Abstract
Background:
Clinical and quality of life outcomes in people living with human immunodeficiency virus (PLWH) are undermined by unhealthy alcohol use (UAU), which is highly prevalent in this population and is often complicated by mental health (MH) or other substance use (SU) comorbidity. In sub-Saharan Africa, evidence-based and implementable treatment options for people with HIV and UAU are needed.
Methods:
We are conducting a hybrid clinical effectiveness-implementation trial at three public-sector HIV clinics in Lusaka, Zambia. Adults with HIV, who report UAU, and have suboptimal HIV clinical outcomes, will be randomized to one of three arms: an alcohol-focused brief intervention (BI), the BI with additional referral to a transdiagnostic cognitive behavioral therapy (Common Elements Treatment Approach [CETA]), or standard of care. The BI and CETA will be provided by HIV peer counselors, a common cadre of lay health worker in Zambia. Clinical outcomes will include HIV viral suppression, alcohol use, assessed by audio computer-assisted self-interview (ACASI) and direct alcohol biomarkers, Phophatidylethanol and Ethyl glucuronide, and comorbid MH and other SU. A range of implementation outcomes including cost effectiveness will also be analyzed.
Conclusion:
Hybrid and 3-arm trial design features facilitate the integrated evaluation of both brief, highly implementable, and more intensive, less implementable, treatment options for UAU among PLWH in sub-Saharan Africa. Use of ACASI and alcohol biomarkers will strengthen understanding of treatment effects.
Keywords: Unhealthy alcohol use, HIV, Sub-Saharan Africa, Phosphatidylethanol, Transdiagnostic therapy
1. Background
Unhealthy alcohol use (UAU) is a significant driver of the HIV epidemic and reduces the clinical and quality of life outcomes of people living with HIV (PLWH) [1–3]. UAU may also contribute to accelerated development of end organ diseases like cardiovascular disease, cancer, and neurocognitive deficits among PLWH [4,5]. Concerningly, the HIV health system is not well-equipped to respond, particularly in sub-Saharan Africa (sSA), where most PLWH receive care [6,7]. Within most HIV programs, the standard of care and typically only alcohol treatments available are alcohol brief interventions (BI). BIs can be clinically and cost-effective for reducing hazardous alcohol use and are often highly implementable [8–10]. However, BIs did not lead to alcohol reduction among PLWH in SSA in several controlled clinical trials [11,12]. One explanation for limited effectiveness of alcohol BIs among PLWH in sSA may be the high burden of comorbid mental health (MH) and other substance use (SU), which BIs are not designed to address [13].
One intervention in sSA that holds promise for treatment of UAU among PLWH is Common Elements Treatment Approach (CETA). CETA is a modular transdiagnostic cognitive behavioral therapy-based protocol that allows a provider, over a course of 6–12 sessions, to treat a range of clinical presentations including substance use, anxiety, depression, and posttraumatic stress [14]. CETA was designed deliberately for low and middle-income countries (LMIC) and features streamlined approaches that permit delivery by lay health workers with no prior mental/behavioral health training [15]. In several non-HIV randomized evaluations, CETA reduced alcohol use and range of behavioral health comorbidities [16,17]. While promising, CETA’s effectiveness and implementation factors among PLWH in sSA require further evaluation. Current HIV care models now minimize the times PLWH must visit their facility; therefore, whether people with HIV and UAU will adequately take up and complete a multi-session intervention like CETA needs to be established.
We now describe the study protocol for a randomized controlled clinical trial among adults with HIV and UAU in Zambia. Using a 3-arm design, the trial will evaluate both CETA and an alcohol BI, based on CETA’s substance use element, for treatment of UAU. A range of HIV, alcohol, and behavioral health outcomes will be assessed. In addition, the trial includes prospective assessment of implementation outcomes, including cost effectiveness analysis, to inform future use.
2. Methods
2.1. Overall study design
The CETA HIV Alcohol Reduction Trial in Zambia (CHARTZ) is a type 1 hybrid effectiveness-implementation trial to compare the effectiveness of CETA and a one-session alcohol BI to standard of care, and to each other, and to measure implementation factors related to the integrated delivery of the interventions (Fig. 1) [18]. The study is guided by a conceptual framework, the modified version of Anderson’s Behavioral Model [19], which has been used to describe the utilization of health services by underserved populations globally. Use of this framework will ensure CHARTZ considers the contextual environment, the healthcare environment, and patient characteristics. Our clinical hypothesis is that both CETA and the BI alone will improve clinical outcomes and CETA will be superior to BI alone, as it can address both UAU and co-occurring and underlying mental and behavioral health comorbidities. Clinical hypotheses will be evaluated through a 3-arm individual randomized clinical trial with the primary outcome of HIV as viral load suppression (VLS), which is the central goal of HIV care. We also hypothesize that both interventions will be acceptable to patients with HIV and UAU and staff at HIV clinics, will be cost effective, and will be considered potentially scalable in Zambia.
Fig. 1.
