This randomized clinical trial determines whether multimodal prehabilitation before colorectal cancer surgery can reduce postoperative complications and enhance functional recovery.
Key Points
Question
Does a 4-week supervised multimodal prehabilitation program before elective resection of nonmetastasized colorectal cancer reduce postoperative complications and enhance functional recovery?
Findings
In this multicenter, international randomized clinical trial that analyzed 251 adults, multimodal prehabilitation resulted in a significant reduction of severe and medical complications. The program also resulted in a statistically significant faster and better postoperative recovery.
Meaning
Patients undergoing resection for nonmetastasized colorectal cancer may benefit from a 4-week multimodal prehabilitation program.
Abstract
Importance
Colorectal surgery is associated with substantial morbidity rates and a lowered functional capacity. Optimization of the patient’s condition in the weeks prior to surgery may attenuate these unfavorable sequelae.
Objective
To determine whether multimodal prehabilitation before colorectal cancer surgery can reduce postoperative complications and enhance functional recovery.
Design, Setting, and Participants
The PREHAB randomized clinical trial was an international, multicenter trial conducted in teaching hospitals with implemented enhanced recovery after surgery programs. Adult patients with nonmetastasized colorectal cancer were assessed for eligibility and randomized to either prehabilitation or standard care. Both arms received standard perioperative care. Patients were enrolled from June 2017 to December 2020, and follow-up was completed in December 2021. However, this trial was prematurely stopped due to the COVID-19 pandemic.
Interventions
The 4-week in-hospital supervised multimodal prehabilitation program consisted of a high-intensity exercise program 3 times per week, a nutritional intervention, psychological support, and a smoking cessation program when needed.
Main Outcomes and Measures
Comprehensive Complication Index (CCI) score, number of patients with CCI score more than 20, and improved walking capacity expressed as the 6-minute walking distance 4 weeks postoperatively.
Results
In the intention-to-treat population of 251 participants (median [IQR] age, 69 [60-76] years; 138 [55%] male), 206 (82%) had tumors located in the colon and 234 (93%) underwent laparoscopic- or robotic-assisted surgery. The number of severe complications (CCI score >20) was significantly lower favoring prehabilitation compared with standard care (21 of 123 [17.1%] vs 38 of 128 [29.7%]; odds ratio, 0.47 [95% CI, 0.26-0.87]; P = .02). Participants in prehabilitation encountered fewer medical complications (eg, respiratory) compared with participants receiving standard care (19 of 123 [15.4%] vs 35 of 128 [27.3%]; odds ratio, 0.48 [95% CI, 0.26-0.89]; P = .02). Four weeks after surgery, 6-minute walking distance did not differ significantly between groups when compared with baseline (mean difference prehabilitation vs standard care 15.6 m [95% CI, −1.4 to 32.6]; P = .07). Secondary parameters of functional capacity in the postoperative period generally favored prehabilitation compared with standard care.
Conclusions and Relevance
This PREHAB trial demonstrates the benefit of a multimodal prehabilitation program before colorectal cancer surgery as reflected by fewer severe and medical complications postoperatively and an optimized postoperative recovery compared with standard care.
Trial Registration
trialregister.nl Identifier: NTR5947
Introduction
With more than 9 million procedures performed each year and an estimated 50% increase by 2040, the risk associated with cancer surgery poses an impressive burden to patients and the health care system.1 Colorectal cancer is third in rank among global cancer incidence rates and was the second leading cause of cancer-related deaths in 2020.2 Despite remarkable advancements in perioperative care, 30-day morbidity did not significantly improve with morbidity rates between 19.7% to 37.4%.3 Furthermore, many patients experience new or worsening impairments, which can persist for months after surgery.4,5 Therefore, reducing the risk of postoperative complications and functional decline is of the utmost importance.
Functional capacity is a strong determinant of a favorable outcome after surgery,6,7 leading to a compelling rationale to improve physical, nutritional, and mental status before surgery. After the diagnosis of colorectal cancer, time to surgery usually comprises several weeks, a salient time ideally suited for multimodal prehabilitation.8,9
Trials that have suggested that prehabilitation may lead to clinically meaningful improvements in the patient’s preoperative condition and surgical outcome10,11,12,13,14,15,16 lack sufficient power and have heterogeneous design.17,18,19,20 To date, recommendations on the implementation of prehabilitation in standard care are weak.21
Therefore, we conducted an international multicenter randomized clinical trial to investigate the effect of a multimodal prehabilitation program on functional capacity recovery and postoperative complications within 30 days after surgery of patients with nonmetastasized colorectal cancer.
Methods
Trial Design and Procedures
The PREHAB trial is an open-label international, multicenter, parallel-arm, randomized, controlled study comparing multimodal prehabilitation with standard preoperative care. The protocol was previously published22 and is available in Supplement 1 and approved by the local institutional review board at each trial site. Participants provided written informed consent before any study-related procedures commenced. An independent data safety monitoring board consisting of an epidemiologist, 2 physicians, and an exercise physiologist monitored the safety and efficacy of the trial. Enrollment began on June 6, 2017, was paused in March 2020 during the COVID-19 pandemic, and was prematurely stopped on December 15, 2020. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.23
The study population consisted of adult patients scheduled for elective surgical resection of primary colorectal cancer. The presence of metastases or another primary tumor, American Society of Anesthesiologists (ASA) score of 4 or higher, chronic kidney failure (creatinine >2.83 mg/dL [to convert to micromoles per liter, multiply by 88.4] or dialysis), indication for abdominoperineal resection, and the impossibility to wait 4 weeks for surgery represented exclusion criteria. Furthermore, patients were not eligible if they had medical conditions and/or cognitive impairments that contraindicated the intervention and/or illiteracy or language barriers. No data on race and ethnicity were collected.
Participating sites were teaching hospitals and secondary or tertiary care centers performing approximately 200 to 300 colorectal resections per year. Seven sites enrolled patients, of which 6 were originally members of an international consortium (eAppendix in Supplement 2).
