Abstract
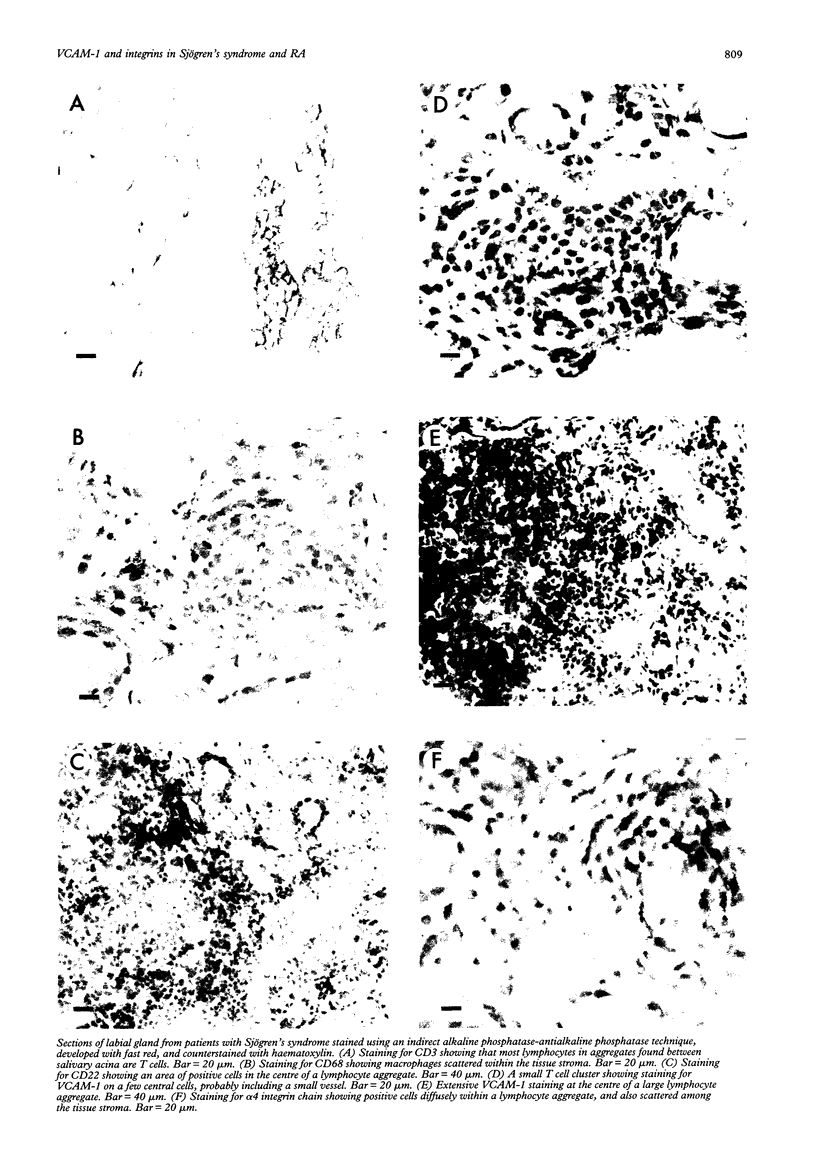
OBJECTIVES--Interactions between vascular cell adhesion molecule 1 (VCAM-1) and its ligand, the alpha 4/beta 1 integrin, have been shown to be important in a number of cellular events in vitro. To assess the importance of such interactions in the development of lymphocytic infiltration in diseased tissue the distribution of the two ligands has been studied immunohistochemically. METHODS--Cryostat sections of labial tissue from patients with Sjögren's syndrome, normal labial tissues, rheumatoid synovia, and normal tonsils were stained using antibodies to VCAM-1, alpha 4 and beta 1 integrin chains, and markers for T cells, B cells, macrophages, and follicular dendritic reticulum cells (FDRCs), visualised using alkaline phosphatase and fast red. RESULTS--Staining patterns for VCAM-1 and integrin chains in lymphocyte aggregates in synovial and labial tissues were similar. VCAM-1 staining was found on both vascular and ramifying dendritic cells at the centre of large T cell aggregates and in all aggregates where there was a central clustering of B cells. VCAM-1 colocalised with, but also extended beyond, staining for the FDRC marker R4/23. Staining for the alpha 4 and beta 1 integrin chains was more widespread than staining for VCAM-1, with no significant increase in staining at sites of maximum VCAM-1 staining. In tonsils VCAM-1 and R4/23 codistributed in germinal centres, but staining for the alpha 4 and beta 1 integrin chains was chiefly seen in T lymphocyte areas. CONCLUSIONS--VCAM-1 may be more important in determining the distribution of B than T lymphocytes in lymphocytic infiltration of non-lymphoid tissue. Unlike the follicles of lymphoid tissue, ectopic follicle-like structures in non-lymphoid tissues may form by immigration of B cells via VCAM-1+ vessels at the centre of T cell aggregates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Jakubowski A., Newman B. M., Rosa M. D., Chi-Rosso G., Lobb R. R. Signaling by vascular cell adhesion molecule-1 (VCAM-1) through VLA-4 promotes CD3-dependent T cell proliferation. Eur J Immunol. 1991 Nov;21(11):2871–2875. doi: 10.1002/eji.1830211132. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Mason D. Y., Pulford K., Falini B., Bliss E., Gatter K. C., Stein H., Clarke L. C., McGee J. O. Immunohistological analysis of human mononuclear phagocytes and dendritic cells by using monoclonal antibodies. Lab Invest. 1986 Mar;54(3):322–335. [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Rice G. E., Bevilacqua M. P., Morimoto C., McIntyre B. W., Rhynhart K., Pober J. S., Nadler L. M. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990 Aug 31;249(4972):1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- Freemont A. J., Jones C. J., Bromley M., Andrews P. Changes in vascular endothelium related to lymphocyte collections in diseased synovia. Arthritis Rheum. 1983 Dec;26(12):1427–1433. doi: 10.1002/art.1780261203. [DOI] [PubMed] [Google Scholar]
- Gerli R., Agea E., Bertotto A., Tognellini R., Flenghi L., Spinozzi F., Velardi A., Grignani F. Analysis of T cells bearing different isotypic forms of the gamma/delta T cell receptor in patients with systemic autoimmune diseases. J Rheumatol. 1991 Oct;18(10):1504–1510. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y., Shimizu H., Yoshida M., Takaya M., Arimori S. T cells bearing gamma/delta T cell receptor and their expression of activation antigen in peripheral blood from patients with Sjögren's syndrome. Clin Exp Rheumatol. 1991 Nov-Dec;9(6):603–609. [PubMed] [Google Scholar]
- Janossy G., Bofill M., Schuurman H. J. Human B-lymphoid differentiation: normal versus malignant. Neth J Med. 1991 Oct;39(3-4):232–243. [PubMed] [Google Scholar]