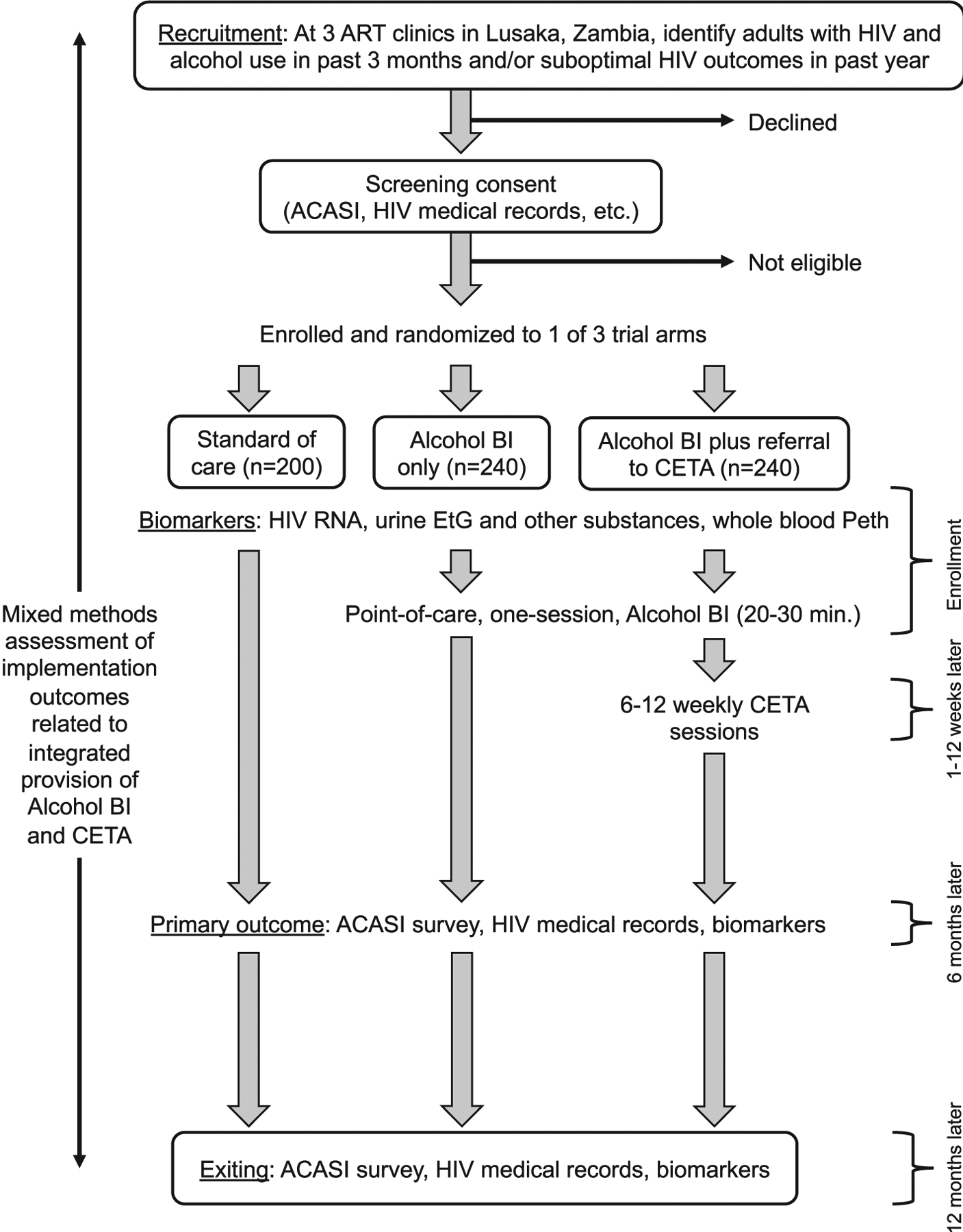
Schema for a 3-arm hybrid clinical effectiveness-implementation trial for unhealthy alcohol use among adults with HIV in Zambia.
During protocol development, we met with officials at the Ministry of Health (MoH), study HIV clinics, and community health leaders in clinic catchment areas. These meetings raised awareness of the study, and helped to refine procedures for recruitment of participants and delivery of interventions. CHARTZ was approved by the University of Alabama at Birmingham Institutional Research Board, the University of Zambia Biomedical Research Ethics Committee, and the National Health Research Authority in Zambia. The trial was registered on ClinicalTrials. gov (NCT05121064; date of registration, November 12, 2021) before participant enrollment. The SPIRIT guidelines for clinical trial protocols were used to present this protocol paper.
2.2. Study population
CHARTZ will recruit 4 participant types: (1) adult patients who are living with HIV, report unhealthy alcohol use, and have suboptimal HIV outcomes; (2) HIV peer counselors providing the trial interventions; (3) HIV clinic staff; (4) key informants in the HIV and substance use health systems in Zambia. Patients, counselors, and clinic staff will be recruited at three large urban public sector HIV clinics in Lusaka.
2.3. Recruitment
2.3.1. Patients
At study clinics, we will solicit referrals of adult patients with HIV who report any degree of alcohol use in the past 3 months, have suboptimal HIV outcomes in the past year, or both. HIV clinic staff will be trained in CHARTZ rationale and eligibility criteria. Patients will be recruited during daily health talks in the waiting area or during ART adherence counseling.
2.3.2. Counselors
HIV peer counselors at study clinics will also participate. HIV peer counselors are lay health workers (usually with 9th to 12th grade education) who have basic psychosocial counseling experience and provide various services at the HIV clinic including patient navigation, HIV testing, filing, and health talks. Based on their CV and a brief interview, 5–6 counselors will be selected per clinic. Selected HIV peer counselors will be trained in the alcohol BI, CETA, and a safety protocol in case of suicidal or homicidal ideations. Training entails a 2-week in person classroom training, a series of practice groups, and ongoing weekly supervision. It is co-led by international clinical psychologists who have master’s or doctorate degree, and local Zambian therapists who are highly trained in CETA; while not required, some local trainers also have advanced degrees. Only when supervised practice groups are completed and the supervisor is satisfied with fidelity to the model will counselors be allowed to provide interventions to trial participants. Trained HIV peer counselors will be invited to optionally be study participants themselves. If agreeable, up to 20 counselors will provide written informed consent for evaluation of their delivery of the intervention and perspectives on implementation.
