Abstract
Background
Screening mammography can detect breast cancer at an early stage. Supporters of adding ultrasonography to the screening regimen consider it a safe and inexpensive approach to reduce false‐negative rates during screening. However, those opposed to it argue that performing supplemental ultrasonography will also increase the rate of false‐positive findings and can lead to unnecessary biopsies and treatments.
Objectives
To assess the comparative effectiveness and safety of mammography in combination with breast ultrasonography versus mammography alone for breast cancer screening for women at average risk of breast cancer.
Search methods
We searched the Cochrane Breast Cancer Group's Specialised Register, CENTRAL, MEDLINE, Embase, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), and ClinicalTrials.gov up until 3 May 2021.
Selection criteria
For efficacy and harms, we considered randomised controlled trials (RCTs) and controlled non‐randomised studies enrolling at least 500 women at average risk for breast cancer between the ages of 40 and 75.
We also included studies where 80% of the population met our age and breast cancer risk inclusion criteria.
Data collection and analysis
Two review authors screened abstracts and full texts, assessed risk of bias, and applied the GRADE approach. We calculated the risk ratio (RR) with 95% confidence intervals (CI) based on available event rates. We conducted a random‐effects meta‐analysis.
Main results
We included eight studies: one RCT, two prospective cohort studies, and five retrospective cohort studies, enrolling 209,207 women with a follow‐up duration from one to three years. The proportion of women with dense breasts ranged from 48% to 100%. Five studies used digital mammography; one study used breast tomosynthesis; and two studies used automated breast ultrasonography (ABUS) in addition to mammography screening. One study used digital mammography alone or in combination with breast tomosynthesis and ABUS or handheld ultrasonography. Six of the eight studies evaluated the rate of cancer cases detected after one screening round, whilst two studies screened women once, twice, or more.
None of the studies assessed whether mammography screening in combination with ultrasonography led to lower mortality from breast cancer or all‐cause mortality. High certainty evidence from one trial showed that screening with a combination of mammography and ultrasonography detects more breast cancer than mammography alone. The J‐START (Japan Strategic Anti‐cancer Randomised Trial), enrolling 72,717 asymptomatic women, had a low risk of bias and found that two additional breast cancers per 1000 women were detected over two years with one additional ultrasonography than with mammography alone (5 versus 3 per 1000; RR 1.54, 95% CI 1.22 to 1.94). Low certainty evidence showed that the percentage of invasive tumours was similar, with no statistically significant difference between the two groups (69.6% (128 of 184) versus 73.5% (86 of 117); RR 0.95, 95% CI 0.82 to 1.09). However, positive lymph node status was detected less frequently in women with invasive cancer who underwent mammography screening in combination with ultrasonography than in women who underwent mammography alone (18% (23 of 128) versus 34% (29 of 86); RR 0.53, 95% CI 0.33 to 0.86; moderate certainty evidence). Further, interval carcinomas occurred less frequently in the group screened by mammography and ultrasonography compared with mammography alone (5 versus 10 in 10,000 women; RR 0.50, 95% CI 0.29 to 0.89; 72,717 participants; high certainty evidence). False‐negative results were less common when ultrasonography was used in addition to mammography than with mammography alone: 9% (18 of 202) versus 23% (35 of 152; RR 0.39, 95% CI 0.23 to 0.66; moderate certainty evidence). However, the number of false‐positive results and necessary biopsies were higher in the group with additional ultrasonography screening. Amongst 1000 women who do not have cancer, 37 more received a false‐positive result when they participated in screening with a combination of mammography and ultrasonography than with mammography alone (RR 1.43, 95% CI 1.37 to 1.50; high certainty evidence). Compared to mammography alone, for every 1000 women participating in screening with a combination of mammography and ultrasonography, 27 more women will have a biopsy (RR 2.49, 95% CI 2.28 to 2.72; high certainty evidence). Results from cohort studies with methodological limitations confirmed these findings.
A secondary analysis of the J‐START provided results from 19,213 women with dense and non‐dense breasts. In women with dense breasts, the combination of mammography and ultrasonography detected three more cancer cases (0 fewer to 7 more) per 1000 women screened than mammography alone (RR 1.65, 95% CI 1.0 to 2.72; 11,390 participants; high certainty evidence). A meta‐analysis of three cohort studies with data from 50,327 women with dense breasts supported this finding, showing that mammography and ultrasonography combined led to statistically significantly more diagnosed cancer cases compared to mammography alone (RR 1.78, 95% CI 1.23 to 2.56; 50,327 participants; moderate certainty evidence). For women with non‐dense breasts, the secondary analysis of the J‐START study demonstrated that more cancer cases were detected when adding ultrasound to mammography screening compared to mammography alone (RR 1.93, 95% CI 1.01 to 3.68; 7823 participants; moderate certainty evidence), whilst two cohort studies with data from 40,636 women found no statistically significant difference between the two screening methods (RR 1.13, 95% CI 0.85 to 1.49; low certainty evidence).
Authors' conclusions
Based on one study in women at average risk of breast cancer, ultrasonography in addition to mammography leads to more screening‐detected breast cancer cases. For women with dense breasts, cohort studies more in line with real‐life clinical practice confirmed this finding, whilst cohort studies for women with non‐dense breasts showed no statistically significant difference between the two screening interventions.
However, the number of false‐positive results and biopsy rates were higher in women receiving additional ultrasonography for breast cancer screening. None of the included studies analysed whether the higher number of screen‐detected cancers in the intervention group resulted in a lower mortality rate compared to mammography alone. Randomised controlled trials or prospective cohort studies with a longer observation period are needed to assess the effects of the two screening interventions on morbidity and mortality.
Plain language summary
Mammography followed by ultrasonography compared to mammography alone for breast cancer screening in women at average risk of breast cancer
What is the issue?
We examined the evidence for and against adding ultrasonography screening to mammograms for women at average risk for breast cancer.
Why is it important?
It is important to weigh the pros and cons of screening because the increased detection of tumours through screening does not necessarily mean that more women will be saved. Evidence shows that mammography in healthy women between the ages of 50 and 69 can detect breast cancer early and reduce the risk of dying from breast cancer. However, mammography is not a perfect tool to detect breast cancer and can miss tumours in some women, particularly those with dense breasts. In these women, the tumour is difficult to distinguish from normal breast tissue on the mammogram. For women with non‐dense breasts, ultrasonography is often routinely performed in addition to mammography to increase the sensitivity of screening.
Gap in the evidence: no study examined the effect of additional ultrasonography screening on death
To determine whether routine screening with mammography and ultrasonography is beneficial, a study (ideally a randomised controlled trial (RCT), that is a study in which participants are randomly assigned to one of two or more treatment groups) comparing whether disease progression and death rates differ between methods is essential. None of the studies, which followed women for one to three years, lasted long enough to determine whether more cancer cases detected during screening with mammography and ultrasonography lead to reductions in disease and death.
How many more cancers are detected by mammography screening with additional ultrasonography?
We found one RCT and seven cohort studies (a type of study in which groups of people are followed over time) that analysed whether the combination of mammography and ultrasonography is more effective than mammography alone for early detection of breast cancer in women at average risk of breast cancer with no symptoms.
The methods of the RCT were sound, and the study represented the best evidence currently available. The study included 72,717 women at average risk for breast cancer, 58% of whom had dense breast tissue. After a two‐year follow‐up, women screened once with a combination of mammography and ultrasonography had two more breast cancers detected per 1000 women compared with women screened with mammography (5.0 versus 3.2 per 1000 women screened).
How effective is additional ultrasound screening in women with dense or non‐dense breasts?
A recent publication analysed a subgroup of the RCT of 19,213 women, and reported results separately for women with dense and non‐dense breasts.
In women with dense breasts, three more breast cancers per 1000 women were detected with mammography and ultrasonography than with mammography alone. This finding was supported by real‐world evidence: the combined result of three cohort studies examining a total of 50,327 women with dense breasts found additional cancers in women with dense breasts when mammography screening was supplemented with ultrasonography. In women with non‐dense breasts, the results of two cohort studies with data from 40,636 women were not consistent with the RCT and found no significant difference in the proportion of cancer cases between the two screening methods.
How many cancer cases were invasive and had lymph nodes involved?
In the RCT, 71% of all tumours identified at screening were classified as invasive, with no significant difference between the two groups. However, the result for the difference between the two groups was imprecise, and our confidence in the result is low. In women with invasive cancer found by mammography screening combined with ultrasonography, lymph nodes were affected in fewer cases than in the group screened by mammography alone (18% (23 of 128) versus 34% (29 of 86)).
Interval cancer: cancer cases detected in the time between screening rounds
The RCT also showed that cancers that were not found during screening examinations (but were found in the time period between examinations) occurred less frequently when screening was performed with a combination of mammography and ultrasonography (5 versus 10 per 10,000) than when screening was performed with mammography alone.
False‐positive and false‐negative rate
The rate of false‐negative results, indicating a negative result when cancer is present, was lower (9% versus 23%) when ultrasonography was performed in addition to mammography. However, the combination of mammography and ultrasound resulted in more false‐positives than mammography alone in women without cancer: 123 versus 86 per 1000 women. Moreover, of 1000 women screened with a combination of mammography and ultrasonography, 27 more needed a biopsy than with mammography alone.
How up‐to‐date is this review?
We searched for studies published up to May 2021.
Conclusion
It is unclear whether or to what extent ultrasonography in addition to mammography screening can reduce the risk of dying from breast cancer therefore ultrasonography should not be used on a routine basis. For women to make an informed decision, we need to assess whether the few additional cancers that can be detected by ultrasonography actually result in a decrease in breast cancer disease and death.
Summary of findings
Summary of findings 1. Mammography in combination with breast ultrasonography versus mammography alone for breast cancer screening in mixed populations of women with dense and non‐dense breasts.
| Mammography + ultrasound compared to mammography alone for breast cancer screening in mixed populations of women with dense and non‐dense breasts | |||||
| Patient or population: breast cancer screening in women with dense and non‐dense breasts Setting: screening Intervention: mammography + ultrasound Comparison: mammography alone | |||||
| Outcomes after 1‐ to 2‐year follow‐up | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with mammography alone | Risk difference with mammography + ultrasound | ||||
| Breast cancer mortality | NR | ⊝⊝⊝⊝ Insufficient | NR | NR | |
| All‐cause mortality | NR | ⊝⊝⊝⊝ Insufficient | NR | NR | |
| Incremental cancer detection: RCT | 72,717 (1 RCT) | ⊕⊕⊕⊕ High | RR 1.54 (1.22 to 1.94) | Study population | |
| 3 per 1000 | 2 more per 1000 (1 to 3 more) | ||||
| Incremental cancer detection: cohort studies | 83,469 (2 cohort studies) |
⊕⊕⊝⊝ Low 1 | RR 1.35 (0.92 to 1.98) | Study population | |
| 4 per 1000 | 1 more per 1000 (0 to 3 more) | ||||
| Incremental detection of invasive cancers: RCT | 301 (1 RCT) | ⊕⊕⊝⊝ Low 2 | RR 0.95 (0.82 to 1.09) | Proportion of invasive cases out of all cancer cases detected by screening | |
| 74 per 100 | 4 fewer per 100 (13 fewer to 7 more) | ||||
| Incremental detection of invasive cancers: cohort studies | 571 (2 cohort studies) | ⊕⊝⊝⊝ Very low 1,3 | RR 1.00 (0.95 to 1.06) | Proportion of invasive cases out of all cancer cases detected by screening | |
| 86 per 100 | 0 fewer per 100 (4 fewer to 5 more) | ||||
| Interval cancer | 72,717 (1 RCT) | ⊕⊕⊕⊕ High | RR 0.50 (0.29 to 0.89) | Study population | |
| 10 per 10,000 | 5 fewer per 10,000 (7 fewer to 1 fewer) | ||||
| Lymph node status | 214 (1 RCT) | ⊕⊕⊕⊝ Moderate 3 | RR 0.53 (0.33 to 0.86) | Proportion of positive lymph node staus among invasive cancer cases | |
| 34 per 100 | 16 fewer per 100 (23 to 5 fewer) | ||||
| False‐positive rate | 70,825 (1 RCT) | ⊕⊕⊕⊕ High | RR 1.43 (1.37 to 1.50) | Proportion of false‐positive results amongst women with no cancer | |
| 86 per 1000 | 37 more per 1000 (32 more to 43 more) | ||||
| False‐negative rate | 354 (1 RCT) | ⊕⊕⊕⊝ Moderate 3 | RR 0.39 (0.23 to 0.66) | Proportion of false‐negative results amongst women with cancer | |
| 23 per 100 | 14 fewer per 100 (8 to 18 fewer) | ||||
| Rate of biopsies | 72,717 (1 RCT) | ⊕⊕⊕⊕ High | RR 2.49 (2.28 to 2.72) | Study population | |
| 18 per 1000 | 27 more per 1000 (23 more to 31 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NR: not reported; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded due to risk of bias in the two retrospective cohort studies (Buchberger 2018; Starikov 2016): in both studies, additional ultrasonography was performed at the explicit request of a radiologist. In the study by Buchberger and colleagues, women with non‐dense breasts were specifically allocated to receive additional ultrasonography. In one of the two studies, no information was provided on familial or genetic breast cancer risk (Starikov 2016). 2Result not statistically significant, downgraded due to imprecision. 3Downgraded due to imprecision.
Summary of findings 2. Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts.
| Mammography + ultrasound compared to mammography alone for breast cancer screening (dense breasts) | |||||
|
Patient or population: breast cancer screening in women at average risk with dense breasts Setting: screening Intervention: mammography + ultrasound Comparison: mammography alone | |||||
| Outcomes after 1‐ to 2‐year follow‐up | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with mammography alone | Risk difference with mammography + ultrasound | ||||
| Incremental cancer detection in women with dense breasts: RCT | 11,390 (1 RCT) | ⊕⊕⊕⊕ High | RR 1.65 (1.0 to 2.72) | Study population | |
| 4 per 1000 | 3 more per 1000 (0 fewer to 7 more) | ||||
| Incremental cancer detection in women with dense breasts: cohort studies | 50,327 (3 cohort studies) | ⊕⊕⊕⊝ Moderate 1 | RR 1.78 (1.23 to 2.56) | Study population | |
| 3 per 1000 | 2 more per 1000 (1 more to 5 more) | ||||
| Incremental detection of invasive cancers | 65 (1 RCT) | ⊕⊕⊝⊝ Low 2 | RR 0.91 (0.67 to 1.24) | Proportion of invasive cases out of all cancer cases detected by screening | |
| 68 per 100 | 6 fewer per 100 (23 fewer to 16 more) | ||||
| Interval cancer | 11,390 (1 RCT) | ⊕⊕⊕⊕ High | RR 0.29 (0.08 to 1.05) | Study population | |
| 2 per 1000 | 1 fewer per 1000 (2 to 0 fewer) | ||||
| False‐positive rate in women with dense breasts | 11,312 (1 RCT) | ⊕⊕⊕⊕ High | RR 1.76 (1.58 to 1.96) | Proportion of false‐positive results amongst women with no cancer | |
| 83 per 1000 | 63 more per 1000 (48 more to 80 more) | ||||
| False‐negative rate in women with dense breasts | 78 (1 RCT) | ⊕⊕⊕⊝ Moderate 3 | RR 0.23 (0.07 to 0.78) | Proportion of false‐negative results amongst women with cancer | |
| 29 per 100 | 23 fewer per 100 (7 to 27 fewer) | ||||
| Biopsy rate in women with dense breasts | 11,390 (1 RCT) | ⊕⊕⊕⊕ High | RR 2.73 (2.24 to 3.34) | Study population | |
| 23 per 1000 | 39 more per 1000 (28 more to 53 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded due to risk of bias in one of the three retrospective cohort studies (Starikov 2016): additional ultrasonography was performed at the explicit request of a radiologist. No information was provided on familial or genetic breast cancer risk. 2Result not statistically significant, downgraded due to imprecision. 3Downgraded due to imprecision.
Summary of findings 3. Mammography in combination with breast ultrasonography versus mammography alone in women with non‐dense breasts.
| Mammography + ultrasound compared to mammography alone for breast cancer screening (non‐dense breasts) | |||||
| Patient or population: breast cancer screening (non‐dense breasts) Setting: screening Intervention: mammography + ultrasound Comparison: mammography alone | |||||
| Outcomes after 1‐ to 2‐year follow‐up | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with mammography alone | Risk difference with mammography + ultrasound | ||||
| Incremental cancer detection for women with non‐dense breasts: RCT | 7823 (1 RCT) | ⊕⊕⊕⊝ Moderate 1 | RR 1.93 (1.01 to 3.68) | Study population | |
| 4 per 1000 | 3 more per 1000 (0 fewer to 10 more) | ||||
| Incremental cancer detection for women with non‐dense breasts: cohort studies | 40,636 (2 cohort studies) | ⊕⊕⊝⊝ Low 2 | RR 1.13 (0.85 to 1.49) | Study population | |
| 4 per 1000 | 1 more per 1000 (1 fewer to 2 more) | ||||
| Interval cancer | 7823 (1 RCT) | ⊕⊕⊕⊕ High | RR 0.22 (0.05 to 1.03) | Study population | |
| 2 per 1000 | 2 fewer per 1000 (0 to 2 fewer) | ||||
| False‐positive rate in women with non‐dense breasts | 7771 (1 RCT) | ⊕⊕⊕⊕ High | RR 1.36 (1.18 to 1.56) | Proportion of false‐positive results amongst women with no cancer | |
| 81 per 1000 | 29 more per 1000 (15 to 45 more) | ||||
| False‐negative rate in women with non‐dense breasts | 52 (1 RCT) | ⊕⊕⊕⊝ Moderate 1 | RR 0.18 (0.04 to 0.74) | Proportion of false‐negative results amongst women with cancer | |
| 39 per 100 | 32 fewer per 100 (10 to 38 fewer) | ||||
| Biopsy rate in women with non‐dense breasts | 7823 (1 RCT) | ⊕⊕⊕⊕ High | RR 2.58 (1.96 to 3.40) | Study population | |
| 18 per 1000 | 28 more per 1000 (17 to 42 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded due to imprecision: absolute risk reduction with wide confidence interval. 2Downgraded due to risk of bias in the two retrospective cohort studies (Buchberger 2018; Starikov 2016): in both studies, additional ultrasonography was performed at the explicit request of a radiologist. In the study by Buchberger and colleagues, women with non‐dense breasts were specifically allocated to receive additional ultrasonography. In one of the two studies, no information was provided on familial or genetic breast cancer risk (Starikov 2016).
Background
Description of the condition
Breast cancer is the most common malignant disease diagnosed in women worldwide, comprising 24.5% of all female cancers in 2020 (Sung 2021). The risk of developing breast cancer increases with age and with certain risk factors, such as dense breasts, a family history of breast or ovarian cancer, and familial breast cancer gene mutations of BRCA1 (BReast CAncer 1, early onset) and BRCA2 (BReast CAncer 2, susceptibility protein). According to the Surveillance, Epidemiology, and End Results (SEER) Cancer Statistics Review 2014 to 2018, the rate of new cases of female breast cancer was 129 per 100,000 women per year, and 20 per 100,000 women died per year (SEER Cancer Statistics). During their lifetime, 12.9% of women will be diagnosed with breast cancer.
Screening with mammography can detect breast cancer at an early stage. Subsequent effective diagnostic pathways and treatment regimens can reduce the burden of breast cancer, most importantly the mortality in women aged 50 to 69 years (Nelson 2016a). A Cochrane Review estimated a relative reduction of mortality from breast cancer of 15%, corresponding to an absolute risk reduction of 0.05% in women aged 50 and older (Gøtzsche 2013). The sensitivity of mammography ranges between 77% and 95%, and the specificity ranges between 94% and 97% (Nelson 2009). The diagnostic accuracy of mammography screening largely depends on the radiographic density of the imaged breasts (Carney 2003). In radiographically dense breasts, non‐calcified breast cancers are more likely to be missed than in fatty breasts. Consequently, some cancers are not detected by mammography screening.
Ultrasonography of the breast is currently not recommended in the screening of women at average risk for breast cancer (CTFPHC 2018; Siu 2016). Most clinical practice guidelines specify ultrasonography of the breast as a supplementary examination for further clarification of ambiguous findings (Albert 2009). The European Guidelines on Quality Assurance in Breast Cancer Screening and Diagnosis do not recommend tailored screening with automated or handheld breast ultrasound in asymptomatic women with high mammographic breast density (European Comission 2021).
Supporters of supplemental ultrasonography to the screening regimen for breast cancer argue that it might be a safe and inexpensive approach to reduce the false‐negative rates of the screening process. However, those opposed are concerned that performing supplemental ultrasonography on women at average risk will also increase the rate of false‐positive findings and can lead to unnecessary biopsies and treatments. Authors of a 2016 systematic review of six observational studies in ultrasonography noted the increased biopsy rate in women with dense breasts and negative mammography who underwent an additional ultrasonography, finding a positive predictive value ranging from 3.0% to 8.3%, meaning that over 90% of positively classified findings were false‐positive (Melnikow 2016).
Description of the intervention
The intervention entails any form of mammography screening (e.g. one view, two views, digital, etc.) with adjunct breast ultrasonography used as a simultaneous screening test. We excluded studies that only included women with negative mammographies because in such a population it would not be possible to compare the efficacy of combining ultrasonography and mammography screening with mammography screening alone. Breast ultrasonography as a diagnostic test following a positive mammogram was not of interest for this systematic review.
To assess the rate of false‐positives, studies that used biopsy as a reference standard were accepted.
How the intervention might work
To increase either sensitivity or specificity, two or more screening tests may be applied in the same individuals. These tests can be used sequentially or simultaneously. Sequential screening tests are applied in a proportion of the population with a specific result of the first screening test. In sequential screening, the post‐test probability of the first screening test becomes the pre‐test probability of the second screening test. The goal of sequential screening is usually to increase sensitivity. By contrast, simultaneous screening applies two (or more) tests to the screened individuals without knowledge of the results of each individual test. The pre‐test probability therefore remains the same for all tests. Because we wanted to evaluate the efficacy of routine breast ultrasonography in addition to mammography screening, we excluded studies that examined only women with a negative mammography result because of the lower yield of cancers detected by ultrasonography after a negative mammogram, resulting in a high number of false‐positives.
Breast ultrasonography is used routinely as a diagnostic measure to distinguish benign from malignant lesions because it can differentiate between cysts and solid tumours and thus lowers the number of indeterminate mammographical findings. A 2015 study found that adding automated breast ultrasonography to screening mammography in a population that included an undefined proportion of women at higher risk for breast cancer resulted in an additional detection of 1.9 cancers per 1000 women screened (95% confidence interval 1.2 to 2.7; P < 0.001) (Brem 2015). Breast ultrasonography as an adjunct screening tool to mammography might therefore also be able to detect cancer lesions that mammography screening misses in a population at average risk of breast cancer.
In women at increased risk for breast cancer, defined by high breast density or other risk factors, several studies have demonstrated that supplemental screening with ultrasonography can increase the detection rates of cancer, particularly in women with dense breasts (Berg 2008; Berg 2012). On mammography, dense breast tissue results in the potential for underlying cancerous and small lesions to be obscured and is an independent risk factor for breast cancer. It is associated with a high risk of interval cancers, that is cancers that become clinically apparent between screening tests (Moshina 2018). Ultrasonography therefore has the potential to detect mammographically occult cancers at an earlier stage and to improve surrogate outcomes such as tumour size and lymph node status, which have been linked to a poor prognosis of breast cancer (Sopik 2018).
Why it is important to do this review
In women with increased risk for breast cancer, adjunct ultrasonography can improve the diagnostic yield of breast cancer screening (Berg 2012). Based on these findings, ultrasonography is sometimes used routinely as an adjunct screening tool in women at average risk. It is unclear whether the use of ultrasonography as an adjunct screening tool in women at average risk corresponds to a reduction in mortality and morbidity (the ultimate goal of any screening programme) or to an increase in screening‐related harms.
Objectives
To assess the comparative effectiveness and safety of mammography in combination with breast ultrasonography versus mammography alone for breast cancer screening for women at average risk of breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
For efficacy and harms we considered randomised controlled trials (RCTs) with either individual or cluster randomisation and any controlled non‐randomised studies with a sample size of at least 500 participants. The only RCT from Japan considered women aged 40 to 49, so for both efficacy and safety, we included cohort studies that considered women up to age 75, for whom international screening recommendations are available due to higher tumour incidence.
We included studies with a follow‐up period of at least one year and that included at least one relevant outcome.
Types of participants
Women between the ages of 40 and 75 years who are at average risk for breast cancer, have not previously had breast cancer, and who participate in a breast cancer screening programme or undergo mammography screening.
We define women at average risk as those who have a lifetime risk of less than 15% or who have dense breasts without any additional risk factors for breast cancer.
We also accepted studies in which 80% of the population matched our age and breast cancer risk inclusion criteria.
Types of interventions
Any form of mammography screening (e.g. one view, two views, digital, tomosynthesis (three‐dimensional (3D)‐mammography), combination of 2D‐ and 3D‐mammography, etc.) with additional breast ultrasonography compared with mammography screening without breast ultrasonography.
Studies that used biopsy as a reference standard were accepted in order to assess the rate of false‐positives and false‐negatives.
Figure 1 depicts the analytic pathway of the research question.
1.
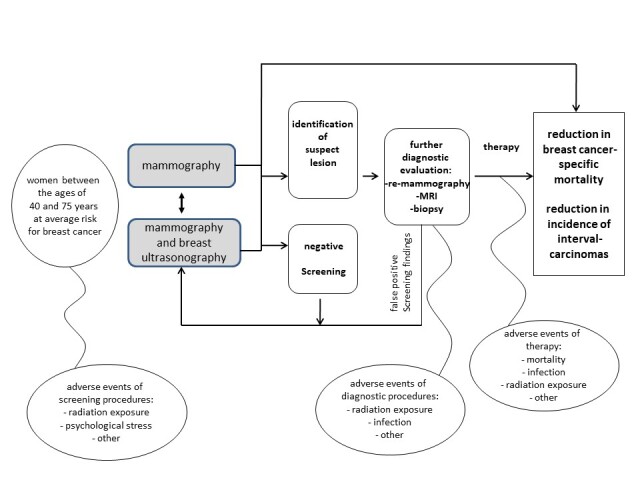
Analytic pathway of the comparative efficacy and risk of harms of mammography screening with and without supplemental ultrasonography.
Types of outcome measures
Primary outcomes
Breast cancer mortality
Secondary outcomes
All‐cause mortality
Incremental cancer detection
Incremental detection of invasive cancers
Interval cancer
Lymph node status
Size of detected cancers
Health‐related quality of life
False‐positive rate
False‐negative rate
Rate of biopsies
Screening‐associated harm (psychological distress, adverse effects caused by subsequent diagnostic or therapeutic interventions, others)
Search methods for identification of studies
Electronic searches
For this 2021 review update, we reassessed the contribution of databases and other resources previously used and removed unnecessary databases and other resources and revised the search strategies. Specifically, for the MEDLINE search strategy, we combined two study design filters: a modified version of the Health Information Research Unit's Therapy filter (Ovid version, best balance of sensitivity and specificity) to identify RCTs (HIRU), and a filter for controlled non‐randomised studies by Waffenschmidt 2020 (Ovid version, the best sensitivity). Additionally, to account for a new technology that had not been included in previous searches, we included new terms for digital breast tomosynthesis in the search strategies.
The new search strategies were designed by our team's information specialist (IK), and peer reviewed by the Cochrane Breast Cancer Group's Information Specialist, prior to the update search being conducted in May 2021.
We searched the following databases:
Cochrane Breast Cancer Group (CBCG) Specialised Register, from 2011 to 10 May 2021;
Cochrane Central Register of Controlled Trials (CENTRAL), from 2011 to Issue 4 of 12, April 2021 (Cochrane Library/Wiley) on 4 May 2021;
Ovid MEDLINE(R) ALL from 2011 to 3 May 2021;
Embase.com (Elsevier) from 2011 to 3 May 2021.
We searched the CBCG Specialised Register on 10 May 2021 using the keywords 'mammography', 'tomosynthesis', 'ultrasonography', and 'screening'. Details of the searched databases and search strategies used by the CBCG Group for the identification of studies and the procedure used to code references are outlined in the CBCG Group's module at breastcancer.cochrane.org/specialised‐register.
We limited all searches to publication dates since 2011 and applied no language restrictions. The search dates overlap with the search strategies of the previous review to account for the new technology of digital breast tomosynthesis, which had not been included in the previous search.
Full search strategies are reported in Appendix 1.
Previous search strategies are reported in Appendix 2 and Appendix 3.
Searching other resources
We manually searched the reference lists of all included studies, pertinent reviews, and background articles on this topic to look for any relevant citations that our searches might have missed.
We searched two clinical trial registries through 4 May 2021 (see Appendix 1 for search strategies):
ClinicalTrials.gov (clinicaltrials.gov/);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (trialsearch.who.it).
Data collection and analysis
Selection of studies
We developed and pilot‐tested literature review forms for abstract and full‐text reviews. For the updated review, each abstract was independently reviewed by two authors on the team (Team: AG, JM, GW, GG, IK, DB, BT, NB, LG). We retrieved full‐text copies of all studies that potentially met the inclusion criteria based on review of the abstract. Studies marked for possible inclusion by either review author underwent full‐text review. In the case of insufficient information to determine inclusion or exclusion, we retrieved the full text of the study and then made the determination. If the needed information in the full‐text article was unclear or missing, we contacted the study authors. Two trained members of the research team independently reviewed each full‐text article for inclusion or exclusion based on the eligibility criteria described above. If both review authors agreed that a study did not meet the eligibility criteria, it was excluded. If the review authors disagreed, they resolved conflicts by discussion and consensus or by consulting a third member of the review team. All results were tracked in an EndNote 20 database (EndNote 20).
Data extraction and management
We designed, pilot‐tested, and used structured data extraction forms to gather pertinent information from relevant articles; this included characteristics of study populations, settings, interventions, comparators, study designs, methods, and results. Trained review authors collected data from each study. A senior review author checked the data for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias, with a third review author consulted in case of disagreement. The aim of our review in relation to RCTs and non‐randomised studies was to assess the effect of assignment to interventions at baseline. To assess the risk of bias in included randomised trials, we used the Cochrane risk of bias tool (RoB 2) as described by the Cochrane Methods Network (Sterne 2019), using a standardised data abstraction form. Outcomes assessed in each study using RoB 2 are listed in Table 4. This tool includes the following domains: randomisation process, deviations from intended interventions, missing data, measurement of the outcomes, and selection of the reported results. RoB 2 includes algorithms with signalling questions for each domain, with the following choices for risk of bias assessment: (1) low risk of bias; (2) some concerns; and (3) high risk of bias.
1. Risk of bias for randomised controlled trial (J‐START): all outcomes.
| Outcomes | Bias arising from the randomisation process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bias due to measurement of outcomes | Bias due to selection of the reported result | Overall |
| Incremental cancer detection | Low | Some concerns | Low | Low | Low | Low |
| Incremental detection of invasive cancer | Low | Some concerns | Low | Low | Low | Low |
| Interval cancer | Low | Some concerns | Low | Low | Low | Low |
| Lymph node status | Low | Some concerns | Low | Low | Low | Low |
| False‐negative rate | Low | Some concerns | Low | Low | Low | Low |
| False‐positive rate | Low | Some concerns | Low | Low | Low | Low |
| Rate of biopsies | Low | Some concerns | Low | Low | Low | Low |
| Justification | Randomisation was performed centrally by an independent research unit. Allocation codes were kept in sealed envelopes and sent to the principal investigator. Baseline characteristics were similarly distributed between the two groups. | Incomplete blinding: because of the nature of the two screening methods, it was not possible to mask participants and study co‐ordinators. To minimise bias, an independent panel unaware of the group assignment performed outcome assessment. | During the course of the study, 0.4% of the initially 72,998 women dropped out at baseline, or data were missing or unknown. The rate of dropouts and missing data was similar in both study groups. | It is unlikely that the detection of cancer cases, usually confirmed by biopsy, in the respective group was influenced by incomplete blinding. | The reported endpoints were published in advance in a study protocol. |
For non‐randomised studies, we assessed risk of bias using the ROBINS‐I tool (Sterne 2016). Outcomes assessed in each study using ROBINS‐I are listed in the Additional tables. This tool includes the following domains: bias due to confounding, participant selection for the study, classification of intervention, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results. Confounding could be caused by an unbalanced distribution of prognostic factors between the groups being compared or by reporting results from two or more rounds of screening, as the pre‐test probability of detecting cancer in the second round is lower if the test result is negative after the first round of screening. ROBINS‐I includes algorithms with signalling questions for each domain, with the following choices for bias risk assessment: (1) low risk of bias; (2) moderate risk of bias; (3) serious risk of bias; and (4) critical risk of bias. We used a standardised data abstraction form to implement ROBINS‐I and assess all relevant domains.
Measures of treatment effect
We extracted data from the original studies to construct 2 x 2 tables. Where multiple studies allowed for quantitative analysis, we calculated the risk ratio (RR) with 95% confidence intervals (CIs) for each outcome using RevMan Web (RevMan Web 2022).
Unit of analysis issues
The unit of analysis was women (not cancer lesions).
Dealing with missing data
We used a modified intention‐to‐treat analysis where data were missing from participants who dropped out of trials before completion. Where data regarding an outcome of interest were not reported, we contacted the study authors to obtain the missing results.
Assessment of heterogeneity
We used the Cochrane Chi2 test (Q‐test) to assess heterogeneity of meta‐analyses. We also used the I2 statistic to estimate the degree of heterogeneity. This measure describes the percentage of total variation across studies that results from heterogeneity rather than chance. We interpreted the importance of any heterogeneity in terms of its magnitude and the direction of effects. We did not use thresholds; instead we adopted the overlapping bands suggested in the Cochrane Handbook for Systematic Reviews of Interventions. For example, we considered an I2 of 0% to 40% as probably not important, 30% to 60% as representing moderate heterogeneity, 50% to 90% as substantial heterogeneity, and 75% to 100% as considerable heterogeneity (Higgins 2019). The result of the Chi2 test is included in the forest plots.
Assessment of reporting biases
We checked trial registries (e.g. ClinicalTrials.gov and WHO ICTRP) to identify completed but unpublished trials.
Due to the small number of studies on breast cancer screening with mammography in combination with breast ultrasound compared with mammography alone, the power of statistical methods to explore publication bias, such as funnel plots, is limited. Because of the low sensitivity to detect publication bias, we did not present funnel plots.
Data synthesis
We analysed the data using RevMan Web (RevMan Web 2022). We pooled data for meta‐analysis where the participant groups were similar and the studies assessed the same treatments with the same comparator and had similar definitions of outcome measures over a similar duration of treatment. The included studies reported unadjusted data. We therefore focused on whether the intervention groups were comparable in terms of breast cancer risk and described whether confounding factors were adequately accounted for in the cohort studies. We used a random‐effects model. We rated the strength of the evidence based on the system developed by the GRADE Working Group.
Subgroup analysis and investigation of heterogeneity
We presented separate results based on breast density. We performed analyses separately for women with dense and non‐dense breasts for the following outcomes: incremental cancer detection rate, incremental invasive cancer detection, interval cancer, lymph node status, false‐positive rate, false‐negative rate, and biopsy rate. We did not present a subgroup analysis, as according to Cochrane Handbook guidelines at least 10 observations should be available for each characteristic modelled.
Sensitivity analysis
When studies at high risk of bias contributed to the evidence, we presented results separately. Separate results associated with a high risk of bias were presented for incremental cancer detection rate in women with mixed populations with dense and non‐dense breasts and for women with dense breasts. In addition, results of the high risk of bias studies were presented for the incremental detection rate of invasive cancer and interval cancer in women with dense breasts.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE system to evaluate the certainty of evidence regarding the comparative effectiveness of screening methods in reducing mortality due to breast cancer or all‐cause mortality, detecting incremental breast cancer and incident interval cancer cases, and determining lymph node status, false‐positive rate, false‐negative rate, and biopsy rate. GRADE criteria for assessing the certainty of the evidence include study limitations, inconsistency of results, indirectness of evidence, imprecision, and publication bias (Balshem 2011).
The GRADE assessment includes four categories: high, moderate, low, and very low certainty evidence. We used GRADEpro GDT to summarise the main findings and to assess the certainty of the evidence (GRADEpro GDT). Results of cohort studies were presented separately in the summary of findings tables.
Results
Description of studies
Results of the search
For the current update of the review, we identified 2164 unique citations from searches and reviews of reference lists. We retrieved 37 full‐text articles for detailed examination. A total of eight studies published in 10 articles (one RCT, J‐START, with secondary analysis of the RCT; two prospective studies (Chough 2020; Giuliano 2013); and five retrospective studies (Buchberger 2018; Chae 2013; Lee 2019; Starikov 2016; Tohno 2013)) met our eligibility criteria. The search results and the flow of the literature for this report are shown in Figure 2.
2.

