Abstract
Introduction:
Chondrocytes perceive and respond to mechanical loading as a signal to regulate their metabolic activity. Joint loading exposes chondrocytes to multiple modes of mechanical stress, including hydrostatic pressure; however, the mechanisms by which chondrocytes sense physiologically-relevant levels of hydrostatic pressure are not well understood. We hypothesized that hydrostatic pressure is transduced to an intracellular signal through mechanosensitive ion channels on the membrane of chondrocytes. The goals of this study were to examine the effect of hydrostatic loading on the development of engineered cartilage tissue and the contribution of mechanosensitive ion channels on these hydrostatic loading effects.
Methods:
Using a 3D model of porcine chondrocytes in agarose, we applied specific chemical inhibitors to determine the role of transient receptor potential (TRP) ion channels TRPV1, TRPV4, TRPC3, and TRPC1 in transducing hydrostatic pressure.
Results:
Hydrostatic loading caused a frequency and magnitude-dependent decrease in sulfated glycosaminoglycans (S-GAG), without changes in DNA content. Inhibiting TRPC3 and TRPV4 decreased S-GAG content; however, only the inhibition of TRPV1 partially attenuated the hydrostatic loading-induced reduction in S-GAG content.
Conclusions:
Our findings indicate that TRPV1 may serve as a transducer of hydrostatic pressure in chondrocytes, and provide further support the role of TRPV4 in regulating chondrocyte anabolism, as well as initial evidence of a role for TRPC3 in chondrogenesis. These findings add to our further understanding of the chondrocyte “channelome” and suggests that a range of ion channels are responsible for mediating the transduction of different biophysical stimuli such as hydrostatic pressure, membrane stretch, or osmotic stress.
Keywords: mechanobiology, osteoarthritis, tissue engineering, capsaicin
Introduction
Osteoarthritis (OA) is a multifactorial chronic disease of multiple origins, characterized by the irreversible degradation of the articular cartilage, as well as pathologic changes in other joint tissues. The precise etiology of OA initiation and progression are not well understood, but the role of excessive and traumatic mechanical forces are implicated in OA pathogenesis due to the early onset of OA upon traumatic joint injuries1,2. Under homeostatic conditions, articular cartilage undergoes millions of cycles of deformational mechanical loading every year. This tissue-scale deformational loading is transduced into distinct mechanical signals that are perceived by the chondrocytes residing within the cartilage, including direct compressive, tensile, and shear strains, as well as osmotic, electrostatic, and hydrostatic pressures that result from the mechanical deformation of the charged and hydrated cartilage tissue. While these different loading conditions are believed to be essential for tissue maintenance3, the specific mechanotransduction mechanisms regulating each of these stimuli, and their roles in chondrocyte homeostasis remain to be determined.
Hydrostatic pressure is one of the critical mechanisms involved in the ability of articular cartilage to withstand high magnitudes of joint loading while allowing for extremely low coefficients of friction and limited tissue deformation4–6. During loading, the low hydraulic permeability of the cartilage tissue prevents the rapid loss of water from the tissue, resulting in the great majority of the applied load to be supported by the high water content present in the tissue, rather than being supported by the solid extracellular matrix5–7. This phenomenon increases in the hydrostatic pressure throughout the tissue, including the chondrocytes8. This pressure is subsequently lost upon the removal of the load, in effect creating a dynamic hydrostatic loading environment present during normal locomotion9,10. Importantly, the role of hydrostatic pressure as a physical factor in modulating chondrocyte physiology can be isolated for study, because physiological levels of hydrostatic pressure can be applied in a uniform manner without inducing confounding physical factors such as fluid flow, electrokinetic effects, or cyclic cell deformation, due to the incompressibility of interstitial water and the extracellular matrix11.
A number of in vitro studies have examined the effects of hydrostatic loading to cartilage tissue explants, isolated chondrocytes, and engineered cartilage tissue systems on cartilage biology as well as stem cell chondrogenesis12–16. In efforts to mimic the dynamic hydrostatic pressure conditions which might be experienced by chondrocytes, numerous studies have evaluated the effect of static or dynamic pressures at a range of amplitudes and frequencies. For primary chondrocytes, a wide range of catabolic and anabolic responses (or no response) have been observed under different hydrostatic pressure loading regimens17. Dynamic hydrostatic pressure was shown to increase the expression of cartilage matrix genes COL2A1 (type II collagen) and ACAN (aggrecan), and the critical chondrogenesis transcription factor SOX9 but had no effect on the expression of type I collagen, suggesting the application of hydrostatic loading specifically alters matrix gene programs18,19. Interestingly, the amplitude of hydrostatic pressure plays an important role in chondrocyte matrix production in the physiologic to supraphysiologic ranges of 2.5 MPa to 50 MPa20. These studies demonstrate the complex role that hydrostatic pressure may exert on chondrocyte physiology, while underscoring lack of detailed knowledge on the mechanisms of chondrocyte mechano-signaling.
Chondrocytes respond to mechanical loading through an array of mechanically-sensitive ion channels and receptors but understanding the contexts and activation modes for many of these mechanosensitive constituents at the molecular scale remain unresolved. Our overarching goal is to understand how mechanical loading of cartilage is deconstructed into distinct mechanical mechanisms which then act on particular mechanically-sensitive ion channels to enact and provoke unique mechano-signaling pathways that influence cartilage development, homeostasis, and disease. Chondrocytes possess a number of mechanically-sensitive ion channels include the Transient Receptor Potential (TRP) family including members TRPV1, TRPV4, TRPC1, TRPC3, TRPC6, and TRPM7 as well as the recently discovered Piezo ion channel family members PEIZO1 and PIEZO221-35. To date, however, the mechanisms by which chondrocytes transduce dynamic hydrostatic pressure to an intracellular signal remain to be determined. Elucidating the role of these mechanosensitive proteins and/or organelles in the role of hydrostatic pressure-induced chondrocyte mechanotransduction is an important step towards gaining a mechanistic understanding of cartilage physiology and pathology.