2.3.3. HIV clinic staff and key informants
At the clinic, up to 20 professional health workers employed by government (nurses, clinicians, clerks), who are working as CHARTZ is implemented, and can speak to implementation factors, will also be invited to be participants. We will also include as participants up to 15 thought leaders, program managers, and policymakers in HIV and substance use in Zambia who can speak to intervention scalability and sustainability.
2.4. Patient eligibility screening, informed consent, and baseline assessment
At referral, a research assistant (RA) will meet with patients to discuss the study and pre-screen for possible eligibility (Table 1). If potentially eligible and agreeable to participate, informed consent will be obtained from the patient for the remaining screening process and inclusion in the trial if eligible. After consent, a unique study number will be issued to the participant and used in all subsequent forms and data. The remaining screening process includes a battery of patient reported outcome (PRO) surveys (Table 2). PROs will be captured using an Audio Computer Assisted Self-Interview (ACASI) on a laptop computer [20]. The ACASI begins with the Alcohol Use Disorders Identification Test. Using an algorithm, if the participant reports no/moderate alcohol use is reported, making her/him ineligible for the trial, remaining items will be skipped. If unhealthy alcohol use, scales for behavioral health comorbidities, (depression, posttraumatic stress, anxiety, other substance use) and health-related quality of life will follow (see Table 2). Screening for suicidal and homicidal ideation is also included. Overall, completion of the ACASI will take as little as 5 min for ineligible patients and around 30–45 min for those who enter the trial.
Table 1.
Study eligibility criteria, by participant type.
| Type | Eligibility criteria |
|---|---|
| Patients in the trial (N = 680) |
Inclusion: age ≥ 18 years old; living with HIV infection; receiving HIV care at study site; ≥ 6 months since ART initiation; hazardous alcohol use plus at least one MH/SU comorbidity or moderate/severe AUD; suboptimal HIV care outcome in past year including late (14+ days) ART drug pickup, HIV VL greater than the lower limit of assay detection, or referral to the ART clinic’s enhanced adherence counseling program Exclusion: plan to relocate out of Lusaka in next 6 months; no access to a telephone; actively suicidal and in need of immediate care; alcohol intoxication or withdrawal requiring immediate care; currently psychotic; participating in another study that would interfere with participation |
| HIV peer counselors providing trial interventions (N = 20) |
Inclusion: age ≥ 18 years old, trained in alcohol BI and/or CETA; experience providing trial interventions including having provided alcohol BI to ≥ 10 participants or xpovided CETA to ≥ 5 participants Exclusion: none |
| HIV clinic staff (N = 20) |
Inclusion: age ≥ 18 years old, working for the Ministry of Health; professional or lay non-study health worker; worked at study site for ≥6 months during implementation of the trial Exclusion: none |
| Key informants (N = 15) |
Inclusion: age ≥ 18 years old, clinic/hospital administrator, program manager, policymaker, or other thought leader in areas of HIV, SU, or MH in Zambia Exclusion: none |
Abbreviations: HIV, human immunodeficiency virus; MH, mental health; SU, substance use; AUD, alcohol use disorder; ART, antiretroviral therapy; CETA, Common Elements Treatment Approach; BI, brief intervention; VL, viral load.
Table 2.
Outcome measures in the CHARTZ trial.
| Outcome | Measure | Description | Interpretation | |
|---|---|---|---|---|
| HIV | HIV VL (Primary) | HIV RNA, copies/ml | HIV RNA concentration in blood reflects viral activity level. Sustained VL below the level of assay detection has both individual and public health (transmission) benefits. | VL will be dichotomized as suppressed (below assay detection) or not suppressed. |
| ART adherence | MPR | MPR is a pharmacy adherence measure [21], which ranges from 0 to 1, and is calculated as the fraction of time a patient did not have medication according to pharmacy records. | MPR will be calculated at 12 months and categorized at >90%, which is a clinically relevant threshold associated with HIV VLS [22]. | |
| Retention in HIV care | Late ART pickup | ART pickups are scheduled and on time pickups reflect retention of a patient in HIV care [23]. | Retention will be defined as never being >28 days late for an ART pickup during follow-up. | |
| Alcohol | Self-reported alcohol use | AUDIT | AUDIT is a 10-item measure of hazardous alcohol use [24,25]. It was previously translated and validated for use in Zambia [26,27]. | AUDIT will form part of trial eligibility if ≥4 for women, ≥8 for men, with a behavioral health comorbidity, and ≥ 12 for women, ≥16 for men without comorbidities. Change in AUDIT score will be analyzed during follow-up. |
| Objective alcohol use | Peth | Peth is an alcohol metabolite that can be detected and quantified on the surface of red blood cells. Its detection and concentration reflects alcohol use in the past 3 weeks [28]. | Participants with underreporting, defined as Peth >8 ng/dl together with AUDIT score of 0, will be excluded in sensitivity analyses. Peth >50 ng/ml will be used to define unhealthy alcohol use [29]. Change in Peth will be analyzed during follow-up. | |
| Objective alcohol use | EtG | EtG is an alcohol metabolite that can be detected in urine 1–5 days after last drink. We will detect it with a commercially available diptest that has a sensitivity of >500 ng/dl [30]. | Participants with underreporting, defined as EtG-positivity together with AUDIT score of 0, will be excluded in sensitivity analyses. | |
| Comorbid behavioral health issues | Depression | CES-D | CES-D is a 20-item measure of depression symptoms [31]. It was previously translated and validated for use in Zambia [26]. | CES-D total score ≥ 16 will be an eligibility criterion for the trial [32]. Change in CES-D score will be analyzed during follow-up. |
