Abstract
Objective
Stepping exergames designed to stimulate physical and cognitive skills can provide important information concerning individuals’ performance. In this study, we investigated the potential of stepping and gameplay metrics to assess the motor-cognitive status of older adults.
Methods
Stepping and gameplay metrics were recorded in a longitudinal study involving 13 older adults with mobility limitations. Game parameters included games’ scores and reaction times. Stepping parameters included length, height, speed, and duration, measured by inertial sensors placed on the shoes while interacting with the exergames. Parameters measured on the first gameplay were correlated against standard cognitive and mobility assessments, including the Montreal Cognitive Assessment (MoCA), gait speed, and the Short Physical Performance Battery. Based on MoCA scores, patients were then stratified into two groups: cognitively impaired and healthy controls. The differences between the two groups were visually inspected, considering their within-game progression over the training period.
Results
Stepping and gameplay metrics had moderate-to-strong correlations with cognitive and mobility performance indicators: faster, longer, and higher steps were associated with better mobility scores; better cognitive games’ scores and reaction times, and longer and faster steps were associated with better cognitive performance. The preliminary visual analysis revealed that the group with cognitive impairment required more time to advance to the next difficulty level, also presenting slower reaction times and stepping speeds when compared to the healthy control group.
Conclusion
Stepping exergames may be useful for assessing the cognitive and motor status of older adults, potentially allowing assessments to be more frequent, affordable, and enjoyable. Further research is required to confirm results in the long term using a larger and more diverse sample.
Keywords: Exergames, serious games, stepping, inertial sensors, motor-cognitive assessment, older adults
Introduction
The aging of the population and the high prevalence of age-related conditions, including cognitive and mobility disorders, are bringing new health, economic, and social challenges.1,2 The global prevalence of cognitive impairment is estimated to be between 5% and 41% in older adults,3 affecting cognitive domains such as memory, executive functions, attention, language, or visuospatial skills.4 Cognitive impairment has been associated with increased risk of dementia and mortality.4,5 It has also been associated with mobility disorders leading to falls, injuries, and loss of independence.6
The screening of cognitive impairment is currently based on paper-and-pencil tests, such as the Montreal Cognitive Assessment (MoCA),7 that require administration by trained health professionals. Besides being performed in unfamiliar settings, these methods are unsuitable for self-administration on a regular basis.8 Abnormal decline is usually detected and intervened late, even though the detection of early signs of impairment is fundamental to prevent and better manage further decline.9,10 Researchers keep joining their efforts to find new strategies for more frequent screening.11
Interactive games constitute a very promising avenue for the unobtrusive screening of cognitive ability. For being played primarily as a form of entertainment, games allow assessments to be more frequent, affordable, and enjoyable.11,12 Many commercial off-the-shelf games incorporate cognitive challenges such as memorizing sequences, making decisions, recognizing patterns, or analyzing complex information. Despite not being primarily designed for this purpose, Mandryk and Birk support that even existing games can be used as biomarkers of cognitive decline, as they generate rich information about players’ performance in multiple cognitive challenges.13 Other games are being purposefully developed for the screening of cognitive disorders. As an example, Tong et al. proposed a serious game featuring the Go/No-Go task, designed for tablets, to measure inhibition ability in older adults in a hospital emergency department.14 The performance of the game correlated significantly with global cognitive performance (as assessed by MoCA scores), being also much easier to administer and fun.14 The scores of another mobile game designed for tablets and based on augmented reality with manipulation of physical objects showed high concurrent validity with the MoCA test in non-demented older adults.15 In another study, two serious games played with the support of custom-designed buttons revealed significantly lower reaction times and accuracy rates in older adults with cognitive impairment.16
Games combining challenging tasks with physical activity, referred to as exergames, have been proposed to improve physical and cognitive parameters in older adults.17,18 Although their effects are traditionally assessed using external outcome measures after gameplay,17,18 parameters extracted directly from the exergames may be used to monitor both the cognitive and the physical state of older adults.19,20 For example, parameters extracted from a balance training exergame employing a three-dimensional movable plate presented moderate-to-high correlations with established motor and cognitive tests in older adults with mild-to-moderate dementia.19 Similar results were obtained for an exergame training program applied to cognitively intact older adults that used a stepping platform to detect multidirectional steps.20 Stepping exergames require the execution of volitional and inhibitory stepping movements, involving the sensory system, information processing skills (such as fast decision-making, dual tasking, and inhibitory response), and neuromuscular control, which are needed to prevent loss of balance and avoid falls in daily living.17,21 Litz et al. showed that game scores and reaction times extracted from a stepping exergame are associated with motor-cognitive performance in older adults with limited functional status, yielding moderate-to-high correlations with cognitive tests, measures of lower extremity function, and dynamic balance.20
In the scope of the project VITAAL (AAL-2017-066), funded by the European Commission through the Active Assisted Living Program, an exergame was developed to stimulate physical and cognitive skills in older adults by providing a multicomponent, simultaneous, training approach.22 VITAAL included exergames for balance and motor-cognitive training focused on attention and executive functions.22 The interaction with the exergame was performed resorting to multidirectional steps that were detected by inertial sensors mounted on the shoes.22 Simultaneously, these inertial sensors allowed the extraction of several steps characteristics, including, their length, height, and duration.23
This study aimed at investigating the potential value of VITAAL exergames as an unobtrusive tool for the assessment of motor-cognitive status in older adults. We investigated the role of stepping characteristics and game metrics extracted during gameplay in a correlational study that compared these metrics with standard assessments of cognition and mobility. In this respect, our hypotheses were that (a) stepping characteristics would significantly correlate with cognition and mobility performance indicators, (b) cognitive games’ metrics would correlate with cognitive performance, and (c) within-game progression would present differences between older adults with and without cognitive impairment.
Methods
A recent work developed a stepping exergame that used inertial sensors to detect multidirectional steps allowing interaction with the game in real time.22 Game data were collected in a longitudinal study and analyzed using a mixed design, comprising a cross-sectional correlational study and a longitudinal analysis. This section describes the methods used to investigate the potential of this exergame as a tool to assess the motor-cognitive status of older adults.
Participants and procedures
This study provides a secondary analysis of data collected under the scope of a Randomized Controlled Pilot Trial (RCT), originally designed to assess the feasibility of the exergame as an intervention for patients with mobility impairment (ClinicalTrials.gov identifier: NCT04587895). The RCT was conducted in a physiotherapy clinic, Physio SPArtos, located in Interlaken, Bern, Switzerland. The inclusion and exclusion criteria are presented in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Aged 60+ years old • Living independently, in a residency dwelling, or with care • Able to stand straight for at least 10 min without aids (to be able to play the exergame) • Visual acuity with correction sufficient to work on a TV screen • Short Physical Performance Battery (SPPB) test score below 10 as indication of impaired mobility24,25 |
• Mobility impairments that did not allow to play the exergame • Severe cognitive impairment (MoCA score below the first percentile according to Thomann et al.26) • Acute or unstable chronic diseases (e.g., recent cardiac infarction, uncontrolled high blood pressure or cardiovascular disease, uncontrolled diabetes) • Orthopedic or neurological diseases that inhibited exergame training • Rapidly progressive or terminal illness • Insufficient knowledge of German • Chronic respiratory disease • Condition or therapy that weakens the immune system • Cancer • Serious obesity (body mass index (BMI) > 40 kg/m2) |
Participants recruited to the RCT were randomly allocated to the control group or to the intervention group. This study has only analyzed the data from the intervention group, who played the exergame three sessions per week (each session lasting about 30 min), during a maximum of 12 weeks (no longer than two weeks of break were allowed). All participants that played the exergame at least once were included in this study. In each session, participants played an individually calculated amount of strength, cognitive, and balance training, that remained the same over the 12-week intervention period. The exergame provided training progression by automatically adjusting difficulty of movements, tasks and/or speed to the participants’ performance.22 Each game was comprised by ten difficulty levels and all participants started at level one. They advanced to the next difficulty level if they achieved scores above 90% or if the average of the last three game scores was above 75%. Average scores below 50% would imply a decrease on the difficulty level of the game.22 Before starting the intervention, all participants were assessed for demographics and medical history, cognitive tests, physical tests, and gait. Tests already administered for the purpose of selecting study participants were not administered again.
