Abstract
Amniotic fluid embolism (AFE) is a rare but potentially fatal complication of pregnancy. Prompt and aggressive resuscitative strategies are crucial in promoting survivability. We present a case of AFE resulting in cardiopulmonary collapse and subsequent cardiac arrest where we were able to safely deliver the baby and resuscitate the mother with veno‐arterial extracorporeal membrane oxygenation and Impella CP—a novel combination known as ECPELLA. We discuss the implications of this approach as a more efficacious strategy in resuscitating AFE‐induced cardiogenic shock and arrest.
Keywords: VA‐ECMO, Impella, Amniotic fluid embolism, Cardiac arrest, Cardiogenic shock, ECPELLA
Introduction
Amniotic fluid embolism (AFE) is a rare but potentially fatal complication of pregnancy that accounts for nearly one in five maternal deaths globally. Of these deaths, nearly half of them occur within 1 h of symptom onset. 1 Therefore, prompt and aggressive resuscitation strategies are crucial in promoting survivability. Unfortunately, traditional approaches of resuscitation fail to provide adequate cardiopulmonary support. Because AFE is a diagnosis of exclusion, it is challenging to properly study and obtain reliable information in terms of incidence, risk factors, managements, and outcomes. 2 The use of veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) has only recently expanded to include such unique patient populations. We present a case of a mother with a relatively uncomplicated pregnancy who developed AFE, resulting in peripartum adult respiratory distress syndrome (ARDS) with subsequent cardiogenic shock and cardiac arrest in her late third trimester. We were able to both safely deliver the baby and successfully resuscitate the mother with the novel combination of VA‐ECMO and Impella CP—a combination known as ECPELLA.
Case report
A 31‐year‐old primigravid female with an uncomplicated pregnancy presented to the emergency room at 35 weeks of gestation for sudden onset shortness of breath. Upon arrival, she was too lethargic to provide history. A set of vital signs revealed an oral temperature of 98.1°F, a blood pressure of 135/118 mmHg, a respiratory rate ~ 40 breaths/min, and heart rate ~ 160 beats per minute. Fetal heart tones were very faint and roughly 90 beats/min (normal: 120–160 beats/min). Frequent uterine contractions were noted. She developed pre‐eclampsia and subsequently rapidly progressed to severe ARDS (Figure 1 ) requiring intubation with mechanical ventilation.
Figure 1.
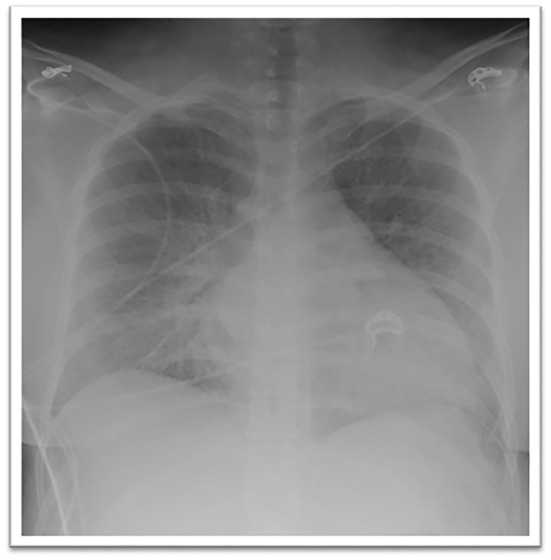
Chest radiograph obtained on initial presentation demonstrating diffuse bilateral opacities and cardiomegaly.
Our initial differential diagnoses at this time included acute myocardial infarction, upper respiratory infection, AFE, aortic or coronary dissection, pulmonary embolism, and septic shock. Thorough assessment of her hemodynamic and laboratory workup including high‐sensitivity troponin, electrocardiography, computed tomographic angiography, and blood cultures allowed us to rule out all the aforementioned differentials except for AFE‐induced shock.
An emergent caesarean section was then performed, and a viable healthy newborn was successfully delivered. Immediately after the infant was delivered, the mother fully decompensated into cardiac arrest. Cardiopulmonary resuscitation (CPR) was successful after 4 min. Bedside transthoracic echocardiogram (TTE) revealed biventricular failure with a severely reduced left ventricular ejection fraction (EF) of 7.1% by Simpson's method (Figure 2 and Supporting Information, Video S1 ).
Figure 2.
