Abstract
Purpose of Review
To provide an up-to-date overview of recent developments in diagnostic methods and therapeutic approaches for chronic wound biofilms and pathogenic microbiota.
Recent Findings
Biofilm infections are one of the major contributors to impaired wound healing in chronic wounds, including diabetic foot ulcers, venous leg ulcers, pressure ulcers, and nonhealing surgical wounds. As an organized microenvironment commonly including multiple microbial species, biofilms develop and persist through methods that allow evasion from host immune response and antimicrobial treatments. Suppression and reduction of biofilm infection have been demonstrated to improve wound healing outcomes. However, chronic wound biofilms are a challenge to treat due to limited methods for accurate, accessible clinical identification and the biofilm’s protective properties against therapeutic agents. Here we review recent approaches towards visual markers for less invasive, enhanced biofilm detection in the clinical setting. We outline progress in wound care treatments including investigation of their antibiofilm effects, such as with hydrosurgical and ultrasound debridement, negative pressure wound therapy with instillation, antimicrobial peptides, nanoparticles and nanocarriers, electroceutical dressings, and phage therapy.
Summary
Current evidence for biofilm-targeted treatments has been primarily conducted in preclinical studies, with limited clinical investigation for many therapies. Improved identification, monitoring, and treatment of biofilms require expansion of point-of-care visualization methods and increased evaluation of antibiofilm therapies in robust clinical trials.
Keywords: Biofilm, Microbiome, Host–pathogen interactions, Chronic wounds, Wound infection, Antimicrobials
Introduction
Biofilms consist of bacteria and fungi organized within a protective layer of extracellular polymeric substance (EPS) matrix comprised of deoxyribonucleic acid (DNA), immunoglobulins, and proteins from both bacteria and host [1]. Recent consensus further defines biofilms as an immunologically protected, genetically diverse microbial community with up to 1,000 times more resistance to antibiotics compared to planktonic bacteria, serving as a source of persistent and recurrent infection [2]. Biofilm infection elicits an inappropriate host inflammatory response, significantly damaging local tissue and skin barrier function. Particularly in chronic wounds, polymicrobial biofilm composition may be influenced by oxidative stress levels in the tissue microenvironment and even by patient genetic variation [3, 4]. Biofilm interaction with the host immune system can lead to persistence of cutaneous inflammatory disease pathogenesis, including in atopic dermatitis [5], acne [6], and hidradenitis suppurativa [7]. Biofilm-driven pathogenesis has been most frequently implicated in chronic wounds including diabetic foot ulcers (DFU), venous leg ulcers (VLU), decubitus or pressure ulcers (PU) and nonhealing surgical wounds [1, 8]. Thus, discussion of diagnostic tools and treatment options herein will focus on wound-based biofilms.
Chronic Wound Microbiome and Host Response
Traditional culture techniques have been found to underestimate the bacterial load and composition of the wound microbiome, especially of anaerobic species. Therefore, microbiome characterization has shifted towards culture-independent techniques such as 16S rRNA sequencing and metagenomic approach [9, 10]. Metagenomic shotgun sequencing of the wound microbiota provides an advantage over 16S sequencing in strain-specific identification, including the ability to discern bacterial isolates with genes providing antibiotic resistance or enterotoxins that correlate to wound healing outcomes [9, 11]. In general, non-healing wounds have lower bacterial diversity than healthy skin [12], with microbial stability correlating to poor clinical outcomes [13••]. Microbiota composition has also been found to differ between ulcer phenotypes based on wound depth, chronicity, and outcomes of healing [10, 14]. In addition to bacteria, 80% of chronic wounds contain fungi, and fungal-bacterial biofilms are associated with poor clinical outcomes [15]. Limited evidence in DFU patients suggests antibiotic treatment does not change overall microbial diversity or abundance [13••]. Antibiotics may even induce a shift in the microbiota to promote virulence factors of pathogenic bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and further delay wound re-epithelialization. These data suggest the need for careful consideration of antibiotic administration and its potential impact on the wound microbiota.
Bacterial biofilms have been shown to trigger an inflammatory response distinct from that of planktonic bacteria, sustaining chronic wound pathogenesis and bacterial infection (Fig. 1) [16••, 17, 18]. Dysfunction of DFU neutrophils may further contribute to inability of host tissue to respond and clear wound biofilm [19]. Stimulated phagocytic evasion, reactive oxygen species (ROS) production, matrix metalloprotease induction, and resulting collagen degradation damage host tissue and contribute to impaired healing [18, 20, 21]. In addition, biofilm infection downregulates tight junction proteins and compromises the skin barrier to cause increased transepidermal water loss [22]. Most recently, S. aureus was identified to have an intracellular niche in DFU epidermal keratinocytes via suppression of innate immune molecule Perforin-2 [23–25]. While it remains to be determined if the intracellular S. aureus contributes to biofilm formation, it has been hypothesized to contribute to persistence of DFU infection. Further studies are needed on the complex interactions in biofilms and subsequent impact on the host chronic wound environment to facilitate therapeutic developments targeting biofilm while also stimulating healing.
Fig. 1.
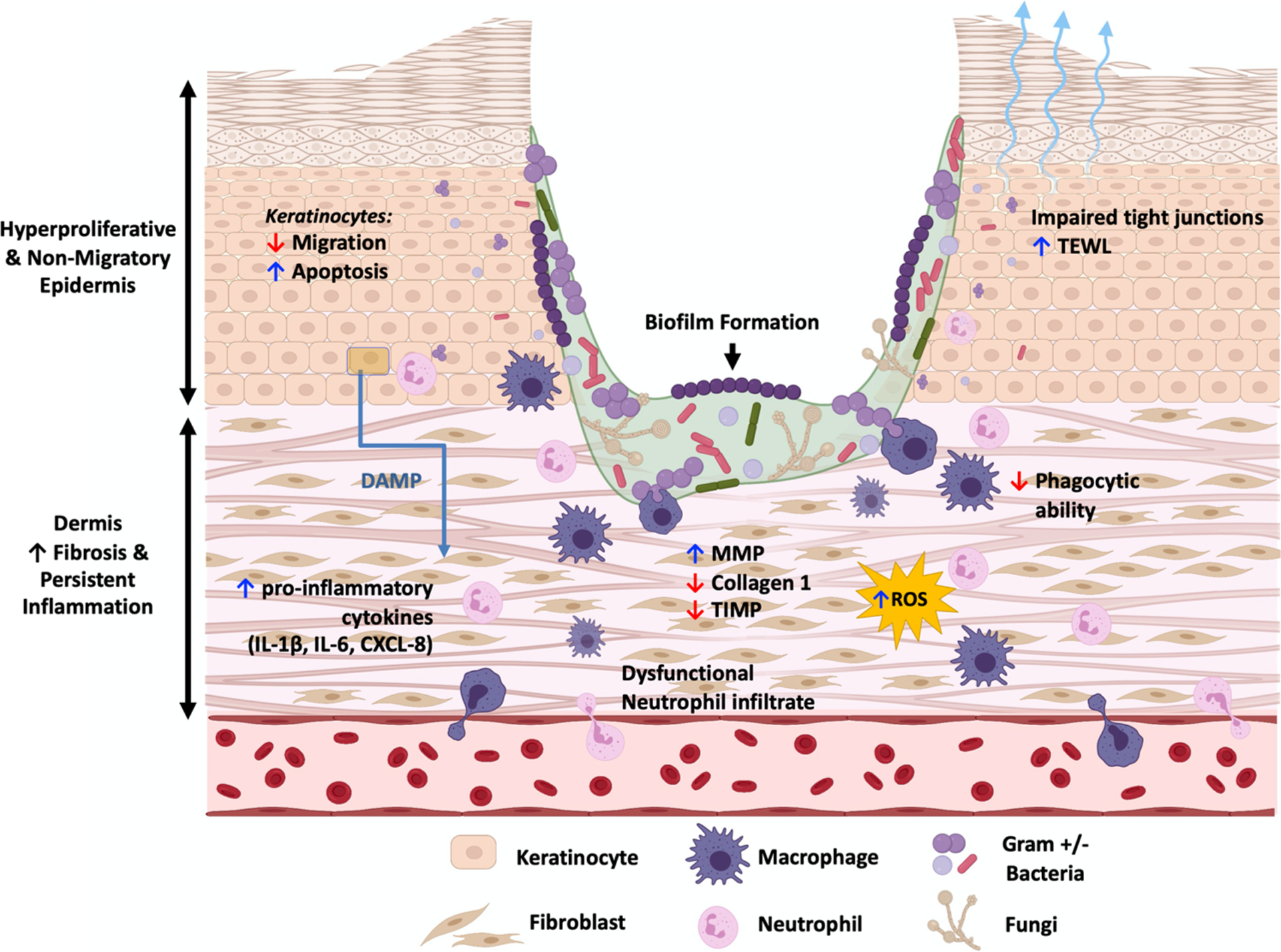
Molecular pathology of chronic wound biofilms. Chronic wounds exhibit a hyperproliferative and non-migratory epidermis, unresolved inflammation, and fibrosis. Biofilm presence, which can incorporate a diverse community of bacteria and fungi, promotes impaired keratinocyte migration, dysregulated inflammatory response, and inflammatory cell dysfunction. Additionally, biofilm damages host tissue through increased neutrophilic reactive oxygen species production, imbalance of metalloproteases and inhibitors, and breakdown of keratinocyte tight junctions. These processes further perpetuate chronic wound pathogenesis. (DAMP, damage-associated molecular patterns; MMP, matrix metalloprotease; ROS, reactive oxygen species; TIMP, tissue inhibitor of matrix metalloprotease; TEWL, transepidermal water loss)
Diagnostics of Chronic Wound Biofilms
Wound biofilms are a therapeutic challenge in part due to limited accurate clinical identification. While only ~ 6% of acute wounds contain a biofilm, prevalence of biofilm in chronic wounds was found to range from 78 to 100% in patient-focused studies [26, 27]. Electron microscopy is the gold standard for biofilm identification; however, it is a labor- and time-intensive process that is not conducive to clinical point-of-care. Previous clinical indicators for wound biofilms such as presence of slough, exudate, and poor granulation tissue formation have demonstrated low correlation with diagnostic accuracy [28].
