Abstract
Melanin deposition is the main cause of skin darkening, which can lead to severe physical and psychological distress, necessitating the development of approaches for preserving skin health and fairness. Tyrosinase (TYR) is the rate-limiting enzyme in melanin synthesis, and its activity directly determines the degree of melanin accumulation in the skin, which in turn affects skin color. Currently, TYR inhibitors derived from natural products are widely used for skin whitening. San-Bai decoction (SBD) is effective for skin whitening and softening, but its mechanism of action, efficacy and high efficiency TYR inhibitors for skin whitening remain poorly understood. Here, we employed systems biology and network pharmacology to analyze the active compounds and targets of SBD, using the follow databases: TCMIP, TCMID, and BATMAN-TCM. Construct a molecular network centered on the regulation of TYR by SBD in skin whitening, using STRING database and cytoscape. Enrichment analysis using KOBAS database and ClusterProfiler. Virtual screening of candidate TYR inhibitors using Molecular Operating Environment software and Amber 18 software. SBD may act through tyrosine metabolism, melanogenesis, and other signaling pathways to regulate TYR activity and inhibit melanogenesis. We identified TYR and ESR1 as possible key targets for the whitening effect of SBD and screened out pentagalloylglucose, 1,3,6-tri-O-galloyl-beta-D-glucose, 1,2,4,6-tetragalloylglucose, and liquiritigenin 4′,7-diglucoside as inhibitors of TYR, in addition to glycyrrhizic acid, pachymic acid methyl ester, nicotiflorin, gamma-sitosterol, and isoliensinine as inhibitors of ESR1. We also performed virtual drug screening of a library of natural small-molecule compounds (19,505 in total) and screened out lycopsamine, 2-phenylethyl b-D-glucopyranoside, and 6-beta-hydroxyhyoscyamine as inhibitors of TYR. We identified natural compounds with the potential for skin whitening through inhibition of TYR, thus advancing research on SBD and its applications.
Keywords: molecular dynamics simulation, network pharmacology, pigment deposition, San-Bai decoction, skin whitening, tyrosinase, virtual drug screening
1. Introduction
The amount of melanin in the skin is directly related to skin darkening, melasma, and pigment deposition.[1] Skin whitening approaches are primarily centered around melanin production, transfer, and metabolism. Tyrosinase (TYR) is a rate-limiting enzyme in melanin synthesis, and its activity directly determines the rate of melanin synthesis and the degree of its accumulation in the skin, which is among the primary causes of skin darkening and pigmentation.[2] Inhibition of TYR activity can reduce melanin synthesis and thus whiten the skin.[3] The discovery and confirmation of safe and effective TYR inhibitors are essential yet challenging aspects in the development of skin-whitening drugs.[4]
Traditional Chinese medicine (TCM) is considered a natural treasure, with the advantages of abundance, high efficacy, and low toxicity. TCM preparations contain a variety of pharmacological components with whitening, age-delaying, and anti-wrinkle effects.[5–8] Arbutin extracted from the TCM lingonberry leaf can block melanin synthesis by inhibiting TYR activity in vivo[9]; it is widely used in skin-whitening products to reduce skin pigment deposition, skin darkening, and melasma. Research on ancient TCM prescriptions as well as the screening and extraction of natural products with TYR-inhibiting activity from TCM herbs holds great potential in the field of skin whitening. Systems biology provides researchers tools that can reveal complex relationships between herbs, active compounds, and targets, while preserving the foundation of traditional TCM. Computer-aided drug design enables the screening of potential drug targets and target lead compounds of TCM origin, saving costs and improving efficiency of the drug development pipeline.[10]
The desire for a beautiful and healthy skin is universal. In the Inner Canon of the Yellow Emperor, it is described that the condition of the human skin is closely associated with the functioning of internal organs. When the internal organs are diseased, the qi and blood are not harmonized, which causes the skin to become rough and blemishes to develop on the face. San-Bai decoction (SBD) is a cosmetic formula handed down from the Ming Dynasty to the present day; it whitens the skin and removes blemishes by harmonizing the qi and blood, regulating the functions of the 5 viscera.[11] SBD consists of Paeoniae radix alba (Baishao), Atractylodis macrocephalae rhizome (Baizhu), Poria (Baifuling), and Glycyrrhizae radix et rhizome (Gancao). Modern pharmacology has shown that SBD contains flavonoids, polysaccharides, triterpenoid saponins, and other active compounds, which can exert various pharmacological effects, such as TYR inhibition, antioxidant activity through radical scavenging, anti-inflammatory effects, and immune stimulation.[12] Among constituent herbs, polysaccharides from Poria and Paeoniae radix alba can substantially inhibit tyrosinase production,[13,14] Atractylodis macrocephalae rhizoma and Glycyrrhizae radix et rhizoma polysaccharides are effective in whitening, repairing sun damage, and wound healing,[5,15] whereas flavonoids act as substrate analogs of tyrosine to inhibit TYR activity and slow melanin formation.[15]
In the present study, network pharmacology, molecular docking, pharmacophore screening, and molecular dynamic simulation were used to construct a molecular regulatory network for the whitening function of SBD via TYR inhibition. This network was found to be involved in several skin-related signaling pathways and TYR metabolic regulatory signaling. In addition, we found that pentagalloylglucose, 1,3,6-tri-O-galloyl-beta-D-glucose, 1,2,4,6-tetragalloylglucose, and liquiritigenin 4′,7-diglucoside in SBD could stably bind to TYR and that glycyrrhizic acid, pachymic acid methyl ester, nicotiflorin, gamma-sitosterol, and isoliensinine in SBD can stably bind to the antioxidant protein Estrogen Receptor 1 (ESR1). We screened a compound library containing 19,505 natural small molecules and identified lycopsamine, 2-phenylethyl b-D-glucopyranoside, and 6-beta-hydroxyhyoscyamine as compounds that stably bind to TYR as potential TYR inhibitors. Our study elucidated the complex mechanism of action for SBD in skin whitening, screening its drug targets and natural active compounds, thereby providing a theoretical basis for modern research on and application of SBD, an ancient TCM. Additionally, we screened natural compounds targeting TYR, thereby providing new directions for drug development.