Study flow diagram.
In the original review, we identified one ongoing RCT (J‐START), which has since become an included study.
Included studies
One RCT, J‐START, with a secondary analysis in 2021, two prospective studies, Chough 2020; Giuliano 2013, and five retrospective cohort studies, Buchberger 2018; Chae 2013; Lee 2019; Starikov 2016; Tohno 2013, with data of altogether 209,207 women assessed the comparative efficacy of mammography in combination with breast ultrasonography versus mammography alone for breast cancer screening. The J‐START, an RCT including 72,717 women, provided the best evidence (J‐START). In a secondary analysis of a subpopulation of the J‐START which included 19,213 women, the results of women with dense and non‐dense breasts were presented separately. Six of the eight studies assessed the sensitivity and specificity of the two screening methods during one screening round. In these studies, a second screening round, if performed, served to detect interval cancers. In the prospective cohort study by Chough 2020 and the retrospective study by Lee 2019, women participated in one, two, or more screening rounds (not specified). The results of the two studies that had screened women more than once are presented separately.
Participants
Participants in the RCT, J‐START, were younger than women included in the cohort studies. In J‐START, ages ranged from 40 to 49, whilst in the cohort studies, the majority of women were between 40 and 70 years old. All studies examined data from asymptomatic women who had undergone breast cancer screening. Six of the eight studies included populations without a personal history of breast cancer; two studies lacked precise population information (Chae 2013; Tohno 2013); and one study did not report the percentage of women with a first‐degree relative who had had breast cancer (Starikov 2016). In the J‐START study, 95.3% of women had no first‐degree relative with breast cancer, 4.6% had one, and 0.1% had more than one first‐degree relative affected by breast cancer (J‐START). In J‐START, 1.3% women had had a benign neoplasm in the past, 2% had breast surgery, and 0.7% had ever had breast inflammation. In the largest retrospective cohort study by Buchberger and colleagues (Buchberger 2018), women with a personal or family history of breast cancer were excluded, whilst in the second‐largest retrospective study by Lee and colleagues (Lee 2019), 13% of the screening examinations were performed on women who had a five‐year breast cancer risk of 2.5% to 3.99%, and 2% on women with a five‐year risk of 3.99% or higher.
The proportion of women with dense breasts in the included studies ranged from 48% to 100%. Three cohort studies were performed exclusively in women with dense breasts (Chae 2013; Chough 2020; Giuliano 2013), and three studies presented separate data for women with dense and non‐dense breasts (Buchberger 2018; J‐START; Starikov 2016).
Interventions
Five studies used digital mammography (Buchberger 2018; Chae 2013; Giuliano 2013; J‐START; Tohno 2013); one study used breast tomosynthesis (Chough 2020); and two studies used automated breast ultrasound (ABUS) in addition to mammography (Chough 2020; Giuliano 2013). One study used either digital mammography alone or in combination with tomosynthesis, and women who were referred to receive additional ultrasonography were examined with ABUS (one‐third) or handheld ultrasonography (Starikov 2016). The second‐largest retrospective study lacked information on the equipment used (Lee 2019).
Outcomes assessed
All studies analysed whether additional cancer cases were detected in women who had mammography screening in combination with ultrasonography compared to mammography screening alone. Five studies reported information about the proportion of interval cancers, the lymph node status of women with breast cancer, and the size of the detected cancers. False‐positive and false‐negative rates of the two screening methods were reported or could be calculated in four studies, and the number of required biopsies as a consequence of screening was reported in three studies. The follow‐up duration of the studies ranged from one to three years.
Excluded studies
We excluded 27 studies. The main reasons for exclusion were study populations that did not meet the eligibility criteria (e.g. women with high risk of breast cancer or women with negative mammography). Reasons for excluding studies after full‐text review are summarised in Characteristics of excluded studies.
Risk of bias in included studies
Detailed risk of bias assessment data are available here.
Risk of bias of randomised controlled trials
We assessed risk of bias for the included RCT with RoB 2 and presented the assessment of risk of bias for the endpoints studied. A risk of bias summary can be found in the corresponding Table 4, Figure 3.
3.
Risk of bias for J‐START.
Overall
Overall, we rated the risk of bias (Figure 3) for incremental cancer detection, incremental invasive cancer detection, interval cancer, lymph node status, size of cancers detected, false‐positive rate, false‐negative rate, and biopsy rate in J‐START as low. The study was methodologically well conducted in most domains, and the likelihood of bias in the results was low (Table 4).
Bias arising from the randomisation process: We judged risk of bias arising from the randomisation process as low for all outcomes assessed. In J‐START, randomisation was performed centrally by an independent research unit. Allocation codes were kept in sealed envelopes and sent to the principal investigator. Baseline characteristics were similarly distributed between the two groups.
Bias due to deviations from intended interventions: We judged risk of bias due to deviations from intended interventions as some concerns for all outcomes assessed. Due to the nature of the two screening methods, it was not possible to mask participants and study co‐ordinators. To minimise bias, an independent panel unaware of the group assignment performed outcome assessment. Due to incomplete blinding, we rated the risk of bias due to deviations from intended interventions as moderate for all outcomes.
Bias due to missing outcome data: We judged risk of bias due to missing outcome data as low for all outcomes assessed. During the course of the study, 0.4% of the initially 72,998 women dropped out at baseline or data were missing or unknown. The rates of dropouts and missing data were similar in both study groups.
Bias due to measurement of outcomes: We judged risk of bias due to measurement of outcomes as low for all reported results. According to our assessment, it is unlikely that the detection of the cancer cases (which is usually confirmed by biopsy) in the respective group was influenced by the incomplete blinding.
Bias due to selection of the reported results: We judged risk of bias due to selection of the reported results as low. The reported endpoints were published in advance in a study protocol.
Risk of bias of non‐randomised controlled trials
We assessed risk of bias for two prospective, Chough 2020; Giuliano 2013, and five retrospective cohort studies, Buchberger 2018; Chae 2013; Lee 2019; Starikov 2016; Tohno 2013, using ROBINS‐I (Sterne 2016).
We have provided a risk of bias assessment for each outcome evaluated as per the tool. The summary and details can be found per outcome in the corresponding tables, as follows.
Incremental cancer detection (Table 5)
Incremental detection of invasive cancer (Table 6)
Interval cancer (Table 7)
Lymph node status (Table 8)
Size of detected cancers (Table 9)
False‐positive rate (Table 10)
False‐negative rate (Table 11)
Rate of biopsies (Table 12)
2. Risk of bias for non‐randomised studies: incremental cancer detection.
| Study | Confounding | Participant selection | Classification of intervention | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported results | Overall |
| Buchberger 2018 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Excluded: women with personal or first‐degree family history | Women with non‐dense breasts received additional ultrasonography per specific radiologist's request. | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chae 2013 | Critical | Critical | Low | Low | Moderate | Low | Low | Serious |
| Potentially included: women with a personal or family history of breast cancer or mutations in breast cancer genes | Women with a family history of breast cancer or who are more health conscious may be more inclined to undergo additional sonography (voluntary self‐financed). | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | Diagnosis and treatment possibly at another hospital, data collection could be incomplete | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chough 2020 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Two rounds of screening, results were presented as the results of a screening round with twice as many women. | No evidence of bias due to selection of participants | Digital breast tomosynthesis, ABUS | No indication for deviations from intended interventions | 14% of women did not get a second screening examination. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Giuliano 2013 | Low | Low | Low | Low | Low | Low | Low | Low |
| Women with dense breasts with no family history of breast cancer or positive BRCA gene | No evidence of bias due to selection of participants | Digital mammography, ABUS | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Lee 2019 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| One to two rounds of screening or more (not specified), data on breast cancer risk were incomplete, it remains unclear whether screening was the indication for the additional ultrasonography for many women. |
No evidence of bias due to selection of participants | No information was provided on whether digital mammography was used or which type of ultrasonography (ABUS, handheld ultrasound) was used. | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Starikov 2016 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Asymptomatic women without a previous history of breast cancer were included, but no information about familial or genetic risk of breast cancer. | Women received additional ultrasonography per specific radiologist's request. | Digital mammography alone or in combination with tomosynthesis, 1/3 automated ABUS, 2/3 handheld ultrasonography | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Tohno 2013 | Critical | Serious | Low | Low | Low | Low | Low | Serious |
| No description of baseline characteristics | Women who did choose an additional ultrasonography differed from women who were screened by mammography only. | Digital mammography, ultrasonography | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting |
ABUS: automated breast ultrasound
3. Risk of bias for non‐randomised studies: incremental detection of invasive cancer.
| Study | Confounding | Participant selection | Classification of intervention | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported results | Overall |
| Buchberger 2018 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Excluded: women with personal or first‐degree family history | Women with non‐dense breasts received additional ultrasonography per specific radiologist's request |
Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chae 2013 | Critical | Critical | Low | Low | Moderate | Low | Low | Serious |
| Potentially included: women with a personal or family history of breast cancer or mutations in breast cancer genes | Women with a family history of breast cancer or who are more health conscious may be more inclined to undergo additional sonography (voluntary self‐financed). | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | Diagnosis and treatment possibly at another hospital, data collection could be incomplete | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chough 2020 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Two rounds of screening, results were presented as the results of a screening round with twice as many women | No evidence of bias due to selection of participants | Digital breast tomosynthesis, ABUS | No indication for deviations from intended interventions | 14% of women did not get a second screening examination. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Giuliano 2013 | Low | Low | Low | Low | Low | Low | Low | Low |
| Women with dense breasts with no family history of breast cancer or positive BRCA gene | No evidence of bias due to selection of participants | Digital mammography, ABUS | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Lee 2019 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| One to two rounds of screening or more (not specified), data on breast cancer risk were incomplete, it remains unclear whether screening was the indication for the additional ultrasonography for many women |
No evidence of bias due to selection of participants | No information was provided on whether digital mammography was used or which type of ultrasonography (ABUS, handheld ultrasound) was used. | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Starikov 2016 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Asymptomatic women without a previous history of breast cancer were included, but no information about familial or genetic risk of breast cancer. | Women received additional ultrasonography per specific radiologist's request. | Digital mammography alone or in combination with tomosynthesis, 1/3 automated ABUS, 2/3 handheld ultrasonography | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting |
ABUS: automated breast ultrasound
4. Risk of bias for non‐randomised studies: interval cancers.
| Study | Confounding | Participant selection | Classification of intervention | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported results | Overall |
| Buchberger 2018 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Excluded: women with personal or first‐degree family history | Women with non‐dense breasts received additional ultrasonography per specific radiologist's request. | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chae 2013 | Critical | Critical | Low | Low | Moderate | Low | Low | Serious |
| Potentially included: women with a personal or family history of breast cancer or mutations in breast cancer genes | Women with a family history of breast cancer or who are more health conscious may be more inclined to undergo additional sonography (voluntary self‐financed). | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | Diagnosis and treatment possibly at another hospital, data collection could be incomplete | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chough 2020 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Two rounds of screening, results were presented as the results of a screening round with twice as many women | No evidence of bias due to selection of participants | Digital breast tomosynthesis, ABUS | No indication for deviations from intended interventions | 14% of women did not get a second screening examination. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Lee 2019 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| One to two rounds of screening or more (not specified), data on breast cancer risk were incomplete, it remains unclear whether screening was the indication for the additional ultrasonography for many women |
No evidence of bias due to selection of participants | No information was provided on whether digital mammography was used or which type of ultrasonography (ABUS, handheld ultrasound) was used. | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting |
ABUS: automated breast ultrasound
5. Risk of bias for non‐randomised studies: lymph node status.
| Study | Confounding | Participant selection | Classification of intervention | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported results | Overall |
| Buchberger 2018 | Low | Moderate | Low | Low | Serious | Low | Low | Serious |
| Excluded: women with personal or first‐degree family history | Women with non‐dense breasts received additional ultrasonography per specific radiologist's request. | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | For 15 cases of mammographically dectected cases, lymph node status was not available. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chough 2020 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Two rounds of screening, results were presented as the results of a screening round with twice as many women | No evidence of bias due to selection of participants | Digital breast tomosynthesis, ABUS | No indication for deviations from intended interventions | 14% of women did not get a second screening examination. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Giuliano 2013 | Low | Low | Low | Low | Low | Low | Low | Low |
| Women with dense breasts with no family history of breast cancer or positive BRCA gene | No evidence of bias due to selection of participants | Digital mammography, ABUS | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Lee 2019 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| One to two rounds of screening or more (not specified), data on breast cancer risk were incomplete, it remains unclear whether screening was the indication for the additional ultrasonography for many women |
No evidence of bias due to selection of participants | No information was provided on whether digital mammography was used or which type of ultrasonography (ABUS, handheld ultrasound) was used. | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting |
ABUS: automated breast ultrasound
6. Risk of bias for non‐randomised studies: size of detected cancers.
| Study | Confounding | Participant selection | Classification of intervention | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported results | Overall |
| Buchberger 2018 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Excluded: women with personal or first‐degree family history | Women with non‐dense breasts received additional ultrasonography per specific radiologist's request. | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chough 2020 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Two rounds of screening, results were presented as the results of a screening round with twice as many women | No evidence of bias due to selection of participants | Digital breast tomosynthesis, ABUS | No indication for deviations from intended interventions | 14% of women did not get a second screening examination. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Giuliano 2013 | Low | Low | Low | Low | Low | Low | Low | Low |
| Women with dense breasts with no family history of breast cancer or positive BRCA gene | No evidence of bias due to selection of participants | Digital mammography, ABUS | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Lee 2019 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| One to two rounds of screening or more (not specified), data on breast cancer risk were incomplete, it remains unclear whether screening was the indication for the additional ultrasonography for many women |
No evidence of bias due to selection of participants | No information was provided on whether digital mammography was used or which type of ultrasonography (ABUS, handheld ultrasound) was used. | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting |
ABUS: automated breast ultrasound
7. Risk of bias for non‐randomised studies: false‐positive rate.
| Study | Confounding | Participant selection | Classification of intervention | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported results | Overall |
| Buchberger 2018 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Excluded: women with personal or first‐degree family history | Women with non‐dense breasts received additional ultrasonography per specific radiologist's request. | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chae 2013 | Critical | Critical | Low | Low | Moderate | low | low | Serious |
| Potentially included: women with a personal or family history of breast cancer or mutations in breast cancer genes | Women with a family history of breast cancer or who are more health conscious may be more inclined to undergo additional sonography (voluntary self‐financed). | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | Diagnosis and treatment possibly at another hospital, data collection could be incomplete | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Giuliano 2013 | Low | Low | Low | Low | Low | Low | Critical | Serious |
| Women with dense breasts with no family history of breast cancer or positive BRCA gene | No evidence of bias due to selection of participants | Digital mammography, ABUS | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | False‐positive results of mammograpy only group not reported. | ||
| Lee 2019 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| One to two rounds of screening or more (not specified), data on breast cancer risk were incomplete, it remains unclear whether screening was the indication for the additional ultrasonography for many women |
No evidence of bias due to selection of participants | No information was provided on whether digital mammography was used or which type of ultrasonography (ABUS, handheld ultrasound) was used. | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting |
ABUS: automated breast ultrasound
8. Risk of bias for non‐randomised studies: false‐negative rate.
| Study | Confounding | Participant selection | Classification of intervention | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported results | Overall |
| Buchberger 2018 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Excluded: women with personal or first‐degree family history | Women with non‐dense breasts received additional ultrasonography per specific radiologist's request. | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Chae 2013 | Critical | Critical | Low | Low | Moderate | Low | Low | Serious |
| Potentially included: women with a personal or family history of breast cancer or mutations in breast cancer genes | Women with a family history of breast cancer or who are more health conscious may be more inclined to undergo additional sonography (voluntary self‐financed). | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | Diagnosis and treatment possibly at another hospital, data collection could be incomplete | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Lee 2019 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| One to two rounds of screening or more (not specified), data on breast cancer risk were incomplete, it remains unclear whether screening was the indication for the additional ultrasonography for many women |
No evidence of bias due to selection of participants | No information was provided on whether digital mammography was used or which type of ultrasonography (ABUS, handheld ultrasound) was used. | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting |
ABUS: automated breast ultrasound
9. Risk of bias for non‐randomised studies: rate of biopsies.
| Study | Confounding | Participant selection | Classification of intervention | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported results | Overall |
| Buchberger 2018 | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Excluded: women with personal or first‐degree family history | Women with non‐dense breasts received additional ultrasonography per specific radiologist's request. | Digital mammography, ultrasonography | Analysis of retrospective data: no deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting | ||
| Lee 2019 | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| One to two rounds of screening or more (not specified), data on breast cancer risk were incomplete, it remains unclear whether screening was the indication for the additional ultrasonography for many women |
No evidence of bias due to selection of participants | No information was provided on whether digital mammography was used or which type of ultrasonography (ABUS, handheld ultrasound) was used. | No indication for deviations from intended interventions | All women analysed. | The biopsy results are unlikely to be biased. | No indication of selective reporting |
ABUS: automated breast ultrasound
Overall, we rated the risk of bias for incremental cancer detection, incremental detection of invasive cancer, interval cancer, lymph node status, size of detected cancer, false‐positive rate, false‐negative rate, and rate of biopsies as moderate to serious in most studies. We assessed the prospective study by Giuliano and colleagues as low risk for cancer detection and serious risk concerning false‐positive results of breast cancer screening. The main reason for downgrading risk of bias was due to a lack of description of possible confounders, that confounding factors were not known for the whole population, or due to the selection of participants into the study which may have been influenced by suspicious findings or other prognostic factors.
Bias due to confounding: We rated the risk of bias due to confounding for the reported outcomes as low in two studies (Buchberger 2018, Giuliano 2013), moderate in three studies (Chough 2020; Lee 2019; Starikov 2016), and critical in two studies (Chae 2013; Tohno 2013). As the reported results could be influenced by prognostic factors in the respective groups, we classified the risk of bias due to confounding similarly for all endpoints depending on the study. In the study by Chough and colleagues and the study by Lee and colleagues, women participated in one or more rounds of screening (Lee 2019: not specified). In the study by Chough and colleagues, all the screenings were combined into a single number without differentiating whether one or two screening examinations took place. In the retrospective cohort study by Lee and colleagues, 3386 women who received 6081 screening examinations with mammography and same‐day ultrasonography were matched with 15,176 women who underwent 30,062 screening exams with mammography alone. Because of the lower pre‐test probability of detecting cancer in the second round if the test result is negative after the first screening round, we classified the risk of bias due to confounding as moderate. In addition, in the study by Lee and colleagues, 13% of the screening examinations were performed on women who had a five‐year breast cancer risk of 2.5% to 3.99%, and 2% on women with a five‐year risk of 3.99% or higher. However, data on breast cancer risk were only available for 92% of the screening examinations. In the study by Starikov (Starikov 2016), asymptomatic women without a previous history of breast cancer were included, but no information about familial or genetic risk of breast cancer was provided. We considered bias due to confounding as critical for two retrospective studies (Chae 2013; Tohno 2013). Chae and colleagues did include asymptomatic women, but did not ascertain whether the women screened included women with a personal or family history of breast cancer or women with mutations in breast cancer genes, who may be more inclined to request additional ultrasonography. Tohno and colleagues included women who attended the institution for the purpose of breast cancer screening but did not describe any population inclusion criteria, so it remains unclear whether confounding factors were present that should have been considered.
Bias in participant selection for the study: We rated the risk of bias due to participant selection for the study for the reported outcomes as low in two prospective studies (Chough 2020; Giuliano 2013), moderate in three retrospective studies (Buchberger 2018; Lee 2019; Starikov 2016), and critical, Chae 2013, or serious, Tohno 2013, in two retrospective studies. As the reported results could be influenced by the selective choice of participants into the respective groups, we classified the risk of bias similarly for all endpoints depending on the study. In two studies, supplemental ultrasonography was performed per specific request of a radiologist (Buchberger 2018; Starikov 2016). In the largest retrospective study, all women with dense breasts received additional ultrasonography, and women with non‐dense breasts received additional ultrasonography based on a radiologist's referral (Buchberger 2018). Women with non‐dense breasts may have been preferentially referred for ultrasonography if mammography showed suspicious or unclear findings. In the retrospective study by Lee and colleagues, it was not verified for a large proportion of women whether screening was the indication for the additional ultrasonography examination (Lee 2019). Data for the ultrasonography cohort were obtained from two registries, of which the screening indication was verified in one registry for 13.7% (728 of 5728) of the examinations. In the second registry, all data were checked and a screening indication for additional ultrasonography was confirmed for 77.9% of the reports. In the other two retrospective studies with a high risk of bias (Chae 2013; Tohno 2013), it remained unclear if women who did choose to undergo an additional ultrasonography differed from women who were screened by mammography only.
Bias in classification of intervention: We rated the risk of bias due to the classification of intervention for the reported outcomes as low in six of the seven cohort studies that described the examinations in the intervention and control groups. Lee 2019 did not provide any information on whether digital mammography was used, or which type of ultrasonography (ABUS, handheld ultrasound) was used. We therefore rated the risk of bias as moderate.
Bias due to deviations from intended interventions: No deviation from the predicted intervention was reported in any of the studies. We therefore rated the risk of bias as low for the reported outcomes.
Bias due to missing data: We rated the risk of bias due to missing data for the reported outcomes as moderate in two cohort studies, Chae 2013; Chough 2020, and as low for most of the outcomes in the five remaining cohort studies. For the outcome lymph node status, we rated the risk of bias due to missing data as serious in Buchberger 2018. In the study by Buchberger and colleagues, lymph node status was not available for 15 mammographically detected cases. In the prospective study by Chough and colleagues (Chough 2020), during the study period of up to three years with annual screenings, 14% of women did not get a second screening examination. As the occurrence of breast cancer is a rare event, cases could have been missed. In Chae 2013, the authors noted that patients may have chosen to be diagnosed and treated in another hospital, and that the cancer registry may have been incomplete.
Bias in measurement of outcomes: As histopathology is the gold standard to identify breast cancer, it is unlikely that the breast cancer rate measurement was influenced by the knowledge of which screening intervention had been performed. We rated the risk of bias for this domain as low for all reported outcomes. In the study by Giuliano and colleagues, the pathologists were not aware of participant assignment and conducted their assessment in a blinded manner (Giuliano 2013).
Bias in selection of the reported results: In the prospective cohort study by Giuliano and colleagues (Giuliano 2013), no data were available on the false‐positive rate of the group of women who underwent mammography alone. A request to the authors of the studies to add missing data has remained unanswered. We therefore rated the risk of bias due to selective reporting as critical for the outcome false‐positive rate reported by Giuliano 2013.
Effects of interventions
See: Table 1; Table 2; Table 3
Breast cancer mortality
No studies reported on breast cancer mortality.
All‐cause mortality
No studies reported on all‐cause mortality.
Incremental cancer detection in mixed populations of women with dense and non‐dense breasts
In J‐START, two more breast cancers cases per 1000 women were detected in the group screened with a combination of mammography and ultrasonography compared with mammography alone (95% confidence interval (CI) 1 to 3 more). During a study period of one year, breast cancer was detected in five of 1000 women (184 of 36,752) screened by mammography and ultrasonography compared with three of 1000 women (117 of 35,965) screened by mammography alone (risk ratio (RR) 1.54, 95% CI 1.22 to 1.94; Analysis 1.1). The certainty of the evidence based on the result of the high‐quality RCT was high (Table 1).
1.1. Analysis.

