Abstract
Background and Objectives
Modified Atkins diet (MAD) has emerged as an adjuvant therapy in drug-resistant epilepsy (DRE). Most studies are in children; there is limited evidence for DRE in adults. This study aimed to investigate whether MAD along with standard drug therapy (SDT) was indeed more effective than SDT alone in reducing seizure frequency and improving psychological outcomes at 6 months in adolescents and adults with DRE (nonsurgical).
Methods
A prospective randomized controlled trial was conducted at tertiary care referral center in India. Persons with DRE aged 10–55 years attending outpatient epilepsy clinics between August 2015 and April 2019, who had more than 2 seizures per month despite using at least 3 appropriate antiseizure medications (ASMs) at their maximum tolerated doses and had not been on any form of diet therapy for the past 1 year, were enrolled. Patients were assessed for the eligibility and randomly assigned to receive SDT plus MAD (intervention arm) or SDT alone (control arm). The primary outcome was >50% reduction in seizure frequency, and the secondary outcomes were quality of life (QOL), behavior, adverse events, and rate of withdrawal at 6 months. Intention-to-treat analysis was performed.
Results
A total of 243 patients were screened for eligibility; 160 patients (80 adults and 80 adolescents) were randomized to either the intervention or control arm. Demographic and clinical characteristics in both groups were comparable at baseline. At 6 months, >50% seizure reduction was seen in 26.2% in the intervention group vs 2.5% in the control group (95% CI 13.5–33.9; p < 0.001). Improvement in QOL was 52.1 ± 17.6 in the intervention group vs 42.5 ± 16.4 in the control group (mean difference, 9.6; 95% CI 4.3 to 14.9, p < 0.001). However, behavior scores could be performed in 49 patients, and improvement was seen in the intervention vs control group (65.6 ± 7.9 vs 71.4 ± 8.1, p = 0.015) at the end of the study. One patient had weight loss; 2 patients had diarrhea.
Discussion
The MAD group demonstrated improvement in all aspects (reduction in seizure frequency and behavioral problems) compared with the control group at the end of the study. MAD is an effective modality in controlling seizures; further research is required to assess its efficacy in terms of biomarkers along with descriptive metabolomics studies.
Trial Registration Information
The clinical trial registry of India: CTRI/2015/07/006048.
Classification of Evidence
This study provides Class III evidence that the MAD increases the probability of seizure reduction in adolescents and adults with DRE.
Epilepsy affects more than 70 million people worldwide, and one-third of persons with epilepsy are resistant to antiseizure medications (ASMs).1
Drug-resistant epilepsy (DRE) is defined by the International League Against Epilepsy as “failure of adequate trials of 2 tolerated, appropriately chosen, and used ASM schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom.”2 Many patients who are not suitable surgical candidates or decline surgery3 have benefited from dietary interventions.4,5
Modified Atkins diet (MAD) aims to provide increase palatability and flexibility with a 1:1 ratio of fat to carbohydrates and protein, as it has around 65% fat, 25% protein, and 10% carbohydrates.6 MAD and low glycemic index diet (LGIT) are hence less restrictive alternatives to the ketogenic diet (KD), as protein and calories are not restricted.7
In previous studies, nearly half the patients with DRE showed >50% seizure reduction on the KD, and about 15%–20% became seizure-free.8 A meta-analysis showed that the combined efficacy rates for freedom from seizures, reduction of seizures by 50% or more, and reduction of seizures below 50% in adults with difficulty to treat epilepsy was 13%, 53%, and 27%, respectively.9 Several studies have shown an efficacy of MAD of at least in 30% of the study patients having >50% reduction in seizure.10-14 The efficacy of MAD has been established and well tolerated in children with DRE.13,15,16 Evidence suggests that MAD may have comparable efficacy but a higher rate of compliance as compared to KD in adults with DRE.17-20 There is an uncertainty as to the best dietary treatment because of a low number of trials in adults with DRE.21,22 Therefore, we chose MAD because of its ease of applicability and better compliance than the KD and the need for randomized controlled trials (RCTs) for assessing long-term outcomes with regard to the response to MAD in a larger cohort, including adolescents and adults with DRE, which are still lacking.
We therefore performed a RCT. Our primary research question was to investigate “whether the addition of MAD (dietary intervention) with on-going standard drug therapy (SDT) is more efficacious in terms of seizure control at 6 months in the nonsurgical patients with DRE?” The secondary objectives were to determine the quality of life, behavior, tolerability of MAD, and their adverse effects at 6 months among adolescents and adults with DRE.
Methods
Trial Design and Oversight
A prospective randomized open-label, blinded endpoint controlled trial with 2 parallel arms design was conducted in the pediatric and adult neurology clinic, All India Institute of Medical Sciences (AIIMS), a tertiary care referral center in New Delhi, India. Eligible participants were randomly assigned to receive the SDT plus MAD or SDT alone in a 1:1 ratio. All the patients underwent clinical evaluations at baseline, 3 months, and 6 months, and outcome assessment was performed at 6 months. Structured formats of seizure-log, ketone-log, food-log, adverse event diary, and schedule of enrollment and timeline of clinical evaluations (eFigure 1A, 1B) are provided in eAppendix 1 (links.lww.com/WNL/C564).