Investigators identified patients at the surgical clinic or during weekly multidisciplinary team meetings. After obtaining written informed consent, the research team assessed eligibility by performing a complete medical history and a cardiopulmonary exercise test (CPET). The conduction and interpretation of CPET results followed clinical guidelines.24
If eligible, participants were randomized on a 1:1 ratio to either prehabilitation or standard care. Randomization was performed by a local investigator via computer-generated random numbers composed of fixed blocks of 8 (ResearchManager25). Groups were stratified for center, neoadjuvant therapy, and tumor location (colon/rectum). Allocation sequence was generated with ResearchManager, and this was concealed from the trained local investigators performing randomization. Investigators and participants were not blinded for group assignment.
Participants standardly underwent functional assessments at baseline, before surgery (approximately 4 weeks after baseline), and 4 and 8 weeks after surgery. A complete list of assessments is available in eTable 1 in Supplement 2.
The local prehabilitation teams directly involved in the study procedures consisted of qualified health care professionals such as physicians, kinesiologists or physiotherapists, dietitians, and psychology-trained personnel. Serious and adverse events related to the intervention were collected.
Multimodal Prehabilitation
Interventions in this trial have been specifically developed for patients awaiting colorectal cancer surgery.26 The intervention consists of a 4-week personalized in-hospital supervised preoperative program including exercise, nutritional, and psychological support. If indicated, a smoking cessation intervention was included. In-hospital entailed outpatient appointments to hospital affiliated training departments or facilities.
The supervised training consisted of a 1-hour session of aerobic and strength exercises 3 times per week with resting days in between. The aerobic part, preferably performed on a bicycle, consisted of a high-intensity interval training using baseline CPET-derived variables. It consisted of 4 intervals of 2-minute high-intensity bouts conducted at 85% to 90% of peak power, alternated with 4 intervals of 4-minute moderate-intensity bouts at 30% of peak power. Resistance exercise consisted of 2 series of 10 repetitions targeting major muscle groups. The intensity was set at 65% to 70% of the calculated baseline indirect 1 repetition maximum (1 RM). Professional strength equipment, body weight, elastic bands, and/or calibrated dumbbells were used.
Based on nutritional assessment and dietary habits, a registered dietitian provided a full nutritional intervention. The program aimed to balance macronutrients and to achieve a daily amount of proteins of 1.5 g per kg. Additionally, participants were provided with a whey protein supplement and were instructed to ingest 30 g within 1 hour after the in-hospital training session and 1 hour before sleeping daily. Vitamin D and multivitamin supplements were also provided.
Anxiety-coping interventions consisted of relaxation techniques and deep breathing exercises provided by psychology-trained personnel in a 1-to-1 session. If a high risk of mental distress was detected by medical history and/or baseline scores of the Generalized Anxiety Disorder 7-item scale of 10 or higher or Patient Health Questionnaire 9-item of 15 or higher, participants were additionally referred to a medical psychologist. A smoking cessation program was offered, if indicated. The program consisted of individual counseling and nicotine replacement therapy.
Standard Care
Participants were treated per local perioperative standard of care. All study sites followed a well-adopted enhanced recovery after surgery (ERAS) pathway to minimize heterogeneity in perioperative care.27 Scheduling of surgery was not affected by study participation. Participants in the standard care group did not receive any additional counseling on exercise, nutrition, or mental health.
Primary Outcomes
The coprimary outcomes were 30-day postoperative complications determined using the Comprehensive Complication Index (CCI) and postoperative 6-minute walking distance (6-MWD) that was measured with the 6-minute walking test (6-MWT).
The CCI is a validated measurement of postoperative morbidity/mortality that weighs all complications using the Clavien-Dindo classification into a sum score ranging from 0 (no complication) to 100 (death).28 Based on our preliminary studies using a similar surgical population,29 a CCI score more than 20 was chosen to define severe complication that impacted postoperative surgical outcome. (This is deviating from the analyses planned in the protocol because the CCI is a zero-inflated highly skewed distribution that cannot be correctly analyzed as a continuous variable with tests for comparing locations. We will include the results of the initially planned analysis among the sensitivity analyses.) Functional capacity was measured with the 6-MWT, which reliably quantified exercise tolerance in the colorectal surgical population.30,31 Our end point was the change in meters walked over the 6 minutes (6-MWD) between baseline and 4 weeks after surgery.
Secondary Outcomes
Prespecified secondary outcomes consisted of surgical and functional end points and health-related quality of life. In addition to the 6-MWD, functional capacity was analyzed by measuring domains of physical, nutritional, and mental status. CPET, a criterion standard test for cardiorespiratory fitness,24 defined the oxygen consumption at the anaerobic threshold (V̇O2AT) and at peak exercise (peak V̇O2). Strength was determined with the indirect 1 RM and handgrip strength. Nutritional status was assessed using the Patient-Generated Subjective Global Assessment.32 Mental health status was measured with the Generalized Anxiety Disorder 7-item scale and the Patient Health Questionnaire 9-item. Finally, health-related quality of life was determined with the European Organisation for Research and Treatment of Cancer, Quality Of Life Of Cancer Patients C30 Module global health status subscale.
These assessments were performed at baseline, preoperatively (approximately 4 weeks after baseline), and 4 and (except for CPET) 8 weeks after surgery. Surgical outcomes, evaluated 30 days after surgery, included length of hospital stay, readmissions, and mortality.
Compliance to the exercise training was assessed as follows. Participants could receive a maximum of 12 supervised sessions (including both baseline and preoperative assessment). When at least 9 of these 12 sessions were completed, participants were considered to be compliant to the exercise intervention (regardless of reasons for missing sessions). Subsequently, the period between baseline 6-MWT and preoperative 6-MWT had to be between 3 and 8 weeks.
Sample Size
Sample size calculation was based on the mean (SD) CCI (10.4 [14]) in our population. We considered a decrease of the CCI of 30% as clinically meaningful. Using an α of 0.05, power of 0.80 (2-sided test), and expecting a dropout rate of 10%, an estimated 714 (357 per arm) sample size was aimed for. This sample size was considered to be sufficient to demonstrate a difference in the coprimary outcome (6-MWD).
