Abstract
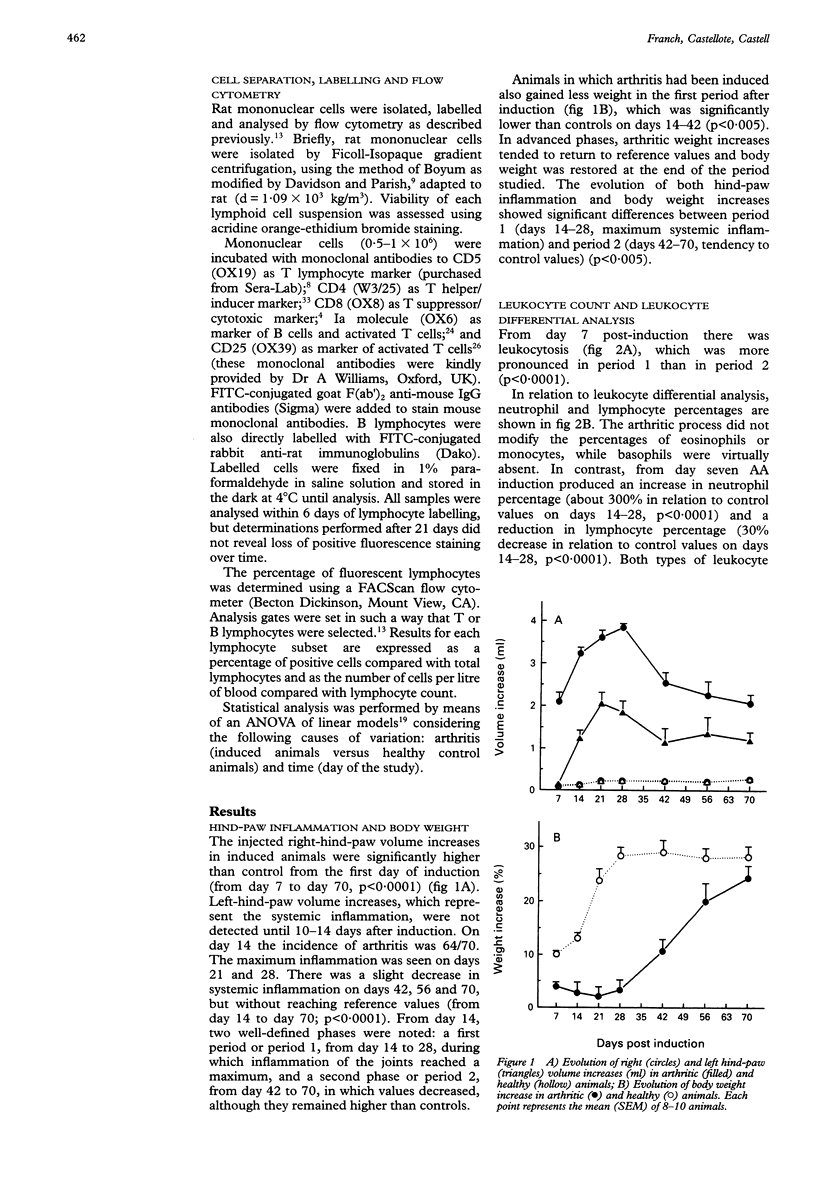
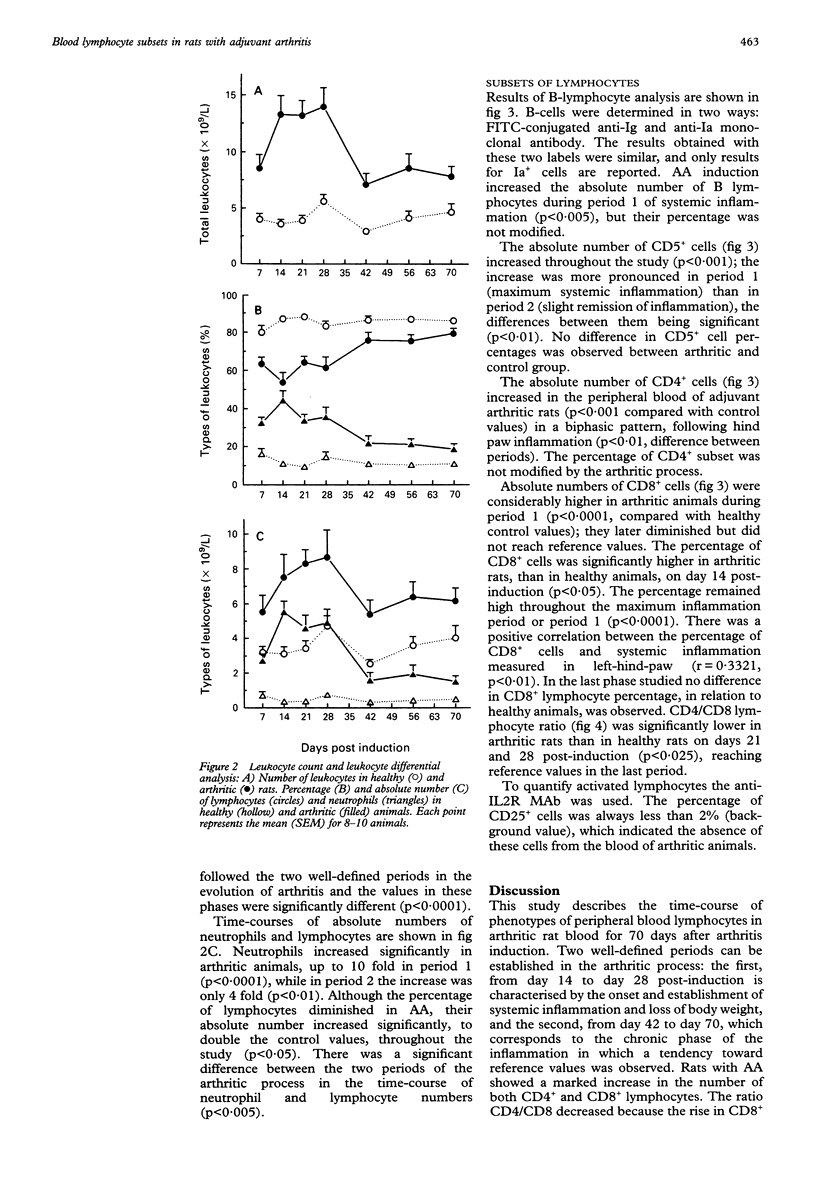
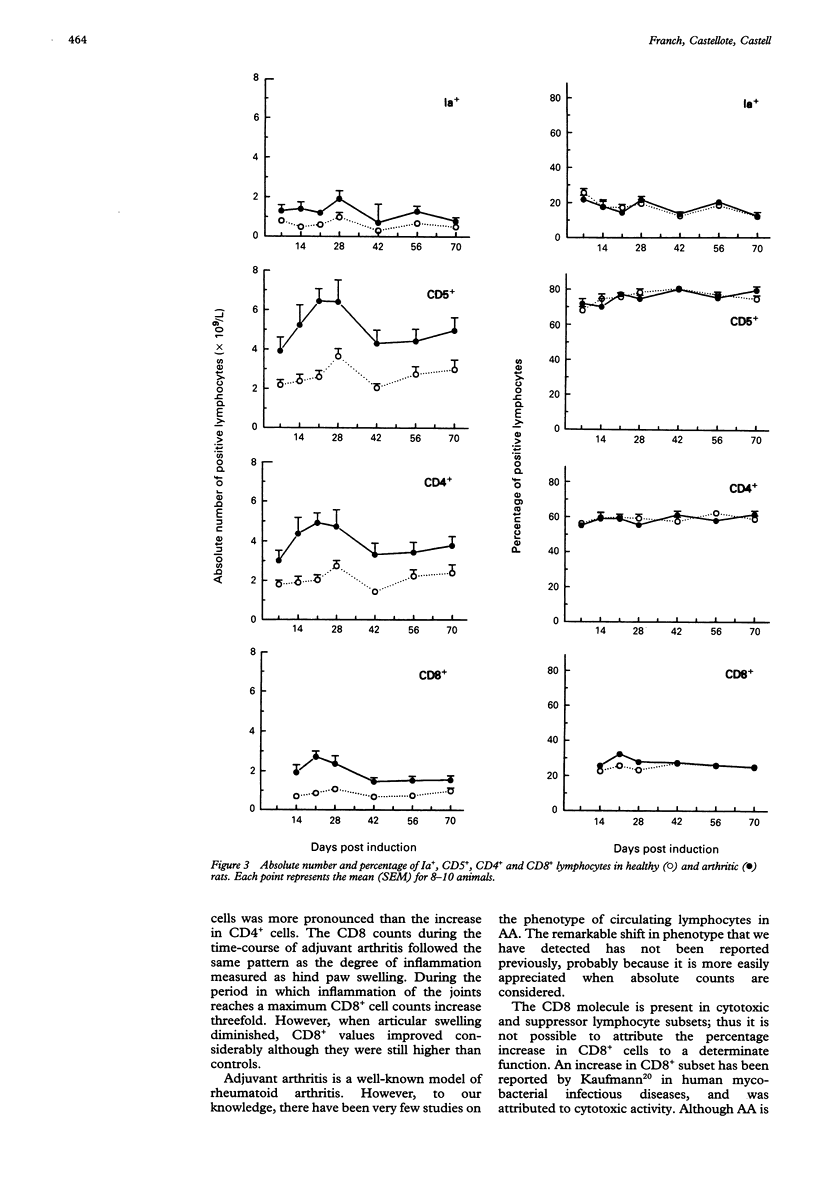
OBJECTIVES--To determine the phenotype of peripheral blood lymphocytes during the time-course of adjuvant arthritis (AA) to detect alterations that could be involved in the pathogenesis of the arthritic process. METHODS--Phenotype analysis was performed on days 7, 14, 21, 28, 42, 56 and 70 after arthritis induction using monoclonal antibodies to CD5, CD4 and CD8 subsets, and flow cytometry. The proportion of activated lymphocytes and lymphocytes was also assessed with monoclonal antibodies to IL-2R (CD25), to Ia antigen and by polyclonal antibodies to rat Ig. RESULTS--Adjuvant arthritis produced leukocytosis with neutrophilia. Rats with AA showed a marked increase in the number of both CD4+ and CD8+ cells. The ratio CD4/CD8 decreased because the rise in CD8+ cells was more pronounced than the increase in CD4+ cells. Changes in lymphocyte counts showed two well-defined periods: the first, from day 14 to day 28, during which the inflammation of the joints reached a maximum and changes in lymphocyte subsets were more pronounced, that is, there was a threefold increase in CD8+ lymphocytes over normal counts, and the second, from day 42 to day 70, in which modified parameters improved considerably but remained different from controls. CONCLUSION--Alterations were detected in the