Advancement of point-of-care diagnostic tools include the most recent developments in visual markers for enhanced biofilm detection in the clinic (Table 1). One of the methods is application of probes targeting bacteria or biofilm components. For example, an enzyme-based hydrogel involving biomarker alkaline phosphatase changes colors from yellow to purple to indicate detection of S. aureus in porcine wound models [29]. Another biofilm detection method is wound blotting and staining. The dyes alcian blue and ruthenium red stain EPS polysaccharides blotted from the biofilm, with alcian blue generally preferred due to quicker staining under a few minutes and increased sensitivity compared to ruthenium red [30]. Beyond hydrogels and dyes, a handheld fluorescence imaging device, MolecuLight i:X, uses violet light to stimulate and detect bacteria in wounds. S. aureus and Escherichia coli emit red fluorescence due to their porphyrin production, while Pseudomonas aeruginosa fluoresces cyan due to production of the fluorescent siderophore pyoverdine [31, 32]. Clinical studies have found MolecuLight i:X to increase bacterial detection fourfold, with a positive predictive value of up to 92.9% for certain species, significantly improving patient care [32, 33••]. Blotting and MolecuLight i:X methods also help localize the distribution of wound infection [30, 34]. With the rapid development of bacterial biofilm identification technologies, visual point-of-care approaches are likely the future of the wound biofilm identification in the clinic.
Table 1.
Novel biofilm identification methods. Mechanism of diagnostic method, advantages, and disadvantages are summarized for each approach
| Diagnostic method | Mechanism | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Electron microscopy | Tissue biopsy processed and viewed under electron microscope, screen for microbial aggregates and EPS | Gold standard; high level of resolution; visualizes both surface and deep layers of biofilm | Requires invasive wound biopsy; detection limited to sampling location | [27, 28] |
| Fluorescent probe biomarkers in hydrogel | Fluorescent probe recognizes biofilm marker (e.g., alkaline phosphatase) and induces color change of hydrogel | Noninvasive; prompt detection with color change within 24 h | Probe recognition selectively limited to bacteria type or species; at proof-of-concept stage | [29] |
| Wound blotting with staining | Blotting the wound transfers biofilm to a nitrocellulose membrane; dyes stain the polysaccharides of biofilm EPS matrix: alcian blue > ruthenium red in sensitivity | Noninvasive; maps biofilm distribution on wound surface; rapid detection with visualization within 2 min | Staining set-up required | [30, 121] |
| Fluorescent imaging (MolecuLight i:X) | 405 nm excitation light emitting diodes shone on wound; fluorescent image captured on device; host tissue appears green, bacteria at > 10^4 CFU/g appear red or cyan | Noncontact, handheld device; rapid detection with image at point of care | Does not distinguish between planktonic and biofilm bacteria | [31–33••, 122] |
Pre-clinical Biofilm Wound Infection Models
Murine Wound Biofilm Models
Murine models are the most common animal models used in scientific research due to low cost of acquisition and maintenance [35]. One benefit is their rapid rate of wound healing, allowing shorter experimental timelines. However, wound healing in murine skin primarily uses contractile forces to close wounds, while human skin primarily utilizes re-epithelialization [35]. To establish biofilm in murine models, wounds are often directly inoculated or infected with pre-formed biofilm [36]. One model utilizes luciferase-expressing bacteria, wherein luminescence released by the breakdown of biofilm EPS and cell lysis signals effects of antimicrobial and biofilm dispersing agents [37]. Biofilm can alternatively be visualized through fluorescent staining or electron microscopy [38]. Diabetic db/db−/− murine models also demonstrate ability to develop wound biofilms under high levels of oxidative stress, using topical antioxidant enzyme inhibitors such as mercaptosuccinic acid and 3-amino-1,2,4-triazole to promote polymicrobial biofilms resembling those of human wounds [3, 39]. While this biofilm model has not been used for pre-clinical assessment of treatments, it has potential for use in future research.
Rabbit Ear Wound Biofilm Model
The rabbit ear is another popular modality to study chronic wound infection as it replicates the ischemia that plays a significant role in developing chronic wounds. An ischemic wound can be created by suturing the arterial blood supply to the rabbit ear and subsequently creating a full-thickness ear wound. The pathogenesis and treatment of infection can then be studied in an ischemic context more closely resembling that of a chronic wound [40]. Like mouse models, rabbits are inexpensive to maintain due to their smaller size and their wounds primarily heal through re-epithelialization [35]. However, anatomical differences still exist from human skin. The dermis is firmly attached to underlying ear cartilage, with an avascular wound base that prevents exact replication of human wound healing. Methods to establish biofilm include monospecies bacterial inoculation into the wound, with impaired wound healing and increased inflammatory cytokines, while topical antibiotics applied 4 days post-wounding to eliminate planktonic bacteria provide improved wound biofilm model in this host [41, 42].
Porcine Wound Biofilm Model
Of all the animal models, the porcine wound healing model is the closest to recapitulating human wound healing. Porcine skin is the most comparable to human skin, with similarities in epidermal thickness, dermal-epidermal thickness ratios, collagen peptides, patterns of hair follicles and blood vessels, and histological location of epidermal keratins 10 and 16, dermal collagen IV, vimentin, and fibronectin [43, 44]. Wound healing also primarily occurs through epithelialization [45]. Drawbacks to experimental use include high costs due to their larger size. However, the large size also allows testing for multiple replicates and therapies within the same experimental animal. In addition to testing multiple novel therapies for biofilm-infected wounds such as antimicrobial nanofiber dressings, electroceutical dressings, and new antibiotics [44], the porcine wound infection model has compared efficacy between different debridement techniques to highlight promising therapeutics that require further investigation in the clinical setting [46].
Novel Therapeutics Against Wound Biofilms
The major challenge in biofilm treatment is that microorganisms are well protected from the host immune response and antimicrobials by several mechanisms including the quorum sensing system, which facilitates bacterial communication to regulate biofilm formation, and the EPS matrix, which impairs treatment penetration and bacterial killing [2]. Successful suppression of biofilm formation significantly improves treatment efficacy and wound healing outcomes. Below we outline current and developing methods against biofilm including physical disruption, targeting EPS or quorum sensing systems, and nanoparticles for enhanced drug delivery or direct bactericidal effects (Table 2).
Table 2.
