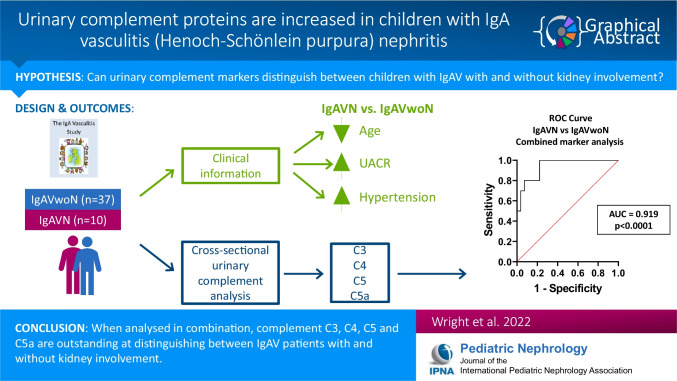
Abstract
Background
Children with immunoglobulin A vasculitis (IgAV Henoch-Schönlein purpura) frequently encounter nephritis (IgAV-N) with 1–2% risk of kidney failure. The pathophysiology of IgAV-N is not fully understood with speculation that complement may contribute. The aim of this study was to identify whether urinary complement proteins are increased in children with IgAV-N.
Methods
A cross-sectional prospective cohort of children with IgAV were recruited together with controls including healthy children and children with systemic lupus erythematosus (SLE). Patients were subdivided according to the presence of nephritis. Urinary C3, C4, C5, and C5a were measured by enzyme-linked immunosorbent assay (ELISA) and corrected for urinary creatinine.
Results
The study included 103 children; 47 with IgAV (37 IgAV without nephritis, IgAVwoN; 10 IgAV-N), 30 SLE and 26 healthy children. Urinary complement C3, C4, and C5 were all statistically significantly increased in all children with IgAV compared to SLE patients (all p < 0.05). In patients with IgAV-N, urinary complement C3, C4, C5, C5a were all statistically significantly increased compared to IgAVwoN (C3 14.65 μg/mmol [2.26–20.21] vs. 2.26 μg/mmol [0.15–3.14], p = 0.007; C4 6.52 μg/mmol [1.30–9.72] vs. 1.37 μg/mmol [0.38–2.43], p = 0.04; C5 1.36 μg/mmol [0.65–2.85] vs. 0.38 μg/mmol [0.03–0.72], p = 0.005; C5a 101.9 ng/mmol [15.36–230.0] vs. 18.33 ng/mmol [4.27–33.30], p = 0.01). Using logistic regression, the urinary complement components produced an outstanding ability to discriminate between patients with and without nephritis in IgAV (AUC 0.92, p < 0.001).
Conclusions
Children with IgAV-N have evidence of increased complement proteins present in their urine that may indicate a pathological role and may allow treatment stratification.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information
Supplementary Information
The online version contains supplementary material available at 10.1007/s00467-022-05747-3.
Keywords: Nephritis, Vasculitis, Immunoglobulin A, Complement, Biomarker, Urine
Introduction
Immunoglobulin A vasculitis (IgAV), formerly known as Henoch-Schönlein purpura, is the most common form of vasculitis in children with an estimated incidence of around 20 cases per 100,000 children per year. It is caused by deposition of aberrantly glycosylated IgA in tissues leading to activation of an autoimmune response [1]. IgAV is self-limiting in 50–70% of cases and most patients will make a complete and full recovery with no intervention. Kidney involvement (termed IgAV nephritis – IgAV-N) is the most damaging consequence of IgAV as it is the only organ affected that is associated with long-term morbidity and carries a 1–2% risk of progression to kidney failure [2, 3]. There are unmet needs in understanding the pathophysiology of IgAV-N and kidney outcomes have not demonstrated any improvement over time [4]. Histologically, glomerular IgA deposition is universally seen and in 90% of cases it is co-located with complement C3. There is growing interest in the role of the complement pathway in IgA-related kidney diseases [1, 5] and in patients with IgA nephropathy (IgAN), reports demonstrate elevated serum concentrations of C3d, C4d, C5b-9, mannose-binding lectin, and mannose-associated serine protease-1 [6–8], such that selective therapeutic inhibition of the lectin and alternative complement pathways are under current evaluation. In other proteinuric kidney diseases, complement activation products are reported to be increased in patients with focal glomerular sclerosis and diabetic nephropathy but not in heavy proteinuria associated with minimal change disease, suggesting that the findings may have an active pathological role in certain inflammatory kidney diseases [9]. In children with IgAV-N, there are limited corresponding data available, apart from histological evidence of glomerular complement deposition (C3, C4 and C5b-9) [10, 11].
