Abstract
Background The relative efficacy and safety of ticagrelor and prasugrel based dual antiplatelet therapy strategies according to the platelet count (PC) in patients with acute coronary syndromes (ACS) have not been defined.
Methods This is a posthoc analysis of the ISAR-REACT 5 trial, in which patients presenting with ACS were randomized to treatment with ticagrelor versus prasugrel. Patients were divided into quartiles according to PC. The primary endpoint was incidence of death, myocardial infarction, or stroke, and the safety endpoint was incidence of BARC (Bleeding Academic Research Consortium) type 3 to 5 bleeding at 12 months.
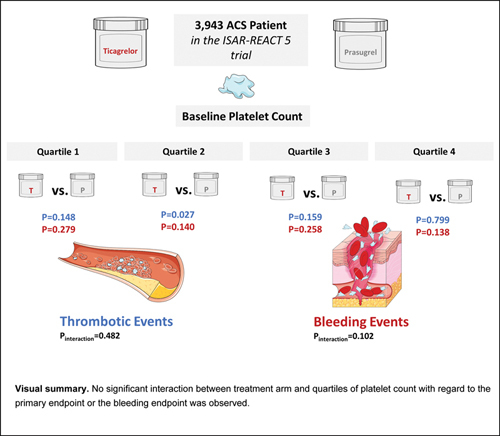
Results A total of 3,943 patients with known PC (997 patients in quartile 1 (Q1), 1,003 in quartile 2 (Q2) [205 ± 10.3 × 10 9 /L], 961 patients in quartile 3 (Q3) [241 ± 11.7 × 10 9 /L], and 982 patients in quartile 4 (Q4) [317 ± 68.6 × 10 9 /L]). There was no significant interaction between treatment arm (ticagrelor vs. prasugrel) and PC group with respect to primary endpoint (Q1: 8.8 vs. 6.3%, hazard ratio [HR] =1.41, 95% confidence interval [CI]: 0.89–2.23; p = 0.148; Q2: 9.9 vs. 5.8%, HR = 1.68, 95% CI: 1.06–2.66; p = 0.027; Q3: 7.8 vs. 5.5%, HR = 1.43, 95% CI: 0.87–2.37; p = 0.159; Q4: 10.1 vs. 10.1%, HR = 1.05, 95% CI: 0.71–1.57; p = 0.799; p for interaction [ p int ] = 0.482) and with respect to bleeding endpoint (Q1: 5.8 vs. 4.2%, HR = 1.41, 95% CI: 0.76–2.63; p = 0.279; Q2: 6.4 vs. 3.7%, HR = 1.62, 95% CI: 0.85–2.06; p = 0.140; Q3: 4.4 vs. 3.0%, HR = 1.53, 95% CI: 0.73–3.18; p = 0.258; Q4: 5.6 vs. 8.5%, HR = 0.67, 95% CI: 0.40–1.14; p = 0.138, p int = 0.102).
Conclusions In this analysis, incidences of ischemic and bleeding events at 12 months are comparable across quartiles of platelet count.
Keywords: trials, antiplatelet therapy, myocardial infarction, acute coronary syndrome
Introduction
Dual antiplatelet therapy (DAPT) is the cornerstone of treatment for patients presenting with an acute coronary syndrome (ACS), in particular those undergoing percutaneous revascularization. 1
Platelet count has been reported to impact on the outcomes of patients with ACS. 2 3 4 5 In particular, a U-shaped association has been reported, with increased risk of all-cause mortality in patients with low and high baseline platelet counts. 6 However, patients with ACS and low platelet count are at particular risk of both mortality and bleeding events. 7 8 9
There are no data on the relative efficacy and safety of ticagrelor- versus prasugrel-based DAPT. The Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 trial showed that prasugrel was superior to ticagrelor in reducing the risk of ischemic events with no increase in the risk of bleeding in patients with ACS predominantly undergoing an invasive management strategy. 10 A platelet function sub-study of the ISAR-REACT 5 trial showed that prasugrel provided more potent platelet inhibition than ticagrelor. 11 However, whether the efficacy or safety of ticagrelor versus prasugrel differs according to the baseline platelet count remains unknown.
The assessment of the relative efficacy and safety of these two potent P2Y 12 inhibitors according to the platelet count may be clinically useful with respect to decision making regarding the optimal antiplatelet therapy regimen in patients with ACS.
Against this background, we performed an analysis of the ISAR-REACT 5 trial 10 to assess whether the efficacy and safety of ticagrelor- versus prasugrel-based DAPT differs according to the baseline platelet count in patients with ACS planned to undergo an invasive management strategy.
Methods
Patients
This is a posthoc analysis of the ISAR-REACT 5 trial in which the efficacy and safety of ticagrelor- versus prasugrel-based DAPT regimens were assessed according to the baseline platelet count. The study design, inclusion and exclusion criteria, and outcomes were previously reported. 10 12 Briefly, the ISAR-REACT 5 trial performed a randomized head-to-head comparison of ticagrelor and prasugrel in patients presenting with ACS (ST-segment elevation myocardial infarction [STEMI], non-ST-segment elevation myocardial infarction [NSTEMI], or unstable angina) planned to undergo an invasive strategy. Eligible patients were randomly assigned in a 1:1 ratio to receive either ticagrelor (loading dose of 180 mg as soon as possible after randomization) or prasugrel (loading dose 60 mg after diagnostic coronary angiography was performed, but before percutaneous coronary intervention (PCI), in patients presenting with NSTE-ACS) or as soon as possible after randomization in patients presenting with STEMI. A platelet count of <100 × 10 9 /L at the time of screening was an exclusion criterion of the ISAR-REACT 5 trial. 10 12 The therapy was continued at a maintenance dose of 90 mg ticagrelor twice daily or 10 mg prasugrel once daily. In patients with a body weight of <60 kg or aged ≥75 years, a maintenance dose of 5 mg of prasugrel once daily was recommended. 13 All patients received a loading dose of 150 to 300 mg of intravenous or chewed aspirin and a maintenance dose of 75 to 100 mg once daily. All patients gave their written informed consent. The study protocol was approved by the local ethics committee, at each participating center and the study conformed to the Declaration of Helsinki. 14 For the purpose of this analysis, patients were divided into four groups based on quartiles of the platelet count. These groups are referred to as patients in the (lower) quartile 1 (Q1), quartile 2 (Q2), quartile 3 (Q3), and (upper) quartile 4 (Q4). Normal platelet count was defined in the range of 140 to 400 × 10 9 /L; platelet count below 140 × 10 9 /L was considered as thrombocytopenia and platelet count beyond 400 × 10 9 /L was considered as thrombocytosis.
Study Endpoints, Follow-Up, and Monitoring
The primary (efficacy) endpoint of this analysis was the composite of death, myocardial infarction (MI), or stroke at 12 months after randomization. The bleeding (safety) endpoint of the study was the 12-month incidence of Bleeding Academic Research Consortium (BARC) type 3 to 5 bleeding. Secondary endpoints included the individual components of the primary endpoint, cardiovascular death, and stent thrombosis (definite or probable). In line with the updated recommendation of the Academic Research Consortium on endpoints in coronary intervention trials, this study assessed all-cause mortality instead of cardiovascular mortality. 15 The definition of MI used in ISAR-REACT 5 trial was adapted from the Third Universal Definition of Myocardial Infarction. 16 Cardiac troponin was used as the preferred biomarker. Creatine kinase (CK)-MB (and CK) values were assessed concurrently and used in the case that troponin values were not available. 17
Detailed definitions of the study outcomes have been reported previously. 10 12 All primary and secondary endpoint events were adjudicated by a blinded event adjudication committee. Clinical follow-up was conducted at 30 days (±10 days), 6 months (±1 month), and 12 months (±1 month). Patients were contacted by telephone, hospital, or outpatient visits or through structured follow-up letters. For all serious adverse events and primary and secondary endpoints, on-site monitoring was performed.
