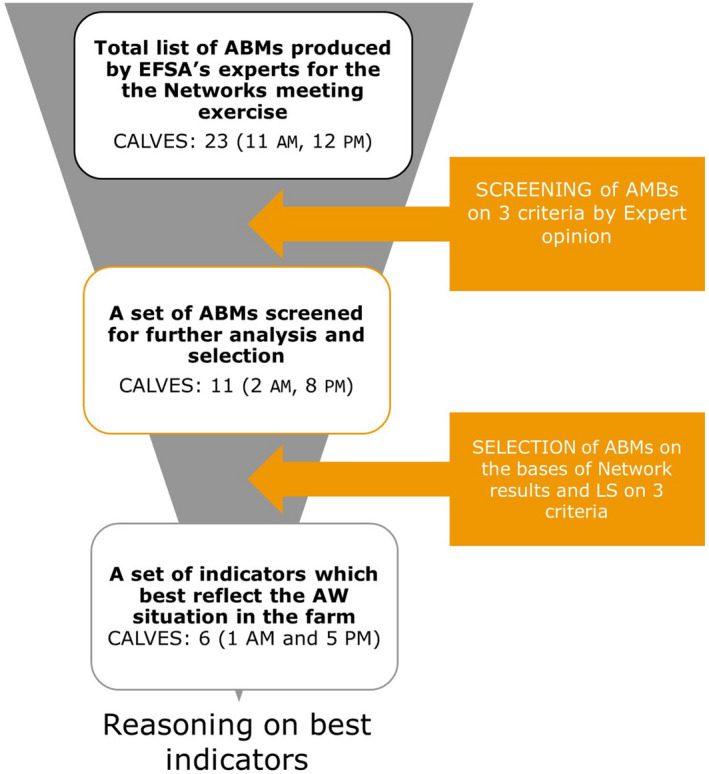
- am: ABMs measured ante‐mortem; pm: ABMs measured post‐mortem; ls: literature search.
Step (i) Screening was carried out through an expert opinion exercise on the initial list of ABMs, on the basis of four (screening) criteria (i.e. questions to answer with a Yes/No option):
Relevance to animal welfare: Is the ABM relevant to the welfare consequences defined in this opinion, and not only to production but also to meat quality aspects?
Relationship with the farm (and not transport or lairage): Is the ABM indicative of a welfare consequence experienced on the farm and not caused or masked by transport, lairage and slaughter?
Existing data in the literature: Do scientific publications describe the ABM detailing methodologies, prevalence and the relation with on‐farm welfare consequences?
Feasibility for large scale collection: Is the ABM already routinely collected or is there evidence that it could be collected in a national programme?
As precautionary principle, if consensus was not reached, the criterion was considered a ‘Yes’. Only ABMs that received a ‘Yes’ for all criteria passed to the second step (Selection). The screening procedure also identified whether the ABM was best assessed before or after slaughter (ante‐ or post‐mortem).
Step (ii) Selection consisted of a ranking of the ABMs based on the three criteria presented below. This was followed by the selection of the most useful ABMs of the purposes of the assessment as described in the Introduction. The approach used for assessing ABMs before and after slaughter in other EFSA F2F mandates (welfare of pigs, broilers and laying hens) was adapted to consider information on current ABM use at national level, as provided at the EFSA AHAW Network meeting (EFSA AHAW Network, 2021). Due to the fact that not all MSs participating in the meeting produced white veal, Criterion (4) of Step 1) was not considered to keep the assessment focused on veal calves and not on other calf categories. This approach considered three criteria:
Welfare consequences (C1): identification of the welfare consequences on farms (see Section 2.2.1) that were associated with the selected ABMs (from the list in Table 37). Each ABM was scored according to the number of different welfare consequences selected.
Technology readiness (C2): each ABM was evaluated for the known level of readiness of an automated system to be adopted by the market, based on the technology readiness scale (Mankins, 1995).
Already used at slaughter (C3): answers provided by MS at the 2021 AHAW Network meeting were considered and complemented with the knowledge from the WG.
For each of these criteria, the WG experts agreed on a score from 0 to 4, where ‘0’ and ‘4’ mean the lowest and the highest possible scores.
Finally, a weight was attributed by consensus to each criterion according to its importance in answering the request of the mandate. The allocated weights were: C1 = 7; C2 = 1.5; C3 = 1.5.
A final score (weighted score) was calculated following the formula below:
After establishing the weighted scores, the best suited ABMs were selected. The full process leading to the final list of ABMs that were selected is summarised in Figure 25, in which the number of ABMs resulting from each step is shown.
3.17.3. Results of the consensus exercise
Of the 23 originally identified ABMs, 11 passed the screening and were submitted to the selection step (Figure 25).
The outcome of the semi‐quantitative consensus exercise is presented in Table 38 in which the specific criteria to select the ABMs at slaughter are reported. The ABMs with the highest scores and considered as the most useful for the purposes described in the introduction of this section were: body condition, carcass condemnations, abomasal lesions, lung lesions (pneumonia and pleuritis), bursitis (swelling) and carcass colour (see Table 38). The cut‐off for determining the ABMs with the highest scores was arbitrary and considered the fact that the mandate requestor had asked for the selection of a limited number of ABMs indicative of welfare on farms.
Table 38.
Scoring of ABMs of veal calves regarding three criteria (C1, C2, C3); scores range from 0 (= absence) to 4 (= high). The weighted score was calculated based on the weight of each criterion given in brackets. The ABMs that were selected are highlighted in grey
| ABM | Assessment |
C1. Welfare consequences |
C2. Technology readiness |
C3. Already measured at slaughter |
Weighted score (1) |
|---|---|---|---|---|---|
|
Body condition |
Ante‐mortem | 4 | 2 | 3 | 3.55 |
|
Carcass condemnations ** |
Post‐mortem | 4 | 1 | 4 | 3.55 |
|
Carcass colour |
Post‐mortem | 3 | 2 | 4 | 3.00 |
| Abomasal lesions | Post‐mortem | 4 | 0 | 1 | 2.95 |
| Lung lesions ‐pneumonia & pleuritis * | Post‐mortem | 3 | 1 | 4 | 2.85 |
| Bursa swelling (hygroma) | Post‐mortem | 3 | 1 | 3 | 2.70 |
| Skin lesions‐wounds/injuries | Ante‐mortem | 2 | 1 | 4 | 2.15 |
| Rumen lesions | Post‐mortem | 2 | 1 | 2 | 1.85 |
| Pericarditis | Post‐mortem | 1 | 0 | 4 | 1.30 |
|
Diarrhoea |
Post‐mortem | 1 | 1 | 1 | 1.00 |
| Nasal discharge | Ante‐mortem | 1 | 0 | 2 | 1.00 |
The final weight was calculated considering the following criterion weights: C1 = 7; C2 = 1.5, C3 = 1.5.
This ABM (lung lesions – pneumonia & pleuritis) is a combination of two ABMs listed in EFSA (2021).
Excluding slaughterhouse contamination.
The following sections present the ABM definitions (as reported in EFSA AHAW Network, 2021), interpretation, current use, considerations for use as standard method and possibilities for automated registration/data collection of each ABM selected by the WG experts.
3.17.4. ABMs
3.17.4.1. Body condition
3.17.4.1.1. Description of the ABM
Definition: Body condition scoring is an assessment of subcutaneous adipose reserves.
3.17.4.1.2. Interpretation
BCS reflects a combination of environmental, management and feed factors. Emaciation or very low BCS may result from, and can be, a sign that animals are being provided with a diet and/or management they cannot cope with. When BCS is low in calves it is due to the fact that they have not gained weight or that they have catabolised their reserves (Boyle and Mee, 2021). Therefore, BCS can be useful as an indirect indicator of health problems such as respiratory disorders. BCS has been used for many years in the management of dairy cows, while it only more recently has been used as a welfare indicator in veal calves (Wilson et al., 2000; Renaud et al., 2018; Deikun et al., 2020; Moser et al., 2020; Boyle and Mee, 2021); Welfare Quality®, 2009. BCS was also used in a recent study organised by the New Zealand Government to identify reasons for mortality and morbidity in dairy calves sent for slaughter (so called ‘bobby calves’), and to identify new welfare indicators in these animals (MPI, 2018).
3.17.4.1.3. Assessment
Timing of assessment: ante‐mortem.
Current use of this ABM. In veal calves, currently BCS is mainly used to identify health problems at farm level and not in the slaughterhouse.
Considerations for use as standard method. BCS in calves can be assessed with different methods with or without palpation of some specific body regions to assess extent of subcutaneous fat and muscle. According to the Welfare Quality Protocol® for veal calves, BCS should be assessed based on quantity of muscle, the estimated body weight and physical appearance, including visibility of the ribs, the extent to which the backbone protrudes and the size of the belly relative to the size of the animal (Welfare Quality®, 2009). According to other authors, fat reserves in calves are best assessed by palpation over the ribs, lumbar spinal processes and tail head (Boyle and Mee, 2021). A five‐point scoring system from excellent to very poor, assessed by visual examination and palpation of bony prominences, has also been described (Wilson et al., 2000). While currently there are no specific technologies for the measurement of calf BCS in slaughterhouses ante‐mortem, carcass conformation data (EUROP system), which is collected systematically post‐mortem, could be used as an indirect indication of BCS, but BCS scoring is preferred because it takes into account the size of the animal relative to the batch and is useful to identify calves in very poor condition.
Possibilities for automation. Evaluating the BCS ante‐mortem is time‐consuming and requires trained evaluators to ensure good interobserver repeatability. There have been several efforts to automate BCS of dairy cows by using image analysis and machine learning techniques with the automated classification methods showing high correlations with manual BCS (Halachmi et al., 2013; Spoliansky et al., 2016; Rodríguez Alvarez et al., 2018; Zhao et al., 2020). Methods using 3D imaging have also been developed for beef cattle (reviewed by Craigie et al., 2012), with good results (Miller et al., 2019).
3.17.4.2. Carcass condemnations
3.17.4.2.1. Description of the ABM
Definition: Carcass and parts that are unfit for use as food (and not caused by the slaughter process), described as: percentage of calves with partial or fully condemn carcass.
During inspection, whole carcasses, parts of carcasses and/or offal that are unfit for human consumption must be condemned for food safety reasons according to EU legislation. The carcass condemnations (or, in the case of partial condemnations, the part/offal that is condemned), must be recorded in all EU slaughterhouses according to the Commission Implementing Regulation (EU) 2019/627. Condemnations are mainly due to signs of disease or injury (Biss et al., 1994; Kozak et al., 2002; Collineau et al., 2022) (except for carcass contamination during the slaughter process, which can be distinguished clearly) and therefore reflect impaired animal welfare. The decision to condemn a carcass can be already determined during the ante‐mortem inspection in case of visibly ill or disabled animals (Collineau et al., 2022). For welfare monitoring purposes, condemnation records should also include animals that are unfit for slaughter due to clinical signs of disease. Ideally, lesions that occurred during transport would not be included in the condemnation rate for on‐farm welfare monitoring purposes.
3.17.4.2.2. Interpretation
Different health issues but also injuries and bruises are reasons for partial or whole carcass condemnation (Biss et al., 1994; Collineau et al., 2022). In a study on condemnation reasons in six million calves slaughtered from 2016 to 2020 in France, peritonitis, pleuritis, emaciation/cachexia, abscesses and haemorrhagic infiltration were the most frequently recorded causes (Collineau et al., 2022). Condemnations thus reflect animal welfare problems on the farm, but transport and lairage conditions can also lead to increased condemnation rates. In addition to condemnation rates, the reason for condemnation is also relevant from the perspective of animal health and welfare surveillance (Vial and Reist, 2015). Furthermore, early and subclinical stages of disease are not detectable by meat inspection, and disease or lesions of low severity do not lead to condemnation and thus only the more severe cases are recorded.
3.17.4.2.3. Assessment
Timing of assessment: post‐mortem.
Current use of this ABM: Condemnations must be recorded in all slaughterhouses following the EU legislation for food safety reasons. There is hardly any information available on the use of these data to assess animal welfare, but there are ongoing projects on this topic (EFSA AHAW Network, 2021).
Considerations for use as standard method: The use of condemnation rates to compare welfare levels across farms requires clear criteria/specifications/terminology (Biss et al., 1994; Vial and Reist, 2015; Collineau et al., 2022) as well as continuing training and auditing of inspectors. For example, in a comprehensive French study, calf condemnation rates varied substantially between regions and slaughterhouses even after controlling for age, sex and breed which may hint to variability in the inspectors' evaluations (Collineau et al., 2022). On a European level, there is a list of conditions leading to condemnation but a harmonised description and specification of lesions and of the consequent decision is lacking (Collineau et al., 2022). Similarly, for pig slaughter, a large variability in recording of condemnations has been reported, as well as differences in terms of terminology used, type, number and use of codes of classification and the use of electronic databases (Alban et al., 2022). Carcass condemnations due to reasons other than health and welfare (e.g. resulting from improper carcass handling or of carcass contamination during slaughter and inspection) would have to be identified and excluded.
Carcass condemnations for use in animal welfare monitoring is best expressed as the number of carcasses condemned (partially or fully) relative to the total number of calves slaughtered (i.e. the proportion of calves with a fully condemned carcass, the proportion of calves with partially condemned carcass and the proportion of calves with a fully or partially condemned carcass). The weight of the entirely condemned carcass or the weight of the condemned carcass parts (in the case of partial condemnation) relative to the weight of carcasses of all slaughtered calves is less informative for animal welfare surveillance compared with the proportion of carcasses fully or partially condemned. Data on underlying causes of condemnations would also be useful for animal welfare monitoring purposes.
3.17.4.2.4. Possibility for automation
The decision for condemnations cannot be automated due to the diversity of underlying pathomorphological findings.
3.17.4.3. Abomasal lesions
3.17.4.3.1. Description of the ABM
Definition: Abomasal erosions, ulcers and scars.
Abomasal lesions comprise alterations of the integrity of the mucosa that can have different levels of severity from superficial erosion to severe perforated ulcer with involvement of the deeper levels of the abomasal wall. The lesions can be signs of acute inflammation, signs of chronic inflammation or scars (Mattiello et al., 2002; Webb et al., 2013; Bus et al., 2019). Abomasal lesions can be found in the pyloric area, can involve the torus pyloricus and/or the fundus, and they can vary in size from little spots to large lesions covering most of the organ. Lesions of different sizes can be distributed all over the organ or involve only one or more specific areas.
3.17.4.3.2. Interpretation
The prevalence of lesions reported in scientific papers exceeds 70% of veal calf abomasa, (reviewed by Bus et al., 2019)). The exact causes for formation of abomasal lesions are not completely understood but risk factors linked with feeding regime have been cited as the most likely contributor (Brščić et al., 2011a,b; Bus et al., 2019). A detailed discussion on abomasal lesions in veal calves can be found in Section 3.3.9.
3.17.4.3.3. Assessment
Timing of assessment: post‐mortem.
Current use of this ABM: Currently, the assessment is mostly done for research purposes. No data on abomasal lesions is routinely collected in abattoir. Severe abomasal lesions may lead to abomasal wall perforation and peritonitis (Hund et al., 2016; Bus et al., 2019), which can result in whole carcass condemnation or condemnation of parts of the carcass. Only if the reason for condemnation is recorded, the prevalence of such severe abomasal lesions can be taken into account.
Considerations for use as standard method: Abomasal lesions are assessed either in the tripery after the detachment of the abomasa from the forestomach or have to be assessed in a dedicated area in order to avoid carcass contamination. Feasibility of the ABM depends on the area where the organ can be assessed after detachment. The assessment could be done a posteriori entering the tripery at the end of the post‐mortem inspection on the slaughter dissection line or in a dedicated area for its assessment.
The assessment can be standardised by descriptions of the areas assessed, classification of the lesions and their sizes. Scoring systems described in the literature for classification of lesions include those described by Wiepkema et al., 1987 (abomasal erosion, ulcer, or scar), Guizzardi et al., 2007 (abomasal inflammation, erosion, ulcer, or scar) or Brščić et al., 2011a,b, which involves counting the number of lesions (regardless of their severity, from erosion to open ulcer) present in the mucosa of the pyloric area and classifying them into one of three different sizes (diameter < 0.5 cm2; 0.5–1 cm2, > 1 cm2). Number of lesions within each size class are counted on 5 levels: 0 = absence of lesions; 1 = presence of 1 lesion; 2 = presence of 2 lesions; 3 = presence of 3 lesions; 4 = presence of 4 or more lesions (Brščić et al., 2011).
3.17.4.3.4. Possibility for automation
It is difficult to automate the identification and classification of abomasal lesions because they require thorough cleaning of the area and careful inspection of the abomasal mucosa.
3.17.4.4. Lung lesions – pneumonia and pleuritis
3.17.4.4.1. Description of the ABM
Definition: Inflammation of the lung tissue with or without an overlying pleurisy and/or inflammation of the pleurae with fibrinous pleural adhesions.
Respiratory tract lesions in veal calves are most often related to bacterial or viral infection. They may be indicative of an acute inflammatory process characterised by tracheitis, congestive pneumonia, bronchopneumonia and hydrothorax – or of a chronic stage with hepatisation (consolidation of lung lobules or even lobes), pleurisy (fibrous or fibrinous adhesions on the lung or between the lung and the chest wall) and abscesses. In post‐mortem examination at the slaughterhouse, chronic lesions, affecting mainly the cranioventral lobes, will be much more common than acute lesions (Lopez and Martinson, 2017; Fernández et al., 2020). Some macroscopic lesions may be indicative of a specific pathogen, but usually the lesions result from a sequence of events caused by multiple agents (see description of bovine respiratory disease in Section 3.2.9). This most common form of pneumonia is usually termed ‘bovine enzootic pneumonia’.
3.17.4.4.2. Interpretation
Respiratory disease in cattle (BRD) is a multifactorial disease resulting from the interplay of farm conditions (e.g. high temperature–humidity index, high ammonia), immunodepression (e.g. stress caused by transport, comingling or overstocking) and infectious agents (Pratelli and Padalino, 2022).
In veal calves, several viruses and bacteria will attain the trachea and lungs, causing inflammation during the first days on the fattening farm, especially if transported and commingled with animals from different sources. In affected calves, cure (after antimicrobial treatment or not) or chronicity will follow. Lesions may remain even after clinical cure or be present in calves that went through subclinical disease. Thus, a high percentage of calves that did not show or only showed mild signs of BRD at the farm, will have lung lesions at slaughter (Brščić et al., 2012; Leruste et al., 2012; Stilwell et al., 2013; Fernández et al., 2020). Chronic catarrhal pneumonia is the most common type found at slaughter (Fernández et al., 2020).
The acute stage of BRD is characterised by the presence of well‐demarcated solid and swollen cranioventral areas (fibrinous bronchopneumonia), with pleural effusion or prominent fibrinous pleuritis with evident vascular reaction, such as congestion or fibrin deposition over the pleura. In the more common chronic bronchopneumonia cases, lesions are variable and depend largely on the agents involved. However, most cases are characterised by well‐demarcated, firm texture and purple to grey consolidated lung parenchyma, with no increase in volume (hepatisation). Sometimes bronchiectasis and pulmonary abscesses are also present (Caswell et al., 2012; Lopez and Martinson, 2017; Murray et al., 2017).
Some pathogens will cause very specific lesions. For example: multifocal necrosis of nasal, pharyngeal, laryngeal, tracheal mucosa in Bovine Herpes Virus 1 (BHV1) infection; peribronchiolar lymphoid hyperplasia (cuffing pneumonia) in Mycoplasma spp.; suppurative bronchopneumonia in infections by Pasteurella multocida or Trueperella pyogenes; interlobular septa distended by oedema, coagulation necrosis and fibrin give a ‘marbling’ aspect to Mannheimia haemolytica or Histophilus somni pneumonia lesions (Divers, 2008; Lopez and Martinson, 2017).
3.17.4.4.3. Assessment
Timing of assessment: post‐mortem.
Current use of this ABM. BRD has a huge impact on the welfare of fattening cattle, including in veal production. Prevalence assessment of pneumonic lesions at the slaughterhouse can provide a good indicator of the prevalence of BRD in veal farms (Radaelli et al., 2008; Brščić et al., 2012; Leruste et al., 2012; Pardon et al., 2013; Fernández et al., 2020) and so has a large potential to be used as an ABM in monitoring calf welfare.
Considerations for use as a standard method. Lung inspection is already done for food safety purposes, with condemnation of the organ when lesions are found. Further classification of type of lesions (e.g. acute/chronic, severity) is not done routinely but would be useful for welfare monitoring purposes. Post‐mortem inspection should provide reliable information on lesion severity, dimension and lesion type (acute or chronic). This should be done by observation, cutting and palpation techniques (EFSA BIOHAZ Panel, 2005). In this way, recording the type of lesion in condemned lungs during the routine carcass inspection, will allow for better understanding of farm environmental conditions, moment of infection and management quality, including accuracy in detecting sick animals and competency in treating these (Wittum et al., 1996; Schneider et al., 2009; Caswell et al., 2012; Fernández et al., 2020).
Some concern may arise from the required detailed inspection of lung lesions by cutting as it may increase the risk of cross‐contamination of the meat with pathogens (EFSA BIOHAZ Panel, 2005). However, adoption of palpation only, instead of palpation and incision, for inspecting lymph nodes and lungs could lead to a lower detection rate and poor characterisation of lung lesions (EFSA BIOHAZ Panel, 2005). The assessment should consider artefacts, such as modification in lung appearance due to the stunning and killing procedure.
A practical classification of macroscopic lung and pleural lesions has been proposed for veal calves (Van der Mei and Van den Ingh, 1987). A pneumonia score is given to each lung using a 4‐point scale: Score 0 for healthy lungs (orange colour with no sign of pneumonia); Score 1 for minimal lesions (one spot of grey/red discoloration); Score 2 for mild or moderate lesions (one larger or several small spots of grey‐red discoloration with a total surface of less than 1 lobe); and Score 3 for severe lesions (grey‐red discoloration area of at least one full lobe and/or presence of abscesses).
The lesions most frequently observed in veal calves that were affected by respiratory disease during the fattening period were consolidated or collapsed parenchyma, lobe‐lobe or lobe‐parietal pleura adhesions, diffuse pleuritis over the lung lobes or involving the margins of the lung or abscesses that should be recorded as small (< 2.5 cm), large (>) 2.5 cm, or diffuse (Leruste et al., 2012).
Possibility for automation. Systems for automatic visual analysis of lung lesions without the handling of carcasses and organs are more developed at pig slaughterhouses than in veal production. A system that can score and grade pleuritis (Trachtman et al., 2020) may be adapted to veal carcasses. Other technology using artificial intelligence to classify enzootic pneumonia‐like lesions in pig carcasses has been proposed (Bonicelli et al., 2021) and may also be adapted to calves. These systems still have to be validated for cattle and particularly for veal calves by comparing results from the standardised manual method currently used in most slaughterhouses.
3.17.4.5. Carcass colour
3.17.4.5.1. Description of the ABM
Definition: Very pale carcass colour associated with low haemoglobin concentration and anaemia.
3.17.4.5.2. Interpretation
Meat colour is mainly determined by the myoglobin content of the sarcoplasm (Purslow et al., 2020). Similar to haemoglobin, myoglobin contains iron, which is involved in the binding of oxygen, and thus the myoglobin concentration also depends on the provision of iron (MacDougall et al., 1973). A pale colour of the carcass/meat is often assumed to result from low haemoglobin levels/anaemia (Ngapo and Gariépy, 2006). However, the correlation between haemoglobin concentration and meat colour has been found to be only moderate (r = 0.65 for correlation with L* values (Barnier et al., 1998); r = 0.30–0.52 for correlation with a* values (Lagoda et al., 2002)) to weak (Scheeder et al., 1999). This may explain why, e.g. a mixed solid feed resulted in higher haemoglobin levels compared with maize grains only, but no relevant differences in carcass colour parameters (Prevedello et al., 2009). Other factors influencing meat/carcass colour include maternal undernutrition (Noya et al., 2022) and the position in the truck when transported to the slaughterhouse (lighter meat colour in calves transported in the front compartment (Van De Water et al., 2003).
3.17.4.5.3. Assessment
Timing of assessment: post‐mortem.
Current use of this ABM: Carcass colour may be assessed visually using colour scales with different numbers of classes, ranging between 3 and 10 classes (e.g. scale with 3 classes, (Räber et al., 2013); Dutch colour scale with 10 classes (Kalf, online). Chromameters provide objective, instrumental measures of the colorimetric characteristics – lightness L*, red index a* and yellow index b*; additionally, the hue angle and chroma values are often reported in meat colour assessments (Girolami et al., 2013). Computer vision systems have also been validated for meat colour assessment (Girolami et al., 2013).
Assessment of carcass colour is routinely carried out in the main veal producing countries for commercial classification purposes. In contrast to meat quality studies, where meat colour is mostly measured in cuts of the M. longissimus dorsi (Bispo et al., 2010), the routine assessment is performed non‐invasively by slaughterhouse operators at the M. abdominis rectus. In the Netherlands, colour of veal calf carcasses is ranked from 1 (very light) to 10 (very dark) (Kalf, online), while in France a 5‐point scale is used (Chanteperdrix, online). In the Netherlands, France and Italy, nowadays carcass colour in most slaughterhouses is evaluated by a chromameter (e.g. Minolta CR400 in France, covering about 65% of the total production), which allows allocation of carcasses to a colour category. Using visual assessments as reference, prediction equations including L*, a* and b* values revealed 83% (5‐point scale; Chanteperdrix, online)) and 79% correct classification (4‐point scale; Vandoni and Sgoifo Rossi, 2009)) with only 1% of classifications deviating more than one category (Chanteperdrix, online). For the 10 point‐scale used in the Netherlands, identical classification was obtained in 50–55% of the samples while 41–44% differed by one category only (Hulsegge et al., 2001). The prediction equations are, however, not always publicly available (e.g. only Vandoni and Sgoifo Rossi, 2009)) provided the coefficients for the equation). While there is evidence in the literature reporting a correlation between in vivo Hb concentrations and carcass colour post‐mortem (Miltenburg et al., 1992; Cozzi et al., 2002), no published data were found on the relationship between carcass colour categories and haemoglobin levels.
Considerations for use as a standard method: Use of subjective colour scales could be easily implemented, but there is a lack of information on reliability (e.g. inter‐observer repeatability) of assessments. Chromameters provide reliable data, but require technical equipment, a standard light source and training of personnel. Carcass colour is evaluated systematically for commercial reasons in abattoirs, but these data are currently not shared by slaughterhouses.
Possibility for automation: The standard use of chromameters in the main veal calf producing countries shows that the measurement of meat colour characteristics can be integrated in the slaughter line under commercial abattoir conditions. While computer vision has been shown to be promising to describe meat colour (Girolami et al., 2013), no studies have been found investigating the automatic assessment of carcass colour through image processing.
3.17.4.6. Bursa swelling (hygroma)
3.17.4.6.1. Description of the ABM
Definition: A hygroma is a fluid filled sac that develops as a result of a pressure injury on the weight‐bearing points of the legs when lying and changing positions.
3.17.4.6.2. Interpretation
Hygromas are most prevalent in the front of the carpal joints and the hock region of the hind limbs, although they can occur in other locations. A hygroma is a specific indicator of resting problems in calves (Brščić et al., 2011). Risk factors were concrete and wooden slatted floors, space allowance ≤ 1.8 m2/calf, and worn or slippery floors, and providing bedding had a preventive effect (Brščić et al., 2011). The frequency of bursitis increased with calf age and was 53% of calves at the end of the fattening period (Brščić et al., 2009).
3.17.4.6.3. Assessment
Timing of assessment: post‐mortem.
Current use of this ABM: Not commonly used but promising indicator due to the fact that it is a specific ABM of resting problems, and it is relatively easy to assess.
Considerations for use as standard method: Identification of bursitis is easier during post‐mortem inspection rather than ante‐mortem because it can be difficult to thoroughly inspect the body and limbs of each calf before slaughter especially when they are in a group. Moreover, body position, tail position and cleanliness of the animals can affect the visibility of bursitis. Variable environmental conditions (e.g. poor lighting and dust) can also limit a reliable ante‐mortem assessment. When present, hygroma can easily be observed on the carcass before skinning (e.g. front of the carpal joints and the hock region of the hind limbs). Harmonisation of the definition of this ABM would be needed for use as a standard method in slaughterhouses. This paragraph is based on expert opinion because to the authors' knowledge there are no published studies on the use of hygroma as a standard ABM at slaughter.
Possibility for automation: There is no published information on the use of automated tools for the detection of hygroma. Camera‐based technologies for the detection of skin lesions are available for other species (e.g. detection of tail lesions in pigs: Brünger et al., 2019; Blömke et al., 2020; detection of pododermatitis in turkeys: Stracke et al., 2022) but there is no indication of the adaptation of such technologies for hygroma detection so far.
3.17.5. Sources of uncertainty in the assessment
The main sources of uncertainty of Specific Scenario 2 were identified following the method described in and are presented in Table 39.
Table 39.
Sources of uncertainty in the assessment of ABM collected in slaughterhouses to monitor the level of on farm welfare of veal calves
| Topic | Sources of uncertainty | Estimated impact on the assessment |
|---|---|---|
| Identification of slaughter ABMs associated with on‐farm welfare |
Few studies published with data on ABMs of veal calf ABMs at slaughter Limited number of studies associating findings at slaughter with actual welfare situation on the farm |
Underestimation or overestimation of welfare consequences |
|
Prioritisation of ABMs associated with several welfare consequences might have excluded measures that relate to a single welfare consequence |
Underestimation of welfare consequences | |
|
Not always possible to determine whether ABMs were already present on‐farm, or if resulted or made worse by transport and lairage |
Underestimation or overestimation of welfare consequences | |
| Current use of ABMs in slaughterhouses |
Scarce published information on current use of ABMs MSs data focused on slaughter data of calves in general rather than veal calves. |
Underestimation or overestimation of use of ABMs |
| Potential for automation | Technology Readiness Level hard to define for some ABMs. There may be technologies being developed, but unknown to the public due to Intellectual Property Rights. | Underestimation or overestimation of the potential for automation |
A limitation of the method used was that the health‐related welfare consequences on farm assessed through the ABMs collected will be underestimated because they do not include the animals that die or get sick and are treated on farm.
3.17.6. Summary conclusions on Specific Scenario 2
Certainty levels were defined according to the methodology described in Section 2.2.5.
While not providing a comprehensive picture, the ABMs body condition score (assessed ante‐mortem), and carcass condemnations, carcass colour, lung lesions, abomasal lesions and bursa swelling (post‐mortem) were selected for the assessment of calf welfare on farm (certainty 90–100%).
Carcass condemnations, lung lesions and abomasal lesions are useful to detect the most prevalent health‐related welfare consequences in veal calves, i.e. respiratory disorders and gastroenteric disorders. These are useful to detect conditions affecting calves over a long period (e.g. gastroenteric disorders causing chronic abomasal lesions) but will be less useful to detect issues that have occurred at the beginning of the fattening period, such as diarrhoea or mild, early respiratory disorders (certainty 90–100%).
The prevalence of the health‐related welfare consequences on farm assessed through the ABMs collected will be underestimated because it does not include the calves that die or get sick and are treated on farm (certainty 90–100%).
Carcass colour, BCS and hygroma reflect issues related to anaemia, general health disorders/inability to cope with rearing conditions, and resting problems, respectively (certainty 90–100%).
There are no available ABMs to be collected at the slaughterhouse to detect problems on the farm related to the inability to perform exploratory and foraging behaviour, or restriction of movement (certainty 90–100%).
The use of the selected ABMs of calf welfare for monitoring is not routinely implemented in EU slaughterhouses, but some are already collected for food safety (such as carcass condemnation rate and presence of lung lesions) or commercial purposes (carcass colour) (certainty 90–100%).
Currently there is no EU‐wide standardisation of collection and recording of such ABMs (certainty 90–100%).
Automated systems for easy and standardised collection of data are unavailable for most ABMs because the technology readiness level of automated monitoring of the ABMs at slaughterhouse for veal calves is currently very low. Carcass colour assessment is the only routinely implemented ABM that is used by abattoir operators; however these data are not accessible (certainty 90–100%).
Implementation of a system to monitor welfare of calves based on the identified ABMs requires harmonisation of assessment methods including reliability testing to allow comparison of data across slaughterhouses, regions and countries and over time. Large variability in the assessment methodologies makes it difficult to compare the currently available data (certainty 90–100%).
3.17.7. Recommendations on Specific Scenario 2
If a monitoring system is to be implemented, data on body condition score, carcass condemnations, carcass colour, abomasal lesions, lung lesions and bursa swelling in calves at slaughter could be collected to identify herds with some of the most common health‐related welfare issues in veal calves. Such data would be useful to benchmark holdings and to inform the need for implementation of preventive measures on farm.
More granular data on the underlying causes of condemnations are recommended.
Data already collected for commercial purposes, such as carcass colour, should be made available to allow incorporation of these ABMs in welfare monitoring systems.
Harmonised systems for data collection and recording should be developed including reliability testing. Systems for the assessment of lung lesions could be developed based on pig inspection methods.
Systems for automatic and continuous assessment of ABMs and data recording should be developed.
For a comprehensive welfare assessment, ABMs collected at slaughter should be complemented with data on behavioural ABMs collected on the farm, and information on on‐farm mortality.
3.18. Specific Scenario 3 – The welfare of dairy calves and the risks associated with limited cow–calf bond
3.18.1. Background
Current EU legislation does not require any contact between the cow and the calf, only demanding that calves receive bovine colostrum within the first six hours of life (Council Directive 2008/119/EC of 18 December 2008). The current practice in dairy farms is indeed to separate cows and calves shortly after birth (here termed ‘conventional system’ or ‘artificial rearing’), but alternative systems allowing contact between cow and calf also exist (CCC systems) (Sirovnik et al., 2020).
3.18.2. Calf rearing systems: dam‐calf rearing, foster cow rearing and artificial rearing
Artificial rearing is the common rearing system on dairy farms and is based on separation of cow and calf immediately or shortly (e.g. 1 h) after birth (e.g. Klein‐Jöbstl et al. (2015)). Following separation, calves are often moved to individual pens; they are fed either whole milk or milk replacer. Calves are sometimes fed milk of lower quality (e.g. milk from medicated cows), although this is not good agricultural practice, and often fed restricted amounts of milk. Motivations for early cow–calf separation mentioned by farmers are financial reasons (harvest of milk that would be otherwise consumed by the calf), easy monitoring of calf's milk intake and prevention of separation stress at a later stage (by preventing the establishment of a bond between dam and calf) (Flower and Weary, 2003). Another cited reason for cow–calf separation at birth is prevention of vertical transmission of disease such as Salmonella Dublin or Mycobacterium avium subsp. paratuberculosis (Johne's disease) (FAWC, 2015). Recent research has investigated whether the financial and health motives for early separation are substantiated by scientific evidence.
As for prevention of Johne's disease, a systematic review examining the efficacy of immediate separation did not find scientific evidence substantiating the efficacy of this practice and noted that early cow–calf separation should not be a substitute for appropriate management of hygiene and overall cleanliness of the maternity/calving pen area (Beaver et al., 2019). While it has to be acknowledged that the efficacy of paratuberculosis control measures is difficult to determine (McAloon et al., 2019), it is expected that CCC facilitates disease transmission (Vass‐Bognár et al., 2022). In fact, increased odds of testing positive to Mycobacterium avium subsp. paratuberculosis were observed in farms practicing suckling with foster cows (Nielsen et al., 2008a,b), suggesting that early cow–calf separation can result in reduced risk of transmission of e.g. Mycobacterium avium subsp. paratuberculosis. Nevertheless, it is unlikely that separating cow and calf at birth as a single measure to control paratuberculosis in a herd will be effective for the control of the disease due to the role that other factors (such as hygiene and colostrum management) have on the transmission of the disease.
Regarding financial reasons and impact on loss of saleable milk in CCC systems, (Mogensen et al., 2022) estimated a loss of 7.1% at a production level of 9,000 kg energy corrected milk/cow/year for the most unrestricted dam‐rearing scenario (full‐time dam‐contact for 13 weeks; estimated intake 1,207 L milk) compared with the most restricted scenario (restricted bucket feeding for 13 weeks; 462 L milk). However, Asheim et al. (2016) concluded that overall reduction in saleable milk observed in CCC systems with a dual‐purpose breed was financially counterbalanced by improved weight gain of calves, health benefits for calves and cows, as well as reduced costs in systems allowing suckling for at least 7 weeks. Suckling until 13 weeks was, however, unprofitable (Asheim et al., 2016).
CCC systems include both rearing by the dam and rearing by a foster cow (Sirovnik et al., 2020). Although there is evidence that CCC systems are beneficial to cow and calf welfare and health (Johnsen et al., 2016; Beaver et al., 2019; Meagher et al., 2019), they are still uncommon and mostly present in organic farms.
In dam rearing, dam and calf have contact for a prolonged period of time, which can vary from weeks to months. A high variation can be found in these systems regarding the length of contact, the daily duration of contact (e.g. full‐time, half day, short periods few times per day), the type of physical contact allowed (e.g. full contact with milk sucking; or partial contact) (Sirovnik et al., 2020; Eriksson et al., 2022). Calf's milk intake and growth will depend on the milk eventually available to the calf.
In foster cow systems, one cow fosters 2–3 (up to 4) calves, either all alien or including her own calf. In general, foster cows are not milked, but exceptions exist (Johnsen et al., 2016). As in dam‐rearing, there are variations regarding type and duration of contact. Generally speaking, this system presents animal welfare benefits compared with artificial rearing because the calf has contact to other calves and adult cows and can perform natural suckling behaviour. However, foster calves may receive less ‘maternal’ care compared with dam‐reared calves (Johnsen et al., 2016; Wieczorreck and Hillmann, 2022). Calf's growth will depend on the number of calves relative to the cow's milk yield (for a review, please refer to Johnsen et al. (2016)).
3.18.3. Assessment scope and assumptions
In this section, the risks of limited cow–calf bond are assessed by outlining the main welfare consequences resulting from lack of contact between dam and offspring. The two main systems (i.e. artificial rearing and CCC rearing) are briefly described and compared, and provide an overview of their respective advantages and disadvantages from a welfare perspective. An estimation of the relationship between length of CCC and inability to perform sucking behaviour was carried out following the F2F model (for more details, please refer to the Data and Methodologies Section).
3.18.4. Welfare consequences of limited cow–calf bond to calves
In a natural setting, the dam often separates from the herd before parturition and stays apart from the herd in the first days after giving birth. When housed in an individual calving pen, calves kept with the dam during the first 24 h after birth show higher vitality, e.g. shorter latency to stand up and suckle (Edwards and Broom, 1982; Metz and Metz, 1986; Lidfors, 1996). In the natural setting, the calf typically ‘hides’ in vegetation, while the dam grazes close by (Vitale et al., 1986) or stands in the vicinity of the calf guarding it (Kiley‐Worthington and Plain, 1983). The dam is therefore the only social partner of the calf during the first days of life, and it is during this period that the maternal‐filial bond is established (reviewed by von Keyserlingk and Weary (2007); Sirovnik et al. (2020)). It is considered that by 4 days post‐partum, the cow–calf bond is fully established (Stěhulová et al., 2008). After the ‘hiding’ period, the dam and calf re‐join the herd, and the calf interacts with same‐age calves in addition to the continued contact with the dam, who remains the main social partner for the first weeks of life, providing maternal care such as nursing and licking (Kiley‐Worthington and Plain, 1983; Wood‐Gush et al., 1984; Vitale et al., 1986; Sato et al., 1987; Jensen, 2011). Cows nurse their calf for around 8–12 months, generally weaning their calf before the next calf is born (Reinhardt and Reinhardt, 1981; Veissier et al., 1990; Bouissou et al., 2001). The duration of contact between the calf and the dam decreases with calf age as the suckling bouts become fewer (Kiley‐Worthington and Plain, 1983; Vitale et al., 1986), but when kept together, the calf remains a preferential social partner of the dam even after weaning and the birth of a new calf (Reinhardt, 1980; Veissier et al., 1990) and it has been reported that some pairs recognise each other even after having been completely separated after weaning for the duration of more than 2 years (Wagner et al., 2012).
