Animal diversity is reflected not only in species numbers but can also include variability in size and fecundity, which the organism's environment can shape. Using a description of individual metabolism, we show how seasonal food availability can explain diversity in body size and reproduction.
Keywords: Bergmann's rule, body size, eNPP, Lack's rule, trait variation, resource availability
Abstract
Animals show a vast array of phenotypic traits in time and space. Such variation patterns have traditionally been described as ecogeographical rules; for example, the tendency of size and clutch size to increase with latitude (Bergmann's and Lack's rules, respectively). Despite considerable research into these variation patterns and their consequences for biodiversity and conservation, the processes behind trait variation remain controversial. Here, we show how food variability, largely set by climate and weather, can drive interspecific trait variation by determining individual energy input and allocation trade-offs. Using a dynamic energy budget (DEB) model, we simulated different food environments, as well as interspecific variability in the parameters for energy assimilation, mobilization and allocation to soma. We found that interspecific variability is greater when the resource is non-limiting in both constant and seasonal environments. Our findings further show that individuals can reach larger biomass and greater reproductive output in a seasonal environment than in a constant environment of equal average resource due to the peaks of food surplus. Our results agree with the classical patterns of interspecific trait variation and provide a mechanistic understanding that supports recent hypotheses which explain them: the resource and the eNPP (net primary production during the growing season) rules. Due to the current alterations to ecosystems and communities, disentangling the mechanisms of trait variation is increasingly important to understand and predict biodiversity dynamics under climate change, as well as to improve conservation strategies.
Introduction
The variation in life-history traits in animal species across temporal and spatial scales is vast (Healy et al., 2019). Despite considerable research describing these traits' patterns of occurrence, a systematic understanding of the underlying mechanisms and processes remains elusive (Gaston et al., 2008). Disentangling and quantifying variation in biological traits is necessary to explain and predict biodiversity dynamics under environmental change (Cardilini et al., 2016; Cabral et al., 2017), and it may aid in answering broad questions that range from the invasive potential of species (Capellini et al., 2015) and the evolution of senescence (Jones et al., 2014), to predicting the influence of stressors on species assemblages (Darling et al., 2012) and improving conservation efforts (Kissling et al., 2018). Hence, it is fundamental to understand the underlying mechanisms giving rise to animal trait variation.
Among animal traits, body size exhibits substantial diversity within orders and narrower clades of animals, and it is thought to play a pivotal role in all individual's ecological and physiological processes (Yom-Tov and Geffen, 2011; Kozlowski et al., 2020). Variation of body size in time and space is assumed to be a product of evolution modulated by the biotic and abiotic environment (Mayr, 1956; Millien et al., 2006; Yom-Tov and Geffen, 2011). Additionally, body size tends to covary with several life-history and morphological traits (Olalla-Tárraga et al., 2019). For example, birds' body size is thought to be positively correlated with clutch size (Jetz et al., 2008; Olalla-Tárraga et al., 2019). Although there is extensive evidence describing and supporting spatial patterns in body size and reproductive output at different biological scales, the processes that underpin their variation are not fully comprehended (Gaston et al., 2008).
The consistent variation in animal traits across time and space, both within and among species or clades, forms the basis of ‘ecogeographical rules’ (Boyer and Jetz, 2010; McNab, 2010). Two of the most frequently explored interspecific patterns are the increase of body size in closely related endotherms (and some ectotherms) with latitude (‘Bergmann's rule’) and the tendency of clutch size to increase with latitude (‘Lack's rule’). In general, empirical evidence in endotherms supports both patterns (e.g. for Bergman's rule see Ashton et al., 2000; Ashton, 2002; Cardillo, 2002; Freckleton et al., 2003; Blackburn and Hawkins, 2004; Rodríguez et al., 2006, 2008; Olalla-Tárraga and Rodríguez, 2007; Ramirez et al., 2008; Olson et al., 2009; Morales-Castilla et al., 2012b, 2012a; Clauss et al., 2013; Torres-Romero et al., 2016; Romano et al., 2020; and reviews in Meiri and Dayan, 2003; Watt et al., 2010; Huston and Wolverton, 2011. For Lack's rule, see Moreau, 1944; Lack, 1947; Ashmole, 1963; Cody, 1966; Ricklefs, 1980; Kulesza, 1990; Iverson et al., 1993; Griebeler and Böhning-Gaese, 2004; Evans et al., 2005; Jetz et al., 2008; Mesquita et al., 2016; Meiri et al., 2020; and review in Boyer et al., 2010. But see Meiri et al., 2004; Medina et al., 2007; Olalla-Tárraga and Rodríguez, 2007; Gohli and Voje, 2016 for contradictory or ambiguous results). Bergmann suggested that a larger body is an adaptation to colder environments because larger organisms have a lower surface–area ratio than smaller organisms, which allows them to conserve heat more effectively (Salewski and Watt, 2017). Lack attributed the larger clutch size in northern species than those in the tropics to a greater food abundance and longer daylight periods during the breeding period (Lack, 1947). These hypotheses, however, remain highly controversial, and several alternative mechanisms have been proposed (Stearns, 1976; Searcy, 1980; Blackburn et al., 1999; Jetz et al., 2008; Pincheira-Donoso, 2010; Meiri, 2011; Olalla-Tárraga, 2011).
Recent hypotheses have explained the variation in body size, reproductive output and life-history traits based on food availability (McNab, 2010; Huston and Wolverton, 2011). In the ‘resource rule’, Bergmann's and Lack's patterns are determined by the size, abundance and food availability (McNab, 2010). The ‘eNPP rule’ (or ‘Geist's rule’) further explains that the factor driving food availability is the global distribution of net primary productivity during the growing season (eNPP) (Geist, 1987; Huston and Wolverton, 2011). Both hypotheses are based on statistical relationships, and agree that, in species or groups of closely related species, the largest and with greater reproductive output will occur where food availability (or eNPP) is highest (McNab, 2010; Huston and Wolverton, 2011). Nevertheless, no clear mechanism has been proposed to explain these hypotheses, and they have not been tested before in a modelling context. Given that habitat degradation poses a great threat to biodiversity, clarifying how food availability and trophic resources may affect species' traits is essential to support conservation actions (Magioli et al., 2019).
According to the resource and the eNPP rules, food availability variation should be the main determinant of energy input (McNab, 2010; Huston and Wolverton, 2011). If food availability is the critical factor determining individual energy allocation, then trait variability among individuals of related species would be minimal when the resource becomes limiting, both in a constant or seasonal environment. Hence, for species with limited phenotypic plasticity, we expect individuals in a low food environment to exhibit a relatively similar body mass and reproductive output compared to a higher food environment, regardless of seasonality.
Research into patterns of trait variation typically exposes the variation using some measure of central tendency in the trait of interest related to particular environmental predictors (Gaston et al., 2008). These patterns are generally approached at three main levels: intraspecific, interspecific and assemblage-based (Gaston et al., 2008; Yom-Tov and Geffen, 2011). Among these, studies considering the interspecific approach are scarcer due to gaps and biases in the knowledge of trait data between species (Gaston et al., 2008). Understanding the effect of environmental predictors on the interspecific variation of body size is complicated by several confounding factors. For example, the use of phenotypic data may lead to the inability to discriminate between genetic (adaptive) and non-genetic (plasticity) sources of variation (Stillwell, 2010). Additionally, the conditions during growth can have a strong effect on body size, for which it would be necessary to control for the birth year (Yom-Tov et al., 2006, 2010; Hersteinsson et al., 2009). A proposed solution to avoid these issues is to conduct common-garden or reciprocal transplant experiments (Yom-Tov and Geffen, 2011). However, these experiments are often not feasible because they involve large samples and individuals' long-term monitoring (Teplitsky and Millien, 2014). For this reason, we adopted a modelling approach that allowed us to investigate interspecific variation in traits by assessing their physiological origin without such confounding effects.
To test our predictions regarding the resource and the eNPP rules, we used the ecophysiological description of the individuals proposed by the Dynamic Energy Budget (DEB) theory (Kooijman, 2010). Using the DEB model, we can mechanistically trace the effect of environmental conditions through individual energy fluxes, and see how they translate into phenotypic traits. In the DEB model, the combination of parameter values define species, and because it has been parameterized for over 3000 animal species (see AmP, 2021 for the complete list), it allowed us to reproduce the natural variation observed in experimental data across species. In this way, we rely on a mechanistic description of the individual metabolism and energy allocation to quantify the environment's effect on across-species traits. We evaluated interspecific variability in genetically-determined physiological characters (represented by the model parameters for assimilation, mobilization and allocation of energy) by carrying out numerical simulations of the DEB model and quantifying the differences in individual traits (i.e. biomass, maturity and reproduction). We considered both constant and seasonal resource conditions in order to provide a complete description of the effect of food availability.
Materials and Methods
To understand the role of resource, we represented interspecific differences with different sets of parameter values and conducted simulations of the DEB model assuming different constant environments. To test our prediction about resource seasonality, we simulated the same interspecific variation in the DEB model but assuming a periodically fluctuating resource. Thus, we evaluated the same set of parameters, each representing a species, both with constant and seasonal resource availability. We then compared the dynamics of two species to highlight the differences due to the resource regime. The Matlab and R scripts used throughout this section are available here: https://gitlab.com/JoanyMarino/deb-resource-and-seasonality.
The standard DEB model
We focus our analysis on the standard DEB model, which is the simplest non-degenerated model implied by DEB theory and the most commonly analysed DEB model (Lika et al., 2011a). The standard DEB model applies to heterotrophic animals, and it supposes that the biomass of an individual is partitioned into reserve energy and structural volume (Kooijman, 2010). Hence, four state variables describe individuals: energy in reserve (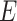 , J), structural volume (
, J), structural volume (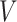 , cm3), cumulative maturity energy (
, cm3), cumulative maturity energy (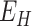 , J) and cumulative reproduction energy (
, J) and cumulative reproduction energy (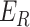 , J). We assume that individuals release the reproduction energy continually as gametes, regardless of the environmental conditions, to neglect its potential contribution to the individuals' biomass. To account for the different possible reproduction strategies, we measure reproductive output as cumulative reproductive energy.
, J). We assume that individuals release the reproduction energy continually as gametes, regardless of the environmental conditions, to neglect its potential contribution to the individuals' biomass. To account for the different possible reproduction strategies, we measure reproductive output as cumulative reproductive energy.
The DEB model assumes that energy from food is assimilated into the reserve (Figure 1) through the assimilation flux (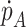 , J/d). The reserve energy is mobilized according to the mobilization flux (
, J/d). The reserve energy is mobilized according to the mobilization flux (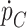 , J/d). A fixed fraction (
, J/d). A fixed fraction (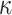 ) of the mobilized reserve is allocated to somatic maintenance and volume growth (
) of the mobilized reserve is allocated to somatic maintenance and volume growth (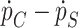 , J/d). The remaining fraction of the mobilized energy (
, J/d). The remaining fraction of the mobilized energy (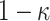 ) is allocated to maturity maintenance and maturation in juvenile individuals or maturity maintenance and reproduction in adults (
) is allocated to maturity maintenance and maturation in juvenile individuals or maturity maintenance and reproduction in adults (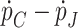 , J/d). We concentrate on ectotherms, thus maintenance costs are considered to be proportional to volume (assuming that the surface-area proportional term is equal to zero). Thus, the temporal dynamic of the individual state variables is:
, J/d). We concentrate on ectotherms, thus maintenance costs are considered to be proportional to volume (assuming that the surface-area proportional term is equal to zero). Thus, the temporal dynamic of the individual state variables is:
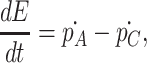 |
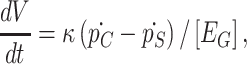 |
(1) |
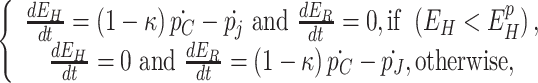 |
where 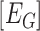 is the volume-specific cost for structure, and the energy fluxes
is the volume-specific cost for structure, and the energy fluxes 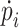 for each process
for each process 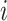 are given in Table 1. The standard DEB model considers organisms with three life stages, as determined by the cumulative maturity level relative to the maturity thresholds parameters,
are given in Table 1. The standard DEB model considers organisms with three life stages, as determined by the cumulative maturity level relative to the maturity thresholds parameters, 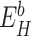 for birth and
for birth and 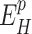 for puberty: embryo (
for puberty: embryo (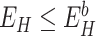 and
and 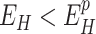 ), juvenile (
), juvenile (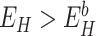 and
and 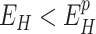 ) and adult (
) and adult (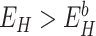 and
and 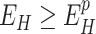 ).
).
Figure 1.
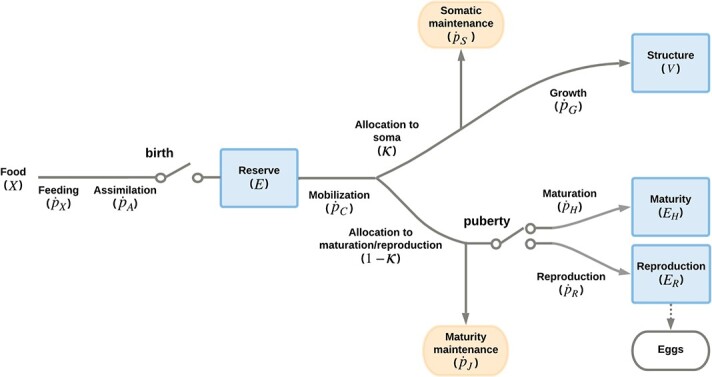
Representation of the standard DEB model (eq. 1). Square boxes denote the state variables, while round boxes represent energy sinks. Lines and arrows correspond to the energy fluxes (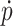 J/d, Table 1). The switches represent metabolic thresholds: birth indicates the start of feeding, while puberty signals the start of energy allocation to reproduction once maturation is complete.