Premature Termination of the Trial
Due to the intercurrent COVID-19 pandemic, continuation of the trial was deemed unsafe and not feasible due to restrictions imposed by governments and downscaling of nonemergent care. The trial was therefore put on hold for an indefinite period of time. As any perspective on full resumption of inclusion was lacking, the principal investigators decided to terminate the trial prematurely.
Data Collection and Monitoring
Data were collected by local investigators or professional data managers and entered into an electronic case report form created in ResearchManager. Due to the behavioral nature of the intervention, blinding was not feasible for either participants, investigators, or health care professionals prescribing or delivering prehabilitation. Outcome assessors who performed medical record reviews to score postoperative complications were blinded. The database was monitored, queried, cleaned, and validated by the Netherlands Comprehensive Cancer Organisation.
Statistical Analyses
Summary statistics were reported per arm. Medians with IQRs for numeric variables, counts and percentages for categorical data, and percentages for missing data were reported. An intention-to-treat approach was used.
The binary outcome variables were analyzed with the Cochran-Mantel-Haenszel test to estimate an odds ratio (OR) corrected for sites. For the cross-sectional numerical outcome variable, a generalized linear model with a gamma distribution and identity link function was used, corrected for site. The longitudinal outcome variables were analyzed with generalized estimating equations using the empirical sandwich estimator, the normal distribution, the identity link function, and an exchangeable variance-covariance working matrix. The factors time and site were treated as fixed effects.
The primary analyses were repeated in a per-protocol analysis, excluding patients with an ASA score of IV, with minimal surgery, from the Danish sites (Eastern Cooperative Oncology Group Performance Scale score >0), and 2 patients in the control who completed the prehabilitation program.
We also performed a Mann-Whitney U test on the CCI scores and compared the occurrence of medical and surgical complications with the Cochran-Mantel-Haenszel test. For the 6-MWD, we conducted a missing not at random imputation. In addition, we repeated the primary analyses on subgroups: high/lower risk (CPET) and colonic/rectal cancer. We applied a significance level of α = 0.05 for all tests. All analyses were conducted with the SAS software package, version 9.4 (SAS Institute).
Results
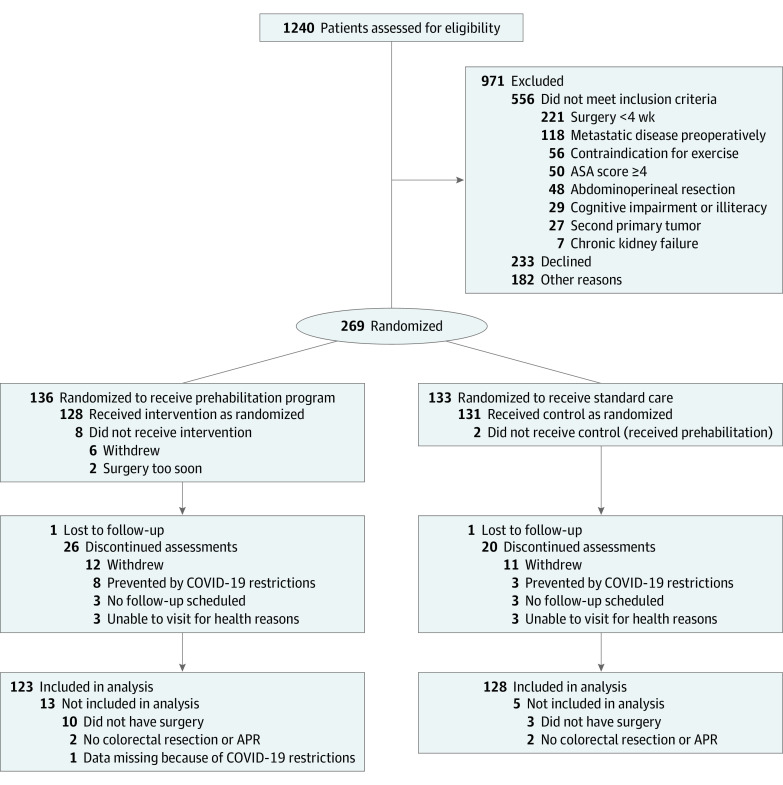
Of 1240 patients screened between June 2017 and December 2020, 269 participants consented to study participation. They were randomly assigned to either prehabilitation or standard care. Follow-up was completed in December 2021. A total of 18 participants were excluded from analysis because they did not undergo colorectal surgery (prehabilitation, n = 2; standard care, n = 2), they did not have any surgery at all (prehabilitation, n = 10; standard care, n = 3), or data were completely missing due to the COVID-19 pandemic (prehabilitation, n = 1) (Figure 1). Hence, 251 participants were included in the intention-to-treat population (prehabilitation, n = 123; standard care, n = 128). Seven of 123 participants (5.7%) experienced adverse events consisting mainly of lightheadedness or nausea due to the exercise intervention (n = 4) or protein supplements (n = 3), respectively. However, no serious adverse events related to the program occurred. A total of 95 of 123 (77.2%; missing n = 5) were considered to be compliant to the exercise intervention.
Figure 1. CONSORT Flow Diagram.
APR indicates abdominoperineal resection; ASA, American Society of Anesthesiologists.
Baseline characteristics are shown in Table 1. The median (IQR) age was 69 (60-76) years. Cancer was located in the colon in 206 of 251 participants (82.1%). Most surgical procedures occurred via a minimal invasive operative technique (234 of 251 participants [93.2%]). Overall baseline functional capacity was similar between groups.