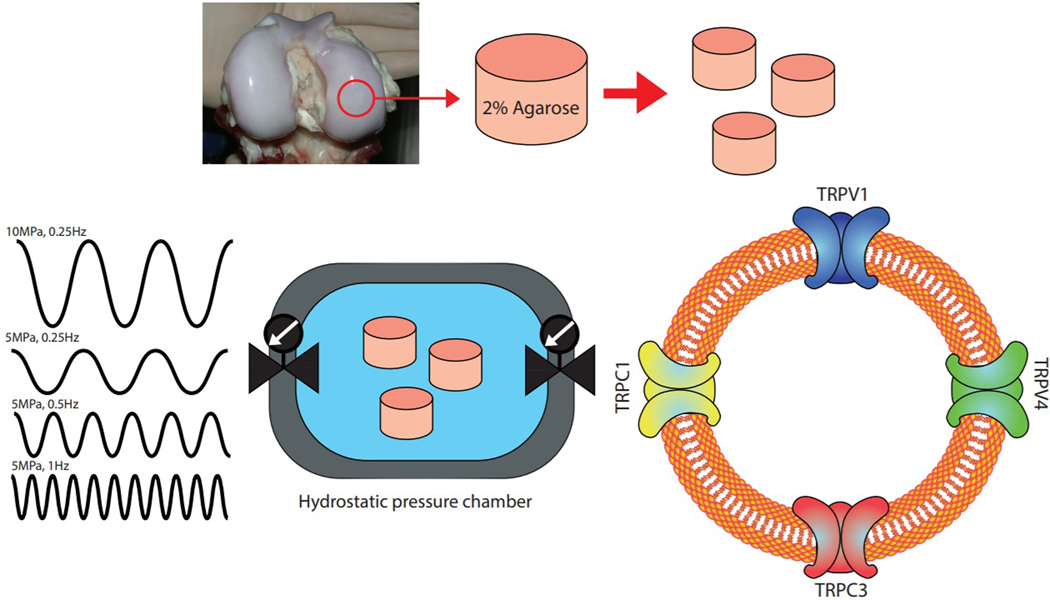
The aim of this study was to investigate the role of several of the TRP family of mechanically-sensitive ion channels in hydrostatic pressure-induced chondrocyte mechanobiology. Using engineered cartilage tissue constructs we first identified the role of dynamic hydrostatic pressure frequency and amplitude on the biosynthesis of sulfated glycosaminoglycans (S-GAG), an important structural molecule synthesized in high levels and throughout life in native cartilage. Engineered cartilage constructs were cultured under hydrostatic loading in the presence or absence of inhibitors of the ion channels TRPV1, TRPV4, TRPC3, and TRPC1 using pharmacologic inhibitors to establish the role of these mechanosensitive factors in hydrostatic pressure-induced S-GAG biosynthesis (Fig. 1).
Figure 1. Schematic of the experimental set up.
Chondrocytes from porcine cartilage were digested and cast into an agarose scaffold to create engineered cartilage constructs. Constructs were loaded in the hydrostatic pressure chamber and tested under different loading regimens and specific TRP channel inhibitors.
Materials and Methods
This study was performed in two sequential experiments. In experiment 1 we characterized the response to tissue engineered cartilage cast using porcine chondrocytes to different amplitudes and frequencies of hydrostatic pressure loading to identify loading regimens that alter chondrocyte metabolic activity and S-GAG accumulation in our engineered cartilage system. In experiment 2 we use the loading regimen identified from experiment 1 to test the role of several putative mechanosensitive ion channels in mediating the hydrostatic loading response using specific chemical inhibitors. For each experiment, a single batch of constructs was tested.
Porcine chondrocyte isolation, tissue-construct casting, and culture
The articular cartilage of porcine stifle joints was harvested on the day of the slaughter and kept in complete media at 37 °C and 5% CO2 for 2 days. Complete media was formulated with high-glucose Dulbecco’s Modified Eagle Media (Gibco), 10% fetal bovine serum (Atlas), 1.5% HEPES (Gibco), 1% penicillin-streptomycin (Gibco), and 1% non-essential amino acid (Gibco). Articular cartilage was digested for 16 h with collagenase (type IV, Sigma) at 37 °C. Cells were isolated by straining through 70 μm filters, followed by counting, centrifuging and washing, and resuspending to achieve a concentration of 60 × 106 cells/ml or 120 × 106 cells/ml before encapsulating in molten agarose (4%, type VIIA, Sigma) and casting to a thickness of 2.34 mm. Cylindrical constructs were punched to create ⊘3 mm × 2.34 mm constructs with a nominal final concentration of 30 or 60 × 106 cells/ml in 2% agarose. Constructs were cultured in complete media supplemented with 50 μg/mL L-ascorbic acid 2-phosphate sesquimagnesium salt and 40 μg/mL L-proline (Sigma) for the duration of the study.
Hydrostatic pressure loading
Hydrostatic pressure loading was performed using a Barozyme HT48 (Pressure Biosciences, South Easton, MA). Samples were loaded into individual tubes and placed in the hydrostatic pressure loading chamber for daily loading per manufacturer’s protocols. Each tube contained 1.5 mL media and was loaded with 6 samples for a daily media supply of ~0.3 mL/million cells/day36. Samples were maintained at 37 °C and loaded for 3 h daily, 5 times per week. In experiment 1, the effects of seeding density (30 or 60 × 106 cells/ml) in engineered cartilage constructs were studied using the following hydrostatic pressure regimens: (a) 0 MPa (control), (b) 5 MPa amplitude, 0.25 Hz, (c) 5 MPa amplitude, 0.5 Hz, (d) 5 MPa amplitude, 1 Hz, and (e) 10 MPa amplitude, 0.25 Hz. Based on the results of experiment 1, the inhibitor studies of experiment 2 were treated with either 0 MPa as an unloaded control regime and 5 MPa at 0.5 Hz as a hydrostatic loading regime. For both sets of experiments (1 and 2), unloaded controls were treated in the same manner as the loaded groups but were not subjected to hydrostatic pressure.
Ion channels inhibitor treatments
To test the role of mechanically-sensitive ion channels to the hydrostatic pressure, we inhibited TRPV4, TRPV1, TRPC3 and TRPC1 cation channels using the selective inhibitors GSK205 (synthesized at the Duke Chemical Synthesis Facility, 10 μM), A 784168 (Tocris Bioscience, 25 nM), Pyr3 (Tocris Bioscience, 3 μM), and MRS 1845 (Tocris Bioscience, 10 μM)29,37–40. Inhibitors were added 15 minutes prior to loading, and constructs were returned to base media after loading. Vehicle controls for each inhibitor were made using either deionized water or dimethyl sulfoxide (DMSO), based on the solvent necessary for inhibitor reconstitution. Exposure to neither the inhibitors nor vehicle induced cell death (Supplemental Fig. 1). Due to the small size of the inhibitors (< 500 Da) and small tissue construct size, we supplemented inhibitors 15 minutes prior to loading which we anticipate is sufficient time for inhibitor transport within the construct.
Biochemical analysis
The wet weight of each construct was recorded for normalizing biochemical content measures and constructs were frozen at −20 °C until digestion. To perform biochemical assays on the engineered cartilage tissues, samples were digested in proteinase K for 16 h at 56 °C. After digestion, the remaining, undigested, agarose scaffold was pulverized and vortexed. To quantify the total amount of S-GAGs, the dimethyl methylene blue assay was performed with standards prepared from shark chondroitin sulfate (Sigma)41. The same assay was used to measure the amount of S-GAG release into the medium after applying the hydrostatic pressure loading. To measure cellularity of each construct, the PicoGreen fluorescent double-stranded DNA assay was used based on manufacturer’s directions (Invitrogen).