Here we concentrate on the welfare consequences linked specifically to the aspect of separation from the dam.
The inability to perform sucking behaviour was identified as one of the most relevant welfare consequences for a calf reared with limited (or no) contact with the dam. The duration of sucking milk in artificial rearing is much shorter per meal and overall (teat bucket: Krohn et al. (1999); AMF: Fröberg et al. (2008); Johns et al. (2011)) compared with dam‐reared calves, which suckle 5–11 times a day during the first weeks, declining to 3–5 daily bouts after 2–3 month (e.g. Fröberg and Lidfors (2009)), with each bout lasting approx. 7 min (e.g. Lidfors et al. (2010)). A short sucking duration may lead to an unfulfilled sucking need and increase the risk of development of abnormal oral behaviours, especially when calves are not fed milk via an artificial teat, and when a low milk allowance is offered. Calves may redirect their sucking behaviour to either objects, such as pen fixtures or, if accessible, to other calves (cross‐sucking). Among group housed calves, cross‐sucking is often directed to the inguinal region, especially the scrotum and udder plant (Keil and Langhans, 2001; Margerison et al., 2003; Roth et al., 2008) (Figure 26) where the active calf's posture resembles the one during sucking the cows' udder. Cross‐sucking and non‐nutritive sucking of objects are considered abnormal, redirected behaviour that indicates thwarted motivation and impairment of welfare (Costa et al., 2016).
Figure 26.

Omphalitis in a calf due to cross‐sucking. © George Stilwell
Prolonged hunger is also relevant in early separation systems, because the amount of milk fed to the calves is often lower than the amount they would ingest if suckling their dam or having ad libitum access to milk when artificially reared (Klein‐Jöbstl et al., 2015; Barth, 2020). Calves with unrestricted contact and ad libitum access to suckle had higher weight gain than artificially reared calves, likely due to the larger amounts of milk that calves ingested (Roth et al., 2009a). In general, the risk of prolonged hunger is considered to be higher in artificial rearing than in CCC systems, but this will depend on access to suckle the dam (e.g. full time, part time or restricted suckling (Nicolao et al., 2022; Roadknight et al., 2022)), number of calves per foster cow and milk allowance in artificial reared calves (see Section 3.2.7.1).
Calves kept in an individual pen experience isolation stress (i.e. stress and negative affective states due to the absence of, or limited social contact with conspecifics). Isolation stress can be prevented by keeping calves together with same‐age calves, which provides social support and stimulates development of social behaviour and social competences (i.e. appropriate social responses) (see Sections 3.2.3 and 3.3.3). However, animals reared in CCC systems exhibited higher social competence (here reflected in demonstrating submissive behaviour towards an older conspecific) compared with animals reared artificially in groups (calves: Buchli et al. (2017); heifers: Wagner et al. (2012)). Long‐term effects of CCC rearing were also detected on reactions to a social challenge test (Wagner et al., 2012, 2013, 2015), and there are some indications that dam‐reared heifers may experience less stress when integrated into the cow herd compared with artificially reared animals (Wagner et al., 2012; Zipp and Knierim, 2020). Nevertheless, further research is needed to better understand the mechanisms behind calf's social development and the potential role of the dam in this.
When separation of cow and calf takes place after they have bonded, calves can experience separation stress. Calves separated immediately after birth do respond to this e.g. by vocalisations (Lidfors, 1996), but there is little response to separation by calves separated before the maternal‐filial bond is fully formed (i.e. at ~ 4 days; Lidfors (1996); Weary and Chua (2000); Stěhulová et al. (2008)) compared with later. The separation stress during and after separation of bonded cow–calf pairs depends on the duration of the contact between dam and calf and on the method of weaning. The response of bonded young dairy calves to abrupt separation at the age of 4–14 days is characterised by restlessness and attempts to re‐establish contact as expressed by more standing and walking in the pen, placing the head outside the pen, more explorative behaviour such as sniffing the walls and the bedding and licking the walls compared with calves separated at birth or within 24 h (Weary and Chua, 2000; Flower and Weary, 2001; Stěhulová et al., 2008). In CCC dairy production systems where the dairy calf and dam have been together for 8–12 weeks, abrupt separation leads to calf responses characterised by increased activity and high pitch calls (Veissier et al., 2013; Johnsen et al., 2015).
Once the bond has been established, increased age at weaning/separation is beneficial: the older beef suckler calves are, the lower are stress reactions (Pérez‐Torres et al., 2016), comparing 3.5‐ and 6.5‐week‐old calves; (Lambertz et al., 2015), comparing 6‐ and 8‐month‐ old calves; comparing 4‐, 10‐ and 26‐week‐old calves (de Souza Teixeira et al., 2021), probably because older calves can more easily rely on solid feed due to a more developed rumen and are thus less dependent on their dam's milk. Similarly, in a full‐time CCC system, the decrease in play behaviour of 36 dairy calves in reaction to weaning was negatively associated with weaning age (ranging from 83–117 days): the older the calves were the lower was the decrease in play behaviour (Susanne Waiblinger, personal communication, 5.4.2022). This likely reflects a decreasing nutritional dependency of milk as calves grow older (Rushen et al., 2016), in line with a lower weight loss after dam separation in the older calves in the study of (Pérez‐Torres et al., 2016).
In addition to age at separation, other factors influencing separation stress include the method of separation and whether this coincides with weaning off milk or not. In abrupt weaning, separation from the dam and weaning off milk happens at once. Minimising the effect of these two stressors by two‐step weaning, i.e. still allowing physical contact but preventing the calf from suckling (either by nose flap or fence line), or gradual weaning (i.e. progressive reduction of contact time until permanent separation), is hypothesised to reduce stress responses in calves. Results from a study with 6.5‐month‐old beef suckler calves separated from their dam by a fence concluded that these calves vocalised about half as much compared with abruptly weaned calves; in addition, they ate, walked and lay down as much as control animals that were kept with their dam. On the other hand, abruptly weaned 6‐month‐old beef calves, ate and lay down less and walked more than controls and fence‐line weaned calves (Price et al., 2003). A similar trend was observed in a study with 10‐week‐old animals: calves fitted with a nose flap to prevent them from suckling during 2 weeks before being separated from their foster cow, vocalised less, walked less and had a lower heart rate after separation compared with calves separated and weaned off milk simultaneously (Loberg et al., 2008). However, nose flaps may cause injuries at the nose septum if left in for too long (Lambertz et al., 2015). Similar positive effects of two‐step separation methods compared with abrupt separation were observed in younger calves; 8‐week‐old dairy calves with fence‐line contact to their dam vocalised less and were less alert compared with calves that were separated from the dam by a solid wall (Johnsen et al., 2015). Also, dairy calves gradually weaned by fence line allowing suckling on the cows' initiative from week 7 to 8, responded less to being fully weaned at 8 weeks than calves weaned by nose flap (Wenker et al., 2022).
However, others argued that such alternative weaning methods should be viewed with caution. (Enríquez et al., 2010) did not observe an overall reduction in behavioural stress responses (i.e. vocalisations, pacing and seeking behaviour) in beef calves weaned by a nose flap or by a fence‐line compared with abruptly weaned calves, but rather a different distribution of these behaviours over time. This indicates that there is not yet a clear understanding of the influence of factors such as age at weaning, duration of nose‐flap/fence‐line period, developmental stage of calves and cow's milk production on calf's response to weaning.
In addition to avoiding or reducing the above‐discussed negative welfare consequences of artificial rearing, prolonged contact with the dam offers unique opportunities for positive affective states and thus positive welfare. The maternal care includes licking, nursing and play behaviours associated with positive affective states and beneficial physiological effects (Lupoli et al., 2001; Uvnäs‐Moberg et al., 2001; Held and Špinka, 2011; Waiblinger et al., 2020).
3.18.5. Welfare consequences of limited cow–calf bond to cows
From the dam's perspective, there may also be negative welfare consequences due to limited or no contact with their calf, but still only few studies have investigated the consequences of separation from the calf to the dam. Peri‐parturient cows have a strong motivation for maternal behaviour (Edwards and Broom, 1982) and a strong motivation to access their calf even when separated shortly after birth (Wenker et al., 2020). Potential beneficial effects of prolonged calf contact to cows are improved health (for review Flower and Weary (2003); Johnsen et al. (2016); Beaver et al. (2019)), increased oxytocin levels in nursing cows (Lupoli et al., 2001) and associated behavioural, physiological and health benefits (Uvnäs‐Moberg et al., 2001). However, more research is warranted.
Separation stress responses were lower in cows separated immediately after birth as compared with cows that had fostered their calves for several days (Stěhulová et al., 2008) or weeks (Flower and Weary, 2001). Two‐step weaning and separation procedures reduced stress at separation also in cows (foster cows: Loberg et al. (2007); dams: Wenker et al. (2022), and a lower response was found with increasing age of the calves (Pérez‐Torres et al., 2016). However, more research is needed to confirm these aspects.
3.18.6. EKE model parameters
An EKE was used to estimate the effect of different degrees of contact between the calf and the cow on cross‐sucking behaviour. A theoretical model integrating the general concept of a ‘non‐exposed’ population and based on expert estimates was applied. The WG based their elicitation on scientific data reported in peer‐reviewed literature and on their expert knowledge. For information on the general methodology please refer to Section 2.2.2.
The ‘inability to perform sucking behaviour’ was considered to be one of the most relevant welfare consequences in calves with limited contact with the dam. A literature search was conducted to gather data from experimental studies on prevalence of cross‐sucking behaviour observed in calves depending on the duration of contact with the dam; these are described below.
While no precise data exist, cross‐sucking is considered to be very prevalent in dairy calves reared artificially. In studies in Austria, approximately two‐thirds of farmers reported that it occurred on their farms (Gugatschka, 2008; Graca, 2016). Cross‐sucking often continues after weaning off milk and can develop into inter‐sucking of the udder of other heifers or cows (Keil et al., 2000; Keil and Langhans, 2001).
In experimental studies, cross‐sucking was not observed among calves with full‐time contact with their dam during the milk feeding period (8 or 12 weeks) (Fröberg and Lidfors, 2009; Roth et al., 2009b) or after weaning off milk (Roth et al., 2009b). In contrast, among calves with twice‐a day contact with the dam for 15 or 30 min, 10–20% of calves performed cross‐sucking (Fröberg et al., 2007; Fröberg et al., 2008; Roth et al., 2009b). Among calves separated within 24 h after birth and reared artificially with restricted milk feeding via a teat, 61–83% of calves cross‐sucked (Fröberg et al., 2008; Fröberg and Lidfors, 2009; Roth et al., 2009b); when calves were fed ad libitum milk 32% of calves cross‐sucked. Calves allowed to suckle their dam for 4 days sucked on objects such as pen fixtures less often and for a shorter duration compared with calves that were separated from their dam immediately after birth, however cross‐sucking was not reduced 3, 6 and 10 weeks after separation from the dam (Krohn et al., 1999). Another study compared the effect of dam‐rearing, foster‐cow rearing and artificial rearing on the occurrence of cross‐sucking. No statistically significant differences were observed between dam‐reared and foster‐reared calves allowed 15 min contact with the cow per day; however, both treatments showed less cross‐sucking than artificially reared calves (Margerison et al., 2003).
A summary on the EKE parameters and components is presented in Table 40 and the systems considered in the EKE in Table 41. In this context, the ABM of interest was the proportion of dairy calves showing cross‐sucking at least once during the period of interest (also referred to as cumulative prevalence in this section).
Table 40.
Summary of EKE parameters and components
| EKE components | Definitions and assumptions |
|---|---|
| Animal category to be considered | Dairy calves up to 12 weeks of age |
| Husbandry system | 4 categories (see Table 41). Period of interest for the EKE was the pre‐weaning period. Weaning was assumed at 12 weeks |
| The exposure variable of interest for the EKE | Duration of contact between calf and dam following birth |
| Welfare consequence at stake for the EKE | Inability to perform suckling behaviour |
| ABM chosen for the exercise | Defined as ‘the proportion of dairy calves showing cross‐sucking over the first 12 weeks of life’. Cross‐sucking was defined as a calf sucking on any body part of another calf (Fröberg et al., 2007) |
| Unexposed population | Defined as calves before weaning, up to 12 weeks, in a cow–calf contact system with permanent access to their own dam (except during times of milking) allowing ad libitum feeding |
| Highly exposed population | Defined as a group of calves separated from their dam within 24 h after birth with restricted (i.e. not ad libitum) milk feeding |
Table 41.
Rearing systems included in the EKE
| Rearing system description | Rationale to select this category for the EKE |
|---|---|
|
Full‐time contact with the cow Milk ad libitum Separation after week 12 |
Cow–calf contact system with prolonged contact; used in organic farming. Some cow–calf systems have longer contact periods |
|
Twice‐a‐day contact with the cow Milk ad libitum Separation after week 10 |
Intermediate system in terms of daily contact duration |
|
Contact with the cow for less than 24 h Milk ad libitum Access to artificial teat (such as teat bucket, teat bars or automatic milk feeder) for the time the milk intake lasts, with no dry teat afterwards |
Conventional separation time, i.e. separated within 24 h after birth. Ad libitum milk not common in practice but effects on cross‐sucking have been described |
|
Contact with the cow for less than 24 h Restricted milk Access to artificial teat (such as teat bucket, teat bars or automatic milk feeder) for the time the milk intake lasts, with no dry teat afterwards |
Conventional system, i.e. separated within 24 h after birth |
3.18.7. Sources of uncertainty in the estimates
Factors other than CCC duration may influence levels of cross‐sucking before weaning, such as amount of milk provided/available, amount of roughage and concentrate provided, outdoor or indoor housing or calf breed. These aspects were considered sources of uncertainty in the estimate because, although it is assumed that they can affect the probability of a calf developing cross‐sucking, their exact impact is not well understood. While there are not always data to confirm these sources of uncertainty, they were hypothesised by the AHAW Panel to be relevant (Table 42).
Table 42.
Sources of uncertainty on the EKE estimate ‘% of calves performing cross‐sucking behaviour’
| Sources of uncertainty in cross‐sucking which effect has not been fully determined by research | Reason |
|---|---|
| Breed | Simmental, Pezzata rossa italiana and Montbeliard breeds are associated with more cross‐sucking behaviour than in dairy type breeds such as Holstein and Brown Swiss |
| Amount of milk available in the udder at contact time (milking process/duration and timing of milking) | Low amount available associated with higher incidence of cross‐sucking |
| Milk allowance (based on % of body weight) | Low milk allowances associated with higher percentage of cross‐sucking |
| Number of teats per calf | A fewer number of teats is expected to result in higher prevalence |
| Access to solid feed | Insufficient access to solid feed associated with higher percentage of cross‐sucking |
| Group size | More cross‐sucking expected in larger groups due to more opportunities to cross‐suck |
| Age difference within group | Higher age heterogeneity associated with higher percentage of cross‐sucking |
| Type of solid feed (quantity of fibre content) | Lower fibre content associated with higher percentage of cross‐sucking |
| Outdoor housing | Indoor associated with higher percentage of cross‐sucking |
| Parity of the dam | 1st parity could increase the risk of cross‐sucking |
| Quality of milk replacer | Higher lactose leads to higher cross‐sucking |
| Automatic milk feeder design | Closing gate associated with lower prevalence |
It is worth noting that calf's age was not considered a source of uncertainty: the cumulative prevalence of cross‐sucking behaviour did not significantly vary over time (Fröberg et al., 2008; Fröberg and Lidfors, 2009). However, studies measuring the duration of cross‐sucking behaviour reported that the duration of the cross‐sucking behaviour decreased as calves grew older (Das et al., 2000; Fröberg et al., 2007).
3.18.8. Results from the EKE model – cross‐sucking cumulative prevalence depending on time spent with the dam
It was estimated that the cumulative prevalence of cross‐sucking in calves is high for early separated calves especially with restricted milk allowance, is reduced in CCC system with short time contact and can be prevented with full‐time cow–calf contact (Figures 27–29).
Figure 27.
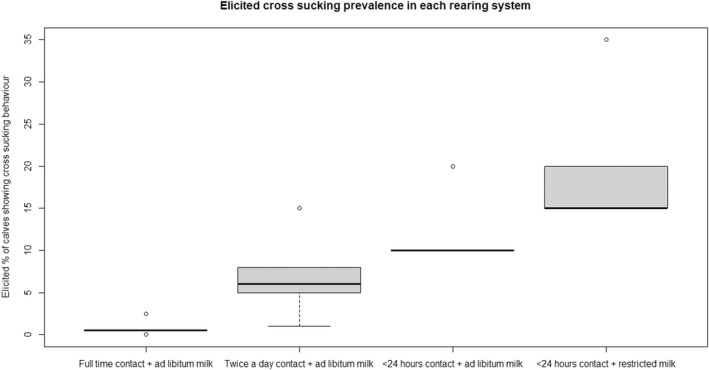
- The box plots represent the median estimate, 25th and 75th percentile of the distribution. The whiskers represent 90% credibility intervals.
Figure C.1.
Classification of relevance of welfare consequences (high, medium, low, no relevance) in calves kept in individual pens in dairy farms
The estimated median value of the proportion of calves showing cross‐sucking behaviour over the preweaning period (between 0 and 12 weeks of age):
in a system allowing full‐time contact between the dam and the calf and ad libitum milk from cow's udder was 1% (90% credibility interval 0–3%).
in CCC systems allowing twice‐a‐day contact between the dam and the calf and ad libitum milk was 14% (90% credibility interval 4–30%).
in artificial rearing systems allowing contact between the dam and the calf for less than 24 h after birth and ad libitum milk from an artificial teat was 30% (90% credibility interval 12–53%).
in artificial rearing systems allowing contact between the dam and the calf for less than 24 h after birth and restricted milk from an artificial teat was 64% (90% credibility interval 38–95%).
Additionally, if calves have full‐time contact with the dam for at least 6 weeks (95% credibility interval 4–8 weeks), cross‐sucking cumulative prevalence does not increase substantially (i.e. by more than 10%) compared with a situation with full contact with the dam for 10 weeks.
3.18.9. Other relevant welfare aspects observed depending on the cow–calf contact duration
A summary of the available evidence on effects on calf's welfare depending on length of contact with the dam is provided in Table 43.
Table 43.
Effects on calf's welfare depending on length of contact with the dam. Some of the positive effects can be observed as early as the first hours of life, while some others require longer contact. Negative effects are mostly linked to separation stress when abrupt separation between cow and calf occurs
| Length of contact between calf and dam | Positive welfare effects for the calf | Negative welfare effects for the calf |
|---|---|---|
| No contact (separation immediately after birth – conventional system) |
No transmission of disease Separation at birth can prevent transmission of salmonellosis because there is significant Salmonella faecal shedding during the first 24 h postpartum (Anderson et al., 2001; House et al., 2001; Holschbach and Peek, 2018) |
More vocalisations Calves vocalised more, licked themselves more at 24, 47 and 72 h pp and moved more at 48 h pp when separated immediately post‐partum (pp) compared with calves separated 4 days pp (Lidfors, 1996) |
| First hours of life (separation within 24 h after birth) | Reduced transmission of disease |
More cross‐sucking Higher proportion of cross‐sucking in calves separated within 24 h after birth and reared with ad libitum milk provided via an artificial teat compared with calves kept with the dam for 12 weeks with ad libitum milk (1% vs 30% cross‐sucking prevalence, as estimated via EKE) Higher diarrhoea prevalence Calves separated at 6 h post‐partum were treated for diarrhoea more days (5.5 ± 1.7) during their first 4 weeks of life compared with calves separated 1 day pp (4 ± 0.7) and 4 days pp (2 ± 1.2) (Weary and Chua, 2000) |
|
Higher calf vitality Reduced latency to defecate and urinate following birth compared with calves separated shortly after birth, probably as a result of anogenital licking by the cow (Metz and Metz, 1986) cited by (Weary and Chua, 2000) Shorter amount of time to first attempt to stand and first successfully stand compared with calves separated immediately post‐partum (Lidfors, 1996) |
– | |
| At least 4 days |
More developed social behaviour Calves that were separated at 4 days after birth spent more time within a two‐meter distance of unfamiliar calves on a test made at 3 and 11 weeks of age when compared with calves that were separated within 24 h (Krohn et al., 1999). Higher weight gain Calves with CCC with or without sucking had better weight gains than calves artificially reared & ad libitum fed compared with calves that had with only a few hours of contact with the dam (Krohn et al., 1999) |
Higher separation stress When separated at 4 days post‐partum calves performed more oral behaviour than calves separated immediately post‐partum; sniffing at the litter, walls, steel bars for about half an hour (Lidfors, 1996); After separation, older calves (4 and 7 days) stood and moved more (p < 0.001), placed their heads outside the pen more often (p < 0.01) and showed more explorative behaviour (i.e. sniffing walls and bedding, p < 0.01; licking walls, p < 0.05) than calves separated on day 1 (Stěhulová et al., 2008). Change in heart rate was more prolonged in calves that were separated on days 4 and 7 compared with calves separated within 24 h (Stěhulová et al., 2008). |
| 7 days |
More developed social behaviour The calves that were separated on day 7 seemed to cope better in a novel situation compared with calves separated within 24 h and 4 days since they demonstrated high activity immediately after they were moved into the group of unfamiliar calves and spent extra time resting during the second day of observations (Stěhulová et al., 2008) |
– |
| 10 days |
Higher weight gain Separation at 14 days when compared with separation at 1 day caused a two to three times increase in weight gain and the effects continued up to four weeks after birth (Flower and Weary, 2001) Separation at 10 days compared with separation at 1 day resulted in weight gain and these effects persisted for up to 2 months (Metz, 1987) after separation. |
– |
| At least 2 weeks |
More developed social behaviour Calves reared with their mothers during the first two weeks of age revealed more intense social behaviour (licking, butting, rubbing of the head) towards unfamiliar calves (Flower and Weary, 2001) which might indicate lesser fearfulness of unknown conspecifics (Krohn et al., 1999) |
Higher separation stress Following separation, calves in the late‐separation treatment (two weeks) moved and placed their heads outside the pen more frequently compared with early‐separated calves (one day) (Flower and Weary, 2001) |
| 3 weeks |
Lower disease prevalence Lower bouts of diarrhoea during the first 3 weeks of life compared with separation at 6 h (Weary and Chua, 2000) |
– |
| No data between 4 and 10 weeks – large uncertainty | ||
| 12 weeks or longer |
Less cross‐sucking Calves separated at 90 days of age showed less cross‐sucking behaviour compared with calves separated within 24 h (p < 0.05) (Roth et al., 2009a) More developed social competence & sociality Heifers kept with their mothers for the first 3 months of life were more socially dominant in adulthood compared with those that were separated immediately from their mother, kept individually and fed from a bucket (Le Neindre and Sourd, 1984) Heifers that had been reared with contact to their mother (full‐time or twice a day contact) for the first 3 months of life displayed greater social competence (used more submissive behaviour, kept more distance to adult cows) compared with heifers that had been separated within the first day of life and reared in dynamic groups of calves (Wagner et al., 2012). Animals reared with contact with their mother for 3 months showed signs of higher sociability both as heifers and primiparous cow (Wagner et al., 2015). Calves reared with contact with the dam for at least 90 days showed more adaptive social behaviour compared with calves housed in peer groups (Buchli et al., 2017) |
– |
| 8 months |
Lower separation stress (vocalisations) Beef calves separated at 8 months showed decreased vocalisations compared with separation at 6 months of age (Lambertz et al., 2015) |
|
In general, it is considered that the positive effects of contact between cow and calf are cumulative; the longer the contact, the more behavioural and health aspects will be positively impacted, such as calf's general activity, health, social behaviour and social competence. For instance, positive effects of contact on calf's vitality can be observed as early as the first hours of life (Lidfors, 1996). Calves that were cared for by their dam after birth stood up, suckled and eliminated earlier compared with calves separated from their dam after birth (Edwards and Broom, 1982; Metz and Metz, 1986; Lidfors, 1996). Other positive effects of contact with the dam at an early age include the social licking, nursing and playing, which are part of the maternal care provided by the dam. Maternal care is associated with release of oxytocin (Uvnäs‐Moberg, 1998; Lupoli et al., 2001); in other species, oxytocin has been demonstrated to stimulate the bonding process between mother and offspring and to reduce stress (Lupoli et al., 2001). Long‐term differences in behavioural and physiological stress reactions to challenges between calves reared artificially or in contact with their mother (Wagner et al., 2015) may at least partly be linked to higher oxytocin levels during rearing; however further studies are necessary to investigate the mechanisms behind this. Only few studies investigated the effects of length of dam‐rearing, but these studies suggest that longer‐term contact, such as at least 2 weeks, impacted positively the development of social behaviour, and that contact for 12 weeks or longer increased social competence (Wagner et al., 2015; Buchli et al., 2017).
Data gaps identified in this assessment include welfare effects of contact with the dam until calves are 3–8 weeks old. There is also a very limited number of studies investigating welfare aspects related with foster cow rearing. There are few studies focusing on the cow's perspective and short and long‐term welfare implications of separation, but these suggest that cows also experience stress at the time of separation from the calf (dam: Weary and Chua (2000); Nicolao et al. (2022); foster cow: Loberg et al. (2007)).
3.18.10. Conclusions on cow–calf contact
Separation of dam and calf immediately after birth is carried out by the great majority of dairy farms (conventional system). This practice prevents calves from experiencing positive effects of contact with the dam, related with vitality, growth, higher resilience to gastroenteric disorders (or diarrhoea) and appropriate development of social competences (certainty 90–100%).
The benefits of CCC for the calf increase with the duration of contact: a positive impact on calf vitality can already be observed after some hours of contact with the dam; a positive effect on weight gain is observed after 4 days of contact; a reduced prevalence of diarrhoea is observed after a contact duration of 2 weeks; and development of social competence improves after 12 weeks (certainty 66–100%). There is a lack of evidence on welfare effects potentially observed in the interval from 3–8 weeks of age (certainty 90–100%).
A negative welfare consequence observed in CCC systems is separation stress, when cow and calf are eventually kept apart. The severity of separation stress depends on calf's age and on the method of separation (certainty 90–100%).
Separation stress is most severe after the cow–calf bond is formed (at 4 days post‐partum) and until 6–10 weeks of age. Hereafter, separation results in fewer reactions the older the calves are (certainty 66–100%). There is a lack of scientific evidence at which age separation responses start to decline (certainty 90–100%).
Following immediate separation from the dam at birth, the negative welfare consequences experienced by a calf depend on the rearing method after separation. Calves kept in groups experience inability to perform natural sucking behaviour (from the udder) and inability to perform play behaviour with the dam; if calves are individually housed, they will also experience isolation stress and inability to perform play behaviour with other calves (certainty 90–100%).
Calves kept with the dam during the pre‐weaning period and allowed to suckle ad libitum from the udder are estimated to show nearly no cross‐sucking (1%; 90% credibility interval 0–3%) compared with calves separated from the dam shortly after birth and provided with a restricted milk allowance fed via an artificial teat (cross‐sucking prevalence of 65%; 90% credibility interval 38–95%).
If the calf cannot be kept with the dam, rearing of the calf with a foster cow prevents the negative welfare consequences related with inability to perform natural sucking behaviour and impaired weight gain (as long as there is not high competition for milk) and promotes development of social competences (certainty 90–100%).
Several data gaps were identified in the assessment. There is limited understanding of calf's responses to separation from the dam when calves are 6–10 weeks of age. There is also very limited published data on cow's responses to separation from the calf. In addition, there is a lack of research on practical strategies for CCC systems, such as duration and frequency of CCC, timing of suckling (i.e. pre‐ or post‐milking), optimum duration of suckling period and weaning strategies (certainty 90–100%).
3.18.11. Recommendations on cow–calf contact
The calf should be kept with the dam for a minimum of ~ 24 h and be housed with another calf after that. This would improve the current situation in which calves are mostly separated from the cow shortly after birth and housed individually after that.
Prolonged cow–calf contact should increasingly be implemented due to the welfare benefits for calf and cow. In the future, calves should have contact with the dam during the whole pre‐weaning period.
The second‐best alternative to dam–calf contact is prolonged contact with a foster cow.
Further research is needed to better understand how to implement CCC in a larger scale and to identify the best options in practice. Research is also needed for defining best practices for foster‐cow rearing.
4. Conclusions and recommendations
For conclusions and recommendations on:
Housing systems, welfare consequences and preventive measures (Common ToR), see Section 3.15.
Individual and group housing (Specific Scenario 1), see Sections 3.16.1.7 and 3.16.1.8.
Space allowance (Specific Scenario 1), see Sections 3.16.2.4 and 3.16.2.5.
Iron (Specific Scenario 1), see Sections 3.16.3.3 and 3.16.3.4.
Fibre (Specific Scenario 1), see Sections 3.17.7, 3.16.4.4 and 3.16.4.5.
ABMs at slaughter (Specific Scenario 2), see Sections 3.17.6 and 3.17.7.
Cow–calf contact (Specific Scenario 3), see Sections 3.18.10 and 3.18.11.
Abbreviations
- ABM
animal‐based measures
- ACTH
adrenocorticotropic hormone
- AHAW
Animal Health and Animal Welfare
- AIP
atypical interstitial pneumonia
- AMF
automatic milk feeder
- BCS
body condition score
- BRD
bovine respiratory disease
- BRSV
bovine respiratory syncytial virus
- BVDV
bovine viral diarrhoea virus
- BW
body weight
- CCC
cow–calf contact
- CRTD
clinical respiratory tract disease
- DM
dry matter
- ECI
European Citizen Initiative
- EKE
Expert Knowledge Elicitation
- ELS
extensive literature searches
- FTPI
failed transfer of passive immunity
- GE
gastroenteric
- NCD
neonatal calf diarrhoea
- NDF
neutral detergent fibre
- NSAID
non‐steroidal anti‐inflammatory drugs
- PCV
packed cell volume
- RBC
red blood cells
- RR
respiration rate
- SO
Scientific Opinion
- THI
temperature–humidity index
- ToR
Terms of Reference
- TRL
Technology Readiness Level
- VFA
volatile fatty acids
- WC
Welfare consequences
- WG
Working Group
- WQ
Welfare Quality
Appendix A – Description of negative affective states and welfare consequences
Table A.1: Description of negative affective states considered in this scientific opinion
| Negative affective state | Description |
|---|---|
| Boredom | Boredom is an unpleasant emotion including suboptimal arousal levels and a thwarted motivation to experience almost anything different or more arousing than the behaviours and sensations currently possible (adapted from Mason and Burn, 2011). |
| Discomfort |
Discomfort can be physical or psychological and is characterised by an unpleasant feeling resulting in a natural response of avoidance or reduction of the source of the discomfort. Pain is one of the causes for discomfort, but not every discomfort can be attributed to pain. Discomfort in non‐communicative patients is assessed and measured via behavioural expression, also used to describe pain and agitation, leading to discomfort being interpreted as pain in some conditions (Ashkenazy and Ganz, 2019). |
| Stress (1) & Distress |
STRESS(1): Stressors are events, internal or external to the body involving real or potential threats to the maintenance of homeostasis. When stressors are present, the body will show stress responses (biological defence to re‐establish homeostasis – for example behavioural, physiological, immunological, cognitive, and emotional). Stress is a state of the body when stress responses are present (Sapolsky, 2002). DISTRESS: Distress is a conscious, negatively valenced, intensified affective motivational state that occurs in response to a perception that current coping mechanisms (involving physiological stress responses) are at risk of failing to alleviate the aversiveness of the current situation in a sufficient and timely manner (McMillan, 2020). |
| Fatigue | Physiological state representing extreme tiredness and exhaustion of an animal (EFSA AHAW Panel, 2020). |
| Fear | The animal experiences an unpleasant emotional affective state induced by the perception of a danger or a potential danger that threaten the integrity of the animal (Boissy, 1995). |
| Frustration | Negatively valenced emotional state consecutive to the impossibility to obtain what is expected or needed. Frustration is very often triggered by restriction of natural behaviours thus resulting in thwarted motivation to perform these behaviours. |
| Pain | An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage (Raja et al., 2020). |
| (1): The term ‘stress’ has been widely used in biology to describe a set of physiological and behavioural changes elicited by aversive stimuli and is strictly speaking not always considered as a negative affective state. However, the term stress is used in the different Scientific Opinions to refer mainly to DISTRESS. | |
The term stress does not describe a negative affective state in itself, but it is mentioned and defined in the table as it is a prerequisite of distress.
Table A.2: Description of specific welfare consequences considered in this scientific opinion
| Welfare consequence | Description |
|---|---|
| Restriction of movement | The animal experiences negative affective states such as pain, fear, discomfort and/or frustration due to the fact that it is unable to move freely or is unable to walk comfortably. |
| Resting problems | The animal experiences negative affective states such as discomfort, fatigue and/or frustration due to the inability to lie or rest comfortably (e.g. inability to perch or due to vibration during transport). |
| Group stress | The animal experiences stress and negative affective states such as pain, fear and/or frustration resulting from a high incidence of aggressive and other types of negative social interactions, often due to hierarchy formation and competition for resources or mates. |
| Handling stress | The animal experiences stress and negative affective states such as pain and/or fear resulting from handling by humans (e.g. moving animals between pens, loading/unloading). |
| Isolation stress | The animal experiences stress and negative affective states such as frustration and/or fear resulting from the absence of or from limited social contact with conspecifics. |
| Separation stress | The animal experiences stress and negative affective states such as fear and/or frustration resulting from separation from conspecifics. |
| Inability to perform comfort behaviour | The animal experiences negative affective states such as discomfort and/or frustration resulting from the thwarting of the motivation to maintain the function and integrity of the integument. |
| Inability to perform exploratory or foraging behaviour | The animal experiences stress and negative affective states such as frustration and/or boredom resulting from the thwarting of the motivation to investigate the environment or to seek for food (i.e. extrinsic and intrinsic exploration). |
| Inability to perform sucking behaviour | The animal experiences negative affective states such as frustration resulting from the thwarting of the motivation to suck from a teat. |
| Inability to chew and ruminate | The animal experiences negative affective states such as frustration resulting from the thwarting of the motivation to ingest sufficient amounts of effective fibres. |
| Inability to perform play behaviour | The animal experiences negative affective states such as frustration resulting from the thwarting of the motivation to engage in social/locomotory or object play. |
| Prolonged hunger | The animal experiences craving or urgent need for food or a specific nutrient, accompanied by an uneasy sensation (a negative affective state), and eventually leading to a weakened condition as metabolic requirements are not met. |
| Prolonged thirst | The animal experiences craving or urgent need for water, accompanied by an uneasy sensation (a negative affective state), and eventually leading to dehydration as metabolic requirements are not met. |
| Heat stress | The animal experiences stress and/or negative affective states such as discomfort and/or distress when exposed to a high effective temperature. |
| Cold stress | The animal experiences stress and/or negative affective states such as discomfort and/or distress when exposed to a low effective temperature. |
| Locomotion disorders (including lameness) | The animal experiences negative affective states such as pain, discomfort and/or distress due to impaired locomotion induced by e.g. bone, joint, skin or muscle damage. |
| Soft tissue lesions and wounds | The animal experiences negative affective states such as pain, discomfort and/or distress due to physical damage to the skin, the feather or underlying tissues, e.g. multiple scratches, open or scabbed wounds, bruises, ulcers or abscesses. This welfare consequence may result from negative social interactions such as aggression, tail‐biting, feather pecking or from damaging environmental features. It also includes intentional mutilations (e.g. beak trimming, de‐toeing, de‐horning, tail docking). |
| Bone lesions (incl. fractures and dislocations) | The animal experiences negative affective states such as pain, discomfort and/or distress due to fractures or dislocations of the bones (excluding those fractures leading to locomotory disorders). |
| Skin disorders (other than soft tissue lesions and integument damage) | The animal experiences negative affective states such as pain, discomfort and/or distress due to e.g. infections (e.g. dermatophytosis/ringworm, pseudomonosis, staphylococcosis, viral diseases), ectoparasites (e.g. mange or red mites), inflammation of the skin or sunburn. |
| Respiratory disorders | The animal experiences negative affective states such as discomfort, pain, air hunger and/or distress due to impaired function or lesion of the lungs or airways. |
| Eye disorders | The animal experiences negative affective states such as discomfort, pain and/or distress due irritation or lesion or lack of function of at least one eye. |
| Gastroenteric disorders | The animal experiences negative affective states such as inappetence, discomfort, pain and/or distress due to impaired function or lesion of the gastrointestinal tract resulting from for example nutritional deficiency, infectious, parasitic or toxigenic agents. |
| Metabolic disorders | The animal experiences negative affective states such as inappetence, weakness, fatigue, discomfort, pain and/or distress due to disturbed metabolism (e.g. acidosis and ketosis), deficiencies in several nutrients (e.g. anaemia) or induced by ectoparasites affecting metabolism (anaemia due to red mites) or poisoning. |
| Umbilical disorders and hernias | The animal experiences negative affective states such as discomfort and/or pain due to an inflammation of the navel or any type of hernias. |
| Soft tissue lesions and integument damage | The animal experiences negative affective states such as pain, discomfort and/or distress due to physical damage to the integument or underlying tissues, e.g. multiple scratches, open or scabbed wounds, bruises, ulcers, abscesses and feather or hair loss. This welfare consequence may result from negative social interactions such as aggression, tail‐biting or feather pecking, from handling or from damaging environmental features, or from mutilation practices (e.g. de‐horning, tail docking). |
Appendix B – Additional information on methodology
Assessment of ABMs
This section provides information on the assessment of ABMs, to complement the information presented in the main text.
Regarding sensitivity and specificity, the assumptions and method described in the EFSA AHAW Panel (2022a) are here reproduced: ‘For sensitivity, the following was considered: the presence of the ABM as its ability to identify animals suffering from the welfare consequence. An ABM that is not systematically present in all animals with the WC will be less sensitive. For specificity, the following was considered: the absence of the ABM as its ability to identify the animals, which are not suffering from the WCs. An ABM that will be present in several WCs will tend to be less specific’.
For instance, bursa swelling (hygroma) is an ABM of resting problems that is specific but not sensitive: it is specific because it is only present if calves experience resting problems and not other welfare consequences (no false positives expected) but it is not sensitive because some of the calves experiencing resting problems will not develop bursa swelling (false negatives expected). In addition to the overall procedure, some additional criteria were defined in this scientific opinion, which are described below.
Two categories were considered when assessing qualitatively sensitivity and specificity: ‘high’ or ‘low’. A justification for high specificity/sensitivity was not included because it was considered that the reasoning was self‐explanatory based on the way the ABM was phrased. In contrast, a justification for low sensitivity and low specificity was provided by giving examples of instances where false negatives or false positives, respectively, can be expected.
