Summary
Background
Ventricular fibrillation (VF) waveform analysis has been proposed as a potential non-invasive guide to optimize timing of defibrillation.
Methods
The AMplitude Spectrum Area (AMSA) trial is an open-label, multicenter randomized controlled study reporting the first in-human use of AMSA analysis in out-of-hospital cardiac arrest (OHCA). The primary efficacy endpoint was the termination of VF for an AMSA ≥ 15.5 mV-Hz. Adult shockable OHCAs randomly received either an AMSA-guided cardiopulmonary resuscitation (CPR) or a standard-CPR. Randomization and allocation to trial group were carried out centrally. In the AMSA-guided CPR, an initial AMSA ≥ 15.5 mV-Hz prompted for immediate defibrillation, while lower values favored chest compression (CC). After completion of the first 2-min CPR cycle, an AMSA < 6.5 mV-Hz deferred defibrillation in favor of an additional 2-min CPR cycle. AMSA was measured and displayed in real-time during CC pauses for ventilation with a modified defibrillator.
Findings
The trial was early discontinued for low recruitment due to the COVID-19 pandemics. A total of 31 patients were recruited in 3 Italian cities, 19 in AMSA-CPR and 12 in standard-CPR, and included in the data analysis. No difference in primary outcome was observed between the two groups. Termination of VF occurred in 74% of patients in the AMSA-CPR compared to 75% in the standard CPR (OR 0.93 [95% CI 0.18–4.90]). No adverse events were reported.
Interpretation
AMSA was used prospectively in human patients during ongoing CPR. In this small trial, an AMSA-guided defibrillation provided no evidence of an improvement in termination of VF.
Trial registration
Funding
European Commission - Horizon 2020; ZOLL Medical Corp., Chelmsford, USA (unrestricted grant); Italian Ministry of Health - Current research IRCCS.
Keywords: Cardiac arrest, Ventricular fibrillation, Amplitude spectrum area, Waveform analysis, Defibrillation
Research in context.
Evidence before this study
Current cardiopulmonary resuscitation (CPR) is based on a fixed, time-based defibrillation strategy. Rhythm analysis and shock delivery (if indicated) are repeated every 2 min requiring cyclical interruptions of chest compressions (CC), with detrimental effects on outcome. A tailored defibrillation strategy should identify treatment priority for each patient, i.e. CC or defibrillation, minimize CC interruptions, speed up the delivery of early effective defibrillation, and reduce the number of ineffective shocks. Real-time waveform analysis has been proposed as a potential non-invasive approach to guide defibrillation during CPR and to prevent the delivery of unsuccessful electrical shocks. Amplitude Spectrum Area (AMSA) is one of the most accurate predictors of defibrillation outcome among several similar algorithms. Despite evidence from numerous animal studies and retrospective analyses of human databases, no prospective clinical investigations with real-time AMSA analysis during CPR have been conducted yet.
Added value of this study
This open-label, multicenter randomized controlled trial reported for the first time the prospective clinical application of an AMSA-guided CPR algorithm. It demonstrated the possibility of measuring and reading AMSA in real-time during CPR in the out-of-hospital setting with the use of an experimental defibrillator. The study also confirmed the association between AMSA and defibrillation outcome in the real clinical scenario. These results are of strong value because they were derived from a randomized study conducted in 3 different cities.
Implications of all the available evidence
The present study finally provided a clinical validation of the capability of AMSA to predict defibrillation success and its ability to potentially guide CPR. Thus, the real-time VF waveform analysis and more specifically the AMSA analysis during CPR is now disclosed to be used in the clinical scenario. Finally, considering all the information derived from this small trial, a larger trial can now be designed.
Introduction
Early defibrillation of ventricular fibrillation (VF) along with high-quality chest compressions (CC) are the main determinants of resuscitation outcome in cardiac arrest patients.1,2 Current cardiopulmonary resuscitation (CPR) guidelines recommend providing a short period of CC while the defibrillator is deployed and ready for rhythm analysis before defibrillation.1,2 After the first shock, CPR should be restarted for 2 min until a new rhythm analysis and another countershock, if indicated.1, 2, 3, 4 However, this standard algorithm is based on a low certainty evidence, remaining the optimal timing of defibrillation in relationship to CC and the priority of intervention, i.e. defibrillation first or CC first, unclear.5, 6, 7, 8
Recently, the International Liaison Committee on Resuscitation (ILCOR) reported that providing a longer CPR interval prior to the first defibrillation does not improve outcome.9 There is also insufficient knowledge about the optimal duration of the CC interval prior to a subsequent defibrillation attempt. No clear benefit from different CPR durations between rhythm checks and shock delivery has been shown.1,9 Thus, delivery of defibrillation attempts has traditionally been recommended on a strict time-based protocol, i.e. every 2-min, without any evaluation of the myocardium pathophysiological pattern, reflected by the VF signal waveform. This may lead to unnecessary CC interruptions for futile defibrillations, potentially worsening post-resuscitation myocardial injury.
Real-time VF waveform analysis has been proposed and advocated as a potential non-invasive approach to guide defibrillation during CPR and to prevent the delivery of unsuccessful high-energy electrical shocks.10, 11, 12, 13, 14, 15 AMplitude Spectrum Area (AMSA) is one of the most accurate predictors of defibrillation outcome among several similar algorithms.12,13,16, 17, 18 Despite evidence from numerous animal studies and retrospective analyses of human data, no prospective clinical investigations with real-time AMSA analysis during CPR have been conducted. Thus, whether VF waveform characteristics can be used to determine the optimal defibrillation strategy remains a knowledge gap, with a clear need for prospective studies.15
The AMSA trial has been conceived as a randomized controlled multicenter clinical trial comparing a new defibrillation strategy guided by AMSA versus standard CPR in out-of-hospital cardiac arrest (OHCA). Due to the early discontinuation of the trial, the AMSA trial turned into a small multicenter randomized clinical study reporting the first-time human use of a real-time AMSA analysis during CPR.