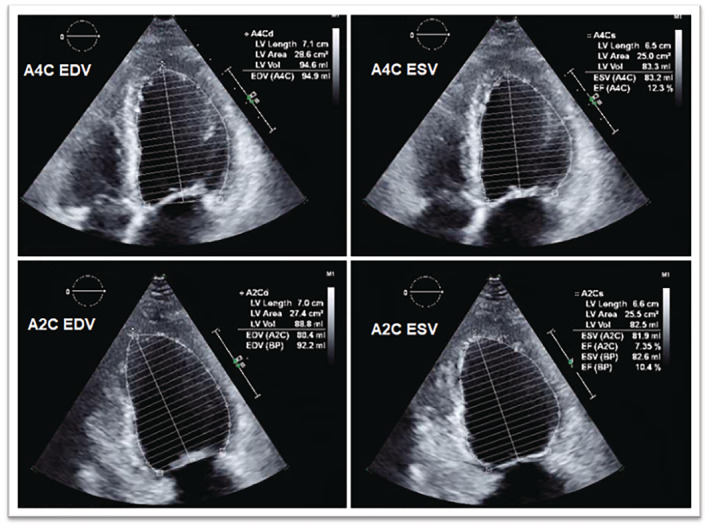
Transthoracic echocardiogram before ECPELLA application demonstrating minimal EF of ~7% once appropriately calculated for using Simpson's method. BP, blood pressure; EDV, end‐diastolic volume; EF, ejection fraction; ESV, end‐systolic volume; LV, left ventricular.
Methods and results
The patient was provided standard therapy for AFE per the Society for Maternal‐Fetal Medicine (SMFM) guidelines. 3 She was being sustained on high‐dose norepinephrine, epinephrine, and vasopressin. She had elevated ventilatory pressures and high oxygen requirements. A right heart catheterization was performed, which revealed no pulsatility in the pulmonary arteries, central venous pressure of 20 mmHg, pulmonary capillary wedge pressure of 17 mmHg, and a cardiac index of 1.8. The decision was then made to perform emergent VA‐ECMO (Figure 3 ) with percutaneous left ventricular decompression using Impella CP (ECPELLA). To do this, we utilized a standard TandemLife system—two minimally invasive large‐bore catheters connected in series with an oxygenator and motorized pump that serve to provide cardiopulmonary bypass. This was prepared per manufacturer's recommendations. While the TandemLife system was being deaired, we expeditiously obtained 14 Fr right femoral arterial access and placed an Impella CP catheter. Thereafter, a 17 Fr left femoral arterial return and 24 Fr multi‐hole right femoral venous drainage cannulas were placed using standard percutaneous access techniques. The Impella CP was turned down from P8 to P3 to function as left ventricular decompression given an EF of 7% during VA‐ECMO implementation. Additionally, 6 Fr antegrade distal perfusion sheaths were placed for bilateral external limb bypasses to ensure limb viability. Finally, standard imaging with transesophageal echocardiography was utilized to confirm correct positioning of the cannulas.
Figure 3.
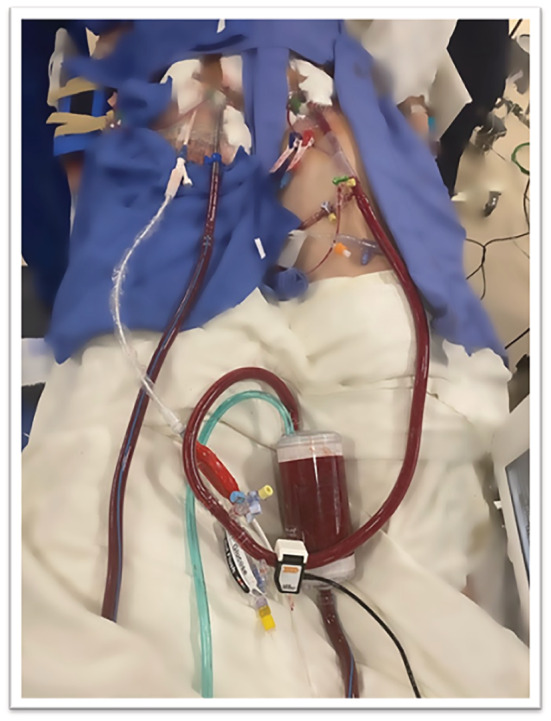
Successful percutaneous cannulation and initiation of veno‐arterial extracorporeal membrane oxygenation with distal perfusion catheters bilaterally to the lower extremities.