J/d, Table 1). The switches represent metabolic thresholds: birth indicates the start of feeding, while puberty signals the start of energy allocation to reproduction once maturation is complete.
Table 1. Energy fluxes (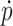 J/d) at each developmental stage. The scaled functional response is
J/d) at each developmental stage. The scaled functional response is 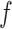 (
(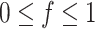 , where 1 is the highest amount of food),
, where 1 is the highest amount of food), 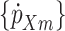 is the maximum surface-area specific ingestion rate (
is the maximum surface-area specific ingestion rate (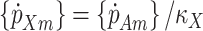 ,
, 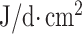 ),
), 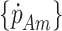 is the maximum surface-area specific assimilation rate (
is the maximum surface-area specific assimilation rate (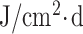 ),
), 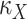 is the assimilation efficiency from food to reserve (dimensionless),
is the assimilation efficiency from food to reserve (dimensionless), 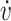 is the energy conductance rate from the energy reserve (
is the energy conductance rate from the energy reserve (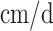 ),
), 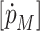 is the volume-specific somatic maintenance cost (
is the volume-specific somatic maintenance cost (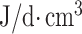 ),
), 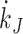 is the maturity maintenance rate coefficient (
is the maturity maintenance rate coefficient (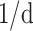 ). Notation: square braces ([ ]) indicate quantities related to structural volume, curly braces ({ }) denote quantities related to structural surface-area, dots ( ˙ ) indicate rates.
). Notation: square braces ([ ]) indicate quantities related to structural volume, curly braces ({ }) denote quantities related to structural surface-area, dots ( ˙ ) indicate rates.
| Flux (J/d) | Embryo | Juvenile | Adult |
|---|---|---|---|
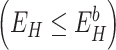
|
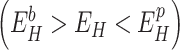
|
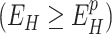
|
|
Feeding, 
|
0 |
|
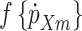
|
Assimilation, 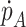
|
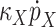
|
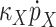
|
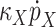
|
Mobilization, 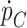
|
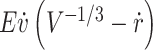
|
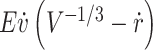
|
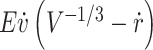
|
Somatic maintenance, 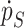
|
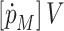
|
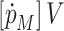
|
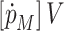
|
Maturity maintenance, 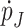
|
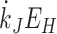
|
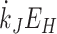
|
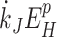
|
The specific growth rate in structural volume is:
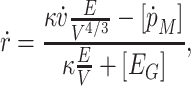 |
(2) |
where 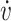 is the energy reserve mobilization rate (
is the energy reserve mobilization rate ( ) and
) and 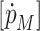 are the volume-specific somatic maintenance costs (
are the volume-specific somatic maintenance costs (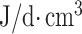 ).
).
In the DEB model, both somatic and maturity maintenance have priority over investment in either growth, maturation, or reproduction. Hence, starvation occurs when mobilized energy does not suffice to cover somatic maintenance (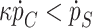 ) or maturity maintenance (
) or maturity maintenance (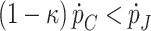 ). The standard DEB model makes no assumptions about these situations, meaning that organisms follow the same dynamics previously outlined when subjected to starvation. Consequently, when there is prolonged starvation, individuals will degrade the structural mass to cover maintenance costs and shrink in size (because the specific growth rate
). The standard DEB model makes no assumptions about these situations, meaning that organisms follow the same dynamics previously outlined when subjected to starvation. Consequently, when there is prolonged starvation, individuals will degrade the structural mass to cover maintenance costs and shrink in size (because the specific growth rate 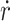 becomes negative).
becomes negative).
DEB theory assumes that the metabolic rates are affected by the organism's temperature, which in ectotherms, particularly aquatic, can be considered equal to environmental temperature (Kooijman, 2010). As a simplification, our simulations did not consider thermoregulation through insulation, modulated by behaviour, or surface colour. Thus, we equated habitat temperature to internal temperature. To increase comparability across species, the model parameters are usually standardized to the reference temperature of 20 °C (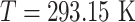 ) through the Arrhenius relationship. For simplicity, we suppose here that the environmental temperature is equal to the reference temperature. Further, because food availability often covaries with environmental temperature, we assume that the temperature remains constant and evaluate the effect of seasonality only in the resource.
) through the Arrhenius relationship. For simplicity, we suppose here that the environmental temperature is equal to the reference temperature. Further, because food availability often covaries with environmental temperature, we assume that the temperature remains constant and evaluate the effect of seasonality only in the resource.
Resource
We assumed that the food density operates directly on the scaled functional response 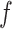 , facilitating contrasting the model's behaviour across different fluctuation regimes (Muller and Nisbet, 2000). To investigate the effect of interspecific differences, we first conducted simulations assuming a constant resource, ranging from scarce (
, facilitating contrasting the model's behaviour across different fluctuation regimes (Muller and Nisbet, 2000). To investigate the effect of interspecific differences, we first conducted simulations assuming a constant resource, ranging from scarce (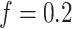 ) to maximum availability (
) to maximum availability (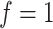 ). We did not evaluate prolonged periods of limited food because this would require making further assumptions on the handling of starvation.
). We did not evaluate prolonged periods of limited food because this would require making further assumptions on the handling of starvation.
To evaluate the effect of resource variability, we assumed a periodically changing functional response, which represents the alternation between two levels of food during the year, similar to a seasonal change. Specifically, the functional response at time 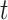 oscillates around the average (
oscillates around the average (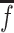 ) with amplitude
) with amplitude 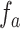 and period equal to the length of 1 year:
and period equal to the length of 1 year:
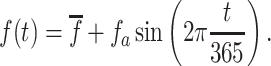 |
(3) |
We only consider variations in the value of the mean scaled functional response for simplicity, keeping the amplitude of the oscillations fixed. To assess the initial resource's effect, we simulated functional responses that start at four different points in the seasonal cycle: maximum resource, intermediate but decreasing resource, minimum resource and intermediate but increasing resource. These different initial conditions of food can be understood as corresponding to individuals born at different times throughout the year.
Interspecific variability and parameter space
Despite the diversity of life-history traits and strategies in the animal kingdom, not all strategies are possible (Healy et al., 2019). In order to select a biologically consistent parameter set, we used the AmP collection (a web repository of species parameterized for DEB models; AmP, 2021) and the routines in the AmPtool (version 03/2020 AmPtool, 2020) MATLAB package (version 9.8, The MathWorks Inc., 2020). We started by taking a subset of the species in the AmP collection (Figure 2, panel 1). Then, we narrowed down the parameter space according to the occurrence frequency of the parameter combinations (Figure 2, panel 3).
Figure 2.
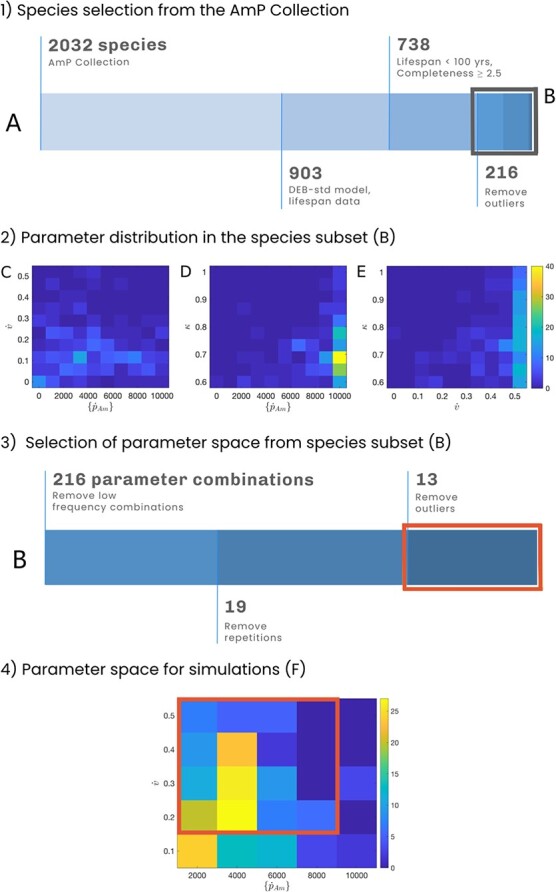
Representation of the selection process that assured biological consistency in the parameter space. Panel 1 shows the species selection in the AmP collection, starting from all the entries (at 03/2020) to a final subset of 216 species (B). The values indicate the number of species at each step. The steps are: i) subset entries modeled with the DEB-std model and containing lifespan data, ii) subset entries with lifespan below 100 years and data completeness equal or greater than 2.5, and iii) remove entries more than 1.5 interquartile ranges above the upper quartile or below the lower quartile of the parameter distribution. In panel 2, the plots show the bivariate distribution of the parameters 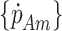 ,
, 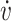 , and
, and 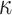 in our 216 species subset (B). For all the plots, the parameters' distribution is not uniform. Hence, all parameter combinations are not equally likely to occur. The colour bar shows the joint frequency of occurrence. Panel 3 shows the selection of the joint parameter space for
in our 216 species subset (B). For all the plots, the parameters' distribution is not uniform. Hence, all parameter combinations are not equally likely to occur. The colour bar shows the joint frequency of occurrence. Panel 3 shows the selection of the joint parameter space for 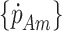 and
and 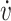 from the species subset B. The values at each step show the number of parameter combinations, starting from 216 to a final subset of 13 parameter combinations (C). The steps are: iv) find the joint distribution of the parameters and remove parameter combinations with a frequency of one, v) remove parameter combinations that are not unique, and vi) remove outlier values. In panel 4, the plot shows the joint distribution of
from the species subset B. The values at each step show the number of parameter combinations, starting from 216 to a final subset of 13 parameter combinations (C). The steps are: iv) find the joint distribution of the parameters and remove parameter combinations with a frequency of one, v) remove parameter combinations that are not unique, and vi) remove outlier values. In panel 4, the plot shows the joint distribution of 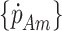 and
and 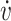 for the 214 parameter combinations most likely to occur in our species subset. The box marks the outer boundary of our parameter space in our parameter combination subset (C). Without repetitions, the parameter space C corresponds to the 16 combinations we evaluated for
for the 214 parameter combinations most likely to occur in our species subset. The box marks the outer boundary of our parameter space in our parameter combination subset (C). Without repetitions, the parameter space C corresponds to the 16 combinations we evaluated for 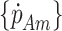 and
and 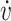 .
.
First, our focus is on the species in the AmP collection (Figure 2, panel 1). We selected the species modelled using the standard DEB model and containing lifespan data. To assure that the values are consistent, we restricted the entries to those with a lifespan 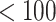 years and data completeness
years and data completeness 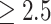 (the data completeness indicates how much data is available to estimate the DEB parameters, ranging from a minimum of 0 when only maximum body weight or size is known, to a maximum of 10 when all aspects of energetics are known; Lika et al., 2011a). Completeness of 2.5 means that there is data for the species on maximum body weight (or size), age, length and weight at birth and puberty and growth in time (Lika et al., 2011a); allowing the estimation of the parameters
(the data completeness indicates how much data is available to estimate the DEB parameters, ranging from a minimum of 0 when only maximum body weight or size is known, to a maximum of 10 when all aspects of energetics are known; Lika et al., 2011a). Completeness of 2.5 means that there is data for the species on maximum body weight (or size), age, length and weight at birth and puberty and growth in time (Lika et al., 2011a); allowing the estimation of the parameters 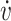 ,
, 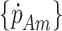 ,
, 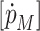 ,
, 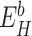 and
and 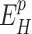 (Kooijman et al., 2008). Then, we removed parameter outliers by excluding values 1.5 interquartile ranges above the upper quartile or below the lower quartile of each parameter distribution, which left 216 species.
(Kooijman et al., 2008). Then, we removed parameter outliers by excluding values 1.5 interquartile ranges above the upper quartile or below the lower quartile of each parameter distribution, which left 216 species.
Next, we concentrated on the parameters of the 216 species subset. We focused our analysis on three parameters that directly relate to concepts of life-history theory: maximum assimilation rate (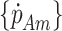 ), which reflects the ability to acquire energy; energy conductance (
), which reflects the ability to acquire energy; energy conductance (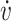 ), which is related to the ‘pace-of-life’ concept; and energy allocation to maturity and reproduction (
), which is related to the ‘pace-of-life’ concept; and energy allocation to maturity and reproduction (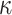 ), which reflects the trade-off between somatic growth and reproduction. All combinations of these three parameters in our subset of species are not equally likely to occur, they may not be biologically realistic, and multiple combinations are repeated (Figure 2, panel 2). For this reason, we calculated the joint distribution of
), which reflects the trade-off between somatic growth and reproduction. All combinations of these three parameters in our subset of species are not equally likely to occur, they may not be biologically realistic, and multiple combinations are repeated (Figure 2, panel 2). For this reason, we calculated the joint distribution of 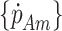 and
and 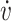 by discretizing their joint range into five intervals and assigning the interval's mean as the parameter value. Subsequently, we removed the combinations of
by discretizing their joint range into five intervals and assigning the interval's mean as the parameter value. Subsequently, we removed the combinations of 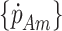 and
and 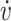 with a low occurrence frequency, leaving the 214 parameter combinations most likely to occur in our species subset. Since these parameter combinations are not unique, we excluded the repetitions, which left 19 unique combinations of
with a low occurrence frequency, leaving the 214 parameter combinations most likely to occur in our species subset. Since these parameter combinations are not unique, we excluded the repetitions, which left 19 unique combinations of 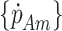 and
and 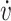 (Figure 2, panel 3). Then, we removed outlier points at the edges of the discrete distribution, leaving a parameter space consisting of 13 combinations of
(Figure 2, panel 3). Then, we removed outlier points at the edges of the discrete distribution, leaving a parameter space consisting of 13 combinations of 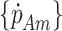 and
and 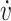 . To increase our analysis's resolution, we added combinations that fell within the parameter space range but had a lower occurrence frequency. Hence, we evaluated
. To increase our analysis's resolution, we added combinations that fell within the parameter space range but had a lower occurrence frequency. Hence, we evaluated 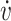 equal to 0.2, 0.3, 0.4 and 0.5, and
equal to 0.2, 0.3, 0.4 and 0.5, and 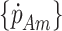 equal to 2000, 4000, 6000 and 8000, which corresponds to 16 different combinations of
equal to 2000, 4000, 6000 and 8000, which corresponds to 16 different combinations of 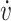 and
and 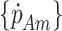 (Figure 2, panel 4).