The aim of this study was to identify whether urine complement proteins are present in children with IgAV, how they compare to another form of glomerulonephritis and whether they are able to distinguish patients with IgAV-N.
Methods
Study cohort
A cross-sectional cohort of patients was recruited for this study. A one-off urine sample was collected at the time of appointment from a cohort of patients with IgA vasculitis and healthy controls obtained as part of the IgA Vasculitis Study (REC: 17/NE/0390) and a cohort of children with SLE and healthy controls from participants within the UK JSLE Cohort Study and Repository (REC: 6/Q1502/77). Informed consent was obtained from all subjects, and if subjects were under the age of 16 years, consent was obtained from a parent and/or legal guardian and assent was obtained from the subject as appropriate. A cohort of age- and sex-matched healthy control participants were used for comparison. For data analysis purposes, healthy controls recruited as part of both studies were pooled as a single control population.
Nephritis classification
Patients within the disease categories (IgAV and SLE) were subdivided according to the presence of nephritis. Nephritis in children with IgAV (termed IgAV-N group) was defined as a urinary albumin to creatinine ratio (ACR) > 30 mg/mmol Cr at the time of sampling. Patients with IgAV who had a normal urine dipstick and/or a urine ACR < 30 mg/mmol Cr at the time of sampling were considered not to have any significant nephritis (termed IgAVwoN). Nephritis in patients with SLE was defined according to the British Isles Lupus Assessment Group (BILAG) 2004 index [12, 13]. Patients with a BILAG score of A or B in their kidney domain (new or worsening hypertension, proteinuria, reduced kidney function, nephrotic syndrome, active urinary sediment and/or active histological evidence of nephritis) were considered to have had nephritis (lupus nephritis, termed LN group) while those with a BILAG score of E in their kidney domain (no previous evidence of nephritis; termed SLEwoLN) were considered not to have LN.
Laboratory methods
Enzyme-linked immunosorbent assay (ELISA) kits were purchased to detect C3, C4, C5, and C5a (Bio-Techne, Abingdon, UK) and were run per the manufacturer’s instructions. Briefly, urine samples were collected as a clean catch sample and immediately transferred to the onsite laboratory for processing. They were spun at 300 g for 5 min to remove any particulate matter and loaded neat onto plates to analyse levels of complement protein in each sample. Urine infection was excluded in all samples prior to analysis either through a urine dipstick that was negative for nitrites and leucocytes or through formal microbiological examination. All urine complement concentrations were corrected for concentration using urinary creatinine measured by the Clinical Chemistry Laboratory at Alder Hey Children's NHS Foundation Trust using the Abbott Enzymatic Creatinine assay on the Abbott Architect Ci8200 (Abbott, Illinois, USA).
Statistical analysis
Urinary complement levels were normalised to urinary creatinine in all instances. Data are expressed as median [range] unless otherwise stated. Statistical analysis was performed using GraphPad Prism 7.01 software programme to compare between the disease cohorts IgAV, SLE and healthy controls and between the subgroups with nephritis (IgAVwoN, IgAV-N, SLEwoLN, LN, healthy controls). Data normality was assessed using Shapiro–Wilk test. Statistical significance was evaluated using Kruskal–Wallis test with Dunn’s post hoc test. Univariate and logistic regression analyses were performed, and receiver operating characteristic (ROC) curves were generated to evaluate the ability of each urinary complement protein to predict the patients with nephritis. The area under the curve (AUC) value was used to assess the strength of the test in distinguishing patients with IgAV-N and defined accordingly (AUC 0.7–0.8 considered acceptable, 0.8–0.9 considered excellent, 0.9–1.0 considered outstanding). A p value of less than 0.05 was considered statistically significant.