2. Materials and Methods
2.1. Herbs, active compounds, and target information of SBD
The 2020 edition of the Chinese Pharmacopoeia[16] was searched for Paeoniae radix alba (Baishao), Atractylodis macrocephalae rhizome (Baizhu), Poria (Baifuling), and Glycyrrhizae radix et rhizome (Gancao) to obtain information on the classification and properties of the 4 herbs, as well as to investigate the efficacy and mechanism of action of SBD in skin whitening. The Integrative Pharmacology-based Research Platform of Traditional Chinese Medicine (TCMIP, http://www.tcmip.cn/TCMIP/index.php),[17] Traditional Chinese Medicines Integrated Database (TCMID, http://bidd.group/TCMID/index.html),[18] and Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine (BATMAN-TCM, http://bionet.ncpsb.org.cn/batman-tcm/)[19] databases were used to determine the active compounds of the herbs in SBD and their targets. Active compounds with quantitative estimate of drug-likeness (QED) > 0.80 in the 4 SBD herbal medicines were selected from the TCMIP database, and the targets of action of these compounds were determined. Active compounds with score cutoff ≥ 20.00 were selected from the TCMID and BATMAN-TCM databases, and the targets of action of these compounds were determined. Finally, the active compounds and their targets obtained from these databases were merged and duplicates were removed.
2.2. Construction of “TYR-PIN” for SBD and enrichment analysis
The targets of SBD were entered into the Search Tool for the Retrieval of Interacting Genes/Proteins (https://cn.string-db.org/).[20] The species was set to Homo sapiens, and the protein–protein interaction network (PPI) of SBD targets was obtained. To investigate the specific association between SBD and TYR, the PPI network was imported into Cytoscape. TYR, targeted by SBD, and the proteins that interact with TYR were selected to construct a TYR–protein–protein interaction network (TYR-PIN) of SBD as a PPI network with TYR as the target at the center. The MCODE module in Cytoscape was used to obtain local high-density regions and construct core subnetworks of TYR-PIN. The biological functions of targets in the core subnetworks of TYR-PIN were searched in the Human Gene Database (GeneCards, https://www.genecards.org/),[21] and a literature review of these targets was performed to select TYR and targets with precise associations with skin whitening as core targets.
Gene ontology (GO) term enrichment of the targets in TYR-PIN was performed using the R package ClusterProfiler, with the biological process (BP), molecular function (MF), and cellular component (CC) modules selected for classification and annotation of the biological functions of protein targets. Kyoto Encyclopedia of Genes and Genomes signaling pathway enrichment was performed using KOBAS-intelligence (KOBAS 3.0, http://bioinfo.org/kobas).[22] Pathway enrichment results with P < .05 were selected to investigate the skin whitening mechanism of SBD targeting TYR-PIN.
2.3. “Active Ingredient-Target Gene” network construction
Cytoscape 3.7.2 software[23] was used to construct an “herb-active compound-core target” network to elucidate the regulatory relationship between active compounds in SBD and TYR-PIN as well as to discover the herbs in SBD and their active compounds that exert whitening effects via core targets.
2.4. Molecular docking validation of core targets and active compounds
Three-dimensional (3D) structures of compounds were downloaded from the National Center for Biotechnology Information (PubChem, https://pubchem.ncbi.nlm.nih.gov/)[24] and ZINC (https://zinc20.docking.org/).[25] Two-dimensional (2D) structures of compounds with confirmed binding capability to core targets were obtained from the DrugBank (https://go.drugbank.com/)[26] and Pubchem databases as positive control drug structures. If only 2D structures were available in the databases, they were converted to 3D structures using Molecular Operating Environment (MOE) v2019.0102 software.
After applying specific criteria (species Homo sapiens, crystal resolution < 3 Å) to screen the RCSB Protein Data Bank (https://www.rcsb.org/),[27] the 3D structures of core proteins were downloaded. Homo sapiens TYR protein structure was downloaded from the AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/).[28,29] Protein structures were preprocessed to remove solvents and inactive ligands using PyMol 1.8. MOE software for molecular docking of preprocessed active compounds, positive control drugs, and core targets. Affinity was used to assess the binding ability between compounds and target proteins; root mean square deviation (RMSD) was used to assess the accuracy of the binding model. The “Ligand Interaction” module was used to analyze interactions between compounds and target proteins.
2.5. Virtual screening of candidate compounds in natural products targeting TYR
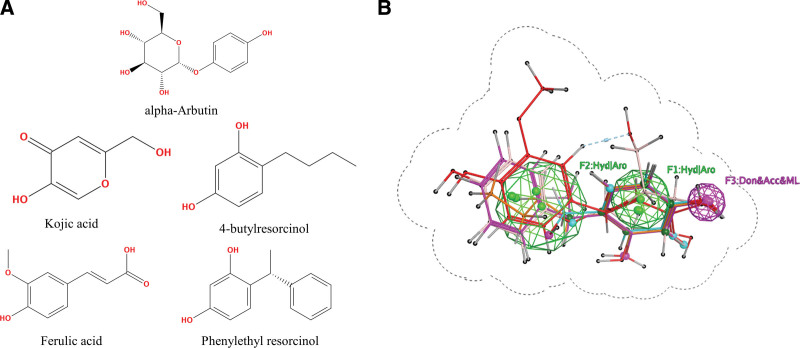
In order to identify natural products that can alleviate melanin deposition and exert skin-whitening effects, we used the “Flexible Alignment” module of MOE software to align the structures of 5 TYR inhibitors (alpha-arbutin, kojic acid, 4-butylresorcinol, ferulic acid, and phenylethyl resorcinol) and evaluated the quality of the aligned models using a score (similarity of the aligned structures and energy stability). “Pharmacophore Query” was used to construct pharmacophores for the aligned models, and the excluded volume was set based on the structure of the aligned models. Fifteen randomly selected compounds and fifteen TYR inhibitors involved in pharmacophore construction were used to construct a validation set for determining the accuracy of the pharmacophore model. Pharmacophore screening was performed using a pharmacophore model with good accuracy to screen a library of natural compounds (totaling 19,505 compounds). Molecular docking of matching compounds with TYR was performed. Finally, potential inhibitors of TYR were screened based on affinity and RMSD values.
2.6. Molecular dynamic simulation validation
Active compounds with good TYR-binding ability in the molecular docking results were selected for molecular dynamic simulation using Amber 18 software. The Amber 99sb force field was selected for 50-ns simulations. The molecular mechanics/Poisson-Boltzmann surface area (MM/PBSA) method was used to calculate the Gibbs free energy of binding of the active compound to the TYR protein and the contribution of amino acid residues at 6 Å from the active compound to the free energy of binding. The Gibbs free energy, RMSD, solvent-accessible surface area (SASA), root mean square fluctuation (RMSF), and energy decomposition analysis were used to assess the binding ability of active compounds to TYR.