Statistical Analysis
Continuous data are presented as mean ± standard deviation or median (25th–75th percentiles) and were compared using the Student's t -test or the Wilcoxon rank sum test. Categorical variables are presented as counts and proportions and were compared using the chi-squared test. The primary endpoint and all-cause death were presented as cumulative incidence(s) and calculated using the Kaplan–Meier method. All other endpoints were presented as cumulative incidence(s) after accounting for the competing risk of death. The comparison between the groups was performed using the Cox proportional hazards model with stratification for the participating center, clinical presentation (ACS with or without ST-segment elevation), and the study treatment group. To estimate the interaction between the treatment arm and platelet count for the study endpoints, an interaction term was entered into the Cox proportional hazards model and a p -value for interaction ( p int ) was calculated. Risk estimates are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). The primary (efficacy) endpoint was analyzed according to the intention-to-treat principle (i.e., including all patients as initially assigned, regardless of the actual treatment received). The bleeding (safety) endpoint was analyzed on a modified intention-to-treat basis (i.e., including all patients who received at least one dose of the study drug, with bleeding assessed for up to 1 week after study drug discontinuation). The statistical analysis was performed using the R 3.6.0 Statistical Software (The R foundation for Statistical Computing, Vienna, Austria). A two-sided p -value of <0.05 was considered to indicate statistical significance.
Results
Patients
Of the 4,018 patients who were randomized to receive either ticagrelor or prasugrel in the ISAR-REACT 5 trial, 3,943 patients had an available platelet count and were included in the current analysis. ISAR-REACT 5 trial was conducted in 23 centers in two countries (Germany and Italy), in 75 patients a baseline platelet count was not available due to local circumstances, which was recognized as protocol deviation.
These patients were categorized into four groups according to quartiles of the platelet count: patients in Q1 of platelet count (mean platelet count: 159 ± 21.9 × 10 9 /L; range: 58–185 × 10 9 /L; n = 997 patients [485 assigned to ticagrelor and 512 assigned to prasugrel]), patients in Q2 of platelet count (mean platelet count: 205 ± 10.3 × 10 9 /L; range: 186–22 × 10 9 /L; n = 1,003 patients [500 assigned to ticagrelor and 503 assigned to prasugrel]), patients in Q3 of platelet count (mean platelet count: 241 ± 11.7 × 10 9 /L; range: 222–262 × 10 9 /L; n = 961 patients [482 assigned to ticagrelor and 479 assigned to prasugrel]), and patients in Q4 of platelet count (mean platelet count: 317 ± 11.7 × 10 9 /L; range: 263–900 × 10 9 /L; n = 982 patients [499 assigned to ticagrelor and 483 assigned to prasugrel]). Of note, 19 patients had a platelet count below 100 × 10 9 /L. The study flow chart is displayed in Fig. 1 .
Fig. 1.
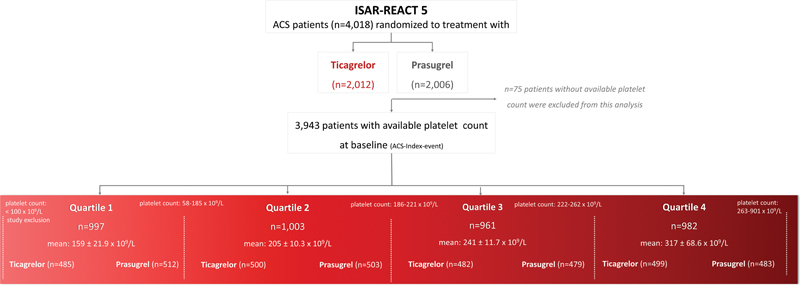
Study flow chart: randomization and grouping of patients according to platelet count quartiles.
Baseline Characteristics in Patients According the Platelet Count
Baseline characteristics in Q1 to Q4 of platelet count are shown in Supplementary Table S1 (available in the online version). Baseline characteristics across quartiles show significant differences. Patients in the upper Q4 of platelet count were younger and more frequent female; had less frequent common cardiovascular risk factors like diabetes mellitus, arterial hypertension, history of prior MI, or PCI, or coronary artery bypass grafting, but were more frequent current smokers and lower creatinine levels; had higher heart rates; and had more frequent a body weight below 60 kg; presented more frequent with STEMI and less frequent with unstable angina.
Procedural and angiographic characteristics in patients in Q1 to Q4 of platelet count are shown in Supplementary Tables S2 and S3 (available in the online version). Regarding angiographic characteristics, patients in Q4 had significantly higher rates of one-vessel disease and lower rates of three-vessel disease. Procedural characteristics were well balanced between quartiles of platelet count, except periprocedural use of aspirin, which was most frequent in Q3.
Baseline Characteristics in Patients According to the Platelet Count and Treatment Arm
Baseline characteristics according to randomized treatment arm in patients and platelet count quartiles are shown in Table 1 . Baseline characteristics were well balanced between prasugrel and ticagrelor across quartiles, except the treatment strategy in Q3, where patients assigned to prasugrel significantly more often received PCI than patients assigned to ticagrelor (87.5 vs. 82.5%; p = 0.028). The angiographic and procedural characteristics according to randomized treatment arm in patients in platelet count quartiles are shown in Tables 2 and 3 . There were no significant differences according to randomized treatment arm, besides total stented length in patients in Q1, which was significantly longer in patients assigned to ticagrelor compared with patients assigned to prasugrel (32.3 mm [ ± 18.4 mm] vs. 29.5 mm [ ± 17.5 mm]; p = 0.028) and type of intervention in Q2, where patients assigned to prasugrel received significantly more often drug-eluting stents compared with patients assigned to ticagrelor (92.6 vs. 86.7%; p = 0.008).
Table 1. Baseline characteristics as per randomized study drug and platelet count quartiles.
| Characteristic | Quartile 1 ( n = 997) |
Quartile 2 ( n = 1,003) |
Quartile 3 ( n = 961) |
Quartile 4 ( n = 982) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ticagrelor ( n = 485) |
Prasugrel ( n = 512) |
p -Value | Ticagrelor ( n = 500) |
Prasugrel ( n = 503) |
p -Value | Ticagrelor ( n = 482) |
Prasugrel ( n = 479) |
p -Value | Ticagrelor ( n = 499) |
Prasugrel ( n = 483) |
p -Value | |
| Age, y | 67.2 ± 11.8 | 66.9 ± 11.3 | 0.631 | 64.5 ± 12.1 | 65.2 ± 12.5 | 0.334 | 63.7 ± 11.4 | 63.4 ± 12.3 | 0.691 | 62.8 ± 12.3 | 62.9 ± 11.8 | 0.973 |
| Sex | 0.171 | 0.607 | 0.412 | 0.520 | ||||||||