The study was approved by the Institutional Ethics Committee of ETH Zurich (registration: 2020-00578) and followed the ethical code for research with human beings as stated by the Declaration of Helsinki. Written informed consent was obtained from all participants. No compensation for participation was given.
Assessments
A health questionnaire was administered to obtain self-reported information about the participants, including, sociodemographic variables (age, gender, weight, height, and education), general health questions (hearing and vision problems), medical history (diagnosed diseases to confirm exclusion criteria), and physical abilities and activity (single question about fear of falling, number of falls in the last six months, and physical activity level). Participants were also asked for video games use and previous experience with exergames.
Global cognitive performance was assessed using the MoCA,27 administered by qualified physiotherapists from Physio SPArtos. MoCA is a widely used and preferred tool for cognitive screening in primary care settings,7 making it suitable for use in this study. This test has a maximum score of 30 points and consists of several tasks organized in seven cognitive domains: visuospatial/executive, naming, attention, language, abstraction, delayed recall, and orientation. The total score is corrected for low education (≤12 years) by adding an extra point.27 Both the total score and the individual domain scores were used in this study.
Functional physical performance was assessed by trained physiotherapists using the Short Physical Performance Battery (SPPB). SPPB consists of three subtests focused on balance, walking, and lower limb strength. SPPB has a maximum score of 12 points,28,29 with scores lower than 10 indicative of mobility impairment.24,25
Gait was measured at participants’ preferred speed, walking along a straight 20 m distance path. To objectively assess gait parameters, inertial sensors were mounted on the shoes, and the method described by Guimarães et al. was employed.30 Gait speed was extracted as a comprehensive measure of individuals’ functional capacity and mobility.31,32
Stepping exergames
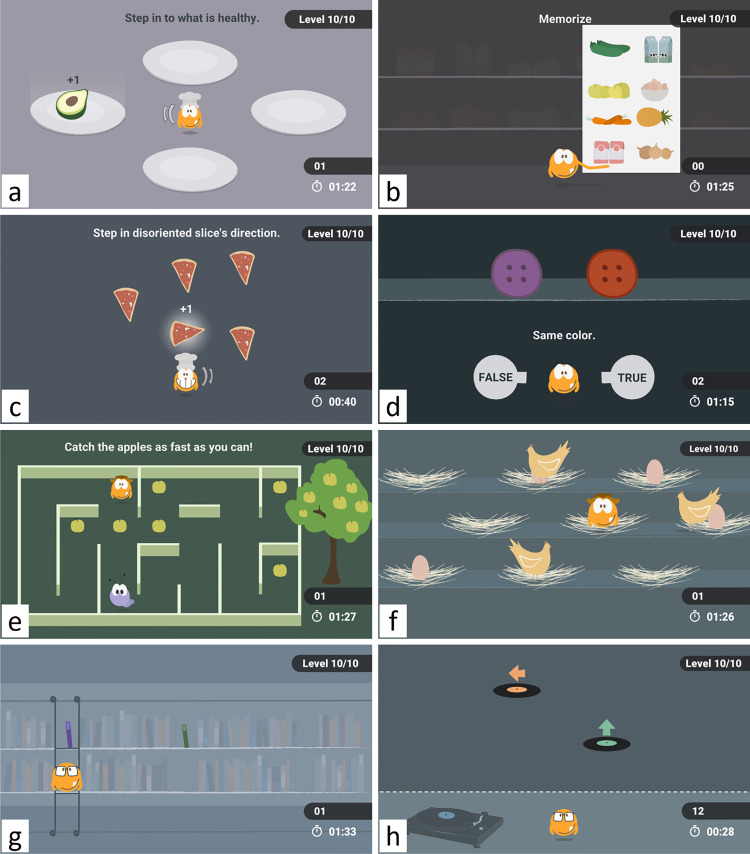
The exergame included eight stepping-based minigames, four of which were designed to stimulate cognition, the other four to stimulate balance (Figure 1). Cognitive games included tasks that challenged specific cognitive domains related to attention and executive functions. The Healthy Snacks game (Figure 1(a)) challenged reaction time and response inhibition control, which were needed to select healthy food as fast as possible while avoiding selection of unhealthy food. The Shopping List game (Figure 1(b)) required short-term memory to memorize and later be able to recognize the list of products previously shown. The Pizza game (Figure 1(c)) challenged selective attention by requiring players to ignore irrelevant distractors (pizza slices pointing in a common direction) and focus on the relevant information (the pizza slice pointing in an opposite direction). Finally, by requiring participants to switch between two cognitive processes—one to interpret the shape, and the other to interpret the color of the objects—the Shopping game (Figure 1(d)) challenged cognitive flexibility. Balance games, that is, Labyrinth (Figure 1(e)), Mommy Chicken (Figure 1(f)), Falling Books (Figure 1(g)), and Music (Figure 1(h)), focused more on the execution of multidirectional steps. Balance games required more frequent steps and transitions between right and left feet to properly command the main character of the game. A full description of the minigames is provided by Guimarães et al.22
Figure 1.
Stepping-based minigames. Cognitive games: (a) Healthy Snacks (cognitive inhibition), (b) Shopping List (short-term memory), (c) Pizza (selective attention), (d) Shopping (cognitive flexibility). Balance games: (e) Labyrinth, (f) Mommy Chicken, (g) Falling Books, and (h) Music.
For each step performed within the games, we recorded its length (maximum displacement in the horizontal plane), height (maximum displacement in the vertical—gravity—axis), and duration (time elapsed since the initiation of the step to foot-contact), as determined by the inertial sensors. These metrics were measured using the methods described by Guimarães et al.23 using wearable inertial measurement units equipped with a triaxial gyroscope and a triaxial accelerometer (Bosch BMI160).30 Averages were then calculated for each game and level played. Step speed was calculated by dividing step length by step duration. For the cognitive games, we have also recorded step reaction times (the time since the presentation of the stimulus until the initiation of the step) and whether it has or has not led to a correct answer within the game. The average reaction time (incl. the average reaction time for the correct and for the incorrect answers) was calculated for the cognitive games and registered for each game and level played. Games scores (a value between 0 and 100% calculated based on the number of correct answers or number of elements picked)22 were also registered for the cognitive games as possible indicators of cognitive performance.
Statistical analysis
The packages pingouin (v0.4.0), StatsModels (v0.13.0), SciPy (v1.7.1), and NumPy (v1.21.5) for Python 3 were used for the statistical analysis. A p-value of less than 0.05 was considered to indicate statistical significance.
Demographic and medical characteristics were summarized using either means and standard deviations, or frequencies and percentages, as appropriate. As height was missing in one participant, its value was replaced by the average.
