Abstract
Background
Cystic lesions of the gnathic bones present challenges in differential diagnosis. This category includes a smorgasbord of odontogenic and non-odontogenic entities that may be reactive or neoplastic in nature. While most cystic jaw lesions are benign, variability in biologic behavior makes distinction between these entities absolutely crucial.
Methods
Review.
Results
Two clinical cases are presented in parallel and are followed by an illustrated discussion of the ten most likely differential diagnoses that should be considered when confronted with a cystic jaw lesion. Strong emphasis is placed on the histologic differences between these entities, empowering readers to diagnose them with confidence. Perhaps even more importantly, the more common diagnostic pitfalls in gnathic pathology are discussed, recognizing that a definitive diagnosis cannot be rendered in every situation. The histologic diagnoses for the two clinical cases are finally revealed.
Conclusion
Cystic lesions of the maxilla and mandible may be odontogenic or non-odontogenic. The most common cystic lesions are the reactive periapical cyst, and the dentigerous cyst (which is developmental in nature). It is important to note that cystic neoplasms also occur in the jaws, and that the presence of inflammation may obscure the diagnostic histologic features of lesions like odontogenic keratocyst and unicystic ameloblastoma. Ancillary testing is of limited diagnostic value in most scenarios. However, both clinical and radiographic information (such as the location, size, duration, associated symptoms, and morphology of the lesion in its natural habitat) are significantly useful.
Keywords: Maxilla, Mandible, Jaws, Odontogenic, Cyst, Differential diagnosis, Review
Introduction
The unique denizens of the maxilla and mandible are, of course, teeth. Odontogenesis, or tooth development, occurs early in life, in two stages: primary teeth are formed first, and are replaced with secondary teeth as our jaws grow and stabilize. The cells that form our teeth are specialized, and tooth buds contain both epithelial and ectomesenchymal components. After odontogenesis is complete, remnants of these original formative structures remain in the gingiva and gnathic bones and are known as odontogenic epithelial rests of Serres and Malassez, respectively. When stimulated by inflammatory or genetic impetuses, these rests may activate to develop odontogenic cysts and tumors. Most odontogenic cysts are reactive and caused by odontogenic infection that travels down the affected tooth’s root canal system into the periapical bone (periapical cyst). The remainder are developmental in nature, and some of these processes are known to be under genetic control (for example, the majority of odontogenic keratocysts exhibit mutations in PTCH1). Accurate classification of odontogenic cysts is essential because of the spectrum of biologic behavior exhibited by these lesions, which directly affects patient treatment and clinical outcomes. While histologic examination remains the most important diagnostic factor, it is essential (albeit a bit unnerving) to note that there is significant histologic overlap between different odontogenic cysts, with inflammation potentially obscuring the true nature of the process. Examination of as much of the cyst lining as possible is strongly encouraged because this increases the likelihood of an accurate diagnosis. To confound matters further, some odontogenic tumors are known to be predominantly cystic: misdiagnosis may occur if the cyst lining epithelium is not scrutinized at high magnification.
The jaws may also house cystic lesions that are not odontogenic in origin. Just a handful of these lesions are true cysts, lined by epithelium (e.g., nasopalatine duct cyst). The simple bone cyst is a degenerative pseudocystic process that is similar histologically to those occurring in non-gnathic bones. The aneurysmal bone cyst is a neoplasm that often shows cystic change but contains a significant solid component as well. While additional non-odontogenic cysts occur in the jawbones, the aforementioned processes are the most common and just they will be discussed in the differential diagnosis.
Clinical Case Descriptions
Case 1
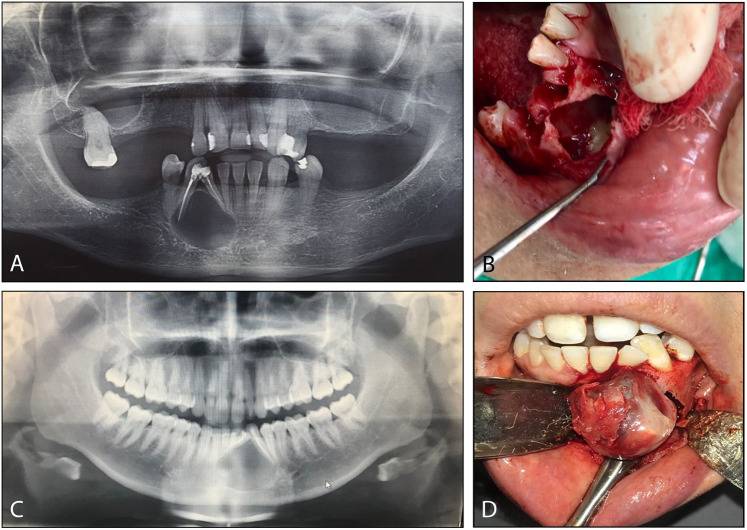
A 56-year-old female presented to her general dentist with a swelling of the right anterior mandible. The lesion gradually increased in size over the past several months and the patient had no pain but noted that her teeth had shifted in position. She had a noncontributory medical history. A panoramic radiograph (Fig. 1A) revealed a well-demarcated unilocular radiolucent lesion situated between the roots of teeth #26 and 27, causing expansion and tooth displacement. Both teeth had been endodontically treated. At surgery, a cystic lesion with a thin lining was curetted out of a bony cavity (Fig. 1B), which contained some whitish debris with a cheese-like consistency. The submitted clinical diagnosis of the removed lesion was radicular (periapical) cyst.
Fig. 1.
Clinical case descriptions. A Panoramic radiograph of Case 1 shows a radiolucent lesion of the anterior mandible, associated with and displacing two teeth. B At surgery in Case 1, a cystic cavity with focal debris was discovered. C Panoramic radiograph of Case 2 reveals a similar well-defined radiolucent lesion of the anterior mandible, associated with two anterior teeth. D At surgery in Case 2, a solid tumor was “shelled out” of a bony cavity with ease
Case 2
A 28-year-old female presented with a nontender swelling of the left anterior mandible, gradually increasing in size over the past 4 months and resulting in tooth displacement. A panoramic radiograph (Fig. 1C) demonstrated a well-delineated unilocular, radiolucent cystic lesion between teeth #22 and 23, with obvious tooth displacement; root canals had been performed on both teeth. A cystic lesion with a thick capsule was relatively easily shelled out of the mandible (Fig. 1D), with intraluminal solid material. The submitted clinical diagnosis was Pindborg tumor.
Discussion of Differential Diagnoses
Odontogenic Cysts
Dentigerous Cyst
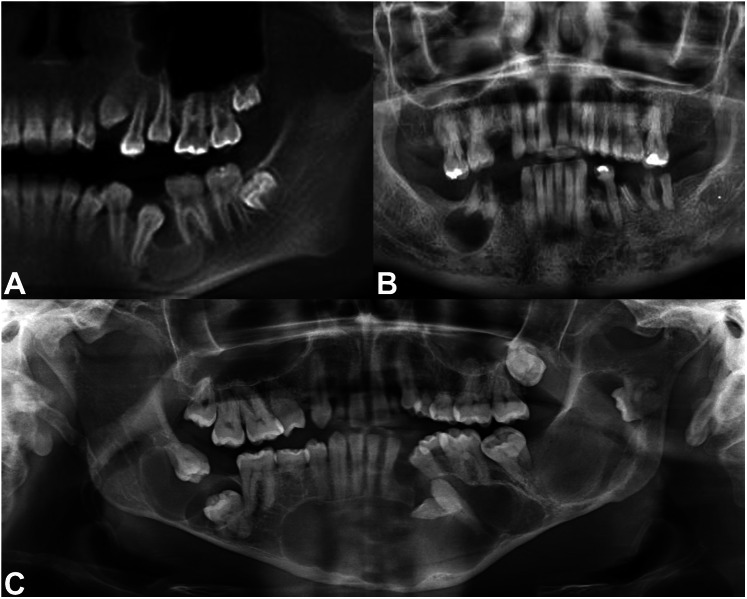
The dentigerous cyst (DC) is a benign odontogenic cyst that is associated with the crown of an unerupted tooth (Fig. 2 A). It is the most common developmental odontogenic cyst (representing 20% of all odontogenic cysts), and often develops around third molars. Less commonly, DC may develop in association with maxillary canine or mandibular premolar teeth [1]. Because persons between the ages of 16 and 25 are routinely screened and are often referred to have their wisdom teeth (3rd molars) removed, DC is seen with increased frequency in this patient population. However, DC may occur in people of any age [2]. In children with mixed dentition, a DC developing around an unerupted permanent tooth may be mistaken for a periapical cyst from the primary tooth. DC appears on radiographs as a well-corticated radiolucency which is usually unilocular. Most patients are asymptomatic, but DCs may become large and cause jaw expansion. By definition, the cystic lining in DC is attached to the cemento-enamel junction, at the neck of the tooth [3].
