Abstract
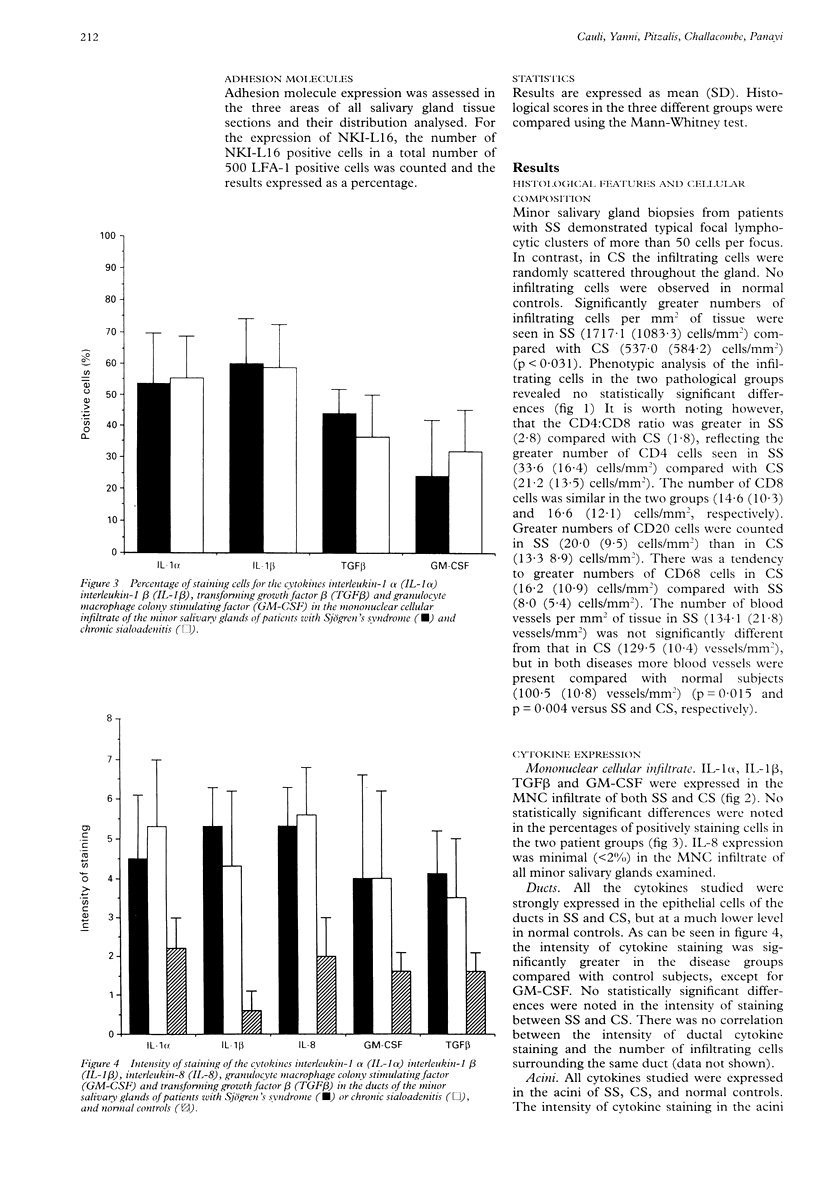
OBJECTIVE--To investigate the role of cytokines and cell adhesion molecules in the pathogenesis of Sjögren's syndrome (SS). METHODS--Using an indirect immunoperoxidase technique we assessed the expression of the cytokines interleukin-1 alpha (IL-1 alpha), interleukin-1 beta (IL-1 beta), interleukin-8 (IL-8), transforming growth factor beta (TGF beta) and granulocyte macrophage colony stimulating factor (GM-CSF), of the adhesion molecules intercellular adhesion molecule-1 (ICAM-1), lymphocyte function associated antigen-1 (LFA-1), the activated molecular form of LFA-1 (NKI-L16), CD2, and LFA-3, and of a panel of cellular markers in the minor salivary glands. RESULTS--In SS and chronic sialoadenitis (CS), the ductal epithelial cells and acini expressed all the cytokines examined. The percentage of glandular mononuclear cells which stained positive for cytokines did not differ significantly between SS and CS. NKI-L16 was detected on 33.6 (SD 10.1)% and 15.3 (4.3)% of LFA-1 cells in SS and CS, respectively (p < 0.002). CONCLUSION--SS and CS did not differ in the pattern of cytokines examined. The characteristic cell clustering seen in the salivary glands in SS may be caused by the upregulation of NKI-L16.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttila H. S., Reitamo S., Erkko P., Ceska M., Moser B., Baggiolini M. Interleukin-8 immunoreactivity in the skin of healthy subjects and patients with palmoplantar pustulosis and psoriasis. J Invest Dermatol. 1992 Jan;98(1):96–101. doi: 10.1111/1523-1747.ep12495817. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Volpini G., Villa L., Nencioni L., Scapigliati G., Nucci D., Antoni G., Matteucci G., Cioli F., Tagliabue A. A monoclonal antibody to the IL-1 beta peptide 163-171 blocks adjuvanticity but not pyrogenicity of IL-1 beta in vivo. J Immunol. 1989 Jul 1;143(1):131–134. [PubMed] [Google Scholar]
- Farahat M. N., Yanni G., Poston R., Panayi G. S. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993 Dec;52(12):870–875. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Bumol T., Fantozzi R., Bone R., Schreiber R. Expression of histocompatibility antigen HLA-DR by salivary gland epithelial cells in Sjögren's syndrome. Arthritis Rheum. 1986 Sep;29(9):1105–1111. doi: 10.1002/art.1780290908. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Howell F. V., Bone R. C., Michelson P. Primary Sjogren syndrome: clinical and immunopathologic features. Semin Arthritis Rheum. 1984 Nov;14(2):77–105. doi: 10.1016/0049-0172(84)90001-5. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Robinson C. A., Curd J. G., Kozin F., Howell F. V. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum. 1986 May;29(5):577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Theofilopoulos A. N., Altman A. Production of interleukin 2 (IL 2) by salivary gland lymphocytes in Sjögren's syndrome. Detection of reactive cells by using antibody directed to synthetic peptides of IL 2. J Immunol. 1985 Nov;135(5):3109–3115. [PubMed] [Google Scholar]
- Lucas C., Bald L. N., Fendly B. M., Mora-Worms M., Figari I. S., Patzer E. J., Palladino M. A. The autocrine production of transforming growth factor-beta 1 during lymphocyte activation. A study with a monoclonal antibody-based ELISA. J Immunol. 1990 Sep 1;145(5):1415–1422. [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A., Shurety W., Caterson B. The quantitation of a native chondroitin sulfate epitope in synovial fluid lavages and articular cartilage from canine experimental osteoarthritis and disuse atrophy. Arthritis Rheum. 1993 Apr;36(4):543–551. doi: 10.1002/art.1780360416. [DOI] [PubMed] [Google Scholar]