| Post-traumatic stress | HTQ | HTQ is a 39-item scale of posttraumatic stress symptoms. It was previously translated and validated for use Zambia [33]. | HTQ average item score ≥ 2.5 will be an eligibility criterion for the trial [34]. Change in HTQ score will be analyzed during follow-up. | |
| Anxiety | GAD-7 | GAD-7 evaluates symptoms of anxiety-related disorders [35]. | GAD-7 total score ≥ 10 points will be an eligibility criterion for the trial. Change in GAD-7 score will be analyzed during follow-up. | |
| Other substance use | ASSIST | ASSIST is a 7-item measure of use, abuse, and dependence symptoms for a range of substance types [36]. The tool ASSIST was previously validated in Zambia [37]. | ASSIST non-alcohol, non-tobacco specific substance involvement score ≥ 27 will be an eligibility criterion for the trial [38]. Change in SSI score will be analyzed during follow-up. | |
| Quality of life | Health-related quality of life | EQ-5D-5L | EQ-5D-5L is a generic measure of health-related quality of life [39]. | Baseline score and change from baseline to outcome timepoints will be used. |
Abbreviations: HIV, human immunodeficiency virus; VL, viral load; ART, antiretroviral therapy; MPR, mediation possession ratio; VLS, viral load suppression; AUDIT, Alcohol Use Disorders Identification Test; Peth, Phosphatidyethanol; EtG, ethyl glucuronide; CES–D, Center for Epidemiological Studies – Depression Scale; HTQ, Harvard Trauma Questionnaire; GAD-7, Generalized Anxiety Disorder Assessment; ASSIST; Alcohol Smoking and Substance Involvement Screening Test.
After completion of ACASI, the final screen will display final trial eligibility (yes/no) and will flag safety if either suicidal or homicidal ideation is reported. If safety is flagged, a study HIV peer counselor will meet the client immediately to discuss the safety issue and make a safety plan. The plan will be discussed over the phone with their supervisor. If necessary, the participant will be brought for additional services. Once safety is addressed, eligible participants will be randomized. For clients who are trial-eligible, ACASI data form the baseline assessment. In addition to ACASI, baseline HIV treatment data (see Table 2) will also be extracted from medical records.
2.5. Randomization/blinding
Randomization of patient participants is conducted by the RA immediately after confirmation of trial eligibility. A statistician will generate lists of randomization ID numbers before trial commencement, and randomization will be stratified by both sex and clinic site. IDs within each stratum were randomly allocated on a ratio 1:1.2:1.2 to SOC, alcohol BI only, or BI plus referral to CETA respectively, using the ralloc procedures in Stata 17 MP8 (StataCorp, College Station, Texas, USA). Randomization will be blocked and RAs will be blind to the blocking sequence. The randomization assignments, for men and women, will be kept sequentially inside sealed opaque envelopes at each site to be opened by the RA at randomization. Only the data analyst, and not the participants and RAs, will be blinded to the assigned arms.
2.6. Blood and urine specimen collection and testing
After randomization, we will also collect baseline urine and blood samples. The RA will test a fresh sample of at least 10 ml of urine for 16 commonly misused substances (see supplementary material), including ethyl glucuronide (EtG) at a threshold of detection of 500 ng/ml, using a commercially available test cup. EtG is an alcohol biomarker that was highly specific in urine for recent (past 3 days) alcohol use [40]. As urine rapid substance use testing is not definitive, results will be for research only and not provided to the participant or the counselor for clinical use. We will also collect 10 ml of blood in an EDTA bottle using venipuncture. A 5-spot (~50 μl per spot) dried blood spot card will be made with a pipette and remaining blood will be used to measure HIV RNA concentration in plasma. HIV VL results will be returned to clinics within 2 weeks and will be used in patient care. Per local guidelines, if VL is >1000 copies/ml, the patient will be referred for EAC. DBS cards will be stored frozen until batch testing for phosphatidylethanol (PEth), an alcohol metabolite on red blood cells that reflects alcohol consumption in past 3 weeks [29].
2.7. Follow-up assessments
During follow-up, trial participants will be exited early in case of withdrawal of consent, death, transfer of HIV care to a distant location that precludes retention, and loss to follow-up. Among those who are retained, follow-up visits will occur at 6 and 12 months post-enrollment, usually in conjunction with scheduled ART medication pickups. At follow-up assessments, a very similar ACASI will be completed, urine will tested for EtG, and blood will be used measure HIV VL and Peth in DBS. At enrollment and each follow-up assessment, participants will receive a transportation reimbursement equivalent to ~6 US dollars.
2.8. Trial arms
2.8.1. Control arm - standard of care
Standard of care at the HIV clinics is ART adherence counseling, which is brief semi-structured one-on-one counseling, lasting 3–10 min, that is focused on HIV medication adherence but includes brief unstructured screening and discussion of alcohol use. ART adherence counseling will be received by all participants, regardless of trial arm, at each ART medication pickup during the study. We previously reported that that ART adherence counseling can have a moderate impact on unhealthy alcohol use [10]. Only HIV peer counselors not trained in study interventions will provide ART adherence counseling. Adherence counseling is documented in the HIV medical record. Imbalance in the number of counseling sessions by trial arm will be tracked for potential adjustment during analysis.