Fig. 2.
Anatomic locations of gnathic cystic lesions. A A dentigerous relationship is exemplified by the cystic lesion seen here, developing around the crown of an impacted tooth 20 in the left posterior mandible. B This well-defined radiolucent lesion is in a periapical location, directly beneath tooth roots. C At times, multiple cystic lesions may occur, as in this panoramic radiograph of a patient with Gorlin syndrome
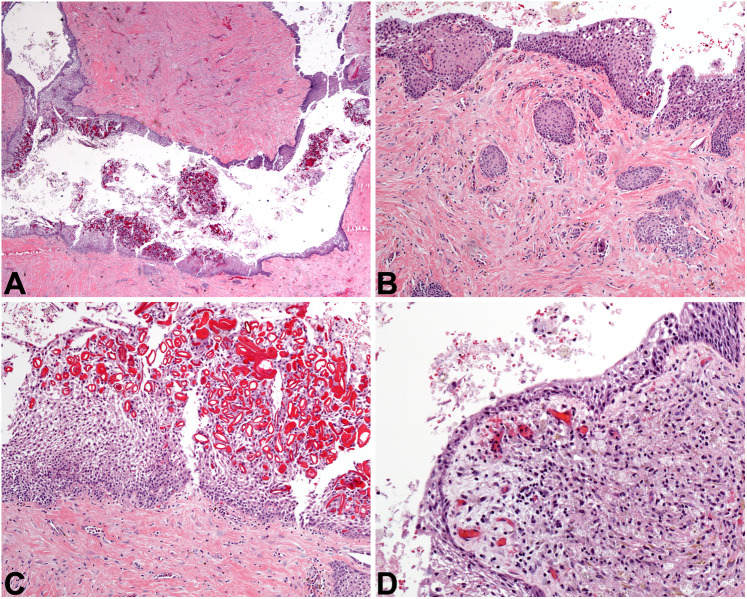
Histologically, DC appears as a squamous-lined cyst, often with inflammation in the fibrous connective tissue wall. The cyst lining epithelium may exhibit some variability in thickness, ranging from 1 to 2 cell layers to 6–8 cell layers thick (Fig. 3A). The interface between the epithelium and the cyst wall is usually flat. However, if the DC is inflamed (as they often are), the squamous epithelium may proliferate and develop rete ridges, acanthosis, and/or spongiosis [2]. Cholesterol clefting may be present if the cyst ruptures. Additionally, DC may contain Rushton bodies (Fig. 3 C) and mucous cells (Fig. 3B); sebaceous cells and cilia are rare [4]. This is a direct reflection of the pluripotentiality of odontogenic epithelial cells. The fibrous connective tissue cyst wall is myxoid to fibrotic (normal dental follicular tissue is myxoid in nature), and odontogenic rests of Malassez may be identified (Fig. 3B). Occasionally, these rests are misinterpreted as primary intraosseous or metastatic carcinoma. However, they are cytologically bland. Dentigerous cysts may become quite large, but they are indolent in nature. Removal of the associated tooth with curettage of the cyst is sufficient treatment, and DC typically does not recur [3, 5].
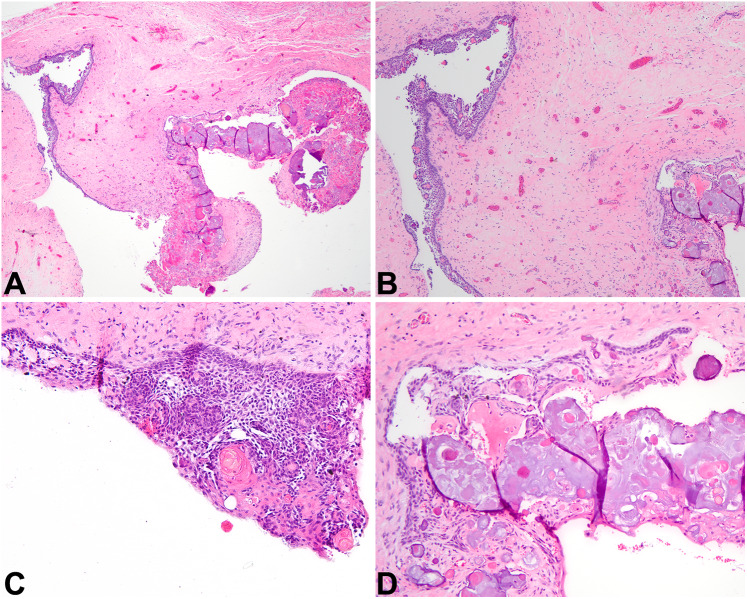
Fig. 3.
Dentigerous cyst and periapical cyst. A This DC at low power demonstrates an epithelial lining and a fibrous connective tissue wall (4x). B Odontogenic rests of Malassez may blend with the cystic lining and may proliferate within the cyst wall of DC or PC, and they may be misinterpreted as invasive squamous cell carcinoma. This DC also exhibits mucous metaplasia (10×). C Rushton bodies are curvilinear eosinophilic formations present in the lining of this DC, but these formations are not specific and may also be found in PC (20×). D A PC is depicted, complete with inflammation, histiocytes, and a thinned epithelial lining (20×)
The key differentials to consider when diagnosing a DC include hyperplastic dental follicle, glandular odontogenic cyst (GOC), odontogenic keratocyst (OKC), and cystic ameloblastoma. A hyperplastic dental follicle occurs when the normal dental follicle around an unerupted tooth grows larger (i.e., greater than 2.5 mm in diameter from the edge of the tooth crown). Hyperplastic dental follicles histologically consist of a myxoid follicular wall with varying amounts of squamous epithelium and reduced enamel epithelium (a residual bilayer of cuboidal cells with eosinophilic cytoplasm that is inactivated after odontogenesis). There is significant overlap with the histologic features of DC, and the distinction is often made based on the clinical size of the lesion (i.e., the lesion may be called DC if the diameter is 5 mm or greater). GOC enters the differential when a DC has uncommon or unusual features, such as mucous cells, cilia, or cuboidal cells. However, a threshold must be met to diagnose a GOC with confidence, and this will be addressed later. OKC has classic histologic features including a palisaded basal layer and “corrugated” parakeratin toward the cyst lumen. Inflamed examples of OKC may be pretty tough to classify, as they lose these distinguishing histologic features. An important question perpetually in the authors’ minds is as follows: “what is the most biologically aggressive lesion you would not want to miss?” As such, the key entity not to miss when considering DC is cystic ameloblastoma. Subtle basal palisading is usually identified in the lining of cystic ameloblastoma, often with a loosening of the cells into a middle stellate reticulum-like layer, with pink keratin toward the luminal surface. However, inflammation may easily obscure these features [6]. Immunohistochemical studies for BRAF V600E and calretinin are useful in distinguishing between DC and cystic ameloblastoma, when these studies are positive [7].
Periapical Cyst
The periapical cyst (PC) or radicular cyst is the most common odontogenic cyst in the jaws. It is reactive in nature, caused by odontogenic infection [5]. When a tooth has a dental cavity (caries) that extends into the pulp chamber, the delicate blood vessels and nerves contained within become infected by the offending bacteria and die. The infection is then transmitted down the root of the tooth through the root canal system and enters the bone at the apex of the tooth. In some instances, the existing odontogenic rests of Malassez within the bone are stimulated by the inflammatory response to the odontogenic infection. These rests may proliferate and form a PC [8]. The same process may be provoked by dental trauma, which may sever the tooth’s blood supply and cause pulpal necrosis. PC is a well-defined radiolucent lesion that is usually seen in adults but may also develop at the apices of cavitated or traumatized primary teeth (Fig. 2B) [1]. The patient may experience pain or swelling, but sometimes these cysts are asymptomatic and appear as incidental findings on dental radiographs. The cyst location is usually periapical to the affected tooth, but a minority are located adjacent to the tooth root because accessory canals may exit the tooth’s root laterally [9].