| Female, no. (%) | 55 (11.3) | 74 (14.5) | 109 (21.8) | 102 (20.3) | 133 (27.6) | 120 (25.1) | 170 (34.1) | 175 (36.2) | ||||
| Diabetes, no. (%) | 117 (24.1) | 138 (27.0) | 0.342 | 114 (22.8) | 105 (20.9) | 0.508 | 103 (21.4) | 85 (17.7) | 0.182 | 117 (23.4) | 99 (20.5) | 0.307 |
| Insulin-treated, no. (%) | 39 (8.0) | 50 (9.8) | 0.399 | 33 (6.6) | 27 (5.4) | 0.490 | 26 (5.4) | 26 (5.4) | >0.999 | 39 (7.8) | 32 (6.6) | 0.557 |
| Current smoker, no. (%) | 129/483 (26.7) | 146/508 (28.7) | 0.520 | 158 (31.6) | 159 (31.6) | >0.999 | 176/480 (36.7) | 158/477 (33.1) | 0.279 | 200/494 (40.5) | 192/482 (39.8) | 0.887 |
| Arterial hypertension, no. (%) | 353/483 (73.1) | 375/511 (73.4) | 0.972 | 358 (71.6) | 353 (70.2) | 0.670 | 334/481 (69.4) | 313 (65.3) | 0.199 | 358 (71.7) | 324/481 (67.4) | 0.155 |
| Hypercholesterolemia, no. (%) | 289/484 (59.7) | 324 (63.3) | 0.275 | 306/499 (61.3) | 292 (58.1) | 0.322 | 291/481 (60.5) | 284/479 (59.4) | 0.782 | 273/498 (54.8) | 250/481 (52.0) | 0.408 |
| Prior myocardial infarction, no. (%) | 89 (18.4) | 110/511 (21.5) | 0.241 | 88 (17.6) | 81 (16.1) | 0.583 | 70/480 (14.6) | 65 (13.6) | 0.720 | 57 (11.4) | 63 (13.0) | 0.498 |
| Prior PCI, no. (%) | 137 (28.2) | 164/511 (32.1) | 0.210 | 123 (24.6) | 114 (22.7) | 0.517 | 98/481 (20.4) | 98 (20.5) | >0.999 | 89 (17.8) | 85 (17.6) | 0.989 |
| Prior CABG, no. (%) | 48 (9.9) | 62/511 (12.1) | 0.306 | 33 (6.6) | 33 (6.6) | >0.999 | 20/481 (4.2) | 19 (4.0) | >0.999 | 14 (2.8) | 15 (3.1) | 0.929 |
| Cardiogenic shock, no. (%) | 9 (1.9) | 8 (1.6) | 0.910 | 7 (1.4) | 7 (1.4) | >0.999 | 8 (1.7) | 7 (1.5) | >0.999 | 6 (1.2) | 12 (2.5) | 0.208 |
| Systolic blood pressure (mmHg) | 142 ± 24.2 | 142 ± 24.1 | 0.957 | 145 ± 25.1 | 144 ± 24.6 | 0.338 | 143 ± 25.5 | 144 ± 25.1 | 0.935 | 143 ± 25.4 | 143 ± 23.7 | 0.600 |
| Diastolic blood pressure (mmHg) | 80.7 ± 14.5 | 80.9 ± 13.2 | 0.876 | 83.2 ± 14.7 | 81.0 ± 13.9 | 0.016 | 81.5 ± 14.1 | 83.3 ± 14.3 | 0.056 | 82.1 ± 14.7 | 82.3 ± 13.8 | 0.846 |
| Heart rate, (beats/min) | 75.7 ± 16.1 | 73.9 ± 15.5 | 0.074 | 75.9 ± 15.6 | 75.0 ± 14.6 | 0.372 | 76.8 ± 15.5 | 76.6 ± 14.7 | 0.779 | 79.2 ± 16.2 | 78.5 ± 16.9 | 0.535 |
| Body mass index, (kg/m 2 ) | 27.8 ± 4.2 | 28.2 ± 4.4 | 0.140 | 28.0 ± 4.5 | 27.8 ± 4.5 | 0.462 | 27.5 ± 4.2 | 27.9 ± 4.4 | 0.161 | 27.8 ± 5.3 | 27.3 ± 4.3 | 0.169 |
| Weight <60 kg | 13/483 (2.7) | 16/504 (3.2) | 0.794 | 25 (5.0) | 29/500 (5.8) | 0.680 | 26/479 (5.4) | 19 (4.0) | 0.363 | 40/496 (8.1) | 29/479 (6.1) | 0.272 |
| Creatinine (µmol/L) | 92.0 ± 29.2 | 93.4 ± 32.8 | 0.487 | 89.5 ± 26.7 | 0.771 | 85.9 ± 25.8 | 86.3 ± 24.5 | 0.816 | 83.4 ± 25.7 | 83.1 ± 26.5 | 0.822 | |
| Diagnosis at admission | 0.739 | 0.946 | 0.612 | 0.980 | ||||||||
| Unstable angina | 77 (15.9) | 86 (16.8) | 72 (14.4) | 69 (13.7) | 56 (11.6) | 63 (13.2) | 42 (8.4) | 42 (8.7) | ||||
| NSTEMI | 232 (47.8) | 252 (49.2) | 235 (47.0) | 240 (47.7) | 225 (46.7) | 210 (43.8) | 231 (46.3) | 221 (45.8) | ||||
| STEMI | 176 (36.3) | 174 (34.0) | 193 (38.6) | 194 (38.6) | 201 (41.7) | 206 (43.0) | 226 (45.3) | 220 (45.5) | ||||
| Coronary angiography | 484 (99.8) | 510 (99.6) | >0.999 | 497 (99.4) | 503 (100) | 0.124 | 480 (99.6) | 478 (99.8) | >0.999 | 496 (99.4) | 481 (99.6) | >0.999 |
| Treatment strategy | 0.907 | 0.699 | 0.028 | 0.474 | ||||||||
| PCI | 395/484 (81.6) | 421 (82.2) | 405 (81.2) | 417 (82.9) | 397/481 (82.5) | 419 (87.5) | 435 (87.3) | 416/482 (86.3) | ||||
| CABG | 12/484 (2.5) | 14 (2.7) | 9 (1.8) | 10 (2.0) | 15/481 (3.1) | 5 (1.0) | 11 (2.2) | 7/482 (1.5) | ||||
| Conservative | 77/484 (15.9) | 77 (15.0) | 85 (17.0) | 76 (15.1) | 69/481 (14.3) | 55 (11.5) | 52 (10.4) | 59/482 (12.2) | ||||
Abbreviations: CABG, coronary artery bypass grafting; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Note: Data are mean ± standard deviation or counts (%).
Missing continuous data:
Q1 quartile: diastolic blood pressure: 4 patients (1 in the ticagrelor group, 3 in the prasugrel group), body mass index: 12 patients (3 in the ticagrelor group, 9 in the prasugrel group), heart rate: 1 patient in the ticagrelor group.
Q2 quartile: diastolic blood pressure: 5 patients (1 in the ticagrelor group, 4 in the prasugrel group), body mass index: 5 patients (2 in the ticagrelor group, 3 in the prasugrel group).
Q3 quartile: body mass index: 5 patients (4 in the ticagrelor group, 1 in the prasugrel group).
Q4 quartile: diastolic blood pressure: 2 patients (1 in each group), heart rate: 1 patient in the prasugrel group, body mass index: 7 patients (3 in the ticagrelor group, 4 in the prasugrel group)
The remaining continuous data were complete.
Table 2. Angiographic data as per randomized study drug and platelet count quartiles.
| Characteristic | Quartile 1 ( n = 997) |
Quartile 2 ( n = 1,003) |
Quartile 3 ( n = 961) |
Quartile 4 ( n = 982) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ticagrelor ( n = 484) |
Prasugrel ( n = 510) |
p -Value | Ticagrelor ( n = 497) |
Prasugrel ( n = 503) |
p -Value | Ticagrelor ( n = 480) |
Prasugrel ( n = 478) |
p -Value | Ticagrelor ( n = 496) |
Prasugrel ( n = 481) |
p -Value | |
| Access site | 0.387 | 0.464 | 0.675 | 0.940 | ||||||||
| Femoral artery | 298 (61.6) | 321 (62.9) | 319 (64.2) | 316 (62.8) | 296 (61.7) | 305 (63.8) | 313 (63.1) | 307 (63.8) | ||||
| Radial artery | 182 (37.6) | 188 (36.9) | 177 (35.6) | 183 (36.4) | 182 (37.9) | 170 (35.6) | 181 (36.5) | 172 (35.8) | ||||
| Other | 4 (0.8) | 1 (0.2) | 1 (0.2) | 4 (0.8) | 2 (0.4) | 3 (0.6) | 2 (0.4) | 2 (0.4) | ||||
| Number of diseased coronary arteries | 0.320 | 0.614 | 0.210 | 0.944 | ||||||||
| No obstructive CAD | 41 (8.5) | 42 (8.2) | 42 (8.5) | 47 (9.3) | 49 (10.2) | 33 (6.9) | 38 (7.7) | 41 (8.5) | ||||
| One-vessel disease | 129 (26.7) | 126 (24.7) | 155 (31.2) | 142 (28.2) | 144 (30.0) | 151 (31.6) | 155 (31.2) | 154 (32.0) | ||||
| Two-vessel disease | 109 (22.5) | 141 (27.6) | 135 (27.2) | 131 (26.0) | 122 (25.4) | 138 (28.9) | 140 (28.2) | 133 (27.7) | ||||
| Three-vessel disease | 205 (42.4) | 201 (39.4) | 165 (33.2) | 183 (36.4) | 165 (34.4) | 156 (32.6) | 163 (32.9) | 153 (31.8) | ||||
| Left ventricular ejection fraction a | 50.8 (11.5) | 51.8 (11.3) | 0.171 | 52.0 (10.9) | 52.2 (11.0) | 0.734 | 52.3 (11.5) | 52.5 (10.3) | 0.832 | 51.1 (11.3) |
51.6 (11.8) | 0.591 |
Abbreviation: CAD, coronary artery disease.