- Rowe D., Griffiths M., Stewart J., Novick D., Beverley P. C., Isenberg D. A. HLA class I and II, interferon, interleukin 2, and the interleukin 2 receptor expression on labial biopsy specimens from patients with Sjögren's syndrome. Ann Rheum Dis. 1987 Aug;46(8):580–586. doi: 10.1136/ard.46.8.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Terauchi K., Shimuta M., Nishiimura S., Yoshino K., Takeuchi T., Tsubota K., Miyasaka N. Expression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren's syndrome. J Clin Lab Anal. 1993;7(3):180–187. doi: 10.1002/jcla.1860070309. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speight P. M., Cruchley A., Williams D. M. Epithelial HLA-DR expression in labial salivary glands in Sjögren's syndrome and non-specific sialadenitis. J Oral Pathol Med. 1989 Mar;18(3):178–183. doi: 10.1111/j.1600-0714.1989.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Springer T. A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989 May 4;339(6219):61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Talal N., Sylvester R. A., Daniels T. E., Greenspan J. S., Williams R. C., Jr T and B lymphocytes in peripheral blood and tissue lesions in Sjögren's syndrome. J Clin Invest. 1974 Jan;53(1):180–189. doi: 10.1172/JCI107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe R., Wadhwa M., Gearing A., Mahon B., Poole S. Sensitive and specific immunoradiometric assays for human interleukin-1 alpha. Lymphokine Res. 1988 Summer;7(2):119–127. [PubMed] [Google Scholar]
- Thrane P. S., Halstensen T. S., Haanaes H. R., Brandtzaeg P. Increased epithelial expression of HLA-DQ and HLA-DP molecules in salivary glands from patients with Sjögren's syndrome compared with obstructive sialadenitis. Clin Exp Immunol. 1993 May;92(2):256–262. doi: 10.1111/j.1365-2249.1993.tb03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Moutsopoulos H. M., Balestrieri G., Bencivelli W., Bernstein R. M., Bjerrum K. B., Braga S., Coll J., de Vita S. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993 Mar;36(3):340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- Yanni G., Whelan A., Feighery C., Quinlan W., Symons J., Duff G., Bresnihan B. Contrasting levels of in vitro cytokine production by rheumatoid synovial tissues demonstrating different patterns of mononuclear cell infiltration. Clin Exp Immunol. 1993 Sep;93(3):387–395. doi: 10.1111/j.1365-2249.1993.tb08190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke G., Strittmatter U., Tees R., Andersen E., Fagg B., Kocher H. P., Schreier M. H. A cocktail of three monoclonal antibodies significantly increases the sensitivity of an enzyme immunoassay for human granulocyte-macrophage colony-stimulating factor. J Immunoassay. 1991;12(2):185–206. doi: 10.1080/01971529108055066. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y., Weder P., Hogervorst F., Verhoeven A. J., van Seventer G., te Velde A. A., Borst J., Keizer G. D., Figdor C. G. Activation of LFA-1 through a Ca2(+)-dependent epitope stimulates lymphocyte adhesion. J Cell Biol. 1991 Jan;112(2):345–354. doi: 10.1083/jcb.112.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]