Table 1. Baseline and Clinical Characteristicsa.
| Characteristic | No. (%) | |
|---|---|---|
| Prehabilitation group (n = 123) | Control group (n = 128) | |
| Age, median (IQR), y | 69 (60-77) | 71 (60-76) |
| Sex | ||
| Female | 61 (49.6) | 52 (40.6) |
| Male | 62 (50.4) | 76 (59.4) |
| Weight, median (IQR), kg | 76.3 (66.9-85.0) | 78.4 (68.6-92.8) |
| Lean body mass, median (IQR), kg | 51.0 (43.5-59.0) | 53.0 (44.0-61.0) |
| Missing | 3 (2.4) | 9 (7.0) |
| Body fat, median (IQR), % | 32.1 (26.0-38.3) | 34.1 (27.5-38.4) |
| Missing | 4 (3.3) | 12 (9.4) |
| BMI, median (IQR) | 26.3 (24.2-30.2) | 27.6 (24.7-31.7) |
| ≥30 | 31 (25.2) | 48 (37.5) |
| ASA | ||
| I | 7 (5.7) | 4 (3.1) |
| II | 85 (69.1) | 97 (75.8) |
| III | 30 (24.4) | 26 (20.3) |
| IVb | 1 (0.8) | 1 (0.8) |
| Comorbidities | ||
| Diabetes | 16 (13) | 30 (23.4) |
| Missing | 13 (10.6) | 17 (13.3) |
| Cardiovascular | 60 (48.8) | 69 (53.9) |
| Missing | 13 (10.6) | 17 (13.3) |
| Pulmonary | 23 (18.7) | 31 (24.2) |
| Missing | 13 (10.6) | 17 (13.3) |
| Charlson Comorbidity Index, median (IQR) | 5 (4-6) | 5 (4-6) |
| Missing | 0 | 1 (0.8) |
| Metabolic status | ||
| CRP, median (IQR), mg/dL | 0.3 (0.1-0.6) | 0.4 (0.2-0.9) |
| Missing | 44 (35.8) | 41 (32.0) |
| Albumin, median (IQR), g/dL | 4.2 (3.9-4.5) | 4.2 (3.9-4.4) |
| Missing | 25 (20.3) | 22 (17.2) |
| Hemoglobin, median (IQR), g/dL | 13.1 (11.3-14.2) | 13.1 (11.0-14.5) |
| Missing | 1 (0.8) | 0 |
| Smoking status | ||
| None | 48 (39.0) | 52 (40.6) |
| Former | 64 (52.0) | 55 (43.0) |
| Current | 11 (8.9) | 21 (16.4) |
| Tumor stage | ||
| 0 | 7 (5.7) | 7 (5.5) |
| I | 39 (31.7) | 36 (28.1) |
| II | 39 (31.7) | 37 (28.9) |
| III | 28 (22.8) | 34 (26.6) |
| IV | 4 (3.3) | 4 (3.1) |
| Unknown | 6 (4.9) | 9 (7.0) |
| Missing | 0 | 1 (0.8) |
| Cancer type | ||
| Colon | 101 (82.1) | 105 (82) |
| Rectum | 22 (17.9) | 23 (18) |
| Neoadjuvant therapy | 1 (0.8) | 3 (2.3) |
| Type of surgery | ||
| Hemicolectomy | ||
| Right | 62 (50.4) | 63 (49.2) |
| Left | 10 (8.1) | 10 (7.8) |
| Colectomy | ||
| Transverse | 2 (1.6) | 0 |
| Subtotal | 2 (1.6) | 3 (2.3) |
| Sigmoid | 17 (13.8) | 24 (18.8) |
| TaTME | 7 (5.7) | 8 (6.3) |
| TEM | 1 (0.8) | 1 (0.8) |
| Low anterior resection | 22 (17.9) | 19 (14.8) |
| Minimal invasive surgeryc | 118 (95.9) | 116 (90.6) |
| Stoma creation | 13 (10.6) | 14 (10.9) |
| Duration of surgery, median (IQR), min | 160.0 (130.0-201.0) | 171.5 (137.0-201.5) |
| Intraoperative blood loss, median (IQR), mLd | 0 (0-50) | 0 (0-50) |
| Missing | 88 (71,5) | 95 (74.2) |
| 6-MWD, median (IQR), m | 514.0 (450.0-578.0) | 512.5 (435.0-572.0) |
| Missing | 1 (0.8) | 2 (1.6) |
| 6-MWD ≥400 m | 100 (81.3) | 106 (82.8) |
| Missing | 1 (0.8) | 2 (1.6) |
| V̇O2AT, median (IQR), mL × kg−1 × min−1 | 12.3 (10.9-15.0) | 12.9 (10.3-15.0) |
| Missing | 14 (11.4) | 17 (13.3) |
| Peak V̇O2, median (IQR), mL × kg−1 × min−1 | 18.0 (15.0-21.9) | 19.0 (15.0-22.3) |
| Missing | 10 (8.1) | 13 (10.2) |
| 1 RM chest press, median (IQR), kg | 15.9 (9.1-28.9) | 18.8 (11.8-30.0) |
| Missing | 5 (4.1) | 32 (25.0) |
| Handgrip strength, median (IQR), kg | 28.0 (21.5-38.0) | 30.5 (23.5-38.0) |
| Missing | 3 (2.4) | 4 (3.1) |
| PG-SGA-SF total score, median (IQR) | 1 (0-4) | 1 (0-3) |
| Missing | 8 (6.5) | 10 (7.8) |
| PHQ-9, median (IQR) | 2 (0-6) | 2 (0-5) |
| Missing | 8 (6.5) | 2 (1.6) |
| GAD-7, median (IQR) | 2 (0-5) | 3 (0-6) |
| Missing | 6 (4.9) | 3 (1.7) |
| EORTC QLQ-C30-global health, median (IQR) | 66 (66-83) | 66 (50-83) |
| Missing | 2 (1.6) | 1 (0.8) |
Abbreviations: 6-MWD, 6-minute walking distance; ASA, American Society of Anesthesiology; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CRP, C-reactive protein; EORTC QLQ-C30, European Organisation for Research And Treatment Of Cancer, Quality Of Life Of Cancer Patients Module; GAD-7, Generalized Anxiety Disorder 7-item scale; peak V̇O2, oxygen consumption at peak exercise; PG-SGA-SF, Patient-Generated Subjective Global Assessment short form; PHQ-9, Patient Health Questionnaire 9-item; 1 RM, indirect 1 repetition maximum; TaTME, transanal total mesorectal excision; TEM, transanal endoscopic microsurgery; V̇O2AT, oxygen consumption at the anaerobic threshold.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; CRP to milligrams per liter, multiply by 10; hemoglobin to grams per liter, multiply by 10.