Results
Patient demographic data and baseline characteristics
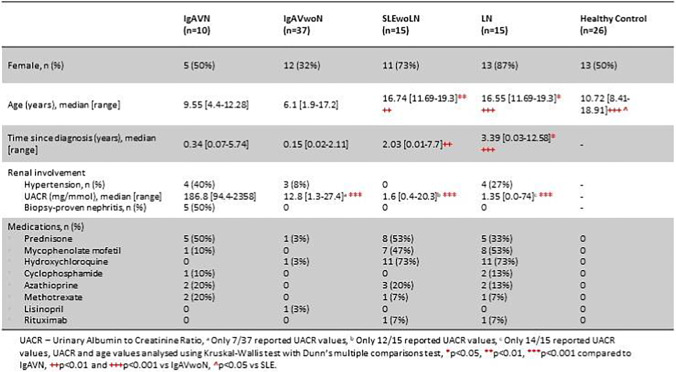
The study included 103 children, consisting of 77 patients with an inflammatory disease and 26 participants who were age- and sex-matched healthy controls. A total of 47 children with IgAV contributed and of these 37 participants were grouped as IgAVwoN and 10 participants were classed as IgAV-N. There were 30 children with SLE consisting of 15 SLE participants without any history of ever having had nephritis (SLEwoLN) and 15 with a history of LN. The demographic data and baseline characteristics are presented in Table 1. As expected, there was an increased proportion of male patients with IgAV and an increased proportion of female patients with SLE, consistent with the overall gender distribution seen for these conditions. SLE patients were significantly older than those with IgAV and healthy controls, consistent with the expected overall age distribution of these conditions. The healthy control cohort was reasonably well-matched in terms of demographics. Patients with IgAV-N had a significantly increased urinary albumin-to-creatinine ratio (UACR) compared to IgAVwoN, SLEwoLN, and LN. Similar types of immunosuppressant therapies were used within the IgAV and SLE cohorts; however, as expected, these were used more frequently in patients with SLE.
Table 1.
Demographic and baseline data for patients included in this study
Urinary complement concentration in all patients with IgAV
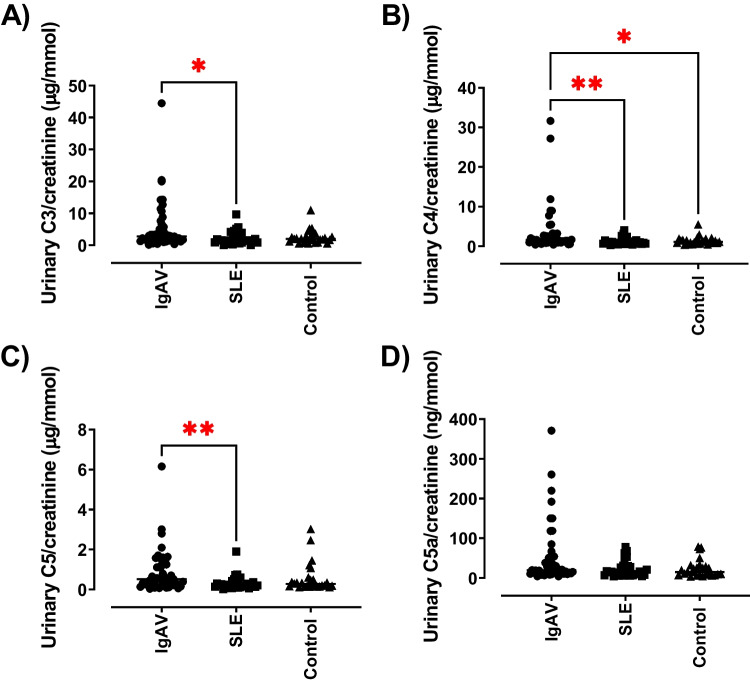
Urinary complement concentrations (C3, C4, C5 and C5a) were assessed in all patients with IgAV and compared to patients with SLE and the healthy control group. The urinary complement C3 concentration was statistically significantly increased in all patients with IgAV compared to patients with SLE (median: IgAV 2.74 μg/mmol [0.15–44.5], SLE 1.52 μg/mmol [0.09–9.66]); p = 0.021), as shown in Fig. 1A. There was no statistically significant difference in the urinary C3 concentrations between patients with IgAV and healthy controls (1.98 μg/mmol [0.49–11.0]). Urinary complement C4 concentrations were also statistically significantly increased in patients with IgAV (median: 1.56 μg/mmol [0.38–31.66]) compared to those with SLE (0.87 μg/mmol [0.25–4.01]; p = 0.001) and with the healthy control participants (1.08 μg/mmol [0.37–5.46]; p = 0.03) (Fig. 1B). There were no statistically significant differences seen between SLE patients and healthy control participants. The urinary complement C5 concentrations were statistically significantly increased in IgAV patients (median: 0.51 μg/mmol [0.03–6.16]) compared to patients with SLE (0.20 μg/mmol [0.02–1.90]; p = 0.008) (Fig. 1C). No statistically significant differences were seen between patients with IgAV and healthy control participants (0.28 μg/mmol [0.10–3.02]) or between patients with SLE and healthy controls. Urinary complement C5a was not statistically significantly different between any of the groups. However, there was a trend toward increased levels in the IgAV group (19.69 ng/mmol [4.27–370.9]) to SLE (14.32 ng/mmol [3.92–78.11]; p = 0.06) (Fig. 1D).