Methods
The study is reported according to CONSORT extended guidelines for pragmatic trials (http://www.consort-statement.org/extensions), and the CONSERVE 2021 statement.19
Ethics and study design
The AMSA trial was originally designed as a phase III multicenter, open-label, efficacy, randomized, controlled clinical trial in OHCA patients, and registered with ClinicalTrials.gov (NCT03237910, registered 3 August 2017). The study protocol was approved by the Ethics Committee AREA 2 Milano, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy (approval no. 189_2017bis, April 20th, 2017). The use of an investigational modified X-Series defibrillator (ZOLL Med. Corp. Chelmsford, MA, USA), with the AMSA algorithm embedded, was notified to the Italian Ministry of Health, according to National regulations. On November 27th 2017, the study protocol was amended to correct the planned sample size. The study was then approved by the Ethics Committees in each participating Center. The trial qualified for exception from informed consent under emergency circumstances. A deferred written consent was then obtained from each patient who survived and regained mental capacity or from a legal surrogate, depending on the circumstances. The patient or each representative had the freedom to withdraw from the study at any time.
A total of 388 patients were planned to be enrolled by the Emergency Medical Systems (EMS) of Milan and Monza (SOREU Metropolitana, Agenzia Regionale Emergenza Urgenza—AREU, Milan), and Bologna (Maggiore Hospital, AUSL, Bologna) in Italy. Länsi-Uusimaa EMS, Helsinki, Finland, joined the trial in January 2020, but did not enroll patients.
The trial started on November 2018 with the training of the EMS personnel. Patients’ enrollment was planned to start April 1st, 2019, but there was a downturn till October 1st, 2019, because the AMSA-equipped X-Series defibrillators underwent a software update by ZOLL Med. Corp., due to an internal error alert evidenced in some devices during the regular daily test. This delay led to 2 consecutive minor protocol amendments to extend the enrollment timeline. Due to the strict inclusion criteria, the enrolment was expected to be slow. Therefore, new centers were under recruitment.
The enrollment plan was then seriously impacted by two COVID-19 pandemic waves that hit the Italian sites (February–August 2020, and October 2020–June 2021).20 The pandemics created a high pressure on the EMS systems, making it impossible to recruit patients as well as new sites.20,21 Indeed, the COVID-19 pandemics affected the system-of-care of OHCA, with longer EMS arrival time, reductions in shockable rhythms, and ultimately worse outcomes.22 The study protocol and timeline were not modified because a reestablishment of the normal EMS operating condition was expected after the end of the first COVID-19 outbreak. However, the recurrence of subsequent pandemic waves with no burden relief on the EMS occurred. It was impossible for the study management to foresee these challenges and on July 27th, 2021, the Steering Committee, with the support from the Data and Safety Monitoring Board (DSMB), formally decided to stop the study for lack of patients’ recruitment over the last 1.5 years. Consequently, the AMSA trial became a small study reporting the first in-human use of real-time AMSA analysis to guide CPR.
Patient population
All adult patients (age ≥18 years old) with an OHCA of presumably cardiac etiology and with a presenting shockable rhythm, i.e. VF or pulseless ventricular tachycardia (VT), were eligible for inclusion. Exclusion criteria were cardiac arrest with age <18 years, presenting non-shockable rhythm, traumatic, presumably non-cardiac cause, presumable irreversible death or known terminal illness, pregnancy, defibrillation delivered by an automated external defibrillator prior to the advanced life support (ALS), enrollment in another clinical or device trial within the previous 30 days, and refused informed consent to the use of data.
Randomization
Upon arrival to the OHCA scene, the physician of the ALS team verified inclusion and exclusion criteria and eligibility for the study and was responsible for enrolment.
The interventions, i.e. AMSA-CPR or standard-CPR were randomly assigned on a 3-month interval to the ALS services using a centrally generated randomization plan, balanced in every center by a stratified randomization scheme. The randomization allocation schedule was computer generated before the start of the trial, by the trial data manger using a proprietary system at the Coordinating Center, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milan, Italy. ALS services were randomized to the intervention with AMSA- or standard-CPR in a 1:1 ratio. The assignment of intervention followed a complete crossover design, so that each cluster (ALS service) was switched from one intervention to the other according to a predefined 3-month period. During the trial, each service had to cross over to each of the treatment groups at least once. The sequence was concealed until the end of the 3-month period, when the new intervention period was assigned.
Real-time AMSA analysis
An X-Series defibrillator with a new software capable to measure and display AMSA values in real-time, was used for the trial (Supplementary Figure S1). Beside AMSA, these X-Series units monitored all other patient vital signs, as regular X-Series devices. The “OneStep CPR electrodes” (ZOLL Med. Corp., MA, USA) were employed for the study. These defibrillator pads presented an acceleration sensor to monitor rate, depth, and pauses of CC.
AMSA calculation was performed in real-time by a built-in software, which acquired the ECG signal from the defibrillator pads and displayed the value continuously, as shown in Supplementary Figure S1. The VF waveform analysis required an artifact-free signal and thus it was performed during the hands-off interval for delivering of two ventilations. For this reason, CPR was performed with a CC:ventilation ratio of 30:2, even in the presence of an established advanced airway. Thus, through the accelerometer sensor located in the defibrillator pads, the software recognized pauses in CC and started AMSA analysis on a 512-point window (2.05 s), such that AMSA value was displayed on the defibrillator screen in approximately 3 s. ECG signals were processed in real-time using the algorithm as previously published.13 Briefly, a 2-Hz high-pass filter was employed to minimize low-frequency artifacts and a 48-Hz low-pass filter to remove interference of ambient noise at higher frequencies. ECG signals were then converted from the time domain to a frequency domain via a fast Fourier transformation (FFT). A Tukey FFT window was used to reduce edge effects. AMSA was calculated as the sum of the products of individual frequencies and their amplitudes: AMSA = ΣAi∙Fi, where Ai represented the amplitude at ith frequency Fi.
The AMSA X-Series defibrillator was used in both study groups, but the AMSA analysis algorithm was turned on only in the AMSA group, while the standard-CPR arm used the defibrillator as a regular X-Series. After each case, the defibrillator data were downloaded and stored in a centralized signal biobank to be reviewed and analyzed.