Since demographics and medical characteristics were obtained in the beginning of the longitudinal study and considering that not all participants played the same number of repetitions of a game level (due to dropout, or due to their performance), we have only analyzed the stepping and game metrics obtained on the first time the participant played the first level of difficulty (the entry-level performance). A correlation analysis was conducted to examine association between gameplay metrics and global physical and cognitive performances. We calculated the partial correlation between step characteristics (step height, step length, step duration, and step speed) and measures of cognitive and physical performance (MoCA total score, SPPB score, and gait speed) while controlling for participants’ height. We have also calculated the partial correlations between cognitive games’ performance metrics (scores and reaction times) and cognitive test scores (MoCA scores without correction for low education and individual domain scores). These correlations were controlled for education as both individual cognitive domains and the total MoCA score may be affected by the educational level.33 We used Spearman's rank correlations because most of the metrics were not normally distributed according to the Shapiro-Wilk test. Significant correlations were interpreted based on their strength as follows: 0.0 to 0.2 (0.0 to −0.2) negligible, 0.2 to 0.4 (−0.2 to −0.4) weak, 0.4 to 0.7 (−0.4 to −0.7) moderate, 0.7 to 0.9 (−0.7 to −0.9) strong, and 0.9 to 1.0 (−0.9 to −1.0) very strong.34
Two groups were formed based on MoCA scores, using the cut-offs proposed by Thomann et al.35 and O’Caoimh et al.,36 as follows: individuals were considered cognitively normal if they had MoCA score ≥ 24 (healthy control group); they were considered cognitively impaired if they had MoCA score < 24 (cognitively impaired group). The differences between groups concerning their progression within the games were analyzed by visually inspecting difficulty levels progression over the intervention period. Time was represented by the number of days since intervention start, rounded to multiples of 5 days for improved data aggregation. The differences in reaction times (for both correct and incorrect answers) and step speed evolution across difficulty levels were also visually represented. Reaction times and step speeds were calculated as average values for each difficulty level, participant, and game. For the comparison between groups, we reported averages and 95% confidence intervals.
Results
A total of 14 participants were allocated to the intervention group, but only the data from 13 participants were analyzed in this study since one patient dropped out before playing any game. Three participants dropped out from the study after having completed a single training session. Two participants have completed 5 and 8 training sessions before dropping out. The eight participants who finished the intervention study have performed between 22 and 32 sessions (the average was 27.4 ± 3.2 sessions).
Descriptive statistics
The demographic and clinical characteristics of the participants are presented in Table 2. The study included participants with an average age of 81.6 ± 7.3 years old, an average MoCA score of 23.5 ± 3.2, and an average gait speed of 0.9 ± 0.2 m/s. Nearly half of the participants fell more than once in the last 6 months. The average gait speed was below 1.0 m/s, possibly denoting an increased risk of falls.37 All participants had a SPPB score below 10 (average of 7.5 ± 1.4) indicating impaired mobility.24,25 Participants had very low experience with the use of video games, and, in particular, with exergames, but they were physically active. Their education spanned from 8 to 24 years (average of 12.0 ± 4.0 years).
Table 2.
Demographic and clinical characteristics of the intervention group.
| Participant characteristics | Intervention group (n = 13) |
|---|---|
| Age (years) | 81.6 ± 7.3 (64–91) |
| Weight (kg) | 71.1 ± 14.3 (52–97) |
| Height (cm) | 165.2 ± 10.1 (148–178) |
| Education (years) | 12.0 ± 4.0 (8–24) |
| Female, n (%) | 8 (61.5) |
| Hearing problems, n (%) | 5 (38.5) |
| Vision problems, n (%) | 4 (30.8) |
| Play video games, n (%) | 1 (7.7) |
| Tried exergames before, n (%) | 2 (15.4) |
| Fear of falling | |
| Never, n (%) | 5 (38.5) |
| Sometimes, n (%) | 5 (38.5) |
| Often, n (%) | 2 (15.4) |
| Always, n (%) | 1 (7.7) |
| Number of falls in the last 6 months | |
| Zero, n (%) | 6 (46.2) |
| One, n (%) | 1 (7.7) |
| More than 1, n (%) | 6 (46.2) |
| Physical activity | |
| >3 times/week, n (%) | 9 (69.2) |
| 1–3 times/week, n (%) | 4 (30.8) |
| 1 time/week, n (%) | 0 (0.0) |
| Never, n (%) | 0 (0.0) |
| MoCA | |
| Total score (0–30) | 23.5 ± 3.2 (20–30) |
| Visuospatial/executive (0–5) | 3.5 ± 1.5 (1–5) |
| Naming (0–3) | 2.9 ± 0.3 (2–3) |
| Attention (0–6) | 5.3 ± 0.8 (4–6) |
| Language (0–3) | 2.1 ± 0.9 (1–3) |
| Abstraction (0–2) | 0.6 ± 0.7 (0–2) |
| Delayed recall (0–5) | 2.6 ± 1.7 (0–5) |
| Orientation (0–6) | 5.8 ± 0.4 (5–6) |
| Physical tests | |
| SPPB score | 7.5 ± 1.4 (4–9) |
| Gait speed (m/s) | 0.9 ± 0.2 (0.6–1.3) |
Data are mean values ± standard deviation (range), except otherwise indicated. MoCA: Montreal Cognitive Assessment; SPPB: Short Physical Performance Battery.
Steps characteristics
The partial correlations between steps characteristics and global physical performance and cognitive tests scores are shown in Table 3. Of the four step characteristics measured, only step length and step speed presented significant correlations with MoCA scores: step length had moderate positive correlations with MoCA scores in two cognitive games (Pizza and Shopping List) and strong positive correlations in two balance games (Mommy Chicken and Music); step speed presented moderate positive correlations with MoCA scores in two cognitive games (Pizza and Shopping List) and a strong positive correlation in a balance game (Mommy Chicken). Although not always significant, the correlations of step length, and speed with MoCA scores were all ≥0.40. Step height and step length were both significantly correlated with SPPB scores in Pizza, Shopping, Books, Labyrinth, and Music; these correlations were all positive and moderate or strong. Speed was significantly correlated with SPPB scores in the same games except Shopping. Additionally, speed had a significant correlation with SPPB scores in Healthy Snacks. The correlations between SPPB scores and step speed were all positive and strong. Of these 15 significant correlations with SPPB scores, only six were also significantly correlated with gait speed, presenting moderate or strong positive association. Most of them were not significant, even though their correlation strengths were still at a high level (>0.40). Step duration was not significantly correlated with any of the global physical performance or cognitive tests scores.
Table 3.