Standard Protocol Approvals, Registrations, and Patient Consents
The institutional ethics committee approved this trial, and written informed consent was obtained from adults, parents, or the legally authorized representatives of the adolescent patients with DRE, before recruitment. This trial was registered with the Clinical Trial Registry of India [(CTRI); ref no. CTRI/2015/07/006048]. The report of the study follows the CONSORT guidelines.23
Participants
The detailed study flowchart is presented in Figure 1. Potential candidates were recruited from the pediatric and neurology epilepsy clinic of tertiary care referral center, New Delhi. We enrolled patients who met the following inclusion criteria: (1) age [10–55 years; adolescents (10 to ≤18 years) and adults (>18–55 years)], (2) DRE who had more than 2 seizures per month despite using at least 3 appropriate ASMs at their maximum tolerated doses,21 and (3) agreed to regular follow-up and maintain their seizure-log. Patients were excluded in the following conditions: (1) surgical candidates, (2) an inborn error of metabolism, clinical suspicion of metabolic disorder4 known as chronic systemic disorder, (3) intake of any dietary therapy in the past, and (4) refusal to give consent. The screening procedure was performed with the assistance of the concerned clinicians (M.T. and S.G.). All patients underwent a 4-week observation period (week -4 to week 0 [the weeks are labeled -4 to 0] [run-in period]). Parents/caregivers were asked to maintain a daily seizure-log by recording the seizure type, duration, and frequency before enrollment. In the run-in period, no special dietary restrictions were advised. All baseline demographic details, biochemical investigations, and clinical details were collected in the paper-based standard case report form and then entered in an excel datasheet after the run-in period.
Figure 1. Study Flowchart.
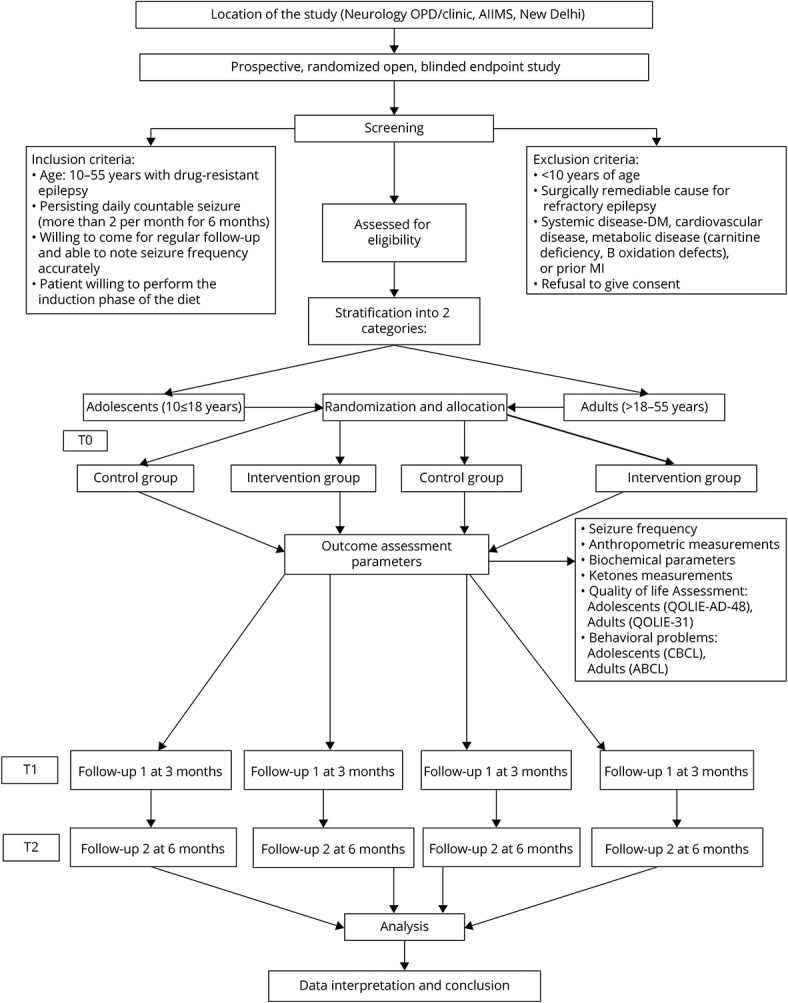
ABCL = Adult Behavior Checklist; CBCL = Child Behavior Checklist.
Randomization and Blinding
Patients were randomly assigned to either of the 2 groups—SDT plus MAD (intervention arm) or SDT alone (control arm). Computer-generated permuted blocks stratified by age group were used to generate a randomization list. Allocation concealment was performed using sealed and serially numbered opaque envelopes. These envelopes were prepared by a person not involved in the study (R.D.). A dietician (M.M.) was directly involved with the diet prescription, and patients and their caregivers were not blinded to the treatment, seizure frequency, and adverse events related to the treatment. The primary outcome assessor (K.K.) was blinded to the treatment allocation. Secondary outcome assessors (S.S. and A.N.), clinicians (M.T. and S.G.), other personnel (R.D.), and statisticians (R.M.P. and A.U.) were also blinded to the group allocation.