Trial interventions
The interventional protocol applied in the AMSA-CPR group is shown in Fig. 1. Upon arrival to the cardiac arrest victim, the ALS rescuers started CC while the defibrillator pads were applied and the AMSA defibrillator was switched on. The first AMSA value was then read on the defibrillator screen (Supplementary Figure S1) and: if AMSA was ≥15.5 mV-Hz, an immediate defibrillation was attempted, followed by 2-min of CPR; if AMSA was <15.5 mV-Hz, defibrillation was not attempted while CPR was performed for 2 min. After completion of the first 2-min CPR cycle, AMSA was read again and: if it was ≤6.5 mV-Hz, the defibrillation was not attempted, and CPR continued for an additional 2-min cycle. Subsequently, if AMSA was >6.5 mV-Hz, an immediate defibrillation shock was delivered, followed by a 2-min CPR cycle. After completion of the second 2-min CPR cycle and till the end of the resuscitative intervention, CPR was then continued based on the standard 2015 ERC guidelines (a defibrillation attempt delivered every 2-min CPR cycle).9 During the whole resuscitation period, AMSA was measured during pauses for ventilation (every 30 CC, approximately every 20–25 s) and in case it was ≥15.5 mV-Hz an immediate defibrillation attempt was anticipated, prior to completing the 2-min cycle (Fig. 1). In the standard-CPR arm, the defibrillation was delivered based on the 2015 European Resuscitation Council (ERC) CPR guidelines9: an immediate defibrillation when the defibrillator was ready, and then a subsequent attempt at the end of each 2-min CPR cycle.
Fig. 1.
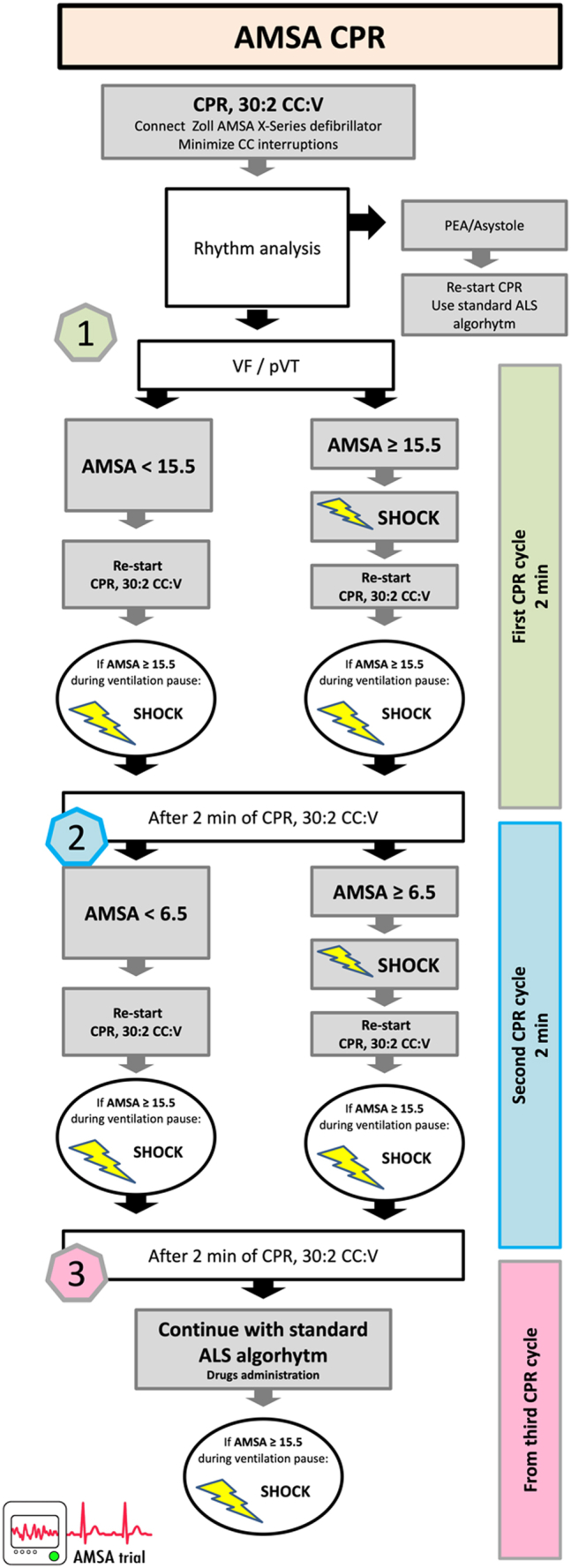
Protocol algorithm in the AMSA arm. CPR, cardiopulmonary resuscitation, CC, chest compression, V, ventilation, VF, ventricular fibrillation, pVT, pulseless ventricular tachycardia, PEA, pulseless electrical activity.
In both groups, rescuers received real-time feedback on the quality of CC and ventilations. Recommended drugs during CPR for shockable rhythms were administered based on 2015 ERC guidelines. Adrenaline was given at the beginning of the 3rd CPR cycle and repeated according to recommendations.9 First dose of amiodarone was given after the 3rd defibrillation attempt, whenever it occurred.
All patients who survived to hospital admission were admitted to the intensive care unit (ICU) and received post-resuscitation care according to local standards of care, based on ERC guidelines.23 Long-term survival was evaluated through a telephone interview by a qualified evaluator, together with neurological recovery by cerebral performance category (CPC 1: a return to normal cerebral function and normal living; CPC 2: cerebral disability but sufficient function for independent activities of daily living; CPC 3: severe disability, limited cognition, inability to carry out independent existence; CPC 4: coma; CPC 5: death or brain death).
Selected AMSA threshold for defibrillation delivery decision
AMSA decisional thresholds, i.e. ≥15.5 mV-Hz to prompt for early defibrillation and <6.5 mV-Hz to defer defibrillation in favor of CPR, were chosen based on an earlier retrospective study conducted by our group in a large clinical database of OHCAs.13 More specifically, an ECG database including 2447 defibrillation attempts from 1050 patients, was used as derivation group, while an additional database, including 1386 defibrillation attempts from 567 patients, served as validation group. The following AMSA thresholds were identified in the validation group: ≥15.5 mV-Hz predicted defibrillation success with a positive predictive value (PPV) of 0.84, while AMSA < 6.5 mV-Hz predicted defibrillation failure with a negative predictive value (NPV) of 0.98.
EMS training
Efforts were made to standardize the interventions among practitioners and study sites. Thus, from November 7th, 2018, to February 28th, 2019, physicians and nurses of the ALS teams in Milan, Monza, and Bologna EMS underwent a training program to familiarize them with the AMSA-defibrillator and to gain confidence with the AMSA interventional protocol (Fig. 1). The training included frontal lessons and hands-on workshops with simulated scenarios on the mannikin. More specifically, training focused on: correct antero-lateral defibrillation pads position; correct hands placement in order to avoid ECG artefacts during AMSA analysis; capability to read AMSA values during pauses for ventilation; recognizing AMSA thresholds and corresponding CPR interventions; protocol algorithm. A training booklet, together with flowcharts and tutorial videos, was also created and circulated among the EMS personnel. In Milan and Monza EMS, 50 training courses were organized for a total of 278 rescuers rotating shifts over 11 ALS services; in Bologna 20 courses were organized for a total of 123 rescuers for 5 ALS services.