Correlations between step characteristics and global physical and cognitive performance tests.
| Game | Step characteristic | MoCA score | SPPB score | Gait speed |
|---|---|---|---|---|
| Healthy Snacks (n = 11) | Height | 0.57 | 0.39 | 0.03 |
| Length | 0.53 | 0.37 | 0.13 | |
| Duration | −0.02 | −0.23 | −0.55 | |
| Speed | 0.41 | 0 . 70* | 0.56 | |
| Pizza (n = 12) | Height | 0.48 | 0.63* | 0.45 |
| Length | 0.60* | 0.73* | 0.61* | |
| Duration | −0.27 | −0.15 | −0.26 | |
| Speed | 0.67* | 0.74** | 0.57 | |
| Shopping (n = 11) | Height | 0.22 | 0.68* | 0.39 |
| Length | 0.40 | 0.69* | 0.45 | |
| Duration | −0.24 | 0.03 | −0.42 | |
| Speed | 0.45 | 0.63 | 0.48 | |
| Shopping List (n = 12) | Height | 0.49 | 0.58 | 0.44 |
| Length | 0.64* | 0.36 | 0.34 | |
| Duration | 0.14 | 0.04 | −0.07 | |
| Speed | 0.64* | 0.52 | 0.50 | |
| Books (n = 13) | Height | 0.30 | 0.64* | 0.65* |
| Length | 0.53 | 0.70* | 0.45 | |
| Duration | −0.38 | −0.30 | −0.46 | |
| Speed | 0.48 | 0.76** | 0.61* | |
| Labyrinth (n = 10) | Height | 0.62 | 0.83** | 0.67* |
| Length | 0.65 | 0.73* | 0.74* | |
| Duration | 0.30 | 0.11 | 0.10 | |
| Speed | 0.66 | 0.81** | 0.63 | |
| Mommy Chicken (n = 12) | Height | 0.58 | 0.53 | 0.25 |
| Length | 0.79** | 0.38 | 0.33 | |
| Duration | −0.08 | −0.06 | −0.43 | |
| Speed | 0.82** | 0.34 | 0.34 | |
| Music (n = 10) | Height | 0.40 | 0.77* | 0.57 |
| Length | 0.78* | 0.67* | 0.56 | |
| Duration | −0.30 | −0.42 | −0.54 | |
| Speed | 0.58 | 0.83** | 0.72* |
Shown are the Spearman partial correlations, adjusted for height. *p < 0.05, **p < 0.01, ***p < 0.001, bold values indicate significance. The number of participants playing each game varied due to patient dropout. MoCA: Montreal Cognitive Assessment; SPPB: Short Physical Performance Battery.
Cognitive games’ metrics
The partial correlations between cognitive games’ metrics and MoCA total and individual domain scores are shown in Table 4. MoCA total score only presented significant correlation with metrics of the Pizza game, namely a moderate negative correlation with the reaction time of correct answers and a moderate positive correlation with game score. The visuospatial/executive component of MoCA was significantly correlated with reaction times in the Healthy Snacks game (strong correlation), with reaction time in the Shopping game (moderate correlation), and with reaction time and score in the Shopping List game (moderate correlation); the correlations were negative for reaction times, and positive for games scores. The abstraction component of MoCA presented strong negative correlation with reaction times and strong positive correlation with the scores of the Pizza game. The remaining components of MoCA (i.e., naming, attention, language, delayed recall, and orientation) did not present any significant correlation with cognitive games’ metrics.
Table 4.
Correlations between cognitive games’ metrics and MoCA scores.
| Game | Game metric | MoCA scorea | MoCA visuospatial/ executive | MoCA naming | MoCA attention | MoCA language | MoCA abstraction | MoCA delayed recall | MoCA orientation |
|---|---|---|---|---|---|---|---|---|---|
| Healthy Snacks (n = 11) | RT | −0.07 | −0.72* | −0.50 | −0.10 | 0.02 | 0.04 | 0.34 | −0.37 |
| RT (correct) | 0.09 | −0.57 | −0.50 | −0.07 | 0.06 | 0.31 | 0.39 | −0.18 | |
| RT (incorrect) | 0.04 | −0.78* | −0.41 | 0.23 | 0.44 | 0.27 | 0.23 | −0.36 | |
| Score | 0.12 | 0.55 | 0.53 | 0.13 | −0.37 | 0.19 | −0.19 | 0.19 | |
| Pizza (n = 12) | RT | −0.48 | −0.17 | —b | −0.44 | −0.47 | −0.86*** | −0.12 | −0.03 |
| RT (correct) | −0.62* | −0.20 | —b | −0.51 | −0.47 | −0.84** | −0.43 | 0.18 | |
| RT (incorrect) | −0.23 | −0.02 | —b | −0.29 | −0.42 | −0.39 | 0.11 | −0.19 | |
| Score | 0.69* | 0.45 | —b | 0.57 | 0.59 | 0.79** | 0.31 | 0.26 | |
| Shopping (n = 11) | RT | −0.19 | −0.38 | −0.10 | −0.33 | −0.21 | −0.41 | 0.18 | 0.05 |
| RT (correct) | −0.55 | −0.66* | −0.51 | −0.61 | −0.11 | −0.47 | −0.05 | 0.05 | |
| RT (incorrect) | 0.19 | −0.31 | −0.32 | 0.20 | −0.25 | 0.66 | 0.32 | −0.04 | |
| Score | 0.33 | 0.32 | 0.30 | 0.53 | 0.21 | 0.39 | 0.02 | −0.02 | |
| Shopping List (n = 12) | RT | −0.31 | −0.65* | —b | −0.34 | 0.19 | −0.47 | 0.09 | 0.23 |
| RT (correct) | −0.05 | −0.52 | —b | 0.01 | 0.33 | −0.27 | 0.28 | 0.13 | |
| RT (incorrect) | −0.14 | −0.48 | —b | −0.13 | −0.23 | −0.28 | 0.12 | −0.35 | |
| Score | 0.27 | 0.67* | —b | 0.36 | −0.04 | 0.44 | −0.23 | −0.15 |
Shown are Spearman partial correlations, adjusted for education. *p < 0.05, **p < 0.01, ***p < 0.001, bold values indicate significance. The number of participants playing each game varied due to patient dropout. aMoCA scores without correction for low education. bCould not be computed because one of the variables was constant. RT: reaction time; MoCA: Montreal Cognitive Assessment.
Game progression
Six participants were considered healthy controls (control group) and seven had cognitive impairment (CI group) according to MoCA. As some participants dropped out from the study or could not reach the most advanced difficulty levels, the number of participants decreased as the difficulty levels progressed. The number of participants playing each game level, stratified by their cognitive status, is shown in Table 5.
Table 5.
Number of participants playing each game level, stratified by their cognitive status.
| Game | Group | Difficulty levels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Healthy Snacks | Control | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 |
| CI | 6 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 3 | |
| Pizza | Control | 6 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 |
| CI | 6 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Shopping | Control | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 |
| CI | 6 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Shopping List | Control | 6 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 |
| CI | 6 | 4 | 3 | 3 | 3 | 3 | 2 | 0 | 0 | 0 | |
| Books | Control | 6 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 4 |
| CI | 7 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | |
| Labyrinth | Control | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 |
| CI | 5 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | |
| Mommy Chicken | Control | 6 | 4 | 4 | 2 | 2 | 1 | 1 | 1 | 1 | 1 |
| CI | 6 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | |
| Music | Control | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 |
| CI | 5 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | |
CI: Cognitive impairment.
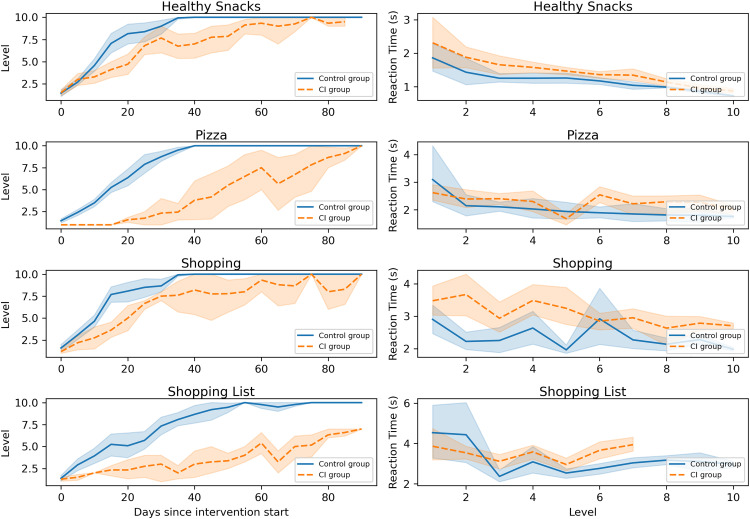
The progression of each group within cognitive games is shown in Figure 2, where we can observe some differences between the two groups. The CI group required, in general, more time than the control group to advance to the next difficulty level (Figure 2–left). Reaction times were generally higher (i.e., slower) in the CI group (Figure 2–right).