Novel anti-biofilm therapeutic approaches. Mode of action, advantages, and disadvantages of various therapeutics are summarized
| Therapeutic method | Mode of action | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| Sharp wound debridement | Scalpel for mechanical removal of bacterial aggregates | Improves healing outcomes, increases susceptibility to antibiotics | Temporary reduction; difficulty accessing deeper layers of infection | [49] |
| Hydrosurgical debridement | High-pressure waterjet for mechanical removal of bacterial aggregates | More efficient compared to sharp surgical debridement | Increased risk of air contamination | [50–52] |
| Ultrasound debridement | Low-frequency ultrasonic waves applied to wound; non-contact or contact application | Preserves viable granulation tissue, reduced slough and exudate | Variety of devices and settings, limited evidence for an optimal setting | [54, 55] |
| NPWTia | Vacuum generates sub-atmospheric pressure in wound area; topical antimicrobials delivered between cycles of negative pressure | Improves healing outcomes; enhanced effect compared to NPWT | Limited patient mobility for up to 22 h; skin irritation around wound due to device adhesion | [58, 60••, 61] |
| AMPsb | Molecules with antimicrobial activity that also modulate host immunity; can promote biofilm dispersal through disrupting quorum sensing and adhesion | Large database of potential natural and synthetic AMPs | Reduced peptide stability in vivo; potential cytotoxicity; potential bacterial evasion in biofilm | [74, 76] |
| Nanotechnology | 3 mechanisms: particles that directly impair bacterial function and biofilm, carriers that deliver antimicrobials into biofilm, particles harnessing energy for physical damage | Diffusion through biofilm; can be designed for selective activation; can carry a variety of molecules | Potential cytotoxic effects depending on active molecule | [80, 85, 90] |
| Honey-based dressing | Bactericidal activity, inhibits bacterial adhesion to extracellular matrix components | Synergistic antibiofilm effect with adjuvant antibiotics | Antimicrobial activity varies between bacterial species | [95, 96, 100] |
| WEDC | Electric field generated by redox reaction across dressing, interfering with bacterial electric signaling for biofilm formation | Less risk of acquiring bacterial resistance | Lack of clinical evidence for antibiofilm efficacy | [105, 106] |
| Micelle matrix gel | Concentrated surfactants disrupt biofilm EPS forces and prevent biofilm formation | Noncytotoxic; less risk of acquiring bacterial resistance | Questionable efficacy against 5. aureus | [108, 109] |
| Xbio™ based gel | Deconstruct EPS matrix’s metallic bonds and polymers, lyses bacteria using osmolarity gradient and surfactant | Healing outcomes superior to broad-spectrum antimicrobials; reduced risk of bacterial resistance | Co-application of antibacterial therapies such as silver may interfere with technology | [108, 110, 111] |
| Phage therapy | Phage virus lyses targets bacterial cells and degrades biofilm matrix | Antimicrobial activity while sparing local microbiota and tissue; customized against specific bacterial strains | Narrow range of efficacy due to specificity; risk of modulating host immune system, resistance, and horizontal transfer of virulence genes | [112, 116••, 117–119] |
NPWTi, negative pressure wound therapy with instillation
AMPs, antimicrobial peptides
WED, wireless electroceutical dressing
Debridement
One of the goals in wound debridement is to reduce bioburden, including necrotic tissue and bacteria, by disrupting the EPS matrix and converting biofilm to planktonic bacteria that is temporarily susceptible to antimicrobial therapy. There are multiple debridement approaches including mechanical, biological (maggot/larval therapy), enzymatic, and ultrasonic methods.
The gold standard to treatment is surgical or conservative sharp wound debridement [2]. However, sharp debridement only temporarily reduces bacterial burden and studies suggest limited removal of microorganisms [22, 47, 48]. Therefore, serial debridement of matured biofilms and use of adjunctive antimicrobial therapy is necessary [2, 49]. Remaining bacteria in deeper layers of the debrided tissue may promote persistent, clinically undetected infection [22]. Hydrosurgical debridement, involving a high-pressure waterjet using up to 15,000 psi, may demonstrate more efficient and precise reduction of bacterial biofilms compared to scalpel use [50]; however, clinical comparison studies have been inconclusive [51]. Additionally, studies have shown significant increased levels of air contamination with bacteria after treatment [50, 52].
Ultrasound debridement involves mechanical low-frequency ultrasonic waves (20–40 kHz) to remove devitalized and necrotic soft tissue while preserving viable tissue [53]. Clinical studies suggest non-contact ultrasonic debridement has limited effect on bacterial burden in wounds compared to contact ultrasound [54]; however, it is hypothesized that effect on wound biofilms is underestimated due to the use of culture-based techniques for identification [55]. Other debridement strategies, including maggot/larvae and enzymatic debridement targeting EPS matrix, have more limited clinical evidence and usage [2]. Lack of visualization of biofilm aggregates is a limitation to complete removal of biofilm during wound debridement, and visual markers discussed above should be utilized to ensure complete removal.
Negative Pressure Wound Therapy
Negative pressure wound therapy (NPWT), also known as vacuum assisted closure (VAC) therapy, utilizes a pump to generate sub-atmospheric pressure in a local area. The negative pressure removes excess exudate, improves blood flow, and reduces bacterial colonization [56]. NPWT has been shown to modulate adhesion factors and quorum sensing systems of S. aureus–and P. aeruginosa–infected murine wounds, causing decrease in biofilm matrix and more scattered colonies [57, 58]. Some clinical reports support bacterial clearance in diabetic wounds [56], but a systematic review of patient wound studies found no change in bacterial burden based on limited evidence, requiring more clinical studies of NPWT’s antimicrobial and anti-biofilm functions [59].
To improve antimicrobial effects, NPWT has been combined with a topical antimicrobial solution delivered to the wound in a regulated, cyclical manner between phases of negative pressure, known as negative pressure wound therapy with instillation (NPWTi). Clinical reports support enhanced effect against bacterial burden compared to just NPWT, particularly in complex wounds such as those with extensive biofilm [58, 60••]. A clinical trial measured decreased nonplanktonic bacteria in chronic ulcers under NPWTi therapy with 0.125% sodium hypochlo-rite solution [61] and other studies demonstrated efficacy with 1% acetic acid, polyhexamethylene biguanide solution, normal saline, and a commercial biofilm-disrupting agent [62–65]. NPWT has been combined with other antimicrobial modalities as well, including silver, with reduced bacterial load in lower extremity wounds of high-velocity trauma patients [66]. Some limitations to NPWT include limited patient mobility due to the patient being attached to the NPWT device, and irritation to peri-wound skin from device adhesion.
Antimicrobial Peptides
Antimicrobial peptides (AMPs) are produced constitutively by many cell types, including resident skin cells, and induced during inflammation or infection, including beta-defensins, cathelicidins (LL-37), and perforin-2 [67]. As widely conserved molecules, AMPs have a broad spectrum of antimicrobial activity and can modulate the host immune system to increase antigen presenting cells, phagocytosis, and suppress inflammatory signaling [67]. They also demonstrate ability to target dormant and intracellular populations, with diminished resistance levels compared to those of antibiotics [24, 68]. However, AMP resistance can eventually develop [69]. Selective antimicrobial activity is also possible through synthetic AMPs containing a binding peptide targeting specific species, which can promote a shift in multispecies biofilm communities to a “healthy” microbiome [70].
Natural and synthetic AMPs specifically act against biofilm by disrupting quorum sensing, inhibiting bacterial adhesion, and promoting biofilm dispersal. One of the first, and most studied, AMPs for anti-biofilm capabilities is LL-37 [71]. LL-37-derived topical gels have demonstrated efficacy against MRSA infection in ex vivo human skin wound models [72]. There are numerous AMPs continuing to be discovered or designed, with databases such as APD (http://aps.unmc.edu/AP/) and DRAMP (http://dramp.cpu-bioinfor.org/) each containing over 2000 entries. Most investigation has been limited to in vitro studies, with a few in vivo animal studies and significantly fewer specifically studying biofilm inhibition.