2.8.2. Experimental arm 1 - alcohol BI
The alcohol BI was adapted from the evidence-based substance use reduction element in CETA and previously pilot tested [13]. Study investigators developed the BI, which has 6 components (see Table 3), with input from local partners working in the HIV health system in Zambia. It was designed for implementation and can be completion in just 20–30 min via a single face-to-face session. CHARTZ is the first randomized evaluation of the BI. For participants assigned to the BI alone or BI plus CETA arms, the RA will refer the participant to a trained HIV peer counselor on the day of enrollment or within the next week if necessary. During the BI, counselors use a structured tool called the Improving Your Health (IYH) worksheet (see supplemental material), which was developed to help counselors structure the session and keep it to the 20–30 min target.
Table 3.
Components of transdiagnostic and alcohol-focused interventions evaluated.
| Common Elements Treatment Approach (CETA) | ||
|---|---|---|
| Psychoeducation/introduction | Program information, normalize symptoms and problems | Psychoeducation; reduce stigma |
| Substance use reduction | CBT and MI merged to set goals and reduce substance use; identification and strategies for ‘drivers’ of substance use | Reduce substance use, increase social support |
| Behavioral activation | Identify and engage in pleasurable activities | Reduce depression symptoms; activate action to engage in helpful programs (i.e., HIV care) |
| Cognitive coping/restructuring | Identify and correct thoughts, feelings, and behaviors; replace unhealthy thoughts with helpful ones in order to feel better and behave in a more healthy, productive way | Reduce depression, anxiety, and trauma-related symptoms; reduce self-blame and stigma; reduce negative thoughts on HIV care; reduce aggressive/violent behavior, reduce risk taking, improve retention and adherence |
| Relaxation | Breathing exercises, imagery, etc. | Reduce anxiety and stress-related symptoms |
| Exposure | Talk about trauma memories or confront fears using gradual desensitization | Reduce trauma and anxiety symptoms |
| Problem solving | Teach a process of steps to solve problems and make healthy decisions | Promote health decision making; skills training for problem solving; improve relationships and communication |
| Alcohol Brief Intervention, based on substance use reduction component of CETA | ||
| Assess/screen for alcohol use | Two-week alcohol timeline follow-back measure | Establish baseline frequency and quantity of alcohol use |
| Understand the impacts of alcohol use | Review core ways alcohol use can negatively impact an individual, family, and the community | Increase client motivation to reduce use by highlighting negative effects; help client understand that positive effects of alcohol use are short-term, the negatives are long-term |
| Explore possibilities for change | Explore potential ways the client would consider changing or reducing their alcohol use | Brainstorm measurable changes the client could make to reduce use |
| Set goals | Set a goal for one way the client could reduce their alcohol use in the next few weeks | Set a measurable target for the client to work toward |
| Identify reasons for alcohol use | Understand client motivations for alcohol use | Use the client’s motivations for alcohol use to determine the best strategies for reducing it |
| Build skills | Teach one coping skill to help the client combat one main reason for alcohol use | Build skills that address reasons for alcohol use |
2.8.3. Experimental arm 2 - BI with referral to CETA
After receiving the alcohol BI as described above, participants assigned to the CETA arm will be linked to the intervention over the next 1–2 weeks. CETA (www.cetaglobal.org) is comprised of 8 modular elements and is tailored to the needs of the client (see Table 3) [14,41]. At weekly supervision meetings, the supervisor will assign newly referred participants to an available HIV peer counselor, preferably the same one that provided the BI, to leverage existing rapport. The supervisor will also provide guidance to the counselor on the treatment plan (i.e., module selection and flow). After assignment, the counselor will phone, or if necessary visit the participant at home, to arrange for the first CETA session. A typically course of CETA entails 6–12 weekly sessions with the same counselor, scheduled at times and locations convenient for both parties. While we expect most CETA sessions will occur in person, telephone-based CETA is also available if requested by the participant. The CETA manual was adapted for telehealth delivery, incorporating best practices in telehealth and recommendations from Zambian health providers. Each session begins with a 27-item Likert scale client monitoring form (CMF; see supplementary material), which gives the counselor feedback on treatment response, and lasts 45–90 min. The CETA course is complete when the planned treatment has been given and a clinical response is observed via the CMF.
2.9. Intervention fidelity tracking
Alcohol BI and CETA session fidelity will be monitored. Supervisors keep logs of BI and CETA session data (ITH, CMF, etc.) and each session is discussed at supervision to reinforce fidelity to the model. If necessary supervisors ask counselors to repeat a session. Supervisors also receive supervision from a CETA trainer/expert, through a weekly call, which is focused on how to supervise the counselors and manage challenging cases [15].
2.10. Trial outcomes
2.10.1. Clinical outcomes
Our primary clinical outcome will be HIV VLS at 6 months post-enrollment. VLS will be defined as HIV RNA concentration below assay detection. If assays with varying levels of detection (such as <40 and < 60 copies/ml) are used, the analysis will consider the highest level of detection as cut-off for the primary outcome. In secondary analysis HIV RNA <1000 copies/ml, widely used in the HIV program, will be considered as VLS. Secondary HIV outcomes at 12 months include VLS, non-retention in HIV care, based on >28 days late for an ART refill, and medical possession ratio adherence <90%. Non-HIV secondary outcomes include change in alcohol use, other substance use, comorbid symptoms of mental illness, and health-related quality of life from enrollment to 6 and 12 months. Change in alcohol use will be based on change in AUDIT score and by alcohol biomarkers. We will examine the change in UAU, defined as Peth >50 ng/ml [29], as well as change in quantitative Peth concentration. We will also re-analyze primary and secondary outcomes after exclusion of patients with false reports of abstinence at 6 and 12 months (i.e., AUDIT score = 0 and either EtG-positive or Peth >8 ng/ml).