Figure 2.
Progression within cognitive games: (left) difficulty levels over time, and (right) reaction times across difficulty levels. Error bands represent 95% confidence intervals.
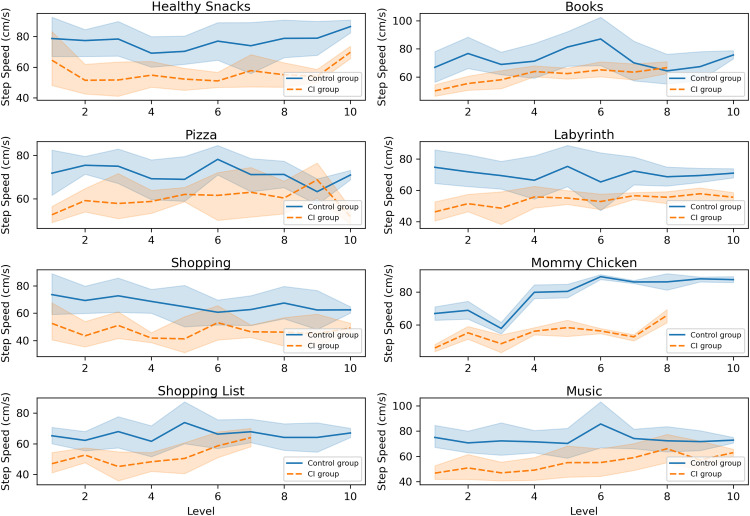
As can be observed in Figure 3, step speed was slower in the CI group, a tendency that spanned across all difficulty levels and all balance and cognitive games.
Figure 3.
Step speed across difficulty levels presented for all balance and cognitive games. Error bands represent 95% confidence intervals.
Discussion
We investigated the potential of stepping and game performance metrics to assess the motor-cognitive status of older adults by evaluating their association with standard cognitive and physical performance metrics. This study was the first evaluating steps characteristics like length, height, or speed using inertial sensors while interacting with exergames and investigating their role as non-invasive indicators of cognitive and physical performance in older adults. In line with our initial hypothesis, several stepping and gameplay metrics presented a significant association with cognition and mobility performance indicators, denoting the potential value of stepping-based exergames as a clinical tool for the screening of motor-cognitive status in older adults.
Association with physical performance
We examined the correlations between stepping metrics and standard tests of physical performance. We anticipated that each exergame and each difficulty level would elicit distinct steps characteristics, due to the different challenges they pose. For this reason, we performed the correlation analysis for each game individually, considering only the metrics obtained the first time the participant played the first level of difficulty. In the results presented by Skjæret-Maroni et al., steps characteristics changed depending on the exergame played, difficulty level, and the number of times the exergame was played.38
In line with our initial hypothesis, several steps characteristics presented significant correlations with physical tests scores (Table 3). Step height, length, and speed presented moderate or strong positive associations with SPPB scores and gait speed in several games, suggesting that the better the mobility of the person, the faster, the larger, and the higher the steps were performed while interacting with balance and cognitive exergames. Step duration was not significantly associated with physical tests scores. Faster speeds—resulting from the simultaneous contribution of increased step lengths and/or decreased steps duration—provided a better indication of physical performance than step duration. Significant correlations were more frequent on balance than cognitive exergames, denoting their potential superior value as indicators of mobility in older adults.
Previous works studied the role of volitional (or voluntary) stepping on mobility performance assessment. Both the maximal step length test and the rapid step test were shown to correlate significantly with measures of mobility performance,39 balance, and fall risk in older adults,21 which is in line with our results. Another study showed that the ability to step rapidly declines with age, being stepping performance also affected by step direction and the need to decide on the most appropriate direction,40 which is also required to play exergames.
Stepping parameters could be extracted directly from the inertial sensors, avoiding the need to use independent motion capture solutions as was the case in the work presented by Skjæret-Maroni et al.38 In fact, most of the existing stepping exergames rely on the use of step-sensitive platforms that are not able to measure these stepping parameters.18,41 As shown in our study, stepping parameters, as measured by inertial sensors, can be used as indicators of mobility in older adults.
Association with cognitive performance
Although cognitive games were not purposefully designed to assess cognition, the tasks within these games were designed to provide some cognitive demand related to attention and executive functions. In line with our initial hypothesis, these games presented significant associations with cognitive performance.
One of the cognitive games (Pizza game) was moderately associated with overall MoCA scores (Table 4), indicating that the higher the game scores and the faster the reaction times, the better the global cognitive performance. Additionally, the Pizza game was strongly associated with the abstraction component of MoCA, being faster reaction times and better scores associated with better performance on abstract reasoning. Within MoCA, the abstraction component refers to a two-item verbal abstraction task.27 Learning this abstract rule requires an efficient allocation of attention, that is, focusing on some aspects of information while ignoring others.42 This ability is usually described as selective attention and may be required to identify the common attributes within the verbal abstraction task.42,43 The Pizza game, designed to challenge selective attention, may also require these abilities to identify the pizza slice that is pointing in the wrong direction. Reflecting on abstraction, philosophers often conceive abstraction as being a form of selective attention.44
As expected, the ability to correctly interpret visual information within the games and the ability to act accordingly depends on visuospatial and executive skills as reflected by the associations with the visuospatial/executive component of MoCA. The games Healthy Snacks, Shopping, and Shopping List were significantly associated with the visuospatial/executive component of MoCA, being better visuospatial/executive skills associated with higher scores and faster reaction times. The visuospatial/executive component of MoCA includes a modified trail-making test, the copy of the cube, and the clock drawing test.27 In the trail-making test, the respondent is asked to draw a line connecting an alternating sequence of numbers and letters.27 Besides visuomotor and visuoperceptual skills, the trail-making test requires mental flexibility to alternate between numbers and letters.45–47 This flexibility of task switching is also demanded by the Shopping game in the ability to switch between shape or color matching.43 To copy the cube, spatial planning and visuomotor coordination are needed,45,48 which makes a parallelism with the abilities required to plan and step in an appropriate direction while interacting with the games. Coordinated visuomotor stepping control is also required to walk safely and avoid falls.49,50 Finally, the performance of the clock drawing task demands planning skills, conceptualization, symbolic representation, and inhibitory control.45,51,52 In our results, Healthy Snacks had stronger correlations with the visuospatial/executive component of MoCA than Shopping and Shopping List games, since it simultaneously required visuoperceptual skills (to rapidly identify the objects), visuomotor coordination (to step in an appropriate direction) and inhibitory response (to avoid selection of unhealthy food).
Although the Shopping List game was designed to challenge short-term memory, it did not present significant associations with the delayed recall component of MoCA. In the Shopping List game, the person had to identify whether the objects shown matched or not the list presented previously. To answer this task, recent memory, in particular, recognition was required.53 In contrast, MoCA asked participants to recall as many items as possible from a list of words, requiring recent memory but, in particular, free recall,53 which differs from the task in the game. Moreover, while the Shopping List game challenges delayed recall through visual memory, MoCA challenges delayed recall through verbal memory, utilizing different and potentially competing cognitive resources.54,55 According to our results, the Shopping List game mainly challenges the ability to recognize and identify the pictures in the shopping list, which is more related to the visuospatial/executive component of MoCA.