In the clinical setting, established treatments include cathelicidin AMPs such as colistin (polymyxin E), polymyxin B, and chlorhexidine [73]. Phase III clinical trials of infected DFU found topical AMP pexiganan acetate to have equivalent results as oral antibiotic ofloxacin in microbial elimination rates and wound healing [74]. Currently intravenous Brilacidin, a host defense peptide mimetic, is being tested in a phase II trial for acute bacterial skin infections (NCT02052388). However, overall, translation of AMPs from preclinical to clinical evaluation has been significantly limited. This may be due to interference of the highly proteolytic host microenvironment and reduced peptide stability in vivo, and AMP cytotoxicity at higher concentrations [75]. Bacteria in biofilms can also enact enzymes, signaling, or resistance genes that allow evasion or inactivation of AMP-mediated bactericidal effects [76, 77]. Peptide stability and antibacterial activity are affected by vehicle of delivery as well, and recent advances in nanostructured antimicrobial peptides (Ns-AMPs) have attempted to improve on such deficiencies [78].
Nanotechnology
One of the largest challenges in biofilm treatment is impaired drug diffusion through the sticky biofilm matrix and dense cellular organization to effectively act on the biofilm structure or pathogenic microbes. Advances in nanotechnology have developed nanoparticles and systems to facilitate diffusion and precision of antibacterial therapies, using particle sizes smaller than biofilm pores and pH sensitivity enabling selective activation in the acidic biofilm microenvironment [79]. These nano-therapies act against biofilm through three main mechanisms: (1) nanoparticles that directly impair bacteria function and biofilm formation, (2) nanocarriers that deliver antimicrobials into biofilm, and (3) physical damage to biofilms.
Nanoparticles made of metal or metal oxide disrupt bacteria function and biofilm formation, including silver, copper, gold, titanium, and zinc [80]. In particular, silver nanoparticles have demonstrated inhibition of quorum sensing virulence factors and biofilm formation, along with antibacterial activity with wound healing in vitro and in vivo [81]. However, there is concern for emerging silver resistance among clinical isolates [82]. A comparative study of metal oxide nanoparticles (ZnO, CuO, and Fe2O3) found zinc oxide to exhibit the most antibacterial effect against multiple bacteria species, and treatment significantly reduced bacterial growth in murine models [83]. However, clinical translation of metal and metal oxide nanoparticles may be limited by cytotoxic effects to host cells such as keratinocytes and fibroblasts [84], and more investigation with in vivo models are needed to address potential nanoparticle toxicity.
The second use of nanoparticles is providing controlled and site-specific delivery of therapeutic agents. Established vehicles such as liposomes and polymeric nanoparticles are reviewed in depth by Forier et al. [85]. Another novel vehicle is vapor nanobubbles, which form around nanoparticles and can locally disturb biofilm integrity to improve antibiotics diffusion [86]. Nanoparticles, lipid-based nano-structures, and nanofiber dressings have demonstrated effective delivery of antibiotics, AMPs, and nitric oxide against biofilms in preclinical models with improved agent stability and action in vivo, although there have been few translated clinical studies thus far [85]. Delivery of photosensitizers into the biofilm for photodynamic therapy (PDT) has effectively impaired P. aeruginosa, MRSA, and S. epidermidis biofilms in vitro [87, 88]. Clinical studies for patients with infected chronic leg ulcers found that PDT significantly decreased bacteria levels, in correlation with wound healing [89••]. However, PDT may also hold cytotoxic effects against human fibroblasts [88].
Biofilms can also be physically disrupted through thermal or enzymatic damage. Irreversible thermal damage is generated by gold or magnetic nanoparticles (γ-Fe2O3 maghemite and Fe3O4 magnetite), which are activated by near-infrared light or alternate magnetic field [90]. These gold or magnetic nanoparticles also can be conjugated with antibiotics, further deepening antibiotic penetration into the biofilm [91]. Enzyme-functionalized nanoparticles can degrade biofilm EPS matrix, demonstrated in vitro against S. aureus biofilms [92].
Combining multiple antimicrobial and antibiofilm molecules into nano-based therapies allows lower doses of adjuvant drugs due to synergistic effects. However, limitations include costly development and lack of clinical use despite the increasing number of new formulations under laboratorial investigation. Further advances in antibiofilm nanotechnology require more focus on evaluating efficacy and biocompatibility of nanoparticles in vivo and in clinical studies, particularly with understanding potential toxicity and metabolism of the nanoparticles in patients.
Novel Dressings
Dressings are a centerpiece of wound care, promoting an environment favorable to healing by maintaining moisture, thermally insulating, allowing gaseous exchange, and, for some dressing materials, controlling microbial growth [93]. Antimicrobial-impregnated dressings, including alginates and silver, are traditionally useful against superficially infected or high-risk wounds [93]. However, bacterial resistance to silver treatment may represent a challenge [82]. Some newer dressing types with specifically antibiofilm properties include honey-based dressing and electroceutical dressings. Manuka honey–based wound dressings are currently U.S. Food and Drug Administration (FDA) cleared for management of chronic wounds and burns and are commercially available [94]. Its bactericidal activity relates to high methylglyoxal content, but is also likely influenced by other components such as low pH, hydrogen peroxide, and phenolic compounds [95]. Biofilm viability is reduced by inhibiting bacterial adhesion to major extracellular matrix components such elastin, fibronectin, and lamin [96], and synergistic antibiofilm effect has been demonstrated with adjuvant antibiotics in vitro [97]. Chronic wound infections also are effectively treated with manuka honey when introduced on scaffolds such as hydrogels and microneedles [98, 99]. However, the honey’s effectiveness varies between bacterial species [95]. A meta-analysis of clinical studies using medicinal honey dressing for DFU found accelerated bacterial clearance rate [100].
The skin naturally contains an electrical gradient, and modulating the host and bacterial electrical forces has become a novel method against biofilm. Presence of an endogenous electric field influences polarization and migration of host cells such as keratinocytes, fibroblasts, and leukocytes [101, 102]. Furthermore, electric signaling plays an important role in bacterial growth, function, and multi-species biofilm formation [103, 104]. Wireless electroceutical dressing (WED), FDA-cleared and available commercially (Procellera®), consists of a matrix embedded with silver and zinc that generates an electric field across the dressing through redox chemical reactions [105]. WED has demonstrated interference of the quorum sensing system, bacterial adherence, and EPS production, inhibiting biofilm-forming bacteria in vitro and disrupting biofilm integrity and biofilm-induced inflammation in vivo [106]. Electronic scaffolds that generate hypochlorous acid also inhibit biofilm formation with minimal damage to surrounding host tissue. Initial clinical studies find electroceutical dressing well tolerated with minimal adverse side effects [106], but investigations have been mostly limited to infected acute wounds [107] (NCT01938066, NCT04079998, NCT00816101) with one clinical trial in progress for biofilm infection in chronic wounds (NCT04794621). In targeting electro-interactions, WED anti-biofilm activity is unlikely to be attenuated by drug resistance from the biofilm-containing bacteria.
Additional topical therapies have emerged with biofilm disruption technology targeting the EPS matrix. Surfactant molecules can disrupt non-covalent forces of microbial aggregates and mature biofilms, playing a role in biofilm detachment and dispersion. A micelle matrix gel (marketed as Plurogel TM, Medline Inc, Northfield, IL) utilizes concentrated surfactant (Poloxamer 188) to impede biofilm development, with potential quorum-sensing interference (reviewed in [108]). Likewise, the proprietary technology of the Xbio product line (marketed as BlastX™, Next Science Inc, Jacksonville, FL), which includes an antimicrobial gel, disrupts metallic bonds and dissolves polymers of the EPS matrix, then uses the product’s high osmolarity and surfactant molecule to promote bacterial cell lysis [108]. Some potential limitations include interference of gel ingredients with use of other antibacterial technologies such as silver. Although direct assessment of biofilm or bacterial aggregates in studies has been limited, a case series of patients with non-healing DFUs demonstrated the ability of micelle matrix gel to reduce microbial load and shift the microbial community composition, and Xbio™ based gel has enhanced healing in multiple randomized control studies of patients with chronic wounds [109–111].