Histologic examination of PC typically reveals fragments of inflamed fibrous connective tissue accompanied by variable amounts of squamous cyst lining epithelium. It is usually stratified and 2–3 cell layers thick. If the inflammation is robust, then the lining may proliferate and/or show spongiosis (Fig. 3D). As in DC, Rushton bodies may be present: this is a nonspecific finding in inflamed odontogenic cysts [10]. The fibrous cyst wall may contain any combination of acute and chronic inflammatory cells, in addition to macrophages and occasional giant cells. Endodontic foreign material may be present if the tooth has been previously endodontically treated. PC is treated by addressing the infected tooth, either by endodontic re-treatment, apicoectomy, or removal of the tooth and curettage of the cyst [11]. If the dead tooth is removed but the PC is not completely excised, it may remain and/or recur as a “residual cyst.”
It is nigh impossible to discern between PC and DC based on histology alone. This distinction requires clinical and radiographic correlation: PC occurs at the apex of a tooth compromised by odontogenic infection or trauma, and DC occurs in a “dentigerous” relationship with an unerupted tooth, surrounding its crown. Periapical granuloma is the term used for granulation tissue at the apex of an infected tooth in which no cyst lining epithelium is identified. The key entity not to miss is OKC, which may occur at any location in the jaws and thus may mimic a PC clinically [5, 9]. Inflammation frequently changes the typical histologic features of OKC, increasing the chance of misdiagnosis.
Odontogenic Keratocyst
OKC is a curious developmental odontogenic cyst with a controversial history regarding its classification [12]. Originally called a cyst, the OKC nomenclature was accepted by the World Health Organization (WHO) for decades until 2005, when it was reclassified as “keratocystic odontogenic tumor.” This change reflected the increasing evidence that OKC represents a cystic neoplasm, with genetic mutations in PTCH identified in up to 93% of sporadically occurring cases [13]. However, in 2017, the term OKC was reinstated by the WHO, and this was continued in the 5th edition in 2022 [14, 15]. While the debate is ongoing, experts agree that OKC is a unique odontogenic cyst with characteristic histologic features, a genetic signature, and a penchant for clinically aggressive behavior and recurrence. Multiple OKCs of the jaws are a classic finding in patients affected by nevoid basal cell carcinoma (Gorlin) syndrome (Fig. 2C) [16].
OKC may occur in patients of any age, with the majority occurring between the 2nd and 4th decades of life. OKCs are identified just a bit more often in males. There is a site predilection for the mandible (especially the posterior region) [1]. Radiographically, OKC is a uni- or multilocular radiolucent lesion that may occur anywhere within the jaws, with or without a direct relationship to teeth. Thus, OKC is an important differential diagnosis to always keep in mind. They may be small and asymptomatic, or they may be quite large, causing discomfort, bony expansion, and clinical swelling. OKCs are known to displace teeth, even occasionally pushing maxillary molars to the inferior orbit [17].
Histologically, OKC demonstrates either a unicystic space or more complex architecture (Fig. 4A). OKCs usually contain luminal thick keratin debris, but this is not a diagnostic requirement [9]. The organized squamous cyst lining epithelium is 6–8 cell layers thick. The interface between the epithelium and the fibrous connective tissue wall is typically flat, with no rete ridge development (Fig. 4B). The basally located cells are cuboidal to columnar in morphology with hyperchromatic nuclei, and they classically exhibit palisading along the basement membrane (Fig. 4C). The luminal surface of the cyst is lined with parakeratin in a bumpy pattern, evoking corrugated cardboard. Mitotic figures are occasionally identified. Other less common findings include cholesterol clefting, Rushton bodies, and mucous cells. Approximately, 20% of OKCs contain satellite cysts and/or rests within the fibrous cyst wall, and these represent additional odontogenic rests that are “along for the ride,” so to speak. In the setting of Gorlin syndrome, these satellite cysts are more common [18]. Very rarely, invasive squamous cell carcinoma may arise from dysplastic change occurring within the cyst lining of OKC [19]. OKC is usually treated by enucleation and curettage, sometimes combined with peripheral ostectomy. Clinicians often marsupialize large OKCs to decompress and shrink them, but most patients tolerate a drain for a limited amount of time [20]. The recurrence rate for OKCs is approximately 30%, requiring that patients undergo long-term monitoring with radiographic evaluation [21].
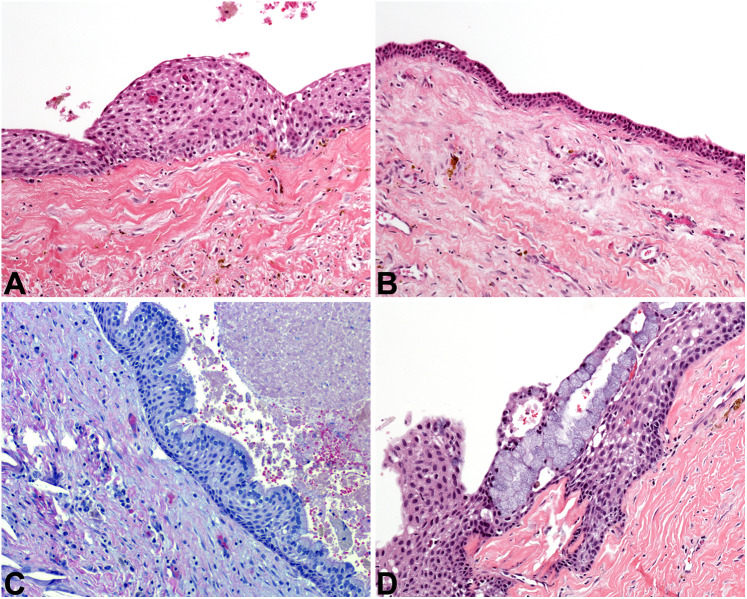
Fig. 4.
Odontogenic keratocyst. A At low power, OKC is overtly cystic, with a lumen filled with pink keratinaceous debris (2x). B Classic OKC lining epithelium is 6–8 cell layers thick, with basal palisading and a corrugated parakeratin surface (10×). C A closer view of a “perfect” OKC, exhibiting the diagnostic features of this cyst (20×). D Classic OKC is demonstrated in the upper left corner of this image, but inflammation completely obscures the diagnostic features of the cystic lining at the right (4x)
OKC should be in the differential for nearly any cystic odontogenic lesion. Identification of the key histologic features described above is essential to this diagnosis. Several distracting factors which may mislead the pathologist should be considered. First and foremost, when OKCs become inflamed or infected, they lose their classic diagnostic features and may appear histologically like DC or unicystic ameloblastoma (discussed later). For example, the squamous lining of OKC may become thicker and more proliferative, developing rounded rete ridges. The basal palisading and corrugated parakeratin at the luminal surface may become less organized or even unrecognizable (Fig. 4D). Aside from DC and unicystic ameloblastoma, the uncommon orthokeratinized odontogenic cyst (OOC) also enters the differential. OOCs produce orthokeratin, and the squamous cyst lining cells have a prominent granular layer. The OOC is indolent in nature, with a recurrence rate of approximately 2%. Foci of orthokeratin may be identified in OKC, but if there is any classic OKC identified, the lesion is best classified as OKC. Because the distinction between OKC and other cystic odontogenic lesions is clinically important, all received tissue should be submitted for histologic examination to maximize the probability of identifying the key features.
Calcifying Odontogenic Cyst
Originally reported in 1962 by Dr. Robert J. Gorlin, the calcifying odontogenic cyst (COC; also known as Gorlin cyst) is a benign developmental odontogenic cyst in the family of ghost cell lesions of the jaws [22]. COC consistently exhibits mutations in CTNNB1, implicating the Wnt/β-catenin signaling pathway in its development [23]. This cyst is somewhat rare and is usually diagnosed in young adults. There is no sex predilection, nor is there a site predilection for either jawbone [24]. While most COC cases are intraosseous in nature, it should be noted that peripheral COCs may occur in the gingiva, presenting as a “bump on the gum” [25].