3. Results
3.1. Analysis of TCM information of SBD
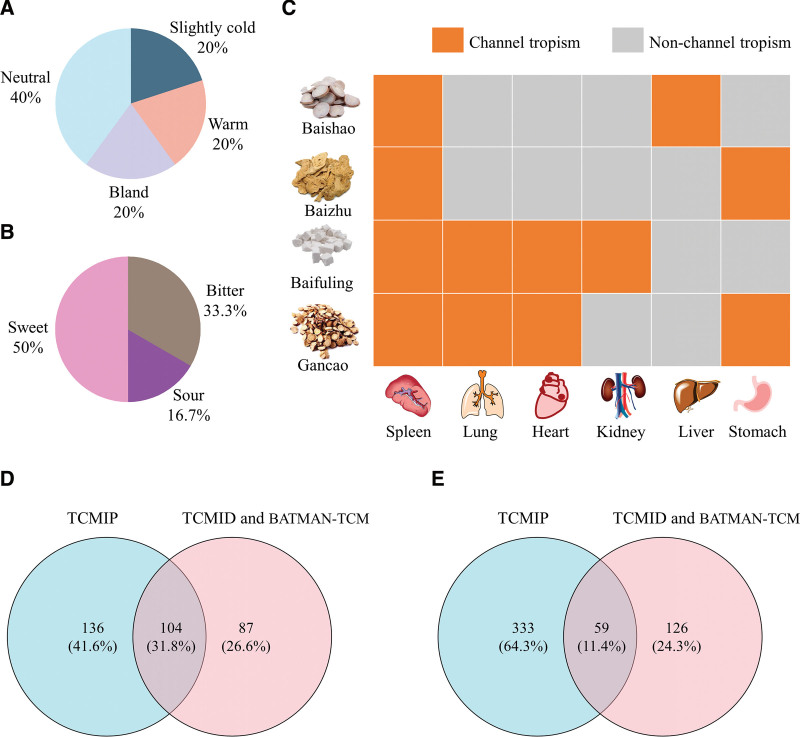
The overall medicinal property of SBD is neutral, with the property of warmth (Fig. 1A), and its flavor is predominantly sweet (Fig. 1B). Paeoniae radix alba (Baishao), Atractylodis macrocephalae rhizome (Baizhu), Poria (Baifuling), and Glycyrrhizae radix et rhizome (Gancao) in SBD are classified to the spleen meridian (Fig. 1C), and thus SBD may exert its skin-whitening effects through the spleen meridian (Table S1, Supplemental Digital Content, http://links.lww.com/MD/I734). The active compounds of SBD were searched and screened using the TCMIP, TCMID, and BATMAN-TCM databases, with 240 active compounds (QED ≥ 0.80) obtained from the TCMIP database and 191 active compounds (score cutoff ≥ 20.00) obtained from the TCMID and BATMAN-TCM databases. Active compounds screened from the 3 databases were merged, and duplicates were removed, yielding 327 active components in SBD (Fig. 1D). The TCMIP database compounds had 392 targets, whereas the TCMID and BATMAN-TCM database compounds had 185 targets, with a total of 518 targets from the 3 databases (Fig. 1E). These were used as the target genes of compounds in SBD for subsequent analysis.
Figure 1.
TCM information of SBD. (A) Percentage of medicinal properties. (B) Percentage of medicinal flavors. (C) Meridian attribution of herbs. (D) Number of active compounds contained in SBD. (E) Number of targets of SBD. BATMAN-TCM = Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine, SBD = San-Bai decoction, TCM = Traditional Chinese medicine, TCMID = Traditional Chinese Medicines Integrated Database, TCMIP = The Integrative Pharmacology-based Research Platform of Traditional Chinese Medicine.
3.2. Construction of SBD “TYR-PIN” and target enrichment analysis
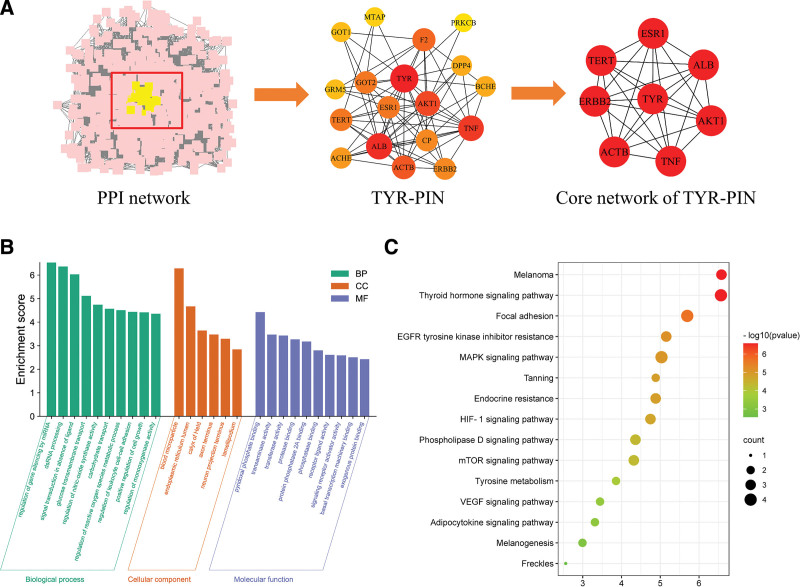
The 518 target genes of SBD were imported into the Search Tool for the Retrieval of Interacting Genes/Proteins database, and a PPI network was constructed (Fig. 2A). TYR and targets interacting with TYR were extracted to construct TYR-PIN, a network of 18 genes (Fig. 2A, TYR-PIN), suggesting that SBD may regulate genes in TYR-PIN to exert skin-whitening and blemish-removal effects. The MCODE module was used to extract the core network of TYR-PIN through which SBD exerts skin-whitening effects (Fig. 2A). The core network contains 8 genes, including TYR, ESR1, TNF, and AKT1. TYR is a key rate-limiting enzyme in melanin synthesis.[2] The estrogen receptor ESR1 is present in human melanocytes, with the binding of estrogen to ESR1 activating second messenger signaling to increase TYR activity and promote pigment synthesis by melanocytes.[7,30–32] Tumor necrosis factor (TNF) regulates lipid metabolism, among other biological processes, and is associated with various diseases such as atopic dermatitis, psoriasis, and sepsis.[33] The AKT1 pathway plays an important role in skin wound healing as well as in delaying and preventing cellular senescence,[34] suggesting that SBD may regulate these targets to treat skin darkening, ameliorate melanin deposition, and delay skin aging.
Figure 2.
Enrichment analysis of the SBD whitening mechanism regulatory network and its targets. (A) PPI network of SBD targets (PPI network), TYR and its interacting proteins (TYR-PIN), and the core network of TYR-PIN (Core network of TYR-PIN). (B) GO enrichment analysis of TYR-PIN function. (C) KEGG enrichment analysis of TYR-PIN signaling pathways. GO = gene ontology, KEGG = Kyoto Encyclopedia of Genes and Genomes, MAPK = Mitogen-Activated Protein Kinase, PPI = protein–protein interaction network, SBD = San-Bai decoction, TYR = tyrosinase, TYR-PIN = TYR–protein–protein interaction network.