phenotype of peripheral blood lymphocytes in AA, which provides an additional marker of disease activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bersani-Amado C. A., Duarte A. J., Tanji M. M., Cianga M., Jancar S. Comparative study of adjuvant induced arthritis in susceptible and resistant strains of rats. III. Analysis of lymphocyte subpopulations. J Rheumatol. 1990 Feb;17(2):153–158. [PubMed] [Google Scholar]
- Binderup L. Decreased T-suppressor cell activity in rats with adjuvant arthritis. Ann Rheum Dis. 1983 Dec;42(6):693–698. doi: 10.1136/ard.42.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Castell M., Castellote M. C., Queralt J. Anti-immunoglobulin antibody detection in adjuvant arthritis by an ELISA technique. Pathol Res Pract. 1986 Dec;181(6):664–667. doi: 10.1016/S0344-0338(86)80041-3. [DOI] [PubMed] [Google Scholar]
- Castell M., Castellote M. C., Queralt J., Barberá G., Torralba A. Evidence of autoantibodies in rats with adjuvant-induced arthritis. Allergol Immunopathol (Madr) 1985 Sep-Oct;13(5):399–403. [PubMed] [Google Scholar]
- Dallman M. J., Thomas M. L., Green J. R. MRC OX-19: a monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur J Immunol. 1984 Mar;14(3):260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W., Tidman N. Analysis of T cell subsets in the peripheral blood and synovial fluid of patients with rheumatoid arthritis by means of monoclonal antibodies. Ann Rheum Dis. 1983 Aug;42(4):357–361. doi: 10.1136/ard.42.