In addition, when describing an ABM, a definition was included. With regard to the interpretation of the meaning of an ABM regarding how it is related with a welfare consequence, it was agreed that when there was a positive relationship between the ABM and the welfare consequence (e.g. more slipping when there is a greater restriction of movement due to slippery floors), no formal interpretation of the ABM was needed. In the remaining cases, some text was added to explain how the ABM relates to the welfare consequence (e.g. no adoption of relaxed lying postures when the calf experiences resting problems).
Methodology to address Specific Scenarios 1 and 3
The methodology used to address Specific Scenarios 1 and 3 is described in detail in Section 3.2 of the ‘EFSA Methodological guidance for the development of animal welfare mandates in the context of the Farm to Fork Strategy’ (EFSA AHAW Panel, 2022a). For simplicity, this model is referred to in this scientific opinion as ‘F2F EKE model’.
This approach enabled to provide quantitative estimates of expected levels of ABMs (i.e. in terms of behaviour or health measures) in situations with different levels of restriction (e.g. low and high space allowances). The general concept underlying the model was the use of normal behaviour/health state under unrestricted conditions/natural exposure as a reference for a ‘natural’ welfare level (including, for instance, the normal occurrence of health problems). This was assumed to avoid setting arbitrary levels of outcome measures as ‘acceptable’.
The text below is part of the publication from EFSA (2022) and here reproduced to provide background information to the reader:
‘The idea of the assessment model is the interpolation of the ABM between a highly exposed population of animals and a non‐exposed population. For the definition of the highly exposed population, extreme exposures are considered (e.g. current minimum allowed space per animal in the EU). For the non‐exposed population farming practices are considered, where the conditions are virtually without exposure, e.g. outdoor farming on wide pasture with virtual no restriction of the space for the animal. If possible, the variation of the ABM within the non‐exposed population is estimated. This variation between animals may be used to interpret the strength of the exposure effect on the average animal.
The underlying assumptions of the model are:
The ABM considered is a valid and sensitive indicator of the welfare of the animals related to the exposure variable.
Since there is no gold standard for animal welfare, it is assumed that the expression of the ABM (i.e. the extent to which a certain behaviour is shown or the occurrence of a certain health disorder) under unexposed conditions (e.g. unlimited space, full cow–calf contact) reflects the natural situation an animal population may experience, which is considered the optimum in terms of animal welfare. The ABM observed under these conditions could be seen as not influenced by exposure to the hazard and work as a control measurement to describe the influence of the exposure. The level of welfare as assessed through this ABM can thus be quantified in relation to optimal welfare, for different degrees of the exposure variable (e.g. ‘what proportion of play behaviour is shown by a calf at different space allowances below unrestricted space?’). Therefore, it is assumed that quantitative recommendations on the exposure variables, as required by the mandates, can be drawn by associating different levels of ABMs expression to different levels of exposure variables that are assessed.
Within a simple interpolation framework, the model involves the estimation of four parameters via EKE to describe the relationship between the exposure variable (hazard) and the ABM considered:
The median ABM in a population of animals subjected to optimal conditions, namely a population not exposed to the hazard (e.g. with no space restriction = situation of reference);
The variation of the ABM in the population of animals not exposed to the hazard (e.g. with no restriction of space);
The greatest degree of exposure to the hazard resulting in no change in the median value of the ABM compared with the value observed in the unexposed population of animals; and
The median value of the ABM in a population of animals under a high exposure to the hazard (e.g. with substantial restriction of space). In order to construct the relationship between exposure and ABM, a regression model can be envisioned according to the complexity. In case of a qualitative assessment the exposure can be described categorical (e.g. different exposure scenarios as crated/non‐crated), and the ABM can be estimated on an ordinal scale (e.g. by scoring). A linear relationship and an ordinal relationship require two questions (ABMs of high and low exposure and eventually ‘in‐between’‐exposure), while categorical relationship requires one question per category.’
The risk assessment model is graphically represented in Figure B.1. The model interpolates the ABM between low and high values of the exposure variable (hazard) by a linear trend (red line). The ‘Range of exposure allowing ABM expression’ (blue range) represents the ABM expression with no significant effect to the average animal and is defined by the hazards (exposure values), which results in ABM values comparable with the variation (80% confidence interval) within a non‐exposed population (green distribution)’. For the estimation of parameters, ‘average’ environmental conditions were considered (e.g. no extreme heat or cold circumstances).
Figure B.1.
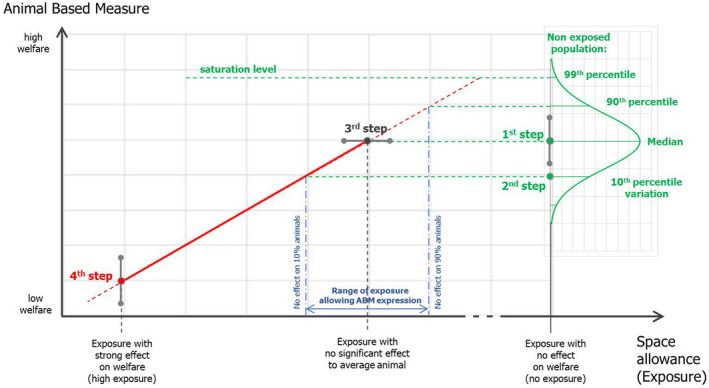
Graphical representation of the risk assessment model used in F2F welfare mandates (F2F EKE model) to express the relationship between exposure and ABMs (EFSA, 2022a). This is an illustration of one case where a linear relationship was assumed
Appendix C – Results of ranking of relevance of welfare consequences in each housing system
Figures C.1, C.2, C.3, C.4, C.5, C.6, C.7, C.8, C.9, C.10, C.11–C.1, C.2, C.3, C.4, C.5, C.6, C.7, C.8, C.9, C.10, C.11 show the ranking of welfare consequences per system. The x axis shows the abbreviated name of the welfare consequence for abbreviations, and the y axis the relevance ranking. Lower values indicate higher relevance of the welfare consequence in that system. Welfare consequences of high, medium, low and no relevance are shown in red, yellow, green and grey, as shown in the top bar of each figure. The final classification was reached via consensus based on a group discussion of the initial, individual ranking by the WG experts. If a welfare consequence was re‐classified during the group discussion, a different colour was assigned (Table C.1).
Figure C.2.
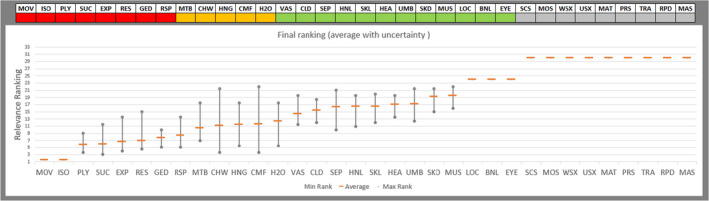
Classification of relevance of welfare consequences (high, medium, low, no relevance) in calves kept in individual pens in veal farms
Figure C.3.
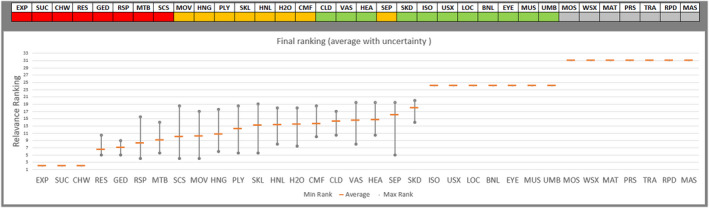
Classification of welfare consequences (high, medium, low, no relevance) in veal calves kept in group housing in small groups
Figure C.4.
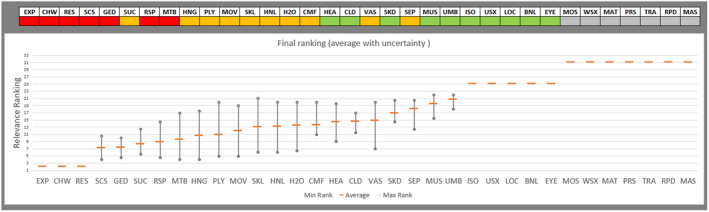
Classification of welfare consequences (high, medium, low, no relevance) in veal calves kept in group housing in large groups
Figure C.5.
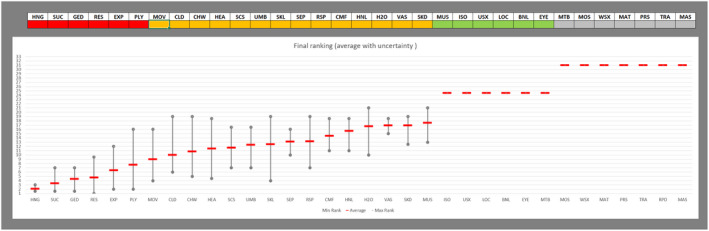
Classification of welfare consequences (high, medium, low, no relevance) in calves kept from birth to weaning with milk feeding via buckets troughs
Figure C.6.
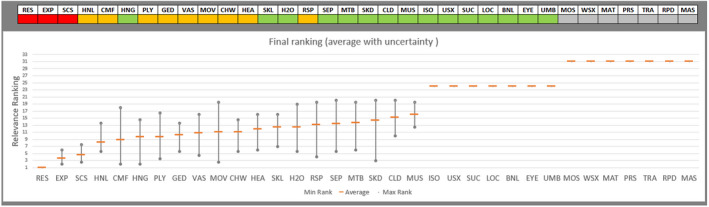
Classification of welfare consequences (high, medium, low, no relevance) in calves kept from weaning to 6 months in fully or partly littered group pens
Figure C.7.
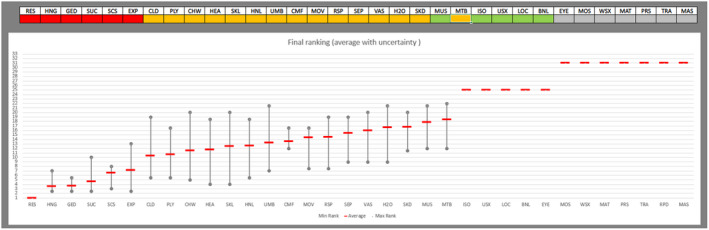
Classification of welfare consequences (high, medium, low, no relevance) of housing calves in large groups and with automatic milk feeding
Figure C.8.
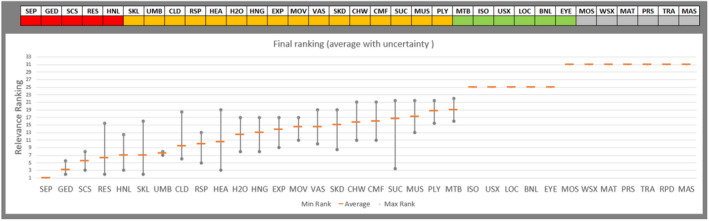
Classification of welfare consequences (high, medium, low, no relevance) in calves kept in systems with cow–calf contact
Figure C.9.
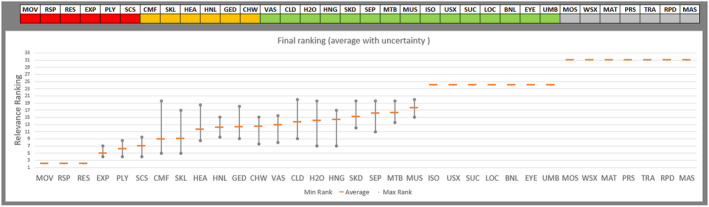
Classification of welfare consequences (high, medium, low, no relevance) in calves kept from weaning to 6 months in fully or partially slatted floor group pens without bedding
Figure C.10.
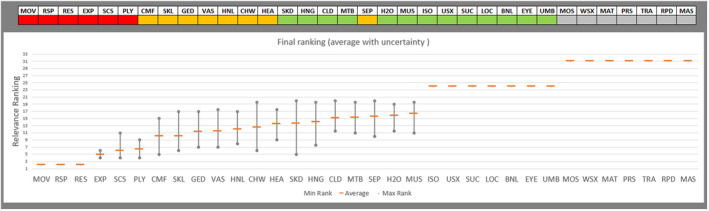
Classification of welfare consequences (high, medium, low, no relevance) in calves kept from weaning to 6 months in group pens with cubicles
Figure C.11.
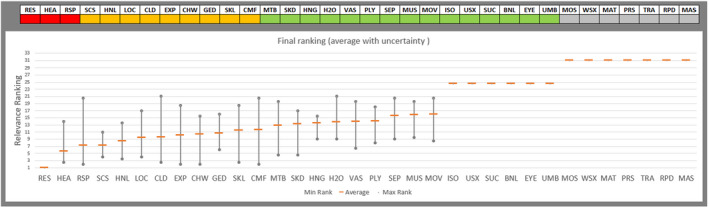
Classification of welfare consequences (high, medium, low, no relevance) in calves kept from weaning to 6 months in outdoor feedlots
Table C.1.
Welfare consequences abbreviations used in Figures C.1, C.2, C.3, C.4, C.5, C.6, C.7, C.8, C.9, C.10, C.11–C.1, C.2, C.3, C.4, C.5, C.6, C.7, C.8, C.9, C.10, C.11
| Abbreviation | Welfare consequence |
|---|---|
| MOV | Restriction of movement |
| RSP | Resting problems |
| SCS | Group stress |
| VAS | Stress from visual and/or auditory stimuli |
| HNL | Handling stress |
| MOS | Motion stress |
| ISO | Isolation stress |
| SEP | Separation stress |
| CMF | Inability to perform comfort behaviour |
| WSX | Inability to perform sexual behaviour |
| USX | Inability to avoid unwanted sexual behaviour |
| EXP | Inability to perform exploratory or foraging behaviour |
| MAT | Inability to express maternal behaviour |
| SUC | Inability to perform sucking behaviour |
| CHW | Inability to chew and ruminate |
| PLY | Inability to perform play behaviour |
| PRS | Predation stress |
| HNG | Prolonged hunger |
| H2O | Prolonged thirst |
| HEA | Heat stress |
| CLD | Cold stress |
| LOC | Locomotion disorders (including lameness) |
| SKL | Skin lesions and wounds |
| TRA | Unfit for Transport |
| BNL | Bone lesions (incl. fractures and dislocations) |
| SKD | Skin disorders (other than pododermatitis or skin lesions) |
| RES | Respiratory disorders |
| EYE | Eye disorders |
| GED | Gastroenteric disorders |
| RPD | Reproductive disorders |
| MAS | Mastitis |
| MTB | Metabolic disorders |
| MUS | Muscle disorders |
| UMB | Umbilical disorders |
Ranking of welfare consequences of keeping calves in individual housing at dairy farms
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance were separation stress, skin lesions, umbilical disorders, cold stress, inability to chew and ruminate, heat stress, prolonged thirst, inability to perform comfort behaviour, resting problems, handling stress, metabolic disorders, stress from visual and/or auditory stimuli, skin disorders (other than skin lesions or pododermatitis) and muscle disorders. Locomotion disorders, bone lesions and eye disorders were considered of low relevance (Figure C.1).
Ranking of welfare consequences of keeping veal calves in individual housing
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are metabolic disorders, inability to chew and ruminate, prolonged hunger, and prolonged thirst. Stress from visual and/or auditory stimuli, cold stress, separation stress, prolonged hunger, umbilical disorders, skin disorders (other than skin lesions or pododermatitis), muscle disorders, locomotion disorders, bone lesions and eye disorders were considered of low relevance (Figure C.2).
Ranking of welfare consequences of keeping veal calves in group housing in small groups
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are restriction of movement, prolonged hunger, inability to perform play behaviour, skin lesions, handling stress, prolonged thirst, and inability to perform comfort behaviour. Cold stress, stress from visual and/or auditory stimuli, heat stress, separation stress, skin disorders, isolation stress, inability to avoid unwanted sexual behaviour, locomotion disorders, bone lesions, eye disorders and muscle disorders were considered of low relevance (Figure C.3).
Ranking of welfare consequences of keeping veal calves in large groups
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are inability to perform sucking behaviour, prolonged hunger, inability to perform play behaviour, restriction of movement, skin lesions, handling stress, prolonged thirst, inability to perform comfort behaviour, stress from visual and/or auditory stimuli and separation stress. Heat stress, cold stress, skin disorders, muscle disorders, umbilical disorders, isolation stress, inability to avoid unwanted sexual behaviour, bone lesions and eye disorders were considered of low relevance (Figure C.4).
Ranking of welfare consequences of keeping calves from birth to weaning in small groups with milk feeding via buckets or troughs
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are cold stress, inability to chew and ruminate, heat stress, skin lesions, handling stress, umbilical disorders, inability to perform comfort behaviour, restriction of movement, resting problems, separation stress, stress from visual and/or auditory stimuli, prolonged thirst, skin disorders and metabolic disorders. Muscle disorders, isolation stress, inability to avoid unwanted sexual behaviour, locomotion disorders and bone lesions were considered of low relevance (Figure C.5).
Ranking of welfare consequences of keeping calves from weaning to six months in fully or partly littered group pens
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are handling stress, inability to perform comfort behaviour, inability to perform play behaviour, gastroenteric disorders, stress from visual and/or auditory stimuli, restriction of movement, inability to chew or ruminate, heat stress or resting problems. Prolonged hunger, skin lesions, prolonged thirst, separation stress, metabolic disorders, skin disorders, cold stress, muscle disorders, isolation stress, inability to avoid unwanted sexual behaviour, inability to perform sucking behaviour, locomotion disorders, bone lesions, eye disorders and umbilical disorders were considered of low relevance (Figure C.6).
Ranking of welfare consequences of keeping calves in group housing in large groups and automatic milk feeding
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are cold stress, inability to perform play behaviour, inability to chew and ruminate, heat stress, skin lesions, handling stress, umbilical disorders, inability to perform comfort behaviour, restriction of movement, resting problems, separation stress, stress from visual and/or auditory stimuli, prolonged thirst, skin disorders and metabolic disorders. Muscle disorders, isolation stress, inability to avoid unwanted sexual behaviour, locomotion disorders and bone lesions were considered of low relevance (Figure C.7).
Ranking of welfare consequences of keeping calves in systems with cow–calf contact
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are skin disorders, umbilical disorders, cold stress, resting problems, heat stress, prolonged thirst, prolonged hunger, inability to perform exploratory or foraging behaviour, restriction of movement, stress from visual and/or auditory stimuli, inability to chew and ruminate, inability to perform comfort behaviour, inability to perform sucking behaviour, muscle disorders and inability to perform play behaviour. Metabolic disorders, isolation stress, inability to avoid unwanted sexual behaviour, locomotion disorders, bone lesions and eye disorders were considered of low relevance (Figure C.8).
Ranking of welfare consequences of keeping calves from weaning to 6 months in fully or partially slatted floor group pens without bedding
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are inability to perform comfort behaviour, skin lesions, heat stress, prolonged hunger, inability to chew or ruminate, stress from visual and/or auditory stimuli, cold stress, prolonged thirst, prolonged hunger, skin disorders. Metabolic disorders, isolation stress, inability to avoid unwanted sexual behaviour, inability to perform sucking behaviour, locomotion disorders, bone lesions, eye disorders and umbilical disorders were considered of low relevance (Figure C.9).
Ranking of welfare consequences of keeping calves from weaning to six months in group pens with cubicles
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are inability to perform comfort behaviour, skin lesions, gastroenteric disorders, stress from visual and/or auditory stimuli, handling stress, inability to chew or ruminate, heat stress and separation stress. Skin disorders, prolonged hunger, cold stress, metabolic disorders, prolonged thirst, muscle disorders, isolation stress, inability to avoid unwanted sexual behaviour, inability to perform sucking behaviour, locomotion disorders, bone lesions, eye disorders and umbilical disorders were considered of low relevance (Figure C.10).
Ranking of welfare consequences of keeping calves from weaning to 6 months in outdoor feedlots
The highly relevant welfare consequences of this system are discussed in the main body of the document. Welfare consequences of medium relevance in this system are group stress, handling stress, locomotion disorders, cold stress, inability to perform exploratory behaviour, gastroenteric disorders, skin lesions, and inability to perform comfort behaviour. Metabolic disorders, skin disorders, locomotion disorders, prolonged hunger, prolonged thirst, separation stress, muscle disorders, restriction of movement, inability to avoid unwanted sexual behaviour, inability to perform sucking behaviour, bone lesions, eye disorders and umbilical disorders were considered of low relevance (Figure C.11).
Appendix D – Summary tables of welfare consequences, ABMs, hazards and preventive measures in each husbandry system
Table D.1: Summary of welfare consequences, ABMs, hazards and preventive measures in individual housing in dairy farms
| Welfare consequence | ABM | Hazard | Preventive or corrective measures of the hazard |
|---|---|---|---|
| Restriction of movement |
Slipping Falling Galloping in unrestricted conditions |
Low space allowance Slatted or slippery floors |
Rubber flooring or provision of bedding on a solid/drained floor Increase space allowance |
| Isolation stress |
Response to standard social approach test Fear response |
Individual housing | Group housing with other calves, and/or keeping contact with the dam |
| Inability to perform sucking behaviour |
Sucking of pen fixtures Cross‐sucking Loss of hair and inflammation of skin in the muzzle/ears area |
Offering milk in open buckets Offering low milk allowances Low dry matter intake and negative energy balance during weaning Removing teat buckets too soon after the milk ration is ingested Breed Separation from dam |
Offering the milk via a teat Increase amount of milk Increase milk feeding frequency Stepwise weaning based on solid feed intake Dam or foster cow rearing |
| Inability to perform play behaviour | No suitable ABMs of individual pens (see text) |
Low space allowances and lack of partner(s) to perform social play Disease, injury, malnutrition Slippery surfaces and dark environments Cold weather Frightening stimuli |
Increase space allowance Environmental changes such as the provision of straw, or other environmental stimuli may stimulate play Provide solid, non‐slip surface |
| Prolonged hunger |
Body condition score Number of vocalisations Restlessness, i.e. increased activity and decreased lying |
Low amount of milk especially before 4 weeks of age Low amount or quality of solid feed (depending on age; at weaning) Low frequency of milk feeding Weaning strategy and age Insufficient amount and quality of the solid feed |
Provide more frequent opportunities to feed, ideally closer to natural feeding pattern Milk feeding corresponding to 20% of body weight until 4 weeks of age Provide feed in amounts and in types that meet not only nutrient requirements but also feelings of satiety |
| Gastroenteric disorders |
Presence of diarrhoea Hair loss in the perineum and hind legs Bloat |
Poor colostrum management Poor hygiene including bedding, teats, buckets; poor biosecurity Proximity to older animals Inadequate positioned or conceived bucket or teat Poor quality milk replacers High stocking rates |
Vaccination of pregnant cows Ensure sufficient (10–12% body weight) and timely (up to 6 h post partum) colostrum intake of high quality Strict hygiene measures Routine (twice daily) monitoring of calves to detect early cases. |
| Inability to perform exploratory or foraging behaviour |
Non‐nutritive oral manipulation Tongue flicks Tongue rolling |
Barren environments Concentrated diets Low feeding frequency/duration |
Provide relevant enrichment, e.g. rubbing fixtures (brushes), enrichment objects, bedding Increase fibre content of diet to increase foraging Make animals work for their feed, e.g. straw rack, and increase feeding frequency Provide access to an outdoor area and pasture |
| Respiratory disorders |
Coughing Respiratory sounds at lung auscultation Rectal temperature Nasal discharge Ocular discharge |
Poor colostrum management Poor ventilation Lack of bedding, especially in cold environments Stressful events Proximity to older animals |
Reduce stress factors (i.e. transport, mutilations, changes in group composition) Ensure good colostrum management and feeding of calves and establish integrated vaccination programs Appropriate ventilation to avoid high ammonia or dust concentrations and adequate temperature‐humidity index Keep calves in small and stable groups |
Table D.2: Summary of welfare consequences, ABMs, hazards and preventive measures in individual pens in veal farms
| Welfare consequence | ABM | Hazard | Preventive measure of the hazard |
|---|---|---|---|
| Restriction of movement |
Slipping Falling |
Low space allowance Slatted or slippery floors |
Increased space allowance Rubber flooring or provision of bedding on a solid/drained floor |
| Isolation stress |
Response to standard social approach test Fear response |
Individual housing Narrow size and position of openings between individual pen preventing contact between calves |
Group housing with other calves |
| Inability to perform play behaviour | No suitable ABMs of individual pens (see text) |
Low space allowances and lack of partner(s) to perform social play Disease, injury, malnutrition Slippery surfaces and dark environments Cold weather Frightening stimuli |
Increase space allowance Environmental changes such as the provision of straw, or other environmental stimuli may stimulate play Provide solid, non‐slip surface |
| Inability to perform sucking behaviour |
Sucking of pen fixtures Loss of hair and inflammation of skin in the navel area Cross‐sucking |
Offering milk in open buckets or a trough Absence of dry teats (rubber teats) to direct sucking behaviour towards |
Offering the milk via a teat, for instance in a teat bucket Increase amount of milk Gradual weaning based on solid feed intake Dam or foster cow rearing |
| Inability to perform exploratory or foraging behaviour |
Non‐nutritive oral manipulation Tongue flicks Tongue rolling |
Barren environment Concentrated diets Low frequency and duration of feeding |
Provide relevant enrichment, e.g. brushes and enrichment objects Provide roughage to increase foraging Increase fibre content of diet to increase foraging Make animals ‘work’ for their feed, e.g. pulling roughage out of rack, and increase feeding frequency Provide access to an outdoor area and pasture |
| Respiratory disorders |
Coughing Nasal and ocular discharge Rectal temperature above 39.7°C Respiratory sounds at lung auscultation |
Large groups Close proximity in the same room of calves originating from different farms and sharing the same air space Long distance transport Poor ventilation and air quality in closed barns High concentration of noxious gases |
Avoid stress‐inducing events, such as long and repeated transport Avoid contact between calves from multiple farm origins Ensure appropriate ventilation to avoid high ammonia or dust concentrations |
| Gastroenteric disorders |
Diarrhoea Hair loss in the perineum and hind legs Bloat |
Low frequency of large milk meals combined with little structure in the solid feed. Stressful events, such as transport to the veal farm Changes in the diet Concentrated diets with small particle size and low abrasive value High concentrate/fibre ratio |
Feed milk in multiple (> 3) smaller meals with a teat allowing for normal extension of the neck. Diet with a high concentrate/fibre ratio Minimise commingling. Vaccination of the dams |
| Resting problems |
Number of lying bouts Time spent in lateral recumbency |
Slatted floor Wet floor Low space allowance per animal Low or high temperature |
Provide bedding or, if not possible, slats with a rubber cover Provide large space allowances Group housing during winter can reduce cold stress and promote adoption of relaxed lying postures for resting Appropriate temperature and humidity to provide suitable thermal comfort |
Table D.3: Summary of welfare consequences, ABMs, hazards and preventive measures in systems keeping veal calves in small groups with milk feeding by bucket/trough
| Welfare consequence | ABM | Hazard | Preventive or corrective measures of the hazard |
|---|---|---|---|
| Inability to perform exploratory or foraging behaviour |
Tongue rolling Non‐nutritive oral manipulation Tongue flicks |
Barren environment Concentrated diets Low frequency of feeding |
Provide relevant enrichment, e.g. rubbing fixtures (brushes), enrichment objects, bedding Increase fibre content of diet to increase foraging Make animals work for their feed, e.g. straw rack, and increase feeding frequency Provide access to an outdoor area and pasture |
| Inability to perform sucking behaviour |
Sucking of pen fixtures Cross‐sucking Loss of hair and inflammation of skin in the muzzle/ears area |
Offering milk in open buckets or a trough Removing teat buckets too soon after the milk ration is ingested Breed Weaning strategy e.g. too early weaning, too low intake of solid feed |
Rear calves with their dam or a foster cow, i.e. allowing the calves to suck milk from an udder Offer the milk via a teat, for instance in a teat bucket rather than via a bucket. Leave the teats with the calves for approx. 20–30 min after the milk is drunk to reduce cross‐sucking Gradual weaning off milk |
| Inability to chew and ruminate | Tongue rolling |
Limited solid feed structure (e.g. concentrates) Restricted solid feed amount Low frequency of feeding |
Provision of roughage for ad lib intake ideally in a long format |
| Respiratory disorders |
Coughing Nasal and ocular discharge High rectal temperature Respiratory sounds at lung auscultation |
Overstocking and large groups sizes Close proximity in the same room of calves originating from different farms and sharing the same air space |
Avoid stress‐inducing events, such as long and repeated transport Avoid contact between calves from multiple farm origins Ensure appropriate ventilation to avoid high ammonia or dust concentrations Limit group size and overstocking Maintain stable groups of similar age and size |
| Gastroenteric disorders |
Abomasal lesions Ruminal plaques Rumen underdevelopment |
Milk replacer‐only diets Abomasal overloading Coarse roughage Little water provision Concentrate diets with small particle size and low abrasive value High concentrate/fibre ratio Commingling of many animals from different origins (for diarrhoea linked to infection) |
Feed milk in multiple (> 3) smaller meals with a teat allowing for normal extension of the neck. Diet with a high concentrate/fibre ratio Provide ad libitum access to solid feed structure. Minimise the mixing of animals from different farms. |
| Resting problems |
Number of lying bouts Time spent in lateral recumbency (H, L) |
Slatted floor of wood or concrete Low space allowance Low or high temperature |
Provide bedding or, if not possible, slats with a rubber cover Provide large space allowances Group housing during winter can reduce cold stress and promote adoption of relaxed lying postures for resting Appropriate temperature and humidity to provide suitable thermal comfort |
| Metabolic disorders | Haemoglobin concentration (H, H) | Low iron content in the diet |
Provision of diet with a high iron content Provision of ad libitum hay |
| Group stress | Aggressive interaction with physical contact |
Low space allowance in general and especially at trough Open trough and no individual feeding place during milk feeding (no fixation) Repeated regrouping |
Individual feeding places with a possibility to fixate calves during milk feeding avoid competition for milk This also makes regrouping due to different speed of drinking milk unnecessary and stability of groups is eased. Regrouping should be avoided as far as possible. Sufficient space for lying enables synchronous resting of calves (Færevik et al., 2008) |
Table D.4: Summary of welfare consequences, ABMs, hazards and preventive measures in systems keeping veal calves in large groups and automatic milk feeding
| Welfare consequence | ABM | Hazard | Preventive measure of the hazard |
|---|---|---|---|
| Inability to perform exploratory or foraging behaviour |
Tongue rolling Non‐nutritive oral manipulation |
Barren environment Concentrated diets Low frequency of feeding/duration |
Provide relevant enrichment, e.g. rubbing fixtures (brushes), enrichment objects, bedding Increase fibre content of diet to increase foraging Make animals work for their feed, e.g. straw rack, and increase feeding frequency Provide access to an outdoor area and pasture |
| Inability to chew and ruminate |
Tongue rolling |
Limited solid feed structure (e.g. concentrates) Restricted solid feed amount Low frequency of feeding |
Ad libitum provision of roughage, ideally in a long format |
| Respiratory disorders |
Coughing Nasal and ocular discharge Rectal temperature above 39.7°C Respiratory sounds at auscultation |
Large groups Close proximity in the same room of calves originating from different farms and sharing the same air space |
Avoid stress‐inducing events, such as long and repeated transport Avoid contact between calves from multiple farm origins Ensure appropriate ventilation to avoid high ammonia or dust concentrations |
| Group stress | Number of aggressive interactions |
High number of animals per automatic milk feeder Open stalls at the automatic milk feeder Low space allowance Regrouping |
Decrease stocking rates Higher space allowance reduces disturbance when resting Low number of animals per automatic milk feeder Avoid regrouping Incorporation of a door that closes the stall when the calf enters the feeding area Structuring the pen into a designated lying area, eventually with further structuring could reduce disturbance |
| Gastroenteric disorders |
Diarrhoea Hair loss in the perineum and hind legs Bloat Abomasal lesions Ruminal plaques Ruminal underdevelopment |
Poor hygiene including bedding, teats, buckets; poor biosecurity Poor colostrum and poor colostrum management Calf stocking density Heterogeneous (size and age) groups Poor quality milk replacers |
Vaccination of pregnant cows Strict hygiene measures Routine (twice daily) monitor of calves to detect early cases Feed milk in multiple (> 3) smaller meals with a teat allowing for normal extension of the neck. Diet with a high concentrate/fibre ratio Provide ad libitum access to solid feed structure |
| Resting problems |
Number of lying bouts Time spent in lateral |
Low space allowance per animal |
Higher space allowance Increase lying area Keep the group stable |
| Metabolic disorders |
Haemoglobin concentration Haemoglobin concentration |
Low iron content in the diet |
Provision of diet with a high iron content Provision of ad libitum hay |
Table D.5: Summary of welfare consequences, ABMs, hazards and preventive measures in systems keeping dairy calves from birth till weaning in in small groups with milk feeding by bucket/trough
| Welfare consequence | ABM | Hazard | Preventive measure of the hazard |
|---|---|---|---|
| Prolonged hunger |
Body condition score Vocalisations Restlessness, i.e. increased activity and decreased lying Attempt to access inaccessible feed |
Low amount of milk, especially before 4 weeks of age Low amount or quality of solid feed (depending on age; at weaning) Low frequency of milk feeding Weaning strategy e.g. too early weaning, too low intake of solid feed |
Provide feed in amounts and in types that meet not only nutrient requirements but also feelings of satiety Milk feeding corresponding to 20% of body weight until 4 weeks of age Feeding milk at least twice a day until at least 4 weeks of age/until gradual weaning is initiated |
| Inability to perform sucking behaviour |
Sucking of pen fixtures Cross‐sucking Loss of hair and inflammation of skin in the navel area |
Offering milk in open buckets Removing teat buckets too soon after the milk ration is ingested Offering low milk allowances Weaning strategy e.g. too early weaning, too low intake of solid feed Breed Separation from dam |
Offering milk via a teat bucket Increase amount of milk Increase milk feeding frequency Stepwise weaning based on solid feed intake Rearing with dam or foster cow Breed selection to avoid the genetic predisposition for the development of cross‐sucking |
| Gastroenteric disorders |
Diarrhoea Hair loss in the perineum and hind legs Bloat |
Poor colostrum management Poor hygiene including bedding, teats, buckets; poor biosecurity Proximity to older animals Inadequately positioned or conceived bucket or teat Poor quality milk replacers High stocking rates |
Vaccination of pregnant cows Ensure sufficient (10–12% body weight) and timely (up to 6 h p.p.) intake of high‐quality colostrum Strict hygiene measures Routine (twice daily) monitoring of calves to detect cases early |
| Respiratory disorders |
Coughing Respiratory sounds at lung auscultation Rectal temperature Nasal discharge Ocular discharge |
Poor colostrum management Poor ventilation Lack of bedding, especially in cold environments Stressful events Proximity to older cattle |
Reduce stress factors (i.e. transport, mutilations, changes in group composition) Ensure good colostrum management and feeding of calves and establish integrated vaccination programs Appropriate ventilation to avoid high ammonia or dust concentrations and adequate temperature‐humidity index Keep calves in small and stable groups |
| Inability to perform exploratory or foraging behaviour |
Non‐nutritive oral manipulation Tongue flicks Tongue rolling |
Barren environment Concentrated diets Low frequency of feeding/duration |
Provide relevant enrichment, e.g. rubbing fixtures (brushes), enrichment objects, bedding Increase fibre content of diet to increase foraging Make animals work for their feed, e.g. straw rack, and increase feeding frequency Provide access to an outdoor area and pasture |
| Inability to perform play behaviour | Time spent in locomotor play | Low space allowance |
Increase space allowance Environmental changes such as the provision of straw, or other environmental stimuli may stimulate play behaviour Provide solid, non‐slip surface |
Table D.6: Summary of welfare consequences, ABMs, hazards and preventive measures in systems keeping calves kept from birth till weaning in large groups and automatic milk feeding
| Welfare consequence | ABM | Hazard | Preventive measure of the hazard |
|---|---|---|---|
| Prolonged hunger |
Body condition score (BCS) Number of vocalisations Restlessness, i.e. increased activity and decreased lying |
Low milk allowance High calf:drinking station ratio Heterogeneous group composition in terms of age |
Provide more frequent opportunities to feed, ideally closer to natural feeding pattern Milk feeding corresponding to 20% of body weight until 4 weeks of age Provide feed in amounts and in types that meet not only nutrient requirements but also feelings of satiety |
| Gastroenteric disorders |
Diarrhoea Hair loss in the perineum and hind legs Bloat Abomasal lesions Ruminal plaques Ruminal underdevelopment |
Poor hygiene including bedding, teats, buckets; poor biosecurity Inadequate positioned or conceived bucket or teat Poor quality milk replacers High stocking rates Poor colostrum and poor colostrum management |
Vaccination of pregnant cows Strict hygiene measures Routine (twice daily) monitor of calves to detect early cases Ensure sufficient (10–12% of body weight) and timely (up to 6 h p.p.) colostrum intake of high quality |
| Inability to perform sucking behaviour |
Sucking of pen fixtures Cross‐sucking (Roth et al., 2009a,b) Loss of hair and inflammation of skin in the navel area |
Offering milk in open buckets (Jensen and Budde, 2006) Offering low milk allowances (Roth et al., 2009a,b) Weaning strategy e.g. too early weaning, too low intake of solid feed Breed Separation from dam. |
Offering milk via a teat Increase amount of milk Increase milk feeding frequency Stepwise weaning based on solid feed intake Rearing with dam or foster |
| Group stress |
Aggressive interactions with physical contact Count displacements from the automatic milk feeder |
High number of calves per automated milk feeder Feeder which cannot be closed or with no lateral barriers Low space allowance per animal Heterogeneous group composition in terms of age |
Low group sizes Low number of animals per feeder Closable feeder Access to teat after milk intake Individual feeding places with a possibility to fixate calves during milk Avoiding regrouping Sufficient space for synchronous lying |
| Inability to perform exploratory or foraging behaviour |
Non‐nutritive oral manipulation Tongue flicks Tongue rolling |
Barren environments Concentrated diets Low feeding frequency/duration |
Provide relevant enrichment, e.g. rubbing fixtures (brushes), enrichment objects, bedding Increase fibre content of diet to increase foraging Make animals work for their feed, e.g. straw rack, and increase feeding frequency Provide access to an outdoor area and pasture |
Table D.7: Summary of welfare consequences, ABMs, hazards and preventive measures in systems with cow–calf contact
| Welfare consequence | ABM | Hazard | Preventive measure of the hazard |
|---|---|---|---|
| Separation stress |
Vocalisations Heart rate after separation Restlessness behaviour |
Simultaneous separation from dam and weaning off milk Young age at weaning Simultaneous separation from the dam and additional change in the social and/or physical environment (separation from other peers and change of housing) |
Separation at an older age (3–4 months) combined with gradual and progressive habituation towards separation. Never remove the milk and the dam at the same time Maintain the social group of calves intact after the separation from the cow(s) |
| Group stress |
Aggression from cow Injurious events Displacements from other calves |
High stocking density, restricted space and lack of a creep area Too many calves per (foster) cow Poor bond between calf and dam/foster cow |
Higher space allowance, avoiding dead ends, bottlenecks and small alleys The number of calves per foster cow need to be adapted to the milk yield of cows and length of suckling period (weaning age) Behaviour and weight gain of calves needs to be monitored regularly by the farmer Freely accessible calf creep area |
| Gastroenteric disorders |
Diarrhoea Hair loss in the perineum and hind legs Bloat |
Failure of adequate colostrum intake Low hygienic levels on farm |
Maternity pens with high hygiene levels Vaccination of pregnant cows Confirmation of adequate sucking to ensure colostrum intake Ensure sufficient (10–12% of body weight) and timely (up to 6 h p.p.) colostrum intake of high quality Routine (twice daily) monitor of calves to detect early cases. |
| Respiratory disorders |
Rectal temperature Respiratory sounds at lung auscultation Coughing Nasal discharge Ocular discharge |
Failure of adequate colostrum intake Poor ventilation Lack of bedding, especially in cold environments Stressful events Proximity to older animals |
Confirmation of adequate sucking to ensure colostrum intake Ensure sufficient (10–12% of body weight) and timely (up to 6 h p.p.) colostrum intake of high quality Vaccinate against respiratory virus and bacteria Prevent commingling of many calves from different origins Ensure appropriate ventilation to avoid high ammonia or dust concentrations Avoid stress‐inducing events, such as long and repeated transport |
| Handling stress |
Injurious and negative events during handling (falling, bumping into something, being hit) Avoidance distance |
Lack of knowledge and training of farmers and stockpeople with respect to appropriate handling Insufficient human‐animal contact during first week of life. Large number of animals per stockperson and thus fewer time per animal High workload |
Appropriate behaviour of humans during handling Gentle human contact during feeding in the first 5 day of life (i.e. in CCC systems bottle feeding of colostrum and assisting calves in suckling their dam) Adapted environmental conditions Training of stockpersons regarding human‐animal relationship |
Table D.8: Summary of welfare consequences, ABMs, hazards and preventive measures in fully slatted floor group pens
| Welfare consequence | ABM | Hazard | Preventive measure of the hazard |
|---|---|---|---|
| Restriction of movement |
Slipping Falling Galloping in unrestricted conditions |
Slippery nature of slats/concrete Poor integrity (state of maintenance) of slats Too wide, uneven or missing slats Low space allowance |
Increase space allowance Provide rubber flooring Provide bedded lying area |
| Resting problems |
Number of lying bouts Time spent in lateral recumbency |
Concrete or slatted floor Low space allowance Commingling and regrouping |
Partial rubberisation or rubber mats on concrete floors Access to a bedded area |
| Respiratory disorders |
Rectal temperature Respiratory sounds at lung auscultation Coughing Nasal discharge Ocular discharge |
Poor colostrum management Poor ventilation Lack of bedding, especially in cold environments Stressful events Proximity to older animals |
Reduce stress factors (i.e. transport, mutilations, changes in group composition) Ensure good colostrum management and feeding of calves and establish integrated vaccination programs Appropriate ventilation to avoid high ammonia or dust concentrations and adequate temperature‐humidity index Keep calves in small and stable groups |
| Inability to perform exploratory or foraging behaviour |
Non‐nutritive oral manipulation Tongue flicks Tongue rolling |
Barren environments Concentrated diets Low feeding frequency/duration |
Provide relevant enrichment, e.g. rubbing fixtures (brushes), enrichment objects, bedding Increase fibre content of diet to increase foraging Make animals work for their feed, e.g. straw rack, and increase feeding frequency Provide access to an outdoor area and pasture |
| Inability to perform play behaviour | No suitable ABMs of individual pens |
Slatted floors Disease, injury, mal‐nutrition, cold weather and frightening stimuli Low space allowances, slippery surfaces and dark environments |
Increased space allowance Non‐slippery rubber coated or straw bedded surfaces Good health and nutrition, as well as thermal comfort |
| Group stress | Aggressive interactions with physical contact |
Low space allowance Regrouping Reduced feed manger area |
Decreasing the total group size and/or increasing the number per pen Higher space allowance Avoid regrouping |
Table D.9: Summary of welfare consequences, ABMs, hazards and preventive measures in in systems keeping calves from weaning to 6 months in fully or partly littered group pens
| Welfare consequence | ABM | Hazard | Preventive measure of the hazard |
|---|---|---|---|
| Respiratory disorders |
Rectal Temperature Respiratory sounds at lung auscultation Coughing Nasal discharge Ocular discharge |
Stress caused by weaning, long distant transport or other stressful events Separation from dam or herd Commingling of unfamiliar animals as well as regrouping familiar animals after a period of separation Lack of ventilation, high temperature‐humidity index and overstocking Poor quality of bedding |
Reduce stress factors (i.e. transport, mutilations, changes in group composition) Keep calves in small and stable groups Ensure good colostrum management and feeding of calves and establish integrated vaccination programs Appropriate ventilation to avoid high ammonia or dust concentrations. Adequate temperature‐humidity index |
| Inability to perform exploratory or foraging behaviour |
Non‐nutritive oral manipulation Tongue flicks Tongue rolling |
Barren environments Concentrated diets Low feeding frequency/duration |
Provide relevant enrichment, e.g. rubbing fixtures (brushes), enrichment objects, bedding Increase fibre content of diet to increase foraging Make animals work for their feed, e.g. straw rack, and increase feeding frequency Provide access to an outdoor area and pasture |
| Group stress | Aggressive interactions with physical contact |
Low space allowance Regrouping Low feed place:animal ratio |
Individual feeding places Avoid competition for milk Avoid regrouping Sufficient space for lying |
Table D.10: Summary of welfare consequences, ABMs, hazards and preventive measures in systems keeping calves from weaning to 6 months in group pens with cubicles
| Welfare consequence | ABM | Hazard | Preventive measure of the hazard |
|---|---|---|---|
| Restriction of movement |
Slipping Falling |
Slippery floors due to slats/concrete in the alleys Cubicle size and design not adapted to calf size |
Cubicle design that allows adoption of comfortable lying postures Space allowance per animal |
| Resting problems |
Number of lying bouts Time spent in lateral recumbency |
|
Provide bedding or, if not possible, slats with a rubber cover Provide large space allowance Appropriate temperature and humidity to provide suitable thermal comfort |
| Respiratory disorders |
Rectal Temperature Respiratory sounds at lung auscultation Coughing Nasal discharge Ocular discharge |
Stress caused by weaning, long distant transport or other stressful events Lack of ventilation, high temperature‐humidity index and overstocking Poor quality of bedding, especially in cold environments Proximity to older animals Poor colostrum management |
Reduce stress factors (i.e. transport, mutilations, changes in group composition) Ensure good colostrum management and feeding of calves and establish integrated vaccination programs Keep calves in small and stable groups of similar age and size Appropriate ventilation to avoid high ammonia or dust concentrations. Adequate temperature‐humidity index |
| Inability to perform exploratory or foraging behaviour |
Non‐nutritive oral manipulation Tongue flicks Tongue rolling |
Barren environments Concentrated diets Low feeding frequency/duration |
Provide relevant enrichment, e.g. rubbing fixtures (brushes), enrichment objects, bedding Increase fibre content of diet to increase foraging Make animals work for their feed, e.g. straw rack, and increase feeding frequency Provide access to an outdoor area and pasture |
| Group stress | Aggressive interactions with physical contact |
Low space allowance in general and especially at trough Open trough and no individual feeding place during milk feeding (no fixation) Repeated regrouping |
Individual feeding places with a possibility to fixate calves during milk feeding avoid competition for milk Regrouping should be avoided as far as possible. Sufficient space for lying enables |
| Inability to perform play behaviour | Time spent in locomotor play |
Injury, malnutrition, cold weather and frightening stimuli Low space allowances Slippery surfaces, concrete floors |
Good health and nutrition Thermal comfort Increase space allowance Non‐slippery rubber coated or straw bedded surfaces |
Table D.11: Summary of welfare consequences, ABMs, hazards and preventive measures in systems keeping calves from weaning to 6 months in outdoor feedlots
| Welfare consequence | ABM | Hazard | Preventive measure of the hazard |
|---|---|---|---|
| Respiratory disorders |
Rectal Temperature Respiratory sounds at lung auscultation Coughing Nasal discharge Ocular discharge |
Heat stress Poor colostrum management Lack of bedding, especially in cold environments Stressful events Proximity to older animals |
Reduce stress factors (i.e. transport, mutilations, changes in group composition) Ensure good colostrum management and feeding of calves and establish integrated vaccination programs Keep calves in small and stable groups of similar age and size Adequate temperature‐humidity index |
| Heat stress |
Respiration rate or panting Body temperature Open mouth breathing Sweating |
High temperature‐humidity index, high solar radiation and low wind speed. Lack of shade especially at the hotter times of the day. Lack of access to cold water, insufficient number water points |
Provision of shades and covered pen areas Provision of sufficient water points and amount |
| Resting problems |
Number of lying bouts Time spent in lateral recumbency |
Lack of bedding lying area Low space allowances |
Higher space allowance Provision of bedded lying area and shelter |
Appendix E – Literature searches
Literature searches were conducted to identify scientific evidence on the elements requested by the ToR. Extensive literature searches (ELSs) were carried out to identify peer‐reviewed publications on welfare implications and associated ABM(s) in relation to the exposure variables identified. Details of the different ELSs are described below. All relevant publications were included in an EndNote x9 Library.