Phage Therapy
Phage therapy uses a virus that can degrade the biofilm by inducing protease synthesis and targeted bacterial cell lysis [112]. Preclinical studies demonstrate efficacy in destroying biofilms and lysing bacteria of specific strains, sometimes using a cocktail of different phages to act on a broad range of bacterial isolates, while sparing the normal skin microbiota and maintaining stability on the human skin [113, 114]. Antibiofilm effects of phage therapy can also be enhanced with combination of other therapeutic treatments such as honey and surgical debridement [112, 115]. Despite being a rather recent development, the bacteriophage approach has several clinical studies supporting efficacy against infected chronic wounds. In one randomized clinical trial, topical application of a bacteriophage cocktail (PP1131) reduced bacterial burden in burn wounds infected by P. aeruginosa [116••]. There are also clinical reports of treating refractory-DFU with commercial anti-staphylococcal bacteriophage [117], and a prospective study of patients with chronic non-healing wounds treated with custom bacteriophages [118]. Topical application of phages has been primarily used to avoid side effects of systematic use. Studies suggest phages may impact the host immune system, thereby promoting bacterial infection in certain situations [119]. Other risks include development of bacterial resistance or horizontal gene transfer, resulting in the phage promoting virulent bacterial genes or transferring the genes to other pathogenic organisms. Phages also have narrow range of efficacy due to their specificity towards individual bacterial strains, although this limitation is combated through cocktail mixtures acting against multiple bacteria strains.
Conclusion
Interaction between microbial infection and host response shapes the formation and maintenance of a pathogenic biofilm. New therapeutic strategies show promise in preclinical studies against biofilm infection. However, preclinical models have limited involvement of host-mediated responses to biofilm infection, and current animal models are primarily acute infected wounds that are not fully analogous to chronic wounds [120], limiting evaluation of ultimate therapeutic efficacy and risks compared to human studies. For many therapeutics already available in the clinical setting, randomized clinical trials have been limited. Biofilm therapeutics with some clinical evidence include debridement, NPWTi, photodynamic therapy, silver-based dressings, electroceuticals, and phage therapy. Direct investigation of biofilm changes is rare, possibly restricted by a currently limited toolbox for accurate biofilm identification and monitoring methods in clinic. Successful translation of antibiofilm therapies from bench to bedside rests on developing standard experimental models and evaluation methods that will ultimately allow us to effectively test biofilm-targeted therapeutics and treat patients.
Acknowledgements
We are thankful to all current and past lab members for continuous inspiration and support. Please note that due to space limitations some relevant work from the field could not be cited. The figure in this manuscript was created using biorender.com. This work is in part supported by R01NR015649, U01DK119085, U24DK122927, and R01AR073614 (MTC).
Footnotes
Conflict of Interest IP research is in part supported by Next Science. MTC research is in part supported by Organogenis.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Versey Z, da Cruz Nizer WS, Russell E, Zigic S, DeZeeuw KG, Marek JE, et al. Biofilm-innate immune interface: contribution to chronic wound formation. Front Immunol. 2021;12: 648554. 10.3389/fimmu.2021.648554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz G, Bjarnsholt T, James GA, Leaper DJ, McBain AJ, Malone M, et al. Global Wound Biofilm Expert P. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25(5):744–57. 10.1111/wrr.12590. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Yang B, Tedesco A, Lebig EGD, Ruegger PM, Xu K, et al. High levels of oxidative stress and skin microbiome are critical for initiation and development of chronic wounds in diabetic mice. Sci Rep. 2019;9(1):19318. 10.1038/s41598-019-55644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tipton CD, Wolcott RD, Sanford NE, Miller C, Pathak G, Silzer TK, et al. Patient genetics is linked to chronic wound microbiome composition and healing. PLoS Pathog. 2020;16(6): e1008511. 10.1371/journal.ppat.1008511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Domenico EG, Cavallo I, Bordignon V, Prignano G, Sperduti I, Gurtner A, et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: a pivotal interplay in the pathogenesis of atopic dermatitis. Sci Rep. 2018;8(1):9573. 10.1038/s41598-018-27421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platsidaki E, Dessinioti C. Recent advances in understanding propionibacterium acnes ( cutibacterium acnes) in acne. F1000Res. 2018;7. 10.12688/f1000research.15659.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ring HC, Bay L, Nilsson M, Kallenbach K, Miller IM, Saunte DM, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176(4):993–1000. 10.1111/bjd.15007. [DOI] [PubMed] [Google Scholar]
- 8.Tomic-Canic M, Burgess JL, O’Neill KE, Strbo N, Pastar I. Skin microbiota and its interplay with wound healing. Am J Clin Dermatol. 2020;21(Suppl 1):36–43. 10.1007/s40257-020-00536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grogan MD, Bartow-McKenney C, Flowers L, Knight SAB, Uberoi A, Grice EA. Research techniques made simple: profiling the skin microbiota. J Invest Dermatol. 2019;139(4):747–52 e1. 10.1016/j.jid.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62(3):923–30. 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalan LR, Meisel JS, Loesche MA, Horwinski J, Soaita I, Chen X, et al. Strain- and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe. 2019;25(5):641–55 e5. 10.1016/j.chom.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbanic S, Shen Y, Lee J, Deacon JM, Chen IA. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes. 2020;6(1):21. 10.1038/s41522-020-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.••.Loesche M, Gardner SE, Kalan L, Horwinski J, Zheng Q, Hodkinson BP, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol. 2017;137(1):237–44. 10.1016/j.jid.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study linkingmicrobiota composition to healing outcomes in patients with chronic wounds.
- 14.Dunyach-Remy C, Salipante F, Lavigne JP, Brunaud M, Demattei C, Yahiaoui-Martinez A, et al. Pressure ulcers microbiota dynamics and wound evolution. Sci Rep. 2021;11(1):18506. 10.1038/s41598-021-98073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalan L, Loesche M, Hodkinson BP, Heilmann K, Ruthel G, Gardner SE, et al. Redefining the chronic-wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. mBio. 2016;7(5). 10.1128/mBio.01058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.••.Secor PR, James GA, Fleckman P, Olerud JE, McInnerney K, Stewart PS. Staphylococcus aureus biofilm and planktonic cultures differentially impact gene expression, mapk phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol. 2011;11:143. 10.1186/1471-2180-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]; Biofilm cultures of Staphylococcus aureus induced a distinct inflammatory response in human keratinocytes that may contribute to chronicity of non healing wounds.