Data analysis
Biochemical contents were normalized to tissue wet weight (converted to DNA content in μg/g tissue or S-GAG content as a mass per wet weight as a percentage, %ww) or normalized for a S-GAG content per cell (μg S-GAG per μg DNA, μg/μg) prior to analysis. For statistical analysis of the influence of different hydrostatic pressure regimens in experiment 1, a one-way ANOVA was used (α=0.05) with each loading regimen constituting an independent factor. For analyzing the effect of hydrostatic loading and inhibitors in experiment 2, a two-way ANOVA (α=0.05) was used where the effect of hydrostatic loading was one independent factor and each inhibitor was a separate independent factor. For S-GAG release, as all the constructs of each group shared the same media, we performed our calculations using standard uncertainty analysis to derive the S-GAG synthesis rate in units of μg S-GAG/d/construct. To determine this rate, regression curves were fit to cumulative μg S-GAG/sample over the time of the study. The regression of each groups provides a slope (representing the rate of S-GAG media loss in units of μg/d /construct), error (standard deviation of this rate), and degrees of freedom. Similarly, the S-GAG content within the constructs at the final time point can be used to estimate an S-GAG accumulation rate also in units of μg/d/construct. Here, we assumed a linear increase in S-GAG accumulation for each construct from day 0 to day 28 and similarly calculated a mean accumulation rate, uncertainty, and degrees of freedom. Summing the S-GAG loss rate (as measured in the media) and S-GAG accumulation rate (as measured in the construct) provides an estimate of total S-GAG synthesis. Uncertainty propagation analysis provides an estimate of the variability of the total synthesis rate. A retention ratio was further computed as the S-GAG accumulation rate divided by the total S-GAG synthesis rate. t-test comparison between each loaded group to the control (0 MPa) and a Bonferroni p-value correction was used to assess groups significantly different than the control. For experimental reasons, select inhibitor treatments were run alongside a single vehicle control group. As our overall objective focused on detecting the effect of hydrostatic loading, the effect the select inhibitor, and the effect of their interaction, our analyses only compared an individual inhibitor treatment and its appropriate vehicle control. Therefore, several comparisons made herein share the same vehicle data.
Results
Experiment 1: Mechanical loading regimens influence S-GAG production
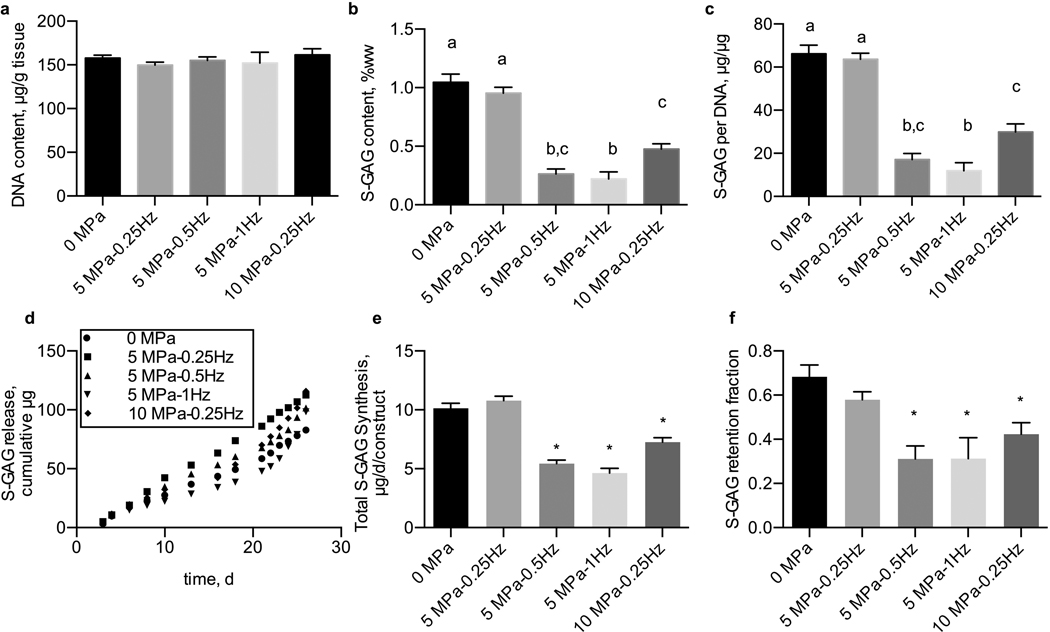
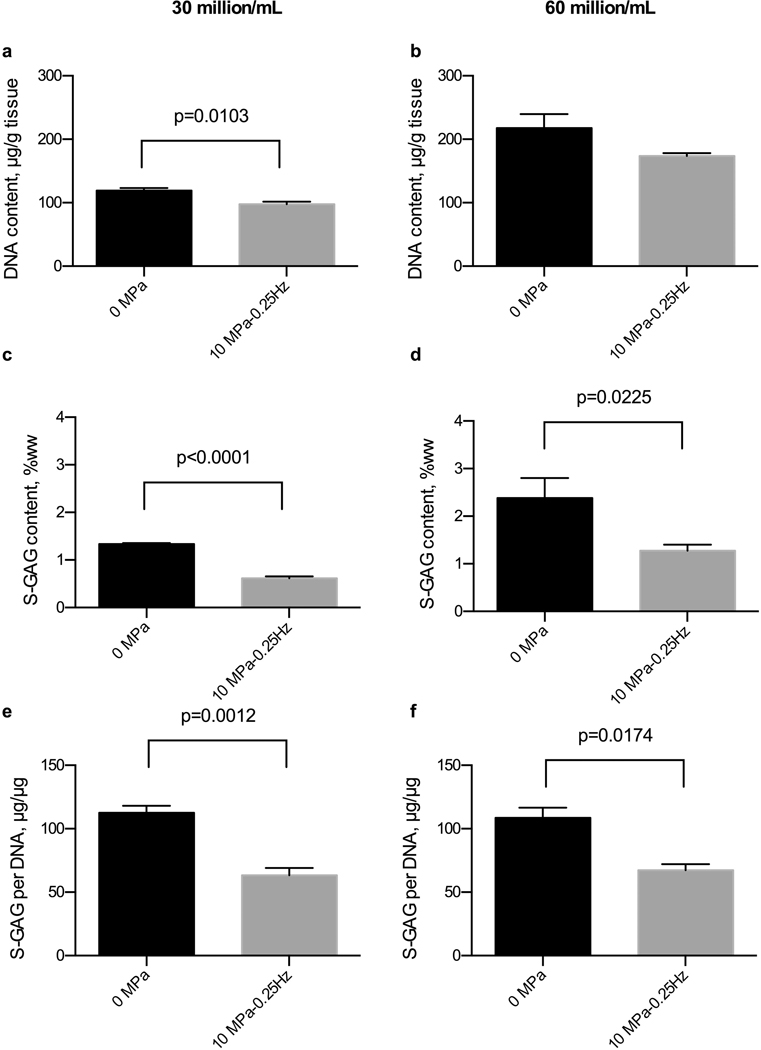
None of hydrostatic pressure regimens significantly influenced the DNA content of tissue constructs (p=0.751) over a long-term, 28-day culture duration (Fig. 2a). The hydrostatic loading regimen did alter S-GAG biosynthesis and accumulation in tissue engineered constructs as measured by the S-GAG content within the tissue constructs (p<0.0001, Fig. 2b) and S-GAG content normalized to the DNA content of each construct (p<0.0001, Fig. 2c). In particular, applying 5 MPa amplitude of hydrostatic loading induced a significant reduction of S-GAG content at frequencies of 0.5 and 1 Hz, but no difference in S-GAG content at 0.25 Hz when compared to unloaded, 0 MPa applied, constructs. These trends were also evident with Safrinin-O staining of histological sections of constructs after 28 days of loading (Supplemental Fig. 2). Also, the lowest amount of total S-GAG (amount released into the medium + construct) and retention fraction was observed in the constructs that were loaded with 5MPa amplitude and the frequencies of 0.5 and 1 Hz (Fig. 2d, 2e). Moreover, the trend of S-GAG release into the medium at different time points showed that by increasing the culture duration, the amount of S-GAG diffusion into the medium will increase (Fig. 2f). Applying a higher loading amplitude, 10 MPa, at 0.25 Hz, also demonstrated a decrease in tissue construct S-GAG content compared to control, unloaded tissue constructs. Similar trends were observed in constructs seeded with 30 or 60 million cells per mL (Fig. 3). Importantly, we did not observe cell death due to hydrostatic loading (Supplemental Fig. 3). Therefore, based on our results from experiment 1, we chose a hydrostatic pressure regimen of 5 MPa at 0.5 Hz in constructs seeded with 30 million cells per mL to proceed with the inhibitor screening of experiment 2.