Sources of information included in the search – Web of Science – Web of Science core collection.
Search strings used in the bibliographic database – The search strings were designed to retrieve relevant publications and to the specific exposure variables (see details below). Restrictions applied in the search string were related to: (i) relevant exposure variable being assessed, and (ii) animal category (calves – bovines younger than 6 months). Language restrictions aimed at identifying only publications with an English abstract and full texts of a language covered in the expertise of the EFSA experts. No document type restrictions were applied in the search string with Primary research, reviews, EFSA outputs, books being considered. The records retrieved from Web of Science were exported to EndNote libraries/Excel files together with the relevant metadata (e.g. title, authors, abstract). Titles and abstracts were screened for relevance, and a conservative approach was taken in order to include papers that seemed relevant. Duplicates were removed when two or more records were identical. Full text publications were screened if title and abstract did not allow assessing the relevance of a paper. The screening was performed by two reviewers (first search, 2020 and one reviewer in 2022).
Based on the reviewed papers, a literature review on each topic was carried out. When it was noted that the search did not retrieve certain publications of relevance already known to the EFSA experts, these papers were added to the list of papers reviewed.
Specific Scenario 1 – ‘Individual housing’
Date: 19 March 2021. All Databases. Advanced search. Timespan: 1990–2021. All languages.
Search string: ((((TS = ((calves OR calf OR veal) AND (hous* OR crate* OR “baby box*” OR “babybox” OR pen) AND (behav* OR welfare) AND (Farm* OR production) NOT (cow) NOT (heifer))))))
Results: 153. Result after screening for relevance: 71
Repetition of search to check whether new relevant papers had been published since:
Date: 24 November 2022. Web of Science Core Collection. Advanced search. Timespan: 20 March 2021–2022. All languages.
Search string: ((((TS = ((calves OR calf OR veal) AND (hous* OR crate* OR “baby box*” OR “babybox” OR pen) AND (behav* OR welfare) AND (Farm* OR production) NOT (cow) NOT (heifer))))))
Results = 46. Result after screening for relevance: 4
Specific Scenario 1 – ‘Space allowance’
Date: 15 March 2021. Web of Science Core Collection. Advanced search. Timespan: 1990–2021. Restriction to English.
Search string (TS = ((calves OR calf OR veal) AND (space allowance OR stocking density)))
Results: 187. Result after screening for relevance: 36
Repetition of search to check whether new relevant papers had been published since:
Date: 24 November 2022. Web of Science Core Collection. Advanced search. Timespan: 2021–2022. Restriction to English.
Search string: (TS = ((calves OR calf OR veal) AND (space allowance OR stocking density)))
Results = 47. Result after screening for relevance: 4
Specific Scenario 1 – ‘Feed (iron and fibres)’
Two search strings were run separately, one focused on iron restriction, and other on other aspects of feed (fibre, solid feed). The search string results were combined, and duplicates removed.
Date: 12 March 2021. Web of Science Core Collection. Advanced search. Timespan: 1990–2021. Restriction to English.
Search string 1: (TS = (((calv* OR calf OR veal*) AND (farm OR production) AND feed* AND (iron OR h?moglobin))))
Search string 2: (TS = ((calv* OR calf OR veal*) AND (farm OR production) AND feed* AND welfare AND (milk OR solid feed OR fibre OR fiber OR concentrate OR roughage OR water OR drink*)))
Results: 278. Result after screening for relevance: 52
Repetition of search to check whether new relevant papers had been published since:
Date: 24 November 2022. Web of Science Core Collection. Advanced search. Timespan: 2021–2022. Restriction to English.
Search string 1: (TS = (((calv* OR calf OR veal*) AND (farm OR production) AND feed* AND (iron OR h?moglobin))))
Search string 2: (TS = ((calv* OR calf OR veal*) AND (farm OR production) AND feed* AND welfare AND (milk OR solid feed OR fibre OR fiber OR concentrate OR roughage OR water OR drink*)))
Results = 84. Result after screening for relevance: 20
Specific Scenario 2 – ‘Carcass colour’
Date: 19 September 2022. Web of Science Core Collection. Advanced search. Timespan: 1975–2022. All languages.
Search string: TS = (“calves” OR “calf”) AND TS = (“carcass*” OR “carcase$”) AND TS = (“colour” OR “color” OR “meat grading” OR “pale”) AND TS = (“slaughter” OR “abattoir” OR “slaughter plant” OR “slaughter line” OR “slaughter factory”) AND TS = (“welf*” OR “health” OR “protection” OR “inspection” OR “meat”) NOT TS = (“econom*” OR “value”)
Results: 53. Result after screening for relevance: 26
Specific Scenario 2 – ‘Abomasal lesions’
Date: 19 September 2022. Web of Science Core Collection. Advanced search. Timespan: 1975–2022. All languages.
Search string: TS = (“calves” OR “calf”) AND TS = (“abomas*”) AND TS = (“lesion*” OR “wound*” OR “ulcer$” OR “scar$”) AND TS = (“slaughter*” OR “abattoir” OR “slaughter plant” OR “slaughter line” OR “slaughter factory”) AND TS = (“welfare” OR “health” OR “protection” OR “inspection”)
Results: 19. Result after screening for relevance: 11
Specific Scenario 2 – exposure variable ‘Bursitis’
Search string: TS = (“calves” OR “calf”) AND TS = (“bursitis” OR “bursa$” OR “carpus swelling”) AND TS = (welfare)
Results: 1. Result after screening for relevance: 1
Specific Scenario 2 – exposure variable ‘Carcass condemnation’
Date: 19 September 2022. Web of Science Core Collection. Advanced search. Timespan: 1975–2022. All languages.
Search string: TS = (“calves” OR “calf”) AND TS = (“carcass*” OR “carcase$” OR “viscera”) AND TS = (“condemnation” OR “trimming” OR “trimmed” OR “condemned”) AND TS = (“slaughter” OR “abattoir” OR “slaughter plant” OR “slaughter line” OR “slaughter factory”) AND TS = (“welf*” OR “health” OR “protection” OR “inspection” OR “meat”) NOT TS = (“econom*” OR “value”)
Results: 9. Result after screening for relevance: 5
Specific Scenario 2 – exposure variable ‘Lung lesions’
Search string: TS = (“calves” OR “calf”) AND TS = (“lung$”) AND TS = (“lesion*” OR “respiratory” OR “wound*” OR “pneumonia” OR “pleuritis” OR “discoloration”) AND TS = (“slaughter*” OR “abattoir” OR “slaughter plant” OR “slaughter line” OR “slaughter factory” OR “necroscopy”) AND TS = (“welfare” OR “health” OR “protection” OR “inspection”) NOT TS = (“econom*” OR “value”)
Results = 21. Result after screening for relevance: 7
Specific Scenario 2 – exposure variable ‘Body condition’
Date: 19 September 2022. Web of Science Core Collection. Advanced search. Timespan: 1975–2022. All languages.
Search string: TS = (“calves” OR “calf”) AND TS = (“body condition” OR “condition” OR “body”) AND TS = (“slaughter*” OR “abattoir” OR “slaughter plant” OR “slaughter line” OR “slaughter factory”) AND TS = (“welfare” OR “health” OR “protection” OR “inspection” OR “meat”) NOT TS = (“society” OR “consumer$” OR “economic$” OR “citizen$”)
Results: 187 Result after screening for relevance: 1
Specific Scenario 3 – Exposure variable cow – calf bond
Date: 16 March 2021. All Databases. Advanced search. Time span: 1990–2021. All languages.
Search string: (((TS = ((calves OR calf OR veal) AND (cow OR mother OR dam OR “maternal deprivation”) AND (bond OR contact OR rearing) AND (behav* OR welfare)))))
Results: 420. Result after screening for relevance: 101
Repetition of search to check whether new relevant papers had been published since:
Date: 24 November 2022. Web of Science Core Collection. Advanced search. Timespan: 1990–2021. All languages.
Search string: (((TS = ((calves OR calf OR veal) AND (cow OR mother OR dam OR “maternal deprivation”) AND (bond OR contact OR rearing) AND (behav* OR welfare)))))
Results = 37. Result after screening for relevance: 8
Appendix F – Data extracted from the literature on play behaviour levels
Different play behaviour definitions were reported in the literature:
Locomotor play defined as ‘galloping’, jumping’ and kicking’, NOT including trotting
Play definition included ‘running’ and ‘trotting’
Play definition included social and ground play in addition to locomotor play
For the purposes of the EKE, locomotor play was defined as ‘galloping’, jumping’ and kicking’, but not trotting. However, all data points from the literature were considered for the elicitation, after being ‘adjusted’ by the working group via expert knowledge. Following extraction from the scientific studies, the data were standardised so they referred to % time spent playing in 24 h (Table F.1).
Table F.1.
Summary of data reported in the scientific literature on locomotor play levels showed by calves in different space allowances
| Paper author, year | Age of animals, dairy/veal calves | Effects observed – quantitative information | Confounders/limitations | Notes |
|---|---|---|---|---|
|
Jensen et al. (1998) (+ page 102 Figures 2 and 3) |
Average of weeks 2, 4 and 6 Dairy calves |
0.0475% total time spent in locomotory play behaviour at 1.4 m 2 per animal |
Straw bedded Group pen with 4 animals N = 48, hereof half in group pens with 4 calves per pen |
Original values were re‐calculated from % active time. Original data obtained from Figures locomotor play is defined as ‘galloping’, jumping’ and kicking’, NOT including trotting |
|
Jensen et al. (1998) (+ page 102, Figures 2 and 3) |
Average of weeks 2, 4 and 6 Dairy calves |
0.0158% total time spent in locomotory play behaviour at 1.4 m 2 per animal |
Straw bedded Individual pens |
original values were re‐calculated from % active time locomotor play is defined as ‘galloping’, jumping’ and kicking’, NOT including trotting |
|
Jensen and Kyhn (2000) (page 40–42) |
Week 5 Dairy calves |
0.0451% total time spent in locomotory play behaviour at 1.5 m 2 per animal |
Straw bedded Group pen with 4 animals (N = 96; 4 treatments (space allowances) |
Original values were re‐calculated from % active time Data was collected in week 7 and 9, but not given per treatment Locomotor play is defined as ‘galloping’, jumping’ and kicking’, NOT including trotting |
|
Jensen and Kyhn (2000) (pp. 40–42) |
Week 5 Dairy calves |
0.0440% total time spent in locomotory play behaviour at 2.2 m 2 per animal |
Straw bedded Group pen with 4 animals |
Locomotor play is defined as ‘galloping’, jumping’ and kicking’, NOT including trotting |
| Sutherland et al. (2014a) (p. 11) |
Week 4 Dairy calves |
0.18% time spent running in home pen/24 h at 1.5 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 24 calves |
The Sutherland estimates of ‘running’ includes ‘trotting’ and are thus not comparable with the estimates of locomotor play of Jensen et al. (1998) and Jensen and Kyhn (2000) ‘running’ includes ‘trotting’ |
| Sutherland et al. (2014a) (p. 11) |
Week 6 Dairy calves |
0.07% time spent running in home pen/24 h at 1.5 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 24 calves |
‘Running’ includes ‘trotting’ |
| Sutherland et al. (2014a) (p. 11) | Average of weeks 4 and 6 | 0.13% time spent running in home pen/24 h at 1.5 m 2 per animal | ||
| Sutherland et al. (2014a) (p. 11) |
Week 4 Dairy calves |
0.17% time spent running in home pen/24 h at 2.0 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 24 calves |
|
| Sutherland et al. (2014a) (p. 11) |
Week 6 Dairy calves |
0.09% time spent running in home pen/24 h at 2.0 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 24 calves |
|
| Sutherland et al. (2014a) (p. 11) | Average of weeks 4 and 6 | 0.13% time spent running in home pen/24 h at 2.0 m 2 per animal | ||
| Sutherland et al. (2014a) (p. 4459) |
Week 2 Dairy calves |
0.2% time spent running in home pen/24 h at 1.5 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 36 calves |
|
| Sutherland et al. (2014a) (p. 4459) |
Week 4 Dairy calves |
0.2% time spent running in home pen/24 h at 1.5 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 36 calves |
|
| Sutherland et al. (2014b) (p. 4459) |
Week 6 Dairy calves |
0.1% time spent running in home pen/24 h at 1.5 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 36 calves |
|
| Sutherland et al. (2014b) (p. 4459) | Average of weeks 2, 4 and 6 | 0.17% time spent running in home pen/24 h at 1.5 m 2 per animal | ‘Running’ includes ‘trotting’ | |
| Sutherland et al. (2014b) (p. 4459) |
Week 2 Dairy calves |
0.2% time spent running in home pen/24 h at 2.0 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 36 calves |
|
| Sutherland et al. (2014b) (p. 4459) |
Week 4 Dairy calves |
0.2% time spent running in home pen/24 h at 2.0 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 36 calves |
|
| Sutherland et al. (2014b) (p. 4459) |
Week 6 Dairy calves |
0.1% time spent running in home pen/24 h at 2.0 m 2 per animal |
Quarry stones (40–60 mm) or sawdust 4 calves per pen, 36 calves |
|
| Sutherland et al. (2014b) (p. 4459) | Average of weeks 2, 4 and 6 | 0.17% time spent running in home pen/24 h at 2.0 m 2 per animal | ||
| Tapki et al. (2006) (p. 15) | Days 4–63 | 2.11% play behaviour (locomotor, social, ground) at 1.5 m 2 per animal | Individually housed, in total 21 animals, 7 per treatment, no SD provided |
Observations were carried out twice a week for 8 h distributed over 24 h using time sampling at 5 min intervals, no SD or similar provided These estimates include also social and ground play in addition to locomotor play |
| Tapki et al. (2006) (p. 15) | Days 4–63 | 2.80% play behaviour (locomotor, social, ground) at 2.25 m 2 per animal | Individually housed, in total 21 animals, 7 per treatment, no SD provided |
See above These estimates include also social and ground play in addition to locomotor play |
|
Jensen et al. (1998) (+ page 102, Figures 2 and 3) |
Average of weeks 2, 4 and 6 Dairy calves |
0.0788% total time spent in locomotory play behaviour at 4 m 2 per animal |
Straw bedded Group pen with 4 animals |
Original values were re‐calculated from % active time Locomotor play is defined as ‘galloping’, jumping’ and kicking’, NOT including trotting |
|
Jensen et al. (1998) (+ page 201, Figures 2 and 3) |
Average of weeks 2, 4 and 6 Dairy calves |
0.0653% total time spent in locomotory play behaviour at 5.4 m 2 per animal |
Straw bedded Individual pens |
Original values were re‐calculated from % active time locomotor play is defined as ‘galloping’, jumping’ and kicking’, NOT including trotting |
|
Jensen and Kyhn (2000) (pp. 40–42) |
Week 5 Dairy calves |
0.0856% total time spent in locomotory play behaviour at 3.0 m 2 per animal |
Straw bedded Group pen with 4 animals |
Locomotor play is defined as ‘galloping’, jumping’ and kicking’, NOT including trotting |
|
Jensen and Kyhn (2000) (pp. 40–42) |
Week 5 Dairy calves |
0.0787% total time spent in locomotory play behaviour at 4.0 m 2 per animal |
Straw bedded Group pen with 4 animals |
Locomotor play is defined as ‘galloping’, jumping’ and kicking’, NOT including trotting |
|
Waiblinger et al. (2020) (p. 145) |
Week 5 Dairy calves | 0.24% total time spent in locomotory play behaviour at 4.6 m 2 per animal |
Straw bedded group pen n = 20 |
Extrapolated from 4 h observations in the afternoon, assuming in total 16 h of observations and a proportion of 0.9 for locomotor play, which was defined as ‘galloping’, jumping’ and kicking’, NOT including trotting |
|
Waiblinger et al. (2020) (p. 145) |
Week 7 Dairy calves |
0.12% total time spent in locomotory play behaviour at 4.2 m 2 per animal |
Straw bedded group pen n = 20 |
See above |
|
Waiblinger et al. (2020) (p. 145) |
Week 12 Dairy calves |
0.14% total time spent in locomotory play behaviour at 5.4 m 2 per animal | Straw bedded group penn = 20 | See above |
|
Waiblinger et al. (2020) (p. 145) |
Average of weeks 5, 7 and 12 Dairy calves |
0.17% total time spent in locomotory play behaviour at on average 4.7 m 2 per animal | Straw bedded group penn = 20 | See above |
|
Waiblinger et al. (2020) (p. 145) |
Week 5 Dairy calves |
0.38% total time spent in locomotory play behaviour at > 10 m 2 per animal |
Straw bedded group pen + access to cow barn, where 90% of play behaviour occurred n = 19 |
See above |
|
Waiblinger et al. (2020) (p. 145) |
Week 7 Dairy calves |
0.31% total time spent in locomotory play behaviour at > 10 m 2 per animal |
Straw bedded group pen + access to cow barn, where 90% of play behaviour occurred n = 19 |
See above |
|
Waiblinger et al. (2020) (page 145) |
Week 12 Dairy calves |
0.35% total time spent in locomotory play behaviour at > 10 m 2 per animal |
Straw bedded group pen + access to cow barn, where 90% of play behaviour occurred n = 19 |
See above |
|
Waiblinger et al. (2020) (p. 145) |
Average of weeks 5, 7 and 12 Dairy calves |
0.35% total time spent in locomotory play behaviour at > 10 m 2 per animal |
Straw bedded group pen + access to cow barn, where 90% of play behaviour occurred n = 19 |
See above |
| Krachun et al. (2009) (p. 74) |
Week 3 Dairy calves |
0.105% (90s, 6 L milk/day) and 0.175–0.21%s (150–180 s; 12 L milk/day) time spent running per day at 3.7 m 2 per animal |
Partly bedded pens Group pen with 9 animals, n = 51 calves in total (17 6 L/day, 34 12 L/day) |
Data obtained from figure; observations for 15 h; night hours not observed because earlier studies suggested that almost no play behaviour takes place during this time, 60s = 0.07% on a 24 h basis ‘Running’ defined as rapid forward movement that lasted 3 s or longer (in real time) and could include instances of jumping or bucking. Both galloping and trotting, as described by Jensen (1999), were included as running. ‘running’ includes ‘trotting’ |
| Krachun et al. (2009) (p. 74) |
Week 5 Dairy calves |
0.134% (115 s, 6 L milk/day) and 0.11% – 0.14% (100–120 s; 12 L milk/day) time spent running per day at 3.7 m 2 per animal |
Partly bedded pens Group pen with 9 animals |
See above ‘running’ includes ‘trotting’ |
| Krachun et al. (2009) (p. 74) |
Week 9 Dairy calves |
0.064% (55 s, 6 L milk/day) and 0.058–0.082% (50–70 s; 12 L milk/day) of time spent running per day at 3.7 m 2 per animal |
Partly bedded pens Group pen with 9 animals |
See above ‘running’ includes ‘trotting’ |
| Krachun et al. (2009) (p. 74) | Average of weeks 3, 5 and 9 | 0.101% (6 L/day) and 0.114%–0.144% (12 L milk/day) time spent running per day at 3.7 m 2 per animal | ||
| Miguel‐Pacheco et al. (2015) (p. 1041) |
Period of 5 days before weaning (which depended on concentrate intake) Dairy calves |
On average 0.042% (36 s) to 0.101% (86.5 s) (range 0.015 (12.5 s) to 0.13% (111.5 s)) of time spent running at 4.2 m 2 per animal |
Partly bedded pens Group pen with 8 animals; 56 animals (12 L/day) |
Night hours not observed; observations for 15 h; night hours not observed because earlier studies because earlier studies suggested that almost no play behaviour takes place during this time, 60s = 0.07% on a 24 h basis Defined locomotor play as running ‘a rapid forward movement that may include jumping, bucking and/or kicking with one or two legs (Jensen et al., 1998; Jensen and Kyhn, 2000), lasting a minimum of 3 s (Krachun et al., 2010. If a calf did two running bouts that were < 3 s apart, they were recorded as part of the same bout’. ‘running’ includes ‘trotting’ |
| Mintline et al. (2012) (p. 103) |
Period of 5 days before weaning (which depended on concentrate intake) Dairy calves |
On average 0.15%, SEM 0.014% (128 s/15 h, SEM 12 s), range 0.023% (20 s/15 h) to 0.35% (300 s/15 h) of time spent running per day at 11.2 m 2 per animal (at individual level) |
Partly bedded pens Group pen with 3 animals, n = 20 |
Night hours not observed; observations for 15 h; night hours not observed because earlier studies because earlier studies suggested that almost no play behaviour takes place during this time, 60s = 0.07% on a 24 h basis Running includes trotting, cantering and gallop ‘running’ includes ‘trotting’ |
| Zobel et al. (2017) (p. 5) | Week 2–3 | 0.15% (2.1 min/18 h; 12:00–08:00; coefficient of variation 66%) of time spent playing (locomotor, social, ground) at 5 m 2 per animal |
Bedded pens 8 group pens with 2 animals |
Includes ‘locomotor play as defined by Jensen et al. (1998) + social play + ground play (i.e. NOT trotting); 60s = 0.07% on a 24 h basis These estimates include also social and ground play in addition to locomotor play, but not trotting |
| Tapki et al. (2006) (p. 15) | Days 4–63 | 2.59% of time spent playing (locomotor, social, ground) at 4 m 2 per animal | Individually housed, in total 21 animals, 7 per treatment, no SD provided |
Observations were carried out twice a week for 8 h distributed over 24 h using time sampling at 5 min intervals, no SD or similar provided These estimates include also social and ground play in addition to locomotor play |
Appendix G – Data extracted from the literature on haemoglobin levels of non‐anaemic calves
Table G.1: Values reported in the literature on haemoglobin concentration of non‐anaemic calves, depending on age (weeks)
| Age (weeks) | Haemoglobin concentration (mmol/L) | Animal category | Sample size | Reference |
|---|---|---|---|---|
| 1 | 7.01 | Crossbreed | 114 | Bouda and Jagoš (1984) |
| 1 | 5.96 | Dairy | 254 | Panousis et al. (2018) |
| 1–2 | 7.07 | Dairy | 141 | Roadknight et al. (2021) |
| 3 | 6.55 | Dairy | 66 | Ježek et al. (2009) |
| 5 | 5.91 | Dairy | 66 | Ježek et al. (2009) |
| 8 | 6.78 | Crossbreed | 114 | Bouda and Jagoš (1984) |
| 2–11 | 5.6–8.7 | Dairy | 40 | Joerling and Doll (2019) |
| 12 | 6.97 | Crossbreed | 114 | Bouda and Jagoš (1984) |
| 16 | 7.11 | Dairy | 66 | Ježek et al. (2009) |
| 24 | 6.71 | Crossbreed | 114 | Bouda and Jagos (1984) |
| 25–30 | 6.39 | Veal (Peter Farm (R)) | 60 | Bokkers and Koene (2001) |
| 25–30 | 5.96 | Veal – Group housing | 60 | Bokkers and Koene (2001) |
| 25–30 | 5.64 | Veal – Individual farm | 60 | Bokkers and Koene (2001) |
Samples collected at slaughterhouse, ante‐mortem.
Appendix H – Nutritional values of feed sources
Table H.1: Nutritional values of feed used in (veal) calves (Source of information: Alimentation des Ruminants ‐ INRA 2018 ‐ ISBN 978–2–27592‐2867‐6, 728 pages, Editions Quae, Versailles (France))
| Dry matter | Crude fibre | Neutral Detergent Fibre | Acid Detergent fibber | ||
|---|---|---|---|---|---|
| ID number INRA | Feed stuff | DM (%) | CF | NDF | ADF |
| ‰ (g/kg) | |||||
| FV 0020 | Grass (permanent) | 16.6% | 244 | 525 | 280 |
| FV 0190 | Grass (permanent) | 21.7% | 323 | 595 | 344 |
| FP 0020 | Straw – wheat | 88.0% | 420 | 798 | 504 |
| FP 0060 | Straw – barley | 88.0% | 420 | 798 | 504 |
| FP 0090 | Straw – oats | 88.0% | 420 | 760 | 470 |
| FF 0550 | Hay | 85.0% | 308 | 604 | 332 |
| FF 0160 | Hay | 85.0% | 317 | 613 | 340 |
| FF 0250 | Hay | 85.0% | 296 | 592 | 322 |
| FF 3330 | Alfalfa hay | 85.0% | 351 | 548 | 352 |
| Corn (maize) silage | 35.0% | 200 | 400 | 204 | |
| CC 0010 | Barley (full grains) | 87.2% | 54 | 215 | 65 |
| CC 0040 | Corn floconné | 86.3% | 26 | 125 | 31 |
| CC 0080 | Oats floconné | 87.6% | 133 | 361 | 165 |
| CC 0140 | Wheat (full grains) | 87.8% | 30 | 159 | 42 |
| CX 0200 | Rapeseed (cake) | 89.0% | 144 | 316 | 208 |
| CX 0250 | Soya been (cake) | 87.5% | 71 | 148 | 88 |
| CP 0010 | Sugerbeet pulp (dehydrated) | 88.8% | 194 | 471 | 238 |
Appendix I – Rumination times of calves fed different amounts of fibre (NDF)
A summary of data extracted from the literature on rumination times of calves fed different amounts of fibre (NDF). Differences between study settings (e.g. indoor/outdoor), period of observation (number of hours per day), animal category (beef/dairy/zebu calves) were discussed and considered. Variables were transformed and standardised when necessary to allow comparisons across studies.
Table I.1: Summary of data extracted from the literature on rumination times of calves fed different amounts of fibre (NDF)
| Reference | Animal age at study start | Feeding type | Amount | NDF in the solid fraction | Percentage of time spent ruminating per 24 h | |||
|---|---|---|---|---|---|---|---|---|
| Study duration | Solid feed | Solid feed | Roughage | NDF in the solid fraction | NDF in the solid fraction | Average over whole study | ||
| Weeks | Weeks | % component | kg DM/period | kg DM/period | %DM | kg DM/day | % | |
| Swanson and Harris (1958) | 1.5 | 15 | Alfalfa‐grass mixed hay, but composition not reported | 21 | ||||
| Hutchison et al. (1962) (on zebu cattle) | 8 | 24 weeks | Pasture – composition not reported | 38 | ||||
| Webb et al. (2012) | 6–10 | 18 | 50% concentrate, 25% straw, 25% maize | 39.0 | 19.50 | 38% | 0.12 | 14 |
| Webb et al. (2012) | 6–10 | 18 | 50% concentrate, 25% straw, 25% maize | 77.5 | 38.74 | 38% | 0.23 | 15 |
| Webb et al. (2012) | 6–10 | 18 | 50% corn, 25% straw, 25% maize | 119.3 | 59.67 | 38% | 0.36 | 21 |
| Webb et al. (2013) | 2 | 18 | Chopped straw | 26.0 | 26.00 | 78% | 0.16 | 13 |
| Webb et al. (2013) | 2 | 18 | Chopped maize | 28.0 | 28.00 | 41% | 0.09 | 6 |
| Webb et al. (2013) | 2 | 18 | Chopped straw | 44.0 | 44.00 | 78% | 0.27 | 14 |
| Webb et al. (2013) | 2 | 18 | Chopped maize | 51.0 | 51.00 | 41% | 0.17 | 9 |
| Webb et al. (2013) | 2 | 18 | Hay | 89.0 | 89.00 | 63% | 0.45 | 16 |
| Webb et al. (2014a,b) | 2 | 18 | 65% maize silage, 30% concentrate, 5% straw | 54.6 | 38.22 | 36% | 0.16 | 16 |
| Webb et al. (2014a,b) | 2 | 18 | 63% concentrate, 25% hay, 8% maize, 4% straw | 293.3 | 108.52 | 34% | 0.80 | 23 |
| Webb et al. (2015) | 2 | 18 | 80% concentrate, 10% maize, 10% straw | 21.4 | 4.28 | 22% | 0.04 | 7 |
| Webb et al. (2015) | 2 | 18 | 50% corn, 25% maize, 25% straw | 21.5 | 10.75 | 37% | 0.06 | 8 |
| Webb et al. (2015) | 2 | 18 | 80% corn, 10% maize, 10% straw | 105.2 | 21.04 | 22% | 0.19 | 7 |
| Webb et al. (2015) | 2 | 18 | 50% corn, 25% maize, 25% straw | 105.5 | 52.75 | 37% | 0.31 | 11 |
| Webb et al. (2015) | 2 | 18 | 80% corn, 10% maize, 10% straw + straw | 105.2 | 21.04 | 22% | 0.19 | 15 |
| Webb et al. (2015) | 2 | 18 | 80% corn, 10% maize, 10% straw | 189.0 | 37.80 | 22% | 0.33 | 9 |
| Webb et al. (2015) | 2 | 18 | 50% corn, 25% maize, 25% straw | 189.7 | 94.85 | 37% | 0.55 | 13 |
| Webb et al. (2015) | 2 | 18 | 71% corn, 20% maize, 9% straw | 272.0 | 78.88 | 25% | 0.53 | 16 |
| Webb et al. (2015) | 2 | 18 | 80% corn, 10% maize, 10% straw | 273.0 | 54.60 | 22% | 0.48 | 9 |
| Webb et al. (2015) | 2 | 18 | 50% corn, 25% maize, 25% straw | 273.8 | 136.90 | 37% | 0.80 | 20 |
| Webb et al. (2015) | 2 | 18 | 71% corn, 20% maize, 9% straw | 294.1 | 85.289 | 25% | 0.57 | 18 |
| Brščić et al. (2014) | 2 | 28.71 | 15% straw, 85% corn grain | 140.0 | 22.5% | 0.16 | 10 | |
| Brščić et al. (2014) | 2 | 28.71 | 72% corn grain, 15% straw, 13% other | 140.0 | 23.1% | 0.16 | 10 | |
| Brščić et al. (2014) | 2 | 28.71 | 83% corn grain, 16% straw, 8% other | 140.0 | 23.2% | 0.16 | 12 | |
| Mattiello et al. (2002) | 1 | 22.86 | 0 (only milk replacer) | 0 | 0% | 0.00 | 4 | |
| Mattiello et al. (2002) | 1 | 22.86 | 250 g/d of wheat straw | 40.00 | 47% | 0.12 | 4 | |
| Mattiello et al. (2002) | 1 | 22.86 | 250 g/d of dried beet pulp | 40.00 | 86% | 0.21 | 7 | |
Appendix J – Definitions of slaughter ABMs (ante and post‐mortem) (Specific Scenario 2)
J.1: Definitions of slaughter ABMs (ante‐ and post‐mortem) used to address Specific Scenario 2
| ABM | Definition |
|---|---|
| Body condition | The body condition reflects body reserves or fat accumulation of an animal. Body condition scoring is used to critically examine the nutritional status of a calf |
| Lameness | Inability to use one or more limbs in a normal manner |
| Skin lesions‐wounds/injuries | Fresh or healed injuries on the skin of the body, which can be scratches, scabs (surface penetration of the epidermis) or wounds (penetration of the muscle tissue). (depending on whether injuries are fresh or healed they can be dated to understand whether they were caused during transport or on the farm) |
| Skin lesions ‐abscesses | Foci of infection with collection of pus that are visible externally to the skin |
| Manure on the body | Presence of manure/faeces on the body |
| Coughing/sneezing | The calf expels air from the lungs with a sudden sharp sound |
| Nasal discharge | Defined as clearly visible flow/discharge from the nostrils; it can be transparent to yellow/green and often is of thick consistency |
| Pumping/Laboured breathing | Heavy and laboured calf breathing |
| Rectal prolapse | Internal tissue extrudes from the rectum |
| Hernia | Protrusion of a bodily structure or organ through the wall that normally contains it, resulting in a lump under the skin |
| Diarrhoea | Loose watery manure below the tail head on both sides of the tail, with the area affected at least the size of a hand |
| Lung lesions ‐pneumonia | Inflammation of the lung tissue with or without an overlying pleurisy |
| Lung lesions –pleurites | Inflammation of the pleurae with fibrinous pleural adhesions |
| Pericarditis | Fibrosis of pericardial sac, with or without the presence of fluid |
| Skin lesions –bruises | An injury (contusion) involving rupture of small blood vessels and discoloration without a break in the overlying skin |
| Abscesses | Foci of infection with collection of pus that may occur internally or externally in the carcass or the organs |
| Bursa (hygroma) | A bursa is a fluid filled sac that develops as a result of a pressure injury on the weight‐bearing points of the legs. Bursae are most prevalent in the front of the carpal joints and the hock region of the hind limbs, although they can occur in other locations |
| Abomasal lesions | Abomasal erosions, ulcers and scars |
| Rumen lesions | Lesions to the rumen mucosa: a hyperkeratosis, plaques and plaques with trapped hair |
| Rumen disorders | The presence of milk in the rumen is cause for concern. Milk in the rumen of calves reflects failure of the oesophageal groove to close properly and to deliver milk directly into the abomasum |
| Intestinal disorders | Enteritis is the most common lesion found in the calf's intestines though congenital defects are found occasionally |
| Carcass colour | Very pale carcass colour associated with low haemoglobin concentration and anaemia |
| Carcass condemnations | Carcass and parts that are unfit for use as food |
| Carcass aspect | Aspect of carcass composition (shape) and fat level |
Annex A
EFSA launched in Autumn 2022 a public consultation to receive input from the scientific community and all interested parties on the draft Scientific Opinion on the Welfare of Calves. The public consultation was open from 29 September to 4 November 2022 and a total of 177 comments were received via the EFSA website. The answers to the comments received and notes explaining how the information provided was incorporated in the scientific opinion, when relevant, is presented in Annex A of this Scientific Opinion.