Microscopic examination of a COC reveals a simple or complex cystic cavity that is lined by epithelial cells arranged in a particular pattern (Fig. 5A, B). The basal cells are cuboidal to columnar in shape, and they show some palisading. Further toward the luminal surface, the epithelial cells become spindled in nature and adopt a web-like arrangement, which is reminiscent of the stellate reticulum (Fig. 5C). The stellate reticulum is a particular functional group of cells in the enamel organ of a normal developing tooth. COC is known for the clusters of “ghost cells” that are embedded in variable quantities within the cyst lining epithelium (Fig. 5C). Ghost cells are circular or polyhedral structures made of homogenous eosinophilic material, with characteristically empty nuclear spaces. Over time, these ghost cells calcify and/or conglomerate together to form small masses (Fig. 5D). Abnormal dentin deposits are often found beneath the epithelial lining, appearing as hyalinized amorphous eosinophilic material. Up to 20% of COCs may be associated with other odontogenic lesions, and the most common of these is the odontoma [9]. Immunostaining for β-catenin will demonstrate nuclear positivity. COC is treated with simple surgical excision, and recurrence is rare [26].
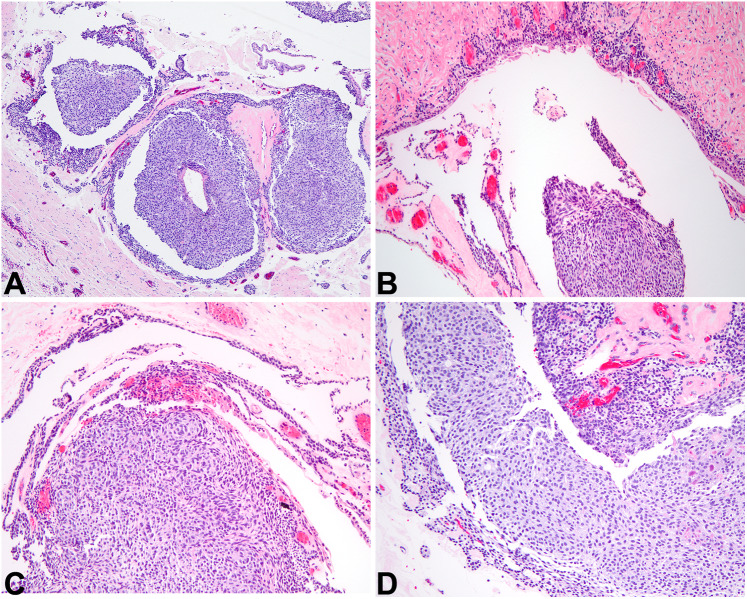
Fig. 5.
Calcifying odontogenic cyst. A At low power, a cyst lining epithelium that is proliferative and contains amorphous material is noted (2x). B The lining epithelium of COC looks reminiscent of cystic ameloblastoma in its pattern (4x). C The proliferative epithelium of COC classically contains eosinophilic anucleate formations called ghost cells (10×). D Over time, ghost cells coalesce together and create amorphous amphophilic material that slowly calcifies (10×)
The differential diagnosis of COC includes other odontogenic ghost cell lesions, in addition to unicystic ameloblastoma. Ghost cells are a very helpful histologic feature in reaching a diagnosis of COC, but they may also be found in dentinogenic ghost cell tumor (DGCT) and ghost cell odontogenic carcinoma (GCOC). Both of these are solid tumors: cystic change may be present focally, if there is any at all. The bulk of DGCT and GCOC is composed of ameloblastomatous islands of basaloid tumor cells, nestled within a dense fibrous stroma. Often, dentinoid material and ghost cells are present. Both tumors may also show a percolating border, occasionally involving skeletal muscle or adjacent soft tissue or bone. Cytologic atypia and mitotic figures are increased in GCOC. If the biopsy is limited, no ghost cells may be identified in an epithelial lining that otherwise resembles unicystic ameloblastoma, making the latter the key entity not to miss in this differential.
Glandular Odontogenic Cyst
The glandular odontogenic cyst (GOC) is an interesting benign odontogenic cyst which exhibits glandular differentiation. GOC is uncommon, accounting for less than 0.5% of all odontogenic cysts [1]. There is no sex predilection, and GOC may occur in patients of all ages, with a peak distribution in the 5th to 7th decades of life. 75% of cases occur in the mandible, with a predilection for the anterior region [27]. Larger tumors often cause bony expansion and swelling, with or without pain, while smaller lesions may be asymptomatic and incidentally found. Imaging findings are nonspecific, with an intrabony well-defined uni- or multilocular radiolucency being observed. Lesions may be periapically located, with scalloping up between tooth roots; or they may be associated with the crown of an impacted tooth [28].
The histologic features were established by Fowler et al. in 2011 [27], and if a cyst has 7 of the 10 described histologic parameters, then it qualifies as a GOC. At low power, larger lesions appear complex, with multiple compartments or cystic spaces. GOC is composed of a cyst lining epithelium of variable thickness, with a flat interface between the epithelium and fibrous cyst wall. Epithelial thickenings or “whorls” are often identified (Fig. 6A). The luminal cells are usually cuboidal to columnar in shape, with eosinophilic cytoplasm (called “hobnail cells”); squamous differentiation is often prominent, as is clear cell change (Fig. 6B). Microcystic spaces and/or mucous cells are usually seen within the lining (Fig. 6B and D). Additional features such as cilia, papillary tufting and apocrine snouting of the hobnail cells toward the lumen of the cyst are also helpful diagnostic features (Fig. 6C) when present. GOCs have been enucleated from the surrounding bone, but with a 30% recurrence rate, en bloc resection with a peripheral bony margin is most often recommended [29].
Fig. 6.
Glandular odontogenic cyst. A In the lining of this GOC, there are epithelial thickenings or “spheres” (10×). B GOC classically exhibits cuboidal cells with eosinophilic cytoplasm, and microcysts are also visible to the upper left of the image (10×). C This GOC shows prominent papillary tuft formation, complete with a ciliated luminal surface (10×). D Variability in thickness of the epithelial lining and abundant mucous cells are seen in this GOC example (20×)
GOC must be considered along with any jaw cyst that has glandular features. Dentigerous cyst may contain mucous cells, cilia, and/or microcystic spaces. Both cysts may occur surrounding the crown of an impacted tooth. However, the glandular differentiation in GOC is usually much more overt, assisting in clinching the diagnosis. The key entity not to miss when considering GOC is central mucoepidermoid carcinoma (MEC), although this is quite rare. There is significant histologic overlap between GOC and MEC, but generally MEC contains smaller cystic spaces and a more prominent pattern of tumor islands contained within a fibrous stroma. Invasion may be quite difficult to assess in low-grade MEC of the gnathic bones. For cases in which the distinction between these entities is not made easily on routine microscopy, performing fluorescence in situ hybridization (FISH) for MAML2 rearrangement helps, as it is negative in GOC [30].
Odontogenic Tumors with Cystic Components
Adenomatoid Odontogenic Tumor
The adenomatoid odontogenic tumor (AOT) is a benign epithelial odontogenic tumor characterized by a proliferative combination of duct-like spaces and epithelial whorls. AOT is uncommon, and accounts for less than 5% of all odontogenic tumors. There is controversy regarding whether AOT represents a hamartoma or a neoplasm [31, 32], with a neoplasm favored at this time. AOT occurs twice as frequently in females, and there is an age predilection for teenagers and young adults. This tumor occurs twice as often in the maxilla, and the anterior region (canine teeth) of the gnathic bones is most commonly affected. AOT usually occurs in a pericoronal fashion, around the crown of an impacted tooth [33, 34]. Patients present with swelling, with imaging showing a well-defined unilocular radiolucent lesion with corticated borders. Small radiopacities may be noted in up to two-thirds of cases. It should be noted that the classic AOT is thickly encapsulated, easily enucleated from the surrounding bone.
Histologically, AOT is delineated by a thick capsule, often with central cystic change (Fig. 7A, B). The bulk of the tumor is made of whorls of spindled epithelial cells called pseudorosettes (Fig. 7C), interspersed with duct-like structures formed by cuboidal to columnar cells with bland nuclei (Fig. 7D). Opposite to the nuclear arrangement characteristic of ameloblastoma, the nuclei of the ductal spaces in AOT are basally located. Additionally, a pattern of anastomosing cords of tumor cells may be identified. Some AOT tumor cells secrete a matrix-like proteinaceous substance that may calcify over time, and this likely represents an attempt at odontogenesis by tumor cells. Cytologic atypia is not common, but occasional mitoses may be identified. AOT is treated via complete enucleation, usually with removal of the involved tooth. The recurrence rate is less than 0.5% [32].