The GO term enrichment analysis of the 18 core targets in TYR-PIN revealed that the genes are primarily involved in oxidative stress and cell growth biological processes, including the regulation of reactive oxygen species metabolic process, positive regulation of cell growth, pyridoxal phosphate binding, regulation of nitric-oxide synthase activity, and protease binding (Fig. 2B). This finding suggests that SBD may play an antioxidant role through these biological pathways to promote the repair of oxidative damage in the skin and delay cellular aging. The Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis of the 18 targets in TYR-PIN revealed that these are primarily involved in signaling pathways associated with melanin synthesis, pigment deposition, and skin damage, including tyrosine metabolism, melanogenesis, melanoma, tanning, and freckle formation (Fig. 2C). This finding suggests that SBD may control tyrosine metabolism and melanin synthesis in the skin by regulating TYR-PIN to regulate oxidative stress damage and skin aging, thus decreasing skin pigmentation and exerting skin-whitening, blemish-removal, and skin-protection effects.
3.3. Construction of a TYR-PIN “herb–active compound–core target” network
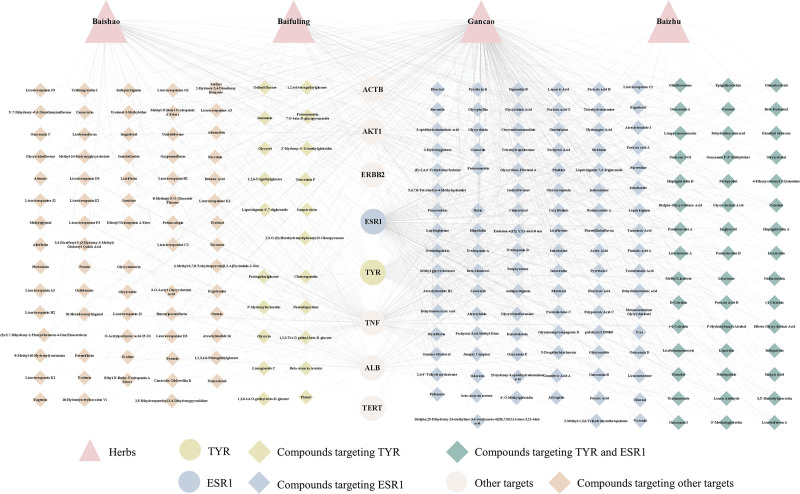
A literature search for targets in the core network of TYR-PIN revealed that TYR and ESR1 are closely associated with melanin deposition.[7,30–32] To discover further active compounds in SBD that target the core factors TYR and ESR1 to exert skin-whitening effects, we used Cytoscape to construct a “herbs–active compounds–targets” network for SBD (Fig. 3). We found 66 active compounds targeting TYR, including pentagalloylglucose, 1,3,6-tri-O-galloyl-beta-D-glucose, 1,2,4,6-tetragalloylglucose, liquiritigenin 4′,7-diglucoside, and 1,2,3-tri-O-galloyl-beta-D-glucose, in addition to 142 active compounds targeting ESR1, including glycyrrhizic acid, pachymic acid methyl ester, nicotiflorin, gamma-sitosterol, and isoliensinine (Fig. 3, Table S2, Supplemental Digital Content, http://links.lww.com/MD/I735). This finding indicates that SBD can exert skin-whitening and blemish-removal effects through multiple herbs, active compounds, and targets.
Figure 3.
The “herb-active compounds–targets” network of SBD. ESR1 = Estrogen Receptor 1, SBD = San-Bai decoction, TYR = tyrosinase.
3.4. Molecular docking validation of core targets and target active compounds
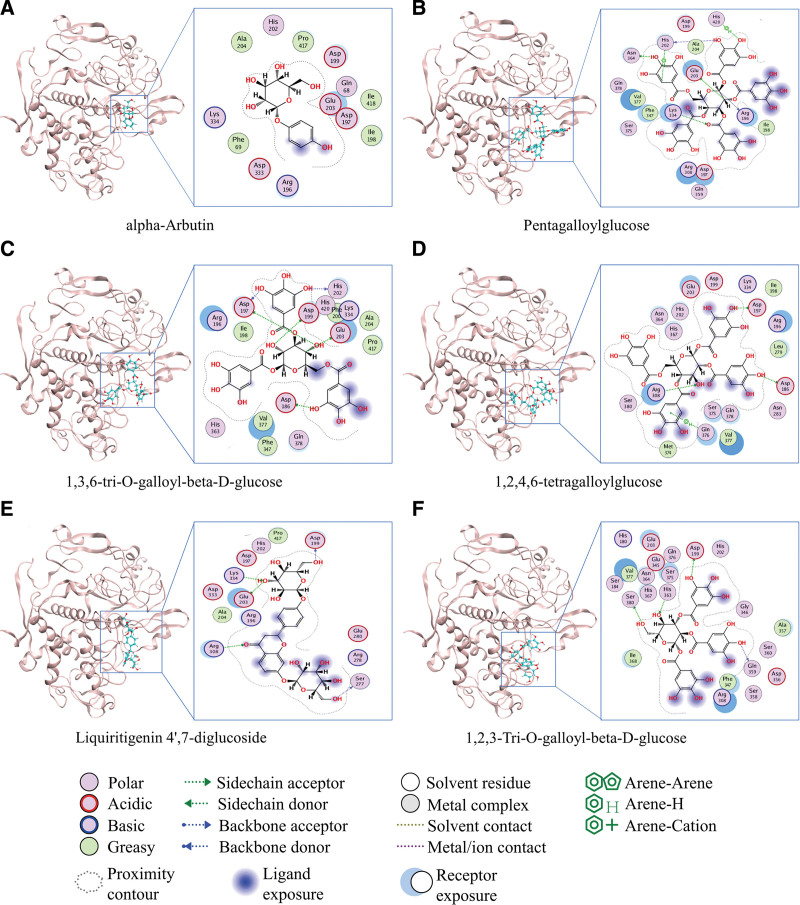
Based on the interaction relationships in the SBD “herbs-active compounds-targets” network, molecular docking was used to validate the ability of active compounds to bind to the core targets TYR and ESR1. Molecular docking of 66 and 142 active compounds for TYR and ESR1, respectively, was performed, and the top 5 compounds with the highest free energy of binding (affinity) were selected as compounds with stable binding. Four of the 5 compounds with binding ability (affinity < −6.83) to TYR were from Paeoniae radix alba (Baishao) (Table 1), and all were galloylglucose analogs primarily composed of β-D-glucose and multiple galloyl groups. The molecular docking pattern of positive control alpha-Arbutin and TYR (Fig. 4A). Pentagalloylglucose forms hydrogen bonds with Arg-196, His-202, Glu-203, Lys-334, and Asn-364 of TYR, in addition to coordination bonds with His-202 and His-420 (Fig. 4B). 1,3,6-Tri-O-galloyl-beta-D-glucose forms hydrogen bonds with Asp-186, Asp-197, Asp-199, His-202, and Glu-203 of TYR (Fig. 4C). 1,2,4,6-Tetragalloylglucose forms hydrogen bonds with Asp-186, Asp-197, and Arg-308 of TYR as well as ligand bonds with Gln-376 (Fig. 4D). 1,2,3-Tri-O-galloyl-beta-D-glucose forms hydrogen bonds with Asp-199, Gln-359, His-363, and Ser-380 of TYR (Fig. 4F). Liquiritigenin 4′,7-diglucoside is derived from Glycyrrhizae radix et rhizome (Gancao) and is a flavonoid derivative composed primarily of β-D-glucose and a flavonoid backbone (2-phenylchromanone), forming hydrogen bonds with Asp-199, Glu-203, Arg-308, and Lys-334 of TYR (Fig. 4E). Multiple compounds interacted with Asp-199, His-202, and Glu-203 of TYR; therefore, it was hypothesized that these sites might be essential for the catalytic activity of TYR. In addition, most of the compounds with good binding ability to ESR1 (affinity < −7.89) were from Glycyrrhizae radix et rhizome (Gancao) and Poria (Baifuling) (Table 1), with multiple interactions of stable binding formed between these compounds and ESR1 (Figure S1, Supplemental Digital Content, http://links.lww.com/MD/I736). Paeoniae radix alba (Baishao), Glycyrrhizae radix et rhizome (Gancao), and Poria (Baifuling) contain multiple active compounds that directly target and modulate TYR and ESR1, suggesting that these compounds may be candidate active compounds that regulate melanin synthesis and exert whitening effects.