- 17.Jeffery Marano R, Jane Wallace H, Wijeratne D, William Fear M, San Wong H, O’Handley R. Secreted biofilm factors adversely affect cellular wound healing responses in vitro. Sci Rep. 2015;5:13296. 10.1038/srep13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pestrak MJ, Chaney SB, Eggleston HC, Dellos-Nolan S, Dixit S, Mathew-Steiner SS, et al. Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLoS Pathog. 2018;14(2): e1006842. 10.1371/journal.ppat.1006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawaya AP, Stone RC, Brooks SR, Pastar I, Jozic I, Hasneen K, et al. Deregulated immune cell recruitment orchestrated by foxm1 impairs human diabetic wound healing. Nat Commun. 2020;11(1):4678. 10.1038/s41467-020-18276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy S, Santra S, Das A, Dixith S, Sinha M, Ghatak S, et al. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann Surg. 2020;271(6):1174–85. 10.1097/SLA.0000000000003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg. 2016;138(3 Suppl):18S–28S. 10.1097/PRS.0000000000002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy S, Elgharably H, Sinha M, Ganesh K, Chaney S, Mann E, et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol. 2014;233(4):331–43. 10.1002/path.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastar I, Marjanovic J, Stone RC, Chen V, Burgess JL, Mervis JS, et al. Epigenetic regulation of cellular functions in wound healing. Exp Dermatol. 2021. 10.1111/exd.14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strbo N, Pastar I, Romero L, Chen V, Vujanac M, Sawaya AP, et al. Single cell analyses reveal specific distribution of antibacterial molecule perforin-2 in human skin and its modulation by wounding and Staphylococcus aureus infection. Exp Dermatol. 2019;28(3):225–32. 10.1111/exd.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastar I, Sawaya AP, Marjanovic J, Burgess JL, Strbo N, Rivas KE, et al. Intracellular Staphylococcus aureus triggers pyroptosis and contributes to inhibition of healing due to perforin-2 suppression. J Clin Invest. 2021. 10.1172/JCI133727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malone M, Bjarnsholt T, McBain AJ, James GA, Stoodley P, Leaper D, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care. 2017;26(1):20–5. 10.12968/jowc.2017.26.1.20. [DOI] [PubMed] [Google Scholar]
- 27.Johani K, Malone M, Jensen S, Gosbell I, Dickson H, Hu H, et al. Microscopy visualisation confirms multi-species biofilms are ubiquitous in diabetic foot ulcers. Int Wound J. 2017;14(6):1160–9. 10.1111/iwj.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oates A, Bowling FL, Boulton AJ, Bowler PG, Metcalf DG, McBain AJ. The visualization of biofilms in chronic diabetic foot wounds using routine diagnostic microscopy methods. J Diabetes Res. 2014;2014: 153586. 10.1155/2014/153586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwynne L, Williams GT, Yan KC, Patenall BL, Gardiner JE, He XP, et al. Tcf-alp: a fluorescent probe for the selective detection of staphylococcus bacteria and application in “smart” wound dressings. Biomater Sci. 2021;9(12):4433–9. 10.1039/d0bm01918f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu YF, Lee TY, Liao WT, Chuan HH, Cheng NC, Cheng CM. Rapid detection of biofilm with modified alcian blue staining: in-vitro protocol improvement and validation with clinical cases. Wound Repair Regen. 2020;28(6):834–43. 10.1111/wrr.12845. [DOI] [PubMed] [Google Scholar]
- 31.Lopez AJ, Jones LM, Reynolds L, Diaz RC, George IK, Little W, et al. Detection of bacterial fluorescence from in vivo wound biofilms using a point-of-care fluorescence imaging device. Int Wound J. 2021;18(5):626–38. 10.1111/iwj.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raizman R, Little W, Smith AC. Rapid diagnosis of pseudomonas aeruginosa in wounds with point-of-care fluorescence imaing. Diagnostics (Basel). 2021;11(2). 10.3390/diagnostics11020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.••.Le L, Baer M, Briggs P, Bullock N, Cole W, DiMarco D, et al. Diagnostic accuracy of point-of-care fluorescence imaging for the detection of bacterial burden in wounds: results from the 350-patient fluorescence imaging assessment and guidance trial. Adv Wound Care (New Rochelle). 2021;10(3):123–36. 10.1089/wound.2020.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent prospective, multi-center controlled study of 350 patients with chronic wounds revealed traditional clinical assessment failed to predict bacterial loads in 85% of infected wounds, while fluorescence imaging significantly increased bacterial detection and influenced patient care across all wound types.
- 34.Farhan N, Jeffery S. Utility of moleculight i: X for managing bacterial burden in pediatric burns. J Burn Care Res. 2020;41(2):328–38. 10.1093/jbcr/irz167. [DOI] [PubMed] [Google Scholar]
- 35.Pastar I, Cao T, Sawaya A, Liang L, Glinos G, Drakulich S, et al. Preclinical models for wound-healing studies. In: Marques A, Reis R, Pirraco R, Cerqueira M, editors. Skin tissue models: Elsevier, Inc; 2017. pp. 223–51. [Google Scholar]
- 36.Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17(3):354–9. 10.1111/j.1524-475X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- 37.Redman WK, Welch GS, Rumbaugh KP. Assessing biofilm dispersal in murine wounds. J Vis Exp. 2021(174). 10.3791/62136. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Fan Q, Guo M, Wu M, Wu S, Shen S, et al. Octenidine dihydrochloride treatment of a meticillin-resistant Staphylococcus aureus biofilm-infected mouse wound. J Wound Care. 2021;30(2):106–14. 10.12968/jowc.2021.30.2.106. [DOI] [PubMed] [Google Scholar]
- 39.Dhall S, Do DC, Garcia M, Kim J, Mirebrahim SH, Lyubovitsky J, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res. 2014;2014: 562625. 10.1155/2014/562625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chien S Ischemic rabbit ear model created by minimally invasive surgery. Wound Repair Regen. 2007;15(6):928–35. 10.1111/j.1524-475X.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 41.Seth AK, Zhong A, Nguyen KT, Hong SJ, Leung KP, Galiano RD, et al. Impact of a novel, antimicrobial dressing on in vivo, pseudomonas aeruginosa wound biofilm: quantitative comparative analysis using a rabbit ear model. Wound Repair Regen. 2014;22(6):712–9. 10.1111/wrr.12232. [DOI] [PubMed] [Google Scholar]
- 42.Park E, Long SA, Seth AK, Geringer M, Xu W, Chavez-Munoz C, et al. The use of desiccation to treat staphylococcus aureus biofilm-infected wounds. Wound Repair Regen. 2016;24(2):394–401. 10.1111/wrr.12379. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9(2):66–76. 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 44.Seaton M, Hocking A, Gibran NS. Porcine models of cutaneous wound healing. ILAR J. 2015;56(1):127–38. 10.1093/ilar/ilv016. [DOI] [PubMed] [Google Scholar]
- 45.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle). 2014;3(7):445–64. 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nusbaum AG, Gil J, Rippy MK, Warne B, Valdes J, Claro A, et al. Effective method to remove wound bacteria: comparison of various debridement modalities in an in vivo porcine model. J Surg Res. 2012;176(2):701–7. 10.1016/j.jss.2011.11.1040. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz JA, Goss SG, Facchin F, Avdagic E, Lantis JC. Surgical debridement alone does not adequately reduce planktonic bioburden in chronic lower extremity wounds. J Wound Care. 2014;23(9):S4, S6, S8 passim. 10.12968/jowc.2014.23.Sup9.S4. [DOI] [PubMed] [Google Scholar]
- 48.Kim PJ, Attinger CE, Bigham T, Hagerty R, Platt S, Anghel E, et al. Clinic-based debridement of chronic ulcers has minimal impact on bacteria. Wounds. 2018;30(5):114–9. [PubMed] [Google Scholar]
- 49.Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, et al. Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J Wound Care. 2010;19(8):320–8. 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]
- 50.Bowling FL, Stickings DS, Edwards-Jones V, Armstrong DG, Boulton AJ. Hydrodebridement of wounds: effectiveness in reducing wound bacterial contamination and potential for air bacterial contamination. J Foot Ankle Res. 2009;2:13. 10.1186/1757-1146-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Ko JH, Secretov E, Huang E, Chukwu C, West J, et al. Comparing the hydrosurgery system to conventional debridement techniques for the treatment of delayed healing wounds: a prospective, randomised clinical trial to investigate clinical efficacy and cost-effectiveness. Int Wound J. 2015;12(4):456–61. 10.1111/iwj.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sonnergren HH, Strombeck L, Aldenborg F, Faergemann J. Aerosolized spread of bacteria and reduction of bacterial wound contamination with three different methods of surgical wound debridement: A pilot study. J Hosp Infect. 2013;85(2):112–7. 10.1016/j.jhin.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Voigt J, Wendelken M, Driver V, Alvarez OM. Low-frequency ultrasound (20–40 khz) as an adjunctive therapy for chronic wound healing: a systematic review of the literature and meta-analysis of eight randomized controlled trials. Int J Low Extrem Wounds. 2011;10(4):190–9. 10.1177/1534734611424648. [DOI] [PubMed] [Google Scholar]
- 54.Kataoka Y, Kunimitsu M, Nakagami G, Koudounas S, Weller CD, Sanada H. Effectiveness of ultrasonic debridement on reduction of bacteria and biofilm in patients with chronic wounds: a scoping review. Int Wound J. 2021;18(2):176–86. 10.1111/iwj.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang YR, Perry J, Cross K. Low-frequency ultrasound debridement in chronic wound healing: a systematic review of current evidence. Plast Surg (Oakv). 2017;25(1):21–6. 10.1177/2292550317693813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vig S, Dowsett C, Berg L, Caravaggi C, Rome P, Birke-Sorensen H, et al. Evidence-based recommendations for the use of negative pressure wound therapy in chronic wounds: steps towards an international consensus. J Tissue Viability. 2011;20 Suppl 1:S1–18. 10.1016/j.jtv.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Li T, Wang G, Yin P, Li Z, Zhang L, Tang P. Adaptive expression of biofilm regulators and adhesion factors of Staphylococcus aureus during acute wound infection under the treatment of negative pressure wound therapy in vivo. Exp Ther Med. 2020;20(1):512–20. 10.3892/etm.2020.8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim PJ, Attinger CE, Crist BD, Gabriel A, Galiano RD, Gupta S, et al. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds. 2015;27(12):S2–19. [PubMed] [Google Scholar]
- 59.Patmo AS, Krijnen P, Tuinebreijer WE, Breederveld RS. The effect of vacuum-assisted closure on the bacterial load and type of bacteria: a systematic review. Adv Wound Care (New Rochelle). 2014;3(5):383–9. 10.1089/wound.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.••.Goss SG, Schwartz JA, Facchin F, Avdagic E, Gendics C, Lantis JC 2nd. Negative pressure wound therapy with instillation (npwti) better reduces post-debridement bioburden in chronically infected lower extremity wounds than npwt alone. J Am Coll Clin Wound Spec. 2012;4(4):74–80. 10.1016/j.jccw.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prospective pilot clinical study showing NPWT with instillation decreased bacterial loads at a clinically significant level, while NPWT did not.