Note: Data are shown as counts (proportion; %) or mean ± standard deviation. Angiographic data were not available for 3 patients in the Q1 quartile (1 in the ticagrelor group, 2 in the prasugrel group), 3 patients in the Q2 quartile (3 in the ticagrelor group), 3 patients in the Q3 quartile (2 in the ticagrelor group, 1 in the prasugrel group), and 5 patients in the Q4 quartile (3 in the ticagrelor group and 2 in the prasugrel group).
Left ventricular ejection fraction was not available in 69 patients in the Q1 quartile (29 in the ticagrelor group and 40 in the prasugrel group), 63 patients in the Q2 quartile (31 in the ticagrelor group and 32 in the prasugrel group), 44 patients in the Q3 quartile (24 in the ticagrelor group and 20 in the prasugrel group), and 43 patients in the Q4 quartile (23 in the ticagrelor group and 20 in the prasugrel group).
Table 3. Procedural characteristics as per randomized study drug and platelet count quartiles.
| Characteristic | Quartile 1 ( N = 816) |
Quartile 2 ( N = 822) |
Quartile 3 ( N = 787) |
Quartile 4 ( N = 851) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ticagrelor ( N = 395) |
Prasugrel ( N = 421) |
p -Value | Ticagrelor ( N = 405) |
Prasugrel ( N = 417) |
p -Value | Ticagrelor ( N = 382) |
Prasugrel ( N = 405) |
p -Value | Ticagrelor ( N = 435) |
Prasugrel ( N = 416) |
p -Value | |
| Target vessel | 0.188 | 0.420 | 0.066 | 0.801 | ||||||||
| Left main coronary artery | 10 (2.5) | 9 (2.1) | 5 (1.2) | 12 (2.9) | 11 (2.9) | 7 (1.7) | 10 (2.3) | 10 (2.4) | ||||
| LAD coronary artery | 177 (44.8) | 166 (39.4) | 170 (42.0) | 174 (41.7) | 176 (46.1) | 181 (44.7) | 198 (45.5) | 176 (42.3) | ||||
| Left circumflex coronary artery | 81 (20.5) | 90 (21.4) | 91 (22.5) | 83 (19.9) | 77 (20.2) | 76 (18.8) | 85 (19.5) | 91 (21.9) | ||||
| Right coronary artery | 120 (30.4) | 138 (32.8) | 132 (32.6) | 143 (34.3) | 107 (28.0) | 138 (34.1) | 139 (32.0) | 134 (32.2) | ||||
| Bypass graft | 7 (1.8) | 18 (4.3) | 7 (1.7) | 5 (1.2) | 11 (2.9) | 3 (0.7) | 3 (0.7) | 5 (1.2) | ||||
| Complex lesion (type B2/C) | 228 (57.7) | 237 (56.3) | 0.733 | 251 (62.0) | 261 (62.6) | 0.913 | 215 (56.3) | 238 (58.8) | 0.527 | 250 (57.5) | 245 (58.9) | 0.725 |
| More than 1 lesion treated | 137 (34.7) | 148 (35.2) | 0.946 | 135 (33.3) | 137 (32.9) | 0.943 | 135 (35.3) | 157 (38.8) | 0.357 | 147 (33.8) | 145 (34.9) | 0.799 |
| TIMI flow grade before the intervention | 0.412 | 0.223 | 0.770 | 0.217 | ||||||||
| 0 | 124 (31.4) | 137 (32.5) | 147 (36.3) | 153 (36.7) | 126 (33.0) | 132 (32.6) | 172 (39.5) | 151 (36.3) | ||||
| 1 | 30 (7.6) | 35 (8.3) | 36 (8.9) | 40 (9.6) | 25 (6.5) | 34 (8.4) | 31 (7.1) | 43 (10.3) | ||||
| 2 | 81 (20.5) | 101 (24.0) | 77 (19.0) | 99 (23.7) | 92 (24.1) | 91 (22.5) | 98 (22.5) | 82 (19.7) | ||||
| 3 | 160 (40.5) | 148 (35.2) | 145 (35.8) | 125 (30.0) | 139 (36.4) | 148 (36.5) | 134 (30.8) | 140 (33.7) | ||||
| TIMI flow grade after the intervention | 0.777 | 0.193 | 0.552 | 0.685 | ||||||||
| 0 | 5 (1.3) | 7 (1.7) | 3 (0.7) | 1 (0.2) | 0 (0.0) | 2 (0.5) | 7 (1.6) | 3 (0.7) | ||||
| 1 | 2 (0.5) | 2 (0.5) | 2 (0.5) | 3 (0.7) | 1 (0.3) | 0 (0.0) | 3 (0.7) | 2 (0.5) | ||||
| 2 | 14 (3.5) | 10 (2.4) | 14 (3.5) | 6 (1.4) | 10 (2.6) | 9 (2.2) | 8 (1.8) | 9 (2.2) | ||||
| 3 | 374 (94.7) | 402 (95.5) | 386 (95.3) | 407 (97.6) | 371 (97.1) | 394 (97.3) | 417 (95.9) | 402 (96.6) | ||||
| Type of intervention | ||||||||||||
| Drug-eluting stent | 355 (89.9) | 378 (89.8) | >0.999 | 351 (86.7) | 386 (92.6) | 0.008 | 361 (94.5) | 383 (94.6) | >0.999 | 393 (90.3) | 373 (89.7) | 0.828 |
| Bare-metal stent | 1 (0.3) | 1 (0.2) | >0.999 | 2 (0.5) | 2 (0.5) | >0.999 | 0 (0.0) | 0 (0.0) | >0.999 | 1 (0.2) | 5 (1.2) | 0.116 |
| Bioresorbable vascular scaffold |
20 (5.1) | 21 (5.0) | >0.999 | 33 (8.2) | 21 (5.0) | 0.097 | 21 (5.5) | 23 (5.7) | >0.999 | 20 (4.6) | 27 (6.5) | 0.290 |
| Drug-eluting balloon | 11 (2.8) | 11 (2.6) | >0.999 | 6 (1.5) | 3 (0.7) | 0.335 | 1 (0.3) | 3 (0.7) | 0.625 | 11 (2.5) | 3 (0.7) | 0.071 |
| Plain balloon angioplasty |
12 (3.0) | 14 (3.3) | 0.973 | 14 (3.5) | 8 (1.9) | 0.250 | 2 (0.5) | 4 (1.0) | 0.687 | 17 (3.9) | 11 (2.6) | 0.400 |
| Maximal stent diameter (mm) | 3.2 (0.5) | 3.2 (0.5) | 0.764 | 3.2 (0.5) | 3.2 (0.5) | 0.522 | 3.2 (0.5) | 3.2 (0.5) | 0.811 | 3.2 (0.5) | 3.2 (0.5) | 0.500 |
| Total stented length (mm) | 32.3 (18.4) | 29.5 (17.5) | 0.028 | 30.9 (17.7) | 31.2 (17.4) | 0.812 | 29.9 (15.0) | 30.3 (16.8) | 0.755 | 30.3 (16.2) |
30.3 (16.2) | 0.998 |
| Successful PCI | 387 (98.0) | 410 (97.4) | 0.746 | 398 (98.3) | 409 (98.1) | >0.999 | 380 (99.5) | 403 (99.5) | >0.999 | 421 (96.8) | 404 (97.1) | 0.933 |
| Periprocedural antithrombotic medication | ||||||||||||
| Aspirin | 345 (87.3) | 369 (87.6) | 0.979 | 354 (87.4) | 375 (89.9) | 0.303 | 355 (92.9) | 380 (93.8) | 0.718 | 399 (91.7) | 372 (89.4) | 0.302 |
| Unfractionated heparin |
369 (93.4) | 398 (94.5) | 0.600 | 383 (94.6) | 388 (93.0) | 0.447 | 361 (94.5) | 375 (92.6) | 0.346 | 410 (94.3) | 393 (94.5) | >0.999 |
| Low-molecular weight-heparin |
20 (5.1) | 18 (4.3) | 0.713 | 16 (3.9) | 18 (4.3) | 0.930 | 10 (2.6) | 14 (3.5) | 0.634 | 23 (5.3) | 13 (3.1) | 0.163 |
| Bivalirudin | 29 (7.3) | 31 (7.4) | >0.999 | 33 (8.2) | 40 (9.6) | 0.545 | 29 (7.6) | 39 (9.6) | 0.373 | 33 (7.6) | 31 (7.5) | >0.999 |
| GPIIb/IIIa inhibitor | 48 (12.2) | 39 (9.3) | 0.222 | 49 (12.1) | 52 (12.5) | 0.955 | 55 (14.4) | 54 (13.3) | 0.742 | 51 (11.7) | 43 (10.3) | 0.592 |
Abbreviations: GPIIb/IIIa, glycoprotein IIb/IIIa; LAD, left anterior descending; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Note: Data are shown as counts (proportions; %) or mean ± standard deviation.