Table 1.
Molecular docking of core targets and their targeted active compounds in San-Bai decoction.
| Target protein | Compound name | Source | Affinity (kcal/mol) | RMSD (Å) |
|---|---|---|---|---|
| TYR (ID:AF-P14679-F1) | alpha-Arbutin | Control | −6.83 | 1.33 |
| Pentagalloylglucose | Baishao | −11.39 | 1.82 | |
| 1,3,6-tri-O-galloyl-beta-D-glucose | Baishao | −9.91 | 1.87 | |
| 1,2,4,6-tetragalloylglucose | Baishao | −9.41 | 1.61 | |
| Liquiritigenin 4’,7-diglucoside | Gancao | −9.31 | 1.62 | |
| 1,2,3-Tri-O-galloyl-beta-D-glucose | Baishao | −8.52 | 1.86 | |
| ESR1 (PDBID: 7RS7) | Progesterone | Control | −7.89 | 1.36 |
| Glycyrrhizic Acid | Gancao | −11.03 | 1.60 | |
| Pachymic Acid Methyl Ester | Baifuling | −10.11 | 1.46 | |
| Nicotiflorin | Gancao | −10.01 | 1.76 | |
| Gamma-Sitosterol | Gancao | −9.97 | 1.57 | |
| Isoliensinine | Gancao | −9.95 | 1.44 |
ESR1 = Estrogen Receptor 1, ID = AlphaFold Protein Structure Database ID, PDBID = RCSB Protein Data Bank ID, RMSD = root mean square deviation, TYR = tyrosinase.
Figure 4.
Molecular docking pattern of TYR with its target active compounds in SBD. SBD = San-Bai decoction, TYR = tyrosinase.
3.5. Virtual screening of TYR inhibitors derived from natural products
TYR inhibitors have been widely used in skin-whitening products. To screen for TYR inhibitors derived from natural products, we performed virtual screening of a library of small molecule compounds from natural products (a total of 19,505 compounds). First, a pharmacophore model was constructed using TYR inhibitors alpha-arbutin, kojic acid, 4-butylresorcinol, ferulic acid, and phenylethyl resorcinol[35–37] (Fig. 5A), which have well-defined pharmacological effects and low toxicity. The pharmacophore model contains 3 key features and an excluded volume with a radius of 1.6 Å (Fig. 5B). In the validation set (which comprised 15 random compounds and 5 TYR inhibitors included in the construction of the pharmacophore), the pharmacophore model matched 4 TYR inhibitors (alpha-arbutin, kojic acid, 4-butylresorcinol, and phenylethyl resorcinol) with good accuracy. The library of small-molecule compounds from natural products was screened using this pharmacophore model, and a total of 1350 were screened out, with a match rate of 6.92% (1350/19,505). Next, molecular docking of the screened compounds with TYR was performed, and 11 compounds were found to bind stably to TYR (affinity < −6.83, RMSD < 2.00), for a screening rate of 0.056% (11/19,505). The top 5 compounds were selected as potential inhibitors of TYR (Table 2, Figure S2, Supplemental Digital Content, http://links.lww.com/MD/I737).
Figure 5.
Pharmacophore model of TYR inhibitors for virtual screening. (A) TYR inhibitors involved in the construction of the pharmacophore model. (B) Pharmacophore model of TYR inhibitors. Pharmacophore properties: Hyd: hydrophobicity; Aro: aromaticity; Don: donor; Acc: acceptor; ML: metallicity. Dashed lines indicate boundaries of excluded volume. TYR = tyrosinase.
Table 2.
Potential tyrosinase inhibitors derived from natural products.
| Compound ID | Compound name | Affinity (kcal/mol) | RMSD (Å) |
|---|---|---|---|
| T5368 | Eicosapentaenoic acid | −7.65 | 1.86 |
| T2725 | Scopolamine HBr | −7.14 | 1.86 |
| TCFN00287 | Lycopsamine | −7.13 | 1.15 |
| NP-002690 | 2-Phenylethyl b-D-glucopyranoside | −7.01 | 1.82 |
| T21532 | 6-beta-Hydroxyhyoscyamine | −6.95 | 1.97 |
RMSD = root mean square deviation.