P values are from the χ2 or Fisher exact test for binary/categorical variables and from the Mann-Whitney U test for continuous variables. Baseline characteristics are of intention-to-treat population (all patients with group allocation, surgery [other than no colorectal cancer or abdominoperineal resection]; n = 251).
Patients were assessed as ASA III at time of inclusion.
Laparoscopic- or robot-assisted.
Missing contains both missing values and participants without any blood loss perioperatively.
Primary Outcomes
Complications
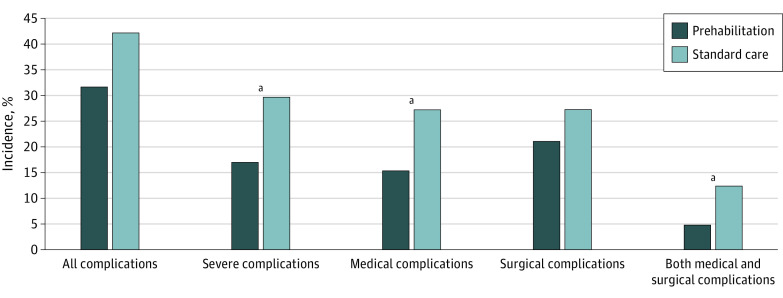
At 30 days after surgery, 149 complications had occurred in 93 of 251 patients (37.1%). The rate of severe complications was significantly lower in patients undergoing prehabilitation compared with standard care (CCI score >20; 21 of 123 [17.1%] vs 38 of 128 [29.7%]; OR, 0.47 [95% CI, 0.26-0.87]; P = .02). The post hoc power was observed to be only 64%. Overall, 39 of 123 patients (31.7%) in prehabilitation experienced complications vs 54 of 128 (42.2%) in standard care (OR, 0.62 [95% CI, 0.37-1.05]; P = .07), with comparable grading (median [IQR] CCI score, prehabilitation: 0 [0-8.7] vs standard care: 0 [0-20.9]; P = .06). Patients encountered fewer medical complications (eg, cardiovascular or respiratory) after prehabilitation compared with standard care (19 of 123 [15.4%] vs 35 of 128 [27.3%]; OR, 0.48 [95% CI, 0.26-0.89]; P = .02). There was no difference in number of surgical complications. Figure 2 and eTable 2 in Supplement 2 summarize the incidence of different types of complications in the 2 study arms. More detailed information on complication type is provided in the eTable 3 in Supplement 2.
Figure 2. Complications Within 30 Days After Surgery.
Complications in the intention-to-treat population (n = 251) are reported as percentage of patients having at least 1 complication, a severe complication (Comprehensive Complication Index score >20), at least 1 medical or surgical complication, and having at least 1 medical and 1 surgical complication.
aP < .05.
Functional Walking Capacity
The mean difference in 6-MWD between the 2 arms 4 weeks postoperatively after correcting for baseline was 15.6 m (95% CI, −1.4 to 32.6; P = .07; Table 2).
Table 2. Six-Minute Walking Distance (6-MWD) Test Assessed 4 Weeks After Surgerya.
| 6-MWD | No. (%) | P value | |
|---|---|---|---|
| Prehabilitation group (n = 123) | Control group (n = 128) | ||
| Median (IQR), m | 536 (470 to 603) | 514 (428 to 568) | .06 |
| Change from baseline to median (IQR), m | 17.5 (−18.0 to 48) | −5.0 (−38 to 34) | |
| Missing | 38 (30.9) | 45 (35.2) | NA |
| Difference from baseline ≥20 m | 39 (31.7) | 27 (21.1) | .05 |
| >400 m 4 wk After surgery | 78 (63.4) | 67 (52.3) | .07 |
Abbreviation: NA, not applicable.
Intention-to-treat population: n = 251.
Secondary Outcomes
Surgical Outcomes
There was no difference in length of hospital stay (median [IQR], 3 [3-5] days in prehabilitation vs 3 [3-4] days in standard care; P = .20) or hospital readmission rate (prehabilitation group: 4 of 123 [3.3%]; standard care group: 8 of 128 [6.3%]; OR, 0.50 [95% CI, 0.15-1.72]; P = .26). Admission to the intensive care unit was less frequent after prehabilitation (4 of 123 [3.3%] in the prehabilitation group vs 14 of 128 [10.9%] in the control group; OR, 0.29 [95% CI, 0.09-0.89]; P = .02). One participant in the prehabilitation group died within 30 days after surgery as a result of colorectal anastomotic leakage.
Functional Outcomes
Figure 3 and eTables 4 and 5 in Supplement 2 display the predetermined secondary functional outcomes over time. Overall, prehabilitation resulted in faster and better recovery of postoperative functional capacity. No statistical differences were found for either nutritional or mental status, nor for health-related quality of life.
Figure 3. Secondary Outcomes.