Annex A is available under the Supporting Information section on the online version of the scientific output.
Supporting information
Outcome of the public consultation on the welfare of calves
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Animal Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Schmidt CG, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Stahl K, Velarde A, Viltrop A, Jensen MB, Waiblinger S, Candiani D, Lima E, Mosbach‐Schulz O, Van der Stede Y, Vitali M and Winckler C, 2023. Scientific Opinion on the welfare of calves. EFSA Journal 2023;21(3):7896, 197 pp. 10.2903/j.efsa.2023.7896
Requestor European Commission
Question number EFSA‐Q‐2020‐00480
Panel members Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, Jose Luis Gonzales Rojas, Christian Gortazar Schmidt, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Hans Spoolder, Karl Stahl, Antonio Velarde, Arvo Viltrop and Christoph Winckler.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The Panel wishes to thank the following for the support provided to this scientific output: WG: Laura Webb; hearing experts: Marta Brščić, Joop Lensink and George Stilwell. EFSA would like to thank Mariana Aires, Simona Bottinelli, Mariana Geffroy and Mimi Kalcheva from EFSA, for all the support provided in this Scientific Opinion. In addition, EFSA would like to thank Hans‐Hermann Thulke and Karen Laing for the support in the activities related with expert opinion. EFSA wishes to acknowledge the European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 22 February 2023
Annex A is available under the Supporting Information section
Notes
OJ L 221, 8.8.1998, p. 23.
OJ L 203, 3.8.1999, p. 53.
OJ L 10, 15.1.2009, p. 7.
OJ L 47, 18.2.2009, p. 5.
OJ L 182, 12.7.2007, p. 19.
OJ L 3, 5.1.2005, p. 1.
OJ L 303, 18.11.2009, p. 1.
References
- Abdelfattah EM, Schutz MM, Lay DC, Marchant‐Forde JN and Eicher SD, 2013. Effect of group size on behavior, health, production, and welfare of veal calves. Journal of Animal Science, 91, 5455–5465. 10.2527/JAS.2013-6308 [DOI] [PubMed] [Google Scholar]
- Absmanner E, Rouha‐Mülleder C, Scharl T, Leisch F and Troxler J, 2009. Effects of different housing systems on the behaviour of beef bulls—an on‐farm assessment on Austrian farms. Applied Animal Behaviour Science, 118, 12–19. 10.1016/j.applanim.2009.02.009 [DOI] [Google Scholar]
- Adcock SJJ and Tucker CB, 2018. The effect of disbudding age on healing and pain sensitivity in dairy calves. Journal of Dairy Science, 101, 10361–10373. 10.3168/jds.2018-14987 [DOI] [PubMed] [Google Scholar]
- Afari N and Buchwald D, 2003. Chronic fatigue syndrome: a review. American Journal of Psychiatry, 160, 221–236. 10.1176/appi.ajp.160.2.221 [DOI] [PubMed] [Google Scholar]
- Alban L, Poulsen MK, Petersen JV, Lindegaard LL, Meinert L, Koch AG and Møgelmose V, 2022. Assessment of risk to humans related to Salmonella from bile on pig carcasses. Food Control, 131, 108415. 10.1016/J.FOODCONT.2021.108415 [DOI] [Google Scholar]
- Allan J, Plate P and Van Winden S, 2020. The effect of iron dextran injection on daily weight gain and haemoglobin values in whole milk fed calves. Animals, 10, 853. 10.3390/ani10050853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LP, Paterson JA, Ansotegui RP, Cecava M and Schmutz W, 2001. The effects of degradable and undegradable intake protein on the performance of lactating first‐calf heifers. Journal of Animal Science, 79, 2224–2232. 10.2527/2001.7982224X [DOI] [PubMed] [Google Scholar]
- Andrighetto I, Gottardo F, Andreoli D and Cozzì Cozzì G, 1999. Effect of type of housing on veal calf growth performance, behaviour and meat quality. Livestock Production Science, 57, 137–145. 10.1016/S0301-6226(98)00170-5 [DOI] [Google Scholar]
- Aschwanden J, Gygax L, Wechsler B and Keil NM, 2009. Loose housing of small goat groups: influence of visual cover and elevated levels on feeding, resting and agonistic behaviour. Applied Animal Behaviour Science, 119, 171–179. 10.1016/J.APPLANIM.2009.04.005 [DOI] [Google Scholar]
- Asheim LJ, Johnsen JF, Havrevoll Ø, Mejdell CM and Grøndahl AM, 2016. The economic effects of suckling and milk feeding to calves in dual purpose dairy and beef farming. Review of Agricultural, Food and Environmental Studies, 97, 225–236. 10.1007/s41130-016-0023-4 [DOI] [Google Scholar]
- Ashkenazy S and Ganz FD, 2019. The differentiation between pain and discomfort: a concept analysis of discomfort. Pain Management Nursing, 20, 556–562. 10.1016/j.pmn.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Autio T, Pohjanvirta T, Holopainen R, Rikula U, Pentikäinen J, Huovilainen A, Rusanen H, Soveri T, Sihvonen L and Pelkonen S, 2007. Etiology of respiratory disease in non‐vaccinated, non‐medicated calves in rearing herds. Veterinary Microbiology, 119, 256–265. 10.1016/j.vetmic.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu LK, Pandey HN and Sahoo A, 2004. Effect of individual versus group rearing on ethological and physiological responses of crossbred calves. Applied Animal Behaviour Science, 87, 177–191. 10.1016/J.APPLANIM.2004.01.006 [DOI] [Google Scholar]
- Bach Knudsen KE, 2001. The nutritional significance of ‘dietary fibre’ analysis. Animal Feed Science and Technology, 90, 3–20. 10.1016/S0377-8401(01)00193-6 [DOI] [Google Scholar]
- Bach A, Ahedo J and Ferrer A, 2010. Optimizing weaning strategies of dairy replacement calves. Journal of Dairy Science, 93, 413–419. 10.3168/JDS.2009-2682 [DOI] [PubMed] [Google Scholar]
- Bähler C, Regula G, Stoffel MH, Steiner A and von Rotz A, 2010. Effects of the two production programs ‘Naturafarm'and ‘conventional'on the prevalence of non‐perforating abomasal lesions in Swiss veal calves at slaughter. Research in Veterinary Science, 88, 352–360. 10.1016/j.rvsc.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Bailly‐Caumette E, Bertelsen M and Jensen MB, 2022. Play behaviour of dam‐reared dairy calves is affected by daily duration of contact with the dam. Proceedings of the 55th Congress of the International Society of Applied Ethology (ISAE 2022), Ohrid. 76 pp. Available online: https://www.applied-ethology.org/res/ISAE_2022_PROCEEDINGS_e-version.pdf [Google Scholar]
- Barnier V, Klont R, Van Dijk A, Eikelenboom G, Hoving‐Bolink A and Smulders F, 1998. Post mortem variation in pH, temperature and colour profiles of electrically stimulated veal carcasses in relation to preslaughter blood haemoglobin content. Proceedings of the 44th International Congress of Meat Science and Technology, Barcelona, Spain, 496–497 pp. [Google Scholar]
- Barth K, 2020. Effects of suckling on milk yield and milk composition of dairy cows in cow–calf contact systems. Journal of Dairy Research, 87, 133–137. 10.1017/S0022029920000515 [DOI] [PubMed] [Google Scholar]
- Beaver A, Meagher RK, von Keyserlingk MAG and Weary DM, 2019. Invited review: a systematic review of the effects of early separation on dairy cow and calf health. Journal of Dairy Science, 102, 5784–5810. 10.3168/JDS.2018-15603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendali F, Bichet H, Schelcher F and Sanaa M, 1999. Pattern of diarrhoea in newborn beef calves in south‐west France. Veterinary Research, 30, 61–74. Available online: https://hal.science/hal-00902557 [PubMed] [Google Scholar]
- Berge A, Besser T, Moore D and Sischo W, 2009. Evaluation of the effects of oral colostrum supplementation during the first fourteen days on the health and performance of preweaned calves. Journal of Dairy Science, 92, 286–295. 10.3168/jds.2008-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini D, Gerardi G, Peli A, Costa LN, Amadori M and Segato S, 2012. The effects of different environmental conditions on thermoregulation and clinical and hematological variables in long‐distance road‐transported calves. Journal of Animal Science, 90, 1183–1191. 10.2527/jas.2011-4113 [DOI] [PubMed] [Google Scholar]
- Bertelsen M and Jensen MB, 2019. Does dairy calves' motivation for social play behaviour build up over time? Applied Animal Behaviour Science, 214, 18–24. 10.1016/J.APPLANIM.2019.02.017 [DOI] [Google Scholar]
- Bhardwaj R, Randhawa C and Randhawa S, 2010. Incidence of iron deficiency in crossbred cow calves reared on pucca floor. Indian journal of animal sciences, 80, 1037. [Google Scholar]
- Bieber A, Walkenhorst M, Eppenstein R, Probst JK, Thüer S, Baki C, Martin B and Neff AS, 2022. Effects of twice a day teat bucket feeding compared to twice a day mother suckling on behaviour, health traits and blood immune parameters in dairy calves and immune parameters in cow's milk. Applied Animal Behaviour Science, 252, 105644. 10.1016/j.applanim.2022.105644 [DOI] [Google Scholar]
- Bispo E, Monserrat L, González L, Franco D and Moreno T, 2010. Effect of weaning status on animal performance and meat quality of Rubia Gallega calves. Meat Science, 86, 832–838. 10.1016/J.MEATSCI.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biss ME, Hathaway SC and Johnstone AC, 1994. Evaluation of the risk of potential bacteraemia in carcasses from very young slaughter calves with localized navel ill. British Veterinary Journal, 150, 377–384. 10.1016/S0007-1935(05)80154-X [DOI] [PubMed] [Google Scholar]
- Blanchard PC, 2012. Diagnostics of dairy and beef cattle diarrhea. Veterinary Clinics: Food Animal Practice, 28, 443–464. 10.1016/j.cvfa.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blömke L, Volkmann N and Kemper N, 2020. Evaluation of an automated assessment system for ear and tail lesions as animal welfare indicators in pigs at slaughter. Meat Science, 159, 107934. 10.1016/j.meatsci.2019.107934 [DOI] [PubMed] [Google Scholar]
- Boissy A, 1995. Fear and fearfulness in animals., 70, 165–191. 10.1086/418981 [DOI] [PubMed] [Google Scholar]
- Boissy A, Veissier I and Roussel S, 2001. Behavioural reactivity affected by chronic stress: an experimental approach in calves submitted to environmental instability. Animal Welfare, 10, S175–S186. 10.1017/S0962728600023605 [DOI] [Google Scholar]
- Boivin X, Le Neindre P and Chupin J, 1992. Establishment of cattle‐human relationships. Applied Animal Behaviour Science, 32, 325–335. 10.1016/S0168-1591(05)80025-5 [DOI] [Google Scholar]
- Bokkers E and Koene P, 2001. Activity, oral behaviour and slaughter data as welfare indicators in veal calves: a comparison of three housing systems. Applied Animal Behaviour Science, 75, 1–15. 10.1016/S0168-1591(01)00175-7 [DOI] [Google Scholar]
- Bokkers E, Leruste H, Heutinck L, Wolthuis‐Fillerup M, van der Werf JT, Lensink B and Van Reenen C, 2009. Inter‐observer and test‐retest reliability of on‐farm behavioural observations in veal calves. Animal Welfare, 18, 381–390. 10.1017/S0962728600000786 [DOI] [Google Scholar]
- Bolt SL, Boyland NK, Mlynski DT, James R and Croft DP, 2017. Pair housing of dairy calves and age at pairing: effects on weaning stress, health, production and social networks. PLoS One, 12, e0166926. 10.1371/journal.pone.0166926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonicelli L, Trachtman AR, Rosamilia A, Liuzzo G, Hattab J, Alcaraz EM, Del Negro E, Vincenzi S, Dondona AC, Calderara S and Marruchella G, 2021. Training convolutional neural networks to score Pneumonia in Slaughtered Pigs. Animals, 11, 3290–3290. 10.3390/ANI11113290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borderas TF, Rushen J, von Keyserlingk MAG and de Passillé AMB, 2009. Automated measurement of changes in feeding behavior of milk‐fed calves associated with illness. Journal of Dairy Science, 92, 4549–4554. 10.3168/JDS.2009-2109 [DOI] [PubMed] [Google Scholar]
- Bouda J and Jagoš P, 1984. Biochemical and hematological reference values in calves and their significance for health control. Acta Veterinaria Brno, 53, 137–142. 10.2754/avb198453030137 [DOI] [Google Scholar]
- Bouissou MF, Boissy A, Pl Neindre and Veissier I, 2001. Social Behaviour in Farm Animals. 113–145. 10.1079/9780851993973.0113 [DOI] [Google Scholar]
- Boyle LA and Mee JF, 2021. Factors affecting the welfare of unweaned dairy calves destined for early slaughter and abattoir animal‐based indicators reflecting their welfare on‐farm. Frontiers in Veterinary Science, 8, 645537. 10.3389/fvets.2021.645537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I and Dalgarno A, 1973. Iron Metabolism in the veal calf: 2.* Iron requirements and the effect of copper supplementation. British Journal of Nutrition, 30, 61–76. 10.1079/BJN19730008 [DOI] [PubMed] [Google Scholar]
- Brown‐Brandl T, Eigenberg R, Nienaber J and Hahn GL, 2005. Dynamic response indicators of heat stress in shaded and non‐shaded feedlot cattle, Part 1: analyses of indicators. Biosystems Engineering, 90, 451–462. 10.1016/j.biosystemseng.2004.12.006 [DOI] [Google Scholar]
- Brown‐Brandl TM, Eigenberg RA and Nienaber JA, 2006. Heat stress risk factors of feedlot heifers. Livestock Science, 105, 57–68. 10.1016/j.livsci.2006.04.025 [DOI] [Google Scholar]
- Brščić M, Gottardo F, Prevedello P, Tessitore E and Cozzi G, 2009. Veal calves' clinical/health status in large groups fed with automatic feeding devices. Italian Journal of Animal Science, 8, 187–189. [Google Scholar]
- Brščić M, Gottardo F, Leruste H, Lensink J, Van Reenen KCG and Cozzi G, 2011a. Prevalence of locomotory system disorders in veal calves and risk factors for occurrence of Bursitis. Agriculturae Conspectus Scientificus, 76, 291–295. 10.1016/j.biosystemseng.2004.12.006 [DOI] [Google Scholar]
- Brščić M, Heutinck LFM, Wolthuis‐Fillerup M, Stockhofe N, Engel B, Visser EK, Gottardo F, Bokkers EAM, Lensink BJ, Cozzi G and Van Reenen CG, 2011b. Prevalence of gastrointestinal disorders recorded at postmortem inspection in white veal calves and associated risk factors. Journal of Dairy Science, 94, 853–863. 10.3168/JDS.2010-3480 [DOI] [PubMed] [Google Scholar]
- Brščić M, Leruste H, Heutinck LFM, Bokkers EAM, Wolthuis‐Fillerup M, Stockhofe N, Gottardo F, Lensink BJ, Cozzi G and Van Reenen CG, 2012. Prevalence of respiratory disorders in veal calves and potential risk factors. Journal of Dairy Science, 95, 2753–2764. 10.3168/JDS.2011-4699 [DOI] [PubMed] [Google Scholar]
- Brščić M, Prevedello P, Cozzi G, Paparella P and Gottardo F, 2010. Concentration of noxious gases in dairy, beef and veal calves farms in Northern Italy. Acta Agraria Kaposváriensis, 14, 111–116. [Google Scholar]
- Brščić M, Prevedello P, Stefani AL, Cozzi G and Gottardo F, 2014. Effects of the provision of solid feeds enriched with protein or nonprotein nitrogen on veal calf growth, welfare, and slaughter performance. Journal of Dairy Science, 97, 4649–4657. 10.3168/JDS.2013-7618 [DOI] [PubMed] [Google Scholar]
- Brščić M, Ricci R, Prevedello P, Lonardi C, De Nardi R, Contiero B, Gottardo F and Cozzi G, 2015. Synthetic rubber surface as an alternative to concrete to improve welfare and performance of finishing beef cattle reared on fully slatted flooring. Animal, 9, 1386–1392. 10.1017/S1751731115000592 [DOI] [PubMed] [Google Scholar]
- Brünger J, Dippel S, Koch R and Veit C, 2019. ‘Tailception’: using neural networks for assessing tail lesions on pictures of pig carcasses. Animal, 13, 1030–1036. 10.1017/S1751731118003038 [DOI] [PubMed] [Google Scholar]
- Buchli C, Raselli A, Bruckmaier R and Hillmann E, 2017. Contact with cows during the young age increases social competence and lowers the cardiac stress reaction in dairy calves. Applied Animal Behaviour Science, 187, 1–7. 10.1016/J.APPLANIM.2016.12.002 [DOI] [Google Scholar]
- Bučková K, Špinka M and Hintze S, 2019. pair housing makes calves more optimistic. Scientific Report RtS, 9, 20246. 10.1038/s41598-019-56798-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus JD, Stockhofe N and Webb LE, 2019. Invited review: abomasal damage in veal calves. Journal of Dairy Science, 102, 943–960. 10.3168/JDS.2018-15292 [DOI] [PubMed] [Google Scholar]
- Calderón‐amor J and Gallo C, 2020. Dairy calf welfare and factors associated with diarrhea and respiratory disease among chilean dairy farms. Animals, 10, 1115. 10.3390/ANI10071115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo‐Lorenzo MS, Hulbert LE, Fowler AL, Louie A, Gershwin LJ, Pinkerton KE, Ballou MA, Klasing KC and Mitloehner FM, 2016. Wooden hutch space allowance influences male Holstein calf health, performance, daily lying time, and respiratory immunity. Journal of Dairy Science, 99, 4678–4692. 10.3168/JDS.2016-10888 [DOI] [PubMed] [Google Scholar]
- Camiloti TV, Fregonesi JA, von Keyserlingk MAG and Weary DM, 2012. Short communication: effects of bedding quality on the lying behavior of dairy calves. Journal of Dairy Science, 95, 3380–3383. 10.3168/jds.2011-5187 [DOI] [PubMed] [Google Scholar]
- Canali E, Verga M, Montagna M and Baldi A, 1986. Social interactions and induced behavioural reactions in milk‐fed female calves. Applied Animal Behaviour Science, 16, 207–215. 10.1016/0168-1591(86)90114-0 [DOI] [Google Scholar]
- Carroll JA and Forsberg NE, 2007. Influence of stress and nutrition on cattle immunity. Veterinary Clinics of North America: Food Animal Practice, 23, 105–149. 10.1016/J.CVFA.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Caswell JL, Hewson J, Slavić Ð, DeLay J and Bateman K, 2012. Laboratory and postmortem diagnosis of bovine respiratory disease. Veterinary Clinics: Food Animal Practice, 28, 419–441. 10.1016/j.cvfa.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers DT, 1959. Grazing behaviour of calves reared at pasture. The Journal of Agricultural Science, 53, 417–424. 10.1017/S0021859600020840 [DOI] [Google Scholar]
- Chamorro MF, Walz PH, Haines DM, Passler T, Earleywine T, Palomares RA, Riddell KP, Galik P, Zhang Y and Givens MD, 2014. Comparison of levels and duration of detection of antibodies to bovine viral diarrhea virus 1, bovine viral diarrhea virus 2, bovine respiratory syncytial virus, bovine herpesvirus 1, and bovine parainfluenza virus 3 in calves fed maternal colostrum or a colostrum‐replacement product. Canadian Journal of Veterinary Research, 78, 81–88. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3962282/ [PMC free article] [PubMed] [Google Scholar]
- Chanteperdrix M, online. Mesure instrumentale de la couleur de la viande de veau. Available online: https://idele.fr/detail-article/mesure-instrumentale-de-la-couleur-de-la-viande-de-veau
- Chase C and Kaushik RS, 2019. Mucosal immune system of cattle all immune responses begin here. Veterinary Clinics of NA: Food Animal Practice, 35, 431–451. 10.1016/j.cvfa.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YI and Yoon KJ, 2014. An overview of calf diarrhea‐infectious etiology, diagnosis, and intervention. Journal of Veterinary Science, 15, 1–17. 10.4142/jvs.2014.15.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua B, Coenen E, Van Delen J and Weary DM, 2002. Effects of pair versus individual housing on the behavior and performance of dairy calves. Journal of Dairy Science, 85, 360–364. 10.3168/JDS.S0022-0302(02)74082-4 [DOI] [PubMed] [Google Scholar]
- Cobb CJ, Obeidat BS, Sellers MD, Pepper‐Yowell AR and Ballou MA, 2014. Group housing of Holstein calves in a poor indoor environment increases respiratory disease but does not influence performance or leukocyte responses. Journal of Dairy Science, 97, 3099–3109. 10.3168/JDS.2013-7823 [DOI] [PubMed] [Google Scholar]
- Collineau E, Corbière F, Darnal S, Holleville N and Salines M, 2022. Analysis of bovine postmortem condemnation data in France: contributions from a comprehensive and standardised information system at the slaughterhouse. Veterinary Record, 191. 10.1002/VETR.1733 [DOI] [PubMed] [Google Scholar]
- Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls (Text with EEA relevance) . Consolidated text. Available at: EUR‐Lex ‐ 02019R0627‐20230109 ‐ EN ‐ EUR‐Lex (europa.eu)
- Conboy MH, Winder CB, Cantor MC, Costa JHC, Steele MA, Medrano‐Galarza C, von Konigslow TE, Kerr A and Renaud DL, 2022. Associations between feeding behaviors collected from an automated milk feeder and neonatal calf diarrhea in group housed dairy calves: a case‐control study. Animals, 12. 10.3390/ani12020170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N, Bennett T and Nordlund K, 2004. Effect of free stall surface on daily activity patterns in dairy cows with relevance to lameness prevalence. Journal of Dairy Science, 87, 2912–2922. 10.3168/jds.S0022-0302(04)73422-0 [DOI] [PubMed] [Google Scholar]
- Cooper M, Arney D and Phillips C, 2007. Two‐or four‐hour lying deprivation on the behavior of lactating dairy cows. Journal of Dairy Science, 90, 1149–1158. 10.3168/jds.S0022-0302(07)71601-6 [DOI] [PubMed] [Google Scholar]
- Cortese VS, 2009. Neonatal immunology. Veterinary Clinics of North America: Food Animal Practice, 25, 221–227. 10.1016/j.cvfa.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JHC, Daros RR, von Keyserlingk MAG and Weary DM, 2014. Complex social housing reduces food neophobia in dairy calves. Journal of Dairy Science, 97, 7804–7810. 10.3168/JDS.2014-8392 [DOI] [PubMed] [Google Scholar]
- Costa JHC, Meagher RK, von Keyserlingk MAG and Weary DM, 2015. Early pair housing increases solid feed intake and weight gains in dairy calves. Journal of Dairy Science, 98, 6381–6386. 10.3168/JDS.2015-9395 [DOI] [PubMed] [Google Scholar]
- Costa JHC, von Keyserlingk MAG and Weary DM, 2016. Invited review: effects of group housing of dairy calves on behavior, cognition, performance, and health. Journal of Dairy Science, 99, 2453–2467. 10.3168/JDS.2015-10144 [DOI] [PubMed] [Google Scholar]
- Costa JH, Cantor MC, Adderley NA and Neave HW, 2019. Key animal welfare issues in commercially raised dairy calves: social environment, nutrition, and painful procedures. Canadian Journal of Animal Science, 99, 649–660. 10.1139/cjas-2019-0031 [DOI] [Google Scholar]
- Council Directive 2008/119/EC of 18 December 2008 laying down minimum standards for the protection of calves . OJ L 10, 11.1.2009, p. 7. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32008L0119
- Council of Europe Standing Committee , 1998. Recommendation concerning cattle adopted by the Standing Committee on 21 October 1998. Available online: http://www.coe.int/t/e/legal_affairs/legal_co-operation/biological_safety_and_use_of_animals/farming/Rec%20cattle%20E.asp
- Cozzi G, Gottardo F, Mattiello S, Canali E, Scanziani E, Verga M and Andrighetto I, 2002. The provision of solid feeds to veal calves: I. Growth performance, forestomach development, and carcass and meat quality. Journal of Animal Science, 80, 357–366. 10.2527/2002.802357x [DOI] [PubMed] [Google Scholar]
- Cozzi G, Brščić M and Gottardo F, 2009. Main critical factors affecting the welfare of beef cattle and veal calves raised under intensive rearing systems in Italy: a review. Italian Journal of Animal Science, 8, 67–80. 10.4081/ijas.2009.s1.67 [DOI] [Google Scholar]
- Craigie C, Navajas E, Purchas R, Maltin C, Bünger L, Hoskin S, Ross D, Morris S and Roehe R, 2012. A review of the development and use of video image analysis (VIA) for beef carcass evaluation as an alternative to the current EUROP system and other subjective systems. Meat Science, 92, 307–318. 10.1016/j.meatsci.2012.05.028 [DOI] [PubMed] [Google Scholar]
- Crouch C, Oliver S and Francis M, 2001. Serological, colostral and milk responses of cows vaccinated with a single dose of a combined vaccine against rotavirus, coronavirus and Escherichia coli F5 (K99). Veterinary Record, 149, 105–108. 10.1136/vr.149.4.105 [DOI] [PubMed] [Google Scholar]
- Curtis G, Argo CM, Jones D and Grove‐White D, 2016. Impact of feeding and housing systems on disease incidence in dairy calves. Veterinary Record, 179, 512–512. 10.1136/vr.103895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack P, 2004. Effect of mass medication with antibiotics at feedlot entry on the health and growth rate of cattle destined for the Australian domestic market. Australian Veterinary Journal, 82:154–156. do 10.1111/j.1751-0813.2004.tb12644.x [DOI] [PubMed] [Google Scholar]
- Dannenmann K, Buchenauer D and Fliegner H, 1985. The behaviour of calves under four levels of lighting. Applied Animal Behaviour Science, 13, 243–258. 10.1016/0168-1591(85)90048-6 [DOI] [Google Scholar]
- Das SM, Redbo I and Wiktorsson H, 2000. Effect of age of calf on suckling behaviour and other behavioural activities of Zebu and crossbred calves during restricted suckling periods. Applied Animal Behaviour Science, 67, 47–57. 10.1016/S0168-1591(99)00115-X [DOI] [PubMed] [Google Scholar]
- Dawkins MS, 1990. From an animal's point of view: Motivation, fitness, and animal welfare. Behavioral and Brain Sciences, 13, 1–9. 10.1017/S0140525X00077104 [DOI] [Google Scholar]
- De Paula Vieira A, von Keyserlingk MAG and Weary DM, 2010. Effects of pair versus single housing on performance and behavior of dairy calves before and after weaning from milk. Journal of Dairy Science, 93, 3079–3085. 10.3168/JDS.2009-2516 [DOI] [PubMed] [Google Scholar]
- De Passillé AM, 2001. Sucking motivation and related problems in calves. Applied Animal Behaviour Science, 72, 175–187. 10.1016/S0168-1591(01)00108-3 [DOI] [PubMed] [Google Scholar]
- De Passillé A and Caza N, 1997. Cross‐sucking by calves occurs after meals and is reduced when calves suck a dry teat. Journal of Dairy Science, 80, 229. [Google Scholar]
- De Passillé AM and Rushen J, 1997. Motivational and physiological analysis of the causes and consequences of non‐nutritive sucking by calves. Applied Animal Behaviour Science, 53, 15–31. 10.1016/S0168-1591(96)01148-3 [DOI] [Google Scholar]
- de Passillé AM and Rushen J, 2016. Using automated feeders to wean calves fed large amounts of milk according to their ability to eat solid feed. Journal of Dairy Science, 99, 3578–3583. 10.3168/jds.2015-10259 [DOI] [PubMed] [Google Scholar]
- de Passillé AM, Jensen MB, Chapinal N and Rushen J, 2010. Technical note: use of accelerometers to describe gait patterns in dairy calves. Journal of Dairy Science, 93, 3287–3293. 10.3168/JDS.2009-2758 [DOI] [PubMed] [Google Scholar]
- De Passillé AM, Borderas TF and Rushen J, 2011. Weaning age of calves fed a high milk allowance by automated feeders: Effects on feed, water, and energy intake, behavioral signs of hunger, and weight gains. Journal of Dairy Science, 94, 1401–1408. 10.3168/JDS.2010-3441 [DOI] [PubMed] [Google Scholar]
- De Paula Vieira A, Guesdon V, de Passillé AM, von Keyserlingk MAG and Weary DM, 2008. Behavioural indicators of hunger in dairy calves. Applied Animal Behaviour Science, 109, 180–189. 10.1016/J.APPLANIM.2007.03.006 [DOI] [Google Scholar]
- De Paula Vieira A, de Passillé AM and Weary DM, 2012. Effects of the early social environment on behavioral responses of dairy calves to novel events. Journal of Dairy Science, 95, 5149–5155. 10.3168/JDS.2011-5073 [DOI] [PubMed] [Google Scholar]
- de Souza Teixeira O, da Rocha MK, Alforma AMP, Fernandes VS, de Oliveira FJ, Corrêa MN, Canozzi MEA, McManus C and Barcellos JOJ, 2021. Behavioural and physiological responses of male and female beef cattle to weaning at 30, 75 or 180 days of age. Applied Animal Behaviour Science, 240, 105339. 10.1016/j.applanim.2021.105339 [DOI] [Google Scholar]
- De Wilt J, 1985. Behaviour and welfare of veal calves in relation to husbandry systems. Master thesis. Wageningen University and Research. Available online: https://library.wur.nl/WebQuery/wurpubs/fulltext/202839 [Google Scholar]
- Deikun LL, Habing GG, Quigley JD and Proudfoot KL, 2020. Health and growth of veal calves provided a fatty acid supplement and a dry teat. Journal of Dairy Science, 103, 4633–4642. 10.3168/JDS.2019-17240 [DOI] [PubMed] [Google Scholar]
- Dennis TS, Suarez‐Mena FX, Hill TM, Quigley JD, Schlotterbeck RL and Hulbert L, 2018. Effect of milk replacer feeding rate, age at weaning, and method of reducing milk replacer to weaning on digestion, performance, rumination, and activity in dairy calves to 4 months of age. Journal of Dairy Science, 101, 268–278. 10.3168/JDS.2017-13692 [DOI] [PubMed] [Google Scholar]
- Diaz M, Van Amburgh M, Smith J, Kelsey J and Hutten E, 2001. Composition of growth of Holstein calves fed milk replacer from birth to 105‐kilogram body weight. Journal of Dairy Science, 84, 830–842. 10.3168/jds.S0022-0302(01)74541-9 [DOI] [PubMed] [Google Scholar]
- Divers TJ, 2008. Respiratory diseases. Rebhun's Diseases of Dairy Cattle, 79. 10.1016/B978-141603137-6.50007-7 [DOI] [Google Scholar]
- Dorny P, Stoliaroff V, Charlier J, Meas S, Sorn S, Chea B, Holl D, Van Aken D and Vercruysse J, 2011. Infections with gastrointestinal nematodes, Fasciola and Paramphistomum in cattle in Cambodia and their association with morbidity parameters. Veterinary Parasitology, 175, 293–299. 10.1016/j.vetpar.2010.10.023 [DOI] [PubMed] [Google Scholar]
- Downey BC, Jensen MB and Tucker CB, 2022. Hay provision affects 24‐h performance of normal and abnormal oral behaviors in individually housed dairy calves. Journal of Dairy Science, 105, 4434–4448. 10.3168/jds.2021-21439 [DOI] [PubMed] [Google Scholar]
- Drackley JK, 2008. Calf nutrition from birth to breeding. Veterinary Clinics of North America: Food Animal Practice, 24, 55–86. 10.1016/J.CVFA.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Drissler M, Gaworski M, Tucker C and Weary D, 2005. Freestall maintenance: effects on lying behavior of dairy cattle. Journal of Dairy Science, 88, 2381–2387. 10.3168/jds.S0022-0302(05)72916-7 [DOI] [PubMed] [Google Scholar]
- Duve LR and Jensen MB, 2011. The level of social contact affects social behaviour in pre‐weaned dairy calves. Applied Animal Behaviour Science, 135, 34–43. 10.1016/J.APPLANIM.2011.08.014 [DOI] [Google Scholar]
- Duve LR and Jensen MB, 2012. Social behavior of young dairy calves housed with limited or full social contact with a peer. Journal of Dairy Science, 95, 5936–5945. 10.3168/JDS.2012-5428 [DOI] [PubMed] [Google Scholar]
- Duve LR, Weary DM, Halekoh U and Jensen MB, 2012. The effects of social contact and milk allowance on responses to handling, play, and social behavior in young dairy calves. Journal of Dairy Science, 95, 6571–6581. 10.3168/JDS.2011-5170 [DOI] [PubMed] [Google Scholar]
- Earley B, Murray M, Farrell J and Nolan M, 2004. Rearing calves outdoors with and without calf jackets compared with indoor housing on calf health and live‐weight performance. Irish Journal of Agricultural and Food Research, 59–67. Available online:. https://www.jstor.org/stable/25562505 [Google Scholar]
- Edwards S, 1983. The behaviour of dairy cows and their newborn calves in individual or group housing. Applied Animal Ethology, 10, 191–198. 10.1016/0304-3762(83)90140-2 [DOI] [Google Scholar]
- Edwards SA and Broom DM, 1982. Behavioural interactions of dairy cows with their newborn calves and the effects of parity. Animal Behaviour, 30, 525–535. 10.1016/S0003-3472(82)80065-1 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Hart A, Maxim L, Siegrist M, Goetz V, da Cruz C, Merten C, Mosbach‐Schulz O, Lahaniatis M, Smith A and Hardy A, 2019. Guidance on communication of uncertainty in scientific assessments. EFSA Journal 2019;17(1):5520, 73 pp. 10.2903/j.efsa.2019.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Network (EFSA Animal Health and Welfare Network) , 2021. The use of animal‐based measures at slaughter for assessing the welfare of calves on farm: EFSA's AHAW Network exercise. EFSA Supporting publication 2021;EN‐7042, 18 pp. 10.2903/SP.EFSA.2021.EN-7042 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Animal Welfare) , 2009. Scientific Opinion on the overall effects of farming systems on dairy cow welfare and disease. EFSA Journal 2009;7(7):1143, 38 pp. 10.2903/J.EFSA.2009.1143 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2012. Scientific Opinion on the welfare of cattle kept for beef production and the welfare in intensive calf farming systems. EFSA Journal 2012;10(5):2669, 45 pp. 10.2903/j.efsa.2012.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2020. Welfare of cattle at slaughter. EFSA Journal 2020;18(11):6275, 55 pp. 10.2903/j.efsa.2020.6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2022a. Methodological guidance for the development of animal welfare mandates in the context of the Farm to Fork Strategy. EFSA Journal 2022;20(7):7403, 29 pp. 10.2903/j.efsa.2022.7403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2022b. Welfare of cattle during transport. EFSA Journal 2022;20(9):7442, 121 pp. 10.2903/j.efsa.2022.7442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2005. Opinion of the Scientific Panel on biological hazards (BIOHAZ) on Revision of Meat Inspection for Beef raised in Integrated Production Systems. EFSA Journal, 2005;3(9):141, 56 pp. 10.2903/j.efsa.2005.141 [DOI] [Google Scholar]
- Egli C and Blum J, 1998. Clinical, haematological, metabolic and endocrine traits during the first three months of life of suckling simmentaler calves held in a cow‐calf operation. Journal of Veterinary Medicine Series A, 45, 99–118. 10.1111/j.1439-0442.1998.tb00806.x [DOI] [PubMed] [Google Scholar]
- Eigenberg RA, Hahn GL, Nienaber JA, Brown‐Brandl TM, Spiers DE and Eigenberg R, 2000. Development of a new respiration rate monitor for cattle. Transactions of the ASAE, 43, 723–728. 10.13031/2013.2755 [DOI] [Google Scholar]
- Ellingsen K, Mejdell CM, Ottesen N, Larsen S and Grøndahl AM, 2016. The effect of large milk meals on digestive physiology and behaviour in dairy calves. Physiology and Behavior, 154, 169–174. 10.1016/j.physbeh.2015.11.025 [DOI] [PubMed] [Google Scholar]
- Engelken TJ, 2020. How does housing influence bovine respiratory disease in confinement cow‐calf operations? Veterinary Clinics: Food Animal Practice, 36, 375–383. 10.1016/j.cvfa.2020.03.011 [DOI] [PubMed] [Google Scholar]
- Enríquez DH, Ungerfeld R, Quintans G, Guidoni AL and Hötzel MJ, 2010. The effects of alternative weaning methods on behaviour in beef calves. Livestock Science, 128, 20–27. 10.1016/J.LIVSCI.2009.10.007 [DOI] [Google Scholar]
- Eriksson H, Fall N, Ivemeyer S, Knierim U, Simantke C, Fuerst‐Waltl B, Winckler C, Weissensteiner R, Pomiès D, Martin B, Michaud A, Priolo A, Caccamo M, Sakowski T, Stachelek M, Spengler Neff A, Bieber A, Schneider C and Alvåsen K, 2022. Strategies for keeping dairy cows and calves together – a cross‐sectional survey study. Animal, 16, 100624. 10.1016/J.ANIMAL.2022.100624 [DOI] [PubMed] [Google Scholar]
- EUROSTAT , 2022. Organic livestock. Available online: https://ec.europa.eu/eurostat/databrowser/view/org_lstspec/default/table?lang=en [Accessed: 29 April 2022].