(Figure 2, panel 4).
Reproduction data is required to estimate the value of 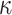 , as well as growth and size at birth and puberty (Lika et al., 2011b). Given that reproduction is often difficult to quantify, many AmP collection entries assume the predefined value of
, as well as growth and size at birth and puberty (Lika et al., 2011b). Given that reproduction is often difficult to quantify, many AmP collection entries assume the predefined value of 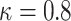 , which results in rapid growth to a large size, long development times and low reproduction. Even when data is available, due to the simplification of seasonality effects in the data, which do not consider the cycles in up- and down-regulation of metabolism, the parameter estimation is likely to result in a high value for
, which results in rapid growth to a large size, long development times and low reproduction. Even when data is available, due to the simplification of seasonality effects in the data, which do not consider the cycles in up- and down-regulation of metabolism, the parameter estimation is likely to result in a high value for 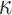 (Kooijman and Lika, 2014). Hence, the AmP collection is biased to high values for
(Kooijman and Lika, 2014). Hence, the AmP collection is biased to high values for 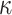 . However, it has been shown that a lower value of
. However, it has been shown that a lower value of 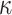 (
(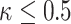 ) is likely to fit growth and reproduction data equally, as well as the larger value, and producing individuals with reduced growth and reproduction (Lika et al., 2011a, 2011b). Here, we chose to evaluate variations around the lower value of
) is likely to fit growth and reproduction data equally, as well as the larger value, and producing individuals with reduced growth and reproduction (Lika et al., 2011a, 2011b). Here, we chose to evaluate variations around the lower value of 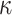 to represent more realistic scenarios where limiting food can alter the reproductive output (Lika and Kooijman, 2003). Thus, we assessed
to represent more realistic scenarios where limiting food can alter the reproductive output (Lika and Kooijman, 2003). Thus, we assessed 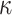 equal to 0.43, 0.51 and 0.58.
equal to 0.43, 0.51 and 0.58.
We consider the rest of the model parameters as constants (Table 2) because previous interspecific comparisons have shown that maintenance costs and structural costs remain largely similar between species (van der Veer et al., 2001; Freitas et al., 2010). To maintain biological consistency among all the parameters, we used the estimated values for Daphnia magna. Moreover, this decision avoids obtaining parameter combinations that were not possible to compute numerically. The parameters of D. magna have been estimated from multiple experiments, reaching a data completeness of 6, which is the highest in the AmP collection. As such, these values are more likely to represent the individual physiology accurately.
Table 2. Parameter values for the simulations of the standard DEB model (equation 1) and the resource (equation 3). Notation: square braces ([ ]) indicate quantities related to structural volume, curly braces ({ }) denote quantities related to structural surface-area, dots ( ˙ ) indicate rates.
| Description | Symbol | Value | Unit |
|---|---|---|---|
| Individual dynamics | |||
| Maximum assimilation rate |
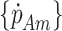
|
[2000, 4000, 6000, 8000] | J/day  cm2 cm2
|
| Assimilation efficiency |
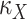
|
0.9 | - |
| Energy conductance rate |
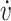
|
[0.2, 0.3, 0.4, 0.5] | cm/day |
| Allocation fraction to soma |
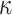
|
[0.43, 0.51, 0.58] | - |
| Somatic maintenance cost |
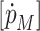
|
1800 | J/day  cm3 cm3
|
| Maturity maintenance coefficient |
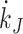
|
0.52 | 1/day |
| Specific cost for structure |
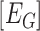
|
4400 | J/cm3 |
| Maturity at birth |
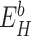
|
0.55 | J |
| Maturity at puberty |
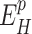
|
1.09 | J |
| Initial energy in the embryo |
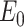
|
0.167 | J |
| Shape coefficient |
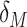
|
1 | - |
| Environmental dynamics | |||
| Environmental temperature |
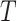
|
293.15 | K |
| Functional response (constant environment) |
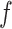
|
[0.2, 0.4, 0.6, 0.8, 1] | - |
| Average functional response (seasonal environment) |
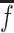
|
[0.4, 0.6, 0.8] | - |
| Functional response (peak) amplitude |
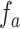
|
0.2 | - |
Parameter space validation
To verify our parameter subset's biological relevance, we examined the AmP collection for species within the parameter space. First, we downloaded the full list of species and parameters from the AmP collection. Then, we used R (version 3.6.2, R Core Team, 2019), to find all the species parameterized with the standard DEB model within the limits of our parameter space for 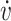 (in the interval [0.15, 0.55]),
(in the interval [0.15, 0.55]), 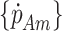 (in [1500, 9500]) and
(in [1500, 9500]) and 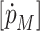 (in [1600, 2000]). We did not restrict the value of
(in [1600, 2000]). We did not restrict the value of 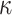 to our parameter subset because the collection is biased towards larger values. To understand how the parameters vary among the validation species, we calculated a coefficient of variation (
to our parameter subset because the collection is biased towards larger values. To understand how the parameters vary among the validation species, we calculated a coefficient of variation (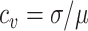 ).
).
Relationship between variables and observable quantities
The state variables in the DEB model are not directly measurable; hence, we transform them into quantities that can be observed across individuals. Specifically, the reserve energy (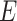 ) plus the structural volume (
) plus the structural volume (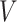 ) constitute the energy fixed in the individual's biomass. Nonetheless, to calculate the biomass as (dry) weight in grams, we need to account for the density and compositions of both variables, as given by:
) constitute the energy fixed in the individual's biomass. Nonetheless, to calculate the biomass as (dry) weight in grams, we need to account for the density and compositions of both variables, as given by:
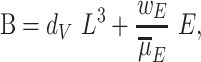 |
(4) |
where 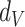 (g/cm3) is the density of the structural volume,
(g/cm3) is the density of the structural volume, 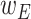 (g/Cmol) is the molar weight of the energy reserve and
(g/Cmol) is the molar weight of the energy reserve and 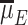 (J/Cmol) is the chemical potential of the energy reserve (equation 3.2 in Kooijman, 2010). These constants are species-specific; for generality, however, we used the standard values:
(J/Cmol) is the chemical potential of the energy reserve (equation 3.2 in Kooijman, 2010). These constants are species-specific; for generality, however, we used the standard values: 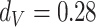 ,
, 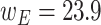 and
and 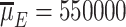 (Kooijman, 2010).
(Kooijman, 2010).
We illustrated the model behaviour using the related Fan-tailed Gerygone, Gerygone flavolateralis and the Grey Warbler, G. igata, as a case study to compare the differences between the constant and seasonal resource environments. For these two species, we transformed the above dry biomass (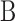 ) into wet biomass (
) into wet biomass (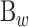 ) assuming the relation
) assuming the relation 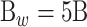 , which is based on observations of water content in fledglings of the Grey Warbler (Gill 1982).
, which is based on observations of water content in fledglings of the Grey Warbler (Gill 1982).
Model analysis
When there are seasonal fluctuations, the model's nonlinearities make it impossible to determine the dynamics analytically (Muller and Nisbet, 2000). For this reason, we addressed our questions through numerical studies. We implemented the DEB model in the R language (version 3.6.2, R Core Team, 2019) and performed the time integrations using the ‘lsoda’ initial value problem solver from the package deSolve (Soetaert et al., 2010). We integrated the model for 3 years, until reaching a steady state or stable limit cycle. When comparing the tropical and temperate species, we used an integration time of 6 years to better reflect the differences that may accumulate over time. For the simulations considering resource variability, we assumed one seasonal cycle per year, i.e. one period of low food availability and one period of high food availability.
Data analysis and model validation
To show the combined effect of the parameters and the resource, we summarized the model simulations at different food levels through scatterplots. We express the energy reserve and the structural volume variables together as the individual's biomass for conciseness. For comparative purposes, in the constant resource environment, we plot the steady-state value of the biomass. In contrast, in the seasonal environment, we show the average values attained after the ontogenetic growth period (transient dynamics) have been discarded. For the cumulative reproduction energy variable, in both environmental scenarios, we show the average reproductive energy attained in the last 2 years of the individual's lifespan. Similarly, for the maturity energy variable, we emphasize the time to reach the puberty threshold in both food environments.
To take into account the relative differences between individuals of different species in our analysis, we computed the relative value of each state variable 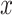 :
:
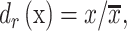 |
(5) |
where 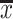 is the mean of all the simulations at the same resource level
is the mean of all the simulations at the same resource level 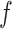 , but considering a different parameter set for
, but considering a different parameter set for 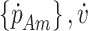 and
and 
At constant resource availability, the equation for energy reserves in (1) leads to the constant:
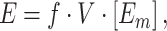 |
(6) |
where 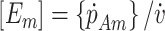 , which is the maximum value of the reserve density (also called the reserve capacity). Thus, to verify our simulations' behaviour, we compared our model predictions for energy reserves at constant resource with the expected values from equation 6.
, which is the maximum value of the reserve density (also called the reserve capacity). Thus, to verify our simulations' behaviour, we compared our model predictions for energy reserves at constant resource with the expected values from equation 6.
Results
Parameter space validation
We found 14 species in the AmP collection modelled with the DEB-std model having parameter values that lie within our parameter space for 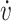 ,
, 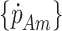 and
and 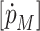 (Figure 3, Supplementary Table 1). These species' parameters have values across all our parameter space for
(Figure 3, Supplementary Table 1). These species' parameters have values across all our parameter space for 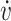 ; however, for
; however, for 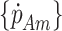 the values are below 8000 (Figure 2, panel 4). All of the species are in the Aves class (superorder Neognathae) likely due to the restriction of selecting entries parameterized with the DEB-std model (which constitute ~45% of the collection) and the high representation of this class in the collection (~21%).
the values are below 8000 (Figure 2, panel 4). All of the species are in the Aves class (superorder Neognathae) likely due to the restriction of selecting entries parameterized with the DEB-std model (which constitute ~45% of the collection) and the high representation of this class in the collection (~21%).
Figure 3.
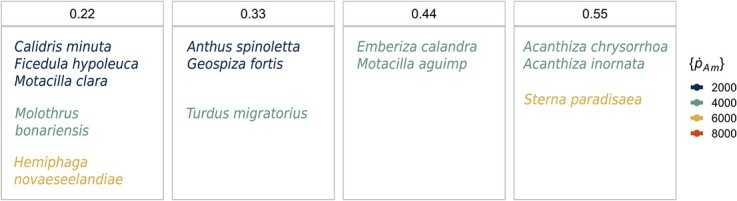
Position of the species within our parameter space for 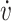 and
and 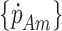 . See Supplementary Table 1 for parameter values and common names.
. See Supplementary Table 1 for parameter values and common names.
Birds that fall within our parameter space are characterized by being small to medium size (from 15.5 g in the European pied flycatcher to 100 g in the Artic tern, except for the New Zealand pigeon at 590 g), terrestrial, flighted and mostly carnivores (mainly insectivores, except for the New Zealand pigeon and the Medium ground finch, which are frugivores or granivores). Relative to the AmP collection (AmP, 2021), the species' parameters are biased to high values of energy conductance and somatic maintenance costs and intermediate to high assimilation values (AmP, 2021). The parameters for 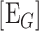 ,
, 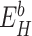 and
and 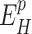 , are all above the limits of our parameter space because they correspond to species of a larger size and longer lifespan than D. magna.
, are all above the limits of our parameter space because they correspond to species of a larger size and longer lifespan than D. magna.
Model validation
Our model's predictions in the constant food environment match the analytical solutions for the reserve density (Supplementary Figure 1), which indicates that the simulations are consistent with the expected results.
Effect of interspecific differences
To test for the consequences of interspecific variability, we conducted simulations in a constant resource environment. We expected that, in the simulations where the resource is limiting, individuals of different species reach a similar size and reproductive output because low food should reduce interspecific variability. We found that a decreasing resource does minimize the consequences of interspecific differences in biomass and reproduction (Figures 4 and 5. See Supplementary Figures 2 and 3 for the differences in reserve energy and structural volume). We found the opposite effect for maturation time, where lower food levels lead to variable development rates (Supplementary Figure 4).
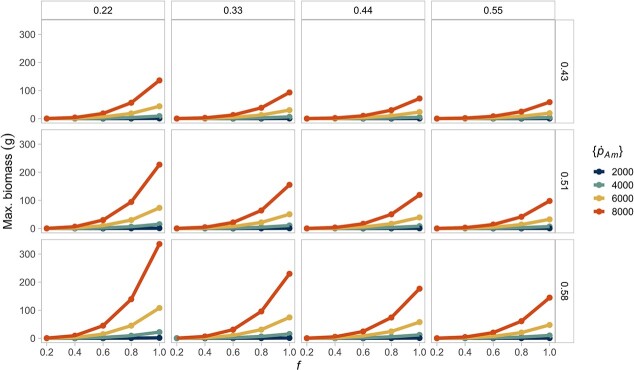
Figure 4.
A decreasing, constant resource (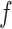 ) reduces interspecific differences in maximum biomass. The largest biomass is attained when individuals combine high assimilation with low energy conductance. The columns show the different values of energy conductance evaluated (
) reduces interspecific differences in maximum biomass. The largest biomass is attained when individuals combine high assimilation with low energy conductance. The columns show the different values of energy conductance evaluated (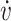 ). The rows represent the fraction of energy allocated to soma (
). The rows represent the fraction of energy allocated to soma (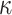 ). The colours of the lines indicate the value of the maximum specific assimilation rate (
). The colours of the lines indicate the value of the maximum specific assimilation rate (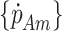 ). Lines of the same colour in each box (equivalent to a parameter combination) represent the same species at different food levels.