After the end of the training period and prior to start patients’ enrollment, EMS teams employed the AMSA-defibrillators (with the AMSA algorithm disabled) for a month (from March 1st to March 31st, 2019) as a device for all the rescue missions, to familiarize users with its use. After the start of enrollment, retraining sessions were planned at 3-month intervals, i.e. prior to the next randomization block.
Länsi-Uusimaa EMS teams underwent the same training program starting from January 2020, then stopped due to trial discontinuation. Two training courses were organized and 20 rescuers, including physicians and paramedics for 2 ALS services, were trained.
Outcomes and safety
The aim of the study was to test the hypothesis that a real-time AMSA analysis during CPR might predict the success of defibrillation and optimize the timing of defibrillation delivery. The primary efficacy outcome of the trial was termination of VF/pulseless VT with achievement of restoration of rhythm associated with spontaneous circulation (ROSC) for an AMSA ≥ 15 mV-Hz. Secondary outcomes included: number of defibrillation attempts and duration of CPR prior to ROSC; high-sensitive cardiac troponin T; short-term and long-term survival with good neurological function at 1 and 6 months; effects of CPR quality on AMSA.
Defibrillation outcome was defined according to established criteria as “termination of VF or defibrillation success” if defibrillation restored an organized rhythm with heart rate ≥40 bpm, potentially associated with ROSC, commencing within 60 s after defibrillation and as “defibrillation failure” if any other rhythm, including VF/VT and asystole or low heart rate <40 bpm, occurred.12,13,24 “Any ROSC” was defined as any documented ROSC in the absence of ongoing CC.25 “Sustained ROSC” was achieved when the spontaneous circulation was maintained up to 20 min and/or till hospital admission. Long-term outcomes included survival to hospital discharge and at 1 and 6 months. Favourable neurological outcome was defined as a CPC score of 1–2.
Information to assess the outcomes were entered by local site principal investigators into a secure web-based CRF Research Electronic Data Capture—REDCap software, hosted at the Mario Negri Institute: https://redcap.marionegri.it. REDCap software platform is designed to support data capture for research studies, providing the data manager 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
In addition, report of any adverse event potentially attributable to the study intervention (i.e. malfunctioning of the AMSA device) had to be sent to the Coordinating Center within 24 h, through the specific form present in the e-CRF. Independent oversight of the safety was provided by a DSMB consisting of three clinical experts, unrelated to the trial. The committee's role was to conduct blinded interim data safety analyses, providing recommendations to the Steering Committee. The scheduled meetings were every 50 patients enrolled in the trial, i.e. approximately every 6 months.
Data were validated by the clinical monitors of the study. When the database was locked, the data manager extracted all the anonymized data and provided them to the statisticians for the analyses.
Sample size
Assuming an incidence of the primary endpoint of 35% in the (historical) control group,13 we calculated that 194 patients per group would be required for the study to have 80% power to detect a minimum clinically meaningful 14% absolute improvement (resulting in an endpoint frequency of 49% in the AMSA group) with a two-sided alpha level of 5%.
Due to the early discontinuation of the trial, the study enrolled only 31 patients (8% of the calculated sample size) and thus was underpowered to show any difference between the treatment groups.
Statistical analyses
The main analyses were performed according to an intention-to-treat approach. Descriptive statistics were reported as counts and percentages for categorical variables and as mean with SD for continuous variables. For comparison between groups, AMSA-CPR vs. Standard-CPR, no p values and testes are provided, and effects are summarized by confidential intervals (CI). Logistic regression analysis was used to compare primary and secondary outcomes. The widths of confidence intervals have not been adjusted for multiplicity, and therefore the intervals should not be used to infer definitive treatment effects for secondary outcomes. No imputation was performed for missing data. All statistical analyses were performed using SPSS version 27 and SAS version 9.4.
Secondary analyses are reported in the Supplementary Statistical Analysis.
Role of the funding source
The study funders (refer to the acknowledgements) had no role in the trial design, in the collection or analysis of the data, or in the writing of the manuscript.
Results
Study population and CPR characteristics
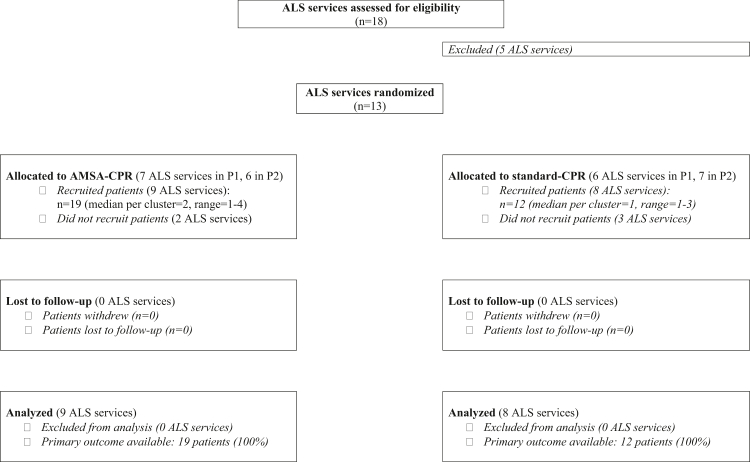
From April 2019 to July 2021, a total of 31 patients were included in the trial and analyzed (Fig. 2), 19 randomized to AMSA-CPR and 12 to standard-CPR. The last patient was enrolled in March 2020, while the last 6-month follow-up ended in May 2020. Baseline characteristics and CPR interventions were similar between the two groups and are summarized in Table 1.
Fig. 2.
Study flowchart. P1, first 3-month period; P2, subsequent 3-month cross-over period.
Table 1.