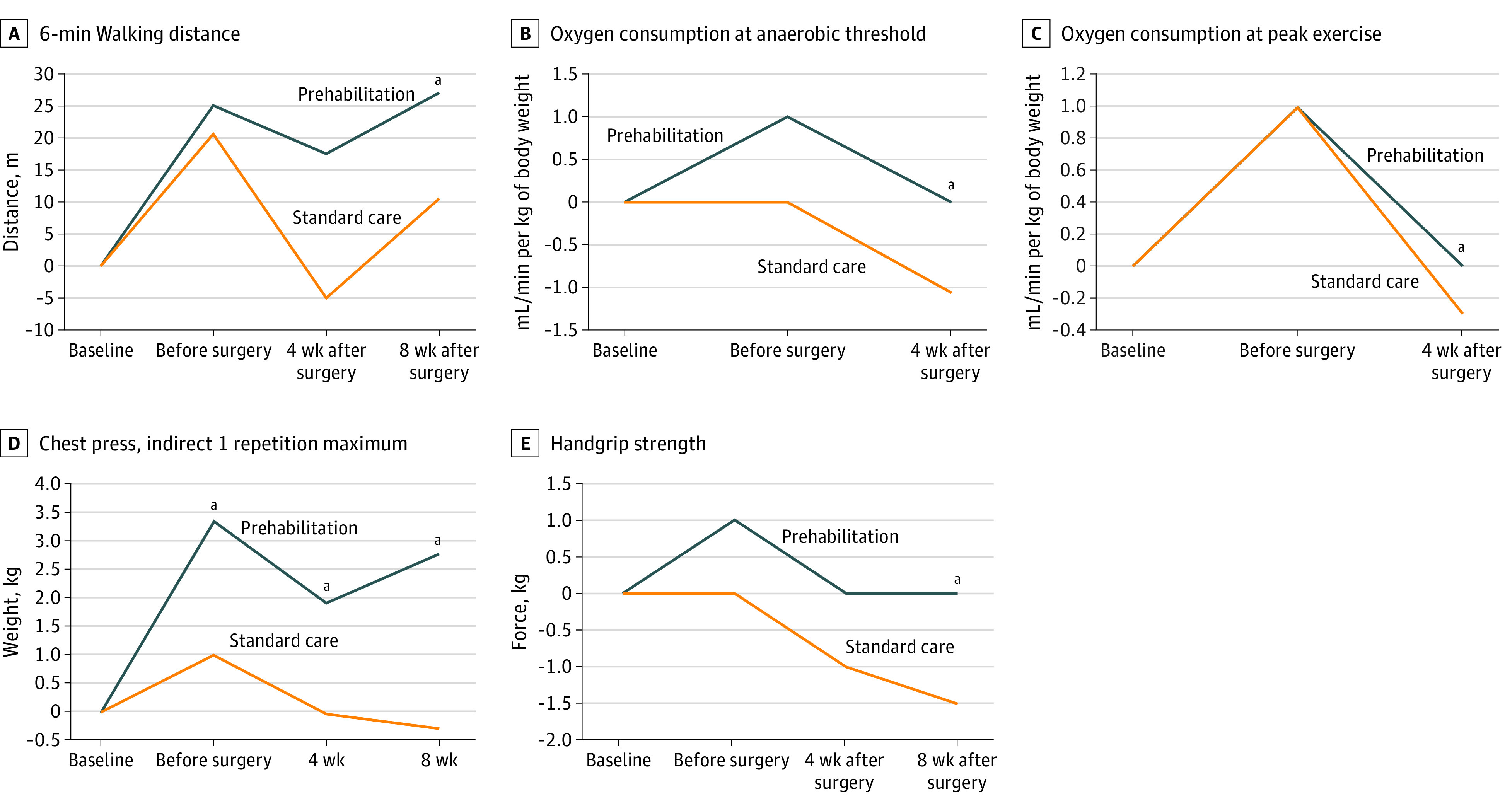
Secondary outcomes for intention-to-treat population (n = 251) are reported. Median change from baseline is reported.
aP < .05 for annotated time point.
Preplanned sensitivity and per-protocol analyses are included in eTable 6 in Supplement 2. The generalized estimating equations analysis of the difference between groups in 6-MWD (corrected for baseline) after the missing not at random imputation confirmed the results of the main analyses.
Discussion
The findings of the present randomized clinical trial showed a reduced rate of severe and medical complications following a 4-week multimodal prehabilitation program. Postoperative walking capacity, 1 RM, CPET, and handgrip strength were greater in the prehabilitation group.
For decades, research has focused on interventions to lower postoperative complications. Prehabilitation aims to increase functional capacity and fasten postoperative recovery. Two recently published randomized clinical trials describe a 50% reduction of complications after prehabilitation in high-risk patients undergoing major abdominal and colorectal surgery.10,33 Meanwhile, other trials studying both unimodal and multimodal prehabilitation programs for colorectal (cancer) surgery did not find any differences regarding complication rate.34,35,36 Such discrepancy might be explained by the low sample size studied and heterogeneous design.
In the present study, the number of severe complications (CCI score >20 considered clinically meaningful) dropped by almost 50% after prehabilitation.22,29 A reduction of medical complications was also reported by others and it aligns with the hypothesis that prehabilitation especially addresses the cardiorespiratory fitness and metabolic balance.37 However, the exact mechanisms are unclear.
Improved cardiorespiratory fitness before surgery is believed to increase physiological reserve and the patient’s resilience to withstand any stress related to surgery.38 Furthermore, higher functional capacity is independently associated with a lower risk of developing postoperative complications in patients with colorectal cancer.29,38,39 While other research groups demonstrated improved 6-MWD or CPET parameters in the preoperative period due to prehabilitation,15,33 the current study only showed a nonsignificant improvement in anaerobic threshold.
An important aim of prehabilitation is to improve functional capacity to enhance postoperative recovery. Three randomized clinical trials containing a similar multimodal prehabilitation program were included in a recently published Cochrane review.20 The review concluded that the data were heterogeneous. However, multimodal prehabilitation may result in an improved functional capacity both preoperatively and postoperatively with mean differences exceeding the clinically meaningful 20-m cutoff level determined with the 6-MWT.32
The current study results show an enhanced recovery of functional capacity in the postoperative period. Although not significant, mean walking distance 4 weeks postoperatively remained above baseline level in the prehabilitation group but seemed to drop beneath baseline results in the control group. The number of patients improving at least 20 m compared with baseline was higher after multimodal prehabilitation. During the assessment 8 weeks after surgery, 6-MWD differed statistically between groups, in favor of prehabilitation.
The V̇O2AT and peak V̇O2 values significantly differed between groups 4 weeks after surgery favoring the prehabilitation group. While the prehabilitation group improved on their baseline test results, the control group did not. In addition, indirect 1 RM results of the chest press and handgrip strength also differed significantly between groups considering the change from baseline during assessment 8 weeks after surgery. All in all, prehabilitation leads to faster recovery to baseline, even exceeding baseline values and this was not seen in the control patients.