The remaining components of MoCA, that is, naming, attention, language, and orientation were not associated with any of the cognitive games’ metrics. Although individual MoCA tests are organized under the subheadings identified in Table 4, tests scores can be grouped using distinct structures to represent the cognitive domains.56,57 The correspondence between each individual test and its assumed cognitive domain is not always robust because each cognitive task requires abilities from multiple cognitive domains.56–58 It is then not surprising that cognitive games challenge multiple cognitive skills, and, for that reason, their scores and metrics may reflect multiple cognitive constructs.15,20 The tasks used to test naming, attention, language, and orientation components within MoCA—that is, a naming task, the digit span forward and backward, letter A tapping, serial-7 subtraction, sentence repetition, letter fluency, and orientation in time and place27—are very distinct from the tasks presented in the games. These components of MoCA were thus not related to the cognitive mechanisms underlying cognitive games.
As hypothesized, stepping characteristics also presented significant associations with cognitive performance (Table 3). Longer step lengths and faster speeds were associated with better global cognitive performance (MoCA scores) in several balance and cognitive games. Previous studies have also identified a relationship between mobility and cognitive performance in older adults, suggesting that gait speed could be used to predict cognitive impairment.32,59–61 Gait metrics determined under dual task (i.e., walking while performing a cognitive task) could identify gait changes related to cognitive impairment that could otherwise go unnoticed.37,62,63 Considering that exergames provide motor-cognitive training (i.e., they require the simultaneous execution of stepping to solve a cognitive challenge), it is not surprising that stepping characteristics may also serve as an indicator of cognitive performance. Like gait parameters measured under single and dual task analysis, stepping characteristics extracted while playing exergames may be used to assess performance on both cognitive and mobility functions.
Due to the low number of participants, we were not able to objectively assess differences between healthy and cognitively impaired groups as determined by MoCA scores. Nevertheless, we observed that step speed was generally lower in the group with cognitive impairment (Figure 3), which is consistent with previous studies on gait analysis.32,37,59–63 This tendency spanned across all difficulty levels and all games. The group with cognitive impairment also required more time to advance to the next difficulty level, being slower to answer to the tasks of the cognitive games (Figure 2). A previous study has examined visuospatial executive function and learning in older adults with and without cognitive impairment. The study showed that relative to healthy controls, individuals with cognitive impairment made more exploratory/learning errors, displaying slower learning curves,64 which is in line with our results. Another study concluded that game progression was slower in older participants, being strongly influenced by age and, thus, sensitive to changes in cognitive ability.65 According to Jakobsen et al., complex reaction time (with inhibition control) can be used to reflect cognitive function in healthy subjects and patients.66 Reaction time slowing has been considered an early sign of cognitive impairment.67 Taken together, the results of these studies strengthen our provisional results indicating that gameplay metrics and the path of progression within the games may be able to differentiate between both groups. Yet, additional tests are needed.
Limitations
The present study was mostly limited by the small number of participants. The correlation analysis performed in this study should be considered of exploratory nature, as it focused on the analysis of the results of the first gameplay and did not include an objective analysis of all difficulty levels. On one side, this entry-level performance can be seen as a reflection of the first impact to gameplay and ensures a fair comparison between participants. In this regard, we need to highlight that all participants had very low previous experience with playing exergames, and, as such, they could all be considered inexperienced users. On the other side, the first impact to gameplay may not entirely reflect the real performance of the player as a simple misunderstanding of the rules or of the interaction with the game can negatively impact players’ performance. Moreover, the lowest difficulty level may not challenge participants as needed to detect cognitive impairment. More advanced difficulty levels could offer a more adequate challenge for a specific patient's performance (avoiding ceiling effects),20 and could potentially strengthen the relationship between variables. Considering that the difficulty levels automatically adapted to each person (to ensure an optimal load in line with performance),68 the number of times the participants played a single difficulty level differed, making it difficult to compare the different levels. A more structured analysis of difficulty levels and repetitions would be needed to more thoroughly assess construct validity, test-retest reliability, and sensitivity to change, as proposed by Litz et al.20
Given the small sample size and the existence of study dropouts, the most complex levels had even lower sample sizes (Table 5) and, as such, lack of statistical power. Therefore, it was not possible for us to objectively evaluate exergame progression and its relation to the motor-cognitive status of the participants. The preliminary visual analysis revealed the potential ability of exergame progression to discriminate between groups with distinct cognitive skills (according to their MoCA scores), however, it requires further investigation.
Although MoCA is the most common and preferable tool for cognitive screening in primary care settings,7 it cannot be considered an alternative for more in-depth neuropsychological assessment, let alone its domain-specific scores.56,58,69 In this study, we used domain-specific scores as presented by MoCA, however, several alternative test grouping schemes are proposed in the literature, reflecting an inconsistent correspondence between individual MoCA tests and their assumed cognitive domains.56,58,69 Our results should, thus, be interpreted with caution.
Finally, our study has only included older adults with mobility limitations (as determined by their SPPB scores) and its results cannot be generalized to other populations. Despite their mobility limitations, they were still quite active and most of them have never or only sometimes experienced fear of falling. A larger and more diverse sample—including participants with more distinct characteristics, higher SPPB scores, and/or cognitive impairment as determined by neuropsychologists—would be needed to better evaluate the generalization of our results and confirm the ability of stepping and exergame metrics to assess the motor-cognitive status of older adults.
Future work
Further research is required to comprehensively evaluate the new assessment approach based on stepping and gameplay metrics measured while playing stepping exergames. We should include a higher number of participants and formally evaluate their cognitive status resorting to expert diagnosis, which should also include potential neurodegenerative conditions and a domain-specific evaluation. Moreover, older adults without mobility limitations should be included to formally assess whether gameplay and stepping metrics are able to differentiate people with and without mobility impairment. More specific measures of mobility (e.g., static and dynamic balance) should be considered as well.
The distinct difficulty levels and the individual progression of the participants should be more thoroughly analyzed to allow more frequent cognitive screening. Future research should address how to differentiate between the effects of learning on a patient's performance and his/her actual cognitive status, particularly relevant when the exergame is available to be played repeatedly at home. Besides its construct validity, we should assess its test-retest reliability and the sensitivity to change in a longitudinal study design purposefully thought to answer all the questions above.
Conclusion
Although stepping exergames were originally designed for the training of physical and cognitive functions, stepping and game performance metrics extracted while playing may also serve as indicators of the motor-cognitive status in older adults.
In this study, we used inertial sensors to extract relevant stepping characteristics like height, length, duration, and speed while interacting with exergames. Moderate-to-strong correlations were obtained between game-derived measures and physical and cognitive performance tests. Faster, longer, and higher steps were associated with better mobility scores. Longer and faster steps were associated with better cognitive scores. Cognitive game metrics (e.g., reaction times and scores) presented significant associations with cognitive performance. People with cognitive impairment seemed to exhibit slower progression within the game, and slower reaction times and step speed when compared to people without cognitive impairment.
Although results in this study should be considered exploratory, they provide some important indications on the potential value of stepping exergames as a clinical tool for the screening of motor-cognitive status in older adults. Exergames like those tested in this work may be able to assess and monitor motor-cognitive function in older adults, potentially allowing assessments to be more frequent, more affordable, and more enjoyable.