3.6. Molecular dynamics simulation validation
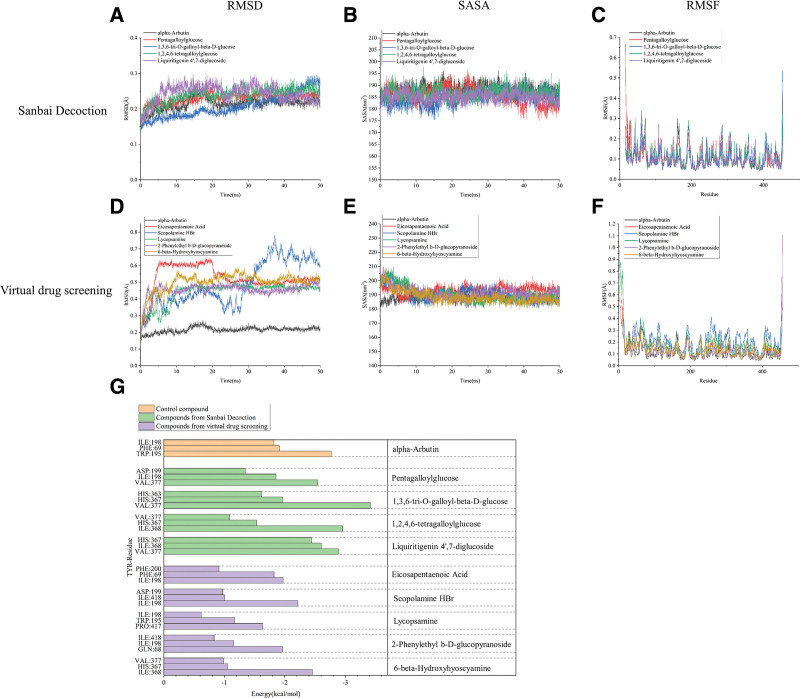
To further elucidate the binding ability between TYR and the active compounds targeting TYR in SBD and the natural product libraries, active compounds having molecular docking results with a free energy of binding to TYR (affinity) of less than −9.00 were selected for molecular dynamic simulation. Alpha-arbutin, a known inhibitor of TYR, was chosen as a control. Among the compounds screened out from SBD, the RMSD values of pentagalloylglucose, 1,3,6-tri-O-galloyl-beta-D-glucose, 1,2,4,6-tetragalloylglucose, and liquiritigenin 4′,7-diglucoside stabilized at 0.15 to 0.25 Å after 10 ns (Fig. 6A), the SASA values stabilized at 180 to 190 nm2 (Fig. 6B), and the Gibbs free energy of binding to TYR was low (<−28.83 kJ/mol) (Table 3). The evaluation indexes were similar to the value of the control compound, suggesting that these compounds in SBD are potential TYR inhibitors. The RMSF and energy decomposition analysis results suggest that the amino acids near the active site of TYR have greater flexibility when TYR binds to these compounds (Fig. 6C), and amino acid residues Ile:198, His:367, and Val:377 provide more energy when compounds are bound to the protein (Fig. 6G), suggesting that these may be the amino acid residues essential for binding between the active compounds and TYR.
Figure 6.
Molecular dynamics simulation of TYR with its potential inhibitors. (A–C) RMSD, SASA, and RMSF of binding between SBD-derived active compounds and TYR. (D–F) RMSD, SASA, and RMSF of binding between the natural product library active compounds and TYR. (G) Amino acid energy decomposition analysis of TYR with its potential. RMSD = root mean square deviation, RMSF = root mean square fluctuation, SASA = solvent-accessible surface area, SBD = San-Bai decoction, TYR = tyrosinase.
Table 3.
Gibbs free energy of tyrosinase with its potential inhibitors.
| Target protein | Compound name | Source | Gibbs free energy (kJ/mol) |
|---|---|---|---|
| TYR (ID:AF-P14679-F1) | alpha-Arbutin | Control | −35.32 |
| Pentagalloylglucose | Baishao | −38.10 | |
| 1,3,6-tri-O-galloyl-beta-D-glucose | Baishao | −32.07 | |
| 1,2,4,6-tetragalloylglucose | Baishao | −41.01 | |
| Liquiritigenin 4′,7-diglucoside | Gancao | −28.83 | |
| Eicosapentaenoic Acid | Virtual screening | −9.86 | |
| Scopolamine HBr | Virtual screening | −25.17 | |
| Lycopsamine | Virtual screening | −17.05 | |
| 2-Phenylethyl b-D-glucopyranoside | Virtual screening | −26.18 | |
| 6-beta-Hydroxyhyoscyamine | Virtual screening | −27.83 |
ID = AlphaFold Protein Structure Database ID, TYR = tyrosinase.
In the library of natural product compounds, the RMSD values of lycopsamine, 2-phenylethyl b-D-glucopyranoside, and 6-beta-hydroxyhyoscyamine obtained from virtual drug screening stabilized at 0.45 to 0.55 Å after 10 ns (Fig. 6D), the SASA values stabilized at 185 to 195 nm2 (Fig. 6E), and the Gibbs free energy of binding to TYR was low (<−17.05 kJ/mol) (Table 3). These molecular dynamic simulation evaluation indexes were similar to those of the control compounds, suggesting that these compounds bind stably to TYR. The RMSF and energy decomposition analysis results suggest that the amino acids at the active site of TYR are more flexible upon the binding of TYR to lycopsamine, 2-phenylethyl b-D glucopyranoside, or 6-beta-hydroxyhyoscyamine (Fig. 6F), with the amino acid residue Ile:198 providing more energy when the protein is bound by compounds, suggesting that Ile:198 is essential for the binding interaction (Fig. 6G).
4. Discussion
Melanin is the main pigment affecting skin color in humans, and its accumulation in melanocytes leads to pigmentation-related skin conditions such as freckles, melasma, and melanoma. Therefore, reducing melanin synthesis is the primary approach for skin whitening and blemish removal.[2] TYR is a key rate-limiting enzyme in melanin synthesis and plays an important role in maintaining the stability of TYR on the melanosomal membrane, thus inhibiting the death of immature melanocytes.[38–40] Currently, common chemical-based skin-whitening products can destroy melanocytes directly but exhibit some cytotoxicity,[41,42] whereas biological skin-whitening agents are safer, yet generally have longer production cycles and are more expensive.[43] Meanwhile, natural skin-whitening products are safe, abundant, and economical[8,42,43]; therefore, TCM formulations hold great potential in skin-whitening research. Based on the TCM theory, the search for safe, nontoxic, and efficient TYR inhibitors derived from Chinese herbs is becoming a popular research topic with promising market prospects.[6]
The traditional formulation SBD consists of 4 Chinese herbs, namely Paeoniae radix alba (Baishao), Atractylodis macrocephalae rhizome (Baizhu), Poria (Baifuling), and Glycyrrhizae radix et rhizome (Gancao). The active compounds of SBD include flavonoids, polysaccharides, and triterpenoid saponins, which have TYR-inhibiting, antioxidant, anti-inflammatory, and immunostimulatory pharmacological effects, inhibiting melanogenesis.[12] The TYR-PIN network regulated by SBD is associated with skin-related signaling pathways (melanoma, tanning, and freckles), and SBD may regulate TYR activity and inhibit melanogenesis through tyrosine metabolism and melanogenesis signaling pathways. In addition, it may regulate the Mitogen-Activated Protein Kinase (MAPK) signaling pathway, one of the most important pathways in aging, to alleviate skin aging. The MAPK pathway can regulate melanin production and levels, alleviating skin inflammatory responses.[44] Several anti-aging drugs have been shown to reduce inflammatory stress damage in the skin by inhibiting MAPK signaling.[45]
The main active compounds in SBD that bind stably with TYR are derived from P. lactiflora, namely pentagalloylglucose, 1,3,6-tri-O-galloyl-beta-D-glucose, 1,2,4,6-tetragalloylglucose, and liquiritigenin 4′,7-diglucoside. In pigmented human skin models, peony extract and paeoniflorin have been found to be efficacious in reducing melanin deposition and skin whitening.[14] All of these active compounds are gallic acid glycosides with anti-aging, skin-whitening, and anti-inflammatory pharmacological effects.[46] Gallic acid can suppress melanin formation by inhibiting tyrosinase,[47] and could reduce immunoglobulin E and TNF-α levels in the serum of an atopic dermatitis mouse model to improve skin inflammation.[48] Gallic acid has also been shown to reduce lipid peroxidation, and thereby ameliorates oxidative stress injury to the skin.[46] Paeoniae radix rubra (Chishao), Chebulae fructus (Hezi) and Canavaliae semen (Daodou) also contain these TYR inhibitors, indicating that these TCM may also have whitening effects (Table S3, Supplemental Digital Content, http://links.lww.com/MD/I738). ESR1 is a prominent protein in the SBD-regulated TYR-PIN network, also associated with mechanisms of melanogenesis,[7,30–32] and compounds that stably bind ESR1 are also present in SBD, namely glycyrrhizic acid, pachymic acid methyl ester, nicotiflorin, gamma-sitosterol, and isoliensinine. In addition, we constructed pharmacophore models with known TYR inhibitors. Based on pharmacophore characteristics, molecular docking, and molecule dynamic simulation, lycopsamine, 2-phenylethyl b-D-glucopyranoside, and 6-beta-hydroxyhyoscyamine were screened out from a library of natural product-derived compounds as active compounds that can stably bind to TYR, thus representing natural TYR inhibitor candidates.