Clinical Outcomes in Patients According to Platelet Count Quartiles
Clinical outcomes in patients of Q1 to Q4 of platelet count are summarized in Table 4 . The primary outcome (death, MI, or stroke) occurred in 313 patients. In patients in the Q1 to Q4 of platelet count, the primary outcome (death, MI, or stroke) occurred in 74 of 997 patients (7.5%), 78 of 1,003 patients (7.9%), 63 of 961 patients (6.7%), and 98 of 982 patients (10.1%), respectively (HR = 0.98; 95% CI [0.71–1.35] Q1 of platelet count versus Q2 of platelet count, HR = 1.13; 95% CI [0.81–1.58] Q1 of platelet count versus Q3 of platelet count, and HR = 0.74; 95% CI [0.54–1.00] Q1 of platelet count versus Q4 of platelet count) ( Fig. 2A ).
Table 4. Clinical outcomes according to platelet count quartiles.
| Quartile 1 ( n = 997) |
Quartile 2 ( n = 1,003) |
Quartile 3 ( n = 961) |
Quartile 4 ( n = 982) |
Q1 vs. Q2 HR [95% CI] |
Q1 vs. Q3 HR [95% CI] |
Q1 vs. Q4 HR [95% CI] |
|
|---|---|---|---|---|---|---|---|
| Primary endpoint (death, myocardial infarction, or stroke) | 74 (7.5) | 78 (7.9) | 63 (6.7) | 98 (10.1) | 0.98 [0.71–1.35] |
1.13 [0.81–1.58] |
0.74 [0.54–1.00] |
| BARC type 3 to 5 bleeding a | 58 (5.9) | 49 (4.9) | 40 (4.2) | 73 (7.5) | 1.29 [0.88–1.89] |
1.50 [1.01–2.27] |
0.81 [0.57–1.15] |
Abbreviations: BARC, Bleeding Academic Research Consortium; CI, confidence interval; HR, hazard ratio.
BARC type 3 to 5 bleeding was analyzed in the intention-to-treat population.
Fig. 2.
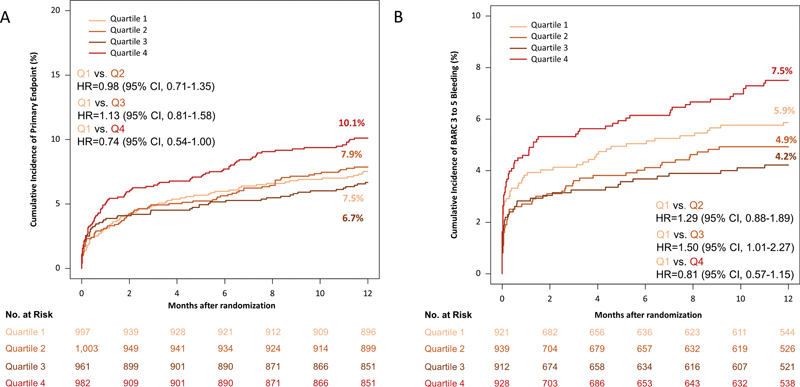
12-Month incidence of the primary (efficacy) endpoint, which was a composite of death, myocardial infarction, and stroke in quartile 1, 2, 3, and 4 of platelet count ( A ) and bleeding (safety) endpoint, which was BARC type 3 to 5 bleeding, in quartile 1, 2, 3, and 4 of platelet count ( B ).
The BARC type 3 to 5 bleeding occurred in 220 patients (analyzed according to intention-to-treat principle). In patients in the Q1 to Q4 of platelet count, bleeding occurred in 58 of 997 patients (5.9%), 49 of 1,003 patients (4.9%), 40 of 961 patients (4.2%), and 73 of 982 patients (7.5%), respectively; (HR = 1.29; 95% CI [0.88–1.89] Q1 of platelet count versus Q2 of platelet count, HR = 1.50; 95% CI [1.01–2.27] Q1 of platelet count versus Q3 of platelet count, and HR = 0.81; 95% CI [0.57–1.15] Q1 of platelet count versus Q4 of platelet count) ( Fig. 2B ).
Clinical Outcomes in Patients According to the Platelet Count and Treatment Arm
Clinical outcomes as per randomized study drug and platelet count quartiles are summarized in Table 5 . The primary outcome (death, MI, or stroke) occurred in 313 patients. In patients in Q1 of platelet count, the primary endpoint occurred in 42 of 485 patients assigned to ticagrelor and in 32 of 512 patients assigned to prasugrel (cumulative incidence: 8.8 vs. 6.3%, HR = 1.41; 95% CI: 0.89–2.23, p = 0.148). In patients in Q2 of platelet count, the primary endpoint occurred in 49 of 500 patients assigned to ticagrelor and in 29 of 503 patients assigned to prasugrel (cumulative incidence: 9.9 vs. 5.8%, HR = 1.68; 95% CI [1.06–2.66], p = 0.027). In patients in Q3 of platelet count, the primary endpoint occurred in 37 of 482 patients assigned to ticagrelor and in 26 of 479 patients assigned to prasugrel (cumulative incidence: 7.8 vs. 5.5%, HR = 1.43; 95% CI [0.87–2.37], p = 0.159) and in patients in Q4 of platelet count, the primary endpoint occurred in 50 of 499 patients assigned to ticagrelor and in 48 of 483 patients assigned to prasugrel (cumulative incidence: 10.1 vs. 10.1%, HR = 1.05; 95% CI [0.71–1.57], p = 0.799). There was no significant interaction between treatment arm (ticagrelor vs. prasugrel) and platelet count group with respect to the primary efficacy outcome ( p int = 0.482). Kaplan–Meier curves of primary outcome are shown in Fig. 3(A–D) .
Table 5. Clinical outcomes as per randomized study drug and platelet count quartiles.
| Characteristic | Quartile 1 ( N = 997) |
Quartile 2 ( N = 1,003) |
Quartile 3 ( N = 961) |
Quartile 4 ( N = 982) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ticagrelor ( N = 485) |
Prasugrel ( N = 512) |
HR [95% CI] |
p -Value | Ticagrelor ( N = 500) |
Prasugrel ( N = 503) |
HR [95% CI] |
p -Value | Ticagrelor ( N = 482) |
Prasugrel ( N = 479) |
HR [95% CI] |
p -Value | Ticagrelor ( N = 499) |
Prasugrel ( N = 483) |
HR [95% CI] |
p -Value | |
| Primary endpoint | 42 (8.8) | 32 (6.3) | 1.41 [0.89–2.23] |
0.148 | 49 (9.9) | 29 (5.8) | 1.68 [1.06–2.66] |
0.027 | 37 (7.8) | 26 (5.5) | 1.43 [0.87–2.37] |
0.159 | 50 (10.1) | 48 (10.1) | 1.05 [0.71–1.57] |
0.799 |
| Death | 27 (5.6) | 19 (3.8) | 1.50 [0.83–2.70] |
0.175 | 13 (2.6) | 17 (3.4) | 0.74 [0.36–1.53] |
0.418 | 21 (4.4) | 13 (2.8) | 1.63 [0.82–3.27] |
0.164 | 27 (5.5) | 23 (4.8) | 1.17 [0.67–2.04] |
0.585 |
| Myocardial infarction | 19 (4.0) | 11 (2.2) | 1.86 [0.88–3.90] |
0.103 | 34 (6.9) | 14 (2.8) | 2.41 [1.29–4.49] |
0.006 | 18 (3.8) | 12 (2.6) | 1.49 [0.72–3.09] |
0.288 | 22 (4.5) | 22 (4.6) | 1.05 [0.58–1.90] |
0.877 |
| Stroke | 4 (0.8) | 7 (1.4) | 0.64 [0.19–2.18] |
0.471 | 8 (1.6) | 2 (0.4) | 3.98 [0.84–18.77] |
0.080 | 3 (0.6) | 4 (0.8) | 0.77 [0.17–3.46] |
0.738 | 5 (1.0) | 6 (1.2) | 0.78 [0.24–2.57] |
0.683 |
| Definite and probable stent thrombosis | 5 (1.0) | 1 (0.2) | 5.05 [0.59–43.27] |
0.140 | 8 (1.6) | 5 (1.0) | 1.59 [0.52–4.87] |
0.418 | 8 (1.7) | 4 (0.8) | 2.05 [0.62–6.81] |
0.242 | 5 (1.0) | 9 (1.9) | 0.56 [0.19–1.67] |
0.297 |
| Definite stent thrombosis | 3 (0.6) | 1 (0.2) | 3.12 [0.32–30.06] |
0.325 | 8 (1.6) | 3 (0.6) | 2.67 [0.71–10.10] |
0.148 | 7 (1.5) | 2 (0.4) | 3.51 [0.73- 16.91] |
0.118 | 4 (0.8) | 5 (1.0) | 0.81 [0.22–3.03] |
0.758 |
| BARC type 3 to 5 bleeding a |
24/481 (5.8) |
17/434 (4.2) |
1.41 [0.76–2.63] |
0.279 | 26/490 (6.4) |
15/447 (3.7) |
1.62 [0.85–3.06] |
0.140 | 18/478 (4.4) |
12/432 (3.0) |
1.53 [0.73–3.18] |
0.258 | 24/495 (5.6) |
34/431 (8.5) |
0.67 [0.40–1.14] |
0.138 |
Abbreviations: BARC, Bleeding Academic Research Consortium; CI, confidence interval; HR, hazard ratio.