Comparison 1: Mammography in combination with breast ultrasonography versus mammography alone in mixed populations of women with dense and non‐dense breasts, Outcome 1: Incremental cancer detection
Consistent with the J‐START, three cohort studies with a mixed population of women with dense and non‐dense breasts and a total of 88,972 participants showed higher detection rates for the combination of mammography and ultrasonography compared with mammography alone (Analysis 1.1.2) (Buchberger 2018; Starikov 2016; Tohno 2013). The pooled risk ratio of two retrospective cohort studies showed that breast cancer was detected in 0.42% (152 of 35,902) of women who received mammography and ultrasonography compared to 0.35% (167 of 47,567) in the mammography alone group (RR 1.35, 95% CI 0.92 to 1.98; I2 = 32%) (Buchberger 2018; Starikov 2016). This corresponds to one more cancer case detected per 1000 women (from 0 fewer to 3 more) with mammography screening and additional ultrasonography than with mammography alone. Adding the study of Tohno and colleagues, with a serious risk of bias, resulted in a similar risk ratio (RR 1.43, 95% CI 0.99 to 2.05; I2 = 28%). However, due to the methodological limitations of cohort studies, we assessed the certainty of the evidence as low.
Lee 2019 studied women who participated in one or more rounds of screening and found no statistically significant difference in cancer detection between the two screening interventions (0.54% (33 of 6081) versus 0.55% (165 of 30,062); RR 0.99, 95% CI 0.68 to 1.44; Analysis 1.1.4). For the outcome incremental cancer detection rate, we classified the risk of bias as moderate for three of the cohort studies (Buchberger 2018; Lee 2019; Starikov 2016), and serious for one cohort study (Tohno 2013).
Incremental cancer detection in women with dense breasts
The secondary analysis of J‐START with 11,390 women with dense breasts showed that the combination of mammography and ultrasonography detected three more breast cancer cases per 1000 women screened (95% CI from 0 fewer to 7 more) than mammography alone (0.71 versus 0.43%; RR 1.65, 95% CI 1.00 to 2.72). The certainty of evidence based on the result of the methodologically well‐conducted secondary analysis of the J‐START study was high (Table 2).
Four cohort studies provided results of 71,191 women with dense breasts (Buchberger 2018; Chae 2013; Giuliano 2013; Starikov 2016). We pooled the results of three cohort studies with a low or unclear risk of bias for key domains, specifically confounding bias and study participant selection (Buchberger 2018; Giuliano 2013; Starikov 2016). The meta‐analysis showed that amongst women with dense breasts, the combination of mammography and ultrasonography detected two more cancer cases (1 to 5 more) per 1000 screened women than mammography alone (0.45 versus 0.30%; RR 1.78, 95% CI 1.23 to 2.56; I2 = 23%; Analysis 2.1.2). Adding the study of Chae and colleagues, rated as a high risk of bias (Analysis 2.2.1), resulted in a higher risk ratio and higher heterogeneity (RR 2.17, 95% CI 1.31 to 3.60; I2 = 61%). It remains unclear whether the study population of Chae and colleagues included women with a personal or family history of breast cancer. The prospective cohort study by Chough 2020 was presented separately, as women participated in one or two screening examinations, resulting in a lower pre‐test probability of detecting cancer at the second screening round (Analysis 2.2.2). Due to methodological limitations, we assessed the certainty of the evidence of the pooled result of the three cohort studies as moderate (Table 2).
2.1. Analysis.