Fig. 1.
Urinary complement concentrations in patients with paediatric inflammatory disease – IgAV and SLE compared to age- and sex-matched controls. Complement concentrations were assessed in urine collected from patients with IgAV, SLE and controls using ELISA. Complement concentrations were normalised to urinary creatinine. (A) Urinary C3/creatinine, (B) Urinary C4/creatinine, (C) Urinary C5/creatinine, and (D) Urinary C5a/creatinine. N = 26–47/group. Data are expressed as median and analysed using Kruskal–Wallis test with Dunn’s multiple comparison test. *P < 0.05 and **P < 0.01
Urinary complement concentration in patients with IgAV nephritis
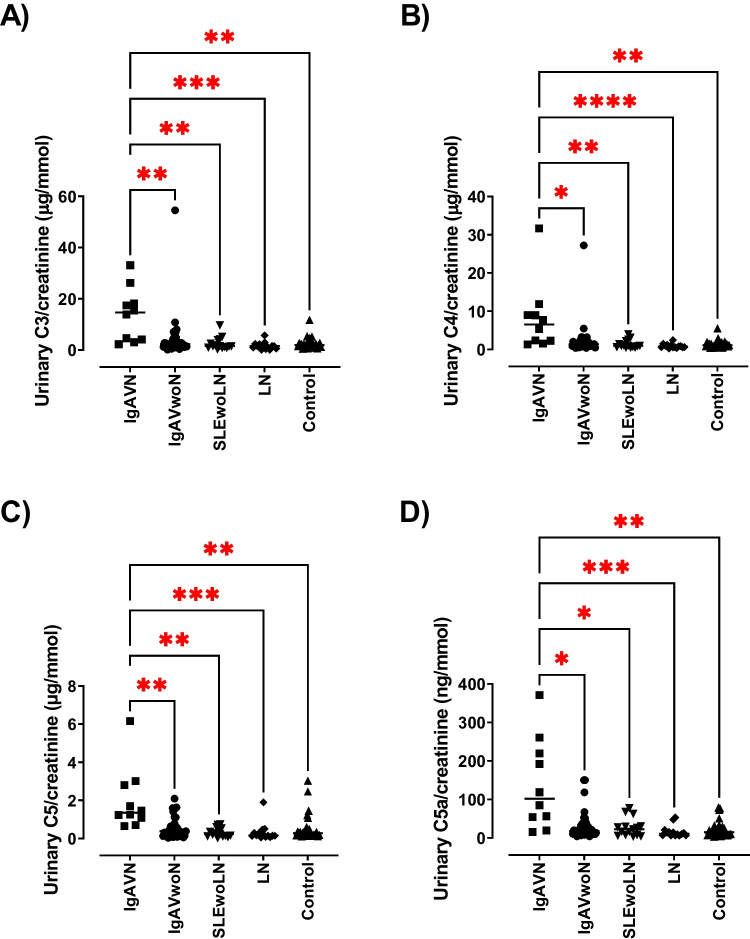
The participants were subdivided according to the presence of nephritis. The concentration of urinary complement proteins (C3, C4, C5 and C5a) was evaluated to see if these could distinguish patients with and without nephritis in IgAV. The urinary complement C3 concentration was statistically significantly increased in patients with IgAV-N (median: 14.65 μg/mmol [2.26–20.21]) compared to patients grouped as IgAVwoN (2.26 μg/mmol [0.15–3.14]; p = 0.007) (Fig. 2A). They were also statistically significantly increased in IgAV-N compared to patients with LN (1.52 μg/mmol [0.54–2.27]; p = 0.0002). The urinary complement C3 concentration was also statistically significantly increased in patients with IgAV-N compared to healthy controls (median: 1.89 μg/mmol [0.49–2.94]; p = 0.003). No other significant differences between the groups were noted. The urinary complement C4 concentration was statistically significantly increased in IgAV-N patients (6.52 μg/mmol [1.30–9.72]) compared to IgAVwoN (1.37 μg/mmol [0.38–2.43]; p = 0.04), and compared to all other groups – SLEwoLN (0.98 μg/mmol [0.47–2.23]; p = 0.007), LN (0.72 μg/mmol [0.25–1.12]; p < 0.0001) and healthy controls (1.08 μg/mmol [0.37–1.57]; p = 0.0012) (Fig. 2B). No other significant differences between groups were noted. Urinary complement C5 concentration was statistically significantly increased in IgAV-N patients (1.36 μg/mmol [0.65–2.85]) compared to IgAVwoN (0.38 μg/mmol [0.03–0.72]; p = 0.005) and all other groups – SLEwoLN (0.24 μg/mmol [0.02–0.55]; p = 0.001), LN (0.16 μg/mmol [0.05–0.33]; p = 0.0001) and healthy controls (0.28 