BARC type 3 to 5 bleeding was analyzed in the intention-to-treat population.
Fig. 3.
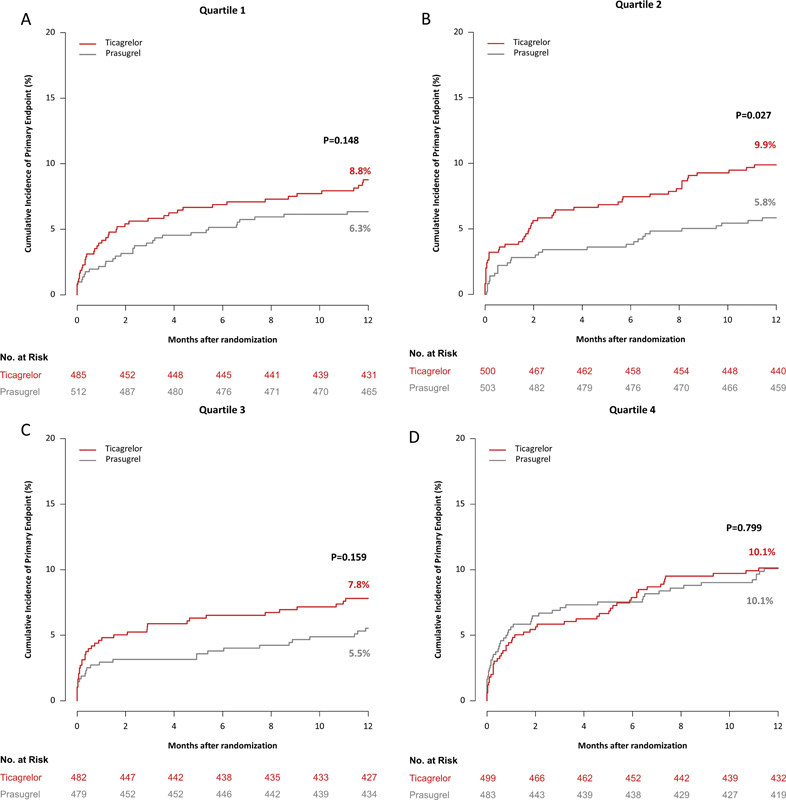
12-Month incidence of the primary (efficacy) endpoint, which was a composite of death, myocardial infarction, and stroke, in patients assigned to ticagrelor and prasugrel in quartile 1 ( A ), 2 ( B ), 3 ( C ), and 4 ( D ) of platelet count.
The BARC type 3 to 5 bleeding occurred in 170 patients (analyzed according to the modified intention-to-treat principle). In patients in Q1 of platelet count, bleeding occurred in 24 of 481 patients assigned to ticagrelor and in 17 of 434 patients assigned to prasugrel (cumulative incidence after accounting for competing risk of death: 5.8 vs. 4.2%, HR = 1.41; 95% CI [0.76–2.63], p = 0.279). In patients in Q2 of platelet count, bleeding occurred in 26 of 490 patients assigned to ticagrelor and in 15 of 447 patients assigned to prasugrel (cumulative incidence after accounting for competing risk of death: 6.4 vs. 3.7%, HR = 1.62; 95% CI [0.85–3.06], p = 0.140). In patients in Q3 of platelet count, bleeding occurred in 18 of 478 patients assigned to ticagrelor and in 12 of 432 patients assigned to prasugrel (cumulative incidence after accounting for competing risk of death: 4.4 vs. 3.0%, HR = 1.53; 95% CI [0.73–3.18], p = 0.258), and in patients in Q4 of platelet count bleeding endpoint occurred in 24 of 495 patients assigned to ticagrelor and in 34 of 431 patients assigned to prasugrel (cumulative incidence after accounting for competing risk of death: 5.6 vs. 8.5%, HR = 0.67; 95% CI [0.40–1.14], p = 0.138). There was no significant interaction between treatment arm (ticagrelor vs. prasugrel) and platelet count group with respect to the bleeding endpoint ( p int = 0.102). Kaplan–Meier curves of bleeding are shown in Fig. 4(A–D) .
Fig. 4.
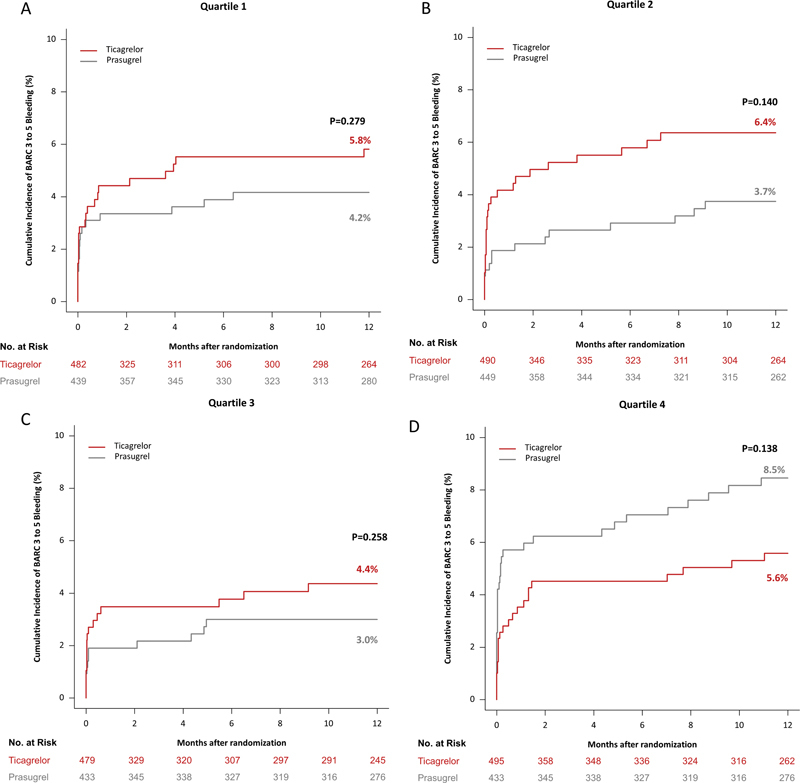
12-Month incidence of the bleeding (safety) endpoint, which was BARC type 3 to 5 bleeding, in patients assigned to ticagrelor and prasugrel in quartile 1 ( A ), 2 ( B ), 3 ( C ), and 4 ( D ) of platelet count.
Clinical outcomes according to prespecified subgroup analyses and study drugs among platelet count quartiles are summarized in Supplementaary Table S4 (available in the online version). The analysis was performed in selected prespecified subgroups, where the number of patients allowed for it in all quartiles.