Comparison 2: Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts, Outcome 1: Incremental cancer detection rate in women with dense breasts
2.2. Analysis.

Comparison 2: Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts, Outcome 2: Incremental cancer detection rate in women with dense breasts: cohort studies with a serious risk of bias or one or more screening rounds
Incremental cancer detection in women with non‐dense breasts
Three studies provided data for 48,459 women with non‐dense breasts (Buchberger 2018; J‐START; Starikov 2016). Regarding cancer detection, the secondary analysis of J‐START with data from 7823 women with non‐dense breasts showed that the combination of mammography and ultrasonography detected three more breast cancer cases per 1000 women screened (95% CI from 0 fewer to 10 more) than mammography alone (0.69 versus 0.36%; RR 1.93, 95% CI 1.01 to 3.68). Due to the imprecision of the result, we assessed the certainty of evidence for the result of the secondary analysis of the J‐START for women with non‐dense breasts as moderate (Table 3).
In contrast to the J‐START result, the two cohort studies of 40,636 women showed no statistically significant difference in detected cancer cases between the combination of mammography screening and ultrasonography and mammography alone (RR 1.13, 95% CI 0.85 to 1.49, I2 = 0%; Analysis 3.1) (Buchberger 2018; Starikov 2016). Due to methodological limitations, we assessed the certainty of the evidence of the pooled result of the two cohort studies as low (Table 3).
3.1. Analysis.

Comparison 3: Mammography in combination with breast ultrasonography versus mammography alone in women with non‐dense breasts, Outcome 1: Incremental cancer detection rate for women with non‐dense breasts
Incremental detection of invasive cancer
Seven of the included studies reported the proportion of invasive cancer (Buchberger 2018; Chae 2013; Chough 2020; Giuliano 2013; J‐START; Lee 2019; Starikov 2016). In J‐START, 71% of all tumours identified by screening were classified as invasive (J‐START). In the mammography in combination with ultrasonography group, 69.6% (128 of 184) of cancer cases were invasive compared to 73.5% (86 of 117) with mammography alone (RR 0.95, 95% CI 0.82 to 1.09). This corresponds to 37 fewer invasive cases per 1000 breast cancer cases (from 132 fewer to 66 more) detected by mammography and ultrasonography compared to mammography alone, with no statistically significant difference between the two screening methods. Due to imprecision, the certainty of the evidence is low that there was no statistically significant difference between the two screening interventions in terms of the proportion of invasive cases out of all cancer cases detected by screening.
Consistent with the results of the J‐START trial, a meta‐analysis of two cohort studies with a mixed population of women with dense and non‐dense breasts showed that the difference between the two screening interventions in terms of the proportion of invasive cases amongst screen‐detected cancers was not statistically significant (Analysis 1.2.2; Table 1; Buchberger 2018; Starikov 2016). Due to the methodological limitations of cohort studies and the imprecise result, we assessed the certainty of evidence of the differences between the two screening methods in the detection of invasive cancer as very low. The study by Lee and colleagues, with one or more rounds of screening, found a lower rate of invasive cancer in women screened with mammography and ultrasound compared to mammography alone, but the difference did not reach statistical significance (Analysis 1.2.3).
1.2. Analysis.

Comparison 1: Mammography in combination with breast ultrasonography versus mammography alone in mixed populations of women with dense and non‐dense breasts, Outcome 2: Incremental detection of invasive cancers
In the secondary analysis of J‐START, the proportion of invasive cancer amongst screen‐detected cancer cases of women with dense breasts was 70.8% (46 of 65), whereas 61.0% (25 of 41) of cancer cases amongst women with non‐dense breasts were invasive, with no statistically significant difference between the two screening interventions (Analysis 2.3.1; Analysis 3.2).
2.3. Analysis.

Comparison 2: Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts, Outcome 3: Incremental detection of invasive cancers in women with dense breasts
3.2. Analysis.

Comparison 3: Mammography in combination with breast ultrasonography versus mammography alone in women with non‐dense breasts, Outcome 2: Incremental detection of invasive cancers in women with non‐dense breasts
Three cohort studies were exclusively performed in women with dense breasts (Chae 2013; Chough 2020; Giuliano 2013). In the prospective study by Giuliano and colleagues, all women had dense breasts and all detected cancer cases were invasive (Analysis 2.3.2). We assessed the results of Chae 2013 as at serious risk of bias; these are presented in Analysis 2.3.3. Women in the prospective study by Chough 2020 participated in one to two screening rounds; the results are presented in Analysis 2.3.4.
Interval cancer
Five studies reported the proportion of interval cancer cases (Buchberger 2018; Chae 2013; Chough 2020; J‐START; Lee 2019). In J‐START, 0.05% (18 of 36,752) of interval cancers occurred in the group screened by mammography in combination with ultrasonography, compared with 0.10% (35 of 35,965) in the group screened by mammography alone (P = 0.034; J‐START). Eighty‐nine per cent (16 of 18) of all interval cancers in the group receiving mammography and additional ultrasonography were invasive compared to 77% (27 of 35) in the group receiving mammography alone (J‐START). The certainty of the evidence based on the result of the high‐quality RCT was high. In the largest retrospective study (Buchberger 2018), screening with a combination of mammography and ultrasonography was performed in all women whose data were evaluated in the study. In a total of 66,680 women, 28 interval cancers occurred (0.04%). In the second largest retrospective cohort study by Lee and colleagues, with one or more screening rounds and 36,143 exams in 18,562 women, fewer interval cancer cases occurred in the mammography plus ultrasonography group, with no statistically significant difference between the two screening methods (Analysis 1.3.2). In the group of women who received mammography in combination with ultrasonography, 1.5 interval cancer cases per 1000 screening examinations were reported, compared to 1.9 in the group with mammography alone (RR 0.67, 95% CI 0.33 to 1.37). Due to the methodological limitations of cohort studies, we assessed the certainty of the evidence as low.
1.3. Analysis.

Comparison 1: Mammography in combination with breast ultrasonography versus mammography alone in mixed populations of women with dense and non‐dense breasts, Outcome 3: Interval cancers
In the secondary analysis of the J‐START (Harada‐Shoji 2021), which examined data from women with dense and non‐dense breasts separately, the proportion of interval cancers was lower when mammographic screening was performed in combination with ultrasonography compared to the group that received only mammographic screening, regardless of breast density (Analysis 2.4.1; Analysis 3.3). In women with dense breasts, one fewer per 1000 (from 2 to 0 fewer) interval cancer cases occurred in the group screened with mammography and ultrasonography than in group with mammography alone (0.1% (3 of 5797) versus 0.2% (10 of 5593); RR 0.29, 95% CI 0.08 to 1.05).
2.4. Analysis.

Comparison 2: Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts, Outcome 4: Interval cancer in women with dense breasts
3.3. Analysis.

Comparison 3: Mammography in combination with breast ultrasonography versus mammography alone in women with non‐dense breasts, Outcome 3: Interval cancer in women with non‐dense breasts
In the Chough 2020 study, in which women participated in one to two screening rounds, no interval cancer cases occurred. Chae 2013, with a serious risk of bias, reported no interval cancer cases in women with dense breasts screened by mammography and ultrasonography, and five cases in the mammography only group (Analysis 2.4.2; 0% (0 of 8359) versus 0.04% (5 of 12,505); RR 0.14, 95% CI 0.01 to 2.46).
Lymph node status in women with screen‐detected breast cancer
The proportion of women with cancer and a positive lymph node status varied in five studies, ranging from 0 to 25% (Buchberger 2018; Chough 2020; Giuliano 2013; J‐START; Lee 2019). J‐START reported that a numerically higher proportion of women with invasive cancer that had been detected by mammography only had a positive lymph node status. Amongst women with invasive cancer that had been detected with mammography screening in combination with ultrasonography, 18% (23 of 128) had a positive lymph node status, compared to 34% (29 of 86) detected by mammography alone (RR 0.53, 95% CI 0.33 to 0.86; Analysis 1.4.1). Due to the imprecise result, we assessed the certainty of the evidence as moderate.
1.4. Analysis.

Comparison 1: Mammography in combination with breast ultrasonography versus mammography alone in mixed populations of women with dense and non‐dense breasts, Outcome 4: Lymph node status in women with screen‐detected breast cancer
No statistically significant differences in the proportion of women with positive lymph nodes amongst screen‐detected cancers were found between the two screening interventions in the cohort studies (Buchberger 2018; Chough 2020; Giuliano 2013; Lee 2019). Due to the methodological limitations of cohort studies and the imprecise result, we assessed the certainty of evidence regarding the lymph node status of women with screen‐detected cancer as very low. In the retrospective study by Lee and colleagues (Lee 2019), with one or more screening rounds, the proportions of women with breast cancer (non‐invasive and invasive) who had positive lymph nodes were similar: 14.6% (6 of 41) versus 16.4% (36 of 219; RR 0.89, 95% CI 0.40 to 1.98; Analysis 1.4.3). In the study by Buchberger, lymph node status was not available for 15 mammographically detected cases. The proportion of women with positive lymph nodes amongst identified cancer cases is presented in Analysis 1.4.2. We assessed risk of bias for the outcome as serious.
Separate data for women with dense and non‐dense breasts are presented in Analysis 2.5 and Analysis 3.4, respectively. These data include a few cases with positive lymph nodes. The lowest proportions of invasive cancer cases with positive lymph node status were reported in two prospective studies. Giuliano and colleagues reported that 2% (1 of 42) of women with breast cancer screened by mammography and ultrasonography had positive lymph nodes compared to 5% (1 of 19) in the mammography only group. In the study by Chough and colleagues, women with dense breasts underwent one to two screening rounds, with all cancer cases (8 of 598) being node‐negative.
2.5. Analysis.

Comparison 2: Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts, Outcome 5: Lymph node status in women with screen‐detected breast cancer
3.4. Analysis.

Comparison 3: Mammography in combination with breast ultrasonography versus mammography alone in women with non‐dense breasts, Outcome 4: Lymph node status in women with screen‐detected breast cancer
Size of detected cancers
Five studies reported tumour sizes (Buchberger 2018; Chough 2020; Giuliano 2013; J‐START; Lee 2019). In J‐START, the mean size of 128 invasive tumours was 15.3 mm (standard deviation (SD) 12.6) in the group screened by mammography and additional ultrasonography compared to 15.1 mm (SD 8.7) of 86 invasive tumours in the mammography alone group (mean difference 0.2, 95% CI −2.65 to 3.05).
All but one prospective cohort study, Giuliano 2013, showed that tumour sizes were comparable irrespective of whether screening was done with mammography and ultrasonography or with mammography alone (Table 13).
10. Size of detected cancers across studies.
| Study | Mammography + ultrasonography | Mammography | Mean difference, P value |
| J‐START 2016 | Mean: 15.3 mm (SD 12.6) in 128 invasive tumours | Mean: 15.1 mm (SD 8.7) in 86 invasive tumours | MD 0.2, 95% CI −2.65 to 3.05 |
| Buchberger 2018 | Median: 14 mm (range: 3 to 32 mm, 25% percentile: 10 mm, 75% percentile: 16 mm) | Median: 14 mm (range: 1 to 41 mm, 25% percentile: 10 mm, 75% percentile: 20 mm) | P = 0.63 |
| Chough 2020 | 0.4 to 1.2 cm | 0.8. to 1.2 cm | Not available |
| Giuliano 2013 | Mean: 14.3 mm | Mean: 21.3 mm | Not available |
| Lee 2019 | ≤ 20 mm: 13 of 20 (65%) > 20 mm: 7 of 20 (35%) | ≤ 20 mm: 96 of 140 (68.6%) > 20 mm: 44 of 140 (31.4%) | Not available |
CI: confidence interval; MD: mean difference; SD: standard deviation
In the prospective study by Giuliano and colleagues (Giuliano 2013), the invasive tumours detected by mammography had a mean size of 21.3 mm, whilst the size of the tumours in the group with additional ultrasound was smaller, measuring 14.3 mm on average (SD not available, no P value). The majority of cancer cases in the group with additional ultrasonography were stage Ia tumours (83%: 35 of 42).
Health‐related quality of life
No studies reported on health‐related quality of life in the respective screening intervention and control groups.
False‐positive rate in mixed populations of women with dense and non‐dense breasts
False‐positive rates in mixed populations could be calculated or were provided in three studies (J‐START and two retrospective studies, Buchberger 2018 and Lee 2019).
J‐START reported a higher proportion of false‐positive results in women screened by mammography and additional ultrasonography than in women screened by mammography alone. Amongst women with no cancer who underwent mammography screening with additional ultrasonography examination, the proportion of false‐positive results was 12.3% (4427 of 35,847) compared with 8.6% (3015 of 34,978) with mammography screening alone (RR 1.43, 95% CI 1.37 to 1.50). The certainty of the evidence based on the result of the high‐quality RCT was high.
The two retrospective cohort studies reported higher false‐positive rates amongst women screened with mammography and ultrasonography than with mammography alone. Due to the methodological limitations of cohort studies and heterogeneous results, we assessed the certainty of evidence regarding false‐positive rates of the two screening methods based on the results of the cohort studies as very low. The largest retrospective study, Buchberger 2018, showed a lower proportion of false‐positive results than J‐START, though the proportion of detected cancer cases was lower. In the study by Buchberger and colleagues, women who were invited annually or biannually received mammography screening in combination with ultrasonography. About 19 per 1000 women had an intermediate mammogram at six months. It can be assumed that the intermediate mammograms at 6 months were used to calculate the specificity of screening at 12 months follow‐up because the specificity was much higher (calculated: 96.0% to 98.7%) than in J‐START (87.8% to 91.4%). Amongst women who were screened by mammography and ultrasonography, the proportion of false‐positives was 3.0% (1959 of 66,382) compared to 2.7% (1760 of 66,382) with mammography alone (RR 1.11, 95% CI 1.04 to 1.19; Analysis 1.5.2).
1.5. Analysis.