Fig. 7.
Adenomatoid odontogenic tumor. A At low power, AOT is an encapsulated cystic tumor filled with whorled epithelial tumor cells (4x). B Without the solid tumor in the lumen, this inflamed AOT lining could be easily mistaken for a reactive or developmental cyst (10×). C AOT is cystic and solid, and the solid component shows evenly spaced and bland epithelial cells arranged in a loosely nested pattern (10×). D Duct-like structures and pseudorosettes are often identified in the solid component of AOT (20×)
The solid portion of AOT is usually well-sampled, so the differential diagnosis is narrow. Occasionally, the pseudorosettes are not prominent, and ameloblastoma enters the differential in those cases. One might also consider COC if there are circular calcifications present within the AOT epithelium; however, AOTs do not contain ghost cells, and nuclear reactivity for β-catenin is negative by immunohistochemistry [35].
Unicystic Ameloblastoma
The unicystic ameloblastoma (UA) is a subtype of ameloblastoma which develops as a singular cystic cavity. While conventional ameloblastoma is quite locally aggressive and has a high recurrence rate, UA behaves in a less aggressive manner, making the distinction essential [36]. UAs associated with impacted teeth occur in younger patients (mean age 16 years) and there is a slight male predilection [37]. UAs not associated with impacted teeth tend to occur in patients in their thirties (mean age 35 years) and there is a slight female predilection. Molars in the posterior mandible are most often affected [38]. Patients present with a swelling of the jaw, usually without pain. Radiographic examination reveals a unilocular radiolucent lesion which may be pericoronal, periapical, or located between tooth roots [38].
Microscopically, UA is composed of a single cystic compartment that is lined by neoplastic epithelial cells (Fig. 8A). The basally oriented columnar cells show palisading and reverse polarity, as is typical of ameloblastoma (Fig. 8B). Additionally, cells toward the lumen become stellate-shaped and more loosely organized, resembling the stellate reticulum of the enamel organ. The luminal surface of the cyst itself is usually squamoid, with homogenous eosinophilic cytoplasm; this creates a “red, white, and blue” pattern (Fig. 8C). Intraluminal tumoral growth may occur, in which the neoplastic epithelium proliferates and forms a plexiform pattern of anastomosing cords within the cystic cavity. Like conventional ameloblastoma, the predominant driving mutation is BRAF p.V600E [39].
Fig. 8.
Unicystic ameloblastoma. A At low power, a relatively nondescript cyst lining is seen adjacent to dense bone (4x). B Upon closer examination, there are subtle palisaded basal cells and a very thin stellate-reticulum-like area, topped at the luminal surface by cells with eosinophilic cytoplasm (10×). C These features are more prominent in this portion of the UA lining, highlighting the necessity to evaluate all of the cystic lining (20×). D Small islands and cords of ameloblastic epithelium are seen in the wall of this cystic ameloblastoma, which for the authors pushes this lesion into the conventional ameloblastoma category (4x)
Previously, the term “mural-type” unicystic ameloblastoma was used, but current guidance places any tumor with mural involvement (Fig. 8D) into the conventional ameloblastoma category [40]. If entirely cystic, UA is often simply enucleated, with long-term follow-up of the patient [36, 37]. The recurrence rate of pure UA after enucleation is between 10 and 20%, compared to enucleation and curettage of conventional ameloblastoma resulting in a 50–90% chance of the lesion recurring [36, 37]. Thus, patients with conventional ameloblastoma require en bloc resection with 1- to 2-cm margins, which is a much more significant surgical procedure [41]. The entire lesion must be histologically examined before rendering a UA diagnosis, and as such UA is not diagnosed reproducibly by incisional biopsy.
UA is the key entity not to miss in the case of any cystic odontogenic lesion (closely followed by OKC). The histologic features of UA may be quite subtle, and thus thorough scrutiny of as much cyst lining epithelium as possible aids in diagnosis of UA, especially if the lesion is at all inflamed. Several other odontogenic cysts come into the differential, including DC, PC, and OKC, among other rarer entities.
Non-odontogenic Cystic Lesions
Nasopalatine Duct Cyst
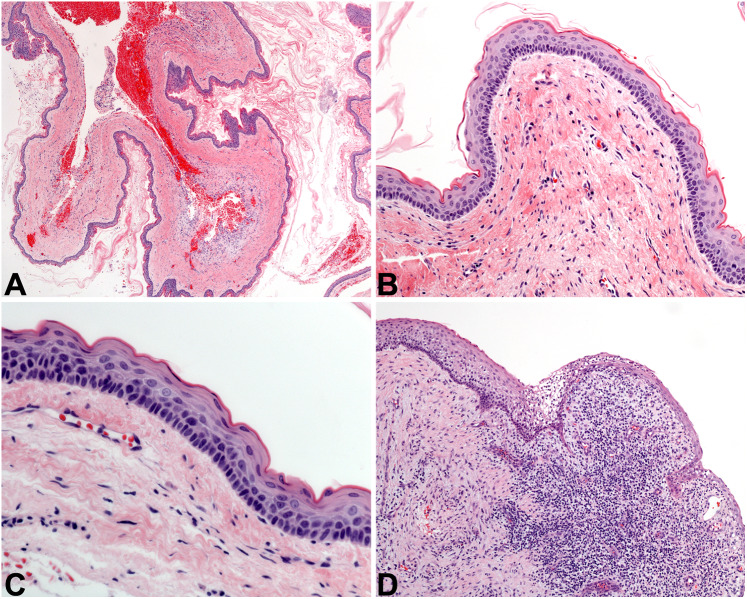
Nasopalatine duct cyst (NPDC) is the most common developmental gnathic cyst that is not odontogenic in nature [42]. This benign cyst originates from the epithelium of the nasopalatine duct, which enters the anterior maxilla at the midline behind the central incisors through the nasopalatine foramen. Affected patients may be of any age, but there is a peak incidence in middle age, and males are affected slightly more often [43]. Most NPDC cases are incidentally found on routine dental radiographic examination, presenting as a heart- or pear-shaped radiolucency measuring between 1 and 2 centimeters, located between the central incisors. Occasionally, the radiolucent lesion may be slightly laterally located [44]. Patients present with an asymptomatic anterior palatal gingival swelling.
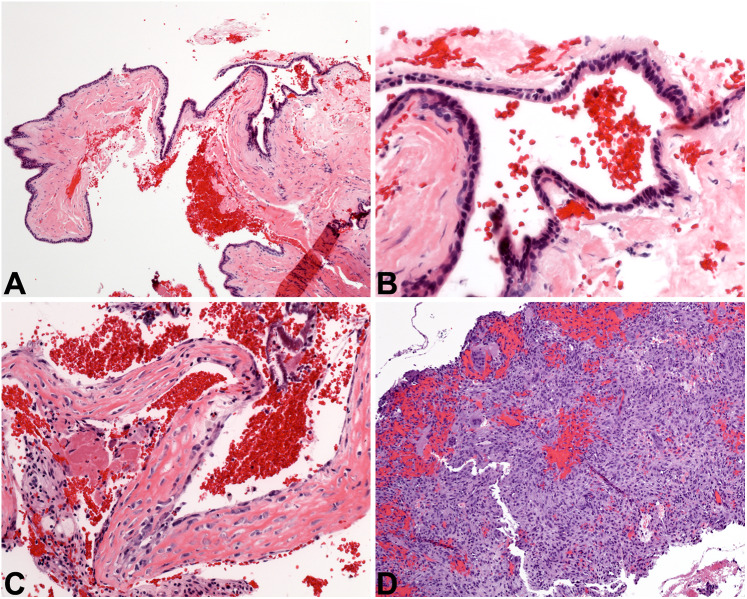
Histologically, NPDC is a cyst lined by a combination of stratified squamous epithelium and respiratory-type epithelium (Fig. 9A). Respiratory epithelium is composed of pseudostratified cuboidal to columnar cells, with or without cilia. Mucous cells may be present, and subepithelial hyalinization is frequently seen (Fig. 9B). The connective tissue of the cyst wall is commonly chronically inflamed, and classically contains larger-caliber nerves and blood vessels. Additionally, salivary gland tissue and/or cartilaginous rests may be identified in the cyst wall, native to the site. NPDC is indolent in nature, and is treated via simple surgical excision from the palatal aspect of the lesion; it does not recur [42].