Figure 2. Effects of dynamic hydrostatic pressure on DNA and S-GAG content in chondrocyte-seeded agarose constructs.
(a) DNA content of tissue engineered cartilage was unchanged by dynamic hydrostatic pressure, while (b) S-GAG content of tissue engineered cartilage and (c) S-GAG content normalized to DNA content were significantly reduced by hydrostatic pressure in a frequency and magnitude-dependent manner. (d) Amount of S-GAG release into the media as a function of culture duration (e) S-GAG retention fraction (f) Total S-GAG content synthesis. Groups not sharing a letter indicate statistically significant differences. n=5–6 per group. Groups with (*) are significantly different from the control (0 MPa).
Figure 3. Effects of cell density and dynamic hydrostatic pressure on DNA and S-GAG content in chondrocyte-seeded agarose constructs.
(a and b) DNA content of tissue engineered cartilage was similar between loading groups for both cell densities, while (c and d) S-GAG content of tissue engineered cartilage and (e and f) S-GAG content normalized to DNA content consistently reduced by hydrostatic loading for both 30 million (n=3–5) and 60 million cell densities (n=2–3).
Experiment 2: Role of mechanosensitive ion channels in transducing hydrostatic pressure
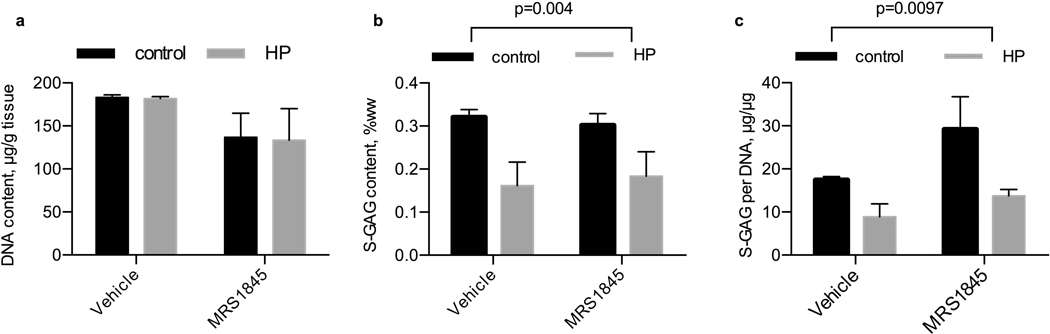
MRS1845 inhibition of TRPC1
We inhibited TRPC1 activity with the inhibitor MRS 1845 (Fig. 4). After 14 days of daily hydrostatic loading, DNA content was not significantly different due to pharmacologic treatment (p=0.055) or loading (p=0.92) while S-GAG content was only significantly lower with the application of hydrostatic loading (p=0.004). Similarly, when S-GAG content is normalized to DNA content as a measure of S-GAG production on a cellularity basis, only the influence of hydrostatic loading was significant (p=0.0097).
Figure 4. Influence of hydrostatic loading and TRPC1 inhibition with MRS 1845 on DNA and S-GAG content.
(a) DNA content per tissue wet weight, (b) S-GAG content per tissue wet weight, and (c) S-GAG content per DNA content in tissue engineered cartilage. Control, no pressure; HP, hydrostatic pressure, n=5–6 per group.
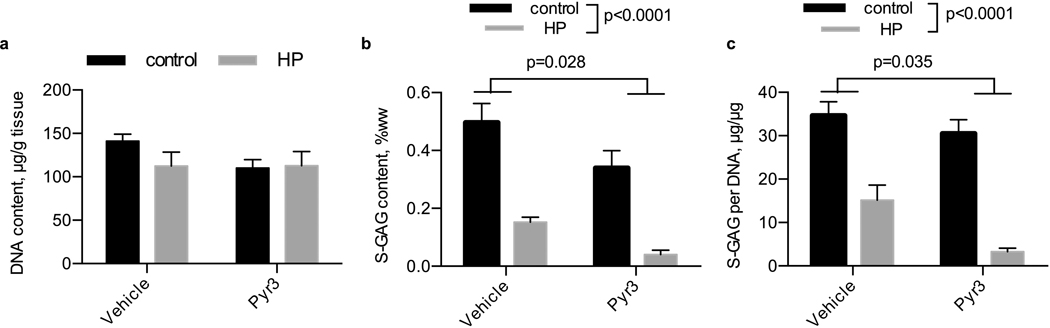
Pyr3 inhibition of TRPC3
We inhibited TRPC3 using the inhibitor Pyr3 (Fig. 5). After 14 days of daily hydrostatic loading, DNA content was similar between all groups. S-GAG content was significantly lower with the application of hydrostatic loading (p<0.0001) and was lower with the Pyr3 inhibitor (p=0.028). When normalized to DNA content, S-GAG content was similarly reduced with the application of hydrostatic loading (p<0.0001) and Pyr3 inhibitor (p=0.035), but the interaction was not significantly altered.
Figure 5. Influence of hydrostatic loading and TRPC3 inhibition with Pyr3 on DNA and S-GAG content.
(a) DNA content per tissue wet weight, (b) S-GAG content per tissue wet weight, and (c) S-GAG content per DNA content in tissue engineered cartilage. Control, no pressure; HP, hydrostatic pressure, n=3–5 per group.