Comparison 1: Mammography in combination with breast ultrasonography versus mammography alone in mixed populations of women with dense and non‐dense breasts, Outcome 5: False‐positive rate: proportion of false‐positive results amongst women with no cancer
Consistent with the results of J‐START, the second largest retrospective study by Lee 2019 reported that the risk of receiving a false‐positive result was 2.2 times higher with screening by mammography in combination with ultrasonography when compared to women who received mammography screening alone (52.0 versus 22.2 per 1000 screens; RR 2.23, 95% CI 1.93 to 2.58). The false‐positive rate when the two screening methods were applied is presented in Analysis 1.5.3.
False‐positive rate in women with dense and non‐dense breasts
Calculations based on numerical data from the secondary analysis of the J‐START showed that in women with dense breasts who had no cancer, the risk of receiving a false‐positive result was 1.76 times higher (14.6% versus 8.3%; RR 1.76, 95% CI 1.58 to 1.96) in the mammography in combination with ultrasonography group compared to mammography screening alone (Analysis 2.6). In women with non‐dense breasts, the risk of receiving a false‐positive result was 1.36 times higher (11.0% versus 8.1%; RR 1.36, 95% CI 1.18 to 1.56; Analysis 3.5). The certainty of the evidence based on the result of the high‐quality RCT was high.
2.6. Analysis.

Comparison 2: Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts, Outcome 6: False‐positive rate in women with dense breasts: proportion of false‐positive results amongst women with no cancer
3.5. Analysis.

Comparison 3: Mammography in combination with breast ultrasonography versus mammography alone in women with non‐dense breasts, Outcome 5: False‐positive rate in women with non‐dense breasts: proportion of false‐positive results amongst women with no cancer
Two cohort studies found higher false‐positive rates in women with dense breasts screened with mammography and ultrasound than with mammography alone (Buchberger 2018; Chae 2013). Due to the hetereogenous results, risk of bias, and methodological limitations of cohort studies, we assessed the certainty of evidence of differences between the two screening methods in terms of the rate of false‐positive results in women with dense breasts to be very low. In the largest retrospective study by Buchberger 2018, amongst women with dense breasts who were screened by mammography and ultrasonography, the proportion of false‐positives was 2.1% (666 of 31,822), compared to 1.2% (385 of 31,822) with mammography alone (Analysis 2.6.2). As previously mentioned, false‐positive rates in the retrospective cohort study by Buchberger were lower than in the RCT because intermediate mammograms may have been included in the calculation of specificity. The retrospective study by Chae and colleagues, with a serious risk of bias, also reported higher false‐positive rates amongst women with dense breasts who were screened with combined mammography and ultrasonography than with mammography alone (5.2% (432 out of 8335) versus 4.1% (517 out of 12,494). The prospective study by Giuliano and colleagues only reported the false‐positive results of mammography screening in combination with ultrasonography, finding that 0.3% (10 out of 3334 women with no cancer) had a positive test result but no cancer (Giuliano 2013).
In women with non‐dense breasts, the largest retrospective cohort study, Buchberger 2018, showed that the proportion of false‐positive results was similar between the two screening methods (3.7% (1293 of 34,560) versus 4.0% (1375 of 34,560); Analysis 3.5.2). In the study by Buchberger and colleagues, women with non‐dense breasts were referred at the specific request of a radiologist, therefore in women with non‐dense breasts, suspicious or unclear mammography results or other factors could have led to additional ultrasonography being performed.
False‐negative rate in mixed populations of women with dense and non‐dense breasts
False‐negative rates in mixed populations could be calculated or were provided in three studies (J‐START and two retrospective studies, Buchberger 2018 and Lee 2019). J‐START and the largest retrospective study by Buchberger showed a lower risk of false‐negative results in women who underwent mammography screening in combination with ultrasonography. In J‐START, 202 women in the group screened with a combination of mammography and ultrasonography had cancer, of which 8.9% (18 of 202) were not detected by screening and were classified as false‐negatives. Amongst women with cancer who underwent mammography screening, 23% (35 of 152) were misclassified as not having cancer (RR 0.39, 95% CI 0.23 to 0.66). Due to the width of the confidence interval, the certainty of evidence based on the result of the J‐START was moderate (Table 1). Consistent with the J‐START study, a similar proportion of false‐negatives amongst screen‐detected cancers was observed in women undergoing mammography screening in combination with ultrasound or mammography alone in the study by Buchberger and colleagues (9.4% (28 out of 298) compared to 21.5% (64 out of 298); RR 0.44, 95% CI 0.29 to 0.66; Analysis 1.6). The retrospective study by Lee 2019, in which women participated in one or more screening rounds, reported no statistically significant difference between the two screening methods (1.5 versus 1.9 per 1000 screenings; RR 0.67, 95% CI 0.33 to 1.37). The percentage of the false‐negative rate when the two screening methods were applied is presented in Analysis 1.6. Due to the methodological limitations and the inconsistent results, we assessed the certainty of evidence based on the cohort study by Lee and colleagues showing no statistically significant difference between the two screening methods in terms of the proportion of false‐negative results in women with cancer to be very low.
1.6. Analysis.

Comparison 1: Mammography in combination with breast ultrasonography versus mammography alone in mixed populations of women with dense and non‐dense breasts, Outcome 6: False‐negative rate: proportion of false‐negative results amongst women with cancer
False‐negative rate in women with dense and non‐dense breasts
J‐START and the largest study, Buchberger 2018, analysed women with dense and non‐dense breasts separately. Chae 2013 only considered women with dense breasts.
A secondary analysis of a subpopulation of the J‐START study showed that women with and without dense breasts had a similarly higher risk of a false‐negative result when examined with mammography alone (Analysis 2.7.1; Analysis 3.6.1). In J‐START, 44 women with dense breasts in the group screened with a combination of mammography and ultrasonography had cancer of which 6.8% (3 of 44) were not detected by screening and were classified as false‐negatives. Amongst women with dense breasts who had cancer and underwent mammography screening alone, 29.4% (10 of 34) were misclassified as not having cancer (RR 0.23, 95% CI 0.07 to 0.78). Due to the width of the confidence interval, the certainty of evidence based on the result of the J‐START was moderate (Table 2). Consistent with J‐START, in the two cohort studies, Buchberger 2018; Chae 2013, with a total of 131 women with dense breasts who had cancer, the risk of false‐negative results was lower with the combination of mammography and ultrasonography compared with mammography screening alone (Analysis 2.7.2).
2.7. Analysis.

Comparison 2: Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts, Outcome 7: False‐negative rate in women with dense breasts: proportion of false‐negative results amongst women with cancer
3.6. Analysis.

Comparison 3: Mammography in combination with breast ultrasonography versus mammography alone in women with non‐dense breasts, Outcome 6: False‐negative rate in women with non‐dense breasts: proportion of false‐negative results amongst women with cancer
Similarly, J‐START showed that the risk of false‐negative results amongst women with non‐dense breasts who had cancer was lower in the group screened with a combination of mammography and ultrasonography than in women who underwent mammography screening (6.9% (2 of 29) versus 39.1% (9 out of 23) were misclassified as not having cancer; RR 0.18, 95% CI 0.04 to 0.74; Analysis 3.6.1). Due to the width of the confidence interval, the certainty of evidence based on the results of the J‐START was moderate (Table 3). Data from the study by Buchberger and colleagues also showed that false‐negative rates in women with non‐dense breasts who had cancer were lower with the combination of mammography and ultrasonography than with mammography alone (5.0% (10 out of 202) versus 13.4% (27 out of 202)).
Due to the methodological limitations and the heterogeneous results, we assessed the certainty of evidence based on the results of the cohort studies as very low.
Rate of biopsies
The J‐START and two retrospective studies reported the proportion of biopsies in both groups (Buchberger 2018; J‐START; Lee 2019). Analogous to the rate of false‐positive results, the proportion of biopsies rate was higher in women who were screened with mammography in combination with ultrasonography compared to mammography alone. In J‐START, 4.5% (1665 of 36,752) of women who received mammography screening and additional ultrasonography underwent a biopsy, compared to 1.8% (655 of 35,965) with mammography only (RR 2.49, 95% CI 2.28 to 2.72). The certainty of the evidence based on the results of the high‐quality RCT was high.
Consistent with this result, both retrospective studies found that the risk of a biopsy after screening was higher when an additional ultrasound was performed. In the retrospective study by Lee and colleagues (Lee 2019), the proportion of biopsies was 57.4 per 1000 screens in the combined mammography and ultrasonography group versus 27.7 per 1000 screens in the mammography only group (RR 2.05, 95% CI 1.79 to 2.34; Analysis 1.7.3). In the study by Lee and colleagues, 15% of the screenings were done on women who had a five‐year breast cancer risk of 2.5% or greater. In the retrospective study by Buchberger and colleagues (Buchberger 2018), biopsy rates were lower than in the J‐START and in the study by Lee and colleagues (mammography plus ultrasonography: 0.9% (623 of 66,680) versus mammography alone: 0.6% (422 of 66,680); Analysis 1.7.2). As mentioned above under 'False‐positive rate in mixed populations of women with dense and non‐dense breasts', it can be assumed that intermediate mammograms at 6 months were used to calculate the specificity of screening at 12 months follow‐up, which could have contributed to the lower biopsy rate at 12 months follow‐up. Due to the heterogeneous results and methodological limitations of the cohort studies, we assessed the certainty of evidence regarding biopsy rates in women screened with mammography in combination with ultrasonography or with mammography alone based on the results of the cohort studies as very low.
1.7. Analysis.

Comparison 1: Mammography in combination with breast ultrasonography versus mammography alone in mixed populations of women with dense and non‐dense breasts, Outcome 7: Rate of biopsies
J‐START and Buchberger 2018 provided separate data for women with dense and non‐dense breasts, showing that for both populations the risk of biopsy was higher with a combination of mammography and ultrasound than with mammography alone (Analysis 2.8; Analysis 3.7).
2.8. Analysis.

Comparison 2: Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts, Outcome 8: Biopsy rate in women with dense breasts
3.7. Analysis.