To our knowledge, the current study is the largest RCT on multimodal prehabilitation for colorectal cancer surgery thus far. The high-intensity supervised in-hospital prehabilitation program is deemed safe, as only 5.7% minor adverse events and no serious adverse events occurred. Mean CCI score and length of hospital stay were relatively low in our population, perhaps partially explained by the large number of right colectomies (49.8%) and the fact that nearly all patients received minimal invasive surgery. The low overall mean CCI score contrasts with other studies.33,35,36 The interobserver variability in reporting CCI score can account for some of the CCI score differences. Length of hospital stay was also considerably lower compared with another study.36 The participating hospitals had ERAS protocols in place for several years and continuously assessed and improved aspects of perioperative care. Implementation of ERAS has substantially improved postoperative outcomes in these hospitals over the past decades. While ERAS interventions mainly focus on perioperative and postoperative factors, prehabilitation is an intuitive addition to this approach.
Limitations
The findings of the current trial should be interpreted with caution. The main limitation is the fact that the prespecified sample size was not reached. Consequently, lack of power may distort the effect of prehabilitation on our primary and main secondary outcomes. Unfortunately, due to the COVID-19 pandemic, trial completion was deemed unfeasible within a reasonable period. It was deemed unsafe for patients to have frequent in-hospital sessions. Therefore, we decided to terminate the study prematurely. Adjusting the protocol to a home-based program was not an option as a large heterogeneity regarding the interventions was anticipated. Additionally, study populations were likely different compared with the pre–COVID-19 period since patients became more inactive due to government restrictions. A delay in treatment as a result of downscaling non–COVID-19 health care might also have resulted in more advanced tumors.40 We did not include an unforeseen circumstances scenario in our study protocol, perhaps a learning point for future trial initiators.
Another limitation is the open-label design of the trial. Participants and the outcome assessors (except for the CCI score) were not blinded, partially due to the nature of the interventions. Risk of performance bias can therefore not be excluded. Finally, missing values were more frequent than anticipated for the coprimary and secondary outcomes, although secondary analyses with imputation confirmed the results of the main analyses.
Conclusions
A 4-week in-hospital multimodal prehabilitation program for patients undergoing elective resection of nonmetastasized colorectal cancer decreased the number of severe and medical postoperative complications and resulted in an enhanced functional recovery after surgery.
Trial protocol and statistical analysis plan
eAppendix. Participating Sites
eTable 1. Overview of Assessments
eTable 2. 30-Days Postoperative Complications
eTable 3. 30-Days Postoperative Complications Specified
eTable 4. Secondary Functional Outcomes
eTable 5. Results of the GEE Analysis
eTable 6. Sensitivity and Per-Protocol Analyses
Nonauthor Collaborators. The PREHAB Study Group nonauthor collaborators
Data sharing statement
References
- 1.Perera SK, Jacob S, Wilson BE, et al. Global demand for cancer surgery and an estimate of the optimal surgical and anaesthesia workforce between 2018 and 2040: a population-based modelling study. Lancet Oncol. 2021;22(2):182-189. doi: 10.1016/S1470-2045(20)30675-6 [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.Sharp SP, Malizia R, Skancke M, et al. A NSQIP analysis of trends in surgical outcomes for rectal cancer: what can we improve upon? Am J Surg. 2020;220(2):401-407. doi: 10.1016/j.amjsurg.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg. 2004;199(5):762-772. doi: 10.1016/j.jamcollsurg.2004.05.280 [DOI] [PubMed] [Google Scholar]
- 5.Stabenau HF, Becher RD, Gahbauer EA, Leo-Summers L, Allore HG, Gill TM. Functional trajectories before and after major surgery in older adults. Ann Surg. 2018;268(6):911-917. doi: 10.1097/SLA.0000000000002659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steffens D, Ismail H, Denehy L, et al. Preoperative cardiopulmonary exercise test associated with postoperative outcomes in patients undergoing cancer surgery: a systematic review and meta-analyses. Ann Surg Oncol. 2021;28(12):7120-7146. doi: 10.1245/s10434-021-10251-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wischmeyer PE, Carli F, Evans DC, et al. ; Perioperative Quality Initiative (POQI) 2 Workgroup . American society for enhanced recovery and perioperative quality initiative joint consensus statement on nutrition screening and therapy within a surgical enhanced recovery pathway. Anesth Analg. 2018;126(6):1883-1895. doi: 10.1213/ANE.0000000000002743 [DOI] [PubMed] [Google Scholar]
- 8.Molenaar CJL, Winter DC, Slooter GD. Contradictory guidelines for colorectal cancer treatment intervals. Lancet Oncol. 2021;22(2):167-168. doi: 10.1016/S1470-2045(20)30738-5 [DOI] [PubMed] [Google Scholar]
- 9.Molenaar CJL, Janssen L, van der Peet DL, Winter DC, Roumen RMH, Slooter GD. Conflicting guidelines: a systematic review on the proper interval for colorectal cancer treatment. World J Surg. 2021;45(7):2235-2250. doi: 10.1007/s00268-021-06075-7 [DOI] [PubMed] [Google Scholar]
- 10.Barberan-Garcia A, Ubré M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg. 2018;267(1):50-56. doi: 10.1097/SLA.0000000000002293 [DOI] [PubMed] [Google Scholar]
- 11.Barker LA, Gray C, Wilson L, Thomson BNJ, Shedda S, Crowe TC. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: a randomised controlled trial. Eur J Clin Nutr. 2013;67(8):802-807. doi: 10.1038/ejcn.2013.117 [DOI] [PubMed] [Google Scholar]
- 12.Bousquet-Dion G, Awasthi R, Loiselle SÈ, et al. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncol. 2018;57(6):849-859. doi: 10.1080/0284186X.2017.1423180 [DOI] [PubMed] [Google Scholar]
- 13.Burden ST, Gibson DJ, Lal S, et al. Pre-operative oral nutritional supplementation with dietary advice versus dietary advice alone in weight-losing patients with colorectal cancer: single-blind randomized controlled trial. J Cachexia Sarcopenia Muscle. 2017;8(3):437-446. doi: 10.1002/jcsm.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937-947. doi: 10.1097/ALN.0000000000000393 [DOI] [PubMed] [Google Scholar]