Discussion
In the current study, we assessed the efficacy and safety of ticagrelor versus prasugrel according to the baseline platelet count in the patients enrolled in the ISAR-REACT 5 trial. The main findings of the current study may be summarized as follows:
There was no significant interaction between the randomized treatment arm and quartiles of platelet count with regard to the incidence of the primary endpoint (death, MI, or stroke) or the bleeding endpoint (BARC type 3–5 bleeding) through to 12-months of follow-up. Thus, the relative efficacy and safety of ticagrelor versus prasugrel appear to differ little according to the baseline platelet count within the range of platelet count investigated in this study.
The incidence of ischemic and bleeding events was comparable in patients across Q1 to Q4 of platelet count up to 12 months of follow-up.
This is the first study to assess the interaction between platelet count and the relative efficacy and safety of ticagrelor and prasugrel based on DAPT strategies in patients with ACS undergoing predominantly an invasive treatment. These results support the findings of the primary trial and suggest that prasugrel may be used in preference to ticagrelor in patients with ACS undergoing an invasive treatment regardless of baseline platelet count. Based upon the available evidence, current ACS guidelines do not recommend to administer routine pretreatment with a P2Y 12 -receptor inhibitor in NSTE-ACS patients in whom coronary anatomy is not known and an early invasive management is planned. Referring to results of the ISAR-REACT 5 trial, in NSTE-ACS patients a prasugrel-based strategy with deferred loading after knowledge of coronary anatomy should be preferred to a ticagrelor-based strategy with routine pretreatment before coronary angiography. 18 In patients aged 75 years or older or in patients weighing less than 60 kg, a reduced maintenance dose of 5 mg prasugrel once daily is recommended by producers, current guidelines, and study results due to increased risk of intracranial or fatal bleeding. 17 18 Current subgroup analysis of the ISAR-REACT 5 trial showed comparable safety and efficacy of a reduced maintenance dose in these patient subgroups. 13
Previous studies have reported on the association between platelet count and the clinical outcomes in patients with ACS. In particular, a U-shaped association has been reported, with an increased risk of mortality observed in patients with low and high platelet counts. 6 Patients with thrombocytopenia have also been reported to have an increased risk of bleeding events. 7 9 However, an analysis of outcomes as per groups based on platelet count had not been performed in an ACS population, where all patients were treated with potent P2Y 12 inhibitor-based DAPT. The current analysis indicates that the incidence of the primary endpoint (death, MI, or stroke) and the bleeding endpoint (BARC type 3–5 bleeding) is comparable in groups according to quartiles of platelet count through to 12 months of follow-up, except patients in Q2, where the primary endpoint was significantly reduced in patients assigned to prasugrel. This effect was mainly driven by a significant reduction of MI in patients treated with prasugrel compared with those treated with ticagrelor.
There are limited data on the impact of platelet count on the relative efficacy and safety of DAPT regimens in patients with ACS. Given that the use of potent P2Y 12 inhibitors has been associated with a reduction in ischemic endpoints but an increase in bleeding in comparison to clopidogrel, it may be of interest to examine the relative efficacy and safety of prasugrel and ticagrelor in the spectrum of platelet counts present in patients enrolled in the ISAR-REACT 5 trial. 19 20 The study cohort of ISAR-REACT 5 had an inherent high ischemic risk at baseline with high rates of diabetes mellitus, arterial hypertension, smoking, and history of coronary revascularization, which may have influenced clinical outcomes at 12 months. Overall, our analysis showed no statistically significant treatment arm (ticagrelor vs. prasugrel)-by-platelet count interaction with respect to the risk for ischemic or bleeding events up to 12 months of follow-up. The incidence of the primary endpoint was numerically lower or at least even in patients assigned to prasugrel in all quartiles, although this was less pronounced in patients in the upper quartile. This appears to be primarily due to the comparable number of MI events in patients assigned to ticagrelor and prasugrel in this group. The bleeding events were numerically higher in patients assigned to ticagrelor in Q1 to Q3 of platelet count compared with prasugrel. Conversely, bleeding events were higher in patients in the upper quartile of platelet count assigned to prasugrel, compared with patients assigned to ticagrelor. However, the treatment-arm-by platelet count interaction with respect to the risk for bleeding did not reach the level of statistical significance ( p int = 0.102). Overall, these data could be interpreted as suggesting that the benefit of prasugrel over ticagrelor observed in the ISAR-REACT 5 trial is less pronounced in the upper platelet count group. Nevertheless, interpretation of the findings of subgroup analysis should be done on the basis of formal interaction testing rather than the HR associated with the treatment effect. 21 22 Furthermore, it should be acknowledged that the division of the patients into quartiles may further reduce the power of the study or introduce errors related to multiple testing. In this regard, the current findings should be seen as exploratory or hypothesis-generating.
Patients with ACS with a high platelet count have been associated with an increased risk of adverse outcomes. 23 It has been suggested that a high platelet count in the setting of ACS may be associated with increased inflammation and in particular with increased levels of interleukin-6 (IL-6), which stimulates megakaryocytes both through an increase in thrombopoietin production in the liver 24 and by acting directly on megakaryocytes to stimulate platelet production. 25 IL-6 has previously been identified as an independent predictor of adverse outcomes in ACS. 26 27 Indeed, it is increasingly recognized that platelets have multiple immunological functions beyond hemostasis and thrombosis and therefore it may be logical to suggest that outcomes may differ in ACS patients with high and low platelet counts. While in the current analysis, the incidence of ischemic and bleeding events was numerically higher in patients in the upper quartile as compared with patients in the lower quartile of platelet count, the differences were not statistically significant. Therefore, our data do not support a clinically meaningful difference in 1-year outcomes in patients with ACS within the investigated platelet count range. A substudy of the PLATO trial reported that the increase in platelet count from 1 to 6 months after an ACS was associated with ischemic/thrombotic events. 28 Another study reported that the addition of platelet count to the PRECISE-DAPT score slightly improves the predictive ability of this score. 29 Of note, in the PRECISE-DAPT study, patients with a lower platelet count within each category had a markedly increased risk of death. In that study, a low platelet count was defined as <150 × 10 9 /L.
Overall, the results of this analysis data support the use of prasugrel in preference to ticagrelor regardless of group based on quartiles of platelet count. However, it is important to consider that patients with a known platelet count of <100 × 10 9 /L were excluded from the ISAR-REACT 5 trial. Thus, these data cannot be extrapolated to patients with thrombocytopenia. In general, there are lack of high-quality data to guide the management of ACS patients with thrombocytopenia. 30 Dedicated randomized controlled trials may are needed to determine the optimal treatment strategy in this challenging group of patients.
The current study has several limitations. The study represents a posthoc analysis of a randomized controlled trial and therefore carries the limitations inherent to this form of analysis and the analysis was not prespecified in the protocol of the primary trial. As such, these findings should be considered hypothesis-generating. In addition, patients with a known platelet count <100 × 10 9 /L at the time of screening were excluded from the ISAR-REACT 5 trial and therefore this analysis cannot provide guidance for these patients at increased risk. In the current analysis, patients were classified as per quartiles of platelet count rather than as per the presence or absence of thrombocytopenia (<150 × 10 9 /L) or thrombocytosis (>450 × 10 9 /L). Thus, many patients within both the lower and upper quartiles of platelet count have platelet count within the generally accepted normal range (150–450 × 10 9 /L). This analysis only reports on outcomes according to the platelet count and platelet function as well as further comprehensive characterization of hemostatic state was not assessed. Further, platelet count was assessed only at one time point (at baseline) and therefore eventual dynamic changes in the platelet count over time remain unaccounted for. In the context of ACS, various parameters and variables are important to stratify patients' risk, and baseline platelet count could only be a supportive tool for higher decision variables such as angiographic findings. Furthermore, several significant differences in baseline characteristics and comorbidities between quartiles may have influenced clinical event rates. Finally, this analysis only reports clinical outcomes out to 12 months, so this could lead to an underestimation of possible late clinical events.
Conclusions
Based on the lack of a significant treatment arm-by-platelet count interaction in terms of the risk for ischemic and bleeding events, it may be concluded that the relative efficacy and safety of ticagrelor versus prasugrel appear to differ little according to the baseline platelet count in patients with ACS predominantly undergoing invasive treatment up to 1 year of follow-up. The incidence of ischemic and bleeding events at 1 year appears to be comparable in patient groups in Q1 to Q4 of platelet count.