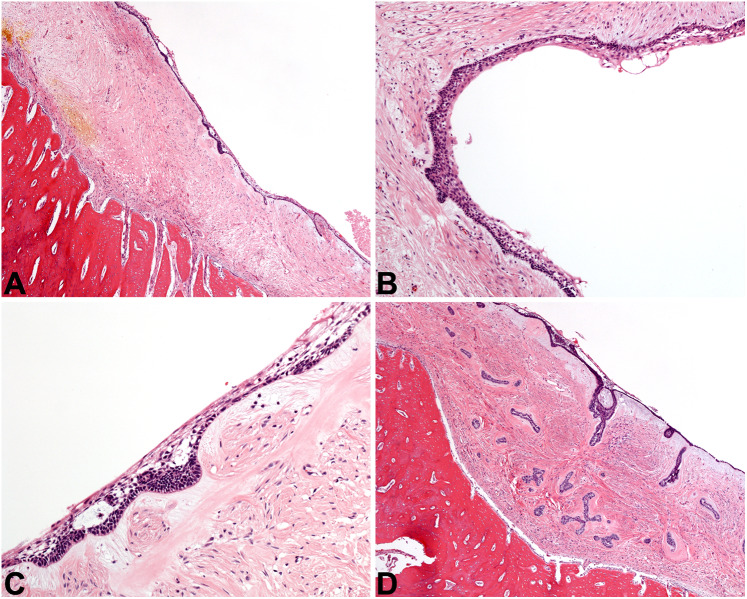
Fig. 9.
Other cystic lesions. A The nasopalatine duct cyst at low power is lined by epithelium of even thickness (4x). B NPDC is lined by cuboidal cells in a simple arrangement, with an associated fibrous cyst wall (20×). C The simple bone cyst is defined by an empty cavity found at surgery, plus this delicate lace-like osteoid with osteoblastic rimming (20×). D The aneurysmal bone cyst is a solid and cellular proliferation of spindled cells admixed with sinusoidal spaces full of blood, graced with giant cells (10×)
Differential diagnoses include other cystic odontogenic lesions, most importantly PC and OKC. PC is usually eliminated by doing some dental sleuthing (i.e., radiographic examination and vitality-testing of adjacent teeth), while OKC is excluded histologically.
Simple Bone Cyst
Simple bone cyst (SBC) is an intrabony pseudocystic process reported in bones all over the body. SBC is an intraosseous cavity with no cyst lining epithelium. It is frequently completely empty, or otherwise may be filled with serous or serosanguinous fluid [45, 46], and is considered a degenerative process. Another name for SBC is “traumatic bone cyst,” but many cases of SBC have been reported in patients with no history of trauma. Most patients with SBC are teenagers or young adults, and there is a significant site predilection for the mandible [47]. Patients are asymptomatic, and the lesion tends to slowly grow in an anteroposterior direction, without causing osseous expansion [46]. SBC is often identified incidentally during routine dental radiographic examination and presents as a well-defined radiolucent lesion which “scallops” up between the tooth roots. As a non-odontogenic entity, teeth are bystanders and are almost always vital. SBC may occur in conjunction with benign fibro-osseous lesions of the jaws [48].
Microscopically, SBC is rather underwhelming and there are no pathognomonic diagnostic features of this lesion. Typically, the clinician curettes the walls of the bony cavity and submits those fragments for evaluation. No epithelial lining is identified. Blood and sometimes focal granulation tissue is present, but the bone is the important tissue to scrutinize. Normal bone may be identified, but the characteristic feature of SBC is an immature osteoid matrix that has a lace-like appearance (Fig. 9 C) [49]. It is important that the surgeon relay the finding of an empty cavity to the pathologist, as this is not a typical feature of other gnathic lesions and helps to narrow the differential considerably. Curettage is completed with the goal of inducing bleeding in the cavity, so that the cavity will eventually fill with granulation tissue which remodels into bone. Bone grafts are done in some cases. The recurrence rate of SBC is very low [50].
With the caveat that excellent surgical sampling is completed and all tissue is evaluated histologically, the absence of an epithelial lining and the surgical finding of an empty cavity excludes other cystic lesions of the jaws. If the surgical and histologic findings do not completely align, a “hedge” diagnosis may be needed, such as “consistent with.”
Aneurysmal Bone Cyst
Aneurysmal bone cyst (ABC) is a benign cystic neoplasm that primarily occurs in the long bones and vertebrae. While common in these locations, less than 2% of ABCs occur in the gnathic bones [45]. ABC may occur as a solitary lesion in the jaws, but up to one quarter of cases are associated with other entities, such as benign fibro-osseous lesions and central giant cell granuloma [51]. Affected patients are usually teenagers or young adults, with a very slight male predilection [52]. Patients present with an expansile mass of the jaw (70% of cases occur in the mandible), with or without pain or paresthesia. Lesions are usually multilocular radiolucencies which cause cortical expansion and occasionally bone perforation. ABC can also displace teeth and cause tooth root resorption. At surgery, ABC appears as a hypervascular spongy mass.
Histologically, ABC is composed of many small sinusoidal spaces filled with red blood cells. No endothelial lining is evident. The bulk of ABC is made up of a proliferation of bland, fibroblast-like spindled cells (Fig. 9D). Large multinucleated giant cells are seen with frequency, and immature osteoid is also often identified. In ABCs that occur secondary to other gnathic lesions, the histologic features of those lesions are also taken into consideration and a hybrid diagnosis is rendered (e.g., “ossifying fibroma with secondary aneurysmal bone cyst formation”). The neoplastic cells of ABC characteristically demonstrate lack of expression of H3.3 p.Gly34Trp by immunohistochemistry, a fact that can be exploited to exclude other giant cell lesions that may occur in the jaws. Genetically, the majority of ABCs have a USP6::CDH11 fusion in the neoplastic spindled cells. While benign, ABCs are neoplasms with significant growth potential. ABC may be conservatively enucleated from the surrounding bone, but patients must be monitored because the recurrence rate is around 15% [53]. More extensive surgical resection of larger lesions is appropriate.
Pertinent differentials for ABC include other giant cell lesions that occur in the gnathic bones. These include cherubism, central giant cell granuloma, and brown tumor of hyperparathyroidism. The clinical history can often help to exclude the former and latter entities. Cherubism is inherited and most cases affect the jaws in a bilaterally symmetric distribution, as opposed to unifocal ABC. Hyperparathyroidism is a systemic condition that manifests as a triad of “stones, bones, and abdominal groans,” and the brown tumor is one of the osseous changes that can occur. Serologic studies for elevated parathyroid hormone will support this diagnosis. The key entities not to miss include neoplasms that have ABC-like degenerative changes associated with them, such as osteoblastoma and osteosarcoma.
Clinical Case Diagnoses
Coming full circle back to our clinical cases, single histologic images for each case are presented in Fig. 10. Case 1 is an odontogenic keratocyst (Fig. 10A), while the diagnosis in Case 2 is an adenomatoid odontogenic tumor (Fig. 10B). Note that endodontic treatments were performed in both cases, which did not resolve either patient’s lesion. This highlights the need for an accurate diagnosis before treatments are rendered. Additionally, the AOT in case 2 is unconventional in location and tooth association. Most importantly, pathologists should use clinical information to narrow down the differential diagnosis for cystic gnathic lesions, supporting the diagnosis with histologic examination. Still, rare cystic lesions may be “fence-sitters” and necessitate diagnostic hedging.
Fig. 10.