Repeat assessment of her hemodynamic post‐ECPELLA revealed a pulmonary capillary wedge pressure of 9 mmHg. The patient was supported as needed with various transfusions, inotropes, and pressors to ensure that mean arterial pressures were kept above 65 mmHg, PaO2 70–100 mmHg, SvO2 > 60%, and central venous pressures 10–15 mmHg. The patient's course was complicated by worsening disseminated intravascular coagulation (DIC) and hemorrhage at the site of cannulation on Day 2, which resolved under 48 h with a series of aggressive transfusions (Supporting Information, Table S2 ). A repeat post‐procedural TTE on Day 3 revealed an improvement of her EF to 25–30% with moderately reduced right ventricular function. Serial echocardiograms continued to show improvement in cardiac function throughout the hospitalization course. By Day 5 on ECPELLA, our patient had total resolution of her ARDS, cardiogenic shock (Supporting Information, Table S3 ), DIC, lactic acidosis, and oliguric renal failure with an EF of 55% (Figure 4 and Supporting Information, Video S2 ). Using weaning protocols and parameters previously described by Ortuno et al., we were able to assess her readiness for decannulation. 4 Our patient was simultaneously weaned off both Impella CP and VA‐ECMO with no complications. She was subsequently discharged to our inpatient rehabilitation unit after continued clinical improvement.
Figure 4.
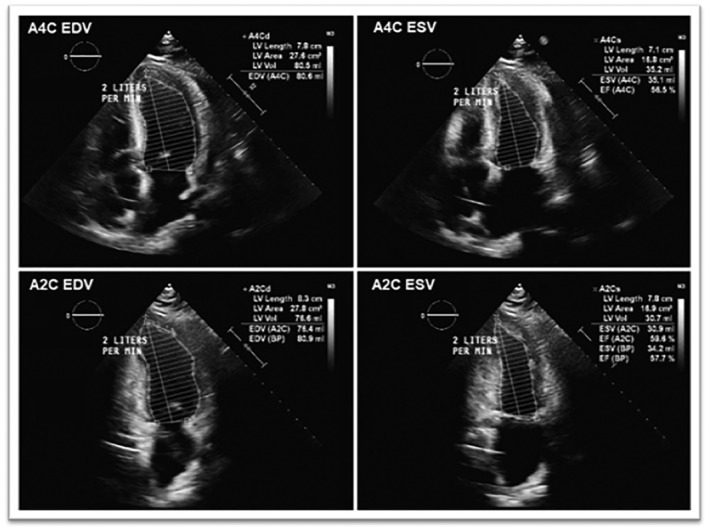
Transthoracic echocardiogram on Day 5 after successful ECPELLA decannulation demonstrating significantly improved EF of 55%. BP, blood pressure; EDV, end‐diastolic volume; EF, ejection fraction; ESV, end‐systolic volume; LV, left ventricular.
Follow‐up and outcomes
Our patient is almost 1 year status‐post ECPELLA resuscitation and doing very well. Her EF remains 65%. She has no cardiopulmonary limitation to date.
Discussion
AFE remains a diagnosis of exclusion primarily based on clinical manifestations. The SMFM issues a Grade 1C recommendation to strongly consider AFE in any peripartum woman who develops sudden cardiopulmonary collapse. 3 To this day, there are no specific diagnostic tests to confirm or rule out AFE. Up until recently, the majority of mothers who developed AFE succumbed to death. Nearly half of these deaths occur within the first hour of incidence. 1 Furthermore, once cardiac arrest occurs, the chances of successful resuscitation and recoverability significantly decrease. Proposed algorithms for cardiac arrest in AFE are centered around immediate delivery of the fetal followed by supportive care for the mother using advanced cardiovascular life support (ACLS) CPR, mechanical ventilation, massive transfusion protocols, and maintenance of hemodynamic stability with inotropes and norepinephrine. 3 Conventional approaches in dealing with AFE with supportive care, mechanical ventilation, dialysis, and pressor support with medications have had very low success rates. 5 We believe aggressive interventions such as VA‐ECMO are necessary in this patient population where the decompensation to acute multiorgan failure is of high probability. With advancements in technology and the recent widespread popularity of VA‐ECMO for CPR, we anticipate the mortality rates to significantly improve if applied in a timely manner. Data regarding the use of extracorporeal membrane oxygenation (ECMO) in peripartum and postpartum women remain extremely sparse and primarily pertain to the use of veno‐venous ECMO (VV‐ECMO) and not VA‐ECMO. 6 To our knowledge, this is the only reported case of AFE and cardiopulmonary collapse that has been treated with the ECPELLA strategy. To date, no trials have included peripartum patients. In other populations, the ECPELLA strategy has shown to result in improved hemodynamics, decrease usage of vasopressors and inotropes, increase likelihood of survival to discharge, and recently has been shown to be associated with reduced mortality. 7 , 8 , 9 We believe our patient's youth, minimal pre‐existing comorbidities, and our team's expeditious implantation were all favourable factors in her outcome and may support the use of such a strategy in these critically ill patients. Given the paucity of data and the difficulty in making such a diagnosis, physicians need to be aware of such presentations and the various approaches to management.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non‐profit sectors.
Supporting information
Table S1: Timeline of pertinent daily lab results.
Table S2: Timeline and quantity of blood product transfusions.