Acknowledgements
The authors would like to thank the physiotherapy clinic Physio SPArtos, namely Jacqueline De Jong and Vincent Becker-Hoff, for their contributions to participant recruitment, data recordings, and data entry. We also thank the collaboration of all volunteers who participated in the study.
Footnotes
Contributorship: VG formulated the research question under the lead of IS and MVC. VG conducted data processing, analysis, and interpretation of the results and produced the first version of the manuscript. IS, MVC, and EdB acted as methodological councils. EdB coordinated the trial. All authors revised and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of ETH Zurich (registration: 2020-00578). Participants provided their written informed consent to take part in this study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the project Smart-Health-4-All (POCI-01-0247-FEDER-046115), co-funded by Portugal 2020, framed under the COMPETE 2020 (Competitiveness and Internationalization Operational Program) and European Regional Development Fund (ERDF) from European Union (EU). It was also supported by the project ConnectedHealth (no. 46858), funded by Competitiveness and Internationalisation Operational Programme (POCI) and Lisbon Regional Operational Programme (LISBOA 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). The data were collected with support by the project VITAAL (AAL-2017-066), funded under the Active Assisted Living Program and co-funded by the European Commission and the National Funding Authorities of Portugal, Switzerland, and Belgium.
Guarantor: VG
ORCID iD: Vânia Guimarães https://orcid.org/0000-0001-8133-0789
References
- 1.Maresova P, Javanmardi E, Barakovic S, et al. Consequences of chronic diseases and other limitations associated with old age – a scoping review. BMC Public Health 2019; 19: 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cylus J, Figueras J, Normand C. Will Population Ageing Spell the End of the Welfare State? A review of evidence and policy options. European Observatory Policy Briefs, Copenhagen (Denmark): European Observatory on Health Systems and Policies, 2019. [PubMed] [Google Scholar]
- 3.Pais R, Ruano L, Carvalho OP, et al. Global cognitive impairment prevalence and incidence in community dwelling older adults—a systematic review. Geriatrics 2020; 5: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med 2013; 29: 753–772. DOI:10.1016/j.cger.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park MH, Kwon DY, Jung JM, et al. Mini-mental status examination as predictors of mortality in the elderly. Acta Psychiatr Scand 2013; 127: 298–304. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Low LF, Schwenk M, et al. Review of gait, cognition, and fall risks with implications for fall prevention in older adults with dementia. Dement Geriatr Cogn Disord 2019; 48: 17–29. [DOI] [PubMed] [Google Scholar]
- 7.Abd Razak M, Ahmad N, Chan Y, et al. Validity of screening tools for dementia and mild cognitive impairment among the elderly in primary health care: a systematic review. Public Health 2019; 169: 84–92. [DOI] [PubMed] [Google Scholar]
- 8.Matsuura T, Sakashita K, Grushnikov A, et al. Statistical analysis of dual-task gait characteristics for cognitive score estimation. Sci Rep 2019; 9: 19927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabbagh M, Boada M, Borson S, et al. Rationale for early diagnosis of mild cognitive impairment (MCI) supported by emerging digital technologies. J Prevent Alzheimer’s Dis 2020; 7: 158–164. DOI: 10.14283/jpad.2020.19 [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen J, Langerman H. Alzheimer’s disease – why we need early diagnosis. Degener Neurol Neuromuscul Dis 2019; 9: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koo BM, Vizer LM. Mobile technology for cognitive assessment of older adults: a scoping review. Innov Aging 2019; 3: 1–14. DOI: 10.1093/geroni/igy038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumsden J, Edwards EA, Lawrence NS, et al. Gamification of cognitive assessment and cognitive training: a systematic review of applications and efficacy. JMIR Serious Games 2016; 4: e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandryk RL, Birk MV. The potential of game-based digital biomarkers for modeling mental health. JMIR Ment Health 2019; 6: e13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong T, Chignell M, Tierney MC, et al. A serious game for clinical assessment of cognitive status: validation study. JMIR serious Games 2016; 4: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boletsis C, McCallum S. Smartkuber: a serious game for cognitive health screening of elderly players. Games Health J 2016; 5: 241–251. [DOI] [PubMed] [Google Scholar]
- 16.Chen YT, Hou CJ, Derek N, et al. Evaluation of the reaction time and accuracy rate in normal subjects, MCI, and dementia using serious games. Appl Sci 2021; 11: 628. [Google Scholar]
- 17.Hauer K, Litz E, Günther-Lange M, et al. Effectiveness and sustainability of a motor-cognitive stepping exergame training on stepping performance in older adults: a randomized controlled trial. Eur Rev Aging Phys Act 2020; 17: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanenburg J, Wild K, Straumann D, et al. Exergaming in a moving virtual world to train vestibular functions and gait; a proof-of-concept-study with older adults. Front Physiol 2018; 9: 988. DOI: 10.3389/fphys.2018.00988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiloth S, Lemke N, Werner C, et al. Validation of a computerized, game-based assessment strategy to measure training effects on motor-cognitive functions in people with dementia. JMIR Serious Games 2016; 4: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litz E, Ball C, Jansen CP, et al. Validation of a motor-cognitive assessment for a stepping exergame in older adults: use of game-specific, internal data stream. Games Health J 2019; 9: 95–107. DOI: 10.1089/g4h.2019.0081 [DOI] [PubMed] [Google Scholar]
- 21.Medell JL, Alexander NB. A clinical measure of maximal and rapid stepping in older women. J Gerontol Series A: Biol Sci Med Sci 2000; 55: M429–M433. [DOI] [PubMed] [Google Scholar]
- 22.Guimarães V, Oliveira E, Carvalho A, et al. An exergame solution for personalized multicomponent training in older adults. Appl Sci 2021; 11: 7986. [Google Scholar]
- 23.Guimarães V, Sousa I, Correia MV. Detection and classification of multidirectional steps for motor-cognitive training in older adults using shoe-mounted inertial sensors. In 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 6926–6929. DOI: 10.1109/EMBC.2019.8856851. [DOI] [PubMed] [Google Scholar]
- 24.Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the short physical performance battery score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol Series A, Biol Sci Med Sci 2009; 64: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernabeu-Mora R, Medina-Mirapeix F, Llamazares-Herrán E, et al. The short physical performance battery is a discriminative tool for identifying patients with COPD at risk of disability. Int J Chron Obstruct Pulmon Dis 2015; 10: 2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomann AE, Goettel N, Monsch RJ, et al. The Montreal cognitive assessment: normative data from a German-speaking cohort and comparison with international normative samples. J Alzheimer’s Dis 2018; 64: 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–M94. [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower- extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995; 332: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guimarães V, Sousa I, Correia MV. Orientation- invariant spatio-temporal gait analysis using foot-worn inertial sensors. Sensors 2021; 21: 3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act 2015; 23: 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grande G, Triolo F, Nuara A, et al. Measuring gait speed to better identify prodromal dementia. Exp Gerontol 2019; 124: 110625. [DOI] [PubMed] [Google Scholar]