Comparison 3: Mammography in combination with breast ultrasonography versus mammography alone in women with non‐dense breasts, Outcome 7: Biopsy rate in women with non‐dense breasts
Screening‐associated harm
The J‐START study reported that no complications or adverse events related to mammography and ultrasonography occurred during the entire screening period. None of the other studies reported on screening‐associated harm such as psychological distress, adverse effects caused by subsequent diagnostic or therapeutic interventions, or other adverse events.
Discussion
Summary of main results
We found one RCT, J‐START, with data from 72,717 women, that analysed the efficacy of mammography screening in combination with ultrasonography compared to mammography screening alone. A recent secondary analysis, Harada‐Shoji 2021, of the same RCT with a subpopulation of 19,213 women provided separate results for women with dense and non‐dense breasts. In addition, we identified two prospective studies, Chough 2020; Giuliano 2013, and five retrospective cohort studies, Buchberger 2018; Chae 2013; Lee 2019; Starikov 2016; Tohno 2013, that were relevant to our review. The results of J‐START and the majority of the cohort studies showed that screening with a combination of mammography and ultrasonography detected more breast cancer cases than mammography alone. In J‐START, which represents the best available evidence, two more breast cancer cases (from 1 to 3 more) per 1000 women were detected with combined mammography and ultrasonography compared to mammography alone after a single screening round (0.50% versus 0.32%). The J‐START study included 57.7% women with dense breasts. Similarly, the secondary analysis of the J‐START, Harada‐Shoji 2021, showed that in women with dense breasts, mammography and ultrasonography detected more breast cancer cases compared to mammography alone. The pooled results of three cohort studies supported this finding and revealed that more cancer cases were detected with the combination of mammography and ultrasonography than with mammography screening alone (Buchberger 2018; Giuliano 2013; Starikov 2016). The certainty of the evidence was high, such that in women with dense breasts, more cancer cases were detected by mammography screening in combination with ultrasonography compared with mammography alone.
Data from the secondary analysis of the J‐START, Harada‐Shoji 2021, revealed that in women with dense breasts, the risk of receiving a false‐positive result was about two times higher with screening by mammography in combination with ultrasonography compared to mammography alone (14.6% versus 8.3%). The proportion of false‐positive results was slightly lower in women with non‐dense breasts (11.0% versus 8.1%). However, due to the imprecision of the result, we assessed the evidence for the benefit of additional ultrasonography for cancer detection in women with non‐dense breasts to be of moderate certainty. Evidence from real‐world data did not confirm this finding: low certainty evidence from two cohort studies found no statistically significant difference between the two screening methods in women with non‐dense breasts (Buchberger 2018; Starikov 2016). In the overall J‐START population of 72,717 women, the effect of additional cancer detection was less with additional ultrasonography than in the dense and non‐dense breast subpopulations by themselves. It appears that more data provide more accurate results, especially in women with non‐dense breasts.
Overall completeness and applicability of evidence
Our systematic review includes one RCT and seven cohort studies that assessed the comparative efficacy of mammography in combination with ultrasonography compared to mammography alone. All studies analysed whether additional cancer cases were detected in women who had mammography screening in combination with ultrasonography compared to mammography screening alone. Six studies provided information about cancer detection in women with dense breasts, and three studies in women with non‐dense breasts. Five studies reported interval cancer cases, the lymph node status of women with breast cancer who were detected by screening, and the size of the detected cancers. Three studies provided information on false‐positive and false‐negative rates and the number of required biopsies. However, the screening methods used in the studies only partially met the requirements of the European guidelines for quality assurance in breast cancer screening and diagnosis (European Commission 2022). For example, the European Commission recommends double reading in mammography screening for early detection of breast cancer, with consensus when expert findings disagree. Mammography was assessed independently by two reviewers in four studies (Chough 2020; Giuliano 2013; J‐START; Tohno 2013), and ultrasonography was also assessed by double reading in three of these four studies (Chough 2020; Giuliano 2013; J‐START). In the largest retrospective cohort study, Buchberger 2018, and in Starikov's retrospective study, Starikov 2016, mammography and ultrasound were assessed by one reviewer. Two studies lacked detailed information on the evaluation process of mammography and ultrasonography (Chae 2013; Lee 2019). J‐START did report that the technologists and physicians involved in the trial completed a two‐day course about screening process standards. Only one study provided any information about the number of screening examinations per year, which is one of the criteria to ensure accurate diagnosis based on breast imaging (Buchberger 2018). The US Food and Drug Administration (FDA) requires evaluating physicians to have performed at least 960 mammographic exams in the 24 months prior to the facility's annual Mammography Quality Standards Act (MQSA) inspection (US FDA ). Five of the eight included studies examined a large number of women per study site. The RCT examined more than 72,000 women from 42 study centres, corresponding to an average of more than 1700 mammograms per year per study centre. (J‐START). Lee 2019 analysed 36,143 screening exams performed in 18,562 women for the matched analysis, but did not specify the number of study sites at which these examinations were conducted. The smallest study, Chough 2020, examined 598 women, and it remains unclear whether the study site met quality requirements.
The RCT differs in several respects from the cohort studies, which may more closely match the real‐world setting. The RCT considered women between 40 and 49 years of age, 23% of whom had never had a mammogram, whereas the retrospective study by Buchberger and colleagues included more than 50% women 50 to 69 years of age. The US Preventive Services Task Force currently recommends biennial mammography screening for women aged 50 to 74 years (Siu 2016), which covers the age groups analysed in the cohort studies. Further, a higher percentage of women from Asian populations were found to have dense breasts compared to other populations (Heller 2015); however, the results of the J‐START study consisted solely of Japanese women and may not be generalisable to other populations. In Buchberger 2018, the proportion of cancer cases in women with dense breasts was lower (2.4 per 1000 women) than in the secondary analysis of J‐START (6.8 per 1000 women). One reason for the different proportions of cancer cases that were detected in the J‐START study versus the largest retrospective cohort study by Buchberger and colleagues can be attributed to the fact that fewer new cases were detected in subsequent rounds of screening amongst women in the retrospective study who had already been screened several times due to the higher age range. In addition, the retrospective study most likely used intermediate mammograms (performed in 19 of 1000 women screened) to calculate the sensitivity and specificity of mammography and ultrasonography.
In the real world, biopsies may be performed more frequently than in an RCT. For example, in the J‐START study, 4.5% of women who received screening mammography and additional ultrasonography underwent a biopsy, compared to 1.8% who received mammography alone. In the retrospective study by Lee and colleagues, the biopsy rate was higher. Biopsies were performed in 57.4 of 1000 women in the group that received mammography and ultrasonography, compared with 27.7 of 1000 women in the group that received mammography alone. One reason for this may be that up to 15% of the screening examinations were performed in women who had a higher five‐year breast cancer risk in the study by Lee and colleagues, which may reflect routine daily clinical practice, since not all participants in screening programmes are comprehensively screened for risk factors in advance.
Quality of the evidence
For the assessment of the certainty of evidence, we primarily relied on the results of the J‐START trial (J‐START; Harada‐Shoji 2021), which represents the best available evidence and was rated as having a low risk of bias. For the majority of the results based on the J‐START total population numbers, we rated the certainty of the evidence as high or moderate due to the precision of the results. No statistically significant difference was found between the two screening methods in the proportion of invasive cancer amongst screen‐detected cancer cases, but the certainty of the evidence was rated low due to imprecision. Based on the secondary analysis of the J‐START, we rated the certainty of evidence as high, finding that in women with dense breasts, more cancers cases are detected by mammography screening in combination with ultrasonography compared with mammography alone. Regarding women with non‐dense breasts, we rated the certainty of evidence as moderate, finding that more cancers cases are detected by mammography screening in combination with ultrasonography compared with mammography alone. The confidence interval of the absolute risk reduction was not sufficiently narrow to achieve a high degree of certainty. The results of the cohort studies were described in relation to J‐START, which was methodologically very well performed.
We assessed the certainty of the evidence from the cohort studies as very low to moderate due to methodological limitations and heterogeneous or imprecise results. The population may differ in terms of breast cancer risk, as not all the retrospective studies adequately described the characteristics of the population, or there may have been specific reasons why women were assigned to additional ultrasonography. For example, Chae 2013 lacked data on the percentage of women with a personal history of breast cancer or whose first‐degree relatives had breast cancer. In two retrospective studies, the selection of women into the study who received ultrasonography in addition to mammography screening may have been influenced by suspicious findings or other factors, as they were referred by physicians (Buchberger 2018; Starikov 2016). Nevertheless, in women with dense breasts, the direction of the effect in the cohort studies was consistent with J‐START. In women with non‐dense breasts, results concerning cancer detection rates in the cohort studies could not confirm the benefit of additional ultrasonography in mammography screening.
Potential biases in the review process
Publication bias is a threat for any systematic review. Although we conducted extensive searches of grey literature in our first search, we cannot be sure that we found every study conducted in this field. For the latest update search, we assessed the contribution of databases and other resources previously used for the retrieval of relevant studies. Concerning grey literature, only study registers had information about potentially relevant unpublished or ongoing studies. For this reason, we limited the update search to the following grey literature sources: WHO ICTRP and ClinicalTrials.gov.
In cases of incomplete or unclear data, we contacted the study authors. Only one author of the RCT answered our questions. Previously unpublished data were published whilst we were finalising our review (Harada‐Shoji 2021).
Due to the small number of studies on the comparative effectiveness and safety of mammography screening in combination with breast ultrasonography versus mammography alone in women at average risk of breast cancer, the validity of statistical methods to explore publication bias, such as funnel plots, was limited.
Agreements and disagreements with other studies or reviews
This is the first systematic review and meta‐analysis assessing the effect of mammography screening in combination with ultrasonography compared to mammography alone with separate meta‐analyses for women with dense and non‐dense breasts.
Breast density refers to the proportion of glandular tissue in the breast, and ranges from a high proportion of fatty tissue to very dense breasts consisting mostly of glandular tissue. In a Danish cohort study, the sensitivity of digital mammography decreased from 78% in BI‐RADS‐1 (Breast Imaging Reporting and Data System) to 47% in women with BI‐RADS‐4 (Lynge 2019). A recent systematic review considering women with dense breasts and an initially negative mammography showed that additional ultrasonography screening results in a higher sensitivity (96%) at the expense of a lower specificity (88%) (Yuan 2020). However, the question is whether breast density is the only criterion to consider when deciding to perform additional imaging. According to a large cohort study (Kerlikowske 2015), high interval cancer rates were observed in women with extremely dense breasts and a five‐year risk of developing breast cancer of 1.67% or greater, and in women with heterogeneously dense breasts and a five‐year risk of 2.49% or greater (BCSC‐5‐year risk: Breast Cancer Surveillance Consortium Risk Calculator). For the majority of women, including those with dense breasts and low risk of breast cancer, who underwent digital mammography, the rate of interval cancer was low. Women with heterogeneously or extremely dense breasts and low‐to‐average risk of breast cancer had interval cancer rates between 0.58 and 0.89 per 1000 mammograms. A risk‐based approach to identify women with dense breasts at high risk for interval cancer may reduce false‐positive results (Kerlikowske 2015).
None of the included studies reported on whether breast cancer screening with mammography and additional ultrasound resulted in a reduced mortality rate. This may be because the observation period was too short to compare death rates or disease progression between the two groups. Simply diagnosing more cases of illness does not necessarily result in lower mortality or less morbidity. A recently published study showed that for every 10,000 women aged 40 years who received annual mammography screening for 23 years, mortality was reduced by five cases compared with no screening (0.34% (182 of 53,883) versus 0.39% (412 of 106,953)) (Duffy 2020). However, the reduction in mortality must be weighed against the harms, such as overdiagnosis, which is considered one of the greatest harms of breast screening. Estimations of overdiagnosis in mammography screening vary depending on the analytic approach of the modelling studies, and range from 11% to 22% (Nelson 2016b). An analysis of the Surveillance, Epidemiology, and End Results (SEER) database looked at the influence of mammography screening on the incidence of small breast cancer tumours with a size of 19 mm or below (Welch 2016). The results showed that the incidence of small‐sized tumours dramatically increased from 82 per 100,000 women (1975 to 1979) before widespread mammography screening was implemented to 244 per 100,000 in the period 2008 to 2012, whilst larger tumours decreased from 145 to 115 per 100,000 women. The authors assumed that some of the larger tumours were detected earlier by screening, and that the increase of the small‐sized tumours that occurred after the implementation of widespread mammography screening were often overdiagnosed breast tumours, which might not have caused any problems during a woman's lifetime. Taking into account that treatment of breast cancer has improved in the last few decades, the authors calculated that mammography screening reduces the mortality rate by eight deaths per 100,000 women. The studies included in our review also showed similar tumour sizes: in the J‐START study, the average size of breast cancer detected by screening was 15 mm, and in the retrospective study by Lee and colleagues, 2/3 of the tumours were 20 mm or less (J‐START; Lee 2019). From a healthcare perspective, weighing benefits against harms is crucial when recommending screening tests. Whether the net effect of screening is harmful or beneficial depends on whether the tumour develops indolently or aggressively, the prevalence in the population, and whether extensive treatment can be avoided by early detection (Esserman 2014). Fast‐growing, aggressive tumours are more often detected not by screening but more commonly between screening exams. The National Comprehensive Cancer Network recommends regular screening or to discuss preventive mastectomy as an option for women with BRCA‐1 or BRCA‐2 mutations (Grade 2 A; Daly 2021).
In the case of indolent tumours, which grow very slowly, do not grow at all, or even regress and would not cause any symptoms throughout life, early detection leads to more harm than good (Esserman 2014). The proportion of indolent tumours detected by breast cancer screening is unclear. Before the introduction of widespread breast cancer screening, ductal carcinoma in situ (DCIS) was rarely diagnosed (van Seijen 2019). DCIS cases account for 25% of all breast cancers detected, and 75% can be detected due to calcifications on mammography, whilst 25% are mammographically occult. DCIS is classified as pre‐ or non‐invasive and is usually treated with breast‐conserving surgery or mastectomy and additional radiotherapy, and in some cases with endocrine therapy. The treatment of DCIS lesions is associated with side effects such as considerable psychological distress, reduced quality of life, and complications of the surgical procedure, although a large proportion of these lesions are unlikely to develop into invasive cancer. To determine whether some people with DCIS are being overtreated, three RCTs are currently underway comparing active surveillance with standard treatment in low‐risk DCIS patients (Elshof 2015; Francis 2015; Hwang 2019). In the J‐START trial, 28% (84 of 301) of cancers detected by screening were non‐invasive and included DCIS and lobular carcinomas (J‐START). But even amongst invasive tumours, there has been an increase since the introduction of screening, especially in those with less aggressive characteristics. It is estimated that 20% of all invasive breast cancers represent overdiagnosed cancer cases (Bleyer 2012). The challenge therefore remains to determine which women might benefit from screening and which women do not need screening. The topic for further research is risk‐based screening to identify women who do not benefit or benefit very little from screening, and in whom harm predominates.
Authors' conclusions
Implications for practice.
Ultrasonography in combination with mammography screening detects more breast cancer cases than mammography alone. However, the proportion of false‐positive results is up to 1.5 times higher than with mammography screening alone. These results were shown by a large, methodologically well‐performed randomised controlled trial and confirmed by large cohort studies.
However, no study has examined the benefit of adding ultrasonography in terms of saving lives or reducing the burden of disease compared with mammography alone. The majority of women undergoing breast cancer screening do not have dense breasts and are at average risk. Similarly, amongst women with dense breasts, the majority have a low risk of developing breast cancer.
At present, no methodologically sound evidence is available justifying the routine use of ultrasonography as an adjunct screening tool in such a population. Even if only a small proportion of screened women will be recalled due to positive ultrasonography findings, the rate of false‐positive results and unnecessary harm caused by subsequent investigations may be unacceptably high given the lack of evidence supporting a gain in health benefits. The US Preventive Services Task Force also concludes that it is not possible to infer from the studies published to date whether the cancers identified by supplemental screening result in improved clinical outcomes and how many of these represent cancers that would not otherwise have become clinically apparent.
Implications for research.
The lack of evidence clearly indicates the need for well‐conducted, controlled studies with outcomes other than cancer detection rates. Ideally, a methodologically sound randomised controlled trial or well‐conducted cohort studies would assess the comparative benefits and risks of mammography alone versus mammography with adjunct ultrasonography in terms of morbidity, screening‐related harms, and mortality, although the consideration of mortality would require a long follow‐up period.
What's new
| Date | Event | Description |
|---|---|---|
| 30 March 2023 | New citation required and conclusions have changed | 8 new studies included |
| 30 March 2023 | New search has been performed | Search for new studies conducted on 03 May 2021 |
History
Protocol first published: Issue 2, 2012 Review first published: Issue 4, 2013
Acknowledgements
We would like to acknowledge Sandra Hummel and Edith Kertesz from the Danube University for their superb administrative support during the update of this review. We also greatly appreciate Megan Van Noord for her help in screening the abstracts for the update of the review, and thank Emma Persad and Michael Winer for proofreading the review.
We wish to thank the following people for their helpful peer review: Bonner Cutting (consumer reviewer), Alamo Breast Cancer Foundation and Komen Advocate‐in‐Science, Kerry Dwan (statistical reviewer), Cochrane Methods Support Unit, UK, and Dr Nehmat Houssami (external clinical reviewer), University of Sydney, Australia; and the Cochrane Breast Cancer Group for editing and reviewing versions. We would also like to thank Peta Skeers and Ava Tan for developing the search strategies, and Lisa Winer for thoroughly copyediting the review.
Appendices
Appendix 1. Search strategies ‐ update search 2021
Ovid MEDLINE(R) ALL 1946 to May 4, 2021
| # | Search | |
| A. Breast cancer | 1 | exp Breast Neoplasms/ |
| 2 | ((breast or mammary) adj2 (cancer* or carcinoma* or neoplas* or tumo?r*)).ti,ab,kf. | |
| 3 | 1 or 2 | |
| B. Mammography | 4 | exp Mammography/ |
| 5 | (mammograph* or mammogram* or tomosynthes*).ti,ab,kf. | |
| 6 | 4 or 5 | |
| A+B | 7 | 3 and 6 |
| C. Ultrasound | 8 | Ultrasonography, Mammary/ |
| 9 | (sonograph* or ultrasound* or ultrasonogr* or echomammogr*).ti,ab,kf. | |
| 10 | 8 or 9 | |
| A+B+C | 11 | 7 and 10 |
| D. Diagnosis | 12 | exp Breast Neoplasms/di [Diagnosis] |
| 13 | "Early Detection of Cancer"/ | |
| 14 | Mass Screening/ | |
| 15 | Early Diagnosis/ | |
| 16 | screening*.ti,ab,kf. | |
| 17 | (screen* or detect* or diagnos*).ti. | |
| 18 | ((screen* or detect* or diagnos*) and (early or asymptomatic*)).ab,kf. | |
| 19 | or/12‐18 | |
| A+B+C+D | 20 | 11 and 19 |
| humans | 21 | limit 20 to "humans only (removes records about animals)" |
| limted to adults | 22 | exp age groups/ not exp adult/ |
| 23 | 21 not 22 | |
| RCT‐Filter | 24 | exp randomized controlled trial/ or (random* or placebo).mp. |
| RCT‐Results | 25 | 23 and 24 |
| cNRS‐Filter | 26 | exp cohort studies/ or exp epidemiologic studies/ or exp clinical trial/ or exp evaluation studies as topic/ or exp statistics as topic/ |
| 27 | ((control and (study or group*)) or (time and factors) or cohort or program or comparative stud* or evaluation studies or survey* or follow‐up* or ci).mp. | |