). Lines of the same colour in each box (equivalent to a parameter combination) represent the same species at different food levels.
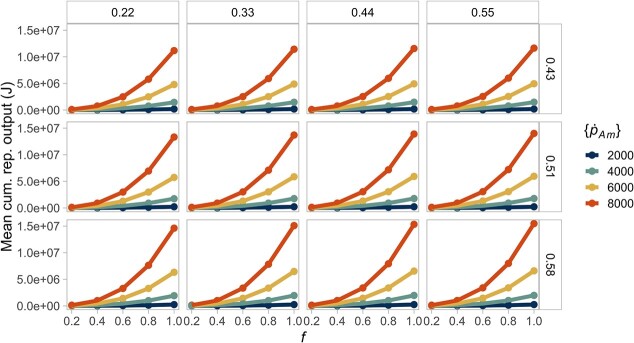
Figure 5.
A declining, constant resource (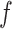 ) reduces interspecific variability in mean cumulative reproductive output. Higher reproductive output is reached when the fraction of energy allocated to soma is high. The columns show the different values of energy conductance evaluated (
) reduces interspecific variability in mean cumulative reproductive output. Higher reproductive output is reached when the fraction of energy allocated to soma is high. The columns show the different values of energy conductance evaluated (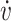 ). The rows represent the fraction of energy allocated to soma (
). The rows represent the fraction of energy allocated to soma (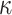 ). The colours of the lines indicate the value of the maximum specific assimilation rate (
). The colours of the lines indicate the value of the maximum specific assimilation rate (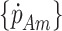 ). Lines of the same colour in each box (equivalent to a parameter combination) represent the same species at different food levels.
). Lines of the same colour in each box (equivalent to a parameter combination) represent the same species at different food levels.
When the resource is non-limiting, a combination of high assimilation but low energy mobilization (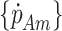 = 8000,
= 8000, 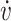 = 0.2) produces individuals with the highest biomass (Figure 4). Here, a lower energy conductance magnifies the interspecific differences since the organisms mobilize less energy from the reserves. However, the fraction of energy allocated to soma does not affect the biomass because the increase in energy and volume is proportional.
= 0.2) produces individuals with the highest biomass (Figure 4). Here, a lower energy conductance magnifies the interspecific differences since the organisms mobilize less energy from the reserves. However, the fraction of energy allocated to soma does not affect the biomass because the increase in energy and volume is proportional.
Interspecific differences are greater in the reproductive output for organisms that combine a large assimilation rate with a high fraction of energy allocation to soma in an environment with high resource availability (Figure 5). This counterintuitive result, where allocating more energy to soma produces higher reproduction (instead of lower reproduction), is caused by the moderate 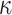 values in our parameter space:
values in our parameter space: 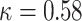 likely to be close to one of the two optimum points of maximum reproductive output as a function of
likely to be close to one of the two optimum points of maximum reproductive output as a function of 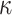 . The energy conductance rate seems not to affect the reproductive output, which may be a consequence of the smaller range evaluated for conductance compared to that of energy assimilation.
. The energy conductance rate seems not to affect the reproductive output, which may be a consequence of the smaller range evaluated for conductance compared to that of energy assimilation.
The maturation time shows increased variability when the resource is scarce (Supplementary Figure 4). The differences in development time are small (between a minimum of 1 day to a maximum of 4 days); however, they are greater for individuals with reduced assimilation and conductance rates at an intermediate value of energy allocation to soma. For example, when food is scarce (f = 0.2) and the energy allocation to soma increases (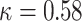 ), a combination of low energy assimilation (
), a combination of low energy assimilation ( = 2000) and mobilization (
= 2000) and mobilization ( = 0.2) produces individuals with the slowest maturation (4 days).
= 0.2) produces individuals with the slowest maturation (4 days).
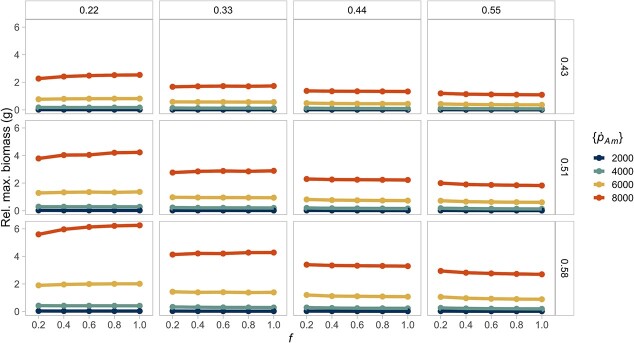
Organisms grow larger at higher food densities, thus resulting in larger absolute differences. However, the relative differences in biomass (Figure 6) are not strictly constant, with higher resource leading to relatively higher biomass when energy conductance is low (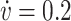 ), but to relatively lower biomass when mobilization is high (
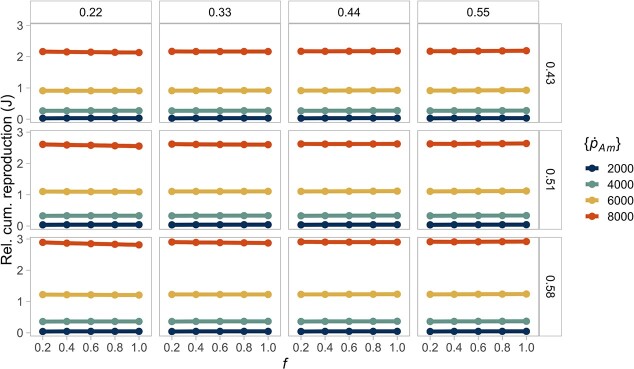
), but to relatively lower biomass when mobilization is high (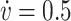 ). These differences in biomass reflect the effect of the energy conductance parameter on the energy reserves (Supplementary Figure 5), while the relative differences in structural volume remain constant (Supplementary Figure 6). As a result, the relative differences are not systematically higher at high food levels, as hypothesized in the introduction, but the effect of food availability is reversed when conductance is high. Yet, this effect does not occur when considering the relative differences in reproduction energy, which show that species' differences are nearly constant across resource levels (Figure 7) or, in the case of maturation time, the differences are small (Supplementary Figure 7). These findings indicate that the resource level mainly has a scaling effect on the reproductive output, but it is not only proportional for the biomass dynamics.
). These differences in biomass reflect the effect of the energy conductance parameter on the energy reserves (Supplementary Figure 5), while the relative differences in structural volume remain constant (Supplementary Figure 6). As a result, the relative differences are not systematically higher at high food levels, as hypothesized in the introduction, but the effect of food availability is reversed when conductance is high. Yet, this effect does not occur when considering the relative differences in reproduction energy, which show that species' differences are nearly constant across resource levels (Figure 7) or, in the case of maturation time, the differences are small (Supplementary Figure 7). These findings indicate that the resource level mainly has a scaling effect on the reproductive output, but it is not only proportional for the biomass dynamics.
Figure 6.
A constant resource (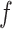 ) partially scales the interspecific differences in biomass. Hence, there are only small relative differences between different food levels for the same species. The columns show the different values of energy conductance evaluated (
) partially scales the interspecific differences in biomass. Hence, there are only small relative differences between different food levels for the same species. The columns show the different values of energy conductance evaluated (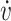 ). The rows represent the fraction of energy allocated to soma (
). The rows represent the fraction of energy allocated to soma (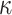 ). The colours of the lines indicate the value of the maximum specific assimilation rate (
). The colours of the lines indicate the value of the maximum specific assimilation rate (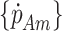 ). Lines of the same colour in each box (equivalent to a parameter combination) represent the same species at different food levels.
). Lines of the same colour in each box (equivalent to a parameter combination) represent the same species at different food levels.
Figure 7.
A constant resource (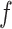 ) scales the interspecific differences in reproductive output. Hence, there are no relative differences between different food levels for the same species. The columns show the different values of energy conductance evaluated (
) scales the interspecific differences in reproductive output. Hence, there are no relative differences between different food levels for the same species. The columns show the different values of energy conductance evaluated (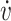 ). The rows represent the fraction of energy allocated to soma (
). The rows represent the fraction of energy allocated to soma (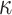 ). The colours of the lines indicate the value of the maximum specific assimilation rate (
). The colours of the lines indicate the value of the maximum specific assimilation rate (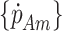 ). Lines of the same colour in each box (equivalent to a parameter combination) represent the same species at different food levels.
). Lines of the same colour in each box (equivalent to a parameter combination) represent the same species at different food levels.
For maturation energy, the relative differences are not identical for each resource level and appear to be larger for a combination of lower resource and energy conductance (Supplementary Figure 7). However, the range of these differences is small and may not reflect significant differences.
Effect of resource variability
We evaluated the consequences of environmental variability by simulating a seasonally-varying resource. As in the environment with a constant resource, absolute interspecific variability in biomass and reproductive output is greater when average food is more abundant (Supplementary Figures 8 to 11). Further, biomass and cumulative reproductive output are independent of the initial resource density (Supplementary Figures 13 to 24), indicating that the individuals can compensate for variations in resource abundance during their lifespan. As expected, the average values for biomass and cumulative reproductive output are larger compared to the same individual in a constant environment with equal mean food availability. We address these results in the next section.
As in the constant environment, the maturation time shows increased variability when the resource is scarce (Supplementary Figure 12). Furthermore, interspecific differences are amplified when the initial resource density is low, compared to simulations where the initial resource is high. Individuals born in an environment that slowly becomes hospitable grow slower and remain small during the first season, particularly if they have a low assimilation rate. In contrast, individuals that start at the onset of a good period grow quickly to a large size, especially when the allocation fraction to soma is large. Yet, these differences in maturation may not be significant, given that the development times are equally short (Supplementary Figures 25 to 27).
Comparing temperate and tropical species: An example
To better understand the resource's effect, we compared the temporal dynamics of one species (given by the parameter set 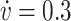 ,
, 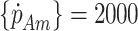 ,
, 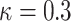 and
and  ) in both a constant and a seasonal resource environment with equal mean resource availability. The dynamics show that, despite having the same parameter values, the individual in the seasonal environment reaches a greater average biomass (Figure 8A) and cumulative reproductive output (Figure 8B) than an individual of the same species in a constant environment with an equal average resource. The resource does not appear to affect the maturation dynamics, given that the maturation threshold parameters are low and can be reached shortly after birth in both environments (Figure 8C). Inspecting the assimilation dynamics shows that the imbalance in the assimilation flux across seasons largely explains the differences in biomass and reproduction between resource environments (Figure 8D). A hypothetical individual of the same species and in the same seasonal environment, in which the structural volume remains constant (once it has reached its maximum volume), has a symmetrical assimilation flux that reflects the oscillations of the resource. In contrast, our simulations show that, when volume is not fixed, an individual can have a larger assimilation flux during the season with high food availability, meaning that it overcompensates the losses of the bad season.
) in both a constant and a seasonal resource environment with equal mean resource availability. The dynamics show that, despite having the same parameter values, the individual in the seasonal environment reaches a greater average biomass (Figure 8A) and cumulative reproductive output (Figure 8B) than an individual of the same species in a constant environment with an equal average resource. The resource does not appear to affect the maturation dynamics, given that the maturation threshold parameters are low and can be reached shortly after birth in both environments (Figure 8C). Inspecting the assimilation dynamics shows that the imbalance in the assimilation flux across seasons largely explains the differences in biomass and reproduction between resource environments (Figure 8D). A hypothetical individual of the same species and in the same seasonal environment, in which the structural volume remains constant (once it has reached its maximum volume), has a symmetrical assimilation flux that reflects the oscillations of the resource. In contrast, our simulations show that, when volume is not fixed, an individual can have a larger assimilation flux during the season with high food availability, meaning that it overcompensates the losses of the bad season.
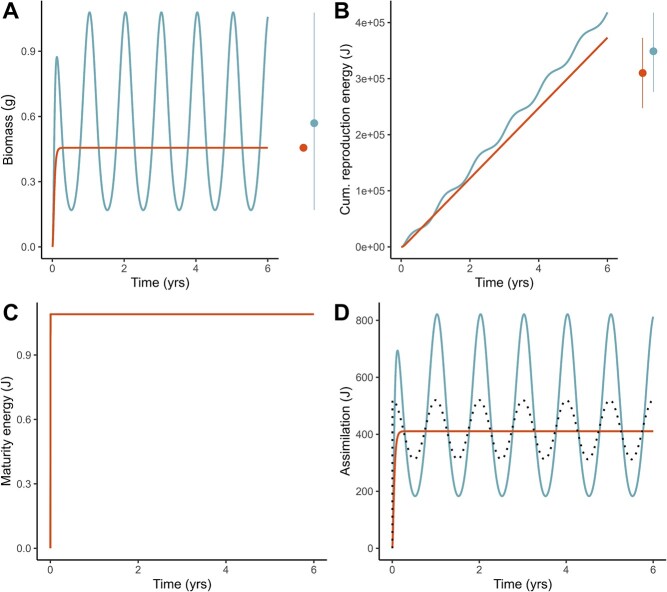
Figure 8.