- 61.Yang C, Goss SG, Alcantara S, Schultz G, Lantis Ii JC. Effect of negative pressure wound therapy with instillation on bioburden in chronically infected wounds. Wounds. 2017;29(8):240–6. [PubMed] [Google Scholar]
- 62.Jeong HS, Lee BH, Lee HK, Kim HS, Moon MS, Suh IS. Negative pressure wound therapy of chronically infected wounds using 1% acetic acid irrigation. Arch Plast Surg. 2015;42(1):59–67. 10.5999/aps.2015.42.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim PJ, Lavery LA, Galiano RD, Salgado CJ, Orgill DP, Kovach SJ, et al. The impact of negative-pressure wound therapy with instillation on wounds requiring operative debridement: pilot randomised, controlled trial. Int Wound J. 2020;17(5):1194–208. 10.1111/iwj.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brinkert D, Ali M, Naud M, Maire N, Trial C, Teot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J. 2013;10(Suppl 1):56–60. 10.1111/iwj.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serena TE, Jalodi O, Serena L, Patel K, Mynti M. Evaluation of the combination of a biofilm-disrupting agent and negative pressure wound therapy: a case series. J Wound Care. 2021;30(1):9–14. 10.12968/jowc.2021.30.1.9. [DOI] [PubMed] [Google Scholar]
- 66.Hahn HM, Lee IJ, Woo KJ, Park BY. Silver-impregnated negative-pressure wound therapy for the treatment of lower-extremity open wounds: a prospective randomized clinical study. Adv Skin Wound Care. 2019;32(8):370–7. 10.1097/01.ASW.0000569116.59534.a6. [DOI] [PubMed] [Google Scholar]
- 67.Chung PY, Khanum R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J Microbiol Immunol Infect. 2017;50(4):405–10. 10.1016/j.jmii.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Spohn R, Daruka L, Lazar V, Martins A, Vidovics F, Grezal G, et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat Commun. 2019;10(1):4538. 10.1038/s41467-019-12364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kubicek-Sutherland JZ, Lofton H, Vestergaard M, Hjort K, Ingmer H, Andersson DI. Antimicrobial peptide exposure selects for staphylococcus aureus resistance to human defence peptides. J Antimicrob Chemother. 2017;72(1):115–27. 10.1093/jac/dkw381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo L, McLean JS, Yang Y, Eckert R, Kaplan CW, Kyme P, et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc Natl Acad Sci U S A. 2015;112(24):7569–74. 10.1073/pnas.1506207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE. Human host defense peptide ll-37 prevents bacterial biofilm formation. Infect Immun. 2008;76(9):4176–82. 10.1128/IAI.00318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haisma EM, Goblyos A, Ravensbergen B, Adriaans AE, Cordfunke RA, Schrumpf J, et al. Antimicrobial peptide p60.4ac-containing creams and gel for eradication of methicillin-resistant Staphylococcus aureus from cultured skin and airway epithelial surfaces. Antimicrob Agents Chemother. 2016;60(7):4063–72. 10.1128/AAC.03001-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lora-Tamayo J, Murillo O, Ariza J. Clinical use of colistin in biofilm-associated infections. Adv Exp Med Biol. 2019;1145:181–95. 10.1007/978-3-030-16373-0_13. [DOI] [PubMed] [Google Scholar]
- 74.Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis. 2008;47(12):1537–45. 10.1086/593185. [DOI] [PubMed] [Google Scholar]
- 75.Starr CG, He J, Wimley WC. Host cell interactions are a significant barrier to the clinical utility of peptide antibiotics. ACS Chem Biol. 2016;11(12):3391–9. 10.1021/acschembio.6b00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sonesson A, Przybyszewska K, Eriksson S, Morgelin M, Kjellstrom S, Davies J, et al. Identification of bacterial biofilm and the Staphylococcus aureus derived protease, staphopain, on the skin surface of patients with atopic dermatitis. Sci Rep. 2017;7(1):8689. 10.1038/s41598-017-08046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golla RM, Mishra B, Dang X, Lakshmaiah Narayana J, Li A, Xu L, et al. Resistome of Staphylococcus aureus in response to human cathelicidin ll-37 and its engineered antimicrobial peptides. ACS Infect Dis. 2020;6(7):1866–81. 10.1021/acsinfecdis.0c00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lam SJ, O’Brien-Simpson NM, Pantarat N, Sulistio A, Wong EH, Chen YY, et al. Combating multidrug-resistant gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat Microbiol. 2016;1(11):16162. 10.1038/nmicrobiol.2016.162. [DOI] [PubMed] [Google Scholar]
- 79.Peulen TO, Wilkinson KJ. Diffusion of nanoparticles in a biofilm. Environ Sci Technol. 2011;45(8):3367–73. 10.1021/es103450g. [DOI] [PubMed] [Google Scholar]
- 80.Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:278–84. 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 81.Mekkawy AI, El-Mokhtar MA, Nafady NA, Yousef N, Hamad MA, El-Shanawany SM, et al. In vitro and in vivo evaluation of biologically synthesized silver nanoparticles for topical applications: effect of surface coating and loading into hydrogels. Int J Nanomedicine. 2017;12:759–77. 10.2147/IJN.S124294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finley PJ, Peterson A, Huckfeldt RE. The prevalence of phenotypic silver resistance in clinical isolates. Wounds. 2013;25(4):84–8. [PubMed] [Google Scholar]
- 83.Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, et al. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol. 2009;129(10):2463–9. 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- 84.Wang M, Lai X, Shao L, Li L. Evaluation of immunoresponses and cytotoxicity from skin exposure to metallic nanoparticles. Int J Nanomedicine. 2018;13:4445–59. 10.2147/IJN.S170745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–23. 10.1016/j.jconrel.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 86.Teirlinck E, Xiong R, Brans T, Forier K, Fraire J, Van Acker H, et al. Laser-induced vapour nanobubbles improve drug diffusion and efficiency in bacterial biofilms. Nat Commun. 2018;9(1):4518. 10.1038/s41467-018-06884-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rout B, Liu CH, Wu WC. Photosensitizer in lipid nanoparticle: a nano-scaled approach to antibacterial function. Sci Rep. 2017;7(1):7892. 10.1038/s41598-017-07444-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Liu Z, Liu H, Chen X, Liu Y, Tan H. Photodynamic inactivation of fibroblasts and inhibition of staphylococcus epidermidis adhesion and biofilm formation by toluidine blue o. Mol Med Rep. 2017;15(4):1816–22. 10.3892/mmr.2017.6184. [DOI] [PubMed] [Google Scholar]
- 89.••.Morley S, Griffiths J, Philips G, Moseley H, O’Grady C, Mellish K, et al. Phase iia randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobial therapy. Br J Dermatol. 2013;168(3):617–24. 10.1111/bjd.12098. [DOI] [PubMed] [Google Scholar]; The first controlled study of photodynamic therapy (PDT) in chronic wounds. This blinded, randomized placebo-controlled phase IIa trial confirmed PDT reduced bacterial load immediately post-treatment, with acceptable safety profile and improved healing outcomes.