Intervention and Control
After the run-in period (−4 weeks), MAD therapy was started on an outpatient basis. Carbohydrate intake was restricted to 20 g/d. The detailed MAD protocol, a standard food exchange list, sample menu, and recipe booklet of standardized recipes including Indian recipe with either 2.5 g or 5 g carbohydrate are provided in eAppendix 2, links.lww.com/WNL/C564. High-fat and low-carbohydrate foods were encouraged; however, proteins were unrestricted. The diet was supplemented with multivitamins and minerals. Parents and caregivers were taught to maintain a daily-log of seizure count, meals consumed in a day, dietary intolerance, and urine ketones (thrice a day) using color-coded keto dipsticks. Average ketosis was calculated after 24 weeks of diet. Any adverse effects (i.e., constipation, diarrhea, weight loss, anorexia, lethargy, vomiting, sleep, disturbance, and hospitalization due to MAD) were noted as per parental/caregivers' interview at each visit at 15 days after the diet initiation, 3 months, and 6 months. Diet compliance was assessed based on carbohydrate consumption recorded in the daily food-log. Consumption of carbohydrates was calculated by using DietCal software.24 Regular telephonic consultation was given weekly to ensure adherence to the diet.
The control group received a normal diet with no specific dietetic inputs. A trained dietician (M.M.) provided counseling to the caregivers along with age and weight-specific dietary charts based on the Recommended Dietary Allowance without any carbohydrate restriction. Prescribed ASMs were not changed during the study period in both the groups. The complete blood count and fasting lipid profile at baseline and follow-up at 6 months were measured. After 6 months, MAD was offered to those who wanted to follow the therapy.
Outcome Measurements
The primary outcome measure was the proportion of patients with greater than 50% seizure reduction (seizure frequency) from baseline to 6 months of follow-up in both groups. Seizure frequency was measured as the average seizures/week in the preceding 4-week period. Secondary outcome measures included tolerability and adverse effects of the diet as per parental/caregivers' reports. We also compared changes in biochemical parameters, QOL, and behavior from baseline to 6 months using QOL in Epilepsy Inventory for Adolescents-48 and QOL in Epilepsy Inventory-31 for adults. Both scales contain questions about health-related quality of life. Child Behavior Checklist and Adult Behavior Checklist scales were used for the behavioral assessments, which were completed by parents/caregivers in each visit. Patients were followed up and assessed using daily seizure-log, food-log, and ketone-log at 1 month, 3 months, and 6 months.
Safety
An independent, external data safety monitoring board (DSMB) (Acknowledgement section) reviewed all the patients' case record files periodically for their safety, efficacy, and adverse events. We followed DSMB guidelines.25
Statistical Analysis
The sample size was calculated based on an anticipated decrease of >50%13,17 for the SDT plus MAD group as compared to the SDT group. Expecting a 30% response rate in the intervention arm and 10% in the control arm, power of 80%, and level of significance 5%, a sample size of 144 (72 each group) was calculated. Considering that 10% of patients might be lost to follow-up at 6 months, 160 patients were enrolled in total.
All statistical analyses were conducted using STATA (Version 14, Stata Corp; College Station, TX). Variables were checked for normal distribution, and frequency (percentage), mean, or median values were used as appropriate. Categorical and continuous variables were computed using the χ2 test/Fisher exact and unpaired t test or the Wilcoxon-Mann-Whitney test. Owing to skewness, the variables (i.e., SGPT and triglycerides) were log-transformed and appropriate test applied. Log-binomial regression was used to see the effect of intervention after adjusting the variables, which were not comparable at baseline. For the primary outcome, percentage reduction on seizure frequency at 6 months was analyzed using effect size (mean or median difference) with 95% CI, and relative risk (RR with 95% CI) was also analyzed to see the risk between the 2 treatment groups. Intention-to-treat (ITT) analysis was performed by including all patients who were enrolled and assigned to an intervention. Patients who could not be contacted at 6 months and their outcome data were missing; the last observation carried forward method was used for primary and secondary outcome analyses. Per-protocol (PP) analysis was performed for patients who assigned the allocation and who adhered to the protocol at 6 months. The effect of diet on seizure reduction was analyzed using worst-case scenario analysis. Adverse effects of the intervention were summarized as number (percentage), and p < 0.05 was considered statistically significant.
The study protocol and statistical analysis plan are available in eSAP 1, links.lww.com/WNL/C563.
Results
Baseline Characteristics
A total of 243 patients with DRE were screened for eligibility between August 2015 and April 2019; 160 patients were enrolled and randomly assigned to the intervention (n = 80) or control (n = 80) group. Fifty-two patients withdrew from the study, and the remaining 108 patients (46: intervention and 62: control) who completed 6 months of follow-up were included for the per-protocol analysis. The reasons for the patient's withdrawal and exclusion from the study are given in the CONSORT chart (Figure 2).
Figure 2. CONSORT Flowchart of the Study.