Individuals of the same species reach larger average biomass (A) and reproduction (B) in a seasonal resource environment (blue lines) relative to the same individual in an environment with an equal mean resource availability (red lines) due to an increase in the assimilation flux (D). In the right panel of (A), for the species in the constant environment, the red point shows the steady-state value of biomass reached at the end of the lifespan. In contrast, for the seasonal environment, the blue point represents the average biomass calculated over the last 4 years (i.e. Years 2 to 6), and lines show the minimum and maximum values. In the right panel of (B), for both species, points represent the average cumulative reproduction energy calculated over the last 4 years, and lines correspond to the minimum and maximum values. The biomass of the individual in the seasonal environment fluctuates according to the resource because the standard DEB model does not consider limits to individuals’ shrinking in size in periods of low food availability. The maturation dynamics (C) are not affected by the resource because the maturation energy threshold parameters are low, and individuals reach puberty shortly after birth. The assimilation flux differs between the individuals in a constant or fluctuating environment (D); the dotted line indicates the expected assimilation flux if only food were varying seasonally but the structural volume (and thus the assimilation surface) remained constant, equal to those of an individual living in a constant environment. For the simulations, we assume 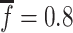 , and the parameter set of the species is:
, and the parameter set of the species is: 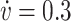 ,
, 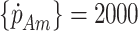 ,
, 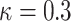 , and
, and  (see other parameter values in Table 2).
(see other parameter values in Table 2).
We further illustrate the model behaviour for the Fan-tailed Gerygone, Gerygone flavolateralis and the Grey Warbler, G. igata, two related species with parameter values close to each other (Figure 5, Supplementary Table 2). Members of the Gerygone genus (Passeriformes, Acanthizidae) are small insectivorous species distributed in the Australasian region (Keast and Recher, 1997), which do not migrate. The Grey Warbler inhabits temperate forests in the South Island of New Zealand, while the Fan-tailed Gerygone dwells in the tropical rainforests and savannahs of New Caledonia and Vanatu (Gill, 1982; Attisano et al., 2019). The species differ slightly in their biomass: 6.45 g for the Grey Warbler (Gill, 1982) and 6.1 g for the Fan-tailed Gerygone (Attisano et al., 2019). The differences in the reproductive output are more pronounced: the Grey Warbler has two broods per year with an average clutch size of four eggs (Gill, 1982); in contrast, the Fan-tailed Gerygone has only one brood per year with a mean of two eggs (Attisano et al., 2019).
As expected, our simulations show that, with similar average food density, the Grey Warbler can reach a similar size and have a greater reproductive output relative to the Fan-tailed Gerygone (Figure 9). These differences in reproduction are more pronounced than in the previous example (Figure 8) because the species differ in their parameter values, mainly on the energy allocated to soma and the cumulative energy at birth and puberty (Supplementary Table 2). Furthermore, the dynamics of the Grey Warbler show pronounced seasonal fluctuations because the standard DEB model does not consider limits to individuals' shrinking in size in periods of low food availability. Consequently, the biomass and reproductive energy of the Grey Warbler fluctuate according to resource availability. Our predictions underestimate both species' average biomass likely because we do not consider that the reproductive energy contributes to the overall weight of the individuals. This assumption allowed us to measure a continuous reproductive output across our simulations, but it may result in biomass underestimation in species that store reproductive energy between discrete reproductive seasons, such as the Grey Warbler or the Fan-tailed Gerygone. Moreover, we assume that the effect of temperature on metabolic rates is negligible. Despite these simplifying assumptions, our forecasts agree with the general pattern between the species and demonstrate the model's behaviour as well as the importance of the resource in contributing to the overall dynamics.
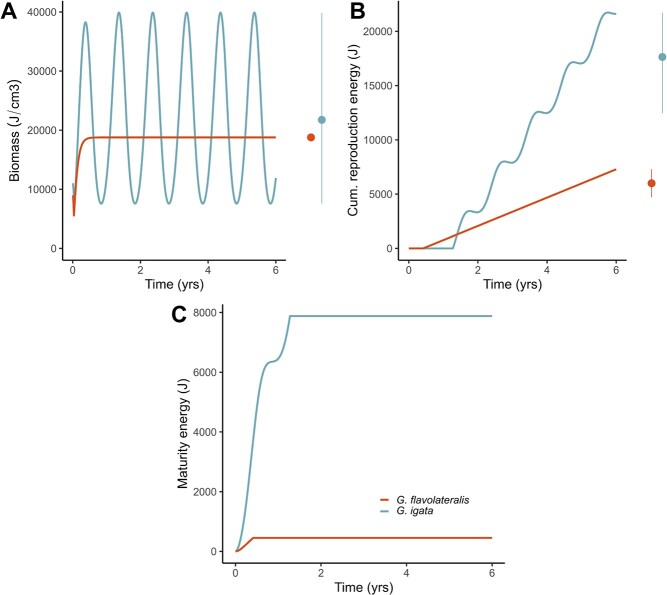
Figure 9.
Simulations for the Grey Warbler (G. igata) in a seasonal environment with an equal mean resource availability as the Fan-tailed Gerygone (G. flavolateralis) in a constant environment show that the Grey Warbler dynamics follow the resource oscillations and reach a greater average wet biomass (A) and reproductive output (B) compared to the Fan-tailed Gerygone. In the right panel of (A), for the species in the constant environment, points show the steady-state value of wet biomass reached at the end of the lifespan. In contrast, for the seasonal environment, points represent the average wet biomass calculated over the last 4 years (i.e. Years 2 to 6), and lines show the minimum and maximum values. In the right panel of (B), for both species, points represent the average cumulative reproduction energy calculated over the last 4 years, and lines correspond to the minimum and maximum values. The Grey Warbler biomass fluctuates according to the resource because the standard DEB model does not consider limits to individuals' shrinking in size in periods of low food availability. Differences in the maturation energy between the species (C) are due to different values of the puberty threshold (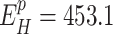 for the Fan-tailed Gerygone and 7880 for the Grey Warbler). The simulations assume
for the Fan-tailed Gerygone and 7880 for the Grey Warbler). The simulations assume 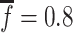 (see parameter values in Supplementary Table 2).
(see parameter values in Supplementary Table 2).
Discussion
We used the DEB model to describe the individual rates of energy acquisition and partitioning, which lead to different life-history traits. Our approach is novel because we used simulations of the DEB model to perform a comparative analysis of 48 different strategies of energy allocation (equivalent to different species) across different environments to evaluate their consequences in body mass and reproductive output. As expected, we found that absolute interspecific differences in biomass and reproduction are more evident when the resource is non-limiting (Figures 4 and 5). The same pattern holds in a seasonal resource environment; moreover, seasonality produces individuals with a greater average biomass and reproductive output relative to the constant environment (Figures 8 and 9). Our results provide a plausible mechanistic explanation to known interspecific patterns of body size and reproductive output variation, and reaffirm the importance of considering the effects of resource availability changes to predict broad biodiversity dynamics.
Non-limiting resource amplifies interspecific variability in biomass and reproduction
Food availability per individual has been proposed as the primary cause of differences in adult body size and biomass, both among and within species, because it underlines organisms' nutritional requirements, which ultimately drive ontogenetic growth (Huston and Wolverton, 2011). Here, we showed that, in general, an abundant resource increases absolute interspecific variability in biomass, regardless of the environment (Figure 4). However, species' relative differences in biomass are not exactly constant, especially at low values of energy mobilization (Figure 6). Although these differences are small, they indicate that the allometric relationships are not strictly proportional. Such disparities in biomass may suggest a larger sensitivity of the model output to parameter combinations with low mobilization and high assimilation at reduced food availability. Nevertheless, our simulations confirmed that, in the DEB model, the resource has partially a scaling effect on the individual's biomass because a larger food availability directly increases the feeding, assimilation, mobilization and somatic maintenance fluxes, which result in a greater growth rate.
Our findings are in line with the central role of food availability on biomass (reflected in Bergmann's rule, sizes in deserts, insular dwarfism, Dehnel's phenomenon and Cope's rule, or more generally the resource rule), by which species become larger or smaller according to the size, abundance and availability of food (McNab, 2010). Although we did not include the effect of environmental temperature, several case studies using the DEB model have previously revealed that food dynamics are more relevant than temperature in determining growth rate and maximum possible size across different taxa (e.g. Cardoso et al., 2006; Freitas et al., 2009; Marn et al., 2019). For example, in two parapatric and genetically distinct populations of loggerhead turtles, a reduction in ultimate size has been shown to be a consequence of constant low food availability (Marn et al., 2019). Thus, within the DEB theory, a reduction in adult size or biomass can be interpreted as a direct consequence of the change in resource and its effect on assimilation (Kearney, 2021), giving further theoretical support to the resource rule.
Animal body size is only one of the traits affected by resource availability; many other characteristics of individuals depend on the extent to which they can be afforded, including maintenance, reproductive output and activity level (McNab, 2010). Moreover, size alone can be considered a determinant of an organism's ecological and physiological properties, such as reproduction (Klingenberg and Spence, 1997; Litchman et al., 2013). Our results indicated that an abundant resource intensifies not only absolute interspecific variability in biomass in the DEB model, but also in reproductive output (Figure 5). Our simulations showed that the resource has a similar effect on the individual's reproductive output, where a greater food availability results in increased cumulative reproduction energy. The limiting effect of food quantity or quality in animal reproduction is well known in the literature (Kozlowski et al., 2020). Nevertheless, this conclusion is not always evident when making interspecific comparisons through the DEB model because of the covariation among parameters. For example, in the aforementioned loggerhead turtles, there is no significant disparity in the reproductive output of populations inhabiting areas with dissimilar food availabilities due to differences in the maturity maintenance and maturity energy thresholds parameters between individuals of the two populations (Marn et al., 2019). However, by fixing these parameters, we have isolated the scaling effect of resource availability on cumulative reproductive output.
In our formulation, we attribute interspecific variation to parameter values. Among the parameters that we evaluated, we found that both maximum assimilation (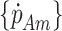 ) and allocation to soma (
) and allocation to soma (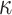 ) have the most important role in promoting interspecific differences in individuals' size and reproduction (Figures 4 and 5). Such effect has previously been recognized among several marine invertebrates and vertebrates, where species differ mostly in their assimilation rate and the energy partitioning to growth and reproduction (van der Veer et al., 2001; Freitas et al., 2010; Marn et al., 2019). Both are parameters that can be considered highly adaptative, and their values are expected to reflect the conditions of the environment where the species evolved (Muller and Nisbet, 2000).
) have the most important role in promoting interspecific differences in individuals' size and reproduction (Figures 4 and 5). Such effect has previously been recognized among several marine invertebrates and vertebrates, where species differ mostly in their assimilation rate and the energy partitioning to growth and reproduction (van der Veer et al., 2001; Freitas et al., 2010; Marn et al., 2019). Both are parameters that can be considered highly adaptative, and their values are expected to reflect the conditions of the environment where the species evolved (Muller and Nisbet, 2000).
Environmental variability increases average biomass and reproduction
Food availability is influenced by biotic and abiotic factors, which can in turn covary, causing resource oscillations in time and space that determine the geographical and temporal changes in body size (Yom-Tov and Geffen, 2006, 2011). By fixing the environmental temperature and evaluating different fluctuating resource scenarios, we showed that the surplus in food availability during one season of the year produces organisms that can reach a greater average biomass relative to the same individual in an environment with an equal average but constant resource (Figures 8A and 9A). Our results provide a mechanism behind the relationship stated in the eNPP rule, in which animals subjected to fluctuations in food availability can do more than compensate for the periods of food deficit during their lifespan: they have an increased assimilation flux during periods of food surplus compared to the assimilation loses during periods of food deficit (Figure 8D), allowing them to gain more body mass (Geist, 1987; Huston and Wolverton, 2011). In the DEB framework, numerical simulations using a DEB-based model have shown that the maximum size rises with the amplitude of the resource cycles rather than to the mean, although the exact mechanism was not specified (Muller and Nisbet, 2000). Thus, our findings are in line with the DEB literature and give further support that indicates that the DEB model offers a mechanistic explanation at the interspecific level for the eNPP rule.
As proposed in the eNPP rule, food fluctuations in quantity and quality have further implications in traits associated with evolutionary fitness, most notably reproduction (Huston and Wolverton, 2011). In our simulations in a seasonal environment, we found that the peaks in food availability combined with overcompensation in the assimilation flux also produce animals with a larger cumulative reproductive output compared to the same individual in an environment that has an equal average constant resource (Figures 8B and 9B). Our results are consistent with previous studies that mention an increase in brood size in birds with latitude (Lack's rule) or, more broadly, eNPP (Boyer et al., 2010). Nonetheless, because of the non-linearities that arise in the DEB model when there are food fluctuations, it has been shown that the total reproductive energy is highly dependent on the organism's energy partitioning as given by the parameter values (Muller and Nisbet, 2000). For example, a decrease in the value of 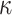 (the energy allocation to soma) implies an increased commitment of energy to reproduction and would be expected to simultaneously decrease size, shorten development and maturation times and increase reproduction allocation (Kearney, 2021). However, as our results illustrate, individuals can also have a higher reproductive output when they allocate a greater fraction of energy to soma. Such behaviour occurs because maximum reproductive output as a function of
(the energy allocation to soma) implies an increased commitment of energy to reproduction and would be expected to simultaneously decrease size, shorten development and maturation times and increase reproduction allocation (Kearney, 2021). However, as our results illustrate, individuals can also have a higher reproductive output when they allocate a greater fraction of energy to soma. Such behaviour occurs because maximum reproductive output as a function of 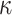 has two optimum values: at intermediate (
has two optimum values: at intermediate (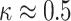 ) and also at high values (
) and also at high values (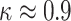 ) (Lika et al., 2011b). Hence, for the parameter combinations that we assessed,
) (Lika et al., 2011b). Hence, for the parameter combinations that we assessed, 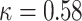 is likely to correspond to that intermediate optimum value that yields a greater reproductive output. Given that we simulated a restricted range of
is likely to correspond to that intermediate optimum value that yields a greater reproductive output. Given that we simulated a restricted range of 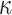 , we would expect that higher values (
, we would expect that higher values (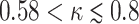 ) produce individuals for which reproduction is reduced because an increase in the energy allocation to soma will also increase the maintenance requirements (Muller and Nisbet, 2000). The pattern of greater reproductive output for increasing values of
) produce individuals for which reproduction is reduced because an increase in the energy allocation to soma will also increase the maintenance requirements (Muller and Nisbet, 2000). The pattern of greater reproductive output for increasing values of 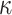 also occurs in the seasonal environments that we evaluated. It has been shown that organisms with low to intermediate values of
also occurs in the seasonal environments that we evaluated. It has been shown that organisms with low to intermediate values of 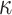 , such as the ones we simulated, reproduce more as the amplitude in food fluctuations increase (Muller and Nisbet, 2000). Thus, depending on the underlying life history of the individual, resource seasonality in the DEB model may increase the reproductive output.