- 90.Hu D, Li H, Wang B, Ye Z, Lei W, Jia F, et al. Surface-adaptive gold nanoparticles with effective adherence and enhanced photothermal ablation of methicillin-resistant staphylococcus aureus biofilm. ACS Nano. 2017;11(9):9330–9. 10.1021/acsnano.7b04731. [DOI] [PubMed] [Google Scholar]
- 91.Nguyen TK, Duong HT, Selvanayagam R, Boyer C, Barraud N. Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy. Sci Rep. 2015;5:18385. 10.1038/srep18385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Devlin H, Fulaz S, Hiebner DW, O’Gara JP, Casey E. Enzyme-functionalized mesoporous silica nanoparticles to target Staphylococcus aureus and disperse biofilms. Int J Nanomedicine. 2021;16:1929–42. 10.2147/IJN.S293190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14(6):449–59. 10.1007/s40257-013-0046-4. [DOI] [PubMed] [Google Scholar]
- 94.Administration USFaD. 510(k) Summary for derma sciences medihoney dressings with active manuka honey. 2008.
- 95.Lu J, Turnbull L, Burke CM, Liu M, Carter DA, Schlothauer RC, et al. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ. 2014;2: e326. 10.7717/peerj.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kot B, Sytykiewicz H, Sprawka I, Witeska M. Effect of manuka honey on biofilm-associated genes expression during methicillin-resistant staphylococcus aureus biofilm formation. Sci Rep. 2020;10(1):13552. 10.1038/s41598-020-70666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu MY, Cokcetin NN, Lu J, Turnbull L, Carter DA, Whitchurch CB, et al. Rifampicin-manuka honey combinations are superior to other antibiotic-manuka honey combinations in eradicating staphylococcus aureus biofilms. Front Microbiol. 2017;8:2653. 10.3389/fmicb.2017.02653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abd El-Malek FF, Yousef AS, El-Assar SA. Hydrogel film loaded with new formula from manuka honey for treatment of chronic wound infections. J Glob Antimicrob Resist. 2017;11:171–6. 10.1016/j.jgar.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Frydman GH, Olaleye D, Annamalai D, Layne K, Yang I, et al. Manuka honey microneedles for enhanced wound healing and the prevention and/or treatment of methicillin-resistant staphylococcus aureus (mrsa) surgical site infection. Sci Rep. 2020;10(1):13229. 10.1038/s41598-020-70186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang C, Guo M, Zhang N, Wang G. Effectiveness of honey dressing in the treatment of diabetic foot ulcers: a systematic review and meta-analysis. Complement Ther Clin Pract. 2019;34:123–31. 10.1016/j.ctcp.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 101.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, et al. Electrical signals control wound healing through phosphatidylinositol-3-oh kinase-gamma and pten. Nature. 2006;442(7101):457–60. 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 102.Banerjee J, Das Ghatak P, Roy S, Khanna S, Sequin EK, Bellman K, et al. Improvement of human keratinocyte migration by a redox active bioelectric dressing. PLoS ONE. 2014;9(3): e89239. 10.1371/journal.pone.0089239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Suel GM. Ion channels enable electrical communication in bacterial communities. Nature. 2015;527(7576):59–63. 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Humphries J, Xiong L, Liu J, Prindle A, Yuan F, Arjes HA, et al. Species-independent attraction to biofilms through electrical signaling. Cell. 2017;168(1–2):200–9 e12. 10.1016/j.cell.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Administration USFaD. Procellera (for professional use) 510(k) summary of safety and effectiveness. 2008.
- 106.Roy S, Prakash S, Mathew-Steiner SS, Das Ghatak P, Lochab V, Jones TH, et al. Disposable patterned electroceutical dressing (ped-10) is safe for treatment of open clinical chronic wounds. Adv Wound Care (New Rochelle). 2019;8(4):149–59. 10.1089/wound.2018.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blount AL, Foster S, Rapp DA, Wilcox R. The use of bioelectric dressings in skin graft harvest sites: a prospective case series. J Burn Care Res. 2012;33(3):354–7. 10.1097/BCR.0b013e31823356e4. [DOI] [PubMed] [Google Scholar]
- 108.Atkin L, Bucko Z, Conde Montero E, Cutting K, Moffatt C, Probst A, et al. Implementing timers: the race against hard-to-heal wounds. J Wound Care. 2019;23(Sup3a):S1–S50. 10.12968/jowc.2019.28.Sup3a.S1. [DOI] [PubMed] [Google Scholar]
- 109.Malone M, Radzieta M, Schwarzer S, Jensen SO, Lavery LA. Efficacy of a topical concentrated surfactant gel on microbial communities in non-healing diabetic foot ulcers with chronic biofilm infections: a proof-of-concept study. Int Wound J. 2021;18(4):457–66. 10.1111/iwj.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim D, Namen Ii W, Moore J, Buchanan M, Hayes V, Myntti MF, et al. Clinical assessment of a biofilm-disrupting agent for the management of chronic wounds compared with standard of care: A therapeutic approach. Wounds. 2018;30(5):120–30. [PubMed] [Google Scholar]
- 111.Wolcott R Disrupting the biofilm matrix improves wound healing outcomes. J Wound Care. 2015;24(8):366–71. 10.12968/jowc.2015.24.8.366. [DOI] [PubMed] [Google Scholar]
- 112.Seth AK, Geringer MR, Nguyen KT, Agnew SP, Dumanian Z, Galiano RD, et al. Bacteriophage therapy for Staphylococcus aureus biofilm-infected wounds: A new approach to chronic wound care. Plast Reconstr Surg. 2013;131(2):225–34. 10.1097/PRS.0b013e31827e47cd. [DOI] [PubMed] [Google Scholar]
- 113.Forti F, Roach DR, Cafora M, Pasini ME, Horner DS, Fiscarelli EV, et al. Design of a broad-range bacteriophage cocktail that reduces pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob Agents Chemother. 2018;62(6). 10.1128/AAC.02573-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holguin AV, Rangel G, Clavijo V, Prada C, Mantilla M, Gomez MC, et al. Phage phipan70, a putative temperate phage, controls pseudomonas aeruginosa in planktonic, biofilm and burn mouse model assays. Viruses. 2015;7(8):4602–23. 10.3390/v7082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oliveira A, Sousa JC, Silva AC, Melo LDR, Sillankorva S. Chestnut honey and bacteriophage application to control pseudomonas aeruginosa and escherichia coli biofilms: evaluation in an ex vivo wound model. Front Microbiol. 2018;9:1725. 10.3389/fmicb.2018.01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.••.Jault P, Leclerc T, Jennes S, Pirnay JP, Que YA, Resch G, et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by pseudomonas aeruginosa (phagoburn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis. 2019;19(1):35–45. 10.1016/S1473-3099(18)30482-1. [DOI] [PubMed] [Google Scholar]; A randomized clinical trial for bacteriophage therapy in infected wounds demonstrating clinical potential and challenges of bacteriophage methods in reducing wound bacterial load.
- 117.Fish R, Kutter E, Wheat G, Blasdel B, Kutateladze M, Kuhl S. Compassionate use of bacteriophage therapy for foot ulcer treatment as an effective step for moving toward clinical trials. Methods Mol Biol. 2018;1693:159–70. 10.1007/978-1-4939-7395-8_14. [DOI] [PubMed] [Google Scholar]
- 118.Patel DR, Bhartiya SK, Kumar R, Shukla VK, Nath G. Use of customized bacteriophages in the treatment of chronic nonhealing wounds: a prospective study. Int J Low Extrem Wounds. 2021;20(1):37–46. 10.1177/1534734619881076. [DOI] [PubMed] [Google Scholar]
- 119.Sweere JM, Van Belleghem JD, Ishak H, Bach MS, Popescu M, Sunkari V, et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science. 2019;363(6434). 10.1126/science.aat9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davis SC, Pastar I. Reply to “questioning the use of an acute porcine wound model to assess anti-biofilm activity of dressings.” Wound Repair Regen. 2020;28(3):429–30. 10.1111/wrr.12795. [DOI] [PubMed] [Google Scholar]
- 121.Nakagami G, Schultz G, Kitamura A, Minematsu T, Akamata K, Suga H, et al. Rapid detection of biofilm by wound blotting following sharp debridement of chronic pressure ulcers predicts wound healing: a preliminary study. Int Wound J. 2020;17(1):191–6. 10.1111/iwj.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rennie MY, Lindvere-Teene L, Tapang K, Linden R. Point-of-care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: a clinical study. J Wound Care. 2017;26(8):452–60. 10.12968/jowc.2017.26.8.452 [DOI] [PubMed] [Google Scholar]