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., Gentry K. C., Mackay I. R., Muirden K. D., Rowley M. Deficiency of the suppressor inducer subset of T lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1987 Aug;30(8):849–856. doi: 10.1002/art.1780300802. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Franch A., Castellote C., Pelegrí C., Tolosa E., Castell M. Blood B, T, CD4+ and CD8+ lymphocytes in female Wistar rats. Ann Hematol. 1993 Sep;67(3):115–118. doi: 10.1007/BF01701732. [DOI] [PubMed] [Google Scholar]
- Franch A., Castellote C., Vilà J. L., Vilaró S., Castell M. Anticytoskeletal autoantibody development in adjuvant arthritis. J Rheumatol. 1994 Mar;21(3):489–497. [PubMed] [Google Scholar]
- Glenn E. M., Gray J., Kooyers W. Chemical changes in adjuvant-induced polyarthritis of rats. Am J Vet Res. 1965 Sep;26(114):1195–1203. [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Goto M., Miyamoto T., Nishioka K., Okumura K. Selective loss of suppressor T cells in rheumatoid arthritis patients: analysis of peripheral blood lymphocytes by 2-dimensional flow cytometry. J Rheumatol. 1986 Oct;13(5):853–857. [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kayashima K., Koga T., Onoue K. Role of T lymphocytes in adjuvant arthritis. I. Evidence for the regulatory function of thymus-derived cells in the induction of the disease. J Immunol. 1976 Nov;117(5 PT2):1878–1882. [PubMed] [Google Scholar]