μg/mmol [0.10–0.51]; p = 0.002) (Fig. 2C). No other significant differences between the groups were noted. Urinary complement C5a concentration was statistically significantly increased in IgAV-N patients (101.9 ng/mmol [15.36–230.0]) compared to patients grouped as IgAVwoN (18.33 ng/mmol [4.27–33.3]; p = 0.01), and all other groups – SLEwoLN (22.81 ng/mmol [3.92–30.79]; p = 0.03), LN (11.28 ng/mmol [6.14–16.88]; p = 0.0004) and healthy controls (14.99 ng/mmol [3.19–29.31]; p = 0.002) (Fig. 2D). No other significant differences between the groups were noted. Interestingly, there were notable outliers seen within the IgAV subgroups (as illustrated in Fig. 2) and these may suggest that certain patients within the nephritis group express a more pronounced complement protein profile.
Fig. 2.
Urinary complement concentrations in patients with paediatric inflammatory disease stratified by kidney involvement – IgAVwoN, IgAV-N, SLEwoLN and LN compared to age- and sex-matched controls. Complement concentrations were assessed in urine collected from patients with IgAVwoN, IgAV-N, SLEwoLN, LN and controls using ELISA. Complement concentrations were normalised to urinary creatinine. (A) Urinary C3/creatinine, (B) Urinary C4/creatinine, (C) Urinary C5/creatinine, and (D) Urinary C5a/creatinine. N = 10–37/group. Data are expressed as median and analysed using Kruskal–Wallis test with Dunn’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001
Urinary complement concentrations distinguishing patients with IgAV-N
Receiver operating characteristic (ROC) curve analyses were used to determine the ability of the complement proteins to discriminate between patients with IgAV-N and those without nephritis. The level of each urinary complement molecule (C3, C4, C5, C5a) was individually excellent at distinguishing between patients with IgAV-N and IgAVwoN (AUC; C3 – 0.085, p = 0.0009; C4 – 0.805, p = 0.0033; C5 – 0.838, p = 0.0012; and C5a – 0.817, p = 0.0024) (Fig. 3). Logistic regression analysis was used to combine all four complement markers into a single biomarker panel and ROC analysis was performed. Combining all four urinary complement components (C3, C4, C5 and C5a) improved the effectiveness of the test to outstanding (AUC; IgAV-N vs. IgAVwoN – 0.919, p < 0.001) (Fig. 4).
Fig. 3.