, such as the ones we simulated, reproduce more as the amplitude in food fluctuations increase (Muller and Nisbet, 2000). Thus, depending on the underlying life history of the individual, resource seasonality in the DEB model may increase the reproductive output.
Resource availability may influence development rates
According to the eNPP rule, the highest ontogenetic growth rates will occur where food availability is highest (Huston and Wolverton, 2011). Consequently, when the resource is limiting, a delay in the maturation and reproduction rates is expected. For example, in two sympatric and sibling insectivorous bat species (genus Myotis) with marked differences in resource supply, there is a delay in the reproduction onset for the species with a lower food availability; however, this difference disappears in years where there is a pulsed input of a secondary resource (Arlettaz et al., 2017). In general, our results suggest that there is a delay in maturation time at lower, constant resource levels (Supplementary Figure 4). In a seasonal environment, our findings indicate that individuals that start their life at different points of the seasonal cycle do not have the same maturation times (Supplementary Figures 25 to 27). More specifically, individuals born during the low food season show a slower development and more variable times to reach puberty, compared to the same organisms born during the high food season (Supplementary Figure 12). Similar results have been described for mussels parameterized with the DEB model (Muller and Nisbet, 2000), highlighting the relevance of birth timing in developmental times when resource oscillates. Nevertheless, in both environmental scenarios, our results may not represent a significant difference as the range of the developmental time variation is small because the parameters that define the maturation thresholds are close to each other and remain constant. Thus, even though we find indications of a possible effect of food level on maturation time, further analyses are needed to reveal the potential variation on development according to the resource in the DEB framework.
Life-history traits and parameter space
By matching our parameter subset to real species, we can explain various patterns within the parameter space. We found that the species in our parameter subset combine an intermediate assimilation rate (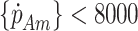 ) with energy conductance ranging from intermediate to high values (
) with energy conductance ranging from intermediate to high values (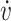 in the interval [0.15, 0.55]), which results in small body weight. In birds, such combination of values for energy conductance and assimilation have been associated with flying adaptations since it usually implies a low reserve density (i.e. a smaller contribution of the energy reserve to biomass; Lika et al., 2014, 2019; Teixeira, 2016; Augustine et al., 2019). Similarly, the energy allocated to soma has a very high average fraction (with a mean of 0.988) and does not exhibit significant variation among species (cv = 0.03 Teixeira, 2016). This combination of parameter values has been suggested to be phylogenetically conserved as selective pressures have mostly driven birds towards larger investments in growth and maintenance, as well as delayed maturation and relatively low production efficiency (Teixeira, 2016; van der Meer et al., 2020). Additionally, most of the species within our parameter space have a carnivore diet, which has been linked to larger assimilation and somatic maintenance rates given that it requires greater enzymatic attack and thick stomach muscles (Ricklefs, 1996; Battley and Piersma, 2005; Teixeira, 2016). Only one species, the New Zealand pigeon, constitutes an exception to the previous pattern, as it is larger and primarily frugivorous. This species has an intermediate assimilation rate combined with a lower mobilization rate, which results in a greater biomass due to a larger energy reserve. In general, our subset of species reflects previous findings that indicate that the mobilization rate is one of the most variable DEB parameters for birds (Teixeira, 2016), possibly suggesting a broad link between assimilation rate and diet.
in the interval [0.15, 0.55]), which results in small body weight. In birds, such combination of values for energy conductance and assimilation have been associated with flying adaptations since it usually implies a low reserve density (i.e. a smaller contribution of the energy reserve to biomass; Lika et al., 2014, 2019; Teixeira, 2016; Augustine et al., 2019). Similarly, the energy allocated to soma has a very high average fraction (with a mean of 0.988) and does not exhibit significant variation among species (cv = 0.03 Teixeira, 2016). This combination of parameter values has been suggested to be phylogenetically conserved as selective pressures have mostly driven birds towards larger investments in growth and maintenance, as well as delayed maturation and relatively low production efficiency (Teixeira, 2016; van der Meer et al., 2020). Additionally, most of the species within our parameter space have a carnivore diet, which has been linked to larger assimilation and somatic maintenance rates given that it requires greater enzymatic attack and thick stomach muscles (Ricklefs, 1996; Battley and Piersma, 2005; Teixeira, 2016). Only one species, the New Zealand pigeon, constitutes an exception to the previous pattern, as it is larger and primarily frugivorous. This species has an intermediate assimilation rate combined with a lower mobilization rate, which results in a greater biomass due to a larger energy reserve. In general, our subset of species reflects previous findings that indicate that the mobilization rate is one of the most variable DEB parameters for birds (Teixeira, 2016), possibly suggesting a broad link between assimilation rate and diet.
Traits associated with differing life histories are usually classified along a ‘fast to slow’ or ‘pace-of-life’ continuum. For example, compared to temperate birds, tropical birds are typically considered as having a ‘slow’ life history, involving small clutch sizes or low annual reproductive output (Moreau, 1944; Kulesza, 1990), as well as slow growth and maturation of nestlings (Ricklefs, 1968, 1976, 2000; Cox and Martin, 2009; Martin et al., 2011; Jimenez et al., 2014). The results of our simulations comparing species in two resource environments agree with the literature, with the species in the seasonal habitat showing a faster growth to a larger body mass and greater reproductive output than the species in the constant habitat (Figures 8 and 9). These differences between tropical and temperate birds have been attributed to trade-offs between investment in either reproduction or maintenance, as mediated by the biotic and abiotic environment (Roff, 1992), which correspond to the species' different parameter values. Nevertheless, our simulations also highlight the effect of the resource in modulating the species traits, which, together with predation risk, has been reported as the main environmental factor affecting growth rate and body size in birds (Bryant and Hails, 1983; Pacheco et al., 2010; Martin et al., 2011; Jimenez et al., 2014).
Model limitations and future work
The DEB theory supposes that the parameters do not change over the individuals' lifespan. Consequently, organisms show a passive phenotypic flexibility in response to resource availability, but there is no plasticity in the physiological mechanisms responsible for development and growth as a response to environmental cues (i.e. active plasticity). To modify this assumption would require discerning all possible changes in the conditions experienced by an organism during ontogeny (Pecquerie and Lika, 2017). However, the current lack of understanding of how parameters may vary during an organism's lifespan limits incorporating such responses into the DEB model (Freitas et al., 2009). Thus, to include active plasticity in the individual energy allocation would require detailed knowledge of the species' long-term seasonal patterns of growth and reproduction.
Quantitative and qualitative changes in food conditions would also be expected to affect individual survivorship and offspring production rate in different ways (Huston and Wolverton, 2011). For example, survival in mice has been shown to decrease in higher but constant feeding levels (Weindruch et al. 1986). In contrast, simulations of the DEB model in a constant environment have suggested an increased lifespan as the resource becomes more abundant (Lika and Kooijman, 2003). When food fluctuates, simulations have shown that individual lifespan is reduced as the oscillations amplitude increase (Muller and Nisbet, 2000). However, we did not consider how resource availability affects individual lifespan or aging because such variations in lifespan are thought to be most significant for large values of 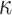 (Muller and Nisbet, 2000; Lika and Kooijman, 2003), which we did not include in our simulations. Nevertheless, it is possible that including survival could impact the resulting interspecific differences. Regarding the reproduction rate, we have not specified how reproductive energy is transformed into quantity and quality of offspring, given that the optimal strategy will depend on the particular environment experienced by each individual and its effect on growth, survival and reproduction (Brown et al., 1993). Further elaborations of our study could incorporate such details, specifying how energy is allocated to survival and production of offspring over the individual's lifetime.
(Muller and Nisbet, 2000; Lika and Kooijman, 2003), which we did not include in our simulations. Nevertheless, it is possible that including survival could impact the resulting interspecific differences. Regarding the reproduction rate, we have not specified how reproductive energy is transformed into quantity and quality of offspring, given that the optimal strategy will depend on the particular environment experienced by each individual and its effect on growth, survival and reproduction (Brown et al., 1993). Further elaborations of our study could incorporate such details, specifying how energy is allocated to survival and production of offspring over the individual's lifetime.
The standard DEB model does not make any assumptions regarding starvation (Kooijman, 2010). For this reason, the individuals in our model can decrease in size to cover maintenance costs when the resource is limiting, and energy reserves are exhausted. This assumption holds within certain limits for many species, including platyhelminths, molluscs, echinoderms and mammals (Genoud, 1988; Downing and Downing, 1993; Wikelski and Thom, 2000; Saló, 2006, Monaco et al., 2014). Yet, other species may respond to food limitation in different ways. Nevertheless, we did not simulate periods of prolonged resource depletion or scarcity that could lead to starvation. Hence, the results of our simulation are still applicable to broad scenarios of seasonal food variation. Understanding other organisms' strategies to survive seasonality without a reduction in structural mass remains an important issue for future research.
We illustrated the consequences of a constant and a seasonal resource by simulating the dynamics of two bird species parameterized for the DEB model. The fluctuations in biomass and reproductive output observed in the simulations with seasonality result from the lack of assumptions regarding starvation. Given that such a decrease in biomass is likely larger than the shrinking birds are actually able to tolerate without dying, our simulations for the Grey Warbler serve mainly to exemplify a possible consequence of a seasonal environment in an organism's physiology. Our results broadly agreed with the general pattern: temperate species can reach a larger reproductive output and slightly greater biomass than the tropical species. However, our inference is hindered because we did not use real estimates of food availability from the species' habitats, and we simplified the abiotic environment only to resource availability, not considering temperature or biotic interactions, for example. Nevertheless, our purpose was to broadly show the different trends between a constant and a seasonal environment in closely related species.
We have shown how a mechanistic approach based on individual energetics can support empirical evidence on cross-specific trait variation. However, the geographical context of such ecological and evolutionary processes is an important component needed to gain a complete understanding of species' trait gradients (Blackburn and Hawkins, 2004; Olalla-Tárraga et al., 2010). Furthermore, any interspecific analysis must consider the phylogenetic non-independence of the data, i.e. closely related species are more similar than distant species (Olalla-Tárraga et al., 2010). Moreover, climate change is predicted to alter the seasonality and periodicity of climatic events (IPCC, 2021) and could thus create a mismatch between physiological adaptations to seasonality and environmental conditions (Durant et al., 2007; Revilla et al., 2014). For these reasons, more complex models that integrate a more accurate multidimensional environment with comparative phylogenetic methods are necessary to make stronger inferences of these eco-evolutionary patterns and processes.
Conclusions
Using the DEB model, we separated the effects of genetically determined physiological traits (represented by DEB model parameters) and resource availability. By fixing the resource, we showed that relative trait differences between species in biomass and reproduction are greater when food is non-limiting. However, the differences regarding biomass are not constant, as at higher levels of energy conductance the pattern is reversed. We found similar results for simulations in a seasonal environment; moreover, resource fluctuations increase the individuals' average biomass and reproductive output due to a greater assimilation flux during the seasons with high resource availability. Our results have potential implications for species of economic interest in which there is a desire to increase the yield in either biomass or reproductive output relative to the food consumption. Furthermore, our findings are a relevant step in forecasting organisms' responses to environmental change. For example, global climate change has been linked to differences in the timing of resource availability and the arrival of migratory species to their feeding grounds. Finally, our simulations offer mechanistic support for patterns of body-size variation between related species (i.e. Bergmann's and Lack's rules, or more generally, the resource and eNPP rules).
Funding
This work was supported by the School of Graduate Studies from Memorial University [baseline fellowship to J.M.], Mitacs [Globalink Research Award to J.M.], and Natural Science and Engineering Research Council of Canada [NSERC Discovery grants 2015-06548 and 2014-05413 to S.C.D. and A.H., respectively].
Conflicts of Interest
The authors have no conflicts to declare.
Data Availability
The data used in this article are available on the AmP repository, at https://www.bio.vu.nl/thb/deb/deblab/add_my_pet/entries_web/Gerygone_igata/Gerygone_igata_res.html and https://www.bio.vu.nl/thb/deb/deblab/add_my_pet/entries_web/Gerygone_flavolateralis/Gerygone_flavolateralis_res.html. The code is available at https://gitlab.com/JoanyMarino/deb-resource-and-seasonality.
Author Contributions
J.M. and C.R. designed the study. J.M. performed the numerical simulations, analysed the data, wrote the manuscript and designed the figures with input from all authors. C.R., S.D. and A.H. supervised the project. All authors discussed the results and commented on the manuscript.
Supplementary Material
Acknowledgments
We thank Starrlight Augustine and Bas Kooijman for their help during the model parameterization for the Gerygone case study. We also acknowledge the members of the Theoretical Biology Laboratory at MUN for their constructive comments on the manuscript. We thank the reviewer for the insightful comments that helped improve the manuscript.
Contributor Information
Joany Mariño, Department of Biology, Memorial University of Newfoundland 45 Arctic Ave., St John’s, Newfoundland, Canada A1C 5S7.
Suzanne C Dufour, Department of Biology, Memorial University of Newfoundland 45 Arctic Ave., St John’s, Newfoundland, Canada A1C 5S7.
Amy Hurford, Department of Biology, Memorial University of Newfoundland 45 Arctic Ave., St John’s, Newfoundland, Canada A1C 5S7; Department of Mathematics and Statistics, Memorial University of Newfoundland. Elizabeth Avenue, St John’s, Newfoundland, Canada A1C 5S7.