5. Conclusion
In this study, we constructed a molecular network centered on the regulation of TYR by SBD in skin whitening and identified potential mechanisms underlying the skin-whitening effects of SBD from a biomolecular network perspective. We found that TYR and ESR1 might be key targets for the skin-whitening effects of SBD, and screened for active compounds in SBD with the ability to stably bind to TYR and ESR1. In addition, the pool of potential TYR inhibitors derived from natural products was expanded using a virtual drug screening approach. The comprehensive utilization of bioinformatic, pharmacological, and biochemistry approaches is expected to generate novel breakthroughs in drug discovery for skin whitening. Nevertheless, the present study had some limitations, such as the limited database of herbal compounds and targets used, as some compounds and targets were not within the scope of the study, in addition to the limited number of natural small-molecule compounds for virtual drug screening. However, the study still provides a theoretical basis for research on SBD and screening for natural products that potentially target TYR and ESR1 for skin-whitening effects.
Author contributions
Conceptualization: Liyuan Li, Yiran Tang, Xin Li, Tao Zhou, Qiuhang Song.
Formal analysis: Liyuan Li, Yiran Tang, Xin Li.
Funding acquisition: Qiuhang Song, Aiying Li.
Methodology: Liyuan Li, Yiran Tang, Xin Li, Tao Zhou.
Software: Liyuan Li, Yiran Tang.
Supervision: Qiuhang Song, Aiying Li.
Validation: Yiran Tang, Xin Li, Tao Zhou.
Visualization: Liyuan Li.
Writing – original draft: Liyuan Li, Yiran Tang, Qiuhang Song.
Writing – review & editing: Qiuhang Song, Aiying Li.
Supplementary Material
Abbreviations:
- AKT1
- threonine kinase 1
- BATMAN-TCM
- Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine
- ESR1
- Estrogen Receptor 1
- GO
- gene ontology
- MAPK
- Mitogen-Activated Protein Kinase
- MOE
- Molecular Operating Environment
- PPI
- protein–protein interaction network
- QED
- quantitative estimate of drug-likeness
- RMSD
- root mean square deviation
- RMSF
- root mean square fluctuation
- SASA
- solvent-accessible surface area
- SBD
- San-Bai decoction
- TCM
- Traditional Chinese medicine
- TCMID
- Traditional Chinese Medicines Integrated Database
- TCMIP
- The Integrative Pharmacology-based Research Platform of Traditional Chinese Medicine
- TNF
- tumor necrosis factor
- TYR
- tyrosinase
- TYR-PIN
- TYR–protein–protein interaction network
This research was funded by Projects of Medical Science Research of Hebei Province (no. 20221482 and 20210258); Project of Hebei Provincial Administration of Traditional Chinese Medicine (no. 2022087); Science and Technology Project of Hebei Provincial Education Department (no. QN2021106); Doctoral Research Funding Project of Hebei University of Chinese Medicine (no. BSZ2020011).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Li L, Tang Y, Li X, Zhou T, Song Q, Li A. Mechanism of skin whitening through San-Bai decoction-induced tyrosinase inhibition and discovery of natural products targeting tyrosinase. Medicine 2023;102:13(e33420).
Contributor Information
Liyuan Li, Email: lay1963@126.com.
Yiran Tang, Email: tangyiran9@163.com.
Xin Li, Email: lay1963@126.com.
Tao Zhou, Email: 442712808@qq.com.
Aiying Li, Email: lay1963@126.com.