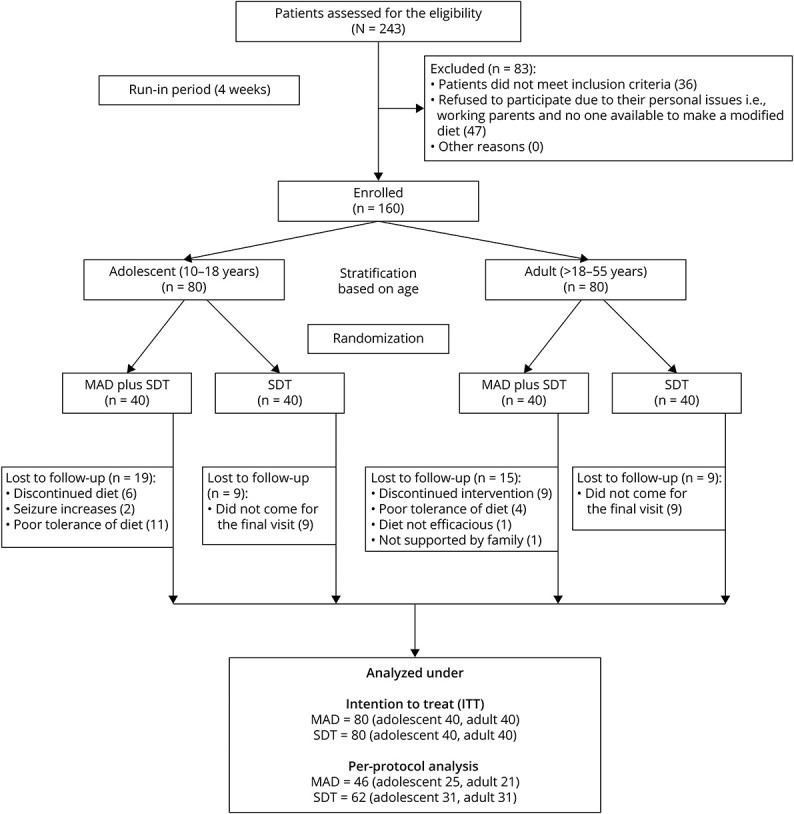
MAD = modified Atkins diet; SDT= standard drug therapy.
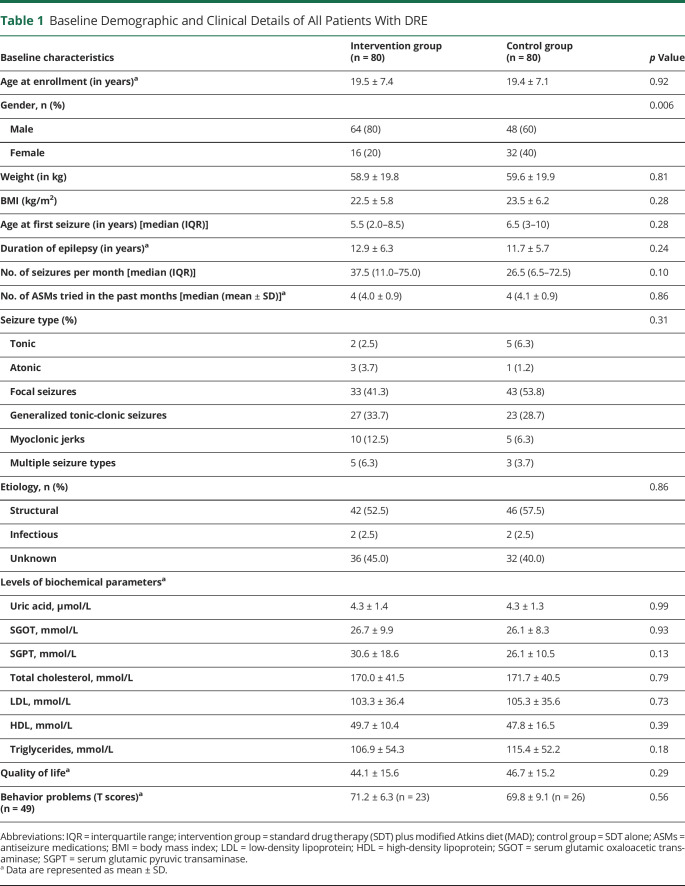
The demographic and clinical characteristics of the patients were comparable at baseline except for gender (p = 0.006) (Table 1). The median of the baseline seizure frequency was similar in both groups (intervention: 16.5 and control: 24.0; p = 0.88). Most of the patients had epilepsy of structural (MAD: 52.5%, SDT: 57.5%) or unknown etiology (MAD: 45.0%, SDT: 40.0%). Most patients were on at least 4 or more ASMs, the frequent of these being levetiracetam (MAD: 60.0%, SDT: 70.0%), valproate (MAD: 75.0%, SDT: 75.0%), and clobazam (MAD: 62.5%, SDT: 55.0%) (eFigure 2, links.lww.com/WNL/C564). Nonvegetarians were higher in both groups (MAD: 65.0%, SDT: 58.7%) as compared to vegetarians (MAD: 35.0%, SDT: 41.2%) (eFigure 3, links.lww.com/WNL/C564).
Table 1.
Baseline Demographic and Clinical Details of All Patients With DRE
Urine ketone levels were moderate to high (40–80 mg/dL) throughout the study period. The mean morning and evening levels of urine ketosis among the patients in the diet group were 58.3 ± 8.0 mg/dL and 62.2 ± 22.6 mg/dL, respectively, indicating satisfactory adherence to the diet. Baseline demographic and clinical details were obtained for adolescents and adults (eTables 1 and 2, links.lww.com/WNL/C564).