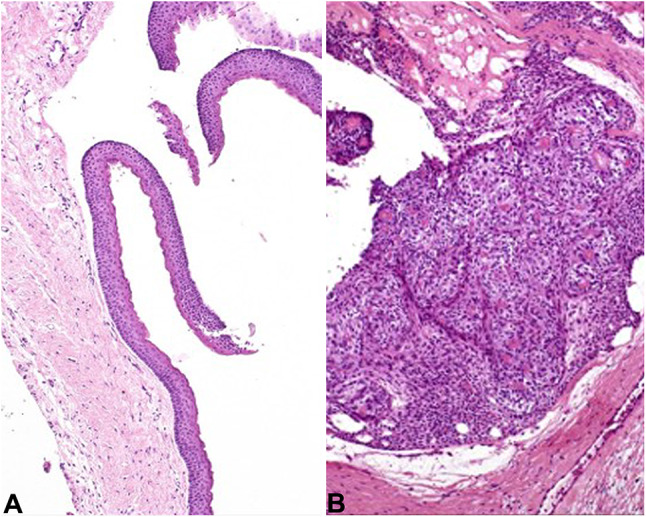
Case diagnoses. A Case 1 represents and OKC with classic features (10×). B Case 2 is an AOT, with solid tumor whorls (10×)
Conclusion
The jaws are home to a wide variety of cystic lesions, both odontogenic and non-odontogenic, and both reactive and neoplastic. The practice of gnathic pathology relies heavily on clinical and radiographic information obtained before tissue is submitted for diagnostic evaluation. Information regarding size, location, relationship to teeth, and radiographic morphology is very useful in narrowing the differential diagnosis. Despite these helpful clues, some cystic odontogenic lesions may prove quite challenging to classify due to histologic overlap. The presence of inflammation is well known to obscure the diagnostic features of some cystic lesions. Additionally, a strong focus on morphology must be emphasized, as immunohistochemical studies rarely aid in diagnosis. Adequate sampling is absolutely essential in diagnosing these entities, recognizing that a qualified diagnosis may be the best we can render on limited material or when overlapping features are seen.
Acknowledgements
The authors would like to sincerely thank Drs. Asma Almazyad, Justin Bishop, Andres Flores-Hidalgo, Jennie Ison, and Scott Steward-Tharp for their generous contributions to this work.
Funding
This review article was not supported by any funding sources.
Data Availability
not applicable.
Code Availability
not applicable.
Declarations
Ethical Approval
No human participants were included in this invited review, but all materials were treated in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
The requirement of consent was waived by the IRB due to the retrospective nature of this invited review. Consent for publication was obtained when personally identifiable information was included.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones AV, Craig GT, Franklin CD. Range and demographics of odontogenic cysts diagnosed in a UK population over a 30-year period. J Oral Pathol Med. 2006;35(8):500–507. doi: 10.1111/j.1600-0714.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 2.Lin HP, Wang YP, Chen HM, Cheng SJ, Sun A, Chiang CP. A clinicopathological study of 338 dentigerous cysts. J Oral Pathol Med. 2013;42(6):462–467. doi: 10.1111/jop.12042. [DOI] [PubMed] [Google Scholar]
- 3.Zhang LL, Yang R, Zhang L, Li W, MacDonald-Jankowski D, Poh CF. Dentigerous cyst: a retrospective clinicopathological analysis of 2082 dentigerous cysts in British Columbia, Canada. Int J Oral Maxillofac Surg. 2010;39(9):878–882. doi: 10.1016/j.ijom.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 4.Takeda Y, Oikawa Y, Furuya I, Satoh M, Yamamoto H. Mucous and ciliated cell metaplasia in epithelial linings of odontogenic inflammatory and developmental cysts. J Oral Sci. 2005;47(2):77–81. doi: 10.2334/josnusd.47.77. [DOI] [PubMed] [Google Scholar]
- 5.Johnson NR, Gannon OM, Savage NW, Batstone MD. Frequency of odontogenic cysts and tumors: a systematic review. J Investig Clin Dent. 2014;5(1):9–14. doi: 10.1111/jicd.12044. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan I, Hirshberg A. The correlation between epithelial cell proliferation and inflammation in odontogenic keratocyst. Oral Oncol. 2004;40(10):985–991. doi: 10.1016/j.oraloncology.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Pereira NB, Pereira KM, Coura BP, Diniz MG, de Castro WH, Gomes CC, et al. BRAFV600E mutation in the diagnosis of unicystic ameloblastoma. J Oral Pathol Med. 2016;45(10):780–785. doi: 10.1111/jop.12443. [DOI] [PubMed] [Google Scholar]
- 8.Lin LM, Huang GT, Rosenberg PA. Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J Endod. 2007;33(8):908–916. doi: 10.1016/j.joen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Bilodeau EA, Collins BM. Odontogenic cysts and neoplasms. Surg Pathol Clin. 2017;10(1):177–222. doi: 10.1016/j.path.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Sedano HO, Gorlin RJ. Hyaline bodies of Rushton. Some histochemical considerations concerning their etiology. Oral Surg Oral Med Oral Pathol. 1968;26(2):198–201. doi: 10.1016/0030-4220(68)90255-7. [DOI] [PubMed] [Google Scholar]
- 11.Lin LM, Ricucci D, Lin J, Rosenberg PA. Nonsurgical root canal therapy of large cyst-like inflammatory periapical lesions and inflammatory apical cysts. J Endod. 2009;35(5):607–615. doi: 10.1016/j.joen.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Soluk-Tekkesin M, Wright JM (2018) The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2017, 4th Edition. Turk Patoloji Derg 34(1) [DOI] [PubMed]
- 13.Stojanov IJ, Schaefer IM, Menon RS, Wasman J, Gokozan HN, Garcia EP, et al. Biallelic PTCH1 inactivation is a Dominant genomic change in sporadic keratocystic odontogenic tumors. Am J Surg Pathol. 2020;44(4):553–560. doi: 10.1097/PAS.0000000000001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright JM, Vered M. Update from the 4th Edition of the World Health Organization classification of Head and Neck Tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol. 2017;11(1):68–77. doi: 10.1007/s12105-017-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vered M, Wright JM. Update from the 5th Edition of the World Health Organization classification of Head and Neck Tumors: odontogenic and maxillofacial bone tumours. Head Neck Pathol. 2022;16(1):63–75. doi: 10.1007/s12105-021-01404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bresler SC, Padwa BL, Granter SR. Nevoid basal cell Carcinoma Syndrome (Gorlin Syndrome) Head Neck Pathol. 2016;10(2):119–124. doi: 10.1007/s12105-016-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vered M, Buchner A, Dayan D, Shteif M, Laurian A. Solid variant of odontogenic keratocyst. J Oral Pathol Med. 2004;33(2):125–128. doi: 10.1111/j.1600-0714.2004.00014.x. [DOI] [PubMed] [Google Scholar]
- 18.Woolgar JA, Rippin JW, Browne RM. A comparative histological study of odontogenic keratocysts in basal cell naevus syndrome and control patients. J Oral Pathol. 1987;16(2):75–80. doi: 10.1111/j.1600-0714.1987.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 19.Ye P, Wei T, Gao Y, Zhang W, Peng X. Primary intraosseous squamous cell carcinoma arising from an odontogenic keratocyst: case series and literature review. Med Oral Patol Oral Cir Bucal. 2021;26(1):e49–e55. doi: 10.4317/medoral.23947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wushou A, Zhao YJ, Shao ZM. Marsupialization is the optimal treatment approach for keratocystic odontogenic tumour. J Craniomaxillofac Surg. 2014;42(7):1540–1544. doi: 10.1016/j.jcms.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Johnson NR, Batstone MD, Savage NW. Management and recurrence of keratocystic odontogenic tumor: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(4):e271–e276. doi: 10.1016/j.oooo.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Gorlin RJ, Pindborg JJ, Odont, Clausen FP, Vickers RA. The calcifying odontogenic cyst–a possible analogue of the cutaneous calcifying epithelioma of Malherbe. An analysis of fifteen cases. Oral Surg Oral Med Oral Pathol. 1962;15:1235–1243. doi: 10.1016/0030-4220(62)90159-7. [DOI] [PubMed] [Google Scholar]
- 23.Sekine S, Sato S, Takata T, Fukuda Y, Ishida T, Kishino M, et al. Beta-catenin mutations are frequent in calcifying odontogenic cysts, but rare in ameloblastomas. Am J Pathol. 2003;163(5):1707–1712. doi: 10.1016/S0002-9440(10)63528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchner A. The central (intraosseous) calcifying odontogenic cyst: an analysis of 215 cases. J Oral Maxillofac Surg. 1991;49(4):330–339. doi: 10.1016/0278-2391(91)90365-S. [DOI] [PubMed] [Google Scholar]