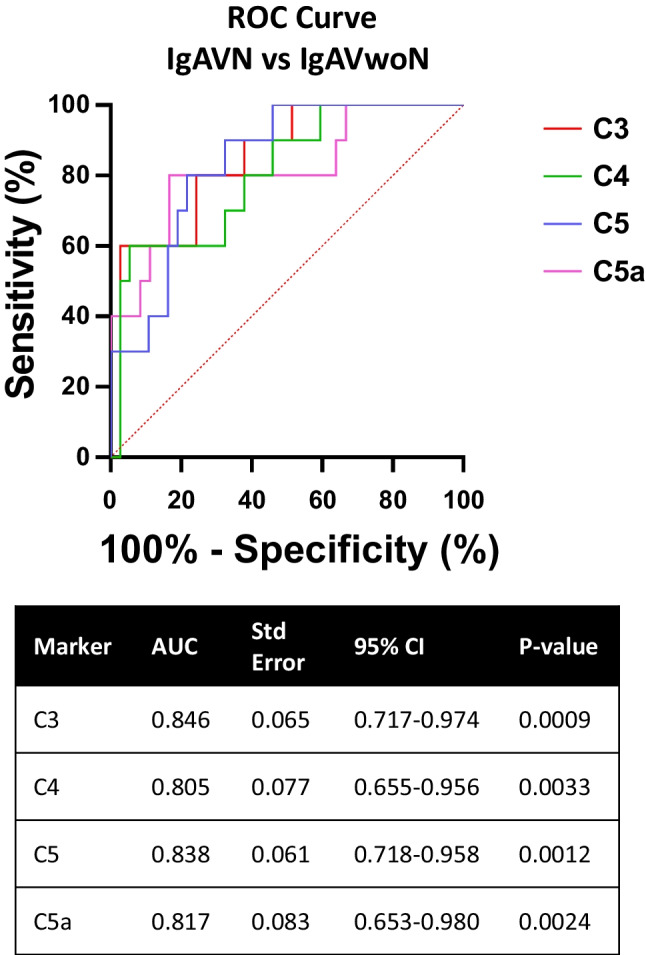
Receiver Operating Characteristic (ROC) curve analysis for IgAV-N patient individual complement concentrations compared to IGAVwoN. Urinary complement concentrations were analysed individually for their sensitivity and 100%—specificity in order to determine their effectiveness at distinguishing between IgAV-N patients vs. IgAVwoN patients
Fig. 4.
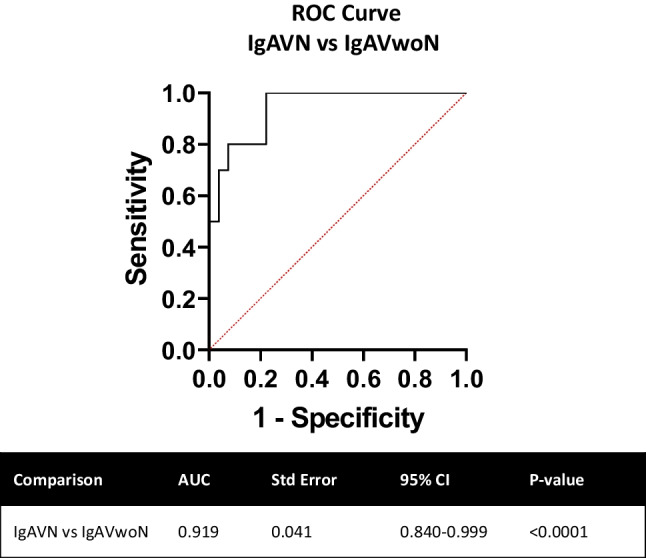
Receiver Operating Characteristic (ROC) curve analysis for IgAV-N patient combined complement concentrations compared to IGAVwoN. Urinary complement concentrations were analysed together using logistic regression analysis for their sensitivity and 100%—specificity in order to determine their effectiveness at distinguishing between IgAV-N patients vs. IgAVwoN patients
Discussion
IgAV is a common paediatric condition and the second most frequent reason to perform a kidney biopsy by paediatric nephrologists. It is well recognised that kidney outcomes from IgAV have failed to improve over time and current treatments lack robust evidence to support their use. Due to growing interest in the role of complement in IgA nephropathy, a condition histologically similar to IgAV, this study aimed to evaluate whether urinary complement proteins can be measured in children with IgAV, how they compare to another form of glomerulonephritis, and if they may indicate the presence of nephritis. This study demonstrated that urinary complement C3, C4, C5, and C5a concentrations were all statistically significantly increased in children with IgAV-N. Each urinary complement marker was individually excellent at distinguishing between those with and without nephritis and when combined, using a logistic regression analysis, their ability to discriminate between children with IgAV with or without nephritis was outstanding (AUC – 0.919).