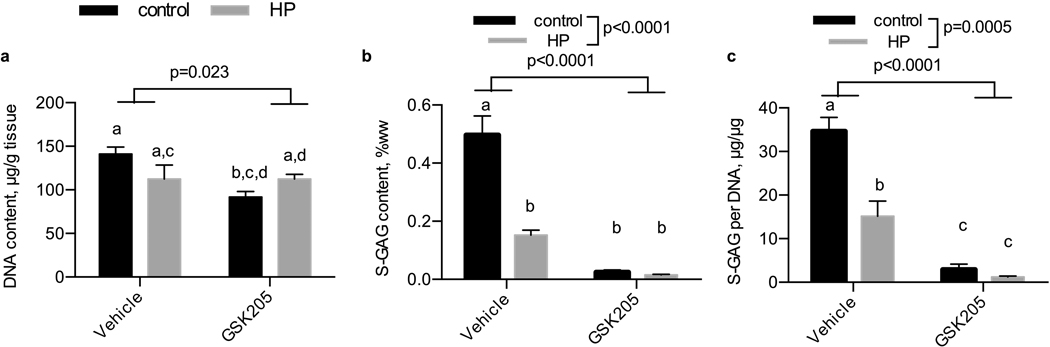
GSK205 inhibition of TRPV4
We inhibited TRPV4 using the inhibitor GSK205 (Fig. 6). After 14 days of daily hydrostatic loading, DNA was lower with the treatment of GSK205. S-GAG content was significantly lower with the application of hydrostatic loading (p<0.0001) and with the GSK205 inhibitor treatment (p<0.0001). Notably, while both the non-loaded and hydrostatic pressure treated samples supplemented with GSK205 were statistically similar (p=0.99), non-loaded and hydrostatic pressure loaded samples were statistically different (p<0.0001), consistent with the characterization from experiment 1. S-GAG content normalized to DNA content was reduced with the application of hydrostatic loading (p=0.0005) and GSK205 inhibitor (p<0.0001).
Figure 6. Influence of hydrostatic loading and TRPV4 inhibition with GSK205 on DNA and S-GAG content.
(a) DNA content per tissue wet weight, (b) S-GAG content per tissue wet weight, and (c) S-GAG content per DNA content in tissue engineered cartilage. Control, no pressure; HP, hydrostatic pressure, n=5 per group.
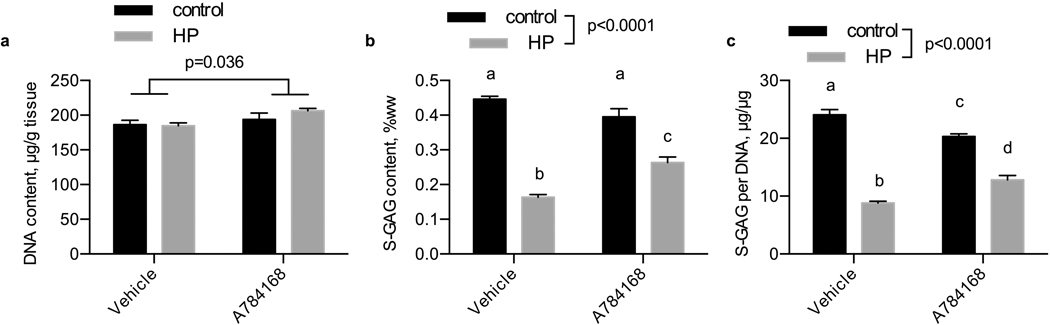
A 784168 inhibition of TRPV1
We used the inhibitor A 784168 to inhibit activity of TRPV1 (Fig. 7). After 14 days of daily hydrostatic loading, DNA was higher with the A 784168 treatment (p=0.036) but unaffected by loading (p=0.397). S-GAG content in engineered cartilage samples was significantly reduced by hydrostatic loading (p<0.0001) but was partially recovered by A 784168 treatment (p=0.136). When normalized to DNA content, engineered cartilage S-GAG content was reduced with the application of hydrostatic loading (p<0.0001) but not by A 784168 (p=0.864). By either measure of S-GAG content, the A 784168 treated group was significantly higher than the untreated group in response to hydrostatic pressure.
Figure 7. Influence of hydrostatic loading and TRPV1 inhibition with A784168 on DNA and S-GAG content.
(a) DNA content per tissue wet weight, (b) S-GAG content per tissue wet weight, and (c) S-GAG content per DNA content in tissue engineered cartilage. Control, no pressure; HP, hydrostatic pressure, n=4 per group.
Discussion
In this study, we analyze the influence of hydrostatic loading on tissue engineered cartilage growth and matrix production and the role of mechanically-sensitive TRP ion channels on this response. We found that increased amplitudes (5–10 MPa) and frequencies (0.5–1 Hz) of dynamic hydrostatic loading induced a consistent response by inhibiting matrix production in our tissue engineered constructs. As mechanically-sensitive ion channels have been shown to play important roles in chondrocyte mechanobiology, we targeted several mechanically-sensitive TRP ion channels to determine if they are responsible for transduction of the signals incurred during hydrostatic pressure loading. Of the various inhibitors we used to target different ion channels, only the inhibition of TRPV1 altered the response to hydrostatic pressure. Together our results suggest that the growth modulating effect caused by hydrostatic loading may be transduced via this channel in the chondrocyte and support the notion that different ion channels are responsible for transducing different biophysical stimuli such as hydrostatic pressure, membrane stretch, or osmotic stress.
Our results on differential HP loading regimes highlighted an interesting synthesis and retention behavior, where constructs exhibited similar S-GAG accumulation, total S-GAG synthesis, and S-GAG retention fraction characteristics. While we believe the mechanism governing differential matrix synthesis rates to be dependent on HP mechanotransduction to the cell, the finding that retention fractions are also differentially regulated by HP suggests other factors could be differentially regulated: (1) HP may also alter the synthesis of binding proteins or other molecules critical for S-GAG retention within the extracellular matrix of engineered cartilage, and/or (2) HP load may be modulating the cellular release of extracellular proteases or other molecules implicated in the loss of the extracellular matrix, and/or (3) HP load may be modulating a cellular-independent effect resulting in the loss of S-GAG, although this cellular-independent mechanism is less clear. Nims et al reported a similar level of S-GAG retention to our measurements here and speculated that S-GAG binding density may be a function of hyaluronan production into the extracellular matrix 42.
We found consistently that dynamic hydrostatic pressure inhibited S-GAG accumulation by chondrocytes in agarose; however, other studies found mixed results by applying the same loading magnitude that was used in this research depending on the duration. Parkkinen et al. also showed that in monolayer culture of bovine chondrocytes, applying the dynamic hydrostatic loading regimen that was used in our study (5MPa, 0.5Hz) for 1.5 h inhibits the S-GAG production as well, however, applying the same load to cartilage explant had the opposite effect43. Moreover, Jortikka et al. found that applying the aforementioned dynamic hydrostatic pressure for 20 h would significantly increase the S-GAG production in bovine chondrocytes in monolayer culture 44. Therefore, it appears that the duration of hydrostatic pressure loading and the culture system of chondrocytes (monolayer (2D) or hydrogel (3D, scaffolds, gels and pellet), and cartilage explants (3D)) have significant effects on the chondrocytes biosynthesis, biochemical properties and differentiation in response to hydrostatic pressure and the results can be completely different by changing any of these conditions18–20,43–60. For instance, collagen type II production seems to be more affected by the pressure magnitude. Dynamic hydrostatic pressure of 10 MPa at 1 Hz has the most effect on its synthesis in 2D culture47,52,53, while lower levels of pressure can increase aggrecan production47,54. On the other hand, in 3D culture, the constructs and the environment are more complicated, therefore, the magnitude, frequency and duration of loading could play an important role in matrix production. Even though physiological levels of hydrostatic pressure with specific durations improved matrix production, high magnitudes of it had negative effects. Increasing the magnitude of static pressure to 20 MPa - 50 MPa in resulted in a significant decrease in S-GAG and collagen production and increased cell apoptosis and the stress response gene heat shock protein 7020,48,49,51,61. Interestingly, in general the cell content of our tissue constructs, as inferred by construct DNA content, did not vary with the application of hydrostatic pressure loading, suggesting the biosynthetic results at high loading pressures (10 MPa) did not induce cell death but just altered cellular metabolism. Furthermore, in our current work we have found TRPV4 and PIEZO1 mechano-sensitive channels provoke inflammatory signaling in chondrocytes62,63 and since high magnitudes of hydrostatic pressure inhibit growth and increase apoptosis, it is possible that these or other channels may be involved in regulating different magnitudes or frequencies of hydrostatic pressure.