- Færevik G, Jensen MB and Bøe KE, 2006. Dairy calves social preferences and the significance of a companion animal during separation from the group. Applied Animal Behaviour Science, 99, 205–221. 10.1016/J.APPLANIM.2005.10.012 [DOI] [Google Scholar]
- Færevik G, Andersen IL, Jensen MB and Bøe KE, 2007. Increased group size reduces conflicts and strengthens the preference for familiar group mates after regrouping of weaned dairy calves (Bos taurus). Applied Animal Behaviour Science, 108, 215–228. 10.1016/j.applanim.2007.01.010 [DOI] [Google Scholar]
- Færevik G, Tjentland K, Løvik S, Andersen IL and Bøe KE, 2008. Resting pattern and social behaviour of dairy calves housed in pens with different sized lying areas. Applied Animal Behaviour Science, 114, 54–64. 10.1016/j.applanim.2008.01.002 [DOI] [Google Scholar]
- Færevik G, Jensen M and Bøe K, 2010. The effect of group composition and age on social behavior and competition in groups of weaned dairy calves. Journal of Dairy Science, 93, 4274–4279. 10.3168/jds.2010-3147 [DOI] [PubMed] [Google Scholar]
- Faulkner PM and Weary DM, 2000. Reducing pain after dehorning in dairy calves. Journal of Dairy Science, 83, 2037–2041. 10.3168/JDS.S0022-0302(00)75084-3 [DOI] [PubMed] [Google Scholar]
- FAWC (Farm Animal Welfare Committee) , 2015. Opinion on the welfare implications of nutritional management strategies for artificially‐reared calves from birth to weaning: Farm Animal Welfare Committee. Available online: Farm Animal Welfare Committee (FAWC) opinion on calf nutrition ‐ GOV.UK (www.gov.uk)
- Fecteau G, Baillargeon P, Higgins R, Paré J and Fortin M, 2002. Bacterial contamination of colostrum fed to newborn calves in Québec dairy herds. The Canadian Veterinary Journal, 43, 523–527. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC341940/ [PMC free article] [PubMed] [Google Scholar]
- Fernández M, Ferreras MDC, Giráldez FJ, Benavides J and Pérez V, 2020. Production significance of bovine respiratory disease lesions in slaughtered beef cattle. Animals, 10, 1770. 10.3390/ANI10101770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower FC and Weary DM, 2001. Effects of early separation on the dairy cow and calf: 2. Separation at 1 day and 2 weeks after birth. Applied Animal Behaviour Science, 70, 275–284. 10.1016/S0168-1591(00)00164-7 [DOI] [PubMed] [Google Scholar]
- Flower FC and Weary DM, 2003. The effects of early separation on the dairy cow and calf. Animal Welfare, 12, 339–348. 10.1017/S0962728600025847 [DOI] [PubMed] [Google Scholar]
- Foster D and Smith GW, 2009. Pathophysiology of diarrhea in calves. Veterinary Clinics of North America: Food Animal Practice, 25, 13–36. 10.1016/j.cvfa.2008.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank NA and Kaneene JB, 1993. Management risk factors associated with calf diarrhea in Michigan dairy herds. Journal of Dairy Science, 76, 1313–1323. 10.3168/jds.S0022-0302(93)77462-7 [DOI] [PubMed] [Google Scholar]
- Frank GH, Briggs RE, Loan RW, Purdy CW and Zehr ES, 2000. Effects of tilmicosin treatment on Pasteurella haemolytica organisms in nasal secretion specimens of calves with respiratory tract disease. American Journal of Veterinary Research, 61, 525–529. 10.2460/ajvr.2000.61.525 [DOI] [PubMed] [Google Scholar]
- Fröberg S and Lidfors L, 2009. Behaviour of dairy calves suckling the dam in a barn with automatic milking or being fed milk substitute from an automatic feeder in a group pen. Applied Animal Behaviour Science, 117, 150–158. 10.1016/J.APPLANIM.2008.12.015 [DOI] [Google Scholar]
- Fröberg S, Aspegren‐Güldorff A, Olsson I, Marin B, Berg C, Hernández C, Galina CS, Lidfors L and Svennersten‐Sjaunja K, 2007. Effect of restricted suckling on milk yield, milk composition and udder health in cows and behaviour and weight gain in calves, in dual‐purpose cattle in the tropics. Tropical Animal Health and Production, 39, 71–81. 10.1007/S11250-006-4418-0 [DOI] [PubMed] [Google Scholar]
- Fröberg S, Gratte E, Svennersten‐Sjaunja K, Olsson I, Berg C, Orihuela A, Galina CS, García B and Lidfors L, 2008. Effect of suckling (‘restricted suckling’) on dairy cows' udder health and milk let‐down and their calves' weight gain, feed intake and behaviour. Applied Animal Behaviour Science, 113, 1–14. 10.1016/J.APPLANIM.2007.12.001 [DOI] [Google Scholar]
- Fujiwara M, Rushen J and de Passillé AM, 2014. Dairy calves' adaptation to group housing with automated feeders. Applied Animal Behaviour Science, 158, 1–7. 10.1016/J.APPLANIM.2014.06.011 [DOI] [Google Scholar]
- Fulton RW, Briggs RE, Payton ME, Confer AW, Saliki JT, Ridpath JF, Burge LJ and Duff GC, 2004. Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1b, BVDV2, bovine herpesvirus‐1, parainfluenza‐3 virus bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half‐life studies and effect on response to vaccination. Vaccine, 22, 643–649. 10.1016/j.vaccine.2003.08.033 [DOI] [PubMed] [Google Scholar]
- Gaillard C, Meagher RK, von Keyserlingk MA and Weary DM, 2014. Social housing improves dairy calves' performance in two cognitive tests. PLoS ONE, 9, e90205. 10.1371/journal.pone.0090205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughan J, Mader T, Holt S, Hahn G and Young B, 2002. Review of current assessment of cattle and microclimate during periods of high heat load. Animal Production in Australian, 24, 77–80. 10.2527/2002.8092373x [DOI] [Google Scholar]
- Gaughan JB, Mader TL, Holt SM and Lisle A, 2008. A new heat load index for feedlot cattle. Journal of Animal Science, 86, 226–234. 10.2527/jas.2007-0305 [DOI] [PubMed] [Google Scholar]
- Gebremedhin KG, Hillman PE, Lee CN, Collier RJ, Willard ST, Arthington JD and Brown‐Brandl TM, 2008. Sweating rates of dairy cows and beef heifers in hot conditions. Transactions of the ASABE, 51, 2167–2178. 10.13031/2013.25397 [DOI] [Google Scholar]
- Gelsinger SL, Coblentz WK, Zanton GI, Ogden RK and Akins MS, 2020. Physiological effects of starter‐induced ruminal acidosis in calves before, during, and after weaning. Journal of Dairy Science, 103, 2762–2772. 10.3168/jds.2019-17494 [DOI] [PubMed] [Google Scholar]
- Girolami A, Napolitano F, Faraone D and Braghieri A, 2013. Measurement of meat color using a computer vision system. Meat Science, 93, 111–118. 10.1016/J.MEATSCI.2012.08.010 [DOI] [PubMed] [Google Scholar]
- Gitter M and Austwick P, 1957. The presence of fungi in abomasal ulcers of young calves. A report of seven cases. Veterinary Record, 69. Available online:. https://www.cabdirect.org/cabdirect/abstract/19601300289 [Google Scholar]
- Godden SM, Lombard JE and Woolums AR, 2019. Colostrum management for dairy calves. Veterinary Clinics: Food Animal Practice, 35, 535–556. 10.1016/j.cvfa.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden PJ and Plummer P, 2010. Control, management, and prevention of bovine respiratory disease in dairy calves and cows. Veterinary Clinics: Food Animal Practice, 26, 243–259. 10.1016/j.cvfa.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozho GN, Plaizier JC, Krause DO, Kennedy AD and Wittenberg KM, 2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. Journal of Dairy Science, 88, 1399–1403. 10.3168/JDS.S0022-0302(05)72807-1 [DOI] [PubMed] [Google Scholar]
- Graca E, 2016. Erhebung zum gegenseitigen Besaugen bei Kälbern der Rasse Fleckvieh in Österreich anhand von Telefonbefragungen. University of Veterinary Medicine, Vienna. 119 pp. [Google Scholar]
- Grace D, Himstedt H, Sidibe I, Randolph T and Clausen P‐H, 2007. Comparing FAMACHA© eye color chart and Hemoglobin Color Scale tests for detecting anemia and improving treatment of bovine trypanosomosis in West Africa. Veterinary Parasitology, 147, 26–39. 10.1016/j.vetpar.2007.03.022 [DOI] [PubMed] [Google Scholar]
- Graf B and Senn M, 1999. Behavioural and physiological responses of calves to dehorning by heat cauterization with or without local anaesthesia. Applied Animal Behaviour Science, 62, 153–171. 10.1016/S0168-1591(98)00218-4 [DOI] [Google Scholar]
- Grandin T, 2014. Animal welfare and society concerns finding the missing link. Meat Science, 98, 461–469. 10.1016/j.meatsci.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Green AC, Lidfors LM, Lomax S, Favaro L and Clark CE, 2021. Vocal production in postpartum dairy cows: Temporal organization and association with maternal and stress behaviors. Journal of Dairy Science, 104, 826–838. 10.3168/jds.2020-18891 [DOI] [PubMed] [Google Scholar]
- Griebel P, Hill K and Stookey J, 2014. How stress alters immune responses during respiratory infection. Animal Health Research Reviews, 15, 161–165. 10.1017/S1466252314000280 [DOI] [PubMed] [Google Scholar]
- Größbacher V, Winckler C and Leeb C, 2018. On‐farm factors associated with cross‐sucking in group‐housed organic Simmental dairy calves. Applied Animal Behaviour Science, 206, 18–24. 10.1016/j.applanim.2018.05.030 [DOI] [Google Scholar]
- Größbacher V, Bučková K, Lawrence AB, Špinka M and Winckler C, 2020. Discriminating spontaneous locomotor play of dairy calves using accelerometers. Journal of Dairy Science, 103, 1866–1873. 10.3168/JDS.2019-17005 [DOI] [PubMed] [Google Scholar]
- Guarnieri E, Fecteau G, Berman J, Desrochers A, Babkine M, Nichols S and Francoz D, 2020. Abomasitis in calves: A retrospective cohort study of 23 cases (2006–2016). Journal of veterinary internal medicine, 34, 1018–1027. 10.1111/jvim.15726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugatschka M, 2008. Erhebung zur Haltung, Gesundheit und Verhaltensproblemen von Kälbern anhand von Fragebögen. University of Veterinary Medicine Vienna. 122 p.. [Google Scholar]
- Guizzardi F, Vanini G, Bellocchio F, Costa A, Comini M, Guizzardi S and Canali E, 2007. Effetto di tre diversi regimi alimentari sull'apparato digestivo di vitelli a carne bianca: rilievi alla macellazione. Large Anim. Rev, 13, 207–210. Available online: https://vetjournal.it/images/archive/pdf_riviste/4087.pdf [Google Scholar]
- Gulliksen S, Jor E, Lie K, Hamnes I, Løken T, Åkerstedt J and Østerås O, 2009a. Enteropathogens and risk factors for diarrhea in Norwegian dairy calves. Journal of Dairy Science, 92, 5057–5066. 10.3168/jds.2009-2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliksen SM, Lie KI, Løken T and Østerås O, 2009b. Calf mortality in Norwegian dairy herds. Journal of Dairy Science, 92, 2782–2795. 10.3168/JDS.2008-1807 [DOI] [PubMed] [Google Scholar]
- Gygax M, Hirni H, Wahlen R, Lazary S and Blum J, 1993. Immune functions of veal calves fed low amounts of iron. Journal of Veterinary Medicine Series A, 40, 345–358. 10.1111/j.1439-0442.1993.tb00638.x [DOI] [PubMed] [Google Scholar]
- Gygax L, Siegwart R and Wechsler B, 2007. Effects of space allowance on the behaviour and cleanliness of finishing bulls kept in pens with fully slatted rubber coated flooring. Applied Animal Behaviour Science, 107, 1–12. 10.1016/J.APPLANIM.2006.09.011 [DOI] [Google Scholar]
- Gygax L, Neuffer I, Kaufmann C, Hauser R and Wechsler B, 2008. Restlessness behaviour, heart rate and heart‐rate variability of dairy cows milked in two types of automatic milking systems and auto‐tandem milking parlours. Applied Animal Behaviour Science, 109, 167–179. 10.1016/j.applanim.2007.03.010 [DOI] [Google Scholar]
- Halachmi I, Klopčič M, Polak P, Roberts D and Bewley J, 2013. Automatic assessment of dairy cattle body condition score using thermal imaging. Computers and electronics in agriculture, 99, 35–40. 10.1016/j.compag.2013.08.012 [DOI] [Google Scholar]
- Hammon H, Liermann W, Frieten D and Koch C, 2020. Importance of colostrum supply and milk feeding intensity on gastrointestinal and systemic development in calves. Animal, 14, s133–s143. 10.1017/S1751731119003148 [DOI] [PubMed] [Google Scholar]
- Hänninen L, De Passillé AM and Rushen J, 2005. The effect of flooring type and social grouping on the rest and growth of dairy calves. Applied Animal Behaviour Science, 91, 193–204. 10.1016/j.applanim.2004.10.003 [DOI] [Google Scholar]
- Hänninen L, Hepola H, Raussi S and Saloniemi H, 2008. Effect of colostrum feeding method and presence of dam on the sleep, rest and sucking behaviour of newborn calves. Applied Animal Behaviour Science, 112, 213–222. 10.1016/j.applanim.2007.09.003 [DOI] [Google Scholar]
- Harris PA, Ellis AD, Fradinho MJ, Jansson A, Julliand V, Luthersson N, Santos AS and Vervuert I, 2017. Review: feeding conserved forage to horses: recent advances and recommendations. Animal, 11, 958–967. 10.1017/S1751731116002469 [DOI] [PubMed] [Google Scholar]
- Hayer JJ, Nysar D, Heinemann C, Leubner CD and Steinhoff‐Wagner J, 2021. Implementation of management recommendations in unweaned dairy calves in western Germany and associated challenges. Journal of Dairy Science, 104, 7039–7055. 10.3168/jds.2020-19829 [DOI] [PubMed] [Google Scholar]
- Heidarpour Bami M, Mohri M, Seifi HA and Alavi Tabatabaee A, 2008. Effects of parenteral supply of iron and copper on hematology, weight gain, and health in neonatal dairy calves. Veterinary Research Communications, 32, 553–561. 10.1007/s11259-008-9058-6 [DOI] [PubMed] [Google Scholar]
- Held SD and Špinka M, 2011. Animal play and animal welfare. Animal Behaviour, 81, 891–899. 10.1016/j.anbehav.2011.01.007 [DOI] [Google Scholar]
- Hemsworth P and Coleman G, 2011. Human‐animal interactions and animal productivity and welfare. Human‐livestock interactions: The stockperson and the productivity and welfare of intensively farmed animals. pp. 47–83. 10.1079/9781845936730.0047 [DOI] [Google Scholar]
- Hepola H, 2003. Milk feeding systems for dairy calves in groups: effects on feed intake, growth and health. Applied Animal Behaviour Science, 80, 233–243. 10.1016/S0168-1591(02)00214-9 [DOI] [Google Scholar]
- Hepola H, Hänninen L, Pursiainen P, Tuure VM, Syrjälä‐Qvist L, Pyykkönen M and Saloniemi H, 2006. Feed intake and oral behaviour of dairy calves housed individually or in groups in warm or cold buildings. Livestock Science, 105, 94–104. 10.1016/J.LIVSCI.2006.04.033 [DOI] [Google Scholar]
- Hepola H, Hänninen L, Raussi S, Pursiainen P, Aarnikoivu A‐M and Saloniemi H, 2008. Effects of providing water from a bucket or a nipple on the performance and behavior of calves fed ad libitum volumes of acidified milk replacer. Journal of Dairy Science, 91, 1486–1496. 10.3168/jds.2007-0500 [DOI] [PubMed] [Google Scholar]
- Herskin MS, Skjøth F and Jensen MB, 2010. Effects of hunger level and tube diameter on the feeding behavior of teat‐fed dairy calves. Journal of Dairy Science, 93, 2053–2059. 10.3168/JDS.2009-2554 [DOI] [PubMed] [Google Scholar]
- Hillman P, Gebremedhin K and Warner R, 1992. Ventilation system to minimize airborne bacteria, dust, humidity, and ammonia in calf nurseries. Journal of Dairy Science, 75, 1305–1312. 10.3168/jds.S0022-0302(92)77881-3 [DOI] [PubMed] [Google Scholar]
- Hillmann E, Bruckmaier R and Buchli C (Newberry RC and Braastad BO), 2019. Animal lives worth living: Proceedings of the 53rd Congress of the International Society for Applied Ethology, Wageningen Academic Publishers. Available online: https://www.applied-ethology.org/res/ISAE%202019%20Congress%20proceedings.pdf#page=333 [Google Scholar]
- Hindhede J, Mogensen L and Sørensen JT, 1999. Effect of group composition and feeding system on behaviour, production and health of dairy heifers in deep bedding systems. Acta Agriculturae Scandinavica, Section A‐Animal Science, 49, 211–220. 10.1080/090647099423962 [DOI] [Google Scholar]
- Hodgson PD, Aich P, Manuja A, Hokamp K, Roche FM, Brinkman FSL, Potter A, Babiuk LA and Griebel PJ, 2005. Effect of stress on viral–bacterial synergy in bovine respiratory disease: novel mechanisms to regulate inflammation. Comparative and Functional Genomics, 6, 244–250. 10.1002/CFG.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson PD, Aich P, Stookey J, Popowych Y, Potter A, Babiuk L and Griebel PJ, 2012. Stress significantly increases mortality following a secondary bacterial respiratory infection. Veterinary Research, 43, 21–21. 10.1186/1297-9716-43-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschbach CL and Peek SF, 2018. Salmonella in dairy cattle. Veterinary Clinics: Food Animal Practice, 34, 133–154. 10.1016/j.cvfa.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath K, Allen A and Miller‐Cushon E, 2020. Effects of access to stationary brushes and chopped hay on behavior and performance of individually housed dairy calves. Journal of Dairy Science, 103, 8421–8432. 10.3168/jds.2019-18042 [DOI] [PubMed] [Google Scholar]
- Hosey G and Melfi V, 2018. Anthrozoology: human‐animal interactions in domesticated and wild animals. Oxford University Press. 10.1093/oso/9780198753629.001.0001 [DOI] [Google Scholar]
- Hostettler‐Allen R, Tappy L and Blum JW, 1993. Enhanced insulin‐dependent glucose utilization in iron‐deficient veal calves. The Journal of Nutrition, 123, 1656–1667. 10.1093/JN/123.10.1656 [DOI] [PubMed] [Google Scholar]
- House JK, Ontiveros MM, Blackmer NM, Dueger EL, Fitchhorn JB, McArthur GR and Smith BP, 2001. Evaluation of an autogenous Salmonella bacterin and a modified live Salmonella serotype Choleraesuis vaccine on a commercial dairy farm. American Journal of Veterinary Research, 62, 1897–1902. 10.2460/ajvr.2001.62.1897 [DOI] [PubMed] [Google Scholar]
- Hubbard K, Stooksbury D, Hahn G and Mader T, 1999. A climatological perspective on feedlot cattle performance and mortality related to the temperature‐humidity index. Journal of Production Agriculture, 12, 650–653. 10.2134/jpa1999.0650 [DOI] [Google Scholar]
- Hulbert LE and Ballou MA, 2012. Innate immune responses and health of individually reared Holstein calves after placement into transition‐pens 23 d after weaning. Journal of Dairy Research, 79, 333–340. 10.1017/S0022029912000271 [DOI] [PubMed] [Google Scholar]
- Hulbert LE and Moisá SJ, 2016. Stress, immunity, and the management of calves. Journal of Dairy Science, 99, 3199–3216. 10.3168/JDS.2015-10198 [DOI] [PubMed] [Google Scholar]
- Hulbert LE, Calvo‐Lorenzo MS, Ballou MA, Klasing KC and Mitloehner FM, 2019. Space allowance influences individually housed Holstein male calves' age at feed consumption, standing behaviors, and measures of immune resilience before and after step‐down weaning. Journal of Dairy Science, 102, 4506–4521. 10.3168/JDS.2018-15368 [DOI] [PubMed] [Google Scholar]
- Hulsegge B, Engel B, Buist W, Merkus G and Klont R, 2001. Instrumental colour classification of veal carcasses. Meat Science, 57, 191–195. 10.1016/S0309-1740(00)00093-0 [DOI] [PubMed] [Google Scholar]
- Hund A, Beer T and Wittek T, 2016. Abomasal ulcers in slaughtered cattle in Austria. Tierarztliche Praxis. Ausgabe G, Grosstiere/nutztiere, 44, 279–285. 10.15653/TPG-150800 [DOI] [PubMed] [Google Scholar]
- Hunt CD and Nielsen FH, 2009. Nutritional aspects of minerals in bovine and human milks. Advanced Dairy Chemistry, 3, 391–456. 10.1007/978-0-387-84865-5_10 [DOI] [Google Scholar]
- Hutchison H, Woof R, Mabon R, Salehe I and Robb J, 1962. A study of the habits of zebu cattle in Tanganyika. The Journal of Agricultural Science, 59, 301–317. 10.1017/S0021859600015379 [DOI] [Google Scholar]
- Hyde RM, Green MJ, Hudson C and Down PM, 2021. Factors associated with daily weight gain in preweaned calves on dairy farms. Preventive Veterinary Medicine, 190, 105320. 10.1016/j.prevetmed.2021.105320 [DOI] [PubMed] [Google Scholar]
- Idris M, Uddin J, Sullivan M, McNeill DM and Phillips CJ, 2021. Non‐invasive physiological indicators of heat stress in cattle. Animals, 11, 71. 10.3390/ani11010071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis IR, Langton S, Forkman B and Lazarus J, 2001. An information primacy model of exploratory and foraging behaviour. Animal Behaviour, 62, 543–557. 10.1006/ANBE.2001.1780 [DOI] [Google Scholar]
- INRA , 2018. Alimentation des Ruminants. Editions Quae, Versailles, France, 728 p. ISBN 978–2–27592‐2867‐6.
- Islam MA, Lomax S, Doughty AK, Islam MR and Clark CE, 2020. Automated monitoring of panting for feedlot cattle: sensor system accuracy and individual variability. Animals, 10, 1518. 10.3390/ani10091518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahani‐Moghadam M, Mahjoubi E, Hossein Yazdi M, Cardoso FC and Drackley JK, 2015. Effects of alfalfa hay and its physical form (chopped versus pelleted) on performance of Holstein calves. Journal of Dairy Science, 98, 4055–4061. 10.3168/JDS.2014-9126 [DOI] [PubMed] [Google Scholar]
- Jelinski MD, Ribble CS, Chirino‐Trejo M, Clark EG and Janzen ED, 1995. The relationship between the presence of Helicobacter pylori, Clostridium perfringens type A, Campylobacter spp, or fungi and fatal abomasal ulcers in unweaned beef calves. The Canadian Veterinary Journal, 36, 379–382. Available online:. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1686945/ [PMC free article] [PubMed] [Google Scholar]
- Jensen MB, 1999. Effects of confinement on rebounds of locomotor behaviour of calves and heifers, and the spatial preferences of calves. Applied Animal Behaviour Science, 62, 43–56. 10.1016/S0168-1591(98)00208-1 [DOI] [Google Scholar]
- Jensen MB, 2001. A note on the effect of isolation during testing and length of previous confinement on locomotor behaviour during open‐field test in dairy calves. Applied Animal Behaviour Science, 70, 309–315. 10.1016/S0168-1591(00)00162-3 [DOI] [PubMed] [Google Scholar]
- Jensen MB, 2004. Computer‐controlled milk feeding of dairy calves: the effects of number of calves per feeder and number of milk portions on use of feeder and social behavior. Journal of Dairy Science, 87, 3428–3438. 10.3168/JDS.S0022-0302(04)73478-5 [DOI] [PubMed] [Google Scholar]
- Jensen MB, 2007. Age at introduction to the group affects dairy calves' use of a computer‐controlled milk feeder. Applied Animal Behaviour Science, 107, 22–31. 10.1016/j.applanim.2006.09.017 [DOI] [Google Scholar]
- Jensen M, 2009. Milk meal pattern of dairy calves is affected by computer‐controlled milk feeder set‐up. Journal of Dairy Science, 92, 2906–2910. 10.3168/jds.2008-1748 [DOI] [PubMed] [Google Scholar]
- Jensen MB, 2011. The early behaviour of cow and calf in an individual calving pen. Applied Animal Behaviour Science, 134, 92–99. 10.1016/J.APPLANIM.2011.06.017 [DOI] [Google Scholar]
- Jensen MB, 2018. The role of social behavior in cattle welfare. Advances in Cattle Welfare, 123–155. 10.1016/B978-0-08-100938-3.00006-1 [DOI] [Google Scholar]
- Jensen M and Budde M, 2006. The effects of milk feeding method and group size on feeding behavior and cross‐sucking in group‐housed dairy calves. Journal of Dairy Science, 89, 4778–4783. 10.3168/jds.S0022-0302(06)72527-9 [DOI] [PubMed] [Google Scholar]
- Jensen MB and Holm L, 2003. The effect of milk flow rate and milk allowance on feeding related behaviour in dairy calves fed by computer controlled milk feeders. Applied Animal Behaviour Science, 82, 87–100. 10.1016/S0168-1591(03)00054-6 [DOI] [Google Scholar]
- Jensen MB and Kyhn R, 2000. Play behaviour in group‐housed dairy calves, the effect of space allowance. Applied Animal Behaviour Science, 67, 35–46. 10.1016/S0168-1591(99)00113-6 [DOI] [PubMed] [Google Scholar]
- Jensen MB and Larsen LE, 2013. The role of social behaviour in cattle welfare. Advances in cattle welfare. Woodhead Publishing. 133 p.. [Google Scholar]
- Jensen MB and Larsen LE, 2014. Effects of level of social contact on dairy calf behavior and health. Journal of Dairy Science, 97, 5035–5044. 10.3168/JDS.2013-7311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MB and Tolstrup RB, 2021. A survey on management and housing of peri‐parturient dairy cows and their calves. Animal, 15, 100388. 10.1016/j.animal.2021.100388 [DOI] [PubMed] [Google Scholar]
- Jensen MB and Vestergaard M, 2021. Invited review: freedom from thirst—do dairy cows and calves have sufficient access to drinking water? Journal of Dairy Science, 104, 11368–11385. 10.3168/jds.2021-20487 [DOI] [PubMed] [Google Scholar]
- Jensen H, Olsen S and Aalbaek B, 1994. Gastrointestinal aspergillosis and zygomycosis of cattle. Veterinary Pathology, 31, 28–36. 10.1177/030098589403100104 [DOI] [PubMed] [Google Scholar]
- Jensen MB, Vestergaard KS, Krohn CC and Munksgaard L, 1997. Effect of single versus group housing and space allowance on responses of calves during open‐field tests. Applied Animal Behaviour Science, 54, 109–121. 10.1016/S0168-1591(96)01183-5 [DOI] [Google Scholar]
- Jensen MB, Vestergaard KS and Krohn CC, 1998. Play behaviour in dairy calves kept in pens: the effect of social contact and space allowance. Applied Animal Behaviour Science, 56, 97–108. 10.1016/S0168-1591(97)00106-8 [DOI] [Google Scholar]
- Jensen MB, De Passillé AM, Von Keyserlingk MAG and Rushen J, 2008. A barrier can reduce competition over teats in pair‐housed milk‐fed calves. Journal of Dairy Science, 91, 1607–1613. 10.3168/JDS.2007-0623 [DOI] [PubMed] [Google Scholar]
- Jensen MB, Duve LR and Weary DM, 2015. Pair housing and enhanced milk allowance increase play behavior and improve performance in dairy calves. Journal of Dairy Science, 98, 2568–2575. 10.3168/JDS.2014-8272 [DOI] [PubMed] [Google Scholar]
- Ježek J, Starič J, Nemec M, Tomaž Z and Klinkon M, 2009. Relationship between blood haemoglobin and serum iron concentrations and heart girth in pre‐weaned dairy calves. Italian Journal of Animal Science, 8, 151–153. 10.4081/ijas.2009.s3.151 [DOI] [Google Scholar]
- Joerling J and Doll K, 2019. Monitoring of iron deficiency in calves by determination of serum ferritin in comparison with serum iron: a preliminary study. Open Veterinary Journal, 9, 177–184. 10.4314/ovj.v9i2.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns J, Wagner K, Waiblinger S, Barth K and Hillmann E, 2011. Hat das Saugen bei der Mutter im Vergleich zum Saugen am Tränkeautomaten für Kälber eine beruhigende Wirkung? Aktuelle Arbeiten zur artgemäßen Tierhaltung 2011: 43. Tagung Angewandte Ehologie bei Nutztieren der DVG, 489, 88–97. [Google Scholar]
- Johnsen JF, Ellingsen K, Grøndahl AM, Bøe KE, Lidfors L and Mejdell CM, 2015. The effect of physical contact between dairy cows and calves during separation on their post‐separation behavioural response. Applied Animal Behaviour Science, 166, 11–19. 10.1016/J.APPLANIM.2015.03.002 [DOI] [Google Scholar]
- Johnsen JF, Zipp KA, Kälber T, Passillé AM, Knierim U, Barth K and Mejdell CM, 2016. Is rearing calves with the dam a feasible option for dairy farms?—Current and future research. Applied Animal Behaviour Science, 181, 1–11. 10.1016/J.APPLANIM.2015.11.011 [DOI] [Google Scholar]
- Johnsen JF, Mejdell CM, Beaver A, de Passillé AM, Rushen J and Weary DM, 2018. Behavioural responses to cow‐calf separation: the effect of nutritional dependence. Applied Animal Behaviour Science, 201, 1–6. 10.1016/j.applanim.2017.12.009 [DOI] [Google Scholar]
- Johnsen JF, Viljugrein H, Bøe KE, Gulliksen SM, Beaver A, Grøndahl AM, Sivertsen T and Mejdell CM, 2019. A cross‐sectional study of suckling calves' passive immunity and associations with management routines to ensure colostrum intake on organic dairy farms. Acta Veterinaria Scandinavica, 61, 1–10. 10.1186/s13028-019-0442-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen JF, Holmøy IH, Nødtvedt A and Mejdell CM, 2021. A survey of pre‐weaning calf management in Norwegian dairy herds. Acta Veterinaria Scandinavica, 63, 20. 10.1186/s13028-021-00587-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MC and Heinrichs AJ, online. Feeding the Newborn Dairy Calf. [Accessed: 9 January 2023]. [Google Scholar]
- Jongman EC, Conley MJ, Borg S, Butler KL and Fisher AD, 2020. The effect of milk quantity and feeding frequency on calf growth and behaviour. Animal Production Science, 60, 944–952. 10.1071/AN19049 [DOI] [Google Scholar]
- Jung J and Lidfors L, 2001. Effects of amount of milk, milk cow and access to a rubber teat on cross‐sucking and non‐nutritive sucking in dairy calves. 10.1016/S0168-1591(01)00110-1 [DOI] [PubMed]
- Kalf , online. Classifying veal. Available online: https://www.kalfhetboek.nl/proces/classificeren-van-kalfsvlees
- Karle BM, Maier GU, Love WJ, Dubrovsky SA, Williams DR, Anderson RJ, Van Eenennaam AL, Lehenbauer TW and Aly SS, 2019. Regional management practices and prevalence of bovine respiratory disease in California's preweaned dairy calves. Journal of Dairy Science, 102, 7583–7596. 10.3168/JDS.2018-14775 [DOI] [PubMed] [Google Scholar]
- Kehoe S, Dechow CD and Heinrichs AJ, 2007. Effects of weaning age and milk feeding frequency on dairy calf growth, health and rumen parameters. Livestock Science, 110, 267–272. 10.1016/j.livsci.2006.11.007 [DOI] [Google Scholar]
- Kehoe SI, Dill‐McFarland KA, Breaker JD and Suen G, 2019. Effects of corn silage inclusion in preweaning calf diets. Journal of Dairy Science, 102, 4131–4137. 10.3168/JDS.2018-15799 [DOI] [PubMed] [Google Scholar]
- Keil NM and Langhans W, 2001. The development of intersucking in dairy calves around weaning. Applied Animal Behaviour Science, 72, 295–308. 10.1016/S0168-1591(00)00207-0 [DOI] [PubMed] [Google Scholar]
- Keil NM, Audigé L and Langhans W, 2000. Factors associated with intersucking in Swiss dairy heifers. Preventive Veterinary Medicine, 45, 305–323. 10.1016/S0167-5877(00)00127-6 [DOI] [PubMed] [Google Scholar]
- Ketelaar de Lauwere C and Smits A, 1991. Spatial requirements of individually housed veal calves of 175 to 300 kg. New Trends in Veal Calf Production EAAP Publ, 52, 49. Available online: https://edepot.wur.nl/317675#page=51 [Google Scholar]
- Ketelaar‐de Lauwere C and Smits A, 1989. Onderzoek naar de uit ethologisch oogpunt minimaal gewenste boxmaten voor vleeskalveren met een gewicht van 175 tot 300 kg: de hoeveelheid ingenomen ruimte en gedragsaspecten. 0169‐2143. Available online: https://library.wur.nl/WebQuery/wurpubs/reports/460642
- Keys JE, Pearson RE and Thompson PD, 1978. Effect of deedbunk stocking density on weight gains and feeding behavior of yearling holstein heifers. Journal of Dairy Science, 61, 448–454. 10.3168/jds.S0022-0302(78)83619-4 [DOI] [PubMed] [Google Scholar]
- Khan M, Weary D and Von Keyserlingk M, 2011. Hay intake improves performance and rumen development of calves fed higher quantities of milk. Journal of Dairy Science, 94, 3547–3553. 10.3168/jds.2010-3871 [DOI] [PubMed] [Google Scholar]
- Kiley‐Worthington M and Plain S, 1983. The behaviour of beef suckler cattle. 10.1007/978-3-0348-6782-5 [DOI]
- Kleen JL, Hooijer GA, Rehage J and Noordhuizen JPTM, 2003. Subacute ruminal acidosis (SARA): a review. Journal of Veterinary Medicine Series A: Physiology Pathology Clinical Medicine, 50, 406–414. 10.1046/J.1439-0442.2003.00569.X [DOI] [PubMed] [Google Scholar]
- Klein‐Jöbstl D, Iwersen M and Drillich M, 2014. Farm characteristics and calf management practices on dairy farms with and without diarrhea: a case‐control study to investigate risk factors for calf diarrhea. Journal of Dairy Science, 97, 5110–5119. 10.3168/jds.2013-7695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein‐Jöbstl D, Arnholdt T, Sturmlechner F, Iwersen M and Drillich M, 2015. Results of an online questionnaire to survey calf management practices on dairy cattle breeding farms in Austria and to estimate differences in disease incidences depending on farm structure and management practices. Acta Veterinaria Scandinavica, 57, 44. 10.1186/s13028-015-0134-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim UI, Nora I and Roth BA, 2015. To be or not to be horned ‐ consequences in cattle. Livestock Science, 179, 29–37. 10.1016/J.LIVSCI.2015.05.014 [DOI] [Google Scholar]
- Koerkamp PG, Metz J, Uenk G, Phillips V, Holden M, Sneath R, Short J, White R, Hartung J and Seedorf J, 1998. Concentrations and emissions of ammonia in livestock buildings in Northern Europe. Journal of Agricultural Engineering Research, 70, 79–95. 10.1006/jaer.1998.0275 [DOI] [Google Scholar]
- Kozak A, Vecerek V, Steinhauserova I, Chloupek P and Pistekova V, 2002. Results of slaughterhouse carcass classification (capable for human consumption, capable for processing and condemned) in selected species of food animals. Veterinarni Medicina‐Praha, 47, 26–31. 10.17221/5799-VETMED [DOI] [Google Scholar]
- Krachun C, Rushen J and de Passillé AM, 2010. Play behaviour in dairy calves is reduced by weaning and by a low energy intake. Applied Animal Behaviour Science, 122, 71–76. 10.1016/j.applanim.2009.12.002 [DOI] [Google Scholar]
- Kristensen NB, Sehested J, Jensen SK and Vestergaard M, 2007. Effect of milk allowance on concentrate intake, ruminal environment, and ruminal development in milk‐fed holstein calves. Journal of Dairy Science, 90, 4346–4355. 10.3168/JDS.2006-885 [DOI] [PubMed] [Google Scholar]
- Krohn C, Foldager J and Mogensen L, 1999. Long‐term effect of colostrum feeding methods on behaviour in female dairy calves. Acta Agriculturae Scandinavica, Section A‐Animal Science, 49, 57–64. 10.1080/090647099421540 [DOI] [Google Scholar]
- Krohn C, Jago J and Boivin X, 2001. The effect of early handling on the socialisation of young calves to humans. Applied Animal Behaviour Science, 74, 121–133. 10.1016/S0168-1591(01)00161-7 [DOI] [Google Scholar]
- Krohn C, Boivin X and Jago J, 2003. The presence of the dam during handling prevents the socialization of young calves to humans. Applied Animal Behaviour Science, 80, 263–275. 10.1016/S0168-1591(02)00230-7 [DOI] [Google Scholar]
- Laarman A and Oba M, 2011. Effect of calf starter on rumen pH of Holstein dairy calves at weaning. Journal of Dairy Science, 94, 5661–5664. 10.3168/jds.2011-4273 [DOI] [PubMed] [Google Scholar]
- Lago A, McGuirk SM, Bennett TB, Cook NB and Nordlund KV, 2006. Calf respiratory disease and pen microenvironments in naturally ventilated calf barns in winter. Journal of Dairy Science, 89, 4014–4025. 10.3168/jds.S0022-0302(06)72445-6 [DOI] [PubMed] [Google Scholar]
- Lagoda H, Wilson L, Henning W, Flowers S and Mills EW, 2002. Subjective and objective evaluation of veal lean color. Journal of animal science, 80, 1911–1916. 10.2527/2002.8071911x [DOI] [PubMed] [Google Scholar]
- Lallès JP, Dréau D, Huet A and Toullec R, 1995. Systemic and local gut‐specific antibody responses in preruminant calves sensitive to soya. Research in Veterinary Science, 59, 56–60. 10.1016/0034-5288(95)90031-4 [DOI] [PubMed] [Google Scholar]
- Lambertz C, Farke‐Röver A and Gauly M, 2015. Effects of sex and age on behavior and weight gain in beef calves after abrupt weaning. Animal Science Journal, 86, 345–350. 10.1111/ASJ.12285 [DOI] [PubMed] [Google Scholar]
- Langel SN, Wark WA, Garst SN, James RE, McGilliard ML, Petersson‐Wolfe CS and Kanevsky‐Mullarky I, 2015. Effect of feeding whole compared with cell‐free colostrum on calf immune status: the neonatal period. Journal of Dairy Science, 98, 3729–3740. 10.3168/JDS.2014-8422 [DOI] [PubMed] [Google Scholar]
- Lava M, Pardon B, Schüpbach‐Regula G, Keckeis K, Deprez P, Steiner A and Meylan M, 2016. Effect of calf purchase and other herd‐level risk factors on mortality, unwanted early slaughter, and use of antimicrobial group treatments in Swiss veal calf operations. Preventive Veterinary Medicine, 126, 81–88. 10.1016/J.PREVETMED.2016.01.020 [DOI] [PubMed] [Google Scholar]
- Le Neindre P, 1989. Influence of rearing conditions and breed on social behaviour and activity of cattle in novel environments. Applied Animal Behaviour Science, 23, 129–140. 10.1016/0168-1591(89)90013-0 [DOI] [Google Scholar]
- Le Neindre P, 1993. Evaluating housing systems for veal calves. Journal of Animal Science, 71, 1345–1354. 10.2527/1993.7151345x [DOI] [PubMed] [Google Scholar]
- Le Neindre P and Sourd C, 1984. Influence of rearing conditions on subsequent social behaviour of Friesian and Salers heifers from birth to six months of age. Applied Animal Behaviour Science, 12, 43–52. 10.1016/0168-1591(84)90095-9 [DOI] [Google Scholar]
- Lensink B, Fernandez X, Cozzi G, Florand L and Veissier I, 2001. The influence of farmers’ behavior on calves’ reactions to transport and quality of veal meat. Journal of Animal Science, 79, 642–652. 10.2527/2001.793642x [DOI] [PubMed] [Google Scholar]
- Leruste H, Brščić M, Heutinck LFM, Visser EK, Wolthuis‐Fillerup M, Bokkers EAM, Stockhofe‐Zurwieden N, Cozzi G, Gottardo F, Lensink BJ and van Reenen CG, 2012. The relationship between clinical signs of respiratory system disorders and lung lesions at slaughter in veal calves. Preventive Veterinary Medicine, 105, 93–100. 10.1016/J.PREVETMED.2012.01.015 [DOI] [PubMed] [Google Scholar]
- Leruste H, Brščić M, Cozzi G, Kemp B, Wolthuis‐Fillerup M, Lensink BJ, Bokkers EAM and van Reenen CG, 2014. Prevalence and potential influencing factors of non‐nutritive oral behaviors of veal calves on commercial farms. Journal of Dairy Science, 97, 7021–7030. 10.3168/JDS.2014-7917 [DOI] [PubMed] [Google Scholar]
- Lidfors LM, 1993. Cross‐sucking in group‐housed dairy calves before and after weaning off milk.