Histologically, evidence of complement deposition is well recognised in IgAV-N, with approximately 74% of patients having histological evidence of C3 deposition identified in studies [10]. The complement system is important in linking the innate and adaptive immunity and it can be activated by different routes, known as the classical pathway, mannose-binding lectin pathway and alternative pathway. These specific pathways lead to a common pathway and the production of the membrane attack complex (C5b-9) causing cell lysis, opsonisation and upregulation of C5a, a neutrophil chemoattractant protein. Evidence of activation of the mannose-binding lectin pathway and/or the alternative complement pathway is reported in the skin and systemically in patients with IgAV [14, 15]. To our knowledge, this is the first report of measuring urinary complement in children with IgAV-N. Previous reports on other proteinuric conditions demonstrate that components of the complement system are able to distinguish between patients with membranous nephropathy and diabetic nephropathy independently of the amount of protein loss [7]. In patients with SLE, systemic complement abnormalities are well described but their precise role is not clearly understood, and surprisingly there are few studies measuring urinary complement in patients with LN [16]. Elevated urinary expression of complement is not found in patients with minimal change nephrotic syndrome suggesting that their presence doesn’t seem to be directly related to heavy proteinuria [9]. This study highlights important findings as urinary complement may represent a reliable biomarker in an easily accessible biofluid to stratify patients with IgAV for potential early therapeutic intervention that may mitigate the onset of nephritis. The clear outliers with increased complement expression highlighted in this study give the impression that there may be certain individuals with nephritis who would benefit the most from this intervention. While urine complement products are not routinely measured in clinical laboratories at present, these can be measured in the pre-clinical and pharmaceutical setting and further studies are required to understand the specific role of complement pathway in the pathogenesis of IgAV-N. As the complement pathway is a target of multiple new therapeutic agents currently under evaluation in clinical trials for many inflammatory kidney diseases, gaining insight into their role in IgAV may provide evidence for broader use of these medications [17].
This study does have limitations. Notably, there were assumptions in presuming a negative urine dipstick represented no proteinuria and therefore microalbuminuria may have been missed. Additionally, it did not demonstrate any significant increase in the urinary complement proteins in children with LN, despite the recognised role that complement has in this disease [18]. This may represent limitations in selecting the LN cohort who were defined as having nephritis according to the kidney domain of the BILAG 2004, and patients with chronic residual proteinuria may have been included, a known limitation of the SLE disease activity index [19]. This is supported by the relatively low concentration of urinary protein seen within the LN cohort, which is unusual in acute LN, and the long time elapsed since diagnosis, suggesting that they may have reflected a cohort of children with previous LN and persisting proteinuria. Other limitations of this study include the cross-sectional nature of the study and the relatively small, heterogeneous cohort of patients that may have limited the generalisability of the findings. It would be difficult to determine whether the urinary complement concentrations were merely increased due to the finding of proteinuria, as patients with nephritis were defined according to the presence of proteinuria and recent reports suggest a complex interplay between proteinuria and its potential to upregulate the tubular expression of complement pathway products [8]. Despite these limitations, our exploratory findings report that urinary complement products are measurable in children with IgAV and our data support a potential role for complement as a contributor to the pathophysiology of IgAV-N. Additional large, longitudinal studies are required to observe a large cohort of children from disease presentation to improvement or the evolution of nephritis to determine the timing of when complement may have its onset in the disease process and to identify when complement could be inhibited as a potential therapeutic target.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the volunteers who consented to participate in this study. This work was partially carried out at the NIHR Alder Hey Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Funding
This work was supported by the UK’s Experimental Arthritis Treatment Centre for Children (supported by Versus Arthritis, Alder Hey Children’s NHS Foundation Trust, the Alder Hey Charity, Alder Hey Children’s Kidney Fund Charity, and the University of Liverpool).
Declarations
Conflict of interest