Primary and Secondary Outcomes
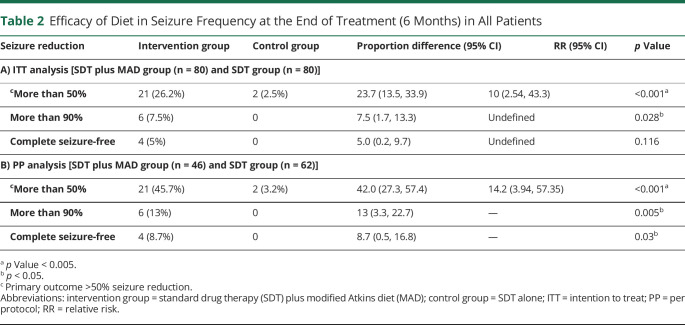
At the end of the study period, the proportion of patients with >50% seizure reduction from baseline was significantly higher in the intervention group (Table 2) as per both ITT [intervention: 26.2%; control: 2.5%, p value: 0.001] and per-protocol analysis (intervention: 45.7%; control: 3.2%, p value: 0.001). It was also observed that >50% seizure reduction in the intervention group was 10 times more as compared to the control group (RR = 10; 95% CI 2.54, 43.3, p = 0.001). In the intervention group, 5.0% (ITT analysis) and 8.7% (PP analysis) of patients were seizure-free at the end of follow-up, whereas none of the patients were seizure-free in the control group. These differences in the seizure freedom rate were statistically significant as per the PP analysis (p = 0.03) and the ITT analysis (Table 2). The median (IQR) percentage reduction in seizure frequency from baseline was found to be significant (p = 0.001) in the intervention group [12.4 (−0.94–50.70)] as compared to the control group [0 (−56.08, 9.45)]. On adjusting variables (i.e., gender), there was 13.8 (95% CI 3.1, 62.6; p = 0.001; ITT analysis) and 24.4 (95% CI 5.24, 113.8, p = 0.001; PP analysis) times more seizure reduction (>50%) observed in the intervention group as compared to the control group. Furthermore, as per ITT analysis, the proportion of adult and adolescent patients having >50% seizure reduction and percentage change in seizure frequency was significantly higher (p = 0.001) in the intervention group as compared to the control group (eFigure4–7, links.lww.com/WNL/C564). As per PP analysis, significant improvement (57.1%; p = 0.001) in seizure reduction was most notable in the adult population of the intervention group vs control group (eTable 3, links.lww.com/WNL/C564).The worst-case scenario analysis revealed no significant improvement (>50% seizure reduction) between the intervention and control groups (eTable 4, links.lww.com/WNL/C564).
Table 2.
Efficacy of Diet in Seizure Frequency at the End of Treatment (6 Months) in All Patients
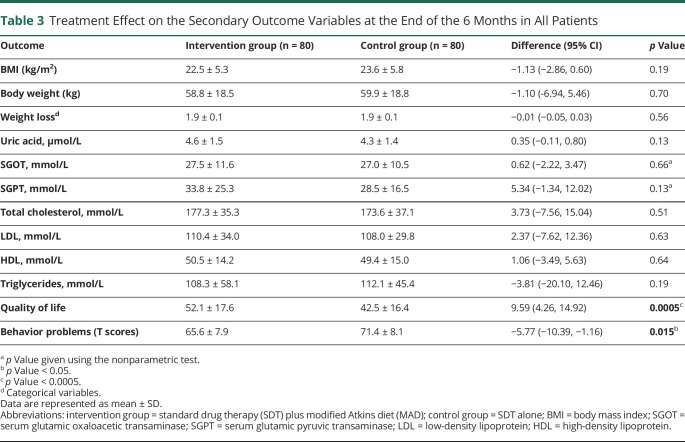
There were no significant differences (p > 0.05) in the mean scores of body weight, weight loss (eTable 5, links.lww.com/WNL/C564), and biochemical profiles between the 2 groups at 6 months (Table 3). There was no change in most biochemical parameters at 6 months on the diet when compared with the baseline in both groups (eFigure 8, A–G). The difference in the QOL and behavior scores was statistically significant (p = 0.0005 and p = 0.015, respectively) in the intervention group as compared to the control group at 6 months (Table 3).
Table 3.
Treatment Effect on the Secondary Outcome Variables at the End of the 6 Months in All Patients
A significant improvement (eTable 6, links.lww.com/WNL/C564) from baseline was noted in the mean score of QOL (baseline: 52.7 ± 11.6 and last follow-up: 58.7 ± 14.2; p = 0.001 [adult]; 35.5 ± 14.3 (baseline) and 45.4 ± 18.3 (follow-up); p = 0.001 [adolescent]) in the intervention group. No significant difference in QOL from baseline was found in adult patients of the control group, whereas significant deterioration was observed in adolescents (baseline: 41.3 ± 15.0 and 6-month follow-up: 33.8 ± 15.3; p = 0.0001). Most of the patients in the intervention group had clinically meaningful increase (6.0 points: adults and ∼10 points: adolescents) in their overall QOL.
Total T scores on the Child Behavior Checklist/Adult Behavior Checklist scales indicated the possible behavior problems at the end of treatment. However, we obsedverd that the behavior problems normalized in the intervention compared to controls. The difference of the mean total behavior T score from baseline to follow-up [8.3, p = 0.0069 (adults); 3.7, p = 0.03 (adolescents)] in the intervention group while in the control group [−3.5, p = 0.0179 (adults); −0.06, p = 0.95 (adolescents)] (eTable 7, links.lww.com/WNL/C564).