- Lidfors LM, 1996. Behavioural effects of separating the dairy calf immediately or 4 days post‐partum. Applied Animal Behaviour Science, 49, 269–283. 10.1016/0168-1591(96)01053-2 [DOI] [Google Scholar]
- Lidfors L and Jensen MB, 2003. Behaviour and welfare of cattle housed in large groups. Applied Animal Behaviour Science, 80, 173. 10.1016/S0168-1591(02)00218-6 [DOI] [Google Scholar]
- Lidfors LM, Jung J and de Passillé AM, 2010. Changes in suckling behaviour of dairy calves nursed by their dam during the first month post‐partum. Applied Animal Behaviour Science, 128, 23–29. 10.1016/j.applanim.2010.09.002 [DOI] [Google Scholar]
- Lindner E, Gingerich K and Miller‐Cushon E, 2021. Effects of early social contact on dairy calf response to initial social grouping and regrouping. Journal of Dairy Science, 104, 10090–10099. 10.3168/jds.2021-20435 [DOI] [PubMed] [Google Scholar]
- Lindner EE, Gingerich KN, Burke KC, Doyle SB and Miller‐Cushon EK, 2022. Effects of social housing on dairy calf social bonding. Animals, 12, 821. 10.3390/ani12070821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindt F and Blum J, 1993. Physical performance of veal calves during chronic iron deficiency anaemia and after acute iron overload 1. Journal of Veterinary Medicine Series A, 40, 444–455. 10.1111/j.1439-0442.1993.tb00651.x [DOI] [PubMed] [Google Scholar]
- Lindt F and Blum J, 1994. Growth performance, haematogical traits, meat variables, and effects of treadmill and transport stress in veal calves supplied different amounts of iron. Journal of Veterinary Medicine Series A, 41, 333–342. 10.1111/j.1439-0442.1994.tb00100.x [DOI] [PubMed] [Google Scholar]
- Loberg J and Lidfors L, 2001. Effect of milkflow rate and presence of a floating nipple on abnormal sucking between dairy calves. Applied Animal Behaviour Science, 72, 189–199. 10.1016/S0168-1591(01)00109-5 [DOI] [PubMed] [Google Scholar]
- Loberg JM, Hernandez CE, Thierfelder T, Jensen MB, Berg C and Lidfors L, 2007. Reaction of foster cows to prevention of suckling from and separation from four calves simultaneously or in two steps. Journal of Animal Science, 85, 1522–1529. 10.2527/jas.2006-813 [DOI] [PubMed] [Google Scholar]
- Loberg JM, Hernandez CE, Thierfelder T, Jensen MB, Berg C and Lidfors L, 2008. Weaning and separation in two steps—A way to decrease stress in dairy calves suckled by foster cows. Applied Animal Behaviour Science, 111, 222–234. 10.1016/J.APPLANIM.2007.06.011 [DOI] [Google Scholar]
- Lopez A and Martinson SA, 2017. Respiratory system, mediastinum, and pleurae. Pathologic basis of veterinary disease, 471. 10.1016/B978-0-323-35775-3.00009-6 [DOI] [Google Scholar]
- Lorenz I, 2021. Calf health from birth to weaning‐an update. Irish Veterinary Journal, 74, 1–8. 10.1186/s13620-021-00185-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz I, Earley B, Gilmore J, Hogan I, Kennedy E and More SJ, 2011. Calf health from birth to weaning. III. Housing and management of calf pneumonia. Irish Veterinary Journal, 64. 10.1186/2046-0481-64-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losinger WC and Heinrichs AJ, 1996. Management variables associated with high mortality rates attributable to respiratory tract problems in female calves prior to weaning. Journal American Veterinary Medical Association, 209, 1756–1759. [PubMed] [Google Scholar]
- Losinger WC and Heinrichs AJ, 1997. Management practices associated with high mortality among preweaned dairy heifers. Journal of Dairy Research, 64, 1–11. 10.1017/S0022029996001999 [DOI] [PubMed] [Google Scholar]
- Lowe D, Steen R, Beattie V and Moss B, 2001. The effects of floor type systems on the performance, cleanliness, carcass composition and meat quality of housed finishing beef cattle. Livestock Production Science, 69, 33–42. 10.1016/S0301-6226(00)00246-3 [DOI] [Google Scholar]
- Lundborg G, Svensson E and Oltenacu P, 2005. Herd‐level risk factors for infectious diseases in Swedish dairy calves aged 0–90 days. Preventive Veterinary Medicine, 68, 123–143. 10.1016/j.prevetmed.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Lupoli B, Johansson B, Uvnäs‐Moberg K and Svennersten‐Sjaunja K, 2001. Effect of suckling on the release of oxytocin, prolactin, cortisol, gastrin, cholecystokinin, somatostatin and insulin in dairy cows and their calves. Journal of Dairy Research, 68, 175–187. 10.1017/S0022029901004721 [DOI] [PubMed] [Google Scholar]
- Lürzel S, Windschnurer I, Futschik A, Palme R and Waiblinger S, 2015. Effects of gentle interactions on the relationship with humans and on stress‐related parameters in group‐housed calves. Animal Welfare, 24, 475–484. 10.7120/09627286.24.4.475 [DOI] [Google Scholar]
- Lürzel S, Windschnurer I, Futschik A and Waiblinger S, 2016. Gentle interactions decrease the fear of humans in dairy heifers independently of early experience of stroking. Applied Animal Behaviour Science, 178, 16–22. 10.1016/j.applanim.2016.02.012 [DOI] [Google Scholar]
- MacDougall D, Bremner I and Dalgarno A, 1973. Effect of dietary iron on the colour and pigment concentration of veal. Journal of the Science of Food and Agriculture, 24, 1255–1263. 10.1002/jsfa.2740241015 [DOI] [PubMed] [Google Scholar]
- Mader TL, Davis M and Brown‐Brandl T, 2006. Environmental factors influencing heat stress in feedlot cattle. Journal of Animal Science, 84, 712–719. 10.2527/2006.843712x [DOI] [PubMed] [Google Scholar]
- Magrin L, Brščić M, Cozzi G, Armato L and Gottardo F, 2020. Prevalence of gastrointestinal, liver and claw disorders in veal calves fed large amounts of solid feed through a cross‐sectional study. Research in Veterinary Science, 133, 318–325. 10.1016/J.RVSC.2020.10.022 [DOI] [PubMed] [Google Scholar]
- Mahendran SA, Wathes DC, Booth RE and Blackie N, 2021. The health and behavioural effects of individual versus pair housing of calves at different ages on a UK commercial dairy farm. Animals, 11, 612. 10.3390/ani11030612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran SA, Wathes DC, Booth RE and Blackie N, 2022. A survey of calf management practices and farmer perceptions of calf housing in UK dairy herds. Journal of Dairy Science, 105, 409–423. 10.3168/jds.2021-20638 [DOI] [PubMed] [Google Scholar]
- Mandel R, Whay HR, Klement E and Nicol CJ, 2016. Invited review: environmental enrichment of dairy cows and calves in indoor housing. Journal of Dairy Science, 99, 1695–1715. 10.3168/jds.2015-9875 [DOI] [PubMed] [Google Scholar]
- Mankins JC, 1995. Technology readiness levels. White Paper, April, 6, 1995. [Google Scholar]
- Marcato F, Van den Brand H, Kemp B and Van Reenen K, 2018. Evaluating potential biomarkers of health and performance in veal calves. Frontiers in Veterinary Science, 5, 133. 10.3389/fvets.2018.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcato F, van den Brand H, Kemp B, Engel B, Wolthuis‐Fillerup M and van Reenen K, 2020. Transport of young veal calves: effects of pre‐transport diet, transport duration and type of vehicle on health, behavior, use of medicines, and slaughter characteristics. Frontiers in Veterinary Science, 7. 10.3389/fvets.2020.576469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcé C, Guatteo R, Bareille N and Fourichon C, 2010. Dairy calf housing systems across Europe and risk for calf infectious diseases. Animal, 4, 1588–1596. 10.1017/S1751731110000650 [DOI] [PubMed] [Google Scholar]
- Margerison JK, Preston TR, Berry N and Phillips CJC, 2003. Cross‐sucking and other oral behaviours in calves, and their relation to cow suckling and food provision. Applied Animal Behaviour Science, 80, 277–286. 10.1016/S0168-1591(02)00231-9 [DOI] [Google Scholar]
- Marshall TS, 2009. Abomasal ulceration and tympany of calves. Veterinary Clinics of North America: Food Animal Practice, 25, 209–220. 10.1016/j.cvfa.2008.10.010 [DOI] [PubMed] [Google Scholar]
- Masmeijer C, Deprez P, van Leenen K, De Cremer L, Cox E, Devriendt B and Pardon B, 2021. Arrival cortisol measurement in veal calves and its association with body weight, protein fractions, animal health and performance. Preventive Veterinary Medicine, 187, 105251. 10.1016/j.prevetmed.2020.105251 [DOI] [PubMed] [Google Scholar]
- Mason GJ and Burn C, 2011. Behavioural restriction. Animal Welfare. 10.1079/9781845936594.0098 [DOI] [Google Scholar]
- Mason GJ and Latham N, 2004. Can't stop, won't stop: is stereotypy a reliable animal welfare indicator? Animal Welfare, 13: S1. Science in the Service of Animal Welfare: Proceedings of the UFAW International Symposium, Edinburgh., 13, S57–S69. 10.1017/S096272860001438X [DOI] [Google Scholar]
- Masset N, Meurens F, Marie M, Lesage P, Lehébel A, Brisseau N and Assié S, 2020. Effectiveness of two intranasal vaccines for the control of bovine respiratory disease in newborn beef calves: A randomized non‐inferiority multicentre field trial. The Veterinary Journal, 263, 105532. 10.1016/j.tvjl.2020.105532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus UJLI, 2014. Caracterização Do Comportamento De Vitelos Em Regime Intensivo Na Procura De Factores De Risco Para Cross‐sucking. Universidade de Lisboa (Portugal). [Google Scholar]
- Mattiello S, Canali E, Ferrante V, Caniatti M, Gottardo F, Cozzi G, Andrighetto I and Verga M, 2002. The provision of solid feeds to veal calves: II. Behavior, physiology, and abomasal damage, 1, 367–375. Available online:. https://academic.oup.com/jas/article/80/2/367/4789313 [DOI] [PubMed] [Google Scholar]
- Mayer C, 2007. Vergleich von Betonspaltenboden, gummimodifizierten Spaltenboden und Buchten mit Einstreu in der Bullenmast unter dem Gesichtspunkt der Tiergerechtheit. Landbauforschung Völkenrode, Bundesforschungsanstalt für Landwirtschaft (FAL). [Google Scholar]
- McAloon CG, Roche S, Ritter C, Barkema HW, Whyte P, More SJ, O'Grady L, Green MJ and Doherty ML, 2019. A review of paratuberculosis in dairy herds—Part 1: Epidemiology. The Veterinary Journal, 246, 59–65. 10.1016/j.tvjl.2019.01.010 [DOI] [PubMed] [Google Scholar]
- McFarlane J, Morris G, Curtis S, Simon J and McGlone J, 1988. Some indicators of welfare of crated veal calves on three dietary iron regimens. Journal of Animal Science, 66, 317–325. 10.2527/jas1988.662317x [DOI] [PubMed] [Google Scholar]
- McMillan FD, 2020. What is distress? A complex answer to a simple question. Mental health and well‐being in animals, CABI Wallingford UK. pp. 140‐155. 10.1079/9781786393401.0140 [DOI] [Google Scholar]
- Meagher RK, Daros RR, Costa JH, Von Keyserlingk MA, Hötzel MJ and Weary DM, 2015. Effects of degree and timing of social housing on reversal learning and response to novel objects in dairy calves. PLOS One, 10, e0132828. 10.1371/journal.pone.0132828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher RK, Beaver A, Weary DM and von Keyserlingk MAG, 2019. Invited review: a systematic review of the effects of prolonged cow–calf contact on behavior, welfare, and productivity. Journal of Dairy Science, 102, 5765–5783. 10.3168/JDS.2018-16021 [DOI] [PubMed] [Google Scholar]
- Medrano‐Galarza C, LeBlanc SJ, DeVries TJ, Jones‐Bitton A, Rushen J, Marie de Passillé A, Endres MI and Haley DB, 2018. Effect of age of introduction to an automated milk feeder on calf learning and performance and labor requirements. Journal of Dairy Science, 101, 9371–9384. 10.3168/JDS.2018-14390 [DOI] [PubMed] [Google Scholar]
- Meganck V, Hoflack G and Opsomer G, 2014. Advances in prevention and therapy of neonatal dairy calf diarrhoea: a systematical review with emphasis on colostrum management and fluid therapy. Acta Veterinaria Scandinavica, 56, 1–8. 10.1186/s13028-014-0075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganck V, Hoflack G, Piepers S and Opsomer G, 2015. Evaluation of a protocol to reduce the incidence of neonatal calf diarrhoea on dairy herds. Preventive Veterinary Medicine, 118, 64–70. 10.1016/j.prevetmed.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S‐H, Zhou H‐B, Li X, Wang M‐X, Kang L‐X, Fu J‐M, Li X, Li X‐T and Zhao Y‐S, 2021. Association between dietary iron intake and serum Ferritin and severe headache or migraine. Frontiers in Nutrition, 8, 685564. 10.3389/fnut.2021.685564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke C, Peer M, Schneider C, Spengler A and Waiblinger S, 2015. Introducing structural elements into the free resting area in loose‐housing systems with horned dairy cows: effects on lying behaviour and cleanliness. Livestock Science, 179, 38–46. 10.1016/j.livsci.2015.05.015 [DOI] [Google Scholar]
- Metz J, 1987. Productivity aspects of keeping dairy cow and calf together in the post‐partum period. Livestock Production Science, 16, 385–394. 10.1016/0301-6226(87)90007-8 [DOI] [Google Scholar]
- Metz J and Metz JHM, 1986. Maternal influence on defecation and urination in the newborn calf. Applied Animal Behaviour Science, 16, 325–333. 10.1016/0168-1591(86)90004-3 [DOI] [Google Scholar]
- Miguel‐Pacheco G, Vaughan A, de Passille A and Rushen J, 2015. Relationship between locomotor play of dairy calves and their weight gains and energy intakes around weaning. Animal, 9, 1038–1044. 10.1017/S1751731115000063 [DOI] [PubMed] [Google Scholar]
- Miller GA, Hyslop JJ, Barclay D, Edwards A, Thomson W and Duthie C‐A, 2019. Using 3D imaging and machine learning to predict liveweight and carcass characteristics of live finishing beef cattle. Frontiers in Sustainable Food Systems, 3, 30. 10.3389/fsufs.2019.00030 [DOI] [Google Scholar]
- Miller‐Cushon EK and DeVries TJ, 2016. Effect of social housing on the development of feeding behavior and social feeding preferences of dairy calves. Journal of Dairy Science, 99, 1406–1417. 10.3168/JDS.2015-9869 [DOI] [PubMed] [Google Scholar]
- Miller‐Cushon EK, Montoro C, Ipharraguerre IR and Bach A, 2014. Dietary preference in dairy calves for feed ingredients high in energy and protein. Journal of Dairy Science, 97, 1634–1644. 10.3168/JDS.2013-7199 [DOI] [PubMed] [Google Scholar]
- Miltenburg G, Wensing T, Smulders F and Breukink H, 1992. Relationship between blood hemoglobin, plasma and tissue iron, muscle heme pigment, and carcass color of veal. Journal of Animal Science, 70, 2766–2772. 10.2527/1992.7092766x [DOI] [PubMed] [Google Scholar]
- Mintline EM, Wood SL, de Passillé AM, Rushen J and Tucker CB, 2012. Assessing calf play behavior in an arena test. Applied Animal Behaviour Science, 141, 101–107. 10.1016/j.applanim.2012.08.006 [DOI] [Google Scholar]
- Mintline EM, Stewart M, Rogers AR, Cox NR, Verkerk GA, Stookey JM, Webster JR and Tucker CB, 2013. Play behavior as an indicator of animal welfare: Disbudding in dairy calves. Applied Animal Behaviour Science, 144, 22–30. 10.1016/j.applanim.2012.12.008 [DOI] [Google Scholar]
- Miqueo E, Torrezan TM, Rocha NB, Paula MR, Silva FLM, Rodrigues PHM and Bittar CMM, 2017. Increase in crude protein content of milk replacers with vegetable protein: effect on health and dairy calves' performance. American Journal of Animal and Veterinary Sciences, 12, 17–25. 10.3844/ajavsp.2017.17.25 [DOI] [Google Scholar]
- Mogensen L, Krohn CC, Sørensen JT, Hindhede J and Nielsen LH, 1997. Association between resting behaviour and live weight gain in dairy heifers housed in pens with different space allowance and floor type. Applied Animal Behaviour Science, 55, 11–19. 10.1016/S0168-1591(97)00041-5 [DOI] [Google Scholar]
- Mogensen L, Krohn C and Foldager J, 1999. Long‐term effect of housing method during the first three months of life on human‐animal relationship in female dairy cattle. Acta Agriculturae Scandinavica, Section A‐Animal Science, 49, 163–171. 10.1080/090647099424079 [DOI] [Google Scholar]
- Mogensen L, Kudahl A, Kristensen T, Bokkers EAM, Webb LE, Vaarst M and Lehmann J, 2022. Environmental impact of dam‐calf contact in organic dairy systems: a scenario study. Livestock Science, 258, 104890. 10.1016/J.LIVSCI.2022.104890 [DOI] [Google Scholar]
- Mohammed SAE‐M, Marouf SAE‐M, Erfana AM, El JKAE‐H, Hessain AM, Dawoud TM, Kabli SA and Moussa IM, 2019. Risk factors associated with E. coli causing neonatal calf diarrhea. Saudi Journal of Biological Sciences, 26, 1084–1088. 10.1016/j.sjbs.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri M, Sarrafzadeh F, Seifi HA and Farzaneh N, 2004. Effects of oral iron supplementation on some haematological parameters and iron biochemistry in neonatal dairy calves. Comparative Clinical Pathology, 13, 39–42. 10.1007/s00580-004-0523-5 [DOI] [Google Scholar]
- Mohri M, Sharifi K and Eidi S, 2007. Hematology and serum biochemistry of Holstein dairy calves: Age related changes and comparison with blood composition in adults. Research in Veterinary Science, 83, 30–39. 10.1016/J.RVSC.2006.10.017 [DOI] [PubMed] [Google Scholar]
- Mohri M, Poorsina S and Sedaghat R, 2010. Effects of parenteral supply of iron on RBC parameters, performance, and health in neonatal dairy calves. Biological Trace Element Research, 136, 33–39. 10.1007/s12011-009-8514-7 [DOI] [PubMed] [Google Scholar]
- Moosavian HR, Mohri M and Seifi HA, 2010. Effects of parenteral over‐supplementation of vitamin A and iron on hematology, iron biochemistry, weight gain, and health of neonatal dairy calves. Food and Chemical Toxicology, 48, 1316–1320. 10.1016/j.fct.2010.02.030 [DOI] [PubMed] [Google Scholar]
- Morand‐Fehr P and Doreau M, 2001. Effects of heat stress on feed intake and digestion in ruminants. Productions Animales (France), 14, 15–27. [Google Scholar]
- Morrison J, Renaud DL, Churchill KJ, Costa JHC, Steele MA and Winder CB, 2021. Predicting morbidity and mortality using automated milk feeders: a scoping review. Journal of Dairy Science, 104, 7177–7194. 10.3168/JDS.2020-19645 [DOI] [PubMed] [Google Scholar]
- Moser L, Becker J, Schüpbach‐regula G, Kiener S, Grieder S, Keil N, Hillmann E, Steiner A and Meylan M, 2020. Welfare assessment in calves fattened according to the “outdoor veal calf” concept and in conventional veal fattening operations in Switzerland. Animals, 10, 1810. 10.3390/ANI10101810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier L, Veissier I and Boissy A, 2005. Behavior, physiology, and performance of bulls mixed at the onset of finishing to form uniform body weight groups. Journal of Animal Science, 83, 1696–1704. 10.2527/2005.8371696x [DOI] [PubMed] [Google Scholar]
- Mounier L, Veissier I, Andanson S, Delval E and Boissy A, 2006. Mixing at the beginning of fattening moderates social buffering in beef bulls. Applied Animal Behaviour Science, 96, 185–200. 10.1016/j.applanim.2005.06.015 [DOI] [Google Scholar]
- MPI MfPI , 2018. Bobby Calf Welfare Across the Supply Chain‐Final Report for Year 1.
- Mueller ML, Cole JB, Sonstegard TS and Van Eenennaam AL, 2019. Comparison of gene editing versus conventional breeding to introgress the POLLED allele into the US dairy cattle population. Journal of Dairy Science, 102, 4215–4226. 10.3168/JDS.2018-15892 [DOI] [PubMed] [Google Scholar]
- Mülleder C and Waiblinger S, 2004. Analyse der Einflussfaktoren auf Tiergerechtheit, Tiergesundheit und Leistung von Milchkühen im Boxenlaufstall auf konventionellen und biologischen Betriebe unter besonderer Berücksichtigung der Mensch‐Tier‐Beziehung. Endbericht zum Forschungsprojekt, 1267, 165. [Google Scholar]
- Murray GM, More SJ, Sammin D, Casey MJ, McElroy MC, O'Neill RG, Byrne WJ, Earley B, Clegg TA and Ball H, 2017. Pathogens, patterns of pneumonia, and epidemiologic risk factors associated with respiratory disease in recently weaned cattle in Ireland. Journal of Veterinary Diagnostic Investigation, 29, 20–34. 10.1177/1040638716674757 [DOI] [PubMed] [Google Scholar]
- Muya M and Nherera F, 2014. Effects of limiting frequency of free access to milk on growth and intake of holstein calves during pre‐and early post‐weaning period. African Journal of Agricultural Research, 9, 2272–2277. 10.5897/AJAR2013.8285 [DOI] [Google Scholar]
- Naylor JM, 2009. Neonatal calf diarrhea. Food Animal Practice, 70. 10.1016/B978-141603591-6.10021-1 [DOI] [Google Scholar]
- Ngapo TM and Gariépy C, 2006. Factors affecting the meat quality of veal. Journal of the Science of Food and Agriculture, 86, 1412–1431. 10.1002/jsfa.2507 [DOI] [Google Scholar]
- Nicolao A, Veissier I, Bouchon M, Sturaro E, Martin B and Pomiès D, 2022. Animal performance and stress at weaning when dairy cows suckle their calves for short versus long daily durations. Animal, 16, 100536. 10.1016/j.animal.2022.100536 [DOI] [PubMed] [Google Scholar]
- Nielsen LH, Mogensen L, Krohn C, Hindhede J and Sørensen JT, 1997. Resting and social behaviour of dairy heifers housed in slatted floor pens with different sized bedded lying areas. Applied Animal Behaviour Science, 54, 307–316. 10.1016/S0168-1591(96)01211-7 [DOI] [Google Scholar]
- Nielsen PP, Jensen MB and Lidfors L, 2008a. Milk allowance and weaning method affect the use of a computer controlled milk feeder and the development of cross‐sucking in dairy calves. Applied Animal Behaviour Science, 109, 223–237. 10.1016/J.APPLANIM.2007.01.015 [DOI] [Google Scholar]
- Nielsen S, Bjerre H and Toft N, 2008b. Colostrum and milk as risk factors for infection with Mycobacterium avium subspecies paratuberculosis in dairy cattle. Journal of Dairy Science, 91, 4610–4615. 10.3168/jds.2008-1272 [DOI] [PubMed] [Google Scholar]
- Nordmann E, Barth K, Futschik A, Palme R and Waiblinger S, 2015. Head partitions at the feed barrier affect behaviour of goats. Applied Animal Behaviour Science, 167, 9–19. 10.1016/j.applanim.2015.03.011 [DOI] [Google Scholar]
- Noya A, Ripoll G, Casasús I and Sanz A, 2022. Long‐term effects of early maternal undernutrition on the growth, physiological profiles, carcass and meat quality of male beef offspring. Research in Veterinary Science, 142, 1–11. 10.1016/J.RVSC.2021.10.025 [DOI] [PubMed] [Google Scholar]
- O'Driscoll K, Von Keyserlingk M and Weary D, 2006. Effects of mixing on drinking and competitive behavior of dairy calves. Journal of Dairy Science, 89, 229–233. 10.3168/jds.S0022-0302(06)72087-2 [DOI] [PubMed] [Google Scholar]
- Olson A, Sischo W, Berge A, Adams‐Progar A and Moore D, 2019. A retrospective cohort study comparing dairy calf treatment decisions by farm personnel with veterinary observations of clinical signs. Journal of Dairy Science, 102, 6391–6403. 10.3168/jds.2018-15623 [DOI] [PubMed] [Google Scholar]
- Overvest MA, Crossley RE, Miller‐Cushon EK and DeVries TJ, 2018. Social housing influences the behavior and feed intake of dairy calves during weaning. Journal of Dairy Science, 101, 8123–8134. 10.3168/JDS.2018-14465 [DOI] [PubMed] [Google Scholar]
- Palczynski LJ, Bleach ECL, Brennan ML and Robinson PA, 2020. Appropriate dairy calf feeding from birth to weaning: “it's an investment for the future”. Animals, 10. 10.3390/ani10010116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panciera RJ, Boileau MJ and Step DL, 2007. Tympany, acidosis, and mural emphysema of the stomach in calves: report of cases and experimental induction. Journal of Veterinary Diagnostic Investigation, 19, 392–395. 10.1177/104063870701900409 [DOI] [PubMed] [Google Scholar]
- Panousis N, Siachos N, Kitkas G, Kalaitzakis E, Kritsepi‐Konstantinou M and Valergakis GE, 2018. Hematology reference intervals for neonatal Holstein calves. Research in veterinary science, 118, 1–10. 10.1016/j.rvsc.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Pardon B, Catry B, Dewulf J, Persoons D, Hostens M, De bleecker K and Deprez P, 2012. Prospective study on quantitative and qualitative antimicrobial and anti‐inflammatory drug use in white veal calves. Journal of Antimicrobial Chemotherapy, 67, 1027–1038. 10.1093/JAC/DKR570 [DOI] [PubMed] [Google Scholar]
- Pardon B, Hostens M, Duchateau L, Dewulf J, De Bleecker K and Deprez P, 2013. Impact of respiratory disease, diarrhea, otitis and arthritis on mortality and carcass traits in white veal calves. BMC Veterinary Research, 9, 1–14. 10.1186/1746-6148-9-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon B, Alliët J, Boone R, Roelandt S, Valgaeren B and Deprez P, 2015. Prediction of respiratory disease and diarrhea in veal calves based on immunoglobulin levels and the serostatus for respiratory pathogens measured at arrival. Preventive Veterinary Medicine, 120, 169–176. 10.1016/j.prevetmed.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen RE, Sørensen JT, Skjøth F, Hindhede J and Nielsen TR, 2009. How milk‐fed dairy calves perform in stable versus dynamic groups. Livestock Science, 121, 215–218. 10.1016/J.LIVSCI.2008.06.007 [DOI] [Google Scholar]
- Pempek JA, Eastridge ML and Proudfoot KL, 2017. The effect of a furnished individual hutch pre‐weaning on calf behavior, response to novelty, and growth. Journal of Dairy Science, 100, 4807–4817. 10.3168/jds.2016-12180 [DOI] [PubMed] [Google Scholar]
- Perez O, de Perez NJ, Poindron P, Le Neindre P and Ravault J, 1985. Relations mère‐jeune et réponse prolactinique à la stimulation mammaire chez la vache: influences de la traite et de l'allaitement libre ou entravé. Reproduction Nutrition Développement, 25, 605–618. 10.1051/rnd:19850502 [DOI] [PubMed] [Google Scholar]
- Pérez‐Torres L, Orihuela A, Corro M, Rubio I, Alonso MA and Galina CS, 2016. Effects of separation time on behavioral and physiological characteristics of Brahman cows and their calves. Applied Animal Behaviour Science, 179, 17–22. 10.1016/J.APPLANIM.2016.03.010 [DOI] [Google Scholar]
- Phillips CJC, 2004. The effects of forage provision and group size on the behavior of calves. Journal of Dairy Science, 87, 1380–1388. 10.3168/JDS.S0022-0302(04)73287-7 [DOI] [PubMed] [Google Scholar]
- Pithua P, Wells SJ, Godden SM and Raizman EA, 2009. Clinical trial on type of calving pen and the risk of disease in Holstein calves during the first 90 d of life. Preventive Veterinary Medicine, 89, 8–15. 10.1016/j.prevetmed.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz S, Ahrens F, Bahrs E, Nüske S and Erhard MH, 2007. Association between floor type and behaviour, skin lesions, and claw dimensions in group‐housed fattening bulls. Preventive Veterinary Medicine, 80, 209–221. 10.1016/j.prevetmed.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Pollmann U, 2003. Einfluss der Strukturierung des Liegebereichs einer Gruppenauslaufhaltung auf das Verhalten der Pferde. Tagungsband der DVG‐Fachgruppe Tierschutzrecht und Tierzucht, Erbpathologie und Haustiergenetik: 71–75.
- Polsky L and von Keyserlingk MAG, 2017. Invited review: effects of heat stress on dairy cattle welfare. Journal of Dairy Science, 100, 8645–8657. 10.3168/jds.2017-12651 [DOI] [PubMed] [Google Scholar]
- Prado M, Prado T, Payton M and Confer A, 2006. Maternally and naturally acquired antibodies to Mannheimia haemolytica and Pasteurella multocida in beef calves. Veterinary Immunology and Immunopathology, 111, 301–307. 10.1016/j.vetimm.2005.10.013 [DOI] [PubMed] [Google Scholar]
- Pratelli A and Padalino B, 2022. Evolving prospects of bovine respiratory diseases and management in feedlot cattle. Frontiers in Veterinary Science, 9. 10.3389/fvets.2022.854844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevedello P, Moro L, Brščić M, Gottardo F and Stefani AL, 2009. Trend overtime of total haemoglobin, iron metabolism and trace minerals in veal calves fed high amounts of two different solid feeds. Italian Journal of Animal Science, 8, 184–186. 10.4081/ijas.2009.s3.184 [DOI] [Google Scholar]
- Prevedello P, Brščić M, Schiavon E, Cozzi G and Gottardo F, 2012. Effects of the provision of large amounts of solid feeds to veal calves on growth and slaughter performance and intravitam and postmortem welfare indicators. Journal of Animal Science, 90, 3538–3546. 10.2527/JAS.2011-4666 [DOI] [PubMed] [Google Scholar]
- Price EO, Harris JE, Borgwardt RE, Sween ML and Connor JM, 2003. Fenceline contact of beef calves with their dams at weaning reduces the negative effects of separation on behavior and growth rate. Journal of Animal Science, 81, 116–121. 10.2527/2003.811116X [DOI] [PubMed] [Google Scholar]
- Probst JK, Neff AS, Leiber F, Kreuzer M and Hillmann E, 2012. Gentle touching in early life reduces avoidance distance and slaughter stress in beef cattle. Applied Animal Behaviour Science, 139, 42–49. 10.1016/j.applanim.2012.03.002 [DOI] [Google Scholar]
- Prodanović R, Nedić S, Radanović O, Milićević V, Vujanac I, Bojkovski J, Kureljušić B, Arsić S, Jovanović L and Kirovski D, 2019. Occurrence of neonatal diarrhea in calves with iron‐deficiency anemia. Veterinarski glasnik, 73, 1–9. 10.2298/VETGL181210011P [DOI] [Google Scholar]
- Purslow PP, Warner RD, Clarke FM and Hughes JM, 2020. Variations in meat colour due to factors other than myoglobin chemistry; a synthesis of recent findings (invited review). Meat Science, 159, 107941. 10.1016/j.meatsci.2019.107941 [DOI] [PubMed] [Google Scholar]
- Quigley J III, Martin K, Bemis D, Potgieter L, Reinemeyer C, Rohrbach B, Dowlen H and Lamar K, 1995. Effects of housing and colostrum feeding on serum immunoglobulins, growth, and fecal scores of Jersey calves. Journal of Dairy Science, 78, 893–901. 10.3168/jds.S0022-0302(95)76703-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räber R, Kaufmann T, Regula G, von Rotz A, Stoffel MH, Posthaus H, Rérat M, Kirchhofer M, Steiner A and Bähler C, 2013. Effects of different types of solid feeds on health status and performance of Swiss veal calves. I. Basic feeding with milk by‐products. Schweiz. Arch. Tierheilkd., 155, 269–281. 10.1024/0036-7281/a000458 [DOI] [PubMed] [Google Scholar]
- Radaelli E, Luini M, Loria GR, Nicholas RAJ and Scanziani E, 2008. Bacteriological, serological, pathological and immunohistochemical studies of Mycoplasma bovis respiratory infection in veal calves and adult cattle at slaughter. Research in Veterinary Science, 85, 282–290. 10.1016/J.RVSC.2007.11.012 [DOI] [PubMed] [Google Scholar]
- Rajabian F, Mohri M and Heidarpour M, 2017. Relationships between oxidative stress, haematology and iron profile in anaemic and non‐anaemic calves. Veterinary Record, 181, 265–265. 10.1136/vr.104179 [DOI] [PubMed] [Google Scholar]
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJM, Jeffrey S, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T and Vader K, 2020. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises, 161, 1976–1982. 10.1097/j.pain.0000000000001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramin A, Asri‐Rezaei S, Paya K, Eftekhari Z, Jelodary M, Akbari H and Ramin S, 2014. Evaluation of anemia in calves up to 4 months of age in Holstein dairy herds. Veteriner Fakültesi Dergisi (Istanbul), 40, 1–6. 10.16988/iuvfd.89026 [DOI] [Google Scholar]
- Rasmussen L, Jensen MB and Jeppesen LL, 2006. The effect of age at introduction and number of milk‐portions on the behaviour of group housed dairy calves fed by a computer controlled milk feeder. Applied Animal Behaviour Science, 100, 153–163. 10.1016/J.APPLANIM.2005.10.019 [DOI] [Google Scholar]
- Rault J‐L, 2012. Friends with benefits: social support and its relevance for farm animal welfare. Applied Animal Behaviour Science, 136, 1–14. 10.1016/j.applanim.2011.10.002 [DOI] [Google Scholar]
- Raussi S, Lensink B, Boissy A, Pyykkonen M and Veissier I, 2003. The effect of contact with conspecifics and humans on calves' behaviour and stress responses. Animal Welfare, 12, 191–204. 10.1017/S096272860002563X [DOI] [Google Scholar]
- Raussi S, Niskanen S, Siivonen J, Hänninen L, Hepola H, Jauhiainen L and Veissier I, 2010. The formation of preferential relationships at early age in cattle. Behavioural Processes, 84, 726–731. 10.1016/j.beproc.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Redbo I, Mossberg I, Ehrlemark A and Ståhl‐Högberg M, 1996. Keeping growing cattle outside during winter: behaviour, production and climatic demand. Animal Science, 62, 35–41. 10.1017/S1357729800014284 [DOI] [Google Scholar]
- Reed LM, Renaud DL and DeVries TJ, 2022. Male dairy calf welfare: a Canadian perspective on challenges and potential solutions. The Canadian Veterinary Journal, 63, 187. Available online:–193. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8759333/ [PMC free article] [PubMed] [Google Scholar]
- Reif J, Gewessler F and Wittek T, 2019. The iron supply status of dairy calves. Wiener Tierärztliche Monatsschrift, 106, 195–202. [Google Scholar]
- Reinhardt V, 1980. Untersuchung zum Sozialverhalten des Rindes: eine zweijährige Beobachtung an einer halb‐wilden Rinderherde (Bos indicus). Springer. [Google Scholar]
- Reinhardt A, 1982. Mock fighting in cattle. Behaviour, 81, 1–12. 10.1163/156853982X00490 [DOI] [Google Scholar]
- Reinhardt V and Reinhardt A, 1981. Natural sucking performance and age of weaning in zebu cattle (Bos indicus). The Journal of Agricultural Science, 96, 309–312. 10.1017/S0021859600066089 [DOI] [Google Scholar]
- Reinhardt V, Mutiso F and Reinhardt A, 1978. Social behaviour and social relationships between female and male prepubertal bovine calves (Bos indicus). Applied Animal Ethology, 4, 43–54. 10.1016/0304-3762(78)90092-5 [DOI] [Google Scholar]
- Reipurth M, Klausen SK, Denwood M, Forkman B and Houe H, 2020. The effect of age when group housed and other management factors on playing and non‐nutritive sucking behaviour in dairy calves: a cross sectional observational study. Acta Veterinaria Scandinavica, 62, 1–13. 10.1186/s13028-020-00562-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud DL, Duffield TF, LeBlanc SJ, Ferguson S, Haley DB and Kelton DF, 2018. Risk factors associated with mortality at a milk‐fed veal calf facility: a prospective cohort study. Journal of Dairy Science, 101, 2659–2668. 10.3168/jds.2017-13581 [DOI] [PubMed] [Google Scholar]
- Renaud DL, Steele MA, Genore R, Roche SM and Winder CB, 2020. Passive immunity and colostrum management practices on Ontario dairy farms and auction facilities: a cross‐sectional study. Journal of Dairy Science, 103, 8369–8377. 10.3168/jds.2020-18572 [DOI] [PubMed] [Google Scholar]
- Renaudeau D, Collin A, Yahav S, De Basilio V, Gourdine JL and Collier RJ, 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Proceedings of the Animal, 6, 707–728. 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Rinnhofer B and Fürst‐Waltl B, 2008. Einflüsse der Haltungsumwelt und der Genetik auf das gegenseitige Besaugen beim Rind, na.