Footnotes
Conflict of Interest D.J.A. reports consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, and Sanofi; and research grants to his institution from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and Scott R. MacKenzie Foundation. H.S. reports honoraria from AstraZeneca, Bayer Vital, MSD SHARP&DOHME, Novartis, Servier, Sanofi-Aventis, Boehringer Ingelheim, Daiichi Sankyo, Amgen, and Pfizer, and consulting fees from AstraZeneca, Amgen, and MSD SHARP&DOHME. S.K. reports consulting and lecture fees from Astra Zeneca, Bristol-Myers Squibb, and Translumina. T.K. is named inventor on a patent application for prevention of restenosis after angioplasty and stent implantation outside the submitted work and received lecture fees from Bayer AG, Pharmaceuticals; M.J. reports speaker fees from Biotronik, personal fees from OrbusNeich, grants and personal fees from Boston Scientific, grants and personal fees from Edwards, personal fees from AstraZeneca, personal fees from Recor, grants from Amgen, not related to the current work.
What is known about this topic?
The ISAR-REACT 5 trial showed that prasugrel was superior to ticagrelor in reducing the risk of ischemic events with no increase in the risk of bleeding in patients with acute coronary syndrome (ACS).
Platelet count has been reported to impact on the outcomes of patients with ACS.
There are no data on the relative efficacy and safety of ticagrelor- versus prasugrel-based DAPT in patients presenting with an ACS and undergoing percutaneous revascularization according to platelet count.
What does this paper add?
This is the first study to assess the interaction between platelet count and the relative efficacy and safety of ticagrelor- and prasugrel-based DAPT strategies in 3,943 patients with ACS undergoing an invasive treatment.
The incidence(s) of ischemic and bleeding events at 1 year appear to be comparable in patients across Q1 to Q4 of platelet count.
Supplementary Material
References
- 1.Angiolillo D J, Galli M, Collet J P, Kastrati A, O'Donoghue M L. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022;17(17):e1371–e1396. doi: 10.4244/EIJ-D-21-00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Małyszczak A, Łukawska A, Dyląg I, Lis W, Mysiak A, Kuliczkowski W. Blood platelet count at hospital admission impacts long-term mortality in patients with acute coronary syndrome. Cardiology. 2020;145(03):148–154. doi: 10.1159/000505640. [DOI] [PubMed] [Google Scholar]
- 3.TIMI Study Group . Ly H Q, Kirtane A J, Murphy S A. Association of platelet counts on presentation and clinical outcomes in ST-elevation myocardial infarction (from the TIMI Trials) Am J Cardiol. 2006;98(01):1–5. doi: 10.1016/j.amjcard.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Mueller C, Neumann F J, Hochholzer W. The impact of platelet count on mortality in unstable angina/non-ST-segment elevation myocardial infarction. Am Heart J. 2006;151(06):12140–1.214E10. doi: 10.1016/j.ahj.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Nikolsky E, Grines C L, Cox D A. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am J Cardiol. 2007;99(08):1055–1061. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 6.Song P S, Ahn K T, Jeong J-O. Association of baseline platelet count with all-cause mortality after acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2021;10(02):176–183. doi: 10.1177/2048872620925257. [DOI] [PubMed] [Google Scholar]
- 7.Wang T Y, Ou F S, Roe M T. Incidence and prognostic significance of thrombocytopenia developed during acute coronary syndrome in contemporary clinical practice. Circulation. 2009;119(18):2454–2462. doi: 10.1161/CIRCULATIONAHA.108.827162. [DOI] [PubMed] [Google Scholar]
- 8.Sinkovič A, Majal M. The impact of thrombocytopenia on outcome in patients with acute coronary syndromes: a single center retrospective study. BioMed Res Int. 2015;2015:907304. doi: 10.1155/2015/907304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakim D A, Dangas G D, Caixeta A. Impact of baseline thrombocytopenia on the early and late outcomes after ST-elevation myocardial infarction treated with primary angioplasty: analysis from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Am Heart J. 2011;161(02):391–396. doi: 10.1016/j.ahj.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 10.ISAR-REACT 5 Trial Investigators . Schüpke S, Neumann F J, Menichelli M. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381(16):1524–1534. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- 11.Mayer K, Bongiovanni D, Karschin V. Ticagrelor or prasugrel for platelet inhibition in acute coronary syndrome patients: the ISAR-REACT 5 trial. J Am Coll Cardiol. 2020;76(21):2569–2571. doi: 10.1016/j.jacc.2020.09.586. [DOI] [PubMed] [Google Scholar]
- 12.Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 Trial Investigators . Schulz S, Angiolillo D J, Antoniucci D. Randomized comparison of ticagrelor versus prasugrel in patients with acute coronary syndrome and planned invasive strategy–design and rationale of the iNtracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 trial. J Cardiovasc Transl Res. 2014;7(01):91–100. doi: 10.1007/s12265-013-9527-3. [DOI] [PubMed] [Google Scholar]
- 13.Menichelli M, Neumann F J, Ndrepepa G. Age- and weight-adapted dose of prasugrel versus standard dose of ticagrelor in patients with acute coronary syndromes : results from a randomized trial. Ann Intern Med. 2020;173(06):436–444. doi: 10.7326/M20-1806. [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 15.Academic Research Consortium . Garcia-Garcia H M, McFadden E P, Farb A. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 consensus document. Circulation. 2018;137(24):2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 16.Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Thygesen K, Alpert J S, Jaffe A S. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 17.ISAR-REACT 5 Trial Investigators . Schüpke S, Neumann F J, Menichelli M. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381(16):1524–1534. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- 18.ESC Scientific Document Group . Collet J P, Thiele H, Barbato E. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 19.PLATO Investigators . Wallentin L, Becker R C, Budaj A. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 20.TRITON-TIMI 38 Investigators . Wiviott S D, Braunwald E, McCabe C H. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Lagakos S W, Ware J H, Hunter D J, Drazen J M. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 22.Drexel H, Pocock S J, Lewis B S. Subgroup analyses in randomized clinical trials: value and limitations. Review #3 on important aspects of randomized clinical trials in cardiovascular pharmacotherapy. Eur Heart J Cardiovasc Pharmacother. 2021;8(03):302–310. doi: 10.1093/ehjcvp/pvab048. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Wu H, Mueller C. Baseline platelet count and clinical outcome in acute coronary syndrome. Circ J. 2012;76(03):704–711. doi: 10.1253/circj.cj-11-0707. [DOI] [PubMed] [Google Scholar]
- 24.Heits F, Stahl M, Ludwig D, Stange E F, Jelkmann W. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. 1999;19(07):757–760. doi: 10.1089/107999099313604. [DOI] [PubMed] [Google Scholar]
- 25.Burstein S A. Effects of interleukin 6 on megakaryocytes and on canine platelet function. Stem Cells. 1994;12(04):386–393. doi: 10.1002/stem.5530120405. [DOI] [PubMed] [Google Scholar]
- 26.Fanola C L, Morrow D A, Cannon C P. Interleukin-6 and the risk of adverse outcomes in patients after an acute coronary syndrome: observations from the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52) trial. J Am Heart Assoc. 2017;6(10):e005637. doi: 10.1161/JAHA.117.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, Deng Z, Li J, Ren Z, Liu F. Meta-analysis of the relationship between interleukin-6 levels and the prognosis and severity of acute coronary syndrome. Clinics (São Paulo) 2021;76:e2690. doi: 10.6061/clinics/2021/e2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PLATO Investigators . Lowenstern A, Storey R F, Neely M. Platelet-related biomarkers and their response to inhibition with aspirin and p2y 12 -receptor antagonists in patients with acute coronary syndrome . J Thromb Thrombolysis. 2017;44(02):145–153. doi: 10.1007/s11239-017-1516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morici N, Tavecchia G A, Antolini L. Use of PRECISE-DAPT score and admission platelet count to predict mortality risk in patients with acute coronary syndrome. Angiology. 2019;70(09):867–877. doi: 10.1177/0003319719848547. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy C P, Steg G, Bhatt D L. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: a clinical conundrum. Eur Heart J. 2017;38(47):3488–3492. doi: 10.1093/eurheartj/ehx531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