Dietary adherence/compliance [median percentage (range)] was 91.07 (87.5–92.85) in the MAD group at 6 months (eFigure 9 and eTable 8, links.lww.com/WNL/C564). No significant adverse effects were observed in patients receiving MAD. However, 1 patient had weight loss; 2 patients had diarrhea (4.3%). The most common adverse effects were constipation, vomiting, diarrhea, lethargy, and anorexia, which resolved by dietary modifications (eTable 9, links.lww.com/WNL/C564).
This study provides Class III evidence that the MAD increases the probability of seizure reduction in adolescents and adults with DRE.
Discussion
In this RCT, we investigated the effect of an add-on MAD therapy on seizure reduction in adolescents and adults with DRE. MAD was found to be more efficacious for reducing seizure frequency than SDT alone. 26.2% of patients had >50% seizure reduction in the intervention group compared with the control group. The result of the present RCT agrees with previous findings,13,15,21,16 and the observed seizure reduction was comparable with previously published reports on MAD with DRE,18,17,26,27 which suggest that MAD for DRE in adults and adolescents is well tolerated; however, data on MAD treatment in adults are limited.21,22 The present cohort demonstrated a lesser reduction in seizure frequency on MAD as compared to another study.17 in adults and in children.13,15,16 This difference could be partially accounted for by the fact that the MAD was started late in our clinical setting. In our study, patients had a duration of epilepsy of more than 10 years on an average and had a median of 37.5 seizures per month in the intervention group and 26.5 in the control group; after having tried an average of 4 different ASMs, most presented with structural (bilateral hypoxic ischemic changes) etiology.
An RCT performed in adults in Iran reported a 35.5% responder (>50% seizure reduction) vs no responder in the control group.21 Another study could not detect a decrease in seizure frequency.22 Both these studies had a relatively low number of participants and a shorter follow-up period.
Our study was conducted in a larger cohort, including adolescents and adults, with a 6-month follow-up. On subgroup analysis, >50% seizure reduction was found in 32.5% of the adult population. Use of exchange list and recipe booklet helped in the initiation of MAD with the flexibility of meal choices and ease of administration, hence an ideal treatment option for low-resource settings.
On PP analysis, we have found similar efficacy of MAD on seizure reduction (45.7%), which is comparable with the observational study10 in contrast to other studies reported.11,21 Worst-case scenario analysis for the missing data was performed, and we observed that there was no significant difference between intervention and control groups for favorable outcome (>50% seizure reduction) and unfavorable outcome (≤50% seizure reduction). The analysis was performed because of the higher dropouts in our study.28,29
Reduction in seizure frequency and QOL improved significantly for the entire population in adults and adolescents in the intervention group. Improvement in nonseizure domains (QOL) was observed in the intervention group as compared to the control group (r = 0.17, p = 0.027), which was significant. The reasons may be probably fewer and lower frequency of seizures that visibly enhanced quality of life. Many other investigators have reported a better QOL without any standard scales, including recent studies on a diet.30-32
Of interest, we have not found a significant difference in weight loss (>10%) in the diet group, which is supported by previous studies.21,18 However, weight loss is more common in adults as reported elsewhere.17 In our study, there was no change in most biochemical parameters at 6 months on the diet when compared with the baseline. None of the patients had hyperuricemia. However, one study reported an increase in the lipid profile over the first 3 months of the diet; these values normalized within a year of treatment, including in patients treated with MAD for more than 3 years.33 Longer follow-up data are required to assess the change in the lipid profile in adults on MAD. Other studies have reported some side effects (i.e., gastrointestinal complaints, dyslipidemias, constipation, and weight loss).21,34,27 Kidney stones31 are a common diet-induced problem in children in the case of diets. None in our study reported renal stones possibly because of adequate liquid consumption during the dietary intervention. Increased seizure frequency was reported in 1 patient. The seizure aggravation in this patient is hard to explain. Others have reported an aggravated seizure frequency when on diet.22,27
We found 32.5% dropouts because of lack of efficacy, nonacceptability of diet, and inability to follow-up (around COVID time). Other reports also show a variation in the dropout rate between 7% and 50%.13,15,21,35,36 We also assessed QOL and behavior using structural scales in the whole cohort along with diet compliance by an expert dietician (M.M.), which added strength to our study. Our study has few limitations; blinding could not be performed with the individuals and dieticians because it required close interaction with patients. Because of resource constraints, the behavior could be assessed only in a subset of patients and not the entire cohort. Compliance to diet is more challenging in long term, especially in adults. However, the tolerance of MAD is much better than high-fat diet (classical KD).15 Daily-logs maintained by caregivers could have missed some seizures, including nocturnal seizures, and runs the risk of introducing subjective errors. A multicentric trial including all primary dietary options such as KD, MAD, and LGIT in older adults with DRE having outcomes of seizure reduction, adverse events, and cognitive effects is required to further validate the results. In addition, a selection bias cannot be ruled out because this was a single-center study.