- Roadknight NW, Courtman NF, Mansell PD, Jongman EC, Loh ZA and Fisher AD, 2021. Biochemistry and hematology reference intervals for neonatal dairy calves aged 5–12 days. Veterinary Clinical Pathology, 50, 278–286. 10.1111/vcp.12955 [DOI] [PubMed] [Google Scholar]
- Roadknight N, Wales W, Jongman E, Mansell P, Hepworth G and Fisher A, 2022. Does the duration of repeated temporary separation affect welfare in dairy cow‐calf contact systems? Applied Animal Behaviour Science, 249, 105592. 10.1016/j.applanim.2022.105592 [DOI] [Google Scholar]
- Rodríguez Alvarez JM, Arroqui M, Mangudo P, Toloza JM, Jatip DE, Rodriguez JM, Zunino Suarez AO, Mateos Diaz CM and Machado C, 2018. Review and analysis of computational techniques and methods for body condition score estimation on cows. Electronic Journal of SADIO (EJS), 17, 48–65. [Google Scholar]
- Roth BA, Hillmann E, Stauffacher M and Keil NM, 2008. Improved weaning reduces cross‐sucking and may improve weight gain in dairy calves. Applied Animal Behaviour Science, 111, 251–261. 10.1016/J.APPLANIM.2007.06.007 [DOI] [Google Scholar]
- Roth BA, Barth K, Gygax L and Hillmann E, 2009a. Influence of artificial vs. mother‐bonded rearing on sucking behaviour, health and weight gain in calves. Applied Animal Behaviour Science, 119, 143–150. 10.1016/j.applanim.2009.03.004 [DOI] [Google Scholar]
- Roth BA, Keil NM, Gygax L and Hillmann E, 2009b. Temporal distribution of sucking behaviour in dairy calves and influence of energy balance. Applied Animal Behaviour Science, 119, 137–142. 10.1016/j.applanim.2009.03.006 [DOI] [Google Scholar]
- Rouha‐Muelleder C, Absmanner E, Kahrer E, Zeiner H, Scharl T, Leisch F, Stanek C and Troxler J, 2012. Alternative housing systems for fattening bulls under Austrian conditions with special respect to rubberised slatted floors. Animal Welfare, 21, 113–126. 10.7120/096272812799129394 [DOI] [Google Scholar]
- Ruis‐Heutinck L, Smits M, Smits A and Heeres J, 2000. Effect of floor and floor area on behaviour and carpal joint lesions in beef bulls. Proceedings of sessions of the EAAP Commission on Animal Management & Health, Improving health and welfare in animal production, The Hague (NL), 21–24 August, EAAP publication No. 102, pp. 29–36. [Google Scholar]
- Rushen J and de Passillé AM, 2012. Automated measurement of acceleration can detect effects of age, dehorning and weaning on locomotor play of calves. Applied Animal Behaviour Science, 139, 169–174. 10.1016/j.applanim.2012.04.011 [DOI] [Google Scholar]
- Rushen J, Wright R, Johnsen JF, Mejdell CM and de Passillé AM, 2016. Reduced locomotor play behaviour of dairy calves following separation from the mother reflects their response to reduced energy intake. Applied Animal Behaviour Science, 177, 6–11. 10.1016/J.APPLANIM.2016.01.023 [DOI] [Google Scholar]
- Saldana D, Jones C, Gehman A and Heinrichs A, 2019. Effects of once‐versus twice‐a‐day feeding of pasteurized milk supplemented with yeast‐derived feed additives on growth and health in female dairy calves. Journal of Dairy Science, 102, 3654–3660. 10.3168/jds.2018-15695 [DOI] [PubMed] [Google Scholar]
- Salter RS, Reuscher KJ and Van Os JM, 2021. Milk‐and starter‐feeding strategies to reduce cross sucking in pair‐housed calves in outdoor hutches. Journal of Dairy Science, 104, 6096–6112. 10.3168/jds.2020-19380 [DOI] [PubMed] [Google Scholar]
- Sandelin A, Härtel H, Seppä‐Lassila L, Kaartinen L, Rautala H, Soveri T and Simojoki H, 2020. Field trial to evaluate the effect of an intranasal respiratory vaccine protocol on bovine respiratory disease incidence and growth in a commercial calf rearing unit. BMC Veterinary Research, 16, 1–10. 10.1186/s12917-020-02294-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santman‐Berends I, de Bont‐Smolenaars A, Roos L, Velthuis A and van Schaik G, 2018. Using routinely collected data to evaluate risk factors for mortality of veal calves. Preventive Veterinary Medicine, 157, 86–93. 10.1016/j.prevetmed.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, 2002. Endocrinology of the stress‐response. In: Becker JB, Breedlove SM, Crews D and McCarthy MM (eds.). Behavioral endocrinology (pp. 409–450). MIT Press. [Google Scholar]
- Sarkozy P, Misley A and Simon F, 1984. Iron deficiency in calves. 3. The survey of consequences of iron deficiency. Magyar Allatorvosok Lapja, Hungary. [Google Scholar]
- Sato S, Wood‐Gush DGM and G W, 1987. Observations on creche behaviour in suckler calves. Behavioural Processes, 15, 333–343. 10.1016/0376-6357(87)90017-9 [DOI] [PubMed] [Google Scholar]
- SCAHAW , 2001. EU Scientific Committee on Animal Health and Animal Welfare Report on the Welfare of Cattle Kept for Beef Production. European Commission, Brussels. Available online:. https://food.ec.europa.eu/system/files/2020-12/sci-com_scah_out54_en.pdf [Google Scholar]
- Schär C, Vidale PL, Lüthi D, Frei C, Häberli C, Liniger MA and Appenzeller C, 2004. The role of increasing temperature variability in European summer heatwaves. Nature, 427, 332–336. 10.1038/nature02300 [DOI] [PubMed] [Google Scholar]
- Scheeder MRL, Becker B and Kreuzer M, 1999. Veal colour and other meat quality characteristics in calves fattened on maize silage and concentrate. Archives Animal Breeding, 42, 535–554. 10.5194/AAB-42-535-1999 [DOI] [Google Scholar]
- Schneider M, Tait R Jr, Busby W and Reecy J, 2009. An evaluation of bovine respiratory disease complex in feedlot cattle: impact on performance and carcass traits using treatment records and lung lesion scores. Journal of Animal Science, 87, 1821–1827. 10.2527/jas.2008-1283 [DOI] [PubMed] [Google Scholar]
- Schnyder P, Schönecker L, Schüpbach‐Regula G and Meylan M, 2019. Effects of management practices, animal transport and barn climate on animal health and antimicrobial use in Swiss veal calf operations. Preventive Veterinary Medicine, 167, 146–157. 10.1016/j.prevetmed.2019.03.007 [DOI] [PubMed] [Google Scholar]
- Schwartz A, 1990. The politics of formula‐fed veal calf production. Journal of the American Veterinary Medical Association, 196, 1578–1586. [PubMed] [Google Scholar]
- Segnalini M, Nardone A, Bernabucci U, Vitali A, Ronchi B and Lacetera N, 2011. Dynamics of the temperature‐humidity index in the Mediterranean basin. International Journal of Biometeorology, 55, 253–263. 10.1007/s00484-010-0331-3 [DOI] [PubMed] [Google Scholar]
- Selman I, McEwan A and Fisher E, 1971. Studies on dairy calves allowed to suckle their dams at fixed times post parttim. Research in Veterinary Science, 12, 1–6. 10.1016/S0034-5288(18)34230-9 [DOI] [PubMed] [Google Scholar]
- Simpson KM, Callan RJ and Van Metre DC, 2018. Clostridial abomasitis and enteritis in ruminants. Veterinary Clinics: Food Animal Practice, 34, 155–184. 10.1016/j.cvfa.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirovnik J, Barth K, De Oliveira D, Ferneborg S, Haskell MJ, Hillmann E, Jensen MB, Mejdell CM, Napolitano F, Vaarst M, Verwer CM, Waiblinger S, Zipp KA and Johnsen JF, 2020. Methodological terminology and definitions for research and discussion of cow‐calf contact systems. Journal of Dairy Research, 87, 108–114. 10.1017/S0022029920000564 [DOI] [PubMed] [Google Scholar]
- Sivula N, Ames T, Marsh W and Werdin R, 1996. Descriptive epidemiology of morbidity and mortality in Minnesota dairy heifer calves. Preventive Veterinary Medicine, 27, 155–171. 10.1016/0167-5877(95)01000-9 [DOI] [Google Scholar]
- Smulders FJ and Algers B, 2009. Welfare of production animals: assessment and management of risks. Wageningen Academic Publishers. 10.3920/978-90-8686-690-8 [DOI] [Google Scholar]
- Somers R, 2012. Play behaviour of dairy and beef calves living in a semi‐natural environment. Utrecht University. Available online:. https://studenttheses.uu.nl/bitstream/handle/20.500.12932/15019/Play%20behaviour%20of%20dairy%20and%20beef%20calves%20-%20Robert%20Somers.pdf?sequence=1&isAllowed=y [Google Scholar]
- Sparke E, Young B, Gaughan J, Holt M and Goodwin P, 2001. Heat load in feedlot cattle. Meat and Livestock Australia, North Sydney, NSW, Australia. [Google Scholar]
- Spoliansky R, Edan Y, Parmet Y and Halachmi I, 2016. Development of automatic body condition scoring using a low‐cost 3‐dimensional Kinect camera. Journal of Dairy Science, 99, 7714–7725. 10.3168/JDS.2015-10607 [DOI] [PubMed] [Google Scholar]
- Stabel J, Hurd S, Calvente L and Rosenbusch R, 2004. Destruction of Mycobacterium paratuberculosis, Salmonella spp., and Mycoplasma spp. in raw milk by a commercial on‐farm high‐temperature, short‐time pasteurizer. Journal of Dairy Science, 87, 2177–2183. 10.3168/jds.S0022-0302(04)70038-7 [DOI] [PubMed] [Google Scholar]
- Stafford KJ and Mellor DJ, 2005. Dehorning and disbudding distress and its alleviation in calves. Veterinary Journal, 169, 337–349. 10.1016/j.tvjl.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Staněk S, Zink V, Doležal O and Štolc L, 2014. Survey of preweaning dairy calf‐rearing practices in Czech dairy herds. Journal of Dairy Science, 97, 3973–3981. 10.3168/jds.2013-7325 [DOI] [PubMed] [Google Scholar]
- Stanley C, Williams C, Jenny B, Fernandez J, Bateman Ii H, Nipper W, Lovejoy J, Gantt D and Goodier G, 2002. Effects of feeding milk replacer once versus twice daily on glucose metabolism in Holstein and Jersey calves. Journal of Dairy Science, 85, 2335–2343. 10.3168/jds.S0022-0302(02)74313-0 [DOI] [PubMed] [Google Scholar]
- Stefanowska J, Swierstra D, Smits A, Berg JVD and Metz J, 2002. Reaction of calves to two flooring materials offered simultaneously in one pen. Acta Agriculturae Scandinavica, Section A‐Animal Science, 52, 57–64. 10.1080/09064700212076 [DOI] [Google Scholar]
- Stěhulová I, Lidfors L and Špinka M, 2008. Response of dairy cows and calves to early separation: effect of calf age and visual and auditory contact after separation. Applied Animal Behaviour Science, 110, 144–165. 10.1016/J.APPLANIM.2007.03.028 [DOI] [Google Scholar]
- Stilwell G and Carvalho RC, 2011. Clinical outcome of calves with failure of passive transfer as diagnosed by a commercially available IgG quick test kit. The Canadian Veterinary Journal, 52, 524. Available online:–526. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3078008/ [PMC free article] [PubMed] [Google Scholar]
- Stilwell G, Lima MS and Broom DM, 2007. Comparing the effect of three different disbudding methods on behaviour and plasma cortisol of calves Comparação do efeito de três métodos de descorna sobre o comportamento e o cortisol plasmatico de vitelas.. Revista Portuguesa Cinecias Veterinaria, 102, 281–288. [Google Scholar]
- Stilwell G, de Carvalho RC, Lima MS and Broom DM, 2009. Effect of caustic paste disbudding, using local anaesthesia with and without analgesia, on behaviour and cortisol of calves. Applied Animal Behaviour Science, 116, 35–44. 10.1016/J.APPLANIM.2008.06.008 [DOI] [Google Scholar]
- Stilwell G, Vieira A, Rodrigues T, Braz M, Carreira M and Carolino N, 2013. Effect of selenium and vitamin E, age and weight on daily weight gain and respiratory disease prevalence in feedlot calves. Proceedings of the 31st World Veterinary Congress Prague, Czech Republic. pp. 184. [Google Scholar]
- Stocker H, Lutz H and Rüsch P, 1999. Clinical, haematological and biochemical findings in milk‐fed calves with chronic indigestion. Veterinary Record, 145, 307–311. 10.1136/vr.145.11.307 [DOI] [PubMed] [Google Scholar]
- Stott G, Marx D, Menefee B and Nightengale G, 1979. Colostral immunoglobulin transfer in calves. IV. Effect of suckling. Journal of Dairy Science, 62, 1908–1913. 10.3168/jds.S0022-0302(79)83522-5 [DOI] [PubMed] [Google Scholar]
- Stracke J, Andersson R, Volkmann N, Spindler B, Schulte‐Landwehr J, Günther R and Kemper N, 2022. Footpad monitoring: reliability of an automated system to assess footpad dermatitis in Turkeys (Meleagris gallopavo) during slaughter. Frontiers in Veterinary Science, 9. 10.3389/fvets.2022.888503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull C and McDonough S, 1994. Multidisciplinary approach to evaluating welfare of veal calves in commercial facilities. Journal of Animal Science, 72, 2518–2524. 10.2527/1994.7292518x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stygar AH, Gómez Y, Berteselli GV, Dalla Costa E, Canali E, Niemi JK, Llonch P and Pastell M, 2021. A systematic review on commercially available and validated sensor technologies for welfare assessment of dairy cattle. Frontiers in Veterinary Science, 8, 634338. 10.3389/fvets.2021.634338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez B, Van Reenen C, Gerrits W, Stockhofe N, Van Vuuren A and Dijkstra J, 2006. Effects of supplementing concentrates differing in carbohydrate composition in veal calf diets: II. Rumen development. Journal of Dairy Science, 89, 4376–4386. 10.3168/jds.S0022-0302(06)72484-5 [DOI] [PubMed] [Google Scholar]
- Sutherland MA, Stewart M and Schütz KE, 2013. Effects of two substrate types on the behaviour, cleanliness and thermoregulation of dairy calves. Applied Animal Behaviour Science, 147, 19–27. 10.1016/j.applanim.2013.04.018 [DOI] [Google Scholar]
- Sutherland MA, Worth GM, Schütz KE and Stewart M, 2014a. Rearing substrate and space allowance influences locomotor play behaviour of dairy calves in an arena test. Applied Animal Behaviour Science, 154, 8–14. 10.1016/j.applanim.2014.02.008 [DOI] [Google Scholar]
- Sutherland MA, Worth GM and Stewart M, 2014b. The effect of rearing substrate and space allowance on the behavior and physiology of dairy calves. Journal of Dairy Science, 97, 4455–4463. 10.3168/JDS.2013-7822 [DOI] [PubMed] [Google Scholar]
- Sutherland M, Worth G, Cameron C, Ross C and Rapp D, 2017. Health, physiology, and behavior of dairy calves reared on 4 different substrates. Journal of Dairy Science, 100, 2148–2156. 10.3168/jds.2016-12074 [DOI] [PubMed] [Google Scholar]
- Svensson C and Liberg P, 2006. The effect of group size on health and growth rate of Swedish dairy calves housed in pens with automatic milk‐feeders. Preventive Veterinary Medicine, 73, 43–53. 10.1016/J.PREVETMED.2005.08.021 [DOI] [PubMed] [Google Scholar]
- Svensson C, Lundborg K, Emanuelson U and Olsson SO, 2003. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf‐level risk factors for infectious diseases. Preventive Veterinary Medicine, 58, 179–197. 10.1016/S0167-5877(03)00046-1 [DOI] [PubMed] [Google Scholar]
- Swanson EW and Harris JD, 1958. Development of Rumination in the Young Calf. Journal of Dairy Science, 41, 1768–1776. 10.3168/JDS.S0022-0302(58)91161-5 [DOI] [Google Scholar]
- Tapki I, 2007. Comparison of two conventional restricted daily milk allowance methods in dairy calf rearing with respect to growth and behavioural responses 1. growth responses. Journal of Animal and Veterinary Advances, 6, 416–420. [Google Scholar]
- Tapkı İ, Şahin A and Önal AG, 2006. Effect of space allowance on behaviour of newborn milk‐fed dairy calves. Applied Animal Behaviour Science, 99, 12–20. 10.1016/j.applanim.2005.09.006 [DOI] [Google Scholar]
- Taschke AC and Fölsch DW, 1997. Ethological, physiological and histological aspects of pain and stress in cattle when being dehorned. Tierarztliche Praxis, 25, 19–27. [PubMed] [Google Scholar]
- Taylor JD, Fulton RW, Lehenbauer TW, Step DL and Confer AW, 2010. The epidemiology of bovine respiratory disease: what is the evidence for predisposing factors? The Canadian Veterinary Journal, 51, 1095. Available online:–1102. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2942046/ [PMC free article] [PubMed] [Google Scholar]
- Telezhenko E and Bergsten C, 2005. Influence of floor type on the locomotion of dairy cows. Applied Animal Behaviour Science, 93, 183–197. 10.1016/j.applanim.2004.11.021 [DOI] [Google Scholar]
- Telezhenko E, Lidfors L and Bergsten C, 2007. Dairy cow preferences for soft or hard flooring when standing or walking. Journal of Dairy Science, 90, 3716–3724. 10.3168/jds.2006-876 [DOI] [PubMed] [Google Scholar]
- Telezhenko E, Magnusson M and Bergsten C, 2017. Gait of dairy cows on floors with different slipperiness. Journal of Dairy Science, 100, 6494–6503. 10.3168/jds.2016-12208 [DOI] [PubMed] [Google Scholar]
- Thomas TJ, Weary DM and Appleby MC, 2001. Newborn and 5‐week‐old calves vocalize in response to milk deprivation. Applied Animal Behaviour Science, 74, 165–173. 10.1016/S0168-1591(01)00164-2 [DOI] [Google Scholar]
- Thomsen PT, Hansen JH and Herskin MS, 2021. Dairy calves show behavioural responses to hot iron disbudding after local anaesthesia with procaine. Veterinary Record, 188, e52. 10.1002/vetr.52 [DOI] [PubMed] [Google Scholar]
- Timmerman HM, Mulder L, Everts H, Van Espen D, Van Der Wal E, Klaassen G, Rouwers S, Hartemink R, Rombouts F and Beynen A, 2005. Health and growth of veal calves fed milk replacers with or without probiotics. Journal of Dairy Science, 88, 2154–2165. 10.3168/jds.S0022-0302(05)72891-5 [DOI] [PubMed] [Google Scholar]
- Torsein M, Lindberg A, Sandgren CH, Waller KP, Törnquist M and Svensson C, 2011. Risk factors for calf mortality in large Swedish dairy herds. Preventive Veterinary Medicine, 99, 136–147. 10.1016/j.prevetmed.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman AR, Bergamini L, Palazzi A, Porrello A, Capobianco Dondona A, Del Negro E, Paolini A, Vignola G, Calderara S and Marruchella G, 2020. Scoring pleurisy in slaughtered pigs using convolutional neural networks. Veterinary Research, 51, 1–9. 10.1186/S13567-020-00775-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranter W and Morris R, 1992. Hoof growth and wear in pasture‐fed dairy cattle. [DOI] [PubMed]
- Trunkfield HR and Broom DM, 1990. The welfare of calves during handling and transport. Applied Animal Behaviour Science, 28, 135–152. 10.1016/0168-1591(90)90050-N [DOI] [Google Scholar]
- Tucker C and Weary D, 2004. Bedding on geotextile mattresses: how much is needed to improve cow comfort? Journal of Dairy Science, 87, 2889–2895. 10.3168/jds.S0022-0302(04)73419-0 [DOI] [PubMed] [Google Scholar]
- Tuomisto L, Huuskonen A, Ahola L and Kauppinen R, 2009. Different housing systems for growing dairy bulls in Northern Finland–effects on performance, behaviour and immune status. Acta Agriculturae Scand Section A, 59, 35–47. 10.1080/09064700902919074 [DOI] [Google Scholar]
- Ude G, Georg H and Schwalm A, 2011. Reducing milk induced cross‐sucking of group housed calves by an environmentally enriched post feeding area. Livestock Science, 138, 293–298. 10.1016/j.livsci.2010.12.004 [DOI] [Google Scholar]
- Ural DA, Ural K, Erdogan H and Gültekin M, 2021. Inter‐and cross‐sucking in simmental and Holstein‐Friesian calves with special interpretation of farm and gender basis. International Journal of Veterinary and Animal Research (IJVAR), 4, 83–86. Available online:. https://ijvar.org/index.php/ijvar/article/view/517 [Google Scholar]
- Uvnäs‐Moberg K, 1998. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology, 23, 819–835. 10.1016/S0306-4530(98)00056-0 [DOI] [PubMed] [Google Scholar]
- Uvnäs‐Moberg K, Johansson B, Lupoli B and Svennersten‐Sjaunja K, 2001. Oxytocin facilitates behavioural, metabolic and physiological adaptations during lactation. Applied Animal Behaviour Science, 72, 225–234. 10.1016/S0168-1591(01)00112-5 [DOI] [PubMed] [Google Scholar]
- Van De Water G , Verjans F and Geers R, 2003. The effect of short distance transport under commercial conditions on the physiology of slaughter calves; pH and colour profiles of veal. Livestock Production Science, 82, 171–179. 10.1016/S0301-6226(03)00010-1 [DOI] [Google Scholar]
- Van der Mei J and Van den Ingh T, 1987. Lung and pleural lesions of veal calves at slaughter and their relationship with carcass weight. Veterinary Quarterly, 9, 203–207. 10.1080/01652176.1987.9694101 [DOI] [PubMed] [Google Scholar]
- Van Metre DC, Tennant BC and Whitlock RH, 2008. Infectious diseases of the gastrointestinal tract. Rebhun's Diseases of Dairy Cattle, 200–200. 10.1016/B978-141603137-6.50009-0 [DOI] [Google Scholar]
- Van Os JM, Mintline EM, DeVries TJ and Tucker CB, 2018. Domestic cattle (Bos taurus taurus) are motivated to obtain forage and demonstrate contrafreeloading. PLOS One, 13, e0193109. 10.1371/journal.pone.0193109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest PJ, Robertson JB and Lewis BA, 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science, 74, 3583–3597. 10.3168/JDS.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Vandoni S and Sgoifo Rossi CA, 2009. Instrumental objective measurement of veal calves carcass colour at slaughterhouse. Italian Journal of Animal Science, 8, 552–554. 10.4081/ijas.2009.s2.552 [DOI] [Google Scholar]
- Vass‐Bognár B, Bakony M, Baumgartner W, Khol JL and Jurkovich V, 2022. Association between calf rearing technology and farm‐level paratuberculosis infection in Hungarian dairy farms. Preventive Veterinary Medicine, 207, 105719. 10.1016/j.prevetmed.2022.105719 [DOI] [PubMed] [Google Scholar]
- Veissier I, Le Neindre P and Trillat G, 1989. The use of circadian behaviour to measure adaptation of calves to changes in their environment. Applied Animal Behaviour Science, 22, 1–12. 10.1016/0168-1591(89)90075-0 [DOI] [Google Scholar]
- Veissier I, Lamy D and Le Neindre P, 1990. Social behaviour in domestic beef cattle when yearling calves are left with the cows for the next calving. Applied Animal Behaviour Science, 27, 193–200. 10.1016/0168-1591(90)90056-J [DOI] [Google Scholar]
- Veissier I, Gesmier V, Le Neindre P, Gautier J‐Y and Bertrand G, 1994. The effects of rearing in individual crates on subsequent social behaviour of veal calves. Applied Animal Behaviour Science, 41, 199–210. 10.1016/0168-1591(94)90023-X [DOI] [Google Scholar]
- Veissier I, Boissy A, de Passillé AM, Rushen J, Van Reenen C, Roussel S, Andanson S and Pradel P, 2001. Calves' responses to repeated social regrouping and relocation. Journal of Animal Science, 79, 2580–2593. 10.2527/2001.79102580x [DOI] [PubMed] [Google Scholar]
- Veissier I, Caré S and Pomiès D, 2013. Suckling, weaning, and the development of oral behaviours in dairy calves. Applied Animal Behaviour Science, 147, 11–18. 10.1016/J.APPLANIM.2013.05.002 [DOI] [Google Scholar]
- Vial F and Reist M, 2015. Comparison of whole carcass condemnation and partial carcass condemnation data for integration in a national syndromic surveillance system: the Swiss experience. Meat Science, 101, 48–55. 10.1016/j.meatsci.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Vitale AF, Tenucci M, Papini M and Lovari S, 1986. Social behaviour of the calves of semi‐wild Maremma cattle, Bos primigenius taurus. Applied Animal Behaviour Science, 16, 217–231. 10.1016/0168-1591(86)90115-2 [DOI] [Google Scholar]
- Völker H and Rotermund L, 2000. Possibilities of oral iron supplementation for maintaining health status in calves. DTW. Deutsche Tierarztliche Wochenschrift, 107, 16–22. [PubMed] [Google Scholar]
- von Keyserlingk MAG and Weary DM, 2007. Maternal behavior in cattle. Hormones and Behavior, 52, 106–113. 10.1016/J.YHBEH.2007.03.015 [DOI] [PubMed] [Google Scholar]
- von Keyserlingk M, Brusius L and Weary D, 2004. Competition for teats and feeding behavior by group‐housed dairy calves. Journal of Dairy Science, 87, 4190–4194. 10.3168/jds.S0022-0302(04)73563-8 [DOI] [PubMed] [Google Scholar]
- von Keyserlingk M, Cunha G, Fregonesi J and Weary D, 2011. Introducing heifers to freestall housing. Journal of Dairy Science, 94, 1900–1907. 10.3168/jds.2010-3994 [DOI] [PubMed] [Google Scholar]
- Wageningen UR Livestock Research , 2010. Animal welfare risk assessment guidelines on housing and management (EFSA Housing Risk). EFSA Supporting Publication 2010;7(11):EN‐87, 283 pp. 10.2903/sp.efsa.2010.EN-87 [DOI] [Google Scholar]
- Wagner K, Barth K, Palme R, Futschik A and Waiblinger S, 2012. Integration into the dairy cow herd: long‐term effects of mother contact during the first twelve weeks of life. Applied Animal Behaviour Science, 141, 117–129. 10.1016/j.applanim.2012.08.011 [DOI] [Google Scholar]
- Wagner K, Barth K, Hillmann E, Palme R, Futschik A and Waiblinger S, 2013. Mother rearing of dairy calves: reactions to isolation and to confrontation with an unfamiliar conspecific in a new environment. Applied Animal Behaviour Science, 147, 43–54. 10.1016/j.applanim.2013.04.010 [DOI] [Google Scholar]
- Wagner K, Seitner D, Barth K, Palme R, Futschik A and Waiblinger S, 2015. Effects of mother versus artificial rearing during the first 12 weeks of life on challenge responses of dairy cows. Applied Animal Behaviour Science, 164, 1–11. 10.1016/j.applanim.2014.12.010 [DOI] [Google Scholar]
- Waiblinger S, Boivin X, Pedersen V, Tosi M‐V, Janczak AM, Visser EK and Jones RB, 2006. Assessing the human–animal relationship in farmed species: a critical review. Applied Animal Behaviour Science, 101, 185–242. 10.1016/j.applanim.2006.02.001 [DOI] [Google Scholar]
- Waiblinger S, Wagner K, Hillmann E and Barth K, 2020. Play and social behaviour of calves with or without access to their dam and other cows. Journal of Dairy Research, 87, 144–147. 10.1017/S0022029920000540 [DOI] [PubMed] [Google Scholar]
- Walker W, Epperson W, Wittum TE, Lord LK, Rajala‐Schultz PJ and Lakritz J, 2012. Characteristics of dairy calf ranches: morbidity, mortality, antibiotic use practices, and biosecurity and biocontainment practices. Journal of Dairy Science, 95, 2204–2214. 10.3168/jds.2011-4727 [DOI] [PubMed] [Google Scholar]
- Wang J, Li J, Wang F, Xiao J, Wang Y, Yang H, Li S and Cao Z, 2020. Heat stress on calves and heifers: a review. Journal of Animal Science and Biotechnology, 11, 1–8. 10.1186/s40104-020-00485-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathes C, Jones C and Webster A, 1983. Ventilation, air hygiene and animal health. The Veterinary Record, 113, 554–559. [PubMed] [Google Scholar]
- Weary DM and Chua B, 2000. Effects of early separation on the dairy cow and calf: 1. Separation at 6 h, 1 day and 4 days after birth. Applied Animal Behaviour Science, 69, 177–188. 10.1016/S0168-1591(00)00128-3 [DOI] [PubMed] [Google Scholar]
- Weaver AD, Atkinson O, Jean GS and Steiner A, 1986. Bovine surgery and lameness. John Wiley & Sons. [Google Scholar]
- Weaver DM, Tyler JW, VanMetre DC, Hostetler DE and Barrington GM, 2000. Passive transfer of colostral immunoglobulins in calves. Journal of Veterinary Internal Medicine, 14, 569–577. 10.1111/j.1939-1676.2000.tb02278.x [DOI] [PubMed] [Google Scholar]
- Webb LE, Bokkers EAM, Engel B, Gerrits WJJ, Berends H and van Reenen CG, 2012. Behaviour and welfare of veal calves fed different amounts of solid feed supplemented to a milk replacer ration adjusted for similar growth. Applied Animal Behaviour Science, 136, 108–116. 10.1016/J.APPLANIM.2011.12.004 [DOI] [Google Scholar]
- Webb LE, Bokkers EAM, Heutinck LFM, Engel B, Buist WG, Rodenburg TB, Stockhofe‐Zurwieden N and van Reenen CG, 2013. Effects of roughage source, amount, and particle size on behavior and gastrointestinal health of veal calves. Journal of Dairy Science, 96, 7765–7776. 10.3168/JDS.2012-6135 [DOI] [PubMed] [Google Scholar]
- Webb LE, Engel B, Berends H, van Reenen CG, Gerrits WJ, de Boer IJ and Bokkers EA, 2014a. What do calves choose to eat and how do preferences affect behaviour? Applied Animal Behaviour Science, 161, 7–19. 10.1016/j.applanim.2014.09.016 [DOI] [Google Scholar]
- Webb LE, Jensen MB, Engel B, Van Reenen CG, Gerrits WJJ, De Boer IJM and Bokkers EAM, 2014b. Chopped or long roughage: what do calves prefer? Using cross point analysis of double demand functions. PLoS ONE, 9, e88778–e88778. 10.1371/JOURNAL.PONE.0088778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb LE, van Reenen CG, Berends H, Engel B, de Boer IJM, Gerrits WJJ and Bokkers EAM, 2015. The role of solid feed amount and composition and of milk replacer supply in veal calf welfare. Journal of Dairy Science, 98, 5467–5481. 10.3168/JDS.2014-8547 [DOI] [PubMed] [Google Scholar]
- Webb L, Van Reenen C, Engel B, Berends H, Gerrits WJ and Bokkers EM, 2017. Understanding oral stereotypies in calves: alternative strategies, hypothalamic–pituitary–adrenal axis (re) activity and gene by environment interactions. Animal, 11, 1054–1062. 10.1017/S1751731116002226 [DOI] [PubMed] [Google Scholar]
- Webb L, Marcato F, Bokkers E, Verwer C, Wolthuis‐Fillerup M, Hoorweg F, van den Brand H, Jensen M and van Reenen C, 2022. Impact of early dam contact on veal calf welfare. Scientific Reports, 12, 22144. 10.1038/s41598-022-25804-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R and Wechsler B, 2001. Reduction in cross‐sucking in calves by the use of a modified automatic teat feeder. Applied Animal Behaviour Science, 72, 215–223. 10.1016/S0168-1591(01)00111-3 [DOI] [PubMed] [Google Scholar]
- Webster A, Saville C, Church B, Gnanasakthy A and Moss R, 1985. The effect of different rearing systems on the development of calf behaviour. British Veterinary Journal, 141, 249–264. 10.1016/0007-1935(85)90061-2 [DOI] [PubMed] [Google Scholar]
- Wechsler B, 2011. Floor quality and space allowance in intensive beef production: a review. Animal Welfare, 20, 497–503. 10.1017/S0962728600003134 [DOI] [Google Scholar]
- Welchman D, Whelehan O and Webster A, 1988. Haematology of veal calves reared in different husbandry systems and the assessment of iron deficiency. The Veterinary Record, 123, 505–510. 10.1136/vr.123.20.505 [DOI] [PubMed] [Google Scholar]
- Welfare Quality® , 2009. Welfare Quality® assessment protocol for cattle. Welfare Quality® Consortium, Lelystad, Netherlands. 180 p.. [Google Scholar]
- Wenker ML, Bokkers EAM, Lecorps B, von Keyserlingk MAG, van Reenen CG, Verwer CM and Weary DM, 2020. Effect of cow‐calf contact on cow motivation to reunite with their calf. Scientific RepoRtS, 10, 14233. 10.1038/s41598-020-70927-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker ML, van Reenen CG, Bokkers EAM, McCrea K, de Oliveira D, Sørheim K, Cao Y, Bruckmaier RM, Gross JJ, Gort G and Verwer CM, 2022. Comparing gradual debonding strategies after prolonged cow‐calf contact: stress responses, performance, and health of dairy cow and calf. Applied Animal Behaviour Science, 253, 105694. 10.1016/j.applanim.2022.105694 [DOI] [Google Scholar]
- Whalin L, Weary DM and von Keyserlingk MAG, 2018. Short communication: Pair housing dairy calves in modified calf hutches. Journal of Dairy Science, 101, 5428–5433. 10.3168/JDS.2017-14361 [DOI] [PubMed] [Google Scholar]
- Whalin L, Weary DM and von Keyserlingk MA, 2021. Understanding behavioural development of calves in natural settings to inform calf management. Animals, 11, 2446. 10.3390/ani11082446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorreck L and Hillmann E, 2022. Ist ammengebundene Aufzucht eine tiergerechte Alternative zu künstlicher Aufzucht von Milchviehkälbern? Proceedings of the Aktuelle Arbeiten zur artgemäßen Tierhaltung, KTBL, Darmstadt, 90–100 pp. [Google Scholar]
- Wiepkema P, Van Hellemond K, Roessingh P and Romberg H, 1987. Behaviour and abomasal damage in individual veal calves. Applied Animal Behaviour Science, 18, 257–268. 10.1016/0168-1591(87)90221-8 [DOI] [Google Scholar]
- Wilson LL, Smith JL, Smith DL, Swanson DL, Drake TR, Wolfgang DR and Wheeler EF, 2000. Characteristics of veal calves upon arrival, at 28 and 84 days, and at end of the production cycle. Journal of Dairy Science, 83, 843–854. 10.3168/JDS.S0022-0302(00)74948-4 [DOI] [PubMed] [Google Scholar]
- Winder CB, Kelton DF and Duffield TF, 2016. Mortality risk factors for calves entering a multi‐location white veal farm in Ontario, Canada. Journal of Dairy Science, 99, 10174–10181. 10.3168/jds.2016-11345 [DOI] [PubMed] [Google Scholar]
- Wittek T, Köchler J and Mader C, 2014. Untersuchungen zur Eisenversorgung von Mastkälbern in Tirol. Wiener Tierärztliche Monatsschrift, 101, 20–24. Available online: https://www.wtm.at/smart_users/uni/user94/explorer/43/WTM/Archiv/2014/WTM_01-02-2014_Artikel_3_Art.1321.pdf [Google Scholar]
- Wittum T, Woollen N, Perino L and Littledike E, 1996. Relationships among treatment for respiratory tract disease, pulmonary lesions evident at slaughter, and rate of weight gain in feedlot cattle. Journal of the American Veterinary Medical Association, 209, 814–818. [PubMed] [Google Scholar]
- Wood‐Gush DG and Vestergaard K, 1989. Exploratory behavior and the welfare of intensively kept animals. Journal of Agricultural Ethics, 2, 161–169. 10.1007/BF01826929 [DOI] [Google Scholar]
- Wood‐Gush DGM and Vestergaard K, 1991. The seeking of novelty and its relation to play. Animal Behaviour, 42, 599–606. 10.1016/S0003-3472(05)80243-X [DOI] [Google Scholar]
- Wood‐Gush DM, Hunt K, Carson K and Dennison SC, 1984. The early behaviour of suckler calves in the field. Biology of Behaviour (Paris), 9, 295–306. [Google Scholar]
- Woolums AR, 2013. BRD in preweaned calves: What's new in risk factors? Proceedings of the American Association of Bovine Practitioners Conference Proceedings, 45–48. 10.21423/aabppro20133780 [DOI] [Google Scholar]
- Woolums AR and Step DL, 2020. Bovine respiratory disease: what's new? Veterinary Clinics: Food Animal Practice, 36, xv–xvi. 10.1016/j.cvfa.2020.04.001 [DOI] [PubMed] [Google Scholar]
- Zhang C, Juniper DT and Meagher RK, 2022. Effects of physical enrichment and pair housing before weaning on growth, behaviour and cognitive ability of calves after weaning and regrouping. Applied Animal Behaviour Science, 249, 105606. 10.1016/j.applanim.2022.105606 [DOI] [Google Scholar]
- Zhao K, Shelley AN, Lau DL, Dolecheck KA and Bewley JM, 2020. Automatic body condition scoring system for dairy cows based on depth‐image analysis. International Journal of Agricultural and Biological Engineering, 13, 45–54. 10.25165/j.ijabe.20201304.5655 [DOI] [Google Scholar]
- Zipp KA and Knierim U, 2020. Physical development, ease of integration into the dairy herd and performance of primiparous dairy cows reared with full whole‐day, half‐day or no mother‐contact as calves. Journal of Dairy Research, 87, 154–156. 10.1017/S002202992000059X [DOI] [PubMed] [Google Scholar]
- Zobel G, Neave HW, Henderson HV and Webster J, 2017. Calves use an automated brush and a hanging rope when pair‐housed. Animals, 7, 84. 10.3390/ani7110084 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Outcome of the public consultation on the welfare of calves