- 15.Minnella EM, Ferreira V, Awasthi R, et al. Effect of two different pre-operative exercise training regimens before colorectal surgery on functional capacity: a randomised controlled trial. Eur J Anaesthesiol. 2020;37(11):969-978. doi: 10.1097/EJA.0000000000001215 [DOI] [PubMed] [Google Scholar]
- 16.Polakowski CB, Kato M, Preti VB, Schieferdecker MEM, Ligocki Campos AC. Impact of the preoperative use of synbiotics in colorectal cancer patients: a prospective, randomized, double-blind, placebo-controlled study. Nutrition. 2019;58:40-46. doi: 10.1016/j.nut.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 17.Bolshinsky V, Li MH, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Dis Colon Rectum. 2018;61(1):124-138. doi: 10.1097/DCR.0000000000000987 [DOI] [PubMed] [Google Scholar]
- 18.Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160(5):1189-1201. doi: 10.1016/j.surg.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 19.Perry R, Herbert G, Atkinson C, et al. Pre-admission interventions (prehabilitation) to improve outcome after major elective surgery: a systematic review and meta-analysis. BMJ Open. 2021;11(9):e050806. doi: 10.1136/bmjopen-2021-050806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molenaar CJL, van Rooijen SJ, Fokkenrood HJ, Roumen RMH, Janssen L, Slooter GD. Prehabilitation versus no prehabilitation to improve functional capacity, reduce postoperative complications and improve quality of life in colorectal cancer surgery. Cochrane Database Syst Rev. 2022;5(5):CD013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS®) society recommendations: 2018. World J Surg. 2019;43(3):659-695. doi: 10.1007/s00268-018-4844-y [DOI] [PubMed] [Google Scholar]
- 22.van Rooijen S, Carli F, Dalton S, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. 2019;19(1):98. doi: 10.1186/s12885-018-5232-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Equator Network. Accessed October 9, 2022. https://www.equator-network.org/reporting-guidelines/consort/
- 24.Levett DZH, Jack S, Swart M, et al. ; Perioperative Exercise Testing and Training Society (POETTS) . Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. 2018;120(3):484-500. doi: 10.1016/j.bja.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 25.Research Manager software. Deventer. Accessed October 9, 2022. https://my-researchmanager.com
- 26.Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. Eur J Surg Oncol. 2018;44(7):919-926. doi: 10.1016/j.ejso.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 27.Fearon KCH, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466-477. doi: 10.1016/j.clnu.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 28.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1-7. doi: 10.1097/SLA.0b013e318296c732 [DOI] [PubMed] [Google Scholar]
- 29.Minnella EM, Liberman AS, Charlebois P, et al. The impact of improved functional capacity before surgery on postoperative complications: a study in colorectal cancer. Acta Oncol. 2019;58(5):573-578. doi: 10.1080/0284186X.2018.1557343 [DOI] [PubMed] [Google Scholar]
- 30.Pecorelli N, Fiore JF Jr, Gillis C, et al. The six-minute walk test as a measure of postoperative recovery after colorectal resection: further examination of its measurement properties. Surg Endosc. 2016;30(6):2199-2206. doi: 10.1007/s00464-015-4478-1 [DOI] [PubMed] [Google Scholar]
- 31.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 32.Thompson KL, Elliott L, Fuchs-Tarlovsky V, Levin RM, Voss AC, Piemonte T. Oncology evidence-based nutrition practice guideline for adults. J Acad Nutr Diet. 2017;117(2):297-310.e47. doi: 10.1016/j.jand.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 33.Berkel AEM, Bongers BC, Kotte H, et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg. 2022;275(2):e299-e306. doi: 10.1097/SLA.0000000000004702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carli F, Bousquet-Dion G, Awasthi R, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. 2020;155(3):233-242. doi: 10.1001/jamasurg.2019.5474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gloor S, Misirlic M, Frei-Lanter C, et al. Prehabilitation in patients undergoing colorectal surgery fails to confer reduction in overall morbidity: results of a single-center, blinded, randomized controlled trial. Langenbecks Arch Surg. 2022;407(3):897-907. doi: 10.1007/s00423-022-02449-0 [DOI] [PubMed] [Google Scholar]
- 36.Onerup A, Andersson J, Angenete E, et al. Effect of short-term homebased pre- and postoperative exercise on recovery after colorectal cancer surgery (PHYSSURG-C): a randomized clinical trial. Ann Surg. 2022;275(3):448-455. doi: 10.1097/SLA.0000000000004901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg. 2020;24(6):1375-1385. doi: 10.1007/s11605-019-04287-w [DOI] [PubMed] [Google Scholar]
- 38.Heldens AFJM, Bongers BC, Lenssen AF, Stassen LPS, Buhre WF, van Meeteren NLU. The association between performance parameters of physical fitness and postoperative outcomes in patients undergoing colorectal surgery: an evaluation of care data. Eur J Surg Oncol. 2017;43(11):2084-2092. doi: 10.1016/j.ejso.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 39.Cuijpers ACM, Heldens AFJM, Bours MJL, et al. Relation between preoperative aerobic fitness estimated by steep ramp test performance and postoperative morbidity in colorectal cancer surgery: prospective observational study. Br J Surg. 2022;109(2):155-159. doi: 10.1093/bjs/znab292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silver JK, Santa Mina D, Bates A, et al. Physical and psychological health behavior changes during the COVID-19 pandemic that may inform surgical prehabilitation: a narrative review. Curr Anesthesiol Rep. 2022;12(1):109-124. doi: 10.1007/s40140-022-00520-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eAppendix. Participating Sites
eTable 1. Overview of Assessments
eTable 2. 30-Days Postoperative Complications
eTable 3. 30-Days Postoperative Complications Specified
eTable 4. Secondary Functional Outcomes
eTable 5. Results of the GEE Analysis
eTable 6. Sensitivity and Per-Protocol Analyses
Nonauthor Collaborators. The PREHAB Study Group nonauthor collaborators
Data sharing statement