Charlotte Récapet, Université de Pau et des Pays de l’Adour, E2S UPPA, INRAE, ECOBIOP. Quartier Ibarron 64210, Saint-Pée-sur-Nivelle, France.
References
- AmP (2021) Online database of DEB parameters, implied properties and referenced underlying data. bio.vu.nl/thb/deb/deblab/add_my_pet/ (last accessed 30 March 2021).
- AmPtool (2020) AmPtool: functions for the visualisation and analysis of patterns in (functions of) parameter values of the add-my-pet (AmP) collection.
- Arlettaz R, Christe P, Schaub M (2017) Food availability as a major driver in the evolution of life-history strategies of sibling species. Ecol Evol 7: 4163–4172. 10.1002/ece3.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmole NP (1963) The regulation of numbers of tropical oceanic birds. Ibis 103b: 458–473. 10.1111/j.1474-919X.1963.tb06766.x. [DOI] [Google Scholar]
- Ashton KG (2002) Patterns of within-species body size variation of birds: strong evidence for Bergmann’s rule: Bergmann’s rule in birds. Glob Ecol Biogeogr 11: 505–523. 10.1046/j.1466-822X.2002.00313.x. [DOI] [Google Scholar]
- Ashton KG, Tracy MC, Queiroz A (2000) Is Bergmann’s rule valid for mammals? Am Nat 156: 390–415. 10.1086/303400. [DOI] [PubMed] [Google Scholar]
- Attisano A, Thiel F, Sato N, Okahisa Y, Bolopo D, Tanaka KD, Kuehn R, Gula R, Ueda K, Theuerkauf J (2019) Breeding biology of the fan-tailed Gerygone Gerygone flavolateralis in relation to parasitism by the shining bronze-cuckoo Chalcites lucidus. J Ornithol 160: 91–103. 10.1007/s10336-018-1592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine S, Lika K, Kooijman SALM (2019) Why big-bodied animal species cannot evolve a waste-to-hurry strategy. J Sea Res 143: 18–26. 10.1016/j.seares.2018.06.002. [DOI] [Google Scholar]
- Battley PF, Piersma T (2005) Adaptive interplay between feeding ecology and features of the digestive tract in birds. In Starck JM, Wang T, eds, Physiological and Ecological Adaptations to Feeding in Vertebrates. Science Publishers, Enfield, New Hampshire, pp. 202–228 [Google Scholar]
- Blackburn TM, Gaston KJ, Loder N (1999) Geographic gradients in body size: a clarification of Bergmann’s rule. Divers Distrib 5: 165–174. 10.1046/j.1472-4642.1999.00046.x. [DOI] [Google Scholar]
- Blackburn TM, Hawkins BA (2004) Bergmann’s rule and the mammal fauna of northern North America. Ecography 27: 715–724. 10.1111/j.0906-7590.2004.03999.x. [DOI] [Google Scholar]
- Boyer AG, Cartron J-LE, Brown JH (2010) Interspecific pairwise relationships among body size, clutch size and latitude: deconstructing a macroecological triangle in birds. J Biogeogr 37: 47–56. 10.1111/j.1365-2699.2009.02175.x. [DOI] [Google Scholar]
- Boyer AG, Jetz W (2010) Biogeography of body size in Pacific island birds. Ecography 33: 369–379. [Google Scholar]
- Brown JH, Marquet PA, Taper ML (1993) Evolution of body size: consequences of an energetic definition of fitness. Am Nat 142: 573–584. 10.1086/285558. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Hails CJ (1983) Energetics and growth patterns of three tropical bird species. The Auk 100: 425–439. 10.1093/auk/100.2.425. [DOI] [Google Scholar]
- Cabral JS, Valente L, Hartig F (2017) Mechanistic simulation models in macroecology and biogeography: state-of-art and prospects. Ecography 40: 267–280. 10.1111/ecog.02480. [DOI] [Google Scholar]
- Capellini I, Baker J, Allen WL, Street SE, Venditti C (2015) The role of life history traits in mammalian invasion success. Ecol Lett 18: 1099–1107. 10.1111/ele.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardilini APA, Buchanan KL, Sherman CDH, Cassey P, Symonds MRE (2016) Tests of ecogeographical relationships in a non-native species: what rules avian morphology? Oecologia 181: 783–793. 10.1007/s00442-016-3590-9. [DOI] [PubMed] [Google Scholar]
- Cardillo M (2002) The life-history basis of latitudinal diversity gradients: how do species traits vary from the poles to the equator? J Anim Ecol 71: 79–87. 10.1046/j.0021-8790.2001.00577.x. [DOI] [Google Scholar]
- Cardoso JFMF, Witte JI, Veer HW (2006) Intra- and interspecies comparison of energy flow in bivalve species in Dutch coastal waters by means of the dynamic energy budget (DEB) theory. J Sea Res 56: 182–197. 10.1016/j.seares.2006.03.011. [DOI] [Google Scholar]
- Clauss M, Dittmann MT, Müller DWH, Meloro C, Codron D (2013) Bergmann′s rule in mammals: a cross-species interspecific pattern. Oikos 122: 1465–1472. [Google Scholar]
- Cody ML (1966) A general theory of clutch size. Evolution 20: 174–184. 10.2307/2406571. [DOI] [PubMed] [Google Scholar]
- Cox WA, Martin TE (2009) Breeding biology of the three-striped warbler in Venezuela: a contrast between tropical and temperate Parulids. Wilson J Ornithol 121: 667–678. 10.1676/08-133.1. [DOI] [Google Scholar]
- Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM, Bellwood D (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15: 1378–1386. 10.1111/j.1461-0248.2012.01861.x. [DOI] [PubMed] [Google Scholar]
- Downing WL, Downing JA (1993) Molluscan shell growth and loss. Nature 362: 506–506. 10.1038/362506a0. [DOI] [Google Scholar]
- Durant J, Hjermann D, Ottersen G, Stenseth N (2007) Climate and the match or mismatch between predator requirements and resource availability. Climate Res 33: 271–283. 10.3354/cr033271. [DOI] [Google Scholar]
- Evans KL, Duncan RP, Blackburn TM, Crick HQP (2005) Investigating geographic variation in clutch size using a natural experiment. Funct Ecol 19: 616–624. 10.1111/j.1365-2435.2005.01016.x. [DOI] [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M (2003) Bergmann’s rule and body size in mammals. Am Nat 161: 821–825. 10.1086/374346. [DOI] [PubMed] [Google Scholar]
- Freitas V, Cardoso JFMF, Lika K, Peck MA, Campos J, Kooijman SALM, Veer HW (2010) Temperature tolerance and energetics: a dynamic energy budget-based comparison of North Atlantic marine species. Phil Trans R Soc B 365: 3553–3565. 10.1098/rstb.2010.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas V, Cardoso JFMF, Santos S, Campos J, Drent J, Saraiva S, Witte JI, Kooijman SALM, Van der Veer HW (2009) Reconstruction of food conditions for Northeast Atlantic bivalve species based on dynamic energy budgets. J Sea Res 62: 75–82. 10.1016/j.seares.2009.07.004. [DOI] [Google Scholar]
- Gaston KJ, Chown SL, Evans KL (2008) Ecogeographical rules: elements of a synthesis. J Biogeogr 35: 483–500. 10.1111/j.1365-2699.2007.01772.x. [DOI] [Google Scholar]
- Geist V (1987) Bergmann’s rule is invalid. Can J Zool 65: 1035–1038. 10.1139/z87-164. [DOI] [Google Scholar]
- Genoud M (1988) Energetic strategies of shrews: ecological constraints and evolutionary implications. Mammal Rev 18: 173–193. 10.1111/j.1365-2907.1988.tb00083.x. [DOI] [Google Scholar]
- Gill BJ (1982) Breeding of the grey warbler Gerygone igata at Kaikoura, New Zealand. Ibis 124: 123–147. 10.1111/j.1474-919X.1982.tb03752.x. [DOI] [Google Scholar]
- Gohli J, Voje KL (2016) An interspecific assessment of Bergmann’s rule in 22 mammalian families. BMC Evol Biol 16: 222. 10.1186/s12862-016-0778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebeler EM, Böhning-Gaese K (2004) Evolution of clutch size along latitudinal gradients: revisiting Ashmole’s hypothesis. Evol Ecol Res 6: 679–694. [Google Scholar]
- Healy K, Ezard THG, Jones OR, Salguero-Gómez R, Buckley YM (2019) Animal life history is shaped by the pace of life and the distribution of age-specific mortality and reproduction. Nat Ecol Evol 3: 1217–1224. 10.1038/s41559-019-0938-7. [DOI] [PubMed] [Google Scholar]
- Hersteinsson P, Yom-Tov Y, Geffen E (2009) Effect of sub-polar gyre, North Atlantic oscillation and ambient temperature on size and abundance in the Icelandic Arctic fox. Glob Change Biol 15: 1423–1433. 10.1111/j.1365-2486.2008.01765.x. [DOI] [Google Scholar]
- Huston MA, Wolverton S (2011) Regulation of animal size by eNPP, Bergmann’s rule, and related phenomena. Ecol Monogr 81: 349–405. 10.1890/10-1523.1. [DOI] [Google Scholar]
- IPCC (2021) Climate Change 2021: The Physical Science Basis, Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
- Iverson JB, Balgooyen CP, Byrd KK, Lyddan KK (1993) Latitudinal variation in egg and clutch size in turtles. Can J Zool 71: 2448–2461. 10.1139/z93-341. [DOI] [Google Scholar]
- Jetz W, Sekercioglu CH, Böhning-Gaese K (2008) The worldwide variation in avian clutch size across species and space. PLoS Biol 6: e303, 2650, 2657, 10.1371/journal.pbio.0060303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez AG, Cooper-Mullin C, Calhoon EA, Williams JB (2014) Physiological underpinnings associated with differences in pace of life and metabolic rate in north temperate and neotropical birds. J Comp Physiol B 184: 545–561. 10.1007/s00360-014-0825-0. [DOI] [PubMed] [Google Scholar]
- Jones OR, Scheuerlein A, Salguero-Gómez R, Camarda CG, Schaible R, Casper BB, Dahlgren JP, Ehrlén J, García MB, Menges ESet al. (2014) Diversity of ageing across the tree of life. Nature 505: 169–173. 10.1038/nature12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney MR (2021) What is the status of metabolic theory one century after Pütter invented the von Bertalanffy growth curve? Biol Rev 96: 557–575. 10.1111/brv.12668. [DOI] [PubMed] [Google Scholar]
- Keast A, Recher HF (1997) The Adaptive Zone of the Genus Gerygone (Acanthizidae) as Shown by Morphology and Feeding Habits. Emu - Austral Ornithology 97: 1–17. 10.1071/mu97001. [DOI] [Google Scholar]
- Kissling WD, Walls R, Bowser A, Jones MO, Kattge J, Agosti D, Amengual J, Basset A, Bodegom PM, Cornelissen JHCet al. (2018) Towards global data products of essential biodiversity variables on species traits. Nat Ecol Evol 2: 1531–1540. 10.1071/mu97001. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Spence J (1997) On the role of body size for life-history evolution. Ecol Entomol 22: 55–68. 10.1046/j.1365-2311.1997.00031.x. [DOI] [Google Scholar]
- Kooijman SALM (2012) Dynamic Energy Budget Theory for Metabolic Organisation, 3rd Edition. Cambridge university press, 10.1017/CBO9780511805400. [DOI] [Google Scholar]
- Kooijman SALM (2013) Waste to hurry: dynamic energy budgets explain the need of wasting to fully exploit blooming resources. Oikos 122: 348–357. 10.1111/j.1600-0706.2012.00098.x. [DOI] [Google Scholar]
- Kooijman SALM, Lika K (2014) Resource allocation to reproduction in animals. Biol Rev 89: 849–859. 10.1111/brv.12082. [DOI] [PubMed] [Google Scholar]
- Kooijman SALM, Sousa T, Pecquerie L, Meer J, Jager T (2008) From food-dependent statistics to metabolic parameters, a practical guide to the use of dynamic energy budget theory. Biol Rev 83: 533–552. 10.1111/j.1469-185X.2008.00053.x. [DOI] [PubMed] [Google Scholar]
- Kozlowski J, Konarzewsk M, Czarnoleski M (2020) Coevolution of body size and metabolic rate in vertebrates: a life-history perspective. Biol Rev 95: 1393–1417. 10.1111/brv.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza G (1990) An analysis of clutch-size in New World passerine birds. Ibis 132: 407–422. 10.1111/j.1474-919X.1990.tb01059.x. [DOI] [Google Scholar]
- Lack D (1947) The significance of clutch-size. Ibis 89: 302–352. 10.1111/j.1474-919X.1947.tb04155.x. [DOI] [Google Scholar]
- Lika K, Augustine S, Kooijman SALM (2019) Body size as emergent property of metabolism. J Sea Res 143: 8–17. 10.1016/j.seares.2018.04.005. [DOI] [Google Scholar]
- Lika K, Augustine S, Pecquerie L, Kooijman SALM (2014) The bijection from data to parameter space with the standard DEB model quantifies the supply-demand spectrum. J Theor Biol 354: 35–47. 10.1016/j.jtbi.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Lika K, Kearney MR, Freitas V, Veer HW, Meer J, Wijsman JWM, Pecquerie L, Kooijman SALM (2011a) The “covariation method” for estimating the parameters of the standard dynamic energy budget model I: philosophy and approach. J Sea Res 66: 270–277. 10.1016/j.seares.2011.07.010. [DOI] [Google Scholar]
- Lika K, Kearney MR, Kooijman SALM (2011b) The “covariation method” for estimating the parameters of the standard dynamic energy budget model II: properties and preliminary patterns. J Sea Res 66: 278–288. 10.1016/j.seares.2011.09.004. [DOI] [Google Scholar]