2.10.2. Implementation outcomes
During the trial, we will prospectively track uptake and completion of interventions in CHARTZ. We will systematically track participant completion of the alcohol BI, uptake of CETA, defined as completion of their first CETA session, time providers dedicate to client tracking/retention in CETA, and CETA completion. Sociodemographic, clinical, and structural factors associated with CETA non-completion by 6 months will be sought using multivariable logistic regression. At the 6-month follow-up visit, a subset of around 50 participants assigned to BI plus referral to CETA will complete a mixed methods survey of implementation outcomes. Implementation measures will focus on the acceptability, appropriateness, cost, and feasibility of CETA and the alcohol BI. We will deliberately include participants who were randomized to but did not complete CETA.
Counselor and supervisor competency and fidelity to the interventions will also be assessed. Counselors will take structured knowledge surveys at 3 time points: after initial trainings, after completion of 1–2 CETA cases, and mid-way through the study. Supervisors will also take a knowledge survey at 3 time points: after initial trainings, after supervising for 3 months, and after supervising for 6 months. In addition, we will conduct role plays with counselors and measure competency using a standardized form. This will be done twice for each counselor during the study.
During the second half of the study, we will also enroll non-patient participants (see Table 1). HIV peer counselors and clinic staff will be invited to participate in focus groups and/or in-depth interviews to explore the acceptability, appropriateness, reach, feasibility, and attitudes, thoughts, feelings, and barriers and facilitators related to implementation. Individual qualitative interviews will be held with key informants, to understand perspectives on intervention sustainability, scale-up, and stakeholder buy-in. Qualitative data will be digitally recorded, translated into English if necessary, and transcribed.
We will also measure cost and cost-effectiveness. Implementation cost per participant (in Zambian Kwacha and U.S. dollars, purchasing parity adjusted) will be estimated for each trial arm using standard micro-costing techniques. We will rely on accounting documents and interviews with administrative and finance staff, supplemented by direct observation at facilities. Routine time and motion studies will collect data on counselors’ specific time on relevant tasks (i.e., provision of BI and CETA sessions, follow-up of clients) by arm. Program management costs such as costs of coordination and fiscal management and regular meetings will also be estimated. Total cost will include expenditures for personnel, recurring supplies, and services (including electricity, water, internet, phone call charges and other utilities), capital expenditure, and building space. Research and routine clinical costs (i.e., costs of HIV care that are covered by the Zambian government, U.S. Presidents Emergency Plan for AIDS Relief, or other parties) will be excluded.
2.11. Data and safety monitoring
The trial will be monitored by a Data Safety Monitoring Board (DSMB). All DSMB members will review and approve the study procedures, as well as procedures for reporting and tracking adverse events, and study progress. Every six months, the DSMB will receive a progress report including enrollment, attrition, and adverse events. If needed, meetings are convened to discuss significant concerns. There are no planned interim analyses or stopping rules given the low risk nature of the interventions.
2.12. Sample size and data analysis
The primary endpoint of the trial is VLS at 6 months. We estimated that in the control arm, 70% of patient participants would have VLS, in those receiving alcohol BI alone this would be 85%, and in those assigned to BI plus CETA it would be 95%. Our sample size calculation included 80% power and was adjusted for three comparisons with an alpha level set to 0.017 and a two-sided Person’s chi-squared test. All three comparisons were considered superiority analyses. We also inflated the sample size by 10% account for patients who transfer out to a non-study clinic or die before the primary outcome and 15% to account for missing HIV VL results. Using the most conservative of the above calculations, we estimated that we would require 200 in SOC, 240 in alcohol BI, and 240 in BI plus CETA, for a total sample size of 680. With that sample size we will be powered for alcohol and other mental and behavioral health comorbidities.
Primary analyses will be intention to treat (ITT). Our primary outcome VLS will be dichotomous. We will use logistic regression model to estimate the effect of intervention (indicator variable). We will perform pairwise comparisons of the margin linear predictions, p-value, and 95% CI, adjusted for Bonferroni correction. Patients with missing data on VLS at 6 months will be excluded from the primary outcome analysis. In a sensitivity analysis, those with missing VL at 6 months will be assumed to have a detectable level. Dichotomous secondary outcomes will be analyzed in an analogous way. The effect of the interventions on changes in alcohol use, from enrollment to 6 and/or 12 months, based on AUDIT score will be analyzed using random-effects logistic regression model. In addition, the proportion with unhealthy alcohol use, defined either by AUDIT or Peth >50 ng/ml, will be compared at 6 and 12 months between arms. Continuous outcomes including AUDIT score will be analyzed using linear regression model or log-normal regression model as appropriate. All analyses will be performed using Stata 17 MP8. To simulate the cost and cost-effectiveness, we will build a state-transition decision-analytic model using TreeAge Pro 2022.R2 (TreeAge® Software, Willamstown, MA). Incremental cost-effectiveness ratios (U.S. dollar/QALY) will be used to assess the interventions’ cost-effectiveness [42,43].
2.13. Trial status
The trial is set to begin enrollment in December 2022 and the enrollment period is expected to last 24 months (i.e., to December 2024). We expect enrollment, treatment, follow-up, and analysis to be completed by mid-2026.