- 25.Buchner A, Merrell PW, Hansen LS, Leider AS. Peripheral (extraosseous) calcifying odontogenic cyst. A review of forty-five cases. Oral Surg Oral Med Oral Pathol. 1991;72(1):65–70. doi: 10.1016/0030-4220(91)90191-E. [DOI] [PubMed] [Google Scholar]
- 26.de Arruda JAA, Schuch LF, Abreu LG, Silva LVO, Monteiro JLG, Pinho RF, et al. A multicentre study of 268 cases of calcifying odontogenic cysts and a literature review. Oral Dis. 2018;24(7):1282–1293. doi: 10.1111/odi.12906. [DOI] [PubMed] [Google Scholar]
- 27.Fowler CB, Brannon RB, Kessler HP, Castle JT, Kahn MA. Glandular odontogenic cyst: analysis of 46 cases with special emphasis on microscopic criteria for diagnosis. Head Neck Pathol. 2011;5(4):364–375. doi: 10.1007/s12105-011-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chrcanovic BR, Gomez RS. Glandular odontogenic cyst: an updated analysis of 169 cases reported in the literature. Oral Dis. 2018;24(5):717–724. doi: 10.1111/odi.12719. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan I, Gal G, Anavi Y, Manor R, Calderon S. Glandular odontogenic cyst: treatment and recurrence. J Oral Maxillofac Surg. 2005;63(4):435–441. doi: 10.1016/j.joms.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Bishop JA, Yonescu R, Batista D, Warnock GR, Westra WH. Glandular odontogenic cysts (GOCs) lack MAML2 rearrangements: a finding to discredit the putative nature of GOC as a precursor to central mucoepidermoid carcinoma. Head Neck Pathol. 2014;8(3):287–290. doi: 10.1007/s12105-014-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichart PA, Philipsen HP, Khongkhunthian P, Sciubba JJ. Immunoprofile of the adenomatoid odontogenic tumor. Oral Dis. 2017;23(6):731–736. doi: 10.1111/odi.12572. [DOI] [PubMed] [Google Scholar]
- 32.Philipsen HP, Reichart PA, Siar CH, Ng KH, Lau SH, Zhang X, et al. An updated clinical and epidemiological profile of the adenomatoid odontogenic tumour: a collaborative retrospective study. J Oral Pathol Med. 2007;36(7):383–393. doi: 10.1111/j.1600-0714.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 33.Philipsen HP, Reichart PA. Adenomatoid odontogenic tumour: facts and figures. Oral Oncol. 1999;35(2):125–131. doi: 10.1016/S1368-8375(98)00111-0. [DOI] [PubMed] [Google Scholar]
- 34.Philipsen HP, Reichart PA, Zhang KH, Nikai H, Yu QX. Adenomatoid odontogenic tumor: biologic profile based on 499 cases. J Oral Pathol Med. 1991;20(4):149–158. doi: 10.1111/j.1600-0714.1991.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 35.Harnet JC, Pedeutour F, Raybaud H, Ambrosetti D, Fabas T, Lombardi T. Immunohistological features in adenomatoid odontogenic tumor: review of the literature and first expression and mutational analysis of beta-catenin in this unusual lesion of the jaws. J Oral Maxillofac Surg. 2013;71(4):706–713. doi: 10.1016/j.joms.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31B(2):86–99. doi: 10.1016/0964-1955(94)00037-5. [DOI] [PubMed] [Google Scholar]
- 37.Philipsen HP, Reichart PA. Unicystic ameloblastoma. A review of 193 cases from the literature. Oral Oncol. 1998;34(5):317–325. doi: 10.1016/S1368-8375(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 38.da Silva YS, Sohal KS, Stoelinga PJW, Grillo R. A meta-analysis on the presentation of Unicystic Ameloblastoma in the jaws and the consequences for their treatment. J Stomatol Oral Maxillofac Surg. 2022;123(5):e433–e8. doi: 10.1016/j.jormas.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Heikinheimo K, Huhtala JM, Thiel A, Kurppa KJ, Heikinheimo H, Kovac M, et al. The Mutational Profile of Unicystic Ameloblastoma. J Dent Res. 2019;98(1):54–60. doi: 10.1177/0022034518798810. [DOI] [PubMed] [Google Scholar]
- 40.Siriwardena B, Tennakoon T, Hunter KD, Tilakaratne WM. Unicystic ameloblastoma: analysis of 370 cases in a single center in Sri Lanka. J Oral Pathol Med. 2018;47(7):706–709. doi: 10.1111/jop.12740. [DOI] [PubMed] [Google Scholar]
- 41.Goh YC, Siriwardena B, Tilakaratne WM. Association of clinicopathological factors and treatment modalities in the recurrence of ameloblastoma: analysis of 624 cases. J Oral Pathol Med. 2021;50(9):927–936. doi: 10.1111/jop.13228. [DOI] [PubMed] [Google Scholar]
- 42.Suter VG, Altermatt HJ, Voegelin TC, Bornstein MM. [The nasopalatine duct cyst–epidemiology, diagnosis and therapy] Schweiz Monatsschr Zahnmed. 2007;117(8):824–839. [PubMed] [Google Scholar]
- 43.Escoda Francoli J, Almendros Marques N, Berini Aytes L, Gay Escoda C. Nasopalatine duct cyst: report of 22 cases and review of the literature. Med Oral Patol Oral Cir Bucal. 2008;13(7):E438–E443. [PubMed] [Google Scholar]
- 44.Jones AV, Franklin CD. An analysis of oral and maxillofacial pathology found in adults over a 30-year period. J Oral Pathol Med. 2006;35(7):392–401. doi: 10.1111/j.1600-0714.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 45.Flores IL, Hamilton ME, Zanchin-Baldissera E, Uchoa-Vasconcelos AC, Chaves-Tarquinio SB, Neutzling-Gomes AP. Simple and aneurysmal bone cyst: aspects of jaw pseudocysts based on an experience of brazilian pathology service during 53 years. Med Oral Patol Oral Cir Bucal. 2017;22(1):e64–e9. doi: 10.4317/medoral.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horne RP, Meara DJ, Granite EL. Idiopathic bone cavities of the mandible: an update on recurrence rates and case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(2):e71–e73. doi: 10.1016/j.oooo.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 47.Lima LB, de Freitas Filho SA, Barbosa de Paulo LF, Servato JP, Rosa RR, Faria PR, et al. Simple bone cyst: description of 60 cases seen at a brazilian school of Dentistry and review of international literature. Med Oral Patol Oral Cir Bucal. 2020;25(5):e616–e25. doi: 10.4317/medoral.23638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chadwick JW, Alsufyani NA, Lam EW. Clinical and radiographic features of solitary and cemento-osseous dysplasia-associated simple bone cysts. Dentomaxillofac Radiol. 2011;40(4):230–235. doi: 10.1259/dmfr/16355120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumhoer D, Smida J, Nathrath M, Jundt G. The nature of the characteristic cementum-like matrix deposits in the walls of simple bone cysts. Histopathology. 2011;59(3):390–396. doi: 10.1111/j.1365-2559.2011.03962.x. [DOI] [PubMed] [Google Scholar]
- 50.Suei Y, Taguchi A, Tanimoto K. Simple bone cyst of the jaws: evaluation of treatment outcome by review of 132 cases. J Oral Maxillofac Surg. 2007;65(5):918–923. doi: 10.1016/j.joms.2006.06.297. [DOI] [PubMed] [Google Scholar]
- 51.Arora SS, Paul S, Arora S, Kapoor V. Secondary jaw aneurysmal bone cyst (JABC)--a possible misnomer? A review of literature on secondary JABCs, their pathogenesis and oncogenesis. J Oral Pathol Med. 2014;43(9):647–651. doi: 10.1111/jop.12132. [DOI] [PubMed] [Google Scholar]
- 52.Motamedi MH, Behroozian A, Azizi T, Nazhvani AD, Motahary P, Lotfi A. Assessment of 120 maxillofacial aneurysmal bone cysts: a nationwide quest to understand this enigma. J Oral Maxillofac Surg. 2014;72(8):1523–1530. doi: 10.1016/j.joms.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Zhou J, Shi J. Clinicopathology and recurrence analysis of 44 Jaw Aneurysmal bone cyst cases: a Literature Review. Front Surg. 2021;8:678696. doi: 10.3389/fsurg.2021.678696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
not applicable.
not applicable.