- Lika K, Kooijman SALM (2003) Life history implications of allocation to growth versus reproduction in dynamic energy budgets. Bull Math Biol 65: 809–834. 10.1016/S0092-8240(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Litchman E, Ohman MD, Kiørboe T (2013) Trait-based approaches to zooplankton communities. J Plankton Res 35: 473–484. 10.1093/plankt/fbt019. [DOI] [Google Scholar]
- Magioli M, Moreira MZ, Fonseca RCB, Ribeiro MC, Rodrigues MG, Ferraz KMPMB (2019) Human-modified landscapes alter mammal resource and habitat use and trophic structure. Proc Natl Acad Sci 116: 18466–18472. 10.1073/pnas.1904384116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marn N, Jusup M, Catteau S, Kooijman SALM, Klanjšček T (2019) Comparative physiological energetics of Mediterranean and North Atlantic loggerhead turtles. J Sea Res 143: 100–118. 10.1016/j.seares.2018.06.010. [DOI] [Google Scholar]
- Martin TE, Lloyd P, Bosque C, Barton DC, Biancucci AL, Cheng Y-R, Ton R (2011) Growth rate variation among passerine species in tropical and temperate sites: an antagonistic interaction between parental food provisioning and nest predation risk. Evolution 65: 1607–1622. 10.1111/j.1558-5646.2011.01227.x. [DOI] [PubMed] [Google Scholar]
- Mayr E (1956) Geographical character gradients and climatic adaptation. Evolution 10: 105–108. 10.2307/2406103. [DOI] [Google Scholar]
- McNab BK (2010) Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia 164: 13–23. 10.1007/s00442-010-1621-5. [DOI] [PubMed] [Google Scholar]
- Medina AI, Martí DA, Bidau CJ (2007) Subterranean rodents of the genus Ctenomys (Caviomorpha, Ctenomyidae) follow the converse to Bergmann’s rule: Converse Bergmann’s rule in Ctenomys. J Biogeogr 34: 1439–1454. 10.1111/j.1365-2699.2007.01708.x. [DOI] [Google Scholar]
- Meiri S (2011) Bergmann’s rule – what’s in a name? Glob Ecol Biogeogr 20: 203–207. 10.1111/j.1466-8238.2010.00577.x. [DOI] [Google Scholar]
- Meiri S, Avila L, Bauer AM, Chapple DG, Das I, Doan TM, Doughty P, Ellis R, Grismer L, Kraus Fet al. (2020) The global diversity and distribution of lizard clutch sizes. Glob Ecol Biogeogr 29: 1515–1530. 10.1111/geb.13124. [DOI] [Google Scholar]
- Meiri S, Dayan T (2003) On the validity of Bergmann’s rule. J Biogeogr 30: 331–351. 10.1046/j.1365-2699.2003.00837.x. [DOI] [Google Scholar]
- Meiri S, Dayan T, Simberloff D (2004) Carnivores, biases and Bergmann’s rule. Biol J Linn Soc 81: 579–588. 10.1111/j.1095-8312.2004.00310.x. [DOI] [Google Scholar]
- Mesquita DO, Costa GC, Colli GR, Costa TB, Shepard DB, Vitt LJ, Pianka ER (2016) Life-history patterns of lizards of the world. Am Nat 187: 689–705. 10.1086/686055. [DOI] [PubMed] [Google Scholar]
- Millien V, Lyons SK, Olson L, Smith FA, Wilson AB, Yom-Tov Y (2006) Ecotypic variation in the context of global climate change: revisiting the rules. Ecol Lett 9: 853–869. 10.1111/j.1461-0248.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- Monaco CJ, Wethey DS, Helmuth B (2014) A dynamic energy budget (DEB) model for the keystone predator Pisaster ochraceus. PloS One 9: e104658. 10.1371/journal.pone.0104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Castilla I, Olalla-Tárraga MÁ, Purvis A, Hawkins BA, Rodríguez MÁ (2012a) The imprint of Cenozoic migrations and evolutionary history on the biogeographic gradient of body size in New World mammals. Am Nat 180: 246–256. 10.1086/666608. [DOI] [PubMed] [Google Scholar]
- Morales-Castilla I, Rodríguez MÁ, Hawkins BA (2012b) Deep phylogeny, net primary productivity, and global body size gradient in birds. Biol J Linn Soc 106: 880–892. 10.1111/j.1095-8312.2012.01917.x. [DOI] [Google Scholar]
- Moreau RE (1944) Clutch-size: a comparative study, with special reference to african birds. Ibis 86: 286–347. 10.1111/j.1474-919X.1944.tb04093.x. [DOI] [Google Scholar]
- Muller EB, Nisbet RM (2000) Survival and production in variable resource environments. Bull Math Biol 62: 1163–1189. 10.1006/bulm.2000.0203. [DOI] [PubMed] [Google Scholar]
- Olalla-Tárraga MÁ (2011) “Nullius in Bergmann” or the pluralistic approach to ecogeographical rules: a reply to Watt et al. (2010). Oikos 120: 1441–1444. 10.1111/j.1600-0706.2011.19319.x. [DOI] [Google Scholar]
- Olalla-Tárraga MÁ, Amado TF, Bini LM, Martínez PA, Morales-Castilla I, Torres-Romero EJ, Villalobos F (2019) Biological traits, phylogeny and human footprint signatures on the geographical range size of passerines (order Passeriformes) worldwide. Glob Ecol Biogeogr geb.12924. 10.1111/geb.12924. [DOI] [Google Scholar]
- Olalla-Tárraga MÁ, Bini LM, Diniz-Filho JAF, Rodríguez MÁ (2010) Cross-species and assemblage-based approaches to Bergmann’s rule and the biogeography of body size in Plethodon salamanders of eastern North America. Ecography. 10.1111/j.1600-0587.2010.06244.x. [DOI] [Google Scholar]
- Olalla-Tárraga MÁ, Rodríguez MÁ (2007) Energy and interspecific body size patterns of amphibian faunas in Europe and North America: anurans follow Bergmann’s rule, urodeles its converse. Glob Ecol Biogeogr 16: 606–617. 10.1111/j.1466-8238.2007.00309.x. [DOI] [Google Scholar]
- Olson VA, Davies RG, Orme CDL, Thomas GH, Meiri S, Blackburn TM, Gaston KJ, Owens IPF, Bennett PM (2009) Global biogeography and ecology of body size in birds. Ecol Lett 12: 249–259. 10.1111/j.1461-0248.2009.01281.x. [DOI] [PubMed] [Google Scholar]
- Pacheco MA, Beissinger SR, Bosque C (2010) Why grow slowly in a dangerous place? Postnatal growth, thermoregulation, and energetics of nestling green-Rumped Parrotlets (Forpus passerinus). The Auk 127: 558–570. 10.1525/auk.2009.09190. [DOI] [Google Scholar]
- Pecquerie L, Lika K (2017) Is reproduction limiting growth? Phys Life Rev 20: 75–77. 10.1016/j.plrev.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Pincheira-Donoso D (2010) The balance between predictions and evidence and the search for universal macroecological patterns: taking Bergmann’s rule back to its endothermic origin. Theory Biosci 129: 247–253. 10.1007/s12064-010-0101-0. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- Ramirez L, Diniz-Filho JAF, Hawkins BA (2008) Partitioning phylogenetic and adaptive components of the geographical body-size pattern of New World birds. Glob Ecol Biogeogr 17: 100–110. [Google Scholar]
- Revilla TA, Encinas-Viso F, Loreau M (2014) (a bit) earlier or later is always better: Phenological shifts in consumer–resource interactions. Theor Ecol 7: 149–162. 10.1007/s12080-013-0207-3. [DOI] [Google Scholar]
- Ricklefs RE (1968) Patterns of growth in birds. Ibis 110: 419–451. 10.1111/j.1474-919X.1968.tb00058.x. [DOI] [Google Scholar]
- Ricklefs RE (1976) Growth rates of birds in the humid New World tropics. Ibis 118: 179–207. 10.1111/j.1474-919X.1976.tb03065.x. [DOI] [Google Scholar]
- Ricklefs RE (1980) Geographical variation in clutch size among passerine birds: Ashmole’s hypothesis. The Auk 97: 38–49. [Google Scholar]
- Ricklefs RE (1996) Morphometry of the digestive tracts of some passerine birds. The Condor 98: 279–292. 10.2307/1369146. [DOI] [Google Scholar]
- Ricklefs RE (2000) Density dependence, evolutionary optimization, and the diversification of avian life histories. The Condor 102: 9–22. 10.1093/condor/102.1.9. [DOI] [Google Scholar]
- Rodríguez MÁ, López-Sañudo IL, Hawkins BA (2006) The geographic distribution of mammal body size in Europe: mammal body size gradient. Glob Ecol Biogeogr 15: 173–181. 10.1111/j.1466-822X.2006.00206.x. [DOI] [Google Scholar]
- Rodríguez MÁ, Olalla-Tárraga MÁ, Hawkins BA (2008) Bergmann’s rule and the geography of mammal body size in the Western hemisphere. Glob Ecol Biogeogr 17: 274–283. 10.1111/j.1466-8238.2007.00363.x. [DOI] [Google Scholar]
- Roff DA (1992) The Evolution of Life Histories: Theory and Analysis. Chapman and Hall, New York [Google Scholar]
- Romano A, Séchaud R, Roulin A (2021) Generalized evidence for Bergmann’s rule: body size variation in a cosmopolitan owl genus. J Biogeogr 48: 51–63. 10.1111/jbi.13981. [DOI] [Google Scholar]
- Salewski V, Watt C (2017) Bergmann’s rule: a biophysiological rule examined in birds. Oikos 126. 10.1111/oik.03698. [DOI] [Google Scholar]
- Saló E (2006) The power of regeneration and the stem-cell kingdom: freshwater planarians (Platyhelminthes). Bioessays 28: 546–559. 10.1002/bies.20416. [DOI] [PubMed] [Google Scholar]
- Searcy WA (1980) Optimum body sizes at different ambient temperatures: an energetics explanation of Bergmann’s rule. J Theor Biol 83: 579–593. 10.1016/0022-5193(80)90190-3. [DOI] [PubMed] [Google Scholar]
- Soetaert K, Petzoldt T, Setzer RW (2010) Solving differential equations in R: package deSolve. J Stat Softw 33: 1–25. 10.18637/jss.v033.i09.20808728 [DOI] [Google Scholar]
- Stearns SC (1976) Life-history tactics: a review of the ideas. Q Rev Biol 51: 3–47. [DOI] [PubMed] [Google Scholar]
- Stillwell RC (2010) Are latitudinal clines in body size adaptive? Oikos 119: 1387–1390. 10.1111/j.1600-0706.2010.18670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CMGL (2016) Application of Dynamic Energy Budget Theory for Conservation Relevant Modelling of Bird Life Histories (Ph.D.). Universidade Técnica de Lisboa & Vrije Universiteit Amsterdam, Lisboa & Amsterdam [Google Scholar]
- Teplitsky C, Millien V (2014) Climate warming and Bergmann’s rule through time: is there any evidence? Evol Appl 7: 156–168. 10.1111/eva.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The MathWorks Inc (2020) MATLAB Release 2020b. Natick, Massachusetts, United States [Google Scholar]
- Torres-Romero EJ, Morales-Castilla I, Olalla-Tárraga MÁ (2016) Bergmann’s rule in the oceans? Temperature strongly correlates with global interspecific patterns of body size in marine mammals. Glob Ecol Biogeogr 25: 1206–1215. 10.1111/geb.12476. [DOI] [Google Scholar]
- Meer J, Donk S, Sotillo A, Lens L (2020) Predicting post-natal energy intake of lesser black-backed gull chicks by dynamic energy budget modeling. Ecol Model 423: 109005. 10.1016/j.ecolmodel.2020.109005. [DOI] [Google Scholar]
- Veer HW, Kooijman SALM, Meer J (2001) Intra- and interspecies comparison of energy flow in North Atlantic flatfish species by means of dynamic energy budgets. J Sea Res 45: 303–320. 10.1016/S1385-1101(01)00061-2. [DOI] [Google Scholar]
- Watt C, Mitchell S, Salewski V (2010) Bergmann’s rule; a concept cluster? Oikos 119: 89–100. 10.1111/j.1600-0706.2009.17959.x. [DOI] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D (1986) The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr 116: 641–654. 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Thom C (2000) Marine iguanas shrink to survive El Niño. Nature 403: 37–38. 10.1038/47396. [DOI] [PubMed] [Google Scholar]
- Yom-Tov Y, Geffen E (2006) Geographic variation in body size: the effects of ambient temperature and precipitation. Oecologia 148: 213–218. 10.1007/s00442-006-0364-9. [DOI] [PubMed] [Google Scholar]
- Yom-Tov Y, Geffen E (2011) Recent spatial and temporal changes in body size of terrestrial vertebrates: probable causes and pitfalls. Biol Rev 86: 531–541. 10.1111/j.1469-185X.2010.00168.x. [DOI] [PubMed] [Google Scholar]
- Yom-Tov Y, Heggberget TM, Wiig Ø, Yom-Tov S (2006) Body size changes among otters, Lutra lutra, in Norway: the possible effects of food availability and global warming. Oecologia 150: 155–160. 10.1007/s00442-006-0499-8. [DOI] [PubMed] [Google Scholar]
- Yom-Tov Y, Roos A, Mortensen P, Wiig Ø, Yom-Tov S, Heggberget TM (2010) Recent changes in body size of the Eurasian otter Lutra lutra in Sweden. Ambio 39: 496–503. 10.1007/s13280-010-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this article are available on the AmP repository, at https://www.bio.vu.nl/thb/deb/deblab/add_my_pet/entries_web/Gerygone_igata/Gerygone_igata_res.html and https://www.bio.vu.nl/thb/deb/deblab/add_my_pet/entries_web/Gerygone_flavolateralis/Gerygone_flavolateralis_res.html. The code is available at https://gitlab.com/JoanyMarino/deb-resource-and-seasonality.