We hypothesized that the response to hydrostatic pressure was mediated by one of the mechanically-sensitive TRP ion channels. Of note, only the inhibition of TRPV1 partially prevented the decrease in S-GAG accumulation caused by hydrostatic pressure. TRPV1, also known as the capsaicin receptor, was the first TRP channel to be identified and cloned and primarily serves as a sensor for heat and pain (nociception) in sensory neurons64. However, in other cell types, TRPV1 serves other sensing functions, alone or in combination with other mechanosensitive channels65–67. In particular, growing evidence suggests that TRPV1 could function as a hydrostatic pressure sensor in the eye68, potentially through interactions with TRPV469. Further work is needed to identify the downstream pathways and potential synergistic interactions of these channels in chondrocytes21. Moreover, based on our results and the polymodal nature of the TRPV1 channel, future studies will be necessary to assess how mechanical versus thermal or pH activation of TRPV1 may induce different cellular signaling pathways and potentially differentially regulate chondrogenesis through TRPV1 modulation.
While we tested a number of TRP channels, it is important to note that other mechanosensitive channels such as TRPC6, TRPM7, and Piezo1/2 may be involved in a hydrostatic pressure response70. But there is currently a lack of commercially-available inhibitors that are selective and specific for these channels. For example, recent studies suggest that the hydrostatic response of immune cells is mediated through the Piezo-family of mechanically sensitive ion channels71. While the pressures they used were far below those examined in our study, and the mechanical environment used is not described (2D/3D), future studies may wish to investigate the role of Piezo ion channels in transducing hydrostatic pressure effects22. Additionally, the inhibitor doses we supplemented were prescribed by established literature in alternative cell types. Future studies may focus on more complete inhibition of the hydrostatic response with inhibitor dosing-response studies and establishing how the channels are activated by a hydrostatic pressure stimuli.
In addition to the potential role of ion channels, several studies have evaluated other intracellular or downstream mechanisms in response to hydrostatic pressure. Nordberg et al. showed that LPR5 and LRP6 mRNA levels increased after applying a dynamic hydrostatic pressure of 7.5 MPa, 1 Hz, 4 hours/day for up to 14 days58. Furthermore, active β-catenin protein expression showed the same trend as LRP5 and increased by applying cyclic hydrostatic pressure. Knight et al. observed that both static (5 MPa) and dynamic (5 MPa, 1 Hz) of hydrostatic loading alters the actin organization and these cytoskeletal changes can be recovered after 1h of applying the pressure72. In addition, another study investigated the changes in the intracellular calcium response of chondrocytes to hydrostatic pressure along with the influence of seeding duration and zonal differences73. Mizuno showed that applying 0.5 MPa of pressure for 5 minutes increases the calcium levels in bovine chondrocytes, and this effect can be inhibited when the cells are treated with gadolinium, intracellular storage blocker (dantrolene), or calcium-free medium. Moreover, this study showed that the middle zone chondrocytes are more responsive to hydrostatic pressure and had the highest calcium influx. Lastly, it was observed that the chondrocytes that were seeded for 2 days did not have any response to hydrostatic pressure, but showed a response after 5 days of seeding.
In summary, we screened a number of TRP channels in an effort to identify the mechanism(s) by which chondrocytes respond to hydrostatic pressure. We targeted mechanosensitive TRP ion channels to evaluate their inhibition of matrix production while cultured under daily hydrostatic loading of 5 MPa at 0.5 Hz. This loading regimen was used because it robustly inhibited S-GAG production of chondrocytes seeded in agarose gel. We hypothesized that by inhibiting mechanosensitive TRP ion channels would recover the S-GAG production present in unloaded engineered cartilage. Interestingly, inhibition of neither TRPV1, TRPV4, TRPC3, and TRPC1 completely inhibited the hydrostatic loading effects on S-GAG production in chondrocytes. The modest influence of TRPV1 suggest future studies may be well targeted on the role of TRPV1 in hydrostatic-mediated effects. Consequently, our pilot study here on the role of mechanically-sensitive TRP channels in hydrostatic pressure-mediated mechanotransduction cascade suggest only a potentially small involvement of the channels and hint to a more complex mechanosensory is involved in the hydrostatic pressure loading response on matrix production of articular cartilage. More studies are required to combine the potential mechanisms to find the actual process by which hydrostatic pressure alters the matrix synthesis in articular cartilage.
Supplementary Material
Acknowledgments
The authors thank Dr. Dennis Carter and Dr. Elizabeth Loboa for ideas and discussions that led to this work.
Funding
This work was supported by the Shriners Hospitals for Children, NSF EAGER Award 1638442, NIH grants AR76665, AG46927, AG15768, AR074240, AR073752, AR074992, and AR072999, and the Arthritis Foundation.
Footnotes
Declaration of Interests
The authors have no competing interests.