- 33.Borda MG, Reyes-Ortiz C, Pérez-Zepeda MU, et al. Educational level and its association with the domains of the Montreal cognitive assessment test. Aging Ment Health 2019; 23: 1300–1306. [DOI] [PubMed] [Google Scholar]
- 34.Overholser BR, Sowinski KM. Biostatistics primer: part 2. Nutr Clin Pract 2008; 23: 76–84. [DOI] [PubMed] [Google Scholar]
- 35.Thomann AE, Berres M, Goettel N, et al. Enhanced diagnostic accuracy for neurocognitive disorders: a revised cut-off approach for the Montreal cognitive assessment. Alzheimer’s Res Therapy 2020; 12: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Caoimh R, Timmons S, Molloy DW. Screening for mild cognitive impairment: comparison of “MCI specific” screening instruments. J Alzheimer’s Dis 2016; 51: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muir SW, Speechley M, Wells J, et al. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture 2012; 35: 96–100. [DOI] [PubMed] [Google Scholar]
- 38.Skjæret-Maroni N, Vonstad EK, Ihlen EAF, et al. Exergaming in older adults: movement characteristics while playing stepping games. Front Psychol 2016; 7: 964. DOI: 10.3389/fpsyg.2016.00964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho Bl, Scarpace D, Alexander NB. Tests of stepping as indicators of mobility, balance, and fall risk in balance-impaired older adults: maximal step length in impaired older adults. J Am Geriatr Soc 2004; 52: 1168–1173. [DOI] [PubMed] [Google Scholar]
- 40.Luchies CW, Schiffman J, Richards LG, et al. Effects of age, step direction, and reaction condition on the ability to step quickly. J Gerontol Series A: Biol Sci Med Sci 2002; 57: M246–M249. [DOI] [PubMed] [Google Scholar]
- 41.Smith ST, Sherrington C, Studenski S, et al. A novel dance dance revolution (DDR) system for in-home training of stepping ability: basic parameters of system use by older adults. Br J Sports Med 2011; 45: 441–445. [DOI] [PubMed] [Google Scholar]
- 42.Plebanek DJ, Sloutsky VM. Selective attention, filtering, and the development of working memory. Dev Sci 2019; 22: e12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diamond A. Executive functions. Annu Rev Psychol 2013; 64: 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bäck A. The concept of abstraction. The Society for Ancient Greek Philosophy Newsletter 2006; 376: 1–14. [Google Scholar]
- 45.Julayanont P, Phillips N, Chertkow H, et al. Montreal Cognitive assessment (MoCA): concept and clinical review. In Larner AJ. (ed.) Cognitive screening instruments. London: Springer London, 2013. pp. 111–151. ISBN 978-1-4471-2451-1 978-1-4471-2452-8. DOI: 10.1007/978-1-4471-2452-8_6. [DOI] [Google Scholar]
- 46.Kortte KB, Horner MD, Windham WK. The trail making test, part B: cognitive flexibility or ability to maintain set? Appl Neuropsychol 2002; 9: 106–109. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Cubillo I, Periáñez J, Adrover-Roig D, et al. Construct validity of the trail making test: role of task- switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc 2009; 15: 438–450. [DOI] [PubMed] [Google Scholar]
- 48.Mori S, Osawa A, Maeshima S, et al. Possibility of using quantitative assessment with the cube copying test for evaluation of visuo-spatial function in patients with Alzheimer’s disease. Progress Rehabil Med 2021; 6: n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patla AE. Understanding the roles of vision in the control of human locomotion. Gait Posture 1997; 5: 54–69. [Google Scholar]
- 50.Young WR, Hollands MA. Evidence for age-related decline in visuomotor function and reactive stepping adjustments. Gait Posture 2012; 36: 477–481. [DOI] [PubMed] [Google Scholar]
- 51.Paula JJd, Miranda DMd, Moraes End, et al. Mapping the clockworks: what does the clock drawing test assess in normal and pathological aging? Arq Neuropsiquiatr 2013; 71: 763–768. [DOI] [PubMed] [Google Scholar]
- 52.Soffer M, Melichercik A, Herrmann N, et al. Time setting errors in the clock drawing test are associated with both semantic and executive deficits. Appl Neuropsychol: Adult 2022: 1–10. DOI: 10.1080/23279095.2021.2023154. [DOI] [PubMed] [Google Scholar]
- 53.Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 2014; 30: 421–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothmayr C, Baumann O, Endestad T, et al. Dissociation of neural correlates of verbal and non-verbal visual working memory with different delays. Behav Brain Funct 2007; 3: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morey CC, Cowan N. When do visual and verbal memories conflict? The importance of working-memory load and retrieval. J Exp Psychol: Learning Memory Cogn 2005; 31: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coen RF, Robertson DA, Kenny RA, et al. Strengths and limitations of the MoCA for assessing cognitive functioning: findings from a large representative sample of Irish older adults. J Geriatr Psychiatry Neurol 2016; 29: 18–24. [DOI] [PubMed] [Google Scholar]
- 57.Freitas S, Simões MR, Marôco J, et al. Construct validity of the Montreal cognitive assessment (MoCA). J Int Neuropsychol Soc: JINS 2012; 18: 242–250. [DOI] [PubMed] [Google Scholar]
- 58.Julayanont P, Brousseau M, Chertkow H, et al. Montreal Cognitive assessment memory index score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer’s disease. J Am Geriatr Soc 2014; 62: 679–684. [DOI] [PubMed] [Google Scholar]
- 59.Beauchet O, Allali G, Montero-Odasso M, et al. Motor phenotype of decline in cognitive performance among community-dwellers without dementia: population-based study and meta-analysis. PloS One 2014; 9: e99318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beauchet O, Blumen HM, Callisaya ML, et al. Spatiotemporal gait characteristics associated with cognitive impairment: a multicenter cross-sectional study, the intercontinental “gait, cognition & decline” initiative. Curr Alzheimer Res 2018; 15: 273–282. [DOI] [PubMed] [Google Scholar]
- 61.Mulas I, Putzu V, Asoni G, et al. Clinical assessment of gait and functional mobility in Italian healthy and cognitively impaired older persons using wearable inertial sensors. Aging Clin Exp Res 2021; 33: 1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Q, Tian C, Tseng B, et al. Gait change in dual task as a behavioral marker to detect mild cognitive impairment in elderly persons: a systematic review and meta-analysis. Arch Phys Med Rehabil 2020; 101: 1813–1821. [DOI] [PubMed] [Google Scholar]
- 63.Bahureksa L, Najafi B, Saleh A, et al. The impact of mild cognitive impairment on gait and balance: a systematic review and meta-analysis of studies using instrumented assessment. Gerontology 2017; 63: 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papp KV, Snyder PJ, Maruff P, et al. Detecting subtle changes in visuospatial executive function and learning in the amnestic variant of mild cognitive impairment. PLoS ONE 2011; 6: e21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonnechère B, Bier JC, Van Hove O, et al. Age-associated capacity to progress when playing cognitive mobile games: ecological retrospective observational study. JMIR Serious Games 2020; 8: e17121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jakobsen LH, Sorensen JM, Rask IK, et al. Validation of reaction time as a measure of cognitive function and quality of life in healthy subjects and patients. Nutrition 2011; 27: 561–570. [DOI] [PubMed] [Google Scholar]
- 67.Andriuta D, Diouf M, Roussel M, et al. Is reaction time slowing an early sign of Alzheimer’s disease? A meta- analysis. Dement Geriatr Cogn Disord 2019; 47: 281–288. [DOI] [PubMed] [Google Scholar]
- 68.Healy AF, Kole JA, Bourne LE. Training principles to advance expertise. Front Psychol 2014; 5: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hendershott TR, Zhu D, Llanes S, et al. Domain-specific accuracy of the Montreal cognitive assessment subsections in Parkinson’s disease. Parkinsonism Relat Disord 2017; 38: 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]