3. Conclusions
CHARTZ will help to advance the screening and treatment of UAU among PLWH in several ways. Results will inform whether and to what degree integration of screening and treatment of co-occurring mental illness is needed when managing UAU among PLWH. The impact of a highly implementable alcohol-focused BI will be determined. Alcohol use, an important secondary outcome, will be measured innovatively with both self-report and biomarkers, allowing us to explore the role of these in clinical care and reduce the impact of reporting bias on trial outcomes. Analyses combining self-report and alcohol biomarkers may also help advocate for the need for objective measures of alcohol use in treatment programs. CHARTZ will also generate data on the prevalence of overlapping behavioral health comorbidities, mediators and moderators of intervention effects, and implementation factors. Although the study focuses on HIV outcomes as primary, which is the current focus of HIV programs in sSA, there is increased focus on health-related quality of life and functional status, which are negatively impacted by the behavioral issues at the heart of CHARTZ. Therefore, results may only increase in relevance as there is shift to outcomes beyond viral suppression [3].
Several limitations warrant discussion. Both interventions tested include an assessment component; therefore, unraveling the separate impacts of assessment versus therapy will not be possible. The dose of CETA varies based on the need of the client; therefore, we will not be able to explore a dose-response relationship in our analysis. CHARTZ will occur in urban Lusaka; thus, implementation outcomes will need to be further evaluated at rural settings. Although we will recruit some clients from clinic ‘late lists’ of clients at risk of loss to follow-up, our recruitment approach focuses on PLWH who come to clinic seeking services. PLWH who are disengaged from care will not be well-represented in CHARTZ, reducing our external validity somewhat. Future studies may consider integrating CETA or BI delivery with community-delivered interventions for PLWH.
Supplementary Material
Funding statement
This study was funded by the National Institute of Alcohol Abuse and Alcoholism at the U.S. National Institutes of Health (P01AA029540 and K01AA026523).
Footnotes
Declaration of Competing Interest
All authors declare they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2023.107116.
Data availability
No data were used for the research described in the article.
References
- [1].Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S, Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings, Prev. Sci 8 (2) (2007) 141. [DOI] [PubMed] [Google Scholar]
- [2].Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N, Rehm J, Causal considerations on alcohol and HIV/AIDS—a systematic review, Alcohol Alcohol. 45 (2) (2010) 159–166. [DOI] [PubMed] [Google Scholar]
- [3].Lazarus JV, Safreed-Harmon K, Barton SE, et al. , Beyond viral suppression of HIV–the new quality of life frontier, BMC Med. 14 (1) (2016) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schouten J, Wit FW, Stolte IG, et al. , Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study, Clin. Infect. Dis 59 (12) (2014) 1787–1797. [DOI] [PubMed] [Google Scholar]
- [5].Langebeek N, Kooij KW, Wit FW, et al. , Impact of comorbidity and ageing on health-related quality of life in HIV-positive and HIV-negative individuals, Aids. 31 (10) (2017) 1471–1481. [DOI] [PubMed] [Google Scholar]
- [6].Hahn JA, Woolf-King SE, Muyindike W, Adding fuel to the fire: alcohol’s effect on the HIV epidemic in sub-Saharan Africa, Current HIV/AIDS Rep. 8 (3) (2011) 172–180. [DOI] [PubMed] [Google Scholar]
- [7].Ferreira-Borges C, Parry CDH, Babor TF, Harmful use of alcohol: a shadow over sub-Saharan Africa in need of workable solutions, Int. J. Environ. Res. Public Health 14 (4) (2017) 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gilbert P, Ciccarone D, Gansky SA, et al. , Interactive “Video Doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings, PLoS One 3 (4) (2008), e1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aharonovich E, Hatzenbuehler ML, Johnston B, et al. , A low-cost, sustainable intervention for drinking reduction in the HIV primary care setting, AIDS Care 18 (6) (2006) 561–568. [DOI] [PubMed] [Google Scholar]
- [10].Asombang M, Helova A, Chipungu J, et al. , Alcohol reduction outcomes following brief counseling among adults with HIV in Zambia: a sequential mixed methods study, PLoS Global Publ. Health 2 (25) (2022), e0000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wandera B, Tumwesigye NM, Nankabirwa JI, et al. , Efficacy of a single, brief alcohol reduction intervention among men and women living with HIV/AIDS and using alcohol in Kampala, Uganda: a randomized trial, J. Int. Assoc. Provid. AIDS Care (JIAPAC) 16 (3) (2017) 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huis in ‘t Veld D, Ensoy-Musoro C, Pengpid S, Peltzer K, Colebunders R, The efficacy of a brief intervention to reduce alcohol use in persons with HIV in South Africa, a randomized clinical trial, PLoS One 14 (8) (2019), e0220799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kane JC, Sharma A, Murray LK, et al. , Efficacy of the common elements treatment approach (CETA) for unhealthy alcohol use among adults with HIV in Zambia: results from a pilot randomized controlled trial, AIDS Behav. 26 (2) (2022) 523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Murray LK, Dorsey S, Haroz E, et al. , A common elements treatment approach for adult mental health problems in low-and middle-income countries, Cogn. Behav. Pract 21 (2) (2014) 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murray LK, Dorsey S, Bolton P, et al. , Building capacity in mental health interventions in low resource countries: an apprenticeship model for training local providers, Int. J. Ment. Heal. Syst 5 (1) (2011) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murray LK, Hall BJ, Dorsey S, et al. , An evaluation of a common elements treatment approach for youth in Somali refugee camps, Global Mental Health. (2018) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kane JC, Glass N, Bolton PA, et al. , Two-year treatment effects of the common elements treatment approach (CETA) for reducing intimate partner violence and unhealthy alcohol use in Zambia, Global Mental Health. (2021) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C, Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact, Med. Care 50 (3) (2012) 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gelberg L, Andersen RM, Leake BD, The behavioral model for vulnerable populations: application to medical care use and outcomes for homeless people, Health Serv. Res 34 (6) (2000) 1273. [PMC free article] [PubMed] [Google Scholar]
- [20].Kane JC, Murray LK, Sughrue S, et al. , Process and implementation of audio computer assisted self-interviewing (ACASI) assessments in low resource settings: a case example from Zambia, Global Mental Health. (2016) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McMahon JH, Jordan MR, Kelley K, et al. , Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring, Clin. Infect. Dis 52 (4) (2011) 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Byrd KK, Hou JG, Hazen R, et al. , Antiretroviral adherence level necessary for HIV viral suppression using real-world data, J. Acquir. Immune Defic. Syndr 82 (3) (2019) 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vinikoor MJ, Schuttner L, Moyo C, et al. , Late refills during the first year of antiretroviral therapy predict mortality and program failure among HIV-infected adults in urban Zambia, AIDS Res. Hum. Retrovir 30 (1) (2014) 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Babor TF, de la Fuente JR, Saunders J, Grant M, The Alcohol Use Disorders Identification Test: Guidelines for use in, 2011.