References
- 1.Goldring MB. Potential Mechanisms of PTOA: Inflammation. Post-Traumatic Arthritis: Springer, 2015. p. 201–209. [Google Scholar]
- 2.Olson SA, Guilak F. Arthritis That Develops After Joint Injury: Is It Post-Traumatic Arthritis or Post-Traumatic Osteoarthritis? Post-Traumatic Arthritis: Springer, 2015. p. 3–6. [Google Scholar]
- 3.Guilak F, Hung C. Basic Orthopaedic Biomechanics and Mechanobiology. 2005. [Google Scholar]
- 4.Armstrong C, Mow V. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. The Journal of bone and joint surgery American volume 1982;64(1):88–94. [PubMed] [Google Scholar]
- 5.Ateshian GA. The role of interstitial fluid pressurization in articular cartilage lubrication. Journal of biomechanics 2009;42(9):1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S, Krishnan R, Nicoll SB, Ateshian GA. Cartilage interstitial fluid load support in unconfined compression. Journal of biomechanics 2003;36(12):1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soltz MA, Ateshian GA. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Annals of biomedical engineering 2000;28(2):150–159. [DOI] [PubMed] [Google Scholar]
- 8.Mow V, Bachrach N, Setton L, Guilak F. Stress, strain, pressure and flow fields in articular cartilage and chondrocytes. Cell mechanics and cellular engineering: Springer, 1994. p. 345–379. [Google Scholar]
- 9.Hodge W, Fijan R, Carlson K, Burgess R, Harris W, Mann R. Contact pressures in the human hip joint measured in vivo. Proceedings of the National Academy of Sciences 1986;83(9):2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. Journal of Orthopaedic Research 2004;22(3):565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachrach NM, Mow VC, Guilak F. Incompressibility of the solid matrix of articular cartilage under high hydrostatic pressures. Journal of biomechanics 1998;31(5):445–451. [DOI] [PubMed] [Google Scholar]
- 12.Finger AR, Sargent CY, Dulaney KO, Bernacki SH, Loboa EG. Differential Effects on Messenger Ribonucleic Acid Expression by Bone Marrow–Derived Human Mesenchymal Stem Cells Seeded in Agarose Constructs Due to Ramped and Steady Applications of Cyclic Hydrostatic Pressure. Tissue engineering 2007;13(6):1151–1158. [DOI] [PubMed] [Google Scholar]
- 13.Nordberg RC, Bodle JC, Loboa EG. Mechanical stimulation of adipose-derived stem cells for functional tissue engineering of the musculoskeletal system via cyclic hydrostatic pressure, simulated microgravity, and cyclic tensile strain. Adipose-Derived Stem Cells: Springer, 2018. p. 215–230. [DOI] [PubMed] [Google Scholar]
- 14.Pattappa G, Zellner J, Johnstone B, Docheva D, Angele P. Cells under pressure–the relationship between hydrostatic pressure and mesenchymal stem cell chondrogenesis. eCells & Materials 2019;2019(37):360–381. [DOI] [PubMed] [Google Scholar]
- 15.Puetzer J, Williams J, Gillies A, Bernacki S, Loboa EG. The effects of cyclic hydrostatic pressure on chondrogenesis and viability of human adipose-and bone marrow-derived mesenchymal stem cells in three-dimensional agarose constructs. Tissue Engineering Part A 2013;19(1–2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angele P, Yoo J, Smith C, Mansour J, Jepsen K, Nerlich M, et al. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. Journal of orthopaedic research 2003;21(3):451–457. [DOI] [PubMed] [Google Scholar]
- 17.Hodder E, Guppy F, Covill D, Bush P. The effect of hydrostatic pressure on proteoglycan production in articular cartilage in vitro: a meta-analysis. Osteoarthritis and Cartilage 2020. [DOI] [PubMed] [Google Scholar]
- 18.Miyanishi K, Trindade MC, Lindsey DP, Beaupré GS, Carter DR, Goodman SB, et al. Dose-and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-β3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue engineering 2006;12(8):2253–2262. [DOI] [PubMed] [Google Scholar]
- 19.Kawanishi M, Oura A, Furukawa K, Fukubayashi T, Nakamura K, Tateishi T, et al. Redifferentiation of dedifferentiated bovine articular chondrocytes enhanced by cyclic hydrostatic pressure under a gas-controlled system. Tissue engineering 2007;13(5):957–964. [DOI] [PubMed] [Google Scholar]
- 20.Hall A, Urban J, Gehl K. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. Journal of orthopaedic research 1991;9(1):1–10. [DOI] [PubMed] [Google Scholar]
- 21.Barrett-Jolley R, Lewis R, Fallman R, Mobasheri A. The emerging chondrocyte channelome. Frontiers in physiology 2010;1:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010;330(6000):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du G, Li L, Zhang X, Liu J, Hao J, Zhu J, et al. Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes. Experimental Biology and Medicine 2020;245(3):180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavenis K, Schumacher C, Schneider U, Eisfeld J, Mollenhauer J, Schmidt-Rohlfing B. Expression of ion channels of the TRP family in articular chondrocytes from osteoarthritic patients: changes between native and in vitro propagated chondrocytes. Molecular and cellular biochemistry 2009;321(1–2):135–143. [DOI] [PubMed] [Google Scholar]
- 25.Lee W, Guilak F, Liedtke W. Role of piezo channels in joint health and injury. Current topics in membranes: Elsevier, 2017. p. 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proceedings of the National Academy of Sciences 2014;111(47):E5114–E5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu D, Qu J, Sun L, Li Q, Ling H, Yang N, et al. Ca2+/Mg2+ homeostasis-related TRPM7 channel mediates chondrocyte hypertrophy via regulation of the PI3K-Akt signaling pathway. Molecular medicine reports 2017;16(4):5699–5705. [DOI] [PubMed] [Google Scholar]
- 28.Mobasheri A, Matta C, Uzielienè I, Budd E, Martín-Vasallo P, Bernotiene E. The chondrocyte channelome: a narrative review. Joint Bone Spine 2019;86(1):29–35. [DOI] [PubMed] [Google Scholar]
- 29.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proceedings of the National Academy of Sciences 2014;111(4):1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 2009;60(10):3028–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian N, Ichimura A, Takei D, Sakaguchi R, Kitani A, Nagaoka R, et al. TRPM7 channels mediate spontaneous Ca2+ fluctuations in growth plate chondrocytes that promote bone development. Sci Signal 2019;12(576):eaaw4847. [DOI] [PubMed] [Google Scholar]
- 32.Sambale M, Intemann J, Pap T, Sherwood J. A homeostatic role for transient receptor potential cation channel (TRPC1) in articular cartilage. Osteoarthritis and Cartilage 2019;27:S175. [Google Scholar]
- 33.Servin-Vences MR, Moroni M, Lewin GR, Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife 2017;6:e21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherwood J, Bertrand J, Seidemann M, Dell’Accio F, Pap T. Activation of the transient receptor potential cation channel TRPC6 is required for chondrocyte phenotypic stability. Osteoarthritis and Cartilage 2016;24:S152–S153. [Google Scholar]
- 35.Somogyi CS, Matta C, Foldvari Z, Juhász T, Katona É, Takács ÁR, et al. Polymodal transient receptor potential vanilloid (TRPV) ion channels in chondrogenic cells. International journal of molecular sciences 2015;16(8):18412–18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nims RJ, Cigan AD, Albro MB, Vunjak-Novakovic G, Hung CT, Ateshian GA. Matrix production in large engineered cartilage constructs is enhanced by nutrient channels and excess media supply. Tissue Engineering Part C: Methods 2015;21(7):747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. Journal of Neuroscience 2006;26(37):9385–9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanju P, Chen Y, Lee W, Yeo M, Lee SH, Romac J, et al. Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Scientific reports 2016;6:26894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenig S, Schernthaner M, Maechler H, Kappe CO, Glasnov TN, Hoefler G, et al. A TRPC3 blocker, ethyl-1-(4-(2, 3, 3-trichloroacrylamide) phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate (Pyr3), prevents stent-induced arterial remodeling. Journal of Pharmacology and Experimental Therapeutics 2013;344(1):33–40. [DOI] [PubMed] [Google Scholar]