Population characteristics by treatment (intention-to-treat).
| Total n = 31 |
AMSA-CPR n = 19 |
Standard-CPR n = 12 |
|
|---|---|---|---|
| Cluster, SOREU, Milan n (%) | 19 (51) | 12 (63) | 7 (58) |
| Gender, male n (%) | 24 (77) | 15 (79) | 9 (75) |
| Age, year | 68 [58–73] | 65 [55–75] | 70 [60–73] |
| Pre-arrest CPC 1, n (%) | 30 (100) | 18 (100) | 12 (100) |
| Charlson index | 3.0 [2.0–4.0] | 3.5 [2.3–5.0] | 3.0 [1.0–4.0] |
| STEMI, n (%) | 12 (39) | 7 (37) | 5 (42) |
| NSTEMI, n (%) | 4 (13) | 1 (5) | 3 (25) |
| No MI, n (%) | 3 (10) | 1 (5) | 2 (17) |
| Unknown,a n (%) | 12 (39) | 10 (53) | 2 (17) |
| Successful PCI, n (%) | 13 (42) | 7 (37) | 6 (50) |
| No PCI, n (%) | 6 (19) | 2 (11) | 4 (33) |
| Unknown°, n (%) | 12 (39) | 10 (53) | 2 (17) |
| Witnessed cardiac arrest, n (%) | |||
| Yes, lay bystander | 22 (71) | 13 (68) | 9 (75) |
| Yes, EMS | 7 (23) | 4 (21) | 3 (25) |
| No | 2 (7) | 2 (11) | 0 |
| Bystander CPR before EMS arrival, n (%) | |||
| Yes, complete | 4 (13) | 3 (16) | 1 (8) |
| Yes, compressions only | 15 (48) | 8 (42) | 7 (58) |
| No | 10 (32) | 6 (32) | 4 (33) |
| Unknown | 2 (7) | 2 (11) | 0 |
| EMS arrival time, min | 10 [7–12] | 10 [9–12] | 10 [6–12] |
| Patients receiving adrenaline, n (%) | 22 (71) | 15 (79) | 7 (58) |
| Patients receiving amiodarone, n (%) | 17 (55) | 14 (74) | 3 (25) |
| Chest compression depth, cm | 4.55 [4.27–5.12] | 4.73 [4.16–5.34] | 4.38 [4.27–4.68] |
| Chest compression rate, CC/min | 123 [112–139] | 129 [112–141] | 117 [113–131] |
| Chest compression fraction, (%) | 73.7 [66.3–78.5] | 71.9 [66.0–81.1] | 74.6 [43.3–78.4] |
Median [IQR] or n (%).
Unknown because patients were not resuscitated.
Seventy-seven percent of patients were male with a median age of 68 years, and in 63% of those admitted alive to the hospital a STEMI was present. Ninety-four percent of cardiac arrests were witnessed, 71% by lay bystanders, while 23% directly by the EMS. Bystander CPR was performed in 61% of patients before EMS arrival, which occurred with a median of 10 min from the emergency call. No differences between AMSA- and standard-CPR groups were observed (Table 1).
CPR quality, i.e. CC depth, rate and CCF, were also similar in the two groups (Table 1). No difference was observed in administration of adrenaline, while 49% more patients in the AMSA group received amiodarone during CPR, compared to those in the standard-CPR arm (OR [95% CI] 8.40 [1.60–44.10], Table 1).
Primary and secondary outcomes
Defibrillation success, i.e. termination of VF with a rhythm associated with ROSC was observed in a similar percentage of patients in both groups, 14/19 (74%) in AMSA-CPR and 9/12 (75%) in standard-CPR, odds ratio 0.93 [95% CI 0.18–4.90], as shown in Table 2.
Table 2.
Outcomes by treatment (intention-to-treat).
| Total n = 31 |
AMSA n = 19 |
Control n = 12 |
OR [95% CI]a | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Defibrillation success, n (%) | 23 (74) | 14 (74) | 9 (75) | 0.93 [0.18–4.90] |
| Secondary endpoints | ||||
| Number of defibrillation attempts | 2 [1–6] | 3 [2–6] | 2 [1–4] | 1.21 [0.90–1.63] |
| CPR duration to sustained ROSC, min | 19 [6–29] | 21 [8–37] | 11 [4–29] | 1.02 [0.96–1.08] |
| Cardiac troponinb | ||||
| 6 h after ROSC | 85 [26–217] | 61 [19–203] | 98 [34–469] | 0.90 [0.25–3.19]a |
| 24 h after ROSC | 167 [64–454] | 159 [42–2268] | 167 [80–1436] | 0.78 [0.18–3.28]a |
| Sustained ROSC, n (%) | 18 (58) | 9 (47) | 9 (75) | 0.30 [0.06–1.47] |
| 24 h survival, n (%) | 18 (58) | 9 (47) | 9 (75) | 0.30 [0.06–1.47] |
| Survival to hospital discharge, n (%) | 14 (45) | 8 (42) | 6 (50) | 0.73 [0.17–3.11] |
| 1-month survival, n (%) | 14 (45) | 8 (42) | 6 (50) | 0.73 [0.17–3.11] |
| 1-month survival with CPC 1–2, n (%) | 12 (39) | 6 (32) | 6 (50) | 0.46 [0.10–2.5] |
| 6-month survival, n (%) | 13 (42) | 7 (37) | 6 (50) | 0.58 [0.14–2.53] |
| 6-month survival with CPC 1–2, n (%) | 12 (39) | 6 (32) | 6 (50) | 0.46 [0.10–2.05] |
Median [IQR] or n (%).
Regression analysis for relation of variable with treatment (univariate for treatment).
Troponin levels were normalized by dividing the obtained value by the ULN (Under Limit of Normality, which varied between 13 and 15 for Troponin T and was 19.8 for Troponin I, values were log-transformed before inclusion in regression analysis).
A total of 121 defibrillations were attempted in the 31 patients enrolled. A median of 3 defibrillation attempts were delivered for each patient over the resuscitation intervention, with no difference between groups (3 in AMSA-CPR vs. 2 in standard-CPR, Table 2).
CPR prior to sustained ROSC lasted in median 19 min and was longer in the AMSA-CPR compared to the standard-CPR (21 min vs. 11 min, OR [95% CI] 1.02 [0.96–1.08], Table 2).
A similar post-resuscitation cardiac troponin release was observed in both groups (Table 2).
No differences in short and long-term secondary outcomes were observed between the AMSA-CPR and standard-CPR groups (Table 2). Sustained ROSC was achieved in 58% of patients and 1-month survival was 45%. Thirty-nine percent of patients were alive at 6 months with a good neurological recovery.
Adverse events
No adverse events potentially related to the intervention were reported (Supplementary Table S1).
Secondary exploratory analyses
In the 19 patients enrolled in the AMSA-CPR group, AMSA was measured in real-time during resuscitation. Secondary analyses specifically performed in this group are reported in the Supplementary Results.