- [25].Saunders JB, Aasland OG, Babor TF, Grant M, Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II, Addiction. 88 (6) (1993) 791–804. [DOI] [PubMed] [Google Scholar]
- [26].Chishinga N, Kinyanda E, Weiss HA, Patel V, Ayles H, Seedat S, Validation of brief screening tools for depressive and alcohol use disorders among TB and HIV patients in primary care in Zambia, BMC Psychiat. 11 (1) (2011) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Inoue S, Chitambi C, Vinikoor MJ, et al. , Testing the validity of the AUDIT-C and AUDIT-3 to detect unhealthy alcohol use among high-risk populations in Zambia: a secondary analysis from two randomized trials, Drug Alcohol Depend. 229 (2021), 109156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hahn JA, Anton RF, Javors MA, The formation, elimination, interpretation, and future research needs of Phosphatidylethanol for research studies and clinical practice, Alcohol. Clin. Exp. Res 40 (11) (2016) 2292–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hahn JA, Dobkin LM, Mayanja B, et al. , Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa, Alcohol. Clin. Exp. Res 36 (5) (2012) 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Anton RF, Commentary on: Ethylglucuronide and ethyl sulfate assays in clinical trials, interpretation 1 and limitations: results of a dose ranging alcohol challenge study and two clinical trials, Alcohol. Clin. Exp. Res 38 (7) (2014) 1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Radloff LS, The CES-D scale: a self-report depression scale for research in the general population, Appl. Psychol. Meas 1 (3) (1977) 385–401. [Google Scholar]
- [32].Vilagut G, Forero CG, Barbaglia G, Alonso J, Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): a systematic review with meta-analysis, PLoS One 11 (5) (2016), e0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kane JC, Van Wyk SS, Murray SM, et al. , Testing the effectiveness of a transdiagnostic treatment approach in reducing violence and alcohol abuse among families in Zambia: study protocol of the violence and alcohol treatment (VATU) trial, Global Mental Health. (2017) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mollica RF, Caspi-Yavin Y, Bollini P, Truong T, Tor S, Lavelle J, The Harvard trauma questionnaire: validating a cross-cultural instrument for measuring torture, trauma, and posttraumatic stress disorder in Indochinese refugees, J. Nerv. Ment. Dis 180 (2) (1992) 111–116. [PubMed] [Google Scholar]
- [35].Spitzer RL, Kroenke K, Williams JB, Lowe B, A brief measure for assessing generalized anxiety disorder: the GAD-7, Arch. Intern. Med 166 (10) (2006) 1092–1097. [DOI] [PubMed] [Google Scholar]
- [36].Humeniuk R, Ali R, Babor TF, et al. , Validation of the alcohol, smoking and substance involvement screening test (ASSIST), Addiction. 103 (6) (2008) 1039–1047. [DOI] [PubMed] [Google Scholar]
- [37].Kane JC, Murray LK, Bass JK, Johnson RM, Bolton P, Validation of a substance and alcohol use assessment instrument among orphans and vulnerable children in Zambia using audio computer assisted self-interviewing (ACASI), Drug Alcohol Depend. 166 (2016) 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Humeniuk R, Henry-Edwards S, Ali R, Poznyak V, Monteiro MG, Organization WH, The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Manual for Use in Primary Care, 2010.
- [39].Ravens-Sieberer U, Wille N, Badia X, et al. , Feasibility, reliability, and validity of the EQ-5D-Y: results from a multinational study, Qual. Life Res 19 (6) (2010) 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Vinikoor MJ, Zyambo Z, Muyoyeta M, Chander G, Saag MS, Cropsey K, Point-of-care urine ethyl glucuronide testing to detect alcohol use among HIV-hepatitis B virus Coinfected adults in Zambia, AIDS Behav. 22 (7) (2018) 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Murray LK, Haroz E, Dorsey S, Kane J, Bolton PA, Pullmann MD, Understanding mechanisms of change: an unpacking study of the evidence-based common-elements treatment approach (CETA) in low and middle income countries, Behav. Res. Ther 130 (2020), 103430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wutzke SE, Shiell A, Gomel MK, Conigrave KM, Cost effectiveness of brief interventions for reducing alcohol consumption, Soc. Sci. Med 52 (6) (2001) 863–870. [DOI] [PubMed] [Google Scholar]
- [43].Edejer TT-T, Baltussen R, Tan-Torres T, et al. , Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis vol. 1, World Health Organization, 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data were used for the research described in the article.