Table S3: Comprehensive flowsheet of our patient's hemodynamic progress on ECPELLA.
Figure S1: Timeline of Lactate and EF Throughout ICU Course.
Video S1: Post‐arrest, pre‐ECPELLA parasternal short‐axis view of TTE revealing a moderately dilated left ventricle and severely reduced systolic function with severe global hypokinesis. Ejection fraction is estimated to be 7%.
Video S2: Repeat TTE on day 5 post‐ECPELLA parasternal short‐axis view of TTE revealing normal left ventricular function with an estimated EF of 55%.
Acknowledgements
The team would like to thank SRMC Structural Heart & Cath Lab team members for all their help and support.
Golzarian, H. , Mariam, A. , Shah, S. R. , Pasley, B. A. , Haq, S. H. , Edgerton, A. R. , Scherger, W. E. , Stallkamp, V. L. , Patel, D. , Laird, A. , Cole, W. C. , and Patel, S. M. (2023) Amniotic fluid embolism‐induced cardiopulmonary collapse successfully treated with combination VA‐ECMO and Impella CP. ESC Heart Failure, 10: 1440–1444. 10.1002/ehf2.14254.
Disclosures: Figure 3 of the manuscript was previously published on Twitter by corresponding author for educational purposes.
References
- 1. DeSimone C. Embolic events of pregnancy. In Complications in Anesthesia, Second ed. W.B. Saunders; 2009. [Google Scholar]
- 2. Knight M, Berg C, Brocklehurst P, Kramer M, Lewis G, Oats J, Roberts CL, Spong C, Sullivan E, van Roosmalen J, Zwart J. Amniotic fluid embolism incidence, risk factors and outcomes: a review and recommendations. BMC Pregnancy Childbirth. 2012; 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pacheco LD, Saade G, Hankins GDV, Clark SL, Society for Maternal‐Fetal Medicine (SMFM) . Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016; 215: B16–B24. [DOI] [PubMed] [Google Scholar]
- 4. Ortuno S, Delmas C, Diehl JL, Bailleul C, Lancelot A, Naili M, Cholley B, Pirracchio R, Aissaoui N. Weaning from veno‐arterial extra‐corporeal membrane oxygenation: which strategy to use? Ann Cardiothorac Surg. 2019; 8: E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaur K, Bhardwaj M, Kumar P, Singhal S, Singh T, Hooda S. Amniotic fluid embolism. J Anaesthesiol Clin Pharmacol. 2016; 32: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Webster CM, Smith KA, Manuck TA. Extracorporeal membrane oxygenation in pregnant and postpartum women: a ten‐year case series. Am J Obstet Gynecol MFM. 2020; 2: 100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel SM, Lipinski J, al‐Kindi SG, Patel T, Saric P, Li J, Nadeem F, Ladas T, Alaiti A, Phillips A, Medalion B, Deo S, Elgudin Y, Costa MA, Osman MN, Attizzani GF, Oliveira GH, Sareyyupoglu B, Bezerra HG. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with Impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J. 2019; 65: 21–28. [DOI] [PubMed] [Google Scholar]
- 8. Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, Cudemus Deseda G, Dabboura S, Eckner D, Eden M, Eitel I, Frank D, Frey N, Funamoto M, Goßling A, Graf T, Hagl C, Kirchhof P, Kupka D, Landmesser U, Lipinski J, Lopes M, Majunke N, Maniuc O, McGrath D, Möbius‐Winkler S, Morrow DA, Mourad M, Noel C, Nordbeck P, Orban M, Pappalardo F, Patel SM, Pauschinger M, Pazzanese V, Reichenspurner H, Sandri M, Schulze PC, H.G. Schwinger R, Sinning J‐M, Aksoy A, Skurk C, Szczanowicz L, Thiele H, Tietz F, Varshney A, Wechsler L, Westermann D. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020; 142: 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Müllerleile K, Colombo A, Sydow K, de Bonis M, Wagner F, Reichenspurner H, Blankenberg S, Zangrillo A, Westermann D. Concomitant implantation of Impella® on top of veno‐arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017; 19: 404–412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Timeline of pertinent daily lab results.
Table S2: Timeline and quantity of blood product transfusions.
Table S3: Comprehensive flowsheet of our patient's hemodynamic progress on ECPELLA.
Figure S1: Timeline of Lactate and EF Throughout ICU Course.
Video S1: Post‐arrest, pre‐ECPELLA parasternal short‐axis view of TTE revealing a moderately dilated left ventricle and severely reduced systolic function with severe global hypokinesis. Ejection fraction is estimated to be 7%.
Video S2: Repeat TTE on day 5 post‐ECPELLA parasternal short‐axis view of TTE revealing normal left ventricular function with an estimated EF of 55%.