- 40.Paez PM, Fulton D, Spreuer V, Handley V, Campagnoni AT. Modulation of canonical transient receptor potential channel 1 in the proliferation of oligodendrocyte precursor cells by the golli products of the myelin basic protein gene. J Neurosci 2011. Mar 9;31(10):3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta (BBA)-General Subjects 1986;883(2):173–177. [DOI] [PubMed] [Google Scholar]
- 42.Nims RJ, Cigan AD, Albro MB, Hung CT, Ateshian GA. Synthesis rates and binding kinetics of matrix products in engineered cartilage constructs using chondrocyte-seeded agarose gels. J Biomech 2014. Jun 27;47(9):2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkkinen J, Ikonen J, Lammi M, Laakkonen J, Tammi M, Helminen H. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Archives of biochemistry and biophysics 1993;300(1):458–465. [DOI] [PubMed] [Google Scholar]
- 44.Jortikka MO, Parkkinen JJ, Inkinen RI, Kärner J, Järveläinen HT, Nelimarkka LO, et al. The role of microtubules in the regulation of proteoglycan synthesis in chondrocytes under hydrostatic pressure. Archives of biochemistry and biophysics 2000;374(2):172–180. [DOI] [PubMed] [Google Scholar]
- 45.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PloS one 2008;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue engineering Part A 2009;15(5):1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikenoue T, Trindade MC, Lee MS, Lin EY, Schurman DJ, Goodman SB, et al. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. Journal of orthopaedic research 2003;21(1):110–116. [DOI] [PubMed] [Google Scholar]
- 48.Lammi MJ, Inkinen R, Parkkinen J, Häkkinen T, Jortikka M, Nelimarkka L, et al. Expression of reduced amounts of structurally altered aggrecan in articular cartilage chondrocytes exposed to high hydrostatic pressure. Biochemical Journal 1994;304(3):723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura S, Arai Y, Takahashi KA, Terauchi R, Ohashi S, Mazda O, et al. Hydrostatic pressure induces apoptosis of chondrocytes cultured in alginate beads. Journal of orthopaedic research 2006;24(4):733–739. [DOI] [PubMed] [Google Scholar]
- 50.Parkkinen JJ, Lammi MJ, Inkinen R, Jortikka M, Tammi M, Virtanen I, et al. Influence of short-term hydrostatic pressure on organization of stress fibers in cultured chondrocytes. Journal of orthopaedic research 1995;13(4):495–502. [DOI] [PubMed] [Google Scholar]
- 51.Parkkinen JJ, Lammi MJ, Pelttari A, Helminen HJ, Tammi M, Virtanen I. Altered Golgi apparatus in hydrostatically loaded articular cartilage chondrocytes. Annals of the rheumatic diseases 1993;52(3):192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith RL, Rusk S, Ellison B, Wessells P, Tsuchiya K, Carter D, et al. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. Journal of orthopaedic research 1996;14(1):53–60. [DOI] [PubMed] [Google Scholar]
- 53.Smith RL, Trindade M, Shida J, Kajiyama G, Vu T. Time-dependent effects of intermittent hydrostatic pressure on articular c on rocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev 2000;37:153–161. [PubMed] [Google Scholar]
- 54.Suh J-K, Baek GH, Arøen A, Malin CM, Niyibizi C, Evans CH, et al. Intermittent sub-ambient interstitial hydrostatic pressure as a potential mechanical stimulator for chondrocyte metabolism. Osteoarthritis and Cartilage 1999;7(1):71–80. [DOI] [PubMed] [Google Scholar]
- 55.Elder S, Fulzele K, McCulley W. Cyclic hydrostatic compression stimulates chondroinduction of C3H/10T1/2 cells. Biomechanics and modeling in mechanobiology 2005;3(3):141–146. [DOI] [PubMed] [Google Scholar]
- 56.Luo Z, Seedhom B. Light and low-frequency pulsatile hydrostatic pressure enhances extracellular matrix formation by bone marrow mesenchymal cells: an in-vitro study with special reference to cartilage repair. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 2007;221(5):499–507. [DOI] [PubMed] [Google Scholar]
- 57.Miyanishi K, Trindade MC, Lindsey DP, Beaupré GS, Carter DR, Goodman SB, et al. Effects of hydrostatic pressure and transforming growth factor-β 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue engineering 2006;12(6):1419–1428. [DOI] [PubMed] [Google Scholar]
- 58.Nordberg RC, Mellor LF, Krause AR, Donahue HJ, Loboa EG. LRP receptors in chondrocytes are modulated by simulated microgravity and cyclic hydrostatic pressure. PloS one 2019;14(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toyoda T, Seedhom BB, Kirkham J, Bonass WA. Upregulation of aggrecan and type II collagen mRNA expression in bovine chondrocytes by the application of hydrostatic pressure. Biorheology 2003;40(1, 2, 3):79–85. [PubMed] [Google Scholar]
- 60.Toyoda T, Seedhom BB, Yao JQ, Kirkham J, Brookes S, Bonass WA. Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis & Rheumatism 2003;48(10):2865–2872. [DOI] [PubMed] [Google Scholar]
- 61.Xu T, Xu G, Gu Z, Wu H. Role of endoplasmic reticulum stress pathway in hydrostatic pressure-induced apoptosis in rat mandibular condylar chondrocytes. Molecular and cellular biochemistry 2017;429(1–2):23–31. [DOI] [PubMed] [Google Scholar]
- 62.Lee W, Nims RJ, Savadipour A, Leddy H, Liu F, McNulty A, et al. Osteoarthritis: articular chondrocyte inflammatory signaling leads to enhanced gene expression and function of mechanotransduction channel Piezo1 as a pathogenic feedforward mechanism. bioRxiv 2020. [Google Scholar]
- 63.Nims R, Pferdehirt L, Ho N, Savadipour A, Lorentz J, Sohi S, et al. A synthetic mechanogenetic gene circuit for autonomous drug delivery in engineered tissues. bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389(6653):816–824. [DOI] [PubMed] [Google Scholar]
- 65.Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Science signaling 2015;8(363):ra15-ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grundy L, Daly DM, Chapple C, Grundy D, Chess-Williams R. TRPV1 enhances the afferent response to P2X receptor activation in the mouse urinary bladder. Scientific reports 2018;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohtsuki T, Shinaoka A, Kumagishi-Shinaoka K, Asano K, Hatipoglu OF, Inagaki J, et al. Mechanical strain attenuates cytokine-induced ADAMTS9 expression via transient receptor potential vanilloid type 1. Experimental cell research 2019;383(2):111556. [DOI] [PubMed] [Google Scholar]
- 68.Sappington RM, Sidorova T, Ward NJ, Chakravarthy R, Ho KW, Calkins DJ. Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress. Channels 2015;9(2):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delamere NA, Shahidullah M, Mathias RT, Gao J, Sun X, Sellitto C, et al. Signaling Between TRPV1/TRPV4 and Intracellular Hydrostatic Pressure in the Mouse Lens. Investigative ophthalmology & visual science 2020;61(6):58–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao R, Afthinos A, Zhu T, Mistriotis P, Li Y, Serra SA, et al. Cell sensing and decision-making in confinement: The role of TRPM7 in a tug of war between hydraulic pressure and cross-sectional area. Science advances 2019;5(7):eaaw7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solis AG, Bielecki P, Steach HR, Sharma L, Harman CC, Yun S, et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019;573(7772):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knight M, Toyoda T, Lee D, Bader D. Mechanical compression and hydrostatic pressure induce reversible changes in actin cytoskeletal organisation in chondrocytes in agarose. Journal of biomechanics 2006;39(8):1547–1551. [DOI] [PubMed] [Google Scholar]
- 73.Mizuno S A novel method for assessing effects of hydrostatic fluid pressure on intracellular calcium: a study with bovine articular chondrocytes. American Journal of Physiology-Cell Physiology 2005;288(2):C329–C337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.