Briefly, mean AMSA was 9.5 ± 1.2 mV-Hz during resuscitation (Supplementary Figure S2). A statistically significantly higher AMSA was observed prior to successful defibrillation attempts compared to failing ones (Supplementary Figure S3).
Defibrillating for an AMSA ≥ 15.5 mV-Hz led to a successful defibrillation in 77% of instances, while defibrillating for an AMSA < 6.5 mV-Hz led to a defibrillation failure in 86% of instances (Supplementary Table S3).
The interval between EMS call and arrival on the scene affected AMSA, with values statistically significantly decreasing during the minutes of untreated cardiac arrest (Supplementary Figures S6A and S7). Similarly, CPR performed prior to EMS arrival affected AMSA (Supplementary Figure S6B).
In the seven patients with a STEMI, AMSA decreased overtime during CPR (Supplementary Figure S6C).
Additional results are in the Supplementary Material.
Discussion
AMSA trial was early discontinued for low recruitment due to the COVID-19 pandemics. In this small multicenter randomized controlled trial, an AMSA-guided defibrillation provided no evidence of an improvement in termination of VF and achievement of ROSC compared to a standard CPR. No benefits were also observed in the secondary long-term outcomes, i.e. survival with neurological recovery, when CPR was guided by AMSA. Nevertheless, this study reported for the first time the real-time analysis of AMSA during CPR and the prospective clinical application, with no adverse events, of an AMSA-guided CPR in the out-of-hospital setting.
In the present trial, an AMSA value ≥15.5 mV-Hz prompted for early defibrillation, while a value <6.5 mV-Hz paused defibrillation in favor of CPR. These AMSA decisional thresholds were derived from an earlier large retrospective study.13 Although the AMSA trial enrolled only 19 patients in the AMSA arm, it almost confirmed the predicting capability of the above thresholds. Indeed, AMSA ≥ 15.5 mV-Hz showed a PPV of 0.77, while AMSA < 6.5 a NPV of 0.86. Thus, this trial reinforced the need for 2 decisional thresholds to achieve high accuracy in discerning for each patient when an interval of CC would be more beneficial than an immediate defibrillation and vice versa.
The AMSA trial failed to demonstrate differences between AMSA-guided vs. standard-CPR. Even though we were not able to complete the planned AMSA study, this small trial remains of high importance. It is the first time real-time AMSA analysis was used in the clinical scenario, testing prospectively AMSA capability to predict defibrillation outcome and guide CPR. The study is a response to a specific knowledge gap outlined initially in the 2015 CPR guidelines and reiterated in 2021.9,26 AMSA values, acquired during ongoing resuscitation in human patients, were confirmed in the secondary analyses to be significantly higher when the defibrillation attempts led to VF termination and ROSC, compared to unsuccessful attempts.
A not statistically significant trend towards shorter CPR duration and greater survival was observed in standard-CPR compared to AMSA-CPR. This unexpected trend might be a consequence of the small sample size together with the lack of experience with the AMSA-CPR algorithm gained by the ALS services. Indeed, prior to trial discontinuation, patient enrollment had a slowdown to allow for the device software update, such that only 1–2 patients were recruited by each cluster (i.e. ALS services with numerous ALS teams rotating shifts). Thus, future larger studies, powered to demonstrate the impact of AMSA on survival and long-term outcome in comparison to standard-CPR, are now needed. Indeed, the results from this study provide a solid basis to better design the next AMSA trial. In addition, redefined and optimized AMSA thresholds may be introduced. The decision of interrupting CC to attempt a defibrillation might be limited to instances when AMSA reaches the threshold of 16 mV-Hz, which in the present study accounted for a PPV >0.8. A threshold <6.5 mV-Hz, as selected in our study, led to 14% of successful defibrillations, although no patients ultimately achieved sustained ROSC. However, an AMSA ≤ 4.5 mV-Hz showed a NPV of 1 and thus it could be safely employed to deny a defibrillation shock throughout the resuscitation intervention.
Of interest is also the relationship observed between the EMS arrival time from the emergency call and the first AMSA measured. AMSA decreased significantly during the interval of untreated cardiac arrest, with a steep decrease from 16.4 to 10.1 mV-Hz after the initial 4 min, as electrophysiological evidence of the progressive increase of myocardial ischemia, moving from the “electrical” phase to the “circulatory” phase of VF.27,28 This is further supported by the higher first AMSA values measured in patients who received bystander CPR prior to EMS arrival compared to those without, as result of a CC-generated coronary blood flow that likely maintained the myocardial energy state.29 These associations may be of additional importance in the view of using real-time AMSA analysis also as a surrogate of the duration of untreated CA and indicator of the vital status of the myocardium.
Interestingly, the secondary analyses showed that in patients with STEMI, AMSA decreased over time during ongoing CPR, despite an initial high value, while it increased in patients without STEMI. The initial high AMSA in STEMI patients can be explained because more than half of these patients were already connected to the defibrillator and monitored by EMS for a suspected STEMI when VF occurred. The initial AMSA measured was therefore high, because coincident with onset of cardiac arrest. However, later on even if CPR was performed, the myocardium was probably not well perfused due to the coronary occlusion, and this likely led to decreases of AMSA.30, 31, 32, 33 Despite the low number of STEMI cases enrolled in this study, data support the hypothesis of a recent retrospective clinical studies on 750 OHCA showing significantly lower AMSA in case of underlying STEMI. Confirmative studies are needed to support another potential application of real-time AMSA analysis for early identification of the underlying arrest cause and for decision of the most appropriate rescue strategy.31,32
The major strength of the study is represented by the novelty of the data. Indeed, for the first time AMSA was used prospectively in human patients during ongoing resuscitation maneuvers. The study showed that a real-time AMSA analysis can be performed during CPR and confirmed the ability of AMSA to predict the success of defibrillation in the real clinical scenario. Further, considering all the information derived from this small trial, a larger study can be planned. Finally, these results are of strong value because they were derived from a randomized controlled trial conducted in 3 different cities.