- Kayashima K., Koga T., Onoue K. Role of T lymphocytes in adjuvant arthritis. II. Different subpopulations of T lymphocytes functioning in the development of the disease. J Immunol. 1978 Apr;120(4):1127–1131. [PubMed] [Google Scholar]
- Larsson P., Holmdahl R., Dencker L., Klareskog L. In vivo treatment with W3/13 (anti-pan T) but not with OX8 (anti-suppressor/cytotoxic T) monoclonal antibodies impedes the development of adjuvant arthritis in rats. Immunology. 1985 Nov;56(3):383–391. [PMC free article] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Nakao H., Eguchi K., Kawakami A., Migita K., Otsubo T., Ueki Y., Shimomura C., Tezuka H., Matsunaga M., Maeda K. Increment of Tal positive cells in peripheral blood from patients with rheumatoid arthritis. J Rheumatol. 1989 Jul;16(7):904–910. [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. Immunology. Antigens for gamma/delta T cells. Nature. 1989 Jun 1;339(6223):342–343. doi: 10.1038/339342a0. [DOI] [PubMed] [Google Scholar]
- Russell A. S. Activated lymphocytes in the peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1990 May;17(5):589–596. [PubMed] [Google Scholar]
- Taurog J. D., Argentieri D. C., McReynolds R. A. Adjuvant arthritis. Methods Enzymol. 1988;162:339–355. doi: 10.1016/0076-6879(88)62089-1. [DOI] [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]