MAD therapy was efficacious, feasible, and well tolerated, with better compliance along with seizure reduction in adolescents and adults with DRE. Reduction of seizure frequency reflected in the improvement of the quality of life in all patients in the intervention group as compared to the control group. Future studies would be needed to identify neurophysiologic and genetic biomarkers associated with MAD response, which may have implications for clinical care by encouraging targeted and earlier use of the MAD and also individualized risk-benefit analysis of the therapeutic diet, which can provide alternative therapy to standard care treatment.
Acknowledgment
This study was part of doctor of philosophy (PhD) research work. The authors acknowledge the support of Department of Biostatistics, AIIMS, for analyzing the data and members who helped in their study: Sanjay Kumar and Anuradha. Special thanks to the Data Safety Monitoring Board (DSMB) members: *Mani Kaliavani, PhD (Biostatistics, AIIMS, India), Sudhir Sarangi, MD, DM (Department of Pharmacology, AIIMS, New Delhi, India), Achal Shrivastava, MD, DM (Dept. of Neurology, AIIMS, New Delhi, India), and Rajesh Sagar (Dept. of Psychiatry, AIIMS, New Delhi, India). The authors also express their gratitude to the patients and their families for participating in this study.
Glossary
- AIIMS
All India Institute of Medical Sciences
- ASMs
antiseizure medications
- DRE
drug-resistant epilepsy
- ILAE
International League Against Epilepsy
- ITT
intention to treat
- MAD
modified Atkins diet
- PP
per protocol
- QOL
quality of life
- RCTs
randomized controlled trials
- SDT
standard drug therapy
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Infographic links.lww.com/WNL/C685
Study Funding
This trial was supported by the Centre of Excellence for Epilepsy (COE)-Phase-II, which is funded by the Department of Biotechnology, Govt. of India (Ref. No.: BT/MED/122/SP24580/2018) for AIIMS, New Delhi, and NBRC, Manesar, Gurgaon (Haryana).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology. 2011;77(10):1005-1012. doi: 10.1212/wnl.0b013e31822cfc90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan P, Arzimanoglou A, Berg AT, et al. . Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2009;51(6):1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- 3.Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol. 2012;8(12):669-677. doi: 10.1038/nrneurol.2012.181 [DOI] [PubMed] [Google Scholar]
- 4.Kossoff EH, Zupec‐Kania BA, Auvin S, et al. ; The Charlie Foundation Matthew's Friends the Practice Committee of the Child Neurology Society. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3(2):175-192. doi: 10.1002/epi4.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JA, Yoon JR, Lee EJ, et al. Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia. 2016;57(1):51-58. doi: 10.1111/epi.13256 [DOI] [PubMed] [Google Scholar]
- 6.Payne NE, Cross JH, Sander JW, Sisodiya SM. The ketogenic and related diets in adolescents and adults-A review: ketogenic and Related Diets in Adolescents and Adults. Epilepsia. 2011;52(11):1941-1948. doi: 10.1111/j.1528-1167.2011.03287.x [DOI] [PubMed] [Google Scholar]
- 7.Kossoff EH, Dorward JL. The modified Atkins diet. Epilepsia. 2008;49(s8):37-41. doi: 10.1111/j.1528-1167.2008.01831.x [DOI] [PubMed] [Google Scholar]
- 8.Martin‐McGill KJ, Jackson CF, Bresnahan R, Levy RG, Cooper PN. Ketogenic Diets for Drug‐resistant Epilepsy. Cochrane Database Syst Rev. 2018. Available from: ncbi.nlm.nih.gov/pmc/articles/PMC6517043/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Yang Y, Wang Y, et al. Ketogenic diet for treatment of intractable epilepsy in adults: a meta‐analysis of observational studies. Epilepsia Open. 2018;3(1):9-17. doi: 10.1002/epi4.12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47(2):421-424. doi: 10.1111/j.1528-1167.2006.00438.x [DOI] [PubMed] [Google Scholar]
- 11.Miranda MJ, Mortensen M, Povlsen JH, Nielsen H, Beniczky S. Danish study of a Modified Atkins diet for medically intractable epilepsy in children: can we achieve the same results as with the classical ketogenic diet?. Seizure 2011;20(2):151-155. doi: 10.1016/j.seizure.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 12.Weber S, Mølgaard C, KarenTaudorf UldallP, Uldall P. Modified Atkins diet to children and adolescents with medical intractable epilepsy. Seizure. 2009;18(4):237-240. doi: 10.1016/j.seizure.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Sankhyan N, Gulati S, Agarwala A. Use of the modified Atkins diet for treatment of refractory childhood epilepsy: a randomized controlled trial. Epilepsia. 2013;54(3):481-486. doi: 10.1111/epi.12069 [DOI] [PubMed] [Google Scholar]