Limitations
We recognize the important limitations of this study. First, the AMSA trial was discontinued for low/no patient enrollment due to the COVID-19 pandemics and thus only a small number of OHCAs were included, i.e. only 8% of the pre-planned sample size. This made the study not powered to demonstrate the study endpoints, i.e. the actual power of the study was 5%, with limit generalizability of the results. Nevertheless, it represents the first report of a prospective clinical application of AMSA during OHCA. Second, AMSA was calculated during pauses in CC to deliver 2 ventilations. The 4 s necessary to deliver the 2 ventilations based on current guidelines were long enough to have AMSA measured and displayed on the defibrillator screen.2 A CPR with a 30:2 CC:ventilation algorithm was therefore needed for an AMSA-guided CPR, even in the presence of an advanced airway. This approach, however, led to similar survival and favorable neurologic outcome, when compared to continuous CC with asynchronized ventilations, in a population of more than 23.000 OHCAs.34 Future technology developments might allow for accurate CC-artifacts filtering and continuous AMSA analysis without pauses in CPR.35 Third, the trial had an open-label design was dictated mainly by the need to perform an investigator-driven trial with limited funding and thus ALS team in the AMSA group could read AMSA over the whole CPR intervention. We aimed to minimize this problem by using outcomes with a low risk of bias, i.e. termination of VF and survival. In addition, a strict protocol for determination of defibrillation outcome was used. The criterion used to define successful defibrillation, therefore, reflected not only VF termination but also the quality of the resulting rhythm and thus offered a more effective identification and discrimination of clinically useful defibrillation outcome predictors. Nevertheless, to overcome the limitation of defining successful defibrillation based on the ECG tracing, in this study the relationship between the last defibrillation attempt and sustained ROSC was also evaluated. Post-defibrillation asystole or pulseless electric activity were not included in the definition of successful defibrillation because, although they may be considered a successful termination of VF, they are not associated with ROSC.13
Conclusions
This trial reported the prospective clinical application of an AMSA-guided CPR. It demonstrated the possibility of measuring and reading AMSA during CPR and provided a clinical validation of the capability of AMSA to predict defibrillation success in the real clinical scenario. However, an AMSA-guided defibrillation provided no evidence of an improvement in termination of VF and ROSC nor in long-term survival.
Contributors
L.R.: Data validation, writing – original draft; F.F.: Data validation, writing– review & editing; F.B.: Investigation, supervision, review & editing; F.S.: Investigation, supervision, review & editing; J.M.T.A.M.: Formal analysis, review & editing; A.B.: Formal analysis, review & editing; M.M.: Investigation, supervision, review & editing; A.M.: Data validation, review & editing; G.G.: Supervision, review & editing; R.F.: Conceptualisation, supervision, review & editing; G.S.: Supervision, review & editing; A.P.: Supervision, review & editing; M.B.S.: Investigation, review & editing; Y.L.: Methodology, review & editing; R.L.: Conceptualisation, funding acquisition, methodology, review & editing; L.W.: Conceptualisation, methodology, review & editing; G.R.: Conceptualisation, funding acquisition, project administration, methodology, writing– review & editing.
All authors read and approved the final version of the manuscript.
Data sharing statement
The data are stored at the Department of Cardiovascular Medicine, Mario Negri Institute for Pharmacological Research in Milan, Italy. Deidentified individual participant data and the data dictionary, study protocol, and informed consent form will be made available for scientific purposes upon formal request and consequent approval of the proposal by the Steering Committee after publication. Requests should be sent to the corresponding author (gristag@gmail.com).
Declaration of interests
MBS received lecture honoraria from BARD Medical (Ireland). All other authors declared no conflicts.
Acknowledgements
The AMSA trial was funded by the Horizon 2020 Program under grant agreement no. 733381 (EU Framework “European Sudden Cardiac Arrest network: towards Prevention, Education and New Treatment—ESCAPE-NET”) plus an additional unrestricted grant from ZOLL Medical Corp., Chelmsford, USA. The study was also partially funded by Italian Ministry of Health - Current research IRCCS.
The authors thank all the physicians and nurses of SOREU Metropolitana, AREU, and AUSL Bologna, who actively participated in the trial.
Finally, author G.R. is extremely grateful to Drs. David Appleby, Annamarie Silver, and Sandeep Pandit (ZOLL Med. Corp., USA) who believed in the AMSA project, and to the tireless Dr. Cristian Costa (ZOLL Med. Corp., Italy), who provided continuous local technical support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104544.
Appendix A. Supplementary data
References
- 1.Panchal A.R., Bartos J.A., Cabañas J.G., et al. Part 3: adult basic and advanced life support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S366–S368. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 2.Olasveengen T.M., Semeraro F., Ristagno G., et al. European Resuscitation Council guidelines 2021: basic life support. Resuscitation. 2021;161:98–114. doi: 10.1016/j.resuscitation.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Cheskes S., Schmicker R.H., Christenson J., et al. Perishock pause: an independent predictor of survival from out-of-hospital shockable cardiac arrest. Circulation. 2011;124:58–66. doi: 10.1161/CIRCULATIONAHA.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwer T.F., Walker R.G., Chapman F.W., Koster R.W. Association between chest compression interruptions and clinical outcomes of ventricular fibrillation out-of-hospital cardiac arrest. Circulation. 2015;132:1030–1037. doi: 10.1161/CIRCULATIONAHA.115.014016. [DOI] [PubMed] [Google Scholar]
- 5.Wik L., Hansen T.B., Fylling F., et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–1395. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 6.Baker P.W., Conway J., Cotton C., et al. Defibrillation or cardiopulmonary resuscitation first for patients with out-of-hospital cardiac arrests found by paramedics to be in ventricular fibrillation? A randomised control trial. Resuscitation. 2008;79:424–431. doi: 10.1016/j.resuscitation.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Stiell I.G., Nichol G., Leroux B.G., et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. N Engl J Med. 2011;365:787–797. doi: 10.1056/NEJMoa1010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olasveengen T.M., Mancini M.E., Perkins G.D., et al. Adult basic life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142:S41–S91. doi: 10.1161/CIR.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 9.Soar J., Böttiger B.W., Carli P., et al. European Resuscitation Council guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Eftestol T., Wik L., Sunde K., Steen P.A. Effects of cardiopulmonary resuscitation on predictors of ventricular fibrillation defibrillation success during out-of-hospital cardiac arrest. Circulation. 2004;110:10–15. doi: 10.1161/01.CIR.0000133323.15565.75. [DOI] [PubMed] [Google Scholar]