- Lasky H. P., Bauer K., Pope R. M. Increased helper inducer and decreased suppressor inducer phenotypes in the rheumatoid joint. Arthritis Rheum. 1988 Jan;31(1):52–59. doi: 10.1002/art.1780310108. [DOI] [PubMed] [Google Scholar]
- Lortan J. E., Roobottom C. A., Oldfield S., MacLennan I. C. Newly produced virgin B cells migrate to secondary lymphoid organs but their capacity to enter follicles is restricted. Eur J Immunol. 1987 Sep;17(9):1311–1316. doi: 10.1002/eji.1830170914. [DOI] [PubMed] [Google Scholar]
- Matthews J. B., Deacon E. M., Kitas G. D., Salmon M., Potts A. J., Hamburger J., Bacon P. A. Primed and naive helper T cells in labial glands from patients with Sjogren's syndrome. Virchows Arch A Pathol Anat Histopathol. 1991;419(3):191–197. doi: 10.1007/BF01626347. [DOI] [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Stein H., Mason D. Y. Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J Clin Pathol. 1983 Feb;36(2):167–175. doi: 10.1136/jcp.36.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Bogue D. T., Ramberg J., Lipsky P. E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991 Nov 1;147(9):2913–2921. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Pelton B. K., Harvey A. R., Denman A. M. The rheumatoid synovial membrane participates in systemic anti-viral immune responses. Clin Exp Immunol. 1985 Dec;62(3):657–661. [PMC free article] [PubMed] [Google Scholar]
- Postigo A. A., Pulido R., Campanero M. R., Acevedo A., García-Pardo A., Corbi A. L., Sanchez-Madrid F., De Landazuri M. O. Differential expression of VLA-4 integrin by resident and peripheral blood B lymphocytes. Acquisition of functionally active alpha 4 beta 1-fibronectin receptors upon B cell activation. Eur J Immunol. 1991 Oct;21(10):2437–2445. doi: 10.1002/eji.1830211021. [DOI] [PubMed] [Google Scholar]
- Schmid U., Helbron D., Lennert K. Development of malignant lymphoma in myoepithelial sialadenitis (Sjögren's syndrome). Virchows Arch A Pathol Anat Histol. 1982;395(1):11–43. doi: 10.1007/BF00443482. [DOI] [PubMed] [Google Scholar]
- Skopouli F. N., Fox P. C., Galanopoulou V., Atkinson J. C., Jaffe E. S., Moutsopoulos H. M. T cell subpopulations in the labial minor salivary gland histopathologic lesion of Sjögren's syndrome. J Rheumatol. 1991 Feb;18(2):210–214. [PubMed] [Google Scholar]
- Szakal A. K., Gieringer R. L., Kosco M. H., Tew J. G. Isolated follicular dendritic cells: cytochemical antigen localization, Nomarski, SEM, and TEM morphology. J Immunol. 1985 Mar;134(3):1349–1359. [PubMed] [Google Scholar]
- Sánchez-Madrid F., De Landázuri M. O., Morago G., Cebrián M., Acevedo A., Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986 Nov;16(11):1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- Wellicome S. M., Thornhill M. H., Pitzalis C., Thomas D. S., Lanchbury J. S., Panayi G. S., Haskard D. O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990 Apr 1;144(7):2558–2565. [PubMed] [Google Scholar]
- Wernick R. M., Lipsky P. E., Marban-Arcos E., Maliakkal J. J., Edelbaum D., Ziff M. IgG and IgM rheumatoid factor synthesis in rheumatoid synovial membrane cell cultures. Arthritis Rheum. 1985 Jul;28(7):742–752. doi: 10.1002/art.1780280704. [DOI] [PubMed] [Google Scholar]
- Wilkinson L. S., Edwards J. C., Poston R. N., Haskard D. O. Expression of vascular cell adhesion molecule-1 in normal and inflamed synovium. Lab Invest. 1993 Jan;68(1):82–88. [PubMed] [Google Scholar]
- Zumla A., Mathur M., Stewart J., Wilkinson L., Isenberg D. T cell receptor expression in Sjögren's syndrome. Ann Rheum Dis. 1991 Oct;50(10):691–693. doi: 10.1136/ard.50.10.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinther-Janssen A. C., Horst E., Koopman G., Newmann W., Scheper R. J., Meijer C. J., Pals S. T. The VLA-4/VCAM-1 pathway is involved in lymphocyte adhesion to endothelium in rheumatoid synovium. J Immunol. 1991 Dec 15;147(12):4207–4210. [PubMed] [Google Scholar]