- 14.Rezaei S, Abdurahman AA, Saghazadeh A, Badv RS, Mahmoudi M. Short-term and long-term efficacy of classical ketogenic diet and modified Atkins diet in children and adolescents with epilepsy: a systematic review and meta-analysis. Nutr Neurosci. 2019;22(5):317-334. doi: 10.1080/1028415x.2017.1387721 [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Goel S, Jain P, Agarwala A, Aneja S. Evaluation of a simplified modified Atkins diet for use by parents with low levels of literacy in children with refractory epilepsy: a randomized controlled trial. Epilepsy Res. 2016;127:152-159. doi: 10.1016/j.eplepsyres.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 16.Sondhi V, Agarwala A, Pandey RM, et al. Efficacy of ketogenic diet, modified Atkins diet, and low glycemic index therapy diet among children with drug-resistant epilepsy: a randomized clinical trial. JAMA Pediatr. 2020;174(10):944-951. doi: 10.1001/jamapediatrics.2020.2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kossoff EH, Rowley H, Sinha SR, Vining EPG. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia 2008;49(2):316-319. doi: 10.1111/j.1528-1167.2007.01256.x [DOI] [PubMed] [Google Scholar]
- 18.Smith M, Politzer N, MacGarvie D, McAndrews MP, del Campo M. Efficacy and tolerability of the Modified Atkins Diet in adults with pharmacoresistant epilepsy: a prospective observational study. Epilepsia 2011;52(4):775-780. doi: 10.1111/j.1528-1167.2010.02941.x [DOI] [PubMed] [Google Scholar]
- 19.Ye F, Li XJ, Jiang WL, Sun HB, Liu J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta-analysis. J Clin Neurol. 2015;11(1):26-31. doi: 10.3988/jcn.2015.11.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic Diets for Drug‐resistant Epilepsy. Cochrane Database Syst Rev. 2021;6. Available from: cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001903.pub5/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zare M, Okhovat AA, Esmaillzadeh A, Mehvari J, Najafi MR, Saadatnia M. Modified Atkins diet in adult with refractory epilepsy: a controlled randomized clinical trial. Iran J Neurol. 2017;16(2):72-77. [PMC free article] [PubMed] [Google Scholar]
- 22.Kverneland M, Molteberg E, Iversen PO, et al. Effect of modified Atkins diet in adults with drug‐resistant focal epilepsy: a randomized clinical trial. Epilepsia. 2018;59(8):1567-1576. doi: 10.1111/epi.14457 [DOI] [PubMed] [Google Scholar]
- 23.Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340(mar23 1):c332. doi: 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DietCal [Internet]. 2021. Available from: dietcal.in/ [Google Scholar]
- 25.Data and Safety Monitoring Board (DSMB) Guidelines. 2022. Available from: nidcr.nih.gov/research/human-subjects-research/toolkit-and-education-materials/interventional-studies/data-and-safety-monitoring-board-guidelines [Google Scholar]
- 26.Green SF, Nguyen P, Kaalund-Hansen K, Rajakulendran S, Murphy E. Effectiveness, retention, and safety of modified ketogenic diet in adults with epilepsy at a tertiary-care centre in the UK. J Neurol. 2020;267(4):1171-1178. doi: 10.1007/s00415-019-09658-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cervenka MC, Terao NN, Bosarge JL, et al. E-mail management of the Modified Atkins Diet for adults with epilepsy is feasible and effective. Epilepsia. 2012;53(4):728-732. doi: 10.1111/j.1528-1167.2012.03406.x [DOI] [PubMed] [Google Scholar]
- 28.Mavridis D, Chaimani A, Efthimiou O, Leucht S, Salanti G. Addressing missing outcome data in meta-analysis. Evid Based Ment Health. 2014:17. [DOI] [PubMed] [Google Scholar]
- 29.Vibha D, Prasad K. How to deal with missing data? Neurol India. 2020;68(4):886. doi: 10.4103/0028-3886.293445 [DOI] [PubMed] [Google Scholar]
- 30.Roehl K, Falco-Walter J, Ouyang B, Balabanov A. Modified ketogenic diets in adults with refractory epilepsy: efficacious improvements in seizure frequency, seizure severity, and quality of life. Epilepsy Behav. 2019;93:113-118. doi: 10.1016/j.yebeh.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 31.Kverneland M, Selmer KK, Nakken KO, Iversen PO, Taubøll E. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav. 2015;53:197-201. doi: 10.1016/j.yebeh.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 32.Bruce S, Devlin A, Air L, Cook L. Changes in quality of life as a result of ketogenic diet therapy: a new approach to assessment with the potential for positive therapeutic effects. Epilepsy Behav. 2017;66:100-104. doi: 10.1016/j.yebeh.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 33.Cervenka MC, Patton K, Eloyan A, Henry B, Kossoff EH. The impact of the modified Atkins diet on lipid profiles in adults with epilepsy. Nutr Neurosci. 2016;19(3):131-137. doi: 10.1179/1476830514y.0000000162 [DOI] [PubMed] [Google Scholar]
- 34.Kossoff EH, Rowley H, Sinha SR, Vining EPG. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia 2008;49(2):316-319. doi: 10.1111/j.1528-1167.2007.01256.x [DOI] [PubMed] [Google Scholar]
- 35.El-Rashidy OF, Nassar MF, Abdel-Hamid IA, et al. Modified Atkins diet vs classic ketogenic formula in intractable epilepsy. Acta Neurol Scand. 2013;128(6):402-408. doi: 10.1111/ane.12137 [DOI] [PubMed] [Google Scholar]
- 36.Kossoff EH, Turner Z, Bluml RM, Pyzik PL, Vining EPG. A randomized, crossover comparison of daily carbohydrate limits using the modified Atkins diet. Epilepsy Behav. 2007;10(3):432-436. doi: 10.1016/j.yebeh.2007.01.012 [DOI] [PubMed] [Google Scholar]