| 28 | 26 or 27 | |
| 29 | comment/ or editorial/ or exp review/ or meta analysis/ or consensus/ or exp guideline/ or hi.fs. or case report.mp. | |
| 30 | 28 not 29 | |
| cNRS‐Results | 31 | 23 and 30 |
| Total (no date limit) | 32 | 25 or 31 |
| since 2016 | 33 | limit 32 to yr="2011 ‐Current" |
Embase.com
| No. | Query |
| #1 | 'breast cancer'/exp |
| #2 | ((breast OR mammary) NEAR/2 (cancer* OR carcinoma* OR neoplas* OR tumo$r*)):ti,ab,kw |
| #3 | #1 OR #2 |
| #4 | 'mammography'/exp/mj |
| #5 | 'mammography system'/exp/mj |
| #6 | mammograph*:ti,ab,kw OR mammogram*:ti,ab,kw OR tomosynthes*:ti,ab,kw |
| #7 | #4 OR #5 OR #6 |
| #8 | #3 AND #7 |
| #9 | 'echomammography'/exp/mj OR 'echomammography device'/exp/mj |
| #10 | sonograph*:ti,ab,kw OR ultrasound*:ti,ab,kw OR ultrasonogr*:ti,ab,kw OR echomammogr*:ti,ab,kw |
| #11 | #9 OR #10 |
| #12 | #8 AND #11 |
| #13 | 'breast cancer'/exp/dm_di |
| #14 | 'early cancer diagnosis'/exp |
| #15 | 'cancer screening'/exp |
| #16 | screening*:ti,ab,kw |
| #17 | screen*:ti OR detect*:ti OR diagnos*:ti |
| #18 | (screen*:ab,kw OR detect*:ab,kw OR diagnos*:ab,kw) AND (early:ab,kw OR asymptomatic*:ab,kw) |
| #19 | #13 OR #14 OR #15 OR #16 OR #17 OR #18 |
| #20 | #12 AND #19 |
| #21 | ('animal'/exp OR 'animal experiment'/de OR 'veterinary study'/exp) NOT 'human'/exp |
| #22 | #20 NOT #21 |
| #23 | 'groups by age'/exp NOT 'adult'/exp |
| #24 | #22 NOT #23 |
| #25 | 'randomized controlled trial'/exp OR random*:ti,ab,kw OR placebo:ti,ab,kw |
| #26 | #24 AND #25 |
| #27 | 'cohort analysis'/exp OR 'clinical trial'/exp OR 'comparative study'/exp |
| #28 | control:ti,ab,kw AND (study:ti,ab,kw OR group*:ti,ab,kw) OR (time:ti,ab,kw AND factors:ti,ab,kw) OR cohort:ti,ab,kw OR program:ti,ab,kw OR 'comparative stud*':ti,ab,kw OR 'evaluation studies':ti,ab,kw OR survey*:ti,ab,kw OR 'follow up*':ti,ab,kw OR ci:ti,ab,kw |
| #29 | #27 OR #28 |
| #30 | 'editorial'/exp OR 'review'/exp OR 'meta analysis'/exp OR 'case report'/exp OR 'practice guideline'/exp OR 'case report':ti,ab,kw |
| #31 | #29 NOT #30 |
| #32 | #24 AND #31 |
| #33 | #26 OR #32 |
| #34 | #33 AND [2011‐2021]/py |
CENTRAL (Cochrane Library/Wiley)
| ID | Search |
| #1 | [mh "Breast Neoplasms"] |
| #2 | ((breast OR mammary) NEAR/2 (cancer* OR carcinoma* OR neoplas* OR tumo?r*)):ti,ab,kw |
| #3 | #1 or #2 |
| #4 | [mh Mammography] |
| #5 | (mammograph* OR mammogram* OR tomosynthes*):ti,ab,kw |
| #6 | #4 or #5 |
| #7 | #3 and #6 |
| #8 | [mh ^"Ultrasonography, Mammary"] |
| #9 | (sonograph* OR ultrasound* OR ultrasonogr* or echomammogr*):ti,ab,kw |
| #10 | #8 or #9 |
| #11 | #7 and #10 |
| #12 | #11 with Publication Year from 2011 to 2021, in Trials |
ClinicalTrials.gov
| Search |
| Other terms: breast OR mammary | Intervention: (mammography OR mammogram OR tomosynthesis) AND (sonography OR ultrasonography OR ultrasound OR echomammography) | Last update posted from 01/01/2011 to 04/04/2021 |
WHO International Clinical Trials Registry Platform (http://ictrptest.azurewebsites.net/Default.aspx)
| Search |
| mammogra* AND sonograph* OR tomosynthes* AND sonograph* OR mammogra* AND ultrasonograph* OR tomosynthes* AND ultrasonograph* OR mammogra* AND ultrasound OR tomosynthes* AND ultrasound OR mammogra* AND echomammograph* OR tomosynthes* AND echomammograph* |
Appendix 2. Search strategies ‐ update search 2016
CENTRAL (via Cochrane Library)
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 breast near cancer* #3 breast near neoplasm* #4 breast near carcinoma* #5 breast near tumour* #6 breast near tumor* #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Mammography] explode all trees #9 mammograph* #10 mammogram #11 #8 or #9 or #10 #12 MeSH descriptor: [Ultrasonography] explode all trees #13 MeSH descriptor: [Ultrasonography, Mammary] explode all trees #14 ultrasonograph* #15 ultrasound #16 #12 or #13 or #14 or #15 #17 MeSH descriptor: [Early Detection of Cancer] explode all trees #18 screen* #19 MeSH descriptor: [Early Diagnosis] explode all trees #20 MeSH descriptor: [Mass Screening] explode all trees #21 #17 and #18 or #19 or #20 #22 #7 and #11 and #16 and #21 Publication Year from 2012 to 2016
MEDLINE (via Ovid)
1 Case‐Control Studies/
2 Control Groups/
3 Matched‐Pair Analysis/
4 Retrospective Studies/
5 ((case* adj5 control*) or (case adj3 comparison*) or control group*).ti,ab.
6 or/1‐5
7 Cohort Studies/
8 Longitudinal Studies/
9 Follow‐Up Studies/
10 Prospective Studies/
11 Retrospective Studies/
12 cohort.ti,ab.
13 longitudinal.ti,ab.
14 prospective.ti,ab.
15 retrospective.ti,ab.
16 or/7‐15
17 randomized controlled trial.pt.
18 controlled clinical trial.pt.
19 randomized.ab.
20 placebo.ab.
21 Clinical Trials as Topic/
22 randomly.ab.
23 trial.ti.
24 (crossover or cross‐over).tw.
25 Pragmatic Clinical Trials as Topic/
26 pragmatic clinical trial.pt.
27 or/17‐26
28 exp Breast Neoplasms/
29 (breast adj6 cancer$).tw.
30 (breast adj6 neoplasm$).tw.
31 (breast adj6 carcinoma$).tw.
32 (breast adj6 tumo?r$).tw.
33 or/28‐32
34 exp Mammography/
35 mammograph$.tw.
36 mammogram.tw.
37 34 or 35 or 36
38 exp Ultrasonography/
39 exp Ultrasonography, Mammary/
40 ultrasonograph$.tw.
41 ultrasound.tw.
42 38 or 39 or 40 or 41
43 exp early diagnosis/
44 exp "Early Detection of Cancer"/
45 exp Mass Screening/
46 screen$.tw.
47 43 or 44 or 45 or 46
48 33 and 37 and 42 and 47
49 limit 48 to yr="2012 ‐Current"
50 6 and 49
51 16 and 49
52 27 and 49
EMBASE (via Ovid)
1 Randomized controlled trial/
2 Controlled clinical study/
3 Random$.ti,ab.
4 randomization/
5 intermethod comparison/
6 placebo.ti,ab.
7 (compare or compared or comparison).ti.
8 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
9 (open adj label).ti,ab.
10 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
11 double blind procedure/
12 parallel group$1.ti,ab.
13 (crossover or cross over).ti,ab.
14 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
15 (assigned or allocated).ti,ab.
16 (controlled adj7 (study or design or trial)).ti,ab.
17 (volunteer or volunteers).ti,ab.
18 human experiment/
19 trial.ti.
20 or/1‐19
21 exp case control study/
22 case control study.ti,ab.
23 ((case control or case base or case matched or retrospective) adj1 (analys* or design* or evaulation* or research or stud* or survey* or trial*)).ti,ab.
24 21 or 22 or 23
25 exp retrospective study/
26 exp prospective study/
27 ((cohort or concurrent or incidence or longitudinal or followup or 'follow up' or prospective or retrospective) adj1 (analys* or design* or evaluation* or research or stud* or survey* or trial*)).ti,ab.
28 25 or 26 or 27
29 exp breast/
30 exp breast disease/
31 (29 or 30) and exp neoplasm/
32 exp breast tumor/
33 exp breast cancer/
34 exp breast carcinoma/
35 (breast$ adj6 (neoplas$ or cancer$ or carcin$ or tumo$ or metasta$ or malig$)).ti,ab.
36 29 or 30 or 31 or 32 or 33 or 34 or 35
37 exp mammography/
38 mammograph$.tw.
39 mammogram.tw.
40 37 or 38 or 39
41 exp echography/
42 ultrasonograph$.tw.
43 exp echomammography/
44 ultrasound.tw.
45 41 or 42 or 43 or 44
46 screen$.tw.
47 exp early diagnosis/
48 exp mass screening/
49 exp cancer screening/
50 46 or 47 or 48 or 49
51 36 and 40 and 45 and 50
52 limit 51 to (embase and yr="2012 ‐Current")
53 20 and 52
54 24 and 52
55 28 and 52
Web of Science
1 TS=(breast) OR TS=(mammary)Indexes=SCI‐EXPANDED, CCR‐EXPANDED Timespan=All years
2 TS=(neoplasm) OR TS=(cancer) OR TS=(carcinoma) OR TS=(tumour) OR TS=(tumor) OR TS=(malignancy)Indexes=SCI‐EXPANDED, CCR‐EXPANDED Timespan=All years
3 #2 AND #1Indexes=SCI‐EXPANDED, CCR‐EXPANDED Timespan=All years
4 TS=(diagnosis) OR TS=(breast screening) OR TS=(mass screening) OR TS=(screening)Indexes=SCI‐EXPANDED, CCR‐EXPANDED Timespan=All years
5 TS=(ultrasonography) OR TS=(breast ultrasonography) OR TS=(mammary ultrasonography)Indexes=SCI‐EXPANDED, CCR‐EXPANDED Timespan=All years
6 TS=(mammography) OR TS=(mammogram)Indexes=SCI‐EXPANDED, CCR‐EXPANDED Timespan=All years
7 #6 AND #5 AND #4 AND #3Indexes=SCI‐EXPANDED, CCR‐EXPANDED Timespan=2012‐2016
BIOSIS Previews
1 TS=(breast) OR TS=(mammary)Indexes=BIOSIS Previews Timespan=All years
2 TS=(neoplasm) OR TS=(cancer) OR TS=(carcinoma) OR TS=(tumour) OR TS=(tumor) OR TS=(malignancy)Indexes=BIOSIS Previews Timespan=All years
3 #2 AND #1Indexes=BIOSIS Previews Timespan=All years
4 TS=(diagnosis) OR TS=(breast screening) OR TS=(mass screening) OR TS=(screening)Indexes=BIOSIS Previews Timespan=All years
5 TS=(ultrasonography) OR TS=(breast ultrasonography) OR TS=(mammary ultrasonography)Indexes=BIOSIS Previews Timespan=All years
6 TS=(mammography) OR TS=(mammogram)Indexes=BIOSIS Previews Timespan=All years
7 #6 AND #5 AND #4 AND #3Indexes=BIOSIS Previews Timespan=All years
WHO ICTRP and ClinicalTrials.gov search strategy
Basic search:
mammography AND ultrasonography AND screening
Appendix 3. Original search strategies 2012
MEDLINE (via OVID) search strategy February 2012
# Searches
1 exp Breast Neoplasms/
2 breast cancer.mp.
3 1 or 2
4 exp Diagnosis/
5 diagnosis.ab,ti,tw.
6 screening.ab,ti,tw.
7 exp Mass Screening/
8 mass screening.ab,ti,tw.
9 exp "Early Detection of Cancer"/
10 4 or 5 or 6 or 7 or 8 or 9
11 3 and 10
12 exp Mammography/
13 mammograph*.ab,ti,tw.
14 mammogram.ab,ti,tw.
15 12 or 13 or 14
16 exp Ultrasonography/
17 exp Ultrasonography, Mammary/
18 breast ultrasonography.mp.
19 mammary ultrasonography.mp.
20 16 or 17 or 18 or 19
21 mammary.ab,ti,tw.
22 breast.ab,ti,tw.
23 21 or 22
24 20 and 23
25 11 and 15 and 24
26 limit 25 to humans
27 limit 26 to (clinical trial, all or clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or clinical trial or comparative study or controlled clinical trial or evaluation studies or meta analysis or multicenter study or randomized controlled trial or "review" or validation studies)
28 ("Single Blind Method" or "Double Blind Method" or "Case Control Study" or "Cohort Study" or "Epidemiologic Study" or "Cross Sectional Study" or "Cross Over Study" or "Follow Up Study" or "Longitudinal Study" or "Prospective Study" or "observational study").mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
29 26 and 28
30 27 or 29
EMBASE (via Embase.com) search strategy February 2012
#25 #24 AND [humans]/lim AND [embase]/lim AND [2008‐2012]/py
#24 #8 AND #12 AND #23
#23 #21 AND #22
#22 'breast cancer screening'
#21 #18 OR #20
#20 #18 AND #19
#19 #15 OR #16 OR #17
#18 #13 OR #14
#17 'breast'/de OR breast AND ultrasonograph*
#16 'breast ultrasonography'/exp OR 'breast ultrasonography'
#15 'ultrasonography'/exp OR ultrasonography
#14 mammograph*
#13 'mammography'/exp OR mammography
#12 #9 OR #10 OR #11
#11 'breast cancer risks'
#10 'breast cancer risk'
#9 'breast neoplasm'
#8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
#7 groups:ab
#6 trial:ab
#5 randomly:ab
#4 placebo:ab
#3 randomi*ed:ab
#2 controlled AND clinical AND trial
#1 randomised AND controlled AND trial
WHO ICTRP search strategy January 2012
Advanced search:
1. Title: mammography in combination with breast ultrasonography versus mammography for breast cancer screening
Recruitment Status: ALL
2. Condition: breast AND (cancer% OR carcinoma% OR neoplas% OR tumour% OR tumor%)
Intervention: (breast mammograph% OR breast ultrasonograph%) AND breast screening
Recruitment Status: ALL
Data and analyses
Comparison 1. Mammography in combination with breast ultrasonography versus mammography alone in mixed populations of women with dense and non‐dense breasts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incremental cancer detection | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 RCT: J‐START | 1 | 72717 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.22, 1.94] |
| 1.1.2 Cohort studies | 2 | 83469 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.92, 1.98] |
| 1.1.3 Cohort study with a serious risk of bias | 1 | 5503 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.80, 6.79] |
| 1.1.4 Cohort study with one or more screening rounds | 1 | 36143 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.68, 1.44] |
| 1.2 Incremental detection of invasive cancers | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 RCT: J‐START | 1 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.09] |
| 1.2.2 Cohort studies | 2 | 571 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.06] |
| 1.2.3 Cohort study with one or more screening rounds | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.59, 1.09] |
| 1.3 Interval cancers | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3.2 Cohort study with one or more screening rounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4 Lymph node status in women with screen‐detected breast cancer | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4.2 Cohort study with a serious risk of bias | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4.3 Cohort study with one ore more screening rounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 False‐positive rate: proportion of false‐positive results amongst women with no cancer | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.2 Cohort studies | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.3 Cohort study with one or more screening rounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 False‐negative rate: proportion of false‐negative results amongst women with cancer | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.2 Cohort study | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.3 Cohort study with one or more screening rounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Rate of biopsies | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.2 Cohort studies | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.3 Cohort study with one or more screening rounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Mammography in combination with breast ultrasonography versus mammography alone in women with dense breasts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incremental cancer detection rate in women with dense breasts | 4 | 61717 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.33, 2.25] |
| 2.1.1 RCT: J‐START | 1 | 11390 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [1.00, 2.72] |
| 2.1.2 Cohort studies | 3 | 50327 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.23, 2.56] |
| 2.2 Incremental cancer detection rate in women with dense breasts: cohort studies with a serious risk of bias or one or more screening rounds | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.1 Cohort study with a serious risk of bias | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.2 Cohort study with one or more screening rounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Incremental detection of invasive cancers in women with dense breasts | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3.2 Cohort study | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3.3 Cohort study with a serious risk of bias | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3.4 Cohort study with one to two screening rounds | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4 Interval cancer in women with dense breasts | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4.2 Cohort study with a serious risk of bias | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5 Lymph node status in women with screen‐detected breast cancer | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5.2 Cohort study | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5.3 Cohort study with one to two screening rounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6 False‐positive rate in women with dense breasts: proportion of false‐positive results amongst women with no cancer | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6.2 Cohort studies | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.7 False‐negative rate in women with dense breasts: proportion of false‐negative results amongst women with cancer | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.7.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.7.2 Cohort studies | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.8 Biopsy rate in women with dense breasts | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.8.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.8.2 Cohort study | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Mammography in combination with breast ultrasonography versus mammography alone in women with non‐dense breasts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Incremental cancer detection rate for women with non‐dense breasts | 3 | 48459 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.92, 1.88] |
| 3.1.1 RCT: J‐START | 1 | 7823 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [1.01, 3.68] |
| 3.1.2 Cohort studies | 2 | 40636 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.85, 1.49] |
| 3.2 Incremental detection of invasive cancers in women with non‐dense breasts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Interval cancer in women with non‐dense breasts | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.4 Lymph node status in women with screen‐detected breast cancer | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5 False‐positive rate in women with non‐dense breasts: proportion of false‐positive results amongst women with no cancer | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.5.2 Cohort study | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.6 False‐negative rate in women with non‐dense breasts: proportion of false‐negative results amongst women with cancer | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.6.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.6.2 Cohort study | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.7 Biopsy rate in women with non‐dense breasts | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.7.1 RCT: J‐START | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.7.2 Cohort study | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Buchberger 2018.
| Study characteristics | |
| Methods | Retrospective cohort study |
| Participants | 66,680 women: 52.1% (34,762) with non‐dense breasts and 47.9% (31,918) with extremely or heterogenously dense breasts Exclusion criteria: women with clinical symptoms, personal or first‐degree family history of breast cancer, previous high‐risk biopsy result Age: 40 to 49 years (46.6%); 50 to 59 years (32.4%); 60 to 69 (21.0%) |
| Interventions | Digital mammography plus sonography: 66,680 women |
| Outcomes | Incremental cancer detection rate Invasive cancers Rate of interval cancers Lymph node status Size of detected cancers False‐positve rate* False‐negative rate* Rate of biopsies 12 months follow‐up |
| Notes | Funding: none |
Chae 2013.
| Study characteristics | |
| Methods | Retrospective cohort study |
| Participants | 20,864 women with dense breasts who had no signs or symptoms of breast abnormalities Age: 22 to 91 years, mean age: 52 years |
| Interventions | Digital mammography: 12,505 women Digital mammography plus sonography: 8359 women |
| Outcomes | Incremental cancer detection rate Invasive cancers Interval cancers Lymph node status Size of detected cancers False‐positive rate False‐negative rate* Rate of biopsies |
| Notes | Funding: Grant 1120410 from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Korea |
Chough 2020.
| Study characteristics | |
| Methods | Prospective cohort study |
| Participants | 600 asymptomatic women, of which 598 completed the study protocol in year one; 513 of these women completed the same set of examinations during a second annual visit. All women had either known heterogeneously dense or extremely dense breasts. Exclusion criteria: known high risk for breast cancer or known or suspected pathogenic BRCA mutation, signs or symptoms of breast disease, recent breast surgery or breast biopsy within the prior 12 months, no prior mammogram within 3 years, prior malignancy other than breast cancer Age: 40 to 75 years |
| Interventions | Digital breast tomosynthesis + ABUS |
| Outcomes | Cancer detection Interval cancer Tumour size Affected lymph nodes |
| Notes | Funding: GE Healthcare |
Giuliano 2013.
| Study characteristics | |
| Methods | Prospective cohort study |
| Participants | 7494 women with dense breasts (50% or greater density based on a Wolf classification), with no family history of breast cancer or positive BRCA gene Women aged < 50 years to > 70 years, 67% between age 50 to 69, median age: 54 to 57 |
| Interventions | Digital mammography: 4076 women Digital mammography + ABUS: 3418 women |
| Outcomes | Cancer detection rate Rate of interval cancers Lymph node status Size of detected cancers False‐positive rate |
| Notes | Funding: not stated |
J‐START.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | 72,717 asymptomatic women with no history of breast cancer, including in‐situ cancer or other cancers in the previous in the previous 5 years, and who had a life expectancy of more than 5 years Age: 40 to 49 years, mean age: 44 years Dense breasts: 57.7% of women (scores of 3 or 4 in the American College of Radiology Breast Imaging Reporting and Data System) Secondary analysis of a subpopulation of the J‐START: 19,213 asymptomatic women with separate data for women with dense and non‐dense breasts |
| Interventions | Mammography: 35,965 Mammography + sonography: 36,752 women |
| Outcomes | Incremental cancer detection rate Incremental detection rate of invasive cancers Rate of interval cancers Lymph node status Size of detected cancers False‐positive rate False‐negative rate Rate of biopsies |
| Notes | Funding: Ministry of Health, Labour and Welfare of Japan |
Lee 2019.
| Study characteristics | |
| Methods | Retrospective cohort study |
| Participants | 18,562 asymptomatic women with no history of breast cancer Age: 30 to 80 years or older; 90% of women were 40 to 69 years old Mammography: 29.0% first‐degree family history of breast cancer; 66.5% with heterogeneously or extremely dense breasts Mammography and ultrasonography: 42.9% first‐degree family history of breast cancer; 74.3% with heterogeneously or extremely dense breasts |
| Interventions | 30,062 mammography examinations in 15,176 women 6081 mammography and ultrasonography examinations in 3386 women |
| Outcomes | Incremental cancer detection rate Incremental detection rate of invasive cancers Rate of interval cancers Lymph node status Size of detected cancers False‐positive rate False‐negative rate Rate of biopsies |
| Notes | Funding: Breast Cancer Surveillance Consortium (P01CA154292); American Cancer Society/Longaberger Company's Horizon of Hope Campaign (ACS 15922 NHLONGGBR), National Cancer Institute (U54CA163303) |
Starikov 2016.
| Study characteristics | |
| Methods | Retrospective cohort study |
| Participants | 16,789 asymptomatic women 40 years of age or older with no previous history of breast cancer; 65% (10,915 of 16,789) women with dense breasts, 35% (5874 of 16,789) women with non‐dense breasts |
| Interventions | 2‐dimensional mammography alone or in combination with tomosynthesis whole breast ultrasonography (1/3 ABUS, 2/3 handheld ultrasonography) |
| Outcomes | Cancer detection rate Recall rate Invasive cancer |
| Notes | Funding: none |
Tohno 2013.
| Study characteristics | |
| Methods | Retrospective cohort study |
| Participants | 5503 women who underwent breast screening Age: 29 years or younger to 70 years or older; 80% of women were 40 to 60 years old |
| Interventions | Digital mammography: 4529 women Digital mammography and ultrasonography: 974 women |
| Outcomes | Incremental cancer detection rate |
| Notes | Funding: none |
*Calculated by the review authors based on data from the publication.
Abbreviations: ABUS: automated breast ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boonlikit 2013 | Ineligible endpoint (agreement between assessments) |
| Brem 2015 | Undefined proportion of women at high risk of breast cancer |
| Chou 2017 | Abstract only |
| Dong 2018 | Ineligible study design (cancer cases were compared to women with no cancer) |
| Dromain 2012 | Ineligible population (women with breast lesions) |