References
- [1].Pillaiyar T, Manickam M, Namasivayam V. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2017;32:403–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chunhakant S, Chaicharoenpong C. Antityrosinase, antioxidant, and cytotoxic activities of phytochemical constituents from Manilkara zapota L. bark. Molecules. 2019;24:2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guo MS, Wu Q, Lai QWS, et al. A prepared platelet-rich plasma extract, namely Self-Growth Colony, inhibits melanogenesis by down-regulating microphthalmia-associated transcription factor in skin melanocyte. J Cosmet Dermatol. 2021;20:3278–88. [DOI] [PubMed] [Google Scholar]
- [4].Liu SC, Sheu ML, Tsai YC, et al. Attenuation of in vitro and in vivo melanin synthesis using a Chinese herbal medicine through the inhibition of tyrosinase activity. Phytomedicine. 2022;95:153876. [DOI] [PubMed] [Google Scholar]
- [5].Huang S, Chen F, Cheng H, et al. Modification and application of polysaccharide from traditional Chinese medicine such as Dendrobium officinale. Int J Biol Macromol. 2020;157:385–93. [DOI] [PubMed] [Google Scholar]
- [6].Mukherjee PK, Biswas R, Sharma A, et al. Validation of medicinal herbs for anti-tyrosinase potential. J Herb Med. 2018;14:1–16. [Google Scholar]
- [7].Wang JY, Wang XQ, Tang Y, et al. [The network pharmacological mechanisms of four anti-vitiligo Uyghur medicines based on Phlegmatic temperament theory]. Zhongguo Zhong Yao Za Zhi. 2018;43:1780–8. [DOI] [PubMed] [Google Scholar]
- [8].Ribeiro AS, Estanqueiro M, Oliveira MB, et al. Main benefits and applicability of plant extracts in skin care products. Cosmetics. 2015;2:48–65. [Google Scholar]
- [9].Park JJ, Hwang SJ, Kang YS, et al. Synthesis of arbutin-gold nanoparticle complexes and their enhanced performance for whitening. Arch Pharm Res. 2019;42:977–89. [DOI] [PubMed] [Google Scholar]
- [10].Luo TT, Lu Y, Yan SK, et al. Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chin J Integr Med. 2020;26:72–80. [DOI] [PubMed] [Google Scholar]
- [11].Li C. Introduction to Medicine. Beijing, China: People’s Medical Publishing House; 2006. [Google Scholar]
- [12].Tao Y, Su D, Du Y, et al. Magnetic solid-phase extraction coupled with HPLC-Q-TOF-MS for rapid analysis of tyrosinase binders from San-Bai decoction by Box–Behnken statistical design. RSC Adv. 2016;6:109730–41. [Google Scholar]
- [13].Lee H, Cha HJ. Poria cocos Wolf extracts represses pigmentation in vitro and in vivo. Cell Mol Biol (Noisy-le-grand). 2018;64:80–4. [PubMed] [Google Scholar]
- [14].Qiu J, Chen M, Liu J, et al. The skin-depigmenting potential of Paeonia lactiflora root extract and paeoniflorin: in vitro evaluation using reconstructed pigmented human epidermis. Int J Cosmet Sci. 2016;38:444–51. [DOI] [PubMed] [Google Scholar]
- [15].Zaid AN, Al Ramahi R. Depigmentation and anti-aging treatment by natural molecules. Curr Pharm Des. 2019;25:2292–312. [DOI] [PubMed] [Google Scholar]
- [16].CPC. Pharmacopoeia of the People’s Republic of China. Beijing, China: China Medical Science Press; 2020. [Google Scholar]
- [17].Xu HY, Zhang YQ, Liu ZM, et al. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019;47:D976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang L, Xie D, Yu Y, et al. TCMID 2.0: a comprehensive resource for TCM. Nucleic Acids Res. 2018;46:D1117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu Z, Guo F, Wang Y, et al. BATMAN-TCM: a bioinformatics analysis tool for molecular mechANism of traditional Chinese medicine. Sci Rep. 2016;6:21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1–33. [DOI] [PubMed] [Google Scholar]
- [22].Bu D, Luo H, Huo P, et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49:W317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim S, Chen J, Cheng T, et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49:D1388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sterling T, Irwin JJ. ZINC 15--Ligand discovery for everyone. J Chem Inf Model. 2015;55:2324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Burley SK, Bhikadiya C, Bi C, et al. RCSB Protein Data Bank: powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021;49:D437–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Varadi M, Anyango S, Deshpande M, et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thornton MJ, Taylor AH, Mulligan K, et al. Oestrogen receptor beta is the predominant oestrogen receptor in human scalp skin. Exp Dermatol. 2003;12:181–90. [DOI] [PubMed] [Google Scholar]
- [31].Bu J, Ma PC, Chen ZQ, et al. Inhibition of MITF and tyrosinase by paeonol-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am J Chin Med. 2008;36:245–63. [DOI] [PubMed] [Google Scholar]
- [32].Bechmann N, Calsina B, Richter S, et al. Therapeutic potential of nitric oxide–releasing selective estrogen receptor modulators in malignant melanoma. J Invest Dermatol. 2022;142:2217–27. [DOI] [PubMed] [Google Scholar]
- [33].Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Somanath PR, Chen J, Byzova TV. Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis. 2008;11:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mann T, Gerwat W, Batzer J, et al. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J Invest Dermatol. 2018;138:1601–8. [DOI] [PubMed] [Google Scholar]
- [36].Kopke D, Muller RH, Pyo SM. Phenylethyl resorcinol smartLipids for skin brightening – Increased loading & chemical stability. Eur J Pharm Sci. 2019;137:104992. [DOI] [PubMed] [Google Scholar]
- [37].Schmaus G, Vielhaber G, Jacobs K, et al. 4-(1-Phenylethyl) 1,3-benzenediol: a new highly potent lightening agent. J Cosmet Sci. 2006;57:197–8. [PubMed] [Google Scholar]
- [38].Lai X, Wichers HJ, Soler-Lopez M, et al. Structure and function of human tyrosinase and tyrosinase-related proteins. Chemistry. 2018;24:47–55. [DOI] [PubMed] [Google Scholar]
- [39].Li C, Chen Q, Wu J, et al. Identification and characterization of two novel noncoding tyrosinase (TYR) gene variants leading to oculocutaneous albinism type 1. J Biol Chem. 2022;298:101922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Feeney MB, Schoneich C. Tyrosine modifications in aging. Antioxid Redox Signal. 2012;17:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Takizawa T, Imai T, Onose J, et al. Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis(2-hydroxypropyl) nitrosamine or N-diethylnitrosamine. Toxicol Sci. 2004;81:43–9. [DOI] [PubMed] [Google Scholar]
- [42].Hashim FJ, Vichitphan S, Han J, et al. Alternative approach for specific tyrosinase inhibitor screening: uncompetitive inhibition of tyrosinase by Moringa oleifera. Molecules. 2021;26:4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ding Y, Jiratchayamaethasakul C, Lee SH. Protocatechuic aldehyde attenuates UVA-induced photoaging in human dermal fibroblast cells by suppressing MAPKs/AP-1 and NF-kappaB signaling pathways. Int J Mol Sci. 2020;21:4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Choi JW, Lee J, Park YI. 7,8-Dihydroxyflavone attenuates TNF-alpha-induced skin aging in Hs68 human dermal fibroblast cells via down-regulation of the MAPKs/Akt signaling pathways. Biomed Pharmacother. 2017;95:1580–7. [DOI] [PubMed] [Google Scholar]
- [46].Monteiro e Silva SA, Calixto GMF, Cajado J, et al. Gallic acid-loaded gel formulation combats skin oxidative stress: development, characterization and ex vivo biological assays. Polymers (Basel). 2017;9:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chaikul P, Khat-Udomkiri N, Iangthanarat K, et al. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB niosome. Eur J Pharm Sci. 2019;131:39–49. [DOI] [PubMed] [Google Scholar]
- [48].Hu G, Zhou X. Gallic acid ameliorates atopic dermatitis-like skin inflammation through immune regulation in a mouse model. Clin Cosmet Investig Dermatol. 2021;14:1675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.