The author LO has previously undertaken expert advisory work for Omeros Corporation and Biocryst Pharmaceuticals Inc., who are developing drugs to target the complement system. All other authors have no competing interests to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oni L, Sampath S. Childhood IgA vasculitis (Henoch Schonlein Purpura)-advances and knowledge gaps. Front Pediatr. 2019;7:257. doi: 10.3389/fped.2019.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rianthavorn P, Chacranon M. Long-term renal outcome in pediatric glomerulonephritis associated with crescent formation. Clin Exp Nephrol. 2018;22:661–667. doi: 10.1007/s10157-017-1498-2. [DOI] [PubMed] [Google Scholar]
- 3.Delbet JD, Hogan J, Aoun B, Stoica I, Salomon R, Decramer S, Brocheriou I, Deschenes G, Ulinski T. Clinical outcomes in children with Henoch-Schonlein purpura nephritis without crescents. Pediatr Nephrol. 2017;32:1193–1199. doi: 10.1007/s00467-017-3604-9. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki Y, Suyama K, Yugeta E, Katayose M, Suzuki S, Sakuma H, Nemoto K, Tsukagoshi A, Nagasawa K, Hosoya M. The incidence and severity of Henoch-Schonlein purpura nephritis over a 22-year period in Fukushima Prefecture, Japan. Int Urol Nephrol. 2010;42:1023–1029. doi: 10.1007/s11255-009-9701-3. [DOI] [PubMed] [Google Scholar]
- 5.Kaartinen K, Safa A, Kotha S, Ratti G, Meri S. Complement dysregulation in glomerulonephritis. Semin Immunol. 2019;45:101331. doi: 10.1016/j.smim.2019.101331. [DOI] [PubMed] [Google Scholar]
- 6.Rizk DV, Maillard N, Julian BA, Knoppova B, Green TJ, Novak J, Wyatt RJ. The emerging role of complement proteins as a target for therapy of IgA nephropathy. Front Immunol. 2019;10:504. doi: 10.3389/fimmu.2019.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araumi A, Osaki T, Ichikawa K, Kudo K, Suzuki N, Watanabe S, Watanabe M, Konta T. Urinary and plasma proteomics to discover biomarkers for diagnosing between diabetic nephropathy and minimal change nephrotic syndrome or membranous nephropathy. Biochem Biophys Rep. 2021;27:101102. doi: 10.1016/j.bbrep.2021.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogrodowski JL, Hebert LA, Sedmak D, Cosio FG, Tamerius J, Kolb W. Measurement of SC5b-9 in urine in patients with the nephrotic syndrome. Kidney Int. 1991;40:1141–1147. doi: 10.1038/ki.1991.326. [DOI] [PubMed] [Google Scholar]
- 9.Morita Y, Ikeguchi H, Nakamura J, Hotta N, Yuzawa Y, Matsuo S. Complement activation products in the urine from proteinuric patients. J Am Soc Nephrol. 2000;11:700–707. doi: 10.1681/ASN.V114700. [DOI] [PubMed] [Google Scholar]
- 10.Celik A, Sarioglu S, Kavukçu S. Comparison of clinical, pathological and long-term renal outcomes of children with Henoch-Schonlein purpura nephritis and IgA nephropathy. Int Urol Nephrol. 2022;54:1925–1932. doi: 10.1007/s11255-021-03063-7. [DOI] [PubMed] [Google Scholar]
- 11.Dumont C, Merouani A, Ducruet T, Benoit G, Clermont MJ, Lapeyraque AL, Phan V, Patey N. Clinical relevance of membrane attack complex deposition in children with IgA nephropathy and Henoch-Schonlein purpura. Pediatr Nephrol. 2020;35:843–850. doi: 10.1007/s00467-019-04445-x. [DOI] [PubMed] [Google Scholar]
- 12.Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce IN, D'Cruz D, Griffiths B, Khamashta M, Maddison P, McHugh N, Snaith M, Teh LS, Yee CS, Zoma A, Gordon C. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44:902–906. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- 13.Smith EMD, Yin P, Jorgensen AL, Beresford MW. Clinical predictors of proteinuric remission following an LN flare - evidence from the UK JSLE cohort study. Pediatr Rheumatol Online J. 2018;16:14. doi: 10.1186/s12969-018-0230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damman J, Mooyaart AL, Bosch T, Seelen MA, Doorn MBV. Lectin and alternative complement pathway activation in cutaneous manifestations of IgA-vasculitis: a new target for therapy? Mol Immunol. 2022;143:114–121. doi: 10.1016/j.molimm.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Endo M, Ohi H, Ohsawa I, Fujita T, Matsushita M. Complement activation through the lectin pathway in patients with Henoch-Schonlein purpura nephritis. Am J Kidney Dis. 2000;35:401–407. doi: 10.1016/s0272-6386(00)70192-2. [DOI] [PubMed] [Google Scholar]
- 16.Manzi S, Rairie JE, Carpenter AB, Kelly RH, Jagarlapudi SP, Sereika SM, Medsger TA, Jr, Ramsey-Goldman R. Sensitivity and specificity of plasma and urine complement split products as indicators of lupus disease activity. Arthritis Rheum. 1996;39:1178–1188. doi: 10.1002/art.1780390716. [DOI] [PubMed] [Google Scholar]
- 17.Willows J, Brown M, Sheerin NS. The role of complement in kidney disease. Clin Med (Lond) 2020;20:156–160. doi: 10.7861/clinmed.2019-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao L, Cunningham PN, Quigg RJ. Complement in lupus nephritis: new perspectives. Kidney Dis (Basel) 2015;1:91–99. doi: 10.1159/000431278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohmura K. Which is the best SLE activity index for clinical trials? Mod Rheumatol. 2021;31:20–28. doi: 10.1080/14397595.2020.1775928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.