| Franchini 2021 | Ineligible population (analysis of women with breast cancer) |
| Huang 2009 | Abstract only |
| Hwang 2015 | Ineligible population (ultrasound in women with negative mammography) |
| Ishida 2014 | Study description J‐START |
| Lee 2017 | Ineligible endpoint (factors affecting the recommendation for supplementary ultrasound examination) |
| Leong 2012 | Ineligible population (ultrasound in women with negative mammography) |
| Melnikow 2016 | Ineligible population (ultrasound in women with negative mammography) |
| Moon 2015 | Ineligible population (ultrasound in women with negative mammography), no cancer detection rate of mammography‐only available (according to author) |
| Nelson 2016 | Ineligible intervention or control (no comparison of combined mammography and ultrasound vs mammography) |
| Sakurai 2014 | Ineligible intervention or control (population‐based screening vs opportunistic screening) |
| Shen 2015 | Ineligible endpoint (results of combined mammography and ultrasound, no results of mammography only group) |
| Skaane 2015 | Ineligible endpoint (agreement between radiologists) |
| Spraque 2015 | Incorrect publication type (cost‐effectiveness analysis based on simulation models) |
| Tagliafico 2016 | Abstract only |
| Weigel 2013 | Ineligible population (women with mammographic abnormalities) |
| Weigert 2015 | Ineligible intervention or control (no mammography only group) |
| Weigert 2017 | Ineligible population (ultrasound in women with negative mammography) |
| Wilczek 2016 | Ineligible population (women with mammographic and sonographic abnormalities) |
| Yaffe 2016 | Ineligible publication (comment on studies about adjunctive ultrasonography) |
Characteristics of ongoing studies [ordered by study ID]
DRKS00019097.
| Study name | DRKS00019097 DIMASOS 2 ‐ Density‐indexed mammographic sonographic breast cancer screening |
| Methods | Cohort study/qualitative study |
| Participants | Women participating in a breast screening programme |
| Interventions | Mammography and ultrasound |
| Outcomes | Cancer detection rate |
| Starting date | 2019 |
| Contact information | |
| Notes |
NCT04097366.
| Study name | Breast Screening ‐ Risk Adaptive Imaging for Density (BRAID) |
| Methods | Randomised controlled study |
| Participants | Women participating in the UK national breast screening programme who have dense breast tissue |
| Interventions | Contrast‐enhanced spectral mammography ABUS Abbreviated MRI |
| Outcomes | Cancer detection rate |
| Starting date | 28 May 2019 |
| Contact information | Fiona Gilbert; fjg28@medschl.cam.ac.uk |
| Notes |
NCT04429269.
| Study name | A comparative study of mammography and ultrasound for breast cancer screening and early diagnosis (MUSD) |
| Methods | Prospective cohort study |
| Participants | Women participating in a breast screening programme |
| Interventions | Mammography and ultrasound screening |
| Outcomes | Sensitivity, specificity, positive predictive value |
| Starting date | 12 June 2020 |
| Contact information | Songjie Shen; shensj@pumch.cn |
| Notes |
Abbreviations: ABUS: automated breast ultrasound; MRI: magnetic resonance imaging
Differences between protocol and review
For the 2021 searches, we completely revised the search strategies and information sources used. To do so, we reviewed the search strategies, information sources, and yield of the previous searches in 2012 and 2016. We then identified the databases and registers that had proved most relevant so far, and only used them for the update search. We modified previous search strategies to improve the balance of sensitivity and precision. The revised search strategies also include terms for digital breast tomosynthesis, a new technology that had not been included in the previous searches.
Regarding the study population, we accepted studies where the mean age or 80% of the population met our age and breast cancer risk inclusion criteria. We also included studies with domains rated as high risk of bias, and included cohort studies to evaluate the efficacy of the screening methods.
We excluded studies that included only women with negative mammography because in such a population it would not be possible to compare the efficacy of combining ultrasonography and mammography screening versus mammography screening alone.
We included studies that did not explicitly mention whether the screening measures met the European guidelines for quality assurance in breast cancer screening and diagnosis (European Commission 2022). We added a paragraph in the Discussion noting to what degree the studies fulfilled the requirements of the European Commission and US Food and Drug Administration.
In the protocol for this review, we cited the Cochrane risk of bias tool and criteria for the assessment of non‐randomised controlled trials for the risk of bias assessment of randomised controlled trials. We changed the tools for the methodological assessment of studies and cite RoB 2 and ROBINS‐I in the current version.
Contributions of authors
Drafted the protocol: GG, AG
Study selection: GG, AG, JM, GW, IK, NB, BT, LG, DB
Extracted data from studies: GG, AG, GW, JM, DB, BT, NB, LG
Entered data into RevMan Web: AG, IK
Carried out the analysis: AG
Interpreted the analysis: GG, AG, TH
Drafted the final review: GG, AG
Disagreement resolution: TH
Updated the review: GG, AG
Sources of support
Internal sources
-
No financial support, Other
The update of the review was revised by the authors without financial support.
External sources
-
Ministry of Health, Austria
The previous version of this Cochrane Review was based on a report funded by the Austrian Ministry of Health. The update version was created without financial support.
Declarations of interest
AG: none known. Works as a general practitioner GW: none known JM: none known. Works as a surgeon BT: none known IK: none known NB: none known. Works as a general practitioner LG: none known DB: none known. Works as a radiologist TH: received a grant, payments for lectures and presentations, and support for attending meetings from Siemens. Works as a radiologist GG: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Buchberger 2018 {published data only}
- Buchberger W, Geiger-Gritsch S, Knapp R, Gautsch K, Oberaigner W. Combined screening with mammography and ultrasound in a population-based screening program. European Journal of Radiology 2018;101:24-9. [DOI] [PubMed] [Google Scholar]
Chae 2013 {published data only}
- Chae EY, Kim HH, Cha JH, Shin HJ, Kim H. Evaluation of screening whole-breast sonography as a supplemental tool in conjunction with mammography in women with dense breasts. Journal of Ultrasound in Medicine 2013;32(9):1573-8. [DOI] [PubMed] [Google Scholar]
Chough 2020 {published data only}
- Chough DM, Berg WA, Bandos AI, Rathfon GY, Hakim CM, Lu AH, et al. A prospective study of automated breast ultrasound screening of women with dense breasts in a digital breast tomosynthesis-based practice. Journal of Breast Imaging 2020;2(2):125-33. [DOI] [PubMed] [Google Scholar]
Giuliano 2013 {published data only}
- Giuliano V, Giuliano C. Improved breast cancer detection in asymptomatic women using 3D-automated breastultrasound in mammographically dense breasts. Clinical Imaging 2013;37(3):480-6. [DOI] [PubMed] [Google Scholar]
J‐START {published data only}
- Harada-Shoji N, Suzuki A, Ishida T, Zheng Y-F, Narikawa-Shiono Y, Sato-tadano A, et al. Evaluation of adjunctive ultrasonography for breast cancer detection among women aged 40-49 years with varying breast density undergoing screening mammography: a secondary analysis of a randomized clinical trial. JAMA Network Open 2021;4(8):e2121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi N, Ishida T, Kawai M, Narikawa Y, Yamamoto S, Sobue T. Randomized controlled trial on effectiveness of ultrasonography screening for breast cancer in women aged 40-49 (J-START): research design. Japanese Journal of Clinical Oncology 2011;41(2):275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi N, Suzuki A, Sobue T, Kawai M, Yamamoto S, Zheng YF, et al, J-START investigator groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet 2016;387(10016):341-8. [DOI] [PubMed] [Google Scholar]
Lee 2019 {published data only}
- Lee JM, Arao RF, Sprague BL, Kerlikowske K, Lehman CD, Smith RA, et al. Performance of screening ultrasonography as an adjunct to screening mammography in women across the spectrum of breast cancer risk. JAMA Internal Medicine 2019;179(5):658-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Starikov 2016 {published data only}
- Starikov A, Drotman M, Hentel K, Katzen J, Min RJ, Arleo EK. 2D mammography, digital breast tomosynthesis, and ultrasound: which should be used for the different breast densities in breast cancer screening? Clinical Imaging 2016;40(1):68-71. [DOI] [PubMed] [Google Scholar]
Tohno 2013 {published data only}
- Tohno E, Umemoto T, Sasaki K, Morishima I, Ueno E. Effect of adding screening ultrasonography to screening mammography on patient recall and cancer detection rates: a retrospective study in Japan. European Journal of Radiology 2013;82(8):1227-30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Boonlikit 2013 {published data only}
- Boonlikit S. Comparison of mammography in combination with breast ultrasonography versus mammography alone for breast cancer screening in asymptomatic women. Asian Pacific Journal of Cancer Prevention 2013;14(12):7731-6. [DOI] [PubMed] [Google Scholar]
Brem 2015 {published data only}
- Brem RF, Tabár L, Duffy SW, Inciardi MF, Guingrich JA, Hashimoto BE, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology 2015;274(3):663-73. [DOI] [PubMed] [Google Scholar]
Chou 2017 {published data only}
- Chou HH, Chen SC. Clinical analysis of the outcome of breast ultrasound after screening mammography as breast imaging reporting and data system (BI-RADS) category. Ultrasound in Medicine and Biology 2017;43:S22. [Google Scholar]
Dong 2018 {published data only}
- Dong H, Huang Y, Song F, Dai H, Liu P, Zhu Y, et al. Improved performance of adjunctive ultrasonography after mammography screening for breast cancer among Chinese females. Clinical Breast Cancer 2018;18(3):e353-61. [DOI] [PubMed] [Google Scholar]
Dromain 2012 {published data only}
- Dromain C, Thibault F, Diekmann F, Fallenberg EM, Jong RA, Koomen M, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results of a multireader, multicase study. Breast Cancer Research 2012;14(3):R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franchini 2021 {published data only}
- Franchini M, Pieroni S, Montrucchio E, Nori Cucchiari J, Di Maggio C, Cassano E, D et al, On Behalf Of The Pink Consortium. The P.I.N.K. study approach for supporting personalized risk assessment and early diagnosis of breast cancer. International Journal of Environmental Research and Public Health 2021;18(5):2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang C, Fann C, Hsu G, Ho M, Chang K, Chen S, et al. A population-based cross-over randomized controlled trial of breast cancer screening with alternate mammography and ultrasound for women aged 40 to 49 years in Taiwan. Cancer Research 2009;69(24 Suppl):73. [Google Scholar]
Hwang 2015 {published data only}
- Hwang JY, Han BK, Ko EY, Shin JH, Hahn SY, Nam MY. Screening ultrasound in women with negative mammography: Outcome analysis. Yonsei Medical Journal 2015;56(5):1352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishida 2014 {published data only}
- Ishida T, Suzuki A, Kawai M, Narikawa Y, Saito H, Yamamoto S, et al. A randomized controlled trial to verify the efficacy of the use of ultrasonography in breast cancer screening aged 40-49 (J-START): 76 196 women registered. Japanese Journal of Clinical Oncology 2014;44(2):134-40. [DOI] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee M, Mariapun S, Rajaram N, Teo S H, Yip CH. Performance of a subsidised mammographic screening programme in Malaysia, a middle-income Asian country. BMC Public Health 2017;17(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leong 2012 {published data only}
- Leong LC, Gogna A, Pant R, Ng FC, Sim LS. Supplementary breast ultrasound screening in Asian women with negative but dense mammograms-a pilot study. Annals of the Academy of Medicine, Singapore 2012;41(10):432-9. [PubMed] [Google Scholar]
Melnikow 2016 {published data only}
- Melnikow J, Fenton JJ, Whitlock EP, Miglioretti DL, Weyrich MS, Thompson JH, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Service Task Force. Agency for Healthcare Research and Quality 2016;126:14-05201-EF-3. [PubMed]
Moon 2015 {published data only}
- Moon HJ, Jung I, Park SJ, Kim MJ, Youk JH, Kim EK. Comparison of cancer yields and diagnostic performance of screening mammography vs. supplemental screening ultrasound in 4394 women with average risk for breast cancer. Ultraschall in der Medizin/European Journal of Ultrasound 2015;36(3):255-63. [DOI] [PubMed] [Google Scholar]
Nelson 2016 {published data only}
- Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive Services Task Force Recommendation. Annals of Internal Medicine 2016;164(4):256-67. [DOI] [PubMed] [Google Scholar]
Sakurai 2014 {published data only}
- Sakurai K, Fujisaki S, Nagashima S, Maeda T, Tomita R, Suzuki S, et al. [Success rate of breast conserving surgery for different breast cancer screening methods]. Gan To Kagaku Ryoho 2014;41(12):1890-1. [PubMed] [Google Scholar]
Shen 2015 {published data only}
- Shen S, Zhou J, Xu J, Zhang B, Duan X, Huang R, et al. A multi-centre randomised trial comparing ultrasound vs. mammography for screening breast cancer in high-risk Chinese women. British Journal of Cancer 2015;112(6):998-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skaane 2015 {published data only}
- Skaane P, Gullien R, Eben EB, Sandhaug M, Schulz-Wendtland R, Stoeblen F. Interpretation of automated breast ultrasound (ABUS) with and without knowledge of mammography: A reader performance study. Acta Radiologica 2015;56(4):404-12. [DOI] [PubMed] [Google Scholar]
Spraque 2015 {published data only}
- Sprague BL, Stout NK, Schechter C, Ravesteyn NT, Cevik M, Alagoz O, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Annals of Internal Medicine 2015;162(3):157-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tagliafico 2016 {published data only}
- Tagliafico A, Calabrese M, Mariscotti G, Durando M, Tosto S, Monetti F, et al. Interim results of the adjunct screening with tomosynthesis or ultrasound in mammography-negative dense breasts (ASTOUND) trial. In: European Journal of Cancer. Vol. 57. 2016:S3-4.
Weigel 2013 {published data only}
- Weigel S, Biesheuvel C, Berkemeyer S, Kugel H, Heindel W. Digital mammography screening: how many breast cancers are additionally detected by bilateral ultrasound examination during assessment? European Radiology 2013;23(3):684-91. [DOI] [PubMed] [Google Scholar]
Weigert 2015 {published data only}
- Weigert J, Steenbergen S. The connecticut experiments second year: ultrasound in the screening of women with dense breasts. The Breast Journal 2015;21(2):175-80. [DOI] [PubMed] [Google Scholar]
Weigert 2017 {published data only}
- Weigert JM. The Connecticut Experiment; The Third Installment: 4 years of screening women with dense breasts with bilateral ultrasound. The Breast Journal 2017;23(1):34-9. [DOI] [PubMed] [Google Scholar]
Wilczek 2016 {published data only}
- Wilczek B, Wilczek H E, Rasouliyan L, Leifland K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. European Journal of Radiology 2016;85(9):1554-63. [DOI] [PubMed] [Google Scholar]
Yaffe 2016 {published data only}
- Yaffe MJ, Jong RA. Adjunctive ultrasonography in breast cancer screening. Lancet 2016;387(10016):313-4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
DRKS00019097 {published data only}
- DRKS00019097. Density-indicated mammographic-sonographic breast cancer screening. https://drks.de/search/en/trial/DRKS00019097 (first received 13 November 2019).
NCT04097366 {published data only}
- NCT04097366. Breast screening - risk adaptive imaging for density breast screening (BRAID). clinicaltrials.gov/show/NCT04097366 (first received 20 September 2019).
NCT04429269 {published data only}
- NCT04429269. A comparative study of mammography and ultrasound for breast cancer screening and early diagnosis. clinicaltrials.gov/ct2/show/NCT04429269 (first received 12 June 2020).
Additional references
Albert 2009
- Albert US, Altland H, Duda V, Engel J, Geraedts M, Heywang-Kobrunner S. 2008 update of the guideline: early detection of breast cancer in Germany. Journal of Cancer Research and Clinical Oncology 2009;135(3):339-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Berg 2008
- Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008;299(18):2151-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 2012
- Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307(13):1394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bleyer 2012
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. The New England Journal of Medicine 2012;367(21):1998-2005. [DOI] [PubMed] [Google Scholar]
Brem 2015
- Brem RF, Tabár L, Duffy SW, Inciardi MF, Guingrich JA, Hashimoto BE, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology 2015;274(3):663-73. [DOI] [PubMed] [Google Scholar]
Carney 2003
- Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Annals of Internal Medicine 2003;138(3):168-75. [DOI] [PubMed] [Google Scholar]
CTFPHC 2018
- Canadian Task Force on Preventive Health Care. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. Canadian Medical Association Journal 2018;190:E1441-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daly 2021
- Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2021;19(1):77-102. [DOI] [PubMed] [Google Scholar]
Duffy 2020
- Duffy S, Vulkan D, Cuckle H, Parmar D, Sheikh S, Smith R, et al. Annual mammographic screening to reduce breast cancer mortality in women from age 40 years: long-term follow-up of the UK Age RCT. Health Technology Assessment 2020;24(55):1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elshof 2015
- Elshof LE, Tryfonidis K, Slaets L, Leeuwen-Stok AE, Skinner VP, Dif N, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ - The LORD study. European Journal of Cancer 2015;51(12):1497-510. [DOI] [PubMed] [Google Scholar]
EndNote 20 [Computer program]
- EndNote. Version 20.1. Clarivate, 30 October 2020. https://endnote.com.
Esserman 2014
- Esserman LJ, Thompson IM, Reid B, Nelson P, Ransohoff DF, Welch HG, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. The Lancet Oncology 2014;15(6):e234-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
European Comission 2021
- Women with high breast density. https://healthcare-quality.jrc.ec.europa.eu/european-breast-cancer-guidelines/dense-breast 3 August 2021.
European Commission 2022
- Organising breast cancer screening programmes. https://healthcare-quality.jrc.ec.europa.eu/european-breast-cancer-guidelines/organisation-of-screening-programme#recs-group-1 8 November 2022.
Francis 2015
- Francis A, Thomas J, Fallowfield L, Wallis M, Bartlett JM, Brookes C, et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. European Journal of Cancer 2015;51(16):2296-303. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 30 November 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gøtzsche 2013
- Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammography. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD001877. [DOI: 10.1002/14651858.CD001877.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heller 2015
- Heller SL, Hudson S, Wilkinson LS. Breast density across a regional screening population: effects of age, ethnicity and deprivation. The British Journal of Radiology 2015;88(1055):20150242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019) The Cochrane Collaboration, 2019. Available from training.cochrane.org/handbook/archive/v6.
HIRU
- Health Information Research Unit. Search Filters for MEDLINE in Ovid Syntax and the PubMed translation. https://hiru.mcmaster.ca/hiru/HIRU_Hedges_MEDLINE_Strategies.aspx accessed 01 May 2021.
Hwang 2019
- Hwang E S, Hyslop T, Lynch T, Frank E, Pinto D, Basila D, et al. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open 2019;9(3):e026797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerlikowske 2015
- Kerlikowske K, Zhu W, Tosteson AN, Sprague BL, Tice JA, Lehman CD, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Annals of Internal Medicine 2015;162(10):673-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynge 2019
- Lynge E, Vejborg I, Andersen Z, Euler-Chelpin M, Napolitano G. Mammographic density and screening sensitivity, breast cancer incidence and associated risk factors in Danish breast cancer screening. Journal of Clinical Medicine 2019;8(11):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melnikow 2016
- Melnikow J, Fenton JJ, Whitlock EP. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Service Task Force. Agency for Healthcare Research and Quality (US). Vol. Evidence Syntheses, No. 126. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343793/: U.S. Preventive Services Task Force, 2016. [PubMed] [Google Scholar]
Moshina 2018
- Moshina N, Sebuødegård D, Lee CI, Akslen LA, Tsuruda KM, Elmore JG, et al. Automated volumetric analysis of mammographic density in a screening setting: worse outcomes for women with dense breasts. Radiology 2018;288(2):343-52. [DOI] [PubMed] [Google Scholar]
Nelson 2009
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, US Preventive Services Task Force. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2009;151(10):727-37, W237-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2016a
- Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Annals of Internal Medicine 2016;164(4):244-55. [DOI] [PubMed] [Google Scholar]
Nelson 2016b
- Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L, US Preventive Services Task Force. Harms of Breast Cancer Screening: Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Annals of Internal Medicine 2016;164(4):256-67. [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
SEER Cancer Statistics
- National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2016. https://seer.cancer.gov/statfacts/html/breast.html accessed 2021.
Siu 2016
- Siu AL. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine 2016;164(4):279-96. [DOI] [PubMed] [Google Scholar]
Sopik 2018
- Sopik V, Narod SA. The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Research and Treatment 2018;170(3):647-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:I4898. [DOI] [PubMed] [Google Scholar]
Sung 2021
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
US FDA
- Compliance guidance: the Mammography Quality Standards Act Final Regulations: preparing for MQSA inspections; final. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/compliance-guidance-mammography-quality-standards-act-final-regulations-preparing-mqsa-inspections 20 August 2018.
van Seijen 2019
- Seijen M, Lips EH, Thompson AM, Nik-Zainal S, Futreal A, Hwang ES, et al. Ductal carcinoma in situ: to treat or not to treat, that is the question. British Journal of Cancer 2019;121(4):285-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waffenschmidt 2020
- Waffenschmidt S, Navarro‐Ruan T, Hobson N, Hausner E, Sauerland S, Haynes RB. Development and validation of study filters for identifying controlled non‐randomized studies in PubMed and Ovid MEDLINE. Research Synthesis Methods 2020;11(5):617-26. [DOI] [PubMed] [Google Scholar]
Welch 2016
- Welch G, Prorok P, O'Malley J, Kramer B. Breast-cancer tumor size, overdiagnosis and mammography screening effectiveness. The New England Journal of Medicine 2016;375:1438-47. [DOI] [PubMed] [Google Scholar]
Yuan 2020
- Yuan WH, Hsu HC, Chen YY, Wu CH. Supplemental breast cancer-screening ultrasonography in women with dense breasts: a systematic review and meta-analysis. British Journal of Cancer 2020;123(4):673-88. [DOI] [PMC free article] [PubMed] [Google Scholar]