- 11.Callaway C.W., Sherman L.D., Mosesso V.N., Jr., Dietrich T.J., Holt E., Clarkson M.C. Scaling exponent predicts defibrillation success for out-of-hospital ventricular fibrillation cardiac arrest. Circulation. 2001;103:1656–1661. doi: 10.1161/01.cir.103.12.1656. [DOI] [PubMed] [Google Scholar]
- 12.Ristagno G., Li Y., Fumagalli F., Finzi A., Quan W. Amplitude spectrum area to guide resuscitation-a retrospective analysis during out-of-hospital cardiopulmonary resuscitation in 609 patients with ventricular fibrillation cardiac arrest. Resuscitation. 2013;84:1697–1703. doi: 10.1016/j.resuscitation.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Ristagno G., Mauri T., Cesana G., et al. Amplitude spectrum area to guide defibrillation: a validation on 1617 patients with ventricular fibrillation. Circulation. 2015;131:478–487. doi: 10.1161/CIRCULATIONAHA.114.010989. [DOI] [PubMed] [Google Scholar]
- 14.Freese J.P., Jorgenson D.B., Liu P.Y., et al. Waveform analysis-guided treatment versus a standard shock-first protocol for the treatment of out-of-hospital cardiac arrest presenting in ventricular fibrillation: results of an international randomized, controlled trial. Circulation. 2013;128:995–1002. doi: 10.1161/CIRCULATIONAHA.113.003273. [DOI] [PubMed] [Google Scholar]
- 15.Berg K.M., Soar J., Andersen L.W., et al. Adult advanced life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142:S92–S139. doi: 10.1161/CIR.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 16.Shanmugasundaram M., Valles A., Kellum M.J., Ewy G.A., Indik J.H. Analysis of amplitude spectral area and slope to predict defibrillation in out of hospital cardiac arrest due to ventricular fibrillation (VF) according to VF type: recurrent versus shock-resistant. Resuscitation. 2012;83:1242–1247. doi: 10.1016/j.resuscitation.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Ristagno G., Bisera J., Tang W., Deng Q., Weil M.H. Electrocardiogram waveforms for monitoring effectiveness of chest compression during cardiopulmonary resuscitation. Crit Care Med. 2008;36:211–215. doi: 10.1097/01.CCM.0000295594.93345.A2. [DOI] [PubMed] [Google Scholar]
- 18.Aiello S., Perez M., Cogan C., et al. Real-time ventricular fibrillation amplitude-spectral area analysis to guide timing of shock delivery improves defibrillation efficacy during cardiopulmonary resuscitation in swine. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orkin A.M., Gill P.J., Ghersi D., et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement. JAMA. 2021;326:257–265. doi: 10.1001/jama.2021.9941. [DOI] [PubMed] [Google Scholar]
- 20.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trentini F., Marziano V., Guzzetta G., et al. The pressure on healthcare system and intensive care utilization during the COVID-19 outbreak in the Lombardy region: a retrospective observational study on 43,538 hospitalized patients. Am J Epidemiol. 2022;191:137–146. doi: 10.1093/aje/kwab252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scquizzato T., Landoni G., Paoli A., et al. Effects of COVID-19 pandemic on out-of-hospital cardiac arrests: a systematic review. Resuscitation. 2020;157:241–247. doi: 10.1016/j.resuscitation.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolan J.P., Soar J., Cariou A., et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines for post-resuscitation care 2015: Section 5 of the European Resuscitation Council guidelines for resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Wu X., Bisera J., Tang W. Signal integral for optimizing the timing of defibrillation. Resuscitation. 2013;84:1704–1707. doi: 10.1016/j.resuscitation.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Gräsner J.T., Wnent J., Herlitz J., et al. Survival after out-of-hospital cardiac arrest in Europe - results of the EuReCa TWO study. Resuscitation. 2020;148:218–226. doi: 10.1016/j.resuscitation.2019.12.042. [DOI] [PubMed] [Google Scholar]
- 26.Soar J., Nolan J.P., Böttiger B.W., et al. European Resuscitation Council guidelines for resuscitation 2015: Section 3. Adult advanced life support. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Weisfeldt M.L., Becker L.B. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288:3035–3038. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 28.Salcido D.D., Menegazzi J.J., Suffoletto B.P., Logue E.S., Sherman L.D. Association of intramyocardial high energy phosphate concentrations with quantitative measures of the ventricular fibrillation electrocardiogram waveform. Resuscitation. 2009;80:946–950. doi: 10.1016/j.resuscitation.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Aiello S.R., Mendelson J.B., Baetiong A., Radhakrishnan J., Gazmuri R.J. Targeted delivery of electrical shocks and epinephrine, guided by ventricular fibrillation amplitude spectral area, reduces electrical and adrenergic myocardial burden, improving survival in swine. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.023956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ristagno G., Tang W., Xu T.Y., Sun S., Weil M.H. Outcomes of CPR in the presence of partial occlusion of left anterior descending coronary artery. Resuscitation. 2007;75:357–365. doi: 10.1016/j.resuscitation.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Hulleman M., Salcido D.D., Menegazzi J.J., et al. Ventricular fibrillation waveform characteristics in out-of-hospital cardiac arrest and cardiovascular medication use. Resuscitation. 2020;151:173–180. doi: 10.1016/j.resuscitation.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 32.Nas J., van Dongen L.H., Thannhauser J., et al. The effect of the localisation of an underlying ST-elevation myocardial infarction on the VF-waveform: a multi-centre cardiac arrest study. Resuscitation. 2021;168:11–18. doi: 10.1016/j.resuscitation.2021.08.049. [DOI] [PubMed] [Google Scholar]
- 33.Indik J.H., Allen D., Gura M., Dameff C., Hilwig R.W., Kern K.B. Utility of the ventricular fibrillation waveform to predict a return of spontaneous circulation and distinguish acute from post myocardial infarction or normal Swine in ventricular fibrillation cardiac arrest. Circ Arrhythm Electrophysiol. 2011;4:337–343. doi: 10.1161/CIRCEP.110.960419. [DOI] [PubMed] [Google Scholar]
- 34.Nichol G., Leroux B., Wang H., et al. Trial of continuous or interrupted chest compressions during CPR. N Engl J Med. 2015;373:2203–2214. doi: 10.1056/NEJMoa1509139. [DOI] [PubMed] [Google Scholar]
- 35.Fumagalli F., Silver A.E., Tan Q., Zaidi N., Ristagno G. Cardiac rhythm analysis during ongoing cardiopulmonary resuscitation using the analysis during compressions with fast reconfirmation technology. Heart Rhythm. 2018;15:248–255. doi: 10.1016/j.hrthm.2017.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.